Abstract
Accumulating evidence suggests that oxidative stress plays an important role in the progression of osteoarthritis (OA), and pyrroloquinoline quinone (PQQ) is considered a strong antioxidant. However, it is unclear whether PQQ can prevent the progression of OA by inhibiting oxidative stress. In this study, anterior cruciate ligament transection (ACLT)-induced OA mice received a diet supplemented with/without PQQ, and were compared with each other and with sham-operated mice. Our results showed that in PQQ-untreated OA mice, articular surfaces collapsed, while the thickness of articular cartilage and the abundance of cartilage matrix protein decreased significantly, whereas PQQ supplementation largely prevented these alterations. We also found that oxidative stress, DNA damage, cellular senescence and the secretion of senescence-associated inflammatory cytokines were increased in PQQ-untreated OA mice compared with sham-operated mice. However, these parameters were obviously rescued in PQQ-treated OA mice. This study demonstrated that PQQ supplementation can prevent ACLT-induced OA by inhibiting oxidative stress, DNA damage, cell senescence and the development of the senescence-associated secretory phenotype.
Keywords: Osteoarthritis, pyrroloquinoline quinone, oxidative stress, chondrocyte senescence
Introduction
Osteoarthritis (OA) is the most common cause of loss of function and reduced ability to complete activities of daily living in elderly adults. Knee OA is a highly prevalent condition among the elderly, characterized by the progressive breakdown of the articular cartilage that influences the articular cartilage, the subchondral bone, and the synovium, and is accompanied by pain, immobility, muscle weakness, and chronic disability [1]. The degeneration can be alleviated by oral anti-inflammatory drugs and intra-articular injection of hyaluronan, but none of these treatments can completely cure the disease except total knee arthroplasty which is invasive and expensive [2]. It is therefore essential to suppress the progression of articular cartilage degeneration at an early stage of the disease.
Among the risk factors involved in the pathological course of OA are aging, obesity, female sex, heredity and injury. However, the pathological mechanism remains unclear. Recently, an increasing number of studies have concluded that OA progression is significantly related to oxidative stress. Antioxidant enzymes, such as SOD, CAT, and GPX, are decreased in OA patients, confirming the role of oxidative stress in the progression of OA [3-5]. When the production of ROS exceeds levels of antioxidant, the expression of lipid peroxidation products and nitrated products is increased in the biological fluids and cartilage of OA patients and in animal models of OA [6,7]. It is commonly believed that when cells including chondrocytes undergo oxidative stress, this triggers activation of the DNA damage response and cell senescence [8]. This has subsequently been linked to the development of OA. Evidence in support of this was provided by extensive staining of a marker of oxidative damage in the degenerated OA cartilage and marked expression of senescent phenotypes when supplementing cultured chondrocytes with ROS which was associated with oxidative stress [9,10].
Senescent cells, referred to as the senescence-associated secretory phenotype (SASP), secrete increased levels of cytokines including IL-1 and IL-6, growth factors and matrix metalloproteinases (MMPs) [11]. More evidence shows that chondrocytes can exhibit features of the SASP which has significant effects on the role of chondrocyte senescence in the development and progression of OA. It is accepted that inflammatory cytokines such as IL-1α, IL-1β, IL-6, MMP-3 and MMP-13 can inhibit the synthesis of type II collagen and aggrecan, and accelerate the degeneration of cartilage and subchondral bone [12,13].
Pyrroloquinoline quinone (PQQ) was initially acted as a redox cofactor of dehydrogenases in the bacterial system, was first identified as a coenzyme for methanol dehydrogenase [14]. During the past decade, abundant evidence has come to light suggesting that PQQ is a strong antioxidant, including acting to ameliorate oxidative stress and lipid peroxidation in the brain of streptozotocin-induced diabetic mice [15], modulating mitochondrial quantity and function in aging rats [16], and exerting long-term protective effects on developmental programming of hepatic lipotoxicity and inflammation in obese mice [17]. Prior study suggests that PQQ exerts a chondroprotective effect during the progression of OA by inhibiting nitric oxide production and metalloproteinase synthesis [18]. Whether PQQ can prevent OA by inhibiting oxidative stress, DNA damage and chondrocyte senescence has not been investigated and any related mechanism is unknown.
To determine the effect of PQQ on knee OA, we established an OA model in mice by transection of the anterior cruciate ligament (ACLT) and then supplementing the ACTL mice with/without PQQ in the diet from 3-weeks-old. The control mice underwent a sham operation and were fed a normal diet. We examined the phenotype of articular cartilage in these mice to investigate whether PQQ can inhibit the progression of OA by inhibiting oxidative stress, DNA damage and chondrocyte senescence.
Materials and methods
Mice
Female C57/BL6J mice were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). They were maintained in a virus- and parasite-free barrier facility and exposed to a 12-h light, 12-h dark cycle. Mice were randomly divided into three groups: 1) sham surgery with a normal diet; 2) ACLT group with a normal diet; 3) ACLT group supplemented with PQQ in the diet from 3 weeks old to 28 weeks old. ACLT mice underwent ACLT surgery at 24 weeks of age to induce mechanical instability-associated OA. The PQQ diet was obtained by supplementing the normal diet with 4 mg PQQ per kg. Sham operations were independently performed. All mice were sacrificed at 28 weeks of age, 1 month after surgery. This study was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University.
