Abstract
Sphingosine kinases (SK1 and SK2) are key, druggable targets within the sphingolipid metabolism pathway that promote tumor growth and pathologic inflammation. A variety of isozyme-selective and dual inhibitors of SK1 and SK2 have been described in the literature, and at least one compound has reached clinical testing in cancer patients. In this chapter, we will review the rationale for targeting SKs and summarize the preclinical and emerging clinical data for ABC294640 as the first-in-class selective inhibitor of SK2.
1. POTENTIAL DRUG TARGETS IN THE SPHINGOLIPID METABOLISM PATHWAY
Sphingolipid metabolism is a key pathway in cancer biology (Hait & Maiti, 2017; Lee & Kolesnick, 2017; Newton, Lima, Maceyka, & Spiegel, 2015; Ogretmen, 2018; Siddique, Li, Chaurasia, Kaddai, & Summers, 2015), in which ceramides, sphingosine, and sphingosine 1-phosphate (S1P) regulate tumor cell death, proliferation, and drug resistance, as well as host angiogenesis, inflammation, and immunity. As indicated in Fig. 1, ceramide is produced by the hydrolysis of sphingomyelin in response to several growth stimulatory (e.g., growth factors and oncoproteins) and inflammatory (e.g., cytokines and radiation) signals. Alternately, ceramide can be synthesized de novo proceeding through the precursor dihydroceramide, which is converted into ceramide by dihydroceramide desaturase (DES1). Ceramide induces apoptosis in tumor cells without disrupting quiescent normal cells (Kolesnick & Fuks, 2003). Ceramide is hydrolyzed by ceramidases to produce sphingosine, which is phosphorylated by sphingosine kinases (SK1 and SK2) to produce S1P. S1P is dephosphorylated by S1P phosphatase 1 and 2 and degraded by S1P lyase, which cleaves S1P yielding phosphoethanolamine and hexadecenal. In addition to intracellular targets, S1P binds to and activates a family of G protein-coupled receptors, i.e., S1P-receptor 1–5 (S1PR1–5), which mediate at least some of the biological activities of this lipid.
Figure 1. A simplified model of sphingolipid metabolism.
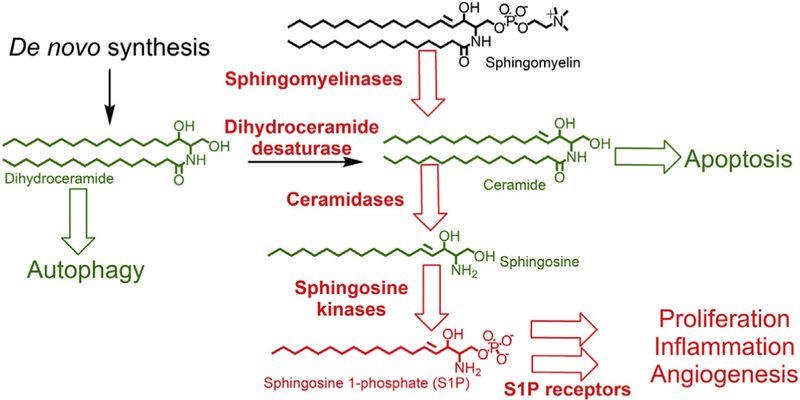
Enzymes and processes that promote tumor growth are shown in red, whereas lipids and processes that inhibit tumor growth are shown in green. Proteins that are currently under consideration as targets for new anticancer drugs include sphingomyelinases, dihydroceramide desaturase (DES1), ceramidases, sphingosine kinases, and sphingosine 1-phosphate (S1P) receptors.
Studies in many cancer cell lines indicate that S1P induces proliferation and protects against ceramide-induced apoptosis. Therefore, a critical balance, i.e., a ceramide/S1P rheostat, has been hypothesized to determine the fate of tumor cells (Spiegel & Milstien, 2002). Sphingolipids also regulate the sensitivities of tumor cells to anticancer drugs (Hendrich & Michalak, 2003; Sietsma, Veldman, & Kok, 2001). For example, ceramide increases apoptosis induced by paclitaxel (Lucci, Han, Liu, Giuliano, & Cabot, 1999), etoposide (Perry & Kolesnick, 2003), and gemcitabine (Guillermet-Guibert et al., 2009; Modrak, Cardillo, Newsome, Goldenberg, & Gold, 2004). Therefore, inhibition of ceramidase or SK is expected to increase tumor chemosensitivity by elevating ceramide levels in the cells. In addition to their direct effects on tumor cells, SKs regulate deleterious inflammation from cytokines such as tumor necrosis factor-alpha (TNFα) and IL-6 (Aoki, Aoki, Ramanathan, Hait, & Takabe, 2016; Chiurchiu, Leuti, & Maccarrone, 2018; Gomez-Munoz et al., 2016; Pettus, Chalfant, & Hannun, 2004; Snider, Orr Gandy, & Obeid, 2010). In particular, S1P is critical for the activation of granulocytes that escalate inflammatory processes in many cancers, especially during chemo- or radiotherapy. Therefore, manipulation of sphingolipid metabolism to elevate ceramide levels and/or to reduce S1P production is an increasingly important approach to the treatment of hyperproliferative and inflammatory diseases, including cancers.
Among the enzymes and receptors that metabolize or interact with sphingolipids, most drug development efforts have focused on inhibition of ceramidases, SKs, or S1PRs. Recent reviews discuss the roles and pharmacology of ceramidases in detail (Coant, Sakamoto, Mao, & Hannun, 2017; Saied & Arenz, 2016; Tan, Pearson, Feith, & Loughran, 2017). Additionally, S1PR biology and a diverse set of compounds that modulate S1PR signaling have been well discussed in several recent reviews (Hait & Maiti, 2017; Huwiler & Zangemeister-Wittke, 2017; Juif, Kraehenbuehl & Dingemanse, 2016; Mao-Draayer, Sarazin, Fox, & Schiopu, 2017; Patmanathan, Wang, Yap, Herr, & Paterson, 2017; Pyne, El Buri, Adams, & Pyne, 2017). Similarly, a number of excellent recent publications describe the molecular properties and functions of SKs (Haddadi, Lin, Simpson, Nassif, & McGowan, 2017; Pyne, Adams, & Pyne, 2016; Siow & Wattenberg, 2011; Song, Zhou, & Sheng, 2017) and provide comprehensive reviews of SK inhibitors (Aurelio et al., 2016; Cao et al., 2018; Hatoum, Haddadi, Lin, Nassif, & McGowan, 2017; Lynch, Thorpe, & Santos, 2016; Pitman, Costabile, & Pitson, 2016; Plano, Amin, & Sharma, 2014; Pyne, Adams, & Pyne, 2017; Pyne, Bittman, & Pyne, 2011; Sanllehi, Abad, Casas, & Delgado, 2016; Santos & Lynch, 2015). This chapter will not duplicate these contributions but rather will discuss some of the key issues that spotlight the potential utility of inhibiting SK activity in cancer patients and describe the preclinical and early clinical data relating to ABC294640, which is the first SK2-targeted drug to reach clinical testing in cancer patients.
2. SPHINGOSINE KINASES AS TARGETS FOR ANTICANCER DRUGS
SKs are important new targets for anticancer drugs for several reasons. First, conversion of sphingosine into S1P is a key site for manipulation of the ceramide/S1P rheostat that regulates tumor cell proliferation and death. Second, the production of S1P in response to inflammatory cytokines is dependent on SK activity (Billich et al., 2005; Hanna et al., 2001; Maines et al., 2008; Mastrandrea, Sessanna, & Laychock, 2005; Nayak et al., 2010; Radeff-Huang et al., 2007; Snider et al., 2010; Xia et al., 1998), typically through nuclear factor-kappa B (NF-kB) (Xia et al., 1998). Because inflammation is a driving force in many types of cancer, suppression of patient systemic or local inflammation is expected to reduce tumor growth. Finally, sphingolipids are likely to play central roles in regulating tumor immunology, although this potential has been sparsely addressed to date (Molino, Tate, McKillop, & Medin, 2017; Rodriguez et al., 2016; Sensken, Nagarajan, Bode, & Graler, 2011). In particular, we hypothesize that inhibition of SK activity will alter signaling through the PD-1/PD-L1 pathway such that the host immune response to the cancer is improved. This postulate is based on the observations that PD-L1 expression in tumor cells is induced by IFN-γ (Cheng et al., 2007; Dondero et al., 2016; Iwai et al., 2002; Muhlbauer et al., 2006), and IFN-γ signaling is dependent on sphingolipid metabolism (Bajwa et al., 2017; Ottenlinger et al., 2016; Seo, Alexander, & Hahm, 2011; Wakita, Nishimura, Tokura, Furukawa, & Takigawa, 1996). Furthermore, Akt- (Abdelhamed, Ogura, Yokoyama, Saiki, & Hayakawa, 2016; Atefi et al., 2014; Dong et al., 2016; Lastwika et al., 2016; Song et al., 2013; Yang et al., 2017; Zhao et al., 2017), NF-kB- (Gowrishankar et al., 2015), and TNFα- (Wang et al., 2017) signaling induce PD-L1 expression, and all of these pathways are regulated by sphingolipid signaling. Therefore, manipulation of tumor immunology using SK inhibitors is an unexplored potential new approach to improved cancer therapy.
2.1. Sphingosine Kinase 1-Derived S1P
SK1 is activated by numerous growth factors and cytokines including epidermal growth factor (EGF), platelet-derived growth factor, vascular endothelial growth factor, and TNFα (Yester, Tizazu, Harikumar, & Kordula, 2011), and by cross-linking of the high affinity receptor for IgE (FcεRI). Receptor stimulation results in ERK1/2-mediated phosphorylation of Ser225 of SK1, thereby increasing its activity and allowing its translocation to the plasma membrane (Pitson et al., 2003). Calcium and integrin-binding protein 1 bind the activated SK1 and facilitate this translocation ( Jarman, Moretti, Zebol, & Pitson, 2010) to the plasma membrane where it interacts with phosphatidylserine (Stahelin et al., 2005). The S1P produced there can be exported from the cell through ATP-binding cassette (ABC) transporters where it can act as an extracellular autocrine or paracrine signaling lipid. S1P binds five different G protein-coupled receptors named S1PR1–5.
S1PR1 couples exclusively to Gαi eliciting a number of responses. Importantly, signaling through S1PR1 is required for lymphocyte egress from lymph nodes and inhibition of SIPR1 function by FTY720 (fingolimod, Gilenya) via internalization sequesters lymphocytes in the lymphoid organs (Matloubian et al., 2004) and provides therapeutic benefit to patients with multiple sclerosis. Cross-linking of FcεRI in response to binding of IgE activates SK1 leading to autocrine signaling of S1P through both S1PR1 and S1PR2. Signaling through S1PR1 in response to FcεRI cross-linking leads to cytoskeleton rearrangement and migration toward the impetus ( Jolly et al., 2004).
