Abstract
Alterations in the molecular mechanisms of cell death are a common feature of cancer. These alterations enable malignant cells to survive intrinsic death signalling leading to accumulation of genetic aberrations and helping them to cope with adverse conditions. Regulated cell death has historically been exclusively associated with classical apoptosis; however, increasing evidence indicates that several alternative mechanisms orchestrate multiple death pathways, such as ferroptosis, entosis, necroptosis and immunogenic cell death, each with distinct underlying molecular mechanisms. Although pharmacological targeting of cell death pathways has been the subject of intensive efforts in recent decades with a dominant focus on targeting apoptosis, the identification of these novel death pathways has opened additional venues for intervention in cancer cells and the immune system. In this mini-review, we cover some recent progress on major recently emerged cell death modalities, emphasizing their potential clinical and therapeutic implications. We also discuss the interplay between cell death and immune response, highlighting the potential of the combination of traditional anticancer therapy and immunocheckpoint blockade. While attempting to stimulate discussion and draw attention to the possible clinical impact of these more recently emerged cell death modalities, we also cover the major progress achieved in translating strategies for manipulation of apoptotic pathways into the clinic, focusing on the attempts to target the anti-apoptotic protein BCL-2 and the tumour suppressor p53.
Keywords: Apoptosis, necroptosis, BCL2, p53, ferroptosis, entosis, skin, cornification
Targeting cell death in human disease
Over the past 20 years, significant efforts of the biomedical scientific community have been dedicated to the development of therapeutic strategies aimed to target cell death signalling pathways in multiple clinical scenarios in which cytoprotection, in the case of ischaemic disorders, or cellular lethality, in oncological conditions, are the desired outcomes 1 2 (Fig. 1). This major investment has led to partial success as exemplified by patients with relapsed or refractory chronic lymphocytic leukaemia (CLL) currently receiving clinical benefit from the treatment with venetoclax, an inhibitor of the anti-apoptotic protein BCL2 3, 4 (Fig. 2b). Given our current knowledge of the molecular mechanisms underlying apoptotic cell death, tilting the balance of this cell death modality today appears possible, and a therapeutic benefit might require a more thorough understanding of the integration of the different cell death modalities adopted or preferred in specific physio-pathological conditions.
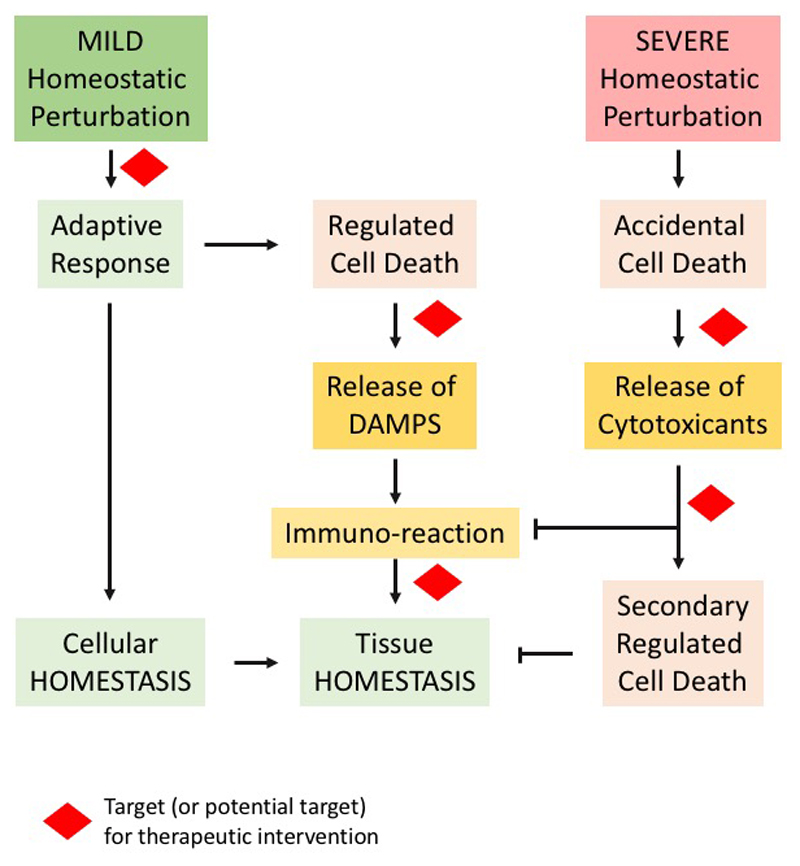
Figure 1. Diagrammatic representation of cell death aspects and therapeutic implications.
Mild alterations of cellular homeostasis, produced by exogenous or endogenous factors, induce an adaptive response to restore homeostasis. Failure of such a response leads to activation of the process of regulated cell death that might (or might not) involve release of danger-associated molecular pattern (DAMP) and trigger an immunoresponse, with the ultimate goal of restoring tissue homeostasis. The adaptive response, executors of the regulated cell death programme and immunoresponse represent or can potentially represent therapeutic targets. Severe homeostatic perturbations lead to accidental cell death that generally involves release of cytotoxic molecules that reiterate the cell death signalling in the tissue. Accidental cell death cannot be therapeutically targeted, but molecules released from the cells succumbing to the primary insult can represent an alternative strategy to pharmacological intervention in these conditions.
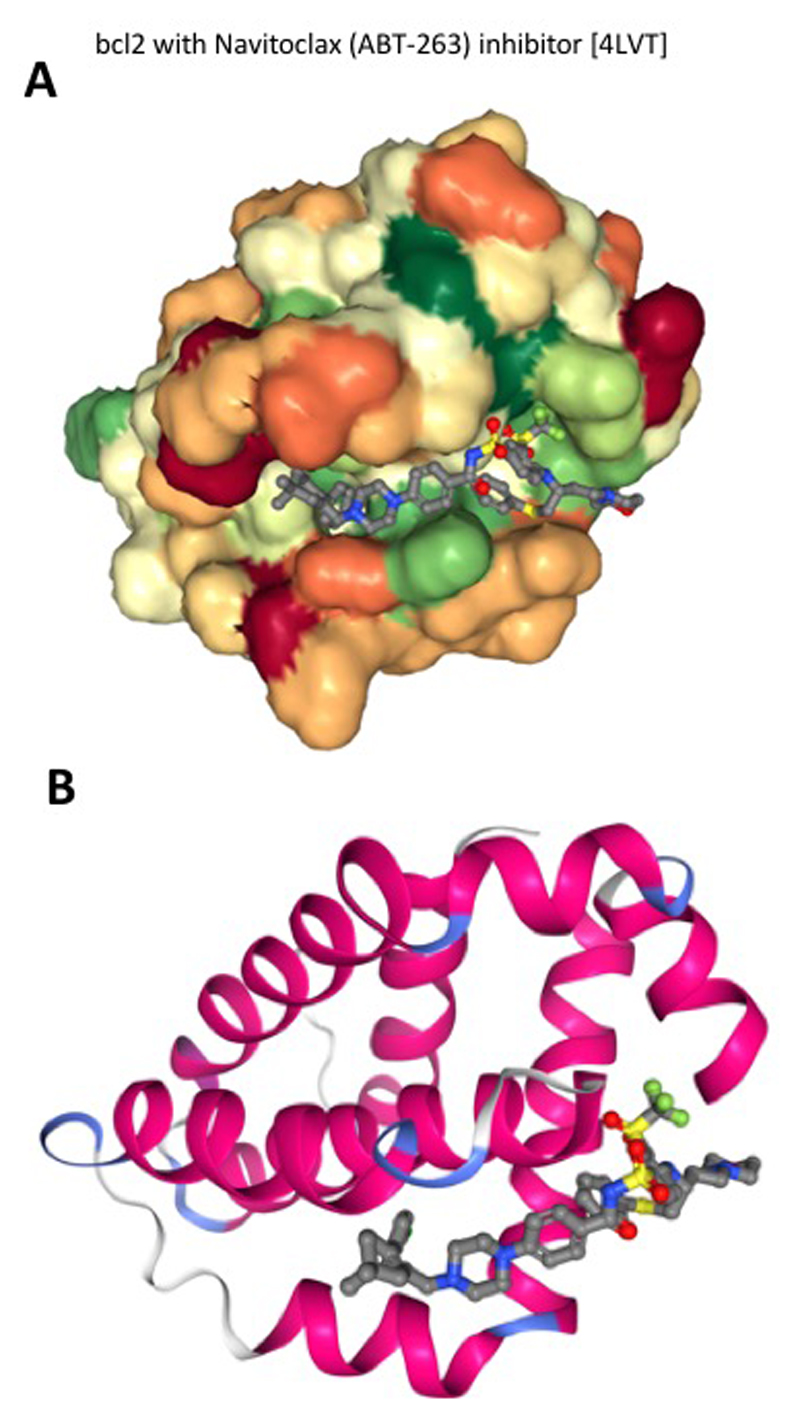
Figure 2. Interaction between Bcl-2 and its inhibitor Navitoclax (ABT-263).
Navitoclax is an oral form of Bcl-2 inhibitor that showed efficacy in BCL2-overexpressing CLL and in follicular lymphoma. Molecular docking analysis shows navitoclax interaction in the binding site of Bcl-2. The inhibitor is shown as a ball and stick, while Bcl-2 is shown in a space filling (a) and ribbon diagram (b) model.
Necroptosis, ferroptosis, and entosis have recently emerged as regulated cell death modalities that execute their death programme following different molecular pathways5. Defining how and whether these mechanisms exert a role in pathological conditions and whether interconnectivity of these signalling and modularity of their execution occur is crucial from a therapeutic standpoint. In this minireview, we provide an overview of the major recently emerged novel cell death modalities emphasizing, where possible, the clinical relevance and therapeutic implications of these molecular signalling pathways. In addition, we discuss the strategies currently in clinical practise or development employed to target cell death, which are mainly confined to targeting apoptotic signalling.
