Abstract
Atherosclerosis, the principal cause of cardiovascular death worldwide, is a pathological disease characterized by fibro-proliferation, chronic inflammation, lipid accumulation, and immune disorder in the vessel wall. As the atheromatous plaques develop into advanced stage, the vulnerable plaques are prone to rupture, which causes acute cardiovascular events, including ischemic stroke and myocardial infarction. Emerging evidence has suggested that atherosclerosis is also an epigenetic disease with the interplay of multiple epigenetic mechanisms. The epigenetic basis of atherosclerosis has transformed our knowledge of epigenetics from an important biological phenomenon to a burgeoning field of cardiovascular research. Here, we provide a systematic and up-to-date overview of the current knowledge of three distinct but interrelated epigenetic processes (including DNA methylation, histone methylation/acetylation, and non-coding RNAs), in atherosclerotic plaque development and instability. Mechanistic and conceptual advances in understanding the biological roles of various epigenetic modifiers in regulating gene expression and functions of endothelial cells (vascular homeostasis, leukocyte adhesion, endothelial-mesenchymal transition, angiogenesis, and mechanotransduction), smooth muscle cells (proliferation, migration, inflammation, hypertrophy, and phenotypic switch), and macrophages (differentiation, inflammation, foam cell formation, and polarization) are discussed. The inherently dynamic nature and reversibility of epigenetic regulation, enables the possibility of epigenetic therapy by targeting epigenetic “writers”, “readers”, and “erasers”. Several Food Drug Administration-approved small-molecule epigenetic drugs show promise in pre-clinical studies for the treatment of atherosclerosis. Finally, we discuss potential therapeutic implications and challenges for future research involving cardiovascular epigenetics, with an aim to provide a translational perspective for identifying novel biomarkers of atherosclerosis, and transforming precision cardiovascular research and disease therapy in modern era of epigenetics.
Keywords: epigenetics, atherosclerosis, DNA methylation, histone modification, non-coding RNA
1. Introduction
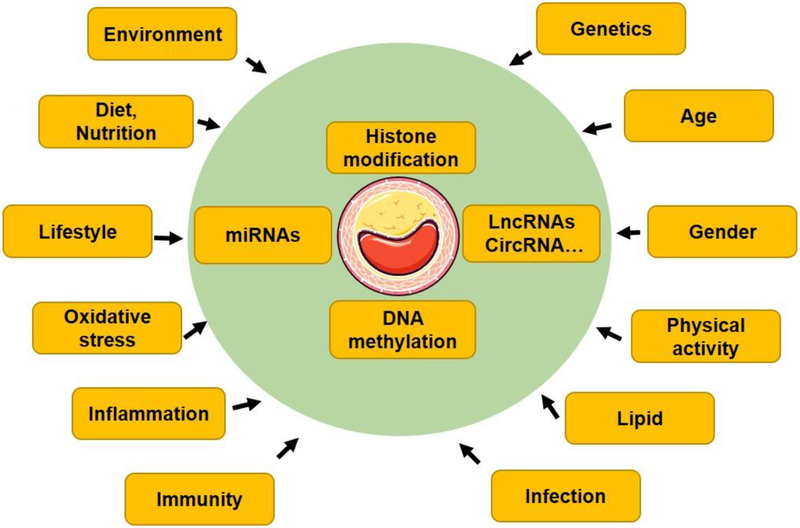
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide (Atlas Writing, et al., 2018; Benjamin, et al., 2018; Fang, Little, & Xu, 2018; S. Xu, Bai, Little, & Liu, 2014; S. Xu, Pelisek, & Jin, 2018; S. Xu, Y. Xu, et al., 2018). According to the 2018 Statistic Report of Heart Disease and Stroke released by the American Heart Association (Benjamin, et al., 2018), CVD causes 17.9 million deaths per year, and this number is expected to increase to >23.8 million by 2030. It is reported that 45.1 percent adults in the United States population have some form of CVD. CVD also inflicts major socioeconomic burdens in society. The direct and indirect costs of CVD and stroke exceed US$1.1 trillion annually (Benjamin, et al., 2018). The pathologies of CVD include atherosclerosis, cardiac hypertrophy, heart failure, hypertension, stroke, valvular heart disease, peripheral artery disease, and other circulatory disease conditions (Benjamin, et al., 2018). Atherosclerosis represents a major component of CVD that preferentially develops at branched or curved regions in medium and large sized arteries (Lusis, 2000). It is a multifactorial disease, which involves chronic inflammation (Libby, Ridker, & Maseri, 2002; Ross, 1999), lipid metabolism and accumulation (Gould, 1951), oxidative stress (Harrison, Griendling, Landmesser, Hornig, & Drexler, 2003), genetic predisposition (Lusis, 2012), immune disorders (Hansson & Hermansson, 2011), epigenetics (S. Xu, J. Pelisek, et al., 2018), and multiple non-genetic risk factors (environmental pollution, smoking, mental health, diet, and lifestyle) (J. Zhong, Agha, & Baccarelli, 2016) (Figure 1). The “multiple hit” hypothesis considers all these insults acting together to initiate atherosclerosis. After initial endothelial injury, endothelial dysfunction occurs, resulting in monocyte adhesion/transmigration/differentiation, lipid uptake, and the formation of “foam cell” (fatty streak). Subsequently, vascular smooth muscle cells (VSMCs) residing in media layer migrate to sub-endothelial space, leading to fibroatheroma formation and atherosclerosis. In conditions of large necrotic core covered by thin fibrous caps, the plaques are prone to rupture (Bentzon, Otsuka, Virmani, & Falk, 2014; Davies, 1996; Finn, Nakano, Narula, Kolodgie, & Virmani, 2010; Little, Osman, & O’Brien, 2008), which lead to several life-threatening conditions, such as ischemic stroke and myocardial infarction (Hansson, Robertson, & Soderberg-Naucler, 2006). Although reducing low-density lipoprotein (LDL) level and cardiovascular events by statins and emerging inhibitors of proprotein convertase subtilisin/kexin type 9 (PCSK9), residual cardiovascular burden remains very high even in the subgroup of patients receiving statin therapy (Reith & Armitage, 2016). This reality underscores the importance to identify novel disease mechanisms and complementary therapeutic approaches; such approaches would need to work along with lipid-lowering therapies to delay the initiation and progression of atherosclerosis.
Figure 1. A conceptual “multiple hit” hypothesis for the development of atherosclerosis.
The “multiple hit” hypothesis consider all these insults acting together on genetically predisposed subjects to induce atherosclerosis.
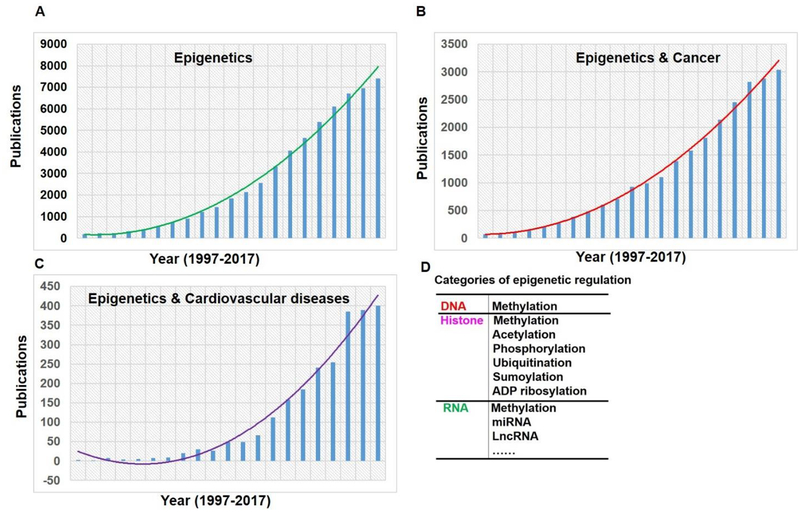
Epigenetics is a rapidly advancing and evolving field of biomedical research. More recently, C. David Allis (Rockefeller University) and Michael Grunstein (University of California, Los Angeles) have been awarded 2018 Albert Lasker Basic Medical Research Award for discoveries elucidating how histone modification influence gene expression (http://www.laskerfoundation.org). Histone and DNA modifications represent the two most common forms of epigenetic regulation, which have far-reaching implications in understanding molecular mechanisms of disease pathogenesis and designing new therapies for various diseases. Emerging evidence in the past two decades has suggested the importance of epigenetic mechanisms as a new layer of biological regulation in CVD. Epigenetics are critically involved in atherosclerosis plaque development and vulnerability (Costantino, et al., 2017). The term of “epigenetics”, was originally coined by Conrad Waddington in 1940s as ‘the branch of biology which studies the causal interactions between genes and their products, which bring the phenotype into being’ (Waddington, 1942). Since then, epigenetics has been considered being involved in embryonic development, imprinting, X chromosome inactivation, and other biological processes (Delcuve, Rastegar, & Davie, 2009). Currently, epigenetics is defined as “studies of heritable changes to the genome that occur independent of alternations in the primary DNA sequence” (Sharma, Kelly, & Jones, 2010). In atherosclerosis, several vascular cells (mainly including endothelial cells, VSMCs, and monocytes/macrophages) harbor global epigenetic alternations, which complement genetic abnormalities. Although much of our knowledge on the importance of epigenetics stem from the cancer research (Sharma, et al., 2010), research interest in cardiovascular epigenetics has significantly increased recently (Baccarelli, Rienstra, & Benjamin, 2010). The pathogenesis of cardiovascular diseases and cancers share many similar mechanisms, such as oxidative stress, inflammation, and susceptibility to common risk factors (Koene, Prizment, Blaes, & Konety, 2016). Starting from the mid-1990s’, there has been a steady growth in the number of publications in epigenetic research (Figure 2A). Cardiovascular epigenetic research has been growing exponentially in recent years (Figure 2B), albeit the increase in the number of cardiovascular publications has lagged behind cancer epigenetic research (Figure 2C). For example, only 3 cardiovascular epigenetic-related articles were published in 1997 and that number was just 49 in 2007. The projected number for 2017 is 401, amounting to near 10-fold increase since 2007.
Figure 2. Trends of cardiovascular epigenetics.
Yearly publication of epigenetic (A), cancer & epigenetic (B), cardiovascular disease & epigenetic (C). PubMed Search was performed on May 18, 2018 using the subject terms (epigenetic) for “epigenetic”; and (cancer AND epigenetic) for cancer epigenetics, and (cardiovascular disease AND epigenetic) for “cardiovascular disease”; Epigenetics (D) covers DNA and RNA methylation, histone modifications (acetylation, methylation, phosphorylation, etc.) and non-coding RNAs (micro-RNA and lncRNA-based mechanisms). These three broad categories of epigenetic modulation are distinct but are interrelated and coordinate to regulate gene expression.
Epigenetic modifications can be broadly categorized into: (1) DNA methylation and emerging RNA methylation; (2) Histone modifications (including methylation, acetylation, phosphorylation, sumoylation, ubiquitination, and ADP ribosylation); and (3) Non-coding RNAs mechanisms, such as microRNAs, and long non-coding RNAs (lncRNAs) (Khyzha, Alizada, Wilson, & Fish, 2017) (Figure 2D). Distinct from genetic mutations, epigenetic alternations are reversible, and susceptible to nutritional and environmental factors, and thus more accessible for modification and/or drug targeting (S. Xu, J. Pelisek, et al., 2018). Epigenetic disorder can be normalized by epigenetic cardiovascular therapies, making targeting epigenetic processes clinically and therapeutically relevant (Baccarelli, et al., 2010). Deepened understanding of the epigenetic basis of atherosclerosis could provide novel insights into mechanisms of atherosclerosis and support the potential of epigenetic mechanisms as druggable targets (Pons, et al., 2009). In this article, we provide a comprehensive review of diverse epigenetic mechanisms and epigenetic-targeted therapies in atherosclerotic plaque development and instability, and we also propose the rationale for developing novel therapeutic strategies by targetting epigenetic processes.
2. Epigenetic mechanisms in atherosclerosis
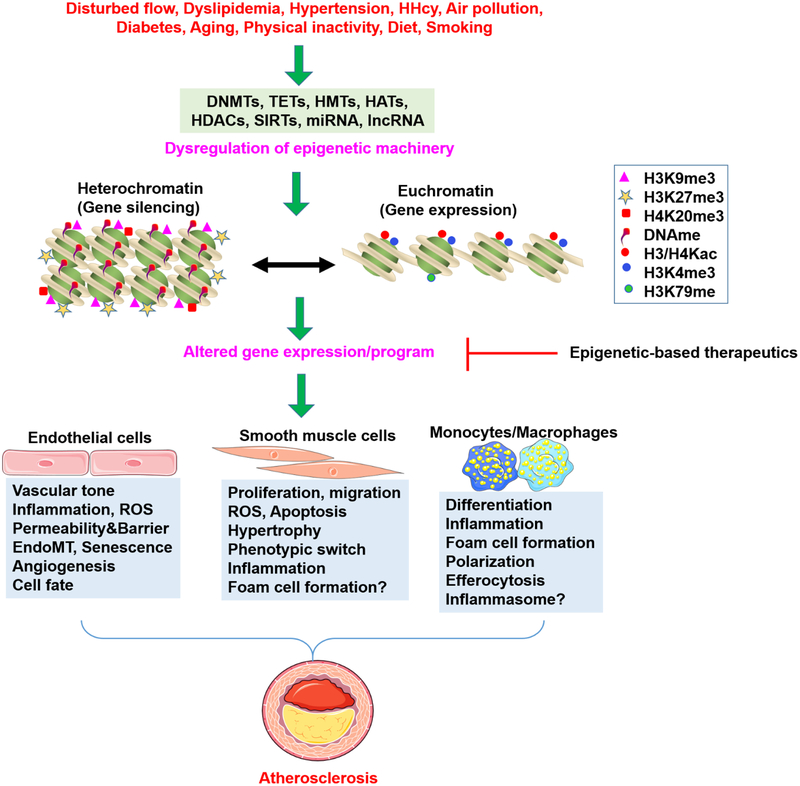
In the realm of epigenetic modifications (Gillette & Hill, 2015; Y. Yang, Hsu, Chen, & Yang, 2018), DNA/RNA methylation, and histone modifications have specific epigenetic “readers”, “writers”, and “erasers”. Epigenetic “readers,” have specialized domains, such as the plant homeodomain finger (which detects methylated histones), and bromo- and extra-terminal domain (BET) (which binds acetyllysine), that bind to various covalent histone modifications. Epigenetic “writers” include DNA methyltransferases (DNMTs, mediating DNA methylation), histone acetyltransferases (HATs, mediating histone acetylation), histone methyltransferases (HMTs, and histone methylation), and protein arginine methyltransferases (PRMTs, mediating arginine methylation). Epigenetic “erasers” include methylcytosine dioxygenase ten-eleven translocation (TET), histone deacetylases (HDACs), and sirtuins. The coexistence and coordinated actions of epigenetic readers, writers, and erasers fine-tune gene expression by recruiting either active epigenetic marks (H3K4me3, H3K36me3, and H3K79me3), or repressive epigenetic marks (such as H3K9me3, H3K27me3, and H4K20me3) to target gene promoters (Figure 3) (T. Zhang, Cooper, & Brockdorff, 2015). The specific role of DNA and histone-modifying enzymes in atherosclerosis and related vascular diseases is summarized in Table 1.
Figure 3. Epigenetic Modifications in the Development of Atherosclerosis.
Multiple disease-associated risk factors lead to dysregulated epigenetic machinery and altered the binding of multiple epigentic marks to target gene promoters. This leads to altered gene expression and cellular phenotypes, which directly drives atherosclerosis. Lysine methylation at H3K4, H3K79, and lysine acetylation of histone 3 and 4 (H3/H4Kac) is associated with euchromatin status and transcriptional activation, whereas methylation at H3K9, H3K27, and H4K20 is related to heterochromatin formation and transcriptional repression (Martin & Zhang, 2005). Altered gene expression/program can be modulated by several categories of epigenetic drugs discussed in this review.
Table 1.
Role of DNA and histone modification enzymes in experimental atherosclerosis and associated vascular diseases in vivo
| Animal models | Target | Phenotype | Reference |
|---|---|---|---|
| DNMT1Tg; ApoE−/− | DNMT1 | ↑ Plaques sizes ↑Macrophage inflammation ↑Increase PPAR-γ methylation ↓PPAR-γ |
(J. Yu, et al., 2016) |
| TET2KD; TET2OE | TET2 | OE↓Neointima hyperplasia KD↑ Neointima hyperplasia |
(R. Liu, et al., 2013) |
| Tet2OE; ApoE−/− Tet2KD; ApoE−/− | TET2 | OE↓ Plaque sizes, inflammation, ↑autophagy KD↑ Plaque sizes, inflammation, ↓autophagy |
(Peng, et al., 2016) |
| TET2ΔMye; LDLr−/− 10% TET2KO BMT | TET2 | ↑ Plaque sizes, IL-β-dependent inflammasome | (Fuster, et al., 2017) |
| TET2ΔHem BMT | TET2 | ↑ Plaque sizes, inflammatory cytokines/chemokines | (Jaiswal, et al., 2017) |
| EZH2OE; ApoE−/− | EZH2 | ↑ Plaque sizes, foam cell formation ↓ ABCA1-dependent cholesterol efflux |
(Lv, et al., 2016) |
| HDAC3KD; ApoE−/− | HDAC3 | ↑ Plaque sizes and vessel rupture in isografted vessels ↓EC survival |
(Zampetaki, et al., 2010) |
| HDAC3−/−; ApoE−/− | HDAC3 | ↓Plaques sizes ↑ Plaque stability |
(Hoeksema, et al., 2014) |
| HDAC9−/−; LDLr−/− | HDAC9 | ↑ Atherosclerotic plaques ↓Cholesterol efflux |
(Cao, Rong, et al., 2014) |
| HDAC9−/−; ApoE−/− | HDAC9 | ↑Plaque sizes and severity | (Azghandi, et al., 2015) |
| SIRT1TgEC; ApoE−/− | SIRT1 | ↓ Plaques sizes ↑Improved EC-dependent vasorelaxation ↑eNOS |
(Q. J. Zhang, et al., 2008) |
| SIRT1+/−; ApoE−/− | SIRT1 | ↑ Plaques sizes ↑NF-kB/LOX-1 pathway ↑Foam cell formation ↑Macrophage/T cell infiltration |
(Stein, Lohmann, et al., 2010) |
| SIRT1+/−; ApoE−/− | SIRT1 | ↑Endothelial activation, ↑ Vascular inflammation, - endothelium-dependent vasorelaxation p-eNOS (S1177), eNOS |
(Stein, Schafer, et al., 2010) |
| SIRT 1Tg; LDLr−/− | SIRT1 | Increased atherosclerotic lesions; Worse lipid profile; Increased Creb deacetylation |
(Qiang, et al., 2011) |
| SIRT1 ΔVSMC; ApoE−/− | SIRT1 | ↑ Atherosclerotic lesions; ↓ Fibrous cap thickness; ↑ VSMC DNA damage and senescence, media degeneration |
(Gorenne, et al., 2013) |
| SIRT1 ΔVSMC; ApoE−/−+Ang-II SIRT1 TgVSMC; ApoE−/−+Ang-II | SIRT1 | KO↑AAA formation and rupture, inflammaging KO↓ CR induced protection against AAA TG↓ AAA formation and rupture, inflammaging |
(H. Z. Chen, et al., 2016; Y. Liu, et al., 2016) |
| SIRT1 ΔVSMC+Ang-II | SIRT1 | ↑Disorganized elastic lamellae ↑Elastin fragmentation ↑ROS production, MMP2/9 activity ↑Aortic stiffness |
(Fry, et al., 2015) |
| SIRT1 T gVSMC+HFHS | ↓Arterial stiffness, inflammation, ROS | (Fry, et al., 2016) | |
| SIRT1ΔEC; ApoE−/− | SIRT1 | ↑ Atherosclerotic lesions | (Wen, et al., 2013) |
| SIRT1ΔMye | SIRT1 | ↑ Insulin resistance ↑Metabolic derangement ↑Hyperacetylation and activation of NF-kB |
(Schug, et al., 2010) |
| SIRT1ΔMye+Ang-II | SIRT1 | ↑ Incidence and severity of AAA ↑ M1 macrophages ↓M2 macrophages |
(Z. Zhang, et al., 2018) |
| SIRT2KD, SrRT2OE LDLr−/− | SIRT2 | OE↓Atherosclerotic lesion KD↑ Atherosclerotic lesion |
(B. Zhang, et al., 2018) |
| SIRT3−/−; LDLr−/− | SIRT3 | -Plaque size -Plaque vulnerability ↑ Weight gain |
(Winnik, et al., 2014) |
| SIRT6+/−; ApoE−/− | SIRT6 | ↑ Atherosclerotic lesion ↑ Necrotic core and unstable plaques |
(Z. Q. Zhang, et al., 2016) |
| SIRT6+/−; ApoE−/− | SIRT6 | ↑ Atherosclerotic lesion ↓ EC-dependent vasorelaxation |
(S. Xu, Yin, et al., 2016) |
| SIRT 6KD; ApoE−/− | SIRT6 | ↑Atherosclerotic lesion ↓EC-dependent vasorelaxation ↑ Unstable plaques ↑EC inflammation and monocyte adhesion |
(Z. Liu, et al., 2016) |
| JMJD1OE, JMJD1KD, balloon injury +HFD+STZ | JMJD1 | OE↑ neointimal hyperplasia KD↓ neointimal hyperplasia |
(J. Chen, et al., 2017) |
| JMJD3 Δmye+BMT (into LDLr−/−) | JMJD3 | ↑ Advanced atherosclerotic lesions ↑ Plaque necrosis -Plaque size |
(Neele, et al., 2018) |
| JMJD1KD+ balloon injury JMJD1KD+ left carotid artery partial ligation |
JMJD3 | ↓Neointimal hyperplasia ↓VSMC proliferation, migration, inflammation |
(Luo, et al., 2018) |
Abbreviations: AAA, abdominal aortic aneurysm; ABCA1, ATP binding cassette subfamily A member 1; Ang-II, angiotensin II; ApoE, apolipoprotein E; BMT, bone marrow transplantation; DNMT, DNA methyltransferase; EC, endothelial cell; eNOS, endothelial nitric oxide synthase; EZH2, enhancer of zeste homolog 2; HDAC, histone deacetylase; Hem, hematopoietic cells; HFHS, high fat high sucrose; JMJD3, JmjC domain-containing protein 3; JMJD1, JmjC domain-containing protein 1; KD, knockdown; KO, knockout; LOX-1, lectin-like oxidized LDL receptor 1; MMP, matrix metalloproteinase; NF-kB, nuclear factor-kappa B; OE, overexpression; PPAR, peroxisome proliferator-activated receptor SIRT, sirtuin; TET2, TET methylcytosine dioxygenase 2; Tg, transgene; VSMC, vascular smooth muscle cells; ΔHem, hematopoietic cell-knockout; ΔMye, myeloid cell-specific knockout; ΔVSMC, vascular smooth muscle cell-specific knockout.
2.1. DNA methylation/demethylation
Several studies reported increased TET1 expression, reduced DNMT1 expression (Greissel, et al., 2015), and decreased global DNA methylation (Aavik, et al., 2015; Wierda, et al., 2015) in atherosclerotic plaques compared to healthy control arteries. However, studies examining global methylation patterns in blood components yield contradictory results. A recent study in Chinese Han population has shown that 5-methylcytosine (5-mC) and DNMT1 expression are decreased in THP-1 macrophage-derived foam cells as well as in circulating leukocytes from patients with coronary artery disease (CAD) (Deng, et al., 2018). While, another study has shown elevated DNMT1 and decreased peroxisome proliferator-activated receptor gamma (PPAR-γ) levels in the monocytes of patients with atherosclerosis (J. Yu, et al., 2016). Potential reasons of these discrepancies include different ethnic groups of patients, different stages of disease, and the type of comparison (diseased artery compared to healthy control artery or disease-free adjacent regions). In the following sections, we will provide a synthesis on the context- and cell type-dependent gene regulation by DNA methylation in vitro and in vivo.
2.1.1. DNA methylation mediated by DNMTs
To date, there are three different DNMTs (i.e., DNMT1, DNMT3a and DNMT3b) in mammals. DNMT1 is the maintenance DNMT during mitosis, which preferentially methylates already hemimethylated DNA, thereby maintaining methylation status in cell replication. DNMT3a and DNMT3b are de novo methylating enzymes which act by directly adding methyl groups to unmethylated DNA (Jeltsch & Jurkowska, 2014). DNA methylation primarily occurs at specific dinucleotide sites (CpG islands). Generally, DNA methylation is a repressive modification that mediates gene silencing by inhibiting the binding of transcription complexes to target gene promoters. DNA methylation in the CpG regions can also promote the binding of methylated DNA binding proteins, such as methyl CpG binding protein 2 (MECP2), thereby repressing gene transcription by reducing the accessibility of promoter sequences to various transcription factors DNA (Jeltsch & Jurkowska, 2014). Generally, DNA methylation is fundamentally implicated in development and disease, including embryonic development, cell identity establishment, genomic imprinting, X chromosome inactivation, and lineage specification (Hanna, Demond, & Kelsey, 2018).
Aberrant DNA methylation is characteristic of many human diseases, such as cancer (Kulis & Esteller, 2010). A recent study (Wei, et al., 2018) has shown increased methylation of the promoter regions of SMAD7 (mothers against decapentaplegic homolog 7) and decreased expression of SMAD7 in atherosclerotic plaques, compared with healthy arteries. The methylation level of SMAD7 gene promoter were positively associated with the level of homocysteine and the risk score of carotid plaques, raising the notion that methylated SMAD7 represents a possible biomarker and therapeutic target for treating atherosclerosis (Wei, et al., 2018). Lectin-like oxidized LDL receptor-1 (LOX-1) is the principal scavenger receptor responsible for oxidized LDL (oxLDL) uptake in endothelial cells, and LOX-1 upregulation is associated with endothelial dysfunction (S. Xu, et al., 2013). DNA hypomethylation of LOX-1 was involved in hyperhomocysteinemia (HHcy)-induced endothelial cell injury. The mechanism is related to toll-like receptor 4 (TLR4)/nuclear factor-kappa B (NF-κB)/DNMT1 pathway (S. C. Ma, et al., 2017). HHcy, also causes hypermethylation of estrogen receptor-alpha (ERα) promoter region, thus allowing VSMCs to proliferate excessively, and thus contribute to the development of atherosclerotic lesions (Y. Huang, et al., 2007). Endothelial nitric oxide (NO) synthase (eNOS, also known as NOS3) is another example that is regulated by DNA methylation. eNOS is an important regulator of vascular homeostasis by regulating NO production via multi-sites phosphorylation in endothelial cells (Heiss & Dirsch, 2014). The expression of eNOS can be regulated at transcriptional, post-transcriptional, translational, and post-translational levels (Forstermann & Sessa, 2012; S. Xu, Liu, et al., 2016). Kruppel-like factor 2 (KLF2), the transcriptional factor of eNOS, is a critical anti-inflammatory and athero-protective transcriptional factor in vascular endothelium (Atkins, et al., 2008; SenBanerjee, et al., 2004). Multiple pro-inflammatory factors, such as lipopolysaccharides (LPS), can induce inflammatory response and KLF2 downregulation. Recent evidence [34] has suggested that epigenetic mechanisms are involved in LPS-induced KLF2 downregulation. Upon LPS stimulation, DNMT1-dependent methylation at 12 CpG sites of KLF2 is increased in HUVECs. Moreover, LPS induced KLF2 downregulation as well as the KLF2 downstream genes (E-selectin, vascular cellular adhesion molecule 1 (VCAM1), eNOS, and thrombomodulin) can be reversed by DNMT1 inhibition [34]. This study suggests that pro-inflammatory LPS stimulation leads to hypermethylation of the KLF2 gene promoter, thereby reducing its gene expression. The above-described studies indicate that complex epigenetic mechanisms coexist to regulate expression and activity of eNOS.
In 2014, three simultaneous studies (Dunn, et al., 2014; Y. Z. Jiang, et al., 2014; J. Zhou, Li, Wang, & Chien, 2014) reported that disturbed blood flow induced atherosclerosis in several murine models via DNMT1 and DNMT3a-dependent DNA methylation alterations of mechanosensitive transcriptional factors Homeobox protein A5, KLF3, and KLF4. Disturbed flow increases DNA methylation of promoters of three endothelial cell marker genes CD31 (cluster of differentiation 31), vWF (von Willebrand factor) and CDH5 (also known as VE-cadherin), but reduces the DNA methylation of distinct promoter regions of mesenchymal genes (CDH2, FSP1, and vimentin) (Lai, et al., 2018). The direct role of DNMTs in atherosclerosis has been demonstrated by the evidence that DNMT1 transgene increases atherosclerosis plaque area in ApoE−/− mice fed an atherogenic diet (J. Yu, et al., 2016). While, pharmacological inhibition of DNMTs by 5-Aza and its analogs inhibits experimental atherosclerosis induced either by atherogenic diets or partial ligation surgery (Cao, Wang, et al., 2014; Dunn, et al., 2014; Zhuang, et al., 2017). A reciprocal regulation between miR-143 and DNMT3a has been reported to mediate VSMC proliferation induced by homocysteine. One the one hand, miR-143 directly targets DNMT3a. One the other hand, increased DNMT3a expression causes the hypermethylation of miR-143 in homocysteine-induced VSMC proliferation (H. P. Zhang, et al., 2016). In addition, a recent study has found that higher methylation levels of cyclin-dependent kinase inhibitor 2A/2B (CDKN2A/2B) increases the risk for aortic arch and coronary artery calcification in patients with ischemic stroke (S. Zhou, et al., 2017; S. Zhou, et al., 2016).
In summary, methylation patterns of promoter regions of a plethora of genes contributing to atherosclerosis undergo significant changes during the disease development. The state of DNA methylation depends upon the expression of DNMTs, whose expression changes in atherosclerosis and is also regulated by various miRNAs. The current findings provide clear evidence of a concordant role and relevance of DNA methylation in atherosclerotic plaque development and progression toward vulnerable lesions.
2.1.2. TET-mediated DNA demethylation
A. TET1 and TET3
DNA demethylation is a counter mechanism for reactivating silenced genes induced by DNMTs. DNA demethylation can be catalyzed by TET methylcytosine dioxygenases family members, including TET1, TET2, and TET3, which convert 5-methylcytosine (5-mC) into 5-hydroxymethylcytosine (5-hmC). TET proteins also oxidize 5-hmC to 5-formylcytosine (5-fC) and 5-carboxylcytosine (5-caC) (X. Wu & Zhang, 2017). Increased expression of TET1 in line with global DNA hypomethylation was observed in advanced carotid atherosclerotic lesions compared with healthy arteries (Greissel, et al., 2015). The exact role of TET1 in atherosclerosis is not known. TET3 has been described being crucial for efficient DNA repair and maintaining genome stability (D. Jiang, Wei, Chen, Zhang, & Li, 2017), however, its role in atherosclerosis has yet to be elucidated.
B. TET2
Increasing evidence in the past several years have suggested an anti-atherosclerotic and vasoprotective role of TET2 (Y. Liu, et al., 2018). For example, Liu et al. (R. Liu, et al., 2013) have elegantly shown that TET2 functions as a master regulator of VSMC plasticity. Specifically, gain- and loss-of-function studies have shown that TET2 overexpression drives a contractile program (myocardin, serum response factor, alpha-smooth muscle actin) in VSMCs, while TET2 depletion activates a dedifferentiation program and induces kruppel like factor 4. Most importantly, TET2 negatively regulates intimal hyperplasia in response to arterial injury in vivo. Interestingly, the TET2 promoter itself can be methylated by DNMT1 in VSMCs, and this abnormal methylation status can be attenuated by 5-aza-2’-deoxycytidine treatment, which in turn increases 5-hmC enrichment in the myocardin gene promoter (Zhuang, et al., 2017).
The role of TET2 in endothelial cell function has also been recognized very recently. Specifically, TET2 expression is downregulated by disturbed blood flow in vitro and during the progression of atherosclerotic lesions in vivo. Compared with laminar blood flow, disturbed flow down-regulated autophagic markers-Beclin-1 and LCII/LCI, which can be reversed by TET2 overexpression. Moreover, TET2 positively regulates eNOS expression, while negatively regulating production of endothelin-1 (ET-1) in endothelial cells, suggesting a potential role of TET2 in maintaining endothelial homeostasis (Q. Yang, et al., 2016). Furthermore, TET2 overexpression decreases, while TET2 short hairpin RNA increases diet-induced atherosclerosis in ApoE−/− mice. The mechanism is related to TET2-mediated demethylation of Beclin-1 gene promoter, which contributed to the downregulation of oxLDL-induced impairment of endothelial cell autophagy and vascular inflammation (intracellular adhesion molecule 1 (ICAM1), VCAM1, interleukin 1 beta (IL-1β), and monocyte chemoattractant protein-1 (MCP1)) (Peng, et al., 2016). Another mechanism of TET2-mediated protection against oxLDL-induced endothelial dysfunction is exerted through activating the cystathionine gamma-lyase (CSE)/hydrogen sulphide (H2S) signaling pathway (which is atheroprotective (S. Xu, Liu, & Liu, 2014)) by promoting demethylation of CSE gene promoter (J. Peng, et al., 2017).
