Key Points
Question
What is the effect of catheter ablation, compared with medical therapy, on quality of life in patients with symptomatic atrial fibrillation?
Findings
In this randomized trial of 2204 patients with atrial fibrillation, catheter ablation, compared with conventional medical therapy, significantly improved quality of life at 1 year as measured by the Atrial Fibrillation Effect on Quality of Life score (mean between-group difference, 5.2 points; patient-level clinically important difference, ≥5 points) and the Mayo AF-Specific Symptom Inventory frequency score (mean between-group difference, −1.7 points; patient-level clinically important difference, ≤−1.6 points) and severity score (mean between-group difference, −1.5 points; patient-level clinically important difference, ≤−1.3 points).
Meaning
In patients with symptomatic atrial fibrillation, catheter ablation, compared with medical therapy, led to clinically important and significant improvements in quality of life at 12 months.
Abstract
Importance
Catheter ablation is more effective than drug therapy in restoring sinus rhythm in patients with atrial fibrillation (AF), but its incremental effect on long-term quality of life (QOL) is uncertain.
Objective
To determine whether catheter ablation is more beneficial than conventional drug therapy for improving QOL in patients with AF.
Design, Setting, and Participants
An open-label randomized clinical trial of catheter ablation vs drug therapy in 2204 symptomatic patients with AF older than 65 years or 65 years or younger with at least 1 risk factor for stroke. Patients were enrolled from November 2009 to April 2016 from 126 centers in 10 countries. Follow-up ended in December 2017.
Interventions
Pulmonary vein isolation, with additional ablation procedures at the discretion of the investigators, for the catheter ablation group (n = 1108) and standard rhythm and/or rate-control drugs selected and managed by investigators for the drug therapy group (n = 1096).
Main Outcomes and Measures
Prespecified co-primary QOL end points at 12 months, including the Atrial Fibrillation Effect on Quality of Life (AFEQT) summary score (range, 0-100; 0 indicates complete disability and 100 indicates no disability; patient-level clinically important difference, ≥5 points) and the Mayo AF-Specific Symptom Inventory (MAFSI) frequency score (range, 0-40; 0 indicates no symptoms and 40 indicates the most severe symptoms; patient-level clinically important difference, ≤−1.6 points) and severity score (range, 0-30; 0 indicates no symptoms and 30 indicates the most severe symptoms; patient-level clinically important difference, ≤−1.3 points).
Results
Among 2204 randomized patients (median age, 68 years; 1385 patients [63%] were men, 946 [43%] had paroxysmal AF, and 1256 [57%] had persistent AF), the median follow-up was 48.5 months, and 1968 (89%) completed the trial. The mean AFEQT summary score was more favorable in the catheter ablation group than the drug therapy group at 12 months (86.4 points vs 80.9 points) (adjusted difference, 5.3 points [95% CI, 3.7-6.9]; P < .001). The mean MAFSI frequency score was more favorable for the catheter ablation group than the drug therapy group at 12 months (6.4 points vs 8.1 points) (adjusted difference, −1.7 points [95% CI, −2.3 to −1.2]; P < .001) and the mean MAFSI severity score was more favorable for the catheter ablation group than the drug therapy group at 12 months (5.0 points vs 6.5 points) (adjusted difference, −1.5 points [95% CI, −2.0 to −1.1]; P < .001).
Conclusions and Relevance
Among patients with symptomatic atrial fibrillation, catheter ablation, compared with medical therapy, led to clinically important and significant improvements in quality of life at 12 months. These findings can help guide decisions regarding management of atrial fibrillation.
Trial Registration
ClinicalTrials.gov Identifier: NCT00911508
This open-label randomized trial compares the effect of pulmonary vein isolation via catheter ablation vs rate and rhythm control using drug therapy on the quality of life of patients with atrial fibrillation.
Introduction
Catheter ablation for atrial fibrillation (AF) was introduced clinically in the 1990s as a last-resort therapy for patients with drug-refractory symptomatic disease.1,2 Over the next 20 years, ablation evolved into a therapeutic option for a broader spectrum of patients with AF. This evolution was due to several important factors, including the achievement of high rates of successful remission of AF, the identification of the importance of pulmonary vein isolation to the success of the procedure, the decrease in complication rates among the higher volume centers, and the growing dissatisfaction with the lack of effective pharmacologic management options for AF rhythm control.3,4,5,6 While the earliest reports speculated that the procedure might be curative,1,5,7 additional experience showed that a substantial minority of patients who underwent ablation experienced recurrent AF if followed up carefully for long enough periods.
As the possibility of a permanent cure for AF with catheter ablation grew less certain, attention turned to the benefits of ablation on quality of life (QOL) associated with AF.8 Some of the early trials that examined the effects of catheter ablation on patients with AF found that ablation was more effective than drug therapy in improving QOL.3,4,9,10 However, these early studies were limited by relatively small sample sizes, a narrow spectrum of patients with AF enrolled (mostly patients with paroxysmal AF), and short duration of follow-up (typically ≤1 year). Hence, the magnitude of QOL benefits provided by ablation across the spectrum of treated participants with AF and the durability of those benefits remain incompletely defined.
The Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial began enrollment in 2009 to test the hypothesis that ablative therapy for AF is more effective than state-of-the-art drug therapies in a broad population of symptomatic but inadequately treated participants with AF. The primary study outcome, a composite measure of death, disabling stroke, serious bleeding, or cardiac arrest, is reported in an accompanying article.11 Comparison of long-term QOL outcomes was a major secondary objective of the CABANA research program and is the subject of this article.
Methods
Patient Population and Primary Clinical Results
Between November 2009 and April 2016, 2204 symptomatic patients with new-onset or undertreated paroxysmal, persistent, or longstanding persistent AF were enrolled at 126 clinical sites in 10 countries. Patients were older than 65 years or 65 years or younger with at least 1 risk factor for stroke. The primary study outcome was a composite measure of death, disabling stroke, serious bleeding, or cardiac arrest. Details of the trial design have been published,12 and the trial organization, CONSORT diagram (eFigure 1 in Supplement 1), and statistical analysis plan (Supplement 2) are provided. All patients provided written informed consent, and study protocol approval was obtained from each site’s institutional review board or ethics committee. Race and ethnicity data were collected as a standard component of a clinical trial supported by the National Institutes of Health (NIH), and the assignment of race/ethnicity was made by the site investigator in conjunction with the patient using standard NIH categories.
Patients were randomized to receive either catheter ablation (pulmonary vein isolation with additional ablation procedures at the discretion of the investigator) or drug therapy (rhythm or rate control, according to investigator discretion) using permuted block design with variable block size (between 2 and 4) stratified by clinical site.
