Abstract
Objectives:
Fibromyalgia syndrome (FMS) is a chronically painful condition whose symptoms are widely reported to be exacerbated by stress. We hypothesized that female patients with FMS differ from healthy female controls in their sympathetic responses, a fact that may unmask important biomarkers and factors that contribute to the etiology of FMS.
Methods:
In a pilot study, blood pressure (BP), skin temperature, thermogenic activity, circulating glucose, and pain sensitivity of 13 patients and 11 controls at room temperature (24°C) were compared to that after exposure to cold (19°C).
Results:
When measured at 24°C, BP, skin temperature, blood glucose and brown adipose tissue (BAT) activity, measured using 18F-fluorodeoxyglucose (FDG) PET/CT, did not differ between controls and patients with FMS. However, after cold exposure (19°C), BP and BAT activity increased in controls but not in FMS patients; skin temperature on the calf and arm decreased in controls more than in FMS patients; and circulating glucose was lower in FMS patients than in controls. Pain sensitivity did not change during the testing interval in response to cold.
Discussion:
The convergence of the effect of cold on four relatively simple measures of thermogenic, cardiovascular and metabolic activity, each regulated by sympathetic activity, strongly indicate that patients with FMS have impaired sympathetic responses to stress that are observable and highly significant even when measured in extraordinarily small sample populations. If insufficient sympathetic responses to stress are linked to FMS, stress may unmask and maximize these potential clinical biomarkers of FMS and be related to its etiology.
Keywords: glucose metabolism, temperature regulation, MRI, positron emission tomography, adipose tissue
INTRODUCTION
Fibromyalgia syndrome (FMS), with a prevalence in the Americas of approximately 1–6%, is characterized by widespread musculoskeletal pain, fatigue, sleep disorder and altered cognitive function that may persist for many years.1 The symptoms are relatively unresponsive to steroidal and non-steroidal anti-inflammatory and analgesic compounds.2–4 Stress enhances symptoms of fibromyalgia,5, 6 as it does many types of pain.7 Circulating glucocorticoids are highly variable but sympathetic activity is enhanced in slightly more than half of these patients even at rest,8 supporting the possibility of a sympathetically driven contribution to the FMS condition.9 Consistent with this, a persistent vasoconstriction is suggested by lower skin temperatures above tender points, areas previously used to characterize FMS.10 Enhanced sympathetic tone appears important as their pain is reduced by experimental injections of anesthetics into stellate ganglia containing sympathetic neurons.11 Whereas stress and cold exacerbate FMS pain,12, 13 warmth temporarily relieves the discomfort.13 In fact, repeated exposure of rodents to cold is proposed as a model of FMS.14
However, most studies of FMS etiology are conducted in the absence of intentionally stressful intervention. Thus, it is not clear how various sympathetic responses are affected in these patients when stressed. Paradoxically, insufficient responses to stress are suggested by the abnormally low sympathetic response to a metabolic stress where an insulin challenge impairs rather than potentiates sympathetic activity in FMS patients.15 Based on this and on reports concerning the effect of temperature on FMS symptoms, we hypothesized that cold influences sympathetic responses in FMS patients differently than in healthy individuals. If true, exposure to stress in future studies may unmask important signs and symptoms of FMS related to sympathetic activity. To test this, we used dietary manipulations and ambient temperatures that enhance sympathetic tone in a pilot study of healthy individuals and compared responses in healthy individuals to those in patients with FMS.
Normally sympathetic activity increases blood pressure (BP), maintains circulating glucose,16 decreases surface temperature to maintain body heat,17 and stimulates non-exercise thermogenesis18 by activating brown adipose tissue (BAT).19 In addition, the volume of BAT in rodents reflects persistent stress as BAT grows in size (recruitment) in response to chronic cold, chronic stress, or diets low in protein.20 In humans, daily 2-h cold exposure at 17°C for 6 weeks increased BAT activity21 but even short-term exposure to cold (10 days) also increased cold-induced glucose uptake in the BAT of patients with type 2 diabetes.21, 22 In contrast, exercise inhibits recruitment of BAT in rodents.23 Similarly in humans, exercise by chronic endurance training inhibits BAT recruitment and improves the symptoms of FMS.24 To date, aerobic and muscle strengthening exercises have been found to be the most effective way of reducing pain and improving global well-being in people with fibromyalgia.25, 26
In this study, we hypothesized that patients with FMS differ from healthy controls in their sympathetic responses to stress, a fact that may unmask important research biomarkers and factors that contribute to the etiology of FMS.
MATERIALS AND METHODS
Participants
Fibromyalgia syndrome (FMS) was diagnosed by the 2010 criteria set forth by the American College of Rheumatology (ACR).27 Fatigue, waking unrefreshed, cognitive symptoms, and the intensity of somatic symptoms in general were each evaluated for symptom severity from 0 (no problem) to 3 (severe, pervasive, continuous, life-disturbing problem). Each of these four evaluations constituted 3 of the possible 12 points on the SS scale. Because FMS patients have a preoccupation with health issues, including a tendency toward somatization disorder and hypochondriasis,28 the information for these evaluations was elicited by specific questions embedded within the Pittsburgh Sleep Quality Index (PSI; raw scores from 0 to 3), the Psychiatric Diagnostic Screening Questionnaire (PDSQ)29 (Yes or No answers), the POMS-SF (Profile of Mood States Short Form) questionnaire30 (raw scores from 0 to 4), and a visual analog scale (raw scores from 0–10) for pain. As summarized in Table 1, the symptom severity (SS) criteria for diagnosing FMS were based on the Profile of Mood State-Short Form (POMS) items 3, 13 and 19. The Pittsburgh sleep survey item 6 and PDSQ item 10 were used as indications of waking unrefreshed. Cognitive symptoms were reflected by PDSQ item 14 and POMS items 15 and 29. Symptoms in general were reflected by the VAS, PDSQ items 1, 102 and 103 as well as POMS item 1, 7 and 16. The WPI and SS scores that characterize each individual of the final FMS patient group are indicated in Table 2.
Table 1.
Criteria Used for the Diagnosis of Fibromyalgia Syndrome (FMS)
| Widespread Pain Index (WPI) (19 pts possible) |
| Subjects were asked to shade areas on a diagram of the human body to indicate where they felt pain. The areas of overlap between those and the 19 areas designated by the ACR to reflect widespread pain consistent with FMS were counted. |
| Somatic Symptom Severity (SS) Scores Total (12 pts possible) |
| Fatigue, waking unrefreshed and cognitive symptoms were each evaluated individually for symptom severity from 0 (no problem) to 3 (severe, pervasive, continuous, life-disturbing problem). This information was elicited by specific questions embedded in the instruments listed below. A positive response in the PDSQ1, a score of 2 or 3 on the Pittsburgh sleep index2, a score of 3 or 4 in the POMS questionnaire3, and a score of 5 or greater in the VAS4 were interpreted as somatic symptoms consistent with FMS. The final category of Symptoms in General describes symptom severity of a wide variety of disorders detected by the instruments described as well as the patient’s medical history. |
| Fatigue Score (3 pts) |
| POMS 3 Have you felt worn out during the past week? |
| POMS 13 Have you felt fatigued during the past week? |
| POMS 19 Have you felt exhausted during the past week? |
| Waking Unrefreshed (3 pts) |
| Pittsburgh sleep #6: During the past month, how would you rate your sleep quality overall |
| PDSQ 10: Did you feel tired out nearly every day during the past 2 weeks? |
| Cognitive Symptoms (3 pts) |
| PDSQ 14: Did you have problems concentrating nearly every day during the past 2 weeks? |
| PDSQ 15: Was decision making more difficult than normal during the past 2 weeks? |
| POMS 29: Did you feel forgetful during the past week? |
| Symptoms in General (3 pts) |
| VAS (5 or greater of 1–10) |
| PDSQ 102: GI problems |
| PDSQ 103: Diffuse aches |
| PDSQ 1: Sad or depressed |
| POMS 7: Sad (if 3 or 4) |
| POMS 1: Tense (if 3 or 4) |
| POMS 16: Nervous (if 3 or 4) |
The Psychiatric Diagnostic Screening Questionnaire (PDSQ) raw scores were Yes or No answers.
The Pittsburgh Sleep Quality Index (PSI) was scored from 0 = very good to 3 = very bad).
The Profile of Mood States-Short Form (POMS) questionnaire has raw scores from 0 to 4.
The visual analog scale (VAS) was scored from 0 to 10 for pain intensity.
Table 2.