In monocytes/macrophages, TET2 regulates monocyte to macrophage differentiation and multiple facets of macrophage function. A DNA methylation dynamics study revealed that during differentiation from human monocytes to macrophages, substantial gene sets related to macrophage identity was dependent on TET2 mediated DNA demethylation (Vento-Tormo, et al., 2016). Li et al (X. Li, et al., 2015) have shown that TET2 gene and protein expression in macrophages is downregulated by oxLDL treatment, concurrent with decreased expression of autophagic markers. TET2 expression was upregulated by LPS treatment, potentially in a NF-κB-dependent manner. By this mechanism, TET2 inhibits LPS-induced macrophage activation via attenuating the expression of interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin 12 (IL-12), and promoting the macrophage phenotypic switch from M1 to M2 subtype (B. Li, Huo, Lin, & Wang, 2017). TET2 also restrains inflammation in murine macrophages and mice (Cull, Snetsinger, Buckstein, Wells, & Rauh, 2017). Recently, TET2 has been shown to selectively regulate macrophage inflammatory gene expression via DNA methylation-independent mechanisms. For example, TET2 recruits histone deacetylase 2 (HDAC2) to repress the transcription of pro-inflammatory gene IL6 via histone deacetylation (Q. Zhang, et al., 2015). In line with the important role of TET2 in regulating vascular cell functions, three seminal studies have recently shown that clonal hematopoietic TET2 deficiency drives atherosclerosis and heart failure in mice by increasing NACHT, LRR and PYD domains-containing protein 3 (NLRP3)/IL-1β-dependent inflammasome activation as well as pro-inflammatory pathways (Fuster, et al., 2017) (Jaiswal, et al., 2017) (Sano, et al., 2018). These evidence suggests the increase of TET2 expression and its demethylase activity by genetic manipulation or pharmacological activation could be exploited as a novel therapeutic strategy to combat atherosclerosis.
In summary, TETs, TET2 in particular, seem to be an important factor regulating the fate of VSMCs, endothelial hemostasis, and proper macrophage functions, which play crucial roles in atherosclerosis. Again, DNA demethylation works in concert with other epigenetic mechanisms, such as histone methylation/acetylation, thus accentuating a rather complex role of epigenetics in vascular diseases.
2.2. Histone modification
Core histone proteins, histone 3 (H3) and histone 4 (H4) in particular, are globular proteins that can be modified by multiple post-translational modifications, for example, acetylation, methylation, ubiquitination, phosphorylation, sumoylation, citrullination, and ADP-ribosylation (Z. Zhang, Wu, Stenoien, & Pasa-Tolic, 2014). In addition, lysine residues on H3 an H4 can be mono-, di-, or trimethylated by diverse histone methyltransferases (HMTs). The biological consequence of histone modification is gene transcription or repression, depending on the site of the modified residues, the type of chromatin remodeling factors, as well as specific type of modification. These modifications regulate the switch of chromatin status from a condensed heterochromatin to an open euchromatin (Bennett & Licht, 2018).
Histone acetyltransferases (HATs) and HDACs regulate the acetylation status of chromatin, while, histone HMTs and demethylases (HDMs) regulate methylation status of chromatin. The intricate interplay between epigenetic writers HATs, epigenetic erasers HDACs, and bromodomain-containing epigenetic readers provides a finely-tuned and reversible gene regulation pathway (Bennett & Licht, 2018). Histone acetylation by HATs (such as p300/CBP) generally promotes gene expression, whereas HDACs exert opposite effects. Sirtuins are class III NAD+-dependent HDACs that impact multiple cellular functions by orchestrating various key biological processes through the deacetylation of a number of histones (i.e., H3K9, H3K18, and H3K56) and non-histone protein substrates which are critically involved in regulating cell senescence, inflammation and metabolism, such as p53, liver X receptor (LXR), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), NF-kB and forkhead box protein O (FOXO) (D’Onofrio, Servillo, & Balestrieri, 2018; Sosnowska, et al., 2017; Vitiello, et al., 2017; Winnik, Auwerx, Sinclair, & Matter, 2015; S. Xu, Bai, & Jin, 2016). In contrast to histone acetylation, the role of histone methylation on gene transcription or repression is more complex and is site-specific. For example, H3K4me3 is an epigenetic mark that leads to gene expression, while H3K9me3, and H3K27me3 lead to gene repression. The histone-writing and -erasing enzymes also interact with DNMTs/TETs to drive context-dependent gene transcription or repression (Bennett & Licht, 2018).
2.2.1. Histone methylation/demethylation
To assess the role of histone methylation and demethylation in atherosclerosis, Greißel et al. (Greissel, et al., 2016; Greissel, et al., 2015) have recently systematically analyzed the expression pattern of histone methylation enzymes (by real-time PCR) and corresponding epigenetic marks (by immunohistochemistry) in human patients with different stages of atherosclerosis. Decreased expression of H3K9me3 and H3K27me3 was observed in plaque-derived VSMCs and inflammatory cells as well as whole atherosclerotic plaques (Greissel, et al., 2015). Moreover, the expression of H3K4me3 and its corresponding methyltransferase MLL2/4 was strongly associated with the severity of atherosclerosis (Greissel, et al., 2016). In the next section, we will discuss the specific role of histone methyltransferase in atherosclerosis.
2.2.1.1. Histone methyltransferases (HMTs)
So far, more than 50 humane lysine histone methyltransferases (HMTs, also named lysine [K] histone methyltransferases, KMTs) have been described. These transferases have high selectivity for specific targeted lysine residues (H3K4, H3K9, H3K27, H3K36, H3K79, and H4K20), as well as the degree of methylation, which is nicely summarized in various review articles (Morera, Lubbert, & Jung, 2016; Peter & Akbarian, 2011; X. Zhang, Wen, & Shi, 2012). In this review, we focused on selected HMTs that were intensively studied in the last three years.
A. Enhancer of zeste 2 (EZH2)
EZH2, the catalytic subunit of PRC2 (polycomb repressive complex 2), is one of the well-studied HMTs in cardiovascular development and diseases. PRC2 is an evolutionarily conserved complex with gene suppressing activities. Other components of PRC2 include Suz12, embryonic ectoderm development (EED), and RbAp48 (K. H. Kim & Roberts, 2016). PRC2 has histone methyltransferase activity and mainly trimethylates histone H3 lysine 27 (i.e. H3K27me3) on target gene promoters, leading to transcriptional silencing. PRC2 is critical for embryonic development, cell differentiation, and multiple human diseases (K. H. Kim & Roberts, 2016). Elevated LDL level is a major risk factor for atherosclerosis. One pro-atherogenic mechanism of LDL is to promote endothelial dysfunction. Kumar et al. (A. Kumar, et al., 2013) observed that LDL and its oxidized form (oxLDL) decrease the expression and activity of endothelial KLF2 via epigenetic mechanisms. LDL induces DNMT1 expression/activity and promotes the binding of MeCP2 and EZH2, whereas decreases the binding of the MEF-2 (myocyte enhancing factor-2), to gene promoters of KLF2 in endothelial cells. Pharmacological inhibition of DNMT1 or knockdown of DNMT1 or EZH2 prevents the downregulation of KLF2 by LDL. This finding suggests that LDL reduces KLF2 expression via enhancing DNA and histone methylation (A. Kumar, et al., 2013). More importantly, EZH2 overexpression exaggerates atherosclerosis lesion development in ApoE−/− mice fed a western-type diet, suggesting EZH2 upregulation drives atherosclerosis development in vivo (Lv, et al., 2016). Mechanistically, EZH2 promotes oxLDL induced foam cell formation in mouse and human macrophages by reducing ABCA1 mRNA and protein expression via DNMT1-mediated DNA methylation. While, pharmacological inhibition of DNMT1 or application of DNMT1 siRNA reversed EZH2 induced ABCA1 downregulation and foam cell formation, suggesting that EZH2 and DNMT1 act in concert to repress ABCA1-dependent cholesterol efflux and promote foam cell formation (Lv, et al., 2016). Similar to LDL, hyperhomocysteinemia (HHcy) is a risk factor for atherosclerosis. After challenging with high-methionine diet, EZH2 and corresponding H3K27me3 levels were increased in ApoE−/− mice. EZH2 overexpression increase, while EZH2 siRNA decrease, global H3K27me3 level and the accumulation of lipids (total cholesterol and triglycerides) in foam cells, suggesting that EZH2 is associated with HHcy-mediated atherosclerosis (Xiaoling, et al., 2016).
In endothelial cells, Tie2-Cre mediated targeted deletion of EZH2 in endothelial cells and hematopoietic cells causes embryonic lethality (Delgado-Olguin, et al., 2014; Neo, et al., 2018). EZH2 silencing in endothelial cells lead to the overrepresentation of genes in the Wnt signaling pathway (Dreger, et al., 2012) as well as the reactivation of vasohibin1, thereby regulating angiogenesis (C. Lu, et al., 2010). Recently, we (S. Xu, Y. Xu, et al., 2018) and others (Maleszewska, Vanchin, Harmsen, & Krenning, 2016) have identified that EZH2 gene and protein expression is downregulated by fluid shear stress (FSS) (which is generated by laminar flow). This EZH2 downregulation is responsible for laminar flow-mediated anti-inflammatory effects (S. Xu, Y. Xu, et al., 2018) and cell quiescence (Maleszewska, et al., 2016). FSS downregulates EZH2 via miR-101. By using next generation RNA-sequencing, insulin like growth factor binding protein 5 was identified as an EZH2 downstream target that mediates anti-inflammatory effects (S. Xu, Y. Xu, et al., 2018). In addition, EZH2 is also upregulated in sepsis rat hearts and LPS-induced cardiac microvascular endothelial cells, and EZH2 downregulation is involved in ulinastatin-induced protection against LPS-induced endothelial cell hyperpermeability and apoptosis (Z. Yu, Rayile, Zhang, Li, & Zhao, 2017).
In VSMCs, EZH2 promotes cell migration and proliferation, while suppressing apoptosis of pulmonary artery VSMC, and regulates the development of pulmonary arterial hypertension (PAH) (Aljubran, et al., 2012). EZH2 also suppresses a differentiation program of VSMCs. EZH2 deficiency reactivates the expression of myocardin and T-box 18, two important regulators of VSMC differentiation (Snitow, Lu, Cheng, Zhou, & Morrisey, 2016). Pharmacological inhibition of EZH2 by EPZ005687 reverses experimental PAH induced by transverse aortic constriction via targeting antioxidant gene superoxide dismutase 1 (SOD1) (Z. L. Shi, et al., 2018). This evidence suggests a potential role of EZH2 in regulating diseases-associated cardiovascular remodeling.
EZH2 also controls LPS-induced macrophage activation and inflammatory responses (X. Zhang, Y. Wang, et al., 2018). In particular, EZH2 mediates LPS-induced myeloid differentiation primary response 88 (MyD88)-dependent pro-inflammatory gene expression in macrophages by epigenetically silencing suppressor of cytokine signaling 3 (SOCS3, an anti-inflammatory gene) and dependent TNF receptor associated factor (TRAF6) ubiquitination. Genetic ablation (EZH2 deficiency) or pharmacological inhibition (by GSK126, a potent and specific inhibitor of EZH2 methyltransferase activity (McCabe, et al., 2012)) of EZH2 reduces the expression of multiple pro-inflammatory genes (IL-6, TNFα, and MCP1) in bone marrow-derived macrophages. Consistent with this evidence, EZH2 depletion also decreases TNFα expression by reducing nuclear expression of NF-κB p65 subunit (Y. Zhang, Q. Zhang, et al., 2018). Both studies highlight the therapeutic potential of EZH2 inhibitors to epigenetically control macrophage activation and treat inflammation-associated diseases (Neele & de Winther, 2018), including atherosclerosis. However, it must be noted that, in addition to functioning as a transcriptional repressor, EZH2 can also act as a transcriptional activator in cancer cells, an effect independent of PRC2-mediated H3K27 trimethylation (K. H. Kim & Roberts, 2016). EZH2 can also directly methylate non-histone substrate proteins, such as androgen receptor and signal transducer and activator of transcription 3 (STAT3), and control gene transcriptional activity or facilitate protein degradation via ubiquitination (K. H. Kim & Roberts, 2016). Tissue specific knockout (or inducible knockout) of EZH2 in endothelial cells, VSMCs, or macrophages are needed to address the tissue specific roles of EZH2 in atherosclerosis. Future studies are also warranted to elucidate direct and indirect effects of EZH2 in various vascular cells.
Taken together, emerging studies have suggested a pivotal role of EZH2 in mature vascular cells, in addition to its crucial functions in the development stage. Thus, EZH2 represents a promising therapeutic target for treatment inflammatory disorders, such as atherosclerosis. In the light of the important role of plaque angiogenesis driving plaque vulnerability (de Vries & Quax, 2016), further studies are warranted to evaluate the potential role of EZH2 in regulating plaque angiogenesis.
B. SET7/9 (SETD7)
Emerging evidence has also shown the involvement of other HMTs in regulating vascular functions. For example, SET7/9, a HMT that specifically monomethylates H3K4 (H3K4me1), and positively regulates inflammatory gene expression in endothelial cells (Keating, et al., 2014) and THP1 cells (Y. Li, et al., 2008), by functioning as a new coactivator of NF-κB. Depletion of SET7/9 by siRNA blocks TNFα induced inflammatory gene expression by inhibiting H3K4me3 and NF-κB p65 recruitment to promoters of MCP1 and TNFα (Y. Li, et al., 2008). Notably, in TNFα-treated monocytes, 25 percent of NF-κB downstream target genes, including H3K27me3 demethylase JmjC domain-containing protein 3 (JMJD3), is reduced by SET7/9 depletion. The final outcome is reduced monocyte adhesion to endothelial cells as well as VSMCs (Y. Li, et al., 2008). SET7/9 knockdown also reduces inflammatory gene upregulation in monocytes stimulated with S100B, an established ligand of receptor of advanced glycation end-products (AGEs) (Y. Li, et al., 2008). In agreement with this evidence, genetic depletion or pharmacological inhibition of SETD7/9 reduces oxidative stress (hydrogen peroxide and cigarette smoking extract) induced expression and production of IL-6 and IL8 by recruiting the binding of H3K4me1 to NF-κB p65 (S. He, Owen, Jelinsky, & Lin, 2015).
In summary, these results indicate that SETD7/9 is both important for delicate regulation of redox and inflammatory status of macrophages. Therefore, SET7/9 seems to play a relevant role in regulating atherosclerosis. However, it remains elusive whether genetic or pharmacological inhibition of SET7/9 (i.e., by Sinefungin (Sasaki, et al., 2016)) ameliorate atherosclerosis in pre-clinical animal models.
C. G9a (Ehmt2)
G9a (also known as Ehmt2) is a HMT responsible for mono-and di-methylation of H3K9 (H3K9me1 and H3K9me2). Depletion (by shRNA) or pharmacological inhibition of G9a (by BIX-01294) inhibits proliferation, and causes cell cycle arrest in human microvascular endothelial cells. This evidence suggests that G9a inhibition could suppress angiogenesis and tumor neovascularization (Wojtala, Macierzynska-Piotrowska, Rybaczek, Pirola, & Balcerczyk, 2018). As a potential therapeutic drug, BIX-01294 has already been shown to inhibit angiogenesis in human hepatocellular carcinoma cells (Oh, et al., 2015). BIX-01294 treatment also inhibits the proliferation and migration of VSMCs (Q. Yang, Lu, Singh, & Raj, 2012). Thus, these results are suggesting the therapeutic potential of G9a inhibitors in treating neointimal hyperplasia, pulmonary hypertension and atherosclerosis.
D. SUV39H1
SUV39H1 is responsible for H3K9 trimethylation (H3K9me3), which is a repressive epigenetic mark that leads to transcriptional silencing. Studies in cultured VSMCs and macrophages demonstrate a protective role of SUV39H1 in regulating vascular functions. For example, SUV39H1 depletion by shRNA increased inflammatory gene expression in normal human VSMCs, while, SUV39H1 overexpression inhibited TNFα induced upregulation of IL6 and MCP1 in VSMCs (Villeneuve, et al., 2008). In macrophages, high glucose treatment decreases protein level of SUV39H1 and global level of H3K9me3, as well as H3K9me3 recruitment to gene promoters of IL6, macrophage inflammatory protein (MIP) 1α, and MIP1β (M. F. Li, et al., 2016). Inhibition of SUV39H1 with chaetocin in the presence or absence of high glucose increases the expression of inflammatory cytokines (IL6, MIP1α, and MIP1β). However, SUV39H1 overexpression decreases the expression of these inflammatory cytokines at basal level and in the presence of high glucose (M. F. Li, et al., 2016). The role of SUV39H1 homolog-SUV39H2 in regulating vascular functions remains unknown. Taken together, this evidence suggests that increased expression of SUV39H1 could possibly confer protective effects against inflammation associated vascular dysfunction. It remains to be investigated whether SUV39H1 can protect against atherosclerosis in pre-clinical models of atherosclerosis.
2.2.1.2. Histone demethylases
Dysregulation of histone methylation by methyltransferases or demethylases are associated with various diseases. Albeit histone methylation has been discovered more than 50 years ago (Murray, 1964), the methylation of lysine residues was considered as irreversible for a long time, until in 2004 Shi et al. reported that a lysine-specific demethylase 1A (KDM1A) demethylates H3K4 (Y. Shi, et al., 2004). Ever since, a plethora of other HDMs has been found in humans (Hyun, Jeon, Park, & Kim, 2017). In this article, we update HDMs described in the last three years in the context of vascular function and atherosclerotic plaque development.
A. UTX (Kdm6a)
Ubiquitously transcribed tetratricopeptide repeat, X chromosome (UTX) is H3K27me3 demethylase that regulates inflammation in macrophages. UTX gene expression was downregulated in macrophages after treatment with LPS (a TLR4 ligand), polyinosinic:polycytidylic acid (Poly (I:C), a TLR3 ligand) or CpG (a TLR9 ligand) (X. Li, et al., 2017). Further studies revealed that UTX depletion inhibits LPS induced IL6 production by increasing the enrichment of H3K27me3 epigenetic mark at IL6 promoter (X. Li, et al., 2017). Consistent with this evidence, UTX was also downregulated after long-term exposure (after 48 h) to particulate matter 2.5 (PM 2.5). PM2.5 increases pro-inflammatory cytokine expression by increasing the binding of H3K4me3 and H3K9me3 to IL6 and interferon beta (IFNβ) promoters, while UTX depletion reduces PM2.5 induced IL6 and IFNβ production (J. H. Ma, et al., 2017). To date, the precise role of UTX in macrophage-derived foam cell formation and atherosclerosis remains unknown.
B. JMJD3 (Kdm6b)
JMJD3 is a specific H3K27me3 demethylase that is increased upon LPS stimulation via an NF-κB-dependent pathway (De Santa, et al., 2007). Interestingly, 70 percent of LPS-responsive genes were reported to be regulated by JMJD3 (De Santa, et al., 2009). Genetic deficiency (De Santa, et al., 2009) or pharmacological inhibition of JMJD3 activity by GSK-J1 inhibits LPS-induced TNFα expression (Kruidenier, et al., 2012). JMJD3 is also highly inducible by serum amyloid A (SAA, acute-phase protein that potently triggers inflammatory response) in macrophages and regulates SAA-induced inflammatory gene expression (Q. Yan, et al., 2014). JMJD3 depletion attenuated SAA-induced expression of pro-inflammatory genes (such as TREM-1) in vitro (cultured macrophages) and in vivo (peritonitis model), along with enrichment of H3K27me3 onto target gene promoters. More importantly, JMJD3 depletion reduces oxLDL-induced foam cell formation in SAA-treated macrophages (Q. Yan, et al., 2014). In addition, TNFα also upregulates JMJD3 expression in monocytes, and silencing of SET7/9 with siRNA reduces TNFα-induced JMJD3 expression (Y. Li, et al., 2008), indicating that SET7/9 dependent H3K4me3 methylation and JMJD3 dependent H3K27me3 demethylation could act in concert in driving pro-inflammatory gene expression. GSK-J4 also reduces cytokine (IFNγ, TNFα, and GM-CSF) production in Natural Killer cells, suggesting a broad pro-inflammatory role of JMJD3 in regulating inflammation (Cribbs, et al., 2018).
During the process of foam cell formation, the pro-fibrotic transcriptome signature is acquired. Using a high-throughput RNA-sequencing approach, Neele et al. (Neele, et al., 2017) recently observed decreased expression of pro-fibrotic genes in macrophage-derived foam cells in JMJD3-deficient cells, indicating the essential role of JMJD3 in regulating the pro-fibrotic transcriptome in foam cells. This study is in line with previous studies supporting the pro-inflammatory role of JMJD3 being a promising target to treat atherosclerosis (Neele, et al., 2017). JMJD3 is also involved in foam cell formation during mycobacterial infection (Holla, et al., 2016). In addition to foam cell formation, JMJD3 expression is increased by interleukin 4 (IL4) and is directly regulated by STAT6 in polarized M2 macrophages (Ishii, et al., 2009). JMJD3 is also essential for macrophage differentiation and M2 macrophage polarization in response to helminth infection and chitin. Mechanistic studies indicate that transcriptional factor interferon regulatory factor 4 (IRF4) was the direct target of JMJD3 that controls the expression of gene sets responsible for M2 macrophages polarization (Satoh, et al., 2010). Taken together, these findings suggest that JMJD3 is important for macrophage differentiation, foam cell formation, and M2 macrophage polarization, which are critical events in atherosclerosis (Satoh, et al., 2010).
In addition to mediating various macrophages functions, JMJD3 is also involved in LPS induced endothelial cell inflammation. LPS stimulation increases the expression and nuclear accumulation of JMJD3 in human endothelial cells (S. Yu, et al., 2017). LPS increases the recruitment of JMJD3, NF-κB, and H3K4me3, while decreasing the binding of H3K27me3 to promoters of multiple pro-inflammatory genes (TNFα, IL6, IL1 β etc), suggesting JMJD3 could synergize with NF-κB to drive inflammatory gene expression. JMJD3 expression can be regulated by anti-inflammatory agents. For example, in brain microvascular endothelial cells stimulated with TNFα, dexamethasone reduces the expression of JMJD3, via recruiting glucocorticoid receptor α and nuclear receptor co-repressor to JMJD3 gene promoters. Dexamethasone also induces the upregulation of claudin 5 and occludin genes (Na, et al., 2017). These findings suggest that dexamethasone could preserve the endothelial cell integrity under inflammatory conditions (Na, et al., 2017). In addition, JMJD3 gene expression and nuclear accumulation is increased in response to oxygen-glucose deprivation/reperfusion injury (OGD/RI) in mouse brain microvascular cells. Mechanistic studies suggest that JMJD3 is involved in OGD/RI induced IL6 upregulation via interaction with NF-κB (p65/p50 subunits) and CCAAT-enhancer-binding protein β (C/EBPβ) at the gene promoter of IL-6. The increased binding of JMJD3 and decreased binding of H3K27me3 to IL6 gene promoter upon OGD/RI, indicates that demethylase activity of JMJD3 is critical for IL6-mediated inflammatory responses (K. Lee, et al., 2012).
These findings open avenues for future therapeutic intervention of inflammation and atherosclerosis by targeting JMJD3 using pharmacological inhibitors or gene deletion. However, a recent study has shown that plaques from LDLr−/− mice transplanted with myeloid cell-specific deletion of JMJD3 bone marrow showed more advanced plaques with increased plaque necrosis (Neele, et al., 2018). Also genetic (siRNA mediated knockdown) or pharmacological inhibition (by cell permeable prodrug GSKJ4) of JMJD3 reduces balloon injury and partial carotid ligation induced neointimal hyperplasia in rodents, suggesting the important role of JMJD3 in vascular remodeling (Luo, et al., 2018). Although mounting evidence suggests that JMJD3 is involved in vascular dysfunction, it remain elusive whether or not pharmacological inhibition of JMJD3 can protect against atherosclerosis in pre-clinical models.
C. JMJD1 (KDM3a)
In a study of the role of H3K9 demethylase JMJD1 (KDM3a) in regulating VSMC function and associated neointimal hyperplasia, JMJD1 expression was increased, while global H3K9me2 level was decreased in diabetic vessels from rats fed high fat diet (J. Chen, et al., 2017). After 4 weeks of balloon injury, JMJD1 overexpression exacerbates, while JMJD1 siRNA attenuates neointima formation in streptozotocin-induced diabetic rats. Mechanistically, JMJD1 regulated the transcription of Angiontensin II receptor 1 and Rho-associated coiled-coil containing protein kinase 2 by reducing H3K9me2 binding to the proximal promoters of both genes (J. Chen, et al., 2017). This study identifies JMJD1 as a novel regulator of VSMC proliferation, migration and formation of neointimal hyperplasia in vivo, providing the first evidence of the role of JMJD1 in vascular remodeling. Since neointimal hyperplasia is a preliminary stage of atherosclerosis, it is interesting to speculate that JMJD1 might represent a promising therapeutic target for treating atherosclerosis at early stage or atherosclerosis occurring in association with hyperglycemia or diabetes.
2.2.2. Histone acetylation/deacetylation
Histone acetylation is catalyzed by HATs, which acetylate conserved lysine residues on histone proteins. In mammalian cells, three categories of HATs have been identified: Gcn5-related N-acetyltransferases (GNAT), MYST, and CREB-binding protein (CBP)/p300 (Roth, Denu, & Allis, 2001). Generally, histone acetylation untightens chromatin structure and thus increases gene expression. Opposite to the functions of HATs, HDACs remove acetyl groups on histone substrates, thus tightening chromatin structure and repressing gene expression. Based on domain structure, biological functions, and sequence homology to the yeast orthologues, HDACs are classified in four classes (Dokmanovic, Clarke, & Marks, 2007) (Matouk & Marsden, 2008): Type I (HDAC1, HDAC2, HDAC3, HDAC8), Type II (IIa: HDAC4, HDAC5, HDAC7, HDAC9; IIb: HDAC6, HDAC10), Type III (Sirtuin 1-Sirtuin 7, NAD+-dependent), and Type IV (HDAC11). The targets of HDACs include core histone proteins and some non-histone proteins. In the next section, we will overview the specific role of HATs (with a focus on p300) and individual HDAC isoforms (including sirtuins) in atherosclerosis in vitro and in vivo.
2.2.2.1. Histone acetylation enzyme p300
In the family of HAT proteins, p300 represents an important transcriptional coactivator and chromatin modifier that regulates gene transcription (Roth, et al., 2001). Based on the current literature, p300 primarily acts pro-atherogenic by interacting with and regulating NF-κB-dependent expression of multiple pro-inflammatory gene in vascular cells (Khyzha, et al., 2017). Clinically relevant, increased levels of acetylated H3K9 and H3K27 are observed in VSMCs, macrophages, and endothelial cells from human advanced atherosclerotic plaques, compared with healthy controls. The level of acetylated H3K9 in VSMCs and macrophages correlates with the extent of plaque severity (Greissel, et al., 2016; Greissel, et al., 2015). 12(S)-hydroxyeicosatetraenoic acid (12(S)-HETE) is an oxidized lipid metabolite derived from 12/15-lipoxygenase that promotes multiple dysfunctional events in VSMCs. 12(S)-HETE promotes H3K9/14Ac recruitment to IL6 and MCP1 gene promoters, which is attenuated by PP2 (an inhibitor of Src) and 12/15-lipoxygenase deficiency (Reddy, Sahar, Villeneuve, Lanting, & Natarajan, 2009). Activation of 5’ AMP-activated protein kinase (AMPK) by tool drug AICAR or a constitutively active mutant of AMPK (AMPK-CA) inhibits p300 acetyltransferase activity by promoting Ser89 phosphorylation, which decreases TNFα-induced p300-mediated acetylation of NF-κB. The outcome is reduced binding of NF-κB to promoters of pro-adhesive molecule VCAM1, and attenuated monocyte adhesion to endothelial cells (Y. Zhang, Qiu, Wang, Zhang, & Xia, 2011). Total or VSMC-specific deletion of KLF15 also increased inflammatory responses, and diet-induced atherosclerosis by altering the level of acetylated NF-κB via interaction with p300 (Y. Lu, et al., 2013). In line with this evidence, cholesterol crystals and 15(S)-HETE induce p300 tyrosine phosphorylation via reactive oxygen species (ROS) production. The phosphorylated p300 promotes STAT1 acetylation and its interaction with PPARγ, thereby inducing CD36-dependent oxLDL uptake and foam cell formation (Kotla & Rao, 2015; Kotla, Singh, & Rao, 2017). While, curcumin, a pharmacological inhibitor of p300 (Balasubramanyam, et al., 2004), has been reported to promote cholesterol efflux and display potent anti-inflammatory effects in macrophages via inhibiting JNK, NF-κB activation, while activating nuclear factor erythroid 2–related factor 2 (Nrf2) and LXRα pathway (T. Liu, et al., 2014; Y. Zhong, Feng, Fan, & Li, 2018). In summary, p300 is an important positive regulator of inflammation, oxidative stress, and macrophage-derived foam cell formation. Thus, p300 seems to be a promising target for reversing multiple cellular dysfunction in atherosclerosis.
2.2.2.2. Histone deacetylases
A. HDAC3
HDAC3 is a member of class I HDAC. It is implicated in the differentiation of endothelial progenitor cells (L. Zeng, et al., 2006). The first examination of HDAC3 in atherosclerotic conditions was performed by Zampetaki et al. (Zampetaki, et al., 2010), who showed that HDAC3 expression is increased in arterial regions of disturbed blood flow. In cultured endothelial cells, disturbed flow induced the phosphorylation of HDAC3 at serine/threonine residues and increase the protein stability of HDAC3. Gain- and loss-of-function assays suggest that HDAC3 interacts and activates Akt phosphorylation and activity, thereby regulating endothelial cell survival. In aortic isografts of ApoE−/− mice depleted with HDAC3 (by shRNA), an increase in atherosclerotic plaque area and mortality rate was observed in shHDAC3-infected grafts (with signs of basement membrane rupture). This finding suggests that HDAC3 is an endothelial cell survival molecule in atherosclerosis development in response to disturbed hemodynamic forces (Zampetaki, et al., 2010). In addition, HDAC3 also inhibits aspirin induced eNOS acetylation, ensuing NO production, and vasorelaxation (Jung, et al., 2010). In macrophages, van den Bossche et al. have shown that HDAC3 inhibition in macrophages renders an atheroprotective phenotype, by increasing histone acetylation and accompanying gene expression of efflux transporters ABCA1 and ABCG1, as well as increasing anti-inflammatory and anti-apoptotic capacities (Van den Bossche, et al., 2014). In human atherosclerotic plaques, HDCA3 is upregulated in ruptured plaques compared to stable plaques. Further study reveals that myeloid cell-specific deletion of HDAC3 (using bone marrow transplantation) favors plaque stability (by increasing plaque collagen content) in LDLR−/− mice fed an atherogenic diet (Hoeksema, et al., 2014). Furthermore, HDAC3-deficient macrophages acquired an anti-inflammatory phenotype and showe less foam cell formation. Moreover, HDAC3 deletion lead to pro-fibrotic program via epigenetic regulation of TGFβ1, which allows VSMCs to generate collagen to stabilize the plaques (Hoeksema, et al., 2014). Taken together, this evidence suggests the necessity to employ macrophage specific deletion of HDAC3 (without influencing the pro-survival effects in endothelial cells) as a promising strategy to prevent atherosclerosis development.
B. HDAC5
HDAC5 belongs to the family of class IIa HDAC. A previous study (X. Xu, et al., 2007) has found that Ang-II stimulates protein kinase D (PKD)-dependent HDAC5 phosphorylation (at Serine259/498 residues), and promotes HDAC5 nuclear export, thus mediating Ang-II-mediated VSMC hypertrophy. Mechanistic studies indicate that HDAC5 is recruited to histone H4 at the smooth muscle-alpha-actin promoter (Yoshida, Gan, & Owens, 2008). Laminar blood flow can also promotes phosphorylation-dependent nuclear export of HDAC5 and derepress the expression of KLF2 and downstream gene eNOS in endothelial cells (Kwon, Wang, Xu, & Jin, 2014; W. Wang, et al., 2010). Although direct evidence of HDAC5 in atherosclerosis is lacking, this evidence suggests that HDAC5 inhibition may confer protective effects against neointimal hyperplasia, and atherosclerosis.
C. HDAC9
HDAC9 belongs to the family of class IIa HDAC. Recent genome-wide association studies have identified several genetic variants of HDAC9 associated with carotid intima-media thickness, peripheral arterial disease, CAD, and ischemic stroke of large vessel (Hacke & Grond-Ginsbach, 2012; Malik, et al., 2017; H. S. Markus, et al., 2013; Matsukura, et al., 2015; Shroff, et al., 2018; X. B. Wang, et al., 2016). By immunohistochemical staining, HDAC9 was mainly expressed in VSMCs and endothelial cells of cerebral and systemic arteries. More importantly, HDAC9 gene expression was upregulated in carotid plaques from patients compared with plaque-free control arteries (H. S. Markus, et al., 2013). Although GWAS shows potential role of HDAC9 in atherosclerosis, the direct evidence of HDAC9 in atherosclerosis was demonstrated by Cao et al. (Cao, Rong, et al., 2014), who observed that HDAC9 expression is induced upon monocyte differentiation to macrophages and systemic deletion or hematopoietic cells-restricted deletion of HDAC9 attenuated atherosclerosis in LDLr−/− mice fed an atherogenic diet. The mechanism is linked to reduction of pro-inflammatory genes, and an increase of M2 macrophage polarization. HDAC9 deletion also promotes cholesterol efflux from macrophages via increasing acetylated H3 and H3K9 to efflux related gene promoter. The pro-atherogenic role of HDAC9 was later confirmed by others using ApoE−/− mice, another well-established model of atherosclerosis (Azghandi, et al., 2015). Studies in cultured cells also show that HDAC9 expression is increased in endothelial cells exposed to apoptosis-inducing dose of oxLDL. HDAC9 depletion reverses oxLDL-induced endothelial cell apoptosis and the inflammatory responses (by reducing TNFα and MCP1 expression) (X. Han, Han, Wang, Shen, & Dong, 2016). These findings collectively suggest that targeted inhibition of HDAC9 represents a novel promising strategy to reduce atherosclerosis.