Anterior cruciate ligament transaction (ACLT)
Mice were anesthetized with chloral hydrate. The right knee joint was exposed through a medial parapatellar approach. The anterior cruciate ligament (ACL) was then transected with micro-scissors. Complete transection was confirmed by a positive anterior drawer sign. The control group underwent arthrotomy without transection of the ACL.
Microcomputed tomography (Micro-CT)
After sacrifice, the right knees were removed and fixed in PLP fixative (2% paraformaldehyde containing 0.075 M lysine and 0.01 M sodium periodate). These samples were then scanned on a micro-CT scanner (Sky Scan 1072 Scanner) using energy of 100 kV, and 98 µA intensity. Three-dimensional (3D) images were generated using the 3D Creator software supplied with the instrument as described previously [19].
Histology
The right knees were removed and dissected free of soft tissue, fixed with PLP fixative at 4°C and processed histologically. Then the knees were decalcified in EDTA glycerol solution for 5 to 7 days at 4°C. The decalcified knees were dehydrated and embedded in paraffin, after which 5 μm sections were cut on a rotary microtome. The sections were stained with 0.1% safranin-O and 0.02% Fast Green or tartrate-resistant acid phosphatase (TRAP) activity according to a previously-described protocol [20] or stained immunohistochemically as described below.
Immunohistochemical staining
Immunohistochemical staining was carried out for type II collagen, 8-hydroxy-2’-deoxyguanosine (8-OHdG), phosphorylation of histone H2AX on Ser139 (γ-H2AX), β-galactosidase (β-gal), p16INK4a, interleukin (IL)-1α, IL-1β, IL-6, matrix metalloproteinase (MMP)-3 and MMP-13 using the avidin-biotin-peroxidase complex technique with affinity-purified rabbit anti-mouse type II collagen antibody (Abcam, Cambridge, MA, USA), 8-OHdG (Abcam), γ-H2AX (Cell Signaling Technology, Danvers, MA, USA), β-gal (Santa Cruz Biotechnology, Santa Cruz, CA, USA), p16INK4a (Abcam), IL-1α (Santa Cruz Biotechnology), IL-1β (Santa Cruz Biotechnology), MMP-3 (Santa Cruz Biotechnology), and MMP-13 (Santa Cruz Biotechnology) following previously-described methods [20].
Western blotting
Proteins were extracted from the articular cartilage of each group of mice. Immunoblotting was carried out as previously described [21]. Primary antibodies against SOD2 (Novus Biological, Centennial, CO, USA), Prdx 1 (Santa Cruz Biotechnology), Prdx 4 (Santa Cruz Biotechnology), γ-H2AX (Cell Signaling Technology), p16INK4a (Abcam), β-actin (Bioworld Technology, St. Louis Park MN, USA) were used. Immunoreactive bands were visualized with ECL chemiluminescence (Amersham Biosciences, Chalfont St. Giles, UK) and analyzed by Scion Image Beta 4.02 (Scion, National Institutes of Health, Bethesda, MD, USA).
Detection of ROS levels
The chondrocytes from articular cartilage were converted into single cell suspensions as follows: tissue was trypsinized, washed with cold PBS, and resuspended in binding buffer, then 106 cells per sample were incubated with 5 µL 2’,7’-dichlorofluorescein diacetate (DCFH-DA, Sigma-Aldrich, St. Louis, MO, USA) for 30 mins in the dark followed by incubation with 10% FBS for 20 mins at 37°C. ROS levels were calculated from mean fluorescence intensity (MFI) measured using a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
RNA isolation and quantitative real-time RT-PCR
RNA was isolated from articular cartilage with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Reverse transcription reactions were performed with the SuperScript First-Strand Synthesis System (Invitrogen) as previously described [22]. To determine the relative expression of genes of interest, quantitative real-time RT-PCR was carried out in an Applied Biosystems Cycler with a SYBR Green PCR reagent kit. The PCR primers were used as described previously [20,21]. GAPDH was used as the internal control for each reaction. All primers were tested for their specificity by conventional PCR before being used for quantitative analysis by real-time RT-PCR. Results were analyzed with SDS 7300 software, and the relative amount of mRNA was calculated after normalization for GAPDH mRNA.
Computer-assisted image analysis
After histochemical or immunohistochemical staining of sections from five mice of each group, images of micrographs from single sections were digitally recorded using a rectangular template, and recordings were processed and analysed using Northern Eclipse image analysis software as described previously [20].
Statistical analysis
All analyses were performed using SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA). Measured data are presented as mean ± SEM. Statistical comparisons were performed using a one-way ANOVA of qualitative data to compare differences between groups. P values < 0.05 were considered statistically significant.
Results
Effect of PQQ on the articular cartilage and subchondral bone of mice with ACLT-induced OA
To examine the effect of PQQ on ACLT-induced OA, we first evaluated the joints of mice using micro-CT. Compared with sham-operated mice, PQQ-untreated ACLT mice were found to have an irregular surface to their articular cartilage; however, dietary PQQ supplementation markedly rescued this problem (Figure 1A). To verify whether alterations of articular imaging were associated with cellular alterations of cartilage and subchondral bone, paraffin sections of knees from mice with or without PQQ supplementation were stained immunohistochemically for type II collagen and histochemically for glycosaminoglycan and TRAP. The results showed that in PQQ-untreated ACLT mice, both the areas stained positive for type II collagen and the intensity of Safranin O staining in the matrix were reduced significantly, while decreased thickness of the articular cartilage and disorganized chondrocytes were observed (Figure 1B, 1C, 1E, 1F). In PQQ-treated ACLT mice, however, most areas of the articular cartilage showed strong staining with Safranin O or type II collagen, while the surface integrity and columnar arrangement of chondrocytes were not disrupted in these joints. These results suggested that the cartilage matrix had not been degraded in PQQ-treated ACLT mice. We also found that TRAP-positive osteoclast numbers in the subchondral bone of PQQ-untreated ACLT mice were increased significantly compared with sham-operated mice (Figure 1D and 1G). However, they were substantially rescued in PQQ-treated ACLT mice. These findings indicated that PQQ prevented cartilage degeneration.
