Abstract
Adverse life events are associated with a constellation of negative health outcomes. Theory and research suggest that the hypothalamic-pituitary-adrenal (HPA) axis acts as one mechanism connecting adverse experiences with negative health outcomes. However, this relation is complicated by the potential for adversity to be associated with both hyperactivity and hypoactivity of the HPA axis, as assessed in both animal and human studies. Over the past decade, methodological advances have enabled the sampling of cortisol stored in hair, which provides a marker of HPA axis activity over a several-month period. The present meta-analysis included 28 studies to assess the strength and direction of the relation between adverse experiences and hair cortisol levels. Analyses were conducted using multilevel modeling (MLM) to quantify the magnitude of effects and mixture modeling to identify distinct subgroups of studies. Results of MLM analyses indicated that the overall effect size was small but significant d=0.213, 95% CI [0.034, 0.397]. There was also significant between-study variance (τ=0.155, 95% CI [0.065, 0.367]). Mixture modeling to identify distinct classes of studies based on effect size and direction resulted in a 2-class model: The first class included four studies with an overall negative and moderate effect size (d=−0.478, 95% CI [−0.639, −0.318]), and the second class included the remaining 24 studies with an overall positive and significant, albeit small, effect size (d=0.141, 95% CI [0.084, 0.199]). Moderator analyses indicated that the strength and direction of the association between adversity and hair cortisol were moderated by features of the adversity exposure (e.g., type of adversity, timing of adversity), characteristics of the samples (e.g., clinical status, racial distribution), and features of the publication (e.g., publication type, geographic region of study). The findings refine our understanding of the long-term impact of adversity on dysregulation of the HPA axis, particularly as reflected in hair cortisol measures.
Keywords: Hair cortisol, Chronic stress, Adversity, Adverse experiences, Trauma, Meta-analysis
1. Introduction
Adverse life events are associated with a plethora of negative mental and physical health outcomes throughout the lifespan (Gallagher et al., 2009; Reynolds, 2013; Turner-Cobb, 2005). The hypothalamic-pituitary-adrenal (HPA) axis is a primary stress system that functions as a mechanism linking stressful life events and adverse health outcomes (e.g., Aguilera, 2011). The brain and body, as well as the stress systems that comprise them, are designed to adaptively respond to internal and external threats. The HPA axis does so by releasing cortisol in response to acute threat and down-regulating the cortisol response, through a negative feedback loop, when no longer under acute treat (Sapolsky et al., 2000). Deleterious health effects are caused by dysregulation of the HPA axis, which can include intense or chronic over-activation or under-activation (Chrousos, 2009), known as allostatic overload (McEwen, 1998).
Until recently, assessing chronic HPA activity was difficult, given that cortisol could only be assessed from saliva, blood serum, or urine, measures that indicate acute (i.e., serum, saliva) or short-term (i.e., 12–24 h; urine) cortisol alterations. Further, the aforementioned means of cortisol assessment are subject to great variability due to circadian rhythms (Spiga et al., 2014), individual differences in state arousal (Dickerson and Kemeny, 2004), and food consumption (Gibson et al., 1999). Recent methodological advances have enabled the sampling of cortisol in hair particles, providing a reliable retrospective measure of HPA axis activity over several months (Russell et al., 2012; Stalder and Kirschbaum, 2012). The average rate of hair growth rate is 1 cm/ month; thus, 3 cm of hair corresponds to hair grown over a three-month period (Wennig, 2000). The process of hair cortisol analyses involves cutting hair from the scalp, mincing the hair, extracting cortisol by methanol, and analyzing cortisol concentrations using either immune assays or liquid chromatography-mass spectrometry (LC/ MS) (Gow et al., 2010; Manenschijn et al., 2011). Although a relatively novel approach, hair cortisol has shown high test-retest reliability (Stalder and Kirschbaum, 2012) and has been validated against salivary (Xie et al., 2011) and urinary cortisol (Sauve et al., 2007). Moreover, the confounders noted above that influence saliva, blood serum, and urine measures do not affect hair cortisol.
The experience of adversity, including maltreatment (abuse and neglect), domestic violence, and exposure to traumatic events, such as accidents, war/combat, and natural disasters, can have an enduring impact on health. These adverse life experiences are associated with a range of negative mental and physical health outcomes, including depression, PTSD, suicide, non-suicidal self-injury, and cardiovascular disease (Chartier et al., 2010; Galea et al., 2005; Kaess et al., 2013; Steptoe and Kivimäki, 2013). Adverse experiences are also associated with atypical cortisol activity (Steudte-Schmiedgen et al., 2016). However, the direction of association between adversity and cortisol has varied, with some studies suggesting that those who have experienced adversity have higher salivary cortisol reactivity (Harkness et al., 2011), as well as higher diurnal salivary cortisol levels (Dozier et al., 2006), while other studies have reported lower salivary cortisol reactivity (Ouellet-Morin et al., 2011) and lower diurnal salivary cortisol levels (Alink et al., 2012).
Similarly, individuals who experience PTSD symptoms following exposure to a traumatic event have been shown to experience both elevated (Bremner et al., 2003; Elzinga et al., 2003) and blunted (Wessa et al., 2006) cortisol patterns. Meta-analytic findings confirm the variability in the direction of effects between PTSD and salivary, plasma, and urinary cortisol levels (Klaassens et al., 2012; Meewisse et al., 2007).
Similar to the adversity-cortisol research noted above, the broader stress literature suggests that chronic stress can cause both hyper- and hypo-activation of the HPA axis (e.g., Miller et al., 2007 for review). Several theories suggest that chronic stress can cause dysregulation in the HPA axis, known as allostatic overload, which can manifest as prolonged hyper-activation or hypo-activation (McEwen, 1998, 2007). Under ideal conditions when cortisol production reaches an optimal level, cortisol binds to glucocorticoid receptors to initiate a negative feedback loop, resulting in the halt of the hormone production (Sapolsky et al., 2000). However, under conditions of chronic or repeated stress, this negative feedback loop is altered, resulting in dysregulation of the HPA axis. Stressors cause an initial hyper-activation of the HPA axis, leading to periods of elevated cortisol that are not counteracted appropriately by the negative feedback loop (Guilliams and Edwards, 2010). Over the long-term, with exposure to prolonged or chronic stress, this hyper-activation causes a number of biological changes throughout the HPA axis (Fries et al., 2005), changes that can eventually result in an under-responsive, hypo-active system (Guilliams and Edwards, 2010). Therefore, the variable findings in the adversity-cortisol literature are consistent with basic research on the hyper- and hypo-activation of the HPA axis under conditions of chronic stress (Chrousos, 2009). Given the complexity in how the HPA axis responds to stress, the present meta-analysis seeks to understand the particular factors that impact whether adversity is associated with hyper-activity or hypo-activity as assessed by hair cortisol concentrations (HCC).
Both timing and type of adversity may be factors that influence whether the relation between adversity and cortisol levels is positive or negative. It is likely that different types of adversity, such as maltreatment, war, or natural disasters have a different impact on HPA activity. Further, many of these kinds of adversity, and in particular maltreatment, vary in nature (i.e., types of maltreatment), intensity, and pervasiveness (i.e., time of onset, duration and frequency of exposure) (Kalmakis and Chandler, 2014), which all have the potential to impact the HPA axis. Data suggest that severe physical neglect is associated with low morning cortisol levels, whereas severe emotional maltreatment is associated with high morning cortisol levels among children in foster care (Bruce et al., 2009). Therefore, in addition to the type of adversity, the subtype of maltreatment might have varying effects on HPA activity. Further, there is evidence that adversity-related changes in HPA activation can vary across development (Bosch et al., 2012; Trickett et al., 2010), with younger children showing elevated cortisol levels compared to adolescents and young adults, who show depressed cortisol levels. In addition, the time since the onset of the stressor can impact the direction of the cortisol response, such that cortisol activity tends to be elevated close to the onset of the stressor but reduces as time passes (Miller et al., 2007). It is noteworthy that the age of the sample, age of adversity, and time since adversity are often confounded in the literature. Taken together, in addition to type of adversity, the subtype of maltreatment (physical, emotional, sexual abuse, or neglect), the age of exposure to adversity, and time since exposure to adversity are important factors to consider.
Given the extensive interest in adversity and HPA activity, a burgeoning literature assessing HCC has emerged. In the hair cortisol literature, findings also vary as to whether adversity is related to increases or decreases in HCC. For example, in a community sample of children, lifetime exposure to trauma was associated with elevated HCC (Simmons et al., 2016). However, in another study, maltreated children exhibited lower HCC compared to non-maltreated children (White et al., 2017). Further, in combat-exposed soldiers, lower HCC predicted more severe PTSD symptoms following traumatic exposure (Steudte-Schmiedgen et al., 2015). Given these differing results, in the context of a number of potential methodological and sample-related contributors, a quantitative synthesis that includes a close examination of potential moderating factors suggested by the existing literature is warranted.
Meta-analysis is the gold-standard for quantitative synthesis. Stalder et al. (2017) recently completed the first meta-analysis of the hair cortisol literature, in which they reviewed studies on HCC to determine the relevant covariates and basic features of HCC. They also explored the relation between HCC and a range of chronic-stress-related measures, including self-reported perceived stress, chronic stress, and mental health problems (e.g., anxiety and mood disorders). Examples of chronic stress assessed included caregiving stress, unemployment, shift work, chronic pain, and surviving an earthquake. Stadler et al. showed that HCC differed by sex (with males having higher HCC), age (with older individuals having higher HCC), BMI (higher BMI associated with higher HCC), and proximal length of hair to scalp (less HCC after first proximal 3-cm hair segment). Regarding chronic and perceived stress, chronic stress was associated with elevated HCC; however, there was not a significant relationship between perceived stress and HCC.
Although Stalder et al. (2017) examined the impact of chronic and perceived stress on HCC, they did not comprehensively explore associations between a range of adverse experiences (e.g., maltreatment, domestic violence, natural disasters, accidents) and HCC. The present meta-analysis seeks to add to the existing literature by focusing specifically on the association between different types of adversity and HCC. To date, studies of adversity and HCC have included many types of adverse experiences. By examining associations between different types of adversity and HCC, as well as exploring relevant covariates and moderators, this meta-analysis seeks to address the variability in the literature regarding the magnitude and direction of effects between adversity and cortisol.
The first objective of the present meta-analysis was to systematically summarize and quantify the strength of the relation between adversity and HCC. A range of adversity types were examined, including maltreatment, domestic violence, and exposure to other traumas, such as accidents, war/combat, and natural disasters. Given the complexities inherent in how the HPA axis responds to chronic stress, our second objective was to examine whether there are differences in magnitude and direction of effect that identify distinct classes of studies assessing the relation between adversity and HCC (e.g., strong and positive relations between adversity and HCC, no relation, or a strong and negative relation). The third objective of this meta-analysis was to explore participant and methodological characteristics that may moderate the strength and direction of observed relations between adversity and HCC.
Potential moderators were chosen based on the extant literature. These moderators included type and timing of adversity, as well as sample characteristics, characteristics of HCC assessment, and publication characteristics. Regarding the type and timing of adversity, we tentatively hypothesized that the type of adversity would be a moderator in that those who were exposed to maltreatment, in particular, would experience blunted HCC levels compared to those who experienced other types of adversity, since maltreatment is often chronic. We also hypothesized that timing of adversity would also be a significant moderator, with those exposed to adversity in childhood showing a significant but negative effect on HCC levels, compared to those not exposed to adversity in childhood, because the potentially severe and persistent activation of the HPA axis in childhood might lead to hypoactivation over time. We hypothesized that more recent adult adversity is associated with elevated HCC levels, consistent with hyper-activation, due to the recency of the adversity. However, one might also predict that the effects of childhood adversity would ‘wash out’ over time, leading to smaller absolute effect sizes, while adult absolute effect sizes would be larger due to recency of the events. Given the aforementioned inconsistencies in the HCC literature, these moderator hypotheses are exploratory in nature.
Importantly, the effects of type and timing of adversity are confounded in the literature, in that all studies of adversity during childhood examine maltreatment, whereas there is more variability in types of adversity occurring during adolescence and adulthood. Therefore timing of adversity could not be separated conclusively from type of adversity. In addition, given the available literature, short duration of adversity could not be reliably differentiated from longer duration of adversity, so no hypotheses are offered regarding duration of adversity.
