Abstract
Background and study aims Recently, histological inflammation has been suggested to be an important predictor of sustained remission or relapse of ulcerative colitis (UC). In this study, we retrospectively compared severity of histological inflammation with endoscopic findings in UC patients with mucosal healing (MH) in the remission maintenance phase, and investigated whether histological healing could be a predictor of sustained remission.
Patients and methods This study included 166 patients with MH in the remission maintenance phase. Endoscopic evaluation was based on the Mayo endoscopic subscore (MES), and MH was defined as MES 0 or 1. Severity of histological inflammation was graded according to the Matts classification. Patients with Matts 1 and 2 were included in the histological healing (HH) group, and those with Matts 3, 4, and 5, in the non-histological healing (NHH) group. In patients with MH, incidence of relapse was compared and analyzed according to severity of histological inflammation.
Results The remission maintenance rate was significantly higher in the MES 0 group than in the MES 1 group ( P = 0.004). The rate was significantly higher in the HH group than in the NHH group ( P = 0.003). Within the MES 1 group, the rate was significantly higher in the HH subgroup than in the NHH subgroup ( P = 0.030).
Conclusions This retrospective study suggests that histological healing can be a predictor of sustained remission in UC patients, and examination of histological inflammation provides useful information for long-term management of UC, particularly in patients with MES 1.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) with unknown etiology that is localized to the colonic mucosa. The disease manifests with symptoms such as abdominal pain, bloody stool, diarrhea, and weight loss and is characterized by repeated relapse and remission. As no curative treatments and strategies exist to date, a realistic treatment goal is to maintain remission for a long period after remission induction 1 2 . However, remission maintenance is often difficult. In recent years, achievement of mucosal healing (MH) has been recognized as an important factor for remission maintenance 3 4 5 6 and shown to lower rates of relapse, hospitalization, and conversion to surgical treatment; reduce healthcare costs; and improve quality of life of patients 3 4 5 6 7 8 9 10 11 12 13 . Moreover, MH has also been reported to contribute to prevention of UC-associated colorectal cancer 14 . Thus, setting a treatment goal to achieve not only clinical remission but also MH is the current standard treatment of UC 3 . However, even among patients with MH, a certain number of patients experience relapse. Various studies have been conducted on prediction of relapse. Recent studies have focused on histological inflammation as a superior indicator of sustained remission to MH. Previous studies have shown that active histological inflammation is observed in approximately 40 % of patients with MH 4 15 16 17 18 . In recent years, the importance of histological healing has attracted attention as an indicator of sustained remission 19 20 . However, no report of any study compared the severity of histological inflammation and endoscopic findings in patients remaining in remission for a long time.
In the current study, we retrospectively compared severity of histological inflammation with endoscopic findings in UC patients with MH in the remission maintenance phase, and investigated whether histological healing could be a predictor of sustained remission.
Patients and methods
Study design and patient population
This retrospective cohort study was conducted in two medical institutions and approved by the ethics committee of Dokkyo Medical University Hospital (approval no. 29002). This study was conducted in accordance with the ethical principles associated with the Declaration of Helsinki and registered in the University Hospital Medical Network Clinical Trials Registry [UMIN000033452]. We provided a means to opt out instead of omitting informed consent, which is a way to guarantee the opportunity for research subjects to notify and publish research information on our website.
The primary endpoint of this study was to examine whether histological healing could be a predictor of sustained remission in UC patients with MH. The second endpoint was to investigate risk factors for relapse (age, sex, affected area, disease duration, smoking rate, and duration of remission before entry). In considering duration of remission before entry, patients were divided into two groups, one maintaining remission for 1 year before entry and one maintaining remission over 1 year (longer term).
To select eligible patients, we retrospectively reviewed medical records of 555 UC patients aged 12 to 86 who had been treated at Dokkyo Medical University Hospital and Japanese Red Cross Ashikaga Hospital between January 2008 and March 2016. Of 207 patients who had remained in clinical remission with only 5-aminosalicylic acid (ASA) for at least 1 year without dose modification, who had been found to have MH by colonoscopy, and who had undergone a biopsy, 166 were confirmed to have consumed 80 % of prescribed medications based on their medical records and included in the current study ( Fig. 1 ). The ASA preparations used were a time-dependent ASA preparation (Pentasa, 2000 – 4000 mg/d; Kyorin Pharmaceutical Co., Ltd., Tokyo, Japan), a pH-dependent ASA preparation (Asacol, 2400 – 3600 mg/d; Zeria Pharmaceutical Co., Ltd., Tokyo, Japan), and salazosulfapyridine (Salazopyrin, 2000 – 4000 mg/d; Pfizer Japan Inc., Tokyo, Japan), which are approved in Japan.
Fig. 1.