D. Other HDACs
HDAC1 (type I HDAC) expression is increased and H3K9 acetylation (H3K9ac) is decreased in the aorta of ApoE−/− mice challenged with high methionine diet to induce hyperhomocysteinemia. HDAC1 overexpression decreased global H3K9ac level and promoted lipid accumulation in foam cells (Q. Zhao, et al., 2017). HDAC2 (type I HDAC) overexpression (but not HDAC 1, 3, or 8) in human aortic endothelial cells suppresses arginase 2 (Arg2) expression, while, HDAC2 depletion by siRNA increase the expression of Arg2. HDAC2 regulates Arg2 by direct binding to Arg2 gene promoter. Overexpression of HDAC2 functionally blocked oxLDL induced impairment of endothelium-dependent vasorelaxation (Pandey, et al., 2014). Similarly, in human aortic endothelial cells, oxLDL increases global level of protein NEDDylation (a post-translational modification of proteins linked to ubiquitination and degradation) and reduces HDAC2 expression. Further studies indicate that HDAC2 is a substrate for NEDD8 conjugation. Whereas, treatment with MLN4924 (an inhibitor of protein NEDDylation), prevented oxLDL induced HDAC2 downregulation and Arg2 upregulation, thereby improves endothelial function (Pandey, et al., 2015). These findings suggest that HDAC2 activation represents a novel therapy for endothelial dysfunction and atherosclerosis. HDAC4 (Class IIa HDAC). A multi-ethnic association study indicates a robust association of a single-nucleotide polymorphism (rs3791398) in HDAC4 with carotid intima/media thickness (Lanktree, Hegele, Yusuf, & Anand, 2009). Further evidence showing the involvement of HDAC4 in neointimal hyperplasia and atherosclerosis is lacking. HDAC6 (Class IIb HDAC) expression/activity was selectively increased in oxLDL-treated human aortic endothelial cells, and selective inhibition of HDAC6 by tubacin and HDAC6 siRNA increased CSE-dependent H2S production, and prevented oxLDL-induced oxidative injury in endothelial cells (Leucker, et al., 2017). This evidence suggests HDAC6 may represent a promising therapeutic target to prevent endothelial dysfunction and atherosclerosis development. The specific role of HDAC6 in atherosclerosis warrants further studies in experimental animal models using genetic and/or pharmacological inhibition of HDAC6.
E. SIRT1
Sirtuins (SIRTs) are class III NAD+-dependent histone deacetylases, which catalyze deacetylation/deacylation reactions on histone and protein substrates. This type of deacetylation generates deacetylated substrate, O-acetyl-ADP-ribose, and nicotinamide (Finkel, Deng, & Mostoslavsky, 2009). Some members of SIRTs also have mono-ADP-ribosylation activity (Hassa, Haenni, Elser, & Hottiger, 2006). SIRTs are activated during calorie restriction and are implicated in many pathophysiological processes, including aging and cell metabolism. To date, seven members of SIRTs have been identified: SIRT1 to SIRT7 (Finkel, et al., 2009). SIRTs share the conserved catalytic domain implicated in substrate deacetylation, but differ as to tissue distribution, intracellular localization, and substrate of choice and cellular functions. SIRT1, SIRT6 and SIRT7 are found mainly in cell nuclei, while SIRT3, SIRT4 and SIRT5 are localized in the mitochondria, and SIRT2 is localized only in the cytosol. Among SIRTs, SIRT1, SIRT2, SIRT3, and SIRT6 provide protective effects against atherosclerosis (D’Onofrio, et al., 2018; Sosnowska, et al., 2017; Vitiello, et al., 2017; Winnik, et al., 2015; S. Xu, Bai, et al., 2016).
SIRT1 is the best characterized nuclear-localized SIRT that has broad cardiovascular protective actions and metabolism-regulating effects (Chang & Guarente, 2014). Well-established substrates of SIRT1 include histones (such as acetylated H3K9, H3K18, and H3K56) and non-histone proteins (such as NF-κB, forkhead transcription factors (FOXOs), p53, peroxisome proliferator-activated receptor-γ co-activator-1α (PGC-1α), LXRα, 66-kDa Src homology 2 domain-containing protein (p66shc), eNOS, and several DNA damage repair proteins (Ku70 and DNA-PK)) (Kitada, Ogura, & Koya, 2016; S. Kumar, Kim, et al., 2017). In the vasculature, SIRT1 negatively regulates vascular inflammation, endothelial dysfunction, VSMCs proliferation and migration, ROS generation, foam cell formation, impaired autophagy, DNA damage response, and senescence, thereby preventing vascular aging, intimal hyperplasia, and atherosclerosis (D’Onofrio, et al., 2018; Sosnowska, et al., 2017; Vitiello, et al., 2017; Winnik, et al., 2015; S. Xu, Bai, et al., 2016). SIRT1 protein expression is decreased in mouse models of atherosclerosis and human patients (Gorenne, et al., 2013). SIRT1 deficient mice in ApoE−/− background increases atherosclerosis development (Stein, Lohmann, et al., 2010; Stein, Schafer, et al., 2010). In addition, endothelial cell-(Wen, et al., 2013), and VSMC-specific (Gorenne, et al., 2013) ablation of SIRT1 increases atherosclerosis development in mice. Macrophage-specific knockout of SIRT1 increased the incidence and severity of abdominal aortic aneurysm formation (induced by Ang-II) by increasing macrophage inflammation and modulating M1/M2 macrophage polarization (Z. Zhang, et al., 2018). Pharmacological inhibition of SIRT1 by EX-527 promotes atherosclerosis in ApoE−/− mice through autophagy impairment (X. Yang, et al., 2017). In contrast, pharmacological activation of SIRT1 by resveratrol (Berbee, et al., 2013; Fukao, et al., 2004; Howitz, et al., 2003; Norata, et al., 2007), SRT1720 (Y. X. Chen, Zhang, Cai, Zhao, & Dai, 2015), SRT3025 (Miranda, et al., 2015) inhibits experimental atherosclerosis. Also, endothelial cell-specific SIRT1 transgene attenuates atherosclerosis in ApoE−/− mice (Q. J. Zhang, et al., 2008). This evidence suggests an overall atheroprotective role of SIRT1 and that SIRT1 activation or overexpression may serve as a novel therapeutic strategy for treating atherosclerosis despite some controversy (Qiang, et al., 2011).
SIRT1 and endothelial function.
SIRT1 exerts endothelial protective effects by increasing eNOS-dependent NO production (Mattagajasingh, et al., 2007), anti-inflammatory pathways (C. W. Liu, et al., 2017; W. Pan, Yu, Huang, & Zhu, 2016), reducing oxidative stress (W. Zhang, Q. Huang, et al., 2017), endoplasmic reticulum (ER) stress (Kassan, et al., 2017), inflammasome activation (Y. Li, et al., 2016; Y. Li, et al., 2017), senescence (R. L. Li, et al., 2016), and improving autophagy dysfunction (J. Liu, et al., 2015; Y. Zhang, et al., 2016). Pharmacological activation of SIRT1 by SRT1720 attenuated LPS-induced lung injury, by decreasing endothelial tight junction permeability (C. Fu, et al., 2018; W. Zhang, Y. Zhang, et al., 2017). Also, SIRT1 activation was responsible for Ginkgolide B mediated anti-inflammatory effects in oxLDL-stimulated human endothelial cells via reducing LOX-1 and ICAM1 expression (Ma, et al., 2013). SIRT1 activation is also responsible for Apelin mediated protective effects against Ang-II mediated endothelial cell senescence (R. Yang, et al., 2018) as well as for chlorogenic acid mediated protective effects against H2O2-induced endothelial apoptosis and mitochondrial dysfunction (Tsai, et al., 2018). Endothelial-mesenchymal transition (EndoMT) is an important mechanism contributing to atherosclerosis (Souilhol, Harmsen, Evans, & Krenning, 2018). SIRT1 inhibits TGFβ-induced EndoMT via direct deacetylation of Smad4 (Z. Li, et al., 2018). SIRT1 activation by red wine polyphenol resveratrol inhibits high glucose-induced oxidative stress, mitochondrial dysfunction and ensuing apoptosis in human endothelial cells (S. Wang, Wang, Zhao, & Li, 2017), underscoring the therapeutic potential of SIRT1 activation in treating the cardiovascular complications of diabetes. SIRT1 expression is increased in circulating monocytes from patients with CAD, associated with upregulation of LOX-1-dependent oxidative stress, apoptosis, and increased monocytes adhesion to endothelial cells (Chan, et al., 2017).
SIRT1 and VSMC function.
In VSMCs, SIRT1 expression is gradually lost during advanced aging process in humans (Thompson, Wagner, & Rzucidlo, 2014). SIRT1 protects against oxidative DNA damage and inhibits atherosclerotic plaque development in hyperlipidemic mice, partially through activating Nijmegen breakage syndrome-1 (NBS-1) (Gorenne, et al., 2013) and 8oxoG DNA glycosylase I (OGG1) (Shah, et al., 2018). In contrast, VSMC-specific SIRT1 transgene attenuates Ang-II as well as injury induced vascular remodeling in mice (L. Li, Zhang, et al., 2011; Z. Liu, et al., 2014). In addition, SIRT1 transgene or pharmacological activation reduces MMP-2 production (induced by platelet activating factor (PAF)) via decreasing expression of PAF receptor in VSMCs, indicating the role of SIRT1 in preventing plaque destabilization (Y. H. Kim, Bae, Lee, Park, & Kim, 2015). Pharmacological activation (by resveratrol and SRT1720) or VSMC-specific overexpression of SIRT1 attenuates arterial stiffness induced by high-fat high-sucrose diet by reducing NF-κB dependent VCAM1 expression and vascular oxidative stress (Fry, et al., 2016). SIRT1 also inhibits Ang-II-induced VSMC hypertrophy (L. Li, Gao, et al., 2011) and associated inflammation, as well as migration of VSMC-derived foam cells after oxLDL stimulation (R. Yang, et al., 2017; M. J. Zhang, et al., 2016).
SIRT1 and macrophage function.
SIRT1 is a master regulator of inflammatory responses in macrophages by chromatin modulation, as macrophage specific deletion of SIRT1 leads to the hyperacetylation of NF-κB and dependent inflammatory genes expression in vitro and in vivo (Schug, et al., 2010). Macrophages from SIRT1+/− ApoE−/− mice show reduced oxLDL uptake and macrophage-derived foam cell formation. The mechanism is related to decreases in LOX-1 expression via suppressing the NF-κB pathway (Stein, Lohmann, et al., 2010). On the other hand, SIRT1 inhibits foam cell formation by deacetylating and activating liver X-receptor (LXR)-dependent ABCA1 and ABCG1 expression, thereby promoting the reverse cholesterol transport (H. T. Zeng, et al., 2013). The net outcome of these effects is the retardation of foam cell formation in macrophages. More recently, Du et al. (C. Du, et al., 2018) have shown that CSE/H2S treatment increases SIRT1 deacetylase activity and activated target proteins p53, p65, and SREBPs, thereby reducing vascular inflammation, inhibiting macrophage cholesterol uptake and hepatic cholesterol synthesis. Mechanistic studies show that CSE/H2S induces SIRT1 sulfhydration at zinc finger domains and prevents SIRT1 degradation (C. Du, et al., 2018), indicating a new mechanism of regulating SIRT1 protein expression/activity via H2S dependent sulfhydration. In addition, SIRT1-mediated effects on upregulating ABCA1-mediated cholesterol efflux and resultant foam cell formation can be induced by several pharmaceutical agents, such as berberine (L. Chi, Peng, Pan, Hu, & Zhang, 2014), curcumin (X. L. Lin, et al., 2015) and Tanshindiol C (Y. Yang, X. Li, et al., 2018). In addition, SIRT1 also promotes efferocytosis of oxLDL-induced apoptotic macrophages via inducing autophagy (B. Liu, Zhang, Guo, Li, & Xu, 2014).
In summary, SIRT1 is a deacetylase that regulates a plethora of important metabolic and physiologic processes including cellular metabolism, stress resistance, apoptosis and senescence. Its upregulation reduces inflammation in atherosclerotic lesions, reverses cholesterol transport, formation of macrophage-derived foam cells, and reduces overall risk for development of cardiovascular diseases (D’Onofrio, et al., 2018; Sosnowska, et al., 2017; Vitiello, et al., 2017; Winnik, et al., 2015; S. Xu, Bai, et al., 2016). In aging research, SIRT1 is one of the so-called longevity markers. The expression of SIRT1 gradually decreases with aging. Thus, SIRT1 activation represents a promising therapeutic strategy to treat multiple cardiovascular disorders including atherosclerosis (Paneni, Diaz Canestro, Libby, Luscher, & Camici, 2017).
F. SIRT2
SIRT2 is a SIRT member localized in the cytosol. Several genetic variants of SIRT2 are associated with acute myocardial infarction (W. Yang, et al., 2017). More recently, Zhang et al. (B. Zhang, Ma, & Xiang, 2018) used gain- and loss-of-function studies to study the role of SIRT2 in atherosclerosis using LDLr−/− mice. The authors observed that SIRT2 attenuates and stabilizes atherosclerotic plaques. SIRT2 overexpression also decreased markers of macrophage infiltration (MOMA-2 staining) and apoptosis (TUNEL staining). Mechanistically, lentivirus-SIRT2 infection reduces the expression of M1 macrophage marker-iNOS, while increases that of M2 macrophage marker Arg1. This new finding suggests an atheroprotective role of SIRT2 by fine-tuning the macrophage polarization process (B. Zhang, et al., 2018). Further studies are warranted to understand the deacetylation targets of SIRT2 in regulating macrophage polarization program.
G. SIRT3
SIRT3 is a SIRT member exclusively localized in mitochondria. SIRT3 regulates multiple aspects of mitochondrial functions, such as mitochondria biogenesis, autophagy and tissue homeostasis, particularly under stress conditions. SIRT3 knockout mice are normal at birth, but exhibit hyperacetylation of multiple mitochondrial proteins, such as manganese-dependent superoxide dismutase (Mn-SOD, also known as SOD2). SIRT3−/− mice have disorder in lipid metabolism, and aberrant hepatic accumulation of triglycerides and acylcarnitines under fasting conditions [17]. SIRT3 protects against exaggerated oxidative stress by directly deacetylating and activating Mn-SOD. However, SIRT3 deficiency only mildly impairs endothelium-dependent relaxation under challenge with an atherogenic diet (Winnik, et al., 2016). Also, in a diet-induced atherosclerosis model in LDLr−/− mice, SIRT3 deficiency does not affect lesion formation, nor plaque instability, despite hepatic protein hyperacetylation in the mitochondria and elevated circulating level of malondialdehyde (an index of lipid peroxidation) (Winnik, et al., 2014). SIRT3 deficiency accelerates thrombus formation by increasing tissue factor activity and the formation of neutrophil extracellular traps in a combined model of carotid thrombosis by a laser and LPS challenge (Gaul, et al., 2018). Of clinical relevance, reduced SIRT3 expression was observed in CD14+ leukocytes from patients with ST-elevation myocardial infarction. Therefore, increasing SIRT3 expression/activity may provide protection against thrombotic complications in patients with myocardial infarction
H. SIRT6
SIRT6 has overlapping cellular localization and biological functions with SIRT1, in terms of regulating endothelial cell senescence, leukocyte adhesion, macrophage-derived foam cell formation, macrophage polarization, lipid metabolism, and inflammatory responses (D’Onofrio, et al., 2018; D’Onofrio, et al., 2015; Sosnowska, et al., 2017; Vitiello, et al., 2017; Winnik, et al., 2015; S. Xu, Bai, et al., 2016). Reduced SIRT6 expression is observed in hypercholestrolemic ApoE−/− mice (Z. Liu, Wang, Huang, Li, & Liu, 2016) as well as in carotid atherosclerotic plaques from patients with atherosclerosis with (Balestrieri, et al., 2015) or without diabetic conditions (Z. Q. Zhang, et al., 2016). SIRT6 alters gene expression by deacetylating epigenetic marks of H3K9, H3K18, and H3K56 on histones and several non-histone protein substrates (D’Onofrio, et al., 2018; D’Onofrio, et al., 2015; Sosnowska, et al., 2017; Vitiello, et al., 2017; Winnik, et al., 2015; S. Xu, Bai, et al., 2016). For example, hepatic SIRT6 deficiency increases LDL-cholesterol in mice by regulating important genes in regulating lipid metabolism, such as PCSK9 and sterol-regulatory element binding protein 2 (SREBP2), by deacetylating H3K9 and H3K56 at promoters of PCSK9 (Tao, Xiong, DePinho, Deng, & Dong, 2013a) and SREBP2 (Tao, Xiong, DePinho, Deng, & Dong, 2013b).
The direct atheroprotective role of SIRT6 in atherosclerotic plaque development has been documented in several animal models of atherosclerosis. SIRT6 heterozygous (SIRT6+/−) mice show an increase in atherosclerotic lesions by upregulating the expression of natural-killer group 2 member D (NKG2D) ligand in macrophages and endothelial cells. This effect leads to the activation of natural killer cells and augmented production of inflammatory cytokines (Z. Q. Zhang, et al., 2016). SIRT6+/−/ApoE−/− and SIRT6 depleted ApoE−/− mice also show increased atherosclerotic lesion area, impaired endothelium-dependent vasodilation, and upregulation of VCAM-1 (Z. Liu, et al., 2016; S. Xu, Yin, et al., 2016; Z. Q. Zhang, et al., 2016). Additional evidence suggests that SIRT6 deacetylates H3K9 at promoters of atherosusceptible gene-tumor necrosis factor superfamily member 4 (TNFSF4) and reduce the expression of TNFSF4 (S. Xu, Yin, et al., 2016).
In vitro, when ECs are exposed high-glucose media, SIRT6 expression was downregulated, accompanied by hyperactivation of NF-κB-dependent pro-inflammatory pathway (Balestrieri, et al., 2015). SIRT6 has been shown to reduce atherosclerosis by inhibiting foam cell formation. Specifically, under oxLDL stimulation, macrophage-derived foam cell formation is reduced by SIRT6 via induction of autophagy and cholesterol efflux. In particular, overexpression of SIRT6 in foam cells increases the levels of ABCA1 and ABCG1, activates cholesterol efflux, and reduces the level of miR-33. Indeed, transfection of miR-33 mimics into cells overexpressing SIRT6 reduces foam cell formation and reverses autophagy flux induction (J. He, et al., 2017). Given that cholesterol efflux is regulated by the LXR/ABCA1 (G1) pathway, and SIRT1 interacts, deacetylates and activates LXR activity (X. Li, et al., 2007) to repress foam cell formation, it merits further exploration whether SIRT6 can also attenuates foam cell formation by deacetylating and activating LXR in the nuclear. In addition to regulating foam cell formation, a recent study has shown that myeloid cell-specific deletion of SIRT6 drives NF-κB hyperactivation, followed by IL6 production and STAT3 activation. This effect drives macrophage polarization toward M1 type, underscoring the important role of macrophage-derived SIRT6 in preventing diet-induced inflammation and insulin resistance (Y. Lee, et al., 2017). Independent of the promising results about the atheroprotective role in atherosclerosis in mice models and in vitro, it still remains to be seen, whether SIRT6 overexpression and/or modulation by specific activators are able to rescue vascular inflammation and retard atherosclerotic plaque development in humans.
To date, there is no literature regarding the direct roles of other SIRTs, such as SIRT4, and SIRT5, and SIRT7 in atherosclerosis. It remains elusive whether these SIRTs also play a role in regulating vascular functions and atherosclerosis.
2.3. RNA-based mechanisms
In addition to DNA methylation and histone modifications, recent researches convincingly demonstrate the diverse biological functions of several RNA-based mechanisms, including non-coding RNAs (ncRNAs). Some ncRNAs contain small open reading frames which encode functional peptides (Gurha & Marian, 2013). Based on size difference, non-coding RNAs are categorized into: (1) small non-coding RNAs (<200 bp), including microRNAs (miRNA), and PIWI-interacting RNAs (piRNA); (2) long non-coding RNAs (lncRNAs, >200 bp) including long intergenic non-coding RNA (lincRNA), circular RNA (circRNAs), natural antisense transcripts (NATs), and enhancer RNAs (eRNAs) (Boon, Jae, Holdt, & Dimmeler, 2016). Mounting evidence has demonstrated that ncRNAs represent another important epigenetic mechanism in the development of atherosclerosis and its vascular complications. The detailed role of ncRNAs in cardiovascular health and diseases is recently reviewed elsewhere (Aryal & Suarez, 2018; A. Das, Samidurai, & Salloum, 2018; Lucas, Bonauer, & Dimmeler, 2018). In this section, we will briefly discuss the updated role of miRNAs, and lncRNAs (including circRNAs) in atherosclerosis.
2.3.1. miRNA
miRNAs are highly conserved, small ncRNAs of 20~40 nucleotides that emerged as key post-transcriptional regulators of gene expression which act by binding to the 3’-untranslated region of target mRNA, thereby degrading mRNA transcripts or blocking protein translation or inducing gene degradation (Gurha & Marian, 2013; Lucas, et al., 2018). DNA methylation and demethylation is also important for miRNA regulation in various types of cancer (Ahmad, Li, Bao, Kong, & Sarkar, 2014). More than a half of all miRNA genes are related with CpG islands, thus susceptible to methylation. Other miRNAs are regulated by histone modifications with or without concurrent DNA methylation (Z. Wang, et al., 2013).
The contribution of miRNAs to atherosclerosis has been extensively investigated in the past decade. As can be seen from Table 2, miRNAs are important in regulating multiple cellular functions involved in atherosclerosis, such as endothelial function/dysfunction, cholesterol metabolism, cell fate, inflammation, and oxidative stress. In macrophages, miRNAs mediate inflammatory responses, cholesterol uptake, cholesterol efflux, monocyte differentiation, and M1/M2 polarization. In ECs, miRNAs regulate inflammation, leukocyte adhesion, eNOS-dependent NO production, and EndoMT. In VSMCs, miRNAs regulate VSMC differentiation, phenotypic switch, calcification, inflammation, apoptosis, proliferation, and migration. Accumulating evidence has also indicated that miRNAs can serve as a novel class of diagnostic or prognostic markers in atherosclerotic plaque progression and instability (Andreou, Sun, Stone, Edelman, & Feinberg, 2015; Feinberg & Moore, 2016; Laffont & Rayner, 2017; Leeper & Maegdefessel, 2018; Madrigal-Matute, Rotllan, Aranda, & Fernandez-Hernando, 2013; Poller, et al., 2017; Schober & Weber, 2016). As circulating miRNAs can be detected in peripheral blood components (such as leukocytes), and urine, miRNAs can be explored as potential biomarkers for the development of atherosclerosis as well as the severity of atherosclerosis (Feinberg & Moore, 2016). These atherorelevant miRNAs can be regulated by different disease-associated stimuli or pharmacological agents. Evidence implicating miRNA in the development of atherosclerosis was recently the subject of multiple excellent systematic reviews elsewhere (Andreou, et al., 2015; Feinberg & Moore, 2016; Laffont & Rayner, 2017; Leeper & Maegdefessel, 2018; Poller, et al., 2017; Schober & Weber, 2016).
Table 2.
Role of miRNAs in the development of atherosclerosis
| Endothelial function and dysfunction (including mechanosensing, vascular tone, inflammation, senescence, apoptosis, pyroptosis, DNA damage, injury, and cell adaptation etc): miR-10a, miR-21, miR-22, miR-27a/b, miR-30–5p, miR-34a, miR-92a, miR101, miR-103, miR-126, miR-135a, miR-142-3p, miR-143/miR-145, miR-146a, miR-155, miR-181b, miR-217, miR-211/222, miR-223, miR-365, miR-383–3p, miR-483, miR-633, miR-712 |
| VSMC phenotypic switch, proliferation and migration, apoptosis, and inflammation: miR-21, miR-26a, miR-29b, miR125b, miR-181a; miR-221/222, miR-143/145 |
| Monocyte differentiation, macrophage inflammation, foam cell formation, and M1/M2 macrophage polarization: miR-19a, miR-21, miR-26, miR-27a, miR-33, miR-98a, miR-106, miR-124, miR-125a, miR-128–1, miR-130b, miR-135a, miR-144, miR-146a, miR-147b, miR-148a, miR-155, miR-183, miR-212, miR-214, and miR-223, miR-301b, miR-302a, miR-let7a, miR-758 |
| Biomarkers of CVD: miR-10b, miR-17, miR-19a, miR-29a, miR-30e-5p, miR-92a, miR-96, miR-122, miR-125a, miR-126, miR-145, miR-150, miR-155, miR-181b, miR-185, miR-211, miR-222, miR-342, miR-378, miR-484 |
|
Cholesterol homeostasis and lipid metabolism: miR-26, miR-27, miR-33, miR-92a, miR-106, miR-122, miR-128–1, miR-144, miR-145, miR-148a, miR-185, miR-223, miR-758 Atherosclerotic plaque stability/vulnerability: miR-19b, miR-21, miR-24, miR-29, miR-33, miR-133b, miR-143, miR-145, miR-155–5p, miR-210, miR-221/miR-222, miR-322, miR-451a, miR-483–5p, miR-494, miR-638, miR-4530, |
Due to space limitations, atherosclerosis related miRNAs (Athero-miR) are summarized numerically based on several recent reviews (A. Das, et al., 2018; Donaldson, Lao, & Zeng, 2018; Feinberg & Moore, 2016; Laffont & Rayner, 2017; Leeper & Maegdefessel, 2018; Lucas, et al., 2018; Poller, et al., 2017; Schober & Weber, 2016; X. Zhang, Price, & Fernandez-Hernando, 2018), and updated with most recent literatures as of the time of submission (Dai, Wu, Dai, Li, & Mehta, 2018; Du, Lu, & Sha, 2018; Eken, et al., 2017; Hartmann, et al., 2016; Jin, et al., 2018; Katano, Nishikawa, Yamada, Yamada, & Mase, 2018; K. Li, Ching, Luk, & Raffai, 2015; P. Li, et al., 2018; S. Li, Q. Geng, et al., 2018; S. Li, et al., 2017; Y. Li, Zhang, & Mao, 2018; Lian, Lv, Yu, & Wang, 2018; Luque, Farwati, Krupinski, & Aran, 2018; S. Ma, et al., 2016; B. Markus, et al., 2016; Miao, Zeng, & Gong, 2018; Natarelli, et al., 2018; Qin, et al., 2018; X. Sun, et al., 2014; X. E. Tang, et al., 2018; Ulrich, et al., 2016; Y. T. Wang, et al., 2017; Wezel, et al., 2015; F. Yang, et al., 2018; Yilmaz, Isbir, Kunt, & Isbir, 2018; Yue, Lv, Zhang, & Kang, 2018; X. Zhong, et al., 2018).
Abbreviations: VSMC, vascular smooth muscle cells; CVD, cardiovascular diseases; miR, micro-RNA.
2.3.2. Long non-coding RNAs (LncRNAs)
LncRNAs represent a family of non-protein coding transcripts > 200 nucleotides, which occupy a large portion of the human genome. lncRNAs have long been considered as “dark matter” of the human genome with unknown biological functions (Uchida & Dimmeler, 2015). A growing body of evidence draws however a conclusion that lncRNAs play critical roles in regulating multiple pathophysiological processes in cardiovascular diseases (A. Das, et al., 2018; Gurha & Marian, 2013; Simion, Haemmig, & Feinberg, 2018; Uchida & Dimmeler, 2015; Weirick, Militello, & Uchida, 2018). A subset of lncRNAs might be exploited as useful biomarkers for cardiovascular diseases. A recent study (Arslan, et al., 2017) has analyzed the expression levels of five lncRNAs known to be associated with CAD, including ANRIL (antisense noncoding RNA in the INK4 locus), MIAT (myocardial infarction associated transcript), MALAT1 (metastasis associated lung adenocarcinoma transcript 1), KCNQ1OT1 (KCNQ1 overlapping transcript 1), and aHIF, by real time-PCR. The authors observed that ANRIL and MIAT expression was higher, while MALAT1 expression was lower in atherosclerotic coronary artery plaques, compared with non-atherosclerotic internal mammary artery. These studies suggest that some lncRNAs are strongly associated with human atherosclerosis, raising the therapeutic possibility to treat atherosclerosis by targeting de-regulated lncRNA expression (Arslan, et al., 2017).
LncRNA can regulate gene expression (either activating or suppressing) via transcriptional, post-transcriptional, and epigenetic mechanisms. Compared with miRNAs, lncRNAs have more diverse functions by acting as chromatin regulators, sponge, decoy, guide, molecular scaffold, and enhancers (Uchida & Dimmeler, 2015). LncRNAs also mediate signal transduction, such as protein phosphorylation, and protein trafficking (Gomes, et al., 2017; Lucas, et al., 2018). Emerging studies in the past 2–3 years have shown that lncRNAs are important regulators of vascular homeostasis and function in cardiovascular health and disease (Table 3). More and more lncRNAs have been identified and functionally characterized in response to pathophysiological stimuli or in different models of vascular disease states. However, our knowledge of the mechanisms whereby these lncRNAs control gene expression and regulate important cellular functions is still in its infancy.
Table 3.
