Abstract
Background:
Given their unique capacity for antigen uptake, processing, and presentation, antigen-presenting cells (APCs) are critical for initiating and regulating innate and adaptive immune responses. We have previously shown the role of nicotinamide adenine dinucleotide (NAD+) in T-cell differentiation independently of the cytokine milieu, whereas the precise mechanisms remained unknown.
Objective:
The objective of this study is to further dissect the mechanism of actions of NAD+ and determine the effect of APCs on NAD+-mediated T-cell activation.
Methods:
Isolated dendritic cells and bone marrow-derived mast cells (MCs) were used to characterize the mechanisms of action of NAD+ on CD4+ T-cell fate in vitro. Furthermore, NAD+ -mediated CD4+ T-cell differentiation was investigated in vivo by using wild-type C57BL/6, MC−/−, MHC class II−/−, Wiskott-Aldrich syndrome protein (WASP)−/−, 5C.C7 recombination-activating gene 2 (Rag2)−/−, and CD11b-DTR transgenic mice. Finally, we tested the physiologic effect of NAD+ on the systemic immune response in the context of Listeria monocytogenes infection.
Results:
Our in vivo and in vitro findings indicate that after NAD+ administration, MCs exclusively promote CD4+ T-cell differentiation, both in the absence of antigen and independently of major APCs. Moreover, we found that MCs mediated CD4+ T-cell differentiation independently of MHC II and T-cell receptor signaling machinery. More importantly, although treatment with NAD+ resulted in decreased MHC II expression on CD11c+ cells, MC-mediated CD4+ T-cell differentiation rendered mice resistant to administration of lethal doses of L monocytogenes.
Conclusions:
Collectively, our study unravels a novel cellular and molecular pathway that regulates innate and adaptive immunity through MCs exclusively and underscores the therapeutic potential of NAD+ in the context of primary immunodeficiencies and antimicrobial resistance. (J Allergy Clin Immunol 2018;142:1894–908.)
Keywords: Nicotinamide adenine dinucleotide, mast cells, T cells, antigen presentation, MHC, T-cell receptor, CD4+ T-cell differentiation, dendritic cells, macrophages, Listeria monocytogenes, Cytokine
Antigen-presenting cells (APCs) play a central role in regulation of the innate and adaptive immune responses.1 APCs have the ability to capture, process, and present antigens through their MHC cell-surface molecules to the T-cell receptor (TCR) to mount an MHC-restricted immune response.2–6 APCs include a myriad of immune cells, such as B cells, neutrophils, macrophages, eosinophils, basophils, and dendritic cells (DCs). Among these populations, DCs are considered the major APCs bridging innate and adaptive immune responses.7,8 The mode of action of DCs is mediated through at least 3 signals: (1) TCR activation, (2) activation of costimulatory molecules, and (3) secretion of chemokines and proinflammatory cytokines.8 Indeed, depletion of CD11c+ DCs has been shown to alter cytotoxic T-lymphocyte responses to infection, as well as CD4+ T-cell activation and antibody production.9 In addition, DCs can also regulate innate and adaptive immune responses by recognizing pathogen-associated molecular patterns (PAMPs), such as microbial nucleic acids, lipoproteins, and carbohydrates, or damage-associated molecular patterns (DAMPs) released from injured cells through intracellular or surface-expressed pattern recognition receptors (PRRs).10–14
Although considered ‘‘atypical’’ APCs, Mast cells (MCs) have been described mainly for their role in allergic and autoimmune responses.15,16 It is well established that MCs are important effector cells in IgE-mediated allergic inflammation, and MCs are also recognized to influence innate and adaptive immune responses.8,16
MC-deficient mice have been shown to exhibit an altered CD4+ T-cell response to infection and in experimental autoimmune encephalomyelitis (EAE), a mouse model for human multiple sclerosis, suggesting that MCs play a role in mediating T-cell responses.17–20 Like DCs, MCs can directly present antigens to T cells in vitro, inducing an antigen-specific clonal expansion of T-cell populations, and are known to express costimulatory molecules and secrete a myriad of chemokines and proinflammatory cytokines.8,15,16,21 However, MCs express MHC class II intracellularly rather than at the cell surface.22 Moreover, MCs have been shown to promote T-cell activation in an antigen-independent manner, and no direct evidence has been provided thus far on the capacity of direct antigen presentation to the TCR.8,23 Thus the mechanisms by which MCs regulate T-cell responses remain unclear and are yet to be determined.
Recently, we have shown the role of nicotinamide adenine dinucleotide (NAD+), a cofactor found in all living cells and nutrients, in T-cell fate regulation.24,25 We have demonstrated that NAD+ was able to regulate CD4+ T-cell differentiation through a novel pathway that is independent of the cytokine environment and well-established transcription factors.25 More recently, we have reported the unique immunosuppressive properties mediated by NAD+ through systemic IL-10 cytokine production.24 Although we characterized the role of NAD+ in regulating T-cell fate, the precise mechanisms of action remain largely unknown.
Here we show that after NAD+ administration, MCs are able to induce exclusively CD4+ T-cell differentiation in vitro and in vivo in the absence of antigen and major APCs. Furthermore, we demonstrate that MC-driven CD4+ T-cell differentiation was independent of MHC class II or TCR activation. Furthermore, when assessing the functional effect of MC-mediated CD4+ T-cell differentiation, we observed that treatment with NAD+ resulted in profound alterations in innate and adaptive immunity and survival outcome after Listeria monocytogenes infection. Collectively, our study unravels a new cellular and molecular pathway regulating innate and adaptive immune responses that is mediated exclusively by MCs.
METHODS
Animals and diphtheria toxin treatment
Eight- to 10-week-old wild-type (WT) C57BL/6 (B6, H2b) mice were purchased from Charles River Laboratories (Wilmington, Mass). MC−/− (WBB6F1/J-KitW/KitW-v/J [KitW/KitW-v] and KitW-sh/HNihrJaeBsmJ [kitWsh/KitWsh]), MHC class II−/− (B6.129S-H2dlAb1-Ea), Wiskott-Aldrich syndrome protein (WASP)−/− (B6.129S6-Wastm1Sbs/J), and CD11b-DTR (B6.FVB-Tg[ITGAM-DTR/EGFP]34Lan/J) mice were purchased from Jackson Laboratory (Bar Harbor, Me). Recombination-activating gene 2 (Rag2)−/− γc−/− (B10; B6-.Rag2tmlFwa II2rgtm1Wjl), Rag2−/− , and 5C.C7 Rag2−/− mice (both on B10.A background) were purchased from Taconic Biosciences (Albany, NY). For CD11b+ cell depletion with diphtheria toxin treatment, CD11b-DTR transgenic mice weighing 25 to 30 g were injected with diphtheria toxin (25 ng/g body weight; Sigma-Aldrich, St Louis, Mo) 24 hours before and 72 hours after beginning NAD+ or PBS administration.
Isolation of mouse naive CD4+CD44−CD62L+ T cells and DCs
Single-cell leukocyte suspensions were obtained from spleens of 8- to 10-week-old C57BL/6 mice, and naive CD4+CD44−CD62L+ T cells were isolated by means of flow cytometry, as described previously.25 For isolation of CD11c+ DCs, single-cell leukocyte suspensions were obtained from spleens of 8- to 10-week-old C57BL/6 WT mice. CD11c+ DCs were then isolated with the EasySep Mouse CD11c Positive Selection Kit (STEMCELL Technologies, Vancouver, British Columbia, Canada), according to the manufacturer’s protocol, followed by cell sorting (CD11c+CD11b+ cells).
L monocytogenes infection
L monocytogenes bacteria (ATCC #35152) were cultured overnight at 37°C in Brain Heart Infusion (Teknova, Hollister, CA) with gentle agitation. Eight- to 10-week-old WT and MC−/− mice were infected intraperitoneally with 0.1 mL of a solution containing 1 × 107 colony-forming units (nonlethal dose) or 1 × 108 colony-forming units (lethal dose) of viable L monocytogenes cells in 0.01 mol/L PBS (pH 7.4). Weight loss and survival after infection were monitored. Before infection, mice were pretreated daily for a period of 5 days with NAD+ (40 mg administered intraperitoneally) or pretreated 5 days before infection and continuously treated daily after infection.
Cultivation of bone marrow-derived mast cells
Bone marrow-derived mast cells (BMMCs) from 8- to 10-week-old C57BL/6J WT mice were obtained by culturing bone marrow cells from femurs and tibias. In short, mice were killed by means of cervical dislocation, intact femurs and tibias were removed, and bone marrow cells were harvested by means of repeated flushing with sterile media. BM cells were cultured in WEHI-3-conditioned medium (containing IL-3) for 90 days, at which time the cells were greater than 95% c-KithighFcɛRIαhigh, as determined by using flow cytometric analysis with PE-Cy7 anti-mouse FcɛRIα (clone MAR-1; eBioscience, San Diego, Calif) and ef450 anti-mouse c-Kit/CD117 (clone 2B8; eBioscience, San Diego, Calif).
