Abstract
Polyacrylamide hydrogels have been widely used in stem cell mechanotransduction studies. Conventional conjugation methods of biochemical cues to polyacrylamide hydrogels suffer from low conjugation efficiency, which leads to poor attachment of human pluripotent stem cells (hPSCs) on soft substrates. In addition, while it is well-established that stiffness-dependent regulation of stem cell fate requires cytoskeletal tension, and is mediated through nuclear translocation of transcription regulator, Yes-associated protein (YAP), the role of biochemical cues in stiffness-dependent YAP regulation remains largely unknown. Here we report a method that enhances the conjugation efficiency of biochemical cues on polyacrylamide hydrogels compared to conventional methods. This modified method enables robust hPSC attachment, proliferation and maintenance of pluripotency across varying substrate stiffness (3 kPa to 38 kPa). Using this hydrogel platform, we demonstrate that varying the types of biochemical cues (Matrigel, laminin, GAG-peptide) or density of Matrigel can alter stiffness-dependent YAP localization in hPSCs. In particular, we show that stiffness-dependent YAP localization is overridden at low or high density of Matrigel. Furthermore, human mesenchymal stem cells display stiffness-dependent YAP localization only at intermediate fibronectin density. The hydrogel platform with enhanced conjugation efficiency of biochemical cues provides a powerful tool for uncovering the role of biochemical cues in regulating mechanotransduction of various stem cell types.
Keywords: hydrogels, stiffness, biochemical cues, stem cells, mechanotransduction, polyacrylamide
1. Introduction
Stem cells reside in a complex multifactorial niche that includes biochemical and mechanical cues[1–3]. Using biomaterials such as hydrogels as an artificial niche, recent studies have shown that stem cells can sense the stiffness of their niche, which in turn modulates stem cell lineage specification[1,4,5]. To elucidate the role of matrix stiffness in regulating stem cell fates, polyacrylamide hydrogels have been widely employed as substrates for stem cell culture given their ease of fabrication and tunable stiffness[6–11]. Substrate stiffness has been shown to regulate cellular adhesion, spreading, proliferation, and differentiation[12–15]. Specifically, substrates with stiffnesses mimicking distinct tissue types induce both adult and pluripotent stem cell (PSC) differentiation toward corresponding tissue lineages[6,16,17].
Stiffness-dependent regulation of stem cell fate requires cytoskeletal tension and is mediated through the activation and localization of the nuclear transcription regulator, Yes-associated protein (YAP)[18]. Previous studies have shown that stem cells cultured on stiff substrate organize F-actin bundles, generate cytoskeletal tension, which leads to translocation of YAP into nucleus for downstream gene activation for osteogenesis[18,19]. However, how varying the types and density of biochemical ligands impact stiffness-induced YAP translocation in stem cells remains unclear. Such gap in knowledge is in part due to the low conjugation efficiency of biochemical cues to polyacrylamide hydrogels, which limits the range of ligand density that can be tested. Unlike human mesenchymal stem cells (hMSCs), human pluripotent stem cells (hPSCs) require higher density of cell adhesion for efficient attachment and spreading. Due to the low protein conjugation efficiency using conventional protocol, hydrogels that support robust attachment of hMSCs were shown to be insufficient in supporting attachment of undifferentiated hPSCs on soft substrate[12,16]. As a result, previous mechanotransduction studies on stem cells mostly utilize hMSCs, and the progress in elucidating mechanotransduction in hPSC is limited due to the lack of biomaterials tool that supports robust hPSC attachment on substrate with tunable stiffness.
To provide cell adhesion cues on polyacrylamide hydrogels with tunable stiffness, current standard method utilizes a heterobifunctional crosslinker, sulfosuccinimidyl-6-(4'-azido-2'-nitrophenylamino) hexanoate (sulfo-SANPAH), to link proteins onto polyacrylamide hydrogels[6,9,20]. While this method supports adhesion of differentiated cells or adult stem cells[6–8], the conjugation efficiency is not high enough to support efficient attachment of human embryonic stem cells on soft polyacrylamide hydrogels coated with Matrigel[12,16]. As a bi-functional crosslinker, sulfo-SANPAH contains an NHS ester group for linking with the primary amine on proteins, and a phenyl azide group that can be photo-activated to react and immobilize to polyacrylamide hydrogel substrate. When activated, phenyl azide undergo ring expansion to form a nucleophile-reactive dehydroazepine, which has high reactivity with nucleophiles such as amines though it can also insert non-selectively at active carbon–hydrogen bonds with substantially lower efficiency[21]. For polyacrylamide hydrogels, the incorporation efficiency using sulfo-SANPAH is low due to the lack of nucleophiles. To enhance the protein conjugation efficiency to polyacrylamide hydrogels, a recent study used 2-pyridinecarboxyaldehyde for conjugating proteins to polyacrylamide hydrogels [22]. Pyridinecarboxyaldehyde was used to replace the NHS ester to improve the stability of the crosslinker. However, this method still uses phenyl azide for immobilization, and the limitation of low efficiency of phenyl azide incorporation onto polyacrylamide hydrogels remains. Furthermore, unreacted excessive aldehyde may lead to non-specific conjugation of other proteins present in the medium onto polyacrylamide hydrogels, causing undesirable fouling effects and making it difficult to interpret cell responses.
Here we report a strategy to substantially improve the conjugation efficiency of biochemical cues onto polyacrylamide hydrogels by introducing primary amines as nucleophiles onto polyacrylamide hydrogels. We hypothesized that addition of primary amine groups to polyacrylamide hydrogels would enhance incorporation of sulfo-SANPAH, thereby enhancing conjugation efficiency of proteins of interests. Conjugation efficiency of proteins on polyacrylamide hydrogel substrates was characterized and compared against the conventional protocol [9]. Subsequently, we examined the effect of the enhanced biochemical coating on the efficiency of supporting undifferentiated hPSC attachment, proliferation and maintenance of pluripotency. We further sought to elucidate the role of varying ligand types and density in modulating stiffness-induced cytoskeletal organization and YAP translocation in hPSCs and hMSCs.
