Abstract
BACKGROUND
Thrombolysis is the standard of care for acute ischemic stroke patients presenting in the appropriate time window. Studies suggest that the risk of recurrent ischemia is lower if carotid revascularization is performed early after the index event. The safety of early carotid revascularization in this patient population is unclear.
OBJECTIVE
To evaluate the safety of carotid revascularization in patients who received thrombolysis for acute ischemic stroke.
METHODS
The Nationwide Inpatient Sample database was queried for patients admitted through the emergency room with a primary diagnosis of carotid stenosis and/or occlusion. Each patient was reviewed for administration of thrombolysis, carotid endarterectomy, (CEA) or carotid angioplasty and stenting (CAS). Primary endpoints were intracerebral hemorrhage (ICH), postprocedural stroke (PPS), poor outcome, and in-hospital mortality. Potential risk factors were examined using univariate and multivariate analyses.
RESULTS
A total of 310 257 patients were analyzed. Patients who received tissue plasminogen activator (tPA) and underwent either CEA or CAS had a significantly higher risk of developing an ICH or PPS than patients who underwent either CEA or CAS without tPA administration. The increased risk of ICH or PPS in tPA-treated patients who underwent carotid revascularization diminished with time, and became similar to patients who underwent carotid revascularization without tPA administration by 7 d after thrombolysis. Patients who received tPA and underwent CEA or CAS also had higher odds of poor outcome and in-hospital mortality.
CONCLUSION
Thrombolysis is a strong risk factor for ICH, PPS, poor outcome, and in-hospital mortality in patients with carotid stenosis/occlusion who undergo carotid revascularization. The increased risk of ICH or PPS due to tPA declines with time after thrombolysis. Delaying carotid revascularization in these patients may therefore be appropriate. This delay, however, will expose these patients to the risk of recurrent stroke. Future studies are needed to determine the relative risks of these 2 adverse events.
Keywords: Carotid stenosis, Intracerebral hemorrhage, Acute cerebral infarction, Thrombolysis, Carotid endarterectomy, Carotid angioplasty and stenting, Tissue plasminogen activator
ABBREVIATIONS
- CAS
carotid angioplasty and stenting
- CEA
carotid endarterectomy
- CI
confidence interval
- ICH
intracerebral hemorrhage
- IV-tPA
intravenous tissue plasminogen activator
- NIS
Nationwide Inpatient Sample
- OR
odds ratio
- PPS
postprocedural stroke
- TIA
transient ischemic attack
- tPA
tissue plasminogen activator
Thrombolysis with intravenous tissue plasminogen activator (IV-tPA) has become a mainstay of treatment for patients presenting with acute ischemic stroke. Its use is increasing due to a number of factors including extension of the therapeutic window from 3 to 4.5 h, the movement toward more regionally coordinated stroke care, and the development of primary stroke centers with expertise in the care of stroke patients including timely delivery of IV-tPA.1-3 As the evidence for aggressive stroke management has accumulated, authors have also reported neurological decline associated with delays between identification of surgical carotid artery disease and carotid revascularization.4,5 These data have been used to recommend early intervention on patients with indications for revascularization.
However, the safety of early carotid revascularization via carotid endarterectomy (CEA) or carotid angioplasty and stenting (CAS) in patients who have received tPA has not been established.
In our previous study, we reported an increased risk of postoperative symptomatic intracerebral hemorrhage (ICH) in patients undergoing CEA after antecedent IV-tPA treatment.6 We performed a retrospective analysis of our single-institution case series of patients undergoing CEA for symptomatic carotid artery stenosis and found that 18.2% (2 of 11) of those receiving antecedent IV-tPA suffered sICH following CEA vs 0.8% (1 of 131) of those not receiving IV-tPA treatment. Others have also examined this issue in small single-institution case series, reporting complication rates of CEA after antecedent IV-tPA as low as 0% and as high as 14.2%.7-12 CAS is an alternate carotid revascularization technique, especially for patients who are poor candidates to undergo CEA. Three case series have examined the safety of early CAS in patients who have received tPA, and did not find any increase in risk of post-CAS ICH.13-15
Given that all available data examining the issue of safety of CEA or CAS in patients recently treated with tPA stem from relatively small studies, we sought to examine this important issue in a wider study of patients with carotid artery disease. To do so, we employed the Nationwide Inpatient Sample (NIS)—the largest all-payer inpatient healthcare database in the United States.
METHODS
Data for the study was generated from the NIS database, years 2002 to 2009. The information contained within the NIS includes diagnoses and procedures (using ICD-9-CM codes), patient demographics, total charges, length of stay, discharge status, and hospital characteristics. It includes information on hospital stays from over 1000 hospitals, representing 20% of all United States community-based inpatient healthcare facilities.16 Data in the NIS are neither identifiable nor private, and therefore this analysis does not meet the federal definition of human subjects research. This study was therefore not subject to Institutional Board Review requirements.
Patient Population
The patient population consisted of hospital stays with a primary diagnosis of occlusion and/or stenosis of a carotid artery with or without mention of cerebral infarction (ICD-9-CM Diagnosis 433.10 or 433.11) and where the patient was admitted as an emergency. Patients were excluded if (1) their length of stay was less than 1 d with an associated discharge to home, (2) mortality was unknown, (3) discharge disposition was unknown, or (4) age was unknown. For each patient, using their ICD-9-CM Diagnosis and Procedure codes, it was determined as to whether they had undergone infusion of tPA (ICD-9-CM Procedure 99.10 or ICD-9-CM Diagnosis V45.88), CEA (ICD-9-CM Procedure 38.12 or 38.02), and/or CAS (ICD-9-CM Procedure 00.63). Based on this classification, a categorical variable was created partitioning patients into 6 unique treatment groups: (1) no intervention, (2) tPA only, (3) CEA only, (4) CEA and tPA, (5) CAS only, or (6) CAS and tPA. All events occurred during the same hospitalization.
