Significance
In the bulk phase, hydroxyl radical from the one-electron transfer and high-valent iron-oxo species from the O-atom transfer compete to be the reactive intermediates in the Fenton and related reactions. In the confined space at a nanoscale, however, the behavior of the Fenton reaction is elusive. Herein, we report an unprecedented singlet oxygen mediated Fenton’s reaction occurred inside carbon nanotube with inner diameter of ∼7 nm, showing exotic catalytic activities, unforeseen adsorption-dependent selectivity, and pH stability for the oxidation of organic compounds. Our results suggest the use of Fenton’s reaction in more scenarios than ever explored.
Keywords: Fenton-like catalysis, confinement, singlet oxygen, nanoparticles, water treatment
Abstract
For several decades, the iron-based Fenton-like catalysis has been believed to be mediated by hydroxyl radicals or high-valent iron-oxo species, while only sporadic evidence supported the generation of singlet oxygen (1O2) in the Haber–Weiss cycle. Herein, we report an unprecedented singlet oxygen mediated Fenton-like process catalyzed by ∼2-nm Fe2O3 nanoparticles distributed inside multiwalled carbon nanotubes with inner diameter of ∼7 nm. Unlike the traditional Fenton-like processes, this delicately designed system was shown to selectively oxidize the organic dyes that could be adsorbed with oxidation rates linearly proportional to the adsorption affinity. It also exhibited remarkably higher degradation activity (22.5 times faster) toward a model pollutant methylene blue than its nonconfined analog. Strikingly, the unforeseen stability at pH value up to 9.0 greatly expands the use of Fenton-like catalysts in alkaline conditions. This work represents a fundamental breakthrough toward the design and understanding of the Fenton-like system under nanoconfinement, might cause implications in other fields, especially in biological systems.
The seminal study by Fenton in 1894 (1) opened the door to create strong oxidants using Fe(II) and H2O2. This ubiquitous industrialized oxidation and its derivatives, i.e., Fenton-like reagents such as Fe(III)/H2O2, photo-/electro- Fenton, have been utilized in a versatile way in various fields including the cognition of biological stress response (2) and sensing (3), chemical analysis (4), molecular synthesis (5), material preparation (6), and environmental remediation (7) in the past century. Notwithstanding the obscure mechanism of Fenton’s reaction (8–10), the main oxidative intermediate species involved in the bulk phase or at aqueous interfaces are proved to be the hydroxyl radicals (HO•) generated through the Haber–Weiss cycle (8, 9) or high-valent iron-oxo species [Fe(IV)=O/Fe(V)=O] produced via the Bray–Gorin mechanism (10, 11).
Recently, thriving researches on nanotechnology and nanomaterial have created tremendous opportunities for reinvestigation of various chemical reactions at the nanoscale, generating new insights on fundamental understanding and tools for various applications (12). For examples, under nanoconfinement provided by carbon nanotube (CNT), molecular-dynamics simulations showed pulse-like water transmission with concerted and rapid motion along the tube axis, as well as tunable channel occupancy and conductivity (13, 14). Similarly, a stable polymorphous crystal formed from ionic liquid [bmim][PF6] inside multiwalled carbon nanotubes (MWCNTs) exhibited a melting point of above 473 K, much higher than that of the unconfined analog (279 K) (15). With respect to RhMn nanoparticles inside CNTs, the electron-deficient interior surface of CNTs was shown to increase the tendency of confined Mn to accept electrons from CO, resulting in one order of magnitude improvement of C2 oxygenate yield from syngas (16).
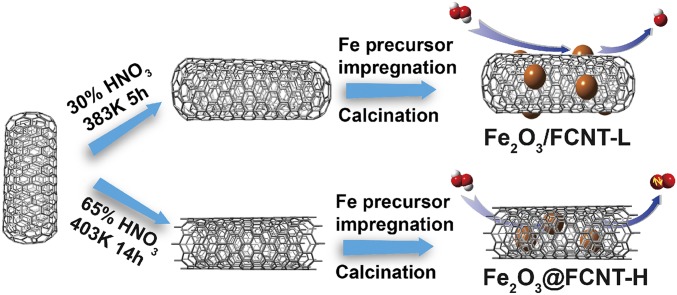
Herein, we report an attempt to understand the behavior of the Fenton reaction under nanoconfinement of CNTs with size of ∼7 nm and demonstrate a singlet oxygen (1O2) mediated pathway. As shown in Fig. 1, two different structures were designed, i.e., distribution of Fe2O3 nanoparticles on the outer surface of CNT (upper route, referred to as Fe2O3/FCNT-L), anchoring of Fe2O3 nanoparticles inside the CNT for nanoconfinement (lower route, referred to as Fe2O3@FCNT-H). The former exhibited traditional Fenton-like reaction pathways with HO• as the main active species; however, an unprecedented reaction pathway with 1O2 as the reactive intermediate was discovered in the latter design. We further used the latter as a catalyst for the oxidation of several organic dyes. Surprisingly, this nanoconfined system selectively oxidized the compounds that could be adsorbed with the reaction rates linearly proportional to the adsorption affinity. For the degradation of a model pollutant methylene blue (MB), the latter catalyst exhibited a remarkably faster kinetics (22.5 times) as well as an exceptionally higher pH stability (pH 5.0–9.0) than its nonconfined analog.
Fig. 1.
Experimental design of this study.