2. Methods
2.1. Literature search and study selection
A comprehensive search was conducted on several databases (PsycINFO, MEDLINE, Social Science Index, EMBASE, Dissertation Abstract International) to obtain published and unpublished research. The following search terms were used: “hair cortisol” and “maltreatment,” “neglect,” “abuse,” “adversity,” “early life stress,” “trauma,” “posttraumatic stress disorder,” “PTSD,” “stress,” “stressor,” “adverse events,” “negative events,” “negative life events,” “life events,” “violence,” “assault,” “rape,” or “accident.” This search included articles published prior to November 2017 that were published in English and used human participants. The literature search yielded 1576 articles, including 590 unique articles. Duplicate articles (e.g., dissertations that were later published) and articles that used the same sample were excluded. When multiple articles used the same sample, the article that included the most outcome data was included in the meta-analysis. In the event that two articles that used the same sample included identical outcome data, the article with the larger sample size was included. Duplicate studies and studies using the same sample were excluded because meta-analyses should have independent study-level effects (Rosenthal, 1991).
Studies were included if they 1) were an empirical study (i.e., not a literature review), 2) included a measure of hair cortisol, 3) included a measure of adversity or trauma (e.g., maltreatment, domestic violence, PTSD following traumatic exposure, non-specific lifetime trauma), and 4) provided outcome data that could be used to compute an effect size. See Fig. 1 for a flow chart of study inclusion. Fourteen studies met the first three requirements but did not provide relevant data to compute an effect size. The corresponding authors of these studies were contacted, and data were requested; five authors provide the requested data. In total, 28 articles were included in the present meta-analysis; 25 were published articles, 2 were dissertations, and 1 was a published abstract.
Fig. 1.
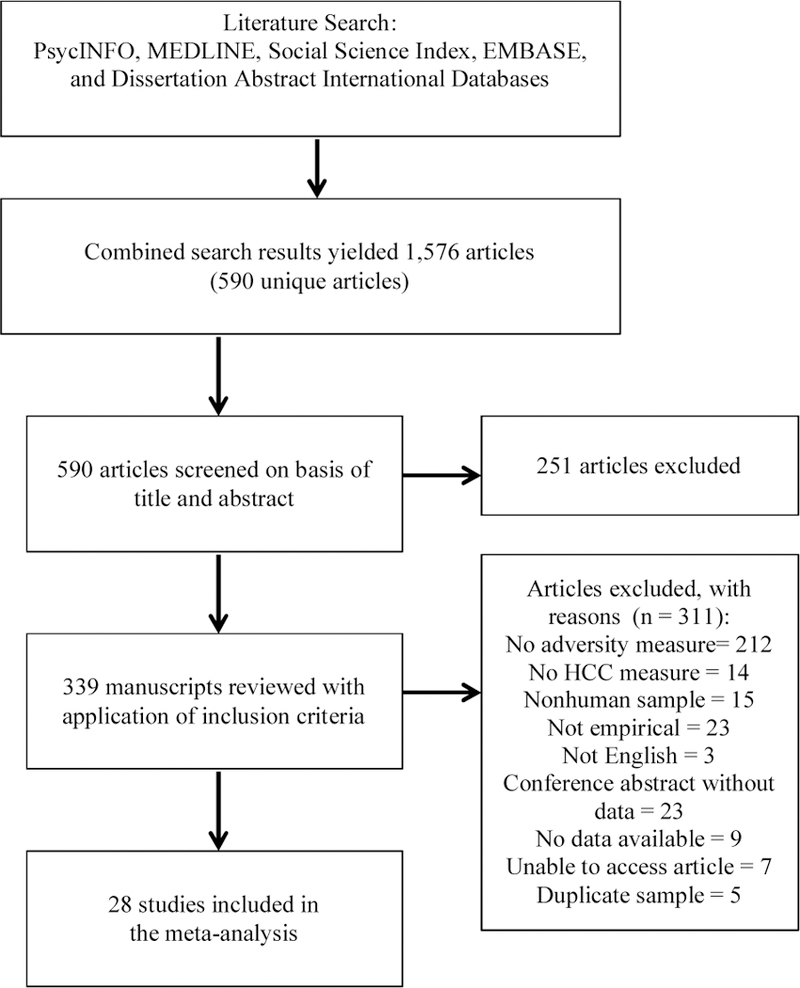
Flow chart for studies included in the meta-analysis. HCC=h air cortisol concentration.
2.1.1. Reliability of study selection and coding study variables
The lead author reviewed all abstracts and full text articles for study inclusion and coded variables for all eligible studies. Two research staff completed reliability checks. First, a research staff reviewed all 590 abstracts/full-text articles for inclusion. There was 97% agreement between the lead author and staff member on which studies to include. Disagreements on 19 articles (3% of total articles) were discussed, and the final inclusion decisions reflect the consensus of both raters. A second staff member coded six (20%) randomly selected studies of the 28 studies that met inclusion criteria. Intraclass correlations across all coded continuous variables ranged from 0.85 to 1.00 (mean=0.96, SD=0.06), and kappas across all coded categorical variables ranged from 0.78 to 1.00 (mean=0.96, SD=0.08). Disagreements were discussed, and the final coding reflects the consensus of both coders.
2.2. Coding of study variables
Several methodological variables were coded to be assessed as potential moderators. These variables included factors associated with the article publication (e.g., publication year, publication status), sample characteristics, hair cortisol measurement, and assessment of adversity.
2.2.1. Article characteristics
As is routine practice for meta-analyses (e.g., Madigan et al., 2016), the geographical region where the study was conducted was coded (North America, Europe, Africa, China, Brazil). In addition, the year of publication and the publication status (peer reviewed article, dissertation) were coded for all studies.
2.2.2. Sample characteristics
Sample characteristics were coded based on the overall sample unless data were provided separately for subgroups within a sample (e.g., trauma-exposed and non-exposed groups). The following participant characteristics were coded: a) the number of individuals in the sample, b) the age category of the sample (i.e., child, adolescent, adult), c) the mean age of participants, d) the sex distribution (i.e., % male), e) the racial composition (i.e., % White), f) the percentage of the sample who smoked, g) the percentage of the sample taking medication, and h) the mean body mass index (BMI) of the sample. The clinical status (clinical diagnosis, no clinical diagnosis) of the sample was also coded. Specifically, studies were classified as including a clinical sample if the primary inclusion criterion for the sample was meeting diagnostic criteria for a psychological disorder (e.g., inpatients with Depressive Disorders) and/or the study provided outcome data for a clinical group compared to a healthy control group. In addition, samples were classified by PTSD diagnosis (PTSD diagnosis, non-PTSD) when relevant data were available.
In addition to the above sample characteristics, we attempted to code education level as a measure of socioeconomic status. However, education level, which was only reported in seven studies, was measured differently across studies. For example, some studies reported number of years of education and others reported the percentage of the sample falling into different educational categories (e.g., less than high school, some college, college, graduate). Given this inconsistent reporting, we were unable to statistically assess education level as a moderator.
2.2.3. Adversity measurement
Factors associated with the assessment of adversity were coded. These factors included the name of the specific questionnaire, the category of adversity (i.e., maltreatment, domestic violence, war/combat exposure, natural disaster, accident, lifetime trauma), the subtype of maltreatment (i.e., emotional, physical, sexual abuse, neglect), the type of measurement (i.e., questionnaire, interview, other), and the time period during which the adversity occurred (i.e., childhood, adulthood). The time period during which the adversity occurred differs from age of sample described above, given that several samples were adults retrospectively reporting experiences of maltreatment that occurred during childhood. We also dichotomized the category of adversity to explore whether maltreatment differed from other types of adversity (i.e., maltreatment vs other adversity). Among studies that assessed maltreatment, all but two (Boeckel et al., 2017; White et al., 2017) retrospectively assessed childhood maltreatment in adolescence or adulthood, in contrast Boeckel et al. (2017) included a sample of children who witnessed domestic violence and White et al. (2017) included a sample of maltreated children, classified based on the maternal report and child protection service records.
It should be noted that maltreatment was the only type of adversity that occurred in childhood (although studies assessed other types of adversity in adolescence). All studies that assessed other forms of adversity (e.g., domestic violence, natural disasters, combat exposure) included adolescent or adult samples only. Therefore, given the age and adversity distributions among studies, it was not possible to definitively separate timing of adversity from type of adversity. Because different types of maltreatment often occur in combination and because repeated experiences of adversity (e.g., maltreatment in childhood and subsequent trauma) likely have unique impact on HPA activity, we also explored the possibility of assessing repeated adversity as a moderator. However, only one study examined the impact of experiencing both maltreatment in childhood and subsequent interpersonal violence (Morris et al., 2017) on HCC; thus, we could not examine number of adversities (or single/multiple adversities) as a moderator. Similarly, we could not explore the age of onset of maltreatment or the duration of maltreatment as this information was not reported in a sufficient number of studies.
2.2.4. Features of hair cortisol samples
Several features related to hair cortisol samples were coded. These features included the type of hair extraction procedure used (i.e., immunoassay [ImA]), liquid chromatography tandem mass spectrometry (LCMS), the length of the hair samples, the time period to which the sample corresponds (e.g., past 3 months), the mean number of times participants washed their hair per week, and information related to hair treatment and coloring.
2.3. Meta-analytic procedures
Individual studies varied in the way in which they presented out-come data. Data were presented as 1) Pearson correlation coefficients between adversity measures and HCC; 2) means and standard deviations of HCC for adversity and control groups, 3) t-tests describing the statistical difference in HCC between adversity and control groups, and 4) odds ratio and confidence limits for adversity and HCC. All outcome data were entered into Comprehensive Meta-Analysis Version 3.0 (CMA; Borenstein et al., 2005) and converted to a standardized point estimate effect size (Cohen’s d), along with the corresponding effect size variance and confidence intervals (CI) (Hedges and Olkin, 1985). Effect sizes were weighted based on the sample sizes. Cohen (1988) classified standardized effect sizes into descriptive categories based on size: d=.20 (small), d= .50 (medium), and d=.80 (large) effects.
All analyses were performed using MPlus version 8.1 (Muthén and Muthén, 2017). Analyses for Objectives 1 and 3 were performed using three-level multilevel models (MLM; Cheung, 2015; Van den Noortgate et al., 2013). Three-level MLM permits the inclusion of multiple effect sizes per study while adequately dealing with the non-independent nature of the data (i.e., effect sizes are nested within studies). Our analyses were performed using a random effect model that assumes that there is one population of effect sizes (i.e., with a mean and a variance that need to be estimated), and permits heterogeneity of effect sizes, which prevents effect size overestimation when heterogeneity in effects is present (Cooper and Hedges, 1994). In contrast with a more typical random effect model which models only the sampling variance of the observed effect size, the three-level MLM decomposes the variances of effect sizes into three components: sampling variance of the observed effect size, within-study variance (i.e., variance between effect sizes within a study), and between-study variance (i.e., variance in effect sizes between studies). In the case of the three-level model for the moderator analyses (Objective 3), the moderators were entered as predictors of variance in effect size. The moderators were either allowed to predict between-study variance (if their values varied across studies but not within studies) or both within-study and between-study variance (if their values varied across and within studies).
These models were estimated using Monte Carlo Markov Chain (MCMC) estimation, as we had few clusters (i.e., each study represents a cluster so that there were 28 clusters). Indeed, MCMC estimation can provide accurate parameter estimates for multilevel models with as few as 20 clusters (Hox et al., 2012). We report model estimates as well as their 95% Credible Interval1 (95% CI) based on higher posterior density. Models were estimated with 50,000 iterations. Model convergence was estimated based on the potential scale reduction and by examining trace plots for irregularities (Hamra et al., 2013; Muthén, 2010).
