Abstract
Purpose:
The purpose of our study is to carry out a Bayesian network meta-analysis comparing the efficacy of different antimicrobial dressings for prevention of catheter-related blood infections (CRBSI) and rank these antimicrobial dressings for practical consideration.
Methods:
We searched the PubMed, Cochrane library, Embase, earlier relevant meta-analysis and reference lists of included studies for randomized controlled trials (RCTs) that compared dressings for prevention of CRBSI. Two authors independently extracted data from each included RCT according to a predesigned Excel spreadsheet and assessed the methodological quality of included RCTs using the Cochrane risk of bias tool. Data was analyzed using the WinBUGS (V.1.4.3) and the Stata (V.15.0).
Results:
Finally, 35 RCTs involving 8494 patients and evaluating 13 dressings were included. Network meta-analysis showed that transparent dressing may be the best way to prevent CRBSI. Suture and bordered polyurethane dressing might have the lowest risk of CRBSI rate per 1000 catheter-days, and sutureless securement device might lead to the lowest incidence of catheter failure.
Conclusions:
This network meta-analysis indicated that transparent dressings may be selected for the prevention of CRBSI in patients with central venous catheters, which is of importance in future research. Although evidence is scant, more attention should be paid to head-to-head comparisons of the most commonly used dressings in this field.
Keywords: antimicrobial dressings, catheter-related bloodstream infections, network meta-analysis
1. Introduction
The central venous catheter (CVC) is an essential device for intensive care, patients with cancer, or patients who need parenteral nutrition. However, catheter-related bloodstream infections (CRBSIs), a major complication of CVCs, are associated with significant morbidity, mortality, and additional medical costs.[1–3] In the United States, CRBSI accounts for an estimated 28,000 deaths and up to $2.3 billion annually;[4,5] in 4 European countries (France, Germany, Italy, and the United Kingdom), it accounts for 14,400 deaths with associated annual costs between €35.9 and €163.9 million.[2] In Australia, a case of CRBSI adds at least AU$14,000 (equivalent to $2010) to the cost of care.[6] In China, the average economic loss per case of CRBSI is about ¥30713.[7,8] CRBSI remains an important problem associated with patient safety in high-income, middle-income, and low-income countries.[8–12]
However, most CRBSIs are preventable. Several measures have been implemented to prevent CRBSI, including maximal barrier precautions during catheter insertion, the use of antiseptic agents, catheter impregnation with antiseptic agents or antibiotics, and education and training of health care workers.[13–17] In recent years, the use of antimicrobial dressings has been demonstrated to significantly reduce the risk of catheter infections.[18] Among them, the chlorhexidine gluconate (CHG)-impregnated dressing is the most commonly used; it is simple to use, breathable, transparent, and has a cost comparable to that of standard film dressing currently used on insertion sites.[19] Other dressings, such as the transparent dressing (TD), bordered polyurethane dressing (BPU), and silver alginate dressing (SAD), are also used to prevent catheter infection.[20–23] It has been suggested that CVC antibacterial dressings can inhibit the colonization of microorganisms on the surface of the catheter and prevent them from spreading into the bloodstream.[24]
Traditional pairwise meta-analysis only allows for the comparison of 2 interventions for CRBSI,[25] and most previous meta-analyses compared the CHG-impregnated dressing with the traditional dressing. However, many of these treatment strategies have not been directly compared in previous randomized controlled trials (RCTs). Therefore, it is difficult to determine the most effective treatment based on direct evidence. Network meta-analyses of existing datasets make it possible to comparatively assess the efficacies of antimicrobial CVC dressings in reducing CRBSI, summarize and interpret the broader picture of the evidence base, and understand the relative merits of the multiple interventions.[26] In this study, we aimed to perform a systematic review and network meta-analysis in order to compare the effectiveness of 13 antimicrobial CVC dressings and different securement devices in terms of preventing CRBSIs.
2. Materials and methods
2.1. Search strategy and selection criteria
This systematic review and meta-analysis was designed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement[27] and Cochrane Collaboration reporting project.[28] We included RCTs that compared antimicrobial dressings and different securement devices for the prevention of CRBSI, the CRBSI rate per 1000 catheter-days, and catheter failure. The RCTs included patients from any inpatient hospital setting with a CVC in place. Studies that evaluated patients with catheters in the vein for ≤48 hours and patients with pacing wires were excluded.
We systematically searched PubMed, the Cochrane Library, and Embase from the date of their inception to June 6, 2018. The search strategy was developed by JHT, who has more than 10 years of experience as an information specialist. We also tracked the references in the included articles and relevant systematic reviews/meta-analyses to identify additional relevant studies. The search strategy was as follows: (CHG-impregnated dressing OR transparent dressing OR sterile dry gauze OR hydrocolloid dressing OR bordered polyurethane dressing OR tissue adhesive OR new-generation transparent dressing OR silver alginate dressing OR standard polyurethane transparent dressing OR adherent dressing OR occlusive dressings OR biguanide disc dressing OR integrated securement-dressing) AND (Catheter Related Infection∗ OR Catheter-Related Infection∗) AND (random∗).
As the present meta-analysis was performed based on previous published studies, no ethical approval and patient consent are required.
2.2. Literature selection and data extraction
Literature search records were imported into EndNoteX8 literature management software (Thomson Reuters, New York, NY). Two researchers (F-PD and J-HT) independently reviewed the title and abstract of the studies and excluded those that clearly did not meet the inclusion criteria. Then the remaining studies were identified by reviewing the full text. Any disagreements were resolved by discussion or through consultation with the third independent examiner. Excluded trials and the reasons for their exclusion were listed and examined by a third reviewer (H-JL).
Two authors (F-PD and J-HT) independently extracted data from each included RCT according to a predesigned Excel spreadsheet created in Microsoft Excel 2016 (Microsoft Corp, Redmond, WA). The items extracted contained study's characteristics (author, year of publication, journal, sample size, type of design, study arms, country where the study was conducted), participant's characteristics (age, details of intervention, hospital day of catheter insertion, site, sex ratio), characteristics of dressing (number of dressing, dressing change, the day of dressing) and outcomes. FPD performed the data extraction and entry, and J-HT was in charge of examining the data.
2.3. Risk of bias in individual studies
The methodological risk of bias of included RCTs was assessed according to the Cochrane Handbook Version 5.1.0,[28] including the method of random sequence generation (selection bias), allocation concealment, blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. Those items were evaluated as being high, low, or unclear risk of bias. We resolved any disagreements through discussion and reached a consensus with the third reviewer (H-JL).
2.4. Statistical analysis
The network meta-analysis was performed and presented by the Stata 15.0 using the mvmeta package. The ranking probabilities of all interventions were used to calculate a summary numerical value: the surface under the cumulative ranking curve (SUCRA) which are expressed as percentages. SUCRA value is 100% for the best treatment and 0% for the worst treatment.[29] We assessed the agreement of direct and indirect evidence by an inconsistency plot. The publication bias in the network was assessed by the comparison adjusted funnel plot. Bayesian network meta-analysis was performed in WinBUGS 1.4.3 by using the Markov chain Monte Carlo method. The priors used in 2 different chains were as follows:
chain 1: treatment effect: (d(k) = 0); SD = 1; mu(i) = 0;
chain 2: treatment effect: (d(k) = −1); SD = 4; mu(i) = −3;
where d(k) = treatment effect of experimental intervention “k” compared with reference and mu(i) = treatment effect of the experimental intervention compared with control in the trial “i”. Model fit was determined based on the deviance information criteria for each outcome measure.[30] Two Markov chains were run simultaneously with different arbitrarily chosen initial values. Convergence was found to be adequate when we generated 20,000 simulations for each chain. These simulations were then discarded as “burn-in” and posterior summaries were based on 100,000 subsequent simulations. The model convergence was assessed by trace plots. The results of all outcomes are reported as means of OR with 95% credible interval (CrI).
