Abstract
Problem:
Among mechanisms triggering onset of parturition, it has been recently postulated that Toll-Like Receptor (TLR)9 engagement by cell-free DNA (cfDNA) triggers inflammation, myometrial contractions and labor in absence of infection. The current study evaluated whether direct (myometrial) or indirect (decidual) TLR9 engagement enhances human myometrial contractility.
Method of Study:
TLR9 expression and cellular localization were surveyed by immunohistochemistry of placenta, fetal membranes, and myometrium in term (gestational age [GA]:>37 weeks) labor (TL, n=7) or term non-labor (TNL, n=7) tissues. Non-pregnant myometrium (n=4) served as reference. TLR9 mRNA expression relative to other TLRs was evaluated through mining of an RNA-seq dataset and confirmed by RT-PCR. Immortalized human myometrial cells (hTERT-HM) were treated with incremental concentrations of TLR9 agonist ODN2395, TNF-α, or LPS. Secreted cytokines were quantified by multiplex immunoassay, and contractility was assessed by an in-gel cell contraction assay (n=9). Induction of hTERT-HM contractility was also evaluated indirectly following exposure to conditioned media from primary term decidual cells (n=4) previously stimulated with ODN2395.
Results:
TLR9 immunostaining in placenta and amniochorion was strongest in decidual cells, but unrelated to labor. TLR9 staining intensity was significantly decreased in TL compared to TNL myometrium (P=0.002). Although total cfDNA in maternal circulation increased in TL (P=0.025 vs. TNL), difference in cffDNA was non-significant. Myometrial TLR9 mRNA levels were unaffected by contractile status and far less abundant than other pro-inflammatory TLRs. hTERT-HM contractility was enhanced by LPS (P=0.002) and TNF-α (P=0.003), but not by ODN2395 (P=0.345) or supernatant of TLR9-stimulated decidual cells.
Conclusion:
Myometrial and decidual TLR9 are unlikely to directly regulate human parturition.
Keywords: TLR9, myometrium, decidua, labor, cytokines, DNA
INTRODUCTION
The molecular signals involved in the onset of human parturition are poorly understood. However, evidence exists that normal term spontaneous labor displays inflammatory features.1,2,3,4 Accordingly, leukocytes infiltrate the myometrium, cervix, and fetal membranes during the onset of labor resulting in production of pro-inflammatory cytokines including interleukin (IL)-1β, IL-6, IL-8 and tumor necrosis factor (TNF)-α.5,6 These cytokines may in turn increase production of prostaglandin E2 PGE2 and cyclooxygenase (COX)-2, and up-regulate oxytocin receptors on myometrial cells, which are necessary steps for initiation of uterine contractions.7,8
Toll-like receptors (TLRs) are membrane-bound proteins that recognize specific pathogen-associated molecular patterns and activate the innate immune system. Several evolutionarily related TLRs, including the TLR9 subfamily (TLR7, TLR8, and TLR9) and TLR3 recognize bacterial and viral nucleic acids and are localized in the endoplasmic reticulum in resting cells.9 Once stimulated by hypomethylated CpG DNA, which is present in DNA viruses, fetal DNA, and in >80% of bacterial genomes, TLR9 activates signal transduction pathways that induce expression of pro-inflammatory cytokines (TNF-α, IL-12, IL-6), chemokines, and other immune response genes through various mechanisms including trafficking to endolysosomal compartments, and activation of MAP kinase and NFκB pathways.10,11,12,13
Several TLRs have been identified in reproductive-specific tissues. Thus, TLRs 2–5 are present in the cervix, whereas spontaneous labor elicits significant decrease in expression of TLR3 and TLR5 transcripts.14,15 Transcripts for TLRs 1–10 have been detected in the human placenta.16 TLR2 and TLR4 and its adaptor molecule MD2, are upregulated in the decidua and chorio-amnion during term labor (TL) and preterm parturition, respectively, suggesting a role for these receptors in initiation of labor.17,18,19 Walsh et al. observed an association between spontaneous preterm labor and significantly higher TLR2 and TLR9 in leukocytes infiltrating maternal decidua accompanied by reduced methylation of both genes.20 In contrast, little information exists concerning the presence of TLRs in human myometrium itself.
In 1997, Lo et al. were the first to report the presence of cell-free fetal (cff)DNA in maternal serum and plasma.21 Subsequently cffDNA increases throughout pregnancy, with the clearest elevation evident in the last 8 weeks.22,23 Several investigators continued to explore the association between cffDNA levels and pregnancy outcomes, with prediction of the risk for preterm birth by elevated cffDNA levels representing a particularly notable area of interest. Elevated levels in maternal circulation between 26–34 weeks gestation of women who delivered prematurely led Leung et al. to suggest that cffDNA may serve as a biomarker for spontaneous preterm birth1 with subsequent studies supporting this suggestion.25,26
TLR9 can be stimulated by synthetic oligodeoxynucleotides containing unmethylated CpG motifs (CpG ODN).27 That fetal DNA is hypomethylated lends plausible support to the premise that cffDNA acts as a ligand for TLR9. Phillippe et al. hypothesized that increased levels of cffDNA activate the innate immune system through stimulation of TLR9, leading to onset of parturition.28 The current study investigated the presence of TLR9 in human laboring and non-laboring myometrium and observed significantly higher TLR9 staining intensity in non-labor versus labor associated myometrium. Analysis of the human myometrium transcriptome showed that TLR9 mRNA was unaffected by its contractile status and was far less abundant than RNA of other TLRs. The potential role of myometrial or decidual TLR9 in eliciting uterine contractions was assessed by stimulating immortalized human myometrial cells or primary decidual cells with CpG ODN. The results indicate that contractility is enhanced by lipopolysaccharides (LPS) and TNF-α, but not by exposure to relevant concentrations of ODN2395 or to the supernatant derived from TLR9-stimulated decidual cells.
MATERIALS AND METHODS
Biological samples and study design
Women were recruited at Yale New Haven Hospital and The Ohio State Wexner Medical Center. This study was approved by the Institutional Review Boards at Yale New Haven Hospital, The Ohio State University Wexner Medical Center, and Nationwide Children’s Hospital, and all patients provided written informed consent. Term gestation was defined as gestational age (GA): ≥37 weeks. GA was established based on last menstrual period confirmed by an ultrasound examination prior to 20 weeks. Eligible women had a singleton fetus without evidence of structural abnormalities at the time of birth or discharge. Exclusion criteria included presence of abnormal karyotype, congenital anomaly, women with maternal medical complications, or viral infection (Hepatitis B-C, HIV).
Using a case control study design, placenta, fetal membranes, myometrium, and serum samples were obtained from patients undergoing primary Cesarean section in the settings of term labor (TL, n=7, GA median [interquartile range]: 40 [39–40] weeks) or term non-labor (TNL, n=7, GA: 39 [39–39] weeks). The demographic and outcome characteristics of the pregnant women providing myometrium samples are presented in Table I. All Cesarean incisions were low-transverse with all full thickness biopsies collected from the upper edge of the hysterotomy. Cesarean indications for TNL specimens included breech presentation and a history of shoulder dystocia or perineal laceration. Indications for the TL group included labor dystocia through failure to descend or failure to progress despite adequate uterine contractions monitored by an intrauterine pressure catheter. Myometrium from reproductive age women obtained at the time of scheduled hysterectomy served as non-pregnant tissue control (n=4). Tissue biopsies were either frozen in liquid nitrogen immediately after delivery or immersed in buffered formalin and processed for paraffin embedding.
