Effective combined antiretroviral therapy regimens have extended survival of persons living with HIV (PLWH). Heavy alcohol consumption is common in PLWH. This overview integrates evidence from clinical and preclinical research to identify salient alcohol-related mechanisms and comorbidities contributing to disease pathogenesis and accelerated aging and senescence in PLWH.
Abstract
Alcohol’s multisystemic effects impact HIV disease pathogenesis and increase the risk for comorbidities in persons living with HIV (PLWH). The increased number of aging PLWH increases the potential for alcohol to enhance the risk for comorbidities. Integration of epidemiological, preclinical and translational studies provide an overarching view of the impact of heavy alcohol consumption on HIV risk, pathogenesis, treatment and burden of disease. The combined insult of HIV infection, heavy alcohol consumption and toxic effects of antiretroviral therapy in aging PLWH poses a public health challenge through increased disease burden that also impacts quality of life and increases health care costs. Herein we provide a brief overview of current knowledge on alcohol’s impact on HIV disease pathogenesis, with focus on aging PLWH.
Short Summary: Effective combined antiretroviral therapy regimens have extended survival of persons living with HIV (PLWH). Heavy alcohol consumption is common in PLWH. This overview integrates evidence from clinical and preclinical research to identify salient alcohol-related mechanisms and comorbidities contributing to disease pathogenesis and accelerated aging and senescence in PLWH.
INTRODUCTION
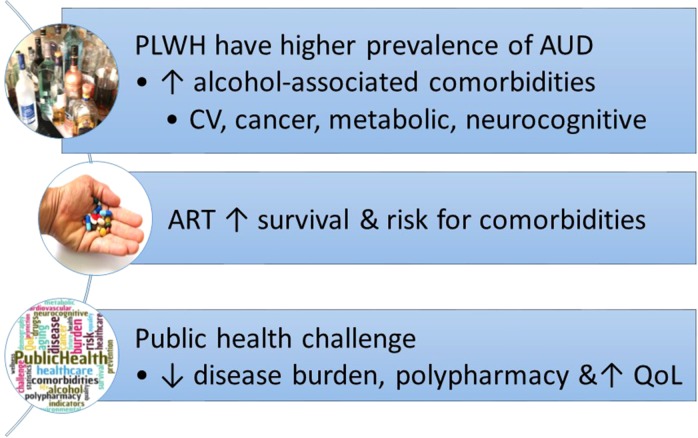
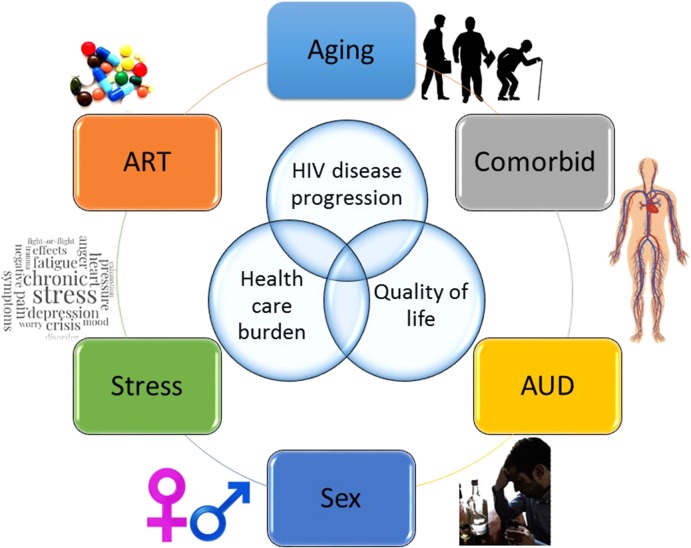
Heavy, or ‘at-risk’, alcohol consumption is defined as consumption of more than 60 and 40 g of ethanol per day (for men and women, respectively) by the World Health Organization (WHO), and by either consumption of more than 14 or 7 drinks per week (for men and women, respectively) or binge drinking (more than 4–5 drinks within a 2-h period) by the National Institute of Alcohol Abuse and Alcoholism (NIAAA). Heavy alcohol consumption is the most common and costly form of substance use disorder in the United States (World Health Organization, 2014; SAMSHA, 2016). Alcohol use is strongly associated with an increased risk of HIV infection (Lefevre et al., 1995) (Fig. 1). Persons living with HIV (PLWH) have a 2–3-fold higher prevalence of alcohol use disorder (AUD) (Conigliaro et al., 2006), and ~8–12% of PLWH are classified as heavy drinkers (Burnam et al., 2001; Galvan et al., 2002). Heavy alcohol consumption can affect disease pathogenesis through direct and indirect interactions with factors including age, stress, sex and access and adherence to antiretroviral therapy (ART) (Fig. 2). In the era of combined ART, HIV infection has become a chronic disease and current estimates indicate that 50% of PLWH in the US are 50 years of age or older (Centers for Disease Control and Prevention, 2016). HIV infection, heavy alcohol consumption and aging independently increase the risk for several comorbidities, including myopathy, cardiovascular disease (CVD), liver cirrhosis, diabetes and pneumonia. Here we provide an overview of salient findings from preclinical and clinical studies and identify areas in need of further investigation.
Fig. 1.
Key aspects of the interaction of chronic risky alcohol consumption on HIV disease.
Fig. 2.
Chronic heavy alcohol consumption impacts HIV pathogenesis through direct and indirect interactions with factors including age, stress, sex and access and adherence to antiretroviral therapy (ART). As a consequence of chronic heavy alcohol consumption; disease progression, health care burden and quality of life are significantly and detrimentally affected.
IMPACT OF ALCOHOL ON HIV DISEASE
Several clinical studies have evaluated the effects of alcohol use on CD4+ T cell counts and viral load, but consistent associations of these clinical markers with at-risk drinking have not always been observed. One prospective longitudinal study showed greater suppression of CD4+ T cell counts in PLWH with frequent alcohol use (Baum et al., 2010), while another reported that heavy alcohol consumption negatively impacted CD4+ cell counts only in ART-naïve subjects (Samet et al., 2007). Other investigations failed to establish an association between heavy alcohol consumption and CD4+ T cell decline, or have shown an indirect effect of alcohol use on CD4+ T cells due to poor ART adherence (Samet et al., 2007; Hahn and Samet, 2010; Wandera et al., 2017; Hahn et al., 2018). Clinical studies have not found a direct, negative effect of alcohol consumption on viral load, however, heavy alcohol consumption has been identified as a significant contributor to poor ART adherence (Braithwaite and Bryant, 2010). Non-adherence to ART is more likely among PLWH that engage in binge drinking than non-drinkers and, consequently, binge drinkers are less likely to achieve viral suppression, potentially increasing morbidity and mortality.
