Abstract
Spinal muscular atrophy (SMA) is an autosomal recessive genetic disorder characterized by loss of motor neurons in the ventral horn of the spinal cord. Clinical features such as progressively lethal respiratory weakness and associated muscle wasting have been extensively studied but less attention has been given to gastrointestinal (GI) dysfunction, which is common symptomatology in SMA patients with 43% constipation, 15% abdominal pain, and 14% meteorism. In the current study, the PrP92-SMN mouse model of SMA was utilized, to complement previous studies in which cells of the Enteric Nervous system (ENS) were susceptible to Smn (survival motor neuron) deficiency and could possibly be the basis of the observed GI symptoms. Necropsy of our mouse model showed impairment in feces excretion and smaller bladder mass, compared to Wild-Type (WT) animals. Along with the reduction in bladder mass, we also observed a decrease in the size of smooth muscles, due to reduction in Cross-Sectional Area (CSA). Interstitial cells of Cajal (ICC) provide important regulatory functions in the GI tract. To investigate if ICC are implicated in Smn deficient-induced colonic dysmotility, we assessed ICC distribution and abundance, by c-Kit, a well-established marker. SMA mice exhibited fewer c-Kit positive cells with altered localization, compared to WT. In conclusion, the observed histopathological abnormalities of our mouse model, can be secondary to SMN deficiency and could possibly underlie the GI symptoms observed in SMA patients. Future therapeutic approaches for SMA, must address not only CNS symptoms, but also non-motor-neuron-related symptoms. The PrP92-SMN mouse model could be a useful model for assessing therapeutic rescue of GI dysfunction in SMA.
Keywords: Spinal muscular atrophy, gastrointestinal dysfunction, smooth muscle atrophy, colon pathology, interstitial cells of Cajal, c-kit
Introduction
Spinal muscular atrophy (SMA) is an autosomal recessive genetic disorder characterized by loss of the anterior horn motor neurons of the spinal cord [1]. This rare neuromuscular disease is caused by mutation in the SMN1 (survival motor neuron) gene that encodes the full-length SMN protein [2,3]. In humans a second form of the SMN gene exists, SMN2 [4]. SMN1 gene differs from SMN2 gene by only 5 nucleotides (nt); more specifically 1 nt at exons 7 and 8 and intron 6 and 2 in intron 7. The nucleotide exchange in exon 7, results in the creation of a new exonic splice silencer (ESS) and consequently 90% of the pre-mRNAs resulting from SMN2 are alternatively spliced, lacking exon 7. A small percentage (10%) of SMN proteins arising from SMN2 are fully functional [5]. The clinical manifestation of SMA includes pediatric progressive muscle wasting and paralysis, eventual respiratory failure [6] or even death based on the maximal motor function achieved [7,8].
In SMA, the pathogenic mechanisms of frequently reported clinical features such as progressively lethal respiratory weakness [6] and other skeletal muscle wasting have been extensively investigated, However, the gastrointestinal dysfunction enuresis and some other autonomic-nerve-associated symptoms like bradycardia hyperhidrosis and abnormal vasodilation that account for a proportion of symptoms in SMA patients [9-12] are rarely studied.
According to the 2007 Consensus Statement for Standard of Care in SMA, patients with spinal muscular atrophy suffer from gastroesophageal dysmotility problems; such as delayed gastric emptying, and potentially life-threatening gastroesophageal reflux (GER) [13]. It has been shown by clinical studies that 4% of patients with SMA type II have been diagnosed with GER and another 7% with GER related symptoms. Furthermore, 43% of patients complain of constipation, 15% had abdominal pain, and 14% had meteorism [14].
In the gastrointestinal (GI) tract, one of the major Enteric Nervous System (ENS) components is Auerbach’s plexus (or myenteric plexus), which provides motor innervation to both longitudinal and circular muscular layers of the GI tract, with parasympathetic and sympathetic input [15,16]. Besides direct innervation of motor neurons to smooth muscle cells, the innervation can also occur through interstitial cells of Cajal (ICC), a type of interstitial cell that interacts with and forms electrical connectivity with neurons and smooth muscle cells [17-20]. ICC provide important regulatory functions in the GI tract including: (a) pacemakers of electrical slow wave activity [21-24], (b) coordination of pacemaker activity and active propagation of slow waves [25-29], (c) neurotransmission by facilitating motor neural innervation from the myenteric ganglion [30], and (d) mechano-sensation to stretch of GI muscles [31-34].
There are different types of ICC: myenteric ICC (MY-ICC) are multipolar shape cells serve as a pacemaker which creates the bioelectrical slow wave potential that leads to contraction of the smooth muscle [35-38]. Intramuscular ICC (IM-ICC) are bipolar shaped cells involved in the stimulation of smooth muscle cells, and that neurotransmitters act through [39-42]. Submucosal interstitial cells of Cajal (SM-ICC) are multipolar shaped cells still thought to be pacemaker cells in the colon [35,43]. It is reasonable to hypothesize that the ENS and ICC innervating the gastrointestinal smooth muscles may be vulnerable to SMN deficiency, as are motor neurons in the spinal cord innervating skeletal muscles.
In the current study, in order to investigate whether ENS (Auerbach’s plexus) and ICC innervating the GI smooth muscle are vulnerable to SMN deficiency, we utilized the SMN2; Smn; PrP92-SMN mutant mice (which we refer to as “SMA” mice). In this strain of mice, SMN production is localized to the CNS. Homozygous SMN2; Smn; PrP92-SMN mice are rescued from the severe SMA phenotype, have significantly increased lifespan (average of 210 days) and have normal lumbar motor neuron root counts and body weight [44]. They can potentially be utilized for studies evaluating SMN restoration only in the brain and spinal cord, thus modeling some of the current human therapeutic strategies. In these mice, we observed significant alterations in micro-pathohistology of the GI tract, including a decrease of smooth muscle cross sectional area in the SMN-deficient bladder and colon. More specially, c-kit positive ICC volume was decreased in the SMN-deficient colon. Both pathological alterations may cause reduced contractile force and problems with neuromuscular activation and transmission, leading to GI dysmotility, as is observed in SMA patients.