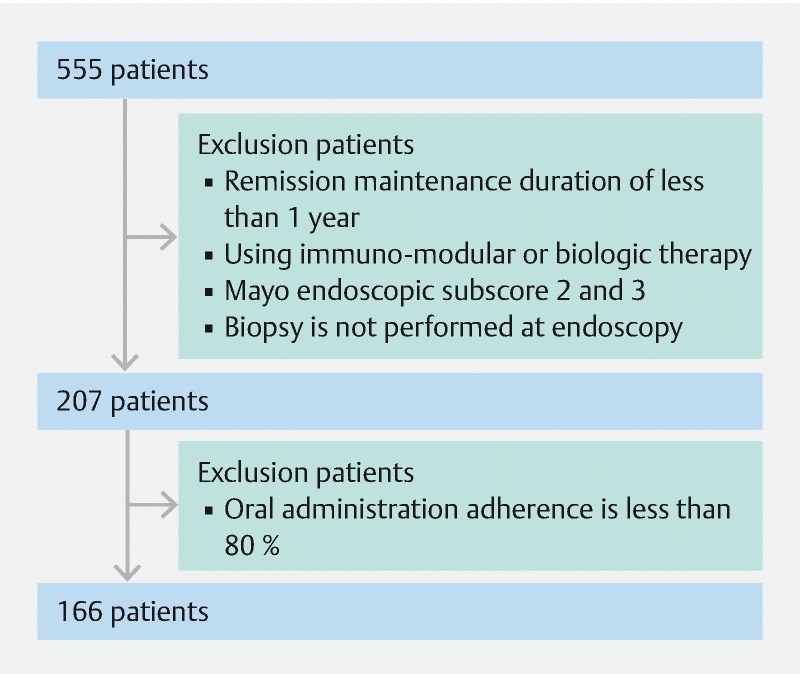
Patient inclusion and exclusion criteria.
Meanwhile, patients who had been treated with immunomodulators or anti-tumor necrosis factor-α drugs were excluded. Clinical remission was defined as Rachmilewitz Clinical Activity Index of 4 points or lower 21 . The strategy for treatment was on the basis of the Japanese clinical guideline for UC 22 . Therefore, clinical evaluation and follow-up for UC patients were the same way in the two institutions.
Endoscopic evaluation
According to the Montreal Classification of UC, the colon was divided into the following three segments 23 24 :
Ulcerative proctitis (E1) (Proctitis type): involvement limited to the rectum (ie, proximal extent of inflammation is distal to the rectosigmoid junction).
Left-Sided UC (E2) (Left-sided type): involvement limited to the portion of the colorectum distal to the splenic flexure.
Extensive UC (E3) (Pancolitis type): involvement extends proximal to the splenic flexure.
Endoscopic evaluation was based on the Mayo endoscopic subscore (MES) 25 26 , where MES 0 (no friability, granularity, and intact vascular pattern) corresponds to normal mucosa and MES 1 (mild erythema or decreased vascular pattern) corresponds to healed mucosa. MH was defined as a state of normal and healed mucosa. In addition, MES 2 (marked erythema, absent vascular pattern, friability, and erosions) and MES 3 (spontaneous bleeding and ulceration) were regarded to correspond to mucosa in the active phase ( Fig. 2 ). Endoscopic findings obtained after at least 1 year of clinical remission were evaluated by two endoscopists, each one selected at the two medical institutions from those with 10 years or more experience with a specialty in IBD who annually treated 200 or more patients with IBD. Endoscopic findings on which the two endoscopists agreed were included in analyses. When they disagreed on evaluation of the findings, cases were scored by assigning one point each to the following three items: presence of redness, visible vascular pattern, and fine mucosal granularity. When the mean of scores calculated by the two endoscopists was two or higher, patients were determined to have MES 1. To evaluate endoscopic findings, scores of the colon segments with the most severe inflammation were used.
Fig. 2.
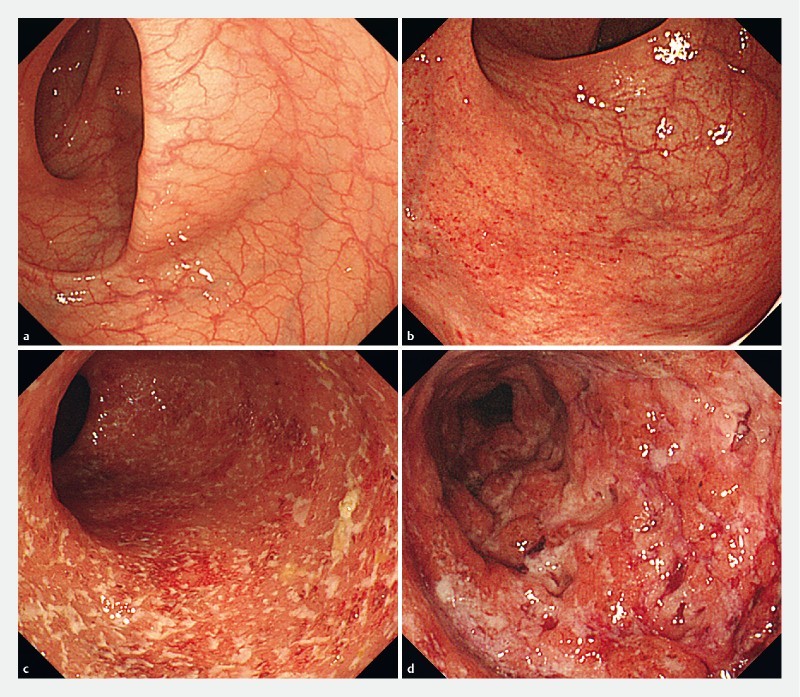
Endoscopic image of Mayo endoscopic subscore (MES). a Endoscopic image of MES 0 (no friability, granularity, and intact vascular pattern). b Endoscopic image of MES 1 (mild erythema or decreased vascular pattern). c Endoscopic image of MES 2 (marked erythema, absent vascular pattern, friability, and erosions). d Endoscopic image of MES 3 (spontaneous bleeding and ulceration).