Figure 1.
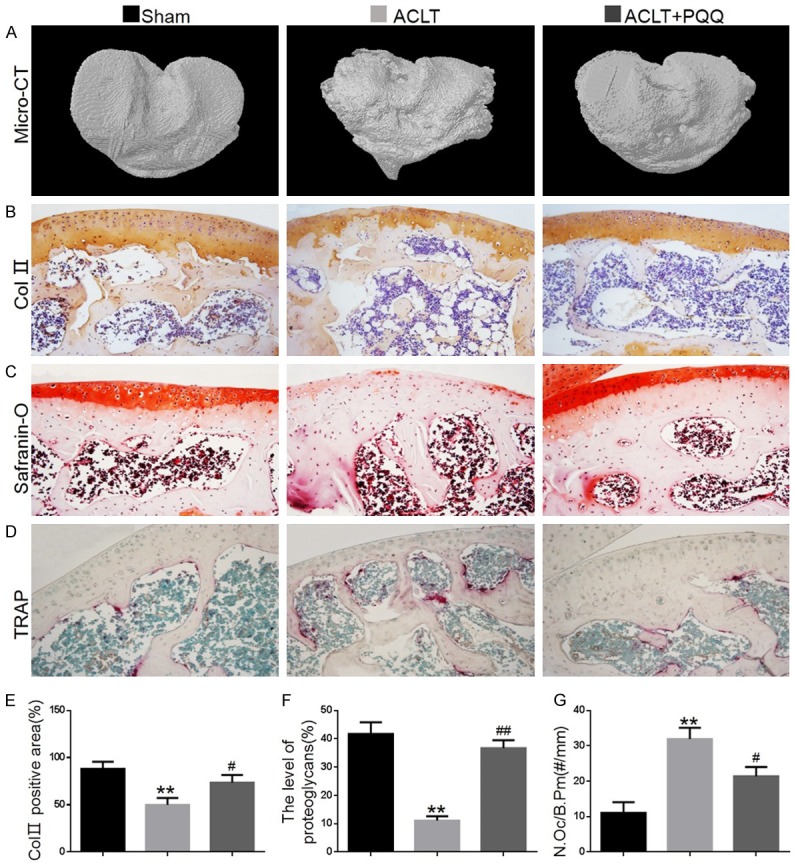
The effect of PQQ on articular cartilage and subchondral bone of ACLT-induced OA mice. (A) Representative Micro-CT-scanned and 3D reconstructed sections of mouse tibial condyles. Representative photomicrographs of paraffin sections of articular cartilage from 28-week-old mice of each group stained (B) immunohistochemically for type II collagen and (C) histochemically for glycosaminoglycan and (D) TRAP. (E) The percentage of the Col II-immunopositive area was determined by image analysis. (F) The level of proteoglycans was determined by image analysis. (G) The number of TRAP-positive osteoclasts (N.Oc) per mm bone perimeter (B.Pm) was measured in the subchondral bone of TRAP-stained condyles. Data are presented as the mean ± SEM of determinations, each data-point was the mean of five specimens. **P < 0.01 versus sham-operated mice. #P < 0.05, ##P < 0.01 versus PQQ-untreated ACLT mice. (B-D) Magnification, 200×.
The effect of PQQ on redox balance in ACLT-induced OA mice
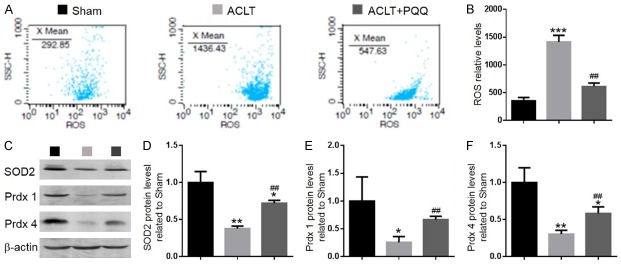
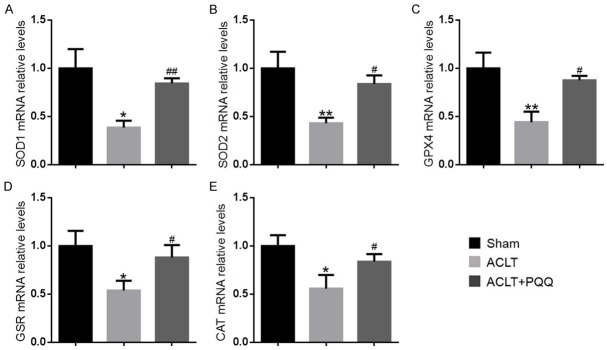
To investigate whether the effect of PQQ on OA was associated with regulation of oxidative stress, we measured the ROS levels and the mRNA levels of antioxidant enzymes in articular cartilage. We found that ROS levels were increased (Figure 2A and 2B), while protein levels (Figure 2C-F) of SOD2, peroxiredoxin 1 (Prdx1) and peroxiredoxin 4 (Prdx4) and mRNA levels of antioxidant enzymes, including SOD1, SOD2, GPX4, GSR and CAT (Figure 3A-E) were significantly reduced in PQQ-untreated ACLT mice compared with the sham-operated group; however, these parameters were rescued significantly in PQQ-treated ACLT mice. These results indicated that PQQ may prevent cartilage degeneration by inhibiting oxidative stress.
