Abstract
Light–dark cycles mimicking natural settings in a zebrafish facility are crucial for maintaining fish with an entrained circadian clock making them an ideal vertebrate model to study such rhythms. However, failure to provide optimal conditions to include complete darkness can lead to a disturbed circadian pacemaker affecting physiology and behavior in zebrafish. To meet building code requirements, the aquatics facility in use was outfitted with EXIT signs emitting a constant light. To determine if light radiating from the EXIT sign has an effect on zebrafish embryo production, 100 fish (1:1 m/f ratio) were split and housed at 10 fish/L. Half were housed directly in front of the EXIT sign, whereas the other half (control) were housed under a true 14-h light–10-h dark cycle. Reproductive success was evaluated by recording fecundity and viability from 10 weekly matings under two light colors: red (640 nm) and green (560 nm). On average the control group spawned twice as many embryos compared to those housed in front of a red EXIT sign, whereas green EXIT sign showed no difference. This suggests the importance of providing a complete dark environment within the night cycle and a recommendation toward dim green EXIT signs to avoid a decline in reproductive performance.
Introduction
The zebrafish (Danio rerio) has become an increasingly popular model for biomedical research for a variety of reasons, including ease of care, high fecundity, rapid external development of optically transparent embryos, and its genetic similarity to humans. These factors provide an important foundation to study development and diseases since there are many human orthologs found in the zebrafish.1 Thousands of adult fish can be housed in narrow bookshelf-like recirculating tanks and provide an efficient use of space differing from other vertebrate models. Adult fish can provide many opportunities for investigation; however, it is the zebrafish embryo that offers unmatched opportunities through a “whole organism” approach for a wide variety of studies.2
While there have been recent advancements in breeding technology to capitalize on zebrafish spawning behavior and efficient collection of embryos,3 the infrastructure of a zebrafish facility, including room temperature, humidity, air exchanges, water quality, and light must provide optimal conditions for maximum efficiency. Established as early as 24 h postfertilization, the circadian rhythm is a daily pattern of rest and activity and is in direct response to light. In animals, clock genes regulate transcription–translation feedback loops that function in response to the 24-h light–24-h dark cycle found in nature. These feedback loops make sure that the animal's circadian rhythm is in sync with the 24-h light–24-h dark cycle found in the natural environment.4 In a laboratory setting, it is important to mimic these natural conditions as much as possible to maintain the health and well being of the zebrafish. Traditionally, zebrafish facilities have a 14-h light–10-h dark cycle to ensure that the natural circadian rhythm of all fish is kept intact. Following a night of sleep deprivation due to suboptimal lighting conditions, larval and adult zebrafish exhibit a higher arousal threshold and increase in daytime rest.5 These environmental changes can have potential negative effects on zebrafish behavior.
Even though a dark cycle can be achieved through timers, complete darkness in a facility can be easily overlooked. Because of the complex nature of large recirculating systems to house fish, many facilities are equipped with touch screen displays and potentially other equipment that may give off light. While EXIT signs are mandatory in certain layouts and must always remain on, they also represent another constant source of unwanted light in a zebrafish facility. According to the 2009 International Building Code, “Exits and exit access doors shall be marked by an approved EXIT sign readily visible from any direction of egress travel… EXIT sign placement shall be such that no point in an exit access corridor or exit passageway is more than 100 feet away.…”6 Therefore, the aim of this study was to determine if the light emitted from EXIT signs placed directly in front of housing tanks impacts embryo production in adult zebrafish.