Primary Endpoints
There were 4 primary end points of interest for the study: ICH, postprocedural stroke (hemorrhagic and/or ischemic; PPS), poor outcome, and in-hospital mortality. ICH was defined by the presence of ICD-9-CM Diagnosis 431. PPS was based on the ICD-9-CM Diagnosis of 997.02 (iatrogenic cerebrovascular infarction or hemorrhage). A dichotomous outcome variable, classifying patients as having a “good” or “poor” outcome, was defined as previously described.17 Good outcome was considered as a discharge to home or rehabilitation facility/hospital. Poor outcome was defined as in-hospital mortality, discharge to either a nursing facility, extended care facility, or hospice, placement of a tracheostomy (ICD-9-CM Procedure: 31.1, 31.2. 31.21, 31.29), and/or placement of a gastrostomy (ICD-9-CM Procedure: 43.1, 43.11, 43.19, 44.32, 44.38, 44.39). In-hospital mortality was provided by the NIS through the variable “Died.”
Statistical Analysis
For each treatment category, the proportion of patients suffering an ICH, poor outcome, and in-hospital mortality was calculated. Assessing the impact of treatment type on each of the primary outcomes was completed initially using a univariate Chi-square analysis. This was followed by a multivariate analysis using a stepwise backward elimination logistical regression model. In both the univariate and multivariate analyses, the odds ratio (OR), with associated 95% confidence intervals (CI), of having an ICH, poor outcome, or in-hospital death was calculated for each of the treatment groups using “No intervention” as the reference. We further calculated the OR of having an ICH, PPS, poor outcome, or in-hospital death making the following comparisons: (1) CEA or CAS with tPA vs CEA or CAS without tPA, (2) CEA and tPA vs CEA only, and (3) CAS and tPA vs CAS only. In addition to these comparisons we analyzed the influence that ICH and PPS had on patients suffering a poor outcome and in-hospital death, again using univariate Chi-square analysis and a multivariate logistical regression model. Lastly, to better understand the impact of the procedure's timing (CEA or CAS) relative to administration of tPA, we calculated the probabilities of suffering an ICH or PPS for days 0 to 15 for patients treated with and without tPA. This was accomplished by using coefficient estimates produced by the logistical regression model.
To control for hospital attributes that could potentially impact our measured outcomes, for each facility we calculated the number of strokes treated per year and the number of CEA and CAS procedures performed each year. These factors as well as hospital bed size, patient age, and presence of the AHRQ Comorbidity Measures (coagulopathy, liver disease, congestive heart failure, chronic lung disease, diabetes, diabetes with chronic complications, hypertension, and renal failure) were used in the multivariate model to control for variability in outcome. All statistical analyses were completed using SAS version 9.3 (SAS Institute Inc, Cary, North Carolina). Statistical significance was defined as P value < .001.
RESULTS
A total of 310 257 patients were identified based on our selection criteria. Intervention occurred in 136 974 (44%) of patients, the breakdown of which can be seen in Table 1 along with patient demographics. (NIS-reporting guidelines prohibit the publication of cell counts less than 10. Therefore, the proportion of patients with each of the AHRQ Comorbidity measures is reported as percentages only.) The mean age at presentation was 72 yr (standard deviation = 26 yr), and 46% were females. The most common AHRQ comorbidity was hypertension, occurring in 77% of patients, followed by diabetes at 26%, and thirdly by chronic lung disease at 18%.
TABLE 1.
Patient Demographics and Potential Risk Factors Among Various Treatment Groups
| Treatment group | |||||||
|---|---|---|---|---|---|---|---|
| Variable | All patients | No intervention | tPA only | CEA only | CEA and tPA | CAS only | CAS and tPA |
| n = 310 257 | n = 173 283 | n = 4427 | n = 120 738 | n = 551 | n = 10 801 | n = 456 | |
| (100%) | (56%) | (1.4%) | (39%) | (0.2%) | (3.5%) | (0.1%) | |
| Age: mean (SD) | 72 (26) | 72 (28) | 67 (31) | 72 (22) | 68 (26) | 72 (23) | 68 (27) |
| Female: n (%) | 141 728 (46) | 84 041 (49) | 1651 (37) | 51 260 (43) | 190 (35) | 4415 (41) | 170 (37) |
| Strokes treated per year: mean (SD) | 108 (195) | 92 (191) | 139 (220) | 125 (183) | 132 (235) | 160 (218) | 202 (290) |
| CEA per year: mean (SD) | 56 (111) | 46 (104) | 57 (108) | 71 (114) | 57 (115) | 62 (105) | 74 (152) |
| CAS per year: mean (SD) | 5 (23) | 4 (20) | 8 (28) | 5 (21) | 8 (31) | 23 (44) | 17 (40) |
| Hospital size: n (%) | |||||||
| Small | 27 916 (9) | 17 065 (10) | 198 (4) | 10 068 (8) | 52 (9) | 529 (5) | NR |
| Medium | 71 541 (23) | 41 519 (24) | 920 (21) | 27 784 (23) | 133 (24) | 1117 (10) | 69 (15) |
| Large | 210 154 (68) | 114 185 (66) | 3295 (75) | 82 797 (69) | 362 (66) | 9132 (85) | 384 (84) |
| AHRQ comorbidity measures: % | |||||||
| Coagulopathy | 2 | 2 | 3 | 1 | 0 | 2 | 4 |
| Liver disease | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Congestive heart failure | 10 | 12 | 10 | 8 | 8 | 11 | 10 |
| Chronic lung disease | 18 | 17 | 15 | 19 | 16 | 19 | 13 |
| Diabetes | 26 | 26 | 21 | 25 | 26 | 27 | 25 |
| Diabetes with chronic complications | 5 | 5 | 3 | 4 | 4 | 5 | 2 |
| Hypertension | 77 | 76 | 73 | 78 | 75 | 76 | 73 |
| Renal failure | 8 | 9 | 7 | 7 | 8 | 10 | 6 |
Outcome: ICH and PPS
A total of 1116 of 310 257 patients (0.4%) experienced an ICH (Table 2). In patients who received “No intervention,” the hemorrhage rate was 0.4%. Across treatment groups this proportion ranged from 0.1% in the “CEA” group to 5.4% in the “CEA and tPA” group. On univariate analysis, in patients who underwent intervention relative to the “No intervention group,” the odds of a patient having an ICH was significantly increased with OR (95% CI) of 11.6 (9.9-13.7), 4.5 (2.4-8.6), and 14.4 (9.5-21.8) in the “tPA only,” “CEA and tPA,” and “CAS and tPA” groups, respectively (Table 3). These data suggest that there is a significant interaction between interventions and the use of tPA. The multivariate logistical regression analysis again demonstrated a significant increase in the odds of a patient having an ICH in the “tPA only,” “CEA and tPA,” and “CAS and tPA” groups compared to “No intervention” (Table 3). The interactive effect between tPA and CAS/CEA was again seen (Table 3).
