Abstract
Study Design:
Systematic review.
Objectives:
Sacral chordomas are rare, primary tumors of the spine, best treated with en bloc resection. The purpose of this study was to assess the literature for resected sacral chordoma and to quantify the prevalence of, risk factors for, and treatment outcomes of local and distant recurrence therein.
Methods:
We searched 5 online databases from January 1980 to May 2016 to find articles that report survival, recurrence outcomes, and/or prognostic factors for the resected sacral chordoma patient population. Characteristics and clinical outcomes of the pooled cohort are reported. Fisher exact tests, unpaired t tests, and one-way analysis of variance were used to investigate patient- and treatment-associated prognostic factors for local and distant recurrence. Survival analyses were performed for time to local recurrence and death. The protocol’s PROSPERO ID is CRD42015024384.
Results:
Fifty-seven studies, with 1235 unique sacral chordoma patients, were included in this review. Local and distant recurrence occurred in 42.6% and 22.4% of patients with adequate follow-up, respectively. Kaplan-Meier overall median survival for patients with and without recurrence were 98 and 209 months after surgery, respectively. Wide surgical margin was associated with a lower rate of local recurrence; and wide surgical margin, female sex, and patient age ≥65 years were associated with lower rates of distant recurrence.
Conclusions:
While surgical margin remains the most significant prognostic factor for local and distant recurrence, combined surgical approach may be associated with local recurrence. Male sex and age <65 years may be associated with distant recurrence. Patients with risk factors for recurrence should undergo close monitoring to maximize survival.
Keywords: sacral chordoma, recurrent chordoma, systematic review, tumors, lumbosacral, sacrum
Introduction
Chordomas are rare, primary bone tumors of the spine believed to originate from the remnants of the embryological notochord. They most frequently arise at the skull base and the sacrum.1 These tumors are classically described to be low-grade and malignant. Chordomas have been recently reported to have a 5-year survival rate of 73% to 86% and a 10-year survival rate of 49% to 71%.2
Surgery persists as the mainstay of chordoma management, as the efficacy of radiation therapy alone remains controversial,3 and the slow-growing nature of chordomas confers a high resistance to chemotherapy.4 Oncologic staging of chordomas describes most as Enneking Stage 1B, indicating en bloc excision with wide margins as the appropriate surgical treatment.4,5 When en bloc excision is not possible due to neural structure involvement or patient fitness, intralesional excision may be appropriate to limit morbidity. Although chordomas have historically proven to be relatively radiation-resistant,4,6 recent promising studies suggest proton beam and carbon ion therapy may contribute to increased local control and improved survival in patients with this disease.7–15
Local recurrence rates following resection of sacral chordoma in large (n ≥ 50) studies range from 38% to 56%,16–21 and distant recurrence rates range from 9% to 33%.6,18–21 In one study with mean follow-up of 8.1 years, local recurrence was associated with a 21-fold increase in risk of tumor-related death and distant recurrence with a 451-fold increase.22 However, due to the low incidence of chordomas (fewer than 1/million/year),2 statistically powerful data on prevalence, risk factors, and treatment outcomes for recurrent sacral chordomas are lacking, with the largest studies to date including fewer than 200 patients. The purpose of this study is to systematically review the literature and pool a large number of patient cohorts describing the resected sacral chordoma population to date and to better estimate the prevalence of, risk factors for, and treatment outcomes of local and distant recurrence therein.
Methods
Search Strategy and Selection Criteria
A systematic review of the literature was performed using PubMed, Embase, CINAHL, Web of Science, and Cochrane, and a review of the bibliographies of relevant articles. The search queries (Supplemental Table S1; available in the online version of the article) were designed to return all articles in English on sacral chordomas that mention surgical treatment published from January 1, 1980, to May 19, 2016. Some patients in these publications had years of presentation back to the 1940s (presentation years listed in Supplemental Table S2). Both individual patient-level data and summary statistics were collected. All patients included in this systematic review underwent surgical treatment for sacral chordoma.
All potentially eligible studies returned by the search strings underwent title and abstract review by 2 authors, and were determined to be eligible or ineligible by consensus, based on inclusion criteria. A third author independently reviewed and verified the decisions. All articles that passed the title and abstract screen underwent full-text review and were subjected to the following exclusion criteria: study size of at least 4 individuals who meet the aforementioned criteria; mean follow-up ≥12 months; adequate description of clinical outcome in terms of local control; separate treatment of resected sacral chordoma patients; fully published, peer-reviewed study; and unique patient cohort. Authors of included studies were not contacted for more detailed information on patients.
Data Analysis
Two-tailed, Fisher’s exact test P values were calculated to compare prevalence of recurrence in subgroups of binary prognostic factors. These analyses were not prespecified, as assessment of the availability of data was first needed to determine feasibility of analysis. Time to recurrence P values for binary prognostic factors were calculated using unpaired, 2-tailed t tests. GraphPad Software was used (GraphPad QuickCalcs) for these calculations. The P values comparing prevalence for the 4 surgical margin groups were calculated using a χ2 test. The time to recurrence P values for surgical margins were calculated using a one-way analysis of variance (ANOVA) test. These calculations were performed in Microsoft Excel (Version 15.24). Relative risk calculations were performed using MedCalc Online.
Cohort-level data was used in the time to recurrence analyses. Mean times to recurrence for each prognostic factor subgroup in each article were used and included in the analyses the appropriate number of times to reflect the number of patients in the subgroup. Patient-level data was used in the survival analyses. All Kaplan-Meier curves were generated using Excel. The Kaplan-Meier log-rank test P value and the Cox proportional hazards regression were calculated using In-Silico Online. Standard deviations provided with pooled cohort means reflect variation in component study means.
Pearson correlation coefficients for case volume analyses were calculated in Microsoft Excel.
Definitions
Margins of a chordoma resection were recorded as follows: wide if the primary study reported the margins to be wide, radical, or greater than 2 cm; marginal if the primary study reported the margins to be marginal, or greater than 0.01 cm and less than 2 cm; intralesional if the primary study reported the margins to be intralesional, positive, inadequate, or Union for International Cancer Control (UICC) category R2;23 wide-contaminated if the primary study reported the margins to be wide-contaminated; and negative if the primary study reported the margins to be negative, tumor-free, or UICC category R0, or reported the resection as clean, adequate, gross total, or complete.
