Abstract
Sphingosine kinase 2 (SPHK2) is a key factor within sphingolipid metabolism, responsible for the conversion of pro-apoptotic sphingosine to the pro-survival sphingosine-1-phosphate. We have previously shown that ABC294640, a first-in-class SPHK2 inhibitor, inhibits growth of cholangiocarcinoma cells. In a Phase I study of ABC294640 in tumors, the best response was achieved in a cholangiocarcinoma patient. These data suggest SPHK2 as a novel therapeutic target of cholangiocarcinoma. However, the antitumor mechanism of ABC294640 in cholangiocarcinoma remains not clear. In the current study, we found that ABC294640 upregulated expression of pro-apoptotic NOXA. In cholangiocarcinoma patients, high NOXA mRNA expression was associated with better overall survival. Also, SPHK2 mRNA expression was negatively correlated with NOXA mRNA expression. NOXA is known to degrade MCL1, an anti-apoptotic BCL2 protein. We showed that ABC294640 directed MCL1 for proteasome degradation. Knockdown of NOXA prevented ABC294640-induced MCL1 degradation and apoptosis. In addition, ABC294640 had a synergistic effect with BCL2/BCL-XL inhibitors ABT-263 and Obatoclax in inhibiting cell growth. Combined treatment with ABC294640 and BCL2/BCL-XL inhibitors induced potent apoptosis. Silencing of MCL1 also potentiated ABT-263-induced cytotoxicity. Furthermore, we found that both SPHK2 and MCL1 protein expression were significantly higher in cholangiocarcinoma than that in nontumoral bile ducts. SPHK2 expression correlated significantly with MCL1 expression. Our study reveals that ABC294640 inhibits cholangiocarcinoma cell growth and sensitizes the antitumor effect of BCL2/BCL-XL inhibitors through NOXA-mediated MCL1 degradation. Combinations of ABC294640 with BCL2/BCL-XL inhibitors may provide novel strategies for the treatment of cholangiocarcinoma.
Keywords: Cholangiocarcinoma, ABC294640, NOXA, MCL1, ABT-263, obatoclax
Introduction
Cholangiocarcinoma is a heterogeneous disease and is often characterized by its aggressive clinical course and poor prognosis. Most of cholangiocarcinoma patients are diagnosed at advanced stages and are not eligible for potential curative therapies (surgical resection or liver transplantation) [1]. Current standard of care for patients at advanced stages is chemotherapy, with limited efficacy. Therefore, there is an urgent and unmet need to develop more effective and less toxic treatments with cholangiocarcinoma.
Sphingosine kinase 2 (SPHK2) is a ubiquitously expressed lipid kinase that catalyze the conversion of the pro-apoptotic sphingosine to the pro-survival sphingosine-1-phosphate (S1P) [2]. SPHK2 has recently been implicated in contributing to neoplastic transformation, tumorigenesis and cancer progression, representing a novel target for cancer therapeutics [3-5]. Recent finding suggests that S1P, probably generated from SPHK2, may contribute to cholangiocarcinoma proliferation and migration [6].
ABC294640 is a first-in-class, orally-administered SPHK2 inhibitor with highly selectivity and low potential off-target inhibition of protein kinases [7]. ABC294640 has shown a good anti-tumor activity in a variety of cancers [4,8-15]. It has a good oral bioavailability and safety profile in preclinical studies [7]. Based on these strong preclinical profiles, a first-in-human phase I trial with ABC294640 was taken to determine the drug’s safety in advanced solid tumors [16]. Of note, one metastatic cholangiocarcinoma patient achieved the best response (sustained partial response, Overall Survival = 20.3 months) [16]. The other two advanced cholangiocarcinoma patients receiving ABC294640 had stable disease (Overall Survival = 17.6 and 16.3 months). We have previous demonstrated that SPHK2 is overexpressed in human cholangiocarcinoma cell lines compared to normal cholangiocytes [17]. In cholangiocarcinoma cells, ABC294640 inhibits proliferation and induces apoptosis [17]. Collectively, both our in vitro study and Phase I clinical study suggests SPHK2 as a potential novel target for the treatment of cholangiocarcinoma. The phase II study of ABC294640 in the treatment of patients with advanced cholangiocarcinoma is ongoing.
Despite the important roles of SPHK2 in cholangiocarcinoma biology, how SPHK2 regulate cholangiocarcinoma cell proliferation and apoptosis remains poorly understood. Here, we showed that SPHK2 specific inhibitor ABC294640 increased transcription of pro-apoptotic NOXA and degradation of pro-survival BCL2 family molecule MCL1 in four human cholangiocarcinoma cell lines (RBE, HCCC9810, HuH28 and HuCCT1). We demonstrated that NOXA and MCL1 played important roles in the regulation of cholangiocarcinoma cells sensitivity to ABC294640 treatment. Furthermore, ABC294640 synergized with BCL2/BCL-XL inhibitors ABT-263 and Obatoclax in inducing cholangiocarcinoma cell death. Our results provide a rationale for clinical drug studies for this combination therapy in cholangiocarcinoma.
Materials and methods
Materials
ABC294640 was purchased from Selleck (Houston, TX, USA). K145, ABT-263 (Navitoclax) and Obatoclax were purchased from Medchemexpress (Monmouth Junction, NJ, USA). Anti-β-actin primary antibody was purchased from Sigma Aldrich (St. Louis, MO, USA). Antibodies against human cleaved PARP (#5625), NOXA (#14766), BCL-XL (#2764) and MCL1 (#5453) were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibody against human BCL2 (SC-7382) was from Santa Cruz (Santa Cruz, CA, USA). Immobilon Western Chemiluminescent HRP detection kit was from Millipore (Burlington, MA, USA). The Bromodeoxyuridine (BrdU) ELISA kit was from Roche (Basel, Switzerland). Cell counting kit-8 (CCK-8) was from Dojindo Laboratories (Kyushu, Japan). Caspase-Glo 3/7 assay kit was from Promega (Madison, WI, USA). cDNA Reverse Transcription Kit and SYBR® Green qPCR detection Kit were from TaKaRA (Tokyo, Japan). RNeasy Plus Mini kit was from QIAGEN (Duesseldorf, Germany). Annexin V-FITC Apoptosis Detection kit was from BD Pharmingen (Franklin Lakes, NJ, USA). Lipofectamine RNAiMAX was from Invitrogen (Carlsbad, CA, USA). Cell culture medium was obtained from Gibco (Grand Island, NY, USA). Fetal bovine serum (FBS) was from Biological Industries (Kibbutz Beit Haemek, Israel). ABC294640 was dissolved in Dimethyl sulfoxide (DMSO) to make a stock solution of 50 mM. K145, ABT-263 and Obatoclax were dissolved in DMSO to make stock solutions of 10 mM.
Cell culture
Four human cholangiocarcinoma cell lines RBE, HCCC9810, HuH28 and HuCCT1 were used. HuH28 and HuCCT1 were provided by Lewis R.Roberts (Mayo Clinic, MN, USA), which were originally obtained from the Japanese Collection of Research Bioresources. RBE and HCCC9810 were obtained from Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China). Cell lines were authenticated using short tandem repeat profiling. All cell lines used were cultured in RPMI 1640 with 10% FBS and maintained at 37°C in the presence of 5% CO2.
BrdU cell proliferation ELISA assay
Cholangiocarcinoma cells were plated in 96-well plates at 3000 cells/well in triplicate. After 24 h, drugs were added and cells were incubated for the indicated time. The BrdU ELISA assay was performed according to the manufacturer’s instructions.
Cell viability assay
Cell viability was detected by CCK-8 assay. Cells were seeded into 96-well plates at 2000-3000 cells/well in triplicate, cultured overnight then treated with drugs for indicated time. The CCK-8 assay was performed as previously described [18]. For analysis of synergy between ABC294640 with ABT-263 and Obatoclax, cells were exposed to single drugs or different drug combinations for 72 h and cell viability was assessed by the CCK-8 assay. The combination index (CI) was determined using CompuSyn software, based on the median-effect model of Chou-Talalay [19]. A CI < 1 indicates synergism.