Human MC line LAD-2 culture
The human MC line LAD-2 was a generous gift from Dr A. Kirshenbaum (National Institutes of Health/National Institute of Allergy and Infectious Diseases). LAD-2 MCs were cultured in serum-free media (StemPro-34 SFM; Life Technologies, Grand Island, NY) supplemented with 2 mmol/L L-glutamine, 100 U/mL penicillin, 50 µg/mL streptomycin, and 100 ng/mL recombinant stem cell factor. LAD-2 cells were tested periodically for expression of Kit and FcɛRI by using flow cytometry.
Cell culture
Isolated naive CD4+ T cells or CD11c+ DCs (1 × 106 cells per well) were cultured in 48-well flat-bottom plates in 0.5 mL of complete RPMI 1640 medium supplemented with 10% FCS, 200 mmol/L L-glutamine, 100 U/mL penicillin/streptomycin, and 4.5 g/L glucose in the presence of 10 µg/mL plate-bound anti-mouse a-CD3 (17A2) and 2 µg/mL soluble a-CD28 (37.51). NAD+ (catalog no. N3014; Sigma-Aldrich) was diluted in PBS and added as indicated. LPS was added at a concentration of 1 µg/mL. All recombinant cytokines and antibodies were purchased from eBioscience. After the indicated day of culture, supernatants and cells were collected and analyzed by means of ELISA and flow cytometry, respectively.
Coculture of mouse naive CD4+ T cells and BMMCs in transwell systems
Noncontacting cocultured cells were prepared as follows: isolated naive CD4+CD44−CD62L+ T cells were plated on the bottom of the 24-well transwell cell culture system (Costar, Cambridge, Mass). BMMCs were cocultured at a ratio of 1:100 in the upper transwell compartment. Cells were stimulated with NAD+ (500 µmol/L) or PBS as a control. Naive CD4+ T cells were cultured in complete media only or in the presence of 10 µg/mL plate-bound anti-mouse α-CD3 (clone 17A2) and 2 µg/mL soluble α-CD28 (clone 37.51). For cell-cell contact experiments, BMMCs and naive CD4+CD44−CD62L+ T cells were cocultured (at a ratio of 1:100) in complete media with NAD+ (500 µmol/L) or PBS as a control. Naive CD4+ T cells were cultured in the presence of 10 µg/mL plate-bound anti-mouse a-CD3 (clone 17A2) and 2 µg/mL soluble a-CD28 (clone37.51) or complete media only. For CD80 blockade, experiments were performed in cell-cell contact conditions, as described above, with a-CD80 neutralizing antibody (clone 16–10A1; eBioscience). Cells were cultured for 96 hours, and CD4+IFN-γ+, CD4+IL-4+, and CD4+IL-17A+ T-cell frequencies were assessed by using flow cytometry.
Isolation and coculture of human naive CD4+ T cells
Human naive CD4+ T cells were isolated from PBMCs by means of density gradient centrifugation with the SepMate kit (STEMCELLTechnologies), followed by the EasySep Human Naïve CD4+ T Cell Isolation Kit (STEMCELL Technologies). Blood was obtained from healthy adult volunteers in accordance with guidelines of and approved by the Institutional Review Board of Beth Israel Deaconess Medical Center. Informed consent was obtained from each volunteer in accordance with the Declaration of Helsinki. Human naive CD4+CD25−CD45RA+CD45RO−CCR7+CD62L+ T cells were then sorted by means of flow cytometry. Naive CD4+ T cells were purified to greater than 98% by using cell sorting. Naive human CD4+ T cells were then plated on the bottom of the 24-well transwell cell culture system (Costar), and LAD-2 cells were cocultured at a ratio of 1:100 in the upper transwell compartment. Cells were stimulated with NAD+ (500 µmol/L) or PBS as a control. Naive CD4+ T cells were cultured in complete media only or in the presence of 10 µg/mL soluble anti-mouse α-CD3 (clone 17A2) and 5 µg/mL soluble α-CD28 (clone 37.51). For cell-cell contact experiments, LAD-2 cells and naive CD4+CD44−CD62L+ T cells were cocultured (at a ratio of 1:100) in complete media with NAD+ (500 µmol/L) or PBS as a control. Naive CD4+ T cells were cultured in the presence of 10 µg/mL soluble anti-mouse α-CD3 (clone 17A2) and 5 µg/mL soluble α-CD28 (clone 37.51) or complete media only. After 96 hours, CD4+ T-cell IFN-γ cytokine production was assessed by using flow cytometry.
DC and macrophage depletion
For DC depletion, WT, Rag2−/−γc−/−, and MC−/− mice were treated intravenously with 0.5 mg of liposomal clodronate (Encapsula NanoSciences, Nashville, Tenn) at days 8, 5, and 1 before NAD+ administration. This regimen ensured depletion of greater than 99% CD11c+ DCs, as described previously.26,27 As a control group, mice were injected with the same amount of isotype-matched rat IgG as control mice.
Flow cytometry
Fluorescence-labeled anti-mouse CD4 (clone GK1.5), CD11b (M1/70), CD11c (N418), CD41 (eBioMWReg30), CD61 (2C9. G3), IL-1β (NJTEN3), IL-4 (11B11), IL-6 (MP5–20F3), IL-10 (JES5–16E3), IL-12/IL-23p40 (C 17.8), IL-17 (eBio17B7), IFN-γ (XMG 1.2), latency-associated peptide (TW7–16B4), and TNF-α (MP6-XT22) were obtained from eBioscience. All antibodies were used at a concentration of 2 to 5 µg per l × l06 cells. To set the gates, flow cytometric dot plots were based on comparison with isotype controls, fluorescence minus one, and permeabilized and unpermeabilized unstained cells. Intracellular staining for IL-1β, IL-4, IL-6, IL-10, IL-12/IL-23p40, IL-17, IFN-γ, latency-associated peptide, and TNF-α was performed according to the manufacturer’s protocols. Cells were fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences, San Jose, Calif). Flow cytometry was performed on a BD FACSCanto II (BD Biosciences) by using standard procedures, and data were analyzed with FlowJo software (TreeStar, Ashland, Ore).
ELISA
Mouse IL-4, IL-17A, and IFN-γ levels were measured with commercial kits (eBioscience), as described previously.24,25
RNA extraction and quantitative PCR
BMMCs from C57BL/6 mice were cultured in the presence of NAD+ (500 µmol/L), LPS, or placebo (PBS). After 24 hours of culture, cells were collected, and mRNA was extracted by using the RNAqueous extraction kit, according to the manufacturer’s protocols (Applied Biosystems, Foster City, Calif). Briefly, cells were homogenized in lysis buffer (total volume of 0.5 mL) and passed through a column. After successive washes, RNA was eluted. For real-time PCR reactions, IL-1α (Mm00439620_ml), IL-β (Mm01336189_m1), IL-4 (Mm00445259_m1), IL-6 (Mm004446190_m1), IL-10 (Mm00439616_m1), IL-12α (Mm00434165_m1), IL-23 (Mm00 518984_m1), TGF-β1 (Mm01178820_m1), TNF-α (Mm00443260_g1), TLR2 (Mm00442346_m1), TLR4 (Mm00445273_m1), CD86 (Mm00 444543_m1), CD80 (Mm00711660_m1), inducible costimulator ligand (Mm00497237_m1), OX40 ligand (Mm00437214_m1), and IL-33 (Mm00 505403_m1) measurements were performed with TaqMan primers and probes from Applied Biosystems. The housekeeping gene glyceraldehye-3-phosphate dehydrogenase (Mm99999915_g1) was used as a control.
RNA sequencing analysis
BMMCs from C57BL/6 mice were cultured in the presence of NAD+ (500 µmol/L), LPS (10 µg/mL; Escherichia coli O127:B8), or placebo (PBS). After 16 hours of culture, cells were collected and RNA was extracted with the RNAqueous extraction kit, according to the manufacturer’s protocols (Applied Biosystems), as described above. cDNAwas obtained by using New England Biolabs kits (NEBNext Ultra Directional RNA Library Prep Kit for Illumina; New England Biolabs, Ipswich, Mass). Briefly, mRNAwas extracted with polyT magnetic beads, and then first- and second-strand syntheses were performed. Once double-strand cDNA was generated, DNA was cleaned up with magnetic beads and then went into library prep. Library preparation was performed by ligating on the P5 and P7 Illumina adaptors along with an index and amplifying the sequencing library by using PCR. The final library was cleaned up by using magnetic beads and made ready for sequencing. FastQ files were aligned against the Ensembl GRCm38.75 genome by using the STAR aligner (version 2.3.1z4) with default parameters.28 Alignment files (BAM format) were filtered to retain only primary alignments (Samtools view-F 0×0100) and inspected for duplication rate with Picard tools MarkDuplicates; for downstream analyses, duplicate reads were not removed because of the high-quality input RNA.29 Reads were quantified at the gene level by using featureCounts with annotated exon features in the Ensembl GRCm38.75 GTF file.30,31 The resulting count matrix was normalized and analyzed for differential expression with DESeq2 software. Ingenuity Pathways Analysis (Ingenuity Systems, Redwood City, Calif) applications were used to generate canonical pathways.
Statistical analysis
Data are presented as means ± SDs. Statistical analysis was done with the tailed Student t test (between 2 groups) and 1-way ANOVA (among multiple groups), where appropriate. Survival was compared by using the log-rank test. P values of less than .05 were considered statistically significant.
