Abstract
Objective
To investigate whether dietary patterns (Mediterranean diet [MedDiet], Dietary Approaches to Stop Hypertension [DASH], and A Priori Diet Quality Score [APDQS]) during adulthood are associated with midlife cognitive performance.
Methods
We studied 2,621 Coronary Artery Risk Development in Young Adults (CARDIA) participants; 45% were black, 57% were female, and mean age was 25 ± 3.5 years at baseline (year 0). Mean diet scores were calculated from diet history at baseline, year 7, and year 20 (mean age 25, 32, and 45 years, respectively). Cognitive function was assessed at years 25 and 30 (mean age 50 and 55 years, respectively). Linear models were used to examine association between tertiles of diet score and change in composite cognitive function and cognitive z scores (verbal memory [Rey Auditory Verbal Learning Test], processing speed [Digit Symbol Substitution Test], and executive function [Stroop Interference test]) and the Montreal Cognitive Assessment (MoCA) at year 30.
Results
DASH was not associated with change in cognitive performance. Higher MedDiet and APDQS scores were associated with less decline in cognitive function (MedDiet: low −0.04, middle 0.03, high 0.03, p = 0.03; APDQS: low −0.04, middle −0.00, high 0.06, p < 0.01) and Stroop Interference (MedDiet: low 0.09, middle −0.06, high −0.03; APDQS: low 0.10, middle 0.01, high −0.09, both p < 0.01). Odds ratios (95% confidence interval) for poor global cognitive function (≥1 SD below mean MoCA score) comparing extreme tertiles of diet scores were 0.54 (0.39–0.74) for MedDiet, 0.48 (0.33–0.69) for APDQS, and 0.89 (0.68–1.17) for DASH.
Conclusion
Greater adherence to MedDiet and APDQS dietary patterns during adulthood was associated with better midlife cognitive performance. Additional studies are needed to define the combination of foods and nutrients for optimal brain health across the life course.
Cognitive impairment is associated with increased risk of mortality, disability, and late-life dementia, as well as high health care costs.1,2 Cognitive decline has been demonstrated in midlife,3 and accumulating data indicate that longitudinal exposure to cardiovascular disease risk factors and sedentary behaviors during adulthood has an adverse effect on midlife cognitive performance.4–6
Diet is a modifiable lifelong exposure, yet few studies have examined whether dietary factors in adulthood influence the risk of cognitive impairment. Dietary patterns such as the Mediterranean diet (MedDiet) and Dietary Approaches to Stop Hypertension (DASH) have shown promise as strategies to slow cognitive decline7–9 and to reduce dementia risk in later life.10,11 However, only a few studies have been conducted, mainly in older populations >60 years of age, and evidence has not been consistent,12,13 in part because of variation between studies in measures of both diet and cognition. Furthermore, most observational studies are limited by a single time point measurement of diet and unlikely to reflect longitudinal variation in habitual dietary intake.14
The Coronary Artery Risk Development in Young Adults (CARDIA) study provides a unique opportunity to investigate diet during early to middle adulthood and change in midlife cognitive function in black and white adults. We aimed to examine associations between 3 heart-healthy diet patterns characterized by the MedDiet,15 the DASH,16 and the CARDIA A Priori Diet Quality Score (APDQS)17 scores and cognitive performance at midlife. We hypothesized that greater long-term adherence to these diet patterns would be associated with preservation of cognitive function.
Methods
Study design
We studied participants enrolled in the CARDIA study, a multicenter longitudinal study of the development of cardiovascular risk factors and disease in 5,115 randomly selected black and white healthy adults 18 to 30 years of age at baseline in 1985 to 1986. The study design and procedures have been previously described.18 Eight follow-up examinations were completed over 30 years: 1987 to 1988 (year 2), 1990 to 1991 (year 5), 1992 to 1993 (year 7), 1995 to 1996 (year 10), 2000 to 2001 (year 15), 2005 to 2006 (year 20), 2010 to 2011 (year 25), and 2015 to 2016 (year 30). Dietary intake was assessed at 3 examinations (baseline and years 7 and 20), and cognitive function was assessed at years 25 and 30 with standard protocols. This study focused on 2,693 participants who completed both cognitive examinations and at least 2 of the 3 dietary assessments. The final analytical cohort comprised 2,621 participants after exclusion of persons with implausible energy intake (<600 or >8,000 kcal/d) (n = 34) and those with missing baseline covariate data (n = 38). Excluded participants were more likely to be female and black and to have lower educational attainment and worse cognitive performance at the year 25 visit (all p < 0.01, data not shown).
Standard protocol approvals, registrations, and patient consents
The study was approved by institutional review boards for the protection of human participants for the CARDIA study sites, and written informed consent was obtained from all participants at each examination.
Diet measure and dietary pattern scores
At the baseline, year 7, and year 20 visits (mean age 25, 32, and 45 years, respectively), dietary intake over the previous month was assessed by a trained interviewer-administered CARDIA diet history.19 Food and beverages consumed were assigned to 1 of 166 created food groups devised by the Nutrition Coordinating Center at the University of Minnesota and based on a modified US Department of Agriculture food grouping system.17,20,21 Reported servings for each food/beverage item were converted to standard serving according to US Department of Agriculture recommendations. Individual food-group intake was calculated as the total number of standard servings reported per day of each food within a given food group.
