Abstract
Treatment of full-thickness osteochondral defects is challenging because of the need to integrate reparative materials with two separate tissue types. This study demonstrates a modular tissue engineering approach, in which multiphase osteochondral tissues are built from hydrogel microbeads (50–150 μm in diameter) into which mesenchymal stromal cells (MSCs) and specific matrix compositions were encapsulated. Chondrogenic microbeads consisted of an agarose base containing a polyelectrolyte complex of chondroitin sulfate and chitosan. Osteogenic microbeads consisted of an agarose base augmented with collagen type 1 and nanoparticulate hydroxyapatite. Modular microbeads were created and predifferentiated separately toward a tissue-specific phenotype and then combined to form biphasic osteochondral constructs. A simple, multiport, perfusion bioreactor was developed to preferentially supply the appropriate medium type to each phase of the osteochondral construct, resulting in tissue development over time in vitro. Cell viability in microbeads was high and tissue-specific differentiation of MSCs in the microbeads was demonstrated. Flow patterns and mixing between medium types in the bioreactor were characterized and used to design the perfusion culture protocol. Over time in culture, constructs showed evidence of both chondrogenic and osteogenic differentiation, with development of a transition zone between phases that mirrored the structure of native tissue. This study therefore demonstrates a modular approach to creating and culturing multiphase tissues in such a way so as to maintain tissue-specific function and also allow development of composite engineered tissues.
Impact Statement
This study describes methods for fabricating, culturing, and characterizing modular microbeads containing progenitor cells that can be used to create osteochondral tissue constructs. Such biphasic engineered tissues were cultured in a low flow rate perfusion bioreactor chamber to maintain tissue-specific differentiation while allowing development of the osteochondral interface.
Keywords: osteochondral, mesenchymal stem cell, 3D cell culture, modular tissue engineering, hydrogels, bioreactor, cartilage
Introduction
In full-thickness osteochondral lesions, damage to an articular surface extends through the cartilage into the underlying bone. The limited healing capacity of cartilage due to a lack of vascularity and a failure to recapitulate native tissue leads to poor outcomes for patients suffering these injuries.1 A tissue engineering approach has the potential to address osteochondral defects by delivering preconditioned cells and materials to the defect site to guide and accelerate the healing process. A variety of strategies have been applied to create engineered osteochondral tissues, including the use of primary chondrocytes or progenitor cells, diffusion or perfusion-based nutrient delivery, and/or multistep scaffold formation, followed by cell seeding.2–10 Building from these studies, we sought to design and characterize a cell-laden two-phase construct that could recapitulate aspects of the osteochondral interface by integrating the following: (1) a family of cell-encapsulating materials that could be optimized for osteogenic or chondrogenic differentiation of stromal progenitor cells, (2) a simple construct production and assembly method, and (3) a system for preferentially perfusing regions of the construct with specific medium types to promote tissue-specific differentiation.
The present study used a modular tissue engineering approach11 to develop agarose-based cell-encapsulating microbeads that can be augmented with specific biomimetic extracellular matrices to promote and sustain chondrocytic or osteoblastic cell phenotypes. The fabrication method is based on our group's experience with water-in-oil emulsification to produce modular microbeads for cell delivery.12–16 Both chondrogenic and osteogenic microbeads were fabricated and then assembled into two-phase osteochondral constructs. To promote tissue-specific differentiation in each phase of the construct, a perfusion system with separate flow channels for chondrogenic and osteogenic media was implemented. The study demonstrates how dual-phase tissues can be fabricated, assembled, and cultured to promote composite tissue maturation.
Materials and Methods
Materials
Sterile stock solutions of chitosan, chondroitin sulfate, and agarose were prepared as described previously.15 Briefly, >90% deacetylated chitosan (UP B 90/500; Novamatrix, Sandvika, Norway) was suspended in distilled water, autoclaved, and then dissolved in acetic acid (final concentrations: 10 mg/mL chitosan, 0.1 N acetic acid). A 20 mg/mL stock of chondroitin sulfate A (C9819; Sigma-Aldrich, St. Louis, MO) was prepared in distilled water. Low-melting-temperature agarose (BP1360; Fisher Scientific, Pittsburgh, PA) was dissolved in distilled water at 20 mg/mL.
Complete medium was made from alpha modification of Minimum Essential Medium (αMEM; Gibco/Thermo Fisher Scientific, Grand Island, NY), 10% fetal bovine serum (FBS; Gibco), and 1% penicillin–streptomycin (Gibco). Chondrogenic medium comprised Dulbecco's Modified Eagle Medium (4.5 g/L glucose; Gibco), 1% penicillin–streptomycin, 1% ITS+ (BD Biosciences, San Jose, CA), 0.35 mM l-proline (Sigma), 0.2 mM l-ascorbic acid 2-phosphate (Sigma), 10 nM dexamethasone (Sigma), 10 ng/mL recombinant human Transforming Growth Factor-Beta 1 (PeproTech, Rocky Hill, NJ), and 1% penicillin–streptomycin. Osteogenic medium consisted of αMEM, 10% FBS, 100 nM dexamethasone, 10 mM β-glycerophosphate (Sigma), and 1% penicillin–streptomycin. Microbead production medium was αMEM plus 0.35 g/L sodium bicarbonate to buffer against ambient carbon dioxide.
