Abstract
Obesity is a major risk factor for the development of illnesses, such as insulin resistance and hypertension, and has become a serious public health problem. Mammals have developed a circadian clock located in the hypothalamic suprachiasmatic nuclei (SCN) that responds to the environmental light-dark cycle. Clocks similar to the one located in the SCN are found in peripheral tissues, such as the kidney, liver, and adipose tissue. The circadian clock regulates metabolism and energy homeostasis in peripheral tissues by mediating activity and/or expression of key metabolic enzymes and transport systems. Knockouts or mutations in clock genes that lead to disruption of cellular rhythmicity have provided evidence to the tight link between the circadian clock and metabolism. In addition, key proteins play a dual role in regulating the core clock mechanism, as well as adipose tissue metabolism, and link circadian rhythms with lipogenesis and lipolysis. Adipose tissues are distinguished as white, brown, and beige (or brite), each with unique metabolic characteristics. Recently, the role of the circadian clock in regulating the differentiation into the different adipose tissues has been investigated. In this review, the role of clock proteins and the downstream signaling pathways in white, brown, and brite adipose tissue function and differentiation will be reviewed. In addition, chronodisruption and metabolic disorders and clinical aspects of circadian adiposity will be addressed.
The role of the circadian clock in regulating adipose tissue function and differentiation and key proteins that play a dual role in regulating clock mechanism and adipose tissue metabolism are addressed.
Essential Points
The circadian clock controls energy homeostasis by regulating circadian expression and/or activity of enzymes, hormones, and transport systems involved in metabolism
Disruption of circadian rhythms leads to obesity and metabolic disorders
The circadian clock regulates the function of and differentiation into white and brown adipose tissue
Key proteins play a dual role in regulating the core clock mechanism as well as adipose tissue metabolism, and link circadian rhythms with lipogenesis, lipolysis, and differentiation
Circadian Rhythms and the Circadian Clock
Organisms on our planet developed an endogenous circadian clock, entrained, or synchronized, by light to exactly 24 hours (1). By the prediction of the day-night cycles, the clock imparts a survival advantage as physiological processes are performed at the appropriate time to find food or mating partners or to avoid predators (1–3). The clock is self-sustained but, in the absence of light, the endogenous clock free-runs, generating cycles of either slightly longer or slightly shorter than 24 hours; hence the term circadian (circadian: circa = about, dies = day). The circadian clock controls numerous cellular, physiological, metabolic, endocrine, and behavioral systems (4, 5). Disruption of this control results in fatigue, disorientation, insomnia, altered hormone profile, and high morbidity, seriously influencing overall health (4, 6–12). Obesity, which is characterized by the excess of fat accumulation in white adipose tissue (WAT), has been related to irregular sleep/wake schedules, high snacking frequency, or social “jet lag,” known to disrupt the circadian clock (13, 14). Bright light at night has also been associated with obesity, as was shown in 100,000 women of the Breakthrough Generations Study (15). All of these studies as a whole strongly suggest that impairment of the circadian system is involved in the etiology of several illnesses (16).
The central circadian clock is located in the anterior hypothalamus and is confined to the suprachiasmatic nuclei (SCN). Photic information perceived by the retina and transmitted via the retinohypothalamic tract synchronizes SCN neurons to coordinate circadian outputs (17–20). Clocks, similar to those found in SCN neurons, are found in peripheral tissues, such as the liver, intestine, heart, adipose tissue, and retina, and in various brain regions (2, 21–23). Five percent to 20% of tissue-specific genes are estimated to exhibit an oscillatory expression profile, emphasizing the circadian control over the function of peripheral tissues (23–32). The SCN clock regulates peripheral rhythms by neuronal connections or secretion of humoral factors (33–35) or indirectly, by driving rhythmic feeding, locomotor activity, and/or body temperature, which in turn, coordinate rhythmic gene expression.
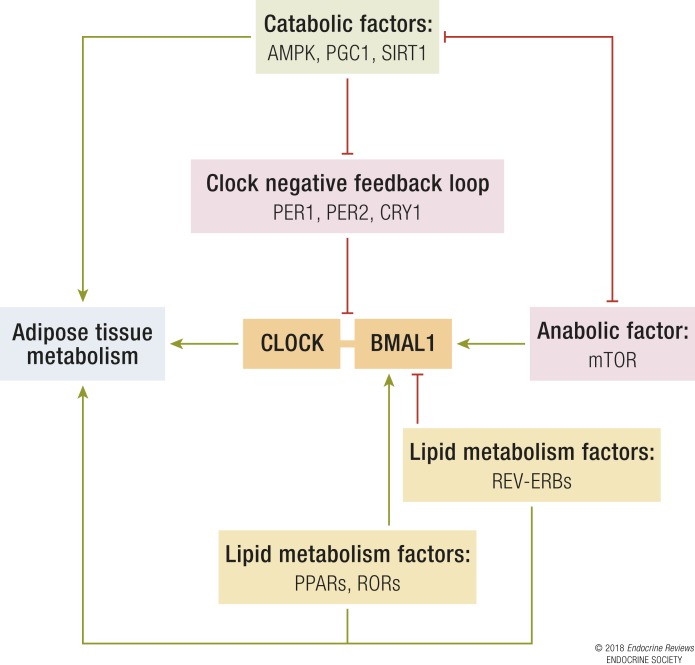
The molecular clock in SCN neurons and peripheral cells is an intracellular mechanism composed of transcription-translation feedback loops (3). The transcription factor circadian locomotor output cycles kaput (CLOCK) dimerizes with brain and muscle aryl hydrocarbon receptor nuclear translocator–like protein 1 (BMAL1), and together, the CLOCK:BMAL1 heterodimer activates transcription by binding to E-box (5′-CACGTG-3′) and E-box-like promoter sequences (2). Among the regulatory targets of CLOCK:BMAL1 are the Period1 and 2 (Per1, Per2) and Cryptochrome1 and 2 (Cry1 and Cry2) genes. Oligomerization and nuclear translocation of periods/cryptochromes (PERs:CRYs) lead to the inhibition of CLOCK:BMAL1-mediated transcription (2, 36) (Fig. 1). The nuclear receptor reverse ERB (REV-ERB) α negatively regulates Bmal1 expression (37), whereas retinoic acid receptor–related orphan receptor (ROR) α (RORα) and RORγ (38) (Fig. 1) positively regulate its expression via ROR response elements (39). All clock genes exhibit 24 hour oscillation in peripheral tissues (40).
Figure 1.
The relationship between the core clock mechanism and metabolic factors. CLOCK and BMAL1 mediate the expression of clock- and clock-controlled genes regulating metabolism. PER1, PER2, and CRY1 serve as the negative-feedback loop that inhibits CLOCK:BMAL1-mediated expression. The catabolic factors SIRT1, PGC1, and AMPK, when activated under low cellular energy levels, relieve the inhibition mediated by the negative-feedback loop. BMAL1 expression is positively regulated by RORs and PPARs and negatively regulated by reverse ERBs (REV-ERBs). mTOR, an anabolic factor, interacts with BMAL1 and suppresses the activity of the catabolic factors. The green arrows and red lines denote possible pathways that activate or inhibit adipocyte metabolism, respectively.
White, Brown, and Beige (or Brite) Adipose Tissue
Classically, adipose tissue has been regarded as a passive reservoir for energy storage, but this traditional point of view is no longer valid. In 1987, adipose tissue was identified as the major site for metabolism of sex steroids (41). Several years later, in 1994, the discovery of the cytokine-like factor leptin (42) entirely changed our perspectives on adipose tissue. This was a revolution in the study of obesity, as for the first time, it was shown that adipose tissue was able to secrete hormones or “adipocytokines,” capable of communicating information from the periphery to the central nervous system. Adipose tissue was no longer considered a passive reservoir of energy; it was an endocrine organ. Adipocytokines or adipokines, such as adiponectin, resistin, tumor necrosis factor α, visfatin, and obestatin, have autoendocrine, paracrine, and endocrine functions. Thus, adipose tissue regulates energy storage and expenditure and influences systemic metabolic homeostasis through production of adipokines (43).
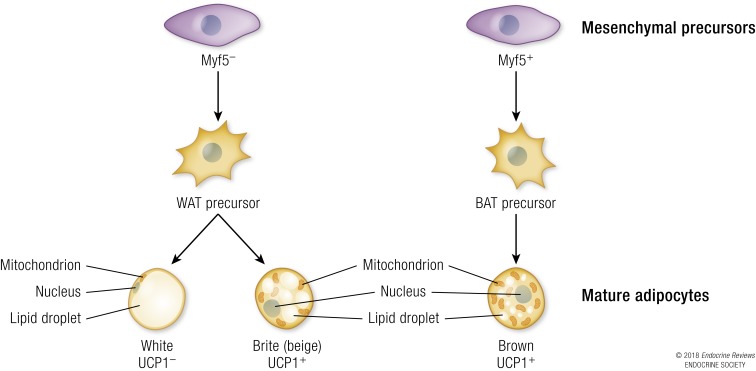
In mammals, at least two types of adipose tissue have been described, WAT and the brown adipose tissue (BAT). WAT and BAT originate from different stem cells, and they have different distribution, gene expression, and function (44) (Fig. 2). Adipocytes with features similar to BAT, found within white adipose depots, are termed beige or brite (brown in white) adipose tissue (44). Nowadays, it is known that the development of obesity depends not only on the balance between food intake and energy expenditure but also on the balance among WAT, BAT, and brite adipose tissue.
