Abstract
It is demonstrated that repeated superovulation has deleterious effects on mouse ovaries and cumulus cells. However, little is known about the effects of repeated superovulation on early embryos. Epigenetic reprogramming is an important event in early embryonic development and could be easily disrupted by the environment. Thus, we speculated that multiple superovulations may have adverse effects on histone modifications in the early embryos. Female CD1 mice were randomly divided into four groups: (a) spontaneous estrus cycle (R0); (b) with once superovulation (R1); (c) with three times superovulation at a 7-day interval (R3) and (d) with five times superovulation at a 7-day interval (R5). We found that repeated superovulation remarkably decreased the fertilization rate. With the increase of superovulation times, the rate of early embryo development was decreased. The expression of Oct4, Sox2 and Nanog was also affected by superovulation in blastocysts. The immunofluorescence results showed that the acetylation level of histone 4 at lysine 12 (H4K12ac) was significantly reduced by repeated superovulation in mouse early embryos (P < 0.01). Acetylation level of histone 4 at lysine 16 (H4K16ac) was also significantly reduced in pronuclei and blastocyst along with the increase of superovulation times (P < 0.01). H3K9me2 and H3K27me3 were significantly increased in four-cell embryos and blastocysts. We further found that repeated superovulation treatment increased the mRNA level of histone deacetylases Hdac1, Hdac2 and histone methyltransferase G9a, but decreased the expression level of histone demethylase-encoding genes Kdm6a and Kdm6b in early embryos. In a word, multiple superovulations alter histone modifications in early embryos.
Introduction
With rapid socio-economic development, more and more young people have reproductive health problems that are induced by many factors such as environmental pollution, unhealthy lifestyle and increasing incidence of chronic diseases. Approximately, 10–15% couples worldwide at childbearing age suffer from infertility and sterility. The use of ARTs (assisted reproductive techniques) for the treatment of human infertility/subfertility is rapidly increasing, although the successful incidence is not as high as expected. Until now, ART is the best treatment for infertility. Approximately, 5–10% of newborn babies each year are produced by ART in some developed countries (Sunderam et al. 2015, European IVF-Monitoring Consortium et al. 2016). Thus, the health of ART children has become a major concern. Previous studies reported that a high frequency of chromosomal abnormalities (Van Steirteghem et al. 2002), rare congenital malformations (Hansen et al. 2002, Bonduelle et al. 2005) and alterations of cognitive and motor development (Stromberg et al. 2002, Kallen et al. 2005) in children may be associated with ART, but how that happens is not clear.
Epigenetic status changes saliently during preimplantation embryo development and gametogenesis in which epigenetic modifications are sensitive to environmental changes (Erhardt et al. 2003, van der Heijden et al. 2005, Wang & Dey 2006, Zhang et al. 2009). In recent years, a number of studies have shown that ART manipulations such as superovulation, vitrification and in vitro culture can induce changes of epigenetic modifications in embryos and fetus (El Hajj & Haaf 2013, Ventura-Junca et al. 2015). For example, superovulation and vitrification alter H4K12ac (histone 4 lysine 12 acetylation) and H3K9ac (histone 3 lysine 9 acetylation) in ICM (inner cell mass) and TE (trophectoderm) (Bakhtari et al. 2014), and superovulation affects DNA methylation pattern of line-1 in blastocyst (Liang et al. 2013). In the clinic, many women would experience more than one exogenous hormone-stimulated cycle before getting a baby. Animal studies have demonstrated that multi-superovulation alters ovarian structure and function in the rhesus monkey, as well as mitochondrial distribution and function in mouse ovaries and cumulus cells (Chao et al. 2005, Dong et al. 2014, Kalthur et al. 2016, Xie et al. 2016). Although the influence of superovulation on DNA methylation is dose dependent (Market-Velker et al. 2010), the effect of repeated superovulation at low dose on epigenetics in embryos is still not well known. So we hypothesized that repeated superovulation may have adverse effects on epigenetic modifications in embryos.
Histone modification is one of the most important epigenetic modifications, which plays a critical role in early embryonic development (Adenot et al. 1997, Li 2002, Morgan et al. 2005, Santos et al. 2005, Bogliotti & Ross 2012, Aoshima et al. 2015). It is demonstrated that H3K4me3 (tri-methylation at histone 3 lysine 4) and H3K27me3 (tri-methylation at histone 3 lysine 27) are reprogrammed in early embryos, which is very important for embryo development (Liu et al. 2016, Zhang et al. 2016). H3K9me2 (di-methylation at histone 3 lysine 9) is an important marker of heterochromatin which can repress the genes expression (Sridharan et al. 2013). H3K9me2 regulates DNA methylation by recruiting PGC7 to chromatin in the early embryos (Nakamura et al. 2012). There is a dynamic change of H3K9me2 in early embryonic development. H4K12ac is associated with cell division (Shang et al. 2016) and is important for chromatin decondensation in zygotes and early embryonic development (van der Heijden et al. 2006, Paradowska et al. 2012). H4K16ac is involved in chromatin structure remodeling (Grigoryan et al. 2018) and early embryonic development (van der Heijden et al. 2006). So we tested the effects of multi-superovulation on histone modifications such as H4K12ac, H4K16ac, H3K27me3 and H3K9me2 in the early embryos. We found that repeated superovulations altered histone modifications in the early embryos. To elucidate how these happen, we further examined the mRNA expression of histone deacetylases (Hdac1, Hdac2), acetylases (Gnc5, Hat1), methyltransferase (G9a) and histone demethylases (Kdm6a, Kdm6b) in the early embryos.
Materials and methods
Ethics statement
Animal care and use were conducted in accordance with the guideline of Qingdao Agricultural University, China. Mice were housed in a temperature-controlled room with proper darkness–light cycles and fed a regular diet. All experiments and the study protocol were approved by the Ethics Committee of Qingdao Agricultural University.
Superovulation
Female CD1 mice (5 weeks of age) were purchased from the Center of Experimental Animals of Qingdao and fed in a temperature- and humidity-controlled room at a light cycle of 12 h light and 12 h darkness. Diet and water were supplied ad libitum. Female CD1 mice were randomly divided into four groups (Fig. 1A): (a) natural estrus cycle (R0); (b) intraperitoneal injection with 8 IU PMSG (Ningbo Hormone Product Co. Ltd., China) and 8 IU hCG (Ningbo Hormone Product Co. Ltd., China) for once (R1); (c) intraperitoneal injection with 8 IU PMSG and 8 IU hCG for three times at a 7-day interval (R3); (d) intraperitoneal injection with 8 IU PMSG and 8 IU hCG for five times at a 7-day interval (R5).
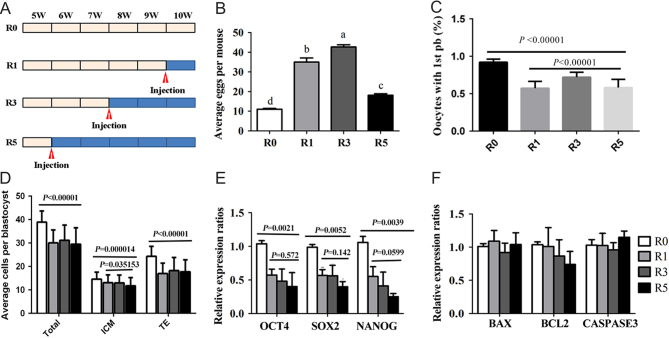
Figure 1.
Effects of multiple superovulations on embryonic development. (A) Schedule of superovulation; (B) average number of eggs retrieved from each mouse after mating with males; (C) the incidence of oocytes with the 1st PB; (D) the cell number of blastocysts. The number of blastocysts tested was 67, 73, 64 and 74 in R0, R1, R3 and R5 groups, respectively. (E and F) Effects of multiple superovulations on embryonic quality were also examined via checking the expression of the genes, such as pluripotency-related genes Oct4, Sox2, Nanog and apoptosis-related genes Bcl2, Bax and Casp3.
