Abstract
Background
Infantile colic is a common disorder in the first months of life, affecting somewhere between 4% and 28% of infants worldwide, depending on geography and definitions used. Although it is self limiting and resolves by four months of age, colic is perceived by parents as a problem that requires action. Pain‐relieving agents, such as drugs, sugars and herbal remedies, have been suggested as interventions to reduce crying episodes and severity of symptoms.
Objectives
To assess the effectiveness and safety of pain‐relieving agents for reducing colic in infants younger than four months of age.
Search methods
We searched the following databases in March 2015 and again in May 2016: CENTRAL, Ovid MEDLINE, Embase and PsycINFO, along with 11 other databases. We also searched two trial registers, four thesis repositories and the reference lists of relevant studies to identify unpublished and ongoing studies.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs evaluating the effects of pain‐relieving agents given to infants with colic.
Data collection and analysis
We used the standard methodological procedures of The Cochrane Collaboration.
Main results
We included 18 RCTs involving 1014 infants. All studies were small and at high risk of bias, often presenting major shortcomings across multiple design factors (e.g. selection, performance, attrition, lack of washout period).
Three studies compared simethicone with placebo, and one with Mentha piperita; four studies compared herbal agents with placebo; two compared sucrose or glucose with placebo; five compared dicyclomine with placebo; and two compared cimetropium ‐ one against placebo and the other at two different dosages. One multiple‐arm study compared sucrose and herbal tea versus no treatment.
Simethicone. Comparison with placebo revealed no difference in daily hours of crying reported for simethicone at the end of treatment in one small, low‐quality study involving 27 infants. A meta‐analysis of data from two cross‐over studies comparing simethicone with placebo showed no difference in the number of of infants who responded positively to treatment (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.73 to 1.23; 110 infants, low‐quality evidence).
One small study (30 participants) compared simethicone with Mentha piperita and found no difference in crying duration, number of crying episodes or number of responders.
Herbal agents. We found low‐quality evidence suggesting that herbal agents reduce the duration of crying compared with placebo (mean difference (MD) 1.33, 95% CI 0.71 to 1.96; three studies, 279 infants), with different magnitude of benefit noted across studies (I² = 96%). We found moderate‐quality evidence indicating that herbal agents increase response over placebo (RR 2.05, 95% CI 1.56 to 2.70; three studies, 277 infants).
Sucrose. One very low‐quality study involving 35 infants reported that sucrose reduced hours spent crying compared with placebo (MD 1.72, 95% CI 1.38 to 2.06).
Dicyclomine. We could consider only one of the five studies of dicyclomine (48 infants) for the primary comparison. In this study, more of the infants given dicyclomine responded than than those given placebo (RR 2.50, 95% CI 1.17 to 5.34).
Cimetropium bromide. Data from one very low‐quality study comparing cimetropium bromide with placebo showed reduced crying duration among infants treated with cimetropium bromide (MD ‐30.20 minutes per crisis, 95% CI ‐39.51 to ‐20.89; 86 infants). The same study reported that cimetropium increased the number of responders (RR 2.29, 95% CI 1.44 to 3.64).
No serious adverse events were reported for all of the agents considered, with the exception of dicyclomine, for which two of five studies reported relevant adverse effects (longer sleep 4%, wide‐eyed state 4%, drowsiness 13%).
Authors' conclusions
At the present time, evidence of the effectiveness of pain‐relieving agents for the treatment of infantile colic is sparse and prone to bias. The few available studies included small sample sizes, and most had serious limitations. Benefits, when reported, were inconsistent.
We found no evidence to support the use of simethicone as a pain‐relieving agent for infantile colic.
Available evidence shows that herbal agents, sugar, dicyclomine and cimetropium bromide cannot be recommended for infants with colic.
Investigators must conduct RCTs using standardised measures that allow comparisons among pain‐relieving agents and pooling of results across studies. Parents, who most often provide the intervention and assess the outcome, should always be blinded.
Plain language summary
Pain‐relieving agents for infantile colic
Review question
Do infants who have colic during the first four months of life benefit from pain‐relieving agents (substances to alleviate/prevent pain) when compared with infants who are given no substance or a placebo (a substance that is identical to the drug but has no active ingredient)?
Background
Infantile colic, which is a common problem in infancy, occurs in the first four months of life in otherwise healthy infants. It is characterised by episodes of excessive crying and often leads to anxiety in parents and in doctors who work with infants.
Pain‐relieving agents, such as drugs (e.g. simethicone, dicyclomine, cimetropium), herbal remedies (e.g. Matricaria recutita, Foeniculum vulgare, Melissa officinalis) and sugar, have been proposed to reduce the symptoms associated with infantile colic, particularly the amount of time spent crying.
Study characteristics
We found 18 randomised controlled trials (studies in which participants were randomly assigned to one of two or more treatment groups) involving 1014 infants with infantile colic. The evidence is current to May 2016.
Infants were eight to 16 weeks old, and males and females were equally represented. All infants had colic, defined in one of two ways. Some studies defined it as inconsolable crying in otherwise healthy infants, lasting longer than three hours per day for more than three days a week for longer than three weeks. Other studies defined colic as attacks of screaming and crying (usually in the afternoon, or in the early evening) during which the infant failed to respond to any amount of comforting by adults.
Four studies explored the effects of simethicone (a drug used to reduce excess gas in the intestinal tract); four studies looked at herbal agents (plant‐derived remedies that might have relaxing properties that reduce cramps and pains in the bowel); two studies looked at sugar; and five studies explored the effects of dicyclomine and two the effects of cimetropium bromide (drugs that relieve bowel muscle spasms). One study compared sucrose and herbal tea in a group of infants who received no treatment for colic.
Sixteen of 18 studies compared the intervention with a placebo. Among the other two studies, one compared simethicone with Mentha piperita, and the other compared two different dosages of cimetropium.
Included studies received funding from different sources: a public institution (two studies), academic funds (one study) and private companies (three studies). Three studies received no funding. Nine studies did not report whether the study received funding. In four studies that reported no funds and no details about funds, private companies supplied the products (pain‐relieving agents).
Key results
Available data provide no evidence that sugar, dicyclomine and cimetropium are effective interventions in the treatment of colic. Some evidence suggests that, compared with placebo or no treatment, herbal agents may reduce crying time. However, because the quality of these studies was very poor and the extent of the benefit observed was variable, these results should be interpreted with caution. The same is true for sugar, dicyclomine and cimetropium, for which we judged the quality of evidence as low or very low.
Studies that tested simethicone reported no benefit from administration of this drug over placebo.
Two studies reported side effects for dicyclomine, for example, difficulty awakening, wide‐eyed state and drowsiness. Studies of other pain‐relieving agents reported no side effects as a result of treatment.
Quality of the evidence
Low‐quality evidence indicates that infants with colic may benefit from treatment with sugar and cimetropium, and that herbal agents may reduce crying time. Moderate‐quality evidence suggests that these agents increase the number of children experiencing improvement in symptoms. Overall, evidence is insufficient to allow firm conclusions about the benefits and side effects of the pain‐relieving agents examined for treatment of crying due to infantile colic.
Summary of findings
Summary of findings for the main comparison. Simethicone versus placebo for infantile colic.
| Simethicone versus placebo for infantile colic | ||||||
| Patient or population: infants with infantile colic Settings: university primary care centre (Sweden) and general paediatric practices (USA) Intervention: simethicone versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Simethicone vs placebo | |||||
| Reduction in crying duration Difference between final values (hours per day of crying) Follow‐up: mean 7 days | Mean crying duration in control groups was 4.37 hours/d | Mean crying duration in intervention groups was 0.13 lower (1.4 lower to 1.14 higher) | MD ‐0.13 (‐1.40 to 1.14) | 27 (1 study) | ⊕⊝⊝⊝ Very lowa,b | ‐ |
| Responders Number of infants who improved after treatment Follow‐up: mean 7 days | Study population | RR 0.95 (0.73 to 1.23) | 110 (2 studies) | ⊕⊕⊝⊝ Lowa,c | ‐ | |
| 591 per 1000 | 561 per 1000 (431 to 727) | |||||
| Moderate | ||||||
| 604 per 1000 | 574 per 1000 (441 to 743) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; MD: mean difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aHigh risk of selection, attrition and reporting bias. bOnly one study with 27 infants. cOnly two studies with 110 infants.
Summary of findings 2. Herbal agents versus placebo for infantile colic.
| Herbal agents versus placebo for infantile colic | ||||||
| Patient or population: patients with infantile colic Settings: multi‐speciality clinics (Russia); university hospitals (Turkey, Italy); primary community‐based clinics (Israel) Intervention: herbal agents versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Herbal agents vs placebo | |||||
| Reduction in crying duration Difference before and after treatment (hours per day of crying) Follow‐up: mean 7 days | Mean reduction in crying duration in control groups was 0.22 hours/d. | Mean reduction in crying duration in intervention groups was 1.33 higher (0.71 to 1.96 higher). | MD 1.33 (0.71 to 1.96) | 279 (3 studies) | ⊕⊕⊝⊝ Lowa,b | ‐ |
| Responders Number of infants who improved after treatment Follow‐up: mean 7 days | Study population | RR 2.05 (1.56 to 2.7) | 277 (3 studies) | ⊕⊕⊕⊝ Moderatec | ‐ | |
| 326 per 1000 | 669 per 1000 (509 to 881) | |||||
| Moderate | ||||||
| 257 per 1000 | 527 per 1000 (401 to 694) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; MD: mean difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aOne study with high risk of selection, performance, detection, reporting and other bias. bVery high heterogeneity (96%). cTwo studies with unclear risk of selection bias.
Summary of findings 3. Sugar versus placebo for infantile colic.
| Sugar versus placebo for infantile colic | ||||||
| Patient or population: infants with infantile colic Settings: university hospital (Turkey) Intervention: sugar versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Sugar vs placebo | |||||
| Reduction in crying duration Difference before and after treatment (hours per day of crying) Follow‐up: mean 7 days | Mean reduction in crying duration in control groups was 0.09 hours/d of crying. | Mean reduction in crying duration in intervention groups was 1.72 higher (1.38 to 2.06 higher). | MD 1.72 (1.38 to 2.06) | 70 (1 study) | ⊕⊝⊝⊝ Very lowa,b | ‐ |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; MD: mean difference. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aHigh risk of selection, performance, detection, reporting and other bias. bOnly one study with 70 infants.
Summary of findings 4. Cimetropium bromide versus placebo for infantile colic.
| Cimetropium bromide versus placebo for infantile colic | ||||||
| Patient or population: infants with infantile colic Settings: university hospital (Italy) Intervention: cimetropium bromide versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cimetropium bromide vsplacebo | |||||
| Reduction in crying duration Difference between final values (minutes per crisis of crying) Follow‐up: mean 3 days | Mean reduction in crying duration in control groups was 47.5 minutes per crisis of crying | Mean reduction in crying duration in intervention groups was 30.2 lower (39.51 to 20.89 lower) | MD ‐30.20 (‐39.51 to ‐20.89) | 86 (1 study) | ⊕⊝⊝⊝ Very lowa,b | ‐ |
| Responders Number of infants who improved after treatment Follow‐up: mean 3 days | Study population | RR 2.29 (1.44 to 3.64) | 86 (1 study) | ⊕⊝⊝⊝ Very lowa,b | ‐ | |
| 326 per 1000 | 746 per 1000 (469 to 1000) | |||||
| Moderate | ||||||
| 326 per 1000 | 747 per 1000 (469 to 1000) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; MD: mean difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aOnly one study with 86 infants. bHigh risk of attrition bias.
