Abstract
Background/Purpose:
To update definitions of multiple joint osteoarthritis (MJOA), and to determine the frequency and impact of MJOA in a community-based cohort.
Methods:
Following PRISMA guidelines and with the help of a professional research librarian, we performed a systematic review in Medline using the terms osteoarthritis, generalized, polyarticular, multiple joint, and multi-joint among others, to obtain articles related to MJOA. A total of 42 articles were included for data extraction based on multiple criteria including the requirement for a clearly stated definition of OA assessed at more than one body site. We assessed frequency of these definitions in the Johnston County OA Project (JoCo OA) cohort as well as outcomes related to general health and physical function.
Results:
A total of 6 clearly stated definitions for MJOA were identified. These definitions were integrated with a list of 24 definitions from our previous systematic review and distilled down to produce 10 literature-derived, operationalized MJOA definitions. Based on these definitions, high frequencies of radiographic (4–74%) and symptomatic (2–52%) MJOA were found in the JoCo OA. Significant detrimental effects were seen on general health and physical function for most definitions.
Conclusions:
We constructed a list of 10 summary MJOA definitions based in the literature that are frequent and associated with important clinical outcomes. These definitions capture some of the variability of MJOA phenotypes and provide a starting point for future analyses of both existing and newly initiated studies.
Keywords: MJOA, generalized osteoarthritis, systematic review
Osteoarthritis (OA), a common and debilitating disease closely associated with increasing age, imposes a large public health burden in the United States. More than 10% of the US adult population (an estimated 27 million US adults) had clinical OA in 2005, and OA was the fourth most common cause of hospitalization in 2009 [1]. On top of this, the obesity epidemic is predicted to push OA prevalence even higher [2].
OA frequently affects multiple joints in an individual [3–6]. This polyarticular subtype is referred to by multiple names in the literature, including multiple joint OA (MJOA) and generalized OA (GOA). Despite the ubiquity of MJOA, it is relatively understudied and has not been extensively characterized epidemiologically; this is likely due to the absence of a widely agreed upon definition of MJOA [6–8].
MJOA imposes a high clinical burden in affected patients, particularly in terms of health-related quality of life (HRQoL), activity limitations [9] and increased disease burden in the individual patient [10–16]. MJOA may represent a distinct etiology from mono-articular OA [9, 17–21] that should be considered separately from single joint OA when assessing for risk factors and associated disease. Our systematic review of the MJOA literature from 1952–2014 yielded MJOA prevalence estimates between 5–25% [6].
Since our initial review, there has been a move away from GOA and favoring MJOA in the literature (PubMed), although there remains a need for standard definition(s) of MJOA to better estimate prevalence, understand systemic outcomes and clinical burden and to contribute to evidence-based clinical practice. Therefore, with this review, we aimed 1) to produce a current, updated, comprehensive list of operationalized MJOA definitions for use by our group and others in further study regarding MJOA risk factors and outcomes [6] and 2) to assess the frequency and impact of these definitions within the Johnston County Osteoarthritis Project (JoCo OA), a large community-based sample with clear criteria for radiographic and symptomatic OA at multiple sites.
Methods
Systematic Review:
To assess recent definitions of MJOA in the literature, we performed an update to a prior systematic review to include articles published between the years 2012–2017. As described in detail in our 2014 systematic review of the same topic [6], a research librarian performed a MEDLINE search on April 18, 2017 to obtain relevant articles (limited to one database as previously noted due to lack of standard terminology). Initial title and abstract reviews were performed by two coauthors (A.E.N. and T.R.G.). Inclusion criteria were a focus on OA and involvement of more than one joint site. When the coauthors disagreed, articles considering more than one joint site underwent full-text review. Subsequent review by A.E.N. and T.R.G. determined which articles would undergo data extraction. Articles were excluded if they: 1) focused on only one joint group (multiple joints within a single site, such as multiple hand joints or tibiofemoral and patellofemoral alone, qualified as one joint group), or 2) focused on a disease process other than OA (for example, chondrocalcinosis, rheumatoid arthritis, septic arthritis, etc.). Through bibliographic review (A.E.N.), three additional articles were identified for data extraction for a total of 42 articles extracted (by T.R.G.) using a previously described methodology [6]. Figure 1 summarizes the search process (for additional details of the original search, please see [6]). Six of these articles included a clear definition of MJOA and were compiled together with the 24 articles identified in our previous systematic review [6]. Definitions of MJOA were extracted from all 30 articles (by T.R.G.) and are listed in Table 1.
Figure 1.
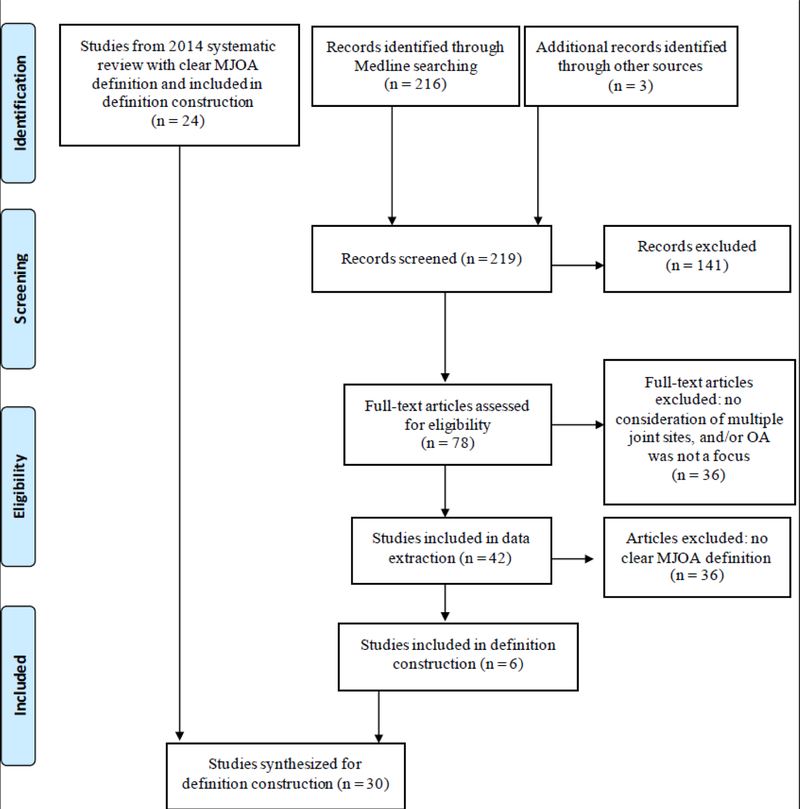
Diagram of literature search
Table 1.