Study approval
Animal care and use were in accordance with the National Institutes of Health and Institutional Animal Care and Use Committee guidelines.
RESULTS
NAD+ requires an intermediary signal to promote CD4+ T-cell differentiation
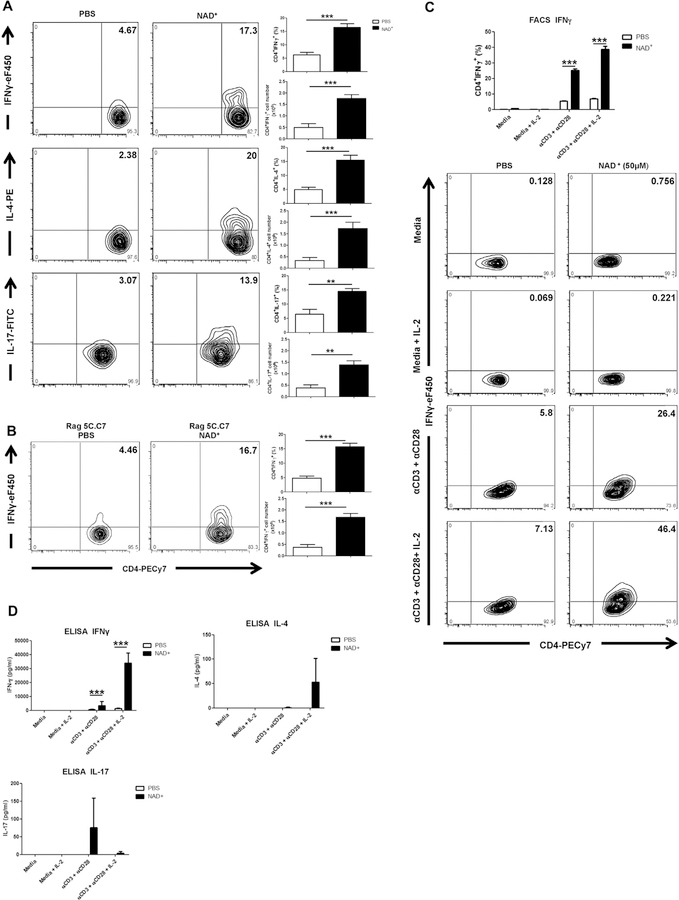
We have demonstrated previously that NAD+ regulated CD4+ T-cell differentiation independently of the cytokine milieu and well-established transcription factors.25 It remains unclear whether NAD+ promotes CD4+ T-cell differentiation by acting directly on CD4+ T cells or through an intermediate cell type. C57BL/6 WT naive mice were treated daily with intraperitoneal injection of NAD+ or a placebo solution (PBS) and CD4+ T-cell responses were assessed to characterize the direct effect of NAD+ on CD4+ T cells in the absence of antigen challenge. After 7 days, mice were killed, and systemic CD4+IFN-γ+, CD4+IL-4+, and CD4+IL-17A+ T-cell frequencies were evaluated by using flow cytometry. Data indicated that NAD+ administration was sufficient to promote a significant increase in frequencies of CD4+IFN-γ+, CD4+IL-4+, and CD4+IL-17+ T cells in vivo (Fig 1, A). Of note, NAD+ administration to Rag2−/− mice adoptively transferred with naive CD4+ T cells from Rag 5C.C7 transgenic mice promoted CD4+IFN-γ+ T-cell differentiation in the absence of moth cytochrome C peptide challenge (Fig 1, B).
FIG 1.
NAD+ induces T-cell activation in vivo and T-cell differentiation in vitro after TCR activation. A, C57BL/6 mice were treated daily with intraperitoneal injection of 40 mg of NAD+ or a placebo solution (PBS). After 7 days, mice were killed, and CD4+ T cells were isolated from spleens. Frequencies and total numbers of CD4+IFN-γ+, CD4+IL-4+, and CD4+IL-17A+ cells were analyzed by using flow cytometry. B, CD4+ T cells (3 × 106) from 5C.C7 RAG-2–deficient mice were injected into Rag2−/− (B10.A background) mice as adoptive transfer recipients. Mice were then treated with NAD+ or PBS for 7 days and subsequently killed to analyze frequencies and total numbers of CD4+IFN-γ+ cells by using flow cytometry. C and D, Sorted naive CD4+CD44−CD62L+ T cells were isolated from spleens of C57BL/6 mice and cultured in complete media alone with α-CD3/α-CD28 with or without IL-2 or in the presence of 50 µmol/L NAD+. After 96 hours, frequencies of CD4+IFN-γ+ cells and IFN-γ, IL-4, and IL-17A cytokine secretion were assessed by using flow cytometry (Fig 1, C) and ELISA (Fig 1, D), respectively. Statistics were as follows: n = 15 (Fig 1, A and B) or n = 10 (Fig 1, C and D). Data were derived from 3 independent sets of experiments. The Student t test and ANOVA were used accordingly to compare groups: **P < .01 and ***P < .001. Data are presented as means ± SDs. FACS, Fluorescein-activated cell sorting; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Thus we next assessed in vitro whether NAD+ directly promotes CD4+ T-cell differentiation and cytokine production. Splenic naive CD4+CD44−CD62L+ T cells from C57BL/6 WT mice were cultured in the presence or absence of α-CD3/α-CD28 with or without IL-2 and in the presence of NAD+ or PBS. After 96 hours of culture, CD4+ T cells were assessed for IFN-γ, IL-4, and IL-17 production by using flow cytometry and ELISA. Consistent with our previous reports, flow cytometry revealed that NAD+ promotes a robust increase of CD4+ IFN-γ+ T-cell frequencies after TCR activation, particularly in the presence of IL-2 (Fig 1, C). These findings were confirmed by means of ELISA, indicating increased IFN-γ, IL-4, and IL-17 production after CD3/CD28 activation (Fig 1, D). Of note, the highest CD4+IL-17+ T-cell frequencies and IL-17 secretion were observed after TCR activation and in the absence of IL-2, which was consistent with previous reports indicating that IL-2 inhibits TH17 development. In contrast, no changes in CD4+IFN-γ+ T-cell frequencies or IFN-γ, IL-4, and IL-17 cytokine production were observed with NAD+ treatment in the absence of TCR activation (Fig 1, C and D). Furthermore, increasing NAD+ concentrations in the absence of TCR activation did not result in a change in CD4+IFN-γ+, CD4+IL-4+, and CD4+IL-17+ T-cell frequencies (see Fig E1, A, in this article’s Online Repository at www.jacionline.org). Collectively, our in vivo data indicate that NAD+ promotes CD4+ T-cell differentiation in the absence of antigen challenge, whereas our in vitro data indicate that NAD+ requires TCR activation, suggesting that NAD+-mediated CD4+ T-cell differentiation observed in C57BL/6 WT naive mice requires an intermediary signal.
NAD+ regulates CD4+ T-cell fate in the absence of major APCs
We next investigated whether other immune cells that are known to activate CD4+ T cells, in particular APCs, are involved in NAD+-mediated CD4+ T-cell differentiation. It has been shown that ATP, a coenzyme, can promote TH17 cells through IL-6, IL-23, and TGF-β cytokine production by CD11c+ cells.32 Because NAD+ also acts as a coenzyme, we first assessed in vitro the effects of NAD+ on CDUc+ DCs. CD11b+CD11c+ DCs were isolated from spleens of C57BL/6 mice and cultured in the presence of increasing NAD+ concentrations or PBS. As a positive control, CD11b+CD11c+ DCs were cultured in the presence of 1 µg/mL LPS. After 16 hours, cells were collected and cytokine expression was quantified by using real-time PCR. Consistent with numerous reports, stimulation of CD11b+CD11c+ cells by LPS resulted in increased mRNA expression levels of IL-1α, IL-1β, IL-6, IL-23, and TNF-α but not IL-12 and a downregulation of TLR4.33,34 More importantly, in the presence of NAD+, CD11c+CD11b+ DCs exhibited increased mRNA expression levels of IL-1α, IL-1β, IL-6, IL-10, IL-12, IL-23, TGF-β1, TNF-α, TLR2, and TLR4 in a dose-dependent manner (see Fig E1, B), suggesting that NAD+ alters CD11b+CD11c+ DC activation.