Results and Discussion
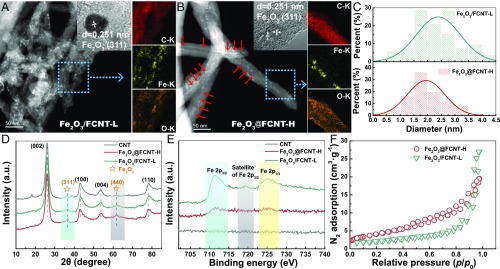
The inner diameters of the CNTs are estimated to be ∼7 nm (SI Appendix, Fig. S1). Representative high-angle dark-field scanning transmission electron microscopy (HADDF-STEM) images with energy-dispersive X-ray spectroscopy (EDX) elemental mapping (Fig. 2 A and B and SI Appendix, Fig. S2) clearly show that in Fe2O3/FCNT-L, nanoparticles are randomly distributed along the direction of CNT; however, in Fe2O3@FCNT-H, the distribution of the nanoparticles is dictated by the center of the CNT (pointed at by the red arrows). The insets of high-resolution transmission electron microscopy (HRTEM) images in both figures show the (311) crystalline lattice of Fe2O3 nanoparticles. The size-distribution histograms of Fe2O3 nanoparticles in both samples in Fig. 2C show the average size to be 2.4 nm (Fe2O3/FCNT-L) and 1.9 nm (Fe2O3@FCNT-H). The mass fractions of Fe2O3 nanoparticles in both samples were determined to be similar, i.e., 2.09% for Fe2O3/FCNT-L and 1.93% for Fe2O3@FCNT-H. The XRD patterns in Fig. 2D show that both Fe2O3/FCNT-L and Fe2O3@FCNT-H exhibit four characteristic diffraction peaks of the (002), (100), (004), and (110) crystal planes of CNTs with typical hexagonal graphite structure. Meanwhile, two weak peaks at 35.9° and 62.3° appear in both samples (Fig. 2D), highly suspected to be the diffraction of (311) and (440) crystal planes of Fe2O3 (Joint Committee on Powder Diffraction Standards 04–0755), which was further confirmed by the sole presence of Fe3+ species by 57Fe Mössbauer spectra of both samples (SI Appendix, Fig. S3) (17, 18).
Fig. 2.
Structure of the catalysts. (A and B) Representative HADDF-STEM images of Fe2O3/FCNT-L and Fe2O3@FCNT-H (Insets) HRTEM images of the Fe2O3 nanoparticles; dexter figures show the EDX elemental mappings of the selected area. (C) Size-distribution histograms of the Fe2O3 nanoparticles in both samples. (D) XRD patterns and (E) Fe 2p XPS spectra of CNT, Fe2O3/FCNT-L and Fe2O3@FCNT-H. (F) N2 adsorption–desorption isotherms of Fe2O3/FCNT-L and Fe2O3@FCNT-H.
The Raman spectra (SI Appendix, Fig. S4) show that in comparison with CNT, the two samples exhibit an adsorption band at around 280 cm−1 after the Fe2O3 loading, which could be assigned to the Eg Fe-O vibration mode (19). The blue shift of this band from 281.2 cm−1 of Fe2O3/FCNT-L to 285.2 cm−1 of Fe2O3@FCNT-H indicates the interaction of Fe2O3 with the inner surface of the CNT in Fe2O3@FCNT-H (19). The Fe 2p XPS spectra in Fig. 2E show surprising difference of the Fe 2p peak intensities between both samples, i.e., much weaker Fe 2p peaks of Fe2O3@FCNT-H than Fe2O3/FCNT-L despite their similar Fe contents. This is probably due to the limited probe depth of the photoelectrons (3–5 nm) for the detection of Fe2O3 inside the CNT. The N2 adsorption–desorption isotherms are shown in Fig. 2F, from which the Brunner–Emmet–Teller (BET)-specific surface areas are calculated to be 197 m2∙g−1 (Fe2O3/FCNT-L) and 206 m2∙g−1 (Fe2O3@FCNT-H), and the BJH pore volumes are 0.59 and 0.43 cm3∙g−1, respectively.
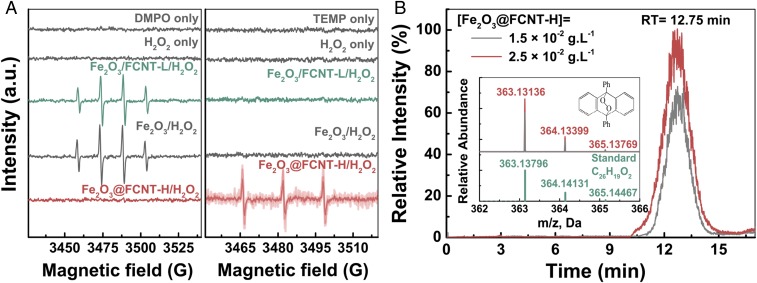
We first identified the generated active species in various systems. The electron spin resonance (ESR) spectra (Fig. 3A) show that, by using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as the trapping agent, one could observe the quadruple peak signals of DMPO-HO• (1:2:2:1, αN = αH = 14.9 G, g = 2.0055) in Fe2O3/FCNT-L/H2O2 and Fe2O3/H2O2 systems, which are expected in traditional Fenton-like systems. On the contrary, the DMPO-HO• signal was not detected in Fe2O3@FCNT-H/H2O2 system at all. Instead, when 2,2,6,6-tetramethyl-4-piperidinol (TEMP) was used as the trapping agent, a triplet peak signal (1:1:1, αN = 16.9 G, g = 2.0054) of 2,2,6,6-tetramethyl-4- piperidinol-N-oxyl radical (TMPN) emerged, indicating the presence of 1O2 (20, 21). Not surprisingly, it was not detected in both Fe2O3/FCNT-L/H2O2 and Fe2O3/H2O2 systems. More credible evidence was discovered based on the determination of indicative products from the specific reactions between classical chemical probe 9,10-diphenylanthracene (DPA) and 1O2 (22). As presented in Fig. 3B, the chromatographic peak of anthracene endoperoxide (DPAO2) appeared in Fe2O3@FCNT-H/H2O2 system and its concentration also increased when the dosage of Fe2O3@FCNT-H increased from 1.5 × 10−2 g∙L−1 to 2.5 × 10−2 g∙L−1, clearly showing the generation of 1O2.
Fig. 3.
Reactive intermediates identification. (A) ESR spectra of different systems using DMPO and TEMP as trapping agents. (B) UHPLC/MS chromatogram of the typical DPAO2 from the oxidation of DPA in Fe2O3@FCNT-H/H2O2 system. Reaction conditions: pH = 5.0, T = 293.2 K, [Fe2O3@FCNT-H] = 1.5 × 10−2 g∙L−1, [H2O2] = 50 mM, [DMPO] = [TEMP] = 50 mM for A, and [DPA] = 10 μM, [ACN] = 50 mM, reaction time = 60 min for B.
