Abstract
Functional changes in spatial genome organization during human development are poorly understood. Here we report a comprehensive profile of nuclear dynamics during human cardiogenesis from pluripotent stem cells by integrating Hi-C, RNA-seq and ATAC-seq. While chromatin accessibility and gene expression show complex on/off dynamics, large-scale genome architecture changes are mostly unidirectional. Many large cardiac genes transition from a repressive to an active compartment during differentiation, coincident with upregulation. We identify a network of such gene loci that increase their association inter-chromosomally, and are targets of the muscle-specific splicing factor RBM20. Genome editing studies show that TTN pre-mRNA, the main RBM20-regulated transcript in the heart, nucleates RBM20 foci that drive spatial proximity between the TTN locus and other inter-chromosomal RBM20 targets such as CACNA1C and CAMK2D. This mechanism promotes RBM20-dependent alternative splicing of the resulting transcripts, indicating the existence of a cardiac-specific trans-interacting chromatin domain (TID) functioning as a splicing factory.
The spatial organization of the genome plays an important but unclearly defined role in gene regulation. Here, the authors integrate Hi-C, RNA-seq and ATAC-seq data to map cardiogenesis from pluripotent stem cells and describe an RBM20-dependent splicing factory assembling the TTN locus with other RBM20 targets.
Introduction
During human development diverse differentiated cell types emerge from a single pluripotent population of cells. This differentiation process requires mechanisms to both activate the gene networks of target lineages and repress those of alternative lineages. The human heart is the first major organ to form and thus in vitro modeling of cardiogenesis using human pluripotent stem cells (hPSCs) provides an ideal system to study the regulation of early fate commitment. Moreover, congenital heart disease, the most common human birth defect, is often caused by mis-regulated transcriptional pathways1. Spatial organization of the genome in 3-D has emerged as a key mechanism by which cells can control regions of chromatin accessibility2,3. Chromosome conformation capture technologies such as Hi-C have identified complex three-dimensional dynamics during development, and abnormal chromosomal architecture has been implicated in a range of diseases, including cancer and limb malformations4–8. While these studies have demonstrated regulated regions of chromosomes, how these transitions occur temporally across multiple stages of human lineage commitment remains to be addressed. Further, to what extent such changes causally impact gene expression is still poorly understood.
We have previously shown that epigenetic modifications are dynamically regulated during cardiomyocyte differentiation of hPSCs9, and that integration of epigenetic data defines general patterns of gene regulation. Here we utilize Hi-C10, which captures higher order chromatin structure, along with ATAC-seq, as a mark of local accessibility, along with RNA-seq during this process of differentiation. We demonstrate new insights into how global chromatin organization changes both coordinately and independently of local changes and how that feeds back on gene regulation. Further we find that genomic regions dynamically regulated during hPSC-cardiomyocyte differentiation resolve to a state that closely resembles that of human fetal cardiomyocytes, indicating these dynamics likely reflect early steps in human cardiogenesis. At the macro level, 19% of the genome changes active (A) vs. inactive (B) compartment state during differentiation. During differentiation the heterochromatic regions pack more tightly, increasing cis interactions. Transcriptional activation of large cardiac genes is associated with B to A transitions, chromatin decompaction and a gain in trans interactions. These properties are exemplified in the TTN (titin) locus, which encodes the largest human protein. In particular, we identify a network of TTN-associated genes co-regulated by the muscle-specific splicing factor RBM2011 that increasingly interact in trans during differentiation. Cross-validation by imaging, and functional experiments using pharmacology or CRISPR/Cas9 gene editing indicate a mechanism whereby RBM20 nuclear foci are nucleated by TTN pre-mRNA and facilitate the interaction of target genes and proper alternative splicing. Overall, this study demonstrates the dynamic interplay between global and local chromatin architecture during human development and exemplifies how this can influence gene expression patterns.
Results
Chromatin structure dynamics during human cardiogenesis
To understand the temporal dynamics of nuclear architecture during cardiac differentiation, we generated highly pure cardiomyocytes (CM; > 90% cTnT+) from undifferentiated RUES2 human embryonic stem cells (hESCs; Fig. 1a, Supplementary Fig. 1a–c). These cells pass through stages representative of early development including mesoderm (MES), and cardiac progenitor (CP), before reaching definitive CMs9 (Supplementary Fig. 1d). We performed in situ DNase Hi-C10 on these stages of differentiation with two independent biological replicates, along with two fetal heart samples (Supplementary Table 1). Chromosome-wide contact maps demonstrate the expected checkerboard pattern, indicative of local associations (topologically associating domains, or TADs) and long-range compartmentalization (A/B compartments) (Fig. 1b). Genome-wide contact maps between whole chromosomes demonstrate that smaller and larger chromosomes tend to self-associate (Supplementary Fig. 2).
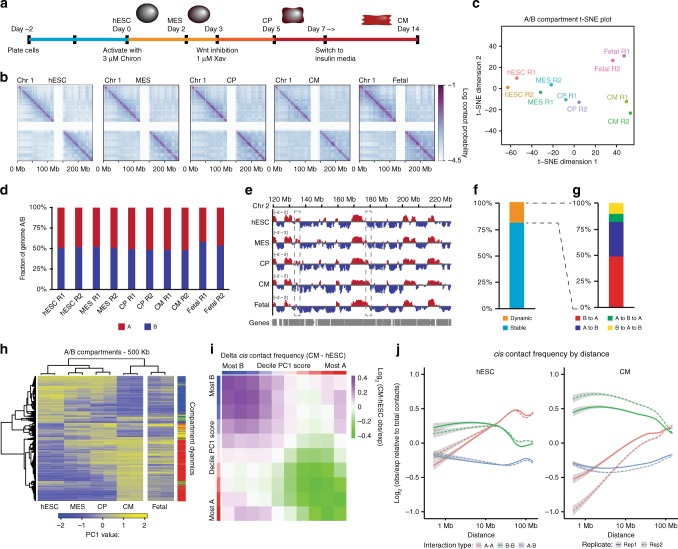
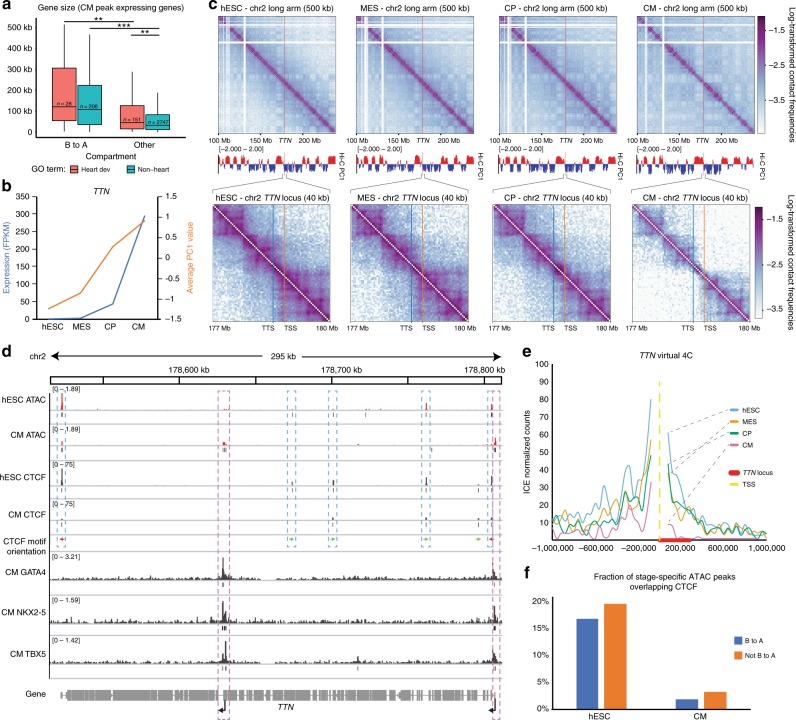
Fig. 1.
Hi-C across cardiac differentiation. a Schematic of the cardiomyocyte differentiation. b Log transformed contact maps of chromosome 1. c t-SNE plot of PC1 scores on the contact matrices. d Fraction of genome in A and B compartment by sample. e PC1 scores for a region of chromosome 2, gray boxes highlight regions transitioning from A to B and B to A. f Genomic regions divided by stable (81%) and switching (19%) A/B compartment (PC1 scores significantly different by one-way ANOVA, p-value < 0.05; n = 2 independent differentiations). g Regions switching A/B compartment divided by types of transitions. A–B (33%), B–A (49%), A–B–A (8%), B–A–B (10%). Two percent of switching regions were A–B–A–B or B–A–B–A and were combined with A–B and B–A. h Heatmap of the PC1 scores of the compartment switching regions. Clustering of rows based on the four time points of differentiation. Dendrogram of columns was ordered to match the temporal status of differentiation. i Delta compartmentalization saddle plot in cis contacts CM vs. hESC. Bins were assigned to ten deciles based on PC1 score, average observed/expected distance-normalized scores for each pair of deciles were calculated. j Distance plot of A–A, B–B, and A–B interactions for hESC and CM, values are normalized to all contacts at a given distance. Data was smoothed using R, raw maps in Supplementary Fig. 3g. Source data are provided as a Source Data file
We computed the first principal component (PC1) from the contact matrix to segregate chromatin bins at 500 kb resolution into A/B compartments, which reflect regions of active and repressive chromatin, respectively12. Using t-SNE to visualize and cluster in two dimensions either PC1 scores or HiC-Rep scores13 closely pairs replicates while generating a differentiation trajectory, demonstrating the reproducibility of the assay (Fig. 1c, Supplementary Fig. 3a, Supplementary Table 2). Fetal heart Hi-C most closely resembles in vitro cardiomyocytes but clusters separately, likely reflective of lower cardiomyocyte purity. Early fetal hearts, while consisting of ~70% cardiomyocytes14, include other cell types such as fibroblasts. Overall the genome is split into ~50% A, ~50% B compartments at each time point (Fig. 1d), and there is little change in the distribution of compartment sizes across differentiation (Supplementary Fig. 3b). The majority of compartment assignments are invariant during differentiation (Fig. 1e–g). However, 19% of the genome changes compartment during differentiation, and hierarchical clustering of dynamic regions recapitulates the differentiation trajectory and clusters cardiomyocytes most closely with fetal heart (Fig. 1h). Most of these changes are unidirectional (B–A or A–B). A small subset exhibits a transitory switch, either A–B–A or B–A–B (Fig. 1g). Together these data show that A/B compartments are dynamic during cardiac differentiation, and that these changes are validated by analyses of fetal hearts.
By integrating the A/B compartment information across differentiation with the interaction contact maps, we noticed that many of the strongest gains in long-range intra-chromosomal (cis) interactions are associated with the B compartment (Supplementary Fig. 3c). This is consistent with prior studies in mouse neuronal specification15, suggesting it may be a general phenomenon of differentiation. Genome-wide analysis shows that stronger cis contacts occur between homotypic regions (A–A or B–B compartments), compared to between heterotypic regions (A–B) (Supplementary Fig. 3d). In the pluripotent state, the strongest cis interactions occur between A compartments, while during differentiation this switches to favor signal between B compartments—a trend supported by patterns in fetal heart (Fig. 1i, Supplementary Fig. 3d, f). This switch occurs as a result of a gain in long-range (>10 Mb) B–B interactions during differentiation (Fig. 1j, Supplementary Fig. 3g), as seen in the contact map. In contrast, inter-chromosomal interactions (trans) favor A–A interactions independent of differentiation state and show no enrichment between B–B contacts (Supplementary Fig. 3e, f). Together these observations suggest a model whereby during differentiation heterochromatic (B compartment) regions condense and pack more tightly, specifically within chromosomes in cis, while inter-chromosomal interactions are most likely to occur between active regions (A compartment) independent of cell state. This is consistent with recent electron micrograph studies showing that heterochromatic regions are more densely packed relative to euchromatic regions in differentiated cells16.
