Abstract
Isocitrate dehydrogenase (IDH) I and II mutations in gliomas cause an abnormal accumulation of 2-hydroxyglutarate (2-HG) in these tumor cells. These mutations have potential prognostic value in that knowledge of the mutation status can lead to improved surgical resection. Information of mutation status obtained by immunohistochemistry or genomic analysis is not available during surgery. We report a rapid extraction nanoelectrospray ionization (nESI) method of determining 2-HG. This should allow the determination of IDH mutation status to be performed intraoperatively, within minutes, using a miniature mass spectrometer. This study demonstrates that the combination of tandem mass spectrometry with low resolution mass spectrometry allows this analysis to be performed with confidence.
Keywords: 2-hydroxyglutarate, glutamate, IDH mutation, tandem mass spectrometry, clinical analysis
Graphical Abstract
Introduction
Gliomas represent about 27% of all primary brain and other central nervous system tumors, while accounting for about 81% of all malignant brain tumors.[1] Patients with high grade gliomas have poor outcomes. Glioblastoma, the most invasive form of glioma, has a five-year survival rate of just 5.5% and there are projected to be 12,760 cases in the US in 2018.[1] Mutations of isocitrate dehydrogenase (IDH) I and II have been found in a majority of World Health Organization (WHO) grade II and III gliomas.[2, 3] Patients with IDH mutations have better outcomes than those with IDH wildtype.[4, 3] Clinical studies have shown that aggressive treatment with radiotherapy and chemotherapy could benefit patients with IDH mutant glioma.[5–7] Maximal surgical resection of IDH1 mutant malignant astrocytomas was also reported to benefit patients.[8] A rapid diagnostic method for intraoperative determination of IDH mutation status is much needed and could potentially improve resection outcome.
IDH 1 and IDH 2 enzymes catalyze the conversion of isocitrate to α-ketoglutarate. Mutant forms of the enzyme catalyze the further reduction of α-ketoglutarate to the oncometabolite 2-hydroxyglutarate (2-HG). The accumulation of 2-HG can reach up to 35 μmol per gram of tumor[9, 10] and the level of 2-HG in glioma can be indicative of IDH mutation status. Measurements of 2-HG in glioma have been demonstrated using magnetic resonance spectroscopy.[11–13] Magnetic resonance spectroscopy detection of 2-HG is non-invasive and can be performed in vivo. However, detection of 2-HG is challenging using conventional 1D magnetic resonance spectroscopy due to spectral overlap with glutamate (GLU) and glutamine, thus 1D-spectral editing or 2D correlation magnetic resonance spectroscopy is necessary to reduce number of false positives.[11]
Mass spectrometry (MS) is a highly sensitive technique for qualitative or quantitative analysis of complex samples. MS is conventionally used in conjunction with liquid or gas chromatography although this requires tedious sample preparations including chromatographic separation. The direct MS analysis of complex samples without any sample preparation is first demonstrated with desorption electrospray ionization (DESI) MS[14] and direct analysis in real time (DART) MS[15]. Since then, numerous new ambient ionization methods have been reported, and comprehensive reviews are available[16–18]. DESI-MS has been applied to mapping of oncometabolites from tissue sections[19], discrimination of glioma, white matter and grey matter based on their lipid profiles[20], intraoperative assessment of tumor margins during glioma resection[21] and detection of microscopic skin cancer[22]. Tissue analysis has also been realized by other ambient ionization methods such as paper spray[23], needle biopsy spray[24], touch spray[25], swab touch spray[26], nano-DESI[27] and liquid microjunction surface sampling probe[28, 29].
Recently, we have developed ion trap based miniature mass spectrometer (Mini MS) systems[30–34] and demonstrated their use for therapeutic drug monitoring[33], illicit drug detection[34], and preclinical pharmacokinetics[35]. These miniature MS instruments take the form of standalone systems without external pumps or gas tanks and fit easily into point-of-care settings such as an operating room. The oncometabolite 2-HG has previously been measured intraoperatively by tandem mass spectrometry using a commercial benchtop mass spectrometer.[36, 19] Here we report using of a Mini MS with extraction nanoelectrospray ionization (extraction nESI) for 2-HG determination. The experiments were done with banked tissue samples as a preliminary study to assess performance of a miniature MS in intraoperative identification of IDH mutation status in glioma tissue biopsies. In this application, GLU and 2-HG were simultaneously isolated and the intensities of their fragments compared to provide a relative measure of 2-HG in tissue. The structures of the analytes and the MS/MS transitions of interest are given in Electronic Supplementary Material (ESM) Table S1.
