Abstract
Behavioral changes in a new environment are often assumed to precede the origins of evolutionary novelties. Here, we examined whether an increase in aggression is associated with a novel scale-eating trophic niche within a recent radiation of Cyprinodon pupfishes endemic to San Salvador Island, Bahamas. We measured aggression using multiple behavioral assays and used transcriptomic analyses to identify differentially expressed genes in aggression and other behavioral pathways across 3 sympatric species in the San Salvador radiation (generalist, snail-eating specialist, and scale-eating specialist) and 2 generalist outgroups. Surprisingly, we found increased behavioral aggression and differential expression of aggression-related pathways in both the scale-eating and snail-eating specialists, despite their independent evolutionary origins. Increased behavioral aggression varied across both sex and stimulus context in both species. Our results indicate that aggression is not unique to scale-eating specialists. Instead, selection may increase aggression in other contexts such as niche specialization in general or mate competition. Alternatively, increased aggression may result from indirect selection on craniofacial traits, pigmentation, or metabolism—all traits which are highly divergent, exhibit signs of selective sweeps, and are affected by aggression-related genetic pathways which are differentially expressed in this system. In conclusion, the evolution of a novel predatory trophic niche within a recent adaptive radiation does not have clear-cut behavioral origins as previously assumed, highlighting the multivariate nature of adaptation and the complex integration of behavior with other phenotypic traits.
Keywords: behavioral ecology, ecological niche, key innovation, lepidophagy, novelty, transcriptomics
Increased aggression does not sufficiently explain the behavioral origins of a novel scale-eating specialist in a recent adaptive radiation of Caribbean pupfishes. Both behavioral and transcriptomic data suggest that scale- and snail-eating pupfish have increased aggression compared to their generalist counterparts. Instead, increased aggression may have resulted from selection in other contexts, or from indirect selection on other traits (i.e., jaw size or pigmentation). In conclusion, the evolution of a novel predatory trophic niche does not have clear-cut behavioral origins as previously assumed.
INTRODUCTION
Evolutionary novelties, such as novel morphological traits or behaviors, allow organisms to perform new functions within new ecological niches, however, their origins are still poorly understood (Pigliucci 2008). For example, in the case of novel resource use, both new behaviors and morphologies are often necessary for organisms to perform new functions. However, the relative importance of behavior and morphology to this new function, and the order in which they evolve is still unknown. Changes in behavior may precede the evolution of novel morphologies, as they can expose organisms to novel environments and selective pressures (Huey et al. 2003; Losos 2010). Investigations of novelty, however, overwhelmingly ignore this possibility (although see: Huey et al. 2003; Losos et al. 2004; Duckworth 2006). Instead, previous studies have focused on novel adaptive morphologies or on how environmental changes expose organisms to new selective pressures (Liem 1973; Barton and Partridge 2000; Janovetz 2005; Hulsey et al. 2008). Changes in behavior may be a plausible origin for novel phenotypes, but to document this we must first understand its variation within and among taxa.
One outstanding example of novelty is lepidophagy (scale-eating) in fishes. Scale-eating has been documented in at least 10 freshwater and 7 saltwater families of fishes and has independently evolved at least 19 times (Sazima 1983; Janovetz 2005; Martin and Feinstein 2014; Nelson et al. 2016; Kolmann et al. 2018). Scale-eating includes both novel morphologies and behaviors. For example, some scale-eaters have premaxillary external teeth for scraping scales (Novakowski et al. 2004), some use aggressive mimicry to secure their prey (Boileau et al. 2015), others sneak scales from fish that they are cleaning (Losey 1979), and still others use ambush tactics to obtain scales (Nshombo et al. 1985). Even though scale-eating is an outstanding example of the convergent evolution of novel trophic ecology across disparate environments and taxa, and scale-eaters display a wide variety of morphologies and behaviors, the evolutionary origins of lepidophagy are still largely unknown.
There are currently 3 hypothesized behavioral origins for scale-eating. First, the algae-grazer hypothesis predicts that scale-eating arises from the incidental ingestion of scales during algae scraping (Fryer et al. 1955; Greenwood 1965; Sazima 1983). Indeed, many scale-eaters are closely related to algae-grazers. For example, many Malawi haplochromine cichlids are algae-scrapers (Greenwood 1965; Fryer and Iles 1972; Ribbink et al. 1983); however, the radiation also includes 2 sister species of scale-eaters (Corematodus shiranus and Corematodus taeniatus) and a second independent origin of scale-eating in Genyochromis mento (Trewavas 1947; Greenwood 1965) within the predominantly rock-dwelling and algae-scraping mbuna cichlids (Fryer and Iles 1972). Similarly, the extinct Lake Victorian scale-eater Haplochromis welcommei was nested within rock-dwelling algae-scrapers (Greenwood 1965). This hypothesis, however, does not address why algae-grazing fish would seek food on the surface of other fish (Greenwood 1965). The second hypothesis, termed the cleaner hypothesis, tries to address this gap by arguing that scale-eating arose from the incidental ingestion of scales during the consumption of ectoparasites from the surface of other fishes (Greenwood 1965; Sazima 1983). One line of evidence supporting this hypothesis is that cleaner fish, which primarily consume ectoparasites, sometimes eat scales. For example, the Hawaiian cleaner wrasse (Labroides phthirophagus) and 2 species of juvenile sea chub (Hermosilla azurea and Girella nigricans) consume both ectoparasites and scales (Losey 1972; Demartini and Coyer 1981; Sazima 1983). The reverse scenario—primarily scale-eating fish who also consume ectoparasites—is less common. In fact, few scale-eating fishes are even closely related to cleaner fish. One of the only examples of this is the spotted piranha (Sarrasalmus marginatus), which was observed cleaning fish-lice from larger species of piranha. Even this example, however, is based only on the observations of 2 individuals (Sazima and Machado 1990). Finally, the aggression hypothesis predicts that scale-eating evolved due to the incidental ingestion of scales during inter- or intraspecies aggression (Sazima 1983). This hypothesis is supported by the fact that 2 characid species of scale-eaters (Probolodus heterostomus and Exodon paradoxus) are closely related to the aggressive Astyanax tetras (Sazima 1983; Kolmann et al. 2018); a similar argument can be made for the scale-eating piranha (Catoprion mento) (Janovetz 2005). Furthermore, Roeboides species facultatively ingest scales during the low-water season when competition for insects is high (Peterson and Winemiller 1997; Peterson and McIntyre 1998). It is thus also possible that increased competition for food resources led to increased aggression and lepidophagy.
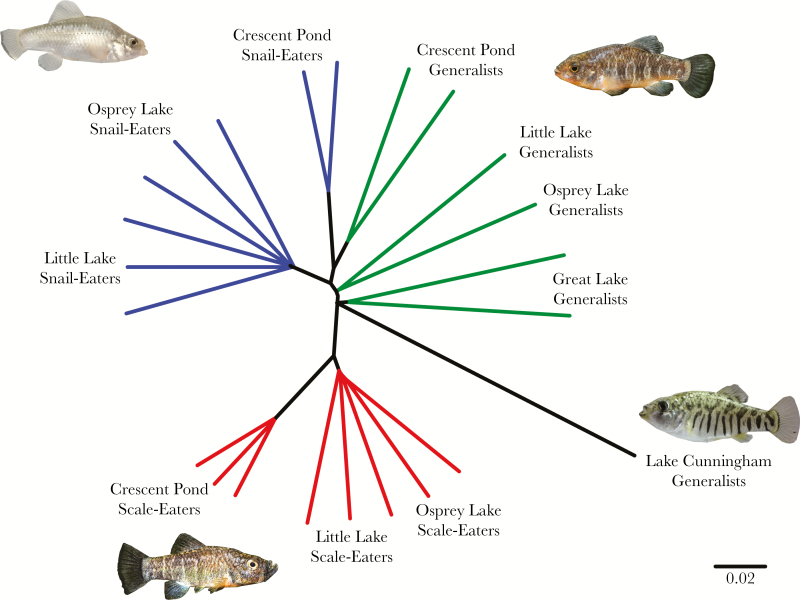
The scale-eating pupfish, Cyprinodon desquamator, is an excellent species for investigating the origins of scale-eating because it is, by far, the youngest scale-eating specialist known. The species is nested within a sympatric adaptive radiation of pupfishes endemic to the hypersaline lakes of San Salvador island, Bahamas (Martin and Wainwright 2011; Martin and Wainwright 2013a). Geological evidence suggests that these hypersaline lakes—and thus the radiation containing the scale-eater—are less than 10,000 years old (Hagey and Mylroie 1995; Martin and Wainwright 2013a; Martin and Wainwright 2013b). In addition to the scale-eating pupfish, the radiation also includes a widespread generalist (C. variegatus) and an endemic snail-eating specialist (C. brontotheroides). Other generalist pupfish lineages (C. variegatus) are also distributed across the Caribbean and western Atlantic Ocean. Despite their shared taxonomy with the San Salvador generalist species, phylogenetic evidence suggests that these generalist populations are outgroups to the San Salvador clade (Martin and Feinstein 2014; Martin 2016; Richards and Martin 2017). Phylogenies based on RADseq data also indicate that scale-eaters form a monophyletic group among lake populations on San Salvador (Figure 1), indicating that the scale-eaters’ most recent common ancestor was most likely an algae-eater (Martin and Feinstein 2014; Lencer et al. 2017). In contrast, snail-eaters clustered with generalists within the same lake, consistent with multiple origins of the snail-eating specialist or extensive introgression with generalists (Martin and Feinstein 2014; Martin 2016). Further evidence of introgression of adaptive alleles fixed in the snail-eating specialist across lakes is consistent with the latter scenario: generalists and snail-eaters are most closely related to each other genome-wide whereas a small number of alleles underlying the snail-eater phenotype have spread among lakes (Figure 1; Richards and Martin 2017; McGirr and Martin 2018). Phylogenies based on RADseq loci and whole-genome data also support a sister relationship between San Salvador generalist populations and snail-eaters across most of the genome. These species are in turn sister to scale-eaters and the San Salvador radiation forms a clade relative to outgroup generalist populations on neighboring islands (Richards and Martin 2017).