Hydroxyapatite (HA) nanoparticles (<200 nm diameter) were used in osteogenic beads and construct phases (Sigma). Microbeads were emulsified in 100 cSt polydimethylsiloxane (PDMS) silicone oil (Clearco Products Co., Inc., Bensalem, PA).
Cell culture
A single pooled population of rat bone marrow mesenchymal stromal cells (MSCs) was used for all cell cultures. Thawed passage-three MSCs that had been previously harvested from four 1-month-old Fisher rats were used as described previously.14 Briefly, marrow flushed from the femora and tibiae was plated at 5 × 105 cells/in2 on 75-cm2 flasks and cultured in MSC growth media consisting of α-MEM (Gibco), 10% FBS (HyClone MSC screened), and penicillin (5000 U/100 mL)/streptomycin sulfate (5 mg/100 mL) (Gibco). Medium changes were performed every 3–4 days. Cells selected for freezing were suspended in 40% FBS, 50% α-MEM, and 10% DMSO in cryogenic tubes and cooled at 1°C/min until −80°C was achieved. The cells were subsequently stored in liquid nitrogen. To thaw, the vials were quickly warmed in a 37°C water bath and resuspended in α-MEM, 10% FBS, and penicillin/streptomycin. Cells were plated at 5 × 105 cells/in2 and medium changes were performed every 3–4 days.
Production and culture of osteogenic and chondrogenic microbeads
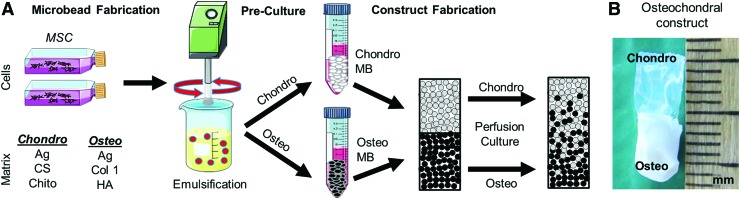
Microbead production was performed using a water-in-oil emulsification process12 shown schematically in Figure 1A. Agarose-based chondrogenic microbeads containing a polyelectrolyte complex (PEC) of chondroitin sulfate and chitosan in a 10:1 mass ratio were produced as described previously.15 Briefly, MSCs (1.0 × 106/mL of aqueous components), FBS, chondroitin sulfate, chitosan, agarose, 0.2 N acetic acid, and microbead production medium were loaded into a two-barreled syringe and injected into PDMS through a 25-gauge needle. Chondroitin sulfate and chitosan were kept in separate syringes to avoid premature PEC formation, which can affect injectability. A two-paddle impeller was used to mix the components at 700 rpm for 6 min at 37°C and then for 30 min on ice to enable gelation of the agarose. Microbeads were aliquoted into vented 15-mL conical tubes (CELLTREAT Scientific Products, Shirley, MA) and precultured for 1 week in either complete or chondrogenic medium.
FIG. 1.
Production of cell-encapsulating microbeads and osteochondral constructs. (A) In vitro-expanded MSCs are combined with hydrogel components and emulsified in silicone oil maintained at 37°C. When the mixing temperature is reduced, the resulting droplets undergo gelation into microbeads that can be collected, washed, and cultured in vented conical tubes. After a 7-day preculture period, osteogenic and chondrogenic microbeads are combined with carrier gels and cast into two-phase osteochondral constructs. (B) A two-phase osteochondral construct showing the chondrogenic phase (top) and osteogenic phase (bottom). MSCs, mesenchymal stromal cells. Color images are available online.
Osteogenic microbeads contained agarose, collagen 1, and HA (final concentrations 16.0, 1.0, and 15.0 mg/mL, respectively). Microbead production was carried out by loading MSCs (1.0 × 106 per mL of aqueous components), FBS, HA nanoparticles suspended in FBS, collagen I, agarose, and 0.1 N NaOH into a syringe and injecting the components into PDMS. Mixing was performed at 800 rpm to reduce collagen aggregation. Osteogenic beads were precultured for 1 week in either complete or osteogenic medium.
Control microbeads consisted of MSCs, FBS, phosphate-buffered saline (PBS), and agarose with component proportions mirroring the osteogenic formulation and production. Control microbeads were precultured for 1 week in complete, osteogenic, or chondrogenic medium.
Two-phase osteochondral construct production
Osteochondral constructs were made by mixing osteogenic or chondrogenic microbeads with agarose-based carrier gels and casting them in two layers within gelatin molds (#3 porcine gelatin drug delivery capsule, Harvard Apparatus, Holliston, MA). Osteogenic layers were created by combining precultured osteogenic microbeads (43% v/v) with an acellular carrier gel of the same initial composition as the microbeads. A chondrogenic layer was poured immediately on top of the osteogenic layer. The chondrogenic layer consisted of precultured chondrogenic microbeads (43% v/v) within a carrier gel of the same composition as the microbeads. The resulting constructs were 4–5 mm in diameter and ∼10 mm long (Fig. 1B). Successful bonding between phases was achieved, resulting in a clearly demarcated physical interface and a construct that could easily be handled with forceps. The two phases remained bonded over 3 weeks of perfusion culture.