Definitions for multiple joint osteoarthritis (MJOA) in the literature
| Author (year), [ref] | MJOA operational definition | Joint sites assessed | Country | Sample size | Female (%) | Reported MJOA frequency |
|---|---|---|---|---|---|---|
| Kellgren (1952), [63] | HN and/or 1st CMC with rOA | CMC, PIP, MTP, K, Hi, Spine | UK | 587 | 92 | 52% of OA patients had HN and CMC OA |
| Lawrence (1969), [64] | ≥3 or ≥5 joint sites with ≥1 joint with rOA | Ha, F, K, Hi, Spine | UK | 1179 | 54 | Non-HN: 3+ joints in 25% men and 20% women; 5+ joints in 9% men and 8% women |
| Solomon (1976), [65] | ≥3 joint sites with rOA | DIP, PIP, MCP, CMC, wrists, 1st MTP, Hi | South Africa | 300 | 71 | HN and 2+ joints in 1 man and 0 women; non-HN and 2+ joints in 6% men and 9% women |
| Doherty (1983), [38] | ≥4 bilateral IP joints with rOA unrelated to trauma | K, Ha | UK | 150 | 15 | 43% |
| Brighton (1985), [66] | ≥3 joint sites with rOA | DIP, PIP, MCP, CMC, MTP | South Africa | 543 | 72 | 4% |
| Price (1987), [51] | Majority of fingers with HN and ≥7 joint sites with rOA | Unspecified | UK | 40 | 100 | 100% (selected MJOA) |
| Doherty (1990), [43] | Polyarticular Ha OA and Heberden (+/−Bouchard) node formation | Ha | No data | No data | No data | No data |
| Waldron (1991), [53] | DIP, 1st CMC, and K with paleopathologic OA | DIP, 1st CMC, K | UK | 968 | 49 | 2% |
| Hopkinson (1992), [45] | ≥4 bilateral Ha rays with rOA, symptomatic or previously symptomatic and HN formation and clinical and/or rOA at other sites | Ha | UK | 255 | 90 | 34% (selected MJOA cases) |
| Hart (1993), [39] | DIP, CMC, and K rOA | DIP, PIP, CMC, K | UK | 985 | 100 | 2% |
| Hordon (1993), [46] | ≥3 joint sites with rOA and/or clinical OA as described in Kellgren ‘52 (above) | DIP, PIP, 1st CMC, 1st MTP, K, Spine, Hi, S, A | UK | 109 | 100 | 100% (selected MJOA) |
| Loughlin (1994), [54] | HN before age 60 and ≥3 other joint sites involved | Unspecified | UK | 133 | No data | 100% of cases (selected for MJOA) |
| Dougados (1996), [49] | Bilateral finger OA (HN or Bouchard nodes bilaterally or bilateral rOA of DIPs or PIPs) or rOA of Spine and both K | DIP, PIP, Spine, K | France | 1021 | No data | 44% (27% bilateral finger and 17% spine and knee OA) |
| Cooper (1996), [37] | Age and threshold-based: for O:E ≥1.5, ≥2 joint sites with rOA needed at age 45–47 but ≥5 at age 63–64 (etc.) | DIP, PIP, CMC, K, Hi | UK | 702 | 100 | 7% overall for O:E 1.5 |
| Gunther (1998), [67] | ≥2 DIP or PIP and ≥1 CMC and 1 K or Hi with rOA | DIP, PIP, CMC, K, Hi | Germany | 809 | 62 | 27% |
| Ushiyama (1998), [68] | ≥3 bilateral IP joints with rOA | Ha, Hi, K | Japan | 383 | 100 | 17% |
| Malaviya (1998), [47] | DIP OA(by ACR criteria) and HN | Ha, K | Kuwait | 69 | 74 | 6% |
| Naito (1999), [42] | ≥3 bilateral IP joints with rOA | Ha, K | Japan | 102 | 100 | 45% |
| Huang (2000), [40] | Ha rOA (≥3 bilateral IP joints with rOA), Hi, and TF rOA | Ha, Hi, K (TF) | Japan | 270 | 100 | 17% |
| Min (2005), [50] | Rotterdam definition: ≥2 joint sites with rOA GARP definition: ≥2 joint sites (out of Ha, Spine, K, or Hi) or at multiple joint sites of the Ha or 1 joint site with sxOA and ≥1 joint site with structural abnormalities | Ha, K, Hi, Spine | Netherlands | 1751 | 58–82% | 23% Rotterdam and 66% GARP |
| Miura (2008), [41] | Bilateral K rOA and lumbar spine rOA | K, Spine | Japan | 518 | 72 | 13% |
| Riyazi (2008), [52] | Same as GARP definition above | Ha, K, Hi, Spine | Netherlands | 727 | Cases=82, controls=64 | 90% had multiple hand joints or 2+ other sites |
| Carroll (2009), [48] | Type I: HN and/or Bouchard nodes with permutations of DIP, PIP, medial K, and 1st MTP involvement Type II: permutations of index and/or middle finger MCP, E, and A OA | Ha, K, F, E, A | Australia | 67 | 85% (Type I), 36% (Type II) | 58% type I and 42% type II |
| Hoogeboom (2010), [44] | All 3: 1) complaints in ≥3 joint sites, 2) ≥2 objective signs of OA in ≥2 joints, 3) limited ADLs (HAQ > 0.5) | Unspecified | Netherlands | 170 | No data | No data |
| Moe (2012), [8] | ≥2 joint sites with clinical OA | Ha, Hi, K | Norway | 408 | 86 | 7% |
| Racaza (2012), [34] | ≥3 joint sites with rOA and pain | K, Spine, Ha, A or F, Hi, S | Philippines | 859 | 75 | 13% |
| Cimmino (2013), [7] | 2 or 3 symptomatic sites | Ha, Hi, K | Italy | 25,445 | 69 | 22% |
| Cuperus (2014), [9] | ≥2 joint sites with objective signs of OA, ≥ 3 joint sites with clinical symptoms, and limited ADLs (HAQ > 0.5) | F (includes A), K, Hi, Spine, N, S, E, Ha (includes wrists) | Netherlands | 147 | 85 | No data |
| Veronese (2016), [35] | ≥2 joints with symptoms or previous OA diagnosis | Ha, Hi, K | Italy | 2,158 | 63 | 34% |
| Park (2017), [36] | ≥3 joint sites with rOA and symptoms including K, Hi, and Spine | K, Hi, Spine | South Korea | 8,976 | 57 | 11% male, 23% female |
HN = Heberden’s node; CMC = carpometacarpal; PIP = proximal interphalangeal; MTP = metatarsophalangeal; K = knee; Hi = hip; Ha = hand; E = elbow; A = ankle; F = foot; MCP = metacarpophalangeal;
S = shoulder; N = neck; rOA = radiographic OA; sxOA = symptomatic OA; GARP = Genetics, Arthrosis, and Progression study
JoCo OA Participants, OA and outcome definitions:
The JoCo OA is a community-based prospective cohort study of OA in African American and Caucasian men and women in Johnston County, North Carolina, and has been described in detail elsewhere, both overall [22] and at the third follow up [23]. This study has been continuously approved by the Institutional Review Boards of the University of North Carolina at Chapel Hill and the Centers for Disease Control and Prevention (primary funder of the JoCo OA). All participants provided written informed consent at the time of recruitment.