Quality of Life Measures
Prespecified Co-primary QOL End Points
The scores of 2 QOL measures were prespecified as co-primary end points: the Atrial Fibrillation Effect on Quality of Life (AFEQT) questionnaire and the Mayo AF-Specific Symptom Inventory (MAFSI). The AFEQT13 is a 21-item, AF-specific, health-related QOL questionnaire designed to assess the effect of AF on patient QOL. The AFEQT produces a summary score (calculated from 18 of the questions) and subscale scores in the following 3 domains: symptoms, daily activities, and treatment concern. Summary and subscale scores range from 0 (complete AF-related disability) to 100 (no AF-related disability). The first AFEQT item (“Are you currently in AF?”) is not included in the scoring. Two additional items regarding treatment satisfaction are not included in the summary score and were not collected for this trial. For AFEQT scales, a score of 5 or more has been identified as a benchmark for a clinically meaningful change in an individual patient.14
The MAFSI was developed as a modification and update of the Bubien-Kay Symptom Checklist.15 A modified MAFSI8 questionnaire, comprised of a 10-item AF symptom checklist that asked about both the frequency and severity of each symptom, was used in this trial. The questionnaire asked participants to indicate the frequency of symptoms over the past month as 0 (never), 1 (rarely), 2 (sometimes), 3 (often), or 4 (always). Item responses were added together for a total frequency score that ranged from 0 (no AF symptoms) to 40 (the most severe AF symptoms). MAFSI severity scores over the past month were recorded as 1 (mild), 2 (moderate), or 3 (extreme). Items were then added together to produce the total severity score, which ranged from 0 (no AF symptoms) to 30 (the most severe AF symptoms). For an individual patient, a clinically meaningful change in the MAFSI score has not previously been established and therefore was considered 1.6 points for the frequency score and 1.3 points for the severity score (approximately one-fourth of the pooled baseline SD).10 MAFSI scores were collected on the trial case report form, which was administered by site coordinators at all follow-up points.
Secondary QOL End Points
Additional QOL instruments used to assess cardiac and health status domains included the Duke Activity Status Index (DASI),16 the Atrial Fibrillation Severity Scale (AFSS),17 the 36-item Short Form Survey (SF-36),18 and the EQ-5D 3 level version (EQ-5D-3L).19 Baseline and follow-up assessments also included the patient’s employment status. For patients who were working, responses to the Stanford Presenteeism Scale20 and the Work Productivity and Activity Impairment Questionnaire21 were collected to evaluate the effect of catheter ablation on the patient’s ability to work. Additional descriptions of these instruments are provided in Supplement 1.
Health-Related QOL Data Collection Methods and Schedule
QOL data were collected via structured interviews at randomization and at months 3 and 12, then every 12 months thereafter (or at the last follow-up visit for participants who prematurely withdrew from the study). A subset of the QOL assessments that included a sampling of questions from the AFEQT, MAFSI, AFSS, SF-36, EQ-5D-3L, Stanford Presenteeism Scale, and Work Productivity and Activity Impairment Questionnaire was also collected at month 6 and every 12 months thereafter. Site coordinators were trained by Duke Clinical Research Institute (DCRI) personnel to perform structured QOL interviews. All baseline interviews were performed by site coordinators. Trained interviewers from the DCRI’s Outcomes Research Group, who were masked to treatment assignment, conducted follow-up interviews via telephone for North American sites. Site coordinators conducted follow-up interviews for sites outside North America.
Statistical Analyses
Primary Analyses
All primary analyses were performed with treatment groups defined by the principle of intention-to-treat (ITT) analyses (as randomized). Descriptive statistics were presented as percentages for discrete variables and median and 25th and 75th percentiles or mean and SD for continuous variables.
The AFEQT and MAFSI end points were analyzed using a repeated-measures mixed model with baseline score and month 3, 12, 24, 36, 48, and 60 responses included as outcome variables, and time, treatment, and time × treatment as fixed effects. Restricted maximum likelihood estimation with an unstructured covariance matrix and Kenward-Roger degrees of freedom approximation was used to model all available data from each patient. No formal imputation procedures were used in our analyses because the mixed-effects model does not require complete data or the same length of follow-up for each patient.
Point estimates for each treatment group and treatment group mean differences (catheter ablation group score − drug therapy group score) with 95% CIs were generated for each follow-up point. The possibility of spurious statistical testing results due to multiple comparisons was addressed in 2 ways. First, the number of formal statistical tests in this report was limited. Two-sided P values are provided for the prespecified co-primary treatment comparisons at 12 months, for the same end points averaged across all follow-up assessments, and for the tests of treatment × covariable interactions. Second, the primary treatment comparison to be tested statistically was prespecified to be the between-treatment group differences at month 12 for the co-primary QOL end points. Where P values are provided as interpretive aids, significance, understood as a heuristic of unexpectedness regarding the consistency of the data with the null hypothesis, was defined as P < .05.22
Secondary analyses examined the treatment effects at months 3, 24, 36, 48, and 60 as well as averaged across the 6 follow-up points to provide an overall mean estimate of effect size. Unadjusted data were displayed as histograms and adjusted data as subgroup plots. The estimated differences and 95% CIs were obtained using the ESTIMATE statement in SAS PROC MIXED software. The 95% CIs around the treatment effect size estimates were used to gauge precision.22 A clinically meaningful difference at the individual patient level refers to a change that patients would both notice and consider important or worthwhile. There are no fully suitable benchmarks for assessing the incremental clinically meaningful differences at the treatment group level.23 Therefore, the patient-level benchmark was used as an informal treatment effect benchmark.
Subgroups
QOL outcomes were compared for the subgroups that were prespecified in the overall trial analysis.12 Baseline tertiles of AFEQT and MAFSI scores for post hoc subgroup analyses were also examined, because the potential to demonstrate a large QOL treatment benefit has been shown in other contexts to be dependent, to an important extent, on the baseline level of impairment present.10 Subgroup treatment effect sizes, CIs, and P values were generated using the ESTIMATE statement in SAS PROC MIXED software and included an interaction between treatment and subgroup and a 3-way interaction among treatment, subgroup, and interval.
Sensitivity Analyses
Per-protocol analyses were performed following the methods established for the clinical analyses of the trial.11 Patients had to undergo ablation within 3 months, 6 months, or 12 months following randomization to be counted as following the ablation protocol. The drug therapy protocol consisted of all patients randomized to the drug therapy group. Patients who crossed over to the catheter ablation group were censored at the time of crossover.
Post Hoc Analyses
To provide a more clinically intuitive sense of the treatment effect at 12 months, we conducted 2 additional post hoc analyses. One analysis examined patterns of QOL scores at baseline and 12 months by severity levels, and the second was a responder analysis. For the severity subgroup analysis, the AFEQT scores at baseline and 12 months were divided into categories corresponding to severely symptomatic from AF (<70), mildly to moderately symptomatic from AF (70-89), or minimally symptomatic from AF or asymptomatic (≥90). For the responder analyses, we compared the proportion of patients in each treatment group whose 12-month AFEQT scores improved from baseline by at least 5, at least 10, at least 15, and at least 20 points, roughly corresponding to values representing from 0.25 SD to 1.0 SD of the baseline score. These comparisons were done using the baseline severity subgroup categories. The responder analysis for the MAFSI frequency score used baseline values of less than 4, 4 to 9, and greater than 9 to indicate minimally symptomatic, mildly to moderately symptomatic, and severely symptomatic from AF, respectively, and responder effect sizes ranging from 0.25 SD (improvement ≥1.6 points) to 1.0 SD (improvement ≥6.4 points).