Materials and methods
Animal breeding and genotyping
Animal studies were performed in accordance with the Institutional Animal Care and Use Committee and approved by the University of Florida. (30 weeks) Male and female mice were used in all studies. Age and sex-matched C57BL/6J were used as controls.
SMN1-deficient, SMN2; Smn; PrP92-SMN mutant mice were utilized as the SMA mouse model. When maintaining a colony, mice heterozygous for the Smn1tm1Msd targeted mutation, homozygous for the SMN2 low copy line 89 transgene, and homozygous for the PrP92-SMN transgene were bred together. Unlike the delta7 mouse, which is homozygous for the Smn1tm1Msd targeted mutation (Smn null allele) and human SMN2 transgene (SMN2 low copy line 89) and exhibits symptoms, neuropathology, and early lethality similar to human type I proximal spinal muscular atrophy (SMA) patients, this strain carries the additional PrP92-SMN transgene (Figure S1). With the mouse prion protein (PrP92) promoter directing full-length human SMN, SMN is expressed at high levels in neurons, but low levels in cardiac muscle, skeletal muscle, kidney, and lower GI tract, with no expression in upper GI tract (Figure S1), liver and spleen [44-46]. With this approach, homozygous SMN2; Smn; Prp92-SMN mice are CNS rescued, have normal lumbar motor neuron root counts and much increased lifespan [44], which should be a good model to investigate the impact of SMN deficiency on organs that have low or no SMN expression.
The genotyping of the mice was completed according to the protocol of The Jackson Laboratory (JAX) [47]. Genomic DNA was extracted from tail clips and amplified with PCR using the following primmers: Common primer, 5’-CTC CGG GAT ATT GGG ATT G-3’; Mutant reverse primer, 5’-GGT AAC GCC AGG GTT TTC C-3’; Wild type reverse primer, 5’-TTT CTT CTG GCT GTG CCT TT-3.
Colon tissue processing
Mice were sacrificed at 30-weeks of age, which is the average reported lifespan (210 days) of these animals [44] and weighed. Various tissues were dissected, weighed and processed. The entire colon was collected, its length was measured and dissected along the mesenteric border. The colon was divided into three pieces: one piece was fixed in ice cold acetone at 4°C for whole mount as previously described [48], another was embedded in optimal cutting temperature (OCT, Fisher HealthCare, US) and frozen in liquid nitrogen-cooled iso-pentane, the rest was snap frozen and stored at -80°C for western blotting.
Histopathology
Transverse and longitudinal cryosections (10 μm) of distal regions of the colon were prepared using a cryostat (Leica, Germany) and were stained with haematoxylin and eosin (H&E). Images were captured on a light microscope (Leica DM4 B, Leica, Germany).
Immunohistochemistry
The preparation and immunostaining for whole mount samples and cryosections were performed according to the protocol from JOVE [48]. All sections were analyzed by immunohistochemistry using the primary and secondary antibodies listed in Table 1. Smooth muscle and bladder CSA was determined on 10 μm cryosections stained with dystrophin. For PGP9.5 primary antibody staining, sections were subjected to antigen retrieval by boiling slides in 10 mM citrate buffer (pH 6.0) for 20 minutes.
Table 1.
Antibodies utilized for immunostaining
| Antibodies | Host | Dilution | Company | Catalog number |
|---|---|---|---|---|
| Primary antibodies | ||||
| PGP 9.5 | Rabbit | 1:1000 | Thermo Scientific | RB-9202 |
| C-kit | Rat | 1:500 | Thermo Scientific | MA5-17836 |
| Dystrophin | Rabbit | 1:1000 | Abcam | AB15277 |
| Secondary antibodies | ||||
| Goat anti-Rabbit IgG, Alexa Fluor 594 | Goat | 1:1000 | Thermo Scientific | A11037 |
| Goat anti-Rat IgG, Alexa Fluor 488 | Goat | 1:1000 | Thermo Scientific | A11006 |
| Goat anti-Rabbit IgG, Alexa Fluor 488 | Goat | 1:1000 | Thermo Scientific | A11034 |
Imaging and quantification
Images were captured on a confocal laser scanning microscope (TCS SP8, Leica, German). All the images were quantified with ImageJ (Fiji 1.0) software. For the smooth muscle, the cellular membrane outline was identified by dystrophin positive pixels (green), and the cross-sectional area (CSA) was determined and qualified by combined use of ‘Trainable weka segmentation 3D’, ‘Binary’ and ‘Particles’ analysis. Similarly, the PGP9.5 positive pixels and DAPI stained nuclei were used to identify Auerbach’s plexuses (enteric nervous ganglia included). The ganglia cells and ganglionic cross section areas were counted and measured. The numbers of ICC, the c-kit positive (green) labeled interstitial cells were counted separately in each anatomical layers of colon tract.
Immunoblot analysis
Tissues extracted for immunoblot analysis were snap frozen in liquid nitrogen and stored at -80°C until further processing. The expression of proteins of interest was measured by western blotting as previously described [49]. Actin was used as loading control in all samples. The blot images were acquired on the LI-COR Imaging System. Quantification of band intensity was analyzed by Image Studio™ Software (LI-COR, USA). All the primary and secondary antibodies are listed in Table 2. Values are expressed as the ratio of protein of interest to loading control.
Table 2.
