Abstract
Objectives
To determine whether pre-existing nephropathy impacts urinary KIM-1 levels, urinary KIM-1 were measured in patients with normal kidney filtration function but either with or without proteinuria. The reference intervals of urinary KIM-1 in adults with normal kidney filtration function but without urine proteinuria were established.
Design and methods
188 urine samples were obtained from adults with normal kidney filtration. 83 of the 188 showed negative urine protein, erythrocytes and leucocytes were used as normal controls. The remaining 105 samples showed at least one abnormal result suggesting possible pre-existing nephropathy. Urinary KIM-1 concentrations were measured using an enzyme-linked immunosorbent assay. Urinary KIM-1 was normalized with urine creatinine concentration. The reference interval for urinary KIM-1 was determined by non-parametric methodology on 147 individuals.
Results
The results showed significantly increased urinary KIM-1 concentration in protein positive (protein +, erythrocyte +/−, leucocyte+/-) samples compared to controls (protein-, erythrocyte -, leucocyte -). Urinary KIM-1 concentrations were significantly higher when proteinuria was at trace concentration (0.25 g/L) and correlated with the severity of proteinuria. The creatinine normalized urinary KIM-1 was significantly higher when urine protein was 1 + to 3+ (0.75–5 g/L). The reference interval for urinary KIM-1 was 0.00 (90%CI: 0-0) to 4.19 (90%CI: 3.11–5.62) μg/L, and for creatinine normalized urinary KIM-1 0.00 (90%CI: 0-0) to 0.58 (90%CI: 0.44–0.74) μg/mmol.
Conclusions
Baseline urinary KIM-1 concentrations were increased when there was detectable urine protein and correlated with its severity. The urinary KIM-1 concentrations should be interpreted with consideration of urine protein levels in individual patients.
Keywords: Urinary KIM-1, Kidney injury, Nephropathy, Reference interval
Highlights
-
•
The correlation of urinary KIM-1 with pre-existing evidence of nephropathy such as proteinuria, hematuria and pyuria was investigated.
-
•
Urinary KIM-1 was normalized to urine creatinine concentration.
-
•
Urinary KIM-1 reference intervals for healthy adults with normal kidney filtration and without proteinuria were established.
-
•
Age and sex effects on the urinary KIM-1 concentration were investigated.
1. Introduction
Kidney injury molecule-1 (KIM-1) was first identified in a rat by Ichimura et al. in 1998 as a type −1 transmembrane protein with an immunoglobulin and mucin domain, upregulated in the renal proximal tubule of the kidney after acute kidney injury (AKI) [1]. It was subsequently shown that extensive KIM-1 expression occurs in proximal tubule cells in patients with confirmed acute tubular necrosis (ATN) [2]. Normalized urinary KIM-1 concentrations were significantly higher in patients with ischemic ATN compared to patients with other forms of AKI [2]. A number of other studies have supported that KIM-1 could be a useful biomarker for early detection and diagnosis of AKI caused by exposure to nephrotoxin, complications of post-cardiac surgery and ischemia. In contrast to non AKI patients, urinary KIM-1 is significantly increased in patients with AKI 2 h after cardiac surgery and may predict AKI with good performance in patients within 24 h of cardiac surgery [3]. Overall, the body of evidence on KIM-1 as a biomarker was sufficient for the Federal Drug Administration and the European Medicines Agency to qualify KIM-1 for preclinical assessment of nephrotoxicity and for clinical evaluation on a case-by-case basis [4].
Recent studies also identified urinary KIM-1 as a biomarker for the assessment of nephropathy in various chronic kidney diseases (CKD). In patients with clinically mild IgA nephropathy, high urinary KIM-1 was linked to relatively severe pathologic changes in renal biopsy [5]. In patients with systemic lupus erythematosus, the number of tissue KIM-1 positive cells strongly correlated with histological findings of glomerular and interstitial inflammation. Urinary KIM-1 concentrations were also significantly correlated with the expression of tissue KIM-1 in systemic lupus erythematosus patients. Further, patients with active lupus nephritis exhibited elevated urinary KIM-1 concentrations compared to inactive lupus nephritis patients. In addition, urinary KIM-1 concentrations were also correlated with severity of proteinuria and tubular damage in active lupus nephritis group [6].
