Abstract
Objective
To investigate whether increased fixed carbidopa doses of 65 or 105 mg (ODM-101/65 and ODM-101/105) in combination with 75, 100, 125, or 150 mg of levodopa and 200 mg of entacapone might improve “off” time in fluctuating Parkinson disease (PD) compared to the standard combination of 4:1 levodopa/carbidopa with the usual 200 mg of entacapone (LCE) during a 4-week treatment period.
Methods
This was a randomized, double-blind, double-dummy, active-controlled, crossover, multicenter, phase II, proof-of-concept study in patients with fluctuating PD.
Results
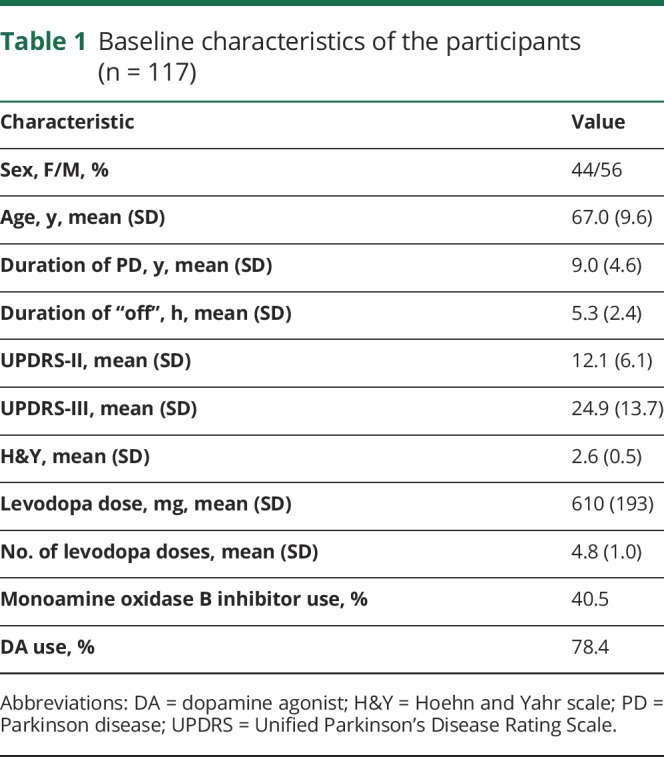
One hundred seventeen patients were randomized into the study (mean age 67.0 years; daily “off” time 5.3 hours; mean daily levodopa dose 610 mg). Carryover-adjusted mean changes from baseline “off” times were during ODM-101/65, −1.53 hours (p = 0.02 vs LCE), during ODM-101/105, −1.57 hours (p = 0.01 vs LCE), and during LCE −0.91 hours. Changes in daily “on” time without dyskinesia were 1.54 hours (p = 0.005 vs LCE), 1.38 hours (p = 0.0214 vs LCE), and 0.69 hours, respectively. Changes in “on” time with troublesome dyskinesia were <0.1 hours and not significantly different between treatments. In patients with high-activity COMT genotypes Val/Met or Val/Val, “off” time was reduced more with ODM-101/65 and ODM-101/105 than with LCE (p = 0.015 and p = 0.006). No difference between the treatments was seen in safety and tolerability. The most common treatment-related adverse effects were nausea, dizziness, drug-effect decrease, and dyskinesia, which were in most cases mild or moderate in severity. Treatment-related serious adverse events were diarrhea (ODM-101/105 and LCE), and myocardial ischemia and blood creatine kinase increase (LCE).
Conclusion
Increasing the dose of carbidopa in combination with levodopa and entacapone should be considered in the treatment of fluctuating PD to improve daily “off” times. Genotyping patients with PD according to COMT activity may improve individual treatment strategies.
ClinicalTrials.gov identifier
Classification of evidence
This study provides Class II evidence that an increased dose of carbidopa improves motor fluctuations when administered with levodopa and entacapone.
Dopa decarboxylase inhibitors (DDCIs) carbidopa and benserazide have been used for decades in Parkinson disease (PD) to reduce peripheral adverse effects and to increase levodopa uptake into the brain.1 It was reported in the early 1980s that changing the levodopa/carbidopa ratio from 10:1 to 5:1 or 4:1 increased the area under the curve (AUC) of levodopa by up to 25%2,3 with improved antiparkinsonian effects in some patients.2,4,5 Thereafter, it has been generally accepted that a 75 to 150-mg daily dose of carbidopa administered over 3 dosages is enough to fully inhibit peripheral dopa decarboxylase (DDC).4,6 Indeed, the Martindale Complete Drug Reference still states that the “full inhibition of peripheral dopa-decarboxylase is reported to be achieved with 70–100 mg of carbidopa daily.”7
The introduction of peripheral catechol-O-methyltransferase (COMT) inhibitors entacapone, tolcapone, and opicapone represented a major advance in the treatment of wearing-off in PD due to their ability to prolong the effect of levodopa.8–10 We hypothesized that adding a COMT inhibitor to levodopa/DDCI therapy might change the balance between the DDC and COMT pathways, making the commonly used 4:1 levodopa/carbidopa ratio insufficient in inhibiting peripheral DDC. Therefore, we increased the dose of carbidopa to 65 or 105 mg with the expectation that this modification might improve the treatment response in fluctuating PD. We also assessed the effect of genetic polymorphisms of COMT as entacapone has shown to inhibit COMT activity, levodopa elimination, and improve clinical efficacy more in patients with PD who have the high-activity COMT genotype Val/Val compared to the low-activity Met/Met genotype.11
Methods
Classification of evidence
This study was designed to investigate the efficacy of 2 increased and fixed carbidopa doses (65 and 105 mg) in combination with the COMT inhibitor entacapone compared with levodopa/carbidopa/entacapone (LCE) in patients with PD who have end-of-dose wearing off. This study provides Class II evidence that an increased dose of carbidopa improves motor fluctuations in patients with PD when administered with levodopa and entacapone (daily carryover-adjusted “off” time −1.53 for 65 mg [p = 0.02 vs LCE] and −1.57 for 105 mg [p = 0.01 vs LCE] compared to −0.91 hours for LCE).