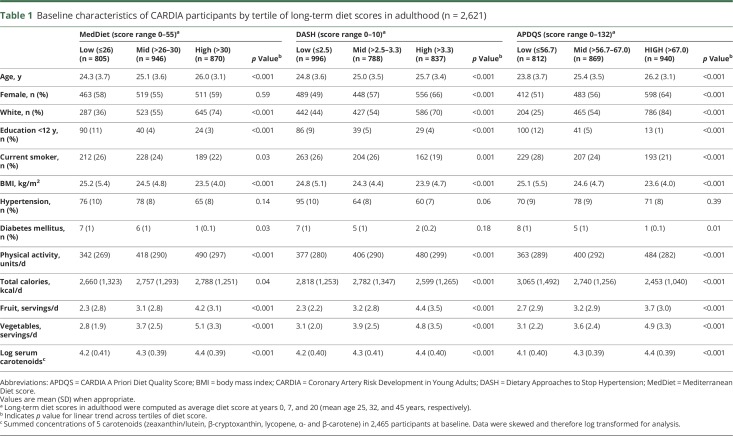
For each time point, individual dietary pattern scores were determined with the MedDiet,15 DASH,16 and APDQS17 scoring systems. Diet scores were computed from predefined food group and nutrient (diet components) criteria for the dietary patterns as decribed.15–17 The main foods and nutrients contributing to each diet score are shown in table 1 available from Dryad (doi.org/10.5061/dryad.443gv60). For each of the 3 diet patterns, individual components contributed equally to the summed score, and the highest possible score indicated higher diet quality or greatest concordance to the dietary pattern.
The MedDiet score15 was calculated from 11 individual diet components scored between 0 and 5 points and then summed for a total score between 0 and 55. Points were assigned monotonically for increasing intake of nonrefined grains, fruits, vegetables, potatoes, legumes, fish, and olive oil and decreasing intake of red meat, poultry, and full-fat dairy. Because information was not available for olive oil, we calculated a ratio of monounsaturated to saturated fatty acid intake and divided this component into sextiles as described previously in a US population.22 We scored the ratio of monounsaturated to saturated fatty acid monotonically from 0 to 5 points, with a ratio ≥2 assigned the highest score in accordance with a Greek MedDiet. Alcohol was scored 0 for nonconsumption or a high intake (>4.5 drinks per day) to a maximum score of 5 for moderate consumption (up to 2 drinks per day).
The DASH diet score16 was calculated from 10 diet components scored 0, 0.5, or 1, depending on frequency of intake. Points were assigned monotonically for increasing intake of total grains, vegetables, fruit, low-fat dairy, legumes, and nuts and decreasing intake of meat, fish and poultry, total fat, saturated fat, sweets, and sodium. Diet component scores were summed for a total score ranging from 0 to 10. In a secondary analysis, we used an alternate version of DASH in which intake of total grains was replaced with whole grains.
The APDQS was derived from classification of 46 foods groups hypothesized to have beneficial, neutral, or adverse health effects.17 The APDQS score, ranging from 0 to 132, was calculated from increasing intake of 20 beneficial components (including fruit, vegetables, legumes, low-fat dairy, fish, and moderate alcohol intake) and decreasing intake of 13 adverse components (including fried foods, salty snacks, desserts, high-fat dairy, and sugar-sweetened soft drinks). Scores for beneficial components increased monotonically from 0 to 4 with higher consumption of reported servings, and scores were reversed for the 13 adverse components.
Our primary predictor was diet during adulthood characterized by the 3 dietary pattern scores. To represent diet patterns over the long term and to reduce within-person variation, we calculated an average score for each of the 3 dietary patterns by taking the mean of the baseline, year 7, and year 20 diet scores.
Cognitive measures
At the year 25 visit, trained interviewers assessed cognitive function using 3 standardized tests: (1) Rey Auditory Verbal Learning Test (RAVLT) to assess verbal learning and memory; the number of words correctly recalled after a 10-minute delay was used in the current analyses (range 0–15), with higher scores indicating better performance; (2) Digit Symbol Substitution Test (DSST) to assess processing speed and executive function (range 0–133), with higher scores for digits correctly substituted indicating better performance; and (3) Stroop test to assess executive function. The Stroop Interference score was obtained by subtracting the score on subtest 2 from the score on subtest 3, with lower scores indicating better performance. A composite cognitive function score was computed by transforming each of the 3 tests to standardized z scores and averaging the summed total.
The cognitive test battery was repeated at the year 30 visit and expanded to include the Montreal Cognitive Assessment (MoCA) to assess global cognitive function with components of attention, executive function, memory, language, visuospatial skills, calculations, and orientation. Scores range from 0 to 30, with higher scores indicating better global cognitive function.
We examined 5-year change in the repeated cognitive z scores (delayed RAVLT, DSST, and Stroop Interference) and in composite cognitive function z score that was computed by averaging change in cognitive z scores (change delayed RAVLT z score − change Stroop Interference z score + change DSST z score/3). Negative z score change values indicated a decline from the initial examination except for the Stroop test, for which a positive z score change reflected a decline in performance.