Figure 2.
The effect of PQQ on oxidative stress in articular cartilage of ACLT-induced OA mice. (A) Representative flow cytometric analysis of ROS levels of articular cartilage chondrocytes from sham, ACLT and ACLT+PQQ mice. (B) Relative fluorescence intensity (RFI) of ROS was calculated and expressed relative to the sham mice. (C) Representative western blots of articular cartilage extracts showing expression of SOD2, peroxiredoxin 1 (Prdx1) and peroxiredoxin 4 (Prdx4); β-actin was used as loading control for the western blots in the sham, ACLT and ACLT+PQQ groups. (D) SOD2, (E) Prdx1, (F) Prdx4 protein levels relative to β-actin protein levels were assessed by densitometric analysis and expressed relative to levels in sham-operated mice. Data are presented as the mean ± SEM of determinations, each data-point was the mean of five specimens. *P < 0.05, **P < 0.01, ***P < 0.001 versus sham mice. ##P < 0.01 versus PQQ-untreated ACLT mice.
Figure 3.
The effect of PQQ on oxidative stress in articular cartilage of ACLT-induced OA mice. Real-time RT-PCR was performed on articular cartilage extracts from 28-week-old mice of each group. Gene expression of (A) SOD1, (B) SOD2, (C) GPX4, (D) GSR and (E) CAT are shown. Messenger RNA expression, assessed by real-time RT-PCR analysis, was calculated as a ratio to the GAPDH mRNA level and expressed relative to levels in sham mice. Data are presented as the mean ± SEM of determinations, each data point is the mean of five specimens. *P < 0.05, **P < 0.01, versus sham mice. #P < 0.05, ##P < 0.01 versus PQQ-untreated ACLT mice.
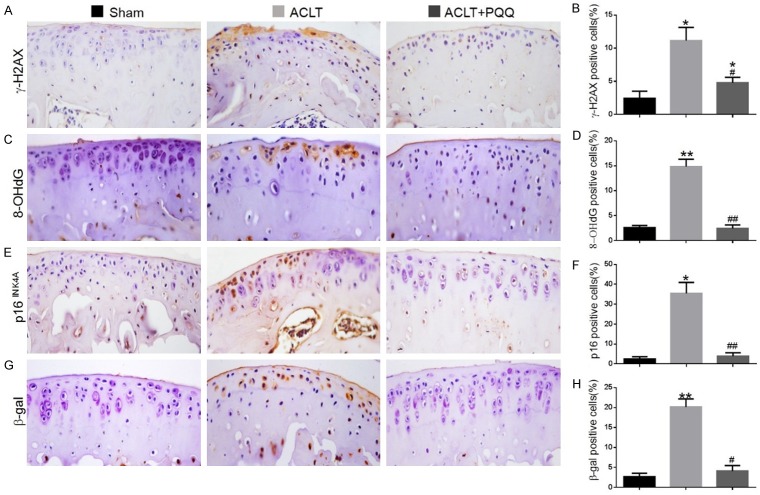
The effect of PQQ on DNA damage and cellular senescence in ACLT-induced OA mice
To investigate whether increased oxidative stress can induce DNA damage and chondrocyte senescence in ACLT-induced OA mice, the DNA damage markers, γ-H2AX and 8-OHdG, and the cellular senescence markers, β-gal and p16INK4a, were examined by immunohistochemical staining in paraffin sections of articular cartilage. Results revealed that the percentages of chondrocytes positive for γ-H2AX (Figure 4A and 4B), 8-OHdG (Figure 4C and 4D), β-gal (Figure 4E and 4F) and p16INK4a (Figure 4G and 4H) and the protein levels of γ-H2AX and p16INK4a (Figure 5A-C) were increased significantly in the articular cartilage of PQQ-untreated ACLT mice compared with the sham-operated mice, whereas these parameters were downregulated dramatically in PQQ-treated ACLT mice.
Figure 4.
The effect of PQQ on DNA damage and cellular senescence in articular cartilage of ACLT-induced OA mice. Representative micrographs of paraffin sections of articular cartilage from 28-week-old mice of each group stained immunohistochemically for (A) γ-H2AX, (C) 8-OHdG, (E) p16INK4a and (G) β-gal. The percentages of (B) γ-H2AX-positive, (D) 8-OHdG-positive, (F) p16INK4a-positive and (H) β-gal-positive cells were determined by image analysis. Data are presented as the mean ± SEM of determinations, each data-point was the mean of five specimens. *P < 0.05, **P < 0.01 versus sham mice. #P < 0.05, ##P < 0.01 versus PQQ-untreated ACLT mice. (A, C, E, G) Magnification, 400×.
Figure 5.