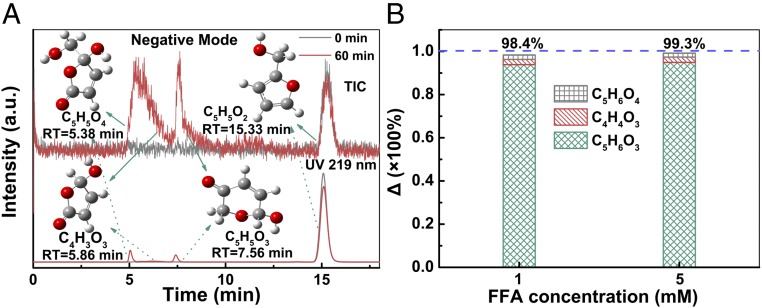
We further evaluated the role of 1O2 in Fe2O3@FCNT-H/H2O2 system by calculating the material balance of furfuryl alcohol (FFA) oxidation (see SI Appendix, Texts S1 and S2 for details). Consistent with the results from previous studies (23, 24), three typical products, C5H6O4 (m/z 129.0181 in negative ionization mode), C4H4O3 (m/z 99.0072 in negative ionization mode), and C5H6O3 (m/z 113.0228 in negative ionization mode) were detected at the retention time of 5.38, 5.86, and 7.56 min, respectively (see Fig. 4A and SI Appendix, Fig. S5 for details). Moreover, a >98% conversion to three classical products from the oxidation of FFA indicated the exclusive role of 1O2 as the reactive intermediate in Fe2O3@FCNT-H/H2O2 system (Fig. 4B). It is accepted that 1O2 could be generated via oxidation, disproportionation, and/or decomposition of peroxy-metal species in the H2O2-based systems catalyzed by halide ions, metal salts, and minerals (e.g., hypochlorite, molybdate, and lanthanum oxide) (25, 26). Nevertheless, the generation of 1O2 in the iron-based Fenton-like reaction was challenged in the bulk phase from both kinetic and thermodynamic points of view (25, 26). Our solid proof of the exclusive role of 1O2 in Fe2O3@FCNT-H/H2O2 system might suggest that the nanoconfinement changes the Fenton-like reaction pathway both kinetically and thermodynamically.
Fig. 4.
The mass balance of FFA oxidation. (A) UHPLC/MS chromatogram of the typical products from the oxidation of FFA in Fe2O3@FCNT-H/H2O2 system. Mass spectra of the products are available in SI Appendix, Fig. S5. (B) The mass balance for the oxidation of FFA in Fe2O3@FCNT-H/H2O2 system. Reaction conditions: pH = 5.0, T = 293.2 K, [Fe2O3@FCNT-H] = 1.5 × 10−2 g∙L−1, [H2O2] = 50 mM, [FFA] = 1/5 mM, [MeOH] = 50 mM, reaction time = 60 min.
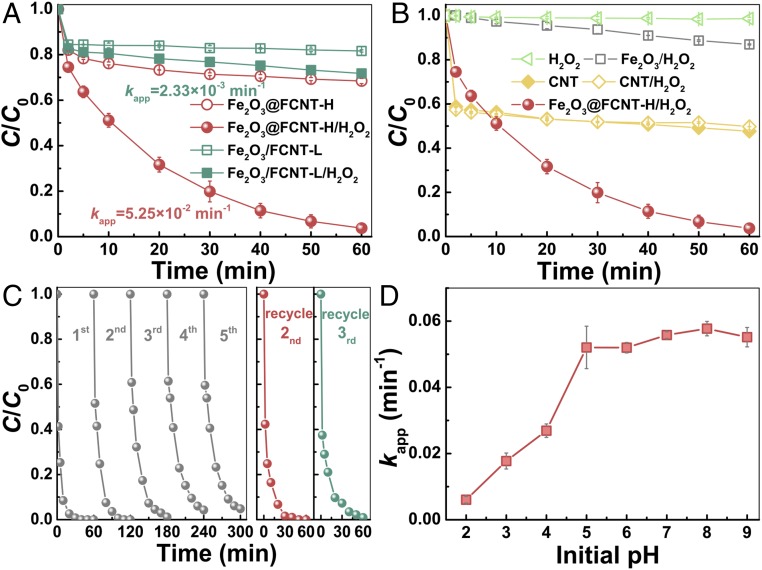
We are intrigued to investigate the catalytic performance of the nanoconfined Fenton’s catalyst. As presented in Fig. 5A, using MB as a model pollutant, one can see that in the absence of H2O2, both samples exhibited noticeable MB removal, presumably due to the adsorption of MB. However, in the presence of H2O2, Fe2O3@FCNT-H exhibited a remarkably higher MB degradation rate with the value of pseudo–first-order constant (kapp) = 5.25 × 10−2 min−1, which is 22.5 times higher than that of Fe2O3/FCNT-L (2.33 × 10−3 min−1) (SI Appendix, Fig. S6). Meanwhile, Fe2O3@FCNT-H/H2O2 is more effective than Fe2O3/FCNT-L/H2O2 in reducing the total organic carbon (25 vs. 15%) in 60 min (SI Appendix, Fig. S7). As shown in Fig. 5B, H2O2 alone has negligible effect on the MB removal even with the presence of Fe2O3; CNT/H2O2 has identical effect as CNT alone due to the adsorption effect (27). Samples of Fe2O3 nanoparticles loaded on various commercial substrates including carboxylated CNTs (CNT-COOH), SBA-15, and graphene oxide (GO) also exhibit significantly lower MB removal activities than Fe2O3@FCNT-H (SI Appendix, Fig. S8). All these results emphasize the key role of the interaction of the Fe2O3 nanoparticles inside the CNT for the surprisingly high MB removal activity of Fe2O3@FCNT-H.
