Abstract
Elevated circulating concentration of endothelial microparticles (MPs) may provide an index of the extent and nature of cellular damage in chronic stroke. The purpose of this study was to determine the circulating concentrations of CD31+/CD42, CD62E+ and CD34+ MPs in chronic stroke subjects, focusing on the effects of chronic stroke by comparing them to both older adults without a history of stroke but with T2DM, as well as older and young healthy controls. Plasma from three groups of sedentary older (50–75 years) men and women (chronic stroke, T2DM or older healthy) as well as a group of younger (18–39 years) healthy controls was isolated from fasting blood and CD31+/CD42b-, CD62E+ and CD34+ MPs were quantified using flow cytometry (n=17/group). Concentrations of CD31+/CD42b- and CD62E+ MPs were higher in the T2DM group (P<0.05), but not chronic stroke, compared to older and younger healthy adults. CD62E+ MP and CD34+ MPs concentrations were elevated in the older compared to younger adults (P<0.05 for both). Sub-analyses excluding chronic stroke subjects who were also diagnosed with diabetes [stroke(diabetes-)] revealed lower CD31+/CD42b- (P<0.05) and CD62E+ (P=0.08) MPs in the stroke(diabetes-) group compared to the T2DM group. CD31+/CD42b- MPs and CD62E+ MPs concentrations were each associated with fasting glucose levels and CD31+/CD42b- MPs also were associated with triglyceride levels. As MPs have been proposed as potential targets for diagnosing, treating and identifying the clinical progression of T2DM, our study provides further support for the use of CD31+/CD42b- and CD62E+ MPs in the clinical progression of T2DM and associated vascular complications.
Keywords: endothelial microparticle, diabetes mellitus, stroke, older age
INTRODUCTION
Cardiovascular disease (CVD) including stroke is the leading cause of death in developed countries but only ~50% of CV events can be explained by traditional biomarkers such as hypercholesterolemia and hypertension (Heidenreich et al., 2011). Plasma microparticles (MPs), small (~0.1 – 1μm) vesicle-like structures formed from the plasma membrane of endothelial cells and other blood-related cells in response to apoptosis or endothelial cell activation (Dignat-George & Boulanger, 2011; Paudel et al., 2016), were recently identified as potential novel biomarkers for CVD risk. MPs express surface markers of their parent cell and are physiologically relevant in chronic stroke because they contain factors such as microRNAs, mRNA, and proteins that can be transferred to other cell types to promote both deleterious or beneficial effects such as endothelial activation and inflammation or endothelial differentiation and survival, respectively (Puddu et al., 2010; Dignat-George & Boulanger, 2011; Paudel et al., 2016). Elevations in MP levels may provide important clinical information regarding the extent of vascular disruption in individuals with chronic stroke. For example, endothelial vasoreactivity as measured by flow-mediated dilation is inversely related to plasma MP levels, independent of age (Esposito et al., 2006b; Chironi et al., 2009). Furthermore, greater carotid-femoral pulse wave velocity measures of arterial stiffness are associated with higher circulating MP concentrations in healthy adults (Wang et al., 2007). Thus, MPs serve as both biomarkers of vascular stress and potentially promote downstream signaling through transport of biological material (Sabatier et al., 2002; Lacroix et al., 2007; Abid Hussein et al., 2008; Chironi et al., 2009; Dignat-George & Boulanger, 2011) or interaction with membrane bound cellular receptors (Zhang et al., 2015).
Although the precise mechanisms contributing to MP release are not completely understood, the circulating concentration and type of MP (i.e. surface markers present) may provide an index of the extent and nature of cellular damage, respectively. For example, one subset of MPs, CD31+/CD42b- MPs, contain endothelial surface markers of non-platelet origin, which in vitro studies indicate are shed by endothelial cells as a result of apoptosis (Horstman et al., 2004; Chironi et al., 2009). High circulating concentrations of CD31+/CD42b- MPs are associated with clinical conditions including hypertension (Preston et al., 2003; Viera et al., 2012) and play a major role in the atherosclerotic process (Paudel et al., 2016). CD62E+ MPs, on the other hand, although also of endothelial origin, are released in response to cellular activation (for example, exposure to elevated levels of TNF-α). While CD62E+ MPs are present even in healthy individuals (Berkmans et al., 2001), elevated MP numbers are seen in various cardiometabolic conditions and in association with cardiovascular risk (Lee et al., 2012; Babbitt et al., 2013). Indeed, Lee et al. found that high CD62E+ MP number in chronic stroke survivors was associated with greater risk of major cardiovascular events and hospitalization within 3 years after their stroke (Lee et al., 2012). Finally, CD34+ MPs are derived from hematopoetic progenitor and endothelial cells. The CD34 surface marker on hematopoietic cells is often associated with pro-angiogenic functions. Like CD34+ hematopoetic cells, CD34+ MPs are elevated in acute coronary syndrome patients compared to those adults with stable angina and healthy controls (Stepien et al., 2012). Despite the few studies suggesting CD34+ MPs as biomarkers with potential pro-angiogenic properties, this MP population has not been well explored. Given the potential roles of these 3 types of circulating MPs in maintaining vascular homeostasis, the study of these MPs in people with chronic stroke and associated risk factors is warranted.
Stroke is particularly prevalent in older adults and is associated with a number of other cardiometabolic complications. Along with macrovascular complications associated with chronic stroke (Ivey et al., 2010), skeletal muscle atrophy of the paretic limb and associated reductions in skeletal muscle capillarization (Prior et al., 2009) often lead to the development of insulin resistance (Prior et al., 2009) and reduced cardiovascular fitness (Ryan et al., 2000; Billinger et al., 2012). Indeed, more than 75% of individuals with chronic stroke have impaired glucose tolerance or type 2 diabetes (T2DM) (Ivey et al., 2006). While there are several studies that have examined the effects of an acute stroke on different MP populations (Cherian et al., 2003; Simak et al., 2004; Simak et al., 2006; Williams et al., 2007; Jung et al., 2009; Chiva-Blanch et al., 2016), only one, to our knowledge, has looked at the MP response in chronic stroke survivors. Lee et al., found that high circulating CD62E+ MPs are associated with greater CVD risk in chronic stroke (Lee et al., 2012) but it is unknown whether this occurs independently of older age or insulin resistance. The purpose of this study was to determine circulating concentrations of CD31+/CD42b-, CD62E+ and CD34+ MPs in chronic stroke patients compared with healthy, young controls, while accounting for potential effects of age and T2DM by also studying groups of healthy older adults and older adults with T2DM. We hypothesized a graduated effect with chronic stroke patients having the highest MP concentrations, followed by T2DM, healthy older and healthy younger controls. We then sought to determine whether traditional CVD risk factors (i.e., plasma lipoprotein-lipid and glucose concentrations) were associated with concentrations of each MP subtype. A greater understanding of factors associated with older age with and without cardiometabolic disease may help establish MPs as novel clinical biomarkers for CVD risk.
METHODS
Ethical Approval
The University of Maryland College Park (UMCP) and the University of Maryland School of Medicine (UM-SOM) Institutional Review Boards approved all study procedures (UM-SOM: #HP00041347, HP00047188, HP00057124, and UMCP: # 343286) and all subjects provided informed verbal and written consent. Study procedures were carried out in accordance with the Declaration of Helsinki except for registration in a research database.