Figure 1.
Neighbor joining tree illustrating the relationships between San Salvador Island species and a Caribbean Island outgroup. Predominant topology from a Saguaro analysis (Zamani et al. 2013) which represents 64% of the genome of generalists (green), snail-eaters (blue), scale-eaters (red), and the Lake Cunningham generalist outgroup (black). Branch lengths represent average number of substitutions per base pair. Figure modified from Richards and Martin 2017.
Here, we investigated the behavioral origins of novelty by examining whether an increase in aggression is associated with the evolution of the scale-eating pupfish. We compared measures of aggression using both behavioral and gene expression data among all 3 sympatric species within the San Salvador clade plus behavioral data for 2 additional generalist outgroups. If the aggression hypothesis is true, we expected to find increased levels of aggressive behavior in scale-eating pupfish, and lower levels of aggressive behavior in snail-eaters, generalists, and outgroups. Similarly, we expected to find differential gene expression in aggression-related pathways between scale-eaters versus generalists, but not between snail-eaters versus generalists. Surprisingly, we found that scale-eaters and snail-eaters both displayed high levels of aggression and exhibited differential expression in several aggression-related pathways during early development.
METHODS
Sampling
Generalist, snail-eating, and scale-eating pupfish were collected by seine from Crescent Pond, Great Lake, Little Lake, Osprey Lake, and Oyster Pond on San Salvador Island, Bahamas in July, 2016 and April, 2018. Generalist outgroups were also collected from Lake Cunningham, New Providence Island (Nassau), Bahamas (hereafter referred to as NAS) and from the coast of North Carolina (Fort Fisher, Cape Fear river drainage; hereafter referred to as NC) in April 2018 and June 2017, respectively. Fishes were housed in 40–80 L tanks in mixed-sex groups at 5–10 ppt salinity in temperatures ranging from 23 to 30 °C. Fish were fed a diet of frozen blood worms, frozen mysis shrimp, or commercial pellet food daily. Wild-caught fish used for assays were held in the laboratory for at least 2 weeks before use in behavioral trials. We only used sexually mature adult fish for behavioral assays as pupfish can be visually sexed at this stage. Furthermore, all fish were in reproductive condition; pupfish mate and lay eggs daily and continually throughout the year after they mature.
Behavioral assays
We used 3 types of behavioral assays to quantify levels of aggression: A mirror assay, a paired aggression assay, and a boldness assay. While mirror assays measured a fish’s level of aggression towards its mirror image, paired aggression assays measured levels of aggression toward another fish. Many species of fish use size as a proxy for aggression, and the mirror assay helps control for this, as the stimulus is the exact same size as the focal individual (Rowland 1989; Buston and Cant 2006). Mirror assays, however, may not accurately detect aggression in some cases (Balzarini et al. 2014). For example, some species use lateral displays of aggression which primarily occur head to tail—a maneuver that is impossible with a mirror image. Additional studies also indicate that mirror tests may not accurately predict aggressive display frequency, duration, or orientation (Elwood et al. 2014; Arnott et al. 2016). We therefore also measured aggression using paired aggression assays which allowed focal fish to display aggression in a more natural fashion. Boldness assays, on the other hand, measured a fish’s willingness to explore a new environment. While this was not a direct measure of aggression per se, many studies have documented a correlation between aggression and boldness; so, we chose to include this measure in our study (Fraser et al. 2001; Rehage and Sih 2004; Sih et al. 2004; Gruber et al. 2017). All available adult wild-caught fish were sampled for the mirror assay (n = 198), but only a subset were randomly sampled for the paired aggression assay (n = 40) and the boldness assay (n = 51).
Mirror assay
We quantified levels of aggression for each pupfish species and sex using mirror tests (Vøllestad and Quinn 2003; Francis 2010). To control for individual size and motivation, we incited aggression using a compact mirror (10 × 14 cm) placed in a 2-L trial tank (25 × 16 × 17 cm). We randomly chose adult fish and isolated each one in 2-L tanks that contained a single bottom synthetic yarn mop for cover and opaque barriers between adjacent tanks. We gave fish at least 12 h to acclimate to their new environment before performing an assay.
During a 5-min focal observation period, we measured 3 metrics as a proxy for aggression: latency to approach mirror image, latency to attack mirror image, and total number of attacks toward the mirror image. A trial began as soon as the mirror was securely lowered into the tank. We measured latency to approach as the time elapsed before an individual approached the mirror to within one-body length. Similarly, we measured latency to attack as the time elapsed before an individual attacked their mirror image for the first time. Finally, we counted the total number of attacks an individual performed during the entirety of the trial. We also measured the standard length of each fish after the trial. To determine the repeatability of this assay, we measured aggression 2 separate times in a subset of our fishes (n = 21). We found significant repeatability for latency to attack and total number of attacks (latency to approach, r2 = 0.02, P = 0.50; latency to attack, r2 = 0.18, P = 0.045; total number of attacks, r2 = 0.36, P = 0.0026). As a control, we also measured latency to approach, latency to attack, and the total number of attacks performed towards the nonreflective side of the mirror (n = 51). We used the same methods as above, but inserted the mirror so that its reverse, nonreflective side faced the fish.
Paired aggression assay
We used a paired aggression assay as a second measurement of aggression for a subset of San Salvador generalists, snail-eaters, and scale-eaters (n = 40; Katzir 1981; Pauers et al. 2008). Paired aggression assays quantified levels of aggression for each species and sex using a conspecific of the same sex, conspecific of the opposite sex, and a heterospecific of the same sex as a stimulus fish. We randomly chose and isolated fish in the same manner as the mirror assay. Fish were again given at least 12 h to acclimate to their new environment before performing an assay. Before an assay, a plastic mesh box (10 × 10 × 10 cm) with mesh size of 0.5 cm was lowered into the tank, and a stimulus fish was placed inside the box, after which the assay began. During the 5-min focal observation period, we measured the focal fish’s latency to approach the stimulus fish (within one-body length), their latency to attack the stimulus fish, and the total number of attacks performed toward the stimulus fish. Each focal fish was given 4 paired aggression assays: 1) stimulus fish was a conspecific of the same sex, 2) stimulus fish was a conspecific of the opposite sex, 3) stimulus fish was a heterospecific of the same sex, and 4) a control with an empty box. Specialists were always given a generalist as the heterospecific stimuli, but generalists were randomly assigned either a snail-eater or a scale-eater. All fish were tested in the same order and were given at least 12 h of rest between assays. We also measured the standard length of each stimulus and focal fish.
Boldness assay
Finally, we conducted a boldness assay to determine the relationship between boldness and aggression in pupfishes (Budaev 1997; Brown et al. 2005; Wilson and Godin 2009). We used a random subset of individuals from the mirror assay for this test (n = 51). Before a trial, a PVC cylinder start box was placed into a 2-L trial tank (25 × 16 × 17 cm). The start box was 12 cm in diameter with a removable screw top and contained a single drilled 3 cm hole for the fish to emerge from (which was blocked with a cork at the start of the trial). At the start of the trial, the top of the start box was removed, and a focal fish was gently placed inside. The top was then secured on the box, and the fish was given 1 min to acclimate. After the acclimation time, the 3 cm hole was unplugged (allowing the fish to emerge from the start box) and the 5-min assay began. We measured the latency of the fish’s head to emerge from the hole, a preliminary behavioral inspection of the outside environment, and the latency of the fish’s tail (i.e., the entire fish) to emerge from the hole as proxies for boldness.
Statistical analyses
We used time-to-event analyses to determine if species and sex were associated with 1) latency to approach mirror image, 2) latency to attack mirror image, and 3) latency to emerge from the start box. We used time-to-event models for time metrics since it allows for right censored data, i.e., individuals who did not approach, attack, or emerge within the 5-min time window are not excluded from the dataset and contributed to Kaplan–Meier estimates (Rich et al. 2010). We used Cox proportional hazards models to analyze time metrics for the boldness assay, paired aggression assays, and the mirror control assay (Survival Package; Therneau 2015). We used a mixed-effects Cox proportional hazards model (coxme package; Therneau 2015) for the mirror assay as the individuals from this assay originated from multiple populations. For each of the above models, we included species and sex as fixed effects and lake population as a random effect for the mirror assay models. Using AICc (Burnham and Anderson 2002), we compared models to equivalent models that also included the interaction between species and sex as a fixed effect, the size of the focal individual (log-transformed) as a covariate, and—where applicable—the size of the stimulus individual (log-transformed) as a covariate. The interaction between species and sex was significant for: 1) the latency to emerge (head) in the boldness assay, 2) the latency to approach in the mirror assay, 3) the latency to approach in the heterospecific assay, and 4) the latency to attack in the same-sex conspecific assay and was therefore retained in those final models. Additionally, the focal fish’s size was a significant covariate for the latency to approach model for the heterospecific assay and the latency to attack model for the mirror assay and was also retained in those models. We used Wald and likelihood ratio tests to determine if species, sex, or their interaction were associated with fishes’ latency to approach, attack, or emerge depending on the assay (Table 1).
Table 1.