Fig. 5.
Catalytic properties of Fe2O3@FCNT-H and Fe2O3/FCNT-L. (A) Plots of MB concentrations versus time of Fe2O3@FCNT-H and Fe2O3/FCNT-L with/without H2O2. (B) Plots of MB concentrations versus time of different systems. (C) Five consecutive MB removal experiments with two more regeneration studies of Fe2O3@FCNT-H. (D) The plot of kapp versus solution pH. Reaction conditions: T = 293.2 K, [MB] = 10 μM, [H2O2] = 50 mM, pH = 5.0 for A–C and 3.0–9.0 for D, [catalysts] (except Fe2O3) = 1.5 × 10−2 g∙L−1 for A, B, and D and 4.0 × 10−2 g∙L−1 for C, [Fe2O3] = 1.0 × 10−1 g∙L−1 in B.
The versatility of Fe2O3@FCNT-H under different conditions is of great general importance. The MB removal by the Fe2O3@FCNT-H/H2O2 system under different conditions is shown in SI Appendix, Fig. S9 with the accompanying discussion. The key information one could acquire is that the generation of 1O2, represented by the apparent MB degradation kinetics, can be effectively elevated by increasing catalyst dosage, H2O2 concentration, and reaction temperature.
We then investigated the reusability and pH stability of Fe2O3@FCNT-H. Fig. 5C shows a slight deactivation of Fe2O3@FCNT-H for five consecutive MB additions, presumably due to either the gradual occupation of active sites or the competitive reaction with 1O2 by the cumulative degradation products (28). However, the catalytic activity was fully recovered after washing with ethanol for later use without detectable ion leaching, suggesting excellent reusability of Fe2O3@FCNT-H. One can see from Fig. 5D that the value of kapp increases with the increase of pH value from 2.0 to 5.0, and unprecedentedly, remains stable as the pH value further increases to 9.0 (SI Appendix, Fig. S10). On the contrary, kapp of Fe2O3/FCNT-L/H2O2 system decreased as the pH value increased from 3.0 to 9.0 (SI Appendix, Fig. S11), which is a common phenomenon observed in traditional Fenton-like systems (29, 30). We discuss the effect of pH on MB removal of our Fe2O3@FCNT-H/H2O2 system in detail (SI Appendix, Part S1). Based on the results of SI Appendix, Figs. S12–S15, we reckon that pH plays a crucial role in the MB removal through affecting the 1O2 generation as well as the MB adsorption. The precise understanding for this unusual phenomenon still needs more elaborate future exploration; nevertheless, it presents a much more improved suitability in a broad pH range than the classical Fenton-like systems.
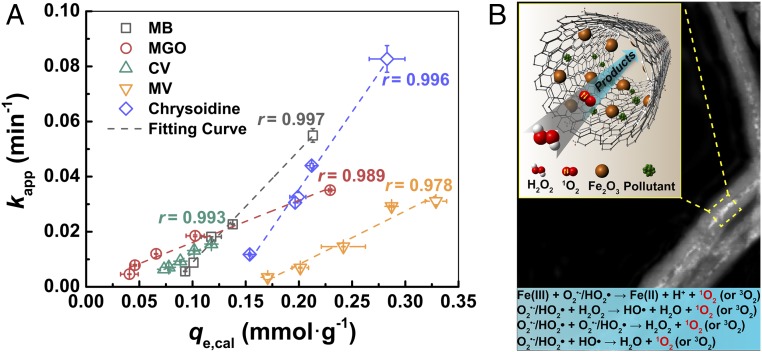
Considering 1O2 is not such a general oxidant as HO•, we list a number of both organic and inorganic species that have been studied previously in 1O2-mediated oxidation systems (SI Appendix, Table S1). In this work, the catalytic degradation of several different compounds [i.e., cationic ones including methyl violet, crystal violet, chrysoidine, and malachite green oxalate; anionic ones including methyl orange (MO) and chromotrope 2R (C2R) and neutral aniline] in Fe2O3@FCNT-H/H2O2 system was carried out to further demonstrate the versatility of Fe2O3@FCNT-H (SI Appendix, Fig. S16). We show that anionic compounds including MO and C2R and neutral aniline could not be effectively adsorbed by Fe2O3@FCNT-H; consequently, only a small fraction was degraded. Those cationic compounds which could be efficiently adsorbed were rapidly degraded. Fig. 6A shows the values of kapp versus qe,cal of Fe2O3@FCNT-H toward various cationic compounds. One can see that the value of kapp is linearly proportional to the value of qe,cal calculated from the pseudo–second-order adsorption kinetics (r > 0.97) (SI Appendix, Fig. S17). This result clearly shows an exotic adsorption-dependent removal feature of Fe2O3@FCNT-H, while the degradation kinetics is normally proportionally to the concentration of HO• and irrelevant to adsorption in traditional Fenton’s reactions (31). As a consequence, our nanoconfined Fenton’s reagent could be used to selectively degrade the compounds which are prone to be adsorbed (SI Appendix, Fig. S18). One might also be interested in the precise location where the pollutant is oxidized by the 1O2, i.e., on the outer surface of CNT or inside the CNT. Although we have our preferential guess of the latter based on the adsorption-dependent process (32–39), we do not have solid proof at this point. We would like to leave this question for further exploration.
Fig. 6.
The role of adsorption and possible mechanism scheme. (A) Plots of kapp versus qe,cal manipulated through the addition of Al3+ for Fe2O3@FCNT-H toward various cationic compounds. Reaction conditions: pH = 5.0, T = 293.2 K, [Fe2O3@FCNT-H] = 1.5 × 10−2 g∙L−1, [target compounds] = 10 μM, [H2O2] = 50 mM, [Al3+] = 0.1–1 mM. (B) Illustration of possible mechanism of pollutants degradation in Fe2O3@FCNT-H/H2O2 system.