Subject characteristics.
| Controls | Age | Height | Weight | BMI | Medications | Past Diagnoses |
|---|---|---|---|---|---|---|
| E001 | 19 | 5’8.5” | 180 | 27.4 | None | None |
| E003 | 18 | 5’3” | 170 | 30.1 | None | None |
| E006 | 21 | 5’8” | 170 | 25.8 | None | None |
| E007 | 33 | 6’0” | 162 | 22.0 | Amoxicillin top | None |
| E008 | 20 | 5’3” | 156 | 26.4 | NuvaRing1 | None |
| E009 | 19 | 5’0” | 135 | 26.4 | None | None |
| E010 | 24 | 5’5” | 128 | 21.3 | Levora2 | None |
| E014 | 26 | 5’8” | 160 | 24.3 | Ibuprofen | None |
| E023 | 31 | 5’8” | 135 | 20.5 | None | Vasovagal syncope |
| E024 | 36 | 5’7” | 165 | 26.0 | Birth control | None |
| E025 | 36 | 5’6” | 140 | 22.1 | Asthma Inhalers3 | None |
| FMS Patients | Age | Height | Weight | BMI | Medications | Past Diagnoses | WPI/SS |
|---|---|---|---|---|---|---|---|
| E011 | 32 | 5’9” | 145 | 21.4 | Tramadol | Endometriosis | 9/5 |
| Triamterene | |||||||
| Zarah4 | |||||||
| Cyclobenzaprine | |||||||
| Bupropion SR | |||||||
| Trazodone prn | |||||||
| Lidocaine oin prn | |||||||
| Lidoderm patch prn | |||||||
| E012 | 47 | 5’2” | 190 | 34.8 | Duloxetine | Hypothyroid, GERD | 11/9 |
| Acyclovir | |||||||
| Levothyroxin | |||||||
| Pantoprazole | |||||||
| E020 | 46 | 5’4” | 138 | 23.7 | Citalopram | None | 7/5 |
| Adderall5 | |||||||
| E021 | 50 | 5’4” | 130 | 22.3 | Gabapentin | Mild Depression | 5/10 |
| Venlafaxine | (6 of 9 on PDSQ) | ||||||
| E027 | 39 | 5’6” | 135 | 21.8 | Duloxetine | None | 17/12 |
| Gabapentin | |||||||
| Zolpidem | |||||||
| E029 | 25 | 5’11” | 165 | 23.0 | Duloxetine | None | 10/11 |
| Gabapentin | |||||||
| E030 | 31 | 5’8” | 205 | 31.2 | Gabapentin | Herniated disc, | 11/9 |
| Lisinopril | bone spurs | ||||||
| Tramadol | |||||||
| Bupropion | |||||||
| Zolpidem | |||||||
| Cyclobenzaprine | |||||||
| E033 | 43 | 5’2” | 113 | 20.7 | Gabapentin | osteoarthritis | 5/9 |
| Cetirizine | |||||||
| E035 | 20 | 5’8” | 127 | 19.3 | Gabapentin | None | 9/12 |
| E037 | 38 | 5’4.5” | 170 | 28.3 | Pregabalin | Allergies | 5/11 |
| Mometasone spray | |||||||
| Azelastine spray | |||||||
| Fexofenadine | |||||||
| Ibuprofen, | |||||||
| Cyclobenzaprine | |||||||
| Montelukast | |||||||
| E038 | 30 | 5’5” | 170 | 28.3 | None | None | 7/6 |
| E039 | 46 | 5’6” | 175 | 28.2 | Gabapentin | None | 10/5 |
| Amitriptyline | |||||||
| E041 | 37 | 5’5” | 125 | 20.8 | Gabapentin | None | 7/5 |
| Birth control |
etonogestrel/ethynyl estradiol vaginal ring
levonorgestrel and ethinyl estradiol tablets
fluticasone/salmeterol and albuterol inhalers
drospirenone/ethinyl estradiol tablets
amphetamine/dextroamphetamine
Diagnostic criteria for fibromyalgia is considered to be satisfied if (1) widespread pain index (WPI)≥7 and somatic symptom severity score (SS)≥5 or if WPI 3–6 and SS≥9; (2) symptoms were present at a similar level for at least 3 months; and (3) the patient does not have a disorder that would otherwise explain the pain. WPI and SS scores defining the final patient group were determined as described in methods. Patient medical records were reviewed by a physician to confirm documentation that the latter two criteria were met. WPI/SS data for volunteers only include those patients who met the diagnostic criteria for FMS.
Subject Selection
Although the study was conducted prior to the 1–18-2017 FDA Final Rule affecting clinical trials in humans, we had previously registered the study under ClinicalTrials.gov identifier NCT01322425 to help recruit subjects and to document the parameters surrounding our study and the feasibility of the approach. All participants were subjected to the same manipulations. Although this study is not a randomized comparison, the details in common with a Consort diagram have been provided in Table 3 and the following text.
Table 3.
Transparent Reporting of Trials
| Event | # Controls | # FMS patients |
|---|---|---|
| Screened*: | 22 | 23 |
| Did not meet inclusion criteria: | 7 | 7 |
| Declined to participate: | 2 | 2 |
| Exposed to first temperature: | 13 | 14 |
| (At random either 24 or 19°C) | ||
| Discontinued study: | 2 | 1 |
| Exposed to second temperature: | 11 | 13 |
| (At random either 24 or 19°C) | ||
| * Screening questions by phone for eligibility for further assessment: | ||
| Gender | F | |
| Age | 18–55 | |
| Premenopausal | yes | |
| Pregnant | No | |
| FMS | Yes or No | |
| Medications | No | |
| Able to comply with study procedures | Yes | |
| Serious medical condition other than FMS | No | |
All subjects heard about the study either from their physician or through advertisements placed in local newspapers; posters hung in home clinics, rheumatology clinics, pain clinics, and common areas throughout the University of Minnesota campus; clinic websites; and Facebook pages. Subjects with FMS were currently being treated for their condition at local family practices as well as rheumatology and pain clinics in the Minneapolis and Saint Paul area. Consent to participate in the study was obtained via a consent process approved by the local institutional review board (IRB) at the University of Minnesota (IRB Study Number 1007M85352). After consent was obtained, each subject participated in a screening visit that included psychiatric screening questionnaires to detect exclusionary conditions and diagnostic criteria for FMS, questionnaires to assess the distribution and intensity of pain, the presence of depression or anxiety, the quality of sleep, and dolorimetric measures of pressure algometry. The results of these measures determined eligibility for the subsequent visits.
Eligible subjects met all inclusion criteria at baseline which included the following: female gender; 18–55 years of age and premenopausal; healthy volunteer or patient diagnosed with FMS; follicular phase of the menstrual period (to minimize major changes in body temperature and other hormone levels; decreased risk of accidental radiation exposure while pregnant; greater mood stability) or on oral contraceptives; ability to comply with study procedures for the entire length of the protocol and to give informed consent (informed consent obtained and signed); and a negative urine pregnancy test within 24 h prior to PET scan visits.
Subjects were excluded if they met any of the following exclusion criteria: Axis I DSM IV diagnoses30 (e.g., major depression, anxiety disorder, drug abuse/dependence, etc.); any serious medical conditions including other rheumatological diseases; acute changes in medication that could affect brain metabolism (e.g., antidepressants, sedatives); use of opioids within 14 days prior to any study visit or non-steroidal anti-inflammatory drugs within 72 h of the beginning of the temperature-intervention study visits; a body mass index (BMI) greater than 35 kg/m2; contraindication to MRI (e.g., claustrophobia, pacemaker, ferromagnetic contamination); pregnancy or breast feeding; exposure to radiation (medical) in prior 12 months; or any condition that, in the investigator’s opinion, made the subject unsuitable for study participation.
Protocol
The study involved a brief pre-screening telephone interview and three visits during a two- to four-week period. Cold was selected as the primary stressor based on reports that it enhances FMS symptoms and also because it is easily applied and controlled. Subjects were scheduled on two separate days on average 18 days apart counterbalancing the order of temperature conditions. Except for dietary details, the entire protocol has been described previously.31 The high fat, low carbohydrate, protein-sparing (HFLCPS) diet consisted of two fried eggs or omelet (without milk), 3 slices of bacon or a fried piece of chicken or turkey, and tea or coffee without milk, sugar, or sweetener. The low fat, low carbohydrate (LFLC) diet consisted of raisin bran cereal, 1% milk, a fruit cup and tea or coffee without milk, sugar, or sweetener.
Questionnaires
During the initial screening, the PSI, a self-rated questionnaire that assesses sleep quality and disturbances over a 1-month time-interval, was administered. In addition, the 7-day physical activity recall; medicine/drug supplement questionnaire; as well as the 24-hour history of food/beverages consumption, medication ingestion, sleep, activity level, and general mood were used to confirm inclusion and exclusion criteria. The PSI was used to quantitate the degree of sleep impairment in the FMS patients. The physical activity questionnaire measured the typical level of current physical activity, which can affect symptoms in FMS subjects and BAT metabolism. Medication history controlled for potential pharmacological effects on FMS physiology, e.g., opiates, steroids, etc. The PDSQ,29 a self-report instrument designed to screen for DSM IV Axis I disorders in psychiatric patients,30 was administered during the screening visit to elicit specific responses for the diagnosis of FMS and for inclusion and exclusion purposes. The POMS-SF (Profile of Mood States-Short Form,32 a 37-item self-report questionnaire, measured mood disturbance in the 6 areas of fatigue-inertia, vigor-activity, tension-anxiety, depression-dejection, anger-hostility, and confusion-bewilderment. Patients were asked to evaluate how they felt during the prescreening visit and ‘right now’, i.e., at the moment the test was administered during the warm and cold interventions.