TABLE 1.
Demographic clinical and outcome characteristics of pregnant women who provided myometrial samples.
| Variable | Term Labor (TL) n = 7 |
Term Non-Labor (TNL) n = 7 |
P value |
|---|---|---|---|
| Clinical characteristics at the time of enrollment | |||
| Maternal age, years † | 31 [18–35] | 28 [27–34] | 0.309 |
| Parity † | 0 [0–0] | 1 [0–1] | 0.054 |
| Uterine contractions ‡ | 7 (100) | 0 (0) | <0.001 |
| Cervical dilatation before surgery, cm † | 8 [5–10] | 1 [1–1] | <0.001 |
| Race § Non-Hispanic white African-American Other |
3 (43) 1 (14) 3 (43) |
4 (57) 1 (14) 2 (29) |
0.842 |
| Clinical characteristics at the time of delivery | |||
| Gestational age at delivery, weeks † | 40 [39–40] | 39 [39–39] | 0.165 |
| Birth weight, grams † | 3300 [2980–3480] | 3060 [2900–3499] | 0.359 |
Data presented as median [interquartile range] and analyzed by Kruskal-Wallis ANOVA on Ranks.
Data presented as n (%) and analyzed by Fisher’s exact test.
Data presented as n (%) and analyzed by Chi square test.
Immunohistochemistry
Five μm tissue sections were deparaffinized in xylene and rehydrated with serial solutions of graded ethanol. Sections were then heated in citrate buffer for antigen retrieval and blocked with 5% donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour at room temperature, followed by incubation with mouse anti-human TLR9 monoclonal antibody (1:250, Abcam, Cat#: ab134368, Cambridge, MA) overnight at 4°C and followed by donkey anti-mouse IgG (1:600, Jackson ImmunoResearch Laboratories) for 1 hour at room temperature. VECTASTAIN Elite ABC complex and Vector Nova Red peroxidase substrate (Vector Laboratories, Burlingame, CA) were used for signal detection. Sections exposed to mouse IgG served as a negative control. TLR9 specific staining was scored semi-quantitatively in a blind fashion by examining six fields per slide and subjectively scoring the intensity of the chromogen deposited into the tissue on a scale from 0 (no staining) to 5 (intense red staining).
Real time PCR and analysis of RNA-seq datasets
Myometrial specimens were flash frozen in liquid nitrogen in the Operating Room and stored at −80°C. Total RNA was extracted from frozen myometrium and placenta specimens using TRIzol reagent (Life Technologies, Grand Island, NY) per the manufacturer’s recommendations. Total RNA integrity was assessed by agarose gel electrophoresis with ethidium bromide labeling in the presence of Protector RNase Inhibitor (Roche, Indianapolis, IN) to visualize intact 28S and 18S rRNA bands and further purified using an RNA Clean-Up and Concentration Kit (Norgen Biotek, Thorold, Ontario, Canada). TLR9 mRNA levels were quantified by real-time PCR using TaqMan probes from Applied Biosystems (Hs00152973_m1) relative to the housekeeping genes β2-microglobulin and RPL30, as previously described.29 In addition, to compare TLR9 levels to other TLRs, we referred to our previously published RNA-seq datasets of human myometrium30 and placenta.31
Measurement of maternal circulating cell-free DNA (cfDNA) and cell-free fetal DNA (cffDNA)
Total serum cfDNA was measured using direct SYBR Gold assay (Invitrogen, Carlsbad, CA), as previously described.32 Briefly, SYBR Gold Nucleic Acid Gel Stain (Invitrogen) was diluted first to 1:1,000 in DMSO, and then further diluted to 1:8 in phosphate buffered saline (PBS). Ten microliters of sample per well was applied to a black 96-well plate to which 40 μL of SYBR Gold stain was added to each well (final dilution 1:10,000). Fluorescence was measured with a 96-well fluorometer (CLARIOstar, BMG Labtech, Cary, NC) at an emission wavelength of 535 nm and an excitation wavelength of 485 nm and concentrations calculated using a standard curve. For assessment of background reading, maternal serum samples were pre-incubated for 5 hours with RNase-free, DNase I (5 PRIME Inc., Gaithersburg, MD), before inactivation with 20 mM ethylenediaminetetraacetic acid. Serum not treated with DNase was used to determine background fluorescence. Each assay was performed in triplicate.
To measure the concentration of cffDNA we used the fetal epigenetic marker RASSF1A, a tumor suppressor gene whose promotor is in reverse methylation state to most genes: hypomethylated in maternal blood cells and hypermethylated in cells of fetal and placental origin. Consequently, methylation-sensitive restriction enzymes will remove maternal sequences while leaving fetal sequences intact and hence able to serve as template for PCR amplification.33 Genomic DNA was extracted from 2-mL of plasma and subjected to digestion with double enzymes as previously described.34 Real-time PCR assays were established for detection of RASSF1A and beta-actin (ACTB) using published primer-probe sets.33 The copy number of RASSF1A in plasma was derived from a standard curve generated from placental DNA of known concentrations using the conversion factor of 6.6 pg DNA per diploid cell.
Myometrial cell culture
The hTERT-HM cells, a telomerase-immortalized myometrial smooth muscle cell line derived from the fundus of a non-pregnant, pre-menopausal woman35 were cultured in Dulbecco modified Eagle/F-12 medium with 10% fetal bovine serum (Invitrogen).
IL-6 immunoassay and V-PLEX inflammatory panel
hTERT-HM cells were seeded onto 96-well plates at a density of 105 cells/well, which resulted in 70% confluence. The cells were left to adhere for 3 hours after which treatments were added to duplicate wells. The following CpG ODNs (InvivoGen, San Diego, CA) were used in 24 hour incubations: ODN2395 (type-C specific TLR9 agonist; 0.5, 1, and 2 μM), negative control for ODN2395 (ODN-CRL, contains GpC nucleotides instead of CpG; 0.5, 1, and 2 μM). As controls, LPS (5 μg/mL, positive control) and endotoxin-free water (vehicle) were used. All agents in this study were diluted 7.5 μL of LPS-free water. Aliquots of 15 μL media were retrieved from each well at 4, 8, and 18 hours and the entire remaining volume collected at 24 hours. IL-6 was measured by immunoassay (eBioscience, Affymetrix, San Diego, CA) in all aliquots to determine the time course of release. The assay was performed according to the manufacturer’s instructions and conditioned media were run in duplicate with a minimal detection concentration of 0.039 pg/mL.
The cell culture medium collected at 24 hours was further analyzed using a multiplex immunoassay (V-PLEX Proinflammatory Panel 1 Human, Meso Scale Discovery, Rockville, MD) that included 10 pro- and anti-inflammatory cytokines: IFN-γ, TNF-α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL12p70, and IL-13. Assays were performed according to the manufacturer’s instructions with minimal modifications and optimization. Briefly, wells of the 96-well plates contained 50 μL of 1:2 diluted sample or high, medium, and low quality control samples supplied by the manufacturer. Plates were incubated at room temperature for 2.5 hours with continuous shaking then washed three times with 1× Wash Buffer. Following addition of SULFO-TAG Detection Antibody Cocktail, plates were incubated for 2 hours with shaking at room temperature, washed, and following addition of 150 μL Read Buffer per well, plates were scanned with a SECTOR Imager 6000 Reader (Meso Scale Discovery). Raw signals generated by the instrument were analyzed with Discovery Workbench 4.0 Software (Meso Scale Discovery). All values are reported in units of pg/mL. Assays performed in duplicate were averaged for analysis. The coefficient of variation for inter- and intra-assay variability was <7% for all assays.