Analyses to identify subclasses of effects (Objective 2) were performed using fixed-effect meta-analytic mixture modeling (Cheung, 2015). Instead of assuming that there is only one population of heterogeneous effect sizes, the fixed effect meta-analytic mixture model assumes that effect sizes are from distinct but homogeneous subpopulations. Heterogeneity in effect sizes across studies is therefore explained by membership in different homogeneous populations, referred to as classes. The appropriate number of classes is identified by comparing models with an increasing number of classes and stopping when either the fit indices become statistically inferior or when classes are too small (Nylund et al., 2007). For the three following fit indices, lower numbers indicate better model fit: Akaike’s Information Criteria (AIC), Bayesian Information Criteria (BIC), and sample size adjusted BIC (aBIC). The BIC is given priority given its good performance at identifying the correct number of classes (Nylund et al., 2007). A BIC difference of 10 is considered strong evidence in favor of the model with the lowest BIC (Nagin, 1999). We also report the Lo-Mendell-Rubin Likelihood Ratio Test (LMR) and the ad hoc adjusted LMR (aLMR), with a significant p-value indicating that a model with k-1 subgroups should be rejected in favor of the model with k classes (Tein et al., 2013). A final index is entropy, which indexes classification accuracy, with values above .80 indicating very good classification accuracy (Tein et al., 2013). These analyses were performed using the robust maximum likelihood estimator, and we report the estimates as well as their 95% Confidence Interval (95% CI). Since one cannot combine three-level models and mixture models in MPlus, we could only use a single effect size per study to examine Objective 2. In the event that a single study provided data for multiple outcomes of interest, these effect sizes were averaged so that there was only one weighted effect size for each individual study for mixture modeling analyses.
2.3.1. Data analytic plan
Analyses were performed in three steps. First, we used a three-level multilevel model to assess the overall magnitude of the association between adversity and hair cortisol, as well as the variance in effect sizes due to within-study and between-study variation (Van den Noortgate et al., 2013). Second, using mixture modeling (Cheung, 2015), we investigated whether there were multiple classes of studies with homogeneous effect sizes. Third, using multilevel modeling, analyses were performed to identify moderators associated with systematic variations in effect size. Moderator analyses were performed based on all effect sizes for which moderator data were present. For continuous moderators, we report the slope indexing the linear association between a moderator and variations in effect size. We also tested non-linear effects by including quadratic slopes. For categorical moderators, it is not possible to compute an omnibus test comparable to the Q statistic when using three-level models in MPlus. Instead, differences in effect sizes were investigated by creating new parameters capturing the difference between two estimates using the MODEL CONSTRAINT command (Muthén and Asparouhov, 2013). Two estimates were considered statistically different if the 95% CI for the new difference parameter did not include zero.
3. Results
3.1. Descriptive statistics
The 28 studies included in this meta-analysis consisted of a total of 3397 participants. Studies varied greatly in terms of sample composition. The average age of participants was 25.06 years old (SD=5.01). On average, samples consisted of 39.05% males and were 40.18% White race. See Table 1 for a description of sample and methodological characteristics of each study.
Table 1.
Reviewed studies, sample characteristics, methods of adversity assessment, and methods of hair extraction for all studies included in the meta-analysis.
| Study | N | Sample Description | Age M (SD) | Sex (% male) | BMI M (SD) | Adversity Indicator | Adversity Classification | Hair Extraction & Length | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Boeckel et al. (2017) | 59 | Mothers and children exposed to interpersonal violence | 35.1 (7.4) | 0% | n/a | Revised Conflict Tactic Scale | Domestic Violence | ImA; 1cm |
| 2 | do Prado et al., 2017 | 57 | Adolescents | 15.3 (1.4) | 42% | 24.6 (2.8) | Childhood Trauma Questionnaire | Child Maltreatment | ImA; 3cm |
| 3 | Etwel et al. (2014) | 39 | Women exposed to the Libyan war | 23.8 (6.2) | 0% | 23.1 (4.8) | Libyan War | War Exposure | ImA; 3cm |
| 4 | Fischer et al. (2016) | 139 | Community sample | 50.6 (14.6) | 28% | 27.5 (6.0) | Childhood Trauma Questionnaire | Child Maltreatment | ImA; 3cm |
| 5 | Ford et al. (2016) | 578 | Adolescents | 14.6 (1.8) | 50% | n/a | Adverse Childhood Experiences | Child Maltreatment | ImA; 3cm |
| 6 | Gao et al. (2014) | 92 | Adult and adolescent earthquake survivors | 30.0 (7.1) | 80% | n/a | Earth Quake | Natural Disaster | LCMS; 3cm |
| 7 | Groer et al. (2015) | 52 | Soldiers | 25.0 | 98% | n/a (n/a) | PTSD Symptom Checklist | War Exposure | ImA; 3cm |
| 8 | Heinze et al. (2016) | 58 | Clinical sample and healthy controls | 20.5 (2.7) | 10% | 22.7 | Childhood Trauma Questionnaire | Child Maltreatment | ImA; 3cm |
| 9 | Hinkelmann et al. (2013) | 84 | Depressed sample and healthy controls | 41.5 (11.0) | 37% | n/a | Childhood Trauma Questionnaire | Child Maltreatment | ImA; 3cm |
| 10 | Hoffman et al. (2017) | 33 | Pregnant women | n/a | n/a | n/a | Adverse Childhood Experiences | Child Maltreatment | n/a |
| 11 | Kaess et al. (2017) | 36 | Clinical sample (youth with Internet Gaming Disorder) | 19.0 (3.4) | 100% | 22.65 (n/a) | Childhood Trauma Questionnaire | Child Maltreatment | ImA; n/a |
| 12 | Kalmakis et al. (2015) | 55 | Undergraduate sample | 19.5 (1.6) | 24% | Adverse Childhood Experiences | Child Maltreatment | ImA; 3cm | |
| 13 | Keenan-Devlin (2015) | 88 | Low-SES youth | 15.2 (2.0) | 50% | 24.0 (6.1) | Adverse Childhood Experiences | Child Maltreatment | ImA; 1cm |
| 14 | Luo et al. (2012) | 84 | Earth quake survivors (half with PTSD) and healthy controls | 14.0 (1.2) | 0% | n/a | Chinese version of the Children’s Revised Impact of Event Scale Structured Clinical Interview for DSM-IV Axis I Disorders |
Natural Disaster | LCMS; 3cm |
| 15 | Mayer (2017) | 74 | Medical interns | 27.41 (2.4) | 44% | 23.02 (3.5) | Childhood Trauma Questionnaire | Child Maltreatment | ImA; 4cm |
| 16 | Melhem et al. (2017) | 115 | Psychiatric inpatients and healthy controls | 22.7 (3.0) | 55% | 23.8 (3.8) | Childhood Trauma Questionnaire | Child Maltreatment | ImA; 3cm |
| 17 | Morris et al. (2017) | 34 | Women exposed to interpersonal violence or child trauma | 24.0 (3.1) | 0% | n/a | Childhood Trauma Questionnaire | Child Maltreatment | ImA; 3cm |
| 18 | Pacella et al. (2017) | 35 | Adults with physical injury | n/a | n/a | n/a | PTSD Symptom Checklist | Physical Injury | ImA; 3cm |
| 19 | Reichl et al. (2016) | 52 | Adolescents engaging in NSSI and healthy controls | 16.3 (1.2) | 8% | n/a | Childhood Experiences of Care and Abuse Interview | Child Maltreatment | ImA; 3cm |
| 20 | Schalinski et al. (2015) | 43 | Clinical outpatients and healthy controls | 34.9 (10.3) | 0% | 26.4 (5.7) | Event checklist Early Trauma Inventory | Lifetime Trauma | ImA; 3cm |
| 21 | Schreier et al. (2015) | 180 | Pregnant women | 31.0 (5.4) | 0% | 25.8 (5.4) | Childhood Trauma Questionnaire | Child Maltreatment | LCMS; 3cm |
| 22 | Schury et al. (2017) | 94 | Mothers of newborns | 32.5 (5.4) | 0% | 24.5 (4.8) | Childhood Trauma Questionnaire | Child Maltreatment | LCMS; 3cm |
| 23 | Steudte et al. (2011) | 27 | PTSD patients and healthy controls | 19.7 (4.5) | 52% | 21.9 (2.1) | Lifetime traumatic events | Lifetime Trauma | ImA; < 3cm |
| 24 | Steudte et al. (2013) | 78 | PTSD patients and healthy controls | 38.7 (12.5) | 8% | 23.7 (3.4) | Childhood Trauma Questionnaire Trauma History Questionnaire Munich Composite International Diagnostic Interview |
Child Maltreatment Lifetime Trauma |
LCMS; 3cm |
| 25 | Steudte-Schmiedgen et al. (2015) | 90 | Soldiers | 27.68 (6.1) | 100% | 25.45(2.7) | Childhood Trauma Questionnaire Traumatic events | Child Maltreatment Lifetime Trauma |
LCMS; 2cm |
| 26 | Trautmann et al. (2018) | 618 | Soldiers | 28.8 (6.2) | 100% | 24.8 (2.7) | Childhood Trauma Questionnaire | Child Maltreatment | ImA; 2cm |
| 27 | Wells et al. (2014) | 217 | Community sample | n/a | 28% | n/a | Intimate Partner Physical Aggression | Domestic Violence | ImA; 2cm |
| 28 | White et al. (2017) | 286 | Maltreated children compared to non-maltreated controls | 9.9 (3.1) | 50% | 50.1 (30.1) | Maternal Maltreatment Classification Interview | Child Maltreatment | ImA; 3cm |
Note: Descriptive information (age, sex, BMI) is provided for the entire sample. If studies provided separate demographic information for subsamples, data were averaged across subsamples. BMI=Body Mass Index; ImA=Immunoassay; LCMS=liquid chromatography tandem mass spectrometry; NSSI=non-suicidal self-injury.
3.2. Overall effect of adversity on hair cortisol
In relation to our first aim, a three-level multi-level model was used to determine the magnitude of the mean effect of adversity on HCC. Here, we used a model without covariates to obtain an average estimate of the outcome (i.e., effect size), as well as its variation both within and across studies. Standardized mean effect sizes representing the impact of adversity on hair cortisol levels for each study are presented in Table 2. Taking into account all studies (k = 28), using all available effect sizes (n = 71), the estimated average effect size was 0.213, 95% CI [0.034, 0.397]. The model also showed that effect sizes were heterogeneous due to variations both within and between studies. The within-study variance (τ=0.034, 95% CI [0.003, 0.108]) was about five times smaller than the between-study variance (τ=0.155, 95% CI [0.065, 0.367]), with 82.7% of the total variance in effect size (0.155/ (0.034+0.155)=0.827). Given the significant heterogeneity in the magnitude and direction of the effects of adversity on HCC, we followed up these overall analyses, first, with mixture modelling to assess the presence of coherent classes of studies with similar effect sizes, and, second, with a series of moderator analyses.
Table 2.
Effect sizes for the association between adversity and hair cortisol concentrations by study and subsample.