Physiologic signs
Core (intra-aural) temperature and blood pressure (BP) were measured using a Spot Vital Signs Model 42MTB (Welch Allyn, Skaneateles Falls, NY). Skin temperatures (forehead, calf, forearm) measured using a Portable Infrared Thermometer Model 153 (Bioseb, Pinellas Park, FL) were taken before and at hourly intervals after entering the temperature-controlled rooms. Heart rate (HR) was monitored periodically throughout the temperature interventions. Comparisons between FMS and control groups were made at the same time relative to the interventions and to the ambient temperature conditions.
Pain Sensitivity
In an effort to detect subtle changes, we explored several characteristics of pain in our subjects in more depth than that required for a diagnosis of FMS in patients. The McGill Pain Questionnaire (MPQ) short form is a scale for describing sensory versus affective pain (0 = none, 3 = severe) with a visual analog scale (VAS) ranging from 0 (no pain) to 100 (highest possible pain). In addition, the present pain index (0 = no pain, 5 = excruciating) allows subjects to circle the number plus descriptive words that best describe their pain. Sensory pain is categorized as those described by throbbing, shooting, stabbing, sharp, cramping, gnawing, hot-burning, aching, heavy, tender and splitting; affective pain is characterized as tiring-exhausting, sickening, fearful and punishing-cruel. Pain thresholds were measured by applying defined forces using a Compact Digital Force Gauge (Wagner Instruments, Greenwich, CT) over the tender sites to serve as dolorimetric measures of pressure algometry. The subject identified the force when pain began, allowing the pain to be rated from 1–10. Measurements were taken before each temperature intervention as well as 1 and 3 h after exposure to cold and warm temperatures. Finally, because the distribution of pain in FMS patients is widespread, the anatomical regions in which pain was spontaneously felt by participants was determined. The number of positive painful sites was identified by the subject on a diagram indicating 45 areas of the body. In addition, a visual analog score allowed subjects to rate the intensity of their overall pain from 0 (no pain) to 10 (worst possible pain). Averages of pain scores were calculated for each group and compared between groups before and after warm and cold exposures.
Rationale for ambient temperature and dietary manipulations
Temperature.
Based on the literature, the temperature and dietary manipulations selected to influence sympathetic activity were based on their ability to influence the activity of BAT, a sympathetically innervated tissue. The cold temperature was selected to avoid maximally stressing subjects and thereby producing a ceiling effect. Rather, we wished to expose them to a moderately cool temperature and diet that would differentiate patients from controls. BAT activity is not typically prominent in clinical FDG PET scans taken while subjects rest at warm room temperatures. However, BAT activity increases markedly after a 2-h exposure to cold 19°C (66.2°F)33, 34 or 16°C (61°F).35 At warm temperatures, BAT activity is variable, reported by one study as practically nonexistent,36 but others report 25% of patients with visible brown adipose37 to more than 80%.38 Given that some BAT is activated at room temperature and most is activated at 16°C, we selected 19°C as it is midway between the 16°C previously used to activate BAT maximally36 and the warmer temperature of 24°C that we used to minimize BAT activity.
Diet.
To decrease the possible visualization of BAT at 24°C (76°F), the HFLCPS diet provided 5 h prior to the scan prevented postprandial hyperglycemia by producing higher concentrations of free fatty acids relative to the concentration of glucose; avoiding competition with FDG uptake; and eliminating the interfering effect of BAT on PET scans when used to evaluate oncological conditions. Through the Randle cycle (glucose-fatty acid cycle), higher concentrations of fatty acids stimulate their oxidation thereby decreasing glucose uptake and flux through glycolysis. By exploiting the Randle cycle, fatty acid loading suppresses glucose metabolism in BAT,39 but not in the brain. Brain mitochondria have 125-fold lower levels of activity of 3-ketoacyl-coenzyme A thiolase than that found in other tissues, thereby minimizing activity of the Randle cycle in the brain during normoglycemia.40 Compared to a group of patients fasted for 24 h, the 5-h HFLCPS diet reduces the incidence of FDG-positive BAT per 100 patients from 6.3 to 2.8.41
PET Scans
Prior studies in humans and animals42, 43 detect BAT primarily in interscapular (rodents) or supraclavicular (humans) regions, but also between skin and muscle, and surrounding gonads and sympathetic ganglia. The measurement of cold-inducible BAT (ci-BAT) with FDG and PET/CT has limitations,44–46 so we used a novel approach and related software to estimate ci-BAT reliably, semi-quantitatively, and efficiently.31 We focused on supraclavicular BAT as the target region because it is the largest store in humans and easy to define and measure.
Analysis of Cortisol and Urocortin I
Blood samples were taken immediately prior to the injection of FDG for the PET scans. Serum cortisol, an adrenal hormone, was measured according to the manufacturer’s recommended protocol using a competitive enzyme immunoassay from R&D Systems (Minneapolis, MN) because it is released in response to stress and has been analyzed extensively in patients with FMS.47 Plasma concentrations of urocortin I were also measured as they have been reported to suppress the sympathetic nervous system,48 whose tonic resting activity is thought to be elevated in FMS.8 Acidic extraction of plasma used C18 Sep-Pak columns; plasma samples were assayed for human urocortin I according to the manufacturer’s recommended protocol using EIA kits from Phoenix Pharmaceuticals, Inc. (Burlingame, CA).
Statistical Analyses
All data in the figures are shown as the mean ± standard error of the mean (SEM). In all analyses, differences were considered statistically significant if the probability that they occurred because of chance alone was less than 5% (P < 0.05). Comparison of the means between two different groups (FMS and controls) was accomplished using an unpaired Student’s t-test whereas comparisons between two values obtained from the same group were made using a paired Student’s t-test. Unless otherwise indicated, all comparisons were two-tailed analyses in normally distributed groups. When statistically different, analyses are summarized in the text as follows (tdf = #, P = #). When more than two values were compared, data were analyzed using a one-way analysis of variance (ANOVA) followed by the post hoc test indicated in the corresponding figure legend. When significantly different, analyses are summarized in the text as follows (Fdf = #, P = #). Correlations were performed using Pearson’s correlation coefficient and considered significant when P was less than 0.05 without correction for multiple comparisons. All statistics were completed using GRAPHPAD-PRISM GRAPHPAD Software (La Jolla CA, USA).
RESULTS
Subjects
Of the 45 volunteers examined, the final subjects used for this study included 11 white female adults without significant medical illness and 13 white female patients with FMS Table 3). Seven controls and 7 FMS patients were excluded at the initial screening visit as they did not meet the inclusion criteria while 2 controls and 2 FMS patients declined to participate. The remaining 13 controls and 14 FMS patients received a single intervention but 3 subjects (2 controls and 1 FMS patient) discontinued prior to completing the second day of testing. The remaining 11 controls and 13 patients with FMS completed the protocol and their data were used for statistical analysis as these numbers met our a priori recruitment and retention criteria for success. The final study was intended as a pilot trial due to the high cost of data collection for each subject. For ethical reasons related to the stress to which subjects were exposed when the significance of the data was already apparent, a follow up study to increase the sample size was not deemed appropriate or necessary.
BMI, indicated in Table 2, did not differ between the two groups (controls = 24.8 kg/m2; FMS = 25.5 kg/m2; unpaired two-tailed Student’s t22 = 0.34, P = 0.735). Controls available were younger (mean = 26 years) than patients with FMS (mean = 37.2 years; unpaired two-tailed Student’s t22 = 3.38, P = 0.0003). However, except for systolic BP, there were no correlations between age and any of the parameters tested. In addition, none of the differences between values obtained in the warm verses cold environment correlated with age.
Diagnosis of FMS
Consistent with 2010 ACR criteria for FMS, we found that individuals suffering from FMS had widespread pain, non-restorative sleep 49 and a preoccupation with health.28 Patients were found to satisfy the diagnostic criteria if the Widespread Pain Index (WPI) was 7 or greater and the somatic symptom severity (SS) score was 5 or greater. If the WPI was 3–6, the SS score needed for a positive diagnosis was 9 or greater, as outlined in Table 1. Subjects were asked to shade areas on a diagram of the human body to indicate the areas where they felt pain. Widespread pain consistent with FMS was found to be reflected by a score of at least 5 of 19 areas designated by the ACR. Patient medical records were examined by a physician (CJT) to ensure that no other diagnosis of a painful or psychiatric condition could account for the occurrence of pain in each patient and to document the long-term nature of the pain complaints. PDSQ tests, used to exclude Axis I pathology and to characterize subjects, detected no major psychiatric disorders in our final cohorts.