These findings increase the potential use of urinary KIM-1 in the diagnosis or prognosis of CKD, but also results in difficulties in the interpretation of urinary KIM-1 when it is used in early detection of AKI. For best laboratory practice it is necessary to determine reference intervals specific to the local patient population, with partitioning by gender and age, where required, according to the recommendations of Clinical and Laboratory Standards Institute (CLSI) guidelines [7]. In most previous clinical studies, the baseline urinary KIM-1 concentration was measured in nominally healthy individuals or individuals who were being treated but did not develop AKI, which does not represent appropriate reference intervals for clinical decision making. In this study we measured and compared urinary KIM-1 concentrations in adults with normal kidney filtration function but with or without pre-existing kidney injury to clarify if they should be considered as different patient populations in the establishment of reference intervals. Moreover, the effect of age and gender on urinary KIM-1 and creatinine normalized urinary KIM-1 concentration was investigated. Finally, we determined urinary KIM-1 reference intervals from subjects with normal kidney filtration function and without proteinuria.
2. Subjects and methods
2.1. Subject selection and urine sample collection
Urine samples were obtained from adults (≥17 year-old) submitting samples for routine urine analysis, between July 2015 and January 2016. Samples were aliquoted into clean tubes and frozen at −20 °C within 4 h of collection for later use. A total of 188 (71 male and 117 female, 17–95 year old) samples were obtained from adults with normal kidney filtration as evidenced on basis of two recent serum creatinine measurements using age- and sex-specific reference limits (male: 54–113 μmol/L, female:37–91 μmol/L). The study was approved by the local Health Research Ethics Authority and the Research Proposals Approval Committee.
2.2. Urine analysis and urine creatinine
Routine urine analysis was done on an Urysis 2400 analyzer (Roche diagnostics) in the hospital core laboratory, using multi-parameter test cassette that measures pH, protein (albumin), glucose, ketones, bilirubin, urobilinogen, nitrite, erythrocyte, leucocyte esterase, and specific gravity. Only urine protein, erythrocyte, leucocyte esterase results that reflecte possible pre-existing nephropathy were recorded for data analysis in this study. The following cutoffs were used to classify results into test-positives. Urine protein positive: trace (0.25 g/L), 1+ (0.75 g/L), 2+ (1.5 g/L) and 3+ (5 g/L). Erythrocyte positive: trace (10Ery/μL), 1+ (25Ery/μL), 2+ (50Ery/μL), 3+ (150Ery/μL) and 4+ (250Ery/μL). Leucocyte esterase positive: trace (25Leu/μL), 1+ (100Leu/μL), 2+ (500Leu/μL). Urinary KIM-1 results were normalized to urine creatinine concentrations determined by analyzing all urine samples within 4 h of collection using an enzymatic assay on the Architect Ci8200 chemistry analyzer (Abbott Laboratories).
2.3. Urinary KIM-1 measurement
The urinary KIM-1 concentrations were measured in duplicate for each sample using the Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems). Manufacturer's instructions were followed in sample preparation, reagent reconstitution and testing procedures. A calibration curve was generated for each testing plate to report urinary KIM-1 in concentration units of μg/L, which were subsequently normalized using μg/mmol creatinine units. The analytical performance of the assay such as linearity, within plate and between plate precision, and recovery were determined to be consistent with manufacturer's claims. The limit of detection was 0.009 μg/L.
2.4. Statistical analysis
The normality of data distribution was assessed by Kolmogorov-Smirnov test. Urinary KIM-1 concentration values were expressed as median with interquartile range. The reference interval was determined following CLSI guideline (C28-A3) [7] by non-parametric method. The outliers were checked by Reed/Dixon method. The KIM-1 concentration between the groups was compared by Mann-Whitney U test of SPSS software version 19.0 for Windows (SPSS Inc. Chicago, USA). P < 0.05 was considered as statistically significant.
3. Results
3.1. Subject general information
A total of 188 urine samples were collected from 117 females and 71 males between 17 and 95 years old. 83 samples showed no evidence of pre-existing nephropathy by urine analysis were negative for urine protein, erythrocytes and leucocytes. The remaining 105 samples showed at least one abnormal result for these three parameters suggesting possible pre-existing nephropathy.
