Abstract
The anaerobic fungus Orpinomyces sp. strain PC-2 produces a broad spectrum of glycoside hydrolases, most of which are components of a high molecular mass cellulosomal complex. Here we report about a cDNA (manA) having 1924 bp isolated from the fungus and found to encode a polypeptide of 579 amino acid residues. Analysis of the deduced sequence revealed that it had a mannanase catalytic module, a family 1 carbohydrate-binding module, and a noncatalytic docking module. The catalytic module was homologous to aerobic fungal mannanases belonging to family 5 glycoside hydrolases, but unrelated to the previously isolated mannanases (family 26) of the anaerobic fungus Piromyces. No mannanase activity could be detected in Escherichia coli harboring a manA-containing plasmid. The manA was expressed in Saccharomyces cerevisiae and ManA was secreted into the culture medium in multiple forms. The purified extracellular heterologous mannanase hydrolyzed several types of mannan but lacked activity against cellulose, chitin, or β-glucan. The enzyme had high specific activity toward locust bean mannan and an extremely broad pH profile. It was stable for several hours at 50 °C, but was rapidly inactivated at 60 °C. The carbohydrate-binding module of the Man A produced separately in E. coli bound preferably to insoluble lignocellulosic substrates, suggesting that it might play an important role in the complex enzyme system of the fungus for lignocellulose degradation.
Keywords: Orpinomyces, anaerobic fungi, mannanase, cellulose-binding module, cellulosome
Résumé:
Le champignon anaérobie Orpinomyces sp. souche PC-2 produit un large spectre d’hydrolases glycosidiques, la plupart faisant partie d’un complexe cellulosomal de masse moléculaire élevée. Nous faisons ici la description d’un ADNc (manA) de 1924 pb isolé du champignon et codant un polypeptide de 579 acides aminés. L’analyse de la séquence déduite a révélé qu’il renfermait un module catalytique de mannanase, un module de liaison aux glucides de la famille 1 et un module d’arrimage non catalytique. Le module catalytique était homologue à des mannanases de champignons aérobies appartenant aux hydrolases glycosidiques que la famille 5 mais n’était pas apparenté à des mannanases isolées précédemment (famille 26) du champignon anaérobie Piromyces. Aucune activité mannanases n’a pu être détectée chez Escherichia coli renfermant un plasmide contenant manA. Le manA fut exprimé chez Saccharomyces cerevisiae et ManA fut secrete dans le milieu culture sous des formes multiples. La mannanase hétérologue extracellulaire purifiée a hydrolysé plusieurs types de mannane mais n’a démontré aucune activité envers le cellulose, la chitine ou le β-glucane. L’enzyme a démontré une activité spécifique élevée envers le mannane de caroube et un profil de pH extrêmement large. Elle etait stable pendant plusieurs heures à 50 °C mais fut rapidement inactivée à 60 °C. Le module de liaison aux glucides de la mannanase A produite séparément chez E. coli s’est lié de préférence à des substrats lignocellulosiques insolubles, indiquant qu’il pourrait jouer un rôle important dans la dégradation du lignocellulose par le système enzymatique complexe du champignon.
Keywords: Orpinomyces, champignons anaérobies, mannanase, module de liaison au cellulose, cellulosome
Introduction
Plant cell wall, an abundant organic matter on earth, is composed of 3 major constituents: cellulose, hemicelluloses, and lignin. The ratio of these components varies depending on the source of cell walls. Hemicelluloses refer to a number of heterologous structures, such as arabinoxylan, mannan, and xyloglucan. These chemically diverse polymers are linked together through physical attachment and chemical bonds. The cross-linking and partially crystalline nature of the matrix offer great resistance to enzymatic degradation. To date, hydrolytic enzymes for the cleavage of almost all chemical bonds found in plant structures have been identified in microbial sources. These enzymes often are modular and, in addition to catalytic domains, they have modules for carbohydrate binding (CBM) and cellulose surface modification and disruption (Bayer et al. 1998; Kataeva et al. 2002; Saloheimo et al. 2000). In addition, especially with anaerobic bacteria and fungi, in which glycoside hydrolases are found in extracellular protein complexes called cellulosomes, the enzymatically active components contain noncatalytic dockerin modules (NCDM) that bind to cohesins of scaffolding proteins (Bayer et al. 1998; Fannuti et al. 1995; Ljungdahl et al. 1998).
Mannanase (endo-1,4-β-mannanase; mannan endo-1,4-β-mannosidase; EC 3.2.1.78) randomly hydrolyzes the 1,4-β-mannosidic bonds of the main chain of glucomannan and galactomannan. On the basis of sequence similarity, microbial β-mannanases are classified into the glycoside hydrolase families 5 and 26 (http://afmb.cnrs-mrs.fr/CAZY/). Among the family 5 members are the mannanases from Trichoderma reesei, Aspergillus aculeatus, and Agaricus bisporus. Although their catalytic modules (CMs) are similar, the presence and location of CBMs vary among them. A mannanase, ManA from Clostridium cellulovorans, belonging to family 5, has a dockerin and is a cellulosomal component. It does not have a CBM (Tamaru and Doi 2000). Clostridium thermocellum produces a mannanase belonging to family 26, which has a dockerin and possibly also a CBM (Halstead et al. 1999). The family 5 mannanase from Caldobacillus cellulovorans has 2 CBMs, one on each side of the CM, and an unknown module (Sunna et al. 2000). There is no conclusive evidence of a fungal mannanase with both a dockerin and a CBM (Bayer et al. 1998; Lynd et al. 2002).
Anaerobic ungi were first described by Orpin (Orpin 1975). At least 17 different anaerobic fungi have been described and classified into 5 genera. The classification was done according to ultrastructural characteristics, type of growth (monocentric or polycentric), and uni-, bi-, or poly-flagellated zoospores. The anaerobic fungi differ from aerobic fungi in that they are very sensitive to oxygen and that instead of mitochondria, they have hydrogenosomes, which are involved in energy metabolism (Ljungdahl et al. 1998; van der Giezen et al. 1997). Many anaerobic fungi are able to completely degrade recalcitrant cell wall materials because of their ability to secrete a broad spectrum of hydrolytic enzymes (Borneman et al. 1989). Some of these enzymes are found free, but most of them are bound to cellulosomal complexes (Fannuti et al. 1995; Steenbakkers et al. 2001). The enzymes of the complexes contain noncatalytic docking modules (NCDM), equivalent to the dockerins of clostridial cellulosomes. But the fungal and bacterial docking modules do not share sequence similarities (Steenbakkers et al. 2001). Evidence for scaffolding polypeptides for the fungal cellulosomes has been presented (Fannuti et al. 1995; Steenbakkers et al. 2001), but genes coding for the scaffolding polypeptides have not been reported.
Orpinomyces sp. strain PC-2, a polycentric fungus isolated from bovine rumen (Borneman et al. 1989), secretes cellulase, β-glucanase, xylanase, feruloyl and p-coumaroyl esterase, acetylxylan esterase, β-glucosidase, and mannanase activities. Many of these enzymes are cellulosomal components (Li et al. 1997a, 1997b; Steenbakkers et al. 2001). Evidence that genes coding for enzymes of anaerobic fungi are of either bacterial or fungal origins has been provided (Ljungdahl et al. 1998).
We report here the isolation of a cDNA coding for ManA of Orpinomyces sp. strain PC-2. Sequence analysis revealed that the enzyme contains 3 modules: a CM, a CBM, and a fungal-type dockerin. The CM with and without the CBM and CBM alone were heterologously expressed and characterized. It is suggested that the complete gene evolved by combination of DNA sequences coding for the various modules.