Histological evaluation
Pathological examination was performed by a pathologist who specialized in pathology of gastrointestinal diseases. Severity of histological inflammation was graded as follows, according to the Matts classification 27 : 1, normal; 2, infiltration of round cells and polymorphonuclear leukocytes into the mucosa and lamina propria; 3, moderate cell infiltration and partial infiltration into the submucosa; 4, crypt abscess and marked cell infiltration of all mucosal layers; 5, erosion, ulceration, mucosal necrosis, and marked cell infiltration. Patients with Matts 1 and 2 were included in the histological healing (HH) group, and those with Matts 3, 4, and 5, in the non-histological healing (NHH) group.
Statistical analyses
Statistical analyses were performed with IBM SPSS Statistics 24 (IBM Japan, Ltd., Tokyo, Japan). The Cohen kappa coefficient (κ) was calculated to determine the agreement rate between the two endoscopists who evaluated the endoscopic findings. The Pearson χ 2 test was performed to compare sex, affected area, endoscopic classification, histological classification, smoking rate, and duration of remission before entry. When the expected value was < 5, the Fisher exact test was performed. The Mann-Whitney U test was performed to compare mean age and mean disease duration. To compare the remission maintenance rate, survival curves were generated using the Kaplan-Meier method and the log-rank test was performed. The Cox proportional hazards model was used to identify predictors of clinical relapse. P < 0.05 indicated statistical significance.
Results
Analysis of study patients
Background characteristics of the 166 study patients are shown in Table 1 . According to the affected areas, proctitis type accounted for 15.1 % of the patients (n = 25), left-sided type for 33.1 % (n = 55), and pancolitis type for 51.8 % (n = 86). Endoscopic findings were graded as MES 0 in 54.8 % of the patients (n = 91) and MES 1 in 45.2 % (n = 75) ( Table 2 ). Regarding the agreement rate of endoscopic evaluation between the two endoscopists, a high Cohen kappa coefficient (κ = 0.73) was obtained, indicating virtually complete agreement. Histological evaluation revealed HH in 73.5 % (n = 122) and NHH in 26.5 % (n = 44) of the patients ( Table 3 ).
Table 1. Background characteristics of the patients.
| Characteristics (n = 166) | |
| Mean age (years) | 48.5 ± 15.3 |
| Sex (male) | 51.2 % (n = 85) |
| Affected area | |
|
15.1 % (n = 25) |
|
33.1 % (n = 55) |
|
51.8 % (n = 86) |
| Endoscopic classification | |
|
54.8 % (n = 91) |
|
45.2 % (n = 75) |
| Histological classification | |
|
73.5 % (n = 122) |
|
26.5 % (n = 44) |
| Mean disease duration (months) | 148.6 ± 99.2 |
| Mean duration of remission (months) | 44.8 ± 25.5 |
| Smoking rate | 3.0 % (n = 5) |
Values are presented as mean ± standard deviation or % (n). MES, Mayo endoscopic subscore; HH, histological healing; NHH, non-histological healing.
Table 2. Comparison of patient characteristics between the MES 0 and MES 1 groups.
| Characteristics (n = 166) | MES 0 (n = 91) | MES 1 (n = 75) | P |
| Mean age (years) | 50.1 ± 15.9 | 46.7 ± 14.5 | NS |
| Sex (male) | 45.1 % (n = 41) | 58.7 % (n = 44) | NS |
| Affected area | NS | ||
|
15.4 % (n = 14) | 14.7 % (n = 11) | |
|
36.3 % (n = 33) | 29.3 % (n = 22) | |
|
48.4 % (n = 44) | 56.0 % (n = 42) | |
| Histological classification | |||
|
91.2 % (n = 83) | 52.0 %(n = 39) | NS |
|
8.8 % (n = 8) | 48.0 % (n = 36) | NS |
| Mean disease duration (months) | 140.4 ± 84.1 | 158.4 ± 114.8 | NS |
| Smoking rate | 2.2 % (n = 2) | 4.0 % (n = 3) | NS |
Values are presented as mean ± standard deviation or % (n). MES, Mayo endoscopic subscore; NS, not significant; HH, histological healing; NHH, non-histological healing.