3.2. Increased concentration of urinary KIM-1 in proteinuria
The urinary KIM-1 concentrations ranged from undetectable to 11.93 μg/L, from undetectable to 4.26 μg/mmol creatinine and were not normally distributed. The urinary KIM-1 concentrations were compared between the following groups clustered using results of routine urine analysis: (1) Protein positive: the group of samples screened positive for urinary protein and/or erythrocytes and/or leucocytes (protein +, erythrocyte +/−, leucocyte+/-); (2) Protein negative: the group with undetectable urinary protein but screened positive for erythrocytes and/or leucocytes (protein-, erythrocyte+/-, leucocyte +/−); and (3) Control: the group which screened negative for urine protein, erythrocyte and leucocyte (protein-, erythrocyte-, leucocyte -) (Table 1). The results showed significantly increased urinary KIM-1 concentration in protein positive samples (group 1) compared to control samples (group 3). A similar trend was observed when samples were normalized for urine creatinine, but without achieving statistical significance. Compared to control samples there was no significant difference in urinary KIM-1 in the urine samples only positive for erythrocytes and/or leucocytes (group 2).
Table 1.
Urinary KIM-1 concentration in urine samples with normal and abnormal urine analysis.
| Number | KIM-1(μg/L) | KIM-1/Cre (μg/mmol) | |
|---|---|---|---|
| Protein positive (Protein +, erythrocyte +/-,leucocyte +/-) | 40 | 1.51(0.78–2.55)** | 0.12(0.08–0.25) |
| Protein negative (Protein-, erythrocyte +/-, leucocyte +/-) | 65 | 0.48(0.14–1.13)## | 0.08(0.03–0.13) |
| Control (Protein-, erythrocyte-, leucocyte -) | 83 | 0.60(0.19–1.26) | 0.09(0.02–0.19) |
Results are expressed as median (interquartile range). (**): P < 0.01 significant difference when compared to control group (Protein-, erythrocyte -, leucocyte-). (##): P < 0.01 significant difference when compared to protein positive group (protein +, erythrocyte+/-, leucocyte +/−). KIM-1: kidney injury molecule-1, KIM-1/Cre: creatinine normalized kidney injury molecule −1.
In addition, the increase of urinary KIM-1 was related to the concentration of urinary protein (Table 2). The urine samples with more protein detected showed higher concentration of KIM-1. For urine samples with positive protein, KIM-1 concentration increased significantly even when the protein in urine was at trace concentration (0.25 g/L). The creatinine normalized urinary KIM-1 was significantly higher when urine protein was 1 + to 3+ (0.75–5 g/L), but not when urine protein was present as trace. Urinary KIM-1 concentrations were not significantly related to erythrocyte and leucocyte positivity if urinary protein was negative.
Table 2.
Association of urinary KIM-1 concentration with the severity of proteinuria, hematuria and pyuria.
| Group | Number | KIM-1(μg/L) | KIM-1/Cre (μg/mmol) |
|---|---|---|---|
| Protein+ (erythrocyte+/-, leucocyte+/-) | |||
| Control | 83 | 0.60(0.19–1.26) | 0.09(0.02–0.19) |
| Trace | 26 | 1.29(0.58–2.24)**## | 0.11(0.05–0.19) |
| 1–3+ | 14 | 1.70(1.27–3.63)** | 0.15(0.09–0.32)* |
| Leucocyte+ (protein-, erythrocyte +/−) | |||
| Control | 83 | 0.60(0.19–1.26) | 0.09(0.02–0.19) |
| Trace | 15 | 0.87(0.07–1.91) | 0.08(0.02–0.27) |
| 1+ | 12 | 0.27(0.06–1.04) | 0.07(0.00–0.11) |
| 2+ | 10 | 0.29(0.18–0.85) | 0.08(0.04–0.17) |
| Erythrocyte+ (protein-, leucocyte+/-) | |||
| Control | 83 | 0.60(0.19–1.26) | 0.09(0.02–0.19) |
| Trace | 14 | 0.30(0.00–0.89) | 0.06(0.00–0.11) |
| 1+ | 14 | 0.76(0.00–1.22) | 0.07(0.00–0.15) |
| 2–4+ | 18 | 0.40(0.15–1.37) | 0.09(0.04–0.19) |
Results are expressed as median (interquartile range). (*): P < 0.05, (**): P < 0.01 significant difference when compared to control group (protein-, erythrocyte-, leucocyte-). (##): P < 0.01 significant difference when compared to protein positive (1–3 + group), KIM-1: kidney injury molecule-1, KIM-1/Cre: creatinine normalized kidney injury molecule-1.