Covariates
Covariates were determined at baseline. Demographics, including age, race, sex, education attainment (maximum number of education years), and smoking, were obtained by self- and interviewer-administered questionnaires. Physical activity was calculated as a total exercise score derived from the self-reported frequency and intensity of 13 activities during the past year.23 Body weight and height were measured with standard clinic techniques, and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Diabetes diagnosis at baseline was defined as fasting glucose level ≥126 mg/dL or taking antidiabetic medication. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or taking antihypertensive medication.
For sensitivity analyses, APOE4 genotype was measured in year 7 blood samples by the method previously described.24 The Center for Epidemiologic Studies Depression (CES-D) questionnaire (score range 0–60) was used to assess midlife depressive symptoms.
Statistical analysis
Participant characteristics by tertile of each dietary score were compared by use of analysis of variance for continuous variables and the χ2 test for dichotomous variables, with corresponding tests for linear trend. Skewed variables were log transformed before analysis.
We used general linear models to examine independent associations between the 3 dietary scores and the cognitive outcomes. Dietary scores were modeled continuously per 1-SD increase and in tertile categories of low, middle, and high score.
We found little evidence of race or sex interaction in relations between dietary scores and change in cognitive measures (p > 0.05); therefore, analyses were performed with data from the full sample. To account for interindividual differences in baseline cognitive performance influencing the rate of cognitive change, the 5-year change in cognitive z scores was adjusted for the initial year 25 z score. Multivariable linear models were further adjusted for the effects of baseline sex, age, race, education attainment (years), BMI, smoking, physical activity, diabetes mellitus, and energy intake that differed according to tertile of diet score.
We also performed logistic regression analyses to examine associations between the 3 dietary patterns and risk of clinically relevant poor cognitive function (defined as 1 SD below the mean MoCA score), adjusting for potential confounders described above.
In sensitivity analyses, we further controlled for APOE4 genotype and CES-D score. All analyses were carried out with SPSS version 22 (IBM Corp, Armonk, NY), and a value of p < 0.05 was considered significant.
Data availability
Anonymized data are available from the CARDIA Coordinating Center (cardia.dopm.uab.edu/contact-cardia). A description of the National Heart, Lung, and Blood Institute policies governing the data and describing access to the data can be found online (cardia.dopm.uab.edu/study-information/nhlbi-data-repository-data).
Results
The mean age of the 2,621 participants at baseline was 25.2 ± 3.5 years; 57% were female; 45% were black; and most (94%) had >12 years of education. Mean MedDiet and DASH scores increased while APDQS score slightly decreased over the 20 years (table 2 available from Dryad, doi.org/10.5061/dryad.443gv60).
The average long-term score for MedDiet was 28.4 ± 4.5 points (range 14.7–44.5), for DASH was 3.0 ± 0.90 points (range 0.8–7.8), and for APDQS was 63.2 ± 11.4 points (range 30.0–98.5). Long-term MedDiet scores positively correlated with both APDQS (r = 0.69, p < 0.01) and DASH (r = 0.60, p < 0.01) long-term scores.
As table 1 shows, participants with highest long-term dietary scores were more likely to be older, white, more highly educated, and physically active; had lower BMIs; and were less likely to smoke compared with those with the lowest scores. Those with higher DASH or APDQS scores were also more likely to be female. Total serum carotenoids (an objective marker of fruit and vegetable consumption) and self-reported fruit and vegetable intake during adulthood increased linearly across tertiles of each diet pattern score (all pTrend < 0.001); fruit and vegetable servings almost doubled between the extreme tertiles for all 3 diet patterns examined.
Table 1.
Baseline characteristics of CARDIA participants by tertile of long-term diet scores in adulthood (n = 2,621)
Diet patterns during adulthood and cognitive performance in midlife
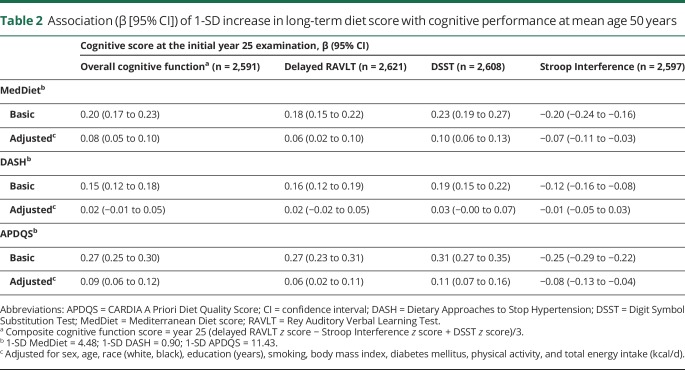
The mean age at the initial cognitive examination was 50.2 years (range 42–59 years). As table 2 shows, long-term scores in each diet pattern were positively associated with better cognitive performance at the initial examination. After adjustment for demographic, lifestyle, health factors, and energy intake, DASH score was no longer associated with initial cognitive performance. However, a 1-SD increase in the MedDiet score (4.5 points) and APDQS score (11.4 points) was associated with better performance in composite cognitive function and all 3 cognitive tests (all p < 0.01).
