Abstract
Bloodstream infection caused by Acinetobacter baumannii has become a major clinical concern, especially multidrug-resistant A baumannii (MDRAB). The aim of this study was to identify the risk factors of nosocomial acquired MDRAB bacteremia and to determine the risk factors related to the mortality of patients with MDRAB bacteremia. Patients with nosocomial acquired A baumannii bacteremia were enrolled between January, 2013 and December, 2017 at the First Affiliated Hospital, School of Medicine, Zhejiang University. Medical records were reviewed, and the clinical and microbial characteristics were collected. Among the 338 patients suffering from A baumannii bacteremia, 274 patients were infected with MDRAB bacteremia. Bacteremia-related mortality was 46.4% for the overall sample; 56.2% for MDRAB bacteremia patients, 4.7% for non-MDRAB bacteremia patients. The identified risk factors for developing MDRAB bacteremia were previous exposure to carbapenems [odds ratio (OR) 5.78, P = .005] and penicillins+β-lactamase inhibitors (OR 4.29, P = .009). Primary bacteremia tended to develop non-MDR bacteremia (OR 0.10, P = .002). The risk factors for MDRAB bacteremia-related mortality were old age (OR 1.02, P = .036), a high Pitt bacteremia score (OR 1.32, P < .001), bacteremia occurring after severe pneumonia (OR 8.66, P < .001), while catheter-related infection (OR 0.47, P = .049) and operations for treating infection (OR 0.51, P = .043) may have a better outcome. Patients with MDRAB had a higher mortality rate. Patients with previous carbapenems and penicillins+β-lactamase inhibitor exposure are at an increased risk of MDRAB bacteremia, whereas patients with primary bacteremia tended to develop non-MDR bacteremia. The risk factors for MDRAB bacteremia-related mortality were old age, a high Pitt bacteremia score, and bacteremia occurring after severe pneumonia, whereas catheter-related infection and operations for the treatment of infection may have a better outcome.
Keywords: Acinetobacter baumannii, bacteremia, MDRAB, multidrug-resistant, risk factors
1. Introduction
Bloodstream infection caused by Acinetobacter baumannii has become a major concern in the clinic.[1,2] The propensity for antimicrobial resistance in A baumannii results in the spread of multidrug-resistant (MDR) phenotypes,[3–5] which may lead to a lack of effective therapeutics,[6] long hospital stay,[7] and high rates of mortality.[8,9] Some studies have reported risk factors associated with MDR acquisition in A baumannii bacteremia,[8,10,11] including the host's condition, prior antimicrobial drug exposure (especially broad-spectrum antibiotics), previous colonization with A baumannii, increased Pitt bacteremia score, being in the intensive care unit (ICU), and recent invasive procedures. The risk factors of mortality of A baumannii bacteremia have been reported in different parts of the world in recent years,[8–10,12,13] including old age, neutropenia, malignancy, surgery before bacteremia, being post-transplantation, severity of illness defined by Pitt bacteremia score or Acute Physiology and Chronic Health Evaluation II score, ICU stay, having a lower level of albumin, respiratory tract as the origin of bacteremia, and inappropriate initial antimicrobial therapy.
For the purpose of prevention and effective treatment of MDR A baumannii (MDRAB) bacteremia, the clinical features, epidemiology, and outcomes of MDRAB bacteremia in our hospital should be reviewed and analyzed. The aim of this study was to identify the risk factors of nosocomial acquired MDRAB bacteremia and to determine the risk factors related to the mortality of patients with MDRAB bacteremia.
2. Methods
2.1. Study design and patient population
This study retrospectively reviewed consecutive in-patients with A baumannii bacteremia between January 1, 2013 and December 31, 2017 at the First Affiliated Hospital, School of Medicine, Zhejiang University, a 2000-bed referral hospital in Hangzhou, China. Adult inpatients hospitalized >3 days with bacteremia due to A baumannii and having symptoms and signs of infection were included in the study. For patients with ≥2 positive blood cultures, only the first episode was selected. No patient was included twice in the study. Patients with positive culture results considered to be due to contaminants as recorded in the case notes were excluded.
