Abstract
Objective
Since 2010, several non-vitamin K antagonist oral anticoagulants (NOACs) have been brought to the U.S. market, yet little is known regarding their evolving adoption for prophylaxis of atrial fibrillation (AF) related stroke. We examined temporal trends in choice of oral anticoagulants (OACs) among incident OAC users with AF and its association with patients’ demographic and clinical characteristics.
Methods
We conducted a serial cross-sectional analysis of medical and pharmacy claims for commercial and Medicare Advantage enrollees in a large, private, U.S. health plan. We identified 112,187 adults with nonvalvular AF starting OACs between October 2010 and March 2017. Multivariable logistic regression was used to examine the associations of patient characteristics with prescription of NOACs vs warfarin. Multinomial logistic regression with generalized logit link function was used to test the association of patient characteristics with choice among NOACs.
Results
The prescription of NOACs has increased dramatically since their introduction in October 2010. In the first quarter of 2017 (2017Q1), 7,502 patients started OACs, of whom 78.9% used NOACs and 21.1% warfarin. For NOACs, 3.8% used dabigatran, 25.0% rivaroxaban, and 50.1% apixaban. In multivariable analyses, factors associated with choice of NOACs vs warfarin included younger age, lower stroke or bleeding risk, fewer comorbidities, higher socioeconomic status, and prescription by cardiologists (all P<0.001). There was no sex difference in likelihood of filling NOACs vs warfarin in 2010Q4–2012 but women had higher odds of starting NOACs (odds ratio=1.19; 95% confidence interval=1.14–1.25) in 2015–2017Q1. Among NOAC users, the odds of apixaban prescription increased with age, female sex, stroke or bleeding risk, and comorbidities (all P<0.05).
Conclusion
NOAC prescriptions have increased substantially among incident OAC users with nonvalvular AF, predominantly driven by increased prescription of apixaban. Warfarin and apixaban were generally preferred for elderly, patients with higher stroke or bleeding risk, and those with more comorbidities.
Keywords: atrial fibrillation, oral anticoagulant, choice, temporal trend
Introduction
Atrial fibrillation (AF), the most common cardiac arrhythmia, increases the risk of ischemic stroke five-fold.1–3 For decades, anticoagulation with warfarin has been the cornerstone of stroke prevention in AF, but its usefulness is limited by a narrow therapeutic window, and need for frequent monitoring and a restricted diet.4 Since 2010, several non-vitamin K antagonist oral anticoagulants (NOACs) without these limitations have been approved by the U.S. Food and Drug Administration (FDA) for preventing stroke in patients with nonvalvular AF (dabigatran in October 2010, rivaroxaban in November 2011, apixaban in December 2012, and edoxaban in January 2015). These drugs have been shown to be superior to warfarin in stroke prevention, and carry a similar overall bleeding risk with reduction in intracranial hemorrhage but an increase in major gastrointestinal bleeding.5–8
Prior studies have examined the uptake of these new drugs and compared their use with that of warfarin since their market availability in the United States in 2010. Younger age, male sex, white race, fewer comorbidities, lower stroke or bleeding risk, and prescription by a cardiologist have been shown to be associated with higher odds of choosing a NOAC over warfarin.9–16 However, most studies focused on dabigatran and rivaroxaban and used data from 2012 and earlier.12–16 Although several studies used more recent data, they included a relatively small sample of patients and especially of those with apixaban.9–11 For example, Desai and colleagues examined 6,893 patients with AF newly prescribed an oral anticoagulant (OAC) between October 2010 and June 2013 in a commercial claims database and identified only 20 new apixaban users.9 Similarly, Ashburner et al found that among patients with AF treated in a large primary care practice network between 2010 and 2015, only 56 patients used apixaban.10 In another study of a national registry involving 4,670 patients with AF treated with an OAC between February 2013 and January 2016, rivaroxaban was the most frequently used OAC.11 Moreover, few studies examined patient characteristics associated with prescription patterns among NOACs.
With increased availability of NOACs and ongoing dissemination of information about these drugs in the U.S., prescribing patterns and factors driving treatment choice may be evolving. Therefore, we examined (1) temporal trends in prescription of warfarin, dabigatran, rivaroxaban, and apixaban from October 2010 through March 2017, (2) the associations of patient demographic characteristics (e.g., age, sex) and clinical factors (e.g., ischemic stroke risk, prescribing specialty) with prescription of a NOAC vs warfarin, and (3) these factors’ associations with choice among NOACs.
Methods
Data Sources
We used data from the OLDW, which includes de-identified medical and pharmacy claims for commercial and Medicare Advantage enrollees in a large, private, U.S. health plan. The database contains longitudinal health information on enrollees of all ages and racial/ethnic groups from geographically diverse regions across the country.17 The claims contain extensive service-level data for physician and hospital services as well as information on each prescription filled such as the drug, the prescriber and his/her clinical specialty.17 This study was exempt from institutional review board approval because it only involved analysis of pre-existing, de-identified data.
Study Participants
The study cohort included patients aged ≥18 years with nonvalvular AF who had a new fill of warfarin, dabigatran, rivaroxaban, or apixaban between October 2010 and March 2017. The first fill date of any of these drugs during that period was designated as the index date. Patients were required to have continuous enrollment in a medical and pharmacy plan for at least 12 months prior to the index date to ensure completeness of claims information. Patients also need to have at least one inpatient or outpatient AF diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 427.31 or ICD-10-CM code I48.0, I48.1, I48.2, or I48.91)10,18–20 within 12 months before the index date. The ICD code performed relatively well with a median positive predictive value of 89% in previous validation studies.18 Patients with AF who did not have a diagnosis or procedure code indicating valvular heart disease9,13 within 12 months preceding the index date were considered as having non-valvular AF, defined as the absence of rheumatic mitral stenosis, a mechanical or bioprosthetic heart valve, or mitral valve repair according to the 2014 American Heart Association (AHA)/ American College of Cardiology (ACC)/Heart Rhythm Society (HRS) Guideline.21
We excluded patients who had an OAC fill within 12 months before the index date, had a diagnosis of deep vein thrombosis or pulmonary embolism within 12 months before the index date, underwent total hip or knee replacement surgery within six weeks prior to the index date, or initiated edoxaban during the study period due to a small sample size of only 167 patients.
Baseline Characteristics
Patient demographic and clinical data included age, sex, race/ethnicity, education status, household net worth, region of residence, prescriber specialty, comorbidity, ischemic stroke risk, bleeding risk, and use of antiplatelets or nonsteroidal anti-inflammatory drugs (NSAIDs). The specialty of providers who prescribed an OAC at the index date was classified as cardiologists, primary care physicians (PCPs), and other providers. To measure the burden of comorbidities, we applied Quan’s enhanced ICD-9-CM and ICD-10-CM coding algorithms for Charlson comorbidities to medical claims within 12 months prior to the index date,22 and then grouped the score into three groups (0–1, 2–3, and ≥4). The Quan’s coding algorithms have been shown to produce similar estimates of comorbidity prevalence in claims data and may outperform the original Deyo coding algorithms.22
Ischemic stroke risk was measured using a CHA2DS2-VASc score, which was calculated by assigning one point each for congestive heart failure, hypertension, diabetes mellitus, vascular diseases, age 65–74 years, and female sex, and two points each for age ≥75 years and previous stroke, transient ischemic attack, or thromboembolism.23 The score ranged from 0 to 9 and was categorized as 0–1, 2–3, and ≥4. Bleeding risk was assessed using a modified HAS-BLED score, which was calculated by giving one point each for hypertension, abnormal renal function, abnormal liver function, stroke, bleeding history or predisposition, age ≥65 years, concomitant use of antiplatelets or NSAIDs, and alcohol abuse.24,25 We did not include the labile international normalized ratio (INR) in the score since INR monitoring is not applicable to NOAC therapy.24,26 The score ranged from 0 to 8 and was classified as 0–1, 2, and ≥3. The comorbidity components of CHA2DS2-VASc and HAS-BLED were identified within 12 months before the index date using ICD codes based on previously used algorithms.27–29
Outcomes
The outcome variables were prescription of a NOAC (dabigatran, rivaroxaban, or apixaban) vs warfarin and choice among the three NOACs at the index date.
Statistical Analysis
We examined baseline characteristics by OAC (warfarin, dabigatran, rivaroxaban, apixaban). Temporal trends in OAC prescriptions were plotted as the proportion of patients starting a specific OAC per quarter for the whole cohort and by selected baseline characteristics.