Due to the multitude of variable behavioral and environmental factors that may modulate disease over the span of HIV infection, it is difficult to accurately assess effects of risky alcohol use on clinical markers of HIV disease in clinical cohorts over short time-spans. Animal model studies using simian immunodeficiency virus (SIV) infected macaques, with well-controlled behavioral and environmental conditions, have provided significant insight on the interaction of HIV and heavy drinking (Amedee et al., 2014). Preclinical studies in the chronic binge alcohol (CBA)-administered SIV macaque have consistently associated increased viral load with alcohol consumption. Additionally, studies using this model (Bagby et al., 2006; Molina et al., 2006, 2008) show a significant temporal acceleration to end-stage disease in the absence of ART, with consistently higher plasma, cerebrospinal fluid and tissue viral loads among CBA-administered animals compared to controls (Poonia et al., 2006; Amedee et al., 2014).
ART leads to a similar reduction in viral load in CBA and control animals, suggesting that, with strict adherence, ART may effectively control viremia (Molina et al., 2014a). However, emerging data suggests that viral expression in reservoirs remains elevated in ART-treated, CBA-administered, SIV-infected macaques (unpublished data), a finding which cannot be explained by decreased adherence and that highlights the possibility that viral reactivation in PLWH may be more common in heavy drinkers than in non-drinkers. In addition, others have shown that SIV continues to replicate in the brain of alcohol-dependent animals, whereas it is undetectable in controls (Kumar et al., 2005). Moreover, recent in vitro studies suggest that ethanol may decrease intracellular efficacy of some ART drugs, such as the integrase strand transfer inhibitor, elvitegravir (Midde et al., 2017). Thus, additional in vivo studies are needed to determine efficacy of ART in viral reservoirs and the impact of alcohol.
ALCOHOL, HIV AND GERIATRIC COMORBIDITIES
Improvement in survival of PLWH is complicated by comorbidities typically associated with advanced age, including osteoporosis, diabetes, CVD, cancer, renal disease and metabolic alterations (Burgess et al., 2015). All of these HIV-associated metabolic comorbidities can be further exacerbated by heavy alcohol consumption (Wand et al., 2007), particularly in the aging population. A significant proportion of PLWH meet criteria for multimorbidity (Kim et al., 2012), particularly PLWH who are older and obese, and have lower CD4+ cell counts. The high rate of comorbidities in PLWH, due in part to high-risk behaviors (e.g. smoking, heavy alcohol consumption), increases morbidity and mortality (Hawkins et al., 2017). These comorbidities, discussed in the following sections, contribute to the development of a frailty syndrome.
Osteoporosis and bone fractures
PLWH have an increased risk of fracture, associated with risk factors of bone fragility like poor nutrition, smoking, alcohol consumption, liver disease and hypogonadism (Compston, 2016). HIV infection in children and adolescents correlates with reduced bone mineral density (BMD) and quality, suggesting that HIV undermines normal bone formation. Further, ART leads to accelerated BMD loss during the initial treatment period and particularly in those with low CD4+ counts and higher plasma HIV viral loads (both of which are associated with heavy alcohol consumption, as discussed above). Heavy alcohol consumption is an established risk factor for low BMD, fractures and osteoporosis, even in the absence of liver failure (Maurel et al., 2012). The underlying mechanisms are multifactorial and include hormonal (decreased androgens, growth hormone and insulin-like growth factor 1), inflammatory (inflammatory cytokines and chemokines) and nutritional (decreased intake of vitamin D and calcium) factors (Luo et al., 2017). In combination, ART and alcohol likely further diminish aging- and sex-related reductions in bone health in PLWH. Recent reports have found associations between alcohol-related diagnoses and between recent, but not lifetime, drinking and BMD in PLWH (Ventura et al., 2017; Womack et al., 2013). This remains an area in need of further investigation.
Cardiovascular disease
Risk of death from acute myocardial infarction independent of traditional CVD risk factors is 1.5–2 times higher in PLWH than in the general population. Lack of virologic suppression, which most frequently occurs with treatment interruption (Kuller et al., 2008) (as frequently reported in alcoholics), increases the risk for atherosclerosis (Triant et al., 2007). However, progression from atherosclerosis to myocardial infarction is faster in PLWH than in controls, even with controlled viremia (Hsue et al., 2012). The proposed underlying mechanisms include chronic inflammation, microbial translocation and mitochondrial dysfunction, which synergize with traditional risk factors for CVD including tobacco and alcohol use, dyslipidemia and obesity (Triant et al., 2007; Kim et al., 2012). An increased risk of CVD in hazardous and dependent drinkers (compared to infrequent and moderate drinkers) has been observed in PLWH (Freiberg et al., 2010). CVD risk has been attributed to the metabolic toxicities of protease inhibitors. More current ART regimens are associated with improved surrogate markers of atherosclerosis (Torriani et al., 2008). However, there is sparse data examining alcohol as a mediator of the increased risk of CVD among PLWH (Kelso et al., 2015).
Metabolic
The incidence of metabolic comorbidities, such as insulin resistance, is high in PLWH (Falutz, 2011), increasing the risk for glucose intolerance and diabetes (Lombo et al., 2015). Although the mechanisms underlying dysglycemia in PLWH remain poorly understood, chronic subclinical inflammation is emerging as a central mechanism of metabolic derangements (Kolter, 2003). The high prevalence of diabetes among PLWH (Kim et al., 2012) is linked to traditional risk factors such as race/ethnicity, smoking status and obesity (De Wit et al., 2008). Risk factors for metabolic comorbidities also include viral proteins and ART therapy. More recent ART regimens reduce metabolic dysregulation. However, heavy alcohol consumption contributes to the development of insulin resistance (Yki-Jarvinen and Nikkila, 1985), lipodystrophy (Cheng et al., 2009) and altered adipokine profiles, which have all been linked to metabolic alterations in obese (Rasouli and Kern, 2008) and ART-treated (Sevastianova et al., 2008) PLWH. Both heavy alcohol consumption (Molina, 2008) and HIV/AIDS (Rimaniol et al., 1996; Alonso et al., 1997) disrupt endocrine mechanisms (i.e. GH/IGF-I system) (Frost et al., 1996) and gonadal hormones (Poretsky et al., 1995) and increase oxidative stress (Israel and Gougerot-Pocidalo, 1997), which could also contribute to metabolic dysregulation in PLWH.