Antibodies utilized for Western blot
| Antibodies | Host | Dilution | Company | Catalog number |
|---|---|---|---|---|
| SMN | mouse | 1:1000 | Cell Signaling Technology | 12976S |
| Goat anti-Mouse IgG, Alexa Fluor 488 | Goat | 1:1000 | Thermo Scientific | A-11001 |
| C-kit | Goat | 1:2000 | Bio-Techne Corporation | AF1356 |
| Rabbit anti-Goat IgG, Alexa Fluor 790 | Rabbit | 1:1000 | Thermo Scientific | A27019 |
| Anti-Actin Antibody, Alexa Fluor 488 | Rabbit | 1:1000 | Merck millipore | ABT1485-AF488 |
Statistical analysis
All data are presented as means ± SEM. All analyses were performed with PRISM 6.0c (GraphPad Software, Inc, USA). A Student’s t test was performed for comparison of 2 groups, and 2-way ANOVA was performed for comparison of ≥ 3 groups to determine significance, followed by Sidak’s multiple comparisons post hoc test. Kolmogorov-Smirnov test was used to compare cumulative distributions of smooth muscle CSA. A P value of < 0.05 was considered significant.
Results
Impaired feces excretion and reduced colorectal smooth muscle fiber size in SMA mice
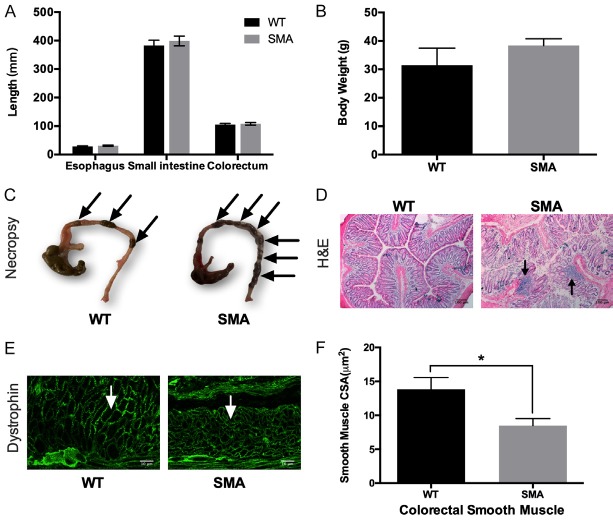
It has been reported that patients with SMA type II have constipation and GI symptoms [14]. To determine the state of the GI system in our animal model, we measured the length of “major” parts of the gastrointestinal system (esophagus, small intestine and colorectum) (Figure 1A) and body weight (Figure 1B). No significant changes were observed in either part of the GI system in our SMA mice (esophagus: 28.52 ± 2.72 mm, small intestine: 382.6 ± 26.7 mm, colorectum: 105.2 ± 5.56 mm; N=2) compared to their controls (esophagus: 31.18 ± 2.58 mm, small intestine: 399.1 ± 24.13 mm, colorectum: 107.9 ± 6.24 mm; N=2). Also, no significance difference was observed in body weight in SMA mice (38.38 ± 6.74 g, N=8), compared to control (31.43 ± 12.01 g, N=4), neither is weight in specific muscles, because the skeletal muscles have similar SMN expression (Figure S1B and S1C). However, increased fecal loading was observed in SMA mice (Figure 1C). To determine the origin of the impaired fecal excretion, transverse sections of SMA mice colon were stained with Hematoxylin and Eosin (H&E) and examined for morphological alterations and possible inflammation. Evaluation of colon sections show the presence of secondary lesions in SMA mice and infiltration by inflammatory cells (Figure 1D). Another possible etiology, of the gastrointestinal problems observed in SMA patients is the involvement of smooth muscles of the GI system. We found that the CSA of smooth muscle in the colon was approximately 40% lower in SMA compared to control mice (Figure 1E, 1F). From the above we conclude, while the PrP92-SMN transgene rescues the SMA motor neuron and CNS phenotypes, dysfunctions of the GI system are still present.
Figure 1.
Anatomical features of the SMN2; Smn; PrP92-SMN mouse (SMA mouse) strain. (A) No difference was observed in Esophagus, Small Intestine and Colorectum lengths or (B) Body weight between SMA and WT mice. (C) The colon was dissected from SMA and WT mice. The arrows points to well-pelleted feces. SMA mice displayed increased numbers of pelleted-feces, indicating constipation. (D) H&E staining of SMA and WT colon. Secondary lesions (arrow) were present in SMA mice. (E) Representative images of colon smooth muscles stained for dystrophin. (F) Quantification of colon smooth muscle CSA. SMA mice have approximately 35% smaller smooth muscle CSA, compared to WT (8.476 ± 1.052 µm2 N=7 vs 13.82 ± 1.735 µm2 N=7, P=0.0218), exhibiting a significant shift in distribution towards smaller CSA fibers (Kolmogorov-Smirnov D=0.2584, P < 0.0001).
SMA mice exhibit reduction in bladder and smooth muscle size
Another area that the role of SMA has been overlooked is enuresis and urinary incontinence. To the best of our knowledge there is just one clinical study that has examined that issue and reported that a third of all children diagnosed with SMA have signs of urinary incontinence [12]. To investigate whether a similar phenomenon is present in our animal model, we measure the bladder weight (mg)/body weight (g) (BD/BW), as a possible indication of the phenomenon. We found that BD/BW of our SMA mice was nearly 50% lower compared to WT mice (Figure 2A). Furthermore, based on immunohistological analysis, SMA mice bladder smooth muscle fibers were by 20% smaller than WT mice (Figure 2B, 2C). The smaller sized smooth muscle fibers may cause poor contraction and can be the etiology for urinary incontinence.
Figure 2.