Table 2.
Association (β [95% CI]) of 1-SD increase in long-term diet score with cognitive performance at mean age 50 years
Diet patterns during adulthood and change in midlife cognitive function
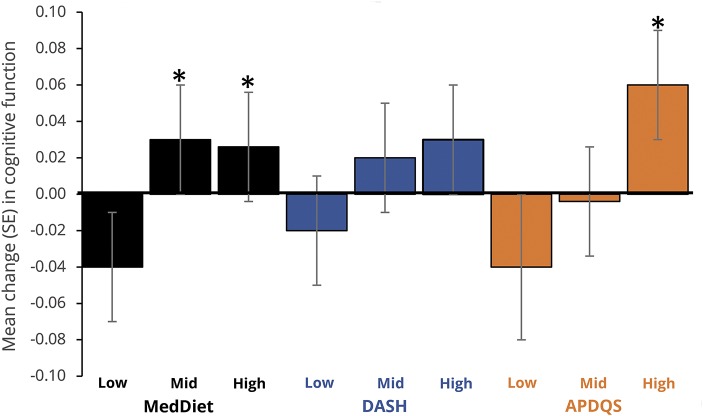
After 5 years of follow-up, small changes were observed in cognitive test scores: RAVLT scores improved on average 0.2 ± 2.6 points, while DSST and Stroop performance declined by 2.5 ± 9.3 and 0.1 ± 9.1 points, respectively. The 5-year change in cognitive function by tertile of long-term diet scores adjusted for demographic, lifestyle, and health factors is shown in figures 1 through 4 and table 3 available from Dryad (doi.org/10.5061/dryad.443gv60).
As figure 1 shows, compared to individuals with low diet scores, those with middle and high MedDiet or APDQS scores had less decline in midlife global cognitive function (p < 0.05 in all multivariable-adjusted models), with a linear relationship observed for both MedDiet (pTrend = 0.03) and APDQS (pTrend = 0.003) dietary patterns. DASH score was not associated with 5-year change in global cognitive function in the adjusted model.
Figure 1. Adjusted mean (SE) 5-year change in midlife cognitive function (50–55 years) by tertile of diet score in adulthood.
APDQS = A Priori Diet Quality Score; DASH = Dietary Approaches to Stop Hypertension; MedDiet = Mediterranean diet; SE = standard error. *Differs (p ≤ 0.05) from low tertile aftercorrection for multiple comparisons (Bonferroni).
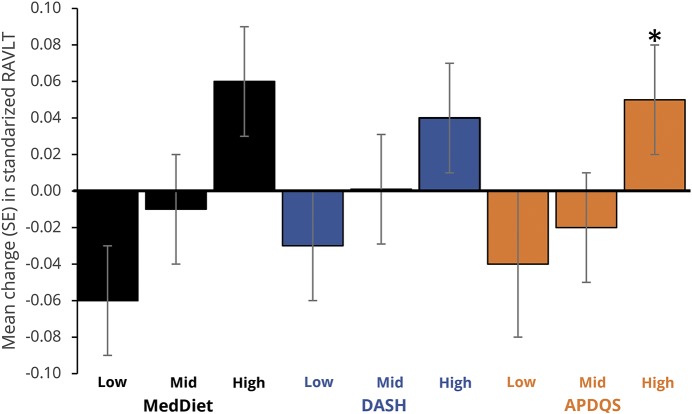
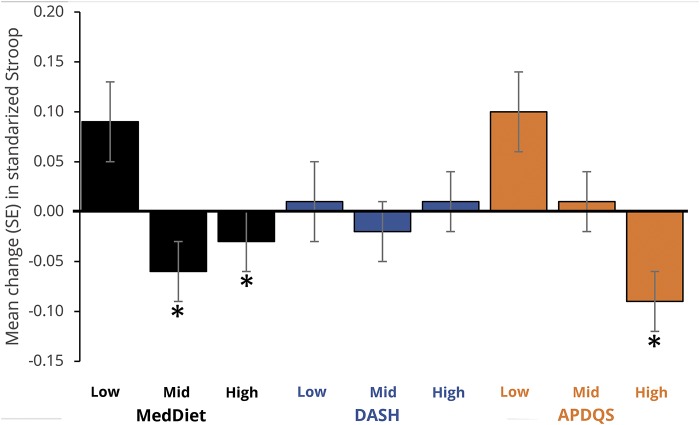
Figures 2 through 4 show the multivariate-adjusted 5-year change in cognitive z scores by tertile of diet score. DASH and APDQS scores were not associated with change in delayed RAVLT, but participants with high MedDiet scores showed improvement in word recall compared to those with low scores (p = 0.05) (figure 2). Change in DSST score did not differ by adherence to the 3 dietary patterns examined (figure 3). Change in Stroop Interference score did not differ by DASH, but participants with higher MedDiet or APDQS scores had faster performance on the Stroop Interference at follow-up (pTrend <0.01 for both) (figure 4).