| Study | Description of separate effects (e.g., subsample/ adversity subtype/ time points) | Effect Size (Cohen’s d) |
||||
|---|---|---|---|---|---|---|
| Estimate | SE | 95% CI | p-value | |||
| 1 | Boeckel et al. (2017) | Mothers | 0.659 | 0.268 | [0.133, 1.185] | .014 |
| Children | 0.294 | 0.263 | [−0.221, 0.809] | .263 | ||
| 2 | do Prado et al. (2017) | T1 | 0.690 | 0.273 | [0.155, 1.225] | .012 |
| T2 | 0.219 | 0.266 | [−0.302, 0.741] | .410 | ||
| T3 | 1.23 | 0.289 | [0.661, 1.794] | < .001 | ||
| 3 | Etwel et al. (2014) | T1- prewar | 0.034 | 0.093 | [−0.150, 0.217] | .720 |
| T2 - postwar | 0.311 | 0.178 | [−0.038, 0.660] | .081 | ||
| 4 | Fischer et al. (2016) | Emotional neglect | −0.065 | 0.242 | [−0.539, 0.410] | .789 |
| Physical neglect | −0.221 | 0.292 | [−0.793, 0.351] | .449 | ||
| Emotional abuse | 0.010 | 0.266 | [−0.512, 0.531] | .971 | ||
| Physical abuse | 0.045 | 0.315 | [−0.572, 0.662] | .887 | ||
| Sexual abuse | 0.165 | 0.238 | [−0.301, 0.630] | .489 | ||
| 5 | Ford et al. (2016) | Witnessing violence | 0.081 | 0.083 | [−0.083, 0.244] | .334 |
| Victim DV | 0.129 | 0.084 | [−0.035, 0.292] | .124 | ||
| Foster care | −0.055 | 0.083 | [−0.219, 0.109] | .510 | ||
| 6 | Gao et al. (2014) | Adults | 1.78 | 0.361 | [1.072, 2.49] | < .001 |
| T1-adolescents | 0.893 | 0.304 | [0.296, 1.49] | .003 | ||
| T2-adolescents | 0.639 | 0.298 | [0.055, 1.22] | .032 | ||
| T3-adolescents | 0.422 | 0.294 | [−0.153, 1.00] | .150 | ||
| 7 | Groer et al. (2015) | 0.797 | 0.308 | [0.194, 1.40] | .010 | |
| 8 | Heinze et al. (2016) | Emotional abuse | −0.486 | 0.412 | [−1.29, 0.321] | .238 |
| Physical abuse | −0.122 | 0.393 | [−0.892, 0.648] | .756 | ||
| Emotional neglect | −0.425 | 0.409 | [−1.23, 0.376] | .298 | ||
| Physical neglect | 0.391 | 0.392 | [−0.377, 1.16] | .318 | ||
| Sexual abuse | 0.268 | 0.396 | [−0.507, 1.04] | .498 | ||
| Total maltreatment | −0.451 | 0.437 | [−1.31, 0.406] | .302 | ||
| 9 | Hinkelmann et al. (2013) | −1.64 | 0.693 | [−2.99, −0.278] | .018 | |
| 10 | Hoffman et al. (2017) | 1.76 | 0.486 | [0.804, 2.71] | < .001 | |
| 11 | Kaess et al. (2017) | Emotional abuse | 0.288 | 0.352 | [−0.401, 0.977] | .413 |
| Physical abuse | 0.261 | 0.351 | [−0.427, 0.949] | .457 | ||
| Sexual abuse | 0.157 | 0.349 | [−0.528, 0.841] | .653 | ||
| Emotional neglect | 0.275 | 0.351 | [−0.414, 0.964] | .434 | ||
| Physical neglect | 0.009 | 0.348 | [−0.673, 0.692] | .978 | ||
| Total maltreatment | 0.279 | 0.352 | [−0.410, 0.968] | .428 | ||
| 12 | Kalmakis et al. (2015) | 0.566 | 0.283 | [0.011, 1.121] | .046 | |
| 13 | Keenan-Devlin (2015) | Girls | 0.904 | 0.343 | [0.233, 1.58] | .006 |
| Boys | −0.169 | 0.330 | [−0.815, 0.478] | .609 | ||
| 14 | Luo et al. (2012) | T1- PTSD & HC | −0.471 | 0.289 | [−1.037, 0.095] | .103 |
| T2- PTSD & HC | 0.623 | 0.292 | [0.052, 1.20] | .033 | ||
| T3- PTSD & HC | 1.16 | 0.307 | [0.562, 1.77] | < .001 | ||
| T1- non-PTSD & HC | 0.241 | 0.286 | [−0.319, 0.802] | .399 | ||
| T2- non−PTSD & HC | 1.24 | 0.310 | [0.634, 1.85] | < .001 | ||
| T3- non-PTSD & HC | 1.29 | 0.312 | [0.679, 1.90] | < .001 | ||
| 15 | Mayer (2017) | T1- pre-internship | 0.673 | 0.250 | [0.182, 1.16] | .007 |
| T2- internship | 0.100 | 0.238 | [−0.366, 0.566] | .674 | ||
| 16 | Melhem et al. (2017) | −0.430 | 0.193 | [−0.808, −0.051] | .026 | |
| 17 | Morris et al. (2017) | Child trauma & IPV | −0.341 | 0.390 | [−1.105, 0.424] | .383 |
| Child trauma only | −0.480 | 0.463 | [−1.39, 0.429] | .301 | ||
| 18 | Pacella et al. (2017) | 0.516 | 0.365 | [−0.199, 1.23] | .157 | |
| 19 | Reichl et al. (2016) | Neglect | 0.231 | 0.297 | [−0.351, 0.814] | .436 |
| Physical abuse | 0.302 | 0.300 | [−0.285, 0.889] | .313 | ||
| Sexual abuse | 0.010 | 0.296 | [−0.570, 0.590] | .973 | ||
| Psychological abuse | 0.181 | 0.349 | [−0.504, 0.866] | .554 | ||
| Total maltreatment | 0.180 | 0.297 | [−0.402, 0.762] | .544 | ||
| 20 | Schalinski et al. (2015) | Hair segment 1 | 0.025 | 0.327 | [−0.615, 0.665] | .939 |
| Hair segment 2 | 0.974 | 0.339 | [0.309, 1.64] | .004 | ||
| 21 | Schreier et al. (2015) | Physical abuse | 0.400 | 0.153 | [0.097, 0.700] | .009 |
| Emotional abuse | 0.084 | 0.151 | [−0.211, 0.379] | .577 | ||
| 22 | Schury et al. (2017) | 0.151 | 0.211 | [−0.263, 0.565] | .475 | |
| 23 | Steudte et al. (2011) | 0.899 | 0.448 | [0.022, 1.78] | .045 | |
| 24 | Steudte et al. (2013) | PTSD & HC, hair 1 | −0.739 | 0.284 | [−1.30, −0.182] | .009 |
| PTSD & HC, hair 2 | −0.838 | 0.287 | [−1.401, −0.276] | .003 | ||
| No-PTSD & HC, hair 1 | −0.634 | 0.282 | [−1.186, −0.081] | .025 | ||
| No-PTSD & HC, hair 2 | −0.697 | 0.283 | [−1.252, −0.141] | .014 | ||
| Total maltreatment | −0.291 | 0.233 | [−0.748, 0.166] | .212 | ||
| Traumatic events | −0.583 | 0.241 | [−1.055, −0.112] | .015 | ||
| 25 | Steudte-Schmiedgen et al. (2015) | Total maltreatment | −0.012 | 0.214 | [−0.432, 0.408] | .955 |
| Traumatic events | −0.163 | 0.215 | [−0.584, 0.259] | .450 | ||
| 26 | Trautmann et al. (2018) | Emotional abuse | 0.140 | 0.081 | [−0.018, 0.299] | .083 |
| Physical abuse | 0.201 | 0.081 | [0.042, 0.360] | .013 | ||
| Emotional neglect | 0.020 | 0.081 | [−0.138, 0.178] | .804 | ||
| Physical neglect | 0.120 | 0.081 | [−0.038, 0.279] | .137 | ||
| Total maltreatment | 0.140 | 0.081 | [−0.018, 0.299] | .083 | ||
| 27 | Wells et al. (2014) | 0.219 | 0.138 | [−0.050, 0.489] | .111 | |
| 28 | White et al. (2017) | −0.345 | 0.120 | [−0.580, −0.110] | .004 | |
Notes: ES = effect size; PTSD = Posttraumatic stress disorder, HC = healthy control; IVP = Interpersonal violence; t = time point.
3.3. Identifying coherent classes across studies
In relation to the second aim, we sought to determine whether there were distinct subpopulations of studies with homogeneous effect sizes. Fit indices of the mixture model with one to four classes are reported in Table 3. The AIC, BIC and aBIC showed substantial improvements when moving from one to two classes, comparatively smaller improvements when moving from two to three classes (i.e., the difference in BIC is less than 10), and showed worse fit when moving from three to four classes. The LMR and aLMR indicated that adding a second and a third class resulted in a significant improvement in fit, whereas this was not the case for the addition of a fourth class. Entropy was very good for the 2-class model and sufficiently good for the 3- and 4-class models. However, the lower entropy for the 3- and 4-class models indicated that the classes were less well separated in the 3- and 4-class models compared to the 2-class model. In other words, there was more uncertainty regarding the class to which each study belonged. In sum, while some indicators suggested that the 3-class model was a better fit than the 2- class model (LMR and LRT), the difference was small (according to the difference in BIC) and classes were less well separated (as indicated by the decrease in both BIC and entropy). Thus, the 2-class model was chosen as the most parsimonious fixed effects model.2
Table 3.
Fit indices of the latent profile analysis with a varying number of classes.
| AIC | BIC | aBIC | Entropy | LMR | aLMR | Class Sizes | |
|---|---|---|---|---|---|---|---|
| 1-class | 177.743 | 179.075 | 175.966 | n/a | n/a | n/a | 28 |
| 2-class | 136.344 | 140.340 | 131.013 | .881 | .0000 | .0000 | 24, 4 |
| 3-class | 129.351 | 136.012 | 120.467 | .709 | .0002 | .0007 | 15, 8, 5 |
| 4-class | 133.249 | 142.575 | 120.811 | .750 | .6412 | .6536 | 15, 8, 5, 0 |
Notes: AIC = Akaike’s Information Criteria; BIC = Bayesian Information Criteria; aBIC = adjusted BIC; LMR = Lo-Mendell-Rubin Likelihood Ratio Test; aLMR = adjusted LMR.
Results best indicated two classes of studies. One class of studies produced negative effects (i.e., more adversity was associated with lower hair cortisol), and the other class of studies produced positive effects (i.e., more adversity was associated with higher hair cortisol). Specifically, the first class included four studies with an overall negative direction of effect and a moderate effect size (d = −0.478, 95% CI [−0.639, −0.318]). The second class included the remaining 24 studies with an overall positive and significant, albeit small, effect size (d=0.141, 95% CI [0.084, 0.199]). These results are consistent with theories that adversity may lead to both hypo- and hyper-activation of the HPA axis (Chrousos, 2009).
The first class included four studies that together produced a medium-sized negative effect. These four studies (Hinkelmann et al., 2013; Melhem et al., 2017 Steudte et al., 2013; White et al., 2017) provided a total of nine effects, of which eight were significantly different from zero. Although there was some heterogeneity, these four studies all assessed forms of maltreatment, some through questionnaires (i.e., CTQ) and others through interviews (e.g., Maternal Maltreatment Classification Interview), providing some support for the hypothesis that maltreatment would be associated with cortisol hyporeactivity. Three of the four studies were based on adult samples retrospectively reporting maltreatment, while the fourth was based on a maltreated child sample (White et al., 2017). Further, the adult sample characteristics varied greatly, including samples of psychiatric patients and individuals with PTSD. The second class included 24 studies that produced a small but significant positive effect. These 24 studies produced a total of 66 effect sizes. Of importance, only 19 of these 66 effects (from 13 unique individual studies) were significantly different from zero (p < .05; Table 2). These studies varied greatly in terms of the types of adversity assessed (maltreatment, domestic violence, exposure to natural disaster) and in the characteristics of the sample (e.g., age, sex). Thus, maltreatment appeared in the group with small positive effects as well, indicating that maltreatment may be associated with significant blunting in some instances but not in others. In subsequent moderation analyses, we further explored whether study or sample characteristics explained the direction and magnitude of effects across all studies.
3.4. Moderators of the relation between adversity and HCC
In relation to the final aim of the meta-analysis, moderator analyses, using multilevel modeling, were performed to identify whether particular study variables explained variations in effect sizes relating adversity to HCC. Both linear effects and quadratic effects were tested. Only linear effects reached significance, so quadratic effects are not reported on further. Complete results of continuous and categorical moderator analyses are reported in Tables 4 and 5, respectively.
Table 4.
Continuous moderators of associations between adversity and hair cortisol.
| Continuous Moderator | k (n) | Slope [95% CI] |
|---|---|---|
| Age (years) | 27 (70) | 0.002 [−0.014, 0.018] |
| Sex (% male) | 27 (70) | −0.002 [−0.007, 0.003] |
| Race (% White) | 15 (37) | −0.006* [−0.012, −0.001] |
| Medication usage (% on medication) | 15 (38) | −0.006 [−0.017, 0.005] |
| Smoking status (% smokers) | 13 (33) | −0.006 [−0.021, 0.009] |
| BMI | 15 (39) | −0.019 [−0.050, 0.021] |
| Length of hair | 26 (65) | 0.022 [−0.132, 0.174] |
| Hair treatment | 9 (26) | −0.005 [−0.025, 0.014] |
| Hair washes | 8 (24) | 0.174 [−0.095, 0.458] |
| Year of publication | 28 (71) | 0.063 [−2.506, 3.918] |
Note. k = number of studies; n = number of effect sizes; CI = credible interval; BMI = Body Mass Index.
Table 5.