Study design and treatments
This was a randomized, double-blind, double-dummy, active-controlled, crossover, multicenter, phase II, proof-of-concept study using the intention-to-treat approach in its analysis. The study was conducted in 24 centers in Germany, Finland, Latvia, Lithuania, and Romania.
Study population
Enrolled patients had idiopathic PD according to the UK brain bank criteria and were at least 30 years of age. The following were inclusion criteria: patients were required to have an average of ≥3.0 hours of “off” time, with a minimum of 0.5 hours of “off” time each day on 3 consecutive days (as assessed with home diaries) with Hoehn and Yahr12 stage 2–4 during “on” time. The main exclusion criteria were severe dyskinesia, severe orthostatic hypotension, currently active hallucinations, a Mini-Mental State Examination score <24, and problematic impulse control disorders.
At study entry, patients were allowed to be on 3 to 8 regular daily doses of levodopa/DDCI with or without entacapone. One evening dose of controlled-release levodopa/DDCI was allowed. Use of soluble levodopa/benserazide preparations up to 4 doses per week (not on diary days) was also allowed as a rescue therapy. All standard levodopa preparations were to be switched to study medications at randomization.
Randomization and masking
The random allocation of treatment sequences to participants' numbers was performed at Orion Pharma by Oracle Clinical Randomization. The design was a 3-period crossover that was stratified by study center and in blocks of 6 treatment sequences according to a Williams13 design. The personnel at the study sites used the Interactive Voice Response System to allocate 3 treatments in randomized order.
To blind the study treatments, a double-dummy technique with matching placebos was used. The ODM-101 tablets were identical. The levodopa dose, however, was unblinded in all ODM-101 and LCE preparations and labeled to ensure the correct individual levodopa dosing for each patient.
None of the persons directly involved in the conduct of the study had access to the treatment codes. The treatment code was opened at Orion Pharma after the study database was locked.
Study conduct
The study consisted of a screening period, 3 treatment periods of 4 weeks each, and a posttreatment period. There were no washout periods between the treatment periods.
The patients' individually optimized daily levodopa regimens were kept stable for at least 2 weeks before randomization. The patients were randomized to receive ODM-101 with 200 mg of entacapone and 65 mg of carbidopa and 75, 100, 125, or 150 mg of levodopa (ODM-101/65), ODM-101 with 200 mg of entacapone and 105 mg of carbidopa and 75, 100, 125, or 150 mg of levodopa (ODM-101/105), and LCE. At each levodopa intake, the patients received either ODM-101/65 or ODM-101/105 and the corresponding placebo for LCE, or LCE and the corresponding placebo for ODM-101.
The levodopa dosage in the study drug (75, 100, 125, or 150 mg) was determined by the patient's individually optimized levodopa regimen (3–8 daily doses including soluble and controlled-release evening formulations if taken regularly). Baseline 75 mg levodopa dosage without entacapone was switched to 75 mg study-drug dosage, 100 mg was switched to 75 mg, 125 mg was switched to 100 mg, 150 mg was switched to 125 mg, and 200 mg was switched to 150 mg. Baseline levodopa dosage with entacapone was switched to the same dosage of the study drug. During the first 2 weeks of each treatment period, the patient's levodopa dosage (but not frequency) was adjusted as needed by the investigator. For the remaining 2 weeks of each treatment period, levodopa dosages were kept stable. The use of tolcapone was prohibited, but stable doses of dopamine agonists (except apomorphine), monoamine oxidase B inhibitors, amantadine, and anticholinergics were allowed.
The patients who had signed informed consent for pharmacogenetic studies (n = 76) were included in a substudy. Blood samples for DNA extraction were taken at the screening visit from these patients and checked for COMT Val158Met polymorphism.
Efficacy variables
The primary efficacy criterion was the change in diary-recorded “off” time during 3 consecutive days from baseline compared to the end of each crossover period. Patients (or caregivers if needed) filled in the diary.14 A concordance training session of at least 2 hours before the baseline visit was performed by all patients. Secondary efficacy variables were diary recorded as “on” time without dyskinesia, “on” time with nontroublesome dyskinesia, and “on” time with troublesome dyskinesia.14 Unified Parkinson's Disease Rating Scale, Parts I–IV (UPDRS I–IV), as well as Schwab and England scores were evaluated by investigators at baseline and at the end of each study period. UPDRS Part III was assessed 30 to 90 minutes after study-drug intake during the presumed “on” stage at each visit. UPDRS Part III was completed after the same study-drug dose of the day. For example, if originally done after the second dose before the randomization visit, then the following assessments were done after the second dose at the end of the treatment periods as well. The study also evaluated the use of electronic home diaries (eDiary) to collect “on/off” times.
Tolerability and safety assessments
Safety was determined by adverse-event documentation, vital signs, ECG, safety laboratory parameters, and physical examination. The occurrence of sleep attacks was separately assessed by asking: “In the past 4 weeks, have you experienced any events in which you fell asleep suddenly or unexpectedly?”
Statistical analysis
For the sample-size calculation, the assumption was to detect a 1-hour decrease in “off” time, calculated as change from baseline, with either dose of ODM-101 compared to LCE with an estimated SD of 2.5. When the significance level was set to 5%, and the power to 80%, the subsequent sample size was estimated to be 13 patients per sequence. Considering up to a 20% dropout rate, 16 patients per treatment sequence were to be randomized, that is, in total approximately 100 patients.