Background
Infants cry for various reasons to express discomfort caused by conditions ranging from benign disorders to life‐threatening illness. Heine 2006 suggested that less than 5% of distressed infants have identifiable medical explanations for their crying. Infantile colic, which is defined as excessive crying in the first few months of life, is a common but poorly understood and often frustrating problem for parents and carers, and is frequently a reason for consultation with paediatricians and community nurses (Freedman 2009).
Description of the condition
Infantile colic represents a clinical condition with a reported incidence from 4% to 28%; this wide range of occurrence seems not to be associated with factors such as nationality and clinical criteria (i.e. gender, socioeconomic class, type of feeding, family history of atopy, and parental smoking) (Lucassen 2001; Lucassen 2015; Vandenplas 2015). Infantile colic is characterised by inconsolable crying, fussing and irritability in an otherwise healthy newborn during the first three months of life. Infant crying tends to occur in the evening and usually increases at six weeks of age, with drawing up of the legs, tension of the body, flushing of the face, painful bowel movements and meteorism (abdominal bloating). The diagnosis is clinical, and the most often cited definition is based on the rule of three, that is, unexplained episodes of paroxysmal crying for longer than three hours per day for three days per week for at least three weeks (Wessel 1954). Many other definitions are available, reflecting different conditions with other risk factors (Reijneveld 2002). Infantile colic shows a wide range of clinical manifestations and can be graded as mild, moderate or severe, but no consensus is known for this classification. The natural history of infantile colic is favourable, and symptoms gradually disappear by around three months of age.
It has been suggested that both biological components (food hypersensitivity/allergy and gut dysmotility) and behavioural factors (psychological and social) may play a role in the development of colic (Gupta 2007). It seems that some infants are predisposed to visceral hypersensitivity and hyperalgesia in the first weeks of life.
Available evidence suggests that infantile colic might have several independent causes, including those listed below (Savino 2007).
Carbohydrate malabsorption, in particular, lactose intolerance due to a relative lactase deficiency (Kanabar 2001).
Food hypersensitivity (cow's milk allergy; Hill 2000; Iacono 2005). Colic might represent an early manifestation of food allergy, although results of studies investigating a link between infant colic and atopy have been conflicting (Gupta 2007; Heine 2006; Iacono 1991). Some infants with moderate or severe symptoms have cow’s milk‐dependent colic that improves after a few days of a hypoallergenic diet. Therefore, in bottle fed babies, a two‐to‐four‐week trial of extensively hydrolysed formulae has been recommended (Fiocchi 2010; Nocerino 2015).
Feeding disorder, that is, disorganised feeding behaviour and lower responsiveness during feeding interaction with mother (Miller‐Loncar 2004).
Dysmotility. Some researchers have suggested that transient dysregulation of the nervous system during development may cause intestinal hypermotility in infants with colic; the predominance of the parasympathetic and the sympathetic nervous system has also been investigated (Garrison 2000; Lucassen 1998; Savino 2002; Weissbluth 1984).
Gut microflora. Lehtonen 1994 first hypothesised that infantile colic may arise from an aberrant gut microbial composition in the first months of life that affects the intestinal fatty acid profile. The role of peculiar intestinal lactobacilli and a particular coliform colonisation pattern has been proposed in the etiopathogenesis of the condition (Savino 2004; Savino 2005b; Savino 2009). More recently, Rhoads demonstrated that gut inflammation and an altered, less diverse fecal flora are seen in infants with colic (Rhoads 2009).
Psychological factors, such as personality disturbance in the child or less than optimal parent–infant interactions (Akman 2006; Canivet 2000; Räihä 2002; Van den Berg 2009; Vik 2009).
Possibly higher rate of night wakening and less nocturnal sleep (Lehtonen 1994b). Data suggest that colic may be associated with disruption and delay in maturation of the circadian rhythm and sleep‐wake organisation, both of which resolve when colic disappears; however, the topic of effects of colic on sleep remains controversial (Sadeh 2009).
Recent hypotheses. Effects of hormone alterations (Savino 2006) and maternal smoking (Canivet 2008) remain to be confirmed.
Infantile colic is a clinical entity with a wide range of presentations and outcomes. Paediatricians should first exclude other underlying diseases through medical examination and should prevent feeding disorders. Then, in light of the favourable clinical course of the condition, healthcare providers should assist parents in adopting safe and well‐tolerated strategies (Savino 2010).
Description of the intervention
Treatment approaches can be grouped into the following categories: pharmacological treatments (e.g. dicyclomine hydrochloride, cimetropium bromide, simethicone), probiotics, complementary therapies (including herbal agents and sucrose), manipulative therapies (including acupuncture), dietary interventions and parental behavioural interventions (Savino 2014). A Cochrane review has examined the effectiveness of manipulative therapies (Dobson 2012); two other Cochrane reviews are ongoing ‐ one on the effectiveness of probiotics (Praveen 2014) and another on dietary modification (Savino 2014b).
This review examines the effectiveness and safety of the following pain‐relieving agents: pharmacological interventions (dicyclomine hydrochloride, cimetropium bromide and simethicone) and complementary therapies (herbal formulations, sucrose or glucose). The development of visceral pain in infancy is a highly complex process with important implications for analgesic policy and clinical management. These agents are aimed at reducing gastrointestinal discomfort, which has been theoretically linked with infantile colic.
Dicyclomine hydrochloride is an anticholinergic drug with antispasmodic activity that is used to relax muscles in the wall of the gut and prevent spasms. Despite some findings of effectiveness in infantile colic, adverse effects have been reported in about 5% of treated infants. Drowsiness, diarrhoea and constipation are most commonly reported, but severe adverse effects, such as apnoea, breathing difficulties, seizures and coma, have also occurred (Edwards 1984; Garriott 1984; Randall 1986; Williams 1984). For this reason, use of anticholinergic drugs is now contraindicated in infants before six months of age (Garrison 2000). Nevertheless, we decided to include dicyclomine in our review for completeness, that is, to perform a comprehensive systematic review that includes all of the agents that have been used or are actually used to treat infant colic, even if one or some of them are not yet recommended because of their ineffectiveness or adverse events.
Cimetropium bromide is an antimuscarinic compound derivative of belladonna with considerable penetration in the blood‐brain barrier. It shows competitive, surmountable antagonism of muscarine receptors of visceral smooth muscle and direct myolytic activity (Bassotti 1987; Imbimbo 1986; Sagrada 1989; Scarpignato 1985; Schiavone 1985). Cimetropium bromide has been well tolerated in infants when administered at the tested dosage. The only registered side effect is increased sleepiness that might be related to pain resolution rather than to central nervous system effects (Savino 2002).
In addition to conventional therapies, the anticholinergic and antiadrenergic activities of some herbal formulations, such as fennel, lemon balm and chamomile, have been proposed to relieve pain (Savino 2005; Weizman 1993).
Simethicone silicone latex, a defoaming agent, is a pharmacological agent that could act as a detergent to reduce the surface tension of bubbles in the intestinal tract, in theory enabling abdominal gas to be expelled more easily. It is safe and may reduce meteorism (abdominal bloating; Metcalf 1994; Sethi 1988).
How the intervention might work
Potential remedies for the management of infantile colic have shown different mechanisms of action; however, the ultimate goal is to relieve pain.
Many years ago, researchers stated that most cases of infantile colic could be explained by colonic hyperperistalsis and increased rectal pressure. In particular, the early literature refers to colic as "hypertonia of infancy". Predominance of the parasympathetic as well as the sympathetic nervous system has been investigated. Indeed, the gastrointestinal tract contains a wide variety of hormones involved in the regulation of intestinal motility (i.e. vasoactive intestinal peptide (VIP), gastrin, motilin (Lothe 1987), and ghrelin). Lothe 1990 hypothesised that motilin, whose serum levels were increased in infants who developed colic, might play a central role in the etiopathogenesis of the condition through its activity in enhancing gastric emptying through increased small‐bowel peristalsis and decreased transit time. In another study, colicky infants presented higher serum levels of motilin and ghrelin compared with their healthy counterparts, suggesting that ghrelin may be implicated in promoting abnormal hyperperistalsis (Savino 2006). These concepts supported the hypothesis of beneficial effects derived from drugs with antispasmodic effects, such as dicyclomine hydrochloride, cimetropium bromide and some herbal formulations. Dicyclomine, which relaxes muscles in the wall of the gut and prevents spasms, has been used in the treatment of infantile colic on the assumption that spasms of intestinal smooth muscle cause colic symptoms (Grunseit 1977; Hwang 1985; Illingworth 1959; Weissbluth 1984). Cimetropium bromide may reduce intestinal sensitivity and hypermotility through its competitive antagonism of muscarine receptors of the visceral smooth muscles and by its spasmolytic activity (Bassotti 1987; Imbimbo 1986; Sagrada 1989; Scarpignato 1985; Schiavone 1985). Fennel, lemon balm and chamomile may be effective in the treatment of infantile colic because of their anticholinergic and antiadrenergic activities. In particular, in animal models, upper gastrointestinal transit has been influenced by the oral administration of an herbal formulation containing extracts from Matricaria recutita flowers (chamomile), Foeniculum vulgare (fennel) and the aerial parts of Melissa officinalis (lemon balm) (Capasso 2007).
Excessive intraintestinal air load, aerophagia and pain, which are characteristic symptoms of colic crying, may be related to increased production of gas in the lower bowel (Sferra 1996). Treem 1994 suggested that colicky infants produce large amounts of gas, probably as the result of colonic bacterial fermentation of malabsorbed dietary carbohydrate, and that they are relieved of symptoms by the passage of gas. Simethicone decreases abdominal distension and discomfort due to excessive gas production through dispersion of gas bubbles from the gastrointestinal tract. For this reason, it has been studied as treatment for colicky infants, with researchers postulating that physical signs during colic episodes, such as bearing down and passage of flatulence, suggest excessive gas (Danielsson 1985; Metcalf 1994; Sethi 1988).
Finally, oral sugar solution has proved to have analgesic and calming effects on newborns (Carbajal 1999; Skogsdal 1997).
Why it is important to do this review
Infantile colic is a frequent but poorly understood and often distressing problem for parents and carers. The favourable clinical course, the range of ways in which it manifests and the day‐to‐day variability in crying time suggest that a well‐tolerated, multi‐factorial and graded strategy should be adopted.
Two systematic reviews have focused on therapeutic interventions for colic (Garrison 2000; Lucassen 2001), but these are now well out‐of‐date. A more recent review, published in 2011, did not include herbal formulations (Hall 2012). A recent Cochrane review examined the effectiveness of manipulative therapies (Dobson 2012); two other Cochrane reviews are ongoing ‐ one on the effectiveness of probiotics (Praveen 2014), and another on dietary modifications (Savino 2014; Savino 2014b). Ultimately, up‐to‐date systematic reviews should seek to inform clinical guidelines for the treatment of infants with colic.