Categorical moderators of associations between adversity and hair cortisol.
| Categorical Moderator | k (n) | d [95% CI] | Significant differences between effect sizes |
|---|---|---|---|
| Age Classification | 28 (71) | None | |
| Adult | 19 (46) | 0.253* [0.024, 0.487] | |
| Adolescent | 7 (23) | 0.188 [−0.197, 0.544] | |
| Child | 2 (2) | −0.120 [−0.745, 0.513] | |
| Clinical Sample | 28 (71) | ||
| Clinical | 9 (28) | −0.049 [−0.334, 0.246] | 1) Clinical vs Non-clinical |
| Non-clinical | 19 (43) | 0.317* [0.122, 0.521] | |
| PTSD Sample | 28 (71) | ||
| PTSD sample | 3 (9) | −0.117 [−0.550, 0.296] | None |
| Non-PTSD sample | 25 (62) | 0.243* [0.062, 0.427] | |
| Adversity Category | 28 (71) | ||
| Natural disaster | 2 (10) | 0.777* [0.226, 1.335] | 1) Natural disaster vs Lifetime |
| Accident | 1 (1) | 0.534 [−0.572, 1.647] | 2) Natural disaster vs Maltreatment |
| War/combat | 2 (3) | 0.382 [−0.273, 1.012] | |
| Domestic violence | 2 (2) | 0.405 [−0.176, 1.016] | |
| Child maltreatment | 19 (46) | 0.132 [−0.058, 0.335] | |
| Lifetime trauma | 4 (9) | −0.069 [−0.459, 0.323] | |
| Maltreatment vs other adversity | 28 (71) | ||
| Maltreatment | 17 (46) | 0.174 [−0.029, 0.389] | None |
| Other | 11 (25) | 0.283* [0.018, 0.554] | |
| Maltreatment Sub-Classification | |||
| Physical abuse | 10 (31) | 0.214 [−0.039, 0.450] | None |
| Emotional abuse | 0.085 [−0.164, 0.322] | ||
| Sexual abuse | 0.152 [−0.200, 0.506] | ||
| Emotional neglect | −0.004 [−0.279, 0.269] | ||
| Physical neglect | 0.094 [−0.163, 0.370] | ||
| Witness DV | 0.144 [−0.168, 0.522] | ||
| Adversity Timing | 28 (71) | ||
| Childhood/adolescence | 22 (55) | 0.169 [−0.028, 0.368] | 1) Adult vs Childhood/Adolescence |
| Adulthood | 6 (7) | 0.596* [0.216, 0.996] | 2) Adult vs Lifetime |
| Lifetime/unspecified time | 4 (9) | −0.042 [−0.440, 0.345] | |
| Adversity Measure | 26 (65) | ||
| PTSD Checklist | 2 (2) | 0.649 [−0.225, 1.538] | None |
| CTS-2 | 2 (3) | 0.334 [−0.394, 1.083] | |
| ACE | 4 (7) | 0.509 [−0.048, 1.105] | |
| CECA | 1 (4) | 0.193 [−0.865, 1.260] | |
| Lifetime trauma | 2 (2) | 0.147 [−0.523, 0.828] | |
| CTQ | 13 (33) | 0.093 [−0.202, 0.391] | |
| THC | 1 (1) | −0.107 [−0.883, 0.678] | |
| Event Checklist | 1 (2) | 0.511 [−0.576, 1.620] | |
| Maltreatment Inventory | 1 (1) | −0.343 [−1.402, 0.682] | |
| PTSD Diagnostic Interview | 2 (10) | −0.132 [−0.688, 0.393] | |
| Adversity Measure Type | 28 (71) | ||
| Questionnaire | 22 (48) | 0.224* [0.023, 0.435] | None |
| Interview | 5 (17) | 0.045 [−0.311, 0.396] | |
| Other | 2 (6) | 0.495 [−0.154, 1.116] | |
| Hair Timing | 26 (65) | ||
| 1 to 2 months | 9 (18) | 0.197 [−0.099, 0.479] | None |
| 3 months | 17 (39) | 0.126 [−0.105, 0.351] | |
| > 3 months | 4 (8) | 0.495* [0.092, 0.902] | |
| Hair Extraction Method | 27 (70) | ||
| ImA | 21 (60) | 0.273 [−0.086, 0.622] | None |
| LCMS | 6 (20) | 0.154 [−0.044, 0.361] | |
| Publication Type | 28 (71) | ||
| Published Abstract | 25 (66) | 1.760* [0.461, 3.028] | 1) Article vs abstract |
| Dissertation | 2 (4) | 0.364 [−0.299, 0.985] | |
| Published Article | 1 (1) | 0.169 [−0.015, 0.348] | |
| Geographic Region | 28 (71) | ||
| North America | 11 (17) | 0.264* [0.014, 0.678] | 1) Europe vs China |
| Europe | 11 (36) | −0.049 [−0.288, 0.182] | 2) Europe vs Brazil |
| Africa | 2 (3) | 0.337 [−0.267, 0.968] | |
| China | 2 (10) | 0.760* [0.264, 1.289] | |
| Brazil | 2 (5) | 0.592* [0.046, 1.136] |
Note. k = number of studies; n = number of effect sizes; CI = credible interval; d = standardized difference in mean; d [95% CI] = indicates if effect size is statistically different from zero; statistical difference between effects=indicates if different categories of each moderator have statistically different effect sizes; ACE=Adverse Childhood Experiences Questionnaire; CTQ=Childhood Trauma Questionnaire; CECA=Childhood Experiences of Care and Abuse Interview; CTS-2 = Conflict Tactic Scale; PTSD=Posttraumatic Stress Disorder; Maltreatment Interview=Maternal Maltreatment Classification Interview; THC=Trauma History Questionnaire; ImA=immunoassay; LCMS=liquid chromatography tandem mass spectrometry.
3.4.1. Study characteristics
While no specific hypotheses were offered related to geographical region, there were two significant moderator effects for the geographical region in which the study was conducted. Studies conducted in Europe had significantly smaller effect sizes than studies from China (difference estimate = −0.808, 95% CI [−1.397, −0.260]) or from Brazil (difference estimate = −0.641, 95% CI [−1.235, −0.049]). Only studies conducted in China, North America, and Brazil produced effects that were significantly different from zero (Table 5). There was also one significant difference as a function of publication type. Effect sizes taken from published abstracts (d=1.760, k =1), were significantly larger than effect sizes from published articles (d=0.169, k=25; difference estimate=1.587, 95% CI [0.334, 2.920]). The year of publication did not significantly moderate the strength of the observed effect (b=0.063, 95% CI [−2.506, 3.918]).
3.4.2. Participant characteristics
No specific hypotheses were offered regarding participant race. However, racial distribution (i.e., % of sample participants who were White) was a significant moderator (b = −0.006, 95% CI [−0.012, −0.001]), such that samples with fewer White participants produced larger positive effects of adversity on HCC. In addition, the clinical status of the sample was a significant moderator, such that non-clinical samples (d = 0.317, k =19) had significantly different effect sizes compared to clinical samples (d = −0.049, k = 9; difference estimate = 0.365, 95% CI [0.046, 0.692]. Only studies that included non-clinical samples produced effects that were significantly different from zero (Table 4). Other sample characteristics, including overall mean age (years) of the participants, age classification (i.e., child, adolescent, adult), sex distribution (% males), percentage of the sample who smoked, percentage taking medication, PTSD diagnostic status, and BMI were not significant moderators (see Tables 4 and 5). It is important to note that the age of the participants in each sample differs from the age of experienced adversity, as examined below, because in a majority of studies adult participants reported adversity experienced in childhood.
3.4.3. Adversity characteristics and adversity measurement
Several adversity-related variables were also explored as potential moderators. First, the type of adversity was hypothesized to impact the direction of effect, such that those who were exposed to maltreatment, in particular, would experience blunted HCC levels compared to those who experienced other types of adversity. This hypothesis received mixed support from the mixture modelling above. When the different categories of adversity were examined as moderators (i.e., natural disaster, accident, war/combat, domestic violence, maltreatment, life-time trauma), only natural disaster effects were significantly different from zero (Table 5). In addition, there were two significant differences within the category of adversity. Samples exposed to natural disasters (d=0.777, k =2) had a significantly larger positive effect size compared both to samples exposed to maltreatment (d=0.132, k =20; difference estimate=0.646, 95% CI [0.053, 1.122]) and to samples exposed to lifetime unspecified trauma (d = −0.069, k = 3; difference estimate=0.852, 95% CI [0.160, 1.494]). However, note that these analyses compute the mean effect size across positive and negative effects, while mixture modelling results above indicated that maltreatment studies fell into two distinct classes, one characterized by moderate negative effects and one by small positive effects.
In addition, we dichotomized adversity as ‘maltreatment’ or ‘all other adversity’ in order to increase the power of this moderator analysis and explore whether maltreatment significantly differed from all other types of adversity. Only ‘other adversity’ effects were significantly different from zero; however, the effects were not significantly different from one another, i.e., maltreatment effects were not significantly smaller than ‘other adversity’ effects.
Moderating effects of subtypes of maltreatment were also examined, including physical, emotional, and sexual abuse, as well as physical and emotional neglect. No maltreatment subtype was significantly associated with hair cortisol and, when the five types of abuse were compared to one another, no differences among maltreatment types were observed.
We also tentatively hypothesized that adversity that occurred in childhood would show a significant but negative relation to HCC levels, compared to those not exposed to adversity in childhood. More recent adult adversity was hypothesized to be associated with significantly higher HCC levels. The time period during which the adversity occurred (i.e., childhood, adulthood, lifetime/unspecified) emerged as a significant moderator. Samples exposed to adversity in adulthood (d = 0.596, k = 6) had a significantly different effect size compared both to samples exposed in childhood (d=0.169, k =22; difference estimate= 0.430, 95% CI [0.028, 0.851]) and to samples with lifetime/ unspecified timing of trauma (d = −0.042, k =4; difference estimate= 0.640, 95% CI [0.093, 1.189]). Further, only samples exposed to adversity in adulthood had effects significantly different from zero (Table 5). There were no differences as a function of the specific adversity measure used (i.e., the specific questionnaires used to measure adversity; see Table 5) or the format of measurement (i.e., interview, questionnaire, other).
3.4.4. Features of hair samples
Several features of the hair cortisol sampling were examined as potential moderators of the adversity-HCC relation, though no specific hypotheses were advanced. The length of hair samples did not moderate results (b=0.022, 95% CI [−0.132, 0.174]). Thus, there were no significant differences by the time period covered by the hair cortisol assessment (1–2 months, 3 months,>3 months; Table 5). However, of these three time periods, only samples that corresponded to greater than 3 months of hair sampled were significantly different from zero (d=0.495).
Based on the eight studies that provided data on hair washing frequency (per week) and hair treatment, neither hair washing frequency (b=0.174, 95% CI [−0.095, 0.458]) nor hair treatment (b = −0.005, 95% CI [−0.025, 0.014]) significantly moderated the relation between adversity and HCC. In addition, no difference was observed as a function of the type of hair extraction procedure used (i.e., ImA or LCMS; Table 5).
4. Discussion
Although hair cortisol is a relatively new measure, a burgeoning literature associating adversity and HCC has emerged over the past decade. Prior research suggests that adversity may be significantly associated with both hyper- and hypo-activity of the HPA axis. In order to evaluate how adversity is related to HCC, this meta-analysis used a wide-ranging definition of adversity, including maltreatment (abuse and neglect), domestic violence, and exposure to other traumatic events, including accidents, war/combat, and natural disasters. The analysis aggregated findings from 28 studies that included 3397 participants from childhood to adulthood and from non-clinical and clinical samples.
The first objective of this meta-analysis was to quantify the magnitude of the effect of adversity on HCC. The first MLM analysis, in which all study effects were included, resulted in a small, albeit significant, positive association between adversity and HCC (d = 0.213, 95% CI [0.034, 0.397]). However, there was significant and large between-study and within-study heterogeneity, indicating significant sources of difference between effect sizes. Given that adversity may be linked to both hyper- and hypo-activity of the HPA axis, potentially resulting in elevated and lowered HCC levels, respectively, pooling negative and positive effects across studies risks yielding a null effect, when in fact there may be two important processes operating with different directions of effect.
Therefore, in relation to the second objective of the study, mixture modeling was used to test whether there were distinct categories of relations between adversity and HCC, that is, subgroups of studies with homogeneous effect sizes. The results of mixture modeling differentiated two coherent classes of effects. The first class included four studies with an overall negative and moderate effect size (d = −0.478), and the second class included the remaining 24 studies with an overall positive, though small, effect size (d=0.141). This finding is consistent with prior research, as well as with theories positing both HPA hypo- and hyper-activity following adversity. For example, adversity is thought to alter the HPA axis, resulting in dysregulation in the form of hyper-activation in the short-term hypo-activation in the long-term (Guilliams and Edwards, 2010).