A/B compartmentalization changes reflect expression dynamics
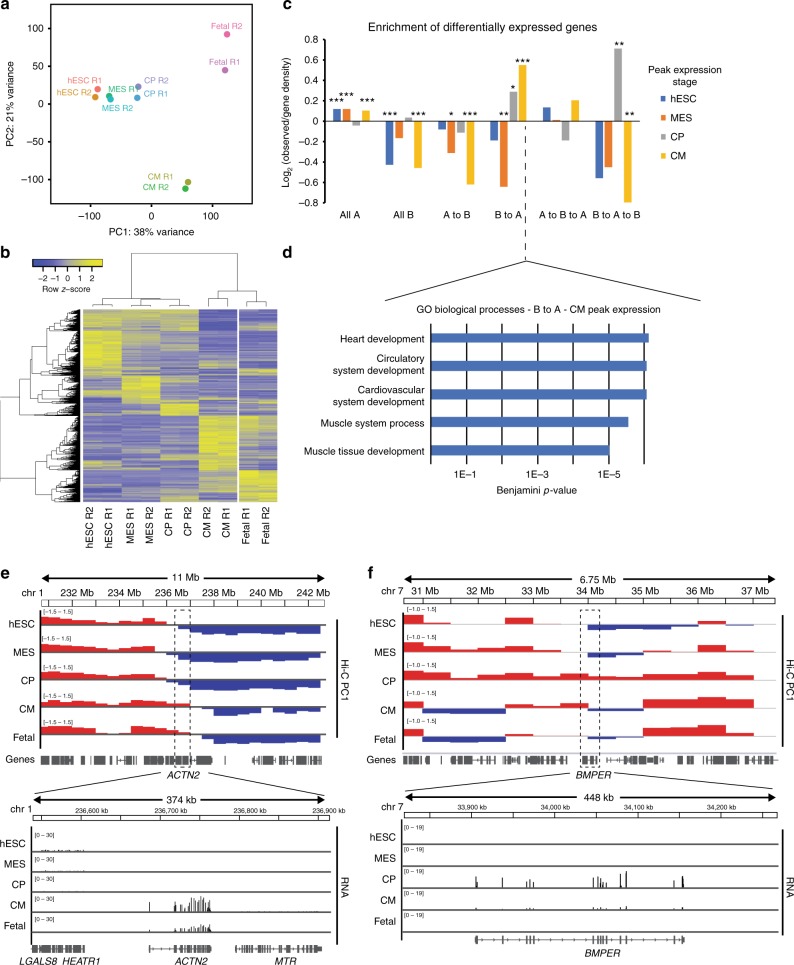
To investigate genes whose expression may be regulated in relationship with compartment changes, we performed RNA-seq on the same stages of differentiation, along with the fetal hearts (Supplementary Table 3). Similar to Hi-C, in principal component analysis samples separated by differentiation state and cardiomyocyte purity (Fig. 2a). In contrast to Hi-C, differentially expressed genes are largely expressed in a cell-type specific manner (Fig. 2b, Supplementary Fig. 4a). While RNA-seq reveals similarities between hESC-CMs and the fetal heart (Fig. 2b), the fetal heart has an increase in the ratio of adult to fetal myosin heavy chain genes (MYH7 and MYH6), suggesting that these mid-gestational cardiomyocytes are more developmentally advanced (Supplementary Fig. 4a). Genes that are upregulated in CPs and CMs are enriched in regions that switch B to A, and underrepresented in A to B transitioning regions (Fig. 2c–e, Supplementary Fig. 4b–c). Gene ontology (GO) analysis reflects that genes upregulated in CMs are enriched in categories related to metabolism along with development and cardiac function (Supplementary Table 4). Focusing on those genes in regions that transition from B to A highly enriches for genes involved in heart development, such as the structural gene alpha-actinin (ACTN2) (Fig. 2d–e, Supplementary Table 5). This suggests that many heart development genes may initially be sequestered in the B compartment and move to A upon activation. Noticeably, even though only 4% of the genome shows two-step dynamics (A–B–A or B–A–B; Fig. 1g), genes that are transiently in the A compartment show peak expression in the CP stage (Fig. 2c). Among these, BMPER and CXCR4 have peak expression in CPs and have important roles in cardiomyocyte function (Fig. 2f, Supplementary Fig. 4d). Gene repression is also associated with compartment switching, including the mesoderm regulator EOMES which switches to B compartment upon downregulation at the CP stage (Supplementary Fig. 4e). Of note, A/B compartment dynamics were largely reproducible in a second hPSC line despite its slower cardiogenic differentiation (Supplementary Note 1 and Supplementary Fig. 5). Taken together, these observations show that dynamic compartment transitions in genomic regions occur coincident with transcriptional regulation, and the observed patterns are consistent between stem cell derivatives and the fetal heart, suggesting important developmental control.
Fig. 2.
Dynamic gene expression correlations with changes in genome architecture. a PCA plot on the replicates of the RNA-seq samples. b Heatmap of the differentially expressed genes during differentiation, clustering of rows based on differentiation time points. c Enrichment of differentially regulated genes by time point of peak expression against A/B compartment dynamics. Log2 values are observed/gene density; p-values by chi-squared test for the indicated gene set overlaps, * < 0.05, ** < 0.01, *** < 0.001. d GO term enrichment in CM peak expression genes in B to A compartments, p-value plotted on log scale. e Gene track of Hi-C PC1 and RNA-seq reads of ACTN2. f Gene track of Hi-C PC1 and RNA-seq reads of BMPER. Source data are provided as a Source Data file
TAD dynamicity is independent from A/B compartmentalization
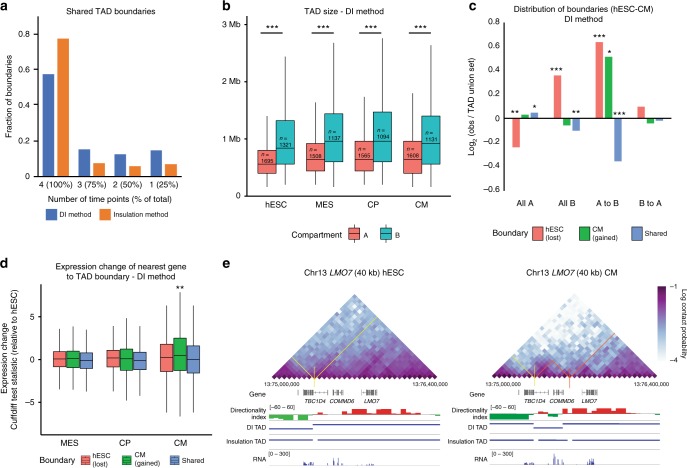
While A/B compartments reflect the global organization of the genome across full chromosomes, local organization can be summarized as topologically associating domains (TADs). We utilized two methods to asses TAD structure across differentiation: directionality index17 and insulation score18 (Fig. 3a, Supplementary Fig. 6a, b, Supplementary Table 6). Using a stringent cutoff, both methods show that a majority of TAD boundaries are constitutive across differentiation (Fig. 3a). While genome-wide TADs range in size between approximately 500 kb and 1 Mb, TADs in the A compartment are smaller in size (Fig. 3b, Supplementary Fig. 6c), which is likely necessary for proper gene regulation in gene-dense areas. Comparing hESCs to CMs, boundaries lost during differentiation are enriched within regions that are constitutive B or switch from A to B (Fig. 3c, Supplementary Fig. 6d). Consistent with prior studies19, we find that TAD boundaries are located near to CTCF peaks in both hESCs and CMs with ~70% of TAD boundaries having a CTCF peak within 1 kb (Supplementary Fig. 6e). Moreover, CTCF peaks are located closer on average to constitutive boundaries rather than differential boundaries by both TAD methods (Supplementary Fig. 6e, chi-sq test, p < 0.001). Together with our previous observation that B domains compact during differentiation, this suggests that as regions compact TAD boundaries may be lost and adjacent regions condense together. In contrast, boundaries that are gained during differentiation are associated with a modest but significant activation of the nearest gene, but are not associated with a transition from inactive to active compartment (Fig. 3c, d, Supplementary Fig. 6f). Examples include, the loci of LMO7 and KCNN2, which change local TAD structure coincident with upregulation of transcription (Fig. 3e and Supplementary Fig. 6g). Overall, the selective acquisition of new boundaries during differentiation is linked to control of gene expression largely independent of A/B compartment changes, while loss of boundaries is not associated with gene regulation and is predominantly associated with heterochromatin compaction.
Fig. 3.
TADs are dynamically regulated independent of A/B compartment changes. a TAD boundaries shared between time points as calculated on the union set of all four time points using both DI and insulation method. b TAD size and number within A and B compartments across differentiation for DI method. Boxplots present the median and 25th and 75th percentile, with the whiskers extending to 1.5 times the inter-quartile range. n = TAD number; p-values by Wilcoxon test, *** < 0.001. c Enrichment of TAD boundaries between hESC and CM state within A/B compartment dynamics for DI method. Log2 values are observed/TAD union set; p-values by chi-squared test for the indicated TAD boundary set overlaps, * < 0.05, ** < 0.01, *** < 0.001. d Expression of nearest gene to TAD boundaries that are either stage specific or shared between hESC and CM for DI method. Box and whisker plots as in panel (b). n = 308 genes for hESC (lost) boundaries, 207 genes for CM (gained) boundaries, and 1326 genes for shared boundaries; p-values by one-sample, two-sided t-test relative to a hypothesized mean value of 0 (no expression change vs hESC), ** < 0.01. e Gene track of DI score, DI-determined TADs and insulation score-determined TADs and RNA, along with a Hi-C heatmap of the COMMD6 and LMO7 locus in hESC and CM. Source data are provided as a Source Data file
Chromatin accessibility and 3D organization are correlated
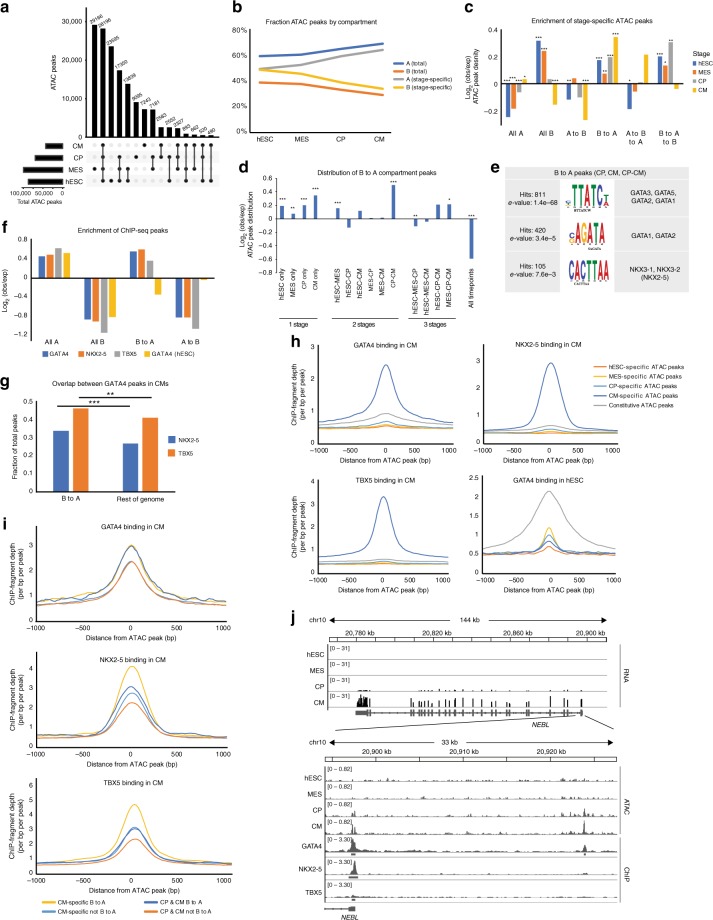
To determine whether changes in higher order organization occur coincident with local changes, we performed ATAC-seq to measure local accessibility across differentiation (Supplementary Fig. 7a, b, Supplementary Table 7). Similar to our previous study using DNase hypersensitivity20, we find a decreasing number of peaks during differentiation (Fig. 4a). During differentiation, there is an increase in the fraction of peaks in A compartment, while peaks being lost are enriched in regions that are constitutively B compartment (Fig. 4b, c, Supplementary Fig. 7c, d). This supports the model of increased heterochromatin packing in CM coincident with decreased accessibility. Similar to the gene expression data, we find that CM-specific ATAC-seq peaks are enriched in regions that transition from B to A and are depleted in A to B regions (Fig. 4c). While we also observe an enrichment of hESC-specific peaks in B to A regions, this is less strong than CP- or CM-specific enrichment and is likely an effect of the overall more open chromatin in hESCs. Stage-specific peaks are on average more distant from TSSs and enriched in motifs corresponding to developmental transcription factors, consistent with possible enhancer activity (Supplementary Fig. 7e, f), in agreement with another recent study21. Interrogating the regions that transition from B to A, these are enriched for peaks present in CP and CM stages and depleted in constitutive peaks (Fig. 4d). The top motifs within these regions match important cardiogenic transcription factors (TF) of the GATA and NKX family (Fig. 4e), which may play pivotal roles in opening up the chromatin during differentiation.
Fig. 4.
Changes in local chromatin accessibility occur coincident with global architecture changes. a ATAC peaks across differentiation. b Fraction of ATAC peaks divided by A and B compartment for stage specific and total peaks. c Enrichment of stage-specific ATAC peaks within A/B compartments. Log2 values are observed/ATAC peak density; p-values by chi-squared test for the indicated ATAC peak set overlaps, * < 0.05, ** < 0.01, *** < 0.001. d Enrichment of peaks within the B to A compartment regions by stage specificity, normalized to ATAC peak density. Plot and statistical analysis as in panel (c). e Motifs from DREME and TOMTOM within CP and CM-specific peaks in B to A compartment bins. f Enrichment of GATA4, NKX2-5, TBX5 (CM), and GATA4 (ESC) ChIP-seq peaks within constitutive A and B regions and B–A and A–B regions. g Overlap of GATA4 peaks with NKX2-5 and TBX5 in B–A regions or the rest of the genome. p-values by chi-squared test for the indicated ChIP-seq peak set overlaps, *** < 0.001. h Metaplot of GATA4, NKX2-5, TBX5 (CM), and GATA4 (ESC) binding across stage specific and constitutive ATAC peaks. Peaks are centered at the mid-point of ATAC peaks and extended ± 1000 bp. i Metaplot of GATA4, NKX2-5, TBX (CM) across CM or CP&CM-specific peaks in B–A regions or the rest of the genome. j Gene track of RNA-seq, ATAC-seq and ChIP-seq reads for NEBL gene and promoter region. Source data are provided as a Source Data file
To explore this possibility in more detail we integrated chromatin organization changes with the binding profiles of these TF. We took advantage of published ChIP-seq datasets from in vitro-differentiated CMs for GATA422, NKX2-523, and TBX522 (another known cardiac TF), as well as hESC ChIP-seq data for GATA424. We find that only the CM ChIPs were enriched in regions that transition from B to A compartment (Fig. 4f). Furthermore, we find a modest but significant increase in the overlap of GATA4 peaks with both NKX2-5 and TBX5 in B to A regions compared with the rest of the genome (Fig. 4g). These observations suggest a cooperative role for these TF in driving chromatin transitions. Examining the enrichment of reads across stage-specific ATAC-seq peaks we find that the CM ChIPs are exclusively enriched at CM-specific peaks (Fig. 4h and Supplementary Fig. 7g). In contrast, GATA4 binding in hESCs is highly enriched in constitutive peaks but not hESC-specific peaks (Fig. 4h), suggesting it may mainly have “housekeeping” roles in this cell type. Given that GATA and NKX motifs were enriched in B to A regions in both CM and CP-CM peaks we examined the enrichment of TF binding across these peaks. Remarkably, GATA4 shows comparable enrichment at CP-CM and CM peaks, while NKX2-5 and TBX5 have greater enrichment at CM-specific peaks. This suggests that GATA4 may be an earlier driver of chromatin transition, perhaps working as a “pioneering” TF, while NKX2-5 and TBX5 may act to further strengthen and reinforce this GATA4-dependent transcriptional network (Fig. 4i). Example of genes co-bound by these TFs are the important sarcomeric proteins NEBL (Fig. 4j) and ACTN2 (Fig. 2e, Supplementary Fig. 7h). Notably, both of these genes transition from B to A compartment during differentiation. Thus, local changes in chromatin accessibility occur coincident with changes in RNA expression and large-scale genome organization.
