Abstract
PURPOSE
To describe perceptions of infertility risk among adult survivors of childhood cancer, to test the concordance of survivors’ risk perceptions and their adult fertility status, and to identify explanatory factors (sociodemographic factors, gonadotoxic treatments, reproductive history, sexual dysfunction) associated with these outcomes.
PATIENTS AND METHODS
Adult childhood cancer survivors (N = 1,067; without children or a history of pregnancies) completed questionnaires that asked about infertility risk perceptions and participated in physical evaluations, including biomarkers of gonadal functioning (eg, semen analysis, blood hormone levels, menses). Multivariable regression models tested associations between explanatory factors and risk perceptions as well as concordance of perceptions and fertility status.
RESULTS
Most childhood cancer survivors (61.9%) perceived themselves at increased risk for infertility, which was significantly associated with sociodemographic factors (older age, white ethnicity, being married/partnered, higher education), gonadotoxic treatments, fertility concerns, previous unsuccessful attempts to conceive, and sexual dysfunction (all P < .05). Laboratory-evaluated impaired gonadal function was found in 24.3% of female and 55.6% of male survivors, but concordance with survivors’ risk perceptions was low (Cohen’s κ < .19). Most survivors with discordant perceptions overestimated risk (ie, perceived being at risk, though fertility status seemed normal; 19.7% of male and 43.6% of female survivors), whereas a minority underestimated risk (ie, perceived no risk but were impaired/infertile; 16.3% of male and 5.3% of female survivors). Factors related to discordance included sociodemographics, gonadotoxic treatments, fertility concerns, and sexual dysfunction (all P < .05).
CONCLUSION
Although childfree survivors may correctly consider themselves at risk for infertility on the basis of their previous treatments, such risk perceptions were discordant from laboratory-evaluated fertility status among many survivors in adulthood. Thus, repeated fertility-related communication throughout survivorship is essential, because treatment-indicated risk does not equal fertility status after treatment. Offering fertility testing to those who were at risk and/or those with fertility-related concerns is recommended.
INTRODUCTION
More than 15,000 children are diagnosed with cancer in the United States every year,1 and more than 80% survive long term into adulthood.2,3 Yet, cure comes at a cost, as 73% to 93% of survivors are diagnosed with one or more chronic health conditions by 30 years after cancer diagnosis.4,5 Endocrine impairments, including fertility problems, are among the most prevalent medical late effects of childhood cancer treatment.6 Infertility rates range from 42% to 66% among male childhood cancer survivors and from 11% to 26% among female childhood cancer survivors; higher rates occur among those who received alkylating agents and/or pelvic radiation.7 Yet, many survivors are unaware of their fertility status,8-12 and impaired fertility or infertility can result in unexpected challenges after treatment.8,13 For example, difficulties establishing romantic relationships,14-18 fertility-related concerns/uncertainty,11,14-16,19-22 and emotional distress23 have been reported among survivors who desire but are unable to have biological children.
Importantly, survivors’ beliefs about their fertility do not necessarily align with their actual fertility status. Qualitative studies indicated that some survivors remain anxious about infertility despite being informed by providers that they should be fertile, whereas others remain hopeful when they are infertile.15,20,21 Survivors may also be told that their fertility is impaired or that test results were ambiguous, which potentially causes uncertainty and leads to a reliance on personal beliefs. Previous quantitative studies focused on either survivors’ self-reports of pregnancies/fertility24-27 or biomarkers of gonadal functioning (eg, hormone levels, semen analysis)28-35 but have not considered survivors’ own fertility-related perceptions. Other studies examined survivors’ infertility risk perceptions in conjunction with gonadotoxic treatment exposure (as a proxy for increased infertility risk) and reported that risk perceptions did not differ by treatment exposure.19,36 Although important, reliance on treatment-indicated risk in fertility counseling falls short, because different responses to therapy yield variability in survivors’ fertility after treatment. Furthermore, previous studies did not assess whether survivors’ fertility-related perceptions align with their actual gonadal functioning. Such considerations are necessary to effectively counsel survivors about their family planning, because having children is an important life goal among many survivors of childhood cancer.25,37-39 Therefore, this study aimed (1.) to describe infertility risk perceptions among childfree adult survivors of childhood cancer and (2.) to examine the concordance of survivors’ risk perceptions with biomarkers of fertility.