Efficacy variables were analyzed by 3 predefined analyses: crossover analysis (primary analysis), crossover analysis with first-order carryover adjustment, and parallel-group analysis for the first period. Carryover is the persistence of a treatment applied during a certain period in a subsequent period of treatment,15 and if simple carryover applies, then Williams squares are optimal designs. The primary evaluations of efficacy variables were analyzed using mixed-effects models for crossover design. The models included baseline, treatment, period, and sequence as fixed effects and participant and residual error as random effects. In addition to crossover analysis, analyses with first-order carryover effect in the model were performed with an additional variable for carryover adjustment. Missing data were not imputed. However, in the statistical analyses, all the available data were utilized using mixed-model theory, where both the patient error stratum (between patients) and the residual stratum (within patients) can be utilized. Statistical methods for the parallel-group analyses were analysis of covariance with the factors of treatment and baseline.
Standard protocol approvals, registrations, and patient consents
For this study, we received approval from the appropriate local and national ethical standards committees. Written informed consents from all patients participating in this study were obtained before the start of study. The clinicaltrials.gov identifier is NCT01766258.
Data availability
The protocol and the statistical analysis plan are available on request. Deidentified participant data are not available for legal and ethical reasons.
Results
Demographics and other baseline characteristics
In total, 137 participants were screened for the study between May 31, 2011, and April 3, 2012, during which 117 were deemed eligible for the study and randomized. One hundred eleven participants completed period 1, 105 period 2, and 101 period 3 of the study. One participant was excluded from the results, leaving in total 100 participants for the final analysis (figure 1). Baseline characteristics are given in table 1.
Figure 1. Study participant disposition and treatment allocations.
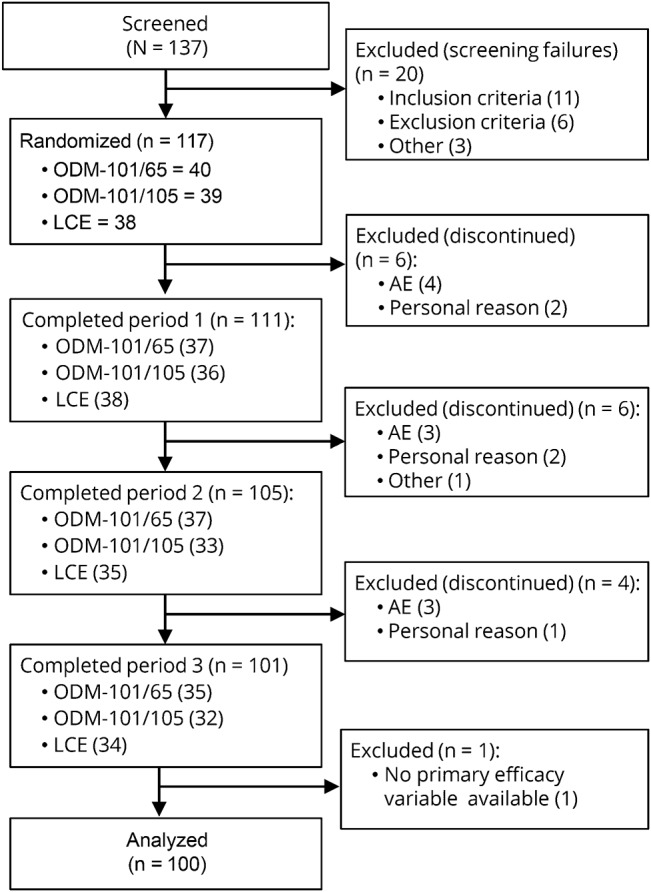
AE = adverse event; LCE = levodopa/carbidopa/entacapone.
Table 1.
Baseline characteristics of the participants (n = 117)
Efficacy variables
Primary efficacy variable: “Off” time
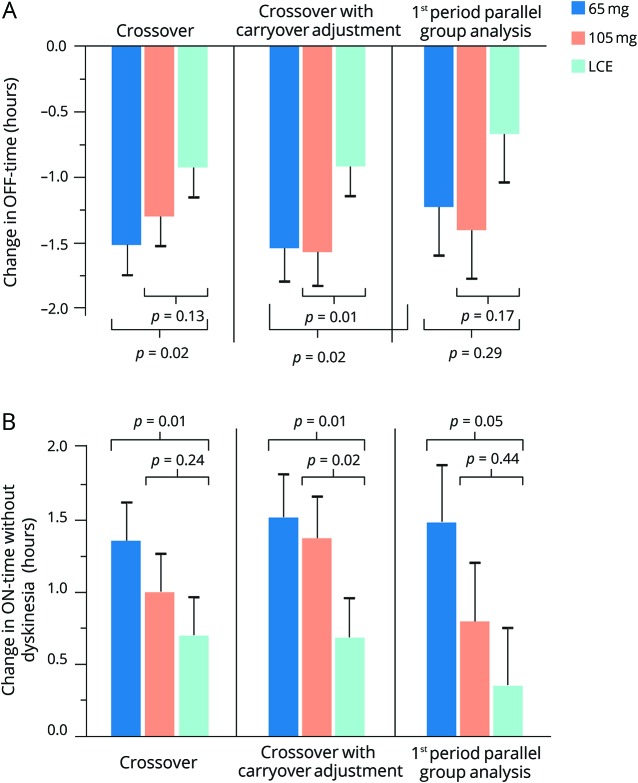
In the primary crossover analysis, the estimated mean changes from baseline in daily “off” time were −1.51 (95% confidence interval: −1.97 to −1.05) for ODM-101/65 mg (n = 107, p = 0.02 vs LCE), −1.29 (−1.75 to −0.83) for ODM-101/105 mg (n = 110, p = 0.13 vs LCE), and −0.92 (−1.38 to −0.47) hours for LCE (n = 110). There was a significant carryover effect (p = 0.048) and therefore a carryover-adjusted statistical model for “off” times was also applied: estimated mean changes in “off” times were −1.53 (−2.05 to −1.01) for ODM-101/65 mg (p = 0.02 vs LCE), −1.57 (−2.08 to −1.05) for ODM-101/105 mg (p = 0.01 vs LCE), and −0.91 (−1.37 to −0.46) hours for LCE. In the first-period parallel-group comparison, the respective changes in “off” time were −1.22 (−1.96 to −0.49) for ODM-101/65 mg (n = 37, p = 0.29 vs LCE), −1.40 (−2.15 to −0.65) for ODM-101/105 mg (n = 36, p = 0.17 vs LCE), and −0.67 (−1.39 to 0.06) hours for LCE (n = 38) (figure 2A).