Role of long non-coding RNA in vascular pathophysiology
| LncRNA | Mechanism | Cell type | Function | Reference |
|---|---|---|---|---|
| LncRNA00113 | p-PI3K/p-Akt/p-mTOR, Bcl-2 | EC, VSMC | ↑Proliferation, ↑ Survival, ↑Migration | (X. Yao, Yan, Zhang, Li, & Wan, 2018) |
| Linc00341 | VCAM1 | EC | ↓Inflammation ↓ Monocyte adhesion |
(T. S. Huang, et al., 2017) |
| SMILR | HAS2 | VSMC | ↓Proliferation | (Ballantyne, et al., 2016) |
| LincRNA-p21 | MDM2/p53 | VSMC, Mφ | ↓Proliferation ↑Apoptosis, ↓Neointimal hyperplasia |
(G. Wu, et al., 2014) |
| MYOSLID | p-smad2, MKL1 | VSMC | ↑VSMC differentiation ↓VSMC proliferation |
(J. Zhao, et al., 2016) |
| XIST | miR-320/NOD2 | EC | ↑oxLDL induced injury, apoptosis | (X. Xu, Ma, Liu, Duan, & Zhang, 2018) |
| SENCR | EC | ↑Mesodermal and endothelial commitment ↑Proliferation, migration and angiogenesis ↑Cell cycle progression |
(Boulberdaa, et al., 2016; H. Sun, Wang, & Song, 2018) | |
| SENCR | Myocardin | VSMC | ↓Smooth muscle cell migration ↑Contractile phenotype |
(Bell, et al., 2014) |
| STEEL | KLF2, eNOS | EC | ↑Angiogenic microvessels | (Man, et al., 2018) |
| MALAT1 | EC | Mechanosensitive | (Qiao, et al., 2016) | |
| MALAT1 | Cell cycle regulators | EC | Hypoxia responsive ↑Angiogenesis |
(Michalik, et al., 2014; X. Zhang, X. Tang, et al., 2018) |
| MALAT1 | β-catenin/CD36 | Mφ | ↑Foam cell formation | (Huangfu, et al., 2018) |
| MALAT1 | Mφ, splenocytes | ↑ Atherosclerosis lesion in MALAT1+/−; ApoE−/− mice ↑ Immune system dysregulation ↓TNFa, iNOS |
(Gast, et al., 2018) | |
| MALAT1 | miR-155 | EC | ↓ox-LDL mediated inflammation and apoptosis | (S. Li, Y. Sun, et al., 2018) |
| Linc00657 | miR-590–3p | EC | ↑Proliferation, migration, and tube formation ↑Angiogenesis |
(M. H. Bao, et al., 2018) |
| MeXis | LXR/ABCA1; DDX17 | Mφ | ↑Cholesterol efflux and atheroprotection | (Sallam, et al., 2018) |
| H19 | miR-148b/WNT/3-catenin | VSMC | ↑Proliferation ↓Apoptosis |
(L. Zhang, et al., 2018a) |
| H19 | miR-130b | Mφ | ↑Lipid accumulation ↑Infammation |
(Y. Han, et al., 2018) |
| H19 | miR-29a | EC | ↑Angiogenesis | (Jia, et al., 2016) |
| H19 | Plasma | Biomarker of CAD risk | (Z. Zhang, et al., 2017) | |
| H19 | p38, NF-kB | EC | ↑Apoptosis ↓Proliferation |
(J. X. Pan, 2017) |
| H19 | EC | Aging | ||
| H19 | HIF1α | VSMC | ↑Abdominal aortic aneurysm ↑VSMC apoptosis |
(D. Y. Li, et al., 2018) |
| LncRNA-RP11–714G18.1 | LRP2BP | EC, VSMC | ↓ Migration ↓ Monocyte adhesion to EC ↓ Angiogenesis ↑ Nitric oxide |
(Y. Zhang, L. Zheng, et al., 2018) |
| TUG1 | miR-133a/FGF1 | VSMC, Mφ | ↑Cell proliferation ↑Migration ↓Apoptosis ↑Atherosclerotic lesion ↑Infammation, hyperlipidemia |
(L. Zhang, et al., 2018b) |
| lincRNA-p21 | LOX-1 | EC | ↑Apoptosis ↑Cholesterol efflux |
(T. Zhou & Chen, 2017) |
| LeXis | LXR/SREBPs | Mφ | ↓Atherosclerotic lesion ↓TC, TG |
(Sallam, et al., 2016; Tontonoz, et al., 2017) |
| Linc00305 | miR-136 | EC | ↑Hypoxia-induced EC apoptosis | (B. Y. Zhang, Jin, & Zhao, 2017) |
| ANRIL | EC | ↑Infammation, apoptosis, coronary atherosclerosis | (Song, et al., 2017) | |
| NEAT1 | CD36, NF-κB | Mφ | ↑Expression upon oxLDL stimulation ↓CD36, DiI-oxLDL uptake ↓p65 phosphorylation, inflammation |
(Huang-Fu, et al., 2018) |
| NEAT1 | miR-128 | Mφ | ↓Proliferation, fapoptosis ↑CD36, oxLDL-induced foam cell formation ↑Inflammation (IL-6, IL-1β, and TNF-α) ↑ROS |
(D. D. Chen, Hui, Zhang, & Chang, 2018) |
| NEAT1 | miR-342–3p | Mφ | ↑Infammation (IL-6, IL-1β, TNFa) ↑CD36-dependent lipid uptake ↑ Lipid accumulation (TC, TG) |
(L. Wang, Xia, Ke, & Zhang, 2018) |
| NEAT1 | WDR5 | VSMC | ↑ Proliferation and migration ↑ Neointima formation |
(Ahmed, et al., 2018) |
| HOTAIR | EC | Decrease in EC from human atherosclerotic plaques ↑Proliferation, migration, ↓Apoptosis ↓oxLDL induced EC injury |
(Y. Peng, et al., 2017) | |
| HOTAIR | miR-330–5p | Mφ | ↑oxLDL induced oxidative stress ↑oxLDL induced inflammation ↑CD36-mediated oxLDL uptake ↑TC, TG |
(J. Liu, Huang, & Ke, 2018) |
| Linc00305 | LIMR/AHRR-NF-κB | Monocytes | ↑Infammation ↑VSMC phenotypic switch |
(D. D. Zhang, et al., 2017) |
| MANTIS | BRG1 | EC | ↑Angiogenic sprouting ↑Alignment of endothelial cells in response to shear stress |
(Leisegang, et al., 2017) |
| HOXC-AS | HOXC6 | Macrophage | ↓Lipid accumulation | (C. Huang, et al., 2016) |
| ANRIL | Pescadillo homologue 1 | VSMC, Mφ | ↑Apoptosis ↓Proliferation ↑Nucleolar stress and p53 activation, |
(Holdt, et al., 2016) |
| HULC | miR-9 | EC | ↓ TNFa-induced apoptosis | (Y. Ma, et al., 2016) |
| MIAT | miR-150–5p | EC | ↑Pathological angiogenesis ↑Diabetes mellitus-induced retinal microvascular dysfunction |
(B. Yan, et al., 2015) |
| RP5–833A20.1 | miR-382–5p/NFIA | Mφ | ↑Cholesterol uptake ↑Infammation ↓ Cholesterol efflux |
(Hu, et al., 2015) |
| Lnc-Ang362 | miR-221 and miR-222 | VSMC | ↑Proliferation | (Leung, et al., 2013) |
| GATA6-AS | LOXL2/periostin and COX2 | EC | ↑EndoMT ↓Vessel formation |
(Neumann, et al., 2018) |
| LEENE (Linc00520) | eNOS | EC | ↑eNOS, NO ↓Inflammation |
(Y. Miao, et al., 2018) |
| HOTTIP | Wnt/β-catenin | EC | ↑Proliferation, migration | (Liao, et al., 2018) |
| HOTTIP | Wnt/b-catenin | EC | ↑Proliferation, migration | (Liao, et al., 2018) |
| XR007793 | STAT2, lmo2, IRF7 | VSMC | ↑Proliferation, migration | (Q. P. Yao, et al., 2017) |
| Dnm3os | Nucleolin and ILF-2 | Mφ | ↑Infammation, phagocytosis, and diabetic vascular complications | (S. Das, et al., 2018) |
| MEG3 | EC | Increase in senescent ECs ↓Angiogenic sprouting |
(Boon, Hofmann, et al., 2016) | |
| MEG3 | VEGFR2 | EC | ↑Migration, angiogenesis | (Ruan, et al., 2018) |
| MEG3 | miR-223/NLRP3 | EC | ↑Pyroptosis | (Guo, et al., 2018) |
| MEG3 | miR-21 | EC | ↓Proliferation and migration ↓Collagen I, V, and proteoglycan expression |
(D. Wu, et al., 2017) |
Abbreviations: ABCA1, ATP binding cassette subfamily A member 1; ANRIL, antisense non-coding RNA at the INK4 locus (also known as CDKN2B antisense RNA 1); CD36, cluster of differentiation 36; DDX17, DEAD-box helicase 17; Dnm3os, DNM3 opposite strand/antisense RNA; EC, endothelial cells; eNOS, endothelial nitric oxide synthase; EndoMT: endothelial to mesenchymal transition; FGF1, fibroblast growth factor 1; GATA6-AS: antisense transcript of GATA6; HAS2, hyaluronan synthase 2; H19, imprinted maternally expressed transcript; HIF1α, hypoxia inducible factor 1, alpha subunit; HOTAIR, HOX transcript antisense RNA; HOTTIP, HOXA transcript at the distal tip; HOXC-AS, HOXC cluster antisense RNA; HULC, hepatocellular carcinoma up-regulated long non-codingRNA; IRF7, Interferon regulatory factor 7; ILF-2, interleukin enhancer-binding factor 2; iNOS, inducible nitric oxide synthase; KLF2, kruppel like factor 2; LEENE, enhancer-associated lncRNA that enhances eNOS expression; LIM domain only 2; LOX-1, lectin-like oxidized LDL receptor 1; LRP2BP, LRP2 binding protein; LOXL2, lysyl oxidase like 2; LXR, liver X receptor; MALAT1, metastasis associated lung adenocarcinoma transcript 1; Mφ, macrophages; MANTIS, lncRNA n342419; MEG3, maternally expressed 3; MDM2, MDM2 proto-oncogene; MIAT, myocardial infarction associated transcript; MKL1, MYOCD-related transcription factor A; MYOSLID, MYOcardin-induced Smooth muscle LncRNA, Inducer of Differentiation; NEAT1, nuclear paraspeckle assembly transcript 1; NFIA, nuclear factor I A; NF-kB, nuclear factor kappa B; NLRP3, NLR family pyrin domain containing 3; NOD2, nucleotide binding oligomerization domain containing 2; SENCR, Smooth muscleand endothelial cell-enriched migration/differentiation-associated long non coding RNA; SMILR, Smooth Muscle Enriched Long Noncoding RNA; STEEL, spliced-transcript endothelial-enriched lncRNA; TC, total cholesterol; TG, triglycerides; TNFa, tumor necrosis factor-alpha; VCAM1, vascular cell adhesion molecule 1; WDR5, WD Repeat Domain 5; VSMC, vascular smooth muscle cells; XIST, X-inactive specific transcript
Like miRNA, lncRNAs not only regulate gene expression but also participate epigenetic regulation like DNA methylation and histone modifications (Khalil, et al., 2009; Voelter-Mahlknecht, 2016). Furthermore, lncRNA expression is tissue and cell specific, and is developmentally regulated. Many lncRNAs are associated with chromatin remodeling complexes to guide them to specific locations inside the genome (Khalil, et al., 2009). PRC2 seems to be particularly involved in establishing heterochromatin states by recruiting H3K27me3 mark to target gene promoters. Consequently, PRC2 together with lncRNA might serve as transcriptional repressing complex by silencing specific genomic loci. Khalil et al. (Khalil, et al., 2009) suggested that lncRNA TUG1 may function in such epigenetic mechanism. The authors propose that following DNA damage, TUG1 is induced through direct binding to p53, which then binds PRC2 and may thus repress various cell-cycle related genes.
In terms of vascular function, LncRNAs regulate endothelial function and dysfunction (such as proliferation, migration, apoptosis, angiogenesis, and EndoMT) (e.g. STEEL (Man, et al., 2018), MALAT1 (Michalik, et al., 2014; X. Zhang, Tang, Hamblin, & Yin, 2018), MEG3 (Boon, Hofmann, et al., 2016; Ruan, et al., 2018), MANTIS (Leisegang, et al., 2017)). LncRNAs also modulate VSMC phenotypes (Simion, et al., 2018) (phenotypic switch, proliferation, migration, apoptosis) (e.g. H19 (L. Zhang, et al., 2018a), ANRIL (Congrains, et al., 2012), SMILR (Ballantyne, et al., 2016), SENCR (Bell, et al., 2014), MYOSLID (J. Zhao, et al., 2016)). A large body of evidence also implicated lncRNAs in regulating monocyte/macrophage functions, such as inflammation (e.g. lincRNA-Cox2 (J. Yu, et al., 2016), linc00305 (D. D. Zhang, et al., 2017), THRIL(Z. Li, et al., 2014)), foam cell formation (e.g. LeXis (Tontonoz, et al., 2017), MeXis (Sallam, et al., 2018), and NEAT1 (Huang-Fu, Cheng, Wang, Li, & Wang, 2018)), and M1/M2 polarization (e.g. GAS5(Ito, Asai, Suzuki, Kobayashi, & Suzuki, 2017)), and lipid handling (e.g. H19 (Y. Han, Ma, Wang, & Wang, 2018)). Several lncRNAs may serve as biomarkers for CAD (e.g. LncRNA-GAS5 (H. P. Zhang, et al., 2016), ENST00000444488.1 (L. Li, et al., 2018), Upperhand (X. Li, et al., 2018), H19 (Z. Zhang, et al., 2017), LIPCAR (Z. Zhang, et al., 2017), LncPPARδ (Cai, Yang, Chen, He, et al., 2016), and CoroMarker (Cai, Yang, Chen, Wu, et al., 2016)). Biological functions and molecular mechanisms of lncRNAs implicated in atherosclerosis from cell culture studies, experimental animal models and human patients are summarized in Table 3. Recently, Miao et al. (Y. Miao, et al., 2018) has shown that an enhancer-associated lncRNA that enhances eNOS expression (LEENE, also known as linc00520) regulate eNOS expression, NO production, and monocyte adhesion. LEENE recruits RNA Pol II to eNOS gene promoter, thereby enhancing eNOS nascent RNA transcription (Y. Miao, et al., 2018). LncRNAs expression can also be modulated by therapeutic agents (such as melatonin (Y. Zhang, X. Liu, et al., 2018) and statins (Y. Miao, et al., 2018)), atherosclerosis-causing stimuli (oxLDL (M. H. Bao, et al., 2018)), and biomechanical factors (such as shear stress (T. S. Huang, et al., 2017) and cyclic strain (Q. P. Yao, et al., 2017)).
Circular RNAs (circRNAs) is a large category of ncRNAs ubiquitously expressed in eukaryotic cells (Szabo & Salzman, 2016). For a long time, the biological functions of circRNAs were underestimated. Deep-scale sequencing has enabled the identification of disease-associated circRNAs. Recently, a circRNA-miRNA-mRNA network has been described in a rabbit model of atherosclerosis (F. Zhang, et al., 2018). This circRNA atlas identified seven dysregulated circRNAs which have primary function in mediating cell adhesion/activation as well as immune response (F. Zhang, et al., 2018). Different from conventional linear RNAs, circRNAs are formed by so called backsplicing, which allow for exonuclease resistance and high stability (Fu, Jiang, Li, Hu, & Guo, 2018). Emerging evidence has shown that circRNAs are involved in the development of atherosclerosis. For example, ANRIL on chromosome 9p21 modulates ribosome RNA maturation and atherosclerosis in humans (Holdt, et al., 2016). ANRIL binds to pescadillo homologue 1 (an essential assembly factor in 60S-preribosome), and affects exonuclease-mediated pre-ribosomal RNA processing and ribosome biogenesis in VSMCs as well as macrophages. By doing so, ANRIL induces p53 activation, causing apoptosis and growth inhibition (Holdt, et al., 2016). Another example is human circ0003575, which is increased in response to oxLDL-stimulation in endothelial cells. Depletion of circ0003575 increases the proliferation and capillary-like tube-forming ability of HUVECs (C. Y. Li, Ma, & Yu, 2017). The role of circRNA in epigenetic regulation of gene expression, as observed for miRNAs or lncRNAs has yet to be elucidated. For a detailed review of the lncRNAs (including circRNAs) in atherosclerosis, we refer readers to several topical reviews recently (A. Das, et al., 2018; Simion, et al., 2018; Weirick, et al., 2018).
The comparison of differentially expressed lncRNAs between control and human atherosclero tic patients, will help identify disease-associated lncRNAs. With the recent advances of novel technologies, such as RNA-sequencing, lncRNA arrays, more atherosclerosis-associated lncRNAs (defined as “Athero-lincs”) are being discovered and biologically characterized. For example, a recent study (L. Li, et al., 2018) has identified over 1000 aberrantly expressed lncRNAs in peripheral blood mononuclear cells isolated from patients with CAD at transcriptome-wide level. Loss of function studies suggest that some of these lncRNAs control the expression of several inflammation-related genes. There have appeared several promising strategies to modulate lncRNA expression to intervene in atherosclerosis, such as using adeno-associated virus (AAV) as expression vectors to deliver lncRNA locally or systemically, or using small interfering RNAs (siRNAs), GapmeRs locked-nucleic acid technology, or CRISPR/cas9-based genome-editing to deplete target lncRNAs. Deepened understanding of lncRNAs in atherosclerosis will provide conceptual and mechanistic insights into pathophysiology of atherosclerosis and accelerate the discovery of novel disease biomarkers and/or therapeutic targets (Simion, et al., 2018). Future studies will be focused on identification of novel “atherolincs” and new therapeutic drugs that target these lncRNAs for atherosclerosis therapeutics.
3. Cardiovascular risk factors and epigenetic regulation
The human epigenome can be influenced by dynamic changes in modifiable risk factors during our life-time to modulate gene expression and functional states of the body. In this regard, multiple cardiovascular risk factors (Figure 4) are associated with DNA methylation and histone modifications (Baccarelli, et al., 2010; J. Zhong, et al., 2016). These risk factors include dyslipidemia (Braun, et al., 2016; Dekkers, et al., 2016; X. Wang, et al., 2017), hyperglycemia (Ling & Groop, 2009), hypertension (Wise & Charchar, 2016), overweight/obesity (Demerath, et al., 2015), hyperhomocysteinemia (S. Zhou, Zhang, & Xu, 2014), aging (Lowe & Raj, 2014), environmental pollution (G. C. Chi, et al., 2016), and lifestyle options (e.g., smoking (Joehanes, et al., 2016), diet (Loche & Ozanne, 2016; Muka, et al., 2016), and physical inactivity (Grazioli, et al., 2017)). In view of the important roles of these risk factors in the development of CVD, AHA adopts “Life’s Simple 7” to track progress toward the 2020 Impact Goal, and these seven factors include not-smoking, physical exercise, a healthy diet regimen, controlled body weight, cholesterol level, blood pressure, and blood glucose level (http://www.heart.org).
Figure 4. Major risk factors for atherosclerosis.
The development of atherosclerosis is influenced by multiple risk factors, which affects different epigenetic mechanisms in atherosclerosis. Some risk factors have effects on transgenerational inheritance and epigenetic memory effects, highlighting the necessity of epigenetic drugs.
4. Trans-generational inheritance and trained immunity
Epigenetic alternations are generally reset during passage through germline transmission. Epigenetic programming can be transmitted to the next generation or even several generations in a gender-specific manner. This phenomenon leads to the occurrence of transgenerational epigenetic inheritance, which can be caused by prenatal/perinatal exposure to environmental factors or alternations of nutritional status (Gabory, Attig, & Junien, 2009). Transgenerational epigenetic inheritance is often very common in the development of atherosclerosis (Leentjens, et al., 2018). For example, ApoE deficiency and postnatal exposure to hypercholesterolemic environment causes altered histone methylation patterns in the vasculature (Alkemade, et al., 2010). Recently, Trenteseaux et al. (Trenteseaux, et al., 2017)evaluated the effect of perinatal hypercholesterolemia on atherosclerosis development in the offspring of ApoE−/− mice. The authors observed that perinatal hypercholesterolemia exacerbates atherosclerosis in their offspring by epigenetically modulating several genes implicated in the metabolism of trimethylamine-N-oxide and bile acids via DNA methylation (Trenteseaux, et al., 2017).
It has been known that monocytes/macrophages can acquire a long-term pro-inflammatory and pro-atherogenic phenotype after prior exposure to inflammatory stimuli via regulating histone methylation alternations at pro-inflammatory gene promoters. This phenomenon is termed ‘trained immunity’ (Bekkering, Joosten, van der Meer, Netea, & Riksen, 2013; Leentjens, et al., 2018). Commonly used in vitro stimuli of monocyte/macrophage training are: oxLDL, β-glucan, and the bacillus Calmette-Guérin vaccine (Bekkering, et al., 2016). In 2006, Groeneweg et al. (Groeneweg, et al., 2006) first observed that, prior treatment with oxLDL increases LPS-induced TNFα and IL6 expression, while it decreases anti-inflammatory cytokine IL10 expression in bone marrow-derived macrophages. However, the epigenetic basis of this observation is unknown. Eight years later, a similar phenomenon was observed in monocytes primed with oxLDL (Bekkering, et al., 2014). Those trained monocytes have an augmented production of a set of inflammatory mediators (including IL6, IL8, IL18, TNFα, MCP1, and MMP2/MMP9) when re-stimulated with TLR ligands 6 days after initial priming. oxLDL training also promotes foam cell formation via increasing the expression of lipid-uptake genes-CD36 and SRA, while decreasing the expression of cholesterol efflux related genes-ABCA1 and ABCG1. Mechanistic studies show increased H3K4me3 in gene promoters of pro-inflammatory genes 6 days after oxLDL. Furthermore, oxLDL-induced pro-inflammatory response is reduced by treatment with a histone methyltransferase inhibitor (Bekkering, et al., 2014). This phenomenon of trained immunity may exist for other priming factors, such as high glucose and AGEs (van Diepen, et al., 2016) or possibly exist in other cell types, such as endothelial cells and VSMCs. For example, El-Osta and colleagues (El-Osta, et al., 2008) reported that transient hyperglycemia exposure (for 16 h) induced long-lasting (6 days after returning to normoglycemia) epigenetic changes in human aortic endothelial cells by inducing SET7-dependent H3K4me1 binding to promoters of genes downstream of NF-κB pathway (MCP1 and VCAM1). In addition, high glucose can induce “senescent memory” in endothelial cells, which can be corrected by treatment with resveratrol and metformin (E. Zhang, et al., 2015).
In addition, feeding with western-type diet also induces NLRP3-dependent trained immunity. A recent report by Christ et al. (Christ, et al., 2018), has shown that myeloid cell responses toward innate stimuli remained increased after diet switch from western diet to normal diet. However, this pattern of systemic inflammation and trained immunity was not seen in NLRP3−/−/LDLr−/− double knockout mice, indicating a central role of NLRP3/IL-1β in mediating trained immunity after western diet feeding. This finding agrees with the promising findings in the recent CANTOS trial, which showed IL1β blockade reduces cardiovascular risk and usher a new direction of new therapeutic interventions for CVD (Ridker, et al., 2017). In agreement with this evidence, mevalonate can induce trained immunity in human monocytes via activation of IGF1 receptor (IGF1R) and mTOR. More clinically relevant, monocytes from patients with mevalonate kinase deficiency (who accumulate mevalonate) recapitulated the trained phenotype. Further, statins, which block mevalonate generation are capable of preventing the trained immunity phenotype (Bekkering, et al., 2018). The identification of mevalonate in regulating trained immunity via epigenetic mechanisms will help the quest for novel therapeutic targets and drugs for treating atherosclerosis. Further studies in this direction are necessary to elucidate the comprehensive role of trained immunity and epigenetic reprograming in atherosclerosis and develop novel pharmacologic targets for disease therapy (Leentjens, et al., 2018).
5. Epigenetic drugs in treating atherosclerosis
The reversible nature of epigenetic modifications (DNA/RNA/histone modifications in particular) has sparked intense interest of ‘epigenetic therapy’ as a treatment option for atherosclerosis. The purpose of epigenetic therapy is to reverse “abnormal” (or disease-causing) epigenetic alternations that occur in atherosclerosis, thus restoring a “normal” cardiovascular epigenome (Sharma, et al., 2010). Many epigenetic drugs have been identified in the past decade that effectively prevented or treated atherosclerosis in several translational animal models, raising the possibility to combat atherosclerosis by targeting epigenetic processes also in humans (Table 4).
Table 4.
Therapeutic drugs that target epigenetic processes in atherosclerosis and associated vascular diseases
| Compound Name | Category | Subjects or Animal models | Pharmacological effects | Reference |
|---|---|---|---|---|
| Resveratrol | Natural SIRT1 activator | 1. ApoE−/−/LDLr−/− mice 2. ApoE−/− mice+HFD 3. ApoE−/− mice+ND 4. ApoE*3-LeidenCETP mice+HCD 5. Fbn1 C1039G/+ Marfan syndrome mouse |
↓Atherosclerosis lesion ↓Thrombosis ↓Inflammatory chemokines ↑Plaque stability ↓Vascular inflammation ↓Aortic root dilation |
(Berbee, et al., 2013; Fukao, et al., 2004; Hibender, et al., 2016; Howitz, et al., 2003; Norata, et al., 2007) |
| SRT1720 | SIRT1 activator | 1. ApoE−/− mice+Ang-II infusion 2. HFHS diet |
↓Atherosclerosis lesion ↓Blood pressure, inflammation ↓NF-κB and STAT3 activation ↓Arterial stiffness |
(Y. X. Chen, et al., 2015; Fry, et al., 2016) |
| SRT2104 | SIRT1 activator | Cigarette Smokers Patients with T2DM | Improved lipid profile ↓Augmentation pressure |
(Venkatasubramanian, et al., 2013; Venkatasubramanian, et al., 2016) |
| SRT3025 | SIRT1 activator | ApoE−/− mice+HFD | ↓Atherosclerosis lesion ↓PCSK9 secretion ↑LDLr expression |
(Miranda, et al., 2015) |
| Icariin | Natural SIRT6 activator | 1. ApoE−/− mice+HFD 2. Rabbits+HFD |
↓Atherosclerosis lesion ↑eNOS/NO-pathway ↓ROS ↓Macrophage infiltration ↓Platelet adhesion and aggregation |
(Y. Chen, et al., 2015; Y. Li, Li, Cole, McLaughlin, & Du, 2018; L. Wang, et al., 2016; Xiao, Sui, & Lu, 2017; W. P. Zhang, Bai, Zheng, Xie, & Yuan, 2013) |
| Fucoidan | Natural SIRT6 activator | ApoE−/− mice+HFD | ↓Atherosclerosis lesion ↓Hepatic steatosis ↓Foam cell accumulation |
(M. K. Rahnasto-Rilla, et al., 2017; Yokota, Nomura, Nagashima, & Kamimura, 2016) |
| Cyanidin | Natural SIRT6 activator | ApoE−/− mice+STZ injection | ↓Atherosclerosis lesion ↑Endothelial repair |
(M. Rahnasto-Rilla, et al., 2018; Y. Zhang, Wang, Wang, Liu, & Xia, 2013) |
| SAHA | HDAC inhibitor | ApoE−/−mice+HFD | ↓Atherosclerosis lesion ↓Monocyte adhesion to EC ↑KLF2 expression |
(Y. Xu, et al., 2017; Ye, Zhao, Lu, Long, & Zhang, 2018) |
| 5-aza-2’-deoxycytidine | DNMT inhib itor | 1. ApoE−/− mice+HFD 2. LDLr−/− mice+HFD 3. ApoE−/− mice+Partial carotid ligation |
↓Atherosclerosis lesion ↓Neointima ↓TET2 methylation ↓Macrophage inflammation and chemotaxis ↓Macrophage ER stress ↓Macrophage adhesion to EC ↑LXRa and PPARγ |
(Cao, Wang, et al., 2014; Dunn, et al., 2014; Zhuang, et al., 2017) |
| Quercetin | Natural DNMT inhib itor | 1. ApoE−/− mice+HFD 2. LDLr−/− mice+HFD |
↓LDL uptake, aggregation ↓LDL oxidation ↓Endothelial dysfunction ↑eNOS and HO1 |
(Hayek, et al., 1997; Leckey, et al., 2010; W. J. Lee, et al., 2005; Shen, et al., 2013) |
| EGCG | Natural DNMT inhibitor | ApoE−/− mice+HFD | ↓Atherosclerosis lesion ↑LXRa and LXRP ↓SREBP1 |
(W. J. Lee, et al., 2005; L. L. Pan, et al., 2018) |
| Curcumin | Natural inhib itor of CBP/p300 | 1. ApoE−/− mice+HFD 2. LDLr−/− mice+HFD |
↓Atherosclerosis lesion ↓TLR4, NF-kB ↓IL-1β, TNFa, VCAM1, ICAM1 |
(Ghosh, et al., 2014; Z. Liu, et al., 2009; S. Zhang, et al., 2018) |
| Statins | EZH2 inhib itor | 1. ApoE*3-Leiden mice+HCD 2. Aged ApoE−/− 3. ApoE−/− mice +HFD 4. LDLr−/− mice+HFD 5. Patients with atherosclerosis |
↓Atherosclerosis lesion ↓Macrophage content ↓TF and MCP1 ↓CD36 and calpain-1 |
(Bea, Blessing, Shelley, Shultz, & Rosenfeld, 2003; Ishikawa, et al., 2014; Kang, Wu, & Li, 2004; Kleemann, et al., 2003; C. P. Lin, et al., 2015; M. Yin, et al., 2017) |
| Vitamin C | TET2 activator | 1. Rabbits+HCD 2. ApoE−/− mice |
↓Atherosclerosis lesion ↓Aortic VEGF, VEGFR2 |
(S. Das, Ray, Snehlata, Das, & Srivastava, 2006; Nespereira, et al., 2003; Qu, et al., 2017; R. Yin, et al., 2013) |
| Valproic acid | HDAC inhib itor | 1. ApoE−/− + STZ 2. LDLr−/− mice+HFD |
↓Atherosclerosis lesion ↓Hepatic GSKβ activity ↓Hepatic GSKβ Activity ↓ER stress |
(Bowes, Khan, Shi, Robertson, & Werstuck, 2009; A. Huang, et al., 2017) |
| Phenylbutyrate | HDAC inhibitor | LDLr−/− mice+HFD | ↓Atherosclerosis lesion ↓Necrotic core size |
(A. Huang, et al., 2017) |
| RVX-208 (Apabetalone) | BET inhib itor | 1. ApoE−/− mice+HFD 2. CAD patients |
↓Atherosclerosis lesion ↓Adverse cardiovascular events ↓Adhesion molecules ↓hsCRP ↓HDL-C |
(Bailey, et al., 2010; Jahagirdar, et al., 2014; McLure, et al., 2013; Nicholls, et al., 2018) |
| (+)-JQ1 | BET inhib itor | 1. LDLr−/− mice+HFD 2. Ang-II infused mice |
↓Atherosclerosis lesion ↓Macrophage and T cell infiltration ↓Leukocyte adhesion ↓Blood pressure |
(Brown, et al., 2014; S. Das, et al., 2017). |
| GSKJ4 | JMJD3 inhibitor | 1. Rats carotid artery balloon injury 2. Mouse left carotid artery partial ligation |
↓Neointimal hyperplasia ↓VSMC proliferation, migration, inflammation |
(Luo, et al., 2018) |
Abbreviations: Ang-II, angiotensin II; ApoE, apolipoprotein E; BET, bromodomain and extra-terminal motif; CAD, coronary artery disease; CD36, cluster of differentiation 36; DNMT, DNA methyltransferase; EC, endothelial cells; EGCG, Epigallocatechin gallate; eNOS, endothelial nitric oxide synthase; ER, endoplasmic reticulum; EZH2, Enhancer of zeste homolog 2; HFD, high fat-diet; GSK-3β; glycogen synthase kinase 3 beta; HCD, high cholesterol diet; HDAC, histone deacetylase; HDL-C, high-density lipoprotein-cholesterol; HFD, high-fat diet; HO1, heme oxygenase 1; hsCRP, high-sensitivity C reactive protein; ICAM1, intercellular adhesion molecule 1; IL-1β, interleukin-1 beta; JMJD3, JmjC domain-containing protein 3; KLF2, kruppel like factor 2; LDL, low-density lipoprotein; LDLr, low density lipoprotein receptor; LXR, liver X receptor; MCP1, monocyte chemoattractant protein-1; NF-kB, nuclear factor-kappa B; NO, nitric oxide; STZ, streptozotocin; T2DM, type 2 diabetes mellitus; TET2, Tet methylcytosine dioxygenase 2; EC, endothelial cells; PPAR, peroxisome proliferator-activated receptor; PCSK9, proprotein convertase subtilisin/kexin type 9; ROS, reactive oxygen species; SAHA, suberanilohydroxamic acid; SIRT, sirtuin; SREBP1, sterol regulatory element-binding protein 1; STAT3, signal transducer and activator of transcription 3; T2DM, type 2 diabetes mellitus; TF, tissue factor; TNFα, tumor necrosis factor-alpha; TLR4, toll like receptor 4; VCAM1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor; VEGFR2, vascular endothelial growth factor receptor 2; VSMC, vascular smooth muscle cells
5.1. DNMT inhibitors (DNMTi)
DNMTi are among the first epigenetic drugs that are used in cancer therapeutics (Sharma, et al., 2010). DNMTi can be classified as nucleoside-based and non-nucleoside inhibitors. 5-Azacytidine and 5-Aza-2’-deoxycytidine are FDA-approved nucleoside-based DNMTi that inhibit DNA methylation in actively dividing cells by trapping DNMTs in the target gene promoters and thus re-activate gene transcription. Both drugs are used for treating myelodysplastic syndromes and acute myeloid leukemia (Sharma, et al., 2010). Several elegant studies have recently shown that 5-Aza-2’-deoxycytidine effectively inhibits atherosclerosis development in several well-established animal models of atherosclerosis, including diet induced atherosclerosis in ApoE−/− mice, LDLr−/− mice and ApoE−/− mice undergoing carotid partial ligation (Cao, Wang, et al., 2014; Dunn, et al., 2014; Zhuang, et al., 2017). One disadvantage of these nucleoside inhibitors is the cytotoxic effect on normal cells as these drugs are irreversibly incorporated into DNA (Sharma, et al., 2010). An alternative approach is to develop nonnucleoside DNMTi, which potently and selectively inhibit DNA methylation by targeting catalytic/cofactor-binding sites of DNMTs or DNMTs gene and protein expression, without being incorporated into DNA (Sharma, et al., 2010). Small-molecule DNMTi of this kind include SGI-1027, RG108 and MG98 (Sharma, et al., 2010) and several natural products (i.e., quercetin (Hayek, et al., 1997; Leckey, et al., 2010; W. J. Lee, Shim, & Zhu, 2005; Shen, et al., 2013), curcumin (Ghosh, Bie, Wang, & Ghosh, 2014; Z. Liu, et al., 2009; S. Zhang, Zou, Li, Zheng, & Feng, 2018) and EGCG (W. J. Lee, et al., 2005; L. L. Pan, et al., 2018)). Although the current literature has proven that some of the naturally occurring non-nucleoside DNMTi are capable of inhibiting atherosclerosis in mice, it is unknown how far their specific mechanism of action and the DNMTs inhibiting effects of these compounds contribute to their atheroprotective effects.
5.2. TET2 activators
Vitamin C (or ascorbic acid) is a classical and potent dietary antioxidant that has been tested both pre-clinically and clinically to treat atherosclerosis. The potential atheroprotective mechanisms of vitamin C include inhibition of LDL oxidation, improving endothelial dysfunction and arterial stiffness, modulation of lipid profile, inhibition of platelet activation, and many others (Lynch, Gaziano, & Frei, 1996). Mounting evidence from animal studies, epidemiological and clinical studies has supported vitamin C supplementation in preventing atherosclerosis progression and reducing the incidence of CVD events, although with some controversy (Lynch, et al., 1996; Moser & Chun, 2016; Salonen, et al., 2003). The antioxidant-independent cardiovascular actions of vitamin C are however remain incompletely understood. Recent studies have demonstrated that vitamin C can also function as an epigenetic drug that activates TET2-dependent DNA demethylation, thereby regulates gene transcription (R. Yin, et al., 2013). The TET2 activating effects of vitamin C have been validated in multiple cell types and in vivo (Agathocleous, et al., 2017; Cimmino, et al., 2017; Qu, et al., 2017). More recently, another potential TET2 activator from natural sources, vicenin-2, has been identified to ameliorate high glucose induced endothelial inflammation (including hyperpermeability, leukocyte adhesion, ROS production, and NF-kB dependent adhesion molecules expression) (Ku & Bae, 2016), as well as LPS induced macrophage inflammation (TNFα, IL1β, and IL18) (Hassan, et al., 2018), underscoring the potential of TET2 activators to prevent atherosclerosis development. Further studies are necessary to validate, whether TET2 activation directly contribute to these compounds-mediated atheroprotection.
5.3. Histone deacetylase inhibitors (HDACi)
HDACi, such as suberoylanilide hydroxamic acid (SAHA), trichostatin A (TsA), phenylbutyrate and valproic acid are widely used in cancer biology and therapeutics (Mottamal, Zheng, Huang, & Wang, 2015). The pharmacological effects of HDACi are mediated through the reactivation of silenced genes by preventing histone deacetylation on target gene promoters. The pan-HDAC inhibitor SAHA, a clinically-used drug for treating T cell cutaneous lymphoma, attenuates vascular inflammation and atherosclerosis lesion development in ApoE−/− mice fed a western-type diet (Y. Xu, et al., 2017). Part of the anti-atherosclerotic mechanism of SAHA is related to pharmacological activation of KLF2, a promising target for treating atherosclerosis (Y. Xu, et al., 2017). In addition, the pan-HDAC inhibitors, phenylbutyrate and valproic acid, which also demonstrated therapeutic effects in attenuating experimental atherosclerosis in mouse models (A. Huang, et al., 2017). Interestingly, both 4-phenylbutyrate and valproic acid also target ER stress and GSK3α/β, which render them as dual function HDACi that confer atheroprotection (A. Huang, et al., 2017). TsA, another pan-HDAC inhibitor, upregulates SR-BI expression and promotes SR-BI-dependent cholesterol efflux (Y. Bao, et al., 2009) and inhibits VSMC proliferation via inducing the expression of p21 (WAF1) expression and cell-cycle arrest (Okamoto, et al., 2006). However, TsA accelerates macrophage infiltration, foam cell formation, and atherosclerosis development in LDLr−/− mice by upregulating scavenger receptor CD36 (Choi, et al., 2005). Probably, the atheroprotective effects of TsA may be surpassed by its pro-atherogenic effects. This phenomenon suggests that HDAC inhibition-dependent and -independent mechanisms must be explored when assessing the pharmacological effects of pan-HDACi.