2.2. Data collection and definition
Medical records were reviewed, and the data on the following parameters were collected: patient characteristics, underlying diseases, primary admission diagnosis, prior exposure to antimicrobial agents, previous immunosuppressant use, previous corticosteroid use, invasive procedure use, source of bacteremia, whether the patient was in the ICU at the time of onset of bacteremia, the patients’ Pitt bacteremia score, treatment after onset of bacteremia, 7-day mortality, 14-day mortality, 28-day mortality, and A baumannii bacteremia-related mortality.
The onset of bacteremia was defined as the day when the blood culture that eventually grew A baumannii was obtained. Chronic lung diseases included chronic obstructive pulmonary disease, bronchiectasis, pulmonary fibrosis, and old pulmonary tuberculosis.[14,15] Chronic kidney disease was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2.[9] Prior exposure to antimicrobial agents was defined as antibiotics for at least 72 hours within a 14-day period before the onset of bacteremia.[2,9] Treatment with other recognized T-cell immunosuppressants, such as cyclosporine, tumor necrosis factor (TNF)-α blockers, specific monoclonal antibodies (such as alemtuzumab), or nucleoside analogs in the 30-day period before the onset of bacteremia was defined as previous immunosuppressant use.[16] Previous corticosteroid use was defined as the use of corticosteroids at a mean minimum dose of 0.3 mg/kg/d of prednisone equivalent for at least 72 hours within a 30-day period before the onset of bacteremia.[16] The source of bacteremia was clarified according to the Centers for Disease Control (CDC) definitions for nosocomial infections (1988).[17] A catheter-associated bacteremia was defined according to the United States Centers for Disease Control and Prevention guidelines.[18] The Pitt bacteremia score was used to assess the severity of acute illness.[19] An appropriate antimicrobial therapy was defined as the administration of at least q antimicrobial agent for at least 72 hours, to which a pathogen was sensitive according to susceptibility tests, within 72 hours of onset of bacteremia, with an approved route and dosage appropriate for end-organ function.[9] Cefoperazone-Sulbactam therapy was defined as intravenous Cefoperazone-Sulbactam (1:1) treatment for at least 72 hours, within 72 hours of onset of bacteremia, with a dosage of at least 2 g every 8 hours. Pneumonia was defined with a confirmatory chest radiograph indicating a new infiltrate, and severe pneumonia was diagnosed according to previous definition: all cases of ventilator-associated pneumonia, requirement for ICU admission, need for vasopressor support, and need for ventilatory support (either invasive or noninvasive).[20] Operations for treating infection include drainage of infection sites, removal or replacement of catheters, and surgical debridement.
Clinical cure and microbiological eradication were measured at 7 days and 14 days after breakthrough A baumannii bacteremia. The evaluation of clinical response was made on the basis of resolution of clinical signs and symptoms, including fever, leukocytosis, C-reactive protein level, and improvement of radiological imagine.[21] Clinical cure was defined as the resolution of presenting symptoms and signs of infection. Clinical improvement was defined as partial improvement of presenting symptoms and signs of infection. Clinical failure was defined as persistence or worsening of presenting symptoms and/or signs of infection. Microbiological eradication was defined as a negative culture for A baumannii in the specimen culture obtained at follow-up; persistence of the pathogen was defined as persistent growth of A baumannii regardless of the clinical outcome of the infection.