2.5. Geometry of the network
A network plot was drawn to describe and present the geometry of the treatment network of comparisons across the trials to ensure that a network meta-analysis would be feasible. Trials were excluded if they were not connected by any treatments. In this network plot, nodes represent different interventions, and edges represent the head-to-head comparisons between interventions. The size of the node reflects the sample size of the intervention, and the thickness of the edge reflects the number of included trials.
3. Results
3.1. Study selection
Six-hundred fifty-eight records were identified from electronic databases, of which 534 duplicates were excluded using EndNoteX8 software and 52 articles were determined to be irrelevant after the first screening. Six references in 4 systematic reviews were reviewed. Finally, 35 RCTs involving 8494 patients were included. The flow diagram (Fig. 1) shows the search results and selection details.
Figure 1.
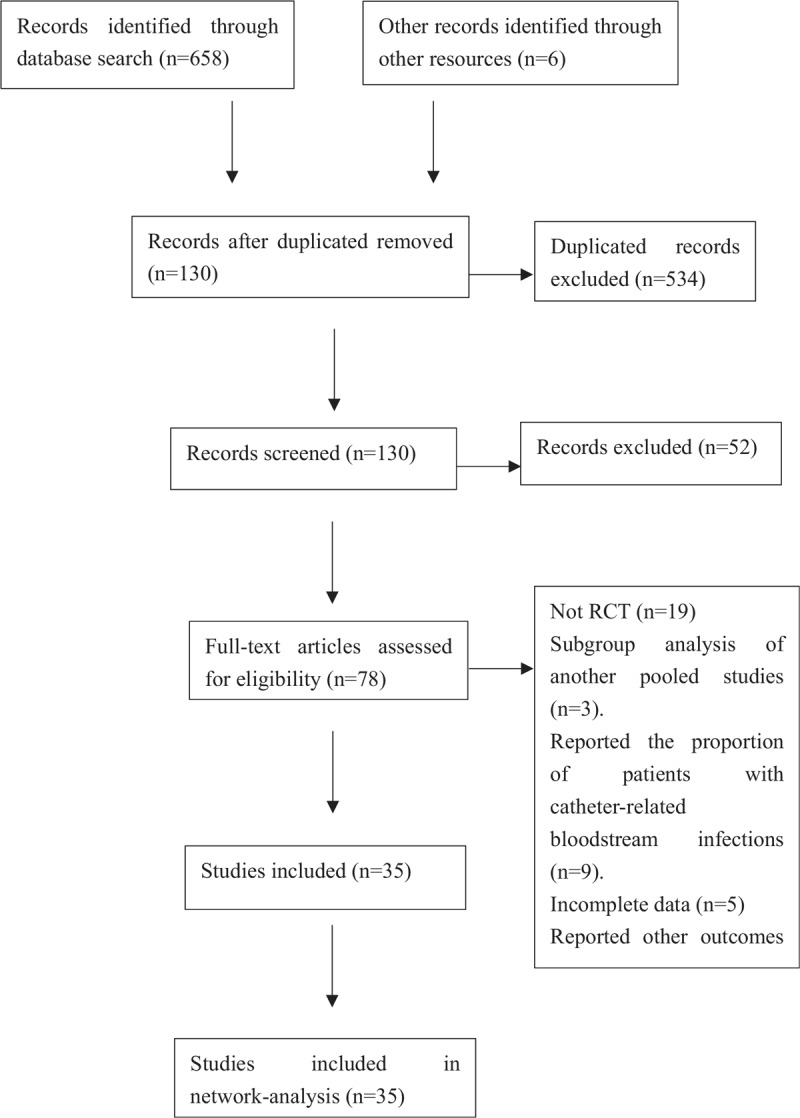
The flow diagrams.
3.2. Characteristics of the included studies
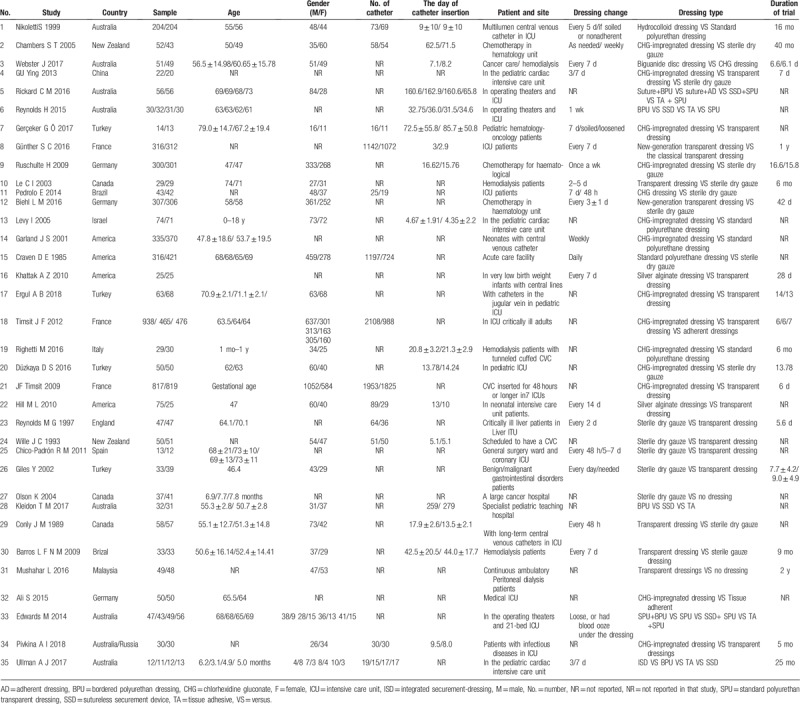
The characteristics of the 35 RCTs are summarized in Table 1. The component studies included 8494 participants. Thirteen kinds of antimicrobial dressings were assessed in the prevention of CRBSI. Of the 35 RCTs, 16 (45.7%) compared the TD with sterile dry gauze (SDG) or other antimicrobial dressings; 13 (37.1%) compared the CHG-impregnated dressing with a standard polyurethane dressing or other antimicrobial dressings; and 22 (62.9%) reported the method of randomization. In the sample population, 4197 (49.4%) of 8494 patients were men. Four (11.4%) of 35 studies randomly assigned participants to 3 or more groups. Of the 35 RCTs, 18 RCTs (5502 participants) studied patients in the intensive care unit, and 8 RCTs (960 participants) studied patients on hemodialysis.
Table 1.
Characteristics of included studies (n = 35).
3.3. Methodological quality of the included studies
Table 2 shows the quality assessment of the studies in this network meta-analysis; the studies with high risk of bias, low risk of bias, and unclear risk of bias are marked in red, green, and yellow, respectively. Twenty-two studies used random sequence generation, and 12 studies used allocation concealment. In 6 studies, the participants, personnel, and outcome assessors were blinded. In 2 trials, the investigators were not blinded, but the outcome assessors were blinded. More than 10% of patients were lost to follow-up, causing high attrition bias. The quality evaluation showed that potential bias was caused by the inadequate random sequence generation and allocation concealment, as well as a lack of blinding of participants and personnel (Fig. 2).