PATIENTS AND METHODS
Design and Participants
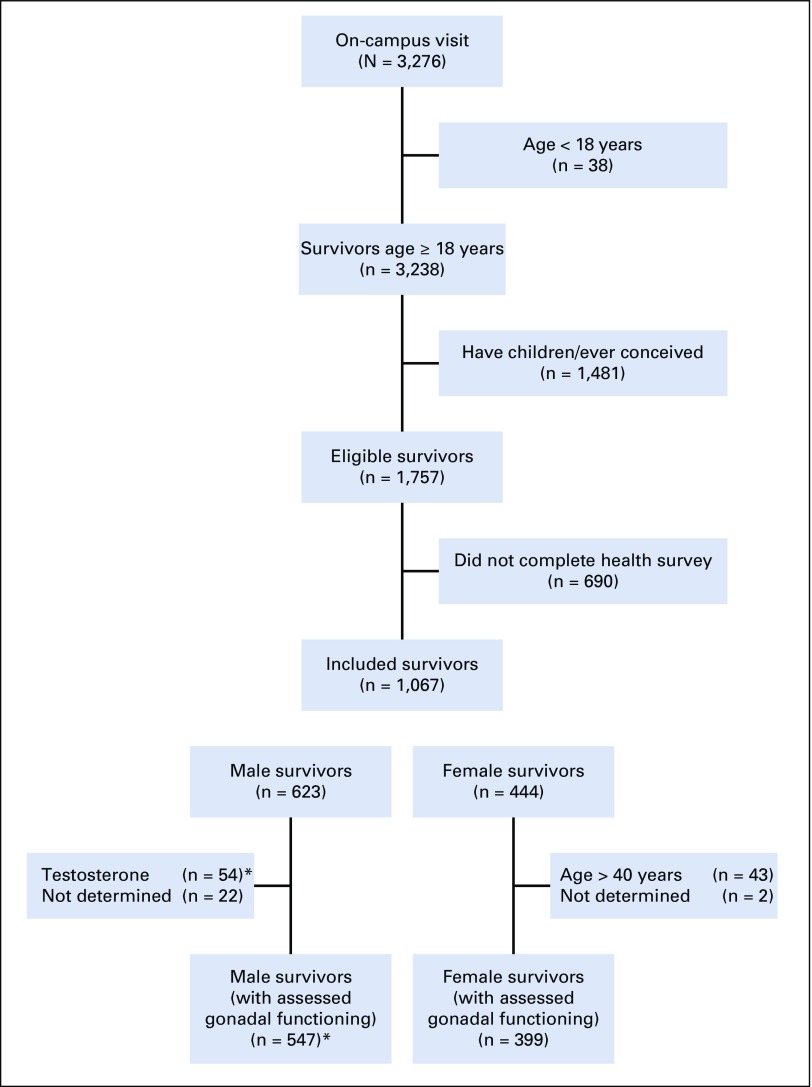
The St Jude Lifetime Cohort Study (SJLIFE) was established for longitudinal evaluations of health outcomes among long-term survivors of childhood cancer treated at St Jude Children’s Research Hospital.40,41 The SJLIFE protocol was approved by the institutional review board, and informed consent was obtained from survivors before participation. Survivors included in these analyses completed an on-campus evaluation before June 2016, were at least 18 years old, were at least 10 years post-diagnosis, and had no biological children or history of conceiving/siring a pregnancy (ie, risk estimates would be unbiased from previous pregnancies). Self-report questionnaires were completed before fertility-related testing during the on-campus visit. A total of 1,757 survivors were eligible, and 1,067 completed the primary outcome (ie, infertility risk perception; Fig 1). These 1,067 survivors were 29.0 years old (standard deviation, 8.0) and 21.9 years (standard deviation, 7.9) after a diagnosis of leukemia (n = 416; 39.0%), solid tumor (n = 342; 32.1%), CNS tumor (n = 156; 14.6%), or lymphoma (n = 153; 14.3%; Tables 1 and 2).
FIG 1.
Flow diagram of eligible and included survivors (n = 1,067). (*) Gonadal functioning of male survivors was determined by semen analysis (n = 309, or 56.5%) or by medical chart information (n = 238, or 43.5%); semen analyses of survivors on testosterone replacement therapy were deemed uninterpretable.
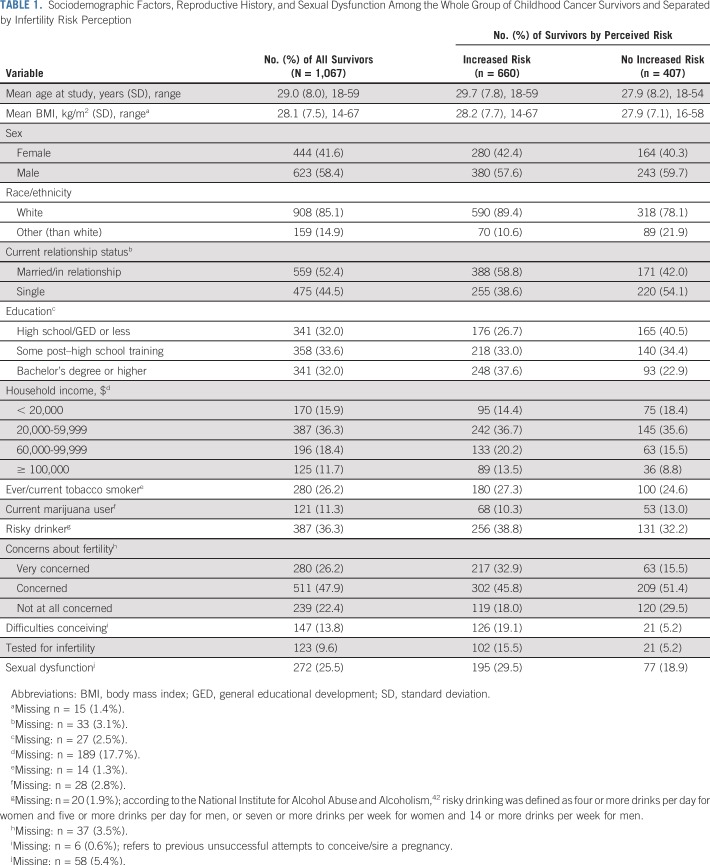
TABLE 1.
Sociodemographic Factors, Reproductive History, and Sexual Dysfunction Among the Whole Group of Childhood Cancer Survivors and Separated by Infertility Risk Perception
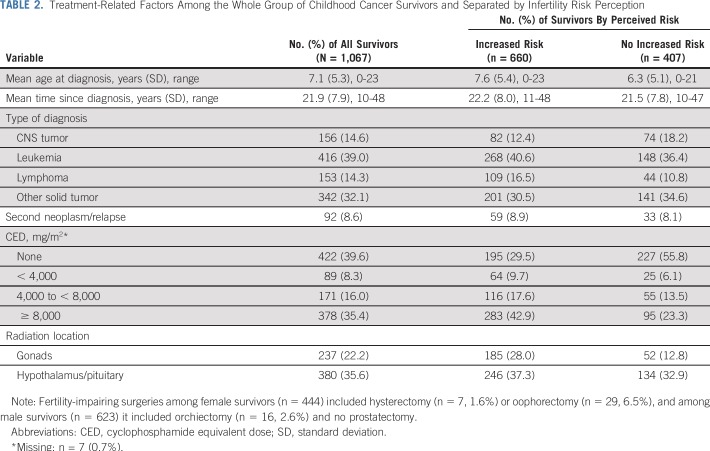
TABLE 2.
