Abstract
Aims/hypothesis
The reasons underlying a greater association of premature mortality with early-onset type 2 diabetes relative to late-onset disease are unclear. We evaluated the clinical characteristics at type 2 diabetes diagnosis and the broad trajectories in cardiometabolic risk factors over the initial years following diagnosis in relation to age at diagnosis.
Methods
Our cohort consisted of 100,606 individuals with newly diagnosed type 2 diabetes enrolled in the Swedish National Diabetes Register from 2002 to 2012. The average follow-up time was 2.8 years. Analyses were performed using a linear mixed-effects model for continuous risk factors and a mixed generalised linear model with a logistic link function for dichotomous risk factors.
Results
The individuals diagnosed at the youngest age (18–44 years) were more often male and had the highest BMI (mean of 33.4 kg/m2) at diagnosis and during follow-up compared with all other groups (those diagnosed at 45–59 years, 60–74 years and ≥75 years; p < 0.05), being ~5 kg/m2 higher than the oldest group. Although HbA1c patterns were similar between all age groups, there was a difference of about 5 mmol/mol (0.45%) between the two groups at 8 years post-diagnosis (p < 0.05). Additionally, individuals diagnosed younger had ~0.7 mmol/l higher triacylglycerol, and ~0.2 mmol/l lower HDL-cholesterol levels at diagnosis relative to the oldest group. Such differences continued for several years post diagnosis. Yet, although more of these younger individuals were receiving oral glucose-lowering agents, other cardioprotective therapies were prescribed less often in this group. Differences in BMI, blood glucose and lipid levels remained with adjustment for potential confounders, including marital status, education and country of birth, and, where relevant, differential treatments by age, and in those with at least 5 years of follow-up.
Conclusions/interpretation
Individuals who develop type 2 diabetes at a younger age are more frequently obese, display a more adverse lipid profile, have higher HbA1c and a faster deterioration in glycaemic control compared with individuals who develop diabetes later in life. These differences largely remain for several years after diagnosis and support the notion that early-onset type 2 diabetes may be a more pathogenic condition than late-onset disease.
Electronic supplementary material
The online version of this article (10.1007/s00125-017-4532-8) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Keywords: Age group, Blood glucose, BMI, Cardiometabolic risk, Cardiovascular disease, Lipids, Premature mortality, Type 2 diabetes

Introduction
The prevalence of diabetes mellitus has been increasing during the past few decades with rapid rises in incidence in the 1990s and early 2000s [1, 2]. Type 2 diabetes is now more frequently diagnosed in young adults and adolescents [3–5]. The obvious reason for this is increasing obesity among young people. In the 1990s, obesity increased by 70% in people aged 18–29 years in the USA and, in the same decade, type 2 diabetes also increased by 70% in people aged 30–39 years [2, 6]. Consequently, in recent years, type 2 diabetes has sometimes been categorised as early-onset diabetes, i.e. people diagnosed before the age of 45, and later-onset (or usual-onset), i.e. people diagnosed ≥45 years of age [7].
Cardiovascular disease (CVD) is a major cause of mortality in individuals with type 2 diabetes [8, 9]. From a cardiovascular perspective, early-onset type 2 diabetes appears to be more aggressive than later-onset disease. For example, when younger people are diagnosed with type 2 diabetes they often already have several risk factors for developing CVD [10–12]. Our research group recently confirmed that younger age at type 2 diabetes diagnosis and poor glycaemic control were individually correlated with excess risk of death from any cause and from CVD [13]. Furthermore, others have shown that early-onset type 2 diabetes is more strongly pathogenic than type 1 diabetes [14]. However, the reasons behind the higher risk of complications in early-onset type 2 diabetes are not fully understood.
Whilst there are some data to suggest that obesity and differential changes in blood glucose may be operating in early-onset type 2 diabetes, a comprehensive study examining major CVD risk factors according to age at diagnosis, as well as examining subsequent trends in risk factor control, is lacking. Therefore, the aims of this study were to evaluate, in relation to age at diagnosis: (1) clinical characteristics at diagnosis; (2) trajectories (time trends) in CVD risk factors over the initial years following type 2 diabetes diagnosis; and (3) whether any apparent differences were inter-related (e.g. BMI vs lipids) or could be easily explained by differential treatments (e.g. statins and glucose-lowering drugs).
Methods
The primary aim of this observational study was to describe how CVD risk factors change over time from diagnosis of type 2 diabetes depending on age at diagnosis in using data from the Swedish National Diabetes Register (NDR). The NDR was launched in 1996 as a nationwide clinical quality register that aims to support and monitor improvements in the quality of diabetes care. It now contains clinical data on about 90% of individuals aged ≥18 years with diabetes (type 1 and type 2) in Sweden. Before inclusion into the NDR informed consent has to be provided by each participant [15, 16]. Ethical approval to conduct this study was acquired from the Regional Ethical Review Board in Gothenburg, Sweden.