Construct perfusion
For perfusion studies, osteochondral constructs were fabricated in the chamber of a 3DKube bioreactor (Kiyatec, Inc., Greenville, SC) shown in Figure 2A. Construct/bioreactor units were perfused with osteogenic and chondrogenic media for 14 or 21 days using an infusion/withdrawal pump system (Fig. 2B) that could be fully contained by a standard cell culture incubator to allow long-term perfusion at 37°C (Fig. 2C). The 3DKube configuration allows perfusion by separate medium types through a set of inlet and outlet ports. In these experiments, chondrogenic medium was perfused through the upper part of the chamber (chondro phase) and osteogenic medium was used in the lower part (osteo phase). This configuration allows each phase to be perfused separately, with limited fluid communication occurring within the bioreactor chambers (Fig. 2D).
FIG. 2.
Perfusion culture chamber and system. (A) A simple, multiport bioreactor chamber was used to fabricate and culture biphasic osteochondral constructs such that osteogenic and chondrogenic media were maintained in separate flow circuits (images courtesy of Kiyatec). (B) The perfusion system after assembly and (C) during active perfusion in a cell culture incubator. (D) Schematic of flow within a bioreactor chamber and construct. Color images are available online.
The perfusion system comprised a syringe pump (KD Scientific, Holliston, MA), sterile 10-mL Luer-Lok syringes (Becton Dickinson, Franklin Lakes, NJ), and Pyrex glass medium reservoirs (Corning, NY), and platinum-cured silicone tubing, polypropylene fittings, and nickel-plated brass valves from Cole-Parmer (Vernon Hills, IL). Air was exchanged between the interior of the pump system and the ambient atmosphere through 0.22-μm pore-size membranes. All wetted components were autoclaved or sterilized with 70% ethanol, as appropriate.
Perfusion system validation
Mixing between flow channels was determined using acellular biphasic constructs and flow rates of 0.24 and 0.01 mL/min. The 0.24 mL/min rate corresponds to an approximate average of flow rates used previously in 3DKube bioreactor studies.17,18 We also used a flow rate of 0.01 mL/min to examine whether a very low perfusion rate was still capable of supporting the microbead constructs. The osteogenic and chondrogenic layers of the acellular scaffolds were of the same composition as the carrier gels described above. Chondrogenic and osteogenic media were simulated using PBS and PBS +10% FBS +3% blue food dye, respectively (McCormick & Company, Inc., Sparks, MD). Mixing between flow circuits was determined by collecting the medium from each circuit and measuring its optical absorbance at 595 nm. Measurements were corrected against a standard curve comprising known dye concentrations.
To verify that media were perfusing the constructs, laser Doppler perfusion imaging (LDPI) was employed to visualize flow within the bioreactor chambers (PIM3; Perimed AB, Järfälla, Sweden). LDPI measurements were performed using an empty bioreactor or one containing either an impermeable construct cast from silicone rubber (Sylgard 184; Dow Corning Corporation, Midland, MI) or an agarose-based permeable construct (16.0 mg/mL final concentration). These conditions permitted the flow to be unobstructed (positive control), fully obstructed in the center of the bioreactor (negative control), or allowed to perfuse through a gel. PBS was used as a flow medium, with 20-nm diameter polystyrene spheres (2 v%) added to enhance the Doppler signal (Thermo Scientific). Perfusion was quantified by measuring mean grayscale intensities of the LDPI image output. Data were corrected against measurements taken under no-flow conditions.
Cell viability
Cell viability within microbeads was determined using a fluorescent live/dead assay (Thermo Fisher) as per the manufacturer's instructions. Images of stained cells were captured using a Nikon Eclipse microscope and fluorescent source (Nikon Instruments, Inc., Melville, NJ). Live and dead cells were counted using ImageJ software and a custom macro (US National Institutes of Health, Bethesda, MD). Cells staining positive for both calcein-AM and ethidium bromide were considered live at the beginning of the assay.
Protein visualization within microbeads
Microbeads were washed in PBS and fixed overnight in buffered zinc formalin (Z-Fix, Anatech Ltd., Battle Creek, MI). The microbeads were washed in 70% ethanol and PBS and then stained with EZBlue (Sigma-Aldrich).
Osteocalcin enzyme-linked immunosorbent assay
Secreted osteocalcin was detected in conditioned medium using an ELISA kit as per the manufacturer's instructions (BTI/Alfa Aesar, Ward Hill, MA). Absorbance was measured at 450 nm using a microplate reader and corrected to a standard curve of known osteocalcin concentrations (BioTek Instruments, Inc., Winooski, VT).
Histology and osteocalcin immunohistochemistry
Microbeads and constructs were washed in PBS and fixed overnight in buffered zinc formalin. Microbead specimens were cast inside 30-mg/mL gelatin disks to facilitate handling using custom-made Delrin molds and Bloom 225 gelatin. The microbead-containing disks were gelled for 10 min at 4°C and then fixed overnight in buffered zinc formalin.
Decalcification of constructs and disks was carried out with 10% EDTA in PBS. Paraffin infiltration of specimens was then performed using an automated tissue processor (Leica Biosystems Inc., Buffalo Grove, IL). Following infiltration, specimens were embedded in paraffin, cut into 6-μm sections on a microtome (Thermo), and mounted on glass slides.
Total collagen content was assessed histologically using Van Gieson's acid fuchsin (Electron Microscopy Sciences, Hatfield, PA). Chondrogenic differentiation was visualized with an Alcian blue–periodic acid-Schiff (PAS) staining kit (Poly Scientific R&D Corp., Bayshore, NY).