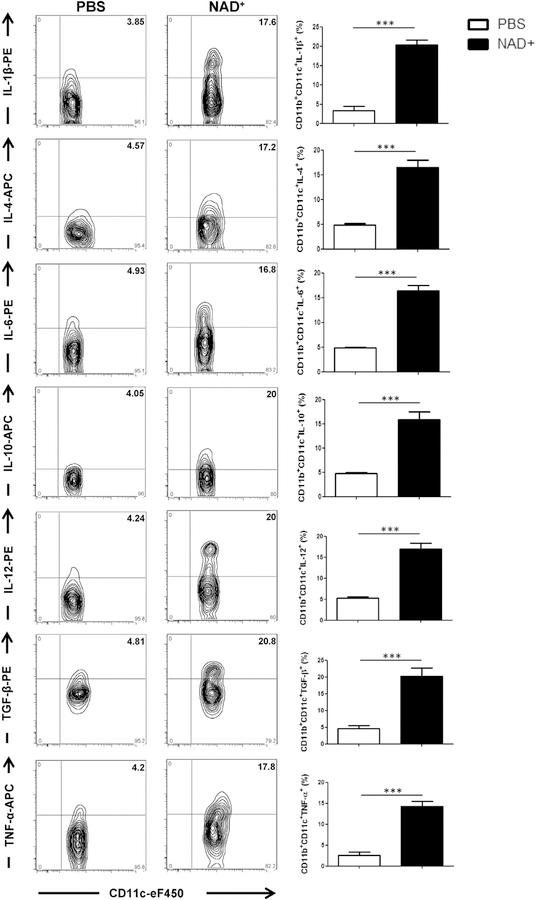
Thus we next assessed whether NAD+ administration can induce CD11c+CD11b+ DC activation in vivo as well. C57BL/ 6 WT mice were treated daily with 40 mg of NAD+ or PBS by means of intraperitoneal injection. Consistent with our in vitro findings, we found that NAD+ -treated WT mice showed increased cytokine production, including IL-1β, IL-4, IL-6, IL-10, IL-12, TGF-β1, and TNF-a, by CD11b+CD11c+ cells when compared with levels in PBS-treated WT mice (Fig 2). This is consistent with a previous study indicating that intracellular NAD+ levels regulate TNF-α cytokine production.35 Taken together, our in vitro and in vivo data indicated that NAD+ promotes CD11b+CD11c+ DC activation and cytokine production and might play a central role in NAD+-mediated CD4+ T-cell differentiation.
FIG 2.
NAD+ treatment induces increased cytokine production by CD11b+CD11c+ DCs in vivo. C57BL/6 mice were treated daily with intraperitoneal injection of 40 mg of NAD+ or a placebo solution (PBS). After 7 days, mice were killed, and CD11b+CD11c+ DCs were isolated from spleens. Frequencies of CD11c+IL-1β+, CD11c+IL-4+, CD11c+IL-6+, CD11c+IL-10+, CD11c+IL-12+, CD11c+TGF-β+, and CD11c+TNF-α+ cells were analyzed by using flow cytometry. Statistics were as follows: n = 15. Data were derived from 3 independent sets of experiments. Data are presented as means ± SDs. The Student t test was used to compare groups: ***P < .001. APC, Allophycocyanin; PE, phycoerythrin.
It is well established that DCs can promote CD4+ T-cell differentiation through cytokine and chemokine release. Moreover, like NAD+ , ATP, a cofactor in energy metabolism, has been shown to enhance IL-6, IL-23, and TGF-β cytokine production by CDUc+ cells and promote a TH17 response.32 Because our results indicated that NAD+ promotes cytokine expression by CD11b+CD11c+ DCs, we tested whether CD4+ T-cell differentiation resulted from NAD+-mediated DC activation. As reported by us and others,26,27 more than 99% of professional phagocytes, including both DCs and macrophages, were depleted in WT mice by means of injection of clodronate liposomes (see Fig E2, A, in this article’s Online Repository at www.jacionline.org). As shown in Fig E2, B, DC depletion did not abolish CD4+IFN-γ+, CD4+IL-4+, and CD4+IL-17+ differentiation. These data suggest that NAD+ regulates CD4+ T-cell differentiation independently of DCs and macrophages.
Although NAD+ -mediated CD4+ T-cell differentiation was not abolished after macrophage and DC depletion, we could not rule out compensation by other APCs, such as B cells. To characterize the role of B cells in NAD+ -mediated CD4+ T-cell differentiation, we used transgenic Rag2−/− γc−/− mice, which lack B, natural killer, and γδ T cells. In addition, Rag2−/− γc−/− mice were subjected to depletion of DCs and macrophages. After depletion (see Fig E2, A), Rag2−/−γc−/− mice received adoptive transfer of naive CD4+CD44−CD62L+ T cells and were subjected to treatment with NAD+ or placebo solution. The results indicated that NAD+ induced a significant increase in numbers of CD4+IFN-γ+, CD4+IL-4+, CD4+IL-17+, and CD4+IL-10+ cells when compared with the control group of mice treated with a placebo solution (see Fig E3, A, in this article’s Online Repository at www.jacionline.org). Moreover, treatment with diphtheria toxin of CD11b-DTR transgenic mice did not abolish CD4+IFN-γ+, CD4+IL-4+, and CD4+IL-17+ differentiation (see Fig E3, B). Taken together, our results indicate that NAD+ promotes CD4+ T-cell differentiation independently of B cells in addition to macrophages and DCs.
NAD+ administration regulates CD4+ T-cell fate through MCs exclusively
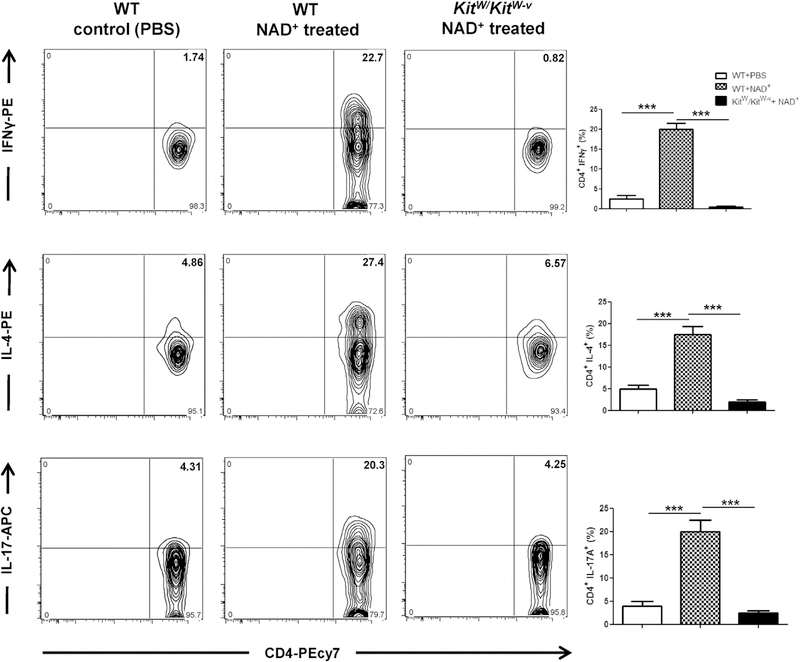
MCs have been mainly described for their role in allergic and autoimmune responses.8,15,16 Although MCs express costimulatory molecules and secrete a myriad of chemokines and proinflammatory cytokines and have been shown to influence T-cell polarization, the mechanisms by which MCs regulate T-cell response remain unclear. Indeed, previous studies have reported that MC-deficient mice display defective CD4+ but also CD8+ T-cell responses after Leishmania major infection36 and in the setting of EAE.37 Furthermore, we have shown previously that NAD+ protects against EAE.25 Thus we next investigated the role of MCs in NAD+ -mediated CD4+ T-cell differentiation. WT and MC-deficient mice (KitW/KitW-v) were treated daily with intraperitoneal injection of NAD+ . Treatment with NAD+ in MC-deficient mice was not able to promote CD4+IFN-γ+, CD4+IL-4+, and CD4+IL-17+ T cells, indicating that MCs were required for NAD+-mediated CD4+ T-cell differentiation (Fig 3).
FIG 3.
MCs play a central role in NAD+ -mediated CD4+ T-cell differentiation. C57BL/6 WT and WBB6F1/JKitw/Kitw-v (MC-deficient) mice were treated daily with intraperitoneal injection of 40 mg of NAD+ or a placebo solution (PBS), as indicated. After 7 days, mice were killed, and systemic levels of CD4+IFN-γ+, CD4+IL-4+, and CD4+IL-17A+ cells were assessed by using flow cytometry. Data were derived from 2 independent experiments (n = 5–10). Data represent means ± SDs. ANOVA was used to compare groups: ***P < .001. APC, Allophycocyanin; PE, phycoerythrin.
Therefore we next sought to dissect the role of MCs in NAD+-mediated T-cell differentiation in vitro. BMMCs were generated, as described in the Methods section. The average yield of MCs (FceRI+c-Kit+ double-positive cells) increased with time and reached greater than 95% on day 90, as shown by using flow cytometry (see Fig E3, C). BMMCs were cultured over 6 weeks to express homogenous levels of KIT and FceRI.8 BMMCs were then directly cocultured with naive CD4+CD44−CD62L+ T cells in the presence of NAD+ or PBS. Moreover, MCs and T cells were cocultured in separate compartments by using a transwell system to determine whether these cells require cell-cell contact. As an additional control, naive CD4+CD44−CD62L+ T cells were activated with α-CD3/α-CD28. The results indicated that in the presence of NAD+, MCs promoted CD4+IFN-γ+, CD4+IL-4+, and CD4+IL-17+ T-cell differentiation in the absence of TCR activation (media plus NAD+ ) and independently of cell-cell contact (see Fig E4 in this article’s Online Repository at www.jacionline.org). Moreover, when MCs and naive CD4+Cd44−CD62L+ T cells were cocultured in the presence of NAD+ and T cells were activated with α-CD3/α-CD28, frequencies of CD4+IFN-γ+ and CD4+IL-17+ T cells increased further (see Fig E4). These results were consistent with our initial findings indicating that cultured naive CD4+CD44−CD62L+ T cells in the presence of NAD+ and α-CD3/α-CD28 promoted CD4+IFN-γ+, CD4+IL-4+, and CD4+IL-17+ (Fig 1, C and D).