Materials and methods
Strains and vectors
Escherichia coli INVαF′ and BL21(DE3) were products of Invitrogen (Carlsbad, Calif.) and Novagen (Madison, Wis.), respectively. Saccharomyces cerevisiae INVSc1 (MAT α his 3-D 1 El 2 trpl-289 ura3-52) and plasmid pYES2 were purchased from Invitrogen Corp. pYES2 possesses ampicillin and tetracycline resistance genes for selection in E. coli, a URA3 gene for high-copy-number maintenance and selection in S. cerevisiae INVSc1, and a GAL1 promoter sequence. Plasmid pET24 (b) was purchased from Novagen. A cDNA library of Orpinomyces sp. strain PC-2 was the same as described before (Chen et al. 1995).
Generation of DNA hybridization probes
To assess the diversity of docking module-containing components of Orpinomyces PC-2 cellulosomes, a PCR-based approach was developed (Steenbakkers et al. 2001) to amplify possible sequences coding for the partial docking modules. A number of distinct DNA sequences were amplified from an Orpinomyces cDNA library (Chen et al. 1995), and 9 were found to be different from those already isolated by activity screening. Degenerate primer designs (Table 1), PCR conditions, cloning and sequencing of PCR products, and analysis of sequence data were done as described by Steenbakkers et al. (2001). One of the sequences, RP2, was reamplified and labeled with digoxigenin (Roche Molecular Biochemicals, Indianapolis, Ind.) by PCR using the same degenerate primers and the RP2-specific PCR clone as template. The incorporation of digoxigenin into PCR products was detected by the increase of sizes on a 4.0% agarose gel.
Table 1.
Oligonucleotides used as PCR primers.
| Primer | Sequencea | Orientation | Amino acid positionb | Restriction sitec |
|---|---|---|---|---|
| NDDCF | 5′-GGIAAITTGGGGIGTIGARAA-3′ | Forward | 518–524 | |
| NDDCR | 5′-TTYTCIACICCCCAITTICC-3′ | Reverse | 562–568 | |
| ManAF | 5′-TGTTCAAACATGGAACCAA-3′ | Forward | 26–32 | |
| ManAR | 5′-CCAAACATCCATACCACCA-3′ | Reverse | 132–137 | |
| PFMan | 5 ′-ACCGAGCTCGATGCATTTCAATAAAGTTAGTG-3′ | Forward | 1–7 | SacI |
| PRMan | 5′-GCTCTAGAGTGGAAGTACTTTTTGCACCTG-3′ | Reverse | 461–468 | XbaI |
| PFMC | 5′-GGAATTCGGATTTAGTTGTGTTTACTA-3′ | Forward | 401–405 | EcoRI |
| PRMC | 5′-GCTAGTGCGGCCGCTTTGGAAGTACTTTTTGCA-3′ | Reverse | 462–469 | NotI |
R represents A and G; Y represents C and T; I represents inosine.
Numbering corresponds to that of Genbank acc. No. AF 177206.
Restriction sites are underlined.
DNA hybridization screening
The digoxigenin labeled RP2 probe was used to screen about 107 plaque-forming units (PFUs) of the Orpinomyces cDNA library. Twenty primary positive plaques were identified and purified into pure clones by a second round. Conditions for hybridization using DIG Easy Hyb buffer (Roche), washing (0.5 × SSC (1 × 0.15 mol/L NaCl plus 0.015 mol/L sodium citrate), 0.1% SDS (sodium dodecyl sulfate), 60 °C), and color development were as described by Roche. The recombinant pBluecript phagemids were excised from positive λZAP clones using a kit provided by Stratagene (La Jolla, Calif.), and the DNA inserts were sequenced as described by Li et al. (1997a). A 5′ region of the clone with the longest insert was amplified by PCR using ManAF and ManAR (Table 1) as primers and labeled with digoxigenin. The new probe was used to isolate the full length cDNA clone (pManA).
Construction of plasmid cassette
Plasmid pYES2 was digested with SacI and XbaI overnight. The digested plasmid was purified using the Geneclean II kit (Bio 101, Inc., La Jolla, Calif.). Primers PFMan and PRMan were synthesized based on the nucleotide sequence of the cloned gene (Table 1). PFMan codes for the first 7 amino acids of the open reading frame (ORF) and had a SacI site attached. PRMan is complementary to the sequence coding for amino acids 461–468, CTA nucleotides (complementary to the UAG stop codon), and a XbaI site attached. The encoded polypeptide should yield ManA without the NCDM. Using PFMan and PRMan as primers and the plasmid isolated from E. coli containing the complete Orpinomyces mannanase cDNA sequence (pManA) as template, the DNA region was amplified by PCR with 30 cycles of denaturation (1 min at 94 °C), annealing (1.0 min at 55 °C), and extension (1.5 min at 72 °C) on a 480 thermocycler (Perkin-Elmer Co., Norwalk, Conn.). PFU polymerase (Stratagene) was used for the PCR reactions. PCR products were purified using the Geneclean kit and digested with SacI and XbaI. Digested DNA fragments were purified and concentrated before they were ligated with the SacI- and XbaI-digested pYES2 using T4 DNA ligase. The plasmid pYES2 containing the insert was named pTmanA.
Transformation of E. coli and propagation of the plasmids
Ligation reactions were performed using a rapid ligation kit (Roche). Transformation was done following instructions of the Original TA Cloning Kit (Invitrogen, Carlsbad, Calif.). Escherichia coli INVαF′ transformants were plated out on Luria–Bertani (LB) plates containing ampicillin (100 μg/mL) and X-Gal (40 μg/mL). White colonies were picked and grown overnight in LB liquid medium containing ampicillin. Plasmids were purified with the spin column kit from Qiagen (Valencia, Calif.). Restriction digestion and PCR amplification were performed to verify the presence of the insert.
Transformation of S. cerevisiae
A single colony of the yeast strain INVSc1 was grown to an OD600 of 1.3 in yeast extract – Bacto-peptone – dextrose (YPD) medium, pH 6.5, containing 1% (w/v) yeast extract, 1% (w/v) bactopeptone, and 1% (w/v) dextrose (Difco). Cells were harvested by centrifugation at 4000g for 5 min at 4 °C and washed twice with ice-cold sterile H2O and twice with ice-cold 1 mol/L sterile sorbitol. The cells were then resuspended in 0.5 mL of 1 mol/L sorbitol. Approximately 5 μg of pYES2 or pTManA was added to transform 40 μL of the yeast cells using an electroporator (Bio-Rad Laboratories, Hercules, Calif.). Transformants were grown in a drop-out base (DOB) medium containing 0.17% (w/v) yeast nitrogen base, 2.0% (w/v) dextrose, 0.08% (w/v) drop out supplements lacking uracil (Bio101, Inc.), as well as 2% (w/v) agarose and 1 mol/L sorbitol. The plates were incubated at 30 °C for 3–5 d.
Induction and expression of manA
Putative transformants were chosen for induction experiments. A single colony of a transformed yeast was transferred to 5 mL of DOB medium containing 0.17% (w/v) yeast nitrogen base, 0.08% (w/v) drop out supplement lacking uracil, and 4% (w/v) raffinose. After growth reached an OD600 of about 1.0, sterile galactose (50%, w/v) was added to a concentration of 2.0% (w/v). During continuous culturing for an additional 40 h at 30 °C, aliquot samples were drawn from the culture for OD600 and enzymatic activity measurements. Cells were harvested by centrifugation (5000g, 20 min) at 4 °C. The supernatant obtained was stored at −20 °C until analyzed. The supernatant from five 500-mL cultures was used for the purification of the mannanase.