S1PR2 couples to multiple Gα subunits, specifically i, q, and 12/13 (Windh et al., 1999). S1PR2 has frequently been associated with vascularization, and S1PR2 null mice exhibit disturbances in the stria vascularis, which forms the vasculature of the inner ear (Kono et al., 2007). S1PR2 was linked to pathological angiogenesis of the eye following ischemic injury by inhibiting the production of endothelial nitric oxide synthetase (eNOS) and promoting the expression of cyclooxygenase-2, thus driving the inflammatory process (Skoura et al., 2007). S1PR2 has been linked to inflammatory processes in other ways as well. Signaling through S1PR2 leads to mast cell degranulation, which leads to anaphylaxis and resultant pulmonary edema in mice. Pretreatment of mice with an S1PR2 antagonist, JTE-013, drastically attenuated anaphylaxis in response to stimuli due to an inhibition of mast cell degranulation (Oskeritzian et al., 2010). Conversely, S1PR2 also seems to be important for recovery from anaphylaxis. SK1 null mice and S1PR2 null mice have delayed clearance of histamine following anaphylaxis. In addition, they had severe hypotension during that time indicating that SK1-derived S1P signaling through S1PR2 impacts blood pressure control and recovery from anaphylaxis (Olivera et al., 2010).
Like S1PR2, S1PR3 couples to Gαi, Gαq, and Gα12/13. Activation of S1PR3 in response to the glucocorticoid dexamethasone in human fibroblasts results in activation of the PI3K/Akt prosurvival pathway, as well as inhibiting apoptosis through increased expression of the antiapoptotic Bcl-2 member Bcl-xL (Nieuwenhuis, Luth, & Kleuser, 2010). Like the other S1P receptors, S1PR3 has been implicated in the inflammatory process. S1P is released from the cell in response to signaling through the protease-activated receptor 1, and this leads to amplification of proinflammatory signaling through S1PR3 (Niessen et al., 2008). S1P signaling through S1PR3 has also been shown to impact vasoconstriction by increasing intracellular Ca2+ concentrations and signaling through Rho kinase in human coronary artery smooth muscle cells (Murakami et al., 2010).
S1PR4 couples to Gαi and Gα12/13 in response to S1P. Less is known about S1PR4, but unlike the other S1P receptors, it has been shown to have immunosuppressive effects. Specifically, signaling through S1PR4 was shown to inhibit the proliferation of T cells and their secretion of effector cytokines while stimulating the release of IL-10, a suppressive cytokine (Wang, Graeler, & Goetzl, 2005).
S1PR5, like S1PR4, couples to both Gαi and Gα12/13 in response to S1P. S1P signaling through S1PR5 was shown to block the migration of oligodendrocyte precursor cells suggesting that S1P is important in cellular communication events involved in brain development (Novgorodov, El-Alwani, Bielawski, Obeid, & Gudz, 2007). Signaling through S1PR5 also inhibits the motility of esophageal cancer cells (Hu et al., 2010). Conversely, signaling through S1PR5 has been shown to be necessary for the egress of natural killer cells from the bone marrow (Mayol, Biajoux, Marvel, Balabanian, & Walzer, 2011). Furthermore, S1P signaling through S1PR5 has been shown to trigger endoplasmic reticulum (ER) stress in the PC3 prostate cancer cell line leading to autophagic cell death (Huang et al., 2014).
SK1-derived S1P also has distinct intracellular functions. TNFa binding to TNF receptor 1 recruits TNF-associated factor 2 (TRAF2) to the intracellular domain of the receptor. TRAF2 can then bind SK1. The S1P produced by SK1 acts as a cofactor for TRAF2 activating its E3 ubiquitin ligase function. TRAF2 mediates the K63 polyubiquitination of receptor interacting protein 1 (RIP1). The polyubiquitination of RIP1 serves as a scaffold for TGFβ-activated kinase 1 and the IkB kinase complex allowing for the activation of NF-kB (Spiegel & Milstien, 2011). Interestingly, TNFα has also been shown to induce the degradation of SK1 in a cathepsin B-dependent manner (Taha et al., 2005).
2.2. Sphingosine Kinase 2-Derived S1P
Like SK1, SK2 catalyzes the phosphorylation of sphingosine; however, SK2 can also phosphorylate two additional sphingoid bases: dihydrosphingosine and phytosphingosine (Gault, Obeid, & Hannun, 2010). Mice with genetic deletion of SK1 (SK1‒/‒) or SK2 (SK2‒/‒) develop normally indicating that the isozymes are able to compensate for one another to some degree. However, knocking out both genes simultaneously is embryonic lethal (Mizugishi et al., 2005). There are five reported splice variants of SK2, and SK2 has been reported to localize to several subcellular compartments including the cytosol (Okada et al., 2005), the ER (Maceyka et al., 2005), and the mitochondria (Strub et al., 2011). However, the primary localization seems to be the nucleus due to a nuclear localization sequence (NLS) within SK2 (Igarashi et al., 2003). Because of the differences in subcellular localization of SK1 and SK2, pools of S1P produced by SK1 and SK2 are likely to have distinct biological functions.
SK2 has been reported to paradoxically inhibit or promote cell growth. SK2 was first reported to suppress growth and promote apoptosis when it was overexpressed. This activity is attributed to a putative BH3 domain within SK2 that is absent in SK1 and which can bind with Bcl-xL preventing its suppression of proapoptotic Bcl-2 family members such as Bax and Bak (Liu et al., 2003). Mutation of a single leucine residue in the BH3 domain of SK2 drastically decreased apoptosis. Alternately, knockdown of SK2 also results in increased apoptosis in glioblastoma cells (Van Brocklyn et al., 2005), colorectal carcinoma cells, and MCF7 breast cancer cells (Sankala et al., 2007). These seemingly conflicting effects are likely attributed to artifacts from the overexpression of SK2 thereby disrupting the regulation of apoptosis and the subcellular distribution of S1P (Sankala et al., 2007).
Like SK1, SK2 is activated by phosphorylation by Erk1/2 (Hait, Bellamy, Milstien, Kordula, & Spiegel, 2007). In the mitochondria, SK2-derived S1P binds to prohibitin 2, which regulates the assembly of complex IV (cytochrome-c oxidase) of the electron transport chain (Strub et al., 2011). S1P derived from SK2 has also been associated with the formation of amyloid plaques in Alzheimer’s disease. The major component of these plaques, amyloid-β, is a cleavage product of the amyloid-β precursor protein (APP). Cleavage of APP is carried out by the β-secretase β-site APP cleaving enzyme 1 (BACE1). SK2-derived S1P has been shown to bind the transmembrane domain of BACE1, which allosterically modifies the catalytic domain increasing its activity (Takasugi et al., 2011). Thus, overexpression of SK2 activity could contribute to Alzheimer’s disease.
Caspase 1-mediated cleavage of SK2 in cells undergoing apoptosis allows for the translocation of a still catalytically active fragment of SK2 to the plasma membrane where it binds phosphatidylserine and mediates the phosphorylation of sphingosine (Weigert et al., 2010). As with SK1-derived S1P, this S1P can be exported from the cell and serve as a chemoattractant for monocytes and macrophages by interacting with S1P receptors (Gude et al., 2008).
The unique nuclear localization of SK2 provides it with a role in epigenetic regulation of gene expression. For example, SK2 has been shown to bind histone H3 and impact its acetylation in the MCF7 breast cancer cell line (Hait et al., 2009). siRNA knockdown of SK2 was reported to decrease the acetylation of H3–K9, H4–K5, and H2B–K12, and addition of S1P to isolated nuclei of siSK2 cells resulted in restoration of acetylation of these three residues. This same study showed that S1P and dihydro-S1P were able to inhibit histone deacetylases 1 and 2, and that siRNA knockdown of SK2 enhanced histone deacetylase (HDAC) activity. Additionally, the authors showed that activation of SK2 by phorbol 12-myristate 13-acetate (PMA, a potent activator of protein kinase C, upstream of ERK1/2-medi-ated SK2 phosphorylation) enhanced the colocalization of SK2 with HDAC1. PMA is a known inducer of the transcription of the cyclin-dependent kinase inhibitor p21 and the transcription factor c-fos. When combined with siRNA knockdown of SK2, PMA failed to induce the transcription of p21 and c-fos. This correlated with a reduction in acetylation of H3 associated with the promoters of p21 and c-fos (Hait et al., 2009). This agreed with this lab’s earlier findings showing knockdown of SK2 prevented doxycycline-induced p21 expression (Sankala et al., 2007). These results are supported by Igarashi et al. who reported that overexpression of SK2 or SK1 with a fused NLS, but not native SK1, causes an inhibition of DNA synthesis (Igarashi et al., 2003). SK2-derived S1P has, thus, been demonstrated to impact gene transcription through regulation of HDAC activity.
Studies of the role of SK2 in carcinogenesis have also provided discordant results, with some research groups finding SK2 to be a tumor suppressor and others a mediator of tumor growth. For example, treatment of SK2‒/‒mice with the carcinogen azoxymethane and the inflammation-inducing toxin dextran sodium sulfate was reported to result in more numerous, larger, and more aggressive tumors than wild-type mice receiving the same treatments. This was reported to coincide with an increase in STAT3, NF-kB, and IL-6 expression in hematopoietic cells of the knockout mice, which drove the colitis and associated cancer. Conversely, SK2 has been described as oncogenic in acute lymphoblastic leukemia (ALL) because knockdown of SK2 or pharmacological inhibition using the inhibitor ABC294640 (discussed below) both attenuated development of leukemia in ALL mouse models (Wallington-Beddoe et al., 2014). This was attributed to a reduction in the expression of c-Myc (and thereby c-Myc target genes) by way of reduced acetylation levels of H3, linking SK2 to the expression this important oncogene. This same study also showed the importance of SK2 in the development of ALL. When BCR/ABL-transduced B-cell progenitor cells were implanted in 29 sublethally irradiated mice, 22 developed ALL with a median survival of 42 days. When the same BCR/ABL translocation was introduced to SK2‒/‒cells, which were then implanted in 29 sublethally irradiated mice, only 16 developed ALL and these experienced a median survival of 58 days. Cells recovered from the SK2‒/‒mice had reduced c-Myc expression compared with their wild-type counterparts (Wallington-Beddoe et al., 2014). The expression of SK2 was linked to actin rearrangement in MCF7 cells with expression promoting a structural rearrangement of actin into membrane ruffles/lamellipodia indicative of a more migratory phenotype (Lim, Sun, Bittman, Pyne, & Pyne, 2011). SK2 expression was also shown to negatively correlate with disease-free survival and overall survival in nonsmall cell lung cancer (NSCLC) patients (Wang et al., 2014). Additionally, inhibition of SK2 has delayed tumor growth in mouse xenograft models of pancreatic, kidney, liver, and colon cancers (Beljanski, Knaak, Zhuang, & Smith, 2011; Beljanski, Lewis, & Smith, 2011; Chumanevich et al., 2010).
Overall, the propensity of data indicates that SK2 is a key mediator of enhanced growth of cancer. It is likely that studies using SK2‒/‒mice do not reflect the normal biology of the critical SK node and clearly do not mimic the pharmacologic inhibition of SK2 because of the absence of the protein, which in and of itself is proapoptotic due to its endogenous BH3 domain. Similarly, studies in which SK2 is overexpressed by transfection are compromised by inappropriate subcellular distribution of SK2 (and its S1P product) and excessive proapoptotic pressure from the elevated presence of BH3 domains.