Primary decidual cell culture
Decidual cells were isolated from term placentas (n=4), grown to confluence in basal medium consisting of phenol red-free DMEM/F12 (Gibco, Grand Island, NY) with 1% Antibiotic-Antimycotic (Gibco) supplemented with 5% charcoal stripped calf serum in 6-well plates and decidualized with 10−8 M β-Estradiol (E2, Sigma-Aldrich, Saint Louis, MO) plus 10−7 M medroxyprogesterone acetate (MPA, Sigma-Aldrich).36 Prior experiments have demonstrated that after 3–4 passages the cultures consist of almost pure decidual cells (>99%).37 After 7 days, decidual cell cultures were washed twice with PBS and switched to a serum-free defined medium consisting of phenol-red free DMEM without serum, and containing steroids (10−8 M E2 plus 10−7 M MPA), 1% ITS+ Premix (BD Biosciences, San Jose, CA), and 10 ng/mL epidermal growth factor (BD Biosciences) for 24 hours. The next day, decidual cell cultures were treated with vehicle (sterile water), 5 μg/mL LPS (L2880, Sigma-Aldrich), ODN2395 (1 μM), or ODN2395 control (1 μM), for 24 hours. Conditioned media was collected and frozen at −80°C for collagen gel contraction assays.
Collagen gel contraction assay
Collagen gel contraction assays were performed as described by Fitzgibbon et al.38 Collagen gels were prepared from sterile bovine Type 1 collagen (Cell Biolabs, San Diego, CA) to a final concentration of 1.5 mg/mL with 100,000 hTERT-HM cells/well and dispensed into wells of 24-well plates containing 1 mL culture medium/well. Cells in collagen gels equilibrated overnight in DMEM supplemented with FBS. The following ligands were added immediately prior to releasing collagen gels from the culture dishes: ODN2395 (0.5–2 μM), ODN-CRL (0.5–2 μM), TNF-α (10 ng/mL; R&D Systems, Minneapolis, MN), and LPS (5 μg/mL).
For TLR9 antagonist experiments, 1 μM concentration for ODN2395 and ODN-CRL was chosen based on the results of the cytokine stimulating experiments that demonstrated an optimal stimulatory effect at this concentration. TNF-α (10 ng/mL) and LPS (5 μg/mL) were used as positive control, non-TLR9 agonists. TLR9 antagonist A151 (1 μM, InvivoGen), an oligodeoxynucleotide containing repeats of the immunosuppressive motif TTAGGG found in mammalian telomeric DNA39 was added 30 minutes prior to agonist treatment and release.
The effects of ODN2395 and ODN-CRL (1 μM) were further tested in the presence of oxytocin (1–100 nM, Sigma-Aldrich). In addition, gel contraction assays were conducted in the presence of conditioned media from cultured term human decidual cells (described above), added just prior to releasing gels. Gel images were captured using ImageJ software (http://rsb.info.nih.gov/ij). For each treatment, collagen contraction was determined in triplicate at a minimum and each experiment was performed at least 3–4 times. Data were interpreted as % change in gel area compared to the water control, which was considered to reflect baseline in-gel contraction. For wells treated with TLR9 agonist and TLR9 antagonist, data were further normalized to contraction resulting from cells treated with ODN-CRL. For cells treated with TLR9 agonist and oxytocin, additional normalization to the size of the gels in the presence of oxytocin alone and/or oxytocin and ODN-CRL were considered as appropriate. For each experiment, sentinel wells with collagen gels, but no cells, were included to exclude any nonspecific shrinking effect during the 24 hour testing time period.
Statistical analysis
Statistical analyses were performed with Sigma Stat, version 2.03 (SPSS Inc., Chicago, IL, USA) and MedCalc (Broekstraat, Belgium) statistical software. Normality testing was performed using the Shapiro-Wilk test, and parametric and non-parametric data were compared using Mann-Whitney rank test or t-test, followed by the Holm-Sidak test. Nonparametric data were also compared using Kruskal-Wallis ANOVA on ranks followed by Dunn’s test. Immunoassay data were transformed logarithmically prior to statistical analysis using 2-way repeated measures (RM) ANOVA. Spearman correlations measured co-linearity between interdependent variables. Chi-square tests were used to make comparisons between proportions. P<0.05 was considered significant throughout the analysis.
RESULTS
Expression of TLR9 in human myometrium
Use of immunohistochemistry revealed that TLR9 is expressed in human myometrium. Staining intensity was higher in biopsies from TNL (Figure 1A) than from TL (Figure 1B) tissues (P<0.001, 1-way ANOVA). At higher magnification, TLR9 was observed to localize to myometrial smooth muscle cells (Figure 1A insert, arrow) and to a population of mononuclear cells likely of hematopoietic origin judging by their perivascular localization (Figure 1A insert, arrowhead). Non-pregnant myometrium displayed an intermediate level of staining (Figure 1C), higher than TL (P=0.007, Figure 1D), but lower than TNL (P=0.045, Figure 1D). Although the most conspicuous staining in non-pregnant myometrium was associated with blood vessel wall, intracellular staining for TLR9 was also detected in smooth muscle cells identified by their elongated shape. In both fetal membranes (Figure 1E&F) and placenta (Figure 1G&H), no differences in TLR9 immunostaining were observed in association with labor status. Among all cellular locations, the most conspicuous TLR9 staining was evident in decidual cells within the fetal membranes (decidua parietalis) and placenta (decidua basalis). Together, results suggest that at the protein level, human myometrium expresses TLR9 and that its presence is higher in TNL and non-pregnant tissues. The decrease in TLR9 protein during labor argues that endogenous TLR9 ligands play a minimal role in sustaining human labor at term.
FIGURE 1.
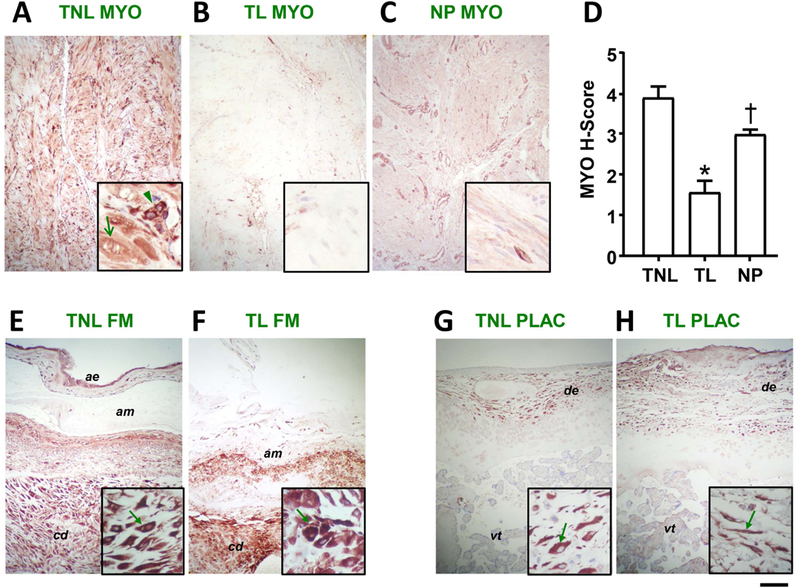
Presence and localization of TLR9 in human myometrium, fetal membrane and placenta. Representative micrographs of human myometrium (A) term non-labor (TNLm n=7), (B) term labor (TL, n=7), and (C) non-pregnant, immunohistochemically stained for TLR9. (D) Results of semi-quantitative scoring of myometrial (MYO) staining intensity (H-Score). Bars represent mean and standard error. Statistical comparison was conducted using 1-way ANOVA followed by Shapiro-Wilk post-hoc tests. Bars marked with symbols are statistically different at P<0.05 (1-way ANOVA with Holms-Sidak post-hoc tests). Representative micrographs of full thickness fetal membranes (FM) from (E) TNL and (F) TL deliveries with matched sections of placenta (G) TNL and (H) TL. Photographs were taken at 100× magnification for panels (scale bar: 100 nm) and 400× for insets (scale bar 25 nm). ae: amnion epithelium; am: amnion; cd: choriodecidua; de: decidual cells; pv: placental villi.
