Abstract
Introduction
In patients older than 70 years there is no valid alternative to progressively introduced substitution therapy. The antiparkinsonian drugs introduced in the last decade to treat Parkinson’s disease, especially in its early phases, promised a comparable efficacy in reducing symptoms to levodopa. In younger patients and/or patients with mild symptoms we hoped to delay the motor complications by postponing the start of levodopa therapy. While these assumptions may not be true for all patients, probably the most important current challenge is the optimal starting moment of levodopa therapy. The aim of the study was to analyze the therapeutical choices during the early phase of Parkinson’s disease in the Neurological Departments of Târgu Mures¸ County Hospital.
Materials and methods
We examined data obtained from hospitalized Parkinson’s disease patients during a 15-year period. According to the duration of the disease we split the patients into two groups, patients with Parkinson’s disease for less than or equal to 5 years and patients with disease duration longer than 5 years, and then analyzed only the former group.
Results
During the examined period, 2,379 patients with Parkinson’s disease were hospitalized, and 1,237 patients had a disease duration shorter than 5 years. In this group, 18 patients had monoamine oxidase inhibitor monotherapy. Also, 665 patients received dopamine agonists, in 120 cases as monotherapy and in 83 patients associated with monoamine oxidase inhibitors. In 521 patients we found only levodopa treatment. A further 481 patients received combined therapy (levodopa with dopamine agonists and/or monoamine oxidase inhibitors).
Conclusion
Treatment strategies for the early stages of Parkinson’s disease in our group were comparable to results from other studies. However, the authors feel that neurologists should use levodopa-sparing drugs with greater courage. Furthermore, if the clinical context is appropriate, physicians should combine substitution therapy with other antiparkinsonian drugs in order to reduce levodopa doses.
Keywords: Parkinson’s disease, levodopa, dopamine agonist
Introduction
The second most common progressive neurodegenerative disease, Parkinson’s disease (PD) is still a public health challenge (1% of the population over the age of 65 years and 5% of 85-year-olds are affected).1 Nevertheless, PD is unique among the neurodegenerative disorders, as it has the highest number of proven symptomatic therapies which may improve the quality of life of patients with PD significantly. However, this also poses challenges to a clinician: how to maximize the level of clinical improvement with minimal dopaminergic side effects/complications. According to our present knowledge, the slowing of the progression of the disease cannot be proved, as there is a lack of generally accepted markers. Thus, the therapeutic recommendations of recent years focus on delaying or alleviating the complications of long-term levodopa (LD) treatment while improving clinical symptoms. Substitution therapy with LD, the current gold standard for PD management, is the most effective tool for clinical correction of dopaminergic tonus.2 Despite the many decades of clinical experience, there is no clear position on how to manage LD treatment (appropriate time, dose and frequency, and monotherapy or its administration associated with other antiparkinsonian drugs). Ideally, therapy should start in a highly individualized way, determined by age and symptom severity at the time of diagnosis, but also taking into account potential associated diseases. In patients diagnosed at the age of 70 years or older, LD has virtually no alternative. In the last decades, several new antiparkinsonian drugs were approved and some of them are already largely used in different stages of PD. Based on clinical experience with newer antiparkinsonian drugs for younger patients (under 60 years), LD-sparing strategies were proposed instead of the early introduction of substitution therapy in an attempt to delay the onset of motor complications.3
There is little evidence from the literature regarding how therapeutic recommendations for early PD management are used in real-life clinical practice. The data collected in our clinic during the last 15 years, regarding the use of selective monoamine oxidase B inhibitors (MAO-Bi), have been partially reported;4 also, our analysis of dopamine agonist (DA) usage is currently in press.
The purpose of our present paper is to analyze the treatment strategies (both regarding the introduction of LD and the usage of alternative therapies, ie, LD-sparing antiparkinsonian drugs) used in the early stages of PD in the 15-year practice database of the Neurological Clinics in Târgu Mures¸, Romania.
Aim and methods
In this retrospective study we analyzed the data of all PD patients diagnosed according to the UK Brain Bank Criteria who were hospitalized in the Neurological Clinics in Târgu Mures¸ between 1 January 2003 and 31 December 2017. It must be noted that the criteria for admission changed significantly during the investigated period. In Romania it was customary to admit to hospital, at least once, every patient with a chronic neurodegenerative disease, especially due to the difficulties imposed by investigation of patients in an outpatient setting (especially regarding imaging techniques). Furthermore, at the time of introduction of DA drugs it was required that a patient be investigated in the Neurology Department of a University Clinic (initially for all DAs, later only for rotigotine). The same was true for the introduction of rasagiline and entacapone. This gradually disappeared as more experience with antiparkinsonian drugs accumulated and the legislation changed. We must note that apomorphine is not available at all and deep brain stimulation (DBS) is available for a very limited number of patients. Furthermore, we do not have access to safinamide, tolcapone, opicapone and extended release amantadine.