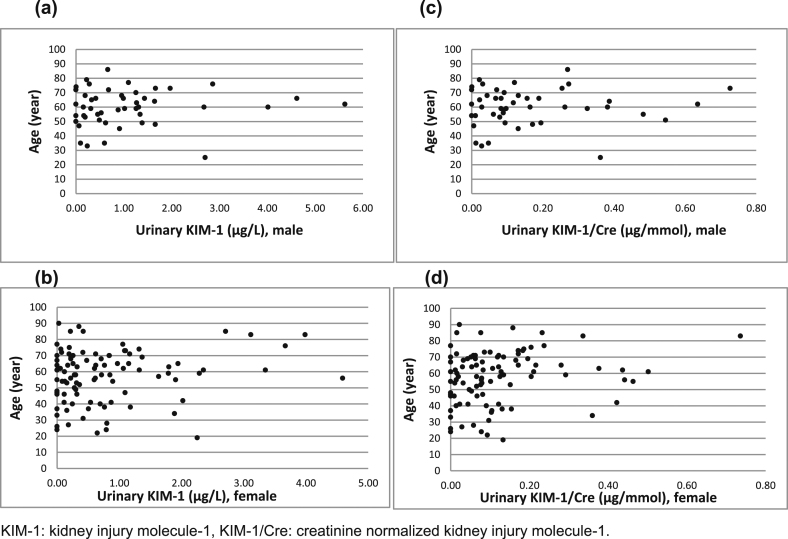
3.3. The effect of age and gender on KIM-1 concentration in urine samples
The concentrations of absolute and urine creatinine normalized urinary KIM-1 were analyzed by age and sex in 147 individuals with normal kidney filtration function and with negative urinary protein, negative or positive erythrocytes or leucocytes (Table 3). No statistically significant age difference of urinary KIM-1 concentration and creatinine normalized KIM-1 concentration in male and female groups were observed in Fig. 1; and there was no significant difference of urinary KIM-1 and creatinine normalized KIM-1 concentration between the genders (KIM-1: p = 0.084, KIM-1/Cre: p = 0.234) (Table 3).
Table 3.
Effect of gender on urinary KIM-1 concentration.
| Age | Female |
Male |
||||
|---|---|---|---|---|---|---|
| Number | KIM-1(μg/L) | KIM-1/Cre (μg/mmol) | Number | KIM-1(μg/L) | KIM-1/Cre (μg/mmol) | |
| 17-95y | 100 | 0.42(0.15–1.07) | 0.08(0.02–0.16) | 47 | 0.68(0.23–1.36) | 0.09(0.03–0.22) |
Results are expressed as median (interquartile range). KIM-1: kidney injury molecule-1, KIM-1/Cre: creatinine normalized kidney injury molecule-1.
Fig. 1.
The distribution of urinary KIM-1 and urine creatinine normalized KIM-1 by age
The median and interquartile range of urinary KIM-1 and creatinine normalized KIM-1 concentrations in 147 adults without proteinuria were 0.54 (0.18–1.18 μg/L) and 0.08 (0.03–0.17 μg/mmol). The laboratory specific reference interval for urinary KIM-1 was 0.00 (90%CI: 0-0) to 4.19 (90%CI: 3.11–5.62)μg/L, and for creatinine normalized urinary KIM-1 0.00 (90%CI: 0-0) to 0.58 (90%CI: 0.44–0.74)μg/mmol.