Experimental
Tissue Samples
Tissue sections (15 μm thickness) were prepared using a cryotome and thaw mounted on superfrost microscope slides (Thermo Fisher Scientific, Waltham, MA). IDH mutation status was determined using immunohistochemistry and genetic sequencing at Indiana University Health Molecular Pathology Laboratory. Adjacent tissue sections and smears were H&E stained using a previously published protocol[20] and blindly evaluated by an expert neuropathologist to provide assessments of tumor cell percentage and diagnosis for each sample.
Extraction nESI
A narrow strip of paper (ca. 0.5 mm wide and 15 mm long) was cut from Whatman 1 filter paper. The paper strip was wiped over the tissue sample (thawed tissue section or intact tissue) to pick up small amounts of material. The sample strip was then inserted into a nanotip (i.d. 0.86 mm, length about 4 cm) preloaded with 10 μL methanol/water (9:1, v/v), which act as both extraction and spray solvent. An acupuncture needle (diameter 0.3 mm, length 4 cm) was inserted into the pulled nanotip to act as a disposable electrode to prevent cross-contamination. Negative 1.3 kV was applied to the needle to initiate nanoelectrospray. Analytes were constantly extracted during the ionization process within the nanotip.
MS Analysis
A linear ion trap Mini MS, Mini β mass spectrometer (PURSPEC Technologies, West Lafayette, IN), was operated in the negative ion mode. The scan function was optimized to isolate and then fragment ions of both m/z 146 and 147 simultaneously by ramping RF voltage while a fixed AC frequency was applied. Ion abundances for the transitions of interest, m/z 147->129 for 2-HG and m/z 146->128 for glutamate (GLU), were recorded. For each sample, 5 spectra were recorded and each spectrum was an average of three scans. Each scan takes 1.7 second, and spectra were saved manually making the total analysis time per sample ca. 1 minute. The scan rate of the Mini MS was 3200 Da/s, ejection AC frequency was 330 kHz.
Additional details on materials, fabrication of nanotips, calculation of IDH mutation score and statistical analysis are given in the Supplementary Information.
Results and Discussion
Extraction nESI for Brain Tissue
Extraction nESI was reported previously for therapeutic drug monitoring in whole blood[37, 38], and we have adapted it here to direct tissue analysis. In this application, deprotonated forms of GLU and 2-HG were isolated simultaneously and the intensities of their fragments compared to provide a relative measure of 2-HG in tissue. The structures and MS/MS spectra of the individual compounds are shown in the ESM (Table S1 and Fig. S1, respectively). Fig. 1 illustrates tissue analysis performed using extraction nESI. On average, the time from sampling to result is ca. 5 min. Typical MS/MS spectra from tissue extracts recorded using the Mini MS are shown in Fig. 2. The scan function for these experiments utilized a wide precursor ion isolation window covering both m/z 146 and m/z 147, which correspond to the deprotonated forms of 2-HG and GLU, and which give product ions at m/z 129 and m/z 128, respectively. MS/MS of the extraction nESI from IDH mutant samples showed fragments from both 2-HG and GLU (Fig. 2 (a)), whereas MS/MS of the extraction from IDH wildtype samples showed only fragments from GLU (Fig. 2(b)).
Fig. 1.