Figure 2. Effect of carbidopa increase on “off” and “on” times.
(A) Mean changes (−SEM) from baseline in daily “off” time. (B) Mean changes (+SEM) from baseline in daily “on” time without dyskinesia. 65 mg = ODM-101/65; 105 mg = ODM-101/105; LCE = levodopa/carbidopa/entacapone.
Predefined, explorative subgroup analyses were also done for “off” time (crossover analyses). The number of patients in different subgroups varied significantly depending on the analysis. Estimated treatment effects between the 2 ODM-101 combinations vs LCE were numerically higher (−0.76 for ODM-101/65 and −0.85 hours for ODM-101/105) in patients who had a higher daily levodopa dose at baseline (mean 812 [SD 144] mg (n = 43) compared to patients with a lower levodopa dose (482 [79] mg [n = 65, −0.49, and 0.08 hours]). In addition, patients taking 5 (n = 38) or more frequent (n = 21) doses of levodopa at baseline had numerically higher treatment differences (−0.97 and −0.86 or −0.70 and −0.86 hours) favoring ODM-101 combinations compared to LCE than patients taking less than 5 doses a day (n = 51, −0.31 and 0.04 hours). Patients who did not have entacapone at baseline (n = 22) had numerically higher treatment differences favoring ODM-101 combinations compared to LCE (−1.43 and −1.37 hours) when compared to those on entacapone at baseline (n = 85, −0.34 and −0.11 hours). Treatment effects were similar (−0.57 and −0.48, −0.66 and −0.58, or −0.51 and −0.10 hours) between ODM-101 combinations and LCE in patients with different durations of “off” time at baseline (<3, 3–5, or >5 hours; n = 8, 47, or 52).
“On” time without dyskinesia
In the primary crossover analysis, the estimated mean changes from baseline in daily “on” time without dyskinesia were 1.37 (0.83, 1.90) for ODM-101/65 mg, 1.01 (0.47, 1.55) for ODM-101/105 mg, and 0.69 (0.16, 1.23) hours for LCE. The respective carryover-adjusted changes were 1.54 (0.93, 2.14) for ODM-101/65 mg (p = 0.005 vs LCE), 1.38 (0.79, 1.98) for ODM-101/105 mg (p = 0.0214 vs LCE), and 0.69 (0.16, 1.23) hours for LCE (figure 2B).
“On” time with nontroublesome dyskinesia
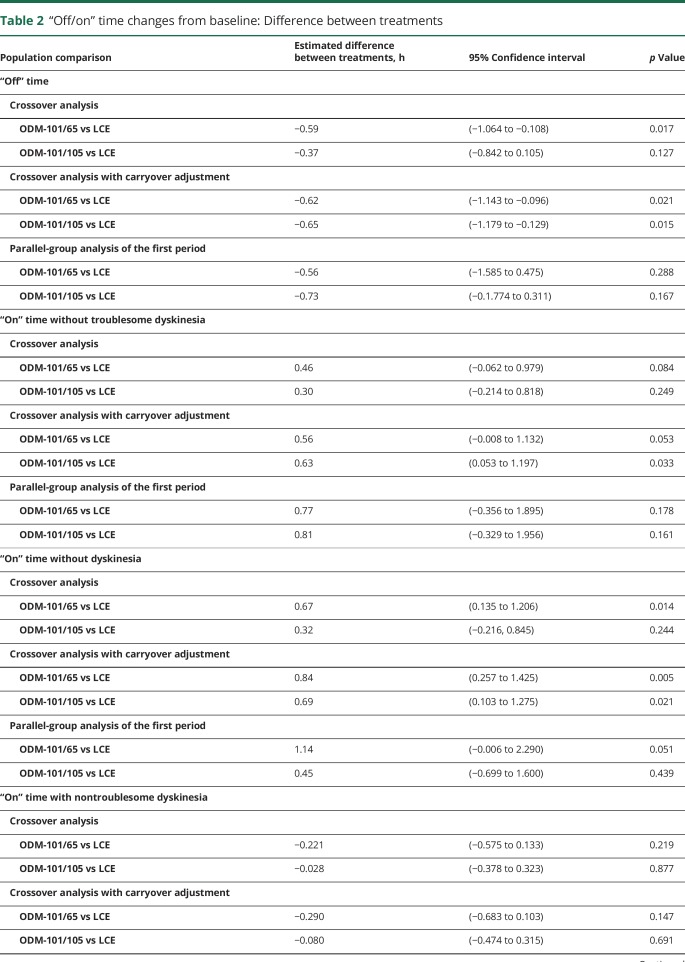
In the primary crossover analysis, the estimated mean changes from baseline in daily “on” time with nontroublesome dyskinesia were 0.09 (−0.33, 0.52) for ODM-101/65 mg, 0.29 (−0.14, 0.71) for ODM-101/105 mg, and 0.32 (−0.11, 0.74) hours for LCE. The respective carryover-adjusted changes were 0.02 (−0.44, 0.49) for ODM-101/65 mg (p = 0.15 vs LCE), 0.23 (−0.23, 0.69) for ODM-101/105 mg (p = 0.69 vs LCE), and 0.69 (0.16, 1.23) hours for LCE (table 2).
Table 2.