Study population
Individuals with newly diagnosed type 2 diabetes who had at least one listing in the NDR between 1 January 2002 and 31 December 2012 were identified. Type 2 diabetes was defined on the basis of epidemiological data: individuals receiving dietary treatment only, oral glucose-lowering agents only, or those diagnosed at ≥40 years of age receiving insulin therapy or insulin and oral glucose-lowering agents. In Sweden, the WHO diagnostic criteria are used to diagnose diabetes [17]. All individuals who did not have information on age at diagnosis of diabetes were excluded. We assessed potential misclassification bias by comparing the concordance between the epidemiological definitions of type 2 diabetes with the clinician’s classification of diabetes type (which is also available in the NDR); we confirmed that 97% of our cohort were also classified as type 2 diabetes by their clinician.
Newly diagnosed individuals in each age category (age at type 2 diabetes diagnosis) were followed until death or end of follow-up on 31 December 2012. The median follow-up was 2.22 years (interquartile range, 0.80–4.31 years; maximum, 10.9 years; mean follow-up was 2.8 years). Thus, we studied individuals who were enrolled in the NDR in the same year that they were diagnosed and we followed them for up to 10 years. Reporting into the NDR is performed approximately annually, which results in multiple measurements being available for each participant. Our final cohort consisted of 100,606 individuals contributing 679,420 observations. We categorised the cohort into the following four age groups (age at type 2 diabetes diagnosis was equal to actual age owing to the inclusion criteria): 18–44 years, 45–59 years, 60–74 years and ≥75 years.
Details on variables
Glycaemic control was estimated based on HbA1c measurements, which were calibrated nationwide with the HPLC Mono-S method and converted into mmol/mol according to the International Federation of Clinical Chemistry [18]. Systolic BP (SBP) and diastolic BP (DBP) were measured in mmHg. BMI was calculated as weight (kg) divided by the height squared (m2). Blood lipid profile was estimated based on LDL-cholesterol, HDL-cholesterol, triacylglycerols and total cholesterol measurements, all measured in mmol/l. Serum creatinine was measured in μmol/l and eGFR was carried out by the Modification of Diet in Renal Disease (MDRD) study equation.
Microalbuminuria was defined as two positive results for three samples obtained within 1 year of each other, with an albumin/creatinine ratio of 3–30 mg/mmol or a urinary albumin clearance of 20–200 μg/min. All variables were assessed from 2002 to 2012. Initial medication data were obtained within 3 months of diagnosis.
Statistical analysis
Continuous dependent variables (e.g. SBP) were analysed using a mixed-effects model that incorporated a random participant effect, fixed effects of age group, time, and a time by age group interaction where time was a categorical variable. This allowed for separate estimates of time trends for each age group. HbA1c observations were analysed starting 1 year after diagnosis to avoid the rapid drop in HbA1c commonly seen during the first year. The models contained adjustment factors, such as sex, BMI, smoking and concomitant treatment subject to convergence, as well as, where relevant, socioeconomic (marital status and education) measures and country of birth (categorised as in Table 1). Complete information on other adjustment variables is available in the ESM Table 1. The mixed-repeated-measures model handles missing data under the assumption of missing at random without relying on crude imputation techniques, such as last value carried forward, and is therefore less prone to bias.
Table 1.
Baseline descriptive statistics stratified by age groups.