5.4. Sirtuin activating compounds (STAC)
Based on the overall atheroprotective functions of SIRT1 and SIRT6, several natural and synthetic SIRT1 and SIRT6 activators have been developed and demonstrated considerable efficacy in attenuating experimental atherosclerosis. One well-studied SIRT1 activator is the wine-derived natural product resveratrol, which displays multiple atheroprotective effects in several mouse models of atherosclerosis, including ApoE−/− mice, ApoE−/−; LDLr−/− mice, and ApoE*3-Leiden.CETP mice (Berbee, et al., 2013; Fukao, et al., 2004; Howitz, et al., 2003; Norata, et al., 2007). Other synthetic STACs, such as SRTT1720 (Y. X. Chen, et al., 2015), SRT3025 (Miranda, et al., 2015), and SRT2104 (Venkatasubramanian, et al., 2013; Venkatasubramanian, et al., 2016) also demonstrate atheroprotective functions. Similar as SIRT1 activators, four SIRT6 activators have been reported to have atheroprotective properties, namely, Icariin (from Chinese medicine Epimedium brevicornum Maxim) (Y. Chen, et al., 2015), Fucoidan (from marine-derived brown seaweeds) (M. K. Rahnasto-Rilla, et al., 2017), Cyanidin (from plant-derived anthocyanidins) (M. Rahnasto-Rilla, et al., 2018), and Fluvastatin (J. H. Kim, Lee, Kim, & Kim, 2018). Since most of these natural products display multiple cardiovascular actions in addition to SIRT activation, it remains to be elucidated, whether the atheroprotective functions are dependent on SIRT activation.
5.5. HMTi (such as EZH2 inhibitors)
The effect of HMT inhibitors (HMTi), such as EZH2 inhibitors, have been intensively explored in cancer therapies. One such EZH2 inhibitor in clinical trial, is GSK343. It is a potent and EZH2 specific inhibitor selectively targeting H3K27me3 in cancer cells (Verma, et al., 2012). GSK343 treatment improves aortic performance in Fbn1C1039G/+ mice (an animal model of Marfan Syndrome), concordant with restoration of SM22α, an important contractile protein expressed in VSMCs (Lino Cardenas, et al., 2018). Several atheroprotective drugs (statins (Ishikawa, et al., 2014), fish oil (Dimri, Bommi, Sahasrabuddhe, Khandekar, & Dimri, 2010), metformin (G. Tang, et al., 2018)) inhibit EZH2 expression in cancer cells and some anti-atherosclerotic treatments (fluid shear stress (Maleszewska, et al., 2016; S. Xu, Y. Xu, et al., 2018)) decrease EZH2 protein expression in endothelial cells. Although most of these findings are performed in cancer cells, the utility of specific or repurposed EZH2 inhibitors in atherosclerosis remains to be assessed. Nevertheless, the current findings reinforce the concept that EZH2 inhibitors may exert cardiovascular protective actions in vivo.
5.6. BET inhibitor
The bromodomain and extra-terminal domain (BET) family of proteins are composed of two tandem bromodomains (“readers” of lysine acetylation) and one extra-terminal domain. Mammalian BET proteins have four members, BRD2, BRD3, BRD4, and BRDT. BET family proteins orchestrate gene transcription through interactions with acetylated lysine residues on target proteins via its bromodomains (Taniguchi, 2016). RVX-208 (also known as apabetalone) is an orally bioavailable and novel epigenetic targeting drug that increases the levels of ApoAI and HDL, thereby increasing HDL functionality (such as cholesterol efflux and anti-inflammatory effects) (Bailey, et al., 2010) by selective binding to BD2 domain (second bromodomain) of BRD4, thereby preventing protein-protein interaction between BET proteins and acetylated lysine residues and transcription factors (McLure, et al., 2013). RVX-208 selectively inhibits LPS induced IL6 expression in U937 cells, but not TNFα mRNA (McLure, et al., 2013). It also inhibits TNFα induced upregulation of MCP1 and VCAM1 in human aortic endothelial cells (Jahagirdar, et al., 2014). Patients receiving RVX-208 have lower levels of circulating inflammatory cytokines and lower incidence of major adverse cardiac events (Gilham, et al., 2016). RVX-208 (150 mg/kg, oral administration, for 12 weeks) also reduces atherosclerosis in hyperlipidemic ApoE−/− by increasing HDL level while decreasing LDL level (Jahagirdar, et al., 2014). (+)-JQ1 is another BET bromodomain inhibitor which has anti-inflammatory effects by displacing BET proteins from chromatin via binding to bromodomain in a competitive manner (Filippakopoulos, et al., 2010). (+)-JQ1 (50 mg/kg, 10 weeks) has been demonstrated to dampen inflammatory responses in activated endothelium and decrease leukocyte adhesion to activated endothelium as well as reduced atherosclerosis in hyperlipidemic ApoE−/− mice (Brown, et al., 2014). In addition, (+)-JQ1 also attenuates Ang-II-induced hypertension, and inflammation in mice (S. Das, et al., 2017). These studies collectively suggests that BET inhibitors could provide potential atheroprotective effects in vivo.
5.7. miRNA-based therapeutics
As discussed earlier, atherosclerosis is a multifactorial disease involving multiple mechanisms and diverse cell types. The nature of this complex regulatory network renders miRNA as a promising therapeutic target for treating atherosclerosis by delivering miRNA mimics or inhibitors locally or systemically (Lucas, et al., 2018). Recent findings from Maegdefessel group have shown the advantage of local delivery of mimics of miR-21 (Jin, et al., 2018) and miR-210 (Eken, et al., 2017) to increase plaque stability and potentially prevent the incidence of atherothrombotic vascular eve nts. However, a major hurdle in the miRNA-based therapeutic field is the question of efficient miRNA delivery strategies. Therefore, development of new vectors (such as nanoparticles, ultrasound-targeted microbubbles etc.) for targeted delivery of miRNAs or miRNA inhibitors to the desired targeted vascular cell type is of the utmost importance to achieve site-specific drug delivery and therapeutic effects in the future (Sharma, et al., 2010).
6. Concluding remarks and future perspectives
The past decade has witnessed unanticipated progress in deciphering various epigenetic mechanisms in gene regulation, with far-reaching implications for understanding cardiovascular development, biology, and diseases. The overall consensus is that individual epigenetic regulation of atherosclerosis is very complex. Cell type- and isoform-specific role of epigenetic enzymes does exist at different stages of atheroprogression. The epigenetic-based hypothesis of atherosclerosis has deepened the understanding of the molecular mechanisms of atherosclerosis, which is traditionally viewed as a chronic inflammatory and lipid disorder with genetic codes as the key determinant (Libby, Tabas, Fredman, & Fisher, 2014). Advances in cardiovascular epigenetics, especially the epigenome-wide association studies help us to appreciate the intricate interplay of epigenetics in regulating lipid metabolism, inflammation, and redox status in atherosclerosis.
Epigenetic processes are often influenced by environmental and lifestyle factors, such as air pollution, physical inactivity, unhealthy diet, and smoking, which are well-known risk factors for CVD (Abdul, Yu, Chung, Jung, & Choi, 2017). Atherosclerotic patients are likely to develop systemic inflammation and cardiovascular complications even after intensive lipid-lowering therapies (by statins). The residual risk of vascular inflammation presents major challenges for treating atherosclerosis. In this scenario, cardiovascular drugs targeting epigenetics hold great promise for cardiovascular therapeutics as they may complement standard statin treatment and PCSK9 inhibitor-based hypolipidemic therapy in clinical practice.
Future directions in the field of cardiovascular epigenetics include:
Exploratory research on novel molecular biomarkers of CVD. It is important to identify specific biomarkers associated with disease (and/or disease severity), such as novel secreted lncRNAs and miRNAs (L. He, Chen, Hao, & Qian, 2018).
New bioinformatic tools to predict the lncRNA-disease associations, such as e.g. LncDisease (J. Wang, et al., 2016) developed by the Cui lab (http://www.cuilab.cn/). These tools are helpful in identifying the biological functions of emerging new lncRNAs in disease prevention, diagnosis and management.
The improvement of precision in cardiovascular medicine. The Institute of Precision Cardiovascular Medicine at AHA (https://precision.heart.org) aims to find a cure for coronary heart disease and improving cardiovascular health. This possibility creates new therapeutic opportunities by modulating disease risk, as well as targeting selected disease through epigenetic mechanisms (Costantino, et al., 2017). Epigenetics may also explain why individuals with similar genetics and risk factors respond differently to treatment. Gender is an important determinant of the incidence of CVD. A recent study (Hassan, et al., 2018) has shown that estrogen can promote phosphorylation-dependent EZH2 degradation via PI3K/Akt pathway, suggesting that sex hormone-dependent modulation of chromatin modifiers could be partially responsible for sex dimorphism.
The role of RNA methylation in atherosclerosis. RNA N6-methyladenosine methylation (m6A) (Yue, Liu, & He, 2015) is an emerging new epigenetic mechanism that has been recently intensively investigated in cancer biology. This new type of modification is dictated by m6A methylation “writers” and can be reversed by demethylases that serve as “erasers”. The role of this new type of RNA modification in cardiovascular biology is unknown and warrants further studies.
New translational animal models for characterizing the pharmacological actions of epigenetic cardiovascular drugs: To date, ApoE−/− or LDLr−/− mice are the two most widely used mouse models for studying atherosclerosis via pharmacological intervention or gene engineering (Emini Veseli, et al., 2017). The development of atherosclerosis in both models can be accelerated by challenging with Western-type diet or high cholesterol diet for several months. The use of other translational pre-clinical models of atherosclerosis, such as partial carotid ligation in ApoE−/− mice (Nam, et al., 2009), or AAV8-mPCSK9D377Y mutant injection (Bjorklund, et al., 2014; Goettsch, et al., 2016; S. Kumar, Kang, Rezvan, & Jo, 2017) will significantly shorten the experimental time frame, and potentially accelerate the discovery of new epigenetic drugs targeting chromatin modifiers.
Research on new epigenetic pharmaceuticals by drug repurposing/repositioning. Besides seeking new therapeutic strategies, identifying new epigenetic targets of FDA-approved small molecule drugs (such as statins which function at least partially through epigenetic mechanisms (Allen & Mamotte, 2017)) and repurposing them as new therapies for atherosclerosis, is potentially a safe and cost-efficient avenue of future cardiovascular drug discovery.
Cocktail or combinatorial epigenetic therapy. Since different epigenetic mechanisms, especially DNA methylation and histone methylation/acetylation coordinate to regulate gene expression networks, HDACi and DNMTi combination therapies are attractive. This type of epigenetic “cocktail” therapy may synergistically derepress atheroprotective gene expression, thereby reducing drug dosages and potentially side effects in atherosclerotic patients. To date, several epigenetic drugs such as DNMTi, EZH2i, BETi, and HDACi have been (or being tested in clinical trials) used in treating cancers (Bennett & Licht, 2018). However, there are up to date very few reports of epigenetics “cocktail” therapy for treating CVD.
Targeting ‘trained immunity’ by epigenetic drugs. Lipid-lowering statins are the cornerstone for treating atherosclerosis. Recent studies (Ridker, et al., 2017) have shown that anti-inflammatory therapy with Canakinumab (a monoclonal antibody that targets IL-1β) reduces recurrent cardiovascular events in high-risk patients, highlighting the importance of targeting inflammation as a second treatment option to improve cardiovascular outcome. Despite these therapeutic advances, residual cardiovascular risk remains very high, which might be due to the “epigenetic memory” effects elicited by abnormal lipid profile and/or pro-inflammatory cytokines in addition to low statin responses in certain human patient populations. The emergence of epigenetic drugs could fill this gap to abrogate the trained immunity effects conferred by pro-atherogenic insults and complement lipid-modulating and anti-inflammatory therapies.
In this review, we timely overviewed the epigenetic mechanisms and targeted therapeutics of atherosclerotic cardiovascular disease. The use of epigenetic drugs in CVD is likely to further energize clinicians, translational scientists, and the pharmaceutical industry to identify new chemical entities that may target selected chromatin “readers”, “writers” and “erasers”. Future epigenetic studies in cardiovascular medicine will improve our understanding of the molecular pathogenesis of CVD and most importantly, facilitate novel biomarker identification, improved disease prevention, and new therapeutic strategies in managing CVD. It can be envisaged that the deepened understanding of epigenetic regulation in cardiovascular health and disease will yield novel therapeutic interventions precisely tailored to patients’ precise disease conditions.
Acknowledgments
This work was supported by grants from NIH (HL09502, HL114570, HL128363 and HL130167 to Z.G.J) and the American Heart Association (AHA) (17GRNT33660671 to Z.G.J) and AHA Career Development Award (18CDA34110359 to S. X.). Figures in this review were made using elements from Servier Medical Arts (https://smart.servier.com/).
Abbreviations
- ABCA1
ATP binding cassette subfamily A member 1
- Ang-II
Angiotensin-II
- ANRIL
CDKN2B antisense RNA 1
- ApoE
Apolipoprotein E
- BET
Bromodomain and extra-terminal motif
- CAD
Coronary artery disease
- CD36
Cluster of differentiation 36
- CSE
Cystathionine gamma-lyase
- CVD
Cardiovascular diseasese
- NOS
Endothelial nitric oxide synthase
- DNMT
DNA methyltransferase
- ET-1
Endothelin-1
- EZH2
Enhancer of zeste homolog 2
- HFD
High fat-diet
- HDAC
Histone deacetylase
- ICAM1
Intercellular adhesion molecule 1
- IL1β
Interleukin-1 beta
- IL6
Interleukin-1
- KLF2
Kruppel like factor 2
- LDL
Low-density lipoproteins
- LDLr
Low density lipoprotein receptor
- LXR
Liver X receptor
- MCP1
Monocyte chemoattractant protein-1
- MMPs
Matrix metalloproteinases
- NF-kB
Nuclear factor-kappa B
- NO
Nitric oxide
- PCSK9
Proprotein convertase subtilisin/kexin type 9
- PPAR
Peroxisome proliferator-activated receptor
- ROS
Reactive oxygen species
- SIRT
Sirtuin
- TET2
Tet methylcytosine dioxygenase 2
- TGFβ
Transforming growth factor-β
- TGFBR
Transforming growth factor-β receptor
- TNFα
Tumor necrosis factor-alpha
- VCAM1
Vascular cell adhesion molecule 1
- VSMCs
Vascular smooth muscle cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare they have no conflicts of interest.
References
- Aavik E, Lumivuori H, Leppanen O, Wirth T, Hakkinen SK, Brasen JH, Beschorner U, Zeller T, Braspenning M, van Criekinge W, Makinen K, & Yla-Herttuala S (2015). Global DNA methylation analysis of human atherosclerotic plaques reveals extensive genomic hypomethylation and reactivation at imprinted locus 14q32 involving induction of a miRNA cluster. Eur Heart J, 36, 993–1000. [DOI] [PubMed] [Google Scholar]
- Abdul QA, Yu BP, Chung HY, Jung HA, & Choi JS (2017). Epigenetic modifications of gene expression by lifestyle and environment. Arch Pharm Res, 40, 1219–1237. [DOI] [PubMed] [Google Scholar]
- Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, Crane GM, Cowin BL, Bruner E, Murphy MM, Chen W, Spangrude GJ, Hu Z, DeBerardinis RJ, & Morrison SJ (2017). Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature, 549, 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A, Li Y, Bao B, Kong D, & Sarkar FH (2014). Epigenetic regulation of miRNA-cancer stem cells nexus by nutraceuticals. Mol Nutr Food Res, 58, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ASI, Dong K, Liu J, Wen T, Yu L, Xu F, Kang X, Osman I, Hu G, Bunting KM, Crethers D, Gao H, Zhang W, Liu Y, Wen K, Agarwal G, Hirose T, Nakagawa S, Vazdarjanova A, & Zhou J (2018). Long noncoding RNA NEAT1 (nuclear paraspeckle assembly transcript 1) is critical for phenotypic switching of vascular smooth muscle cells. Proc Natl Acad Sci U S A, 115, E8660–e8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljubran SA, Cox R Jr., Tamarapu Parthasarathy P, Kollongod Ramanathan G, Rajanbabu V, Bao H, Mohapatra SS, Lockey R, & Kolliputi N (2012). Enhancer of zeste homolog 2 induces pulmonary artery smooth muscle cell proliferation. PLoS One, 7, e37712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkemade FE, van Vliet P, Henneman P, van Dijk KW, Hierck BP, van Munsteren JC, Scheerman JA, Goeman JJ, Havekes LM, Gittenberger-de Groot AC, van den Elsen PJ, & DeRuiter MC (2010). Prenatal exposure to apoE deficiency and postnatal hypercholesterolemia are associated with altered cell-specific lysine methyltransferase and histone methylation patterns in the vasculature. Am J Pathol, 176, 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SC, & Mamotte CDS (2017). Pleiotropic and Adverse Effects of Statins-Do Epigenetics Play a Role? J Pharmacol Exp Ther, 362, 319–326. [DOI] [PubMed] [Google Scholar]
- Arslan S, Berkan O, Lalem T, Ozbilum N, Goksel S, Korkmaz O, Cetin N, & Devaux Y (2017). Long non-coding RNAs in the atherosclerotic plaque. Atherosclerosis, 266, 176–181. [DOI] [PubMed] [Google Scholar]
- Aryal B, & Suarez Y (2018). Non-coding RNA regulation of endothelial and macrophage functions during atherosclerosis. Vascul Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins GB, Wang Y, Mahabeleshwar GH, Shi H, Gao H, Kawanami D, Natesan V, Lin Z, Simon DI, & Jain MK (2008). Hemizygous deficiency of Kruppel-like factor 2 augments experimental atherosclerosis. Circ Res, 103, 690–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas Writing G, Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, Blum M, Pinto F, & Vardas P (2018). European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur Heart J, 39, 508–579. [DOI] [PubMed] [Google Scholar]
- Azghandi S, Prell C, van der Laan SW, Schneider M, Malik R, Berer K, Gerdes N, Pasterkamp G, Weber C, Haffner C, & Dichgans M (2015). Deficiency of the stroke relevant HDAC9 gene attenuates atherosclerosis in accord with allele-specific effects at 7p21.1. Stroke, 46, 197–202. [DOI] [PubMed] [Google Scholar]
- Baccarelli A, Rienstra M, & Benjamin EJ (2010). Cardiovascular epigenetics: basic concepts and results from animal and human studies. Circ Cardiovasc Genet, 3, 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D, Jahagirdar R, Gordon A, Hafiane A, Campbell S, Chatur S, Wagner GS, Hansen HC, Chiacchia FS, Johansson J, Krimbou L, Wong NC, & Genest J (2010). RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol, 55, 2580–2589. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, & Kundu TK (2004). Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem, 279, 51163–51171. [DOI] [PubMed] [Google Scholar]
- Balestrieri ML, Rizzo MR, Barbieri M, Paolisso P, D’Onofrio N, Giovane A, Siniscalchi M, Minicucci F, Sardu C, D’Andrea D, Mauro C, Ferraraccio F, Servillo L, Chirico F, Caiazzo P, Paolisso G, & Marfella R (2015). Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: effects of incretin treatment. Diabetes, 64, 1395–1406. [DOI] [PubMed] [Google Scholar]
- Ballantyne MD, Pinel K, Dakin R, Vesey AT, Diver L, Mackenzie R, Garcia R, Welsh P, Sattar N, Hamilton G, Joshi N, Dweck MR, Miano JM, McBride MW, Newby DE, McDonald RA, & Baker AH (2016). Smooth Muscle Enriched Long Noncoding RNA (SMILR) Regulates Cell Proliferation. Circulation, 133, 2050–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao MH, Li GY, Huang XS, Tang L, Dong LP, & Li JM (2018). Long Noncoding RNA LINC00657 Acting as a miR-590–3p Sponge to Facilitate Low Concentration Oxidized Low-Density Lipoprotein-Induced Angiogenesis. Mol Pharmacol, 93, 368–375. [DOI] [PubMed] [Google Scholar]
- Bao Y, Yang Y, Wang L, Gao L, Jiang W, Wang L, Si S, & Hong B (2009). Identification of trichostatin A as a novel transcriptional up-regulator of scavenger receptor BI both in HepG2 and RAW 264.7 cells. Atherosclerosis, 204, 127–135. [DOI] [PubMed] [Google Scholar]
- Bea F, Blessing E, Shelley MI, Shultz JM, & Rosenfeld ME (2003). Simvastatin inhibits expression of tissue factor in advanced atherosclerotic lesions of apolipoprotein E deficient mice independently of lipid lowering: potential role of simvastatin-mediated inhibition of Egr-1 expression and activation. Atherosclerosis, 167, 187–194. [DOI] [PubMed] [Google Scholar]
- Bekkering S, Arts RJW, Novakovic B, Kourtzelis I, van der Heijden C, Li Y, Popa CD, Ter Horst R, van Tuijl J, Netea-Maier RT, van de Veerdonk FL, Chavakis T, Joosten LAB, van der Meer JWM, Stunnenberg H, Riksen NP, & Netea MG (2018). Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell, 172, 135–146e139. [DOI] [PubMed] [Google Scholar]
- Bekkering S, Blok BA, Joosten LA, Riksen NP, van Crevel R, & Netea MG (2016). In Vitro Experimental Model of Trained Innate Immunity in Human Primary Monocytes. Clin Vaccine Immunol, 23, 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkering S, Joosten LA, van der Meer JW, Netea MG, & Riksen NP (2013). Trained innate immunity and atherosclerosis. Curr Opin Lipidol, 24, 487–492. [DOI] [PubMed] [Google Scholar]
- Bekkering S, Quintin J, Joosten LA, van der Meer JW, Netea MG, & Riksen NP (2014). Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler Thromb Vasc Biol, 34, 1731–1738. [DOI] [PubMed] [Google Scholar]
- Bell RD, Long X, Lin M, Bergmann JH, Nanda V, Cowan SL, Zhou Q, Han Y, Spector DL, Zheng D, & Miano JM (2014). Identification and initial functional characterization of a human vascular cell-enriched long noncoding RNA. Arterioscler Thromb Vasc Biol, 34, 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, & Muntner P (2018). Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. [DOI] [PubMed] [Google Scholar]
- Bennett RL, & Licht JD (2018). Targeting Epigenetics in Cancer. Annu Rev Pharmacol Toxicol, 58, 187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzon JF, Otsuka F, Virmani R, & Falk E (2014). Mechanisms of plaque formation and rupture. Circ Res, 114, 1852–1866. [DOI] [PubMed] [Google Scholar]
- Berbee JF, Wong MC, Wang Y, van der Hoorn JW, Khedoe PP, van Klinken JB, Mol IM, Hiemstra PS, Tsikas D, Romijn JA, Havekes LM, Princen HM, & Rensen PC (2013). Resveratrol protects against atherosclerosis, but does not add to the antiatherogenic effect of atorvastatin, in APOE*3-Leiden.CETP mice. J Nutr Biochem, 24, 1423–1430. [DOI] [PubMed] [Google Scholar]
- Bjorklund MM, Hollensen AK, Hagensen MK, Dagnaes-Hansen F, Christoffersen C, Mikkelsen JG, & Bentzon JF (2014). Induction of atherosclerosis in mice and hamsters without germline genetic engineering. Circ Res, 114, 1684–1689. [DOI] [PubMed] [Google Scholar]
- Boon RA, Hofmann P, Michalik KM, Lozano-Vidal N, Berghauser D, Fischer A, Knau A, Jae N, Schurmann C, & Dimmeler S (2016). Long Noncoding RNA Meg3 Controls Endothelial Cell Aging and Function: Implications for Regenerative Angiogenesis. J Am Coll Cardiol, 68, 2589–2591. [DOI] [PubMed] [Google Scholar]
- Boon RA, Jae N, Holdt L, & Dimmeler S (2016). Long Noncoding RNAs: From Clinical Genetics to Therapeutic Targets? J Am Coll Cardiol, 67, 1214–1226. [DOI] [PubMed] [Google Scholar]
- Boulberdaa M, Scott E, Ballantyne M, Garcia R, Descamps B, Angelini GD, Brittan M, Hunter A, McBride M, McClure J, Miano JM, Emanueli C, Mills NL, Mountford JC, & Baker AH (2016). A Role for the Long Noncoding RNA SENCR in Commitment and Function of Endothelial Cells. Mol Ther, 24, 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes AJ, Khan MI, Shi Y, Robertson L, & Werstuck GH (2009). Valproate attenuates accelerated atherosclerosis in hyperglycemic apoE-deficient mice: evidence in support of a role for endoplasmic reticulum stress and glycogen synthase kinase-3 in lesion development and hepatic steatosis. Am J Pathol, 174, 330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun KV, Voortman T, Dhana K, Troup J, Bramer WM, Troup J, Chowdhury R, Dehghan A, Muka T, & Franco OH (2016). The role of DNA methylation in dyslipidaemia: A systematic review. Prog Lipid Res, 64, 178–191. [DOI] [PubMed] [Google Scholar]
- Brown JD, Lin CY, Duan Q, Griffin G, Federation A, Paranal RM, Bair S, Newton G, Lichtman A, Kung A, Yang T, Wang H, Luscinskas FW, Croce K, Bradner JE, & Plutzky J (2014). NF-kappaB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell, 56, 219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Yang Y, Chen X, He D, Zhang X, Wen X, Hu J, Fu C, Qiu D, Jose PA, Zeng C, & Zhou L (2016). Circulating “LncPPARdelta” From Monocytes as a Novel Biomarker for Coronary Artery Diseases. Medicine (Baltimore), 95, e2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Yang Y, Chen X, Wu G, Zhang X, Liu Y, Yu J, Wang X, Fu J, Li C, Jose PA, Zeng C, & Zhou L (2016). Circulating ‘lncRNA OTTHUMT00000387022’ from monocytes as a novel biomarker for coronary artery disease. Cardiovasc Res, 112, 714–724. [DOI] [PubMed] [Google Scholar]
- Cao Q, Rong S, Repa JJ, St Clair R, Parks JS, & Mishra N (2014). Histone deacetylase 9 represses cholesterol efflux and alternatively activated macrophages in atherosclerosis development. Arterioscler Thromb Vasc Biol, 34, 1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Wang X, Jia L, Mondal AK, Diallo A, Hawkins GA, Das SK, Parks JS, Yu L, Shi H, Shi H, & Xue B (2014). Inhibiting DNA Methylation by 5-Aza-2’-deoxycytidine ameliorates atherosclerosis through suppressing macrophage inflammation. Endocrinology, 155, 4925–4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SH, Hung CH, Shih JY, Chu PM, Cheng YH, Lin HC, & Tsai KL (2017). SIRT1 inhibition causes oxidative stress and inflammation in patients with coronary artery disease. Redox Biol, 13, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, & Guarente L (2014). SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab, 25, 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DD, Hui LL, Zhang XC, & Chang Q (2018). NEAT1 contributes to ox-LDL-induced inflammation and oxidative stress in macrophages through inhibiting miR-128. J Cell Biochem. [DOI] [PubMed] [Google Scholar]
- Chen HZ, Wang F, Gao P, Pei JF, Liu Y, Xu TT, Tang X, Fu WY, Lu J, Yan YF, Wang XM, Han L, Zhang ZQ, Zhang R, Zou MH, & Liu DP (2016). Age-Associated Sirtuin 1 Reduction in Vascular Smooth Muscle Links Vascular Senescence and Inflammation to Abdominal Aortic Aneurysm. Circ Res, 119, 1076–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang J, Yang J, Xu L, Hu Q, Xu C, Yang S, & Jiang H (2017). Histone demethylase KDM3a, a novel regulator of vascular smooth muscle cells, controls vascular neointimal hyperplasia in diabetic rats. Atherosclerosis, 257, 152–163. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sun T, Wu J, Kalionis B, Zhang C, Yuan D, Huang J, Cai W, Fang H, & Xia S (2015). Icariin intervenes in cardiac inflammaging through upregulation of SIRT6 enzyme activity and inhibition of the NF-kappa B pathway. Biomed Res Int, 2015, 895976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YX, Zhang M, Cai Y, Zhao Q, & Dai W (2015). The Sirt1 activator SRT1720 attenuates angiotensin II-induced atherosclerosis in apoE(−)/(−) mice through inhibiting vascular inflammatory response. Biochem Biophys Res Commun, 465, 732–738. [DOI] [PubMed] [Google Scholar]
- Chi GC, Liu Y, MacDonald JW, Barr RG, Donohue KM, Hensley MD, Hou L, McCall CE, Reynolds LM, Siscovick DS, & Kaufman JD (2016). Long-term outdoor air pollution and DNA methylation in circulating monocytes: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health, 15, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Peng L, Pan N, Hu X, & Zhang Y (2014). The anti-atherogenic effects of berberine on foam cell formation are mediated through the upregulation of sirtuin 1. Int J Mol Med, 34, 1087–1093. [DOI] [PubMed] [Google Scholar]
- Choi JH, Nam KH, Kim J, Baek MW, Park JE, Park HY, Kwon HJ, Kwon OS, Kim DY, & Oh GT (2005). Trichostatin A exacerbates atherosclerosis in low density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol, 25, 2404–2409. [DOI] [PubMed] [Google Scholar]
- Christ A, Gunther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Bassler K, Klee K, Schulte-Schrepping J, Ulas T, Moorlag S, Kumar V, Park MH, Joosten LAB, Groh LA, Riksen NP, Espevik T, Schlitzer A, Li Y, Fitzgerald ML, Netea MG, Schultze JL, & Latz E (2018). Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell, 172, 162–175e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino L, Dolgalev I, Wang Y, Yoshimi A, Martin GH, Wang J, Ng V, Xia B, Witkowski MT, Mitchell-Flack M, Grillo I, Bakogianni S, Ndiaye-Lobry D, Martin MT, Guillamot M, Banh RS, Xu M, Figueroa ME, Dickins RA, Abdel-Wahab O, Park CY, Tsirigos A, Neel BG, & Aifantis I (2017). Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell, 170, 1079–1095e1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congrains A, Kamide K, Katsuya T, Yasuda O, Oguro R, Yamamoto K, Ohishi M, & Rakugi H (2012). CVD-associated non-coding RNA, ANRIL, modulates expression of atherogenic pathways in VSMC. Biochem Biophys Res Commun, 419, 612–616. [DOI] [PubMed] [Google Scholar]
- Costantino S, Libby P, Kishore R, Tardif JC, El-Osta A, & Paneni F (2017). Epigenetics and precision medicine in cardiovascular patients: from basic concepts to the clinical arena. Eur Heart J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs A, Hookway ES, Wells G, Lindow M, Obad S, Oerum H, Prinjha RK, Athanasou N, Sowman A, Philpott M, Penn H, Soderstrom K, Feldmann M, & Oppermann U (2018). Inhibition of histone H3K27 demethylases selectively modulates inflammatory phenotypes of natural killer cells. J Biol Chem, 293, 2422–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull AH, Snetsinger B, Buckstein R, Wells RA, & Rauh MJ (2017). Tet2 restrains inflammatory gene expression in macrophages. Exp Hematol, 55, 56–70e13. [DOI] [PubMed] [Google Scholar]
- D’Onofrio N, Servillo L, & Balestrieri ML (2018). SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection. Antioxid Redox Signal, 28, 711–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio N, Vitiello M, Casale R, Servillo L, Giovane A, & Balestrieri ML (2015). Sirtuins in vascular diseases: Emerging roles and therapeutic potential. Biochim Biophys Acta, 1852, 1311–1322. [DOI] [PubMed] [Google Scholar]
- Dai Y, Wu X, Dai D, Li J, & Mehta JL (2018). MicroRNA-98 regulates foam cell formation and lipid accumulation through repression of LOX-1. Redox Biol, 16, 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Samidurai A, & Salloum FN (2018). Deciphering Non-coding RNAs in Cardiovascular Health and Disease. Front Cardiovasc Med, 5, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Ray R, Snehlata, Das N, & Srivastava LM (2006). Effect of ascorbic acid on prevention of hypercholesterolemia induced atherosclerosis. Mol Cell Biochem, 285, 143–147. [DOI] [PubMed] [Google Scholar]
- Das S, Reddy MA, Senapati P, Stapleton K, Lanting L, Wang M, Amaram V, Ganguly R, Zhang L, Devaraj S, Schones DE, & Natarajan R (2018). Diabetes Mellitus-Induced Long Noncoding RNA Dnm3os Regulates Macrophage Functions and Inflammation via Nuclear Mechanisms. Arterioscler Thromb Vasc Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Senapati P, Chen Z, Reddy MA, Ganguly R, Lanting L, Mandi V, Bansal A, Leung A, Zhang S, Jia Y, Wu X, Schones DE, & Natarajan R (2017). Regulation of angiotensin II actions by enhancers and super-enhancers in vascular smooth muscle cells. Nat Commun, 8, 1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MJ (1996). Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation, 94, 2013–2020. [DOI] [PubMed] [Google Scholar]