Figure 2.
Lineage of white, brown, and “brite/beige” adipocytes from mesenchymal stem cells and their cellular and molecular markers. Myf5, myogenic factor 5.
WAT
This is the most abundant type of adipose tissue in mammals—and the main energy reservoir. It stores dietary energy as triacylglycerides (TAGs) in unilocular lipid droplets and secretes a huge number of hormones and cytokines that regulate metabolism and insulin resistance (43, 45). After meals, WAT uptakes dietary fats and carbohydrates from the circulation and, through lipogenesis, converts them to TAGs (46). During fasting or physical activity, lipolysis ensues, and TAGs are broken down into free fatty acids (FFAs) and glycerol that are released to the circulation to supply the needs of other tissues (46).
BAT
This tissue contains multilocular lipid droplets and high mitochondrial density and is able to convert chemical energy into heat (47). Cold stimulation leads to the release of norepinephrine at innervated sites within the BAT, which leads to lipolysis of TAGs and an increase in cellular FFAs. FFAs are subsequently transported to the mitochondria and serve as substrates for β-oxidation. Heat is generated using uncoupling protein 1 (UCP1), a protein that is localized on the inner membrane of mitochondria and uncouples electron transport from adenosine triphosphate production (47). The resulting energy derived from substrate oxidation is dissipated as heat. In addition to its key role in maintaining body temperature under cold stress, the metabolic function of BAT is important for global energy balance, insulin sensitivity, and lipid metabolism (48–50). Developmentally, BAT is typified by the activation of myogenic factor 5 and early paired box 7 expression (51, 52), which encode two transcription factors that mark myogenic precursor cells. Late paired box 7 expression marks cells that are predominantly restricted to the skeletal muscle lineage (52, 53).
Brite (beige) adipose tissue
Unlike WAT, which is characterized by the presence of few mitochondria that are devoid of UCP1, beige adipocytes, found within WAT, express UCP1 and are mitochondrial rich. These adipocytes are distinct from BAT but still have a thermogenic capacity by responding to cold exposure to dissipate heat through UCP1 (54).
The Circadian Clock and Metabolism
The robust and coordinated expression of clock genes in peripheral tissues, such as liver and adipose tissue, mediates the activity of nuclear receptors, enzymes, hormones, and transporters involved in carbohydrate, lipid, and protein metabolism (21, 30, 55–61). In turn, animal studies have shown that a high-fat diet may contribute to the development of obesity and insulin resistance via alterations in the circadian period of locomotor activity rhythms and changes in the oscillation of clock genes in adipose and liver tissue (62–65). In humans, results suggest that the modulation of the dietary fat and carbohydrate content alters the function of the central and peripheral circadian clocks in humans (66). The mutual influence of the clock and metabolic regulation is achieved as a result of the participation of key catabolic, anabolic, and lipid metabolic factors in the core clock mechanism (Fig. 1).
REV-ERBs and RORs
Two important families that link the core clock mechanism with lipid metabolism are REV-ERBs and RORs. These factors are vital for adipocyte differentiation (67), lipogenesis, and lipid storage and exhibit striking circadian rhythms (68, 69). In addition to their metabolic role, REV-ERBs are the negative and RORs the positive regulators of Bmal1 expression (37, 38, 70, 71) (Fig. 1). In turn, the CLOCK:BMAL1 heterodimer regulates the expression of Rev-erbs and Rors (37, 38, 72). Diet-induced obese mice, treated with a REV-ERB agonist, showed reduced fat mass and improved dyslipidemia and hyperglycemia (73). In addition, a REV-ERB agonist suppressed mouse orexinergic gene expression, whereas REV-ERBβ-deficient mice had increased orexinergic transcripts (74). In this line, mice deficient of Rev-erbα develop higher adiposity on regular chow and a high-fat diet, supposedly as a result of increased fat uptake by adipose tissue (75). Nevertheless, in BAT, Rev-erbα seems to have an opposite role on adiposity, as deletion of Rev-erbα markedly improved the thermogenic response to cold, and physiological induction of UCP1 by cold temperatures is preceded by rapid downregulation of Rev-erbα (76). As REV-ERBs negatively regulate Bmal1 expression, the role of BMAL1 in WAT and BAT function merits further study.
Peroxisome proliferator–activated receptors
Peroxisome proliferator–activated receptors (PPARs) are a nuclear receptor family consisting of three isoforms in mammals (α, β/δ, and γ), which are differentially expressed among tissues (77). PPARs play a key role in the transcription of genes involved in lipid and glucose metabolism upon binding of endogenous FFAs (78, 79). PPARα is abundant in the liver, BAT, heart, and kidney, whereas PPARγ is expressed in the adipose tissue and PPARβ/δ throughout the body (80). PPARα agonists, such as fibrates, are clinically proven lipid-lowering drugs, whereas PPARγ ligands, such as thiazolidinediones, improve glycemic control via insulin sensitization in patients with type 2 diabetes (81). PPARs are connected to the core clock mechanism, as their expression is mediated by the CLOCK:BMAL heterodimer. In turn, PPARα activates Bmal1 expression (Fig. 1) by binding to the peroxisome-proliferator response element (PPRE) (82–84). The role of PPARs in the circadian regulation has been demonstrated through experiments performed with PPARα agonists that showed advanced locomotor activity and feeding daily rhythms in mice (85), whereas experiments in PPARγ deletion showed dampened behavioral and cellular circadian rhythms (86).
PPARγ coactivator 1
The PPARγ coactivator 1 (PGC1) is a family of transcriptional coactivators that induce mitochondrial oxidative metabolism. Indeed, PGC-1α is a catabolic factor whose expression is induced in the liver during starvation, in BAT during cold exposure, and in skeletal muscle during physical exercise (87). PGC-1α null mice show abnormal diurnal rhythms of body temperature, activity, and metabolic rate, as a result of disrupted clock and metabolic gene expression (88). Moreover, PGC1 family members exhibit circadian expression, and in turn, PGC1α stimulates the expression of Bmal1, Clock, Per2, and Rev-erbα through coactivation of the ROR family (88, 89).
Adenosine 5′-monophosphate–activated protein kinase
Adenosine 5′-monophosphate–activated protein kinase (AMPK) is a sensor of the energy status within cells whose phosphorylation activates cellular catabolism (90, 91). When activated, AMPK phosphorylates and hence, activates casein kinase I ε, which leads to PER degradation (92). AMPK also phosphorylates and as a result, destabilizes CRY1 in mouse fibroblasts (93) (Fig. 1). PER and CRY degradation relieves CLOCK:BMAL1 inhibition, leading to a phase advance in the circadian expression in some tissues (63). Indeed, metformin, an indirect AMPK activator, leads to alterations of the circadian phase in a tissue-specific manner (94).
Sirtuin 1
Sirtuin 1 (SIRT1), a nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase, is a catabolic factor involved in transcriptional silencing (95, 96). SIRT1 is connected to the core clock mechanism by interacting with CLOCK to deacetylate BMAL1 and PER2 (97, 98). Deacetylated PER2 is further phosphorylated and degraded, relieving CLOCK:BMAL1 inhibition (Fig. 1). Hepatic circadian transcriptome analyses revealed that differently from SIRT1, SIRT6 interacts with CLOCK:BMAL1 and controls their recruitment to gene promoters. In addition, SIRT6 controls circadian chromatin recruitment of sterol regulatory element-binding protein 1, resulting in the cyclic regulation of genes involved in fatty acid and cholesterol metabolism (99). It turns out that the CLOCK:BMAL1 heterodimer, as well as SIRT1, regulate the circadian expression of nicotinamide phosphoribosyltransferase, a rate-limiting enzyme in the NAD+ salvage pathway, therefore enabling circadian synthesis of the coenzyme required for SIRT1 activity (100, 101). SIRT1 activity is also enhanced by AMPK, which increases cellular NAD+ levels (102). High levels of NAD+ inhibit DNA binding of CLOCK:BMAL1 (103).
Mammalian target of rapamycin
Mammalian target of rapamycin (mTOR) is an anabolic factor involved in protein synthesis; integrates the input from multiple upstream pathways, including insulin, growth factors, and mitogens; and functions as a sensor of cellular nutrient and energy levels (104). The mTOR pathway is regulated by light in the SCN (105). One of the key factors in the mTOR pathway, protein 70 S6 kinase 1, rhythmically phosphorylates BMAL1, allowing it to both associate with the translational machinery and stimulate circadian oscillations of protein synthesis (106) (Fig. 1). Thus, the mTOR signaling pathway, in addition to its role in protein translation, is linked to the circadian clock mechanism.
Chronodisruption and Metabolic Disorders
Chronodisruption is defined as the chronic desynchronization of the 24-hour rhythms, resulting in adverse health effects (107). More specifically, chronodisruption occurs when the synchronization between external environmental cues and internal physiological processes is lost. This can either result in a total loss of rhythmicity, a reduction in rhythm amplitudes, or phase differences between the SCN and peripheral clocks (108). The inter-relations between the key metabolic factors mentioned previously and the core clock mechanism may explain why metabolic chronodisruption is detrimental.