Results
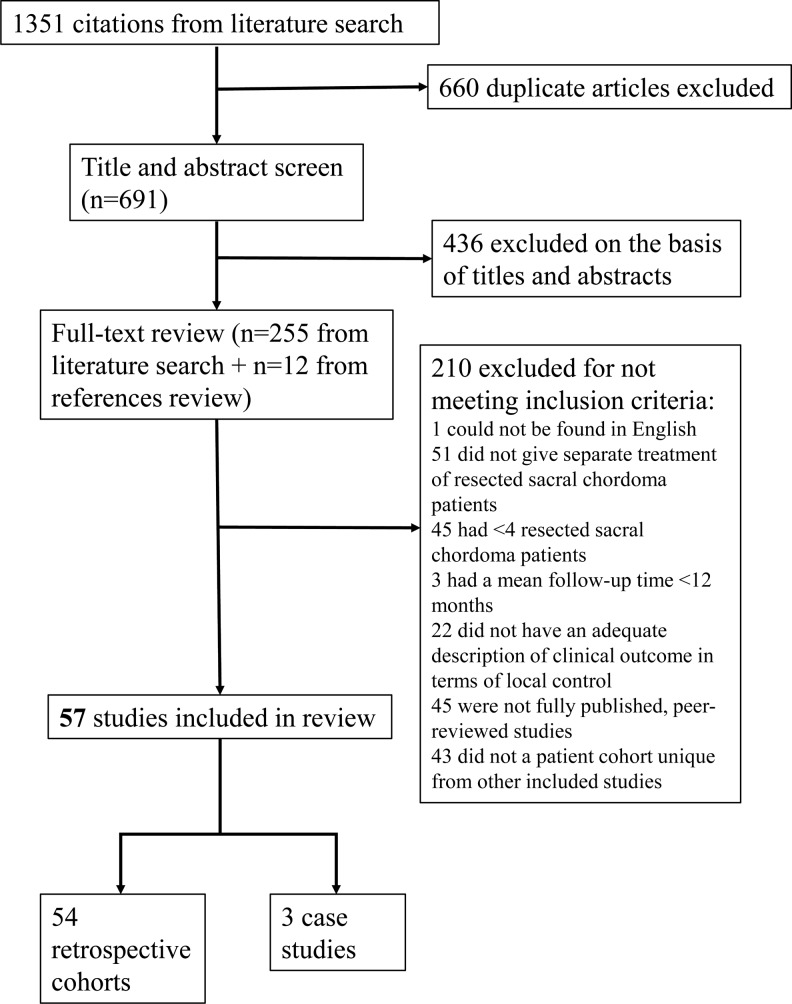
A literature search was performed using 5 online databases. After exclusion based on the established eligibility criteria, 57 articles were included in the analysis, with a total of 1235 surgical sacral chordoma patients (Figure 1). These studies originated from many different medical departments, with 34 first authors affiliated with orthopedic surgery or oncology, 8 affiliated with general surgery, 6 with neurosurgery, 3 with surgical oncology, and 6 from various other departments including pathology and anesthesiology.
Figure 1.
CONSORT diagram detailing inclusion and exclusion criteria. Fifty-seven articles were included in the analysis, with a total of 1235 surgical sacral chordoma patients.
Among the patients for whom gender was known, 62% were male, giving a male-to-female ratio of 1.6:1 (Table 1). The mean age at diagnosis of sacral chordoma was 56.1 ± 5.4 (range 13-85). The primary symptom was pain (87%), followed by palpable mass (50%) and various neurologic symptoms (15% to 30%). The mean follow-up time for the cohort was 72.0 ± 27.5 months.
Table 1.
Characteristics of Patients in Pooled Cohorta.
| Characteristic | % (n) of Patients |
|---|---|
| Total patients, n | 1235 |
| Sex, n = 1081 | |
| Male | 62.0% (670) |
| Female | 38.0% (411) |
| Ratio (male to female) | 1.6 |
| Age, n = 800 | |
| Mean | 56.1 ± 5.4 |
| Range | 13-85 |
| Previous sacral surgery, n = 601 | 20.5% (123) |
| Symptoms at presentation | |
| Pain, n = 308 | 86.7% (267) |
| Mass/swelling, n = 177 | 50.3% (89) |
| Bowel dysfunction, n = 171 | 27.5% (47) |
| Bladder dysfunction, n = 180 | 18.9% (34) |
| Neuropathic pain, n = 124 | 16.1% (20) |
| Neurologic symptoms: unspecified or other, n ==190 | 21.1% (40) |
| Follow-up, n = 956 | |
| Mean (months) | 72.0 ± 27.5 |
| Range (years) | 0-34 |
Abbreviation: n, number of patients at risk (in studies reporting characteristic).
aA total of 1235 surgical sacral chordoma patients were included. Among the patients for whom gender was known, 62% were male, giving a male-to-female ratio of 1.6:1. The mean age at diagnosis of sacral chordoma was 56.1 ± 5.4 (range 13-85).
Approximately 95% of patients underwent biopsy or had had previous sacral surgery before resection at the institution of primary treatment (Table 2). The mean operating time on these sacral chordomas was 9.2 ± 4.5 hours (1.2-26). Mean estimated blood loss was 2991 ± 2841 mL. Fifty-five percent of patients underwent a posterior-only approach to the resection, 45% underwent a combined approach, and one case was anterior only. The mean reported diameter of the sacral chordomas was 10.5 ± 2 cm (2.8-30), and 61% of the tumors extended to S2 or above.
Table 2.
Tumor and Surgery Characteristicsa.
| Characteristic | % (n) of Patients |
|---|---|
| Biopsy preoperative, n = 458 | 94.8% (434) |
| Biopsy type, n = 271 | |
| Previous surgery | 25.1% (68) |
| Needleb | 52.8% (143) |
| Open | 22.1% (60) |
| Enneking staging, n = 61 | |
| 1B | 96.7% (59) |
| 3A | 1.6% (1) |
| 3B | 1.6% (1) |
| Operating time (hours), n = 225 | |
| Mean | 9.2 ± 4.5 |
| Range | 1.2-26 |
| Estimated blood loss (mL), n = 273 | |
| Mean | 2991 ± 2841 |
| Range | 100-28 800 |
| Blood transfusion (units), n = 60 | |
| Mean | 8.7 ± 1.5 |
| Range | 0-35 |
| Approach, n = 803 | |
| Posterior only | 54.9% (441) |
| Anterior only | 0.1% (1) |
| Combined A/P | 45.0% (361) |
| Proximal extentc, n = 574 | |
| L5 | 2.1% (12) |
| S1 | 19.2% (110) |
| S2 | 39.9% (229) |
| S3 | 24.7% (142) |
| S4 | 11.3% (65) |
| S5 or below | 2.8% (16) |
| Maximum tumor diameter (cm), n = 254 | |
| Mean | 10.5 ± 2.0 |
| Range | 2.8-30 |
| Tumor volume (mL), n = 261 | |
| Mean | 519 ± 146 |
| Range | 4-4532 |
Abbreviations: n, number of patients at risk (in studies reporting characteristic).
aApproximately 95% of patients underwent biopsy or had had previous sacral surgery before resection at the institution of primary treatment.
bFine-needle aspiration, computed tomography–guided needle, tru-cut needle, core needle.
cExtent of tumor or of resection.
Out of nearly 1000 surgeries, 81% had negative margins (39% wide, 22% marginal, 20% unspecified negative; Table 3). Seventeen percent were intralesional or had positive margins, and 2% had wide margins following contamination. Sixteen percent of cases had a complication that required reoperation; indications for reoperation include operative debridement, repair of intestinal fistula, hardware revision after loss of fixation, and correction of persistent cerebrospinal fluid leak.24–26
Table 3.