Based on the therapeutic recommendations of the final report of 2,379 patients, we analyzed the specificities of the age-dependent usage of several antiparkinsonian drugs in the early stages of PD. First, we created two groups of patients based on the time since PD diagnosis: longer than 5 years and less than or equal to 5 years.4 In our earlier studies we showed that reliable documentation of treatment strategies for PD is possible, especially with regard to the date of LD therapy initiation. This type of division is also justified by the clinical observation that the upper limit of the efficacy of LD-sparing drug combinations is also around 4–5 years.5 Patients with uncertainties regarding differential diagnosis, such as initial significant cognitive decline/dementia, severe depression and multiple lacunar infarctions confirmed by imaging studies, and any cases where LD was only a therapeutic test, were not included in this study.
Results
In the examined period, 2,379 patients with PD received hospital care. The study group’s main characteristics as well as the specificities of the treatment used in the early stages of PD are summarized in Table 1. In the case of patients with multiple hospital referrals we used the data from the first final report. In 489 cases, the beginning of the disease could not be established reliably. Out of the 1,237 patients diagnosed in 5 years or less, 18 were treated with MAO-Bi monotherapy and the treatment strategy of 665 patients included DA drugs either as monotherapy (in 120 cases) or associated with MAO-Bi (83 cases). In 521 patients LD monotherapy was used; also, an additional 481 patients were treated with LD in combination with DA and/or MAO-Bi. We also analyzed the treatment strategies used within different age groups (monotherapy or combination of specified antiparkinsonian drugs). These results are summarized in Figure 1. Significant differences regarding LD monotherapy or combined therapy were not found in the different age groups (Figure 2). In the age group below 50 years the LD doses were lower when used as monotherapy versus combination therapy (464.7±47.55 mg vs 501.5±44.26 mg, all values as mean±standard error of the mean). The opposing trend was observed in patients with age between 50 and 65 years (587.9±38.55 mg vs 551.3±18.1 mg). However, the only significant difference between LD doses was in the patients over 65 years when comparing monotherapy versus combined therapy (514.8±13.83 mg vs 545.9±15.28 mg, p=0.0037). The distribution of treatment strategies by gender is shown in Figure 3. A chi-square test was used to evaluate potential differences between men and women but the differences were not significant statistically (p=0.23).
Table 1.
Summarized results
| Characteristic | Value |
|---|---|
| Total: n (%) | 2,379 (100%) |
| Men | 1,249 (52.5%) |
| Women | 1,130 (47.5%) |
| Average age (mean±SD) | |
| Total | 67.23±10.42 years |
| Time since PD diagnosis ≤5 years | 65.31±10.51 years |
| Time since PD diagnosis ≥5 years | 67.05±10.42 years |
| Time since PD diagnosis unknown | 72.35±8.38 years |
| Time since PD diagnosis | |
| ≤5 years: n (%) | 1,237 (52.0%) |
| ≥5 years: n (%) | 653 (27.4%) |
| Not known: n (%) | 489 (20.6%) |
| Treatment strategies in case of PD diagnosed in ≤5 years | |
| MAO-Bi monotherapy | 18/1,237 (1.5%) |
| DA monotherapy | 120/1,237 (9.7%) |
| LD monotherapy | 521/1,237 (42.1%) |
| DA+MAO-Bi | 83/1,237 (6.7%) |
| Combined treatment | 481/1,237 (38.8%) |
| No treatment | 14/1,237 (1.1%) |
| Average age in case of different treatment strategies | |
| MAO-Bi monotherapy | 55.21±9.25 years |
| DA monotherapy | 59.87±11.34 years |
| LD monotherapy | 69.01±9.04 years |
| DA+MAO-Bi | 57.71±9.32 years |
| Combined treatment | 64.15±9.77 years |
| No treatment | 78.92±4.21 years |
Abbreviations: PD, Parkinson’s disease; MAO-Bi, monoamine oxidase B inhibitors; DA, dopamine agonist; LD, levodopa; combined treatment, LD±DA±MAO-Bi.
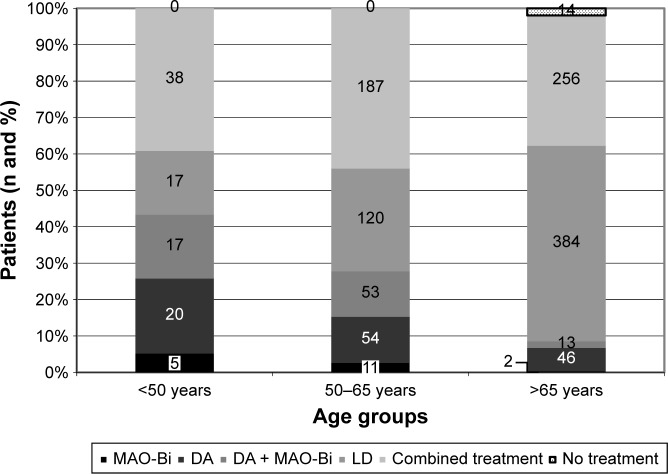
Figure 1.