A follow up visit was conducted from 2013–15 that included 908 participants, of which 32% were men and 34% were African American, with a mean age of 72 years and mean body mass index (BMI) of 31 kg/m2. Frequencies of the compiled MJOA definitions, utilizing both radiographic and symptomatic (radiographs plus symptoms) approaches, were assessed within this cohort (n=904 with data to determine MJOA type) to provide an estimate of the prevalence in the general population. Specifically, for definitions requiring a number of sites (e.g., 3 or more involved sites), we specified unique sites, such that involvement of the bilateral knees counted as a single site rather than two joints.
At the 2013–15 visit, all participants underwent radiographs of the posteroanterior (PA) hands, PA fixed flexion knees using a Synaflexer positioning frame, supine anteroposterior (AP) pelvis, lateral lumbosacral spine, AP and lateral ankle and dorsoplantar foot, graded by a single musculoskeletal radiologist as previously described [24–27]. Radiographic OA (rOA) was defined as a Kellgren-Lawrence grade (KLG) of 2 [28] or more at the distal interphalangeal (DIP), proximal interphalangeal (PIP), carpometacarpal (CMC), tibiofemoral, and femoroacetabular joints. At the spine, rOA was defined as disc space narrowing (grade 1 or more [29]) and osteophyte (OST) (grade 2 or more) in at least one vertebral level. Ankle rOA was defined as a KLG ≥ 2 at the tibiotalar joint. Using the La Trobe radiographic atlas, [30] foot rOA was defined as ≥2 OST or joint space narrowing (JSN) in at least 1 of 5 joints:1st metatarsophalangeal, 1st cuneo-metatarsal, 2nd cuneo-metatarsal, navicular-1st cuneiform, talo-navicular.
For each site (i.e., hands, hips, knees, back, ankles, feet), the participant addressed whether or not they experienced ―pain, aching, or stiffness‖ in that site on most days. The clinical examination included determination of tenderness and bony enlargement (i.e. IP nodes) at each of the 30 hand joints. To assess reliability, this examination was independently performed by two trained and experienced staff members in a subset of 40 randomly selected participants with proportional agreement ranging from 0.57 to 0.97 for nodes and 0.86 to 0.97 for tenderness (Supplemental Table). For the hips, knees, back, ankles, and feet, symptomatic OA (sxOA) was defined as the presence of symptoms and rOA by these definitions in the same joint site. For the hand, if individual joints were included (e.g., DIP, PIP), sxOA was defined as tenderness and rOA in each joint, and for nodes as present or absent in that joint.
Finally, we assessed two general measures of health outcomes in relation to MJOA status: the first question of the SF-36 [31], ―In general, would you say your health is:‖ dichotomized as excellent/very good/good vs fair/poor, and the Patient Reported Outcomes Measurement Information System (PROMIS®) Physical Function Scale (Short Form 10a version 1.0, [32, 33]). Chi square tests for the former, and t-tests for the latter, were used to determine differences in the distributions among individuals meeting and not meeting the various MJOA definitions.
Results
Search and articles:
A total of 42 articles were included in data extraction for the updated review. These studies comprised 29 large cohorts (total n~56,000), 7 clinical series (total n~28,000), 1 systematic review (n=79 studies) and 2 meta-analyses (total n~980,000) across 14 countries/5 continents (Europe, North America, Africa, Asia and Australia) and 5 years (2012–2017).
Of the 30 articles from which operationalized definitions were extracted, 24 originated from the 2014 systematic review and 6 from the 2017 review. The 98 articles reviewed in 2014 included, 24 unique large cohorts (n=30,223) and numerous clinical series (n=9,252), across 22 countries spanning 5 continents (North America, Europe, Africa, Asia, and Australia), and 60 years (1952– 2012) [6]. Of the 6 most recent articles, 4 clinical series (n~26,900) were included along with 2 observational studies (n~11,100) across 5 countries/2 continents (Europe and Asia) and 5 years (2012–2017).
Joint sites and definitions:
The sites assessed and OA definitions at each site varied, but most often included the hands, knees and hips. Six of the 42 studies [7–9, 34–36] stated a clear definition for MJOA. No two of these definitions were exactly the same; however, all required either ≥2 or ≥3 joint sites affected, without strict requirements for involvement of specific joint sites. No definitions included a supporting rationale. Diagnostic criteria for OA varied and included consideration of American College of Rheumatology criteria, assessment of KLG for rOA, clinical evaluation, and magnetic resonance imaging (MRI). Twelve of the 30 studies with operationalized definitions based their OA findings on radiographic criteria [37–42], 8 used clinical criteria such as symptoms and physical exam findings [7–9, 43–47], 8 studies integrated both approaches [34–36, 48–52], 1 used paleopathologic evidence [53], and 1 was unclear [54]. Estimates of the frequencies of MJOA, as defined by each individual author, in these studies ranged from 7–34% (Table 1). In at least 4 articles the authors used alternate terms such as ―polyarticular OA‖ [34, 35] and ―generalized OA‖ [8, 34] to denote multiple joint involvement.
Although the remaining 36 studies provided no definition for MJOA, 12 articles collected data for OA disease characteristics in multiple joints within the individual [10–17, 20, 55–57]. For example, one study tallied the number of painful joint regions to assess the extent of disabling OA at the ―person level‖ (as opposed to the regional level) [13]. In another study, ―clinical OA-number of sites‖ was included as a categorical variable [10]. By inclusion of these data, investigators assessed clinical burden in those with MJOA in terms of pain, frailty, HRQoL, and limitations in activities of daily living (ADL).