Characteristics of the studies in the two classes were consistent with this broad prediction in that the four studies with negative effects on HCC all included measures of maltreatment beginning in childhood (three retrospective reports from adulthood, one current protective service involvement/parent report in childhood). Conversely, studies of adult experiences of trauma were clustered among the studies with positive effects on HCC. However, 16 studies of childhood maltreatment were also included in the class of 24 studies with small positive effects. Thus, it will be important to identify additional factors associated with childhood maltreatment that differentiate maltreated individuals with hypo- versus hyper-reactivity. For example, the chronicity and severity of maltreatment may contribute to variability (Gunnar et al., 2001). A prior meta-analysis found that in agency-referred samples, child maltreatment was associated with blunted wake-up salivary cortisol levels (Bernard et al., 2017), but no associations were found when maltreatment was self-reported, suggesting a measure- specific or severity-specific finding. Other factors such as the perpetrator of maltreatment (e.g., caregiver or stranger) and compensating environmental supports (e.g., positive family environment; peer relationships) might contribute to the HPA axis response to maltreatment (McCrory et al., 2010). Many of these factors were not reported frequently enough in the HCC literature to include in the current analyses and should be considered in future work.
To address the significant heterogeneity in effect sizes and the distinct positive and negative effect size classes, we evaluated several factors that might moderate the relation between adversity and HCC in a series of moderator analyses (Objective 3).
4.1. Features of adversity as moderator
The age at which adversity occurred moderated the relation between adversity and HCC, such that adversity that occurred in adulthood produced significantly different effects than adversity experienced during childhood/adolescence or adversity that occurred during a non-specific time frame. This finding is in line with the results of the mixture modelling indicating that the studies of childhood maltreatment fell into two distinct classes, with one characterized by a small overall positive effect and the other by a robust negative effect. While the moderation effect alone might indicate simply that more recent adversity produces larger elevations in cortisol, the mixture modelling further indicates that the age at which adversity is experienced does not produce a single linear attenuation process based on time elapsed since the adversity. Instead, age of adversity appears to be associated with the occurrence of two distinct processes with opposite directions of effect. Thus, results suggest that adversity is best understood as associated with diverging effect sizes, which include both positive and negative effects.
In regard to variation based on adversity type, results indicated that the specific type of adversity impacted the magnitude of effects. Studies of natural disasters produced different effects compared both to studies of maltreatment and studies of unspecified types of trauma (see Table 5). However, when maltreatment was compared to all other types of adversity (i.e., a dichotomous comparison), maltreatment did not differ significantly from other forms of adversity. Again, as noted above, in a subgroup of studies maltreatment was associated with lower hair cortisol levels (i.e., studies that comprise the first class from the mixture modeling), which might act to reduce the mean effect size associated with maltreatment in these moderation analyses.
Although we conducted separate analyses for the conceptually distinct effects of age and type of adversity, it should be noted that age of adversity and type of adversity were highly confounded in the existing literature. Of the 22 studies classified as assessing adversity during childhood/adolescence, 20 assessed effects of childhood maltreatment and only two assessed effects of a natural disaster (experienced in adolescence). In contrast, all studies of adversity experienced during adulthood assessed effects of other forms of adversity (domestic violence, natural disaster, etc.). Therefore, given the distributions of sample age, timing, and types of adversity in the existing literature, the moderator results can only reliably assess the distinction between maltreatment occurring in childhood/adolescence and other forms of trauma occurring in adulthood.
Due to the paucity of studies, we could not assess reliably whether other types of adversity in childhood (e.g., exposure to natural disasters, accidents) yield significant effects on HCC. In addition, in 17 of 19 studies of childhood maltreatment, maltreatment was assessed through adult retrospective report encompassing all of childhood. Only two studies assessed childhood maltreatment during childhood, through parent report and protective service involvement. Thus, we also do not have enough data to evaluate whether maltreatment assessed in childhood would yield the same pattern of effects as that yielded here by cumulative childhood maltreatment reported in adulthood.
Some prior evidence also suggests that the impact of adversity on the HPA axis varies based on stage of development at exposure (Bosch et al., 2012; Trickett et al., 2010). However, except for the two child samples included, the studies that explored adversity in relation to HCC in child samples either did not include relevant data in the manuscript or used a broader definition of adversity (e.g., parental separation, bullying) and thus were not included here. Further studies are needed assessing HCC in childhood, both in relation to experiences of maltreatment and in relation to other forms of adversity, to better differentiate age of occurrence from type of adversity and to elucidate developmental effects.
Prior research has also shown differences among maltreatment subtypes on salivary cortisol (e.g., Cicchetti and Rogosch, 2001), such that emotional maltreatment is associated with high morning cortisol levels whereas physical neglect is associated with low morning cortisol levels (Bruce et al., 2009). Many of these studies of salivary cortisol were conducted in childhood. Among studies on HCC included in the present analyses, seven studies provided outcome data for separate types of maltreatment (see Table 2), but all seven studies were adolescent or adult samples retrospectively reporting childhood maltreatment. In the current analyses that compared effects of abuse, neglect, and witnessing domestic violence on HCC, none of the maltreatment subtypes differed. Thus, cortisol effects associated with different types of maltreatment may be more evident when assessed in childhood compared to adolescence/adulthood (as done here), or effects may be more evident in salivary than hair measures. Longitudinal research is needed to assess subtypes of maltreatment across childhood and adolescence in association with both hair and salivary cortisol to further examine how types of maltreatment impact regulation of the HPA axis.
Notably, in all the above analyses of childhood maltreatment, there are potential issues of validity related to adult retrospective reporting of childhood adversity. Although a review of the literature concluded that false positive reports are rare, there are substantial false negative reports (i.e., non-reporting of abuse) (Hardt and Rutter, 2004). Thus, adult retrospective reports of maltreatment may underrepresent adversity and should be interpreted with caution, particularly when recalling very early experiences or experiences that are heavily reliant on interpretation (Hardt and Rutter, 2004).
4.2. Sample characteristics
As stated by Russell et al. (2012), “there is a dearth of knowledge on the effect of factors such as ethnicity, age, [and] sex…on hair cortisol content” (p. 598). Given other recent meta-analytic work on this issue (Stalder et al., 2017), it was not our aim to examine main effects of sample characteristics on HCC. Instead, we explored whether sample characteristics moderated associations between adversity and HCC. First, we found that the clinical status of the sample moderated results, such that clinical samples had significantly smaller effects compared to non-clinical samples, and only non-clinical samples produced effects that were significantly different from zero (Table 5). We did not find that samples with a PTSD diagnosis differed from samples without a PTSD diagnosis.
While the moderator analyses tell us that the overall mean effects were smaller among clinical groups, they do not tell us whether effects in clinical samples were more likely to yield significant negative effects, suggesting hypo-reactivity of the HPA axis. Notably, the mixture modelling did place three clinical samples within the class with a moderate negative effect on HCC (all also reported childhood maltreatment). This indicates that adversity in clinical samples (similar to maltreated samples) might be more likely to manifest as hypo-reactivity of the HPA axis. However, this interpretation is tentative, given that several clinical samples were also classified in the small positive effect-size class. In addition, given the overlap in studies using clinical samples and assessing maltreatment, it is difficult to disentangle the extent to which psychopathology, maltreatment, or an interaction between psychopathology and maltreatment were contributing to HPA hypo-reactivity.
In relation to this point, in prior studies using salivary cortisol, there is evidence that different mental health disorders are associated with different forms of dysregulation in HPA axis functioning (e.g., depression with elevated cortisol and PTSD with lowered cortisol; Knorr et al., 2010; Wahbeh and Oken, 2013). Prior research has also found interacting effects of abuse and depression on HPA-axis activity (Heim et al., 2001). In response to a CRH (corticotropin releasing hormone) challenge, women with a history of abuse but no depression had relatively higher ACTH responses whereas women with depression (with or without abuse history) had relatively lower ACTH responses, and those with depression and abuse had the most blunted cortisol responses. This suggests that although abuse may lead to HPA alterations, the nature of these alterations may change in interaction with psychiatric symptoms. Thus, the clinical composition of studies should be considered when interpreting HCC results. Unfortunately, current literature does not allow exploration of how particular kinds of psychopathology, with or without abuse, relate to HCC. More studies are needed to compare effects of psychopathology, maltreatment, and their additive and interactive effects on HCC.
We also found that race moderated findings, such that samples with more non-White participants produced a larger positive relation between adversity and HCC. Prior studies have documented variation in HCC by race/ethnicity. For example, in a study of pregnant women, Black women were found to have higher HCC than White women across all trimesters of pregnancy, and Hispanic women were found to have higher HCC than non-Hispanic White women in the second and third trimesters of pregnancy (Schreier et al., 2015). Some researchers (see Brunst et al., 2014) have proposed that different races may respond differentially to stress exposures via a variety of possible mechanisms, including genetic, lifestyle, and social differences. However, it is also possible that in the present analyses race may be confounded by a third unmeasured variable, such as cumulative environmental risk (e.g., income, education, refugee status, etc.). Future research is needed to explore further how race relates to an array of other risk variables in moderating associations between adversity and HCC.
We also explored whether participant age at the time of study participation (i.e., child, adolescent, adult) moderated results and did not find evidence of moderation. Importantly, as noted earlier, age of participant differed from age of experienced adversity (child/adolescent, adult, not specified) because in 18 studies adolescent or adult participants reported on adversity experienced in childhood. Thus, these findings should be interpreted cautiously.
There were several other sample characteristics that did not moderate adversity-HCC associations, including sex, medication use, smoking status, and BMI. One limitation of comparing these participant factors across studies is that studies varied greatly in their reporting of such factors. In fact, a number of sample characteristics (i.e., % White, % medication, % smokers, BMI) were reported in fewer than 20 studies. Given that this literature is in its infancy, despite the nonsignificant associations found here, future research should continue to explore how sample characteristics are associated with HCC and whether such characteristics moderate the relation between adversity and HCC.
4.3. Features of hair samples
We also explored whether the association between adversity and HCC varied based on qualities of the hair samples. Prior meta-analytic findings showed that HCC declines by almost 30% from the first proximal 3-cm hair segment to the second most proximal 3-cm hair segment (Stalder et al., 2017). The length of hair samples and corresponding time period assessed by hair samples (i.e., 1 to 2 months, 3 months, > 3 months) are overlapping variables. However, given that length is a continuous variable and time period is a categorical variable, they were not entirely overlapping. First, we found that only hair samples corresponding to > 3 months produced moderator effect sizes significantly different from zero (d=0.495; Table 5). However, moderator effects were not significant when comparing the three different time periods of hair sampling (i.e., 1 to 2 months, 3 months,>3 months) or when examining hair length as a continuous moderator (Table 4). Thus, it is unclear whether hair length or the time period indexed by the hair sample moderates the link between adversity and HCC, and future research should continue to explore hair length and corresponding time period as potential moderators of HCC effects.
We also did not find that hair washing frequency or hair treatment moderated effects of adversity on HCC. In contrast, prior meta-analytic work (Stalder et al., 2017) found that hair-washing frequency was negatively associated with HCC. It is possible that the nonsignificant effect here is related to the limited power in these moderation analyses, as only eight studies provided information regarding hair washing. However, to date, no mechanism has been advanced to explain why hair washing might affect HCC or the relation between HCC and adversity. It is also possible that other sample characteristics that are associated with hair washing frequency, such as race, culture, or country, may be confounding these findings. Taken together, results suggest that hair washing frequency and hair treatment might be associated with HCC levels but do not specifically impact the association between adversity and HCC.
4.4. Theoretical considerations related to HPA activation
The results from both the multi-level mixture analyses and the moderator analyses can be understood in the context of theories of HPA activation. The two classes of effect sizes that emerged from the mixture modelling suggest that adversity may be associated with both HPA axis hyper-activation and hypo-activation. Hyper-activation may be due to sensitization of the HPA axis following stress, resulting in a more reactive stress system. For example, heightened activity of the amygdala results in increased stimulation of the paraventricular nucleus of the hypothalamus (McEwen, 2007), contributing to greater HPA activity. Heightened activation of the HPA axis can lead to depletion of neurochemicals responsible for cortisol production (e.g., CRH receptor down-regulation; Heim et al., 2000), leading to HPA hypo-activity over time (Guilliams and Edwards, 2010).