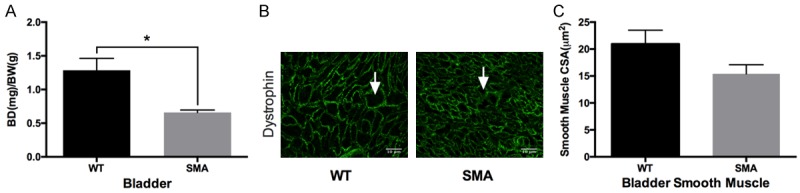
The bladder morphology of SMA mice. A. Normalized bladder mass to body mass. SMA mouse bladder is 50% smaller as compared to WT (0.6589 ± 0.03764 N=7 vs 1.285 ± 0.1773 N=7, P=0.0015). B. Representative images of bladder smooth muscles stained for dystrophin. C. Quantification of bladder smooth muscle CSA. No difference was observed in bladder smooth muscle CSA between SMA and WT (15.41 ± 1.691 µm2 N=5 vs 20.94 ± 2.557 µm2 N=5, P=0.1086), but with a significant shift in distribution towards smaller CSA smooth muscle cells (Kolmogorov-Smirnov D=0.1647, P < 0.0001).
SMA mice have a lower volume of c-kit positive ICC in colon
The GI tract is innervated by extrinsic (sympathetic, parasympathetic and visceral afferent) axons and intrinsic neurons of the enteric nervous systems (ENS). Defects of these innervations or ICC facilitation may cause GI dysmotility [50-55]. In the current study, we examined enteric neurons and ICC in our animal model. Neuron density in the colon was identified and quantified by PGP9.5 staining. ICC abundance and distribution were determined by immunoblot and staining, respectively [48]. Examining the cryosections from serosa to mucosa, we found that no significant difference was observed in the mesh formed by PGP9.5-positive nerve cells, between SMA and WT mice. However, SMA mice exhibited 50% fewer c-Kit positive stained ICCs compared to WT mice (Figure 3A-J). The reduction of c-Kit protein levels in the SMA mice was confirmed by immunoblot (Figure 3K, 3L). Whole-mount immunofluorescence at three different tract layers (Myenteric (MY), Intramuscular (IM) and submucosa (SM)) (Figure 4), showed that there is a lower abundance (approximately 50%) of c-kit positive ICC in SMA mice compared to controls. These findings could be an indication that ICC may play a role in facilitating motor neural innervation from the myenteric plexus, and SMN deficiency might cause c-kit positive ICC maldevelopment and reduced abundance impacting neurotransmission.
Figure 3.
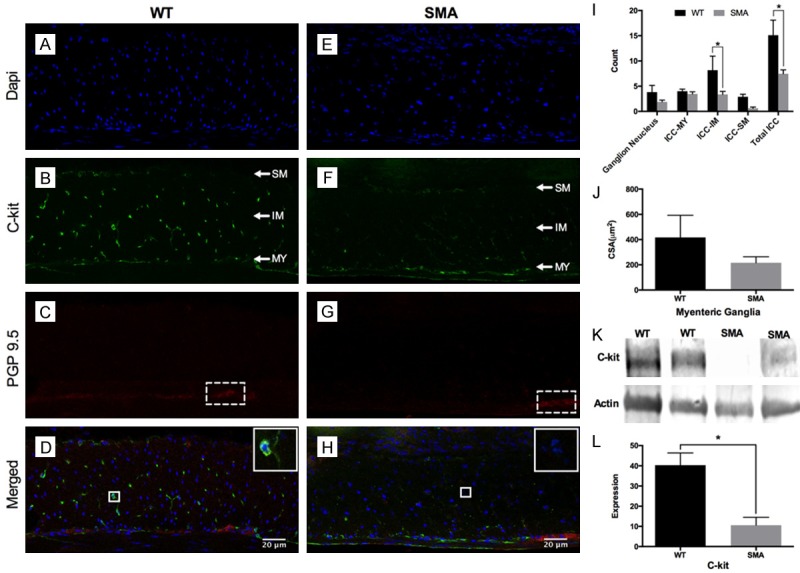
Histological investigation on distribution and abundance of ENS and ICC. (A-H) are representative images of enteric neurons/ganglions and ICC in colons from WT and SMA mice. Enteric neurons were stained with the neuronal marker PGP9.5 (Red), ICC with c-Kit (Green) and cell nuclei were stained with DAPI (Blue). (J) The WT mice have a non-significantly larger ganglionic CSA (417.2 ± 175.3 µm2, N=6 vs 216.2 ± 47.72 µm2, N=10, P=0.1916) and ganglionic nuclei number (3.82 ± 1.335, N=6 vs 1.89 ± 0.372, N=10, P=0.7390) than SMA mice; (I) The SMA mice had a decreased trend for c-kit positive ICC compared to WT mice in the MY and SM layers, and presented significantly decreased numbers in the IM layer (3.38 ± 0.593, N=10 vs 8.18 ± 2.779, N=6, P=0.0206) and a striking 50% reduction in total ICC numbers (7.47 ± 0.779, N=10 vs 15.10 ± 2.997, N=6, P < 0.0001). (K) The immunoblots of c-Kit protein and actin. (L) Semi-quantification of c-Kit protein relative to an actin loading control. The c-kit protein level is four-fold higher in WT colon tissues compared to the SMA mice (40.31 ± 6.025 N=5 vs 10.61 ± 3.892 N=5, P=0.0032), consistent with the immunofluorescence imaging results.
Figure 4.