2. Materials and methods
2.1. Fabrication of polyacrylamide hydrogels coated with varying types and dosage of biochemical cues.
Polyacrylamide hydrogels with three stiffnesses (3, 14 and 38 kPa) were fabricated by mixing acrylamide solution (Sigma A4058,40% (v/v)) and N,N′-methylenebisacrylamide solution (Sigma M1533, 2% (v/v)) to achieve the final concentrations of acrylamide (8% (v/v)) and bisacrylamide (0.08, 0.2, 0.48% (v/v)). To enhance the efficiency of protein conjugation, 2-aminoethyl methacrylate (Aldrich 516155, 15 mM in deionized water) was added to the polyacrylamide precursor solution. To initiate photocrosslinking, photoinitiator 2-hydroxy-1-[4-(2-hydroxyethoxy) phenyl]-2-methyl-1-propanone (Irgacure 2959, Ciba, 0.05% (w/v)) was also added to the precursor solution. Hydrogel precursor solution (65 μl) was loaded between two 15 mm glass coverslips and exposed to light (365 nm, 4 mW/cm2) for 5 min. After removing the bottom coverslip, the top coverslip-gel composite was rinsed in phosphate-buffered saline (PBS, pH 7.4, Life Technologies 10010). The hydrogel surface was subsequently modified with the heterobifunctional crosslinker sulfo-SANPAH (Life Technologies 22589, 0.83 mg/ml in PBS) to introduce NHS groups via exposure to light (365 nm, 4 mW/cm2) for 5 min. Next, the hydrogels were quickly washed with PBS and incubated overnight at 37 °C with solutions of the following protein types and concentrations: Matrigel (BD Biosciences 356230), 1:40 dilution in Knockout DMEM:F12 (Life Technologies 12660); rhLaminin (Corning 354221), 15 μg/ml in PBS; mouse laminin (Sigma L2020), 15 μg/ml in PBS; recombinant human vitronectin (Life Technologies A14700), 5 μg/ml in PBS; human collagen IV (Sigma 6745), 15 μg/ml in PBS; or human fibronectin (BD Biosciences 354008), 1, 4, 7, 10 μg/ml in PBS. Matrigel was also further diluted to 1:400 and 1:1000 to examine its dosage effect on modulating hPSC mechanosensing. For GAG peptide-conjugated polyacrylamide hydrogels, same process was followed but the crosslinker was switched from sulfo-SANPAH to sulfo-MBS (Life Technologies 22312, 10 mM in PBS). Sulfo-MBS solution (200 μl) was added and incubated for 30 min at 37 °C to introduce maleimide groups. Then, the gels were washed with PBS three times for 10 min. GAG-peptide solution (CGKKQRFRHRNRKG, Biomatik Corp., 1 mM in PBS, 150 μl) was loaded onto the hydrogels and incubated for 30 min at 37 °C. Hydrogels were washed with PBS to remove unconjugated peptide, then incubated with cysteine solution (0.1 M in PBS, 200 μl) for 30 min at 37 °C to block unreacted maleimide groups. After overnight incubation at 37 °C, hydrogels modified with various biochemical cues were washed with PBS and used for characterization or cell plating.
2.2. Mechanical testing.
The stiffnesses of polyacrylamide hydrogels were measured using atomic force microscopy (Park System NX10). Hydrogels were immersed in PBS and indented with a colloidal cantilever (CP-PNP-SiO, NanoAndMore) with a force constant of 0.08 N/m at an indentation speed of 1 μm/s. Young’s modulus was calculated by fitting the force-indentation profile (200 pN – 2 nN) to a Hertzian model[23].
2.3. Characterizing the effect of primary amine addition on protein conjugation.
To evaluate the efficiency of protein conjugation, FITC-BSA (Life Technologies A23015, 1 mg/ml in PBS) was used as a model protein, and was coated onto hydrogel with three stiffnesses (3, 14 and 38 kPa) following the protocol described above. To determine the effect of primary amine addition on protein conjugation efficiency, FITC-BSA was also conjugated onto polyacrylamide hydrogels with tunable stiffness following the conventional protocol without primary amine[9]. For fibronectin dosage experiment, immunostaining was performed on hydrogels coated with various fibronectin concentrations (1, 4, 7 and 10 μg/ml) using our modified protocol. Fibronectin coated with conventional protocol was also included as a control using 0 or 10 μg/ml protein. After overnight incubation with protein solution, the hydrogels were washed extensively with PBS. To visualize the fibronectin incorporation onto the hydrogels, hydrogels were stained for fibronectin according to the immunocytochemistry protocol below. Hydrogels were imaged in a gel imaging system using Alexa 488 (Bio-Rad ChemiDoc™ MP with Blue Epi Illumination). To analyze the images, a region of interest was selected and applied to each hydrogel to measure the fluorescence intensity of each hydrogel (n=3 per group) and the average of the mean gray value was plotted using Fiji.
2.4. Cell culture.
A human embryonic stem cell line, H9 (WiCell Research Institute) was used as a model cell line for hPSCs. H9 cells were expanded on Matrigel (1:40 in Knockout DMEM:F12)-coated tissue culture plates in mTeSR1 medium (Stemcell Technologies 05857). For plating cells on polyacrylamide hydrogels, H9 cells were detached from Matrigel-coated tissue culture plastic using Accutase (Stemcell Technologies 07920) for 5 min at 37 °C. Cells were resuspended in mTeSR1 medium and centrifuged at 200 × g for 5 min. Cells were then resuspended in mTeSR1 medium supplemented with thiazovivin (Stemgent, 2 uM in mTeSR1) and plated on the hydrogels at 8,000 cells/cm2. Cells were incubated at 37 °C with 5% CO2, and the medium was refreshed with mTeSR1 medium without thiazovivin daily. Human MSCs were purchased from Lonza and expanded in growth medium composed of Dulbecco’s Modified Eagle Medium (Gibco), fetal bovine serum (10% v/v, Gibco), penicillin-streptomycin (1% v/v, ThermoFisher Scientific), and recombinant human fibroblast growth factor-basic (10 ng/ml, Peprotech). Passage 6 hMSCs were plated at 2,500 cells/cm2 on the hydrogels. All stem cells were cultured on hydrogels coated with various biochemical cues and stiffnesses for 2 days before being fixed for outcome analyses using immunostaining.
2.5. Immunocytochemistry.
Cells were fixed with 4% paraformaldehyde for 15 min at room temperature, washed three times with washing buffer (0.1% Tween-20), and permeabilized with 1% Triton X-100 for 30 min at room temperature. Cells were then incubated with blocking buffer (3% BSA and 2% goat serum in PBS) for 30 min at room temperature. The following primary antibodies were used including rabbit Oct4 (Abcam 19857, 5 μg/ml), mouse Tra1-60 (Abcam 16288, 1:50 dilution), mouse YAP (Santa Cruz Biotechnology sc-101199, 1:300 dilution), rabbit Ki-67 (Abcam ab15580, 1:500 dilution), mouse vinculin (Abcam 18058, 1:100 dilution), rabbit fibronectin (Abcam ab2413, 1:100 dilution). Cells were incubated with primary antibody solution overnight at 4 °C or 1 h at room temperature on a shaker. Cells were washed with washing buffer for 5 min at room temperature three times, then incubated with secondary antibodies for 1 h at room temperature on a shaker. The following secondary antibodies were used including Alexa 488 Goat-antimouse (Invitrogen A11001, 1:300 dilution), rhodamine goat-anti-rabbit (Millipore AP132, 1:300 dilution). For cytoskeletal staining, cells were incubated with rhodamine-phalloidin (Sigma P1951, 1:100 dilution) for 1 h at room temperature. Cell nuclei were stained using Hoechst dye (Cell Signaling Technology 4082S, 2 μg/ml in blocking buffer). Results were imaged using a confocal microscope (40x oil immersion, Leica SP8 confocal system) and analyzed using open source Fiji software. To quantify nuclear YAP localization within a cell, we used the method outlined previously[24]. Briefly, percentage of nuclear YAP was assessed by measuring the fluorescent intensity of a region of the nucleus and that of a region with the equal size in the cytoplasm. The corresponding Hoechst staining image was used to delineate the nuclear regions. Quantifications were performed with at least 14 cells per group.