3. SPHINGOSINE KINASE INHIBITORS
Because of the importance of S1P in growth signaling, a great deal of research has been done with the aim of inhibiting S1P signaling. Numerous therapeutic strategies have been developed that target either one or both SKs, as well as those that inhibit S1PR signaling. As indicated above, these SK inhibitors have been extensively reviewed elsewhere, so only a few compounds are highlighted here. The earliest example of an SK inhibitor is N,N-dimethylsphingosine (DMS), a naturally occurring N-methyl derivative of sphingosine that inhibits both SK1 and SK2 with Kis of ~ 16 and ~ 14 µM, respectively (Gao, Peterson, Smith, & Smith, 2012). Dimethylsphingosine inhibits SK activity in human platelets preventing platelet aggregation in response to exogenous S1P (Yatomi et al., 1996) and induces apoptosis in leukemic and colonic carcinoma cell lines ( Jendiroba, Klostergaard, Keyhani, Pagliaro, & Freireich, 2002; Sweeney et al., 1996). Two additional sphingosine analogs, L-threo-dihydrosphingosine and N,N,N-trimethyl-sphingosine, have also been described as SK inhibitors; however, they also inhibit protein kinase C, which may be responsible for the observed phenotype (Canals & Hannun, 2013). The first nonlipid SK inhibitors were described in 2003, and the compound called SKI-II in particular has been widely used to probe SK involvement in many cell processes (French et al., 2003). SKI-II inhibits both isoforms with Kis of 16 µM (SK1) and 8 µM (SK2) (Gao et al., 2012), but it has also been shown to inhibit DES1 (Cingolani et al., 2014). Treatment of prostate cancer and breast cancer cells with SKI-II induces apoptosis in vitro (Antoon, Meacham, et al., 2011; Leroux et al., 2007) and in vivo (French et al., 2006). The compound PF-543, which is over 100-fold more selective for SK1 than SK2, has a Ki of 3.6 nM for SK1 (Schnute et al., 2012). Though it does not affect the growth of 1483 HNSCC cells, it was able to inhibit the formation of S1P in human whole blood ex vivo by >90% compared to control (Schnute et al., 2012). SK1-I is a specific competitive inhibitor of the ATP-binding site of SK1 with a Ki of 10 mM that has been shown to induce apoptosis in the U937 and Jurkat leukemic cell lines (Paugh et al., 2008). Treatment of these cells with SK1-I decreased ERK1/2 and Akt phosphorylation. Additionally, treatment of AML xenograft bearing mice with SK1-I reduced tumor growth (Paugh et al., 2008). SK1-I also decreased Akt activation and increased apoptosis in glioblastoma cell lines and slowed glioblastoma xenograft growth (Kapitonov et al., 2009). Importantly, in a murine model of breast cancer, treatment with SK1-I reduced metastasis and decreased overall tumor burden (Nagahashi et al., 2012), which was attributed to diminished angiogenesis caused by the suppression of circulating S1P. FTY720 is a structural analog of sphingosine. Following its phosphorylation by SK2, the resultant phospho-FTY720 can inhibit SK1 or exit the cell via ABC transporters to bind S1P receptors (except S1PR2). Binding results in the internalization of the receptor and prolonged receptor downregulation muting extracellular S1P signaling (Nagahashi et al., 2014). Prevention of S1P receptor signaling, mainly that of S1PR1, has a dramatic clinical impact in preventing the release of lymphocytes from lymphoid tissue thereby attenuating the autoimmune response responsible for multiple sclerosis (Allende, Dreier, Mandala, & Proia, 2004; Nagahashi et al., 2014). An analog, (R)-FTY720 methyl ester, was reported to have specific inhibitory activity against SK2, which prevented actin rearrangement in MCF7 cells in response to S1P thereby inhibiting motility (Lim et al., 2011).
3.1. Preclinical Development of ABC294640, the First-in-Class Inhibitor of SK2
Developed by Apogee Biotechnology Corporation, 3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)amide (ABC294640, Fig. 2) is an inhibitor of SK2 that is competitive with respect to sphingosine and therefore does not have off-target effects on protein kinases (French et al., 2010; Gao et al., 2012). In tissue culture, ABC294640 reduces S1P levels (French et al., 2010; Gao et al., 2012; Maines, French, Wolpert, Antonetti, & Smith, 2006; Maines et al., 2008), suppresses the proliferation of many tumor cell lines, and inhibits tumor cell migration concomitant with loss of microfilaments. ABC294640 depletes S1P and elevates ceramide in tumor cells, suppresses signaling through pERK, pAkt, and NF-kB, and promotes autophagy and/or apoptosis in a broad range of cancer cells (Antoon, White, et al., 2011; Beljanski, Knaak, & Smith, 2010; Ding et al., 2016; French et al., 2010; Gao et al., 2012; Qin et al., 2014; Schrecengost, Keller, Schiewer, Knudsen, & Smith, 2015). Importantly, ABC294640 downregulates the expression of c-Myc in a variety of tumor cell lines (Lewis, Voelkel-Johnson, & Smith, 2016; Schrecengost et al., 2015; Venant et al., 2015; Venkata et al., 2014; Wallington-Beddoe et al., 2014) and also reduces androgen receptor expression in prostate cancer cells (Schrecengost et al., 2015; Venant et al., 2015). In addition to SK2, ABC294640 inhibits DES1, which accounts for the marked increases in dihydroceramides in cells treated with the drug (French et al., 2010; Venant et al., 2015). ABC294640 is synergistically cytotoxic with gemcitabine toward pancreatic cancer cell lines and results in decreased expression of both RRM2 and MYC, decreased S780 phosphorylation of Rb, and increased acetylation of H3-K9 and p21 (Lewis et al., 2016). The reduction in Rb phosphorylation is consistent with studies by Kraveka et al. who demonstrated that inhibition of DES1 is associated with reduction of Rb phosphorylation and suppression of cell cycle progression (Kraveka et al., 2007). Fig. 2 describes the current model in which ABC294640 dually inhibits SK2 and DES1 resulting in inhibition of multiple signaling pathways that promote tumor growth.
Figure 2. Mechanism for Suppression of Tumor Growth by ABC294640.
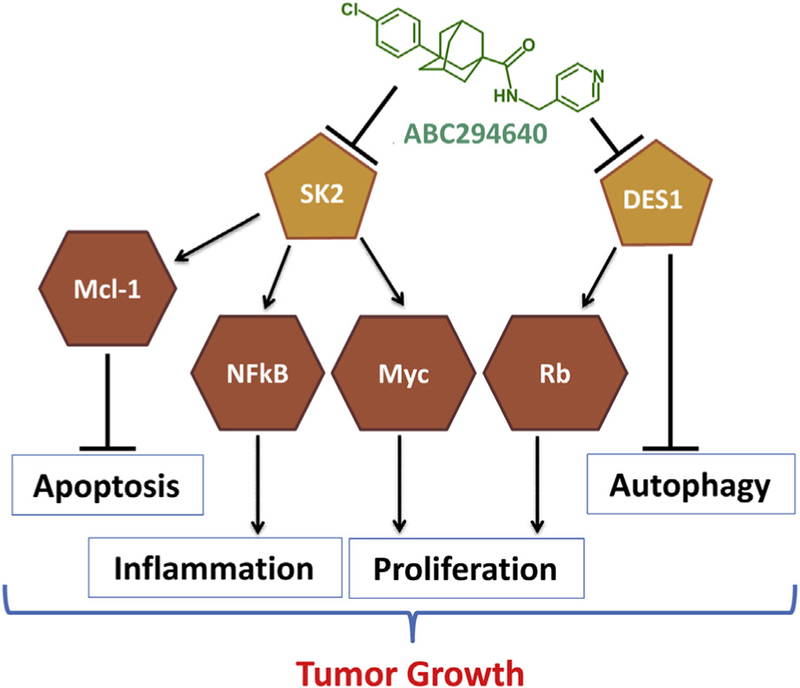
ABC294640 has similar potency toward sphingosine kinase (SK2) and dihydroceramide desaturase (DES1). Inhibition of SK2 activity causes downregulation of the expression of myeloid cell leukemia 1 (Mcl-1) and c-Myc and suppresses the activation of nuclear factor-kappa B (NF-kB), thereby promoting apoptosis and inhibiting proliferation and inflammation. In parallel, inhibition of DES1 by ABC294640 promotes autophagy and promotes Rb phosphorylation resulting in suppression of proliferation.
As summarized below, ABC294640 has therapeutic activity in diverse mouse tumor models both alone and in combination with other anticancer drugs (Antoon, White, Driver, Burow, & Beckman, 2012; Antoon et al., 2010; Antoon, White, et al., 2011; Beljanski et al., 2010; Beljanski, Knaak, et al., 2011; Beljanski, Lewis, et al., 2011; Dai et al., 2017; Dai, Smith, Foroozesh, Miele, & Qin, 2018; French et al., 2010; Qin et al., 2014; Schrecengost et al., 2015; Venant et al., 2015; Venkata et al., 2014; Wallington-Beddoe et al., 2014; Xu et al., 2018; Xun et al., 2015; Zhou, Chen, & Yu, 2018), as well as a number of rodent inflammation models (Fitzpatrick, Green, Frauenhoffer, et al., 2011; Fitzpatrick, Green, Maines, & Smith, 2011; Liu et al., 2012; Maines et al., 2008; Maines, Fitzpatrick, Green, Zhuang, & Smith, 2010; Maines et al., 2006; Poti et al., 2012; Shi et al., 2012) not discussed here. Additionally, ABC294640 diminished tumor incidence and multiplicity in the azoxymethane/dextran sulfate sodium model of colon carcinogenesis (Chumanevich et al., 2010). Antitumor activity is associated with accumulation of ABC294640 in the tumors, reduction of tumor S1P levels, and induction of apoptosis (French et al., 2010). Intratumoral concentrations of ABC294640 can exceed the IC50 for tumor cell cytotoxicity by as much as 25-fold (French et al., 2010), and ABC294640 reduces plasma S1P levels ~ 50% at therapeutically efficacious doses (Beljanski, Lewis, et al., 2011). Preclinical studies demonstrated an excellent oral bioavailability and safety profile for ABC294640, with no hematologic or major organ toxicity (French et al., 2010).
The in vivo anticancer activity of ABC294640 was first shown by inhibition of tumor growth in a syngeneic JC (breast cancer) xenograft model in BALB/c mice (French et al., 2010). In additional studies in breast cancer models, ABC294640 has been shown to bind to the antagonist ligand-binding domain of the estrogen receptor thereby inhibiting the growth of the estrogen receptor positive breast cancer cell line MCF7 both in vitro and in vivo (Antoon et al., 2010). ABC294640 was also effective at inducing apoptotic cell death in triple-negative MCF7 cells (MCF7-TN-R) by diminishing prosurvival signaling through the NF-kB pathway (Antoon, White, et al., 2011). In vitro levels of apoptosis were further increased by addition of either etoposide or doxorubicin after pretreatment with ABC294640. Additionally, Ki67 staining was decreased in MCF7-TN-R orthotopic tumors treated with ABC294640 when compared to control. However, ABC294640 is ineffective against the luminal, endocrine-resistant MDA-MB-361 breast cancer cell line, although the drug did reduce Bcl-2 in MCF7 tumors (Antoon et al., 2012).