Screening and standard assessments
Subjects were recruited from the greater Baltimore and Washington DC area. All subjects were sedentary completing ≤ 20 min of exercise on ≤ 2 days per week. Chronic stroke, T2DM and older healthy subjects were sedentary men and women 50–75 years old (the latter postmenopausal for >2 years and not prescribed hormone replacement therapy). Chronic stroke subjects had suffered an ischemic stroke >3 months prior to testing and had completed all conventional physical therapy. T2DM subjects either had a diagnosis of type 2 diabetes, a HbA1C value of >6.5%, or a fasting glucose >126 mg/dL on two or more occasions (Association, 2018). Chronic stroke and T2DM subjects were not excluded due to other chronic conditions including high blood pressure, hyperlipidemia or hyperglycemia and these conditions and accompanying medications were documented. Healthy older adults were of similar age to the chronic stroke and T2DM groups but had blood pressure, and plasma lipid and glucose levels in the normal ranges. Younger adults were sedentary men and women 18–39 years old. Younger women were all tested during the early follicular phase of their menstrual cycle. Exclusion criteria for the healthy groups were: smoking, known CVD, type 1 or type 2 diabetes, chronic obstructive pulmonary disease, current treatment for active cancer, systolic blood pressure ≥ 130 mmHg; diastolic blood pressure ≥ 90 mmHg; serum total cholesterol ≥ 200 mg/dL; low density lipoprotein-cholesterol (LDL-C) ≥ 130 mg/dL; high-density lipoprotein-cholesterol (HDL-C) ≤ 35 mg/dL; or fasting glucose ≥ 100 mg/dL.
Peak Oxygen Consumption
Peak oxygen consumption (VO2peak) was measured by indirect calorimetry (Quark, Cosmed USA, Chicago, IL) during a graded treadmill exercise test on a motorized treadmill. Subjects walked at a constant velocity with the grade initially set to 0% and increasing 4% after the first 2 minutes and 2% every 2 min thereafter to volitional exhaustion, as reported previously (Ivey et al., 2007; Prior et al., 2009). Tests were terminated at the subject’s request or according to criteria set forth by American College of Sports Medicine (Pescatello et al., 2014). Subjects were allowed handrail support but were instructed to minimize handrail use to that necessary to maintain balance. VO2peak was defined as the highest oxygen consumption value obtained in the last minute of exercise.
Fasted blood draw
Subjects reported to the lab the morning after an overnight (12-hr) fast. Subjects were asked to refrain from food, alcohol, caffeine until after the blood draw. Height, weight and seated blood pressure were recorded after arriving to the lab. Blood was collected in tubes containing 15% potassium EDTA; plasma was isolated for measurement of lipoprotein-lipid levels and plasma for MP measures was isolated and stored at −80˚ C until future analysis.
Blood sample analyses
Plasma glucose levels were measured in duplicate using the glucose oxidase method (2300 STAT Plus. YSI. Yellow Springs. Ohio. USA). Plasma triglyceride (TG) and cholesterol were analyzed by enzymatic methods (Hitachi mofel-917 analyzer). HDL-C was measured in the supernatant after precipitation with dextran sulfate and LDL-C was calculated using the Friedewald equation (Friedewald et al., 1972): [LDL-C] = [total cholesterol]- [(TG/5 + HDL-C)]
Plasma microparticles
MP quantification and analysis was performed as previously described (Jenkins et al., 2011) with minor modifications. Briefly, plasma samples were thawed and platelet-poor plasma was obtained through centrifugation. Platelet-poor plasma was then further centrifuged to obtain cell-free plasma (CFP), which was used for MP analyses. 50uL of CFP was incubated with anti-CD31-phycoerythrin (PE) and anti-CD42b-fluorescein isothiocyanate (FITC), anti-CD62E-PE or anti-CD34-FITC (BD Biosciences) for 30 minutes. Samples were then fixed with 2% paraformaldehyde and diluted with filtered PBS before flow cytometric analysis.
Microparticle Quantification and Analysis
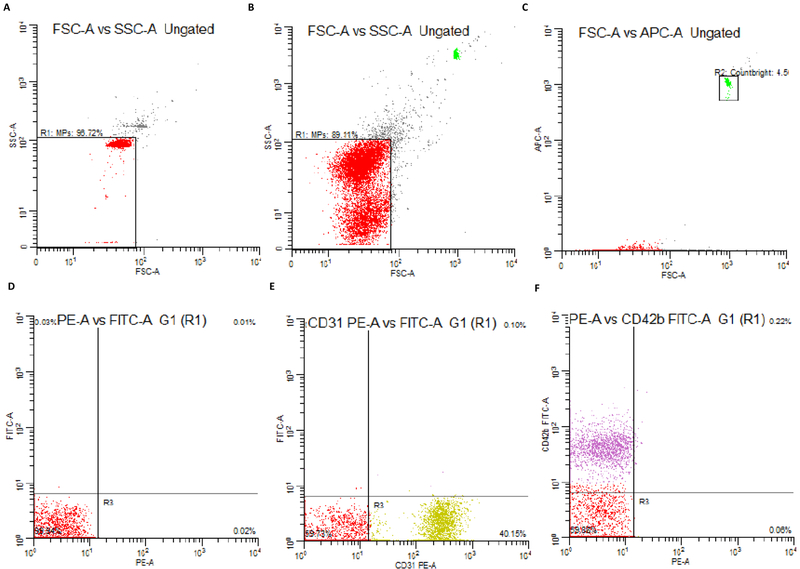
MP quantification was performed using an LSR II flow cytometer with a lower detection limit of 0.5 μm. Samples were each analyzed for 3 minutes at a medium flow rate. Countbright Absolute Counting Beads (ThermoFisher Scientific) were added to each sample immediately before quantification and ultra-pure water was run for 1 minute in between each sample. MPs were defined as CD31+CD42b-, CD62E+ or CD34+ events between 0.5–1.0 μm, excluding exosomes and other small microvesicles from analyses (Gould & Raposo, 2013). A logarithmic scale was used for forward scatter signal, side scatter signal and each fluorescent channel and 0.9 μm standard precision NIST traceable polystyrene particle beads (Polysciences, Inc) were used for size calibration and forward scatter signal. Proper color and size controls were used to distinguish true events from background noise. Examples of controls and gating strategies can be found in Figure 1. MPs were quantified using Winlist 6.0 (Verity Software). The concentration of MPs was calculated using Countbright Absolute Counting Beads (ThermoFisher Scientific).
Figure 1.
Examples of gating strategies for flow cytometry analyses. a-b) NIST beads are used to create gates which delineate events <1.0 μm c) Countbright beads are used to ensure a consistent number of events for each sample and are used to calculate MP concentration. d) unstained controls, e) PE color control and f) FITC color controls
Statistical Analysis
Statistical analyses were completed using IBM SPSS version 21 (Armonk, NY). Assumptions of homoscedasticity and normality were evaluated for all measures. MP data were log-transformed for statistical analyses due to a non-normal distribution of residuals in raw data. Differences among groups were compared using ANOVAs. For our main analyses, power calculations using previous cross-sectional comparisons of MPs in populations with various levels of CVD risk factors (Preston et al., 2003; Lansford et al., 2016) estimated a sample size of n=11/group to yield detectable differences and 80% power. As forty-one percent of chronic stroke subjects were also diagnosed with diabetes mellitus, a sub-analysis was performed to distinguish effects of chronic stroke and T2DM by dividing the chronic stroke group into stroke(diabetes+) and stroke(diabetes-). Pearson correlation coefficients were calculated for log values of plasma MP and values of physical fitness, blood lipids, blood pressure, body mass index (BMI), and glucose. Factors that significantly correlated with MPs were further assessed in a multivariable regression. Statistical significance was accepted at P≤0.05. MP data are expressed as geometric means and 95% confidence limits; remaining data are expressed as means ± standard deviation.