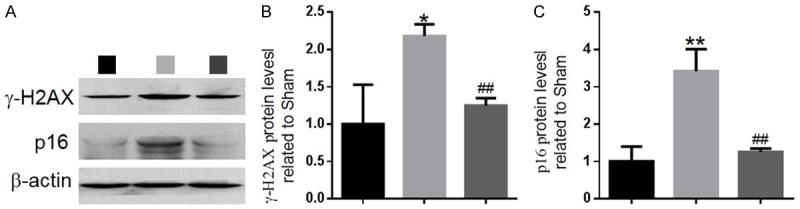
The effect of PQQ on DNA damage and cellular senescence in articular cartilage of ACLT-induced OA mice. (A) Representative western blots of articular cartilage extracts analysed for expression of γ-H2AX and p16INK4a. β-actin was used as loading control for western blots in the sham, ACLT and ACLT+PQQ mice. (B) γ-H2AX and (C) p16INK4a protein levels relative to β-actin protein levels were assessed by densitometric analysis and expressed relative to levels in sham mice. Data are presented as the mean ± SEM of determinations; each data-point is the mean of five specimens. *P < 0.05, **P < 0.01 versus sham mice. ##P < 0.01 versus PQQ-untreated ACLT mice.
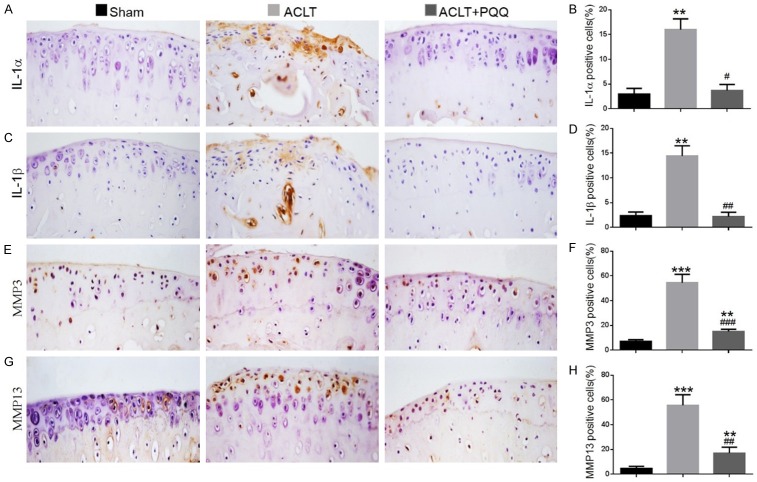
Effect of PQQ on the production of senescence-associated inflammatory cytokines in ACLT-induced OA mice
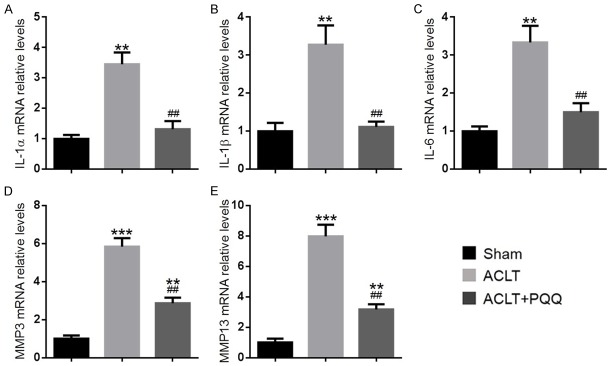
To determine whether increased cellular senescence could induce senescence-associated inflammatory cytokine production in chondrocytes, the expression of inflammatory cytokines was examined in articular cartilage by immunohistochemistry and real-time RT-PCR. Compared with the sham group, the percentage of chondrocytes positive for IL-1α (Figure 6A and 6B), IL-1β (Figure 6C and 6D), MMP-3 (Figure 6E and 6F), and MMP-13 (Figure 6G and 6H), and the gene expression levels of IL-1α, IL-1β, IL-6, MMP-3, and MMP-13 (Figure 7A-E) were all upregulated significantly in articular cartilage of PQQ-untreated ACLT mice. However, they were markedly rescued by PQQ supplementation.
Figure 6.
The effect of PQQ on the secretion of senescence-associated inflammatory cytokines in articular cartilage of ACLT-induced OA mice. Representative micrographs of paraffin sections of articular cartilage from 28-week-old mice of each group stained immunohistochemically for (A) IL-1α, (C) IL-1β, (E) MMP-3 and (G) MMP-13. The percentages of (B) IL-1α-positive, (D) IL-1β-positive, (F) MMP-3-positive and (H) MMP-13-positive cells were determined by image analysis. Data are presented as the mean ± SEM of determinations; each data-point is the mean of five specimens. **P < 0.01, ***P < 0.001 versus sham mice. #P < 0.05, ##P < 0.01, ###P < 0.001 versus PQQ-untreated ACLT mice. (A, C, E, G) Magnification, 400×.
Figure 7.
The effect of PQQ on the secretion of senescence-associated inflammatory cytokines in the articular cartilage of ACLT-induced OA mice. Real-time RT-PCR was performed on extracts of articular cartilage from 28-week-old mice of each group to investigate gene expression of (A) IL-1α, (B) IL-1β, (C) IL-6, (D) MMP-3 and (E) MMP-13. Messenger RNA expression, assessed by real-time RT-PCR analysis, was calculated as a ratio to the GAPDH mRNA level and shown relative to levels in sham mice. Data are presented as the mean ± SEM of determinations, each data-point is the mean of five specimens. **P < 0.01, ***P < 0.001 versus sham mice. ##P < 0.01 versus PQQ-untreated ACLT mice.