CBM production by E. coli
To produce the CBM, 2 oligonucleotides, PFMC and PRMC, containing EcoRI and NotI restriction sites, respectively, were synthesized (Table 1). With these as primers and pManA as template, PCR produced a DNA fragment coding for a polypeptide of 69 amino acid residues (401–469) of ManA. The fragment was cloned into pET24 (b) digested with EcoRI and NotI. The polypeptide was 6× His tagged at the C-terminus to facilitate purification. Sequencing of the nucleotide confirmed the lack of unwanted mutation. A plasmid with the CBM coding sequence inserted was named pCBM and was used for the transformation of E. coli BL21 (DE3). Transformed cells were grown on LB medium containing 50 μg/mL kanamycin. The presence of recombinant CBM production was verified by comparing protein binding patterns among cells transformed with pET24 (b) with or without the insert.
Mannanase and CBM purification
Saccharomyces cerevisiae cells transformed with pTmanA were grown in raffinose YPD medium (5 × 500 mL) to an OD600 of 1.0. Galactose was added to 2.0% (w/v), and the cultures were shaken for an additional 30 h. Cultures were harvested and cell debris removed by centrifugation at 5000g for 20 min. The supernatant (2200 mL) was concentrated to 30 mL using an ultrafiltration cell equipped with a 10-kDa cutoff membrane (PM10; Millipore, Bedford, Mass.) and dialyzed against 20 mmol/L Tris–HCl, pH 7.0 (starting buffer). A fast protein liquid chromatography (FPLC) system was used for all of the column chromatographic steps. The concentrated sample was applied to a HiPrep 16/10 Q XL column equilibrated with the starting buffer. The column was washed with 500 mL of the same buffer. The mannanase was then eluted with a 400-mL linear gradient of NaCl from 0 to 1 mol/L. Fractions containing about 80% of the mannanase activity were combined, concentrated, dialyzed against the starting buffer, and then applied to an equilibrated gel filtration column packed with Superdex 75 (Pharmacia). The fractions displaying activity after being applied to both columns were analyzed by SDS – polyacrylamide gel electrophoresis (SDS–PAGE) to assess the purity of the mannanase.
To purify the CBM fusion protein, an E. coli culture (2.0 L) was grown to an OD600 of 1.0, induced with 1 mmol/L of isopropyl-β-D-thiogalactopyranoside, and then grown for 5 h. The cells recovered by centrifugation (5000g, 20 min) were washed with 20 mmol/L sodium phosphate buffer, pH 7.0, containing 0.5 mol/L NaCl (starting buffer), and then disrupted using a french press. Cell debris was removed by centrifugation. All purification steps were done at 4 °C, except for the FPLC column chromatography, which was performed at room temperature. Clear supernatant was applied onto a Ni – nitrilotriacetic acid (NTA) agarose (Qiagen Inc., Valencia, Calif.) column equilibrated with the starting buffer. The column was washed with 20 mmol/L sodium phosphate (0.5 mol/L NaCl, pH 6.0). Proteins were eluted by a gradient of 0 0.5 mol/L imidazole, pH 6.0. Fractions containing the CBM were combined, concentrated by precipitation with ammonium sulfate (60% saturation), and the precipitated protein was dialyzed against 20 mmol/L Tris–HCl buffer, pH 7.5, containing 0.1 mol/L NaCl. Dialyzed CBM was further purified by gel-exclusion chromatography on a Superdex-200 prepacked column (Pharmacia Biotech, Piscataway, N.J.). Eluted CBM was concentrated using Centricon 3 concentrator (Amicon, Inc., Beverly, Mass.) and stored at 4 °C. Purity of the CBM during purification was monitored by gradient (8%–16%) SDS–PAGE.
Enzyme activity and protein determination
Mannanase activity was routinely measured using 1% (w/v) locust bean mannan (Sigma) dissolved in 50 mmol/L sodium citrate buffer, pH 6.0, as substrate. The assay mixture contained 50 μL of an appropriately diluted enzyme solution and 200 μL of the substrate. Reaction mixtures were incubated for 15 min at 50 °C. Reducing sugars were measured by the dinitrosalicylic acid procedure of Miller (Miller 1959). Xylanase, CMCase, and filter paper hydrolase activities were measured in the presence of 1% xylan, 1% C, and 20 mg of filter paper in 200 μL of 50 mmol/L sodium citrate buffer, pH 6.0. One unit of enzyme is defined as the amount required for the generation of 1 μmol/min of reducing sugars under the assay conditions.
Insoluble polysaccharide binding assay
Binding of the CBM to insoluble polysaccharides (10 g/L) was done at room temperature in 1.5-mL microcentrifuge tubes. The CBM (18 nmol) was mixed with carbohydrates in 50 mmol/L sodium citrate buffer, pH 6.0, in a final volume of 0.5 mL. Tube contents were continuously mixed by rotation. After equilibration for 2 h, the carbohydrates were removed by centrifugation at 10 000g for 10 min. Centrifugation was repeated twice to ensure the removal of all of the carbohydrates. The amount of unbound protein was determined. The amount of bound protein was calculated as the difference between the initial amount of protein and unbound protein. Each data point is the mean of 5 replicates.
Affinity electrophoresis
Affinity electrophoresis using polyacrylamide gels with soluble polysaccharide ligands was performed as described earlier (Kataeva et al. 2001; Tomme et al. 2000). The polysaccharides were incorporated into the gel at a concentration of 0.2% prior to polymerization. A control gel without polysaccharides was prepared and run simultaneously. Electrophoresis was at 4 °C and 150 V for 3 h.
Analytical methods
Gel (10%) electrophoresis (SDS–PAGE) was done according to Laemmli (1970). Molecular-weight protein standards (Bio-Rad) were used as markers. Electrophoresis was performed in a Mini-Protein II cell, and gels were stained with Coomassie Brilliant Blue R-250 (Fairbanks et al. 1971). Mannanase active bands were visualized in a zymogram gel containing 0.5% mannan (Locust bean gum) as substrate after staining with 1.0% Congo Red as done before (Chen et al. 1997).
Protein was determined with the Coomassie Protein Assay Reagent (Pierce, Rockford, Ill.) using bovine serum albumin as standard. In CBM binding experiments, protein concentrations were determined on the basis of A278 values. Molar absorption coefficient of the CBM (calculated from tryptophan and tyrosine content) was 9140 M−1cm−1.
The metal content of the CBM was determined by plasma emission spectrophotometry (Jarrell-Ash 965 ICP). Each data point was a mean of 3 replicates.
The pH optimum of ManA was determined by performing assays with 1% mannan as substrate at 50 °C in 0.1 mol/L sodium acetate (pH 3.8–5.6) and 0.1 mol/L sodium phosphate (pH 5.8–8.2). Enzyme stability at different pH values was determined by measuring the residual activity after incubating the enzyme for 24 h at 4 °C with the buffers above. The effect of the temperature on mannanase activity was determined by assaying the enzyme at temperatures from 30 to 65 °C. The stability of the ManA activity at various temperatures was determined by incubating the enzyme in 100 mmol/L sodium phosphate buffer, pH 6.0, from 30 min to 8 h at 50 and 55 °C. During the time course, samples were withdrawn and tested for remaining activity.
Nucleotide sequence accession No.
The nucleotide sequence reported in this paper was submitted to Genbank nucleotide sequence databases under access No. AF177206.
Results
Isolation of Orpinomyces manA
In addition to the corresponding regions coding for celA, celB, celC, celD, celE, and xynA, 11 novel docking module specific sequences of Orpinomyces PC-2 were obtained by PCR amplification and cloning (Steenbakkers et al. 2001). Among the 11 novel sequences, the insert in pRP2 was reamplified, labeled with digoxigenin, and used as hybridization probe for screening of the cDNA library. Restriction analysis by EcoRI digestion of the excised positive plasmids revealed that the longest insert was 1.8 kb. It did not contain a full-length ORF. Therefore, a 300-bp region at the 5′ region of the longest clone was amplified using primers ManAF and ManAR (Table 1) and labeled with digoxigenin. Using the new amplified sequence as a probe, 9 positive λ plaques were isolated from the library and were used to recover the full length cDNA (pManA).
