Abstract
Shigella-infected bacillary dysentery or commonly known as Shigellosis is a leading cause of morbidity and mortality worldwide. The gradual emergence of multidrug resistant Shigella spp. has triggered the search for alternatives to conventional antibiotics. Phage therapy could be one such suitable alternative, given its proven long term safety profile as well as the rapid expansion of phage therapy research. To be successful, phage therapy will need an adequate regulatory framework, effective strategies, the proper selection of appropriate phages, early solutions to overcome phage therapy limitations, the implementation of safety protocols, and finally improved public awareness. To achieve all these criteria and successfully apply phage therapy against multidrug resistant shigellosis, a comprehensive study is required. In fact, a variety of phage-based approaches and products including single phages, phage cocktails, mutated phages, genetically engineered phages, and combinations of phages with antibiotics have already been carried out to test the applications of phage therapy against multidrug resistant Shigella. This review provides a broad survey of phage treatments from past to present, focusing on the history, applications, limitations and effective solutions related to, as well as the prospects for, the use of phage therapy against multidrug resistant Shigella spp. and other multidrug resistant bacterial pathogens.
Keywords: Multidrug-resistant, Phage therapy, Bacillary dysentery, Shigella
Introduction
Shigella is one of the key pathogens responsible for the diarrhoeal disease generally known as bacillary dysentery and more specifically as shigellosis. Until recently, about 55 serotypes belonging to four species of Shigella (Shigella dysenteriae, Shigella boydii, Shigella flexneri and Shigella sonnei) have been identified as being responsible for shigellosis and death worldwide (The et al., 2016). Shigella is transmitted efficiently in low doses through direct or indirect human faecal contamination due to poor hygienic conditions (Weissman et al., 1975). Different food products like salads, soft cheese, vegetables and meat products are usually associated with this type of outbreak. Other indirect routes of infections including ingestion of Shigella-contaminated food and water, contact via fomites (such as drinking devices, eating utensil and other inanimated objects) and certain modes of sexual intercourse (Morgan et al., 2006; Okame et al., 2012). Different housefly-like vectors which can physically transport infected feaces, are also known to play a vital role in spreading the disease. Antibiotics have so far been the most common therapeutic agents against dysentery. However, the gradual emergence of drug resistant Shigella has caused growing concern of the long-term efficiency of antibiotics. The drug resistant characteristics of Shigella have been reported since 1940s, and have led to the increasing emergence of multidrug resistant strains over the past few decades. The development of new antibiotics to combat these new strains is time consuming, laborious and costly. Moreover, no effective vaccine is available to prevent shigellosis, which is thus a serious global medical and social problem (Arias & Murray, 2009; Deris et al., 2013; Magiorakos et al., 2012; WHO, 2014).
Increasing antibiotic resistance and the lack of new agents in development make imperative the development of alternative or complementary approaches to antibiotics for treating common bacterial pathogens, including Shigella. Currently, phage therapy appears to be a viable option. This therapy has already been utilized in some Eastern European countries with good safety records without significant side effects (Kutter, 2009). For example, phage therapy was successfully used to treat Russian soldiers during and after the World War II (Kutter et al., 2010). Slopek et al. (1987) also reported a 91–100% success rate for cases of phage treatment in Poland against diseases of the digestive system and alimentary tract. In addition, a study in the USA used phages to treat typhoid patients by intravenously injecting phages to modulate immune responses (Desranleau, 1949). No serious side effects were reported. A clinical trial project named ‘Phagoburn’ focused on the efficacy of phage therapy for treating burn wounds patients infected by Pseudomonas aeruginosa and Escherichia coli. It was the first potential multi-centre, randomised, single blind and well organized clinical trial of phage therapy in the world (Pherecydes Pharma, 2015). This clinical trial was soundly based on Good Manufacturing and Good Clinical Practices and the results gained through the clinical trial indicated significant developments regarding the regulatory framework of phage therapy (Pherecydes Pharma, 2015). Recently, a novel phage therapy has successfully treated patients with multidrug-resistant Acinetobacter baumannii infections. The treatment was jointly conducted by the University of California San Diego, School of Medicine, the U.S. Navy Medical Research Center and the Texas A&M University (LaFee & Buschman, 2017).
Phage therapy was originally introduced a century ago, almost 10 years earlier than antibiotic therapy. It has however never outperformed the latter due to several reasons, including the often confusing and inconsistent results of phage therapy trials, the lack of reproducibility, problems in ensuring the administration of appropriate doses, and the limited availability of genetic information on phages (Wittebole, De Roock & Opal, 2014). As a result, many researchers, especially from the USA and Western Europe, gradually lost interest in phage therapy research.
Fortunately, some researchers in Eastern Europe continued to study phage therapy, and performed a significant number of phage therapy trials and treatments (Smith & Huggins, 1983). Although the issue on phage resistant bacteria is a concern, this issue should not be a major concern especially when it is compared with the bacterial resistance to antibiotic. The main reason is because the growth of phages are shadowing bacterial growth, thus they mutate at the same rate as bacteria. Furthermore, with huge number of available phages, there will be certainly another phage or phages that are able to invade the resistant, mutated bacteria (Inal, 2003). Over the years, the advancement of knowledge and technology on phage therapy in Eastern Europe has become the beacon of new hope for exploring the application of phage therapy against multidrug resistant bacteria. The Eliava Institute in Tibilissi, Georgia and the Hirsfield Institute in Wroclaw, Poland are among well-known medical research institutes for phage-based therapeutics.
This review surveys the extent of outbreaks of shigellosis and their effects, and then investigates the treatments of Shigella spp. using both antibiotic and phage therapy. It contains a chronological description of the emergence of Shigella spp. as a multidrug resistant pathogen, as well as outlining the limitations of antibiotics against multidrug resistant bacterial strains. It goes on to discuss the problems and limitations of phage therapy from the past to the present, together with the recent developments of this therapy as an alternative to antibiotic treatment. It highlights some potential solutions and future directions for the use of phage therapy against drug resistant bacterial pathogens, especially Shigella spp. Finally, this review explains why scientists and policymakers should revisit phage therapy, in a positive and progressive manner, in order to find effective cures for drug resistant bacteria.
Survey methodology
In order to provide a clear picture to readers, we performed a comprehensive literature study covering Shigella and shigellosis; multidrug-resistant bacterial pathogens and the related emerging challenges with antibiotic treatments and the development of new antibiotics; and finally the use of bacteriophages and phage therapy against Shigella and Shigella-like microorganisms. “PubMed”, “Scopus” and “Google” search engines were used to search for journal articles using specific key words: Shigella, Shigellosis and phage therapy. Our study describes clearly why and how phage therapy can be a viable alternative or complementary treatment to antibiotics, in particular against Shigella and Shigella-like organisms. To ensure that our review was comprehensive, logically organized and balanced, we reviewed in chronological order a very broad range of relevant articles published from the time of the discovery of Shigella in 1896 up to the present (2018).
Shigella, Shigellosis and outbreaks
Shigellosis caused by Shigella is endemic, and is one of the main causes of mortality and morbidity in all age groups in both developing and developed countries. It is particularly prevalent in children between 0 and 5 years in developing countries (Bardhan et al., 2010; Wen et al., 2012). Shigellae are Gram-negative, nonmotile, rod-shaped facultative anaerobic and non-spore-forming bacteria. Shigella was first discovered by a Japanese scientist, Kiyoshi Shiga, in 1897 (Shiga, 1898; Trofa et al., 1999). The Shigella spp. discovered by him was Shigella dysenteriae. The Shigella genus was soon expanded with the discovery of Shigella flexneri in 1899 (Flexner, 1900), followed by Shigella sonnei in 1906 and Shigella boydii in 1921 (Barceloux, 2008; Shiga, 1936). These four species of Shigellae are further subdivided into different serotypes, based on their type-specific antigens (15 for S. dysenteriae; 19 for S. flexneri; 20 for S. boydii, and 1 serotype for S. sonnei) (The et al., 2016).
Currently, S. flexneri is the main cause of bacillary dysentery in countries with low-income economies, particularly in sub-Saharan Africa and Asia, accounting for almost two third of all Shigella infections in these areas. On the other hand, S. sonnei is the most common pathogen in high-income or transitional countries, especially in North America and Europe, accounting for up to 80% of all Shigella infections in this zone (Gu et al., 2012a). Previously, a multicenter study on Shigellosis conducted in six Asian countries (Pakistan, China, Bangladesh, Vietnam, Thailand and Indonesia) reported S. flexneri as the most frequent isolated Shigella spp. (68%), except in Thailand (Von Seidlein et al., 2006).
In contrast, shigellosis caused by the species S. dysenteriae and S. boydii has in recent years been reported in less than 5% cases globally. Interestingly, S. dysenteriae was the main cause of dysentery more than 100 years ago, but the incidence of this pathogen is now quite rare (Bardhan et al., 2010; Gu et al., 2012a). In the late 19th and early 20th centuries, S. dysenteriae caused numerous outbreaks. It then disappeared for unknown reasons, although S. dysenteriae type 1 reappeared as an epidemic in 1968 in Central America, Asia and Africa (Gangarosa et al., 1970; Pal, 1984; Rahaman et al., 1975; Ries et al., 1994). Later, the prevalence of S. dysenteriae was replaced by S. flexneri, which in turn was gradually replaced by S. sonnei (Kostrzewski, 1968; Martin, Pollard & Feldman, 1983). Occurrences of S. boydii have meanwhile been reported on the Indian subcontinent and Latin America, but have been infrequent in other regions of the world (Fernandez-Prada et al., 2004; Niyogi, 2005; Rolfo et al., 2011).
Outbreaks of Shigella are common, and have been reported widely. For instance, a serious outbreak occurred between 2014 and 2015 in California, with the causative agent being Shigella sonnei (Kozyreva et al., 2016). At the same time, the frequency of occurrence and severity of shigellosis outbreaks varied greatly between different regions and countries. In Morobe Province on the northern coast of Papua New Guinea, approximately 1,200 cases and five deaths were reported as shigellosis caused by the S. flexneri serotype 2 (Benny et al., 2014), while fifty-five cases of shigellosis were reported in Taiwan caused by S. flexneri 2a, S. sonnei and S. flexneri 3b (Ko et al., 2013). In Bangladesh, a total of 10,827 isolates were identified between 2001 and 2011, with the predominant spp. detected being S. flexneri, followed by S. sonnei, S. boydii and S. dysenteriae, respectively (Ud-Din et al., 2013). In Sichuan Province (China), about 96 students in a rural elementary school suffered from shigellosis after drinking untreated well water; the causal organism identified in this case was S. flexneri 2b (He et al., 2012). In another outbreak in Parison city (Iran), 701 inmates experienced severe diarrhea caused by S. flexneri serotype 3a (Hosseini & Kaffashian, 2010). Two outbreaks were reported in Sweden in 2009, caused by S. dysenteriae (Löfdahl et al., 2009) and S. sonnei, that affected air travelers departing from Hawaii (Gaynor et al., 2009). In Austria, a foodborne outbreak of shigellosis was caused by S. sonnei (Kuo et al., 2009), and Shigella spp. (Müller et al., 2009) was reported in Denmark.
The above examples show that outbreaks of shigellosis caused by Shigella have been occurring frequently all over the world, from developing to developed countries, with the predominant causative spp. being S. flexneri and S. sonnei.
Multidrug resistant Shigella spp.
Multidrug resistant bacterial pathogens impose critical challenge for clinical and pharmaceutical research due to their potentially severe impact on human health. The Infectious Disease Society of America (IDSA) is extremely concerned about the worrying growth in microbial pathogens and antibiotic resistance in the USA and elsewhere in the world (Spellberg et al., 2008). This antibiotic resistance is caused by both bacterial and social factors, such as high mutation frequencies coupled with the exchange of genetic information by bacteria; the misuse or overuse of antibiotics by human beings; and increasing population densities and global migratory movements by animals and people (Huijbers et al., 2015; Liu et al., 2016).
The acquisition of antibiotic resistance in bacteria is due to genetic exchanges via horizontal gene transfer involving three mechanisms (i.e., random transformation, transduction and conjugation). Uptake of small fragments of DNA by bacteria occurs during transformation, while transduction encompasses transfer of DNA (via bacteriophages) from one bacterium into another, and conjugation involves transfer of DNA through sexual pili involving cell-to-cell contact. The newly acquired recipients which were susceptible previously can express resistance due to the resistant genes acquired from the resistant donor (Frost et al., 2005; Oliveira et al., 2017). Moreover, the presence of R factors (plasmids) may play a major role in developing new serotypes which can foster antibacterial resistance (Tanaka et al., 1969). From the beginning of the antibiotic era, tetracycline, ampicillin, chloramphenicol, nalidixic acid and trimethoprim-sulfamethoxazole were used to treat Shigellosis. As Shigella developed increasing resistance to these agents, more recently ciprofloxacin, ceftriaxone and azithromycin have served as the mainstays of shigellosis treatments. However, the growing resistances of Shigella spp. against these antibiotics have also been studied and reported (Table 1) (Klontz & Singh, 2015).