Discussion
OA is the most prevalent disease characterized by degeneration of articular cartilage, causing pain and physical disability [22]. Advancing age and trauma are the main risk factors for the development of OA. Finding effective therapies to slow OA progression is therefore especially critical.
Multiple pathways have been linked to OA. Oxidative stress is known to be detrimental to many cells and has been described as an important factor during disease and with aging. A large number of studies have concluded that oxidative stress has a role in the progression of OA, and oxidative stress has been found to be elevated in OA patients [5,23]. Chondrocytes express a variety of antioxidants which can protect cells from oxidative stress damage, including CAT, SOD and GPX. Alterations in the levels of these enzymes and increased levels of lipid peroxides cause loss of homeostasis in the maintenance of healthy articular cartilage that leads to the pathological degeneration of articular cartilage in OA with increasing age [24,25]. This study examined whether oxidative stress was increased in ACLT-induced OA mice. Results showed that ROS levels were increased and mRNA levels of endogenous antioxidants, including SOD1, SOD2, GPX4, GSR and CAT, were significantly downregulated in articular cartilage of ACLT-induced OA mice. Consequently, increased oxidative stress may contribute to ACLT-induced OA.
Pyrroloquinoline quinine (PQQ) has been shown to act as an antioxidant, modulating the oxidative stress response of living cells in vivo. It has been suggested that PQQ is a potent neuroprotective nutrient that functions as a scavenger of ROS, especially superoxide [26]. In the same way as the antioxidant NAC, PQQ treatment reversed the increase in the ROS level of skeletal muscle atrophy samples [27]. Recently, several reports have revealed that PQQ plays an important role in rescuing osteoporosis by upregulating anti-oxidant ability and inhibiting oxidative stress [19,28]. In the current study, PQQ treatment largely ameliorated the phenotype of OA and reduced oxidative stress. These results were consistent with previous studies that suggested that PQQ participates in the antioxidant mechanism and protects cells from oxidative damage.
There is accumulating evidence for age-related oxidative stress in many tissues, and oxidative stress has been found to induce DNA damage and cell senescence [29,30]. Chondrocyte senescence is triggered by oxidative damage and contributes to the abnormal inflammatory environment present in OA [31]. Compared with chondrocytes isolated from normal cartilage, articular chondrocytes from older adults and OA chondrocytes positively express a variety of senescence-associated markers, including telomere attrition, activated DNA damage response (DDR), SA-β-gal activity, and increased p16INK4a expression [32-35]. In the current study, the DNA damage markers 8-OHdG and γ-H2AX, and the cellular senescence markers, β-gal and p16INK4a, were increased in the articular cartilage of PQQ-untreated ACLT mice. These results suggested that increased oxidative stress in ACLT-induced OA can trigger DNA damage, ultimately leading to chondrocyte senescence.
Senescent cells can exhibit a complex proinflammatory response termed the senescence-associated secretory phenotype (SASP) that enables them to communicate with other cells, as well as the microenvironment, stimulating neighbouring cells to senesce [36]. There is evidence that chondrocytes can exhibit features of the SASP, which has a pivotal effect on the role of chondrocyte senescence in OA. In our study, we found that the percentage of IL-1α-, IL-1β-, MMP-3- and MMP-13-positive chondrocytes and the gene expression levels of IL-1α, IL-1β, IL-6, MMP-3, and MMP-13 were all increased significantly in articular cartilage from PQQ-untreated ACLT mice compared to the sham group. Chondrocyte SASP including production of proinflammatory cytokines and matrix-degrading proteases can disturb metabolism and enhance catabolism of cartilage in OA. Accumulating studies have demonstrated that IL-1β in articular cartilage can induce the production of proinflammatory cytokines such as IL-6 and of chemokines such as IL-8 to amplify and perpetuate the OA process [37,38]. Levels of IL-1β and IL-6 are elevated in the synovial fluid and cartilage of OA patients and can reduce expression of type II collagen and stimulate the release of MMPs [39,40]. Senescent cells also secrete increased levels of some MMPs. It has been reported that MMP-13 serves as a major mediator of type II collagen cleavage, and immunostaining of MMP-3 and MMP-13 has been shown to increase in cartilage with aging [12]. Our results also found that the expression of IL-1α, IL-1β, IL-6, MMP-3 and MMP-13 was increased dramatically in ACLT-induced OA mice. These secreted products of the SASP may lead to breakdown of the cartilage matrix including type II collagen and glycosaminoglycan, thus resulting in articular cartilage degradation.
A large number of studies have illustrated that inhibiting cell senescence may be an effective therapy for OA. Selective clearance of the senescent cells from in vitro cultures of chondrocytes isolated from OA patients reduced expression of senescence and inflammatory markers while also increasing expression of extracellular matrix proteins in cartilage tissue [41,42]. In our study, PQQ supplementation efficiently suppressed the expression of cell senescence markers and the development of the SASP. These results suggested that PQQ could be a new therapy with the ability to slow or stop the progression of OA.