Myometrial TLR9 expression by RT-PCR and relative expression to other TLRs by RNA-seq
Myometrial expression of TLR9 was low compared to placenta (Figure 2A). Furthermore, in both tissues TLR9 expression was ~4 orders of magnitude less abundant than expression of the housekeeping genes used for relative quantitation. To further estimate relative abundance of TLR9 compared to that of other TLRs, we analyzed our previously published RNA-seq dataset of human TNL (n=5) and TL (n=5) myometrium.30 Among all TLRs (TLR1–10), the sequence counts corresponding to TLR9 transcripts were the lowest (Figure 2B). The highest sequence counts were registered for TLR4 (309-fold greater than TLR9) followed by TLR2 (170-fold greater than TLR9) (Figure 2B). A statistically significant increase in transcript abundance associated with labor status was observed only for TLR2 (P=0.029) and TLR8 (P=0.046). Significant decreases were associated with labor for both TLR3 (P=0.008) and TLR7 (P=0.004).
FIGURE 2.
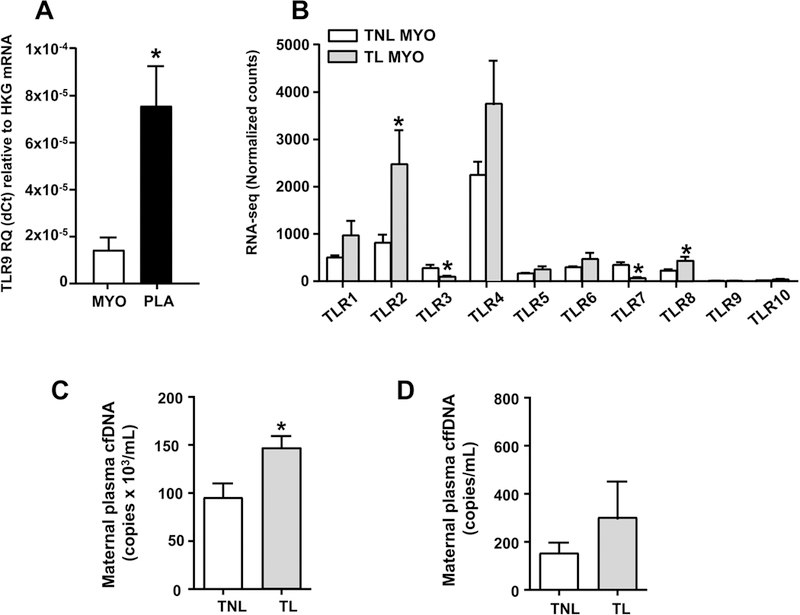
Human myometrial TLR9 expression by RT-PCR, relative expression to other TLRs by RNA-seq, and serum cfDNA and cffDNA levels in maternal circulation. (A) Relative quantitation (RQ) by real-time RT-PCR of TLR9 mRNA expression in myometrium and villous placenta relative to expression of housekeeping genes (HKG). (B) mRNA expression by RNA sequencing (RNA-seq) for the 10 known TLRs (TLR1-TLR10) in term non-labor (TNL, n=5) and labor (TL, n=5) myometrium. (C) Genomic equivalents of total cell-free DNA (cfDNA) and (D) cell-free fetal DNA (cffDNA) in maternal circulation in term women not in labor (TNL) or in active labor (TL). Results are shown as mean and standard error. The asterisk (*) denotes statistically significant difference at P<0.05 (Student t-test).
Maternal cfDNA and cffDNA levels
The total concentration of cfDNA in maternal circulation was significantly higher in women experiencing labor compared to those not in labor (Figure 2C, P=0.025). However, the difference in cffDNA did not reach statistical significance (Figure 2D, P=0.364).
Effect of TLR9 stimulation on cytokine release by hTERT-HM cells
As shown in Figure 3A, TLR9 stimulation with ODN2395 (1 and 2 μM) induced a transient, but significant increase in IL-6 concentration in culture medium that peaked at 4 hours before returning to basal levels (2-way RM ANOVA P=0.016 for 1 uM and P=0.015 for 2 μM). The lower concentration (0.5 μM) exerted no effect above the level elicited by ODN-CRL. By comparison, TLR4 stimulation with LPS increased IL-6 release at 4 hours compared to cells treated with vehicle (Figure 3B, P=0.004) and IL-6 continued to increase throughout the assay (P<0.001 at 8, 18, and 24 hours). Expanding the panel of cytokines measured at 24 hours by multiplex immunoassay revealed similar results. Accordingly, no significant increases were observed in any of the 10 cytokines after exposure to either 0.5 or 2 μM TLR9 agonist (Figure 3C). The sole significant change from ODN-CRL was a decrease in IFN-γ in response to the highest ODN2395 concentration (Figure 3D). This result contrasted with exposure of hTERT-HM cells to LPS, which induced the release of all of the cytokines displayed in the panel over vehicle alone (Figure 3E, 2-way ANOVA, P=0.002 for IL-13 and P<0.001 for all other cytokines).
FIGURE 3.