Because the study was performed at multiple sites, we also performed a post hoc ITT mixed-model analysis adjusted for enrolling site and enrolling site × treatment group interaction as random effects.
All analyses were performed using SAS software, version 9.4 or later (SAS Institute).
Results
Baseline Characteristics and QOL Data Collection Rates
The median age of the 2204 patients enrolled in the trial was 68 years, 1385 (62.8%) were men, 2025 (91.9%) were white, 946 (42.9%) had paroxysmal AF, and 1257 (57.1%) had persistent AF (Table 1). Median (25th, 75th percentiles) follow-up duration was 48.5 (29.9, 62.1) months. Of the 2204 patients, 1108 were randomized to the catheter ablation group and 1096 were randomized to the drug therapy group (eFigure 1 in Supplement 1).
Table 1. Baseline Characteristics of Patients With Atrial Fibrillation (AF) in the CABANA Trial.
| Baseline Characteristics | All Patients, No. (%) |
|---|---|
| No. | 2204 |
| Age, y | |
| Median (25th, 75th percentiles) | 68 (62, 72) |
| <65 | 766 (34.8) |
| 65-74 | 1130 (51.3) |
| ≥75 | 308 (13.9) |
| Sex | |
| Men | 1385 (62.8) |
| Women | 819 (37.2) |
| Racea | |
| White | 2025 (91.9) |
| Black/African American | 77 (3.5) |
| Otherb | 98 (4.4) |
| Hispanic/Latino ethnicity | 225 (10.2) |
| BMI, median (25th, 75th percentiles) | 30 (27, 35) |
| AF severityc | |
| 0 (Least severe) | 223 (10.2) |
| 1 | 339 (15.5) |
| 2-3 | 1486 (67.8) |
| 4 (Most severe) | 143 (6.5) |
| Heart function severityd | |
| Class I (Least severe) | 279 (12.8) |
| Class II/III (Most severe) | 776 (35.5) |
| Medical historye | |
| Hypertension or LVH | 1851 (84.0) |
| Hypertension | 1776 (80.6) |
| LVH | 662 (40.3) |
| Diabetes | 561 (25.5) |
| Sleep apnea | 508 (23.1) |
| Coronary artery disease | 424 (19.2) |
| Congestive heart failure | 337 (15.3) |
| Family history of AF | 252 (11.5) |
| Prior CVA or TIA | 220 (10.0) |
| Prior CVA | 126 (5.7) |
| Ejection fraction ≤35%f | 69 (4.5) |
| Thromboembolic events | 90 (4.1) |
| CHA2DS2-VASc,g median (25th, 75th percentile) | 3.0 (2.0, 4.0) |
| 0-1 (Lowest risk) | 395 (17.9) |
| 2 | 562 (25.6) |
| 3 | 637 (28.9) |
| 4 | 329 (14.9) |
| ≥5 (Highest risk) | 279 (12.7) |
| Arrhythmia History | |
| Years since onset of AF, median (25th, 75th percentile) | 1.1 (0.3, 3.9) |
| Type of AF at enrollmenth | |
| Paroxysmal | 946 (42.9) |
| Persistent | 1042 (47.3) |
| Longstanding persistent | 215 (9.8) |
| Prior hospitalization for AF | 874 (39.7) |
| Prior direct cardioversion | 809 (36.7) |
| History of atrial flutter | 298 (13.7) |
| Prior ablation for atrial flutter | 108 (4.9) |
| Rhythm control therapyi | |
| 1 Rhythm control drug | 850 (81.9) |
| ≥2 Rhythm control drugs | 188 (18.1) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CVA, cerebrovascular accident; LVH, left ventricular hypertrophy; TIA, transient ischemic attack.
Race/ethnicity was determined by the site investigator in conjunction with the patient based on predefined categories defined by the National Institutes of Health.
Asian, American Indian/Alaskan Indian, Hawaiian or other Pacific Islander, and multiracial.
Based on the Canadian Cardiovascular Society Severity of Atrial Fibrillation Scale. Range, 0 to 4; 0 indicates asymptomatic and 4 indicates the most severe symptoms.
Based on New York Heart Association classification. Class I is the least severe and class IV is the most severe symptom of heart failure.
Medical history was obtained by patient report and electronic health record review and recorded on the case report form.
Of 1530 patients.
The CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 years [doubled], diabetes, stroke/TIA/thromboembolism [doubled], vascular disease [prior myocardial infarction, peripheral artery disease, or aortic plaque], age 65-75 years, sex category [female]) ranges from 0 to 9; 0 represents the lowest risk and 9 represents the highest risk.
Paroxysmal: AF episode lasting ≥1 h in duration that terminates spontaneously within 7 d or cardioversion is performed within 48h of AF onset; persistent: AF episode sustained for ≥7 d or cardioversion is performed more than 48 h after AF onset; longstanding persistent: continuous AF of duration >1 y.
Current or past use of rhythm control therapy reported at the time of enrollment.
Of a possible 20 461 patient QOL questionnaires from baseline through 60 months, 18 436 (90.1%) were collected. For the co-primary end points (AFEQT and MAFSI scores), data from at least 1 questionnaire at 12 months were collected from 1883 of 2074 possible questionnaires (90.8%). At month 60, data were collected from 711 of 897 possible questionnaires (79.3%). The number of expected questionnaires excluded questionnaires from patients who died or withdrew from the trial. The rate of expected but missing QOL questionnaires did not differ by treatment group at baseline or any follow-up interval (eTable 1 in Supplement 1).