Materials and Methods
Set up and fish (red EXIT sign)
Zebrafish used in this study were 10-month-old AB wild-type strain (AB1). Fish (50 males, 50 females) were randomly selected from a single population and split into two groups of 50 each (25 males, 25 females). The fish in each experimental group were housed in 1.1-L tanks at a density of 10 fish (5 males, 5 females) per tank. All fish were fed ∼315 mg of GEMMA Micro 300 (Skretting) per day and kept on a 14-h light–10-h dark cycle. During the 14 h light phase, a dual 56 W fluorescent light fixture containing Sylvania T5 fluorescent tubes at 3500 and 5000 kelvin provided daylight conditions throughout the facility. One group of five 1.1-L tanks were placed on the rack and directly in front of the closest EXIT sign (Philips Chloride, 45V series), 1.09 m away, with a constantly emitting LED red light at a wavelength of 639 nm. The location of this EXIT sign was predetermined during the onset of room layout, keeping in mind rack location and height, and based on one out of two means of egress out of the aquatics facility. The other five tanks in the control group were placed on a separate rack where light from the EXIT sign would not penetrate the tank, which allowed for complete darkness during the dark cycle. Both groups were placed equidistant (0.50 m) from the daylight fluorescent fixture.
Spawning trials
After a 2-week acclimation period, each group of fish was set up to spawn in an iSpawn™ (Tecniplast) keeping male and female fish separated overnight. The following morning the separator was removed and fish from each iSpawn were allowed to spawn for a 30-min interval. The embryos spawned by each group were collected and counted. Embryo viability of each clutch was calculated by saving 100 embryos from each group in two Petri dishes of 50 each and counted the following day. The spawning trials were repeated for a total 10 weeks keeping set up and spawn day consistent throughout.
Set up, fish and spawning trials (green EXIT sign)
The red EXIT sign was replaced with a green EXIT sign (Philips Chloride, 45V series) with a constantly emitting LED green light at a wavelength of 560 nm. Using a separate population of 8-month-old AB wild-type fish (AB2), the same methodology as described above was used to separate and house two groups in the identical locations as the previous trial. Keeping the methods consistent with the above trials under red light, these fish were allowed to acclimate for 2 weeks and then spawned weekly for a 10-week period. Fish set up, spawning, embryo collection, and viability were analyzed as described above.
Results
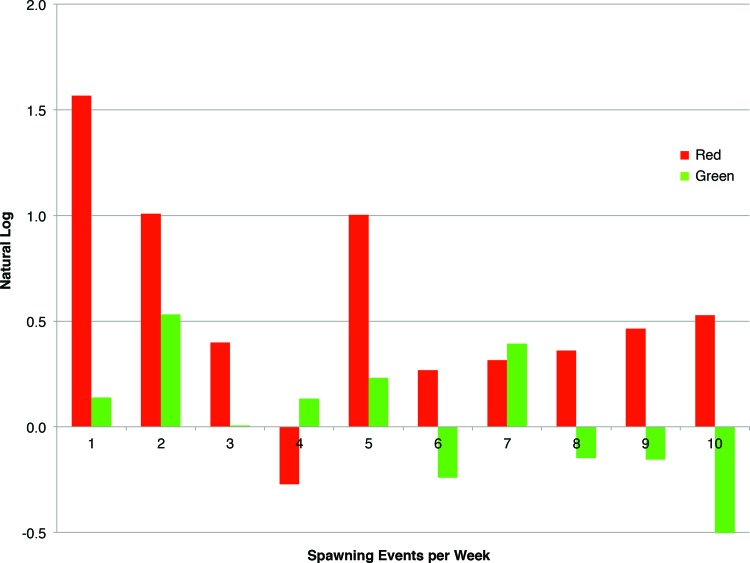
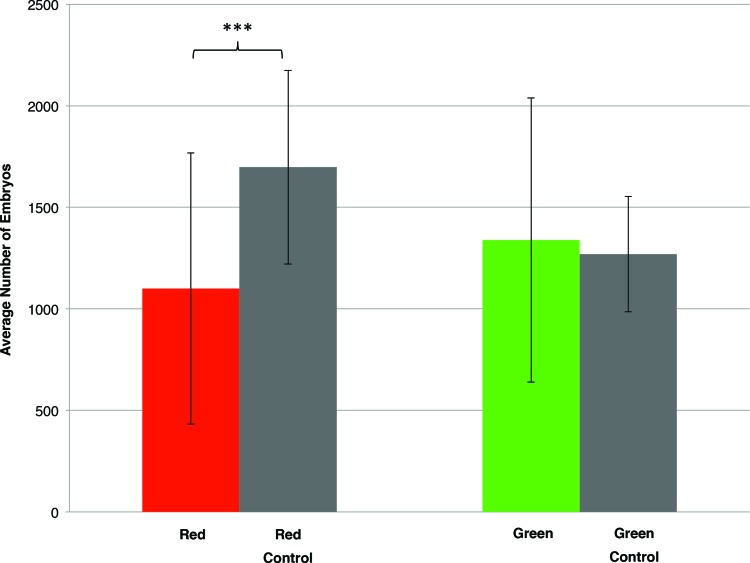