Treatment Details and Complicationsa.
| Characteristic | % (n) of Patients |
|---|---|
| Neoadjuvant therapy, n = 179 | |
| Radiation | 15.6% (28) |
| Chemotherapy | 2.8% (5) |
| Surgical margin, n = 991 | |
| Wideb | 39.1% (387) |
| Marginalc | 21.8% (216) |
| Wide-contaminated | 1.9% (19) |
| Intralesionald | 17.2% (170) |
| Negative (not specified)e | 20.1% (199) |
| Adjuvant therapy, n = 780 | |
| Radiation | 37.1% (289) |
| Chemotherapy | 1.8% (14) |
| Complication requiring reoperation, n = 335 | 15.8% (53) |
| Wound complications, n = 552 | |
| Dehiscence | 19.2% (106) |
| Infection | 17.2% (95) |
Abbreviation: n, number of patients at risk (in studies reporting characteristic).
aOut of nearly 1000 surgeries, 81% had negative margins (39% wide, 22% marginal, 20% unspecified negative).
bReported as wide, radical, or >2 cm from tumor.
cReported as marginal or >0.01 cm and <2 cm from tumor.
dReported as intralesional, positive, inadequate, or R2.
eReported as negative, tumor-free, R0, clean, adequate, gross total, or complete.
The most common adjuvant medical therapy for these patients was radiation, which was used preoperatively in 16% of reported cases and postoperatively in 37% of cases. In reporting these practices, it is important to note that usage rates of radiotherapy varied widely from center to center. Some articles report use of radiation in all sacral chordoma patients,27–30 while others did not augment surgical treatment in any case.14,31–33 The most common practice was to use radiotherapy sparingly, selecting the candidates deemed most likely to benefit from its use.19,20,22,24,25,33–54 Chemotherapy was uncommon across all studies.
Out of 1229 patients with local control follow-up in this study, 43% experienced a local recurrence (Table 4). The mean time to local recurrence was 40 ± 19 (1-228) months after surgery. Metastases were half as prevalent as local recurrence, with only 22% of subjects demonstrating distant recurrence at a mean time after surgery of 60 ± 30 (0-228) months. The most common location for distant recurrence was the lung, accountable for 69% of metastatic lesions with known location. Regarding long-term prognosis, just over a quarter (27%) of all surgically treated sacral chordoma patients died from their chordoma by the end of follow-up. However, nearly another quarter (24%) were continuously disease-free throughout follow-up. An additional 30% had no evidence of remaining chordoma and another 16% were alive with disease at the end of follow-up.
Table 4.
Disease Control and Outcomesa.
| Variable | % (n) of Patients |
|---|---|
| Local recurrence event, n = 1229 | 42.6% (523) |
| Local recurrence, time to (months), n = 275 | |
| Time to, mean | 40 ± 19 |
| Time to, range | 1-228 |
| Distant recurrence event, n = 921 | 22.4% (206) |
| Distant recurrence, time to (months), n = 75 | |
| Time to, mean | 60. ± 30. |
| Time to, range | 0-228 |
| Location of distant recurrence, nb = 127 | |
| Lung | 68.5% (87) |
| Liver | 6.3% (8) |
| Spine | 8.7% (11) |
| Elsewhere | 16.5% (21) |
| Oncologic status at end of follow-up, n = 776 | |
| Continuously disease free | 23.7% (184) |
| No evidence of disease | 29.5% (229) |
| Alive with disease | 15.6% (121) |
| Dead of disease/treatment | 26.7% (207) |
| Dead of other causes | 3.7% (29) |
| Lost to follow-up | 0.8% (6) |
Abbreviation: n, number of patients at risk (in studies reporting characteristic).
aOf 1229 patients with local control follow-up in this study, 43% experienced a local recurrence. The mean time to local recurrence was 40 ± 19 (1-228) months after surgery.
bNumber of metastases (not necessarily patients) with known location.
Three factors were found to be significantly associated with local recurrence: history of previous sacral surgery, surgical margin, and adjuvant therapy (Table 5). Patients with a history of sacral surgery had significantly greater local recurrence compared with patients receiving sacral surgery for the first time (61.3% compared with 38.4%, P = .0002). Resection margins were found to be statistically significant in recurrence prognosis as well (P < .0001), with patients who received wide, marginal, intralesional, and wide-contaminated margins experiencing local recurrence at rates of 25.6%, 44.4%, 57.0%, and 64.7%, respectively. Patients who received adjuvant therapy had higher rates of local recurrence compared with those who did not receive adjuvant therapy (53.5% vs 42.3%, P = .0147). Finally, patients who underwent surgery with combined anterior-posterior approach had a strong trend toward greater local recurrence compared with patients treated by posterior approach only (45.6% vs 35.2%, P = .0505).
Table 5.
Univariate Analyses for Prognostic Factors for Recurrencea.
| Variable | Local Recurrence Event % (LR/Total) | P | Time to LR (Months) | P | Distant Recurrence Event % (DR/Total) | P | Time to DR (Months) | P |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Male | 47.2% (153/324) | .0601 | 37 | .7441 | 27.8% (54/194) | .0265 | 51 | .7582 |
| Female | 38.8% (80/206) | 38 | 16.4% (19/116) | 54 | ||||
| Age | ||||||||
| <65 | 46.0% (165/359) | .6742 | 39 | .0433 | 25.7% (61/237) | .0304 | 55 | .0457 |
| ≥65 | 43.4% (53/122) | 30 | 13.8% (11/80) | 38 | ||||
| Neurologic symptomsb | ||||||||
| Present | 46.7% (28/60) | 1.000 | 25 | .0028 | 16.3% (8/49) | .6064 | 47 | c |
| Absent | 45.3% (24/53) | 38 | 21.3% (10/47) | 30 | ||||
| Surgical approach | ||||||||
| Combined | 45.6% (77/169) | .0505 | 49 | <.0001 | 24.3% (26/107) | .0963 | 47 | .7293 |
| Posterior | 35.2% (64/182) | 26 | 15.2% (19/125) | 43 | ||||
| Previous surgery | ||||||||
| Yes | 60.0% (57/95) | .0004 | 50 | .2964 | 37.0% (10/27) | .2244 | 54 | .8425 |
| No | 37.8% (90/238) | 41 | 24.1% (27/112) | 52 | ||||
| Surgical margin | ||||||||
| Wide | 25.6% (80/313) | <.0001 | 47 | .1034 | 16.4% (23/140) | <.0001 | 66 | .6724 |
| Marginal | 44.4% (59/133) | 37 | 40.0% (24/60) | 56 | ||||
| Wide-contaminated | 64.7% (11/17) | 44 | 75.0% (3/4) | 39 | ||||
| Intralesional | 57.0% (73/128) | 35 | 52.5% (32/61) | 52 | ||||
| Proximal extent | ||||||||
| Above S3 | 44.3% (120/271) | 1.000 | 42 | .5819 | 27.0% (38/141) | .1984 | 58 | .0193 |
| S3 or below | 44.5% (85/191) | 45 | 18.6% (16/86) | 35 | ||||
| Adjuvant therapyd | ||||||||
| Used | 53.5% (115/215) | .0147 | 39 | .0014 | 23.2% (29/125) | .0338 | 50 | .0512 |
| Not used | 42.3% (121/286) | 25 | 12.6% (17/135) | 31 |
Abbreviations: LR, local recurrence; DR, distant recurrence.
aThree factors were found to be significantly associated with local recurrence: history of previous sacral surgery, surgical margin, and adjuvant therapy.
bIncludes sensory deficit, motor deficit, saddle anesthesia, difficulty walking, neuropathic pain, bowel dysfunction, bladder dysfunction.
cNot enough subjects to perform a statistical analysis.
dIncludes radiation therapy, chemotherapy, cryosurgery.