Treatment strategies as a function of age in patients diagnosed with Parkinson’s disease for less than 5 years.
Note: Numbers in columns represent number of patients.
Abbreviations: MAO-Bi, monoamine oxidase B inhibitors; DA, dopamine agonist; LD, levodopa; combined treatment, LD±DA±MAO-Bi.
Figure 2.
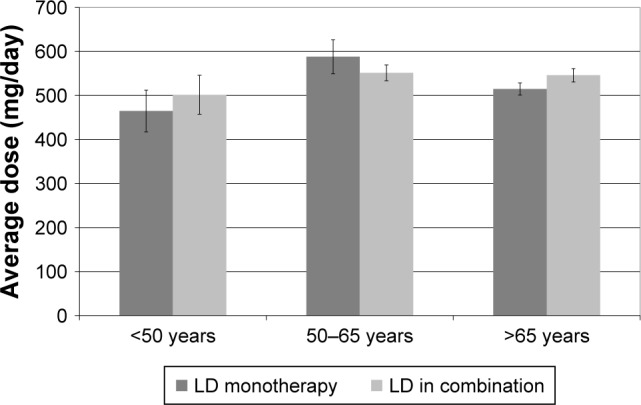
Average treatment dose of levodopa.
Note: Chart shows mean doses and standard error of mean.
Abbreviations: LD, levodopa; LD in combination, LD±dopamine agonist± monoamine oxidase B inhibitors.
Figure 3.
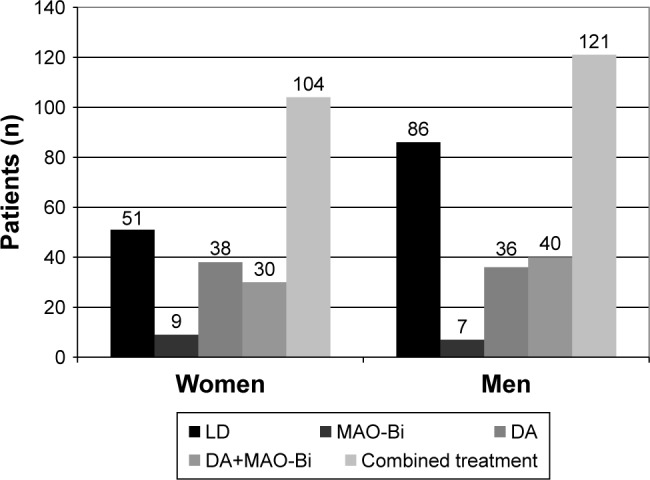
Treatment strategies as a function of gender in patients with Parkinson’s disease diagnosed before the age of 65 years.
Abbreviations: LD, levodopa; MAO-Bi, monoamine oxidase B inhibitors; DA, dopamine agonist; combined treatment, LD±DA±MAO-Bi.
Discussion
There are several symptomatic therapies for PD with efficacy proven both by clinical trials and decades-long medical practice. Clinically, the most effective and cheapest method for restoring dopaminergic tonus is substitution therapy with LD formulations.6 The major disadvantage of LD is that, after 4–6 years of use, about 40% of patients will experience motor fluctuations and dyskinesia. It must be noted that the relationship of dyskinesia with PD duration and LD treatment is still under discussion.7 Nevertheless, based on clinical observations it seems that in many cases if the therapy is started too early and with high doses, these complications may appear earlier and can be more severe, especially in younger patients. This recognition served as the basis for the dynamically changing therapeutic guidelines of the last decades, which in addition to improving symptoms, now also focus on delaying and alleviating the motor and non-motor complications of long-term LD treatment.3,5,8 The fact that by the time clinical symptoms appear the dopamine levels of the nigrostriatal system have dropped by 70%–80% proves that there is a number of compensating mechanisms.8 With the progress of the underlying neurodegenerative processes, the aberrant metabolism of LD to dopamine and its non-physiological release from the serotoninergic system, as a false neurotransmitter, contribute to abnormal stimulation of the already hypersensitive nigrostriatal dopaminergic receptors and induce the aberrant plasticity that will eventually lead to dyskinesia.8,9 The understanding of these complex processes has several important practical consequences as well: there is an increased appreciation of the endogenous, ie, still existing, dopamine level and the improvement of the pharmacodynamic efficacy of exogenous dopamine. Selective MAO-Bi may be suitable for both of the above purposes.5,6,8 On the other hand, DAs “short-circuit” the complex adaptation mechanisms and the nigrostriatal neurons damaged by the neurodegenerative processes by direct stimulation of postsynaptic dopaminergic receptors.10 The short and long-term significance of this recognition cannot yet be fully assessed. The clinical utility of selective MAO-Bi and DAs in early and late PD has been suggested by many clinical studies.6,8,10–12 They can be used even in the advanced stages of the disease, after the introduction of device-aided therapies.13–16
The mechanisms involved in the development of motor complications are only partially known. Motor fluctuations are likely to be related to the short half-life of LD as well as its irregular bioavailability, whereas dyskinesia may appear as a result of a complex, multifactorial, pre and post-synaptic abnormal plasticity that can be determined by the severity of the disease, the striatal degeneration rate, the LD doses used, the age of the patient, gender and body weight as well as the duration of the substitution treatment.17 A better understanding of these factors and taking into account the already mentioned compensation mechanisms can provide the basis for LD-sparing strategies that may delay motor complications. In addition to the unavoidable onset of motor complications, the physician’s efforts are further undermined by the progressive reduction of LD’s therapeutic efficacy as well as the frequent side effects. In addition to the dynamically changing therapeutic guidelines, clinical trials with third-generation MAO-Bi and catechol-O-methyltransferase inhibitors are intended to mitigate/delay these disadvantages.18–21