Cell Death Modalities
The longest studied mechanisms of cell death is skin cornification6, 7, which involves a p53 family member, namely, p638. In contrast to accidental cell death associated with catastrophic exposure of cells to severe physical, chemical or mechanical insults, regulated cell death is the result of the activation of defined signal transduction molecular mechanisms, implying that, in theory, such death modes can be pharmacologically or genetically manipulated. All the different modalities of regulated cell death maintain the purpose of responding to microenvironment perturbations to promote cellular and organismal homeostasis in both physiological and pathological conditions, providing obvious advantages to multicellular organisms9 (Fig. 1).
The best studied and defined mechanism of regulated cell death is apoptosis. Apoptosis is characterized by nuclear fragmentation, chromatin condensation, cytoplasmic shrinkage and plasma membrane blebbing. This process results in the accumulation of apoptotic bodies (intact small vesicles) that are neatly cleared by phagocytic cells through a process known as efferocytosis, which is classically viewed as a non-inflammatory mode of cell disposal. At the molecular level, apoptosis is categorized into two major pathways, the extrinsic and intrinsic pathways that mainly differ based on the original trigger that initiates the cascade of events. The transcriptional factor p53 and its family members p63 and p73 are major sensors of cellular stress leading to intrinsic apoptosis10–13 (Fig. 3a). Cellular damage, such as genotoxic stress, can enable stabilization of p53 that subsequently promotes a transcriptional programme aimed to promote repair and fidelity or otherwise kill the damaged cells14. In addition, non-cell autonomous mechanisms have also been associated with p53 tumour suppression15–17 and partially shared by the family members18–24. For many years, this mechanism was thought to be responsible for p53 tumour suppression; however, recent evidence highlighted larger complexity in the p53 tumour suppressor network 25–27. Numerous studies been dedicated to review different aspects of these cell death modalities, dissecting many different mechanistic details. In this section of the article, we wish to dedicate more attention to the less characterized regulated cell death modalities and discuss their therapeutic implications.
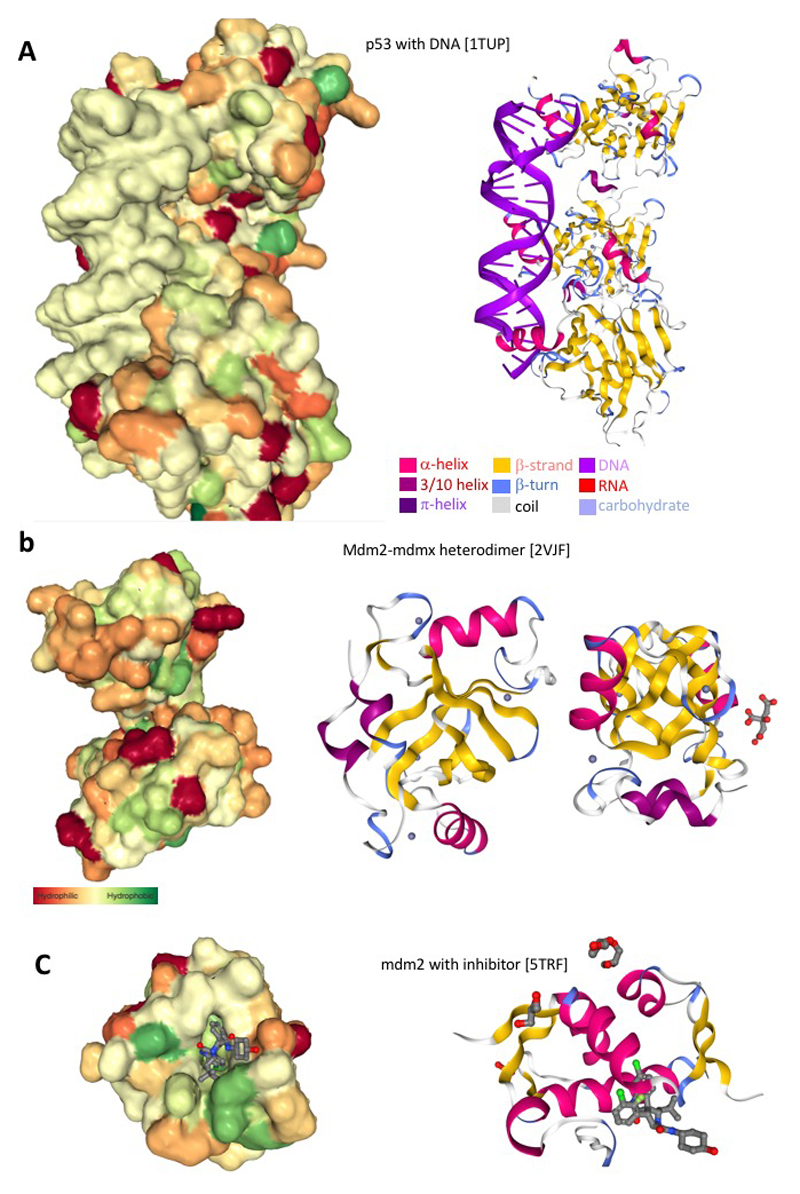
Figure 3. p53 and MDM2 biochemical interactions.
a, Genotoxic stress leads to activation of p53 that in turn results in p53 tetramer interaction with DNA and regulation of the p53-dependent transcriptional programme. This includes upregulation of the pro-apoptotic genes PUMA and Noxa. Panel a depicts the space filling (left) and ribbon diagram (right) model of the p53 interaction on the genomic locus of the pro-apoptotic gene PUMA. b, p53 activation is largely regulated at the level of protein stability by direct interaction with the MDM2/MDMX E3 ubiquitin ligase. Genotoxic stress produces post-translational modification of p53 that inhibits interaction with MDM2. Panel b depicts the space filling (left) and ribbon diagram (right) model of the MDM2/MDMX heterodimer. c, Pharmacologic approaches have been addressed to artificially promote activation of p53 in human cancer by inhibiting the interaction of p53-MDM2. Panel c depicts the space filling (left) and ribbon diagram (right) model of MDM2 inhibitor in the binding pocket of MDM2.
Necroptosis
Necroptosis is a form of regulated cell death generally manifested with a necrotic morphology, which is initiated by signals from the microenvironment that can be detected by specific death receptors, such as TNFR1 and FAS 28–33, or receptors for pathogen recognition, such as DAI, TLR3 and TLR4 34 35 36, 37. Engagement of death receptors triggers the activation of the receptor-interacting protein kinase 1 (RIPK1), which autophosphorylates and recruits RIPK3 through RIP homotypic interaction motif (RHIM) domains present on both kinases 38. Then, activation of necroptosis critically depends on the sequential activation by RIPK3 of the mixed lineage kinase domain-like pseudokinase (MLKL) (provided that CASP8 is inactive)39, 40. Once activated, MLKL translocates onto the plasma membrane, inducing its rupture and subsequent cell death with release of the intracellular content including pro-inflammatory cytokines41 and many types of disease-associated molecular patterns (DAMPs) into the microenvironment.
Necroptosis was originally associated with adaptive functions upon failing responses to stress; however, increasing evidence indicates that it also participates in developmental programmes by ensuring the elimination of potentially defective organisms and in T-cell homeostasis42–45. Triggering regulated necrosis emerged as a new possible antitumoural strategy given that one of the hallmarks of cancer is the blockade or evasion of apoptosis46. Necroptosis can bypass apoptosis resistance and consequentially kill cancer cells. Moreover, the release of DAMPs in combination with cytokines and chemokines make cells dying through this programme immunogenic and able to potentially induce antitumour immunity47–49. This regulated cell death modality can become especially important for the design of novel cancer treatments.
Beyond cancer, for which boosting necroptosis could be beneficial, necroptosis is associated with a variety of human diseases, including atherosclerosis, pancreatitis, inflammatory bowel disease, and neurodegenerative diseases, such as amyotrophic lateral sclerosis, multiple sclerosis and Alzheimer’s50. In these settings of inflammatory and degenerative diseases, targeting key necroptotic players represents a promising strategy. Indeed, the small molecule necrostatin-1 (Nec-1) targets RIPK1 and dramatically inhibits TNFR1-driven necroptosis51, 52. Nec-1 is protective in various ischaemic and neurodegenerative diseases53, 54.
Ferroptosis
Similar to necroptosis, ferroptosis manifests with a necrotic morphotype, but this regulated cell death programme is initiated by specific perturbations of the intracellular redox homeostasis associated with iron availability55–58. The major molecular mechanism leading to ferroptotic cell death is severe lipid peroxidation, and it is potentially associated with a consistent release of immunostimulatory DAMPs59, 60. Alterations in reduced glutathione (GSH) synthesis and restoration are the main causes associated with initiation of ferroptosis. Inhibitors of oxidized GSH (GSSG)-GSH turn over, such as RSL3, and de novo synthesis of GSH, such as Erastin, modulate ferroptosis56, 61, 62. Accordingly, intracellular imbalances in glutamine and cysteine, which are required for the synthesis of intracellular GSH, also activate this iron-dependent cell death modality 63 64 65, 66.
Implications for human therapy associated with ferroptosis might exhibit relevance in the glutamine addiction observed in cancer cells. For example, triple-negative breast carcinoma (TNBC) displays a severe glutamine addiction related to its ability to drive cystine uptake via cystine/glutamate antiporter system xc− 67, 68. The antiporter xc− is a target of Erastin that indeed is thought to trigger ferroptosis by influencing cystine/cysteine intracellular balance and thus indirectly affecting the activity of the GSH-dependent enzyme glutathione peroxidase 4 (GPX4). Xc− may therefore constitute a therapeutic target in this cancer setting. Interestingly, the tumour suppressor function of p53 is partially attributed to its ability to induce ferroptosis through inhibition of the Xc− system 69, 70; however, the role of p53 in ferroptosis is highly context dependent 71. Beyond carcinogenesis, ferroptosis is linked to the pathological cell death associated with neurodegenerative diseases, brain haemorrhage or injury, ischaemia-reperfusion injury, and kidney degeneration57. To successfully target ferroptosis in different clinical settings, its contribution to necroinflammation and immune cell activation must be thoroughly dissected.