In addition, the moderation results related to the time period in which adversity occurred and the type of adversity experienced can also be understood in the context of HPA axis development and allostatic load. Prior research suggests that adversity experienced during sensitive periods of development can produce unique alterations to the allostatic systems and resulting HPA axis activity (Danese and McEween, 2012). For example, childhood adversity can result in biological embedding that leads to enduring modifications in the maturation and responsiveness of allostatic systems (Danese and McEwen, 2012). Adversity during stress-sensitive periods when brain plasticity is high may have a particularly large impact on allostatic load (Ganzel and Morris, 2011). The time period of adversity may also influence the direction of effects on HPA activity (Ganzel and Morris, 2011). For example, one study found that CRH was higher in adult women who experienced childhood abuse after the age of 6 compared to those who experienced abuse before the age of 6 (Heim et al., 2008). Therefore, age at the time of adversity might impact the direction of effects on subsequent HPA axis development. As noted earlier, research is needed to separate effects of timing of adversity from type of adversity, as both might have a unique impact on allostatic changes.
4.5. Strengths and limitations
This meta-analysis has many strengths, including the range of adversity assessed (i.e., maltreatment, domestic violence, war/combat, natural disaster, accident), the breadth of assessment measures captured (i.e., a range of interviews and questionnaires), and the examination of several adversity, hair, and participant moderators. Despite its many strengths, this meta-analysis has some limitations.
First, this meta-analysis is constrained by the methodological variability of the studies included. Because studies varied greatly in the measures utilized and the metrics used to examine similar moderators, comparing variables across studies was challenging. For example, although many studies provided the racial/ethnic distribution of the sample, the way in which the distributions were reported varied greatly (e.g., % White, % minority). We chose to report the percentage of the sample who were White because this was the most frequently used indicator of race/ethnicity. However, it is an estimate that does not capture the richness of the possible racial distributions within each sample.
Second, there are other factors that are theoretically important to the observed effect, but which we were not able to assess given that those factors were not assessed in enough individual studies. For example, the time elapsed since an adverse event occurred likely influences both the strength and direction of effects. However, such timing information was not reported in many individual studies. In addition, timing may be difficult to ascertain given that many types of adversity may be chronic, intermittent, or ongoing. In addition, prior research suggests that the developmental timing of maltreatment has unique implications for the development of the HPA axis (Gunnar and Hostinar, 2015; Hostinar et al., 2015). However, there were not enough studies available to allow us to test whether the developmental timing of maltreatment (e.g., early childhood versus adolescence) influences the direction and magnitude of effects on HCC. Further, it is likely that timing of adversity experience and time lapse since adversity occurred were confounding each other given that many samples were adolescents/adults retrospectively reporting adversity that occurred during childhood. Future research that includes samples across the lifespan who vary in amounts of time since exposure are needed to elucidate the potentially separable effects of developmental stage at occurrence of adversity and time lapsed since adversity. Finally, examining the joint impact of childhood adversity and subsequent adversity on HCC may have revealed important insights. However, only one study included data for groups who experienced both childhood trauma and subsequent interpersonal violence (Morris et al., 2017). We urge future researchers to assess the impact of multiple forms of adversity on HCC to uncover the potential impact of repeated adversity on the HPA axis.
In addition, we were not able to determine whether specific kinds of psychopathology differentially moderated effects because few studies provided data for separate types of psychopathology. In the developmental context, only White et al. (2017) provided descriptive statistics for a sample with distinct internalizing/externalizing problems. We encourage future researchers to provide data on psychopathology in their samples to permit the examination of multiple forms of psychopathology as moderators of the effect of adversity on HCC.
Also, data for some of the moderators assessed (% White, % taking medication, % smokers, BMI, hair treatment, hair washing frequency) were provided in a limited number of studies. Based on MCMC estimation guidelines, moderators should include 20 or more clusters. Thus, these moderator analyses should be interpreted with caution.
It should also be noted that the current meta-analysis included two studies (*Groer et al., 2015; *Pacella et al., 2017) that derived their effects from the number of PTSD symptoms in association with HCC. Importantly, effects derived from PTSD symptom measures were not significantly different from effects derived from other kinds of adversity measures (difference estimate=0.475, CI [−0.364, 1.258]). These two studies were included to maximize the number and representativeness of the studies contributing to the analyses. However, the inclusion of these two studies should be considered when interpreting results.
Finally, while we comprehensively explored the impact of individual factors as moderators of the relation between HCC and adversity, we were unable to examine the interactions among moderators. Given the complex profiles of individuals who have experienced adversity, future research is needed to examine more complex moderation models. Future studies should also explore potential protective factors and their interactions with risk factors as possible moderators of adversity-HCC associations.
5. Conclusion
The present meta-analysis demonstrates that adversity is significantly related to hair cortisol levels. Results point to coherent classes of effects in the literature, with one class indexing a significant, albeit small, increase in HPA activity consistent with hyperactivity, and another indexing a robust and significant negative effect on HPA activity, consistent with hypoactivity in response to adversity. Notably, all studies with significant negative effects of adversity on HCC included a measure of childhood maltreatment. These findings underscore a particular need for studies across development in order to map how the HPA system responds to different forms of adversity during different periods of development.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
When using MCMC, (Bayesian) credible intervals are reported instead of (frequentist) confidence intervals. With a large prior variance (as is the case here) and large samples, both approaches should lead to comparable results, but the former are more accurate in smaller samples (Muthén and Asparouhov, 2012)
The 3-class model separated the positive class into two: a larger class with a small and positive effect size (n = 15, d = .109, 95% CI [0.068, 0.149]) and a smaller class with a larger effect size (n = 15, d = .706, 95% CI [0.473, 0.940]). One additional study (Morris et al., 2017) was also included in the negative class.
* indicates studies included in the current meta-analysis
References3
- Aguilera G, 2011. HPA axis responsiveness to stress: implications for healthy aging. Exp. Gerontol 46 (2–3), 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alink LRA, Cicchetti D, Kim J, Rogosch FA, 2012. Longitudinal associations among child maltreatment, social functioning, and cortisol regulation. Dev. Psychol 48, 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Frost A, Bennett CB, Lindhiem O, 2017. Maltreatment and diurnal cortisol regulation: a meta-analysis. Psychoneuroendocrinology 78, 57–67. [DOI] [PubMed] [Google Scholar]
- *Boeckel MG, Viola TW, Daruy-Filho L, Martinez M, Grassi-Oliveira R, 2017. Intimate partner violence is associated with increased maternal hair cortisol in mother–child dyads. Compr. Psychiatry 72, 18–24. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Rothstein D, Cohen J, 2005. Comprehensive Meta-Analysis: a Computer Program for Research Synthesis (version 2.0) [computer Software] Biostat, Englewood. [Google Scholar]
- Bosch NM, Riese H, Reijneveld SA, Bakker MP, Verhulst FC, Ormel J, Oldehinkel AJ, 2012. Timing matters: long term effects of adversities from prenatal period up to adolescence on adolescents’ cortisol stress response. The TRAILS study. Psychoneuroendocrinology 37, 1439–1447. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Heninger G, 2003. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology 28 (6), 733–750. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S, 2009. Morning cortisol levels in pre-school‐aged foster children: differential effects of maltreatment type. Dev. Psychobiol 51, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunst KJ, Enlow MB, Kannan S, Carroll KN, Coull BA, Wright RJ, 2014. Effects of prenatal social stress and maternal dietary fatty acid ratio on infant temperament: Does race matter? Epidemiology (Sunnyvale, Calif.) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier MJ, Walker JR, Naimark B, 2010. Separate and cumulative effects of adverse childhood experiences in predicting adult health and health care utilization. Child Abuse Negl 34, 454–464. [DOI] [PubMed] [Google Scholar]
- Cheung MWL, 2015. Meta-analysis: A Structural Equation Modeling Approach John Wiley & Sons. [Google Scholar]
- Chrousos GP, 2009. Stress and disorders of the stress system. Nat. Rev. Endocrinol 5, 374–381. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, 2001. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Dev. Psychopathol 13 (4), 783–804. [PubMed] [Google Scholar]
- Cohen J, 1988. A power primer. Psychol. Bull 112, 155–159. [DOI] [PubMed] [Google Scholar]
- Cooper H, Hedges L (Eds.), 1994. The Handbook of Research Synthesis Russell Sage, New York. [Google Scholar]
- Danese A, McEwen BS, 2012. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav 106 (1), 29–39. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, 2004. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull 130, 355–391. [DOI] [PubMed] [Google Scholar]
- *do Prado CH, Grassi-Oliveira R, Daruy-Filho L, Wieck A, Bauer ME, 2017. Evidence for immune activation and resistance to glucocorticoids following childhood maltreatment in adolescents without psychopathology. Neuropsychopharmacology 42, 2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, Levine S, 2006. Foster children’s diurnal production of cortisol: an exploratory study. Child Maltreat 11, 189–197. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CG, Vermetten E, van Dyck R, Bremner JD, 2003. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology 28 (9), 1656. [DOI] [PubMed] [Google Scholar]
- *Etwel F, Russell E, Rieder MJ, Van Uum SH, Koren G, 2014. Hair cortisol as a biomarker of stress in the 2011 Libyan war. Clin. Investig. Med 37, 403–408. [DOI] [PubMed] [Google Scholar]
- *Fischer S, Duncko R, Papadopoulos A, Hatch SL, Hotopf M, Cleare AJ, 2016. Sociodemographic, lifestyle, and psychosocial determinants of hair cortisol–Evidence from a south London community sample. Psychoneuroendocrinology 76, 144–153. [DOI] [PubMed] [Google Scholar]
- *Ford JL, Boch SJ, McCa\rthy D, 2016. Feasibility of hair collection for cortisol measurement in population research on adolescent health. Nurs. Res 65, 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH, 2005. A new view on hypocortisolism. Psychoneuroendocrinology 30, 1010–1016. [DOI] [PubMed] [Google Scholar]
- Galea S, Nandi A, Vlahov D, 2005. The epidemiology of post-traumatic stress disorder after disasters. Epidemiol. Rev 27, 78–91. [DOI] [PubMed] [Google Scholar]
- Gallagher P, Reid KS, Ferrier IN, 2009. Neuropsychological functioning in health and mood disorder: modulation by glucocorticoids and their receptors. Psychoneuroendocrinology 34, S196–S207. [DOI] [PubMed] [Google Scholar]
- Ganzel BL, Morris PA, 2011. Allostasis and the developing human brain: explicit consideration of implicit models. Dev. Psychopathol 23 (4), 955–974. [DOI] [PubMed] [Google Scholar]
- *Gao W, Zhong P, Xie Q, Wang H, Jin J, Deng H, Lu Z, 2014. Temporal features of elevated hair cortisol among earthquake survivors. Psychophysiology 51, 319–326. [DOI] [PubMed] [Google Scholar]
- Gibson EL, Checkley S, Papadopoulos A, Poon L, Daley S, Wardle J, 1999. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosom. Med 61 (2), 214–224. [DOI] [PubMed] [Google Scholar]
- Gow R, Thomson S, Rieder M, Van Uum S, Koren G, 2010. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci. Int 196, 32–37. [DOI] [PubMed] [Google Scholar]
- *Groer MW, Kane B, Williams SN, Duffy A, 2015. Relationship of PTSD symptoms with combat exposure, stress, and inflammation in American soldiers. Biol. Res. Nurs 17, 303–310. [DOI] [PubMed] [Google Scholar]