Also, 6 studies discussed the hypothesis that MJOA arises from a systemic etiology, based on clinical and/or biomolecular findings such as metabolic syndrome, fat-free mass, serum S100A8/A9 (proinflammatory protein) levels, serum hyaluronic acid (sHA) levels, and subchondral bone attrition [9, 17–21]. For example, in a retrospective cadaveric study of 710 skeletons, sHA levels were found to have a positive correlation with the number of involved joints [20]. Additionally, in the Cohort Hip and Cohort Knee (CHECK) Study, patients with progression of hand OA had a higher risk for radiographic change at the knee than those without hand OA progression, suggesting a systemic process [58]. Conversely, two studies argued against the hypothesis of a common pathogenic process behind MJOA due to associations with indicators of joint and whole-body health that differed based on joint site affected [17, 18]. For example, a systematic review (n=6,673) found that knee OA, both alone and together with hand OA, was associated with weight and fat-free mass, while hand OA alone was associated with metabolic syndrome. This was interpreted as suggesting the co-occurrence of knee and hand OA does not derive from a common pathogenic mechanism [17].
Current definitions:
After literature review, 10 definitions were constructed to summarize and represent the 30 existing MJOA definitions across studies in a way that could be operationalized by joint site, number of joints involved, and diagnostic criteria (radiographic vs. symptomatic) (Table 2). In our search, two general categories emerged that could be used to classify each definition. These were: 1) minimum number of involved joint sites, and 2) site of involvement. With our 10 definitions, we sought to encompass the variability in the two categories across the 30 existing definitions. For instance, we represented a minimum involved joint site category by requiring varying numbers of joint involvement across definitions, from at least 2 to at least 5 joint sites affected. This reflected the variation in the literature: some studies only required 2 or more joints while others required as many as 5 or more joints to be considered MJOA. As for the category representing site of involvement, the MJOA definitions from the 30 articles ranged from site-unspecified, to lower body only, to upper and lower body, to OA solely in the hands.Of note, as these definitions were constructed based on pattern recognition, the quantity of definitions created was arbitrary; we stopped once we felt all 30 existing definitions were represented.
Table 2.
Operationalized multiple joint (MJOA) definitions based on literature review and frequencies of these definitions in the Johnston County Osteoarthritis Project (JoCo OA; 2013–2015 follow-up visit, n=904)
| MJOA- | Joint sites included in the definition | JoCo OA frequencies |
References* | |
|---|---|---|---|---|
| rOA | sxOA | |||
| 1 | ≥1 IP node and ≥2 other sites (hip, knee, spine, ankle, or foot) | 503 (56) | 441 (49) | [67], [51] |
| 2 | ≥2 IP and ≥1 CMC and knee or hip | 228 (26) | 139 (16) | [67], [39] |
| 3 | ≥5 joint sites (DIP, PIP, CMC, hip, knee, spine, ankle, or foot) | 230 (26) | 165 (18) | [64], [34] |
| 4 | ≥2 lower body joint sites (hip, knee, spine, ankle, or foot) | 565 (63) | 345 (38) | [7–9, 35, 44, 50] |
| 5 | Knee or hip and 1 other joint site (spine, ankle, or foot) | 489 (55) | 466 (52) | Same as MJOA-4 |
| 6 | ≥3 sites (hip, knee, spine, ankle, or foot) | 228 (25) | 147 (16) | [7, 9, 34, 36, 44, 46, 64–66] |
| 7 | Bilateral knees and spine | 24 (4) | 14 (2) | [41, 49] |
| 8 | ≥3 joint sites (DIP, PIP, CMC, hip, knee, spine, ankle, or foot) | 606 (67) | 390 (43) | [7, 9, 34, 36, 39, 40, 44, 46, 53, 64, 65, 67] |
| 9 | ≥1 CMC and bilateral nodes | 289 (32) | 120 (13) | [63], [49] |
| 10 | ≥3 IPs or bilateral nodes | 663 (74) | 135 (15) | [68], [42], [38] |
References on which the definition was based
rOA = radiographic OA (KLG≥2 except as noted below1); sxOA = symptomatic OA (symptoms + rOA in same site); KLG = Kellgren-Lawrence grade; knee=tibiofemoral joint; ankle=tibiotalar joint; DIP = distal interphalangeal; PIP = proximal interphalangeal; CMC = carpometacarpal
Exceptions:
rOA of spine: DSN ≥1 and ≥2 OST together in ≥1 vertebral level; rOA of foot: ≥2 OST or JSN in at least 1 of 5 joints (1st metatarsophalangeal, 1st cuneo-metatarsal, 2nd cuneo-metatarsal, navicular-1st cuneiform, talo-navicular); DSN = disc space narrowing; OST = osteophyte; JSN = joint space narrowing
The 10 constructed definitions are numbered arbitrarily for ease of reference; for example, MJOA-1 refers to MJOA definition #1, not to number of joint sites involved. As shown in Table 2, of the 10 definitions, 4 focused on the lower body only (MJOA 4–7), 4 comprised the lower body plus hand (MJOA 1–3 and 8), and 2 included hand involvement only (MJOA 9–10). Lower body joint sites considered included hip, knee, spine, ankle, or foot. Hand joint sites specified included DIP (distal interphalangeal), PIP (proximal interphalangeal), CMC (carpometacarpal), and nodes in the IP (interphalangeal) joints.
Frequencies of each MJOA definition within the JoCo OA cohort are also listed in Table 2. As noted in the methods, since the studies varied regarding OA criteria, we applied both rOA and sxOA criteria for each MJOA definition. For rOA, these numbers ranged from as low as 4% for MJOA-7 (bilateral knees and spine) to as high as 74% for MJOA-10 (more than 3 IPs or bilateral nodes), with a median frequency of 50%. For sxOA, the numbers ranged from 2% for MJOA-7 to 52% for MJOA-5 (knee or hip and one of spine, ankle, or foot) with a median frequency of 24%.
Finally, we considered the impact of MJOA in the JoCo OA cohort, using outcomes of general health and PROMIS physical function (Table 3). For the general health question, the proportion of individuals reporting fair/poor health was significantly higher among those meeting criteria for radiographic MJOA-4 or 6 (focused on lower body OA), and for essentially all of the symptomatic MJOA definitions (excepting only MJOA-5 and 10). For the PROMIS Physical Function measure, where higher scores reflect better function (range 10–50), the decrements with MJOA were even more apparent for both radiographic (significantly poorer function for all definitions but MJOA-7 and 10) and symptomatic (all significant) MJOA definitions.
Table 3.