Entosis
Entosis is a mechanism involving engulfment of viable cells by non-phagocyte cells of the same or a different cell type defined as homotypic or heterotypic entosis, respectively 72–74. This form of cellular cannibalism has been observed in healthy and malignant mammalian tissues72. The current understanding of the underlying mechanism suggests that the internalization of entotic cells involves a process of cell invasion rather than a canonical mechanism of phagocytosis72, 75. Cell-in-cell invasion is promoted by the cellular junctions between the engulfing and entotic cell, involving E-cadherin (also known as cadherin 1, CDH1) and catenin alpha 176. The Rho-associated coiled-coil containing protein kinase 1 (ROCK1), ROCK2, Ras homologue family member A (RHOA), and diaphanous-related formin 1 (DIAPH1) promote contraction of the cell cytoskeleton that results in engulfment 75, 77, 78. Once engulfed, entotic cells are often eliminated by a BCL2/Caspase-independent cell death programme that generally requires a specific autophagy-related process commonly known as LC3-associated phagocytosis (LAP)79.
To date, three main mechanisms triggering entosis have been characterized, including matrix de-adhesion, aberrant mitosis and glucose deprivation, each corresponding to well-known cancer hallmarks (anchorage independence, deregulated proliferation and metabolic stress, respectively), suggesting that different cancer cell features can induce entotic cell killing and cannibalism80. Indeed, entotic cell death has been observed in several cancer types76. Interestingly, chemical inhibition of ROCK abrogates entosis, favouring the anchorage-independent growth of malignant cells and indicating that entosis can act as an oncosuppressor mechanism76. Conversely, entosis can promote tumour progression by inducing a non-genetic route to aneuploidization and polyploidization 81–83. Considering such a dual role of entosis in cancer, potential therapeutic strategies must carefully consider when and how to act on this delicate balance towards entotic host/inner cell survival.
Cell Death as a therapeutic target: BCL2 and p53
Targeting cell death pathways has been the subject of intensive efforts in the past decades with most studies focusing on the mechanisms regulating apoptosis84, 85, the best characterized programme of cell demise, and particularly BCL2 and p53. Approximately 30 years after their initial discovery, the long road to the clinic culminated successfully, as mentioned above, with the recent FDA approval of the orally bioavailable and highly selective BCL2 inhibitor, venetoclax, for relapsed or refractory CLL. These results pave the way for further development of agents, such as small molecules BH3 mimetics, which displace pro-apoptotic BCL2 family proteins from the constraint of pro-survival members 86. Consistent with the fact that tumour suppressor genes are more difficult to target with drugs, approaches able to reactivate p53 function and provide significant advances in the standard of care for patients have been more difficult to achieve. Here, we briefly review some aspects of BCL2 and p53 targeting, report current approaches using these strategies in interventional clinical trials and highlight the challenges epitomized by these two important regulators of apoptosis for the successful translation of therapeutic approaches targeting cell death.
BCL2 inhibition
BCL2 pro- and anti-apoptotic family member interaction establishes the apoptotic threshold regulating the life/death decisions87. BCL2 is overexpressed in a variety of human cancers through either chromosomal alteration or other mechanisms, such as deregulation of BCL2-targeting microRNAs86, 88–91. Most tumours indeed bear high levels of one or more pro-survival family member or carry mutations impairing the induction of pro-apoptotic members, such as PUMA and NOXA, which are normally activated by p5325, 92, 93. Nonetheless, cancers retain the bulk of the apoptotic machinery and are therefore prone for killing induced by BCL2 homology 3 (BH3) mimetics, agents mimicking the BH3 domains of pro-apoptotic family members, which neutralize their anti-apoptotic siblings by binding their surface hydrophobic grooves. In fact, cancer cells with elevated expression of pro-survival factors such as BCL2 are prone to undergo apoptosis, indicating that the their ‘addiction’ to pro-survival factors make them more susceptible than normal cells. The first BH3 mimetic compound targeting BCL2, ABT-737 (developed by AbbVie), exhibited low solubility and oral bioavailability compared with its orally bioavailable derivative navitoclax (ABT-263) (Fig. 2). However, upon binding, BCL-XL, which regulates platelet lifespan, also caused acute thrombocytopaenia, which limited their application. Nonetheless, navitoclax exhibited efficacy in BCL2-overexpressing CLL and in follicular lymphoma94. In both diseases, the combined use of rituximab, an antibody recognizing the CD20 antigen expressed on the majority of mature B-cells, increased response rates95.
Further optimization of the lead compound led to venetoclax (ABT-199), the potent selective BCL2 inhibitor that exerts antitumour activity while sparing platelets. Venetoclax is currently used against some CLL forms as mentioned above96. Beyond CLL, venetoclax has achieved favourable responses as monotherapy in mantle cell lymphoma and to a minor extent in follicular lymphoma, myeloma, diffuse large B-cell lymphomas and acute myeloid leukaemia. In the latter, tumours bearing mutations of isocitrate dehydrogenase 1 or 2 were found to be BCL2 dependent, facilitating patient stratification97. Interestingly, positive results also emerged from venetoclax used in combination with anti-CD20 antibodies and/or chemotherapy in CLL and B-cell lymphomas; ibrutinib, which inhibits Bruton tyrosine kinase in CLL and mantle cell lymphomas; the proteasome inhibitor bortezomib in multiple myeloma and rituximab in relapsed CLL. Moreover, the drug development pipeline is further enriched with other selective inhibitors for the main pro-survival family members BCL2, BCL-XL and MCL1: S5574698, (BCL2 selective); WEHI-5397899 and its more potent derivatives A-1155463 and A-1331852 100 (BCL-XL selective); S63845 101, S64315, AMG-176, AZD-5991 (MCL1 selective inhibitors) and APG-1252 (BCL2 and BCL-XL inhibitor). Inhibiting MCL1 is particularly promising given that this anti-apoptotic protein is highly expressed in various cancer types and likely mediates resistance to navitoclax and venetoclax by complementing BCL2 function through binding common targets 3, 102, 103. However, MCL1 is also implicated in other cell functions; therefore, it will be crucial to identify the optimal therapeutic setting and window for the application of MCL1 inhibitors, similarly to BCL-XL inhibitors, for which a ramped dosing controls thrombocytopaenia.
A myriad of studies (115 retrieved for venetoclax alone on clinicaltrials.gov) are currently evaluating the possible efficacy of these agents in clinical trials. Most of these studies involve haematological malignancies, but many others are assessing a possible treatment of various solid tumours. Here, we list a series of selected on-going, interventional, phase I–III clinical studies using BCL2 as a target (Table 1) from which results are expected in the next few years. However, this list is not meant to be exhaustive, and other trials can be retrieved searching for specific drugs and/or disease. Overall, BH3 mimetics offer various advantages as anticancer therapeutics. In particular, they act on a universal apoptotic pathway downstream of p53; thus, BH3 mimetics are potentially effective in most cancer types3, 104, 105. Moreover, BH3 profiling, as proposed by Letai106, could help to identify tumours sensitive to specific BH3 mimetics, guiding their use in the clinical practise. Interestingly, BCL2 exhibits intriguing connections with autoimmune diseases, suggesting that evaluation of BH3 mimetics for a possible repurposing in autoimmune pathologies is worthy of investigation107.
Table 1. Selected Phase I–III clinical studies currently evaluating interventional BCL2-based therapeutic approaches.