Immunohistochemistry (IHC) was used to detect osteocalcin bound to the microbead and construct matrix. The procedure was performed using an anti-mouse HRP-DAB staining kit (R&D Systems, Inc., Minneapolis, MN). Briefly, dewaxed sections were exposed to a commercial antigen retrieval reagent for 10 min at 90–92°C (R&D Systems). Next, sections were blocked and subsequently incubated overnight at 4°C in a mouse anti-rat osteocalcin primary antibody (R&D Systems) in tris-buffered saline (TBS). Control sections were incubated in TBS–BSA–FBS only. The specificity of detection was verified using controls exposed to the secondary antibody only.
Following incubation in the anti-mouse secondary antibody, the slides were exposed to the DAB chromogen system and then rinsed in absolute ethanol, cleared in xylene, and cover slipped.
Collagen type 2 was also detected in the constructs using IHC. The primary antibody (II-II6B3, Developmental Studies Hybridoma Bank, Iowa City, IA) was used at a concentration of 4 μg/mL.
Statistical analysis
Experiments were conducted with at least three biological replicates and assays were performed using two to three experimental replicates. Unless otherwise noted, statistical comparisons were made using one-way analysis of variance (ANOVA), followed by Dunnett's T3 post hoc test (SPSS, International Business Machine Corp., Armonk, NY). A value of p ≤ 0.05 was considered significant. Data are presented as mean ± standard deviation.
Results
Microbead formation and cell viability
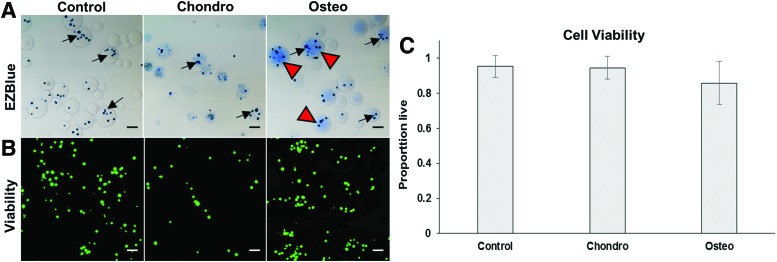
Water-in-oil emulsification resulted in cell-encapsulating spherical microbeads 50–150 μm in diameter. In this study, chondrogenic and agarose-only control microbeads were produced as described previously.15 However, osteogenic microbeads were a novel formulation containing a biomimetic complex of HA and collagen I within the microbead matrix. The presence of HA permitted a homogeneous distribution of collagen, as visualized using EZBlue protein staining (Fig. 3A). When HA was omitted from the mixture, the collagen tended to agglomerate into fibrillary patches, reducing the homogeneity of incorporation into the microbeads. Cell viability in all microbead types was ∼85–95% within 24 h of production, with no significant differences between microbead formulations (Fig. 3B, C).
FIG. 3.
Microbead morphology and viability of encapsulated cells after 1 day in culture. (A) EZBlue staining reveals spherical microbeads encapsulating embedded cells (arrows; scale bar = 100 μm). Blue staining shows protein content within osteogenic microbeads (arrowheads). (B) Viability staining indicates a very large proportion of live cells (green) compared with dead cells (red). (C) Quantification of vital staining showed high cell viability in all microbead formulations. Color images are available online.
Osteogenic differentiation in microbeads
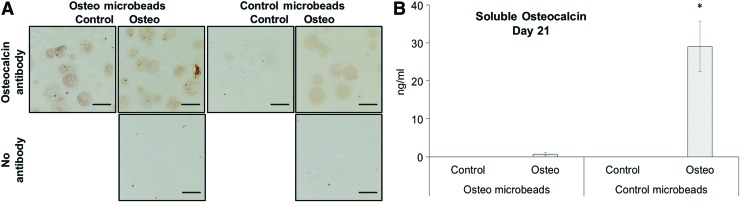
Osteocalcin was employed as a specific marker of osteoblast differentiation in osteogenic and control microbeads. IHC was used to visualize osteocalcin that was bound to the microbead matrix (Fig. 4A). Osteocalcin was detected throughout the interior of osteogenic microbeads cultured in either osteogenic or control conditions. These results suggest that osteocalcin is produced by cells within osteogenic microbeads and remains sequestered in the HA/collagen 1 matrix, as would be expected for a matricellular protein. Furthermore, the production of osteocalcin by osteogenic microbeads when cultured in control medium suggests that the microenvironment within these microbeads may promote osteogenic differentiation in the absence of supplementation with dexamethasone and β-glycerophosphate.
FIG. 4.
Osteocalcin expression in osteogenic microbeads. (A) IHC revealed osteocalcin (brown) within osteogenic microbeads cultured under both osteogenic and control conditions (scale bar = 100 μm). (B) Osteocalcin was detected through ELISA in conditioned medium of control (agarose only) microbeads cultured under osteogenic conditions (*p ≤ 0.05 compared with other groups). ELISA, enzyme-linked immunosorbent assay; IHC, immunohistochemistry. Color images are available online.
The levels of secreted osteocalcin in supernatant media were also measured using the enzyme-linked immunosorbent assay (Fig. 4B). Significantly greater osteocalcin levels were detected in conditioned media from control (agarose-only) microbeads cultured in osteogenic conditions compared with all other groups. Osteocalcin production in agarose-only microbeads indicates that our medium supplementation was sufficient to promote osteogenic differentiation. These results also provide further evidence that collagen type 1 and HA added to the microbead matrix serve to sequester osteocalcin within the microbeads.