2.6. Quantification of H9 cell number over time.
To examine the effect of substrate stiffness on H9 proliferation, H9 cells were cultured on Matrigel-coated hydrogels with tunable stiffness (3, 14 and 38 kPa) and tissue culture plastic (TCP). Total DNA amount was quantified daily for up to 3 days. Briefly, H9 cells were detached from the substrate using Accutase and collected via centrifugation for 5 min at 200 × g. The supernatant was removed and cells were resuspended in Ultrapure RNase/DNase-free distilled water (Life Technologies 10977). Samples were frozen at −80 °C for 30 min, then thawed at room temperature for 30 min. The freeze-thaw cycle was repeated three times in total. After the last freeze-thaw cycle, the samples were centrifuged at 200 × g for 5 min and DNA-containing supernatant was saved for total DNA quantification via PicoGreen assay (Life Technologies P7589).
2.7. Statistics.
Data are presented as mean ± standard error of means. For between-group comparisons, data were analyzed with GraphPad Prism 6.01 using one-way ANOVA by Tukey’s multiple comparisons test or two-way ANOVA by Bonferroni’s multiple comparisons test. Confidence intervals were kept at 95%, and P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Primary amine enhanced the protein conjugation efficiency on polyacrylamide hydrogels
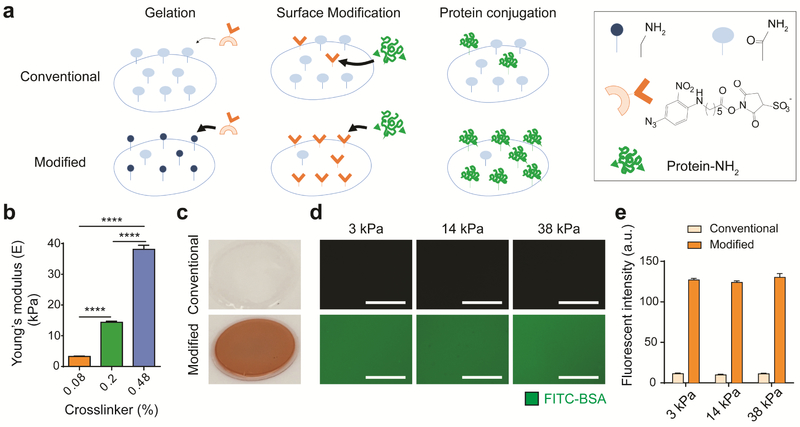
To enhance the conjugation efficiency of sulfo-SANPAH on polyacrylamide hydrogel substrates, primary amine groups were introduced on the hydrogel surface using 2-aminoethyl methacrylate. Given its nucleophilicity, the primary amine group has higher binding efficiency with the nitrophenyl azide group in sulfo-SANPAH than the amide group in unmodified polyacrylamide (Figure 1a). Using atomic force microscopy and rheological measurements, we selected three hydrogel formulations that captured a stiffness range (3, 14, and 38 kPa) comparable to that used in previous investigations (Figure 1b, Figure S1a)[12,16,17]. Furthermore, we confirmed that introducing primary amines by adding aminoethyl methacrylate into polyacrylamide precursor did not change the stiffness of the hydrogel (Figure S1b). Polyacrylamide hydrogels conjugated using conventional method showed clear color. In contrast, our modified method resulted in orange color of polyacrylamide hydrogels after sulfo-SANPAH incubation, indicating a much higher level of sulfo-SANPAH incorporation (Figure 1c). To quantify the amount of protein conjugation, fluorescein isothiocyanate-bovine serum albumin (FITC-BSA) was used as a model protein for conjugation. The modified polyacrylamide hydrogels led to 10-fold higher amount of fluorescence signals than conventional method (Figure 1d,e), suggesting enhanced sulfo-SANPAH incorporation through primary amine. In addition, we also collected the supernatant containing unconjugated sulfo-SANPAH and FITC-BSA from modified polyacrylamide hydrogels and conventional polyacrylamide hydrogels after overnight incubation. The supernatant was yellow from modified polyacrylamide hydrogels and orange from unmodified hydrogels (Figure S1c), further confirming that our modified method incorporated more sulfo-SANPAH onto the hydrogel.
Figure 1. Modified polyacrylamide hydrogel with primary amines significantly enhances protein conjugation efficiency regardless of hydrogel stiffness.