Clock gene mutants and knockouts demonstrate the most compelling linkage between metabolic disorders and the circadian clock (see Table 1). Mice with a mutated Clock gene (ClockΔ19 mice) have a dampened diurnal feeding rhythm and are obese, and they develop a metabolic syndrome of hyperleptinemia, hyperlipidemia, hepatic steatosis, and hyperglycemia (109). A combination of the ClockΔ19 mutation with the leptin knockout (ob/ob) leads to significantly heavier mice than the ob/ob phenotype (110), reiterating the contribution of clock disruption to the obese phenotype (55, 57, 111). Similarly, compared with wild-type mice, Per2−/− mice fed a high-fat diet developed substantial obesity (112).
Table 1.
Clock Gene Mutations in Mice and Genetic Variants in Humans and Their Effect on Metabolism
| Clock Gene | Mutant Mice | Metabolic Alterations in Mutant Mice | Human Genetic Variant | Metabolic Alterations in Humans |
|---|---|---|---|---|
| CLOCK | ClockΔ 19 (109) | Hyperphagic and obese, develop a metabolic syndrome of hyperleptinemia, hyperlipidemia, hepatic steatosis, and hyperglycemia | CLOCK SNPs [rs3749474, rs4580704, and rs1801260 (3111 T > C)] (123–129) | Obesity, metabolic syndrome, higher energy and fat intake, higher risk of developing diabetes and hypertension, alterations in the autonomic nervous system |
| BMAL1 | Bmal1−/− (113) | Suppressed diurnal variations in glucose and triglycerides and abolished gluconeogenesis | BMAL2 rs7958822 (131) | High risk of type 2 diabetes development in obese patients |
| CRYs | Cry1/2(−/−) (116–120) | Increased insulin secretion and lipid storage in adipose tissue, elevation of proinflammatory cytokines | CRY1 rs2287161 (135) | Increased insulin resistance in carriers of the risk variant who have high intake of carbohydrates |
| PER2 | Per2−/− (112) | On high-fat diet, eat as much during the light period as the dark period, and develop substantial obesity | PER2 SNPs (rs2304672 C > G and rs4663302C > T) (130) | Abdominal obesity, several obesogenic behaviors |
| REV-ERBα | Rev-erbα−/− (133) | Reduced spontaneous locomotor activity | REV-ERBα rs2314339 (132–134) | Obesity resulting from decreased physical activity |
Bmal1−/− knockout mice exhibit suppressed diurnal changes in triglycerides and glucose levels, as well as no gluconeogenesis (113). Hyperinsulinemic-euglycemic clamps showed that Bmal1−/− mice exhibit no circadian rhythm in insulin action (114). Adipocyte-specific deletion of Bmal1 resulted in obesity in mice. As adipocytes signal the levels of stored energy to the brain, Bmal1 deletion led to changes in the expression of hypothalamic neuropeptides that regulate appetite, emphasizing the role of the adipocyte clock in the temporal organization of energy regulation (115).
In mouse studies, circadian changes in hepatic Cry1/2 expression were sufficient to modulate gluconeogenesis (116), emphasizing the important role that CRY proteins play in regulating hepatic glucose output (117). In addition, Cry1/2−/− mice had an increased vulnerability to high-fat diet–induced obesity that correlated with increased insulin secretion and lipid storage in adipose tissue (118, 119) and constitutive elevation of proinflammatory cytokines (120). SCN mutant mice showed a substantial number of rhythmic transcripts (121). Thus, although it is known that the SCN clock regulates lipolytic and lipogenic processes (122), recent findings in tissue-specific knockouts indicate that peripheral clocks play a crucial role in regulation of whole-body energy homeostasis.
Consistent with these findings, genetic polymorphisms in human clock genes have been associated with metabolic alteration (Table 1). Although mutations are rare in humans, it is rather common to have a single nucleotide polymorphism (SNP) that underlies differences in our vulnerability to disease.
CLOCK
It was first demonstrated that several variants at CLOCK were associated with obesity, especially abdominal obesity (123). Later that year, it was confirmed that CLOCK could play a relevant role in the development of the metabolic syndrome, type 2 diabetes, and cardiovascular disease (124). Subsequently, we demonstrated that several genetic variants in clock genes were related to obesity and related diseases, such as metabolic syndrome. For example, CLOCK SNPs [rs3749474, rs4580704, and rs1801260 (3111 T > C)] were associated with obesity and insulin resistance-related variables (125). Systolic and diastolic blood pressure values were also strongly associated with SNP rs4580704 (125). Furthermore, these genetic variants were associated with energy intake (126). Minor alleles ate more, specifically fat, and were more obese, whereas minor allele carriers (A) of CLOCK rs4580704 showed decreased risk of developing diabetes (31% lower) and hypertension (46% lower) than noncarriers (125). Some of these associations were functionally explained by the presence of a polymorphism involving a change in the structure of the mRNA leading to a change in gene expression (127). In addition, people carrying CLOCK 3111 C (risk C carriers: including TC and CC) are more likely to be obese and exhibit greater difficulties in losing weight. Further studies performed to understand the connections between CLOCK rs1801260 (3111 T > C) and resistance to weight loss showed that risk carriers (C) displayed substantial abnormalities at the daily rhythms of wrist temperature and activity. These abnormalities included the following: (1) lower amplitude, (2) greater rhythm fragmentation, (3) less stable patterns, and (4) significantly decreased circadian function. C carriers were also less active, started their activities later in the morning, and were sleepier during the day, showing a delayed acrophase (maximum expression) that characterizes “evening-type” subjects (128). Moreover, C carriers have higher parasympathetic activity during daytime (when it is supposed to be low) than TT carriers and reduced daily rhythms of autonomic nervous system function (129). These changes correlated with weight-loss resistance, as low sympathetic activity and high parasympathetic activity reduce energy expenditure and predispose onset of obesity (Mona Lisa theory) (136).
PER2
PER2 SNPs (rs2304672 C > G and rs4663302 C > T) have been associated with abdominal obesity (130). In particular, PER2 rs2304672 C > G minor allele carriers G (6% of the population) displayed several obesogenic behaviors, such as an increased attrition of the weight-loss treatment, increased frequency of snacking, stress while dieting, eating while bored, and skipping breakfast, when compared with noncarriers (130).
BMAL2
BMAL2 SNPs have also been associated with a high risk of developing type 2 diabetes in obese patients. The AG and AA genotypes of BMAL2 rs7958822 showed significantly higher odds ratios for type 2 diabetes than the GG genotype among obese men and women. There were no substantial associations between BMAL1 rs11022775 or rs2290035 genotypes and type 2 diabetes (131).
REV-ERBα
REVERBα rs2314339 has been associated with obesity in two independent populations: Mediterranean and North American. However, as opposed to other clock gene variants, which are associated with obesity through changes in dietary intake, REVERBα rs2314339 is associated with obesity through a decrease in physical activity (132). These findings were consistent with those obtained in experimental animals showing that Rev-erbα−/− mice showed a significantly lower spontaneous locomotor activity than their wild-type littermates (133). However, mice lacking Rev-erbα displayed marked hyperactivity and impaired response habituation when introduced to a new environment (134).
CRY1
Genetic variants at CRY1 have been connected to glucose metabolism. Meta-analysis performed in two independent populations (Mediterranean and European-origin North American) indicated that an increase in carbohydrate intake was associated with a substantial increase in homeostasis model assessment of insulin resistance, fasting insulin, and a decrease in quantitative insulin sensitivity check index, only among individuals homozygous for the minor C allele at CRY1 rs2287161. These findings support the notion that there is a strong link between the circadian system and glucose metabolism and suggest the importance of this CRY1 locus for insulin resistance and diabetes risk (135).
Meal Timing, Chronodisruption, and Metabolic Alterations
Several behaviors also help to explain the connections between chronodisruption and metabolic alterations. Meal timing, such as restricting food to a particular time of day, defined as time-restricting feeding, has profound effects on the behavior and physiology of animals. Two to 4 hours before the meal, the animals display food anticipatory behavior, which is demonstrated by an increase in locomotor activity, body temperature, corticosterone secretion, gastrointestinal motility, and activity of digestive enzymes (137–140), all known output systems of the biological clock. Moreover, the changing of the time of food intake to unusual times, as happens when nocturnal mice are fed a high-fat diet, only during the 12-hour sleep (light) phase, is associated with an increase in weight gain (141). In humans, unusual feeding time produces a disruption of the circadian system, which might produce unhealthy consequences (142). The timing of food intake may have an important role in obesity (141), as well as in weight loss and glucose metabolism (143). For example, mice with adipocyte-specific deletion of Bmal1 fed a high-fat diet during the light period gained significantly more weight compared with mice fed during the dark period. Thus, disruption of the adipocyte clock leads to obesity without an overall increase in daily caloric intake when mice are fed during the inactive phase (115). In humans, late eating has been shown to be predictive of weight loss difficulties during a 20-week dietary intervention conducted in 420 obese and overweight individuals (144). The effect was independent of the total 24-hour caloric intake (144). In addition, insulin sensitivity was lower in late eaters compared with early eaters. Moreover, a 12-week clinical study showed that those subjects assigned to high caloric intake during dinner lost significantly less weight than those assigned to high caloric intake during breakfast. Interestingly, there were no substantial differences in the total amount of food or in energy expenditure, as assayed by a physical activity questionnaire (145).