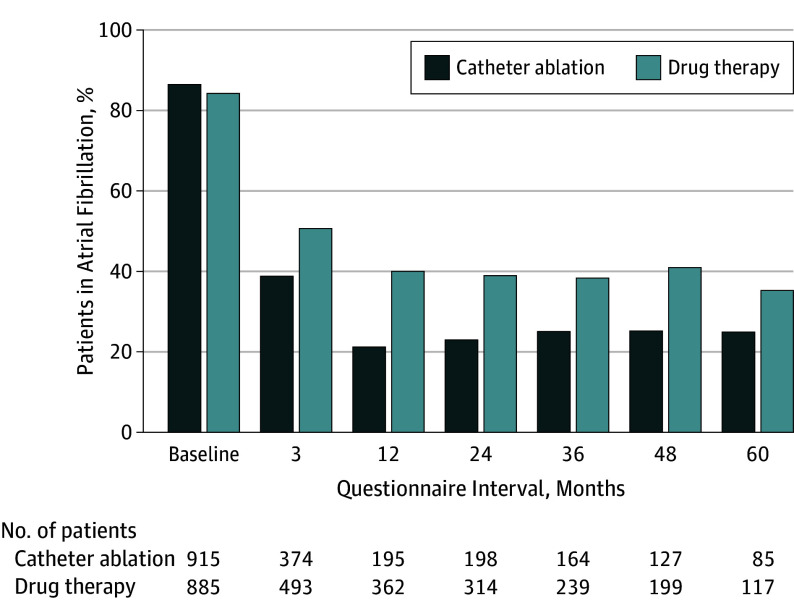
At baseline, 915 of 1064 (86.0%) patients in the catheter ablation group and 885 of 1057 (83.7%) in the drug therapy group self-reported being in atrial fibrillation currently or within the past month (Figure 1). By month 12, this rate dropped to 195 of 926 (21.1%) in the catheter ablation group and 362 of 910 (39.8%) in the drug therapy group. At 60 months, 85 of 344 patients (24.7%) in the catheter ablation group and 117 of 334 patients (35.0%) in the drug therapy group reported AF within the past month.
Figure 1. Patients Who Reported Being in Atrial Fibrillation Currently or Within the Past Month.
Responses to the first item in the Atrial Fibrillation Effect on Quality of Life instrument.
Quality of Life Outcomes
Prespecified Principal Patient-Reported Quality of Life End Points
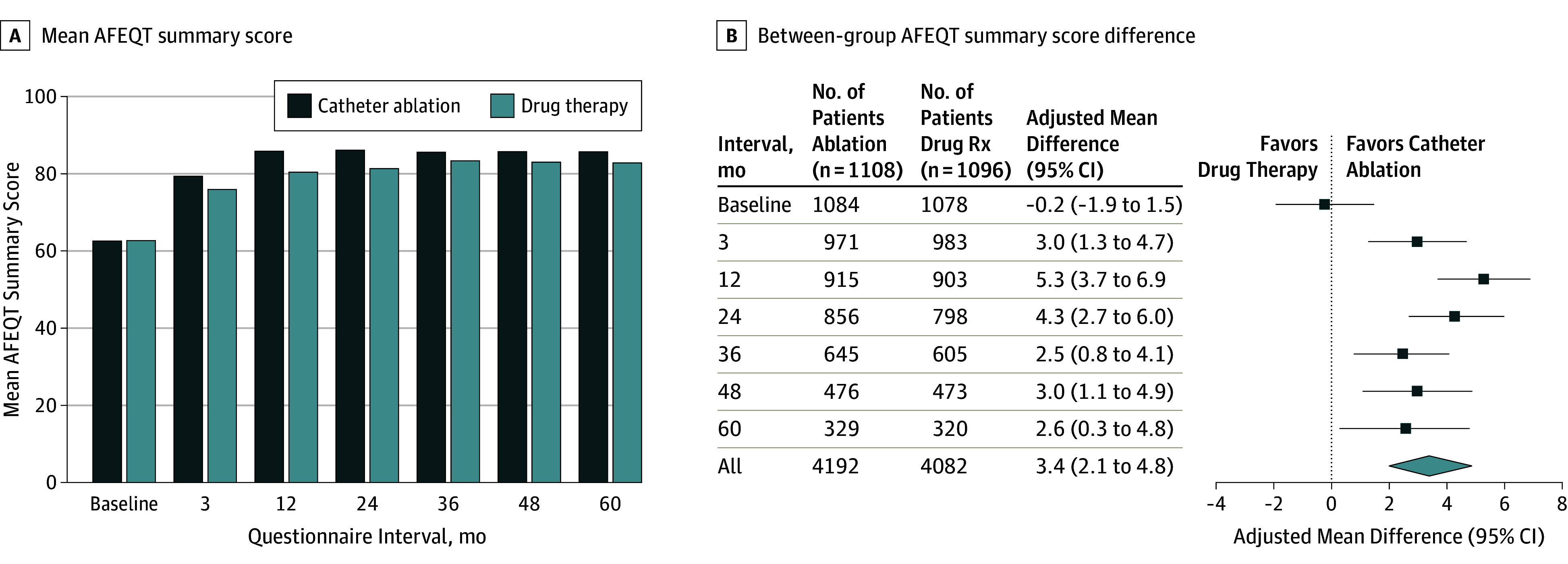
The mean AFEQT summary score (range, 0-100; a higher score indicates a lower level of AF-related disability) baseline values were 62.9 points in the catheter ablation group and 63.1 points in the drug therapy group (Table 2 and Figure 2). At 12 months, the mean summary scores were 86.4 points in the catheter ablation group and 80.9 points in the drug therapy group. The mean AFEQT summary score difference at 12 months, favoring catheter ablation, was 5.3 points (95% CI, 3.7-6.9; P < .001). At the 5-year follow-up, the mean AFEQT summary score was 3.4 points higher in the catheter ablation group than in the drug therapy group (95% CI, 2.1-4.8; P < .001). All 3 component scores for the AFEQT (symptoms, daily activities, and treatment concern) showed comparable patterns of benefit in favor of catheter ablation (eTable 2 in Supplement 1).
Table 2. Prespecified Primary Quality of Life Measures by Intention-to-Treat Analysis in the CABANA Trial.