Based on the previous results and discussion, a possible catalytic pathway of Fe2O3@FCNT-H/H2O2 system for pollutant degradation is illustrated as a scheme in Fig. 6B. Since both light and oxygen do not contribute to the process (SI Appendix, Fig. S19), we assert that H2O2 is the only source for 1O2 generation in Fe2O3@FCNT-H/H2O2 system, via the gain of two electrons by H2O2 to generate 1O2. There are three possible oxidation pathways of H2O2, a one-step two-electron transfer and two-step one-electron transfer with or without the contribution of high-valent iron-oxo species. The one-step two-electron transfer involving the reduction of Fe(III) to Fe(I) only exists in Fe-catalyzed cross-coupling reactions theoretically (40). The two-step one-electron transfer involving the oxidation of H2O2 by high-valent iron-oxo species depicted by Collins and coworkers could also be excluded because the pivotal intermediate could not be detected (see DMSO quenching experiment for the detection of high-valent iron-oxo species in SI Appendix, Fig. S13) (41). In contrast, the two-step one-electron transfer following the Haber–Weiss cycle is the most likely pathway for 1O2 generation. In the first step, the Fe(III) species on the surface of the Fe2O3 nanoparticles inside CNT is reduced by H2O2 to produce HO2•/O2·−, which can be further supported by the obvious shift to the lower region by 0.7 eV with the addition of H2O2 in in situ X-ray absorption near edge structure (XANES) analysis as depicted in SI Appendix, Fig. S20. The nanoconfinement by CNT poses strong electronic interaction with Fe2O3 nanoparticles due to the electron-deficient concave surface, facilitating this process compared with the Fe2O3 nanoparticles outside (42–44). The second step deals with several possibilities contributing to the oxidation of HO2•/O2·− to form 1O2, since we have demonstrated HO2•/O2·− to be the key intermediate for 1O2 generation (SI Appendix, Part S1 and Figs. S12–S14). (i) One is the oxidation by Fe(III) in the favored spin state, regulated by the confined space and the unique electronic structure of CNTs (25). (ii) The second is the radical–radical reactions including HO2•/O2·− recombination and the reaction between HO2•/O2·− and HO• (45–48). The Gibbs free energy for HO2•/O2·− recombination is −6.4 kcal⋅mol−1 at pH 7, indicating its thermodynamic spontaneity (45). However, we could not obtain even tiny evidence for the presence of HO• in Fe2O3@FCNT-H/H2O2 system by chemical quenching or trapping experiment (Fig. 3A and SI Appendix, Fig. S13), which is probably due to either the slow diffusion of quenching molecules or the accelerated radical recombination (49). Consequently, we do not exclude the possible contribution of HO• in the 1O2 generation but we reckon its contribution is small. (iii) The third is the reaction between HO2•/O2·− and H2O2 (50). The reaction constant is ∼3–10 magnitude smaller than those in (i) and (ii) (SI Appendix, Table S2); however, its contribution to the 1O2 generation might be compensated by significantly higher H2O2 concentration than radicals. These three possible reactions could be well accelerated via the enrichment as well as the limited migration of the as-formed radicals in the confined space (37, 48, 49). Another key role of the nanoconfinement in Fe2O3@FCNT-H/H2O2 system is suspected to largely improve the selectivity of these reactions to produce 1O2, rather than O2 in the traditional bulk systems (51).
In conclusion, by loading ∼2 nm Fe2O3 nanoparticles inside the confined space of CNT with inner diameter of ∼7 nm, we are able to create a Fenton-like catalyst Fe2O3@FCNT-H showing completely different catalytic pathway from traditional Fenton-like catalyst Fe2O3/FCNT-L, in which Fe2O3 nanoparticles are loaded on the outer surface of CNT. The 1O2 radical was observed as the main reactive intermediate generated in Fe2O3@FCNT-H/H2O2 system, other than HO• that was generated in Fe2O3/FCNT-L/H2O2 system and has been accepted as the main active species in the Fenton-like systems for many years. Meanwhile, a 22.5× faster MB degradation kinetics in Fe2O3@FCNT-H/H2O2 than Fe2O3/FCNT-L/H2O2 was obtained. Moreover, Fe2O3@FCNT-H exhibited surprisingly high stability over a broad pH range from 5.0 to 9.0, while it is almost impossible to utilize traditional Fenton-like catalyst under alkaline conditions. We also demonstrated that the oxidation rates of different pollutants in the Fe2O3@FCNT-H/H2O2 system are highly dependent on the adsorption affinity between the pollutants and the catalyst. Our work has presented a delicate design of Fenton’s catalyst using nanoconfinement for pollutant removal with exceptional activity and pH suitability, and also, has laid a milestone toward the mechanistic understanding of the Fenton-like reactions under nanoconfinement. We hope our work could stimulate the researchers to use nanoconfined Fenton’s reaction in various applications especially biological systems to realize selective and efficient oxidation.
Methods
The chemicals and materials used in the experiments are described in SI Appendix, Text S3.
Preparation of Catalysts.
The catalysts used in this work were synthesized according to a slightly modified method (42, 43). Pristine MWCNTs (pCNT) were first refluxed in concentrated HNO3 solution (65 wt %) at 403 ± 5 K under magnetic stirring for 14 h. After being filtered and washed with ultrapure water until the pH value of the filtrate reached ∼6.1, the solids were ultrasonically dispersed in the ultrapure water and lyophilized (Freezone 2.5; Labconco) for better dispersion of the particles (denoted as FCNT-H). Sample FCNT-L was prepared by refluxing pCNT in HNO3 solution (30 wt %) at 383 ± 5 K for 5 h. Sample of CNT-COOH was prepared by refluxing commercial sample in HCl solution (38 wt %) at 343 K for 12 h to remove residual metal impurities. Fe(NO3)3.9H2O (0.361 g) was dissolved in 250 mL acetone to serve as an iron precursor. Afterward, each of 0.20 g supporter (i.e., FCNT-H, FCNT-L, CNT-COOH, SBA-15, and GO) was dispersed in 20 mL Fe(III)/acetone solution, respectively. The mixed solution was first stirred for 1 h and then treated by ultrasonication for 4 h, while temperature was kept constant at 293.2 ± 0.3 K using a thermostat (THD-5015; Tianheng). As the slow evaporation of acetone, Fe(III) was deposited on the inner or outer surface of the host materials. The as-obtained solid was gradually heated to 413 K in air and kept for 10 h. After being washed with ultrapure water, we obtained samples of Fe2O3@FCNT-H, Fe2O3/FCNT-L, Fe2O3/CNT-COOH, Fe2O3/SBA-15, and Fe2O3/GO, respectively. The Fe loading of the catalysts was 2 wt % unless else stated. The unsupported iron oxides and FCNT-H for comparison were prepared using a similar method without adding FCNT-H or iron precursor, respectively. Catalysts were stored as the form of aqueous suspensions via direct ultrasonication for 20 min. To maintain homogeneity, the stock suspension was periodically treated by ultrasonication for 10 min before use.