A growing body of evidence indicates that ABC294640 may be particularly effective for the treatment of prostate cancer. Initially, ABC294640 was also shown to inhibit proliferation in androgen-resistant PC-3 and LNCaP prostate cancer cell lines (Gestaut, Antoon, Burow, & Beckman, 2014). Schrecengost et al. (2015) then showed that ABC294640 abrogates signaling pathways requisite for prostate cancer growth and proliferation. Of particular importance, ABC294640 treatment of early-stage and advanced prostate cancer models caused downregulation of c-Myc and androgen receptors (both full-length in LNCaP cells and the splice variant in 22Rv1 cells) expression and activity. This corresponded with significant inhibition of growth, proliferation, and cell-cycle progression. Finally, oral administration of ABC294640 was found to dramatically impede xenograft tumor growth. Studies by Venant et al. provided further confirmation of activity toward prostate cancer cells in association with decreased expression of both c-Myc and androgen receptors (Venant et al., 2015). Furthermore, that study confirmed that ABC294640 inhibits DES1 activity resulting in marked increases in dihydroceramides both in tissue culture and in xenografts. Studies by McNaughton et al. examined the effects of SKI-II and ABC294640 in androgen-independent LNCaP-AI prostate cancer cells and demonstrated that these compounds suppress both SK1 and DES1 by inducing their proteolysis (McNaughton, Pitman, Pitson, Pyne, & Pyne, 2016). Overall, the combined effects of ABC294640 on SK1, SK2, DES1, and androgen receptors in androgen-sensitive and castrate-resistant prostate cancer cells and xenografts build a strong rationale for examining the therapeutic effects of ABC294640 in prostate cancer patients.
ABC294640 has been shown to promote autophagic cell death in xenografts of A-498 (kidney cancer) cells in SCID mice as evidenced by in-creases in Beclin-1 and the cleavage of the microtubule-associated proteins 1A/1B light chain 3A (LC3). This same study demonstrated a decrease of MAPK and Akt pathway activation in response to ABC294640 in vivo (Beljanski et al., 2010). Similarly, ABC294640 caused pronounced apoptosis, cell-cycle arrest, and inhibition of NSCLC growth in vitro and in vivo (Dai et al., 2018). This is consistent with a previous report that ABC294640 enhances the antitumor effects of TRAIL in NSCLC cells, likely by enhancing the translocation of death receptor 4/5 to the cell membrane (Yang et al., 2015). Leili et al. demonstrated that ABC294640 enhances doxorubicin-stimulated apoptosis in NSCLC cells, and that this is associated with suppression of the expression of survivin (Leili, Nasser, Nadereh, Siavoush, & Pouran, 2018). Also using lung cancer cells in vitro, Guan et al. demonstrated that apoptosis induced by ABC294640 can be suppressed by overexpression of glucosylceramide synthase (GCS) or enhanced by the GCS inhibitor PDMP (Guan, Liu, Yan, & Zhou, 2016). Studies by Xun et al. using transformed and primary colorectal cancer cells demonstrated that these cells are also sensitive to growth inhibition by ABC294640 that could be amplified by combination with 5-fluorouracil and cisplatin and attenuated by cotreatment with a JNK inhibitor (Xun et al., 2015). Similarly, ABC294640 promoted apoptosis in skin squamous cell carcinoma cells in vitro and inhibited tumor growth in vivo associated with inhibition of Akt and JNK signaling (Zhou et al., 2018).
The potential use of ABC294640 in treating cancers of hematopoietic origin has also been demonstrated. Treatment of primary effusion lymphoma (PEL) cells positive for Kaposi’s sarcoma-associated herpesvirus (KSHV) (BCBL-1 cell line) with ABC294640 induced dose-dependent apoptosis that corresponded with decreases in phosphorylation of ERK1/2, Akt, and NF-kB (Qin et al., 2014). When these cells were injected into NOD/SCID mice, ABC294640 treatment delayed disease progression as indicated by spleen size, weight gain, and ascites volume. ABC294640 treatment vastly attenuated the pathogenesis of KSHV+ PEL (Qin et al., 2014). Mechanistically, elevation of dihydroceramides was shown to induce apoptosis in KSHV-infected PEL cells through activation of KSHV lytic gene expression (Dai et al., 2015). Finally, impressive activity of ABC294640 toward virally induced cancers both in vitro and in vivo was also demonstrated by Dai, et al. who found that KSHV long-term-infected immortalized endothelial cells are efficiently killed by ABC294640, whereas noninfected cells were not (Dai et al., 2017).
ABC294640 has anticancer activity in models of multiple myeloma (MM). Treatment of both immortalized MM cell lines and freshly isolated CD138+ myeloma cells from myeloma patients resulted in inhibition of proliferation (Venkata et al., 2014). ABC294640 increased caspase 3 activation and caspase 9 cleavage in OPM1 MM cells indicative of apoptotic cell death. Treatment of MM cell lines with ABC294640 also increased mRNA and protein expression of the proapoptotic Bcl-2 family member Noxa, which is regulated by p53. The same study additionally showed that ABC294640 promotes proteosomal degradation of myeloid cell leukemia 1 (Mcl-1), a Bcl-2 family protein overexpressed in the majority of MM patients. Thus, ABC294640 shifts the balance of these apoptotic mediators in the direction of apoptosis. ABC294640 also enhanced the proteosomal degradation of c-Myc in the MM cells. Additionally, the authors demonstrated that ABC294640 induced apoptosis of MM cells even in the presence of bone marrow stromal cells, which support the growth of MM cells. These findings prompted in vivo studies using a luciferase expressing MM.1S cell line xenograft where ABC294640 suppressed tumor growth (Venkata et al., 2014).
ABC294640 has also been evaluated in preclinical testing against ALL (Wallington-Beddoe et al., 2014). ABC294640 inhibited proliferation and induced cell death in ALL cell lines and ALL patient samples but, importantly, not bone marrow mononuclear cells. Cell death in this instance was attributed to a mixed etiology, being caspase-independent but not completely autophagic. Next, a gene expression profile of the impact of ABC294640 on gene expression was compiled, which revealed an inhibition of c-Myc and c-Myc-regulated genes. MYC downregulation in response to ABC294640 treatment was confirmed by qRT-PCR. This same study showed that inhibition of SK2 by ABC294640 prevented S1P-mediated inhibition of HDAC activity resulting in a reduction in the association of c-Myc with histone 3 acetylated on lysine 9 and thereby a reduction in c-Myc expression. Xenografts of ALL in NOD/SCID mice treated with ABC294640 had significantly less disease than vehicle control following 21 days of treatment (Wallington-Beddoe et al., 2014).
Combination of ABC294640 with other anticancer drugs has shown promising results. Combination of ABC294640 with sorafenib resulted in synergistic cell killing in A-498 kidney carcinoma and BxPC-3 pancreatic adenocarcinoma cells by means of apoptosis, and the combination attenuated the growth of xenografts of each cell line in SCID mice to a greater degree than either drug alone (Beljanski, Knaak, et al., 2011). This same combination proved effective in two hepatocellular carcinoma xenografts with the mode of cell death being autophagic (Beljanski, Lewis, et al., 2011). As with the A-498 and BxPC-3 cells, ABC294640 decreased the levels of p-Erk1/2 provoking the idea that ABC294640-mediated cell death could come as a result of diminished MAPK signaling. Similarly, combination of ABC294640 with sorafenib caused synergistic apoptosis of cholangiocarcinoma cells, and this was associated with marked suppression of STAT3 phosphorylation (Ding et al., 2016). Combination of ABC294640 with the microtubule stabilizer paclitaxel was shown to greatly increase caspase-9 signaling and apoptosis when compared with either drug alone (White, Chan, Antoon, & Beckman, 2013). ABC294640 has also been combined with doxorubicin, vincristine, imatinib, and bortezomib in ALL cells (Wallington-Beddoe et al., 2014). Only additive effects were observed with the DNA intercalating agent doxorubicin and the mitosis inhibitor vincristine. However, synergistic cell death was produced with the protea-some inhibitor bortezomib and the multityrosine kinase inhibitor (i.e., Bcr-Abl inhibitor) imatinib when either was combined with ABC294640 (Wallington-Beddoe et al., 2014). ABC294640 treatment of cervical carcinoma cells promoted apoptosis that was amplified by combination with the BCL-2 inhibitor GDC-0199 both in vitro and in vivo (Xu et al., 2018).
3.2. Phase I Clinical Trial of ABC294640
A phase I clinical trial of ABC294640 in patients with advanced solid tumors has been completed (Britten et al., 2017). ABC294640 was administered orally on a continuous schedule, with 28 days constituting a Cycle. Tumors were reimaged every two Cycles (8 weeks), and patients were allowed to continue receiving the drug if there was no disease progression by RECIST tumor imaging criteria. Twenty-one patients received the drug in this phase I trial. The first patient on study at 250 mg qd developed grade 4 hyperglycemia in the setting of rapidly progressing pancreatic cancer; however, no other patients experienced possibly drug-related hyperglycemia. Among the four patients enrolled at 750 mg bid, one ovarian cancer patient had dose-limiting grade 3 nausea and vomiting, and two patients were unable to complete Cycle 1 due to diverse possibly drug-related toxicities. One patient at the expanded 500 mg dose level was dose reduced at Cycle 3 due to grade 1 adverse events. The other five patients at 500 mg bid tolerated the drug well, and the 500 mg bid dose level was established as the recommended phase II dose. Across all dose levels, the most common drug-related toxicities were nausea, fatigue, vomiting, and diarrhea. In addition, 2 patients experienced psychiatric dis-orders including agitation/anxiety, mood changes, and/or hallucinations; and 5 patients experienced grade 1–2 nervous system disorders, including dizziness, dysarthria, dysgeusia, dysesthesia, memory loss, muscle spasms, paresthesia, somnolence, and/or spasticity that resolved on discontinuation of ABC294640.
Among the patients evaluable by RECIST, 1 (6%, a patient with refractory cholangiocarcinoma) had a Partial Response, 6 (38%) had Stable Disease, and 9 (56%) had Progressive Disease as their best response. Subjects included a patient with advanced hepatocellular carcinoma who lived for 10 months after completing 4 cycles; a patient with recurrent metastatic bladder cancer who received 12 cycles; and a patient with advanced cholangiocarcinoma who received 18 cycles.
Pharmacokinetic (PK) profiling was conducted on Days 1 and 28 of Cycle 1, and data from patients treated with 500 mg of ABC294640 are shown in Fig. 3. After oral dosing, plasma levels of ABC294640 typically peaked at 1–2 h, and then declined with a halftime of clearance of ~ 4 h. The peak plasma concentrations (Cmax) and AUCs within a cohort were generally similar. Notably, the Cmax and AUC values for most patients were not significantly different between Day 1 and Day 28, indicating a lack of metabolic adaptation to the drug. Importantly, Cmax levels of ABC294640 in patients receiving 500 mg were in the range expected to have therapeutic activity. Specifically, antitumor activity in mouse models occurs at ABC294640 doses of 25–50 mg/kg, which results in plasma Cmax levels of ~3.5 µg/mL (French et al., 2010). Patients receiving a 500 mg dose of ABC294640 had Cmax levels averaging 16.4 µM (Fig. 3), with 9 of 12 profiles exceeding the threshold of 9 µM. Patients given 250 mg of ABC294640 exceeded the Cmax threshold approximately 50% of the time, whereas all patients receiving 750 mg of ABC294640 reached this level. Because the t1/2s for clearance are equal in mice and humans, the drug exposure in patients at 500 mg and higher dose levels is expected to be sufficient to inhibit SK2 in the tumors.
Figure 3. Pharmacokinetics of ABC294640 in humans.