- De Santa F, Narang V, Yap ZH, Tusi BK, Burgold T, Austenaa L, Bucci G, Caganova M, Notarbartolo S, Casola S, Testa G, Sung WK, Wei CL, & Natoli G (2009). Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J, 28, 3341–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, & Natoli G (2007). The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell, 130, 1083–1094. [DOI] [PubMed] [Google Scholar]
- de Vries MR, & Quax PH (2016). Plaque angiogenesis and its relation to inflammation and atherosclerotic plaque destabilization. Curr Opin Lipidol, 27, 499–506. [DOI] [PubMed] [Google Scholar]
- Dekkers KF, van Iterson M, Slieker RC, Moed MH, Bonder MJ, van Galen M, Mei H, Zhernakova DV, van den Berg LH, Deelen J, van Dongen J, van Heemst D, Hofman A, Hottenga JJ, van der Kallen CJ, Schalkwijk CG, Stehouwer CD, Tigchelaar EF, Uitterlinden AG, Willemsen G, Zhernakova A, Franke L, t Hoen PA, Jansen R, van Meurs J, Boomsma DI, van Duijn CM, van Greevenbroek MM, Veldink JH, Wijmenga C, van Zwet EW, Slagboom PE, Jukema JW, & Heijmans BT (2016). Blood lipids influence DNA methylation in circulating cells. Genome Biol, 17, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcuve GP, Rastegar M, & Davie JR (2009). Epigenetic control. J Cell Physiol, 219, 243–250. [DOI] [PubMed] [Google Scholar]
- Delgado-Olguin P, Dang LT, He D, Thomas S, Chi L, Sukonnik T, Khyzha N, Dobenecker MW, Fish JE, & Bruneau BG (2014). Ezh2-mediated repression of a transcriptional pathway upstream of Mmp9 maintains integrity of the developing vasculature. Development, 141, 4610–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerath EW, Guan W, Grove ML, Aslibekyan S, Mendelson M, Zhou YH, Hedman AK, Sandling JK, Li LA, Irvin MR, Zhi D, Deloukas P, Liang L, Liu C, Bressler J, Spector TD, North K, Li Y, Absher DM, Levy D, Arnett DK, Fornage M, Pankow JS, & Boerwinkle E (2015). Epigenome-wide association study (EWAS) of BMI, BMI change and waist circumference in African American adults identifies multiple replicated loci. Hum Mol Genet, 24, 4464–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Huang W, Peng C, Gao J, Li Z, Qiu X, Yang N, Yuan B, & Zheng F (2018). Genomic 5-mC contents in peripheral blood leukocytes were independent protective factors for coronary artery disease with a specific profile in different leukocyte subtypes. Clin Epigenetics, 10, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri M, Bommi PV, Sahasrabuddhe AA, Khandekar JD, & Dimri GP (2010). Dietary omega-3 polyunsaturated fatty acids suppress expression of EZH2 in breast cancer cells. Carcinogenesis, 31, 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokmanovic M, Clarke C, & Marks PA (2007). Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res, 5, 981–989. [DOI] [PubMed] [Google Scholar]
- Donaldson CJ, Lao KH, & Zeng L (2018). The salient role of microRNAs in atherogenesis. J Mol Cell Cardiol, 122, 98–113. [DOI] [PubMed] [Google Scholar]
- Dreger H, Ludwig A, Weller A, Stangl V, Baumann G, Meiners S, & Stangl K (2012). Epigenetic regulation of cell adhesion and communication by enhancer of zeste homolog 2 in human endothelial cells. Hypertension, 60, 1176–1183. [DOI] [PubMed] [Google Scholar]
- Du C, Lin X, Xu W, Zheng F, Cai J, Yang J, Cui Q, Tang C, Cai J, Xu G, & Geng B (2018). Sulfhydrated Sirtuin-1 Increasing Its Deacetylation Activity Is an Essential Epigenetics Mechanism of Anti-Atherogenesis by Hydrogen Sulfide. Antioxid Redox Signal. [DOI] [PubMed] [Google Scholar]
- Du XJ, Lu JM, & Sha Y (2018). MiR-181a inhibits vascular inflammation induced by ox-LDL via targeting TLR4 in human macrophages. J Cell Physiol, 233, 6996–7003. [DOI] [PubMed] [Google Scholar]
- Dunn J, Qiu H, Kim S, Jjingo D, Hoffman R, Kim CW, Jang I, Son DJ, Kim D, Pan C, Fan Y, Jordan IK, & Jo H (2014). Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest, 124, 3187–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eken SM, Jin H, Chernogubova E, Li Y, Simon N, Sun C, Korzunowicz G, Busch A, Backlund A, Osterholm C, Razuvaev A, Renne T, Eckstein HH, Pelisek J, Eriksson P, Gonzalez Diez M, Perisic Matic L, Schellinger IN, Raaz U, Leeper NJ, Hansson GK, Paulsson-Berne G, Hedin U, & Maegdefessel L (2017). MicroRNA-210 Enhances Fibrous Cap Stability in Advanced Atherosclerotic Lesions. Circ Res, 120, 633–644. [DOI] [PubMed] [Google Scholar]
- El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, & Brownlee M (2008). Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med, 205, 2409–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini Veseli B, Perrotta P, De Meyer GRA, Roth L, Van der Donckt C, Martinet W, & De Meyer GRY (2017). Animal models of atherosclerosis. Eur J Pharmacol, 816, 3–13. [DOI] [PubMed] [Google Scholar]
- Fang J, Little PJ, & Xu S (2018). Atheroprotective Effects and Molecular Targets of Tanshinones Derived From Herbal Medicine Danshen. Med Res Rev, 38, 201–228. [DOI] [PubMed] [Google Scholar]
- Feinberg MW, & Moore KJ (2016). MicroRNA Regulation of Atherosclerosis. Circ Res, 118, 703–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, & Bradner JE (2010). Selective inhibition of BET bromodomains. Nature, 468, 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Deng CX, & Mostoslavsky R (2009). Recent progress in the biology and physiology of sirtuins. Nature, 460, 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AV, Nakano M, Narula J, Kolodgie FD, & Virmani R (2010). Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol, 30, 1282–1292. [DOI] [PubMed] [Google Scholar]
- Forstermann U, & Sessa WC (2012). Nitric oxide synthases: regulation and function. Eur Heart J, 33, 829–837, 837a–837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry JL, Al Sayah L, Weisbrod RM, Van Roy I, Weng X, Cohen RA, Bachschmid MM, & Seta F (2016). Vascular Smooth Muscle Sirtuin-1 Protects Against Diet-Induced Aortic Stiffness. Hypertension, 68, 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry JL, Shiraishi Y, Turcotte R, Yu X, Gao YZ, Akiki R, Bachschmid M, Zhang Y, Morgan KG, Cohen RA, & Seta F (2015). Vascular Smooth Muscle Sirtuin-1 Protects Against Aortic Dissection During Angiotensin II-Induced Hypertension. J Am Heart Assoc, 4, e002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C, Hao S, Xu X, Zhou J, Liu Z, Lu H, Wang L, Jin W, & Li S (2018). Activation of SIRT1 ameliorates LPS-induced lung injury in mice via decreasing endothelial tight junction permeability. Acta Pharmacol Sin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Jiang Z, Li T, Hu Y, & Guo J (2018). Circular RNAs in hepatocellular carcinoma: Functions and implications. Cancer Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao H, Ijiri Y, Miura M, Hashimoto M, Yamashita T, Fukunaga C, Oiwa K, Kawai Y, Suwa M, & Yamamoto J (2004). Effect of trans-resveratrol on the thrombogenicity and atherogenicity in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Blood Coagul Fibrinolysis, 15, 441–446. [DOI] [PubMed] [Google Scholar]
- Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA, & Walsh K (2017). Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science, 355, 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabory A, Attig L, & Junien C (2009). Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol, 304, 8–18. [DOI] [PubMed] [Google Scholar]
- Gast M, Rauch BH, Nakagawa S, Haghikia A, Jasina A, Haas J, Nath N, Jensen L, Stroux A, Bohm A, Friebel J, Rauch U, Skurk C, Blankenberg S, Zeller T, Prasanth KV, Meder B, Kuss A, Landmesser U, & Poller W (2018). Immune system-mediated atherosclerosis caused by deficiency of long noncoding RNA MALAT1 in ApoE−/− mice. Cardiovasc Res. [DOI] [PubMed] [Google Scholar]
- Gaul DS, Weber J, Van Tits LJ, Sluka S, Pasterk L, Reiner MF, Calatayud N, Lohmann C, Klingenberg R, Pahla J, Vdovenko D, Tanner FC, Camici GG, Eriksson U, Auwerx J, Mach F, Windecker S, Rodondi N, TF LUS, Winnik S, & Matter CM (2018). Loss of Sirt3 accelerates arterial thrombosis by increasing formation of neutrophil extracellular traps and plasma tissue factor activity. Cardiovasc Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SS, Bie J, Wang J, & Ghosh S (2014). Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerancein LDLR−/− mice--role of intestinal permeability and macrophage activation. PLoS One, 9, e108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilham D, Wasiak S, Tsujikawa LM, Halliday C, Norek K, Patel RG, Kulikowski E, Johansson J, Sweeney M, & Wong NC (2016). RVX-208, a BET-inhibitor for treating atherosclerotic cardiovascular disease, raises ApoA-I/HDL and represses pathways that contribute to cardiovascular disease. Atherosclerosis, 247, 48–57. [DOI] [PubMed] [Google Scholar]
- Gillette TG, & Hill JA (2015). Readers, writers, and erasers: chromatin as the whiteboard of heart disease. Circ Res, 116, 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goettsch C, Hutcheson JD, Hagita S, Rogers MA, Creager MD, Pham T, Choi J, Mlynarchik AK, Pieper B, Kjolby M, Aikawa M, & Aikawa E (2016). A single injectionof gain-of-function mutant PCSK9 adeno-associated virus vector induces cardiovascular calcification in mice with no genetic modification. Atherosclerosis, 251, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes CPC, Spencer H, Ford KL, Michel LYM, Baker AH, Emanueli C, Balligand JL, & Devaux Y (2017). The Function and Therapeutic Potential of Long Non-coding RNAs in Cardiovascular Development and Disease. Mol Ther Nucleic Acids, 8, 494–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenne I, Kumar S, Gray K, Figg N, Yu H, Mercer J, & Bennett M (2013). Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation, 127, 386–396. [DOI] [PubMed] [Google Scholar]
- Gould RG (1951). Lipid metabolism and atherosclerosis. Am J Med, 11, 209–227. [DOI] [PubMed] [Google Scholar]
- Grazioli E, Dimauro I, Mercatelli N, Wang G, Pitsiladis Y, Di Luigi L, & Caporossi D (2017). Physical activity in the prevention of human diseases: role of epigenetic modifications. BMC Genomics, 18, 802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greissel A, Culmes M, Burgkart R, Zimmermann A, Eckstein HH, Zernecke A, & Pelisek J (2016). Histone acetylation and methylation significantly change with severity of atherosclerosis in human carotid plaques. Cardiovasc Pathol, 25, 79–86. [DOI] [PubMed] [Google Scholar]
- Greissel A, Culmes M, Napieralski R, Wagner E, Gebhard H, Schmitt M, Zimmermann A, Eckstein HH, Zernecke A, & Pelisek J (2015). Alternation of histone and DNA methylationin human atherosclerotic carotid plaques. Thromb Haemost, 114, 390–402. [DOI] [PubMed] [Google Scholar]
- Groeneweg M, Kanters E, Vergouwe MN, Duerink H, Kraal G, Hofker MH, & de Winther MP (2006). Lipopolysaccharide-induced gene expression in murine macrophages is enhanced by prior exposure to oxLDL. J Lipid Res, 47, 2259–2267. [DOI] [PubMed] [Google Scholar]
- Guo L, Akahori H, Harari E, Smith SL, Polavarapu R, Karmali V, Otsuka F, Gannon RL, Braumann RE, Dickinson MH, Gupta A, Jenkins AL, Lipinski MJ, Kim J, Chhour P, de Vries PS, Jinnouchi H, Kutys R, Mori H, Kutyna MD, Torii S, Sakamoto A, Choi CU, Cheng Q, Grove ML, Sawan MA, Zhang Y, Cao Y, Kolodgie FD, Cormode DP, Arking DE, Boerwinkle E, Morrison AC, Erdmann J, Sotoodehnia N, Virmani R, & Finn AV (2018). CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J Clin Invest, 128, 1106–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke W, & Grond-Ginsbach C (2012). Commentary on a GWAS: HDAC9 and the risk for ischaemic stroke. BMC Med, 10, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Han X, Wang Z, Shen J, & Dong Q (2016). HDAC9 regulates ox-LDL-induced endothelial cell apoptosis by participating in inflammatory reactions. Front Biosci (Landmark Ed), 21, 907–917. [DOI] [PubMed] [Google Scholar]
- Han Y, Ma J, Wang J, & Wang L (2018). Silencing of H19 inhibits the adipogenesis and inflammation response in ox-LDL-treated Raw264.7 cells by up-regulating miR-130b. Mol Immunol, 93, 107–114. [DOI] [PubMed] [Google Scholar]
- Hanna CW, Demond H, & Kelsey G (2018). Epigenetic regulation in development: is the mouse a good model for the human? Hum Reprod Update, 24, 556–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK, & Hermansson A (2011). The immune system in atherosclerosis. Nat Immunol, 12, 204–212. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Robertson AK, & Soderberg-Naucler C (2006). Inflammation and atherosclerosis. Annu Rev Pathol, 1, 297–329. [DOI] [PubMed] [Google Scholar]
- Harrison D, Griendling KK, Landmesser U, Hornig B, & Drexler H (2003). Role of oxidative stress in atherosclerosis. Am J Cardiol, 91, 7A–11A. [DOI] [PubMed] [Google Scholar]
- Hartmann P, Zhou Z, Natarelli L, Wei Y, Nazari-Jahantigh M, Zhu M, Grommes J, Steffens S, Weber C, & Schober A (2016). Endothelial Dicer promotes atherosclerosis and vascular inflammation by miRNA-103-mediated suppression of KLF4. Nat Commun, 7, 10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassa PO, Haenni SS, Elser M, & Hottiger MO (2006). Nuclear ADP-ribosylation reactions in mammalian cells: where are we today and where are we going? Microbiol Mol Biol Rev, 70, 789–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan N, Ali A, Withycombe C, Ahluwalia M, Al-Nasseri RH, Tonks A, & Morris K (2018). TET-2 up-regulation is associated with the anti-inflammatory action of Vicenin-2. Cytokine, 108, 37–42. [DOI] [PubMed] [Google Scholar]
- Hayek T, Fuhrman B, Vaya J, Rosenblat M, Belinky P, Coleman R, Elis A, & Aviram M (1997). Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler Thromb Vasc Biol, 17, 2744–2752. [DOI] [PubMed] [Google Scholar]
- He J, Zhang G, Pang Q, Yu C, Xiong J, Zhu J, & Chen F (2017). SIRT6 reduces macrophage foam cell formation by inducing autophagy and cholesterol efflux under ox-LDL condition. FEBS J, 284, 1324–1337. [DOI] [PubMed] [Google Scholar]
- He L, Chen Y, Hao S, & Qian J (2018). Uncovering novel landscape of cardiovascular diseases and therapeutic targets for cardioprotection via long noncoding RNA-miRNA-mRNA axes. Epigenomics, 10, 661–671. [DOI] [PubMed] [Google Scholar]
- He S, Owen DR, Jelinsky SA, & Lin LL (2015). Lysine Methyltransferase SETD7 (SET7/9) Regulates ROS Signaling through mitochondria and NFE2L2/ARE pathway. Sci Rep, 5, 14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss EH, & Dirsch VM (2014). Regulation of eNOS enzyme activity by posttranslational modification. Curr Pharm Des, 20, 3503–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibender S, Franken R, van Roomen C, Ter Braake A, van der Made I, Schermer EE, Gunst Q, van den Hoff MJ, Lutgens E, Pinto YM, Groenink M, Zwinderman AH, Mulder BJ, de Vries CJ, & de Waard V (2016). Resveratrol Inhibits Aortic Root Dilatation in the Fbn1C1039G/+ Marfan Mouse Model. Arterioscler Thromb Vasc Biol, 36, 1618–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeksema MA, Gijbels MJ, Van den Bossche J, van der Velden S, Sijm A, Neele AE, Seijkens T, Stoger JL, Meiler S, Boshuizen MC, Dallinga-Thie GM, Levels JH, Boon L, Mullican SE, Spann NJ, Cleutjens JP, Glass CK, Lazar MA, de Vries CJ, Biessen EA, Daemen MJ, Lutgens E, & de Winther MP (2014). Targeting macrophage Histone deacetylase 3 stabilizes atherosclerotic lesions. EMBO Mol Med, 6, 1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, Kohlmaier A, Herbst A, Northoff BH, Nicolaou A, Gabel G, Beutner F, Scholz M, Thiery J, Musunuru K, Krohn K, Mann M, & Teupser D (2016). Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun, 7, 12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holla S, Prakhar P, Singh V, Karnam A, Mukherjee T, Mahadik K, Parikh P, Singh A, Rajmani RS, Ramachandra SG, & Balaji KN (2016). MUSASHI-Mediated Expression of JMJD3, a H3K27me3 Demethylase, Is Involved in Foamy Macrophage Generation during Mycobacterial Infection. PLoS Pathog, 12, e1005814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, & Sinclair DA (2003). Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature, 425, 191–196. [DOI] [PubMed] [Google Scholar]
- Hu YW, Zhao JY, Li SF, Huang JL, Qiu YR, Ma X, Wu SG, Chen ZP, Hu YR, Yang JY, Wang YC, Gao JJ, Sha YH, Zheng L, & Wang Q (2015). RP5–833A20.1/miR-382–5p/NFIA-dependent signal transduction pathway contributes to the regulation of cholesterol homeostasis and inflammatory reaction. Arterioscler Thromb Vasc Biol, 35, 87–101. [DOI] [PubMed] [Google Scholar]
- Huang-Fu N, Cheng JS, Wang Y, Li ZW, & Wang SH (2018). Neat1 regulates oxidized low-density lipoprotein-induced inflammation and lipid uptake in macrophages via paraspeckle formation. Mol Med Rep, 17, 3092–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A, Young TL, Dang VT, Shi Y, McAlpine CS, & Werstuck GH (2017). 4-phenylbutyrate and valproate treatment attenuates the progression of atherosclerosis and stabilizes existing plaques. Atherosclerosis, 266, 103–112. [DOI] [PubMed] [Google Scholar]
- Huang C, Hu YW, Zhao JJ, Ma X, Zhang Y, Guo FX, Kang CM, Lu JB, Xiu JC, Sha YH, Gao JJ, Wang YC, Li P, Xu BM, Zheng L, & Wang Q (2016). Long Noncoding RNA HOXC-AS1 Suppresses Ox-LDL-Induced Cholesterol Accumulation Through Promoting HOXC6 Expression in THP-1 Macrophages. DNA Cell Biol, 35, 722–729. [DOI] [PubMed] [Google Scholar]
- Huang TS, Wang KC, Quon S, Nguyen P, Chang TY, Chen Z, Li YS, Subramaniam S, Shyy J, & Chien S (2017). LINC00341 exerts an anti-inflammatory effect on endothelial cells by repressing VCAM1. Physiol Genomics, 49, 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Peng K, Su J, Huang Y, Xu Y, & Wang S (2007). Different effects of homocysteine and oxidized low density lipoprotein on methylation status in the promoter region of the estrogen receptor alpha gene. Acta Biochim Biophys Sin (Shanghai), 39, 19–26. [DOI] [PubMed] [Google Scholar]
- Huangfu N, Xu Z, Zheng W, Wang Y, Cheng J, & Chen X (2018). LncRNA MALAT1 regulates oxLDL-induced CD36 expression via activating beta-catenin. Biochem Biophys Res Commun, 495, 2111–2117. [DOI] [PubMed] [Google Scholar]
- Hyun K, Jeon J, Park K, & Kim J (2017). Writing, erasing and reading histone lysine methylations. Exp Mol Med, 49, e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, Carson W. F. t., Cavassani KA, Li X, Lukacs NW, Hogaboam CM, Dou Y, & Kunkel SL (2009). Epigenetic regulation of the alternatively activated macrophage phenotype. Blood, 114, 3244–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Hayashi H, Kinoshita K, Abe M, Kuroki H, Tokunaga R, Tomiyasu S, Tanaka H, Sugita H, Arita T, Yagi Y, Watanabe M, Hirota M, & Baba H (2014). Statins inhibittumor progression via an enhancer of zeste homolog 2-mediated epigenetic alteration in colorectal cancer. Int J Cancer, 135, 2528–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito I, Asai A, Suzuki S, Kobayashi M, & Suzuki F (2017). M2b macrophage polarization accompanied with reduction of long noncoding RNA GAS5. Biochem Biophys Res Commun, 493, 170–175. [DOI] [PubMed] [Google Scholar]
- Jahagirdar R, Zhang H, Azhar S, Tobin J, Attwell S, Yu R, Wu J, McLure KG, Hansen HC, Wagner GS, Young PR, Srivastava RA, Wong NC, & Johansson J (2014). A novel BET bromodomain inhibitor, RVX-208, shows reduction of atherosclerosis in hyperlipidemic ApoE deficient mice. Atherosclerosis, 236, 91–100. [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, & Ebert BL (2017). Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med, 377, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch A, & Jurkowska RZ (2014). New concepts in DNA methylation. Trends Biochem Sci, 39, 310–318. [DOI] [PubMed] [Google Scholar]
- Jia P, Cai H, Liu X, Chen J, Ma J, Wang P, Liu Y, Zheng J, & Xue Y (2016). Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett, 381, 359–369. [DOI] [PubMed] [Google Scholar]
- Jiang D, Wei S, Chen F, Zhang Y, & Li J (2017). TET3-mediated DNA oxidation promotes ATR-dependent DNA damage response. EMBO Rep, 18, 781–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YZ, Jimenez JM, Ou K, McCormick ME, Zhang LD, & Davies PF (2014). Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-Like Factor 4 promoter in vitro and in vivo. Circ Res, 115, 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Li DY, Chernogubova E, Sun C, Busch A, Eken SM, Saliba-Gustafsson P, Winter H, Winski G, Raaz U, Schellinger IN, Simon N, Hegenloh R, Matic LP, Jagodic M, Ehrenborg E, Pelisek J, Eckstein HH, Hedin U, Backlund A, & Maegdefessel L (2018). Local Delivery of miR-21 Stabilizes Fibrous Caps in Vulnerable Atherosclerotic Lesions. Mol Ther, 26, 1040–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joehanes R, Just AC, Marioni RE, Pilling LC, Reynolds LM, Mandaviya PR, Guan W, Xu T, Elks CE, Aslibekyan S, Moreno-Macias H, Smith JA, Brody JA, Dhingra R, Yousefi P, Pankow JS, Kunze S, Shah SH, McRae AF, Lohman K, Sha J, Absher DM, Ferrucci L, Zhao W, Demerath EW, Bressler J, Grove ML, Huan T, Liu C, Mendelson MM, Yao C, Kiel DP, Peters A, Wang-Sattler R, Visscher PM, Wray NR, Starr JM, Ding J, Rodriguez CJ, Wareham NJ, Irvin MR, Zhi D, Barrdahl M, Vineis P, Ambatipudi S, Uitterlinden AG, Hofman A, Schwartz J, Colicino E, Hou L, Vokonas PS, Hernandez DG, Singleton AB, Bandinelli S, Turner ST, Ware EB, Smith AK, Klengel T, Binder EB, Psaty BM, Taylor KD, Gharib SA, Swenson BR, Liang L, DeMeo DL, O’Connor GT, Herceg Z, Ressler KJ, Conneely KN, Sotoodehnia N, Kardia SL, Melzer D, Baccarelli AA, van Meurs JB, Romieu I, Arnett DK, Ong KK, Liu Y, Waldenberger M, Deary IJ, Fornage M, Levy D, & London SJ (2016). Epigenetic Signatures of Cigarette Smoking. Circ Cardiovasc Genet, 9, 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SB, Kim CS, Naqvi A, Yamamori T, Mattagajasingh I, Hoffman TA, Cole MP, Kumar A, Dericco JS, Jeon BH, & Irani K (2010). Histone deacetylase 3 antagonizes aspirin-stimulated endothelial nitric oxide production by reversing aspirin-induced lysine acetylation of endothelial nitric oxide synthase. Circ Res, 107, 877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Wu Y, & Li X (2004). Effects of statin therapy on the progression of carotid atherosclerosis: a systematic review and meta-analysis. Atherosclerosis, 177, 433–442. [DOI] [PubMed] [Google Scholar]
- Kassan M, Vikram A, Kim YR, Li Q, Kassan A, Patel HH, Kumar S, Gabani M, Liu J, Jacobs JS, & Irani K (2017). Sirtuin1 protects endothelial Caveolin-1 expression and preserves endothelial function via suppressing miR-204 and endoplasmic reticulum stress. Sci Rep, 7, 42265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano H, Nishikawa Y, Yamada H, Yamada K, & Mase M (2018). Differential Expression of microRNAs in Severely Calcified Carotid Plaques. J Stroke Cerebrovasc Dis, 27, 108–117. [DOI] [PubMed] [Google Scholar]
- Keating ST, Ziemann M, Okabe J, Khan AW, Balcerczyk A, & El-Osta A (2014). Deep sequencing reveals novel Set7 networks. Cell Mol Life Sci, 71, 4471–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, Regev A, Lander ES, & Rinn JL (2009). Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A, 106, 11667–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khyzha N, Alizada A, Wilson MD, & Fish JE (2017). Epigenetics of Atherosclerosis: Emerging Mechanisms and Methods. Trends Mol Med, 23, 332–347. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee JM, Kim JH, & Kim KR (2018). Fluvastatin activates sirtuin 6 to regulate sterol regulatory element-binding proteins and AMP-activated protein kinase in HepG2 cells. Biochem Biophys Res Commun, 503, 1415–1421. [DOI] [PubMed] [Google Scholar]
- Kim KH, & Roberts CW (2016). Targeting EZH2 in cancer. Nat Med, 22, 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Bae JU, Lee SJ, Park SY, & Kim CD (2015). SIRT1 attenuates PAF-induced MMP-2 production via down-regulation of PAF receptor expression in vascular smooth muscle cells. Vascul Pharmacol, 72, 35–42. [DOI] [PubMed] [Google Scholar]
- Kitada M, Ogura Y, & Koya D (2016). The protective role of Sirt1 in vascular tissue: its relationship to vascular aging and atherosclerosis. Aging (Albany NY), 8, 2290–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann R, Princen HM, Emeis JJ, Jukema JW, Fontijn RD, Horrevoets AJ, Kooistra T, & Havekes LM (2003). Rosuvastatin reduces atherosclerosis development beyond and independent of its plasma cholesterol-lowering effect in APOE*3-Leiden transgenic mice: evidence for antiinflammatory effects of rosuvastatin. Circulation, 108, 1368–1374. [DOI] [PubMed] [Google Scholar]
- Koene RJ, Prizment AE, Blaes A, & Konety SH (2016). Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation, 133, 1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotla S, & Rao GN (2015). Reactive Oxygen Species (ROS) Mediate p300-dependent STAT1 Protein Interaction with Peroxisome Proliferator-activated Receptor (PPAR)-gamma in CD36 Protein Expression and Foam Cell Formation. J Biol Chem, 290, 30306–30320. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kotla S, Singh NK, & Rao GN (2017). ROS via BTK-p300-STAT1-PPARgamma signaling activation mediates cholesterol crystals-induced CD36 expression and foam cell formation. Redox Biol, 11, 350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruidenier L, Chung CW, Cheng Z, Liddle J, Che K, Joberty G, Bantscheff M, Bountra C, Bridges A, Diallo H, Eberhard D, Hutchinson S, Jones E, Katso R, Leveridge M, Mander PK, Mosley J, Ramirez-Molina C, Rowland P, Schofield CJ, Sheppard RJ, Smith JE, Swales C, Tanner R, Thomas P, Tumber A, Drewes G, Oppermann U, Patel DJ, Lee K, & Wilson DM (2012). A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature, 488, 404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku SK, & Bae JS (2016). Vicenin-2 and scolymoside inhibit high-glucose-induced vascular inflammation in vitro and in vivo. Can J Physiol Pharmacol, 94, 287–295. [DOI] [PubMed] [Google Scholar]
- Kulis M, & Esteller M (2010). DNA methylation and cancer. Adv Genet, 70, 27–56. [DOI] [PubMed] [Google Scholar]
- Kumar A, Kumar S, Vikram A, Hoffman TA, Naqvi A, Lewarchik CM, Kim YR, & Irani K (2013). Histone and DNA methylation-mediated epigenetic downregulation of endothelial Kruppel-like factor 2 by low-density lipoprotein cholesterol. Arterioscler Thromb Vasc Biol, 33, 1936–1942. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kang DW, Rezvan A, & Jo H (2017). Accelerated atherosclerosis development in C57Bl6 mice by overexpressing AAV-mediated PCSK9 and partial carotid ligation. Lab Invest, 97, 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kim YR, Vikram A, Naqvi A, Li Q, Kassan M, Kumar V, Bachschmid MM, Jacobs JS, Kumar A, & Irani K (2017). Sirtuin1-regulated lysine acetylation of p66Shc governs diabetes-induced vascular oxidative stress and endothelial dysfunction. Proc Natl Acad Sci U S A, 114, 1714–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon IS, Wang W, Xu S, & Jin ZG (2014). Histone deacetylase 5 interacts with Kruppel-like factor 2 and inhibits its transcriptional activity in endothelium. Cardiovasc Res, 104, 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont B, & Rayner KJ (2017). MicroRNAs in the Pathobiology and Therapy of Atherosclerosis. Can J Cardiol, 33, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai B, Li Z, He M, Wang Y, Chen L, Zhang J, Yang Y, & Shyy JY (2018). Atheroprone flow enhances the endothelial-to-mesenchymal transition. Am J Physiol Heart Circ Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanktree MB, Hegele RA, Yusuf S, & Anand SS (2009). Multi-ethnic genetic association study of carotid intima-media thickness using a targeted cardiovascular SNP microarray. Stroke, 40, 3173–3179. [DOI] [PubMed] [Google Scholar]
- Leckey LC, Garige M, Varatharajalu R, Gong M, Nagata T, Spurney CF, & Lakshman RM (2010). Quercetin and ethanol attenuate the progression of atherosclerotic plaques with concomitant up regulation of paraoxonase1 (PON1) gene expression and PON1 activity in LDLR−/− mice. Alcohol Clin Exp Res, 34, 1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Na W, Lee JY, Na J, Cho H, Wu H, Yune TY, Kim WS, & Ju BG (2012). Molecular mechanism of Jmjd3-mediated interleukin-6 gene regulation in endothelial cells underlying spinal cord injury. J Neurochem, 122, 272–282. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Shim JY, & Zhu BT (2005). Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol, 68, 1018–1030. [DOI] [PubMed] [Google Scholar]
- Lee Y, Ka SO, Cha HN, Chae YN, Kim MK, Park SY, Bae EJ, & Park BH (2017). Myeloid Sirtuin 6 Deficiency Causes Insulin Resistance in High-Fat Diet-Fed Mice by Eliciting Macrophage Polarization Toward an M1 Phenotype. Diabetes, 66, 2659–2668. [DOI] [PubMed] [Google Scholar]
- Leentjens J, Bekkering S, Joosten LAB, Netea MG, Burgner DP, & Riksen NP (2018). Trained Innate Immunity as a Novel Mechanism Linking Infection and the Development of Atherosclerosis. Circ Res, 122, 664–669. [DOI] [PubMed] [Google Scholar]
- Leeper NJ, & Maegdefessel L (2018). Non-coding RNAs: key regulators of smooth muscle cell fate in vascular disease. Cardiovasc Res, 114, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisegang MS, Fork C, Josipovic I, Richter FM, Preussner J, Hu J, Miller MJ, Epah J, Hofmann P, Gunther S, Moll F, Valasarajan C, Heidler J, Ponomareva Y, Freiman TM, Maegdefessel L, Plate KH, Mittelbronn M, Uchida S, Kunne C, Stellos K, Schermuly RT, Weissmann N, Devraj K, Wittig I, Boon RA, Dimmeler S, Pullamsetti SS, Looso M, Miller FJ Jr., & Brandes RP (2017). Long Noncoding RNA MANTIS Facilitates Endothelial Angiogenic Function. Circulation, 136, 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucker TM, Nomura Y, Kim JH, Bhatta A, Wang V, Wecker A, Jandu S, Santhanam L, Berkowitz D, Romer L, & Pandey D (2017). Cystathionine gamma-lyase protects vascular endothelium: a role for inhibition of histone deacetylase 6. Am J Physiol Heart Circ Physiol, 312, H711–h720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Trac C, Jin W, Lanting L, Akbany A, Saetrom P, Schones DE, & Natarajan R (2013). Novel long noncoding RNAs are regulated by angiotensin II in vascular smooth muscle cells. Circ Res, 113, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Huo Y, Lin Z, & Wang T (2017). [DNA hydroxymethylase 10–11 translocation 2 (TET2) inhibits mouse macrophage activation and polarization]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi, 33, 1165–1170. [PubMed] [Google Scholar]
- Li CY, Ma L, & Yu B (2017). Circular RNA hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells proliferation and angiogenesis. Biomed Pharmacother, 95, 1514–1519. [DOI] [PubMed] [Google Scholar]
- Li DY, Busch A, Jin H, Chernogubova E, Pelisek J, Karlsson J, Sennblad B, Liu S, Lao S, Hofmann P, Backlund A, Eken SM, Roy J, Eriksson P, Dacken B, Ramanujam D, Dueck A, Engelhardt S, Boon RA, Eckstein HH, Spin JM, Tsao PS, & Maegdefessel L (2018). H19 Induces Abdominal Aortic Aneurysm Development and Progression. Circulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Ching D, Luk FS, & Raffai RL (2015). Apolipoprotein E enhances microRNA-146a in monocytes and macrophages to suppress nuclear factor-kappaB-driven inflammation and atherosclerosis. Circ Res, 117, e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Gao P, Zhang H, Chen H, Zheng W, Lv X, Xu T, Wei Y, Liu D, & Liang C (2011). SIRT1 inhibits angiotensin II-induced vascular smooth muscle cell hypertrophy. Acta Biochim Biophys Sin (Shanghai), 43, 103–109. [DOI] [PubMed] [Google Scholar]