Acinetobacter baumannii bacteremia-related death was considered if ≥1 of the following criteria were present: blood cultures were positive for A baumannii bacteremia at the time of death; death occurred before resolution of signs and symptoms of A baumannii bacteremia; and death occurred within 14 days after breakthrough A baumannii bacteremia, without another explanation.[22]
2.3. Bacterial isolates and identification
The isolates from blood were identified by the Vitek GNI-card (bioMérieux, Marcy-l’Étoile, France) as Acinobacter calcoaceticus–A baumannii complex. Susceptibility testing of the A baumannii isolates was performed using the agar dilution method as defined by the Clinical Laboratory Standards Institute.[23] The susceptibility results were interpreted in accordance with the Clinical and Laboratory Standards Institution. MDRAB is defined as nonsusceptibility to at least 1 agent in 3 or more antimicrobial categories. These A baumannii isolates were further defined as MDRAB or non-MDRAB according to the international expert proposal for interim standard definition for Acinetobacter spp.[24]
2.4. Statistical analysis
The mean and standard deviation were calculated for continuous variables with a normal distribution, and median and interquartile range (IQR) was calculated for those with a non-normal distribution. Student test and the Mann–Whitney test were used to compare continuous variables, and the chi-square test or Fisher exact test was used for independent binomial variables according to the number of observations. A P value of <.05 was considered as statistically significant. All variables with P < .05 in the univariate analysis were included in the logistic regression for multivariate analysis. Statistics were performed with the Statistical Package for Social Science (IBM SPSS (V.19), Chicago, IL).
3. Results
During the 60-month study period, 338 patients were diagnosed with A baumannii bacteremia and were included in our study. Of all the enrolled patients, 235 (69.5%) were males, and the mean age was 62.1 years old (ranging from 22 to 98 years). The median hospital stay until isolation of A baumannii was 13 days, and the median total hospital day was 27.5 days. The 7-day mortality, 14-day mortality, and 28-day mortality rates were 32.8%, 44.1%, and 50.0%, respectively. A baumannii bacteremia-related mortality was 46.4%. The A baumannii isolates included 274 (81.1%) MDRAB and 64 (18.9%) non-MDRAB isolates. The MDRAB was most isolated in January and December, whereas non-MDRAB was isolated in August and September (Fig. 1). Antibiotic susceptibility rate: Gentamycin 36.3%, Tobramycin 41.3%, Amikacin 64.7%, Imipenem 22.7%, Ciprofloxacin 23.7%, Levofloxacin 26.8%, Piperacillin-tazobactam 19.3%, cefoperazone-sulbactam 21.7%, Ampicillin-sulbactam 21.7%, Ceftriaxone 3.4%, Ceftazidime 20.1%, Cefepime 22.7%, Trimethoprim-sulphamethoxazole 37.4%, Tigecycline 79.2%.
Figure 1.

Number of Acinetobacter baumannii isolates recovered from patients from January, 2013 through December, 2017 by month. The isolated month distribution of MDRAB differed significantly from non-MDRAB. For MDRAB isolates, the most common months were January and December, whereas for non-MDRAB isolates, the most common months were August and September. MDRAB = multidrug-resistant Acinetobacter baumannii.
3.1. Severity and outcomes of MDRAB and non-MDRAB bacteremia
Variables related to the clinical presentation of A baumannii bacteremia were analyzed and are listed in Table 1.
Table 1.
Severity and outcomes of MDRAB and non-MDRAB, and risk factors for development of MDRAB bacteremia.