Zygote collection and embryo culture
Females were mated with fertile males to produce zygotes at the last time of hCG injection. The same males were used in each treatment, and all the males were purchased from the Center of Experimental Animals of Qingdao. If the virginal plug was observed in the next morning, females were killed by cervical dislocation 20 h after hCG administration. Fertilized eggs surrounded with cumulus cells were collected from oviduct ampulla. After that, cumulus cells were removed using 1 mg/mL hyaluronidase in the M2 medium. To collect fertilized eggs of R0, pre-estrus mice were selected and mated with males. Then, fertilized eggs from the above four groups were cultured in KSOM + AA culture medium under liquid paraffin oil at 37°C with 5% CO2. We obtained PN4 (8 h) fertilized eggs, two-cell-stage embryos (24 h), four-cell-stage embryos (48 h) and blastocysts (84 h and 108 h).
Antibodies and immunofluorescence
Antibodies for detecting H4K12ac (catalog No. 39927) and H4K16ac (catalog No. 39727) were purchased from Santa Cruz Biotechnology. The antibody of H3K9me2 was purchased from Bioworld Technology (catalog No. BS7234) and H3K27me3 was purchased from Abcam (catalog No. ab6002). Antibodies of FITC (catalog No. FITC1) and Cy3 (catalog No. L0419) were purchased from Sigma. Immunofluorescence was performed according to previous protocols (Ma et al. 2014). Briefly, embryos were fixed in 4% paraformaldehyde for 40 min and then treated with 0.5% Triton X-100 for 20 min after three washes using washing buffer (PBS with 0.1% Tween 20 and 0.01% Triton-100). After blocking using 1% BSA for 1 h, embryos were incubated with the primary antibody (1:50) overnight at 4°C. After washing five times using washing buffer, embryos were then incubated with the secondary antibody (1:200) for 2 h at room temperature. Then, embryos were incubated with Hoechst 33342 (propidium iodide/4,6-diamino-2-phenylindole) for 20 min to counterstain DNA after washing three times using washing buffer. Finally, the embryos were mounted on glass slides with DABCO and examined using a laser scanning confocal microscope (Leica TCS SP5).
Quantitative fluorescence intensity
Stained embryos were scanned using the Leica TCS SP5 confocal microscope. For each antibody detection, the same excitation wavelength was used, including FITC excitation wavelength of 488 nm, Cy3 excitation wavelength of 561 nm. We unified the background value in the process of laser scanning. To quantify fluorescence intensity, we put all the Z-stacks together. The fluorescence intensity was analyzed and treated using ImageJ.
Calculation of blastocyst cells
Cell number of blastocysts was counted as previously described (Van der Elst et al. 1998). Briefly, blastocysts were incubated in rabbit anti-mouse serum for 30 min, and then incubated in solution supplemented with 1:5 guinea-pig serum and PI for 5 min. After that, blastocysts were fixed using 4% paraformaldehyde for 40 min, and then treated with 0.5% Triton X-100 for 20 min. Then, blastocysts were incubated with Hoechst 33342 for 20 min after washing three times using washing buffer. Finally, the blastocysts were mounted on glass slides with DABCO. The TE and ICM cells were stained in red and blue, respectively. Blastocysts were scanned using a laser scanning confocal microscope (Leica TCS SP5). The cell numbers were counted using ImageJ.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using EZ-10 Spin Column Total RNA Isolation Kit (Sangon Biotech, Shanghai, China) according to the manufacturer’s instruction. A total of 50–150 embryos were collected for the RNA extraction of each sample, depending on their developmental stages. cDNA was synthesized using the HiScript IIQ RT SuperMix (Vazyme, Nanjing, China) according to the manufacturer’s instructions. The synthesized cDNA was used as the template for qRT-PCR or stored at −80°C until used. Primers were shown in Table 1. qRT-PCR was carried out using the Applied Biosystems 7500 Sequence Detection System. Amplification was performed in the 20 μL volume containing 10 μL of SYBR Green Master Mix (Vazyme), 0.4 μL of primers (10 mM), 0.4 μL of ROX Reference Dye 2, 2 μL of cDNA and 7.2 μL of RNase free H2O. PCR amplification conditions were as follows: the reaction was initiated at 95°C for 10 min, followed by 40 cycles of denaturing at 95°C for 15 s, annealing at 60°C for 30 s and extension at 72°C for 20 s. Values were normalized against the expression level of GAPDH (reference). Relative expression values were calculated with the 2−ΔΔCt method. Values of gene expression were means of three replicates.
Table 1.
The sequence of primers used in RT-PCR.
| Gene | Primer (5′–3′) | Size (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| Hdac1 | TTCCAACATGACCAACCA | AGCATCCTCAAGTTCTCAA | 78 |
| Hdac2 | CCAGAACACTCCAGAATA | CATCTCCACTGTCTTCAT | 131 |
| G9a | TCATCTGCGAGTATGTAG | CGAAGAGGTAAGAATCATC | 75 |
| Kdm6a (UTX) | CAGTATAAGTTAGCAGTGGAA | GCGTTCTCAGAAGACAAT | 157 |
| Kdm6b (Jmjd3) | GACGAGCCTGCCTACTAC | TGCCATTCTCACTTGTAACG | 76 |
| Hat1 | AAGTGTAACACCAACACAGCA | CGAAAGCAGTTTCATCATCCCC | 127 |
| Gcn5 | AAGGCCAATGAAACCTGCAAG | CTCACAGCTACGGCACAACTC | 117 |
| Oct4 | GGCTTCAGACTTCGCCTCC | AACCTGAGGTCCACAGTATGC | 211 |
| Sox2 | GCGGAGTGGAAACTTTTGTCC | CGGGAAGCGTGTACTTATCCTT | 157 |
| Nanog | TCTTCCTGGTCCCCACAGTTT | GCAAGAATAGTTCTCGGGATGAA | 100 |
| Bax | ATGCGTCCACCAAGAAGCTGAG | CCCCAGTTGAAGTTGCCATCAG | 166 |
| Bcl2 | ATGATAACCGGGAGATCGTG | GACGGTAGCGACGAGAGAAG | 294 |
| Casp 3 | GACTGGGATGAACCACGACCC | TCTGACTGGAAAGCCGAAAC | 205 |
| Gapdh | GACAAAATGGTGAAGGTCGGT | GAGGTCAATGAAGGGGTCG | 120 |
Statistical analysis
The expression of genes, cell numbers and fluorescence intensity were represented as mean ± s.d. Differences were evaluated by one-way analysis of variance. The differences of data that were presented as a percentage were calculated with one-way ANOVA test. If the P value was <0.05, the difference between groups was considered significant.
Results
Repeated superovulation reduced the potency of early embryonic development
Zygotes were collected from oviducts 20 h after hCG injection and cultured in vitro to monitor the developmental process of early embryos. The average number of eggs per mouse (including fertilized and unfertilized) was higher in superovulated groups compared to that of R0 group, but it was decreased with the increase of superovulation times (P < 0.01, Fig. 1B). However, it appeared that the decrease in the percentage of oocytes with the first polar body (1st PB) was a consequence of repeated superovulation (P < 0.00001). Superovulation times had adverse effects on the percentage of oocytes with the 1st PB (Fig. 1C, P < 0.00001). The rate of pronucleus was higher in R0 group than that in R1, R3 and R5 groups (P < 0.01, Table 2). With the increase of superovulation times, there was a significant decrease in the rate of pronucleus formation in R1, R3 and R5 groups (P < 0.05, Table 2).
Table 2.