Results of 1) mixed-effect Cox proportional hazards models, 2) Cox proportional hazards models, 3) GLMMs, and 4) GLMs describing aggression-related behaviors
| Metric | Assay | Predictor | df | χ 2 | P |
|---|---|---|---|---|---|
| a) Latency to approach | Mirror | Species | 4 | 6.02 | 0.2 |
| Sex | 1 | 0.01 | 0.91 | ||
| Species:Sex | 4 | 9.67 | 0.046 | ||
| Conspecific same sex | Species | 2 | 1.87 | 0.39 | |
| Sex | 1 | 1.83 | 0.18 | ||
| Conspecific opposite sex | Species | 2 | 0.55 | 0.76 | |
| Sex | 1 | 0.14 | 0.71 | ||
| Heterospecific | Species | 2 | 0.05 | 0.98 | |
| Sex | 1 | 1.3 | 0.25 | ||
| Size | 1 | 5.02 | 0.025 | ||
| Species:Sex | 2 | 8.26 | 0.016 | ||
| Mirror control | Species | 4 | 2.67 | 0.61 | |
| Sex | 1 | 3.33 | 0.07 | ||
| Paired aggression control | Species | 2 | 1.58 | 0.45 | |
| Sex | 1 | 0.37 | 0.55 | ||
| b) Latency to attack | Mirror | Species | 4 | 5.18 | 0.27 |
| Sex | 1 | 3.37 | 0.07 | ||
| Size | 1 | 6.22 | 0.01 | ||
| Conspecific same sex | Species | 2 | 3.49 | 0.18 | |
| Sex | 1 | 1.77 | 0.18 | ||
| Species:Sex | 2 | 7.37 | 0.025 | ||
| Conspecific opposite sex | Species | 2 | 2.45 | 0.29 | |
| Sex | 1 | 0.13 | 0.72 | ||
| Heterospecific | Species | 2 | 7.34 | 0.026 | |
| Sex | 1 | 6.86 | 0.009 | ||
| Mirror control | Species | 4 | 3.89 | 0.42 | |
| Sex | 1 | 0.81 | 0.37 | ||
| Paired aggression control | Species | 2 | 2.6 | 0.27 | |
| Sex | 1 | 0.02 | 0.9 | ||
| c) Total number of attacks | Mirror | Species | 4 | 12.96 | 0.01 |
| Sex | 1 | 7.73 | 0.005 | ||
| Size | 1 | 3.8 | 0.051 | ||
| Species:Sex | 4 | 14.37 | 0.006 | ||
| Conspecific same sex | Species | 2 | 6.6 | 0.037 | |
| Sex | 1 | 4.53 | 0.033 | ||
| Species:Sex | 2 | 6.19 | 0.045 | ||
| Conspecific opposite sex | Species | 2 | 3.52 | 0.17 | |
| Sex | 1 | 0.08 | 0.78 | ||
| Heterospecific | Species | 2 | 13.46 | 0.001 | |
| Sex | 1 | 0.68 | 0.41 | ||
| Mirror control | Species | 4 | 7.78 | 0.1 | |
| Sex | 1 | 1.62 | 0.2 | ||
| Paired aggression control | Species | 2 | 0 | 1 | |
| Sex | 1 | 0 | 1 | ||
| Size | 1 | 0.23 | 0.64 | ||
| Species:sex | 2 | 0 | 1 | ||
| d) Latency to emerge (head) | Boldness | Species | 4 | 0.48 | 0.98 |
| Sex | 1 | 0.28 | 0.6 | ||
| Species:sex | 4 | 7.02 | 0.14 | ||
| e) Latency to emerge (tail) | Boldness | Species | 4 | 5.1 | 0.28 |
| Sex | 1 | 6.33 | 0.01 |
Significant predictors are indicated in bold.
We used generalized linear models (GLM) or generalized linear mixed models (GLMM) to analyze the total number of attacks performed for each assay. For the 1) same-sex conspecific assay, 2) opposite sex conspecific assay, and 3) heterospecific assay, we used GLMs with a negative binomial distribution to analyze the total number of attacks. We modeled species and sex as fixed effects for these models. For the mirror assay, however, we used a GLMM with a negative binomial distribution. Here, we modeled species and sex as fixed effects and population as a random effect. We modeled the total number of attacks during controls for 1) the mirror assays and 2) the paired aggression assay, using GLMs with a Poisson distribution, and included species and sex as fixed effects. Using AICc, we compared each of these models to equivalent models which also included the interaction between species and sex as a fixed effect, the size of the focal individual (log scale) as a covariate, and—for paired aggression assays—the size of the stimulus individual (log scale) as a covariate. We found models including the interaction between species and sex best explained the data for the: 1) control for the paired aggression assay model, 2) the conspecific of the same-sex assay model, and 3) the mirror assay model, and were thus retained in the final models. Additionally, models including size of the focal individual significantly improved the fit of the paired aggression assay model and the mirror assay model and were thus retained in the final models. We used Wald and likelihood ratio tests to determine if species, sex, or their interaction significantly affected the total number of attacks performed during assays (Table 1).
One caveat is that we did not correct for phylogeny in any of these models. While correcting for phylogeny is important when hierarchical species relationships exist (Felsenstein 1985), this is not the case for the recently diverged San Salvador clade which is best explained by a network of interconnected populations with extensive gene flow. Indeed, numerous admixture events in addition to the maximum likelihood phylogeny were supported by Treemix (Pickrell and Pritchard 2012) population admixture graphs (Martin 2016); similarly, only 82% of the genome supported a monophyletic relationship for San Salvador species (Richards and Martin 2017). Importantly, populations of the scale-eating and snail-eating specialists were never most closely related to each other. When so few regions of the genome underlie phenotypic differences, these species can be viewed as a set of populations with substantial evidence for gene flow.
Finally, we made direct comparisons between groups for all time and count metrics using bootstrap resampling methods with replacement (10,000 replicates; boot package; Canty and Ripley 2017). For right censored time metrics, we calculated the median survival time for each group of interest (Bewick et al. 2004). Median survival times represent the timepoint at which 50% of the group experienced an event (i.e., approached, attacked or emerged). Lower medians indicate that the event occurred quickly while a median of > 300 indicates that 50% of the group never experienced the event (and is therefore right censored). For count data (i.e., attacks), we simply calculated the mean for each group. Finally, we calculated the bias-corrected and accelerated bootstrap 95% confidence intervals for each mean and median (Haukoos and Lewis 2005). All analyses were performed in R (R Core Team 2018).
Early developmental genes affecting differences in aggression between species
We searched a previously published dataset of 15 San Salvador pupfish transcriptomes to identify differentially expressed genes between each generalist and specialist pair annotated for behavioral effects (which had not previously been examined [McGirr and Martin 2018]). Purebred F1 and F2 offspring from the 3 species found on San Salvador Island were raised in a common garden laboratory environment. Larvae were euthanized in an overdose of MS-222 at 8–10 days postfertilization (dpf), immediately preserved in RNAlater (Ambion, Inc.), and stored at −20 °C after 24 h at 4 °C. Total mRNA was extracted from whole larvae for: 6 generalists, 6 snail-eaters, and 3 scale-eaters (RNeasy kits, Qiagen). The KAPA-stranded mRNA-seq kit (KAPA Biosystems 2016) was used to prepare libraries at the High Throughput Genomic Sequencing Facility at UNC Chapel Hill. Stranded sequencing on an Illumina HiSeq 4000 resulted in 363 million raw reads that were aligned to the Cyprinodon variegatus reference genome (NCBI, C. variegatus Annotation Release 100, total sequence length = 1,035,184,475; number of scaffold = 9259, scaffold N50 = 835,301; contig N50 = 20,803; Lencer et al. 2017). We removed adaptors and low-quality reads (Phred score <20) using Trim Galore (v. 4.4, Babraham Bioinformatics).
Aligned reads were mapped to annotated features using STAR (v. 2.5(33)), with an average read depth of 309× per individual and read counts were generated across annotated features using the featureCounts function from the Rsubread package (Liao et al. 2013). We then used MultiQC to assess mapping and count quality (Ewels et al. 2016). DEseq2 (Love et al. 2014, v. 3.5) was used to normalize counts and to complete pairwise comparisons between snail-eaters versus generalists and between scale-eaters versus generalists. Genes with fewer than 2 read counts or low normalized counts (determined by DESeq2) were discarded (Love et al. 2014). Finally, we compared normalized posterior log fold change estimates between groups using a Wald test (Benjamini-Hochberg correction), and found: 1) 1014 differentially expressed genes between snail-eaters versus generalists and 2) 5982 differentially expressed genes between scale-eaters versus generalists (McGirr and Martin 2018).
We performed gene ontology (GO) enrichment analyses for differentially expressed genes using resources from the GO Consortium (geneontology.org; Ashburner et al. 2000; The Gene Ontology Consortium 2017). We identified 1-way and reciprocal best hit zebrafish orthologs for genes differentially expressed between 1) snail-eaters versus generalists (n = 722) and 2) scale-eaters versus generalists (n = 3966) using BlastP (Shah et al. 2018). While a reciprocal best hit method is more powerful for identifying true orthologs, it often misses orthologs in lineages which have experienced genome duplication events, such as teleost fishes (Dalquen and Dessimoz 2013). Hence, we used both approaches to identify possible orthologs.