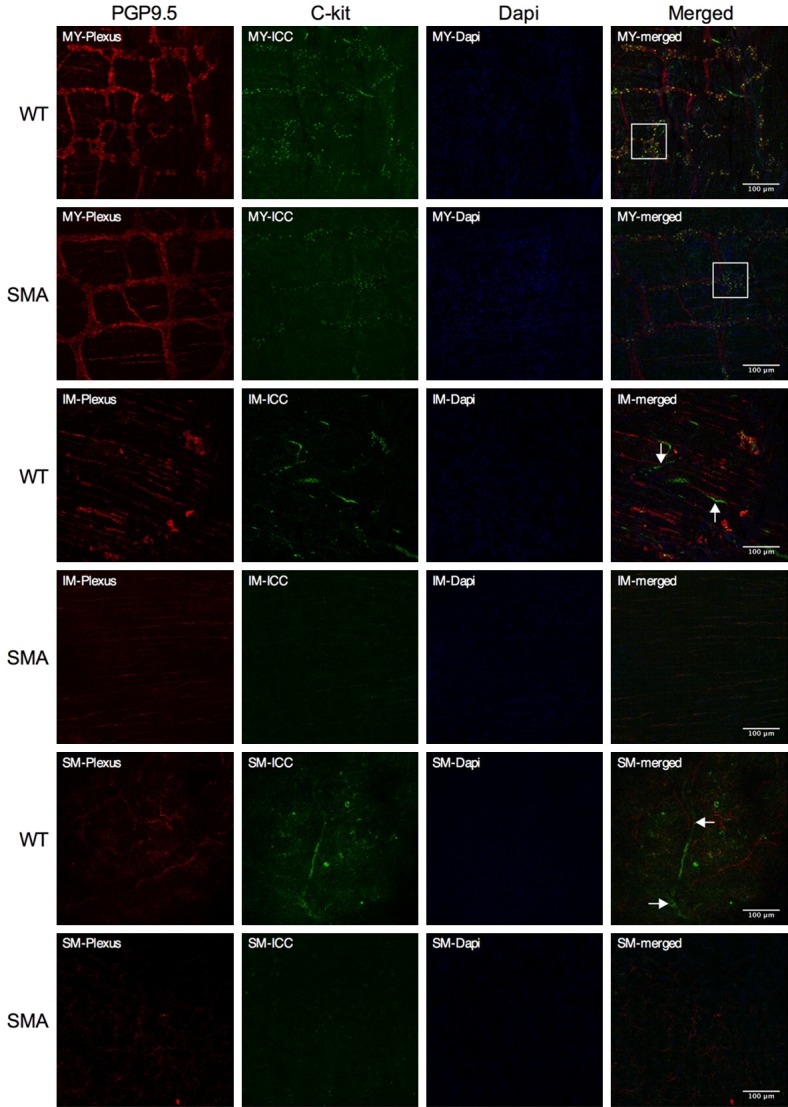
Whole mount immunofluorescence of colon. Whole mount of colon with PGP9.5 (Red) staining for neurons, c-Kit (Green) staining for ICC and with DAPI (Blue) staining for cell nuclei in three focal planes: (Myenteric (MY), Intramuscular (IM) and submucosa (SM): Compared to the SMA mice, the WT mice present stronger green intensity of c-kit positive ICC (indicated by white solid square) surrounding the myenteric ganglion (12.47 ± 0.01 vs 9.990 ± 0.01, P < 0.0001). Both in the intramuscular (IM) and submucosa (SM) layers, stronger staining, larger c-kit positive fibers (white arrows) were found in WT mice, paralleling the PGP 9.5 positive structures, as compared to SMA mice.
Discussion
Spinal Muscular Atrophy patients present with GI-related symptomatology, which include gastroesophageal reflux, constipation and delayed gastric emptying. These phenotypes have received limited attention in SMA animal model studies. In the current study, 30-week old Prnp92-SMN mice (SMA mice) were used for investigation, allowing organs to be larger and better developed for histological analysis than in Delta 7 mice, which have an average survival of only 13 days [56]. Using the PrP92-SMN mice model of SMA, that exhibits a mild phenotype due to CNS and motor neuron rescue, we were able to examine the phenotypical and morphological alterations in the GI tract associated with the loss of SMN.
We found that the body mass of WT and SMA (PrP92-SMN) mice had no significant difference (Figure 1B), in contrast to two other studies that examined the GI phenotype of SMA mice [57,58]. The reason for that discrepancy can be attributed to PrP92-promoter used to CNS specific expression for SMN. Motor neuron rescue in the spinal cord prevents the skeletal muscle atrophy caused by denervation. Also, some of the mice were found to have leaky expression from the PrP92-SMN transgene outside of the neuronal compartment, especially in skeletal muscles (Figure S1C). This may be another reason for skeletal muscle restoration and no weight loss. However, leaky expression was not present in GI tissues such as colon, stomach and small intestine tissues (Figure S1C).
In a previous study [58], SMN deficiency was found to lead to disrupted ENS signaling to the smooth muscle of colon with a 10-fold greater contractile response with high-frequency electrical field stimulation. However, no neurodegeneration was found to be associated with abnormal ENS signaling in that study. This is consistent with our observation of no significant differences in ganglionic nucleus number and CSA were in our study. But this observation is contrary to a study in Taiwanese SMA mice that exhibited an increase of enteric neuron numbers in the small intestine [57]. Limited clinic data from 8 infants with Werdnig-Hoffmann disease (SMA type I) showed low values of neural tissue in the myenteric plexus in the small intestine and colon [59].
ICC [60] connects motor neurons to local effector cells, such as smooth muscle cells. The current study examines the impact of SMN deficiency on smooth muscle and ICC in SMA. A reduction of c-Kit positive ICC was observed in SMA mice, suggesting that SMN deficiency could lead to loss of ICC that resembles loss of motor neurons in spinal cord. ICC was previously found to integrate excitatory and inhibitory neurotransmission with slow-wave activity to orchestrate peristaltic motor activity [61], therefore ICC loss could be a potential reason for the abnormal ENS signaling in SMA mice. In addition, decreased smooth muscle CSA was seen in colon smooth muscle in our SMA mice. This muscular alteration could be directly caused by SMN-deficiency, as is the case in skeletal muscle, where it has been reported that SMN-deficiency impairs myotube formation by altering myogenic gene expression and focal adhesion dynamics that affect muscle cell migration and organization of the actin cytoskeleton [62]. The muscular alterations might also be the result of denervation resulting from the c-kit positive ICC loss.