Combined surgical approach and use of adjuvant therapy were both found to be significantly associated with longer time to local recurrence. Age ≥65 years and presence of neurologic symptoms were also both associated with shorter time to local recurrence, despite having no association with recurrence prevalence.
Four factors were found to be significantly associated with distant recurrence: sex, age, surgical margin, and use of adjuvant therapy. Males had a higher rate of distant recurrence than females (27.8% vs 16.4%, P = .0265), and patients <65 years of age had a higher rate of distant recurrence than patients ≥65 years of age (25.7% vs 13.8%, P = .0304). Wide, marginal, intralesional, and wide-contaminated surgical margins were associated with 16.4%, 40.0%, 52.5%, and 75.0% rates of distant recurrence, respectively (P < .0001). Last, the use of adjuvant therapy was associated with a 23.2% rate of distant recurrence, which is significantly higher than the 12.6% rate for patients who did not receive adjuvant therapy (P = .0338).
Age and proximal extent of tumor were also both significantly associated with time to distant recurrence. Patients <65 years of age demonstrated a longer mean time to distant recurrence compared with patients ≥65 years of age, and higher proximal extent of tumor (above S3) was associated with a longer mean time to distant recurrence than lower proximal extent.
Patients who were treated with a surgery with wide margins had very significantly lower rates of both local and distant recurrence compared with patients with any other surgical margin classification (Table 6). Marginal margins were associated with a lower local recurrence rate than intralesional margins (P = .0477), though not a lower distant recurrence rate.
Table 6.
Surgical Margins Pairwise Comparisonsa.
| Surgical Margin | Local Recurrence P Value | Distant Recurrence P Value |
|---|---|---|
| Wide versus | ||
| Marginal | .0001 | .0005 |
| Wide-contaminated | <.0001 | <.0001 |
| Intralesional | .0011 | .0187 |
| Not wide | <.0001 | <.0001 |
| Marginal versus | ||
| Wide-contaminated | .1286 | .3019 |
| Intralesional | .0477 | .2033 |
| Wide-contaminated versus | ||
| Intralesional | .6099 | .6177 |
| Wide/marginal versus | ||
| Contaminated/intralesional | <.0001 | <.0001 |
aPatients who were treated with a surgery with wide margins had very significantly lower rates of both local and distant recurrence compared with patients with any other surgical margin classification.
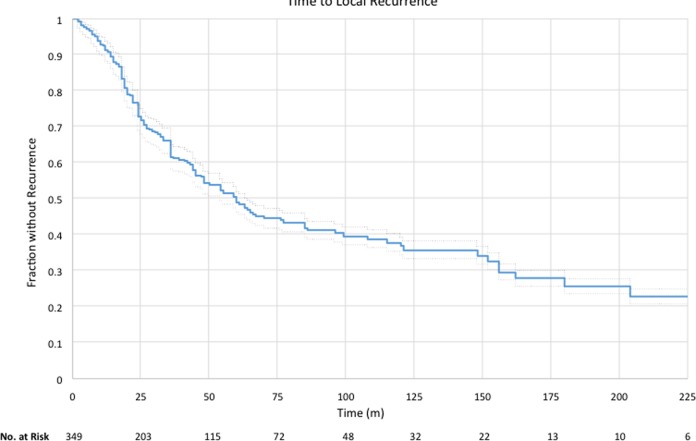
Patient-level data on follow-up, recurrence status, and time to recurrence (as applicable) were available for 349 patients. Of the 349 patients, 169 (48.4%) had a recurrence. Mean time to recurrence was 37 ± 36 months (range 2-204), and Kaplan-Meier median was 59 months (Figure 2).
Figure 2.
Kaplan-Meier curve for the 349 patients for whom specific recurrence data were available. Mean time to recurrence was 37 ± 36 months (range 2-204), and Kaplan-Meier median was 59 months.
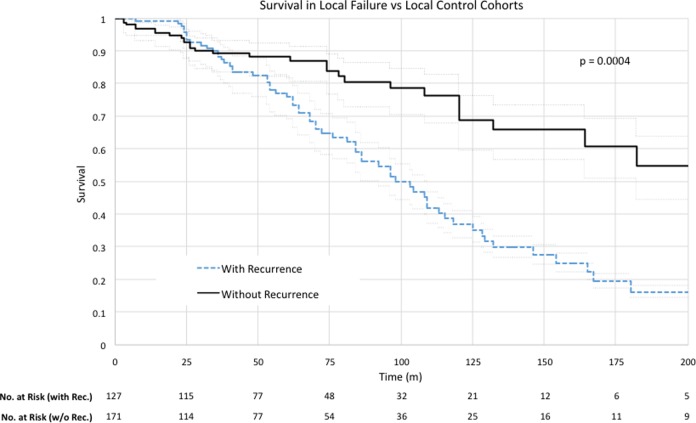
There were 298 individuals for whom time to recurrence (as applicable), total follow-up time, and survival status at the end of the study were known. Of these 298 patients, 127 (42.6%) had a local recurrence. A Kaplan-Meier analysis looking at survival in the local recurrence cohort versus the local control cohort is presented in Figure 3. Median survival in the local recurrence group was 98 months, compared with 209 months in the local control group (P = .0005). Survival after surgery at 5, 10, and 15 years for the local control cohort was 88%, 69%, and 61%, respectively, compared with 76%, 37%, and 16% for the local failure cohort. The Cox proportional hazard regression for local recurrence was 2.04 (95% confidence interval = 1.34-3.08).
Figure 3.
Kaplan-Meier survival analysis for the local recurrence cohort and local control cohort. Median survival in the local recurrence group was 98 months, compared with 209 months in the local control group (P = .0005).
After data extraction, we performed an overview analysis to investigate the impact of institution caseload on recurrence rate. We used the years over which the databases were queried and the number of patients that met inclusion criteria for this review in order to approximate the number of sacral chordoma resections each institution performed per year. There were 9 single-institution studies that averaged fewer than one resection every 2 years by this metric (“low-volume”), and 6 single-institution studies that averaged more than 3 resections per year (“high-volume”). Two centers meeting the initial criteria for low-volume were excluded for incomplete reporting, leaving 7 studies for this analysis.
Numbers of cases per year varied from 0.22 to 12.8. Of the 39 patients seen at low-volume centers, 24 (61.5%) had a local recurrence (institution recurrence rates ranged from 20% to 75%). Of the 364 patients seen at high-volume centers, 150 (41.2%) had a local recurrence (range 7.7% to 55.2%). This difference was statistically significant (P = .0173). Mean known time to recurrence for patients treated at low-volume institutions was 23.0 months, compared with 41.6 months for high-volume institutions (P < .0001). Pearson correlation coefficients for caseload versus local recurrence rate and caseload versus time to local recurrence were −0.175 and 0.0626, respectively (Supplemental Figure 1).
Discussion
Recurrence is often considered the most important prognostic factor for the surgically treated sacral chordoma patient.68 However, studies comprehensively evaluating recurrence in sacral chordoma are generally small and underpowered. This study was carried out to systematically review the literature and pool patient cohorts to describe the resected sacral chordoma population and to estimate the prevalence of, risk factors for, and treatment outcomes of local and distant recurrence therein.