| Variable | Missing data, n (%) | Overall | 18–44 years of age | 45–59 years of age | 60–74 years of age | ≥75 years of age |
|---|---|---|---|---|---|---|
| n | 100,606 | 8642 | 30,907 | 45,269 | 15,788 | |
| Follow-up time (years) | 2.8 (2.5) | 2.5 (2.5) | 2.9 (2.6) | 2.9 (2.5) | 2.7 (2.3) | |
| Sex, women | 0 (0.0) | 44,925 (44.7) | 3442 (39.8) | 11,695 (37.8) | 19,865 (43.9) | 9923 (62.9) |
| Age (years) | 0 (0.0) | 62.0 (12.3) | 38.3 (5.6) | 53.1 (4.2) | 66.2 (4.1) | 80.4 (4.4) |
| HbA1c | 8208 (8.2) | |||||
| mmol/mol | 49.2 (11.0) | 51.5 (14.6) | 50.4 (12.3) | 48.2 (9.7) | 48.5 (9.4) | |
| % | 6.7 (1.0) | 6.9 (1.3) | 6.8 (1.1) | 6.6 (0.9) | 6.6 (0.9) | |
| >53 mmol/mol | 16, 462 (17.6) | 1782 (32.4) | 6001 (28.0) | 6360 (20.0) | 2319 (20.8) | |
| BMI (kg/m2) | 25,701 (25.5) | 30.6 (5.7) | 33.4 (7.3) | 31.5 (5.8) | 30.2 (5.2) | 28.3 (4.7) |
| BMI >30 kg/m2 | 25,701 (25.5) | 36,437 (48.6) | 4131 (65.3) | 12,964 (55.7) | 15,753 (46.3) | 3589 (31.9) |
| SBP (mmHg) | 18,480 (18.4) | 137.3 (17.3) | 128.4 (15.3) | 134.9 (16.5) | 139.1 (16.9) | 141.6 (18.3) |
| Systolic hypertension (>140 mmHg) | 18,480 (18.4) | 26,766 (32.6) | 982 (14.8) | 6737 (26.9) | 13,502 (36.2) | 5545 (42.0) |
| DBP (mmHg) | 18,480 (18.4) | 79.7 (10.0) | 80.5 (10.5) | 82.0 (9.9) | 79.4 (9.6) | 75.6 (9.8) |
| Diastolic hypertension (>90 mmHg) | 18,480 (18.4) | 7918 (9.6) | 809 (12.2) | 3494 (14.0) | 3109 (8.3) | 506 (3.8) |
| Total cholesterol (mmol/l) | 33,648 (33.4) | 5.3 (1.2) | 5.3 (1.2) | 5.4 (1.2) | 5.2 (1.1) | 5.1 (1.1) |
| Hypercholesterolaemia (>6.2 mmol/l) | 33,648 (33.4) | 12,208 (18.2) | 939 (17.7) | 4401 (20.8) | 5519 (17.6) | 1349 (14.8) |
| LDL-cholesterol (mmol/l) | 42,691 (42.4) | 3.1 (1.0) | 3.2 (1.0) | 3.2 (1.0) | 3.1 (1.0) | 3.0 (1.0) |
| HDL-cholesterol (mmol/l) | 40,893 (40.6) | 1.2 (0.4) | 1.1 (0.3) | 1.2 (0.4) | 1.3 (0.4) | 1.3 (0.4) |
| Triacylglycerol (mmol/l) | 40,037 (39.8) | 2.0 (1.4) | 2.4 (1.8) | 2.2 (1.6) | 1.9 (1.2) | 1.7 (0.9) |
| Microalbuminuriaa | 51,166 (50.9) | 6110 (12.4) | 492 (11.6) | 1820 (11.6) | 2732 (12.2) | 1066 (15.1) |
| eGFR (ml/min) | 22,951 (22.8) | 84.8 (25.2) | 105.8 (26.9) | 93.5 (25.3) | 81.8 (21.1) | 67.0 (19.9) |
| Smoker | 22,205 (22.1) | 14,257 (18.2) | 1540 (24.1) | 6117 (25.2) | 5875 (16.5) | 725 (5.9) |
| Medication useb | ||||||
| Antihypertensive agents | 7845 (7.8) | 57,686 (62.2) | 1801 (23.1) | 14,499 (51.2) | 29,630 (70.5) | 11,756 (80.4) |
| Statins | 7927 (7.9) | 35,582 (38.4) | 1285 (16.3) | 9575 (33.6) | 19,029 (45.4) | 5693 (39.5) |
| No medication | 0 (0.0) | 52,491 (52.2) | 3824 (44.2) | 14,094 (45.6) | 24,533 (54.2) | 10,040 (63.6) |
| Oral glucose-lowering agents | 0 (0.0) | 38,582 (38.3) | 3973 (46.0) | 13,197 (42.7) | 16,894 (37.3) | 4518 (28.6) |
| Insulin | 0 (0.0) | 5589 (5.6) | 525 (6.1) | 2031 (6.6) | 2194 (4.8) | 839 (5.3) |
| Insulin + oral glucose-lowering agents | 0 (0.0) | 3944 (3.9) | 320 (3.7) | 1585 (5.1) | 1648 (3.6) | 391 (2.5) |
| Marital status | 448 (0.4) | |||||
| Single | 18,283 (18.3) | 4099 (47.5) | 8155 (26.5) | 5212 (11.6) | 817 (5.2) | |
| Married | 53,276 (53.2) | 3587 (41.6) | 15,933 (51.7) | 26,678 (59.2) | 7078 (45.3) | |
| Separated | 17,917 (17.9) | 910 (10.5) | 6194 (20.1) | 9042 (20.1) | 1771 (11.3) | |
| Widowed | 10,682 (10.7) | 35 (0.4) | 549 (1.8) | 4152 (9.2) | 5946 (38.1) | |
| Country of birth | 0 (0.0) | |||||
