Abstract
Neurodegenerative disorders of ageing (NDAs) such as Alzheimer’s disease, Parkinson’s disease, frontotemporal dementia, Huntington’s disease and amyotrophic lateral sclerosis represent a major socio-economic challenge in view of their high prevalence yet poor treatment. They are often called proteinopathies owing to the presence of misfolded and aggregated proteins that lose their physiological roles and acquire neurotoxic properties. One reason underlying the accumulation and spread of oligomeric forms of neurotoxic proteins is insufficient clearance by the autophagic–lysosomal network. Several other clearance pathways are likewise compromised in NDAs: chaperone-mediated autophagy, the ubiquitin–proteasome system, extracellular clearance by proteases and extrusion into the circulation via the blood–brain barrier and glymphatic system. This article focuses on emerging mechanisms for enhancing neurotoxic protein clearance, a strategy that may curtail the onset and slow the progression of NDAs.
Neurodegenerative disorders of ageing [G] (NDAs) include Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD) and related tauopathies. They are ultimately fatal, have no disease-modifying therapies and are associated with an increasing socioeconomic burden due to their rising incidence. These ‘proteinopathies [G]’ display complex and partly distinctive pathophysiological profiles, yet all share a cardinal feature: accumulation of aberrantly processed and misfolded proteins such as amyloid-β [G] (Aβ), tau [G], α-synuclein [G], TAR DNA-protein 43 [G] (TDP-43) and mutant forms of huntingtin (Htt) [In NDAs, these proteins lose their physiological roles, aggregate and acquire novel neurotoxic functions1, and an impairment of elimination is implicated in their buildup and spread1–5.
As summarized in Figure 1, several endogenous mechanisms are involved in neurotoxic protein clearance. The glymphatic system [G] and the blood–brain barrier [G] (BBB) extrude neurotoxic proteins from the extracellular space, interstitial fluid (ISF) and cerebrospinal fluid (CSF), where they may also be degraded by proteases or phagocytosed by microglia and astrocytes. Within neurons and other cell types, intracellular elimination of neurotoxic proteins is predominantly effected by the ubiquitin–proteasome system (UPS) or by autophagy, a process by which superfluous or potentially dangerous cytoplasmic material is delivered to lysosomes [G] for degradation Three basic types of autophagy are recognised (Figure 2)3,4: microautophagy, in which cytosolic material is directly engulfed by invaginations of lysosomes; chaperone-mediated autophagy (CMA), which involves translocation of non-membrane bound, chaperone-captured substrates across the lysosomal membrane, and macroautophagy, which involves sequestration of cytosolic material into de novo synthesized, double-membrane-bound autophagosomes that deliver their contents to lysosomes for digestion. The whole process, from the formation of the autophagosome isolation membrane to cargo digestion in the lysosome, is referred to as autophagic flux (Box 1). Macroautophagy is far better characterized than the other two types, so we use the term autophagy to refer to macroautophagy from this point on unless otherwise specified.
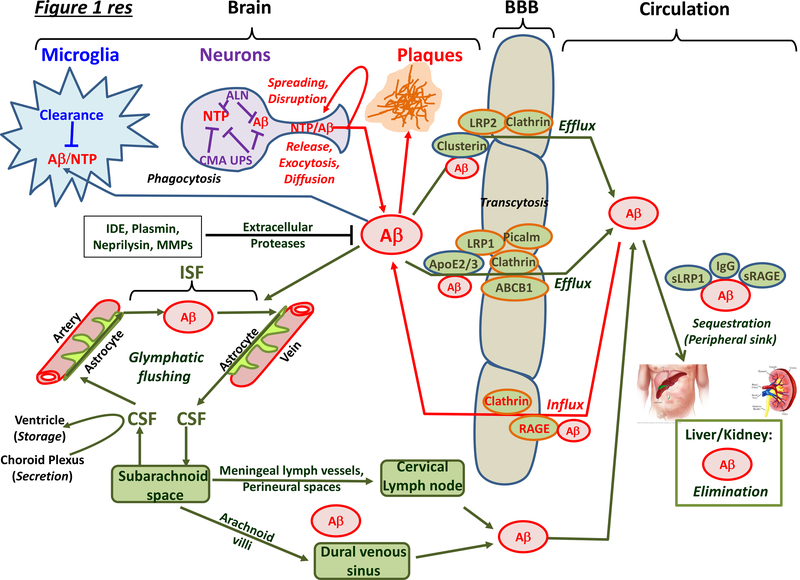
Figure 1 |. Overview of intracelluar and extracellular mechanisms for the clearance of neurotoxic proteins from the brain.
Neurotoxic proteins (NTPs) are eliminated by a broad suite of specific and non-specific mechanisms in neurons, glial cells and endothelial/vascular smooth muscle cells of vessels. The three major modes of intracellular clearance — the autophagic–lysosomal network (ALN), chaperone-mediated autophagy (CMA) and the ubiquitin–proteasome system (UPS) — are shown for neurons but they are also active in other cells such as microglia. Under conditions of inflammation, proteasomal β-subunits in glia are switched and substrate specificity changes: the precise role of these ‘immunoproteasomes’ — specialized in peptide production for antigen presentation — for neurotoxic protein elimination in NDAs is debated8. Clearance also occurs in the extracellular space, the interstitial fluid (ISF) of the brain parenchyma that surrounds neurons, and the cerebrospinal fluid (CSF) with which the ISF exchanges. Intraneuronal mechanisms of clearance are illustrated for NTPs in general, but only Aβ42 is shown for extracellular clearance, since the vast majority of currently available data is for this NTP. Extracellular pools of NTPs are derived from passive diffusion, active release from terminals, extrusion by exocytosis, and dispersion upon cell death. NTPs disrupt neuronal and synaptic function and are taken up by other neurons and glial cells (‘spreading’). Therapeutically relevant proteases degrading NTPs include endothelin-converting enzyme and insulin degrading enzyme (IDE) (mainly cytosolic), neprilysin and matrix metalloproteinases (MMP) (intracellular and extracellular), and plasmin (mainly extracellular). NTPs that escape glial capture and proteases are driven into the circulation. First, blood–brain barrier (BBB) localised receptors and transporters actively eject them into the blood, including P-glycoproteins such as ABCB1 transporters and low-density lipoprotein receptor related protein 1 (LRP1). Conversely, the receptor for advanced glycation end-product (RAGE) receptor returns Aβ into the CNS. Similar mechanisms operate at the blood–CSF barrier in the choroid plexus; for example, LRP2 transfer of transthyretin-bound Aβ from CSF into blood. Second, transfer of NTPs to the periphery is mediated through the glymphatic system. CSF runs along the peri-arterial space, transverses aquaporin 4 receptor-bearing circumvascular astrocytes to enter the ISF. Convective flow driven by arterial pulsing flushes NTPs via glial cells and the peri-venous space back into the CSF. Glymphatic-cleared, CSF-derived NTPs mainly reach the circulation mainly via the cervical lymph nodes, but also via the dural venous sinus. Within the blood, specific proteins sequester Aβ, such as the soluble fragment of LRP1 and immunoglobulins (IgG). NTPs are ultimately eliminated in the kidneys and liver. Abbreviation not in main text or above: s, soluble.
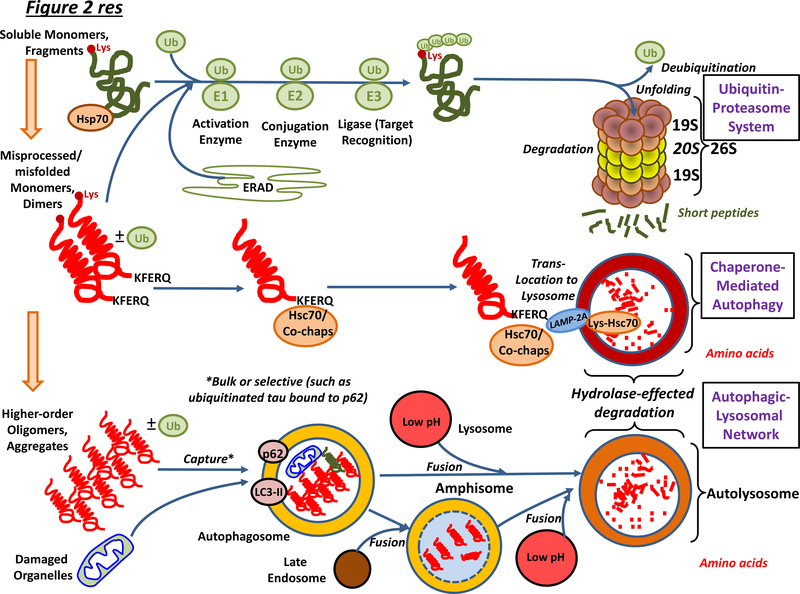
Figure 2 |. Overview of intracellular mechanisms for the elimination of neurotoxic proteins from neurons and other classes of cell in the brain.
Within neurons and other classes of cell, the UPS and CMA clear non-aggregated forms of neurotoxic protein, and the UPS also deals with substrates of endoplasmic reticulum-associated degradation (ERAD) of incorrectly-folded proteins. Proteins destined for the proteasome are poly-ubiquinated and guided to the proteasome by chaperones. They are deubiquinated by Rpn11 once committed to entering the proteosome pore: other deubiquitinases such as USP14 may rescue them before entry49. Unfolding is followed by degradation. The CMA operates on proteins bearing a KFERQ-like motif. This sequence is found in, for example, tau but not Aβ. Hsc70 recognises the KFERQ sequence and, together with co-chaperones, transports the protein to the LAMP2A receptor on lysosomes: LAMP2A then coordinates protein translocation into the lumen. The ALN is the major system for removing misfolded, higher-order, aggregated proteins as well as damaged organelles. Autophagosomes bearing cargo fuse with acidic lysosomes, leading to degradation of contents. In addition, some autophagosomes fuse with late endosomes. The resultant amphisomes then likewise fuse with lysosomes. See also Figure 3. Abbreviation not in main text: Co-chap, co-chaperone; Lys, lysine and Ub, ubiquitin,
Box 1 |. Autophagic-lysosomal flux and its measurement: cellular and animal models.
Characterisation of the ALN and its therapeutic restitution in NDAs necessitates accurate interpretation of autophagic states both in vitro and in vivo10,23. While electron microscopy has traditionally been used to observe key features of autophagosomes, recently introduced approaches allow for more refined analysis of the ALN: for example, whether increases in autophagosome number (the most common measure undertaken) reflect an increase in their synthesis or, rather, decreased ALN flux23.
Since membrane-bound LC3-II (called Atg8 in zebrafish) is covalently conjugated to phosphotidylethanolamine on the outer and inner autophagosomal membranes (Figure 3), its expression and localisation is widely used to track autophagic kinetics. Calculating the ratio of LC3-II to tubulin is a popular method for measuring cellular autophagosome levels by immunoblot17. Green fluorescent protein (GFP)-tagged LC3 has proven especially useful for quantifying autophagosomes, but self-aggregation of cytosolic GFP-LC3 and the quenching of GFP fluorescence in acidic lysosomes complicates interpretation in cytological assays23. To overcome GFP quenching, tandem constructs containing GFP and an acid-resistant red fluorescent protein (DsRed or mCherry) can be used to discriminate autophagosomes (and amphisomes) from autolysosomes (Figure 3). To show that increased levels of LC3-II genuinely represent accelerated ALN flux, it is useful to use compounds such as bafilomycin A or chloroquine, which neutralise lysosomal pH and produce an additive elevation in LC3-II levels under conditions where ALN flux is indeed high. Levels of p62 or other cargo acceptors are also useful readouts: a decrease in p62 often accompanies accelerated autophagic flux, while its accumulation may indicate a decrease. Potential variables that complicate this measure include proteasomal degradation of p62, alterations in transcription and reduced protein synthesis in degenerating cells315. Therefore, parallel monitoring of p62 mRNA levels and UPS status is recommended229. Phospho-specific antibodies that detect activation states of key autophagy-regulatory kinases like AMPK, mTORC1 and Ulk1 are also instructive indicators of ALN status.
As regards in vivo models, Zebrafish (Dano rio) larvae are transparent and permit visualization of ALN reporters such as GFP-LC3-II constructs and neurotoxic proteins316. Furthermore, targeted gene transduction, deletion or editing can easily be performed by morpholinos and the “CRISPR/Cas” system. Comparatively high-throughput screening can also be undertaken with compounds added to water that are absorbed transdermally103. For example, stimulating autophagy and TFEB nuclear translocation by trifluoperazine prevented neuronal loss in PINK1-deficient zebrafish317. Fruitflies (Drosophila melanogaster) are also useful. They can be rendered autophagy-deficient, resulting in spontaneous neurodegeneration, while restoration of autophagy is neuroprotective in PINK1 mutants318. In addition, genetic tools are available for manipulating each step of ALN disruption, while somatic, mutant clones in subsets of specific neurons permit evaluation of ALN status in impacted cells surrounded by wild-type tissue319. Drosophila have also been used to validate the effects of drugs regulating the ALN: for example, rapamycin had beneficial effects in a polyglutamine model of HD133. Nonetheless, mice remain the most common, in vivo, preclinical model for modulation of the ALN in NDAs23 and a broad range of pharmacological agents has been studied, as summarized in Table 2. Apart from the brain, retinal tissue has also proven instructive; for example, in evaluating axonal transport of acidic vesicles to lysosomes312,320.
Finally, for in vitro and in vivo studies of the ALN, overexpression of mutant proteins associated with NDAs is often used as a model of proteinopathy burden. However, this may not faithfully recapitulate sporadic forms of disease and the importance of other factors influencing the ALN, such as ER stress, the cytosolic and mitophagic UPR (Suppl Box 3) and diminished energy supply, should be borne in mind25,57,58,99,321.
In this article, we first summarize the key aspects of the autophagic–lysosomal network (ALN), CMA and the UPS, then outline the nature of their disruption in NDAs. We then consider opportunities and challenges for intervening via these systems with the goal of clearing neurotoxic proteins in NDAs Owing to its predilection for aggregated forms of neurotoxic proteins, as well as damaged organelles that also build up in NDAs, the ALN is an especially attractive target for disease modification and consequently a major focus of this article However, it is unlikely that modulation of the ALN will prove to be a panacea1,4,5, and opportunities for harnessing non-ALN driven mechanisms of clearance for course alteration of NDAs are discussed as well2,3. We also review mechanisms for the clearance of extracelluar neurotoxic proteins and strategies for their therapeutic enhancement Finally, we analyse over-arching issues for the characterization and development of therapies to promote neurotoxic protein clearance in NDAs
The autophagic–lysosomal network
Crucial role in clearing aggregated proteins
Autophagy is a phylogenetically-conserved mechanism crucial for the intracellular clearance of burdensome proteins in all cell types, including neurons. Furthermore, astrocytes and several subtypes of microglia play important roles in the phagocytosis and subsequent autophagic elimination of extracellular pools of neurotoxic proteins6–8 In addition to bulk clearance of cytoplasmic contents, dedicated autophagy receptors promote sequestration of specific misfolded and/or aggregated proteins, damaged organelles, aggresomes [G], stress granules [G], peroxisomes [G], endoplasmic reticulum (ER)/Golgi components, lipids, ribosomes, polysaccharides and nucleic acids4,9. LC3-II and adaptor/scaffold receptor proteins such as optineurin and p62 recruit discrete classes of protein to autophagosomes10. Other scaffolds include Nix, BNIP1 and prohibitin-2 for dysfunctional mitochondria (Box 2)4,9–11. Ubiquitin-dependent and non-ubiquitin-dependent autophagy occurs, with ubiquitination of tau and other neurotoxic proteins enhancing capture by autophagic receptors such as p62. Post-translational modifications such as acetylation (e.g., of Htt) may favour ALN degradation, but await further evaluation12.
Box 2 |. Defective mitophagy and its restoration for treatment of NDAs.
Mitochondria support the high energetic costs of a complex and dynamic neuronal architecture, synaptic transmission and, last but not least, operation of the ALN. Indeed, mitochondrial function and the ALN are reciprocally interlinked. For example, generation of radical oxygen species and ATP depletion induce the ALN via AMPK which will, in turn, eliminate damaged mitochondria21,322. In fact, there are several quality control mechanisms that preserve healthy mitochondrial populations: fusion and fission cycles to redistribute mitochondrial content and isolate damaged mitochondria; chaperones for ensuring maturation and folding of mitochondrial proteins; proteases for degrading misfolded mitochondrial constituents; lysosome-dependent pathways for destruction of damaged mitochondria; and a specific mode of UPR that preserves mitochondrial proteostasis57,255,323.
Mitophagy refers to a type of macroautophagy that leads to degradation of mitochondria (Figure 2)9,70,323. While crucial for many developmental programmes, mitophagy has a more generalized, protective role in preventing the accumulation of reactive oxygen species and the release of pro-apoptotic factors. Of particular significance to NDAs is a stress-responsive, mitochondrial degradation cascade co-regulated by two genes known to be mutated in familial PD: the mitochondrial kinase, PINK1 and the E3 ubiquitin ligase, Parkin69,70. This cascade, driven by PINK1-dependent activation of Parkin and ubiquitylation of proteins in dysfunctional mitochondria, is a well-characterised pathway of mitochondrial clearance, and studies using fluorescent reporter systems to track mitochondria in autophagosomes and lysosomes have highlighted its important role in neurons324. PINK1 may also clear damaged mitochondria independently of Parkin by recruiting autophagy receptors like optineurin: for example, in AD where PINK1 appears to be deficient325.
Whether driven by the PINK1/Parkin system or other ubiquitin-dependent or independent mechanisms, mitophagy decreases with age. Furthermore, while mitophagy may be compensatorily augmented at the onset of NDAs, in later phases it is generally disrupted9,75,323. There is a complex interplay between protein aggregation, mitochondrial dysfunction and mitophagy. Aggregation-prone proteins, such as Aβ, SOD-1 variants and α-synuclein, are imported into mitochondria326. This may reflect an adaptive mechanism, using mitochondria to clear aggregates255. However, in the long run, aggregation-prone proteins such as α-synuclein provoke mitochondrial dysfunction and block mitochondrial protein import. Stimulating mitophagy may thus improve both mitochondrial function and cytosolic proteostasis58,255,326.
As for pharmacological approaches for promoting mitophagy in NDAs327, some are common to those inducing cytosolic autophagy. More specifically, several strategies aim to activate PINK1/Parkin-driven mitophagy, for example, by the neo-substrate, kinetin triphosphate, which enhances PINK1 kinase activity328. Small-molecule transcriptional activators of Parkin have also been proposed329. Other approaches use iron chelators to induce PINK1/Parkin-independent mitophagy. The ubiquitin-specific deubiquitinase, USP30, negatively regulates the initiation of Parkin-mediated removal of damaged mitochondria: its structurally distinct features compared with other deubiquitinases are encouraging interest as a Parkin-related drug target227,330. Two other deubiquitinases, USP8 (delays Parkin binding to damaged mitochondria) and USP15 (suppresses Parkin-driven mitophagy) are also under scrunity as targets for promoting mitophagy in NDAs217.
The inner mitochondrial membrane protein prohibitin 2 directly binds LC3-II to target ruptured mitochondria for degradation and is depleted in human PD brain11. Since prohibitin 2 overexpression is protective in cellular models of PD, it is an interesting target for potential therapy331. Compounds that stabilise Nrf2 are also of interest, since Nrf2 triggers Parkin-independent mitophagy by a mechanism involving activation of p62332. Replenishment of nicotinamide, which declines with age56, may promote mitochondrial clearance by activating sirtuin-1-driven mitophagy333. Furthermore, in promoting mitochondrial proteostasis, nicotinamide derivatives opposed the deposition of Aβ in cellular and mouse models of AD58. The plant flavanol kaempferol induces autophagy and exerts protective effects on mitochondria; for example, against toxins triggering PD-like dysfunction. Its actions involve induction of Akt upstream of mTORC1334. Other natural compounds, such as urolithin A, promote mitophagy by mechanisms that remain to be determined335. Finally, lifestyle factors, such as exercise and intermittent fasting, favour mitochondrial and neuronal health by a combination of mechanisms that include the stimulation of mitophagy9,25,164,207.
Autophagy can be constitutive or inducible, rapidly adapting to alterations in the internal and external environment of cells. Flexibility is important for maintaining normal brain function and for ensuring a constant supply of recycled amino acids, sugars, lipids and other products of ALN-mediated catabolism3,13. That autophagy serves an essential housekeeping role is demonstrated by genetic ablation of autophagy-related genes [G] (Atg). For example, mice with neuron-specific Atg7 or Atg5 deletions develop early post-natal neurodegeneration14, while knockdown of Beclin 1 (the mammalian orthologue of yeast Atg6 [exacerbates the vulnerability of hippocampal neurons to energy deprivation15. Moreover, post-mitotic neurons cannot dilute harmful proteins via mitosis, so they are uniquely vulnerable to impairment of clearance1,3,5,16–18.
Maintaining efficient ALN flux (Box 1) requires coordination of a suite of modulatory proteins and phospholipids (Figure 3)3,10 Changes in their amount, stoichiometry and function are characteristic of NDAs1–3,5,10,18–20.
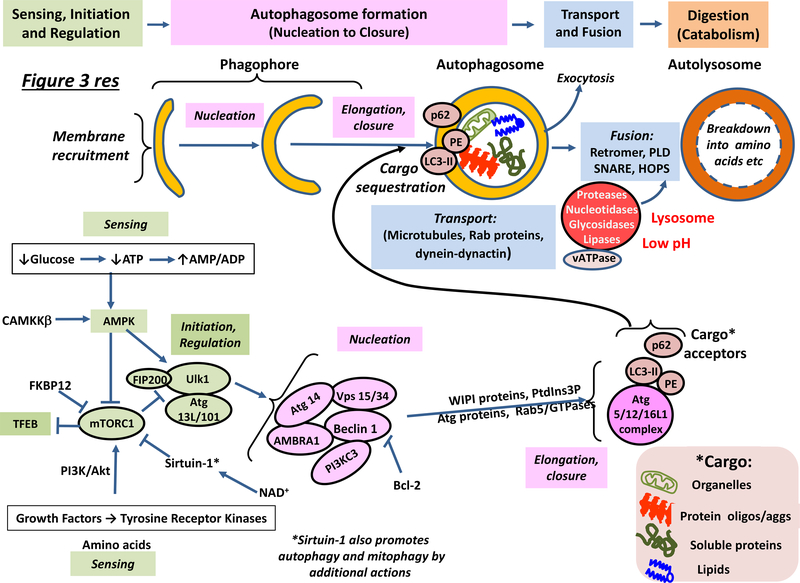
Figure 3 |. Organization, operation and regulation of the autophagic–lysosomal network.
The top part of the figure illustrates the sequence of steps associated with operation of the ALN, while the bottom part shows the main regulatory proteins involved, focusing on potential targets for pharmacotherapy. ‘Sensing’ — both extrinsic (for example, glucose levels) and intrinsic (e.g. for example, ATP/AMP levels) — can determine whether or not autophagy is initiated by activation of AMPK and/or inhibition of mTORC1, which leads to TFEB-driven transcription of ALN-requisite proteins. The pre-autophagosome (phagophore) structure first emerges from diverse membrane sources, and its formation is promoted by Atg9 (not shown). Nucleation is accomplished with the help of a complex cluster of proteins. Phosphatidylinositol-3-phosphate (PtdIns3P) is recognised by WIPI (WD-repeat-protein-interacting-with-phosphoInositides) proteins that help induce autophagosome elongation in association with several classes of Atg protein and small GTPases such as Rab5. With the aid of LC3 and cargo acceptors, autophagosomes take up cytoplasmic material such as aggregated proteins and dysfunctional mitochondria (Box 2). Autophagosomes and other autophagic vesicles are transported with the help of dynactin and dynein along microtubules towards acidic lysosomes. Autophagosomes fuse with lysosomes containing resident hydrolases that degrade their contents into amino acids, sugars and lipids for recycling. Exosomal release/secretion of neurotoxic proteins (“exocytosis)”) may occur upon reduced ALN flux and accumulation of autophagosomes. For details, see main text. Abbreviations not in main text or Glossary: FIP, family interacting protein; HOPS; Homotypic fusion and protein sorting complex; NAD+, nicotinamide adenine dinucleotide; PE, phosphoethanolamine; PI3K/Akt: phosphoinositol-3-kinase/atypical kinase and PLD, phospholipase D.
Operation and regulation of the ALN
Sensing, initiation and regulation of ALN induction.
The heterotrimeric serine/threonine kinase, AMP-regulated kinase [G] (AMPK), and mammalian target of rapamycin complex [G] (mTORC1) respectively trigger and repress autophagy and mitophagy (Figure 3, Box 2)3,10,20–23. Unc-51-like kinase (Ulk1) is primarily an autophagy-initiating protein3,10,19, as is mTORC1-suppressed transcription factor EB (TFEB), which orchestrates the synthesis of lysosomal and other proteins critical for maintaining ALN flux20–23. Since the class III deacetylase, sirtuin 1, requires nicotinamide adenine dinucleotide [G] to sustain its activity, this positive regulator of autophagy may also be considered as a sensor24.
Intrinsic sensors detect changes in intracellular levels of glucose, amino acids, fatty acids, AMP, inositol triphosphate (IP3), cytosolic Ca2+, reactive oxygen species and metabolic intermediates such as acetyl coenzyme A [G] (Box 2)5,13,19,21,23,25. For example, decreased glucose availability and impaired mitochondrial respiration compromise ATP production, leading to elevated levels of AMP and ADP, which allosterically activate the γ-subunit of AMPK21. Extrinsic sensing occurs via drug-targetable mechanisms at the plasma membrane. First, receptor tyrosine kinases converge onto mTOR1, AMPK or the Beclin 1–Vps 34 complex (Figure 3) to modulate autophagy following stimulation by growth factors10,26. Second, G-protein coupled receptors (GPCRs) and ion-channel coupled receptors control autophagy via signalling pathways that likewise modulate AMPK and mTORC127–29. GPCR-mediated generation of cAMP can negatively regulate autophagy via, for example, protein kinase A (PKA)-mediated phosphorylation of Atg proteins27,29,30. Third, specific classes of cytokine and cytokine receptor also modulate autophagy, although events in the brain remain poorly defined23.