Treatment-Related Factors Among the Whole Group of Childhood Cancer Survivors and Separated by Infertility Risk Perception
Measures
Infertility risk perceptions.
As part of the SJLIFE Men’s/Women’s Health Questionnaire, survivors were asked to rate whether they perceived themselves at risk for infertility compared with women/men their age without a history of cancer. Answer categories included five options that were dichotomized into perceptions of no increased risk (ie, much less, slightly less, or about the same) and perceptions of increased risk for infertility (ie, slightly more or much more).
Explanatory factors.
Potential covariates included self-reported sociodemographic factors, including age at evaluation, sex, ethnicity, relationship status, education, household income, tobacco use, risky drinking, and marijuana use, along with on-campus measured body mass index. (Table 1 lists descriptive statistics and categorizations.) Treatment-related factors were obtained from medical records and included age at diagnosis, type of diagnosis, second neoplasm or relapse, gonadotoxic chemotherapy (expressed as cyclophosphamide equivalent dose [CED]),43 radiation directed at gonads and/or hypothalamus/pituitary, and any fertility-impairing surgeries (Table 2). Survivors also self-reported aspects of reproductive history, including concerns about fertility, unsuccessful attempts to conceive/sire a pregnancy, and previous fertility testing (Table 1). Finally, sexual dysfunction was defined as moderate to severe sexual problems that impaired sexual activities; it was measured with subscales of the International Index of Erectile Dysfunction44 among men and with the Sexual Functioning Questionnaire45 among women. Specifically, the International Index of Erectile Dysfunction erectile functioning subscale (six items) assessed difficulties getting and maintaining an erection on a 5-point Likert-type scale (almost never through almost always; 1 through 5). Scores were summed and dichotomized into presence (ie, scores ≤ 21)46 versus absence of erectile dysfunction. Similarly, the sexual problems subscale of the Sexual Functioning Questionnaire listed nine problems that impair sexuality (eg, vaginal dryness, pain during intercourse). If female survivors endorsed any problem as occurring 75% of the time or always and/or if it interrupted sexual activities, they were categorized as experiencing sexual dysfunction (Table 1).
Male gonadal functioning.
Fertility status in male survivors was primarily determined through semen analysis (n = 309; Fig 1). Male survivors were characterized as having normospermia (if > 15 million sperm/mL, > 40% motility, and ≥ 4% normal forms by strict Krueger morphology),47 oligospermia (if values fell outside these ranges), or azoospermia (if no sperm was identified). These were dichotomized into subfertile/infertile (oligospermic, azoospermic) versus fertile (normospermic). For survivors who did not complete semen analyses (n = 238), morning plasma levels of follicle stimulating hormone (FSH), luteinizing hormone (LH), and testosterone were reviewed (collected during SJLIFE visit) along with recorded diagnoses of hypogonadism on the basis of the Common Terminology Criteria for Adverse Events, version 4.03. If this information was inconclusive, medical records were reviewed (n = 144) to evaluate whether survivors had previous/repeated evaluations of FSH, LH, and/or testosterone, whether they were receiving testosterone replacement therapy, and/or whether they had other conditions that potentially influenced their fertility (Fig 1). Assays for normative hormone levels were 2.0 to 9.2 IU/L (for FSH), 1.2 to 7.0 IU/L (for LH), and 249 to 846 ng/dL (for testosterone).48
Female gonadal functioning.
Gonadal functioning in female survivors was determined through medical chart reviews that were based on previously established criteria.49 Information to determine fertility status included the following: physician diagnosis of premature ovarian insufficiency; Common Terminology Criteria for Adverse Events grade for primary or central hypogonadism; absence of spontaneous onset of menarche (after treatment); menstrual cycles (in combination with contraceptive use) at study participation; and levels of FSH, LH, and estradiol (collected during SJLIFE visit). Fertility status was dichotomized into subfertile/infertile versus fertile. Female survivors older than age 40 years (n = 43 [9.8%] of 444) were excluded from these subanalyses, because they may have experienced natural menopause (Fig 1).50
Statistical Analyses
Descriptive statistics and missing data were examined for all variables. Household income had the highest proportion of missing values (17.7%; other variables had < 5.4% missing). Therefore, a 20-iterartion multiple imputation was conducted for household income using a fully conditional specification method,51 conditioned on educational level. Subsequently, a three-stepped approach was used to build regression models for infertility risk perception (Aim 1). First, associations among all explanatory factors and this outcome were tested using univariable analyses. Covariates significant at P values < .2 were retained to include a broad net of potentially relevant variables. Second, the least absolute shrinkage and selection operator (LASSO) was used to select covariates. For each imputed data set, 10-fold cross-validations were performed; and λ, the regularization weight, which resulted in minimal cross-validation error, was used to select covartiates. Those with coefficients greater than 0 in at least five of the 10-fold cross-validations were considered, and covariates selected in at least 80% of the 20 imputed data sets were retained for multivariable models. Third, multivariable models that used modified Poisson regression52 were fitted using the selected covariates. Models were further simplified by removing covariates with P values > .1 without significant effect on the model fit, as assessed by quasi-likelihood under the independence model criterion (QIC). If household income was selected by LASSO, results of the 20 imputed data sets were combined and aggregated using PROC MIANALYZE in SAS (SAS Institute, Cary, NC) to conduct statistical inferences. Final results are presented as risk ratios (RRs) and 95% CIs.