Animal aggression has previously been categorized, and includes intermale aggression, maternal aggression, sex-related aggression, and territorial aggression (Moyer 1971; Wilson 2000; Nelson and Chiavegatto 2001). Furthermore, previous studies have found differential gene expression in the context of intermale aggression, female-female aggression, and maternal aggression, (Nelson and Trainor 2007). We then compared the reciprocal best hit and 1-way best hit zebrafish orthologs to gene ontologies in the similar categories of: aggressive behavior (GO: 0002118), intermale aggressive behavior (GO: 0002121), maternal aggressive behavior (GO:0002125), maternal care behavior (GO: 0042711), and territorial aggressive behavior (GO: 0002124; AmiGo; Ashburner et al. 2000; Carbon et al. 2009; The Gene Ontology Consortium 2017). Steroid hormones, like vasopressin, androgens, and estradiol, have also been linked to changes in aggression (Nelson and Chiavegatto 2001; Nelson and Trainor 2007), so we also searched gene ontologies for those 3 hormone pathways. Thus, we performed an exhaustive and unbiased search of all aggression and parental care genes differentially expressed relative to the generalist in any tissue during the early development of each specialist species.
RESULTS
Behavioral assays
Scale-eaters and snail-eaters are more aggressive than generalists
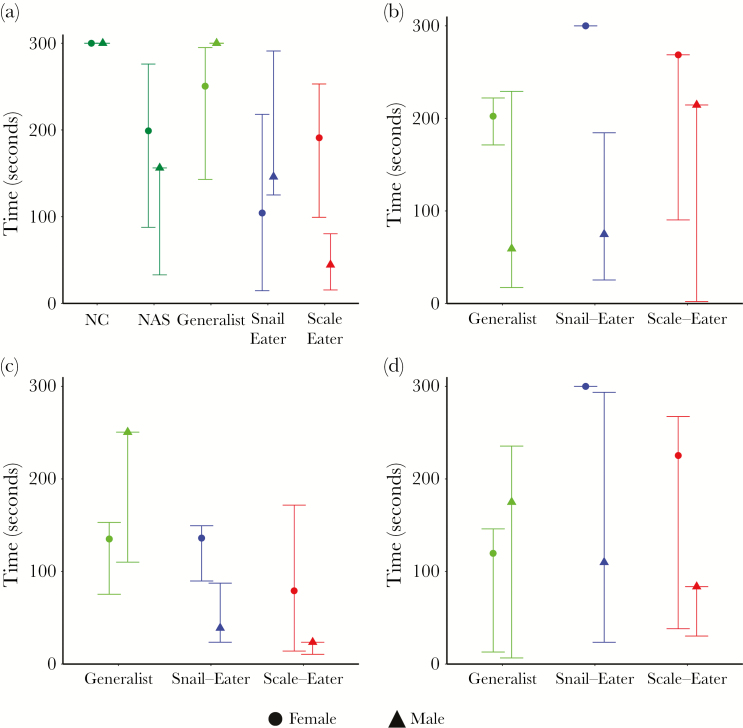
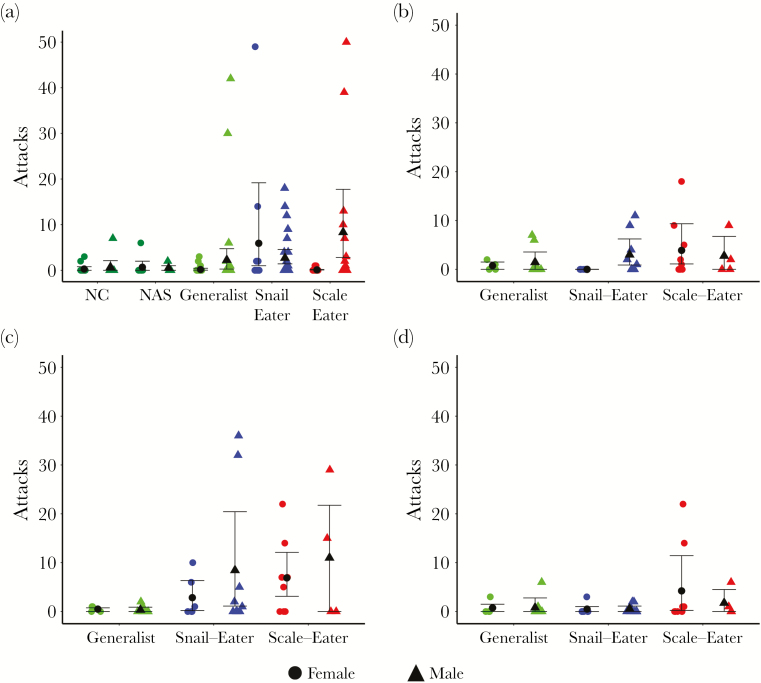
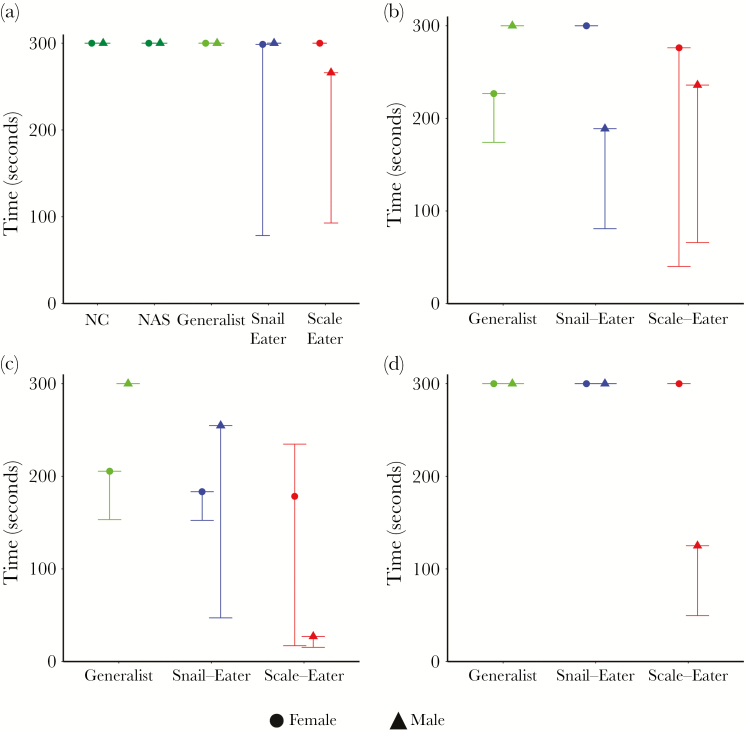
Both scale-eaters and snail-eaters exhibited increased aggression compared to their generalist counterparts. Male scale- and snail-eaters approached their mirror image significantly quicker than NC and San Salvador generalists (Table 1, a; Figure 2a), and attacked their mirror image significantly more than NAS generalists (Table 1, c; Figure 4a). Female snail-eaters also attacked their mirror image significantly more than generalists from NC and San Salvador (Table 1, c; Figure 4a). We saw a similar pattern when fish were presented with conspecific or heterospecific live fish stimuli. Male scale- and snail-eaters approached heterospecific fish significantly more quickly than San Salvador generalists (Table 1, a; Figure 2c), and attacked male conspecifics significantly more quickly than did generalists (Table 1, b; Figure 3b). Scale- and snail-eaters also attacked heterospecific fish significantly more quickly and performed more total attacks towards heterospecific fish than did generalists (Table 1, a and c; Figures 2c and 3c).
Figure 2.
Median and 95% CI’s (BCa) for latency to approach: (a) mirror image, (b) same-sex conspecific, (c) heterospecifics, or (d) opposite sex conspecific.
Figure 4.
Mean number and 95% CI’s (BCa) for attacks performed towards: (a) mirror image, (b) same-sex conspecific, (c) heterospecifics, or (d) opposite sex conspecific.
Figure 3.
Median and 95% CI’s (BCa) for latency to attack: (a) mirror image, (b) same-sex conspecific, (c) heterospecifics, or (d) opposite sex conspecific.
Aggression is sex dependent, but not consistent across species
We also found that levels of aggression varied across sexes, but that the pattern was not consistent across species. While male scale- and snail-eaters were consistently more aggressive than their female counterparts, female generalists were more aggressive than males. Both male scale- and snail-eaters showed increased aggression during assays in which they faced stimuli similar to themselves (i.e., mirror assays and same-sex conspecific assays). Scale-eater males approached their mirror image more quickly and performed more total attacks toward their mirror image than female scale-eaters (Table 1, a and c; Figures 1a and 3a). Similarly, male snail-eaters attacked male conspecifics more quickly and performed more total attacks toward male conspecifics than females did toward female conspecifics (Table 1, b and c; Figures 2b and 3b). Generalist females, however, approached their mirror image more quickly than generalist males (Table 1, a; Figure 2a), and attacked female conspecifics quicker than males attacked male conspecifics (Table 1, b; Figure 3b).
Aggression varies across different behavioral assays
Not only did aggression vary between species and sex, but it also varied across behavioral assays. While female generalists and scale-eaters attacked female conspecifics quicker than snail-eaters (Table 1, b; Figure 3b), female snail-eaters performed more total attacks toward their mirror image than either of these groups (Table 1, c; Figure 4a). Similarly, male scale-eaters only exhibited increased aggression compared to snail-eater males when approaching their mirror image or a heterospecific stimulus fish (Table 1, 1a; Figures 2a and c).
Boldness did not vary across species
Unlike aggression, boldness did not vary across species. Latency for their head to emerge from the start box did not vary by sex, species, nor their interaction (Table 1, d). Further, the latency for the tail to emerge also did not vary by species (Table 1, e). It did, however, significantly vary by sex (Table 1, e), with male fish fully emerging from the start box about 6 times quicker than female fish (median male time: 42.23 [17.33, 131.67]; median female time: 253.05 [112.06, 288.28]). Propensity to approach or attack novel objects also did not vary by species, sex, or their interaction in both our control mirror and control paired aggression assays (Table 1, a–c).