| Sweden | 81,545 (81.1) | 5839 (67.6) | 23,267 (75.3) | 38,412 (84.9) | 14,027 (88.8) | |
| Europe (excl. Sweden) | 10,612 (10.5) | 710 (8.2) | 3441 (11.1) | 5047 (11.1) | 1414 (9.0) | |
| Rest of world | 8449 (8.4) | 2093 (24.2) | 4199 (13.6) | 1810 (4.0) | 347 (2.2) | |
| Education | 1881 (1.9) | |||||
| Elementary school | 36,743 (37.2) | 1881 (22.2) | 8099 (26.5) | 17,687 (39.8) | 9076 (59.6) | |
| College level | 44,029 (44.6) | 4719 (55.8) | 15,862 (51.9) | 18,939 (42.6) | 4509 (29.6) | |
| Upper/secondary school | 17,953 (18.2) | 1858 (22.0) | 6617 (21.6) | 7827 (17.6) | 1651 (10.8) |
All data are based on the first observation after diagnosis, except for HbA1c where the first observation 1 year after diagnosis was used
All continuous variables are represented as mean (SD). Dichotomous variables are represented as number of individuals (%).
aMicroalbuminuria was defined as two positive results for three samples obtained within 1 year of each other, with an albumin/creatinine ratio of 3–30 mg/mmol or a urinary albumin clearance of 20–200 μg/min
bInitial medication data were obtained within 3 months of diagnosis
The graphical representation of the data is based on estimated and observed yearly averages with 95% CI for up to 8 years after diagnosis. Sensitivity analyses, adjusted for confounders, were conducted for individuals with at least 5 years of follow-up (ESM Fig. 2a–e), which included adjustments for the confounders shown in ESM Table 1. Further sensitivity analyses were additionally adjusted for marital status, education and country of birth.
A p value <0.05 was considered to be statistically significant and, since no adjustment for multiple comparisons has been made, the interpretations should focus on the overall patterns rather than the outcomes of single hypothesis tests. All statistical analyses were conducted with SAS (version 9.4; www.sas.com/en_gb/software/sas9.html, accessed 10 November 2017) and R (version 3.2.3) (https://www.rstudio.com/products/rstudio/download/; https://cran.hafro.is/; both accessed 10 November 2017).
Results
Baseline characteristics and risk factor differences
Table 1 shows baseline characteristics in the overall cohort stratified by age group. Baseline was defined as the participant’s first observation in the NDR at year of diagnosis. Women represented 44.7% of the total cohort. The mean age (years) in each age group was 38.3, 53.1, 66.2 and 80.4. At diagnosis, the youngest group contained proportionately more men, had a higher mean BMI by about 5 kg/m2 and a higher mean HbA1c of ~3 mmol/mol (0.27%) compared with the oldest group. The youngest group also had the highest triacylglycerol and the lowest HDL-cholesterol levels of all the groups. However, this group had the lowest SBP and one of the highest DBP compared with the other age groups. In terms of treatments, statin use was lowest in youngest age group, being almost three times lower than rates of use in the 60–74 years age group. In contrast, oral glucose-lowering agent use was higher in the youngest group at 46% vs 29% in the oldest group. In addition, antihypertensive agent use was considerably lower in the youngest group at 23% vs 80% in the oldest group. Considerably more of the younger cohort were from countries outside Europe. Men had higher HbA1c levels at diagnosis compared with women, but women had higher BMI (ESM Table 2).