4. Discussion
This study examined baseline urinary KIM-1 concentrations in adults with normal kidney filtration but with or without pre-existing nephropathy. Urinary KIM-1 concentrations were significantly higher when urine protein was detected and increased with the severity of urine proteinuria but not hematuria or pyuria. No significant effect of age and sex on urinary KIM-1 concentration was observed. A laboratory specific reference interval for urinary KIM-1 in adults with normal kidney function and without proteinuria was established.
KIM-1 presents at very low concentration in healthy normal kidneys. It is expressed in proliferating bromodeoxyuridine-positive and dedifferentiated vimentin-positive epithelial cells in regenerating proximal tubules and is recognized as a biomarker for early detection of kidney tubular damage [1,2,12]. The increase of urinary KIM-1 has been observed to correlate with proteinuria or albuminuria in diabetic patients [[8], [9], [10]]. Urine KIM-1 is increased in type 2 diabetic patients even with normal (urinary albumin/creatinine ratio <10 mg/g creatinine) or mildly (urinary albumin/creatinine ratio 10–30 mg/g creatinine) increased albuminuria [8]. Positive correlation was found between KIM-1 and urine albumin and urine albumin/creatinine ratio in type 2 diabetic patients (r = 0.479 P < 0.001, r = 0.400, P < 0.001 respectively) [9]. Urinary KIM-1 concentration was significantly associated with the regression of microalbuminuria over the subsequent 2 years [10]. Moreover, in patients with type 1 diabetes and proteinuria, baseline blood KIM-1 concentration strongly predicted the rate of eGFR loss and risk of ESRD during 5–15 years of follow-up, after adjustment for baseline urinary albumin/creatinine ratio, eGFR, and HbA1C [11]. These studies indicate that urinary KIM-1 concentration is elevated when urine microalbumin is normal or mildly increased and associated with progress of chronic kidney injury. In this study we measured urinary KIM-1 in adults with normal kidney filtration but with pre-existing nephropathy indicated by positive urine protein and/or erythrocyte and/or leucocyte in routine urine analysis. The results showed that urinary KIM-1 significantly increased when urine protein was at even trace concentrations, and the KIM-1 concentrations increased with the degree of urine proteinuria. Although, the creatinine normalized urinary KIM-1 concentrations also increased, it was to a lesser degree as absolute KIM-1 concentrations. No increase in KIM-1 concentrations was observed if hematuria or pyuria was present without significant proteinuria. The urinary KIM-1 could be 2–3 times higher in patients with urine protein positive than patients with negative urine protein. Therefore, when using urinary KIM-1 to early detect AKI, patient baseline urinary KIM-1 should be interpreted carefully with appropriate reference intervals or cutoff values if patient is possible with chronic kidney diseases. Routine urine analysis is not a sensitive method for detecting urine albumin, however it has a fast turnaround time, can be used for POCT, and provide preliminary information to facilitate decisions on whether KIM-1 measurement would be useful.
The effect of age and sex on urinary KIM-1 was investigated in at least five studies since 2013 and reference intervals were established for urinary KIM-1 in different population. For healthy full term newborns at first or second day of life, urinary KIM-1 correlated with gestational age but did not correlate with birth weight and gender. Median urinary KIM-1 was reported as 1.326 ng/mL for female and 1.248 ng/mL for male[13]. In healthy infants aged 1 day to 1 year old urinary KIM-1 concentrations were substantially lower in almost all 106 subjects (median 0.08 ng/mL) and not related to age, sex or ethnicity [14]. However, a study by McWilliam including 120 and 171 healthy children recruited in USA and UK respectively, showed creatinine normalized urinary KIM-1 concentrations to be significantly associated with age and ethnicity but not with sex. Urinary KIM-1 also showed diurnal variation, with higher concentrations found in morning samples. The mean urinary KIM-1 concentrations were 0.43 ng/mg creatinine in the UK group and 0.18 ng/mg creatinine in the USA group, which might be due to the difference of participant ethnicity mix and the time at sample collection [15]. Similarly, Bennett MR et al. [16] established the reference intervals for urinary KIM-1 for 368 healthy children ages 3–18 years old and showed an overall median of urinary KIM-1 concentration of 0.410 ng/mL. The urinary KIM-1 concentration did not show significant difference between male and female groups. However, urinary KIM-1 was significantly higher in 15–18 year old children (0.5156 ng/mL) when compared to 3–5 year (0.3368 ng/mL) and 5–10 year olds (0.3864 ng/mL). The reference intervals established by Pennemans V et al. included 338 healthy, non-smoking subjects between 0 and 95 year old. In their study, the subjects with elevated alpha 1- microglobulin values that indicating tubular damage were excluded. Both age and gender differences were found in urinary absolute KIM-1 results [17]. Including adults from 17 to 95 year old with normal kidney function and without proteinuria that excluded glomerular and tubular kidney damage, our reference intervals did not show statistical significance on the increase of urinary KIM-1 concentrations with age increasing. The differences in male versus female urinary KIM-1 concentrations also failed to achieve statistical significance. The impact of differences in the number of male (47) versus female (100) subjects on this finding is unclear. Furthermore, there was no significant difference on creatinine normalized urinary KIM-1 based on age or sex. The currently reference intervals of urinary KIM-1 cannot be standardized due to subject population, methodology and the statistical analysis used in the calculation of reference intervals. Laboratory specific reference intervals for urinary KIM-1 need to be established with subjects excluded chronic kidney diseases before the test is used in the laboratory service.