Neurocognitive impairment
Alcohol and HIV have potentially overlapping and additive, or synergistic, effects on neurocognitive function. In the ART era, the prevalence of HIV-associated dementia (HAD) has declined (Heaton et al., 2010), but neurocognitive impairment in the form of asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorder (MND) is still prevalent in up to 50% of PLWH (Woods et al., 2009; Heaton et al., 2010; Saylor et al., 2016). Although the severity of cognitive impairments has decreased, incidence of ANI has increased (Heaton et al., 2010) and the domains affected have shifted, resulting in a lower percentage of PLWH with deficits in verbal domains, information processing and motor coordination, but more learning and executive function impairment (Heaton et al., 2011; Sacktor et al., 2016). Theses shifts in cognitive deficits may reflect differential vulnerabilities of brain regions to HIV-associated neuropathology. Site-specific structural changes have been identified at the whole brain level in PLWH, with ventricular enlargement, thinning of the cortex, and decreased volume of the frontal cortex and caudate being associated with cognitive impairment (Rosenbloom et al., 2010). Metabolic dysfunction, CVD and poor ART adherence may aggravate cognitive decline, particularly in aging PLWH (Levine et al., 2016; Canizares et al., 2014). Alcohol-associated alterations in micronutrient deficits, metabolic homeostasis and polydrug use can all increase the risk of neurocognitive impairment (Gongvatana et al., 2014) and remain an understudied area of research.
Geriatric syndromes
Geriatric syndromes are multifactorial health conditions typically occurring with advanced age, and ranging from incontinence to dementia to impaired physical function to polypharmacy. Data suggest that HIV itself leads to precocious aging, geriatric comorbidities and frailty (Freiberg et al., 2013; Womack et al., 2013). These have commonalities with recognized HIV-associated comorbidities including cachexia and sarcopenia, low bone density, neurocognitive impairment, depression and viral (particularly hepatitis) coinfection (Brothers et al., 2014). Hazardous alcohol use in PLWH is associated with an elevated risk of phenotypic frailty (Piggott et al., 2013).
Cellular and immune aging
Early on, it was recognized that HIV infection is associated with immunosenescence resulting from chronic immune activation driven by HIV-1 continuous virion replication, production of pro-inflammatory cytokines, a rise in lymphocyte proliferation and cell death (Meyaard et al., 1992), and an imbalance in Th1/Th2 responses (Romagnani et al., 1994). Our preclinical and clinical studies show significantly increased peripheral blood CD8+ T cell activation and immunosenescence as compared to baseline levels in CBA, non-ART-treated, SIV-infected macaques (Katz et al., 2015). Moreover, the frequency of activated immunosenescent CD8+ T cells positively correlated with alcohol use disorder identification test (AUDIT) scores in PLWH (Katz et al., 2015). Heavy alcohol consumption has also been linked with accelerated cellular aging as reflected by shorter telomere length (Pavanello et al., 2011). Telomeres, DNA–protein protective structures at the ends of each chromosome, undergo continuous loss with each cell division, decreasing in length as cells approach senescence and/or apoptosis. In the MacArthur Health Aging Study, alcohol use predicted loss of telomere length (Epel et al., 2009); and in the Helsinki Businessman Study, lifetime drinking predicted shorter telomeres (Strandberg et al., 2012). Thus, alcohol appears to hasten cellular aging in the general population and this appears to be exacerbated in PLWH. Therefore, biological age and cellular senescence associated with heavy alcohol consumption are likely to synergize to increase the risk for comorbidities and development of geriatric syndromes in PLWH. As the number of aging PLWH continues to rise, diagnosis and management of comorbidities will be of equal importance to achieving control of viremia, and decreasing alcohol use in this vulnerable population.
MECHANISMS IMPLICATED IN ALCOHOL-HIV PATHOPHYSIOLOGY
Several mechanisms involved in alcohol-induced tissue and organ injury are likely to be responsible for the increased risk of comorbidities, morbidity and mortality in PLWH with AUD. Oxidative stress, mitochondrial injury and epigenetic modifications are among the most salient mechanisms that are potential therapeutic targets.
Chronic inflammation
Chronic inflammatory states correlate with higher viremia, disease progression and clinical sequela of chronic HIV infection (Appay and Kelleher, 2016). Additive or synergistic effects of alcohol and HIV on systemic inflammation promote HIV replication (Bagasra et al., 1996). Although ART can effectively reduce or eliminate plasma HIV levels, low-level viral replication and the expression of viral proteins in discrete tissue reservoir sites may contribute to the inflammatory state and further exacerbate immune dysfunction.
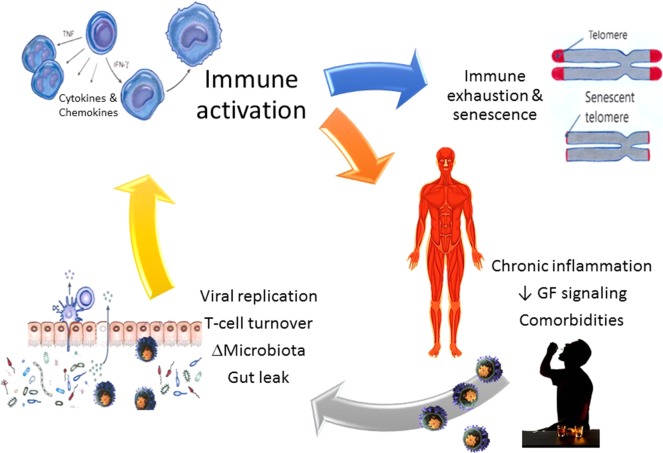
Evidence supporting the link between enhanced viral replication and chronic inflammation and immune dysfunction is derived from preclinical studies in ART-naïve SIV-infected macaques. Those studies showed markedly increased rates of proliferation/activation of gastrointestinal (GI) submucosa CD4+ and CD8+ T cells and decreased total numbers of T cells (Veazey et al., 2015), which correlated with increased SIV expression in CBA-administered animals (Poonia et al., 2006). Similarly, heightened vaginal pro-inflammatory milieu in CBA-administered female SIV-infected macaques coincided with greater viral replication Loganantharaj et al. (2014). Relevant to overall disease, chronic intestinal inflammation and the associated immune activation promotes viral replication, compromises the integrity of the gut mucosal barrier, and increases the potential for microbial translocation (Fig. 3).
Fig. 3.
Integrating findings from clinical and preclinical studies, the current thinking is that chronic heavy alcohol consumption by persons living with HIV (PLWH) promotes increased viral replication, lymphocyte turnover, dysbiosis and gut barrier leak, resulting in translocation of toxins and bacterial products into the systemic circulation. Together, toxins and increased viral replication promote immune activation leading to a state of immune exhaustion and senescence, and chronic systemic inflammation, tissue injury and increased risk for development of comorbidities.
The GI tract is the primary target for early HIV infection, viral expansion and CD4+ T cell loss (Veazey et al., 1998). The early phase of HIV infection is associated with marked gut inflammation and barrier leak (Gori et al., 2008). Abundant evidence, including work from our group, now supports a pivotal role for the GI tract microbiota as a regulator of host processes and homeostasis (Samuelson et al., 2017). The microbiota, through production of specific nutrients and biomediators (e.g. short chain fatty acids), maintains epithelial integrity. Preclinical models have clearly demonstrated the essential role of intestinal microorganisms in the development and control of both local and systemic immunity. During HIV infection, the normal microbiota of the alimentary tract is perturbed (Vazquez-Castellanos et al., 2015; Williams et al., 2016). Similarly, alcohol leads to bacterial overgrowth and alters the alimentary tract microbial community composition (Leclercq et al., 2014).