We conducted multivariable logistic regression to examine the associations of patient demographic and clinical characteristics with prescription of a NOAC vs warfarin and reported the odds ratios (ORs) and 95% confidence intervals (CIs). The analysis was stratified by calendar period (October 2010–2012 [the period prior to the FDA approval of apixaban], 2013–2014 [the early period when all three NOACs were available], and 2015-March 2017). Next, we performed multinomial logistic regression with generalized logit link function to test the associations of patient characteristics with choice among NOACs and reported the ORs and 95% CIs, restricting analyses to when all three NOACs were FDA-approved for AF. The analysis was stratified by calendar period (2013–2014, and 2015-March 2017).
Because CHA2DS2-VASc includes patient age, sex, and commodities, some of which were also included in HAS-BLED and Charlson comorbidity index (CCI), we conducted separate models for these variables to avoid potential multicollinearity. The main model included patient age, sex, race/ethnicity, education, household net worth, region of residence, CCI score, comorbidity components of CHA2DS2-VASc and HAS-BLED that were not already included in CCI, and prescriber specialty. The second model included the CHA2DS2-VASc score but excluded patient age, sex, and comorbidities that were already included in the score. The third model included the HAS-BLED score but excluded patient age and comorbidities that were already included in the score. The models were also controlled for a linear time term (calendar quarter) to capture temporal trends in patient characteristics and OAC choice.
Statistical significance was set at P < 0.05 and all tests were two-tailed. SAS version 9.4 (SAS Institute Inc) was used for all analyses.
Results
Cohort Description and Baseline Characteristics
The final cohort included 112,187 patients with nonvalvular AF newly prescribed an OAC between October 2010 and March 2017 (Figure 1). Of these, 50,713 (45.2%) started warfarin, 13,776 (12.3%) dabigatran, 24,188 (21.6%) rivaroxaban, and 23,510 (21.0%) apixaban.
Figure 1.
Study cohort
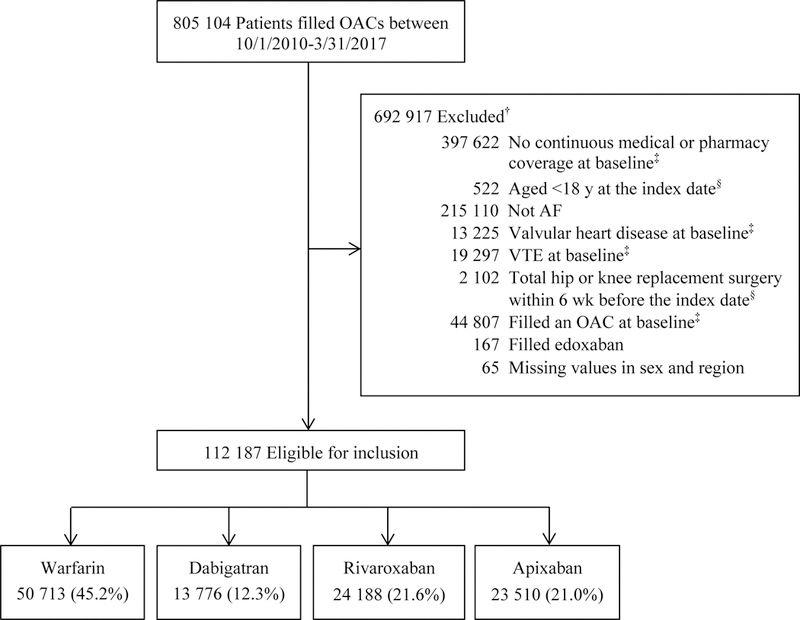
† Exclusions applied sequentially. Patients could be excluded for more than one reason.
‡ At baseline refers to within 12 months before the index date.
§ The index date refers to the first fill date of an OAC between October 2010 and March 2017.
OAC indicates oral anticoagulant; AF, atrial fibrillation; VTE, venous thromboembolism.
Overall, incident warfarin users were oldest (mean age 73.2 years) with the highest mean CCI (3.0), CHA2DS2-VASc (4.3), and HAS-BLED score (2.8), followed by incident apixaban users (Table 1). Incident dabigatran users were youngest (mean age 67.9 years) with the lowest mean CCI (2.1), CHA2DS2-VASc (3.4), and HAS-BLED score (2.3). Moreover, incident apixaban users included the highest proportion of women (46.8%), followed by incident warfarin users (43.5%); incident dabigatran users included the lowest proportion of women (37.2%). Compared with patients newly prescribed a NOAC, those newly prescribed warfarin were less likely to have a bachelor degree, have high household net worth, have a cardiologist prescriber, use NSAIDs and live in the South area, but were more likely to live in the Midwest area.
Table 1.
Baseline characteristics of patients with AF by OAC, October 2010 to March 2017
| Characteristics | Warfarin (n=50 713) | Dabigatran (n=13 776) | Rivaroxaban (n=24 188) | Apixaban (n=23 510) | Total (n=112 187) |
|---|---|---|---|---|---|
| Age, y, mean (SD) | 73.2 (10.0) | 67.9 (11.5) | 69.4 (11.5) | 72.4 (10.9) | 71.6 (10.9) |
| Age, n (%) | |||||
| 18–64 y | 9 231 (18.2) | 5 120 (37.2) | 7 666 (31.7) | 5 291 (22.5) | 27 308 (24.3) |
| 65–74 y | 15 658 (30.9) | 4 272 (31.0) | 7 880 (32.6) | 7 402 (31.5) | 35 212 (31.4) |
| ≥ 75 y | 25 824 (50.9) | 4 384 (31.8) | 8 642 (35.7) | 10 817 (46.0) | 49 667 (44.3) |
| Sex, n (%) | |||||
| Male | 28 678 (56.6) | 8 658 (62.9) | 14 486 (59.9) | 12 509 (53.2) | 64 331 (57.3) |
| Female | 22 035 (43.5) | 5 118 (37.2) | 9 702 (40.1) | 11 001 (46.8) | 47 856 (42.7) |
| Race/ethnicity, n (%) | |||||
| Non-Hispanic white | 40 430 (79.7) | 11 131 (80.8) | 19 089 (78.9) | 18 093 (77.0) | 88 743 (79.1) |
| Non-Hispanic black | 4 279 (8.4) | 999 (7.3) | 1 854 (7.7) | 2 161 (9.2) | 9 293 (8.3) |
| Hispanic | 2 151 (4.2) | 550 (4.0) | 1 248 (5.2) | 1 218 (5.2) | 5 167 (4.6) |
| Asian | 793 (1.6) | 251 (1.8) | 527 (2.2) | 472 (2.0) | 2 043 (1.8) |
| Unknown | 3 060 (6.0) | 845 (6.1) | 1 470 (6.1) | 1 566 (6.7) | 6 941 (6.2) |
| Education, n (%) | |||||
| ≤ High school | 20 866 (41.2) | 4 987 (36.2) | 7 525 (31.1) | 7 390 (31.4) | 40 768 (36.3) |
| Some college | 24 163 (47.7) | 6 385 (46.4) | 12 428 (51.4) | 11 987 (51.0) | 54 963 (49.0) |
| ≥ Bachelor degree | 4 504 (8.9) | 2 082 (15.1) | 3 620 (15.0) | 3 253 (13.8) | 13 459 (12.0) |
| Unknown | 1 180 (2.3) | 322 (2.3) | 615 (2.5) | 880 (3.7) | 2 997 (2.7) |
| Household net worth, n (%) | |||||
| Low (< $250,000) | 22 670 (44.7) | 5 428 (39.4) | 8 959 (37.0) | 8 966 (38.1) | 46 023 (41.0) |
| Medium ($250,000-$499,999) | 14 059 (27.7) | 3 762 (27.3) | 6 721 (27.8) | 6 198 (26.4) | 30 740 (27.4) |
| High (≥ $500,000) | 9 689 (19.1) | 3 552 (25.8) | 6 228 (25.8) | 5 766 (24.5) | 25 235 (22.5) |