Validation of perfusion system and development of testing parameters
A perfusion culture system was developed to maintain cell viability and promote the desired differentiated phenotypes within the constructs over time in culture. The system allowed perfusion with both chondrogenic and osteogenic media, with each being targeted to the appropriate phase of the microbead construct. The system was designed to ensure perfusion across each phase of the construct and to limit mixing between the chondrogenic and osteogenic flow circuits.
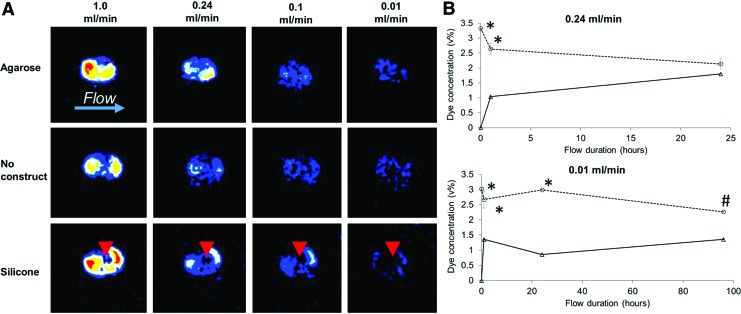
Perfusion of the constructs in the 3DKube bioreactor chamber was verified using LDPI to visualize flow within the chamber at rates of 0.01–1.0 mL/min (Fig. 5A). The results revealed perfusion across and within the agarose constructs even at the lowest flow rate of 0.01 mL/min. The perfusion pattern was similar to that of empty bioreactor chambers. In contrast, perfusion of chambers containing an impermeable silicone plug was concentrated around the edges of the plug as the flow was forced around this obstruction. These results verified that perfusion occurs in agarose constructs even at low flow rates, a prerequisite for maintenance of cell viability and phenotype.
FIG. 5.
Perfusion system characterization. (A) Visualization of flow using LDPI shows perfusion occurring in the center of the agarose constructs while verifying that the impermeable silicone constructs obstructed the flow (arrowheads). Red color indicates greater perfusion. (B) At a pump rate of 0.24 mL/min, mixing between the two flow circuits was largely achieved within 24 h. At 0.01 mL/min, flow separation was maintained for ∼96 h (*p ≤ 0.05 compared with other groups, #p ≤ 0.1). LDPI, laser Doppler perfusion imaging. Color images are available online.
Mixing between flow circuits was quantified by adding a dye to the osteogenic circuit and then monitoring the concentration of the dye in both circuits over time (Fig. 5B). At a 0.24 mL/min flow rate, substantial mixing between the two circuits was achieved within 1 h. The degree of mixing increased over time, with no significant differences in dye concentration between the circuits after 24 h. In contrast, at 0.01 mL/min, a burst of mixing also occurred within 1 h; however, after 96 h of flow, the dye concentration in the chondrogenic circuit remained at ∼60% that of the osteogenic circuit (p = 0.08). These results suggest that a lower flow rate can delay cross talk between the two flow circuits and, in turn, help maintain different cell lineages within biphasic constructs.