Gene expression
Three thousand nine hundred and sixty-six genes were differentially expressed between scale-eaters versus generalists and 722 genes were differentially expressed between snail-eaters versus generalists. We found differentially expressed genes within ontologies for maternal care behavior, the estradiol hormone pathway, and the androgen hormone pathway (Table 2). None of these ontologies were significantly over-represented in either species comparison, which were instead enriched for cranial skeleton, metabolism, and pigmentation genes (McGirr and Martin 2018).
Table 2.
List of all differentially expressed genes in aggression and parental-care pathways at 8–10 dpf between: a) snail-eaters vs. generalists and b) scale-eaters vs. generalists
| Species comparison | Gene | log2 fold change | GO pathway |
|---|---|---|---|
| a) Snail-eater vs. generalist | |||
| rnf14 | −0.53 | Androgen | |
| crebrf | −0.7 | Maternal care | |
| b) Scale-eater vs. generalist | |||
| hdac6* | −0.84 | Androgen | |
| med12 | −0.98 | Androgen | |
| med16 | 1.24 | Androgen | |
| ncoa1 | 1.27 | Androgen | |
| rnf14 | −1.07 | Androgen | |
| crebrf | −1.41 | Maternal care | |
| esr1 | −0.95 | Estradiol | |
The 2 genes differentially expressed in both comparisons are highlighted in bold. Asterisks indicate genes which were differentially expressed using both 1-way and reciprocal best hits approaches. All remaining genes were identified using 1-way best hits.
Despite over 1000 differentially expressed genes from whole larvae at this developmental stage, only 2 genes were associated with aggression-related ontologies in the snail-eater versus generalist comparison (Table 2, a) and only 7 genes were associated with aggression-related ontologies in the scale-eater versus generalist comparison (Table 2, b) using 1-way best hits. Furthermore, these comparisons shared 2 genes in common: a transcriptional coactivator, which interacts with androgen receptors (rnf14) and a DNA binding transcription factor involved in glucocorticoid receptor regulation (crebrf) (Kang et al. 1999; Martyn et al. 2012). While both specialists showed differential expression in androgen and maternal care-related pathways when compared to the generalist, scale-eaters additionally showed differential expression in the estradiol hormone pathway. When using a reciprocal best hits approach, only a single gene, hdac6, was associated with aggression-related ontologies in the scale-eater versus generalist comparison. However, the primary function of this gene is histone deacetylation, and it is conserved across flies and mammals, which could explain why it was the sole result of the conservative reciprocal best hits approach (Perry et al. 2017).
DISCUSSION
The origins of novelty have overwhelmingly been examined from a morphological perspective, often ignoring behavior’s potential role (but see: Sol and Lefebvre 2000; Duckworth 2006; Zuk et al. 2006). Here, we used both behavioral and gene expression data to investigate whether increased aggression contributed to the origin of scale-eating in Caribbean pupfishes. We expected to find increased levels of aggression in scale-eaters compared to generalist and snail-eating pupfish species. Contrary to these predictions, however, both snail-eaters and scale-eaters showed increased levels of aggression compared to generalist species. Our gene expression data supported these findings; both scale-eaters and snail-eaters showed differential expression of genes involved in several aggression-related pathways during larval development. We also found that aggression varied between and within sexes, and contexts. Our data therefore does not support the aggression hypothesis as the sole origin of scale-eating in pupfishes. Instead, selection may have favored increased levels of aggression in other contexts, such as mate competition or trophic specialization in general. Increased levels of aggression could have also arisen indirectly due to selection for other behaviors or traits, including several differentially expressed genes involved in both aggression and craniofacial morphology (e.g., med12).
Only a few previous studies have directly investigated the behavioral origins of novelty. The Pacific field cricket (Teleogryllus oceanicus)—which exhibits a novel silent morph—is one of the few examples of evolutionary novelty with a behavioral origin (Zuk et al. 2006; Tinghitella and Zuk 2009; Bailey et al. 2010). Increased brain size in birds has also been linked to behavioral shifts and novelty. Birds that display innovative feeding behaviors have larger brains and are more successful at invading novel environments (Nicolakakis and Lefebvre 2000; Sol and Lefebvre 2000; Overington et al. 2009). Likewise, the role of behavior in evolutionary novelty has also been explored in western bluebirds (Sialia mexicana; Duckworth 2006) and Anolis lizards (Losos et al. 2004, 2006). Despite the growing empirical evidence of behavior’s role in evolutionary innovation, a consensus has not yet been reached on whether behavior ultimately drives or inhibits novelty. Furthermore, studies that investigate behavioral origins of novelty rarely do so using both behavioral and genetic approaches. In this study, however, we were able to leverage our gene expression data to gain some mechanistic insight into the divergent origins of increased behavioral aggression in each specialist species.
While both our behavioral and transcriptomic analyses provided evidence of increased aggression in both trophic specialist species, contrary to our expectations, there are a few caveats. First, aggression and aggression-related pathways were not enriched terms in our GO analysis. Instead, we found enrichment for cranial skeleton, metabolism, and pigmentation terms (McGirr and Martin 2018). However, gene expression differences are biologically relevant even if they are not enriched among all processes. Here, we used whole-larval tissue at a timepoint of 8–10 dpf to detect several genes and pathways that were differentially expressed between pupfish species within the San Salvador radiation. This sampling timepoint provides valuable insight which other methods may not afford. For example, gene expression differences (especially in behavioral pathways) are often transient in adults and can be attributed to factors such as diet, sex, dominance status, reproductive state, or mood (McGraw et al. 2003; Aubin-Horth et al. 2007; Rosvall 2013). Instead, by examining early larval stages our gene expression analyses provide insight into species-specific differences in aggression-related genetic pathways established during an early developmental timepoint. Second, while we used one-way and reciprocal best hits to determine potential orthology between pupfish and zebrafish many studies have found neofunctionalization of paralogs—meaning that functions may not always be retained (Braasch et al. 2006; Douard et al. 2008; Cortesi et al. 2015). Nonetheless, we found surprising congruence between our behavioral and transcriptomic data supporting the conclusions of increased aggression in both San Salvador specialists due to different aggression-related genetic pathways.
New hypotheses for varying levels of aggression within a sympatric radiation of pupfishes
Increased aggression due to specialization
If increased levels of aggression are not associated with scale-eating, then what explains this variation between species? One possibility is that selection may have directly favored increased aggression in the context of dietary specialization. Aggression may be positively correlated with traits associated with specialization (Genner et al. 1999; Peiman and Robinson 2010; Blowes et al. 2013), suggesting that specialists should show increased levels of aggression compared to generalists. Increased levels of aggression have been documented in specialist butterflyfishes (chaetodontids; Blowes et al. 2013), a specialist surfperch (Embiotoca lateralis; Holbrook and Schmitt 1992), and even observed in game theory simulations (Chubaty et al. 2014).
Alternatively, aggression may be increased in specialists due to competition for food. For example, species of Roeboides turn to scale-eating during low-water seasons when competition for insects rises (Peterson and Winemiller 1997; Peterson and McIntyre 1998). However, pupfish inhabit hypersaline lakes connected to the ocean which do not experience seasonal fluctuations in water levels (Hagey and Mylroie 1995). Instead, variation in abundance of pupfish over the year could lead to increased competition for food (Martin and Wainwright 2013a, 2013b, 2013c). Competition for food may also explain increased aggression in snail-eaters. Although snail-eating pupfish consume the largest proportion of snails in their diet (22–30%; Martin and Wainwright 2013a), generalist pupfish also consume snails in low quantities (0.03–4%; Martin and Wainwright 2013c). Furthermore, generalists comprise 92–94% of the pupfish population (Martin and Wainwright 2013c), indicating that snail-eaters may compete with generalists for food items regularly. It is possible that snail-eaters developed increased aggression to protect their food source from generalists.
Another possibility is that increased aggression may be associated with colonizing a novel niche. Aggression is often tightly correlated with boldness in a phenomenon termed the aggressiveness-boldness syndrome (Sih et al. 2004). Many studies have shown that increased boldness in species such as cane toads, mosquitofish, and Trinidadian killifish leads to increased dispersal into novel habitats or niches (Fraser et al. 2001; Rehage and Sih 2004; Gruber et al. 2017). This relationship indicates that increased aggression may be an incidental effect of selection for increased boldness and occupation of a novel niche. However, our measures of boldness did not show any variation across species, and instead indicated that males were bolder than females.
This relationship between aggression and specialization is also supported by our transcriptomic data. First, both genes differentially expressed in our snail-eater versus generalist analysis were also differentially expressed in our scale-eater versus generalist analysis (rnf14 and crebrf). Second, rnf14, a coactivator of androgen receptors, is also associated with metabolism suggesting that it may be the specialized diets of snail- and scale-eaters which led to their increased aggression (Michael et al. 2011). This is consistent with the significant amount of parallel expression in both specialists in genetic pathways associated with metabolism and the increased nitrogen consumption and enrichment in both specialists (McGirr and Martin 2018). While increased aggression may be important for a specialized diet or occupying a novel niche neither of these hypotheses explain the variation in aggression between sexes.
Increased aggression due to mating system
Increased aggression may be favored in the context of courtship or mate competition. It is well documented across multiple taxa that the sex with the higher potential reproductive rate should have increased levels of aggression as they must compete more heavily for access to mates (Clutton-Brock and Parker 1992; Andersson 1994; Jennions and Petrie 1997). Normally, males have higher potential reproductive rates since mating is energetically cheap for them (Trivers 1972). Cyprinodon pupfishes follow this pattern since they mate in a lekking system and do not provide parental care (Gumm 2012). Our behavioral measures of aggression support this; both male scale- and snail-eaters showed increased levels of aggression compared to their female counterparts.