TABLE 2.
Incidence of Events for Treatment Groups
| Event | ||||
|---|---|---|---|---|
| Treatment | ICH | PPS | Poor outcome | Mortality |
| All patients (n = 310 257) | 1116 (0.4%) | NA | 47 835 (15.4%) | 7464 (2.4%) |
| No intervention (n = 173 283) | 676 (0.4%) | NA | 38 582 (22.3%) | 5835 (3.4%) |
| tPA only (n = 4427) | 191 (4.3%) | NA | 1727 (39%) | 591 (13.3%) |
| CEA only (n = 120 738) | 137 (0.1%) | 1369 (1.1%) | 6453 (5.3%) | 786 (0.7%) |
| CEA and tPA (n = 551) | 10 (1.7%) | 43 (7.9%) | 104 (18.9%) | 24 (4.4%) |
| CAS only (n = 10 801) | 78 (0.7%) | 143 (1.3%) | 813 (7.5%) | 158 (1.5%) |
| CAS and tPA (n = 456) | 24 (5.4%) | 23(5.1%) | 157 (34.3%) | 70 (15.4%) |
TABLE 3.
Comparisons of Treatments to No Intervention. Odds Ratio of Event with 95% Confidence Intervals
| Event | |||
|---|---|---|---|
| Treatment | ICH | Poor outcome | Mortality |
| Univariate Chi-square analysis | |||
| No intervention (n = 173 283; Reference) | 1.00 | 1.00 | 1.00 |
| tPA only (n = 4427) | 11.6 (9.9-13.7) | 2.2 (2.1-2.4) | 4.5 (4.1-4.9) |
| CEA only (n = 120 738) | 0.3 (0.2-0.3) | 0.2 (0.2-0.2) | 0.2 (0.2-0.2) |
| CEA and tPA (n = 551) | 4.5 (2.4-8.6) | NS | NS |
| CAS only (n = 10 801) | 1.9 (1.5-2.3) | 0.3 (0.3-0.3) | 0.4 (0.4-0.5) |
| CAS and tPA (n = 456) | 14.4 (9.5-21.8) | 1.8 (1.5-2.2) | 5.2 (4.0-6.7) |
| Multivariate logistical regression analysis | |||
| No intervention (n = 173 283; Reference) | 1.00 | 1.00 | 1.00 |
| tPA only (n = 4427) | 8.7 (7.3-10.3) | 2.5 (2.4-2.7) | 3.8 (3.4-4.2) |
| CEA only (n = 120 738) | 0.3 (0.2-0.4) | 0.2 (0.2-0.2) | 0.2 (0.2-0.2) |
| CEA and tPA (n = 551) | 4.0 (2.1-7.5) | NS | NS |
| CAS only (n = 10 801) | 1.6 (1.2-2.0) | 0.3 (0.3-0.3) | 0.3 (0.3-0.4) |
| CAS and tPA (n = 456) | 9.0 (5.9-13.8) | 1.9 (1.5-2.3) | 3.5 (2.6-4.6) |
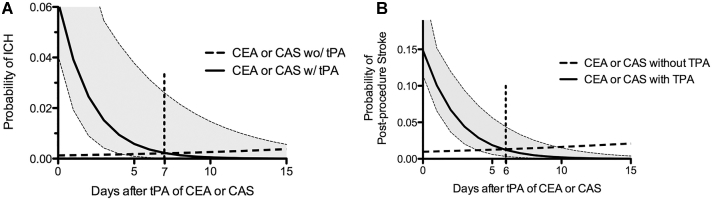
Results comparing the impact of tPA on the development of an ICH and PPS are shown in Table 4. Patients who underwent either CEA or CAS following tPA were at a significantly increased risk for developing both ICH (OR 20.8) and PPS (OR 7.2) compared to those who did not receive tPA. This effect was persistent in both univariate and multivariate analyses. Further analysis, comparing CEA and CAS groups separately, again demonstrated a strong effect of tPA on the development of an ICH or PPS (Table 4). The impact of treatment day relative to tPA infusion on probability of development of ICH and PPS is shown in Figure 1. Comparing interventions with and without tPA, the probabilities of an ICH equilibrate on day 7 (0.2%) and the probabilities of PPS equilibrate on day 6 (1%).
TABLE 4.
Comparisons of Treatments With and Without tPA. Odds Ratio of Event With 95% Confidence Intervals
| Event | ||||
|---|---|---|---|---|
| Treatment | ICH | PPS | Poor outcome | Mortality |
| Univariate analysis | ||||
| Treatment with tPA vs treatment without tPA | 20.8 (13.6-31.8) | 7.2 (5.6-9.5) | 6.1 (5.2-7.2) | 13.7 (10.7-17.7) |
| (CEA and tPA) vs CEA only | 15.5 (8.0-30.1) | 7.6 (5.5-10.4) | 4.2 (3.4-5.2) | 7.1 (4.7-10.8) |
| (CAS and tPA) vs CAS only | 7.8 (4.8-12.3) | 4.0 (2.6-6.3) | 6.4 (5.2-7.9) | 12.2 (9.0-16.5) |
| Multivariate analysis | ||||
| Treatment with tPA vs treatment without tPA | 11.1 (7.0-17.5) | 7.5 (5.7-9.9) | 5.6 (4.6-6.7) | 11.2 (8.1-15.4) |
| (CEA and tPA) vs CEA only | 12.7 (6.5-24.7) | 7.3 (5.3-10.0) | 3.7 (3.0-4.7) | 4.1 (2.6-6.4) |
| (CAS and tPA) vs CAS only | 5.8 (3.6-9.3) | 3.7 (2.3-5.8) | 6.0 (4.8-7.5) | 10.8 (7.8-15.1) |
FIGURE.