Table 2.
Risk of bias of included studies (n = 35).
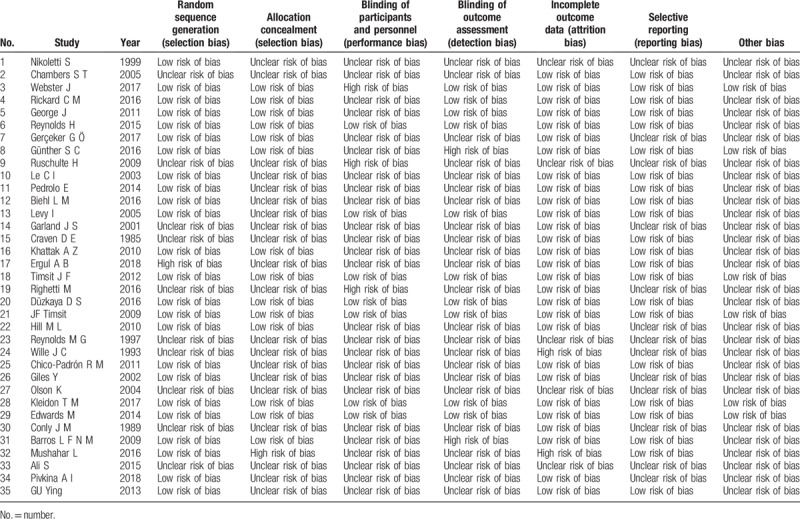
Figure 2.
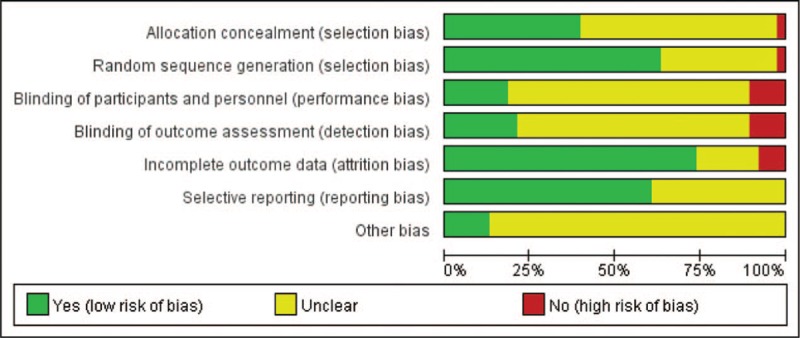
Assessment of risk of bias of the included trials. Note: The number of lost follow-ups greater than 10% of the total number is defined as the risk of high bias when judges the incomplete outcome data (attrition bias).
3.4. Results of the network meta-analysis
Figure 3 shows the network structure of the comparisons among the different interventions for the outcomes. The lines between the intervention nodes indicate the direct comparisons made within randomized trials. The width of the lines is proportional to the number of trials comparing each pair of interventions. The size of each node is proportional to the number of randomly assigned participants (e.g., the sample size). A star geometry is formed between all of these network plots. The missing links between active interventions reflect the lack of direct comparisons.
Figure 3.
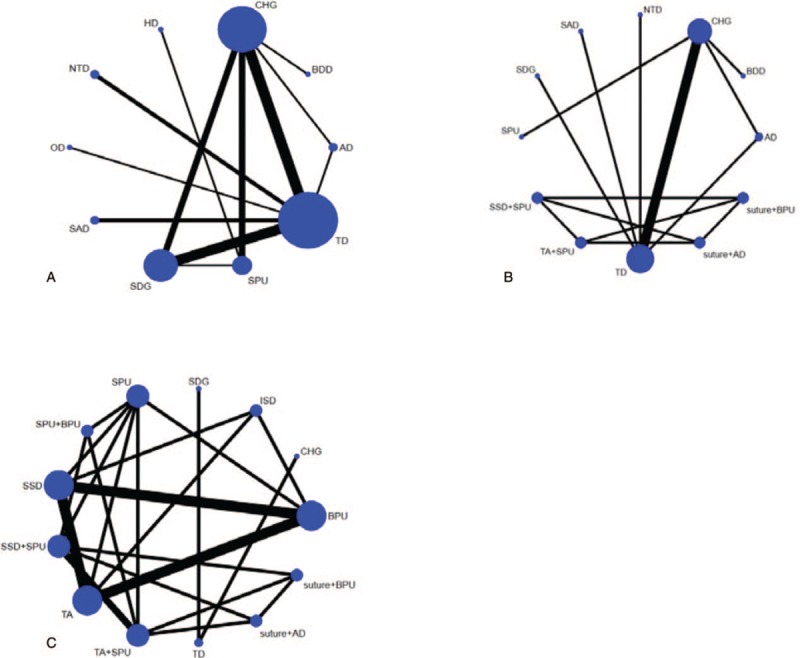
Network plot of evidence for CRBSI (A), CRBSI rate per 1000 days (B), Catheter failure (C). CRBSI = catheter-related blood infections.
3.5. Primary outcome: CRBSI
The results of the network meta-analysis comparing 10 antimicrobial dressings are shown in Fig. 3A. Twenty-five RCTs[19,20,22,23,31–41,44–47,53,54–58] (7090 patients) reported the incidence of CRBSI and included all 10 dressings (CHG, BDD, AD, TD, SPU, SDG, SAD, OD, NTD, and HD). The result indicated that TD (odds ratio [OR] 0.35, 95% CrI 0.14, 0.89) was statistically significantly more effective than the other dressings in preventing CRBSI. The indirect comparison of reported interventions for CRBSI according to WinBUGS 1.4.3 is presented in Table 3 .
Table 3.
The indirect comparison in CRBSI.

3.6. Secondary outcome: CRBSI rate per 1000 catheter-days
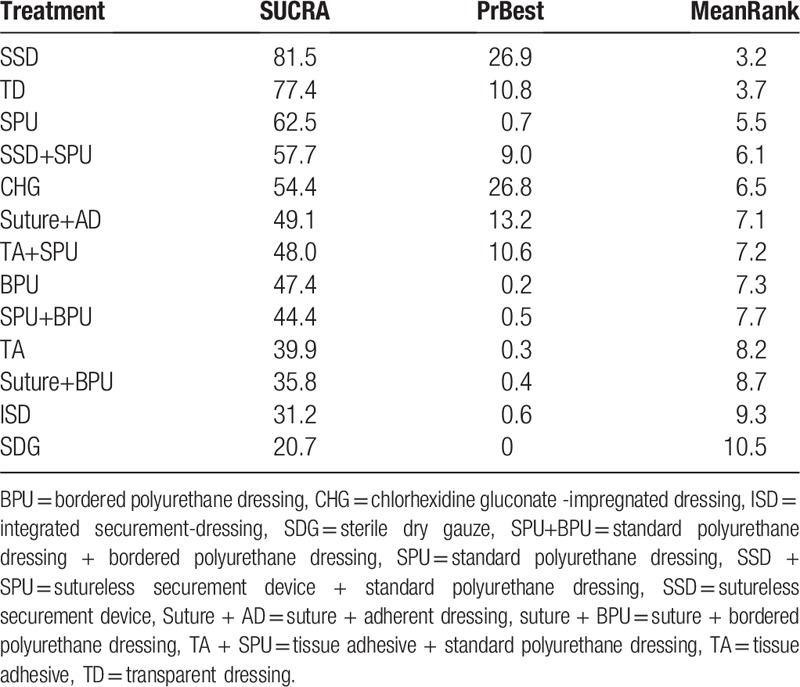
Ten RCTs[19,20,31,39–41,43,49,54,51] (1497 participants) reported the CRBSI rate per 1000 catheter-days. Suture + BPU (OR 0.34, 95% CrI 0.27, 0.43) was statistically significantly more effective than dressings alone in reducing the CRBSI rate per 1000 catheter-days. Moreover, BDD can also obviously reduce the CRBSI rate per 1000 catheter-days when compared with CHG (OR 0.64, 95% CrI 0.43, 0.93). Eight RCTs[20–22,42,48,50,52,59] reported catheter failure. The result indicated that SSD (OR 0.35, 95% CrI 0.14, 0.89) was statistically significantly more effective than other dressings in reducing catheter failure.