We also found some support for this in our gene expression data. In our scale-eater versus generalist comparison, we found differential gene expression of the esr1 gene which encodes an estrogen receptor. Differential expression of this gene has been linked to aggression, territoriality, mate-seeking behavior, and even parental care (Tuttle 2003; Horton et al. 2013, 2014; Hashikawa et al. 2016). However, differential expression of esr1 was only observed in the scale-eater versus generalist comparison and not between snail-eaters versus generalists. Crebrf, a regulatory factor which is differentially expressed in both scale- and snail-eaters versus generalists, has also been associated with lack of maternal care in mice (Martyn et al. 2012). Although all 3 species exhibit a lekking mating system, there may be quantitative differences in male competition and degree of lekking among species and lake populations (C.H.M., personal observation).
Increased aggression due to indirect selection
Alternatively, aggression may have increased via selection on other traits. For example, melanin production and aggression are physiologically linked via the melanocortin system (Cone 2005; Price et al. 2008). This association has been documented across a wide array of vertebrates and suggests that selection for increased melanin pigmentation in other contexts (e.g., mate choice or camouflage) may incidentally increase aggression (McGraw et al. 2003; Ducrest et al. 2008; Price et al. 2008). Indeed, territorial male scale-eating pupfish exhibit jet black breeding coloration, unique among Cyprinodon, and snail-eating pupfish exhibit the lightest male breeding coloration of any Cyprinodon species (Martin and Wainwright 2013a). Similarly, selection for morphological traits may also indirectly increase aggression. We found differential gene expression between scale-eater versus generalist pupfish in the med12 gene, which is annotated for the androgen pathway (Table 2, b). Med12 is a mediator complex subunit which codes for a thyroid hormone receptor associated protein. Mutations in this gene have not only been linked to craniofacial defects, but also to a slender body shape (Philibert and Madan 2007; Risheg et al. 2007; Ding et al. 2008; Vulto-van Silfhout et al. 2013). C. desquamator show extreme craniofacial features, including enlarged oral jaws and a fusiform body that may be beneficial for scale-eating with an estimated 4 moderate-effect quantitative trait loci all increasing oral jaw size, consistent with directional selection on this trait (Martin et al. 2017). Thus, it is intriguing that selection for increased jaw size or body elongation may have indirectly selected for increased aggression in this species. Given the enlarged oral jaws of most scale-eating species, this may be a general mechanism indirectly contributing to increased aggression in scale-eaters depending on how frequently this genetic pathway is modified.
Multimodal signals for aggression
An additional finding of this study is that pupfish aggression varies not only across species and sex, but also across context. This was especially surprising when comparing the results of our mirror assay to the results of the conspecifics of the same-sex assays. These assays are arguably the most similar (i.e., stimuli are conspecifics of the same sex), and we expected that the results should also be similar. However, this was not true for female snail- or scale-eaters. Female snail-eaters had very low rates of approaching and attacking female conspecifics (Figures 2b, 3b, and 4b), but they readily approached and attacked their mirror image (Figures 2a, 3a, and 4a). This could suggest that snail-eaters need more than visual cues to identify conspecifics. Female snail-eaters also approached and attacked their mirror image at the same rates as heterospecific stimulus fish (Figures 2c, 3c, and 4c), suggesting that they misidentified their mirror image as a heterospecific fish. Female scale-eaters, on the other hand, attacked conspecific stimuli significantly quicker and more often than their mirror image (Figures 3a,b, and 4a,b), and they approached and attacked heterospecifics at the same rate and frequency as conspecifics. This could suggest that, like snail-eaters, female scale-eaters also need multiple signals to determine potential competition or prey. Multiple studies have documented that the use of multiple cues leads to greater accuracy in con- and heterospecific identification (Rand and Williams 1970; Hankison and Morris 2003; Ward and Mehner 2010). Höjesjö et al. (2015) also found that the use of multiple cues was additive for females, but not for males. However, many of these studies focus on identification in the context of mating—not in the context of aggression.
Conclusion
Our study surprisingly suggests that the aggression hypothesis is not a sufficient explanation for the origins of an exceptional trophic innovation, scale-eating in pupfish. Instead, increased aggression in both specialists indicates that aggression may perform a more general function in dietary specialization or occupation of a novel niche. Alternatively, increased aggression may be an indirect effect of selection on other ecological or sexual traits. Specifically, the aggression-boldness syndrome, the melanocortin system, increased protein metabolism, or selection for oral jaw size could all have indirectly increased aggression. Future studies should investigate whether aggression is adaptive for scale- and snail-eating in pupfishes.
FUNDING
This research was kindly funded by NSF CAREER 1749764 and NIH 1R01DE027052-01A1 grants to C.H.M.
Acknowledgments
We thank the BEST commission and the Ministry of Agriculture of the Commonwealth of the Bahamas for permission to conduct this research and export specimens; E. Tibbetts for helpful comments on the manuscript; the Gerace Research Centre and Troy Dexter for logistical support during fieldwork; and the Vincent J. Coates Genomics Sequencing Laboratory and Functional Genomics Laboratory at UC Berkeley, supported by NIH S10 OD018174 Instrumentation Grant, for performing RNAseq library preparation and sequencing. All laboratory behavioral and sampling protocols were approved by the University of North Carolina at Chapel Hill IACUC (protocol# 15–179.0).
AUTHOR CONTRIBUTIONS
M.E.S. and C.H.M. conceptualized the project; M.E.S. and J.A.M. collected data and performed all analyses; M.E.S. wrote the original draft; M.E.S., J.A.M., and C.H.M. reviewed and edited drafts; and C.H.M. provided funding.
Data accessibility: Analyses reported in this article can be reproduced using the data provided by St. John et al. 2018. Genomic and transcriptomic raw sequence reads are deposited at the NCBI BioProject database (Title: Craniofacial divergence in Caribbean Pupfishes. Accession: PRJNA391309).
REFERENCES
- Andersson MB. 1994. Sexual selection. Princeton (NJ): Princeton University Press. [Google Scholar]
- Arnott G, Beattie E, Elwood RW.. 2016. To breathe or fight? Siamese fighting fish differ when facing a real opponent or mirror image. Behav Processes. 129:11–17. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. 2000. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 25:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin-Horth N, Desjardins JK, Martei YM, Balshine S, Hofmann HA.. 2007. Masculinized dominant females in a cooperatively breeding species. Mol Ecol. 16:1349–1358. [DOI] [PubMed] [Google Scholar]
- Bailey NW, Gray B, Zuk M.. 2010. Acoustic experience shapes alternative mating tactics and reproductive investment in male field crickets. Curr Biol. 20:845–849. [DOI] [PubMed] [Google Scholar]
- Balzarini V, Taborsky M, Wanner S, Koch F, Frommen JG.. 2014. Mirror, mirror on the wall: the predictive value of mirror tests for measuring aggression in fish. Behav Ecol Sociobiol. 68:871–878. [Google Scholar]
- Barton N, Partridge L.. 2000. Limits to natural selection. Bioessays. 22:1075–1084. [DOI] [PubMed] [Google Scholar]
- Bewick V, Cheek L, Ball J.. 2004. Statistics review 12: survival analysis. Crit Care. 8:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blowes SA, Pratchett MS, Connolly SR.. 2013. Heterospecific aggression and dominance in a guild of coral-feeding fishes: the roles of dietary ecology and phylogeny. Am Nat. 182:157–168. [DOI] [PubMed] [Google Scholar]
- Boileau N, Cortesi F, Egger B, Muschick M, Indermaur A, Theis A, Büscher HH, Salzburger W.. 2015. A complex mode of aggressive mimicry in a scale-eating cichlid fish. Biol Lett. 11:20150521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch I, Salzburger W, Meyer A.. 2006. Asymmetric evolution in two fish-specifically duplicated receptor tyrosine kinase paralogons involved in teleost coloration. Mol Biol Evol. 23:1192–1202. [DOI] [PubMed] [Google Scholar]
- Brown C, Jones F, Braithwaite V.. 2005. In situ examination of boldness-shyness traits in the tropical poeciliid, Brachyraphis episcopi. Anim Behav. 70:1003–1009. [Google Scholar]
- Budaev SV. 1997. Alternative styles in the European wrasse, Symphodus ocellatus: boldness-related schooling tendency. Environ Biol Fishes. 49:71–78. [Google Scholar]
- Burnham KP, Anderson DR.. 2002. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. New York (NY): Springer-Verlag. [Google Scholar]
- Buston PM, Cant MA.. 2006. A new perspective on size hierarchies in nature: patterns, causes, and consequences. Oecologia. 149:362–372. [DOI] [PubMed] [Google Scholar]