Objectives
To assess the effectiveness and safety of pain‐relieving agents for reducing colic in infants younger than four months of age.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Infants younger than four months of age at enrolment who had infantile colic, as confirmed by a physician. Infantile colic is defined as a prolonged period of crying for no apparent reason in an otherwise healthy infant. For inclusion in this review, we accepted all definitions of excessive crying, and both breast fed and bottle fed infants were eligible.
We excluded studies of infants with crying of normal duration.
Types of interventions
We included any pain‐relieving agent used for the treatment of infant colic, that is, pharmacological interventions (dicyclomine, cimetropium bromide, simethicone) and complementary interventions (herbal formulations, sucrose or glucose). These agents could be compared with placebo or with no treatment. We also included studies that compared two different agents against each other and we performed separate analyses.
Types of outcome measures
Primary outcomes
Reduction in crying duration (post‐treatment vs baseline)* (available data may be continuous, for example, hours per day, or dichotomous, for example, reduction under a threshold defined by trialists)
Responders* (dichotomous outcome), defined as proportions of participants who showed improvement by the end of treatment, according to the measures used by study authors
Secondary outcomes
Reduction in frequency of crying episodes (post‐treatment vs baseline)* (available data may be continuous, for example, hours per day, or dichotomous, for example, reduction under a threshold defined by trialists)
Parental or family quality of life, including measures of parental stress, anxiety or depression (continuous outcome)
Sleeping time, that is, change in duration of peaceful sleeping (post‐treatment vs baseline)* (continuous outcome)
Parental satisfaction, measured by Likert scales or on a numerical rating scale (NRS) (continuous outcome)
Adverse effects: constipation, vomiting, apnoea, apparent life‐threatening events (ALTEs) and lethargy* (dichotomous outcome)
Timing of outcome assessment: We included outcomes evaluated after completion of any treatment protocol (i.e. any period, any number of treatments) and at later follow‐up, if reported.
*We included those outcomes marked with an asterisk (*) in Table 1, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2008).
Search methods for identification of studies
We ran the initial searches in April 2012 with no limitations by date, language or publication type. We updated the searches in April 2014, and added an age filter to the strategies for CENTRAL, Ovid MEDLINE and Embase to reduce the number of irrelevant records. We updated the searches most recently on 27 March 2015, and on 16 May 2016. We reported details about each set of searches in Appendix 1, and reported search strategies for each source in Appendix 2.
Electronic searches
We searched the electronic databases listed below.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4) in the Cochrane Library (searched 16 May 2016), which includes the Cochrane Developmental, Psychosocial and Learning Problems Group Specialised Register.
MEDLINE Ovid (1946 to May week 1 2016).
MEDLINE(R) In‐Process & Other Non‐Indexed Citations Ovid (13 May 2016).
Embase Ovid (1980 to 2016 week 20).
PsycINFO Ovid (1806 to May week 2 2016).
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to current).
Science Citation Index Web of Science (SCI; 1970 to 16 May 2016).
Social Science Citation Index Web of Science (SSCI; 1970 to 16 May 2016).
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S; 1970 to 16 May 2016).
Conference Proceedings Citation Index ‐ Social Sciences & Humanities Web of Science (CPCI‐SS&H; 1970 to 16 May 2016).
Cochrane Database of Systematic Reviews (CDSR; 2016, Issue 5) in the Cochrane Library (searched 16 May 2016).
Database of Abstracts of Reviews of Effects (DARE; 2016, Issue 2) in the Cochrane Library (searched 16 May 2016).
WorldCat (limited to theses and dissertations; www.worldcat.org; searched 17 May 2016).
HOMEOINDEX (Virtual Health Library; bvsalud.org/en; searched 17 May 2016).
LILACS (Latin American and Caribbean Health Science Information Database; Virtual Health Library; lilacs.bvsalud.org/en; searched 16 May 2016).
Networked Digital Library of Theses and Dissertations (SCIRUS) (NDLTD; all available years up to 2012. Not available via SCIRUS after 2012. Searched again via search.ndltd.org/index.php on 17 May 2016).
IBECS (Virtual Health Library; bvsalud.org/en; searched 17 May 2016).
ClinicalTrials.gov (clinicaltrials.gov; searched 17 May 2016).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; who.int/ictrp/en; searched 17 May 2016).
TROVE (limited to Australian theses; trove.nla.gov.au; searched to 27 March 2015).
DART‐Europe E‐theses Portal (www.dart‐europe.eu/basic‐search.php; searched to 27 March 2015).
Searching other resources
We evaluated bibliographies of articles identified through the electronic searches to look for additional published and unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (FS, VT) independently screened titles and abstracts yielded by the searches, discarding irrelevant records. Review authors then retrieved the full text of all potentially eligible articles to assess them independently against the inclusion criteria. We resolved discrepancies through discussion and, when necessary, by consultation with a third review author (EB). If information was not forthcoming, or if we were unable to resolve the dispute, we approached the Cochrane Developmental, Psychosocial and Learning Problems Group (CDPLPG) editorial base for advice.
Data extraction and management
We developed data extraction forms a priori, as per recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We extracted the information listed below.
Methods: study design, setting, duration, recruitment procedures, risk of bias (such as sequence generation, allocation concealment, blinding of outcome assessors, evaluation of success of blinding).
Participants: source of participants, inclusion/exclusion criteria, total number at baseline, total number at completion, definition of 'colic' applied, diagnostic criteria applied, age at onset of colic, age at commencement of intervention, evaluation of potential effects of confounding characteristics (e.g. age, gender, breast fed or bottle fed).
Interventions and controls: number of groups, intervention(s) applied, frequency and duration of treatment, total number of treatments, permitted co‐interventions, evaluation of potential therapeutic value of sham/placebo.
Outcomes: list of outcomes assessed, definitions used, values for mean and standard deviation (SD) at baseline and at time points defined by the study protocol (or change from baseline measures, if given).
Results: measures at end of protocol, follow‐up data (including means, SDs, standard errors and confidence intervals (CIs) for continuous data and frequencies for dichotomous data), withdrawals, loss to follow‐up.
Other: references to other relevant studies, points to follow up on with study authors, comments from review authors, key conclusions of the study (of study authors), other comments from review authors.
Two review authors (FS, VT) extracted data independently using the data extraction form. The third review author (EB) resolved disagreements.
We used the latest version of Review Manager (RevMan) software (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors (FS, VT) independently evaluated each study for risk of bias using the criteria recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b) for the domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other potential threats to validity. For each included study, review authors rated each domain as having low, high or unclear risk of bias, and then compared their grading. In the case of differently scored items, the two review authors tried to reach agreement by discussion. If this was not possible, we discussed disagreements with the rest of the team until consensus was reached. Review authors were not blinded to the titles of journals nor to the identities of study authors, as they are familiar with the field. We provide in Appendix 3 a detailed description of the criteria used to judge risk of bias for each domain.
In this context, parents often administered the intervention. Thus, we primarily assessed the risk of bias associated with blinding of participants and personnel on the likelihood that such blinding was sufficient to ensure that parents had no knowledge of which intervention the infant received. We considered blinding of participants to be unnecessary in this population of young infants.
We considered as outcome assessors both parents and those who interpreted the crying diaries (paediatrician, nurse).
Measures of treatment effect
Dichotomous data
For dichotomous data, we calculated effect sizes as risk ratios (RRs) with their associated 95% CIs and probability values (P values), when possible. When the RR did not straddle the position of null effect, we pooled dichotomous data and calculated the number needed to treat for an additional beneficial outcome (NNTB) and the associated 95% CI.
Continuous data
For continuous outcomes, we presented mean differences (MDs) in change scores or final values, according to available data, and 95% CIs. If studies used different scales to measure the same outcome, we used the standardised mean difference (SMD) to standardise the MD to a uniform scale.
Unit of analysis issues
For each included study, we determined whether the unit of analysis was appropriate for the unit of randomisation and the design of each study (in other words, whether the number of observations matched the number of randomised 'units') (Deeks 2008).
Studies with multiple treatment arms
When we found multi‐arm studies, we combined results across all eligible intervention (pain‐relieving agents) arms, making single, pair‐wise comparisons, but we divided the sample size for common comparator arms proportionately across each comparison (Higgins 2008c). This simple approach allowed the use of standard software (including RevMan 2014) and prevented inappropriate double‐counting of individuals. When such a strategy prevented investigation of potential sources of heterogeneity, we analysed each pain‐relieving agent separately.
Cross‐over studies
In randomised cross‐over studies, individuals receive each intervention sequentially in random order. One problem with this design involves the risk of carry‐over effect, which occurs when the first treatment affects the second. To reduce the carry‐over effect, cross‐over studies usually include a washout period, that is, a stage after the first treatment but before the second treatment during which time is given for the active effects of the first treatment to wear off before the new treatment is begun. Inadequate washouts are seen when the carry‐over effect exceeds the washout period. For this review, we considered a minimum of one day to be an adequate washout period for cross‐over studies.
We used the inverse variance method, as recommended by Elbourne 2002, to include data from cross‐over studies with an adequate washout period. To take account of the correlation between the two study periods, we calculated the correlation co‐efficient between periods for each study (Savino 2012). When the correlation co‐efficient could not be obtained, we used data from the first period only. For continuous data, no studies reported the SD of a paired t‐test, and for binary data, only one of the included studies with a planned washout period reported the number of participants who responded to both treatments (Metcalf 1994). Consequently, we decided to analyse cross‐over trials as if they were parallel‐group trials. This approach, even if it is not the most correct, is conservative, as it overestimates the variability between study periods. Furthermore, we conducted separate meta‐analyses for both cross‐over and parallel‐group trials, thus avoiding the unit of analysis error.
For cross‐over studies with an inadequate washout period, we used data from the first period only. If data from the first period were not available, we did not incorporate these studies into the meta‐analysis.
Dealing with missing data
For missing continuous data, we estimated SDs from other available data, such as standard errors, or we imputed them using the methods described in Higgins 2011c. We made no assumptions about loss to follow‐up, and we based our analyses on participants who completed the trial.
For missing dichotomous outcomes, we investigated the effects of dropout and exclusion by conducting analyses of worst‐case versus best‐case scenarios.
If we noted a discrepancy between the number randomised and the number analysed in each treatment group, we calculated and reported the percentage lost to follow‐up for each group.
For all included studies, we analysed available data. When we observed that data were missing, we recorded this on the data collection form and reported it in the 'Risk of bias' table (beneath the Characteristics of included studies tables), and in the Discussion section of the review, we considered the extent to which the missing data could alter our results and conclusions.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the distribution of important participant factors (e.g. age) across trials, interventions and outcomes. We assessed methodological heterogeneity by comparing the distribution of important trial factors (e.g. study design, risk of bias (such as randomisation concealment, blinding of outcome assessment), losses to follow‐up).
We assessed statistical heterogeneity by examining the I² statistic (Deeks 2008), a quantity that describes the proportion of variation in point estimates that is due to variability across studies rather than to sampling error. We interpreted the I² statistic as recommended in the latest version of Higgins 2011c, as follows.
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: may represent considerable heterogeneity.
We also evaluated the CI for the I² statistic.
In addition, we employed a Chi² test of homogeneity to determine the strength of the evidence that heterogeneity was genuine, and used Tau² to assess between‐study variability.