Tables 1 to 4 serve to characterize the surgical sacral chordoma population and disease course. In Table 1, the mean age at diagnosis of sacral chordoma is reported to be 56, but with a low end of the range at 13. Most chordomas present late in life due to characteristically slow growth over years; however, within the current model of embryologic origin,1 it is not unanticipated that rare pediatric patients could present secondary to the etiology of the disease. In their large epidemiologic study, McMaster et al found that less than 2% of sacral chordoma patients were age 3 to 25 at diagnosis.2
In Table 2, the mean estimated blood loss (EBL) across all patients is reported to be ∼3 L (range 0.1-28.8 L). Although there are several examples of individual cases with high EBL, there is no single study in this review that reports a mean EBL greater than 9 L; therefore, higher EBLs could be a product of particularly difficult cases (increased tumor size, increased tumor vascularity, invasion of critical structures, etc). The case with the highest EBL in our analysis comes from Ozaki et al, who report an anterior/posterior resection of a 624 mL chordoma lasting 11.3 hours, and extending up to S1.46 Localio et al note that a higher EBL is associated with staged resections over single-phase surgeries.55
The majority of patients underwent a posterior-only or a combined anterior/posterior approach; however, 1 patient in our analysis underwent an anterior-only approach. This patient comes from the study by Chung Rong et al, who do not specify their indication for this surgical plan. The authors write, “As for operative methods, the abdominal/sacral approach was used in 6 cases; and the abdominal approach was chosen in one case.”39 On analysis, a combined anterior-posterior approach to resection trended strongly toward association with an increased rate of local recurrence compared to a posterior-only approach (relative risk [RR] = 1.30, 95% confidence interval = CI 1.00-1.68), which is similar to a previous study by Kayani et al, which also trends in that direction.19 While some studies traditionally suggest using a combined approach to resect chordomas that extend above S3,18,19,25,46,56,57 some report that a posterior-only approach to all tumors that do not extend above the lumbosacral junction is both feasible and safe.40,51 The debate over the safest and most effective surgical approach to treat spinal chordoma patients is not new and is not unique to sacral chordoma surgery.58 Surgical planning should take into account surgeon experience and comfort, tumor anatomy, patient surgical history, the patient’s neurologic status, surgical risks, and potential for improving quantity and quality of life.
A history of sacral surgery was also associated with an increased rate of local recurrence compared with no history (RR = 1.59, 95% CI = 1.26-2.00). This finding is consistent with existing literature that reproducibly associates history of sacral surgery with both increased rate of recurrence and decreased survival.16,18,22,28,59,60
Patients in this study with neurologic symptoms experienced, on average, 13 fewer months of local-disease-free survival (95% CI = 5-22 months) than those without neurologic symptoms. In a 2015 study, Varga et al60 found no association between preoperative motor deficit and local recurrence rates, but found motor deficit to be associated with decreased survival (P < .01). These results suggest that neurologic symptoms might be indicative of more aggressive disease or reflect deeper invasion and a more difficult resection.
Male sex was found to be significantly associated with an increased rate of distant recurrence (RR = 1.70, 95% CI = 1.06-2.71). This is in contrast to existing literature, which finds no association.18,20,38 Males may present with more advanced disease due to differences in male and female pelvic anatomy or behavioral trends. Younger age (<65) was associated with a significantly higher rate of distant recurrence (RR = 1.87, 95% CI = 1.04-3.38), which is also in contrast to existing literature;18,60 however, in this study, the mean follow-up time for those under 65 was 6 ± 2.5 years, and for those 65 and over was only 4.5 ± 2.1 years. Thus, the association of young age with higher distant recurrence rates observed by this study can likely be attributed to the confounding impacts of longer survival and follow-up times in a younger population.
In the current study, surgical margin was found to be the most significant predictor of both local (RR for non-wide margins = 2.01, 95% CI = 1.61-2.51) and distant (RR = 2.87, 95% CI = 1.89-4.36) recurrence. These results corroborate conclusions in the existing literature.16,18–20,22,25,38,54,57,60–62
Last, this study found adjuvant therapy to be associated with a significantly higher rate of both local (RR = 1.26, 95% CI = 1.05-1.52) and distant (RR = 1.84, 95% CI = 1.07-3.18) recurrence. A probable confounding factor in this analysis is a selection bias for the tumors that were treated with adjuvant therapy: tumors with positive resection margins, complex anatomy, large size, protracted clinical course, and aggressive histology were likely more often treated with adjuvant therapy than simple tumors. It is also possible that the damage some forms of adjuvant therapy cause to structures local to the resection site makes adjacent tissue more susceptible to tumor reinvasion. Some previous studies investigating the matter have found no prognostic significance of adjuvant therapy with respect to recurrence.18,20,38,60 Kayani et al,19 however, found adjuvant radiotherapy to be associated with higher rate of local recurrence (P < .01), and trending toward a higher rate of distant recurrence (P = .09), with no effect on survival (P = .32). Yet there are also many studies that advocate for the use of adjuvant therapy, particularly proton beam and carbon ion modalities, in the treatment of chordoma, citing primarily improved disease-free survival.14,16,44,50,54 Efficacy notwithstanding, sacral chordomas can be difficult radiotherapy targets due to the presence of adjacent sensitive neurologic structures and potential interference from reconstructive hardware.4,6
The current article confirms reports in the literature that local recurrence is a significant prognostic factor for survival,4,22,57 as the median survival after surgery for patients with local recurrence was less than half the survival of patients without local recurrence (98 months vs 209 months, P = .0005; Figure 2).
The results in this study inform clinical practice in several crucial ways. First, a long time course and relatively high rate of recurrence for this disease necessitates long-term follow-up. Furthermore, a high percentage of metastases that are pulmonary (Table 4) indicates that chest imaging should be a fundamental component of these visits. Regarding treatment, given the strength and reproducibility of association between history of sacral surgery and adverse outcomes, adequate margins in the first operation should be considered essential to care, and debulking surgeries should be avoided if possible. A predilection of distant recurrence for males over females might support a clinical practice of more frequent follow-up in males, although further studies investigating the mechanism behind this trend would be valuable.
The high degree of significance of association between surgical margin and all outcomes of interest in this study confirms the existing principle that the achievement of a wide surgical margin is the single most important contribution a medical team can make to the prognosis of a sacral chordoma patient. However, this must be balanced against the increased morbidity associated with sacrificing neural or other critical structures to achieve that goal.63 Resection at the level of S3 and above begins to substantially affect bladder and bowel continence, and nerve resections at the level of L5 and S1 can begin to compromise ambulatory function.18,49,64,65 In addition to increasing the risk for colostomy, bowel incontinence has been independently associated with longer hospital stay and longer wound-healing duration.64 In a cohort of 42 patients who underwent sacrectomy for sacral chordoma, Schwab et al found that nearly 3 out of 4 patients reported postoperative sexual dysfunction, bowel dysfunction (including colostomies), or the need to self-catheterize to void.49 With regard to short-term morbidity for sacral surgery, in the current review, 16% of patients required reoperation for a complication.