| Unknown | 4 295 (8.5) | 1 034 (7.5) | 2 280 (9.4) | 2 580 (11.0) | 10 189 (9.1) |
| Prescriber specialty, n (%) | |||||
| Cardiologist | 14 308 (28.2) | 7 298 (53.0) | 11 343 (46.9) | 10 648 (45.3) | 43 597 (38.9) |
| Primary care physician | 20 604 (40.6) | 3 341 (24.3) | 6 000 (24.8) | 5 419 (23.1) | 35 364 (31.5) |
| Other/unknown | 15 801 (31.2) | 3 137 (22.8) | 6 845 (28.3) | 7 443 (31.7) | 33 226 (29.6) |
| Region of residence, n (%) | |||||
| Northeast | 7 603 (15.0) | 1 970 (14.3) | 3 769 (15.6) | 3 624 (15.4) | 16 966 (15.1) |
| Midwest | 20 397 (40.2) | 3 726 (27.1) | 7 268 (30.1) | 6 871 (29.2) | 38 262 (34.1) |
| South | 17 001 (33.5) | 6 428 (46.7) | 10 414 (43.1) | 10 511 (44.7) | 44 354 (39.5) |
| West | 5 712 (11.3) | 1 652 (12.0) | 2 737 (11.3) | 2 504 (10.7) | 12 605 (11.2) |
| CCI, mean (SD) | 3.0 (2.5) | 2.1 (2.1) | 2.2 (2.2) | 2.7 (2.4) | 2.7 (2.4) |
| CCI, n (%) | |||||
| 0–1 | 16 435 (32.4) | 6 723 (48.8) | 11 239 (46.5) | 8 992 (38.3) | 43 389 (38.7) |
| 2–3 | 15 748 (31.1) | 4 264 (31.0) | 7 462 (30.9) | 7 287 (31.0) | 34 761 (31.0) |
| ≥ 4 | 18 530 (36.5) | 2 789 (20.3) | 5 487 (22.7) | 7 231 (30.8) | 34 037 (30.3) |
| CHA2DS2-VASc, mean (SD) | 4.3 (1.8) | 3.4 (1.9) | 3.5 (1.8) | 4.1 (1.8) | 4.0 (1.9) |
| CHA2DS2-VASc, n (%) | |||||
| 0–1 | 3 151 (6.2) | 2 267 (16.5) | 3 353 (13.9) | 2 062 (8.8) | 10 833 (9.7) |
| 2–3 | 13 070 (25.8) | 4 980 (36.2) | 8 604 (35.6) | 6 996 (29.8) | 33 650 (30.0) |
| ≥ 4 | 34 492 (68.0) | 6 529 (47.4) | 12 231 (50.6) | 14 452 (61.5) | 67 704 (60.4) |
| HAS-BLED, mean (SD) | 2.8 (1.3) | 2.3 (1.3) | 2.4 (1.2) | 2.7 (1.3) | 2.7 (1.3) |
| HAS-BLED, n (%) | |||||
| 0–1 | 6 572 (13.0) | 3 586 (26.0) | 5 391 (22.3) | 3 675 (15.6) | 19 224 (17.1) |
| 2 | 14 699 (29.0) | 4 401 (32.0) | 7 843 (32.4) | 6 918 (29.4) | 33 861 (30.2) |
| ≥ 3 | 29 442 (58.1) | 5 789 (42.0) | 10 954 (45.3) | 12 917 (54.9) | 59 102 (52.7) |
| Comorbidities, n (%) | |||||
| Congestive heart failure | 20 741 (40.9) | 3 915 (28.4) | 7 064 (29.2) | 8 071 (34.3) | 39 791 (35.5) |
| Hypertension | 44 353 (87.5) | 11 404 (82.8) | 20 164 (83.4) | 20 446 (87.0) | 96 367 (85.9) |
| Diabetes mellitus | 19 602 (38.7) | 4 247 (30.8) | 7 639 (31.6) | 8 063 (34.3) | 39 551 (35.3) |
| Prior stroke/TIA/TE | 8 268 (16.3) | 1 566 (11.4) | 2 619 (10.8) | 3 374 (14.4) | 15 827 (14.1) |
| Myocardial infarction | 7 886 (15.6) | 1 367 (9.9) | 2 494 (10.3) | 2 957 (12.6) | 14 704 (13.1) |
| Vascular disease | 27 681 (54.6) | 6 098 (44.3) | 10 659 (44.1) | 11 757 (50.0) | 56 195 (50.1) |
| Abnormal renal function | 11 725 (23.1) | 1 527 (11.1) | 3 074 (12.7) | 4 581 (19.5) | 20 907 (18.6) |
| Abnormal liver function | 2 954 (5.8) | 705 (5.1) | 1 349 (5.6) | 1 428 (6.1) | 6 436 (5.7) |
| Bleeding history or predisposition | 20 370 (40.2) | 3 845 (27.9) | 7 086 (29.3) | 8 124 (34.6) | 39 425 (35.1) |
| Alcohol abuse | 1 465 (2.9) | 413 (3.0) | 847 (3.5) | 738 (3.1) | 3 463 (3.1) |
| History of medication use, n (%) | |||||
| Antiplatelets | 6 796 (13.4) | 1 733 (12.6) | 3 040 (12.6) | 3 450 (14.7) | 15 019 (13.4) |
| NSAIDs | 9 290 (18.3) | 3 025 (22.0) | 5 333 (22.1) | 5 012 (21.3) | 22 660 (20.2) |
| Year, n (%) | |||||
| 2010Q4 | 2 974 (5.9) | 261 (1.9) | 0 (0.0) | 0 (0.0) | 3 235 (2.9) |
| 2011 | 10 093 (19.9) | 4 923 (35.7) | 39 (0.2) | 0 (0.0) | 15 055 (13.4) |
| 2012 | 9 776 (19.3) | 3 733 (27.1) | 2 577 (10.7) | 0 (0.0) | 16 086 (14.3) |
| 2013 | 8 548 (16.9) | 1 996 (14.5) | 5 170 (21.4) | 1 140 (4.8) | 16 854 (15.0) |
| 2014 | 6 149 (12.1) | 934 (6.8) | 5 286 (21.9) | 3 324 (14.1) | 15 693 (14.0) |
| 2015 | 6 210 (12.2) | 618 (4.5) | 4 577 (18.9) | 6 067 (25.8) | 17 472 (15.6) |
| 2016 | 5 383 (10.6) | 1 022 (7.4) | 4 666 (19.3) | 9 219 (39.2) | 20 290 (18.1) |
| 2017Q1 | 1 580 (3.1) | 289 (2.1) | 1 873 (7.7) | 3 760 (16.0) | 7 502 (6.7) |
AF indicates atrial fibrillation; OAC, oral anticoagulant; CCI, Charlson comorbidity index; TIA, transient ischemic attack; TE, thromboembolism; NSAID, nonsteroidal anti-inflammatory drug.
Overall Temporal Trends
The proportion of incident OAC users for NOACs increased rapidly from 8.1% in the fourth quarter of 2010 (2010Q4) to 78.9% in the first quarter of 2017 (2017Q1), and surpassed that of warfarin in the third quarter of 2013 (2013Q3) (Figure 2). Dabigatran prescription peaked in 2011Q4 when 37.4% of the patients initiated the drug. From then on, its prescription reduced sharply, reaching 3.2% in 2015Q1, and then, the trend leveled out throughout the rest of the study period. Concurrent with the decreasing prescription of dabigatran, rivaroxaban prescription increased and peaked in 2014Q2 with 34.9% of the patients prescribed the drug. The prescription of apixaban surpassed dabigatran in 2013Q4, surpassed rivaroxaban in 2015Q1, and surpassed warfarin in 2015Q3. From 2015Q3 onwards, apixaban became the most prescribed OAC for AF. In 2017Q1, apixaban accounted for 50.1% of new OAC prescriptions, followed by rivaroxaban (25.0%) and warfarin (21.1%).
Figure 2.
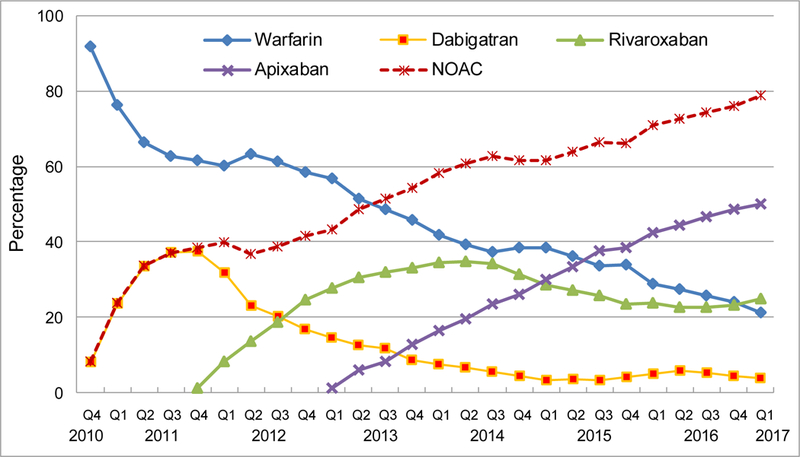
Quarterly trends in oral anticoagulant prescriptions for atrial fibrillation, from the fourth quarter of 2010 (2010Q4) to the first quarter of 2017 (2017Q1)
Y-axis refers to the percentage of incident OAC users prescribed a specific OAC.