In conclusion, this study highlighted that the increase of urinary KIM-1 in the patient with pre-existing nephropathy is related to the severity of proteinuria. Patient baseline urinary KIM-1 should be interpreted with caution if patients have chronic kidney diseases when urinary KIM-1 is used for early detection of AKI. Different reference intervals or cutoff values may need to be determined for patients with versus without pre-existing nephropathy. Urine protein should be assessed to distinguish subjects with versus without proteinuria when establishing reference intervals for urinary KIM-1, and might be considered as an adjunct test to determine whether the increase of KIM-1 is of clinical significance.
Conflict of interest
The authors declare no conflict of interest.
Funding
The study was funded by Health Care Foundation Research Funding, Eastern Health Authority, 2013. The founding sponsors had no role in the design and completion of the study and the publication.
Acknowledgements
The authors thank the technologists in the Core Lab of St. Clare's Mercy Hospital and the Renal Lab of Health Sciences Center, Eastern Health Authority for their great assistance in sample collection and measurement.
References
- 1.Ichimura T., Bonventre J.V., Bailly V., Wei H., Hession C.A., Cate R.L., Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998 Feb 13;273(7):4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 2.Han W.K., Bailly V., Abichandani R., Thadhani R., Bonventre J.V. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002 Jul;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y., Don-Wauchope A.C. The clinical utility of kidney injury molecule 1 in the prediction, diagnosis and prognosis of acute kidney injury: a systematic review. Inflamm. Allergy - Drug Targets. 2011 Aug;10(4):260–271. doi: 10.2174/187152811796117735. [DOI] [PubMed] [Google Scholar]
- 4.Dieterle F., Sistare F., Goodsaid F., Papaluca M., Ozer J.S., Webb C.P., Baer W., Senagore A., Schipper M.J., Vonderscher J., Sultana S., Gerhold D.L., Phillips J.A., Maurer G., Carl K., Laurie D., Harpur E., Sonee M., Ennulat D., Holder D., Andrews-Cleavenger D., Gu Y.Z., Thompson K.L., Goering P.L., Vidal J.M., Abadie E., Maciulaitis R., Jacobson-Kram D., Defelice A.F., Hausner E.A., Blank M., Thompson A., Harlow P., Throckmorton D., Xiao S., Xu N., Taylor W., Vamvakas S., Flamion B., Lima B.S., Kasper P., Pasanen M., Prasad K., Troth S., Bounous D., Robinson-Gravatt D., Betton G., Davis M.A., Akunda J., McDuffie J.E., Suter L., Obert L., Guffroy M., Pinches M., Jayadev S., Blomme E.A., Beushausen S.A., Barlow V.G., Collins N., Waring J., Honor D., Snook S., Lee J., Rossi P., Walker E., Mattes W. Renal biomarker qualification submission: a dialog between the FDA-EMEA and predictive safety testing consortium. Nat. Biotechnol. 2010 May;28(5):455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 5.Bieniaś B., Zajączkowska M., Borzęcka H., Sikora P., Wieczorkiewicz-Płaza A., Wilczyńska B. Early markers of tubulointerstitial fibrosis in children with idiopathic nephrotic syndrome: preliminary report. Medicine (Baltim.) 2015 Oct;94(42):e1746. doi: 10.1097/MD.0000000000001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nozaki Y., Kinoshita K., Yano T., Shiga T., Hino S., Niki K., Kishimoto K., Funauchi M., Matsumura I. Estimation of kidney injury molecule-1 (Kim-1) in patients with lupus nephritis. Lupus. 2014 Jul;23(8):769–777. doi: 10.1177/0961203314526292. [DOI] [PubMed] [Google Scholar]
- 7.CLSI (C28-A3): Defining, Establishing and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline, third ed.