Pain Sensitivity Tests
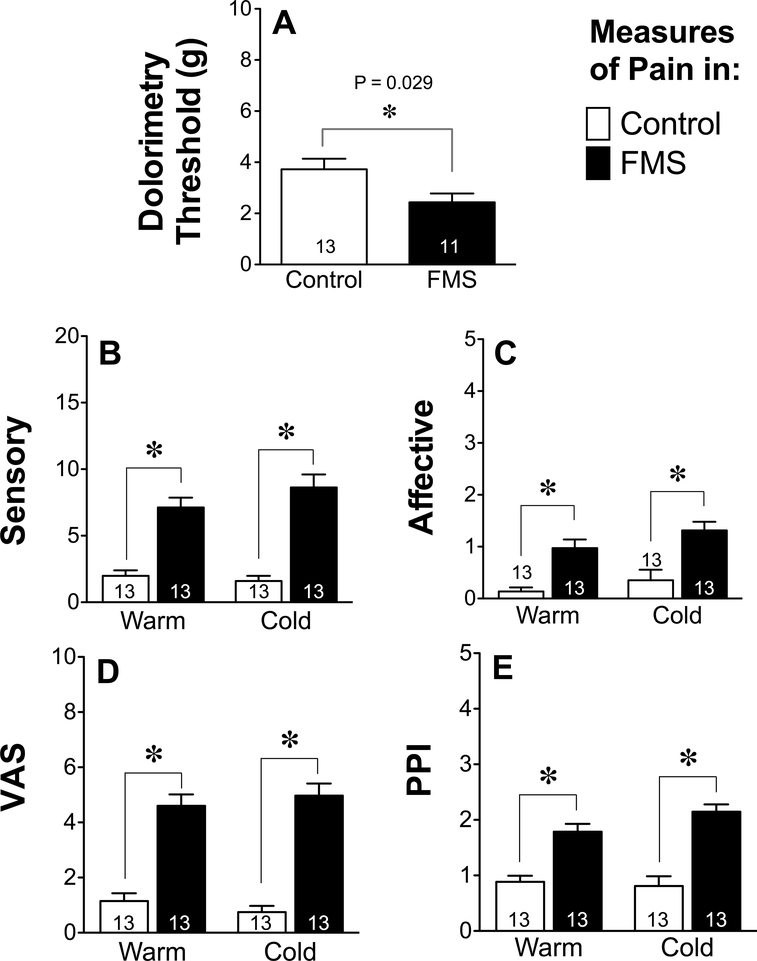
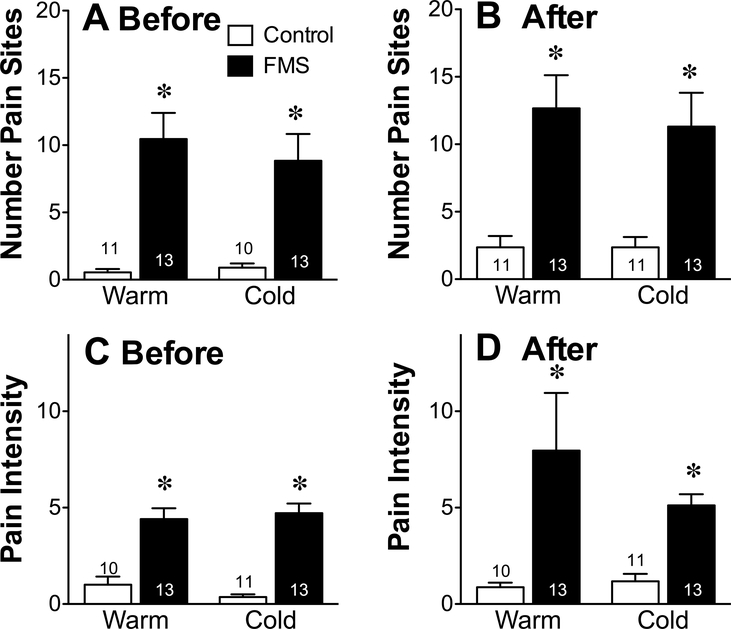
Exposure to cold had no effect on any of the pain measures used. Tender point sensitivity of FMS patients was greater than that of healthy controls when compared in either a warm or cold environment. Based on pressure algometry values, there was a consistent difference between FMS patients and healthy controls (t22 = 2.33, P = 0.029) when measured using dolorimetric testing (Fig. 1A), lower values reflecting a reduced threshold to tactile pain and a greater sensitivity to mechanical pain than controls. Consistent with dolorimetry test results, FMS patients also scored higher on both the MPQ sensory (Fig. 1B, Warm: t24 = 6.053, P < 0.0001, Cold: t24 = 6.65, P < 0.0001) as well as the affective pain scales than controls (Fig. 1C, Warm: t24 = 4.58, P = 0.0001, Cold: t23 = 3.64, P = 0.001). The distribution of pain, as indicated by the number of sites on their body (up to 45) that subjects considered painful before each temperature intervention (Fig. 2A), was scored by FMS patients to be much greater than that of healthy controls (ANOVA followed by Tukey’s multiple comparison test, F3,46 = 12.89, P < 0.0001) and these values remained unchanged within each group by the end of each intervention (Fig. 2B). The intensity of that pain, as indicated by an additional visual analog measure of pain from zero (no pain) to 10 (worst possible pain) (Fig. 2C) was scored by FMS patients to be much higher than that of healthy controls before exposure to each temperature intervention (F3,46 = 27.41, P < 0.0001), and these values also remained unchanged within each group by the end of each intervention (Fig. 2D).
Figure 1.
Pain-related measurements across groups (FMS, Controls) and temperatures (Warm, Cold) show FMS patients experience more pain than controls under both temperature conditions. The pain threshold was reflected in measures of the mean ± SEM dolorimetry values obtained in patients (A). These values did not differ in individuals or in groups when recorded before, during or after exposure to either hot or cold. The McGill pain questionnaire (MPQ) further characterized sensory (B) and affective pain (C) as well as results from the visual analog scale (VAS) (D) and present pain index (PPI) (E). Individual and group values of each parameter did not differ in response to exposure to either cold or warm temperatures. Values in panels A-E were therefore averaged for each individual and represent the average (± SEM) of all time-points of each group at each temperature and analyzed using an unpaired two-tailed Student’s t-test using a cutoff of P < 0.05 for significance.
Figure 2.
Distribution and intensity of pain. The mean ± SEM number of painful sites identified on a diagram of 45 possible areas of the body (A and B) and a VAS of that pain (C and D) were assessed before (A and C) and immediately after (B and D) exposure to the warm and cold temperatures. The values of healthy controls (Control) were compared to that of patients with FMS using a two-tailed unpaired Student’s t-test with a P < 0.05 as a cutoff for statistical significance, as indicated by the asterisk.
Physiologic Parameters
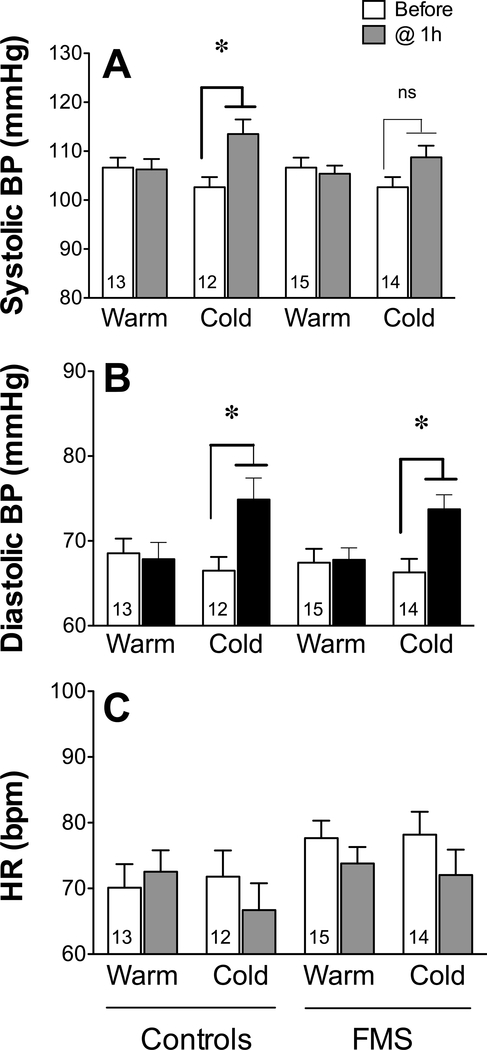
Both systolic and diastolic BP (Fig. 3A, 3B) and HR (Fig. 3C) were identical between the FMS and control groups before temperature challenge as well as after 1 h of exposure to warmth. Exposure to 1 h of cold increased systolic BP in healthy controls (paired two-tailed Student’s t-test t11 = 4.036, P = 0.002) but not in patients with FMS (t11 = 2.006, P = 0.07) at any time during and after the intervention. In contrast, by 1 h after cold exposure, the diastolic BP increased in both controls (t11 = 3.628, P = 0.004) and patients with FMS (t12 = 4.167, P = 0.0013) and remained elevated throughout.