Risk factor trajectories
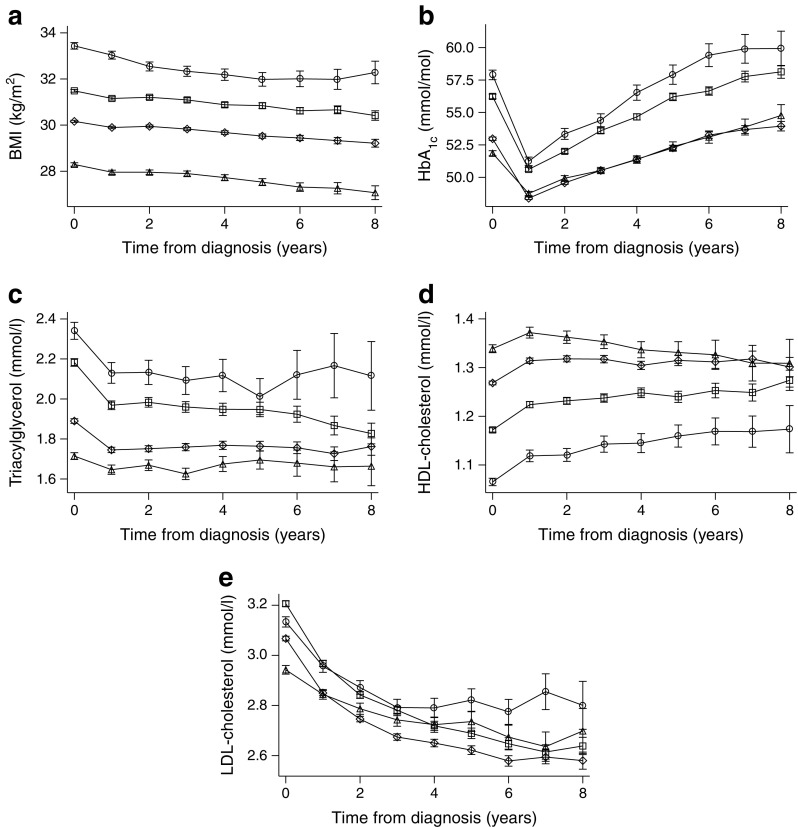
The trajectories of cardiometabolic risk factors over time by age at type 2 diabetes diagnosis are presented in Fig. 1. The significantly (p < 0.05) higher BMI level observed in the youngest age group at diagnosis was sustained over time relative to the other groups (Fig. 1a). A widening and significant (p < 0.05) difference in HbA1c was observed over time, with a difference of ~5 mmol/mol (0.45%) at 8 years between the youngest vs two oldest age groups (Fig. 1b).
Fig. 1.
Yearly averages (95% CI) stratified by age group for (a) BMI, (b) HbA1c, (c) triacylglycerol, (d) HDL-cholesterol and (e) LDL-cholesterol. White circles, 18–44 years old; plus sign (+), 45–59 years old; crosses (×), 60–74 years old; white triangles, ≥75 years old. All analyses, p < 0.05 where 95% CI do not overlap
Lipid levels remained significantly (p < 0.05) different between age groups, with the youngest group showing continually higher triacylglycerol levels (by ~0.5–0.6 mmol/l) compared with the two oldest age groups, and lower HDL-cholesterol levels compared with all other age groups (although this latter difference narrowed over time) (Fig. 1c,d). The differences in LDL-cholesterol levels between age groups widened over time; even though levels in all groups declined this widening probably reflects more frequent use of statins in the older age groups (Fig. 1e).
In summary, individuals that developed type 2 diabetes at a younger age had greater aggregated exposure (i.e. AUC) to higher BMI, higher blood glucose levels and abnormal lipids compared with those who developed diabetes at an older age.
Other risk factors (including BP, renal function, total cholesterol and albuminuria) also changed over time (ESM Fig. 1a–e), but most were as expected. The use of statins and antihypertensive agents was lowest in the youngest age group at diagnosis and this was sustained, at least in the early years following diagnosis (ESM Fig. 1f,g). Finally, number of current smokers was highest in the youngest age group and this did not change over time (ESM Fig. 1h).
Sensitivity analyses in individuals with at least 5 years follow-up
When we restricted analyses to only include individuals with at least 5 years follow-up and adjusted for key confounders for each of the main risk factors as depicted in ESM Table 1, the findings were broadly similar to those reported for the entire cohort with unadjusted analyses (see ESM Fig. 2a–e). For example, the trajectory of blood glucose levels after the first year was significantly (p < 0.05) greater in the youngest group relative to the older groups, leading to the highest blood glucose levels observed in this group at year 6, despite adjustment for diabetes medication and BMI. Likewise, differences in lipid abnormalities persisted despite adjustment for sex, BMI and lipid-lowering therapy. Further analyses adjusting for marital status, education and country of birth did not materially change the pattern of results (ESM Fig. 3a–e).
Discussion
To the best of our knowledge, this study of 100,606 individuals with newly diagnosed type 2 diabetes is the largest and perhaps the first to compare in detail trajectories on several cardiometabolic risk factors between individuals with type 2 diabetes grouped according to age of diagnosis in a European (white) population, complementing and extending data from a recent study in China [19]. The results from our study show that age at diagnosis of type 2 diabetes is an important aspect of risk factor control with regard to adiposity, blood glucose and lipid levels; these factors were all worse in individuals diagnosed at a younger age and throughout the early years following diagnosis, during which the use of glucose-lowering medication was higher in this group relative to older individuals. Moreover, such differences appear not to be accounted for by obvious confounders including differential drug therapy rates, sex, smoking and, where relevant, BMI differences by age group. The results were also maintained when we adjusted for socioeconomic variables and, in particular, country of birth. Therefore, our findings support the notion that early-onset type 2 diabetes may be a more pathogenic condition per se than later-onset type 2 diabetes, helping to explain the observation of greater life-years lost when diabetes is diagnosed at a younger age, as recently reported [20, 21].