Nucleotide and deduced amino acid sequences of manA
The DNA fragment obtained consisted of a total of 1924 nucleotides. It contained an open reading frame of 1740 bp coding for a polypeptide of 579 amino acid residues. The calculated mass of the polypeptide was 64 425 Da. A putative translation start codon (ATG) was assigned based on the fact that the preceding region was highly A+T rich, contained no ATG codon, and the amino-terminal region of the deduced polypeptide had the characteristics of a signal peptide (von Heijne 1986). The G+C content of the entire cDNA sequence and the ORF were 31.8% and 34.4%, respectively, while that of the 5′ and 3′ noncoding region was 17.6%, in agreement with other genes of the fungus (Chen et al. 1995; Li et al. 1997a). No long poly(A) tail at the 3′ end was found. The nucleotide sequence between NDDCF and NDDCR in pRP2 matched perfectly with nucleotides 1709–1819 (amino acid residues 525–561) of the manA cDNA and the corresponding regions of all the partial cDNA clones. It suggests that there is no other gene highly homologous to manA in the fungal genome.
Modular structure and similarity analysis of ManA
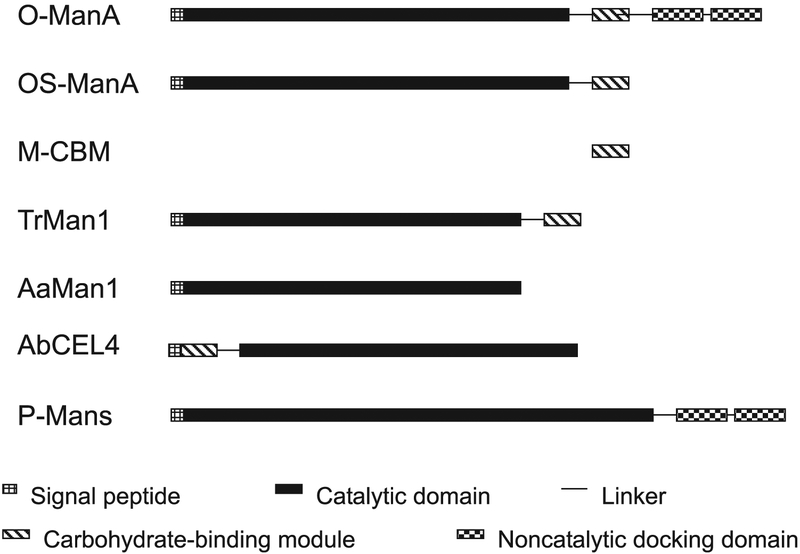
Similarity search with the full length and various regions of the deduced ManA amino acid sequence using FASTA and BLAST revealed that ManA has a modular structure, as illustrated in Fig. 1. Starting with the N-terminus, there is a putative signal peptide of about 20 amino acid residues rich in nonpolar side chains. It is followed by about a 350-amino-acid residue, CM, a CBM, a linker, and 2 NCDMs. The CM of ManA belongs to glycoside hydrolase family 5, which includes hydrolases of bacteria, fungi, and plants. ManA has about 30% sequence identity with the CMs of mannanases from T. reesei (Stålbrand et al. 1995), Aspergillus aculeatus (Christgau et al. 1994), and Agaricus bisporus (Yagüe et al. 1997) (Fig. 2). It is also homologous to endoglucanases, xylanases, and other mannanases of family 5 glycoside hydrolases. Residues corresponding to Glu-192 and Glu-313 of ManA are highly conserved among family 5 glycoside hydrolases and shown to be involved in catalysis (Maccarón et al. 1993; Stålbrand et al. 1995; Fig. 2). However, the CM of ManA has several short insertions and is longer than those of the enzymes from the aerobic fungi. The functional significances of these insertions in ManA or deletions in the mannanases of aerobic fungi are not known.
Fig. 1.
Illustration of module organizations of some mannanases from various microbial sources. O-ManA, Orpinomyces mannanase A; OS-ManA, Orpinomyces mannanase A expressed in S. cerevisiae with NCDM truncated; M-CBM, Orpinomyces mannanase A CBM expressed in E. coli; TrMan1, T. reesei mannanase 1 (Stålbrand et al. 1995); AaMan1, Aspergillus aculeatus mannanase 1 (Christgau et al. 1994); AbCEL4, Agaricus bisporus CEL4 (Tang et al. 2001; Yagüe et al. 1997); P-Mans, mannanases of the anaerobic fungus Piromyces equi (Fannuti et al. 1995; Millward-Sadler et al. 1996).
Fig. 2.
Amino acid sequence alignment of the catalytic domains of fungal mannanases belonging to family 5 glycoside hydrolases of Orpinomyces PC-2 (O-ManA), T. reesei (TrMan1; Stålbrand et al. 1995), Aspergillus aculeatus (AaMan1; Christgau et al. 1994), and Agaricus bisporus (AbCEL4; Yagüe et al. 1997).
The amino acid sequence spanning residues 406–463 showed homology to CBMs of fungal hydrolases belonging to CBM family 1 (Tomme et al. 1995; Fig. 3). CBMs of family 1, which are composed of about 40 amino acid residues, bind to insoluble substrates with varying reversibility, and form flat binding surfaces accessible to crystalline substrates (Carrard and Linder 1999; Linder and Teeri 1996). Three conserved aromatic residues (corresponding to Tyr-5, Tyr-31, and Tyr-32 of T. reesei CBHI CBM; Linder and Teeri 1996) form the binding surface. A glutamine (Gln-34 of T. reesei CBHI CBM) stabilizes Tyr-32 (Kraulis et al. 1989; Mattinen et al. 1998). In the CBM of ManA, 2 of the 3 corresponding aromatic residues (Tyr-458 and Tyr-459) and the glutamine (Gln-461) are conserved (Fig. 3).
Fig. 3.
Alignment of the Orpinomyces mannanase CBM (O-ManA) with those from T. reesei mannanase (T-Man1; Stålbrand et al. 1995), cellobiohydrolase I (T-CbhI; Shoemaker et al. 1983), cellobiohydrolase II (T-CbhII; Teeri et al. 1987), endoglucanase I (T-EngI; Penttilä et al. 1986), endoglucanase II (T-EngII; Saloheimo et al. 1988), and endoglucanase V (T-EngV; Saloheimo et al. 1994). Identical amino acids are in bold.
Following the CBM of ManA is a Thr- and Ser-rich linker, which connects to a typical NCDM at the C-terminus. This module was used for the cloning of the manA cDNA and its partial sequence was reported as NCDM-PCR2 (Steenbakkers et al. 2001). NCDM sequences of anaerobic fungi have been placed into 3 groups according to their number and conservation of Cys residues. The ManA NCDM belongs to Group B.
Expression of manA in, and secretion of, the ManA from S. cerevisiae
In contrast with xynA, celA, celB, celC, and axeA cDNAs (Blum et al. 1999; Li et al. 1997a, 1997b), active mannanase was not expressed in E. coli harboring the plasmid containing the full-length manA cDNA. To confirm that the cDNA codes for a mannanase and to study its enzymatic properties, S. cerevisiae was used as an expression host. The region (Fig. 1) coding for the putative signal peptide, CM, and CBM (amino acid residues 1–500) was inserted into pYES2. Yeast transformed with the plasmid produced active mannanase in both cell-associated and extracellular fractions. The yeast cells transformed with the plasmid with and without the manA insertion grew similarly in shake flask cultures (Fig. 4), indicating that the ManA insert had no negative effect on the yeast cells. The production level of the mannanase by the yeast cells was about 1.15 U/mL after 30 h induction by galactose. The amount of ManA was about 6 μg/mL, considering that the specific activity of the enzyme is 179 U/mg (see below). A much higher level (150 μg/mL) was obtained using similar conditions when a T. reesei mannanase gene was expressed in S. cerevisiae (Stålbrand et al. 1995).