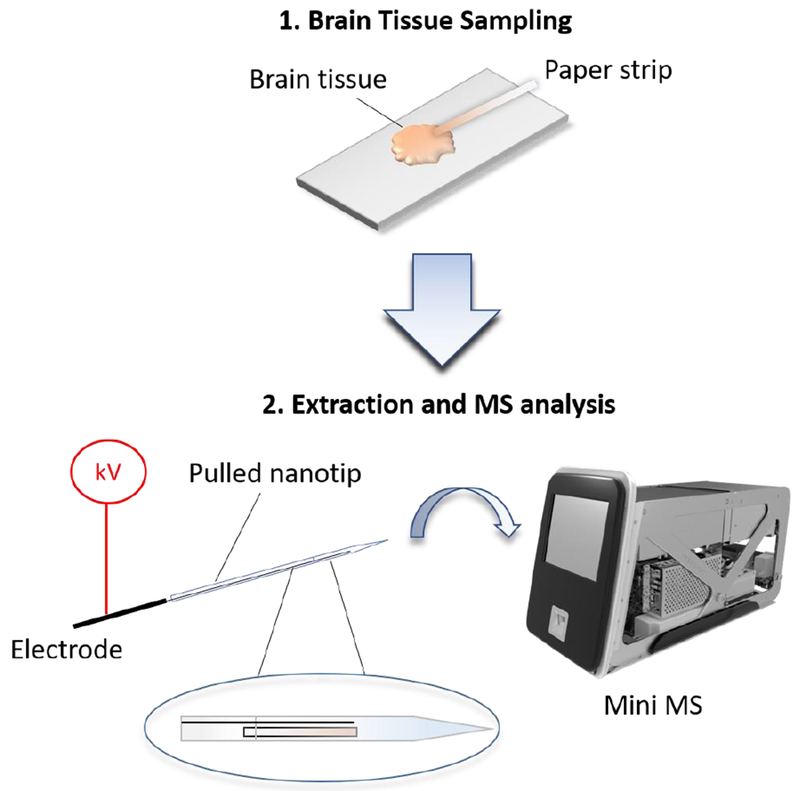
Extraction nanoelectrospray of brain tissue
Fig. 2.
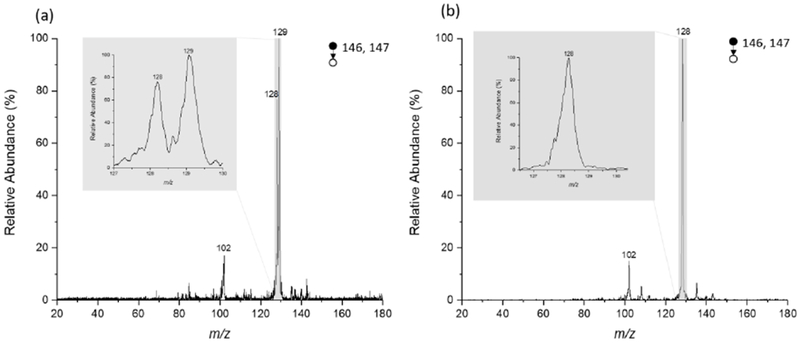
MS/MS spectra recorded using Mini MS (a) IDH mutant glioma and (b) IDH wildtype glioma. Note that peak at m/z 129 only occurs in IDH mutant tissue
GLU is an abundant brain metabolite and has been reported[39] to have lower levels in IDH mutant gliomas compared to IDH wild-type (3.23 ± 1.27 mM in IDH mutant and 5.22 ± 1.36 mM in IDH wildtype). The combined assessment of 2-HG and GLU for IDH mutation assessment improves prediction compared to using 2-HG alone.[39] Using endogenous GLU instead of isotope labeled internal standard as a reference for 2-HG simplifies the assay.
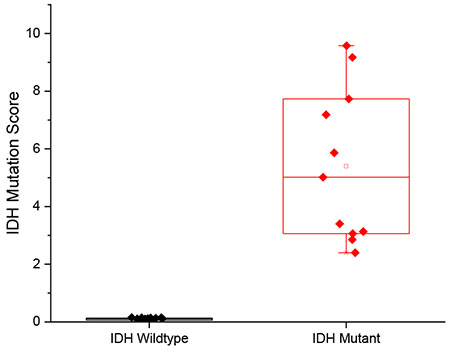
An IDH mutation score was obtained by calculating the ratio of product ion intensities, m/z 129 intensity divided by m/z 128 intensity, with isotopic corrections for contributions of the natural C13 glutamate fragment ion to signal at m/z 129. A higher IDH mutation score indicates more 2-HG relative to GLU. Since IDH mutations result in accumulation of 2-HG in tissue and decreased levels of GLU, we hypothesized that a high IDH mutation score would be indicative of an IDH mutant glioma.
Analysis of Tissue Sections
We analyzed 39 glioma tissue cryosections (29 IDH wild-type and 10 IDH mutant) prepared from banked glioma tissues, collected from 29 human subjects, as an initial evaluation of the method. The IDH mutation scores ranged from 0.04 to 0.16 for IDH wildtype and from 0.28 to 6.83 for IDH mutant samples (ESM Table S2). The distribution of IDH mutation scores was significantly higher in the IDH mutant glioma samples relative to the IDH wild-type gliomas, and the distributions were statistically different (p=0.0025, ESM Fig. S2). IDH mutant samples with low tumor cell percentage (TCP) had lower IDH mutation scores compared to IDH mutant samples with high TCP (ESM Fig. S2). This trend has been reported previously[19] and is recapitulated in our results.