In conclusion, our study demonstrated that PQQ played a preventive and protective role in ACLT-induced OA by inhibiting oxidative stress, DNA damage, cell senescence and SASP secretion. Our results from this study provided experimental evidence of another pathway for the clinical application of PQQ, suggesting that PQQ could be considered as a potential therapeutic agent for OA prevention and treatment.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (81500682 to L.C.). And we thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Newberry SJ, FitzGerald J, SooHoo NF, Booth M, Marks J, Motala A, Apaydin E, Chen C, Raaen L, Shanman R, Shekelle PG. Treatment of osteoarthritis of the knee: an update review. Rockville MD. 2017 [PubMed] [Google Scholar]
- 2.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004;427(Suppl):S6–15. doi: 10.1097/01.blo.0000143938.30681.9d. [DOI] [PubMed] [Google Scholar]
- 3.Soran N, Altindag O, Cakir H, Celik H, Demirkol A, Aksoy N. Assessment of paraoxonase activities in patients with knee osteoarthritis. Redox Rep. 2008;13:194–198. doi: 10.1179/135100008X308911. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Moreno M, Soto-Hermida A, Pertega S, Oreiro N, Fernandez-Lopez C, Rego-Perez I, Blanco FJ. Mitochondrial DNA (mtDNA) haplogroups and serum levels of anti-oxidant enzymes in patients with osteoarthritis. BMC Musculoskelet Disord. 2011;12:264. doi: 10.1186/1471-2474-12-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altindag O, Erel O, Aksoy N, Selek S, Celik H, Karaoglanoglu M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol Int. 2007;27:339–344. doi: 10.1007/s00296-006-0247-8. [DOI] [PubMed] [Google Scholar]
- 6.Karan A, Karan MA, Vural P, Erten N, Tascioglu C, Aksoy C, Canbaz M, Oncel A. Synovial fluid nitric oxide levels in patients with knee osteoarthritis. Clin Rheumatol. 2003;22:397–399. doi: 10.1007/s10067-003-0761-y. [DOI] [PubMed] [Google Scholar]
- 7.Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 8.Lim HD, Kim YS, Ko SH, Yoon IJ, Cho SG, Chun YH, Choi BJ, Kim EC. Cytoprotective and anti-inflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J Pineal Res. 2012;53:225–237. doi: 10.1111/j.1600-079X.2012.00991.x. [DOI] [PubMed] [Google Scholar]
- 9.Dai SM, Shan ZZ, Nakamura H, Masuko-Hongo K, Kato T, Nishioka K, Yudoh K. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Semin Arthritis Rheum. 2006;54:818–831. doi: 10.1002/art.21639. [DOI] [PubMed] [Google Scholar]
- 10.Yudoh K, Nguyen vT, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther. 2005;7:R380–391. doi: 10.1186/ar1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Gene Dev. 2014;28:99–114. doi: 10.1101/gad.235184.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu W, Billinghurst RC, Pidoux I, Antoniou J, Zukor D, Tanzer M, Poole AR. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Semin Arthritis Rheum. 2002;46:2087–2094. doi: 10.1002/art.10428. [DOI] [PubMed] [Google Scholar]
- 13.Loeser RF. The role of aging in the development of osteoarthritis. Trans Am Clin Climatol Assoc. 2017;128:44–54. [PMC free article] [PubMed] [Google Scholar]
- 14.Stites TE, Mitchell AE, Rucker RB. Physiological importance of quinoenzymes and the O-quinone family of cofactors. J Nutr. 2000;130:719–727. doi: 10.1093/jn/130.4.719. [DOI] [PubMed] [Google Scholar]
- 15.Kumar N, Kar A. Pyrroloquinoline quinone ameliorates oxidative stress and lipid peroxidation in the brain of streptozotocin-induced diabetic mice. Can J Physiol Pharmacol. 2015;93:71–79. doi: 10.1139/cjpp-2014-0270. [DOI] [PubMed] [Google Scholar]
- 16.Singh AK, Pandey SK, Saha G, Gattupalli NK. Pyrroloquinoline quinone (PQQ) producing Escherichia coli Nissle 1917 (EcN) alleviates age associated oxidative stress and hyperlipidemia, and improves mitochondrial function in ageing rats. Exp Gerontol. 2015;66:1–9. doi: 10.1016/j.exger.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Jonscher KR, Stewart MS, Alfonso-Garcia A, DeFelice BC, Wang XX, Luo Y, Levi M, Heerwagen MJ, Janssen RC, de la Houssaye BA, Wiitala E, Florey G, Jonscher RL, Potma EO, Fiehn O, Friedman JE. Early PQQ supplementation has persistent long-term protective effects on developmental programming of hepatic lipotoxicity and inflammation in obese mice. FASEB J. 2017;31:1434–1448. doi: 10.1096/fj.201600906R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao R, Wang S, Xia X, Wang Y, Cao Y, Huang Y, Xu X, Liu Z, Liu P, Tang X, Liu C, Shen G, Zhang D. Pyrroloquinoline quinone slows down the progression of osteoarthritis by inhibiting nitric oxide production and metalloproteinase synthesis. Inflammation. 2015;38:1546–1555. doi: 10.1007/s10753-015-0129-x. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Li J, Zhang H, Wang H, Yin G, Miao D. Pyrroloquinoline quinone prevents testosterone deficiency-induced osteoporosis by stimulating osteoblastic bone formation and inhibiting osteoclastic bone resorption. Am J Transl Res. 2017;9:1230–1242. [PMC free article] [PubMed] [Google Scholar]