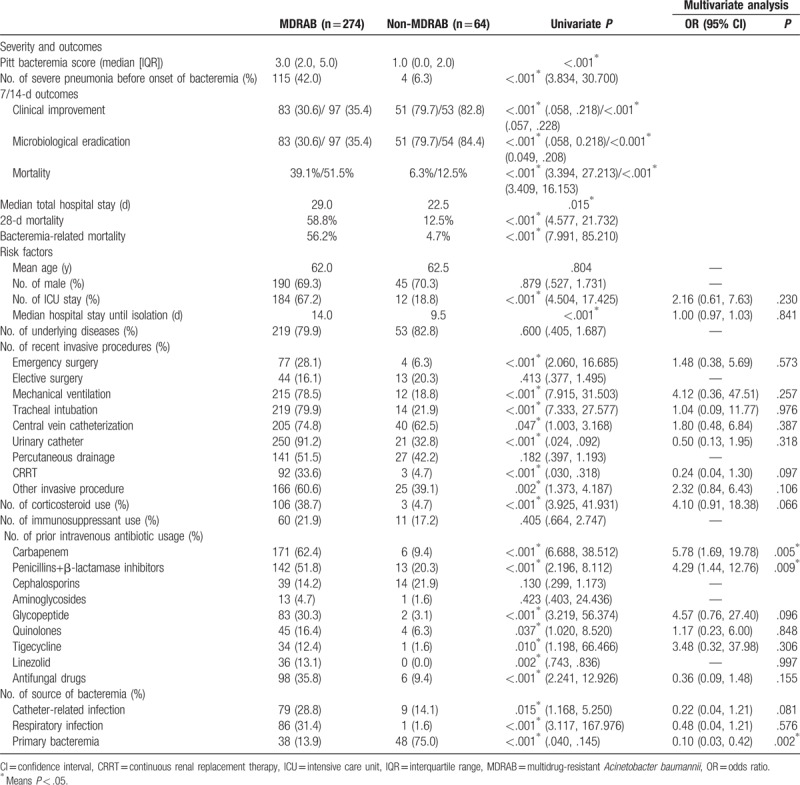
Compared with non-MDRAB bacteremia, MDRAB bacteremia patients had a serious condition at the time of onset of bacteremia, a longer hospital stay, and poor outcomes. In particular, the attributed mortality in MDRAB bacteremia was significantly higher than in non-MDRAB bacteremia (56.2% vs 4.7%).
3.2. Risk factors for development of A baumannii bacteremia
Demographic and clinical characteristic data of patients with MDRAB and non-MDRAB bacteremia are shown in Table 1.
Cases of MDRAB bacteremia resulting from catheter-related and respiratory infection were significantly more prevalent than those of non-MDRAB bacteremia, whereas primary bacteremia was more common in cases of non-MDRAB bacteremia.
Using univariate analysis, factors associated with MDRAB bacteremia included ICU stay, a longer hospital stay before isolation of A baumannii, emergency surgery, mechanical ventilation, tracheal intubation, central vein catheterization, urinary catheter, continuous renal replacement therapy (CRRT) and other recent invasive procedures, prior use of corticosteroid, carbapenem, penicillins+β-lactamase inhibitors, glycopeptide, quinolones, tigecycline, linezolid and antifungal drugs, and catheter-related infection, respiratory infection and primary bacteremia as source of bacteremia. Patients with MDRAB bacteremia were similar to patients with non-MDRAB bacteremia with respect to age, sex, underlying diseases, elective surgery, and percutaneous drainage tube before onset of bacteremia, prior use of immunosuppressant, cephalosporins and aminoglycosides, and other infection as sources of bacteremia (including surgical wound infection, intra-abdominal infection, central nervous system, skin and soft tissue infection, P > .05, data not shown).
Multivariate analysis showed that the risk factors for developing MDRAB bacteremia were previous exposure to carbapenems (odds ratio [OR] 5.78, P = .005) and penicillins+β-lactamase inhibitors (OR 4.29, P = .009). Primary bacteremia tended to develop non-MDR bacteremia (OR 0.10, P = .002).
3.3. Risk factors for MDRAB bacteremia-related mortality
Due to the significant bacteremia-related mortality rates between patients with MDRAB or non-MDRAB bacteremia, analysis was conducted separately.
As shown in Table 2, in patients with MDRAB bacteremia, using univariate analysis, factors independently associated with bacteremia-related outcomes included old age, catheter-related infection, respiratory infection, higher Pitt bacteremia score, bacteremia occurring after severe pneumonia, adequate dosage of cefoperazone-sulbactam therapy, and operations for treatment of infections.
Table 2.
MDR Acinobacter baumannii bacteremia-related mortality.