Fig. 4.
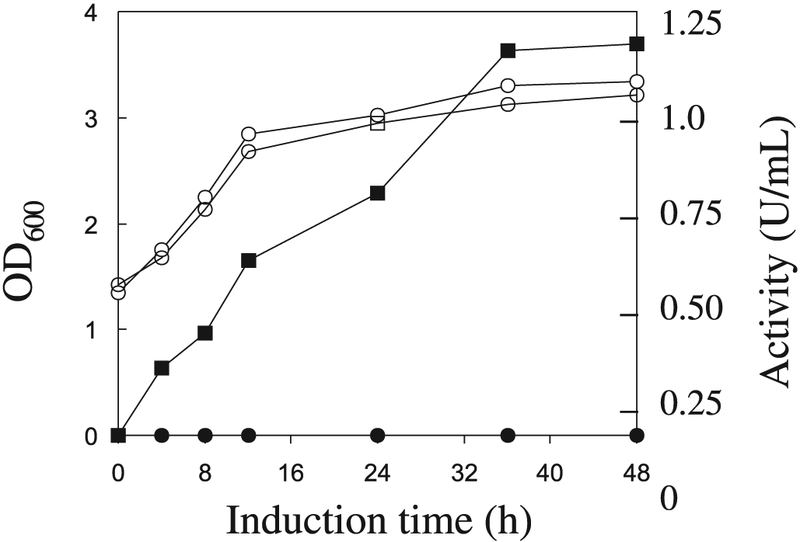
Mannanase production by S. cerevisiae transformed with pManA after galactose induction. An aliquot of an overnight culture grown in DOB medium was used to inoculate raffinose – YPD medium. After growth to an OD600 of 1.0, sterile galactose was added. Samples were withdrawn at time points shown in the figure. Levels of OD600 (open symbols) and extracellular mannanase activity (filled symbols) are shown for the transformants containing pManA and pYES2.
Purification and analysis of the ManA
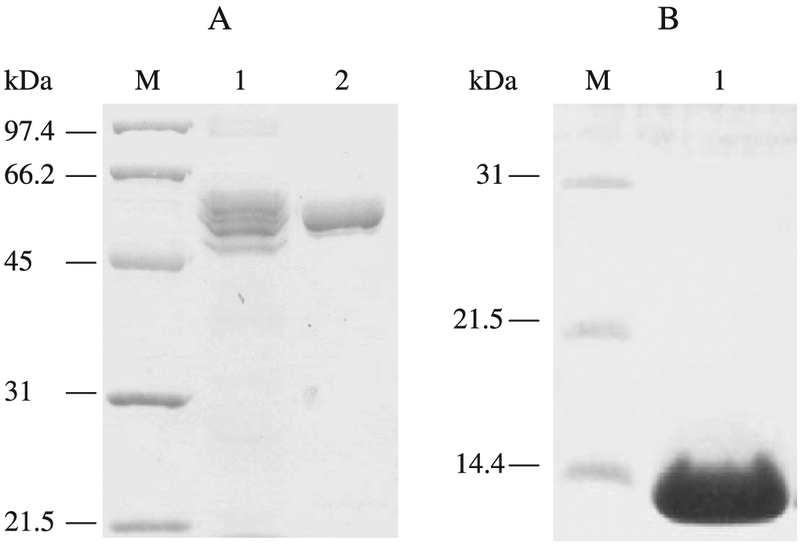
A crude mannanase preparation from the culture medium after 30 h of induction by galactose was concentrated and analyzed by zymogram after separation on SDS–PAGE. Although a major activity band (52 kDa) was revealed, other minor bands from 50 to 70 kDa (data not shown) of activity were also visualized on the gel, suggesting that the secreted mannanase might be glycosylated at various degrees, and (or) various truncated forms of the mannanase were secreted. A total of 3 N-glycosylation sites were found within the whole sequence, and 2 of them are located within the CM region. A total of 3 N-glycosylation sites were found within the whole sequence and 2 of those are within the CM region. Purification of the mannanase using ion exchange and gel filtration chromatography from 2.0 L of culture medium yielded 2.3 mg of purified mannanase that appeared as a single band of 55 kDa on SDS–PAGE gel (Fig. 5A).
Fig. 5.
SDS–PAGE analysis of the Orpinomyces ManA produced in S. cerevisiae and ManA CBM produced in E. coli. Gel was stained with Coomassie Brilliant Blue R-250 and destained before photographs were taken. Lane M, low molecular weight standards (Bio-Rad); Lane 1 of Panel A, 40-μg proteins of yeast culture supernatant; Lane 2 of Panel A, 10 μg ManA purified from yeast culture supernatant; Lane 1 of Panel B; 15 μg ManA CBM purified from E. coli cell lysate.
Properties of the ManA
Among soluble substrates, the enzyme had the highest activity toward locust bean mannan (138.1 U/mg), followed by ivory nut mannan (46.7 U/mg), and oat spelt xylan (23.5 U/mg) (Table 2). It did not hydrolyze barley β-glucan or carboxymethylcellulose. Using various concentrations of locust bean mannan, a Lineweaver–Burk plot of activity levels over substrate concentrations revealed that the Km and Vmax of the enzyme were 3.4 mg/mL and 179.1 U/mg, respectively. With locust bean mannan, the enzyme was active from pH 3.8 to 8.0 at 50 °C. The optimum pH was from 4.6. to 7.2 (Fig. 6). The enzyme was stable at room temperature from pH 3.8 to 9.5 for 24 h. At pH 6.0, the highest activity was around 50 °C when the enzyme was assayed between 20 and 65 °C. The enzyme maintained about 70% of its activity for 8 h when stored at pH 6.0 and 50 °C, but after 1 h at 55 °C, only 50% of the activity remained. At 60 °C, the mannanase activity was lost within 2 min.
Table 2.
Substrate specificity of the recombinant ManA.
| Substrate | Activitya (μmol·min−1·mg−1) |
|---|---|
| Mannan (Locust bean) | 138.1 |
| Mannan (Ivory nut) | 46.7 |
| Xylan (Oat spelt) | 23.5 |
| β-Glucan (Barley) | NDb |
| Carboxymethylcellulose | ND |
Activity was measured at 50 °C in pH 6.0 sodium citrate buffer (50 mmol/L) with a substrate concentration of 1.0% (w/v).
ND, activity level less than 1.0% of that obtained using locust bean mannan.
Fig. 6.
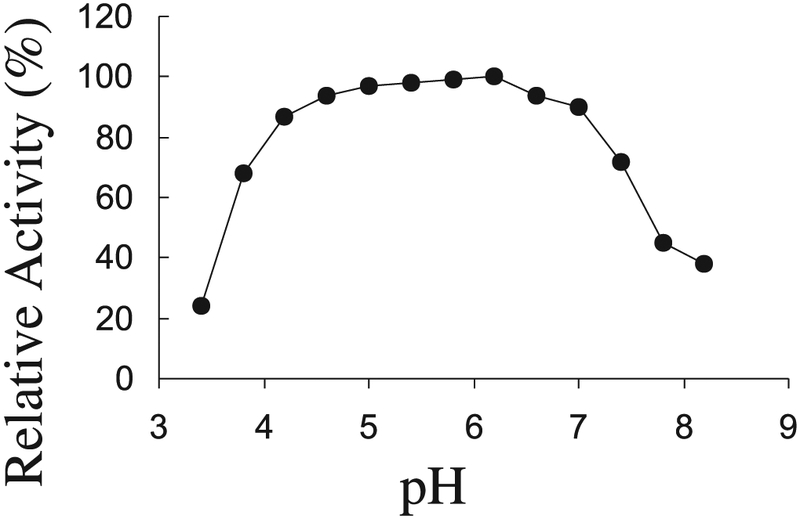
Effect of pH on the activity of ManA assayed at 50 °C. For details see “Materials and methods” section.