Many of the tissue sections analyzed in this study are sections adjacent to those used in a previous study in which 2-HG was quantified using direct infusion ESI-MS/MS[40]. The IDH mutation scores obtained from the Mini MS samples are highly correlated to the previously published quantitative results. A coefficient of determination (R2) of 0.9229 was obtained for the five IDH mutant tissue sections, plotted as red points in Fig. 3. Meanwhile, the 2-HG concentrations of IDH wildtypes were below limits of detection for both methods, hence the 23 blue points group at the lower left corner of the plot. The high correlation seen indicates that higher IDH mutation scores are related to higher concentrations of 2-HG in the tissue. The correlation is indicative only because the data are being compared with those of another study on adjacent tissue samples[40]. The comparison assumes that the distribution of 2-HG is homogeneous throughout the tissue and no sample deterioration occurred on storage.
Fig. 3.
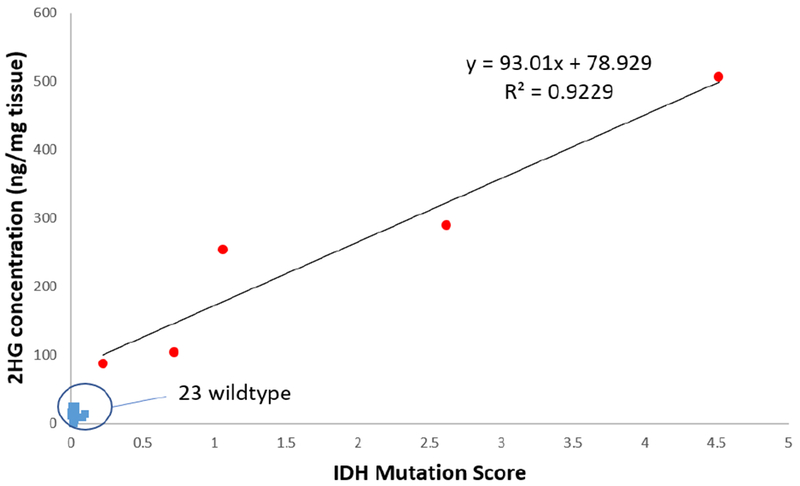
Correlation between concentration of 2-HG determined by triple quad MS analysis [40] and IDH mutation scores determined using the Mini MS [this work] from an adjacent tissue section
Analysis of Bulk Tissues
To further evaluate the method for intraoperative diagnostic purposes, we analyzed frozen bulk tissue biopsies as an intermediate step to fresh tissue analysis. Tissue biopsies were originally collected during surgeries in 1.5 mL centrifuge tubes and kept frozen at −80 °C. After thawing, each tissue piece was touched using a paper strip and analyzed with extraction nESI using the Mini MS. Reproducibility was examined by sampling three times from the same position on the same tissue biopsy. The results are presented in ESM Table S3. The average relative standard deviation was 29.0 ± 3.6 % for three medium or high TCP IDH mutant tissues and 24.8 ± 11.4 % for five IDH wildtype tissues.
A total of 44 biopsies from 15 subjects were analyzed using extraction nESI with Mini MS. The IDH mutation status and IDH mutation scores are summarized in ESM Table S4. The IDH wildtype tissues had IDH mutation scores below 0.15 regardless of TCP, whereas all IDH mutant samples had IDH mutation scores above 0.40 (p = 1.06 E-6). Among the 28 IDH mutant tissue biopsies, 12 were low TCP tissues, 5 were medium TCP tissues and 11 were high TCP tissues. The IDH mutation scores of these samples are compared in ESM Fig. S3.
The TCP can significantly impact the diagnosis of IDH mutation; low TCP samples have fewer tumor cells and correspondingly lower quantities of 2-HG. In Fig. 4, IDH mutation scores of 11 IDH mutant with high TCP and 16 IDH wildtype tissue biopsies are compared. The difference in IDH mutation scores between the highest-scored IDH wildtype and the lowest-scored IDH mutant with high TCP was 16 times (0.15 vs. 2.40). The difference in IDH mutation scores between the highest-scored IDH wildtype and the lowest-scored IDH mutant with low TCP was less than 3 times (0.15 vs. 0.40). Some low TCP samples have relatively high scores (ESM Fig. S3, up to 5.17), which may reflect TCP variations in tissue. However, discrimination of IDH mutants from IDH wildtypes is achieved regardless of their TCP.