Table 1. First use of antibiotics for Shigella treatment and initial reporting of resistance.
| Name of drug | Beginning period | Place and initial report of resistance | References |
|---|---|---|---|
| Sulfonamide | 1930s | Philippine Islands (1946) Japan (1952–1957) Israel (1953–1955) USA (1961–1964) |
Cheever (1946), Haltalin & Nelson (1965), Marberg, Altmann & Eshkol-Bruck (1958), Mitsuhashi (1969), Mitsuhashi (1971) and Mitsuhashi et al. (1960) |
| Ampicillin | Late 1960s–1970s | New Zealand (1974) Bangladesh (1974) Mexico city (1976) |
Olarte, Filloy & Galindo (1976), Rahaman et al. (1974) and Smith, Bremner & Datta (1974) |
| Rimethoprim– sulfamethoxazole | 1970s | Brazil (1980) Canada (1980) Korea (1981) India (1981) Finland (1975–1982) Bangladesh (1979–1983) |
Chun, Seol & Suh (1981), Finlayson (1980), Heikkilä et al. (1990), Macaden & Bhat (1985), Taylor, Keystone & Devlin (1980) and Zaman et al. (1983) |
| Furazolidone | 1970s | Dallas, USA (1972) India (1984) |
Bose et al. (1984) and Lexomboon et al. (1972) |
| Nalidixic acid | 1980s | Zaire (1982) India (1984) Bangladesh (1986) Burundi (1990) |
Bhardwaj & Panhotra (1985), Munshi et al. (1987), Ries et al. (1994) and Rogerie et al. (1986) |
| Pivmecillinam | 1970s | Bangladesh (2000–2012) | Klontz et al. (2014) |
| Fluoroquinolone | Late 1980s–1990s | India (1984) | Bose et al. (1984) |
| Azithromycin | 1990s–2000s | India (2006–2011) Netherlands (2012) |
Bhattacharya et al. (2014) and Hassing et al. (2014) |
| Ceftriaxone | 1990s–2000s | Korea (2000) Vietnam (2000–2002) India (2006–2011) USA (2003–2012) |
Bhattacharya et al. (2014), Pai et al. (2001) and Vinh et al. (2009) |
There have been numerous reports of single drug resistance, cross-resistance and multidrug resistance in Shigella worldwide, and such cases are growing in both frequency and diversity on a daily basis. In a study, approximately 1,376 Shigella isolates were collected from the Foodborne Diseases Active Surveillance Network (FoodNet) and were tested in the US National Antimicrobial Resistance Monitoring System (NARMS) between 2000 and 2010 (Shiferaw et al., 2012). Among the tested isolates, 74% proved to be ampicillin resistant, followed by 58% that were streptomycin resistant, 36% trimethoprim-sulfamethoxazole (TMP-SMX) resistant, 32% sulfamethoxazole-sulfisoxazole resistant, 28% tetracycline resistant, 2% nalidixic acid resistant, and 0.5% ciprofloxacin resistant. Moreover, around 5% of these strains showed multiple resistances to ampicillin, streptomycin, chloramphenicol, tetracycline and sulfamethoxazole-sulfisoxazole (Shiferaw et al., 2012).
In 2002, S. dysenteriae type 1 isolates were identified in Eastern India that showed resistance to all available antibiotics, including norfloxacin and ciprofloxacin but with the exception of ofloxacin (Sur, Niyogi & Sur, 2003). In the following year, similar types of isolates were detected in Bangladesh that were resistant to all common antibiotics, including ofloxacin (Naheed et al., 2004). In addition, about 200 S. sonnei isolates were identified in Bangladesh that demonstrated a wide range of resistance against frequently used antibiotics, such as ampicillin, mecillinam , ciprofloxacin, nalidixic acid and trimethoprim-sulfamethoxazole, at ratios of 9.5, 10.5, 17, 86.5, and 89.5%, respectively (Ud-Din et al., 2013). More recently, a study in Iran reported high frequency of resistance against trimethoprim/sufamethoxazole, ampicillin, cefotaxime and nalidixic acid (80, 85, 63 and 47%, respectively), in 85 Shigella spp isolated from 211 positive stool cultures of children with gastroenteritis (Mahmoudi et al., 2017).
In the annual report of the National Salmonella, Shigella & Listeria Reference Laboratory (NSSLRL-2014, https://www.researchgate.net/publication/280804929), 93% of the 45 Shigella isolates listed were identified as multi-drug resistant (Delappe, King & Cormican, 2014). The degree and prevalence of resistance to azithromycin, fluoroquinolones and ceftriaxone do vary considerably between different regions of the world (Bhattacharya et al., 2014). In particular, one study demonstrated that Shigella exhibited far higher levels of resistance to nalidixic acid and ciprofloxacin in Asia-Africa than those in Europe-America: 33.6% and 5.0% respectively, or 10.5 and 16.7 times higher (Gu et al., 2012a).
In summary, it is extremely difficult to delimit the geographic range of drug resistant strains of Shigella or to control the disease through a single antibiotic, because of the dissemination of resistant pathogens through multiple vectors and the continuous emergence of new serotypes.
Medical treatments for Shigellosis
A number of treatments of bacillary dysentery are commonly used. The World Health Organization (WHO) recommends the use of oral rehydration therapy, together with zinc supplements, for 10–14 days. The administration of zinc during shigellosis reduces the duration and frequency of expelling loose stools (Nichter, Acuin & Vargas, 2008; UNICEF & World Health Organization, 2006). The WHO also suggests the use of effective antimicrobials against clinically suspected shigellosis (Christopher et al., 2009). In practice, beta-lactams (amoxicillin, ampicillin, ceftriaxone, cefixime, and pivmecillinam), quinolones (nalidixic acid, ciprofloxacin, norfloxacin, and ofloxacin), macrolides (erythromycin and azithromycin) and other antibiotics (sulfonamides, tetracycline, furazolidone, and cotrimoxazole) are commonly used to treat Shigella dysentery. This development, together with the unavailability of the Food and Drug Administration (FDA) approved vaccines, have led researchers to seek alternative treatments against drug resistant bacterial pathogens (Katz et al., 2004; Wu et al., 2011). Administration of antimicrobial peptides and antibiotic cocktails are promising replacements, however, these alternatives may eventually suffer a similar fate as the current treatment (Worthington & Melander, 2013). Conversely, bacteriophages have potentials to be used as an alternative to antibiotics, because phages have different modes of action and they could be rapidly ‘trained’ on ancestral bacterial strains via successive passages, as well as their capability to defeat bacterial resistance by evolving in situ mutations (Betts et al., 2013).
Hence, phage therapy could be the best option for treating shigellosis, because it has been shown to work against Shigella spp. Phage treatment also has the additional advantage of causing less disruption to gut flora than antibiotic treatment (Kutter et al., 2010). Moreover, experimental anti-dysentery trials using phages have been successfully conducted over several decades in Eastern Europe (Kutter, 2009).
Early history of phage therapy
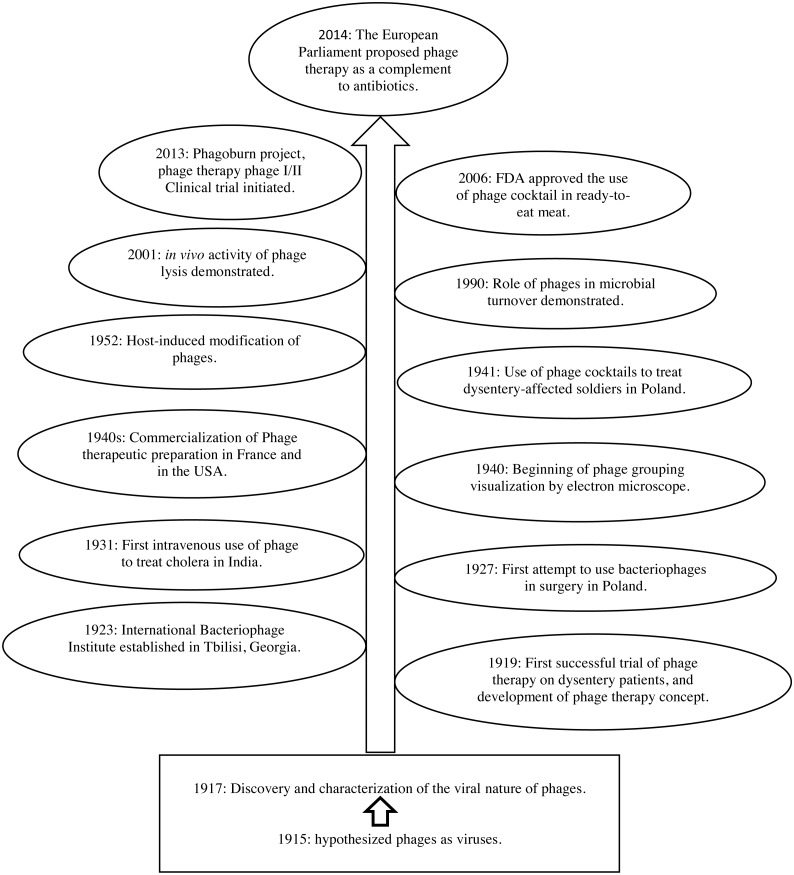
In the beginning of the twentieth century, Twort (1915) and D’Herelle (1917) independently discovered Bacteriophages (Nobrega et al., 2015). In addition, d’Herelle and his co-workers isolated phages with lytic activity against pathogenic bacteria, including Shigella spp., and developed the idea of “phage therapy” meaning the prophylactic and/or therapeutic use of these substances (D‘Herelle, 1923). Bacteriophages were then subsequently used in medicine from 1919 onwards—before the invention of the first antibiotic (penicillin). Figure 1 summarizes important milestones in the development of phage research.
Figure 1. Milestones in phage therapy research, adapted from Elbreki et al. (2014) and Salmond & Fineran (2015).
In the early stages, expectations were particularly high with regard to Shiga-phages (phages against Shigella), due to their success in treating dysentery patients safely. This success inspired the commercialization of therapeutic phages to treat bacterial infections in humans (Eaton & Bayne-Jones, 1934; Krueger & Scribner, 1941). However, at that time, scientists did not fully understand the mechanisms behind the treatment, and in particular how the phages killed the bacteria. Besides, the outcomes of phage treatment were inconsistent. Moreover, the introduction of antibiotics in the 1940s to treat a broader range of infections led to a reduction in phage therapy research (Matsuzaki et al., 2014). Despite the success of phage therapy in a number of Eastern European countries, it remained largely neglected in Western Europe due to the inconsistent results, the lack of a specific regulatory framework, and the complicated procedures for patenting phages (Verbeken et al., 2014).
Current phage therapy
More recently, interest in phage therapy has increased dramatically, and the use of phages in controlling bacterial infections has regained popularity, as well as those unanswered questions of phage therapy are now gradually being addressed (Fischetti, 2001; Stone, 2002; Summers, 2001). By looking back to the pre-antibiotic era, scientists aim to resurrect phages as an antidote to antibiotic resistant pathogens, as well as to solve other medical, agricultural, food safety and environmental problems. Nowadays, the availability of sophisticated molecular tools, the growing understanding of phage control techniques, and the evaluation experiences of Eastern European researchers have all widened the possibility of phage therapy applications. In Eastern Europe, phages have been administered orally (tablets or liquid), topically, rectally and intravenously for almost 90 years with no serious side effects have been reported (Sulakvelidze, Alavidze & Morris, 2001). As a result of these developments, phage therapy has attracted increasing attention as a potential alternative solution in treating antibiotic-resistant bacteria. Six clinics in five different countries (the US, the UK, the Republic of Georgia, Poland and Belgium) are now offering phage therapy for treating diseases (Table 2).