Persistent GI disease/inflammation and loss of mucosal integrity, accompanied by microbial product translocation into the systemic circulation, is associated with HIV disease progression and mortality as supported by clinical and preclinical studies (Dandekar et al., 2010). PLWH have increased biomarkers of microbial translocation, lipopolysaccharide and its receptor (soluble CD14), and bacterial 16 S ribosomal RNA (rRNA) gene, compared with uninfected persons. This increased antigenic burden results in immune activation and chronic inflammation, culminating in immune exhaustion and senescence (Dinh et al., 2015). Alcohol increases intestinal permeability and translocation of bacterial components (Keshavarzian et al., 2009). Collectively, these data indicate that the GI tract is a central point at which HIV, heavy alcohol use and immune activation intersect, and therefore may promote the immune senescence observed as a result of HIV infection (Desai and Landay, 2010; Chou et al., 2013). Preliminary analyses by our group have implicated alcohol-associated dysbiosis as contributory to immune activation in PLWH.
Oxidative stress
Alcohol metabolism is a significant source of reactive oxygen species (ROS) and shifts cellular redox state. Oxidative stress, resulting from either an excess production of ROS or a depletion of tissue reducing antioxidant equivalents, contributes to alcohol- and HIV-mediated tissue injury (Molina et al., 2014b) and promotes HIV-1 replication (Kumar et al., 2012). Oxidative stress activates lymphocytes and accentuates chronic inflammation and lipid peroxidation (Aukrust et al., 2003), producing the cellular damage associated with HIV, heavy alcohol consumption (Fernandez-Sola et al., 2007; Molina et al., 2014a,b) and ART. Moreover, mitochondrial energy generation through oxidative phosphorylation also generates ROS that form adducts with proteins, DNA and lipids, causing cellular damage. Our studies show that CBA administration results in depletion of antioxidant capacity, decreased expression of super oxide dismutase 2 (SOD2) (a potent mitochondrial antioxidant enzyme), and altered gene regulatory networks that regulate oxidative stress. Together, these mechanisms contribute to accentuated skeletal muscle wasting at end-stage disease in SIV-infected macaques (Molina et al., 2008; Lecapitaine et al., 2011; Simon et al., 2015; Duplanty et al., 2017). Whether similar defects in mitochondrial antioxidant capacity occur in other tissues, like the liver, remains to be investigated.
Mitochondrial dysfunction
Excessive ROS production and/or defective antioxidant capacity play a role in mitochondrial dysfunction and mitochondrial DNA (mtDNA) mutations (Calvani et al., 2013). Mitochondrial dysfunction due to heavy alcohol consumption occurs in the liver (Hoek et al., 2002), skeletal muscle (Simon et al., 2017b) and cardiac muscle (Steiner and Lang, 2017) and is characterized by mitochondrial genome degradation in the brain, heart and skeletal muscle and by decreased mitochondrial biogenesis (mitogenesis). HIV infection also induces mitochondrial damage (Ferri et al., 2000) and long-term ART has been linked to mitochondrial toxicity and impairment of genes responsible for mitogenesis (Barve et al., 2010). Nucleoside reverse-transcriptase inhibitors (NRTIs) in particular have been shown to significantly impair mitochondrial homeostasis (Lewis, 2003) although newer agents, like emtricitabine (FTC) (Venhoff et al., 2007), have less mitochondrial toxicity. Despite known mitochondrial dysfunction with AUD, HIV and ART, few studies have elucidated the interactions between alcohol and ART. Recently, our group demonstrated that CBA impairs expression of genes associated with mitochondrial biogenesis, mitophagy and protection from ROS at end-stage disease in non-ART-administered, SIV-infected macaques (Duplanty et al., 2017). We believe that mitochondrial dysfunction contributes to accelerated HIV disease progression including decreased whole body insulin sensitivity (Ford et al., 2016), decreased myogenic potential (Simon et al., 2017a) and chronic sensory peripheral neuropathy (Bennett et al., 2014).
Altered growth factor signaling
Both alcohol and HIV can alter growth factor expression and signaling, critical mechanisms regulating protein turnover and proliferation in many cell types, including skeletal muscle (Steiner et al., 2015). In PLWH, particularly those with lipodystrophy, significant alterations in the growth hormone (GH)–insulin-like growth factor 1 (IGF-1) axis have been reported (Grinspoon et al., 1998). However, the synergism between AUD and HIV is not well described. Both alcohol and HIV can suppress neurotrophic factors critical for neuronal growth and survival (Bachis et al., 2012; Boyadjieva and Sarkar, 2013; Fields et al., 2014). Brain-derived neurotropic factor (BDNF) is a key growth factor with mechanistic and therapeutic implications in AUD or HIV infection (Davis, 2008) and its expression is lower in the cortex, hippocampus and striatum of PLWH with HAD compared to that of uninfected controls (Bachis et al., 2012). Neuronal apoptosis resulting from HIV infection in experimental models is associated with reduced BDNF expression (Nosheny et al., 2004). Moreover, HIV protein gp120-mediated decreases in neurogenesis can be rescued by BDNF overexpression (Avraham et al., 2014), supporting a role for BDNF in HIV-associated cognitive dysfunction. Similarly, alcoholics have lower plasma BDNF levels than non-alcoholics (Joe et al., 2007) and preclinical models show reduced BDNF expression and phosphorylated TrkB (Raivio et al., 2012) following alcohol administration. Our exploratory microarray analysis shows that CBA administration in SIV-infected macaques upregulates hippocampal genes involved in immune function and dysregulates expression of genes involved in neurogenesis (Maxi et al., 2016), which we believe may result from impaired BDNF signaling cascade. Exploring the potential of targeting BDNF expression in PLWH with cognitive impairment, either pharmacologically or through interventions like aerobic exercise, are promising areas for further research.
Epigenetic alterations
Epigenetic alterations include histone acetylation or deacetylation, DNA methylation, and gene regulation by non-coding RNAs (ncRNAs) and these modifications are achieved by histone acetyl transferases or histone deacetylases (HATs, HDACs), and DNA methyltransferases (DNMTs). Alcohol-mediated gene expression changes are regulated by epigenetic mechanisms in several tissues including liver, brain and immune system (Shukla and Lim, 2013). Products of alcohol metabolism such as acetaldehyde, acetate, acetyl-CoA, ROS, as well as non-oxidative products, such as phosphatidylethanol (PEth) and fatty acid ethyl ester (FAEE) (Molina et al., 2014b), can induce tissue-specific epigenetic changes including histone acetylation (H3AcK9) (Kim and Shukla, 2006) and alterations in non-coding microRNAs (miRNAs).