The introduction of LD, in a strictly individualized manner, should be based on the following important aspects: age of the patient and disease duration at the time of diagnosis, degree of dysfunction, cognitive status, possible comorbidities and drug side effects. There is no uniform, generally accepted recommendation on the timing of the introduction and the optimal dosage of LD. In the case of older patients, ie, over 70 years old, if mild cognitive decline and/or other comorbidities are present, small doses of gradually introduced LD treatment are considered rational pharmacotherapy. In patients under the age of 60 years, early substitution therapy should be carefully considered based on the patient’s age and the patient’s personal option. According to current therapeutic recommendations the age of 65 years is considered a “milestone” for “levodopa-sparing” treatment strategies.3,5 Both DAs and selective MAO-Bi as well as their combination could be a valid therapeutic option. Most of the practicing neurologists consider that the efficacy of LD-sparing treatment strategies is significantly reduced after a 5-year treatment period, which is why we chose this timeframe as the basis of our present study. Nevertheless, clinical studies have shown that in about 35% of patients pramipexole or ropinirole monotherapy was still an efficient alternative even after 5 years of treatment.17
There is little evidence in the literature about the therapeutic strategies used in everyday (real-life) practice. The interpretation of current results is also fuzzy due to the fact that several articles are using data based on the market share of some antiparkinsonian drugs and various budgetary parameters. The adequate interpretation of the latter by the clinician is often cumbersome.22–24 Although the scope of drugs used in PD is rather limited in other diseases, the existence of “other” options/suggestions, especially for anticholinergics and DAs, may call into question the accuracy of the data from these studies. Furthermore, potential diagnostic mistakes should be considered, especially if the diagnosis or treatment is not the attribute of a neurologist. The drug support scheme in some countries that sometimes limits the use of more expensive MAO-Bi and DAs can significantly affect the use of several antiparkinsonian drugs.22 Against this background, it is reasonable to assume that studies based on the market share of individual drugs do not reflect real-life clinical practice but only current therapeutic tendencies. One good example would be the study published in 2014 by Pitcher et al,25 regarding the use of antiparkinsonian medication in New Zealand, that showed the marked increase of several antiparkinsonian agents once funding was available in this country. Also, only a small part of the articles dealing with the subject detail the therapeutic strategies used in the early stages of PD.10,26 In a study that investigated PD in Southwest China, DAs were used as initial monotherapy in 39.4% of patients diagnosed under the age of 65 years, and 21.5% in those aged 65 years and over; LD monotherapy was the most common in both age groups: 43.5% and 73.1%, respectively.27 In the same study, MAO-Bi were used as initial monotherapy in 10.3% of patients under the age of 65 years and in 3.3% of those over 65 years.27 However, in a recent study published in 2017, the rate of application of DAs was 67.26%, despite the fact that DAs are being described as a decisive cost-increasing factor. Data from this article also show that the proportion of MAO-Bi is 16.38%, which is lower than the proportion found in Germany, with a MAO-Bi application rate of 26.9%.28 According to a 2013 study of PD in the Scandinavian countries, DAs are more common in Norway (37% of examined patients) than in Sweden (16%). Combined therapies were used in 68% of cases in Sweden and 72% in Norway. The most common combination used in Sweden was LD+pramipexole (14%); the same association in Norway was “only” 7% (the combination of LD+selegiline was more common, at 10%). Since rasagiline has been classified in this study as “others” (together with the LD-carbidopa intestinal gel), the role of MAO-Bi is difficult to evaluate.29 In a study published in 2016 by Peretz et al26 regarding patients with selegiline or rasagiline monotherapy in Israel, the authors did not find any significant difference between the time required for the introduction of dopaminergic therapy (DA or LD); however, the association of rasagiline with pramipexole significantly delayed the introduction of substitution therapy. An article in 2015 that evaluated the treatment of 16,785 PD patients in the United States analyzed the changes/trends regarding each of the antiparkinsonian drugs. The proportion of DAs in 2001 was 21.7%, and this increased to 31.2% in 2006 but fell again to 27% in 2012.30 DA monotherapy in Taiwan represented 2.25% of prescriptions in 2004 and then increased to 4.85% in 2011 in younger patients. Also, the percentage of DA monotherapy prescribed was almost twofold higher compared to the older-aged group.31
There are hardly any data on LD doses used in PD’s early stages. In a study published in 2005 by Möller et al,32 data from questionnaires completed by 6,620 patients were used. Patients were divided into two groups based on the duration of the disease: longer than 7 years and shorter than 7 years. In patients with a history of less than 7 years of PD the average LD dose was 466 mg/day, significantly lower than the 726 mg/day in patients with disease duration longer than 7 years. Compiling data from our database yielded similar values (Figure 2).