Based on the perfusion pattern and flow mixing characterization, subsequent experiments were performed using a pump rate of 0.01 mL/min. This flow rate limited cross talk across channels, permitted measurable perfusion within the constructs, and allowed medium changes to occur at 4-day intervals to conserve supplies of costly medium components.
Formation of an osteochondral interface within constructs
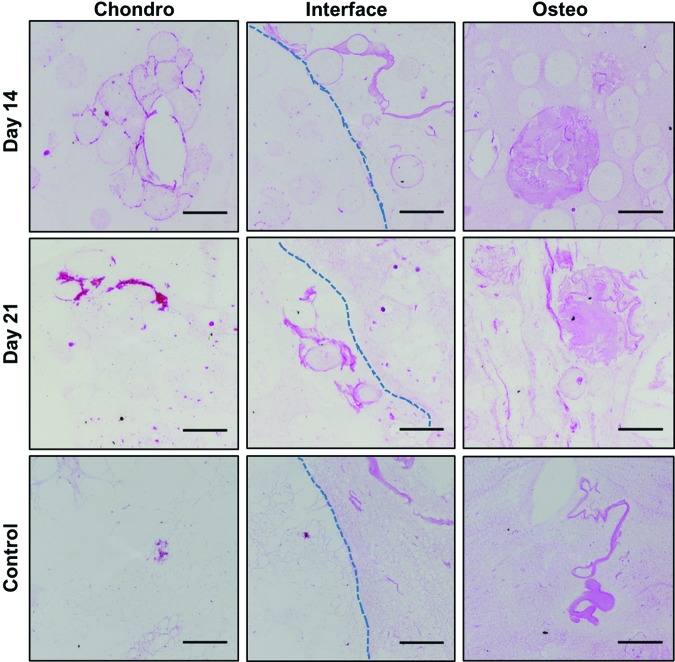
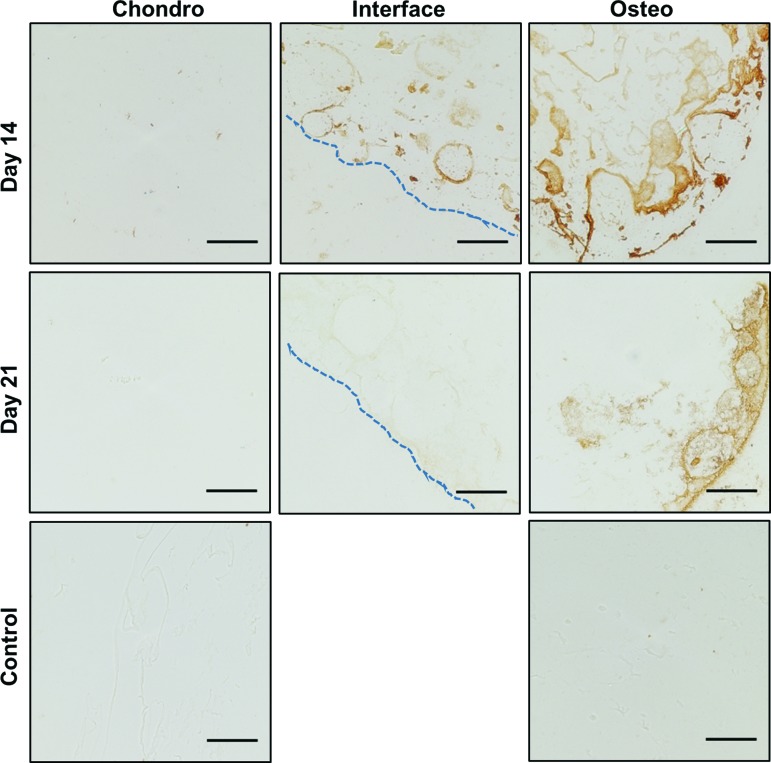
Total collagen content within the constructs was visualized using Van Gieson's acid fuchsin (Fig. 6). Regions of collagen were visible within the osteogenic phases of constructs at 14 and 21 days of perfusion. Collagen was also observed within the interiors of microbeads at both time points. These images indicate maintenance of a collagenous extracellular matrix (ECM) in the osteogenic phases consisting of collagen 1 and HA over 3 weeks of perfusion culture. Within the chondrogenic phases, areas of de novo collagen formation were noted around the microbead perimeters, although as expected, the bulk matrix exhibited little collagen staining. Staining for osteocalcin production (Fig. 7) further confirmed osteogenic differentiation of cells in the bone phase of constructs. Particularly intense staining was found near the construct perimeters as well as in localized areas distributed throughout the matrix, further suggesting that osteocalcin was bound by the matrix.
FIG. 6.
Total collagen content visualized histologically by Van Gieson's acid fuchsin (pink staining). Dotted lines mark the physical interface between phases (scale bar = 100 μm). Color images are available online.
FIG. 7.
Osteogenic differentiation in constructs as indicated by osteocalcin IHC (brown staining). Dotted lines mark the physical interface between phases (scale bar = 100 μm). Color images are available online.
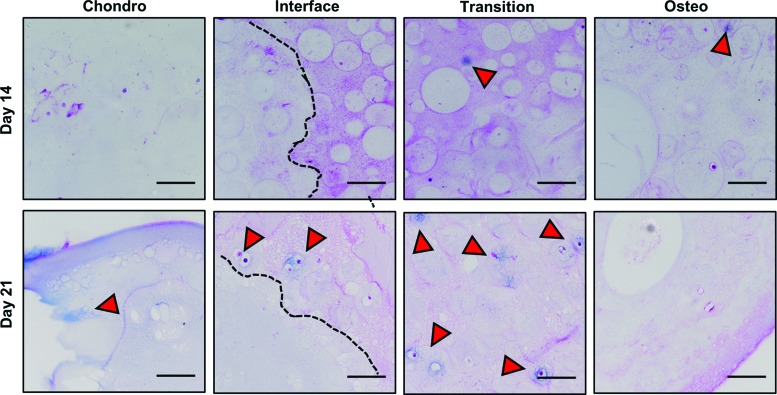
Chondrogenic differentiation was assessed using Alcian blue-PAS staining for glycosaminoglycan production (Fig. 8). At day 14 of perfusion culture, little positive staining was observed in the chondrogenic phase. Interestingly, at this time point, there was some positive blue staining for chondrocytes in the osteogenic phase, particularly in the transition zone adjacent to the chondro-osteo interface. By day 21, positive staining in the chondrogenic phase was observed, and chondrocytic differentiation remained evident in the transition zone. These results suggest that chondrogenic differentiation took place in a region of the constructs that received both chondrogenic and osteogenic signals, although osteogenic differentiation did not span across the construct interface.
FIG. 8.
Chondrogenic differentiation as indicated by Alcian blue-PAS staining (arrowheads). Dotted lines mark the physical interface between phases (scale bar = 100 μm). PAS, periodic acid-Schiff. Color images are available online.