Epigenetic mechanisms can also impact HIV disease at multiple levels. Histone deacetylation and methylation of the HIV promotor region in cells of gut-associated lymphoid tissue (GALT) and the central nervous system (CNS) (Friedman et al., 2011) are proposed mechanisms of HIV gene silencing (du Chéné et al., 2007), thus facilitating viral persistence. Interestingly, DNA methylation patterns in PLWH mimic those of chronologically older uninfected individuals (Gross et al., 2016; Nelson et al., 2017). miRNA modulation of viral protein expression favoring latency has also been reported (Nair et al., 2004). How alcohol-induced epigenomic modulation of host gene expression contributes to viral persistence remains unexplored. However, CD8 T cells isolated from PLWH display downregulated immunomodulatory miRNA expression in viremic progressors compared to elite controllers and uninfected controls (Egana-Gorrono et al., 2016). Whether alcohol regulates HIV replication or latency via epigenetic mechanisms warrants further investigation and is being pursued by our group.
Circulating miRNAs may serve as biomarkers of disease. Alterations in brain (Yelamanchili et al., 2010) and plasma miR (Witwer et al., 2011) profiles in SIV-infected macaques appear to modulate disease progression. Recent studies from our group identified highly sensitive and specific microRNA signatures distinguishing PLWH with cognitive impairment from those without impairment (Wyczechowska et al., 2017). Altered miR expression profiles in PLWH may impact gene regulatory networks involved in HIV-associated neurocognitive dysfunction (Noorbakhsh et al., 2010; Tatro et al., 2010). Support for epigenetic mechanisms contributing to alcohol-induced pathogenesis is provided by our findings of decreased myoblast differentiation potential associated with a decrease in muscle-specific miR-206 expression, which targets histone deacetylase 4, a known suppressor of myogenic genes (Simon et al., 2017a). Moreover, recent findings suggest decreased anti-HIV miRNAs (miR-27 and miR-181) expression in peripheral blood mononuclear cells (PBMCs) of SIV-infected macaques that could be further modulated by alcohol and require further investigation. Additional mechanisms contributing to persistent CD4+ viral expression in reservoirs should be explored to gain mechanistic insights into disease progression and potential therapeutic targets. Further identification of miRs as potential biomarkers of disease progression and indicators of response to interventions should be a focus of future studies.
SUMMARY
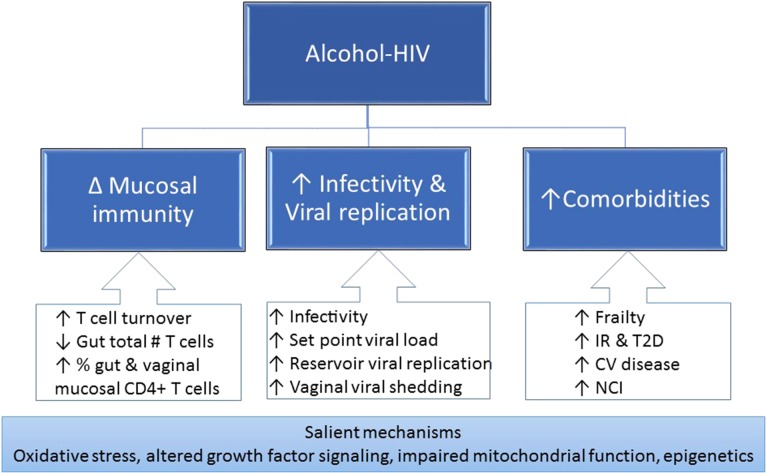
Both preclinical and clinical findings strongly suggest that heavy alcohol consumption can exacerbate HIV pathogenesis through alterations in mucosal immunity, increased viral replication, chronic immune activation and inflammation (Fig. 4). The state of chronic immune activation and inflammation increases the risk of geriatric conditions including frailty, osteoporosis, sarcopenia, insulin resistance and diabetes, CVD and neurocognitive impairment. Additional salient mechanisms include oxidative stress, altered growth factor signaling, impaired mitochondrial homeostasis and epigenetic modifications.
Fig. 4.
The alcohol-mediated mechanisms of exacerbated HIV pathogenesis include alterations in mucosal immunity and increased viral infectivity and replication, leading to chronic immune activation and inflammation promoting frailty-related comorbidities. IR, insulin resistance; T2D, type 2 diabetes; CV, cardiovascular; NCI, neurocognitive impairment.
PERSPECTIVES
Alcohol’s multifactorial effects on organ systems imposes an additional risk factor for development of comorbidities in PLWH. In an aging PLWH population, heavy alcohol consumption accelerates senescence and the onset of frailty, decreasing quality of life despite the prolonged survival from HIV disease with effective ART. Improved understanding of the pathophysiology of alcohol-associated comorbidities in PLWH will require increased transdisciplinary efforts. We propose that development of biomedical approaches to restore alcohol-induced impaired organ function should be coupled with evidence-based interventions to decrease alcohol use in this vulnerable population.
ACKNOWLEDGEMENTS
The authors acknowledge the investigators, trainees and research staff affiliated with the LSUHSC Comprehensive Alcohol-HIV/AIDS Research Center for their scientific contributions to the study of alcohol interactions with SIV/HIV. The authors are grateful for editorial assistance from Rebecca Gonzales.
FUNDING
Work by the authors is supported by the National Institutes of Health awards (P60 AA009803, UH2 AA026198, and UH2 AA026226).
CONFLICTS OF INTEREST STATEMENT
None declared.