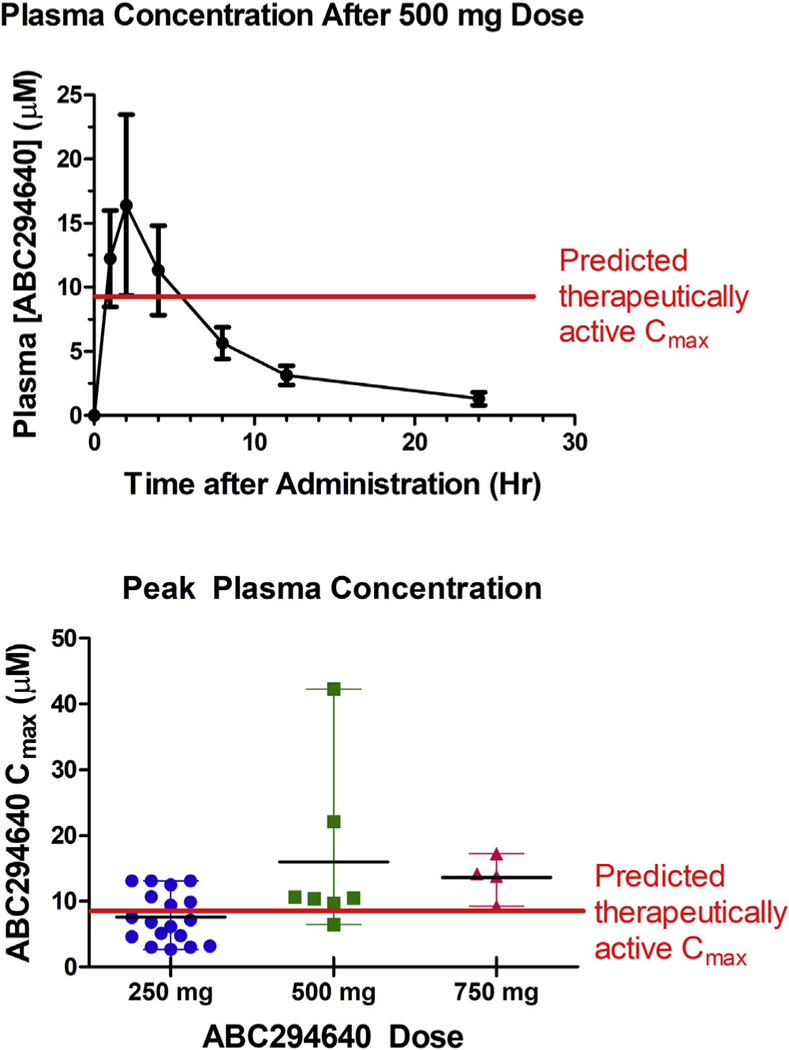
Top Panel. Plasma was isolated from patients given 500 mg of ABC294640 on either Day 1 or Day 28 of Cycle 1 and levels of ABC294640 were quantified using a GLP LC/MS method. Values represent the mean ± SEM for 7 data sets. Antitumor activity in mouse models occurs at ABC294640 doses ≥ 25 mg/kg, which provides plasma Cmax levels of 9 µM. The horizontal line indicates the Cmax predicted to have antitumor activity based on the mouse tumor models. Bottom Panel. Cmax values are shown for patient samples collected on either Day 1 or Day 28 (there is no change in Cmax or AUC in this period). The horizontal line indicates the Cmax predicted to have antitumor activity based on the mouse tumor models.
The most direct pharmacodynamic biomarkers for inhibition of SK2 and DES1 by ABC294640 are S1P and dihydroceramide levels in the tumor. However, tumor biopsies were not obtained in the phase I clinical trial, so sphingolipids in the plasma were measured as a biomarker for ABC294640. In the patients, ABC294640 treatment caused rapid decreases in plasma S1P levels with a parallel increase in plasma dihy-droC16-ceramide levels (Fig. 4). In general, plasma S1P reached a minimum approximately 12 h after ABC294640 treatment and recovered to baseline by 24 h. This is consistent with the clearance profile of the drug and supports the BID dosing schedule going forward into phase II clinical trials. The maximum S1P decreases for the 250, 500, and 750 mg cohorts were 51 ± 6% (n = 10), 46 ± 9% (n = 5), and 54 ± 14% (n = 2), respectively, indicating that ABC294640 exposures attained with the 250 mg dose are sufficient to optimally inhibit S1P generation in the patients.
Figure 4. Effect of ABC294640 treatment on plasma sphingosine 1-phosphate (S1P) levels.
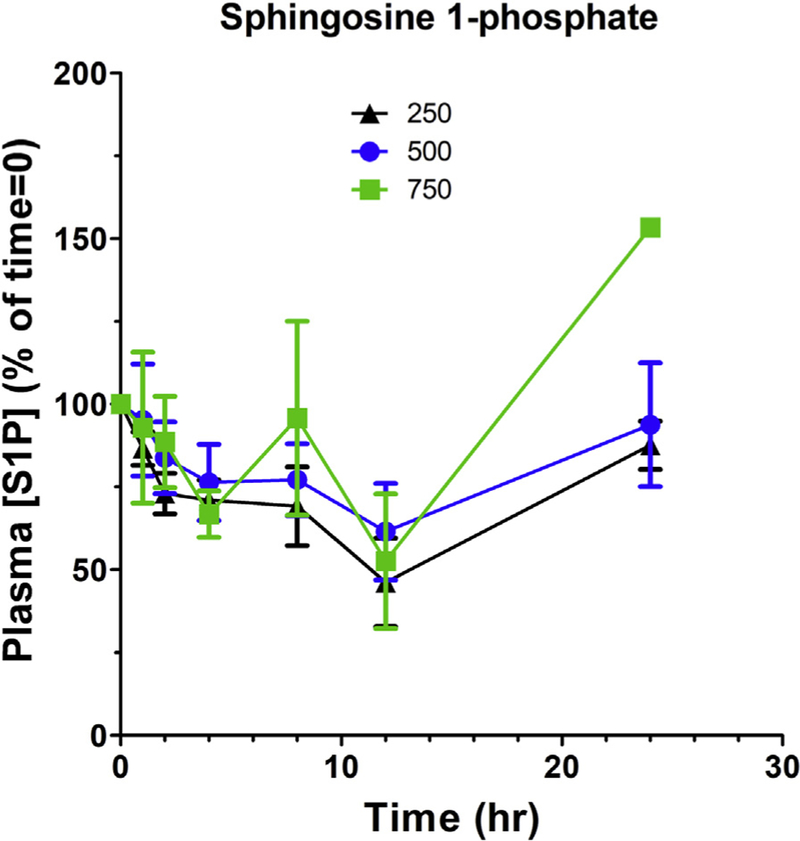
S1P concentrations were normalized to the Time 0 levels for individual patients receiving 250 mg ( ), 500 mg (
), 500 mg ( ), or 750 mg (
), or 750 mg ( ) of ABC294640.
) of ABC294640.
4. CONCLUSIONS
Continuing research on the sphingolipid metabolism pathway is providing increasing support for targeted disruption of key enzymes in this pathway for the treatment of cancer and inflammatory diseases. Important differences in the biology and pharmacology of SK1 and SK2 have been revealed by insightful basic and translational research and provide rationale for continuing the movement of novel inhibitors into clinical testing. Ultimately, the efficacies and safety of these new drugs will reveal whether or not targeting sphingolipid metabolism provides therapeutic benefit to patients. Clinical testing of ABC294640 is continuing with trials in patients having MM, cholangiocarcinoma, or hepatocellular carcinoma currently underway.
ACKNOWLEDGMENTS
Financial support for the authors was provided by grant P01 CA203628.
REFERENCES
- Abdelhamed S, Ogura K, Yokoyama S, Saiki I, & Hayakawa Y (2016). AKT-STAT3 pathway as a downstream target of EGFR signaling to regulate PD-L1 expression on NSCLC cells. Journal of Cancer, 7(12), 1579–1586. 10.7150/jca.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allende ML, Dreier JL, Mandala S, & Proia RL (2004). Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. Journal of Biological Chemistry, 279(15), 15396–15401. 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- Antoon JW, Meacham WD, Bratton MR, Slaughter EM, Rhodes LV, Ashe HB, et al. (2011). Pharmacological inhibition of sphingosine kinase isoforms alters estrogen receptor signaling in human breast cancer. Journal of Molecular Endocrinology, 46(3), 205–216. 10.1530/JME-10-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoon JW, White MD, Driver JL, Burow ME, & Beckman BS (2012). Sphingosine kinase isoforms as a therapeutic target in endocrine therapy resistant luminal and basal-A breast cancer. Experimental Biology and Medicine (Maywood), 237(7), 832–844. 10.1258/ebm.2012.012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoon JW, White MD, Meacham WD, Slaughter EM, Muir SE, Elliott S, et al. (2010). Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology, 151(11), 5124–5135. 10.1210/en.2010-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoon JW, White MD, Slaughter EM, Driver JL, Khalili HS, Elliott S, et al. (2011). Targeting NFkB mediated breast cancer chemoresistance through selective inhibition of sphingosine kinase-2. Cancer Biology & Therapy, 11(7), 678–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki M, Aoki H, Ramanathan R, Hait NC, & Takabe K (2016). Sphingosine-1-phos-phate signaling in immune cells and inflammation: Roles and therapeutic potential. Mediators of Inflammation, 2016, 8606878 10.1155/2016/8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, et al. (2014). Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clinical Cancer Research, 20(13), 3446–3457. 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurelio L, Scullino CV, Pitman MR, Sexton A, Oliver V, Davies L, et al. (2016). From sphingosine kinase to dihydroceramide desaturase: A structure-activity relationship (SAR) study of the enzyme inhibitory and anticancer activity of 4-((4-(4-Chlorophenyl) thiazol-2-yl)amino)phenol (SKI-II). Journal of Medicinal Chemistry, 59(3), 965–984. 10.1021/acs.jmedchem.5b01439. [DOI] [PubMed] [Google Scholar]
- Bajwa A, Huang L, Kurmaeva E, Ye H, Dondeti KR, Chroscicki P, et al. (2017). Sphingosine kinase 2 deficiency attenuates kidney fibrosis via IFN-gamma. Journal of the American Society of Nephrology: JASN, 28(4), 1145–1161. 10.1681/ASN.2016030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beljanski V, Knaak C, & Smith CD (2010). A novel sphingosine kinase inhibitor induces autophagy in tumor cells. The Journal of Pharmacology and Experimental Therapeutics, 333(2), 454–464. 10.1124/jpet.109.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beljanski V, Knaak C, Zhuang Y, & Smith CD (2011). Combined anticancer effects of sphingosine kinase inhibitors and sorafenib. Investigational New Drugs, 29(6), 1132–1142. 10.1007/s10637-010-9452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beljanski V, Lewis CS, & Smith CD (2011). Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Cancer Biology & Therapy, 11(5), 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Mechtcheriakova D, Natt F, Huesken D, & Baumruker T (2005). Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: Function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cellular Signalling, 17(10), 1203–1217. [DOI] [PubMed] [Google Scholar]
- Britten CD, Garrett-Mayer E, Chin SH, Shirai K, Ogretmen B, Bentz TA, et al. (2017). A phase I study of ABC294640, a first-in-class sphingosine kinase-2 inhibitor, in patients with advanced solid tumors. Clinical Cancer Research, 23(16), 4642–4650. 10.1158/1078-0432.CCR-16-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals D, & Hannun YA (2013). Novel chemotherapeutic drugs in sphingolipid cancer research. Handbook of Experimental Pharmacology, 211–238. 10.1007/978-3-7091-1368-4_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Ji C, Zhou Y, Huang W, Ni W, Tong X, et al. (2018). Sphingosine kinase inhibitors: A patent review. International Journal of Molecular Medicine, 41(5), 2450–2460. 10.3892/ijmm.2018.3505. [DOI] [PubMed] [Google Scholar]
- Cheng X, Zhao Z, Ventura E, Gran B, Shindler KS, & Rostami A (2007). The PD-1/PD-L pathway is up-regulated during IL-12-induced suppression of EAE mediated by IFN-gamma. Journal of Neuroimmunology, 185(1–2), 75–86. 10.1016/j.jneuroim.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiu V, Leuti A, & Maccarrone M (2018). Bioactive lipids and chronic inflammation: Managing the fire within. Frontiers in Immunology, 9, 38 10.3389/fimmu.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumanevich AA, Poudyal D, Cui X, Davis T, Wood PA, Smith CD, et al. (2010). Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis, 31(10), 1787–1793. 10.1093/carcin/bgq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani F, Casasampere M, Sanllehi P, Casas J, Bujons J, & Fabrias G (2014). Inhibition of dihydroceramide desaturase activity by the sphingosine kinase inhibitor SKI II. The Journal of Lipid Research, 55(8), 1711e1720 10.1194/jlr.M049759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coant N, Sakamoto W, Mao C, & Hannun YA (2017). Ceramidases, roles in sphingolipid metabolism and in health and disease. Advances in Biological Regulation, 63, 122–131. 10.1016/j.jbior.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Bai A, Smith CD, Rodriguez PC, Yu F, & Qin Z (2017). ABC294640, a novel sphingosine kinase 2 inhibitor, induces oncogenic virus-infected cell autophagic death and represses tumor growth. Molecular Cancer Therapeutics, 16(12), 2724–2734. 10.1158/1535-7163.MCT-17-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Smith CD, Foroozesh M, Miele L, & Qin Z (2018). The sphingosine kinase 2 in-hibitor ABC294640 displays anti-non-small cell lung cancer activities in vitro and in vivo. International Journal of Cancer, 142(10), 2153–2162. 10.1002/ijc.31234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Trillo-Tinoco J, Bai A, Chen Y, Bielawski J, Del Valle L, et al. (2015). Ceramides promote apoptosis for virus-infected lymphoma cells through induction of ceramide synthases and viral lytic gene expression. Oncotarget, 6(27), 24246–24260. 10.18632/oncotarget.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Chaiteerakij R, Moser CD, Shaleh H, Boakye J, Chen G, et al. (2016). Antitumor effect of the novel sphingosine kinase 2 inhibitor ABC294640 is enhanced by inhibition of autophagy and by sorafenib in human cholangiocarcinoma cells. Oncotarget 10.18632/oncotarget.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondero A, Pastorino F, Della Chiesa M, Corrias MV, Morandi F, Pistoia V, et al. (2016). PD-L1 expression in metastatic neuroblastoma as an additional mechanism for limiting immune surveillance. OncoImmunology, 5(1), e1064578 10.1080/2162402X.2015.1064578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Lv H, Li W, Song Z, Li L, Zhou S, et al. (2016). Co-expression of PD-L1 and p-AKT is associated with poor prognosis in diffuse large B-cell lymphoma via PD-1/PD-L1 axis activating intracellular AKT/mTOR pathway in tumor cells. Oncotarget, 7(22), 33350–33362. 10.18632/oncotarget.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick LR, Green C, Frauenhoffer EE, French KJ, Zhuang Y, Maines LW, et al. (2011). Attenuation of arthritis in rodents by a novel orally-available inhibitor of sphingosine kinase. Inflammopharmacology, 19(2), 75–87. 10.1007/s10787-010-0060-6. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LR, Green C, Maines LW, & Smith CD (2011). Experimental osteoarthritis in rats is attenuated by ABC294640, a selective inhibitor of sphingosine kinase-2. Pharmacology, 87(3–4), 135–143. 10.1159/000323911. [DOI] [PubMed] [Google Scholar]