RESULTS
Subject characteristics
Subject characteristics can be found in Table 1. By design, the chronic stroke, T2DM and healthy older groups were significantly older than the younger group; these three groups also had higher BMI values compared with the younger adults (P<0.05). There were no significant differences in age or BMI among the three older groups (chronic stroke, T2DM and healthy older). All older groups exhibited significantly lower absolute and relative VO2peak compared with younger adults, with chronic stroke subjects having the lowest VO2peak values (P<0.05). Both HbA1c and fasting glucose were highest in the T2DM group and all three older groups exhibited significantly higher glucose levels compared with healthy younger adults. HbA1c levels were 7.5 ± 0.85% in the stroke(diabetes+) group and 5.5 ± 0.12% in stroke(diabetes-) group, which are similar to their T2DM and healthy older counterparts, respectively. The T2DM group had significantly higher triglycerides and significantly lower HDL-C levels compared with both healthy younger and older adults (P<0.05 for both). All subjects in the chronic stroke group and T2DM group were on at least one statin and/or blood pressure medication and 88% of subjects diagnosed with T2DM were prescribed medications to control diabetes (Table 2).
Table 1.
Subject Characteristics
| Stroke (n=17) |
T2DM (n=17) |
Healthy Older (n=17) |
Healthy Younger (n=17) |
|
|---|---|---|---|---|
| Sex (M/F) | 13/4 | 14/3 | 13/4 | 14/3 |
| Age (years) | 65 ± 8.9* | 61 ± 6.2* | 62 ± 6.9* | 27 ± 4.6 |
| Weight (kg) | 90.1 ± 19.5 | 99.7 ± 22.9 | 92.8 ± 14.6 | 72.8 ± 11.2 |
| BMI (kg/m2) | 29 ± 4.1* | 32 ± 5.7* | 30 ± 5.3* | 24 ± 2.8 |
| HbA1c (%) | 6.3 ± 1.8 | 7.2 ± 1.1# | 5.5 ± 0.3 | - |
| Glucose (mg/dL) | 107 ± 35.3*$ | 140 ± 34.4*# | 94 ± 7.9* | 86 ± 7.3 |
| Total cholesterol (mg/dL) | 162 ± 69.8 | 155 ± 31.7# | 186 ± 30.2 | 172 ± 25.5 |
| HDL-C (mg/dL) | 50 ± 20.5 | 38 ± 11.8*# | 51 ± 13.2 | 60 ± 20 |
| LDL-C (mg/dL) | 91 ± 54.8 | 88 ± 27.3# | 118 ± 23.8* | 97 ± 21.7 |
| Triglycerides (mg/dL) | 102 ± 50 | 138 ± 65.3*# | 83 ± 42.3 | 74 ± 30.7 |
| SBP (mmHg) | 125 ± 16.1 | 131 ± 11# | 119 ± 17 | 124 ± 13.2 |
| DBP (mmHg) | 72 ± 8.2 | 76 ± 10.5 | 74 ± 7.8 | 78 ± 6.4 |
| MAP (mmHg) | 90.2 ± 8.2 | 94.6 ± 8.8 | 89.3 ± 8.8 | 93.1 ± 7.8 |
| VO2peak (L/min) | 1.7 ± 0.7*$# | 2.5 ± 0.6* | 2.5 ± 0.6* | 3.2 ± 0.9 |
| VO2peak (ml/kg/min) | 18.7 ± 4.5*$# | 25 ± 4.9* | 26 ± 7.5* | 44 ± 8.9 |
Means ± SD
significantly different than healthy younger (P<0.05)
significantly different than healthy older (P<0.05)
significantly different than T2DM (P<0.05)
Table 2.
Medications
| Stroke | T2DM | Healthy Older | Healthy Younger | |
|---|---|---|---|---|
| Medication, n (%) | ||||
| Statin | 9 (53) | 11 (65) | 1 (4) | 0 |
| Other lipid medication | 4 (24) | 6 (35) | 0 | 0 |
| Blood pressure medication | 14 (82) | 10 (59) | 1 (4) | 0 |
| Diuretic | 2 (12) | 2 (12) | 0 | 0 |
| Diabetes medication | 7 (41) | 14 (82) | 0 | 0 |
| Insulin | 2 (12) | 3 (18) | 0 | 0 |
| Blood thinner | 5 (29) | 4 (24) | 0 | 0 |
| Heartburn/GERD medication | 3 (18) | 4 (24) | 1 (4) | 0 |
| Aspirin | 10 (59) | 6 (35) | 2 (12) | 0 |
| Other NSAID | 4 (24) | 4 (24) | 3 (18) | 0 |
| Bronchodilator | 2 (12) | 0 | 2 (12) | 0 |
| Antidepressant | 6 (35) | 2 (12) | 0 | 0 |
| Anti-seizure medication | 4 (24) | 0 | 0 | 0 |
| Glaucoma medication | 2 (12) | 3 (18) | 0 | 0 |
| Muscle relaxant | 3 (18) | 1 (4) | 0 | 0 |
| Bladder/kidney medication | 2 (12) | 2 (12) | 0 | 0 |
| Prostate hypertrophy medication | 3 (18) | 1 (4) | 0 | 0 |
| Prednisone | 1 (4) | 1 (4) | 0 | 0 |
Data represent total number of subjects taking each medication and percent of total from each group; n (%)
Microparticle Concentrations
CD31+/CD42b- Microparticles
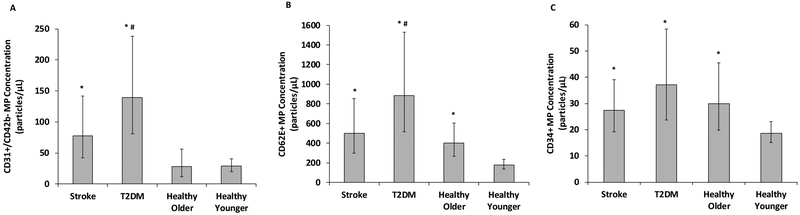
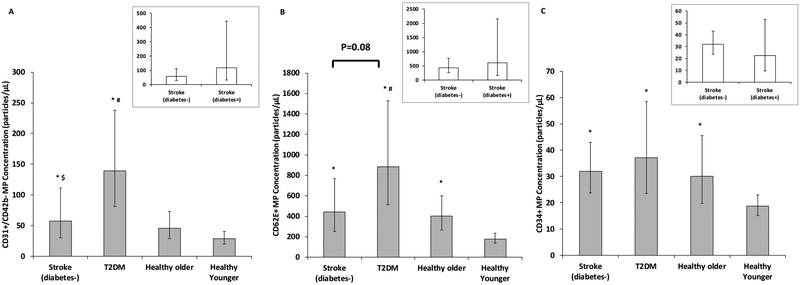
CD31+/CD42b- MP concentration was relatively low in young adults and not different in healthy older adults (P=0.13); however, CD31+/CD42b- MP concentrations from chronic stroke subjects were 170% higher than younger adults (P=0.04). Additionally, T2DM subjects had 384% and 204% greater CD31+/CD42b- MP concentrations than healthy young (P=0.008) and older adults (P=0.02), respectively (Figure 2A). Analyses of chronic stroke subjects with and without diabetes indicated that the stroke(diabetes+) had 105% higher CD31+/CD42b- MP concentrations compared with stroke(diabetes-), although this did not reach statistical significance (P=0.20). However, sub-analyses revealed that the T2DM group had 140% more CD31+/CD42b- MPs compared with chronic stroke(diabetes-) group (P=0.03; Figure 3A).