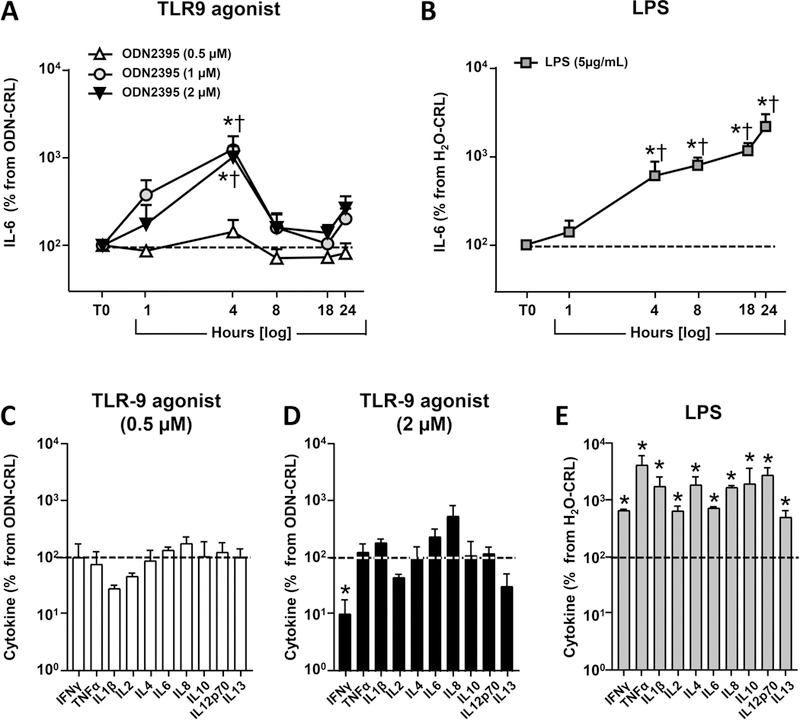
Effects of TLR stimulation on cytokine secretion by hTERT-HM cells. Time-course of IL-6 concentration in culture medium after challenge with (A) increasing concentrations of the TLR9 agonist ODN2395 (0.5, 1, or 2 μM) or (B) LPS (5 μg/mL). At each time point and for each independent experiment (n=4), IL-6 concentrations in wells with ODN2395 were normalized to IL-6 concentration in wells treated with the control oligonucleotide (ODN-CRL, has GpC nucleotides instead of CpG) and illustrated by the dashed horizontal line marking 100% baseline level. IL-6 concentrations in wells with LPS were normalized to levels measured in wells treated with the equivalent volume of endotoxin-free water (vehicle for LPS) which represents their baseline. *P<0.05 vs. baseline †P<0.05 vs. t=0 hours (T0), 2-way repeated measures ANOVA followed by post-hoc Holms-Sidak tests. (C) Concentration of a panel of cytokines measured by multiplex immunoassay in medium of hTERT-HM cells collected after 24 hours stimulation with low dose, 0.5 μM) or (D) high dose (2 μM) of TLR agonist ODN2395. (E) Cytokine levels after stimulation with LPS (5 μg/mL). Data is shown as mean and standard error. *P<0.05 vs. baseline (1-way repeated measures ANOVA with correction for multiple comparisons using the Holms-Sidak method).
Effect of TLR9 stimulation on basal hTERT-HM contraction
Representative photographs of the gel contraction assays are presented in Figure 4A. Over the course of 24 hours, the gel disks progressively shrink proportional to contraction of the cells embedded in the gel matrix. Both LPS and TNF-α accelerated the contraction of the gels compared to vehicle-treated cells (Figure 4B). The difference in gel area attained statistically significance at 6 hours (2-way RM ANOVA, P=0.035 and P=0.033, respectively). In comparison, TLR9 agonist ODN2395 (1 μM) did not affect the contraction of hTERT-HM cells embedded in collagen gels (Figrue 4B). As shown in Figure 4C, normalizing the gel area to the size of the gels treated with ODN-CRL revealed that both ODN2395 and its antagonist A151 elicited less contraction. At 24 hours, this effect attained significance for A151 (P<0.001 vs 0 hours and P=0.002 vs ODN-CRL). This effect was eliminated by challenging hTERT-HM cells with ODN2395 after pre-treatment with the antagonist (P=0.226 vs ODN-CRL, Figure 4C).
FIGURE 4.
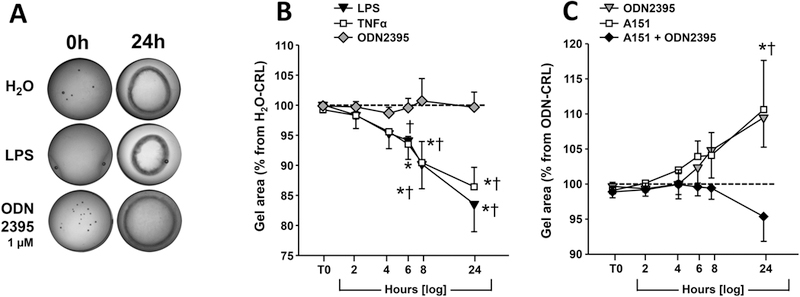
Effect of TLR stimulation on contractility of hTERT-HM cells embedded in collagen gels. (A) Representative gel contraction assay after 24 h treatment with water (vehicle), LPS (5 μg/mL) or ODN2395 (1 μM). (B) Results of collagen gel contraction assays after treatment with the TLR9 agonist ODN2395 (1 μM), LPS (5 μg/mL), TNF-α (10 ng/mL). Data was normalized to control wells which received water diluent and represent a common baseline for each independent experiment (n=6). (C) Results of collagen gel contraction assay after exposure to TLR9 agonist ODN2395 (1 μM), TLR9 antagonist A151 (1 μM), or their combination. Data was normalized to equivalent gels in each independent experiment (n=3) treated with ODN-CRL and considered as baseline. Data is shown as mean and standard error. *P<0.05 vs. baseline †P<0.05 vs. t=0 hours (T0), 2-way repeated measures ANOVA followed by post-hoc Holms-Sidak tests to correct for multiple comparisons.
Effect of TLR9 stimulation on oxytocin-induced hTERT-HM contraction
To further examine the potential for TLR9 stimulation to inhibit human myometrial contractility, collagen gels with embedded hTERT-HM cells were exposed to oxytocin. While no significant effect was observed in response to 10 nM oxytocin, treatment with 100 nM oxytocin resulted in significantly smaller average gel area at 2 hours (2-way RM ANOVA, P=0.008), 4 hours (P=0.005), and at all subsequent times analyzed from 6 to 24 hours (P<0.001 for all) compared to gels exposed to the equivalent water volume (Figure 5A).
FIGURE 5.
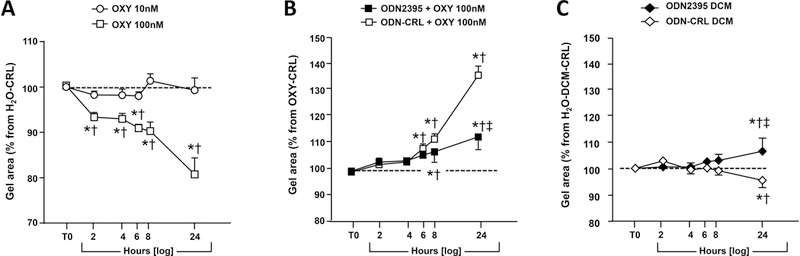
TLR9-mediated effects on oxytocin-induced contractility and decidual cell condition medium (DCM) on hTERT-HM cells embedded in collagen gels. (A) Contractility of hTERT-HM cells was enhanced by exposure to 100 ng/mL but not by 10 ng/mL of oxytocin. Data was normalized to gels treated with endotoxin-free water. (B) Effect of ODN2395 (CpG ODN) and ODN-CRL (GpC ODN) in the presence of 100 ng/mL oxytocin. Baseline reflects area of equivalent gels treated with 100 ng/mL oxytocin which was used for normalization for each individual experiment (n=4). (C) Indirect effects of decidual cell TLR9 stimulation on contraction of hTERT-HM cells embedded in collagen gels. Primary decidual cells were treated in culture for 1 μM ODN2395 or ODN-CRL (GpC-ODN, 1 μM) and DCM collected at 24 hours. Collagen gels with embedded hTERT-HM cells were then exposed to DCM for up to 24 hours. Percent gel contraction was normalized to baseline representing gels incubated in conditioned medium from decidual cells treated with equivalent volume of endotoxin-free water. The entire experiment was repeated 3 times. Data is shown as mean and standard error. *P<0.05 relative to baseline; †P<0.05 relative to t=0 hours (T0); ‡P<0.05 vs. ODN-CRL (2-way repeated measures ANOVA followed by post-hoc Holms-Sidak tests).