Early embryonic development in vitro. Data are presented as percent ± s.d.
| n | 8 h | 24 h | 48 h | 84 h | 108 h | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pronucleus | Death | Block | 2-Cell | Death | Block | 4-Cell | Blastocyst | |||
| R0 | 1956 | 91.33 ± 4.14a | 1.79 ± 1.51c | 1.73 ± 1.34c | 97.95 ± 2.52a | 8.83 ± 6.64b | 3.18 ± 2.60b | 94.55 ± 3.32a | 26.57 ± 6.44a | 82.92 ± 8.19a |
| R1 | 4195 | 50.40 ± 5.19b | 4.51 ± 4.89c | 8.16 ± 3.87a | 91.85 ± 2.99b | 28.55 ± 6.39a | 6.78 ± 3.85b | 80.14 ± 5.37b | 12.34 ± 3.49b | 51.98 ± 8.16b |
| R3 | 4973 | 66.44 ± 6.15c | 12.03 ± 5.70b | 4.11 ± 2.77b | 91.73 ± 5.17b | 27.53 ± 7.93a | 14.53 ± 5.66a | 77.03 ± 5.13b | 5.53 ± 2.03c | 44.76 ± 10.46c |
| R5 | 2025 | 42.73 ± 9.45d | 21.04 ± 6.74a | 8.01 ± 4.35a | 85.39 ± 7.56c | 11.85 ± 9.71b | 11.85 ± 9.71ab | 59.06 ± 9.31c | 8.68 ± 4.94bc | 37.72 ± 10.69d |
Different letters mean P value <0.05 between groups. Pronucleus (%) = no. of eggs with pronucleus/total; no. of eggs embryo (%) = no. of embryos/no. of eggs with pronucleus.
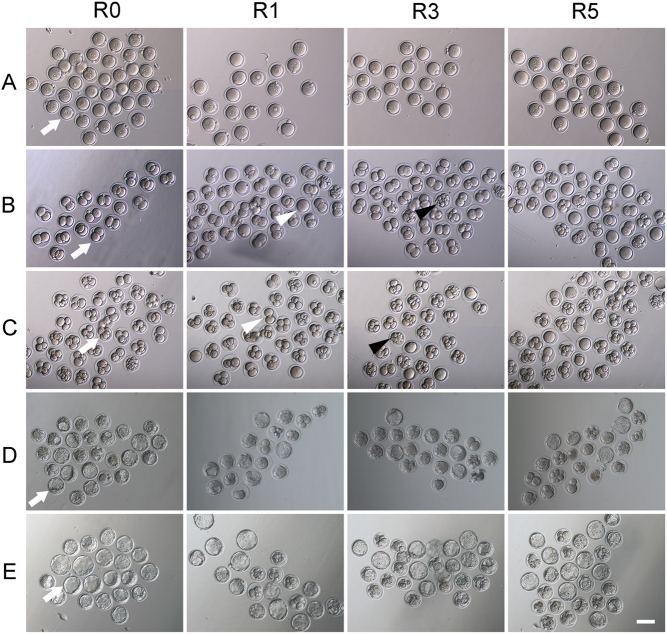
The rate of pronucleus of R0 group was 91.3 ± 4.14%, while pronucleus rates were 50.4 ± 5.19%, 66.4 ± 6.15% and 42.7 ± 9.45% in R1, R3 and R5 groups, respectively. The cleavage rate was also significantly decreased with the increase of superovulation times (P < 0.05, Table 2). The incidence of two-cell embryos (Fig. 2B) in R5 group (85.4 ± 7.56%) was obviously lower than that in R0 group (97.9 ± 2.52%, P < 0.01), but it was similar between R1 group (91.9 ± 2.99%) and R3 group (91.7 ± 5.12%, P = 0.943). With the increase of superovulation times, the incidence of four-cell embryos had a significantly decreasing trend (P < 0.01). A similar trend was observed at the blastocyst stage (P < 0.01). The four-cell embryo rate of R0, R1, R3 and R5 groups were 94.6 ± 3.32%, 80.1 ± 5.37%, 77.0 ± 5.13% and 59.1 ± 9.31%, respectively. The frequency of blastocyst in R0 group was 82.9 ± 8.19%, which was significantly higher than that in R1, R3 or R5 group (52.0 ± 8.16%, 44.8 ± 10.46% and 37.7 ± 10.69%, respectively). Meanwhile, embryos derived from superovulation treatment had a higher incidence of death and developmental block during in vitro culture (Fig. 2 and Table 2). We also found that the cell number of blastocyst was significantly affected by superovulation, and ICM cell number was significantly decreased with the increase of superovulation times (P = 0.035153, Fig. 1D). To further understand the effects of superovulation on embryonic development, we investigated the expression of Oct4, Sox2 and Nanog. The expression levels of these genes were similar among R1, R3 and R5 groups, although it was lower compared to R0 group (Fig. 1E). However, there was a decreased trend of these genes expression with the increase of superovulation times (Fig. 1E). The expression of genes related to apoptosis, such as Bax, Bcl2 and Casp 3 was not affected by superovulation in blastocysts (Fig. 1F). These results suggest that repeated superovulation may reduce embryonic development potential and embryonic quality in a superovulation time-dependent manner.
Figure 2.
Representative images of in vitro-cultured embryos. Embryonic morphology images were acquired at 8, 24, 48, 84 and 108 h of culture. (A) Prokaryotic embryos at the PN4 phase; (B) two-cell stage embryos; (C) four-cell stage embryos; (D and E) blastocysts at 84 h and 108 h. Arrow indicates normal embryos at different stages, black arrowhead indicates dead embryos and white arrowhead refers to the blocked embryos. Scale bars = 100 μm.
Repeated superovulation altered histone acetylation level in the early embryos
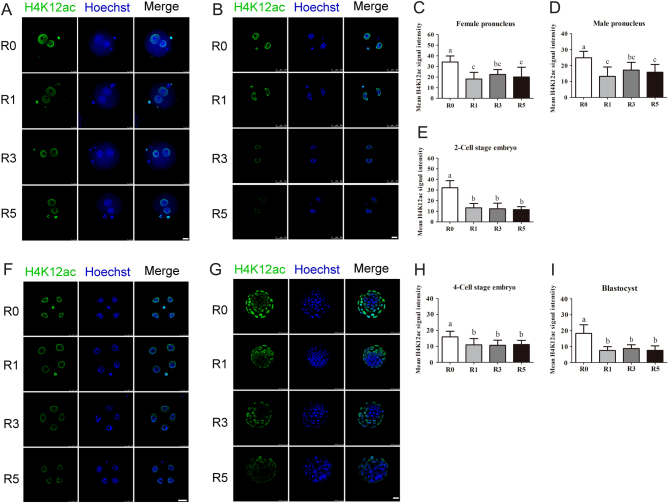
Proper histone modification is crucial for early embryo development, so we used immunofluorescence to examine the histone acetylation levels of H4K12ac and H4K16ac. The fluorescence intensity of H4K12ac was significantly stronger in R0 group than that in R1, R3 and R5 groups at the pronuclear stage (Fig. 3A). Similar results were observed at two-cell, four-cell and blastocyst stages (Fig. 3D, F and G). We further quantified the relative fluorescence intensity of H4K12ac and found that the relative fluorescence intensity of H4K12ac was lower in embryos of R1, R3 and R5 group compared to that of R0 group (P < 0.05), but there was no significant difference among R1, R3 and R5 groups (Fig. 3B, C, E, H and I).
Figure 3.
Acetylation levels of H4K12 in the early embryos. Fluorescence intensity of H4K12ac in early embryos was examined using confocal microscopy. (A) Pronuclear embryos at the PN4 phase, n = 42 (R0), n = 45 (R1), n = 44 (R3), n = 40 (R5); (B and C) average fluorescence intensity of pronucleus; (D) two-cell stage embryos, n = 52 (R0), n = 50 (R1), n = 57 (R3), n = 49 (R5); (E) average fluorescence intensity of two-cell stage embryos; (F) four-cell stage embryos, n = 39 (R0), n = 43 (R1), n = 45 (R3), n = 41 (R5); (H) blastocysts, n = 49 (R0), n = 47 (R1), n = 43 (R3), n = 45; (G and I) average fluorescence intensity of 4-cell stage embryos and blastocysts. Scale bars (prokaryotic embryos, four-cell stage embryos, blastocysts) = 25 μm. Scale bars (two-cell stage embryos) = 50 μm. Data present as mean ± s.d. Different letters indicate P < 0.05 between groups.