The ManA CBM binds to insoluble substrates
The ManA CBM (Fig. 1) was over-expressed in E. coli and purified to homogeneity (Fig. 5B). No metal ions were detected when the purified CBM was analyzed by plasma emission spectroscopy. Among the many polysaccharides used for binding assay, the CBM bound strongly to acid-swollen cellulose, Avicel PH-105, bacterial crystalline cellulose, filter paper, dewaxed cotton, chitin, and insoluble oat spelt xylan, but failed to bind to soluble oat spelt xylan, carboxymethylcellulose, arabinan, pectic galactan, laminarin, or lichenan (Table 3).
Table 3.
Binding of the ManA CBM to polysaccharides.
| Polysaccharide | Liquid state | Relative affinity |
|---|---|---|
| Acid swollen cellulose | Insoluble | +++ |
| Avicel PH-105 | Insoluble | + |
| Bacterial crystalline cellulose | Insoluble | + + |
| Insoluble oat spelt xylana | Insoluble | +++ |
| Soluble oat spelt xylana | Soluble | NB |
| Chitin | Insoluble | +++ |
| Filter paper | Insoluble | + |
| Dewaxed cotton | Insoluble | ++ |
| Carboxymethyl cellulose | Soluble | NB |
| Soluble birchwood xylana | Soluble | NB |
| Arabinan | Soluble | NB |
| Pectic galactan | Soluble | NB |
| Laminarin | Soluble | NB |
| Lichenan | Soluble | NB |
Note: Reaction mixtures contained 10 mg polysaccharide and 18.3 nmol CBM in 0.5 mL sodium citrate buffer (50 mmol/L), pH 6.0. Adsorption scale:
more than 50% adsorbed;
20%−49% adsorbed;
5%-19% adsorbed; NB, less than 5% adsorbed.
Soluble and insoluble xylan preparations were obtained by stirring 2.0% (w/v) xylan suspension for 1 h and separating the 2 fractions by centrifugation at 5000g for 15 min.
Discussion
To isolate genes coding for cellulosomal components of anaerobic fungi, several approaches have been employed. The most straightforward has been screening fungal cDNA expression libraries made in E. coli for various enzymatic activities. This has achieved the isolation of many genes coding for hydrolases of the anaerobic fungi. Many of the enzymes contain NCDMs and they are associated with the cellulosomal complexes produced by the anaerobic fungi (Fannuti et al. 1995; Ljungdahl et al. 1998), whereas some are devoid of the NCDM and therefore act individually. Examples of the former are Neocallimastix patriciarum XylA (Gilbert et al. 1992) and Orpinomyces CelA, CelB, CelC, CelE, and XynA (Li et al. 1997a, 1997b; Chen et al. 1998) and those of the later are Orpinomyces CelF (Chen et al. 2003), LicA (Chen et al. 1997), AxeA (Blum et al. 1999), and BglA (Li et al. 2004). The use of polyclonal antibodies against the Piromyces cellulosomes have been proven useful for the isolation of genes encoding a noncatalytic cellulo-somal component (Freelove et al. 2001). In addition, a PCR-based approach has been developed for the isolation of genes coding for NCDM-containing components (Steenbakkers et al. 2001). With this method, 16 and 19 distinct NCDM sequences were found in Orpinomyces strain PC-2 and Piromyces strain E2, respectively. Of these, 1 from Piromyces and 7 from Orpinomyces were identified with enzymes previously isolated from cDNA expression libraries. The other sequences are presumably part of genes coding for novel cellulosomal components. On the basis of these sequences, we have successfully isolated some full-length cDNA sequences from the Orpinomyces library. They code for 3 new cellulases (unpublished) and the ManA that is reported here.
The CM of ManA from Orpinomyces belongs to family 5 glycoside hydrolases. This family contains diverse enzymes hydrolyzing β-glycosidic bonds of cellulose, mannan, and xylan. The Orpinomyces ManA has the same modular structure as that of T. reesei, except that it has 2 NCDMs after the CBM separated by a linker (Fig. 1). In contrast, the 3 mannanases of the anaerobic fungus Piromyces (Ali et al. 1995; Fannuti et al. 1995; Millward-Sadler et al. 1996)) contain 2 NCDMs and a CM of family 26 glycoside hydrolases. The family 26 mannanases had previously been found only in bacteria. One may propose that the Orpinomyces ManA has a fungal origin, whereas the Piromyces mannanase genes are of bacterial origin. Though there are no more debates regarding being placed as eukaryotes, anaerobic fungi have acquired genes from bacterial sources. Lateral exchanges of genetic resources between fungi and bacteria seem common and are probably due to their close interaction within the rumen and intestinal ecosystems (Ljungdahl et al. 1998). Typical examples of genes common with bacterial sources are those coding for the Orpinomyces LicA (Chen et al. 1997) and the 3 Piromyces mannanases (Fannuti et al. 1995; Millward-Sadler et al. 1996). Genes coding for hydrolases commonly found in fungi are Neocallimastix celA (Denman et al. 1996) and Orpinomyces celF (Chen et al. 2003), as well as manA reported here. The presence of these genes reflects the diversity of the genetic background of hydrolytic enzymes in anaerobic fungi for plant cell wall degradation.
The CBM of Orpinomyces ManA belongs to family 1 (Tomme et al. 1995, http://afmb.cnrs-mrs.fr/~cazy/CAZY/). The solution structures of 2 family 1 CBMs (formerly cellulose-binding domains or CBDs) of T. reesei cellobio-hydrolase I (Kraulis et al. 1989) and endoglucanase I (Mattinen et al. 1998) form flat binding faces with 1 glutamine and 3 aromatic residues for binding to the crystalline cellulose surface. In ManA CBM at the C-terminal end, glutamine and tyrosine residues are aligned, whereas a tyrosine located at the N-terminal end is not aligned with those of the Trichoderma CBMs (Fig. 3). The binding of CBHI CBM is reversible, whereas that of CBHII CBM is irreversible (Carrard and Linder 1995; Linder and Teeri 1996). However, the binding preferences of the ManA CBM reported here are similar to those of both CBHI and CBHII CBMs. Thus, the ManA CBM binds preferably to insoluble polysaccharides that include both cellulose and hemicellulose structures. In fact, the module displays a higher affinity to crystalline cellulose and insoluble xylan than to mannan. This implies that the module within the complex plays a larger role than just helping the hydrolysis of mannan within the wall structures. Rather, it may facilitate the anchorage of the whole complex to the network surface of lignocellulosic structures. It can be noted that the Piromyces noncatalytic protein NCP1 (Freelove et al. 2001) contains 2 novel CBMs belonging to CBM family 29 and a docking module but is devoid of a CM. The CBMs of the NCP1 bind preferably to soluble polysaccharides. CelA of N. patriciarum and CelF of Orpinomyces have family I CBMs. However, they lack docking modules, are not cellulosomal, and act individually in the hydrolysis of plant cell wall constituents (Chen et al. 2003; Denman et al. 1996). Many bacterial cellulosomal catalytic components have CBMs in addition to dockerins (Bayer et al. 1998), but none of them has a family 1 CBM. In terms of fungal cellulosomes, a N. patriciarum xylanase was found to have a CBM and a NCDM (Liu et al. 1999), but the order of the CBM and the NCDM is reversed in comparison with that in ManA reported here.