Like DCs, MCs express costimulatory molecules and secrete a myriad of cytokines that are known to regulate innate and adaptive immune responses. Thus to determine whether MCs mediate CD4+ T-cell differentiation through costimulatory molecules, cytokines, or both, MCs were treated with NAD+ in vitro, and mRNA levels of OX40 ligand, inducible costimulator ligand, CD80, CD86, TNF-α, IL-4, IL-6, and IL-33 were measured by using real-time PCR. NAD+ induced a modest increase in CD80 and IL-33 and a decrease in IL-4 mRNA expression levels by MCs (see Fig E5, A, in this article’s Online Repository at www.jacionline.org). It is well established that CD80 can either promote or inhibit activation of naive T cells by binding to CD28 or cytotoxic T lymphocyte-associated antigen 4, respectively. Therefore to assess the role of CD80 in NAD+-MC-mediated CD4+ T-cell differentiation, blockade of CD80 with a neutralizing antibody was performed in vitro by using our cell-cell contact coculture system. Our findings indicated that CD80 blockade did not reduce CD4+IFN-γ+ or CD4+IL-17+ T-cell frequencies when compared with an isotype control (see Fig E5, B and C). Of note, CD80 blockade resulted in increased CD4+IL-4+ T-cell frequencies (see Fig E5, D), suggesting that CD80 can play an inhibitory effect on MC-mediated IL-4 cytokine production. Collectively, our findings suggest that MC-mediated CD4+ T-cell differentiation does not require cell-cell contact or involvement of conventional costimulatory molecules, such as CD80.
MC-mediated CD4+ T-cell differentiation is conserved in the human MC line LAD-2
We next investigated whether this novel pathway is conserved in human subjects and whether human MCs could regulate human CD4+ T-cell differentiation as well. Naive CD4+ T cells were isolated from healthy donors and cocultured in direct contact or in our transwell system with LAD-2 cells, a well-established human MC line. Similar to murine BMMCs, cocultures were performed in the presence of NAD+ or PBS and with or without α-CD3/α-CD28. Flow cytometry indicated that in the presence of NAD+, human MCs promoted CD4+IFN-γ+ T-cell differentiation (see Fig E6, A, in this article’s Online Repository at www.jacionline.org). Consistent with our murine BMMC data, human MCs were able to promote THl polarization in the absence of cell-cell contact. Taken together, these results suggest that the MC-mediated CD4+ T-cell differentiation pathway through MCs is conserved in human subjects as well.
Unique gene expression profile by MCs after NAD+ activation
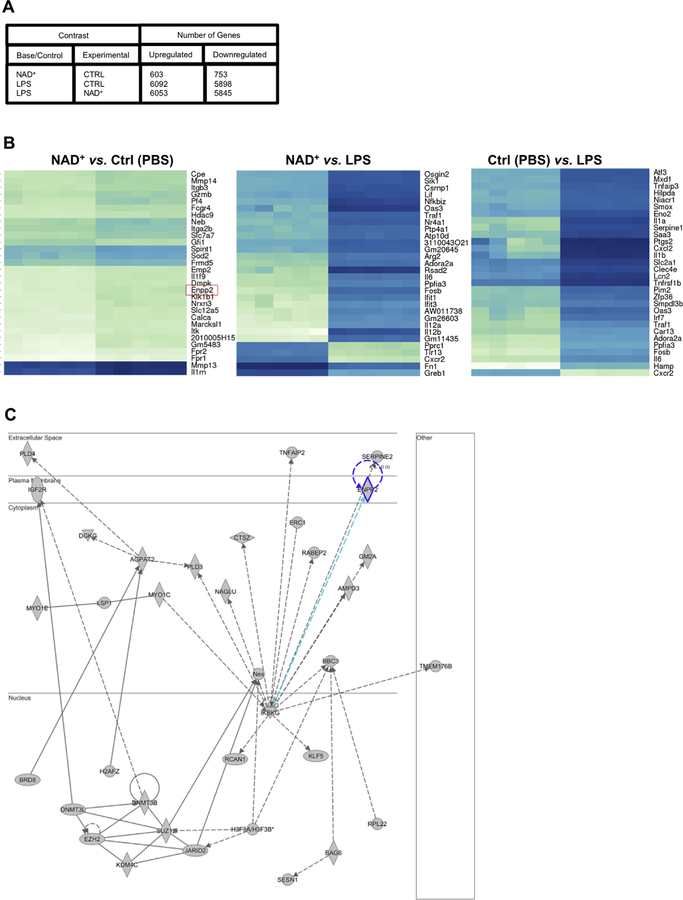
To unravel a potential signaling pathway involved in MC-mediated CD4+ T-cell differentiation, we next performed an RNA-sequencing analysis on BMMCs that were cultured for l6 hours in the presence of NAD+, LPS, or PBS. As shown in Fig 4, A, the results indicated that NAD+ significantly upregulated 603 genes and downregulated 753 genes (with a P value of less than .05) when compared with MCs treated with PBS. Moreover, when compared with LPS conditions, changes in the number of genes marked as differentially expressed were dramatic. When comparing LPS versus NAD+ conditions, 6053 genes were significantly upregulated, and 5845 were found to be downregulated, suggesting that NAD+ signaling machinery is distinct from LPS stimulation. As expected, dramatic changes in gene expression were observed when comparing PBS with LPS treatment (Fig 4, A).
FIG 4.
Unique MC gene expression profile after NAD+ activation. BMMCs from C57BL/6 mice were cultured in the presence of NAD+ (500 µmol/L), LPS (10 µg/mL; Escherichia coli O127:B8), or placebo (PBS). After 16 hours of culture, cells were collected and RNA was extracted for RNA sequencing analysis. A, Differential gene expression. B, Gene heat map expression profile. C, Ingenuity Pathway Analysis.
To elucidate potential genes involved in MC-mediated CD4+ T-cell differentiation, we investigated the role of the most upregulated genes in NAD+ versus PBS conditions and compared them with gene expression characteristics in presence of LPS (Fig 4, B). Among the first 10 genes upregulated in PBS versus NAD+ conditions, a robust upregulation of autotaxin (Enpp2), dystrophia myotonica protein kinase (DMPK), and FERM domain containing 5 (Frmd5) were specific to NAD+ conditions when compared with PBS or LPS treatment. Both DMPK38,39 and FRMD540 proteins have been implicated in stabilizing cell membranes and cytoskeletons. Autotaxin has been shown to play a role in T-cell activation and chemotaxis,41,42 and MCs have been shown to be able to produce autotaxin.43 Furthermore, Ingenuity Pathway Analysis predicted significant perturbations by NAD+ of other signaling molecules involved in MC activation, such as TNFAIP2 or IKBKG (NEMO; Fig 4, C). It is well established that the ectoenzyme autotaxin generates lysophosphatidic acid (LPA), a potent lipid mediator that acts on a series of specific G proteincoupled receptors through hydrolysis of lysophosphatidylcholine.41 LPA can be produced by a myriad of different cell types that include postmitotic neurons, adipocytes, MCs, and other lymphoid cells. Autotaxin has been recently described as regulating cytokine production, and autotaxin-LPA pathways have been shown to play a critical role in asthmatic patients.44 Both human and murine T cells express LPA receptors,45,46 and LPA has been found to inhibit TCR-mediated calcium mobilization.47
Collectively, our results indicate that MC activation by NAD+ triggers a unique gene expression profile that is distinct from LPS stimulation and suggests the existence of an alternative pathway that remains to be determined.
MCs induce T-cell differentiation in MHC class II−/− and WASP−/− mice after NAD+ administration
Our findings indicate that NAD+ promotes CD4+ T-cell differentiation through MCs and in the absence of antigen and TCR activation. Thus we next sought to determine the role of MHC class II and TCR molecules in NAD+ -MC-mediated CD4+ T-cell differentiation. MHC class II−/− mice were treated daily with NAD+ or a placebo solution (PBS). After 7 days, flow cytometry showed that NAD+ was able to promote a significant increase in CD4+IFN-γ+, CD4+IL-4+, and CD4+IL-17+ T cells in MHC class II-deficient mice (see Fig E6, B).
In addition, WASP−/− mice, which are known to have an altered TCR activation and cytokine production, in particular IFN-γ,48 were used to assess the role of TCR in NAD+-MC-mediated CD4+ T-cell differentiation. Thus WASP−/− mice were treated daily with intraperitoneal injection of NAD+ or PBS to assess whether NAD+-MC-mediated CD4+ T-cell differentiation could promote IFN-γ production also in the context of immunodeficiency. The results indicated that NAD+ was able to promote a robust increase in CD4+IFN-γ+, CD4+IL-4+, and CD4+IL-17+ T-cell frequencies in WASP−/− mice when compared with the control group (see Fig E6, C). Collectively, our results suggest that NAD+-MC-mediated CD4+ T-cell differentiation is functional, even in the absence of MHC class II or with altered TCR activation.