| Study description | Condition | Phase | Clinical Trial Identifier |
|---|---|---|---|
| Venetoclax (ABT-199) and combination strategies in haematological malignancies and solid tumours | |||
| Study of venetoclax | Patients with relapsed or refractory Waldenström macroglobulinemia | II | NCT02677324 |
| Venetoclax in combination with standard intensive AML induction/consolidation therapy with FLAG-IDA | Newly diagnosed or relapsed/refractory AML | Ib/II | NCT03214562 |
| Venetoclax in combination with the mIDH1 Inhibitor ivosidenib (AG120) | IDH1-mutated haematologic malignancies | Ib/II | NCT03471260 |
| BCL2 inhibitor venetoclax (ABT-199) in combination with obinutuzumab and ibrutinib | Relapsed, refractory, or previously untreated CLL | I/II | NCT02427451 |
| Study of ibrutinib in combination with venetoclax (ABT-199) | Relapsed/refractory mantle cell lymphoma | I/Ib | NCT02419560 |
| Study of venetoclax in combination with obinutuzumab and bendamustine as front line therapy | High tumour burden follicular lymphoma | II | NCT03113422 |
| Study comparing the efficacy of venetoclax + fulvestrant vs. fulvestrant | Patients with oestrogen receptor-positive, Her2-negative locally advanced or metastatic breast cancer who experienced recurrence or progression during or after CDK4/6 inhibitors | II | NCT03584009 |
| Study of duvelisib and venetoclax | Relapsed or refractory CLL or SLL | I/II | NCT03534323 |
| Study of venetoclax (ABT-199) in combination with liposomal vincristine | Relapsed or refractory T-Cell or B-Cell ALL | Ib/II | NCT03504644 |
| Venetoclax in combination with ublituximab and umbralisib (TGR-1202) | Relapsed or refractory CLL/SLL | I/II | NCT03379051 |
| Study of the combination of venetoclax with chemotherapy as frontline therapy | Older patients with ALL | Ib | NCT03319901 |
| Trial evaluating combination of atezolizumab with venetoclax and obinutuzumab | Relapsed or refractory lymphomas | II | NCT03276468 |
| Trial of venetoclax in combination with R-ICE (V+RICE) chemotherapy | Relapsed or refractory diffuse large B-cell lymphoma | I | NCT03064867 |
| Study of venetoclax in combination with dose-adjusted EPOCH-R | Patients with Richter Syndrome | II | NCT03054896 |
| Study of venetoclax in combination with carfilzomib and dexamethasone | Relapsed or refractory multiple myeloma | II | NCT02899052 |
| Trial of obinutuzumab in combination with venetoclax | Previously, untreated follicular lymphoma | I | NCT02877550 |
| Study of bortezomib and dexamethasone in combination with either venetoclax or placebo | Relapsed or refractory multiple myeloma sensitive or naïve to proteasome inhibitors | III | NCT02755597 |
| Navitoclax (ABT-263) and combination strategies in solid tumours | |||
| MEK inhibitor trametinib in combination with navitoclax | KRAS or NRAS mutation-positive advanced solid tumours | Ib/II | NCT02079740 |
| Navitoclax in combination with sorafenib tosylate (Nexavar) | Relapsed or refractory solid tumours | I | NCT02143401 |
| Study of AZD9291 in combination with navitoclax | Patients with EGFR-positive previously treated advanced or metastatic non-small cell lung cancer | Ib | NCT02520778 |
| Study of dabrafenib, trametinib, and navitoclax | Patients with BRAF mutant melanoma or metastatic unresectable solid tumours | I/II | NCT01989585 |
| Other BCL2 targeting approaches | |||
| Dose-escalation study of the orally administered selective BCL2 inhibitor S55746 | Refractory or relapsed CLL and B-cell non-Hodgkin lymphoma | I | NCT02920697 |
| Dose-escalation study of the orally administered selective BCL2 inhibitor S55746 as monotherapy | AML or high or very high risk myelodysplastic syndrome | I | NCT02920541 |
| Study of the safety, pharmacokinetic and pharmacodynamic properties of intravenously administered APG-1252 highly potent BCL2 family inhibitor | Small cell lung cancer or advanced solid tumours | I |
NCT03387332; NCT03080311 |
| Study of PNT2258 | Patients with relapsed or refractory diffuse large B-cell lymphoma | II | NCT02226965 |
The table reports selected studies that used BCL2 as a target for treatment retrieved from a search of clinical trials.gov (NIH, US National Library of Medicine). Other terms: BCL2; Status: Recruiting/Active, not recruiting/Enrolling by invitation; Study type: Interventional Studies; Phase: I, II, III. We did not report studies using BCL2 as biomarker for patient selection (NCT03132584; NCT02706405; NCT03418038; NCT03103971; NCT03038672) or studies that were not cancer related.
ALL: Acute Lymphocytic Leukaemia; AML: Acute Myeloid Leukaemia; CML: Chronic Myelogenous Leukaemia; FLAG-IDA: fludarabine, cytarabine, granulocyte colony-stimulating factor – idarubicin; SLL: Small Lymphocytic Lymphoma.
Pharmacological targeting of p53
Restoring the function of TP53, the most frequently altered gene in human cancers, has been an obvious goal for cancer therapy that has been addressed by disparate strategies108, 109. Alterations in p53 can result in loss of protein function, leading to development of an unstable genome, evasion of apoptosis and gain of function activities that confer a survival advantage. Despite a variety of underlying mechanisms, both processes foster cancer development and progression25, 110. Among the first clinical approaches to reactivate p53, a retrovirus carrying the wt TP53 gene was directly injected into non-small cell lung cancers in 1996, successfully inducing apoptosis and tumour regression/stabilization in six of the nine treated patients111. However, concerns were raised about the use of retroviruses, paving the way for the use of adenoviral vectors, such as the recombinant adenoviral human TP53 vector gendicine, which was approved in China in 2003 for the treatment of head and neck cancer in combination with radiotherapy112. Given the low transduction efficiency of TP53-expressing adenoviruses, replicating viruses have been developed and engineered to selectively replicate in TP53-deficient tumours, such as dl-1520 (Onyx-015). In addition, its derivative H101 in combination with chemotherapy was approved in China for treatment of late-stage refractory nasopharyngeal cancer113. Various phase II/III studies failed to demonstrate efficient activity of Onyx-015 mostly due to inefficient systemic delivery and limited intratumoural dissemination, setting the stage for next generation approaches, including mesenchymal and neural stem cells as delivery vehicles 114, 115. Recently, administration of p53 has been attempted through the scL nanocomplex (SGT-53), and results from a first-in-man Phase I clinical trial demonstrated that the compound is well tolerated, exhibits anticancer activity and reaches metastatic lesions116. Ablation of the p53 negative regulator MDM2 leads to p53-dependent cell death117. Strategies to reactivate endogenous wt p53 in tumours in which it is not mutated have been attempted by targeting its MDM2 and MDMX through the use of peptides and small molecules (Fig. 3b,c). Among the first, peptides designed to mimic p53 and block the p53 binding site of MDM2 through steric hindrance have been recently optimized trough ‘stapling’ via introducing non-natural amino acids into the peptide that increases affinity, half-life and cellular uptake 113, 118.
The archetype of small molecules acting as MDM2 inhibitors are the nutlins. Nutlin-3a was first developed in 2004119 and exhibited efficacy against various cancer types. However, both poor bioavailability and high toxicities hindered its clinical use. Nonetheless, many studies are investigating the use of nutlin and its derivative compounds in combination strategies for a variety of tumours (reviewed in 115, 118 for both pre-clinical and clinical approaches). Among these new drugs, spirooxindoles, such as SAR405838, and piperidinones, such as AMG-232, are being tested in clinical trials for their safety (NCT01636479 and NCT01985191 both completed) and efficacy in various solid and haematological tumours, respectively (NCT03107780; NCT03041688; NCT02110355; NCT02016729/completed; NCT01723020/completed;). As resistance to MDM2 inhibition might arise from MDMX overexpression, dual MDM2/MDMX or small molecule inhibitors of MDMX have also been pursued. Currently, nine compounds are under clinical trial assessment (recently reviewed in 120). Other approaches indirectly targeting p53 are directed against p53-regulating microRNAs; p53 upstream regulators, such as agents that block p53 acetylation; p53 vaccination with a mixture of synthetic p53-derived peptides or through its expression in dendritic cells; and use of synthetic lethal strategies, i.e., pharmacologically forcing p53-defective cells, which have a faulty G1/S checkpoint in response to DNA damage and thus rely on the G2/M checkpoint, into a lethal G2/M transition (all approaches reviewed in 113).
Given that p53 gain of function mutants, which have been identified in 42% of cases across twelve cancer types121–124, have such a high impact on cancer, the development of numerous strategies aimed at reactivating wild-type p53 function in mutated cancers has been stimulated125. Small molecules that restore p53 DNA binding include PRIMA and its derivative APR-246, CP31398, Ellipticine analogues and JNJ26854165113. Interestingly, APR-246 exhibited positive results in a phase I/IIa clinical trial including patients with refractory haematological or prostate cancer126 and is currently under investigation in six clinical trials in combination strategies for oesophageal cancer (NCT02999893), myelodysplasies (NCT03588078/not yet recruiting and NCT03072043), melanoma (NCT03391050), and ovarian cancer (NCT03268382 and NCT02098343). Remarkably, APR-246 is also able to restore the function of p63 mutations that are associated with several human diseases118. Other approaches in this direction are promising, such as the use of Zn2+ chelators and others (all extensively reviewed118, 127). Here, a list of selected currently active interventional phase I–III clinical studies targeting p53, including some of the approaches overviewed above, is provided in Table 2. Hopefully, these studies will soon provide information on their potential as new therapeutic avenues for a variety of cancer patients.
Table 2. Selected Phase I–III clinical studies currently evaluating interventional p53-based therapeutic approaches.