Compartmentalization controls large cardiac-specific genes
Given the strong enrichment of CM-upregulated genes in the regions of the genome that transition from B to A, we sought to determine whether there are specific characteristics of these genes. Regions that transition from B to A have lower gene density (Supplementary Fig. 8a), suggesting these genes may be bigger or more isolated and therefore exert a stronger influence on their region of the genome. Indeed, upregulated genes in B to A regions are larger than other upregulated genes, independent of GO term (Fig. 5a). In contrast, heart development genes show greater separation from the nearest gene promoters (start site to start site), independent of compartment dynamics or gene size (Supplementary Fig. 8b, c). Using expression data from 37 adult tissues25 we find that those genes that are upregulated and go from B to A are more heart-enriched compared with other upregulated genes (Supplementary Fig. 8d). Together, this supports a model in which expression of large lineage-specific cardiac genes is regulated by changes in chromatin architecture, as they are initially insulated in B compartment and transition to A upon activation. On the other hand, other developmental genes are regulated by different mechanisms that act more locally, which is facilitated by their promoter isolation.
Fig. 5.
TTN locus is spatially regulated during differentiation. a Gene size of upregulated genes peaking in CM stage subdividing either B to A compartment or heart development genes (GO term). Boxplots present the median and 25th and 75th percentile, with the whiskers extending to 1.5 times the inter-quartile range. n = gene number; p-values by Wilcoxon test, ** < 0.01, *** < 0.001. b PC1 value of the TTN locus and the FPKM expression levels across differentiation. c Log transformed contact maps of the long arm of chromosome 2 containing the TTN locus at 500 kb resolution and a zoomed in view at 40 kb resolution, along with the PC1 values. d Gene track of the ATAC-seq signal, CTCF ChIP-seq signal (ESC and CM) and motif orientation, and GATA4, NKX2-5, and TBX5 (CM) ChIP-seq signal across the TTN locus. Dynamic ATAC peaks are highlighted, including increase (in red) at the promoters and decreases (in blue). e Virtual 4C of the TTN promoter at 40 kb resolution. f Fraction of stage-specific ATAC peaks (ESC and CM) overlapping CTCF peaks in the corresponding time point in B–A regions and the rest of the genome. Source data are provided as a Source Data file
Dynamic regulation of TTN by 3D structural changes in cis
A prominent example of the regulation of large cardiac genes just described is titin, the largest protein in the human genome, which when mutated is the leading cause of familial dilated cardiomyopathy (DCM)26. The TTN locus transitions from B to A, coincident with an opening up of the local chromatin structure and upregulation of transcription (Fig. 5b, c). There is a corresponding increase in accessibility at the two primary TTN TSSs, both the full-length transcript as well as a shorter isoform, termed Cronos TTN27 (Fig. 5d). Both isoforms have important developmental roles as mutations downstream of the Cronos TSS are significantly more deleterious than those only in the long isoform27. Both TSS-associated ATAC peaks are marked by the cardiac TF GATA4, NKX2-5, and TBX5 (Fig. 5d). Interestingly, we also observe peaks of accessibility within TTN that decrease during differentiation, all of which overlap CTCF peaks28. Using CTCF ChIP-seq data from hESCs and CMs28, we find that the CTCF sites that overlap hESC ATAC peaks show decreased occupancy during differentiation. With the exception of one CTCF site located at the transcriptional termination site, the CTCF motifs on TTN are orientated in a convergent direction, predictive of the presence of intragenic looping29. Indeed, during differentiation there is a decreased interaction between the TTN TSS and the TTN gene body, while there is little change involving the upstream region (Fig. 5e). Together this suggests that intragenic looping may be a mechanism to maintain silencing of TTN within the B compartment in hESCs, and that this is potentially mediated through CTCF30. Genome-wide we find a much greater overlap of hESC-specific ATAC peaks and hESC CTCF binding, compared with ATAC peaks that are CM-specific and CM CTCF binding, and this trend is not dramatically different between regions that go B to A and the rest of the genome (Fig. 5f). Thus while cardiac transcription factors are enriched in gained ATAC peaks and in B to A regions (Fig. 4g), CTCF is enriched in hESC-specific peaks and may be important to maintain differentiation permissive chromatin.
TTN interacts in trans with multiple RBM20-regulated loci
Having analyzed in detail the regulation of the TTN locus in cis, we explored the possibility that its genomic interactions with other chromosomes (in trans) could also be of regulatory importance. Interestingly, coincident with TTN’s move to the A compartment there is a switch in trans interactions from an enrichment with B compartment to A (Fig. 6a, hESC median Z-score A: −0.26 B: −0.07, CM median Z-score A: 0.19, B: −0.29). Genes upregulated and within the enriched trans contacts include factors involved in cell surface signaling and muscle contraction (Supplementary Table 8). Two of those TTN-associated cardiac genes are CACNA1C (which encodes the central subunit of the L-type calcium channel) and CAMK2D (which encodes a calcium/calmodulin dependent kinase that regulates excitation– contraction coupling; Supplementary Fig. 8e). All of these genes are subject to extensive alternative splicing, and are known targets of the muscle-specific splicing regulator RBM2011 (Fig. 6b). RBM20 controls tissue-specific isoform expression of several cardiac proteins mainly by promoting exclusion of selected exons or by controlling mutually exclusive exon selection, which is mediated by direct binding of RBM2011,31,32. Mutations in RBM20 are a relatively common cause of familial DCM, indicating its key importance in cardiac homeostasis33. Notably, while RBM20 is present throughout the interphase nucleus of muscle cells, it forms two clearly identifiable foci which overlap with newly synthetized TTN mRNA32. Thus, we hypothesized that the interaction of TTN with CACNA1C and CAMK2D could indicate the existence of a broader network of trans-interacting RBM20 targets.
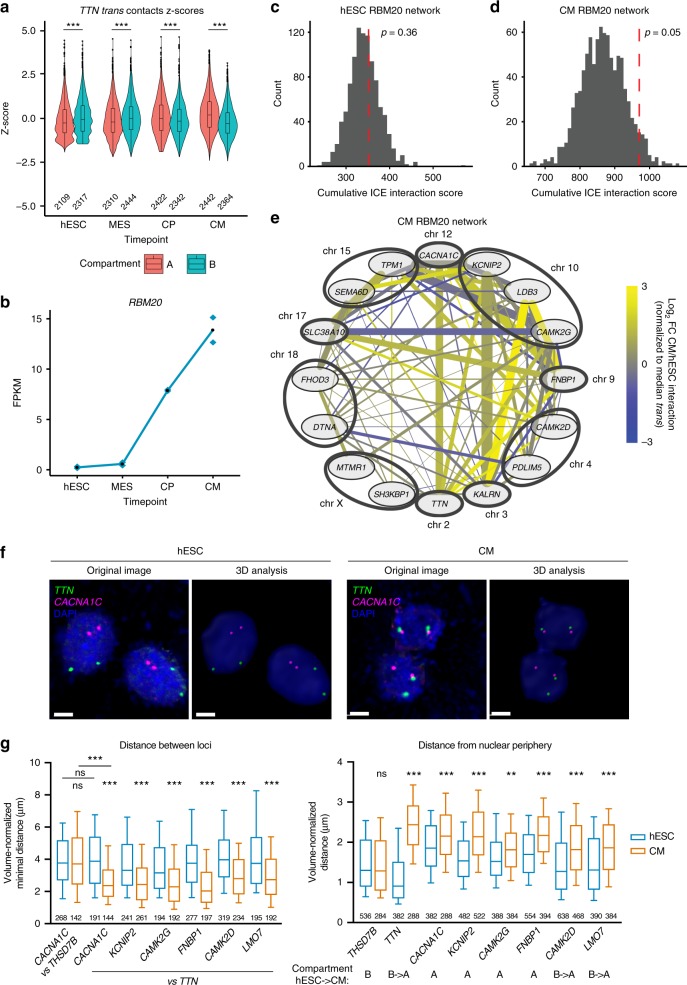
Fig. 6.
TTN locus becomes associated with RBM20 target genes. a Trans contacts of TTN by compartment, Z-scored by time point at 500 kb resolution. Boxplots within violin plots present the median and 25th and 75th percentile, with the whiskers extending to whole data range. The number of genomic bins is indicated; p-values by Wilcoxon test, *** < 0.001. b FPKM values for RBM20 across differentiation. c, d Association of upregulated RBM20 target genes in hESCs (c) and CM (d). Dashed red line indicates cumulative sum of ICE normalized Hi-C reads between target genes in trans. Histogram represents the background model of 1000 random permutations of selected genes from similar chromosomal distribution. The resulting random shuffling p-value is indicated. e Network of upregulated RBM20 genes, line thickness proportional to contact score, only scores greater than median trans contact displayed. f Representative 3D DNA FISH images in hESC and CM, and 3D reconstructions after spot calling processing (nuclei counterstained with DAPI); scale bars: 5 µm. g On the left, normalized minimum distance per diploid cell between the indicated loci (the number of cells is indicated). On the right, normalized distance of each locus from the nuclear periphery (the number of loci is indicated); A/B compartment transitions based on Hi-C data are reported. Box and whiskers plots present aggregated data from two independent cultures, and indicate median, 25th and 75th percentile, and the 10–90 percentile range. p-values by Kruskal–Wallis test followed by Dunn’s multiple comparisons vs hESC (unless otherwise indicated); ns ≥ 0.05; ** < 0.01; *** < 0.001. Source data are provided as a Source Data file
To computationally test this, we focused on a set of 16 CM-upregulated genes whose spicing is regulated by RBM20 in both human and rat hearts11. These genes show a significant Hi-C association in CMs but not in hESCs (Fig. 6c–e). To control for the possibility that this could be simply the result of compartment switching, we repeated the analysis with only those genes that are constitutively in the A compartment against a comparable background gene set and still observed a gain in association (Supplementary Fig. 8f). We validated these findings by 3D DNA FISH for TTN and its top five trans-interacting genes within the RBM20 network (CACNA1C, CAMK2D, CAMK2D, KCNIP2, and FNBP1; Fig. 6f, g). We also included LMO7 because while this gene was not present in the set of RBM20 targets we originally considered, it is a well-established RBM20 target34 which shows strong interaction with TTN in CM. Remarkably, each of these loci moves significantly closer to the TTN locus when hESCs differentiate into CMs (Fig. 6f, g and Supplementary Fig. 9a). Accordingly, these loci were found in proximity to TTN in 33–58% of all CM (compared with 8–24% in hESCs; Supplementary Fig. 9a). On the contrary, differentiation does not affect the distance between CACNA1C and THSD7B, a negative control region on the same chromosome as TTN but located in a constitutive B domain ~40 Mb away (Fig. 6g). This indicates that the increased vicinity between TTN and CACNA1C in CM does not result from mere changes in the position of chromosome territories. Interestingly, these same 3D FISH experiments showed that the position of TTN and the other tested loci relative to the nuclear periphery closely correlate with their compartment status as predicted by Hi-C data (Fig. 6g). In particular, during differentiation TTN moves from the nuclear periphery to the interior, suggesting that this resides in a heterochromatic lamin-associated domain in hESCs, and moves into euchromatic nucleoplasm coincident with its B to A transition in cardiomyocytes (Fig. 6f, g). CAMK2D and LMO7 show a similar strong change in localization which matches their movement from B to A, while there is only a limited effect on the distance from the nuclear periphery for genes found always in the A compartment. Collectively, these observations indicate that as multiple RBM20 targets are upregulated they migrate toward the center of the nucleus, where they increase their proximity to the TTN locus.