The physiological explanation for the metabolic alterations related to late eating is that when feeding patterns are changed, for example, during shift work in humans or by means of restricted access to chow in rodents, this has profound effects on peripheral oscillator regulation, whereas SCN rhythms remain largely unaltered (146, 147). Indeed, in animal models, it has been demonstrated that temporal feeding restriction can change the phase of circadian gene expression in peripheral cell types by up to 12 hours, while leaving the phase of cyclic gene expression in the SCN unaffected (146). Likewise, a recent in-laboratory study has demonstrated that a 5-hour delay in meals leads to a 1.5-hour delay in the circadian rhythm of the clock gene PER2 in adipose tissue, whereas no significant changes were found in the central clock (148).
Circadian Regulation of Adipose Tissue in Health and Disease
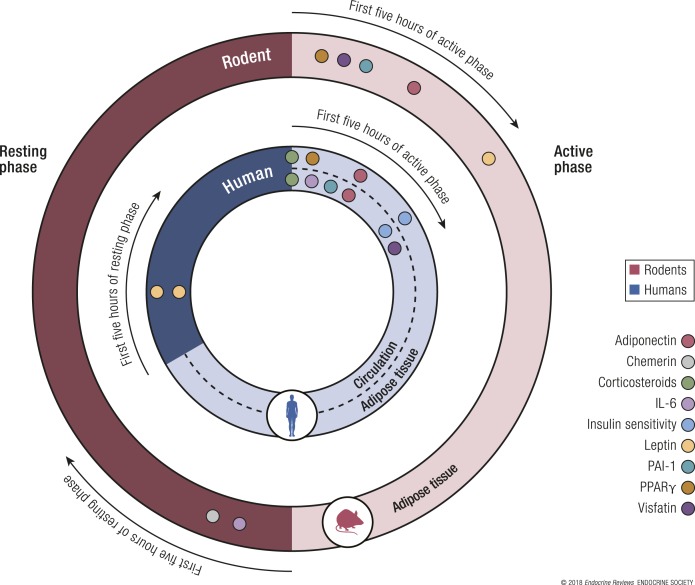
As all peripheral tissues, the adipocyte clock mechanism regulates cellular functions (30). More importantly, these circadian rhythms persist in the absence of the SCN, for example, in adipose tissue explant cultures (149). To ensure proper nutrient/energy flux and substrate use by the organism, the circulating levels of secreted adipokines, such as leptin and adiponectin—key players in the regulation of energy metabolism—display an oscillatory profile with a 24-hour period (122) (Fig. 3). Indeed, it was shown that BAT glucose uptake in mice and humans reveals a diurnal rhythm that could, in part, be generated by signals from plasma hormones (150, 151). Alterations of this timing may have important detrimental, metabolic consequences. Interestingly, the circadian rhythms of adipokine expression/secretion differ, depending on the location of the adipose tissue, as substantial differences were found both in their relative acrophase and amplitude between subcutaneous and visceral adipose tissues (152). Analyses of various genes implicated in metabolic processes, such as energy intake and expenditure, insulin resistance, adipocyte differentiation, dyslipidemia, and body fat distribution, indicated that circadian rhythmicity followed a predictable physiological pattern, particularly for subcutaneous depots (25, 152). Visceral depots also show a higher amplitude for all glucocorticoid-related genes, and (152) it secretes more proinflammatory factors than subcutaneous adipose tissue (153). However, induction of lipolysis is easier in visceral fat depots, as a result of the increased sensitivity to catecholamines (154).
Figure 3.
Temporal expression of adipocytokines in rodents and humans. The peak expression of each adipokine is depicted. Representation of the acrophase or timing of the maximum levels of adipokine expression in human adipose tissue: comparison with human circulating levels and with rodent adipose tissue mRNA levels. Data are represented as the 5 hours of active and resting phase in both humans and rodents, as this is the timing when most adipokines peak. IL-6, interleukin 6; PAI-1, plasminogen activator inhibitor 1; PPAR, peroxisome proliferator–activated receptor.
Leptin
The adipocyte-derived leptin, which acts at specific receptors in the hypothalamus to suppress appetite and increase metabolism, is extremely important in obesity. Plasma leptin levels are circadian, with leptin peaking early in the nonactive phase, i.e., during the early dark phase in diurnal animals, such as monkeys and humans (155, 156), and during the early-to-midlight phase in nocturnal animals, such as rats and mice (157, 158) (Fig. 3). SCN ablation was shown to eliminate leptin circadian rhythmicity in rodents, suggesting that the central circadian clock regulates leptin expression (157). In obese subjects, leptin retains diurnal variation in release but with lower amplitude (159), suggesting that blunted circadian variation may play a role in leptin resistance and obesity (160). If exogenous leptin was continuously applied, then desynchronized feeding during the light phase failed to cause excessive weight gain in ob/ob mice, but if it was applied in a timed pulse during the light phase, then obesity ensued (161). Thus, desynchronized feeding can affect the endogenous rhythm of leptin to influence metabolic signals that lead to weight gain.
Adiponectin
In human adipose tissue, the expression of adiponectin achieved its zenith (maximum) during the morning, which could be implicated in the maximal withdrawal of fatty acids and the improvement in glucose tolerance at that time of the day (162). As adiponectin promotes insulin sensitivity, its acrophase should precede insulin sensitivity, which reaches its maximum level at noon (152, 163, 164). PPARγ, which participates both in the clock mechanism (Fig. 1) and adiponectin expression, could also be related to adiponectin circadian expression. In fact, the high expression of PPARγ during the morning is consistent with results obtained in nocturnal mammals (56) and could be influencing the further increase in adiponectin expression and the increase in insulin sensitivity during this time of the day.
Corticosteroids
It has been described that in all species, the maximum of circulating corticosteroid rhythms occurs just before or at the onset of activity (165, 166). Over the course of the day, they fall, reaching low or undetectable levels 1 hour or 2 hours before bedtime. In human adipose tissue, the antiphase relationship between leptin and glucocorticoids receptors (Fig. 3) (152) is reasonable, considering that both hormones are strongly inter-related and they exert opposite functions in food-intake regulation. Whereas leptin displays an anorexigenic role, glucocorticoids increase appetite (orexigenic function). Indeed, plasma leptin ultradian pulses were inversely correlated with those of adrenocorticotropic hormone and cortisol (164).
Insulin
Adipose tissue lipogenesis is highly regulated by insulin. Intake of sugars, such as glucose, leads to elevated insulin concentrations in the blood, which in turn, increases glucose uptake by upregulating glucose transporter 4 expression on adipocyte membranes (167). This process increases the substrate for glycolysis and consequently, fatty acid biosynthesis in adipocytes (168). As mentioned previously, insulin sensitivity varies according to time of day, with decreased values in the evening and at night in humans. Indeed, it was demonstrated that insulin sensitivity in human subcutaneous adipose tissue displays a circadian rhythm and that it reaches its maximum (acrophase) around noon, with sensitivity 54% higher than during midnight (Fig. 3). In contrast, no circadian rhythms in insulin sensitivity were detected in visceral adipose tissue (163). Mechanisms responsible for the diurnal variation in insulin sensitivity warrant further study.
Role of the Circadian Clock in Adipose Tissue Differentiation
Currently, it is known that key clock proteins not only regulate the “time-specific” functions in adipose tissue, but they also play a direct role in adipogenesis in both WAT and BAT. However, some of the reported results are still contradictory.
WAT adipogenesis
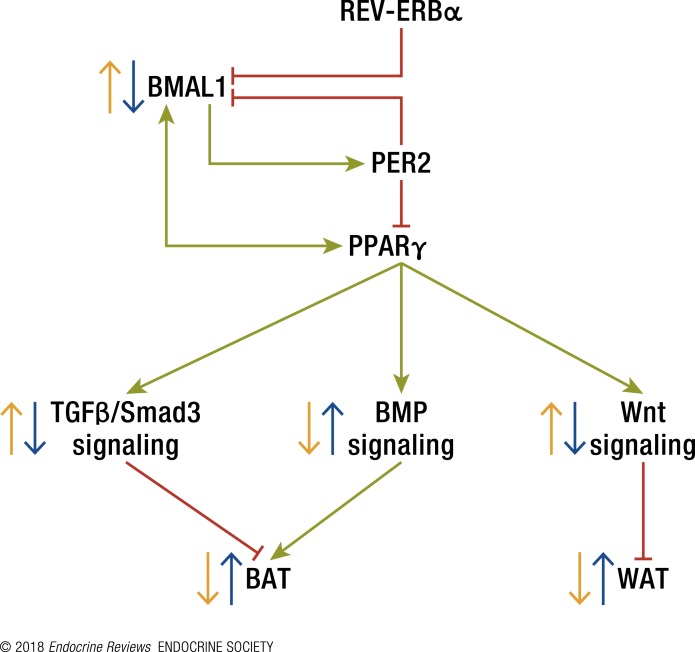
PER2, a negative regulator of BMAL1 expression, was found to interact with PPARγ and repress its proadipogenic activity (169) (Fig. 4). Indeed, PER2-deficient mice display an altered lipid metabolism with drastic reduction of total TAG and nonesterified fatty acids. PER2 exerts its inhibitory function by blocking PPARγ recruitment to target promoters and thereby inhibiting transcriptional activation (169). As mentioned previously, PPARγ expression is positively regulated by the CLOCK:BMAL1 heterodimer, and it positively regulates Bmal1 gene expression. Indeed, as mentioned previously, an agonist for REV-ERB, which negatively regulates Bmal1 expression, suppressed mouse orexinergic gene expression, whereas REV-ERBβ-deficient mice had increased orexinergic transcripts (74). In addition, cell-based studies demonstrate that activation of REV-ERBα with either synthetic or natural (heme) ligands is required for adipocyte differentiation (158). Thus, PPARγ repression by PER2 and activation of REV-ERB, which lead to BMAL1 downregulation, would be expected to attenuate adipogenesis. However, surprisingly, knockdown of BMAL1 led to increased adipogenesis, adipocyte hypertrophy, and obesity in mice and promoted adipogenic differentiation in preadipocyte and mesenchymal stem cells (115, 170, 171) (Fig. 4). This was achieved by downregulation of genes in the canonical Wnt pathway, an evolutionarily conserved pathway that regulates crucial aspects of cell-fate determination (172). The Wnt pathway is also known to suppress adipogenesis, as BMAL1 binds to the promoter of genes at the Wnt pathway to increase their transcription. These contradictory results could stem from a change in the levels of the involved proteins throughout the adipogenic process. Further studies are needed to delineate the role of BMAL1 in adipogenesis and how other clock regulators fit into this mechanism.