- French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, et al. (2003). Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Research, 63(18), 5962–5969. [PubMed] [Google Scholar]
- French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, & Smith CD (2006). Antitumor activity of sphingosine kinase inhibitors. The Journal of Pharmacology and Experimental Therapeutics, 318(2), 596–603. 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, et al. (2010). Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. The Journal of Pharmacology and Experimental Therapeutics, 333(1), 129–139. 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Peterson YK, Smith RA, & Smith CD (2012). Characterization of isoenzyme-selective inhibitors of human sphingosine kinases. PLoS One, 7(9), e44543 10.1371/journal.pone.0044543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault CR, Obeid LM, & Hannun YA (2010). An overview of sphingolipid metabolism: From synthesis to breakdown. Advances in Experimental Medicine and Biology, 688, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestaut MM, Antoon JW, Burow ME, & Beckman BS (2014). Inhibition of sphingosine kinase-2 ablates androgen resistant prostate cancer proliferation and survival. Pharmacological Reports: PR, 66(1), 174–178. 10.1016/j.pharep.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Gomez-Munoz A, Presa N, Gomez-Larrauri A, Rivera IG, Trueba M, & Ordonez M (2016). Control of inflammatory responses by ceramide, sphingosine 1-phosphate and ceramide 1-phosphate. Progress in Lipid Research, 61, 51–62. 10.1016/j.plipres.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Gowrishankar K, Gunatilake D, Gallagher SJ, Tiffen J, Rizos H, & Hersey P (2015). Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-kappaB. PLoS One, 10(4), e0123410 10.1371/journal.pone.0123410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S, Liu YY, Yan T, & Zhou J (2016). Inhibition of ceramide glucosylation sensitizes lung cancer cells to ABC294640, a first-in-class small molecule SphK2 inhibitor. Biochemical and Biophysical Research Communications, 476(4), 230–236. 10.1016/j.bbrc.2016.05.102. [DOI] [PubMed] [Google Scholar]
- Gude DR, Alvarez SE, Paugh SW, Mitra P, Yu J, Griffiths R, et al. (2008). Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. The FASEB Journal, 22(8), 2629–2638. 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillermet-Guibert J, Davenne L, Pchejetski D, Saint-Laurent N, Brizuela L, Guilbeau-Frugier C, et al. (2009). Targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug. Molecular Cancer Therapeutics, 8(4), 809–820. 10.1158/1535-7163.MCT-08-1096. [DOI] [PubMed] [Google Scholar]
- Haddadi N, Lin Y, Simpson AM, Nassif NT, & McGowan EM (2017). “Dicing and splicing” sphingosine kinase and relevance to cancer. International Journal of Molecular Sciences, 18(9). 10.3390/ijms18091891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. (2009). Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science, 325(5945), 1254–1257. 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Bellamy A, Milstien S, Kordula T, & Spiegel S (2007). Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. Journal of Biological Chemistry, 282(16), 12058–12065. 10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
- Hait NC, & Maiti A (2017). The role of sphingosine-1-phosphate and ceramide-1-phosphate in inflammation and cancer. Mediators of Inflammation, 2017, 4806541 10.1155/2017/4806541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna AN, Berthiaume LG, Kikuchi Y, Begg D, Bourgoin S, & Brindley DN (2001). Tumor necrosis factor-alpha induces stress fiber formation through ceramide production: Role of sphingosine kinase. Molecular Biology of the Cell, 12(11), 3618–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatoum D, Haddadi N, Lin Y, Nassif NT, & McGowan EM (2017). Mammalian sphingosine kinase (SphK) isoenzymes and isoform expression: Challenges for SphK as an oncotarget. Oncotarget, 8(22), 36898–36929. 10.18632/oncotarget.16370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich AB, & Michalak K (2003). Lipids as a target for drugs modulating multidrug resistance of cancer cells. Current Drug Targets, 4(1), 23–30. [DOI] [PubMed] [Google Scholar]
- Hu WM, Li L, Jing BQ, Zhao YS, Wang CL, Feng L, et al. (2010). Effect of S1P5 on proliferation and migration of human esophageal cancer cells. World Journal of Gastroenterology: WJG, 16(15), 1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Chang CL, Tang CH, Lin YC, Ju TK, Huang WP, et al. (2014). Extrinsic sphingosine 1-phosphate activates S1P5 and induces autophagy through generating endoplasmic reticulum stress in human prostate cancer PC-3 cells. Cellular Signalling, 26(3), 611–618. 10.1016/j.cellsig.2013.11.024. [DOI] [PubMed] [Google Scholar]
- Huwiler A, & Zangemeister-Wittke U (2017). The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: Recent findings and new perspectives. Pharmacology & Therapeutics 10.1016/j.pharmthera.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, & Nakamura S (2003). Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. Journal of Biological Chemistry, 278(47), 46832–46839. 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, & Minato N (2002). Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immuno-therapy by PD-L1 blockade. Proceedings of the National Academy of Sciences of the United States of America, 99(19), 12293–12297. 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman KE, Moretti PA, Zebol JR, & Pitson SM (2010). Translocation of sphingosine kinase 1 to the plasma membrane is mediated by calcium- and integrin-binding protein 1. Journal of Biological Chemistry, 285(1), 483–492. 10.1074/jbc.M109.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendiroba DB, Klostergaard J, Keyhani A, Pagliaro L, & Freireich EJ (2002). Effective cytotoxicity against human leukemias and chemotherapy-resistant leukemia cell lines by N-N-dimethylsphingosine. Leukemia Research, 26(3), 301–310. [DOI] [PubMed] [Google Scholar]
- Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, et al. (2004). Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. The Journal of Experimental Medicine, 199(7), 959–970. 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juif PE, Kraehenbuehl S, & Dingemanse J (2016). Clinical pharmacology, efficacy, and safety aspects of sphingosine-1-phosphate receptor modulators. Expert Opinion on Drug Metabolism and Toxicology, 12(8), 879e895 10.1080/17425255.2016.1196188. [DOI] [PubMed] [Google Scholar]
- Kapitonov D, Allegood JC, Mitchell C, Hait NC, Almenara JA, Adams JK, et al. (2009). Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Research, 69(17), 6915–6923. 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick R, & Fuks Z (2003). Radiation and ceramide-induced apoptosis. Oncogene, 22(37), 5897–5906. 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, et al. (2007). Deafness and stria vascularis defects in S1P2 receptor-null mice. Journal of Biological Chemistry, 282(14), 10690–10696. 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- Kraveka JM, Li L, Szulc ZM, Bielawski J, Ogretmen B, Hannun YA, et al. (2007). Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. Journal of Biological Chemistry, 282(23), 16718–16728. 10.1074/jbc.M700647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, et al. (2016). Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Research, 76(2), 227–238. 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- Lee WK, & Kolesnick RN (2017). Sphingolipid abnormalities in cancer multidrug resistance: Chicken or egg? Cellular Signalling, 38, 134–145. 10.1016/j.cellsig.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leili H, Nasser S, Nadereh R, Siavoush D, & Pouran K (2018). Sphingosine kinase-2 inhibitor ABC294640 enhances doxorubicin-induced apoptosis of NSCLC cells via altering survivin expression. Drug Research, 68(1), 45–53. 10.1055/s-0043-117181. [DOI] [PubMed] [Google Scholar]
- Leroux ME, Auzenne E, Evans R, Hail N Jr., Spohn W, Ghosh SC, et al. (2007). Sphingolipids and the sphingosine kinase inhibitor, SKI II, induce BCL-2-independent apoptosis in human prostatic adenocarcinoma cells. The Prostate, 67(15), 1699–1717. 10.1002/pros.20645. [DOI] [PubMed] [Google Scholar]
- Lewis CS, Voelkel-Johnson C, & Smith CD (2016). Suppression of c-Myc and RRM2 expression in pancreatic cancer cells by the sphingosine kinase-2 inhibitor ABC294640. Oncotarget, 7(37), 60181–60192. 10.18632/oncotarget.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KG, Sun C, Bittman R, Pyne NJ, & Pyne S (2011). (R)-FTY720 methyl ether is a specific sphingosine kinase 2 inhibitor: Effect on sphingosine kinase 2 expression in HEK 293 cells and actin rearrangement and survival of MCF-7 breast cancer cells. Cellular Signalling, 23(10), 1590–1595. 10.1016/j.cellsig.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, et al. (2003). Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. Journal of Biological Chemistry, 278(41), 40330e40336 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- Liu Q, Rehman H, Shi Y, Krishnasamy Y, Lemasters JJ, Smith CD, et al. (2012). Inhibition of sphingosine kinase-2 suppresses inflammation and attenuates graft injury after liver transplantation in rats. PLoS One, 7(7), e41834 10.1371/journal.pone.0041834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucci A, Han TY, Liu YY, Giuliano AE, & Cabot MC (1999). Modification of ceramide metabolism increases cancer cell sensitivity to cytotoxics. International Journal of Oncology, 15(3), 541–546. [DOI] [PubMed] [Google Scholar]