Table 3. Comparison of patient characteristics between the HH and NHH groups.
| Characteristics (n = 166) | HH group (n = 122) | NHH group (n = 44) | P |
| Mean age (years) | 48.7 ± 15.2 | 48.0 ± 15.9 | NS |
| Sex (male) | 49.2 % (n = 60) | 56.8 % (n = 25) | NS |
| Affected area | NS | ||
|
15.6 % (n = 19) | 13.6 % (n = 6) | |
|
34.4 % (n = 42) | 29.6 % (n = 13) | |
|
50.0 % (n = 61) | 56.8 % (n = 25) | |
| Endoscopic classification | |||
|
68.0 % (n = 83) | 18.2 %(n = 8) | NS |
|
32.0 % (n = 39) | 81.8 % (n = 36) | NS |
| Mean disease duration (months) | 146.3 ± 102.3 | 154.9 ± 91.1 | NS |
| Smoking rate | 2.4 % (n = 3) | 4.5 % (n = 2) | NS |
Values are presented as mean ± standard deviation or % (n). HH, histological healing; NHH, non-histological healing; NS, not significant; MES: Mayo endoscopic subscore.
Of patients with MES 0, 91.2 % (83/91) achieved HH, whereas 8.8 % (8/91) had NHH. Meanwhile, 52.0 % (39/75) of patients with MES 1 achieved HH and 48.0 % (36/75) had NHH.
Mucosal healing and relapse-free survival
When the MES 0 and MES 1 groups were compared and analyzed, the remission maintenance rate was higher in the MES 0 group, with a statistically significant difference (hazard ratio, 4.484; 95 % confidence interval, 1.474 – 13.642; P = 0.004) ( Fig. 3 ).
Fig. 3.
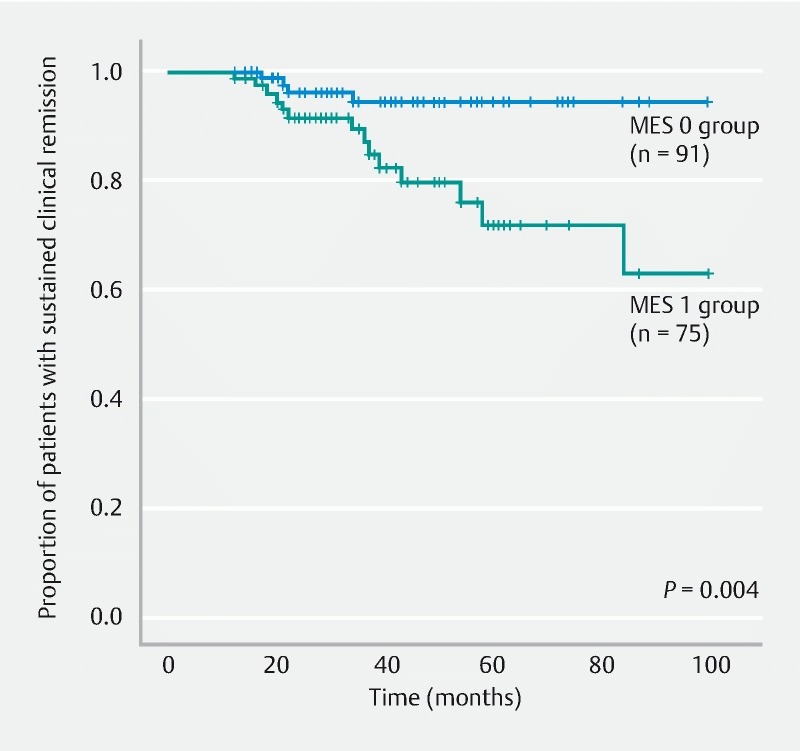
Comparison of remission maintenance rates between the MES 0 and MES 1 groups. MES, Mayo endoscopic subscore.
Histological healing and relapse-free survival
The HH and NHH groups were compared and analyzed. The remission maintenance rate was higher in the HH group, with a statistically significant difference (hazard ratio, 3.866; 95 % confidence interval, 1.497 – 9.982; P = 0.003) ( Fig. 4 ). Furthermore, when the HH and NHH subgroups within the MES 0 group were compared and analyzed, no significant difference was observed in the remission maintenance rate (hazard ratio, 0.042; 95 % confidence interval, 0.000 – 66635; P = 0.502) ( Fig. 5 ). Moreover, when the HH and NHH subgroups within the MES 1 group were compared and analyzed, the remission maintenance rate was higher in the HH subgroup, with a statistically significant difference (hazard ratio, 3.744; 95 % confidence interval, 1.041 – 13.466; P = 0.030) ( Fig. 6 ). Meanwhile, comparison of the HH subgroups of the MES 0 and MES 1 groups did not reveal any significant difference in the remission maintenance rate (hazard ratio, 1.640; 95 % confidence interval, 0.367 – 7.337; P = 0.512) ( Fig. 7 ).
Fig. 4.