Annexin V-FITC apoptosis assay
Cells were seeded in 6-well plates at 105 cells/well and treated with varying concentrations of ABC294640 alone or in combination with ABT-263 and Obatoclax for 72 h. Apoptosis was assessed using the Annexin V-FITC Apoptosis Detection kit and performed according to the manufacturer’s instructions. Data were analyzed using FlowJo software.
Caspase 3/7 activity assay
Caspase 3/7 activity was analyzed using the Caspase-Glo 3/7 assay kit according to the manufacturer’s instructions. 3000 cells were seeded into 96-well white opaque plates and a corresponding optically clear 96-well plate and then allowed to adhere overnight. The next day, cells were treated with varying concentrations of indicated drugs for 48 h. At the end of the incubation time, Caspase-Glo reagent was added to each well. Plates were gently mixed and incubated for 1 h at room temperature. The luminescence was then measured in a GloMax Luminometer (Promega, Madison, WI, USA). The corresponding 96-well clear plate was used to measure the relative number of viable cells with the CCK-8 assay. Caspase 3/7 activity was normalized to viable cell number.
Western immunoblotting
Equivalent amounts of protein were separated on a 4-20% Tris-HCl gel and transferred to PVDF membranes. Membranes were probed with the appropriate primary antibodies. Blots were then incubated with horseradish peroxidase-conjugated secondary antibodies and signals were visualized using the HRP detection kit. β-actin was used as a loading control. Quantitation of the signal was performed using Image J software.
Real-time qPCR
MRNA was extracted using the RNeasy Plus Mini kit and cDNA was synthesized from 500 ng mRNA using High Capacity cDNA Reverse Transcription Kits according to the manufacturer’s instructions. Quantitative real-time PCR was done with the Real-time PCR System (Roche, Basel, Switzerland) using the SYBR qPCR detection Kit. 18S was used as the internal control. The primers used were listed in Table 1.
Table 1.
The sequences of primers for real-time RT-PCR
| Gene | Primer sequence | |
|---|---|---|
| NOXA (PMAIP1) | Forward | CTGGAAGTCGAGTGTGCTACT |
| Reverse | TCAGGTTCCTGAGCAGAAGAG | |
| MCL1 | Forward | CATTTCTTTTGGTGCCTTTGTG |
| Reverse | CCAGTCCCGTTTTGTCCTTAC | |
| BCL2 | Forward | CATGTGTGTGGAGAGCGTCAA |
| Reverse | GCCGGTTCAGGTACTCAGTCA | |
| BCL-XL | Forward | GACAAGGAGATGCAGGTATTGG |
| Reverse | TCCCGTAGAGATCCACAAAAGT | |
| BIM | Forward | TAAGTTCTGAGTGTGACCGAGA |
| Reverse | GCTCTGTCTGTAGGGAGGTAGG | |
| BAD | Forward | CCCAGAGTTTGAGCCGAGTG |
| Reverse | CCCATCCCTTCGTCGTCCT | |
| BAX | Forward | CACCAGCTCTGAGCAGATCATGAAG |
| Reverse | GCGGCAATCATCCTCTGCAG | |
| BAK | Forward | CCCAGGACACAGAGGAGGTTT |
| Reverse | GCCTCCTGTTCCTGCTGATG | |
| BID | Forward | GGAGGAGGACCGGAACAGG |
| Reverse | GAAAGACATCACGGAGCAAGGAC | |
| BIK | Forward | TCTGCAATTGTCACCGGTTA |
| Reverse | TTGAGCACACCTGCTCCTC | |
| 18S | Forward | TTGGAGGGCAAGTCTGGTG |
| Reverse | CCGCTCCCAAGATCCAACTA | |
RNA interfering
SiRNA targeting human NOXA (5’-GGGUAUCUGUAUAGAUAAUTT-3’ and 5’-AUUAUCUAUACAGAUACCCTT-3’) and MCL1 (5’-GAUUAUCUCUCGGUACCUUTT-3’ and 5’-AAGGUACCGAGAGAUAAUCTT-3’) were synthesized by GenePharma (Shanghai, China). For gene knockdown experiments, cells were plated in 6-well plates 24 h before transfection and were then transfected with 50 nM of siRNA using Lipofectamine RNAiMAX, as per the manufacturer’s protocol. Cells were used at 48 h after transfection for further experiments.
Immunohistochemistry (IHC) assay
The cholangiocarcinoma tissue microarray slides (HBiDC122Su01) were obtained from SHANGHAI OUTDO BIOTECH (Shanghai, China). The microarray was built by SHANGHAI OUTDO BIOTECH using tissues from National Human Genetic Resources Sharing Service Platform (2005DKA21300). The study methodologies conformed to the standards set by the Declaration of Helsinki. IHC was performed to determine the protein expression of SPHK2 and MCL1 in cholangiocarcinoma and nontumoral surrounding intrahepatic bile ducts using the primary antibodies of SPHK2 from Abgent (AP7238b, San Diego, CA, USA) and MCL1 from Abcam (ab32087, Cambridge, MA, USA). Staining intensity was graded as follows: 0 (negative), 1 (weak staining), 2 (moderate staining) and 3 (strong staining). The percentage of staining was graded as follows: 0 (no positive cells), 1 (< 25% positive cells), 2 (25%-50% positive cells), 3 (50%-75% positive cells) and 4 (≥ 75% positive cells). The total score was calculated by combining the two parameters. Immunohistochemical staining was analyzed by two pathologists in a blinded manner.
Dataset analysis
Publicly available cholangiocarcinoma dataset GSE26566 was downloaded and used to analyze the correlation of SPHK2 and NOXA [20]. Moreover, publicly available data generated by The Cancer Genome Atlas (TCGA) Research Network (http://cancergenome.nih.gov/) was used to perform survival-outcome analyses [21].
Statistical analysis
All statistical tests were conducted with GraphPad Prism 6.0. The half maximal inhibitory concentration (IC50) was calculated using nonlinear regression analysis in Prism 6.0. Parametric Student’s t test or nonparametric Mann-Whitney test were used to compare two groups. In experiments involving more than two groups, one-way ANOVA with a Turkey post hoc test was used. Results were considered statistically significant at P < 0.05.
Results
ABC294640 inhibits proliferation and induces apoptosis of RBE and HCCC9810 cells
Previous data from our team showed that ABC294640 decreases the proliferation of six cholangiocarcinoma cell lines (HuH28, HuCCT1, WITT, EGI-1, OZ and LIV27) [17]. In the current study, we evaluated its effect on two additional cholangiocarcinoma cell lines RBE and HCCC9810. Cholangiocarcinoma cells were exposed to increasing concentrations of ABC294640 for 72 h and cell proliferation was evaluated by BrdU ELISA assay. ABC294640 dose-dependently inhibited RBE and HCCC9810 cell proliferation with IC50 33.03 μM and 42.49 μM respectively (Figure 1A). To characterize ABC294640-induced cytotoxicity, apoptotic cell death was assessed by Annexin V/PI double staining. Decrease in cell viability and increase in apoptosis were observed in both RBE and HCCC9810 cells after 50 μM ABC294640 treatment for 72 h (Figure 1B and 1C), consistent with our previous study using other cholangiocarcinoma cell lines. Collectively, these data further prove that SPHK2 may play a role in the regulation of cholangiocarcinoma proliferation and apoptosis.
Figure 1.