For Aim 2, the concordance of survivors’ risk perceptions and biomarkers of gonadal function was tested using Cohen’s κ, for which coefficients less than 0.20 were considered poor.53 Survivors either accurately appraised their infertility risk, overestimated risk (ie, they were fertile but perceived an increased risk), or underestimated risk (ie, they were subfertile/infertile but perceived no risk). Descriptive statistics are presented, and factors associated with over- and underestimations of risk (v accurately considered risk) were tested. The same three-stepped approach as described in the previous paragraph was used to arrive at final multivariable models; results are presented as RRs and 95% CIs. Statistical software packages used in this study were SAS 9.4 (SAS Institute) for multiple imputations and for univariable and multivariable analyses, whereas R3.4.2 was used for LASSO (package “glmnet”).54
RESULTS
Infertility Risk Perceptions
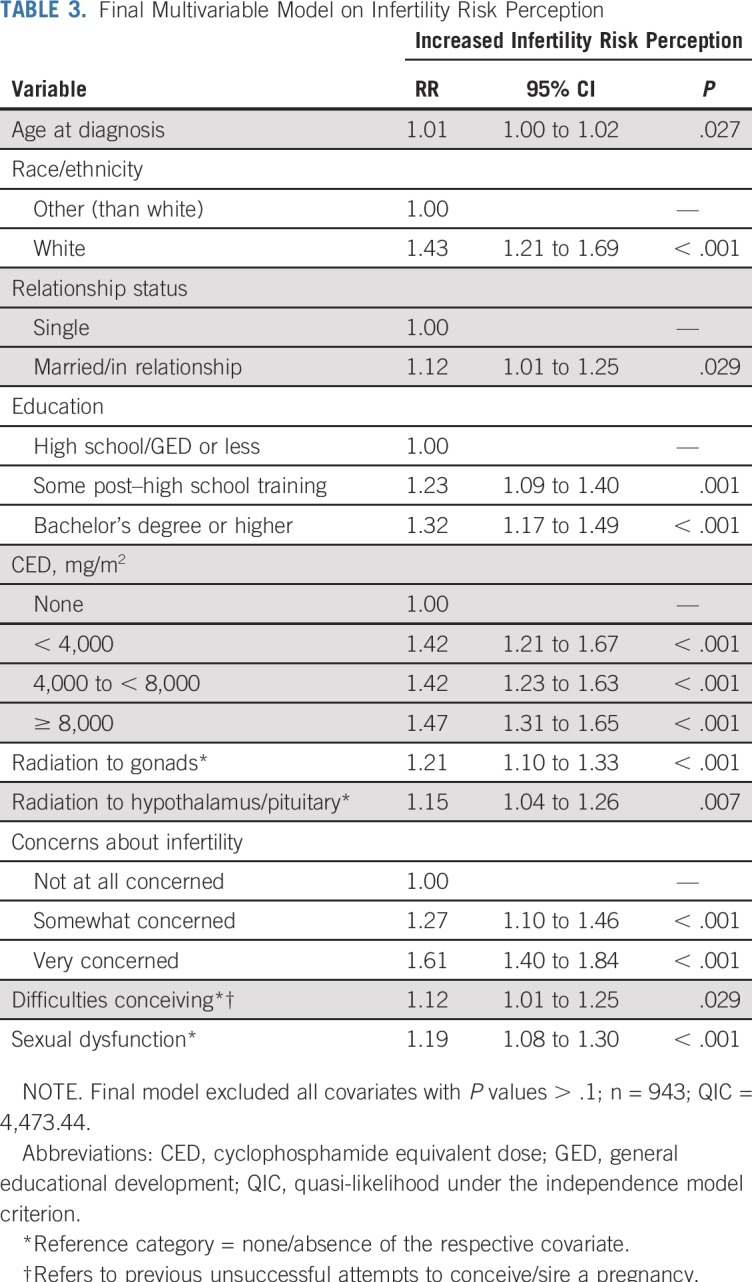
Almost two thirds of survivors (n = 660; 61.9%) perceived an increased risk for infertility relative to peers with no cancer history (v perceptions of no increased risk by n = 407 survivors, 38.1%), which did not differ by sex (P = .491). The final regression model (Table 3) indicated that sociodemographic factors associated with increased risk perceptions were older age at diagnosis (RR, 1.01; 95% CI, 1.00 to 1.02; P = .027), white ethnicity (RR, 1.43; 95% CI, 1.21 to 1.69; P < .001), married or partnered status (RR, 1.12; 95% CI, 1.01 to 1.25; P < .029), and a high school diploma (RR, 1.23; 95% CI, 1.09 to 1.40; P = .001) or bachelor’s degree (RR, 1.32; 95% CI, 1.17 to 1.49; P < .001) relative to less than high school diploma. In addition, treatment-related factors associated with increased risk perceptions included any CED (relative to none; RR, 1.42 to 1.47; P < .001; Table 3), radiation to gonads (RR, 1.21; 95% CI, 1.10 to 1.33; P < .001), and radiation to the hypothalamus (RR, 1.15; 95% CI, 1.04 to 1.26; P = .007). Reports of any concerns about fertility relative to none (RR, 1.61 and RR, 1.27; P < .001; Table 3), previous unsuccessful attempts to conceive (RR, 1.20; 95% CI, 1.08 to 1.33; P < .001), and sexual dysfunction (RR, 1.19; 95% CI, 1.08 to 1.30; P < .001) were also related to increased infertility risk perceptions (Table 3).
TABLE 3.
Final Multivariable Model on Infertility Risk Perception
Risk Perceptions Versus Laboratory-Evaluated Gonadal Function
Male survivors.