In a previous study, macrophage infiltration was observed in the small intestine from the Taiwanese SMA mouse model [57]. Another animal study [63] reported that under the pro-inflammatory conditions of the muscularis-associated GI obstruction, the release of bioactive substances from activated resident macrophages may affect smooth muscle contractility. In addition, under these conditions, both the number and the function of neighboring ICC may also be affected [63,64]. Thus, the macrophage infiltration in SMA colon tract could be a potential contributor to the ICC volume reduction and smooth muscle atrophy of the colon from SMA mice. Therefore, for the SMN deficiency-associated observed GI symptoms (smooth muscle atrophy, ICC loss, and macrophage infiltration), we cannot distinguish which are the primary pathological alterations due to SMN loss and which are secondary consequences. But in the future, cellular level studies could help determine if these defects are primary or secondary in SMN-depleted smooth muscle cells, ICC and macrophage cells.
In addition to GI symptoms, children with SMA types I and II have a high rate of urinary incontinence [12]. This has been identified as “stress incontinence” due to striated muscles weakness and atrophy in the pelvic floor muscles and the external urethral sphincter, but not the attributed to smooth muscles of the detrusor [12]. However, in our study, we observed a 50% lower bladder weight/body weight and 20% smaller bladder smooth muscle fibers in the SMA-deficient mice, suggesting that SMN deficiency may cause poor contraction of the smooth muscle may contribute to overflow incontinence.
In SMA, besides the well-known motor-neuron-related symptoms, symptoms like GI dysmotility, bradycardia, and hyperhidrosis make up a non-motor-neuron-related symptomatic group. The histopathological abnormalities we found in colon and bladder of SMA mice, ICC loss and smooth muscle atrophy secondary to SMN deficiency, could be responsible for the pathological changes in SMA patients. In the future, the therapies for SMA will need to not only target the CNS and motor neurons, but also address such non-motor-neuron-related symptoms. The mice we described herein will be a useful model for testing the ability of therapies to rescue GI dysfunction at different post-natal stages.
Acknowledgements
This study was supported by a grant from the SMA Foundation. YY thanks all of his colleagues of the UF Myology Institute, with a special thanks to his uncle, Dr. Zhaohui Yang. He motivated the medical career of YY, and was valued member of our lab from September, 2015 until his untimely death on December 17, 2016. This article was motivated by his observations and is dedicated to his memory.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Lunn MR, Wang CH. Spinal muscular atrophy. Lancet. 2008;371:2120–2133. doi: 10.1016/S0140-6736(08)60921-6. [DOI] [PubMed] [Google Scholar]
- 2.Hahnen E, Forkert R, Marke C, Rudnik-Schöneborn S, Schönling J, Zerres K, Creavin T, Wirth B. Molecular analysis of candidate genes on chromosome 5q13 in autosomal recessive spinal muscular atrophy: evidence of homozygous deletions of the SMN gene in unaffected individuals. Hum Mol Genet. 1995;4:1927–1933. doi: 10.1093/hmg/4.10.1927. [DOI] [PubMed] [Google Scholar]
- 3.Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 4.Rochette C, Gilbert N, Simard L. SMN gene duplication and the emergence of the SMN2 gene occurred in distinct hominids: SMN2 is unique to Homo sapiens. Hum Gene. 2001;108:255–266. doi: 10.1007/s004390100473. [DOI] [PubMed] [Google Scholar]
- 5.Gavrilov DK, Shi X, Das K, Gilliam TC, Wang CH. Differential SMN2 expression associated with SMA severity. Nat Genet. 1998;20:230–231. doi: 10.1038/3030. [DOI] [PubMed] [Google Scholar]
- 6.Tunas MC, Santos LS, Cuinas MB, Nunez AR. Spinal muscular atrophy and respiratory failure. How do primary care paediatricians act in a simulated scenario. An Pediatr (Barc) 2015;83:336–340. doi: 10.1016/j.anpedi.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Dubowitz V. Chaos in the classification of SMA: a possible resolution. Neuromuscul Disord. 1995;5:3–5. doi: 10.1016/0960-8966(94)00075-k. [DOI] [PubMed] [Google Scholar]
- 8.Zerres K, Rudnik-Schöneborn S. Natural history in proximal spinal muscular atrophy: clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol. 1995;52:518–523. doi: 10.1001/archneur.1995.00540290108025. [DOI] [PubMed] [Google Scholar]
- 9.Arai H, Tanabe Y, Hachiya Y, Otsuka E, Kumada S, Furushima W, Kohyama J, Yamashita S, Takanashi JI, Kohno Y. Finger cold-induced vasodilatation, sympathetic skin response, and R-R interval variation in patients with progressive spinal muscular atrophy. J Child Neurol. 2005;20:871–875. doi: 10.1177/08830738050200110301. [DOI] [PubMed] [Google Scholar]
- 10.Bach JR. Medical considerations of long-term survival of Werdnig-Hoffmann disease. Am J Phys Med Rehabil. 2007;86:349–355. doi: 10.1097/PHM.0b013e31804b1d66. [DOI] [PubMed] [Google Scholar]
- 11.Davis RH, Godshall BJ, Seffrood E, Marcus M, LaSalle BA, Wong B, Schroth MK, Swoboda KJ. Nutritional practices at a glance: spinal muscular atrophy type I nutrition survey findings. J Child Neurol. 2014;29:1467–1472. doi: 10.1177/0883073813503988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Von Gontard A, Laufersweiler-Plass C, Backes M, Zerres K, Rudnik-Schöneborn S. Enuresis and urinary incontinence in children and adolescents with spinal muscular atrophy. BJU Int. 2001;88:409–413. doi: 10.1046/j.1464-410x.2001.02341.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang CH, Finkel RS, Bertini ES, Schroth M, Simonds A, Wong B, Aloysius A, Morrison L, Main M, Crawford TO. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22:1027–1049. doi: 10.1177/0883073807305788. [DOI] [PubMed] [Google Scholar]