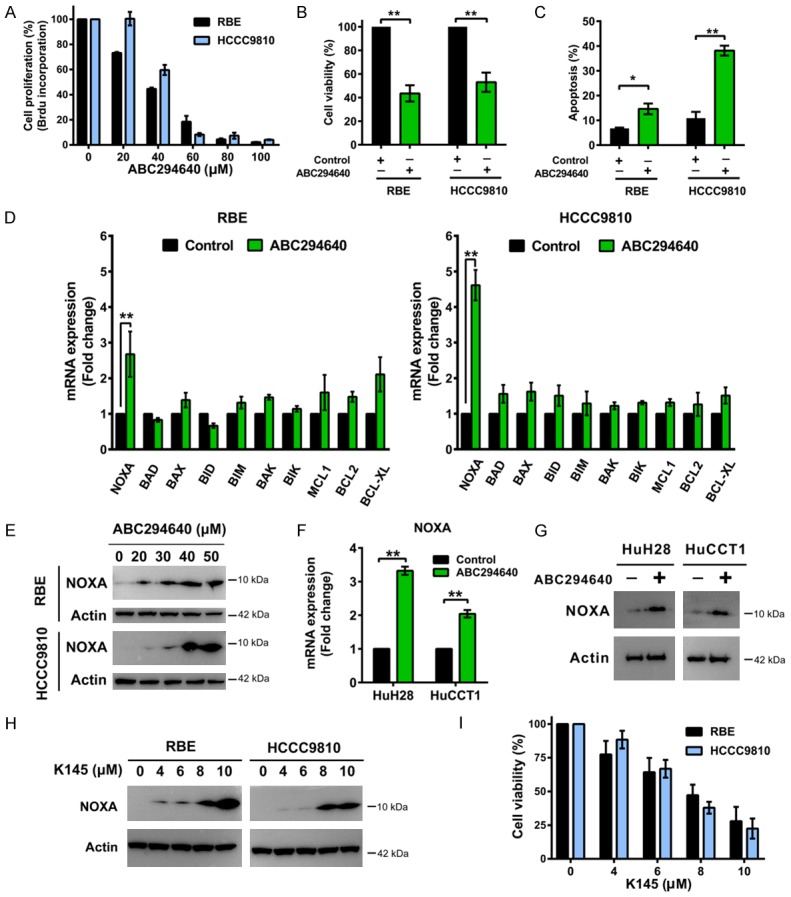
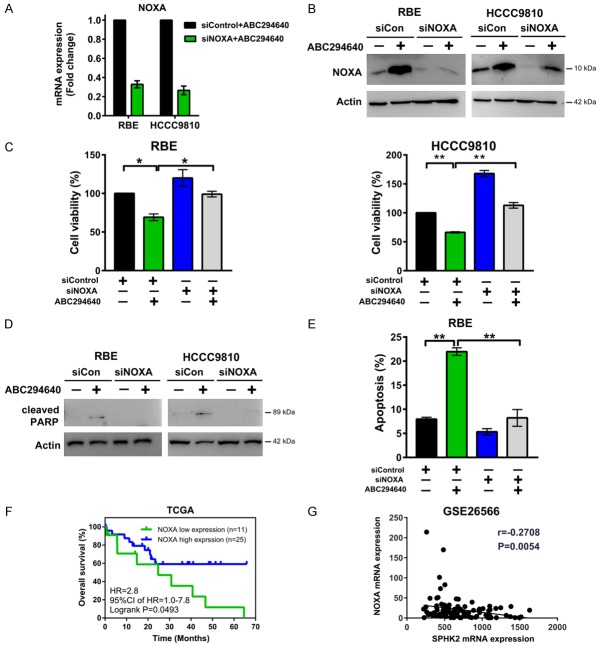
SPHK2 inhibition suppresses cholangiocarcinoma cell growth, induces apoptosis and upregulates NOXA expression. A. RBE and HCCC9810 cells were treated with ABC294640 for 72 h and cell proliferation was quantified by BrdU ELISA assay. B. Cells were treated with ABC294640 at 50 μM for 72 h and cell viability was determined by CCK-8 assay. C. Cells were treated with ABC294640 at 50 μM for 72 h and cell apoptosis was then measured by Annexin V-FITC/PI labeling followed by flow cytometry. D. Real-time qPCR analysis of BCL2 family mRNA level in RBE and HCCC9810 cells treated with 50 μM ABC294640 or no drug control for 24 h. E. Western immunoblotting analysis of NOXA protein levels in RBE and HCCC9810 cells treated with different concentrations of ABC294640 for 24 h. Data shown represents 3 independent experiments. F. Real-time qPCR analysis of NOXA mRNA level in HuH28 and HuCCT1 cells treated with 50 μM ABC294640 for 24 h. G. Western immunoblotting analysis of NOXA protein levels in HuH28 and HuCCT1 cells treated with 50 μM ABC294640 or no drug control for 24 h. Data shown represents 3 independent experiments. H. Western immunoblotting analysis of NOXA protein levels in RBE and HCCC9810 cells treated with different concentrations of K145 for 24 h. Data shown represents 2 independent experiments. I. RBE and HCCC9810 cells were treated with different concentrations of K145 for 72 h and cell viability were determined by CCK-8 assay. Quantitative analysis from 3 independent experiments (Student’s t test; data are shown as mean ± SEM; *P < 0.05, **P < 0.01) are shown.
ABC294640 induces pro-apoptotic NOXA expression
The BCL2 protein family, which includes both pro-apoptotic and anti-apoptotic proteins, is a major regulator of cell apoptosis [22]. To investigate the underlying molecular mechanism by which SPHK2 regulates cholangiocarcinoma cell survival and apoptosis, we first evaluated the expression of several common genes in the BCL2 family in RBE and HCCC9810 cells, including NOXA, BAX, BAK, BID, BIM, BAD, BIK, MCL1, BCL2 and BCL-XL, using real-time qPCR. We observed significant induction of NOXA (PMAIP1) mRNA levels when cells were treated by 50 μM ABC294640 for 24 h in both RBE and HCCC9810 cells (Figure 1D). Also, ABC294640 dose-dependently increased the protein level of NOXA in these two cell lines (Figure 1E). Upregulation of NOXA mRNA and protein level by ABC294640 was also observed in the other two cholangiocarcinoma cell lines (HuH28 and HuCCT1) (Figure 1F and 1G). K145 is another recently reported SPHK2 specific inhibitor. It does not inhibit SPHK1 with a concentration up to 10 μM [23]. Likewise, treatment of RBE and HCCC9810 cells with K145 showed a dose- dependent increase in NOXA expression (Figure 1H), confirming this NOXA inducing mechanism is mediated through SPHK2. Consistent with its effects on NOXA, K145 blocked cell survival in a dose-dependent manner (Figure 1I). Our results showed that SPHK2 inhibition upregulated pro-apoptotic NOXA expression in cholangiocarcinoma cells.
NOXA plays important role in regulation of cholangiocarcinoma cell apoptosis to SPHK2 inhibition
To determine whether NOXA is a critical component in defining the therapeutic efficacy of ABC294640, we functionally silenced NOXA mRNA expression and assessed the response of RBE and HCCC9810 cells to treatment. Silencing of NOXA by RNAi greatly reduced the NOXA mRNA and protein expression with cholangiocarcinoma cells exposed to ABC294640 (Figure 2A and 2B). Further analysis showed that loss of NOXA expression effectively protected cholangiocarcinoma cells from cell death induced by ABC294640 in both RBE and HCCC9810 cells (Figure 2C-E). We then determined the clinical relevance of NOXA expression levels to cholangiocarcinoma patient outcome through analyzing the TCGA dataset of cholangiocarcinoma patient samples [21]. Kaplan-Meier survival analysis revealed that patients with high tumor NOXA mRNA expression levels was associated with longer overall survival (P < 0.05) (Figure 2F). In addition, SPHK2 mRNA expression is negatively correlated with NOXA mRNA expression in the cholangiocarcinoma dataset GSE26566 (P < 0.01) [20] (Figure 2G). Together, these data suggest a role for NOXA in enabling cytotoxicity after SPHK2 inhibition in cholangiocarcinoma cells.
Figure 2.