Characterization of Catalysts.
The loaded Fe content was determined by an atomic absorption spectrophotometer (AA-7000; Shimadzu) after acidic digestion overnight using the concentrated HCl solution assisted by ultrasonic treatment in a sealed polytetrafluoroethylene (PTFE) bottle. The crystalline structures of the catalysts were determined by XRD (D-MAX Rapid-II; Rigaku) using Mo Kα radiation (λ = 0.7093 Å). For better analysis and comparison with the data from XRD in Cu Kα radiation, the obtained XRD spectra were transferred according to the Bragg equation (SI Appendix, Text S4). The analysis of surface properties was conducted using N2 adsorption–desorption test at 77 K (NOVA3000; Quantachrome). XPS (K-Alpha; Thermo) was used to determine the element chemical states near the surface region. The 57Fe Mössbauer spectra were recorded by proportional counter on Topologic 500 spectrometer (MFD-500AV) with 57Co (Rh) as a λ-ray source at room temperature. The morphologies were examined by TEM (TECNAI G2 F20; FEI) at an accelerating voltage of 200 kV. STEM was performed to characterize the dispersion and configuration of the catalysts. Individual heavy atoms can be discerned in the HAADF images. Moreover, STEM-EDX was conducted to map the abundance of C, O, and Fe in the catalysts. Raman spectra were recorded with a LabRAM Aramis Raman spectrometer (Horiba Scientific) with an Ar+ laser at 532 nm. In situ XANES analysis was carried out at room temperature in Beijing Synchrotron Radiation Facility with Fe foil and Fe2O3 as reference, K-edge energies of which were located at 7,112.1 and 7,126.6 eV, respectively.
Examination of Catalysts.
The degradation experiments were conducted in a 50-mL conical flask under magnetic stirring at 293.2 ± 0.3 K. Reactions were initiated by simultaneous addition of the catalyst suspension and H2O2 into the solution containing target dyes. Adsorption experiments were also conducted in a 50-mL conical flask on a rotary shaker at 150 rpm at 293.2 ± 0.3 K. The experiment was initiated after adding the catalyst suspension to the solutions of various target compounds with/without Al3+ ions (0.1–1 mM) at pH 5.0 unless specified. The pH values of solutions were adjusted by diluted HNO3 and NaOH. Reaction aliquots were periodically taken and filtered through a membrane (0.22 μm) to remove the solid catalysts for analysis. The detailed experimental conditions are presented in the legend of each figure. To test the stability and reusability of Fe2O3@FCNT-H, a 1.75 L reactor was used to avoid the loss of catalyst during recycle (see SI Appendix, Text S5 for details). The used catalysts were collected by suction filtration and simply washed with ethanol and ultrapure water.
The concentrations of organic dyes were analyzed at the maximum absorption wavelength by a UV-vis spectrophotometer (T6; Beijing Persee) (SI Appendix, Table S3). An ultrahigh-performance liquid chromatography (UHPLC, Ultimate 3000; Thermo) with a symmetry C18 column was used to analyze FFA, aniline, and 4-CP (SI Appendix, Table S4). TOC was measured by subtracting the concentration of inorganic carbon from the total carbon on a Shimadzu TOC-VCPH analyzer (Japan) and the quantification was based on a standard calibration in the range of 0–3 mg∙L−1. The concentration of leached iron species was determined through 1,10-phenanthroline method after reduction by hydroxylamine hydrochloride with the limit of detection of 0.36 μM. ESR spectra of DMPO-HO•, DMPO-OOH, and TMPN were obtained with an ESR A300 spectrometer (Bruker). The detailed operation methods are presented in SI Appendix, Text S6. A Thermo U3000 ultrahigh performance liquid chromatography coupled with a Thermo Q-Exactive Focus MS with an electron spray ionization source was employed to identify the intermediate products from DPA oxidation and FFA oxidation (see SI Appendix, Text S1 for details). The concentration of products from FFA oxidation was calculated as described in SI Appendix, Text S2.
Supplementary Material
Acknowledgments
The authors are grateful to Beijing Synchrotron Radiation Facility for XANES analysis and the High Performance Computing Center of Nanjing University for doing the numerical calculations in this paper on its blade cluster system. We thank Prof. Xiulian Pan, Dalian Institute of Chemical Physics, for the advice on catalyst preparation and Prof. Peng Wang, College of Engineering and Applied Sciences, Nanjing University, for the help in TEM and STEM-EDX characterization. Dr. Chao Shan provided insightful discussion during the revision. This study was financially supported by Natural Science Foundation of China (Grant 21177059/51761165011), National Key R&D Program of China (Grant 2016YFA0203104), and Natural Science Foundation of Jiangsu Province (Grant BK20160653).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819382116/-/DCSupplemental.