3.7. Rank probability
Rank probability analysis (Table 4, Fig. 4) indicated that TD had the highest probability of reduction of incidences of CRBSI (SUCRA = 92.5%), followed by HD (SUCRA = 69.8%) and SAD (SUCRA = 67.4%). Suture + BPU (SUCRA = 62.0%) had the largest probability of being the best treatment in the reduction of the CRBSI rate per 1000 days, followed by BDD (SUCRA = 61.6%) (Table 5, Fig. 5). SSD had the highest probability of being the best treatment in terms of catheter failure (SUCRA = 81.5%), followed by TD (SUCRA = 77.4%). The results of rank probability analysis are presented in Table 6.
Table 3 (Continued).
The indirect comparison in CRBSI.
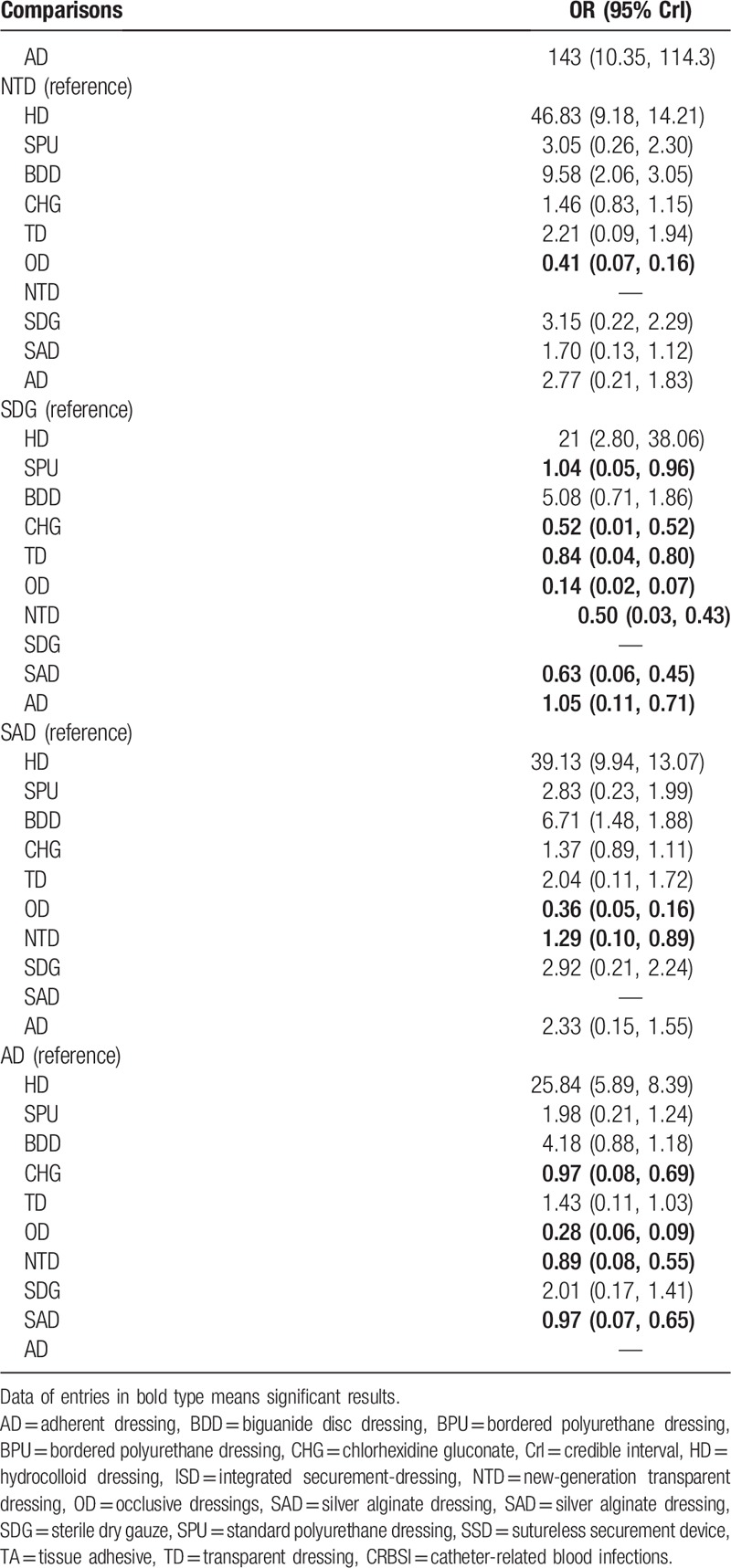
Figure 4.
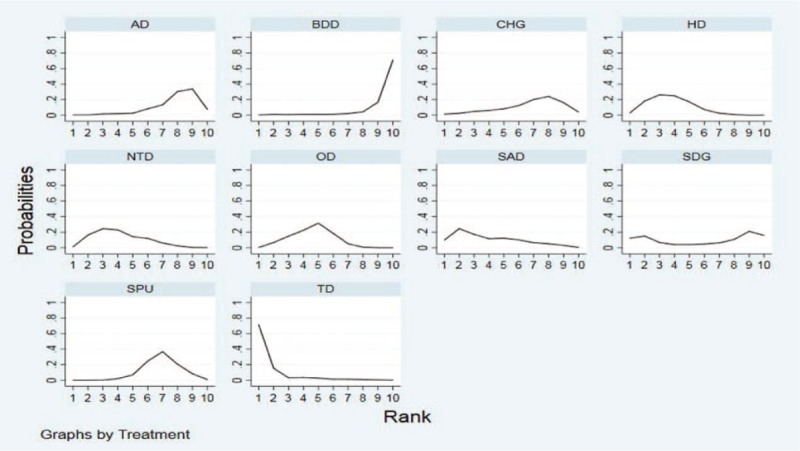
Rank of CRBSI. AD = adherent dressing, BDD = biguanide disc dressing, CHG = chlorhexidine gluconate, HD = hydrocolloid dressing, NTD = new-generation transparent dressing, OD = occlusive dressings, SAD = silver alginate dressing, SDG = sterile dry gauze, SPU = standard polyurethane dressing, TD = transparent dressing, CRBSI = catheter-related blood infections.
Table 4.
Rank probability of CRBSI (SUCRA/%).