SPHK2 regulates cholangiocarcinoma cell apoptosis via NOXA. A. Cells were transfected with 50 nM Control siRNA (siCon) or NOXA siRNA (siNOXA) for 48 h and then treated with 50 μM ABC294640 for 48 h. NOXA mRNA level was analyzed by Real-time qPCR. B. Cells were transfected with 50 nM Control siRNA (siCon) or NOXA siRNA (siNOXA) for 48 h and then treated with 50 μM ABC294640 for 24 h. NOXA protein level was analyzed by Western immunoblotting. C-E. Cells were transfected with 50 nM Control siRNA (siCon) or NOXA siRNA (siNOXA) for 48 h and then treated with 50 μM ABC294640 for 72 h. Cell viability was determined by CCK-8 assay. Apoptosis was analyzed by PARP cleavage through Western immunoblotting in both cell lines and by Annexin V-FITC/PI labeling followed by flow cytometry in RBE cells. F. Kaplan-Meier curves showing the overall survival rate of 36 patients in the TCGA dataset according to the expression status of NOXA. G. Correlation between SPHK2 mRNA expression and NOXA mRNA expression in a cholangiocarcinoma dataset (GSE26566). The r and P values were determined by Pearson correlation analysis. Quantitative analysis from 3 independent experiments (one-way ANOVA with a Turkey post hoc test; data are shown as mean ± SEM; *P < 0.05, **P < 0.01) are shown.
Pro-apoptotic effects of SPHK2 inhibition are mediated by MCL1 degradation
Cholangiocarcinoma cells frequently overexpress the anti-apoptotic BCL2 family member MCL1 to inhibit apoptosis and promote cell survival [24]. Because previous study shows that NOXA can promote intrinsic apoptosis through proteasomal degradation of the anti-apoptotic protein MCL1 [25,26], we examined the changes in protein levels of MCL1 and along with two other pro-survival BCL2 family members BCL2 and BCL-XL after ABC294640 treatment. After 24 h treatment, ABC294640 dose-dependently reduced protein levels of MCL1 without decreasing the expression of BCL2 and BCL-XL (Figure 3A and 3B). Notably, this loss of MCL1 protein was not associated with the reduction of MCL1 mRNA expression (Figure 1D), suggestive of post-transcriptional regulation of MCL1 expression. Instead, we observed that MCL1 mRNA was slightly elevated by ABC294640, which may compensate for its protein loss. We then tested if ABC294640 treatment promoted MCL1 degradation in a proteasome-dependent manner. RBE and HCCC9810 cells were treated with ABC294640 alone, proteasome inhibitor MG132 alone or ABC294640 in combination of MG132 for 12 h. Indeed, MG132 protected MCL1 from degradation induced by ABC294640 treatment (Figure 3C). Similar effects of ABC294640 on MCL1 expression were observed in two other cholangiocarcinoma cell lines (HuH28 and HuCCT1) tested (Figure 3D and 3E). Following knock down of NOXA, MCL1 degradation was in part rescued (Figure 3F and 3G). These data suggested that ABC294640 decreased pro-survival MCL1 expression probably through NOXA mediated proteasome degradation.
Figure 3.
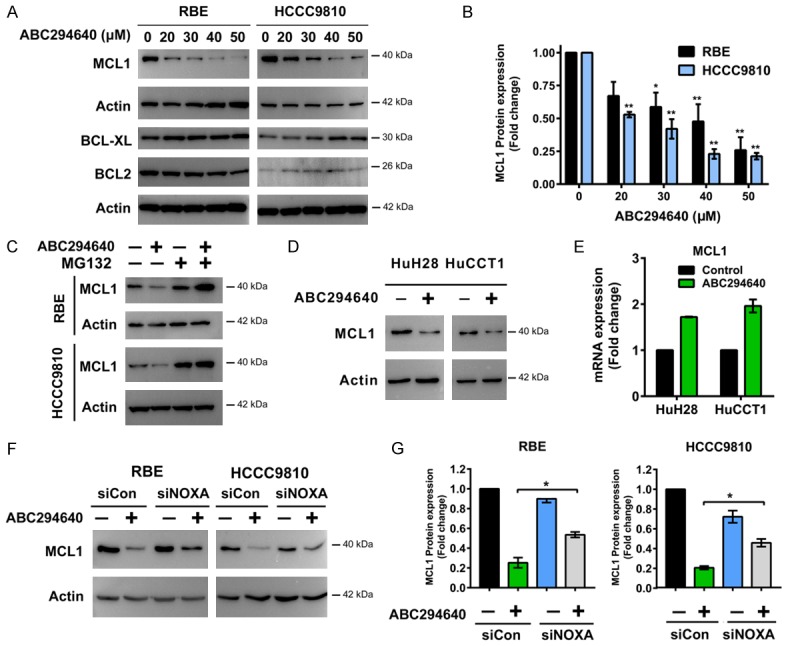
ABC294640 induces degradation of pro-survival protein MCL1. A, B. Western immunoblotting analysis of MCL1, BCL-XL and BCL2 protein levels in RBE and HCCC9810 cells treated with different concentrations of ABC294640 for 24 h. MCL1 protein level following ABC294640 treatment for 24 h was quantified using Image J. Quantitative analysis from three independent experiments (one-way ANOVA with a Turkey post hoc test; data are shown as mean ± SEM; *P < 0.05, **P < 0.01) are shown. C. RBE and HCCC9810 cells treated with no drug control buffer or Proteasome inhibitor MG132 (200 nM) for 2 h, followed by treatment with 50 μM of ABC294640 for additional 12 h. Whole cell lysate was prepared and analyzed for MCL1 expression by Western immunoblotting. Result shown represents 2-3 independent experiments. D. Western immunoblotting analysis of MCL1 protein levels in HuH28 and HuCCT1 cells treated with 50 μM of ABC294640 for 24 h. Result shown represents 3 independent experiments. E. Real-time qPCR analysis of MCL1 mRNA levels in HuH28 and HuCCT1 cells treated with 50 μM of ABC294640 for 24 h. Data are shown as mean ± SEM from 3 independent results. F. Cells were transfected with 50 nM Control siRNA (siCon) or NOXA siRNA (siNOXA) for 48 h and then treated with 50 μM ABC294640 for 24 h. MCL1 protein level was analyzed by Western immunoblotting. Result shown represents 2-3 independent experiments. G. MCL1 protein level of previous experiment was quantified using Image J. Quantitative analysis from 2-3 independent experiments (one-way ANOVA with a Turkey post hoc test; data are shown as mean ± SEM; *P < 0.05) are shown.
ABC294640 synergizes with BCL2/BCL-XL inhibitors ABT-263 and Obatoclax in inducing cholangiocarcinoma cell death
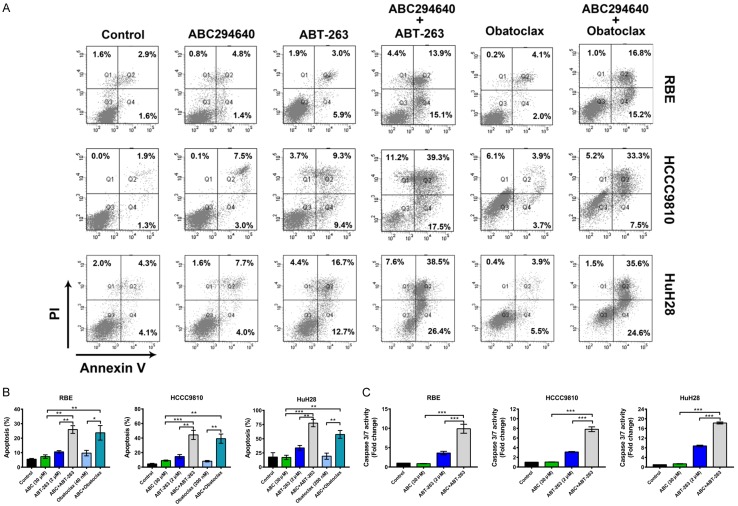
The BH3-mimetic ABT-263, which inhibits the other pro-survival BCL2 family proteins (BCL2, BCL-XL and BCL-W), but not MCL1, shows promise for treating tumors which have low dependency on MCL1 for cell survival [27]. Cholangiocarcinoma cells appear resistant to ABT-263 due to elevated expression of MCL1 [28]. Therefore, we examined whether targeting MCL1 levels via SPHK2 inhibition could sensitize cholangiocarcinoma cells to ABT-263. Indeed, we found that sub-cytotoxic combinations of ABC294640 and ABT-263 induced synergistic cell death in all three cholangiocarcinoma cells (RBE, HCCC9810 and HuH28) tested (Figure 4A, CI < 1). Then, we extended our investigations to another BH3-mimetic Obatoclax. Obatoclax differs from ABT-263 in that in addition to targeting BCL2/BCL-XL/BCL-W, it also targets MCL1 and Bfl-1. Similar to ABT-263, obvious synergism was observed between Obatoclax and ABC294640 (Figure 4B, CI < 1). Apoptosis induction by ABT-263 and Obatoclax was also markedly enhanced by ABC294640 in all three cholangiocarcinoma cells assessed by Annexin V/PI staining assay (Figure 5A and 5B). Additionally, combined inhibition of SPHK2 and BCL2/BCL-XL induced a strong increase in Caspase 3/7 activity (Figure 5C). Taken together, ABC294640 acted synergistically with ABT-263 and Obatoclax in reducing cell viability and inducing apoptosis in cholangiocarcinoma cells.