Assessment of reporting biases
To minimise publication bias, we attempted to obtain the results of unpublished studies to compare results extracted from published journal reports with results from other sources (including correspondence).
Data synthesis
When interventions were similar in terms of type of pain‐relieving agent, type of outcome assessed and type of colic, we grouped these studies and synthesised their results in a meta‐analysis. We presented results for each combination of pain‐relieving agent and assessed outcome and colic type, except in studies for which no data were provided.
Because we assumed that clinical heterogeneity was very likely to impact the results of our review, given the wide breadth and types of interventions included, we combined studies by using a random‐effects model, regardless of statistical evidence of heterogeneity for effect sizes. We calculated all overall effects by using the inverse variance method. We converted continuous data to MD, and if different scales were used, we first computed SMD, then overall MD and overall SMD (Schünemann 2008). If both a continuous outcome and a dichotomous outcome were available for a particular outcome, we included only the continuous outcome in the primary analysis. If some studies reported an outcome as a dichotomous measure and others used a continuous measure for the same construct, we converted results of the former from an odds ratio (OR) to an SMD (Deeks 2011), provided that we could assume the underlying continuous measure had approximated a normal or logistical distribution (otherwise, we carried out two separate analyses).
We carried out statistical analyses by using RevMan 2014.
Summary of findings table
We summarised the evidence in 'Summary of findings' tables and provided summary estimates of absolute and relative effects (see Table 1; Table 2; Table 3; and Table 4). We included a rating (ranging from very low to high) of our confidence in the estimate of effect for the overall quality of evidence for each outcome, as assessed via the GRADE approach (Guyatt 2008; Guyatt 2013). We used an iterative, electronic correspondence discussion process to reach consensus on factors that affect confidence in the estimate of effects (including risk of bias, i.e. design and study limitations; imprecision; indirectness (directness in the GRADE approach includes generalisability and applicability); inconsistency of results, i.e. heterogeneity; magnitude of effect; and issues of residual plausible confounding); and in evidence rating.
Subgroup analysis and investigation of heterogeneity
We performed no subgroup analyses because we included too few studies in each comparison, making subgroup analyses impossible or non‐informative.
Subgroup analyses archived for future updates of this review can be found in Appendix 4 and in our protocol (Savino 2012).
Sensitivity analysis
We conducted sensitivity analyses to determine whether findings were sensitive to restriction of analyses to studies judged to be at low risk of bias for blinded assessment of the primary outcome. When sensitivity analyses confirmed results of the main analysis, we regarded results of the review with a higher degree of certainty.
We did not conduct planned sensitivity analyses to investigate the impact of missing data on results because the percentage of missing data was low in all included studies (ranging from 0% to 16.7%; see Table 5). These sensitivity analyses, which have been archived for future updates of this review, can be found in Appendix 4 and in our protocol (Savino 2012).
1. Summary of study characteristics.
| Study | N°infants enrolled | N° infants analysed | Mean age (SD) in weeks at study entry | Male (%) | Intervention/control | Loss to follow‐up (%) (intervention/control) | Study treatment duration (in days) | Study design | Outcomes |
| Akçam 2006 | 30 | 25 | 9.1 (5.9) | 40 | Glucose (30%)/placebo | 16.7 | 4 | Randomised, cross‐over | Responders Adverse effects |
| Alexandrovich 2003 | 125 | 121 | 4.3 (1.1) | 45.5 | Fennel seed oil emulsion/placebo | 4.6/1.7 | 7 | Randomised, parallel‐arm | Responders (as relief of colic symptoms) Cumulative crying time (hours/week) Number of doses/d Consumed mL/d Adverse effects |
| Alves 2012 | 30 | 30 | 4.7 (1.6) | 45.5 | Simethicone/herbal agents (Mentha piperita) | 0 | 7 | Randomised, cross‐over | Responders (as improvement in symptoms) Frequency of colic episodes (daily) Daily colic duration (minutes) Duration of colic Adverse effects |
| Arikan 2008 | 175 | 105* | 8.24 (2.88) | 55.5 | Herbal tea or sucrose/no treatment | 0 | 7 | Randomised, parallel‐arm | Crying time (hours/d) |
| Blomquist 1983 | 18 | 18 | Range 2 to 14 | 44.4 | Dicyclomine plus sugar/placebo | 0 | 7 | Randomised, cross‐over | Responders (as improvement in colic severity) Frequency of crying episodes |
| Danielsson 1985 | 27 | 27 | 4.8 (range 2 to 8) | 44.4 | Simethicone/placebo | 0 | 7 | Quasi‐randomised, cross‐over | Responders Crying time (hours/d) Time sleeping (hours/d) Number of feedings Number of stools |
| Gomirato 1989 | 40 | 40 | 4.4 | 40 | Cimetropium bromide (1.2 mg/kg)/cimetropium bromide (2.0 mg/kg) | 0 | 14 | Randomised, parallel‐arm | Responders (as improvement in symptoms) Number of crying episodes/d Duration of longest episodes (minutes) Adverse effects |
| Grunseit 1977 | 25 | 22 | 5.4 (range 3 to 12) | 40.9 | Dicyclomine/placebo | 12.0 | 7 | Randomised, cross‐over | Score of symptoms (as continuous measure) |
| Hwang 1985 | 30 | 30 | 4 to 5 | Not reported | Dicyclomine/placebo | 0 | 7 | Quasi‐randomised, cross‐over | Responders (as improvement in symptoms) Crying time (hours/d) Sleeping time (hours/d) Adverse effects |
| Illingworth 1959 | 16 | 16 | < 8 | Not reported | Dicyclomine/placebo | 0 | 7 | Randomised, cross‐over | Score of symptoms Responders (as improvement in symptoms) |
| Markestad 1997 | 19 | 19 | 7.3 (3.4) | 68.4 | Sucrose (12%)/placebo | 0 | 8 to 12 | Randomised, cross‐over | Responders (as improvement in symptoms) Parental satisfaction |
| Metcalf 1994 | 83 | 83 | Not reported | 49.4 | Simethicone/placebo | 0 | 7 (3 to 10) | Randomised, cross‐over | Responders (as improvement in symptoms) |
| Montaseri 2013 | 60 | 50 | 8 (4 to 16) | 50 | Fumaria extract/placebo | 17.0 | 7 | Quasi‐randomised | Frequency of crying attack Crying duration Frequency of waking up (all as categorical variables) |
| Savino 2002 | 97 | 86 | Range 2 to 8 | Not reported | Cimetropium bromide/placebo | 9.8/13.0 | 3 | Randomised, parallel‐arm | Responders Adverse effects |
| Savino 2005 | 93 | 88 | 4.3 (1.5) | 46.6 | Herbal tea/placebo | 4.7/6.0 | 7 | Randomised, parallel‐arm | Responders Crying time (minutes/d) Adverse effects |
| Sethi 1988 | 26 | 26 | Range 1 to 12 | 40 | Simethicone/placebo | 0 | 7 | Randomised, cross‐over | Frequency of crying (daily) Amplitude of crying attack |
| Weissbluth 1984 | 48 | 48 | 5 | 58 | Dicyclomine/placebo | 0 | 14 | Randomised, parallel‐arm | Responders Adverse effects |
| Weizman 1993 | 72 | 68 | 3.3 (1.2) | 38.6 | Herbal tea/placebo | 8.3/2.8 | 7 | Randomised, parallel‐arm | Responders (as colic eliminated) Frequency of nocturnal awakenings Colic improvement score |
*105 infants analysed for the three groups included in this review.
SD: standard deviation.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies.
Results of the search
The electronic search identified 1306 records up to 16 March 2016.
After removing duplicates, we identified 1060 potentially relevant records. Two review authors (FS, VT) screened titles and abstracts for relevance and excluded 1032 records. We retrieved full‐text reports of the remaining 28 records and assessed these against the inclusion/exclusion criteria (Criteria for considering studies for this review). We excluded nine studies (see Excluded studies) and identified one ongoing study (see Characteristics of ongoing studies), leaving 18 eligible studies that contributed to 19 comparisons (see Characteristics of included studies). A third independent review author (EB) screened reports of studies in which FS collaborated as a study author.
See Figure 1 for the study flow diagram.
1.

Study flow diagram.
Included studies
Eighteen studies involving 1014 infants met the inclusion criteria for this review (see Characteristics of included studies). The selected studies were conducted between 1959 (Illingworth 1959) and 2013 (Montaseri 2013).
Study design
All studies were RCTs. We found no quasi‐RCTs. Ten of 18 studies (56%) were cross‐over trials (Akçam 2006; Alves 2012; Blomquist 1983; Danielsson 1985; Grunseit 1977; Hwang 1985; Illingworth 1959; Markestad 1997; Metcalf 1994; Sethi 1988).
Setting
Eleven studies were conducted in Europe (Akçam 2006; Arikan 2008; Blomquist 1983;Danielsson 1985; Gomirato 1989; Hwang 1985; Illingworth 1959; Markestad 1997; Savino 2002; Savino 2005; Sethi 1988), three in America (Alves 2012; Metcalf 1994; Weissbluth 1984), two in Asia (Montaseri 2013; Weizman 1993), one in Russia (Alexandrovich 2003) and one in Australia (Grunseit 1977).
Most of the studies were performed in children's hospitals (Alexandrovich 2003; Alves 2012; Arikan 2008; Gomirato 1989; Illingworth 1959; Markestad 1997; Savino 2002; Savino 2005, Weissbluth 1984), four in primary care clinics (Grunseit 1977; Hwang 1985; Montaseri 2013; Weizman 1993) and the remaining five in general practitioner and paediatric outpatient clinics (Akçam 2006; Blomquist 1983; Danielsson 1985; Metcalf 1994; Sethi 1988).
Participants
The number of participants randomised to intervention and control groups ranged from 18 (Blomquist 1983) to 175 (Akçam 2006).
Participant age ranged from about one week (Sethi 1988) to 16 weeks (Montaseri 2013). Two studies did not provide the ages of enrolled infants (Metcalf 1994; Savino 2002).
Definition of colic
The definition of infant colic most commonly used within the broader literature is that given by Wessel 1954: "inconsolable crying for more than three hours per day for more than three days a week for more than three weeks". A total of 13 of the 18 included studies used this definition (Akçam 2006; Alexandrovich 2003; Alves 2012; Arikan 2008; Gomirato 1989; Hwang 1985; Markestad 1997; Metcalf 1994; Montaseri 2013; Savino 2002; Savino 2005; Weissbluth 1984; Weizman 1993), and some used minor modifications or more specific definitions (Akçam 2006; Alexandrovich 2003; Hwang 1985; Markestad 1997; Metcalf 1994; Montaseri 2013). Grunseit 1977 defined infant colic as "post‐prandial attacks of screaming and crying, unabated by maternal comforting, vomiting and sleep disturbance", and Illingworth 1959 reported that "the diagnosis was based on rhythmical attacks of screaming in the evenings in well, thriving babies who were gaining not less than seven oz per week during the period of observation, screaming unabated when the baby was picked up". The three remaining studies provided no definition of infant colic (Blomquist 1983; Danielsson 1985; Sethi 1988).
Pain‐relieving agents
Pain‐relieving agents varied across studies.