OAC indicates oral anticoagulant; NOAC, non-vitamin K antagonist oral anticoagulants, including dabigatran, rivaroxaban, and apixaban.
Temporal Trends by Age Group
NOACs were adopted into clinical practice much faster among patients aged 18–64 years; the prescription of warfarin was surpassed by dabigatran in 2011Q3, by rivaroxaban in 2013Q2, and by apixaban in 2014Q3 (Figure 3). In contrast, the prescription of dabigatran or rivaroxaban had never overtaken warfarin during the study period among patients aged ≥75 years. From 2015Q3, NOACs accounted for over 80% of new OAC prescriptions among patients aged 18–64 years, but had never exceeded 80% of new OAC prescriptions over the study period among patients aged ≥65 years. Nevertheless, apixaban prescription increased fastest among patients aged ≥75 years, reaching 52.4% in 2017Q1 in contrast with less than half among those aged <75 years.
Figure 3.
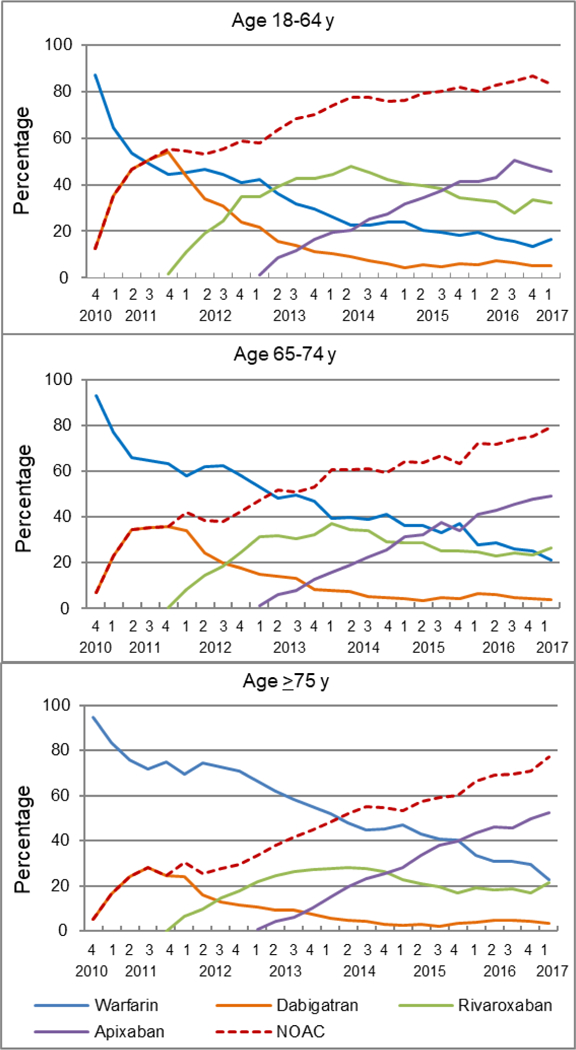
Quarterly trends in oral anticoagulant prescriptions for atrial fibrillation by age, from the fourth quarter of 2010 (2010Q4) to the first quarter of 2017 (2017Q1)
Y-axis refers to the percentage of incident OAC users prescribed a specific OAC.
OAC indicates oral anticoagulant; NOAC, non-vitamin K antagonist oral anticoagulant, including dabigatran, rivaroxaban, and apixaban.
Temporal Trends by Sex
A higher proportion of women (93.9%) than men (90.7%) were prescribed warfarin in 2010Q4 (Figure 4). However, because NOAC prescriptions increased more rapidly among women than men, by 2017Q1, a lower proportion of women (19.7%) than men (22.2%) were prescribed warfarin. The higher rate of the increasing NOAC prescription in women vs men was attributable to apixaban; the proportion of women treated with apixaban was, on average, 4 percentage points higher than that of men in 2015–2017Q1. In 2017Q1, 53.3% of women were prescribed apixaban while only 47.4% of men were prescribed the drug.
Figure 4.
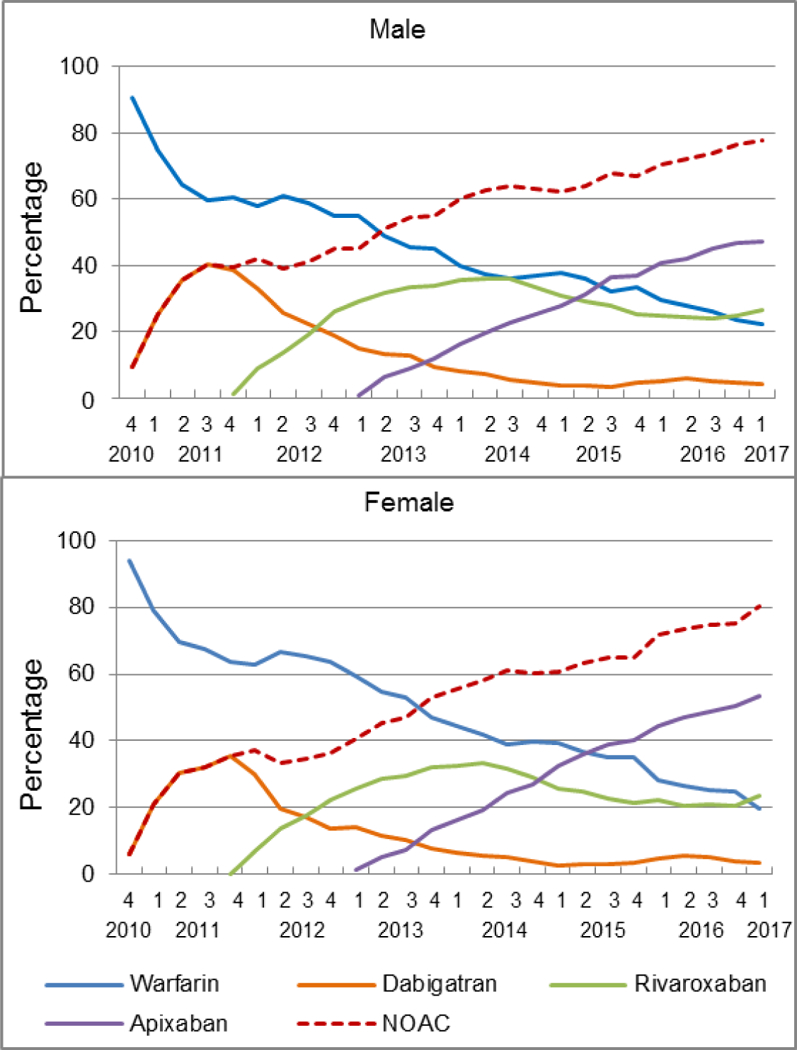
Quarterly trends in oral anticoagulant prescriptions for atrial fibrillation by sex, from the fourth quarter of 2010 (2010Q4) to the first quarter of 2017 (2017Q1)
Y-axis refers to the percentage of incident OAC users prescribed a specific OAC.
OAC indicates oral anticoagulant; NOAC, non-vitamin K antagonist oral anticoagulant, including dabigatran, rivaroxaban, and apixaban.
Temporal Trends by Ischemic Stroke Risk
The uptake of NOACs was slower among patients with the CHA2DS2-VASc score ≥4, and by 2015Q4, warfarin was still the most prescribed OAC in this group (39.8%) (Figure 5). In contrast, warfarin became the second most prescribed OAC in 2014Q1 among patients with the CHA2DS2-VASc score of 2–3 and in 2011Q3 among those with the CHA2DS2-VASc score of 0–1. Nevertheless, among patients with the CHA2DS2-VASc score ≥4, apixaban became the most prescribed OAC from 2016Q1, and accounted for 51.8% of new OAC prescriptions in 2017Q1, 4.4 and 5.1 percentage points higher than those with the CHA2DS2-VASc score of 0–1 and 2–3, respectively.
Figure 5.