- Canty A, Ripley B.. 2017. boot: Bootstrap R (S-Plus) Functions. R package version 1.3–20. Available from: https://CRAN.R-project.org/package=boot. [Google Scholar]
- Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S; AmiGO Hub; Web Presence Working Group 2009. AmiGO: online access to ontology and annotation data. Bioinformatics. 25:288–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubaty AM, Ma BO, Stein RW, Gillespie DR, Henry LM, Phelan C, Palsson E, Simon FW, Roitberg BD.. 2014. On the evolution of omnivory in a community context. Ecol Evol. 4:251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH, Parker GA.. 1992. Potential reproductive rates and the operation of sexual selection. Q Rev Biol. 67:437–456. [Google Scholar]
- Cone RD. 2005. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 8:571–578. [DOI] [PubMed] [Google Scholar]
- Cortesi F, Musilová Z, Stieb SM, Hart NS, Siebeck UE, Malmstrøm M, Tørresen OK, Jentoft S, Cheney KL, Marshall NJ, et al. 2015. Ancestral duplications and highly dynamic opsin gene evolution in percomorph fishes. Proc Natl Acad Sci USA. 112:1493–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalquen DA, Dessimoz C.. 2013. Bidirectional best hits miss many orthologs in duplication-rich clades such as plants and animals. Genome Biol Evol. 5:1800–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demartini EE, Coyer JA.. 1981. Cleaning and Scale-Eating in Juveniles of the Kyphosid Fishes, Hermosilla azurea and Girella nigricans. Copeia. 785–789. [Google Scholar]
- Ding N, Zhou H, Esteve PO, Chin HG, Kim S, Xu X, Joseph SM, Friez MJ, Schwartz CE, Pradhan S, et al. 2008. Mediator links epigenetic silencing of neuronal gene expression with x-linked mental retardation. Mol Cell. 31:347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douard V, Brunet F, Boussau B, Ahrens-Fath I, Vlaeminck-Guillem V, Haendler B, Laudet V, Guiguen Y.. 2008. The fate of the duplicated androgen receptor in fishes: a late neofunctionalization event?BMC Evol Biol. 8:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth RA. 2006. Aggressive behaviour affects selection on morphology by influencing settlement patterns in a passerine bird. Proc Biol Sci. 273:1789–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducrest AL, Keller L, Roulin A.. 2008. Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends Ecol Evol. 23:502–510. [DOI] [PubMed] [Google Scholar]
- Elwood RW, Stoilova V, McDonnell A, Earley RL, Arnott G.. 2014. Do mirrors reflect reality in agonistic encounters? A test of mutual cooperation in displays. Anim Behav. 97:63–67. [Google Scholar]
- Ewels P, Magnusson M, Lundin S, Käller M.. 2016. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 32:3047–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. 1985. Phylogenies and the comparative method. Am Nat Am Nat. 125:1–15. [Google Scholar]
- Francis RC. 2010. Temperament in a fish: a longitudinal study of the development of individual differences in aggression and social rank in the Midas cichlid. Ethology. 86:311–325. [Google Scholar]
- Fraser DF, Gilliam JF, Daley MJ, Le AN, Skalski GT.. 2001. Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. Am Nat. 158:124–135. [DOI] [PubMed] [Google Scholar]
- Fryer G, Greenwood PH, and Trewavas E.. 1955. Scale-eating Habits of African Cichlid Fishes. Nature. 175:1089–1090. [Google Scholar]
- Fryer G, Iles TD.. 1972. The cichlid fishes of the Great Lakes of Africa: their biology and evolution. Vol. 23 Edinburgh (UK): Oliver & Boyd. [Google Scholar]
- Genner MJ, Turner GF, Hawkins SJ.. 1999. Resource control by territorial male cichlid fish in Lake Malawi. J Anim Ecol. 68:522–529. [Google Scholar]
- Greenwood PH. 1965. Two new species of Haplochromis (Pisces, Cichlidae) from Lake Victoria. J Nat Hist Ser 13. 8:303–318. [Google Scholar]
- Gruber J, Brown G, Whiting MJ, Shine R.. 2017. Geographic divergence in dispersal-related behaviour in cane toads from range-front versus range-core populations in Australia. Behav Ecol Sociobiol. 71:38. [Google Scholar]
- Gumm JM. 2012. Sex recognition of female-like sneaker males in the Comanche Springs pupfish, Cyprinodon elegans. Anim Behav. 83:1421–1426. [Google Scholar]
- Hagey FM, Mylroie JE.. 1995. Pleistocene lake and lagoon deposits, San Salvador island, Bahamas. Spec Pap Soc Am. 77–90. [Google Scholar]
- Hankison SJ, Morris MR.. 2003. Avoiding a compromise between sexual selection and species recognition: female swordtail fish assess multiple species-specific cues. Behav Ecol. 14:282–287. [Google Scholar]
- Hashikawa K, Hashikawa Y, Falkner A, Lin D.. 2016. The neural circuits of mating and fighting in male mice. Curr Opin Neurobiol. 38:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukoos JS, Lewis RJ.. 2005. Advanced statistics: bootstrapping confidence intervals for statistics with “difficult” distributions. Acad Emerg Med. 12:360–365. [DOI] [PubMed] [Google Scholar]
- Höjesjö J, Axelsson M, Dahy R, Gustavsson L, Johnsson JI.. 2015. Sight or smell? Behavioural and heart rate responses in subordinate rainbow trout exposed to cues from dominant fish. PeerJ. 3:e1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook SJ, Schmitt RJ.. 1992. Causes and consequences of dietary specialization in surfperches : patch choice and intraspecific competition. Ecology. 73:402–412. [Google Scholar]
- Horton BM, Hu Y, Martin CL, Bunke BP, Matthews BS, Moore IT, Thomas JW, Maney DL.. 2013. Behavioral characterization of a white-throated sparrow homozygous for the ZAL2(m) chromosomal rearrangement. Behav Genet. 43:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton BM, Hudson WH, Ortlund EA, Shirk S, Thomas JW, Young ER, Zinzow-Kramer WM, Maney DL.. 2014. Estrogen receptor α polymorphism in a species with alternative behavioral phenotypes. Proc Natl Acad Sci USA. 111:1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey RB, Hertz PE, Sinervo B.. 2003. Behavioral drive versus behavioral inertia in evolution: a null model approach. Am Nat. 161:357–366. [DOI] [PubMed] [Google Scholar]
- Hulsey CD, Roberts RJ, Lin AS, Guldberg R, Streelman JT.. 2008. Convergence in a mechanically complex phenotype: detecting structural adaptations for crushing in cichlid fish. Evolution. 62: 1587–1599. [DOI] [PubMed] [Google Scholar]
- Janovetz J. 2005. Functional morphology of feeding in the scale-eating specialist Catoprion mento. J Exp Biol. 208:4757–4768. [DOI] [PubMed] [Google Scholar]
- Jennions MD, Petrie M.. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev Camb Philos Soc. 72:283–327. [DOI] [PubMed] [Google Scholar]
- Kang HY, Yeh S, Fujimoto N, Chang C.. 1999. Cloning and characterization of human prostate coactivator ARA54, a novel protein that associates with the androgen receptor. J Biol Chem. 274:8570–8576. [DOI] [PubMed] [Google Scholar]
- Katzir G. 1981. Aggression by the damselfish Dascyllus aruanus L. Towards conspecifics and heterospecifics. Anim Behav. 29:835–841. [Google Scholar]
- Kolmann MA, Huie JM, Evans K, Summers AP.. 2018. Specialized specialists and the narrow niche fallacy: a tale of scale-feeding fishes. R Soc Open Sci. 5:171581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer ES, Warren WC, Harrison R, McCune AR.. 2017. The Cyprinodon variegatus genome reveals gene expression changes underlying differences in skull morphology among closely related species. BMC Genomics. 18:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W.. 2013. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 41:e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF. 1973. Evolutionary Strategies and Morphological Innovations: cichlid Pharyngeal Jaws. Syst Zool. 22:425. [Google Scholar]
- Losey GS. 1972. The ecological importance of cleaning symbiosis. Copeia. 820–833. [Google Scholar]
- Losey GS. 1979. Fish cleaning symbiosis: proximate causes of host behavior. Anim Behav. 27:669–685. [Google Scholar]
- Losos JB. 2010. Adaptive radiation, ecological opportunity, and evolutionary determinism. American Society of Naturalists E. O. Wilson award address. Am Nat. 175:623–639. [DOI] [PubMed] [Google Scholar]
- Losos JB, Schoener TW, Langerhans RB, Spiller DA.. 2006. Rapid temporal reversal in predator-driven natural selection. Science. 314:1111. [DOI] [PubMed] [Google Scholar]
- Losos JB, Schoener TW, Spiller DA.. 2004. Predator-induced behaviour shifts and natural selection in field-experimental lizard populations. Nature. 432:505–508. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S.. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CH. 2016. The cryptic origins of evolutionary novelty: 1000-fold faster trophic diversification rates without increased ecological opportunity or hybrid swarm. Evolution. 70:2504–2519. [DOI] [PubMed] [Google Scholar]
- Martin CH, Erickson PA, Miller CT.. 2017. The genetic architecture of novel trophic specialists: larger effect sizes are associated with exceptional oral jaw diversification in a pupfish adaptive radiation. Mol Ecol. 26:624–638. [DOI] [PubMed] [Google Scholar]
- Martin CH, Feinstein LC.. 2014. Novel trophic niches drive variable progress towards ecological speciation within an adaptive radiation of pupfishes. Mol Ecol. 23:1846–1862. [DOI] [PubMed] [Google Scholar]