Discussion
The overall goal of this study was to develop module-based osteochondral tissue constructs and a perfusion culture system to maintain such multiphase tissues. A key feature of the modular approach is that the modules can be cultured separately to promote tissue-specific differentiation, but can then be combined into multiphase constructs to produce more complex tissues. The chondrogenic microbeads used in this study were developed previously15 and have been shown to support chondrogenic differentiation of MSCs in culture. The osteogenic microbeads were a new formulation also based on an agarose matrix, but functionalized with a biomimetic combination of type 1 collagen and HA nanoparticles. Type 1 collagen and HA are, respectively, the predominant protein and inorganic extracellular matrix components of native bone.19 Collagen promotes MSC attachment and integrin-mediated differentiation, both of which contribute to an osteoblastic phenotype.20 HA offers biocompatibility while enabling osteoinduction and conduction.21
Osteogenic predifferentiation of microbeads resulted in production of osteocalcin, a specific marker of osteoblastic differentiation, over 21 days in culture. Interestingly, osteogenic microbeads expressed osteocalcin in response to both control and osteogenic media, suggesting that the microbead matrix alone induces differentiation. In contrast, control (pure agarose) microbeads expressed osteocalcin only in response to osteogenic medium. In addition, our results showed that osteogenic microbeads sequestered osteocalcin within the matrix, whereas control microbeads released this protein into the surrounding medium. An apparent reduction in osteocalcin staining at the location of the physical interface between the two phases (Fig. 7) may be due to a number of factors. It is possible that by the later time point, MSCs were of a more mature osteogenic phenotype and/or that there was advancement of an induced osteochondral interface into the original osteo phase.
We incorporated HA into the microbead matrix because it is known that the Ca2+ and PO3− ions present in this mineral bind proteins and other factors.22 Therefore, we expected that addition of HA would improve incorporation of collagen into the microbeads during production and would subsequently provide a substrate for serum proteins that could enhance cell attachment in the otherwise nonadherent agarose. It is likely that an additional benefit of HA was its ability to sequester osteocalcin within the microbeads rather than permitting it to become solubilized in the media. The binding affinity of osteocalcin to HA is well documented23 and this mineral constituent may therefore also provide a means of retaining other ECM components produced by cells encapsulated within osteoblastic microbeads.
This study was particularly focused on creating biphasic osteochondral constructs that had distinct tissue phases to match the function of the native tissue, and which could be used to repair full-thickness cartilage defects. The bone-mimicking osteogenic phase was designed to promote osseointegration with the subchondral bone through known osteoconductive effects of collagen/HA nanocrystal composites.24 The chondrogenic phase was designed to integrate with the surrounding cartilage and provide a continuous articular surface. The method of fabricating the biphasic construct was simple and produced a construct with an initially distinct interface between the chondrogenic and osteogenic phases. Since agarose-based microbeads are not highly self-adherent, a surrounding support gel was used to provide structure and shape to the construct.
Dual perfusion of osteochondral constructs in vitro was accomplished using a simple bioreactor chamber that allowed separate inlet and outlet flows for chondrogenic and osteogenic culture media. This configuration allowed each phase of the construct to be perfused with the appropriate medium type7 rather than compromising on a single cocktail medium formulation that supports both types of differentiation.4,5 Our characterization showed that the medium types could be substantially sequestered to specified regions of the construct between medium changes and supported development of the biphasic tissue. Over 2–3 weeks in culture, the interface between the phases gradually developed a region of overlapping chondrogenic and osteogenic differentiation, that is, a transition zone between tissue types, as is seen in native cartilage–bone interfaces. Interestingly, this zone was located within the original osteogenic phase, between the initial interface and a more distal region of generally exclusive osteogenesis. It is possible that the collagen and HA in the osteogenic phase allowed adsorption of chondrogenic factors in the area that evolved into the transition zone. This transition zone mirrors the structure of mature articular cartilage, in which a calcified cartilage layer25 is interlaced with trabecular bone, forming an interdigitated interface that connects the cartilage to the underlying bone.2,26
As with our prior work,12,15,16 these modules and constructs employ agarose as a base biomaterial that is functionalized through the addition of cells and tissue-specific matrix components. Agarose is a natural polysaccharide with excellent handling qualities, high biocompatibility, and an extensive history of use as a biomaterial for tissue engineering applications, including neurogenesis, angiogenesis, wound healing, and osteochondral tissue repair.27 The human body cannot degrade agarose enzymatically,28 which allows this material to persist in the wound location and support tissue repair in some applications. However, agarose can eventually be cleared from the mammalian body through mechanisms that are not well understood.28 Agarose has been used in a variety of in vivo applications, and if desired, it may be possible to modulate agarose degradation through the local introduction of exogenous agarases29 or by creating modified agarose polymers that are susceptible to hydrolysis or proteolytic degradation.
Conclusion
This study demonstrated that distinct chondrogenic and osteogenic tissue modules could be fabricated and cultured to promote tissue-specific differentiation. These modules were differentiated separately in static culture and could then be combined into a biphasic osteochondral construct. Low flow rate perfusion culture of osteochondral constructs in a simple multiport bioreactor chamber allowed preferential exposure of each tissue phase to appropriate conditions to promote tissue development. Over time in culture, a transition zone developed at the osteochondral interface, which mimicked aspects of the native tissue. These experiments demonstrate a modular tissue engineering strategy and provide methods for fabrication, characterization, and perfusion culture of multiphase engineered tissues.
Acknowledgments
Research reported in this publication was supported, in part, by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R21AR062709 (to J.P.S.), R01AR062636 (to J.P.S.), and P30AR069620 (Michigan Integrative Musculoskeletal Health Core Center). In addition, we would like to thank Dr. Andrew Putnam at the University of Michigan's Department of Biomedical Engineering for the use of the LDPI system.
Disclosure Statement
No competing financial interests exist.