Figure 2. Adjusted mean (SE) 5-year change in midlife delayed RAVLT (50–55 years) by tertile of diet score in adulthood.
APDQS = A Priori Diet Quality Score; DASH = Dietary Approaches to Stop Hypertension; MedDiet = Mediterranean diet; RAVLT = Rey Auditory Verbal Learning Test; SE = standard error. *Differs (p ≤ 0.05) from low tertile aftercorrection for multiple comparisons (Bonferroni).
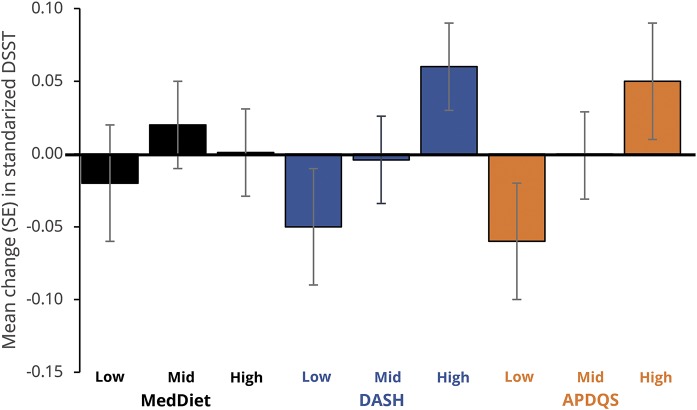
Figure 3. Adjusted mean (SE) 5-year change in midlife DSST (50–55 years) by tertile of diet score in adulthood.
APDQS = A Priori Diet Quality Score; DASH = Dietary Approaches to Stop Hypertension; DSST = Digit Symbol Substitution Test; MedDiet = Mediterranean diet; SE = standard error.
Figure 4. Adjusted mean (SE) 5-year change in midlife Stroop Interference (50–55 years) by tertile of diet score in adulthood.
APDQS = A Priori Diet Quality Score; DASH = Dietary Approaches to Stop Hypertension; MedDiet = Mediterranean diet; SE = standard error. *Differs (p ≤ 0.05) from low tertile after correction for multiple comparisons (Bonferroni).
Further adjustment for APOE4 status or CES-D score slightly attenuated the associations, but the overall findings were unchanged. Associations between alternate long-term DASH score and midlife cognitive performance were similar to those reported for the long-term DASH score (data not shown). When we repeated the analyses by replacing the long-term diet scores with the baseline or year 20 diet scores as predictor variables (table 4 available from Dryad, doi.org/10.5061/dryad.443gv60), the baseline scores were not associated with 5-year change in either midlife global cognitive function or individual cognitive domains. There was no association between year 20 DASH score and change in midlife global cognitive function. However, MedDiet and APDQS scores at year 20 showed slightly stronger inverse associations with 5-year global cognitive decline than the long-term diet scores (p < 0.01 in all multivariable-adjusted models). Higher MedDiet score at year 20 was associated with faster performance on Stroop Interference and less decline in word recall and DSST scores (all p = 0.02); higher DASH score at year 20 was associated with less decline in DSST score (p = 0.007); and higher APDQS score at year 20 was associated with faster performance on Stroop Interference (p = 0.001).
Diet patterns during adulthood and risk of clinically poor cognitive global function in midlife
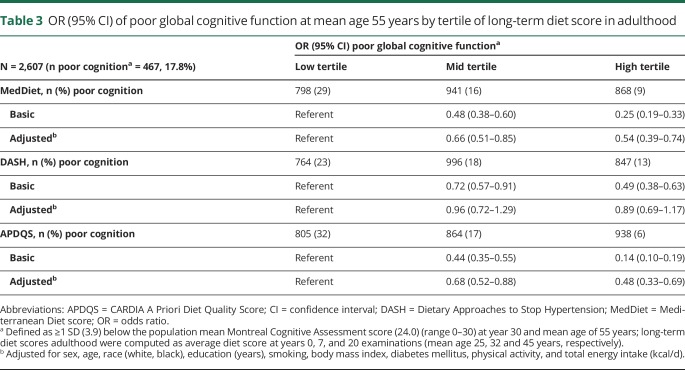
Clinically relevant poor global cognitive function, defined as >1 SD (3.9 points) below the population mean MoCA score, was documented in almost 18% of the sample. Table 3 shows that individuals in the high and middle tertiles of long-term MedDiet, DASH, or APDQS scores were less likely to have poor global cognitive function compared to those with low diet scores (odds ratios [ORs] for the high vs low MedDiet, DASH, and APDQS tertiles 0.25 [95% confidence interval (CI) 0.19–0.33], 0.49 [95% CI 0.38–0.63], 0.14 [95% CI 0.10–0.19], respectively). After adjustment for demographic, lifestyle, and health factors, only the MedDiet and APDQS scores were independently and linearly associated with a lower risk of poor global cognitive performance. Individuals in the middle and high APDQS tertiles had a respective 32% (OR 0.68 [95% CI 0.52–0.88]) and 52% (OR 0.48 [95% CI 0.33–0.69]) lower risk of poor cognitive function relative to those in the low APDQS tertile (pTrend <0.001). The associations were similar for participants scoring in the middle and high vs low tertile of MedDiet (OR 0.66 [95% CI 0.51–0.85] and OR 0.54 [95% CI–0.39, 0.74], respectively). Similar to the DASH score, the alternate DASH score was not related to risk of poor global cognitive function. In adjusted models, the OR was 0.95 (95% CI 0.70–1.23) and 0.90 (95% CI 0.70–1.17) for middle and high tertiles, respectively, compared to low tertile of alternate DASH score.