Table 2. Main features and activities of Phage therapy centers.
| Name of center | Country | Main features and activities |
|---|---|---|
| Center for Phage Therapy | Poland | Since 1980, specific bacteriophages have been used to treat over 1,500 patients with suppurative bacterial infections, where routine antibiotic therapy has failed (http://www.iitd.pan.wroc.pl). |
| Eliava Phage Therapy Center | Georgia | A network of eight laboratories have developed bacteriophage preparations for fighting against dangerous and antibiotic-resistant superbugs (http://www.mrsaphages.com). |
| Novomed | Georgia | Effective treatment delivery through phage therapy in many areas of medicine, drawing on the expertise of local physicians. Treatments are available not only to local Georgians, but also to foreign patients, especially those with chronic wounds, osteomyelitis or other types of acute and chronic infections (http://www.phagetherapy.com). |
| Phage Therapy Center | Georgia | Provides excellent treatment for patients who have bacterial infections and are difficult/non-healing, chronic, drug-resistant or have not responded to conventional antibiotic therapies (http://www.phagetherapycenter.com). |
| Phage International Inc. | The United States | Treats patients with chronic, drug-resistant or difficult to treat infections (http://www.phageinternational.com) |
There are 11 US and international biotechnology companies, as well as a number of academic investigators currently working in the field of bacteriophage technology and products. These companies and researchers are utilising bacteriophages in the food processing industry and for the treatment of human diseases. For example, US companies such as Intralytix and Novolytics are using bacteriophages as biotechnology tools and as platform technologies (http://www.dreamingrock.com/viridax/eviridax/cphage.htm). The FDA and the United States Department of Agriculture (USDA) have marked a milestone in phage research by approving three phage products, each comprising a “cocktail” of phages, to target and kill bacteria. The first FDA-approved phage product was ListShield™ (Intralytix, Baltimore, MD, USA) which can be used as a food additive against Listeria monocytogenes in ready-to-eat meat and poultry (Mead et al., 2006). The second product, EcoShield™ (Intralytix, Baltimore, MD, USA), can be sprayed on red meat, in order to kill Escherichia coli (particularly strain O157:H7) before the meat is ground into hamburgers (Abuladze et al., 2008; Scallan et al., 2011). The third phage product, called SalmoFresh™ (Intralytix, Baltimore, MD, USA), which acts against Salmonella enterica was approved as a food processing aid. It is used for the treatment of fresh and processed vegetables, fruits, seafood such as shellfish and fish. Lately, another phage preparation- ShigaShield™ (Intralytix, Baltimore, MD, USA), is currently undergoing FDA and USDA reviews for the GRAS (Generally Recognized As Safe) status (GRN672). According to the report by Soffer et al. (2017) this Shigella phage product, ShigaShield™ is able to reduce Shigella levels in various foods experimentally contaminated with a S. sonnei strain. Novolytics has the aim to lead in the utilisation of bacteriophage as a treatment for bacterial infections. Currently, the company’s most promising products NOVO12, a phage cocktail, administered as a form of gel for topical treatment of MRSA (Methicillin-resistant Staphylococcus aureus) infections (http://www.cobrabio.com/News/June-2013/Cobra-Biologics-and-Novolytics-Unveil-Successful-D).
The European Union (EU) also shows support for phage therapy research. In 2013, a project entitled ‘Phagoburn’, aimed at exploring the efficacy of phage therapy in protecting patients of burn wounds against severe bacterial infection, was funded by European Commission (Matsuzaki et al., 2014). In April 2014, the European Parliament passed a resolution in favour of prioritizing the development of phage therapy as a complement to antibiotic therapy in order to combat antibiotic resistance (Council of Europe, 2014). This is an important milestone fostering phage therapy research and development, but more time is needed to see its practical impact in future.
Phage biology and phage-host interaction
Bacteriophages exhibit four known life cycles inside the bacterial host: lysogenic, pseudo-lysogenic, chronic and lytic infection (Drulis-Kawa et al., 2012; Weinbauer, 2004). For phage therapy, the main interest has always been focused on lytic phages particularly the families of Podoviridae, Myoviridae and Siphoviridae. There are also a few reports on the applications of filamentous phages and cubic phages in phage therapy (Drulis-Kawa et al., 2012). For any type of life cycle, the initial step of an infection is the recognition and binding of a phage to a host receptor, which is facilitated by the phage receptor binding protein (RBP). The host specificity of bacteriophages towards different bacterial cells depends on different RBPs (Le et al., 2013). Three types of host receptors in Salmonella were identified by Shin et al. (2012): flagella, BtuB (outer membrane protein up taken by vitamin B12) and lipopolysaccharide-related O-antigen. Transmission electron microscopy analysis showed that the phages from Podoviridae family use O-antigen of LPS as a receptor while phages from Siphoviridae family use flagella (BtuB) as a receptor. Most frequently, mutations of these receptors caused the host cells resistant to these phages (Shin et al., 2012). The recognition of phage to host cells and the subsequent binding of RBPs and host receptors stimulate a spectrum of the probable phage-bacteria interactions (Wittebole, De Roock & Opal, 2014). After binding to host receptors, phages inject its DNA into the host cell via inducing a pore in the host cell wall and leaving behind their capsids outside the bacterial cell. Before lysis of bacterial cell occurs, packing and assembly of phages take place. Finally, release of phage progenies from the hosts. Different phage enzymes (murein synthesis inhibitors, lysins and holins) are then involved for helping the release of phage progenies into the extracellular environment (Weinbauer, 2004). In molecular aspect, when a phage invades a susceptible bacterial cell, its nucleic acid enters the cell and induces production cycle of the phage. The cell is converted to a phage factory. Some of the components of the biosynthetic apparatus involved in bacterial growth and metabolisms (such as ribosomes and ATP generators) are no longer performing their normal tasks during the phage production cycle (Campbell, 2003). It is known that while bacteria can evolve to become resistant to phages, phages can also develop new mechanisms to infect the resistant bacteria. Hosseinidoust, Tufenkji & Van De Ven (2013) demonstrated that resistance development is linked to changes in bacterial fitness and alteration of virulence determinants that are usually maintained in the absence of the agent to which the bacteria confer resistance. The alteration of phenotypic characteristics is associated with changes in gene regulation levels.
Phage therapy vs antibiotic treatment
Treating multidrug resistant Shigella spp. by a new antibiotic or a new combination of antibiotics tends to be more complicated than treating it with a phage or phage cocktail (Khatun et al., 2011). Generally, phages are environmentally friendly. In both cases, the clinical trial is expensive but it is usually quicker and less expensive to select, isolate and identify phages than to develop a new antibiotic, which can take a longer period (Matsuzaki et al., 2005; Weber-Dąbrowska, Mulczyk & Górski, 2001). Secondary infections may happen but very rare and minimal in phage therapy compared to antibiotics. The term secondary infection during phage therapy is due to the interaction between phage and bacteria, which can cause superinfection immunity or superinfection exclusion. Alternatively, secondary infection can also be described equivalently to superinfection or coinfection, which can result in phage-on-phage parasitism, genetic exchange between phages as well as various partial reductions in phage productivity that have been termed as partial/mutual exclusion, or the depressor effect (Abedon, 2015). With respect to antibiotics, it can cause secondary infection by attacking the normal flora of patients, in addition to the targeted pathogens (Table 3). In addition, phage resistance is less of a concern than antibiotic resistance, because phages can mutate and evolve naturally to counter phage-resistant bacteria (Ho, 2001; Matsuzaki et al., 2005). Moreover, the phage resistance development can be mitigated by using phage cocktails (combinations of multiple phages) and/or by applying phages in conjunction with antibiotics as therapy (Ho, 2001; Kutateladze & Adamia, 2010). The differences between phage therapy and antibiotic treatments are summarized in Table 3.
Table 3. Comparison between phage therapy and antibiotic treatment.
| Feature | Phage | Antibiotic |
|---|---|---|
| Host specificity | Very specific to their host cells: usually affect primarily or exclusively the targeted bacterial species (Chernomordik, 1989). | Can target a wide range of pathogenic microbes. Can therefore be used when the exact disease-causing pathogen is unknown. However, this can lead to the emergence of new drug resistant pathogens (Sulakvelidze, Alavidze & Morris, 2001). |
| Mode of action | Bacteriophages replicate exponentially as long as the specific bacteria they are targeting are available in abundance. They replicate at the site of infection and are available where they are most needed (Smith & Huggins, 1982). | Antibiotics are metabolized and then expelled from the body, and do not necessarily concentrate at the site of infection (Sulakvelidze, Alavidze & Morris, 2001). |
| Side effects | Generally the side effects are less than the antibiotic treatment. No or very few side effects have been described (Sulakvelidze, Alavidze & Morris, 2001). | Due to their non-specificity to the host, antibiotics destroy commensal microflora. This can lead to several side effects, including allergies, intestinal disorders and secondary infections (Inal, 2003; Lehmann, 1999). |
| Time and cost for new development | The selection of new phages against drug resistant or phage resistant bacteria is a comparatively rapid process which can be carried out in days or weeks (Sulakvelidze, Alavidze & Morris, 2001). Sometimes, it also takes longer period and extra cost for safety approval and in vivo trial. | The development of a new antibiotic against antibiotic resistant bacteria is not only time-consuming, but can also cost millions of dollars for clinical trials, and so may not be cost-effective (Chopra et al., 1997; Silver & Bostian, 1993). |
| Dose administration | Repeated dose administration is not always essential , because the phage reproduces until the target bacterium is destroyed (Inal, 2003). | Most cases require repeated dose administration. |
| Application range | In spite of some negative effects, the range of applications of bacteriophages is broader: they can, for example, be applied as protective materials in food supplements, the milk industry, pharmacology, toothpastes, cleaning solutions and so on (Veiga-Crespo & Villa, 2010). | The application ranges of antibiotics are restricted and narrower. |
One of the advantages of phages is that they have much fewer side effects. In fact, the prolonged use of phages to treat human infections in Eastern Europe has not elicited any allergic reactions, nor have animal trials in Western Europe revealed any unusual histological changes, mortality or morbidity when phages were administered orally, intravenously or intramuscularly (Biswas et al., 2002; Carlton et al., 2005; Merril et al., 1996). Indeed, intakes of the T4 phage up to 105 PFU (Plaque Forming Unit) have not caused any secondary effect (Bruttin & Brüssow, 2005). The intravenous injection of purified phages has not produced any side effect in either HIV-infected individuals (Fogelman et al., 2000), healthy volunteers (Ochs et al., 1993), or other patients with immunodeficiency diseases (Ochs, Davis & Wedgwood, 1971). Phage therapy has been successfully used to treat antibiotic resistant infections in the Southwest Regional Wound Care Centre in Texas (Clark & March, 2006), while biodegradable patches impregnated with phages have also been applied to patients with prolonged infections in Georgia (Fischetti, Nelson & Schuch, 2006).
In summary, as antibacterial agents, phages have a number of properties that make them a compelling alternative to antibiotics. Moreover, most of the concerns associated with phage therapy should be manageable through a combination of appropriate phage selection, effective formulations, and clear knowledge and expertise on how to prepare and apply phages (Loc-Carrillo & Abedon, 2011).
Phage therapy for controlling Shigella
There is a historic relationship between Shigella spp. and the discovery of phages. The first application of phage against human infections was conducted by d’Herelle in 1919 to treat the symptoms of dysentery. He injected an anti-dysentery phage into a patient with severe dysentery (10–12 bloody stools per day). The patient made a rapid recovery, displaying no symptoms shortly after receiving the phage therapy (Summers, 1999). This pioneering experiment of d’Herrelle led to many successful applications of this therapy against dysentery, which were reported in scientific articles over the subsequent 20 years. For instance, in the US state of Maryland, Shigella flexneri was identified in dysentery-affected children, and phage therapy was given orally and rectally in doses ranging from 5 to 1,300 ml (Davison, 1922). In one successful example, Spence & McKinley (1924) treated shigellosis patients through the oral administration of 10 ml phages, which substantially reduced their mortality rate and length of stay in the hospital (10% and 5.8 days, respectively) when compared to a control group in another hospital (40% and 12.8 days). Another example, Querangal des Essarts (1933) treated a bacillary dysentery patient in France with a polyvalent Shiga-Flexner bacteriophage through the oral administration of 5–10 ml of phages with alkaline water during an outbreak on board two ships at the port of Brest in 1933. The results were remarkable, with blood and mucus rapidly ceased (2nd or 3rd day) and the stools reverted to normal on the 4th day. The same physician also stopped an outbreak of dysentery by the prophylactic administration of bacteriophages among newborns at a holiday camp (Goodridge, 2013).
On the other hand, there have also been some failures, mainly due to the late administration of the therapy. Vaill & Morton (1937) reported that out of 200 cases of dysentery treated with bacteriophages in New Jersey (USA), only 22 cases were successful (Vaill & Morton, 1937). Johnston, Ebbs & Kaake (1933) treated 70 infants aged less than 2 years old using 1 ounce of bacteriophage per hour, and found that the clinical course of dysentery was not improved as what they expected with this therapy. These lower success rates may be due to the fact that the trials used a strain-specific bacteriophage, and the phage only effective against 17 out of 94 bacterial strains which is approximately 20% of bacterial strains tested in vitro (Johnston, Ebbs & Kaake, 1933). The British army conducted a phage therapy research in the Middle East and the experiment was divided into four small scales, of which two were reported as unsatisfactory results. The unimpressive result of the third one was published in the British Medical Journal. The last experiment was administrated judiciously and among the 32 enrolled cases, the control cases, and phages treatment cases were 18 and 14, respectively. The outcomes of this research did not show any remarkable result but a marginally better improvement of the treated cluster than the control cluster (Boyd & Portnoy, 1944; Goodridge, 2013).