“Off/on” time changes from baseline: Difference between treatments
“On” time with troublesome dyskinesia
At the baseline of each treatment period, the duration of “on” time with troublesome dyskinesia varied between 0.68 and 0.73 hours. The changes from baseline were <0.1 hours for all 3 treatments in the crossover analyses with or without carryover adjustments. All comparisons between the treatments (including first-period parallel comparison) were statistically nonsignificant (table 2).
“Off” time by COMT enzyme rs4680 genotype
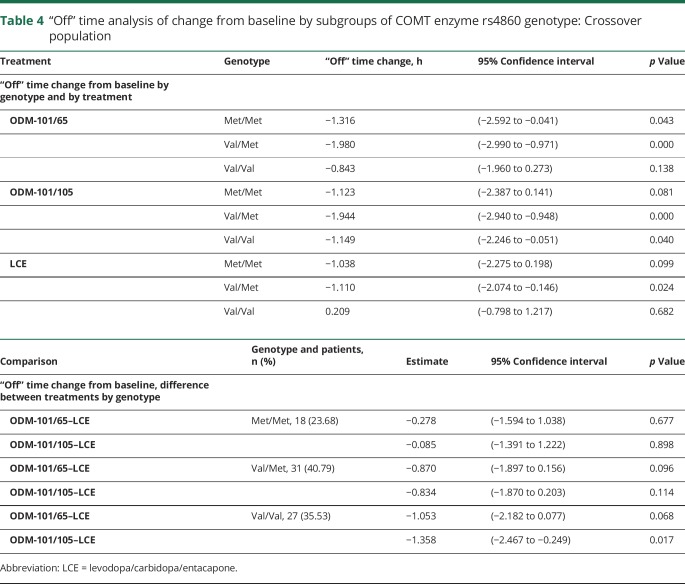
Val158Met genotyping data were available for 76 of 117 patients of whom 18 (24%) presented a low-COMT-activity Met/Met genotype, 31 (41%) were of heterozygous Val/Met, and 27 (36%) of homozygous Val/Val genotype. “Off” time from baseline was reduced significantly more during ODM-101/65 and ODM-101/105 treatments than during LCE treatment (p = 0.015 and p = 0.006) in patients with high-COMT-activity Val/Met or Val/Val genotypes. In patients with the low-COMT-activity genotype Met/Met, no such differences were seen (p = 0.679 and p = 0.890; table 3). “Off” time reduction in patients with the Val/Val genotype was seen with the ODM-101/65 and ODM-101/105 treatments but not with the LCE treatment (table 4).
Table 3.
Carbidopa effect on “off” times by patient COMT genotype
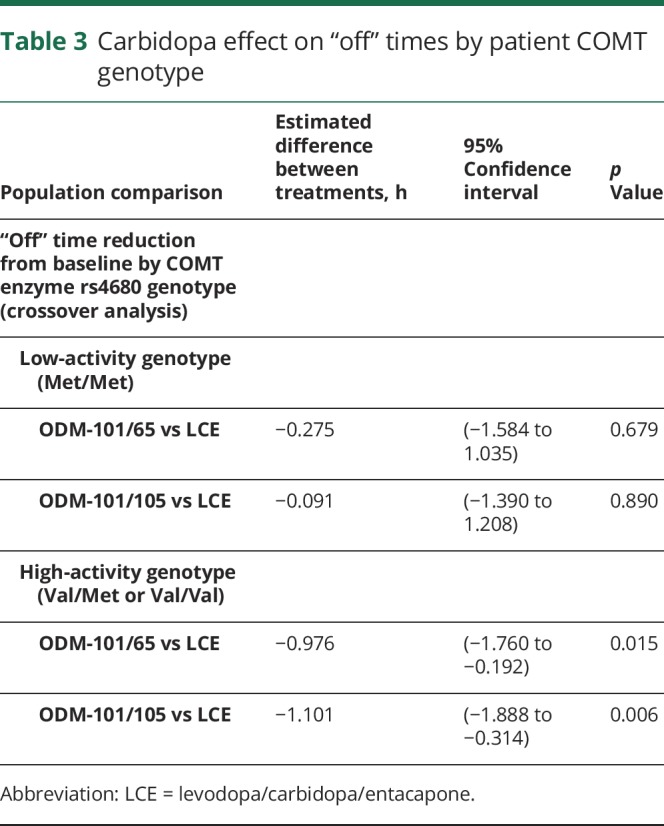
Table 4.
“Off” time analysis of change from baseline by subgroups of COMT enzyme rs4860 genotype: Crossover population
UPDRS Parts I–IV and Schwab and England
There were small differences between LCE and the 2 ODM-101 combinations in UPDRS Parts I–IV scores. UPDRS-I changes from baseline were −0.03, −0.09, and 0.07 for ODM-101/65, ODM-101/105, and LCE. UPDRS II–IV changes were −1.54, −1.62, and −1.19; −3.74, −4.13, and −3.95; and −0.72, −0.59, and −0.64, respectively. All comparisons with the 3 predefined analyses were statistically nonsignificant. The same was the case for the Schwab and England score (data not shown).
Daily DDCI doses during the study treatments
The daily carbidopa doses (mean [10th, 90th percentiles]) derived from the study medications were 115 (100, 150) mg, 298 (260, 390) mg, and 479 (420, 630) mg during LCE, ODM-101/65, and ODM-101/105 treatments, respectively. Total daily DDCI doses (carbidopa and benserazide) including controlled-release preparations not switched to study medications were 123 (100, 175) mg, 308 (260, 400) mg, and 488 (420, 630) mg during LCE, ODM-101/65, and ODM-101/105 treatments, respectively.