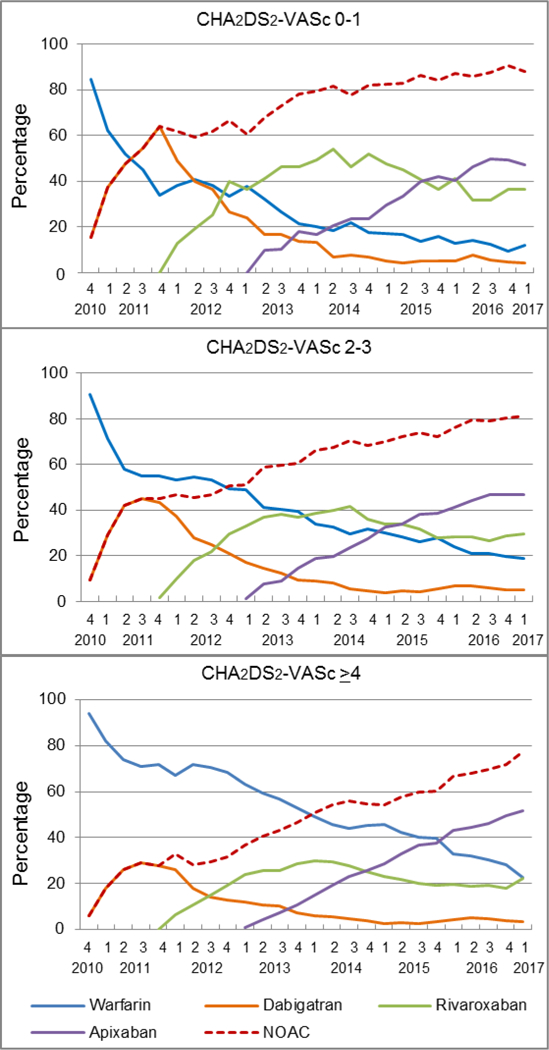
Quarterly trends in oral anticoagulant prescriptions for atrial fibrillation by ischemic stroke risk, from the fourth quarter of 2010 (2010Q4) to the first quarter of 2017 (2017Q1)
Y-axis refers to the percentage of incident OAC users prescribed a specific OAC.
OAC indicates oral anticoagulant; NOAC, non-vitamin K antagonist oral anticoagulant, including dabigatran, rivaroxaban, and apixaban.
Temporal Trends by Prescriber Specialty
Compared with PCPs, cardiologists adopted NOACs into their clinical practice much quicker (Figure 6). Their prescription of warfarin was overtaken by dabigatran in 2011Q3, by rivaroxaban in 2013Q2, and by apixaban in 2014Q2. Conversely, the prescription of dabigatran or rivaroxaban by PCPs had never surpassed warfarin during the study period, but more apixaban than warfarin began to be prescribed by PCPs in 2016Q2. In 2016–2017Q1, more than 51% of cardiologists prescribed apixaban while less than 46% of PCPs prescribed the drug.
Figure 6.
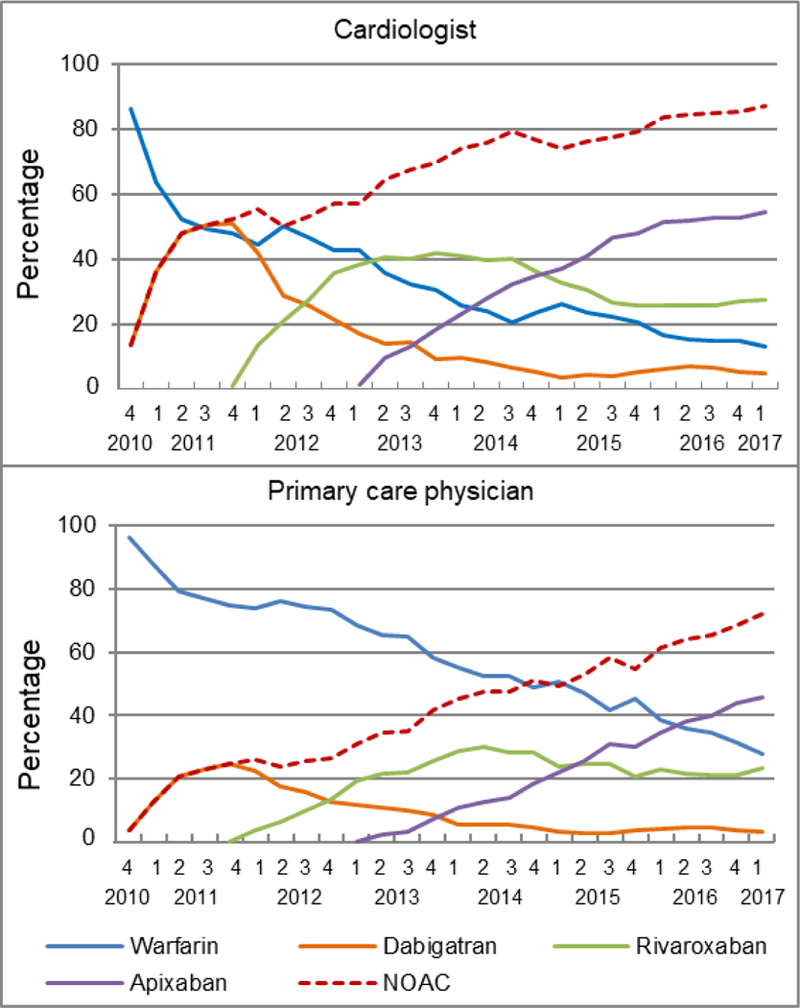
Quarterly trends in oral anticoagulant prescriptions for atrial fibrillation for cardiologists and primary care physicians, from the fourth quarter of 2010 (2010Q4) to the first quarter of 2017 (2017Q1)
Y-axis refers to the percentage of incident OAC users prescribed a specific OAC.
OAC indicates oral anticoagulant; NOAC, non-vitamin K antagonist oral anticoagulant, including dabigatran, rivaroxaban, and apixaban.
Factors Associated with Prescription of NOACs vs Warfarin
In the multivariable logistic regression analysis, patients were significantly less likely to receive a NOAC vs warfarin with advanced age, higher CHA2DS2-VASc, HAS-BLED, or CCI scores, lower socioeconomic status, and prescription by a PCP (vs cardiologist) in all three periods (Table 2). For example, compared with patients aged 18–64 years, patients aged ≥75 years were 56% and 51% less likely to initiate a NOAC vs warfarin in 2010Q4–2012 and 2015–2017Q1, respectively. Compared with cardiologists, PCPs were 64% and 59% less likely to prescribe a NOAC vs warfarin for incident OAC users in 2010Q4–2012 and 2015–2017Q1, respectively.
Table 2.
Factors associated with prescription of NOACs vs warfarin for incident OAC users in 2010Q4–2012, 2013–2014, and 2015–2017Q1
| Characteristics | 2010Q4–2012 (n=34 376) | 2013–2014 (n=32 547) | 2015–2017Q1 (n=45 264) |
|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | |||
| Age† | |||
| 18–64 y | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 65–74 y | 0.59 (0.56–0.63) | 0.56 (0.52–0.59) | 0.54 (0.50–0.57) |
| ≥ 75 y | 0.44 (0.41–0.47) | 0.44 (0.41–0.47) | 0.49 (0.46–0.52) |
| Sex† | |||
| Male | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Female | 1.00 (0.95–1.06) | 1.06 (1.01–1.11) | 1.19 (1.14–1.25) |
| Race/ethnicity† | |||
| Non-Hispanic white | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Non-Hispanic black | 0.86 (0.78–0.95) | 0.96 (0.88–1.04) | 0.95 (0.88–1.03) |
| Hispanic | 0.97 (0.85–1.11) | 0.93 (0.83–1.04) | 1.01 (0.91–1.11) |
| Asian | 1.01 (0.81–1.27) | 1.13 (0.96–1.33) | 1.22 (1.05–1.43) |
| Unknown | 1.01 (0.91–1.13) | 1.01 (0.89–1.15) | 1.08 (0.96–1.21) |
| Education† | |||
| ≤ High school | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Some college | 1.15 (1.08–1.22) | 1.13 (1.07–1.19) | 1.05 (1.00–1.11) |
| ≥ Bachelor degree | 1.66 (1.51–1.82) | 1.43 (1.31–1.57) | 1.43 (1.31–1.56) |
| Unknown | 1.47 (1.22–1.77) | 1.10 (0.86–1.40) | 0.95 (0.80–1.13) |
| Household net worth† | |||
| Low (< $250,000) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Medium ($250,000-$499,999) | 1.18 (1.11–1.26) | 1.09 (1.02–1.15) | 1.05 (0.99–1.11) |
| High (≥ $500,000) | 1.30 (1.20–1.40) | 1.31 (1.22–1.41) | 1.36 (1.27–1.45) |
| Unknown | 0.97 (0.87–1.09) | 1.05 (0.96–1.16) | 1.08 (0.99–1.17) |
| Region of residence† | |||
| Northeast | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Midwest | 0.76 (0.69–0.83) | 0.69 (0.64–0.74) | 0.71 (0.67–0.76) |
| South | 1.32 (1.22–1.44) | 1.40 (1.30–1.50) | 1.58 (1.48–1.69) |
| West | 1.01 (0.92–1.12) | 0.95 (0.86–1.04) | 0.93 (0.85–1.01) |
| Charlson comorbidity index† | |||
| 0–1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2–3 | 0.79 (0.75–0.84) | 0.81 (0.77–0.86) | 0.87 (0.82–0.92) |
| ≥ 4 | 0.56 (0.52–0.60) | 0.58 (0.55–0.62) | 0.65 (0.61–0.69) |
| Prescriber specialty† | |||
| Cardiologist | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Primary care physician | 0.36 (0.34–0.38) | 0.35 (0.33–0.37) | 0.41 (0.39–0.44) |
| Other/unknown | 0.49 (0.46–0.52) | 0.42 (0.39–0.44) | 0.48 (0.46–0.51) |
| Calendar quarter† | 1.17 (1.16–1.18) | 1.15 (1.13–1.16) | 1.12 (1.11–1.13) |
| CHA2DS2-VASc‡ | |||
| 0–1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2–3 | 0.66 (0.61–0.71) | 0.61 (0.55–0.67) | 0.54 (0.49–0.60) |
| ≥ 4 | 0.41 (0.38–0.44) | 0.41 (0.37–0.45) | 0.39 (0.35–0.44) |
| HAS-BLED§ | |||
| 0–1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 | 0.67 (0.62–0.71) | 0.69 (0.64–0.75) | 0.66 (0.61–0.72) |
| ≥ 3 | 0.55 (0.51–0.58) | 0.54 (0.50–0.58) | 0.55 (0.51–0.59) |
NOAC indicates non-vitamin K antagonist oral anticoagulant; OAC, oral anticoagulant.