Probability of ICH A and PPS B after CEA or CAS with time from thrombolysis. ICH—intracerebral hemorrhage, CEA—carotid endarterectomy, CAS—carotid angioplasty and stenting, tPA—tissue plasminogen activator.
Covariates used in the multivariate model found to be significant predictors of ICH are listed in Table 5. From these, there was a small decrease in the chance of having a hemorrhage related to a hospital's volume of CEA and CAS procedures. Both showed a 7% decrease in the odds of having a hemorrhage for every 10 additional procedures performed.
TABLE 5.
Multivariate Logistic Regression Analysis of Various Potential Risk Factors for ICH, PPS, Poor Outcome, and Mortality. Odds Ratio with 95% Confidence Intervals
| Variable | ICH | PPS | Poor outcome | Mortality |
|---|---|---|---|---|
| Presence of ICH | NA | NA | 6.2 (5.7-6.8) | 16.3 (14.0-19.0) |
| Occurrence of PPS | NA | NA | ||
| Age units of 10 | NS | 1.06 (1.01-1.10) | 1.5 (1.51-1.53) | 1.2 (1.1-1.2) |
| Strokes treated per year units of 10 | 1.05 (1.04-1.06) | 1.04 (1.03-1.04) | 1.05 (1.04-1.05) | 1.02 (1.02-1.03) |
| CEA per year units of 10 | 0.93 (0.92-0.95) | 0.96 (0.95-0.98) | 0.92 (0.91-0.92) | 0.97 (0.96-0.98) |
| CAS per year units of 10 | 0.93 (0.89-0.97) | NS | 0.83 (0.81-0.84) | NS |
| Hospital size | ||||
| Medium compared to Small | NS | NS | 0.94 (0.90-0.98) | NS |
| Large compared to Small | NS | 0.83 (0.70-0.99) | 0.95 (0.92-0.99) | 1.2 (1.1-1.3) |
| AHRQ comorbidity measures | ||||
| Coagulopathy | 2.8 (2.4-3.4) | 3.0 (2.3-3.7) | 1.7 (1.6-1.9) | 2.9 (2.6-3.2) |
| Liver disease | NS | NS | 1.6 (1.4-1.8) | NS |
| Congestive heart failure | 1.1 (1.01-1.3) | 1.4 (1.2-1.6) | 1.8 (1.8-1.9) | 2.4 (2.3-2.6) |
| Chronic lung disease | 1.2 (1.1-1.3) | 1.3 (1.1-1.4) | 1.1 (1.1-1.2) | NS |
| Diabetes | NS | NS | 1.1 (1.1-1.1) | 0.90 (0.85-0.95) |
| Diabetes with chronic complications | NS | NS | 1.1 (1.1-1.2) | 0.68 (0.60-0.77) |
| Hypertension | 0.77 (0.71-0.84) | 0.76 (0.69-0.84) | 0.80 (0.78-0.82) | 0.65 (0.62-0.68) |
| Renal failure | 1.2 (1.01-1.3) | 1.2 (1.01-1.4) | 1.1 (1.1-1.2) | 1.2 (1.1-1.3) |
Outcome: Poor Outcome and In-Hospital Mortality
Poor outcome occurred in 47 835 (15.4%) of 310 257 patients and 7464 (2.4%) died during hospitalization. Table 1 shows the proportions of patients within the individual treatment groups with a poor outcome and in-hospital mortality. On univariate and multivariate analyses, only the “tPA” and “CAS and tPA” groups demonstrated increased risk of having a poor outcome or mortality relative to the “No intervention” patients (Tables 3 and 4). However, an interactive effect between CEA/CAS and tPA existed (Tables 3 and 4). Relative to “CEA,” the “CEA and tPA” group were 3.7 (3.0-4.7) times more likely to have a poor outcome and 4.1 (2.6-6.4) times more likely to suffer an in-hospital mortality. Relative to “CAS,” the “CAS and tPA” group were 6.0 (4.8-7.5) times more likely to have a poor outcome and 10.8 (7.8-15.1) times more likely to suffer an in-hospital mortality.
From the multivariate analysis, the presence of an ICH was a significant predictor of poor outcome and mortality with ORs of 6.2 (5.7-6.8) and 16.3 (14.0 and 19.0), respectively (Table 5). Other covariates found to be significant predictors of poor outcome and mortality are shown in Table 5.
DISCUSSION
Due to the increasing use of thrombolysis for acute ischemic stroke and the accumulating evidence supporting the role of early carotid revascularization for moderate or severe symptomatic carotid stenosis, the number of patients being evaluated for CEA or CAS soon after thrombolysis for ischemic stroke has risen. However, the evidence regarding the safety of early CEA or CAS in patients who have received thrombolysis is limited and conflicting. Regarding CEA, we recently reported that the risk of ICH after CEA is significantly increased in patients who have received antecedent tPA (18.2% vs 0.8%). 6 However, this study was limited by inclusion of patients from a single institution and a consequent small sample size. In the present study, we sought to more definitively establish the link between thrombolysis and post-CEA ICH by examining a larger patient population, the NIS. In addition, we evaluated the effect of thrombolysis on the risk of ICH after CAS—an alternative carotid revascularization procedure. We found that patients who received tPA had significantly higher odds of experiencing an ICH, PPS, poor outcome, and in-hospital mortality after CEA or CAS than patients who did not receive tPA. We also found that the increased risk of ICH or PPS after CEA or CAS in patients who received tPA decreased with time, and became similar to that of patients who did not receive tPA by 7 d from thrombolysis.