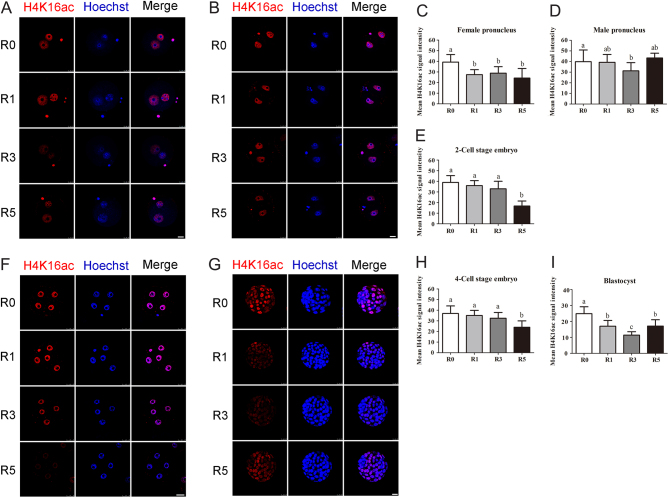
We found that H4K16ac was not affected by multiple superovulations in paternal pronucleus except for R3 group having a weaker fluorescence and relative low fluorescence intensity (P < 0.05; Fig. 4A and C). But the H4K16ac level was clearly decreased in maternal pronucleus (P < 0.05; Fig. 4C). At the two-cell and four-cell stages, the fluorescence level of H4K16ac in R5 group was obviously weaker than that in R0, R1 or R3 group (Fig. 4D and F). The relative fluorescence intensity of H4K16ac was also significantly lower in R5 group compared to that in R0, R1 or R3 group (P < 0.01; Fig. 4E and H). Blastocysts from superovulated mice had a lower H4K16ac level compared to that in R0 group (P < 0.01; Fig. 4G and I). These results indicate that superovulation obviously alters histone acetylation of H4K12 and H4K16 in preimplantation embryos and the effect of superovulation on histone acetylation of H4K16 shows a superovulation time-dependent manner.
Figure 4.
Acetylation levels of H4K16 in the early embryos. Fluorescence intensity of H4K16ac in early embryos was tested using confocal microscopy. (A) Pronuclear embryos at the PN4 phase, n = 44 (R0), n = 43 (R1), n = 42 (R3), n = 42 (R5); (B and C) average fluorescence intensity of pronucleus. (D) two-cell stage embryos, n = 50 (R0), n = 55 (R1), n = 52 (R3), n = 53 (R5); (E) average fluorescence intensity of two-cell stage embryos; (F) four-cell stage embryos, n = 37 (R0), n = 44 (R1), n = 50 (R3), n = 47 (R5); (H) blastocyst embryos, n = 50 (R0), n = 46 (R1), n = 47 (R3), n = 50; (G and I) average fluorescence intensity of four-cell stage embryos and blastocysts. Scale bars = 25 μm. Data present as mean ± s.d. Different letters indicate P < 0.05 between groups.
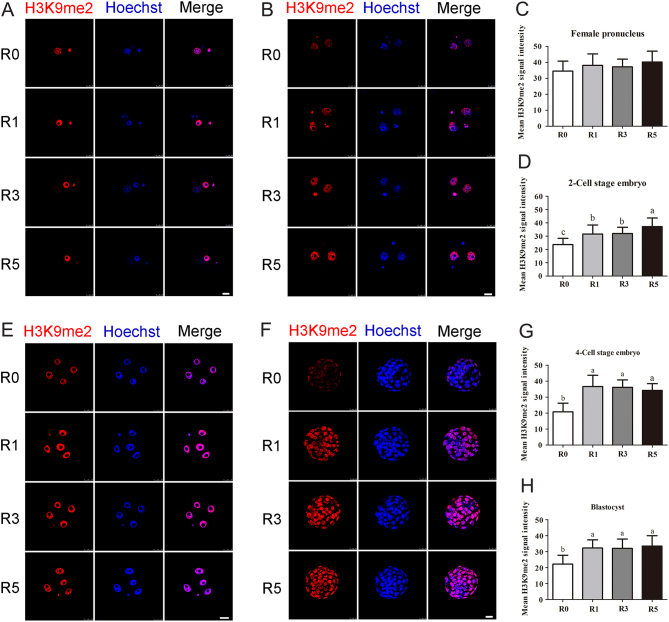
Repeated superovulation altered di-methylation of H3K9 and tri-methylation of H3K27 in the early embryos
We further examined the effects of repeated superovulation on histone methylations. At the pronuclear phase, there was no difference of di-methylation of H3K9 (H3K9me2) level in maternal pronucleus among R0, R1, R3 and R5 groups (Fig. 5A and C). At the two-cell embryo stage, the H3K9me2 level was significantly increased with the increase of superovulation times (P < 0.05; Fig. 5B and D). Although H3K9me2 level was higher in 4-cell embryos and blastocysts, there was no difference among R1, R3 and R5 groups (P > 0.05; Fig. 5E, F, G and H).
Figure 5.
Di-methylation of H3K9 level in the early embryos. Fluorescence intensity of H3K9me2 in the early embryos was tested using confocal microscopy. (A) Pronuclear embryos at the PN4 phase, n = 47 (R0), n = 47 (R1), n = 49 (R3), n = 50 (R5); (B) average fluorescence intensity of pronucleus; (C) two-cell stage embryos, n = 51 (R0), n = 52 (R1), n = 52 (R3), n = 54 (R5); (D) average fluorescence intensity of two-cell stage embryos; (E) four-cell stage embryos, n = 39 (R0), n = 41 (R1), n = 44 (R3), n = 43 (R5); (F) average fluorescence intensity of four-cell stage embryos; (G) blastocysts, n = 47 (R0), n = 47 (R1), n = 47 (R3), n = 43; (H) average fluorescence intensity of blastocysts. Scale bars = 25 μm. Data present as mean ± s.d. Different letters indicate P < 0.05 between groups.
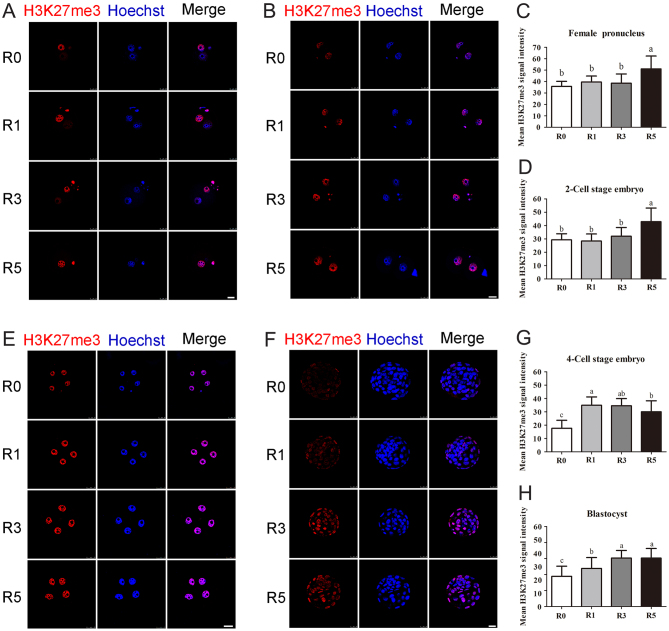
There was a significant increase for tri-methylation of H3K27 (H3K27me3) in R5 group at the pronucleus and two-cell embryo stages (P < 0.05; Fig. 6A, B, C and D). When embryos developed to the four-cell and blastocyst stages, the tri-methylation level of H3K27 was still higher in embryos experienced superovulation (P < 0.05; Fig. 6E, F, G and H), and the changes of the H3K27me3 level was at a superovulation time-dependent manner. It can be concluded from these results that superovulation has deleterious effects on H3K9me2 and H3K27me3 in early embryonic development. Furthermore, the effect of superovulation on H3K27me3 is in a superovulation-time-dependent manner.
Figure 6.