Figure 2.
Comparison of a) CD31+/CD42b-, b) CD62E+ and c) CD34+ microparticle (MP) concentrations in individuals with chronic stroke, type 2 diabetes mellitus (T2DM), and healthy older and younger individuals (n=17/group). Raw data are presented but were log-transformed for analyses. Figures represent mean and 95% confidence limits. * indicates significantly different than healthy younger adults (P<0.05); # indicates significantly different than healthy older adults (P<0.05)
Figure 3.
Comparison of a) CD31+/CD42b-, b) CD62E+ and c) CD34+ microparticle (MP) concentrations in chronic stroke(diabetes-) (n=10), type 2 diabetes mellitus (T2DM)(n=17), and healthy older (n=17) and younger individuals (n=17). Inset figures represent comparison between chronic stroke(diabetes+) (n=7) and chronic stroke(diabetes-) (n=10). Raw data are presented but were log-transformed for analyses. Figures represent mean and 95% confidence limits. * indicates significantly different than healthy younger adults (P<0.05); # indicates significantly different than healthy older adults (P<0.05); $ indicates significantly different than T2DM (P<0.05)
CD62E+ Microparticles
Compared with young adults, CD62E+ MP concentrations were significantly higher in all older groups (122%, 179% and 393%, in healthy (P=0.03), chronic stroke (P=0.02) and T2DM subjects (P=0.005), respectively; Figure 2B). Furthermore, CD62E+ MP concentrations were higher in the T2DM group compared with healthy older adults (P=0.03, Figure 2B). Analyses of chronic stroke subjects with and without diabetes indicated that the stroke(diabetes+) had 37% more CD62E+ MPs compared with stroke(diabetes-), although this did not reach statistical significance (P=0.23). Sub-analyses indicated a tendency for 101% higher CD62E+ MPs in the T2DM compared with the stroke(diabetes-) (P=0.08; Figure 3B).
CD34+ Microparticles
There was an effect of age on CD34+ MP concentrations, with healthy older adults (P=0.02), chronic stroke (P=0.01), and T2DM (P=0.01) subjects having significantly higher CD34+ MP concentrations than healthy younger individuals (61%, 47% and 99% higher, respectively; Figure 2C). No additional effect of T2DM or chronic stroke was observed (P=0.36 and P=0.38 for both). CD34+ MP concentrations did not differ between stroke subjects with and without diabetes and sub-analyses of stroke(diabetes-) were consistent with findings from original analyses (P=0.92; Figure 3C).
Relationships among MP concentrations and CVD risk factors
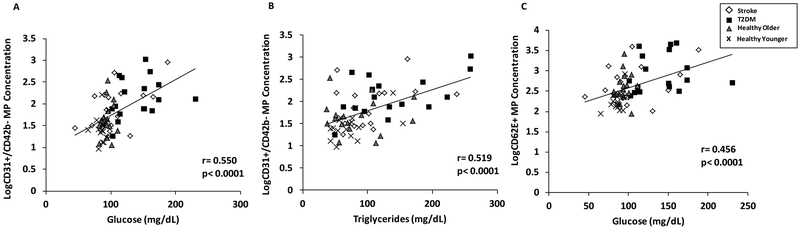
CD31+/CD42b- MP concentrations directly correlated with glucose, triglycerides and BMI (r = 0.35–0.55; P<0.001 for all) and inversely correlated with HDL cholesterol (r = −0.35; P<0.001). When these factors were entered into a regression analysis, significant correlations between only CD31+/CD42b- MPs and glucose and triglycerides persisted (Figure 4A–B; P<0.001 for both). Similarly, CD62E+ MP concentrations significantly correlated with plasma glucose, triglycerides and BMI (r = 0.32–0.46; P<0.001 for all) and inversely with HDL cholesterol (r = −0.32; P<0.001. When these factors were entered into a regression analysis only glucose remained significantly correlated with CD62E+ MP concentrations (Figure 4C; P<0.001). There was no significant correlation between and CD34+ MP concentration and any factor measured (r = │0.03–0.18│; P>0.05 for all).
Figure 4.
Correlations between CD31+/CD42b- microparticles and a) fasting glucose or b) triglycerides, and c) between CD62E+ microparticles and glucose. Log-transformed MP data are presented.
DISCUSSION
MPs are potential biomarkers for endothelial impairment in older age and chronic disease; however, it was previously unknown whether the chronic effects of stroke, T2DM or older age account for elevations in MPs. In this study, we demonstrate that elevated concentrations of CD31+/CD42b- and CD62E+ MPs appear to be driven by T2DM but not chronic stroke. We also show that older age results in further elevations in CD62E+ MP concentrations, while CD34+ MP concentrations are affected by older age alone. Additionally, we found that CD31+/CD42b- MPs and CD62E+ MPs concentrations were each associated with fasting glucose levels and that CD31+/CD42b- MPs also were associated with triglyceride levels. This is not surprising, as high plasma glucose and triglyceride levels coincide with level of T2DM control and are associated with increased CVD risk. Our findings provide further support for the potential use of MPs as novel biomarkers for assessing the clinical progression of CVD risk in older age.
Chronic stroke is associated with long-term vascular consequences that extend far beyond the cerebrum into the periphery (Alexandrova & Bochev, 2005; Billinger et al., 2012; Banerjee & Chimowitz, 2017) and include reduced endothelial vasoreactivity (Ivey et al., 2010) and capillary rarefaction (Prior et al., 2009), most notably in the paretic limb. These vascular complications could, in part, be attributed to MPs with pro-inflammatory effects as we hypothesized that chronic stroke would have elevated MP concentrations compared with other groups. As expected, all three MP subtypes were higher in the chronic stroke group compared with the younger adults. However, we found no differences in any of the three MP subtypes between chronic stroke and the other two older groups (T2DM or healthy older) in our primary analyses. In this study, the degree of hemiparesis was not quantified in the individuals with chronic stroke, but it is possible that MP and other outcomes could vary within this population as a function of the degree of hemiparesis. Impaired glucose metabolism is highly prevalent in chronic stroke (Ivey et al., 2007; Prior et al., 2009) and 42% of the subjects in our chronic stroke group were also diagnosed with type 2 diabetes. Therefore, we performed additional analyses in which we excluded all chronic stroke subjects who were also diagnosed with diabetes (i.e., stroke(diabetes-) vs. T2DM group). Interestingly, the stroke(diabetes-) group had significantly lower CD31+/CD42b- and there was a trend for lower CD62E+ MPs compared with the T2DM group. Our findings indicate that the presence of T2DM, and not the effects of chronic stroke itself, appears to explain the elevations in CD31+/CD42b- and CD62E+ MPs in the present report.