Figure 4.
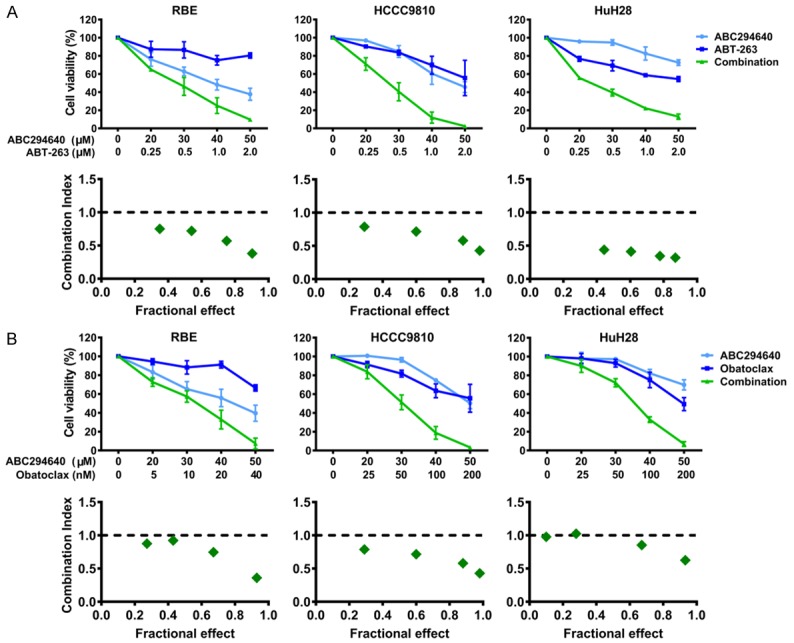
ABC294640 acts synergistically with BH3-mimetics in inhibiting cholangiocarcinoma cell growth. A. RBE, HCCC9810 and HuH28 cells were treated with various concentrations of ABC294640 or ABT-263 alone or their combination for 72 h, then the cell viability was analyzed by CCK-8 assay. The results are presented as mean ± SEM from 3-4 independent experiments. The combination index (CI) was determined using the Chou-Talalay Method. CI < 1 indicates that the interaction between ABC294640 and ABT-263 was synergistic. B. RBE, HCCC9810 and HuH28 cells were treated with various concentrations of ABC294640 or Obatoclax alone or their combination for 72 h, then the cell viability was analyzed by CCK-8 assay. The results are presented as mean ± SEM from 3-4 independent experiments. CI was determined using the Chou-Talalay Method. CI < 1 indicates that the interaction between ABC294640 and Obatoclax was synergistic.
Figure 5.
ABC294640 potentiates apoptosis induced by BH3-mimetics in cholangiocarcinoma cells. A, B. RBE, HCCC9810 and HuH28 cells were treated with ABC294640 (ABC) alone or in combination with ABT-263 or Obatoclax at indicated concentrations for 72 h. Cell apoptosis was measured by Annexin V-FITC/PI staining followed by flow cytometry. C. RBE, HCCC9810 and HuH28 cells were treated with ABC294640 alone or in combination with ABT-263 at indicated concentration for 48 h. Cell apoptosis was determined by the Caspase 3/7 activity assay. Quantitative analysis from 3 independent experiments (one-way ANOVA with a Turkey post hoc test; data are shown as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001) are shown.
Silencing of MCL1 enhances the antitumor effects of ABT-263
We knocked down MCL1 by specific siRNA and observed that silencing MCL1 potently sensitized RBE and HCCC9810 cells to the cytotoxic effects of ABT-263 (Figure 6A and 6B), proving that MCL1 levels are key regulators in ABT-263 mediated apoptosis and ABC294640 may sensitize cholangiocarcinoma cells to BCL2/BCL-XL inhibitors mainly through inhibition of MCL1.
Figure 6.
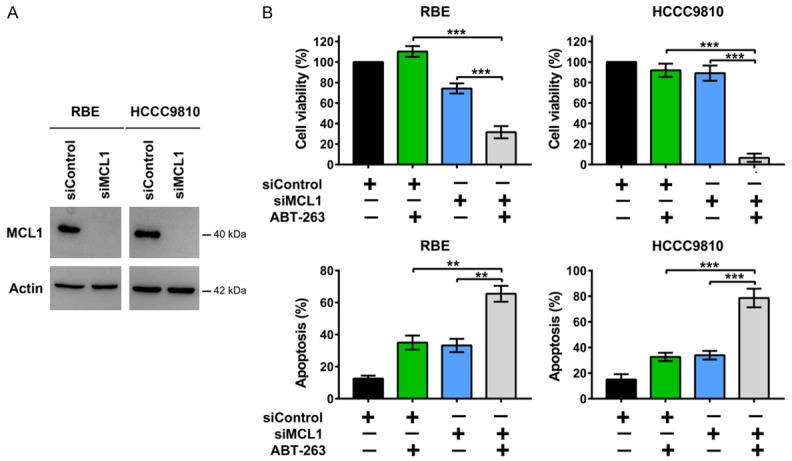
Silencing of MCL1 enhances the antitumor effects of ABT-263. A. Western immunoblotting analysis of MCL1 protein levels in RBE and HCCC9810 cells transfected with siRNA for 48 h. Data shown represents 2 independent experiments. B. RBE and HCCC9810 cells were transfected with control siRNA or MCL1 siRNA for 48 h and then treated with ABT-263 at indicated concentrations for 48 h. Cell viability was determined by CCK-8 assay. Cell apoptosis was measured by Annexin V-FITC/PI staining followed by flow cytometry. Quantitative analysis from 3 independent experiments (one-way ANOVA with a Turkey post hoc test; data are shown as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001) are shown.
SPHK2 and MCL1 are overexpressed in cholangiocarcinoma and has a positive correlation
We have done immunohistochemistry analysis of SPHK2 and MCL1 in cholangiocarcinoma tissue microarray slides. We found that both SPHK2 and MCL1 protein expression were significantly higher in cholangiocarcinoma than that in nontumoral bile ducts (Figure 7A-D). Additionally, SPHK2 expression had a significant correlation with MCL1 expression in the cholangiocarcinoma tissue microarray (r = 0.4218, P < 0.0001) (Figure 7E).
Figure 7.