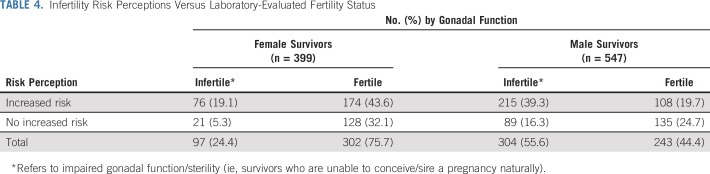
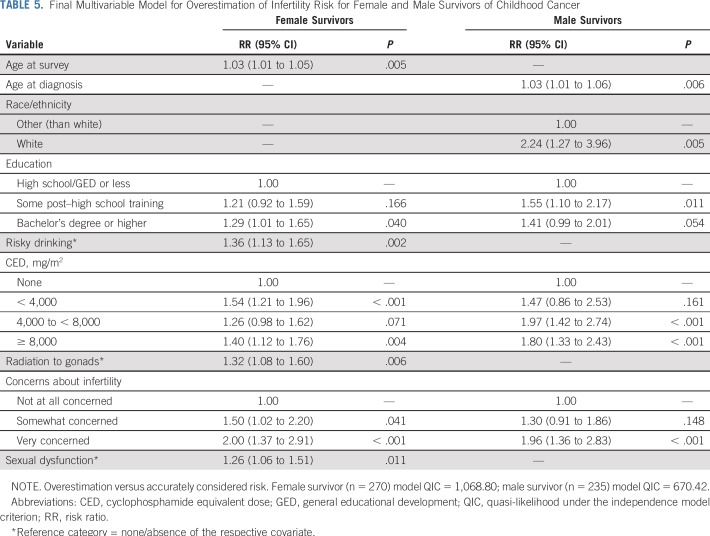
More than half of all male survivors (n = 304; 55.6%) were classified as having impaired gonadal function or being sterile, of whom 70.7% (n = 215 of 304) accurately perceived themselves at increased risk. Less than one fifth of all male survivors overestimated their risk (n = 108; 19.7%), whereas 16.3% underestimated their risk (n = 89; Table 4); and concordance with laboratory-evaluated fertility status was poor (κ = 0.19). Multivariable regression (Table 5) indicated that men who overestimated their risk were older at diagnosis (RR, 1.03; 95% CI, 1.01 to 1.06; P = .006), were more often white (RR, 2.24; 95% CI, 1.27 to 3.96; P = .005), received some post–high school education (relative to high school only: RR, 1.55; 95% CI, 1.10 to 2.17; P = .012), received CEDs greater than 4,000 mg/m2 (RR, 1.80 and RR, 1.96; P < .001; Table 5), and were very concerned about their fertility (RR, 1.96; 95% CI, 1.36 to 2.83; P < .001) relative to men who did not overestimate their infertility risk.
TABLE 4.
Infertility Risk Perceptions Versus Laboratory-Evaluated Fertility Status
TABLE 5.
Final Multivariable Model for Overestimation of Infertility Risk for Female and Male Survivors of Childhood Cancer
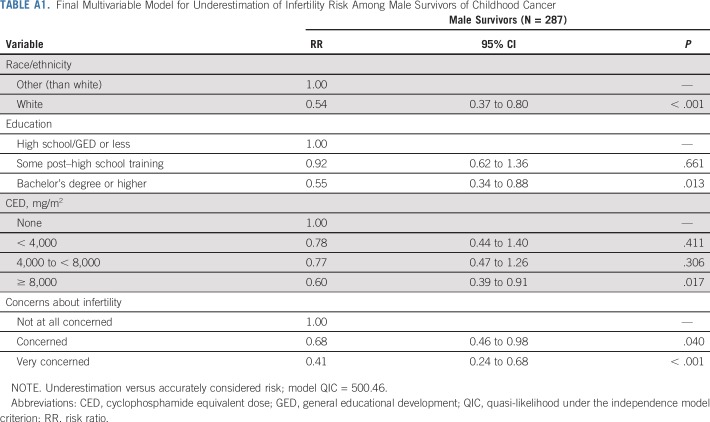
Accordingly, multivariable models identified the same factors, apart from age at diagnosis, for underestimation of risk in the opposite direction. Thus, male survivors who were white, received the highest education or highest CED, or reported concerns about their fertility were less likely to underestimate risk (Appendix Table A1, online only).
Note that distributions of discordant risk perceptions among male survivors whose fertility status was determined through semen analyses were comparable to those of the full sample. Of those analyzed, 71.2% were classified as infertile (29.5%, oligospermia; 41.7%, azoospermia); and 70.4% accurately perceived an increased infertility risk, whereas 13.6% overestimated their risk.
Female survivors.
A total of 97 female survivors younger than age 40 years (24.3%) were classified as infertile, of whom 78.4% (n = 76 of 97) considered themselves at increased risk for infertility. However, overall concordance of risk estimates with laboratory-evaluated gonadal function was poor (κ = 0.14): almost half of female survivors (n = 174; 43.6%) overestimated their risk (Table 4). Multivariable regression (Table 5) indicated that women who overestimated risks were older (RR, 1.03; 95% CI, 1.01 to 1.05; P = .005), had a bachelor’s degree or higher (relative to high school only: RR, 1.29; 95% CI, 1.01 to 1.65; P = .040), were more often risky drinkers (RR, 1.36; 95% CI, 1.13 to 1.65; P = .002), had received varying CEDs (relative to none: RR, 1.54 and RR, 1.40; P < .004; Table 5), had received radiation to the gonads (RR, 1.32; 95% CI, 1.08 to 1.60; P = .006), reported some or great concern about their fertility (RR, 1.50 and RR, 2.00, respectively; P < .041), and/or reported sexual dysfunction (RR, 1.26; 95% CI, 1.06 to 1.51; P = .011; Table 5) relative to women who accurately considered themselves at risk.
Similar analyses for female survivors who underestimated risk (n = 21) could not be reported. Final models did not converge because of limited sample size.
DISCUSSION
This is, to our knowledge, the first study to systematically highlight the intersection of self-reported infertility risk perceptions and actual fertility status in a large cohort of childfree adult survivors of childhood cancer. Consistent with previous research,19,36 most survivors perceived themselves at increased risk for infertility. Yet, although female survivors tend to be infertile at lower rates than male survivors,7,55 both sexes had similar risk perceptions. Consequently, many female, but also male, survivors overestimated their risk for infertility, which highlights that many survivors draw incorrect conclusions about their current fertility potential. Infertility risk perceptions that are discordant from actual gonadal function after treatment can have adverse implications for physical and/or emotional health among survivors.