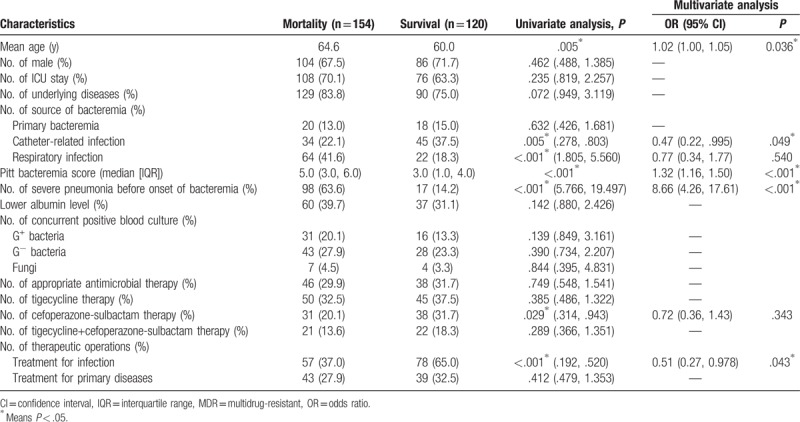
Multivariate analysis showed that old age (OR 1.02, P = .036), a high Pitt bacteremia score (OR 1.32, P < .001), bacteremia occurring after severe pneumonia (OR 8.66, P < .001) were risk factors for MDRAB bacteremia-related mortality, whereas catheter-related infection (OR 0.47, P = .049) and operations for treatment of infection (OR 0.51, P = .043) may have a better outcome.
4. Discussion
Bloodstream infection caused by MDRAB has become a major concern in the clinic. In this study, we sought to conclude the clinical features and outcomes of MDRAB and non-MDRAB bacteremia, identify the risk factors for developing MDRAB bacteremia, and MDRAB bacteremia-related mortality.
Gram-negative bacteria, including A baumannii, are increasingly recognized as exhibiting seasonal trends in bloodstream infection incidence; that is, significantly higher rates of A baumannii bacteremia were observed during the summer months.[25–29] However, in our study, the A baumannii strains were mostly isolated in winter months (January and December), whereas non-MDRAB strains were isolated in late summer months (August and September). These results can influence clinical diagnosis and empiric antibiotic treatment.
In contrast to non-MDRAB bacteremia, MDRAB bacteremia had a notably higher attributed mortality.[2,30,31] Our results also demonstrated that MDRAB bacteremia had poor outcomes including a serious condition at the time of onset of bacteremia, a longer hospital stay, a lower clinical cure rate, a lower microbiological eradication rate, and higher mortalities. The total attributed mortality rate of A baumannii bacteremia and MDRAB was 46.4% and 56.2%, respectively, which was similar to previously reported rates of 44.8% and 59.4%[2] in a hospital in northern China.
Given the notably high mortality of MDRAB bacteremia, we reviewed the risk factors for developing MDRAB bacteremia. Antibiotic exposure is 1 of the most frequently reported risk factors for MDRAB colonization or infection,[10,11,32,33] and the use of carbapenems, third-generation cephalosporins, and β-lactams has been reported. Antibiotic therapy facilitates the emergence of new resistant mutants or the proliferation of antibiotic-resistant A baumannii by exerting selective pressure.[34] Compatible with these reports, we revealed that previous exposure to carbapenems and penicillins+β-lactamase inhibitors were independent risk factors for developing MDRAB bacteremia. As our results showed, recent invasive procedures related to a higher incidence of MDRAB acquisition.[11,33] Invasive procedures may promote the cross-transmission and invasiveness of pathogens; moreover, invasive procedures partly reflect the severity of the illness.
Our results are consistent with previous studies showing that respiratory infections and intravascular catheters were the most frequent sources of nosocomial A baumannii bloodstream infections.[22,35] A matched case-control study found no significant difference in the portals of entry of bacteremia between MDRAB and non-MDRAB bacteremia.[11] However, our study revealed that MDRAB bacteremia mostly resulted from catheter-related and respiratory infections, whereas most non-MDRAB bacteremia were primary bacteremia. Our unmatched design may have an effect on these univariate analysis results.
A number of diverse factors have been investigated as potential risk factors for attributable mortality in MDRAB bacteremia patients.[8–10,12,13] As expected, old age and a high Pitt bacteremia were independent risk factors for mortality, whereas catheter-related infection and operations for treatment of infection were protective factors. Removing the tube can remove the cause of catheter-related infection directly, so patients had a better prognosis when catheter-related infection was the source of bacteremia. Moreover, we found that bacteremia occurred after severe pneumonia was another risk factor; this can be explained that MDRAB bacteremia complicated with severe pneumonia demonstrated a poor condition of patients.