Table 3.
OR (95% CI) of poor global cognitive function at mean age 55 years by tertile of long-term diet score in adulthood
In sensitivity analyses, we adjusted the models for the presence of the APOE4 allele. Associations were slightly attenuated, but there was little notable difference to the overall pattern of findings. Results were not appreciably altered when we removed 102 participants who scored <17 points on the MoCA from the analyses (data not shown), nor when we adjusted the models further for midlife depression score, midlife obesity, or midlife hypertension. Furthermore, when we repeated the analysis with the baseline and year 20 diet scores (table 5 available from Dryad doi.org/10.5061/dryad.443gv60), observed risk estimates were attenuated for baseline diet scores but similar for year 20 diet scores in the fully adjusted models.
Discussion
In this study, we examined longitudinal associations between 3 dietary patterns during adulthood and cognitive performance in midlife. Our findings in a large sample of biracial adults indicate that greater long-term adherence to MedDiet or APDQS, but not DASH, was associated with less decline in global cognitive function at midlife. Higher long-term MedDiet and APQDS scores were particularly associated with less decline in midlife executive function. Mean verbal memory scores increased at follow-up in this population. This could be explained by a performance gain or a “practice effect” carried over from the initial test, which is especially likely with memory tests.25 Even so, higher MedDiet scores were associated with small improvement in verbal memory score. Furthermore, individuals with the highest adherence to MedDiet or APDQS had a 46% to 52% lower risk of poor global cognitive function after adjustment for demographic, health, and lifestyle covariates. Overall, our findings indicate that long-term adherence to a dietary pattern consistent with the MedDiet and APDQS and general dietary recommendations for heart health26 is associated with better midlife cognitive function.
The MedDiet and DASH diets have been linked to lower risk of cognitive decline7,8 and dementia,10,11 but findings from epidemiologic studies have been conflicting.12,13 Prior studies have tended to examine relations between diet and cognition in older, predominately white populations without considering earlier life dietary exposures. Our findings build on an earlier CARDIA study27 that reported positive associations between APDQS score and midlife cognitive performance measured at 1 time point. Given that progression from normal cognition to cognitive impairment can take years to manifest, maintaining a healthy diet over the life course may offer accruing cognitive protection. Longitudinal investigations of midlife dietary patterns and cognition later in life are limited but generally support this assertion; for example, a healthy dietary pattern in midlife has been associated with better global cognition and verbal memory scores28 and reduced risk of dementia29 in older adults, and long-term adherence to the MedDiet was associated with better overall cognitive function but not with cognitive decline in educated women.30 In the current study, baseline diet scores at an average age of 25 years were not related to change in midlife cognitive function, while the more recent diet scores, particularly the MedDiet score, at an average age of 45 years tended to be more strongly associated with cognitive performance in middle-age. Few dietary intervention studies have been conducted; however, modest improvement in cognitive function with MedDiet31 and calorie-restricted DASH32 interventions has been reported in middle-aged to older adults at increased vascular risk. It is not yet known whether dietary modification in early to midlife can affect the rate of cognitive decline during aging.
The mechanisms by which diet can influence midlife cognitive function are not clear but likely to involve oxidative stress, inflammatory, and vascular disease pathways that contribute to accelerated cognitive decline and dementia.33 Antioxidants from foods (e.g., fruit, vegetables, legumes, and nuts) and beverages (tea, red wine) protect against oxidative damage and can alter inflammatory processes.34 Antioxidant-rich diets, particularly the MedDiet and DASH diet, are shown to reduce markers of oxidative stress,35,36 and in CARDIA, the APDQS score has been inversely related to oxidative stress.37 Furthermore, dietary components (polyphenols and omega-3 fatty acids, fruits, and vegetables) have anti-inflammatory properties with likely synergistic or additive effects in vivo38 that can decrease markers of systemic inflammation.39 It is well established that diet can modify several vascular risk factors, including hypertension, dyslipidemia, obesity, and insulin resistance,40 as well as endothelial function41 and risk of developing cardiovascular disease.26,40 The MedDiet has demonstrated reduced cardiovascular events, particularly rates of stroke, in adults at high cardiovascular risk.42
In contrast to MedDiet and APDQS scores, higher DASH diet scores were not associated with global cognitive function or executive function. Dietary patterns characterized by MedDiet, DASH, and APDQS scores share similar profiles but also have unique components that may account for different strengths of association observed across the cognitive domains. DASH uniquely limits sodium intake; however, APDQS also accounts for foods considered high in sodium, including salty snacks and processed foods. Moderate alcohol is recommended in both MedDiet and APDQS but is not considered in the DASH score system, suggesting that moderate alcohol intake as part of a healthy diet could be relevant for brain health in midlife. The role of alcohol in brain health is not clear, but moderate alcohol may exert protective effects on cognitive function either directly by enhancing acetylcholine release in the hippocampus43 or indirectly by mediating vascular risk reduction via decreased platelet aggregation or modification of serum lipids.44
This study had several strengths, including 30 years of follow-up data and 5-year change in 3 aspects of cognitive testing in a relatively large sample with adequate representation of both men and women and blacks and whites. Other strengths were the detailed and repeated measures of diet to capture long-term dietary exposure and the repeated cognitive measures using validated neuropsychological tests to determine change in cognition across several domains. A further advantage was that dietary exposure was determined early in adulthood and before the assessment of cognitive function, thus minimizing the possibility of reverse causation.