The Orpinomyces ManA produced by S. cerevisiae hydrolyzes the β-1,4-mannosyl chain of locust bean and ivory nut mannan. It lacks activity against other structurally related polysaccharides, such as cellulose, β-glucan, or xylan, indicating that it is an endo-type mannanase. In contrast, many cellulases display substantial levels of activity against mannan substrates (Biely and Tenkanen 1998). The Vmax of the enzyme on locust been mannan, 179 U/mg, is somewhat higher than that (113 and 103 U/mg) of 2 mannanases purified from T. reesei (Harjunp et al. 1995). A unique feature of the ManA is that it has an extremely broad pH optimum (Fig. 6), and this may prove to be attractive for several industrial applications.
Acknowledgements
Support by grant DE-FG02-93ER20127 for work done at University of Georgia from the U.S. Department of Energy to LGL is gratefully acknowledged.
Footnotes
Names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and the use of the name by USDA implies no approval of the product to the exclusion of others that may also be suitable.
Contributor Information
Eduardo A. Ximenes, Laboratorio De Enzimologia, Departmento De Biologia Celular, Universidade De Brasilia, Asa Norte, Brasilia-DF-Brazil 70910-900, Brazil.
Huizhong Chen, Division of Microbiology, National Center for Toxicological Research, U.S. Food and Drug Administration, 3900 NCTR Road, Jefferson, AR 72079-9502, USA..
Irina A. Kataeva, Department of Biochemistry and Molecular Biology and Center for Biological Resource Recovery, The University of Georgia, Athens, GA 30602-7229, USA.
Michael A. Cotta, Fermentation Biotechnology Research Unit, National Center for Agricultural Utilization Research, USDA2/ARS, 1815 N. University Street, Peoria, IL 61604, USA.
Carlos R. Felix, Laboratorio De Enzimologia, Departmento De Biologia Celular, Universidade De Brasilia, Asa Norte, Brasilia-DF-Brazil 70910-900, Brazil.
Lars G. Ljungdahl, Department of Biochemistry and Molecular Biology and Center for Biological Resource Recovery, The University of Georgia, Athens, GA 30602-7229, USA.
Xin-Liang Li, Fermentation Biotechnology Research Unit, National Center for Agricultural Utilization Research, USDA2/ARS, 1815 N. University Street, Peoria, IL 61604, USA..
References
- Ali BRS, Zhou LQ, Graves FM, Freedman RB, Black GW, Gilbert HJ, and Hazlewood GP 1995. Cellulases and hemi-cellulases of the anaerobic fungus Piromyces constitute a multiprotein cellulose-binding complex and are encoded by multigene families. FEMS Microbiol. Lett 125: 15–22. [DOI] [PubMed] [Google Scholar]
- Bayer EA, Shimon LJ, Shoham Y, and Lamed R 1998. Cellulosomes-structure and ultrastructure. J. Struct. Biol 124: 221–234. [DOI] [PubMed] [Google Scholar]
- Biely P, and Tenkanen M 1998. Enzymology of hemicellulose degradation In Trichoderma and Gliocladium. Vol. 2 Edited by Harman GE and Kubicek CP. Taylor and Francis, London: pp. 25–47. [Google Scholar]
- Blum DL, Li X-L, Chen H, and Ljungdahl LG 1999. Characterization of an acetyl xylan esterase from the anaerobic fungus Orpinomyces sp. strain PC-2. Appl. Environ. Microbiol 65: 3990–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borneman WS, Akin DE, and Ljungdahl LG 1989. Fermentation products and plant cell wall-degrading enzymes produced by monocentric and polycentric anaerobic fungi. Appl. Environ. Microbiol 55: 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrard G, and Linder M 1999. Widely different off rates of two closely related cellulose-binding domains from Trichoderma reesei. Eur. J. Biochem 262: 637–643. [DOI] [PubMed] [Google Scholar]
- Chen H, Li X-L, and Ljungdahl LG 1995. A cyclophilin from the polycentric anaerobic rumen fungus Orpinomyces sp. strain PC-2 is highly homologous to vertebrate cyclophilin B. Proc. Natl. Acad. Sci. U.S.A 92: 2587–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Li X-L, and Ljungdahl LG 1997. Sequencing of an 1,3–1,4-β-D-glucanase (lichenase) from the anaerobic fungus Orpinomyces sp. strain PC-2. Properties of the enzyme expressed in E. coli and evidence that the gene has bacterial origin. J. Bacteriol 179: 6028–6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Li X-L, Blum DL, and Ljungdahl LG 1998. Two genes of the anaerobic fungus Orpinomyces sp. strain PC-2 encoding cellulases with endoglucanase activities may have arisen by gene duplication. FEMS Microbiol. Lett 159: 63–68. [DOI] [PubMed] [Google Scholar]
- Chen H, Li X-L, Blum DL, Ximenes EA, and Ljungdahl LG 2003. CelF of Orpinomyces PC-2 has an intron and encodes a cellulase (CelF), containing a carbohydrate binding module. Appl. Biochem. Biotechnol. 105–108: 775–785. [DOI] [PubMed] [Google Scholar]
- Christgau S, Kauppinen S, Vind J, Kofod LV, and Dalboge H 1994. Expression, cloning, purification and characterization of a β-mannanase from Aspergillus aculeatus. Biochem. Mol. Biol. Int 33: 917–925. [PubMed] [Google Scholar]
- Denman S, Xue GP, and Patel B 1996. Characterization of a Neocallimastix patriciarum cellulase cDNA (celA) homologous to Trichoderma reesei cellobiohydrolase II. Appl. Environ. Microbiol 62: 1889–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G, Steak TE, and Wallach DFH 1971. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry, 10: 2606–2616. [DOI] [PubMed] [Google Scholar]
- Fannuti G, Ponyi T, Black GW, Hazlewood GP, and Gilbert HJ 1995. The conserved non-catalytic 40-residue sequence in cellulases and hemicellulases from anaerobic fungi functions as protein docking domain. J. Biol. Chem 270: 29314–29322. [DOI] [PubMed] [Google Scholar]
- Freelove AC, Bolam DN, White P, Hazlewood GP, and Gilbert HJ 2001. A novel carbohydrate-biding protein is a component of the plant cell wall-degrading complex of Piromyces equi. J. Biol. Chem 276: 43010–43017. [DOI] [PubMed] [Google Scholar]
- Gilbert HJ, Hazlewood GP, Laurie JI, Orpin CG, and Xue GP 1992. Homologous catalytic domains in a rumen fungal xylanase: evidence for gene duplication and prokaryotic origin. Mol. Microbiol 6: 2065–2072. [DOI] [PubMed] [Google Scholar]
- Halstead JR, Vercoe PE, Gilbert HJ, Davidson K, and Hazlewood GP 1999. A family 26 mannanase produced by Clostridium thermocellum as a component of the cellulosome contains a domain which is conserved in mannanases from anaerobic fungi. Microbiology, 145: 3101–3108. [DOI] [PubMed] [Google Scholar]
- Harjunp V, Teleman A, Siika-Aho M, and Drakenberg T 1995. Kinetic and stereochemical studies of mano-oligosaccharide hydrolysis catalyzed by β-mannanases from Trichoderma reesei. Eur. J. Biochem. 234: 278–283. [DOI] [PubMed] [Google Scholar]
- Kataeva IA, Seidel RD III, Li X-L, and Ljungdahl LD 2001. Properties and mutation analysis of the CelK cellulose-binding domain from the Clostridium thermocellum cellulosome. J. Bacteriol 183: 1552–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataeva IA, Seidel RD III, Shah A, West LT, Li X-L, and Ljungdahl LG 2002. The fibronectin type-3-like repeat fromClostridium thermocellum cellobiohydrolase CbhA promotes hydrolysis of cellulose by modifying its surface. Appl. Environ. Microbiol 68: 4292–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraulis PJ, Lore GM, Nilges M, Jones T, Pettersson G, Knowles J, and Gronenborn AM 1989. Determination of the three-dimensional solution structure of the C-terminal domain of cellobiohydrolase I from Trichoderma reesei. A study using nuclear magnetic resonance and hybrid distance geometry-dynamical simulated annealing. Biochemistry, 28: 7241–7257. [DOI] [PubMed] [Google Scholar]