AMPK is central to several mechanisms that trigger autophagy — most importantly, phosphorylation-activation of Ulk1/2 (Ser317 and Ser777) and phosphorylation-inhibition of mTORC121,31 [Conversely, mTORC1 inhibits Ulk1/2 by Ser757 phosphorylation3,4,31. mTORC1 also restrains autophagy by preventing nuclear translocation of TFEB20. Other transcription factors that positively regulate autophagy include Forkhead-Box O1 and O322. Conversely, repression is effected by STAT3 (signal transducer and activator of transcription 3) and, possibly, ZKSCAN3, although its role has been disputed22,32. Sirtuin 1 is activated by AMPK-mediated increases in nicotinamide: it drives the ALN by inhibition of mTORC1, induction of Forkhead-O1/O3, and activation of key regulatory proteins such as Atg5, Atg7 and LC3. These actions comprise part of a broad palette of sirtuin-1-mediated neuroprotective effects in NDAs24.
Autophagosome formation, cargo sequestration and delivery to lysosomes.
Activation of Ulk1 triggers autophagosome nucleation through phosphorylation-activation of Beclin 1 within the autophagy-specific Vps 34 kinase complex10 (Figure 3). LC3 and other family members such as GABARAP covalently conjugate with phosphatidylethanolamine and assist in elongation of the isolation membrane and closure of autophagosomes1,3,10,33. They also serve as docking sites for autophagy receptors that selectively capture ALN substrates (Box 1)3.
Compared to glia, the complex structure of neurons complicates ALN degradation of neurotoxic proteins1,8,10,18. Autophagosomes formed in synaptic terminals and neurites must be retrogradely transported with the aid of microtubules and dynein–dynactin motor complexes to the perikarya where lysosomal fusion occurs10,16,34. Indeed, many autophagosomes fuse with late endolysomal compartments containing membrane-localised Rab7 protein [G] (a GTPase) and lysosome-associated membrane protein (LAMP)1 before reaching the perikaryon. This implies that the ALN process is partly intiatiated in advance of fusion with mature lysosomes and full luminal acidification, a process completed upon arrival in the perikaryon (Figure 2)10,16,34,35.
Autolysosome formation is facilitated by the retromer complex, itself retrogradely transported to cell bodies36,37. SNARE [G] proteins and the homotypic fusion and vacuole-protein sorting complex bridge mature autophagosomes/amphisomes to lysosomes to initiate fusion4,19. Rab proteins and LAMP1/2 collectively aid in autophagosome maturation and lysosomal fusion, which is also dependent on membrane constituents such as phospholipase D1 [G], phosphoinositols and other phospholipids such as cholesterol10,19,38.
Lysosomal digestion of cargo.
Autophagosomes fuse with lysosomes that provide the hydrolases required for cargo degradation3,4,9,39. Hydrolases are dependent on a low pH, and lysosomal acidification is promoted by vacuolar-type H+-ATPase complex (v-ATPase), which pumps protons into the lysosomal lumen. The electrogenic potential created by proton import is mediated by multiple ion channels that influence lysosomal pH40. Underpinning the importance of acidity, digestion can be halted by v-ATPase inhibitors such as bafilomycin A41 and lysosmotropic basic amphiphiles such as chloroquine that alkalinize the lysosomal lumen42. Furthermore, a deficiency of lysosomal cathepsins (B, L and D etc) prevents protein degradation and leads to accumulation of undigested cargo16,17,39. Lysosomal dysfunction blocks flux across the entire ALN, as evidenced by lysosomal storage diseases [G] (LSDs) such as Niemann-Pick Type C [G] that manifest with neuropathological phenotypes (Suppl Box 1)43.
In addition to ALN function, the importance of maintaining lysosomal activity reflects a broader role in, for example, regulation of cytosolic Ca2+ and energy homeostasis44.
Chaperone-mediated autophagy
Like macroautophagy [CMA is important for amino-acid recycling during periods of poor nutrient availability but, in contrast, it involves transfer of protein substrates for degradation into the lysosomal lumen without enclosure by any membrane structure (Figure 2)45–47. With the help of heat shock protein 90 (Hsp90) and other co-chaperones, heat shock cognate protein 70 [G] (Hsc70) recognises soluble, cytosolic proteins bearing a KFERQ [G] or equivalent motif and guides them to the transmembrane LAMP2A receptor1–5,10,47. The substrate complex binds to the cytosolic tail of LAMP2A, leading to LAMP2A stabilization and oligomerization: following unfolding of the protein cargo, it is then translocated into the lysosomal lumen. This process is aided by a specific, low pH-dependent lysosomal form of Hsc70 (Lysine-Hsc70), which promotes dissociation of the LAMP2A multimer so that the monomeric form is again available for substrate recognition and import. The level of LAMP2A determines the rate of CMA.
In contrast to the ALN, CMA is not devoted to the degradation of higher-order neurotoxic proteins and aggregates, but it is important for clearing oxidized proteins. Tau, α-synuclein and TDP-43 are substrates for CMA degradation, as well as APP but not Aβ42 itself3,45–47,48. Htt is not efficiently cleared by CMA, and the same appears to hold for its fragments, mutant and post-translationaly modified forms, although the precise role of CMA in Htt elimination remains to be more fully defined2,45–47.
The ubiquitin–proteasome system
The UPS mainly targets soluble and monomeric proteins rather than aggregates, using a process involving Hsp70 and the sequential actions of three classes of ubiquitin ligase (E1, E2, and E3). They effect the addition onto targeted proteins of ubiquitin residues, often as polyubiquitin chains, at single or multiple lysine sites (Figure 2)2,3,8,48,49. Ubiquitinated substrates are recognised by the 19S regulatory particle of the UPS complex. After binding to the Rpn subunits of the 19S ring, ubiquitin motifs are removed by three enzymes, Usp14, Uch37 and Rpn11. Rpn11 removes ubiquitination chains only after substrates are committed to destruction, whereas Ups14 and probably Uch37 act before commitment and hence can rescue substrates49. Following removal of ubiquitin moieties, proteins are unfolded by the Rpt1–6 subunits (ATPases) of the 19S component. The substrate then passes the α-subunit gate of the 20S core particle to enter its central β-subunit, which possesses peptidase activity (trypsin, chymotrypsin and caspase-like) and effects proteolysis.
In addition to ubiquitinated substrates, the UPS can also handle oxidized proteins, which may accumulate under conditions of cellular stress8,50. Furthermore, as well as cytosolic proteins, the UPS degrades mitochondrial proteins that build up upon failure of mitochondrial import or sorting51. It also operates in the nucleus. Interestingly, the UPS is important for elimination of tau and other neurotoxic proteins in post-synaptic dendritic compartments (a key site of spreading), where it plays a more general role favouring synaptic plasticity, dendritogenesis and memory formation49,52. Susceptibility of neurotoxic proteins to ubiquitination is modified by phosphorylation and other post-translational modifications3,8,49,51.
Impaired intracellular protein clearance
Neurons adopt multiple strategies to deal with potentially dangerous proteins. With the aid of chaperones such as Hsp70, anomalously configured proteins may be refolded or, if clumped in aggregates, disassociated2,3,53. Neurotoxic proteins may also be sequestered in insoluble tangles (for example, as with tau) or in microtubule-associated aggresomes2,4. This intracellular lock-up may, at least initially, be neuroprotective, but continuing accumulation eventually poses a threat to cells, underscoring the importance of elimination2,4. While clearance systems are, at least initially, recruited in NDAs, they eventually become unable to cope with the additional neurotoxic burden (Table 1)1,5,9,18,54,55. The partly common and partly disease-specific patterns of ALN, CMA and UPS disruption in NDAs are superimposed upon a generalized, age-related decline in clearance both in neurons and in other cell types such as microglia1,2,7,18,46,47,55,56. Insufficient neuronal ALN flux is frequently manifested by lysosomal accumulation of lipofuscin [G] 18.
Table 1:
Neurodegenerative disorders of ageing: major clinical and pathophysiological features, disruption of proteostasis, and impairment of neurotoxic protein clearance.
| Disease (age of onset) % Familial Main risk genes related to poor clearance |
Clinical and pathophysiological phenotype | Disruption of proteostasis | Autophagic-lysosomal network impairment | Impairment of CMA and of the UPS | Impairment in other modes of neurotoxic protein clearance |
|---|---|---|---|---|---|
| Alzheimer’s (usually over 70) ca. 5% APOE4, APP, PS1, PICALM, TREM2 |
Cognitive deficits; psychiatric symptoms; disorganized language; disrupted sleep/circadian rhythms. Neurodegener-ation (entorhinal cortex, medial temporal lobe, hippocampus etc); ↓axonal transport; axonal and synaptic degeneration; altered microglial phenotype. | Aβ oligomers disrupt neurones, synapses, aggravate tau toxicity; Aβ aggregates in extra-cellular plaques/vessels; aberrant tau cleavage, post-translational marking, folding and oligomerisation; ↑tau release and spreading; intra-cellular tau tangles (with p62 and other Ub-proteins). α-syn neuropathology in subpopulation. | ↓Sirtuin-1; ↓Neuronal ALN flux; ↓Autophagosome maturation, transport (MAPT) and fusion with lysosomes; ↓APP loading (PICALM); APP and C-terminal fragment accumulation in endo-lysosomes; ↓Lysosomal acidity and digestion (PS-1/2, APP ApoE4); ↓Glial ALN (TREM2, ApoE4). ↓Mitophagy (PS1). | ↓ CMA (disrupted by Aβ/tau aggregates); Anomalous mutant tau at LAMP2A impedes CMA; ↓ UPS clearance (perturbed by Aβ and tau oligomers); FKBP51 binds Hsp90 to interfere with UPS substrate loading. | ↓Proteolytic Aβ clearance (↓IDE, Neprilysin, Plasmin); ↓BBB clearance of Aβ and, probably, tau (↓LRP1; ↓P-glycoprotein; ↑RAGE); ↓Aβ provision to BBB (ApoE4); ↓glymphatic clearance of Aβ and, probably, tau. |
| Parkinson’s (usually over 60) ca. 5–15% SNCA, PINK1, GBA, PARK2, LRRK2, PARK9, UCH-L1 |
Motor impairment (poor gait, rigidity, bradykinesia, tremor); ↓olfaction; gastrointestinal problems; cognitive deficits; pain; depression; prodromal RBD. Neuronal loss (Dopaminergic cells in SNPC etc). | α-Syn inclusions and Lewy Bodies (contain lipids, α-syn, Tau, other neurotoxic proteins, ubiquitin); ↑α-syn release and spreading in brain - possibly earlier, in gut. Tau neuropathology in subpopulation. | Many α-syn related anomalies of ALN: ATG9 mislocalisation; ↓Formation, maturation, axonal transport and lysosomal fusion of autophagosomes;↓Lysosomal function (LRRK2, PARK9, GBA); ↓beclin 1 (LRRK2); ↓Mitophagy (PINK1, PARK2). | ↓LAMP2A/Hsc70 levels; ↓ CMA activity (aggregated α-syn and mutant forms of α-syn and LRRK2 block); Slow α-syn dissociation from LAMP2A.↓UPS clearance (aggregates and mutant forms of α-syn block); Impaired α-syn traffic to UPS (UCH-L1). | ↓BBB α-syn clearance; likely ↓α-syn elimination by glymphatic system. |
| Frontotemporal dementia (~40–60) ca 10–15% MAPT, C9ORF72, GRN, VCP, FUS, TARDBP, TREM2, CHMP2B, TMEM106, UBQLN2 |
Cognitive impairment; altered personality; mood and language deficits; cell loss prominently in inferior frontal and anterior temporal cortices, asymmetrically or bilaterally. | Misfolded and aggregated forms of tau, TDP-43 and/or (more rarely) FUS; Often found with p62 and ubiquitin in inclusions. | Autophagosome accumulation; ↓Cargo loading into autophagosomes by p62; ↓Axonal autophagosome transport (MAPT); ↓Endosomal trafficking (CHMP2B); Lysosomal dysfunction (GRN, TMEM106); ↓Glial ALN flux (TREM2). | ↓CMA and UPS clearance (impeded by aggregates of tau, TDP-43 and FUS); poly-GA aggregates (caused by C9orf72 mutations) sequester and stall proteasomes; p62 dysfunction. | Not well defined, but likely similarities to AD as regards altered BBB permeability and ↓ glymphatic flow. |
| Amyotrophic lateral sclerosis (~50–60) ca 10% SOD1, TARDBP, FUS, C9ORF72, VCP, SQSTM1, UBQLN2, OPTN, TBK1, DCTN, GRN, TREM2 |
Motor impairment (cramps, muscle weakness, spasticity); cognitive impairment; mood disturbances (especially late-phase); ventral horn motoneuron loss; brainstem and cortical neuron degeneration. | Misfolded and aggregated TDP-43 and (more rarely) SOD1 and FUS inclusions in brain, spinal cord and motoneurons; inclusions may contain ubiquitin and ubiquitin-ligases. | Mainly ↓ALN, but if cellular stress severe, high ALN may actually be detrimental; ↓Autophagosome maturation (C9ORF72); ↓Cargo loading (SQSTM1, UBQLN2, OPTN, TBK1); ↓Autophagosome retrograde transport (DCTN, C9ORF72); ↓Lysosomal function (CHMP2B/GRN); ↓Glial ALN flux (TREM2). | Aggregated proteins block proteasome; ↓Hsp70 and Hsp40; ↓ Provision of SOD1 and other proteins for UPS degradation (VCP); ↓ CMA clearance of TDP-43. | BBB disruption; ↓glymphatic flow may impede efflux of neurotoxic proteins. |
| Huntington (~30–50) Inherited (ca. 8–10% = de novo mutations) HTT |
Motor dysfunction (chorea, dystonia, slurred speech); cognitive impairment; sleep disturbances; basal ganglia neuron loss, especially striatal medium spinal neurons; disruption of corticostriatal pathway; failure of axonal transport. | Aggregates of mutant (excess CAG repeat number) Htt; mutant Htt inclusions with ubiquitin, beclin 1, mTOR1, p62 and other cargo-loading proteins; Mutant Htt and fragments of Htt are cytotoxic. | Mutant Htt poor substrate of and disrupts ALN - and mitophagy; interference with beclin-1; ↓Autophagosome formation and cargo recognition/loading; ↓Axonal transport of autophagosomes. | Mutant Htt poor substrate of CMA and UPS; LAMP2A and Hsc70 initially upregulated, but CMA less efficient in late stages; Possible ↓ UPS (blocked by mutant forms of Htt?); ↓Hsp70. | BBB disruption due to accumulation of Htt, but role in Htt clearance uncertain; potential ↓glymphatic clearance to establish. |
Clearance mechanisms are recruited early in disease, yet eventually become dysfunctional and/or inadequate to cope with neurotoxic burden. Not all changes can be shown, and NDAs are associated with neuroinflammation/immune deregulation, glial anomalies, disruption of cerebral bioenergetics, mitochondrial dysfunction and ER/oxidative stress. Several variants of frontotemporal dementia (FTD) include behavioural, progressive non-fluent aphasia and semantic forms. ALS shares common pathological hallmarks and risk genes with FTD like C9orf72 (Chromosome 9 Open Reading Frame 72). This and other NDA-associated risk genes linked to impaired clearance, are indicated in column one. Examples of genes/proteins incriminated in pathological processes are given in columns 3–6. APOE4 (apolipoprotein E4); PARK9 (ATPase13A2); CHMP2B (chromatin-modifying protein 2B); DCTN1 (dynactin); FUS (Fused in sarcoma); GBA1 (β-glucocerebrosidase); GRN (progranulin); HTT (huntingtin); LRRK2 (leucine-rich repeat kinase 2); MAPT (microtubule association protein, tau); OPTN (optineurin); PARK2 (parkin); PICALM (phosphatidylinositol binding clathrin assembly protein); PINK1 (PTEN-induced putative kinase 1); PS (presenilin); SNCA (α-synuclein); SOD1 (superoxide dismutase 1); SQSTM1 (sequestome 1, p62); TBK1 (TANK-binding kinase 1); TARDBP (TAR DNA binding Protein 43); TMEM106, transmembrane Protein 106B; TREM2 (triggering receptor expressed on myeloid cells 2); UBQLN2 (ubiquilin 2); UCH-L1, Ubiquitin carboxy-terminal hydrolase L1 (a deubiquitinase) and VCP (valosin-containing protein). Aβ refers to Aβ42 and related neurotoxic fragments of APP. See text for further information and citations. Abbreviations not above or in text: FKBP, FK-binding protein; SNPC, substantia nigra, pars compacta and RBD, rapid eye movement sleep behavioural disorder.
For optimisation of therapy in NDAs, accurate interpretation of the causes of impaired elimination of neurotoxic proteins is paramount. This is challenging since it may be a repercussion of upstream anomalies such as protein overproduction, misfolding or an excessive cytosolic unfolded protein response [G] (UPR) (Suppl Box 2)57. Furthermore, it is difficult to identify the exact nature of UPS, CMA and ALN dysfunction [G] (Box 1). While inadequate ALN flux is a common problem for all NDAs, under certain conditions ALN overactivity may contribute to pathology and even autosis [G] 4 in ALS (Suppl Box 3).
The following paragraphs and Table 1 summarize the complex patterns of defective neurotoxic protein clearance seen in specific classes of NDAs.
Alzheimer’s disease
While induced in the early phase of AD1,3,47,58, ALN, UPS and CMA-mediated clearance eventually becomes overwhelmed and impaired. First, autophagosomes and autophagic vacuoles indicative of failed maturation, transport and/or fusion with lysosomes are abundant, particularly in dystrophic neurites. Their accumulation may be linked to impaired lysosomal elimination of cargo18. Second, while decreases in Beclin 1 levels in AD remain to be confirmed, sirtuin 1 expression is diminished24. Third, apolipoprotein E4 [G] allele (ApoE4), a major risk allele for sporadic AD, is associated with increased generation and accumulation of Aβ4259,60. ApoE4 slows lysosomal Aβ42 clearance and, like Aβ42 itself, destabilizes lysosomal membranes. In addition to decreased degradation, one consequence is leakage of asparaginyl endopeptidase into the cytosol, where it generates toxic fragments of tau61. Moreover, ApoE4 impairs the elimination of Aβ42 and tau by astrocytes and microglia, additionally compromised by decreased activity of TREM2 (triggering receptor expressed on myeloid cells 2)7,62. Fourth, genetic mutations and anomalies of presenilin 1 [G], a dominant-negative gene linked to AD, are associated with reduced lysosomal v-ATPase-mediated acidification40,63, a compromised ALN and deficient mitophagy64. Presenilin-2, likewise an autosomal-dominant risk gene, is enriched in late endosomes/lysosomes, where its dysfunction provokes lysosomal accumulation of insoluble Aβ42 and possibly tau65. Fifth, mutations in amyloid precursor protein [G] (APP), similarly disrupt endosomal and lysosomal function, in part due to accumulation of the β-secretase-generated, carboxyl-terminal and Aβ42-containing fragment of APP called C9966. Sixth, Aβ42 compromises the function of AMPK to impede initiation of the ALN67. Finally, Aβ42 obstructs the UPS and CMA47,68. Both aggregates and mutant forms of tau likewise block the proteasome, and its efficacy for degrading hyperphosphorylated and oligomeric tau is reduced compared to the physiological form3,55,68. Finally, while physiological tau possesses KFERQ motifs and is degraded by CMA, aggregates, mutant forms and fragments interfere with CMA45,47.
Parkinson’s disease
Disrupted proteostasis is also a major feature of PD, with the efficiency of ALN, CMA, UPS and other modes of clearance compromised by multiple cellular anomalies. First, autosomal-recessive forms of early-onset PD are associated with mutations in phosphatase and tensin homolog-induced putative kinase (PINK1) and the E3 ubiquitin ligase Parkin: [G] these mutations lead to deficits in the mitophagic removal of damaged mitochondria (Box 2)69,70. Second, the GTPase leucine-rich repeat kinase 2 (LRRK2) is the most commonly “mutated” protein in late-onset, familial PD. Its role is complex, with both loss and gain of function mutations. Some of these lead to an impairment of the ALN due to reduced activation of Beclin 1; another repercussion may be altered processing of APP, providing an unexpected link to AD69,71–73. Third, α-synuclein mutations, triplication or excess amplify the ALN burden, interfere with autophagosome formation and irreversibly disrupt the lysosomal membrane1,3,44,56. Fourth, homozygous mutations of lysosomal β-glucocerebrosidase provoke the LSD, Gaucher’s disease [G], which is linked to decreased ALN flux, α-synuclein accumulation and a five-fold increase in risk for PD (Suppl Box 1)43. Decreased β-glucocerebrosidase activity also occurs in sporadic PD, leading to the build-up of glucosides, lipid dyshomeostasis, poor clearance of α-synuclein and impaired lysosomal activity43,74,75. Fifth, defects in several genes disrupt lysosomal acidification40. For example, disruption of the ATPase ATP13A2 (PARK9), which is also depleted in sporadic PD, leads to lysosomal alkalisation and digestive failure76 together with accumulation of α-synuclein and other ubiquinated proteins76–78. Sixth, aggregates and mutant forms of α-synuclein disrupt the proteasome in dopaminergic neurons. Furthermore, mutations in Parkin and several other genes are linked to reduced UPS activity2,56,69,79,80. Finally, oligomeric and mutant forms of α-synuclein impair LAMP2A-mediated cargo transport for CMA, while levels of both LAMP2A and Hsc70 are reduced in PD brain45,47,55,80. In addition, CMA is disrupted by several genetic mutations occurring in PD, including LRRK2 (2,3,45–47,55,69,80). CMA dysfunction in PD favours the accumulation of α-synuclein and leads to inactivation of the dopaminergic neuron survival factor, MEF2D (2,45,47,55).
Frontotemporal dementia
As FTD was initially associated with tau mutations, it was originally considered a “tauopathy”81,82. However, in light of common risk genes such as p62 (Sequestome1) and C9ORF72 (chromosome 9 open reading frame 72), FTD is increasingly linked to ALS82,83. Genetic anomalies in FTD are closely related to a deficient ALN, and, like ALS, the disease is characterised by aggregates containing tau, TDP43, Fused-in-Sarcoma and other ubiquitinated proteins insufficiently cleared by the ALN82,84. Aggregates interfere with the UPS to create a vicious circle that further overloads the ALN1,18,55,56,68,84. Recently, it was found that poly-glycine/alanine tracts linked to mutant forms of the C9ORF72 gene form twisted ribbon aggregates that sequester and stall the activity of proteasomes85. MAPT (tau) is a distinctive risk gene for FTD versus ALS, and dissociation of tau from microtubules disrupts retrograde transport of autophagosomes to the lysosome81,82. In addition, lysosomal dysfunction and loss of acidification is caused by tau fragments and a deficit of progranulin40,82,83,86, while an interrelated deficiency of endosomal trafficking is linked to mutations in CHMP 2B (charged multivesicular body protein 2B) as well as C9ORF7282,83.
Amyotrophic lateral sclerosis
ALS shares many causal genes with FTD, including p62, CHMP2B, TBK1 (tank-binding kinase 1), optineurin and others associated with deficits in ALN and mitophagy. For example, mutations in optineurin and TBK1 interfere with cargo loading82,84,87. Mutations in C9ORF72 (the most prevalent risk gene for familial ALS and FTD) are likewise linked to disruption of the ALN, including interference with dynactin–dynein coordinated transport of autophagosomes along axons of motor neurons to the perikarya82,88. They also lead to deregulation of Rab-GTPases and a failure of autophagosome elongation89. Paradoxically, however, certain anomalies of C9ORF72 may stimulate the ALN and, under conditions of severe cellular stress, high ALN activity may be detrimental (Suppl Box 3)48,88,90. In any event, depending on their genetic profiles, ALS patients reveal aggregates of risk gene-encoded proteins like TDP-43, optineurin, Fused in Sarcoma and/or superoxide dismutase (SOD1) [G] 48,82,84,87,89. Aggregated SOD1 and TDP-43 disrupt CMA and the UPS — with the latter also impaired by mutations in the C9ORF72 gene2,8,47,48,55,85,91. Thus, mirroring other classes of NDA, a failure to clear neurotoxic proteins is characteristic of ALS48,82,84.
Huntington’s disease
In this autosomal-dominant, polyglutamine disorder, an increase in CAG-expansion repeats [G] in the HTT gene encoding Htt protein magnifies its propensity to oligomerise2,3,55,80. Mutant Htt is cleared by autophagy, but it compromises the ALN because of decreased poor cargo loading and impaired autophagosome formation and transport55,56,68,92. Furthermore, ALN disruption in the striatum (a region strongly affected in HD) involves altered activity of the striatal-specific Beclin 1 and Htt-interacting protein Rhes93,94. In addition, loss of physiological Htt and abnormal polyQ-Htt perturb neuronal cilia — important sites of cellular communication and signaling that reciprocally interact with autophagic mechanisms controlling their formation and growth92. CMA only poorly handles mutant and post-translationally modified forms of Htt, which interfere with its activity2,45,47,95. While LAMP2A and Hsc70 are upregulated in early HD to compensate for decreased ALN clearance, CMA eventually fails in parallel with neuronal loss47,96. The status of the UPS in HD is currently unclear, but it only poorly cleaves mutant forms of Htt (and other polyglutamine tracts), while animal models suggest that it is impaired in HD [which would further lead to reduced clearance of Htt97.
Enhancing clearance by the ALN
Ultimately, any strategy that improves protein quality control and reduces excessive generation, aberrant processing and/or abnormal folding of neurotoxic proteins should moderate the ALN burden and facilitate clearance. For example, agents that promote folding of nascent proteins, prevent misfolding, refold aberrantly configured proteins, dissociate aggregates, counter ER stress and/or blunt an excessive UPR might pre-empt the build-up of neurotoxic proteins (Suppl Box 2)1,2,54,56,57,84,98–100. However, the present review focuses on strategies for elimination of neurotoxic proteins (Table 2 and Figure 4). It should be noted that the precise mechanisms of drug action are not invariably well-defined4 and that certain agents exert multiple beneficial (or deleterious) actions. For example, methylene blue counters tau oligomerization as well as promoting autophagy (Suppl Table 1)101,102. In addition, several agents such as resveratrol interact at multiple nodes of the ALN. Indeed, future drugs designed to act in a multi-modal manner may prove to be the most effective for enhancing neurotoxic protein clearance in NDAs.