When hTERT-HM cells were challenged with ODN2395 (1 μM) or ODN-CRL (1 μM) in the presence of 100 nM oxytocin, a contractile inhibitory effect of ODN2395 relative to oxytocin alone was observed at 8 and 24 hours (2-way RM ANOVA P=0.026 and P<0.001, respectively, Figure 5B). However, this contractile inhibitory effect was also observed for gels treated with ODN-CRL which attained significance at 6 hours and remained significant at 8 and 24 hours (P<0.001). At 8 and 24 hours, gels exposed to oxytocin and ODN-CRL contracted significantly less than gels exposed to oxytocin and ODN2395 (P=0.033 and P<0.001, respectively) and both contracted less than gels exposed to 100 mM oxytocin alone as noted above. These results support the existence of a contractile inhibitory effect of these oligodeoxynucleotides on uterine myocytes albeit not mediated by TLR9.
Effect of conditioned medium from TLR9 stimulated decidual cells on hTERT-HM contraction
That the current immunohistochemistry experiments identified decidual cells as the most prominent expressor of TLR9 prompted exploration of an indirect stimulation scenario whereby primary decidual cells were first exposed to ODN2395 or ODN-CRL and then hTERT-HM exposed to decidual cell conditioned medium. The results of these experiments were normalized to the effect of decidual cell conditioned medium alone and are presented in Figure 5C. No differences were seen up to 8 hours. At the 24-hour time point, exposure to ODN-CRL induced a small but significant contraction as displayed by a decrease in the gel area (P=0.009), whereas ODN2395 produced less contraction both relative to water treatment of decidual cells (P=0.006) and to their treatment with ODN-CRL (P<0.001). These result support a distinct effect of ODN2395 compared to ODN-CRL that appears more likely to reflect a pro-relaxant rather than a pro-contractile effect.
DISCUSSION
There is growing interest in the role played by cffDNA in pregnancy. The source of cffDNA in maternal circulation is primarily of placental origin,40 and the release of cffDNA is considered to be part of physiologic syncytiotrophoblast turnover.41 Several studies, not stratified by labor status, demonstrated that the fetus also contributes to the circulating cfDNA pool. 20,21 The previously reported increased methylation ratio occurring during TL indicated that mother also makes a significant contribution to the total cfDNA.42 The above observations raise the relevant question as to what is the role and functional relevance of cfDNA in maternal circulation prior to or during human labor?
Use of immunohistochemistry detected the presence of TLR9 in human myometrium which was significantly down-regulated in TL, compared to TNL. Differing from the original hypothesis, our current finding implies that availability of the myometrial TLR9 system is inversely correlated with human myometrial contractions. In contrast with oxytocin, as well as in vitro administration of CpG ODN did not stimulate contraction of hTERT-HM cells. Interestingly, a high concentration of CpG ODN reversed oxytocin’s contractile effect. This result was also observed for the GpC ODN control. While the results presented in this study do not define the mechanism responsible for this effect, a reasonable suggestion is that GpC ODN may interfere with the role of oxytocin receptor in activating non-specific inflammatory pathways, regulation of miRNA expression, as well as the crosstalk between oxytocin and G protein-coupled receptors.43 Another possibility is that CpG and GpC motifs function as utero-relaxant agents independent of TLR9, which is in line with previous in-vitro observations that GpC ODN acting through TLR7 displayed immunosuppressive properties by allowing plasmacytoid dentritic cells to assist with generation of FOXP3+ T cells.44 While further studies are required to demonstrate that these mechanisms are active during human parturition, the current observation that TLR7 mRNA expression is significantly higher in the quiescent myometrium implies that prior to labor, GpC ODN may activate TLR7 and stimulate uterine quiescence by maintaining a state of immunosuppression. Nevertheless, based on the experimental results obtained in the current study alone does not support the conclusion that myometrial TLR9 is an important player in the initiation of human parturition.
Unlike myometrium, expression of placental villous and fetal membranes TLR9 did not vary with labor status. Unexpectedly, TLR9 immunostaining was most intense in the decidual cells, contrasting with an earlier study by Walsh et al., who reported that in the decidua most staining is carried by the infiltrating leukocytes.20 This discrepancy could be the result of use of different antibodies. We used a monoclonal antibody, whereas Walsh et al. used a polyclonal antibody.20 Because we verified the specificity of our antibody, we are confident in our data demonstrating expression of TLR9 by human decidua. Nonetheless, similar to our results, others demonstrated TLR9 is expressed in non-immune cells including trophoblasts and decidual cells.45,46,47 Interestingly, contraction of hTERT-HM cells was not indirectly elicited by stimulation of decidual cells with CpG ODN. Rather, conditioned medium from CpG ODN-stimulated primary term decidual cells suppressed hTERT-HM contractility significantly at 24 hours. Yet, we cannot exclude this result as an artifact from carryover ODN. These data, again, underline a potential minimal role of TLR9 in triggering human myometrial contractions.
The concept that cffDNA can bind to and stimulate TLR9 has biologic plausibility as DNA containing unmethylated CpG motifs is present in fetal DNA.27,28 We could not identify any study prior to ours that directly measured the concentration of cffDNA in maternal circulation in relation with term labor status. Our finding of no significant difference in directly measured cffDNA in labor concurs with the study by Herrera el al., who by measuring total cfDNA and methylation ratio concluded that term labor associates with a larger contribution of maternally derived cfDNA.42 Nevertheless, because in our study total cfDNA was found to increase during labor and there was a measurable presence of cffDNA in the maternal circulation, it is still possible for TLR9 engagement to play a role in tissues other than myometrium (e.g. placenta). Experimental animal models demonstrated that fetal resorption occurred following injection of hypomethylated human cffDNA into wild-type mice.48 This phenomenon was not observed following injection of human adult DNA (normal methylation).48 When cffDNA was injected into TLR9−/− mice, animals had normal gestational sacs and delivered normal healthy fetuses, indicating that fetal resorption is dependent on TLR9. In the TLR9−/− mice there was absent cellular infiltrate, TNF-α, and IL-6 staining at the placental site. Conversely, injection of cffDNA in wild-type mice, elicited local uterine inflammation, but no maternal systemic inflammatory response. Collectively, murine studies paint a picture of responses deleterious to pregnancy in which the trigger is unmethylated CpG containing DNA, the switch is TLR9, and outcomes are influenced by the cytokine response.
Innate immunity has fundamental roles creating immunologic tolerance at the maternal-fetal interface.49 Some proposed that at the maternal-fetal interface TLRs not only help to maintain pregnancy early on, but also promote pregnancy-associated complications including preeclampsia and preterm birth in response to excessive cytokine production.50,51 In our experiments, stimulation of hTERT-HM cells with CpG ODN promoted a transient induction of secreted IL-6 in a dose and time dependent fashion, indicating TLR9 activation of a system who’s viability was tested in comparison to that of LPS. However, in comparison with LPS, TLR9’s response was short-lived, implying TLR9’s functional relevance could be highly dependent on its interaction with antigen presenting cells such as B cells and dendritic cells, which were not present in our in-vitro experimental model.52 Despite minimal involvement of TLR9 in myometrial contractility, pattern recognition receptors have important roles in endometrial inflammation, and innate immunity.53 These roles are central to the defense of the endometrium against pathogens, most probably post-delivery of the fetus. Further studies are necessary to test these hypotheses.