- Laemmli UK 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Li X-L, Chen H, and Ljungdahl LG 1997a. Monocentric and polycentric anaerobic fungi produce structurally related cellulases and xylanases. Appl. Environ. Microbiol 63: 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-L, Chen H, and Ljungdahl LG 1997b. Two cellulases, CelA and CelC, from the polycentric fungus Orpinomyces sp. strain PC-2 contain N-terminal docking domains for a cellulase-hemicellulase complex. Appl. Environ. Microbiol 63: 4721–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-L, Ljungdahl LG, Ximenes EA, Chen H, Felix CR, Cotta MA, and Dien BS 2004. Properties of a recombinant β-glucosidase from the polycentric anaerobic fungus Orpinomyces PC-2 and its application for cellulose hydrolysis. Appl. Biochem. Biotechnol 113: 233–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder M, and Teeri TT 1996. The cellulose-binding domain of the major cellobiohydrolase of Trichoderma reesei exhibits true reversibility and a high exchange rate on crystalline cellulose. Proc. Natl. Acad. Sci. U.S.A 93: 12251–12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J-H, Selinger BL, Tsai C-F, and Cheng K-J 1999. Characterization of a Neocallimastix patriciarum xylanase gene and its product. Can. J. Microbiol 45: 970–974. [DOI] [PubMed] [Google Scholar]
- Ljungdahl LG, Li X-L, and Chen H 1998. Evidence in anaerobic bacteria of transfer of genes between them and from aerobic fungi, bacteria, and animal hosts In Thermophiles: the keys to molecular evolution and the origin of life. Edited by Wiegel J and Adams MW. Taylor and Frances Inc., Philadelphia, PA, U.S.A. pp. 187–197. [Google Scholar]
- Lynd LR, Weimer PJ, van Zyl WH, and Pretorius IS 2002. Microbial cellulose utilization: fundamental and biotechnology. Microbiol. Mol. Biol. Rev 66: 506–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarón R, van Beeuman J, Henrissat B, de la Mata I, and Claeyssens M 1993. Identification of an essential carboxylic group in the active site of endoglucanase III from Trichoderma reesei. FEBS Lett. 316: 137–140. [DOI] [PubMed] [Google Scholar]
- Mattinen M-L, Linder K, Drakenberg T, and Annila A 1998. Solution structure of the cellulose-binding domain of endo-glucanase I from Trichoderma reesei and its interaction with cello-oligosaccharides. Eur. J. Biochem 256: 279–286. [DOI] [PubMed] [Google Scholar]
- Miller GL 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Biochem 31: 426–428. [Google Scholar]
- Millward-Sadler SJ, Hall J, Black GW, Hazlewood GP, and Gilbert HJ 1996. Evidence that Piromyces gene family encoding endo-1,4-mannanases arose through gene duplication. FEMS Microbiol. Lett 141: 183–188. [DOI] [PubMed] [Google Scholar]
- Orpin GC 1975. Studies on the rumen flagellate Neocallimastix frontalis. J. Gen. Microbiol 91: 249–262. [DOI] [PubMed] [Google Scholar]
- Penttilä M, Lechtovaara P, Nevalainen H, Bhikhabhai R, and Knowles J 1986. Homology between cellulase genes of Trichoderma reesei: complete nucleotide sequence of the endoglucanase gene. Gene, 45: 253–263. [DOI] [PubMed] [Google Scholar]
- Saloheimo M, Lechtovovaara P, Penttilä M, Teeri TT, Ståhlberg J, Johansson G, Petterson G, Claeysens M, Tomme P, and Knowles JKC 1988. EGIII, a new endo-glucanase from Trichoderma reesei: the characterization of both gene and enzyme. Gene, 63: 11–21. [DOI] [PubMed] [Google Scholar]
- Saloheimo A, Henrissat B, Hoffren AM, Teleman O, and Penttilå M 1994. A novel, small endoglucanase gene, egl5, from Trichoderma reesei isolated by expression in yeast. Mol. Microbiol 13: 219–228. [DOI] [PubMed] [Google Scholar]
- Saloheimo M, Paloheimo M, Hakola S, Pere J, Swanson B, Nyyssönen E, Bhatia A, Ward M, and Pettlilå M 2000. Swollenin, a Trichoderma reesei protein with sequence similarity to the plant expansins, exhibits disruption activity on cellulosic materials. Eur. J. Biochem 269: 4202–4211. [DOI] [PubMed] [Google Scholar]
- Shoemaker S, Schweickart V, Ladner M, Gelfand D, Kwok S, Myambo K, and Innis M 1983. Molecular cloning of exo-cellobiohydrolase I derived from Trichoderma reesei strain L27. Bio/Technology, 1: 691–699. [Google Scholar]
- Stålbrand H, Saloheimo A, Vehmaanperä J, Henrissat B, and Pentillä M 1995. Cloning and expression in Saccharomyces cerevisiae of a Trichoderma reesei β-mannanase gene containing a cellulose binding domain. Appl. Environ. Microbiol 61: 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbakkers PJ, Li X-L, Ximenes EA, Arts JG, Chen H, Ljungdahl LG, and Op Den Camp HJM 2001. Noncatalytic docking domains of cellulosomes of anaerobic fungi. J. Bacteriol 183: 5325–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunna A, Gibbs MD, Chin CWJ, Nelson PJ, and Bergquist PL 2000. A gene encoding a novel multidomain β-1,4-mannanase from Caldobacillus cellulovorans and action of the recombinant enzyme on kraft pulp. Appl. Environ. Microbiol 66: 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaru Y, and Doi RH 2000. The engl gene cluster of Clostridium cellulovorans contains a gene for cellulosomal ManA. J. Bacteriol 182: 244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CM, Waterman LD, Smith MH, and Thurston CF 2001. The cel4 gene of Agaricus bisporus encodes a β-mannanase. Appl. Environ. Microbiol 67: 2298–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeri TT, Lechtovaara P, Kauppinnen S, Salovuori I, and Knowles JKC 1987. Homologous domains in Trichoderma reesei cellulolytic enzymes: gene sequencing and expression of cellobiohydrolase II. Gene, 51: 43–52. [DOI] [PubMed] [Google Scholar]
- Tomme R, Warren RAJ, Miller RC Jr., Kilburn DG, and Gilkes NR 1995. Cellulose-binding domains: classification and properties In Enzymatic degradation of insoluble carbohydrates. Edited by Saddler JN and Penner MH, American Chemical Society, Washington, D.C. U.S.A. pp. 142–163. [Google Scholar]
- Tomme P, Boraston A, Kormos JF, Warren AJ, and Kilburn DG 2000. Affinity electrophoresis for the identification and characterization of soluble binding by carbohydrate-binding modules. Enzyme Microb. Technol 27: 453–458. [DOI] [PubMed] [Google Scholar]
- van der Giezen M, Rechinger KB, Svendsen I, Durand R, Hirt RP, Fevre M, Embley TM, and Prins RA 1997. A mitochondrial-like targeting signal on the hydrogenosomal malic enzyme from the anaerobic fungus Neocallimastix frontalis: support for the hypothesis that hydrogenosomes are modified mitochondria. Mol. Microbiol 23: 11–21. [DOI] [PubMed] [Google Scholar]
- von Heijne G 1986. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 14: 4683–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagüe E, Mehak-Zunic M, Morgan L, Wood DA, and Thurston CF 1997. Expression of CEL2 and CEL4, two proteins from Agaricus bisporus with similarity to fungal cellobiohydrolase I and β-mannanase, respectively, is regulated by carbon source. Microbiology, 143: 239–244. [DOI] [PubMed] [Google Scholar]