Fig. 4.
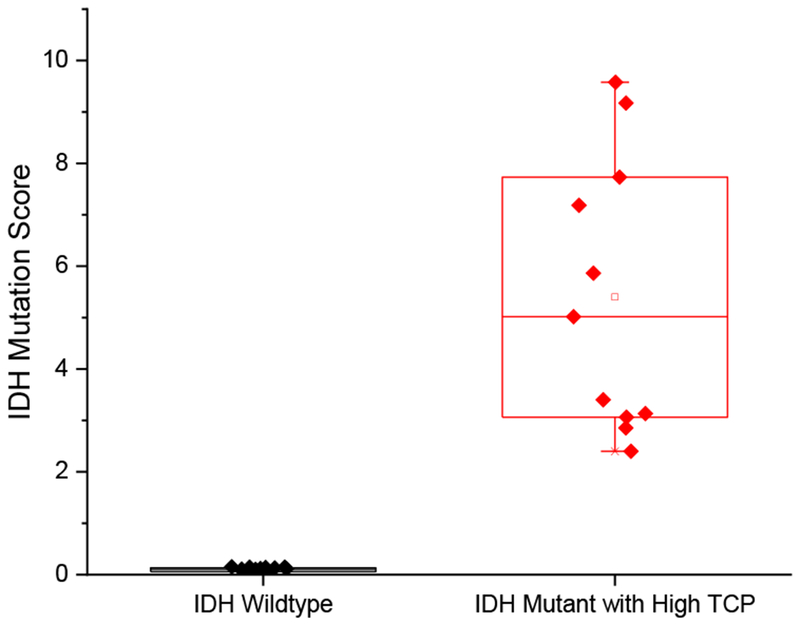
Comparison of IDH mutation scores of IDH wildtype (16 samples) and IDH mutant with high TCP (11 samples)
Conclusions
We demonstrate a method for rapid assessment of IDH mutation status of banked human gliomas samples using extraction nESI with a portable Mini MS. The Mini MS instrument used in this study provided reliable diagnostic information regarding IDH mutation status with a much smaller footprint compared to conventional bench-top mass spectrometers, thus being more suitable for an operating room. The average sampling to result time was 5 minutes. Bulk tissues of IDH wildtypes and IDH mutants with high TCP were differentiated with a 16-fold difference in IDH mutation scores. These merits suggest that the methodology could enable assessment of IDH mutation status of glioma biopsies at point-of-care during brain surgery. In our follow-up study, we will evaluate this methodology in the intraoperative environment.
Supplementary Material
Acknowledgement
The authors gratefully acknowledge funding from Grant #UL1TR002529 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award; from the National Institute of Biomedical Imaging and Bioengineering, NIH Grant R21EB015722; from the National Institute of Allergy And Infectious Diseases, NIH Grant R01AI122298; and from the Purdue University Center for Cancer Research Small Grants Program. The authors thank Dr. Mahua Dey, Dr. Charles Kulwin, Dr. James Miller, Dr. Troy Payner, Dr. Mitesh Shah, Dr. Scott Shapiro and Dr. Aaron A. Cohen-Gadol at Goodman Campbell Brain and Spine (Indianapolis, IN) for providing tissue samples, and Dr. Eyas M. Hattab at University of Louisville for pathology analysis. PURSPEC Technologies Inc. is acknowledged for providing the miniature mass spectrometry system and for technical support. Support from the Purdue University Center, Cancer Research Small Grants Program, is gratefully acknowledged.
Footnotes
Compliance with Ethical Standards
Banked tissue samples were obtained from the Methodist Research Institute Biorepository in Indianapolis in accordance with approved Institutional Review Board (IRB) protocols at Indiana University School of Medicine (IUSM) (IRB #1410015344). Tissues for bulk tissue analysis were prospectively obtained from human subjects undergoing tumor resection for suspected glioma at Indiana University Department of Neurosurgery, Goodman Campbell Brain and Spine Institute, after they had provided written informed consent to participate in the research study, following an IUSM IRB approved protocol (IRB #1410342262).