Daily NAD+ treatment protects against lethal doses of L monocytogenes through MCs exclusively
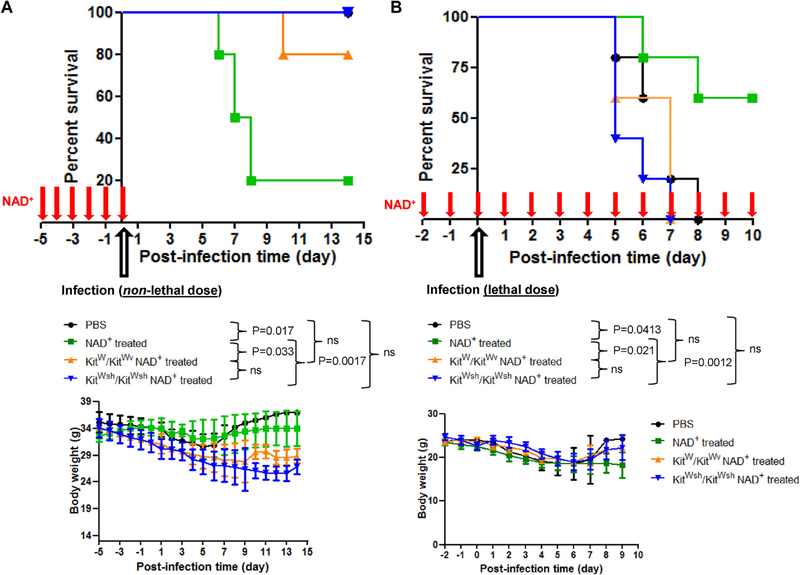
WT mice were pretreated for 5 consecutive days with NAD+ before infection with a nonlethal dose of L monocytogenes to test whether regulation of innate and adaptive immune cells through MCs after NAD+ administration had a physiologic effect. In addition, a control group of animals was pretreated with PBS before infection. Mice treated with PBS had only a modest loss of weight, did not exhibit signs of lethargy or gained weight 7 days after infection, and showed l00% survival (Fig 5, A) when infected with a nonlethal dose of L monocytogenes. In contrast, mice pretreated with NAD+ before infection (nonlethal dose) had a more pronounced loss of weight, exhibited lethargy, and were very susceptible to infection with an 80% mortality rate (Fig 5, A).
FIG 5.
MCs mediate protection against lethal doses of L monocytogenes during daily NAD+ administration. C57BL/6 WT, KitW/KitW-v, and kitWsh/KitWsh MC-deficient mice were pretreated for 5 consecutive days with daily intraperitoneal injections of NAD+ (40 mg) or placebo solution (PBS). A, Mice were then infected with an intraperitoneal nonlethal dose (1 × 107 colony-forming units) of viable L monocytogenes. B, C57BL/6 WT mice were pretreated for 5 consecutive days with NAD+ (40 mg administered intraperitoneally) or placebo solution (PBS) before infection. WT mice were then treated daily with NAD+ (40 mg administered intraperitoneally) or placebo solution (PBS) after a lethal dose (1 × 108 colony-forming units) of viable L monocytogenes. Weight loss and survival after infection were monitored. Data were derived from 3 independent experiments (n = 5–10 per group). Data represent means ± SDs. ns, Not significant. ANOVA and log-rank tests were used to compare groups, respectively.
Thus we tested whether MCs play a role in L monocytogenes infection survival outcome after NAD+ administration. Indeed, KitW/KitWv MC-deficient mice showed improved survival compared with NAD+-treated WT mice. In contrast to WT mice pretreated with NAD+ , KitW/KitWv transgenic mice had only a modest loss of weight and did not exhibit signs of lethargy. More importantly, KitW/KitWv mice pretreated with NAD+ showed an 80% survival rate (Fig 5, A), confirming our in vivo and in vitro findings and indicating that MCs play a central role in the capacity of NAD+ to regulate innate and adaptive immunity. Furthermore, we tested another set of MC-deficient mice (KitWsh/KitWsh) in the context of L monocytogenes infection. Similar to KitW/KitWv mice, KitWsh/KitWsh mice exhibited a 100% survival rate with no sign of lethargy. Collectively, NAD+-mediated CD4+ T-cell differentiation dramatically altered the immune responses through MCs and significantly affected physiologic functions of protection against bacterial infection.
Our results indicated that pretreatment with NAD+ promoted TH1, TH2, and TH17 CD4+ T-cell subsets. Because TH1 has been shown to play a protective role in L monocytogenes infection,35 we initially expected NAD+ pretreatment to protect mice after bacterial infection. However, we could not rule out that continuous NAD+ treatment might be required to sustain a robust THl response. Thus we assessed whether prolonged and continuous treatment with NAD+ could provide protection against L monocytogenes infection. As shown in Fig 5, B, WT mice continuously treated with NAD+ exhibited resistance (>60% survival rate) to even lethal doses of L monocytogenes when compared with the control group of mice that exhibited a 100% mortality rate. Consistent with our previous findings, NAD+-mediated protection against lethal doses of L monocytogenes was abolished in both KitW/KitWv and KitWsh/KitWsh MC−/− mice. We have shown previously that NAD+ regulates CD4+ T-cell differentiation through a pathway distinct of well-established transcription factors.25 In addition, we showed that NAD+ altered dramatically differentiated TH1 and TH2 CD4+ T cells by repressing their cytokine production and transcription factors.25 More importantly, second stimulation of differentiated TH1 and TH2 CD4+ T cells was profoundly altered when they were cultured in the presence of NAD+ after first stimulation.25
Thus we next investigated whether pretreatment with NAD+ altered the classical CD4+ T-cell differentiation pathway,36 specifically in CD4+IFN-γ+ T cells that have been shown to be essential for host resistance to L monocytogenes infection. We assessed whether NAD+ altered frequencies of CD4+ T-bet+ T cells. Mice pretreated with NAD+ had lower frequencies and total numbers of CD4+IFN-γ+T-bet+ cells when compared with mice treated with a placebo solution (see Fig E7, A, in this article’s Online Repository at www.jacionline.org). Although treatment with NAD+ resulted in downregulation of T-bet expression, CD4+ T cells from mice treated with NAD+ mounted robust IFN-γ production (see Fig E7, B), which was consistent with our previous studies.24,25 Although CD4+IFN-γ+ T-cell responses remained similar in NAD+- and placebo-treated mice, continuous treatment with NAD+ promoted host protection against a lethal dose of L monocytogenes. In addition, NAD+ affected the regulation and function of other important immune cells, such as MCs and, more importantly, DCs. Thus we investigated whether NAD+ promotes IFN-γ production that is not mediated by CD4+ T cells. Our results indicated that mice treated with NAD+ exhibited a significant increase in IFN-γ production by non-CD4+ T cells, suggesting that NAD+ can promote IFN-γ production by other non-CD4+ lymphocytes (see Fig E7, C). Taken together, our results indicate that continuous treatment with NAD+ promotes robust IFN-γ production and protects against lethal doses of L monocytogenes through MCs exclusively.
Pretreatment with NAD+ renders mice susceptible to sublethal doses of L monocytogenes by dampening the MCH class II-TCR pathway
Our data indicate that mice pretreated with NAD+ are susceptible to sublethal doses of L monocytogenes, whereas continuous treatment protects against lethal doses. Thus we next investigated how pretreatment with NAD+ rendered mice susceptible to a sublethal dose of bacteria.
Because antigen presentation through the MHC class II cell-surface molecule is crucial in CD4+ T-cell responses and our results indicated that NAD+ promotes CD4+ T-cell differentiation in the absence of MHC class II molecules, we investigated the effect of NAD+ pretreatment on MHC class II expression of CD11b+CD11c+ DCs after L monocytogenes infection. The results indicated that pretreatment with NAD+ resulted in a dramatic decrease in MHC class II expression when compared with the control group (see Fig E7, D), indicating that NAD+ dampens antigen presentation capacities of DCs after L monocytogenes infection. WT mice pretreated for 5 consecutive days with NAD+ before infection with a nonlethal dose of L monocytogenes had a more pronounced loss of weight, exhibited lethargy, and were very susceptible to infection with an 80% mortality rate (Fig 5, A). In contrast, WT mice continuously treated with NAD+ exhibited resistance (>60% survival rate) to even lethal doses of L monocytogenes when compared with the control group of mice that exhibited a 100% mortality rate (Fig 5, B).
Collectively, our results indicate that pretreatment with NAD+ will trigger the NAD+-MC pathway while dampening the APC-MHC class II-TCR signaling machinery, suggesting that discontinuous administration of NAD+ can subject the animals to an immunodeficient state that renders them susceptible to sublethal doses of L monocytogenes. Collectively, our results indicate that NAD+ profoundly alters, in the absence of antigen, innate and adaptive immune responses through MCs exclusively and independently from the classical DC/MHC class II antigen presentation machinery that results in significant physiologic changes (Fig 6).