We identified 310 257 patients with carotid stenosis and/or occlusion who were admitted emergently, and these patients were divided into 6 treatment groups (see Table 1). The incidence of ICH in tPA-treated patients (who did not undergo any revascularization procedure; “tPA only” group) was 4.8%. This is comparable to the 6.4% incidence of ICH in the NINDS-tPA trial, as well as the 5.9% rate of ICH in a pooled analysis of 6 randomized controlled trials of intravenous tPA for stroke patients.18 On both univariate and multivariate analyses, patients in the “CEA and tPA” group had higher odds of experiencing an ICH when compared to patients in the “CEA only” group (see Table 4). The incidence of ICH was 1.7% in patients in the “CEA and tPA” group vs 0.1% in patients in the “CEA only” group. In previously reported single-institution case series including our own, the incidence of ICH in tPA-treated patients who subsequently underwent CEA ranges from 0% to 18.2%.6-12,14,19 In our prior publication, we pooled our institutional data with the then published case series7-12 to calculate an ICH rate of 5.4% for patients treated who received tPA prior to CEA. For patients who did not receive antecedent tPA, the incidence of post-CEA ICH was 0.8% in our previous retrospective study,6 and 0.2% to 0.6% in other large studies of patients who underwent CEA.20-23 When taken together, these data strongly suggest that the incidence of ICH after CEA in patients who have recently received tPA is substantially higher than that in patients who did not receive tPA. Similarly, in the present study, on both univariate and multivariate analyses, patients in the “CAS and tPA” group had higher odds of experiencing an ICH when compared to patients in the “CAS only” group. The incidence of ICH was 5.4% in patients in the “CAS and tPA” group vs 0.7% in patients in the “CAS only” group. This is in contrast to 3 prior studies that examined the incidence of ICH after “early” CAS in patients who received thrombolysis, where a post-CAS ICH rate of 0% was reported (0 out of 4 CAS patients, 0 out of 6 CAS patients, and 0 out of 6 CAS patients, respectively).13-15 This difference is likely due to the very low sample size of the 3 prior studies, which rendered them underpowered.
The second endpoint in our analysis was PPS. Patients in the “CEA and tPA” group had higher incidence of PPS than patients in the “CEA only” group (7.9 vs 1.1%, Table 2). On both univariate and multivariate analyses, patients in the “CEA and tPA” group had significantly higher odds of experiencing a PPS (multivariate OR = 7.3, Table 4) than patients in the “CEA only” group. Similarly, patients in the “CAS and tPA” group had significantly higher incidence (5.3% vs 1.3%, Table 2) of PPS, and odds of experiencing PPS (multivariate OR = 3.7, Table 4) when compared to patients in the “CAS only” group. This finding is consistent with a recent NIS analysis24 which demonstrated that patients who received tPA on the day of carotid revascularization (CEA or CAS) had significantly higher odds (OR = 11.2) of experiencing a PPS. However, our study clarifies that the higher odds of PPS is not confined to patients receiving tPA on the day of carotid revascularization, but likely extends to those undergoing carotid revascularization as late as 7 d after tPA administration.
The final 2 endpoints examined in our analysis were in-hospital mortality and poor outcome. We found that the in-hospital mortality rate of tPA-treated patients who did not undergo carotid revascularization (ie, “tPA only” group) was 13.4%. This rate is similar to past NIS analyses where stroke patients who received thrombolysis had mortality rates of 10.1% to 11.4%.25,26 Our analysis also found that patients in the “CEA and tPA” and “CAS and tPA” groups had significantly higher incidence (Table 2) and odds (Table 4) of in-hospital mortality and poor outcome than patients in the “CEA only” and “CAS only” groups, respectively. This association was also found on multivariate analysis in “CAS and tPA” patients. This association, however, did not reach significance on multivariate analysis in “CAS and CEA” patients (see Table 3), which might reflect the greater incidence of postprocedure ICH in CAS patients (OR = 9.0) vs CEA patients (OR = 4.0). Overall, our findings related to the impact of tPA treatment on in-hospital mortality and poor outcome in patients undergoing carotid revascularization are consistent with the recent publication by Villwock et al24 who found that patients who receive tPA on the day of carotid revascularization have a higher odds of in-hospital mortality (OR = 3.9).24 Our study, however, clarifies that the higher odds of in-hospital mortality is not confined to patients receiving tPA on the day of carotid revascularization, but likely extends to those undergoing carotid revascularization as late as 7 d after tPA administration. Our study also extends this observation beyond the outcome of mortality, as poor patient outcome overall was also linked to tPA treatment.
While current guidelines suggest that carotid revascularization be performed early (within 2 wk) in patients with stable deficits after a transient ischemic attack (TIA) or nondisabling stroke, the optimum interval between onset of symptoms and carotid revascularization is not well defined, particularly for patients who have received thrombolysis.27,28 In this study, we found that the increased odds of experiencing a PPS or ICH in tPA-treated patients who undergo carotid revascularization decreases with time after tPA administration and becomes similar to those who undergo carotid revascularization without antecedent tPA administration by days 6 and 7 after tPA administration, respectively (Figure 1). This finding is consistent with previously published retrospective studies (including ours) that have observed that all post-CEA ICH in tPA-treated patients occurred when CEA was performed within 3 d after thrombolysis,6,7,12 and the recent NIS study that demonstrated increased odds of PPS and in-hospital mortality when CEA or CAS was performed on the day of tPA administration.24 Although a few studies have suggested that early CEA or CAS is safe after thrombolysis,8-14 the majority of these studies had a median interval of 6 to 11 d from thrombolysis to carotid revascularization procedure.8,9,11,12,14 This suggests that the increased risk of ICH or PPS after CEA or CAS in tPA-treated patients was mitigated at the time of carotid revascularization in a large proportion of patients included in these studies owing to the more delayed nature of their CEA or CAS. In aggregate, the accumulated published case series6-12,14 and the present NIS analysis strongly suggest that caution should be exercised when considering very early CEA or CAS in acute ischemic stroke patients recently treated with tPA. At our institution, we have implemented the following protocol in an effort to mitigate this risk: (1) we perform a repeat computed tomography or magnetic resonance imaging study following IV-tPA to determine if asymptomatic hemorrhage has occurred; and (2) we delay CEA for at least a week in patients without radiographic evidence for post-tPA hemorrhage and delay CEA for at least 2 wk in patients with radiographic evidence for post-tPA hemorrhage. We have not experienced a post-CEA symptomatic ICH since instituting this protocol. Importantly, however, before this protocol could be extended into a treatment guideline, additional validation of our findings in a prospective data set will be required. A randomized trial of early vs delayed CEA or CAS could be considered.