a Schematic of polyacrylamide hydrogel conjugation. On polyacrylamide hydrogels fabricated by the conventional method, only a few amide groups ( ) react with photoactivated nitrophenyl azide groups (
) react with photoactivated nitrophenyl azide groups ( ) from the sulfo-SANPAH crosslinker, leading to low protein conjugation efficiency. When primary amine groups (
) from the sulfo-SANPAH crosslinker, leading to low protein conjugation efficiency. When primary amine groups ( ) are introduced into the polyacrylamide hydrogel surface, photoactivated nitrophenyl azide groups dominantly react with primary amines, thereby enhancing the amount of NHS groups (
) are introduced into the polyacrylamide hydrogel surface, photoactivated nitrophenyl azide groups dominantly react with primary amines, thereby enhancing the amount of NHS groups ( ) from the sulfo-SANPAH crosslinker on the surface for conjugation of amine-containing proteins. b Young’s moduli of three polyacrylamide hydrogel groups determined via atomic force microscopy. Data are mean values; standard error of the mean is given as error bars. (n=18, ****p<0.0001, One-way ANOVA by Tukey’s multiple comparisons test) c Photograph of polyacrylamide hydrogels after sulfo-SANPAH incorporation. The orange color of the modified polyacrylamide hydrogel indicates high incorporation of orange sulfo-SANPAH, whereas the conventional hydrogel remains clear. d Fluorescence image of FITC-BSA incorporation into polyacrylamide hydrogels fabricated with the conventional or modified protocol. With the same exposure time, the hydrogel with primary amine groups more efficiently conjugated FITC-BSA than the hydrogel without primary amine groups. Scale bar: 100 μm. e Quantification of fluorescence of FITC-BSA on conventional or modified polyacrylamide hydrogels of three stiffnesses. Modified polyacrylamide hydrogels with primary amine groups show a 10-fold higher fluorescence intensity than hydrogels without primary amine groups. Data are mean values; error bars are standard error of the mean. (n=3)
) from the sulfo-SANPAH crosslinker on the surface for conjugation of amine-containing proteins. b Young’s moduli of three polyacrylamide hydrogel groups determined via atomic force microscopy. Data are mean values; standard error of the mean is given as error bars. (n=18, ****p<0.0001, One-way ANOVA by Tukey’s multiple comparisons test) c Photograph of polyacrylamide hydrogels after sulfo-SANPAH incorporation. The orange color of the modified polyacrylamide hydrogel indicates high incorporation of orange sulfo-SANPAH, whereas the conventional hydrogel remains clear. d Fluorescence image of FITC-BSA incorporation into polyacrylamide hydrogels fabricated with the conventional or modified protocol. With the same exposure time, the hydrogel with primary amine groups more efficiently conjugated FITC-BSA than the hydrogel without primary amine groups. Scale bar: 100 μm. e Quantification of fluorescence of FITC-BSA on conventional or modified polyacrylamide hydrogels of three stiffnesses. Modified polyacrylamide hydrogels with primary amine groups show a 10-fold higher fluorescence intensity than hydrogels without primary amine groups. Data are mean values; error bars are standard error of the mean. (n=3)
3.2. Polyacrylamide hydrogels with enhanced protein conjugation support robust hPSC attachment, proliferation, and self-renewal on hydrogels across a broad range of stiffnesses
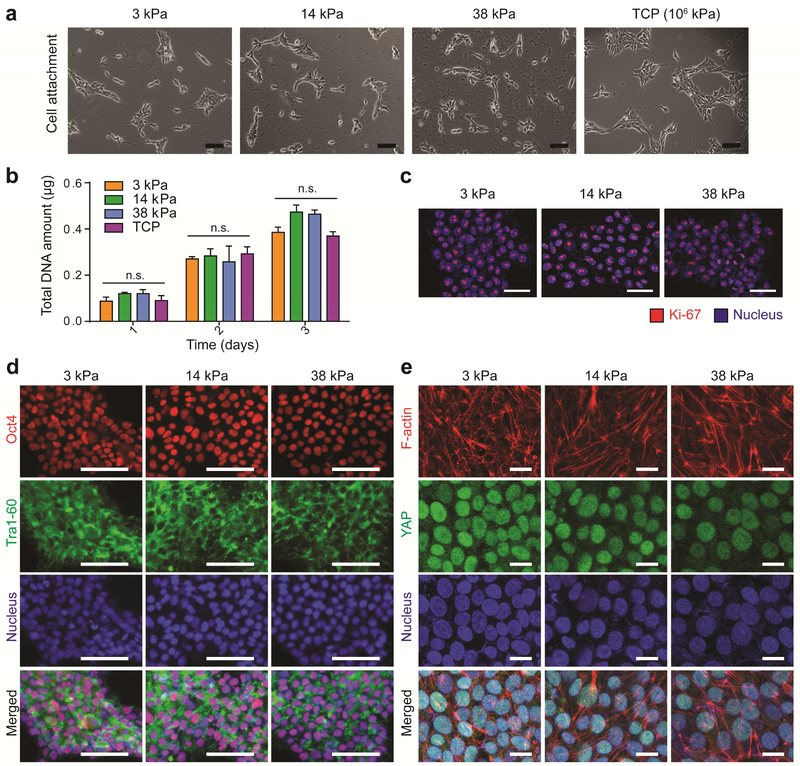
To examine the effect of substrate stiffness on hPSC fates, we chose a widely used human embryonic stem cell line (H9) as a model cell line for hPSCs. Various ECM proteins have been used to help hPSC attachment and self-renewal on tissue culture plastic including Matrigel, recombinant human laminin α5β2γ1, mouse laminin, recombinant human vitronectin, and human collagen IV[25–27]. We chose Matrigel due to its efficacy to support H9 attachment with high efficiency and reproducibility (Figure S2). When undifferentiated H9 cells were plated on polyacrylamide hydrogels with enhanced Matrigel coating, H9 attachment was robust and comparable across the range of stiffnesses tested (3–38 kPa, tissue culture plastic ~ GPa) (Figure 2a). In agreement with bright-field images (Figure 2a), DNA assay confirmed comparable number of cells attached at day 1 on all four hydrogels tested (Figure 2b). Given that high proliferation capacity is a hallmark of undifferentiated hPSCs, the effect of substrate stiffness on cell proliferation was examined by quantifying total cell number and the percentage of cells undergoing active division. Regardless of substrate stiffness, the proliferation of H9 cells on hydrogels coated with Matrigel were comparable to those grown on tissue culture plastic until confluency (day 3) (Figure 2b). In addition, 100% of H9 cells were undergoing active division, as shown by Ki-67 expression[28], regardless of substrate stiffness (Figure 2c).
Figure 2. Polyacrylamide hydrogels with enhanced Matrigel conjugation induce comparable levels of hPSC attachment, proliferation, and maintenance of pluripotency regardless of substrate stiffness.
a Bright-field image of H9 cells attached to Matrigel-coated hydrogel substrates (3, 14, 38 kPa) and tissue culture plastic (TCP) at day 1 in growth medium (mTeSR). All hydrogel groups enabled robust H9 cell attachment. Scale bar: 100 μm. b Effect of substrate stiffness on cell proliferation. Changes in amount of total DNA content over time confirm comparable H9 cell attachment and proliferation across three stiffnesses. Data are mean values; error bars are standard error of the mean. Groups did not significantly differ according to two-way ANOVA by Bonferroni’s multiple comparisons test. (n=3, n.s. not significant) c Immunostaining of Ki-67, a marker of actively proliferating cells, revealed that H9 cell proliferation was not influenced by substrate stiffness (day 2, mTeSR). Scale bar: 50 μm. d Expression of pluripotency markers (Oct4, Tra1-60) was maintained regardless of substrate stiffness (day 2, mTeSR). Scale bar: 100 μm. e Regardless of substrate stiffness, actin robustly polymerized into filaments and YAP translocated into the nucleus. Scale bar: 20 μm.