Despite the increased morbidity that can be associated with wide resection, the morbidities associated with recurrence can also be numerous. These include an approximately halving of predicted survival, the need for further treatments with or without surgery and associated complications of that treatment, additional time and financial cost to the patient, and new anxiety and distress that comes with recurrence of cancer. Thus, as with any medical treatment, the planning of the resection of a sacral chordoma demands consideration of the characteristics of the individual disease in addition to the individualized goals of therapy for the patient. A nuanced discussion of benefits and risks, uncertainties of outcome, and personal values of the patient should serve as the centerpiece of surgical planning. A team of multidisciplinary professionals including orthopedic surgery, neurosurgery, general surgery, plastic surgery, and radiation oncology should be consulted as needed.
Beyond specialty collaboration, it is possible that experience plays a role in clinical outcomes for these sometimes technically demanding resections. The results from a small analysis in this study show a significantly higher rate of local recurrence in patients treated at institutions that treat fewer than one sacral chordoma patient every 2 years compared with those treated at institutions that treat more than 3 sacral chordomas per year (RR = 1.49, 95% CI = 2.74-24.4). Treatment at a low-volume center was also associated with shorter time to local recurrence (23 months vs 42 months, P < .0001). However, caseload does not appear to be associated with either local recurrence rate or time to local recurrence, as Pearson coefficients of correlation for both were close to 0. These results suggest a threshold effect, such that treatment at an institution that performs fewer than one sacral chordoma surgery every 2 years might have a detrimental impact on local recurrence.
Overall, the role of radiation and other adjuvant therapy in the treatment of sacral chordomas remains uncertain. While recent studies investigating proton beam8,13,66 and carbon ion therapy13,14 suggest a potential future role for these modalities in chordoma management, we advocate for further research to better characterize the subpopulation most likely to benefit from adjuvant treatment. Additionally, although use of chemotherapy is currently limited and largely confined to particular cases of unresectable19,20 or recurrent50 disease, we look forward to the results of continued investigation into novel targeted therapies such as imatinib, which has shown promise in arresting chordoma progression.67,68 In all, sacral chordoma carries with it an uncertain but not necessarily dire prognosis.
As with any systematic review, publication bias raises concern for self-selection of patients and of treatment institutions/level of care. Variability in definitions and types of data reported from study to study represented a constant extraction challenge (further study of this disease would benefit from continued maintenance of an international database, such as that established by AOSpine International60). Furthermore, all studies included in this review were retrospective, most likely secondary to the extremely low prevalence of sacral chordomas. As such, all studies included in the analysis were at high risk for bias, primarily derived from incomplete outcomes data, absence of a control population, and differential losses to follow up. Regarding statistical analysis, one-way ANOVA and unpaired t tests looking at times to recurrence used cohort-level information, slightly artificially decreasing the standard error and associated P values for each time-based analysis compared with a data set with patient-level information. Finally, while a multivariate analysis is typically preferred for association studies, low availability of individual-level data, interstudy discrepancy regarding which characteristics were reported, and high collinearity of many of the reported variables made a meaningful and statistically powerful multivariate analysis unfeasible.
Some less commonly reported factors were excluded from analysis in this study and are worth mention. Other characteristics that have been previously associated with increased local or distant recurrence of sacral chordoma include larger tumor size,16,19,20,69 surrounding muscle invasion,25 sacroiliac joint involvement,3 low CD40 expression,33 and open procedure for biopsy.51 In addition, patient genotype may play a prognostic role in mortality associated with spinal column chordomas.70 For the reader’s consideration, valuable discussions of previously investigated prognostic factors are available from Angelini et al,16 Kayani et al,19 and Varga et al,60 while an important discussion on the management of recurrent chordoma is offered by Ailon et al.71
Conclusions
Despite the consensus in the literature that local control is an essential determinant of long-term survival for patients with sacral chordoma, large studies investigating factors and outcomes for recurrence in sacral chordoma are lacking. Fifty-seven studies consisting of 1235 unique sacral chordoma patients were included in this review. Wide surgical margin was associated with lower rates of local recurrence, and wide surgical margin, female sex, and patient age ≥65 years were associated with lower rates of distant recurrence. Additionally, posterior surgical approach trended strongly toward association with lower rates of local recurrence, and treatment at a very low caseload institution might be associated with a higher rate of local recurrence. To the authors’ knowledge, this is the largest systematic review describing contributing factors, outcome, and prognosis for patients with surgically treated sacral chordoma. Looking forward, there is a need for large, multicenter, randomized investigations into the efficacy of novel adjuvant treatments to be used in conjunction with surgery for management of this disease.
Supplemental Material
Supplemental Material, GSJ741114_suppl_mat for Local and Distant Recurrence in Resected Sacral Chordomas: A Systematic Review and Pooled Cohort Analysis by Daniel Kerekes, C. Rory Goodwin, A. Karim Ahmed, Jorrit-Jan Verlaan, Chetan Bettegowda, Nancy Abu-Bonsrah, and Daniel M. Sciubba in Global Spine Journal
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C. Rory Goodwin: UNCF Merck Postdoctoral Fellow and has received an award from the Burroughs Wellcome Fund. Daniel Kerekes: None. A. Karim Ahmed: Has received an award from the NREF Medical Student Summer Research Fellowship. Jorrit-Jan Verlaan: None. Chetan Bettegowda: None. Nancy Abu-Bonsrah: None. Daniel M. Sciubba: Consultant for Medtronic, Globus, DePuy, Styrker, and Orthofix.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: The supplemental material is available in the online version of the article.