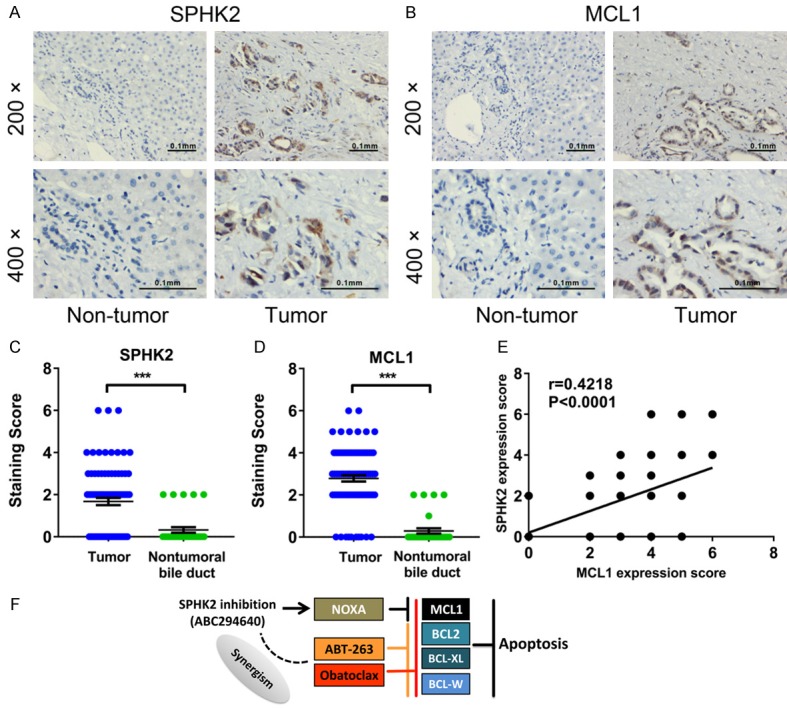
SPHK2 and MCL1 are overexpressed in cholangiocarcinoma and has a positive correlation. A, B. Immunohistochemical staining with anti-SPHK2 and anti-MCL1 antibodies were performed on 89 human cholangiocarcinoma tissues and 31 adjacent non-tumor tissues. Representative images were shown (200× and 400×). C, D. Semi-quantitative analysis of immunohistochemical staining showed that SPHK2 and MCL1 protein expression were upregulated in cholangiocarcinoma in the tissue microarray. Mann-Whitney test were used to compare two groups. ***P < 0.001. E. The expression level of SPHK2 showed a significant positive correlation with MCL1 expression in the tissue microarray. The r and P values were determined by Spearman correlation analysis. F. Schematic representation of the mechanisms of action of ABC294640 in cholangiocarcinoma cells.
Discussion
S1P has been proposed to contribute to tumor proliferation, migration and angiogenesis in a number of cancers including cholangiocarcinoma. As S1P is converted by SPHK1 and SPHK2, both two isoforms can be therapeutic target in cholangiocarcinoma. SK1-I is the specific inhibitor of SPHK1 [29]. Chen et al demonstrated that SK1-I has potent antiproliferative activity in cholangiocarcinoma in vitro and in vivo [30]. The findings of this study and our study suggest that both SPHK1 and SPHK2 have important roles in inducing cholangiocarcinoma cell survival. Liu at al analyzed mRNA levels of SPHK1 and SPHK2 in rat and human cholangiocarcinoma cell lines and found that SPHK2 mRNA expression being predominant over that of SPHK1 in the cholangiocarcinoma cells, suggesting SPHK2 may play a relatively more important role than SPHK1 in cholangiocarcinoma [6]. However, SPHK1 inhibitors will provide additional opportunities to discover effective therapies in cholangiocarcinoma.
We have previously shown that SPHK2 is overexpressed in cholangiocarcinoma cell lines and its inhibition suppresses cholangiocarcinoma cell proliferation and survival [17]. In this study, we provide new mechanistic insights regarding how SPHK2 regulate cholangiocarcinoma cell survival. These data indicate that (1) SPHK2 negatively regulates pro-apoptotic NOXA mRNA expression; (2) SPHK2 promotes MCL1 stabilization probably through NOXA inhibition, providing resistance to cell death induced by BH3- mimetics ABT-263 and Obatoclax. These findings are summarized and illustrated in Figure 7F and discussed in greater detail below.
ABC294640 is a competitive inhibitor of SPHK2 with respect to sphingosine, which has minimal off-target effects. In vitro, it does not inhibit SPHK1 at concentration up to at least 100 μM [7]. ABC294640 depletes S1P, elevates ceramide and suppresses ERK and AKT proliferative signaling in tumor cells [10,31]. Recently, ABC294640 has also been shown to down-regulate oncogenic c-Myc expression in a variety of tumor cell lines through both transcriptional and post-transcriptional mechanisms [4,9,10,14].
Here, we identified selective induction of pro-apoptotic NOXA mRNA expression after SPHK2 inhibition in cholangiocarcinoma cell lines. NOXA knockdown afforded significant protection from ABC294640-induced apoptosis, demonstrating the critical role of NOXA in the cytotoxicity of ABC294640. Furthermore, high NOXA mRNA expression within cholangiocarcinoma tumors correlates with longer overall survival in the TCGA dataset. An inverse correlation was also observed between SPHK2 and NOXA mRNA expression in cholangiocarcinoma patients. These data suggest NOXA may be a critical downstream target of SPHK2.
Pro-survival BCL2 family protein MCL1 is the well-known target of NOXA. Previous studies have demonstrated that when NOXA is expressed, it is localized at the mitochondria via its mitochondrial targeting domain [25]. Then MCL1 is recruited from the cytosol to the mitochondria by binding to the BH3 domain of NOXA, which initiates MCL1 phosphorylation and subsequent ubiquitination triggering proteasome-mediated degradation [25,32]. Consistent with it, we subsequently found that SPHK2 inhibition in cholangiocarcinoma cells resulted in loss of MCL1 through proteasome-dependent degradation, whereas BCL2 and BCL-XL expression were unaffected. Many studies have shown that MCL1 has a critical role in a variety of hematological malignancies and solid tumors including cholangiocarcinoma [33,34]. In particular, MCL1 has been implicated in drug resistance in cholangiocarcinoma [24,35]. In addition, MCL1 is frequently amplified in cholangiocarcinoma [36,37]. We and others previously have shown that MCL1 is critical for cholangiocarcinoma survival [18,24]. In the current study, we found that MCL1 was overexpressed in cholangiocarcinoma. Thus, our new finding is of particular interest.
Targeting anti-apoptotic proteins BCL2, BCL-XL and BCL-W with BH3-mimetics such as ABT-263 represents one of the most promising antitumor drugs [27]. However, ABT-263 has shown limited success against cancers with high MCL1 expression [28,38]. Obatoclax, another BH3-mimetic, which inhibits MCL1 in addition to BCL2/BCL-XL/BCL-W, has been limited by its neurologic toxicity [27]. Therefore, many groups have developed combinational therapies whereby BCL2/BCL-XL inhibitors were combined with inhibitors of MCL1. Targeting these cooperative survival signaling pathways may overcome drug resistance, improve therapeutic efficacy and reduce side effects [25,39-42]. Previous study using short hairpin RNA-targeted knockdown of MCL1 sensitized KMCH cholangiocarcinoma cells to ABT-263 mediated apoptosis [28]. Therefore, we further explored whether targeting SPHK2 would be similarly effective in restoring cholangiocarcinoma cell susceptibility to BCL2/BCL-XL inhibitors. Indeed, ABC294640 synergized with BH3-mimetics ABT-263 and Obatoclax in inhibiting cell survival and inducing cell apoptosis in three cholangiocarcinoma cell lines. As a first-in-class SPHK2 inhibitor, ABC294640 shows promise against cholangiocarcinoma in both in vitro experiments and first-in-human clinical trial. As we demonstrated that targeting SPHK2 with ABC294640 and BCL2/BCL-XL inhibitors induced synergistic lethality in cholangiocarcinoma, these rational combinational therapies may prove more efficacious for the treatment of cholangiocarcinoma.
S63845 is a specific MCL1 inhibitor [34]. S63845 has been shown to have combination effect with BCL-2 inhibitor ABT-199 or BCL-2/BCL-XL inhibitor ABT-263 in different cancer models [43,44]. Thus, S63845 may also have synergistic effect with BCL2/BCL-XL inhibitors in cholangiocarcinoma. However, ABC294640 also provide an apoptotic stimulus through inhibition AKT, STAT3 or other survival signaling pathways. Therefore, ABC294640 may be superior to MCL1 inhibitors in the combination therapy. In any case, the availability of MCL1 inhibitors will provide new opportunities to discover efficacious combinations that may be effective in cholangiocarcinoma.