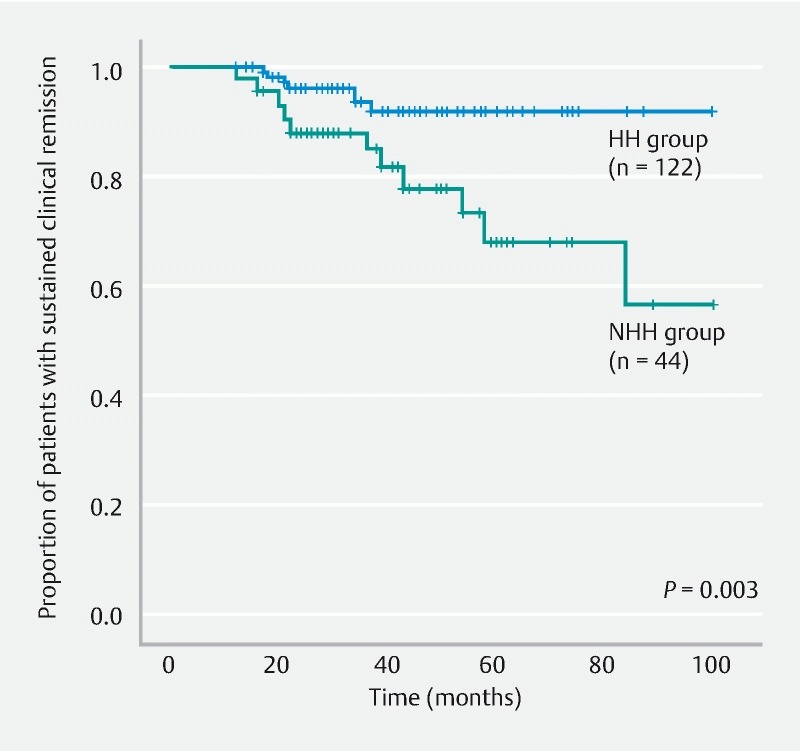
Comparison of remission maintenance rates between the HH and NHH groups. HH, histological healing; NHH, non-histological healing.
Fig. 5.
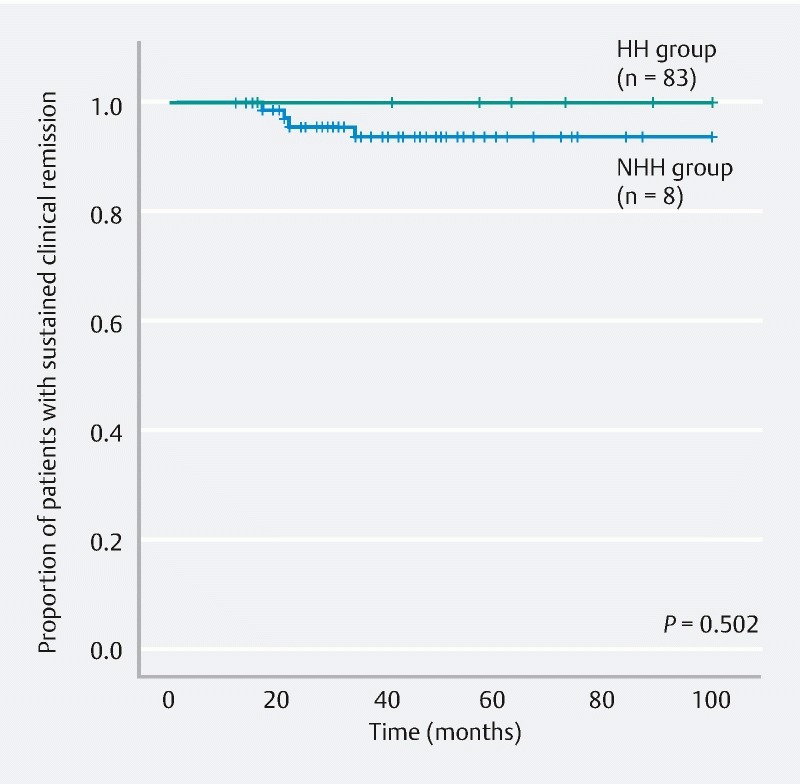
Comparison of remission maintenance rates between the HH and NHH subgroups within the MES 0 group. HH, histological healing; NHH, non-histological healing; MES, Mayo endoscopic subscore.
Fig. 6.