A widely accepted view is that the female gender is an independent predisposing factor for the development of dyskinesia. In light of this opinion an interesting question refers to the use of LD depending on the patient’s gender, especially in patients diagnosed with PD before the age of 65 years. Data from our database revealed that LD monotherapy was used, in this age group, more frequently in men; however, this result was not statistically significant (Figure 3). A similar tendency can be observed in Möller et al’s study: men received a significantly higher dose of LD (644 mg) than women (539 mg).32
The general neurologist, ie, not a movement disorders specialist, considers the DAs antiparkinsonian effect to be inferior to that of LD and somewhat stronger than MAO-Bi, and in everyday clinical practice the latter is more commonly used as adjuvant therapy instead of a real LD-sparing alternative. Their judgment is not significantly improved by the better therapeutic adherence, although this has been demonstrated by a number of studies.33,34 If compliance is poor, the clinical condition of patients deteriorates more rapidly. Consequently, the quality of life of both patients and relatives is reduced and the health expenditure increases. In the early stage of the disease, when a single dose of long-acting DA or MAO-Bi, or a combination of these, is sufficient to alleviate symptoms, the patient’s interaction is greatest.30,35 It is likely that this also explains the more common rasagiline usage found in our analysis.4
The decision of a clinician to choose LD or DA as an introductory therapy is influenced by a number of factors but a more detailed analysis of this decision (including, but not limited to, the advantages and disadvantages as well as the short and long-term side effects of individual DAs) goes beyond the scope of this study. LD therapy is limited by the appearance of motor complications, which in turn may lead to an unjustified phobia regarding LD and the overuse of DAs, whereas the use of DAs is complicated mainly by the non-motor complications, such as impulse control disorders, daytime sleepiness and sudden sleep.36–42 Because our study looks at a long period it is hard to retroactively statistically assess how much of the clinical decisions was influenced by the early optimism (as reflected in the initial studies) regarding the potential disease-modifying effect as well as the magnitude of the clinical efficacy of DAs.
An important practical aspect refers to the generally accepted view that DAs have a significant antidepressant effect. This view was initially based on the results of mostly open, non-double-blind, placebo-controlled studies but later similar results were found in well-designed clinical studies of pramipexole and rotigotine.43–45 This may explain the more frequent use of pramipexole and later rotigotine in our patients (in press article).
In our study, 14 (1.1%) out of the 1,237 early stage PD patients did not start therapy at the time of diagnosis; this probably may be explained by the relatively high mean age of the group (78.92±4.21 years) and/or the possible presence of comorbidities (Figure 1). We also need to highlight the relatively high proportion of young (under 65 years of age) patients with LD monotherapy. In everyday clinical practice it is not uncommon to observe that once MAO-Bi or DA monotherapy is not efficient anymore and substitution therapy is unavoidable due to the progression of the disease, the therapist will switch directly to LD monotherapy. This happens in spite of the presumed fact that the combined therapy may favor the aforementioned compensatory mechanisms, delay the onset of motor complications and, additionally, allow the use of lower LD doses.46,47 Therefore, we interpret as a negative trend the fact that in our study, in all age groups, the proportion of patients treated with combined therapy (LD±DA±MAO-Bi) was below 50%. In patients treated with LD from the start, if the severity of the clinical picture forces us to change therapy, the introduction of DAs as complementary treatment can result in similar improvement as the sole increase of LD dosage, but with a considerably lower risk of dyskinesia. According to a study published in 2010, the proportion of patients who developed dyskinesia was 3% in patients receiving ropinirole-supplemented therapy versus 17% in the case of patients with an elevated dose of LD.48
We consider our examined sample representative. The University Teaching Hospital of Târgu Mures¸, Romania, with its Neurological Clinics, draws patients from a relatively large geographical area (around eight counties). Besides the international guidelines regarding the treatment of PD there is a national therapeutical guideline, which is enforced both nationally and locally. Nevertheless, in Romania, and more generally in Eastern Europe, due to mostly financial constraints new antiparkinsonics are firstly used with a latency of perhaps 1–2 years; also, several new drugs are still not available (safinamide, tolcapone, opicapone). According to the current Romanian legislation, medical treatment of PD is free of charge, but during the investigated period the prescription of several drugs required approval of regional professional committees (such as rasagiline, rotigotine, entacapone). This resulted in a significant increase in hospital admissions, as both the accuracy of the diagnosis and the reasonableness of the given treatment required proper documentation. We should also emphasize that in Romania the diagnosis of PD, start of therapy as well as tracking of therapy effectiveness are the sole competence of the neurologist.4 In contrast, according to the aforementioned US article, neurological testing is not mandatory in PD’s early stages; also, according to the study involving Scandinavian countries, nearly one in four PD cases is seen only by general practitioners, geriatricians or related field physicians.29,30 This is similar to Israel, at least regarding the clarification of diagnosis and beginning of therapy. However, in this case the younger the patient, the greater the likelihood that a neurologist will get involved.26 Based on the large number of patients hospitalized during the 15 years analyzed, we believe that our analysis is a true reflection of the therapeutic trends of PD in Romania as well as Eastern Europe.