Electronic home diaries
This study also evaluated the feasibility of using electronic home diaries. All patients started with the electronic system. Forty patients (35.4%) reported that they were able to use electronic diaries, whereas 72 patients (63.7%) were not able to use the system. No response was given by 1 patient. The main reasons for patients being unable to complete electronic diaries were “other” (25 patients), PD motor status (24 patients), and technical problems with the device (20 patients). Only 37 patients (32.7%) were willing to use an electronic diary in future studies. Diaries were completed with the electronic device by 29.7% of patients (28.8%, 34.0%, and 28.0% of diaries during treatment with ODM-101/65, ODM-101/105, and LCE, respectively). Patients not able to use electronic diaries were allowed to use traditional paper diaries. The treatment effects were comparable between patients using electronic and paper diaries (data not shown).
Tolerability and safety
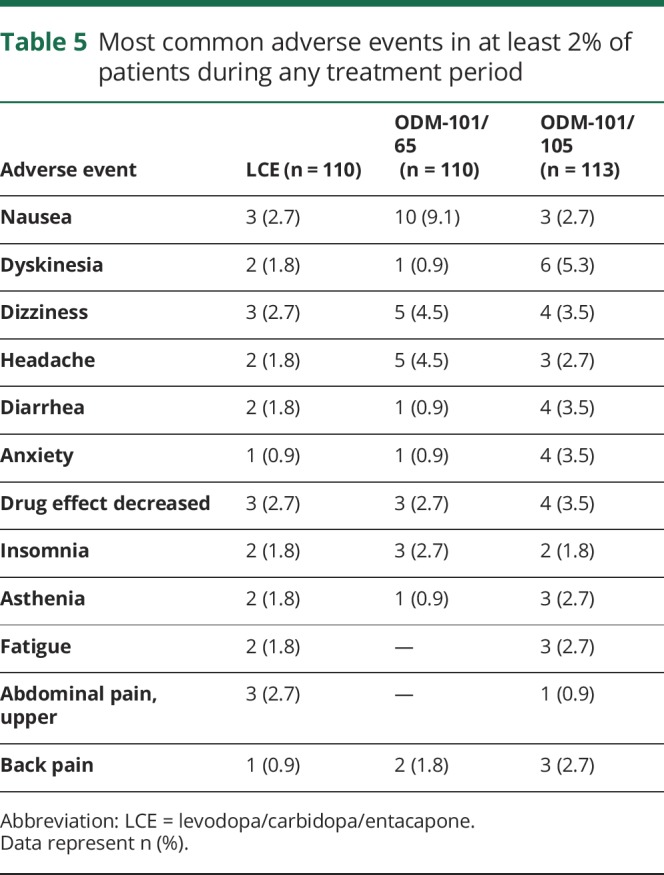
One hundred one patients (86.3%) completed all 3 crossover periods. One hundred ten, 113, and 110 patients were exposed to ODM-101/65, ODM-101/105, and LCE, of whom 30 (27.3%), 30 (26.5%), and 27 (24.5%) were reported to have treatment-related adverse events, respectively. Of these events, 95.8% were mild or moderate in severity, and the most common (related and unrelated) are presented in table 5.
Table 5.
Most common adverse events in at least 2% of patients during any treatment period
Seven patients experienced a total of 11 serious adverse events (4 related and 7 unrelated) during the treatment periods: 1 patient (back pain) during the ODM-101/65 period, 4 patients (cataract, cataract operation complication, diarrhea, bronchospasm, myocardial infarction leading to death) during the ODM-101/105 period, and 3 patients (myocardial ischemia, creatine kinase [CK] increase, hypertension, diarrhea) during the LCE period. Five (fall, drug effect decreased, nausea, anxiety, anxiety), 4 (dyskinesia, bradykinesia, diarrhea, death), and 1 (myocardial ischemia) patients discontinued the study because of an adverse event during ODM-101/65, ODM-101/105, and LCE treatment, respectively.
The death during ODM-101/105 treatment (76-year-old man with a history of hypertension and asthma) due to a severe myocardial infarction was judged as not treatment-related by the investigator. Two patients were reported to have had treatment-related serious adverse events: 1 patient with myocardial ischemia and CK increase during LCE treatment and 1 patient with diarrhea with both LCE and ODM-101/105 treatment.
There were no differences in vital signs, ECG, or laboratory tests between the 3 treatments. Four patients reported sudden sleep attacks during the screening period, and 6, 4, and 4 patients during ODM-101/65, ODM-101/105, and LCE treatments, respectively.
Discussion
In the present study, the tested carbidopa doses (both 65 and 105 mg), which were both higher than the currently applied 4:1 ratios used in marketed levodopa preparations and in LCE, generally improved both “off” and “on” times in patients with fluctuating PD compared to LCE. In predefined carryover-adjusted crossover and parallel-group comparisons (of the first treatment period), ODM-101 combinations with either 65 or 105 mg of carbidopa resulted in approximately 0.6 to 0.7 hours better “off” times compared to LCE.
Based on explorative subgroup analyses of “off” time, estimated treatment effects between the 2 ODM-101 combinations vs LCE were higher in patients who at baseline received a higher daily levodopa dose, took at least 5 doses of levodopa, and did not receive entacapone. However, subgroup analyses must be interpreted cautiously, because the number of patients in the different subgroups varied considerably and the analyses were not corrected for carryover effects. Differences in “off” time decrease between LCE and ODM-101 combinations were largely of similar magnitude than differences in daily “on”-time increase, without patients suffering more from either troublesome or nontroublesome dyskinesias. Patients who were homozygous or heterozygous for the high-activity COMT gene had a more pronounced treatment effect following the carbidopa dose increase. These results may be explained by the results of a pilot trial investigating the effect of high- and low-activity COMT polymorphism measured in erythrocytes, which showed enhanced effects of entacapone on the pharmacodynamics and pharmacokinetics of levodopa in patients with the high-COMT-activity genotype.11 This finding may explain the clinical variation observed in patients with PD when starting entacapone and may be relevant for future application of COMT inhibitors in general as a step to precision medicine.