The importance of timely and appropriate antimicrobial therapy for MDRAB bacteremia is well known.[36–39] However, similar to some studies,[12,40,41] our results revealed that the use of appropriate antimicrobial therapy was not found to be associated with the mortality of MDRAB bacteremia. Cefoperazone-Sulbactam can overcome β-lactamase-mediated resistance and restore the activity of the co-formulated β-lactams and possesses intrinsic activity against Acinetobacter species.[42] Tigecycline has become a promising option for the treatment of MDRAB infections.[43] Tigecycline/sulbactam combination has synergy and partial synergy activity to A baumannii isolates.[44,45] Nevertheless, our results showed that therapy regimes containing tigecycline or Cefoperazone-Sulbactam, even a combination of both, had no significant effect on the mortality of MDRAB bacteremia. The ineffective antimicrobial treatment partially causes high mortality in our study.
5. Conclusions
In conclusion, patients with MDRAB had a higher mortality rate, patients with previous carbapenems and penicillins+β-lactamase inhibitor exposure, and patients who received other recent invasive procedures are at an increased risk of MDRAB bacteremia. The risk factors for MDRAB bacteremia-related mortality were old age, a high Pitt bacteremia score, and bacteremia occurring after severe pneumonia, whereas catheter-related infection and operations for the treatment of infection may have a better outcome. Further research should determine the effective antimicrobial treatment of MDRAB bacteremia.
Author contributions
Conceptualization: Hua Zhou.
Data curation: Hua Zhou, Yake Yao, Bingquan Zhu, Yiqi Fu, Yunsong Yu.
Formal analysis: Yake Yao, Bingquan Zhu, Yunsong Yu.
Funding acquisition: Hua Zhou, Bingquan Zhu, Danhong Ren.
Investigation: Hua Zhou.
Methodology: Hua Zhou.
Project administration: Hua Zhou.
Resources: Qing Yang.
Software: Bingquan Zhu, Danhong Ren, Yiqi Fu.
Supervision: Jianying Zhou.
Writing - original draft: Hua Zhou, Yake Yao.
Footnotes
Abbreviations: CDC = Centers for Disease Control, CRRT = continuous renal replacement therapy, ICU = intensive care unit, MDRAB = multidrug-resistant A baumannii, OR = odds ratio, TNF = tumor necrosis factor.
HZ and YY contributed equally to this work.
This work was supported by a research grant from the Natural Science Foundation of Zhejiang Province (LY16H190004 and LQ18H190001), grants from Health and family planning commission of Zhejiang Province (2015RCA009 and 2016KYA075), grant from Zhejiang Province Public Warfare Technology Applied Research Program (2015C33278).
The authors report no conflicts of interest.
References
- [1].Smolyakov R, Borer A, Riesenberg K, et al. Nosocomial multi-drug resistant Acinetobacter baumannii bloodstream infection: risk factors and outcome with ampicillin-sulbactam treatment. J Hosp Infect 2003;54:32–8. [DOI] [PubMed] [Google Scholar]
- [2].Guo N, Xue W, Tang D, et al. Risk factors and outcomes of hospitalized patients with blood infections caused by multidrug-resistant Acinetobacter baumannii complex in a hospital of Northern China. Am J Infect Control 2016;44:e37–9. [DOI] [PubMed] [Google Scholar]
- [3].Akova M. Epidemiology of antimicrobial resistance in bloodstream infections. Virulence 2016;7:252–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Michalopoulos A, Falagas ME. Treatment of Acinetobacter infections. Expert Opin Pharmacother 2010;11:779–88. [DOI] [PubMed] [Google Scholar]
- [5].Mahgoub S, Ahmed J, Glatt AE. Completely resistant Acinetobacter baumannii strains. Infect Control Hosp Epidemiol 2002;23:477–9. [DOI] [PubMed] [Google Scholar]
- [6].Marchaim D, Levit D, Zigron R, et al. Clinical and molecular epidemiology of Acinetobacter baumannii bloodstream infections in an endemic setting. Future Microbiol 2017;12:271–83. [DOI] [PubMed] [Google Scholar]