- 14.Messina S, Pane M, De Rose P, Vasta I, Sorleti D, Aloysius A, Sciarra F, Mangiola F, Kinali M, Bertini E. Feeding problems and malnutrition in spinal muscular atrophy type II. Neuromuscul Disord. 2008;18:389–393. doi: 10.1016/j.nmd.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Gabella G. Fine structure of the myenteric plexus in the guinea-pig ileum. J Anat. 1972;111:69. [PMC free article] [PubMed] [Google Scholar]
- 16.Wood J. Physiology of the enteric nervous system. Physiology of the Gastrointestinal Tract. 1994;1:423–482. [Google Scholar]
- 17.Imaizumi M, Hama K. An electron microscopic study on the interstitial cells of the gizzard in the love-bird (Uroloncha domestica) Z Zellforsch Mikrosk Anat. 1969;97:351–357. doi: 10.1007/BF00968841. [DOI] [PubMed] [Google Scholar]
- 18.Faussone PM, Cortesini C, Romagnoli P. Ultrastructure of the tunica muscularis of the cardial portion of the human esophagus and stomach, with special reference to the so-called Cajal’s interstitial cells. Arch Ital Anat Embriol. 1977;82:157–177. [PubMed] [Google Scholar]
- 19.Daniel E, Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol. 1984;246:G305–G315. doi: 10.1152/ajpgi.1984.246.3.G305. [DOI] [PubMed] [Google Scholar]
- 20.Rumessen JJ, Mikkelsen HB, Thuneberg L. Ultrastructure of interstitial cells of Cajal associated with deep muscular plexus of human small intestine. Gastroenterology. 1992;102:56–68. doi: 10.1016/0016-5085(92)91784-2. [DOI] [PubMed] [Google Scholar]
- 21.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hulzinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 23.Ördög T, Ward SM, Sanders KM. Interstitial cells of Cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518:257–269. doi: 10.1111/j.1469-7793.1999.0257r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alberti E, Mikkelsen HB, Wang X, Diaz M, Larsen JO, Huizinga JD, Jimenez M. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1499–G1510. doi: 10.1152/ajpgi.00136.2006. [DOI] [PubMed] [Google Scholar]
- 25.Dickens EJ, Hirst G, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirst G, Edwards F. Generation of slow waves in the antral region of guinea-pig stomach--a stochastic process. J Physiol. 2001;535:165–180. doi: 10.1111/j.1469-7793.2001.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kito Y, Fukuta H, Suzuki H. Components of pacemaker potentials recorded from the guinea pig stomach antrum. Pflugers Arch. 2002;445:202–217. doi: 10.1007/s00424-002-0884-z. [DOI] [PubMed] [Google Scholar]
- 28.Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003;553:803–818. doi: 10.1113/jphysiol.2003.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kito Y, Ward SM, Sanders KM. Pacemaker potentials generated by interstitial cells of Cajal in the murine intestine. Am J Physiol Cell Physiol. 2005;288:C710–C720. doi: 10.1152/ajpcell.00361.2004. [DOI] [PubMed] [Google Scholar]
- 30.Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol. 2010;588:4621–4639. doi: 10.1113/jphysiol.2010.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strege PR, Ou Y, Sha L, Rich A, Gibbons SJ, Szurszewski JH, Sarr MG, Farrugia G. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1111–G1121. doi: 10.1152/ajpgi.00152.2003. [DOI] [PubMed] [Google Scholar]
- 32.Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci U S A. 2005;102:14913–14918. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin J, Chen JD. Roles of interstitial cells of Cajal in regulating gastrointestinal motility: in vitro versus in vivo studies. J Cell Mol Med. 2008;12:1118–1129. doi: 10.1111/j.1582-4934.2008.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forrest AS, Hennig GW, Jokela-Willis S, Park CD, Sanders KM. Prostaglandin regulation of gastric slow waves and peristalsis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1180–G1190. doi: 10.1152/ajpgi.90724.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takaki M. Gut pacemaker cells: the interstitial cells of Cajal (ICC) J Smooth Muscle Res. 2003;39:137–161. doi: 10.1540/jsmr.39.137. [DOI] [PubMed] [Google Scholar]
- 36.Hirst GD, Edwards FR. Role of interstitial cells of Cajal in the control of gastric motility. J Pharmacol Sci. 2004;96:1–10. doi: 10.1254/jphs.crj04002x. [DOI] [PubMed] [Google Scholar]
- 37.Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 38.Hennig GW, Spencer NJ, Jokela-Willis S, Bayguinov PO, Lee H, Ritchie LA, Ward SM, Smith TK, Sanders KM. ICC-MY coordinate smooth muscle electrical and mechanical activity in the murine small intestine. Neurogastroenterol Motil. 2010;22:e138–51. doi: 10.1111/j.1365-2982.2009.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirst G, Ward S. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward S, Sanders K. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol Motil. 2004;16:112–117. doi: 10.1111/j.1743-3150.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 42.Kito Y. The functional role of intramuscular interstitial cells of Cajal in the stomach. J Smooth Muscle Res. 2011;47:47–53. doi: 10.1540/jsmr.47.47. [DOI] [PubMed] [Google Scholar]
- 43.Plujà L, Albertí E, Fernández E, Mikkelsen HB, Thuneberg L, Jiménez M. Evidence supporting presence of two pacemakers in rat colon. Am J Physiol Gastrointest Liver Physiol. 2001;281:G255–G266. doi: 10.1152/ajpgi.2001.281.1.G255. [DOI] [PubMed] [Google Scholar]
- 44.Gavrilina TO, McGovern VL, Workman E, Crawford TO, Gogliotti RG, DiDonato CJ, Monani UR, Morris GE, Burghes AH. Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle-specific SMN expression has no phenotypic effect. Hum Mol Genet. 2008;17:1063–1075. doi: 10.1093/hmg/ddm379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Xu G, Slunt HH, Gonzales V, Coonfield M, Fromholt D, Copeland NG, Jenkins NA, Borchelt DR. Coincident thresholds of mutant protein for paralytic disease and protein aggregation caused by restrictively expressed superoxide dismutase cDNA. Neurobiol Dis. 2005;20:943–952. doi: 10.1016/j.nbd.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 46. http://www.proteinatlas.org/ENSG00000171867-PRNP/tissue.