Descriptive analyses of General Health and PROMIS Physical Function outcomes for each radiographic (rOA) and symptomatic (sxOA) MJOA definition in the JoCo OA
| Outcomes by rOA status | Outcomes by sxOA status | ||||
|---|---|---|---|---|---|
| MJOA- | General health1 Fair/poor n/N (%) | PROMIS Physical Function2 n; Mean (SD) | General health1 Fair/poor n/N (%) | PROMIS Physical Function2 n; Mean (SD) | |
| 1 | Yes | 119/503 (23.7) | 500; 38.1 (9.4)** | 124/441 (28.1)** | 438; 35.3 (9.2)** |
| No | 73/396 (18.4) | 390; 39.7 (9.2) | 68/458 (14.8) | 452; 42.2 (8.1) | |
| 2 | Yes | 50/228 (21.9) | 228; 36.4 (9.5)** | 39/139 (28.1)* | 139; 33.3 (9.0)** |
| No | 139/665 (20.9) | 656; 39.7 (9.1) | 150/754 (19.9) | 745; 39.9 (9.0) | |
| 3 | Yes | 56/230 (24.3) | 230; 36.5 (9.7)** | 50/165 (30.3)** | 165; 33.9 (9.3)** |
| No | 137/674 (20.4) | 663; 39.6 (9.1) | 143/739 (19.4) | 728; 39.9 (9.0) | |
| 4 | Yes | 135/565 (23.9)** | 562; 38.1 (9.5)** | 103/345 (29.9)** | 342; 35.0 (9.1)** |
| No | 57/336 (17.0) | 330; 39.9 (9.0) | 89/556 (16.0) | 550; 41.2 (8.6) | |
| 5 | Yes | 114/489 (23.3) | 486; 38.2 (9.4)** | 110/466 (23.6) | 463; 38.1 (9.4)** |
| No | 75/405 (18.5) | 399; 39.9 (8.8) | 79/428 (18.5) | 422; 39.9 (8.8) | |
| 6 | Yes | 65/228 (28.5)** | 227; 37.0 (9.6)** | 52/147 (35.4)** | 146; 34.1 (9.3)** |
| No | 127/673 (18.9) | 665; 39.4 (9.2) | 140/754 (18.6) | 746; 39.7 (9.1) | |
| 7 | Yes | 7/24 (29.2) | 23; 36.1 (11.4) | 6/14 (42.9)* | 13; 30.9 (10.0)** |
| No | 117/605 (19.3) | 599; 39.8 (8.8) | 118/615 (19.2) | 609; 39.9 (8.8) | |
| 8 | Yes | 136/606 (22.4) | 603; 37.9 (9.3)** | 110/390 (28.2)** | 387; 35.0 (9.2)** |
| No | 57/298 (19.2) | 290; 40.6 (9.1) | 83/516 (16.2) | 506; 41.7 (8.4) | |
| 9 | Yes | 69/289 (23.9) | 288; 36.7 (9.5)** | 39/120 (32.5)** | 119; 32.3 (9.5)** |
| No | 120/601 (20.0) | 593; 39.9 (9.1) | 150/770 (19.5) | 762; 39.9 (8.9) | |
| 10 | Yes | 133/663 (20.1) | 659; 38.5 (9.3) | 36/135 (26.7) | 135; 33.9 (9.7)** |
| No | 60/238 (25.2) | 232; 39.7 (9.5) | 156/764 (20.4) | 755; 39.7 (9.0) | |
General health (n=903): fair/poor vs. excellent/very good/good, chi-square tests were used to compare those meeting, to those not meeting, each definition of MJOA for rOA and sxOA
Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function (n=893): higher scores indicate better function (range 10–50), t-tests were used to compare those meeting, to those not meeting, each definition of MJOA for rOA and sxOA
p value<=0.05
p value <=0.01
Discussion
This review, synthesizing the literature on MJOA from 1952–2017, and building upon our prior review, indicates continued wide use of varied MJOA definitions. However, the high frequencies of MJOA-1, MJOA-4, MJOA-5, and MJOA-8 (rOA > 55% and sxOA > 38%) in the large community-based cohort suggest that MJOA is highly prevalent in the population. Meeting most MJOA definitions, especially symptomatic ones, was associated with participant-reported perceptions of general poor health and poor physical function as assessed by a PROMIS measure; these relationships indicate a high burden of disease related to MJOA.
It is not possible to make direct comparisons across studies for MJOA frequency given the considerable variation in population (by age, gender, geography, etc.). In general, it appeared that frequencies for both rOA and sxOA in JoCo OA were generally higher than in the source population for a given definition. These trends could be due to the higher mean age (72 years) and BMI (31 kg/m2) of this particular JoCo OA sample, thus increasing the likelihood of OA in our sample as compared to the source studies. Additionally, the incongruity between MJOA frequencies in JoCo OA and the studies in question may be partly explained by the different diagnostic criteria by which each study defined OA. Finally, our definitions were meant to represent generalizations of the source definitions and thus do not perfectly correspond with single literature definitions, complicating direct frequency comparisons.
Disease burden may differ based on location of joint site involvement. For instance, multiple studies have shown lower body OA—OA in the lumbar spine, hips, knees, ankles, and/or feet— to be exceptionally debilitating. OA is a primary indication for total knee arthroplasty which in 2010 was the most frequent inpatient procedure performed on adults aged 45 and older [59]. Additionally, in a 2014 study, those with MJOA noted that activity limitations involving their lower extremities, such as walking, were most important in daily life [9]. This principle of joint site involvement applies to the upper extremities as well; in the Rotterdam Study, rOA of the first CMC joint had a stronger association with hand pain than did rOA in the other hand joints [60]. Further, multiple studies describe an association between increasing disease burden and number of joints involved. In the North Staffordshire OA Project (n=18,474), an increased number of OA affected joint sites correlated with increased risk of disabling disease, defined as OA with pain interfering with an individual’s normal work [61]. In an observational study by Carlesso (n=2,455), odds of frailty and pre-frailty increased as number of joint sites with OA increased [10].
We recognize that not all cohorts have or wish to collect the data to define MJOA in all 10 ways noted in Table 2, and this is certainly not our intent. By including a range of literature-supported definitions, we hope to make it feasible for most cohorts to define MJOA in at least one of these categories, allowing comparison to the literature and an improved capability to incorporate MJOA into studies of prevalence and outcomes. As additional studies are done, this will allow a better understanding of the impact of specific definitions, better informing the choice of an optimal definition (or multiple) for a given study. For example, we have used MJOA1–4 in work exploring hypermobility and its effect on lower extremity OA [62].