REFERENCES
- Alonso K, Pontiggia P, Medenica R, et al. (1997) Cytokine patterns in adults with AIDS. Immunol Invest 26:341–50. [DOI] [PubMed] [Google Scholar]
- Amedee AM, Nichols WA, Robichaux S, et al. (2014) Chronic alcohol abuse and HIV disease progression: studies with the non-human primate model. Curr HIV Res 12:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Kelleher AD (2016) Immune activation and immune aging in HIV infection. Curr Opin HIV AIDS 11:242–9. [DOI] [PubMed] [Google Scholar]
- Aukrust P, Müller F, Svardal AM, et al. (2003) Disturbed glutathione metabolism and decreased antioxidant levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy—potential immunomodulatory effects of antioxidants. J Infect Dis 188:232–8. [DOI] [PubMed] [Google Scholar]
- Avraham HK, Jiang S, Fu Y, et al. (2014) The cannabinoid CB(2) receptor agonist AM1241 enhances neurogenesis in GFAP/gp120 transgenic mice displaying deficits in neurogenesis. Br J Pharmacol 171:468–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Avdoshina V, Zecca L, et al. (2012) Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J Neurosci 32:9477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, Bachman SE, Jew L, et al. (1996) Increased human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells induced by ethanol: potential immunopathogenic mechanisms. J Infect Dis 173:550–8. [DOI] [PubMed] [Google Scholar]
- Bagby GJ, Zhang P, Purcell JE, et al. (2006) Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin Exp Res 30:1781–90. [DOI] [PubMed] [Google Scholar]
- Barve S, Kapoor R, Moghe A, et al. (2010) Focus on the liver: alcohol use, highly active antiretroviral therapy, and liver disease in HIV-infected patients. Alcohol Res Health 33:229–36. [PMC free article] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, et al. (2010) Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses 26:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ, Doyle T, Salvemini D (2014) Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat Rev Neurol 10:326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyadjieva NI, Sarkar DK (2013) Cyclic adenosine monophosphate and brain-derived neurotrophic factor decreased oxidative stress and apoptosis in developing hypothalamic neuronal cells: role of microglia. Alcohol Clin Exp Res 37:1370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, Bryant KJ (2010) Influence of alcohol consumption on adherence to and toxicity of antiretroviral therapy and survival. Alcohol Res Health 33:280–7. [PMC free article] [PubMed] [Google Scholar]
- Brothers TD, Kirkland S, Guaraldi G, et al. (2014) Frailty in people aging with human immunodeficiency virus (HIV) infection. J Infect Dis 210:1170–9. [DOI] [PubMed] [Google Scholar]
- Burgess MJ, Zeuli JD, Kasten MJ (2015) Management of HIV/AIDS in older patients-drug/drug interactions and adherence to antiretroviral therapy. HIV AIDS (Auckl) 7:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnam MA, Bing EG, Morton SC, et al. (2001) Use of mental health and substance abuse treatment services among adults with HIV in the United States. Arch Gen Psychiatry 58:729–36. [DOI] [PubMed] [Google Scholar]
- Calvani R, Joseph AM, Adhihetty PJ, et al. (2013) Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem 394:393–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canizares S, Cherner M, Ellis RJ (2014) HIV and aging: effects on the central nervous system. Semin Neurol 34:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2016) HIV Surveillance Report Vol. 27, 2015. Published November 2016. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (2 February 2018, date last accessed).
- Cheng DM, Libman H, Bridden C, et al. (2009) Alcohol consumption and lipodystrophy in HIV-infected adults with alcohol problems. Alcohol 43:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JP, Ramirez CM, Wu JE, et al. (2013) Accelerated aging in HIV/AIDS: novel biomarkers of senescent human CD8+ T cells. PLoS One 8:e64702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston J. (2016) HIV infection and bone disease. J Intern Med 280:350–8. [DOI] [PubMed] [Google Scholar]
- Conigliaro J, Justice AC, Gordon AJ, et al. (2006) Role of alcohol in determining human immunodeficiency virus (HIV)-relevant outcomes: a conceptual model to guide the implementation of evidence-based interventions into practice. Med Care 44:S1–6. [DOI] [PubMed] [Google Scholar]
- Dandekar S, George MD, Baumler AJ (2010) Th17 cells, HIV and the gut mucosal barrier. Curr Opin HIV AIDS 5:173–8. [DOI] [PubMed] [Google Scholar]
- Davis MI. (2008) Ethanol-BDNF interactions: still more questions than answers. Pharmacol Ther 118:36–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit S, Sabin CA, Weber R, et al. (2008) Incidence and risk factors for new-onset diabetes in HIV-infected patients: the data collection on adverse events of anti-HIV drugs (D:A:D) study. Diabetes Care 31:1224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S, Landay A (2010) Early immune senescence in HIV disease. Curr HIV/AIDS Rep 7:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh DM, Volpe GE, Duffalo C, et al. (2015) Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis 211:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Chéné I, Basyuk E, Lin YL, et al. (2007) SUV39H1 and HP1gamma are responsible for chromatin-mediated HIV-1 transcriptional silencing and post-integration latency. EMBO J 26:424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplanty AA, Simon L, Molina PE (2017) Chronic binge alcohol-induced dysregulation of mitochondrial-related genes in skeletal muscle of simian immunodeficiency virus-infected rhesus macaques at end-stage disease. Alcohol Alcohol 52:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egana-Gorrono L, Guardo AC, Bargallo ME, et al. (2016) MicroRNA profile in CD8+ T-lymphocytes from HIV-infected individuals: relationship with antiviral immune response and disease progression. PLoS One 11:e0155245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Merkin SS, Cawthon R, et al. (2009) The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany NY) 1:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falutz J. (2011) HIV infection, body composition changes and related metabolic complications: contributing factors and evolving management strategies. Curr Opin Clin Nutr Metab Care 14:255–60. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sola J, Preedy VR, Lang CH, et al. (2007) Molecular and cellular events in alcohol-induced muscle disease. Alcohol Clin Exp Res 31:1953–62. [DOI] [PubMed] [Google Scholar]
- Ferri KF, Jacotot E, Blanco J, et al. (2000) Mitochondrial control of cell death induced by HIV-1-encoded proteins. Ann N Y Acad Sci 926:149–64. [DOI] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Langford TD, et al. (2014) Role of neurotrophic factor alterations in the neurodegenerative process in HIV associated neurocognitive disorders. J Neuroimmune Pharmacol 9:102–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford SM, Simon L, Vande Stouwe C, et al. (2016) Chronic binge alcohol administration impairs glucose-insulin dynamics and decreases adiponectin in asymptomatic simian immunodeficiency virus-infected macaques. Am J Physiol Regul Integr Comp Physiol 311:R888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg MS, Chang CC, Kuller LH, et al. (2013) HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg MS, Mcginnis KA, Kraemer K, et al. (2010) The association between alcohol consumption and prevalent cardiovascular diseases among HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr 53:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Cho WK, Chu CK, et al. (2011) Epigenetic silencing of HIV-1 by the histone H3 lysine 27 methyltransferase enhancer of Zeste 2. J Virol 85:9078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost RA, Fuhrer J, Steigbigel R, et al. (1996) Wasting in the acquired immune deficiency syndrome is associated with multiple defects in the serum insulin-like growth factor system. Clin Endocrinol 44:501–14. [DOI] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, et al. (2002) The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV cost and services utilization study. J Stud Alcohol 63:179–86. [DOI] [PubMed] [Google Scholar]
- Gongvatana A, Morgan EE, Iudicello JE, et al. (2014) A history of alcohol dependence augments HIV-associated neurocognitive deficits in persons aged 60 and older. J Neurovirol 20:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori A, Tincati C, Rizzardini G, et al. (2008) Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. J Clin Microbiol 46:757–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon S, Corcoran C, Stanley T, et al. (1998) Effects of androgen administration on the growth hormone-insulin-like growth factor I axis in men with acquired immunodeficiency syndrome wasting. J Clin Endocrinol Metab 83:4251–6. [DOI] [PubMed] [Google Scholar]