The mechanism causing increased risk of ICH after CEA or CAS in patients undergoing antecedent tPA treatment remains unclear, though several possibilities exist. One potential but unlikely explanation is that a direct anticoagulant effect of tPA increases the risk of ICH after carotid revascularization procedure. IV-tPA, however, has a half-life of only 3 to 5 min;29 and IV-tPA-induced ICH in ischemic stroke patients typically occurs within 36 h of thrombolysis.30 In contrast, all reported tPA-treated patients who suffered ICH after CEA presented outside these time windows (for review, see Vellimana et al6). Thus, a direct anticoagulant effect as the underlying mechanism appears unlikely. A second more plausible explanation relates to a well-described phenomenon in which about 5% of ischemic stroke patients develop asymptomatic microhemorrhage(s) after IV-tPA treatment.31 This subset of tPA-treated patients would likely have substantial risk for postprocedural ICH given that patients undergoing CEA or CAS routinely receive intraprocedural heparin and often experience significant intra- and postprocedural alterations in cerebral hemodynamics. Screening with magnetic resonance imaging techniques to identify asymptomatic microhemorrhages may help risk-stratify tPA-treated patients when considering early CEA or CAS. A third potential explanation involves a well-documented downstream molecular effect of tPA—augmentation of matrix metalloproteinase-9 expression in cerebral vessels.32,33 In fact, enhancement in matrix metalloproteinase-9 activity has been implicated in post-tPA hemorrhagic transformation of ischemic infarcts.34 It is therefore possible that alterations in cerebral hemodynamics after CEA or CAS could deleteriously impact cerebral vessels made vulnerable by the enhanced expression of matrix metalloproteinase-9 caused by tPA treatment. A fourth potential explanation could be tPA-induced dysfibrinogenemia that leads to a prolonged increase in hemorrhagic risk beyond multiple half-lives of tPA. Which of these potential mechanisms are ultimately responsible, however, will require further investigation.
Limitations
Our study has several limitations. First, the NIS is a large administrative database, and therefore prone to coding errors that could have led to missing or incorrect diagnosis and/or procedure codes. However, given the large sample size of the database, these potential errors would constitute only a small percentage of the study population and would have been randomly distributed, and are therefore highly unlikely to influence our results. Second, this study is retrospective, and nonrandomized, and therefore lacks the strength of a prospective randomized controlled trial. However, this limitation is partly offset by its large sample size. Third, our study was designed to examine outcomes in patients with carotid stenosis who experienced a TIA or an ischemic stroke. It is therefore possible that the groups which included patients who received tPA (ie, tPA only, CEA only, CAS only) had only ischemic stroke patients while groups with patients who did not receive tPA (ie, no intervention, CEA only, CAS only) had both TIA and ischemic stroke patients. However, since we excluded patients who were discharged home within 1 d after admission, it is likely that a significant proportion of patients in all groups of the study had an ischemic stroke. Fourth, it is possible that the inferior outcomes observed in tPA-treated patients who undergo carotid revascularization compared to those who undergo carotid revascularization without antecedent tPA is secondary to the lower pretreatment baseline status in tPA-treated patients. However, since patients with poor clinical status due to large strokes would not undergo early carotid revascularization, the sicker tPA-treated patients were likely not included in the study population, and therefore the comparison groups were equivalent. Fifth, the NIS database does not provide adequate data to assess other potentially confounding variables such as surgeon experience, degree of carotid stenosis, use of antiplatelet agents/anticoagulants, and blood pressure control. However, given the overwhelming number of patients that the current study reports, the inclusion of other significant variables such as hospital size, stroke volume, CEA volume, and CAS volume, and the fact that this analysis corroborates our previous case series, we feel that these limitations have likely been mitigated.
CONCLUSION
Our analysis of the NIS suggests an increased risk of ICH, PPS, poor outcome, and in-hospital mortality in patients with carotid stenosis/occlusion who receive thrombolysis and undergo CEA or CAS during the same hospitalization. The increased risk of ICH and PPS in patients undergoing CEA or CAS after thrombolysis becomes comparable to those who undergo CEA or CAS without antecedent thrombolysis, by 7 d after thrombolysis. A prospective randomized controlled trial could be pursued to investigate the relative risks of ICH compared to recurrent stroke. Until that data are available, we feel that our NIS finding—when coupled with available data from single-institution case series—indicates recent tPA treatment should be considered when determining optimal timing for carotid revascularization in appropriately selected ischemic stroke patients.
Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
COMMENT
The use of intravenous tissue plasminogen activator (IV-tPA) to treat patients who have suffered acute, ischemic stroke has increased since the expansion of the therapeutic window from 3 to 4.5 hours from symptom onset.1,2 However, relatively little data exists to guide surgeons regarding the timing after which carotid revascularization, via either carotid endarterectomy (CEA) or carotid artery stenting (CAS), may be safely performed following administration of IV-tPA.3 The authors previously demonstrated that IV-tPA increases the risk of intracerebral hemorrhage following carotid endarterectomy in patients with symptomatic carotid artery stenosis.2 Yet, the aforementioned study, similar to the limited number of comparable reports,3,4 suffers from retrospective, single-center, and small cohort design. Therefore, in the current study, the authors queried the National Inpatient Sample database to investigate whether patients who were admitted through the emergency department for symptomatic carotid stenosis who underwent CEA or CAS after administration of IV-tPA were at higher risk of peri-procedural morbidity and mortality as compared to those patients who did not receive IV-tPA.