Limitations in our study stem largely from the lack of clear definitions, uniformity in diagnostic criteria, and consistency of assessed joint sites in the literature, which limited our ability to incorporate quantitative methods in our definition construction. Since some decisions were therefore based on pattern recognition and opinion, reproducibility of our results may be limited (i.e., another group might generate different definitions using the same literature base). Additionally, stringent thresholds were used to classify OA, such as the KL cutoff of 2. Another limitation is the lack of studies specifically designed to define MJOA. Only one of 30 studies was designed for this purpose [37]. Finally, we cannot generalize our results to ages below 55 as the included JoCo participants ranged from 55–94 years old, with a mean age of 72 years.
Strengths of this study include its use of two systematic reviews encompassing 65 years of literature. Additionally, our ability to assess frequencies of definitions and general health impact in a large community-based cohort (JoCo OA) provides preliminary results to understand disease presence and burden in the general population. Although the qualitative process of MJOA definition construction is listed as a limitation above, there are some benefits to these methods; principally, inclusion of varying requirements for joint site and number allows representation of a broad spectrum of disease and clinical burden.
Further research should focus on evaluating these literature-based MJOA definitions in other large cohorts to assess generalizability and impact on OA and other health-related outcomes. Additionally, MJOA should be assessed along with outcomes and risk factors to better understand its clinical relevance.
Conclusion
We have generated 10 clearly defined literature-based definitions of frequently occurring MJOA. In a community-based cohort, these definitions are associated with clinically relevant participant-reported outcomes including poor general health, greater OA symptoms and worse physical function. As in 2014, there is still little consensus in the literature concerning the definition of MJOA as a condition. These 10 definitions capture some of the variability of MJOA phenotypes and represent a starting point for future analysis into its frequency and associations in relevant populations.
Supplementary Material
Acknowledgements
We would like to thank Christiane Voisin, research librarian, and the staff and participants of the Johnston County Osteoarthritis Project.
Funding Sources
Ms. Gullo’s work was funded by the Medical Student Training in Aging Research program through NIA 2-T35-AG038047-08, and a Rheumatology Research Foundation Medical Student Research Preceptorship. This work was also supported in part by: NIAMS P60 AR049465, R01 AR067743, Association of Schools of Public Health/Centers for Disease Control and Prevention S043 and S3486, and CDC U01DP006266.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy L, Helmick CG: The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs 2012, 112(3 Suppl 1):S13–19. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Jordan JM: Epidemiology of osteoarthritis. Clin Geriatr Med 2010, 26(3):355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraus VB, Jordan JM, Doherty M, Wilson AG, Moskowitz R, Hochberg M, Loeser R, Hooper M, Renner JB, Crane MM et al. : The Genetics of Generalized Osteoarthritis (GOGO) study: study design and evaluation of osteoarthritis phenotypes. Osteoarthritis Cartilage 2007, 15(2):120–127. [DOI] [PubMed] [Google Scholar]
- 4.Nelson AE, Renner JB, Schwartz TA, Kraus VB, Helmick CG, Jordan JM: Differences in multijoint radiographic osteoarthritis phenotypes among African Americans and Caucasians: The Johnston County Osteoarthritis project. Arthritis Rheum 2011, 63(12):3843–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson AE, Golightly YM, Renner JB, Schwartz TA, Kraus VB, Helmick CG, Jordan JM: Brief report: differences in multijoint symptomatic osteoarthritis phenotypes by race and sex: the Johnston County Osteoarthritis Project. Arthritis Rheum 2013, 65(2):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson AE, Smith MW, Golightly YM, Jordan JM: “Generalized osteoarthritis”: a systematic review. Semin Arthritis Rheum 2014, 43(6):713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cimmino MA, Scarpa R, Caporali R, Parazzini F, Zaninelli A, Sarzi-Puttini P: Body mass and osteoarthritic pain: results from a study in general practice. Clin Exp Rheumatol 2013, 31(6):843–849. [PubMed] [Google Scholar]
- 8.Moe RH, Grotle M, Kjeken I, Hagen KB, Kvien TK, Uhlig T: Disease impact of hand OA compared with hip, knee and generalized disease in specialist rheumatology health care. Rheumatology (Oxford) 2013, 52(1):189–196. [DOI] [PubMed] [Google Scholar]
- 9.Cuperus N, Vliet Vlieland TP, Mahler EA, Kersten CC, Hoogeboom TJ, van den Ende CH: The clinical burden of generalized osteoarthritis represented by self-reported health-related quality of life and activity limitations: a cross-sectional study. Rheumatol Int 2015, 35(5):871–877. [DOI] [PubMed] [Google Scholar]
- 10.Carlesso LC, Hawker GA, Waugh EJ, Davis AM: Disease-specific pain and function predict future pain impact in hip and knee osteoarthritis. Clin Rheumatol 2016, 35(12):2999–3005. [DOI] [PubMed] [Google Scholar]
- 11.Castell MV, van der Pas S, Otero A, Siviero P, Dennison E, Denkinger M, Pedersen N, Sanchez-Martinez M, Queipo R, van Schoor N et al. : Osteoarthritis and frailty in elderly individuals across six European countries: results from the European Project on OSteoArthritis (EPOSA). BMC Musculoskelet Disord 2015, 16:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira D, Severo M, Santos RA, Barros H, Branco J, Lucas R, Costa L, Ramos E: Knee and hip radiographic osteoarthritis features: differences on pain, function and quality of life. Clin Rheumatol 2016, 35(6):1555–1564. [DOI] [PubMed] [Google Scholar]
- 13.Wen L, Shin MH, Kang JH, Yim YR, Kim JE, Lee JW, Lee KE, Park DJ, Kim TJ, Park YW et al. : The relationships between bone mineral density and radiographic features of hand or knee osteoarthritis in older adults: data from the Dong-gu Study. Rheumatology (Oxford) 2016, 55(3):495–503. [DOI] [PubMed] [Google Scholar]