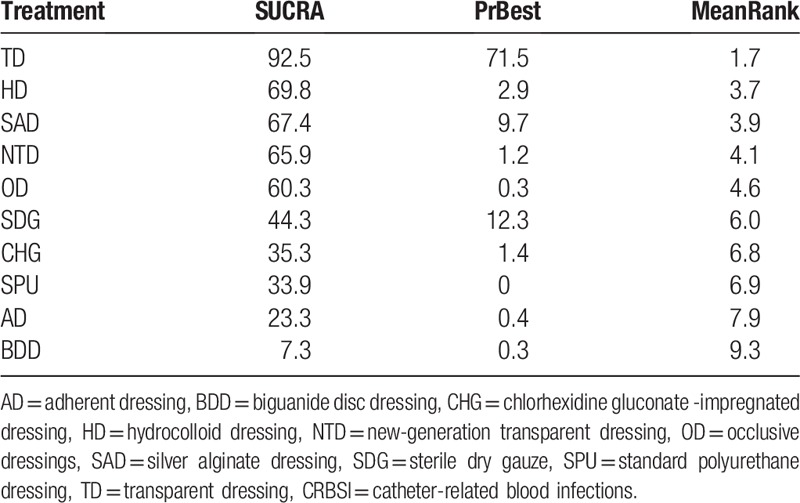
Figure 5.
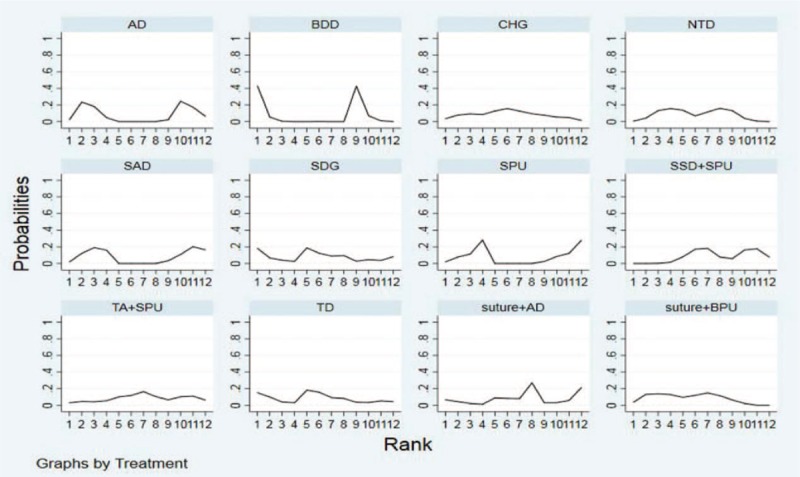
Rank of CRBSI rate per 1000 days. AD = adherent dressing, BDD = biguanide disc dressing, BPU = bordered polyurethane dressing, CHG = chlorhexidine gluconate-impregnated dressing, NTD = new-generation transparent dressing, SAD = silver alginate dressing, SDG = sterile dry gauze, SPU = standard polyurethane dressing, SSD = sutureless securement device, TA = tissue adhesive, TD = transparent dressing, CRBSI = catheter-related blood infections.
Table 5.
Rank probability of CRBSI rate per 1000 days (SUCRA/%).
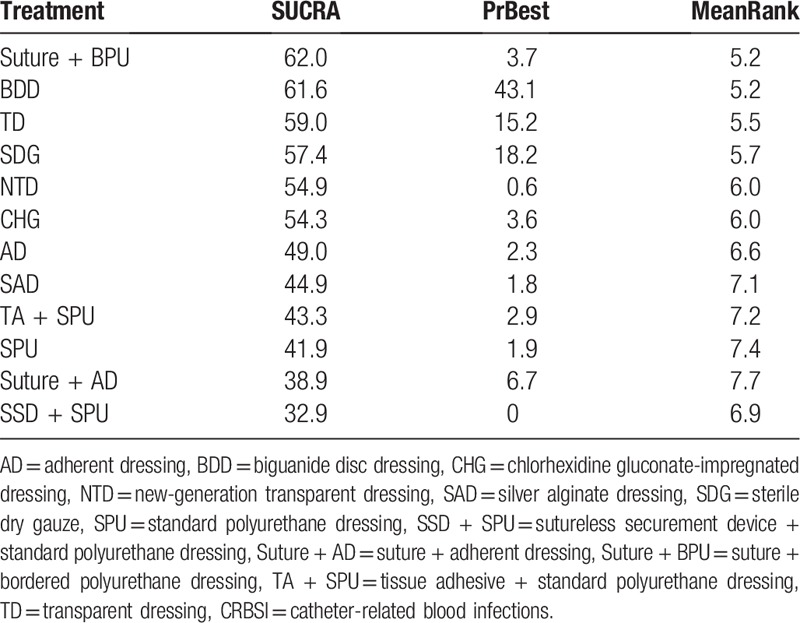
Table 6.
Rank probability of catheter failure (SUCRA/%).
3.8. Publication bias
We drew comparison-adjusted funnel plots for all outcomes (Fig. 6). Different colors correspond to different comparisons. The results showed that the probability of publication bias was very small for the included studies.
Figure 6.
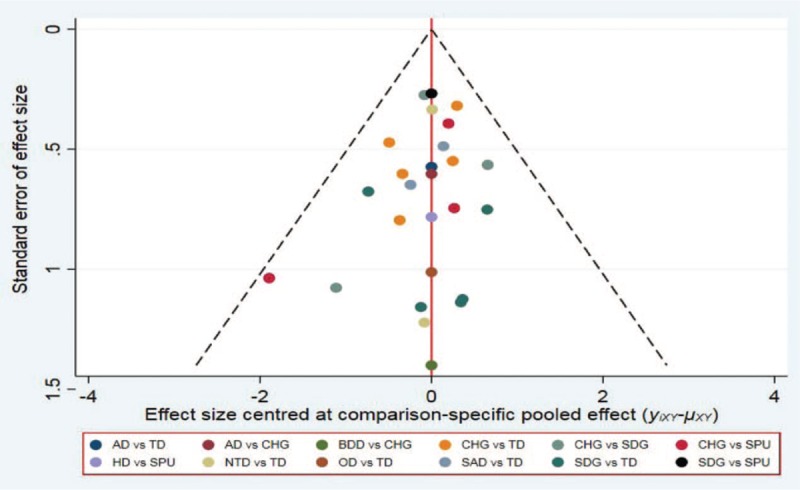
Comparison-adjusted funnel plot of CRBSI. CRBSI = catheter-related blood infections.
4. Discussion
Our network meta-analysis collected the currently available RCTs to assess the effectiveness of 13 antimicrobial CVC dressings and different securement devices for the prevention of CRBSI. According to the results of the rank probability analysis, TD appeared to be the most effective in reducing CRBSI among the 13 antimicrobial CVC dressings. In this network meta-analysis, no significant difference in the intervention effect was detected with respect to the CRBSI rate per 1000 days or catheter failure for all included dressings. Based on the results of the rank probability analysis, suture + BPU was associated with the lowest CRBSI rate per 1000 days, and SSD was associated with the lowest catheter failure. When the differences in the effect size of different treatments are small, the clinical decision about the choice of treatments can be recommended based on the results of probability ranking.[60]
In this network meta-analysis, 35 RCTs were included and involved 8494 patients, and 13 antimicrobial dressings were assessed for the prevention of CRBSI. In the treatment of CRBSI, the result of the indirect comparison indicated that HD was better than SPU, BDD, CHG, TD, OD, NTD, SDG, SAD, and AD. SPU was better than CHG, TD, OD, NTD, SAD, and AD. BDD was better than CHG, TD, OD, and NTD. CHG was better than TD, OD, NTD, and SAD. TD was better than CHG, OD, NTD, SAD, and AD. NTD was better than OD, and SDG was better than SPU, CHG, TD, OD, SAD, and AD. SAD has advantages over OD and NTD, whereas AD has advantages over CHG, OD, and SAD. These differences were statistically significant.