Simethicone was used in four studies (Alves 2012; Danielsson 1985; Metcalf 1994; Sethi 1988).
Herbal formulations were used in four studies (Alexandrovich 2003; Montaseri 2013; Savino 2005; Weizman 1993).
Sucrose or glucose was used in three studies (Akçam 2006; Arikan 2008; Markestad 1997).
Dicyclomine was used in five studies (Blomquist 1983; Grunseit 1977; Hwang 1985; Illingworth 1959; Weissbluth 1984).
Cimetropium bromide (a drug that is distributed only in Italy and in Corea) was used in two studies (Gomirato 1989; Savino 2002).
Herbal tea was used in one study (Arikan 2008).
Control conditions
In all but three studies, the control arm was given placebo. Gomirato 1989 evaluated two different dosages of cimetropium bromide (1.2 mg/kg vs 2.0 mg/kg); Arikan 2008 compared sucrose or herbal tea versus no treatment; and Alves 2012 compared simethicone medication against Mentha piperita.
Duration and frequency of treatments
Treatment schedules varied among studies.
Ten studies lasted for 14 days. Infants received the first treatment for seven days, then crossed to the other treatment group for the next seven days (Danielsson 1985; Gomirato 1989; Grunseit 1977; Hwang 1985; Illingworth 1959; Markestad 1997; Metcalf 1994; Sethi 1988; Weissbluth 1984; Weizman 1993).
Two studies administered treatment for one week (Arikan 2008; Montaseri 2013).
One study lasted for eight days (Akçam 2006). Infants were administered treatment or placebo for four days, then were transferred over to the other study treatment arm for the next four days.
One study delivered treatment over a three‐week period: one week before enrolment to measure crying time followed by two weeks of treatment (Alexandrovich 2003).
Infants enrolled in one study received Mentha piperita for one week, then after three days of washout received simethicone for the next seven days (Alves 2012).
One study had a 15‐day duration consisting of one week of treatment and one day of washout, then cross‐over, followed by seven days of placebo (Blomquist 1983).
One study provided three days of treatment (Savino 2002).
One study lasted for 10 days: After three days of observation, infants were treated with an herbal agent or with placebo for a period of one week (Savino 2005).
Outcomes
Primary outcomes
All studies provided data on at least one primary outcome (e.g. reduction in crying duration, responders).
Table 6 shows details on different definitions of responders as given by different study authors.
2. Responders' definitions.
| Author | Responders' definitions (as reported in the article) | Notes on definitions (as considered in the review) |
| Akçam 2006 | At each visit, parents described the effect of the last treatment on a scale of 6: 0 = 'getting worse', 1 = 'no improvement', 2 = 'mild improvement', 3 = 'moderate improvement' and 4 = 'completely well after each dose'. Responders were infants with improvement classified as 2, 3 or 4. | ‐ |
| Alexandrovich 2003 | Relief of colic symptoms, which were defined as decrease in cumulative crying to < 9 hours/week | ‐ |
| Alves 2012 | Responses were classified as slightly improved, greatly improved and completely improved. | From the results, all included patients seemed to show improvement. We considered as responders infants who had "completely improved" ‐ these infants had colic cessation. |
| Arikan 2008 | N/A | |
| Blomquist 1983 | Parents' ratings of the effects of infantile colic: excellent, good, moderate, worsening | Data from diagram 2: We considered as responders infants with excellent or good effects |
| Danielsson 1985 | Number of infants with improvement in symptoms (defined as better or much better) | ‐ |
| Gomirato 1989 | In the Methods section, improvement or worsening of symptoms was classified as follows: ‐ 2 crisis longer than baseline (> 60 minutes); ‐ 1 crisis longer than baseline (within 30 to 60 minutes); 0 = no improvement; + 1 crisis shorter than baseline (within 30 to 60 minutes); + 2 crisis shorter than baseline (> 60 minutes). In the Results section, only percentages of infants with excellent, good, moderate or poor improvement were reported. | We considered as responders infants with excellent or good improvement. |
| Grunseit 1977 | Pooled scoring symptoms (including postprandial crying, postprandial vomiting, sleeping disturbance); for each symptom, 0 = no symptom, 1 = mild, 2 = moderately severe, 3 = severe | ‐ |
| Hwang 1985 | Number of infants who improved receiving treatment or placebo | ‐ |
| Illingworth 1959 | Results were graded from ‐ 3 to + 3: + 1 the child was slightly better, + 2 definitively better but still had some discomfort, + 3 the infant was very greatly improved and free from symptoms. ‐ 1 the infant was slightly worse. | In the Results section, infants with + 3 seemed to be considered as responders. |
| Markestad 1997 | Methods section: Parents described effects of the last treatment on a scale from 5 ‘getting worse’ through ‘no improvement’, ‘some improvement’, ‘marked improvement’ and ‘complete stop of crying after each dose’. Results section: No details on obtained effect were provided ‐ only the number of infants who responded to sucrose or to placebo. | ‐ |
| Metcalf 1994 | A 5‐point scale was used to identify the child’s symptoms as definitely better or symptom‐free (+ 2), possibly better (+ 1), the same (0), possibly worse (‐ 1) or definitely worse (‐ 2). Responders to simethicone or to placebo were infants judged by the carer to have had a positive response (+ 1, + 2) only to simethicone or only to placebo. | ‐ |
| Montaseri 2013 | N/A | ‐ |
| Savino 2002 | Therapy was considered efficacious if crying ended within 15 minutes after administration of compounds. Responders were children who stopped crying within this time. The cutoff of 15 minutes was derived from the minimal crying time of each crisis before treatment. | ‐ |
| Savino 2005 | Therapy was considered efficacious if crying time was reduced by ≥ 50% per day; responders were infants who had such a reduction in crying time. | ‐ |
| Sethi 1988 | N/A | ‐ |
| Weissbluth 1984 | Number of participants without colic. Colic was defined on the basis of the Wessel definition (crying < 3 hours/d or crying > 3 hours/d for < 3 days/week). | ‐ |
| Weizman 1993 | Number of participants without colic. Colic was defined on the basis of the Wessel definition (crying < 3 hours/d or crying > 3 hours/d for < 3 days/week). | ‐ |
N/A: not applicable, because this outcome was not assessed in the study.
Excluded studies
We excluded eight full‐text articles: three because they were not experimental studies (Barr 1999; Benjamins 2013; Koonce 2011); two because the comparison was not eligible (Oggero 1994; Savino 2007); two because they were not comparative clinical studies (Becker 1988; NCT00655083); and one because the participants were not eligible (NCT01532518).
Ongoing studies
One trial was ongoing and compared two different dosages of nepadutant versus placebo (NCT01258153).
Risk of bias in included studies
We have provided details of 'Risk of bias' assessments in Characteristics of included studies tables, Figure 2 and Figure 3.
2.
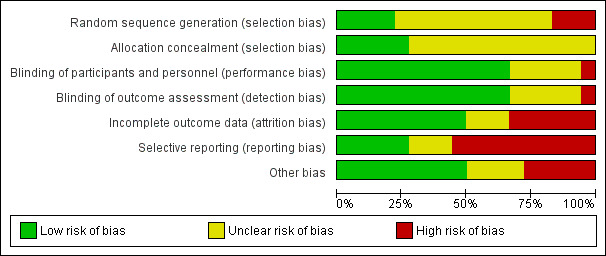
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
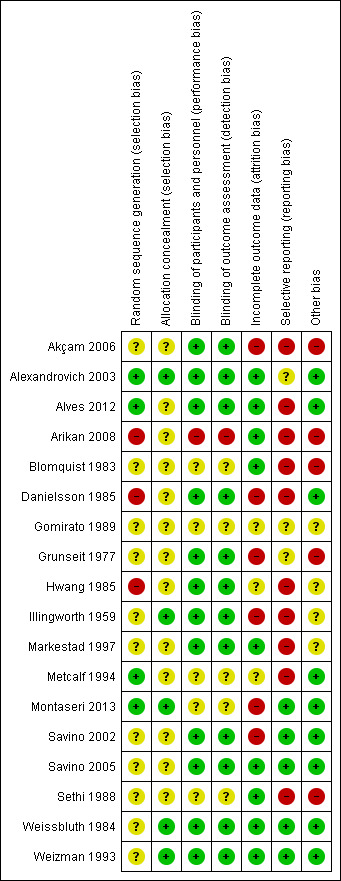
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We deemed the method of random sequence generation to be adequate in four studies (Alexandrovich 2003; Alves 2012; Metcalf 1994; Montaseri 2013); we rated these studies as having low risk of bias for this domain.
Eleven studies did not report information on sequence generation; we rated these as having unclear risk of bias (Akçam 2006; Blomquist 1983; Gomirato 1989; Grunseit 1977; Illingworth 1959; Markestad 1997; Savino 2002; Savino 2005; Sethi 1988; Weissbluth 1984; Weizman 1993).
We judged three studies as having high risk of selection bias because random sequence generation was not adequate (Arikan 2008; Danielsson 1985; Hwang 1985).
Regarding allocation concealment, five studies used an independent person to allocate participants to groups; we judged these as having low risk of bias for this domain (Alexandrovich 2003; Illingworth 1959; Montaseri 2013; Weissbluth 1984; Weizman 1993). All other studies provided no information on the method used to conceal allocation to study arms; we rated these as having unclear risk of bias (Akçam 2006; Alves 2012; Arikan 2008; Blomquist 1983; Danielsson 1985; Gomirato 1989; Grunseit 1977; Hwang 1985; Markestad 1997; Metcalf 1994; Savino 2002; Savino 2005; Sethi 1988).
Blinding
We considered blinding of parents as blinding of personnel because parents administered the treatment to their infants, completed the crying diaries and described the condition of the infant. We considered parents who completed the crying diaries, as well as those responsible for interpreting the crying diaries (in these situations, usually nurse or paediatrician), as outcome assessors.
Five studies provided no information on blinding of parents; we rated these studies as having unclear risk of performance and detection bias (Blomquist 1983; Gomirato 1989; Metcalf 1994; Montaseri 2013; Sethi 1988).
One study did not blind parents owing to the nature of the treatments compared (one of four treatment groups received massage) (Arikan 2008). We considered this study to be at high risk of performance and detection bias.
All other studies blinded parents to the treatment administered to their infant; we considered these studies to have low risk for performance and detection bias.
Incomplete outcome data
We judged six studies as having high risk of attrition bias (Akçam 2006; Danielsson 1985; Grunseit 1977; Illingworth 1959; Montaseri 2013; Savino 2002).
The articles for three studies provided insufficient details on the numbers of participants randomised and analysed; we judged these studies to have unclear risk of attrition bias (Gomirato 1989; Hwang 1985; Metcalf 1994).
We judged all other studies as having low risk of attrition bias because study authors reported no withdrawals, or because dropouts were few, dropouts were balanced between groups and reasons for dropout were reported.
Selective reporting
Three studies did not clearly specify the outcomes in the Methods section (Alexandrovich 2003; Gomirato 1989; Grunseit 1977); we judged these studies as having unclear risk of reporting bias. We judged 10 studies to have high risk of bias because study authors did not report the results for all outcomes mentioned in the Methods (Arikan 2008), or, for cross‐over studies, they did not report results separately for the first study period and the end of the study (Akçam 2006; Alves 2012; Blomquist 1983; Danielsson 1985; Hwang 1985; Illingworth 1959; Markestad 1997; Metcalf 1994; Sethi 1988).