- Li L, Wang L, Li H, Han X, Chen S, Yang B, Hu Z, Zhu H, Cai C, Chen J, Li X, Huang J, & Gu D (2018). Characterization of LncRNA expression profile and identification of novel LncRNA biomarkers to diagnose coronary artery disease. Atherosclerosis, 275, 359–367. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang HN, Chen HZ, Gao P, Zhu LH, Li HL, Lv X, Zhang QJ, Zhang R, Wang Z, She ZG, Zhang R, Wei YS, Du GH, Liu DP, & Liang CC (2011). SIRT1 acts as a modulator of neointima formation following vascular injury in mice. Circ Res, 108, 1180–1189. [DOI] [PubMed] [Google Scholar]
- Li MF, Zhang R, Li TT, Chen MY, Li LX, Lu JX, & Jia WP (2016). High Glucose Increases the Expression of Inflammatory Cytokine Genes in Macrophages Through H3K9 Methyltransferase Mechanism. J Interferon Cytokine Res, 36, 48–61. [DOI] [PubMed] [Google Scholar]
- Li P, Zhong X, Li J, Liu H, Ma X, He R, & Zhao Y (2018). MicroRNA-30c-5p inhibits NLRP3 inflammasome-mediated endothelial cell pyroptosis through FOXO3 down-regulation in atherosclerosis. Biochem Biophys Res Commun. [DOI] [PubMed] [Google Scholar]
- Li RL, Lu ZY, Huang JJ, Qi J, Hu A, Su ZX, Zhang L, Li Y, Shi YQ, Hao CN, & Duan JL (2016). SRT1720, a SIRT1 specific activator, protected H2O2-induced senescent endothelium. Am J Transl Res, 8, 2876–2888. [PMC free article] [PubMed] [Google Scholar]
- Li S, Geng Q, Chen H, Zhang J, Cao C, Zhang F, Song J, Liu C, & Liang W (2018). The potential inhibitory effects of miR19b on vulnerable plaque formation via the suppression of STAT3 transcriptional activity. Int J Mol Med, 41, 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Lee C, Song J, Lu C, Liu J, Cui Y, Liang H, Cao C, Zhang F, & Chen H (2017). Circulating microRNAs as potential biomarkers for coronary plaque rupture. Oncotarget, 8, 48145–48156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Sun Y, Zhong L, Xiao Z, Yang M, Chen M, Wang C, Xie X, & Chen X (2018). The suppression of ox-LDL-induced inflammatory cytokine release and apoptosis of HCAECs bylong non-coding RNA-MALAT1 via regulating microRNA-155/SOCS1 pathway. Nutr Metab Cardiovasc Dis. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Q, Shi Q, Liu Y, Zhao K, Shen Q, Shi Y, Liu X, Wang C, Li N, Ma Y, & Cao X (2017). Demethylase Kdm6a epigenetically promotes IL-6 and IFN-beta production in macrophages. J Autoimmun, 80, 85–94. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang S, Blander G, Tse JG, Krieger M, & Guarente L (2007). SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell, 28, 91–106. [DOI] [PubMed] [Google Scholar]
- Li X, Zhao Z, Gao C, Rao L, Hao P, Jian D, Li W, Tang H, & Li M (2018). Identification of a peripheral blood long non-coding RNA (Upperhand) as a potential diagnostic marker of coronary artery disease. Cardiol J, 25, 393–402. [DOI] [PubMed] [Google Scholar]
- Li X, Zhou Y, Yu C, Yang H, Zhang C, Ye Y, & Xiao S (2015). Paeonol suppresses lipid accumulation in macrophages via upregulation of the ATPbinding cassette transporter A1 and downregulation of the cluster of differentiation 36. Int J Oncol, 46, 764–774. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Cole A, McLaughlin S, & Du W (2018). Icariin improves Fanconi anemia hematopoietic stem cell function through SIRT6-mediated NF-kappa B inhibition. Cell Cycle, 17, 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, Ren B, & Natarajan R (2008). Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem, 283, 26771–26781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang P, Yang X, Wang W, Zhang J, He Y, Zhang W, Jing T, Wang B, & Lin R (2016). SIRT1 inhibits inflammatory response partly through regulation of NLRP3 inflammasome in vascular endothelial cells. Mol Immunol, 77, 148–156. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang X, He Y, Wang W, Zhang J, Zhang W, Jing T, Wang B, & Lin R (2017). Negative regulation of NLRP3 inflammasome by SIRT1 in vascular endothelial cells. Immunobiology, 222, 552–561. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang K, & Mao W (2018). Inhibition of miR34a prevents endothelial cell apoptosis by directly targeting HDAC1 in the setting of atherosclerosis. Mol Med Rep, 17, 4645–4650. [DOI] [PubMed] [Google Scholar]
- Li Z, Chao TC, Chang KY, Lin N, Patil VS, Shimizu C, Head SR, Burns JC, & Rana TM (2014). The long noncoding RNA THRIL regulates TNFalpha expression through its interaction with hnRNPL. Proc Natl Acad Sci U S A, 111, 1002–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang F, Zha S, Cao Q, Sheng J, & Chen S (2018). SIRT1 inhibits TGF-beta-induced endothelial-mesenchymal transition in human endothelial cells with Smad4 deacetylation. J Cell Physiol. [DOI] [PubMed] [Google Scholar]
- Lian Z, Lv FF, Yu J, & Wang JW (2018). The anti-inflammatory effect of microRNA-383–3p interacting with IL1R2 against homocysteine-induced endothelial injury in rat coronary arteries. J Cell Biochem. [DOI] [PubMed] [Google Scholar]
- Liao B, Chen R, Lin F, Mai A, Chen J, Li H, Xu Z, & Dong S (2018). Long noncoding RNA HOTTIP promotes endothelial cell proliferation and migration via activation of the Wnt/beta-catenin pathway. J Cell Biochem, 119, 2797–2805. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, & Maseri A (2002). Inflammation and atherosclerosis. Circulation, 105, 1135–1143. [DOI] [PubMed] [Google Scholar]
- Libby P, Tabas I, Fredman G, & Fisher EA (2014). Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res, 114, 1867–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CP, Huang PH, Lai CF, Chen JW, Lin SJ, & Chen JS (2015). Simvastatin Attenuates Oxidative Stress, NF-kappaB Activation, and Artery Calcification in LDLR−/− Mice Fed withHigh Fat Diet via Down-regulation of Tumor Necrosis Factor-alpha and TNF Receptor 1. PLoS One, 10, e0143686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin XL, Liu MH, Hu HJ, Feng HR, Fan XJ, Zou WW, Pan YQ, Hu XM, & Wang Z (2015). Curcumin enhanced cholesterol efflux by upregulating ABCA1 expression through AMPK-SIRT1-LXRalpha signaling in THP-1 macrophage-derived foam cells. DNA Cell Biol, 34, 561–572. [DOI] [PubMed] [Google Scholar]
- Ling C, & Groop L (2009). Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes, 58, 2718–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lino Cardenas CL, Kessinger CW, MacDonald C, Jassar AS, Isselbacher EM, Jaffer FA, & Lindsay ME (2018). Inhibition of the methyltranferase EZH2 improves aortic performance in experimental thoracic aortic aneurysm. JCI Insight, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Osman N, & O’Brien KD (2008). Hyperelongated biglycan: the surreptitious initiator of atherosclerosis. Curr Opin Lipidol, 19, 448–454. [DOI] [PubMed] [Google Scholar]
- Liu B, Zhang B, Guo R, Li S, & Xu Y (2014). Enhancement in efferocytosis of oxidized low-density lipoprotein-induced apoptotic RAW264.7 cells through Sirt1-mediated autophagy. Int J Mol Med, 33, 523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CW, Sung HC, Lin SR, Wu CW, Lee CW, Lee IT, Yang YF, Yu IS, Lin SW, Chiang MH, Liang CJ, & Chen YL (2017). Resveratrol attenuates ICAM-1 expression and monocyte adhesiveness to TNF-alpha-treated endothelial cells: evidence for an anti-inflammatory cascade mediated by the miR-221/222/AMPK/p38/NF-kappaB pathway. Sci Rep, 7, 44689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Bi X, Chen T, Zhang Q, Wang SX, Chiu JJ, Liu GS, Zhang Y, Bu P, & Jiang F (2015). Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression. Cell Death Dis, 6, e1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Huang GQ, & Ke ZP (2018). Silence of long intergenic noncoding RNA HOTAIR ameliorates oxidative stress and inflammation response in ox-LDL-treated human macrophages by upregulating miR-330–5p. J Cell Physiol. [DOI] [PubMed] [Google Scholar]
- Liu R, Jin Y, Tang WH, Qin L, Zhang X, Tellides G, Hwa J, Yu J, & Martin KA (2013). Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation, 128, 2047–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Li C, Sun H, Luo T, Tan Y, Tian D, & Guo Z (2014). Curcumin inhibits monocyte chemoattractant protein-1 expression and enhances cholesterol efflux by suppressing the c-Jun N-terminal kinase pathway in macrophage. Inflamm Res, 63, 841–850. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peng W, Qu K, Lin X, Zeng Z, Chen J, Wei D, & Wang Z (2018). TET2: A Novel Epigenetic Regulator and Potential Intervention Target for Atherosclerosis. DNA Cell Biol, 37, 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang TT, Zhang R, Fu WY, Wang X, Wang F, Gao P, Ding YN, Xie Y, Hao DL, Chen HZ, & Liu DP (2016). Calorie restriction protects against experimental abdominal aortic aneurysms in mice. J Exp Med, 213, 2473–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang J, Huang E, Gao S, Li H, Lu J, Tian K, Little PJ, Shen X, Xu S, & Liu P (2014). Tanshinone IIA suppresses cholesterol accumulation in human macrophages: role of heme oxygenase-1. J Lipid Res, 55, 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang J, Huang X, Li Z, & Liu P (2016). Deletion of sirtuin 6 accelerates endothelial dysfunction and atherosclerosis in apolipoprotein E-deficient mice. Transl Res, 172, 18–29.e12. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xie Z, Jones W, Pavlovicz RE, Liu S, Yu J, Li PK, Lin J, Fuchs JR, Marcucci G, Li C, & Chan KK (2009). Curcumin is a potent DNA hypomethylation agent. Bioorg Med Chem Lett, 19, 706–709. [DOI] [PubMed] [Google Scholar]
- Loche E, & Ozanne SE (2016). Early nutrition, epigenetics, and cardiovascular disease. Curr Opin Lipidol, 27, 449–458. [DOI] [PubMed] [Google Scholar]
- Lowe D, & Raj K (2014). Premature aging induced by radiation exhibits pro-atherosclerotic effects mediated by epigenetic activation of CD44 expression. Aging Cell, 13, 900–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, Armaiz-Pena GN, Hu W, Stone RL, Munkarah A, Ravoori MK, Shahzad MM, Lee JW, Mora E, Langley RR, Carroll AR, Matsuo K, Spannuth WA, Schmandt R, Jennings NB, Goodman BW, Jaffe RB, Nick AM, Kim HS, Guven EO, Chen YH, Li LY, Hsu MC, Coleman RL, Calin GA, Denkbas EB, Lim JY, Lee JS, Kundra V, Birrer MJ, Hung MC, Lopez-Berestein G, & Sood AK (2010). Regulation of tumor angiogenesis by EZH2. Cancer Cell, 18, 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang L, Liao X, Sangwung P, Prosdocimo DA, Zhou G, Votruba AR, Brian L, Han YJ, Gao H, Wang Y, Shimizu K, Weinert-Stein K, Khrestian M, Simon DI, Freedman NJ, & Jain MK (2013). Kruppel-like factor 15 is critical for vascular inflammation. J Clin Invest, 123, 4232–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Bonauer A, & Dimmeler S (2018). RNA Therapeutics in Cardiovascular Disease. Circ Res, 123, 205–220. [DOI] [PubMed] [Google Scholar]
- Luo X, Yang D, Wu W, Long F, Xiao C, Qin M, Law BY, Suguro R, Xu X, Qu L, Liu X, & Zhu YZ (2018). Critical role of histone demethylase JMJD3 in the regulation of neointima formation following vascular injury. Cardiovasc Res. [DOI] [PubMed] [Google Scholar]
- Luque A, Farwati A, Krupinski J, & Aran JM (2018). Association between low levels of serum miR-638 and atherosclerotic plaque vulnerability in patients with high-grade carotid stenosis. J Neurosurg, 1–8. [DOI] [PubMed] [Google Scholar]
- Lusis AJ (2000). Atherosclerosis. Nature, 407, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis AJ (2012). Genetics of atherosclerosis. Trends Genet, 28, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv YC, Tang YY, Zhang P, Wan W, Yao F, He PP, Xie W, Mo ZC, Shi JF, Wu JF, Peng J, Liu D, Cayabyab FS, Zheng XL, Tang XY, Ouyang XP, & Tang CK (2016). Histone Methyltransferase Enhancer of Zeste Homolog 2-Mediated ABCA1 Promoter DNA Methylation Contributes to the Progression of Atherosclerosis. PLoS One, 11, e0157265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SM, Gaziano JM, & Frei B (1996). Ascorbic acid and atherosclerotic cardiovascular disease. Subcell Biochem, 25, 331–367. [DOI] [PubMed] [Google Scholar]
- Ma JH, Song SH, Guo M, Zhou J, Liu F, Peng L, & Fu ZR (2017). Long-term exposure to PM2.5 lowers influenza virus resistance via down-regulating pulmonary macrophage Kdm6a and mediates histones modification in IL-6 and IFN-beta promoter regions. Biochem Biophys Res Commun, 493, 1122–1128. [DOI] [PubMed] [Google Scholar]
- Ma L, Liu X, Zhao Y, Chen B, Li X, & Qi R (2013). Ginkgolide B reduces LOX-1 expression by inhibiting Akt phosphorylation and increasing Sirt1 expression in oxidized LDL-stimulated human umbilical vein endothelial cells. PLoS One, 8, e74769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Tian XY, Zhang Y, Mu C, Shen H, Bismuth J, Pownall HJ, Huang Y, & Wong WT (2016). E-selectin-targeting delivery of microRNAs by microparticles ameliorates endothelial inflammation and atherosclerosis. Sci Rep, 6, 22910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SC, Hao YJ, Jiao Y, Wang YH, Xu LB, Mao CY, Yang XL, Yang AN, Tian J, Zhang MH, Jin SJ, Xu H, Jiang YD, & Zhang HP (2017). Homocysteineinduced oxidative stress through TLR4/NFkappaB/DNMT1mediated LOX1 DNA methylation in endothelial cells. Mol Med Rep, 16, 9181–9188. [DOI] [PubMed] [Google Scholar]
- Ma Y, Huang D, Yang F, Tian M, Wang Y, Shen D, Wang Q, Chen Q, & Zhang L (2016). Long Noncoding RNA Highly Upregulated in Liver Cancer Regulates the Tumor Necrosis Factor-alpha-Induced Apoptosis in Human Vascular Endothelial Cells. DNA Cell Biol, 35, 296–300. [DOI] [PubMed] [Google Scholar]
- Maleszewska M, Vanchin B, Harmsen MC, & Krenning G (2016). The decrease in histone methyltransferase EZH2 in response to fluid shear stress alters endothelial gene expression and promotes quiescence. Angiogenesis, 19, 9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Dau T, Gonik M, Sivakumar A, Deredge DJ, Edeleva EV, Gotzfried J, van der Laan SW, Pasterkamp G, Beaufort N, Seixas S, Bevan S, Lincz LF, Holliday EG, Burgess AI, Rannikmae K, Minnerup J, Kriebel J, Waldenberger M, Muller-Nurasyid M, Lichtner P, Saleheen D, Rothwell PM, Levi C, Attia J, Sudlow CL, Braun D, Markus HS, Wintrode PL, Berger K, Jenne DE, & Dichgans M (2017). Common coding variant in SERPINA1 increases the risk for large artery stroke. Proc Natl Acad Sci U S A, 114, 3613–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HSJ, Sukumar AN, Lam GC, Turgeon PJ, Yan MS, Ku KH, Dubinsky MK, Ho JJD, Wang JJ, Das S, Mitchell N, Oettgen P, Sefton MV, & Marsden PA (2018). Angiogenic patterning by STEEL, an endothelial-enriched long noncoding RNA. Proc Natl Acad Sci U S A, 115, 2401–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus B, Grote K, Worsch M, Parviz B, Boening A, Schieffer B, & Parahuleva MS (2016). Differential Expression of MicroRNAs in Endarterectomy Specimens Taken from Patients with Asymptomatic and Symptomatic Carotid Plaques. PLoS One, 11, e0161632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus HS, Makela KM, Bevan S, Raitoharju E, Oksala N, Bis JC, O’Donnell C, Hainsworth A, & Lehtimaki T (2013). Evidence HDAC9 genetic variant associated with ischemic stroke increases risk via promoting carotid atherosclerosis. Stroke, 44, 1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, & Zhang Y (2005). The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol, 6, 838–849. [DOI] [PubMed] [Google Scholar]
- Matouk CC, & Marsden PA (2008). Epigenetic regulation of vascular endothelial gene expression. Circ Res, 102, 873–887. [DOI] [PubMed] [Google Scholar]
- Matsukura M, Ozaki K, Takahashi A, Onouchi Y, Morizono T, Komai H, Shigematsu H, Kudo T, Inoue Y, Kimura H, Hosaka A, Shigematsu K, Miyata T, Watanabe T, Tsunoda T, Kubo M, & Tanaka T (2015). Genome-Wide Association Study of Peripheral Arterial Disease in a Japanese Population. PLoS One, 10, e0139262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, & Irani K (2007). SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A, 104, 14855–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, Liu Y, Graves AP, Della Pietra A 3rd, Diaz E, LaFrance LV, Mellinger M, Duquenne C, Tian X, Kruger RG, McHugh CF, Brandt M, Miller WH, Dhanak D, Verma SK, Tummino PJ, & Creasy CL (2012). EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature, 492, 108–112. [DOI] [PubMed] [Google Scholar]
- McLure KG, Gesner EM, Tsujikawa L, Kharenko OA, Attwell S, Campeau E, Wasiak S, Stein A, White A, Fontano E, Suto RK, Wong NC, Wagner GS, Hansen HC, & Young PR (2013). RVX-208, an inducer of ApoA-I in humans, is a BET bromodomain antagonist. PLoS One, 8, e83190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Zeng H, & Gong H (2018). microRNA-212 promotes lipid accumulation and attenuates cholesterol efflux in THP-1 human macrophages by targeting SIRT1. Gene, 643, 55–60. [DOI] [PubMed] [Google Scholar]
- Miao Y, Ajami NE, Huang TS, Lin FM, Lou CH, Wang YT, Li S, Kang J, Munkacsi H, Maurya MR, Gupta S, Chien S, Subramaniam S, & Chen Z (2018). Enhancer-associated long non-coding RNA LEENE regulates endothelial nitric oxide synthase and endothelial function. Nat Commun, 9, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalik KM, You X, Manavski Y, Doddaballapur A, Zornig M, Braun T, John D, Ponomareva Y, Chen W, Uchida S, Boon RA, & Dimmeler S (2014). Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res, 114, 1389–1397. [DOI] [PubMed] [Google Scholar]
- Miranda MX, van Tits LJ, Lohmann C, Arsiwala T, Winnik S, Tailleux A, Stein S, Gomes AP, Suri V, Ellis JL, Lutz TA, Hottiger MO, Sinclair DA, Auwerx J, Schoonjans K, Staels B, Luscher TF, & Matter CM (2015). The Sirt1 activator SRT3025 provides atheroprotection in Apoe−/− mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. Eur Heart J, 36, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morera L, Lubbert M, & Jung M (2016). Targeting histone methyltransferases and demethylases in clinical trials for cancer therapy. Clin Epigenetics, 8, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MA, & Chun OK (2016). Vitamin C and Heart Health: A Review Based on Findings from Epidemiologic Studies. Int J Mol Sci, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottamal M, Zheng S, Huang TL, & Wang G (2015). Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules, 20, 3898–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muka T, Koromani F, Portilla E, O’Connor A, Bramer WM, Troup J, Chowdhury R, Dehghan A, & Franco OH (2016). The role of epigenetic modifications in cardiovascular disease: A systematic review. Int J Cardiol, 212, 174–183. [DOI] [PubMed] [Google Scholar]
- Murray K (1964). THE OCCURRENCE OF EPSILON-N-METHYL LYSINE IN HISTONES. Biochemistry, 3, 10–15. [DOI] [PubMed] [Google Scholar]
- Na W, Shin JY, Lee JY, Jeong S, Kim WS, Yune TY, & Ju BG (2017). Dexamethasone suppresses JMJD3 gene activation via a putative negative glucocorticoid response element and maintains integrity of tight junctions in brain microvascular endothelial cells. J Cereb Blood Flow Metab, 37, 3695–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, & Jo H (2009). Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol, 297, H1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarelli L, Geissler C, Csaba G, Wei Y, Zhu M, di Francesco A, Hartmann P, Zimmer R, & Schober A (2018). miR-103 promotes endothelial maladaptation by targeting lncWDR59. Nat Commun, 9, 2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neele AE, & de Winther MPJ (2018). Repressing the repressor: Ezh2 mediates macrophage activation. J Exp Med, 215, 1269–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neele AE, Gijbels MJJ, van der Velden S, Hoeksema MA, Boshuizen MCS, Prange KHM, Chen HJ, Van den Bossche J, van Roomen C, Shami A, Levels JHM, Kroon J, Lucas T, Dimmeler S, Lutgens E, & de Winther MPJ (2018). Myeloid Kdm6b deficiency results in advanced atherosclerosis. Atherosclerosis, 275, 156–165. [DOI] [PubMed] [Google Scholar]
- Neele AE, Prange KH, Hoeksema MA, van der Velden S, Lucas T, Dimmeler S, Lutgens E, Van den Bossche J, & de Winther MP (2017). Macrophage Kdm6b controls the pro-fibrotic transcriptome signature of foam cells. Epigenomics, 9, 383–391. [DOI] [PubMed] [Google Scholar]
- Neo WH, Booth CAG, Azzoni E, Chi L, Delgado-Olguin P, de Bruijn M, Jacobsen SEW, & Mead AJ (2018). Cell-extrinsic hematopoietic impact of Ezh2 inactivation in fetal liver endothelial cells. Blood, 131, 2223–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nespereira B, Perez-Ilzarbe M, Fernandez P, Fuentes AM, Paramo JA, & Rodriguez JA (2003). Vitamins C and E downregulate vascular VEGF and VEGFR-2 expression in apolipoprotein-E-deficient mice. Atherosclerosis, 171, 67–73. [DOI] [PubMed] [Google Scholar]
- Neumann P, Jae N, Knau A, Glaser SF, Fouani Y, Rossbach O, Kruger M, John D, Bindereif A, Grote P, Boon RA, & Dimmeler S (2018). The lncRNA GATA6-AS epigenetically regulates endothelial gene expression via interaction with LOXL2. Nat Commun, 9, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls SJ, Ray KK, Johansson JO, Gordon A, Sweeney M, Halliday C, Kulikowski E, Wong N, Kim SW, & Schwartz GG (2018). Selective BET Protein Inhibition with Apabetalone and Cardiovascular Events: A Pooled Analysis of Trials in Patients with Coronary Artery Disease. Am J Cardiovasc Drugs, 18, 109–115. [DOI] [PubMed] [Google Scholar]
- Norata GD, Marchesi P, Passamonti S, Pirillo A, Violi F, & Catapano AL (2007). Anti-inflammatory and anti-atherogenic effects of cathechin, caffeic acid and trans-resveratrol in apolipoprotein E deficient mice. Atherosclerosis, 191, 265–271. [DOI] [PubMed] [Google Scholar]
- Oh SY, Seok JY, Choi YS, Lee SH, Bae JS, & Lee YM (2015). The Histone Methyltransferase Inhibitor BIX01294 Inhibits HIF-1alpha Stability and Angiogenesis. Mol Cells, 38, 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Fujioka Y, Takahashi A, Takahashi T, Taniguchi T, Ishikawa Y, & Yokoyama M (2006). Trichostatin A, an inhibitor of histone deacetylase, inhibits smooth muscle cell proliferation via induction of p21(WAF1). J Atheroscler Thromb, 13, 183–191. [DOI] [PubMed] [Google Scholar]
- Pan JX (2017). LncRNA H19 promotes atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur Rev Med Pharmacol Sci, 21, 322–328. [PubMed] [Google Scholar]
- Pan LL, Wu Y, Wang RQ, Chen JW, Chen J, Zhang Y, Chen YJ, Geng M, Xu ZD, Dai M, Li JH, Wang W, & Zhang ZZ (2018). (−)-Epigallocatechin-3-Gallate Ameliorates Atherosclerosis and Modulates Hepatic Lipid Metabolic Gene Expression in Apolipoprotein E Knockout Mice: Involvement of TTC39B. Front Pharmacol, 9, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Yu H, Huang S, & Zhu P (2016). Resveratrol Protects against TNF-alpha-Induced Injury in Human Umbilical Endothelial Cells through Promoting Sirtuin-1-Induced Repression of NF-KB and p38 MAPK. PLoS One, 11, e0147034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey D, Hori D, Kim JH, Bergman Y, Berkowitz DE, & Romer LH (2015). NEDDylation promotes endothelial dysfunction: a role for HDAC2. J Mol Cell Cardiol, 81, 18–22. [DOI] [PubMed] [Google Scholar]
- Pandey D, Sikka G, Bergman Y, Kim JH, Ryoo S, Romer L, & Berkowitz D (2014). Transcriptional regulation of endothelial arginase 2 by histone deacetylase 2. Arterioscler Thromb Vasc Biol, 34, 1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneni F, Diaz Canestro C, Libby P, Luscher TF, & Camici GG (2017). The Aging Cardiovascular System: Understanding It at the Cellular and Clinical Levels. J Am Coll Cardiol, 69, 1952–1967. [DOI] [PubMed] [Google Scholar]
- Peng J, Tang ZH, Ren Z, He B, Zeng Y, Liu LS, Wang Z, Wei DH, Zheng XL, & Jiang ZS (2017). TET2 Protects against oxLDL-Induced HUVEC Dysfunction by Upregulating the CSE/H2S System. Front Pharmacol, 8, 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Yang Q, Li AF, Li RQ, Wang Z, Liu LS, Ren Z, Zheng XL, Tang XQ, Li GH, Tang ZH, Jiang ZS, & Wei DH (2016). Tet methylcytosine dioxygenase 2 inhibits atherosclerosis via upregulation of autophagy in ApoE−/− mice. Oncotarget, 7, 76423–76436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Meng K, Jiang L, Zhong Y, Yang Y, Lan Y, Zeng Q, & Cheng L (2017). Thymic stromal lymphopoietin-induced HOTAIR activation promotes endothelial cell proliferation and migration in atherosclerosis. Biosci Rep, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter CJ, & Akbarian S (2011). Balancing histone methylation activities in psychiatric disorders. Trends Mol Med, 17, 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poller W, Dimmeler S, Heymans S, Zeller T, Haas J, Karakas M, Leistner DM, Jakob P, Nakagawa S, Blankenberg S, Engelhardt S, Thum T, Weber C, Meder B, Hajjar R, & Landmesser U (2017). Non-coding RNAs in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur Heart J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons D, de Vries FR, van den Elsen PJ, Heijmans BT, Quax PH, & Jukema JW (2009). Epigenetic histone acetylation modifiers in vascular remodelling: new targets for therapy in cardiovascular disease. Eur Heart J, 30, 266–277. [DOI] [PubMed] [Google Scholar]
- Qiang L, Lin HV, Kim-Muller JY, Welch CL, Gu W, & Accili D (2011). Proatherogenic abnormalities of lipid metabolism in SirT1 transgenic mice are mediated through Creb deacetylation. Cell Metab, 14, 758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C, Meng F, Jang I, Jo H, Chen YE, & Zhang J (2016). Deep transcriptomic profiling reveals the similarity between endothelial cells cultured under static and oscillatory shear stress conditions. Physiol Genomics, 48, 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin B, Shu Y, Long L, Li H, Men X, Feng L, Yang H, & Lu Z (2018). MicroRNA-142–3p Induces Atherosclerosis-Associated Endothelial Cell Apoptosis by Directly Targeting Rictor. Cell Physiol Biochem, 47, 1589–1603. [DOI] [PubMed] [Google Scholar]
- Qu K, Ma XF, Li GH, Zhang H, Liu YM, Zhang K, Zeng JF, Lei JJ, Wei DH, & Wang Z (2017). Vitamin C down-regulate apo(a) expression via Tet2-dependent DNA demethylation in HepG2 cells. Int J Biol Macromol, 98, 637–645. [DOI] [PubMed] [Google Scholar]
- Rahnasto-Rilla M, Tyni J, Huovinen M, Jarho E, Kulikowicz T, Ravichandran S, V AB, Ferrucci L, Lahtela-Kakkonen M, & Moaddel R (2018). Natural polyphenols as sirtuin 6 modulators. Sci Rep, 8, 4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnasto-Rilla MK, McLoughlin P, Kulikowicz T, Doyle M, Bohr VA, Lahtela-Kakkonen M, Ferrucci L, Hayes M, & Moaddel R (2017). The Identification of a SIRT6 Activator from Brown Algae Fucus distichus. Mar Drugs, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MA, Sahar S, Villeneuve LM, Lanting L, & Natarajan R (2009). Role of Src tyrosine kinase in the atherogenic effects of the 12/15-lipoxygenase pathway in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol, 29, 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith C, & Armitage J (2016). Management of residual risk after statin therapy. Atherosclerosis, 245, 161–170. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, & Glynn RJ (2017). Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med, 377, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Ross R (1999). Atherosclerosis--an inflammatory disease. N Engl J Med, 340, 115–126. [DOI] [PubMed] [Google Scholar]
- Roth SY, Denu JM, & Allis CD (2001). Histone acetyltransferases. Annu Rev Biochem, 70, 81–120. [DOI] [PubMed] [Google Scholar]
- Ruan W, Zhao F, Zhao S, Zhang L, Shi L, & Pang T (2018). Knockdown of long noncoding RNA MEG3 impairs VEGF-stimulated endothelial sprouting angiogenesis via modulating VEGFR2 expression in human umbilical vein endothelial cells. Gene, 649, 32–39. [DOI] [PubMed] [Google Scholar]
- Sallam T, Jones M, Thomas BJ, Wu X, Gilliland T, Qian K, Eskin A, Casero D, Zhang Z, Sandhu J, Salisbury D, Rajbhandari P, Civelek M, Hong C, Ito A, Liu X, Daniel B, Lusis AJ, Whitelegge J, Nagy L, Castrillo A, Smale S, & Tontonoz P (2018). Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding RNA. Nat Med, 24, 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam T, Jones MC, Gilliland T, Zhang L, Wu X, Eskin A, Sandhu J, Casero D, Vallim TQ, Hong C, Katz M, Lee R, Whitelegge J, & Tontonoz P (2016). Feedback modulation of cholesterol metabolism by the lipid-responsive non-coding RNA LeXis. Nature, 534, 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen RM, Nyyssonen K, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Rissanen TH, Tuomainen TP, Valkonen VP, Ristonmaa U, Lakka HM, Vanharanta M, Salonen JT, Poulsen HE, & Antioxidant Supplementation in Atherosclerosis Prevention, S. (2003). Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: the Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation, 107, 947–953. [DOI] [PubMed] [Google Scholar]
- Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, Fuster JJ, & Walsh K (2018). Tet2-Mediated Clonal Hematopoiesis Accelerates Heart Failure Through a Mechanism Involving the IL-1beta/NLRP3 Inflammasome. J Am Coll Cardiol, 71, 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Doi S, Nakashima A, Irifuku T, Yamada K, Kokoroishi K, Ueno T, Doi T, Hida E, Arihiro K, Kohno N, & Masaki T (2016). Inhibition of SET Domain-Containing Lysine Methyltransferase 7/9 Ameliorates Renal Fibrosis. J Am Soc Nephrol, 27, 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh T, Honma K, Matsuyama T, Yui K, Tsujimura T, Standley DM, Nakanishi K, Nakai K, & Akira S (2010). The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol, 11, 936–944. [DOI] [PubMed] [Google Scholar]
- Schober A, & Weber C (2016). Mechanisms of MicroRNAs in Atherosclerosis. Annu Rev Pathol, 11, 583–616. [DOI] [PubMed] [Google Scholar]
- Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, & Li X (2010). Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol, 30, 4712–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA Jr., Garcia-Cardena G, & Jain MK (2004). KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med, 199, 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Gray K, Figg N, Finigan A, Starks L, & Bennett M (2018). Defective Base Excision Repair of Oxidative DNA Damage in Vascular Smooth Muscle Cells Promotes Atherosclerosis. Circulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Kelly TK, & Jones PA (2010). Epigenetics in cancer. Carcinogenesis, 31, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Ward NC, Hodgson JM, Puddey IB, Wang Y, Zhang D, Maghzal GJ, Stocker R, & Croft KD (2013). Dietary quercetin attenuates oxidant-induced endothelial dysfunction and atherosclerosis in apolipoprotein E knockout mice fed a high-fat diet: a critical role for heme oxygenase-1. Free Radic Biol Med, 65, 908–915. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, & Shi Y (2004). Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell, 119, 941–953. [DOI] [PubMed] [Google Scholar]
- Shi ZL, Fang K, Li ZH, Ren DH, Zhang JY, & Sun J (2018). EZH2 Inhibition Ameliorates Transverse Aortic Constriction-Induced Pulmonary Arterial Hypertension in Mice. Can Respir J, 2018, 9174926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff N, Ander BP, Zhan X, Stamova B, Liu D, Hull H, Hamade FR, Dykstra-Aiello C, Ng K, Sharp FR, & Jickling GC (2018). HDAC9 Polymorphism Alters Blood Gene Expression in Patients with Large Vessel Atherosclerotic Stroke. Transl Stroke Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simion V, Haemmig S, & Feinberg MW (2018). LncRNAs in vascular biology and disease. Vascul Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitow M, Lu M, Cheng L, Zhou S, & Morrisey EE (2016). Ezh2 restricts the smooth muscle lineage during mouse lung mesothelial development. Development, 143, 3733–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CL, Wang JP, Xue X, Liu N, Zhang XH, Zhao Z, Liu JG, Zhang CP, Piao ZH, Liu Y, & Yang YB (2017). Effect of Circular ANRIL on the Inflammatory Response of Vascular Endothelial Cells in a Rat Model of Coronary Atherosclerosis. Cell Physiol Biochem, 42, 1202–1212. [DOI] [PubMed] [Google Scholar]