| Study description | Condition | Phase | Clinical Trial Identifier |
|---|---|---|---|
| Mechanism of action: compounds that bind to MDM2 or mutant p53 | |||
| Neoadjuvant AMG-232 concurrent with preoperative radiotherapy | wt p53 soft tissue sarcoma | Ib | NCT03217266 |
| Study of MDM2 Inhibitor AMG-232 | Newly diagnosed GBM harbouring unmethylated MGMT promoters and wt TP53 or recurrent GBM | I | NCT03107780 |
| Study of AMG-232 in combination with decitabine | Relapsed, refractory, or newly diagnosed wt TP53 AML | Ib | NCT03041688 |
| Study evaluating AMG-232 combined with trametinib and dabrafenib or trametinib | Adult patients with metastatic cutaneous melanoma | Ib/IIa | NCT02110355 |
| APR-246 in combination with carboplatin/PLD chemotherapy vs. carboplatin/PLD chemotherapy alone (PiSARRO) | Platinum sensitive recurrent high-grade serous ovarian cancer with mutated p53 | Ib/II | NCT02098343 |
| APR-246 in combination with PLD chemotherapy (PiSARRO-R) | Platinum-resistant high grade serous ovarian cancer (positive for p53 nuclear expression by IHC) | II | NCT03268382 |
| Dose-escalation study evaluating the efficacy of APR-246, in combination with standard chemotherapy (cisplatin and 5-FU) | Platinum resistant advanced and metastatic oesophageal or gastro-oesophageal junction cancers | Ib/II | NCT02999893 |
| Study to investigate the safety and clinical activity of APR-246 in combination with dabrafenib | BRAF V600 mutant unresectable and/or metastatic cutaneous melanoma resistant to dabrafenib/trametinib combination | I/II | NCT03391050 |
| Study to evaluate the safety and efficacy of APR-246 in combination with azacitidine | TP53 mutant myeloid neoplasms | Ib/II | NCT03072043 |
| Dose-escalation study of imidazolopyrrolidinone analogue p53-MDM2 inhibitor HDM201 | Selected advanced solid and haematological wt TP53 tumours | I | NCT02143635 |
| Dose-escalation study of HDM201 | Adult patients with advanced solid and haematological wt TP53 tumours | I | NCT02143635 |
| Study of oral HDM201 in combination with oral LEE011 | Adult patients with liposarcoma | Ib/II | NCT02343172 |
| Study of PKC inhibitor LXS196 antitumour activity as a single agent and in combination with HDM201 | Metastatic uveal melanoma | I | NCT02601378 |
| Dose escalation study of oral CGM097, a p53/HDM2-interaction inhibitor | Selected advanced solid tumours with wt p53 | Ib/II | NCT01760525 |
| Dose escalation study of oral CGM097, a p53/HDM2-interaction inhibitor | Adult patients with selected advanced solid tumours | I | NCT01760525 |
| Study of the safety, pharmacokinetic and pharmacodynamic properties of orally administered APG-115 | Advanced solid tumours or lymphomas | I | NCT02935907 |
| Multiple ascending dose study of the oral MDM2 inhibitor DS-3032b | Advanced solid tumours or lymphomas | I | NCT01877382 |
| Dose escalation study of DS-3032b | AML, ALL, CML in blast phase, or High-Risk MDS | I | NCT02319369 |
| Study of DS-3032b | Relapsed and/or refractory multiple myeloma | I | NCT02579824 |
| Trial of Anti-PD-L1 atezolizumab With MEK1/2 Inhibitor cobimetinib or MDM2 antagonist idasanutlin | Metastatic ER+ breast cancer | Ib/II | NCT03566485 |
| Study of idasanutlin with cytarabine versus cytarabine plus placebo | Relapsed or refractory AML | III | NCT02545283 |
| Idasanutlin in combination with ixazomib and dexamethasone | 17p deleted, relapsed multiple myeloma | I/II | NCT02633059 |
| Dose-escalation study of BI 907828 | Adult patients with wt TP53 enriched advanced solid tumours and expansion in patients with MDM2 amplified advanced solid tumours | Ia/Ib | NCT03449381 |
| Study to determine the safety and tolerability of the stapled peptide ALRN-6924 | Advanced solid tumours or lymphomas expressing wt p53 | I/IIa | NCT02264613 |
| Study of COTI-2 - orally available third generation thiosemicarbazone and activator of mutant forms of the p53 | Advanced or recurrent gynaecologic malignancies and HNSCC | I | NCT02433626 |
| TP53 vaccination and gene therapy approaches | |||
| Evaluation of Ad-p53 in combination with capecitabine (Xeloda) or Anti-PD1 | Unresectable liver metastases of CRC and other solid tumours, recurrent HNSCC and primary hepatic cancers with known disease progression on standard therapy | I/II | NCT02842125 |
| Study of Ad-p53 transduced DC Vaccine in Combination With 1-methyl-D-tryptophan in | Metastatic solid tumours and invasive breast cancer | I/II | NCT01042535 |
| Vaccine therapy with Ad-p53-infected autologous DCs in combination with neoadjuvant or adjuvant chemotherapy and adjuvant radiotherapy | Women with p53-overexpressing stage III breast cancer | Ib/II | NCT00082641 |
| Ad-p53 DCs in combination with chemotherapy with or without all trans RA | Patients with extensive stage small cell lung cancer | II | NCT00617409 |
| Study to evaluate efficacy and safety of Ad-p53 in combination with nivolumab versus nivolumab alone | Recurrent HNSCC | II | NCT03544723 |
| Combination immunotherapy with ipilimumab and nivolumab plus a DC based p53 vaccine | Relapsed small cell lung cancer | II | NCT03406715 |
| Study of a p53MVA vaccine in combination with pembrolizumab | Solid tumours (bearing TP53 Mutation) that failed prior therapy | I | NCT02432963 |
| Study of metastatic cancer that overexpress p53 using lymphodepleting conditioning followed by infusion of anti-p53 TCR-gene engineered lymphocytes and DC vaccination | Progressive or recurrent metastatic cancer | II | NCT00704938 |
| Study of a tumour-targeted IL-2 fusion protein, ALT-801, capable of binding a tumour associated p53 peptide presented in the context of HLA-A2 | Patients with Bacillus Calmette-Guerin failure non-muscle invasive bladder cancer | Ib/II | NCT01625260 |
| First-in-human clinical study with RNA-immunotherapy combination of IVAC_W_bre1_uID and IVAC_M_uID for individualized tumour therapy (RNA based vaccine) | Triple-negative breast cancer Patients | I | NCT02316457 |
| Study of SGT-53 in combination with topotecan and cyclophosphamide | Paediatric patients with recurrent or refractory solid tumours | I | NCT02354547 |
| Study of SGT-53 plus temozolomide | Recurrent GBM | II | NCT02340156 |
| Study of SGT-53 plus gemcitabine/nab-paclitaxel | Metastatic pancreatic cancer | II | NCT02340117 |
The table reports selected studies that used p53-based approaches retrieved from a search of clinical trials.gov (NIH, US National Library of Medicine). Other terms: p53 or MDM2/HDM2; Status: Recruiting/Active, not recruiting/Enrolling by invitation; Study type: Interventional Studies; Phase: I, II, III. We did not report studies using p53 as a biomarker for patient selection (such as NCT03149679 ‘The p53 Colorectal Cancer Trial’; NCT02965950 ‘The p53 Breast Cancer Trial’; NCT02042989; NCT03144804; NCT03077243; NCT02734537), tumour classification/biomarker, or readout of treatment.
Ad-p53: adeno virus expressing p53; ALL: Acute Lymphocytic Leukaemia; AML: Acute Myelogenous Leukaemia; CML: Chronic Myelogenous Leukaemia; CRC: Colorectal Carcinoma; DC: dendritic cell; GBM: glioblastoma multiforme; HLA-A2: major histocompatibility complex, class I, A2; HNSCC: Head and Neck Squamous Cell Carcinoma; MDS: myelodysplastic syndrome; MGMT: O-6-methylguanine-DNA methyltransferase; MVA: modified vaccinia Ankara; PKC: Protein Kinase C; PLD: Pegylated Liposomal Doxorubicin Hydrochloride; RA: retinoic acid; 5-FU: 5-fluorouracil.
Despite such an arsenal of compounds and strategies, re-establishing p53 function in the clinical setting has proven difficult mainly owing to lack of efficacy, resistance development, side effects and shortfalls in defining first which subset of patients would have more likely benefited specific approaches. In particular, many of these compounds exhibited both on-target and off-target effects, and this feature coupled with the difficulty in determining the outcome of p53 activated response further complicated the design of suitable trials. Indeed, although early studies suggested that p53 tumour suppressor function relies on its ability to induce cell cycle arrest, senescence or apoptosis, new studies challenged this paradigm. Beyond the complexity due to the type/amount of stress required to elicit a p53 response, the context dependence, the ability to interact and/or regulate hundreds of genes, the possible overlapping function of family members, and the different gain-of-function-specific mutants, p53 also regulates many autonomous and non-autonomous additional processes, including metabolism, autophagy, stem cell reprogramming, fertility, invasion metastasis and longevity. These findings indicate that we need to achieve p53-mediated tumour suppression without promoting ageing in the clinical setting25, 108, 128. Moreover, recent studies indicate that some of the tumour suppressor mechanisms of p53 might be related to its role in other programmed cell death pathways, such as necroptosis 129 and ferroptosis56, suggesting that a careful dissection of all the aspects connected to the recently identified mechanisms of cell death is needed to establish how and in what clinical setting we can target these pathways.
Immunogenic cell death and Immunotherapy
Immunogenic cell death refers to all the forms of regulated cell death that stimulates a T cell-dependent immune response specific for dead cell-derived antigens. Immunogenic cell death indeed requires that dying cells activate adaptive responses associated with the expression and secretion of DAMPs in the microenvironment. A number of currently employed and well-established chemotherapeutics can elicit immunogenic cell death, including anthracyclines, mitoxantrone, bleomycin, bortezomib, and cyclophosphamide. Apoptosis, necroptosis and in theoretical terms also ferroptosis can stimulate activation of the immune system. Tumour transplantation experiments in immunocompetent BALB/c mice have demonstrated that doxorubicin treatment stimulated an immune response that was abolished by the presence of the pan-caspase inhibitor z-Vad130. Analogous experiments have demonstrated similar immunostimulatory capacities in processes of necroptotic cell death131.
The relevance of immunogenic cell death in clinical setting lies on the ability of this process of reactivating physiological anticancer immunity. DAMPs released by dying cancer cells favour the recruitment, activation and interaction with T lymphocytes, thus impairing the immunoevasion that is often at the basis of tumour development and progression. The combination of immunostimulating anticancer therapy and the immunocheckpoint blockade have subsequently become crucial132.
Antibodies blocking the cytotoxic T lymphocyte–associated protein 4 (CTLA-4) or the programmed cell death 1 (PD-1) pathway, either alone or in combination, can elicit an immune response from pre-existence antitumour T cells that were limited by these specific immune checkpoints. This resulted in unprecedented rates of long-lasting tumour responses in patients with a variety of cancers. In chemotherapy-induced immunogenic cell death, secretion of DAMPs by dying cancer cells facilitates recruitment of tumour-infiltrating dendritic cells, resulting in immunogenic phagocytosis133. These mechanisms suggest a potential synergism between traditional chemotherapy and immunocheckpoint blockade134.
The success of immunotherapy and its combinations relies on pre-existing levels of antitumour immune cells. Traditional and more modern anticancer approaches, such as chemotherapy, radiotherapy and oncogene-targeted therapies, might have the potential to reshape the tumour microenvironment, including the immune content, and therefore further promote responses to immune checkpoint blockade135.
With the recent success of cancer immunotherapy, it is imperative to invest in more research on the mechanisms of immunogenic cell death to identify routes for overcoming unresponsiveness and preventing acquisition of resistance.
Concluding Remarks
This recent discovery of novel modalities of regulated cell death opened an entirely new therapeutic perspective for the field. However, the clear contributions of the individual mechanisms for human disease are not clear, and a general consensus has not always been reached in the field. Two major approaches should be adopted to potentially succeed in therapeutically targeting cell death. The first approach should aim to develop strategies designed to switch cell death modalities rather than enhancing or abrogating the execution of a specific cell death programme 136–139. A second approach should aim to develop agents that intercept DAMPs or regulate DAMP-dependent signalling pathways 140, 141.