Figure 4.
The relationship between the core clock mechanism and differentiation signaling pathways in BAT and WAT. BMAL1 activates the transcription of PPARγ and PER2. PER2 inhibits BMAL1 activity, and PPARγ increases BMAL1 expression. Wnt signaling represses WAT adipogenesis, whereas TGFβ/Smad3 signaling represses BAT formation. BMP signaling induces BAT formation. Reduced levels of BMAL1 lead to increased WAT and BAT formation.
BAT adipogenesis
BMAL1 inhibits adipogenesis and thermogenic capacity (173). Global ablation of BMAL1 in mice and adipocyte-selective inactivation increase brown fat mass and cold tolerance. BMAL1 inhibits brown adipogenesis through direct transcriptional control of key components of the cellular differentiation signaling pathways of transforming growth factor β (TGFβ) and bone morphogenetic protein (BMP); activation of TGFβ/Smad3 or blockade of BMP pathways suppresses enhanced differentiation in BMAL1-deficient brown adipocytes (173). REV-ERBα, the negative regulator of BMAL1 expression, promotes BAT development and adipogenesis. As opposed to BMAL1, REV-ERBα represses key components of the TGFβ signaling pathway (174), an inhibitory pathway of brown fat development (175, 176). Thus, in WAT, BMAL1 primarily regulates the canonical Wnt cascade, whereas in BAT, regulation of the TGFβ pathway is predominant (Fig. 4). Whether the effects of BMAL1 and REV-ERBα are pleotropic or demonstrate the physiological significance of the clock in BAT thermogenesis remain to be determined.
Future Perspectives
Research during the last years has focused on the characterization of the molecular mechanisms by which circadian clocks impact adipose tissue physiology. Synchronization of the molecular clocks in the different types of adipose tissues with the rest of the body still merits further investigation. The connections of circadian genetic variants and obesity now help to understand the importance of the circadian system in adipose-related illnesses, such as obesity. More importantly, they may help to design individual therapies to obesity, based on the circadian system, to increase the effectiveness of the treatments. Identification of certain compounds, capable of manipulating clock function at the cellular level, is critical to reprogram adipose tissue physiology.
Despite the immense knowledge achieved in the relevance of the internal clock in health and disease, there is still a big gap between this molecular knowledge and clinical practice. As explained previously, a big effort has been made in the last years to connect the results observed in experimental models (animals, tissues, cells) to humans. Currently, large-scale epidemiological studies are repeatedly demonstrating that the alteration of the circadian system has an impact on health and is associated with several adipose tissue-related metabolic illnesses, such as obesity, metabolic syndrome, or diabetes. Nevertheless, the success in translating this knowledge to the clinical practice is still limited.
To achieve this goal, it is necessary to: (1) increase the understanding of the importance of the circadian regulation of the endocrine system in medical practice; (2) develop new tools that help to assess the circadian system performance in clinical practice; and (3) design new therapies based on the regulation of the circadian system to increase the effectiveness of treatments of adipose tissue-related pathologies. Salivary samples, which may be noninvasively collected at several time points throughout the day, are useful to determine the daily pattern of hormones that connect the circadian system and adipose tissue. In addition, chronodisruption scores to assess the metabolic syndrome in the clinical practice (177) or the use of devices that allow continuous measurement of activity, body or wrist skin temperature, or heart rates may help to assess daily patterns in the habitual life of the patient (178, 179) and as a result, the circadian system performance.
In conclusion, timing is a crucial aspect in adipose tissue metabolism to ensure the correct accumulation of fat during periods of excess energy or TAG mobilization when energy requirements are not achieved. Therefore, different adipokines have to be secreted at the correct time to confer the proper endocrine, paracrine, or autocrine function. The current knowledge of the presence of an internal clock in the different adipose tissue types—white, brown, and beige—and its connection with key elements in metabolism may help us to achieve a better understanding of adipose tissue function and to design novel strategies to combat obesity.
Acknowledgments
Financial Support: This work was supported in part by the Spanish Government of Investigation, Development and Innovation (SAF2018-84135-R), including Fondo Europeo de Desarrollo Regional co-funding, and by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK105072 (to M.G.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations
- AMPK
adenosine 5′-monophosphate–activated protein kinase
- BAT
brown adipose tissue
- BMAL1
brain and muscle aryl hydrocarbon receptor nuclear translocator–like protein 1
- BMP
bone morphogenetic protein
- CLOCK
circadian locomotor output cycles kaput
- CRY
cryptochrome
- FFA
free fatty acid
- mTOR
mammalian target of rapamycin
- NAD
nicotinamide adenine dinucleotide
- PER
period
- PER1
period 1
- PER2
period 2
- PGC1
peroxisome proliferator–activated receptor γ coactivator 1
- PPAR
peroxisome proliferator–activated receptor
- REV-ERB
reverse ERB
- ROR
retinoic acid receptor–related orphan receptor
- SCN
suprachiasmatic nuclei
- SIRT1
sirtuin 1
- SNP
single nucleotide polymorphism
- TAG
triacylglyceride
- TGFβ
transforming growth factor β
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
References
- 1. Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417(6886):329–335. [DOI] [PubMed] [Google Scholar]
- 2. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. [DOI] [PubMed] [Google Scholar]
- 3. Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms. 2003;18(3):250–260. [DOI] [PubMed] [Google Scholar]
- 4. Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354(6315):994–999. [DOI] [PubMed] [Google Scholar]
- 5. Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18(3):164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gibson EM, Williams WP III, Kriegsfeld LJ. Aging in the circadian system: considerations for health, disease prevention and longevity. Exp Gerontol. 2009;44(1-2):51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111(1):41–50. [DOI] [PubMed] [Google Scholar]
- 8. Filipski E, King VM, Li X, Granda TG, Mormont MC, Claustrat B, Hastings MH, Lévi F. Disruption of circadian coordination accelerates malignant growth in mice. Pathol Biol (Paris). 2003;51(4):216–219. [DOI] [PubMed] [Google Scholar]
- 9. Davis S, Mirick DK. Circadian disruption, shift work and the risk of cancer: a summary of the evidence and studies in Seattle. Cancer Causes Control. 2006;17(4):539–545. [DOI] [PubMed] [Google Scholar]
- 10. Montagnana M, Salvagno GL, Lippi G. Circadian variation within hemostasis: an underrecognized link between biology and disease? Semin Thromb Hemost. 2009;35(1):23–33. [DOI] [PubMed] [Google Scholar]
- 11. Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119(11):1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jung-Hynes B, Reiter RJ, Ahmad N. Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. J Pineal Res. 2010;48(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Foster RG, Peirson SN, Wulff K, Winnebeck E, Vetter C, Roenneberg T. Sleep and circadian rhythm disruption in social jetlag and mental illness. Prog Mol Biol Transl Sci. 2013;119:325–346. [DOI] [PubMed] [Google Scholar]
- 14. Touitou Y. Adolescent sleep misalignment: a chronic jet lag and a matter of public health. J Physiol Paris. 2013;107(4):323–326. [DOI] [PubMed] [Google Scholar]
- 15. McFadden E, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. The relationship between obesity and exposure to light at night: cross-sectional analyses of over 100,000 women in the Breakthrough Generations Study. Am J Epidemiol. 2014;180(3):245–250. [DOI] [PubMed] [Google Scholar]
- 16. Garaulet M, Ordovás JM, Madrid JA. The chronobiology, etiology and pathophysiology of obesity. Int J Obes. 2010;34(12):1667–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14(4):697–706. [DOI] [PubMed] [Google Scholar]
- 18. Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell. 1997;91(6):855–860. [DOI] [PubMed] [Google Scholar]
- 19. Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1(8):708–713. [DOI] [PubMed] [Google Scholar]
- 20. Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63(1):647–676. [DOI] [PubMed] [Google Scholar]
- 21. Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107(7):855–867. [DOI] [PubMed] [Google Scholar]
- 22. Froy O, Chapnik N. Circadian oscillation of innate immunity components in mouse small intestine. Mol Immunol. 2007;44(8):1954–1960. [DOI] [PubMed] [Google Scholar]
- 23. Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am J Physiol Heart Circ Physiol. 2006;290(1):H1–H16. [DOI] [PubMed] [Google Scholar]