- Lynch KR, Thorpe SB, & Santos WL (2016). Sphingosine kinase inhibitors: A review of patent literature (2006e2015). Expert Opinion on Therapeutic Patents, 26(12), 1409–1416. 10.1080/13543776.2016.1226282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, et al. (2005). SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. Journal of Biological Chemistry, 280(44), 37118–37129. 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- Maines LW, Fitzpatrick LR, French KJ, Zhuang Y, Xia Z, Keller SN, et al. (2008). Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Digestive Diseases and Sciences, 53(4), 997–1012. 10.1007/s10620-007-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines LW, Fitzpatrick LR, Green CL, Zhuang Y, & Smith CD (2010). Efficacy of a novel sphingosine kinase inhibitor in experimental Crohn’s disease. Inflammopharmacology, 18(2), 73–85. 10.1007/s10787-010-0032-x. [DOI] [PubMed] [Google Scholar]
- Maines LW, French KJ, Wolpert EB, Antonetti DA, & Smith CD (2006). Pharmacologic manipulation of sphingosine kinase in retinal endothelial cells: Implications for angiogenic ocular diseases. Investigative Ophthalmology & Visual Science, 47(11), 5022–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao-Draayer Y, Sarazin J, Fox D, & Schiopu E (2017). The sphingosine-1-phosphate receptor: A novel therapeutic target for multiple sclerosis and other autoimmune diseases. Clinical Immunology, 175, 10–15. 10.1016/j.clim.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrandrea LD, Sessanna SM, & Laychock SG (2005). Sphingosine kinase activity and sphingosine-1 phosphate production in rat pancreatic islets and INS-1 cells: Response to cytokines. Diabetes, 54(5), 1429e1436. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. (2004). Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature, 427(6972), 355–360. 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Mayol K, Biajoux V, Marvel J, Balabanian K, & Walzer T (2011). Sequential desensitization of CXCR4 and S1P5 controls natural killer cell trafficking. Blood, 118(18), 4863–4871. 10.1182/blood-2011-06-362574. [DOI] [PubMed] [Google Scholar]
- McNaughton M, Pitman M, Pitson SM, Pyne NJ, & Pyne S (2016). Proteasomal degradation of sphingosine kinase 1 and inhibition of dihydroceramide desaturase by the sphingosine kinase inhibitors, SKi or ABC294640, induces growth arrest in androgen-independent LNCaP-AI prostate cancer cells. Oncotarget, 7(13), 16663–16675. 10.18632/oncotarget.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, & Proia RL (2005). Essential role for sphingosine kinases in neural and vascular development. Molecular and Cellular Biology, 25(24), 11113–11121. 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrak DE, Cardillo TM, Newsome GA, Goldenberg DM, & Gold DV (2004). Synergistic interaction between sphingomyelin and gemcitabine potentiates ceramide-mediated apoptosis in pancreatic cancer. Cancer Research, 64(22), 8405–8410. [DOI] [PubMed] [Google Scholar]
- Molino S, Tate E, McKillop WM, & Medin JA (2017). Sphingolipid pathway enzymes modulate cell fate and immune responses. Immunotherapy, 9(14), 1185–1198. 10.2217/imt-2017-0089. [DOI] [PubMed] [Google Scholar]
- Muhlbauer M, Fleck M, Schutz C, Weiss T, Froh M, Blank C, et al. (2006). PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. Journal of Hepatology, 45(4), 520–528. 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Murakami A, Takasugi H, Ohnuma S, Koide Y, Sakurai A, Takeda S, et al. (2010). Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: Investigation based on a new S1P3 receptor antagonist. Molecular Pharmacology, 77(4), 704e 10.1124/mol.109.061481. [DOI] [PubMed] [Google Scholar]
- Nagahashi M, Hait NC, Maceyka M, Avni D, Takabe K, Milstien S, et al. (2014). Sphingosine-1-phosphate in chronic intestinal inflammation and cancer. Advances in Biological Regulation, 54, 112e120 10.1016/j.jbior.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, et al. (2012). Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Research, 72(3), 726–735. 10.1158/0008-5472.CAN-11-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D, Huo Y, Kwang WX, Pushparaj PN, Kumar SD, Ling EA, et al. (2010). Sphingosine kinase 1 regulates the expression of proinflammatory cytokines and nitric oxide in activated microglia. Neuroscience, 166(1), 132–144. 10.1016/j.neuroscience.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Newton J, Lima S, Maceyka M, & Spiegel S (2015). Revisiting the sphingolipid rheostat: Evolving concepts in cancer therapy. Experimental Cell Research, 333(2), 195–200. 10.1016/j.yexcr.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, et al. (2008). Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature, 452(7187), 654–658. 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis B, Luth A, & Kleuser B (2010). Dexamethasone protects human fibroblasts from apoptosis via an S1P3-receptor subtype dependent activation of PKB/Akt and Bcl XL. Pharmacological Research: The Official Journal of the Italian Pharmacological Society, 61(5), 449–459. 10.1016/j.phrs.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Novgorodov AS, El-Alwani M, Bielawski J, Obeid LM, & Gudz TI (2007). Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. The FASEB Journal, 21(7), 1503e1514 10.1096/fj.06-7420com. [DOI] [PubMed] [Google Scholar]
- Ogretmen B (2018). Sphingolipid metabolism in cancer signalling and therapy. Nature Reviews Cancer, 18(1), 33–50. 10.1038/nrc.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOkada T, Ding G, Sonoda H, Kajimoto T, Haga Y, Khosrowbeygi A, et al. (2005). Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. Journal of Biological Chemistry, 280(43), 36318–36325. 10.1074/jbc.M504507200. [DOI] [PubMed] [Google Scholar]
- Olivera A, Eisner C, Kitamura Y, Dillahunt S, Allende L, Tuymetova G, et al. (2010). Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. Journal of Clinical Investigation, 120(5), 1429–1440. 10.1172/JCI40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskeritzian CA, Price MM, Hait NC, Kapitonov D, Falanga YT, Morales JK, et al. (2010). Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. The Journal of Experimental Medicine, 207(3), 465–474. 10.1084/jem.20091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenlinger FM, Mayer CA, Ferreiros N, Schreiber Y, Schwiebs A, Schmidt KG, et al. (2016). Interferon-Beta increases plasma ceramides of specific chain length in multiple sclerosis patients, unlike fingolimod or natalizumab. Frontiers in Pharmacology, 7, 412 10.3389/fphar.2016.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patmanathan SN, Wang W, Yap LF, Herr DR, & Paterson IC (2017). Mechanisms of sphingosine 1-phosphate receptor signalling in cancer. Cellular Signalling, 34, 66–75. 10.1016/j.cellsig.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Paugh SW, Paugh BS, Rahmani M, Kapitonov D, Almenara JA, Kordula T, et al. (2008). A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood, 112(4), 1382–1391. 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DK, & Kolesnick RN (2003). Ceramide and sphingosine 1-phosphate in anti-cancer therapies. Cancer Treatment and Research, 115, 345–354. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Chalfant CE, & Hannun YA (2004). Sphingolipids in inflammation: Roles and implications. Current Molecular Medicine, 4(4), 405–418. [DOI] [PubMed] [Google Scholar]
- Pitman MR, Costabile M, & Pitson SM (2016). Recent advances in the development of sphingosine kinase inhibitors. Cellular Signalling, 28(9), 1349–1363. 10.1016/j.cellsig.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, et al. (2003). Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. The EMBO Journal, 22(20), 5491–5500. 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plano D, Amin S, & Sharma AK (2014). Importance of sphingosine kinase (SphK) as a target in developing cancer therapeutics and recent developments in the synthesis of novel SphK inhibitors. Journal of Medicinal Chemistry, 57(13), 5509–5524. 10.1021/jm4011687. [DOI] [PubMed] [Google Scholar]
- Poti F, Bot M, Costa S, Bergonzini V, Maines L, Varga G, et al. (2012). Sphingosine kinase inhibition exerts both pro- and anti-atherogenic effects in low-density lipoprotein receptor-deficient (LDL-R(−/−)) mice. Thrombosis & Haemostasis, 107(3), 552–561. 10.1160/TH11-08-0583. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Adams DR, & Pyne S (2017). Sphingosine kinase 2 in autoimmune/inflammatory disease and the development of sphingosine kinase 2 inhibitors. Trends in Pharmacological Sciences, 38(7), 581–591. 10.1016/j.tips.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, El Buri A, Adams DR, & Pyne S (2017). Sphingosine 1-phosphate and cancer. Advances in Biological Regulation 10.1016/j.jbior.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Pyne S, Adams DR, & Pyne NJ (2016). Sphingosine 1-phosphate and sphingosine kinases in health and disease: Recent advances. Progress in Lipid Research, 62, 93–106. 10.1016/j.plipres.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Pyne S, Bittman R, & Pyne NJ (2011). Sphingosine kinase inhibitors and cancer: Seeking the golden sword of hercules. Cancer Research, 71(21), 6576–6582. 10.1158/0008-5472.can-11-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Dai L, Trillo-Tinoco J, Senkal C, Wang W, Reske T, et al. (2014). Targeting sphingosine kinase induces apoptosis and tumor regression for KSHV-associated primary effusion lymphoma. Molecular Cancer Therapeutics, 13(1), 154–164. 10.1158/1535-7163.MCT-13-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeff-Huang J, Seasholtz TM, Chang JW, Smith JM, Walsh CT, & Brown JH (2007). Tumor necrosis factor-alpha-stimulated cell proliferation is mediated through sphingosine kinase-dependent Akt activation and cyclin D expression. Journal of Biological Chemistry, 282(2), 863–870. [DOI] [PubMed] [Google Scholar]
- Rodriguez YI, Campos LE, Castro MG, Aladhami A, Oskeritzian CA, & Alvarez SE (2016). Sphingosine-1 phosphate: A new modulator of immune plasticity in the tumor microenvironment. Frontiers in Oncologyront Oncol, 6, 218 10.3389/fonc.2016.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saied EM, & Arenz C (2016). Inhibitors of ceramidases. Chemistry and Physics of Lipids, 197, 60–68. 10.1016/j.chemphyslip.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Sankala HM, Hait NC, Paugh SW, Shida D, Lepine S, Elmore LW, et al. (2007). Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Research, 67(21), 10466e10474 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- Sanllehi P, Abad JL, Casas J, & Delgado A (2016). Inhibitors of sphingosine-1-phos-phate metabolism (sphingosine kinases and sphingosine-1-phosphate lyase). Chemistry and Physics of Lipids, 197, 69–81. 10.1016/j.chemphyslip.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Santos WL, & Lynch KR (2015). Drugging sphingosine kinases. ACS Chemical Biology, 10(1), 225–233. 10.1021/cb5008426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnute ME, McReynolds MD, Kasten T, Yates M, Jerome G, Rains JW, et al. (2012). Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. The Biochemical Journal, 444(1), 79–88. 10.1042/BJ20111929. [DOI] [PubMed] [Google Scholar]