It is reasonable to optimistically envisage the targeting of cell death as a promising approach for human cancer and generally for several human disorders. On the other hand, considerable effort has been made to develop strategies to target cell death for clinical purposes; however, it appears clear that additional studies are still required to devise the most efficient strategies.
Table 3. Examples of Phase I–III clinical studies currently evaluating cancer immunotherapy drugs.
| Study description | Condition | Phase | Clinical Trial Identifier |
|---|---|---|---|
| Combine TACE and Autologous Tcm Immunotherapy | Hepatocellular Carcinoma | I | NCT03575806 |
| Autologous Tcm cells immunotherapy | Urinary Bladder Neoplasm | II | NCT03389438 |
| Immunotherapy (Nivolumab, Atezolizumab) Plus Radiotherapy | Metastatic Renal Cell Carcinoma Metastatic Urothelial Carcinoma |
II | NCT03115801 |
| Epstein-Barr Virus Specific Immunotherapy | Nasopharyngeal Carcinoma | II | NCT00834093 |
| Intra-tumoral T4 immunotherapy | Head and Neck Cancer | I | NCT01818323 |
| Docetaxel, Gemcitabine, Pemetrexed HyperAcute®-Lung Immunotherapy Drug |
Non-small Cell Lung Cancer Progression of Non-small Cell Lung Cancer Non-small Cell Lung Cancer Recurrent |
II/III | NCT01774578 |
| Anti-PD-1 antibody DC-CIK Immunotherapy Thermotron RF-8EX |
Mesothelioma, Malignant | I/II | NCT03393858 |
| DC-CIK Immunotherapy Capecitabine Monotherapy |
Breast Cancer | II | NCT02491697 |
| Atezolizumab | Non-Small-Cell Lung | II | NCT03102242 |
| Nivolumab NK immunotherapy |
Malignant Solid Tumour | I/II | NCT02843204 |
| anti-PD-1/PD-L1 immunotherapy Radiation therapy at 9.5Gy |
Metastatic Cancer | II | NCT02843165 |
The table reports selected studies that used p53-based approaches retrieved from a search of clinical trials.gov (NIH, US National Library of Medicine).
Acknowledgements
This work has been supported by the Medical Research Council, UK; grants from Associazione Italiana per la Ricerca contro il Cancro (AIRC): AIRC 2014 IG15653 renewed in 2018-2022 (to G.M.), AIRC 5xmille MCO9979 (to G.M.).
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
G Melino 0000-0001-9428-5972
N Di Daniele 0000-0001-7671-0015
S Grelli 0000-0003-1028-3203
I Amelio 0000-0003-0739-325X
F Pentimalli 0000-0003-4740-6801
References
- 1.Dorn GW., 2nd Novel pharmacotherapies to abrogate postinfarction ventricular remodeling. Nat Rev Cardiol. 2009;6(4):283–91. doi: 10.1038/nrcardio.2009.12. [DOI] [PubMed] [Google Scholar]
- 2.Galluzzi L, Morselli E, Kepp O, Kroemer G. Targeting post-mitochondrial effectors of apoptosis for neuroprotection. Biochim Biophys Acta. 2009;1787(5):402–13. doi: 10.1016/j.bbabio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov. 2017;16(4):273–284. doi: 10.1038/nrd.2016.253. [DOI] [PubMed] [Google Scholar]
- 4.Valdes-Mas R, Gutierrez-Abril J, Puente XS, Lopez-Otin C. Chronic lymphocytic leukemia: looking into the dark side of the genome. Cell Death Differ. 2016;23(1):7–9. doi: 10.1038/cdd.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6(4):328–40. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 7.Rivetti di Val Cervo P, Lena AM, Nicoloso M, Rossi S, Mancini M, Zhou H, et al. p63-microRNA feedback in keratinocyte senescence. Proc Natl Acad Sci U S A. 2012;109(4):1133–8. doi: 10.1073/pnas.1112257109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Candi E, Amelio I, Agostini M, Melino G. MicroRNAs and p63 in epithelial stemness. Cell Death Differ. 2015;22(1):12–21. doi: 10.1038/cdd.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galluzzi L, Bravo-San Pedro JM, Kepp O, Kroemer G. Regulated cell death and adaptive stress responses. Cell Mol Life Sci. 2016;73(11–12):2405–10. doi: 10.1007/s00018-016-2209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez I, Tournillon AS, Prado Martins R, Karakostis K, Malbert-Colas L, Nylander K, et al. p53-mediated suppression of BiP triggers BIK-induced apoptosis during prolonged endoplasmic reticulum stress. Cell Death Differ. 2017;24(10):1717–1729. doi: 10.1038/cdd.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kastenhuber ER, Lowe SW. Putting p53 in Context. Cell. 2017;170(6):1062–1078. doi: 10.1016/j.cell.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rufini A, Niklison-Chirou MV, Inoue S, Tomasini R, Harris IS, Marino A, et al. TAp73 depletion accelerates aging through metabolic dysregulation. Genes Dev. 2012;26(18):2009–14. doi: 10.1101/gad.197640.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vikhreva P, Melino G, Amelio I. p73 Alternative Splicing: Exploring a Biological Role for the C-Terminal Isoforms. J Mol Biol. 2018;430(13):1829–1838. doi: 10.1016/j.jmb.2018.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon H, Brauning B, Fainer I, Ben-Nissan G, Rabani S, Goldfinger N, et al. Post-translational regulation of p53 function through 20S proteasome-mediated cleavage. Cell Death Differ. 2017;24(12):2187–2198. doi: 10.1038/cdd.2017.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charni M, Molchadsky A, Goldstein I, Solomon H, Tal P, Goldfinger N, et al. Novel p53 target genes secreted by the liver are involved in non-cell-autonomous regulation. Cell Death Differ. 2016;23(3):509–20. doi: 10.1038/cdd.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tschaharganeh DF, Xue W, Calvisi DF, Evert M, Michurina TV, Dow LE, et al. p53-dependent Nestin regulation links tumor suppression to cellular plasticity in liver cancer. Cell. 2014;158(3):579–92. doi: 10.1016/j.cell.2014.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153(2):449–60. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napoli M, Flores ER. The p53 family orchestrates the regulation of metabolism: physiological regulation and implications for cancer therapy. Br J Cancer. 2017;116(2):149–155. doi: 10.1038/bjc.2016.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amelio I, Melino G. The p53 family and the hypoxia-inducible factors (HIFs): determinants of cancer progression. Trends Biochem Sci. 2015;40(8):425–34. doi: 10.1016/j.tibs.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Amelio I, Inoue S, Markert EK, Levine AJ, Knight RA, Mak TW, et al. TAp73 opposes tumor angiogenesis by promoting hypoxia-inducible factor 1alpha degradation. Proc Natl Acad Sci U S A. 2015;112(1):226–31. doi: 10.1073/pnas.1410609111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Memmi EM, Sanarico AG, Giacobbe A, Peschiaroli A, Frezza V, Cicalese A, et al. p63 Sustains self-renewal of mammary cancer stem cells through regulation of Sonic Hedgehog signaling. Proc Natl Acad Sci U S A. 2015;112(11):3499–504. doi: 10.1073/pnas.1500762112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salah Z, Melino G, Aqeilan RI. Negative regulation of the Hippo pathway by E3 ubiquitin ligase ITCH is sufficient to promote tumorigenicity. Cancer Res. 2011;71(5):2010–20. doi: 10.1158/0008-5472.CAN-10-3516. [DOI] [PubMed] [Google Scholar]
- 23.Sundvall M, Korhonen A, Paatero I, Gaudio E, Melino G, Croce CM, et al. Isoform-specific monoubiquitination, endocytosis, and degradation of alternatively spliced ErbB4 isoforms. Proc Natl Acad Sci U S A. 2008;105(11):4162–7. doi: 10.1073/pnas.0708333105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marini A, Rotblat B, Sbarrato T, Niklison-Chirou MV, Knight JRP, Dudek K, et al. TAp73 contributes to the oxidative stress response by regulating protein synthesis. Proc Natl Acad Sci U S A. 2018;115(24):6219–6224. doi: 10.1073/pnas.1718531115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14(5):359–70. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM, et al. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell. 2011;145(4):571–83. doi: 10.1016/j.cell.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mello SS, Valente LJ, Raj N, Seoane JA, Flowers BM, McClendon J, et al. A p53 Super-tumor Suppressor Reveals a Tumor Suppressive p53-Ptpn14-Yap Axis in Pancreatic Cancer. Cancer Cell. 2017;32(4):460–473 e6. doi: 10.1016/j.ccell.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vercammen D, Vandenabeele P, Beyaert R, Declercq W, Fiers W. Tumour necrosis factor-induced necrosis versus anti-Fas-induced apoptosis in L929 cells. Cytokine. 1997;9(11):801–8. doi: 10.1006/cyto.1997.0252. [DOI] [PubMed] [Google Scholar]
- 29.Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W, et al. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998;188(5):919–30. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–21. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawar M, Busov B, Chandrasekhar A, Yao J, Zacks DN, Besirli CG. FAS apoptotic inhibitory molecule 2 is a stress-induced intrinsic neuroprotective factor in the retina. Cell Death Differ. 2017;24(10):1799–1810. doi: 10.1038/cdd.2017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Messemaker TC, Mikkers HMM, Huizinga TW, Toes REM, van der Helm-van Mil AHM, Kurreeman F. Inflammatory genes TNFalpha and IL6 display no signs of increased H3K4me3 in circulating monocytes from untreated rheumatoid arthritis patients. Genes Immun. 2017;18(3):191–196. doi: 10.1038/gene.2017.20. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen TN, Baaklini S, Koukouikila-Koussounda F, Ndounga M, Torres M, Pradel L, et al. Association of a functional TNF variant with Plasmodium falciparum parasitaemia in a congolese population. Genes Immun. 2017;18(3):152–157. doi: 10.1038/gene.2017.13. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288(43):31268–79. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiser WJ, Upton JW, Mocarski ES. Viral modulation of programmed necrosis. Curr Opin Virol. 2013;3(3):296–306. doi: 10.1016/j.coviro.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7(4):302–13. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith LM, Weissenburger-Moser LA, Heires AJ, Bailey KL, Romberger DJ, LeVan TD. Epistatic effect of TLR-1, -6 and -10 polymorphisms on organic dust-mediated cytokine response. Genes Immun. 2017;18(2):67–74. doi: 10.1038/gene.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3(115):re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- 39.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39(3):443–53. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 40.Witt A, Vucic D. Diverse ubiquitin linkages regulate RIP kinases-mediated inflammatory and cell death signaling. Cell Death Differ. 2017;24(7):1160–1171. doi: 10.1038/cdd.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anovazzi G, Medeiros MC, Pigossi SC, Finoti LS, Souza Moreira TM, Mayer MP, et al. Functionality and opposite roles of two interleukin 4 haplotypes in immune cells. Genes Immun. 2017;18(1):33–41. doi: 10.1038/gene.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38(2):209–23. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Dara L, Liu ZX, Kaplowitz N. Questions and controversies: the role of necroptosis in liver disease. Cell Death Discov. 2016;2 doi: 10.1038/cddiscovery.2016.89. 16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chou FC, Kuo CC, Chen HY, Chen HH, Sytwu HK. DNA demethylation of the TIM-3 promoter is critical for its stable expression on T cells. Genes Immun. 2016;17(3):179–86. doi: 10.1038/gene.2016.6. [DOI] [PubMed] [Google Scholar]