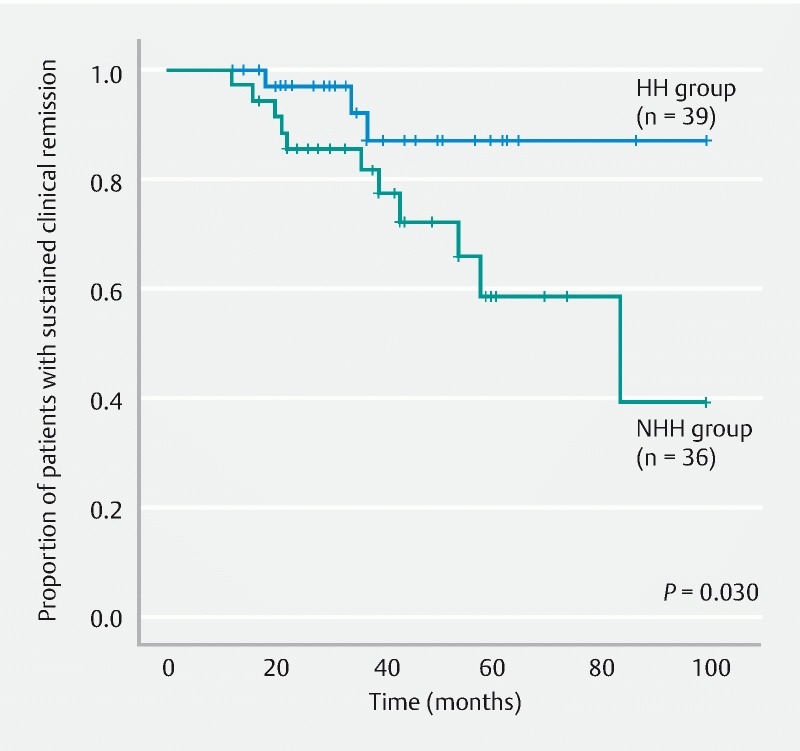
Comparison of remission maintenance rates between the HH and NHH subgroups within the MES 1 group. HH, histological healing; NHH, non-histological healing; MES, Mayo endoscopic subscore.
Fig. 7.
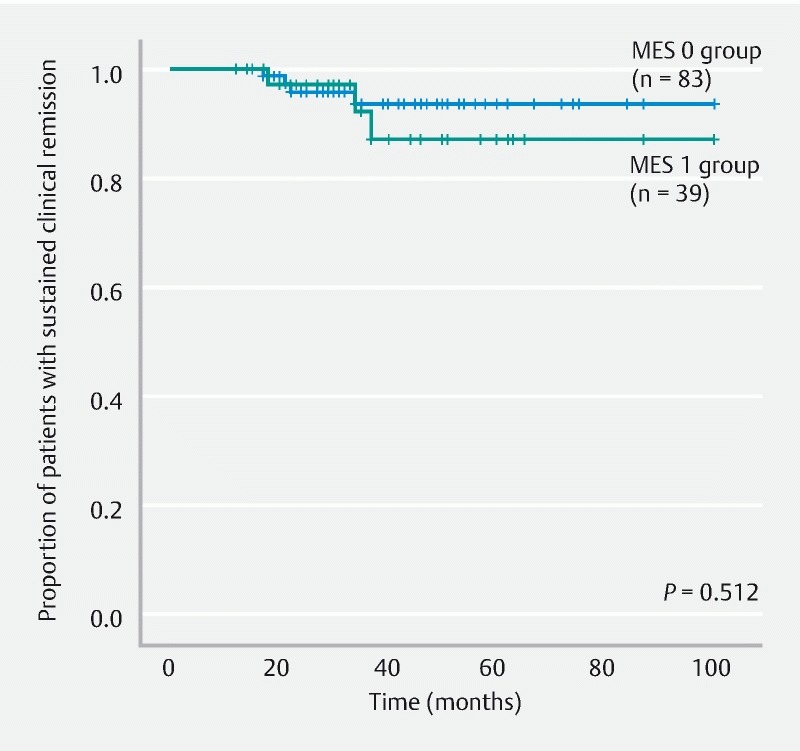
Comparison of the remission maintenance rates between the MES 0 and MES 1 subgroups within the HH group. MES, Mayo endoscopic subscore; HH, histological healing.
Risk factors
Age, sex, affected area, disease duration, smoking rate, and duration of remission before entry were analyzed, but none was identified to be a significant factor for predicting presence or absence of relapse ( Table 4 ).
Table 4. Analysis of risk factors for relapse.
| Risk factor | Hazard ratio (95 % CI) | P value |
| Age | 0.988 (0.956 – 1.021) | 0.455 |
| Sex (male) | 1.056 (0.397 – 2.808) | 0.914 |
| Affected area | NS | |
|
– | 0.811 |
|
0.744 (0.150 – 3.690) | 0.717 |
|
1.248 (0.436 – 3.571) | 0.680 |
| Disease duration | 1.000 (0.996 – 1.005) | 0.876 |
| Smoking rate | – | 0.999 |
| MES0 vs MES1 | 4.484 (1.474 – 13.642) | 0.004 |
| HH vs NHH | 3.866 (1.497 – 9.982) | 0.003 |
| MES0 (HH vs NHH) | 0.042 (0.000 – 66635) | 0.502 |
| MES1 (HH vs NHH) | 3.744 (1.041 – 13.466) | 0.030 |
| HH (MES0 vs MES1) | 1.640 (0.367 – 7.337) | 0.512 |
| Duration of remission before entry | 1.110 (0.414 – 2.973) | 0.836 |
CI, confidence interval; MES, Mayo endoscopic subscore; HH, histological healing; NHH, non-histological healing.