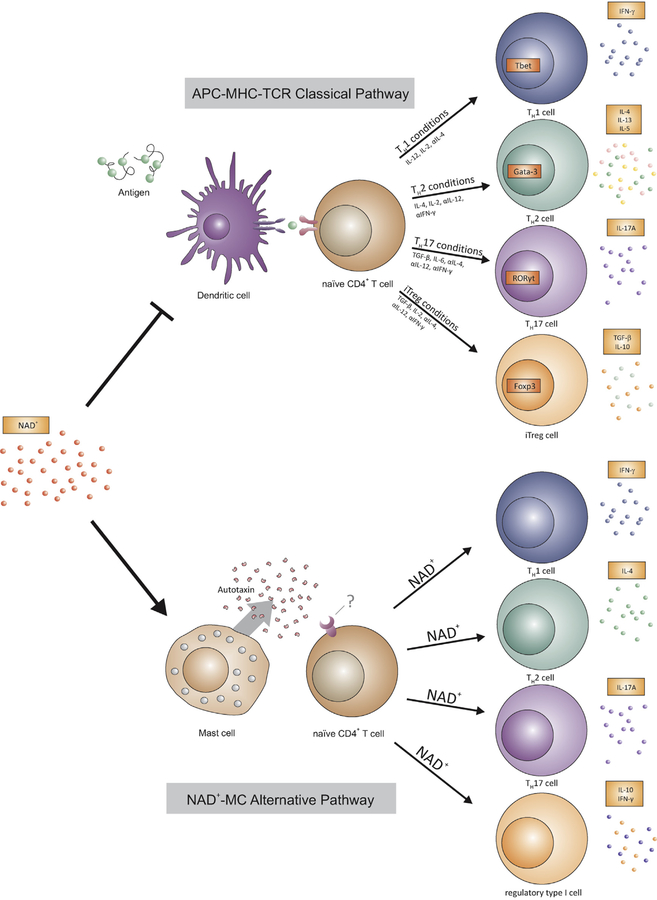
FIG 6.
NAD+ regulates T-cell differentiation through a novel MC-dependent signaling pathway. MC-mediated CD4+ T-cell differentiation after NAD+ administration does not require antigen presentation through major APCs and MHC class II.
DISCUSSION
Our previous studies have demonstrated a novel role for NAD+ in regulating T-cell fate in the context of antigen-specific responses.24,25 However, whether NAD+ mechanisms of action operate in an antigen-independent manner and whether NAD+ affects other immune cells remained unknown. APCs, in particular DCs, have been considered the most potent immune cells to prime naive CD4+ T cells. In addition to their capacity for antigen processing and presentation to the TCR through MHC class II cell-surface molecules, DCs can regulate CD4+ T-cell activation through costimulatory molecules and a myriad of secreted cytokines and chemokines.8 Moreover, it has been reported that recognition of conserved PAMPs, such as microbial nucleic acids, lipoproteins, and carbohydrates, or DAMPs, which are released from injured, through intracellular or distinct PRRs expressed on DCs contribute to pathogen-specific CD4+ T-cell responses12 Thus DCs and antigen presentation are regarded as fundamentally required and play a central role in CD4+ T-cell activation and differentiation and are considered the bridge between innate and adaptive immune responses.1,7,8,49 These observations are widely supported by studies based on transgenic animals with alteration in MHC class II, TCR, and DC depletion and confirmed in immunodeficient patients.50,51
Here we demonstrated that NAD+ , a natural cofactor, induces CD4+ differentiation in the absence of antigen presentation, MHC class II expression, and absence of TCR activation. Furthermore, we demonstrate that effects of NAD+ are not mediated by major APCs, including DCs, but through MCs exclusively. Moreover, our study indicated that major costimulatory molecules are not involved in NAD+-MC-mediated CD4+ T-cell differentiation, suggesting that MCs deliver T-cell stimulation through an alternative pathway. This is in line with previous studies indicating that MCs cannot prime naive CD4+ T cells, most likely because of an altered costimulatory molecule expression.8 Furthermore, previous studies have reported that MCs express MHC class II molecules; however, it has been found to be sequestered mainly in lysosomal intracellular compartments.22 This is consistent with our findings indicating that NAD+-MC-mediated CD4+ T-cell differentiation does not require antigen presentation or MHC class II molecules.
The cofactor ATP is well recognized for its role as a source of high energy and in cellular metabolism; however, when released from cells after cellular damage, ATP acts as a DAMP signal. DAMP signaling is mainly mediated through the PRRs that are expressed on APCs, in particular DCs and macrophages.52 Although the primary function of PRRs is to mediate innate immune responses to pathogen invasion and tumors, increasing evidence has shown their role in the development of autoimmune and chronic inflammation52 by promoting TH17 responses. ATP, for instance, has been shown to convert CD4+CD25+Foxp3+ regulatory T cells into TH17 cells and to promote colitis by enhancing the TH17 proinflammatory subset in the lamina propria through DCs.32,53 Similar to ATP, NAD+, which is also a cofactor, has been described for its role in energy metabolism and more recently in aging.54–56 In contrast to ATP, NAD+-mediated innate and adaptive immune regulation was intermediated by MCs and was independent of major APCs, including DCs and macrophages. Moreover, our previous studies have underscored the robust immunosuppressive properties of NAD+ by promoting a systemic increase in IL-10 cytokine levels through regulatory type l cells.24,25 We have also shown that NAD+ regulates CD4+ T-cell fate in the absence of major transcription factors. Indeed, NAD+ was able to promote a robust TH1 response in the absence of T-bet, a transcription factor considered indispensable for TH1 differentiation and IFN-g production.25 In contrast to proinflammatory responses induced by DAMPs, including ATP, NAD+ protects against autoimmune diseases and promotes allograft survival.24,25 Furthermore, our RNA-sequencing analysis indicated that treatment with NAD+ resulted in upregulation of a distinct set of genes when compared with LPS, a prototypical PAMP, suggesting that NAD+ triggered a different signaling pathway that remains yet to be determined in detail.
In line with our previous reports, NAD+ treatment downregulated T-bet expression but did not alter CD4+IFN-γ1 T-cell responses when compared with those in the control group of mice. Although CD4+IFN-γ+ T-cell responses remained similar in NAD+ and placebo-treated mice, continuous treatment with NAD+ conferred host protection against L monocytogenes. Previous studies have underscored the critical role of IFN-γ in host protection against L monocytogenes.9,57 Thus NAD+ might mediate host protection by promoting IFN-γ production by other immune cells, such as CD8+ cells or innate lymphoid cells. Although our study emphasizes the potent role of NAD+ on many immune cells, such as CD4+ T cells, MCs, and DCs, its effect on other important immune cells, such as B cells, CD8+ cells, and innate lymphoid cells, remain yet to be determined, and its role in other inflammatory conditions or infections, including viral infections, requires further investigation.
More importantly, our study unravels a novel cellular mechanism that regulates CD4+ T-cell differentiation, a process known to play a central role in many inflammatory conditions.58 NAD+-mediated immune changes in the absence of antigen had a robust physiologic effect after bacterial infection. Indeed, NAD+-pretreated mice were highly susceptible to even a nonlethal dose of bacterial infection that resulted in high mortality. In contrast, maintenance of NAD+ signaling machinery by continuous treatment with NAD+ conferred host protection after infection. More importantly, NAD+ treatment altered T-bet expression and MHC class II expression on CD11c+ DCs, suggesting that the NAD+ -MC-mediated CD4+ T-cell differentiation pathway might not only promote robust host protection against bacterial infection but also might be distinct from the classical DC-MHC class II-TCR pathway. Indeed, with clodronate administration, DTR transgenic and MC-deficient mice (KitW/KitW-v), and BMMCs, we demonstrated that NAD+-MC-mediated CD4+ T-cell differentiation is independent of the classical DC-MHC class II-TCR pathway. More importantly, our data indicated that in the presence of NAD+, MCs mediated differentiation of both murine and human CD4+ T cells. These findings suggest that NAD+ can pave the way for novel therapies in the context of microbial infections or primary immunodeficiencies that are characterized by altered DC-MHC class II-TCR signaling machinery.
In summary, our study demonstrates the emerging role of NAD+ as a new paradigm in innate and adaptive immune responses with its unique property as an MC-mediated immune regulator that is distinct from the classical APC-MHC class II-TCR pathway.
Supplementary Material
Key messages.
NAD+ alone regulates CD4+ T-cell fate in the absence of antigen.
NAD+ regulates CD4+ T-cell fate independently of major APCs and MHC-TCR signaling machinery.
Mast cells exclusively regulate CD4+ T-cell differentiation after NAD+ administration.
Acknowledgments
Supported by National Institutes of Health grants R01NS073635 and R01MH110438 (to A.V.), R01 HL096795 and U01 HL126497 (to I.G.), and R01AG039449 (to S.G.T.). H.R.C.B. was supported by the Swiss Society of Cardiac Surgery. M.A.d.l.F was supported by FIS-ISCIII (grant PI10/02 511) and Fundaci_on Ram_on Areces (CIVP16A1843).