Interestingly the antagonist, ODN A151 alone suppressed myometrial cell contractility, but not in the presence of CpG ODN, suggesting that its suppressive DNA motifs (TTAGGG) cannot entirely suppress the effect of TLR9 stimulation. The effects of ODN A151 extend beyond TLR9-mediated cytokine production.54,55 The mammalian telomeric TTAGGG motif repeated in ODN A151 abrogated CpG-stimulated IL-6 secretion, whereas mutagenesis of this suppressive motif or removal from telomeres of fetal, placental, and adult murine DNA enhanced proinflammatory cytokine secretion.56,57 Fetal DNA contains both stimulatory (hypomethylated CpG) and inhibitory (telomeric motifs) further complicating the signaling interplay, and potentially explaining the results of our ODN A151 experiments in the presence or absence of oxytocin.
One plausible explanation for the discrepancy of our findings with previous work is that our experiments were performed with cultured human myometrium and decidual cells versus earlier experiments in mice.48 There are significant differences between mouse and human immune system development, activation, and response to microbial pathogens, and these differences include TLRs and their ligands. It has been proposed that evolution and adaptation of mice and humans to different microbial environments modified TLR cell-type specificity, its ligand specificity and promoter regulation.58,59 Therefore, it warrants consideration that a given murine response may not extrapolate directly to humans. In fact, several studies have shown species-specific differences in distribution and function of TLRs (including TLR9) and other immune cells, between mice and humans.60,61,62 Decidua is composed of several cell types (stroma, glandular cells, leukocytes), whereas cultured primary term decidual cells used in our studies were purposely cleaned-up of other types of cells.37 Although our previous studies have shown that these decidual cells respond to pro-inflammatory stimuli mimicking chorioamnionitis,37 failure to elicit a response to TLR9 stimulation could reflect an incomplete complement of other cell types normally present in decidua.
In summary, our results suggest myometrial, placental, and decidual TLR9 appear to have minimal direct or indirect role in eliciting human myometrial cell contractility in-vitro. Engagement of TLR9 elicits a transitory inflammatory effect that may have a minimal role in triggering the forceful and sustained myometrial contractions required for human parturition.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the help provided by Dr. Dennis Lewandowski in formatting and editing the manuscript. They would like to acknowledge the fellows, research nurses and patients that provided consent for this study.
FUNDING: Research on this project was supported by internal funds of the Center for Perinatal Research of the Research Institute at Nationwide Children’s Hospital, Columbus, OH. In addition, funds from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/NICHD) R01HD062007 were used to support collection and processing of biospecimens used in this study. The funding sources had no involvement in study design, interpretation of data, writing of the report or decision to submit the paper for publication.
LIST OF ABBREVIATIONS
- GA
gestational age
- TLR
Toll-like receptor
- cf
cell-free
- TNL
term non-labor
- TL
term labor
- ODN
oligodeoxynucleotide
Footnotes
DISCLOSURE
The authors have no financial conflicts of interest.
REFERENCES
- 1.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci 2009;16:206–215. [DOI] [PubMed] [Google Scholar]
- 2.Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labor is an inflammatory process. Hum Reprod 1999;14:229–236. [PubMed] [Google Scholar]
- 3.Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Lye SJ. Myometrial immune cells contribute to term parturition, preterm labor and post-partum involution in mice. J Cell Mol Med 2013;17:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudley DJ, Collmer D, Mitchell MD, Trautman MS. Inflammatory cytokine mRNA in human gestational tissues: implications for term and preterm labor. J Soc Gynecol Investig 1996;3:328–335. [PubMed] [Google Scholar]
- 5.Young A, Thomson AJ, Ledingham MA, Jordan F, Greer IA, Norman JE. Immunolocalization of pro-inflammatory cytokines in myometrium, cervix and fetal membranes during human parturition at term. Biol Reprod 2002;66:445–449. [DOI] [PubMed] [Google Scholar]
- 6.Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labor at term. Mol Hum Reprod 2003;9:41–45. [DOI] [PubMed] [Google Scholar]
- 7.Todd HM, Dundoo VL, Gerber WR, Cwiak CA, Baldassare JJ, Hertelendy F. Effect of cytokines on prostaglandin E2 and prostacyclin production in primary cultures of human myometrial cells. J Matern Fetal Med 1996;5:161–167. [DOI] [PubMed] [Google Scholar]
- 8.Rauk PN, Friebe-Hoffmann U, Winebrenner LD, Chiao JP. Interleukin-6 up-regulates the oxytocin receptor in cultured uterine smooth muscle cells. Am J Reprod Immunol 2001;45:148–153. [DOI] [PubMed] [Google Scholar]
- 9.Wagner H The immunobiology of the TLR9 subfamily. Trends Immunol 2004;25:381–386. [DOI] [PubMed] [Google Scholar]
- 10.Latz E, Schoenemeyer A, Visintin A, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 2004;5:190–198. [DOI] [PubMed] [Google Scholar]
- 11.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol 2006;7:49–56. [DOI] [PubMed] [Google Scholar]
- 12.Beutler BA. TLRs and innate immunity. Blood 2009;113:1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll like receptors. Nat Immunol 2010;11:373–84. [DOI] [PubMed] [Google Scholar]
- 14.Hassan SS, Romero R, Haddad R, et al. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2006;195:778–786. [DOI] [PubMed] [Google Scholar]
- 15.Ackerman WE, Buhimschi IA, Snedden A, et al. Comparative transcriptomics analysis of human cervix and myometrium using next-generation RNA sequencing (RNAseq). #263. Am J Obstet Gynecol 2018;218:S168. [Google Scholar]
- 16.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull 2005;28:886–892. [DOI] [PubMed] [Google Scholar]
- 17.Holmlund U, Cebers G, Dahlfors AR, et al. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology 2002;107:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YM, Romero R, Chaiworapongsa T, et al. Toll-like receptor-2 and −4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol 2004;191:1346–1355. [DOI] [PubMed] [Google Scholar]
- 19.Dulay AT, Buhimschi CS, Zhao G, et al. Buhimschi. Amniotic Fluid Soluble Myeloid Differentiation-2 (sMD-2) as Regulator of Intra-amniotic Inflammation in Infection-induced Preterm Birth. Am J Reprod Immunol 2015l73:507–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh SW, Chumble AA, Washington SL, Archer KJ, Sahingur SE, Strauss JF 3rd. Increased expression of toll-like receptors 2 and 9 is associated with reduced DNA methylation in spontaneous preterm labor. J Reprod Immunol 2017;121:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo YM, Corbetta N, Chamberlain PF, et al. Presence of fetal DNA in maternal plasma and serum. Lancet 1997;350:485–487. [DOI] [PubMed] [Google Scholar]
- 22.Lo YM, Tein MS, Lau TK, et al. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am J Hum Genet 1998;62:768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birch L, English CA, O’Donoghue K, Barigye O, Fisk NM, Keer JT. Accurate and robust quantification of circulating fetal and total DNA in maternal plasma from 5 to 41 weeks gestation. Clin Chem 2005;51:312–320. [DOI] [PubMed] [Google Scholar]