Conflict of Interest
Zheng Ouyang is the founder of PURSPEC Technologies Inc.
References
- 1.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol 2017;19(suppl_5):v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131(6):803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 3.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009;360(8):765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 2010;120(6):707–18. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 5.Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med 2016;374(14):1344–55. doi: 10.1056/NEJMoa1500925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 2013;31(3):337–43. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairncross JG, Wang M, Jenkins RB, Shaw EG, Giannini C, Brachman DG et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol 2014;32(8):783–90. doi: 10.1200/JCO.2013.49.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beiko J, Suki D, Hess KR, Fox BD, Cheung V, Cabral M et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol 2014;16(1):81–91. doi: 10.1093/neuonc/not159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–44. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller JJ, Shih HA, Andronesi OC, Cahill DP. Isocitrate dehydrogenase-mutant glioma: Evolving clinical and therapeutic implications. Cancer. 2017;123(23):4535–46. doi: 10.1002/cncr.31039. [DOI] [PubMed] [Google Scholar]
- 11.Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med 2012;4(116):116ra4. doi: 10.1126/scitranslmed.3002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med 2012;18(4):624–9. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pope WB, Prins RM, Albert Thomas M, Nagarajan R, Yen KE, Bittinger MA et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol 2012;107(1):197–205. doi: 10.1007/s11060-011-0737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takats Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306(5695):471–3. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 15.Cody RB, Laramee JA, Durst HD. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem 2005;77(8):2297–302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 16.Monge ME, Harris GA, Dwivedi P, Fernandez FM. Mass spectrometry: recent advances in direct open air surface sampling/ionization. Chem Rev 2013;113(4):2269–308. doi: 10.1021/cr300309q. [DOI] [PubMed] [Google Scholar]
- 17.Alberici RM, Simas RC, Sanvido GB, Romao W, Lalli PM, Benassi M et al. Ambient mass spectrometry: bringing MS into the “real world”. Anal Bioanal Chem 2010;398(1):265–94. doi: 10.1007/s00216-010-3808-3. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Gamez G, Zenobi R. What can we learn from ambient ionization techniques? J Am Soc Mass Spectrom. 2009;20(11):1947–63. doi: 10.1016/j.jasms.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Santagata S, Eberlin LS, Norton I, Calligaris D, Feldman DR, Ide JL et al. Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery. Proc Natl Acad Sci U S A. 2014;111(30):11121–6. doi: 10.1073/pnas.1404724111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarmusch AK, Pirro V, Baird Z, Hattab EM, Cohen-Gadol AA, Cooks RG. Lipid and metabolite profiles of human brain tumors by desorption electrospray ionization-MS. Proc Natl Acad Sci U S A. 2016;113(6):1486–91. doi: 10.1073/pnas.1523306113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pirro V, Alfaro CM, Jarmusch AK, Hattab EM, Cohen-Gadol AA, Cooks RG. Intraoperative assessment of tumor margins during glioma resection by desorption electrospray ionization-mass spectrometry. Proc Natl Acad Sci U S A. 2017;114(26):6700–5. doi: 10.1073/pnas.1706459114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margulis K, Chiou AS, Aasi SZ, Tibshirani RJ, Tang JY, Zare RN. Distinguishing malignant from benign microscopic skin lesions using desorption electrospray ionization mass spectrometry imaging. Proc Natl Acad Sci U S A. 2018;115(25):6347–52. doi: 10.1073/pnas.1803733115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Manicke NE, Yang Q, Zheng L, Shi R, Cooks RG et al. Direct analysis of biological tissue by paper spray mass spectrometry. Anal Chem 2011;83(4):1197–201. doi: 10.1021/ac103150a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Cooks RG, Ouyang Z. Biological tissue diagnostics using needle biopsy and spray ionization mass spectrometry. Anal Chem 2011;83(24):9221–5. doi: 10.1021/ac202626f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerian KS, Jarmusch AK, Cooks RG. Touch spray mass spectrometry for in situ analysis of complex samples. Analyst. 