- 47. https://www2.jax.org/protocolsdb/f?p=116:5:0::NO:5:P5_MASTER_PROTOCOL_ID PJC, 008212.
- 48.Chen Y, Shamu T, Chen H, Besmer P, Sawyers CL, Chi P. Visualization of the interstitial cells of cajal (ICC) network in mice. J Vis Exp. 2011 doi: 10.3791/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vassilakos G, Lei H, Yang Y, Puglise J, Matheny M, Durzynska J, Ozery M, Bennett K, Spradlin R, Bonanno H. Deletion of muscle IGF-I transiently impairs growth and progressively disrupts glucose homeostasis in male mice. FASEB J. 2019;33:181–194. doi: 10.1096/fj.201800459R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okamoto E, Ueda T. Embryogenesis of intramural ganglia of the gut and its relation to Hirschsprung’s disease. J Pediatr Surg. 1967;2:437–443. [Google Scholar]
- 51.Webster W. Embryogenesis of the enteric ganglia in normal mice and in mice that develop congenital aganglionic megacolon. Development. 1973;30:573–585. [PubMed] [Google Scholar]
- 52.He CL, Burgart L, Wang L, Pemberton J, Young-Fadok T, Szurszewski J, Farrugia G. Decreased interstitial cell of Cajal volume in patients with slow-transit constipation. Gastroenterology. 2000;118:14–21. doi: 10.1016/s0016-5085(00)70409-4. [DOI] [PubMed] [Google Scholar]
- 53.Wedel T, Spiegler J, Soellner S, Roblick UJ, Schiedeck TH, Bruch HP, Krammer HJ. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. 2002;123:1459–1467. doi: 10.1053/gast.2002.36600. [DOI] [PubMed] [Google Scholar]
- 54.Rolle U, Piotrowska AP, Nemeth L, Puri P. Altered distribution of interstitial cells of Cajal in Hirschsprung disease. Arch Pathol Lab Med. 2002;126:928–933. doi: 10.5858/2002-126-0928-ADOICO. [DOI] [PubMed] [Google Scholar]
- 55.Jain D, Moussa K, Tandon M, Culpepper-Morgan J, Proctor DD. Role of interstitial cells of Cajal in motility disorders of the bowel. Am J Gastroenterol. 2003;98:618–624. doi: 10.1111/j.1572-0241.2003.07295.x. [DOI] [PubMed] [Google Scholar]
- 56.Le TT, Pham LT, Butchbach ME, Zhang HL, Monani UR, Coovert DD, Gavrilina TO, Xing L, Bassell GJ, Burghes AH. SMNΔ7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum Mol Genet. 2005;14:845–857. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- 57.Sintusek P, Catapano F, Angkathunkayul N, Marrosu E, Parson SH, Morgan JE, Muntoni F, Zhou H. Histopathological defects in intestine in severe spinal muscular atrophy mice are improved by systemic antisense oligonucleotide treatment. PLoS One. 2016;11:e0155032. doi: 10.1371/journal.pone.0155032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gombash SE, Cowley CJ, Fitzgerald JA, Iyer CC, Fried D, McGovern VL, Williams KC, Burghes AH, Christofi FL, Gulbransen BD. SMN deficiency disrupts gastrointestinal and enteric nervous system function in mice. Hum Mol Genet. 2015;24:3847–3860. doi: 10.1093/hmg/ddv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galvis DA, Ang SM, Wells TR, Landing BH, Romansky SG. Microdissection study of the myentric plexus in acardia, ataxia-telangiectasia, cystic fibrosis, extrahepatic biliary atresia, pediatric AIDS and Werdnig-Hoffmann disease. Pediatr Pathol. 1992;12:385–395. doi: 10.3109/15513819209023317. [DOI] [PubMed] [Google Scholar]
- 60.Thuneberg L. One hundred years of interstitial cells of Cajal. Microsc Res Tech. 1999;47:223–238. doi: 10.1002/(SICI)1097-0029(19991115)47:4<223::AID-JEMT2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 61.Klein S, Seidler B, Kettenberger A, Sibaev A, Rohn M, Feil R, Allescher HD, Vanderwinden JM, Hofmann F, Schemann M. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat Commun. 2013;4:1630. doi: 10.1038/ncomms2626. [DOI] [PubMed] [Google Scholar]
- 62.Bricceno KV, Martinez T, Leikina E, Duguez S, Partridge TA, Chernomordik LV, Fischbeck KH, Sumner CJ, Burnett BG. Survival motor neuron protein deficiency impairs myotube formation by altering myogenic gene expression and focal adhesion dynamics. Hum Mol Genet. 2014;23:4745–4757. doi: 10.1093/hmg/ddu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Won K, Suzuki T, Hori M, Ozaki H. Motility disorder in experimentally obstructed intestine: relationship between muscularis inflammation and disruption of the ICC network. Neurogastroenterol Motil. 2006;18:53–61. doi: 10.1111/j.1365-2982.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- 64.Chang IY, Glasgow NJ, Takayama I, Horiguchi K, Sanders KM, Ward SM. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001;536:555–568. doi: 10.1111/j.1469-7793.2001.0555c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.