Under the two EXIT sign colors, the total number of live embryos spawned was used to compare the differences between fish housed in front of the light emitting sign versus a control group. The individual spawning events for the 10-week period is shown in Figure 1. In Figure 2, the averages of these 10 individual experiments are summarized. In front of the red emitting EXIT sign, the AB1 population spawned an average 1106 live embryos compared to the control, which spawned 1698 live embryos. This significant difference represents a 56% increase in embryo production with a significant p = 0.01. In front of the green emitting EXIT sign, the AB2 population spawned no significant difference compared to the control (average 1339 vs. 1269, respectively, p = 0.72). Under all four treatment groups (two controls, red EXIT and green EXIT) there was no difference in embryo viability.
FIG. 1.
Increase in total live embryo count from two AB wild-type populations of fish housed in front of a red versus green emitting EXIT sign compared to the control group. Under Red light, the average over a 10-week trial represents a statistically significant difference and a 56% increase in embryos spawned. Under Green light, no significant differences in embryos spawned were observed over a 10-week trial. Color images available online at www.liebertpub.com/zeb
FIG. 2.
Average number of live embryos spawned in a 30-min interval for the 10-week trial under the various conditions. Red and Green represent two AB wild-type populations of fish housed facing the EXIT sign. The control group represents a true 10-h dark cycle. Asterisks indicate a significant difference in reproductive performance (t-test, p < 0.05). Color images available online at www.liebertpub.com/zeb
Discussion
In combining genetics and behavior, zebrafish are an incredibly useful biomedical model for studying the visual system and the circadian clock. Previous studies have focused on the effects of light on larval growth, development, and behavior in fish.4,7,8 However, this is one of the first studies to address the effects of light and its impact on adult zebrafish as it relates to spawning and reproduction.
By using reproductive performance of zebrafish we were able to compare the effects of two wavelengths of mandatory light versus those fish housed under a true 14-h light–10-h dark cycle. Our results show a high potential for disruption to the circadian cycle for fish housed in front of a red emitting light resulting in a decreased embryo production. Although under the same housing, feeding and spawning conditions we did not see a significant difference in embryo production for fish housed in front of a green emitting light. The EXIT signs emit light at a relatively low intensity and there were no observed differences in behavior for groups of fish housed in front of light compared to those housed away from light. Even behavior for those fish housed in front of red light was no different to those housed in front of green light. However, there is a strong connection between the zebrafish circadian clock and spawning and reproductive behavior. Typically the most pronounced peak of embryo production occurs at dawn or immediately after illumination in domesticated fish.9 Although the location of where fish spawned in each trial was under the true 14-h light–10-h dark cycle, the affected fish were disturbed enough under their housing condition (red light) to show a significant decline in embryo production during the 10-week spawning trial.