For example, survivors who assume that they are at risk despite a fertility status that seems normal may become anxious about their family planning, which could result in unnecessary worry and distress and thus affect their emotional health. Also, such misperceptions could affect survivors’ physical health if they have unplanned pregnancies,11 increased use of emergency contraception,56 and/or acquire sexually transmitted diseases secondary to engaging in unprotected sexual behaviors. In contrast, survivors with impaired fertility who perceive themselves at no increased risk also raise concern. Fortunately, this subgroup was relatively small among both female and male survivors (< 17%), but these survivors are prone to emotional difficulties, distress, and/or relationship problems if they plan on having children but discover they are infertile.8,11,12,14-16,20
Importantly, survivors’ risk perceptions can be addressed by health care providers throughout survivorship. Typically, survivors’ infertility risk is estimated on the basis of previous treatment exposure, and, as demonstrated in this study, survivors who received greater gonadotoxic treatment (ie, higher CED, radiation to gonads) perceived higher risk. This suggests that survivors were accurately counseled and/or educated themselves about their infertility risk. However, treatment-indicated risk does not directly translate into adult fertility status (as also shown in our overestimation analyses). In addition, the fertility potential of survivors may decline more rapidly. Although many female survivors were deemed fertile at the time of evaluation, they may experience premature ovarian insufficiency, which underscores the importance of repeated fertility-related education and counseling throughout survivorship.
Other factors identified in this study (eg, sexual dysfunction, risky drinking) warrant additional exploration, because underlying mechanisms/relations with infertility perceptions remain unclear. It may be speculated that female survivors with sexual dysfunction interpret their symptoms (and potential other physical sequelae) as impairment of their fertility or ability to carry a pregnancy.15 This again underscores the importance of educating survivors about their reproductive health.56,57
Addressing survivors’ fertility status after treatment will continue to be a salient issue in the future. Although there are many efforts to increase fertility preservation at diagnosis, preservation is not viable for every patient or family, and preservation methods remain experimental for prepubertal patients. Preservation options will continue to improve and should be considered in future research. However, and irrespective of whether patients undergo fertility preservation at diagnosis, many survivors will desire clarity about their fertility status later in life. This will inform whether they may need to use or could discard cryopreserved specimens and enable informed decisions about protected sexual behaviors and/or family planning.
Although this is the first study, to our knowledge, that combines survivors’ self-reported infertility risk perceptions and laboratory-evaluated gonadal functioning, several limitations should be considered. We excluded survivors with a history of conceiving/siring a pregnancy, because pregnancies are typically interpreted as indicators of intact fertility. As such, our findings apply to childfree survivors, and future studies should examine whether survivors with children experience fertility-related difficulties/distress during family planning, whether they are in need of counseling, and/or whether they used assisted reproductive technologies. Furthermore, we combined subfertile and infertile survivors into one group. This decision was made to use a more inclusive approach to highlight survivors who could experience difficulties siring/conceiving a pregnancy naturally. Reliance on hormonal data to assess fertility is another limitation, which may have resulted in misclassification of participants and under-reporting of infertility. In women, the presence of spontaneous onset of and/or regular menses is not necessarily indicative of intact fertility and may be distorted by contraceptive use. Additional markers may facilitate ovarian function assessments (eg, anti-Mullerian hormone levels, antral follicle count), but such evaluations were not part of SJLIFE. In men, hormone levels are less reliable than semen analysis results for the evaluation of reproductive function. However, proportions of discordant risk ratings were similar in men with semen analysis and the whole group of male survivors. Furthermore, participation in research about sensitive issues (ie, fertility testing, questionnaires about intimate issues) may induce selection bias. Nevertheless, the presented sample did not differ from the 690 nonrespondents with regard to age at participation, age at diagnosis, or type of diagnosis, but more men than women refused to complete questionnaires (P = .004). Finally, conducting research to assess fertility status requires debriefing of survivors (ie, communication of test results), which was performed after questionnaire completion in this study.
To conclude, this study demonstrated that most childfree adult survivors of childhood cancer consider themselves at risk for infertility, which was related to various sociodemographic, gonadotoxic, and reproductive factors, as well as sexual dysfunction. Risk perceptions were often discordant from laboratory-evaluated gonadal functioning, and most survivors overestimated—rather than underestimated—risk. Addressing fertility in survivorship seems particularly important for survivors of childhood cancer, because young age at diagnosis often precludes them from participating in fertility preservation and/or fertility-related discussions with providers. Nevertheless, and even if patients preserved sperm/oocytes, many survivors may be left questioning their fertility status after treatment. Providers should educate and counsel survivors based on treatment-indicated risk, yet they should emphasize that estimated risk does not simply translate into adult fertility status. Survivors’ personal goals about family planning should also be taken into consideration. Thus, survivors at risk, those who show initial signs of fertility problems (eg, irregular menses), those who desire/request more fertility-related information, and/or those who are experiencing fertility-related uncertainties, worries, or distress should be offered fertility testing. This could range from interpretation of blood hormone levels to semen analysis/antral follicle counts and/or referrals to reproductive specialists. By providing such services, a reduction in misperceptions and emotional burden may be achieved. Furthermore, such discussions may promote opportunities for fertility preservation among survivors with impaired gonadal functioning (eg, female survivors with reduced or declining ovarian reserve; male survivors with severe oligospermia). Activities of this nature will expand family building options for childhood cancer survivors and increase opportunities for biological parenthood among those who desire biological children.
APPENDIX
TABLE A1.
Final Multivariable Model for Underestimation of Infertility Risk Among Male Survivors of Childhood Cancer
Footnotes
Podcast by Dr Schover
The views expressed in this article are the authors’ own and not an official position of the institution or funder.
Supported by the National Cancer Institute Grant No. CA195547 (L.L.R. and M.M.H.); the Cancer Center Support (CORE) Grant No. CA21765 to St Jude’s Research Hospital; and the American Lebanese Syrian Associated Charities.