- 24. Kornmann B, Preitner N, Rifat D, Fleury-Olela F, Schibler U. Analysis of circadian liver gene expression by ADDER, a highly sensitive method for the display of differentially expressed mRNAs. Nucleic Acids Res. 2001;29(11):E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12(7):540–550. [DOI] [PubMed] [Google Scholar]
- 26. Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol. 2002;12(7):551–557. [DOI] [PubMed] [Google Scholar]
- 27. Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–320. [DOI] [PubMed] [Google Scholar]
- 28. Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417(6884):78–83. [DOI] [PubMed] [Google Scholar]
- 29. Kita Y, Shiozawa M, Jin W, Majewski RR, Besharse JC, Greene AS, Jacob HJ. Implications of circadian gene expression in kidney, liver and the effects of fasting on pharmacogenomic studies. Pharmacogenetics. 2002;12(1):55–65. [DOI] [PubMed] [Google Scholar]
- 30. Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55(4):962–970. [DOI] [PubMed] [Google Scholar]
- 31. Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O’Neill JS, Wong GK, Chesham J, Odell M, Lilley KS, Kyriacou CP, Hastings MH. Circadian orchestration of the hepatic proteome. Curr Biol. 2006;16(11):1107–1115. [DOI] [PubMed] [Google Scholar]
- 32. McCarthy JJ, Andrews JL, McDearmon EL, Campbell KS, Barber BK, Miller BH, Walker JR, Hogenesch JB, Takahashi JS, Esser KA. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics. 2007;31(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, Weitz CJ. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294(5551):2511–2515. [DOI] [PubMed] [Google Scholar]
- 34. Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417(6887):405–410. [DOI] [PubMed] [Google Scholar]
- 35. Kraves S, Weitz CJ. A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Nat Neurosci. 2006;9(2):212–219. [DOI] [PubMed] [Google Scholar]
- 36. Froy O, Chang DC, Reppert SM. Redox potential: differential roles in dCRY and mCRY1 functions. Curr Biol. 2002;12(2):147–152. [DOI] [PubMed] [Google Scholar]
- 37. Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110(2):251–260. [DOI] [PubMed] [Google Scholar]
- 38. Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43(4):527–537. [DOI] [PubMed] [Google Scholar]
- 39. Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37(2):187–192. [DOI] [PubMed] [Google Scholar]
- 40. Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96(2):271–290. [DOI] [PubMed] [Google Scholar]
- 41. Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr. 1987; 45(1, Suppl)277–282. [DOI] [PubMed] [Google Scholar]
- 42. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. [DOI] [PubMed] [Google Scholar]
- 43. Aldhahi W, Hamdy O. Adipokines, inflammation, and the endothelium in diabetes. Curr Diab Rep. 2003;3(4):293–298. [DOI] [PubMed] [Google Scholar]
- 44. Chen Y, Pan R, Pfeifer A. Fat tissues, the brite and the dark sides. Pflugers Arch. 2016;468(11-12):1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53(4):619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lafontan M. Advances in adipose tissue metabolism. Int J Obes. 2008;32(Suppl 7)S39–S51. [DOI] [PubMed] [Google Scholar]
- 47. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. [DOI] [PubMed] [Google Scholar]
- 48. Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123(8):3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123(1):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17(2):200–205. [DOI] [PubMed] [Google Scholar]
- 51. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48(7):424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang W, Seale P. Control of brown and beige fat development. Nat Rev Mol Cell Biol. 2016;17(11):691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Froy O. Metabolism and circadian rhythms--implications for obesity. Endocr Rev. 2010;31(1):1–24. [DOI] [PubMed] [Google Scholar]
- 56. Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126(4):801–810. [DOI] [PubMed] [Google Scholar]
- 57. Garaulet M, Madrid JA. Chronobiological aspects of nutrition, metabolic syndrome and obesity. Adv Drug Deliv Rev. 2010;62(9-10):967–978. [DOI] [PubMed] [Google Scholar]
- 58. La Fleur SE, Kalsbeek A, Wortel J, Buijs RM. A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J Neuroendocrinol. 1999;11(8):643–652. [DOI] [PubMed] [Google Scholar]
- 59. La Fleur SE. Daily rhythms in glucose metabolism: suprachiasmatic nucleus output to peripheral tissue. J Neuroendocrinol. 2003;15(3):315–322. [DOI] [PubMed] [Google Scholar]
- 60. Davidson AJ, Castañón-Cervantes O, Stephan FK. Daily oscillations in liver function: diurnal vs circadian rhythmicity. Liver Int. 2004;24(3):179–186. [DOI] [PubMed] [Google Scholar]
- 61. Ramsey KM, Marcheva B, Kohsaka A, Bass J. The clockwork of metabolism. Annu Rev Nutr. 2007;27(1):219–240. [DOI] [PubMed] [Google Scholar]
- 62. Pendergast JS, Branecky KL, Yang W, Ellacott KL, Niswender KD, Yamazaki S. High-fat diet acutely affects circadian organisation and eating behavior. Eur J Neurosci. 2013;37(8):1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barnea M, Madar Z, Froy O. High-fat diet delays and fasting advances the circadian expression of adiponectin signaling components in mouse liver. Endocrinology. 2009;150(1):161–168. [DOI] [PubMed] [Google Scholar]
- 64. Barnea M, Madar Z, Froy O. High-fat diet followed by fasting disrupts circadian expression of adiponectin signaling pathway in muscle and adipose tissue. Obesity (Silver Spring). 2010;18(2):230–238. [DOI] [PubMed] [Google Scholar]
- 65. Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414–421. [DOI] [PubMed] [Google Scholar]
- 66. Pivovarova O, Jürchott K, Rudovich N, Hornemann S, Ye L, Möckel S, Murahovschi V, Kessler K, Seltmann AC, Maser-Gluth C, Mazuch J, Kruse M, Busjahn A, Kramer A, Pfeiffer AF. Changes of dietary fat and carbohydrate content alter central and peripheral clock in humans. J Clin Endocrinol Metab. 2015;100(6):2291–2302. [DOI] [PubMed] [Google Scholar]
- 67. Chawla A, Lazar MA. Induction of Rev-ErbA alpha, an orphan receptor encoded on the opposite strand of the alpha-thyroid hormone receptor gene, during adipocyte differentiation. J Biol Chem. 1993;268(22):16265–16269. [PubMed] [Google Scholar]
- 68. Bray MS, Young ME. Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte. Obes Rev. 2007;8(2):169–181. [DOI] [PubMed] [Google Scholar]
- 69. Torra IP, Tsibulsky V, Delaunay F, Saladin R, Laudet V, Fruchart JC, Kosykh V, Staels B. Circadian and glucocorticoid regulation of Rev-erbalpha expression in liver. Endocrinology. 2000;141(10):3799–3806. [DOI] [PubMed] [Google Scholar]
- 70. Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, Liddle C, Auwerx J, Downes M, Panda S, Evans RM. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485(7396):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lau P, Nixon SJ, Parton RG, Muscat GE. RORalpha regulates the expression of genes involved in lipid homeostasis in skeletal muscle cells: caveolin-3 and CPT-1 are direct targets of ROR. J Biol Chem. 2004;279(35):36828–36840. [DOI] [PubMed] [Google Scholar]
- 72. Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, Hashimoto S. A transcription factor response element for gene expression during circadian night. Nature. 2002;418(6897):534–539. [DOI] [PubMed] [Google Scholar]
- 73. Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R, Yoo SH, Takahashi JS, Butler AA, Kamenecka TM, Burris TP. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485(7396):62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Amador A, Wang Y, Banerjee S, Kameneka TM, Solt LA, Burris TP. Pharmacological and genetic modulation of REV-ERB activity and expression affects orexigenic gene expression [published correction appears in PLos One 2016;11(5):e0156367]. PLoS One. 2016;11(3):e0151014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Delezie J, Dumont S, Dardente H, Oudart H, Gréchez-Cassiau A, Klosen P, Teboul M, Delaunay F, Pévet P, Challet E. The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 2012;26(8):3321–3335. [DOI] [PubMed] [Google Scholar]
- 76. Gerhart-Hines Z, Feng D, Emmett MJ, Everett LJ, Loro E, Briggs ER, Bugge A, Hou C, Ferrara C, Seale P, Pryma DA, Khurana TS, Lazar MA. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature. 2013;503(7476):410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20(5):649–688. [DOI] [PubMed] [Google Scholar]
- 78. Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405(6785):421–424. [DOI] [PubMed] [Google Scholar]
- 79. Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116(3):571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137(1):354–366. [DOI] [PubMed] [Google Scholar]
- 81. Chen L, Yang G.. PPARs integrate the mammalian clock and energy metabolism. PPAR Res. 2014;2014:653017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Oishi K, Shirai H, Ishida N. CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor alpha (PPARalpha) in mice. Biochem J. 2005;386(3):575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Inoue I, Shinoda Y, Ikeda M, Hayashi K, Kanazawa K, Nomura M, Matsunaga T, Xu H, Kawai S, Awata T, Komoda T, Katayama S. CLOCK/BMAL1 is involved in lipid metabolism via transactivation of the peroxisome proliferator-activated receptor (PPAR) response element. J Atheroscler Thromb. 2005;12(3):169–174. [DOI] [PubMed] [Google Scholar]