Among the strengths of our study we highlight the accuracy of the diagnosis, as only patients admitted to the university clinic are included; the large number of cases and the long evaluated period. All these factors, when considered together, provide a more realistic picture of the therapeutic decisions that are made in everyday practice when compared to a clinical trial that uses strict inclusion criteria. The retrospective method, however, has its limitations: there are unknown or missing data, and only a cross-sectional view of PD is provided as there is no information about patient follow-up, efficacy of the treatment and potential side effects. Also, there is no national database against which our data could be compared.
Conclusions
The therapeutic strategies used in the early stages of PD in the study period are similar to those found in the literature. In the opinion of the authors, neurologists treating this disease should, with due diligence, apply a greater proportion of different LD-sparing combinations, especially if they are not financially burdensome. Additionally, if the severity of the clinical image requires substitution therapy, the use of combined therapies (MAO-Bi and/or DAs) can significantly reduce LD doses and thus the requirement to use the minimum efficient LD dose can be achieved.
Statement of ethics
This study enrolled patients admitted to the Neurological Clinics in Târgu Mures¸. According to national legislation, all patients had to sign the patient consent form of the teaching hospital. Furthermore, the study was approved by the Ethics Committee of the University of Medicine and Pharmacy from Târgu Mures¸, approval no 94/19.05.2017 (https://www.umfst.ro/universitate/comisii-de-etica/comisia-de-etica-a-cercetarii-stiintifice/avize/2017.html).
Acknowledgments
The authors are thankful for the help offered by Fazakas Péter Alpár, Grieb Levente Gábor, Szo˝cs Ildikó and Szatmári Szabolcs Jr. This work was supported by the joint project of Studium-Prospero and the Hungarian Academy of Sciences, project no 138/2017.01.26.
Footnotes
Author contributions
Szász JA, Orbán-Kis K, Constantin VA, and Szatmári S contributed to data gathering and analysis and publication writing and editing. Péter C, Bíró I, Mihály I, Szegedi K, and Balla A contributed to data gathering and analysis. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Szász JA reports consultancy for and speaking honoraria from Abbvie, Novartis, Boehringer-Ingelheim, UCB, Lund-beck, GSK, Pfizer. Constantin VA reports speaking honoraria for Abbvie. The authors report no conflicts of interest in this work.
References
- 1.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Oertel W, Schulz JB. Current and experimental treatments of Parkinson disease: A guide for neuroscientists. J Neurochem. 2016;139:325–337. doi: 10.1111/jnc.13750. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira JJ, Katzenschlager R, Bloem BR, et al. Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur J Neurol. 2013;20(1):5–15. doi: 10.1111/j.1468-1331.2012.03866.x. [DOI] [PubMed] [Google Scholar]
- 4.Szász J, Constantin V, Fazakas P, et al. The role of selective monoamine oxidase B inhibitors in the therapeutic strategy of Parkinson’s disease in the neurology clinics of Tirgu Mures county emergency clinical hospital [A szelektív monoaminoxidáz-B-gátlók helye a Parkinson-kór kezelési straté] Orv Hetil. 2017;158:2023–2028. doi: 10.1556/650.2017.30914. [DOI] [PubMed] [Google Scholar]
- 5.Horstink M, Tolosa E, Bonuccelli U, et al. Review of the therapeutic management of Parkinson’s disease. Report of a joint task force of the European federation of neurological societies (EFNS) and the movement disorder society-European section (MDS-ES). Part II: late (complicated) Parkinson’s disease. Eur J Neurol. 2006;13(11):1186–1202. doi: 10.1111/j.1468-1331.2006.01548.x. [DOI] [PubMed] [Google Scholar]
- 6.Dezsi L, Vecsei L. Monoamine Oxidase B Inhibitors in Parkinson’s disease. CNS Neurol Disord Drug Targets. 2017;16(4):425–439. doi: 10.2174/1871527316666170124165222. [DOI] [PubMed] [Google Scholar]
- 7.Cilia R, Akpalu A, Sarfo FS, et al. The modern pre-levodopa era of Parkinson’s disease: insights into motor complications from sub-Saharan Africa. Brain. 2014;137(10):2731–2742. doi: 10.1093/brain/awu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schapira AHV. Monoamine Oxidase B Inhibitors for the treatment of Parkinson’s disease. CNS Drugs. 2011;25(12):1061–1071. doi: 10.2165/11596310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Aviles-Olmos I, Kefalopoulou Z, Foltynie T. Understanding and prevention of “therapy-” induced dyskinesias. Parkinsons Dis. 2012;2012:640815. doi: 10.1155/2012/640815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schapira AH, McDermott MP, Barone P, et al. Pramipexole in patients with early Parkinson’s disease (PROUD): a randomised delayed-start trial. Lancet Neurol. 2013;12(8):747–755. doi: 10.1016/S1474-4422(13)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olanow CW, Kieburtz K, Leinonen M, et al. A randomized trial of a low-dose Rasagiline and Pramipexole combination (P2B001) in early Parkinson’s disease. Mov Disord. 2017;32(5):783–789. doi: 10.1002/mds.26941. [DOI] [PubMed] [Google Scholar]