Next, we evaluated the effect of substrate stiffness on the maintenance of H9 cell pluripotency by immunostaining of Oct4 and Tra1-60, two pluripotency markers of hPSCs. When H9s were cultured on hydrogels with high density of Matrigel coating, all cells stained positive for both pluripotency markers on all three hydrogel formulations with stiffnesses ranging from 3 kPa to 38 kPa (Figure 2d). We then examined the organization of filamentous actin (F-actin) and the localization of YAP when hPSCs were cultured on modified soft hydrogels (3 kPa) versus stiff hydrogels (38 kPa). As expected, H9 cells grown on stiff hydrogels (38 kPa) showed filamentous F-actin and translocated YAP into the nucleus (Figure 2e). Interestingly, cells grown on soft substrate (3 kPa) also assembled actin filaments and translocated YAP into the nucleus (Figure 2e). These results suggest that when plated on hydrogels with high density of Matrigel, hPSCs sense soft substrate as if they were cultured on a stiff substrate.
3.3. Stiffness-dependent YAP regulation in stem cells depends on the biochemical ligand density
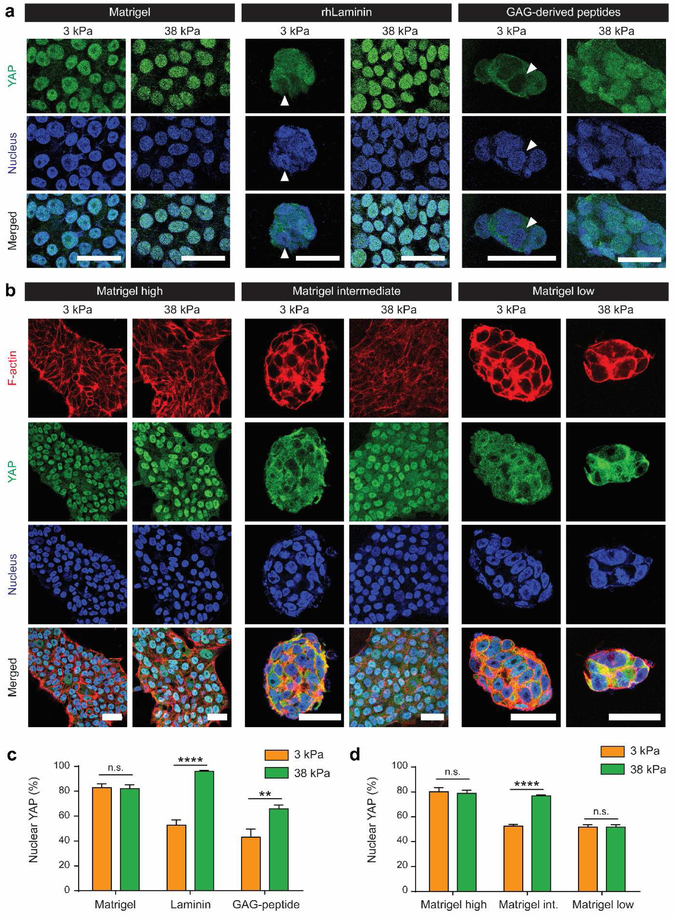
Previous reports examined the effect of substrate stiffness on hPSC self-renewal using Matrigel or GAG-derived peptide as biochemical cues on polyacrylamide hydrogels[12,17]. We chose three types of biochemical cues including Matrigel and GAG-derived peptides from the previous reports, and recombinant human laminin-521 (rhLaminin). We included rhLaminin because it is a major component of Matrigel and supports H9 attachment on hydrogel substrate (Figure S2). Sulfo-SANPAH was used to conjugate Matrigel and rhLaminin, and m-maleimidobenzoyl-N-hydroxysulfosuccinimide ester (sulfo-MBS) was used to conjugate cysteine-containing GAG peptides. When H9 cells were cultured on Matrigel-conjugated substrates using our enhanced conjugation protocol, H9 cells exhibited high nuclear YAP on both soft (3 kPa) and stiff (38 kPa) hydrogels. In contrast, when H9 cells were cultured on hydrogels modified with rhLaminin- or GAG-peptide, stiffness-induced YAP localization was observed: cytoplasmic YAP localization when cultured on soft hydrogels and nuclear YAP localization when cultured on stiff hydrogels (Figure 3a, c). Since rhLaminin is one component of Matrigel and GAG-peptide is a short peptide from full-length glycoprotein, vitronectin[12], we further asked a question on potential role of biochemical ligand density in YAP translocation. To examine the effect of varying ligand density on stiffness-induced YAP translocation in H9 cells, we cultured H9 cells on polyacrylamide hydrogels coated with varying concentration of Matrigel. Interestingly, stiffness-induced YAP nuclear translocation in hPSCs was only observed when cultured on hydrogels coated with intermediate concentration of Matrigel (Figure 3b, d). In contrast, when H9 cells were cultured on hydrogels coated with low or high density of Matrigel, YAP regulation is dominated by biochemical cues and stiffness-induced YAP translocation response disappeared. Specifically, low Matrigel density always induced low nuclear YAP whereas high Matrigel density always induced high nuclear YAP (Figure 3b, d). F-actin staining revealed that nuclear localization pattern of YAP in response to substrate stiffness and ligand density correlated with spreading and morphology of H9 cells (Figure 3b). Taken together, our findings demonstrate that YAP regulation and mechanotransduction of hPSCs depend not only on hydrogel stiffness, but also on the density of biochemical cues.
Figure 3. Type and concentration of biochemical cues alter stiffness-dependent cytoskeletal organization and YAP regulation in hPSCs.