Tri-methylation of H3K27 level in the early embryos. Fluorescence intensity of H3K27me3 in the early embryos was examined using confocal microscopy. (A) Pronuclear embryos at the PN4 phase, n = 50 (R0), n = 50 (R1), n = 49 (R3), n = 50 (R5); (B) average fluorescence intensity of female pronucleus; (C) two-cell stage embryos, n = 53 (R0), n = 54 (R1), n = 54 (R3), n = 54 (R5); (D) average fluorescence intensity of two-cell stage embryos; (E) four-cell stage embryos, n = 44 (R0), n = 44 (R1), n = 44 (R3), n = 43 (R5); (F) average fluorescence intensity of four-cell stage embryos; (G) blastocysts, n = 46 (R0), n = 47 (R1), n = 46 (R3), n = 43; (H) average fluorescence intensity of blastocysts. Scale bars = 25 μm. Data present as mean ± s.d. Different letters indicate P < 0.05 between groups.
Multi-superovulation altered mRNA expressions of relative genes in the early embryos
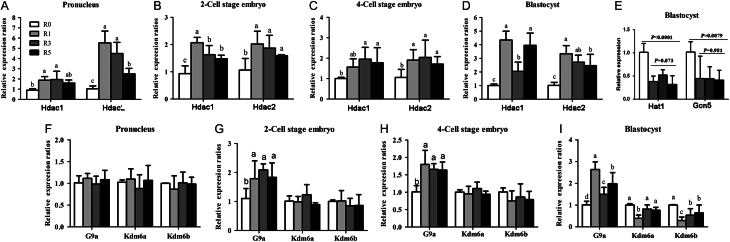
We further examined mRNA expressions of genes encoding histone deacetylases HDAC1/2, acetylases GCN5 and HAT1, histone methyltransferases G9a and histone demethylases KDM6a/b, which regulate histone acetylation and methylation level at different stages of embryonic development.
At the pronuclear stage, the mRNA expression of both histone deacetylases Hdac1 and Hdac2 was higher in R1, R3 and R5 groups than in R0 group (P < 0.05; Fig. 7A). But the increased fold in R5 group was lower than that in R1 and R3 groups (Fig. 7A). At the two-cell embryo stage, the expression of Hdac1 and Hdac2 was also higher in R1, R3 and R5 groups than in R0 group, and the increased fold had a decreasing trend with increasing times of superovulation (P < 0.05; Fig. 7B). The higher expression level of Hdac1 and Hdac2 was maintained to the four-cell embryo (P < 0.05) and blastocyst (P < 0.05) stages. There was also a decreased trend of the mRNA expression level of Hdac1 and Hdac2 with increased superovulation times in four-cell embryos and blastocysts though the difference was not significant (P > 0.05; Fig. 7C and D). Meanwhile, we found that the expression of Gcn5 and Hat1 in blastocysts was similar among R1, R3 and R5 groups, but it was lower when compared to that in R0 group (Fig. 7E). These indicate that deacetylases may play a key role in the decrease of histone acetylation with the increase of superovulation times.
Figure 7.
Expressions of Hdac1, Ddac2, G9a, Kdm6a and Kdm6b in the early embryos. The expressions of Hdac1, Ddac2, G9a, Kdm6a and Kdm6b in the early embryos at different stages were examined using qRT-PCR. (A) Expressions of Hdac1 and Hdac2 in the PN4 phase embryos (150 embryos, n = 3); (B) expressions of Hdac1 and Hdac2 in two-cell stage embryos (130 embryos, n = 3); (C) expressions of Hdac1 and Hdac2 in four-cell stage embryos (130 embryos, n = 3); (D) expressions of Hdac1 and Hdac2 in blastocysts (50 embryos, n = 3); (E) expressions of acetylases, such as Hat1 and Gcn5; (F) expressions of G9a, Kdm6a and Kdm6b in the PN4 phase embryos (150 embryos, n = 3); (G) expressions of G9a, Kdm6a and Kdm6b in two-cell stage embryos (130 embryos, n = 3); (H) expressions of G9a, Kdm6a and Kdm6b in four-cell stage embryos (130 embryos, n = 3); (I) expressions of G9a, Kdm6a and Kdm6b in blastocysts (50 embryos, n = 3). Data present as mean ± s.d. Different letters indicate P < 0.05 between groups.
There was no significant change in the expression of G9a, Kdm6a and Kdm6b among the R1, R3 and R5 groups at the pronuclear stage (P > 0.05; Fig. 7F). At the two-cell and four-cell embryo stages, the expression of G9a was significantly increased in R1, R3 and R5 groups compared to that of R0 group (P < 0.05; Fig. 7G and H). The expression of Kdm6a and Kdm6b was not affected by superovulation (Fig. 7G and H) at the two-cell and the four-cell embryo stages. In blastocysts, superovulation increased the expression of G9a in R1, R3 and R5 groups, but with the increase of superovulation times, there was a significantly decreased trend of G9a expression, especially for R3 group (P < 0.05; Fig. 7I). The expression of Kdm6a and Kdm6b was reduced by superovulation in blastocysts, especially Kdm6b in R1, R3 and R5 groups (P < 0.05; Fig. 7I). These results indicate that multi-superovulation alters histone modifications by influencing the expression of histone deacetylases, methyltransferase and demethylases in mouse early embryos.
Discussion
Superovulation is one of the most important technologies in ART and is widely used in human and animals to get more available oocytes. In the clinic, many women experience more than one exogenous hormone stimulation cycle to obtain a healthy baby. Similarly, repeated superovulation is also used in animals to increase the utilization rate of good females. But studies have shown that ovarian stimulation has an adverse effect on granulosa cell apoptosis (Tarin et al. 2001, Combelles & Albertini 2003) and mitochondrial copy number in cumulus cells (Xie et al. 2016). Superovulation can also lead to changes in cytoplasmic distribution of organelles in oocytes, spindle abnormalities, abnormal expression of octamer-binding transcription factor 4 (Oct4) and a decrease of oocyte development potential (Kalthur et al. 2016). Van Blerkom and Davis confirmed that repeated ovarian stimulation in mice significantly increases the frequency of spindle defects and causes chromosome errors (Wilding et al. 2001). Superovulation also alters nuclear maturation, cAMP in oocytes, ultrastructure of oocytes and the expression of Epab and Pabpc1 (Hyttel et al. 1989, Wang et al. 2006, Dadarwal et al. 2015, Ozturk et al. 2016). Furthermore, a recently published paper demonstrated that repeated superovulation decreases 5-methylcytosine level in mouse oocytes (Xiao et al. 2019). The compromised oocyte quality may be an important reason for the decrease in the percentage of oocytes with the 1st PB and the rate of pronucleus at a superovulation time-dependent manner. For example, a recent study found repeated superovulation disturbs spindle organization and chromosome alignment during oocyte maturation (Xiao et al. 2019). Previous studies showed that superovulation increased the percentage of immature oocytes and decreased the fertilization rate, pronuclear rate and embryonic developmental potential (Ishibashi & Aoki 1977, Evans & Armstrong 1984, Sartori et al. 2010, Kon et al. 2014, Taiyeb et al. 2017). To avoid the effects of males on embryonic development, we used the same WT males in our experiments in each group. Our data also suggest that multiple superovulations decrease early embryonic development potentials, such as a lower blastocyst rate and a higher incidence of arrested embryos. In the human being, ART increases the risk of miscarriage, preterm delivery and low birth weight (De Geyter et al. 2006), which suggests that embryonic development potential may be compromised by ART manipulation. These results demonstrate that superovulation decreases the embryonic developmental potential.