| Follow-up Point | Catheter Ablation | Drug Therapy | Mean Adjusted Difference (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Median (25th, 75th percentile) | Mean (SD) | No. | Median (25th, 75th percentile) | Mean (SD) | No. | ||
| AFEQT Summary Score a | |||||||
| Baseline | 63 (48, 79) | 62.9 (20.5) | 1084 | 63 (48, 80) | 63.1 (20.6) | 1078 | −0.2 (−1.9 to 1.5) |
| 3 mo | 85 (69, 95) | 79.8 (18.6) | 971 | 81 (63, 94) | 76.5 (20.4) | 983 | 3.0 (1.3 to 4.7) |
| 12 mo | 94 (80, 99) | 86.4 (16.5) | 915 | 86 (69, 96) | 80.9 (18.5) | 903 | 5.3 (3.7 to 6.9)b |
| 24 mo | 94 (78, 99) | 86.6 (16.2) | 856 | 88 (71, 98) | 81.8 (18.9) | 798 | 4.3 (2.7 to 6.0) |
| 36 mo | 93 (77, 99) | 86.1 (15.8) | 645 | 90 (76, 98) | 83.9 (17.3) | 605 | 2.5 (0.8 to 4.1) |
| 48 mo | 92 (78, 99) | 86.2 (15.9) | 476 | 89 (74, 98) | 83.5 (17.7) | 473 | 3.0 (1.1 to 4.9) |
| 60 mo | 93 (77, 100) | 86.2 (16.2) | 329 | 90 (75, 98) | 83.3 (18.6) | 320 | 2.6 (0.3 to 4.8) |
| All follow-up | 91 (76, 98) | 84.8 (17.0) | 4192 | 87 (70, 97) | 80.9 (19.0) | 4082 | 3.4 (2.1 to 4.8)b |
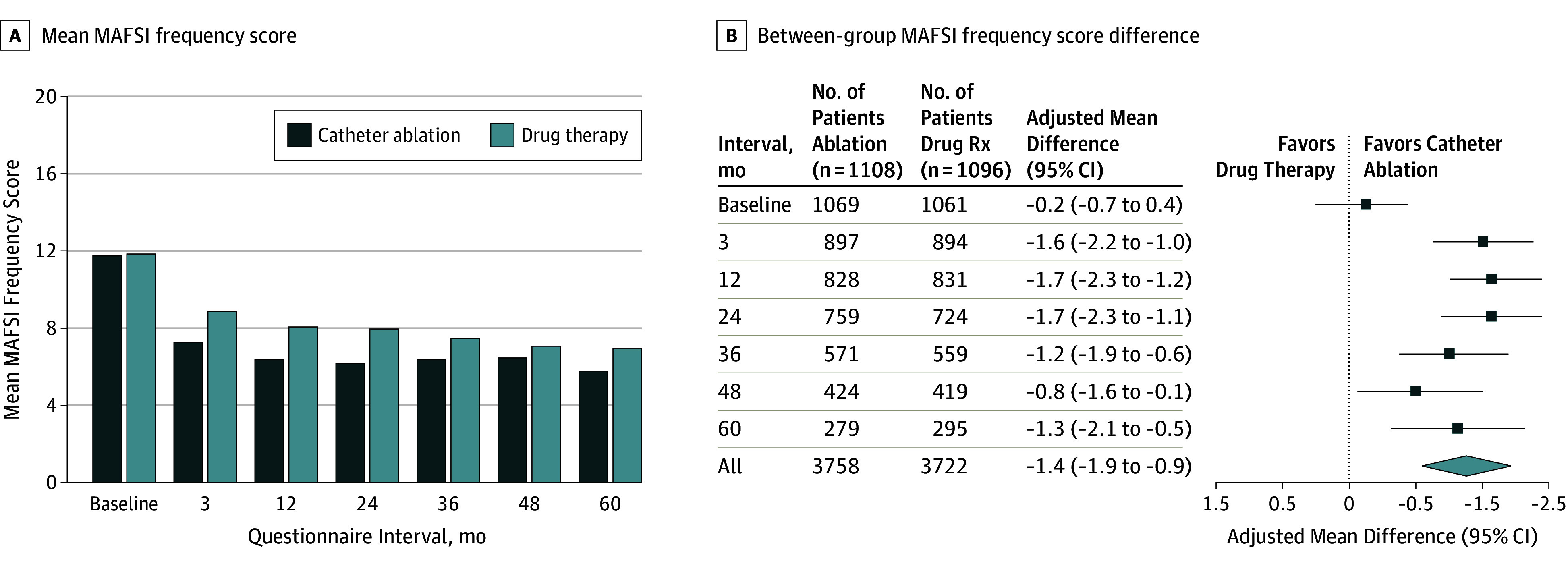
| MAFSI Frequency Score c | |||||||
| Baseline | 11 (7, 16) | 11.8 (6.2) | 1069 | 11 (7, 16) | 11.9 (6.4) | 1061 | −0.2 (−0.7 to 0.4) |
| 3 mo | 6 (2, 11) | 7.3 (5.9) | 897 | 8 (3, 13) | 8.9 (6.5) | 894 | −1.6 (−2.2 to −1.0) |
| 12 mo | 5 (1, 10) | 6.4 (6.0) | 828 | 7 (3, 12) | 8.1 (6.3) | 831 | −1.7 (−2.3 to −1.2)b |
| 24 mo | 5 (1, 10) | 6.2 (5.7) | 759 | 7 (3, 12) | 8.0 (6.5) | 724 | −1.7 (−2.3 to −1.1) |
| 36 mo | 5 (2, 10) | 6.4 (5.9) | 571 | 6 (2, 12) | 7.5 (6.4) | 559 | −1.2 (−1.9 to −0.6) |
| 48 mo | 5 (1, 10) | 6.5 (6.1) | 424 | 6 (2, 11) | 7.1 (6.2) | 419 | −0.8 (−1.6 to −0.1) |
| 60 mo | 4 (1, 9) | 5.8 (5.7) | 279 | 5 (2, 10) | 7.0 (6.3) | 295 | −1.3 (−2.1 to −0.5) |
| All follow-up | 5 (2, 10) | 6.5 (5.9) | 3758 | 7 (3, 12) | 8.0 (6.4) | 3722 | −1.4 (−1.9 to −0.9)b |
| MAFSI Severity Score d | |||||||
| Baseline | 9 (6, 13) | 9.3 (4.9) | 1066 | 9 (5, 13) | 9.3 (5.1) | 1056 | −0.1 (−0.5 to 0.4) |
| 3 mo | 5 (2, 9) | 5.8 (4.7) | 891 | 6 (3, 11) | 7.0 (5.2) | 892 | −1.3 (−1.8 to −0.9) |
| 12 mo | 4 (1, 8) | 5.0 (4.7) | 827 | 5 (2, 10) | 6.5 (5.1) | 830 | −1.5 (−2.0 to −1.1)b |
| 24 mo | 4 (1, 8) | 4.9 (4.5) | 757 | 5 (2, 9) | 6.2 (5.1) | 722 | −1.3 (−1.7 to −0.8) |
| 36 mo | 4 (1, 8) | 5.0 (4.5) | 569 | 5 (2, 9) | 5.8 (5.0) | 559 | −1.0 (−1.5 to −0.5) |
| 48 mo | 4 (1, 8) | 5.0 (4.7) | 423 | 5 (2, 9) | 5.5 (4.8) | 419 | −0.7 (−1.3 to −0.2) |
| 60 mo | 3 (1, 7) | 4.6 (4.7) | 279 | 4 (2, 8) | 5.6 (4.9) | 295 | −1.0 (−1.7 to −0.4) |
| All follow-up | 4 (1, 8) | 5.1 (4.7) | 3746 | 5 (2, 9) | 6.3 (5.1) | 3717 | −1.1 (−1.5 to −0.8)b |
Abbreviations: AFEQT, Atrial Fibrillation Effect on Quality of Life; MAFSI, Mayo AF-Specific Symptom Inventory.
Score range: 0-100; score <70 indicates severely symptomatic; 70-89, mildly to moderately symptomatic; ≥90, minimally symptomatic or asymptomatic.
P <.001; statistical significance testing results provided only for 12-mo interval and all follow-up.
Score range:0-40; score >9 indicates severely symptomatic; 4-9, mildly to moderately symptomatic; <4, minimally symptomatic or asymptomatic.
Score range: 0-40; higher scores indicate higher severity of symptoms.