We considered all other studies to have low risk of reporting bias.
Other potential sources of bias
We judged four cross‐over studies as having high risk of other bias because investigators planned no washout period (Akçam 2006; Blomquist 1983; Grunseit 1977; Sethi 1988); and one study with a parallel‐group design as having high risk of bias because of imbalance in relevant characteristics at baseline (Arikan 2008).
We judged three cross‐over studies as having unclear risk of other bias because study authors provided no information about the washout period (Hwang 1985; Illingworth 1959; Markestad 1997); one parallel‐group study as having unclear risk of other bias because baseline differences between participants could not be excluded, as no such details were reported (Gomirato 1989); and all other studies as having low risk of bias in this domain.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Below, we present results grouped by pain‐relieving agent and outcome. We excluded from the meta‐analyses six cross‐over studies that provided no information about the washout period and did not report first period data (Akçam 2006; Blomquist 1983; Hwang 1985; Illingworth 1959; Markestad 1997; Sethi 1988). We provide a narrative description of these studies.
Comparison 1. Simethicone versus placebo
Three cross‐over studies with 136 infants were available for this comparison (Danielsson 1985; Metcalf 1994; Sethi 1988).
Primary outcomes
Reduction in crying duration
One study with 27 infants assessed the efficacy of simethicone for crying duration (Danielsson 1985) and reported final crying values only (i.e. without change scores).
Simethicone did not differ significantly from placebo as regards daily crying duration (daily hours of difference: MD ‐0.13, 95% CI ‐1.40 to 1.14; very low‐quality evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1 Simethicone versus placebo, Outcome 1 Reduction in crying duration.
Responders
Two cross‐over studies involving 110 infants analysed the number of infants who responded positively to treatment (Danielsson 1985; Metcalf 1994). Infants treated with simethicone did not have a significantly higher probability of responding to this agent than those treated with placebo (RR 0.95, 95% CI 0.73 to 1.23; tau² = 0.01, I² = 19%; low‐quality evidence; Analysis 1.2; Figure 4).
1.2. Analysis.
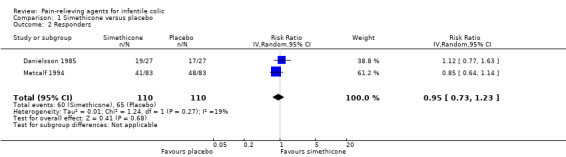
Comparison 1 Simethicone versus placebo, Outcome 2 Responders.
4.
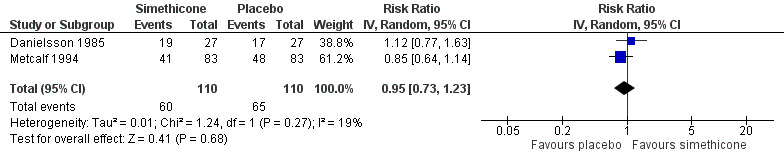
Forest plot of comparison: 1 Simethicone versus placebo, outcome: 1.2 Responders.
Secondary outcomes
Reduction in frequency of crying episodes
Sethi 1988 performed a cross‐over study without a washout period. Study authors reported a significant difference between active treatment and placebo in favour of simethicone after four days of treatment (P < 0.05). As stated above, study authors did not report results by treatment period and arm, but stated that the order of administration did not affect the results of treatment or placebo.
Parental or family quality of life, Sleeping time, parental satisfaction,
No studies assessed these outcomes.
Adverse effects
One study (Sethi 1988) involving 26 infants reported no adverse effects. The other two studies provided no data on adverse effects.
Comparison 2. Herbal agents versus placebo or no intervention
We included in this comparison five parallel‐group studies with 397 infants (Alexandrovich 2003; Arikan 2008; Montaseri 2013; Savino 2005; Weizman 1993).
Primary outcomes
Reduction in crying duration
Alexandrovich 2003, Arikan 2008 and Savino 2005 assessed crying duration. Montaseri 2013 also reported data for crying duration but reported the frequency of infants crying for less than one hour, between one and three hours and longer than three hours. Consequently, we were not able to include these data in the meta‐analysis.
For Analysis 2.1, we derived the correlation co‐efficient from Arikan 2008 and used it to calculate the SD of the mean reduction for Alexandrovich 2003 and Savino 2005, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). From this analysis (Analysis 2.1; Figure 5), we obtained an overall estimate, which favoured herbal formulations over placebo (MD 1.33, 95% CI 0.71 to 1.96; tau² = 0.29, I² = 96%; 279 infants; low‐quality evidence), indicating a significant difference in crying of more than one hour each day (P < 0.0001).
2.1. Analysis.
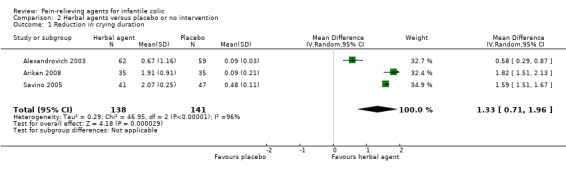
Comparison 2 Herbal agents versus placebo or no intervention, Outcome 1 Reduction in crying duration.
5.
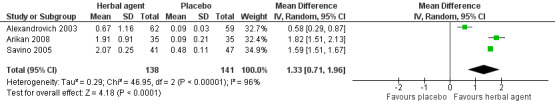
Forest plot of comparison: 2 Herbal agents versus placebo, outcome: 2.1 Reduction in crying duration.
Although the studies included in this analysis reported a statistically significant result in favour of herbal agents, the magnitude of the benefit differed across studies (tau² = 0.29, I² = 96%), probably because of the heterogeneity of the included population. In fact, these trials included children with high variability in the duration of crying at baseline (e.g. Alexandrovich 2003: 1.89 ± 0.25; Savino 2005: 3.33 ± 0.29 hours/d; Arikan 2008: 4.86 ± 1.43 hours/d). We could not exclude the risk of selection bias from two studies (Arikan 2008; Savino 2005), and we found that the risk of performance, detection and reporting bias was high in Arikan 2008.
Sensitivity analysis
When we restricted the analysis to studies in which parents were blinded (Alexandrovich 2003; Savino 2005), results remained statistically significant and favoured herbal agents (MD 1.09, 95% CI 0.11 to 2.08; tau² = 0.50, I = 98%²; 209 infants; Analysis 2.2).
2.2. Analysis.

Comparison 2 Herbal agents versus placebo or no intervention, Outcome 2 Sensitivity: reduction in crying duration.
Responders
Results of a meta‐analysis of the three studies reporting on responders to treatment (Alexandrovich 2003; Savino 2005; Weizman 1993) suggest benefit for herbal agents over placebo (RR 2.05, 95% CI 1.56 to 2.70; 277 infants; moderate‐quality evidence; Analysis 2.3; Figure 6). Heterogeneity among studies was low (tau² = 0.01, I² = 13%), but we cannot rule out selection bias due to insufficient information from Savino 2005 and Weizman 1993.
2.3. Analysis.

Comparison 2 Herbal agents versus placebo or no intervention, Outcome 3 Responders.
6.
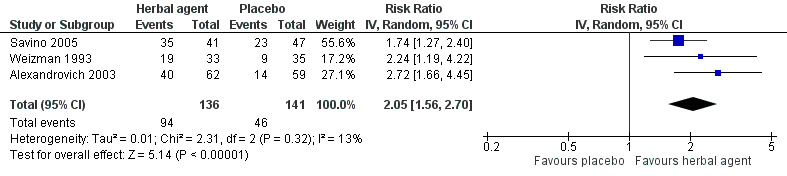
Forest plot of comparison: 2 Herbal agents versus placebo, outcome: 2.2 Responders.
Secondary outcomes
Reduction in frequency of crying episodes
Only Montaseri 2013 reported the frequency of crying episodes, but reported frequency of attacks of a particular duration: less than one hour, between one and three hours and longer than three hours. Study authors reported a statistically significant difference between the two treatment arms in favour ofFumaria extract (P < 0.05); the frequency of episodes longer than one hour seemed to be reduced in the treatment arm.
Parental or family quality of life, sleeping time, parental satisfaction
No studies assessed these outcomes.
Adverse effects
Two studies reported data on adverse effects: Alexandrovich 2003 found no adverse effects. Savino 2005 reported the following adverse effects among participants who received herbal agents: vomiting (n = 8), sleepiness (n = 2), constipation (n = 4), inappetence (n = 1) and cutaneous reactions (n = 1) (see Table 7), and the following adverse effects among those given placebo: vomiting (n = 2), sleepiness (n = 1), restlessness (n = 1), inappetence (n = 3) and constipation (n = 5).
3. Results for adverse events.
| Study | Experimental treatment | Control | Number of participants analysed (treatment/control) | Number of adverse events in treatment arm | Types of adverse events in treatment arm (number) | Number of adverse events in control arm | Types of adverse events in control arm (number) | Notes |
| Akçam 2006 | Glucose (30%) | Placebo | 25/25 | 0 | ‐ | 0 | ‐ | No adverse effect |
| Alexandrovich 2003 | Fennel (oil emulsion) | Placebo | 62/59 | 0 | ‐ | 0 | ‐ | No adverse effect |
| Alves 2012 | Simethicone | Mentha | 30/30 | 0 | ‐ | 0 | ‐ | No adverse effect |
| Arikan 2008 | Sucrose (12%) or herbal tea (fennel) | No treatment | 35/35/35 | UK | ‐ | UK | ‐ | ‐ |
| Blomquist 1983 | Dicyclomine plus sugar | Placebo | 18/18 | UK | ‐ | UK | ‐ | ‐ |
| Danielsson 1985 | Simethicone | Placebo | 27/27 | UK | ‐ | UK | ‐ | ‐ |
| Gomirato 1989 | Cimetropium bromide 2.0 mg/kg | Cimetropium bromide 1.2 mg/kg | 20/20 | 4 | Constipation (4) | 0 | ‐ | ‐ |
| Grunseit 1977 | Dicyclomine | Placebo | 22/22 | 3 | 1 | Constipation (2), loose motions (1) | Constipation (1) | ‐ |
| Hwang 1985 | Dicyclomine | Placebo | 30/30 | 4 | Drowsy (4) | 1 | Drowsy (1) | ‐ |
| Illingworth 1959 | Dicyclomine | Placebo | 16/16 | UK | ‐ | UK | ‐ | ‐ |
| Markestad 1997 | Sucrose (12%) | Placebo | 19/19 | UK | ‐ | UK | ‐ | ‐ |
| Metcalf 1994 | Simethicone | Placebo | 83/83 | UK | ‐ | UK | ‐ | ‐ |
| Montaseri 2013 | Fumaria extract | Placebo | 26/24 | UK | ‐ | UK | ‐ | ‐ |
| Savino 2002 | Cimetropium bromide | Placebo | 43/43 | 23 | Meteorism (8); vomiting (1); sleepiness (7); inappetence (1); cutaneous reactions (3); constipation (3) | 19 | Meteorism (12); sleepiness (1); restlessness (1); constipation (5) | ‐ |
| Savino 2005 | Herbal tea (chamomile/fennel/balm‐mint) | Placebo | 41/47 | 16 | Vomiting (8); sleepiness (2); inappetence (1); cutaneous reactions (1); constipation (4) | 12 | Vomiting (2); sleepiness (1); restlessness (1); inappetence (3); constipation (5) | ‐ |
| Sethi 1988 | Simethicone | Placebo | 26/26 | 0 | ‐ | 0 | ‐ | No adverse effect |
| Weissbluth 1984 | Dicyclomine | Placebo | 24/24 | 2 | Longer sleep (1); wide‐eyed (1) | 0 | ‐ | ‐ |
| Weizman 1993 | Herbal tea (chamomile/vervain/licorices/fennel/balm‐mint) | Placebo | 33/35 | UK | ‐ | UK | ‐ | ‐ |
UK: unknown.