References
- 1.Fenton HJH. Oxidation of tartaric acid in presence of iron. J Chem Soc Trans. 1894;65:899–910. [Google Scholar]
- 2.Liu Q, et al. A Fenton reaction at the endoplasmic reticulum is involved in the redox control of hypoxia-inducible gene expression. Proc Natl Acad Sci USA. 2004;101:4302–4307. doi: 10.1073/pnas.0400265101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JW, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006;440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- 4.Shelor CP, et al. Fenton digestion of milk for iodinalysis. Anal Chem. 2011;83:8300–8307. doi: 10.1021/ac202165e. [DOI] [PubMed] [Google Scholar]
- 5.Chen MS, White MC. A predictably selective aliphatic C-H oxidation reaction for complex molecule synthesis. Science. 2007;318:783–787. doi: 10.1126/science.1148597. [DOI] [PubMed] [Google Scholar]
- 6.Feng G, et al. Accelerated crystallization of zeolites via hydroxyl free radicals. Science. 2016;351:1188–1191. doi: 10.1126/science.aaf1559. [DOI] [PubMed] [Google Scholar]
- 7.Brillas E, Sirés I, Oturan MA. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev. 2009;109:6570–6631. doi: 10.1021/cr900136g. [DOI] [PubMed] [Google Scholar]
- 8.Walling C. Fenton’s reagent revisited. Acc Chem Res. 1975;8:125–131. [Google Scholar]
- 9.Pignatello JJ, Oliveros E, MacKay A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol. 2006;36:1–84. [Google Scholar]
- 10.Enami S, Sakamoto Y, Colussi AJ. Fenton chemistry at aqueous interfaces. Proc Natl Acad Sci USA. 2014;111:623–628. doi: 10.1073/pnas.1314885111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bossmann SH, et al. New evidence against hydroxyl radicals as reactive intermediates in the thermal and photochemically enhanced Fenton reactions. J Phys Chem A. 1998;102:5542–5550. [Google Scholar]
- 12.Miners SA, Rance GA, Khlobystov AN. Chemical reactions confined within carbon nanotubes. Chem Soc Rev. 2016;45:4727–4746. doi: 10.1039/c6cs00090h. [DOI] [PubMed] [Google Scholar]
- 13.Hummer G, Rasaiah JC, Noworyta JP. Water conduction through the hydrophobic channel of a carbon nanotube. Nature. 2001;414:188–190. doi: 10.1038/35102535. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Pan X, Zhang S, Han X, Bao X. Diffusion of water inside carbon nanotubes studied by pulsed field gradient NMR spectroscopy. Langmuir. 2014;30:8036–8045. doi: 10.1021/la500913r. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Wu G, Sha M, Huang S. Transition of ionic liquid [bmim][PF6] from liquid to high-melting-point crystal when confined in multiwalled carbon nanotubes. J Am Chem Soc. 2007;129:2416–2417. doi: 10.1021/ja067972c. [DOI] [PubMed] [Google Scholar]
- 16.Pan X, et al. Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles. Nat Mater. 2007;6:507–511. doi: 10.1038/nmat1916. [DOI] [PubMed] [Google Scholar]
- 17.Zboril R, Mashlan M, Petridis D. Iron(III) oxides from thermal processes-synthesis, structural and magnetic properties, Mössbauer spectroscopy characterization, and applications. Chem Mater. 2002;14:969–982. [Google Scholar]
- 18.Fierro G, Moretti G, Ferraris G, Andreozzi GB. A Mössbauer and structural investigation of Fe-ZSM-5 catalysts: Influence of Fe oxide nanoparticles size on the catalytic behaviour for the NO-SCR by C3H8. Appl Catal B. 2011;102:215–223. [Google Scholar]
- 19.Chen W, Pan X, Bao X. Tuning of redox properties of iron and iron oxides via encapsulation within carbon nanotubes. J Am Chem Soc. 2007;129:7421–7426. doi: 10.1021/ja0713072. [DOI] [PubMed] [Google Scholar]
- 20.Moan J, Wold E. Detection of singlet oxygen production by ESR. Nature. 1979;279:450–451. doi: 10.1038/279450a0. [DOI] [PubMed] [Google Scholar]
- 21.Song B, Wang G, Tan M, Yuan J. A europium(III) complex as an efficient singlet oxygen luminescence probe. J Am Chem Soc. 2006;128:13442–13450. doi: 10.1021/ja062990f. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto S, Martinez GR, Martins APB, Medeiros MHG, Di Mascio P. Direct evidence of singlet molecular oxygen [O2 (1Δg)] production in the reaction of linoleic acid hydroperoxide with peroxynitrite. J Am Chem Soc. 2003;125:4510–4517. doi: 10.1021/ja029262m. [DOI] [PubMed] [Google Scholar]
- 23.Braun AM, et al. (2+4)‐Cycloaddition with singlet oxygen. 17O‐investigation of the reactivity of furfuryl alcohol endoperoxide. Photochem Photobiol. 1999;70:868–874. [Google Scholar]
- 24.Halladja S, Ter Halle A, Aguer JP, Boulkamh A, Richard C. Inhibition of humic substances mediated photooxygenation of furfuryl alcohol by 2,4,6-trimethylphenol. Evidence for reactivity of the phenol with humic triplet excited states. Environ Sci Technol. 2007;41:6066–6073. doi: 10.1021/es070656t. [DOI] [PubMed] [Google Scholar]
- 25.Evans DF, Upton MW. Studies on singlet oxygen in aqueous solution. Part 4. The ‘spontaneous’ and catalysed decomposition of hydrogen peroxide. J Chem Soc Dalton Trans. 1985;12:2525–2529. [Google Scholar]
- 26.Aubry JM. Search for singlet oxygen in the decomposition of hydrogen peroxide by mineral compounds in aqueous solutions. J Am Chem Soc. 1986;17:5844–5849. [Google Scholar]
- 27.Pignatello JJ, Mitch WA, Xu W. Activity and reactivity of pyrogenic carbonaceous matter toward organic compounds. Environ Sci Technol. 2017;51:8893–8908. doi: 10.1021/acs.est.7b01088. [DOI] [PubMed] [Google Scholar]