Figure 2. Atrial Fibrillation Effect on Quality of Life (AFEQT) Summary Scores .
The mean MAFSI frequency score (range, 0-40; a lower score indicates less frequent symptoms) values, based on ITT analysis, at baseline and 12 months were 11.8 points and 6.4 points, respectively, in the catheter ablation group and 11.9 points and 8.1 points, respectively, in the drug therapy group. The mean difference in MAFSI frequency scores at 12 months was −1.7 points, favoring catheter ablation (95% CI, −2.3 to −1.2; P < .001) (Table 2 and Figure 3). Among all follow-up intervals, the mean MAFSI frequency score difference was −1.4 points (95% CI, −1.9 to −0.9; P < .001). The mean MAFSI severity score (range, 0-30; a lower score indicates less severe symptoms) values, based on ITT analysis, at baseline and 12 months were 9.3 points and 5.0 points, respectively, in the catheter ablation group and 9.3 points and 6.5 points, respectively, in the drug therapy group. The mean MAFSI severity score difference at 12 months showed a higher level of improvement in the catheter ablation group compared with the drug therapy group (difference, −1.5 points [95% CI, −2.0 to −1.1]; P < .001) (Table 2). Among all follow-up intervals, the mean MAFSI severity score difference was −1.1 points (95% CI, −1.5 to −0.8).
Figure 3. Mayo Atrial Fibrillation–Specific Symptom Inventory (MAFSI) Frequency Scores .
Secondary Quality of Life End Points
Secondary QOL end points, including the DASI, SF-36 scales, AFSS, and the EQ-5D scores, showed the same general patterns of treatment difference seen with the AFEQT and MAFSI scores (eTables 3-6 in Supplement 1). At baseline, 35% of trial patients were employed. At 12 months, 32% of patients in the catheter ablation group and 34% of patients in the drug therapy group reported current employment. Additional employment and productivity measures are reported in eTable 7 and eTable 8 in Supplement 1.
Subgroup Analysis
In the AFEQT, there were no treatment × covariable interactions 12 months after randomization in the clinically prespecified subgroups (eFigure 2 in Supplement 1). In particular, there were no significant differences in treatment effect according to site location (only North America masked QOL follow-up, rest of the world unmasked QOL follow-up) (P = .23). For the MAFSI frequency score, there was a statistically significant interaction between treatment and the subgroup with hypertension and left ventricular hypertrophy (P = 0.02; eFigure 3 in Supplement 1). The magnitude of the difference in AFEQT scores for this subgroup interaction was proportionately smaller and not statistically significant.
Sensitivity Analysis
A total of 102 of 1108 patients (9%) randomized to the catheter ablation group did not undergo the procedure, while 301 of 1092 patients (28%) randomized to the drug therapy group crossed over to catheter ablation at a median of 368 days.11 To explore the potential effects of patients who crossed over, a prespecified per-protocol analysis was performed, in which the catheter ablation group included the 969 patients who had the procedure within the first 6 months following randomization and the drug therapy group started with all patients randomized to receive drug therapy and censored patients who subsequently crossed over to catheter ablation at the time of crossover. Alternate time points (ie, 3 months and 12 months) were used to define the catheter ablation cohort but did not materially alter the results.
The 6-month per-protocol analysis (using a 6-month window from randomization to define a protocol catheter ablation procedure) showed a pattern of incremental treatment benefit in the catheter ablation group compared with the drug therapy group, based on mean AFEQT summary scores, that was evident at the 12-month follow-up (difference, 6.8 points [95% CI, 5.2-8.5]) and consistent at the 60-month follow-up (difference, 5.6 points [95% CI, 3.0-8.1]) (eTable 9 and eFigure 4 in Supplement 1). The 3-month and 12-month per-protocol analyses also showed similar treatment benefit for the catheter ablation group (eTables 10 and 11 in Supplement 1). A similar pattern of benefit favoring catheter ablation was seen in the 6-month per-protocol analysis based on mean MAFSI frequency scores at the 12-month follow-up (difference, −1.9 points [95% CI, −2.5 to −1.3]) and at the 60-month follow-up (difference, −1.8 points [95% CI, −2.8 to −0.9]) (eTable 12 and eFigure 5 in Supplement 1). The 3-month and 12-month per-protocol analyses showed similar results (eTable 13 and eTable 14 in Supplement 1).
Post Hoc Analyses
The benefit of catheter ablation varied as a function of the baseline AFEQT score (eFigure 6 in Supplement 1). For patients in the lowest AFEQT tertile (score range, 0-55), the mean score in the catheter ablation group was 7.7 points higher than in the drug therapy group at 12 months (95% CI, 5.1-10.3), while the middle tertile (score range, 55.1-74) had an difference of 5.3 points (95% CI, 2.7-7.9), and the highest tertile (score range, 74.1-100) had the smallest difference (2.7 points [95% CI, 0.2-5.2]; P = .023). No consistent relationship was found between baseline MAFSI frequency score tertiles and 12-month catheter ablation treatment benefit (eFigure 7 in Supplement 1).
Of 2162 patients in both groups at baseline, 1302 (60%) were severely symptomatic, 655 (30%) were mildly to moderately symptomatic, and 205 (10%) were minimally symptomatic or asymptomatic, based on AFEQT responses. At 12 months, 11% more patients in the drug therapy group than the catheter ablation group were severely symptomatic and 14% more patients in the catheter ablation group than the drug therapy group were minimally symptomatic or asymptomatic (eFigure 8 in Supplement 1).
Responder analyses were used to examine the sensitivity of the treatment benefit from catheter ablation to the criteria used to define a clinically important difference in AFEQT and MAFSI scores. In patients whose baseline scores were in the severely symptomatic or mildly to moderately symptomatic categories, the incremental QOL benefits from ablation were not sensitive to the definition used to define a clinically important improvement (eFigure 9 and eFigure 10 in Supplement 1). The 205 of 2162 patients (10%) who had baseline scores in the minimally symptomatic category showed no difference by treatment group and the smallest improvements over the first 12 months of follow-up.
Adjustment for site and the site-treatment interaction as random effects had no effect on estimates of treatment effect (eTable 15 in Supplement 1).
Discussion
In this international multicenter randomized trial, catheter ablation provided incremental symptomatic and QOL benefits over drug therapy that were clinically important and statistically significant for patients with AF. In addition to the incremental benefits produced by catheter ablation, patients in both treatment groups demonstrated clinically important improvements of approximately 20% to 50% in AF symptoms and QOL scores relative to baseline values.