As a commonly used antiseptic agent, CHG has been used in intensive care units (ICU) for routine daily care, and its use has been shown to decrease the incidence of CRBSI.[61–63] Some studies have shown that CHG-impregnated dressings reduce the incidence of CRBSI.[62,64,65] The study by Karpanen suggested that CHG-impregnated dressings have detectable antimicrobial activity for up to 7 days, and the sustained release of CHG from the dressing increases with time, which may reduce the microbial load at the catheter insertion site, thereby reducing the risk of CRBSI.[64] However, these studies only compared 2 dressings. Based on the largest evidence and network meta-analysis of antimicrobial CVC dressings, our findings indicated that TD is the most effective in preventing CRBSI, which may also be of benefit with arterial catheters.[42]
Recently, Chong et al[66] performed a network meta-analysis to assess the comparative efficacy of antimicrobial CVC impregnations in reducing CRBSI in adults, and they included 60 studies with 17,255 catheters. Their study suggested that the minocycline-rifampicin-impregnated CVC is most effective for preventing CRBSI. In addition, the placement of dressings is important. In our network meta-analysis, we found that suture combined with bordered polyurethane dressing (similar to SPU dressings but with a tough, adhesive fabric border) had the largest probability of being the best treatment in the CRBSI rate per 1000 catheter-days than dressings alone. Therefore, combining 2 or more dressings and securement devices might be considered a more rational and optimal approach to reducing the CRBSI rate per 1000 catheter-days.
One study[42] confirmed that catheter failure is an ongoing, significant problem, and that antimicrobial dressings and securement devices are priority areas for improvement. Recently, in the study by Roethlisberger et al,[67] the dressing was stapled to the skin along its edges with a surgical stapler to minimize the risk of detachment and dislocation.
The trial by Chan et al[68] compared standard care with 3 innovative dressing and securement methods in 121 adult patients receiving acute care. Their findings also showed that securement devices combined with dressings are superior to standard care alone in terms of CRBSI, the catheter failure rate, product costs, and patient and staff satisfaction. Those results are similar to our study's findings. Our network meta-analysis showed that SSD might lead to the lowest incidence of catheter failure, followed by TD, SPU, and SSD + SPU. However, a 4-arm randomized controlled trial[21] with 123 patients in the operating theater and intensive care found that the application of SSD was harder than other interventions. SSD requires a multistep procedure to apply, which likely led to a longer application time compared with the other interventions, and lower satisfaction ratings. Therefore, improving the application of SSD in the future is very important.
The methodological quality of the included RCTs was moderate to high. The sample sizes of the included RCTs ranged from 25 to 1879 patients. One study indicated that small to moderately sized trials have stronger effect estimates than larger trials.[69] Therefore, we used a comparison-adjusted funnel plot to assess the bias of small-study effects. The results showed that the probability of bias of the included studies was low.
There were some limitations in our study. First, a number of the meta-analyses published focused on the efficacy and safety of different dressings for CRBSI.[27–29] However, it has been difficult to directly compare the clinical efficacy among dressings in RCTs and traditional meta-analysis. Second, there is either a high or an unclear risk of bias for 1 or more of the quality elements we assessed, and the included trials had wide confidence intervals. Third, the number of included studies and the sample size were small. In particular, the comparisons of HD and SPU, BDD and CHG, as well as OD and TD were performed based on only one RCT, so the potential for bias should be considered. Finally, reporting of the included studies themselves was incomplete. The majority of the trials failed to specify the method of randomization, use appropriate allocation concealment procedures, and ensure blinding of relevant personnel, which are methodological limitations that may affect the results. However, our study also had strength. To the best of our knowledge, this is the first network meta-analysis to comprehensively investigate various outcomes of different dressings and securement devices to reduce the incidence of CRBSI.
5. Conclusion
We compared 13 antimicrobial dressings for preventing CRBSIs through a Bayesian network meta-analysis. The use of this method has enabled us to provide new information on the relative effectiveness of antimicrobial dressings for the management of CRBSIs and catheter failure. Based on the results of the network meta-analysis and probability ranking analysis, TD may be the best way to prevent CRBSIs, a sutureless securement device might lead to the lowest incidence of catheter failure, and suture and bordered polyurethane dressing might have the lowest CRBSI rate per 1000 catheter-days. Therefore, this network meta-analysis is important for future research, and it highlights the need for properly designed RCTs and more head-to-head comparisons of the most commonly used dressings in this field. Currently, there is scant evidence, mainly indirect, and mostly from small trials with a risk of unclear bias.
Author contributions
Data curation: fangping dang.
Formal analysis: Jin-Hui Tian.
Funding acquisition: Hui-Ju Li.
Methodology: Jin-Hui Tian.
Project administration: Hui-Ju Li.
Software: fangping dang, Jin-Hui Tian.
Supervision: Jin-Hui Tian.
Validation: Jin-Hui Tian.
Visualization: Jin-Hui Tian.
Writing – original draft: fangping dang.
Writing – review & editing: fangping dang.
Footnotes
Abbreviations: AD = adherent dressing, BDD = biguanide disc dressing, BPU = bordered polyurethane dressing, CHG = chlorhexidine gluconate, CrI = credible interval, CVC = central venous catheter, HD = hydrocolloid dressing, ICU = intensive care unit, ISD = integrated securement-dressing, NTD = new-generation transparent dressing, OD = occlusive dressings, OR = odds ratio, RCTs = randomized controlled trials, SAD = silver alginate dressing, SDG = sterile dry gauze, SPU = standard polyurethane dressing, SSD = sutureless securement device, SUCRA = the surface under the cumulative ranking curve, TA = tissue adhesive, TD = transparent dressing.
The authors have no conflicts of interest to disclose.