The results came from models that adjusted for all these variables and comorbidity components of CHA2DS2-VASc and HAS-BLED that were not already included in Charlson comorbidity index.
The results came from models that adjusted for race/ethnicity, education, household net worth, region of residence, prescriber specialty, calendar quarter, and comorbidity components of Charlson comorbidity index and HAS-BLED that were not already included in CHA2DS2-VASc.
The results came from models that adjusted for sex, race/ethnicity, education, household net worth, region of residence, prescriber specialty, calendar quarter, and comorbidity components of Charlson comorbidity index and CHA2DS2-VASc that were not already included in HAS-BLED.
The association between sex and prescription of a NOAC vs warfarin changed over time. Male and female had a similar likelihood of filling a NOAC vs warfarin in 2010Q4–2012, whereas women had higher odds of NOAC prescriptions in 2013–2014 (OR=1.06, 95% CI=1.01–1.11) and 2015–2017Q1 (OR=1.19, 95% CI=1.14–1.25).
Factors Associated with Choice among NOACs
In the multinomial logistic regression analysis, apixaban was generally preferred over dabigatran or rivaroxaban as a new prescription for elderly, women, and patients with higher CHA2DS2-VASc, HAS-BLED, or CCI scores in both periods (Table 3). For example, compared with patients aged 18–64 years, patients aged ≥75 years were less likely to initiate dabigatran (OR=0.75, 95% CI=0.65–0.85 in 2013–2014; OR=0.69, 95% CI=0.61–0.78 in 2015–2017Q1) or rivaroxaban (OR=0.84, 95% CI=0.76–0.92 in 2013–2014; OR=0.64, 95% CI=0.60–0.69 in 2015–2017Q1) relative to apixaban. Women had lower odds of receiving dabigatran (OR=0.85, 95% CI=0.76–0.94 in 2013–2014; OR=0.85, 95% CI=0.77–0.94 in 2015–2017Q1) or rivaroxaban (OR=0.90, 95% CI=0.84–0.98 in 2013–2014; OR=0.84, 95% CI=0.80–0.89 in 2015–2017Q1) vs apixaban.
Table 3.
Factors associated with choice among NOACs for incident NOAC users in 2013–2014 and 2015–2017Q1
| Dabigatran vs Apixaban |
Rivaroxaban vs Apixaban |
|||
|---|---|---|---|---|
| Characteristics | 2013–2014 (n=7 394) | 2015–2017Q1 (n=20 975) | 2013–2014 (n=14 920) | 2015–2017Q1 (n=30 162) |
| Odds Ratio (95% Confidence Interval) | ||||
| Age† | ||||
| 18–64 y | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 65–74 y | 0.96 (0.84–1.09) | 0.88 (0.78–1.00) | 0.96 (0.87–1.06) | 0.83 (0.78–0.89) |
| ≥ 75 y | 0.75 (0.65–0.85) | 0.69 (0.61–0.78) | 0.84 (0.76–0.92) | 0.64 (0.60–0.69) |
| Sex† | ||||
| Male | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Female | 0.85 (0.76–0.94) | 0.85 (0.77–0.94) | 0.90 (0.84–0.98) | 0.84 (0.80–0.89) |
| Race/ethnicity† | ||||
| Non-Hispanic white | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Non-Hispanic black | 0.94 (0.79–1.13) | 0.79 (0.65–0.95) | 0.88 (0.77–1.00) | 0.87 (0.80–0.96) |
| Hispanic | 0.98 (0.77–1.24) | 1.18 (0.97–1.44) | 1.04 (0.88–1.24) | 1.19 (1.07–1.33) |
| Asian | 1.48 (1.07–2.05) | 1.41 (1.05–1.89) | 1.18 (0.91–1.53) | 1.18 (1.00–1.39) |
| Unknown | 0.99 (0.76–1.28) | 1.18 (0.92–1.50) | 1.02 (0.84–1.23) | 1.11 (0.98–1.26) |
| Education† | ||||
| ≤ High school | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Some college | 1.03 (0.91–1.16) | 0.99 (0.88–1.11) | 1.06 (0.97–1.16) | 1.01 (0.95–1.07) |
| ≥ Bachelor degree | 0.97 (0.81–1.16) | 0.80 (0.66–0.96) | 1.01 (0.88–1.15) | 0.97 (0.89–1.06) |
| Unknown | 1.10 (0.66–1.83) | 0.71 (0.49–1.02) | 1.09 (0.74–1.61) | 1.00 (0.83–1.20) |
| Household net worth† | ||||
| Low (< $250,000) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Medium ($250,000-$499,999) | 0.90 (0.79–1.02) | 0.88 (0.77–0.99) | 1.01 (0.92–1.12) | 1.04 (0.98–1.11) |
| High (≥ $500,000) | 0.76 (0.66–0.88) | 0.89 (0.77–1.03) | 0.93 (0.83–1.03) | 0.97 (0.90–1.04) |
| Unknown | 0.92 (0.75–1.12) | 1.06 (0.89–1.27) | 0.90 (0.77–1.04) | 1.03 (0.94–1.14) |
| Region of residence† | ||||
| Northeast | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Midwest | 0.75 (0.64–0.87) | 0.74 (0.64–0.86) | 0.93 (0.82–1.04) | 1.00 (0.93–1.08) |
| South | 0.53 (0.46–0.61) | 0.75 (0.66–0.86) | 0.83 (0.74–0.93) | 0.80 (0.75–0.86) |
| West | 0.60 (0.50–0.72) | 0.74 (0.62–0.90) | 0.72 (0.62–0.83) | 0.93 (0.85–1.03) |
| Charlson comorbidity index† | ||||
| 0–1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2–3 | 0.94 (0.83–1.06) | 0.98 (0.88–1.10) | 0.91 (0.84–1.00) | 0.91 (0.86–0.96) |
| ≥ 4 | 0.83 (0.72–0.96) | 0.77 (0.67–0.88) | 0.87 (0.78–0.96) | 0.75 (0.70–0.80) |
| Prescriber specialty† | ||||
| Cardiologist | 1.00 (reference) | 1.00 (reference) | 1 [Reference] | 1 [Reference] |
| Primary care physician | 2.02 (1.78–2.29) | 0.97 (0.86–1.10) | 1.68 (1.53–1.85) | 1.26 (1.19–1.34) |
| Other/unknown | 1.35 (1.19–1.53) | 0.84 (0.75–0.94) | 1.33 (1.22–1.46) | 1.08 (1.03–1.15) |
| Calendar quarter† | 0.61 (0.59–0.62) | 0.98 (0.96–1.00) | 0.75 (0.74–0.76) | 0.93 (0.92–0.94) |
| CHA2DS2-VASc‡ | ||||
| 0–1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2–3 | 0.75 (0.64–0.89) | 1.04 (0.88–1.24) | 0.82 (0.72–0.92) | 0.84 (0.77–0.91) |
| ≥ 4 | 0.64 (0.55–0.76) | 0.77 (0.65–0.91) | 0.67 (0.59–0.76) | 0.62 (0.57–0.67) |
| HAS-BLED§ | ||||
| 0–1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 | 0.88 (0.76–1.01) | 0.90 (0.78–1.03) | 0.91 (0.82–1.02) | 0.85 (0.79–0.92) |
| ≥ 3 | 0.78 (0.68–0.90) | 0.80 (0.70–0.92) | 0.86 (0.77–0.96) | 0.69 (0.64–0.74) |
NOAC indicates non-vitamin K antagonist oral anticoagulant.