- [7].Sunenshine RH, Wright MO, Maragakis LL, et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 2007;13:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee HY, Chen CL, Wu SR, et al. Risk factors and outcome analysis of Acinetobacter baumannii complex bacteremia in critical patients. Crit Care Med 2014;42:1081–8. [DOI] [PubMed] [Google Scholar]
- [9].Liu CP, Shih SC, Wang NY, et al. Risk factors of mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia. J Microbiol Immunol Infect 2016;49:934–40. [DOI] [PubMed] [Google Scholar]
- [10].Liu Q, Li W, Du X, et al. Risk and prognostic factors for multidrug-resistant Acinetobacter baumannii complex bacteremia: a retrospective study in a tertiary hospital of west China. PloS One 2015;10:e0130701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shih MJ, Lee NY, Lee HC, et al. Risk factors of multidrug resistance in nosocomial bacteremia due to Acinetobacter baumannii: a case-control study. J Microbiol Immunol Infect 2008;41:118–23. [PubMed] [Google Scholar]
- [12].Gu Z, Han Y, Meng T, et al. Risk factors and clinical outcomes for patients with Acinetobacter baumannii bacteremia. Medicine 2016;95:e2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nutman A, Glick R, Temkin E, et al. A case-control study to identify predictors of 14-day mortality following carbapenem-resistant Acinetobacter baumannii bacteraemia. Clin Microbiol Infect 2014;20:O1028–34. [DOI] [PubMed] [Google Scholar]
- [14].Liu YM, Lee YT, Kuo SC, et al. Comparison between bacteremia caused by Acinetobacter pittii and Acinetobacter nosocomialis. J Microbiol Immunologyd Infect 2017;50:62–7. [DOI] [PubMed] [Google Scholar]
- [15].Lee YT, Kuo SC, Yang SP, et al. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin Infect Dis 2012;55:209–15. [DOI] [PubMed] [Google Scholar]
- [16].De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control 1988;16:128–40. [DOI] [PubMed] [Google Scholar]
- [18].O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011;52:e162–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents 1999;11:7–12. [DOI] [PubMed] [Google Scholar]
- [20].Dallas J, Kollef M. Severe hospital-acquired pneumonia: a review for clinicians. Curr Infect Dis Rep 2009;11:349–56. [DOI] [PubMed] [Google Scholar]
- [21].Garnacho-Montero J, Amaya-Villar R, Gutierrez-Pizarraya A, et al. Clinical efficacy and safety of the combination of colistin plus vancomycin for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Chemotherapy 2013;59:225–31. [DOI] [PubMed] [Google Scholar]
- [22].Lee JY, Kang CI, Ko JH, et al. Clinical features and risk factors for development of breakthrough gram-negative bacteremia during carbapenem therapy. Antimicrob Agents Chemother 2016;60:6673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 27th informational supplement. CLSI document M100-S16.Wayne, PA: Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- [24].Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–81. [DOI] [PubMed] [Google Scholar]
- [25].Eber MR, Shardell M, Schweizer ML, et al. Seasonal and temperature-associated increases in gram-negative bacterial bloodstream infections among hospitalized patients. PloS One 2011;6:e25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Perencevich EN, McGregor JC, Shardell M, et al. Summer peaks in the incidences of gram-negative bacterial infection among hospitalized patients. Infect Control Hosp Epidemiol 2008;29:1124–31. [DOI] [PubMed] [Google Scholar]
- [27].Gales AC, Jones RN, Forward KR, et al. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997-1999). Clin Infect Dis 2001;32 suppl 2suppl 2:S104–113. [DOI] [PubMed] [Google Scholar]