Light and circadian control
Common to most teleosts, zebrafish are diurnal resulting in low levels of melatonin during the day followed by high levels of melatonin at night.10–12 In zebrafish, this endogenous rhythm is controlled in large part by the pineal system, a photosensory organ, and the retina, which contain circadian oscillators that regulate melatonin synthesis.10 As early as 18 h postfertilization, melatonin receptors are expressed in zebrafish larvae and by 24 h postfertilization, the pineal gland secrets melatonin and is sensitive to light.5,13 By 5 days postfertilization, the retina contains all cell types of a vertebrate eye including photoreceptors, which are further subdivided into cones and rods. Zebrafish are unique in their precise arrangement of cones laid out in a mosaic pattern and are subdivided into spectral subtypes based on peak sensitivity: ultraviolet–blue during larval stages and the addition of green–red developing later as juveniles.4,14,15 These are the first steps to establishing a circadian rhythm in zebrafish.
A review of several teleost fish reported a decline in melatonin synthesis at night during illumination of chromatic light. Experiments using light, from pulses to continuous, during the night cycle have been demonstrated to suppress and even completely eliminate melatonin synthesis.16,17 More specifically, a difference in measured concentrations of nocturnal plasma melatonin after groups of European Sea Bass were exposed to a pulse of light at the same intensity (6 μW/cm2), but at different wavelengths. Interestingly, the pulse of red light (640 nm) caused a greater reduction of naturally occurring levels of nocturnal plasma melatonin than the pulse of green light (544 nm).18 In fact, studies manipulating photoperiod by using variations of continuous and natural light in cod have been shown to delay spawning, reduce egg size, and reduce fecundity.4,7
In our study, light intensity from both red and green EXIT signs 1.09 m away from the light source was measured using a Wide Range Digital Light Meter (Mannix DLM-1337). Even though wavelength emitted differs in spectrum, we found that both EXIT signs emit the same intensity of light (0.08 fc), suggesting a difference in how light is perceived. While melatonin was not measured in the present study, this likely suggests an affect by the spectral quality causing stress and/or the inability to reset their circadian clock necessary to ensure a synchronized 24-h cycle. 4 In the natural setting, spectral composition is altered by the refraction of light by water, weather, and natural variations of time of day (dusk and dawn) and season.12 As light enters the water column it is perceived by fish through the retina and the pineal organ through overlying tissue of the skull. Different species perceive light differently as light penetrates through the skull, however, long wavelength light has been found to be far more effective at penetrating the skull and into these photoreceptive cells than shorter wavelengths.19 This further underscores the intricate relationship with specific physiological characteristics related to the circadian system such that it regulates the production and release of melatonin into circulation.4
Conclusion
As options for optimal facility design and layout are considered, special attention must be focused on light given off by electrical devices, especially as zebrafish and its reproductive potential are increasingly used in biomedical research. This attention must be given to all stages of zebrafish development.8 Further studies on wavelength and its effect on disrupting the circadian cycle causing decreased spawning in zebrafish may be important in further understanding a wide variety of husbandry and anatomical functions, including the sensitivity of the pineal system, the retina, stress factors, optimal housing conditions, spawning and reproduction, and many more. From this study it is possible to conclude that a completely dark environment is a vital environmental signal regulating optimal rhythmicity of melatonin synthesis that can have a significant impact on spawning and reproduction in zebrafish. If EXIT signs are required to act as guides to points of egress, then dim green light should be the preferred color of choice to allow for a stable circadian rhythm.
Acknowledgments
The authors would like to thank Avik Choudhuri, Paul Tighe, and Matthew Fickett for their thorough review of the article. A special thanks to Matthew F. for his insights throughout the entire study.
Disclosure Statement
No competing financial interests exist.