TTN trans contacts need transcription-dependent RBM20 foci
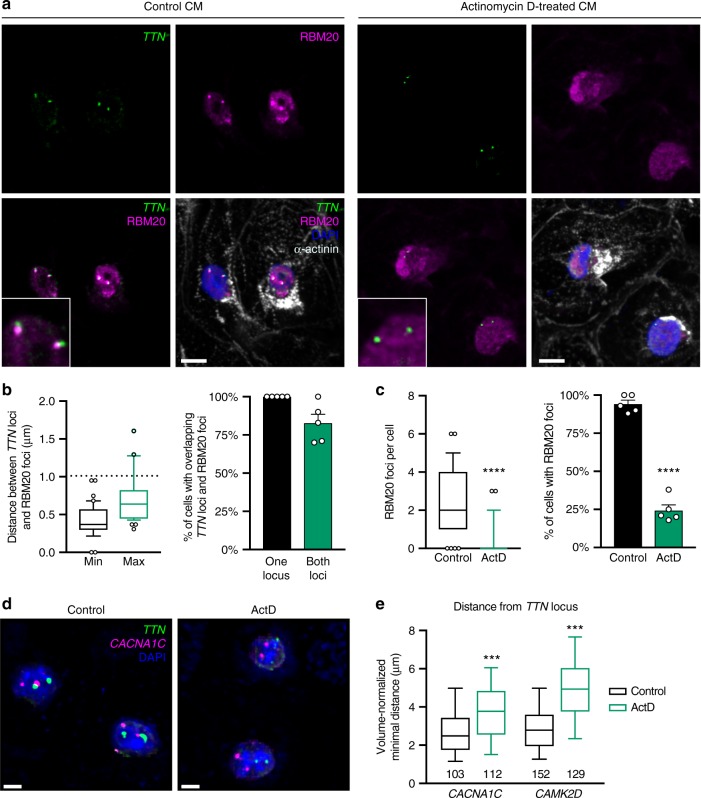
To functionally test the hypothesis that RBM20 forms a splicing factory involving multiple loci found on different chromosomes, we first took advantage of pharmacology. It was previously shown that RBM20 foci in HL-1 immortalized mouse cardiomyocytes are transcription-dependent32, which offers an avenue to test the effect of their disruption. We confirmed that RBM20 forms foci also in CM, and that these closely co-localize with the TTN loci (Fig. 7a, b). Treatment with the transcription inhibitor Actinomycin D leads to a rapid loss of such RBM20 foci in most CM (Fig. 7a–c). Remarkably, this correlates with a strong reduction in the proximity between TTN and both CACNA1C and CAMK2D, while the localization of these loci relative to nuclear periphery was either unaffected or only modestly affected (Fig. 7d, e and Supplementary Fig. 9b). Thus active transcription is necessary for the establishment and/or maintenance of these trans interactions, but not for localization of the individual loci within the nucleoplasm.
Fig. 7.
TTN trans interactions are transcription-dependent. a Representative results of immunofluorescence for RBM20 and α-actinin combined with DNA FISH for the TTN locus in CM (immunoFISH; nuclei counterstained with DAPI); scale bars: 10 µm. Insets show magnified views. Cells were maintained in standard culture conditions or treated with 5 µM Actinomycin D. b Quantification of distance relationships between RBM20 foci and TTN loci in control conditions. Center-to-center distance below 1 µM (twice the radius of 3D-resconstructed spots) was used to determine overlap (see Methods). On the left, box and whiskers plots showing median, 25th and 75th percentile, and the 10–90 percentile range plus outliers; n = 40 diploid cells. On the right mean ± s.e.m.; n = 5 field of views. c Quantification of RBM20 foci in control and Actinomycin D-treated CM. Graphs are as described for panel (b) except that on the left n = 64 and 55 cells for CTR and ActD, respectively. Note that all CM were considered, including polyploid cells with more than two RBM20 foci. p values by Mann–Whitney test (left) or Welch’s t-test (right); **** < 0.0001. d Representative 3D FISH images of control and Actinomycin D-treated CM; scale bars: 5 µm. e Normalized minimum distance per diploid CM between TTN and the indicated loci (the number of cells is reported). Box and whiskers plots show median, 25th and 75th percentile, and the 10–90 percentile range. p-values by Kruskal–Wallis test followed by Dunn’s multiple comparisons vs Control; *** < 0.001. Source data are provided as a Source Data file
The TTN pre-mRNA nucleates an RBM20 splicing factory
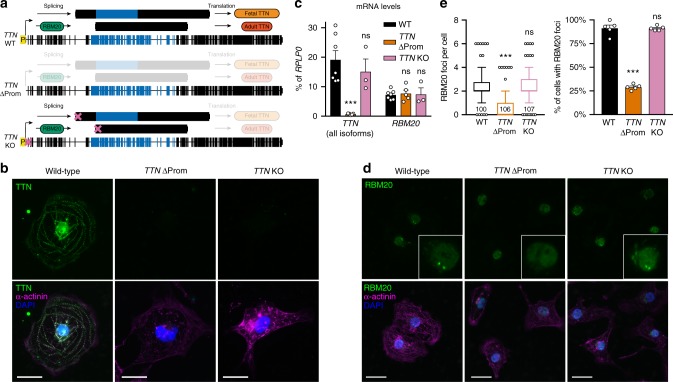
To further probe the mechanism behind the formation of RBM20 foci and of the trans chromatin interactions involving TTN, we generated a number of gene-edited lines using CRISPR/Cas9 (Supplementary Fig. 10a–h). First, we deleted the TTN promoter to test the effect of abolishing its transcription (Fig. 8a; referred to as TTN ∆Prom). As a control, we inserted a nonsense mutation in the first coding exon of TTN, which is predicted to interfere only with mRNA translation but not with TTN transcription nor splicing (Fig. 8a; referred as TTN KO). We confirmed that both mutations abolish titin protein expression (Fig. 8b), while only TTN ∆Prom impairs steady state mRNA levels (Fig. 8c). Remarkably, while RBM20 is expressed at normal levels in TTN ∆Prom and localized throughout the nucleus, the number of RBM20 foci is strongly reduced (Fig. 8c–e). On the contrary, the localization of RBM20 is normal in TTN KO CM (Fig. 8c–e). This indicates that the TTN mRNA is specifically required for the nucleation of RBM20 into foci, which can be explained by fact that TTN contains the highest number of RBM20 binding sites among all of its targets (more than 100 locations compared with an average of ~1.7 for other genes34).
Fig. 8.
TTN transcription is required for nucleation of RBM20 into foci. a Schematic of the hESC gene editing strategies used to test the role of transcription at the TTN locus. ∆Prom: promoter (P) deletion; KO: functional knockout by frameshift mutation (indicated by the X). Aspects of TTN biogenesis predicted to be impaired by each modification are indicated by increased transparency (loss of transcription following promoter deletion; loss of translation following knockout). Alternatively spliced exons regulated by RBM20 are shown in blue. b Representative immunofluorescence results in CM derived from the indicated hESC lines (nuclei counterstained with DAPI); scale bars: 10 µm. c RT-qPCR in CM derived from the indicated hESC lines. Expression is relative to the housekeeping gene RPLP0 and mean ± s.e.m. is shown. n = 3, 5, or 7 independent differentiations for TTN KO, TTN ΔProm, and WT, respectively. d As in panel (b); insets show magnified views. e Quantification of RBM20 foci in CM derived from the indicated hESC lines. On the left, box and whiskers plots showing median, 25th and 75th percentile, and the 10–90 percentile range plus outliers; the number of cells is indicated (non-diploid CM were included in the analysis). On the right mean ± s.e.m.; n = 5 field of views. All p-values in this figure are calculated vs WT by one-way ANOVA followed by Holm-Sidak’s multiple comparisons (for (c) and the right graph in (e)) or by Kruskal–Wallis test followed by Dunn’s multiple comparisons (for the left graph in (e)); ns ≥ 0.05; *** < 0.01. Source data are provided as a Source Data file
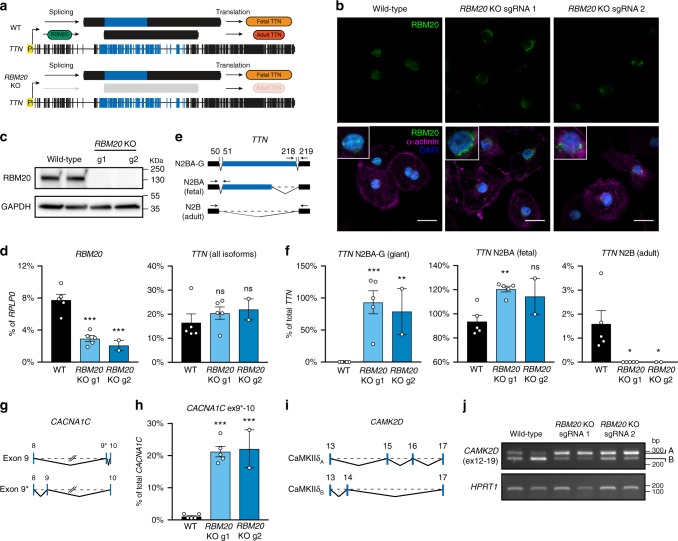
As a second genetic model, we knocked out RBM20 itself (Fig. 9a). Previous studies have examined the effect of DCM-causing heterozygous point mutations in RBM20 using hPSC-CM35,36, but the functional effect of complete RBM20 loss of function in this context remains unknown. By targeting the second exon of RBM20 with two independent single guide RNAs (sgRNAs) we abolished nuclear expression of RBM20 in CM (Fig. 9b). While the RBM20 antibody showed some perinuclear staining in RBM20 KO CM, western blot indicated complete loss of full-length RBM20 with no detectable truncated protein (Fig. 9c and Supplementary Fig. 11). To validate the knockout from a functional standpoint we examined the expression of TTN splice variants. Two major developmentally-regulated TTN isoform exists: the fetal N2BA isoform, which includes a number of alternatively spliced exons encoding PEVK elastic domains and leads to a long and compliant protein, and the adult N2B isoform, which lacks such PEVK exons and is stiffer37. This developmental switch is physiologically important to increase the stiffness of the developing myocardium as it sustains higher blood pressure. Previous findings in Rbm20 knockout rats and mice demonstrated that Rbm20 is required for the exclusion of PEVK exons11,38, and that complete loss of function of Rbm20 leads to expression a non-physiological giant form of TTN, named N2BA-G. We confirmed that while RBM20 KO CMs express normal levels of TTN mRNA, the transcript is entirely represented by the N2BA-G isoforms, while the N2B isoform is undetectable (Fig. 9d–f). We also examined alternative splicing of CACNA1C and CAMK2D, and confirmed that RBM20 KO affects the choice of alternative exons for both transcript (Fig. 9g–j). For CACNA1C, this results in inclusion of exon 9*, which is associated with hyperpolarization of L-type calcium channel39. For CAMK2D, this leads to an increased ratio of the CaMKIIδA isoform, which is found at the intercalated disks and T tubules, over the CaMKIIδB isoform, which is nuclear localized40. Overall, these findings validated the functional knockout of RBM20 in CM.
Fig. 9.
Knockout of RBM20 dysregulates alternative splicing in hESC-derived cardiomyocytes. a Predicted effects of the knockout of RBM20 on TTN biogenesis (loss of RBM20-dependent alternative exon exclusion). Refer to the legend of Fig. 8a for the abbreviations. b Representative immunofluorescence results in CM derived from wild-type or RBM20 knockout hESCs generated with two independent CRISPR/Cas9 single guide RNAs (sgRNAs). Nuclei are counterstained with DAPI, and insets show magnified views; scale bars: 10 µm. c Western blot validation of RBM20 knockout (uncropped images shown in Supplementary Fig. 11). g: sgRNA. d RT-qPCR in CM derived from the indicated hESC lines. Expression is relative to the housekeeping gene RPLP0 and mean ± s.e.m. is shown. n = 5 (WT and RBM20 KO g1) or 2 (RBM20 KO g2) independent differentiations. e Expected major TTN isoforms in CM. Exons predicted to be excluded by RBM20 are in blue, and arrows indicate the location of RT-qPCR primers. f As in panel (d), but expression of the indicated TTN isoform is relative to the total amount of TTN. g Expected CACNA1C isoforms due to RBM20-dependent alternative exon selection. RBM20 is predicted to favor inclusion of exon 9 over exon 9*. h As in panel (d), but expression of the CACNA1C exon 9* isoform is relative to the total amount of CACNA1C. i Expected CAMK2D isoforms due to RBM20-dependent alternative exon selection. RBM20 is predicted to favor inclusion of exon 14 over exons 15–16. j Semiquantiative RT-PCR in CM for the indicated CAMK2D isoforms and the housekeeping gene HPRT1; n = 2 independent differentiations. All p-values in this figure are vs WT and calculated by one-way ANOVA followed by Holm-Sidak’s multiple comparisons (ns ≥ 0.05; * < 0.05; ** < 0.01; *** < 0.001). Source data are provided as a Source Data file
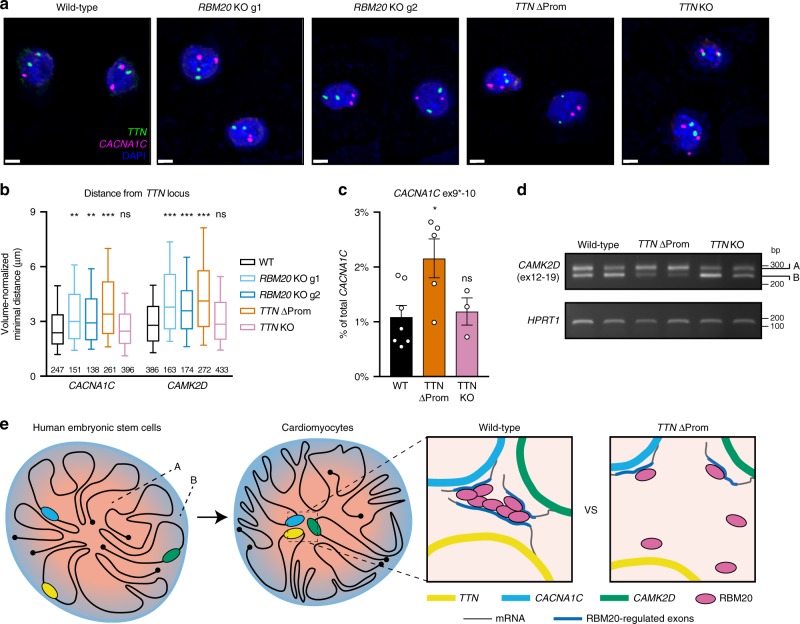
Having developed these cellular models, we tested the effect of each genetic perturbation with regards to the interaction of TTN with RBM20 target loci. Remarkably, we observed that TTN’s interaction with CACNA1C and CAMK2D is significantly reduced in both RBM20 KO and TTN ∆Prom CM, while TTN KO CM behave similarly to wild types (Fig. 10a, b and Supplementary Fig. 9c). Further, the localization of these loci relative to the nuclear periphery is either unaffected or only modestly affected, indicating that RBM20 is not required for the transition from the nuclear periphery (Supplementary Fig. 9c). Thus, these multiple trans chromatin interactions involving the TTN locus require both the expression of RBM20 and the transcription of TTN mRNA to nucleate RBM20 into foci.