Figure 3.
Physiologic changes before and during exposure to two ambient temperatures. Values represent the mean ± SEM systolic blood pressure (BP) (A); diastolic BP (B); and heart rate (HR) (C) before and 1 h after exposure to warm and cold environments in controls and in patients with FMS. All groups A-C were analyzed using paired two-tailed Student’s t-test with a cutoff of P < 0.05 for significance, as indicated by the asterisk (significant) or ‘ns’ (not significant).
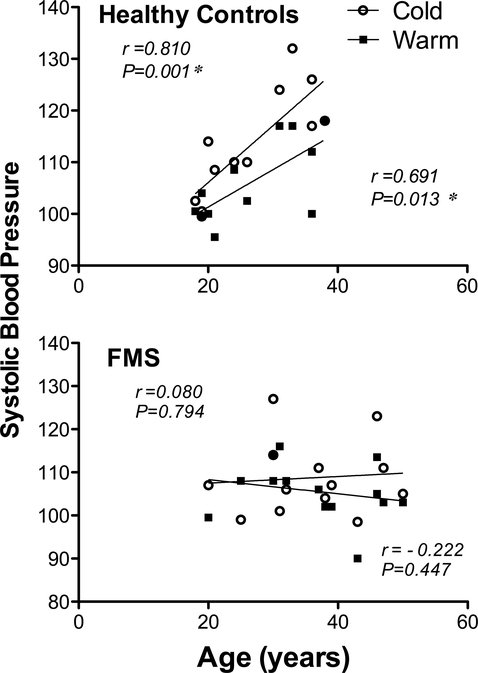
Because of the unavoidable difference in the mean age of our two groups, we also examined the relationship between BP and age. Systolic BP in control subjects increased with age when measured in either the warm (r = 0.691, P = 0.013) or cold (r = 0.810, P = 0.001) environment (Fig. 4) whereas FMS patients differed from controls by not correlating with age at either warm (r = −0.222, P = 0.447) or cold (r = 0.080, P = 0.794) temperatures (Fig. 4).
Figure 4.
Systolic BP after exposure to two ambient temperatures relative to subject age. Values represent age and systolic BP in controls and in patients with FMS 1 h after exposure to warm or cold environments. Pearson’s correlational analyses compared individual ages of subjects with their corresponding systolic BP values for each group 1 h after exposure to either warm or cold environment with a cutoff of P < 0.05 for significance, as indicated by the asterisk.
Circulating Stress Hormones
The concentrations of circulating cortisol and urocortin in healthy controls did not differ from those of FMS patients after exposure to a warm environment. In addition, circulating cortisol did not differ after exposure to 3.5 h of a cold environment (meancontrol = 69 ± 16; meanFMS = 49 ± 7, Student’s unpaired two-tailed t-test t16 = 0.946, P = 0.358). Similarly, the concentration of urocortin did not differ after exposure to a cold environment (meancontrol = 6.5 ± 0.5; meanFMS = 5.6 ± 0.8, t14 = 1.003, P = 0.333).
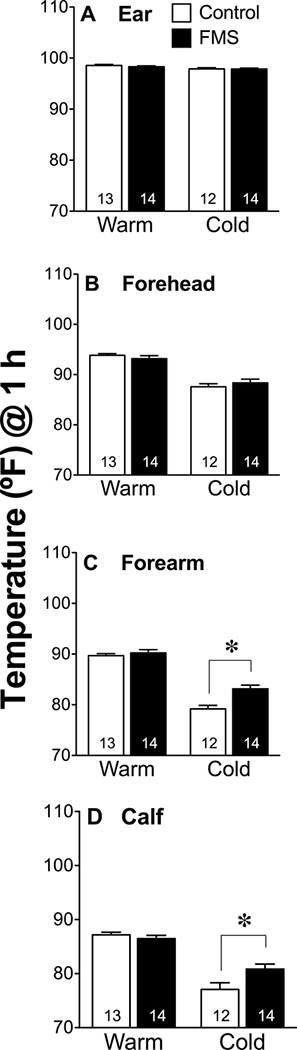
Body Temperatures
Body temperatures did not differ between groups prior to cold exposure and all subjects had lower skin temperatures on their extremities (arm and calf) after 3 h of cold (Fig. 5). Core body temperatures and forehead temperatures (Fig. 5A, 5B) did not decrease in response to cold exposure and did not differ between groups. However, after cold exposure distal skin temperatures of patients with FMS were not as low as controls on the arm (Fig. 5C, t23 = 3.84, P = 0.0008) and on the calf (Figure 5D, t23 = 2.475, P = 0.021). Only 4 out of 11 healthy control participants reported shivering in response to the cold whereas 12 out of 13 FMS patients reported shivering during the cold exposure. The average room temperatures recorded during testing of FMS patients (Cold = 61.7°F ± 0.3; Warm = 75.7°F ± 0.2, n = 13) did not differ from that during their respective test of healthy controls (Cold = 61.6°F ± 0.3; Warm = 75.8°F ± 0.3, n = 12, unpaired, two-tailed Student’s t-test P < 0.05).
Figure 5.
Skin temperatures 1 h after warm and cold exposures. Values indicate the mean ± SEM temperature of skin in the ear (A), on the forehead (B), over the forearm (C), and on the calf (D) in controls and patients with FMS. Groups were compared using an unpaired, two-tailed Student’s t-test with a cutoff of P < 0.05 for significance, as indicated by the asterisk.
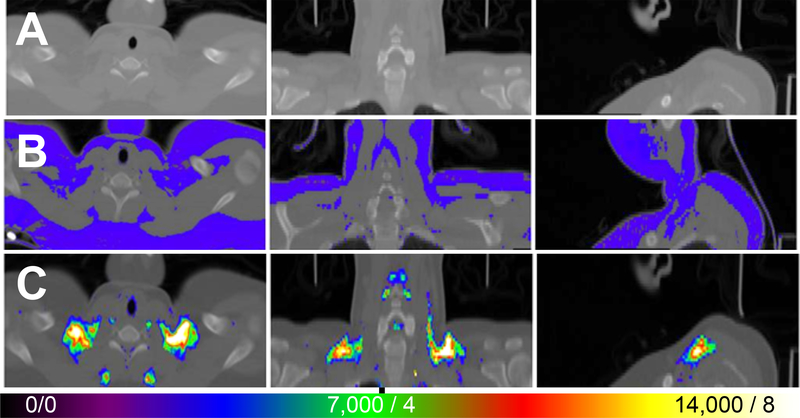
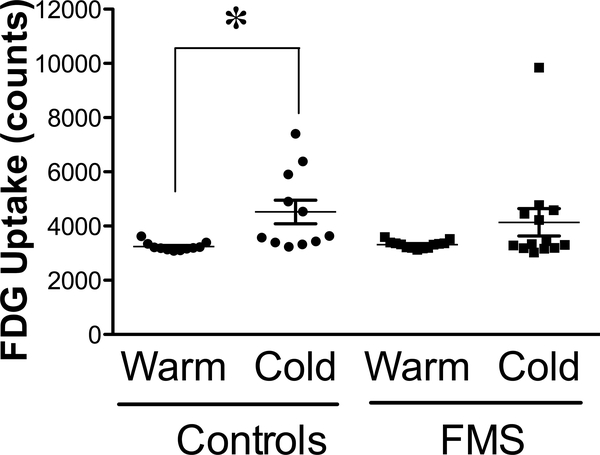
Brown Adipose Tissue Activity
Based on the importance of BAT during responses to stress, we examined ci-BAT using FDG PET/CT to explore whether the cold intervention induced thermogenesis that was sufficient to increase BAT activity in healthy control individuals and thus explore possible differences between thermoregulation in FMS and control groups following cold exposure (Fig. 6A-C). Under warm conditions, BAT activity was consistently minimal and similar in both control and FMS subjects (Fig. 7). After cold exposure, BAT activity was much more variable within and between groups, especially within the values for FMS patients, where there was also a tendency to take up less FDG than controls despite a large outlier that was not removed from the data [Wilcoxon Mann-Whitney test: n1 = 10; n2 = 12; U = 86.0; P (two-tailed) = 0.09]. BAT activity of FMS patients was not increased by exposure to cold (two-tailed paired Student’s t-test t12 = 1.644, P = 0.126), whereas that of healthy control subjects was (t10 = 2.909, P = 0.002). The volume of ci-BAT was not significantly different across groups and FDG uptake in a warm environment was the same across groups, reflecting good reliability.