- 14.Englund M, Haugen IK, Guermazi A, Roemer FW, Niu J, Neogi T, Aliabadi P, Felson DT: Evidence that meniscus damage may be a component of osteoarthritis: the Framingham study. Osteoarthritis Cartilage 2016, 24(2):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Kruijf M, Verlinden VJ, Huygen FJ, Hofman A, van der Geest JN, Uitterlinden AG, Bierma-Zeinstra SM, Ikram MA, van Meurs JB: Chronic joint pain in the lower body is associated with gait differences independent from radiographic osteoarthritis. Gait Posture 2015, 42(3):354–359. [DOI] [PubMed] [Google Scholar]
- 16.Queen RM, Sparling TL, Schmitt D: Hip, Knee, and Ankle Osteoarthritis Negatively Affects Mechanical Energy Exchange. Clin Orthop Relat Res 2016, 474(9):2055–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastick AN, Belo JN, Runhaar J, Bierma-Zeinstra SM: What Are the Prognostic Factors for Radiographic Progression of Knee Osteoarthritis? A Meta-analysis. Clin Orthop Relat Res 2015. [DOI] [PMC free article] [PubMed]
- 18.Huetink K, Stoel BC, Watt I, Kloppenburg M, Bloem JL, Malm SH, Van’t Klooster R, Nelissen RG: Identification of factors associated with the development of knee osteoarthritis in a young to middle-aged cohort of patients with knee complaints. Clin Rheumatol 2015, 34(10):1769–1779. [DOI] [PubMed] [Google Scholar]
- 19.Wesseling J, Bastick AN, ten Wolde S, Kloppenburg M, Lafeber FP, Bierma-Zeinstra SM, Bijlsma JW: Identifying Trajectories of Pain Severity in Early Symptomatic Knee Osteoarthritis: A 5-year Followup of the Cohort Hip and Cohort Knee (CHECK) Study. J Rheumatol 2015, 42(8):1470–1477. [DOI] [PubMed] [Google Scholar]
- 20.Boiwka AV, Bajwa NS, Toy JO, Eubanks J, Ahn NU: Lumbar degenerative disc disease and tibiotalar joint arthritis: a 710-specimen postmortem study. Am J Orthop (Belle Mead NJ) 2015, 44(4):E100–105. [PubMed] [Google Scholar]
- 21.Van Spil WE, Welsing PM, Bierma-Zeinstra SM, Bijlsma JW, Roorda LD, Cats HA, Lafeber FP: The ability of systemic biochemical markers to reflect presence, incidence, and progression of early-stage radiographic knee and hip osteoarthritis: data from CHECK. Osteoarthritis Cartilage 2015, 23(8):1388–1397. [DOI] [PubMed] [Google Scholar]
- 22.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, Fang F, Schwartz TA, Abbate LM, Callahan LF et al. : Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol 2007, 34(1):172–180. [PubMed] [Google Scholar]
- 23.Lateef S, Golightly YM, Renner JB, Jordan JM, Nelson AE: A Cross-sectional Analysis of Radiographic Ankle Osteoarthritis Frequency and Associated Factors: The Johnston County Osteoarthritis Project. J Rheumatol 2017, 44(4):499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jordan JM, Linder GF, Renner JB, Fryer JG: The impact of arthritis in rural populations. Arthritis Care Res 1995, 8(4):242–250. [DOI] [PubMed] [Google Scholar]
- 25.Goode AP, Nelson AE, Kraus VB, Renner JB, Jordan JM: Biomarkers reflect differences in osteoarthritis phenotypes of the lumbar spine: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage 2017, 25(10):1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin J, Barbour KE, Murphy LB, Nelson AE, Schwartz TA, Helmick CG, Allen KD, Renner JB, Baker NA, Jordan JM: Lifetime Risk of Symptomatic Hand Osteoarthritis: The Johnston County Osteoarthritis Project. Arthritis Rheumatol 2017, 69(6):1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraus VB, Kilfoil TM, Hash TW 2nd, McDaniel G, Renner JB, Carrino JA, Adams S: Atlas of radiographic features of osteoarthritis of the ankle and hindfoot. Osteoarthritis Cartilage 2015, 23(12):2059–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kellgren JH, Lawrence JS: Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957, 16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burnett S, Hart DJ, Cooper C, Spector TD: A radiographic atlas of osteoarthritis London: Springer; 1994. [Google Scholar]
- 30.Menz HB, Munteanu SE, Landorf KB, Zammit GV, Cicuttini FM: Radiographic evaluation of foot osteoarthritis: sensitivity of radiographic variables and relationship to symptoms. Osteoarthritis Cartilage 2009, 17(3):298–303. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE: SF-36 health survey : manual and interpretation guide Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 32.White DK, Master H: Patient-Reported Measures of Physical Function in Knee Osteoarthritis. Rheum Dis Clin North Am 2016, 42(2):239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fries JF, Krishnan E, Rose M, Lingala B, Bruce B: Improved responsiveness and reduced sample size requirements of PROMIS physical function scales with item response theory. Arthritis Res Ther 2011, 13(5):R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Racaza GZ, Salido EO, Penserga EG: Clinical profile of Filipino patients with osteoarthritis seen at two arthritis clinics. Int J Rheum Dis 2012, 15(4):399–406. [DOI] [PubMed] [Google Scholar]
- 35.Veronese N, Trevisan C, De Rui M, Bolzetta F, Maggi S, Zambon S, Musacchio E, Sartori L, Perissinotto E, Crepaldi G et al. : Association of Osteoarthritis With Increased Risk of Cardiovascular Diseases in the Elderly: Findings From the Progetto Veneto Anziano Study Cohort. Arthritis Rheumatol 2016, 68(5):1136–1144. [DOI] [PubMed] [Google Scholar]
- 36.Park JH, Hong JY, Han K, Suh SW, Park SY, Yang JH, Han SW: Prevalence of symptomatic hip, knee, and spine osteoarthritis nationwide health survey analysis of an elderly Korean population. Medicine (Baltimore) 2017, 96(12):e6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper C, Egger P, Coggon D, Hart DJ, Masud T, Cicuttini F, Doyle DV, Spector TD: Generalized osteoarthritis in women: pattern of joint involvement and approaches to definition for epidemiological studies. J Rheumatol 1996, 23(11):1938–1942. [PubMed] [Google Scholar]
- 38.Doherty M, Watt I, Dieppe P: Influence of primary generalised osteoarthritis on development of secondary osteoarthritis. Lancet 1983, 2(8340):8–11. [DOI] [PubMed] [Google Scholar]
- 39.Hart DJ, Spector TD: Cigarette smoking and risk of osteoarthritis in women in the general population: the Chingford study. Ann Rheum Dis 1993, 52(2):93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J, Ushiyama T, Inoue K, Kawasaki T, Hukuda S: Vitamin D receptor gene polymorphisms and osteoarthritis of the hand, hip, and knee: acase-control study in Japan. Rheumatology (Oxford) 2000, 39(1):79–84. [DOI] [PubMed] [Google Scholar]