- 24.Leung TN, Zhang J, Lau TK, Hjelm NM, Lo YM. Maternal plasma fetal DNA as a marker for preterm labour. Lancet 1998;352:1904–1905. [DOI] [PubMed] [Google Scholar]
- 25.Farina A, LeShane ES, Romero R, et al. High levels of fetal cell-free DNA in maternal serum: a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol 2005;193:421–425. [DOI] [PubMed] [Google Scholar]
- 26.Jakobsen TR, Clausen FB, Rode L, Dziegiel MH, Tabor A. High levels of fetal DNA are associated with increased risk of spontaneous preterm delivery. Prenat Diagn 2012;32:840–845. [DOI] [PubMed] [Google Scholar]
- 27.Bauer S, Kirschning CJ, Häcker H, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA 2001;98:9237–9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillippe M Cell-free Fetal DNA–A Trigger for Parturition. N Engl J Med 2014;370:2534–2536. [DOI] [PubMed] [Google Scholar]
- 29.Lee SY, Buhimschi IA, Dulay AT, et al. IL-6 trans-signaling system in intra-amniotic inflammation, preterm birth, and preterm premature rupture of the membranes. J Immunol 2011;186:3226–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackerman WE 4th, Buhimschi IA, Brubaker D, et al. Integrated microRNA and mRNA network analysis of the human myometrial transcriptome in the transition from quiescence to labor. Biol Reprod 2018;98:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ackerman WE 4th, Buhimschi IA, Eidem HR, et al. Comprehensive RNA profiling of villous trophoblast and decidua basalis in pregnancies complicated by preterm birth following intra-amniotic infection. Placenta 2016;44:23–33. [DOI] [PubMed] [Google Scholar]
- 32.Goldshtein H, Hausmann MJ, Douvdevani A. A rapid direct fluorescent assay for cell-free DNA quantification in biological fluids. Ann Clin Biochem 2009;46:488–494. [DOI] [PubMed] [Google Scholar]
- 33.Chan KC, Ding C, Gerovassili A, et al. Hypermethylated RASSF1A in maternal plasma: A universal fetal DNA marker that improves the reliability of noninvasive prenatal diagnosis. Clin Chem 2006;52:2211–2218. [DOI] [PubMed] [Google Scholar]
- 34.Zhao F, Wang J, Liu R, et al. Quantification and application of the placental epigenetic signature of the RASSF1A gene in maternal plasma. Prenat Diagn 2010;30:778–782. [DOI] [PubMed] [Google Scholar]
- 35.Condon J, Yin S, Mayhew B, et al. Telomerase immortalization of human myometrial cells. Biol Reprod 2002;67:506–514. [DOI] [PubMed] [Google Scholar]
- 36.Lockwood CJ, Murk W, Kayisli UA, et al. Progestin and thrombin regulate tissue factor expression in human term decidual cells. J Clin Endocrinol Metab 2009;94:2164–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lockwood CJ, Arcuri F, Toti P, et al. Tumor necrosis factor-alpha and interleukin-1beta regulate interleukin-8 expression in third trimester decidual cells: implications for the genesis of chorioamnionitis. Am J Pathol 2006;169:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzgibbon J, Morrison JJ, Smith TJ, O’Brien M. Modulation of human uterine smooth muscle cell collagen contractility by thrombin, Y-27632, TNF alpha and indomethacin. Reprod Biol Endocrinol 2009;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flierl U, Nero TL, Lim B, et al. Phosphorothioate backbone modifications of nucleotide-based drugs are potent platelet activators. J Exp Med 2015;212:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bianchi DW. Circulating fetal DNA: its origin and diagnostic potential-a review. Placenta 2004;25:S93–S101. [DOI] [PubMed] [Google Scholar]
- 41.Tjoa ML, Cindrova-Davies T, Spasic-Boskovic O, Bianchi DW, Burton GJ. Trophoblastic oxidative stress and the release of cell-free feto-placental DNA. Am J Pathol 2006;169:400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herrera CA, Stoerker J, Carlquist J, et al. Cell-free DNA, inflammation, and the initiation of spontaneous term labor. Am J Obstet Gynecol 2017;217:583.e1–583.e8. [DOI] [PubMed] [Google Scholar]
- 43.Kim SH, Bennett PR, Terzidou V. Advances in the role of oxytocin receptors in human parturition. Mol Cell Endocrinol 2017;449:56–63. [DOI] [PubMed] [Google Scholar]
- 44.Volpi C, Fallarino F, Bianchi R, et al. A GpC-rich oligonucleotide acts on plasmacytoid dendritic cells to promote immune suppression. J Immunol 2012;189:2283–2289. [DOI] [PubMed] [Google Scholar]
- 45.Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol 2010;63:587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patni S, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. Expression and activity of Toll-like receptors 1–9 in the human term placenta and changes associated with labor at term. Biol Reprod 2009;80:243–248. [DOI] [PubMed] [Google Scholar]
- 47.Krikun G, Lockwood CJ, Abrahams VM, Mor G, Paidas M, Guller S. Expression of Toll-like receptors in the human decidua. Histol Histopathol 2007;22:847–854. [DOI] [PubMed] [Google Scholar]
- 48.Scharfe-Nugent A, Corr SC, Carpenter SB, et al. TLR9 provokes inflammation in response to fetal DNA: mechanism for fetal loss in preterm birth and preeclampsia. J Immunol 2012;188:5706–5712. [DOI] [PubMed] [Google Scholar]
- 49.Bulmer JN, Pace D, Ritson A. Immunoregulatory cells in human decidua: morphology, immunohistochemistry and function. Reprod Nutr Dev 1988;28:1599–1613. [DOI] [PubMed] [Google Scholar]
- 50.Patni S, Flynn P, Wynen LP, et al. An introduction to Toll-like receptors and their possible role in the initiation of labour. BJOG 2007;114:1326–1334. [DOI] [PubMed] [Google Scholar]
- 51.Panda B, Panda A, Ueda I, et al. Dendritic cells in the circulation of women with preeclampsia demonstrate a pro-inflammatory bias secondary to dysregulation of TLR receptors. J Reprod Immunol 2012;94:210–215. [DOI] [PubMed] [Google Scholar]
- 52.Kaisho T, Akira S. Regulation of dendritic cell function through Toll-like receptors. Curr Mol Med 2003;3:373–385. [DOI] [PubMed] [Google Scholar]
- 53.Sheldon IM, Bromfield JJ. Innate immunity in the human endometrium and ovary. Am J Reprod Immunol. 2011;66 Suppl 1:63–71. [DOI] [PubMed] [Google Scholar]
- 54.Kaminski JJ, Schattgen SA, Tzeng TC, Bode C, Klinman DM, Fitzgerald KA. Synthetic oligodeoxynucleotides containing suppressive TTAGGG motifs inhibit AIM2 inflammasome activation. J Immunol 2013;191:3876–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shirota H, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides inhibit Th1 differentiation by blocking IFN-gamma- and IL-12-mediated signaling. J Immunol 2004;173:5002–5007. [DOI] [PubMed] [Google Scholar]
- 56.Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, Klinman DM. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol 2003;171:1393–1400. [DOI] [PubMed] [Google Scholar]
- 57.Goldfarb IT, Adeli S, Berk T, Phillippe M. Fetal and Placental DNA Stimulation of TLR9: A Mechanism Possibly Contributing to the Pro-inflammatory Events During Parturition. Reprod Sci 2017;25:788–796. [DOI] [PubMed] [Google Scholar]
- 58.Rehli M Of mice and men: species variations of Toll-like receptor expression. Trends Immunol 2002;23:375–378. [DOI] [PubMed] [Google Scholar]
- 59.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004;172:2731–2738. [DOI] [PubMed] [Google Scholar]
- 60.Haehnel V, Schwarzfischer L, Fenton MJ, Rehli M. Transcriptional regulation of the human Toll-like receptor 2 gene in monocytes and macrophages. J Immunol 2002;168:5629–5637. [DOI] [PubMed] [Google Scholar]
- 61.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol 2002;168:4531–4537. [DOI] [PubMed] [Google Scholar]
- 62.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med 2001;194:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