The authors studied outcomes in 6 patient groups: those who did not undergo intervention, those who received IV-TPA only, CEA only, CEA and IV-tPA, CAS only, and CAS and IV-tPA. Multivariate, logistic regression techniques were employed after identification of risk factors associated with morbidity and mortality on the basis of univariate analysis. Univariate analysis revealed increased risk of intraparenchymal hemorrhage (IPH) in the IV-tPA, CEA and IV-tPA, and CAS and IV-tPA groups when compared to the “no intervention” group. These results were confirmed on multivariate analysis. More importantly, when compared against CEA or CAS alone, either procedure completed after administration of IV-tPA resulted in increased post-procedural IPH and stroke risk, as well as poor functional outcome and in-hospital mortality. While several covariates, such as hospital stroke volume and various patient-specific comorbidities were also associated with poor outcomes, the effect of these factors was not as robust as the administration of IV-tPA. Lastly, the authors evaluated the risk of post-procedural IPH and stroke after IV-tPA as a function of time and showed that the probability of the former drops to 0.2% by post-stroke day 7, while the probability of the latter fell to 1% by day 6. On the basis of these data, the authors have instituted a protocol in their center whereby CEA or CAS is delayed for 7 days in setting of normal, post-tPA imaging and 14 days if post-tPA imaging reveals intracranial hemorrhage.
The strengths of this study are several. The sample size is large (n = 310 257), which mitigates many of the weaknesses of the retrospective design. In addition, the statistical methods are robust and the research question is both poignant and well answered. The limitations of the study are primarily those intrinsic to retrospective study protocols (ie, selection bias) and large, nationalized, patient databases (ie, errors in data entry). The authors suggest that a prospective, randomized trial to determine the optimal timing for carotid revascularization following administration of IV-tPA in the setting of patients admitted to the hospital with carotid stenosis is warranted. While interesting, a prospective study design may better answer a more nuanced question that was unable to be addressed by these data. The authors culled all patients admitted with a primary diagnosis of carotid stenosis/occlusion with or without cerebral infarction. The current status of the literature does not support a trial of CEA or CAS with IV-tPA versus without in the setting of a radiographically documented ischemic stroke on the basis of lack of equipoise. However, a thought-provoking question, primed for a prospective, randomized trial is whether hyperacute carotid revascularization (ie, <7 days after ictus) in the setting of TIA or non-disabling stroke with severe, ipsilateral carotid stenosis is safe and reduces recurrent ischemic events when compared to delayed intervention (ie, >7 days from ictus). Indeed, a recent cohort study of such patients treated with dual antiplatelet therapy and CEA with median delay of 8 days from symptom onset demonstrated favorable results.5
Brian M. Howard
C. Michael Cawley
Atlanta, Georgia
References
- 1. doi: 10.1161/STROKEAHA.109.192535. Del Zoppo GJ, Saver JL, Jauch EC, Adams HP Jr., American Heart Association Stroke C. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke; A Journal of Cerebral Circulation. 2009;40(8):2945-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. doi: 10.1227/NEU.0000000000000261. Vellimana AK, Yarbrough CK, Blackburn S, et al. Intravenous tissue-type plasminogen activator therapy is an independent risk factor for symptomatic intracerebral hemorrhage after carotid endarterectomy. Neurosurgery. 2014;74(3):254-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Naylor AR. Thrombolysis and expedited carotid revascularization. The Journal of Cardiovascular Surgery. 2015;56(2):159-164. [PubMed] [Google Scholar]
- 4. doi: 10.1016/j.ejvs.2014.08.012. Mandavia R, Qureshi MI, Dharmarajah B, Head K, Davies AH. Safety of carotid intervention following thrombolysis in acute ischaemic stroke. European Journal of Vascular and Endovascular Surgery: The Official Journal of the European Society for Vascular Surgery. 2014;48(5):505-512. [DOI] [PubMed] [Google Scholar]
- 5. doi: 10.1016/j.ejvs.2015.07.019. Batchelder A, Hunter J, Cairns V, Sandford R, Munshi A, Naylor AR. Dual antiplatelet therapy prior to expedited carotid surgery reduces recurrent events prior to surgery without significantly increasing peri-operative bleeding complications. European Journal of Vascular and Endovascular Surgery: The Official Journal of the European Society for Vascular Surgery. 2015;50(4):412-419. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1. Del Zoppo GJ, Saver JL, Jauch EC, Adams HP. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association Stroke. 2009;40(8):2945-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Derdeyn CP, Panagos PD. Stroke center certification: where are we in 2010? J NeuroInterv Surg. 2010;2(1):41-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dirks M, Niessen LW, Van Wijngaarden JDH et al. Promoting thrombolysis in acute ischemic stroke. Stroke. 2011;42(5):1325-1330. [DOI] [PubMed] [Google Scholar]
- 4. Fairhead JF, Mehta Z, Rothwell PM. Population-based study of delays in carotid imaging and surgery and the risk of recurrent stroke. Neurology. 2005;65(3):371-375. [DOI] [PubMed] [Google Scholar]
- 5. Johansson EP, Wester P. Delay from symptoms to carotid endarterectomy. J Intern Med. 2008;263(4):404-411. [DOI] [PubMed] [Google Scholar]