Comparison 3. Sugar versus placebo or no intervention
Three studies addressed this comparison (Akçam 2006; Arikan 2008; Markestad 1997). Two studies involving 50 infants used a cross‐over design in comparing sucrose or glucose solution with placebo (Akçam 2006; Markestad 1997), whereas Arikan 2008 (70 infants) used a parallel‐group design to compare sucrose solution with no intervention control. Data from the two cross‐over trials provided no information about the washout period and so could not be pooled in any meta‐analysis.
Primary outcomes
Reduction in crying duration
Only Arikan 2008 reported results in terms of crying duration. Compared with no treatment, sugar (i.e. glucose; 30%) reduced crying duration by more than one hour (MD 1.72 hours/d, 95% CI 1.38 to 2.06; 70 infants; very low‐quality evidence; Analysis 3.1). This difference is of large magnitude, but we could not exclude selection, performance, detection and reporting bias.
3.1. Analysis.
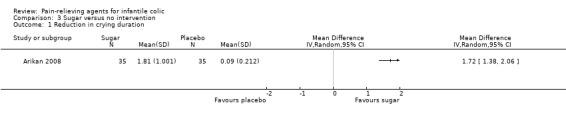
Comparison 3 Sugar versus no intervention, Outcome 1 Reduction in crying duration.
Responders
Akçam 2006 reported moderate improvement for six of 25 infants (24%) in the glucose group compared with three of 25 (12%) in the placebo group. Investigators reported marked improvement for five of 25 (20%) infants in the glucose group compared with one of 25 (4%) in the placebo group.
In Markestad 1997, 12 of 19 (63%) infants experienced an ameliorating effect on crying when treated with sucrose, and relapse when treated with placebo. One infant did not improve with either solution, and one responded better when given placebo (1/19; 5%). For five infants, it was not possible to determine specific effects because relapse did not occur when the solution was changed.
Secondary outcomes
No studies of sugar for infantile colic assessed its impact on frequency of crying episodes, parental or family quality of life, sleeping time of infants, and parental satisfaction.
Adverse effects
Only Akçam 2006 reported information on adverse effects, specifically stating that no adverse effects were registered. See Table 7.
Comparison 4. Dicyclomine versus placebo
Five studies addressed this comparison. Four used a cross‐over design (Blomquist 1983; Grunseit 1977; Hwang 1985; Illingworth 1959) and included 89 infants; one used a parallel‐group design and included 48 infants (Weissbluth 1984). We could not pool data from cross‐over trials because they failed to provide information on the washout period and did not reveal first period data by treatment arm (Blomquist 1983; Hwang 1985; Illingworth 1959), or because they presented data in a way that was not useful (Grunseit 1977).
Primary outcomes
Reduction in crying duration
Only Hwang 1985 analysed the efficacy of dicyclomine in terms of crying duration. This cross‐over study (N = 30) provided no information about the washout period and did not report data for the first and second treatment periods separately. Mean hours of crying per day were reported as 3.3 (SD 3.0) during dicyclomine treatment and 4.3 (SD 3.9) during placebo treatment.
Responders
One parallel‐group study involving 48 infants (Weissbluth 1984) reported a significant result in favour of dicyclomine (RR 2.50, 95% CI 1.17 to 5.34; Analysis 4.1). However, we could not rule out the risk of selection bias.
4.1. Analysis.
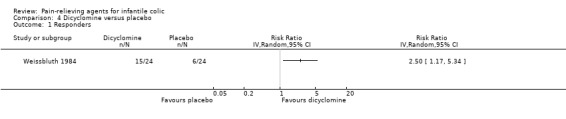
Comparison 4 Dicyclomine versus placebo, Outcome 1 Responders.
Grunseit 1977 reported a total score for symptom improvement (crying, vomiting and sleep disturbance) by treatment arm and by study period, but did not report the frequency with which infants showed improvement or worsening of their initial condition. Study authors stated that their analysis suggests that dicyclomine significantly reduced the frequency of pooled symptoms compared with placebo (P < 0.025).
Blomquist 1983 reported that in 12 (of 18) cases, investigators rated the solution with dicyclomine hydrochloride as having the best effect. In four of these cases, they rated the solutions as having an equal effect, and in two cases, they rated the solution without dicyclomine hydrochloride as having the best effect.
Hwang 1985 reported that 25 infants improved when receiving dicyclomine, and 17 when receiving placebo (P < 0.05).
Illingworth 1959 reported that four (of 16) infants receiving placebo and 12 (of 20) receiving dicyclomine achieved a score of plus three (i.e. showed improvement).
Secondary outcomes
No studies assessed the secondary outcomes of reduction in frequency of crying episodes, parental or family quality of life and parental satisfaction.
Sleeping time
Hwang 1985 provided data on sleeping time but provided no information about a washout period in this cross‐over study.
Time spent sleeping was 13.3 hours (SD 3.6) with placebo administration and 13.8 hours (SD 3.7) with administration of dicyclomine.
Adverse effects
Two of five studies that compared dicyclomine versus placebo reported significant adverse effects (longer sleep 4%, wide‐eyed state 4%, drowsy 13%; in the placebo group, only one case of drowsiness (2%) was reported; see Table 7) (Hwang 1985; Weissbluth 1984). Grunseit 1977 reported side effects for three babies: One mother reported loose motions during the last days of dicyclomine, one reported constipation in both periods and one reported constipation during dicyclomine only.
Comparison 5. Cimetropium bromide versus placebo
Only one parallel‐group study involving 86 infants examined this comparison (Savino 2002).
Primary outcomes
Reduction in crying duration
This study found a significant, clinically relevant difference in crying duration of ‐30.20 minutes per crisis (95% CI ‐39.51 to ‐20.89; very low‐quality evidence) and was at low risk for performance, detection and reporting bias, but we could not rule out risks of selection and attrition bias.
Responders
This study found a significant result favouring cimetropium bromide (RR 2.29, 95% CI 1.44 to 3.64; very low‐quality evidence), suggesting that infants treated with cimetropium bromide are twice as likely to experience improvement in their symptoms.
Secondary outcomes
Reduction in frequency of crying episodes
This study found no significant difference between the two arms in frequency of daily colic episodes (MD ‐0.40, 95% CI ‐1.50 to 0.70; 86 infants; Analysis 5.3).
5.3. Analysis.
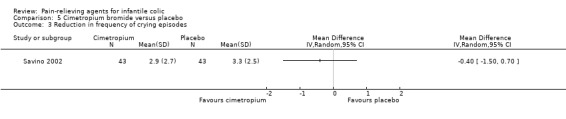
Comparison 5 Cimetropium bromide versus placebo, Outcome 3 Reduction in frequency of crying episodes.
Parental or family quality of life, sleeping time, parental satisfaction
This study did not assess these outcomes.
Adverse effects
This study reported 23 adverse events in the cimetropium bromide arm and 19 in the placebo arm. In the cimetropium bromide arm, study authors reported meteorism (n = 8), vomiting (n = 1), sleepiness (n = 7), inappetence (n = 1), cutaneous reactions (n = 3) and constipation (n = 3); in the placebo arm, they reported meteorism (n = 12), sleepiness (n = 1), restlessness (n = 1) and constipation (n = 5). See Table 7 for details.
Comparison 6. Cimetropium bromide 1.2 mg/kg versus cimetropium bromide 2.0 mg/kg
We included in this comparison one parallel‐group study involving 40 infants (Gomirato 1989).
Primary outcomes
Reduction in crying duration
Gomirato 1989 reported the duration of the longest daily crying episode, providing only baseline and final values but no change scores. Even though this was a randomised, parallel‐arm trial, we could not consider the final values because the two groups of infants differed at baseline. Study authors reported that crying duration decreased from 99 minutes (SD 10 minutes) to 28 minutes (SD nine minutes) after one week of treatment, and to five minutes (SD three minutes) after two weeks of treatment with the lower dose; and from 121 minutes (SD 11 minutes) to 55 minutes (SD seven minutes) after one week of treatment, and to 15 minutes (SD nine minutes) after two weeks of treatment with the higher dose. Study authors concluded that differences between the two schedules for this parameter were not statistically significant.
Responders
This study reported percentages of infants with excellent, good, moderate and poor improvement by treatment group. They detected no differences between arms regarding the percentage of infants with excellent or good improvement in symptoms (85% of cases in both arms).
Secondary outcomes
Reduction in frequency of crying episodes
This study reported the frequency of crying episodes before and after treatment. As baseline values were similar between the two arms in terms of crying episodes, we considered the final value difference between the two dosages of cimetropium bromide. The difference between final values (MD ‐0.50, 95% CI ‐0.68 to ‐0.32; Analysis 6.1) in episodes of crying per day favoured 1.2 mg/kg over 2.0 mg/kg.
6.1. Analysis.
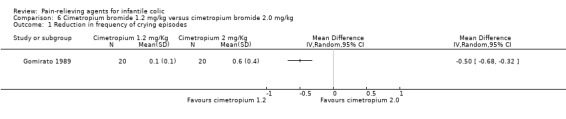
Comparison 6 Cimetropium bromide 1.2 mg/kg versus cimetropium bromide 2.0 mg/kg, Outcome 1 Reduction in frequency of crying episodes.
Parental or family quality of life, sleeping time, parental satisfaction
This study did not assess these outcomes.
Adverse effects
Four patients (10%) in the higher dosage arm had constipation, and those in the lower dosage arm reported no adverse events (see Table 7).
Comparison 7. Simethicone versus herbal agents (Mentha piperita)
We included in this comparison one cross‐over study involving 30 infants (Alves 2012).
Primary outcomes
Reduction in crying duration
We found no significant differences between simethicone and Mentha piperita (MD ‐0.01, 95% CI ‐0.11 to 0.09; Analysis 7.1).
7.1. Analysis.
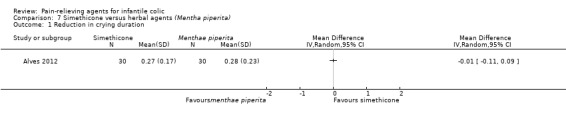
Comparison 7 Simethicone versus herbal agents (Mentha piperita), Outcome 1 Reduction in crying duration.
Responders
We found no significant differences between simethicone and Mentha piperita (RR 1.08, 95% CI 0.59 to 1.97; Analysis 7.2).
7.2. Analysis.

Comparison 7 Simethicone versus herbal agents (Mentha piperita), Outcome 2 Responders.
Secondary outcomes
Reduction in frequency of crying episodes
We found no significant differences between simethicone and Mentha piperita (MD ‐0.20, 95% CI ‐0.48 to 0.08; Analysis 7.3); the difference between simethicone and Mentha piperita was less than one episode of crying per day.