- Guilliams TG, Edwards L, 2010. Chronic stress and the HPA axis. The standard 9, 1–12. [Google Scholar]
- Gunnar MR, Hostinar CE, 2015. The social buffering of the hypothalamic–pituitary–adrenocortical axis in humans: developmental and experiential determinants. Soc. Neurosci 10, 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Morison SJ, Chisholm K, Schuder M, 2001. Salivary cortisol levels in children adopted from Romanian orphanages. Dev. Psychopathol 13, 611–628. [DOI] [PubMed] [Google Scholar]
- Hamra G, MacLehose R, Richardson D, 2013. Markov chain Monte Carlo: an introduction for epidemiologists. Int. J. Epidemiol 42 (2), 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, Rutter M, 2004. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J. Child Psychol. Psychiatry 45 (2), 260–273. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Stewart JG, Wynne-Edwards KE, 2011. Cortisol reactivity to social stress in adolescents: role of depression severity and child maltreatment. Psychoneuroendocrinology 36, 173–181. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I, 1985. Statistical Methods for Meta-analysis Academic Press, San Diego, CA. [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH, 2000. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 25, 1–35. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB, 2001. Altered pituitary–adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry 158, 575–581. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB, 2008. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33 (6), 693–710. [DOI] [PubMed] [Google Scholar]
- *Heinze K, Lin A, Reniers RL, Wood SJ, 2016. Longer-term increased cortisol levels in young people with mental health problems. Psychiatry Res 236, 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hinkelmann K, Muhtz C, Dettenborn L, Agorastos A, Wingenfeld K, Spitzer C, Otte C, 2013. Association between childhood trauma and low hair cortisol in depressed patients and healthy control subjects. Biol. Psychiatry 74, e15–e17. [DOI] [PubMed] [Google Scholar]
- *Hoffman MC, Davis EP, Ross RG, 2017. 596: maternal childhood trauma is associated with both maternal and newborn perinatal stress. Am. J. Obstet. Gynecol 216, S351–S352. [Google Scholar]
- Hostinar CE, Johnson AE, Gunnar MR, 2015. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Dev. Sci 18, 281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hox JJ, van de Schoot R, Matthijsse S, 2012. How few countries will do? Comparative survey analysis from a Bayesian perspective. Surv. Res. Methods 6 (July 2), pp. 87–93. [Google Scholar]
- Kaess M, Parzer P, Mattern M, Plener PL, Bifulco A, Resch F, Brunner R, 2013. Adverse childhood experiences and their impact on frequency severity, and the individual function of nonsuicidal self-injury in youth. Psychiatry Res 206, 265–272. [DOI] [PubMed] [Google Scholar]
- *Kaess M, Parzer P, Mehl L, Weil L, Strittmatter E, Resch F, Koenig J, 2017. Stress vulnerability in male youth with Internet Gaming Disorder. Psychoneuroendocrinology 77, 244–251. [DOI] [PubMed] [Google Scholar]
- Kalmakis KA, Chandler GE, 2014. Adverse childhood experiences: towards a clear conceptual meaning. J. Adv. Nurs 70, 1489–1501. [DOI] [PubMed] [Google Scholar]
- *Kalmakis KA, Meyer JS, Chiodo L, Leung K, 2015. Adverse childhood experiences and chronic hypothalamic–pituitary–adrenal activity. Stress 18, 446–450. [DOI] [PubMed] [Google Scholar]
- *Keenan-Devlin L, 2015. The weight of structural violence:syndemic stress and obesity among black urban youth in the US. Diss. Abstr. Int
- Klaassens ER, Giltay EJ, Cuijpers P, van Veen T, Zitman FG, 2012. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: a meta-analysis. Psychoneuroendocrinology 37, 317–331. [DOI] [PubMed] [Google Scholar]
- *Knorr U, Vinberg M, Kessing LV, Wetterslev J, 2010. Salivary cortisol in depressed patients versus control persons: a systematic review and meta-analysis. Psychoneuroendocrinology 35, 1275–1286. [DOI] [PubMed] [Google Scholar]
- *Luo H, Hu X, Liu X, Ma X, Guo W, Qiu C, Hannum G, 2012. Hair cortisol level as a biomarker for altered hypothalamic-pituitary-adrenal activity in female adolescents with posttraumatic stress disorder after the 2008 Wenchuan earthquake. Biol. Psychiatry 72, 65–69. [DOI] [PubMed] [Google Scholar]
- Madigan S, Brumariu LE, Villani V, Atkinson L, Lyons-Ruth K, 2016. Representational and questionnaire measures of attachment: a meta-analysis of relations to child internalizing and externalizing problems. Psychol. Bull 142, 367. [DOI] [PubMed] [Google Scholar]
- Manenschijn L, Koper JW, Lamberts SWJ, van Rossum EFC, 2011. Evaluation of a method to measure long term cortisol levels. Steroids 76, 1032–1036. [DOI] [PubMed] [Google Scholar]
- *Mayer SE, 2017. Examining the Relationships between Chronic Stress, HPA Axis Activity, and Depression in a Prospective and Longitudinal Study of Medical Internship. Dissertations. Abstracts International
- McCrory E, De Brito SA, Viding E, 2010. Research review: the neurobiology and genetics of maltreatment and adversity. J. Child Psychol. Psychiatry 51 (10), 1079–1095. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 1998. Protective and damaging effects of stress mediators. N. Engl. J. Med 338, 171–179. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2007. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev 8, 873–904. [DOI] [PubMed] [Google Scholar]
- Meewisse M-L, Reitsma JB, de Vries G-J, Gersons BPR, Olff M, 2007. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br. J. Psychiatry 191, 387–392. [DOI] [PubMed] [Google Scholar]
- *Melhem NM, Munroe S, Marsland A, Gray K, Brent D, Porta G, Driscoll H, 2017. Blunted HPA axis activity prior to suicide attempt and increased inflammation in attempters. Psychoneuroendocrinology 77, 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Miller GE, Chen E, Zhou ES, 2007. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull 133, 25. [DOI] [PubMed] [Google Scholar]
- Morris M, Mielock A, Abelson J, Rao U, 2017. 20 Psychobiology of cumulative trauma: hair cortisol as a risk marker for stress exposure. Biol. Psychiatry 81, S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B, 2010. Bayesian Analysis in Mplus: A Brief Introduction Unpublished manuscript 203 www.statmodel.com/download/IntroBayesVersion. [Google Scholar]
- Muthén B, Asparouhov T, 2012. Bayesian structural equation modeling: a more flexible representation of substantive theory. Psychol. Methods 17, 313–335. [DOI] [PubMed] [Google Scholar]
- Muthén B, Asparouhov T, 2013. BSEM measurement invariance analysis. Mplus Web Notes 17, 1–48. [Google Scholar]
- Muthén LK, Muthén B, 2017. Mplus User’s Guide, 8th ed. Muthén, Los Angeles: Muthén &. [Google Scholar]
- Nagin DS, 1999. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol. Methods 4, 139–157. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthén BO, 2007. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct. Equ. Model 14 (4), 535–569. [Google Scholar]
- Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, Arseneault L, 2011. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biol. Psychiatry 70, 1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Pacella ML, Hruska B, Steudte-Schmiedgen S, George RL, Delahanty DL, 2017. The utility of hair cortisol concentrations in the prediction of PTSD symptoms following traumatic physical injury. Soc. Sci. Med 175, 228–234. [DOI] [PubMed] [Google Scholar]
- *Reichl C, Heyer A, Brunner R, Parzer P, Völker JM, Resch F, Kaess M, 2016. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology 74, 203–211. [DOI] [PubMed] [Google Scholar]
- Reynolds RM, 2013. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis—2012 curt richter award winner. Psychoneuroendocrinology 38, 1–11. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, 1991. Effect sizes: pearson’s correlation, its display via the BESD, and alternative indices. Am. Psychol 46, 1086–1087. [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S, 2012. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 37, 589–601. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU, 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev 21, 55–89. [DOI] [PubMed] [Google Scholar]
- Sauve B, Koren G, Walsh G, Tokmakejian S, Van Uum SHM, 2007. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin. Invest. Med 30, E183–E191. [DOI] [PubMed] [Google Scholar]
- *Schalinski I, Elbert T, Steudte-Schmiedgen S, Kirschbaum C, 2015. The cortisol paradox of trauma-related disorders: lower phasic responses but higher tonic levels of cortisol are associated with sexual abuse in childhood. PLoS One 10, e0136921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Schreier HM, Enlow MB, Ritz T, Gennings C, Wright RJ, 2015. Childhood abuse is associated with increased hair cortisol levels among urban pregnant women. J. Epidemiol. Community Health 69, 1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Schury K, Koenig AM, Isele D, Hulbert AL, Krause S, Umlauft M, Guendel H, 2017. Alterations of hair cortisol and dehydroepiandrosterone in mother-infant-dyads with maternal childhood maltreatment. BMC Psychiatry 17, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JG, Badcock PB, Whittle SL, Byrne ML, Mundy L, Patton JC, Olsson JC, Allen NB, 2016. The lifetime experience of traumatic events is associated with hair cortisol concentrations in community-based children. Psychoneuroendocrinology 63, 276–281. [DOI] [PubMed] [Google Scholar]
- Spiga F, Walker JJ, Terry JR, Lightman SL, 2014. HPA axis-rhythms. Compr. Physiol 4, 1273–1298. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, 2012. Analysis of cortisol in hair–state of the art and future directions. Brain Behav. Immun 26, 1019–1029. [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, Miller R, 2017. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology 77, 261–274. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kivimäki M, 2013. Stress and cardiovascular disease: an update on current knowledge. Annu. Rev. Public Health 34, 337–354. [DOI] [PubMed] [Google Scholar]
- *Steudte S, Kolassa IT, Stalder T, Pfeiffer A, Kirschbaum C, Elbert T, 2011. Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology 36, 1193–1200. [DOI] [PubMed] [Google Scholar]
- *Steudte S, Kirschbaum C, Gao W, Alexander N, Schönfeld S, Hoyer J, Stalder T, 2013. Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biol. Psychiatry 74, 639–646. [DOI] [PubMed] [Google Scholar]
- *Steudte-Schmiedgen S, Stalder T, Schönfeld S, Wittchen HU, Trautmann S, Alexander N, Kirschbaum C, 2015. Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology 59, 123–133. [DOI] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S, Kirschbaum C, Alexander N, Stadler T, 2016. An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: Insight from recent hair cortisol findings. Neurosci. Biobehav. Rev 69, 124–135. [DOI] [PubMed] [Google Scholar]
- Tein JY, Coxe S, Cham H, 2013. Statistical power to detect the correct number of classes in latent profile analysis. Struct. Equ. Model. A Multidiscip. J 20 (4), 640–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Trautmann S, Muehlhan M, Kirschbaum C, Wittchen HU, Höfler M, Stalder T, Steudte‐Schmiedgen S, 2018. Biological stress indicators as risk markers for increased alcohol use following traumatic experiences. Addict. Biol 23, 281–290. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW, 2010. Attenuation of cortisol across development for victims of sexual abuse. Dev. Psychopathy 22, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner-Cobb J, 2005. Psychological and stress hormone correlates in early life: a key to HPA-axis dysregulation and normalisation. Stress. Int. J. Biol. Stress 8, 47–57. [DOI] [PubMed] [Google Scholar]
- Van den Noortgate W, López-López JA, Marín-Martínez F, Sánchez-Meca J, 2013. Three-level meta-analysis of dependent effect sizes. Behav. Res. Methods 45 (2), 576–594. [DOI] [PubMed] [Google Scholar]
- Wahbeh H, Oken B, 2013. Salivary cortisol lower in posttraumatic stress disorder. J. Trauma. Stress 26, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Wells S, Tremblay PF, Flynn A, Russell E, Kennedy J, Rehm J, Graham K, 2014. Associations of hair cortisol concentration with self-reported measures of stress and mental health-related factors in a pooled database of diverse community samples. Stress 17, 334–342. [DOI] [PubMed] [Google Scholar]
- Wennig R, 2000. Potential problems with the interpretation of hair analysis results. Forensic Sci. Int 107, 5–12. [DOI] [PubMed] [Google Scholar]
- Wessa M, Rohleder N, Kirschbaum C, Flor H, 2006. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology 31, 209–215. [DOI] [PubMed] [Google Scholar]
- *White LO, Ising M, von Klitzing K, Sierau S, Michel A, Klein AM, Uhr M, 2017. Reduced hair cortisol after maltreatment mediates externalizing symptoms in middle childhood and adolescence. J. Child Psychol. Psychiatry 58, 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie QZ, Gao W, Li JF, Qiao T, Jin J, Deng HH, Lu ZH, 2011. Correlation of cortisol in 1-cm hair segment with salivary cortisol in human: hair cortisol as an endogenous biomarker. Clin. Chem. Lab. Med 49, 2013–2019. [DOI] [PubMed] [Google Scholar]