To activate the zygotic genome, chromatin structure remodeling is required after fertilization. Epigenetic modifications play a pivotal role in regulating chromatin structure (Eckersley-Maslin et al. 2018). It is demonstrated that zygotic genome activation (ZGA) is regulated by histone modifications, miRNAs, DNA methylation, and so on (Zenk et al. 2017, Eckersley-Maslin et al. 2018). H4K12ac is an important histone modification in sperm and it is inherited by paternal pronucleus after fertilization (van der Heijden et al. 2006). For the maternal pronucleus, there is an increase of H4K12ac until the fusion of maternal and paternal pronuclei (Vieweg et al. 2015). H4K12ac is enriched at CTCF-binding sites and transcription start sites of genes involved in the developmental processes (Arpanahi et al. 2009) and gene activation in early embryos (Paradowska et al. 2012). For example, genes activated by H4K12ac at the four-cell embryo stage are mainly associated with gene expression, histone fold and DNA-dependent transcription. Genes activated by H4K12ac at the eight-cell embryo and blastocyst stage are involved in developmental processes (Paradowska et al. 2012). H4K16ac is another crucial acetylation at lysine 16 of histone 4 which can disrupt high-order chromatin structure and activate gene transcription in vivo and in vitro (Wu et al. 2011). H4K16ac is also very important for embryogenesis in humans and mice (Gupta et al. 2008, Lin et al. 2013). Histone deacetylases of HDAC1 and HDAC2 are important enzymes regulating histone acetylation and early embryo development (Ma & Schultz 2016). When fertilized embryos are treated with an inhibitor of HDAC, the blastocyst rate is significantly reduced (Ma et al. 2001). In the present study, we tested the mRNA expressions of Hdac1 and Hdac2 in embryos at different stages and found that the expression of Hdac1 and Hdac2 was higher in the early embryos from superovulation groups. This indicates that the abnormal expression of Hdac1 and Hdac2 may play a key role in the reduced H4K12ac and H4K16ac levels in early embryos.
Histone methylation is another important histone modification which plays an important role in the early embryos. H3K9me2 widely exists in the genome and undergoes great changes in cell differentiation (Lienert et al. 2011, Chen et al. 2012). In preimplantation mouse embryos, H3K9me2 has an important contribution to silencing retrotransposon to protect the genomes (Hatanaka et al. 2015). PGC7 suppresses the conversion of 5mC to 5hmC in early embryos via binding to histone H3K9me2, too (Nakamura et al. 2012). We found that repeated superovulation increased H3K9me2 in early embryos, which may be caused by the increase of G9a (Tachibana et al. 2002). H3K27me3 locates at the promoter regions of genes involved in developmental processes and represses genes’ expression in the early embryos (Shpargel et al. 2012). In early embryo development, there is a dynamic change of H3K27me3 at different stages. In blastocyst, H3K27me3 is lower than in other stage embryos (Liu et al. 2016). But the signal of H3K27me3 in early embryos derived from superovulation was significantly higher compared to that of non-stimulated mouse embryos. The increase of H3K27me3 was in a superovulation time-dependent manner. The dynamic change of H3K27me3 in the early embryos is mainly regulated by histone demethylases KDM6 and JmjC (Jumonji-C) (Shpargel et al. 2012). Therefore, the decreased expression of Kdm6a and Kdm6b may be essential for the higher signal of H3K27me3 in the early embryos.
Our data suggest that the alteration of histone modifications in early embryos may affect chromatin structure remodeling, which regulates ZGA. After fertilization, ZGA is the most important event as it initiates embryonic development. Embryos would be blocked at the two-cell stage in mice if the zygotic genome is not activated after fertilization. If ZGA is compromised, it would decrease embryonic development potentials, such as a lower blastocyst rate and a higher incidence of blocked embryos (Jachowicz et al. 2017). Therefore, it is plausible that multiple superovulations may affect embryonic quality and development through influencing chromatin structure and ZGA.
In summary, we found that repeated superovulations altered histone modifications in early embryos via increasing the expressions of Hdac1, Hdac2 and G9a and decreasing the expressions of Kdm6a and Kdm6b. The alteration of histone modifications may play a pivotal role in compromised embryonic development. However, it is not clear how multiple superovulations regulate gene expressions and histone modifications. Furthermore, what pathways mediate the alteration of histone modifications in this model remains ambiguous. It is demonstrated that the ovarian structure and mitochondrial function in cumulus cells are affected by multiple superovulations. Therefore, the dysfunction of mitochondria in cumulus cells might play an important role in mediating the effects of multiple superovulations on epigenetic marks. More studies are needed to investigate the mechanism underlying the effect of repeated superovulations on embryonic development and offspring health.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the funds of the National Natural Science Foundation of China (81401198, 31872312) and High-level Personnel Scientific Research Fund of Qingdao Agricultural University (1116008, 6631113337).
Author contribution statement
Tang S B designed the study, analyzed data and wrote the manuscript; Yang L L organized the charts; Zhang T T interpreted the data; Yin S, Luo S M and Shen W participated in the conception of study design and revised the manuscript; Ge Z J and Sun Q Y conceived the study design, participated in data analysis and interpretation.
Acknowledgements
The authors acknowledge Sun Yan-Li, Cui Ying-Xue, Li Wei-Dong and other staff working in College of Life Sciences, Institute of Reproductive Sciences, Qingdao Agricultural University for their contribution to this work.
References
- Adenot PG, Mercier Y, Renard JP, Thompson EM. 1997. Differential H4 acetylation of paternal and maternal chromatin precedes DNA replication and differential transcriptional activity in pronuclei of 1-cell mouse embryos. Development 124 4615–4625. [DOI] [PubMed] [Google Scholar]
- Aoshima K, Inoue E, Sawa H, Okada Y. 2015. Paternal H3K4 methylation is required for minor zygotic gene activation and early mouse embryonic development. EMBO Reports 16 803–812. ( 10.15252/embr.201439700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpanahi A, Brinkworth M, Iles D, Krawetz SA, Paradowska A, Platts AE, Saida M, Steger K, Tedder P, Miller D. 2009. Endonuclease-sensitive regions of human spermatozoal chromatin are highly enriched in promoter and CTCF binding sequences. Genome Research 19 1338–1349. ( 10.1101/gr.094953.109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhtari A, Rahmani HR, Bonakdar E, Jafarpour F, Asgari V, Hosseini SM, Hajian M, Edriss MA, Nasr-Esfahani MH. 2014. The interfering effects of superovulation and vitrification upon some important epigenetic biomarkers in mouse blastocyst. Cryobiology 69 419–427. ( 10.1016/j.cryobiol.2014.09.379) [DOI] [PubMed] [Google Scholar]
- Bogliotti YS, Ross PJ. 2012. Mechanisms of histone H3 lysine 27 trimethylation remodeling during early mammalian development. Epigenetics 7 976–981. ( 10.4161/epi.21615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonduelle M, Wennerholm UB, Loft A, Tarlatzis BC, Peters C, Henriet S, Mau C, Victorin-Cederquist A, Van Steirteghem A, Balaska A. et al. 2005. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Human Reproduction 20 413–419. ( 10.1093/humrep/deh592) [DOI] [PubMed] [Google Scholar]