Figure 6.
FDG uptake in PET/CT of a healthy subject at cool temperature. CT scan shows radiodensity of neck and upper chest to just below clavicle (A). Template (blue) indicates all fat superimposed on CT (B). Template was based on radiodensity difference of fat from non-fat tissues (e.g., muscle, lung). FDG uptake in PET/CT scan of control subject resting for 2.5 h in cold (62°F or 16.8°C) (C). Color scale indicates greater uptake with hotter colors. The threshold was set at 3,500 counts (i.e., blue, normalized to whole brain uptake of 20,000) or SUV = 2 based on body weight. Maximum or greater uptake indicated by white color indicating 14,000 counts or SUV 8.
Figure 7.
Quantification of BAT activity after exposure to cool (62°F or 16.8°C) and warm (76°F or 24.4°C) ambient temperatures, as visualized using methods illustrated in Figure 5. Values reflect the mean ± SEM of FDG uptake in BAT in healthy controls (n = 10) and in patients with FMS (n = 12). One outlier occurred in the FMS patients in the cool condition and was retained for statistical analysis. Data were compared within groups after exposure to warm and to cold environments using paired two-tailed Student’s t-test with a cutoff of P < 0.05 for significance, as indicated by the asterisk (significant) or ‘ns’ (not significant).
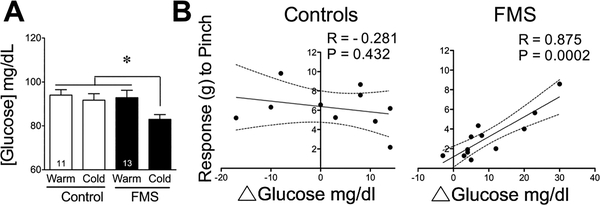
Blood Glucose
Circulating blood glucose did not differ between groups prior to cold exposure or after 2 h in a warm environment (Fig. 8A). Although all subjects had blood glucose concentrations within the normal range, after exposure to 2 h of cold, patients with FMS had lower blood glucose values than control subjects (F3,40 = 3.392, P = 0.027). Although gabapentinoids may decrease blood glucose, comparison of FMS patients taking them to those who do not, indicated that their mean blood glucose did not differ due to gabapentinoids. When the magnitude of individual differences in circulating glucose were compared to the intensity of their corresponding pain values measured using dolorimetry, there was a significant and positive correlation (Fig. 8B) (Pearson’s correlation coefficient, r = 0.875, F1,10 = 32.6, P = 0.0002) between the pain threshold and the ability to maintain a normal circulating glucose in the face of cold in FMS patients. No such correlation was observed in healthy controls (r = −0.281, F1,8 = 0.684, P = 0.432).
Figure 8.
Circulating blood glucose and its relationship to dolorimetric measures of pain threshold. Values depict the mean ± SEM concentration of glucose measured in blood samples taken immediately prior to CT scans of both groups after exposure to warm and cold environments (A). These values were compared using ANOVA using a cutoff of P < 0.05 for significance, as indicated by the asterisk. Individual changes in values for glucose (B) reflect the difference in concentrations between warm and cold environments. Using Pearson’s correlational analyses, these glucose values were compared within groups to their corresponding pain assessments obtained when tested by dolorimetric analyses (as used in figure 1). Correlation coefficients (R) and P values were calculated for each group with a P < 0.05 as the cutoff for significance.
DISCUSSION
This study tests the hypothesis that female patients with FMS differ from healthy female controls in their sympathetic responses to stress, a fact that may unmask important biomarkers and factors that contribute to the etiology of FMS. A cold stressor was used to elicit sympathetic activation. After verifying the ability of temperature to influence these sympathetic responses in healthy controls and comparing their responses to those in patients with FMS, we found that while BP, skin temperature, circulating glucose and BAT activity did not differ between the two groups in a warm environment, they all differed in relatively mild cold stress. These responses suggest that readily straightforward and simple measures of sympathetic activity in response to stress reflect differences between even small groups of patients compared to controls, and that cold stress might be exploited to explore other parameters potentially related to the etiology of FMS.
Subjects
The MPQ and pressure algometry confirmed that the pain experienced by our cohort of FMS patients at the time of testing was widespread, a major criterion for the diagnosis of FMS. In addition, the sleep disturbances and non-restorative sleep reported by our FMS patients are characteristic of patients with FMS.49 The results from PDSQ and POMS survey in FMS patients reflect a preoccupation with health28 and fatigue49 that characterize this condition. Together these data are consistent with a diagnosis of FMS based on the 2010 ACR criteria.
We hypothesized that stress might exacerbate symptoms of FMS, unmasking potential research-relevant biomarkers of FMS and parameters linked to the etiology of this condition. Although we found that exposure to a cold environment did not potentiate their reported pain, it did influence several responses differently in patients than in healthy controls, providing several convergent lines of evidence that sympathetic responses to cold stress are compromised in patients with FMS.
Pain
Prior to temperature interventions, FMS patients were more sensitive to pain than controls regardless of the instrument used to assess pain sensitivity. However, exposure to the stress of a colder environment elicited no additional change in pain thresholds, pain intensity, or pain distribution. It is unlikely that the measures of pain used were limited by having reached a ceiling response in warm conditions, thereby obscuring increases in hyperalgesia, as we rarely recorded maximal responses. Stress may simply make patients more aware of their pain, increasing their discomfort. Alternatively, the time-interval studied may not be long enough to capture post stress-induced increases in pain sensitivity. Additional studies may resolve this by expanding the time-course of pain measurements.
Blood Pressure (BP)
In a warm environment, BP did not differ between patients with FMS and healthy controls. After exposure to cold, systolic BP increased in controls but not in patients with FMS. If BP responses in the older FMS patients were influenced by age, one would expect BP to be higher rather than lower than those in the younger control group. Instead, FMS patients had an identical mean systolic BP as controls at the warmer temperature and lower mean systolic BP than controls at the colder temperature, the opposite of what would be expected if the data were the result of the age differences. Diastolic pressure did not differ between groups at either temperature. These data provide one line of evidence that stress-induced sympathetic activity in patients with FMS is less than that in controls. This is somewhat paradoxical as sympathetic tone has been reported to be elevated in patients with FMS in the absence of intentional stress8 in spite of normal concentrations of circulating catecholamines, like norepinephrine and epinephrine.15 However, consistent with our data, insulin-induced stress increases epinephrine more in healthy controls than in patients with FMS,15 supporting less rather than more sympathetic activity in response to stress.
Brown Adipose Tissue
Not only is FMS more common in women than men, women also have more BAT, suggesting a greater thermogenic capacity.36 BAT is usually not active in a warm environment unless subjected to stress or overeating. In keeping with this, BAT activity did not vary greatly within groups or between groups when measured in a warm environment and a HFLCPS diet, conditions that minimize thermogenesis. Thus, BAT is not perceptibly enhanced by the tonically heightened sympathetic tone previously reported in unstressed FMS patients.50 In contrast, cold exposure plus a LFLC diet, conditions that together enhance and permit maximal BAT activity, generate heat by uncoupling oxidative metabolism from that producing ATP. This was reflected in healthy controls by an increased uptake of FDG into BAT reflecting sympathetically-induced thermogenesis. However, these same conditions failed to increase BAT activity in patients with FMS, consistent with an attenuated sympathetic response to stress.
Thermogenesis is decreased by feedback inhibition transmitted by primary afferent C-fibers projecting from BAT to the spinal cord where they release substance P. This attenuates the generation of heat in BAT51 and initiates cooling responses.52 Even in the absence of intentional stress, patients with FMS have elevated concentrations of substance P in their cerebrospinal fluid.53, 54 Although this is widely attributed to release from nociceptors, its origin is unknown as nociceptive and thermoregulatory pathways converge at several sites, including the spinal cord, enabling them to influence each other.55 As a result, substance P may negatively impact thermogenesis while positively impacting pain sensitivity. Nerve growth factor (NGF), a neurotrophic factor that enhances synthesis of substance P and sensitizes nociceptors, also supports growth of sympathetic nerves. It is noteworthy that NGF is increased in the CSF of patients with FMS,56 potentially increasing pain and decreasing thermogenesis by its influence on substance P.