References
- [1].Olaechea PM, Palomar M, Álvarez-Lerma F, et al. Morbidity and mortality associated with primary and catheter-related bloodstream infections in critically ill patients. Rev Esp Quimioter 2013;26:21–9. [PubMed] [Google Scholar]
- [2].Tacconelli E, Smith G, Hieke K, et al. Epidemiology, medical outcomes and costs of catheter-related bloodstream infections in intensive care units of four European countries: literature- and registry-based estimates. J Hosp Infect 2009;72:97–103. [DOI] [PubMed] [Google Scholar]
- [3].Barnett AG, Page K, Campbell M, et al. The increased risks of death and extra lengths of hospital and ICU stay from hospital-acquired bloodstream infections: a case-control study. BMJ Open 2013;3:e003587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dudeck MA, Edwards JR, Allenbridson K, et al. National Healthcare Safety Network report, data summary for 2013, Device-associated Module. Am J Infect Control 2015;43:206–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sagana R, Hyzy RC. Achieving zero central line–associated bloodstream infection rates in your intensive care unit. Crit Care Clin 2013;29:1–9. [DOI] [PubMed] [Google Scholar]
- [6].Halton KA, Cook D, Paterson DL, et al. Cost-effectiveness of a central venous catheter care bundle. PLos One 2010;5:2742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu Y, Cao Y, Zhou C, et al. Case-control study on economic loss of central catheter-related blood flow infection. Chin Hosp J Infect Sci 2018;28:2615–7. [Google Scholar]
- [8].Peng S, Lu Y. Clinical epidemiology of central venous catheter-related bloodstream infections in an intensive care unit in China. J Crit Care 2013;28:277–83. [DOI] [PubMed] [Google Scholar]
- [9].Rosenthal VD, Maki DG, Salomao R, et al. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Ann Int Med 2006;145:582. [DOI] [PubMed] [Google Scholar]
- [10].Leblebicioglu H, Erben N, Rosenthal VD, et al. International Nosocomial Infection Control Consortium (INICC) national report on device-associated infection rates in 19 cities of Turkey, data summary for 2003-2012. Ann Clin Microbiol Antimicrob 2014;13:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wa DSP, Algra A, Mf VDB, et al. Systematic literature review and meta-analysis of the burden of healthcare-associated infections (HAI) in Southeast Asia. Clin Infect Dis 2015;60:614–9. [DOI] [PubMed] [Google Scholar]
- [12].Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. Am J Infect Control 2008;36:2725–32. [DOI] [PubMed] [Google Scholar]
- [13].Hu KK, Lipsky BA, Veenstra DL, et al. Using maximal sterile barriers to prevent central venous catheter–related infection: a systematic evidence-based review. Am J Infect Control 2004;32:142–6. [DOI] [PubMed] [Google Scholar]
- [14].Chaiyakunapruk N, Veenstra DL, Lipsky BA, et al. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Acc Curr J Rev 2002;136:792. [DOI] [PubMed] [Google Scholar]
- [15].Gnass SA, Barboza L, Bilicich D, et al. Prevention of central venous catheter–related bloodstream infections using non-technologic strategies. Infect Control Hosp Epidemiol 2004;25:675–7. [DOI] [PubMed] [Google Scholar]
- [16].Falagas ME, Fragoulis K, Bliziotis IA, et al. Rifampicin-impregnated central venous catheters: a meta-analysis of randomized controlled trials. J Antimicrob Chemother 2007;59:359–69. [DOI] [PubMed] [Google Scholar]
- [17].Musu M, Finco G, Mura P, et al. Controlling catheter-related bloodstream infections through a multi-centre educational programme for intensive care units. J Hosp Infect 2017;97:275–81. [DOI] [PubMed] [Google Scholar]
- [18].Kawamura H, Takahashi N, Takahashi M, et al. Differences in microorganism growth on various dressings used to cover injection sites: inspection of the risk of catheter-related bloodstream infections caused by gram-negative bacilli. Surg Today 2014;44: 2339–2344. [DOI] [PubMed] [Google Scholar]
- [19].Webster J, Larsen E, Marsh N, et al. Chlorhexidine gluconate or polyhexamethylene biguanide disc dressing to reduce the incidence of central-line-associated bloodstream infection: a feasibility randomized controlled trial (the CLABSI trial). J Hosp Infect 2017;96:223–8. [DOI] [PubMed] [Google Scholar]
- [20].Günther SC, Schwebel C, Hamidfar-Roy R, et al. Complications of intravascular catheters in ICU: definitions, incidence and severity. A randomized controlled trial comparing usual transparent dressings versus new-generation dressings (the ADVANCED study). Int Care Med 2016;42:1753–65. [DOI] [PubMed] [Google Scholar]
- [21].Reynolds H, Taraporewalla K, Tower M, et al. Novel technologies can provide effective dressing and securement for peripheral arterial catheters: a pilot randomised controlled trial in the operating theatre and the intensive care unit. Aust Crit Care 2015;28:140–8. [DOI] [PubMed] [Google Scholar]
- [22].Hill ML, Baldwin L, Slaughter JC, et al. A silver-alginate-coated dressing to reduce peripherally inserted central catheter (PICC) infections in NICU patients: a pilot randomized controlled trial. J Perinatol 2010;30:469–73. [DOI] [PubMed] [Google Scholar]
- [23].Kleidon TM, Ullman AJ, Gibson V, et al. A pilot randomized controlled trial of novel dressing and securement techniques in 101 pediatric patients. J Vasc Interv Radiol 2017;28:1548.e1–56.e1. [DOI] [PubMed] [Google Scholar]
- [24].O’Grady N, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011;52:e162–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Safdar N, O’Horo JC, Ghufran A, et al. Chlorhexidine-impregnated dressing for prevention of catheter-related bloodstream infection: a meta-analysis. Crit Care Med 2014;42:1703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018;391:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Int Med 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [28].Higgins JPT, Green S. Cochrance handbook for systematic reviews of interventions version 5.1.0 [EB/OL]. The Cochrane Collaboration, 2011, Available at: http://www.cochrane-handbook.org (accessed May 16, 2013). [Google Scholar]
- [29].Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [30].Shriner D, Yi N. Deviance information criterion (DIC) in Bayesian multiple QTL mapping. Comput Stat Data Anal 2009;53:1850–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gerçeker GÖ, Yardimci F, Aydinok Y. Randomized controlled trial of care bundles with chlorhexidine dressing and advanced dressings to prevent catheter-related bloodstream infections in pediatric hematology-oncology patients. Eur J Oncol Nurs 2017;28:14–20. [DOI] [PubMed] [Google Scholar]
- [32].Wille JC, Blussé vOAA, Thewessen EA. A comparison of two transparent film-type dressings in central venous therapy. J Hosp Infect 1993;23:113–21. [DOI] [PubMed] [Google Scholar]
- [33].Pedrolo E, Danski MTR, Vayego SA. Chlorhexidine and gauze and tape dressings for central venous catheters: a randomized clinical trial. Rev Lat Am Enfermagem 2014;22:764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Biehl LM, Huth A, Panse J, et al. A randomized trial on chlorhexidine dressings for the prevention of catheter-related bloodstream infections in neutropenic patients. Ann Oncol 2016;27:1916–22. [DOI] [PubMed] [Google Scholar]
- [35].Levy I, Katz J, Solter E, et al. Chlorhexidine-impregnated dressing for prevention of colonization of central venous catheters in infants and children: a randomized controlled study. Pediatr Infect Dis J 2005;24:676–9. [DOI] [PubMed] [Google Scholar]
- [36].Garland JS, Alex CP, Mueller CD, et al. A randomized trial comparing povidone-iodine to a chlorhexidine gluconate-impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics 2001;107:1431–6. [DOI] [PubMed] [Google Scholar]