- Chao HT, Lee SY, Lee HM, Liao TL, Wei YH, Kao SH. 2005. Repeated ovarian stimulations induce oxidative damage and mitochondrial DNA mutations in mouse ovaries. Annals of the New York Academy of Sciences 1042 148–156. ( 10.1196/annals.1338.016) [DOI] [PubMed] [Google Scholar]
- Chen X, Skutt-Kakaria K, Davison J, Ou YL, Choi E, Malik P, Loeb K, Wood B, Georges G, Torok-Storb B. et al. 2012. G9a/GLP-dependent histone H3K9me2 patterning during human hematopoietic stem cell lineage commitment. Genes and Development 26 2499–2511. ( 10.1101/gad.200329.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combelles CM, Albertini DF. 2003. Assessment of oocyte quality following repeated gonadotropin stimulation in the mouse. Biology of Reproduction 68 812–821. ( 10.1095/biolreprod.102.008656) [DOI] [PubMed] [Google Scholar]
- Dadarwal D, Honparkhe M, Dias FC, Alce T, Lessard C, Singh J. 2015. Effect of superstimulation protocols on nuclear maturation and distribution of lipid droplets in bovine oocytes. Reproduction, Fertility and Development 27 1137–1146. ( 10.1071/RD13265) [DOI] [PubMed] [Google Scholar]
- De Geyter C, De Geyter M, Steimann S, Zhang H, Holzgreve W. 2006. Comparative birth weights of singletons born after assisted reproduction and natural conception in previously infertile women. Human Reproduction 21 705–712. ( 10.1093/humrep/dei378) [DOI] [PubMed] [Google Scholar]
- Dong G, Guo Y, Cao H, Zhou T, Zhou Z, Sha J, Guo X, Zhu H. 2014. Long-term effects of repeated superovulation on ovarian structure and function in rhesus monkeys. Fertility and Sterility 102 1452.e1–1457.e1. ( 10.1016/j.fertnstert.2014.07.739) [DOI] [PubMed] [Google Scholar]
- Eckersley-Maslin MA, Alda-Catalinas C, Reik W. 2018. Dynamics of the epigenetic landscape during the maternal-to-zygotic transition. Nature Reviews: Molecular Cell Biology 19 436–450. ( 10.1038/s41580-018-0008-z) [DOI] [PubMed] [Google Scholar]
- El Hajj N, Haaf T. 2013. Epigenetic disturbances in in vitro cultured gametes and embryos: implications for human assisted reproduction. Fertility and Sterility 99 632–641. ( 10.1016/j.fertnstert.2012.12.044) [DOI] [PubMed] [Google Scholar]
- Erhardt S, Su IH, Schneider R, Barton S, Bannister AJ, Perez-Burgos L, Jenuwein T, Kouzarides T, Tarakhovsky A, Surani MA. 2003. Consequences of the depletion of zygotic and embryonic enhancer of zeste 2 during preimplantation mouse development. Development 130 4235–4248. ( 10.1242/dev.00625) [DOI] [PubMed] [Google Scholar]
- European IVF-Monitoring Consortium (EIM), European Society of Human Reproduction and Embryology (ESHRE), Kupka MS, D’Hooghe T, Ferraretti AP, de Mouzon J, Erb K, Castilla JA, Calhaz-Jorge C, De Geyter Ch. et al. 2016. Assisted reproductive technology in Europe, 2011: results generated from European registers by ESHRE. Human Reproduction 31 233–248. ( 10.1093/humrep/dev319) [DOI] [PubMed] [Google Scholar]
- Evans G, Armstrong DT. 1984. Reduction in fertilization rate in vitro of oocytes from immature rats induced to superovulate. Journal of Reproduction and Fertility 70 131–135. ( 10.1530/jrf.0.0700131) [DOI] [PubMed] [Google Scholar]
- Grigoryan A, Guidi N, Senger K, Liehr T, Soller K, Marka G, Vollmer A, Markaki Y, Leonhardt H, Buske C. et al. 2018. Lamina/C regulates epigenetic and chromatin architecture changes upon aging of hematopoietic stem cells. Genome Biology 19 189. ( 10.1186/s13059-018-1557-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Guerin-Peyrou TG, Sharma GG, Park C, Agarwal M, Ganju RK, Pandita S, Choi K, Sukumar S, Pandita RK. et al. 2008. The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Molecular and Cellular Biology 28 397–409. ( 10.1128/MCB.01045-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Kurinczuk JJ, Bower C, Webb S. 2002. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. New England Journal of Medicine 346 725–730. ( 10.1056/NEJMoa010035) [DOI] [PubMed] [Google Scholar]
- Hatanaka Y, Inoue K, Oikawa M, Kamimura S, Ogonuki N, Kodama EN, Ohkawa Y, Tsukada Y, Ogura A. 2015. Histone chaperone CAF-1 mediates repressive histone modifications to protect preimplantation mouse embryos from endogenous retrotransposons. PNAS 112 14641–14646. ( 10.1073/pnas.1512775112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyttel P, Greve T, Callesen H. 1989. Ultrastructure of oocyte maturation and fertilization in superovulated cattle. Progress in Clinical and Biological Research 296 287–297. [PubMed] [Google Scholar]
- Ishibashi I, Aoki H. 1977. Effects of gonadotrophins on maturation and ovulation of oocytes in adult rats. Reproduction 22 130–138. ( 10.1262/jrd1955.22.130) [DOI] [Google Scholar]
- Jachowicz JW, Bing X, Pontabry J, Boskovic A, Rando OJ, Torres-Padilla ME. 2017. LINE-1 activation after fertilization regulates global chromatin accessibility in the early mouse embryo. Nature Genetics 49 1502–1510. ( 10.1038/ng.3945) [DOI] [PubMed] [Google Scholar]
- Kallen B, Finnstrom O, Nygren KG, Otterblad Olausson P. 2005. In vitro fertilization in Sweden: maternal characteristics. Acta Obstetricia and Gynecologica Scandinavica 84 1185–1191. ( 10.1111/j.0001-6349.2005.00858.x) [DOI] [PubMed] [Google Scholar]
- Kalthur G, Salian SR, Nair R, Mathew J, Adiga SK, Kalthur SG, Zeegers D, Hande MP. 2016. Distribution pattern of cytoplasmic organelles, spindle integrity, oxidative stress, octamer-binding transcription factor 4 (Oct4) expression and developmental potential of oocytes following multiple superovulation. Reproduction, Fertility and Development 28 2027–2038. ( 10.1071/RD15184) [DOI] [PubMed] [Google Scholar]
- Kon H, Hokao R, Shinoda M. 2014. Fertilizability of superovulated eggs by estrous stage-independent PMSG/hCG treatment in adult Wistar-Imamichi rats. Experimental Animals 63 175–182. ( 10.1538/expanim.63.175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E. 2002. Chromatin modification and epigenetic reprogramming in mammalian development. Nature Reviews Genetics 3 662–673. ( 10.1038/nrg887) [DOI] [PubMed] [Google Scholar]
- Liang XW, Cui XS, Sun SC, Jin YX, Heo YT, Namgoong S, Kim NH. 2013. Superovulation induces defective methylation in line-1 retrotransposon elements in blastocyst. Reproductive Biology and Endocrinology 11 69. ( 10.1186/1477-7827-11-69) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienert F, Mohn F, Tiwari VK, Baubec T, Roloff TC, Gaidatzis D, Stadler MB, Schubeler D. 2011. Genomic prevalence of heterochromatic H3K9me2 and transcription do not discriminate pluripotent from terminally differentiated cells. PLoS Genetics 7 e1002090. ( 10.1371/journal.pgen.1002090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Conti M, Ramalho-Santos M. 2013. Histone variant H3.3 maintains a decondensed chromatin state essential for mouse preimplantation development. Development 140 3624–3634. ( 10.1242/dev.095513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang C, Liu W, Li J, Li C, Kou X, Chen J, Zhao Y, Gao H, Wang H. et al. 2016. Distinct features of H3K4me3 and H3K27me3 chromatin domains in pre-implantation embryos. Nature 537 558–562. ( 10.1038/nature19362) [DOI] [PubMed] [Google Scholar]
- Ma P, Schultz RM. 2016. HDAC1 and HDAC2 in mouse oocytes and preimplantation embryos: specificity versus compensation. Cell Death and Differentiation 23 1119–1127. ( 10.1038/cdd.2016.31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Svoboda P, Schultz RM, Stein P. 2001. Regulation of zygotic gene activation in the preimplantation mouse embryo: global activation and repression of gene expression. Biology of Reproduction 64 1713–1721. ( 10.1095/biolreprod64.6.1713) [DOI] [PubMed] [Google Scholar]
- Ma XS, Wang XG, Qin L, Song CL, Lin F, Song JM, Zhu CC, Liu HL. 2014. De novo DNA methylation of the paternal genome in 2-cell mouse embryos. Genetics and Molecular Research 13 8632–8639. ( 10.4238/2014.October.27.2) [DOI] [PubMed] [Google Scholar]