A major secondary objective of the CABANA research program was to compare the effects of catheter ablation with drug therapy on long-term patient-reported outcomes in the 2204 patients with AF randomized in the trial. Interpretation of QOL data can be challenging because the measures used are often unfamiliar to both clinicians and patients.10,24 Interpretive benchmarks can be established for patient-level assessments but may not be suitable for treatment group–level comparisons in a clinical trial because the mean treatment difference can be achieved in several very different ways (eg, every patient in the catheter ablation group has the mean level of improvement, some patients have large improvements while others improve modestly or not at all).23 For both co-primary QOL end points in this study, the 12-month prespecified comparisons showed a mean treatment effect size for ablation that was equal to the patient-level benchmark, implying that a substantial proportion of patients had sizable, clinically important incremental improvements after catheter ablation relative to drug therapy.
For both MAFSI and AFEQT summary scores, treatment benefits from catheter ablation tended to be maximal at 12 months and showed some modest attenuation at or after 24 months. Similar patterns were seen for most of the secondary QOL instruments. At least 2 possible explanations for the smaller effect at later follow-up points are possible. One explanation is that more patients experienced recurrent AF at the time of the later study follow-up, which reduced some of the benefit of catheter ablation evident at 12 months. However, mean AFEQT scores in the catheter ablation group between 12 months and 60 months did not decrease. Instead, drug therapy group scores increased modestly over time. The second possibility, suggested by these patterns, is that patients who crossed over from the drug therapy group attenuated the benefits of the catheter ablation group. In support of this possible explanation, the per-protocol analysis comparison showed treatment differences that were slightly higher at 12 months than the ITT analysis comparisons, and the pattern of late attenuation was no longer evident.
Two sources of possible bias exist when QOL outcomes are measured in an unmasked trial. First, the patient may be biased in his or her self-assessments because of expectations tied to knowledge of the treatment received. To control for this possibility, sham or placebo treatment groups may be helpful. Sham AF ablation has not been attempted, to our knowledge, and its feasibility is uncertain. With regard to what can be done with currently available data to address this issue, future analyses are planned to examine the extent to which QOL changes after ablation are concordant with electrocardiographic monitoring of disease activity. Second, the outcome assessment process may be biased because of knowledge of treatment group assignments. In CABANA, QOL outcomes for North American patients were performed centrally by telephone interviewers at DCRI who were unaware of each patient’s treatment assignment. Assessments for patients outside of North America were done by local site coordinators who had access to treatment assignment information. No significant difference in the treatment effect size was seen according to a prespecified treatment × location interaction.
A number of previous trials have compared the effects of catheter ablation vs drug therapy on QOL in patients with AF. These studies varied in the type of AF that was examined, they were much smaller than CABANA, and they had much shorter follow-up.5,6 QOL was assessed in most trials with the SF-36 and a version of the Bubien-Kay Symptom Checklist.10 Most of these trials, but not all,4 found that ablation had a significant benefit on QOL in patients with AF.3,9
To make interpretation of the trial findings more clinically intuitive, 3 post hoc analyses were performed. First, defining subgroups of AFEQT scores by tertiles at baseline showed that the greater the extent of AF-related baseline impairment, as reflected in the AFEQT scores, the greater the incremental improvement provided by catheter ablation over drug therapy (eFigure 6 in Supplement 1). Second, defining interpretive categories for AFEQT showed that at baseline, 60% of patients in both groups were severely symptomatic, 30% were mildly to moderately symptomatic, and 10% were minimally symptomatic or asymptomatic (eFigure 8 in Supplement 1). At 12 months, 56% of patients in the catheter ablation group and 42% in the drug therapy group were minimally symptomatic or asymptomatic. Thus, the catheter ablation group produced 14% more patients who achieved complete or near-complete relief of their AF symptoms. In addition, responder analyses were conducted for both AFEQT and MAFSI scores, varying the size of the improvement required to define a clinically important difference from 0.25 SD to 1.0 SD, and found that the incremental benefits of ablation were robust even when a clinically important difference was defined conservatively (eFigure 9 and eFigure 10 in Supplement 1).
Limitations
This study has several limitations. First, the treatment groups were not masked. Second, QOL studies often generate a multitude of possible comparisons because multiple instruments can be used, with each measured repeatedly over the course of study follow-up. A 3-part strategy was employed to ensure the results were robust: (1) 2 co-primary QOL end points (AFEQT and MAFSI scores) and 1 follow-up point (12 months) were prespecified to serve as the primary QOL statistical comparisons, (2) the effects of catheter ablation vs drug therapy on the AFEQT and MAFSI scores averaged over the 60-month follow-up period were compared to provide robust secondary statistical comparisons, and (3) all other comparisons of primary treatment effects were performed using estimates of adjusted effect size and precision (95% CIs) without P values. In those comparisons, consistency of patterns of treatment effects over time and across instruments was considered more important than individual time point comparisons that might meet criteria for statistical significance. Third, missing follow-up data create the potential for biased estimates of treatment effects. In large international trials, even with the exercise of great diligence, some amount of missingness is unavoidable when assessing nonfatal outcomes, particularly outcomes requiring the active participation of study participants. In the present study, 2 factors made serious biases due to missing QOL end point data very unlikely: (1) no differences were observed by treatment group in the rates of missing data up to 5 years, and (2) overall rates of follow-up co-primary end point QOL data collection were 91% at 12 months and 79% at 5 years, and results were stable over time.
Conclusions
Among patients with symptomatic atrial fibrillation, catheter ablation, compared with medical therapy, led to clinically important and significant improvements in quality of life at 12 months. These findings can help guide decisions regarding management of atrial fibrillation.