2014;139(11):2714–20. doi: 10.1039/c4an00548a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirro V, Llor RS, Jarmusch AK, Alfaro CM, Cohen-Gadol AA, Hattab EM et al. Analysis of human gliomas by swab touch spray-mass spectrometry: applications to intraoperative assessment of surgical margins and presence of oncometabolites. Analyst. 2017;142(21):4058–66. doi: 10.1039/c7an01334e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laskin J, Heath BS, Roach PJ, Cazares L, Semmes OJ. Tissue imaging using nanospray desorption electrospray ionization mass spectrometry. Anal Chem 2012;84(1):141–8. doi: 10.1021/ac2021322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang F, Guo C, Ma X, Zhang J, Su Y, Tian R et al. Rapid In Situ Profiling of Lipid C horizontal lineC Location Isomers in Tissue Using Ambient Mass Spectrometry with Photochemical Reactions. Anal Chem 2018;90(9):5612–9. doi: 10.1021/acs.analchem.7b04675. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Rector J, Lin JQ, Young JH, Sans M, Katta N et al. Nondestructive tissue analysis for ex vivo and in vivo cancer diagnosis using a handheld mass spectrometry system. Sci Transl Med 2017;9(406). doi: 10.1126/scitranslmed.aan3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao L, Song Q, Patterson GE, Cooks RG, Ouyang Z. Handheld rectilinear ion trap mass spectrometer. Anal Chem 2006;78(17):5994–6002. doi: 10.1021/ac061144k. [DOI] [PubMed] [Google Scholar]
- 31.Gao L, Sugiarto A, Harper JD, Cooks RG, Ouyang Z. Design and characterization of a multisource hand-held tandem mass spectrometer. Anal Chem 2008;80(19):7198–205. doi: 10.1021/ac801275x. [DOI] [PubMed] [Google Scholar]
- 32.Ouyang Z, Cooks RG. Miniature mass spectrometers. Annu Rev Anal Chem 2009;2:187–214. doi: 10.1146/annurev-anchem-060908-155229. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Chen TC, Ren Y, Hendricks PI, Cooks RG, Ouyang Z. Mini 12, miniature mass spectrometer for clinical and other applications--introduction and characterization. Anal Chem 2014;86(6):2909–16. doi: 10.1021/ac403766c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendricks PI, Dalgleish JK, Shelley JT, Kirleis MA, McNicholas MT, Li L et al. Autonomous in situ analysis and real-time chemical detection using a backpack miniature mass spectrometer: concept, instrumentation development, and performance. Anal Chem 2014;86(6):2900–8. doi: 10.1021/ac403765x. [DOI] [PubMed] [Google Scholar]
- 35.Pu F, Zhang W, Bateman KP, Liu Y, Helmy R, Ouyang Z. Using miniature MS system with automatic blood sampler for preclinical pharmacokinetics study. Bioanalysis. 2017;9(21):1633–41. doi: 10.4155/bio-2017-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alfaro CM, Pirro V, Keating MF, Hattab EM, Cooks RG, Cohen-Gadol AA. Intraoperative assessment of isocitrate dehydrogenase mutation status in human gliomas using desorption electrospray ionization-mass spectrometry. J Neurosurg 2019:1–8. doi: 10.3171/2018.8.JNS181207. [DOI] [PubMed] [Google Scholar]
- 37.Espy RD, Teunissen SF, Manicke NE, Ren Y, Ouyang Z, van Asten A et al. Paper spray and extraction spray mass spectrometry for the direct and simultaneous quantification of eight drugs of abuse in whole blood. Anal Chem 2014;86(15):7712–8. doi: 10.1021/ac5016408. [DOI] [PubMed] [Google Scholar]
- 38.Ren Y, Liu J, Li L, McLuckey MN, Ouyang Z. Direct Mass Spectrometry Analysis of Untreated Samples of Ultralow Amounts Using Extraction Nano-Electrospray. Anal Methods. 2013;5(23). doi: 10.1039/C3AY41149D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagashima H, Tanaka K, Sasayama T, Irino Y, Sato N, Takeuchi Y et al. Diagnostic value of glutamate with 2-hydroxyglutarate in magnetic resonance spectroscopy for IDH1 mutant glioma. Neuro Oncol 2016;18(11):1559–68. doi: 10.1093/neuonc/now090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yannell KE, Smith K, Alfaro CM, Jarmusch AK, Pirro V, Cooks RG. N-Acetylaspartate and 2-Hydroxyglutarate Assessed in Human Brain Tissue by Mass Spectrometry as Neuronal Markers of Oncogenesis. Clin Chem 2017;63(11):1766–7. doi: 10.1373/clinchem.2017.279364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