The results came from models that adjusted for all these variables and comorbidity components of CHA2DS2-VASc and HAS-BLED that were not already included in Charlson comorbidity index.
The results came from models that adjusted for race/ethnicity, education, household net worth, region of residence, prescriber specialty, calendar quarter, and comorbidity components of Charlson comorbidity index and HAS-BLED that were not already included in CHA2DS2-VASc.
The results came from models that adjusted for sex, race/ethnicity, education, household net worth, region of residence, prescriber specialty, calendar quarter, and comorbidity components of Charlson comorbidity index and CHA2DS2-VASc that were not already included in HAS-BLED.
PCPs were more likely than cardiologists to prescribe rivaroxaban (OR=1.68, 95% CI=1.53–1.85) or dabigatran (OR=2.02, 95% CI=1.78–2.29) vs apixaban in 2013–2014, but the strength of the associations significantly decreased (OR=1.26, 95% CI=1.19–1.34 for rivaroxaban) or became insignificant (OR=0.97, 95% CI=0.86–1.10 for dabigatran) in 2015–2017Q1.
Discussion
In this large retrospective study of patients with nonvalvular AF starting OAC therapy between October 2010 and March 2017, we found that the proportion of incident OAC users receiving NOAC prescriptions increased dramatically immediately following their introduction into the market, accounting for four fifths of incident OAC users in 2017Q1. Apixaban has become the most frequently prescribed OAC since 2015Q3 and accounted for half of the incident OAC prescriptions in 2017Q1. Compared with warfarin, NOACs were less likely to be prescribed to elderly, patients with higher stroke or bleeding risk, patients with more comorbidities, and those with lower socioeconomic status but more likely to be prescribed by a cardiologist. Among incident NOAC users, older age, female sex, higher stroke or bleeding risk, and more comorbidities were associated with higher odds of apixaban prescription.
Our finding of a substantial increase in incident apixaban prescription over time is consistent with studies from Denmark,30 Norway,31 and Sweden32 that reported an increasing initiation of apixaban, which became the most used OAC for AF in 2015. Although the European Society of Cardiology guidelines recommend the use of a NOAC over warfarin33,34 while the AHA/ACC/HRS guideline offers no such preference,21 physicians in both the U.S. and Europe tended to prescribe more NOACs than warfarin over time. In addition, we found that the prescription of apixaban rose with increasing age, in concordance with a recently published review that recommended apixaban as first choice of OAC therapy for patients with AF older than 75 years.35 It is possible that the prescribing pattern of apixaban may be influenced by a perception of favorable safety profiles of the drug for patients with AF regardless of age.28,36,37 For example, Halvorsen et al reported that apixaban was associated with a lower risk of stroke or systemic embolism, major bleeding, and death compared with warfarin in patients with AF of all ages, with an even greater benefit with advanced age.36
The sharp reduction in dabigatran prescription one year after its FDA approval was likely due to the introduction of rivaroxaban and apixaban. Nevertheless, it may be more likely to reflect physician reaction to early case reports of myocardial infarction and fatal bleeding,38–40 FDA warnings of significant risk of bleeding and gastrointestinal side effects, concerns over the validity of the data and outcomes from the original clinical trial used for FDA approval, and litigations against the drug which were settled for $650m.41,42 Although a recent study found that relative effectiveness and safety with dabigatran vs warfarin was in line with that reported in the trial,43 how this finding affects dabigatran prescription is not yet known. Moreover, about 80% of dabigatran is eliminated by the kidney, higher than 27% and 36% renal elimination for apixaban and rivaroxaban, respectively.44 Therefore, physicians may be more reluctant to prescribe dabigatran to patients with impaired renal function, a condition often found among elderly and comorbid patients. In addition, although idarucizumab, a reversal agent for dabigatran, was approved by the FDA in October 2015,45 our results showed that dabigatran prescription did not increase afterwards. This is contrary to the assumption that a NOAC would be more commonly prescribed if there was an available antidote.11–13,46,47
We found that the association between female sex and prescription of a NOAC vs warfarin changed over time, from no significant association in 2010Q4–2012 to a positive association in 2013–2017Q1. This may be related to a slightly higher rate of increasing prescription of apixaban over time among women than men. A prior U.S. study using registry data in 2010–2014 also found that NOAC use increased at a slightly higher rate in women relative to men.48 According to a meta-analysis, compared with men, women treated with a NOAC had a lower risk of major bleeding while those treated with warfarin had a higher risk of stroke and systemic embolism, suggesting an increased net clinical benefit of NOACs compared with warfarin in preventing stroke among women with AF.49
Our finding that cardiologists were more likely to prescribe a NOAC vs warfarin is consistent with prior studies in the U.S.11,12,16 This finding is not surprising given that cardiologists are specialists most commonly involved in care for patients with AF. They are possibly more familiar with the literature about NOAC use and have more experience in prescribing a NOAC, and thus, may have a higher level of comfort with NOAC prescriptions.50 What is unique from our finding is that the strength of the positive association between prescriber specialty (PCPs vs cardiologists) and the prescription of rivaroxaban or dabigatran vs apixaban decreased or became insignificant over time.
Our study has several limitations. First, our analysis of OAC prescriptions among commercial and Medicare Advantage beneficiaries may not represent treatment decisions for all patients with AF in the U.S. However, our cohort consisted of a large diverse patient population across various geographic regions, and thus, our findings of the associations between patient characteristics and OAC choice may be generalizable to the insured patients in AF in the country. Second, although we included a variety of relevant confounders in our analysis, we cannot rule out the possibility of unobserved factors, such as formulary restrictions, and physician and patient preferences, which may be associated with the selection of an OAC. Third, the claims we used may have coding errors that led to misclassifications of patients with nonvalvular AF. However, the ICD codes for nonvalvular AF were commonly used in studies using claims and registry data.10,18–20 Fourth, although we were able to identify OAC prescriptions from the claims, we do not know whether the patients actually took these drugs. Finally, because claims do not include information on the use of over-the-counter drugs, the proportion of patients prescribed antiplatelets or NSAIDs may be underestimated. Nevertheless, the underestimation is expected to be non-differential across different OAC groups.
In conclusion, NOAC prescriptions have increased substantially among patients with nonvalvular AF starting OAC therapy. Clinical practice tended to respond rapidly to evidence of effectiveness and harm associated with apixaban and dabigatran. Apixaban has become the most prescribed OAC eventually and was mainly prescribed to elderly, women, patients with higher stroke or bleeding risk, and those with more comorbidities. The association of certain patient characteristics such as sex and prescriber specialty with OAC choice changed over time. This finding together with increased availability of NOACs underscores the need for careful, ongoing surveillance of the use of OACs in different patient populations in clinical practice and for longitudinal effectiveness and safety analyses of these drugs.
Acknowledgments
Sources of Funding
Dr. Zhu is supported by a Career Development Award from the Agency for Healthcare Research and Quality (Grant No. 1 K01 HS024737–01).
Footnotes
Conflict of interest statement
Dr. Alexander is Chair of FDA’s Peripheral and Central Nervous System Advisory Committee, serves as a paid advisor to QuintilesIMS, serves on the advisory board of MesaRx Innovations, is a member of OptumRx’s National P&T Committee; and holds equity in Monument Analytics. Dr. Nazarian is a consultant to Biosense Webster, Siemens, St Jude Medical, and CardioSolv and receives research grants from Biosense Webster, Siemens, and Imricor. These arrangements have been reviewed and approved by Johns Hopkins University and the University of Pennsylvania, in accordance with respective conflict of interest policies.
Contributor Information
Junya Zhu, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, OptumLabs, Visiting Fellow, Cambridge, MA.