- 41.Miura H, Kawano T, Takasugi S, Manabe T, Hosokawa A, Iwamoto Y: Two subtypes of radiographic osteoarthritis in the distal interphalangeal joint of the hand. J Orthop Sci 2008, 13(6):487–491. [DOI] [PubMed] [Google Scholar]
- 42.Naito K, Takahashi M, Kushida K, Suzuki M, Ohishi T, Miura M, Inoue T, Nagano A: Measurement of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in patients with knee osteoarthritis: comparison with generalized osteoarthritis. Rheumatology (Oxford) 1999, 38(6):510–515. [DOI] [PubMed] [Google Scholar]
- 43.Doherty M, Pattrick M, Powell R: Nodal generalised osteoarthritis is an autoimmune disease. Ann Rheum Dis 1990, 49(12):1017–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoogeboom TJ, Stukstette MJ, de Bie RA, Cornelissen J, den Broeder AA, van den Ende CH: Non-pharmacological care for patients with generalized osteoarthritis: design of a randomized clinical trial. BMC Musculoskelet Disord 2010, 11:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hopkinson ND, Powell RJ, Doherty M: Autoantibodies, immunoglobulins and Gm allotypes in nodal generalized osteoarthritis. Br J Rheumatol 1992, 31(9):605–608. [DOI] [PubMed] [Google Scholar]
- 46.Hordon LD, Stewart SP, Troughton PR, Wright V, Horsman A, Smith MA: Primary generalized osteoarthritis and bone mass. Br J Rheumatol 1993, 32(12):1059–1061. [DOI] [PubMed] [Google Scholar]
- 47.Malaviya AN, Shehab D, Bhargava S, Al-Jarallah K, Al-Awadi A, Sharma PN, Al-Ghuriear S, Al-Shugayer A: Characteristics of osteoarthritis among Kuwaitis: a hospital-based study. Clin Rheumatol 1998, 17(3):210–213. [DOI] [PubMed] [Google Scholar]
- 48.Carroll GJ, Breidahl WH, Jazayeri J: Confirmation of two major polyarticular osteoarthritis (POA) phenotypes--differentiation on the basis of joint topography. Osteoarthritis Cartilage 2009, 17(7):891–895. [DOI] [PubMed] [Google Scholar]
- 49.Dougados M, Nakache JP, Gueguen A: Criteria for generalized and focal osteoarthritis. Rev Rhum Engl Ed 1996, 63(9):569–575. [PubMed] [Google Scholar]
- 50.Min JL, Meulenbelt I, Riyazi N, Kloppenburg M, Houwing-Duistermaat JJ, Seymour AB, Pols HA, van Duijn CM, Slagboom PE: Association of the Frizzled-related protein gene with symptomatic osteoarthritis at multiple sites. Arthritis Rheum 2005, 52(4):1077–1080. [DOI] [PubMed] [Google Scholar]
- 51.Price T, Hesp R, Mitchell R: Bone density in generalized osteoarthritis. J Rheumatol 1987, 14(3):560–562. [PubMed] [Google Scholar]
- 52.Riyazi N, Rosendaal FR, Slagboom E, Kroon HM, Breedveld FC, Kloppenburg M: Risk factors in familial osteoarthritis: the GARP sibling study. Osteoarthritis Cartilage 2008, 16(6):654–659. [DOI] [PubMed] [Google Scholar]
- 53.Waldron HA: Prevalence and distribution of osteoarthritis in a population from Georgian and early Victorian London. Ann Rheum Dis 1991, 50(5):301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loughlin J, Irven C, Fergusson C, Sykes B: Sibling pair analysis shows no linkage of generalized osteoarthritis to the loci encoding type II collagen, cartilage link protein or cartilage matrix protein. Br J Rheumatol 1994, 33(12):1103–1106. [DOI] [PubMed] [Google Scholar]
- 55.Visser AW, de Mutsert R, le Cessie S, den Heijer M, Rosendaal FR, Kloppenburg M: The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: the NEO study. Ann Rheum Dis 2015, 74(10):1842–1847. [DOI] [PubMed] [Google Scholar]
- 56.Raja R, Dube B, Hensor EM, Hogg SF, Conaghan PG, Kingsbury SR: The clinical characteristics of older people with chronic multiple-site joint pains and their utilisation of therapeutic interventions: data from a prospective cohort study. BMC Musculoskelet Disord 2016, 17:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma L, Chmiel JS, Almagor O, Dunlop D, Guermazi A, Bathon JM, Eaton CB, Hochberg MC, Jackson RD, Kwoh CK et al. : Significance of preradiographic magnetic resonance imaging lesions in persons at increased risk of knee osteoarthritis. Arthritis Rheumatol 2014, 66(7):1811–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Spil WE, Jansen NW, Bijlsma JW, Reijman M, DeGroot J, Welsing PM, Lafeber FP: Clusters within a wide spectrum of biochemical markers for osteoarthritis: data from CHECK, a large cohort of individuals with very early symptomatic osteoarthritis. Osteoarthritis Cartilage 2012, 20(7):745–754. [DOI] [PubMed] [Google Scholar]
- 59.Williams SN, Wolford ML, Bercovitz A: Hospitalization for Total Knee Replacement Among Inpatients Aged 45 and Over: United States, 2000–2010. NCHS Data Brief 2015(210):1–8. [PubMed] [Google Scholar]
- 60.Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW: Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis 2005, 64(5):682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas E, Peat G, Croft P: Defining and mapping the person with osteoarthritis for population studies and public health. Rheumatology (Oxford) 2014, 53(2):338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gullo TR, Golightly YM, Flowers P, Jordan JM, Renner JB, Schwartz TA, Kraus VB, Hannan MT, Cleveland RJ, Nelson AE: Joint hypermobility and multijoint osteoarthritis in a community-based cohort. J Am Geriatr Soc 2018, 66(S2):S291. [Google Scholar]
- 63.Kellgren JH, Moore R: Generalized osteoarthritis and Heberden’s nodes. Br Med J 1952, 1(4751):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lawrence JS: Generalized osteoarthrosis in a population sample. Am J Epidemiol 1969, 90(5):381–389. [DOI] [PubMed] [Google Scholar]
- 65.Solomon L, Beighton P, Lawrence JS: Osteoarthrosis in a rural South African Negro population. Ann Rheum Dis 1976, 35(3):274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brighton SW, de la Harpe AL, Van Staden DA: The prevalence of osteoarthrosis in a rural African community. Br J Rheumatol 1985, 24(4):321–325. [DOI] [PubMed] [Google Scholar]
- 67.Gunther KP, Sturmer T, Sauerland S, Zeissig I, Sun Y, Kessler S, Scharf HP, Brenner H, Puhl W: Prevalence of generalised osteoarthritis in patients with advanced hip and knee osteoarthritis: the Ulm Osteoarthritis Study. Ann Rheum Dis 1998, 57(12):717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ushiyama T, Ueyama H, Inoue K, Nishioka J, Ohkubo I, Hukuda S: Estrogen receptor gene polymorphism and generalized osteoarthritis. J Rheumatol 1998, 25(1):134–137. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