Fig. 10.
RBM20 foci promote alternative splicing of genes interacting with TTN in trans. a Representative 3D DNA FISH images in CM derived from the indicated hESC lines (nuclei counterstained with DAPI); scale bars: 5 µm. b Normalized minimum distance per diploid CM between TTN and the indicated loci (the number of cells is reported). Box and whiskers plots show median, 25th and 75th percentile, and the 10–90 percentile range. p-values by Kruskal–Wallis test followed by Dunn’s multiple comparisons vs WT; ns ≥ 0.05; ** < 0.01; *** < 0.001. c RT-qPCR in CM derived from the indicated hESC lines. Expression of the CACNA1C exon 9* isoform is relative to the total amount of CACNA1C and mean ± s.e.m. is shown. n = 3, 5, or 7 independent differentiations for TTN KO, TTN ΔProm, and WT, respectively. p-values calculated by one-way ANOVA followed by Holm-Sidak’s multiple corrections vs WT; ns ≥ 0.05; * < 0.05. d Semiquantiative RT-PCR in CM for the indicated CAMK2D isoforms and the housekeeping gene HPRT1; n = 2 independent differentiations. e Proposed model for the regulation of global and local chromatin organization during human cardiogenesis. Upon differentiation the heterochromatin compacts while large cardiac genes such as TTN transition from the inactive to the active compartment. Transcription of TTN nucleates foci of its splicing regulator RBM20 leading to a trans-interacting chromatin domain (TID) involving other RBM20 targets. This mechanism promotes alternative splicing of the resulting transcripts, and can be disrupted by preventing TTN transcription. Source data are provided as a Source Data file
Finally, we investigated whether disruption of this mechanism affects alternative splicing of RBM20 targets by profiling RBM20-dependent isoform expression in TTN ∆Prom CM. Notably, both CACNA1C exon 9* and CaMKIIδA are upregulated in these conditions, compared with both wild-type CM and TTN KO CM (Fig. 10c, d). Based on these collective observations, we conclude that TTN, CACNA1C, and CAMK2D are part of a RBM20-dependent trans-interacting chromatin domain, and that this mechanism controls their alternative splicing (Fig. 10e).
Discussion
This study provides a comprehensive integration of Hi-C, RNA-seq, and ATAC-seq across a defined time course of human hPSC differentiation into cardiomyocytes, which demonstrates the dynamic interplay of local and global chromatin structure on gene regulation during cell fate transitions. Moreover, we confirm that in vitro derived human cardiomyocytes are similar to their in vivo counterparts with respect to genome architecture and gene expression, supporting their use in developmental and disease modeling. We find that ~19% of the genome transitions either from active (A) to repressive (B) compartment or the reverse. Regions that transition from B compartment to A include many large cardiac genes, and this transition occurs coincident with an increase in local accessibility and increased transcription. While we do not find a strong association between genes that transition from A to B compartment with downregulation, this could reflect a mechanism to permanently silence genes related to alternative lineages which were never activated. These findings are in line with recent Hi-C analyses during mouse cardiogenesis41, indicating that dynamic compartmentalization is a conserved mechanism for gene regulation. Active regions are associated both in cis and trans and have high chromatin accessibility and transcription throughout differentiation. In contrast, heterochromatin is relatively accessible in hESCs compared with differentiated cells, but this compacts during differentiation. Heterochromatin compaction coincides with loss of ATAC peaks and TAD boundaries while long-range Hi-C signal increases. This process is similar to that resulting from CTCF or cohesin depletion, which results in loss of local TAD structure but does not alter compartmentalization, and in fact strengthens long-range interactions42,43. Thus, we speculate that loss or decrease of CTCF/cohesin activity in B compartment along with a gain in heterochromatin proteins during differentiation may provide the driving force behind compaction.
Mis-regulation of the spatial organization of the genome has been implicated in a host of diseases44. Approximately 1% of live births manifest congenital heart disease (CHD), which is often caused by mutations in transcription factors or chromatin modifiers1. Furthermore, over 500 loci are associated with risk of cardiovascular diseases (CVD) in the adult, and a large fraction of these are intergenic regions with likely regulatory functions involving long-range chromatin interactions. Therefore, the use of genomics resources such as those described here represents a valuable approach to clarify the molecular bases of CHD and CVD. Recent examples of this approach include using promoter capture Hi-C45–47 or deeply sequenced Hi-C48 to prioritize functional targets of CVD associations, and combining Hi-C from non-cardiac cells with GWAS analysis to identify gene modifiers associated with high risk of Tetralogy of Fallot49. Overall, by providing a detailed genome-wide chromatin organization map in differentiating cardiomyocytes together with extensive gene expression and chromatin accessibility data, our work will represent a valuable resource for the study of both cardiac development and disease.
We observed that trans interactions are enriched within A compartments. We suspected that these interactions were clues to gene regulatory mechanisms, which we explored in detail for the key cardiac gene TTN. This led us to identify a novel 3D chromatin feature specific to cardiomyocytes: the spatial coalescence of multiple genes from different chromosomes into foci marked by the splicing regulator RBM20. This mechanism relies on the newly-transcribed TTN pre-mRNA, which has a large number of RBM20 binding sites and thus appears to function as a scaffold to nucleate RBM20 foci. The concentration of RBM20 and multiple target loci results in more efficient RBM20-mediated regulation of alternative splicing of its other target transcripts, and it may reciprocally enhance the efficiency of TTN RNA splicing. RBM20 has been shown to affect splicing in a concentration-dependent manner, and to compete with the splicing regulator PTB4, which binds to the same RNA motif and antagonizes its function31. Thus, foci of highly concentrated RBM20 are expected to promote its activity by increasing binding to the target transcripts. We propose that this property explains why RBM20-dependent alternative splicing is less effective when RBM20 foci are disrupted due to deletion of the TTN promoter. It is possible that this mechanism may be similarly dysregulated in certain forms of hereditary or acquired cardiac disease and could lead to aberrant alternative splicing of key proteins involved in excitation–contraction coupling such as CACNA1C and CAMK2D, similarly to what has been described for mutations in RBM20 itself50. For instance, heterozygous mutations in GATA4 that cause CHD lead to reduced transcription of TTN22 (in agreement with the presence of a strong GATA4 binding site on the TTN TSS), which could weaken RBM20 foci and thus indirectly affect splicing of other RBM20 targets. Another interesting observation is that the N2BA TTN isoform normally repressed by RBM20 is upregulated in ischemic heart disease and in dilated cardiomyopathy51,52. This suggests weakening of the RBM20-dependent splicing factory, which could influence a number of other RBM20 targets. Thus, modulation of RBM20 activity or localization could offer a therapeutic avenue to correct aberrant alternative splicing in CHD and CVD.
Generalizing our findings beyond the cardiomyocyte context, we propose that the RBM20-dependent splicing factory is an example of trans-interacting chromatin domains (TIDs) functionally involved in the regulation of gene expression (similarly to what achieved in cis by TADs). While trans interactions in Hi-C datasets are generally poorly explored due to our limited understanding of their significance, another well-established example of a TID-like structure has been described in mouse olfactory neurons, in which the transcription factor LHX2 and adaptor protein LDB1 organize a multi-chromosomal super-enhancer to enable activation of a single olfactory receptor gene53. A wide array of nuclear speckles exists, in which factors that regulate transcription or various aspects of mRNA metabolism are highly concentrated54. By analogy to our findings for RBM20 foci, we hypothesize that many other TIDs exist in association to such nuclear speckles or to other yet to be determined subnuclear structures. Overall, we submit that the nature of TIDs and their properties such as stability, size, heterogeneity, regulation, and cell specificity represent promising avenues of future investigation.
Methods
Cell culture
RUES2 hESCs (RUESe002-A; WiCell) and WTC-11 hiPSCs (a gift of Bruce R. Conklin; also available from Coriell #GM25256) were maintained on recombinant human Laminin-521 matrix (rhLaminin521; Biolamina) at 0.5 μg cm−2 with Essential 8 (E8) media (ThermoFisher) and passaged as single cells with Versene (ThermoFisher). Cells were tested to be karyotypically normal by conventional G-banding. hESC differentiation was conducted with modifications from a published method55. In total, 4 × 105 cells in E8 with 10 μM Y-27632 (Tocris) were seeded per well of a 12-well dish that was pre-coated with 2 μg cm−2 rhLaminin521 (denoted day −2). After 24 h media was changed to E8 with 1 μM CHIR-99021 (Cayman), denoted day −1. On day 0 media was changed to RPMI (ThermoFisher) with 500 μg mL−1 BSA (Sigma, A9418) +213 μg mL−1 ascorbic acid (Sigma, A8960), denoted RBA media, supplemented with 3 μM CHIR-99021. Samples for day 0 were collected prior to media change. Day 2 samples were collected 48 h later. Wells for further differentiation were left unchanged until day 3, when media was changed to RBA plus 1 μM Xav-939 (Tocris). On day 5, samples were either collected or changed to RBA without inhibitors. On day 7 media was changed to RPMI plus B27 (Thermo Fisher, 17504044), with further media changes every other day until day 14 samples were collected. hiPSC differentiation was conducted according to this same protocol with the following optimizations: on day −2, 1.5 × 105 cells were seeded per well of a 12-well dish; on day 0, RBA was supplemented with 6 μM CHIR-99021; on day 3, RBA was supplemented with 2 μM Wnt-C59 (Selleckchem).
Fetal heart samples
Fetal Heart samples were collected by the Laboratory of Developmental Biology at the University of Washington (UW), in accordance with IRB approval from the UW. Collected hearts were washed in PBS and flash frozen in liquid N2 and stored at −80 °C prior to further analysis.
In situ DNase Hi-C
Hi-C was performed on 2–3 × 106 cells from each time point from two independent differentiations according to the published protocol10. Cells were fixed in the dish with fresh RPMI supplemented with 2% formaldehyde (with methanol) while in gentle orbital rotation for 10 min, and then quenched with 1% 2.5 M Glycine for 5 min at room temperature followed by 15 min at 4 °C. Cells were then treated with Trypsin (0.05%, ThermoFisher) for 5 min at 37 °C, rinsed in RPMI with 10% FBS, and scraped off the plate. Cells were washed once with PBS and flash frozen at stored at −80 °C until library prep. For fetal heart samples, frozen hearts were first homogenized with a tissue dismembrator (2000 RPM, 1 min) treated with Trypsin for 5 min at 37 °C, and then subjected to cell lysis. Whole heart samples were lysed in 500 μL buffer (10 mM Tris-HCl, pH 8.0, 10 mM NaCl, 0.2% Igepal CA-630, 1× Protease Inhibitor) and then dounce homogenized 30 times with a tight pestle. Cell culture samples were lysed directly in 500 μL lysis buffer. Samples were treated similarly from here forward. Nuclei were first resuspended in 300 μL DNase buffer with 0.2% SDS plus MnCl2 and incubated at 37 °C for 60 min with periodic vortexing, and then 300 μL DNase buffer with 2% Triton X-100 and RNase A were added and incubated for another 10 min. Seven units of DNase (ThermoFisher EN0525) were added and incubated for 7 min at RT. The reaction was stopped with 30 μL of 0.5 M EDTA and 15 μL 10% SDS. Nuclei were spun down and resuspended in 150 μL water and added with 300 μL AMPure XP beads (Beckman) to irreversibly bind nuclei. In situ reactions were performed for end repair (T4 DNA Polymerase, ThermoFisher EP0062, and Klenow, ThermoFisher EP0052) and dA-tailing (Klenow exo-, ThermoFisher EP0422) prior to overnight ligation of biotin adapters at 16 °C. After incubation nuclei were washed twice with AMPure buffer (20% PEG in 2.5 M NaCl) followed by two washes with 80% ethanol to remove un-ligated adapter. Nuclei were then treated with PNK to phosphorylate the adapters and ligation reaction was performed for 4 h at RT with shaking. Nuclei were then treated with proteinase K overnight at 62 °C, and DNA was precipitated with 3 μL glycogen, 3 M Na-acetate (pH 5.2) and isopropanol for 2 h at −80 °C. DNA was resuspended in 100 μL water and purified with 100 μL of AMPure beads. Biotin pull-down was performed on the purified DNA to isolate adapter containing DNA. Myone C1 beads (ThermoFisher 65001) were mixed with DNA for 30 min at RT with rotation. Following this, samples were washed four times with B&W buffer (5 mM Tris-HCl pH 8.0, 0.5 mM EDTA, 1 M NaCl, 0.05% Tween-20). DNA was then treated on beads for end repair (ThermoFisher K0771) and dA-tailing (Klenow Exo-), four washes with B&W buffer and two washes with TE were performed between each reaction. Sequencing Y-adapters were then ligated for 1 h at RT. Libraries were amplified between 9 and 12 cycles with Kapa HiFi ReadyStart Master Mix (KK2602) with barcode-containing primers. Libraries were then purified with 0.8 volumes of Ampure XP beads and quantified with a Qubit for sequencing.