Our results suggest that SPHK2 may regulate cholangiocarcinoma cell survival through inhibition of NOXA expression and MCL1 proteasomal degradation. Our data also provide preliminary insight into the possible use of SPHK2 inhibitor ABC294640 in combination with BCL2/BCL-XL inhibitors such as ABT-263 and Obatoclax to improve the treatment of cholangiocarcinoma.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81602095, No. 81672935 and No. 81472756), the Natural Science Foundation from the Department of Science and Technology of Jiangsu Province (No. BK20160111), the Fundamental Research Funds for the Central Universities (No. 021414380140). We thank the Hongyan Wu and Jun Yang from Department of Pathology (Nanjing Drum Tower Hospital) for their technical assistance of Immunohistochemistry.
Disclosure of conflict of interest
None.
References
- 1.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evangelisti C, Evangelisti C, Buontempo F, Lonetti A, Orsini E, Chiarini F, Barata JT, Pyne S, Pyne NJ, Martelli AM. Therapeutic potential of targeting sphingosine kinases and sphingosine 1-phosphate in hematological malignancies. Leukemia. 2016;30:2142–2151. doi: 10.1038/leu.2016.208. [DOI] [PubMed] [Google Scholar]
- 3.Weigert A, Schiffmann S, Sekar D, Ley S, Menrad H, Werno C, Grosch S, Geisslinger G, Brune B. Sphingosine kinase 2 deficient tumor xenografts show impaired growth and fail to polarize macrophages towards an anti-inflammatory phenotype. Int J Cancer. 2009;125:2114–2121. doi: 10.1002/ijc.24594. [DOI] [PubMed] [Google Scholar]
- 4.Wallington-Beddoe CT, Powell JA, Tong D, Pitson SM, Bradstock KF, Bendall LJ. Sphingosine kinase 2 promotes acute lymphoblastic leukemia by enhancing MYC expression. Cancer Res. 2014;74:2803–2815. doi: 10.1158/0008-5472.CAN-13-2732. [DOI] [PubMed] [Google Scholar]
- 5.Neubauer HA, Pham DH, Zebol JR, Moretti PA, Peterson AL, Leclercq TM, Chan H, Powell JA, Pitman MR, Samuel MS, Bonder CS, Creek DJ, Gliddon BL, Pitson SM. An oncogenic role for sphingosine kinase 2. Oncotarget. 2016;7:64886–64899. doi: 10.18632/oncotarget.11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu R, Zhao R, Zhou X, Liang X, Campbell DJ, Zhang X, Zhang L, Shi R, Wang G, Pandak WM, Sirica AE, Hylemon PB, Zhou H. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology. 2014;60:908–918. doi: 10.1002/hep.27085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, Upson JJ, Green CL, Keller SN, Smith CD. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther. 2010;333:129–139. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoon JW, White MD, Slaughter EM, Driver JL, Khalili HS, Elliott S, Smith CD, Burow ME, Beckman BS. Targeting NFkB mediated breast cancer chemoresistance through selective inhibition of sphingosine kinase-2. Cancer Biol Ther. 2011;11:678–689. doi: 10.4161/cbt.11.7.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkata JK, An N, Stuart R, Costa LJ, Cai H, Coker W, Song JH, Gibbs K, Matson T, Garrett-Mayer E, Wan Z, Ogretmen B, Smith C, Kang Y. Inhibition of sphingosine kinase 2 downregulates the expression of c-Myc and Mcl-1 and induces apoptosis in multiple myeloma. Blood. 2014;124:1915–1925. doi: 10.1182/blood-2014-03-559385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrecengost RS, Keller SN, Schiewer MJ, Knudsen KE, Smith CD. Downregulation of critical oncogenes by the selective SK2 inhibitor ABC294640 hinders prostate cancer progression. Mol Cancer Res. 2015;13:1591–1601. doi: 10.1158/1541-7786.MCR-14-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venant H, Rahmaniyan M, Jones EE, Lu P, Lilly MB, Garrett-Mayer E, Drake RR, Kraveka JM, Smith CD, Voelkel-Johnson C. The sphingosine kinase 2 inhibitor ABC294640 reduces the growth of prostate cancer cells and results in accumulation of dihydroceramides in vitro and in vivo. Mol Cancer Ther. 2015;14:2744–2752. doi: 10.1158/1535-7163.MCT-15-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xun C, Chen MB, Qi L, Tie-Ning Z, Peng X, Ning L, Zhi-Xiao C, Li-Wei W. Targeting sphingosine kinase 2 (SphK2) by ABC294640 inhibits colorectal cancer cell growth in vitro and in vivo. J Exp Clin Cancer Res. 2015;34:94. doi: 10.1186/s13046-015-0205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Yang C, Zhang S, Mei Z, Shi M, Sun S, Shi L, Wang Z, Wang Y, Li Z, Xie C. ABC294640, a sphingosine kinase 2 inhibitor, enhances the antitumor effects of TRAIL in non-small cell lung cancer. Cancer Biol Ther. 2015;16:1194–1204. doi: 10.1080/15384047.2015.1056944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis CS, Voelkel-Johnson C, Smith CD. Suppression of c-Myc and RRM2 expression in pancreatic cancer cells by the sphingosine kinase-2 inhibitor ABC294640. Oncotarget. 2016;7:60181–60192. doi: 10.18632/oncotarget.11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai L, Bai A, Smith CD, Rodriguez PC, Yu F, Qin Z. ABC294640, a novel sphingosine kinase 2 inhibitor induces oncogenic virus infected cell autophagic death and represses tumor growth. Mol Cancer Ther. 2017;16:2724–2734. doi: 10.1158/1535-7163.MCT-17-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Britten CD, Garrett-Mayer E, Chin SH, Shirai K, Ogretmen B, Bentz TA, Brisendine A, Anderton K, Cusack SL, Maines LW, Zhuang Y, Smith CD, Thomas MB. A phase I study of ABC294640, a first-in-class sphingosine kinase-2 inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2017;23:4642–4650. doi: 10.1158/1078-0432.CCR-16-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding X, Chaiteerakij R, Moser CD, Shaleh H, Boakye J, Chen G, Ndzengue A, Li Y, Zhou Y, Huang S, Sinicrope FA, Zou X, Thomas MB, Smith CD, Roberts LR. Antitumor effect of the novel sphingosine kinase 2 inhibitor ABC294640 is enhanced by inhibition of autophagy and by sorafenib in human cholangiocarcinoma cells. Oncotarget. 2016;7:20080–20092. doi: 10.18632/oncotarget.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding X, Zhang B, Pei Q, Pan J, Huang S, Yang Y, Zhu Z, Lv Y, Zou X. Triptolide induces apoptotic cell death of human cholangiocarcinoma cells through inhibition of myeloid cell leukemia-1. BMC Cancer. 2014;14:271. doi: 10.1186/1471-2407-14-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 20.Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, Conner EA, Gillen MC, Roskams T, Roberts LR, Factor VM, Thorgeirsson SS. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–1031. e1015. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farshidfar F, Zheng S, Gingras MC, Newton Y, Shih J, Robertson AG, Hinoue T, Hoadley KA, Gibb EA, Roszik J, Covington KR, Wu CC, Shinbrot E, Stransky N, Hegde A, Yang JD, Reznik E, Sadeghi S, Pedamallu CS, Ojesina AI, Hess JM, Auman JT, Rhie SK, Bowlby R, Borad MJ Cancer Genome Atlas Network. Zhu AX, Stuart JM, Sander C, Akbani R, Cherniack AD, Deshpande V, Mounajjed T, Foo WC, Torbenson MS, Kleiner DE, Laird PW, Wheeler DA, McRee AJ, Bathe OF, Andersen JB, Bardeesy N, Roberts LR, Kwong LN. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;18:2780–2794. doi: 10.1016/j.celrep.