Discussion
The International Organization for the Study of Inflammatory Bowel Disease defines MH as “an absence of friability, bleeding, erosions, or ulcerations in all endoscopically observable segments of the intestinal mucosa” 2 . Based on previous studies, the remission maintenance rate is high in patients achieving MH 3 4 5 6 7 8 9 10 11 12 13 14 . MES and Matts classification have never been fully validated, but do represent two of the most widely used scores to quantify endoscopic and histologic activity, respectively. MES is widely used to evaluate the mucosa, and MES 0 and MES 1 correspond to endoscopic MH 6 . However, no consistent views have been reached on whether the remission maintenance rate differs between patients with MES 0 and MES 1 7 10 28 . The current study revealed a higher remission maintenance rate in the MES 0 group than in the MES 1 group, indicating that if the mucosal condition is endoscopically confirmed as MES 0, probability of relapse can be expected to be low. Meanwhile, approximately 30 % of patients with MES 1 experienced relapse in 60 months. Even in patients with endoscopically confirmed MH, estimating the probability of relapse separately in those with MES 0 and MES 1 seemed necessary.
In recent years, histological healing has been considered to be a much superior indicator of remission maintenance to MH. It has also been suggested that not only MH but also resolution of histological inflammation is associated with decreases in relapse rate, hospitalization rate, steroid use, surgery rate, and risk of UC-associated colorectal cancer 4 15 16 17 18 19 20 29 30 31 32 33 . Riley et al. demonstrated in a study of 82 UC patients that the rate of relapse within 12 months was significantly higher in patients with histological findings of acute inflammatory cell infiltration, crypt abscess, and goblet cell depletion 15 . Bitton et al. reported in a study of 74 UC patients in clinical and endoscopic remission that presence of basal plasmacytosis detected by rectal biopsy is an independent predictor of relapse of UC 17 . Bryant et al. reported that, compared with endoscopic MH, histological healing is more useful for predicting development of severe acute colitis requiring remission induction with corticosteroids or hospitalization 19 . Feagins et al. studied histological findings from 51 UC patients in clinical remission and reported that presence of basal lymphoplasmacytic tumor, epithelial erosion/ulceration, and moderate to severe structural changes is significantly useful for predicting clinical relapse at 6 and 12 months. Compared with assessment of only endoscopic findings, assessment of histological findings was reported to be more useful for predicting relapse 20 . Christensen et al. reported that histological healing can be used as a clinical endpoint for UC patients and that histological evaluation should be a part of endoscopic evaluation as an indicator of activity of IBD 33 .
We investigated the importance of assessing histological healing in patients who had remained in clinical remission for a long time and had been found to have MH by colonoscopy. Our study yielded results comparable to those of previous studies. Within the MES 1 group, the HH subgroup showed a higher remission maintenance rate than the NHH subgroup. Meanwhile, within the HH group, the remission maintenance rates were comparable between the MES 0 and MES 1 subgroups. These results indicated that if histological inflammation is graded as Matts 1 or 2 in patients with MES 1, their remission maintenance rate is comparable with that in patients with MES 0.
In the current study, risk factors for relapse of UC were also investigated through analyses of secondary endpoints. On this issue, various studies have been conducted. Bitton et al. reported that early relapse is associated with younger age, short duration of remission before their investigation, and frequency of prior relapses 17 . Meanwhile, Christensen et al. reported that age, sex, smoking, disease duration, and affected area did not show any significant difference as factors for remission maintenance 33 . No consistent views have been reached. The current study analyzed age, sex, affected area, disease duration, smoking rate, and duration of remission before entry, but none was a significant risk factor for relapse. However, because the current study was retrospective, lifestyle habits were not rigorously assessed. We hope that future prospective studies may provide new findings.
As for the limitations of the current study, we did not assess effects of remission induction techniques (use of steroids, tacrolimus, biological drugs, etc.), doses and types of 5-ASA preparations, application of topical drugs, or lack of details on maintenance treatments for patients in endoscopic/histologic remission.
Another limitation was the retrospective study design. However, because no other study has been conducted on patients in sustained remission, our study presumably provided an indicator for predicting relapse in such patients.
Conclusion
In conclusion, although MES 0 and MES 1 are often regarded as corresponding to MH, this study suggests that histological healing could be a predictor of sustained remission in UC patients. Particularly in patients with MES 1, examination of histological inflammation seemed useful for long-term management of UC. To apply the results of the current study to clinical practice, we recommend that well-designed multicenter prospective studies be conducted. Furthermore, we believe that it would be better to design similar research in Crohn's disease in the future.