Our data on the relationship between age at diagnosis and cardiometabolic risk factors meaningfully extend previous studies [22, 23] as it is contemporary and multiple-fold larger in terms of size. Our results also hold potential clinical relevance. With respect to hyperglycaemia, despite a higher rate of glucose-lowering agent use in younger (i.e. ≤45 years of age) compared with older individuals (Table 1), younger individuals had higher HbA1c levels and worse trajectories over time, so their aggregated exposure to hyperglycaemia is worse from the point of diagnosis (and potentially prior to diagnosis if diagnosed later). Therefore, total exposure to hyperglycaemia is clearly higher in younger individuals and, since hyperglycaemia is a strong and independent predictor for microvascular complications and has long-term consequences for CVD in individuals with type 2 diabetes [13, 24, 25], the risk of complications driven in part by hyperglycaemia should be higher in this group. Younger individuals may experience delayed diabetes diagnoses owing to a lower likelihood of opportunistic screening, but the poorer HbA1c trajectories observed in this group despite similar or greater prescription rates for glucose-lowering medications suggest that undertreatment is unlikely to be the only reason for poor glycaemic control over time. Clearly, younger individuals are more frequently obese and their aggregated BMI over time is higher than in older individuals, which is possibly related to higher blood glucose levels over time; however, our sensitivity analyses suggest that blood glucose levels remain higher in the younger group even with adjustment for BMI (ESM Fig. 2b) and with adjustment for other factors including country of birth (ESM Fig. 3b). Of course, genetic factors may also be of greater importance for early-onset type 2 diabetes.
Dyslipidaemia, characterised by low HDL-cholesterol and elevated triacylglycerol levels, is a risk factor for CVD [25]. In this study, younger individuals had lower HDL-cholesterol levels and higher triacylglycerol levels at diagnosis, in accordance with most previous studies [22, 23, 26]; although Hillier et al, in a much smaller study, did not observe a difference in triacylglycerol levels between individuals with early-onset and later-onset type 2 diabetes [23]. Whilst recent evidence indicates that higher triacylglycerol levels are causally linked to CVD [27], other strong evidence indicates earlier hyperlipidaemia (as measured by either LDL-cholesterol or non-HDL-cholesterol—also higher in younger individuals in the present study) is associated with long-term harm [28]. These collective findings broadly concur with greater obesity in younger individuals, although, once again, lipid differences remained with adjustments for BMI and lipid-lowering therapy use, which was lower in younger individuals in the early years following diagnosis and did not catch up with older groups for at least several years. This is notable since these younger individuals may have more to profit from preventive treatments, such as statins, in terms of gain of life-years free from CVD events [29].
The poorer BMI, HbA1c and lipid profiles observed in the younger individuals helps to explain why early-onset type 2 diabetes may confer a very high lifetime risk of CVD and premature death compared with later-onset disease [13, 19, 30]. It is clear that the relative hazard of developing CVD is much greater in individuals with early-onset than later-onset disease, compared with age-matched individuals without diabetes [7]. There have been increasing calls [29, 31] for earlier treatment of cardiometabolic risk factors for individuals with high lifetime risks of CVD to enhance survival benefits; younger individuals with type 2 diabetes fit these criteria well.
The strength of this study is that it is a nationwide study which included virtually all individuals with newly diagnosed type 2 diabetes in Sweden within the study period, and is thus representative of the general diabetes population. This is in contrast to smaller observational studies. However, we fully accept some, often inevitable, limitations. For example, our study only included Swedish nationals; whilst our results should be relevant to many high-income countries with high proportions of individuals of European descent, our work needs to be replicated in other ethnic groups. We also recognise that the overall mean length of follow-up was modest, but note that the same trends were observed in individuals who had at least 5 years of follow-up (ESM Fig. 2a–e). In addition, we cannot rule out the probability of missing BMI values from individuals with normal or close to normal BMI because these individuals may be less likely to have their BMI recorded, which could lead to a potential bias that would in fact underestimate, rather than overestimate, diagnosis-related BMI differentials by age, as well as changes in BMI over time. However, our BMI data fit well with recent findings in a Scottish diabetes dataset, lending some external validity [32]. Finally, we acknowledge that higher HbA1c levels at diagnosis in younger individuals is due, in part, to a greater delay in diagnosis compared with older individuals, perhaps because younger individuals are less likely to be captured by opportunistic screening. However, blood glucose levels remained higher over time, even after diagnosis, in those under 45 years of age (despite more of this group being on glucose-lowering therapy), potentially suggesting some innate contribution to higher risk blood glucose profiles.