References
- 1. Gulluoglu S, Turksoy O, Kuskucu A, Ture U, Bayrak OF. The molecular aspects of chordoma. Neurosurg Rev. 2016;39:185–196. [DOI] [PubMed] [Google Scholar]
- 2. McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12:1–11. [DOI] [PubMed] [Google Scholar]
- 3. Pennicooke B, Laufer I, Sahgal A, et al. Safety and local control of radiation therapy for chordoma of the spine and sacrum: a systematic review. Spine (Phila Pa 1976). 2016;41:S186–S192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sciubba DM, Cheng JJ, Petteys RJ, Weber KL, Frassica DA, Gokaslan ZL. Chordoma of the sacrum and vertebral bodies. J Am Acad Orthop Surg. 2009;17:708–717. [DOI] [PubMed] [Google Scholar]
- 5. Jawad MU, Scully SP. Classifications in brief: Enneking classification: benign and malignant tumors of the musculoskeletal system. Clin Orthop Relat Res. 2010;468:2000–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casali PG, Stacchiotti S, Sangalli C, Olmi P, Gronchi A. Chordoma. Curr Opin Oncol. 2007;19:367–370. [DOI] [PubMed] [Google Scholar]
- 7. Chen YL, Liebsch N, Kobayashi W, et al. Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine (Phila Pa 1976). 2013;38:E930–E936. [DOI] [PubMed] [Google Scholar]
- 8. DeLaney TF, Liebsch NJ, Pedlow FX, et al. Long-term results of phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol. 2014;110:115–122. [DOI] [PubMed] [Google Scholar]
- 9. Holliday EB, Mitra HS, Somerson JS, et al. Postoperative proton therapy for chordomas and chondrosarcomas of the spine: adjuvant versus salvage radiation therapy. Spine (Phila Pa 1976). 2015;40:544–549. [DOI] [PubMed] [Google Scholar]
- 10. Imai R, Kamada T, Tsuji H, et al. Effect of carbon ion radiotherapy for sacral chordoma: results of phase I-II and phase II clinical trials. Int J Radiat Oncol Biol Phys. 2010;77:1470–1476. [DOI] [PubMed] [Google Scholar]
- 11. Imai R, Kamada T, Sugahara S, Tsuji H, Tsujii H. Carbon ion radiotherapy for sacral chordoma. Br J Radiol. 2011;84:S48–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Indelicato DJ, Rotondo RL, Begosh-Mayne D, et al. A prospective outcomes study of proton therapy for chordomas and chondrosarcomas of the spine. Int J Radiat Oncol Biol Phys. 2016;95:297–303. [DOI] [PubMed] [Google Scholar]
- 13. Mima M, Demizu Y, Jin D, et al. Particle therapy using carbon ions or protons as a definitive therapy for patients with primary sacral chordoma. Br J Radiol. 2014;87:20130512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishida Y, Kamada T, Imai R, et al. Clinical outcome of sacral chordoma with carbon ion radiotherapy compared with surgery. Int J Radiat Oncol Biol Phys. 2011;79:110–116. [DOI] [PubMed] [Google Scholar]
- 15. Uhl M, Welzel T, Jensen A, et al. Carbon ion beam treatment in patients with primary and recurrent sacrococcygeal chordoma. Strahlenther Onkol. 2015;191:597–603. [DOI] [PubMed] [Google Scholar]
- 16. Angelini A, Pala E, Calabrò T, Maraldi M, Ruggieri P. Prognostic factors in surgical resection of sacral chordoma. J Surg Oncol. 2015;112:344–351. [DOI] [PubMed] [Google Scholar]
- 17. Chandawarkar RY. Sacrococcygeal chordoma: review of 50 consecutive patients. World J Surg. 1996;20:717–719. [DOI] [PubMed] [Google Scholar]
- 18. Ji T, Guo W, Yang R, Tang X, Wang Y, Huang L. What are the conditional survival and functional outcomes after surgical treatment of 115 patients with sacral chordoma? Clin Orthop Relat Res. 2017;475:620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kayani B, Sewell MD, Tan KA, et al. Prognostic factors in the operative management of sacral chordomas. World Neurosurg. 2015;84:1354–1361. [DOI] [PubMed] [Google Scholar]
- 20. Radaelli S, Stacchiotti S, Ruggieri P, et al. Sacral chordoma: long-term outcome of a large series of patients surgically treated at two reference centers. Spine (Phila Pa 1976). 2016;41:1049–1057. [DOI] [PubMed] [Google Scholar]
- 21. Xie C, Whalley N, Adasonla K, Grimer R, Jeys L. Can local recurrence of a sacral chordoma be treated by further surgery? Bone Joint J. 2015;97-B:711–715. [DOI] [PubMed] [Google Scholar]
- 22. Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88:2122–2134. [DOI] [PubMed] [Google Scholar]
- 23. Wittekind C, Compton C, Quirke P, et al. A uniform residual tumor (R) classification: integration of the R classification and the circumferential margin status. Cancer. 2009;115:3483–3488. [DOI] [PubMed] [Google Scholar]
- 24. Dubory A, Missenard G, Lambert B, Court C. Interest of laparoscopy for “en bloc” resection of primary malignant sacral tumors by combined approach: comparative study with open median laparotomy. Spine (Phila Pa 1976). 2015;40:1542–1552. [DOI] [PubMed] [Google Scholar]
- 25. Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87:2211–2216. [DOI] [PubMed] [Google Scholar]
- 26. Randall RL, Bruckner J, Lloyd C, Pohlman TH, Conrad EU., 3rd Sacral resection and reconstruction for tumors and tumor-like conditions. Orthopedics. 2005;28:307–313. [DOI] [PubMed] [Google Scholar]
- 27. Makhdoomi R, Ramzan A, Khursheed N, et al. Clinicopathological characteristics of chordoma: an institutional experience and a review of the literature. Turk Neurosurg. 2013;23:700–706. [DOI] [PubMed] [Google Scholar]
- 28. Park L, Delaney TF, Liebsch NJ, et al. Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys. 2006;65:1514–1521. [DOI] [PubMed] [Google Scholar]
- 29. Schoenthaler R, Castro JR, Petti PL, Baken-Brown K, Phillips TL. Charged particle irradiation of sacral chordomas. Int J Radiat Oncol Biol Phys. 1993;26:291–298. [DOI] [PubMed] [Google Scholar]
- 30. Zabel-du Bois A, Nikoghosyan A, Schwahofer A, et al. Intensity modulated radiotherapy in the management of sacral chordoma in primary versus recurrent disease. Radiother Oncol. 2010;97:408–412. [DOI] [PubMed] [Google Scholar]
- 31. Böhm B, Milsom JW, Fazio VW, Lavery IC, Church JM, Oakley JR. Our approach to the management of congenital presacral tumors in adults. Int J Colorectal Dis. 1993;8:134–138. [DOI] [PubMed] [Google Scholar]
- 32. Furlani S, Cagol P, Zanella A, Lise M. Sacrococcygeal chordoma. Surg Italy. 1980;10(4):290–297. [Google Scholar]
- 33. Li X, Wang S, Chen Y, Liu G, Yang X. Overexpression of CD40 in sacral chordomas and its correlation with low tumor recurrence. Onkologie. 2013;36:567–571. [DOI] [PubMed] [Google Scholar]
- 34. Aguiar Júnior S, Andrade WP, Baiocchi G, et al. Natural history and surgical treatment of chordoma: a retrospective cohort study. Sao Paulo Med J. 2014;132:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahmed AR. Safety margins in resection of sacral chordoma: analysis of 18 patients. Arch Orthop Trauma Surg. 2009;129:483–487. [DOI] [PubMed] [Google Scholar]
- 36. Asavamongkolkul A, Waikakul S. Wide resection of sacral chordoma via a posterior approach. Int Orthop. 2012;36:607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Atalar H, Selek H, Yildiz Y, Sağlik Y. Management of sacrococcygeal chordomas. Int Orthop. 2006;30:514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen KW, Yang HL, Lu J, Liu JY, Chen XQ. Prognostic factors of sacral chordoma after surgical therapy: a study of 36 patients. Spinal Cord. 2010;48:166–171. [DOI] [PubMed] [Google Scholar]