Hi-C processing
Samples were paired-end sequenced on an Illumina NextSeq 500 in a high output run with 150 cycles (75 for each end). Fastq files were mapped to the hg38 genome using BWA-MEM with default parameters, mapping each end of the read pairs individually. Primary mapping sequences were extracted, and then the mapped files were processed through HiC-Pro56, filtering for MAPQ score >30, and excluding read pairs that mapped within 1 kb, to generate valid pairs and ICE balanced matrices at 500 and 40 kb resolution56. Downstream analysis for female RUES2 hESCs excluded the X chromosome due to the confounding factor of X inactivation, while sex chromosomes were included for male WTC-11 hiPSCs. Heatmaps for all Hi-C data were generated through Cooler (https://github.com/mirnylab/cooler), based on the raw counts as cooler includes its own ICE balancing, and were used only for visualization purposes using default parameters and log values on the contact matrices, without diagonals. Virtual 4C data were extracted from ICE balanced matrices. HiC-Rep scores were calculated using a resolution of 500 kb with a max distance of 5 Mb and h = 113.
The valid pairs file from HiC-Pro (after filtering for distance and MAPQ score) was used as input into HOMER57 for eigenvalue decomposition of the contact maps to calculate PC1 (A/B) scores at 500 kb resolution and with no additional windowing (super-resolution also set at 500 kb). Bins were assigned to A compartment if their average value between replicates were > 0 and B if < 0. Visualization of sample similarity by PC1 scores was performed with the t-sne function in R. Gene tracks were generated in IGV58. Switching A/B compartments were determined by a one-way ANOVA p-value < 0.05 (two replicates per time point, across the four time points of differentiation) and at least one time point having an average PC1 score > 0 and at least one < 0. A–B–A–B and B–A–B–A transitions represented 2% of the switching regions and were combined with A–B and B–A transitioning regions for downstream analysis. Saddle plots were generated by assigning each bin to its corresponding percentile value and dividing the genome into ten sets of deciles. Each interaction was normalized to the average score at the corresponding distance for cis interactions or to the average of all trans interactions and assigned to the pair of deciles based on the two bins. The plot represents the log2 average value for pairs of deciles. The change between CM and hESC or hiPSC is the log2 value of the difference. For the distance curves by compartment, each interaction was assigned to A–A, B–B, or A–B based on the pairs of bins and then the average interaction score for a given distance was normalized to the average interaction score for all pairs of contacts for that distance. For presentation, data are plotted on a log scale. For smoothing, the R function geom_smooth from ggplot259 was used with a loess smoothing function.
TADs were determined using two distinct methods: (1) Directionality Index (DI) method and domain call pipeline17; (2) insulation score method18 at 40 kb resolution. Similarity in TAD boundaries (edges of assigned TADs) between samples were quantified by Jaccard Index (number of shared boundaries divided by union set of boundaries). For stringent analysis between replicates, boundaries were considered shared if there was a boundary within ± 80 kb in the other sample. Given that TAD boundaries cluster by replicate we used TAD calls on the merged samples for each condition for subsequent analyses. For comparison between time points boundaries were considered shared if there was a boundary within ± 200 kb, time point specific boundaries were identified by the lack of a shared boundary with the respective comparison. For comparison between A/B compartments and TADs we used to bedtools intersect -u -f 0.51 -a <TADs.bed> -b <compartment.bed> to assign the TADs to a specific compartment, and the majority compartment if it spans a boundary. For differential and shared boundaries between hESC and CM we used bedtools intersect -u -f 0.51 -a <TADs_boundary.bed> -b <compartment.bed>, p-values were calculated based on a chi-squared test between the distribution of differential or shared TAD boundaries versus the distribution of the union set of TAD boundaries.
For trans-interaction analysis reads from the two replicates were pooled. For analysis of a specific locus, all inter-chromosomal ICE balanced counts at 500 kb resolution were extracted for the bin of interest and then Z-scored after filtering out the top 0.5% values. Z-scores were assigned to A/B compartments based on their Hi-C PC1 score for the given time point. P-values between time points were determined by the Wilcoxon test in R. Network association for RBM20 was determined by totaling the ICE balanced counts at 500 kb resolution for all associated trans contacts within the set of 16 genes, excluding the counts between genes on the same chromosome. Bin assignment was determined by the location of the gene promoter. The background model was based on a set of 1000 random permutations, each time selecting a new set of genes with the same number of genes per chromosome as the seed set, similar to an earlier report60, and totaling the respective ICE balanced counts. Visualization of the network was generated with Cytoscape61. Edge widths are proportional to the ICE balanced counts. Only edges with a count greater than the median trans count for CM are presented. Edge color is scaled to the log2 fold change of CM versus hESC of values divided by the median trans count for the respective time point.
RNA-seq
RNA-seq libraries were prepared from total RNA using the TruSeq Stranded Total RNA Ribo-Zero H/M/R kit (Illumina RS-122-2201). RNA-seq libraries were paired-end sequenced on a Illumina NextSeq 500 in a high output run with 150 cycles (75 for each end). Reads were mapped to hg38 using STAR with default parameters62 and then quantified and processed through the Cufflinks suite63. Differential expressed genes exhibited a q-value < 0.05 for a pairwise comparison and were expressed at least 5 FPKM in one time point. Clustering and data processing were performed with the CummeRbund suite in R. For gene tracks, values were normalized to reads per million with Bedtools64.
Enrichment of differential genes with A/B compartments was normalized to reflect the genome-wide gene density as calculated by the number of genes in each type of compartment transition (A–B, B–A, etc.). P-values were calculated by a chi-squared test between the total number of genes in a type of compartment transition relative to the number of differentially expressed genes within that region. For example comparing the number of genes in B to A regions with the number of genes upregulated at CM. Gene ontology was conducted with DAVID65. Gene sizes and gene distances were calculated from the Ensembl hg38 annotation. P-values were calculated by the Wilcoxon test in R. For comparison of genes across adult tissues including adult heart from the EMBL Protein Atlas, RNA expression values were downloaded from EMBL-EBI (E-MTAB-2836). For each gene, expression was ranked by tissue from 1 (highest expression value tissue) to 37 (lowest expression value tissue). Genes were then sub-divided first by whether they were upregulated in CMs and second by whether they were in B to A regions or other genomic regions. Cumulative distribution functions were plotted in R, and p-values determined by K-S test.
ATAC-seq
ATAC-seq was performed according to the protocol from the Greenleaf lab66. Briefly, cells were harvested from cell culture and lysed in lysis buffer (10 mM Tris-Cl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.1% Igepal CA-630, 1× Protease inhibitor, Sigma). In total, 5 × 104 nuclei were washed in lysis buffer and resuspended in 25 μL TD buffer, 24 μL lysis buffer, 1 μL TDE1 enzyme from the Nextera DNA Prep kit (Illumina FC-121-1030). The reaction was incubated for 30 min at 55 °C and DNA was then extracted with a MinElute PCR Purfication kit (Qiagen 28004). DNA was amplified using NEBNext High-Fidelity 2× Master Mix (M0541L) and quantified with a Qubit. All samples were pooled with Nextera primers and paired-end sequenced on a Illumina NextSeq 500 in a high-output run with 150 cycles (75 for each end).
ATAC-seq processing
Sequenced reads were mapped with BWA-MEM to hg38 with default parameters for paired-end reads. Mapped reads were filtered for their primary mapping location and a MAPQ score >30. Duplicate reads were removed with Picard Tools (available at http://broadinstitute.github.io/picard/). Hotspots were called with MACS2 using the parameters “--nomodel -q 0.01 --shift -100 --extsize 200”. The intersection and union sets were determined by the Bedtools intersect function64. Quantification was performed with a custom python HTSeq script67 that treats each read of the paired-end reads separately. Normalization and correlation was of ATAC hotspots was performed with DESeq268. The overlap figure was generated with the UpSetR package69. Motif analysis was performed with DREME and Tomtom using a cut-off of E-value < 0.0570. Enrichment analysis with types of compartment dynamics was calculated based on the expected distribution given the distribution of the union set of all ATAC peaks. P-values were calculated based on chi-squared test between counts in a given compartment dynamic for stage-specific peaks and total ATAC peaks. Gene tracks were normalized to reads per million and produced in IGV.
ChIP-seq processing
Reads were downloaded from GEO for the corresponding samples: GATA4 hESC24 (GSE61475); GATA4 and TBX5 CM22 (GSE85628); NKX2-5 CM23 (GSE89457). Peak files were downloaded from GEO and lifted over to hg38 using UCSC liftover. Gene tracks were generated by mapping sequenced to hg38 with BWA-MEM with default parameters for single end reads. Duplicate reads were removed with Picard Tools (available at http://broadinstitute.github.io/picard/). Genome coverage was calculated with bedtools genomeCoverageBed program with an extension of 200 bp. Coverage over ATAC peaks was calculated with HOMER annotatePeaks.pl using -hist for cumulative plots and -ghist for heatmaps. CTCF data were downloaded from ENCODE (https://www.encodeproject.org) for hESC CTCF (ENCSR000AMF) and CM CTCF (ENCSR713SXF). Pseudoreplicated idr thresholded peaks were used for peak calls and fold change over control was used for gene tracks.
Gene editing
Single guide RNAs (sgRNAs) for CRISPR/Cas9 were identified using GuideScan71 to achieve a specificity score higher than 0.7. Two sgRNAs were designed up- and downstream of the TTN promoter with the goal of deleting this region upon co-delivery. Individual sgRNAs were designed toward exon 2 of TTN and exon 2 of RBM20 with the aim of inducing knockout indels leading to nonsense mutations following repair of double-strand breaks by non-homologous end joining (NEHJ). The 20 bp sgRNA targeting sequences (no PAM) were: TTN prom up 5′-GTGAAACTCTACTTAGAGGG-3′; TTN prom down 5′-TTTAAGGGAATCAACTGCTG-3′; TTN ex2 5′-GCAGCCGTTACAAAGCGTTG-3′; RBM20 ex2-1 5′-AGGGTCCGGGGCCTCGTGTC-3′; RBM20 ex2-2 5′-CGGGCAGTGTGACCTATGAA-3′. These sequences were cloned as double stranded oligonucleotides in pSpCas9(BB)-2A-Puro (PX459) V2.0 (a gift from Feng Zhang; Addgene plasmid #62988). All sgRNAs were verified by Sanger sequencing.
In total, 3 × 105 RUES2 hESCs were seeded per well of 6-well dish in E8 with 10 μM Y-27632 (dishes pre-coated with 2 μg cm−2 rhLaminin521), and immediately transfected with 3 μg of sgRNA-containing plasmid using 9 μL of GeneJuice (Millipore). To delete the TTN promoter, 1.5 μg of each of the two sgRNA was co-transfected. After 16 h, cells were rinsed in PBS, and fed with E8 containing 10 μM Y-27632 and 0.5 μg mL−1 puromycin dihydrochloride (Sigma-Aldrich) to enrich for transfected hESCs. After 24 h, media was replaced with conventional E8 and changed daily. After 72 h, hESCs were dispersed to single cells using Versene and seeded at a low density of 5 × 103 cells per 100 mm culture dish in E8 with 10 μM Y-27632. After daily media changes with E8 for 7–10 days, clonal lines were isolated by picking well-separated individual colonies and expanded for genotyping.
Genomic DNA was extracted using the QIAGEN DNeasy Blood & Tissue Kit, and used as template for PCR with LongAmp Taq (NEB). TTN promoter deletion was assessed using primers located ~400 bp up- and downstream to the CRISPR/Cas9 cut sites (fw 5′-ATGCCATCTATGGGGGTGGAA-3′ and rev 5′-CTTTGGGATGAACAACTTTCTGTGT-3′). The resulting product was run on an 1% Agarose-TBE gel and detected by ethidium bromide staining, and clones with homozygous deletion of the ~1.7 kb TTN promoter fragment were isolated (Supplementary Fig. 10b). The deletion was further confirmed by Sanger sequencing of the PCR product. TTN and RBM20 knockout was assessed by Sanger sequencing of genomic PCRs for TTN exon 2 (fw 5′-GGAGAAACGTGTGTCTCTGCTA-3′; rev 5′-GTGTTGGACTAATTTTCCGAAGTG-3′; 316 bp amplicon) and RBM20 exon 2 (fw 5′-GTCAGTAACCCGAACCCTCTG-3′; rev 5′-TTGGTTCCTCGGGGTCGTA-3′; 841 bp amplicon). Clones with homozygous mutations predicted to lead to premature truncation of the resulting protein were isolated (Supplementary Fig. 10c, f). All the gene-edited hESC lines maintained a normal kayotype throughout the procedure as assessed by standard G-banding.
3D DNA FISH
hESC-CMs were obtained as described above. At day 14 of differentiation, CMs were rinsed in PBS, dissociated to single cells with 0.25% Trypsin in Versene for 5–7 min at 37 °C, washed twice in RPMI 20% FBS, and frozen in CryoStor CS10 (Sigma-Aldrich) at a density of 1 × 107 cells mL−1. In preparation for 3D DNA FISH, CMs were thawed and seeded at a density of 2 × 105 cells cm−2 on rhLaminin521-coated dishes in RPMI-B27 with 10 μM Y-27632 and 5% FBS. After 16 h, cells were fed with fresh RPMI-B27 and cultured for 4 days to allow for CM recovery (indicated by synchronous beating). CM were metabolically selected by 4 days of culture in DMEM without glucose and pyruvate but supplemented with 4 μM lactate. This well-established procedure efficiently removed contaminating non-CM cells (which do not tolerate lactate metabolic selection), thus highly enriching for CM (> 95% as assessed by Flow Cytometry for cTnT and immunofluorescence for α-actinin). CM were then cultured in RPMI-B27 for 4 more days before being collected for 3D DNA FISH.