Nonetheless, phage therapy has been successful in most cases. In 1938, Haler reported the phage treatment of a dysentery epidemic caused by Shigella sonnei in which the patients were administered with bacteriophages three times daily and the epidemic ceased after two days and no further case was observed for a year (Haler, 1938). In Poland (1941), 10 ml of local phage mixture containing sodium bicarbonate in a half cup of tea or coffee was found effective against Shigella infection (Kliewe & Helmreich, 1941). It has also been shown, in 1945, that the effective proportion of a phage can be diluted up to a 1:10 ratio of phage-bacterium injection. In a bacterial challenge experiment, mortality can be prevented with phage treatment up to 4 days before the challenge or with maximum 3 h delay after the challenge (Morton & Engley Jr, 1945). In 1957, the Hirszfeld Institute of Immunology and Experimental Therapy (HIIET) in Poland applied phages to treat shigellosis and other infectious diseases caused by antibiotic resistant bacteria, which were untreatable by conventional antibiotics (Sulakvelidze, Alavidze & Morris, 2001). In the 1960s, a clinical trial was conducted extensively to evaluate the efficacy of phage therapy against shigellosis (Babalova et al., 1968). This study was performed in Tbilisi, Georgia in which 30,769 children were involved. The children, aged between 6 months to 7 years old were divided into two groups, with one group being given tablet made of dried Shigella phages and the other group a placebo, orally once a week, for each child. These children were monitored for 109 days and the results showed that the occurrence of dysentery was nearly a fourfold higher in the children given placebos than those treated with phages (Babalova et al., 1968). In another investigation reported in 1984, Anpilov & Prokudin (1984) demonstrated that the phage-mediated preventive treatment of shigellosis produced a ten-fold reduction in the incidence of dysentery among the phage-treated patients. Miliutina & Vorotyntseva (1993) conducted an experiment on phage therapy and a combined phage-antibiotics treatment on shigellosis and salmonellosis in 1993. They observed that the combined phage-antibiotics treatment was more effective in some cases as compared to the antibiotics treatment alone.
There are many articles reporting successful treatments of Shigellosis in 21st century. The efficacy of phages against multidrug resistant Streptococi and Pseudomonas as well as some antibiotic resistant Enterobacteriaceae family members, including the genera of Shigella, Salmonella, Serratia, Escherichia, Klebsiella and Proteus, have been investigated (Kumari, Harjai & Chhibber, 2010). These studies have largely confirmed the viability of phage therapy as a treatment for gastrointestinal distress, particularly for Shigella. Zhang, Wang & Bao (2013) studied the ability of Shigella-specific phages and phage cocktails to inhibit Shigella spp in chicken products. They concluded that phages with higher concentration (3 × 108 PFU/g) could lyse bacteria more effectively in comparison to phages with lower concentration (1 × 108 PFU/g), and that the Shigella-specific phages were able to significantly reduce or eliminate Shigella spp. in the edible chicken products.
In summary, from the very beginning to the present day, the success rate of phage therapy against Shigellosis has been promising. An intensive and extensive studies of anti-Shigella phages could therefore help to identify alternative treatments for the increasing number of drug resistant bacteria, and hence reduce the pressure to find new antibiotics. In the longer term, greater use of phage therapy could help to reduce the emergence of new multidrug resistant bacterial strains.
Limitations, solutions and prospects for phage therapy
Despite all the advantages of phage therapy, it is still a long way from being the “magic bullet” for treating infections, because many parameters (e.g., frequency and duration of treatment, route of administration and optimal dose) have yet to be determined precisely through clinical trials (Wittebole, De Roock & Opal, 2014).
The major limitations of phage therapy are summarized below based on the reports from a few research groups (Hermoso, García & García, 2007; Kutter & Gowrishankar, 2001; Matsuzaki et al., 2014; Nilsson, 2014):
-
I.
A narrow host range as well as serotype specificity (which might reduce effectiveness and coverage).
-
II.
A single phage is inadequate for treating illnesses caused by multiple bacteria.
-
III.
The release of various pro-inflammatory components (endotoxins and peptidoglycans) from bacteria lysed by phages might cause problems in the human body.
-
IV.
There is a possibility that resistant bacteria might emerge after treating with phages, however phages can evolve and adapt to combat resistant bacteria.
-
V.
Complicated pharmacokinetics and pharmacodynamics of phage treatments and interference by anti-phage antibodies.
In addition, there are other problems associated with patenting, manufacturing, and administration which often create obstacles for development of phage therapy. The lack of a definite regulatory outline reflecting individualized therapies, or difficulties for the pharmaceutical companies to register intellectual properties for phage and phage products are some of the major problems in phage therapy (Nobrega et al., 2015; Young & Gill, 2015). The eventual success of phage therapy will largely depend on the development of appropriate strategies to overcome these limitations. In addition, adequate regulatory framework must be created, appropriate safety protocols have to be implemented and the general acceptance of public towards phage treatment is needed (Nobrega et al., 2015).
Several initiatives have been taken to overcome the limitations in phage therapy. Phage cocktails have been formulated, consisting of several phages with complementary features (different receptors) which can play a vital role to overcome the limitations of a single phage with its narrow host range (Chan & Abedon, 2012; Chan, Abedon & Loc-Carrillo, 2013; Goodridge, 2010). In addition, phage cocktails containing different types of phages potentially capable of combating the same species and strains of bacteria could reduce the emergence of bacteria resistant to phage (Chan & Abedon, 2012; Gu et al., 2012b; Potera, 2013). A complementary approach proposed by Friman et al. (2016), where phage cocktails can also be modified by including not only various phages, but also in vitro evolved phages from different evolutionary time points (Friman et al., 2016). Moreover, the host range of phages can be broadened by engineering their genomes to express endosialidase (Ackermann, 2001) and by substituting the gene encoding putative host binding proteins (Yoichi et al., 2005). In addition, the challenges of phage therapy may be overcome by producing genetically modified phages (recombined-phage genomes, site-directed mutagenesis, selected spontaneous mutants or phage display techniques) (Chhibber & Kumari, 2012; Dąbrowska et al., 2014; Moradpour & Ghasemian, 2011). Mutated phages could also be used to overcome bacterial resistance as well as to prevent the human immune system against phages (Matsuzaki et al., 2014).
The efficacy of phage therapy could be enhanced by utilizing the antimicrobial synergy between phages and antibiotics. Torres-Barceló et al. (2016) found a strong synergistic effect on bacterial population density by applying treatment with combination of antibiotics and phages. Their results indicated that phages not only could contribute in managing the level of antibiotic resistance but also limit the consequences of bacterial virulence evolution. In another study with experimentally challenged mice, Mai et al. (2015) demonstrated that a combination of phage cocktail (5 Shigella specific bacteriophages) and an antibiotic (ampicillin), designated as ShigActive™, was able to decrease Shigella counts effectively. They did not observe any deleterious side effects of phage application during this study, and the impact of phage cocktail on the normal gut microbiota was much lesser than that caused by the treatment with generally recommended antibiotics (Mai et al., 2015).
This synergistic effect could hasten cell lysis and allow phages to spread more quickly (Comeau et al., 2007; Ryan et al., 2012). Thus antibiotics conjugated to phages could enable the delivery of antibiotics to specific cells and cause an increase in local drug concentrations (Yacoby & Benhar, 2008). At the same time, the antibiotic resistance of bacteria could be minimized by applying phages to inject sensitizing alleles of the mutated genes (e.g., rpsL and gyrA) for restoring drug efficacy. For instance, temperate phages have been used to reverse antibiotic resistance of pathogenic bacteria by lysogenizing the genes gyrA and rpsL in which both conferred sensitivity in a dominant fashion to two antibiotics, nalidixic acid and streptomycin, respectively. This made the bacterial pathogens sensitive to antibiotics prior to host infection (Edgar et al., 2012). In addition, the incorporation of genes that inhibit stress responses, improve drug uptake or repress biofilm production can enhance the antibiotic sensitivity of E. coli (Lu & Collins, 2009).
A number of foodborne pathogens from the family Enterobacteriaceae including Shigella, contain prophages which encodes the Shiga-like toxin, a major virulence factor. In S. flexneri the O-antigen modification (serotype conversion) is a key virulence determining factor, which is introduced by temperate bacteriophages (Allison & Verma, 2000). A careful screening of the phage genome for virulence genes would help to minimize the risk of phage engineering. Another approach for the safe use of phage therapy is to use the viral gene products (endolysins) instead of the whole phage particles (Fischetti, 2005; Nelson et al., 2012; Schmelcher, Donovan & Loessner, 2012). The use of gene products might eliminate the risk of phages giving toxic properties to bacteria (Hermoso, García & García, 2007) and thus reduce the risk of resistance developing (Borysowski, Weber-Dąbrowska & Górski, 2006; Nelson et al., 2012; Schmelcher, Donovan & Loessner, 2012).
Another important limitation of bacteriophage therapy is the capability of phages to act only on outside of bacterial cell and the risk of being attacked by the in vivo anti-phage antibodies (Singla et al., 2016). To overcome these risks, Singla et al. (2016) used liposome as a delivery vehicle for phages. This study reinforced the growing interest to apply phage treatment as a means to target multiple drug resistant (MDR) bacterial infections, as the encapsulation of phages has increased the efficiency to overcome most of the difficulties and problems related to the clinical use (both in vitro and in vivo) of phages (Singla et al., 2016).
As things now stand, most of the drawbacks of phage therapy have been overcome to a lesser or greater degree, and phages are now capable of being successfully incorporated into the era of multi drug resistant treatment. As further steps, next-generation sequencing could be employed to determine genomic DNA sequences from multiple phage products, which could reduce further the risks of phage therapy by eliminating harmful genes and gene products (Matsuzaki et al., 2014). Recently the whole genomes of all five lytic bacteriophages of the cocktail ShigaShield™ have been sequenced and analysed, and no undesirable genes have been found, including those listed in the US Code for Federal Regulations (40 CFR Ch1) (Soffer et al., 2017). In addition, the multi-route administration of phages (intramuscular, intravenous, intraperitoneal, subcutaneous, intranasal and oral) would broaden the use of phage therapy as a potential agent in the future. Moreover, the prophylactic use of phages and the development of vaccines using phages or phage products would open up a new dimension for the prevention of antibiotic resistant pathogens (Chanishvili, 2012; Morello et al., 2011). In addition, the active participation of dysentery patients and a large scale trial of phage therapy against multidrug resistant shigellosis and other dysenteries would enhance the acceptance of phage therapy as a common treatment. Finally, it is essential to build up public awareness of phage therapy as well as expand the availability of phages and phage therapy centres in order to expand and exploit this potentially fruitful innovation.
Conclusion
Phage therapies for Shigella spp. and other pathogenic bacteria have been studied and applied for about a century, but phage therapy as an antibacterial treatment in general has not received much attention due to lack of clinical knowledge and public awareness of phages. However, given that the development of novel antibiotics is laborious, time-consuming and costly, it makes eminent sense to seek alternative antimicrobial approaches to combat drug resistant pathogens. While it inevitably has some drawbacks, phage-based biocontrol and bacteriophage therapy are very promising approaches to combat the challenge of pathogenic bacterial infections, particularly when the search for new antibiotics is stagnating. The potential of phage therapy has been acknowledged and revisited by many scientists over the last few decades, and there has been a rejuvenation of research into phage therapy. Moreover, phages have many unexploited potentials as an alternative to antibiotics, both due to the range of intrinsic variation in their mode of action, also due to almost unlimited variety of phages and their ability to evolve in situ to successfully deal with bacterial resistance. The FDA has approved bacteriophages as GRAS and allowed the application of phages as food additives in 2006, which is a significant boost to phage therapy research.
Nevertheless, the therapeutic application of phages still requires extensive studies, judiciously performed clinical trials, and importantly well-defined regulatory guidelines. Currently, phage therapy is encouraged in many parts of the world because policymakers consider growing MDR as a serious health problem. This awareness should further encourage researchers to study the biological properties of phages, which eventually increases their safety and efficacy. Furthermore, genetically modified phages could help to solve the issues of patent filing and as a result increase the interest of pharmaceutical and biotechnology companies to produce phage-products. Finally, cocktails of natural phages and genetically modified phages could open new perspectives for successful phage therapy in the future, particularly against the major challenge of Shigella and Shigella-like multidrug resistant bacteria.
Funding Statement
This study was supported by Research Grants PG 046-2015B, RG 347-15AFR from the University of Malaya, Malaysia and a Ministry of Education Grant from Bangladesh. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Swee-Seong Tang conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft, manuscript writing, reviewing and editing.