- 12.Shill HA, Stacy M. Update on ropinirole in the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2009;5(1):33–36. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L355664604%5Cnhttp://www.dovepress.com/getfile.php?fileID=4098. [PMC free article] [PubMed] [Google Scholar]
- 13.Antonini A, Poewe W, Chaudhuri KR, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat Disord. 2017;45:13–20. doi: 10.1016/j.parkreldis.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Băjenaru O, Ene A, Popescu BO, et al. The effect of levodopa-carbidopa intestinal gel infusion long-term therapy on motor complications in advanced Parkinson’s disease: a multicenter Romanian experience. J Neural Transm. 2016;123(4):407–414. doi: 10.1007/s00702-015-1496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juhász A, Aschermann Z, Ács P, et al. Levodopa/carbidopa intestinal gel can improve both motor and non-motor experiences of daily living in Parkinson’s disease: an open-label study. Parkinsonism Relat Disord. 2017;37:79–86. doi: 10.1016/j.parkreldis.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Szász J, Constantin V, Orbán-Kis K, et al. Characteristics of dopaminergic treatments in advanced Parkinson’s before levodopa-carbidopa intestinal gel infusion: data from 107 tested patients. Mov Disord. 2018;33:171. [Google Scholar]
- 17.Schapira AHV, Olanow CW. Drug selection and timing of initiation of treatment in early Parkinson’s disease. Ann Neurol. 2009;64(S2):S47–S55. doi: 10.1002/ana.21460. [DOI] [PubMed] [Google Scholar]
- 18.Scott LJ. Opicapone: A review in Parkinson’s disease. Drugs. 2016;76(13):1293–1300. doi: 10.1007/s40265-016-0623-y. [DOI] [PubMed] [Google Scholar]
- 19.Borgohain R, Szasz J, Stanzione P, et al. Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov Disord. 2014;29(2):229–237. doi: 10.1002/mds.25751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borgohain R, Szasz J, Stanzione P, et al. Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson’s disease. Mov Disord. 2014;29(10):1273–1280. doi: 10.1002/mds.25961. [DOI] [PubMed] [Google Scholar]
- 21.Borgohain R, Szasz J, Stanzione P, et al. First 2-year, controlled study to assess safinamide as add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov Disord. 2011;26(2):120–121. doi: 10.1002/mds.25751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosa MM, Ferreira JJ, Coelho M, Freire R, Sampaio C. Prescribing patterns of antiparkinsonian agents in Europe. Mov Disord. 2010;25(8):1053–1060. doi: 10.1002/mds.23038. [DOI] [PubMed] [Google Scholar]
- 23.Morrish P. Prescribing in Parkinson’s disease: a story of hope and adverse events. Pract Neurol. 2012;12(5):335–340. doi: 10.1136/practneurol-2012-000210. [DOI] [PubMed] [Google Scholar]
- 24.Osinaga EA, Inchaurregui LCA, Ikobaltzeta IE, Alonso NB, Del Pozo JG. A pharmacoepidemiological study of the consumption of antiparkinson drugs in the basque autonomous community (Spain) (1992–2004) Parkinsonism Relat Disord. 2007;13(8):500–504. doi: 10.1016/j.parkreldis.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Pitcher TL, MacAskill MR, Anderson TJ. Trends in antiparkinsonian medication use in New Zealand: 1995–2011. Parkinsons Dis. 2014;2014:379431. doi: 10.1155/2014/379431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peretz C, Segev H, Rozani V, et al. Comparison of Selegiline and Rasagiline therapies in Parkinson Disease: a real-life study. Clin Neuropharmacol. 2016;39(5):227–231. doi: 10.1097/WNF.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Chen D, Song W, et al. Survey on general knowledge on Parkinson’s disease in patients with Parkinson’s disease and current clinical practice for Parkinson’s disease among general neurologists from Southwest China. Clin Neurol Neurosurg. 2014;118:16–20. doi: 10.1016/j.clineuro.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Yang J-X, Chen L. Economic burden analysis of Parkinson’s disease patients in China. Parkinsons Dis. 2017;2017:1–7. doi: 10.1155/2017/8762939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skogar O, Nilsson M, Törnhage C-J, Lökk J. National surveys: a way to manage treatment strategies in Parkinson’s disease? Pharmaceutical prescribing patterns and patient experiences of symptom control and their impact on disease. J Multidiscip Healthc. 