- Schrecengost RS, Keller SN, Schiewer MJ, Knudsen KE, & Smith CD (2015). Downregulation of critical oncogenes by the selective SK2 inhibitor ABC294640 hinders prostate cancer progression. Molecular Cancer Research, 13(12), 1591–1601. 10.1158/1541-7786.MCR-14-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensken SC, Nagarajan M, Bode C, & Graler MH (2011). Local inactivation of sphingosine 1-phosphate in lymph nodes induces lymphopenia. The Journal of Immunology: Official Journal of the American Association of Immunologists, 186(6), 3432e3440 10.4049/jimmunol.1002169. [DOI] [PubMed] [Google Scholar]
- Seo YJ, Alexander S, & Hahm B (2011). Does cytokine signaling link sphingolipid metabolism to host defense and immunity against virus infections? Cytokine & Growth Factor Reviews, 22(1), 55e61 10.1016/j.cytogfr.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Rehman H, Ramshesh VK, Schwartz J, Liu Q, Krishnasamy Y, et al. (2012). Sphingosine kinase-2 inhibition improves mitochondrial function and survival after hepatic ischemia-reperfusion. Journal of Hepatology, 56(1), 137–145. 10.1016/j.jhep.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique MM, Li Y, Chaurasia B, Kaddai VA, & Summers SA (2015). Dihydro-ceramides: From bit players to lead actors. Journal of Biological Chemistry, 290(25), 15371e 15379. 10.1074/jbc.R115.653204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sietsma H, Veldman RJ, & Kok JW (2001). The involvement of sphingolipids in multidrug resistance. The Journal of Membrane Biology, 181(3), 153–162. [DOI] [PubMed] [Google Scholar]
- Siow D, & Wattenberg B (2011). The compartmentalization and translocation of the sphingosine kinases: Mechanisms and functions in cell signaling and sphingolipid metabolism. Critical Reviews in Biochemistry and Molecular Biology, 46(5), 365–375. 10.3109/10409238.2011.580097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, & Hla T (2007). Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. Journal of Clinical Investigation, 117(9), 2506–2516. 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider AJ, Orr Gandy KA, & Obeid LM (2010). Sphingosine kinase: Role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie, 92(6), 707e715 10.1016/j.biochi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DD, Zhou JH, & Sheng R (2017). Regulation and function of sphingosine kinase 2 in diseases. Histology & Histopathology 10.14670/hh-11-939,11939. [DOI] [PubMed] [Google Scholar]
- Song M, Chen D, Lu B, Wang C, Zhang J, Huang L, et al. (2013). PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PLoS One, 8(6), e65821 10.1371/journal.pone.0065821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, & Milstien S (2002). Sphingosine 1-phosphate, a key cell signaling molecule. Journal of Biological Chemistry, 277(29), 25851–25854. [DOI] [PubMed] [Google Scholar]
- Spiegel S, & Milstien S (2011). The outs and the ins of sphingosine-1-phosphate in immunity. Nature Reviews Immunology, 11(6), 403e415 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahelin RV, Hwang JH, Kim JH, Park ZY, Johnson KR, Obeid LM, et al. (2005). The mechanism of membrane targeting of human sphingosine kinase 1. Journal of Biological Chemistry, 280(52), 43030–43038. 10.1074/jbc.M507574200. [DOI] [PubMed] [Google Scholar]
- Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, et al. (2011). Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. The FASEB Journal, 25(2), 600–612. 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney EA, Sakakura C, Shirahama T, Masamune A, Ohta H, Hakomori S, et al. (1996). Sphingosine and its methylated derivative N,N-dimethylsphingosine (DMS) induce apoptosis in a variety of human cancer cell lines. International Journal of Cancer, 66(3), 358–366. . [DOI] [PubMed] [Google Scholar]
- Taha TA, Kitatani K, Bielawski J, Cho W, Hannun YA, & Obeid LM (2005). Tumor necrosis factor induces the loss of sphingosine kinase-1 by a cathepsin B-dependent mechanism. Journal of Biological Chemistry, 280(17), 17196–17202. 10.1074/jbc.M413744200. [DOI] [PubMed] [Google Scholar]
- Takasugi N, Sasaki T, Suzuki K, Osawa S, Isshiki H, Hori Y, et al. (2011). BACE1 activity is modulated by cell-associated sphingosine-1-phosphate. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(18), 6850–6857. 10.1523/JNEUROSCI.6467-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SF, Pearson JM, Feith DJ, & Loughran TP Jr. (2017). The emergence of acid ceramidase as a therapeutic target for acute myeloid leukemia. Expert Opinion on Therapeutic Targets, 21(6), 583–590. 10.1080/14728222.2017.1322065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, & Prior TW (2005). Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: Roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. Journal of Neuropathology and Experimental Neurology, 64(8), 695–705. [DOI] [PubMed] [Google Scholar]
- AVenant H, Rahmaniyan M, Jones EE, Lu P, Lilly MB, Garrett-Mayer E, et al. (2015). The sphingosine kinase 2 inhibitor ABC294640 reduces the growth of prostate cancer cells and results in accumulation of dihydroceramides in vitro and in vivo. Molecular Cancer Therapeutics, 14(12), 2744–2752. 10.1158/1535-7163.MCT-15-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkata JK, An N, Stuart R, Costa LJ, Cai H, Coker W, et al. (2014). Inhibition of sphingosine kinase 2 downregulates the expression of c-Myc and Mcl-1 and induces apoptosis in multiple myeloma. Blood, 124(12), 1915–1925. 10.1182/blood-2014-03-559385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakita H, Nishimura K, Tokura Y, Furukawa F, & Takigawa M (1996). Inhibitors of sphingolipid synthesis modulate interferon (IFN)-gamma-induced intercellular adhesion molecule (ICAM)-1 and human leukocyte antigen (HLA)-DR expression on cultured normal human keratinocytes: Possible involvement of ceramide in biologic action of IFN-gamma. The Journal of Investigative Dermatology, 107(3), 336–342. [DOI] [PubMed] [Google Scholar]
- Wallington-Beddoe CT, Powell JA, Tong D, Pitson SM, Bradstock KF, & Bendall LJ (2014). Sphingosine kinase 2 promotes acute lymphoblastic leukemia by enhancing MYC expression. Cancer Research, 74(10), 2803–2815. 10.1158/0008-5472.CAN-13-2732. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li J, Li G, Li Y, Xu C, Li M, et al. (2014). Prognostic significance of sphingosine kinase 2 expression in non-small cell lung cancer. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine, 35(1), 363–368. 10.1007/s13277-013-1051-1. [DOI] [PubMed] [Google Scholar]
- Wang W, Graeler MH, & Goetzl EJ (2005). Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. The FASEB Journal, 19(12), 1731–1733. 10.1096/fj.05-3730fje. [DOI] [PubMed] [Google Scholar]
- Wang X, Yang L, Huang F, Zhang Q, Liu S, Ma L, et al. (2017). Inflammatory cytokines IL-17 and TNF-alpha up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunology Letters, 184, 7–14. 10.1016/j.imlet.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert A, Cremer S, Schmidt MV, von Knethen A, Angioni C, Geisslinger G, et al. (2010). Cleavage of sphingosine kinase 2 by caspase-1 provokes its release from apoptotic cells. Blood, 115(17), 3531–3540. 10.1182/blood-2009-10-243444. [DOI] [PubMed] [Google Scholar]
- White MD, Chan L, Antoon JW, & Beckman BS (2013). Targeting ovarian cancer and chemoresistance through selective inhibition of sphingosine kinase-2 with ABC294640. Anticancer Research, 33(9), 3573–3579. [PubMed] [Google Scholar]
- Windh RT, Lee MJ, Hla T, An S, Barr AJ, & Manning DR (1999). Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. Journal of Biological Chem-istry, 274(39), 27351–27358. [DOI] [PubMed] [Google Scholar]
- Xia P, Gamble JR, Rye KA, Wang L, Hii CS, Cockerill P, et al. (1998). Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proceedings of the National Academy of Sciences of the United States of America, 95(24), 14196–14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Jin L, Yang B, Wang L, Xia Z, Zhang Q, et al. (2018). The sphingosine kinase 2 inhibitor ABC294640 inhibits cervical carcinoma cell growth. Oncotarget, 9(2), 2384–2394. 10.18632/oncotarget.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun C, Chen MB, Qi L, Tie-Ning Z, Peng X, Ning L, et al. (2015). Targeting sphingosine kinase 2 (SphK2) by ABC294640 inhibits colorectal cancer cell growth in vitro and in vivo. Journal of Experimental & Clinical Cancer Research, 34, 94 10.1186/s13046-015-0205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yang C, Zhang S, Mei Z, Shi M, Sun S, et al. (2015). ABC294640, a sphingosine kinase 2 inhibitor, enhances the antitumor effects of TRAIL in non-small cell lung cancer. Cancer Biology & Therapy, 16(8), 1194–1204. 10.1080/15384047.2015.1056944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Huang F, Mei J, Wang X, Zhang Q, Wang H, et al. (2017). Posttranscriptional control of PD-L1 expression by 17beta-estradiol via PI3K/Akt signaling pathway in ERalpha-positive cancer cell lines. International Journal of Gynecological Cancer, 27(2), 196–205. 10.1097/IGC.0000000000000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatomi Y, Ruan F, Megidish T, Toyokuni T, Hakomori S, & Igarashi Y (1996). N,N-dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry, 35(2), 626–633. 10.1021/bi9515533. [DOI] [PubMed] [Google Scholar]
- Yester JW, Tizazu E, Harikumar KB, & Kordula T (2011). Extracellular and intra-cellular sphingosine-1-phosphate in cancer. Cancer and Metastasis Reviews, 30(3–4), 577–597. 10.1007/s10555-011-9305-0. [DOI] [PubMed] [Google Scholar]
- Zhao L, Li C, Liu F, Zhao Y, Liu J, Hua Y, et al. (2017). A blockade of PD-L1 produced antitumor and antimetastatic effects in an orthotopic mouse pancreatic cancer model via the PI3K/Akt/mTOR signaling pathway. OncoTargets and Therapy, 10, 2115–2126. 10.2147/OTT.S130481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Chen J, & Yu H (2018). Targeting sphingosine kinase 2 by ABC294640 inhibits human skin squamous cell carcinoma cell growth. Biochemical and Biophysical Research Communications, 497(2), 535–542. 10.1016/j.bbrc.2018.02.075. [DOI] [PubMed] [Google Scholar]