- 8.de Carvalho J.A., Tatsch E., Hausen B.S., Bollick Y.S., Moretto M.B., Duarte T., Duarte M.M., Londero S.W., Premaor M.O., Comim F.V., Delanghe J.R., Moresco R.N. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as indicators of tubular damage in normoalbuminuric patients with type 2 diabetes. Clin. Biochem. 2016 Feb;49(3):232–236. doi: 10.1016/j.clinbiochem.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Aslan O., Demir M., Koseoglu M. Kidney injury molecule levels in type 2 diabetes mellitus. J. Clin. Lab. Anal. 2016 Nov;30(6):1031–1036. doi: 10.1002/jcla.21976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaidya V.S., Niewczas M.A., Ficociello L.H., Johnson A.C., Collings F.B., Warram J.H., Krolewski A.S., Bonventre J.V. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-β-D-glucosaminidase. Kidney Int. 2011 Feb;79(4):464–470. doi: 10.1038/ki.2010.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabbisetti V.S., Waikar S.S., Antoine D.J., Smiles A., Wang C., Ravisankar A., Ito K., Sharma S., Ramadesikan S., Lee M., Briskin R., De Jager P.L., Ngo T.T., Radlinski M., Dear J.W., Park K.B., Betensky R., Krolewski A.S., Bonventre J.V. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J. Am. Soc. Nephrol. 2014 Oct;25(10):2177–2186. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaidya V.S., Ramirez V., Ichimura T., Bobadilla N.A., Bonventre J.V. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am. J. Physiol. Renal. Physiol. 2006 Feb;290(2):F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 13.Kamianowska M., Szczepański M., Kulikowska E.E., Bebko B., Wasilewska A. Do serum and urinary concentrations of kidney injury molecule-1 in healthy newborns depend on birth weight, gestational age or gender? J. Perinatol. 2017 Jan;37(1):73–76. doi: 10.1038/jp.2016.169. [DOI] [PubMed] [Google Scholar]
- 14.Zwiers A.J., de Wildt S.N., de Rijke Y.B., Willemsen S.P., Abdullahi N.S., Tibboel D., Cransberg K. Reference intervals for renal injury biomarkers neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in young infants. Clin. Chem. Lab. Med. 2015 Jul;53(8):1279–1289. doi: 10.1515/cclm-2014-1020. [DOI] [PubMed] [Google Scholar]
- 15.McWilliam S.J., Antoine D.J., Sabbisetti V., Pearce R.E., Jorgensen A.L., Lin Y., Leeder J.S., Bonventre J.V., Smyth R.L., Pirmohamed M. Reference intervals for urinary renal injury biomarkers KIM-1 and NGAL in healthy children. Biomark. Med. 2014;8(10):1189–1197. doi: 10.2217/bmm.14.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett M.R., Nehus E., Haffner C., Ma Q., Devarajan P. Pediatric reference ranges for acute kidney injury biomarkers. Pediatr. Nephrol. 2015 Apr;30(4):677–685. doi: 10.1007/s00467-014-2989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pennemans V., Rigo J.M., Faes C., Reynders C., Penders J., Swennen Q. Establishment of reference values for novel urinary biomarkers for renal damage in the healthy population: are age and gender an issue? Clin. Chem. Lab. Med. 2013 Sep;51(9):1795–1802. doi: 10.1515/cclm-2013-0157. [DOI] [PubMed] [Google Scholar]