AUTHOR CONTRIBUTIONS
Conception and design: Vicky Lehmann, Wassim Chemaitilly, Daniel M. Green, Deo Kumar Srivastava, Melissa M. Hudson, James L. Klosky
Collection and assembly of data: Vicky Lehmann, Wassim Chemaitilly, Lu Lu, Leslie L. Robison, Melissa M. Hudson, James L. Klosky
Data analysis and interpretation: All authors
Provision of study material or patients: Leslie L. Robison, Melissa M. Hudson, James L. Klosky
Administrative support: Leslie L. Robison, Melissa M. Hudson, James L. Klosky
Financial support: Leslie L. Robison, Melissa M. Hudson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Gonadal Functioning and Perceptions of Infertility Risk Among Adult Survivors of Childhood Cancer: A Report From the St Jude Lifetime Cohort Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
William H. Kutteh
Speakers' Bureau: AbbVie, Natera
Melissa M. Hudson
Consulting or Advisory Role: Coleman Supportive Oncology Initiative for Children with Cancer, Oncology Research Information Exchange Network, Princess Máxima Center
James L. Klosky
Employment: InTown Physical Therapy (I)
Research Funding: Merck Sharp & Dohme
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Cancer Institute Cancer statistics, 2017. https://www.cancer.gov/about-cancer/understanding/statistics
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. https://seer.cancer.gov/csr/1975_2014/
- 3.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374:833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 5.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skinner R, Wallace WHB, Levitt GA. Long-term follow-up of people who have survived cancer during childhood. Lancet Oncol. 2006;7:489–498. doi: 10.1016/S1470-2045(06)70724-0. [DOI] [PubMed] [Google Scholar]
- 7.Chemaitilly W, Cohen LE. Diagnosis of endocrine disease: Endocrine late-effects of childhood cancer and its treatments. Eur J Endocrinol. 2017;176:R183–R203. doi: 10.1530/EJE-17-0054. [DOI] [PubMed] [Google Scholar]
- 8.Green D, Galvin H, Horne B. The psycho-social impact of infertility on young male cancer survivors: A qualitative investigation. Psychooncology. 2003;12:141–152. doi: 10.1002/pon.622. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Mersereau JE. A pilot study about female adolescent/young childhood cancer survivors’ knowledge about reproductive health and their views about consultation with a fertility specialist. Palliat Support Care. 2015;13:1251–1260. doi: 10.1017/S147895151400114X. [DOI] [PubMed] [Google Scholar]
- 10.Hohmann C, Borgmann-Staudt A, Rendtorff R, et al. Patient counselling on the risk of infertility and its impact on childhood cancer survivors: Results from a national survey. J Psychosoc Oncol. 2011;29:274–285. doi: 10.1080/07347332.2011.563344. [DOI] [PubMed] [Google Scholar]
- 11.Zebrack BJ, Casillas J, Nohr L, et al. Fertility issues for young adult survivors of childhood cancer. Psychooncology. 2004;13:689–699. doi: 10.1002/pon.784. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann V, Nahata L, Ferrante AC, et al. Fertility-related perceptions and impact on romantic relationships among adult survivors of childhood cancer. J Adolesc Young Adult Oncol. 2018;7:409–414. doi: 10.1089/jayao.2017.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schover LR, Brey K, Lichtin A, et al. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002;20:1880–1889. doi: 10.1200/JCO.2002.07.175. [DOI] [PubMed] [Google Scholar]
- 14.Thompson AL, Long KA, Marsland AL. Impact of childhood cancer on emerging adult survivors’ romantic relationships: A qualitative account. J Sex Med. 2013;10:65–73. doi: 10.1111/j.1743-6109.2012.02950.x. [DOI] [PubMed] [Google Scholar]
- 15.Benedict C, Shuk E, Ford JS. Fertility issues in adolescent and young adult cancer survivors. J Adolesc Young Adult Oncol. 2016;5:48–57. doi: 10.1089/jayao.2015.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson J, Jervaeus A, Lampic C, et al. ‘Will I be able to have a baby?’ Results from online focus group discussions with childhood cancer survivors in Sweden. Hum Reprod. 2014;29:2704–2711. doi: 10.1093/humrep/deu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann V, Grönqvist H, Engvall G, et al. Negative and positive consequences of adolescent cancer 10 years after diagnosis: An interview-based longitudinal study in Sweden. Psychooncology. 2014;23:1229–1235. doi: 10.1002/pon.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackie E, Hill J, Kondryn H, et al. Adult psychosocial outcomes in long-term survivors of acute lymphoblastic leukaemia and Wilms’ tumour: A controlled study. Lancet. 2000;355:1310–1314. doi: 10.1016/S0140-6736(00)02112-7. [DOI] [PubMed] [Google Scholar]
- 19.Gilleland Marchak J, Elchuri SV, Vangile K, et al. Perceptions of infertility risks among female pediatric cancer survivors following gonadotoxic therapy. J Pediatr Hematol Oncol. 2015;37:368–372. doi: 10.1097/MPH.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 20.Crawshaw MA, Sloper P. Swimming against the tide: The influence of fertility matters on the transition to adulthood or survivorship following adolescent cancer. Eur J Cancer Care (Engl) 2010;19:610–620. doi: 10.1111/j.1365-2354.2009.01118.x. [DOI] [PubMed] [Google Scholar]
- 21.Frederick NN, Recklitis CJ, Blackmon JE, et al. Sexual dysfunction in young adult survivors of childhood cancer. Pediatr Blood Cancer. 2016;63:1622–1628. doi: 10.1002/pbc.26041. [DOI] [PubMed] [Google Scholar]
- 22.Parry C. Embracing uncertainty: An exploration of the experiences of childhood cancer survivors. Qual Health Res. 2003;13:227–246. doi: 10.1177/1049732302239600. [DOI] [PubMed] [Google Scholar]