a Effects of the type of biochemical cue on hPSC mechanotransduction. rhLaminin- and GAG-derived peptides-conjugated hydrogels rescued stiffness-dependent YAP localization. White arrowhead indicates nucleus that is negative for YAP expression. Scale bar: 20 μm. b Concentration-dependent effect of Matrigel on hPSC mechanosensing of substrate stiffness. Mechanosensing of bulk hydrogel stiffness appeared only with an intermediate amount of Matrigel coating (1:400). In contrast, high Matrigel conjugation (1:40) led hPSCs to sense a ‘stiff’ environment and low Matrigel conjugation (1:1000) led to sensing of a ‘soft’ microenvironment regardless of ‘bulk’ hydrogel stiffness. Scale bar: 20 μm. c,d Quantification of nuclear YAP localization. Data are mean values; error bars are standard error of the mean. (n=14, **p<0.01, ****p<0.0001, two-way ANOVA by Bonferroni’s multiple comparisons test)
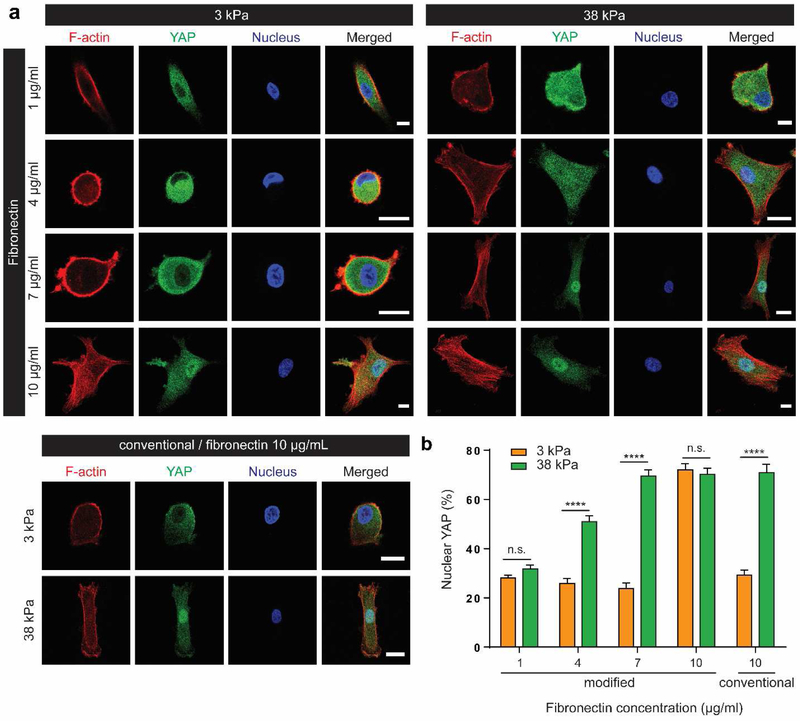
In addition to hPSCs, we also examined hMSCs to assess whether ligand density impacts stiffness-induced YAP localization and mechanotransduction in adult multipotent stem cells. For mechanotransduction studies of hMSCs, fibronectin has been widely used as a biochemical cue to support cell adhesion[6,8,19,29]. Four concentrations of fibronectin solution were used for conjugation (1, 4, 7, 10 μg/ml). To quantify the amount of fibronectin incorporated onto hydrogels, HiLyte™ 488-labeled fibronectin was used to correlate fluorescent intensity to fibronectin amount (Figure S3). Our results showed that ~ 50-130% more fibronectin was incorporated using our modified method compared to the conventional method when the same concentration of fibronectin solution was used (10 μg/ml) (Figure S3c). Using the standard curve (Fig. S3b), we calculated that increasing the concentration of fibronectin solution (1 – 10 μg/ml) resulted in non-linear increase in the amount of incorporated fibronectin (~ 5 – 140 ng/cm2). Similar to the trend observed in hPSCs (Figure 3), stiffness-induced YAP nuclear translocation in hMSCs was only observed when hMSCs were cultured on hydrogels coated with intermediate concentration of fibronectin (~ 10 – 67 ng/cm2) (Figure 4). Regardless of stiffness tested (3, 38kPa), low fibronectin density (< 10 ng/cm2) induced low nuclear YAP whereas high fibronectin density (>100 ng/cm2) induced high nuclear YAP (Figure 4a, b, S3). It is important to note that protein concentration in the solution used for conjugation does not reflect the actual amount of proteins incorporated as it is dependent on conjugation efficiency. Since the actual fibronectin incorporated using conventional method is lower, hMSCs displayed stiffness-dependent YAP localization when polyacrylamide hydrogels were coated with higher concentration of fibronectin solution (10 μg/ml) (Figure 4). Together, these results further support that adding 2-aminoethyl methacrylate help enhance the incorporation efficiency of fibronectin.
Figure 4. Concentration of fibronectin alters stiffness-dependent cytoskeletal organization and YAP regulation in hMSCs.
a Concentration-dependent effect of fibronectin on hMSC sensing of substrate stiffness. While high fibronectin coating (10 μg/ml) led hMSCs to sense a ‘stiff’ environment, low fibronectin coating (1 μg/ml) led to sensing of a ‘soft’ microenvironment regardless of hydrogel stiffness. In the groups with 4 or 7 μg/ml fibronectin, YAP regulation was dependent on substrate stiffness, as shown in the conventional polyacrylamide hydrogel groups. Scale bar: 20 μm. b Quantification of nuclear YAP localization. Data are mean values; error bars are standard error of the mean. (n=14, ****p<0.0001, two-way ANOVA by Bonferroni’s multiple comparisons test. n.s. not significant)
Given it has been demonstrated that cell shape can modulate YAP activity[18], we examined the correlation between cell spreading and YAP localization in response to varying ligand density. Our results show that high cell spreading (over ~ 1400 μm2) generally correlates with YAP nuclear translocation (Figure S5).
4. Discussion
Substrate stiffness can modulate cell behaviors including spreading, migration, and differentiation[6,7,30]. It has been well established that cells use integrin-mediated adhesions to bind to various ECM ligands, and exert tractional forces against these adhesions to probe the physical properties of the cell niche[14,31]. Previous studies have shown that varying integrin type, density and spatial distribution can modulate how a cell responds to the matrix stiffness[31–33]. A recent study has identified YAP as an important transcriptional regulator in translating stiffness signal[18]. While cell responses to changes in ECM stiffness as a function of ligand presentation (type, density, and/or clustering) have been widely appreciated and documented in the literature for quite some time[31–33], how varying ligands modulate YAP translocation remains largely unknown. The present study seeks to examine whether stiffness-induced YAP localization also depends on types and concentration of biochemical ligands. To answer this question, we first develop a method to fabricate polyacrylamide hydrogel substrate with high protein conjugation efficiency and broad biochemical coating range. Specifically, we show that introduction of primary amine to polyacrylamide hydrogels significantly enhances the conjugation efficiency of ECM proteins compared to conventional protocol (Figure 1, S1, S3). Using sulfo-SANPAH or sulfo-MBS as crosslinkers, we demonstrate efficient conjugation of several biochemical cues including Matrigel, rhLaminin, fibronectin, and GAG-derived peptide that have been used for previous stem cell mechanotransduction studies. The enhanced protein conjugation allowed robust attachment of undifferentiated hPSCs, even on soft polyacrylamide hydrogel substrates, thereby enabling mechanotransduction studies of hPSCs as well as hMSCs. Given that primary amine groups are widely utilized for conjugation with numerous chemical groups including NHS esters and phenyl azides[21], this method enables the conjugation of a variety of proteins or peptides of interest. We further characterized the homogeneity of incorporated ligand using image analysis, and showed fibronectin incorporation was homogeneous (Figure S4a-c). In contrast, hydrogel surface conjugated with high density of Matrigel™ (1:40 dilution) was inhomogeneous (Figure S4d,e). Unlike fibronectin, Matrigel™ is composed of 60% laminin, 30% collagen IV, 8% entactin, and hundreds of other proteins. We speculate the inhomogeneity may have resulted from physical interactions among proteins within Matrigel, which can form bundles in the solution before get conjugated to hydrogel surface. In fact, previous studies using collagen I coating also typically showed inhomogeneous bundle formation on the hydrogel surface [29,8]. Furthermore, entactin is known to act as a crosslinking molecule to bridge laminin and collagen IV in basement membrane. At high concentration, different components within Matrigel™ may crosslink, which further increases the inhomogeneity.