There were some limitations to consider in this study. We were unable to incorporate olive oil as a beneficial component in the derived MedDiet score,15 which may have underestimated the association of this dietary pattern on cognitive decline. The observed effect sizes between diet scores and change in cognitive function were small but of a magnitude similar to that of effects observed for relations between vascular and genetic risk factors and cognitive function in midlife.5,45 Despite being able to adjust our analysis for important confounders measured during adulthood, residual confounding by unidentified confounders cannot be excluded. Furthermore, our findings may not be generalizable beyond black and white US adults in midlife.
This study shows that the MedDiet and APDQS dietary patterns are associated with better overall cognitive performance in midlife. Additional investigations are needed to define the combination of foods and nutrients for optimal brain health, but our findings lend support to heart-healthy dietary patterns high in fruit, vegetables, and legumes; moderate in nuts, fish, and alcohol; and low in meat for neuroprotection in midlife. Further prospective studies are required to understand how the duration of dietary exposure influences risk of cognitive impairment across the life course in different populations to help inform the nature and timing of dietary interventions targeted for brain health.
Glossary
- APDQS
A Priori Diet Quality Score
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- CES-D
Center for Epidemiologic Studies Depression
- CI
confidence interval
- DASH
Dietary Approaches to Stop Hypertension
- DSST
Digit Symbol Substitution Test
- MedDiet
Mediterranean diet
- MoCA
Montreal Cognitive Assessment
- OR
odds ratio
- RAVLT
Rey Auditory Verbal Learning Test
Footnotes
Editorial, page 645
CME Course: NPub.org/cmelist
Author contributions
C.T. McEvoy: conception, design, data acquisition, analysis and interpretation, statistical analysis, drafting and editing the manuscript. T. Hoang: design, interpretation of data, editing the manuscript and revising it critically for intellectual content. S. Sidney and L.M. Steffen: editing the manuscript and revising it critically for intellectual content. D.R. Jacobs, Jr.: data acquisition (diet) and design, editing the manuscript and revising it critically for intellectual content. J.M. Shikany and J.T. Wilkins: editing the manuscript and revising it critically for intellectual content. K. Yaffe: study supervision, conception, design, data interpretation and analysis, editing the manuscript and revising it critically for intellectual content, final manuscript approval.
Study funding
Dr. McEvoy is supported by a Beeson-CARDI Fellowship from the American Federation of Aging Research. CARDIA is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute and the Intramural Research Program of the National Institute on Aging, as well as by a grant (R01-HL-053560).
Disclosure
C. McEvoy, T. Hoang, S. Sidney, and L. Steffen report no disclosures relevant to the manuscript. D. Jacobs, Jr. is consultant to the California Walnut Commission. J. Shikany and J. Wilkins report no disclosures relevant to the manuscript. K. Yaffe is a Senate member of the German Center for Neurodegenerative Diseases and the Beeson Advisory Committee. She serves on Data Safety Monitoring Board for Takeda, Eli Lilly, and an NIH-sponsored study. Go to Neurology.org/N for full disclosures.