- Gross AM, Jaeger PA, Kreisberg JF, et al. (2016) Methylome-wide analysis of chronic HIV infection reveals five-year increase in biological age and epigenetic targeting of HLA. Mol Cell 62:157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JA, Cheng DM, Emenyonu NI, et al. (2018) Alcohol use and HIV disease progression in an antiretroviral naive cohort. J Acquir Immune Defic Syndr. 10.1097/QAI.0000000000001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JA, Samet JH (2010) Alcohol and HIV disease progression: weighing the evidence. Curr HIV/AIDS Rep 7:226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins KL, Brown TT, Margolick JB, et al. (2017) Geriatric syndromes: new frontiers in HIV and sarcopenia. AIDS 31:S137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, et al. (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, et al. (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek JB, Cahill A, Pastorino JG (2002) Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology 122:2049–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsue PY, Scherzer R, Hunt PW, et al. (2012) Carotid intima-media thickness progression in HIV-infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. J Am Heart Assoc 1:jah3–e000422. 10.1161/JAHA.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel N, Gougerot-Pocidalo MA (1997) Oxidative stress in human immunodeficiency virus infection. Cell Mol Life Sci 53:864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe KH, Kim YK, Kim TS, et al. (2007) Decreased plasma brain-derived neurotrophic factor levels in patients with alcohol dependence. Alcohol Clin Exp Res 31:1833–8. [DOI] [PubMed] [Google Scholar]
- Katz PS, Siggins RW, Porretta C, et al. (2015) Chronic alcohol increases CD8+ T-cell immunosenescence in simian immunodeficiency virus-infected rhesus macaques. Alcohol 49:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso NE, Sheps DS, Cook RL (2015) The association between alcohol use and cardiovascular disease among people living with HIV: a systematic review. Am J Drug Alcohol Abuse 41:479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A, Farhadi A, Forsyth CB, et al. (2009) Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol 50:538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Shukla SD (2006) Acute in vivo effect of ethanol (binge drinking) on histone H3 modifications in rat tissues. Alcohol Alcohol 41:126–32. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Westfall AO, Chamot E, et al. (2012) Multimorbidity patterns in HIV-infected patients: the role of obesity in chronic disease clustering. J Acquir Immune Defic Syndr 61:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter DP. (2003) Current concepts of metabolic abnormalities in HIV patients: focus on lipodystrophy. AIDS Read 13:S5–13. [PubMed] [Google Scholar]
- Kuller LH, Tracy R, Belloso W, et al. (2008) Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Jin M, Ande A, et al. (2012) Alcohol consumption effect on antiretroviral therapy and HIV-1 pathogenesis: role of cytochrome P450 isozymes. Expert Opin Drug Metab Toxicol 8:1363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Perez-Casanova AE, Tirado G, et al. (2005) Increased viral replication in simian immunodeficiency virus/simian-HIV-infected macaques with self-administering model of chronic alcohol consumption. J Acquir Immune Defic Syndr 39:386–90. [DOI] [PubMed] [Google Scholar]
- Lecapitaine NJ, Wang ZQ, Dufour JP, et al. (2011) Disrupted anabolic and catabolic processes may contribute to alcohol-accentuated SAIDS-associated wasting. J Infect Dis 204:1246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Matamoros S, Cani PD, et al. (2014) Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A 111:E4485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre F, O’leary B, Moran M, et al. (1995) Alcohol consumption among HIV-infected patients. J Gen Intern Med 10:458–60. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Quach A, Moore DJ, et al. (2016) Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. J Neurovirol 22:366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis W. (2003) Defective mitochondrial DNA replication and NRTIs: pathophysiological implications in AIDS cardiomyopathy. Am J Physiol Heart Circ Physiol 284:H1–9. [DOI] [PubMed] [Google Scholar]
- Loganantharaj N, Nichols WA, Bagby GJ, et al. (2014) The effects of chronic binge alcohol on the genital microenvironment of simian immunodeficiency virus-infected female rhesus macaques. AIDS Res Hum Retroviruses 30:783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombo B, Alkhalil I, Golden MP, et al. (2015) Prevalence of metabolic syndrome in patients with HIV in the era of highly active antiretroviral therapy. Conn Med 79:277–81. [PubMed] [Google Scholar]
- Luo Z, Liu Y, Liu Y, et al. (2017) Cellular and molecular mechanisms of alcohol-induced osteopenia. Cell Mol Life Sci 74:4443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel DB, Boisseau N, Benhamou CL, et al. (2012) Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int 23:1–16. [DOI] [PubMed] [Google Scholar]
- Maxi JK, Dean M, Zabaleta J, et al. (2016) Chronic binge alcohol administration dysregulates hippocampal genes involved in immunity and neurogenesis in simian immunodeficiency virus-infected macaques. Biomolecules 6:E43. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyaard L, Otto SA, Jonker RR, et al. (1992) Programmed death of T cells in HIV-1 infection. Science 257:217–9. [DOI] [PubMed] [Google Scholar]
- Midde NM, Sinha N, Lukka PB, et al. (2017) Alterations in cellular pharmacokinetics and pharmacodynamics of elvitegravir in response to ethanol exposure in HIV-1 infected monocytic (U1) cells. PLoS One 12:e0172628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE. (2008) Alcohol—intoxicating roadblocks and bottlenecks in hepatic protein and lipid metabolism. Am J Physiol Endocrinol Metab 295:E1–2. [DOI] [PubMed] [Google Scholar]
- Molina PE, Amedee AM, Veazey R, et al. (2014. a) Chronic binge alcohol consumption does not diminish effectiveness of continuous antiretroviral suppression of viral load in simian immunodeficiency virus-infected macaques. Alcohol Clin Exp Res 38:2335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, Gardner JD, Souza-Smith FM, et al. (2014. b) Alcohol abuse: critical pathophysiological processes and contribution to disease burden. Physiology (Bethesda) 29:203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, Lang CH, Mcnurlan M, et al. (2008) Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol Clin Exp Res 32:138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, Mcnurlan M, Rathmacher J, et al. (2006) Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcohol Clin Exp Res 30:2065–78. [DOI] [PubMed] [Google Scholar]
- Nair MP, Mahajan S, Hewitt R, et al. (2004) Association of drug abuse with inhibition of HIV-1 immune responses: studies with long-term of HIV-1 non-progressors. J Neuroimmunol 147:21–5. [DOI] [PubMed] [Google Scholar]
- Nelson KN, Hui Q, Rimland D, et al. (2017) Identification of HIV infection-related DNA methylation sites and advanced epigenetic aging in HIV-positive, treatment-naive U.S. veterans. AIDS 31:571–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorbakhsh F, Ramachandran R, Barsby N, et al. (2010) MicroRNA profiling reveals new aspects of HIV neurodegeneration: caspase-6 regulates astrocyte survival. FASEB J 24:1799–12. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Acquas E, et al. (2004) Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci 20:2857–64. [DOI] [PubMed] [Google Scholar]
- Pavanello S, Hoxha M, Dioni L, et al. (2011) Shortened telomeres in individuals with abuse in alcohol consumption. Int J Cancer 129:983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott DA, Muzaale AD, Mehta SH, et al. (2013) Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One 8:e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonia B, Nelson S, Bagby GJ, et al. (2006) Intestinal lymphocyte subsets and turnover are affected by chronic alcohol consumption: implications for SIV/HIV infection. J Acquir Immune Defic Syndr 41:537–47. [DOI] [PubMed] [Google Scholar]
- Poretsky L, Can S, Zumoff B (1995) Testicular dysfunction in human immunodeficiency virus-infected men. Metabolism 44:946–53. [DOI] [PubMed] [Google Scholar]
- Raivio N, Tiraboschi E, Saarikoski ST, et al. (2012) Brain-derived neurotrophic factor expression after acute administration of ethanol. Eur J Pharmacol 687:9–13. [DOI] [PubMed] [Google Scholar]
- Rasouli N, Kern PA (2008) Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab 93:S64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimaniol AC, Zylberberg H, Zavala F, et al. (1996) Inflammatory cytokines and inhibitors in HIV infection: correlation between interleukin-1 receptor antagonist and weight loss. AIDS 10:1349–56. [DOI] [PubMed] [Google Scholar]
- Romagnani S, Del Prete G, Manetti R, et al. (1994) Role of TH1/TH2 cytokines in HIV infection. Immunol Rev 140:73–92. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Sullivan EV, Pfefferbaum A (2010) Focus on the brain: HIV infection and alcoholism: comorbidity effects on brain structure and function. Alcohol Res Health 33:247–57. [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Seaberg E, et al. (2016) Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 86:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Cheng DM, Libman H, et al. (2007) Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr 46:194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMSHA (Substance Abuse and Mental Health Services Administration) (2016) 2015 National Survey on Drug Use and Health Rockville, MD. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm#tab2-41b (2 February 2018, date last accessed).