T2DM is associated with disturbances in metabolism and vascular function (Deng et al., 2016) which could be partially attributable to concentrations of specific MPs. We found that CD31+/CD42b- and CD62E+ MPs were highest in subjects with T2DM compared with healthy older and younger groups. Other studies have also found that CD31+/CD42b- MP levels are higher in men with T2DM compared with controls (Esposito et al., 2007; Leroyer et al., 2008) and that they are predictive of macrovascular complications in T2DM (Jung et al., 2011). However, there are currently few studies demonstrating alterations in CD62E+ MPs in subjects with T2DM (Esposito et al., 2007). Our findings suggest that MPs associated with endothelial apoptosis and activation are more elevated in T2DM than in chronic stroke or older age alone. MPs are released in response to a variety of inflammatory stimuli including hyperglycemia and dyslipidemia (Dignat-George & Boulanger, 2011; Jansen et al., 2013) and a chronic inflammatory environment in the setting of T2DM may promote the development and release of these MPs. Once released, MPs may further exacerbate this condition by transporting bioactive cellular content to other cell types or binding to other target cell receptors and interfering with cell signaling. Indeed, one study found that MPs promote insulin resistance by influencing insulin signaling pathways or by interfering with glucose tranporter-4 translocation and blocking glucose uptake by the cell (Zhang et al., 2015). Thus, elevations in CD31+/CD42b- and CD62E+ MPs may be a result of, as well as a contributor to, T2DM and its complications.
While various MP concentrations have been implicated as a consequence and potential contributor to various age-related cardiometabolic diseases, the concentrations of different MPs in older vs. younger adults has not been well explored. Animal studies in mice demonstrate that older mice exhibit more leukocyte-derive MPs compared with young mice (McDonald et al., 2010), but studies in non-human primates found that age does not correlate with the number of different endothelial MPs (Knight et al., 2013). Similarly, Gustafson et al., found no relationship between age and CD62E+ MP concentrations (Gustafson et al., 2015) in healthy adults. To our knowledge, this is the first study to demonstrate elevations in CD62E+ and CD34+ MP concentrations in older compared with younger adults. These findings suggest that endothelial activation resulting in the release of CD62E+ MPs may contribute to the age-related decline in vascular function. In the present report, all healthy older adults were sedentary. As regular aerobic exercise training can attenuate the age-related decline in endothelial function (Seals et al., 2011; DeVan & Seals, 2012), future studies should investigate whether elevations in CD62E+ and CD34+ MPs are mitigated in older chronically active adults compared with sedentary adults.
The origin and effect of CD34+ MPs has been less explored in the literature compared with other MP populations. CD34+ is best known as a progenitor cell marker but can also be present on endothelial cells. In an atherogenic environment, CD34+ progenitor cells can take on characteristics of foam cells in the development of atherosclerosis, which may result in MP release (Daub et al., 2006). Thus, elevations in CD34+ MPs observed with in the older groups may be indicative of chronic low-grade inflammation associated with older age. Alternatively, CD34+ exosomes have been found in vitro to contain angiogenesis-promoting miRNAs (Sahoo & Losordo, 2014; Mathiyalagan et al., 2017). Although the size and mechanisms of release differ between exosomes and MPs, potential similarities in cargo between the two extracellular vesicles suggest that perhaps the elevations in CD34+ MPs are serving in a compensatory capacity to offset the elevations in CD62E+ MPs also associated with older age. Our findings suggest a need for further studies to better clarify the role of CD34+ MPs observed with older age.
In an effort to gain insight into systemic factors that may be involved in MP release from the endothelium, we performed regression analyses among the three MP subtypes and levels of physical fitness, plasma lipids, BMI, and glucose, and found that CD31+/CD42b- and CD62E+ MPs directly correlated with fasting glucose levels. These findings are congruent with previous in vitro work demonstrating that a high glucose environment induces endothelial cell apoptosis (Baumgartner-Parzer et al., 1995) and endothelial activation (Videm & Albrigtsen, 2007) as well as in vivo findings that hyperglycemia is associated with greater CVD risk (Tabit et al., 2010). Additionally, we found that triglyceride levels directly correlated with CD31+/CD42b- MP concentrations, which supports hypertriglyceridemia’s known association with endothelial impairments (Lundman et al., 2003). The present findings are congruent with other reports of direct associations between CD31+/CD42b- MP and triglyceride levels (Ferreira et al., 2004; Amabile et al., 2014), and extend those findings by demonstrating elevated MPs in subjects with more chronic, lower-grade elevations in fasting triglycerides. Conversely, regression analyses found no association between CD62E+ and fasting triglyceride levels. In the current study, the T2DM group exhibits the highest triglyceride and glucose levels of all groups. Although triglyceride levels are still within the normal range, this is likely pharmacologically induced as all T2DM subjects were prescribed statins, potentially influencing our results. It seems likely that a combination of various metabolic imbalances (elevated glucose, triglycerides and lower HDL-C) contribute to endothelial apoptosis which lead to greater MP release in the T2DM group.
There are several differences between the current and previous studies on MPs. First, subjects in our study represent a wider range of age and health including chronic stroke and T2DM. Additionally, it is important to note that 76% of chronic stroke subjects and all T2DM subjects in our study were on some form of diabetes medication, statin or lipid lowering medication. Some medications have been found to influence specific MP concentrations with studies indicating that statins both increase (Diamant et al., 2017) or decrease (Tramontano et al., 2004) different MPs in vitro and some medications resulting in lower MP concentrations in individuals with diabetes and hypertension (Nomura et al., 2005; Esposito et al., 2006a; Nomura et al., 2007; Nomura et al., 2009). Due to their necessity in these populations we were unable to withhold medications for longer than the 12-hour fasting period and the use of medications may have influenced some of our findings. More research is needed to better understand the effects of individual medications and the combined effects of multiple medications on MP concentrations. Finally, it is worth noting that HDL-C levels in our subjects were surprisingly high, especially in the chronic stroke group, with only T2DM subjects having significantly lower HDL-C levels compared with healthy younger adults. This is likely a result of statin usage in the chronic stroke population. HDL cholesterol is involved in the transport of cholesterol from the arteries; thus, it is possible that the relatively high HDL-C levels attenuated endothelial impairment and MP production in the chronic stroke population.
A strength of this study is the balance for sex across groups, although the relatively low female participation prevented our ability to examine sex differences in MPs concentrations from each group. Some recent studies have begun to examine these issues, although the sex differences remain unclear with some studies reporting lower and others higher resting levels of CD62E+ MPs in sedentary younger women compared with men (Durrer et al., 2015; Gustafson et al., 2015). In addition, some studies are beginning to explore MP responses to an acute bout of aerobic exercise and find differential responses between men and women in CD62E+ (Durrer et al., 2015; Lansford et al., 2016; Shill et al., 2018) and CD34+ MPs (Lansford et al., 2016), suggesting sex differences in responses to vascular stressors with exercise. Thus, future studies specifically designed to examine mechanisms related to sex differences in various populations and in response to an exercise or nutritional intervention might provide better indication as to the use of MPs as sex-specific biomarkers of CVD risk.