3D DNA FISH was performed with modifications from a published protocol72. Unless otherwise indicated, all steps were performed at room temperature. Poly-Lysine slides (Sigma-Aldrich, P0425) were pre-coated with 0.5 or 2 μg mL−1 rhLaminin521 (for hESCs or CMs, respectively) dispersed on a ~2 cm2 spot outlined by a hydrophobic pen for 2 h at 37 °C. Cultures were rinsed in PBS, dissociated to single cells with TrypLE at 37 °C, washed in culture media, and plated in culture media supplemented with 10 μM Y-27632 at a density of 40,000 cells per spot onto the pre-coated slides. Cells were allowed to attach to for 4 h at 37 °C. For transcription inhibition experiments, 5 µM Actinomycin D was added to the culture media both 3 h before collecting the cells and during the seeding procedure. Cells were fixed in 4% PFA and then quenched in 0.1 M Tris-HCl pH 7.4 for 10 min each. Cells were subsequently permeablized in 0.1% Saponin/0.1% Triton X-100 in PBS for 10 min, followed by two washes in PBS. Cells were equilibrated in 20% Glycerol/PBS for 20 min, and stored overnight in 50% Glycerol/PBS at −20 °C.
FISH probes were prepared using the Vysis Spectrum Nick Translation Kit (Vysis, 32-801300) from BACs obtained from the BACPAC Resources Center: CH17-275G10 (TTN); CH17-236B15 (THSD7B); CH17-82H11 (CACNA1C); CH17-266P23 (CAMK2D); CH17-473L14 (CAMK2G); CH17-468A13 (FNBP1); CH17-200I17 (KCNIP2); CH17-147J13 (LMO7). BACs were purified using the QIAGEN Plasmid Midi Kit, and validated by PCR with two primer sets for the expected genomic region. Two micrograms of BAC DNA was combined with either SpectrumGreen (Vysis, 30-803000) or SpectrumOrange (Vysis, 30-803200) and labeled according to manufacturer’s protocol for 4 h at 15 °C followed by inactivation at 70 °C for 10 min. Probes were first purified with the QIAGEN PCR purification kit and then precipitated with 40 μL Salmon Sperm DNA (Thermo Fisher 15632-011), 60 μL Human Cot-1 DNA (Thermo Fisher 15279-011), 15 μL sodium acetate (3 M, pH 5.5), and 390 μL 100% Ethanol for 2 h at −20 °C. Probes were spun down at 16,000 × g for 30 min at 4 °C and resuspended in 50 μL Hybridization Buffer (50% formamide, 2× SSC, 10% dextran sulfate). Probes were stored at −20 °C.
For hybridization, slides were removed from −20 °C and equilibrated in 20% Glycerol/PBS for 20 min. Slides were submerged in liquid nitrogen and then allowed to thaw; this procedure was repeated for three cycles. Slides were washed twice in PBS and incubated in 0.1 M HCl for 30 min. Slides were washed once in PBS then permeabilized in 0.5% Saponin/0.5% Triton X-100/PBS for 30 min followed by two more washes in PBS. Cells were then equilibrated in 50% formamide/2× SSC for 10 min. Five microliters of green- and orange-labeled FISH probes were combined and put onto a coverslip which was inverted onto the slide. Slides were sealed with rubber cement and air dried in the dark for 30 min. Slides were incubated for 10 min at 78 °C to denature the DNA, then overnight at 37 °C for hybridization. After incubation the rubber cement was removed and slides were washed in (1) 50% formamide/2× SSC 15 min at 45 °C; (2) 0.2× SSC 15 min at 63 °C; (3) 2× SSC 5 min at 45 °C; (4) 2× SSC 5 min at RT; (5) PBS 5 min at RT. Slides were then counterstained with DAPI (5 μg mL−1 in 2× SSC, ThermoFisher D3571) for 2 min and washed twice with PBS. Coverslips were mounted with 10 μL Slow Fade Glow (Thermo Fisher, S36936) and sealed with nail polish.
We captured images with a Yokogawa W1 spinning disk confocal mounted on an inverted Nikon Eclipse TiE base and a CFI PlanApo VC 60× Water Immersion lens (NA 1.2). Sample excitation was via full field exposure from 100 mW 405-, 490-, and 561-nm lasers. Sample emission was captured sequentially through Chroma Technology filter sets ET455/50, ET525/35, and ET605/52 by an Andor iXon EM + EMCCD camera. Z-stacks were acquired at a step size of 0.3 μm to cover a z-distance of 15 μm (51 stacks). Twenty randomly selected field of views (FOV) were imaged at a separation of two FOV from each other. Automated image analysis was performed with Imaris software (version 9.2.0; Bitplane). Background was removed with a Gaussian filter (9 μm for DAPI and 1 μm for FISH signals, based on the average size of each feature). Nuclei boundaries were defined with the surface feature with a surface detail of 1 μm (all other parameters as default; auto thresholding based on surface quality). FISH images were first masked based on the nuclear boundaries to retain only the nuclear signal, then loci were identified with the spot calling feature. One-micrometer-wide spots were determined based on the average size of FISH signal (all other parameters were set as default; auto thresholding based on spot quality). The distances between the various features was determined by applying the distance transformation function. The statistics for surfaces and spots were processed using a custom Python script which extracted and analyzed information only for diploid nuclei (containing exactly two green and two orange spots. At least 100 cells per conditions were analyzed (corresponding to at least 10 FOVs), and the exact number is indicated in each figure. Distances between loci and between a locus and the nuclear periphery were calculated relative to the center of 1-μm-wide FISH spots, and were normalized by the average hESC nuclear volume of based on the cube root of the cell volume for each cell (Xnorm = Xraw × average volume1/3 × volume−1/3). Proximity between two loci in a given cell was defined as the minimal distance between one pair being less than twice the diameter of a FISH spot (2 μm). In the representative pictures reported in the Figures, the orange FISH signal is depicted in magenta to the benefit of color blind readers.
ImmunoFISH
Immunofluorescence combined with DNA FISH was performed according to a published method73. CMs were plated on slides prepared for FISH analyses as described above. Upon removal from storage at −20 °C in 50% Glycerol/PBS, slides were washed two times in PBS for 5 min, then permeabilized and blocked in PBS with 0.1% Triton X-100 and 5% normal goat serum for 30 min. Primary antibodies (anti-RBM20 rabbit polyclonal at 1:200 dilution, ThermoFisher #PA5-57404; anti-sarcomerinc α-actinin mouse monoclonal [EA-53] at 1:300 dilution, Abcam #ab9465) were diluted in antibody buffer (PBS with 0.1% Triton X-100 and 1% normal goat serum) and incubated overnight at 4 °C in a humid chamber. Following three washes with PBS for 5 min, secondary antibodies (goat anti-rabbit Alexa Fluor 647 and goat anti-mouse Alexa Fluor 594, both used at 1 μg mL−1, ThermoFisher) were diluted in antibody buffer and incubated for 1 h at room temperature in the dark. Following three washes with PBS for 5 min, slides were fixed with 3% PFA in PBS for 10 min. Slides were washed two times in 2× SSC, denatured in 50% formamide 2× SSC pH 7.2 for 30 min at 80 °C, and washed three times with ice cold 2× SSC. Ten microliters of the TTN green-labeled FISH probe was pipetted on a coverslip that was then inverted onto the slide. Slides were sealed with rubber cement, air dried in the dark for 30 min, and hybridized overnight at 42 °C. After removing the rubber cement, slides were washed three times in 50% formamide and 50% 2× SSC, pH 7.2, at 42 °C. Slides were then counterstained with DAPI (5 μg mL−1 in 2× SSC) for 2 min and washed twice with PBS. Coverslips were mounted with 10 μL Slow Fade Glow and sealed with nail polish.
ImmunoFISH were imaged as described for 3D DNA FISH, and subjected to the same automated image analysis using Imaris to identify nuclei boundaries and TTN loci. The RBM20 immunostaining signal was processed analogously to FISH signal to identify nuclear foci with the spot calling feature. One-micrometer-wide spots were determined based on the average size of RBM20 foci (all other parameters were set as default; autothresholding was based on spot quality). The distance transformation function was applied to compute the distance between the center of 1-μm-wide TTN loci and RBM20 foci in diploid cells. An overlap between these two features in a given diploid cell was defined as their distance being less than the diameter of a spot (1 μm).
Immunofluorescence
CMs were processed as just described for immunoFISH, but after secondary antibody incubation slides were counterstained with DAPI (5 μg mL−1 in PBS), washed three times in PBS for 5 min, and mounted. The anti-RBM20 and anti-sarcomerinc α-actinin antibodies were diluted as described for immunoFISH, while the anti-titin rabbit polyclonal antibody (Myomedix; #Z1Z2) was used at a concentration of 0.3 μg mL−1.
Flow cytometry
CM were collected at day 14 of differentiation for flow cytometry analysis. Briefly, cells were washed with PBS and treated with TrypLE (ThermoFisher) for 5–10 min at 37 °C and collected in RPMI. Cells were spun down and resuspended in 200 μL 4% paraformaldehyde for 10 min at RT. Cells were spun down and resuspended in 500 μL PBS + 5% FBS and split into two tubes for staining. Tubes spun 2,000 rpm × 5 min and were resuspended in 50 μL PBS + 5% FBS + 0.75% saponin with either 0.5 μL mouse IgG1 (eBioscience 14-4714) or 0.5 μL mouse cTnT (ThermoFisher MS-295) and incubated for 30 min at RT. After incubation 150 μL PBS + 5% FBS + 0.75% saponin were added and samples were spun down and washed with 200 μL PBS + 5% FBS + 0.75% saponin. Both sets were resuspended after washing in 50 μL PBS + 5% FBS + 0.75% saponin with 0.25 μL goat anti-mouse PE secondary (Jackson 115-116-072) and incubated for 30 min RT in the dark. Following secondary 150 μL PBS + 5% FBS were added and samples were spun down and washed with 200 μL PBS + 5% FBS. Samples were then stored in 400 μL PBS + 5% FBS + 100 μL 4% paraformaldehyde at 4 °C until analysis. Samples were run on a BD FACSCanto II and data were acquired with the BD FACSDIVA software (both from BD Biosciences). Analysis was performed with FloJo version 10 software. Only samples >75% cTnT positive were used for downstream applications.
RT-qPCR and semiquantitative RT-PCR
Cell samples were collected in RLT buffer and homogenized with a Qiashredder (QIAGEN) before RNA purification with the RNeasy Mini Kit (QIAGEN) including on-column DNase digestion. RNA from whole hearts was extracted with RNeasy Fibrous Tissue Kit (QIAGEN). cDNA was generated from RNA with M-MLV RT (ThermoFisher) and random hexamer priming. RT-qPCR was performed in technical triplicate using the SYBR Select MasterMix (ThermoFisher) and run on a 7900HT Fast Real-Time PCR system (Applied Biosystems), all according to the manufacturer’s instructions. Gene expression was calculated using the ∆Ct method as 2(Ct gene – Ct housekeeping), and the housekeeping gene is indicated in the Figure legends. For semiquantitative RT-PCR, 10 ng of cDNA was subjected to 25 cycles of amplification using GoTaq Flexi DNA Polymerase (Promega) in a 25 μL reaction. Ten microliters of the PCR product were run on a 2% Agarose-TBE gel and detected by ethidium bromide staining. Oligonucleotide primer sequences for RT-qPCR and semiquantiative PCR are listed in Supplementary Table 9.
Western blot
Protein lysates were obtained with RIPA buffer containing protease inhibitors. Following clarification of the lysate by centrifugation and assessment of protein centrifugation by BCA assay, samples were diluted in Laemmli buffer and boiled. Twenty micrograms of protein were electrophoretically separated using 4–20% Mini-PROTEAN TGX Precast Protein Gels (Bio-Rad). Proteins were transferred to PVDF membranes and blocked in PBS with 0.1% Tween-20 (PBST) and 5% bovine serum albumin (BSA). The anti-RBM20 rabbit polyclonal primary antibody (ThermoFisher #PA5-57404) was diluted at 1:500 in PBST 1% BSA and incubated overnight at 4 °C. Membranes were washed three times in PBST for 10 min at room temperature, incubated for 1 h at room temperature with goat anti-rabbit HRP-conjugated secondary antibody, and washed three times in PBST for 10 min. Chemiluminescent reaction was initiated by incubation with SuperSignal West Pico Chemiluminescent Substrate (ThermoFisher), and images were acquired using a ChemiDoc Imaging System (Bio-Rad) in “high resolution” mode. Before re-probing for the housekeeping protein GAPDH (mouse monoclonal [6C5] diluted at 1:5000; Abcam #8245) according to the same protocol but using goat anti-mouse HRP-conjugated secondary antibody, membranes were treated with Restore Plus western blot stripping buffer (ThermoFisher), washed three times, and re-blocked.
Statistical analyses
Standard statistical analyses were performed using R or Prism 6 (GraphPad). The type and number of replicates, the statistical test used, and the test results are described in the figure legends. All statistical tests employed were two-tailed. No experimental samples were excluded from the statistical analyses. Sample size was not pre-determined through power calculations, and no randomization or investigator blinding approaches were implemented during the experiments and data analyses.