The use of electronic diaries did not affect the results. However, only approximately a third of the patients were willing to use electronic diaries, making the used electronic diaries an unviable option for recording “on” and “off” times.
In healthy controls and in the presence of COMT inhibition, increasing carbidopa doses up to 65 or 105 mg with each levodopa dose elevates levodopa exposure and minimum concentrations (data on file, Orion Pharma). Levodopa concentration has been earlier shown to correlate with symptom control in PD, and in this study, we saw similar clinical effects of increased levodopa concentrations.16 In studies with other levodopa treatments, increased levodopa exposure has been shown to correlate with clinical efficacy.17–20 In randomized studies, “off” time was reduced about 1 hour more with test treatments than with placebo but no significant difference was found when compared with entacapone.20
In a recent clinical study in levodopa-treated patients with fluctuating PD, the daily carbidopa dose was increased from 75 to 450 mg.6 The study failed to find differences between the 2 carbidopa doses in the AUC of tapping/walking speed, tremor, and dyskinesia scores. However, 11 patients reported clinical effects during the outpatient phases suggesting that they may have had increased levodopa levels during the treatment period with higher carbidopa. In any case, the daily carbidopa dose of 450 mg appeared to be safe.
The mean daily carbidopa (or DDCI) doses in the present study were approximately 120 mg during LCE treatment. The same doses were approximately 300 and 480 mg during the 2 ODM-101 combinations with 65 mg and 105 mg of carbidopa, respectively. However, the clinical effects on “on” and “off” times were similar between the ODM-101 combinations. This may be attributable to the fact that differences in repeated-dose levodopa pharmacokinetics were relatively small between 65 and 105 mg doses of carbidopa (data on file).
The safety and tolerability of the LCE and ODM-101 combinations were acceptable. Reported adverse events were those commonly seen in PD studies and most of them were mild or moderate in severity. Discontinuations were numerically higher during ODM-101 combinations compared to LCE. One patient died during ODM-101/105 due to severe treatment-unrelated myocardial infarction and 1 patient experienced myocardial ischemia during LCE treatment. Myocardial ischemia, blood CK increase, and diarrhea on LCE, and diarrhea on ODM-101/105 were reported as related serious adverse events. Vital signs, ECG, and laboratory tests did not include any new findings. Seven patients reported sleep attacks without any differences between the treatments. The limitations of this study include a short duration of treatment with only 4 weeks of treatment in each period. Therefore, long-term efficacy and possible long-term safety aspects of increased carbidopa dosages remain to be determined. As this is a pilot, crossover, phase II trial, carryover effects may interfere with some treatment effects because of the design.
It is known that the pharmacokinetics of levodopa correlates well with motor symptoms in patients with PD who have end-of-dose motor fluctuations.21 Repeated-dose pharmacokinetic studies in patients with PD and healthy controls have shown that entacapone increases daily levodopa AUC by approximately 30% to 35% when administered with levodopa/carbidopa in a 4:1 ratio.22,23 Based on a pooled analysis of 4 phase III entacapone trials, this increase in levodopa exposure by entacapone has translated into improved (placebo vs entacapone) daily “on/off” times at 6 months by an average of 0.8 hours.24 Although no direct comparisons can be made, the present study with ODM-101 combinations demonstrated that increased carbidopa doses administered with levodopa and entacapone almost doubled the treatment effect on “on/off” times previously shown between entacapone and placebo.24
In closing, we report that higher doses of carbidopa administered with levodopa and entacapone can improve daily “on” and “off” times in a clinically meaningful manner compared to the currently used LCE combination. The present study also repeats the earlier finding that daily carbidopa doses up to about 500 mg do not worsen parkinsonism.6 These results need to be confirmed in longer, randomized, parallel-arm studies.
Acknowledgment
The PARPOC study group: Finland: Juha-Pekka Erälinna, Esko Kinnunen, Vilho Myllylä, Osmo Pammo; Germany: Georg Ebersbach, Karla Eggert, Siegfried Muhlack, Walter Paulus, Christoph Schrader, Martin Sommer; Latvia: Nela Aleksejenko, Zita Danevica, Jelena Sorokina; Lithuania: Daiva Rastenyte, Lina Malciene; Romania: Corina Roman Filip, Manuela Rodica Marceanu, Mihaela Simu, Joszef Szasz. Finland: Aila Holopainen, Valtteri Aho, Alec Mushunje, Outi Reinholm, Priteesh Chotai, Orion Pharma. The authors thank Timothy Wilson, PhD, MA, for providing editorial support. Country principal investigators: Tapani Jolma, Porin Lääkäritalo, Pori, Finland; Ligita Smeltere, Health Centre 4, Riga, Latvia; Valmantas Budrys, Vilnius University Hospital, Vilnius, Lithuania; Ana Campeanu, Institutul Clinic Fundeni, Bucharest, Romania.
Glossary
- AUC
area under the curve
- CK
creatine kinase
- COMT
catechol-O-methyltransferase
- DDC
dopa decarboxylase
- DDCI
dopa decarboxylase inhibitor
- LCE
levodopa/carbidopa/entacapone
- PD
Parkinson disease
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Editorial, page 597
Class of Evidence: NPub.org/coe
Author contributions
C. Trenkwalder contributed to study design, clinical conduct, analysis and interpretation of data, and edited the manuscript and approved it for publication. M. Kuoppamäki contributed to study design, protocol writing, analysis and interpretation of the data, literature search, study reporting, and manuscript writing and editing. M. Vahteristo contributed to study design, protocol writing, statistical analysis and interpretation of data, and revision of the manuscript. T. Müller contributed to clinical conduct and interpretation of data, and edited the manuscript and approved it for publication. J. Ellmén contributed to study design, study conduct, data analysis and interpretation, study reporting, and manuscript writing and editing.
Study funding
This study was fully funded by Orion Corporation, Orion Pharma.