G. Caleb Alexander, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD.
Saman Nazarian, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, The University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Jodi B. Segal, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD.
Albert W. Wu, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD.
REFERENCES
- 1.Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA 2015;313:1950–1962. [DOI] [PubMed] [Google Scholar]
- 2.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med 2003;349:1019–1026. [DOI] [PubMed] [Google Scholar]
- 3.Moss JD, Cifu AS. Management of anticoagulation in patients with atrial fibrillation. JAMA 2015;314:291–292. [DOI] [PubMed] [Google Scholar]
- 4.Mega JL. A new era for anticoagulation in atrial fibrillation. N Engl J Med 2011;365:1052–1054. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 7.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 8.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 9.Desai NR, Krumme AA, Schneeweiss S, et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation--quality and cost implications. Am J Med 2014;127:1075–1082.e1. [DOI] [PubMed] [Google Scholar]
- 10.Ashburner JM, Singer DE, Lubitz SA, Borowsky LH, Atlas SJ. Changes in use of anticoagulation in patients with atrial fibrillation within a primary care network associated with the introduction of direct oral anticoagulants. Am J Cardiol 2017;120:786–791. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg BA, Shrader P, Thomas L, et al. Factors associated with non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with new-onset atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT-AF II). Am Heart J 2017;189:40–47. [DOI] [PubMed] [Google Scholar]
- 12.AbuDagga A, Stephenson JJ, Fu AC, Kwong WJ, Tan H, Weintraub WS. Characteristics affecting oral anticoagulant therapy choice among patients with non-valvular atrial fibrillation: a retrospective claims analysis. BMC Health Serv Res 2014;14:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lauffenburger JC, Farley JF, Gehi AK, Rhoney DH, Brookhart MA, Fang G. Factors driving anticoagulant selection in patients with atrial fibrillation in the United States. Am J Cardiol 2015;115:1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoof N, Schnee J, Schneider G, et al. Characteristics of patients with non-valvular atrial fibrillation using dabigatran or warfarin in the US. Curr Med Res Opin 2014;30:795–804. [DOI] [PubMed] [Google Scholar]
- 15.Baik SH, Hernandez I, Zhang Y. Evaluating the initiation of novel oral anticoagulants in Medicare beneficiaries. J Manag Care Spec Pharm 2016;22:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes 2012;5:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.OptumLabs. OptumLabs and OptumLabs Data Warehouse (OLDW) Descriptions and Citation Cambridge Mnp, June 2017. PDF. Reproduced with permission from OptumLabs. [Google Scholar]
- 18.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf 2012;21:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao X, Abraham NS, Sangaralingham LR, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc 2016;5:e003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu AYX, Malo S, Svenson LW, Wilton SB, Hill MD. Temporal trends in the use and comparative effectiveness of direct oral anticoagulant agents versus warfarin for nonvalvular atrial fibrillation: a Canadian population-based study. J Am Heart Assoc 2017;6:e007129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–76. [DOI] [PubMed] [Google Scholar]
- 22.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 23.Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olesen JB, Lip GY, Hansen PR, et al. Bleeding risk in ‘real world’ patients with atrial fibrillation: comparison of two established bleeding prediction schemes in a nationwide cohort. J Thromb Haemost 2011;9:1460–1467. [DOI] [PubMed] [Google Scholar]
- 25.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 26.Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ 2015;350:h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gundlund A, Staerk L, Fosbøl EL, et al. Initiation of anticoagulation in atrial fibrillation: which factors are associated with choice of anticoagulant? J Intern Med 2017;282:164–174. [DOI] [PubMed] [Google Scholar]
- 28.Noseworthy PA, Yao X, Abraham NS, Sangaralingham LR, McBane RD, Shah ND. Direct comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in non-valvular atrial fibrillation. Chest 2016;150:1302–1312. [DOI] [PubMed] [Google Scholar]
- 29.Dukanovic A, Staerk L, Fosbøl EL, Gadsbøll K, Gislason GH, Olesen JB. Predicted risk of stroke and bleeding and use of oral anticoagulants in atrial fibrillation: Danish nationwide temporal trends 2011–2016. Thromb Res 2017;160:19–26. [DOI] [PubMed] [Google Scholar]
- 30.Staerk L, Fosbøl EL, Gadsbøll K, et al. Non-vitamin K antagonist oral anticoagulation usage according to age among patients with atrial fibrillation: temporal trends 2011–2015 in Denmark. Sci Rep 2016;6:31477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kjerpeseth LJ, Ellekjær H, Selmer R, Ariansen I, Furu K, Skovlund E. Trends in use of warfarin and direct oral anticoagulants in atrial fibrillation in Norway, 2010 to 2015. Eur J Clin Pharmacol 2017;73:1417–1425. [DOI] [PubMed] [Google Scholar]
- 32.Komen J, Forslund T, Hjemdahl P, Andersen M, Wettermark B. Effects of policy interventions on the introduction of novel oral anticoagulants in Stockholm: an interrupted time series analysis. Br J Clin Pharmacol 2017;83:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camm AJ, Pinto FJ, Hankey GJ, Andreotti F, Hobbs FD, Writing Committee of the Action for Stroke Prevention Alliance. Non-vitamin K antagonist oral anticoagulants and atrial fibrillation guidelines in practice: barriers to and strategies for optimal implementation. Europace 2015;17:1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 35.Diener HC, Aisenberg J, Ansell J, et al. Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: part 2. Eur Heart J 2017;38:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halvorsen S, Atar D, Yang H, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J 2014;35:1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lip GY, Mitchell SA, Liu X, et al. Relative efficacy and safety of non-vitamin K oral anticoagulants for non-valvular atrial fibrillation: network meta-analysis comparing apixaban, dabigatran, rivaroxaban and edoxaban in three patient subgroups. Int J Cardiol 2016;204:88–94. [DOI] [PubMed] [Google Scholar]
- 38.Harper P, Young L, Merriman E. Bleeding risk with dabigatran in the frail elderly. N Engl J Med 2012;366:864–866. [DOI] [PubMed] [Google Scholar]
- 39.Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 2012;172:397–402. [DOI] [PubMed] [Google Scholar]
- 40.Legrand M, Mateo J, Aribaud A, et al. The use of dabigatran in elderly patients. Arch Intern Med 2011;171:1285–1286. [DOI] [PubMed] [Google Scholar]
- 41.Cohen D Concerns over data in key dabigatran trial. BMJ 2014;349:g4747. [DOI] [PubMed] [Google Scholar]
- 42.Cohen D Dabigatran: how the drug company withheld important analyses. BMJ 2014;349:g4670. [DOI] [PubMed] [Google Scholar]
- 43.Go AS, Singer DE, Toh S, et al. Outcomes of dabigatran and warfarin for atrial fibrillation in contemporary practice: a retrospective cohort study. Ann Intern Med 2017;167:845–854. [DOI] [PubMed] [Google Scholar]
- 44.Di Lullo L, Ronco C, Cozzolino M, et al. Nonvitamin K-dependent oral anticoagulants (NOACs) in chronic kidney disease patients with atrial fibrillation. Thromb Res 2017;155:38–47. [DOI] [PubMed] [Google Scholar]
- 45.Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015;373:511–520. [DOI] [PubMed] [Google Scholar]
- 46.Komen J, Forslund T, Hjemdahl P, Wettermark B. Factors associated with antithrombotic treatment decisions for stroke prevention in atrial fibrillation in the Stockholm region after the introduction of NOACs. Eur J Clin Pharmacol 2017;73:1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker D, Wilsmore B, Narasimhan S. Adoption of direct oral anticoagulants for stroke prevention in atrial fibrillation. Intern Med J 2016;46:792–797. [DOI] [PubMed] [Google Scholar]
- 48.Thompson LE, Maddox TM, Lei L, et al. Sex differences in the use of oral anticoagulants for atrial fibrillation: a report from the National Cardiovascular Data Registry (NCDR®) PINNACLE Registry. J Am Heart Assoc 2017;6:e005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pancholy SB, Sharma PS, Pancholy DS, Patel TM, Callans DJ, Marchlinski FE. Meta-analysis of gender differences in residual stroke risk and major bleeding in patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Am J Cardiol 2014;113:485–490. [DOI] [PubMed] [Google Scholar]
- 50.Huang C, Siu M, Vu L, Wong S, Shin J. Factors influencing doctors’ selection of dabigatran in non-valvular atrial fibrillation. J Eval Clin Pract 2013;19:938–943 [DOI] [PubMed] [Google Scholar]