Skin Temperature
Ear and forehead skin temperatures did not differ between groups at either temperature. However, when subjected to cold, skin temperature on distal appendages (arm and calf) of all subjects decreased, but less so in FMS patients. This exposes different abilities of the two groups to defend against cold. Higher skin temperatures in patients with FMS during cold cannot be attributed to greater thermogenesis,57 given the compromised response of BAT to cold. A greater percentage of FMS patients shivered than controls, leading to heat generation. However, the major regulator of skin temperature is blood flow. Circulation in skin is regulated by arteriole-venule shunts that divert the flow of blood from capillaries to venules. Sympathetic activity constricts these shunts to prevent heat loss whereas activation of sensory nerves by lowered surface temperatures induces dilation to maintain blood flow. Our results suggest that FMS patients have either a less robust sympathetic response to stress (less vasoconstriction), consistent with our BP data, and/or greater sensory nerve activity (more vasodilatation), consistent with the greater density of sensory innervation of arteriole-venule shunts in glabrous skin of patients with FMS.58
Blood glucose
Sympathetic activity transiently increases circulating blood glucose. The heightened sympathetic tone of FMS patients at rest does not influence resting blood glucose differently in FMS patients than in controls. After exposure to cold, blood glucose was lower in the FMS group than controls, albeit never in the range that constitutes hypoglycemia. Insufficient sympathetic responses can explain this inability to maintain circulating glucose during stress. Differences in glucose cannot explain the PET results because the relatively lower unlabeled glucose concentration in FMS patients would compete less with radiotracer uptake. Less competition would increase, rather than decrease, the uptake of FDG in BAT. Thus, FMS patients had less rather than more uptake of label in BAT depots despite, rather than because of, their lower circulating glucose.
Correlation between Circulating Glucose and Pain
Although the degree of patients’ hyperalgesia did not increase in response to stress, their nociceptive sensitivity measured by algometry correlated well with differences in the concentration of glucose in warm compared to cold conditions. Neither pain sensitivity nor blood glucose correlated with any other parameter measured. Greater decreases in blood glucose corresponded to lower sensitivities to pain, suggesting that it is the maintenance of higher glucose values during stress that is linked to hyperalgesia in patients. Future studies must distinguish whether causal relationships exist.
Sympathetic Regulation
Circulating cortisol did not differ due to cold, in agreement with the reported variability of glucocorticoids in FMS.59 Instead, insufficient sympathetic activity in patients with FMS during cold likely results from decreased adrenergic activity (epinephrine) like that in response to insulin-induced stress.15 Impaired adrenal responses to stress may account for abnormal BP, skin temperature, BAT activity and circulating glucose in stressed patients. Deficiencies in epinephrine release may be due to less activation of the adrenal or insufficient phenylethanolamine-N-methyl transferase (PMNT), the synthesizing enzyme for epinephrine in the cytosol of adrenal medullary cells.60–62 Impaired sympathetic responses to cold in FMS patients may seem at odds with their greater shivering. However, shivering-induced and non-shivering-induced thermogenesis are separate systems as shivering follows the somatomotor system and not the sympathetic pathway.63
Limitations
This study’s sample size is small, and almost half the subjects enrolled either did not meet all criteria after initial screening, declined to participate, or did complete the entire study. This factor limits generalizability. The large number of patient dropouts after initial enrollment will need consideration for future work. The control subjects available were younger than patients with FMS. In spite of this, we found no correlations between age and any of the parameters measured in these studies. In addition, none of the differences between study values obtained in the warm verses cold environment correlated with age. Together this suggests that the difference in age did not contribute to differences between FMS and control groups. This study did not use advanced techniques for cooling such as whole-body, ice-water, circulating suits to more precisely control the cooling stress; some degree of shivering occurred. The limitations of FDG PET for the study of BAT metabolism was reviewed previously.31
CONCLUSIONS
In healthy controls, cold stress increased BP, BAT activity, maintained circulating glucose and decreased distal skin temperatures. In contrast, FMS patients had no increase in systolic BP or BAT activity, decreased circulating glucose, and higher distal skin temperatures than healthy controls. Differences in these characteristics were not present at warm temperatures. This provides four convergent lines of evidence suggesting attenuated sympathetic responses to stress in FMS patients. While sympathetic tone may be excessive in FMS patients at rest, our data indicate that cold stress recruits less sympathetic activity in these patients than in controls. In future studies of FMS, stress may be useful to unmask elements related to its etiology and simultaneously confirm these easily measured clinical parameters as potential research biomarkers of FMS.
Supplementary Material
Acknowledgments
Funding Sources that require acknowledgment
This work was supported by a grant to AAL and JVP from NIH from the National Institutes on Arthritis and Musculoskeletal and Skin Diseases [AR056092], to JVP from the Veterans Administration [5I01CX000501], to JVP from the Clinical and Translational Science Award (CTSA) UL1TR000114, the Department of Veterans Affairs, and to AAL, JVP and JDP from the University of Minnesota Graduate School.
Additional Acknowledgments
We thank our subjects for their generosity. All human studies were approved and monitored by the Institutional Review Board of the University of Minnesota (IRB Study #1007M85352) in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects gave written informed consent prior to their inclusion in the study. The scripts for the analysis of PET scans are available upon request from Joel T. Lee.
References Cited
- 1.Queiroz LP. Worldwide epidemiology of fibromyalgia. Current pain and headache reports 2013;17:356. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P and et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–72. [DOI] [PubMed] [Google Scholar]
- 3.Fischer S, Doerr JM, Strahler J, Mewes R, Thieme K and Nater UM. Stress exacerbates pain in the everyday lives of women with fibromyalgia syndrome--The role of cortisol and alpha-amylase. Psychoneuroendocrinology 2016;63:68–77. [DOI] [PubMed] [Google Scholar]
- 4.D’Arcy Y, Kraus S, Clair A and Kiley D. Fibromyalgia: Timely diagnosis and treatment options. Nurse Pract 2016;41:37–43. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Lavin M. Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia. Arthritis Res Ther 2007;9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaeroy H, Qiao ZG, Morkrid L and Forre O. Altered sympathetic nervous system response in patients with fibromyalgia (fibrositis syndrome). J Rheumatol 1989;16:1460–5. [PubMed] [Google Scholar]
- 7.Abdallah CG and Geha P. Chronic Pain and Chronic Stress: Two Sides of the Same Coin? Chronic Stress (Thousand Oaks) 2017;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez-Martinez LA, Mora T, Vargas A, Fuentes-Iniestra M and Martinez-Lavin M. Sympathetic nervous system dysfunction in fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: a review of case-control studies. J Clin Rheumatol 2014;20:146–50. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Lavin M, Vidal M, Barbosa RE, Pineda C, Casanova JM and Nava A. Norepinephrine-evoked pain in fibromyalgia. A randomized pilot study [ISRCTN70707830]. BMC Musculoskelet Disord 2002;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeschonneck M, Grohmann G, Hein G and Sprott H. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford) 2000;39:917–21. [DOI] [PubMed] [Google Scholar]
- 11.Bengtsson A and Bengtsson M. Regional sympathetic blockade in primary fibromyalgia. Pain 1988;33:161–7. [DOI] [PubMed] [Google Scholar]
- 12.Juuso P, Skar L, Olsson M and Soderberg S. Living with a double burden: Meanings of pain for women with fibromyalgia. Int J Qual Stud Health Well-being 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith BW, Tooley EM, Montague EQ, Robinson AE, Cosper CJ and Mullins PG. Habituation and sensitization to heat and cold pain in women with fibromyalgia and healthy controls. Pain 2008;140:420–8. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyori M and Ueda H. Prolonged gabapentin analgesia in an experimental mouse model of fibromyalgia. Mol Pain 2008;4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler GK, Kinsley BT, Hurwitz S, Mossey CJ and Goldenberg DL. Reduced hypothalamic-pituitary and sympathoadrenal responses to hypoglycemia in women with fibromyalgia syndrome. Am J Med 1999;106:534–43. [DOI] [PubMed] [Google Scholar]
- 16.Haman F, Peronnet F, Kenny GP, Massicotte D, Lavoie C, Scott C and Weber JM. Effect of cold exposure on fuel utilization in humans: plasma glucose, muscle glycogen, and lipids. J Appl Physiol (1985) 2002;93:77–84. [DOI] [PubMed] [Google Scholar]