Because the present study was limited to correlation analyses of relationships among CD31+/CD42b- and CD62E+ MP concentrations and triglycerides or glucose, it cannot provide specific mechanistic evidence of the effects of these factors on MPs (or vice versa). Future studies are needed to investigate molecular pathways involved in dislipidemia and hyperglycemia, how these relate to MP production, and potential mechanisms through which MPs may further be involved in disease pathology to fully understand the complex roles of different MP subtypes. Additionally, we are unable to present the timeframe since diagnosis of T2DM for all our subjects, which might play a role in the extent of vascular stress and consequently, MP concentration. Future work documenting the concentration of different MP subtypes at different stages of T2DM diagnosis would allow for a better understanding of the disease process and how MPs are involved. Recent work has demonstrated that diet, specifically low-carbohydrate diet, can affect MP concentrations in T2DM (Francois et al., 2018). Subjects in the current study arrived fasted but diet was not controlled leading up to their visit which may have affected our outcomes. This current study was designed as a cross-sectional analysis of the levels of three MP subtypes. Further classification of MPs including analyses of MP content and activity as well as their direct effects on endothelial function and whether this can be modified are necessary to fully understand the nature and actions of these factors. It should be acknowledged that plasma was obtained using EDTA anticoagulant. Although ACD anticoagulant is preferred (Golanski et al., 1996; Jayachandran et al., 2012; Gyorgy et al., 2014), we (Jenkins et al., 2011) and others (Babbitt et al., 2013) have successfully quantified MPs using EDTA plasma. Thus, while this may limit our ability to compare specific concentrations of MPs to some other studies, observations of trends for differences in MP levels among similar populations are possible. The samples used in this study were collected on different dates and stored frozen prior to analysis which can influence MP concentrations (Dey-Hazra et al., 2010). The storage times varied within all groups, but were not likely different among groups. However, analyses of MP degradation would allow for a better understanding of whether potential differences in storage times affected our outcomes. Finally, we acknowledge that the sub-analyses of stroke(diabetes-) included a lower sample size, and thus, may not be adequately powered. This may explain why significant differences between the stroke (diabetes+) and stroke (diabetes-) groups were not detected. While these findings may prove physiologically significant, larger studies are needed to confirm the effects of stroke with and without the presence of diabetes.
In conclusion, our study demonstrates that elevations in concentrations of CD31+/CD42b- and CD62E+ MP are much more pronounced in T2DM, but not chronic stroke and demonstrates, for the first time, elevations in CD62E+ and CD34+ MP concentrations in older vs. younger adults. Furthermore, we demonstrate relationships between fasting glucose and CD31+/CD42b- and CD62E+ MPs and triglycerides and CD31+/CD42b- MPs. As various MPs have been proposed as potential targets for diagnosing, treating and identifying the clinical progression and complications of T2DM, our study provides further support for the use of CD31+/CD42b- and CD62E+ MPs in examining these issues. Specific MPs, perhaps combined with other established biomarkers of vascular health such as endothelial progenitor cells, may eventually be used to determine the balance between endogenous vascular injury and repair in different populations to provide a better indicator of the clinical progression of CVD.
NEW FINDINGS.
What is the central question of this study?
What is the effect of chronic stroke on circulating microparticle populations, accounting for potential effects of age and type 2 diabetes?
What is the main finding and its importance?
Elevated concentrations of CD31+/CD42b- and CD62E+ microparticles appear to be driven by type 2 diabetes but not chronic stroke and are associated with fasting glucose and triglyceride levels. Older age results in elevations in CD62E+ and CD34+ microparticle concentrations. These microparticles have been proposed as potential targets for diagnosing, treating and identifying the clinical progression and complications of type 2 diabetes.
ACKNOWLEDGEMENTS
RQL was supported by the NIH T32 (HL007698). This study was supported by a Veterans Affairs (VA) Maryland Exercise and Robotics Center for Excellence (MERCE) Pilot Award (RQL), VA Senior Research Career Scientist Award (ASR), NIH R01-AG030075 (ASR), Career Development Award Number IK2 RX-000944 from the United States (U.S) Department of VA Rehabilitation R&D (Rehab RD) Service (MCS), K23- AG040775 (NIH and the American Federation for Aging Research; SJP), (Department of VA; SJP), and the Baltimore VA Geriatric Research, Education and Clinical Center (GRECC).
Abbreviations
- BMI
body mass index
- CVD
cardiovascular disease
- FITC
fluorescein isothiocyanate
- HDL-C
high density lipoprotein cholesterol
- LDL-C
low density lipoprotein cholesterol
- MP
microparticles
- PE
phycoerythrin
- T2DM
Type 2 Diabetes Mellitus
- TG
triglyceride
- VO2peak
Peak oxygen consumption
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Abid Hussein MN, Boing AN, Biro E, Hoek FJ, Vogel GM, Meuleman DG, Sturk A & Nieuwland R (2008). Phospholipid composition of in vitro endothelial microparticles and their in vivo thrombogenic properties. Thromb Res 121, 865–871. [DOI] [PubMed] [Google Scholar]
- Alexandrova ML & Bochev PG (2005). Oxidative stress during the chronic phase after stroke. Free Radical Biology and Medicine 39, 297–316. [DOI] [PubMed] [Google Scholar]
- Amabile N, Cheng S, Renard JML, M.G., Ghorbani A, McCabe E, Griffin G, Guerin C, Ho JE, Shaw SY, Cohen KS, Vasan RS, Tedgui A, Boulanger CM & Wang TJ (2014). Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. European Heart Journal 35, 2972–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AD (2018). Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 41 (Supplement 1), S13–S27. [DOI] [PubMed] [Google Scholar]
- Babbitt DM, Diaz KM, Feairheller DL, Sturgeon KM, Perkins AM, Veerabhadrappa P, Williamson ST, Kretzschmar J, Ling C, Lee H, Grimm H, Thakkar SR, Crabbe DL, Kashem MA & Brown MD (2013). Endothelial activation microparticles and inflammation status improve with exercise training in african americans. Int J Hypertens 2013, 538017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee C & Chimowitz MI (2017). Stroke Caused by Atherosclerosis of the Major Intracranial Arteries. Circulation Research 120, 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner-Parzer SM, Wagner L, Pettermann M, Grillari J, Gessl A & Waldausl W (1995). High-Glucose-Triggered Apoptosis in Cultured Endothelial Cells. Diabetes 44, 1323–1327. [DOI] [PubMed] [Google Scholar]
- Berkmans RJ, Nieuwland R, Boing AN, Romijn FPHTM, Hack CE & Sturk A (2001). Cell-derived Microparticles Circulate in Healthy Humans and Support Low Grade Thrombin Generation. Thromb Haemost 85, 639–646. [PubMed] [Google Scholar]