- Martin CH, Wainwright PC.. 2011. Trophic novelty is linked to exceptional rates of morphological diversification in two adaptive radiations of Cyprinodon pupfish. Evolution. 65:2197–2212. [DOI] [PubMed] [Google Scholar]
- Martin CH, Wainwright PC.. 2013a. A remarkable species flock of cyprinodon pupfishes endemic to San Salvador Island, Bahamas. Bull Peabody Museum Nat Hist. 54:231–241. [Google Scholar]
- Martin CH, Wainwright PC.. 2013b. Multiple fitness peaks on the adaptive landscape drive adaptive radiation in the wild. Science. 339:208–211. [DOI] [PubMed] [Google Scholar]
- Martin CH, Wainwright PC.. 2013c. On the measurement of ecological novelty: scale-eating pupfish are separated by 168 my from other scale-eating fishes. PLoS One. 8:e71164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn AC, Choleris E, Gillis DJ, Armstrong JN, Amor TR, McCluggage AR, Turner PV, Liang G, Cai K, Lu R.. 2012. Luman/CREB3 recruitment factor regulates glucocorticoid receptor activity and is essential for prolactin-mediated maternal instinct. Mol Cell Biol. 32:5140–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr JA, Martin CH.. 2018. Parallel evolution of gene expression between trophic specialists despite divergent genotypes and morphologies. Evol Lett. 2:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw KJ, Dale J, Mackillop EA.. 2003. Social environment during molt and the expression of melanin-based plumage pigmentation in male house sparrows (Passer domesticus). Behav Ecol Sociobiol. 53:116–122. [Google Scholar]
- Michael D, Soi S, Cabera-Perez J, Weller M, Alexander S, Alevizos I, Illei G, Chiorini J.. 2011. Microarray analysis of sexually dimorphic gene expression in human minor salivary glands. Oral Dis. 17:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer KE. 1971. The physiology of hostility. Oxford (England): Markham. [Google Scholar]
- Nelson RJ, Chiavegatto S.. 2001. Molecular basis of aggression. Trends Neurosci. 24:713–719. [DOI] [PubMed] [Google Scholar]
- Nelson JS, Grande TC, Wilson MVH.. 2016. Fishes of the World. Hoboken (NJ): John Wiley & Sons. [Google Scholar]
- Nelson RJ, Trainor BC.. 2007. Neural mechanisms of aggression. Nat Rev Neurosci. 8:536–546. [DOI] [PubMed] [Google Scholar]
- Nicolakakis N, Lefebvre L.. 2000. Forebrain size and innovation rate in European birds: feeding, nesting and confounding variables. Behaviour. 137:1415–1429. [Google Scholar]
- Novakowski GC, Fugi R, Hahn NS.. 2004. Diet and dental development of three species of Roeboides (Characiformes: Characidae). Neotrop Ichthyol. 2:157–162. [Google Scholar]
- Nshombo M, Yanagisawa Y, Nagoshi M.. 1985. Scale-eating in Perissodus microlepis (Cichlidae) and of its food habits with growth. Japanese J Ichthyol. 32:66–73. [Google Scholar]
- Overington SE, Morand-Ferron J, Boogert NJ, Lefebvre L.. 2009. Technical innovations drive the relationship between innovativeness and residual brain size in birds. Anim Behav. 78:1001–1010. [Google Scholar]
- Pauers MJ, Kapfer JM, Fendos CE, Berg CS.. 2008. Aggressive biases towards similarly coloured males in Lake Malawi cichlid fishes. Biol Lett. 4:156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiman KS, Robinson BW.. 2010. Ecology and evolution of resource-related heterospecific aggression. Q Rev Biol. 85:133–158. [DOI] [PubMed] [Google Scholar]
- Perry S, Kiragasi B, Dickman D, Ray A.. 2017. The role of histone deacetylase 6 in synaptic plasticity and memory. Cell Rep. 18:1337–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CC, McIntyre P.. 1998. Ontogenetic diet shifts in Roeboides affinis with morphological comparisons. Environ Biol Fishes. 53:105–110. [Google Scholar]
- Peterson CC, Winemiller KO.. 1997. Ontogenic diet shifts and scale-eating in Roeboides dayi, a Neotropical characid. Environ Biol Fishes. 49:111–118. [Google Scholar]
- Philibert RA, Madan A.. 2007. Role of MED12 in transcription and human behavior. Pharmacogenomics. 8:909–916. [DOI] [PubMed] [Google Scholar]
- Pickrell JK, Pritchard JK.. 2012. Inference of population splits and mixtures from genome-wide allele frequency data. PLoS Genet. 8:e1002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigliucci M. 2008. What, if Anything, Is an Evolutionary Novelty?Philos Sci. 75:887–898. [Google Scholar]
- Price AC, Weadick CJ, Shim J, Rodd FH.. 2008. Pigments, patterns, and fish behavior. Zebrafish. 5:297–307. [DOI] [PubMed] [Google Scholar]
- Rand AS, Williams EE.. 1970. An estimation of redundancy and information content of anole dewlaps. Am Nat. 104:99–103. [Google Scholar]
- R Core Team 2018. R: a language and environment for statistical computing. Vienna (Austria): R Found. Stat. Comput; https//www.R-project.org/. 0:{ISBN} 3-900051-07-0. [Google Scholar]
- Rehage JS, Sih A.. 2004. Dispersal behavior, boldness, and the link to invasiveness: a comparison of four Gambusia species. Biol Invasions. 6:379–391. [Google Scholar]
- Ribbink AJ, Marsh BA, Marsh AC, Ribbink AC, Sharp BJ.. 1983. A preliminary survey of the cichlid fishes of rocky habitats in Lake Malawi. South African J Zool. 18:149–310. [Google Scholar]
- Rich JT, Neely JG, Paniello RC, Voelker CC, Nussenbaum B, Wang EW.. 2010. A practical guide to understanding Kaplan-Meier curves. Otolaryngol Head Neck Surg. 143:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards E, Martin C.. 2017. Adaptive introgression from distant Caribbean islands contributed to the diversification of a microendemic radiation of trophic specialist pupfishes. PLOS Genet. 13:e1006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risheg H, Graham JM, Clark RD, Rogers RC, Opitz JM, Moeschler JB, Peiffer AP, May M, Joseph SM, Jones JR, et al. 2007. A recurrent mutation in MED12 leading to R961W causes Opitz-Kaveggia syndrome. Nat Genet. 39:451. [DOI] [PubMed] [Google Scholar]
- Rosvall KA. 2013. Proximate perspectives on the evolution of female aggression: good for the gander, good for the goose. Philos. Trans. R. Soc. B Biol. Sci. 368:20130083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland WJ. 1989. The effects of body size, aggression and nuptial coloration on competition for territories in male threespine sticklebacks, Gasterosteus aculeatus. Anim Behav. 37:282–289. [Google Scholar]
- Sazima I. 1983. Scale-eating in characoids and other fishes. Environ Biol Fishes. 9:87–101. [Google Scholar]
- Sazima I, Machado FA.. 1990. Underwater observations of piranhas in western Brazil. Environ Biol Fishes. 28:17–31. [Google Scholar]
- Shah N, Nute MG, Warnow T, Pop M.. 2018. Misunderstood parameter of NCBI BLAST impacts the correctness of bioinformatics workflows. Bioinformatics. doi: 10.1093/bioinformatics/bty833 [DOI] [PubMed] [Google Scholar]
- Sih A, Bell AM, Johnson JC, Ziemba RE.. 2004. Behavioral syndromes: an integrative overview. Q Rev Biol. 79:241–277. [DOI] [PubMed] [Google Scholar]
- Sol D, Lefebvre L.. 2000. Behavioural flexibility predicts invasion success in birds introduced to New Zealand. Oikos. 90:599–605. [Google Scholar]
- St. John ME, McGirr JA, Martin CH.. 2018. Data from: the behavioral origins of novelty: did increased aggression lead to scale-eating in pupfishes?Dryad Digital Repository. http://dx.doi.org/ 10.5061/dryad.0vt58q0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Gene Ontology Consortium 2017. Expansion of the Gene Ontology knowledgebase and resources. Nucleic Acids Res. 45:D331–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau T. 2015. A package for survival analysis in S. Version 2.38. Available from: https://CRAN.R-project.org/package=survival. [Google Scholar]
- Tinghitella RM, Zuk M.. 2009. Asymmetric mating preferences accommodated the rapid evolutionary loss of a sexual signal. Evolution. 63:2087–2098. [DOI] [PubMed] [Google Scholar]
- Trewavas E. 1947. An example of mimicry in fishes. Nature. 159:120. [DOI] [PubMed] [Google Scholar]
- Trivers RL. 1972. Parental investment and sexual selection. Cambridge (MA): Biol. Lab. Harvard Univ; Vol. 136. [Google Scholar]
- Tuttle EM. 2003. Alternative reproductive strategies in the white-throated sparrow: behavioral and genetic evidence. Behav Ecol. 14:425–432. [Google Scholar]
- Vøllestad LA, Quinn TP.. 2003. Trade-off between growth rate and aggression in juvenile coho salmon, Oncorhynchus kisutch. Anim Behav. 66:561–568. [Google Scholar]
- Vulto-van Silfhout AT, de Vries BBA, van Bon BWM, Hoischen A, Ruiterkamp-Versteeg M, Gilissen C, Gao F, van Zwam M, Harteveld CL, van Essen AJ, et al. 2013. Mutations in MED12 cause X-linked Ohdo syndrome. Am J Hum Genet. 92:401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AJW, Mehner T.. 2010. Multimodal mixed messages: the use of multiple cues allows greater accuracy in social recognition and predator detection decisions in the mosquitofish, Gambusia holbrooki. Behav Ecol. 21:1315–1320. [Google Scholar]
- Wilson EO. 2000. Sociobiology. Cambridge: Harvard University Press. [Google Scholar]
- Wilson ADM, Godin J-GJ.. 2009. Boldness and behavioral syndromes in the bluegill sunfish, Lepomis macrochirus. Behav Ecol. 20:231–237. [Google Scholar]
- Zamani N, Russell P, Lantz H, Hoeppner MP, Meadows JR, Vijay N, Mauceli E, di Palma F, Lindblad-Toh K, Jern P, et al. 2013. Unsupervised genome-wide recognition of local relationship patterns. BMC Genomics. 14:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk M, Rotenberry JT, Tinghitella RM.. 2006. Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol Lett. 2:521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]