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu K, Guo TL, Hait NC, Allegood J, Parikh HI, Xu W, Kellogg GE, Grant S, Spiegel S, Zhang S. Biological characterization of 3-(2-amino-ethyl)-5-[3-(4-butoxyl-phenyl)-propylidene]-thiazolidine-2,4-dione (K145) as a selective sphingosine kinase-2 inhibitor and anticancer agent. PLoS One. 2013;8:e56471. doi: 10.1371/journal.pone.0056471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taniai M, Grambihler A, Higuchi H, Werneburg N, Bronk SF, Farrugia DJ, Kaufmann SH, Gores GJ. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004;64:3517–3524. doi: 10.1158/0008-5472.CAN-03-2770. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima W, Hicks MA, Tanaka N, Krystal GW, Harada H. Noxa determines localization and stability of MCL-1 and consequently ABT-737 sensitivity in small cell lung cancer. Cell Death Dis. 2014;5:e1052. doi: 10.1038/cddis.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres-Adorno AM, Lee J, Kogawa T, Ordentlich P, Tripathy D, Lim B, Ueno NT. Histone deacetylase inhibitor enhances the efficacy of MEK inhibitor through NOXA-Mediated MCL1 degradation in triple-negative and inflammatory breast cancer. Clin Cancer Res. 2017;23:4780–4792. doi: 10.1158/1078-0432.CCR-16-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billard C. BH3 mimetics: status of the field and new developments. Mol Cancer Ther. 2013;12:1691–1700. doi: 10.1158/1535-7163.MCT-13-0058. [DOI] [PubMed] [Google Scholar]
- 28.Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, Werneburg NW, Gustafson MP, Dietz AB, Roberts LR, Sirica AE, Gores GJ. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897–907. doi: 10.1158/0008-5472.CAN-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paugh SW, Paugh BS, Rahmani M, Kapitonov D, Almenara JA, Kordula T, Milstien S, Adams JK, Zipkin RE, Grant S, Spiegel S. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood. 2008;112:1382–1391. doi: 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen MH, Yen CC, Cheng CT, Wu RC, Huang SC, Yu CS, Chung YH, Liu CY, Chang PM, Chao Y, Chen MH, Chen YF, Chiang KC, Yeh TS, Chen TC, Huang CY, Yeh CN. Identification of SPHK1 as a therapeutic target and marker of poor prognosis in cholangiocarcinoma. Oncotarget. 2015;6:23594–23608. doi: 10.18632/oncotarget.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin Z, Dai L, Trillo-Tinoco J, Senkal C, Wang W, Reske T, Bonstaff K, Del Valle L, Rodriguez P, Flemington E, Voelkel-Johnson C, Smith CD, Ogretmen B, Parsons C. Targeting sphingosine kinase induces apoptosis and tumor regression for KSHV-associated primary effusion lymphoma. Mol Cancer Ther. 2014;13:154–164. doi: 10.1158/1535-7163.MCT-13-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guikema JE, Amiot M, Eldering E. Exploiting the pro-apoptotic function of NOXA as a therapeutic modality in cancer. Expert Opin Ther Targets. 2017;21:767–779. doi: 10.1080/14728222.2017.1349754. [DOI] [PubMed] [Google Scholar]
- 33.Gores GJ, Kaufmann SH. Selectively targeting Mcl-1 for the treatment of acute myelogenous leukemia and solid tumors. Genes Dev. 2012;26:305–311. doi: 10.1101/gad.186189.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, Chanrion M, Kelly GL, Gong JN, Moujalled DM, Bruno A, Csekei M, Paczal A, Szabo ZB, Sipos S, Radics G, Proszenyak A, Balint B, Ondi L, Blasko G, Robertson A, Surgenor A, Dokurno P, Chen I, Matassova N, Smith J, Pedder C, Graham C, Studeny A, Lysiak-Auvity G, Girard AM, Grave F, Segal D, Riffkin CD, Pomilio G, Galbraith LC, Aubrey BJ, Brennan MS, Herold MJ, Chang C, Guasconi G, Cauquil N, Melchiore F, Guigal-Stephan N, Lockhart B, Colland F, Hickman JA, Roberts AW, Huang DC, Wei AH, Strasser A, Lessene G, Geneste O. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538:477–482. doi: 10.1038/nature19830. [DOI] [PubMed] [Google Scholar]
- 35.Meng F, Yamagiwa Y, Ueno Y, Patel T. Over-expression of interleukin-6 enhances cell survival and transformed cell growth in human malignant cholangiocytes. J Hepatol. 2006;44:1055–1065. doi: 10.1016/j.jhep.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, Zuo M, Zinner R, Hong D, Meric-Bernstam F, Janku F, Crane CH, Mishra L, Vauthey JN, Wolff RA, Mills G, Javle M. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One. 2014;9:e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross JS, Wang K, Gay L, Al-Rohil R, Rand JV, Jones DM, Lee HJ, Sheehan CE, Otto GA, Palmer G, Yelensky R, Lipson D, Morosini D, Hawryluk M, Catenacci DV, Miller VA, Churi C, Ali S, Stephens PJ. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist. 2014;19:235–242. doi: 10.1634/theoncologist.2013-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suryani S, Carol H, Chonghaile TN, Frismantas V, Sarmah C, High L, Bornhauser B, Cowley MJ, Szymanska B, Evans K, Boehm I, Tonna E, Jones L, Manesh DM, Kurmasheva RT, Billups C, Kaplan W, Letai A, Bourquin JP, Houghton PJ, Smith MA, Lock RB. Cell and molecular determinants of in vivo efficacy of the BH3 mimetic ABT-263 against pediatric acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2014;20:4520–4531. doi: 10.1158/1078-0432.CCR-14-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucas KM, Mohana-Kumaran N, Lau D, Zhang XD, Hersey P, Huang DC, Weninger W, Haass NK, Allen JD. Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clin Cancer Res. 2012;18:783–795. doi: 10.1158/1078-0432.CCR-11-1166. [DOI] [PubMed] [Google Scholar]
- 40.Faber AC, Farago AF, Costa C, Dastur A, Gomez-Caraballo M, Robbins R, Wagner BL, Rideout WM 3rd, Jakubik CT, Ham J, Edelman EJ, Ebi H, Yeo AT, Hata AN, Song Y, Patel NU, March RJ, Tam AT, Milano RJ, Boisvert JL, Hicks MA, Elmiligy S, Malstrom SE, Rivera MN, Harada H, Windle BE, Ramaswamy S, Benes CH, Jacks T, Engelman JA. Assessment of ABT-263 activity across a cancer cell line collection leads to a potent combination therapy for small-cell lung cancer. Proc Natl Acad Sci U S A. 2015;112:E1288–1296. doi: 10.1073/pnas.1411848112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knorr KL, Schneider PA, Meng XW, Dai H, Smith BD, Hess AD, Karp JE, Kaufmann SH. MLN4924 induces Noxa upregulation in acute myelogenous leukemia and synergizes with Bcl-2 inhibitors. Cell Death Differ. 2015;22:2133–2142. doi: 10.1038/cdd.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, Nimmer P, Jin S, Smith M, Xiao Y, Kovar P, Tanaka A, Bruncko M, Sheppard GS, Wang L, Gierke S, Kategaya L, Anderson DJ, Wong C, Eastham-Anderson J, Ludlam MJ, Sampath D, Fairbrother WJ, Wertz I, Rosenberg SH, Tse C, Elmore SW, Souers AJ. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax) Cell Death Dis. 2015;6:e1590. doi: 10.1038/cddis.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arai S, Jonas O, Whitman MA, Corey E, Balk SP, Chen S. Tyrosine kinase inhibitors increase MCL1 degradation and in combination with BCLXL/BCL2 inhibitors drive prostate cancer apoptosis. Clin Cancer Res. 2018;24:5458–5470. doi: 10.1158/1078-0432.CCR-18-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grundy M, Balakrishnan S, Fox M, Seedhouse CH, Russell NH. Genetic biomarkers predict response to dual BCL-2 and MCL-1 targeting in acute myeloid leukaemia cells. Oncotarget. 2018;9:37777–37789. doi: 10.18632/oncotarget.26540. [DOI] [PMC free article] [PubMed] [Google Scholar]