Sudhangshu Kumar Biswas conceived and designed the experiments, prepared figures and/or tables, approved the final draft, manuscript writing, table and figure design and editing.
Wen Siang Tan, Ananda Kumar Saha and Bey-Fen Leo authored or reviewed drafts of the paper, approved the final draft, manuscript reviewing and editing.
Data Availability
The following information was supplied regarding data availability:
This is a literature review article. No raw data was generated.
References
- Abedon (2015).Abedon ST. Bacteriophage secondary infection. Virologica Sinica. 2015;30(1):3–10. doi: 10.1007/s12250-014-3547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuladze et al. (2008).Abuladze T, Li M, Menetrez MY, Dean T, Senecal A, Sulakvelidze A. Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157: H7. Applied and Environmental Microbiology. 2008;74:6230–6238. doi: 10.1128/AEM.01465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann (2001).Ackermann H-W. Frequency of morphological phage descriptions in the year 2000. Archives of Virology. 2001;146(5):843–857. doi: 10.1007/s007050170120. [DOI] [PubMed] [Google Scholar]
- Allison & Verma (2000).Allison GE, Verma NK. Serotype-converting bacteriophages and O-antigen modification in Shigella flexneri. Trends in Microbiology. 2000;8:17–23. doi: 10.1016/s0966-842x(99)01646-7. [DOI] [PubMed] [Google Scholar]
- Anpilov & Prokudin (1984).Anpilov L, Prokudin A. Preventive effectiveness of dried polyvalent Shigella bacteriophage in organized collective farms. Voenno-meditsinskii Zhurnal. 1984;5:39–40. [PubMed] [Google Scholar]
- Arias & Murray (2009).Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. New England Journal of Medicine. 2009;360:439–443. doi: 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]
- Babalova et al. (1968).Babalova E, Katsitadze K, Sakvarelidze L, Imnaishvili N, Sharashidze T, Badashvili V, Kiknadze G, Mei˘pariani A, Gendzekhadze N, Machavariani E. Preventive value of dried dysentery bacteriophage. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii. 1968;45:143–145. [PubMed] [Google Scholar]
- Barceloux (2008).Barceloux DG. Medical toxicology of natural substances: foods, fungi, medicinal herbs, plants, and venomous animals. Hoboken: John Wiley & Sons; 2008. [Google Scholar]
- Bardhan et al. (2010).Bardhan P, Faruque A, Naheed A, Sack DA. Decreasing shigellosis-related deaths without Shigella spp.-specific interventions, Asia. Emerging Infectious Diseases. 2010;16:1718–1723. doi: 10.3201/eid1611.090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benny et al. (2014).Benny E, Mesere K, Pavlin BI, Yakam L, Ford R, Yoannes M, Kisa D, Abdad M, Menda L, Greenhill AR. A large outbreak of shigellosis commencing in an internally displaced population, Papua New Guinea, 2013. Western Pacific Surveillance and Response. 2014;5(3):18–21. doi: 10.5365/WPSAR.2014.5.2.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts et al. (2013).Betts A, Vasse M, Kaltz O, Hochberg ME. Back to the future: evolving bacteriophages to increase their effectiveness against the pathogen Pseudomonas aeruginosa PAO1. Evolutionary Applications. 2013;6(7):1054–1063. doi: 10.1111/eva.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj & Panhotra (1985).Bhardwaj G, Panhotra B. Which chemotherapy for Shigella dysentery? Indian Journal of Pediatrics. 1985;52:451–452. doi: 10.1007/BF02751013. [DOI] [PubMed] [Google Scholar]
- Bhattacharya et al. (2014).Bhattacharya D, Bhattacharya H, Thamizhmani R, Sayi D, Reesu R, Anwesh M, Kartick C, Bharadwaj A, Singhania M, Sugunan A. Shigellosis in Bay of Bengal Islands, India: clinical and seasonal patterns, surveillance of antibiotic susceptibility patterns, and molecular characterization of multidrug-resistant Shigella strains isolated during a 6-year period from 2006 to 2011. European Journal of Clinical Microbiology & Infectious Diseases. 2014;33:157–170. doi: 10.1007/s10096-013-1937-2. [DOI] [PubMed] [Google Scholar]
- Biswas et al. (2002).Biswas B, Adhya S, Washart P, Paul B, Trostel AN, Powell B, Carlton R, Merril CR. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infection and Immunity. 2002;70:204–210. doi: 10.1128/IAI.70.1.204-210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysowski, Weber-Dąbrowska & Górski (2006).Borysowski J, Weber-Dąbrowska B, Górski A. Bacteriophage endolysins as a novel class of antibacterial agents. Experimental Biology and Medicine. 2006;231:366–377. doi: 10.1177/153537020623100402. [DOI] [PubMed] [Google Scholar]
- Bose et al. (1984).Bose R, Nashipuri J, Sen P, Datta P, Bhattacharya S, Datta D, Sen D, Bhattacharya M. Epidemic of dysentery in West Bengal: clinicians’enigma. The Lancet. 1984;324:1160–1160. doi: 10.1016/s0140-6736(84)91600-3. [DOI] [PubMed] [Google Scholar]
- Boyd & Portnoy (1944).Boyd J, Portnoy B. Bacteriophage therapy in bacillary dysentery. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1944;37:243–262. doi: 10.1016/S0035-9203(44)90037-4. [DOI] [PubMed] [Google Scholar]
- Bruttin & Brüssow (2005).Bruttin A, Brüssow H. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrobial Agents and Chemotherapy. 2005;49:2874–2878. doi: 10.1128/AAC.49.7.2874-2878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell (2003).Campbell A. The future of bacteriophage biology. Nature Reviews Genetics. 2003;4:471–477. doi: 10.1038/nrg1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton et al. (2005).Carlton R, Noordman W, Biswas B, De Meester E, Loessner M. Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regulatory Toxicology and Pharmacology. 2005;43:301–312. doi: 10.1016/j.yrtph.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Chan & Abedon (2012).Chan BK, Abedon ST. 1 phage therapy pharmacology: phage cocktails. Advances in Applied Microbiology. 2012;78:1–23. doi: 10.1016/B978-0-12-394805-2.00001-4. [DOI] [PubMed] [Google Scholar]
- Chan, Abedon & Loc-Carrillo (2013).Chan BK, Abedon ST, Loc-Carrillo C. Phage cocktails and the future of phage therapy. Future Microbiology. 2013;8:769–783. doi: 10.2217/fmb.13.47. [DOI] [PubMed] [Google Scholar]
- Chanishvili (2012).Chanishvili N. A literature review of the practical application of bacteriophage research. New York: Nova Biomedical Books; 2012. [Google Scholar]
- Cheever (1946).Cheever F. Dysentery outbreak aboard naval vessels in San Pedro Bay, Philippine Islands. Naval Medical Bulletin. 1946;46:479–494. [PubMed] [Google Scholar]
- Chernomordik (1989).Chernomordik A. Bacteriophages and their therapeutic-prophylactic use. Meditsinskaia Sestra. 1989;48:44–47. [PubMed] [Google Scholar]
- Chhibber & Kumari (2012).Chhibber S, Kumari S. Bacteriophages. Vol. 158. India: InTechOpen 571; 2012. Application of therapeutic phages in medicine; pp. 139–570. [Google Scholar]
- Chopra et al. (1997).Chopra I, Hodgson J, Metcalf B, Poste G. The search for antimicrobial agents effective against bacteria resistant to multiple antibiotics. Antimicrobial Agents and Chemotherapy. 1997;41:497–503. doi: 10.1128/AAC.41.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher et al. (2009).Christopher PR, David KV, John SM, Sankarapandian V. Antibiotic therapy for Shigella dysentery. The Cochrane Library. 2009;4:CD006784. doi: 10.1002/14651858.CD006784.pub2. [DOI] [PubMed] [Google Scholar]
- Chun, Seol & Suh (1981).Chun D, Seol SY, Suh MH. Transferable resistance to trimethoprim in Shigella. Journal of Infectious Diseases. 1981;143:742–742. doi: 10.1093/infdis/143.5.742. [DOI] [PubMed] [Google Scholar]
- Clark & March (2006).Clark JR, March JB. Bacteriophages and biotechnology: vaccines, gene therapy and antibacterials. Trends in Biotechnology. 2006;24:212–218. doi: 10.1016/j.tibtech.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Comeau et al. (2007).Comeau AM, Tétart F, Trojet SN, Prere M-F, Krisch H. Phage-antibiotic synergy (PAS): β-lactam and quinolone antibiotics stimulate virulent phage growth. PLOS ONE. 2007;2:e799. doi: 10.1371/journal.pone.0000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council of Europe (2014).Council of Europe . Phage therapy a public health issue-motion for a resolution. Council of Europe; Strasbourg: 2014. pp. 1–2. [Google Scholar]
- Dąbrowska et al. (2014).Dąbrowska K, Kaźmierczak Z, Majewska J, Miernikiewicz P, Piotrowicz A, Wietrzyk J, Lecion D, Hodyra K, Nasulewicz-Goldeman A, Owczarek B. Bacteriophages displaying anticancer peptides in combined antibacterial and anticancer treatment. Future Microbiology. 2014;9:861–869. doi: 10.2217/fmb.14.50. [DOI] [PubMed] [Google Scholar]
- Davison (1922).Davison WC. The bacteriolysant therapy of bacillary dysentery in children: therapeutic application of bacteriolysants; d’Herelle’s phenomenon. American Journal of Diseases of Children. 1922;23:531–534. doi: 10.1001/archpedi.1922.01910420062011. [DOI] [Google Scholar]
- Delappe, King & Cormican (2014).Delappe N, King J, Cormican M. Galway: National Salmonella, Shigella & Listeria Reference Laboratory: Department of Bacteriology at the Clinical Science Institute, NUI Galway, Irelandhttps://www.researchgate.net/publication/280804929. [21 July 2017];NSSLRL Report 2014. 2014
- Deris et al. (2013).Deris JB, Kim M, Zhang Z, Okano H, Hermsen R, Groisman A, Hwa T. The innate growth bistability and fitness landscapes of antibiotic-resistant bacteria. Science. 2013;342:1–26. doi: 10.1126/science.1237435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desranleau (1949).Desranleau J-M. Progress in the treatment of typhoid fever with Vi bacteriophages. Canadian Journal of Public Health/Revue Canadienne de Sante’e Publique. 1949;40:473–478. [PubMed] [Google Scholar]
- D’Herelle (1917).D’Herelle F. Sur un microbe invisible antagoniste des bacilles dysentériques. Comptes-rendus de l’Académie des Sciences de Paris. 1917;165:373–375. [Google Scholar]
- D‘Herelle (1923).D‘Herelle F. Autolysis and bacteriophagis. Journal of State Medicine. 1923;31:461–466. [Google Scholar]
- Drulis-Kawa et al. (2012).Drulis-Kawa Z, Majkowska-Skrobek G, Maciejewska B, Delattre A-S, Lavigne R. Learning from bacteriophages-advantages and limitations of phage and phage-encoded protein applications. Current Protein and Peptide Science. 2012;13:699–722. doi: 10.2174/138920312804871193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton & Bayne-Jones (1934).Eaton MD, Bayne-Jones S. Bacteriophage therapy: review of the principles and results of the use of bacteriophage in the treatment of infections. Journal of the American Medical Association. 1934;103:1847–1853. doi: 10.1001/jama.1934.72750500003009. [DOI] [Google Scholar]
- Edgar et al. (2012).Edgar R, Friedman N, Molshanski-Mor S, Qimron U. Reversing bacterial resistance to antibiotics by phage-mediated delivery of dominant sensitive genes. Applied and Environmental Microbiology. 2012;78:744–751. doi: 10.1128/AEM.05741-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbreki et al. (2014).Elbreki M, Ross RP, Hill C, O’Mahony J, McAuliffe O, Coffey A. Bacteriophages and their derivatives as biotherapeutic agents in disease prevention and treatment. Journal of Viruses. 2014;2014:1–20. [Google Scholar]