- Billinger SA, Coughenour E, MacKay-Lyons MJ & Ivey FM (2012). Reduced Cardiorespiratory Fitness after Stroke: Biological Consequences and Exercise-Induced Adaptations. Stroke Research and Treatment 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian P, Hankey GJ, Eikelboom JW, Thom J, Baker RI, McQuillan A, Staton J & Yi Q (2003). Endothelial and platelet activation in acute ischemic stroke and its etiological subtypes. Stroke 34, 2132–2137. [DOI] [PubMed] [Google Scholar]
- Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet J & Tedgui A (2009). Endothelial Microparticles in Diseases. Cell Tissue Res 335, 143–151. [DOI] [PubMed] [Google Scholar]
- Chiva-Blanch G, Suades R, Crespo J, Pena E, Padro T, Jimenez-Xarrie E, Marti-Fabregas J & Badimon L (2016). Microparticle Shedding from Neural Progenitor Cells and Vascular Compartment Cells Is Increased in Ischemic Stroke. PLoS One 11, e0148176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub K, Langer H, Seizer P, Stellos K, May AE, Goyal P, Bigalke B, Schonberger T, Geisler T, Siegel-Axel D, Oostendorp RAJ, Lindemann S & Gawaz M (2006). Platelets induce differentiation of human CD34 progenitor cells into foam cells and endothelial cells. FASEB 20, E1935–E1944. [DOI] [PubMed] [Google Scholar]
- Deng F, Wang S & Zhang L (2016). Endothelial Microparticles Act as Novel Diagnostic and Therapeutic Biomarkers of Diabetes and Its Complications: A Literature Review. BioMed Research International 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVan AE & Seals DR (2012). Vascular health in the ageing athlete. Exp Physiol 97, 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey-Hazra E, Hertel B, Kirsch T, Woywodt A, Lovric S, Haller H, Haubitz M & Erdbruegger U (2010). Detection of circulating microparticles by flow cytometry: influence of centrifugation, filtration of buffer, and freezing. Vasc Health Risk Manag 6, 1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant M, Tushuizen ME, Abid-Hussein MN, Hau CM, Böing AN, Sturk A & Nieuwland R (2017). Simvastatin-induced endothelial cell detachment and microparticle release are prenylation dependent. Thrombosis and Haemostasis 100, 489–497. [PubMed] [Google Scholar]
- Dignat-George F & Boulanger CM (2011). The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 31, 27–33. [DOI] [PubMed] [Google Scholar]
- Durrer C, Robinson E, Wan Z, Martinez N, Hummel ML, Jenkins NT, Kilpatrick MW & Little JP (2015). Differential impact of acute high-intensity exercise on circulating endothelial microparticles and insulin resistance between overweight/obese males and females. PLoS One 10, e0115860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K, Ciotola M & Giugliano D (2006a). Pioglitazone reduces endothelial microparticles in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 26, 1926. [DOI] [PubMed] [Google Scholar]
- Esposito K, Ciotola M, Giugliano F, Schisano B, Improta L, Improta MR, Beneduce F, Rispoli M, De Sio M & Giugliano D (2007). Endothelial microparticles correlate with erectile dysfunction in diabetic men. International Journal of Impotence Research 19, 161–166. [DOI] [PubMed] [Google Scholar]
- Esposito K, Ciotola M, Schisano B, Gualdiero R, Sardelli L, Misso L, Giannetti G & Giugliano D (2006b). Endothelial Microparticles Correlate with Endothelial Dysfunction in Obese Women. The Journal of Clinical Endocrinology and Metabolism 9, 3676–3679. [DOI] [PubMed] [Google Scholar]
- Ferreira AC, Peter AA, Mendez AJ, J JJ, Mauro LM, Chirinos JA, Ghany R, Virani S, Garcia S, H LL, Purow J, J W, Ahn YS & Marchena E (2004). Postprandial Hypertriglyceridemia Increases Circulating Levels of Endothelial Cell Microparticles. Circulation 110, 3599–3603. [DOI] [PubMed] [Google Scholar]
- Francois ME, Myette-Cote E, Bammert TD, Durrer C, Neudorf H, DeSouza CA & Little JP (2018). Carbohydrate restriction with postmeal walking effectively mitigates postprandial hyperglycemia and improves endothelial function in type 2 diabetes. Am J Physiol Heart Circ Physiol 314, H105–H113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI & Fredrickson DS (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18, 499–502. [PubMed] [Google Scholar]
- Golanski J, Pietrucha T, Baj Z, Greger J & Watala C (1996). Molecular insights into the anticoagulant-induced spontaneous activation of platelets in whole blood-carious anticoagulants are not equal. Thrombosis Research 83, 199–216. [DOI] [PubMed] [Google Scholar]
- Gould SJ & Raposo G (2013). As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson CM, Shepherd AJ, Miller VM & Jayachandran M (2015). Age- and sex-specific differences in blood-borne microvesicles from apparently healthy humans. Biology of Sex Differences 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorgy B, Paloczi K, Kovacs A, Barabas E, Beko G, Varnai K, Pallinger E, Szabo-Taylor K, Szabo T, Kiss AA, Falus A & Buzas EI (2014). Improved circulating microparticle analysis in acid-citrate dextrose (ACD) anticoagulant tube. Thrombosis Research 133, 285–292. [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ, American Heart Association Advocacy Coordinating C, Stroke C, Council on Cardiovascular R, Intervention, Council on Clinical C, Council on E, Prevention, Council on A, Thrombosis, Vascular B, Council on C, Critical C, Perioperative, Resuscitation, Council on Cardiovascular N, Council on the Kidney in Cardiovascular D, Council on Cardiovascular S, Anesthesia, Interdisciplinary Council on Quality of C & Outcomes R (2011). Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123, 933–944. [DOI] [PubMed] [Google Scholar]
- Horstman L, Jy W, Jimenez J & Ahn Y (2004). Endothelial Microparticles as Markers of Endothelial Dysfunction. Frontiers in Bioscience 9, 1118–1135. [DOI] [PubMed] [Google Scholar]
- Ivey FM, Hafer-Macko CE, Ryan AS & Macko RF (2010). Impaired Leg Vasodilatory Function After Stroke: Adaptations with Treadmill Exercise Training. Stroke 41, 2913–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey FM, Ryan AS, Hafer-Macko CE, Garrity BM, Sorkin JD, Goldberg AP & Mackio RF (2006). High prevalence of abnormal glucose metabolism and poor sensitivity of fasting plasma glucose in the chronic phase of stroke. Cerebrovascular diseases 22, 368–371. [DOI] [PubMed] [Google Scholar]
- Ivey FM, Ryan AS, Hafer-Macko CE, Goldberg AP & Macko RF (2007). Treadmill Aerobic Training Improves Glucose Tolerance and Indices of Insulin Sensitivity in Disabled Stroke Survivors: A Priliminary Report. Stroke 38, 2752–2758. [DOI] [PubMed] [Google Scholar]
- Jansen F, Yang X, Franklin BS, Hoelscher M, Schmitz T, Bedorf J, Nickenig G & Werner N (2013). High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovascular Research 98, 94–106. [DOI] [PubMed] [Google Scholar]
- Jayachandran M, Miller VM, Heit JA & Owen WG (2012). Methodology for Isolation, Identification and Characterization of Microvesicles in Peripheral Blood. J Immunol Methods 31, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins NT, Landers RQ, Thakkar SR, Fan X, Brown MD, Prior SJ, Spangenburg EE & Hagberg JM (2011). Prior endurance exercise prevents postprandial lipaemia-induced increases in reactive oxygen species in circulating CD31+ cells. J Physiol 589, 5539–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K, Chu K, Lee S, Bahn J, Kim J, Kim M, Lee S & Roh J (2011). Risk of macrovascular complications in type 2 diabetes mellitus: endothelial microparticle profiles. Cerebrovascular diseases 31, 485–493. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Lee ST, Park HK, Bahn JJ, Kim DH, Kim JH, Kim M, Kun Lee S & Roh JK (2009). Circulating endothelial microparticles as a marker of cerebrovascular disease. Ann Neurol 66, 191–199. [DOI] [PubMed] [Google Scholar]