References
- 1.Brittijn SA, Duivesteijn SJ, Belmamoune M, Bertens LFM, Bitter W, Debruijn JD, et al. Zebrafish development and regeneration: new tools for biomedical research. Int J Dev Biol 2009;53:835–850 [DOI] [PubMed] [Google Scholar]
- 2.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet 2007;8:353–367 [DOI] [PubMed] [Google Scholar]
- 3.Adatto I, Lawrence C, Thompson M, Zon LI. A new system for the rapid collection of large numbers of developmentally staged zebrafish embryos. PLoS One 2011;6:e21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villamizar N, Vera LM, Foulkes NS, Sánchez-Vázquez FJ. Effect of lighting conditions on zebrafish growth and development. Zebrafish 2013;11:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhdanova IV. Sleep in zebrafish. Zebrafish 2006;3:215–226 [DOI] [PubMed] [Google Scholar]
- 6.Section 1011.1. Excerpted from the 2009 International Building Code; Copyright 2009. International Code Council, Washington, DC; Reproduced with permission. All rights reserved. Available at www.ICCSAFE.org Accessed October30, 2013 [Google Scholar]
- 7.Villamizar N, Blanco-Vives B, Migaud H, Davie A, Carboni S, Sánchez-Vázquez FJ. Effects of light during early larval development of some aquacultured teleosts: a review. Larvi 2009—Proceedings 5th Fish Shellfish Larviculture Symposium Gent, Belgium, September 2009, 2011, volume 315, pp. 86–94 [Google Scholar]
- 8.Bilotta J. Effects of abnormal lighting on the development of zebrafish visual behavior. Behav Brain Res 2000;116:81–87 [DOI] [PubMed] [Google Scholar]
- 9.Spence R, Fatema MK, Reichard M, Huq KA, Wahab MA, Ahmed ZF, et al. The distribution and habitat preferences of the zebrafish in Bangladesh. J Fish Biol 2006;69:1435–1448 [Google Scholar]
- 10.Cahill GM. Circadian regulation of melatonin production in cultured zebrafish pineal and retina. Brain Res 1996;708:177–181 [DOI] [PubMed] [Google Scholar]
- 11.Ekstrom P, Meissl H. The pineal organ of teleost fishes. Rev Fish Biol Fish 1997;7:199–284 [Google Scholar]
- 12.Falcón J, Migaud H, Muñoz-Cueto JA, Carrillo M. Current knowledge on the melatonin system in teleost fish. Gen Comp Endocrinol 2010;165:469–482 [DOI] [PubMed] [Google Scholar]
- 13.Kazimi N, Cahill GM. Development of a circadian melatonin rhythm in embryonic zebrafish. Dev Brain Res 1999;117:47–52 [DOI] [PubMed] [Google Scholar]
- 14.Allison WT, Barthel LK, Skebo KM, Takechi M, Kawamura S, Raymond PA. Ontogeny of cone photoreceptor mosaics in zebrafish. J Comp Neurol 2010;518:4182–4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson J, Schmitt EA, Hárosi FI, Reece RJ, Dowling JE. Zebrafish ultraviolet visual pigment: absorption spectrum, sequence, and localization. Proc Natl Acad Sci U S A 1993;90:6009–6012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolliet V, Falcón J, Ali MA. Regulation of melatonin secretion by light in the isolated pineal organ of the white sucker (Catostomus commersoni). J Neuroendocrinol 1995;7:535–542 [DOI] [PubMed] [Google Scholar]
- 17.Ziv L, Tovin A, Strasser D, Gothilf Y. Spectral sensitivity of melatonin suppression in the zebrafish pineal gland. Exp Eye Res 2007;84:92–99 [DOI] [PubMed] [Google Scholar]
- 18.Bayarri M, Madrid J, Sánchez-Vázquez F. Influence of light intensity, spectrum and orientation on sea bass plasma and ocular melatonin. J Pineal Res 2002;32:34–40 [DOI] [PubMed] [Google Scholar]
- 19.Migaud H, Taylor Jf, Taranger Gl, Davie A, Cerdá-Reverter Jm, Carrillo M, et al. A comparative ex vivo and in vivo study of day and night perception in teleosts species using the melatonin rhythm. J Pineal Res 2006;41:42–52 [DOI] [PubMed] [Google Scholar]