- Fernandez-Prada et al. (2004).Fernandez-Prada C, Venkatesan M, Franco A, Lanata C, Sack R, Hartman A, Spira W. Molecular epidemiology of Shigella flexneri in a diarrhoea-endemic area of Lima, Peru. Epidemiology and Infection. 2004;132:303–316. doi: 10.1017/S0950268803001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson (1980).Finlayson M. Shigella sonnei resistant to cotrimoxazole. Canadian Medical Association Journal. 1980;123:718–721. [PMC free article] [PubMed] [Google Scholar]
- Fischetti (2001).Fischetti VA. Phage antibacterials make a comeback. Nature Biotechnology. 2001;19:734–735. doi: 10.1038/90777. [DOI] [PubMed] [Google Scholar]
- Fischetti (2005).Fischetti VA. Bacteriophage lytic enzymes: novel anti-infectives. Trends in Microbiology. 2005;13:491–496. doi: 10.1016/j.tim.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Fischetti, Nelson & Schuch (2006).Fischetti VA, Nelson D, Schuch R. Reinventing phage therapy: are the parts greater than the sum? Nature Biotechnology. 2006;24:1508–1511. doi: 10.1038/nbt1206-1508. [DOI] [PubMed] [Google Scholar]
- Flexner (1900).Flexner S. The etiology of tropical dysentery. The British Medical Journal. 1900;2:917–920. [Google Scholar]
- Fogelman et al. (2000).Fogelman I, Davey V, Ochs HD, Elashoff M, Feinberg MB, Mican J, Siegel JP, Sneller M, Lane HC. Evaluation of CD4 + T cell function in vivo in HIV-infected patients as measured by bacteriophage phiX174 immunization. Journal of Infectious Diseases. 2000;182:435–441. doi: 10.1086/315739. [DOI] [PubMed] [Google Scholar]
- Friman et al. (2016).Friman VP, Soanes-Brown D, Sierocinski P, Molin S, Johansen HK, Merabishvili M, Pirnay JP, De Vos D, Buckling A. Pre-adapting parasitic phages to a pathogen leads to increased pathogen clearance and lowered resistance evolution with Pseudomonas aeruginosa cystic fibrosis bacterial isolates. Journal of Evolutionary Biology. 2016;29:188–198. doi: 10.1111/jeb.12774. [DOI] [PubMed] [Google Scholar]
- Frost et al. (2005).Frost LS, Leplae R, Summers AO, Toussaint A. Mobile genetic elements: the agents of open source evolution. Nature Reviews Microbiology. 2005;3:722–732. doi: 10.1038/nrmicro1235. [DOI] [PubMed] [Google Scholar]
- Gangarosa et al. (1970).Gangarosa EJ, Perera DR, Mata LJ, Mendizábal-Morris C, Guzmán G, Reller LB. Epidemic Shiga bacillus dysentery in Central America. II. Epidemiologic studies in 1969. The Journal of Infectious Diseases. 1970;122(3):181–190. doi: 10.1093/infdis/122.3.181. [DOI] [PubMed] [Google Scholar]
- Gaynor et al. (2009).Gaynor K, Park S, Kanenaka R, Colindres R, Mintz E, Ram P, Kitsutani P, Nakata M, Wedel S, Boxrud D. International foodborne outbreak of Shigella sonnei infection in airline passengers. Epidemiology and Infection. 2009;137:335–341. doi: 10.1017/S0950268807000064. [DOI] [PubMed] [Google Scholar]
- Goodridge (2010).Goodridge LD. Designing phage therapeutics. Current Pharmaceutical Biotechnology. 2010;11(1):15–27. doi: 10.2174/138920110790725348. [DOI] [PubMed] [Google Scholar]
- Goodridge (2013).Goodridge LD. Bacteriophages for managing Shigella in various clinical and non-clinical settings. Bacteriophage. 2013;3:e25098. doi: 10.4161/bact.25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu et al. (2012a).Gu B, Cao Y, Pan S, Zhuang L, Yu R, Peng Z, Qian H, Wei Y, Zhao L, Liu G. Comparison of the prevalence and changing resistance to nalidixic acid and ciprofloxacin of Shigella between Europe–America and Asia–Africa from 1998 to 2009. International Journal of Antimicrobial Agents. 2012a;40:9–17. doi: 10.1016/j.ijantimicag.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Gu et al. (2012b).Gu J, Liu X, Li Y, Han W, Lei L, Yang Y, Zhao H, Gao Y, Song J, Lu R. A method for generation phage cocktail with great therapeutic potential. PLOS ONE. 2012b;7:e31698. doi: 10.1371/journal.pone.0031698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haler (1938).Haler D. Use of the bacteriophage in an outbreak of dysentery. British Medical Journal. 1938;2:698–700. doi: 10.1136/bmj.2.4056.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltalin & Nelson (1965).Haltalin KC, Nelson JD. In vitro susceptibility of shigellae to sodium sulfadiazine and to eight antibiotics. Journal of the American Medical Association. 1965;193:705–710. doi: 10.1001/jama.1965.03090090011002. [DOI] [PubMed] [Google Scholar]
- Hassing et al. (2014).Hassing R, Melles D, Goessens W, Rijnders B. Case of Shigella flexneri infection with treatment failure due to azithromycin resistance in an HIV-positive patient. Infection. 2014;42:789–790. doi: 10.1007/s15010-014-0594-4. [DOI] [PubMed] [Google Scholar]
- He et al. (2012).He F, Han K, Liu L, Sun W, Zhang L, Zhu B, Ma H. Shigellosis outbreak associated with contaminated well water in a rural elementary school: Sichuan Province, China, June, 2009, 7–16. PLOS ONE. 2012;7:e47239. doi: 10.1371/journal.pone.0047239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä et al. (1990).Heikkilä E, Siitonen A, Jahkola M, Fling M, Sundström L, Huovinen P. Increase of trimethoprim resistance among Shigella species, 1975–1988: analysis of resistance mechanisms. Journal of Infectious Diseases. 1990;161:1242–1248. doi: 10.1093/infdis/161.6.1242. [DOI] [PubMed] [Google Scholar]
- Hermoso, García & García (2007).Hermoso JA, García JL, García P. Taking aim on bacterial pathogens: from phage therapy to enzybiotics. Current Opinion in Microbiology. 2007;10:461–472. doi: 10.1016/j.mib.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Ho (2001).Ho K. Bacteriophage therapy for bacterial infections: rekindling a memory from the pre-antibiotics era. Perspectives in Biology and Medicine. 2001;44:1–16. doi: 10.1353/pbm.2001.0006. [DOI] [PubMed] [Google Scholar]
- Hosseini & Kaffashian (2010).Hosseini MJ, Kaffashian AR. An outbreak of shigellosis due to Shigella flexneri serotype 3a in a prison in Iran. Archives of Iranian Medicine. 2010;13:413–416. [PubMed] [Google Scholar]
- Hosseinidoust, Tufenkji & Van De Ven (2013).Hosseinidoust Z, Tufenkji N, Van De Ven TG. Predation in homogeneous and heterogeneous phage environments affects virulence determinants of Pseudomonas aeruginosa. Applied and Environmental Microbiology. 2013;79:2862–2871. doi: 10.1128/AEM.03817-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers et al. (2015).Huijbers PM, Blaak H, De Jong MC, Graat EA, Vandenbroucke-Grauls CM, De Roda Husman AM. Role of the environment in the transmission of antimicrobial resistance to humans: a review. Environmental Science & Technology. 2015;49:11993–12004. doi: 10.1021/acs.est.5b02566. [DOI] [PubMed] [Google Scholar]
- Inal (2003).Inal JM. Phage therapy: a reappraisal of bacteriophages as antibiotics. Arch ivum Immunologiae Et Therapiae Experimentalis-English Edition. 2003;51:237–244. [PubMed] [Google Scholar]
- Johnston, Ebbs & Kaake (1933).Johnston MM, Ebbs J, Kaake MJ. Laboratory section: “baderiophage therapy in acute intestinal infection”. Canadian Public Health Journal. 1933;24:443–446. [Google Scholar]
- Katz et al. (2004).Katz DE, Coster TS, Wolf MK, Trespalacios FC, Cohen D, Robins G, Hartman AB, Venkatesan MM, Taylor DN, Hale TL. Two studies evaluating the safety and immunogenicity of a live, attenuated Shigella flexneri 2a vaccine (SC602) and excretion of vaccine organisms in North American volunteers. Infection and Immunity. 2004;72:923–930. doi: 10.1128/IAI.72.2.923-930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatun et al. (2011).Khatun F, Faruque A, Koeck J, Olliaro P, Millet P, Paris N, Malek M, Salam M, Luby S. Changing species distribution and antimicrobial susceptibility pattern of Shigella over a 29-year period (1980–2008) Epidemiology and Infection. 2011;139:446–452. doi: 10.1017/S0950268810001093. [DOI] [PubMed] [Google Scholar]
- Kliewe & Helmreich (1941).Kliewe H, Helmreich W. Ueber ruhrbakteriophagen, München. med. Wchnschr. 1941;88:617–619. [Google Scholar]
- Klontz et al. (2014).Klontz EH, Das SK, Ahmed D, Ahmed S, Chisti MJ, Malek MA, Faruque ASG, Klontz KC. Long-term comparison of antibiotic resistances in Vibrio cholerae O1 and Shigella spp. between urban and rural Bangladesh. Clinical Infectious Diseases. 2014;58(9):e133–e136. doi: 10.1093/cid/ciu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klontz & Singh (2015).Klontz KC, Singh N. Treatment of drug-resistant Shigella infections. Expert Review of Anti-infective Therapy. 2015;13:69–80. doi: 10.1586/14787210.2015.983902. [DOI] [PubMed] [Google Scholar]
- Ko et al. (2013).Ko C-F, Lin N-T, Chiou C-S, Wang L-Y, Liu M-C, Yang C-Y, Lee Y-S. Infrequent cross-transmission of Shigella flexneri 2a strains among villages of a mountainous township in Taiwan with endemic shigellosis. BMC Infectious Diseases. 2013;13:1. doi: 10.1186/1471-2334-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrzewski (1968).Kostrzewski J. Changes in the epidemiology of dysentery in Poland and the situation in Europe. Archivum Immunologiae Et Therapiae Experimentalis. 1968;16:429–451. [PubMed] [Google Scholar]
- Kozyreva et al. (2016).Kozyreva VK, Jospin G, Greninger A, Watt JP, Eisen JA, Chaturvedi V. Recent outbreaks of shigellosis in california caused by two distinct populations of Shigella sonnei with increased virulence or fluoroquinolone resistance. BioRxiv. 2016:063818. doi: 10.1128/mSphere.00344-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger & Scribner (1941).Krueger AP, Scribner EJ. The bacteriophage: its nature and its therapeutic use. Journal of the American Medical Association. 1941;116:2269–2277. doi: 10.1001/jama.1941.62820200013011. [DOI] [Google Scholar]
- Kumari, Harjai & Chhibber (2010).Kumari S, Harjai K, Chhibber S. Topical treatment of Klebsiella pneumoniae B5055 induced burn wound infection in mice using natural products. The Journal of Infection in Developing Countries. 2010;4:367–377. [PubMed] [Google Scholar]
- Kuo et al. (2009).Kuo H-W, Kasper S, Jelovcan S, Höger G, Lederer I, König C, Pridnig G, Luckner-Hornischer A, Allerberger F, Schmid D. A food-borne outbreak of Shigella sonnei gastroenteritis, Austria, 2008. Wiener Klinische Wochenschrif. 2009;121:157–163. doi: 10.1007/s00508-008-1141-7. [DOI] [PubMed] [Google Scholar]
- Kutateladze & Adamia (2010).Kutateladze M, Adamia R. Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends in Biotechnology. 2010;28:591–595. doi: 10.1016/j.tibtech.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Kutter (2009).Kutter E. Bacteriophage therapy: past and present. In: Schaecter M, editor. Encyclopedia of microbiology. Elsevier; Oxford: 2009. pp. 258–266. [Google Scholar]
- Kutter et al. (2010).Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon ST. Phage therapy in clinical practice: treatment of human infections. Current Pharmaceutical Biotechnology. 2010;11:69–86. doi: 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- Kutter & Gowrishankar (2001).Kutter E, Gowrishankar J. Book review-//phage therapy: bacteriophage as natural, self-replicating and self-limiting antibiotics. Journal of Genetics. 2001;80:117–117. doi: 10.1007/BF02728337. [DOI] [Google Scholar]