In summary, individuals with early-onset type 2 diabetes are more obese at diagnosis and for several years afterwards, have higher blood glucose levels, and a faster deterioration of glycaemic control following 1 year of diagnosis compared with individuals diagnosed at an older age. They also have more atherogenic lipid profiles. Since such differences remained with adjustment for obvious confounders, including, where relevant, glucose- or lipid-lowering treatments and country of birth, our results support the notion that early-onset type 2 diabetes is a more pathogenic condition than later-onset disease. Irrespective of the reasons for worse adiposity, lipid and glycaemic risk factor profiles in the younger age group, these real-life data suggest a need for more aggressive management of type 2 diabetes in younger individuals to lessen life-years lost in this high-risk group.
Electronic supplementary material
(PDF 940 kb)
Acknowledgements
Some preliminary versions of the analyses conducted with more age groups were presented in A.O. Steinarsson’s BSc thesis. We thank all the data-collecting clinicians and staff at the Swedish National Diabetes Register. We thank Liz Coyle for her excellent technical assistance in the preparation of this article.
Abbreviations
- CVD
Cardiovascular disease
- DBP
Diastolic BP
- NDR
The Swedish National Diabetes Register
- SBP
Systolic BP
Author contributions
All authors contributed to study concept. AOS conducted the research and wrote the manuscript. AR contributed to the statistical analysis and the discussion, and reviewed/edited the manuscript. SG contributed to the discussion and reviewed/edited the manuscript. SF led the statistical analysis and contributed to the writing of the paper. AMS conducted the research and contributed to the discussion and writing of the paper. NS reviewed/edited the manuscript and supervised the revisions. All authors gave final approval of the version to be published. NS is the guarantor of this work.
Funding
This work was supported by grants from the Swedish Association of Local Authorities and Regions and by Region Västra Götaland in Sweden.
Data availability
The data that support the findings of this study are available from the corresponding author in anonymised form upon reasonable request.
Duality of interest
NS has consulted for Boehringer Ingelheim, Novo Nordisk, Janssen and Eli Lilly, and received grant support from AstraZeneca. All other authors declare no conflict of interest associated with this study.
Contributor Information
Soffia Gudbjörnsdottir, Email: soffia.gudbjornsdottir@medic.gu.se.
Naveed Sattar, Email: naveed.sattar@glasgow.ac.uk.
References
- 1.González ELM, Johansson S, Wallander M-A, Rodríguez LAG. Trends in the prevalence and incidence of diabetes in the UK: 1996-2005. J Epidemiol Community Health. 2009;63:332–336. doi: 10.1136/jech.2008.080382. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, et al. Diabetes trends in the U.S.: 1990-1998. Diabetes Care. 2000;23:1278–1283. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 3.Alberti G, Zimmet P, Shaw J, et al. Type 2 diabetes in the young: the evolving epidemic: the International Diabetes Federation Consensus Workshop. Diabetes Care. 2004;27:1798–1811. doi: 10.2337/diacare.27.7.1798. [DOI] [PubMed] [Google Scholar]
- 4.Koopman RJ, Mainous AG, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med. 2005;3:60–63. doi: 10.1370/afm.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grinstein G, Muzumdar R, Aponte L, et al. Presentation and 5-year follow-up of type 2 diabetes mellitus in African-American and Caribbean-Hispanic adolescents. Horm Res. 2003;60:121–126. doi: 10.1159/000072523. [DOI] [PubMed] [Google Scholar]
- 6.Mokdad AH, Serdula MK, Dietz WH, et al. The spread of the obesity epidemic in the United States, 1991-1998. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 7.Hillier TA, Pedula KL. Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care. 2003;26:2999–3005. doi: 10.2337/diacare.26.11.2999. [DOI] [PubMed] [Google Scholar]
- 8.Gordon-Dseagu VLZ, Shelton N, Mindell J. Diabetes mellitus and mortality from all-causes, cancer, cardiovascular and respiratory disease: evidence from the Health Survey for England and Scottish Health Survey cohorts. J Diabetes Complicat. 2014;28:791–797. doi: 10.1016/j.jdiacomp.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Jansson SPO, Andersson DKG, Svärdsudd K. Mortality and cardiovascular disease outcomes among 740 patients with new-onset type 2 diabetes detected by screening or clinically diagnosed in general practice. Diabet Med. 2016;33:324–331. doi: 10.1111/dme.13019. [DOI] [PubMed] [Google Scholar]