Footnotes
Competing interests None
References
- 1.Kornbluth A, Sachar D B. Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–523. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 2.D'Haens G, Sandborn W J, Feagan B G et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 3.Froslie K F, Jahnsen J, Moum B A et al. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 4.Truelove S C, Richards W C. Biopsy studies in ulcerative colitis. Br Med J. 1956;1:1315–1318. doi: 10.1136/bmj.1.4979.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neurath M F, Travis S P. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619–1635. doi: 10.1136/gutjnl-2012-302830. [DOI] [PubMed] [Google Scholar]
- 6.Colombel J F, Rutgeers P, Reinisch W et al. Early mucosal healing with infliximab is associated with improved longterm clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 7.Meucci G, Fasoli R, Saibeni S et al. Prognostic significance of endoscopic remission in patients with active ulcerative colitis treated with oral and topical mesalazine: a prospective, multicenter study. Inflamm Bowel Dis. 2012;18:1006–1010. doi: 10.1002/ibd.21838. [DOI] [PubMed] [Google Scholar]
- 8.Ardizzone S, Andrea C, Piergiorgio D et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2011;9:483–489. doi: 10.1016/j.cgh.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Gibson P R, Vaizey C, Black C M et al. Relationship between disease severity and quality of life and assessment of health care utilization and cost for ulcerative colitis in Australia: a cross-sectional, observational study. J Crohns Colitis. 2014;8:598–606. doi: 10.1016/j.crohns.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi F, Tominaga K, Kanamori A et al. Timing for dose-down of 5-ASA depends on mucosal status with ulcerative colitis. Scand J Gastroenterol. 2016;51:827–834. doi: 10.3109/00365521.2016.1141315. [DOI] [PubMed] [Google Scholar]
- 11.Sandborn W J, Rutgeerts P, Feagan B G et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009;137:1250–1260. doi: 10.1053/j.gastro.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 12.Feagan B G, Reinisch W, Rutgeerts P et al. The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol. 2007;102:794–802. doi: 10.1111/j.1572-0241.2007.01094.x. [DOI] [PubMed] [Google Scholar]
- 13.Peyrin-Biroulet L, Sandborn W, Sands B E et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 14.Boal Carvalho P, Cotter J. Mucosal healing in ulcerative colitis: a comprehensive review. Drugs. 2017;77:159–173. doi: 10.1007/s40265-016-0676-y. [DOI] [PubMed] [Google Scholar]
- 15.Riley S A, Mani V, Goodman M J et al. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32:174–178. doi: 10.1136/gut.32.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bessissow T, Lemmens B, Ferrante M et al. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol. 2012;107:1684–1692. doi: 10.1038/ajg.2012.301. [DOI] [PubMed] [Google Scholar]
- 17.Bitton A, Peppercorn M A, Antonioli D A et al. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology. 2001;120:13–20. doi: 10.1053/gast.2001.20912. [DOI] [PubMed] [Google Scholar]
- 18.Park S, Abdi T, Gentry M et al. Histological disease activity as a predictor of clinical relapse among patients with ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2016;111:1692–1701. doi: 10.1038/ajg.2016.418. [DOI] [PubMed] [Google Scholar]
- 19.Bryant R V, Burger D C, Delo J et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut. 2016;65:408–414. doi: 10.1136/gutjnl-2015-309598. [DOI] [PubMed] [Google Scholar]
- 20.Feagins L A, Melton S D, Iqbal R et al. Clinical implications of histologic abnormalities in colonic biopsy specimens from patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis. 2013;19:1477–1482. doi: 10.1097/MIB.0b013e318281f4ae. [DOI] [PubMed] [Google Scholar]
- 21.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuoka K, Kobayashi T, Ueno F et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53:305–353. doi: 10.1007/s00535-018-1439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverberg M S, Satsangi J, Ahmad T et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19:5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 24.Satsangi J, Silverberg M S, Vermeire S et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeder K W, Tremaine W J, Ilstrup D M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 26.Scherl E J, Pruitt R, Gordon G L et al. Safety and efficacy of a new 3.3 g b.i.d. tablet formulation in patients with mild-to-moderately-active ulcerative colitis: a multicenter, randomized, double-blind, placebo-controlled study. Am J Gastroenterol. 2009;104:1452–1459. doi: 10.1038/ajg.2009.83. [DOI] [PubMed] [Google Scholar]
- 27.Matts S G. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q J Med. 1961;30:393–407. [PubMed] [Google Scholar]
- 28.Nakarai A, Kato J, Hiraoka S et al. Prognosis of ulcerative colitis differs between patients with complete and partial mucosal healing, which can be predicted from the platelet count. World J Gastroenterol. 2014;20:18367–18374. doi: 10.3748/wjg.v20.i48.18367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin D T, Huo D, Kinnucan J A et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol. 2013;11:1601–1608. doi: 10.1016/j.cgh.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta R B, Harpaz N, Itzkowitz S et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hefti M M, Chessin D B, Harpaz N H et al. Severity of inflammation as a predictor of colectomy in patients with chronic ulcerative colitis. Dis Colon Rectum. 2009;52:193–197. doi: 10.1007/DCR.0b013e31819ad456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peyrin-Biroulet L, Bressenot A, Kampman W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin Gastroenterol Hepatol. 2014;12:929–934. doi: 10.1016/j.cgh.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Christensen B, Hanauer S B, Erlich J et al. Histologic normalization occurs in ulcerative colitis and is associated with improved clinical outcomes. Clin Gastroenterol Hepatol. 2017;15:1557–1564. doi: 10.1016/j.cgh.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