- 45.Marwaha AK, Panagiotopoulos C, Biggs CM, Staiger S, Del Bel KL, Hirschfeld AF, et al. Pre-diagnostic genotyping identifies T1D subjects with impaired Treg IL-2 signaling and an elevated proportion of FOXP3(+)IL-17(+) cells. Genes Immun. 2017;18(1):15–21. doi: 10.1038/gene.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–75. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 48.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 49.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4(6):469–78. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 50.Ng GZ, Sutton P. The MUC1 mucin specifically inhibits activation of the NLRP3 inflammasome. Genes Immun. 2016;17(3):203–6. doi: 10.1038/gene.2016.10. [DOI] [PubMed] [Google Scholar]
- 51.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–9. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 52.Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol. 2014;35:24–32. doi: 10.1016/j.semcdb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 53.Zhao H, Jaffer T, Eguchi S, Wang Z, Linkermann A, Ma D. Role of necroptosis in the pathogenesis of solid organ injury. Cell Death Dis. 2015;6:e1975. doi: 10.1038/cddis.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, Cao H, Li J, Wang B, Zhang P, Dong Zhang X, et al. Autophagy induced by DAMPs facilitates the inflammation response in lungs undergoing ischemia-reperfusion injury through promoting TRAF6 ubiquitination. Cell Death Differ. 2017;24(4):683–693. doi: 10.1038/cdd.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dixon SJ. Ferroptosis: bug or feature? Immunol Rev. 2017;277(1):150–157. doi: 10.1111/imr.12533. [DOI] [PubMed] [Google Scholar]
- 56.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369–79. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fearnhead HO, Vandenabeele P, Vanden Berghe T. How do we fit ferroptosis in the family of regulated cell death? Cell Death Differ. 2017;24(12):1991–1998. doi: 10.1038/cdd.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A. 2014;111(47):16836–41. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol. 2016;11(11):977–985. doi: 10.1038/nnano.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234–45. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3(3):285–96. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 63.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brigelius-Flohe R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830(5):3289–303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 65.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hayano M, Yang WS, Corn CK, Pagano NC, Stockwell BR. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23(2):270–8. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Timmerman LA, Holton T, Yuneva M, Louie RJ, Padro M, Daemen A, et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell. 2013;24(4):450–65. doi: 10.1016/j.ccr.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muir A, Danai LV, Gui DY, Waingarten CY, Lewis CA, Vander Heiden MG. Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. Elife. 2017;6 doi: 10.7554/eLife.27713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Charni M, Aloni-Grinstein R, Molchadsky A, Rotter V. p53 on the crossroad between regeneration and cancer. Cell Death Differ. 2017;24(1):8–14. doi: 10.1038/cdd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2018 doi: 10.1016/j.freeradbiomed.2018.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krishna S, Overholtzer M. Mechanisms and consequences of entosis. Cell Mol Life Sci. 2016;73(11–12):2379–86. doi: 10.1007/s00018-016-2207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Florey O, Gammoh N, Kim SE, Jiang X, Overholtzer M. V-ATPase and osmotic imbalances activate endolysosomal LC3 lipidation. Autophagy. 2015;11(1):88–99. doi: 10.4161/15548627.2014.984277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perez E, Bergmann A. Intercellular cannibalism fuels tumor growth. Cell Death Differ. 2017;24(5):759–760. doi: 10.1038/cdd.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang M, Ning X, Chen A, Huang H, Ni C, Zhou C, et al. Impaired formation of homotypic cell-in-cell structures in human tumor cells lacking alpha-catenin expression. Sci Rep. 2015;5 doi: 10.1038/srep12223. 12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131(5):966–79. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 77.Purvanov V, Holst M, Khan J, Baarlink C, Grosse R. G-protein-coupled receptor signaling and polarized actin dynamics drive cell-in-cell invasion. Elife. 2014;3 doi: 10.7554/eLife.02786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun Q, Cibas ES, Huang H, Hodgson L, Overholtzer M. Induction of entosis by epithelial cadherin expression. Cell Res. 2014;24(11):1288–98. doi: 10.1038/cr.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Florey O, Kim SE, Sandoval CP, Haynes CM, Overholtzer M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biol. 2011;13(11):1335–43. doi: 10.1038/ncb2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Durgan J, Florey O. Cancer cell cannibalism: Multiple triggers emerge for entosis. Biochim Biophys Acta. 2018;1865(6):831–841. doi: 10.1016/j.bbamcr.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 81.Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376(22):2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 82.Krajcovic M, Johnson NB, Sun Q, Normand G, Hoover N, Yao E, et al. A non-genetic route to aneuploidy in human cancers. Nat Cell Biol. 2011;13(3):324–30. doi: 10.1038/ncb2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krajcovic M, Overholtzer M. Mechanisms of ploidy increase in human cancers: a new role for cell cannibalism. Cancer Res. 2012;72(7):1596–601. doi: 10.1158/0008-5472.CAN-11-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amelio I, Melino G, Knight RA. Cell death pathology: cross-talk with autophagy and its clinical implications. Biochem Biophys Res Commun. 2011;414(2):277–81. doi: 10.1016/j.bbrc.2011.09.080. [DOI] [PubMed] [Google Scholar]
- 85.Rufini A, Melino G. Cell death pathology: the war against cancer. Biochem Biophys Res Commun. 2011;414(3):445–50. doi: 10.1016/j.bbrc.2011.09.110. [DOI] [PubMed] [Google Scholar]
- 86.Zanesi N, Balatti V, Bottoni A, Croce CM, Pekarsky Y. Novel insights in molecular mechanisms of CLL. Curr Pharm Des. 2012;18(23):3363–72. doi: 10.2174/138161212801227104. [DOI] [PubMed] [Google Scholar]
- 87.Hantusch A, Brunner T, Frickey T, Rehm M. Bcl-2-Ome - a database and interactive web service for dissecting the Bcl-2 interactome. Cell Death Differ. 2017;24(1):192. doi: 10.1038/cdd.2016.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pekarsky Y, Zanesi N, Croce CM. Molecular basis of CLL. Semin Cancer Biol. 2010;20(6):370–6. doi: 10.1016/j.semcancer.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nana-Sinkam P, Croce CM. MicroRNAs in diagnosis and prognosis in cancer: what does the future hold? Pharmacogenomics. 2010;11(5):667–9. doi: 10.2217/pgs.10.57. [DOI] [PubMed] [Google Scholar]
- 90.Fouque A, Lepvrier E, Debure L, Gouriou Y, Malleter M, Delcroix V, et al. The apoptotic members CD95, BclxL, and Bcl-2 cooperate to promote cell migration by inducing Ca(2+) flux from the endoplasmic reticulum to mitochondria. Cell Death Differ. 2016;23(10):1702–16. doi: 10.1038/cdd.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pihan P, Carreras-Sureda A, Hetz C. BCL-2 family: integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017;24(9):1478–1487. doi: 10.1038/cdd.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lowe JM, Nguyen TA, Grimm SA, Gabor KA, Peddada SD, Li L, et al. The novel p53 target TNFAIP8 variant 2 is increased in cancer and offsets p53-dependent tumor suppression. Cell Death Differ. 2017;24(1):181–191. doi: 10.1038/cdd.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Belle JI, Petrov JC, Langlais D, Robert F, Cencic R, Shen S, et al. Repression of p53-target gene Bbc3/PUMA by MYSM1 is essential for the survival of hematopoietic multipotent progenitors and contributes to stem cell maintenance. Cell Death Differ. 2016;23(5):759–75. doi: 10.1038/cdd.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30(5):488–96. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roberts AW, Advani RH, Kahl BS, Persky D, Sweetenham JW, Carney DA, et al. Phase 1 study of the safety, pharmacokinetics, and antitumour activity of the BCL2 inhibitor navitoclax in combination with rituximab in patients with relapsed or refractory CD20+ lymphoid malignancies. Br J Haematol. 2015;170(5):669–78. doi: 10.1111/bjh.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–8. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 97.Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong WJ, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med. 2015;21(2):178–84. doi: 10.1038/nm.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Casara P, Davidson J, Claperon A, Le Toumelin-Braizat G, Vogler M, Bruno A, et al. S55746 is a novel orally active BCL-2 selective and potent inhibitor that impairs hematological tumor growth. Oncotarget. 2018;9(28):20075–20088. doi: 10.18632/oncotarget.24744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lessene G, Czabotar PE, Sleebs BE, Zobel K, Lowes KN, Adams JM, et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nat Chem Biol. 2013;9(6):390–7. doi: 10.1038/nchembio.1246. [DOI] [PubMed] [Google Scholar]