References
- 1.Teng E, Tassniyom K, Lu PH. Reduced quality-of-life ratings in mild cognitive impairment: analyses of subject and informant responses. Am J Geriatr Psychiatry 2012;20:1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer's Association Report 2017: Alzheimer's disease facts and figures. Alzheimers Demen 2017;13:325–373. [Google Scholar]
- 3.Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 2012;344:d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis JP, Loria CM, Launer LJ, et al. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol 2013;73:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 2014;129:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoang TD, Reis J, Zhu N, et al. Effect of early adult patterns of physical activity and television viewing on midlife cognitive function. JAMA Psychiatry 2016;73:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology 2014;83:1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van de Rest O, Berendsen AA, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr 2015;6:154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feart C, Samieri C, Barberger-Gateau P. Mediterranean diet and cognitive health: an update of available knowledge. Curr Opin Clin Nutr Metab Care 2015;18:51–62. [DOI] [PubMed] [Google Scholar]
- 10.Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement 2015;11:1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh B, Parsaik AK, Mielke MM, et al. Association of Mediterranean diet with mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis 2014;39:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haring B, Wu C, Mossavar-Rahmani Y, et al. No association between dietary patterns and risk for cognitive decline in older women with 9-year follow-up: data from the Women's Health Initiative Memory Study. J Acad Nutr Diet 2016;116:921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsson E, Karlström B, Kilander L, et al. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J Alzheimers Dis 2015;43:109–119. [DOI] [PubMed] [Google Scholar]
- 14.Naska A, Lagiou A, Lagiou P. Dietary assessment methods in epidemiological research: current state of the art and future prospects. F1000Res 2017;6:926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panagiotakos DB, Pitsavos C, Arvaniti F, et al. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med 2007;44:335–340. [DOI] [PubMed] [Google Scholar]
- 16.Folsom AR, Parker ED, Harnack LJ. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am J Hypertens 2007;20:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sijtsma FP, Meyer KA, Steffen LM, et al. Longitudinal trends in diet and effects of sex, race, and education on dietary quality score change: the Coronary Artery Risk Development in Young Adults study. Am J Clin Nutr 2012;95:580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 19.McDonald A, Van Horn L, Slattery M, et al. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc 1991;91:1104–1112. [PubMed] [Google Scholar]
- 20.Mursu J, Steffen L, Meyer K, Duprez D, Jacobs D. Diet quality indices and mortality in postmenopausal women: the Iowa Women's Health Study. Am J Clin Nutr 2013;98:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs D, Tapsell L. Food synergy: the key to a healthy diet. Proc Nutr Soc 2013;72:200–206. [DOI] [PubMed] [Google Scholar]
- 22.Park YM, Zhang J, Steck SE, et al. Obesity mediates the association between mediterranean diet consumption and insulin resistance and inflammation in US adults. J Nutr 2017;147:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidney S, Jacobs DR Jr, Haskell WL, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Epidemiol 1991;133:1231–1245. [DOI] [PubMed] [Google Scholar]
- 24.Kamboh MI, Ferrell RE, Kottke B. Genetic studies of human apolipoproteins, V: a novel rapid procedure to screen apolipoprotein E polymorphism. J Lipid Res 1988;29:1535–1543. [PubMed] [Google Scholar]
- 25.Hawkins KA, Dean D, Pearlson GD. Alternative forms of the Rey Auditory Verbal Learning Test: a review. Behav Neurol 2004;15:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Horn L, Carson JA, Appel LJ, et al. Recommended dietary pattern to achieve adherence to the American Heart Association/American College of Cardiology (AHA/ACC) guidelines: a scientific statement from the American Heart Association. Circulation 2016;134:e505–e529. [DOI] [PubMed] [Google Scholar]
- 27.Zhu N, Jacobs DR, Meyer KA, et al. Cognitive function in a middle aged cohort is related to higher quality dietary pattern 5 and 25 years earlier: the CARDIA study. J Nutr Health Aging 2015;19:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kesse-Guyot E, Andreeva VA, Jeandel C, Ferry M, Hercberg S, Galan P. A healthy dietary pattern at midlife is associated with subsequent cognitive performance. J Nutr 2012;142:909–915. [DOI] [PubMed] [Google Scholar]
- 29.Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife healthy-diet index and late-life dementia and Alzheimer's disease. Dement Geriatr Cogn Dis Extra 2011;1:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samieri C, Okereke OI, E Devore E, Grodstein F. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nutr 2013;143:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valls-Pedret C, Sala-Vila A, Serra-Mir M, et al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med 2015;175:1094–1103. [DOI] [PubMed] [Google Scholar]
- 32.Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension 2010;55:1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorelick PB, Furie KL, Iadecola C, et al. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke 2017;48:e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajendran P, Nandakumar N, Rengarajan T, et al. Antioxidants and human diseases. Clin Chim Acta 2014;436:332–347. [DOI] [PubMed] [Google Scholar]
- 35.Fitó M, Guxens M, Corella D, et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med 2007;167:1195–1203. [DOI] [PubMed] [Google Scholar]
- 36.Asemi Z, Samimi M, Tabassi Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effects of DASH diet on lipid profiles and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: a randomized clinical trial. Nutrition 2014;30:1287–1293. [DOI] [PubMed] [Google Scholar]
- 37.Meyer KA, Sijtsma FP, Nettleton JA, et al. Dietary patterns are associated with plasma F(2)-isoprostanes in an observational cohort study of adults. Free Radic Biol Med 2013;57:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minihane AM, Vinoy S, Russell WR, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr 2015;114:999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets 2014;14:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation 2016;133:187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marin C, Ramirez R, Delgado-Lista J, et al. Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am J Clin Nutr 2011;93:267–274. [DOI] [PubMed] [Google Scholar]
- 42.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 43.Letenneur L, Larrieu S, Barberger-Gateau P. Alcohol and tobacco consumption as risk factors of dementia: a review of epidemiological studies. Biomed Pharmacother 2004;58:95–99. [DOI] [PubMed] [Google Scholar]
- 44.Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health 2014;14:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results from the National Institute of Mental Health's BIOCARD study. Neuropsychology 2005;19:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data are available from the CARDIA Coordinating Center (cardia.dopm.uab.edu/contact-cardia). A description of the National Heart, Lung, and Blood Institute policies governing the data and describing access to the data can be found online (cardia.dopm.uab.edu/study-information/nhlbi-data-repository-data).