Table 2:
Pharmacotherapeutic strategies for promoting intracellular clearance: actions in cellular and animal models of neurodegenerative disorders of aging.
| Agent | Clinical indication (or other use), and mechanistic influence on clearance mechanisms | Influence on neurotoxic proteins: In vitro procedures | Influence on neurotoxic proteins: In vivo models | |
|---|---|---|---|---|
| Autophagy activators: modulation of sensing, initiation and regulation | ||||
| AMPK facilitation | Antihypertensives | α2-adrenergic agonists/AC inhibition, ↓AC-AMP/↑AMPK | PC12: ↓α-syn (Syn A53T) /↓Htt (HttQ74)103 | Mice: ↓Htt, ↑motor function (Htt82Q)104 |
| Clonidine, Rilmenidine | ||||
| Calpastatin, Calpeptin | Investigational compounds (endogenous peptides) | Calpain inhibitors: ↑AMP/AMPK induction, ↓ cleavage Atg proteins | SK-N-SH: ↓Htt (HttQ74)103 | Drosophila: ↓Htt, ↓neurodegeneration (HttQ46)54 Mice: ↓Htt aggregates, ↑motor function (Htt171–82Q)54; ↓motoneuron loss (SOD1G93A)107, ↓tauopathy (JNPL3-MAPTP301L)106 |
| AICAR | Experimental agent. Potential treatment for myocardial ischaemia | AMP analogue -allosteric inducer of AMPK | N2a: ↑AMPK108; Glia: ↓toxicity(Aβ/LPS)109; SH-SY5Y: ↓α-syn (wild-type protein)110 | - |
| A-769662 | Experimental agent | Allosteric AMPK inducer | Striatal neurones/mouse fibroblasts: ↑LC3 and p62, ↓mHtt and ↑cell viability111 | - |
| Resveratrol | Polyphenol found in grapes etc (dietary supplement). Clinical evaluation in AD, MCI | CaMKK2 potentiator, upstream of AMPK; Upstream inducer of Sirtuin-1 | N2a: ↑AMPK108; ↓Aβ (APP695)114; Cortical neurones: ↓Aβ (J20)114 | C. elegans: ↓polyglutamine (HttQ128)115; Mice: ↓Aβ (APP/PS1)114 |
| Metformin | Antidiabetic. Clinical evaluation for MCI | AMPK activator | SH-SY5Y: ↓α-syn110; ↓tau phosphorylation117, ↓Aβ toxicity118 | Mice: ↓TH neuronal loss, ↑motor function (MPTP)119 |
| Trehalose | Disaccharide. Abiotic stress protectant. Food-additive | Glucose transporter inhibitor, ↑AMP/AMPK activator | PC12 ↓α-syn (A30P/A53T) / ↓Htt (Q74)121; Cortical neurones: ↓tau (TauRDΔK280)122 | Mice: SOD1 (SOD1G93A)120; ↓Htt (R6/2- Htt150Q)124, ↓tauopathy (PS19-MAPTP301S)125, ↓Aβ (APP/PS1)123 |
| Lithium | Mood stabiliser, anti-epileptic Evaluated in FTD and ALS | ↓Inositol monophosphate, AMPK activator? | SK-N-SH: ↓Htt (HttQ74)126 | Mice: ↑survival (SOD1G93A)128; ↓tau/filaments, ↑motor function, ↑autophagy (JNPL3)127 |
| Methylene blue | Dye. Treatment of methemoglobinemia. Development for AD/FTD (various formulations) | AMPK activator, ↑beclin 1 (also inhibitor of tau aggregation) | HT-22: ↑AMPK, ↓cell death (serum deprivation)102; Organoypic Hippocampal Slice/Neurones: ↓tau (JNPL3, MAPTP301L)101 | Mice: ↓tau (JNPL3)101 |
| Calcitriol (Vitamin D metabolite) | Treatment of Ca2++ deficiency. | CaMKK2 potentiator upstream of AMPK | - | Mice: ↓neurodegeneration (C57BL/6/MPTP)129 |
| mTOR1 Inhibition | Macrolide. Immunosuppressant (organ transplants). Potential chemotherapy | mTOR1 inhibitor | PC12: ↓α-syn (MPTP)130, ↓Htt (HttQ74)131 Cortical neurones: ↓FUS, ↓stress granule (FUSR521C)132 |
Drosophila: ↓Htt,↓neurodegeneration (HttQ74)133; Mice: ↓Aβ/tau (3XTgAD)136, ↓TDP43/p62 (FTLD-U/TDP43)134 and ↓neuronal loss (MPTP)135 |
| Rapamycin | ||||
| Temsirolimus | Renal cell carcinoma | mTOR1/2 inhibitor | SH-SY5Y: ↓hyperphosphorylated tau (okadaic acid)137 | Mice ↓tau (MAPTP301S)137, ↓α-syn/ neuroprotection(MPTP)138, ↓Ataxin3 (Ataxin3Q70)139;↓Htt/ ↑motor skills (R6/2)133 |
| Curcumin | Tumeric extract. Food colour. Dietary supplement. Clinically evaluated in MCI | Indirect mTOR1 repressor, p300 HAT inhibition causing Atg deactylation | SH-SY5Y: ↓α-syn aggregation (SynA53T) 142,143; DA neurones: ↑neuroprotection (rotenone)141 | Mice: ↓Aβ aggregation (Tg2576)146, ↓tau dimers (hTau)145, ↓α-syn (GFP-Syn)144 |
| Fisetin | Plant polyphenol. Anti-oxidant | mTOR1-dependent activator of TFEB | Cortical Neurones: ↓phospho-tau149 | Mice: ↓Aβ (APP/PS1)150 |
| Nilotinib | Resistant chronic myelogenous leukemia. Clinically evaluated in PD | C-Abl kinase inhibitor, upstream recruitment of mTOR1 | M17: ↓TDP43 (GFP-TDP43)154 | Mice: ↓α-syn, ↑motor function (SynA53T)153, ↓TDP43 (TDP43)154 |
| Sirtuin1 facilitation | Vitaminin in food. Treatment of niacin deficiency. Clinically evaluated in AD | Nicotinamide adenine dinucleotide precursor/sirtuin1 promoter, Atg deacetylation | Cortical Neurones: ↓Aβ toxicity (Aβ25–35/1–42)158 | Mice: ↓Aβ and tau (3XTgAD)159 |
| Nicotinamide | ||||
| Cilostazol | Treatment of intermittent claudication. Platelet aggregation inhibitor. | Phosphodiesterase 3 inhibitor, Upstream recruiter of Sirtuin-1 | N2a: ↓Aβ (APPSWE); ↑AMPK, ↓mTOR1, ↑autophagosomes, ↑cathepsin B108 | Mice: ↓Aβ, ↓phospho and acetylated-tau; ↑ cognition (icv Aβ25–35)162,163 |
| Spermidine | Natural polyamine. Potential promoter of longevity | p300 HAT Inhibitor, Atg and Histone H3 deacetylator, ↑Beclin 1 | Cortical Neurones/PC12: ↑survival, ↓toxicity (staurosporine)168 | Drosophila: ↑motor function (α-syn) 170; C. elegans: ↓α-syn toxicity (UAS-GAL4-α-syn)170; ↓TDP-43 (FTLD-U)169 |
| Autophagy activators: enhanced autophagosome formation | ||||
| Isorhynchophylline | Plant alkaloid. Investigational compound | ↑Beclin 1 | DA Neurones/N2a: ↓α-syn (SynWT, SynA53T, SynA30P)175 | - |
| Auten-99 | Investigational compound | ↑ PtdnIns3P activity (via Jumpy phosphatase inhibition) | SH-SY5Y: ↑survival (H2O2)181 | Drosophila: ↓neurodegeneration, ↓p62 (ParkinR275W)181 |
| Enhancers of autophagosome fusion/transport | ||||
| Paclitaxel, Epothilone D | Chemotherapy of several cancers (Paclitaxel). Potential treatment for cancer (Epothilone) | ↑Cytoskeletal/microtubule transport of autophagosomes | SH-SY5Y: ↓Aβ-mediated cytoskeletal destabilization and ER stress (Aβ25–35)182 | Mice: ↓tau (PS19, TauP301S)183 |
| Enhancers of lysosomal digestion | ||||
| 2-Hydroxypropyl-β-cyclodextrin | Investigational compound. (binds cholesterol) | TFEB inducer; ↓endolysosomal cholesterol; ↓lysosomal pH; ↑ABCB1 transporters (astrocytes) | H4: ↓α-syn aggregates(α-syn-GFP)195; N2a: ↓Aβ (APPSWE)173 | Mice: ↓tau, ↓Aβ plaques, ↑memory (Tg19959/CRND8)173 |
| Clioquinol | Anti-fungal, anti-protozoal drug | Zinc (and iron) chelator; Increased lysosomal acidification. | Fibroblasts: ↓α-syn(ATP13a2/PARK9 knockdown)78 | Mice: ↓Aβ(Tg2576)197 |
| GZ/667161, GZ/SAR402671 | Investigational compounds, Clinically evaluated in PD | Inhibitors of glucosylceramide synthesis, substrate reducers | - | Mice: ↓α-syn/ubiquitin/tau, ↑memory(GBAD409V)199 |
| Miglustat | Gaucher’s disease, Niemann-Pick Type C1 disease | Inhibitor of glucosylceramide synthesis, substrate reducer | Mesencephalic Neurones: ↓lipid accumulation in lysosomes (MPTP+ conditural-β-epoxide)75 | Mice: ↓substrate storage, ↑longevity (MPTP)75 |
| Ambroxol | Secretolytic for respiratory diseases. Clinically evaluated in PD and Gaucher’s disease | Chaperone: aids β-glucocerebrosidase transport to lysosome | Dopaminergic Neurons: ↓α-syn (GBAN370S)200 | Drosophila: ↓ER stress (GBAN370S,L444P)201; Mice:↓α-syn (SNCAXSNCAKOtm1Nbm)202 |
| NCGC607 | Salicyclic acid derivative. Investigational compound | Chaperone: aids transport of β-glucocerebrosidase to lysosome - no catalytic inhibition | Dopaminergic neurons from Gaucher’s patients: ↓glycolipids, ↓α-syn (GBAN370S+/+, GBAN370S/c.84dupG)203 | - |
| HEP14 | Investigational compound | Protein Kinase C-mediated TFEB activation and possibly ZKSCAN3 inhibition | - | Mice: ↓Aβ(APP/PS1)151 |
| Facilitators of proteosomal (UPS-mediated) degradation | ||||
| Arimoclomol | Niemann-Pick Type C1 disease. Clinical evaluation for ALS | Heat Shock Factor 1 stabilizer, ↑Hsp70 chaperone production | Motoneurones: ↑survival (staurosporine, H2O2)211 | Mice: ↓SOD1, ↓motor loss, ↑longevity (SOD1G93A)212 |
| IU1/IU1–47 | Investigational compounds | USP14 (deubiquitinase) inhibitors | Cortical Neurones: ↓tau, Ub-proteins (Prostaglandin J2)215; ↑tau degradation and ↑ALN flux216 | - |
| Geldanamycin | Antibiotic. Potential anti-tumorigenic | Hsp90 inhibitor ↑Hsp70 chaperone activity | M17: ↓tau (tau transfected)219; H4: ↓α-syn (α-syn-YFP complementation)220 | Drosophila: ↓ α-syn (α-synA306/504) 202 Drosophila: ↓insoluble (HttQ93)222; Mice: ↓tau (JNPL3)219 |
| 17-AAG | Investigational compound. Potential anti-tumorigenic | Hsp90 inhibitor (improved brain entry), ↑Hsp70 chaperone activity | H4: ↓α-syn oligomers (α-syn-YFP complementation)220 | Mice: ↓ Aβ and ↓synaptic toxicity/memory impairment (Tg2576)223,224, ↓tau (JNP3L)224 |
| HSP990 | Investigational compound | Hsp90 inhibitor, HSF1 promoter, ↑Hsp70 chaperone activity | - | Mice: ↓Htt aggregates, ↑motor performance (R6/2)225 |
| Rolipram | Investigational compound. Potential use in auto-immune disorders | Phosphodiesterase inhibitor, ↑Protein Kinase A-mediated proteasome phosphorylation | Cortical Neurones: ↓Aβ/α-syn synaptic damage (human brain extract)228 | Mice: ↓tau, ↓ubiquitin, ↑improved cognition (rTg4510, JNPL3)229 |
| PD169316 | Investigational compound | p38 MAPK inhibitor, ↓p38 MAPK proteasome phosphorylation | ↓α-syn (wild-type protein)233 | - |
↓Indicates reduced, and ↑ increased levels. Cell line/species is followed by drug action in procedure/model. SK-N-SH, its sub-line SH-SY5Y and M17 are human neuroblastoma cell lines, H4 is a human neuroglioma cell line, and RPE denotes human retinal pigmented cells. Pheochromocytoma-12 (PC12) and neuro 2a (N2a) are mouse neuroblastoma cell lines, while HT-22 is a mouse hippocampal cell line. Cells were transfected with mutant protein, treated with Aβ peptides, or exposed to cytotoxic stressors like serum deprivation, okadaic acid (phosphatase inhibitor), rotenone (mitochondrial complex I inhibitor), staurosporine (protein kinase A/C inhibitor), hydrogen peroxide (H2O2), lipopolysaccharide (pro-inflammatory) or prostaglandin J2 (neurotoxic). Mutant protein variants in superscripts: e.g., SynA53T. YFP signifies yellow-fluorescent protein tagged (fluoresce when oligomerised). For in vivo models, Table shows overexpression of mutant neurotoxic proteins, in some cases tagged with Green Fluorescent Protein (GFP) for visualization. Models employing transgenes and/or mutations (superscript) listed as, e.g., R6/2-Htt150. Transgenic models for polyglutamine disorders express pro-aggregant proteins bearing multiple CAG repeats. For example, the R6/2 HD mouse expresses exon 1 of the human HTT gene containing 144–150 CAG repeats. In a model of Joseph-Machado disease, mice overexpressed Ataxin 3(Q70)) with 70 CAG repeats. TDP43 and FUS (Fused in Sarcoma) refer to mice overexpressing these proteins as models for FTD and/or ALS. FLTD-U mice show Ubiquitin-inclusions upon TDP43 overexpression. The SOD1 mutant mouse, G93A, is a model of ALS. Tau (MAP gene)-based models related to FTD (and AD) include mice with P301L (JNPL3 line) or P301S (PS19 line) mutations. RTg4510 mice have regulatable tau (P301L) expression. HTau signifies overexpression of human, wild-type tau. Mouse models for AD are based on overexpression of Tau and/or APP (Swedish and Swedish/Indiana) mutations: Tg2576 mice overexpress mutant APP (isoform 695) with the Swedish mutation (KM670/671NL); J20, TgCRND8 and Tg19959 mice overexpress mutant APP with Swedish plus Indiana (V717F) mutations; APP/PS1 mice bear APP-Swedish plus PS1-L166P mutations; 3XTgAD mice contain 3 mutations (APP-Swedish, PS1-M146L and tau-P301L) and 5XFAD mice encode 3 APP mutations (Swedish, Florida and London) plus 2 PS1 mutations (M146L and L286V). Models for PD are overexpression of wild-type or mutant (A53T, A30P) human α-synuclein, in one case on a α-syn knockout background (SNCAKOtm1Nbm). R275W is a mitophagy-linked Parkin (PARK2) mutant mouse. GBA (β-glucocerebrosidase) mice embrace lines with natural (N370S and L444P) and induced mutations (D409V). Lesion-based models of PD employed the dopaminergic neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), okadaic acid or H2O2. Abbreviations not above or in text: CaMKK2, Calmodulin Kinase Kinase 2; DA, dopaminergic; HAT, Histone acetyl transferase; MAP Kinase, Mitogen Activated Protein Kinase; MCI, Mild cognitive Impairment; PE, phosphotidylethanolamine; PrP, prion protein; PS, presenilin; and PtdIns, phosphatidyl-inositol-3-kinase.
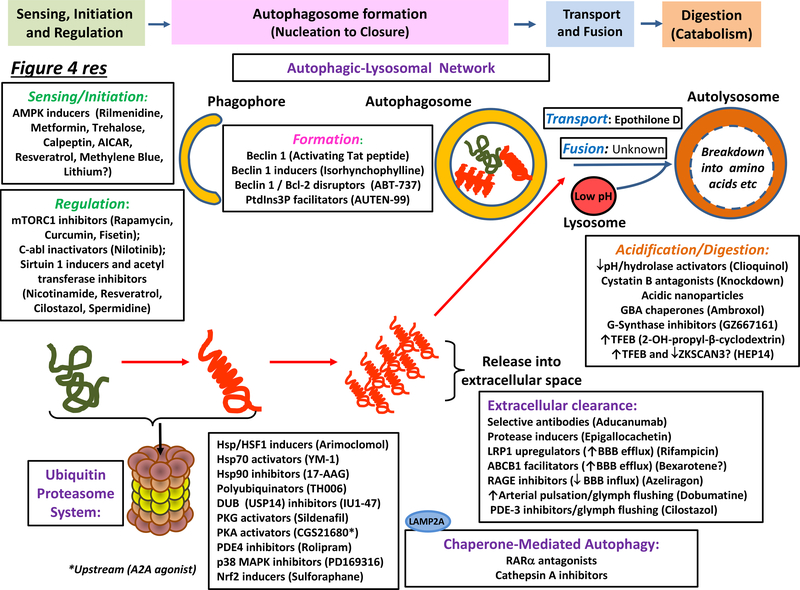
Figure 4 |. Major molecular sites of action of agents that enhance neurotoxic protein clearance in neurodegenerative disorders of aging.
Representative agents are shown for diverse modes of intracellular (the autophagic–lysosomal network (ALN) and the ubiquitin–proteasome system (UPS), extracellular (immunotherapy and protease-driven) and vascular (blood–brain barrier (BBB) extrusion and glymphatic) clearance. The principal loci of drug actions are depicted, yet precise mechanisms of action remain to be more fully deciphered for many drugs while several agents like resveratrol act at multiple sites (main text). As illustrated, a broad range of drugs exert their actions via AMPK, mTORC1 or sirtuin 1 (which also influences downstream events such as autophagosome formation). Some agents exert their effects via other components of the ALN, up to and including lysosomal catabolism. In addition, ambroxol acts as a chaperone to help transport β−glucocerebrosidase to lysosomes. Diverse class of agent likewise promote UPS activity, including chaperones that assist in protein refolding and triage, modulators of proteasomal phosphorylation, and agents acting via the transcription factor, Nrf2, to induce coordinated synthesis of proteasomal subunits. Extraneuronal clearance of full-length, truncated, post-translationally-modified, monomeric and/or higher-order neurotoxic proteins can be promoted by: stimulating proteases like neprilysin; immunotherapies targeting specific neurotoxic proteins; and increasing BBB-mediated and glymphatic extrusion into the circulation. For details, see main text. Abbreviations not in main text or Figure 3: AT, acetyl transferase; DUB, deubiquitinase; GBA; β−glucocerebrosidase; G-synthase, glucoceramide synthase; PDE, phosphodiesterase; PKA/G, protein kinases A/G and RAR, retinoid acid receptor.
The following paragraphs mainly relate to classical small-molecules drugs: innovative treatment modalities for reinforcing clearance are outlined in Box 3.
Box 3 |. Novel modalities for enhancing neurotoxic protein clearance.
Classical small-molecule agents (such as those that are compatible with Lipinski’s rule of five for orally available drugs ) may not be suitable for some targets such as protein–protein interfaces and lipids. They are also not ideal for discrete delivery to specific brain regions. Here, we overview a suite of novel modalities for eliminating neurotoxic proteins in NDAs.
Protein–protein interactions such as Beclin–BCL-2 can be disrupted by using a peptide that binds to one protein partner. The peptide itself is linked to a short, basic, arginine-rich sequence (derived from the HIV Tat protein) to improve cell penetrance A Tat–Beclin 1 construct triggered autophagy and cleared polyglutamine expansion protein aggregates in vitro174, while also promoting long-term memory in rats336.
Aptamers are small oligonucleotides that recognise specific proteins. They offer another chemically distinctive strategy for modulating clearance. Using this technology, the de-ubiquitinase, USP1449,217 could be inhibited to facilitate tau clearance214. Inhibiting ubiquitin carboxyl-terminal hydrolase 37, another proteosome-linked de-ubiquitinase, may also facilitate proteasomal clearance of neurotoxic proteins337. Similarly, aptamers moderated the ALN burden by blocking the misfolding and oligomerisation of tau338 and α-synuclein339.
Numerous classes of miRNA are deregulated in NDAs165, including an increase of miR-34a in AD, which neutralizes mRNAs encoding sirtuin 1 and TREM2165. Conversely, miR-132, which likewise interacts with sirtuin 1, is down-regulated in AD165. Another example is the loss of miR-124 in a lesion model of PD340. Selective targeting of miRNAs in NDAs is becoming possible using modified oligonucleotides such as antagomiRs, locked nucleic acids and miRNA sponges165. In addition, stabilized antisense oligonucleotides are showing promise not only for silencing miRNAs like miR-34, but also for knocking out or altering the aberrant splicing of specific neurotoxic/aggregating protein such as tau, mutant Htt, CRorf72 and SOD1341.
PROTACs permit selective proteosomal elimination of unwanted proteins. They are composed of two motifs joined by a linker: one recognises a specific protein such as tau236, whereas the other encodes an E3-ligase binding site234. This allows the target protein to be poly-ubiquitinated, captured and degraded by proteasomes (and the ALN): addition of TAT-like motifs can increase efficacy234. In the 3XTgAD mouse model, PROTACs moderated levels of tau in the cortex and hippocampus, suggesting target engagement in key pathological regions234. Interestingly, PROTACs may also be useful for orienting proteins towards CMA since the E3-ligase binding site can be substituted by a “KFERQ” CMA-recognition motif. This approach was used to clear α-synuclein in vitro233. Smaller PROTAC variants offer improved stability, higher potency and better structure–activity relationships342.
Restoring lysosomal acidification using poly(DL-lactide-co-glycolide) acidic nanoparticles proved neuroprotective in preclinical models of PD343. Although they are poorly brain-penetrant, nanoparticles with improved pharmacokinetic profiles are being developed. Encouragingly, intranasal delivery reduced 6-hydroxydopamine-induced neurotoxicity in rats344. Another dimension of nanotechnology is represented by engineered nanorods which, when internalized by Hela cells, accelerated the ALN and cleared Htt aggregates in synergy with trehalose via a mTORC1/ERK-signalling pathway: in vivo actions and safety remain to be established345.
One strategy for locally enhancing intracellular clearance is virally-produced gene delivery to the pathological site, avoiding autophagic induction in ‘healthy’ areas346. A target protein might be expressed in restricted areas using neuronal-type-specific promoters, like the dopamine transporter in dopaminergic neurons347. Invasiveness of delivery is a drawback, but peripheral administration employing exosomes together with the use of focused ultrasound to favour local BBB passage may offer a solution348. The latter approach enhanced access of siRNA to the striatum for knocking down mutant Htt300. Further, localised clearance was achieved with striatal lentivirus transfer of the proteasome activator PA28γ that binds the 20S subunit to form an immunoproteasome. It enhanced clearance and improved motor performance in an Htt mouse model349. Another example is provided by intranigral gene delivery of Beclin 1 or TFEB that stimulated the ALN and alleviated pathology in α-synuclein overexpressing mice350.
Finally, recurrent exposure of mice to a non-invasive, 40Hz flicker regime that entrained GABA interneuron-driven oscillations in visual cortex reduced Aβ40/42 load: this resulted from a suppression of amyloidogenesis and a shift in microglial activation status, leading to enhanced uptake and clearance351.
Modulators of sensing, initiation and regulation
Direct and indirect activators of AMPK-induced autophagy.
Ligands inhibiting GPCRs coupled to the AC–cAMP–PKA axis are potential activators of AMPK27,29. Indeed, clonidine and rilmenidine, two Gi/o coupled α2-adrenoceptor and imidazoline1 receptor agonists, stimulate autophagy and clear Htt in cellular103 and animal models of HD104. Although their precise mechanisms of action await further elucidation21,103,104, there may be a role for calpains 1 and 2. These Ca2+-activated cysteine proteases are elevated in ageing and proteolytically generate various neurotoxic peptides54,81. They stimulate the AC–cAMP–PKA axis to inhibit AMPK by activation of GSα103. Genetic knockdown of calpain1 or 2 or overexpression of its endogenous inhibitor, calpastatin, increased autophagy and cleared aggregates in SK-N-SH cells overexpressing a mutant form of Htt103. Efficacy was also seen in mutant Drosophila and mouse models of HD54. Calpeptin, a cell-permeable calpain inhibitor, can also reduce Htt proteinopathy via induction of autophagy103,105. Calpain inhibition by calpastatin or pharmacological agents also confers neuroprotective effects in other NDAs models, including enhanced clearance of tau, α-synuclein and SOD154,106,107.
The aminoimidazole derivative, AICAR, undergoes intracellular transformation to an AMP analog that triggers AMPK-mediated autophagy21,108. It conferred neuroprotection upon exposure of astrocytes to Aβ or oxidative stress109 and countered α-synuclein toxicity in cultured rat neurons110. Another direct facilitator of AMPK, A769662, elicited autophagy and reduced the burden of Htt in a striatal cell line derived from knock-in mice expressing a humanized form of mutant Htt (exon 1 containing 7 polyglutamine repeats111). Selenium deficits have been linked to AD, so it is interesting that selenomethionine boosted ALN flux from AMPK recruitment through autophagosome formation to lysosomal degradation in the 3xTgAD mouse model112.
The ‘anti-ageing’ agent resveratrol is thought to indirectly recruit AMPK via activation of calmodulin-kinase-kinase-β which, acting in synergy with Ca2, exerts its effects via Thr172 phosphorylation113. This action, amongst others (below), is involved in its reduction of Aβ levels in N2a cells and neurons114 and the elimination of Aβ and Htt in animal models of AD and HD114,115.
The anti-diabetic drug metformin, a prototypical activator of AMPK, induced autophagy and increased longevity in mice116. Like AICAR, metformin abrogated α-synuclein toxicity in primary cultures of cortical neurons, although the precise contribution of autophagy requires clarification110. Moreover, reductions in levels of hyperphosphorylated tau and Aβ were seen in metformin-treated neurons117,118, while it blunted neuronal loss in a neurochemical-lesion model of PD in mice119.
The di-glucose derivative trehalose inhibits the SLC2A family of glucose transporters to promote AMPK-induced autophagy and reduce neurotoxic protein load, although it also exerts other actions downstream in the ALN4,120. Trehalose promoted autophagy and reduced disease progression in a SOD1 mouse model of ALS120. It also proved effective in cellular models of PD, HD and AD,121,122 as well as in mouse models of HD, AD and tauopathies, where it cleared aggregates, reduced neurodegeneration and ameliorated motor and cognitive performance123–125.
Lithium ions inhibit inositol monophosphatase to deplete inositol phosphate-3. This mechanism may be involved in its promotion of autophagy and reduction in cellular levels of α-synuclein, SOD1, Htt and tau126, amelioration of motor function in a P301L mouse model of tauopathy127, and slowing of disease progression in SOD1 mice128. However, its precise mechanism of action awaits further elucidation126.