Disclosure
C. Trenkwalder received honoraria from Novartis, Benevolent, Orion, and the European Brain Council for consulting and from UCB and Grünenthal as speaker. M. Kuoppamäki was an employee of and his salary was supported by Orion Pharma. M. Vahteristo is an employee of and his salary was supported by Orion Pharma. T. Müller received honoraria from Bial, Zambon, and Orion as speaker and consultant in the past. J. Ellmén is an employee of and his salary was supported by Orion Pharma. Go to Neurology.org/N for full disclosures.
References
- 1.Gershanik OS. Improving L-dopa therapy: the development of enzyme inhibitors. Mov Disord 2015;30:103–113. [DOI] [PubMed] [Google Scholar]
- 2.Cedarbaum JM, Kutt H, Dhar AK, Watkins FH, McDowell FH. Effect of supplemental carbidopa on bioavailability of L-dopa. Clin Neuropharmacol 1986;9:153–159. [DOI] [PubMed] [Google Scholar]
- 3.Kaakkola S, Männistö PT, Nissinen E, Vuorela R, Mäntylä R. The effect of an increased ratio of carbidopa to levodopa on the pharmacokinetics of levodopa. Acta Neurol Scand 1985;72:385–391. [DOI] [PubMed] [Google Scholar]
- 4.Hoehn MM. Increased dosage of carbidopa in patients with Parkinson's disease receiving low doses of levodopa. A pilot study. Arch Neurol 1980;37:146–149. [DOI] [PubMed] [Google Scholar]
- 5.Tourtellotte WW, Syndulko K, Potvin AR, Hirsch SB, Potvin JH. Increased ratio of carbidopa to levodopa in treatment of Parkinson's disease. Arch Neurol 1980;37:723–726. [DOI] [PubMed] [Google Scholar]
- 6.Brod LS, Aldred JL, Nutt JG. Are high doses of carbidopa a concern? A randomized, clinical trial in Parkinson's disease. Mov Disord 2012;27:750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweetman SC, editor. Martindale: The Complete Drug Reference. London: Pharmaceutical Press; 2011:893. [Google Scholar]
- 8.Männistö P, Kaakkola S. Rationale for selective COMT inhibitors as adjuncts in the drug treatment of Parkinson's disease. Pharmacol Toxicol 1990;66:317–323. [DOI] [PubMed] [Google Scholar]
- 9.Parkinson Study Group. Entacapone improves motor fluctuations in levodopa-treated Parkinson's disease patients. Parkinson Study Group. Ann Neurol 1997;42:747–755. [DOI] [PubMed] [Google Scholar]
- 10.Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society evidence-based medicine review update: treatments for the motor symptoms of Parkinson's disease. Mov Disord 2011;26:S2–S41. [DOI] [PubMed] [Google Scholar]
- 11.Corvol JC, Bonnet C, Charbonnier-Beaupel F, et al. The COMT Val158Met polymorphism affects the response to entacapone in Parkinson's disease: a randomized crossover clinical trial. Ann Neurol 2011;69:111–118. [DOI] [PubMed] [Google Scholar]
- 12.Hoehn M, Yahr M. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 13.Williams EJ. Experimental designs balanced for the estimation of residual effects of treatments. Aust J Sci Res 1949;2:149–168. [Google Scholar]
- 14.Hauser RA, Friedlander J, Zesiewicz TA, et al. A home diary to assess functional status in patients with Parkinson's disease with motor fluctuations and dyskinesia. Clin Neuropharmacol 2000;23:75–81. [DOI] [PubMed] [Google Scholar]
- 15.Senn SJ. Cross-over Trials in Clinical Research. Chichester: Wiley; 1993. [Google Scholar]
- 16.Ruottinen HM, Rinne UK. A double-blind pharmacokinetic and clinical dose-response study of entacapone as an adjuvant to levodopa therapy in advanced Parkinson's disease. Clin Neuropharmacol 1996;19:283–296. [DOI] [PubMed] [Google Scholar]
- 17.Hauser RA, Ellenbogen AL, Metman LV, et al. Crossover comparison of IPX066 and a standard levodopa formulation in advanced Parkinson's disease. Mov Disord 2011;26:2246–2252. [DOI] [PubMed] [Google Scholar]
- 18.Hauser RA, Hsu A, Kell S, et al. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson's disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol 2013;12:346–356. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira JJ, Rocha JF, Falcão A, et al. Effect of opicapone on levodopa pharmacokinetics, catechol-O-methyltransferase activity and motor fluctuations in patients with Parkinson's disease. Eur J Neurol 2015;22:815–825. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira JJ, Lees A, Rocha JF, et al. Opicapone as an adjunct to levodopa in patients with Parkinson's disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol 2016;15:154–165. [DOI] [PubMed] [Google Scholar]
- 21.Tolosa ES, Martin WE, Cohen HP, et al. Patterns of clinical response and plasma dopa levels in Parkinson's disease. Neurology 1975;25:177–183. [DOI] [PubMed] [Google Scholar]
- 22.Kuoppamäki M, Korpela K, Marttila R, et al. Comparison of pharmacokinetic profile of levodopa throughout the day between levodopa/carbidopa/entacapone and levodopa/carbidopa when administered four or five times daily. Eur J Clin Pharmacol 2009;65:443–455. [DOI] [PubMed] [Google Scholar]
- 23.Kaakkola S, Teräväinen H, Ahtila S, et al. Entacapone in combination with standard or controlled-release levodopa/carbidopa: a clinical and pharmacokinetic study in patients with Parkinson's disease. Eur J Neurol 1995;2:341–347. [DOI] [PubMed] [Google Scholar]
- 24.Kuoppamäki M, Vahteristo M, Ellmén J, et al. Pooled analysis of phase III with entacapone in Parkinson's disease. Acta Neurol Scand 2014;130:239–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The protocol and the statistical analysis plan are available on request. Deidentified participant data are not available for legal and ethical reasons.