Using our modified method, we uncovered the crucial role of ligand density in stiffness-dependent YAP regulation. For example, when hPSCs were cultured on the substrate with low concentration of Matrigel, we observed low levels of nuclear YAP localization regardless of substrate stiffness. A similar trend was observed in hMSCs as well, when hydrogels were coated with low concentration of fibronectin. In sum, our results reveal that the biochemical ligand density can influence YAP regulation and even override the effect of stiffness. To further examine whether ligand density alters YAP nuclear translocation through cell spreading, cell projection area was quantified. Our results showed increased cell spreading generally correlated with YAP nuclear translocation (Figure 4, S5a). This is in an agreement with a previous study where an increase in spreading of hMSCs strongly correlated with increasing nuclear YAP expression[18]. However, there were some exceptions for hMSCs cultured on stiff hydrogels with low density of fibronectin (38 kPa, FN 1 μg/ml), which showed cell spreading but cytoplasmic YAP (Figure S5b). It is well established that the stiffness-sensing mechanism is a complex, dynamic process involving F-actin organization, focal adhesion stabilization, actomyosin contractility, and cytoskeletal tension generation, which is required for YAP nuclear translocation[14,18,24,34–37]. We further demonstrate that high nuclear YAP groups (3kPa-FN10 μg/ml and 38kPa-4,7,10 μg/ml) can induce vinculin localization (Figure S6). This suggests that ligand density cooperates together with stiffness to induce formation of focal adhesion complexes, organize F-actin bundles, and generate cytoskeletal tension. The molecular mechanism of how biochemical ligand density can alter stiffness-mediated YAP regulation needs further investigation.
Lastly, we are aware of the ongoing controversy about using the term of “mesenchymal stem cells (MSCs)”, which refer to a highly heterogenous cell population[38]. Future studies in the field will further characterize these cells to more accurately define their origin, biological functions, and potential therapeutic uses. Despite the limitations, we used the term “mesenchymal stem cells” in this manuscript to maintain continuity in the literature.
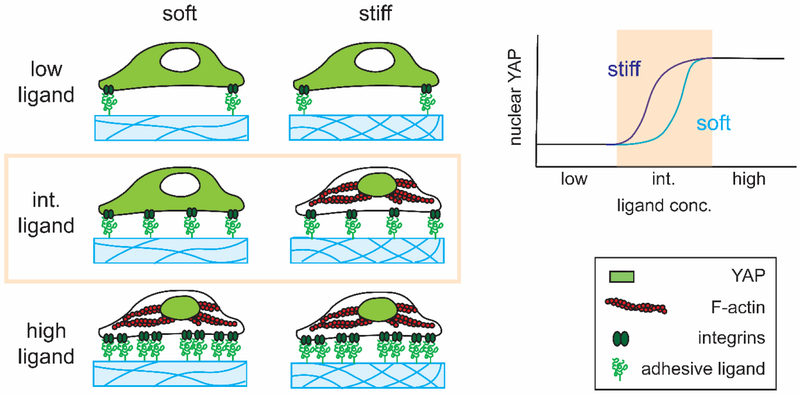
In summary, the current study demonstrates that YAP nuclear translocation is not solely dictated by substrate stiffness but is significantly influenced by biochemical ligands through which cells adhere to the underlying substrate (Figure 5). This finding reiterates that cells sense their mechanical environment via dynamic and complex processes. Our modified polyacrylamide hydrogels with enhanced conjugation of biochemical cues serve as a powerful tool for supporting efficient adhesion of both hPSCs and hMSCs, and can be used to enable future mechanistic studies to further elucidate the role of biochemical cues in modulating mechanotransduction and differentiation of various stem cell types.
Figure 5. A summary of interplay of stiffness and ligand density in YAP regulation in stem cells.
On a hydrogel substrate with intermediate concentration of biochemical cues, stem cell mechanotransduction depends on substrate stiffness, and increasing substrate stiffness will increase YAP nuclear localization. However, when the concentration of biochemical cues is too low (low ligand) or too high (saturated), biochemical cues will override the effect of substrate stiffness in stem cell mechanotransduction. Regardless of substrate stiffness, low ligand concentration will result in low nuclear YAP whereas high ligand concentration will result in high nuclear YAP.
Supplementary Material
Acknowledgments
The authors acknowledge NIH R01DE024772 (F.Y.), NSF CAREER award CBET-1351289 (F.Y.), California Institute for Regenerative Medicine Tools and Technologies Award RT3-07804 (F.Y.), a Stanford Chem-H Institute Biomaterials Seed grant (F.Y.), the Stanford Bio-X Interdisciplinary Initiative Program (F.Y.), and a Stanford Child Health Research Institute Faculty Scholar Award (F.Y.). S.L. and A. S. thank the Bio-X fellowships from the Stanford Bio-X program for support. S. L. also acknowledges NIH (SIG number 1S10OD01058001A1) for usage of the Leica SP8 confocal system.