Other compounds that act through AMPK activation include the anti-aggregant, methylene blue (Suppl Box 1), which elevated levels of Beclin 1, p62 and LC3, induced autophagy and suppressed tau in organotypic neuronal cultures and a mouse model of FTD101,102. In addition, calcitriol (the active metabolite of vitamin D3) elicited AMPK-dependent autophagy in a neurochemical lesion-induced model of PD129.
Modulators of mTORC1 and its transcriptional control of the ALN.
One major strategy for promoting autophagy is relief of repression by mTORC1. This kinase is classically inactivated by rapamycin that binds to the modulatory protein FKBP12 (12-kDa FK506-binding protein). Enhancing autophagy with rapamycin reduced levels of α-syn, Fused-in-Sarcoma and Htt130–132. It also diminished polyglutamine aggregates and countered motor impairment in a Drosophila model of HD133. In addition, rapamycin abrogated pathology in murine models of AD and FTD, as well as countering neuronal loss in MPTP-treated mice134–136. Likewise, temsirolimus reduced the accumulation of phosphorylated tau in SH-SY5Y cells and P301S tauopathy mice137. It also removed cellular aggregates of mutant Htt and improved motor performance in a mouse model of HD, reduced α-synuclein aggregation and afforded neuroprotection in a lesion-based model of PD, and depleted mutant ataxin 3 in a mouse model of supraspinal cerebellar ataxia 3133,138,139. Interestingly, several ‘small-molecule enhancers of rapamycin’ promoted autophagy and eliminated Htt in cellular and Drosophila models, but the precise role of mTORC1 in their actions remains to be clarified140.
The natural product curcumin induced macroautophagy and neuroprotected rotenone-treated dopaminergic neurons141 as well as accelerating elimination of mutant A53T-α-synuclein by repression of mTORC1 in a cellular model of early-onset PD, although it also exerts other actions such as modulation of protein acetylation and aggregation142,143. Pro-autophagic effects of curcumin are reflected in improved function, as well as reduced levels of α-synuclein aggregates144 and Aβ/tau oligomers in cellular and animal models of PD and AD145,146.
Inasmuch as phosphorylation by mTORC1 blocks translocation of TFEB from lysosomes to nuclei, mTORC1 inhibitors should promote the coordinated synthesis of proteins driving the ALN20,22,147. Indeed, TFEB over-expression reduced amyloid plaques in a APP/PS1 mouse model148. Moreover, the flavonol fisetin stimulated autophagic degradation of phosphorylated tau in cortical neurons via mTORC1-dependent activation of TFEB and the cytoprotective transcription factor, nuclear factor erythroid-2-related factor 2 (Nrf2)149. Fisetin also reduced Aβ accumulation in an APP/PS1 mice model of AD150. Thus, mTORC1 — and, possibly, AMPK via poorly characterised cascades21 — represent options for stimulating TFEB. It remains, nonetheless, a challenging target for induction22,151.
C-ABL tyrosine kinase is a proto-oncogene that negatively regulates autophagy, partly acting upstream of the Akt–mTORC1 axis. It is over-activated in AD and tauopathies such as FTD152. Inactivation of c-ABL with brain-penetrant nilotinib conferred neuroprotective autophagy in mouse models of PD153. It also reduced aggregates in cell and mouse models expressing TDP-43 protein154. Nilotinib recently underwent a Phase I safety study for treatment of PD155.
Modulators of sirtuin-1 and inhibitors of acetyl transferases.
Activity of the deacetylase sirtuin 1 declines with age, partially due to limited availability of its co-factor, nicotinamide24,56,156. Therefore, it is interesting that nicotinamide and its analogues promoted autophagic removal of damaged mitochondria in fibroblasts157 and reduced Aβ toxicity in rat cortical neurons158. They also improved mitochondrial energy generation and, partly as a consequence, reduced plaques in Aβ-expressing neuronal cells and AD mice, while improving cognitive function58. Nicotinamide analogues similarly slowed cognitive decline and neuropathology in a 3xTgAD mouse model of AD159.
Resveratrol can stimulate sirtuin 1 via AMPK (see above), and it also possesses an AMPK-independent mode of sirtuin 1 recruitment that participates in blunting of the neurotoxicity of Aβ25–35 fragments in PC12 cells160. This possibly involves a role for the DNA-repair protein, poly(ADP-ribose)polymerase 1 (PARP1). Its pharmacological inhibition elevates levels of the substrate, nicotinamide, with an enhancement of mitochondrial energy generation contributing to neuroprotective properties in an animal model of AD160,161.
Cilostazol (a phosphodiesterase-3 inhibitor) clears Aβ42 from neuronal cell lines by promoting autophagy, upregulating Beclin 1, Atg5 and LC3, down-regulating mTORC1, and inducing lysosomal cathepsin B: these actions of cilostazol involve activation of sirtuin 1 as well as upstream Tyr-172 phosphorylation of AMPK108,162,163. Cilostazol improved cognition and reduced levels of A42 and hyperphosphorylated tau following intracerebroventricular injection of Aβ(25–35) into mice162,163.
Protein deacetylation, as effected by inducers of sirtuin 1, is of broader relevance to the ALN, as reflected in activation of Atg gene transcription20,24,164. Furthermore, acetyl transferases such as p300 are druggable20,165 and their inhibition (by garnicol) protected against autophagic deficits in a rodent model of PD166. Another p300 inhibitor, spermidine, has attracted attention by virtue of its autophagy-related increase in longevity164,167. Spermidine inhibited the acetylation of Atg proteins 7, 11 and 15 as well as that of histone 3, while inducing Beclin 1 via blockade of its cleavage through caspase 3168. Spermidine also decreased disease progression in a mouse model of FTD169 and reduced α-synuclein toxicity in C. elegans170. Depletion of acetyl coenzyme A would be worth exploring in models of NDAs171. Underpinning interest in inhibitors of acetyl transferase, p300 expression is increased in AD brain and involved in the aberrant acetylation of tau165,167,172,173.
Inducers of autophagosome formation
As outlined in Box 3, the cell-permeable peptide, Tat-Beclin 1 [G], acts at the Beclin 1–Vsp 34 complex to increase autophagy and promote the clearance of Htt aggregates in cell lines174. In addition, the plant-derived alkaloid isorhynchophylline upregulated Beclin 1 independently of mTORC1 and promoted autophagic clearance of α-synuclein, although its precise mechanism of action remains to be clarified175. Beclin 1 bears a BH3 element on its N-terminus that is subject to inhibition by the anti-apoptotic protein, B-cell lymphoma (BCL)-219,165,176. Disruption of this BCL-2–Beclin 1 complex is an alternative approach for promoting autophagy, as achieved in mouse fibroblasts by the BH3 mimetic ABT-737177. A knockin, gain-of-function Beclin 1 mutant with reduced repression by BCL-2 also increased autophagy, promoted Aβ sequestration and improved cognition in a 5XFAD mouse model of AD: this pattern of effects was reproduced with ML246, a novel autophagy potentiator with an uncertain mode of action178. Other potential approaches to Beclin 1 activation include inhibitors of (tau-phosphorylating) cyclin-dependent kinase 5179.
The multi-modal agent resveratrol induced the expression of Atg4 and promoted autophagosome formation. This led to accelerated degradation of polyQ-Htt aggregates and protected SH-SY5Y cells from toxicity180. An unusual approach to augmenting autophagosome formation is represented by brain-penetrant ‘autophagy enhancer-99’ (AUTEN-99), which blocks Jumpy, a phosphatase that inhibits the phosphotidyl-inositol-3-kinase-mediated generation of the autophagosome membrane (Figure 3). Auten-99 augmented autophagic flux in isolated neurons, increased markers of autophagy in mouse brain and slowed neurodegeneration in Drosophila models of PD and HD181.
Promoters of autophagosome transport and lysosomal fusion
Disruption of cytoskeletal networks and loss of microtubule function in NDAs compromises the transport of autophagosomes, late endosomes, amphisomes and retromers to perikaryal lysosomes, and hence impedes degradation of neurotoxic proteins34–36. Accumulation of autophagosomes and lysosomes in axonal swellings is associated with local APP processing into Aβ42, as well as plaque formation16,34. The microtubule stabilizers paclitaxel and epothilone A countered Aβ42-induced cytoskeletal disruption — and moderated excessive UPR — in neurons182. Furthermore, epothilone D countered microtubule disruption and cognitive deficits in aged P301S/P19 AD mice183. However, it is unclear to what extent these agents promote ALN in the perikaryon, and a risk of cytoskeletal over-rigidity should not be neglected. Thus, mechanisms that promote microtubule/actin dynamics and cytoskeletal shuttling of autophagosomes/endosomes to lysosomes present alternative strategies for evaluation184.
Several other, potentially targetable mechanisms might also aid autophagosome delivery to (and fusion with) lysosomes185. These include Rab and Rab-effector proteins which facilitate the assembly of Synataxin17–SNARE complexes critical for fusion186. Interestingly, genetic or pharmacological activation of Rab5 countered neurodegeneration in mouse C9orf72 models of ALS and FTD187. There is also growing interest in the stabilization of retromers for promoting fusion. This appears feasible based on modulation of their role in diverting APP out of endosomes and hence curtailing its cleavage into Aβ4237,188. Finally, inducers of histone deacetylase 6, broadly implicated in cytosolic transport and the fusion of autophagosomes, might be an option3.
Facilitators of lysosomal digestion
Maintaining optimal intraluminal acidity is critical for activating lysosomal hydrolases and digesting cargo. There are several ways that a loss of lysosomal acidity in NDAs might be countered. First, lysosomal acidification could be favoured by stabilised cAMP analogues: in human fibroblasts bearing a presenilin 1 mutation, cAMP acidified lysosomes and augmented the availability of cathepsins189. Second, the TFEB inducer 2-hydroxypropyl-β-cyclodextrin promoted the acidity of lysosomes in neurons190. Third, acidic nanoparticles such as polylactic acid and poly(lactide)co-glycolide increase acidification (Box 3). Fourth, activation of the lysosomal Ca2+ channel transient receptor potential mucolipin-1 with a synthetic agonist (ML-SA1) increased intralysosomal Ca2+ and lowered pH191,192. Other approaches include the enhancement of v-ATPase activity, and countering deficiencies in progranulin activity40,63,86,193–195.
Dysfunction of PARK9 (ATP13a2) leads to an imbalance in the handling of zinc, a disruption of lysosomal activity and accumulation of α-synuclein77. Clioquinol, which acts as a metal chelator, reverses these deficits and may reinforce lysosomal function (and acidification) in NDAs where the regulation of zinc and other metals is abnormal77,196. Indeed, clioquinol countered disruption of autophagy by chloroquine in retinal cells, reduced Aβ42 accumulation in CHO cells expressing APP and mutant presenilin 1, and diminished amyloid-misfolding and aggregation in Tg2576 AD mice196,197. Cystatin B and C are endogenous antagonists of the cysteine-active site on lysosomal cathepsins and their genetic down-regulation ameliorated deficits in lysosomal proteolysis, synaptic plasticity and amyloid clearance in TgCNRD8 AD mice198. Pharmacological blockers of cystatins are currently being sought. In addition, upregulation of retromer complex might stimulate provision of hydrolases to the lysosome37,188.
Lysosomal enzyme replacement is an established treatment for several primary LSDs: for example, β-glucocerebrosidase supplementation for type I (non-neuropathic) Gaucher’s disease (Suppl Box 1)43. Due to BBB impermeability, enzyme supplementation does not appear promising in PD. However, inhibition of substrate (glucosylceramide) synthesis by brain-penetrant GZ/667161 and GZ/SAR402671 reversed synucleinopathy in A53T-SNCA mice199. Another glycosphingolipid synthesis blocker, miglustat,43 showed activity in cellular and in vivo models of PD75, although its ability to downregulate target sphingolipids in the brain is limited.
One might also act upstream to promote lysosomal function by accelerating the import of functional enzymes. β-glucocerebrosidase again provides a good example. Ambroxol acts as a molecular chaperone to promote folding of β-glucocerebrosidase and aid its transit from the ER to lysosomes43. It increased expression of β-glucocerebrosidase, normalised autophagy and accelerated degradation of α-synuclein in a stem-cell model of dopaminergic neurons derived from PD patients bearing mutations for β-glucocerebrosidase200. Ambroxol, which also decreased ER stress in Drosophila201, reduced α-synuclein levels in overexpressing, transgenic mice202. It is being evaluated for use in idiopathic PD (Suppl Table 1). A downside of ambroxol is that it occludes the catalytic site of β-glucocerebrosidase, but novel agents like NCGC607 avoid this untoward effect203. Intriguingly, while enhancement of β-glucocerebrosidase conferred therapeutic benefit in animal models of PD, its inhibition by conduritol-β-epoxide was beneficial in a mouse model ALS, underpinning the apparently distinctive nature of ALS as regards ALN function and energy balance (Suppl Box 3)90.
Finally, a more global approach for harnessing lysosomal activity would be the induction of TFEB20,22. Harnessing TFEB by 2-hydroxypropyl-β-cyclodextrin promoted clearance of proteolipid aggregates and α-synuclein in a cellular model of PD195,204. It also augmented the elimination of Aβ in a Tg19959/CRND8 mouse model of AD173. The protein kinase C activator HEP14 stimulated nuclear translocation of TFEB to boost lysosomal gene transcription and reduced Aβ plaques in APP/PS1 AD mouse brains151. Modulation of DNA methylation and post-translational histone marking offer further opportunities for transcriptional control of lysosomal activity, while miRNAs could intervene at the level of translation (Box 3)20,165.
Clinical studies of agents that modulate the ALN
Some of the above-discussed agents have been clinically evaluated, alone or in association, in NDAs (Suppl Table 1). For example, metformin for cognitive function and energetic status in AD; resveratrol for functional decline and Aβ load in AD; rilmenidine for motor performance in HD; and ambroxol for β-glucocerebrosidase activity and motor function in PD. To date, despite some positive observations, unequivocal proof for symptomatic improvement and/or course-altering effects has not been provided for any drug (Suppl Table 1). Nonetheless, long-term effects remain under study, no medication that specifically and exclusively induces the ALN has as yet been therapeutically characterized, and proof of target engagement in clinical trials remains challenging. Hence, it is premature to draw conclusions as regards therapeutic efficacy.
In fact, the anti-oxidant edavarone, which decreased autophagy in ischaemic brain and macrophages205, was recently authorized for use in a subset of ALS patients (Suppl Box 3)206. This appears paradoxical, but fits with the suggestion that high ALN flux is detrimental under conditions of severe cellular stress in ALS90. Whether decreased ALN flux is genuinely implicated in its clinical actions remains to be confirmed(Suppl Box 3)3,206.
Caloric restriction and exercise mimetics for promoting ALN clearance
Anti-ageing and lifespan-extending benefits of ‘caloric restriction mimetics’ expressed across a range of multicellular organisms are related, at least in part, to the induction of AMPK and sirtuin 1, leading to promotion of autophagy21,24,164,207. These mimetics are generally safe yet encompass drugs that reduce ATP availability by interfering with cerebral/neuronal glucose uptake. This may pose problems because compromised neuronal energy is itself a risk factor for NDAs like AD and PD25,164. Nonetheless, efforts to find well-tolerated, autophagy-inducing mimetics are continuing164 and clinical trials should prove instructive25,164. Furthermore, there is increasing interest in pharmacological exercise mimics that exert putative neuroprotective properties via the modulation of AMPK, mTORC1, beclin 1 and other regulators of the ALN21,207.
Enhancing clearance by the UPS and CMA
Opportunities for pharmacological manipulation of the UPS and CMA in NDAs are less well-established than those for the ALN, but there are encouraging routes of progress2,45–47,55,56,68. Furthermore, the UPS inhibitors bortezmib, carfilzomib and ixazomib are approved for the treatment of multiple myeloma, indicating that clinical application of UPS modulators is possible3.
Facilitation of chaperones acting on client proteins
One approach for reinforcing the UPS focuses on agents that target chaperones involved in the handling and recognition of neurotoxic proteins2,68,208. Of particular interest is Hsp70 which interacts with the E3 ubiquitin ligase CHIP to aid ubiquitination of proteins destined for proteasomal destruction208. Hsp70 binds to heat shock factor 1 [G] (HSF1) and, under conditions of neurotoxic protein stress, their dissociation leads to mutual activation, with HSF1 driving transcriptional generation of Hsp70 and other chaperones that facilitate proteostasis208,209. Hsp70 also exerts a more general role in the refolding and disassociation of aggregated proteins2,3.
One promising agent is the hydroxylamine derivative arimoclomol, which increases the activity of Hsp70 by augmenting transcriptional activity of HSF1210. Arimoclomol rescued cultured motoneurons from oxidative stress and from the pro-apoptotic actions of staurosporine211. It also mediated the removal of mutant SOD1 aggregates and improved motor function in a mouse model of ALS212. Supporting interest in arimocomol, it mimicked recombinant Hsp70 in reversing lysosomal pathology in fibroblasts from patients with LSDs (Suppl Box 3). In an alternative approach, the rhodocyanine derivative YM-1 allosterically promoted the activity of Hsp70 to enhance degradation of polyglutamine (polyQ) proteins: these findings suggest potential utility in HD213. Furthermore, Hsp70 has been co-administered with inhibitors (IU1 and its more potent derivative, IU1–47) of the deubiquitinating enzyme USP14 to enhance proteasomal degradation of tau214–216. USP14 inhibitors act by preventing deubiquitination rescue of tau and other UPS substrates such as TDP43 and Ataxin-3. They may also effect allosteric changes in proteasomal subunits217. Interestingly, USP14 inhibitors promote the ubiquitination activation of Beclin 1 to recruit the ALN216
Hsp90 counters the effects of Hsp70 by forming a complex with it to impede substrate ubiquitination: it likewise exerts a suppressive influence on HSF1210,218. Amongst compounds that inhibit Hsp90, geldanamycin promoted elimination of both hyperphosphorylated tau and oligomeric α-synuclein in cell lines219,220. Moreover, geldanamycin reduced Lewy-like bodies221 and Htt aggregates in Drosophila neurites222 and reduced tau in AD mice219. The less cytotoxic analogue of geldanamycin, 17-AAG, has improved brain penetrance. It decreased Aβ levels,223 improved memory224 and lowered tau in transgenic AD mice224. 17-AAG also reduced α- synuclein oligomers in H4 cells220. Another Hsp90 inhibitor, HSP990, has shown promise in lowering Htt aggregates and improving motor performance in two mouse models of HD225
Modulation of the phosphorylation status of the proteasome
Numerous classes of kinase phosphorylate the proteasome68,226,227. Phosphodiesterase inhibitors protect cAMP from degradation to recruit protein kinase A and boost UPS activity. Accordingly, rolipram protected rat cortical neurons from Aβ-induced synaptic disruption228. Furthermore, in a transgenic tau mouse model of FTD in which 26S proteasomal activity was impaired, rolipram attenuated markers of tauopathy, improved memory and protected synaptic integrity by strengthening protein kinase A-mediated phosphorylation of the Rpn6 component of the 26S proteasomal subunit229,230. Rpn6 activation may also be involved in the anti-ageing effects of caloric restriction56,164. Interestingly, resveratrol inhibits phosphodiesterase 4, suggesting that proteasomal recruitment may be yet another component of its global impact on neurotoxic protein clearance113. One concern with phosphodiesterase inhibitors/protein kinase A inducers is their huge range of targets (including AMPK), but it may be possible to target proteasome-specific isoforms. Furthermore, acting upstream of cAMP is an alternative strategy. Chronic administration of CGS21680, a selective agonist of AC-coupled adenosine 2A receptors, restored proteasomal activity in cellular and murine models for HD via protein kinase A-mediated Ser-120 phosphorylation of the Rtp6 component of the 19S subunit231.
Another kinase that activates the proteasome (Rpt6 subunit) — and directs it to dendritic spines — is calmodulin-dependent kinase II227. Its recruitment may account for proteasomal activation by the GABAA receptor antagonist, bicuculline52,232. Protein kinase G similarly activates the proteasome, and inhibition of cGMP breakdown by sildenafil reduced neurotoxic protein aggregation in cardiomyocytes, encouraging studies in NDAs68,226,227. P38 mitogen-activated protein kinase indirectly influences the phosphorylation status of the proteasome, probably via cAMP signalling3,68,226,227. P38 depletion, or its blockade by PD169316, accelerated the degradation of ubiquinated proteins, promoted α-synuclein clearance and improved cell survival233.
Phosphorylation is a dynamic process, and small-molecule inhibitors of the nuclear proteasome phosphatase UBLCP1 suggest that calcineurin and other phosphatases represent hitherto-unexploited targets for enhancing UPS-driven clearance of neurotoxic proteins227.
Selective elimination of specific classes of neurotoxic protein
An important question is whether the UPS can specifically clear neurotoxic proteins while safeguarding those that function normally. Several strategies are under exploration. The first is targeted protein degradation with small molecules, which can be achieved by various compounds — including proteolysis targeting chimeras (PROTACS) and phthalimides that bind to E3 ubiquitin ligases and the protein of interest, thereby promoting UPS-driven degradation234,235,236 (These strategies are conceptually analogous, as described in this review http://www.nature.com/articles/nrd.2016.211) (Box 3). Certain agents amplify PROTAC-mediated breakdown of α-synuclein233, while other classes of bifunctional ligand bind a target protein plus Hsp70 to direct UPS degradation235. Alternatively, target proteins can be bound by agents bearing bulky, hydrophobic adamantyl tags that provoke conformational instability and encourage proteasomal elimination234. Second, the cytosolic antibody receptor tripartite motif protein 21 binds to protein-coupled antibodies, then recruits the UPS for substrate degradation. This has been demonstrated for tau and could be adapted for degradation of other classes of neurotoxic protein237. Third, cellular inhibitor of apoptosis protein specifically binds mutant SOD1 and drives it to proteasomal degradation. This provides another potential path to discrete elimination of unwanted proteins in NDAs238.
Control of transcription factors generating UPS components
The transcription factors Nrf1 and Nrf2 are both substrates of proteasomal degradation, as well as inducers of proteasomal synthesis, and the latter has been specifically linked to NDAs239,240. Furthermore, Nrf2 is a master regulator of the anti-oxidant response and drives synthesis of lysosomal and anti-inflammatory proteins in addition to 26S proteasome components149. Translocation of Nrf2 to the nucleus is promoted by triterpenoid derivatives that counter the ageing-related diminution of UPS activity241. In addition, sulforaphane elevates proteasome levels in vivo by inducing Nrf2, protects neurons against oxidative stress, and has been proposed for the treatment of HD242. Several other agents promote the proteolytic competence of proteasomes and facilitate clearance of Aβ and/or tau in cellular models, including betulinic acid. Enhanced transcription has been implicated in their actions, but this remains to be clarified242. Finally, mirroring its inhibitory influence on the ALN, mTORC1 suppresses the UPS by impeding the formation and assembly of proteasomal subunits. Correspondingly, pharmacological blockade of mTOR may promote UPS degradation as well as ALN elimination of neurotoxic proteins243.
Enhancement of CMA-mediated clearance
Some mechanisms outlined above for the UPS, such as increasing chaperone-driven delivery of client proteins to degradative machinery, are also relevant to the CMA47,48,95. In fact, specific induction of CMA has received little attention, possibly since the rate-limiting element LAMP2A has, to date, proven intractable for small-molecule chemistry. Nonetheless, over-expression of LAMP2A accelerated CMA clearance of α-synuclein and afforded protection of dopaminergic neurons45, and several routes to potential pharmacological exploitation may be mentioned. First, cathepsin A cleaves LAMP2A, resulting in its lysosomal degradation, so selective inhibitors of cathepsin A should reinforce CMA39,47,48. Second, LAMP2A is stored in cholesterol-rich membrane regions: hence, cholesterol depletion might enhance transfer to regions where it is functionally active46. Third, the dynamics of the LAMPA2A-client protein translocation complex are (oppositely) controlled by mTORC2 and the phosphatase PHLPP1, offering potential targets for augmenting CMA244. Fourth, CMA is under the negative control of retinoic acid receptor-α and their blockade by synthetic, all-trans retinoic acid derivatives resulted in upregulation of CMA, including the activity of LAMP2A245. Mouse fibroblasts treated with these agents showed improved resistance to combined over-expression of α-synuclein and oxidative stress245.
Importance of early intervention
There are, then, emerging opportunities for intensifying the elimination of neurotoxic proteins by the UPS and CMA47,68,227. However, it is important that they are homeostatically regulated since — mirroring the ALN — excess activity is potentially dangerous241. As the UPS and CMA are disrupted by neurotoxic proteins like Aβ42 and tau, their early and preventative reinforcement may be critical. UPS potentiation might be particularly efficacious when enacted in dendritic sites of neurotoxic protein spreading to counteract NDA-related deficits in synaptic plasticity and learning1,3,5,8,47,52,68,227.
Interplay between the ALN, CMA and the UPS: therapeutic relevance
As pointed out above, there is evidence of coordinated regulation of the ALN and UPS via mTORC11,3,5,243. Furthermore, studies of a mutant tau allele that increases the risk for FTD and AD showed that upregulating the ALN compensated for the impairment of proteosomal activity246. This finding underscores the reciprocal interplay between these clearance systems3. Indeed, the ALN can ‘sense’ UPS failure and compensates by upregulating its own activity. For example, proteasomal failure exacerbates ER stress and leads via the UPR to the expression of sestrin 2, which recruits AMPK to down-regulate mTORC1 upstream of the ALN; Nrf2 is also upregulated3. Supporting the relevance of sestrin 2, it protects dopaminergic neurons from the neurotoxin, rotenone, via AMPK-transduced autophagy247. Sestrin 2 overexpression also prompted mTORC1-dependent autophagy in cortical neurons in a presenilin-knockout model of AD248. Proteasomal degradation of Ulk1, LC3 and other ALN regulatory proteins may prevent ALN over-activity, an observation of particular relevance to ALS (Suppl Box 3)3. By analogy, subunits of the catalytic core of the proteasome are regulated by CMA-mediated degradation47,55.
Impaired extracellular protein clearance
Exosomal liberation of neurotoxic proteins from neurons
When intracellular pathways of protection against neurotoxic proteins prove insufficient, neurons may alleviate the burden of harmful proteins by discharging them into the extracellular space. This may be a self-preservation mechanism and an attempt to acquire glial support for elimination. However, the ‘release’ of neurotoxic proteins contributes to trans-cerebral spread of pathology. That is, abnormal conformers of proteins originating in donor cells enter recipient cells to promote protein misfolding and disrupt clearance, diffusing in a domino, snowball-like fashion across the brain81,249.