- 20.Shen M, Luo Y, Niu Y, Chen L, Yuan X, Goltzman D, Chen N, Miao D. 1,25(OH)2D deficiency induces temporomandibular joint osteoarthritis via secretion of senescence-associated inflammatory cytokines. Bone. 2013;55:400–409. doi: 10.1016/j.bone.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Chen L, Zhi C, Shen M, Sun W, Miao D, Yuan X. 1,25(OH)2D3 deficiency induces colon inflammation via secretion of senescence-associated inflammatory cytokines. PLoS One. 2016;11:e0146426. doi: 10.1371/journal.pone.0146426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 23.Altay MA, Erturk C, Bilge A, Yapti M, Levent A, Aksoy N. Evaluation of prolidase activity and oxidative status in patients with knee osteoarthritis: relationships with radiographic severity and clinical parameters. Rheumatol Int. 2015;35:1725–1731. doi: 10.1007/s00296-015-3290-5. [DOI] [PubMed] [Google Scholar]
- 24.Poole AR. Can serum biomarker assays measure the progression of cartilage degeneration in osteoarthritis? Semin Arthritis Rheum. 2002;46:2549–2552. doi: 10.1002/art.10586. [DOI] [PubMed] [Google Scholar]
- 25.Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 2016;1862:576–591. doi: 10.1016/j.bbadis.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Hara H, Hiramatsu H, Adachi T. Pyrroloquinoline quinone is a potent neuroprotective nutrient against 6-hydroxydopamine-induced neurotoxicity. Neurochem Res. 2007;32:489–495. doi: 10.1007/s11064-006-9257-x. [DOI] [PubMed] [Google Scholar]
- 27.Qiu J, Fang Q, Xu T, Wu C, Xu L, Wang L, Yang X, Yu S, Zhang Q, Ding F, Sun H. Mechanistic role of reactive oxygen species and therapeutic potential of antioxidants in denervation- or fasting-induced skeletal muscle atrophy. Front Physiol. 2018;9:215. doi: 10.3389/fphys.2018.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Chen N, Miao D. Pyrroloquinoline quinone plays an important role in rescuing Bmi-1(-/-) mice induced developmental disorders of teeth and mandible-anti-oxidant effect of pyrroloquinoline quinone. Am J Transl Res. 2018;10:40–53. [PMC free article] [PubMed] [Google Scholar]
- 29.Rai P, Onder TT, Young JJ, McFaline JL, Pang B, Dedon PC, Weinberg RA. Continuous elimination of oxidized nucleotides is necessary to prevent rapid onset of cellular senescence. Proc Natl Acad Sci U S A. 2009;106:169–174. doi: 10.1073/pnas.0809834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan H, Suzuki T, Aizawa K, Miyagawa K, Nagai R. Ataxia telangiectasia mutated (ATM)-mediated DNA damage response in oxidative stress-induced vascular endothelial cell senescence. J Biol Chem. 2010;285:29662–29670. doi: 10.1074/jbc.M110.125138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin JA, Klingelhutz AJ, Moussavi-Harami F, Buckwalter JA. Effects of oxidative damage and telomerase activity on human articular cartilage chondrocyte senescence. J Gerontol A Biol Sci Med Sci. 2004;59:324–337. doi: 10.1093/gerona/59.4.b324. [DOI] [PubMed] [Google Scholar]
- 32.Erusalimsky JD, Kurz DJ. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp Gerontol. 2005;40:634–642. doi: 10.1016/j.exger.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, Clark IM. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 34.Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci. 2001;56:B172–179. doi: 10.1093/gerona/56.4.b172. [DOI] [PubMed] [Google Scholar]
- 35.Zhou HW, Lou SQ, Zhang K. Recovery of function in osteoarthritic chondrocytes induced by p16INK4a-specific siRNA in vitro. Rheumatology. 2004;43:555–568. doi: 10.1093/rheumatology/keh127. [DOI] [PubMed] [Google Scholar]
- 36.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henrotin YE, De Groote DD, Labasse AH, Gaspar SE, Zheng SX, Geenen VG, Reginster JY. Effects of exogenous IL-1 beta, TNF alpha, IL-6, IL-8 and LIF on cytokine production by human articular chondrocytes. Osteoarthritis Cartilage. 1996;4:163–173. doi: 10.1016/s1063-4584(96)80012-4. [DOI] [PubMed] [Google Scholar]
- 38.Lotz M, Terkeltaub R, Villiger PM. Cartilage and joint inflammation. Regulation of IL-8 expression by human articular chondrocytes. J Immunol. 1992;148:466–473. [PubMed] [Google Scholar]
- 39.Shakibaei M, Schulze-Tanzil G, John T, Mobasheri A. Curcumin protects human chondrocytes from IL-l1beta-induced inhibition of collagen type II and beta1-integrin expression and activation of caspase-3: an immunomorphological study. Ann Anat. 2005;187:487–497. doi: 10.1016/j.aanat.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Poree B, Kypriotou M, Chadjichristos C, Beauchef G, Renard E, Legendre F, Melin M, Gueret S, Hartmann DJ, Mallein-Gerin F, Pujol JP, Boumediene K, Galera P. Interleukin-6 (IL-6) and/or soluble IL-6 receptor down-regulation of human type II collagen gene expression in articular chondrocytes requires a decrease of Sp1. Sp3 ratio and of the binding activity of both factors to the COL2A1 promoter. J Biol Chem. 2008;283:4850–4865. doi: 10.1074/jbc.M706387200. [DOI] [PubMed] [Google Scholar]
- 41.Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeon OH, David N, Campisi J, Elisseeff JH. Senescent cells and osteoarthritis: a painful connection. J Clin Invest. 2018;128:1229–1237. doi: 10.1172/JCI95147. [DOI] [PMC free article] [PubMed] [Google Scholar]