2013;6:239–247. doi: 10.2147/JMDH.S44451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crispo JAG, Fortin Y, Thibault DP, et al. Trends in inpatient anti-parkinson drug use in the USA, 2001–2012. Eur J Clin Pharmacol. 2015;71(8):1011–1019. doi: 10.1007/s00228-015-1881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu WM, Wu RM, Chang CH, Lin JW, Liu YC, Lin CH. National trends of Antiparkinsonism treatment in Taiwan: 2004–2011. Parkinsons Dis. 2016;2016:1859321. doi: 10.1155/2016/1859321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Möller JC, Körner Y, Dodel RC, et al. Pharmacotherapy of Parkinson’s disease in Germany. J Neurol. 2005;252(8):926–935. doi: 10.1007/s00415-005-0784-1. [DOI] [PubMed] [Google Scholar]
- 33.Haycox A, Armand C, Murteira S, Cochran J, François C. Cost effectiveness of Rasagiline and Pramipexole as treatment strategies in early Parkinson’s disease in the UK setting. Drugs Aging. 2009;26(9):791–801. doi: 10.2165/11316770-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs N, Janszky J, Nagy F. Cost effectiveness of Rasagiline and Pramipexole as treatment Strategies in early Parkinson’s disease in the UK setting. Drugs Aging. 2011;28(2):161–162. doi: 10.2165/80-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 35.Yun JY, Kim YE, Yang HJ, Kim HJ, Jeon B. Twice-daily versus once-daily Pramipexole extended release dosage Regimens in Parkinson’s disease. Parkinsons Dis. 2017;2017:8518929. doi: 10.1155/2017/8518929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parkinson Study Group CALM Cohort Investigators Long-term effect of initiating Pramipexole vs Levodopa in early Parkinson disease. Arch Neurol. 2009;66(5):563–570. doi: 10.1001/archneur.66.1.nct90001. [DOI] [PubMed] [Google Scholar]
- 37.Antonini A, Calandrella D. Once-daily pramipexole for the treatment of early and advanced idiopathic Parkinson’s disease: implications for patients. Neuropsychiatr Dis Treat. 2011;7(1):297–302. doi: 10.2147/NDT.S10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Biase L, Brittain JS, Shah SA, et al. Tremor stability index: A new tool for differential diagnosis in tremor syndromes. Brain. 2017;140(7):1977–1986. doi: 10.1093/brain/awx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szatmari S, Illigens BM-W, Siepmann T, Pinter A, Takats A, Bereczki D. Neuropsychiatric symptoms in untreated parkinson’s disease. Neuropsychiatr Dis Treat. 2017;13:815–826. doi: 10.2147/NDT.S130997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinson V, Gaines K. Adjunctive therapy in Parkinson’s disease: the role of rasagiline. Neuropsychiatr Dis Treat. 2012:285–294. doi: 10.2147/NDT.S25142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Tan LC-S. Revisiting the medical management of Parkinson’s disease: levodopa versus Dopamine Agonist. [Accessed, 2018];Curr Neuropharmacol. 2016 14(4):356–363. doi: 10.2174/1570159X14666151208114634. http://www.ncbi.nlm.nih.gov/pubmed/26644151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schröder S, Kuessner D, Arnold G, Zöllner Y, Jones E, Schaefer M. Do neurologists in Germany adhere to the national Parkinson’s disease guideline? Neuropsychiatr Dis Treat. 2011;7(1):103–110. doi: 10.2147/NDT.S8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyons KE, Pahwa R. Outcomes of Rotigotine clinical trials. Neurol Clin. 2013;31(3):S51–S59. doi: 10.1016/j.ncl.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Barone P, Poewe W, Albrecht S, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(6):573–580. doi: 10.1016/S1474-4422(10)70106-X. [DOI] [PubMed] [Google Scholar]
- 45.Leentjens AFG. The role of Dopamine Agonists in the treatment of depression in patients with Parkinson’s disease. Drugs. 2011;71(3):273–286. doi: 10.2165/11585380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 46.Talati R, Baker WL, Patel AA, Reinhart K, Coleman CI. Adding a dopamine agonist to preexisting levodopa therapy vs. levodopa therapy alone in advanced Parkinson’s disease: a meta analysis. Int J Clin Pract. 2009;63(4):613–623. doi: 10.1111/j.1742-1241.2009.02027.x. [DOI] [PubMed] [Google Scholar]
- 47.Poewe WH, Rascol O, Quinn N, et al. Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson’s disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol. 2007;6(6):513–520. doi: 10.1016/S1474-4422(07)70108-4. [DOI] [PubMed] [Google Scholar]
- 48.Watts RL, Lyons KE, Pahwa R, et al. Onset of dyskinesia with adjunct ropinirole prolonged-release or additional levodopa in early Parkinson’s disease. Mov Disord. 2010;25(7):858–866. doi: 10.1002/mds.22890. [DOI] [PubMed] [Google Scholar]