Reporting Summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
Thank you to members of the Murry lab for helpful discussions during the development of this project and especially to Xiulan Yang for assistance with obtaining samples and Katie Mitzelfelt and Silvia Marchianò for experimental support. We thank members of the Shendure and Noble labs and in particular Ruolan Qiu and Kate Cook for assistance with Hi-C technology and analysis. Flow cytometry was done with assistance from UW Cell Analysis Facility in the Department of Immunology. We would like to acknowledge the Mike and Lynn Garvey Cell Imaging Lab at UW and its director Dale Hailey for assistance with sample imaging and analysis. We thank members of the Birth Defects Research Laboratory for assistance in obtaining human heart tissue. A.B. is funded by an EMBO Long-Term Fellowship, ALTF 448-2017. P.A.F. is funded through Experimental Pathology of Cardiovascular Disease training grant, NIH T32 HL007312. This work is part of the NIH 4D Nucleome consortium (NIH U54 DK107979, to C.E.M., W.S.N. and J.S.), with additional support from P01 GM081619, R01 HL128362, and the Foundation Leducq Transatlantic Network of Excellence (to C.E.M.). The Birth Defects Research Laboratory is supported by NIH grant R24 HD000836.
Author contributions
A.B. designed, performed and analyzed FISH, pharmacology, and gene editing experiments, performed Hi-C on hiPSCs, and wrote the manuscript. P.A.F. designed and performed Hi-C, ATAC-seq, and RNA-seq experiments on hESCs, performed most bioinformatics analyses, and wrote the manuscript. V.R. contributed to experimental design and execution and assisted in writing the manuscript. G.B. and G.G.Y. contributed to high-throughput sequence analysis. H.R. and L.P. contributed to experimental design and supervised the experiments. W.S.N. and J.S. contributed to study design and supervision. C.E.M. conceived and supervised the study, obtained research funding and contributed to writing the manuscript.
Data availability
All data for Hi-C, RNA-seq and ATAC-seq is available on Gene Expression Omnibus accession number GSE106690. Source data for gel images is provided in Supplementary Fig. 11.The data for graphical representations in the Figures and Supplementary Figures is provided in the Source Data File.
Code availability
Custom code is available on github at https://github.com/pfields8/Fields_et_al_2018/.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks Benoit Bruneau and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alessandro Bertero, Paul A. Fields.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-09483-5.
References
- 1.Bruneau BG. Signaling and transcriptional networks in heart development and regeneration. Cold Spring Harb. Perspect. Biol. 2013;5:a008292. doi: 10.1101/cshperspect.a008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieberman-Aiden E, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Fan C, Zheng Y, Li C. Dynamic chromatin accessibility modeled by Markov process of randomly-moving molecules in the 3D genome. Nucleic Acids Res. 2017;45:e85. doi: 10.1093/nar/gkx086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franke M, et al. Formation of new chromatin domains determines pathogenicity of genomic duplications. Nature. 2016;538:265–269. doi: 10.1038/nature19800. [DOI] [PubMed] [Google Scholar]
- 5.Javierre BM, et al. Lineage-specific genome architecture links enhancers and non-coding disease variants to target gene promoters. Cell. 2016;167:1369–1384.e19. doi: 10.1016/j.cell.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitt AD, et al. A compendium of chromatin contact maps reveals spatially active regions in the human genome. Cell Rep. 2016;17:2042–2059. doi: 10.1016/j.celrep.2016.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon JR, et al. Chromatin architecture reorganization during stem cell differentiation. Nature. 2015;518:331–336. doi: 10.1038/nature14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser J, et al. Hierarchical folding and reorganization of chromosomes are linked to transcriptional changes in cellular differentiation. Mol. Syst. Biol. 2015;11:852–852. doi: 10.15252/msb.20156492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paige SLL, et al. A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell. 2012;151:221–232. doi: 10.1016/j.cell.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramani V, et al. Mapping 3D genome architecture through in situ DNase Hi-C. Nat. Protoc. 2016;11:2104–2121. doi: 10.1038/nprot.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo W, et al. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat. Med. 2012;18:766–773. doi: 10.1038/nm.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imakaev M, et al. Iterative correction of Hi-C data reveals hallmarks of chromosome organization. Nat. Methods. 2012;9:999–1003. doi: 10.1038/nmeth.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang T, et al. HiCRep: assessing the reproducibility of Hi-C data using a stratum-adjusted correlation coefficient. Genome Res. 2017;27:1939–1949. doi: 10.1101/gr.220640.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P, Pu WT. Recounting cardiac cellular composition. Circ. Res. 2016;118:368–370. doi: 10.1161/CIRCRESAHA.116.308139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonev B, et al. Multiscale 3D genome rewiring during mouse neural development. Cell. 2017;171:557–572.e24. doi: 10.1016/j.cell.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ou HD, et al. ChromEMT: visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science. 2017;357:eaag0025. doi: 10.1126/science.aag0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon JR, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane E, et al. Condensin-driven remodelling of X chromosome topology during dosage compensation. Nature. 2015;523:240–244. doi: 10.1038/nature14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao SSP, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stergachis AB, et al. Developmental fate and cellular maturity encoded in human regulatory DNA landscapes. Cell. 2013;154:888–903. doi: 10.1016/j.cell.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, et al. Genome-wide temporal profiling of transcriptome and open chromatin of early cardiomyocyte differentiation derived from hiPSCs and hESCs. Circ. Res. 2017;121:376–391. doi: 10.1161/CIRCRESAHA.116.310456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ang YS, et al. Disease model of GATA4 mutation reveals transcription factor cooperativity in human cardiogenesis. Cell. 2016;167:1734–1749.e22. doi: 10.1016/j.cell.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson DJ, et al. NKX2-5 regulates human cardiomyogenesis via a HEY2 dependent transcriptional network. Nat. Commun. 2018;9:1373. doi: 10.1038/s41467-018-03714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsankov AM, et al. Transcription factor binding dynamics during human ES cell differentiation. Nature. 2015;518:344–349. doi: 10.1038/nature14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uhlén M, et al. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 26.Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc. Res. 2008;77:637–648. doi: 10.1016/j.cardiores.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Zou J, et al. An internal promoter underlies the difference in disease severity between N- and C-terminal truncation mutations of Titin in zebrafish. eLife. 2015;4:e09406. doi: 10.7554/eLife.09406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Wit E, et al. CTCF binding polarity determines chromatin looping. Mol. Cell. 2015;60:676–684. doi: 10.1016/j.molcel.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Dowen JM, et al. Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell. 2014;159:374–387. doi: 10.1016/j.cell.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dauksaite V, Gotthardt M. Molecular basis of titin exon exclusion by RBM20 and the novel titin splice regulator PTB4. Nucleic Acids Res. 2018;46:5227–5238. doi: 10.1093/nar/gky165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, Guo W, Dewey CN, Greaser ML. Rbm20 regulates titin alternative splicing as a splicing repressor. Nucleic Acids Res. 2013;41:2659–2672. doi: 10.1093/nar/gks1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas J, et al. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur. Heart J. 2015;36:1123–1135. doi: 10.1093/eurheartj/ehu301. [DOI] [PubMed] [Google Scholar]
- 34.Maatz H, et al. RNA-binding protein RBM20 represses splicing to orchestrate cardiac pre-mRNA processing. J. Clin. Invest. 2014;124:3419–3430. doi: 10.1172/JCI74523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyles SP, et al. Modeling structural and functional deficiencies of RBM20 familial dilated cardiomyopathy using human induced pluripotent stem cells. Hum. Mol. Genet. 2016;25:254–265. doi: 10.1093/hmg/ddv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Streckfuss-Bömeke K, et al. Severe DCM phenotype of patient harboring RBM20 mutation S635A can be modeled by patient-specific induced pluripotent stem cell-derived cardiomyocytes. J. Mol. Cell. Cardiol. 2017;113:9–21. doi: 10.1016/j.yjmcc.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Roberts, A. M. et al. Integrated allelic, transcriptional, and phenomic dissection of the cardiac effects of titin truncations in health and disease. Sci. Transl. Med.7, 270ra6 (2015). [DOI] [PMC free article] [PubMed]
- 38.Khan MAF, et al. RBM20 regulates circular RNA production from the titin gene. Circ. Res. 2016;119:996–1003. doi: 10.1161/CIRCRESAHA.116.309568. [DOI] [PubMed] [Google Scholar]
- 39.Liao P, et al. Smooth muscle-selective alternatively spliced exon generates functional variation in Cav1.2 calcium channels. J. Biol. Chem. 2004;279:50329–50335. doi: 10.1074/jbc.M409436200. [DOI] [PubMed] [Google Scholar]
- 40.Gray CBB, Brown JH. CaMKIIdelta subtypes: localization and function. Front. Pharmacol. 2014;11:5–15. doi: 10.3389/fphar.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nothjunge S, et al. DNA methylation signatures follow preformed chromatin compartments in cardiac myocytes. Nat. Commun. 2017;8:1667. doi: 10.1038/s41467-017-01724-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nora EP, et al. Targeted degradation of CTCF decouples local insulation of chromosome domains from genomic compartmentalization. Cell. 2017;169:930–944. doi: 10.1016/j.cell.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao SSP, et al. Cohesin loss eliminates all loop domains. Cell. 2017;171:305–320.e24. doi: 10.1016/j.cell.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krumm, A. & Duan, Z. Understanding the 3D genome: emerging impacts on human disease. Semin. Cell Dev. Biol. 10.1016/j.semcdb.2018.07.004 (2018). [DOI] [PMC free article] [PubMed]
- 45.Montefiori LE, et al. A promoter interaction map for cardiovascular disease genetics. eLife. 2018;7:1–35. doi: 10.7554/eLife.35788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilsbach R, et al. Distinct epigenetic programs regulate cardiac myocyte development and disease in the human heart in vivo. Nat. Commun. 2018;9:391. doi: 10.1038/s41467-017-02762-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choy MK, et al. Promoter interactome of human embryonic stem cell-derived cardiomyocytes connects GWAS regions to cardiac gene networks. Nat. Commun. 2018;9:2526. doi: 10.1038/s41467-018-04931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, Y. et al. 3D Chromatin architecture remodeling during human cardiomyocyte differentiation reveals a novel role of HERV-H in demarcating chromatin domains. bioRxiv 485961, 10.1101/485961 (2018).
- 49.Guo T, et al. Genome-wide association study to find modifiers for tetralogy of fallot in the 22q11.2 deletion syndrome identifies variants in the GPR98 locus on 5q14.3. Circ. Cardiovasc. Genet. 2017;10:e001690. doi: 10.1161/CIRCGENETICS.116.001690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van den Hoogenhof MMG, et al. RBM20 mutations induce an arrhythmogenic dilated cardiomyopathy related to disturbed calcium handling. Circulation. 2018;138:1130–1342. doi: 10.1161/CIRCULATIONAHA.117.031420. [DOI] [PubMed] [Google Scholar]
- 51.Nagueh SF, et al. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–162. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 52.Neagoe C, et al. Titin isoform switch in ischemic human heart disease. Circulation. 2002;106:1333–1341. doi: 10.1161/01.CIR.0000029803.93022.93. [DOI] [PubMed] [Google Scholar]
- 53.Monahan K, Horta A, Lomvardas S. LHX2- and LDB1-mediated trans interactions regulate olfactory receptor choice. Nature. 2019;565:448–453. doi: 10.1038/s41586-018-0845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galganski L, Urbanek MO, Krzyzosiak WJ. Nuclear speckles: molecular organization, biological function and role in disease. Nucleic Acids Res. 2017;45:10350–10368. doi: 10.1093/nar/gkx759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burridge PW, et al. Chemically defined generation of human cardiomyocytes. Nat. Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Servant N, et al. HiC-Pro: an optimized and flexible pipeline for Hi-C data processing. Genome Biol. 2015;16:259. doi: 10.1186/s13059-015-0831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wickham, H. Ggplot2: Elegrant Graphics for Data Analysis. (Springer, New York, 2009).
- 60.Witten DM, Noble WS. On the assessment of statistical significance of three-dimensional colocalization of sets of genomic elements. Nucleic Acids Res. 2012;40:3849–3855. doi: 10.1093/nar/gks012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 66.Buenrostro J, et al. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 2015;22:134–139. doi: 10.1002/0471142727.mb2129s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lex A, Gehlenborg N, Strobelt H, Vuillemot R, Pfister H. UpSet: visualization of intersecting sets. Ieee. Trans. Vis. Comput. Graph. 2014;20:1983–1992. doi: 10.1109/TVCG.2014.2346248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailey TL. DREME: motif discovery in transcription factor ChIP-seq data. Bioinformatics. 2011;27:1653–1659. doi: 10.1093/bioinformatics/btr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perez AR, et al. GuideScan software for improved single and paired CRISPR guide RNA design. Nat. Biotechnol. 2017;35:347–349. doi: 10.1038/nbt.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bolland DJ, King MR, Reik W, Corcoran AE, Krueger C. Robust 3D DNA FISH using directly labeled probes. J. Vis. Exp. 2013;15:1–8. doi: 10.3791/50587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaumeil J, Chow J, Augui S, Heard E. Combined immunofluorescence, RNA fluorescent in situ hybridization to study chromatin changes, transcriptional activity, nuclear organization. Methods Mol. Biol. 2008;463:297–308. doi: 10.1007/978-1-59745-406-3_18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data for Hi-C, RNA-seq and ATAC-seq is available on Gene Expression Omnibus accession number GSE106690. Source data for gel images is provided in Supplementary Fig. 11.The data for graphical representations in the Figures and Supplementary Figures is provided in the Source Data File.
Custom code is available on github at https://github.com/pfields8/Fields_et_al_2018/.