Exosomes [G] are involved in the release of tau, APP/Aβ−42 and α-synuclein. Accordingly, they are linked to the progression of NDAs 55,77,81,250,251. Intriguingly, when the ALN is overwhelmed and cargo accumulates, a process of ‘autophagic’ exocytosis participates in the neuronal liberation of neurotoxic proteins. This discharge of neurotoxic proteins adds to the extracellular burden from dying cells, accelerates spreading, and underpins the imortance of clearance mechanisms extrinsic to neurons250,252. In this light, capture and digestion of extracellular proteins by glial cells is primordial7,8. However, there exist several other, therapeutically pertinent mechanisms for ridding the brain of extracellular pools of neurotoxic proteins.
Clearance of neurotoxic proteins by proteases in the extracellular space
Neurons and glia contain diverse classes of protease, and they are localized in all those compartments where neurotoxic proteins accumulate — cytosol, mitochondria and even the nucleus39,253–256. However, certain intracellular proteases in the cytosol generate toxic fragments, notably of tau (calpains and caspases) and Htt (matrix metalloproteinases (MMPs)39,257. Accordingly, their inhibition rather than induction is of interest for the treatment of disorders such as AD and Huntington’s disease. Indeed, the inducible (extracellular) proteases most relevant to promoting neurotoxic protein clearance in NDAs are actively secreted by neurons and glia, located on exosomes and/or expressed on plasma membranes (Figure 1)254. They include several classes of MMP, neprilysin, insulin-degrading enzyme (IDE) and plasmin253,256,258,259.
Aβ42 and amylin (a pancreas-derived, AD-associated protein found in brain) are substrates for degradation by IDE, which also irreversibly ‘traps’ Aβ42 and α−synuclein, preventing their aggregation and promoting ALN and UPS elimination259. Cerebral levels of IDE are reduced in early AD and in mouse models of AD while, mirroring AD amyloidosis, Aβ42 accumulates in mice genetically depleted of IDE. In a vicious circle, Aβ42 itself decreases IDE expression, although it may prompt its release from glia254,259. IDE also degrades and prevents the formation of α-synuclein fibrils259. By analogy to IDE, neprilysin catabolizes Aβ42 and its loss in mouse models of AD and patients alike also contributes to levels Aβ42 accumulation253,256,260.
Another Aβ42-degrading protease, plasmin, is derived from inactive plasminogen by the actions of tissue-type plasminogen activator (urokinase), which is used to treat stroke. It is secreted by neurons (and possibly glia) into the extracellular space. Like IDE and neprilysin, plasmin degrades Aβ42 and blocks Aβ42-induced toxicity, suggesting that the decrease in its levels in AD is involved in the evolution of AD254,256,261. Plasmin also degrades α-synuclein to retard intercellular spreading262.
Interestingly, certain isoforms of MMPs cleave fibrillar as well as monomeric Aβ42254, while extracellular α-synuclein is also a substrate for MMP-3256,258. Another protease with pharmacotherapeutic potential is angiotensin-converting enzyme, which contributes, albeit less prominently, to degradation of neurotoxic proteins in NDAs263. Finally, the extracellular and intracellular serine protease neurosin (kallikrein 6) cleaves α-synuclein. Levels are reduced in Lewy body dementia and, based on lentivirus transduction studies, it is a potential treatment for clearing α-synuclein in PD264.
Clearance of neurotoxic proteins by the blood–brain barrier and the glymphatic system
In AD, HD and other NDAs, disruption of the structure and function of the dynamically regulated BBB is driven, at least in part, by detrimental actions of neurotoxic proteins such as Aβ42. This permits the otherwise-restricted entry of immune cells and toxic substances into the brain. In addition, the active elimination of neurotoxic proteins like Aβ42 and tau (possibly encapsulated in exosomes) from the brain may be compromised (Table 1 and Figure 1)265–273.
Dysregulation of BBB integrity is serious since it normally transfers neurotoxic proteins to the circulation using both generalized and specialized receptors and transporters (Figure 1) 265–267,270–272. In addition, proteins are degraded by vascular smooth muscle and endothelial cells of the BBB itself265,271,272. In ageing, AD and PD, a diminution of BBB-localized P-glycoprotein efflux transporters compromises elimination of neurotoxic proteins267,273. There are also decreases of low-density lipoprotein receptor-related protein1 (LRP1) transporters in AD, whereas receptor for advanced glycolation end-products (RAGE) receptors are induced. These changes would respectively contribute to retention in, and return of, Aβ42 to the brain270–272. An ApoE4 genotype in AD exacerbates poor Aβ42 clearance by reducing its transport to the BBB and diminishing efflux270–272.
Arterial pulsing aids CSF/ISF flow in flushing out interstitial extraneuronal proteins via the complementary glymphatic system (Figure 1)265,269,274,275. Its regulation is not well understood, but roles for aquaporin 4 water channels, other astrocytic mechanisms and noradrenaline have been documented265,276,277. Deletion of aquaporin 4 in astrocytes markedly reduced glymphatic flow and aggravated Aβ42 accumulation in a genetic mouse model of AD276,278, while aquaporin-4 expression is altered in the ageing, AD and PD brain276,277. Loss of sleep has been linked to an impairment of glymphatic clearance and Aβ42 accumulation274. This is significant since “rapid eye-movement sleep-behavior disorder” is the most robust predictor of PD, while insomnia and anomolous sleep patterns occur in other NDAs like early-onset AD, where disrupted sleep is correlated with alterations in Aβ levels279.
Enhancing extracellular clearance
Increasing protease-driven degradation
Overexpression of neprilysin or IDE reduces levels of Aβ42 and amyloid plaque burden in senescence-accelerated mice256. As regards pharmacological manipulation, substances such as epigallocatechin and somatostatin promote the expression, secretion and — allosterically —catalytic activity of IDE and neprilysin in parallel with an increase in the degradation of Aβ peptides259,280. Furthermore, expression of progranulin in the hippocampus of AD mice reduces the density of amyloid plaques by enhancing the activity of neprilysin281. Epigenetic regulation of neprilysin at the level of histones, as exemplified by valproate, offers another potential approach to proteolytic potentiation253. As regards other proteases, augmentation of plasmin clearance by blockade of the plasminogen inhibitor PAI-1 (the expression of which increases with ageing and in murine models of AD) reduced Aβ levels and restored memory deficits in mouse models of AD261,282.
These observations underscore the interest in proteases as targets for degradation of neurotoxic proteins253. Furthermore, several agents mentioned above, such as resveratrol and curcumin, induce IDE and/or neprilysin, suggesting a contribution to their actions253. Nonetheless, structure–activity relationships for small molecules that enhance the catalytic activity (or production) of proteases are not well-characterised253,283. Furthermore, there are issues of substrate specificity. For example, IDE degrades insulin and glucagon as well as Aβ42 and interacts with many other proteins, including the proteasome259. Neprilysin targets a range of substrates such as atrial natriuretic peptides and substance P, and inhibitors are employed in the therapy of heart failure,253 while MMP activators exert deleterious as well as beneficial effects, reflecting their influence on microglia and the BBB258,284. Additional questions centre on whether any protease inducer alone could comprehensively and enduringly clear the burden of neurotoxic proteins in NDAs.
Thus, further work is needed to determine to what extent potentiation of extracellular, glial and endothelial/BBB-localized proteases is a viable strategy for safely enhancing neurotoxic protein clearance in NDAs253,259.
Immunotherapies for neurotoxic protein sequestration
Immunotherapies [G] for neurotoxic protein clearance in NDAs have been pursued for over a decade. As reviewed elsewhere81,285, the most advanced approach is currently antibodies for sequestering extracellular pools of Aβ and tau (AD) or α−synuclein (PD)7,286. BBB antibody penetration is limited, but they may generate a ‘peripheral sink’ in addition to exerting actions centrally. Although Aβ-immunotherapy has not yet yielded an approvable treatment (examples of phase III trial failures include AN1792 (ClinicalTrials.gov registration number NCT00676143) and bapineuzumab (NCT00112073), more refined cohort selection, amyloid imaging for selection of early-disease patients, and the use of monoclonal antibodies derived from human patients such as aducanamab in MCI (NCT01397539, NCT02782975 and NCT02434718) and recruiting for Phase III (NCT02484547 and NCT0247780) offers hope for progress287.
There are at least 5 antibodies under investigation for clearing tau, including a Phase II trial (NCT02880956) for C2N8E12 in AD288. Another trial (NCT02985879) is underway in post-cerebral palsy employing a single-chain antibody. This is the second tau-based Phase II trial after AADvac-1 (NCT02579252) to use an active immunotherapy approach288. Passive tau immunity approaches are also being tested using antibodies specific for [the PHF1 (Ser396/Thr404) epitope (ACI-35; ISRCTN13033912) and Ser409 epitope (RG1600; NCT03289143)81,288. Targeting extracellular tau to block intercellular spreading249 should preclude the need for high antibody inclusion into cells. Antibodies such as PRX002289 have also shown promise for reducing extracellular α-synuclein and propagation of pathology, and Phase I testing has been completed (NCT02157714 and NCT02095171)285.
Potential problems should not be ignored, including the deposition of immune-complexes in vascular tissue, inaccessibility of tau in exosomes, and antibody-driven import of Aβ into the brain. Nonetheless, employing more effective antibodies and appropriate biomarkers, there are still reasonable prospects for achieving course-alteration with immunotherapy.
Improving BBB-mediated and glymphatic transfer to the circulation
The BBB is equipped with potentially targetable transporter proteins, channels and receptors (Figure 1)265–267,270–273. Inhibition of the α-secretase ADAM10 was found to drive LRP1-mediated extrusion of Aβ42 into the circulation290. In addition, LRP1 might be indirectly modulated by aquaporin 4 channels276–278 and epigenetically via miRNAs165. Further, a hydroxymethylglutaryl-coenzyme-A inhibitor, fluvastatin, upregulated LRP1 in the BBB to provoke Aβ42 extrusion291. The antibiotic rifampicin likewise promoted Aβ42 clearance by inducing BBB-localised LRP1 and P-glycoproteins273,292. Whether LRP1-driven uptake of Aβ42 by microglia (and hepatocytes) is involved in the favourable effects of LRP1 up-regulation remains to be clarified271. Interestingly, both fuvastatin and rifampicin have additional actions — including a probable induction of the ALN — that contribute to beneficial actions in models of AD291,293. As for RAGE receptors, their blockade should temper re-entry of Aβ into the brain, and exert anti-inflammatory properties294,295. However, despite promising improvement in cognition in a Phase II trial296, a Phase III study with azeliragon (TTP488) in AD recently failed (NCT02080364; 02916056) (http://ir.vtvtherapeutics.com/phoenix.zhtml?c=254081&p=irol-newsArticle&ID=2341681). Interestingly, resveratrol downregulated RAGE as well as MMP-9 — actions related to decreased hippocampal load of Aβ42297. Finally, at least in murine models of AD, agonists of retinoid-X receptors induce the BBB-localized P-glycoprotein ABCB1 transporter, and this may account for bexarotene-mediated Aβ clearance from the brains of AD mice298. Data with bexarotene remain controversial, but the principle of acting via BBB-localised transporters to encourage neurotoxic protein extrusion is clearly valid.
Focused ultrasound therapy has mainly been used to enhance the entry of proteins and vectors into the brain. For example, siRNA probes for knocking down Htt or, in principle, genes encoding clearance-promoting mechanisms299,300. However, it acts bi-directionally, so CNS-to-periphery transfer of neurotoxic proteins might likewise be accelerated. By targeting selective brain areas such as the hippocampus/entorhinal cortex in AD, neurotoxic proteins could be driven into the periphery. Safety is obviously an issue, but it is reassuring that gap junctions close within 6 hours or less301.
Activation of aquaporin 4 channels on perivascular astrocytes to aid the glymphatic elimination of cerebral Aβ and other toxic proteins is a potential strategy for stimulating clearance. Both antagonists as well as positive modulators have been identified, so this seems “chemically” feasible269,272,275,278. A contrasting approach is represented by dobutamine, which stimulates arterial pulsation and the perivascular/glymphatic CSF flushing of neurotoxic proteins from the ISF via lymphatic conduits into the blood269,275. Deposition of Aβ42 in cerebral vessels impairs vascular function-flexibility and is accompanied by an upregulation of phosphodiesterase 3 in smooth muscle cells302. Cilostazol, a phosphodiesterase 3 inhibitor clinically approved for peripheral vascular disease (and an UPS activator), restored vascular reactivity, increased perivascular drainage of Aβ and promoted cognitive performance in a mouse model of cerebral β-amyloidogenesis302. Intriguingly, a retrospective clinical analysis suggested that cilostazol (added onto donepezil abrogates cognitive decline in patients with modest dementia303. Adrenergic mechanisms influence ISF volume and hence neurotoxic protein clearance274, and additional pharmacological opportunities for promoting glymphatic efflux will probably emerge from an improved understanding of its regulation by astrocytic, neurotransmitter and other mechanisms269,272,274.
Disruption of sleep impedes glymphatic clearance of neurotoxic proteins, so encouraging sleep hygiene should promote CSF/ISF transfer to the periphery274,275. The atypical antidepressant and sleep-promoting agent trazodone is of interest since it normalized an over-protracted UPR and accordingly reversed pathology in animal models of tauopathies (Suppl Box 2)99. Other therapies that favour sleep in NDAs may improve glymphatic clearance of proteotoxic substrates and hence abate disease progression265,269,279. Interestingly, alcohol displays a J-shaped curve, with low/high consumption respectively enhancing/reducing glymphatic function, and moderating/aggravating the risk of dementia304.
Finally, in a recent study in human subjects, peritoneal dialysis cleared peripheral Aβ from the circulation, while parallel experiments in APP/PS1 mice showed that peritoneal dialysis reduced ISF and brain Aβ load and ameliorated behavioural deficits305.
Therapeutic perspectives and open questions
Accumulation of neurotoxic proteins unquestionably contributes to the onset and progression of NDAs. Accordingly, agents that promote their elimination are attractive as potential therapeutic agents. Nonetheless, several issues remain to be resolved prior to successful and safe clinical exploitation.
First, improved knowledge of the causes, characteristics and chronology of poor clearance in NDAs, and of similarities and differences amongst them, would be important for clarifying which therapeutic strategy is best adapted to the treatment of specific classes of NDA and subsets of patients. This would also help determine the optimal mode, timing, pattern and dosage of treatment4.
Second, it is important to better understand the interplay between neurotoxic protein clearance and other pathophysiological processes, such as neuroinflammation. Moreover, hub proteins such as AMPK, mTORC1 and sirtuin 1 affect both the ALN and manifold other processes implicated in NDAs, such as epigenetic regulation and energy homeostasis21,24,25,306,307. Hence, drugs that modulate their activity may have beneficial and/or deleterious actions beyond their influence on clearance. Indeed, potential side-effects should not be ignored. This is exemplified by mTORC1 antagonists such as rapamycin, which possess immune-suppressive actions and affect memory formation, although studies in oncology and neurodevelopmental disorders are reassuring5,307.
Third, numerous mechanisms remain to be pharmacologically harnessed. These include receptor tyrosine kinases for the ALN and “upstream” GPCRs potentially for all modes of elimination26,27,29. For the ALN, additional targets include the Vps34 complex, histone deacetylase 63, Rab proteins implicated in autophagosome–lysosome fusion186 and v-ATPase, crucial for lysosomal acidification40. There has been much recent progress towards manipulation of the UPS, whereas exploitation of the CMA remains a major challenge2,3,45–47,68,80. For certain targets, novel platforms such as PROTACS, aptamers and RNA probes, as well as nanoparticles and nucleic acid-based therapeutics, may prove useful (Box 3). Novel technologies will also be of importance for achieving the specific clearance of neurotoxic versus “normal” proteins, and for directing actions to discrete cells and brain regions, such as dopaminergic pathways in PD8,45. Further research is needed to confirm, clarify and potentially exploit the role of glymphatic clearance in the elimination of neurotoxic proteins in NDAs308. Another line of research could focus on the blood–CSF-barrier, which has parallels and differences to the BBB, is affected in ageing. It also represents a potential site for acceleration of neurotoxic protein elimination; its contribution to clearance of Aβ42 is diminished in AD269,272,309,310. 309,310,272.
Fourth, to improve the preclinical characterization of candidate medicines, we need more refined cellular and animal models, including induced pluripotent stem cells from patients (Box 1)1,3,4,10,23. This will help to determine precisely which components of the ALN, CMA and UPS are affected by specific classes of medication, and to quantify their influence on overall ALN flux. Improved models should also help determine the influence of therapeutic agents on clearance in discrete classes of neuron in comparison to astrocytes and microglia, which may well require contrasting modes of manipulation. Improved models and measures should also facilitate the development of translational readouts for clinical trials. Studies of the multi-functional ALN promoter and aggregation inhibitor, methylene blue, exemplify challenges faced in patient selection, trial design, dose–response relationships, readouts of efficacy and optimal time of intervention (Suppl Table 1).
Fifth, improved clearance may well have a broad therapeutic time-window, yet early treatment would be advantageous, especially as regards reinforcement of the UPS and CMA before aggregation predominates. Hence, reliable biomarkers of clearance will be important for detecting pre-symptomatic subjects for early intervention81,311. Biomarkers are likewise crucial for demonstration of target engagement and as surrogate signals of disease-slowing and long-term efficacy. While we cannot directly monitor ALN, CMA or UPS in human brain, quantification of CSF and plasma levels of neurotoxic proteins like Aβ42 and tau is instructive. Furthermore, imaging of neurotoxic protein load is helping enrollment of subjects into clinical trials311. In addition, retinal imaging offers a window on cerebral clearance of tau312 while biomarkers of neurovascular flow from the brain to the circulation are under development265,275.
Sixth, the therapeutic strategies evoked herein are pertinent to other classes of NDA. For example, Machado-Joseph disease (spinocerebellar ataxia type-3) is an autosomal-dominant, polyglutamine disease provoked by over-repetition of a CAG sequence in the ataxin3 gene. The mutant protein destabilizes beclin 194. Accordingly, studies in transgenic mice and fibroblasts from patients suggest that reinforcing beclin 1-dependent ALN flux would be beneficial313,314. Blockade of mTOR1 to induce autophagy (and the UPS) may likewise be useful.
Finally, reinforcing clearance might best be undertaken in association with other strategies like suppression of protein misfolding, amelioration of cerebral energetics, or moderation of neuroinflammation2,3,7,25,164,181,. Drug associations or multi-target agents possessing complementary mechanisms of action are both viable options. In addition, therapies for promoting neurotoxic protein clearance will probably prove most effective when used in conjunction with lifestyle changes such as improved sleep hygiene, exercise and a healthy diet.
Concluding comments
An excessive neurotoxic protein load is a core pathophysiological feature underlying and driving NDAs. Amongst several potential strategies for alleviating this burden, an enhancement of clearance is particularly attractive in view of the range of options available, and because insufficent elimination is itself implicated in the pathogenesis of NDAs. While challenges remain, ALN, CMA, UPS, proteolytic, neurovascular and lymphatic mechanisms of clearance offer potentially important strategies for preventing the onset and progression of diverse classes of NDA.
Supplementary Material
Acknowledgements
The authors would like to thank San-Michel Rivet for help in the preparation of the Figures, Karen Duff, Ross Jeggo, Marie-Claude Potier and Clot Mannoury la Cour for helpful comments on the manuscript, as well as Muriel Galliot and her colleague in the IDRS Documention Department for provision of papers relevant to this article. This article is based upon a small, focused Congress which was supported by an unrestricted grant from “Advances in Neuroscience for Medical Innovation”, affiliated to the Institut de recherche Servier.
Abbreviations
- Ca2+
intracellular cytosolic calcium
- Aβ
amyloid-β-protein
- AD
Alzheimer’s disease
- ALN
autophagic-lysosomal network
- ALS
amyotrophic lateral sclerosis
- AMPK
AMP-kinase
- ApoE4
apolipoprotein Epsilon 4 allele
- APP
amyloid precursor protein
- Atg
autophagy-related gene
- BBB
blood brain barrier
- Bcl
B-cell lymphoma
- CMA
chaperone-mediated autophagy
- CSF
cerebrospinal Fluid
- ER
endoplasmic reticulum
- FTD
frontotemporal dementia
- GFP
green fluorescent protein
- GPCR
G-protein coupled receptor
- HD
Huntington’s disease
- Hsc
heat shock cognate
- HSF
Heat Shock Factor
- Hsp
heat shock protein
- Htt
Huntington protein
- ISF
interstitial fluid
- LAMP
lysosome-associated membrane protein
- LC3
microtubule-associated proteins 1A/1B light chain 3B
- LRP1
low density lipoprotein receptor-related protein 1
- LSD
lysosomal storage disease
- MMP
matrix metalloproteinase
- mTORC1
mammalian target of rapamycin complex 1
- NDA
neurodegenerative disease associated with ageing
- Nrf2
nuclear factor erythroid 2–related factor 2
- PINK1
PTEN-induced putative kinase 1
- PROTAC
proteolysis-targeting chimeric molecules
- SOD1
superoxide dismutase
- TDP-43
Transactive response DNA protein-43
- TFEB
transcription factor EB
- TREM2
triggering receptor expressed on myeloid cells 2
- Ulk1
unc-51-like kinase 1
- UPR
Unfolded protein response
- UPS
ubiquitin proteasome system
- v-ATPase
vacuolar-type H+-ATPase
- Vps
vacuolar protein sorting-associated protein
Glossary
- Neurodegenerative disorders of ageing (NDA)
A suite of neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis and frontotemporal dementia that typically are diagnosed in the elderly. Most cases are sporadic, but rare forms are associated with mutations (Table 1). Huntington’s disease is an exception in being purely genetic and having a somewhat earlier onset at 30–50 years of age.
- Proteinopathy
General term for disorders characterised by the buildup of excess, anomalously-marked, misfolded and/or aggregated neurotoxic proteins like Aβ, tau or α-synuclein.
- Amyloid-β
The major neurotoxic product of APP processing, including amyloid-β42, that deposits into extracellular plaques in Alzheimer’s disease. It is toxic as a soluble monomer or low-order oligomers by, for example, disrupting synaptic transmission, damaging mitochondria and impeding proteosomal clearance.
- Tau
A protein that stabilizes axonal microtubules. It is prone to cleavage, hyperphosphorylation and other modifications that trigger and/or follow microtubule dissociation. This leads to misfolding, oligomerisation, synaptic mislocalization and inter-neuronal spreading. Aggregates, fibrils and initracellular neurofibrillary tangles are also formed.
- α-Synuclein
A phospholipid-binding protein abundant in pre-synaptic terminals and involved in the release and regulation of synaptic vesicles. α-synuclein is a major component of Lewy bodies (protein and lipid aggregates) in Parkinson’s disease. Its spread and accumulation in dopaminergic cell bodies and other cell types is a typical feature of the disease.
- TAR DNA protein-43
A normally nuclear protein that is associated with FTD and ALS. In these diseases, it is found in the cytoplasm, where it aggregates.
- Glymphatic system
CSF-driven mechanism for flushing extracellular pools of neurotoxic protein into the circulation: it involves perivascular drainage, astrocytes and the lymph system.
- Blood–brain barrier
Physical and functional barrier that isolates the brain from the rest of the body. Certain nutrients, lipid vesicles and small molecules enter, yet it excludes toxic elements that may damage the brain. It also ejects neurotoxic proteins and other unwanted material. Active transfer of neurotoxic proteins from the brain to the periphery involves specific classes of receptor and transporter.
- Aggresomes
Microtubule-associated inclusions located in the perinuclear region that contain mainly oligomeric, aggregated and ubiquitinated neurotoxic proteins together with p62 and chaperones that aid in their formation. Often generated when UPS activity is insufficient. Protective when short-lived, yet may be harmful in the long-term and can morph into Lewy bodies in PD. Cleared by the ALN
- Stress granules
Non-membrane enclosed, cytoplasmic agglomerates of ribonucleoproteins that store and protect mRNA during short-term cellular stress. Chaperones such as Hsp70 are involved in assembly and unfolding. In NDAs, neurotoxic proteins prolong the presence of stress granules and decrease their solubility, leading to aggregation or transformation into aggresomes
- Peroxisomes
Small (100 nm–1 μM) organelles which oxidize long-chain fatty acids and aid in detoxification. They can be generated by budding-off the endoplasmic reticulum and replicate via fission. Pexophagy refers to the autophagy of peroxisomes.
- Lysosomes
An acidic compartment for the degradation of proteins and other cellular constituents. Their breakdown yields products like amino acids, sugars and lipids, which are recycled. Christian de Duve received the Nobel Prize in Physiology or Medicine for their discovery in 1974.
- Autophagy-related genes
Genes and the molecular machinary for autophagy were characterised in yeast by Y. Ohsumi (Nobel prize in Physiology or Medicine, 2016) and others. The associated genes, identified using mutants, were originally termed Apg1–15, yet Atg is now used. In view of conservation across species, this terminology is used for genes/proteins that regulate autophagy in humans as well.
- AMP kinase
5’-adenosine monophosphate-activated protein kinase, an enzyme involved in energy and nutrient sensing. When activated, AMPK triggers glucose uptake, lipogenesis and triglyceride synthesis. It is a major protein for sensing ATP deficits and initiating the autophagic-lysosomal network.
- Mammalian target of rapamycin
Multi-tasking serine/threonine protein kinase that inhibits autophagy, mitophagy and proteosomal degradation. It also has other roles in, for example, controlling mRNA translation and protein synthesis. Comprises part of a complex (mTORC1) together with several other regulatory and effector proteins.
- Nicotinamide adenine dinucleotide
Dinucleotide co-enzyme necessary for energy generation in all types of cell. It is a co-factor for activation of sirtuin 1, and is required for operation of the ALN. The oxidised and active form is NAD+
- Acetyl coenzyme A
Cofactor involved in protein, carbohydrate and lipid metabolism. it is formed during glycolysis. It provides the acetyl used by acetyl transferases like p300 to acetylate Agt proteins, histones and other substrates such as tau
- Rab proteins
Members of the Ras superfamily of monomeric G-proteins that participate in vesicular trafficking, vesicle formation, vesicle movement (actin/tubulin-mediated) and vesicular fusion, as in autophagosomal fusion with lysosomes.
- SNARE
SNARE (Soluble N-ethylmaleamide-sensitive factor Attachment protein REceptor) refers to a complex of proteins including Synaptobrevin, Syntaxin, “SNAP-25” and Synaptogamin. SNARE contributes to vesicle fusion by “zippering” a donor vesicle (like an autophagosome) onto the recipient compartment (like the lysosome).