- [28].McDonald LC, Banerjee SN, Jarvis WR. Seasonal variation of Acinetobacter infections: 1987-1996. Nosocomial Infections Surveillance System. Clin Infect Dis 1999;29:1133–7. [DOI] [PubMed] [Google Scholar]
- [29].Richet H. Seasonality in Gram-negative and healthcare-associated infections. Clin Microbiol Infect 2012;18:934–40. [DOI] [PubMed] [Google Scholar]
- [30].Lee NY, Lee HC, Ko NY, et al. Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol 2007;28:713–9. [DOI] [PubMed] [Google Scholar]
- [31].Gkrania-Klotsas E, Hershow RC. Colonization or infection with multidrug-resistant Acinetobacter baumannii may be an independent risk factor for increased mortality. Clin Infect Dis 2006;43:1224–5. [DOI] [PubMed] [Google Scholar]
- [32].Falagas ME, Kopterides P. Risk factors for the isolation of multi-drug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa: a systematic review of the literature. J Hosp Infect 2006;64:7–15. [DOI] [PubMed] [Google Scholar]
- [33].Chopra T, Marchaim D, Johnson PC, et al. Risk factors and outcomes for patients with bloodstream infection due to Acinetobacter baumannii-calcoaceticus complex. Antimicrob Agents Chemother 2014;58:4630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Donskey CJ. Antibiotic regimens and intestinal colonization with antibiotic-resistant gram-negative bacilli. Clin Infect Dis 2006;43suppl 2:S62–69. [DOI] [PubMed] [Google Scholar]
- [35].Jang TN, Lee SH, Huang CH, et al. Risk factors and impact of nosocomial Acinetobacter baumannii bloodstream infections in the adult intensive care unit: a case-control study. J Hosp Infect 2009;73:143–50. [DOI] [PubMed] [Google Scholar]
- [36].Metan G, Sariguzel F, Sumerkan B. Factors influencing survival in patients with multi-drug-resistant Acinetobacter bacteraemia. Eur J Intern Med 2009;20:540–4. [DOI] [PubMed] [Google Scholar]
- [37].Falagas ME, Kasiakou SK, Rafailidis PI, et al. Comparison of mortality of patients with Acinetobacter baumannii bacteraemia receiving appropriate and inappropriate empirical therapy. J Antimicrob Chemother 2006;57:1251–4. [DOI] [PubMed] [Google Scholar]
- [38].Cisneros JM, Reyes MJ, Pachon J, et al. Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin Infect Dis 1996;22:1026–32. [DOI] [PubMed] [Google Scholar]
- [39].Rodriguez-Bano J, Pascual A, Galvez J, et al. [Acinetobacter baumannii bacteremia: clinical and prognostic features]. Enferm Infecc Microbiol Clin 2003;21:242–7. [DOI] [PubMed] [Google Scholar]
- [40].Ng TM, Teng CB, Lye DC, et al. A multicenter case-case control study for risk factors and outcomes of extensively drug-resistant Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol 2014;35:49–55. [DOI] [PubMed] [Google Scholar]
- [41].Wareham DW, Bean DC, Khanna P, et al. Bloodstream infection due to Acinetobacter spp: epidemiology, risk factors and impact of multi-drug resistance. Eur J Clin Microbiol Infect Dis 2008;27:607–12. [DOI] [PubMed] [Google Scholar]
- [42].Viehman JA, Nguyen MH, Doi Y. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs 2014;74:1315–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Morfin-Otero R, Dowzicky MJ. Changes in MIC within a global collection of Acinetobacter baumannii collected as part of the Tigecycline Evaluation and Surveillance Trial, 2004 to 2009. Clin Ther 2012;34:101–12. [DOI] [PubMed] [Google Scholar]
- [44].Ni W, Cui J, Liang B, et al. In vitro effects of tigecycline in combination with colistin (polymyxin E) and sulbactam against multidrug-resistant Acinetobacter baumannii. J Antibiot (Tokyo) 2013;66:705–8. [DOI] [PubMed] [Google Scholar]
- [45].Deveci A, Coban AY, Acicbe O, et al. In vitro effects of sulbactam combinations with different antibiotic groups against clinical Acinetobacter baumannii isolates. J Chemother 2012;24:247–52. [DOI] [PubMed] [Google Scholar]