- 100.Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SK, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med. 2015;7(279):279ra40. doi: 10.1126/scitranslmed.aaa4642. [DOI] [PubMed] [Google Scholar]
- 101.Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538(7626):477–482. doi: 10.1038/nature19830. [DOI] [PubMed] [Google Scholar]
- 102.Bose P, Gandhi V, Konopleva M. Pathways and mechanisms of venetoclax resistance. Leuk Lymphoma. 2017;58(9):1–17. doi: 10.1080/10428194.2017.1283032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carrington EM, Zhan Y, Brady JL, Zhang JG, Sutherland RM, Anstee NS, et al. Anti-apoptotic proteins BCL-2, MCL-1 and A1 summate collectively to maintain survival of immune cell populations both in vitro and in vivo. Cell Death Differ. 2017;24(5):878–888. doi: 10.1038/cdd.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peperzak V, Slinger E, Ter Burg J, Eldering E. Functional disparities among BCL-2 members in tonsillar and leukemic B-cell subsets assessed by BH3-mimetic profiling. Cell Death Differ. 2017;24(1):111–119. doi: 10.1038/cdd.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reinhart R, Rohner L, Wicki S, Fux M, Kaufmann T. BH3 mimetics efficiently induce apoptosis in mouse basophils and mast cells. Cell Death Differ. 2018;25(1):204–216. doi: 10.1038/cdd.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Montero J, Stephansky J, Cai T, Griffin GK, Cabal-Hierro L, Togami K, et al. Blastic Plasmacytoid Dendritic Cell Neoplasm Is Dependent on BCL2 and Sensitive to Venetoclax. Cancer Discov. 2017;7(2):156–164. doi: 10.1158/2159-8290.CD-16-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Molinier-Frenkel V, Mestivier D, Castellano F. Alterations of the immunosuppressive IL4I1 enzyme activity induced by naturally occurring SNP/mutations. Genes Immun. 2016;17(2):148–52. doi: 10.1038/gene.2015.55. [DOI] [PubMed] [Google Scholar]
- 108.Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol. 2015;16(7):393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 109.Abdul Razak FR, Diepstra A, Visser L, van den Berg A. CD58 mutations are common in Hodgkin lymphoma cell lines and loss of CD58 expression in tumor cells occurs in Hodgkin lymphoma patients who relapse. Genes Immun. 2016;17(6):363–6. doi: 10.1038/gene.2016.30. [DOI] [PubMed] [Google Scholar]
- 110.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26(12):1268–86. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roth JA, Nguyen D, Lawrence DD, Kemp BL, Carrasco CH, Ferson DZ, et al. Retrovirus-mediated wild-type p53 gene transfer to tumors of patients with lung cancer. Nat Med. 1996;2(9):985–91. doi: 10.1038/nm0996-985. [DOI] [PubMed] [Google Scholar]
- 112.Wilson JM. Gendicine: the first commercial gene therapy product. Hum Gene Ther. 2005;16(9):1014–5. doi: 10.1089/hum.2005.16.1014. [DOI] [PubMed] [Google Scholar]
- 113.Merkel O, Taylor N, Prutsch N, Staber PB, Moriggl R, Turner SD, et al. When the guardian sleeps: Reactivation of the p53 pathway in cancer. Mutat Res. 2017;773:1–13. doi: 10.1016/j.mrrev.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 114.Crompton AM, Kirn DH. From ONYX-015 to armed vaccinia viruses: the education and evolution of oncolytic virus development. Curr Cancer Drug Targets. 2007;7(2):133–9. doi: 10.2174/156800907780058862. [DOI] [PubMed] [Google Scholar]
- 115.Stegh AH. Targeting the p53 signaling pathway in cancer therapy - the promises, challenges and perils. Expert Opin Ther Targets. 2012;16(1):67–83. doi: 10.1517/14728222.2011.643299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Senzer N, Nemunaitis J, Nemunaitis D, Bedell C, Edelman G, Barve M, et al. Phase I study of a systemically delivered p53 nanoparticle in advanced solid tumors. Mol Ther. 2013;21(5):1096–103. doi: 10.1038/mt.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fouchecourt S, Livera G, Messiaen S, Fumel B, Parent AS, Marine JC, et al. Apoptosis of Sertoli cells after conditional ablation of murine double minute 2 (Mdm2) gene is p53-dependent and results in male sterility. Cell Death Differ. 2016;23(3):521–30. doi: 10.1038/cdd.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khoo KH, Verma CS, Lane DP. Drugging the p53 pathway: understanding the route to clinical efficacy. Nat Rev Drug Discov. 2014;13(3):217–36. doi: 10.1038/nrd4236. [DOI] [PubMed] [Google Scholar]
- 119.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 120.Espadinha M, Barcherini V, Lopes EA, Santos MMM. An Update On Mdmx And Dual Mdm2/X Inhibitors. Curr Top Med Chem. 2018 doi: 10.2174/1568026618666180604080119. [DOI] [PubMed] [Google Scholar]
- 121.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alexandrova EM, Moll UM. Depleting stabilized GOF mutant p53 proteins by inhibiting molecular folding chaperones: a new promise in cancer therapy. Cell Death Differ. 2017;24(1):3–5. doi: 10.1038/cdd.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Freed-Pastor WA, Mizuno H, Zhao X, Langerod A, Moon SH, Rodriguez-Barrueco R, et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148(1–2):244–58. doi: 10.1016/j.cell.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kehrloesser S, Osterburg C, Tuppi M, Schafer B, Vousden KH, Dotsch V. Intrinsic aggregation propensity of the p63 and p73 TI domains correlates with p53R175H interaction and suggests further significance of aggregation events in the p53 family. Cell Death Differ. 2016;23(12):1952–1960. doi: 10.1038/cdd.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aggarwal M, Saxena R, Sinclair E, Fu Y, Jacobs A, Dyba M, et al. Reactivation of mutant p53 by a dietary-related compound phenethyl isothiocyanate inhibits tumor growth. Cell Death Differ. 2016;23(10):1615–27. doi: 10.1038/cdd.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lehmann S, Bykov VJ, Ali D, Andren O, Cherif H, Tidefelt U, et al. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol. 2012;30(29):3633–9. doi: 10.1200/JCO.2011.40.7783. [DOI] [PubMed] [Google Scholar]
- 127.Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18(2):89–102. doi: 10.1038/nrc.2017.109. [DOI] [PubMed] [Google Scholar]
- 128.Xia Q, Wang M, Yang X, Li X, Zhang X, Xu S, et al. Autophagy-related IRGM genes confer susceptibility to ankylosing spondylitis in a Chinese female population: a case-control study. Genes Immun. 2017;18(1):42–47. doi: 10.1038/gene.2016.48. [DOI] [PubMed] [Google Scholar]
- 129.Tu HC, Ren D, Wang GX, Chen DY, Westergard TD, Kim H, et al. The p53-cathepsin axis cooperates with ROS to activate programmed necrotic death upon DNA damage. Proc Natl Acad Sci U S A. 2009;106(4):1093–8. doi: 10.1073/pnas.0808173106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Inoue H, Tani K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. 2014;21(1):39–49. doi: 10.1038/cdd.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guo ZS, Liu Z, Bartlett DL. Oncolytic Immunotherapy: Dying the Right Way is a Key to Eliciting Potent Antitumor Immunity. Front Oncol. 2014;4:74. doi: 10.3389/fonc.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350(6263):972–8. doi: 10.1126/science.aad0779. [DOI] [PubMed] [Google Scholar]
- 134.Garg AD, Romano E, Rufo N, Agostinis P. Immunogenic versus tolerogenic phagocytosis during anticancer therapy: mechanisms and clinical translation. Cell Death Differ. 2016;23(6):938–51. doi: 10.1038/cdd.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33(17):1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Galluzzi L, Bravo-San Pedro JM, Demaria S, Formenti SC, Kroemer G. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nat Rev Clin Oncol. 2017;14(4):247–258. doi: 10.1038/nrclinonc.2016.183. [DOI] [PubMed] [Google Scholar]
- 137.Dudek AM, Garg AD, Krysko DV, De Ruysscher D, Agostinis P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev. 2013;24(4):319–33. doi: 10.1016/j.cytogfr.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 138.Vanden Berghe T, Kalai M, van Loo G, Declercq W, Vandenabeele P. Disruption of HSP90 function reverts tumor necrosis factor-induced necrosis to apoptosis. J Biol Chem. 2003;278(8):5622–9. doi: 10.1074/jbc.M208925200. [DOI] [PubMed] [Google Scholar]
- 139.Liang J, Yan R, Chen G, Feng J, Wu WW, Ren W, et al. Downregulation of ZBTB24 hampers the G0/1- to S-phase cell-cycle transition via upregulating the expression of IRF-4 in human B cells. Genes Immun. 2016;17(5):276–82. doi: 10.1038/gene.2016.18. [DOI] [PubMed] [Google Scholar]
- 140.Vandenabeele P, Vandecasteele K, Bachert C, Krysko O, Krysko DV. Immunogenic Apoptotic Cell Death and Anticancer Immunity. Adv Exp Med Biol. 2016;930:133–49. doi: 10.1007/978-3-319-39406-0_6. [DOI] [PubMed] [Google Scholar]
- 141.Hernandez C, Huebener P, Schwabe RF. Damage-associated molecular patterns in cancer: a double-edged sword. Oncogene. 2016;35(46):5931–5941. doi: 10.1038/onc.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]