- Phospholipase D
Enzyme involved in the transformation of various lipids: it participates in the fusion of autophagosomes with lysosomes
- Lysosomal storage disorders
Diseases resulting from genetic mutations that lead to failure of lysosomal digestion and consequent accumulation of lipids, proteins and other non-digested material. Their pathology is not restricted to the brain and the age of onset is much earlier than for sporadic, age-related neurodegenerative disorders
- Niemann-Pick Type C disease
Lysosomal storage disorder triggered by a defect in the NPC1 gene responsible for cholesterol transport. Patients often display Aβ42 and tau pathology, underpinning parallels to AD in which cholesterol transport is likewise disrupted
- Hsc70
Hsc70 (Heat shock cognate 70 kDa protein) is a constitutively-expressed chaperone also known as Heat Shock Protein Family A member 8 which effects ATP-dependent nascent/unfolded protein folding. It specifically recognizes proteins with an exposed KFERQ-like sequence and delivers them to LAMP2A on lysosomes where, aided by other proteins, substrates are translocated to the lumen for degradation by CMA
- KFERQ
The KFERQ motif on a protein is the principal criterion for capture followed by CMA. Q refers to glutamine, although this sometimes may be an asparagine (N). The other residues are acidic (D), basic (K, R) or basic/hydrophobic (F). There are, however, variations and post-translational modification can modify susceptibility of proteins bearing a KFERQ signal for CMA.
- Lipofuscin
Pigmented cellular inclusion composed of undigested lysosomal contents, including oxidised and cross-linked proteins. This electron-dense, autofluorescent material is characteristic of ageing and NDAs, and can be seen in all types of cerebral cell.
- Unfolded protein response (UPR)
Protective response to help cells recover from cellular and ER stress. Acts via three key effector proteins to modify gene transcription/mRNA translation. The UPR interrupts bulk protein synthesis, promotes the generation of chaperones for protein folding, and increases degradation of misfolded proteins. Over-activation and protracted engagement of the UPR is harmful for neurons and implicated in NDAs
- ALN dysfunction
Underactive autophagy — term used when rates of autophagosome formation and cargo sequestration decrease below basal levels, or fail to upregulate sufficiently under stress. Impaired autophagy — lysosomal delivery, fusion or digestion of autophagosomes is compromised. Overactive autophagy — over-production of autophagosomes and excess ALN activity; can lead to autosis
- Autosis
Autophagy-mediated cell death mediated principally by the Na+/K+-ATPase pump. This can occur with prolonged and excessive autophagy. It is triggered by hypoxia–ischemia (as in stroke or traumatic brain injury), but its occurrence in NDAs is uncertain
- Apolipoprotein Epsilon 4 (ApoE4)
Robust genetic risk factor for AD compared with the more common ApoE2 and E3 alleles. ApoE is secreted by astrocytes and binds lipids such as cholesterol, which are carried to neurons. Also involved in transport of cholesterol-bound Aβ to the blood-brain barrier (ApoE4 is less efficient than ApoE2/3), and in driving synthesis of Aβ42 (ApoE4 is more potent than ApoE2/3).
- Presenilin-1
Catalytic unit of the γ-secretase complex that processes APP into β-amyloid. Mutations are associated with familial AD, and in part reflect altered APP processing. In addition, reduced lysosomal acidification and ALN function may be involved due to mutant presenilin-1-driven deficits in maturation and translocation of vATPase subunits to the lysosome
- Amyloid precursor protein
Transmembrane protein highly expressed in neurons and involved in maintaining cell–cell contact. Successive cleavage by β- and γ-secretases results in the formation of APP terminal fragments like C99, as well as Aβ42 and related species of neurotoxic peptide
- Parkin
Component of the E3 ubiquitin ligase complex that binds to its partner PINK1 to facilitate the autophagic removal of dysfunctional mitochondria that have lost their membrane potential.
- Gaucher’s disease
Primary, autosomal-recessive lysosomal storage disease caused by mutations in the GBA1 gene, which encodes β-glucocerebrosidase. There is a 5-fold higher risk for PD in affected carriers. The activity of β-glucocerebrosidase is impaired in a sub-population of non-familial PD patients, many of whom have genetic mutations related to lysosomal disruption
- Superoxide dismutase (SOD1)
Mitochondrial enzyme dedicated to the reduction of free radicals (reactive oxygen species). SOD1 mutations and dysfunction are seen in a subset of patients with amyotrophic lateral sclerosis.
- CAG-expansion repeats
Proteins containing multiple CAG repeats (CAG encodes glutamine (symbol “Q”). When the number of CAG repeats is supra-normal (for example, >35 for Htt protein), proteins aggregate, provoke cellular damage and trigger inherited, polyglutamine (polyQ) diseases such as Huntington’s disease, spinocerebellar ataxia 3/Joseph-Machado disease (ataxin-3 protein), and spinal and bulbar muscular atrophy (androgen receptor protein)
- TAT–beclin 1
A synthetic peptide comprising 11 amino acids of the Human Immunodeficiency Virus Tat protein transduction domain, a diglycine linker and a (commonly 11-mer) sequence derived from amino acids 267–284 of beclin 1. It is cell-penetrant and triggers ALN-mediated neurotoxic protein clearance without causing cytotoxicity, although higher concentrations may carry the risk of autosis
- Heat shock factor 1 (HSF1)
Protein that occurs as a monomer in the nucleus and cytoplasm, being repressed by heat shock proteins such as Hsp70. Following disruption of proteostasis, heat shock proteins dissociate from HSF1 in order to aid protein-folding. HSF1 then trimerizes and acts as a transcription factor to increase production of Hsp70 and other neuroprotective proteins.
- Exosome
Small (30–150nm), ceramide-rich vesicles formed from cytosolic endosomes, multivesicular bodies and lysosomes. Released with contents (proteins, lipids, sugars and nucleic acids) into extracellular space upon fusion with plasma membrane. Contribute to spread of neurotoxic proteins. Exosomes in CSF, blood and urine are stable and useful as biomarkers.
- Immunotherapy
A biological therapy that passively or actively boosts the body’s natural defenses. Specific classes of antibody aim to neutralise neurotoxic proteins such as Aβ42 or tau. Entrance to the brain is limited, but they may also act as a peripheral sink for neurotoxic proteins in the circulation. In the brain, antibodies probably act for the most part extrinsically to neurons
References
- 1.Menzies FM et al. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron 93, 1015–1034, doi: 10.1016/j.neuron.2017.01.022 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Ciechanover A & Kwon YT Protein Quality Control by Molecular Chaperones in Neurodegeneration. Front Neurosci 11, 185, doi: 10.3389/fnins.2017.00185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dikic I Proteasomal and Autophagic Degradation Systems. Annu Rev Biochem 86, 193–224, doi: 10.1146/annurev-biochem-061516-044908 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Galluzzi L et al. Molecular definitions of autophagy and related processes. EMBO J 36, 1811–1836, doi: 10.15252/embj.201796697 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR & Kroemer G Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov, 16, 487–511. doi: 10.1038/nrd.2017.22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrer I Diversity of astroglial responses across human neurodegenerative disorders and brain aging. Brain Pathol 27, 645–674, doi: 10.1111/bpa.12538 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh FL, Hansen DV & Sheng M TREM2, Microglia, and Neurodegenerative Diseases. Trends Mol Med 23, 512–533, doi: 10.1016/j.molmed.2017.03.008 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Jansen AH, Reits EA & Hol EM The ubiquitin proteasome system in glia and its role in neurodegenerative diseases. Front Mol Neurosci 7, 73, doi: 10.3389/fnmol.2014.00073 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerr JS et al. Mitophagy and Alzheimer’s Disease: Cellular and Molecular Mechanisms. Trends Neurosci 40, 151–166, doi: 10.1016/j.tins.2017.01.002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molino D, Zemirli N, Codogno P & Morel E The Journey of the Autophagosome through Mammalian Cell Organelles and Membranes. J Mol Biol 429, 497–514, doi: 10.1016/j.jmb.2016.12.013 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Wei Y, Chiang WC, Sumpter R Jr., Mishra P & Levine B Prohibitin 2 Is an Inner Mitochondrial Membrane Mitophagy Receptor. Cell 168, 224–238 e210, doi: 10.1016/j.cell.2016.11.042 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khaminets A, Behl C & Dikic I Ubiquitin-Dependent And Independent Signals In Selective Autophagy. Trends Cell Biol 26, 6–16, doi: 10.1016/j.tcb.2015.08.010 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Banerjee K, Munshi S, Frank DE & Gibson GE Abnormal Glucose Metabolism in Alzheimer’s Disease: Relation to Autophagy/Mitophagy and Therapeutic Approaches. Neurochem Res 40, 2557–2569, doi: 10.1007/s11064-015-1631-0 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komatsu M et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169, 425–434 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fekadu J & Rami A Beclin-1 Deficiency Alters Autophagosome Formation, Lysosome Biogenesis and Enhances Neuronal Vulnerability of HT22 Hippocampal Cells. Molecular neurobiology 53, 5500–5509, doi: 10.1007/s12035-015-9453-2 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Sato Y & Nixon RA Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. J Neurosci 31, 7817–7830, doi: 10.1523/JNEUROSCI.6412-10.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boland B et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci 28, 6926–6937 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nixon RA The role of autophagy in neurodegenerative disease. Nat Med 19, 983–997, doi: 10.1038/nm.3232 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Rubinsztein DC, Codogno P & Levine B Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 11, 709–730, doi: 10.1038/nrd3802 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fullgrabe J, Klionsky DJ & Joseph B The return of the nucleus: transcriptional and epigenetic control of autophagy. Nat Rev Mol Cell Biol 15, 65–74, doi: 10.1038/nrm3716 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Herzig S & Shaw RJ AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol, 19, 121–135, doi: 10.1038/nrm.2017.95 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fullgrabe J, Ghislat G, Cho DH & Rubinsztein DC Transcriptional regulation of mammalian autophagy at a glance. J Cell Sci 129, 3059–3066, doi: 10.1242/jcs.188920 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Klionsky DJ et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12, 1–222, doi: 10.1080/15548627.2015.1100356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubbard BP & Sinclair DA Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci 35, 146–154, doi: 10.1016/j.tips.2013.12.004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camandola S & Mattson MP Brain metabolism in health, aging, and neurodegeneration. EMBO J 36, 1474–1492, doi: 10.15252/embj.201695810 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraser J, Cabodevilla AG, Simpson J & Gammoh N Interplay of autophagy, receptor tyrosine kinase signalling and endocytic trafficking. Essays Biochem 61, 597–607, doi: 10.1042/EBC20170091 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wauson EM, Dbouk HA, Ghosh AB & Cobb MH G protein-coupled receptors and the regulation of autophagy. Trends Endocrinol Metab 25, 274–282, doi: 10.1016/j.tem.2014.03.006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondratskyi A, Kondratska K, Skryma R, Klionsky DJ & Prevarskaya N Ion channels in the regulation of autophagy. Autophagy, 14, 3–21, doi: 10.1080/15548627.2017.1384887 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Todd N & Thathiah A The role of GPCRs in neurodegenerative diseases: avenues for therapeutic intervention. Curr Opin Pharmacol 32, 96–110, doi: 10.1016/j.coph.2017.02.001 (2017). [DOI] [PubMed] [Google Scholar]
- 30.He C & Klionsky DJ Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43, 67–93, doi: 10.1146/annurev-genet-102808-114910 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Kundu M, Viollet B & Guan KL AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13, 132–141, doi: 10.1038/ncb2152 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan H, Yan Y, Liu C & Finkel T The role of ZKSCAN3 in the transcriptional regulation of autophagy. Autophagy 13, 1235–1238, doi: 10.1080/15548627.2017.1320635 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuboyama K et al. The ATG conjugation systems are important for degradation of the inner autophagosomal membrane. Science 354, 1036–1041, doi: 10.1126/science.aaf6136 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Gowrishankar S, Wu Y & Ferguson SM Impaired JIP3-dependent axonal lysosome transport promotes amyloid plaque pathology. J Cell Biol 216, 3291–3305, doi: 10.1083/jcb.201612148 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maday S Mechanisms of neuronal homeostasis: Autophagy in the axon. Brain Res 1649, 143–150, doi: 10.1016/j.brainres.2016.03.047 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tammineni P, Jeong YY, Feng T, Aikal D & Cai Q Impaired axonal retrograde trafficking of the retromer complex augments lysosomal deficits in Alzheimer’s disease neurons. Hum Mol Genet 26, 4352–4366, doi: 10.1093/hmg/ddx321 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berman DE, Ringe D, Petsko GA & Small SA The use of pharmacological retromer chaperones in Alzheimer’s disease and other endosomal-related disorders. Neurotherapeutics 12, 12–18, doi: 10.1007/s13311-014-0321-y (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martens S, Nakamura S & Yoshimori T Phospholipids in Autophagosome Formation and Fusion. J Mol Biol, 2016 Oct 27. pii: S0022–2836(16)30455–7. doi: 10.1016/j.jmb.2016.10.029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaminskyy V & Zhivotovsky B Proteases in autophagy. Biochim Biophys Acta 1824, 44–50, doi: 10.1016/j.bbapap.2011.05.013 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Colacurcio DJ & Nixon RA Disorders of lysosomal acidification-The emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res Rev 32, 75–88, doi: 10.1016/j.arr.2016.05.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauvezin C, Nagy P, Juhasz G & Neufeld TP Autophagosome-lysosome fusion is independent of V-ATPase-mediated acidification. Nat Commun 6, 7007, doi: 10.1038/ncomms8007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu S & Nixon RA in Lysosomes: Biology, Diseases, and Therapeutics, pp 315–356 (John Wiley & Sons, Inc., 2016). [Google Scholar]
- 43.Platt FM Emptying the stores: lysosomal diseases and therapeutic strategies. Nat Rev Drug Discov, 17, 133–150, doi: 10.1038/nrd.2017.214 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Settembre C, Fraldi A, Medina DL & Ballabio A Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol 14, 283–296, doi: 10.1038/nrm3565 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xilouri M & Stefanis L Chaperone mediated autophagy to the rescue: A new-fangled target for the treatment of neurodegenerative diseases. Mol Cell Neurosci 66, 29–36, doi: 10.1016/j.mcn.2015.01.003 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Catarino S, Pereira P & Girao H Molecular control of chaperone-mediated autophagy. Essays Biochem 61, 663–674, doi: 10.1042/EBC20170057 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Kaushik S & Cuervo AM The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 2018. April 6. doi: 10.1038/s41580-018-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Medinas DB, Valenzuela V & Hetz C Proteostasis disturbance in amyotrophic lateral sclerosis. Hum Mol Genet 26, R91–R104, doi: 10.1093/hmg/ddx274 (2017). [DOI] [PubMed] [Google Scholar]
- 49.de Poot SAH, Tian G & Finley D Meddling with Fate: The Proteasomal Deubiquitinating Enzymes. J Mol Biol 429, 3525–3545, doi: 10.1016/j.jmb.2017.09.015 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonet-Costa V, Pomatto LC & Davies KJ The Proteasome and Oxidative Stress in Alzheimer’s Disease. Antioxid Redox Signal 25, 886–901, doi: 10.1089/ars.2016.6802 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wrobel L et al. Mistargeted mitochondrial proteins activate a proteostatic response in the cytosol. Nature 524, 485–488, doi: 10.1038/nature14951 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Hegde AN Proteolysis, synaptic plasticity and memory. Neurobiol Learn Mem 138, 98–110, doi: 10.1016/j.nlm.2016.09.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolfe KJ, Ren HY, Trepte P & Cyr DM Polyglutamine-rich suppressors of huntingtin toxicity act upstream of Hsp70 and Sti1 in spatial quality control of amyloid-like proteins. PLoS One 9, e95914, doi: 10.1371/journal.pone.0095914 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menzies FM et al. Calpain inhibition mediates autophagy-dependent protection against polyglutamine toxicity. Cell Death Differ 22, 433–444, doi: 10.1038/cdd.2014.151 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciechanover A & Kwon YT Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med 47, e147, doi: 10.1038/emm.2014.117 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vilchez D, Saez I & Dillin A The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun 5, 5659, doi: 10.1038/ncomms6659 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Mollereau B et al. Adaptive preconditioning in neurological diseases - therapeutic insights from proteostatic perturbations. Brain Res 1648, 603–616, doi: 10.1016/j.brainres.2016.02.033 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sorrentino V et al. Enhancing mitochondrial proteostasis reduces amyloid-beta proteotoxicity. Nature, 552, 187–193, doi: 10.1038/nature25143 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang YA, Zhou B, Wernig M & Sudhof TC ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Abeta Secretion. Cell 168, 427–441 e421, doi: 10.1016/j.cell.2016.12.044 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zlokovic BV Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol 70, 440–444, doi: 10.1001/jamaneurol.2013.2152 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Z, Xie M & Ye K Asparagine endopeptidase is an innovative therapeutic target for neurodegenerative diseases. Expert Opin Ther Targets 20, 1237–1245, doi: 10.1080/14728222.2016.1182990 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simonovitch S et al. Impaired Autophagy in APOE4 Astrocytes. J Alzheimers Dis 51, 915–927, doi: 10.3233/JAD-151101 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Lee JH et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141, 1146–1158, doi:S0092–8674(10)00544–1 [pii] 10.1016/j.cell.2010.05.008 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin-Maestro P et al. Mitophagy Failure in Fibroblasts and iPSC-Derived Neurons of Alzheimer’s Disease-Associated Presenilin 1 Mutation. Front Mol Neurosci 10, 291, doi: 10.3389/fnmol.2017.00291 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sannerud R et al. Restricted Location of PSEN2/gamma-Secretase Determines Substrate Specificity and Generates an Intracellular Abeta Pool. Cell 166, 193–208, doi: 10.1016/j.cell.2016.05.020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lauritzen I et al. Intraneuronal aggregation of the beta-CTF fragment of APP (C99) induces Abeta-independent lysosomal-autophagic pathology. Acta Neuropathol 132, 257–276, doi: 10.1007/s00401-016-1577-6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seixas da Silva GS et al. Amyloid-beta oligomers transiently inhibit AMP-activated kinase and cause metabolic defects in hippocampal neurons. J Biol Chem 292, 7395–7406, doi: 10.1074/jbc.M116.753525 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Myeku N, Duff K Targeting the 26S proteasome to protect against proteotoxic diseases. Trends Mol Med 24, 15–29, doi: 10.1016/j.molmed.2017.11.006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corti O, Lesage S & Brice A What genetics tells us about the cause and mechanisms of Parkinson’s disease: Physiol Rev Transm Suppl, 91, 1161–1128 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Youle RJ & Narendra DP Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12, 9–14, doi: 10.1038/nrm3028 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen ZC et al. Phosphorylation of amyloid precursor protein by mutant LRRK2 promotes AICD activity and neurotoxicity in Parkinson’s disease. Sci Signal 10, doi: 10.1126/scisignal.aam6790 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Giaime E et al. Age-Dependent Dopaminergic Neurodegeneration and Impairment of the Autophagy-Lysosomal Pathway in LRRK-Deficient Mice. Neuron 96, 796–807 e796, doi: 10.1016/j.neuron.2017.09.036 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manzoni C et al. mTOR independent regulation of macroautophagy by Leucine Rich Repeat Kinase 2 via Beclin-1. Sci Rep 6, 35106, doi: 10.1038/srep35106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aflaki E, Westbroek W & Sidransky E The Complicated Relationship between Gaucher Disease and Parkinsonism: Insights from a Rare Disease. Neuron 93, 737–746, doi: 10.1016/j.neuron.2017.01.018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noelker C et al. Glucocerebrosidase deficiency and mitochondrial impairment in experimental Parkinson disease. Journal of the neurological sciences 356, 129–136, doi: 10.1016/j.jns.2015.06.030 (2015). [DOI] [PubMed] [Google Scholar]
- 76.Bento CF, Ashkenazi A, Jimenez-Sanchez M & Rubinsztein DC The Parkinson’s disease-associated genes ATP13A2 and SYT11 regulate autophagy via a common pathway. Nat Commun 7, 11803, doi: 10.1038/ncomms11803 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kong SM et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes alpha-Synuclein externalization via exosomes. Hum Mol Genet 23, 2816–2833, doi: 10.1093/hmg/ddu099 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Tsunemi T & Krainc D Zn(2)(+) dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum Mol Genet 23, 2791–2801, doi: 10.1093/hmg/ddt572 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zondler L et al. Proteasome impairment by alpha-synuclein. PLoS One 12, e0184040, doi: 10.1371/journal.pone.0184040 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sala G, Marinig D, Arosio A & Ferrarese C Role of Chaperone-Mediated Autophagy Dysfunctions in the Pathogenesis of Parkinson’s Disease. Front Mol Neurosci 9, 157, doi: 10.3389/fnmol.2016.00157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li C & Gotz J Tau-based therapies in neurodegeneration: opportunities and challenges. Nat Rev Drug Discov, 16, 863–883, doi: 10.1038/nrd.2017.155 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Gao FB, Almeida S & Lopez-Gonzalez R Dysregulated molecular pathways in amyotrophic lateral sclerosis-frontotemporal dementia spectrum disorder. EMBO J 36, 2931–2950, doi: 10.15252/embj.201797568 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gotzl JK, Lang CM, Haass C & Capell A Impaired protein degradation in FTLD and related disorders. Ageing Res Rev 32, 122–139, doi: 10.1016/j.arr.2016.04.008 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Ramesh N & Pandey UB Autophagy Dysregulation in ALS: When Protein Aggregates Get Out of Hand. Front Mol Neurosci 10, 263, doi: 10.3389/fnmol.2017.00263 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo Q et al. In Situ Structure of Neuronal C9orf72 Poly-GA Aggregates Reveals Proteasome Recruitment. Cell 172, 696–705 e612, doi: 10.1016/j.cell.2017.12.030 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tanaka Y et al. Progranulin regulates lysosomal function and biogenesis through acidification of lysosomes. Hum Mol Genet 26, 969–988, doi: 10.1093/hmg/ddx011 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Oakes JA, Davies MC & Collins MO TBK1: a new player in ALS linking autophagy and neuroinflammation. Mol Brain 10, 5, doi: 10.1186/s13041-017-0287-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nassif M, Woehlbier U & Manque PA The Enigmatic Role of C9ORF72 in Autophagy. Front Neurosci 11, 442, doi: 10.3389/fnins.2017.00442 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ji YJ, Ugolino J, Brady NR, Hamacher-Brady A & Wang J Systemic deregulation of autophagy upon loss of ALS- and FTD-linked C9orf72. Autophagy 13, 1254–1255, doi: 10.1080/15548627.2017.1299312 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henriques A et al. Inhibition of beta-Glucocerebrosidase Activity Preserves Motor Unit Integrity in a Mouse Model of Amyotrophic Lateral Sclerosis. Sci Rep 7, 5235, doi: 10.1038/s41598-017-05313-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin G, Mao D & Bellen HJ Amyotrophic Lateral Sclerosis Pathogenesis Converges on Defects in Protein Homeostasis Associated with TDP-43 Mislocalization and Proteasome-Mediated Degradation Overload. Curr Top Dev Biol 121, 111–171, doi: 10.1016/bs.ctdb.2016.07.004 (2017). [DOI] [PubMed] [Google Scholar]
- 92.Kaliszewski M, Knott AB & Bossy-Wetzel E Primary cilia and autophagic dysfunction in Huntington’s disease. Cell Death Differ 22, 1413–1424, doi: 10.1038/cdd.2015.80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mealer RG, Murray AJ, Shahani N, Subramaniam S & Snyder SH Rhes, a striatal-selective protein implicated in Huntington disease, binds beclin-1 and activates autophagy. J Biol Chem 289, 3547–3554, doi: 10.1074/jbc.M113.536912 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ashkenazi A et al. Polyglutamine tracts regulate beclin 1-dependent autophagy. Nature, 545, 118–111,doi: 10.1038/nature22078 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bauer PO et al. Harnessing chaperone-mediated autophagy for the selective degradation of mutant huntingtin protein. Nat Biotechnol 28, 256–263, doi: 10.1038/nbt.1608 (2010). [DOI] [PubMed] [Google Scholar]
- 96.Xilouri M, Vogiatzi T, Vekrellis K, Park D & Stefanis L Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS One 4, e5515, doi: 10.1371/journal.pone.0005515 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Her LS et al. The Differential Profiling of Ubiquitin-Proteasome and Autophagy Systems in Different Tissues before the Onset of Huntington’s Disease Models. Brain Pathol 25, 481–490, doi: 10.1111/bpa.12191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pakos-Zebrucka K et al. The integrated stress response. EMBO Rep 17, 1374–1395, doi: 10.15252/embr.201642195 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Halliday M et al. Repurposed drugs targeting eIF2alpha-P-mediated translational repression prevent neurodegeneration in mice. Brain, 140,1768–1773, doi: 10.1093/brain/awx074 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mogk A, Bukau B & Kampinga HH Cellular Handling of Protein Aggregates by Disaggregation Machines. Mol Cell 69, 214–226, doi: 10.1016/j.molcel.2018.01.004 (2018). [DOI] [PubMed] [Google Scholar]