- 17.Charkoudian N Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc 2003;78:603–12. [DOI] [PubMed] [Google Scholar]
- 18.Rothwell NJ and Stock MJ. Effects of denervating brown adipose tissue on the responses to cold, hyperphagia and noradrenaline treatment in the rat. J Physiol 1984;355:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannon B and Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews 2004;84:277–359. [DOI] [PubMed] [Google Scholar]
- 20.Kuroshima A, Habara Y, Uehara A, Murazumi K, Yahata T and Ohno T. Cross adaption between stress and cold in rats. Pflugers Arch 1984;402:402–8. [DOI] [PubMed] [Google Scholar]
- 21.Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T and Saito M. Recruited browin adipose tissue as an antiobesity agent in humans. J Clin Invest 2013;123:3404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanssen MJ, van der Lans AA, Brans B, Hoeks J, Jardon KM, Schaart G, Mottaghy FM, Schrauwen P and van Marken Lichtenbelt WD. Short-term Cold Acclimation Recruits Brown Adipose Tissue in Obese Humans. Diabetes 2016;65:1179–89. [DOI] [PubMed] [Google Scholar]
- 23.Hoeger Bement MK, Weyer A, Hartley S, Drewek B, Harkins AL and Hunter SK. Pain perception after isometric exercise in women with fibromyalgia. Arch Phys Med Rehabil 2011;92:89–95. [DOI] [PubMed] [Google Scholar]
- 24.Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EB, van der Lans AA, Broeders EP, Mottaghy FM, Schrauwen P and van Marken Lichtenbelt WD. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes (Lond) 2015;39:1696–702. [DOI] [PubMed] [Google Scholar]
- 25.Sosa-Reina MD, Nunez-Nagy S, Gallego-Izquierdo T, Pecos-Martin D, Monserrat J and Alvarez-Mon M. Effectiveness of Therapeutic Exercise in Fibromyalgia Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. BioMed research international 2017;2017:2356346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busch AJ, Webber SC, Brachaniec M, Bidonde J, Bello-Haas VD, Danyliw AD, Overend TJ, Richards RS, Sawant A and Schachter CL. Exercise therapy for fibromyalgia. Current pain and headache reports 2011;15:358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB and Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–10. [DOI] [PubMed] [Google Scholar]
- 28.McDermid AJ, Rollman GB and McCain GA. Generalized hypervigilance in fibromyalgia: evidence of perceptual amplification. Pain 1996;66:133–44. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman M and Sheeran T. Screening for principal versus comorbid conditions in psychiatric outpatients with the Psychiatric Diagnostic Screening Questionnaire. Psychol Assess 2003;15:110–4. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman M The psychiatric diagnostic screening questionnaire: manual. Western Psychological Services, 2002. [Google Scholar]
- 31.Pardo JV, Lee JT, Larson RC, Thuras P and Larson AA. Automated quantitation of cold-inducible human brown adipose tissue with FDG PET/CT with application to fibromyalgia. Am J Nucl Med Mol Imaging 2017;7:24–32. [PMC free article] [PubMed] [Google Scholar]
- 32.Shacham S A shortened version of the Profile of Mood States. J Pers Assess 1983;47:305–6. [DOI] [PubMed] [Google Scholar]
- 33.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y and Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009;58:1526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zukotynski KA, Fahey FH, Laffin S, Davis R, Treves ST, Grant FD and Drubach LA. Constant ambient temperature of 24 degrees C significantly reduces FDG uptake by brown adipose tissue in children scanned during the winter. Eur J Nucl Med Mol Imaging 2009;36:602–6. [DOI] [PubMed] [Google Scholar]
- 35.Lichtenbelt WDV, Vanhommerig JW, Smulders NM, Drossaerts JMAFL, Kemerink GJ, Bouvy ND, Schrauwen P and Teule GJJ. Cold-Activated Brown Adipose Tissue in Healthy Men. New England Journal of Medicine 2009;360:1500–1508. [DOI] [PubMed] [Google Scholar]
- 36.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM and Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobert N, Menzel C, Hamscho N, Wordehoff W, Kranert WT and Grunwald F. Atypical thoracic and supraclavicular FDG-uptake in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Q J Nucl Med Mol Imaging 2004;48:33–8. [PubMed] [Google Scholar]
- 38.Rousseau C, Bourbouloux E, Campion L, Fleury N, Bridji B, Chatal JF, Resche I and Campone M. Brown fat in breast cancer patients: analysis of serial (18)F-FDG PET/CT scans. Eur J Nucl Med Mol Imaging 2006;33:785–91. [DOI] [PubMed] [Google Scholar]
- 39.Frayn KN. The glucose-fatty acid cycle: a physiological perspective. Biochem Soc Trans 2003;31:1115–9. [DOI] [PubMed] [Google Scholar]
- 40.Yang SY, He XY and Schulz H. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J Biol Chem 1987;262:13027–32. [PubMed] [Google Scholar]
- 41.Williams G and Kolodny GM. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. AJR Am J Roentgenol 2008;190:W151–6. [DOI] [PubMed] [Google Scholar]
- 42.Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A and Kolodny GM. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab 2015;21:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauwens M, Wierts R, van Royen B, Bucerius J, Backes W, Mottaghy F and Brans B. Molecular imaging of brown adipose tissue in health and disease. Eur J Nucl Med Mol Imaging 2014;41:776–91. [DOI] [PubMed] [Google Scholar]
- 44.Borga M, Virtanen KA, Romu T, Leinhard OD, Persson A, Nuutila P and Enerback S. Brown adipose tissue in humans: detection and functional analysis using PET (positron emission tomography), MRI (magnetic resonance imaging), and DECT (dual energy computed tomography). Methods Enzymol 2014;537:141–59. [DOI] [PubMed] [Google Scholar]
- 45.Gifford A, Towse TF, Walker RC, Avison MJ and Welch EB. Human brown adipose tissue depots automatically segmented by positron emission tomography/computed tomography and registered magnetic resonance images. Journal of visualized experiments : JoVE 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruth MR, Wellman T, Mercier G, Szabo T and Apovian CM. An automated algorithm to identify and quantify brown adipose tissue in human 18F-FDG-PET/CT scans. Obesity (Silver Spring) 2013;21:1554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riva R, Mork PJ, Westgaard RH, Ro M and Lundberg U. Fibromyalgia syndrome is associated with hypocortisolism. Int J Behav Med 2010;17:223–33. [DOI] [PubMed] [Google Scholar]
- 48.Charles CJ, Jardine DL, Nicholls MG, Rademaker MT and Richards AM. Urocortin 1 exhibits potent inhibition of cardiac sympathetic nerve activity in conscious sheep. J Hypertens 2008;26:53–60. [DOI] [PubMed] [Google Scholar]
- 49.Moldofsky H The significance, assessment, and management of nonrestorative sleep in fibromyalgia syndrome. CNS Spectr 2008;13:22–6. [DOI] [PubMed] [Google Scholar]
- 50.Sarzi-Puttini P, Atzeni F, Diana A, Doria A and Furlan R. Increased neural sympathetic activation in fibromyalgia syndrome. Ann N Y Acad Sci 2006;1069:109–17. [DOI] [PubMed] [Google Scholar]
- 51.Hori T. Capsaicin and central control of thermoregulation. Pharmacol Ther 1984;26:389–416. [DOI] [PubMed] [Google Scholar]
- 52.Dib B Thermoregulatory behaviour induced by intrathecal injection of substance P in the rat. Eur J Pharmacol 1987;133:147–53. [DOI] [PubMed] [Google Scholar]
- 53.Russell IJ, Orr MD, Littman B, Vipraio GA, Alboukrek D, Michalek JE, Lopez Y and MacKillip F. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum 1994;37:1593–601. [DOI] [PubMed] [Google Scholar]
- 54.Vaeroy H, Helle R, Forre O, Kass E and Terenius L. Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: new features for diagnosis. Pain 1988;32:21–6. [DOI] [PubMed] [Google Scholar]
- 55.Larson AA, Pardo JV and Pasley JD. Review of overlap between thermoregulation and pain modulation in fibromyalgia. The Clinical journal of pain 2014;30:544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giovengo SL, Russell IJ and Larson AA. Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia. J Rheumatol 1999;26:1564–9. [PubMed] [Google Scholar]
- 57.Hofmann WE, Liu X, Bearden CM, Harper ME and Kozak LP. Effects of genetic background on thermoregulation and fatty acid-induced uncoupling of mitochondria in UCP1-deficient mice. J Biol Chem 2001;276:12460–5. [DOI] [PubMed] [Google Scholar]
- 58.Albrecht PJ, Hou Q, Argoff CE, Storey JR, Wymer JP and Rice FL. Excessive peptidergic sensory innervation of cutaneous arteriole-venule shunts (AVS) in the palmar glabrous skin of fibromyalgia patients: implications for widespread deep tissue pain and fatigue. Pain Med 2013;14:895–915. [DOI] [PubMed] [Google Scholar]
- 59.Neeck G and Crofford LJ. Neuroendocrine perturbations in fibromyalgia and chronic fatigue syndrome. Rheum Dis Clin North Am 2000;26:989–1002. [DOI] [PubMed] [Google Scholar]
- 60.Wurtman RJ, Pohorecky LA and Baliga BS. Adrenocortical control of the biosynthesis of epinephrine and proteins in the adrenal medulla. Pharmacol Rev 1972;24:411–26. [PubMed] [Google Scholar]
- 61.Wright A and Jones IC. Chromaffin tissue in the lizard adrenal gland. Nature 1955;175:1001–2. [DOI] [PubMed] [Google Scholar]
- 62.Coupland RE. On the morphology and adrenaline-nor-adrenaline content of chromaffin tissue. J Endocrinol 1953;9:194–203. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura K and Morrison SF. Central efferent pathways for cold-defensive and febrile shivering. J Physiol 2011;589:3641–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.