eFigure 1: Overall CABANA Trial CONSORT Diagram
eTable 1: Data Collection in the CABANA Trial Quality of Life Study
eTable 2: AFEQT Component Scores: Symptoms, Daily Activities, and Treatment Concern
eTable 3: Duke Activity Status Score
eTable 4: SF-36 Scores
eTable 5: Toronto Atrial Fibrillation Severity Score (AFSS) Scores
eTable 6: EQ-5D Scores
eTable 7: Stanford Presenteeism Scale (SPS-6) Scores
eTable 8: Work Productivity And Impairment (WPAI) Scores
eTable 9: AFEQT Scores in the 6-month Per Protocol Analysis
eTable 10: AFEQT Scores in the 3-month Per Protocol Analysis
eTable 11: AFEQT Scores in the 12-month Per Protocol Analysis
eTable 12: MAFSI Scores in the 6-month Per Protocol Analysis
eTable 13: MAFSI Scores in the 3-month Per Protocol Analysis
eTable 14: MAFSI Scores in the 12-month Per Protocol Analysis
eTable 15: AFEQT Scores with Adjustment for Site and the Site-Treatment Interaction as Random Effects
eFigure 2: AFEQT Summary Scores in Pre-Specified Subgroups by Intention-to-Treat
eFigure 3: MAFSI Frequency Scores in Pre-Specified Subgroups by Intention-to-Treat
eFigure 4: AFEQT Summary Scores in the 6-Month Per Protocol Analysis
eFigure 5: MAFSI Frequency Scores in the 6-Month Per Protocol Analysis
eFigure 6: AFEQT Scores by Baseline Tertiles by Intention-to-Treat
eFigure 7: MAFSI Scores by Baseline Tertiles by Intention-to-Treat
eFigure 8: AFEQT Summary Scores Illustrative Changes
eFigure 9: AFEQT Summary Score Responder Analysis
eFigure 10: MAFSI Frequency Score Responder Analysis
Statistical analysis plan
Data sharing statement
References
- 1.Jaïs P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997;95(3):572-576. doi: 10.1161/01.CIR.95.3.572 [DOI] [PubMed] [Google Scholar]
- 2.Haïssaguerre M, Gencel L, Fischer B, et al. Successful catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 1994;5(12):1045-1052. doi: 10.1111/j.1540-8167.1994.tb01146.x [DOI] [PubMed] [Google Scholar]
- 3.Cosedis Nielsen J, Johannessen A, Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367(17):1587-1595. doi: 10.1056/NEJMoa1113566 [DOI] [PubMed] [Google Scholar]
- 4.Morillo CA, Verma A, Connolly SJ, et al. ; RAAFT-2 Investigators . Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311(7):692-700. doi: 10.1001/jama.2014.467 [DOI] [PubMed] [Google Scholar]
- 5.Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293(21):2634-2640. doi: 10.1001/jama.293.21.2634 [DOI] [PubMed] [Google Scholar]
- 6.Oral H, Pappone C, Chugh A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354(9):934-941. doi: 10.1056/NEJMoa050955 [DOI] [PubMed] [Google Scholar]
- 7.Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol. 2006;48(11):2340-2347. doi: 10.1016/j.jacc.2006.08.037 [DOI] [PubMed] [Google Scholar]
- 8.Wokhlu A, Monahan KH, Hodge DO, et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol. 2010;55(21):2308-2316. doi: 10.1016/j.jacc.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 9.Wilber DJ, Pappone C, Neuzil P, et al. ; ThermoCool AF Trial Investigators . Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303(4):333-340. doi: 10.1001/jama.2009.2029 [DOI] [PubMed] [Google Scholar]
- 10.Mark DB. Assessing quality-of-life outcomes in cardiovascular clinical research. Nat Rev Cardiol. 2016;13(5):286-308. doi: 10.1038/nrcardio.2016.10 [DOI] [PubMed] [Google Scholar]
- 11.Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial [published online March 15, 2019]. JAMA. doi: 10.1001/jama.2019.0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Packer DL, Mark DB, Robb RA, et al. ; CABANA Investigators . Catheter ablation versus antiarrhythmic drug therapy for atrial fibrillation (CABANA) trial: study rationale and design. Am Heart J. 2018;199:192-199. doi: 10.1016/j.ahj.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spertus J, Dorian P, Bubien R, et al. Development and validation of the atrial fibrillation effect on quality-of-life (AFEQT) questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4(1):15-25. doi: 10.1161/CIRCEP.110.958033 [DOI] [PubMed] [Google Scholar]
- 14.Simon DN, Thomas LE, O’Brien EC, et al. Clinically important difference in the atrial fibrillation effect on quality-of-life (AFEQT) score: results from the ORBIT-AF registry. Circ Cardiovasc Qual Outcomes. 2018;9(suppl 2):A130. [Google Scholar]
- 15.Bubien RS, Knotts-Dolson SM, Plumb VJ, Kay GN. Effect of radiofrequency catheter ablation on health-related quality of life and activities of daily living in patients with recurrent arrhythmias. Circulation. 1996;94(7):1585-1591. doi: 10.1161/01.CIR.94.7.1585 [DOI] [PubMed] [Google Scholar]
- 16.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64(10):651-654. doi: 10.1016/0002-9149(89)90496-7 [DOI] [PubMed] [Google Scholar]
- 17.Dorian P, Paquette M, Newman D, et al. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J. 2002;143(6):984-990. doi: 10.1067/mhj.2002.122518 [DOI] [PubMed] [Google Scholar]
- 18.Ware J Jr, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 19.EuroQol Group . EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 20.Koopman C, Pelletier KR, Murray JF, et al. Stanford presenteeism scale: health status and employee productivity. J Occup Environ Med. 2002;44(1):14-20. doi: 10.1097/00043764-200201000-00004 [DOI] [PubMed] [Google Scholar]
- 21.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353-365. doi: 10.2165/00019053-199304050-00006 [DOI] [PubMed] [Google Scholar]
- 22.Mark DB, Lee KL, Harrell FE Jr. Understanding the role of P values and hypothesis tests in clinical research. JAMA Cardiol. 2016;1(9):1048-1054. doi: 10.1001/jamacardio.2016.3312 [DOI] [PubMed] [Google Scholar]
- 23.Guyatt GH, Juniper EF, Walter SD, Griffith LE, Goldstein RS. Interpreting treatment effects in randomised trials. BMJ. 1998;316(7132):690-693. doi: 10.1136/bmj.316.7132.690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coon CD, Cappelleri JC. Interpreting change in scores on patient-reported outcome instruments. Ther Innov Regul Sci. 2016;50(1):22-29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1: Overall CABANA Trial CONSORT Diagram
eTable 1: Data Collection in the CABANA Trial Quality of Life Study
eTable 2: AFEQT Component Scores: Symptoms, Daily Activities, and Treatment Concern
eTable 3: Duke Activity Status Score
eTable 4: SF-36 Scores
eTable 5: Toronto Atrial Fibrillation Severity Score (AFSS) Scores
eTable 6: EQ-5D Scores
eTable 7: Stanford Presenteeism Scale (SPS-6) Scores
eTable 8: Work Productivity And Impairment (WPAI) Scores
eTable 9: AFEQT Scores in the 6-month Per Protocol Analysis
eTable 10: AFEQT Scores in the 3-month Per Protocol Analysis
eTable 11: AFEQT Scores in the 12-month Per Protocol Analysis
eTable 12: MAFSI Scores in the 6-month Per Protocol Analysis
eTable 13: MAFSI Scores in the 3-month Per Protocol Analysis
eTable 14: MAFSI Scores in the 12-month Per Protocol Analysis
eTable 15: AFEQT Scores with Adjustment for Site and the Site-Treatment Interaction as Random Effects
eFigure 2: AFEQT Summary Scores in Pre-Specified Subgroups by Intention-to-Treat
eFigure 3: MAFSI Frequency Scores in Pre-Specified Subgroups by Intention-to-Treat
eFigure 4: AFEQT Summary Scores in the 6-Month Per Protocol Analysis
eFigure 5: MAFSI Frequency Scores in the 6-Month Per Protocol Analysis
eFigure 6: AFEQT Scores by Baseline Tertiles by Intention-to-Treat
eFigure 7: MAFSI Scores by Baseline Tertiles by Intention-to-Treat
eFigure 8: AFEQT Summary Scores Illustrative Changes
eFigure 9: AFEQT Summary Score Responder Analysis
eFigure 10: MAFSI Frequency Score Responder Analysis
Statistical analysis plan
Data sharing statement