- 101.Congdon EE et al. Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy 8, 609–622, doi: 10.4161/auto.19048 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xie L et al. Methylene blue induces macroautophagy through 5’ adenosine monophosphate-activated protein kinase pathway to protect neurons from serum deprivation. Front Cell Neurosci 7, 56, doi: 10.3389/fncel.2013.00056 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Williams A et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat Chem Biol 4, 295–305 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rose C et al. Rilmenidine attenuates toxicity of polyglutamine expansions in a mouse model of Huntington’s disease. Hum Mol Genet 19, 2144–2153, doi: 10.1093/hmg/ddq093 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sarkar S, Ravikumar B, Floto RA & Rubinsztein DC Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ 16, 46–56 (2009). [DOI] [PubMed] [Google Scholar]
- 106.Rao MV et al. Specific calpain inhibition by calpastatin prevents tauopathy and neurodegeneration and restores normal lifespan in tau P301L mice. J Neurosci 34, 9222–9234, doi: 10.1523/JNEUROSCI.1132-14.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rao MV, Campbell J, Palaniappan A, Kumar A & Nixon RA Calpastatin inhibits motor neuron death and increases survival of hSOD1(G93A) mice. J Neurochem 137, 253–265, doi: 10.1111/jnc.13536 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park SY et al. Cilostazol Modulates Autophagic Degradation of beta-Amyloid Peptide via SIRT1-Coupled LKB1/AMPKalpha Signaling in Neuronal Cells. PLoS One 11, e0160620, doi: 10.1371/journal.pone.0160620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ayasolla KR, Singh AK & Singh I 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) attenuates the expression of LPS- and Abeta peptide-induced inflammatory mediators in astroglia. J Neuroinflammation 2, 21, doi: 10.1186/1742-2094-2-21 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dulovic M et al. The protective role of AMP-activated protein kinase in alpha-synuclein neurotoxicity in vitro. Neurobiol Dis 63, 1–11, doi: 10.1016/j.nbd.2013.11.002 (2014). [DOI] [PubMed] [Google Scholar]
- 111.Walter C et al. Activation of AMPK-induced autophagy ameliorates Huntington disease pathology in vitro. Neuropharmacology 108, 24–38, doi: 10.1016/j.neuropharm.2016.04.041 (2016). [DOI] [PubMed] [Google Scholar]
- 112.Zhang ZH et al. Selenomethionine Mitigates Cognitive Decline by Targeting Both Tau Hyperphosphorylation and Autophagic Clearance in an Alzheimer’s Disease Mouse Model. J Neurosci 37, 2449–2462, doi: 10.1523/JNEUROSCI.3229-16.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park SJ et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148, 421–433, doi: 10.1016/j.cell.2012.01.017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vingtdeux V et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem 285, 9100–9113, doi:M109.060061 [pii] 10.1074/jbc.M109.060061 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parker JA et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet 37, 349–350, doi: 10.1038/ng1534 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Martin-Montalvo A et al. Metformin improves healthspan and lifespan in mice. Nat Commun 4, 2192, doi: 10.1038/ncomms3192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kickstein E et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci U S A 107, 21830–21835, doi: 10.1073/pnas.0912793107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen B, Teng Y, Zhang X, Lv X & Yin Y Metformin Alleviated Abeta-Induced Apoptosis via the Suppression of JNK MAPK Signaling Pathway in Cultured Hippocampal Neurons. Biomed Res Int 2016, 1421430, doi: 10.1155/2016/1421430 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patil SP, Jain PD, Ghumatkar PJ, Tambe R & Sathaye S Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience 277, 747–754, doi: 10.1016/j.neuroscience.2014.07.046 (2014). [DOI] [PubMed] [Google Scholar]
- 120.Castillo K et al. Trehalose delays the progression of amyotrophic lateral sclerosis by enhancing autophagy in motoneurons. Autophagy 9, 1308–1320, doi: 10.4161/auto.25188 (2013). [DOI] [PubMed] [Google Scholar]
- 121.Sarkar S, Davies JE, Huang Z, Tunnacliffe A & Rubinsztein DC Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 282, 5641–5652 (2007). [DOI] [PubMed] [Google Scholar]
- 122.Kruger U, Wang Y, Kumar S & Mandelkow EM Autophagic degradation of tau in primary neurons and its enhancement by trehalose. Neurobiol Aging 33, 2291–2305, doi: 10.1016/j.neurobiolaging.2011.11.009 (2012). [DOI] [PubMed] [Google Scholar]
- 123.Du J, Liang Y, Xu F, Sun B & Wang Z Trehalose rescues Alzheimer’s disease phenotypes in APP/PS1 transgenic mice. J Pharm Pharmacol 65, 1753–1756, doi: 10.1111/jphp.12108 (2013). [DOI] [PubMed] [Google Scholar]
- 124.Tanaka M et al. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med 10, 148–154 (2004). [DOI] [PubMed] [Google Scholar]
- 125.Schaeffer V & Goedert M Stimulation of autophagy is neuroprotective in a mouse model of human tauopathy. Autophagy 8, 1686–1687, doi: 10.4161/auto.21488 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sarkar S et al. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 170, 1101–1111 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shimada K et al. Long-term oral lithium treatment attenuates motor disturbance in tauopathy model mice: implications of autophagy promotion. Neurobiol Dis 46, 101–108, doi: 10.1016/j.nbd.2011.12.050 (2012). [DOI] [PubMed] [Google Scholar]
- 128.Fornai F et al. Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 105, 2052–2057, doi: 10.1073/pnas.0708022105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li H et al. Biochemical protective effect of 1,25-dihydroxyvitamin D3 through autophagy induction in the MPTP mouse model of Parkinson’s disease. Neuroreport 26, 669–674, doi: 10.1097/WNR.0000000000000401 (2015). [DOI] [PubMed] [Google Scholar]
- 130.Webb JL, Ravikumar B, Atkins J, Skepper JN & Rubinsztein DC Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278, 25009–25013 (2003). [DOI] [PubMed] [Google Scholar]
- 131.Ravikumar B, Duden R & Rubinsztein DC Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet 11, 1107–1117 (2002). [DOI] [PubMed] [Google Scholar]
- 132.Ryu HH et al. Autophagy regulates amyotrophic lateral sclerosis-linked fused in sarcoma-positive stress granules in neurons. Neurobiol Aging 35, 2822–2831, doi: 10.1016/j.neurobiolaging.2014.07.026 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Ravikumar B et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36, 585–595 (2004). [DOI] [PubMed] [Google Scholar]
- 134.Wang IF, Tsai KJ & Shen CK Autophagy activation ameliorates neuronal pathogenesis of FTLD-U mice: a new light for treatment of TARDBP/TDP-43 proteinopathies. Autophagy 9, 239–240, doi: 10.4161/auto.22526 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu K, Shi N, Sun Y, Zhang T & Sun X Therapeutic effects of rapamycin on MPTP-induced Parkinsonism in mice. Neurochem Res 38, 201–207, doi: 10.1007/s11064-012-0909-8 (2013). [DOI] [PubMed] [Google Scholar]
- 136.Caccamo A, Majumder S, Richardson A, Strong R & Oddo S Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem 285, 13107–13120, doi:M110.100420 [pii] 10.1074/jbc.M110.100420 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jiang T et al. Temsirolimus attenuates tauopathy in vitro and in vivo by targeting tau hyperphosphorylation and autophagic clearance. Neuropharmacology 85, 121–130, doi: 10.1016/j.neuropharm.2014.05.032 (2014). [DOI] [PubMed] [Google Scholar]
- 138.Siracusa R et al. Neuroprotective Effects of Temsirolimus in Animal Models of Parkinson’s Disease. Mol Neurobiol, 55, 2403–2419, doi: 10.1007/s12035-017-0496-4 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Menzies FM et al. Autophagy induction reduces mutant ataxin-3 levels and toxicity in a mouse model of spinocerebellar ataxia type 3. Brain 133, 93–104, doi: 10.1093/brain/awp292 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sarkar S et al. Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat Chem Biol 3, 331–338 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Satish Bollimpelli V & Kondapi AK Differential sensitivity of immature and mature ventral mesencephalic neurons to rotenone induced neurotoxicity in vitro. Toxicol In Vitro 30, 545–551, doi: 10.1016/j.tiv.2015.09.006 (2015). [DOI] [PubMed] [Google Scholar]
- 142.Pandey N, Strider J, Nolan WC, Yan SX & Galvin JE Curcumin inhibits aggregation of alpha-synuclein. Acta Neuropathol 115, 479–489, doi: 10.1007/s00401-007-0332-4 (2008). [DOI] [PubMed] [Google Scholar]
- 143.Jiang TF et al. Curcumin ameliorates the neurodegenerative pathology in A53T alpha-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. J Neuroimmune Pharmacol 8, 356–369, doi: 10.1007/s11481-012-9431-7 (2013). [DOI] [PubMed] [Google Scholar]
- 144.Spinelli KJ, Osterberg VR, Meshul CK, Soumyanath A & Unni VK Curcumin Treatment Improves Motor Behavior in alpha-Synuclein Transgenic Mice. PLoS One 10, e0128510, doi: 10.1371/journal.pone.0128510 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ma QL et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J Biol Chem 288, 4056–4065, doi: 10.1074/jbc.M112.393751 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang F et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280, 5892–5901, doi: 10.1074/jbc.M404751200 (2005). [DOI] [PubMed] [Google Scholar]
- 147.Medina DL et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 17, 288–299, doi: 10.1038/ncb3114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xiao Q et al. Neuronal-Targeted TFEB Accelerates Lysosomal Degradation of APP, Reducing Abeta Generation and Amyloid Plaque Pathogenesis. J Neurosci 35, 12137–12151, doi: 10.1523/JNEUROSCI.0705-15.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim S et al. Fisetin stimulates autophagic degradation of phosphorylated tau via the activation of TFEB and Nrf2 transcription factors. Sci Rep 6, 24933, doi: 10.1038/srep24933 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hori Y et al. A Food and Drug Administration-approved asthma therapeutic agent impacts amyloid beta in the brain in a transgenic model of Alzheimer disease. J Biol Chem 290, 1966–1978, doi: 10.1074/jbc.M114.586602 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li Y et al. Protein kinase C controls lysosome biogenesis independently of mTORC1. Nat Cell Biol 18, 1065–1077, doi: 10.1038/ncb3407 (2016). [DOI] [PubMed] [Google Scholar]
- 152.Schlatterer SD, Acker CM & Davies P c-Abl in neurodegenerative disease. J Mol Neurosci 45, 445–452, doi: 10.1007/s12031-011-9588-1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hebron ML, Lonskaya I & Moussa CE Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of alpha-synuclein in Parkinson’s disease models. Hum Mol Genet 22, 3315–3328, doi: 10.1093/hmg/ddt192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wenqiang C et al. Parkin-mediated reduction of nuclear and soluble TDP-43 reverses behavioral decline in symptomatic mice. Hum Mol Genet 23, 4960–4969, doi: 10.1093/hmg/ddu211 (2014). [DOI] [PubMed] [Google Scholar]
- 155.Pagan F et al. Nilotinib Effects in Parkinson’s disease and Dementia with Lewy bodies. J Parkinsons Dis 6, 503–517, doi: 10.3233/JPD-160867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Satoh A, Imai SI & Guarente L The brain, sirtuins, and ageing. Nat Rev Neurosci 18, 362–374, doi: 10.1038/nrn.2017.42 (2017). [DOI] [PubMed] [Google Scholar]
- 157.Kang HT & Hwang ES Nicotinamide enhances mitochondria quality through autophagy activation in human cells. Aging Cell 8, 426–438, doi: 10.1111/j.1474-9726.2009.00487.x (2009). [DOI] [PubMed] [Google Scholar]
- 158.Wu MF, Yin JH, Hwang CS, Tang CM & Yang DI NAD attenuates oxidative DNA damages induced by amyloid beta-peptide in primary rat cortical neurons. Free Radic Res 48, 794–805, doi: 10.3109/10715762.2014.907889 (2014). [DOI] [PubMed] [Google Scholar]
- 159.Liu D et al. Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol Aging 34, 1564–1580, doi: 10.1016/j.neurobiolaging.2012.11.020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Deng H & Mi MT Resveratrol Attenuates Abeta25–35 Caused Neurotoxicity by Inducing Autophagy Through the TyrRS-PARP1-SIRT1 Signaling Pathway. Neurochem Res 41, 2367–2379, doi: 10.1007/s11064-016-1950-9 (2016). [DOI] [PubMed] [Google Scholar]
- 161.Martire S et al. Bioenergetic Impairment in Animal and Cellular Models of Alzheimer’s Disease: PARP-1 Inhibition Rescues Metabolic Dysfunctions. J Alzheimers Dis 54, 307–324, doi: 10.3233/JAD-151040 (2016). [DOI] [PubMed] [Google Scholar]
- 162.Park SH et al. Protective effect of the phosphodiesterase III inhibitor cilostazol on amyloid beta-induced cognitive deficits associated with decreased amyloid beta accumulation. Biochem Biophys Res Commun 408, 602–608, doi: 10.1016/j.bbrc.2011.04.068 (2011). [DOI] [PubMed] [Google Scholar]
- 163.Lee HR et al. Attenuation of beta-amyloid-induced tauopathy via activation of CK2alpha/SIRT1: targeting for cilostazol. J Neurosci Res 92, 206–217, doi: 10.1002/jnr.23310 (2014). [DOI] [PubMed] [Google Scholar]
- 164.Madeo F, Pietrocola F, Eisenberg T & Kroemer G Caloric restriction mimetics: towards a molecular definition. Nat Rev Drug Discov 13, 727–740, doi: 10.1038/nrd4391 (2014). [DOI] [PubMed] [Google Scholar]
- 165.Millan MJ Linking deregulation of non-coding RNA to the core pathophysiology of Alzheimer’s disease: An integrative review. Prog Neurobiol, 156, 1–68, doi: 10.1016/j.pneurobio.2017.03.004 (2017). [DOI] [PubMed] [Google Scholar]
- 166.Park G et al. Regulation of Histone Acetylation by Autophagy in Parkinson Disease. J Biol Chem 291, 3531–3540, doi: 10.1074/jbc.M115.675488 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Madeo F, Eisenberg T, Pietrocola F & Kroemer G Spermidine in health and disease. Science 359, doi: 10.1126/science.aan2788 (2018). [DOI] [PubMed] [Google Scholar]
- 168.Yang Y et al. Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death Dis 8, e2738, doi: 10.1038/cddis.2017.161 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang IF et al. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc Natl Acad Sci U S A 109, 15024–15029, doi: 10.1073/pnas.1206362109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Buttner S et al. Spermidine protects against alpha-synuclein neurotoxicity. Cell Cycle 13, 3903–3908, doi: 10.4161/15384101.2014.973309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Marino G et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol Cell 53, 710–725, doi: 10.1016/j.molcel.2014.01.016 (2014). [DOI] [PubMed] [Google Scholar]
- 172.Aubry S et al. Assembly and interrogation of Alzheimer’s disease genetic networks reveal novel regulators of progression. PLoS One 10, e0120352, doi: 10.1371/journal.pone.0120352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yao J et al. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J Exp Med 209, 2501–2513, doi: 10.1084/jem.20121239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shoji-Kawata S et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature 494, 201–206, doi: 10.1038/nature11866 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lu JH et al. Isorhynchophylline, a natural alkaloid, promotes the degradation of alpha-synuclein in neuronal cells via inducing autophagy. Autophagy 8, 98–108, doi: 10.4161/auto.8.1.18313 (2012). [DOI] [PubMed] [Google Scholar]
- 176.Di Rita A & Strappazzon F AMBRA1, a Novel BH3-Like Protein: New Insights Into the AMBRA1-BCL2-Family Proteins Relationship. Int Rev Cell Mol Biol 330, 85–113, doi: 10.1016/bs.ircmb.2016.09.002 (2017). [DOI] [PubMed] [Google Scholar]
- 177.Pedro JM et al. BAX and BAK1 are dispensable for ABT-737-induced dissociation of the BCL2-BECN1 complex and autophagy. Autophagy 11, 452–459, doi: 10.1080/15548627.2015.1017191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rocchi A et al. A Becn1 mutation mediates hyperactive autophagic sequestration of amyloid oligomers and improved cognition in Alzheimer’s disease. PLoS Genet 13, e1006962, doi: 10.1371/journal.pgen.1006962 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Salminen A et al. Impaired autophagy and APP processing in Alzheimer’s disease: The potential role of Beclin 1 interactome. Prog Neurobiol 106–107, 33–54, doi: 10.1016/j.pneurobio.2013.06.002 (2013). [DOI] [PubMed] [Google Scholar]
- 180.Vidoni C, Secomandi E, Castiglioni A, Melone MAB & Isidoro C Resveratrol protects neuronal-like cells expressing mutant Huntingtin from dopamine toxicity by rescuing ATG4-mediated autophagosome formation. Neurochem Int, doi: 10.1016/j.neuint.2017.05.013 (2017). [DOI] [PubMed] [Google Scholar]
- 181.Kovacs T et al. The small molecule AUTEN-99 (autophagy enhancer-99) prevents the progression of neurodegenerative symptoms. Sci Rep 7, 42014, doi: 10.1038/srep42014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Seyb KI, Ansar S, Bean J & Michaelis ML beta-Amyloid and endoplasmic reticulum stress responses in primary neurons: effects of drugs that interact with the cytoskeleton. J Mol Neurosci 28, 111–123 (2006). [DOI] [PubMed] [Google Scholar]
- 183.Zhang B et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J Neurosci 32, 3601–3611, doi: 10.1523/JNEUROSCI.4922-11.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kast DJ & Dominguez R The Cytoskeleton-Autophagy Connection. Curr Biol 27, R318–R326, doi: 10.1016/j.cub.2017.02.061 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Coutts AS & La Thangue NB Regulation of actin nucleation and autophagosome formation. Cell Mol Life Sci 73, 3249–3263, doi: 10.1007/s00018-016-2224-z (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang Z et al. The Vici Syndrome Protein EPG5 Is a Rab7 Effector that Determines the Fusion Specificity of Autophagosomes with Late Endosomes/Lysosomes. Mol Cell 63, 781–795, doi: 10.1016/j.molcel.2016.08.021 (2016). [DOI] [PubMed] [Google Scholar]
- 187.Shi Y et al. Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat Med 24, 313–325, doi: 10.1038/nm.4490 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mecozzi VJ et al. Pharmacological chaperones stabilize retromer to limit APP processing. Nat Chem Biol 10, 443–449, doi: 10.1038/nchembio.1508 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Coffey EE, Beckel JM, Laties AM & Mitchell CH Lysosomal alkalization and dysfunction in human fibroblasts with the Alzheimer’s disease-linked presenilin 1 A246E mutation can be reversed with cAMP. Neuroscience 263, 111–124, doi: 10.1016/j.neuroscience.2014.01.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Moruno-Manchon JF et al. TFEB ameliorates the impairment of the autophagy-lysosome pathway in neurons induced by doxorubicin. Aging (Albany NY) 8, 3507–3519, doi: 10.18632/aging.101144 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang W et al. Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation. Proc Natl Acad Sci U S A 112, E1373–1381, doi: 10.1073/pnas.1419669112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bae M et al. Activation of TRPML1 clears intraneuronal Abeta in preclinical models of HIV infection. J Neurosci 34, 11485–11503, doi: 10.1523/JNEUROSCI.0210-14.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kao AW, McKay A, Singh PP, Brunet A & Huang EJ Progranulin, lysosomal regulation and neurodegenerative disease. Nat Rev Neurosci 18, 325–333, doi: 10.1038/nrn.2017.36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Arrant AE, Onyilo VC, Unger DE & Roberson ED Progranulin Gene Therapy Improves Lysosomal Dysfunction and Microglial Pathology Associated with Frontotemporal Dementia and Neuronal Ceroid Lipofuscinosis. J Neurosci 38, 2341–2358, doi: 10.1523/JNEUROSCI.3081-17.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kilpatrick K, Zeng Y, Hancock T & Segatori L Genetic and chemical activation of TFEB mediates clearance of aggregated alpha-synuclein. PLoS One 10, e0120819, doi: 10.1371/journal.pone.0120819 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Seo BR, Lee SJ, Cho KS, Yoon YH & Koh JY The zinc ionophore clioquinol reverses autophagy arrest in chloroquine-treated ARPE-19 cells and in APP/mutant presenilin-1-transfected Chinese hamster ovary cells. Neurobiol Aging 36, 3228–3238, doi: 10.1016/j.neurobiolaging.2015.09.006 (2015). [DOI] [PubMed] [Google Scholar]
- 197.Cherny RA et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 30, 665–676 (2001). [DOI] [PubMed] [Google Scholar]
- 198.Sun B et al. Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer’s disease. Neuron 60, 247–257, doi: 10.1016/j.neuron.2008.10.001 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sardi SP et al. Glucosylceramide synthase inhibition alleviates aberrations in synucleinopathy models. Proc Natl Acad Sci U S A 114, 2699–2704, doi: 10.1073/pnas.1616152114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang SY, Beavan M, Chau KY, Taanman JW & Schapira AH A Human Neural Crest Stem Cell-Derived Dopaminergic Neuronal Model Recapitulates Biochemical Abnormalities in GBA1 Mutation Carriers. Stem Cell Reports 8, 728–742, doi: 10.1016/j.stemcr.2017.01.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sanchez-Martinez A et al. Parkinson disease-linked GBA mutation effects reversed by molecular chaperones in human cell and fly models. Sci Rep 6, 31380, doi: 10.1038/srep31380 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Migdalska-Richards A, Daly L, Bezard E & Schapira AH Ambroxol effects in glucocerebrosidase and alpha-synuclein transgenic mice. Ann Neurol 80, 766–775, doi: 10.1002/ana.24790 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Aflaki E et al. A New Glucocerebrosidase Chaperone Reduces alpha-Synuclein and Glycolipid Levels in iPSC-Derived Dopaminergic Neurons from Patients with Gaucher Disease and Parkinsonism. J Neurosci 36, 7441–7452, doi: 10.1523/JNEUROSCI.0636-16.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Song W, Wang F, Lotfi P, Sardiello M & Segatori L 2-Hydroxypropyl-beta-cyclodextrin promotes transcription factor EB-mediated activation of autophagy: implications for therapy. J Biol Chem 289, 10211–10222, doi: 10.1074/jbc.M113.506246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Duan WJ et al. A SIRT3/AMPK/autophagy network orchestrates the protective effects of trans-resveratrol in stressed peritoneal macrophages and RAW 264.7 macrophages. Free Radic Biol Med 95, 230–242, doi: 10.1016/j.freeradbiomed.2016.03.022 (2016). [DOI] [PubMed] [Google Scholar]
- 206.Writing G & Edaravone ALSSG Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. The Lancet. Neurology 16, 505–512, doi: 10.1016/S1474-4422(17)30115-1 (2017). [DOI] [PubMed] [Google Scholar]
- 207.Li S & Laher I Exercise Pills: At the Starting Line. Trends Pharmacol Sci 36, 906–917, doi: 10.1016/j.tips.2015.08.014 (2015). [DOI] [PubMed] [Google Scholar]
- 208.Lackie RE et al. The Hsp70/Hsp90 Chaperone Machinery in Neurodegenerative Diseases. Front Neurosci 11, 254, doi: 10.3389/fnins.2017.00254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Neef DW, Jaeger AM & Thiele DJ Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat Rev Drug Discov 10, 930–944, doi: 10.1038/nrd3453 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kalmar B, Lu CH & Greensmith L The role of heat shock proteins in Amyotrophic Lateral Sclerosis: The therapeutic potential of Arimoclomol. Pharmacology & therapeutics 141, 40–54, doi: 10.1016/j.pharmthera.2013.08.003 (2014). [DOI] [PubMed] [Google Scholar]
- 211.Kalmar B & Greensmith L Activation of the heat shock response in a primary cellular model of motoneuron neurodegeneration-evidence for neuroprotective and neurotoxic effects. Cell Mol Biol Lett 14, 319–335, doi: 10.2478/s11658-009-0002-8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kieran D et al. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med 10, 402–405, doi: 10.1038/nm1021 (2004). [DOI] [PubMed] [Google Scholar]
- 213.Wang AM et al. Activation of Hsp70 reduces neurotoxicity by promoting polyglutamine protein degradation. Nat Chem Biol 9, 112–118, doi: 10.1038/nchembio.1140 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lee JH et al. Facilitated Tau Degradation by USP14 Aptamers via Enhanced Proteasome Activity. Sci Rep 5, 10757, doi: 10.1038/srep10757 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kiprowska MJ et al. Neurotoxic mechanisms by which the USP14 inhibitor IU1 depletes ubiquitinated proteins and Tau in rat cerebral cortical neurons: Relevance to Alzheimer’s disease. Biochim Biophys Acta 1863, 1157–1170, doi: 10.1016/j.bbadis.2017.03.017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Boselli M et al. An inhibitor of the proteasomal deubiquitinating enzyme USP14 induces tau elimination in cultured neurons. J Biol Chem, 292, 19209–19221, doi: 10.1074/jbc.M117.815126 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Harrigan JA, Jacq X, Martin NM & Jackson SP Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat Rev Drug Discov 17, 57–78, doi: 10.1038/nrd.2017.152 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang B et al. A CNS-permeable Hsp90 inhibitor rescues synaptic dysfunction and memory loss in APP-overexpressing Alzheimer’s mouse model via an HSF1-mediated mechanism. Mol Psychiatry 22, 990–1001, doi: 10.1038/mp.2016.104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Petrucelli L et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet 13, 703–714, doi: 10.1093/hmg/ddh083 (2004). [DOI] [PubMed] [Google Scholar]
- 220.Danzer KM et al. Heat-shock protein 70 modulates toxic extracellular alpha-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J 25, 326–336, doi: 10.1096/fj.10-164624 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Auluck PK & Bonini NM Pharmacological prevention of Parkinson disease in Drosophila. Nat Med 8, 1185–1186, doi: 10.1038/nm1102-1185 (2002). [DOI] [PubMed] [Google Scholar]