- [37].Craven DE, Lichtenberg DA, Kunches LM, et al. A randomized study comparing a transparent polyurethane dressing to a dry gauze dressing for peripheral intravenous catheter sites. Infect Control Hosp Epidemiol 1985;6:361–6. [DOI] [PubMed] [Google Scholar]
- [38].Ergul AB, Gokcek I, Ozcan A, et al. Use of a chlorhexidine-impregnated dressing reduced catheter-related bloodstream infection caused by Gram-positive microorganisms. Pak J Med Sci 2018;34:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Timsit JF, Mimoz O, Mourvillier B, et al. Randomized controlled trial of chlorhexidine dressing and highly adhesive dressing for preventing catheter-related infections in critically ill adults. Am J Respir Crit Care Med 2012;186:1272–8. [DOI] [PubMed] [Google Scholar]
- [40].Righetti M, Palmieri N, Bracchi O, et al. Tegaderm™ CHG dressing significantly improves catheter-related infection rate in hemodialysis patients. J Vasc Access 2016;17:417–22. [DOI] [PubMed] [Google Scholar]
- [41].Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. J Am Med Assoc 2009;53:247–8. [DOI] [PubMed] [Google Scholar]
- [42].Edwards M, Rickard CM, Rapchuk I, et al. A pilot trial of bordered polyurethane dressings, tissue adhesive and sutureless devices compared with standard polyurethane dressings for securing short-term arterial catheters. Crit Care Resuscitation 2014;16:175–83. [PubMed] [Google Scholar]
- [43].Khattak AZ, Ross R, Ngo T, et al. A randomized controlled evaluation of absorption of silver with the use of silver alginate (Algidex) patches in very low birth weight (VLBW) infants with central lines. J Perinatol 2010;30:337–42. [DOI] [PubMed] [Google Scholar]
- [44].Düzkaya DS, Sahiner NC, Uysal G, et al. Chlorhexidine-impregnated dressings and prevention of catheter-associated bloodstream infections in a pediatric intensive care unit. Crit Care Nurse 2016;36:e1. [DOI] [PubMed] [Google Scholar]
- [45].Chico-Padrón RM, Carrión-García L, Delle-Vedove-Rosales L, et al. Comparative safety and costs of transparent versus gauze wound dressings in intravenous catheterization. J Nurs Care Qual 2011;26:371–6. [DOI] [PubMed] [Google Scholar]
- [46].Reynolds MG, Tebbs SE, Elliott TSJ. Do dressings with increased permeability reduce the incidence of central venous catheter related sepsis? Intens Crit Care Nurs 1997;13:26–9. [DOI] [PubMed] [Google Scholar]
- [47].Ruschulte H, Franke M, Gastmeier P, et al. Prevention of central venous catheter related infections with chlorhexidine gluconate impregnated wound dressings: a randomized controlled trial. Ann Hematol 2009;88:267–72. [DOI] [PubMed] [Google Scholar]
- [48].Ali SM, Koul SS, Memon MI, et al. Comparison of chlorhexidine-based dressing versus simple occlusive dressing in preventing catheter related bloodstream infections at medical ICU in a resource constraint setting. Intens Care Med Exp 2015;3:A812. [Google Scholar]
- [49].Rickard CM, Edwards M, Spooner AJ, et al. A 4-arm randomized controlled pilot trial of innovative solutions for jugular central venous access device securement in 221 cardiac surgical patients. J Crit Care 2016;36:35–42. [DOI] [PubMed] [Google Scholar]
- [50].Mushahar L, Mei LW, Yusuf WS, et al. Exit-site dressing and infection in peritoneal dialysis: a randomized controlled pilot trial. Perit Dial Int 2016;36:135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gu Y, Yuan J, Hu J, et al. Comparison of the effects and costs of three kinds of application in venous central venous maintenance. Chin J Nurs 2013;48:510–3. [Google Scholar]
- [52].Giles Y, Aksoy M, Tezelman S. What really affects the incidence of central venous catheter-related infections for short-term catheterization. Acta Chir Belg 2002;102:256–8. [DOI] [PubMed] [Google Scholar]
- [53].Nikoletti S, Leslie G, Gandossi S, et al. A prospective, randomized, controlled trial comparing transparent polyurethane and hydrocolloid dressings for central venous catheters. Am J Infect Control 1999;27:488–96. [DOI] [PubMed] [Google Scholar]
- [54].Pivkina AI, Gusarov VG, Blot SI, et al. Effect of an acrylic terpolymer barrier film beneath transparent catheter dressings on skin integrity, risk of dressing disruption, catheter colonisation and infection. Intens Crit Care Nurs 2018;46:17–23. [DOI] [PubMed] [Google Scholar]
- [55].Barros LFNM, Arênas VG, Bettencourt ARC, et al. Evaluation of two types of dressings used on central venous catheters for hemodialysis. Acta Paulista Enfermagem 2009;22:481–6. [Google Scholar]
- [56].Olson K, Rennie RP, Hanson J, et al. Evaluation of a no-dressing intervention for tunneled central venous catheter exit sites. J Infusion Nurs 2004;27:37–44. [DOI] [PubMed] [Google Scholar]
- [57].Le Corre I, Delorme M, Cournoyer S. A prospective, randomized trial comparing a transparent dressing and a dry gauze on the exit site of long term central venous catheters of hemodialysis patients. J Vasc Access 2003;4:56–61. [PubMed] [Google Scholar]
- [58].Ullman AJ, Cooke ML, Mitchell M, et al. Dressings and securement devices for central venous catheters (CVC). Cochrane Database Syst Rev 2015;20139:CD010367.1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Conly JM, Grieves K, Peters B. A prospective, randomized study comparing transparent and dry gauze dressings for central venous catheters. J Infect Dis 1989;159:310–9. [DOI] [PubMed] [Google Scholar]
- [60].Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Statist Sci 1992;7:457–72. [Google Scholar]
- [61].Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis 2008;46:274–81. [DOI] [PubMed] [Google Scholar]
- [62].Ho KM, Litton E. Use of chlorhexidine-impregnated dressing to prevent vascular and epidural catheter colonization and infection: a meta-analysis. J Antimicrob Chemother 2006;58:281–7. [DOI] [PubMed] [Google Scholar]
- [63].Loewenthal M, Dobson P, Boyle M. Chlorhexidine 2% and choice of transparent dressing increase skin reactions at central venous catheter insertion sites. Am J Infect Control 2016;44:1712–4. [DOI] [PubMed] [Google Scholar]
- [64].Marsh N, Webster J, Mihala G, et al. Devices and dressings to secure peripheral venous catheters: a Cochrane systematic review and meta-analysis. Int J Nurs Stud 2017;67:12–9. [DOI] [PubMed] [Google Scholar]
- [65].Scheithauer S, Lewalter K, Schröder J, et al. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection 2014;42:155–9. [DOI] [PubMed] [Google Scholar]
- [66].Chong HY, Lai NM, Apisarnthanarak A, et al. Comparative efficacy of antimicrobial central venous catheters in reducing catheter-related bloodstream infections in adults: abridged Cochrane systematic review and network meta-analysis. Clin Infect Dis 2017;64suppl_2:S131–40. [DOI] [PubMed] [Google Scholar]
- [67].Roethlisberger M, Moffa G, Fisch U, et al. Effectiveness of a chlorhexidine dressing on silver-coated external ventricular drain associated colonization/infection-a prospective single-blinded randomized controlled clinical trial (EVDAI-study). Clin Infect Dis 2018;67:1868–77. [DOI] [PubMed] [Google Scholar]
- [68].Chan RJ, Northfield S, Larsen E, et al. Central venous Access device Securement And Dressing Effectiveness for peripherally inserted central catheters in adult acute hospital patients (CASCADE): a pilot randomised controlled trial. Trials 2017;18:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dechartres A, Trinquart L, Boutron I, et al. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ 2013;346:f2304. [DOI] [PMC free article] [PubMed] [Google Scholar]