- 23.Schover LR. Psychosocial aspects of infertility and decisions about reproduction in young cancer survivors: A review. Med Pediatr Oncol. 1999;33:53–59. doi: 10.1002/(sici)1096-911x(199907)33:1<53::aid-mpo10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 24.Wasilewski-Masker K, Seidel KD, Leisenring W, et al. Male infertility in long-term survivors of pediatric cancer: A report from the childhood cancer survivor study. J Cancer Surviv. 2014;8:437–447. doi: 10.1007/s11764-014-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinmuth S, Liebeskind AK, Wickmann L, et al. Having children after surviving cancer in childhood or adolescence: Results of a Berlin survey. Klin Padiatr. 2008;220:159–165. doi: 10.1055/s-2008-1073143. [DOI] [PubMed] [Google Scholar]
- 26.Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2677–2685. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green DM, Kawashima T, Stovall M, et al. Fertility of male survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28:332–339. doi: 10.1200/JCO.2009.24.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green DM, Liu W, Kutteh WH, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. Lancet Oncol. 2014;15:1215–1223. doi: 10.1016/S1470-2045(14)70408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen EC, Müller J, Schmiegelow K, et al. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab. 2003;88:5307–5314. doi: 10.1210/jc.2003-030352. [DOI] [PubMed] [Google Scholar]
- 30.Kenney LB, Laufer MR, Grant FD, et al. High risk of infertility and long-term gonadal damage in males treated with high dose cyclophosphamide for sarcoma during childhood. Cancer. 2001;91:613–621. doi: 10.1002/1097-0142(20010201)91:3<613::aid-cncr1042>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 31.Rendtorff R, Hohmann C, Reinmuth S, et al. Hormone and sperm analyses after chemo- and radiotherapy in childhood and adolescence. Klin Padiatr. 2010;222:145–149. doi: 10.1055/s-0030-1249658. [DOI] [PubMed] [Google Scholar]
- 32.Balcerek M, Reinmuth S, Hohmann C, et al. Suspected infertility after treatment for leukemia and solid tumors in childhood and adolescence. Dtsch Arztebl Int. 2012;109:126–131. doi: 10.3238/arztebl.2012.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Casteren NJ, van der Linden GH, Hakvoort-Cammel FG, et al. Effect of childhood cancer treatment on fertility markers in adult male long-term survivors. Pediatr Blood Cancer. 2009;52:108–112. doi: 10.1002/pbc.21780. [DOI] [PubMed] [Google Scholar]
- 34.Brignardello E, Felicetti F, Castiglione A, et al. Gonadal status in long-term male survivors of childhood cancer. J Cancer Res Clin Oncol. 2016;142:1127–1132. doi: 10.1007/s00432-016-2124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hudson MM. Reproductive outcomes for survivors of childhood cancer. Obstet Gynecol. 2010;116:1171–1183. doi: 10.1097/AOG.0b013e3181f87c4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilleland Marchak J, Seidel KD, Mertens AC, et al. Perceptions of risk of infertility among male survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2018;124:2447–2455. doi: 10.1002/cncr.31343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown MC, Pearce MS, Bailey S, et al. The long-term psychosocial impact of cancer: The views of young adult survivors of childhood cancer. Eur J Cancer Care (Engl) 2016;25:428–439. doi: 10.1111/ecc.12380. [DOI] [PubMed] [Google Scholar]
- 38.Klosky JL, Simmons JL, Russell KM, et al. Fertility as a priority among at-risk adolescent males newly diagnosed with cancer and their parents. Support Care Cancer. 2015;23:333–341. doi: 10.1007/s00520-014-2366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geue K, Richter D, Schmidt R, et al. The desire for children and fertility issues among young German cancer survivors. J Adolesc Health. 2014;54:527–535. doi: 10.1016/j.jadohealth.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for classification and severity-grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26:666–674. doi: 10.1158/1055-9965.EPI-16-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Institute of Alcohol Abuse and Alcoholism Drinking levels defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
- 43.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 45.Syrjala KL, Schroeder TC, Abrams JR, et al. Sexual function measurement and outcomes in cancer survivors and matched controls. J Sex Res. 2000;37:213–225. [Google Scholar]
- 46.Schover LR, Canada AL, Yuan Y, et al. A randomized trial of internet-based versus traditional sexual counseling for couples after localized prostate cancer treatment. Cancer. 2012;118:500–509. doi: 10.1002/cncr.26308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 48.Chemaitilly W, Li Z, Huang S, et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: A report from the St Jude Lifetime Cohort study. J Clin Oncol. 2015;33:492–500. doi: 10.1200/JCO.2014.56.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chemaitilly W, Li Z, Krasin MJ, et al. Premature ovarian insufficiency in childhood cancer survivors: A report from the St Jude Lifetime Cohort Study. J Clin Endocrinol Metab. 2017;102:2242–2250. doi: 10.1210/jc.2016-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11:391–410. doi: 10.1093/humupd/dmi012. [DOI] [PubMed] [Google Scholar]
- 51.Van Buuren S, Brand JP, Groothuis-Oudshoorn CG, et al. Fully conditional specification in multivariate imputation. J Stat Comput Simul. 2006;76:1049–1064. [Google Scholar]
- 52.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 53.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 54.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 55.Brignardello E, Felicetti F, Castiglione A, et al. Endocrine health conditions in adult survivors of childhood cancer: The need for specialized adult-focused follow-up clinics. Eur J Endocrinol. 2013;168:465–472. doi: 10.1530/EJE-12-1043. [DOI] [PubMed] [Google Scholar]
- 56.Medica ACO, Stark SS, Hadnott TN, et al. Use of emergency contraception among female young adult cancer survivors. Fertil Steril. 2018;109:1114–1120.e1. doi: 10.1016/j.fertnstert.2018.02.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubinsak LA, Christianson MS, Akers A, et al. Reproductive health care across the lifecourse of the female cancer patient. Support Care Cancer. 2019;27:23–32. doi: 10.1007/s00520-018-4360-5. [DOI] [PubMed] [Google Scholar]