- 222.Agrawal N et al. Identification of combinatorial drug regimens for treatment of Huntington’s disease using Drosophila. Proc Natl Acad Sci U S A 102, 3777–3781, doi: 10.1073/pnas.0500055102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chen Y et al. Hsp90 chaperone inhibitor 17-AAG attenuates Abeta-induced synaptic toxicity and memory impairment. J Neurosci 34, 2464–2470, doi: 10.1523/JNEUROSCI.0151-13.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ho SW et al. Effects of 17-allylamino-17-demethoxygeldanamycin (17-AAG) in transgenic mouse models of frontotemporal lobar degeneration and Alzheimer’s disease. Translational neurodegeneration 2, 24, doi: 10.1186/2047-9158-2-24 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Labbadia J et al. Altered chromatin architecture underlies progressive impairment of the heat shock response in mouse models of Huntington disease. The Journal of clinical investigation 121, 3306–3319, doi: 10.1172/JCI57413 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Guo X, Huang X & Chen MJ Reversible phosphorylation of the 26S proteasome. Protein Cell 8, 255–272, doi: 10.1007/s13238-017-0382-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.VerPlank JJS & Goldberg AL Regulating protein breakdown through proteasome phosphorylation. Biochem J 474, 3355–3371, doi: 10.1042/BCJ20160809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bate C & Williams A cAMP-Inhibits Cytoplasmic Phospholipase A(2) and Protects Neurons against Amyloid-beta-Induced Synapse Damage. Biology (Basel) 4, 591–606, doi: 10.3390/biology4030591 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Myeku N et al. Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling. Nat Med 22, 46–53, doi: 10.1038/nm.4011 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lokireddy S, Kukushkin NV & Goldberg AL cAMP-induced phosphorylation of 26S proteasomes on Rpn6/PSMD11 enhances their activity and the degradation of misfolded proteins. Proc Natl Acad Sci U S A 112, E7176–7185, doi: 10.1073/pnas.1522332112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lin JT et al. Regulation of feedback between protein kinase A and the proteasome system worsens Huntington’s disease. Mol Cell Biol 33, 1073–1084, doi: 10.1128/MCB.01434-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Djakovic SN et al. Phosphorylation of Rpt6 regulates synaptic strength in hippocampal neurons. J Neurosci 32, 5126–5131, doi: 10.1523/JNEUROSCI.4427-11.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Leestemaker Y et al. Proteasome Activation by Small Molecules. Cell Chem Biol 24, 725–736 e727, doi: 10.1016/j.chembiol.2017.05.010 (2017). [DOI] [PubMed] [Google Scholar]
- 234.Crew AP et al. Identification and Characterization of Von Hippel-Lindau-Recruiting Proteolysis Targeting Chimeras (PROTACs) of TANK-Binding Kinase 1. J Med Chem, 61, 583–598, doi: 10.1021/acs.jmedchem.7b00635 (2017). [DOI] [PubMed] [Google Scholar]
- 235.Collins I, Wang H, Caldwell JJ & Chopra R Chemical approaches to targeted protein degradation through modulation of the ubiquitin-proteasome pathway. Biochem J 474, 1127–1147, doi: 10.1042/BCJ20160762 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chu TT et al. Specific Knockdown of Endogenous Tau Protein by Peptide-Directed Ubiquitin-Proteasome Degradation. Cell Chem Biol 23, 453–461, doi: 10.1016/j.chembiol.2016.02.016 (2016). [DOI] [PubMed] [Google Scholar]
- 237.Clift D et al. A Method for the Acute and Rapid Degradation of Endogenous Proteins. Cell, 171, 1692–1706, doi: 10.1016/j.cell.2017.10.033 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Choi JS et al. cIAPs promote the proteasomal degradation of mutant SOD1 linked to familial amyotrophic lateral sclerosis. Biochem Biophys Res Commun 480, 422–428, doi: 10.1016/j.bbrc.2016.10.065 (2016). [DOI] [PubMed] [Google Scholar]
- 239.Vangala JR, Sotzny F, Kruger E, Deshaies RJ & Radhakrishnan SK Nrf1 can be processed and activated in a proteasome-independent manner. Curr Biol 26, R834–R835, doi: 10.1016/j.cub.2016.08.008 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Pajares M, Cuadrado A & Rojo AI Modulation of proteostasis by transcription factor NRF2 and impact in neurodegenerative diseases. Redox Biol 11, 543–553, doi: 10.1016/j.redox.2017.01.006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tsakiri EN et al. Proteasome dysfunction in Drosophila signals to an Nrf2-dependent regulatory circuit aiming to restore proteostasis and prevent premature aging. Aging Cell 12, 802–813, doi: 10.1111/acel.12111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Opattova A, Cente M, Novak M & Filipcik P The ubiquitin proteasome system as a potential therapeutic target for treatment of neurodegenerative diseases. Gen Physiol Biophys 34, 337–352, doi: 10.4149/gpb_2015024 (2015). [DOI] [PubMed] [Google Scholar]
- 243.Rousseau A & Bertolotti A An evolutionarily conserved pathway controls proteasome homeostasis. Nature 536, 184–189, doi: 10.1038/nature18943 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Arias E et al. Lysosomal mTORC2/PHLPP1/Akt Regulate Chaperone-Mediated Autophagy. Mol Cell 59, 270–284, doi: 10.1016/j.molcel.2015.05.030 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Anguiano J et al. Chemical modulation of chaperone-mediated autophagy by retinoic acid derivatives. Nat Chem Biol 9, 374–382, doi: 10.1038/nchembio.1230 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lopez A et al. A152T tau allele causes neurodegeneration that can be ameliorated in a zebrafish model by autophagy induction. Brain 140, 1128–1146, doi: 10.1093/brain/awx005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hou YS et al. Sestrin2 Protects Dopaminergic Cells against Rotenone Toxicity through AMPK-Dependent Autophagy Activation. Mol Cell Biol 35, 2740–2751, doi: 10.1128/MCB.00285-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chen YS, Chen SD, Wu CL, Huang SS & Yang DI Induction of sestrin2 as an endogenous protective mechanism against amyloid beta-peptide neurotoxicity in primary cortical culture. Exp Neurol 253, 63–71, doi: 10.1016/j.expneurol.2013.12.009 (2014). [DOI] [PubMed] [Google Scholar]
- 249.Shafiei SS, Guerrero-Munoz MJ & Castillo-Carranza DL Tau Oligomers: Cytotoxicity, Propagation, and Mitochondrial Damage. Front Aging Neurosci 9, 83, doi: 10.3389/fnagi.2017.00083 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Valdinocci D, Radford RA, Siow SM, Chung RS & Pountney DL Potential Modes of Intercellular alpha-Synuclein Transmission. Int J Mol Sci 18, doi: 10.3390/ijms18020469 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Laulagnier K et al. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol Life Sci, 75, 757–773, doi: 10.1007/s00018-017-2664-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wu JW et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 19, 1085–1092, doi: 10.1038/nn.4328 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jha NK et al. Impact of Insulin Degrading Enzyme and Neprilysin in Alzheimer’s Disease Biology: Characterization of Putative Cognates for Therapeutic Applications. J Alzheimers Dis 48, 891–917, doi: 10.3233/JAD-150379 (2015). [DOI] [PubMed] [Google Scholar]
- 254.Baranello RJ et al. Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. Current Alzheimer research 12, 32–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ruan L et al. Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 543, 443–446, doi: 10.1038/nature21695 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Saido T & Leissring MA Proteolytic degradation of amyloid beta-protein. Cold Spring Harb Perspect Med 2, a006379, doi: 10.1101/cshperspect.a006379 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Miller JP et al. Matrix metalloproteinases are modifiers of huntingtin proteolysis and toxicity in Huntington’s disease. Neuron 67, 199–212, doi: 10.1016/j.neuron.2010.06.021 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Brkic M, Balusu S, Libert C & Vandenbroucke RE Friends or Foes: Matrix Metalloproteinases and Their Multifaceted Roles in Neurodegenerative Diseases. Mediators Inflamm 2015, 620581, doi: 10.1155/2015/620581 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kurochkin IV, Guarnera E & Berezovsky IN Insulin-Degrading Enzyme in the Fight against Alzheimer’s Disease. Trends Pharmacol Sci, 39, 49–58, doi: 10.1016/j.tips.2017.10.008 (2017). [DOI] [PubMed] [Google Scholar]
- 260.Maetzler W et al. Neprilysin activity in cerebrospinal fluid is associated with dementia and amyloid-beta42 levels in Lewy body disease. J Alzheimers Dis 22, 933–938, doi: 10.3233/JAD-2010-101197 (2010). [DOI] [PubMed] [Google Scholar]
- 261.Jacobsen JS et al. Enhanced clearance of Abeta in brain by sustaining the plasmin proteolysis cascade. Proc Natl Acad Sci U S A 105, 8754–8759, doi: 10.1073/pnas.0710823105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kim KS et al. Proteolytic cleavage of extracellular alpha-synuclein by plasmin: implications for Parkinson disease. J Biol Chem 287, 24862–24872, doi: 10.1074/jbc.M112.348128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Saito S & Ihara M New therapeutic approaches for Alzheimer’s disease and cerebral amyloid angiopathy. Front Aging Neurosci 6, 290, doi: 10.3389/fnagi.2014.00290 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Spencer B et al. Lentivirus mediated delivery of neurosin promotes clearance of wild-type alpha-synuclein and reduces the pathology in an alpha-synuclein model of LBD. Mol Ther 21, 31–41, doi: 10.1038/mt.2012.66 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 265.Tarasoff-Conway JM et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 11, 457–470, doi: 10.1038/nrneurol.2015.119 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Drouin-Ouellet J et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: Potential implications for its pathophysiology. Ann Neurol 78, 160–177, doi: 10.1002/ana.24406 (2015). [DOI] [PubMed] [Google Scholar]
- 267.Cabezas R et al. Astrocytic modulation of blood brain barrier: perspectives on Parkinson’s disease. Front Cell Neurosci 8, 211, doi: 10.3389/fncel.2014.00211 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shi M et al. CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimers Dement 12, 1125–1131, doi: 10.1016/j.jalz.2016.04.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sun BL et al. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog Neurobiol, 2017 Sep 10. pii: S0301–0082(17)30062-X. doi: 10.1016/j.pneurobio.2017.08.007 (2017). [DOI] [PubMed] [Google Scholar]
- 270.Zlokovic BV Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12, 723–738, doi: 10.1038/nrn3114 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kanekiyo T & Bu G The low-density lipoprotein receptor-related protein 1 and amyloid-beta clearance in Alzheimer’s disease. Front Aging Neurosci 6, 93, doi: 10.3389/fnagi.2014.00093 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ueno M et al. Blood-brain barrier and blood-cerebrospinal fluid barrier in normal and pathological conditions. Brain Tumor Pathol 33, 89–96, doi: 10.1007/s10014-016-0255-7 (2016). [DOI] [PubMed] [Google Scholar]
- 273.Bartels AL Blood-brain barrier P-glycoprotein function in neurodegenerative disease. Curr Pharm Des 17, 2771–2777 (2011). [DOI] [PubMed] [Google Scholar]
- 274.Xie L et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377, doi: 10.1126/science.1241224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Iliff JJ et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34, 16180–16193, doi: 10.1523/JNEUROSCI.3020-14.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lan YL et al. Aquaporin 4 in astrocytes is a target for therapy in Alzheimer’s disease. Curr Pharm Des, doi: 10.2174/1381612823666170714144844 (2017). [DOI] [PubMed] [Google Scholar]
- 277.Hoshi A et al. Expression of Aquaporin 1 and Aquaporin 4 in the Temporal Neocortex of Patients with Parkinson’s Disease. Brain Pathol 27, 160–168, doi: 10.1111/bpa.12369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Xu Z et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Abeta accumulation and memory deficits. Mol Neurodegener 10, 58, doi: 10.1186/s13024-015-0056-1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Jiang H et al. RBD and Neurodegenerative Diseases. Mol Neurobiol 54, 2997–3006, doi: 10.1007/s12035-016-9831-4 (2017). [DOI] [PubMed] [Google Scholar]
- 280.Yamamoto N et al. Epigallocatechin gallate induces extracellular degradation of amyloid beta-protein by increasing neprilysin secretion from astrocytes through activation of ERK and PI3K pathways. Neuroscience 362, 70–78, doi: 10.1016/j.neuroscience.2017.08.030 (2017). [DOI] [PubMed] [Google Scholar]
- 281.Van Kampen JM & Kay DG Progranulin gene delivery reduces plaque burden and synaptic atrophy in a mouse model of Alzheimer’s disease. PLoS One 12, e0182896, doi: 10.1371/journal.pone.0182896 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Bi Oh S, Suh N, Kim I & Lee JY Impacts of aging and amyloid-beta deposition on plasminogen activators and plasminogen activator inhibitor-1 in the Tg2576 mouse model of Alzheimer’s disease. Brain Res 1597, 159–167, doi: 10.1016/j.brainres.2014.11.042 (2015). [DOI] [PubMed] [Google Scholar]
- 283.Nalivaeva NN, Belyaev ND, Zhuravin IA & Turner AJ The Alzheimer’s amyloid-degrading peptidase, neprilysin: can we control it? Int J Alzheimers Dis 2012, 383796, doi: 10.1155/2012/383796 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Spampinato SF, Merlo S, Sano Y, Kanda T & Sortino MA Astrocytes contribute to Abeta-induced blood-brain barrier damage through activation of endothelial MMP9. J Neurochem 142, 464–477, doi: 10.1111/jnc.14068 (2017). [DOI] [PubMed] [Google Scholar]
- 285.Kingwell K Zeroing in on neurodegenerative alpha-synuclein. Nat Rev Drug Discov 16, 371–373, doi: 10.1038/nrd.2017.95 (2017). [DOI] [PubMed] [Google Scholar]
- 286.Wes PD, Sayed FA, Bard F & Gan L Targeting microglia for the treatment of Alzheimer’s Disease. Glia 64, 1710–1732, doi: 10.1002/glia.22988 (2016). [DOI] [PubMed] [Google Scholar]
- 287.Sevigny J et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 537, 50–56, doi: 10.1038/nature19323 (2016). [DOI] [PubMed] [Google Scholar]
- 288.Panza F et al. Tau-based therapeutics for Alzheimer’s disease: active and passive immunotherapy. Immunotherapy 8, 1119–1134, doi: 10.2217/imt-2016-0019 (2016). [DOI] [PubMed] [Google Scholar]
- 289.Schenk DB et al. First-in-human assessment of PRX002, an anti-alpha-synuclein monoclonal antibody, in healthy volunteers. Mov Disord 32, 211–218, doi: 10.1002/mds.26878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Shackleton B, Crawford F & Bachmeier C Inhibition of ADAM10 promotes the clearance of Abeta across the BBB by reducing LRP1 ectodomain shedding. Fluids Barriers CNS 13, 14, doi: 10.1186/s12987-016-0038-x (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Shinohara M et al. Reduction of brain beta-amyloid (Abeta) by fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFs) and Abeta clearance. J Biol Chem 285, 22091–22102, doi: 10.1074/jbc.M110.102277 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Qosa H, Abuznait AH, Hill RA & Kaddoumi A Enhanced brain amyloid-beta clearance by rifampicin and caffeine as a possible protective mechanism against Alzheimer’s disease. J Alzheimers Dis 31, 151–165, doi: 10.3233/JAD-2012-120319 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Umeda T et al. Rifampicin is a candidate preventive medicine against amyloid-beta and tau oligomers. Brain 139, 1568–1586, doi: 10.1093/brain/aww042 (2016). [DOI] [PubMed] [Google Scholar]
- 294.Wan W et al. Abeta(1–42) oligomer-induced leakage in an in vitro blood-brain barrier model is associated with up-regulation of RAGE and metalloproteinases, and down-regulation of tight junction scaffold proteins. J Neurochem 134, 382–393, doi: 10.1111/jnc.13122 (2015). [DOI] [PubMed] [Google Scholar]
- 295.Deane R et al. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest 122, 1377–1392, doi: 10.1172/JCI58642 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Burstein AH et al. Effect of TTP488 in patients with mild to moderate Alzheimer’s disease. BMC Neurol 14, 12, doi: 10.1186/1471-2377-14-12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zhao HF et al. Resveratrol decreases the insoluble Abeta1–42 level in hippocampus and protects the integrity of the blood-brain barrier in AD rats. Neuroscience 310, 641–649, doi: 10.1016/j.neuroscience.2015.10.006 (2015). [DOI] [PubMed] [Google Scholar]
- 298.Corona AW, Kodoma N, Casali BT & Landreth GE ABCA1 is Necessary for Bexarotene-Mediated Clearance of Soluble Amyloid Beta from the Hippocampus of APP/PS1 Mice. J Neuroimmune Pharmacol 11, 61–72, doi: 10.1007/s11481-015-9627-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Fan CH, Lin CY, Liu HL & Yeh CK Ultrasound targeted CNS gene delivery for Parkinson’s disease treatment. J Control Release 261, 246–262, doi: 10.1016/j.jconrel.2017.07.004 (2017). [DOI] [PubMed] [Google Scholar]
- 300.Burgess A, Huang Y, Querbes W, Sah DW & Hynynen K Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J Control Release 163, 125–129, doi: 10.1016/j.jconrel.2012.08.012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.McMahon D, Bendayan R & Hynynen K Acute effects of focused ultrasound-induced increases in blood-brain barrier permeability on rat microvascular transcriptome. Sci Rep 7, 45657, doi: 10.1038/srep45657 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Maki T et al. Phosphodiesterase III inhibitor promotes drainage of cerebrovascular beta-amyloid. Ann Clin Transl Neurol 1, 519–533, doi: 10.1002/acn3.79 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ihara M et al. Cilostazol add-on therapy in patients with mild dementia receiving donepezil: a retrospective study. PLoS One 9, e89516, doi: 10.1371/journal.pone.0089516 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Lundgaard I et al. Beneficial effects of low alcohol exposure, but adverse effects of high alcohol intake on glymphatic function. Sci Rep 8, 2246, doi: 10.1038/s41598-018-20424-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Jin WS et al. Peritoneal dialysis reduces amyloid-beta plasma levels in humans and attenuates Alzheimer-associated phenotypes in an APP/PS1 mouse model. Acta Neuropathol, 134, 207–220, doi: 10.1007/s00401-017-1721-y (2017). [DOI] [PubMed] [Google Scholar]
- 306.Domise M & Vingtdeux V AMPK in Neurodegenerative Diseases. EXS 107, 153–177, doi: 10.1007/978-3-319-43589-3_7 (2016). [DOI] [PubMed] [Google Scholar]
- 307.Millan MJ An epigenetic framework for neurodevelopmental disorders: from pathogenesis to potential therapy. Neuropharmacology 68, 2–82, doi: 10.1016/j.neuropharm.2012.11.015 (2013). [DOI] [PubMed] [Google Scholar]
- 308.Smith AJ & Verkman AS The “glymphatic” mechanism for solute clearance in Alzheimer’s disease: game changer or unproven speculation? FASEB J, 32, 543–551, doi: 10.1096/fj.201700999 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Gonzalez-Marrero I et al. Choroid plexus dysfunction impairs beta-amyloid clearance in a triple transgenic mouse model of Alzheimer’s disease. Front Cell Neurosci 9, 17, doi: 10.3389/fncel.2015.00017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Alvira-Botero X & Carro EM Clearance of amyloid-beta peptide across the choroid plexus in Alzheimer’s disease. Curr Aging Sci 3, 219–229 (2010). [DOI] [PubMed] [Google Scholar]
- 311.Jeromin A & Bowser R Biomarkers in Neurodegenerative Diseases. Adv Neurobiol 15, 491–528, doi: 10.1007/978-3-319-57193-5_20 (2017). [DOI] [PubMed] [Google Scholar]
- 312.Nguyen CTO et al. Retinal biomarkers provide “insight” into cortical pharmacology and disease. Pharmacol Ther 175, 151–177, doi: 10.1016/j.pharmthera.2017.02.009 (2017). [DOI] [PubMed] [Google Scholar]
- 313.Nascimento-Ferreira I et al. Beclin 1 mitigates motor and neuropathological deficits in genetic mouse models of Machado-Joseph disease. Brain 136, 2173–2188, doi: 10.1093/brain/awt144 (2013). [DOI] [PubMed] [Google Scholar]
- 314.Onofre I et al. Fibroblasts of Machado Joseph Disease patients reveal autophagy impairment. Sci Rep 6, 28220, doi: 10.1038/srep28220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Song P et al. Parkin promotes proteasomal degradation of p62: implication of selective vulnerability of neuronal cells in the pathogenesis of Parkinson’s disease. Protein Cell 7, 114–129, doi: 10.1007/s13238-015-0230-9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Martin-Jimenez R, Campanella M & Russell C New zebrafish models of neurodegeneration. Current Neurology Neuroscience Rep 15, 33, doi: 10.1007/s11910-015-0555-z (2015). [DOI] [PubMed] [Google Scholar]
- 317.Zhang Y et al. Rescue of Pink1 Deficiency by Stress-Dependent Activation of Autophagy. Cell Chem Biol 24, 471–480.e474, doi: 10.1016/j.chembiol.2017.03.005 (2017). [DOI] [PubMed] [Google Scholar]
- 318.Wang T, Lao U & Edgar BA TOR-mediated autophagy regulates cell death in Drosophila neurodegenerative disease. J Cell Biol 186, 703–711, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Hewitt VL & Whitworth AJ Mechanisms of Parkinson’s Disease: Lessons from Drosophila. Curr Top Dev Biol 121, 173–200, doi: 10.1016/bs.ctdb.2016.07.005 (2017). [DOI] [PubMed] [Google Scholar]
- 320.Miyake S, Takihara Y, Yokota S, Takamura Y & Inatani M Effect of Microtubule Disruption on Dynamics of Acidic Organelles in the Axons of Primary Cultured Retinal Ganglion Cells. Curr Eye Res, 43, 77–83, doi: 10.1080/02713683.2017.1370117 (2017). [DOI] [PubMed] [Google Scholar]
- 321.Fouillet A et al. ER stress inhibits neuronal death by promoting autophagy. Autophagy 8, 915–926, doi: 10.4161/auto.19716 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Palikaras K, Daskalaki I, Markaki M & Tavernarakis N Mitophagy and age-related pathologies: Development of new therapeutics by targeting mitochondrial turnover. Pharmacol Ther 178, 157–174, doi: 10.1016/j.pharmthera.2017.04.005 (2017). [DOI] [PubMed] [Google Scholar]
- 323.Martinez-Vicente M Neuronal Mitophagy in Neurodegenerative Diseases. Front Mol Neurosci 10, 64, doi: 10.3389/fnmol.2017.00064 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ashrafi G, Schlehe JS, LaVoie MJ & Schwarz TL Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol 206, 655–670, doi: 10.1083/jcb.201401070 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Du F et al. PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer’s disease. Brain 140, 3233–3251, doi: 10.1093/brain/awx258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Di Maio R et al. Alpha-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci Transl Med 8, 342ra378, doi: 10.1126/scitranslmed.aaf3634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Georgakopoulos ND, Wells G & Campanella M The pharmacological regulation of cellular mitophagy. Nat Chem Biol 13, 136–146, doi: 10.1038/nchembio.2287 (2017). [DOI] [PubMed] [Google Scholar]
- 328.Hertz NT et al. A neo-substrate that amplifies catalytic activity of parkinson’s-disease-related kinase PINK1. Cell 154, 737–747, doi: 10.1016/j.cell.2013.07.030 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hasson SA et al. Chemogenomic profiling of endogenous PARK2 expression using a genome-edited coincidence reporter. ACS Chem Biol 10, 1188–1197, doi: 10.1021/cb5010417 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Gersch M et al. Mechanism and regulation of the Lys6-selective deubiquitinase USP30. Nat Struct Mol Biol, 24, 920–930, doi: 10.1038/nsmb.3475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Dutta D et al. EphrinA2 regulates clathrin mediated KSHV endocytosis in fibroblast cells by coordinating integrin-associated signaling and c-Cbl directed polyubiquitination. PLoS pathogens 9, e1003510, doi: 10.1371/journal.ppat.1003510 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.East DA et al. PMI: a DeltaPsim independent pharmacological regulator of mitophagy. Chem Biol 21, 1585–1596, doi: 10.1016/j.chembiol.2014.09.019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Jang SY, Kang HT & Hwang ES Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J Biol Chem 287, 19304–19314, doi: 10.1074/jbc.M112.363747 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wu B et al. Succinate-induced neuronal mitochondrial fission and hexokinase II malfunction in ischemic stroke: Therapeutical effects of kaempferol. Biochim Biophys Acta, 1863, 2307–2318, doi: 10.1016/j.bbadis.2017.06.011 (2017). [DOI] [PubMed] [Google Scholar]
- 335.Ryu D et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med 22, 879–888, doi: 10.1038/nm.4132 (2016). [DOI] [PubMed] [Google Scholar]
- 336.Hylin MJ et al. A role for autophagy in long-term spatial memory formation in male rodents. J Neurosci Res 96, 416–426, doi: 10.1002/jnr.24121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Lee JH & Lee MJ Isolation and Characterization of RNA Aptamers against a Proteasome-Associated Deubiquitylating Enzyme UCH37. Chembiochem a European journal of chemical biology 18, 171–175, doi: 10.1002/cbic.201600515 (2017). [DOI] [PubMed] [Google Scholar]
- 338.Kim JH et al. Inhibitory RNA Aptamers of Tau Oligomerization and Their Neuroprotective Roles against Proteotoxic Stress. Molecular pharmaceutics 13, 2039–2048, doi: 10.1021/acs.molpharmaceut.6b00165 (2016). [DOI] [PubMed] [Google Scholar]
- 339.Tsukakoshi K, Abe K, Sode K & Ikebukuro K Selection of DNA aptamers that recognize alpha-synuclein oligomers using a competitive screening method. Analytical chemistry 84, 5542–5547, doi: 10.1021/ac300330g (2012). [DOI] [PubMed] [Google Scholar]
- 340.Wang H et al. MiR-124 Regulates Apoptosis and Autophagy Process in MPTP Model of Parkinson’s Disease by Targeting to Bim. Brain Pathol 26, 167–176, doi: 10.1111/bpa.12267 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Schoch KM & Miller TM Antisense Oligonucleotides: Translation from Mouse Models to Human Neurodegenerative Diseases. Neuron 94, 1056–1070, doi: 10.1016/j.neuron.2017.04.010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wurz RP et al. A “Click Chemistry Platform” for the Rapid Synthesis of Bispecific Molecules for Inducing Protein Degradation. J Med Chem, 61, 453–461, doi: 10.1021/acs.jmedchem.6b01781 (2017). [DOI] [PubMed] [Google Scholar]
- 343.Bourdenx M et al. Nanoparticles restore lysosomal acidification defects: Implications for Parkinson and other lysosomal-related diseases. Autophagy 12, 472–483, doi: 10.1080/15548627.2015.1136769 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Gambaryan PY, Kondrasheva IG, Severin ES, Guseva AA & Kamensky AA Increasing the Efficiency of Parkinson’s Disease Treatment Using a poly(lactic-co-glycolic acid) (PLGA) Based L-DOPA Delivery System. Exp Neurobiol 23, 246–252, doi: 10.5607/en.2014.23.3.246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Popp L & Segatori L Differential autophagic responses to nano-sized materials. Curr Opin Biotechnol 36, 129–136, doi: 10.1016/j.copbio.2015.08.016 (2015). [DOI] [PubMed] [Google Scholar]
- 346.Hernandez D et al. Regulation of presynaptic neurotransmission by macroautophagy. Neuron 74, 277–284, doi: 10.1016/j.neuron.2012.02.020 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Han Y, Khodr CE, Sapru MK, Pedapati J & Bohn MC A microRNA embedded AAV alpha-synuclein gene silencing vector for dopaminergic neurons. Brain Res 1386, 15–24, doi: 10.1016/j.brainres.2011.02.041 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Carpentier A et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med 8, 343re342, doi: 10.1126/scitranslmed.aaf6086 (2016). [DOI] [PubMed] [Google Scholar]
- 349.Jeon J, Kim W, Jang J, Isacson O & Seo H Gene therapy by proteasome activator, PA28gamma, improves motor coordination and proteasome function in Huntington’s disease YAC128 mice. Neuroscience 324, 20–28, doi: 10.1016/j.neuroscience.2016.02.054 (2016). [DOI] [PubMed] [Google Scholar]
- 350.Decressac M et al. TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proc Natl Acad Sci U S A 110, E1817–1826, doi: 10.1073/pnas.1305623110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Iaccarino HF et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540, 230–235, doi: 10.1038/nature20587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.