- Knight DR, Smith AH, Schroeder RL, Huang C, Beebe DA, Sokolowski SA & Wang M (2013). Effects of age on noninvasive assessments of vascular function in nonhuman primates: implications for translational drug discovery. Journal of Translational Medicine 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix R, Sabatier F, Mialhe A, Basire A, Pannell R, Borghi H, Robert S, Lamy E, Plawinski L, Camoin-Jau L, Gurewich V, Angles-Cano E & Dignat-George F (2007). Activation of plasminogen into plasmin at the surface of endothelial microparticles: a mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood 110, 2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansford KA, Shill DD, Dicks AB, Marshburn MP, Southern WM & Jenkins NT (2016). Effect of acute exercise on circulating angiogenic cell and microparticle populations. Exp Physiol 101, 155–167. [DOI] [PubMed] [Google Scholar]
- Lee S, Chu K, HJung K, Kim JB, Moon H, Bahn J, Im W, Sunwoo J, Moon J, Kim M, Lee SK & Roh J (2012). Circulating CD62E+ Microparticles and Cardiovascular Outcomes. PLoS One 7, e35713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroyer AS, Tedgui A & Boulanger CM (2008). Microparticles and type 2 diabetes. Diabetes and Metabolism 34, S27–S31. [DOI] [PubMed] [Google Scholar]
- Lundman P, Eriksson MJ, Silveira A, Hansson L, Pernow J, Ericsson C, Hamsten A & Tornvall P (2003). Relation of Hypertriglyceridemia to Plasma Concentrations of Biochemical Markers of Inflammation and Endothelial Activation (C-Reactive Protein, Interleukin-6, Soluble Adhesion Molecules, von Willebrand Factor, and Endothelin-1). American Journal of Cerdiology 9, 1128–1131. [DOI] [PubMed] [Google Scholar]
- Mathiyalagan P, Liang Y, Kim D, Misener S, Thorne T, Kamide CE, Klyachko E, Losordo DW, Hajjar RJ & Sahoo S (2017). Angiogenic Mechanisms of Human CD34+ Stem Cell Exosomes in the Repair of Ischemic Hindlimb. Circ Res 120, 1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AP, Meier TR, Hawley AE, Thibert JN, Farris DM, Wrobleski SK, Henke PK, Wakefield TW & Myers DD (2010). Aging is associated with impaired thrombus resolution in a mouse model of stasis induced thrombosis. Thrombosis Research 125, 72–78. [DOI] [PubMed] [Google Scholar]
- Nomura S, Inami N, Kimura Y, Omoto S, Shouzu A, Nishikawa M & Iwasaka T (2007). Effect of nifedipine on adiponectin in hypertensive patients with type 2 diabetes mellitus. J Hum Hypertens 21, 38–44. [DOI] [PubMed] [Google Scholar]
- Nomura S, Shouzu A, Omoto S, Inami N, Ueba T, Urase F & Maeda Y (2009). Effects of eicosapentaenoic acid on endothelial cell-derived microparticles, angiopoietins and adiponectin in patients with type 2 diabetes. J Atheroscler Thromb 16, 83–90. [DOI] [PubMed] [Google Scholar]
- Nomura S, Shouzu A, Omoto S, Nishikawa M & Iwasaka T (2005). Benidipine improves oxidized LDL-dependent monocyte and endothelial dysfunction in hypertensive patients with type 2 diabetes mellitus. J Hum Hypertens 19, 551–557. [DOI] [PubMed] [Google Scholar]
- Paudel KR, Panth N & Kim D (2016). Circulating Endothelial Microparticles: A Key Hallmark of Athersclerosis Progression. Scientifica 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescatello LS, Arena R, Riebe D & Thompson PD (2014). American College of Sports Medicine Guidelines for Exercise Testing and Prescription, 9th Ed Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- Preston RA, Jy W, Jimenez JJ, Mauro LM, H LL, Valle M, Aime G & Ahn YS (2003). Effects of Severe Hypertension on Endothelial and Platelet Microparticles. Hypertension 41, 211–217. [DOI] [PubMed] [Google Scholar]
- Prior SJ, McKenzie MJ, Joseph LJ, Ivey FM, Macko RF, Hafer-Macko CE & Ryan AS (2009). Reduced skeletal muscle capillarization and glucose intolerance. Microcirculation 16, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddu P, Puddu GM, Cravero E, Muscari S & Muscari A (2010). The involvement of circulating microparticles in inflammation, coagulation and cardiovascular diseases. Can J Cardiol 26, s140–e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AS, Dobrovolny L, Silver KH, Smith G & Macko RF (2000). Cardiovascular Fitness After Stroke: Role of Muscle Mass and Gait Deficit Severity. Journal of Stroke and Cerebrovascular Diseases 9, 185–191. [DOI] [PubMed] [Google Scholar]
- Sabatier F, Roux V, Anfosso F, Camoin L, Sampol J & Dignat-George F (2002). Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood 99, 3962–3970. [DOI] [PubMed] [Google Scholar]
- Sahoo S & Losordo DW (2014). Exosomes and Cardiac Repair After Myocardial Infarction. Circulation Research 114, 333–344. [DOI] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL & Donato AJ (2011). Aging and vascular endothelial function in humans. Clin Sci (Lond) 120, 357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shill DD, Lansford KA, Hempel HK, Call JA, Murrow JR & Jenkins NT (2018). Effect of Exercise Intensity on Circulating Microparticles in Men and Women. Experimental Physiology. [DOI] [PubMed] [Google Scholar]
- Simak J, Gelderman MP, Yu H, Wright V & Baird AE (2006). Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J Thromb Haemost 4, 1296–1302. [DOI] [PubMed] [Google Scholar]
- Simak J, Holada K, Risitano AM, Zivny JH, Young NS & Vostal JG (2004). Elevated circulating endothelial membrane microparticles in paroxysmal nocturnal haemoglobinuria. Br J Haematol 125, 804–813. [DOI] [PubMed] [Google Scholar]
- Stepien E, Stankiewicz E, Zalewski J, Godlewski J, Zmud K & Wybranska I (2012). Number of Microparticles Generated During Acute Myocardial Infarction and Stable Angina Correlates with Platelet Activation. Archives of Medical Research 43, 13–35. [DOI] [PubMed] [Google Scholar]
- Tabit CE, Chung WB, Hamburg NM & Vita JA (2010). Endothelial dysfunction in diabetes mellitus: Molecular mechanisms and clinical implications. Rev Endocr Metab Disord 11, 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontano AF, O’Leary J, Black AD, Muniyappa R, Cutaia MV & El-Sherif N (2004). Statin decreases endothelial microparticle release from human coronary artery endothelial cells: implication for the Rho-kinase pathway. Biochem Biophys Res Commun 320, 34–38. [DOI] [PubMed] [Google Scholar]
- Videm V & Albrigtsen M (2007). Soluble ICAM-1 and VCAM-1 as Markers of Endothelial Activation. Scandinavian Journal of Immunology 67, 532–531. [DOI] [PubMed] [Google Scholar]
- Viera AJ, Mooberry M & Key NS (2012). Microparticles in cardiovascular disease pathophysiology and outcomes. Journal of the American Society of Hypertension 6, 243–252. [DOI] [PubMed] [Google Scholar]
- Wang J, Huang Y, Wang Y, Xu M, Wang L, Wang S & Tao J (2007). Increased Circulating CD31/CD42 Microparticles Are Associated With Impaired Systemic Artery Elasticity in Healthy Subjects. American Journal of Hypertension 20, 957–964. [DOI] [PubMed] [Google Scholar]
- Williams JB, Jauch EC, Lindsell CJ & Campos B (2007). Endothelial microparticle levels are similar in acute ischemic stroke and stroke mimics due to activation and not apoptosis/necrosis. Acad Emerg Med 14, 685–690. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shi L, Mei H, Zhang J, Zhu Y, Han X & Zhu D (2015). Inflamed macrophage microvesicles induce insulin resistance in human adipocytes. Nutrition and Metabolism 12. [DOI] [PMC free article] [PubMed] [Google Scholar]