- Sosnowska B, Mazidi M, Penson P, Gluba-Brzozka A, Rysz J, & Banach M (2017). The sirtuin family members SIRT1, SIRT3 and SIRT6: Their role in vascular biology and atherogenesis. Atherosclerosis, 265, 275–282. [DOI] [PubMed] [Google Scholar]
- Souilhol C, Harmsen MC, Evans PC, & Krenning G (2018). Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc Res, 114, 565–577. [DOI] [PubMed] [Google Scholar]
- Stein S, Lohmann C, Schafer N, Hofmann J, Rohrer L, Besler C, Rothgiesser KM, Becher B, Hottiger MO, Boren J, McBurney MW, Landmesser U, Luscher TF, & Matter CM (2010). SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis. Eur Heart J, 31, 2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S, Schafer N, Breitenstein A, Besler C, Winnik S, Lohmann C, Heinrich K, Brokopp CE, Handschin C, Landmesser U, Tanner FC, Luscher TF, & Matter CM (2010). SIRT1 reduces endothelial activation without affecting vascular function in ApoE−/− mice. Aging (Albany NY), 2, 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Wang S, & Song M (2018). Long noncoding RNA SENCR alleviates the inhibitory effects of rapamycin on human umbilical vein endothelial cells. Mol Med Rep, 18, 1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, He S, Wara AKM, Icli B, Shvartz E, Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK, Croce K, & Feinberg MW (2014). Systemic delivery of microRNA-181b inhibits nuclear factor-kappaB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circ Res, 114, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo L, & Salzman J (2016). Detecting circular RNAs: bioinformatic and experimental challenges. Nat Rev Genet, 17, 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Guo J, Zhu Y, Huang Z, Liu T, Cai J, Yu L, & Wang Z (2018). Metformin inhibits ovarian cancer via decreasing H3K27 trimethylation. Int J Oncol, 52, 1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XE, Li H, Chen LY, Xia XD, Zhao ZW, Zheng XL, Zhao GJ, & Tang CK (2018). IL-8 negatively regulates ABCA1 expression and cholesterol efflux via upregulating miR-183 in THP-1 macrophage-derived foam cells. Cytokine. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y (2016). The Bromodomain and Extra-Terminal Domain (BET) Family: Functional Anatomy of BET Paralogous Proteins. Int J Mol Sci, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Xiong X, DePinho RA, Deng CX, & Dong XC (2013a). FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression. J Biol Chem, 288, 29252–29259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Xiong X, DePinho RA, Deng CX, & Dong XC (2013b). Hepatic SREBP-2 and cholesterol biosynthesis are regulated by FoxO3 and Sirt6. J Lipid Res, 54, 2745–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AM, Wagner R, & Rzucidlo EM (2014). Age-related loss of SirT1 expression results in dysregulated human vascular smooth muscle cell function. Am J Physiol Heart Circ Physiol, 307, H533–541. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Wu X, Jones M, Zhang Z, Salisbury D, & Sallam T (2017). Long Noncoding RNA Facilitated Gene Therapy Reduces Atherosclerosis in a Murine Model of Familial Hypercholesterolemia. Circulation, 136, 776–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenteseaux C, Gaston AT, Aguesse A, Poupeau G, de Coppet P, Andriantsitohaina R, Laschet J, Amarger V, Krempf M, Nobecourt-Dupuy E, & Ouguerram K (2017). Perinatal Hypercholesterolemia Exacerbates Atherosclerosis Lesions in Offspring by Altering Metabolism of Trimethylamine-N-Oxide and Bile Acids. Arterioscler Thromb Vasc Biol, 37, 2053–2063. [DOI] [PubMed] [Google Scholar]
- Tsai KL, Hung CH, Chan SH, Hsieh PL, Ou HC, Cheng YH, & Chu PM (2018). Chlorogenic Acid Protects Against oxLDL-Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol Nutr Food Res, 62, e1700928. [DOI] [PubMed] [Google Scholar]
- Uchida S, & Dimmeler S (2015). Long noncoding RNAs in cardiovascular diseases. Circ Res, 116, 737–750. [DOI] [PubMed] [Google Scholar]
- Ulrich V, Rotllan N, Araldi E, Luciano A, Skroblin P, Abonnenc M, Perrotta P, Yin X, Bauer A, Leslie KL, Zhang P, Aryal B, Montgomery RL, Thum T, Martin K, Suarez Y, Mayr M, Fernandez-Hernando C, & Sessa WC (2016). Chronic miR-29 antagonism promotes favorable plaque remodeling in atherosclerotic mice. EMBO Mol Med, 8, 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche J, Neele AE, Hoeksema MA, de Heij F, Boshuizen MC, van der Velden S, de Boer VC, Reedquist KA, & de Winther MP (2014). Inhibiting epigenetic enzymes to improve atherogenic macrophage functions. Biochem Biophys Res Commun, 455, 396–402. [DOI] [PubMed] [Google Scholar]
- van Diepen JA, Thiem K, Stienstra R, Riksen NP, Tack CJ, & Netea MG (2016). Diabetes propels the risk for cardiovascular disease: sweet monocytes becoming aggressive? Cell Mol Life Sci, 73, 4675–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian S, Noh RM, Daga S, Langrish JP, Joshi NV, Mills NL, Hoffmann E, Jacobson EW, Vlasuk GP, Waterhouse BR, Lang NN, & Newby DE (2013). Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. J Am Heart Assoc, 2, e000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian S, Noh RM, Daga S, Langrish JP, Mills NL, Waterhouse BR, Hoffmann E, Jacobson EW, Lang NN, Frier BM, & Newby DE (2016). Effects of the small molecule SIRT1 activator, SRT2104 on arterial stiffness in otherwise healthy cigarette smokers and subjects with type 2 diabetes mellitus. Open Heart, 3, e000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vento-Tormo R, Company C, Rodriguez-Ubreva J, de la Rica L, Urquiza JM, Javierre BM, Sabarinathan R, Luque A, Esteller M, Aran JM, Alvarez-Errico D, & Ballestar E (2016). IL-4 orchestrates STAT6-mediated DNA demethylation leading to dendritic cell differentiation. Genome Biol, 17, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SK, Tian X, LaFrance LV, Duquenne C, Suarez DP, Newlander KA, Romeril SP, Burgess JL, Grant SW, Brackley JA, Graves AP, Scherzer DA, Shu A, Thompson C, Ott HM, Aller GS, Machutta CA, Diaz E, Jiang Y, Johnson NW, Knight SD, Kruger RG, McCabe MT, Dhanak D, Tummino PJ, Creasy CL, & Miller WH (2012). Identification of Potent, Selective, Cell-Active Inhibitors of the Histone Lysine Methyltransferase EZH2. ACS Med Chem Lett, 3, 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, & Natarajan R (2008). Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A, 105, 9047–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello M, Zullo A, Servillo L, Mancini FP, Borriello A, Giovane A, Della Ragione F, D’Onofrio N, & Balestrieri ML (2017). Multiple pathways of SIRT6 at the crossroads in the control of longevity, cancer, and cardiovascular diseases. Ageing Res Rev, 35, 301–311. [DOI] [PubMed] [Google Scholar]
- Voelter-Mahlknecht S (2016). Epigenetic associations in relation to cardiovascular prevention and therapeutics. Clin Epigenetics, 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH (1942). The epigenotype. Endeavour, 1, 18–20. [Google Scholar]
- Wang J, Ma R, Ma W, Chen J, Yang J, Xi Y, & Cui Q (2016). LncDisease: a sequence based bioinformatics tool for predicting lncRNA-disease associations. Nucleic Acids Res, 44, e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, Liu J, Deng D, Lau CW, Wan S, Ai D, Mak KK, Tong KK, Kwan KM, Wang N, Chiu JJ, Zhu Y, & Huang Y (2016). Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. [DOI] [PubMed] [Google Scholar]
- Wang L, Xia JW, Ke ZP, & Zhang BH (2018). Blockade of NEAT1 represses inflammation response and lipid uptake via modulating miR-342–3p in human macrophages THP-1 cells. J Cell Physiol. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang J, Zhao A, & Li J (2017). SIRT1 activation inhibits hyperglycemia-induced apoptosis by reducing oxidative stress and mitochondrial dysfunction in human endothelial cells. Mol Med Rep, 16, 3331–3338. [DOI] [PubMed] [Google Scholar]
- Wang W, Ha CH, Jhun BS, Wong C, Jain MK, & Jin ZG (2010). Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood, 115, 2971–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang S, Yao G, Yu D, Chen K, Tong Q, Ye L, Wu C, Sun Y, Li H, Hermann DM, Doeppner TR, Jin F, Dai Y, & Wu J (2017). Identification of the histone lysine demethylase KDM4A/JMJD2A as a novel epigenetic target in M1 macrophage polarization induced by oxidized LDL. Oncotarget, 8, 114442–114456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Han YD, Sabina S, Cui NH, Zhang S, Liu ZJ, Li C, & Zheng F (2016). HDAC9 Variant Rs2107595 Modifies Susceptibility to Coronary Artery Disease and the Severity of Coronary Atherosclerosis in a Chinese Han Population. PLoS One, 11, e0160449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Wang YH, Ma YT, Fu ZY, Yang YN, Ma X, Li XM, Adi D, Liu F, & Chen BD (2017). ACAT-1 gene polymorphism is associated with increased susceptibility to coronary artery disease in Chinese Han population: a case-control study. Oncotarget, 8, 89055–89063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yao H, Lin S, Zhu X, Shen Z, Lu G, Poon WS, Xie D, Lin MC, & Kung HF (2013). Transcriptional and epigenetic regulation of human microRNAs. Cancer Lett, 331, 1–10. [DOI] [PubMed] [Google Scholar]
- Wei L, Zhao S, Wang G, Zhang S, Luo W, Qin Z, Bi X, Tan Y, Meng M, Qin J, Qin H, Tian D, & Zhang A (2018). SMAD7 methylation as a novel marker in atherosclerosis. Biochem Biophys Res Commun, 496, 700–705. [DOI] [PubMed] [Google Scholar]
- Weirick T, Militello G, & Uchida S (2018). Long Non-coding RNAs in Endothelial Biology. Front Physiol, 9, 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Chen Z, Zhang F, Cui X, Sun W, Geary GG, Wang Y, Johnson DA, Zhu Y, Chien S, & Shyy JY (2013). Ca2+/calmodulin-dependent protein kinase kinase beta phosphorylation of Sirtuin 1 in endothelium is atheroprotective. Proc Natl Acad Sci U S A, 110, E2420–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wezel A, Welten SM, Razawy W, Lagraauw HM, de Vries MR, Goossens EA, Boonstra MC, Hamming JF, Kandimalla ER, Kuiper J, Quax PH, Nossent AY, & Bot I (2015). Inhibition of MicroRNA-494 Reduces Carotid Artery Atherosclerotic Lesion Development and Increases Plaque Stability. Ann Surg, 262, 841–847; discussion 847–848. [DOI] [PubMed] [Google Scholar]
- Wierda RJ, Rietveld IM, van Eggermond MC, Belien JA, van Zwet EW, Lindeman JH, & van den Elsen PJ (2015). Global histone H3 lysine 27 triple methylation levels are reduced in vessels with advanced atherosclerotic plaques. Life Sci, 129, 3–9. [DOI] [PubMed] [Google Scholar]
- Winnik S, Auwerx J, Sinclair DA, & Matter CM (2015). Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J, 36, 3404–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnik S, Gaul DS, Preitner F, Lohmann C, Weber J, Miranda MX, Liu Y, van Tits LJ, Mateos JM, Brokopp CE, Auwerx J, Thorens B, Luscher TF, & Matter CM (2014). Deletion of Sirt3 does not affect atherosclerosis but accelerates weight gain and impairs rapid metabolic adaptation in LDL receptor knockout mice: implications for cardiovascular risk factor development. Basic Res Cardiol, 109, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnik S, Gaul DS, Siciliani G, Lohmann C, Pasterk L, Calatayud N, Weber J, Eriksson U, Auwerx J, van Tits LJ, Luscher TF, & Matter CM (2016). Mild endothelial dysfunction in Sirt3 knockout mice fed a high-cholesterol diet: protective role of a novel C/EBP-beta-dependent feedback regulation of SOD2. Basic Res Cardiol, 111, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise IA, & Charchar FJ (2016). Epigenetic Modifications in Essential Hypertension. Int J Mol Sci, 17, 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtala M, Macierzynska-Piotrowska E, Rybaczek D, Pirola L, & Balcerczyk A (2018). Pharmacological and transcriptional inhibition of the G9a histone methyltransferase suppresses proliferation and modulates redox homeostasis in human microvascular endothelial cells. Pharmacol Res, 128, 252–263. [DOI] [PubMed] [Google Scholar]
- Wu D, Huang RT, Hamanaka RB, Krause M, Oh MJ, Kuo CH, Nigdelioglu R, Meliton AY, Witt L, Dai G, Civelek M, Prabhakar NR, Fang Y, & Mutlu GM (2017). HIF-1alpha is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen C, Cai Y, Huang H, Yang Y, Liu Y, Xu Z, He D, Zhang X, Hu X, Pinello L, Zhong D, He F, Yuan GC, Wang DZ, & Zeng C (2014). LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation, 130, 1452–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, & Zhang Y (2017). TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet, 18, 517–534. [DOI] [PubMed] [Google Scholar]
- Xiao HB, Sui GG, & Lu XY (2017). Icariin improves eNOS/NO pathway to prohibit the atherogenesis of apolipoprotein E-null mice. Can J Physiol Pharmacol, 95, 625–633. [DOI] [PubMed] [Google Scholar]
- Xiaoling Y, Li Z, ShuQiang L, Shengchao M, Anning Y, Ning D, Nan L, Yuexia J, Xiaoming Y, Guizhong L, & Yideng J (2016). Hyperhomocysteinemia in ApoE−/− Mice Leads to Overexpression of Enhancer of Zeste Homolog 2 via miR-92a Regulation. PLoS One, 11, e0167744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Bai P, & Jin ZG (2016). Sirtuins in Cardiovascular Health and Diseases. Trends Endocrinol Metab, 27, 677–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Bai P, Little PJ, & Liu P (2014). Poly(ADP-ribose) polymerase 1 (PARP1) in atherosclerosis: from molecular mechanisms to therapeutic implications. Med Res Rev, 34, 644–675. [DOI] [PubMed] [Google Scholar]
- Xu S, Liu B, Yin M, Koroleva M, Mastrangelo M, Ture S, Morrell CN, Zhang DX, Fisher EA, & Jin ZG (2016). A novel TRPV4-specific agonist inhibits monocyte adhesion and atherosclerosis. Oncotarget, 7, 37622–37635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Liu Z, & Liu P (2014). Targeting hydrogen sulfide as a promising therapeutic strategy for atherosclerosis. Int J Cardiol, 172, 313–317. [DOI] [PubMed] [Google Scholar]
- Xu S, Ogura S, Chen J, Little PJ, Moss J, & Liu P (2013). LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cell Mol Life Sci, 70, 2859–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Pelisek J, & Jin ZG (2018). Atherosclerosis Is an Epigenetic Disease. Trends Endocrinol Metab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Xu Y, Yin M, Zhang S, Liu P, Koroleva M, Si S, Little PJ, Pelisek J, & Jin ZG (2018). Flow-dependent epigenetic regulation of IGFBP5 expression by H3K27me3 contributes to endothelial anti-inflammatory effects. Theranostics, 8, 3007–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Yin M, Koroleva M, Mastrangelo MA, Zhang W, Bai P, Little PJ, & Jin ZG (2016). SIRT6 protects against endothelial dysfunction and atherosclerosis in mice. Aging (Albany NY), 8, 1064–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ha CH, Wong C, Wang W, Hausser A, Pfizenmaier K, Olson EN, McKinsey TA, & Jin ZG (2007). Angiotensin II stimulates protein kinase D-dependent histone deacetylase 5 phosphorylation and nuclear export leading to vascular smooth muscle cell hypertrophy. Arterioscler Thromb Vasc Biol, 27, 2355–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ma C, Liu C, Duan Z, & Zhang L (2018). Knockdown of long noncoding RNA XIST alleviates oxidative low-density lipoprotein-mediated endothelial cells injury through modulation of miR-320/NOD2 axis. Biochem Biophys Res Commun. [DOI] [PubMed] [Google Scholar]
- Xu Y, Xu S, Liu P, Koroleva M, Zhang S, Si S, & Jin ZG (2017). Suberanilohydroxamic Acid as a Pharmacological Kruppel-Like Factor 2 Activator That Represses Vascular Inflammation and Atherosclerosis. J Am Heart Assoc, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li YJ, Tao ZF, Song YC, Chen Q, & Jiang Q (2015). lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res, 116, 1143–1156. [DOI] [PubMed] [Google Scholar]
- Yan Q, Sun L, Zhu Z, Wang L, Li S, & Ye RD (2014). Jmjd3-mediated epigenetic regulation of inflammatory cytokine gene expression in serum amyloid A-stimulated macrophages. Cell Signal, 26, 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Chen Q, He S, Yang M, Maguire EM, An W, Afzal TA, Luong LA, Zhang L, & Xiao Q (2018). miR-22 Is a Novel Mediator of Vascular Smooth Muscle Cell Phenotypic Modulation and Neointima Formation. Circulation, 137, 1824–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Li X, Li R, Peng J, Wang Z, Jiang Z, Tang X, Peng Z, Wang Y, & Wei D (2016). Low Shear Stress Inhibited Endothelial Cell Autophagy Through TET2 Downregulation. Ann Biomed Eng, 44, 2218–2227. [DOI] [PubMed] [Google Scholar]
- Yang Q, Lu Z, Singh D, & Raj JU (2012). BIX-01294 treatment blocks cell proliferation, migration and contractility in ovine foetal pulmonary arterial smooth muscle cells. Cell Prolif, 45, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Fang W, Liang J, Lin C, Wu S, Yan S, Hu C, & Ke X (2018). Apelin/APJ axis improves angiotensin II-induced endothelial cell senescence through AMPK/SIRT1 signaling pathway. Arch Med Sci, 14, 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Zhu X, Duan ZJ, Li YY, Wang YP, & Yang K (2017). [Sirt1 inhibited oxidized low-density lipoprotein induced smooth muscle cells inflammatory response]. Zhonghua Yi Xue Za Zhi, 97, 1659–1663. [DOI] [PubMed] [Google Scholar]
- Yang W, Gao F, Zhang P, Pang S, Cui Y, Liu L, Wei G, & Yan B (2017). Functional genetic variants within the SIRT2 gene promoter in acute myocardial infarction. PLoS One, 12, e0176245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wei J, He Y, Jing T, Li Y, Xiao Y, Wang B, Wang W, Zhang J, & Lin R (2017). SIRT1 inhibition promotes atherosclerosis through impaired autophagy. Oncotarget, 8, 51447–51461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hsu PJ, Chen YS, & Yang YG (2018). Dynamic transcriptomic m(6)A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res, 28, 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li X, Peng L, An L, Sun N, Hu X, Zhou P, Xu Y, Li P, & Chen J (2018). Tanshindiol C inhibits oxidized low-density lipoprotein induced macrophage foam cell formation via a peroxiredoxin 1 dependent pathway. Biochim Biophys Acta, 1864, 882–890. [DOI] [PubMed] [Google Scholar]
- Yao QP, Xie ZW, Wang KX, Zhang P, Han Y, Qi YX, & Jiang ZL (2017). Profiles of long noncoding RNAs in hypertensive rats: long noncoding RNA XR007793 regulates cyclic strain-induced proliferation and migration of vascular smooth muscle cells. J Hypertens, 35, 1195–1203. [DOI] [PubMed] [Google Scholar]
- Yao X, Yan C, Zhang L, Li Y, & Wan Q (2018). LncRNA ENST00113 promotes proliferation, survival, and migration by activating PI3K/Akt/mTOR signaling pathway in atherosclerosis. Medicine (Baltimore), 97, e0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Zhao X, Lu Y, Long B, & Zhang S (2018). Varinostat alters gene expression profiles in aortic tissues from ApoE−/− mice. Hum Gene Ther Clin Dev. [DOI] [PubMed] [Google Scholar]
- Yilmaz SG, Isbir S, Kunt AT, & Isbir T (2018). Circulating microRNAs as Novel Biomarkers for Atherosclerosis. In Vivo, 32, 561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M, Liu Q, Yu L, Yang Y, Lu M, Wang H, Luo D, Rong X, Tang F, & Guo J (2017). Downregulations of CD36 and Calpain-1, Inflammation, and Atherosclerosis by Simvastatin in Apolipoprotein E Knockout Mice. J Vasc Res, 54, 123–130. [DOI] [PubMed] [Google Scholar]
- Yin R, Mao SQ, Zhao B, Chong Z, Yang Y, Zhao C, Zhang D, Huang H, Gao J, Li Z, Jiao Y, Li C, Liu S, Wu D, Gu W, Yang YG, Xu GL, & Wang H (2013). Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc, 135, 10396–10403. [DOI] [PubMed] [Google Scholar]
- Yokota T, Nomura K, Nagashima M, & Kamimura N (2016). Fucoidan alleviates high-fat diet-induced dyslipidemia and atherosclerosis in ApoE(shl) mice deficient in apolipoprotein E expression. J Nutr Biochem, 32, 46–54. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Gan Q, & Owens GK (2008). Kruppel-like factor 4, Elk-1, and histone deacetylases cooperatively suppress smooth muscle cell differentiation markers in response to oxidized phospholipids. Am J Physiol Cell Physiol, 295, C1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Qiu Y, Yang J, Bian S, Chen G, Deng M, Kang H, & Huang L (2016). DNMT1-PPARgamma pathway in macrophages regulates chronic inflammation and atherosclerosis development in mice. Sci Rep, 6, 30053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Chen X, Xiu M, He F, Xing J, Min D, & Guo F (2017). The regulation of Jmjd3 upon the expression of NF-kappaB downstream inflammatory genes in LPS activated vascular endothelial cells. Biochem Biophys Res Commun, 485, 62–68. [DOI] [PubMed] [Google Scholar]
- Yu Z, Rayile A, Zhang X, Li Y, & Zhao Q (2017). Ulinastatin protects against lipopolysaccharide-induced cardiac microvascular endothelial cell dysfunction via downregulation of lncRNA MALAT1 and EZH2 in sepsis. Int J Mol Med, 39, 1269–1276. [DOI] [PubMed] [Google Scholar]
- Yue Y, Liu J, & He C (2015). RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev, 29, 1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Lv W, Zhang L, & Kang W (2018). MiR-147b influences vascular smooth muscle cell proliferation and migration via targeting YY1 and modulating Wnt/beta-catenin activities. Acta Biochim Biophys Sin (Shanghai). [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Zeng L, Margariti A, Xiao Q, Li H, Zhang Z, Pepe AE, Wang G, Habi O, deFalco E, Cockerill G, Mason JC, Hu Y, & Xu Q (2010). Histone deacetylase 3 is critical in endothelial survival and atherosclerosis development in response to disturbed flow. Circulation, 121, 132–142. [DOI] [PubMed] [Google Scholar]
- Zeng HT, Fu YC, Yu W, Lin JM, Zhou L, Liu L, & Wang W (2013). SIRT1 prevents atherosclerosis via liverXreceptor and NFkappaB signaling in a U937 cell model. Mol Med Rep, 8, 23–28. [DOI] [PubMed] [Google Scholar]
- Zeng L, Xiao Q, Margariti A, Zhang Z, Zampetaki A, Patel S, Capogrossi MC, Hu Y, & Xu Q (2006). HDAC3 is crucial in shear- and VEGF-induced stem cell differentiation toward endothelial cells. J Cell Biol, 174, 1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Ma Y, & Xiang C (2018). SIRT2 decreases atherosclerotic plaque formation in low-density lipoprotein receptor-deficient mice by modulating macrophage polarization. Biomed Pharmacother, 97, 1238–1242. [DOI] [PubMed] [Google Scholar]
- Zhang BY, Jin Z, & Zhao Z (2017). Long intergenic noncoding RNA 00305 sponges miR-136 to regulate the hypoxia induced apoptosis of vascular endothelial cells. Biomed Pharmacother, 94, 238–243. [DOI] [PubMed] [Google Scholar]
- Zhang DD, Wang WT, Xiong J, Xie XM, Cui SS, Zhao ZG, Li MJ, Zhang ZQ, Hao DL, Zhao X, Li YJ, Wang J, Chen HZ, Lv X, & Liu DP (2017). Long noncoding RNA LINC00305 promotes inflammation by activating the AHRR-NF-kappaB pathway in human monocytes. Sci Rep, 7, 46204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang E, Guo Q, Gao H, Xu R, Teng S, & Wu Y (2015). Metformin and Resveratrol Inhibited High Glucose-Induced Metabolic Memory of Endothelial Senescence through SIRT1/p300/p53/p21 Pathway. PLoS One, 10, e0143814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Zhang R, Zhang X, Wu Y, Li X, Zhang S, Hou W, Ding Y, Tian J, Sun L, & Kong X (2018). Comprehensive analysis of circRNA expression pattern and circRNA-miRNA-mRNA network in the pathogenesis of atherosclerosis in rabbits. Aging (Albany NY). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HP, Wang YH, Cao CJ, Yang XM, Ma SC, Han XB, Yang XL, Yang AN, Tian J, Xu H, Zhang MH, & Jiang YD (2016). A regulatory circuit involving miR-143 and DNMT3a mediates vascular smooth muscle cell proliferation induced by homocysteine. Mol Med Rep, 13, 483–490. [DOI] [PubMed] [Google Scholar]
- Zhang L, Cheng H, Yue Y, Li S, Zhang D, & He R (2018a). H19 knockdown suppresses proliferation and induces apoptosis by regulating miR-148b/WNT/beta-catenin in ox-LDL-stimulated vascular smooth muscle cells. J Biomed Sci, 25, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Cheng H, Yue Y, Li S, Zhang D, & He R (2018b). TUG1 knockdown ameliorates atherosclerosis via up-regulating the expression of miR-133a target gene FGF1. Cardiovasc Pathol, 33, 6–15. [DOI] [PubMed] [Google Scholar]
- Zhang MJ, Zhou Y, Chen L, Wang X, Pi Y, Long CY, Sun MJ, Chen X, Gao CY, Li JC, & Zhang LL (2016). Impaired SIRT1 promotes the migration of vascular smooth muscle cell-derived foam cells. Histochem Cell Biol, 146, 33–43. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X, Zhao D, Liu Y, Wang C, Zhang X, Su X, Liu J, Ge W, Levine RL, Li N, & Cao X (2015). Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature, 525, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, & Liang CC (2008). Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res, 80, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zou J, Li P, Zheng X, & Feng D (2018). Curcumin Protects against Atherosclerosis in Apolipoprotein E-Knockout Mice by Inhibiting Toll-like Receptor 4 Expression. J Agric Food Chem, 66, 449–456. [DOI] [PubMed] [Google Scholar]
- Zhang T, Cooper S, & Brockdorff N (2015). The interplay of histone modifications - writers that read. EMBO Rep, 16, 1467–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Huang Q, Zeng Z, Wu J, Zhang Y, & Chen Z (2017). Sirt1 Inhibits Oxidative Stress in Vascular Endothelial Cells. Oxid Med Cell Longev, 2017, 7543973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhang Y, Guo X, Zeng Z, Wu J, Liu Y, He J, Wang R, Huang Q, & Chen Z (2017). Sirt1 Protects Endothelial Cells against LPS-Induced Barrier Dysfunction. Oxid Med Cell Longev, 2017, 4082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WP, Bai XJ, Zheng XP, Xie XL, & Yuan ZY (2013). Icariin attenuates the enhanced prothrombotic state in atherosclerotic rabbits independently of its lipid-lowering effects. Planta Med, 79, 731–736. [DOI] [PubMed] [Google Scholar]
- Zhang X, Price NL, & Fernandez-Hernando C (2018). Non-coding RNAs in lipid metabolism. Vascul Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tang X, Hamblin MH, & Yin KJ (2018). Long Non-Coding RNA Malat1 Regulates Angiogenesis in Hindlimb Ischemia. Int J Mol Sci, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang Y, Yuan J, Li N, Pei S, Xu J, Luo X, Mao C, Liu J, Yu T, Gan S, Zheng Q, Liang Y, Guo W, Qiu J, Constantin G, Jin J, Qin J, & Xiao Y (2018). Macrophage/microglial Ezh2 facilitates autoimmune inflammation through inhibition of Socs3. J Exp Med, 215, 1365–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wen H, & Shi X (2012). Lysine methylation: beyond histones. Acta Biochim Biophys Sin (Shanghai), 44, 14–27. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao X, Zhu W, Liu Z, Liu H, Zhou Y, Cao Y, Liu C, & Xie Y (2016). Resveratrol Enhances Autophagic Flux and Promotes Ox-LDL Degradation in HUVECs via Upregulation of SIRT1. Oxid Med Cell Longev, 2016, 7589813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu X, Bai X, Lin Y, Li Z, Fu J, Li M, Zhao T, Yang H, Xu R, Li J, Ju J, Cai B, Xu C, & Yang B (2018). Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. J Pineal Res, 64. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Qiu J, Wang X, Zhang Y, & Xia M (2011). AMP-activated protein kinase suppresses endothelial cell inflammation through phosphorylation of transcriptional coactivator p300. Arterioscler Thromb Vasc Biol, 31, 2897–2908. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang X, Wang Y, Liu Y, & Xia M (2013). Supplementation of cyanidin-3-O-beta-glucoside promotes endothelial repair and prevents enhanced atherogenesis in diabetic apolipoprotein E-deficient mice. J Nutr, 143, 1248–1253. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Q, Gui L, Cai Y, Deng X, Li C, Guo Q, He X, & Huang J (2018). Let-7e inhibits TNF-alpha expression by targeting the methyl transferase EZH2 in DENV2-infected THP-1 cells. J Cell Physiol. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zheng L, Xu BM, Tang WH, Ye ZD, Huang C, Ma X, Zhao JJ, Guo FX, Kang CM, Lu JB, Xiu JC, Li P, Xu YJ, Xiao L, Wu Q, Hu YW, & Wang Q (2018). LncRNA-RP11–714G18.1 suppresses vascular cell migration via directly targeting LRP2BP. Immunol Cell Biol, 96, 175–189. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Gao W, Long QQ, Zhang J, Li YF, Liu DC, Yan JJ, Yang ZJ, & Wang LS (2017). Increased plasma levels of lncRNA H19 and LIPCAR are associated with increased risk of coronary artery disease in a Chinese population. Sci Rep, 7, 7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wu S, Stenoien DL, & Pasa-Tolic L (2014). High-throughput proteomics. Annu Rev Anal Chem (Palo Alto Calif), 7, 427–454. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu J, Liu Y, Wang T, Pei J, Cheng L, Hao D, Zhao X, Chen HZ, & Liu DP (2018). Mouse macrophage specific knockout of SIRT1 influences macrophage polarization and promotes angiotensin II-induced abdominal aortic aneurysm formation. J Genet Genomics, 45, 25–32. [DOI] [PubMed] [Google Scholar]
- Zhang ZQ, Ren SC, Tan Y, Li ZZ, Tang X, Wang TT, Hao DL, Zhao X, Chen HZ, & Liu DP (2016). Epigenetic regulation of NKG2D ligands is involved in exacerbated atherosclerosis development in Sirt6 heterozygous mice. Sci Rep, 6, 23912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhang W, Lin M, Wu W, Jiang P, Tou E, Xue M, Richards A, Jourd’heuil D, Asif A, Zheng D, Singer HA, Miano JM, & Long X (2016). MYOSLID Is a Novel Serum Response Factor-Dependent Long Noncoding RNA That Amplifies the Vascular Smooth Muscle Differentiation Program. Arterioscler Thromb Vasc Biol, 36, 2088–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Li S, Li N, Yang X, Ma S, Yang A, Zhang H, Yang S, Mao C, Xu L, Gao T, Yang X, Zhang H, & Jiang Y (2017). miR-34a Targets HDAC1-Regulated H3K9 Acetylation on Lipid Accumulation Induced by Homocysteine in Foam Cells. J Cell Biochem, 118, 4617–4627. [DOI] [PubMed] [Google Scholar]
- Zhong J, Agha G, & Baccarelli AA (2016). The Role of DNA Methylation in Cardiovascular Risk and Disease: Methodological Aspects, Study Design, and Data Analysis for Epidemiological Studies. Circ Res, 118, 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Li P, Li J, He R, Cheng G, & Li Y (2018). Downregulation of microRNA34a inhibits oxidized lowdensity lipoproteininduced apoptosis and oxidative stress in human umbilical vein endothelial cells. Int J Mol Med, 42, 1134–1144. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Feng J, Fan Z, & Li J (2018). Curcumin increases cholesterol efflux via heme oxygenase1mediated ABCA1 and SRBI expression in macrophages. Mol Med Rep, 17, 6138–6143. [DOI] [PubMed] [Google Scholar]
- Zhou J, Li YS, Wang KC, & Chien S (2014). Epigenetic Mechanism in Regulation of Endothelial Function by Disturbed Flow: Induction of DNA Hypermethylation by DNMT1. Cell Mol Bioeng, 7, 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Cai B, Zhang Z, Zhang Y, Wang L, Liu K, Zhang H, Sun L, Cai H, Lu G, Liu X, & Xu G (2017). CDKN2B Methylation and Aortic Arch Calcification in Patients with Ischemic Stroke. J Atheroscler Thromb, 24, 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Zhang Y, Wang L, Zhang Z, Cai B, Liu K, Zhang H, Dai M, Sun L, Xu X, Cai H, Liu X, Lu G, & Xu G (2016). CDKN2B methylation is associated with carotid artery calcification in ischemic stroke patients. J Transl Med, 14, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Zhang Z, & Xu G (2014). Notable epigenetic role of hyperhomocysteinemia in atherogenesis. Lipids Health Dis, 13, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, & Chen X (2017). Long intergenic noncoding RNA p21 mediates oxidized LDLinduced apoptosis and expression of LOX1 in human coronary artery endothelial cells. Mol Med Rep, 16, 8513–8519. [DOI] [PubMed] [Google Scholar]
- Zhuang J, Luan P, Li H, Wang K, Zhang P, Xu Y, & Peng W (2017). The Yin-Yang Dynamics of DNA Methylation Is the Key Regulator for Smooth Muscle Cell Phenotype Switch and Vascular Remodeling. Arterioscler Thromb Vasc Biol, 37, 84–97. [DOI] [PubMed] [Google Scholar]