- 28.Snyder BER, et al. Mechanism of selective benzene hydroxylation catalyzed by iron-containing zeolites. Proc Natl Acad Sci USA. 2018;115:12124–12129. doi: 10.1073/pnas.1813849115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Xu X, Xu J, Han Y. Iron oxychloride (FeOCl): An efficient fenton-like catalyst for producing hydroxyl radicals in degradation of organic contaminants. J Am Chem Soc. 2013;135:16058–16061. doi: 10.1021/ja409130c. [DOI] [PubMed] [Google Scholar]
- 30.Pouran SR, Raman AAA, Wan MAWD. Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J Clean Prod. 2014;64:24–35. [Google Scholar]
- 31.Navalon S, Dhakshinamoorthy A, Alvaro M, Garcia H. Heterogeneous fenton catalysts based on activated carbon and related materials. ChemSusChem. 2011;4:1712–1730. doi: 10.1002/cssc.201100216. [DOI] [PubMed] [Google Scholar]
- 32.Dujardin E, Ebbesen TW, Hiura H, Tanigaki K. Capillarity and wetting of carbon nanotubes. Science. 1994;265:1850–1852. doi: 10.1126/science.265.5180.1850. [DOI] [PubMed] [Google Scholar]
- 33.Yang H, Zhang L, Zhong L, Yang Q, Li C. Enhanced cooperative activation effect in the hydrolytic kinetic resolution of epoxides on [Co(salen)] catalysts confined in nanocages. Angew Chem Int Ed Engl. 2007;46:6861–6865. doi: 10.1002/anie.200701747. [DOI] [PubMed] [Google Scholar]
- 34.Lan Y, Yang L, Zhang M, Zhang W, Wang S. Microreactor of Pd nanoparticles immobilized hollow microspheres for catalytic hydrodechlorination of chlorophenols in water. ACS Appl Mater Interfaces. 2010;2:127–133. doi: 10.1021/am900622p. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Guan Z, Li M, Yang Q, Li C. Enhancement of the performance of a platinum nanocatalyst confined within carbon nanotubes for asymmetric hydrogenation. Angew Chem Int Ed Engl. 2011;50:4913–4917. doi: 10.1002/anie.201006870. [DOI] [PubMed] [Google Scholar]
- 36.Pan X, Bao X. The effects of confinement inside carbon nanotubes on catalysis. Acc Chem Res. 2011;44:553–562. doi: 10.1021/ar100160t. [DOI] [PubMed] [Google Scholar]
- 37.Li B, et al. Hydration of epoxides on [CoIII(salen)] encapsulated in silica-based nanoreactors. Angew Chem Int Ed. 2012;124:11685–11689. doi: 10.1002/anie.201203774. [DOI] [PubMed] [Google Scholar]
- 38.Liu C, et al. Yolk-shell Fe0@SiO2 nanoparticles as nanoreactors for fenton-like catalytic reaction. ACS Appl Mater Interfaces. 2014;6:13167–13173. doi: 10.1021/am503063m. [DOI] [PubMed] [Google Scholar]
- 39.Zeng T, Zhang X, Wang S, Niu H, Cai Y. Spatial confinement of a Co3O4 catalyst in hollow metal-organic frameworks as a nanoreactor for improved degradation of organic pollutants. Environ Sci Technol. 2015;49:2350–2357. doi: 10.1021/es505014z. [DOI] [PubMed] [Google Scholar]
- 40.Hu L, Chen H. Substrate-dependent two-state reactivity in iron-catalyzed alkene [2+2] cycloaddition reactions. J Am Chem Soc. 2017;139:15564–15567. doi: 10.1021/jacs.7b06086. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh A, et al. Catalase-peroxidase activity of iron(III)-TAML activators of hydrogen peroxide. J Am Chem Soc. 2008;130:15116–15126. doi: 10.1021/ja8043689. [DOI] [PubMed] [Google Scholar]
- 42.Chen W, Pan X, Willinger MG, Su DS, Bao X. Facile autoreduction of iron oxide/carbon nanotube encapsulates. J Am Chem Soc. 2006;128:3136–3137. doi: 10.1021/ja056721l. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Fan Z, Pan X, Bao X. Effect of confinement in carbon nanotubes on the activity of Fischer-Tropsch iron catalyst. J Am Chem Soc. 2008;130:9414–9419. doi: 10.1021/ja8008192. [DOI] [PubMed] [Google Scholar]
- 44.Chen W, et al. Enhanced visible-light activity of titania via confinement inside carbon nanotubes. J Am Chem Soc. 2011;133:14896–14899. doi: 10.1021/ja205997x. [DOI] [PubMed] [Google Scholar]
- 45.Koppenol WH. Reactions involving singlet oxygen and the superoxide anion. Nature. 1976;262:420–421. doi: 10.1038/262420a0. [DOI] [PubMed] [Google Scholar]
- 46.Khan AU, Kasha M. Singlet molecular oxygen in the Haber-Weiss reaction. Proc Natl Acad Sci USA. 1994;91:12365–12367. doi: 10.1073/pnas.91.26.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivanova IP, et al. Mechanism of chemiluminescence in Fenton reaction. J Biophys Chem. 2012;3:88–100. [Google Scholar]
- 48.Dou X, et al. Production of superoxide anion radicals as evidence for carbon nanodots acting as electron donors by the chemiluminescence method. Chem Commun. 2013;49:5871–5873. doi: 10.1039/c3cc41145a. [DOI] [PubMed] [Google Scholar]
- 49.Foley S, et al. Radiolysis of confined water: Production and reactivity of hydroxyl radicals. Angew Chem Int Ed Engl. 2004;44:110–112. doi: 10.1002/anie.200460284. [DOI] [PubMed] [Google Scholar]
- 50.MacManus-Spencer LA, McNeill K. Quantification of singlet oxygen production in the reaction of superoxide with hydrogen peroxide using a selective chemiluminescent probe. J Am Chem Soc. 2005;127:8954–8955. doi: 10.1021/ja052045b. [DOI] [PubMed] [Google Scholar]
- 51.Turro NJ. From boiling stones to smart crystals: Supramolecular and magnetic isotope control of radical-radical reactions in zeolites. Acc Chem Res. 2000;33:637–646. doi: 10.1021/ar980103a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.