Footnotes
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Murphy WL, McDevitt TC, Engler AJ, Materials as stem cell regulators, Nat Mater. 13 (2014) 547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Madl CM, Heilshorn SC, Engineering Hydrogel Microenvironments to Recapitulate the Stem Cell Niche, Annu Rev Biomed Eng. 20 (2018) 21–47. doi: 10.1146/annurev-bioeng-062117-120954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lutolf MP, Gilbert PM, Blau HM, Designing materials to direct stem-cell fate, Nature. 462 (2009) 433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Smith LR, Cho S, Discher DE, Stem Cell Differentiation is Regulated by Extracellular Matrix Mechanics, Physiology (Bethesda). 33 (2018) 16–25. doi: 10.1152/physiol.00026.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vining KH, Mooney DJ, Mechanical forces direct stem cell behaviour in development and regeneration, Nature Reviews Molecular Cell Biology. 18 (2017) 728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Engler AJ, Sen S, Sweeney HL, Discher DE, Matrix elasticity directs stem cell lineage specification, Cell. 126 (2006) 677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [7].Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D, Substrate compliance versus ligand density in cell on gel responses, Biophys. J 86 (2004) 617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ, Interplay of matrix stiffness and protein tethering in stem cell differentiation, Nature Materials. 13 (2014) 979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tse JR, Engler AJ, Preparation of hydrogel substrates with tunable mechanical properties, Curr Protoc Cell Biol. Chapter 10 (2010) Unit 10.16. doi: 10.1002/0471143030.cb1016s47. [DOI] [PubMed] [Google Scholar]
- [10].Eroshenko N, Ramachandran R, Yadavalli VK, Rao RR, Effect of substrate stiffness on early human embryonic stem cell differentiation, J Biol Eng. 7 (2013) 7. doi: 10.1186/1754-1611-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, Chen X, Roberts CJ, Stevens MM, Substrate stiffness affects early differentiation events in embryonic stem cells, Eur Cell Mater. 18 (2009) 1–13; discussion 13-14. [DOI] [PubMed] [Google Scholar]
- [12].Musah S, Morin SA, Wrighton PJ, Zwick DB, Jin S, Kiessling LL, Glycosaminoglycan-Binding Hydrogels Enable Mechanical Control of Human Pluripotent Stem Cell Self-Renewal, ACS Nano. 6 (2012) 10168–10177. doi: 10.1021/nn3039148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM, Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture, Science. 329 (2010) 1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pelham RJ, l Wang Y, Cell locomotion and focal adhesions are regulated by substrate flexibility, Proc. Natl. Acad. Sci. U.S.A 94 (1997) 13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rowlands AS, George PA, Cooper-White JJ, Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation, Am. J. Physiol., Cell Physiol 295 (2008) C1037–1044. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- [16].Musah S, Wrighton PJ, Zaltsman Y, Zhong X, Zorn S, Parlato MB, Hsiao C, Palecek SP, Chang Q, Murphy WL, Kiessling LL, Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification, Proc Natl Acad Sci U S A. 111 (2014) 13805–13810. doi: 10.1073/pnas.1415330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Keung AJ, Asuri P, Kumar S, Schaffer DV, Soft microenvironments promote the early neurogenic differentiation but not self-renewal of human pluripotent stem cells, Integr Biol (Camb). 4 (2012) 1049–1058. doi: 10.1039/c2ib20083j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Digabel JL, Forcato M, Bicciato S, Elvassore N, Piccolo S, Role of YAP/TAZ in mechanotransduction, Nature. 474 (2011) 179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- [19].Yang C, Tibbitt MW, Basta L, Anseth KS, Mechanical memory and dosing influence stem cell fate, Nat Mater. 13 (2014) 645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang YL, Pelham RJ, Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells, Meth. Enzymol 298 (1998) 489–496. [DOI] [PubMed] [Google Scholar]
- [21].Bioconjugate Techniques, Elsevier, 2013. doi: 10.1016/C2009-0-64240-9. [DOI] [Google Scholar]
- [22].Lee JP, Kassianidou E, MacDonald JI, Francis MB, Kumar S, N-terminal specific conjugation of extracellular matrix proteins to 2-pyridinecarboxaldehyde functionalized polyacrylamide hydrogels, Biomaterials. 102 (2016) 268–276. doi: 10.1016/j.biomaterials.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Discher DE, Janmey P, Wang Y, Tissue Cells Feel and Respond to the Stiffness of Their Substrate, Science. 310 (2005) 1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- [24].Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Roux A-LL, Shanahan CM, Trepat X, Navajas D, Garcia-Manyes S, Roca-Cusachs P, Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores, Cell. 171 (2017) 1397–1410.e14. doi: 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- [25].Rodin S, Antonsson L, Niaudet C, Simonson OE, Salmela E, Hansson EM, Domogatskaya A, Xiao Z, Damdimopoulou P, Sheikhi M, Inzunza J, Nilsson A-S, Baker D, Kuiper R, Sun Y, Blennow E, Nordenskjöld M, Grinnemo K-H, Kere J, Betsholtz C, Hovatta O, Tryggvason K, Clonal culturing of human embryonic stem cells on laminin-521/E-cadherin matrix in defined and xeno-free environment, Nat Commun. 5 (2014) 3195. doi: 10.1038/ncomms4195. [DOI] [PubMed] [Google Scholar]
- [26].Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK, Feeder-free growth of undifferentiated human embryonic stem cells, Nat. Biotechnol 19 (2001) 971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- [27].Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, Lebrin F, Kats P, Hochstenbach R, Passier R, Sonnenberg A, Mummery CL, Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin, Stem Cells. 26 (2008) 2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- [28].Scholzen T, Gerdes J, The Ki-67 protein: from the known and the unknown, J. Cell. Physiol 182 (2000) 311–322. doi:. [DOI] [PubMed] [Google Scholar]
- [29].Trappmann B, Gautrot JE, Connelly JT, Strange DGT, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, Spatz JP, Watt FM, Huck WTS, Extracellular-matrix tethering regulates stem-cell fate, Nature Materials. 11 (2012) 642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- [30].Peyton SR, Putnam AJ, Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion, Journal of Cellular Physiology. 204 (2005) 198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- [31].Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ, Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate, Nature Materials. 9 (2010) 518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rajagopalan P, Marganski WA, Brown XQ, Wong JY, Direct Comparison of the Spread Area, Contractility, and Migration of balb/c 3T3 Fibroblasts Adhered to Fibronectin- and RGD-Modified Substrata, Biophys J. 87 (2004) 2818–2827. doi: 10.1529/biophysj.103.037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cavalcanti-Adam EA, Volberg T, Micoulet A, Kessler H, Geiger B, Spatz JP, Cell Spreading and Focal Adhesion Dynamics Are Regulated by Spacing of Integrin Ligands, Biophysical Journal. 92 (2007) 2964–2974. doi: 10.1529/biophysj.106.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Geiger B, Bershadsky A, Pankov R, Yamada KM, Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk, Nat. Rev. Mol. Cell Biol 2 (2001) 793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- [35].Holle AW, Tang X, Vijayraghavan D, Vincent LG, Fuhrmann A, Choi YS, del Álamo JC, Engler AJ, In situ mechanotransduction via vinculin regulates stem cell differentiation, Stem Cells. 31 (2013) 2467–2477. doi: 10.1002/stem.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Haase K, Al-Rekabi Z, Pelling AE, Mechanical cues direct focal adhesion dynamics, Prog Mol Biol Transl Sci. 126 (2014) 103–134. doi: 10.1016/B978-0-12-394624-9.00005-1. [DOI] [PubMed] [Google Scholar]
- [37].Elosegui-Artola A, Oria R, Chen Y, Kosmalska A, Pérez-González C, Castro N, Zhu C, Trepat X, Roca-Cusachs P, Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity, Nature Cell Biology. 18 (2016) 540–548. doi: 10.1038/ncb3336. [DOI] [PubMed] [Google Scholar]
- [38].Sipp D, Robey PG, Turner L, Clear up this stem-cell mess, Nature. 561 (2018) 455. doi: 10.1038/d41586-018-06756-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.