- 10.Bell RA, Mayer-Davis EJ, Beyer JW, et al. Diabetes in non-Hispanic white youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(Suppl 2):S102–S111. doi: 10.2337/dc09-S202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29:1300–1306. doi: 10.2337/dc05-2470. [DOI] [PubMed] [Google Scholar]
- 12.Zeitler P, Hirst K, Pyle L, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tancredi M, Rosengren A, Svensson A-M, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373:1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 14.Wong J, Constantino M, Yue DK. Morbidity and mortality in young-onset type 2 diabetes in comparison to type 1 diabetes: where are we now? Curr Diab Rep. 2015;15:566. doi: 10.1007/s11892-014-0566-1. [DOI] [PubMed] [Google Scholar]
- 15.Eliasson B, Gudbjörnsdottir S. Diabetes care – improvement through measurement. Diabetes Res Clin Pract. 2014;106:S291–S294. doi: 10.1016/S0168-8227(14)70732-6. [DOI] [PubMed] [Google Scholar]
- 16.Rawshani A, Landin-Olsson M, Svensson AM, et al. The incidence of diabetes among 0-34 year olds in Sweden: new data and better methods. Diabetologia. 2014;57:1375–1381. doi: 10.1007/s00125-014-3225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO (2006) Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Available at: www.who.int/diabetes/publications/diagnosis_diabetes2006/en/. Accessed 11 Oct 2017
- 18.Hoelzel W, Weykamp C, Jeppsson JO, et al. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004;50:166–174. doi: 10.1373/clinchem.2003.024802. [DOI] [PubMed] [Google Scholar]
- 19.Huo X, Gao L, Guo L, et al. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol. 2016;4:115–124. doi: 10.1016/S2213-8587(15)00508-2. [DOI] [PubMed] [Google Scholar]
- 20.Wright AK, Kontopantelis E, Emsley R, et al. Life expectancy and cause-specific mortality in type 2 diabetes: a population-based cohort study quantifying relationships in ethnic subgroups. Diabetes Care. 2017;40:338–345. doi: 10.2337/dc16-1616. [DOI] [PubMed] [Google Scholar]
- 21.Emerging Risk Factors Collaboration. SRK S, Kaptoge S, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatunic M, Burns N, Finucane F, et al. Contrasting clinical and cardiovascular risk status between early and later onset type 2 diabetes. Diab Vasc Dis Res. 2005;2:73–75. doi: 10.3132/dvdr.2005.012. [DOI] [PubMed] [Google Scholar]
- 23.Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care. 2001;24:1522–1527. doi: 10.2337/diacare.24.9.1522. [DOI] [PubMed] [Google Scholar]
- 24.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niskanen L, Turpeinen A, Penttilä I, Uusitupa MIJ. Hyperglycemia and compositional lipoprotein abnormalities as predictors of cardiovascular mortality in type 2 diabetes: a 15-year follow-up from the time of diagnosis. Diabetes Care. 1998;21:1861–1869. doi: 10.2337/diacare.21.11.1861. [DOI] [PubMed] [Google Scholar]
- 26.Aguilar-Salinas CA, Rojas R, Gómez-Pérez FJ, et al. Prevalence and characteristics of early-onset type 2 diabetes in Mexico. Am J Med. 2002;113:569–574. doi: 10.1016/S0002-9343(02)01314-1. [DOI] [PubMed] [Google Scholar]
- 27.Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration. Sarwar N, Sandhu MS, et al. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634–1639. doi: 10.1016/S0140-6736(10)60545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navar-Boggan AM, Peterson ED, D’Agostino RB, et al. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation. 2015;131:451–458. doi: 10.1161/CIRCULATIONAHA.114.012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.JBS3 Board (2014) Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart 100:ii1-ii67 [DOI] [PubMed]
- 30.Song SH, Hardisty CA. Early onset type 2 diabetes mellitus: a harbinger for complications in later years – clinical observation from a secondary care cohort. QJM. 2009;102:799–806. doi: 10.1093/qjmed/hcp121. [DOI] [PubMed] [Google Scholar]
- 31.Sniderman AD, Thanassoulis G, Williams K, Pencina M. Risk of premature cardiovascular disease vs the number of premature cardiovascular events. JAMA Cardiol. 2016;1:492–494. doi: 10.1001/jamacardio.2016.0991. [DOI] [PubMed] [Google Scholar]
- 32.Logue J, Walker JJ, Colhoun HM, et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54:3003–3006. doi: 10.1007/s00125-011-2313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 940 kb)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author in anonymised form upon reasonable request.