- Market-Velker BA, Zhang L, Magri LS, Bonvissuto AC, Mann MR. 2010. Dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner. Human Molecular Genetics 19 36–51. ( 10.1093/hmg/ddp465) [DOI] [PubMed] [Google Scholar]
- Morgan HD, Santos F, Green K, Dean W, Reik W. 2005. Epigenetic reprogramming in mammals. Human Molecular Genetics 14 R47–R58 R47–R58. ( 10.1093/hmg/ddi114) [DOI] [PubMed] [Google Scholar]
- Nakamura T, Liu YJ, Nakashima H, Umehara H, Inoue K, Matoba S, Tachibana M, Ogura A, Shinkai Y, Nakano T. 2012. PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature 486 415–419. ( 10.1038/nature11093) [DOI] [PubMed] [Google Scholar]
- Ozturk S, Yaba-Ucar A, Sozen B, Mutlu D, Demir N. 2016. Superovulation alters embryonic poly(A)-binding protein (Epab) and poly(A)-binding protein, cytoplasmic 1 (Pabpc1) gene expression in mouse oocytes and early embryos. Reproduction, Fertility and Development 28 375–383. ( 10.1071/RD14106) [DOI] [PubMed] [Google Scholar]
- Paradowska AS, Miller D, Spiess AN, Vieweg M, Cerna M, Dvorakova-Hortova K, Bartkuhn M, Schuppe HC, Weidner W, Steger K. 2012. Genome wide identification of promoter binding sites for H4K12ac in human sperm and its relevance for early embryonic development. Epigenetics 7 1057–1070. ( 10.4161/epi.21556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos F, Peters AH, Otte AP, Reik W, Dean W. 2005. Dynamic chromatin modifications characterise the first cell cycle in mouse embryos. Developmental Biology 280 225–236. ( 10.1016/j.ydbio.2005.01.025) [DOI] [PubMed] [Google Scholar]
- Sartori R, Bastos MR, Wiltbank MC. 2010. Factors affecting fertilisation and early embryo quality in single- and superovulated dairy cattle. Reproduction, Fertility and Development 22 151–158. ( 10.1071/RD09221) [DOI] [PubMed] [Google Scholar]
- Shang WH, Hori T, Westhorpe FG, Godek KM, Toyoda A, Misu S, Monma N, Ikeo K, Carroll CW, Takami Y. et al. 2016. Acetylation of histone H4 lysine 5 and 12 is required for CENP-A deposition into centromeres. Nature Communications 7 13465. ( 10.1038/ncomms13465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpargel KB, Sengoku T, Yokoyama S, Magnuson T. 2012. UTX and UTY demonstrate histone demethylase-independent function in mouse embryonic development. PLoS Genetics 8 e1002964. ( 10.1371/journal.pgen.1002964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan R, Gonzales-Cope M, Chronis C, Bonora G, McKee R, Huang C, Patel S, Lopez D, Mishra N, Pellegrini M. et al. 2013. Proteomic and genomic approaches reveal critical functions of H3K9 methylation and heterochromatin protein-1gamma in reprogramming to pluripotency. Nature Cell Biology 15 872–882. ( 10.1038/ncb2768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg B, Dahlquist G, Ericson A, Finnstrom O, Koster M, Stjernqvist K. 2002. Neurological sequelae in children born after in-vitro fertilisation: a population-based study. Lancet 359 461–465. ( 10.1016/S0140-6736(02)07674-2) [DOI] [PubMed] [Google Scholar]
- Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, Barfield WD. & Centers for Disease, and Prevention 2015. Assisted reproductive technology surveillance – United States, 2013. MMWR Surveillance Summaries 64 1–25. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, Fukuda M, Takeda N, Niida H, Kato H. et al. 2002. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes and Development 16 1779–1791. ( 10.1101/gad.989402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiyeb AM, Muhsen-Alanssari SA, Dees WL, Hiney J, Kjelland ME, Kraemer DC, Ridha-Albarzanchi MT. 2017. Improvements in oocyte competence in superovulated mice following treatment with cilostazol: ovulation of immature oocytes with high developmental rates. Biochemical Pharmacology 137 81–92. ( 10.1016/j.bcp.2017.04.019) [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Perez-Albala S, Cano A. 2001. Cellular and morphological traits of oocytes retrieved from aging mice after exogenous ovarian stimulation. Biology of Reproduction 65 141–150. [DOI] [PubMed] [Google Scholar]
- Van der Elst J, Amerijckx Y, Van Steirteghem A. 1998. Ultra-rapid freezing of mouse oocytes lowers the cell number in the inner cell mass of 5 day old in-vitro cultured blastocysts. Human Reproduction 13 1595–1599. ( 10.1093/humrep/13.6.1595) [DOI] [PubMed] [Google Scholar]
- van der Heijden GW, Dieker JW, Derijck AA, Muller S, Berden JH, Braat DD, van der Vlag J, de Boer P. 2005. Asymmetry in histone H3 variants and lysine methylation between paternal and maternal chromatin of the early mouse zygote. Mechanisms of Development 122 1008–1022. ( 10.1016/j.mod.2005.04.009) [DOI] [PubMed] [Google Scholar]
- van der Heijden GW, Derijck AA, Ramos L, Giele M, van der Vlag J, de Boer P. 2006. Transmission of modified nucleosomes from the mouse male germline to the zygote and subsequent remodeling of paternal chromatin. Developmental Biology 298 458–469. ( 10.1016/j.ydbio.2006.06.051) [DOI] [PubMed] [Google Scholar]
- Van Steirteghem A, Bonduelle M, Devroey P, Liebaers I. 2002. Follow-up of children born after ICSI. Human Reproduction Update 8 111–116. ( 10.1093/humupd/8.2.111) [DOI] [PubMed] [Google Scholar]
- Ventura-Junca P, Irarrazaval I, Rolle AJ, Gutierrez JI, Moreno RD, Santos MJ. 2015. In vitro fertilization (IVF) in mammals: epigenetic and developmental alterations. Scientific and bioethical implications for IVF in humans. Biological Research 48 68. ( 10.1186/s40659-015-0059-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieweg M, Dvorakova-Hortova K, Dudkova B, Waliszewski P, Otte M, Oels B, Hajimohammad A, Turley H, Schorsch M, Schuppe HC. et al. 2015. Methylation analysis of histone H4K12ac-associated promoters in sperm of healthy donors and subfertile patients. Clinical Epigenetics 7 31. ( 10.1186/s13148-015-0058-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Dey SK. 2006. Roadmap to embryo implantation: clues from mouse models. Nature Reviews Genetics 7 185–199. ( 10.1038/nrg1808) [DOI] [PubMed] [Google Scholar]
- Wang Y, Ock SA, Chian RC. 2006. Effect of gonadotrophin stimulation on mouse oocyte quality and subsequent embryonic development in vitro. Reproductive Biomedicine Online 12 304–314. ( 10.1016/S1472-6483(10)61002-4) [DOI] [PubMed] [Google Scholar]
- Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G. 2001. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Human Reproduction 16 909–917. ( 10.1093/humrep/16.5.909) [DOI] [PubMed] [Google Scholar]
- Wu SF, Zhang H, Cairns BR. 2011. Genes for embryo development are packaged in blocks of multivalent chromatin in zebrafish sperm. Genome Research 21 578–589. ( 10.1101/gr.113167.110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao P, Nie J, Wang X, Lu K, Lu S, Liang X. 2019. Melatonin alleviates the deterioration of oocytes from mice subjected to repeated superovulation. Journal of Cellular Physiology Epub. ( 10.1002/jcp.28018) [DOI] [PubMed] [Google Scholar]
- Xie JK, Wang Q, Zhang TT, Yin S, Zhang CL, Ge ZJ. 2016. Repeated superovulation may affect mitochondrial functions of cumulus cells in mice. Scientific Reports 6 31368. ( 10.1038/srep31368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenk F, Loeser E, Schiavo R, Kilpert F, Bogdanovic O, Iovino N. 2017. Germ line-inherited H3K27me3 restricts enhancer function during maternal-to-zygotic transition. Science 357 212–216. ( 10.1126/science.aam5339) [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang F, Kou Z, Zhang Y, Gao S. 2009. Defective chromatin structure in somatic cell cloned mouse embryos. Journal of Biological Chemistry 284 24981–24987. ( 10.1074/jbc.M109.011973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Zheng H, Huang B, Li W, Xiang Y, Peng X, Ming J, Wu X, Zhang Y, Xu Q. et al. 2016. Allelic reprogramming of the histone modification H3K4me3 in early mammalian development. Nature 537 553–557. ( 10.1038/nature19361) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a