- LaFee & Buschman (2017).LaFee S, Buschman H. UC San Diego News Center, University of California, USANovel phage therapy saves patient with multidrug-resistant bacterial infection. 2017
- Le et al. (2013).Le S, He X, Tan Y, Huang G, Zhang L, Lux R, Shi W, Hu F. Mapping the tail fiber as the receptor binding protein responsible for differential host specificity of Pseudomonas aeruginosa bacteriophages PaP1 and JG004. PLOS ONE. 2013;8(7):e68562. doi: 10.1371/journal.pone.0068562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann (1999).Lehmann PF. In: Manual of clinical microbiology. Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Vol. 146. 1999. pp. 107–108. (Mycopathologia). [Google Scholar]
- Lexomboon et al. (1972).Lexomboon U, Mansuwan P, Duangmani C, Benjadol P, M’cMinn MT. Clinical evaluation of co-trimoxazole and furazolidone in treatment of shigellosis in children. British Medical Journal. 1972;3:23–26. doi: 10.1136/bmj.3.5817.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2016).Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. The Lancet Infectious Diseases. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- Loc-Carrillo & Abedon (2011).Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage. 2011;1:111–114. doi: 10.4161/bact.1.2.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfdahl et al. (2009).Löfdahl M, Ivarsson S, Andersson S, Långmark J, Plym-Forshell L. An outbreak of Shigella dysenteriae in Sweden, May-2009, with sugar snaps as the suspected source. Euro Surveillance: Bulletin Europeen Sur Les Maladies Transmissibles: European Communicable Disease Bulletin. 2009;14:29–35. doi: 10.2807/ese.14.28.19268-en. [DOI] [PubMed] [Google Scholar]
- Lu & Collins (2009).Lu TK, Collins JJ. Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4629–4634. doi: 10.1073/pnas.0800442106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaden & Bhat (1985).Macaden R, Bhat P. The changing pattern of resistance to ampicillin and co-trimoxazole in Shigella serotypes in Bangalore, southern India. Journal of Infectious Diseases. 1985;152:1348–1348. doi: 10.1093/infdis/152.6.1348. [DOI] [PubMed] [Google Scholar]
- Magiorakos et al. (2012).Magiorakos AP, Srinivasan A, Carey R, Carmeli Y, Falagas M, Giske C, Harbarth S, Hindler J, Kahlmeter G, Olsson-Liljequist B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- Mahmoudi et al. (2017).Mahmoudi S, Pourakbari B, Moradzadeh M, Eshaghi H, Ramezani A, Haghi Ashtiani MT, Keshavarz Valian S, Mamishi S. Prevalence and antimicrobial susceptibility of Salmonella and Shigella spp. among children with gastroenteritis in an Iranian referral hospital. Microbial Pathogenesis. 2017;109:45–48. doi: 10.1016/j.micpath.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Mai et al. (2015).Mai V, Ukhanova M, Reinhard MK, Li M, Sulakvelidze A. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage. 2015;5(4):e1088124. doi: 10.1080/21597081.2015.1088124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marberg, Altmann & Eshkol-Bruck (1958).Marberg K, Altmann G, Eshkol-Bruck A. Observations on resistance to sulfadiazine and antibiotics in shigellosis. The American Journal of Tropical Medicine and Hygiene. 1958;7:51–57. doi: 10.4269/ajtmh.1958.7.51. [DOI] [PubMed] [Google Scholar]
- Martin, Pollard & Feldman (1983).Martin J, Pollard RA, Feldman RA. Shigella infections in the United States, 1974–1980. Journal of Infectious Diseases. 1983;147:771–775. doi: 10.1093/infdis/147.4.771. [DOI] [PubMed] [Google Scholar]
- Matsuzaki et al. (2005).Matsuzaki S, Rashel M, Uchiyama J, Sakurai S, Ujihara T, Kuroda M, Ikeuchi M, Tani T, Fujieda M, Wakiguchi H. Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. Journal of Infection and Chemotherapy. 2005;11:211–219. doi: 10.1007/s10156-005-0408-9. [DOI] [PubMed] [Google Scholar]
- Matsuzaki et al. (2014).Matsuzaki S, Uchiyama J, Takemura-Uchiyama I, Daibata M. Perspective: the age of the phage. Nature. 2014;509:S9–S9. doi: 10.1038/509S9a. [DOI] [PubMed] [Google Scholar]
- Mead et al. (2006).Mead P, Dunne E, Graves L, Wiedmann M, Patrick M, Hunter S, Salehi E, Mostashari F, Craig A, Mshar P. Nationwide outbreak of listeriosis due to contaminated meat. Epidemiology and Infection. 2006;134:744–751. doi: 10.1017/S0950268805005376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril et al. (1996).Merril CR, Biswas B, Carlton R, Jensen NC, Creed GJ, Zullo S, Adhya S. Long-circulating bacteriophage as antibacterial agents. Proceedings of the National Academy of Sciences of the United States of Americ. 1996;93:3188–3192. doi: 10.1073/pnas.93.8.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliutina & Vorotyntseva (1993).Miliutina L, Vorotyntseva N. Current strategy and tactics of etiotropic therapy of acute intestinal infections in children. Antibiotiki i Khimioterapiia: Antibiotics and Chemoterapy [sic]/Ministerstvo Meditsinskoi i Mikrobiologicheskoi Promyshlennosti SSSR. 1993;38:46–53. [PubMed] [Google Scholar]
- Mitsuhashi (1969).Mitsuhashi S. The R factors. The Journal of Infectious Diseases. 1969;119:89–100. doi: 10.1093/infdis/119.1.89. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi (1971).Mitsuhashi S. Epidemiology and genetics of R factors. Annals of the New York Academy of Sciences. 1971;182:141–152. doi: 10.1111/j.1749-6632.1971.tb30653.x. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi et al. (1960).Mitsuhashi S, Harada K, Kameda M, Suzuki M, Egawa R. On the drug-resistance of enteric bacteria. 3. Transmission of the drug-resistance from Shigella to F–or Hfr strains of E. coli K-12. The Japanese Journal of Experimental Medicine. 1960;30:301–306. [PubMed] [Google Scholar]
- Moradpour & Ghasemian (2011).Moradpour Z, Ghasemian A. Modified phages: novel antimicrobial agents to combat infectious diseases. Biotechnology Advances. 2011;29:732–738. doi: 10.1016/j.biotechadv.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Morello et al. (2011).Morello E, Saussereau E, Maura D, Huerre M, Touqui L, Debarbieux L. Pulmonary bacteriophage therapy on Pseudomonas aeruginosa cystic fibrosis strains: first steps towards treatment and prevention. PLOS ONE. 2011;6(2):e16963. doi: 10.1371/journal.pone.0016963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan et al. (2006).Morgan O, Crook P, Cheasty T, Jiggle B, Giraudon I, Hughes H, Jones S-M, Maguire H. Shigella sonnei outbreak among homosexual men, London. Emerging Infectious Diseases. 2006;12(9):1458–1460. doi: 10.3201/eid1209.060282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton & Engley Jr (1945).Morton HE, Engley Jr FB. The protective action of dysentery bacteriophage in experimental infections in mice. Journal of Bacteriology. 1945;49:245–255. doi: 10.1128/jb.49.3.245-255.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller et al. (2009).Müller L, Jensen T, Petersen R, Mølbak K, Ethelberg S. Imported fresh sugar peas as suspected source of an outbreak of Shigella sonnei in Denmark, April-May 2009. Eurosurveillance. 2009;14(24):19241. doi: 10.2807/ese.14.24.19241-en. [DOI] [PubMed] [Google Scholar]
- Munshi et al. (1987).Munshi M, Haider K, Rahaman M, Sack D, Ahmed Z, Morshed M. Plasmid-mediated resistance to nalidixic acid in Shigella dysenteriae type 1. The Lancet. 1987;330(8556):P419–P421. doi: 10.1016/S0140-6736(87)90957-3. [DOI] [PubMed] [Google Scholar]
- Naheed et al. (2004).Naheed A, Kalluri P, Talukder K, Faruque A, Khatun F, Nair G, Mintz E, Breiman R. Fluoroquinolone-resistant Shigella dysenteriae type 1 in northeastern Bangladesh. The Lancet Infectious Diseases. 2004;4(10):607–608. doi: 10.1016/S1473-3099(04)01143-0. [DOI] [PubMed] [Google Scholar]
- Nelson et al. (2012).Nelson DC, Schmelcher M, Rodriguez-Rubio L, Klumpp J, Pritchard DG, Dong S, Donovan DM. Endolysins as antimicrobials. Advances in Virus Research. 2012;83:299–365. doi: 10.1016/B978-0-12-394438-2.00007-4. [DOI] [PubMed] [Google Scholar]
- Nichter, Acuin & Vargas (2008).Nichter M, Acuin CS, Vargas A. Geneva: World Health Organizationhttp://whqlibdoc.who.int/publications/2008/9789241596473_eng.pdf Introducing zinc in a diarrhoeal disease control programme: guide to conducting formative research. 2008
- Nilsson (2014).Nilsson AS. Phage therapy—constraints and possibilities. Upsala Journal of Medical Sciences. 2014;119:192–198. doi: 10.3109/03009734.2014.902878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi (2005).Niyogi SK. Shigellosis. Journal of Microbiology. 2005;43(2):133–143. [PubMed] [Google Scholar]
- Nobrega et al. (2015).Nobrega FL, Costa AR, Kluskens LD, Azeredo J. Revisiting phage therapy: new applications for old resources. Trends in Microbiology. 2015;23:185–191. doi: 10.1016/j.tim.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Ochs, Davis & Wedgwood (1971).Ochs HD, Davis SD, Wedgwood RJ. Immunologic responses to bacteriophage ϕX 174 in immunodeficiency diseases. Journal of Clinical Investigation. 1971;50:2559. doi: 10.1172/JCI106756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs et al. (1993).Ochs HD, Nonoyama S, Zhu Q, Farrington M, Wedgwood RJ. Regulation of antibody responses: the role of complement and adhesion molecules. Clinical Immunology and Immunopathology. 1993;67:S33–S40. [PubMed] [Google Scholar]
- Olarte, Filloy & Galindo (1976).Olarte J, Filloy L, Galindo E. Resistance of Shigella dysenteriae type 1 to ampicillin and other antimicrobial agents: strains isolated during a dysentery outbreak in a hospital in Mexico City. Journal of Infectious Diseases. 1976;133(5):572–575. doi: 10.1093/infdis/133.5.572. [DOI] [PubMed] [Google Scholar]
- Okame et al. (2012).Okame M, Adachi E, Sato H, Shimizu S, Kikuchi T, Miyazaki N, Koga M, Nakamura H, Suzuki M, Oyaizu N. Shigella sonnei outbreak among men who have sex with men in Tokyo. Japanese Journal of Infectious Diseases. 2012;65:277–278. doi: 10.7883/yoken.65.277. [DOI] [PubMed] [Google Scholar]
- Oliveira et al. (2017).Oliveira PH, Touchon M, Cury J, Rocha EP. The chromosomal organization of horizontal gene transfer in bacteria. Nature Communications. 2017;8(1):841. doi: 10.1038/s41467-017-00808-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai et al. (2001).Pai H, Choi E-H, Lee H-J, Hong JY, Jacoby GA. Identification of CTX-M-14 extended-spectrum β-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. Journal of Clinical Microbiology. 2001;39(10):3747–3749. doi: 10.1128/JCM.39.10.3747-3749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal (1984).Pal S. Epidemic bacillary dysentery in West Bengal, India, 1984. The Lancet. 1984;1(8392):1462. doi: 10.1016/s0140-6736(84)91948-2. [DOI] [PubMed] [Google Scholar]
- Pherecydes Pharma (2015).Pherecydes Pharma Evaluation of phage therapy for the treatment of Escherichia coli and Pseudomonas aeruginosa wound infections in burned patients (PHAGOBURN) 2015. [15 June 2016]. ClinicalTrials gov. http://1 usa gov/1YtJ43U .