- 39. Chung Rong C, Hsu CH, Chen JS, Wang JY. Sacrococcygeal chordoma. Asian J Surg. 1996;29:152–156. [Google Scholar]
- 40. Clarke MJ, Dasenbrock H, Bydon A, et al. Posterior-only approach for en bloc sacrectomy: clinical outcomes in 36 consecutive patients. Neurosurgery. 2012;71:357–364. [DOI] [PubMed] [Google Scholar]
- 41. Eid AS, Chang UK, Lee SY, Jeon DG. The treatment outcome depending on the extent of resection in skull base and spinal chordomas. Acta Neurochir (Wien). 2011;153:509–516. [DOI] [PubMed] [Google Scholar]
- 42. Froehlich EV, Scheipl S, Lazàry A, et al. Expression of ezrin, MMP-9, and COX-2 in 50 chordoma specimens: a clinical and immunohistochemical analysis. Spine (Phila Pa 1976). 2012;37:E757–E767. [DOI] [PubMed] [Google Scholar]
- 43. Housari G, González M, Calero P, Beni R, Lobo E. Sacral chordoma: management of a rare disease in a tertiary hospital. Clin Transl Oncol. 2013;15:327–330. [DOI] [PubMed] [Google Scholar]
- 44. Moojen WA, Vleggeert-Lankamp CL, Krol AD, Dijkstra SP. Long-term results: adjuvant radiotherapy in en bloc resection of sacrococcygeal chordoma is advisable. Spine (Phila Pa 1976). 2011;36:E656–E661. [DOI] [PubMed] [Google Scholar]
- 45. Osaka S, Osaka E, Kojima T, Yoshida Y, Tokuhashi Y. Long-term outcome following surgical treatment of sacral chordoma. J Surg Oncol. 2014;109:184–188. [DOI] [PubMed] [Google Scholar]
- 46. Ozaki T, Hillmann A, Winkelmann W. Surgical treatment of sacrococcygeal chordoma. J Surg Oncol. 1997;64:274–279. [DOI] [PubMed] [Google Scholar]
- 47. Prabhakaran PS, Misra S, Kannan V, et al. Sacral chordomas: a 10-year study. Australas Radiol. 1998;42:42–46. [DOI] [PubMed] [Google Scholar]
- 48. Puri A, Agarwal MG, Shah M, et al. Decision making in primary sacral tumors. Spine J. 2009;9:396–403. [DOI] [PubMed] [Google Scholar]
- 49. Schwab JH, Healey JH, Rose P, Casas-Ganem J, Boland PJ. The surgical management of sacral chordomas. Spine (Phila Pa 1976). 2009;34:2700–2704. [DOI] [PubMed] [Google Scholar]
- 50. Thieblemont C, Biron P, Rocher F, et al. Prognostic factors in chordoma: role of postoperative radiotherapy. Eur J Cancer. 1995;31A:2255–2259. [DOI] [PubMed] [Google Scholar]
- 51. Waisman M, Kligman M, Roffman M. Posterior approach for radical excision of sacral chordoma. Int Orthop. 1997;21:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xue-Song L, Chao Y, Kai-Yong Y, Si-Qing H, Heng Z. Surgical excision of extensive sacrococcygeal chordomas assisted by occlusion of the abdominal aorta. J Neurosurg Spine. 2010;12:490–496. [DOI] [PubMed] [Google Scholar]
- 53. Yang YK, Chan CM, Zhang Q, Xu HR, Niu XH. Computer navigation-aided resection of sacral chordomas. Chin Med J (Engl). 2016;129:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. York JE, Kaczaraj A, Abi-Said D, et al. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery. 1999;44:74–79. [DOI] [PubMed] [Google Scholar]
- 55. Localio SA, Eng K, Ranson JH. Abdominosacral approach for retrorectal tumors. Ann Surg. 1980;191:555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gennari L, Azzarelli A, Quagliuolo V. A posterior approach for the excision of sacral chordoma. J Bone Joint Surg Br. 1987;69:565–568. [DOI] [PubMed] [Google Scholar]
- 57. Hanna SA, Aston WJ, Briggs TW, Cannon SR, Saifuddin A. Sacral chordoma: can local recurrence after sacrectomy be predicted? Clin Orthop Relat Res. 2008;466:2217–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Demirkiran G, Dede O, Karadeniz E, Olgun D, Ayvaz M, Yazici M. Anterior and posterior vertebral column resection versus posterior-only technique: a comparison of clinical outcomes and complications in congenital kyphoscoliosis. Clin Spine Surg. 2017;30:285–290. [DOI] [PubMed] [Google Scholar]
- 59. Ruggieri P, Angelini A, Ussia G, Montalti M, Mercuri M. Surgical margins and local control in resection of sacral chordomas. Clin Orthop Relat Res. 2010;468:2939–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Varga PP, Szövérfi Z, Fisher CG, et al. Surgical treatment of sacral chordoma: prognostic variables for local recurrence and overall survival. Eur Spine J. 2015;24:1092–1101. [DOI] [PubMed] [Google Scholar]
- 61. Cheng EY, Ozerdemoglu RA, Transfeldt EE, Thompson RC., Jr Lumbosacral chordoma. Prognostic factors and treatment. Spine (Phila Pa 1976). 1999;24:1639–1645. [DOI] [PubMed] [Google Scholar]
- 62. Hulen CA, Temple HT, Fox WP, Sama AA, Green BA, Eismont FJ. Oncologic and functional outcome following sacrectomy for sacral chordoma. J Bone Joint Surg Am. 2006;88:1532–1539. [DOI] [PubMed] [Google Scholar]
- 63. van Wulfften Palthe OD, Houdek MT, Rose PS, et al. How does the level of nerve root resection in en bloc sacrectomy influence patient-reported outcomes? Clin Orthop Relat Res. 2017;475:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guo Y, Palmer JL, Shen L, et al. Bowel and bladder continence, wound healing, and functional outcomes in patients who underwent sacrectomy. J Neurosurg Spine. 2005;3:106–110. [DOI] [PubMed] [Google Scholar]
- 65. Andreoli F, Balloni F, Bigiotti A, et al. Anorectal continence and bladder function. Effects of major sacral resection. Dis Colon Rectum. 1986;29:647–652. [DOI] [PubMed] [Google Scholar]
- 66. Rotondo RL, Folkert W, Liebsch NJ, et al. High-dose proton-based radiation therapy in the management of spine chordomas: outcomes and clinicopathological prognostic factors. J Neurosurg Spine. 2015;23:788–797. [DOI] [PubMed] [Google Scholar]
- 67. Stacchiotti S, Longhi A, Ferraresi V, et al. Phase II study of imatinib in advanced chordoma. J Clin Oncol. 2012;30:914–920. [DOI] [PubMed] [Google Scholar]
- 68. Hindi N, Casali PG, Morosi C, et al. Imatinib in advanced chordoma: a retrospective case series analysis. Eur J Cancer. 2015;51:2609–2614. [DOI] [PubMed] [Google Scholar]
- 69. Dhawale AA, Gjolaj JP, Holmes L, Jr, Sands LR, Temple HT, Eismont FJ. Sacrectomy and adjuvant radiotherapy for the treatment of sacral chordomas: a single-center experience over 27 years. Spine (Phila Pa 1976). 2014;39:E353–E359. [DOI] [PubMed] [Google Scholar]
- 70. Bettegowda C, Yip S, Lo SFL, et al. Spinal column chordoma: prognostic significance of clinical variables and T (brachyury) gene SNP rs2305089 for local recurrence and overall survival. Neurooncology. 2017;19:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ailon T, Torabi R, Fisher CG, et al. Management of locally recurrent chordoma of the mobile spine and sacrum: a systematic review. Spine (Phila Pa 1976). 2016;41:S193–S198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, GSJ741114_suppl_mat for Local and Distant Recurrence in Resected Sacral Chordomas: A Systematic Review and Pooled Cohort Analysis by Daniel Kerekes, C. Rory Goodwin, A. Karim Ahmed, Jorrit-Jan Verlaan, Chetan Bettegowda, Nancy Abu-Bonsrah, and Daniel M. Sciubba in Global Spine Journal