- 84. Canaple L, Rambaud J, Dkhissi-Benyahya O, Rayet B, Tan NS, Michalik L, Delaunay F, Wahli W, Laudet V. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol. 2006;20(8):1715–1727. [DOI] [PubMed] [Google Scholar]
- 85. Gutman R, Barnea M, Haviv L, Chapnik N, Froy O. Peroxisome proliferator-activated receptor α (PPARα) activation advances locomotor activity and feeding daily rhythms in mice. Int J Obes. 2012;36(8):1131–1134. [DOI] [PubMed] [Google Scholar]
- 86. Yang G, Jia Z, Aoyagi T, McClain D, Mortensen RM, Yang T. Systemic PPARγ deletion impairs circadian rhythms of behavior and metabolism. PLoS One. 2012;7(8):e38117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li S, Lin JD. Transcriptional control of circadian metabolic rhythms in the liver. Diabetes Obes Metab. 2015;17(Suppl 1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447(7143):477–481. [DOI] [PubMed] [Google Scholar]
- 89. Grimaldi B, Sassone-Corsi P. Circadian rhythms: metabolic clockwork. Nature. 2007;447(7143):386–387. [DOI] [PubMed] [Google Scholar]
- 90. Carling D. AMP-activated protein kinase: balancing the scales. Biochimie. 2005;87(1):87–91. [DOI] [PubMed] [Google Scholar]
- 91. Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase--development of the energy sensor concept. J Physiol. 2006;574(1):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Um JH, Yang S, Yamazaki S, Kang H, Viollet B, Foretz M, Chung JH. Activation of 5′-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. J Biol Chem. 2007;282(29):20794–20798. [DOI] [PubMed] [Google Scholar]
- 93. Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326(5951):437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Barnea M, Haviv L, Gutman R, Chapnik N, Madar Z, Froy O. Metformin affects the circadian clock and metabolic rhythms in a tissue-specific manner. Biochim Biophys Acta. 2012;1822:1796–1806. [DOI] [PubMed] [Google Scholar]
- 95. Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20(21):2913–2921. [DOI] [PubMed] [Google Scholar]
- 96. Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20(7):325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134(2):317–328. [DOI] [PubMed] [Google Scholar]
- 98. Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Masri S, Rigor P, Cervantes M, Ceglia N, Sebastian C, Xiao C, Roqueta-Rivera M, Deng C, Osborne TF, Mostoslavsky R, Baldi P, Sassone-Corsi P. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell. 2014;158(3):659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324(5927):654–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, Bass J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342(6158):1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293(5529):510–514. [DOI] [PubMed] [Google Scholar]
- 104. Li F, Yin Y, Tan B, Kong X, Wu G. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids. 2011;41(5):1185–1193. [DOI] [PubMed] [Google Scholar]
- 105. Cao R, Lee B, Cho HY, Saklayen S, Obrietan K. Photic regulation of the mTOR signaling pathway in the suprachiasmatic circadian clock. Mol Cell Neurosci. 2008;38(3):312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lipton JO, Yuan ED, Boyle LM, Ebrahimi-Fakhari D, Kwiatkowski E, Nathan A, Güttler T, Davis F, Asara JM, Sahin M. The circadian protein BMAL1 regulates translation in response to S6K1-mediated phosphorylation. Cell. 2015;161(5):1138–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Erren TC, Reiter RJ. Defining chronodisruption. J Pineal Res. 2009;46(3):245–247. [DOI] [PubMed] [Google Scholar]
- 108. Laermans J, Depoortere I. Chronobesity: role of the circadian system in the obesity epidemic. Obes Rev. 2016;17(2):108–125. [DOI] [PubMed] [Google Scholar]
- 109. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Oishi K, Ohkura N, Wakabayashi M, Shirai H, Sato K, Matsuda J, Atsumi G, Ishida N. CLOCK is involved in obesity-induced disordered fibrinolysis in ob/ob mice by regulating PAI-1 gene expression. J Thromb Haemost. 2006;4(8):1774–1780. [DOI] [PubMed] [Google Scholar]
- 111. Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134(5):728–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, Aguilera G, Abel ED, Chung JH. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150(5):2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2(11):e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH. Circadian disruption leads to insulin resistance and obesity. Curr Biol. 2013;23(5):372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, Bradfield CA, Vaughan CH, Eiden M, Masoodi M, Griffin JL, Wang F, Lawson JA, Fitzgerald GA. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012;18(12):1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16(10):1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hatori M, Panda S. CRY links the circadian clock and CREB-mediated gluconeogenesis. Cell Res. 2010;20(12):1285–1288. [DOI] [PubMed] [Google Scholar]
- 118. Barclay JL, Shostak A, Leliavski A, Tsang AH, Jöhren O, Müller-Fielitz H, Landgraf D, Naujokat N, van der Horst GT, Oster H. High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am J Physiol Endocrinol Metab. 2013;304(10):E1053–E1063. [DOI] [PubMed] [Google Scholar]
- 119. Froy O. A CRY for help to fight fat. Am J Physiol Endocrinol Metab. 2013;304(11):E1129–E1130. [DOI] [PubMed] [Google Scholar]
- 120. Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci USA. 2012;109(31):12662–12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kolbe I, Husse J, Salinas G, Lingner T, Astiz M, Oster H. The SCN clock governs circadian transcription rhythms in murine epididymal white adipose tissue. J Biol Rhythms. 2016;31(6):577–587. [DOI] [PubMed] [Google Scholar]
- 122. Shostak A, Husse J, Oster H. Circadian regulation of adipose function. Adipocyte. 2013;2(4):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sookoian S, Gemma C, Gianotti TF, Burgueño A, Castaño G, Pirola CJ. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr. 2008;87(6):1606–1615. [DOI] [PubMed] [Google Scholar]
- 124. Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes. 2008;32(4):658–662. [DOI] [PubMed] [Google Scholar]
- 125. Garaulet M, Lee YC, Shen J, Parnell LD, Arnett DK, Tsai MY, Lai CQ, Ordovas JM. CLOCK genetic variation and metabolic syndrome risk: modulation by monounsaturated fatty acids. Am J Clin Nutr. 2009;90(6):1466–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Garaulet M, Corbalán MD, Madrid JA, Morales E, Baraza JC, Lee YC, Ordovas JM. CLOCK gene is implicated in weight reduction in obese patients participating in a dietary programme based on the Mediterranean diet. Int J Obes. 2010;34(3):516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Garaulet M, Lee YC, Shen J, Parnell LD, Arnett DK, Tsai MY, Lai CQ, Ordovas JM. Genetic variants in human CLOCK associate with total energy intake and cytokine sleep factors in overweight subjects (GOLDN population). Eur J Hum Genet. 2010;18(3):364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bandín C, Martinez-Nicolas A, Ordovás JM, Ros Lucas JA, Castell P, Silvente T, Madrid JA, Garaulet M. Differences in circadian rhythmicity in CLOCK 3111T/C genetic variants in moderate obese women as assessed by thermometry, actimetry and body position. Int J Obes. 2013;37(8):1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lo MT, Bandin C, Yang HW, Scheer FA, Hu K, Garaulet M. CLOCK 3111T/C genetic variant influences the daily rhythm of autonomic nervous function: relevance to body weight control. Int J Obes (Lond). 2018;42(2):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Garaulet M, Corbalán-Tutau MD, Madrid JA, Baraza JC, Parnell LD, Lee YC, Ordovas JM. PERIOD2 variants are associated with abdominal obesity, psycho-behavioral factors, and attrition in the dietary treatment of obesity. J Am Diet Assoc. 2010;110(6):917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yamaguchi M, Uemura H, Arisawa K, Katsuura-Kamano S, Hamajima N, Hishida A, Suma S, Oze I, Nakamura K, Takashima N, Suzuki S, Ibusuki R, Mikami H, Ohnaka K, Kuriyama N, Kubo M, Tanaka H; Japan Multi-institutional Collaborative Cohort (J-MICC) Study Group . Association between brain-muscle-ARNT-like protein-2 (BMAL2) gene polymorphism and type 2 diabetes mellitus in obese Japanese individuals: a cross-sectional analysis of the Japan Multi-institutional Collaborative Cohort Study. Diabetes Res Clin Pract. 2015;110(3):301–308. [DOI] [PubMed] [Google Scholar]