- Samuelson DR, Shellito JE, Maffei VJ, et al. (2017) Alcohol-associated intestinal dysbiosis impairs pulmonary host defense against Klebsiella pneumoniae. PLoS Pathog 13:e1006426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, et al. (2016) HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol 12:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevastianova K, Sutinen J, Kannisto K, et al. (2008) Adipose tissue inflammation and liver fat in patients with highly active antiretroviral therapy-associated lipodystrophy. Am J Physiol Endocrinol Metab 295:E85–91. [DOI] [PubMed] [Google Scholar]
- Shukla SD, Lim RW (2013) Epigenetic effects of ethanol on the liver and gastrointestinal system. Alcohol Res 35:47–55. [PMC free article] [PubMed] [Google Scholar]
- Simon L, Ford SM, Song K, et al. (2017. a) Decreased myoblast differentiation in chronic binge alcohol-administered simian immunodeficiency virus-infected male macaques: role of decreased miR-206. Am J Physiol Regul Integr Comp Physiol 313:R240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Hollenbach AD, Zabaleta J, et al. (2015) Chronic binge alcohol administration dysregulates global regulatory gene networks associated with skeletal muscle wasting in simian immunodeficiency virus-infected macaques. BMC Genomics 16:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Jolley SE, Molina PE (2017. b) Alcoholic myopathy: pathophysiologic mechanisms and clinical implications. Alcohol Res 38:207–17. [PMC free article] [PubMed] [Google Scholar]
- Steiner JL, Gordon BS, Lang CH (2015) Moderate alcohol consumption does not impair overload-induced muscle hypertrophy and protein synthesis. Physiol Rep 3:e12333 10.14814/phy2.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JL, Lang CH (2017) Etiology of alcoholic cardiomyopathy: mitochondria, oxidative stress and apoptosis. Int J Biochem Cell Biol 89:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg TE, Strandberg AY, Saijonmaa O, et al. (2012) Association between alcohol consumption in healthy midlife and telomere length in older men. The Helsinki Businessmen Study. Eur J Epidemiol 27:815–22. [DOI] [PubMed] [Google Scholar]
- Tatro ET, Scott ER, Nguyen TB, et al. (2010) Evidence for alteration of gene regulatory networks through microRNAs of the HIV-infected brain: novel analysis of retrospective cases. PLoS One 5:e10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torriani FJ, Komarow L, Parker RA, et al. (2008) Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol 52:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, Lee H, Hadigan C, et al. (2007) Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Castellanos JF, Serrano-Villar S, Latorre A, et al. (2015) Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol 8:760–72. [DOI] [PubMed] [Google Scholar]
- Veazey RS, Amedee A, Wang X, et al. (2015) Chronic binge alcohol administration increases intestinal T-cell proliferation and turnover in rhesus macaques. Alcohol Clin Exp Res 39:1373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, Demaria M, Chalifoux LV, et al. (1998) Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427–31. [DOI] [PubMed] [Google Scholar]
- Venhoff N, Setzer B, Melkaoui K, et al. (2007) Mitochondrial toxicity of tenofovir, emtricitabine and abacavir alone and in combination with additional nucleoside reverse transcriptase inhibitors. Antivir Ther 12:1075–85. [PubMed] [Google Scholar]
- Ventura AS, Winter MR, Heeren TC, et al. (2017) Lifetime and recent alcohol use and bone mineral density in adults with HIV infection and substance dependence. Medicine (Baltimore) 96:e6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand H, Calmy A, Carey DL, et al. (2007) Metabolic syndrome, cardiovascular disease and type 2 diabetes mellitus after initiation of antiretroviral therapy in HIV infection. AIDS 21:2445–53. [DOI] [PubMed] [Google Scholar]
- Wandera B, Tumwesigye NM, Nankabirwa JI, et al. (2017) Hazardous alcohol consumption is not associated with CD4+ T-cell count decline among PLHIV in Kampala Uganda: a prospective cohort study. PLoS One 12:e0180015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B, Landay A, Presti RM (2016) Microbiome alterations in HIV infection a review. Cell Microbiol 18:645–51. [DOI] [PubMed] [Google Scholar]
- Witwer KW, Sarbanes SL, Liu J, et al. (2011) A plasma microRNA signature of acute lentiviral infection: biomarkers of central nervous system disease. AIDS 25:2057–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack JA, Goulet JL, Gibert C, et al. (2013) Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin Infect Dis 56:1498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, et al. (2009) Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev 19:152–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2014) Global Status Report on Alcohol and Health. Geneva: World Health Organization, 2014. [Google Scholar]
- Wyczechowska D, Lin HY, Laplante A, et al. (2017) A miRNA signature for cognitive deficits and alcohol use disorder in persons living with HIV/AIDS. Front Mol Neurosci 10:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelamanchili SV, Chaudhuri AD, Chen LN, et al. (2010) MicroRNA-21 dysregulates the expression of MEF2C in neurons in monkey and human SIV/HIV neurological disease. Cell Death Dis 1:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Jarvinen H, Nikkila EA (1985) Ethanol decreases glucose utilization in healthy man. J Clin Endocrinol Metab 61:941–5. [DOI] [PubMed] [Google Scholar]