- Potera (2013).Potera C. Phage renaissance: new hope against antibiotic resistance. Environmental Health Perspectives. 2013;121(2) doi: 10.1289/ehp.121-a48. a48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querangal des Essarts (1933).Querangal des Essarts J. Le bactériophage dans une épidémie de dysenterie bacillaire (Applications thérapeutiques et prophylactiques) Bulletin of the Exotic Pathology Society. 1933;26:979–981. [Google Scholar]
- Rahaman et al. (1974).Rahaman MM, Huq I, Dey C, Kibriya AG, Curlin G. Ampicillin-resistant Shiga bacillus in Bangladesh. The Lancet. 1974;303(7854):P406–P407. doi: 10.1016/s0140-6736(74)93171-7. [DOI] [PubMed] [Google Scholar]
- Rahaman et al. (1975).Rahaman MM, Khan MM, Aziz K, Islam MS, Kibriya AG. An outbreak of dysentery caused by Shigella dysenteriae type 1 on a coral island in the Bay of Bengal. Journal of Infectious Diseases. 1975;132(1):15–19. doi: 10.1093/infdis/132.1.15. [DOI] [PubMed] [Google Scholar]
- Ries et al. (1994).Ries AA, Wells JG, OlivoIa D, Ntakibirora M, Nyandwi S, Ntibakivayo M, Ivey CB, Greene KD, Tenover FC, Wahlquist SP. Epidemic Shigella dysenteriae type 1 in Burundi: panresistance and implications for prevention. Journal of Infectious Diseases. 1994;169(5):1035–1041. doi: 10.1093/infdis/169.5.1035. [DOI] [PubMed] [Google Scholar]
- Rogerie et al. (1986).Rogerie F, Ott D, Vandepitte J, Verbist L, Lemmens P, Habiyaremye I. Comparison of norfloxacin and nalidixic acid for treatment of dysentery caused by Shigella dysenteriae type 1 in adults. Antimicrobial Agents and Chemotherapy. 1986;29:883–886. doi: 10.1128/AAC.29.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfo et al. (2011).Rolfo F, Marin GH, Silberman M, Pattin J, Giugnio S, Gatti B, Bettiol M, Rigoni A. Epidemiological study of shigellosis in an urban area of Argentina. The Journal of Infection in Developing Countries. 2011;6:324–328. doi: 10.3855/jidc.1977. [DOI] [PubMed] [Google Scholar]
- Ryan et al. (2012).Ryan EM, Alkawareek MY, Donnelly RF, Gilmore BF. Synergistic phage-antibiotic combinations for the control of Escherichia coli biofilms in vitro. FEMS Immunology & Medical Microbiology. 2012;65(2):395–398. doi: 10.1111/j.1574-695X.2012.00977.x. [DOI] [PubMed] [Google Scholar]
- Salmond & Fineran (2015).Salmond GP, Fineran PC. A century of the phage: past, present and future. Nature Reviews Microbiology. 2015;13:777–786. doi: 10.1038/nrmicro3564. [DOI] [PubMed] [Google Scholar]
- Scallan et al. (2011).Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States—major pathogens. Emerging Infectious Diseases. 2011;17(1):715. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelcher, Donovan & Loessner (2012).Schmelcher M, Donovan DM, Loessner MJ. Bacteriophage endolysins as novel antimicrobials. Future Microbiology. 2012;7:1147–1171. doi: 10.2217/fmb.12.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiferaw et al. (2012).Shiferaw B, Solghan S, Palmer A, Joyce K, Barzilay EJ, Krueger A, Cieslak P. Antimicrobial susceptibility patterns of Shigella isolates in foodborne diseases active surveillance network (foodnet) sites, 2000–2010. Clinical Infectious Diseases. 2012;54:S458–S463. doi: 10.1093/cid/cis230. [DOI] [PubMed] [Google Scholar]
- Shiga (1898).Shiga K. Ueber den Erreger der dysenterie in Japan. Zentralbl Bakteriol Mikrobiol Hyg (Vorläufige Mitteilung) 1898;23:599–600. [Google Scholar]
- Shiga (1936).Shiga K. The trend of prevention, therapy and epidemiology of dysentery since the discovery of its causative organism. New England Journal of Medicine. 1936;215:1205–1211. doi: 10.1056/NEJM193612242152602. [DOI] [Google Scholar]
- Shin et al. (2012).Shin H, Lee J-H, Kim H, Choi Y, Heu S, Ryu S. Receptor diversity and host interaction of bacteriophages infecting Salmonella enterica serovar Typhimurium. PLOS ONE. 2012;7(8):e43392. doi: 10.1371/journal.pone.0043392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver & Bostian (1993).Silver L, Bostian K. Discovery and development of new antibiotics: the problem of antibiotic resistance. Antimicrobial Agents and Chemotherapy. 1993;37:377–383. doi: 10.1128/AAC.37.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla et al. (2016).Singla S, Harjai K, Katare OP, Chhibber S. Encapsulation of bacteriophage in liposome accentuates its entry in to macrophage and shields it from neutralizing antibodies. PLOS ONE. 2016;11(4):e0153777. doi: 10.1371/journal.pone.0153777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopek et al. (1987).Slopek S, Weber-Dabrowska B, Dabrowski M, Kucharewicz-Krukowska A. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981–1986. Archivum Immunologiae et Therapiae Experimentalis. 1987;35:569–583. [PubMed] [Google Scholar]
- Smith, Bremner & Datta (1974).Smith J, Bremner D, Datta N. Ampicillin resistance of Shigella sonnei. Antimicrobial Agents and Chemotherapy. 1974;6:418–421. doi: 10.1128/AAC.6.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith & Huggins (1982).Smith HW, Huggins M. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. Microbiology. 1982;128:307–318. doi: 10.1099/00221287-128-2-307. [DOI] [PubMed] [Google Scholar]
- Smith & Huggins (1983).Smith HW, Huggins M. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. Microbiology. 1983;129(8):2659–2675. doi: 10.1099/00221287-129-8-2659. [DOI] [PubMed] [Google Scholar]
- Soffer et al. (2017).Soffer N, Woolston J, Li M, Das C, Sulakvelidze A. Bacteriophage preparation lytic for Shigella significantly reduces Shigella sonnei contamination in various foods. PLOS ONE. 2017;12(3):e0175256. doi: 10.1371/journal.pone.0175256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg et al. (2008).Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J, America IDSo The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clinical Infectious Diseases. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- Spence & McKinley (1924).Spence RC, McKinley EB. The therapeutic value of the bacteriophage in treatment of bacillary dysentery. Southern Medical Journal. 1924;17:563–571. doi: 10.1097/00007611-192408000-00005. [DOI] [Google Scholar]
- Stone (2002).Stone R. Stalin’s forgotten cure. Science. 2002;298(5594):728–731. doi: 10.1126/science.298.5594.728. [DOI] [PubMed] [Google Scholar]
- Sulakvelidze, Alavidze & Morris (2001).Sulakvelidze A, Alavidze Z, Morris JG. Bacteriophage therapy. Antimicrobial Agents and Chemotherapy. 2001;45:649–659. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers (1999).Summers WC. Felix dHerelle and the origins of molecular biology. USA: Yale University Press; 1999. [Google Scholar]
- Summers (2001).Summers WC. Bacteriophage therapy. Annual Reviews in Microbiology. 2001;55:437–451. doi: 10.1146/annurev.micro.55.1.437. [DOI] [PubMed] [Google Scholar]
- Sur, Niyogi & Sur (2003).Sur D, Niyogi S, Sur S. Multidrug-resistant Shigella dysenteriae type-1: forerunners of a new epidemic strain in Eastern India? Emerging Infectious Diseases. 2003;9:404–405. doi: 10.3201/eid0903.020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka et al. (1969).Tanaka T, Nagai Y, Hashimoto H, Mitsuhashi S. Distribution of R factors among Shigella strains isolated in Japan. Microbiology and Immunology. 1969;13:187–191. doi: 10.1111/j.1348-0421.1969.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Taylor, Keystone & Devlin (1980).Taylor D, Keystone J, Devlin H. Resistance to trimethoprim and other antibiotics in Ontario shigellae. The Lancet. 1980;1:426–426. [PubMed] [Google Scholar]
- The et al. (2016).The HC, Thanh DP, Holt KE, Thomson NR, Baker S. The genomic signatures of Shigella evolution, adaptation and geographical spread. Nature Reviews Microbiology. 2016;14:235–250. doi: 10.1038/nrmicro.2016.10. [DOI] [PubMed] [Google Scholar]
- Torres-Barceló et al. (2016).Torres-Barceló C, Franzon B, Vasse M, Hochberg ME. Long-term effects of single and combined introductions of antibiotics and bacteriophages on populations of Pseudomonas aeruginosa. Evolutionary Applications. 2016;9:583–595. doi: 10.1111/eva.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofa et al. (1999).Trofa AF, Ueno-Olsen H, Oiwa R, Yoshikawa M. Dr. Kiyoshi Shiga: discoverer of the dysentery bacillus. Clinical Infectious Diseases. 1999;29:1303–1306. doi: 10.1086/313437. [DOI] [PubMed] [Google Scholar]
- Twort (1915).Twort FW. An investigation on the nature of ultra-microscopic viruses. The Lancet. 1915;186:1241–1243. doi: 10.1016/S0140-6736(01)20383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ud-Din et al. (2013).Ud-Din AI, Wahid SU, Latif HA, Shahnaij M, Akter M, Azmi IJ, Hasan TN, Ahmed D, Hossain MA, Faruque AS. Changing trends in the prevalence of Shigella species: emergence of multi-drug resistant Shigella sonnei biotype g in Bangladesh. PLOS ONE. 2013;8:e82601. doi: 10.1371/journal.pone.0082601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF & World Health Organization (2006).UNICEF. World Health Organization Implementing the new recommendations on the clinical management of diarrhoea: guidelines for policy makers and programme managers. 2006. http://apps.who.int/iris/bitstream/10665/43456/1/9241594217_eng.pdf http://apps.who.int/iris/bitstream/10665/43456/1/9241594217_eng.pdf
- Vaill & Morton (1937).Vaill S, Morton GL. Bacteriophage therapy in bacillary dysentery. Translational Research. 1937;22:594–600. [Google Scholar]
- Veiga-Crespo & Villa (2010).Veiga-Crespo P, Villa TG. Advantages and disadvantages in the use of antibiotics or phages as therapeutic agents. Enzybiotics: Antibiotic Enzymes as Drugs and Therapeutics. 2010:27–58. [Google Scholar]
- Verbeken et al. (2014).Verbeken G, Huys I, Pirnay J-P, Jennes S, Chanishvili N, Scheres J, Górski A, De Vos D, Ceulemans C. Taking bacteriophage therapy seriously: a moral argument. BioMed Research International. 2014;2014:1–8. doi: 10.1155/2014/621316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh et al. (2009).Vinh H, Baker S, Campbell J, Hoang NVM, Loan HT, Chinh MT, Anh VTC, Diep TS, Schultsz C, Farrar J. Rapid emergence of third generation cephalosporin resistant Shigella spp. in southern Vietnam. Journal of Medical Microbiology. 2009;58:281–283. doi: 10.1099/jmm.0.002949-0. [DOI] [PubMed] [Google Scholar]
- Von Seidlein et al. (2006).Von Seidlein L, Kim DR, Ali M, Lee H, Wang X, Thiem VD, Chaicumpa W, Agtini MD, Hossain A, Bhutta ZA. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLOS Medicine. 2006;3:e353. doi: 10.1371/journal.pmed.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Dąbrowska, Mulczyk & Górski (2001).Weber-Dąbrowska B, Mulczyk M, Górski A. Inflammation. USA: Springer; 2001. Bacteriophage therapy of bacterial infections: an update of our institute’s experience; pp. 201–209. [PubMed] [Google Scholar]
- Weinbauer (2004).Weinbauer MG. Ecology of prokaryotic viruses. FEMS Microbiology Reviews. 2004;28:127–181. doi: 10.1016/j.femsre.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Weissman et al. (1975).Weissman J, Schmerler A, Gangarosa E, Marier R, Lewis J. Shigellosis in day-care centres. The Lancet. 1975;305:88–90. doi: 10.1016/S0140-6736(75)91086-7. [DOI] [PubMed] [Google Scholar]
- Wen et al. (2012).Wen X-M, Wu Y-G, Bian F-Z, Sun Y-G, Zheng X-F, Zhang Y-F, Jiang C, Yuan G-Y. High prevalence of atypical class 1 integrons and class 2 integrons in multi-drug resistance Shigella flexneri isolated from China. African Journal of Microbiology Research. 2012;6:6987–6993. [Google Scholar]
- Wittebole, De Roock & Opal (2014).Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014;5:226–235. doi: 10.4161/viru.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2014).World Health Organization (WHO) Antimicrobial resistance: global report on surveillance. WHO Press; Geneva: 2014. [Google Scholar]
- Worthington & Melander (2013).Worthington RJ, Melander C. Combination approaches to combat multidrug-resistant bacteria. Trends in Biotechnology. 2013;31:177–184. doi: 10.1016/j.tibtech.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2011).Wu T, Grassel C, Levine MM, Barry EM. Live attenuated Shigella dysenteriae type 1 vaccine strains overexpressing shiga toxin B subunit. Infection and Immunity. 2011;79:4912–4922. doi: 10.1128/IAI.05814-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoby & Benhar (2008).Yacoby I, Benhar I. Targeted filamentous bacteriophages as therapeutic agents. Expert Opinion on Drug Delivery. 2008;5:321–329. doi: 10.1517/17425247.5.3.321. [DOI] [PubMed] [Google Scholar]
- Yoichi et al. (2005).Yoichi M, Abe M, Miyanaga K, Unno H, Tanji Y. Alteration of tail fiber protein gp38 enables T2 phage to infect Escherichia coli O157: H7. Journal of Biotechnology. 2005;115:101–107. doi: 10.1016/j.jbiotec.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Young & Gill (2015).Young R, Gill JJ. Phage therapy redux—What is to be done? Science. 2015;350:1163–1164. doi: 10.1126/science.aad6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman et al. (1983).Zaman K, Yunus M, Baqui AH, Hossain K, Khan M. Co-trimoxazole-resistant Shigella dysenteriae type 1 outbreak in a family in rural Bangladesh. The Lancet. 1983;322:796–797. doi: 10.1016/s0140-6736(83)92332-2. [DOI] [PubMed] [Google Scholar]
- Zhang, Wang & Bao (2013).Zhang H, Wang R, Bao H. Phage inactivation of foodborne Shigella on ready-to-eat spiced chicken. Poultry Science. 2013;92:211–217. doi: 10.3382/ps.2011-02037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
This is a literature review article. No raw data was generated.