Abbreviations used
- APC
Antigen-presenting cell
- BMMC
Bone marrow-derived mast cell
- DAMP
Damage-associated molecular pattern
- DC
Dendritic cell
- EAE
Experimental autoimmune encephalomyelitis
- LPA
Lysophosphatidic acid
- MC
Mast cell
- NAD+
Nicotinamide adenine dinucleotide
- PAMP
Pathogen-associated molecular pattern
- PRR
Pattern recognition receptor
- Rag2
Recombination-activating gene 2
- TCR
T-cell receptor
- WASP
Wiskott-Aldrich syndrome protein
- WT
Wild-type
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–52. [DOI] [PubMed] [Google Scholar]
- 2.Zinkernagel RM, Doherty PC. The discovery of MHC restriction. Immunol Today 1997;18:14–7. [DOI] [PubMed] [Google Scholar]
- 3.Haskins K, Kappler J, Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. Annu Rev Immunol 1984;2:51–66. [DOI] [PubMed] [Google Scholar]
- 4.Exley M, Terhorst C, Wileman T. Structure, assembly and intracellular transport of the T cell receptor for antigen. Semin Immunol 1991;3:283–97. [PubMed] [Google Scholar]
- 5.Zinkernagel RM, Doherty PC. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature 1974;251:547–8. [DOI] [PubMed] [Google Scholar]
- 6.Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature 1974;248:701–2. [DOI] [PubMed] [Google Scholar]
- 7.Lemos MP, Esquivel F, Scott P, Laufer TM. MHC class II expression restricted to CD8alpha1 and CD11b+ dendritic cells is sufficient for control of Leishmania major. J Exp Med 2004;199:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol 2014;14:719–30. [DOI] [PubMed] [Google Scholar]
- 9.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, et al. In vivo depletion of CDllcl dendritic cells abrogates priming of CD81 T cells by exogenous cell-associated antigens. Immunity 2002;17:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolerance Matzinger P., danger, and the extended family. Annu Rev Immunol 1994;12:991–1045. [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997; 388:394–7. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol 2015;16:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998;282:2085–8. [DOI] [PubMed] [Google Scholar]
- 14.Poltorak A, Smirnova I, He X, Liu MY, Van Huffel C, McNally O, et al. Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol Dis 1998; 24:340–55. [DOI] [PubMed] [Google Scholar]
- 15.Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol 2008;26:705–39. [DOI] [PubMed] [Google Scholar]
- 16.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol 2010;10:440–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urb M, Sheppard DC. The role of mast cells in the defence against pathogens. PLoS Pathog 2012;8:e1002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayed BA, Walker ME, Brown MA. Cutting edge: mast cells regulate disease severity in a relapsing-remitting model of multiple sclerosis. J Immunol 2011; 186:3294–8. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Nourbakhsh B, Safavi F, Li K, Xu H, Cullimore M, et al. Kit (W-sh) mice develop earlier and more severe experimental autoimmune encephalomyelitis due to absence of immune suppression. J Immunol 2011;187:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piconese S, Costanza M, Musio S, Tripodo C, Poliani PL, Gri G, et al. Exacerbated experimental autoimmune encephalomyelitis in mast-cell-deficient Kit W-sh/W-sh mice. Lab Invest 2011;91:627–41. [DOI] [PubMed] [Google Scholar]
- 21.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol 2005;6:135–42. [DOI] [PubMed] [Google Scholar]
- 22.Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell 1997;8: 2631–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tkaczyk C, Villa I, Peronet R, David B, Chouaib S, Mecheri S. In vitro and in vivo immunostimulatory potential of bone marrow-derived mast cells on Band T-lymphocyte activation. J Allergy Clin Immunol 2000;105:134–42. [DOI] [PubMed] [Google Scholar]
- 24.Elkhal A, Rodriguez Cetina Biefer H, Heinbokel T, Uehara H, Quante M, Seyda M, et al. NAD(+) regulates Treg cell fate and promotes allograft survival via a systemic IL-10 production that is CD4(+) CD25(+) Foxp3(+) T cells independent. Sci Rep 2016;6:22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tullius SG, Biefer HR, Li S, Trachtenberg AJ, Edtinger K, Quante M, et al. NAD(+) protects against EAE by regulating CD4(+) T-cell differentiation. Nat Commun 2014;5:5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberhuber R, Heinbokel T, Cetina Biefer HR, Boenisch O, Hock K, Bronson RT, et al. CD11c+ dendritic cells accelerate the rejection of older cardiac transplants via interleukin-17A. Circulation 2015;132:122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 2007;447:92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics 2009;25:2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol 2010;11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014;30: 923–30. [DOI] [PubMed] [Google Scholar]
- 32.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature 2008;455:808–12. [DOI] [PubMed] [Google Scholar]
- 33.Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol 2000; 164:3476–9. [DOI] [PubMed] [Google Scholar]
- 34.Roses RE, Xu S, Xu M, Koldovsky U, Koski G, Czerniecki BJ. Differential production of IL-23 and IL-12 by myeloid-derived dendritic cells in response to TLR agonists. J Immunol 2008;181:5120–7. [DOI] [PubMed] [Google Scholar]
- 35.Van Gool F, Galli M, Gueydan C, Kruys V, Prevot PP, Bedalov A, et al. Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat Med 2009;15:206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurer M, Lopez Kostka S, Siebenhaar F, Moelle K, Metz M, Knop J, et al. Skin mast cells control T cell-dependent host defense in Leishmania major infections. FASEB J 2006;20:2460–7. [DOI] [PubMed] [Google Scholar]
- 37.Gregory GD, Robbie-Ryan M, Secor VH, Sabatino JJ Jr, Brown MA. Mast cells are required for optimal autoreactive T cell responses in a murine model of multiple sclerosis. Eur J Immunol 2005;35:3478–86. [DOI] [PubMed] [Google Scholar]
- 38.Lam LT, Pham YC, Nguyen TM, Morris GE. Characterization of a monoclonal antibody panel shows that the myotonic dystrophy protein kinase, DMPK, is expressed almost exclusively in muscle and heart. Hum Mol Genet 2000;9:2167–73. [DOI] [PubMed] [Google Scholar]
- 39.Kaliman P, Catalucci D, Lam JT, Kondo R, Gutierrez JC, Reddy S, et al. Myotonic dystrophy protein kinase phosphorylates phospholamban and regulates calcium uptake in cardiomyocyte sarcoplasmic reticulum. J Biol Chem 2005;280: 8016–21. [DOI] [PubMed] [Google Scholar]
- 40.Hu J, Niu M, Li X, Lu D, Cui J, Xu W, et al. FERM domain-containing protein FRMD5 regulates cell motility via binding to integrin beta5 subunit and ROCK1. FEBS Lett 2014;588:4348–56. [DOI] [PubMed] [Google Scholar]
- 41.Knowlden S, Georas SN. The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J Immunol 2014;192:851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knowlden SA, Capece T, Popovic M, Chapman TJ, Rezaee F, Kim M, et al. Regulation of T cell motility in vitro and in vivo by LPA and LPA2. PLoS One 2014;9:e101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori K, Kitayama J, Aoki J, Kishi Y, Shida D, Yamashita H, et al. Submucosal connective tissue-type mast cells contribute to the production of lysophosphatidic acid (LPA) in the gastrointestinal tract through the secretion of autotaxin (ATX)/ lysophospholipase D (lysoPLD). Virchows Arch 2007;451:47–56. [DOI] [PubMed] [Google Scholar]
- 44.Park GY, Lee YG, Berdyshev E, Nyenhuis S, Du J, Fu P, et al. Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am J Respir Crit Care Med 2013;188:928–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, et al. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther 2006;318:619–28. [DOI] [PubMed] [Google Scholar]
- 46.Goetzl EJ, Kong Y, Voice JK. Cutting edge: differential constitutive expression of functional receptors for lysophosphatidic acid by human blood lymphocytes. J Immunol 2000;164:4996–9. [DOI] [PubMed] [Google Scholar]
- 47.Oda SK, Strauch P, Fujiwara Y, Al-Shami A, Oravecz T, Tigyi G, et al. Lysophosphatidic acid inhibits CD8 T cell activation and control of tumor progression. Cancer Immunol Res 2013;1:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu CH, et al. Wiskott Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity 1998;9:81–91. [DOI] [PubMed] [Google Scholar]
- 49.Lemos MP, Fan L, Lo D, Laufer TM. CD8alpha+ and CD11b+ dendritic cell-restricted MHC class II controls Th1 CD4+ T cell immunity. J Immunol 2003; 171:5077–84. [DOI] [PubMed] [Google Scholar]
- 50.Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med 2011;365:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007; 449:419–26. [DOI] [PubMed] [Google Scholar]
- 52.Mills KH. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol 2011;11:807–22. [DOI] [PubMed] [Google Scholar]
- 53.Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal 2011;4:ra12. [DOI] [PubMed] [Google Scholar]
- 54.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Cohen H, Lin SS, et al. Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J Biol Chem 2002;277: 18881–90. [DOI] [PubMed] [Google Scholar]
- 55.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol 2016; 17:679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013;155:1624–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersson A, Dai WJ, Di Santo JP, Brombacher F. Early IFN-gamma production and innate immunity during Listeria monocytogenes infection in the absence of NK cells. J Immunol 1998;161:5600–6. [PubMed] [Google Scholar]
- 58.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 2010;28:445–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.