- 132. Garaulet M, Smith CE, Gomez-Abellán P, Ordovás-Montañés M, Lee YC, Parnell LD, Arnett DK, Ordovás JM. REV-ERB-alpha circadian gene variant associates with obesity in two independent populations: Mediterranean and North American. Mol Nutr Food Res. 2014;58(4):821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MK, Paquet C, Delhaye S, Shin Y, Kamenecka TM, Schaart G, Lefebvre P, Nevière R, Burris TP, Schrauwen P, Staels B, Duez H. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med. 2013;19(8):1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jager J, O’Brien WT, Manlove J, Krizman EN, Fang B, Gerhart-Hines Z, Robinson MB, Klein PS, Lazar MA. Behavioral changes and dopaminergic dysregulation in mice lacking the nuclear receptor Rev-erbα. Mol Endocrinol. 2014;28(4):490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Dashti HS, Smith CE, Lee YC, Parnell LD, Lai CQ, Arnett DK, Ordovás JM, Garaulet M. CRY1 circadian gene variant interacts with carbohydrate intake for insulin resistance in two independent populations: Mediterranean and North American. Chronobiol Int. 2014;31(5):660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Messina G, De Luca V, Viggiano A, Ascione A, Iannaccone T, Chieffi S, Monda M. Autonomic nervous system in the control of energy balance and body weight: personal contributions. Neurol Res Int. 2013;2013:639280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Saito M, Murakami E, Suda M. Circadian rhythms in disaccharidases of rat small intestine and its relation to food intake. Biochim Biophys Acta. 1976;421(1):177–179. [DOI] [PubMed] [Google Scholar]
- 138. Honma KI, Honma S, Hiroshige T. Critical role of food amount for prefeeding corticosterone peak in rats. Am J Physiol. 1983;245(3):R339–R344. [DOI] [PubMed] [Google Scholar]
- 139. Comperatore CA, Stephan FK. Entrainment of duodenal activity to periodic feeding. J Biol Rhythms. 1987;2(3):227–242. [DOI] [PubMed] [Google Scholar]
- 140. Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17(4):284–292. [DOI] [PubMed] [Google Scholar]
- 141. Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring). 2009;17(11):2100–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, Scheer FA. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci USA. 2015;112(17):E2225–E2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Garaulet M, Gómez-Abellán P. Timing of food intake and obesity: a novel association. Physiol Behav. 2014;134:44–50. [DOI] [PubMed] [Google Scholar]
- 144. Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FA. Timing of food intake predicts weight loss effectiveness [published correction appears in Int J Obes (Lond) 2013;37(4):624]. Int J Obes (Lond). 2013;37(4):604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Jakubowicz D, Barnea M, Wainstein J, Froy O.. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring). 2013;21(12):2504–2512. [DOI] [PubMed] [Google Scholar]
- 146. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291(5503):490–493. [DOI] [PubMed] [Google Scholar]
- 148. Wehrens SM, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ, Johnston JD. Meal timing regulates the human circadian system. Curr Biol. 2017;27(12):1768–1775.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Gómez-Santos C, Gómez-Abellán P, Madrid JA, Hernández-Morante JJ, Lujan JA, Ordovas JM, Garaulet M. Circadian rhythm of clock genes in human adipose explants. Obesity (Silver Spring). 2009;17(8):1481–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. van der Veen DR, Shao J, Chapman S, Leevy WM, Duffield GE. A diurnal rhythm in glucose uptake in brown adipose tissue revealed by in vivo PET-FDG imaging. Obesity (Silver Spring). 2012;20(7):1527–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lee P, Bova R, Schofield L, Bryant W, Dieckmann W, Slattery A, Govendir MA, Emmett L, Greenfield JR. Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metab. 2016;23(4):602–609. [DOI] [PubMed] [Google Scholar]
- 152. Garaulet M, Ordovás JM, Gómez-Abellán P, Martínez JA, Madrid JA. An approximation to the temporal order in endogenous circadian rhythms of genes implicated in human adipose tissue metabolism. J Cell Physiol. 2011;226(8):2075–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2(4):367–373. [DOI] [PubMed] [Google Scholar]
- 154. Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med. 1995;27(4):435–438. [DOI] [PubMed] [Google Scholar]
- 155. Kalra SP, Bagnasco M, Otukonyong EE, Dube MG, Kalra PS. Rhythmic, reciprocal ghrelin and leptin signaling: new insight in the development of obesity. Regul Pept. 2003;111(1-3):1–11. [DOI] [PubMed] [Google Scholar]
- 156. Downs JL, Urbanski HF. Aging-related sex-dependent loss of the circulating leptin 24-h rhythm in the rhesus monkey. J Endocrinol. 2006;190(1):117–127. [DOI] [PubMed] [Google Scholar]
- 157. Kalsbeek A, Fliers E, Romijn JA, La Fleur SE, Wortel J, Bakker O, Endert E, Buijs RM. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142(6):2677–2685. [DOI] [PubMed] [Google Scholar]
- 158. Sukumaran S, Almon RR, DuBois DC, Jusko WJ. Circadian rhythms in gene expression: relationship to physiology, disease, drug disposition and drug action. Adv Drug Deliv Rev. 2010;62(9–10):904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Licinio J. Longitudinally sampled human plasma leptin and cortisol concentrations are inversely correlated. J Clin Endocrinol Metab. 1998;83(3):1042. [DOI] [PubMed] [Google Scholar]
- 160. Heptulla R, Smitten A, Teague B, Tamborlane WV, Ma YZ, Caprio S. Temporal patterns of circulating leptin levels in lean and obese adolescents: relationships to insulin, growth hormone, and free fatty acids rhythmicity. J Clin Endocrinol Metab. 2001;86(1):90–96. [DOI] [PubMed] [Google Scholar]
- 161. Arble DM, Vitaterna MH, Turek FW. Rhythmic leptin is required for weight gain from circadian desynchronized feeding in the mouse. PLoS One. 2011;6(9):e25079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Gómez-Abellán P, Gómez-Santos C, Madrid JA, Milagro FI, Campion J, Martínez JA, Ordovás JM, Garaulet M. Circadian expression of adiponectin and its receptors in human adipose tissue. Endocrinology. 2010;151(1):115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Carrasco-Benso MP, Rivero-Gutierrez B, Lopez-Minguez J, Anzola A, Diez-Noguera A, Madrid JA, Lujan JA, Martínez-Augustin O, Scheer FA, Garaulet M. Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity. FASEB J. 2016;30(9):3117–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88(6):2838–2843. [DOI] [PubMed] [Google Scholar]
- 165. Peterson RE. Plasma corticosterone and hydrocortisone levels in man. J Clin Endocrinol Metab. 1957;17(10):1150–1157. [DOI] [PubMed] [Google Scholar]
- 166. García-Prieto MD, Tébar FJ, Nicolás F, Larqué E, Zamora S, Garaulet M. Cortisol secretary pattern and glucocorticoid feedback sensitivity in women from a Mediterranean area: relationship with anthropometric characteristics, dietary intake and plasma fatty acid profile. Clin Endocrinol (Oxf). 2007;66(2):185–191. [DOI] [PubMed] [Google Scholar]
- 167. Kiehn JT, Tsang AH, Heyde I, Leinweber B, Kolbe I, Leliavski A, Oster H. Circadian rhythms in adipose tissue physiology. Compr Physiol. 2017;7(2):383–427. [DOI] [PubMed] [Google Scholar]
- 168. Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3(4):267–277. [DOI] [PubMed] [Google Scholar]
- 169. Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, Granneman JG, Piomelli D, Leff T, Sassone-Corsi P. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010;12(5):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Guo B, Chatterjee S, Li L, Kim JM, Lee J, Yechoor VK, Minze LJ, Hsueh W, Ma K. The clock gene, brain and muscle Arnt-like 1, regulates adipogenesis via Wnt signaling pathway. FASEB J. 2012;26(8):3453–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Shimba S, Ogawa T, Hitosugi S, Ichihashi Y, Nakadaira Y, Kobayashi M, Tezuka M, Kosuge Y, Ishige K, Ito Y, Komiyama K, Okamatsu-Ogura Y, Kimura K, Saito M. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS One. 2011;6(9):e25231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Nam D, Guo B, Chatterjee S, Chen MH, Nelson D, Yechoor VK, Ma K. The adipocyte clock controls brown adipogenesis through the TGF-β and BMP signaling pathways. J Cell Sci. 2015;128(9):1835–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Nam D, Chatterjee S, Yin H, Liu R, Lee J, Yechoor VK, Ma K. Novel function of Rev-erbα in promoting brown adipogenesis. Sci Rep. 2015;5(1):11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Koncarevic A, Kajimura S, Cornwall-Brady M, Andreucci A, Pullen A, Sako D, Kumar R, Grinberg AV, Liharska K, Ucran JA, Howard E, Spiegelman BM, Seehra J, Lachey J. A novel therapeutic approach to treating obesity through modulation of TGFβ signaling. Endocrinology. 2012;153(7):3133–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, Sun P, Lonning S, Skarulis M, Sumner AE, Finkel T, Rane SG. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab. 2011;14(1):67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Corbalán-Tutau MD, Gómez-Abellán P, Madrid JA, Canteras M, Ordovás JM, Garaulet M. Toward a chronobiological characterization of obesity and metabolic syndrome in clinical practice. Clin Nutr. 2015;34(3):477–483. [DOI] [PubMed] [Google Scholar]
- 178. Martinez-Nicolas A, Ortiz-Tudela E, Rol MA, Madrid JA. Uncovering different masking factors on wrist skin temperature rhythm in free-living subjects. PLoS One. 2013;8(4):e61142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Ortiz-Tudela E, Martinez-Nicolas A, Campos M, Rol MA, Madrid JA. A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLOS Comput Biol. 2010;6(11):e1000996. [DOI] [PMC free article] [PubMed] [Google Scholar]