References
- 1. Hunziker E.B. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis Cartilage 7, 15, 2015 [DOI] [PubMed] [Google Scholar]
- 2. Allan K.S., Pilliar R.M., Wang J., Grynpas M.D., and Kandel R.A. Formation of biphasic constructs containing cartilage with a calcified zone interface. Tissue Eng 13, 167, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Dormer N.H., Singh M., Wang L., Berkland C.J., and Detamore M.S. Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Ann Biomed Eng 38, 2167, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grayson W.L., Bhumiratana S., Grace Chao P.H., Hung C.T., and Vunjak-Novakovic G. Spatial regulation of human mesenchymal stem cell differentiation in engineered osteochondral constructs: effects of pre-differentiation, soluble factors and medium perfusion. Osteoarthritis Cartilage 18, 714, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng H.W., Luk K.D., Cheung K.M., and Chan B.P. In vitro generation of an osteochondral interface from mesenchymal stem cell-collagen microspheres. Biomaterials 32, 1526, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Caldwell D.J., Rao R.R., and Stegemann J.P. Assembly of discrete collagen–Chitosan microenvironments into multiphase tissue constructs. Adv Healthc Mater 2, 673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuiper N.J., Wang Q.G., and Cartmell S.H. A perfusion co-culture bioreactor for osteochondral tissue engineered plugs. J Biomater Tissue Eng 4, 162, 2014 [Google Scholar]
- 8. Levingstone T.J., Thompson E., Matsiko A., Schepens A., Gleeson J.P., and O'Brien F.J. Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomater 32, 149, 2016 [DOI] [PubMed] [Google Scholar]
- 9. Zhang T., Zhang H., Zhang L., et al. . Biomimetic design and fabrication of multilayered osteochondral scaffolds by low-temperature deposition. manufacturing and thermal-induced phase-separation techniques. Biofabrication 9, 025021, 2017 [DOI] [PubMed] [Google Scholar]
- 10. Brown W.E., Huey D.J., Hu J.C., and Athanasiou K.A. Functional self-assembled neocartilage as part of a biphasic osteochondral construct. PLoS One 13, e0195261, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nichol J.W., and Khademhosseini A. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter 5, 1312, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Batorsky A., Liao J., Lund A.W., Plopper G.E., and Stegemann J.P. Encapsulation of adult human mesenchymal stem cells within collagen-agarose microenvironments. Biotechnol Bioeng 92, 492, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Wang L., Rao R.R., and Stegemann J.P. Delivery of mesenchymal stem cells in chitosan/collagen microbeads for orthopedic tissue repair. Cells Tissues Organs 197, 333, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wise J.K., Alford A.I., Goldstein S.A., and Stegemann J.P. Comparison of uncultured marrow mononuclear cells and culture-expanded mesenchymal stem cells in 3D collagen-chitosan microbeads for orthopedic tissue engineering. Tissue Eng Part A 20, 210, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daley E.L.H., Coleman R.M., and Stegemann J.P. Biomimetic microbeads containing a chondroitin sulfate/chitosan polyelectrolyte complex for cell-based cartilage therapy. J Mater Chem B 3, 7920, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rioja A.Y., Daley E.L.H., Habif J.C., Putnam A.J., and Stegemann J.P. Distributed vasculogenesis from modular agarose-hydroxyapatite-fibrinogen microbeads. Acta Biomater 55, 144, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Portalska K.J., Chamberlain M.D., Lo C., van Blitterswijk C., Sefton M.V., and de Boer J. Collagen modules for in situ delivery of mesenchymal stromal cell-derived endothelial cells for improved angiogenesis. J Tissue Eng Regen Med 10, 363, 2016 [DOI] [PubMed] [Google Scholar]
- 18. Ward A., Quinn K.P., Bellas E., Georgakoudi I., and Kaplan D.L. Noninvasive metabolic imaging of engineered 3D human adipose tissue in a perfusion bioreactor. PLoS One 8, e55696, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3, S131, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mizuno M., Fujisawa R., and Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol 184, 207, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Zhou H., and Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater 7, 2769, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Hauschka P.V., and Wians F.H. Jr. Osteocalcin-hydroxyapatite interaction in the extracellular organic matrix of bone. Anat Rec 224, 180, 1989 [DOI] [PubMed] [Google Scholar]
- 23. Hoang Q.Q., Sicheri F., Howard A.J., and Yang D.S. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature 425, 977, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Itoh S., Kikuchi M., Takakuda K., et al. . The biocompatibility and osteoconductive activity of a novel hydroxyapatite/collagen composite biomaterial, and its function as a carrier of rhBMP-2. J Biomed Mater Res 54, 445, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Sophia Fox A.J., Bedi A., and Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sports Health 1, 461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shimomura K., Moriguchi Y., Murawski C.D., Yoshikawa H., and Nakamura N. Osteochondral tissue engineering with biphasic scaffold: current strategies and techniques. Tissue Eng Part B Rev 20, 468, 2014 [DOI] [PubMed] [Google Scholar]
- 27. Zarrintaj P., Manouchehri S., Ahmadim Z., et al. . Agarose-based biomaterials for tissue engineering. Carbohydr Polym 187, 66, 2018 [DOI] [PubMed] [Google Scholar]
- 28. Emans P.J., van Rhijn L.W., Welting T.J., et al. . Autologous engineering of cartilage. Proc Natl Acad Sci USA 107, 3418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ng K.W., Kugler L.E., Doty S.B., Ateshian G.A., and Hung C.T. Scaffold degradation elevates the collagen content and dynamic compressive modulus in engineered articular cartilage. Osteoarthritis Cartilage 17, 220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]