- 6. Vellimana AK, Yarbrough CK, Blackburn S et al. Intravenous tissue-type plasminogen activator therapy is an independent risk factor for symptomatic intracerebral hemorrhage after carotid endarterectomy. Neurosurgery. 2014;74(3):254-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartoli MA, Squarcioni C, Nicoli F et al. Early carotid endarterectomy after intravenous thrombolysis for acute ischaemic stroke. Eur J Vasc Endovasc Surg. 2009;37(5):512-518. [DOI] [PubMed] [Google Scholar]
- 8. Crozier JEM, Reid J, Welch GH, Muir KW, Stuart WP. Early carotid endarterectomy following thrombolysis in the hyperacute treatment of stroke. Br J Surg. 2011;98(2):235-238. [DOI] [PubMed] [Google Scholar]
- 9. Leseche G, Alsac J, Houbbalah R et al. Carotid endarterectomy in the acute phase of stroke-in-evolution is safe and effective in selected patients. J Vasc Surg. 2012;55(3):701-707. [DOI] [PubMed] [Google Scholar]
- 10. Mcpherson CM, Woo D, Cohen PL et al. Early carotid endarterectomy for critical carotid artery stenosis after thrombolysis therapy in acute ischemic stroke in the middle cerebral artery editorial comment. Stroke. 2001;32(9):2075-2080. [DOI] [PubMed] [Google Scholar]
- 11. Rathenborg LK, Jensen LP, Baekgaard N, Schroeder TV. Carotid endarterectomy after intravenous thrombolysis for acute cerebral ischaemic attack: is it safe? Eur J Vasc Endovasc Surg. 2013;45(6):573-577. [DOI] [PubMed] [Google Scholar]
- 12. Yong YP, Saunders J, Abisi S et al. Safety of carotid endarterectomy following thrombolysis for acute ischemic stroke. J Vasc Surg. 2013;58(6):1671-1677. [DOI] [PubMed] [Google Scholar]
- 13. Endo S, Kuwayama N, Hirashima Y, Akai T, Nishijima M, Takaku A. Results of urgent thrombolysis in patients with major stroke and atherothrombotic occlusion of the cervical internal carotid artery. AJNR Am J Neuroradiol. 1998;19(6):1169-1175. [PMC free article] [PubMed] [Google Scholar]
- 14. Koraen-Smith L, Troeng T, Bjorck M et al. Urgent carotid surgery and stenting may be safe after systemic thrombolysis for stroke. Stroke. 2014;45(3):776-780. [DOI] [PubMed] [Google Scholar]
- 15. Sallustio F, Koch G, Rocco A et al. Safety of early carotid artery stenting after systemic thrombolysis: a single center experience. Stroke Research and Treatment. 2012;2012:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. HCUP Databases. Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2002-2009. Available at: www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed January 8, 2014. [PubMed] [Google Scholar]
- 17. Washington CW, Derdeyn CP, Dacey RG, Dhar R, Zipfel GJ. Analysis of subarachnoid hemorrhage using the Nationwide Inpatient Sample: the NIS-SAH severity score and outcome measure. J Neurosurg. 2014;121(2):482-489. [DOI] [PubMed] [Google Scholar]
- 18. Hacke W, Donnan G, Fieschi C.. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet North Am Ed. 2004;363(9411):768-774. [DOI] [PubMed] [Google Scholar]
- 19. Azzini C, Gentile M, De Vito A et al. Very early carotid endarterectomy after intravenous thrombolysis. Eur J Vasc Endovasc Surg. 2016;51(4):482-486. [DOI] [PubMed] [Google Scholar]
- 20. Piepgras DG, Morgan MK, Sundt TM, Yanagihara T, Mussman LM. Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg. 1988;68(4):532-536. [DOI] [PubMed] [Google Scholar]
- 21. Solomon RA, Loftus CM, Quest DO, Correll JW. Incidence and etiology of intracerebral hemorrhage following carotid endarterectomy. J Neurosurg. 1986;64(1):29-34. [DOI] [PubMed] [Google Scholar]
- 22. Ferguson GG, Eliasziw M, Barr HWK et al. The North American symptomatic carotid endarterectomy trial: surgical results in 1415 patients. Stroke. 1999;30(9):1751-1758. [DOI] [PubMed] [Google Scholar]
- 23. Stromberg S, Gelin J, Osterberg T et al. Very urgent carotid endarterectomy confers increased procedural risk. Stroke. 2012;43(5):1331-1335. [DOI] [PubMed] [Google Scholar]
- 24. Villwock MR, Singla A, Padalino DJ, Deshaies EM. Stenting versus endarterectomy and the impact of ultra-early revascularization for emergent admissions of carotid artery stenosis. J Stroke Cerebrovasc Dis. 2014;23(9):2341-2349. [DOI] [PubMed] [Google Scholar]
- 25. Bateman BT, Schumacher HC, Boden-Albala B et al. Factors associated with in-hospital mortality after administration of thrombolysis in acute ischemic stroke patients: an analysis of the nationwide inpatient sample 1999 to 2002. Stroke. 2006;37(2):440-446. [DOI] [PubMed] [Google Scholar]
- 26. Dubinsky R, Lai S-M. Mortality of stroke patients treated with thrombolysis: analysis of nationwide inpatient sample. Neurology. 2006;66(11):1742-1744. [DOI] [PubMed] [Google Scholar]
- 27. Brott TG, Halperin JL, Abbara S.. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Stroke. 2011;42(8):e464-e540. [DOI] [PubMed] [Google Scholar]
- 28. Jauch EC, Saver JL, Adams HP et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. [DOI] [PubMed] [Google Scholar]
- 29. Chandler WL, Alessi MC, Aillaud MF, Henderson P, Vague P, Juhan-Vague I. Clearance of Tissue Plasminogen Activator (TPA) and TPA/Plasminogen Activator Inhibitor Type 1 (PAI-1) Complex : relationship to elevated TPA antigen in patients with high PAI-1 activity levels. Circulation. 1997;96(3):761-768. [DOI] [PubMed] [Google Scholar]
- 30. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28(11):2109-2118. [DOI] [PubMed] [Google Scholar]
- 31. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1587. [DOI] [PubMed] [Google Scholar]
- 32. Ning M, Furie KL, Koroshetz WJ et al. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006;66(10):1550-1555. [DOI] [PubMed] [Google Scholar]
- 33. Tsuji K, Aoki T, Tejima E et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36(9):1954-1959. [DOI] [PubMed] [Google Scholar]
- 34. Montaner J, Molina CA, Monasterio J et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107(4):598-603. [DOI] [PubMed] [Google Scholar]