7.3. Analysis.

Comparison 7 Simethicone versus herbal agents (Mentha piperita), Outcome 3 Reduction in frequency of crying episodes.
Parental or family quality of life, sleeping time, parental satisfaction
This study did not assess these outcomes.
Adverse effects
Researchers reported no adverse effects.
Comparison 8. Sugar versus herbal agents
Arikan 2008 used a parallel‐group design (N = 70) and included three groups that were eligible for our review (sugar, herbal agents and no‐treatment).
Primary outcomes
Reduction in crying duration
This study found no significant differences between herbal agents (herbal tea) and sugar (i.e. glucose; 30%) in reducing crying duration (MD ‐0.10 hours/d, 95% CI ‐0.55 to 0.35; 70 infants; Analysis 8.1 ).
8.1. Analysis.
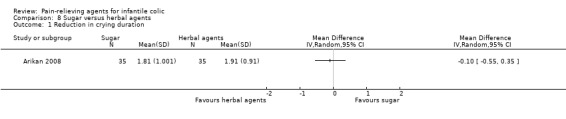
Comparison 8 Sugar versus herbal agents, Outcome 1 Reduction in crying duration.
Responders
This study did not assess this outcome for this comparison.
Secondary outcomes
This study provided no data relevant to our secondary outcomes of reduction in frequency of crying episodes, parental or family quality of life, sleeping time, or parental satisfaction and no information on adverse events.
Discussion
Summary of main results
This review included 18 studies enrolling a total of more than one thousand infants, and evaluated the effects of several pain‐relieving agents (i.e. simethicone, herbal remedy, sugar, dicyclomine, cimetropium bromide) in the treatment of infant colic. Researchers provided no evidence of beneficial effects on crying duration or on responders with simethicone. Herbal agents (i.e. extract of Matricaria recutita, Foeniculum vulgare and Melissa officinalis; fennel seed emulsion; Fumaria extract; and herbal tea preparation) were associated with reductions in crying duration compared with placebo or no treatment, and with improvement in symptoms, compared with placebo. However, the quality of the evidence is low or moderate. Researchers reported some side effects in relation to herbal agents, such as constipation, vomiting and inappetence, which, again, limits our confidence in their use.
All studies that compared sucrose, glucose, dicyclomine and cimetropium bromide versus placebo found evidence of low or very low quality favouring the active intervention, with limited sample sizes; however, on average, infants treated with the active agent were more likely than those treated with placebo to respond to treatment. We had expected to see half of these studies reporting results in the opposite direction. Only two studies on dicyclomine, a medicine that is well known to cause adverse events, reported adverse events such as longer sleep, wide‐eyed state and drowsiness in a small percentage of infants. Only one study on sugar reported specifically that no adverse effects were registered.
The study on cimetopium reported a total of 23 adverse events in the cimetropium bromide arm and a total of 19 adverse events in the placebo arm. Meteorism and constipation were slightly more common in the placebo group, and sleepiness was more common in the cimetropium group. Three participants in the cimetropium group had cutaneous reactions.
None of the included studies assessed the outcomes of parental or family quality of life, sleeping time and parental satisfaction.
See Table 1; Table 2; Table 3; and Table 4 for main comparisons.
Overall completeness and applicability of evidence
Results of this review were derived from trials that, in general, were poorly designed, conducted and reported.
Even though the included studies were conducted in both university clinics and primary care hospitals, and in different countries, the applicability of retrieved evidence to clinical practice is limited: Most trials were outdated, primary studies did not report causes of infantile colic and heterogeneity was evident among the colic definitions and outcome measures used. Studies most often included small samples, and because of incomplete reporting that made some studies almost uninformative, we were not able to pool results of the included studies in a meta‐analysis. Consequently, the number of infants included within each comparison, including meta‐analyses, was low, ranging from 27 (for the comparison of simethicone vs placebo, for the outcome of reduction in crying duration) to 279 (for the comparison of herbal agents vs placebo or no treatment for responders). Moreover, as stated above, none of the included studies assessed parental quality of life, even though validated questionnaires are available (Sung 2014 recently applied such measures in a randomised controlled trial of infant colic); thus investigators failed to recognise the negative impact that infant colic can have on parents' feelings. In fact, the persistence of this clinical condition has the potential to damage future relationships between infants and their parents (Pauli‐Pott 2000). These drawbacks also emerged during a recent systematic review of outcome measures reported in trials of infant colic (Steutel 2014).
Given these drawbacks and the consequent low quality of the evidence, our results should be interpreted with caution. Even if some results look positive (for dicyclomine, herbal agents, sugar and cimetropium bromide), the reader may conclude that the advantages associated with these interventions are simply due to bias. Thus, our interpretation is conservative: Based on available evidence, we cannot recommend herbal agents, sugar, dicyclomine and cimetropium bromide, and we believe that simethicone is no better than placebo or Mentha.
With the exception of dicyclomine, included studies reported no major adverse effects. For dicyclomine, two of five included studies reported significant adverse effects over placebo (longer sleep, wide‐eyed state, drowsiness). Some case reports within the biomedical literature have described side effects of dicyclomine (Goldman 2004; Steinherz 2004; Walker 1988; Williams 1984). Williams 1984 reported respiratory symptoms (such as shortness of breath, breathing difficulty, breathlessness, respiratory collapse and apnoeas), as well as seizures, syncope, asphyxia, pulse rate fluctuations, muscular hypotonia and coma, in infants younger than three months of age. In some cases, symptoms happened within minutes after ingestion and lasted up to 20 to 30 minutes. Some studies reported two cases of sudden infant death among infants aged three months or younger who were given dicyclomine, and described two infants as having excessively high dicyclomine blood levels (Randall 1986; Walker 1988). Although no causal relationship has been established between dicyclomine and adverse events, dicyclomine is contraindicated in infants younger than six months (Goldman 2004; Steinherz 2004), and its use has been banned in this population. We agree with this position, given that no clinical trials have been performed since 1985 (Garrison 2000; Lucassen 1998; Savino 2007b).
Quality of the evidence
Using the GRADE approach, we judged the overall quality of evidence on the effectiveness of pain‐relieving agents in infants with colic as ranging from very low to moderate.
Study limitations/risks of bias
Many identified studies were old, and the quality of reporting and conduct was poor. We judged only 25% of the included studies as having low risk of selection bias and could not consider about 30% of studies as having low risk of performance and detection bias because investigators did not blind parents or provided insufficient information on blinding. We judged only 50% of studies as having low risk of attrition bias, and we detected selective reporting of results in about 60%.
We did not assess indirectness because population, interventions and outcomes were those under consideration in our review, although we must point out that the included studies assessed response to treatment (i.e. improved symptoms) in different ways.
The small number of studies included for each comparison, the variety of interventions assessed and the diversity of measures used to assess relevant outcomes led to findings of imprecision in results for most comparisons.
For the primary outcome 'reduction in crying duration', results were inconsistent for the comparison of herbal agents versus placebo (I² = 96%). This is probably due to the different study settings reported, although all studies showed results in favour of herbal agents.
We could not evaluate publication bias because we included few trials in each meta‐analysis.
Overall, we judged the quality of evidence as ranging from very low to low for simethicone, from low to moderate for herbal agents and very low for both sugar and cimetropium bromide.
Potential biases in the review process
We conducted comprehensive searches, including extensive searches of the grey literature, to identify all relevant studies.
To avoid biases, two review authors (FS, VT) independently evaluated study eligibility, extracted data and assessed risk of bias; we resolved disagreements by discussion with the rest of the team until consensus was reached. For the two studies in which one review author (FS) was involved (Savino 2002; Savino 2005), two other review authors (EB, VT) who did not participate in these studies evaluated study eligibility, extracted data and assessed risk of bias.
Our review deviated from our protocol with regards to the types of interventions that we would include in the review and the definitions of outcomes used. We deemed that these changes were necessary, so we might better describe available evidence on pain‐relieving agents for infant colic. We have described these changes in the section Differences between protocol and review.
Finally, this review received no direct funding, although review authors acknowledged assistance received from their associates and institutions (please see Acknowledgements and Sources of support sections below).
Agreements and disagreements with other studies or reviews
We found five systematic reviews evaluating pain‐relieving agents (dicyclomine, simethicone, cimetropium bromide, herbal agents, sugars) for the treatment of colicky infants (Cohen‐Silver 2009; Garrison 2000; Hall 2012; Lucassen 1998; Perry 2011).
Garrison 2000 and Lucassen 1998 in two systematic reviews of treatments for infant colic reported that some evidence suggests effectiveness of herbal tea and dicyclomine in relieving colic symptoms. However, these review authors suggest that use of more objective outcome measures could reduce the potential for bias.
An earlier review (Cohen‐Silver 2009) reported that simethicone was not superior to placebo in reducing symptoms of colic. Anticholinergic medications, such as dicyclomine hydrochloride and dicycloverine, have been shown to be effective in reducing peristaltic bowel movements. Unfortunately, these agents are associated with several adverse effects, including loose bowel movements, accidental overdose of medication and the appearance of patients as dopey, wide‐eyed and excessively sleepy.
Perry 2011 performed a systematic review of all complementary and alternative medicines and nutritional supplements for the treatment of infantile colic. Authors of this review, which included some of the trials considered in the present review, concluded that they found encouraging results for fennel extract, mixed herbal tea and sugar solutions, but stressed that these trials had major limitations, such as small sample size, poor quality of reporting and no mention of adverse effects.
Most recently, the systematic review Hall 2012 and the clinical review DTB 2013 stated that review authors found poor scientific evidence to support the use of simethicone, dicyclomine hydrochloride and cimetropium bromide. Furthermore, severe adverse effects of dicyclomine hydrochloride, including respiratory distress and seizures, have led to withdrawal of its licence for use in infants younger than six months (Roberts 2004).
Authors' conclusions
Implications for practice.
We have concluded that:
no robust conclusions can be drawn on the effectiveness of pain‐relieving agents for the treatment of infant colic because evidence is sparse and is prone to bias; currently only a few studies with small sample sizes, most of them now old and with serious limitations, are available;
simethicone is not effective in reducing crying time or improving symptoms when compared with placebo, which means that no evidence suggests it is suitable for use as a pain‐relieving agent; and
compared with placebo, herbal agents, sugar, dicyclomine and cimetropium bromide may be beneficial in reducing crying time or relieving other symptoms of colic, but the quality of evidence is low or very low. Moreover, results for dicyclomine, sugar and cimetropium bromide are based on only one study. Also, relevant side effects have been reported for dicyclomine, and it has been banned from the market.
Implications for research.
Current evidence on the effectiveness of pain‐relieving agents for infantile colic is based on studies that generally are small and methodologically prone to bias.
Additional randomised controlled trials are needed: Outcomes of crying time per day, parental quality of life and sleeping time, as well as adverse events, should be assessed by standardised measures that allow comparison and pooling of results across studies. In addition, parents ‐ those who provide the intervention and assess the outcome ‐ should always be blinded.
In planning new clinical trials, researchers should adopt a standard definition of infantile colic, such as the definition proposed by the Rome Co‐ordinating Committee III (Hyman 2006), which included the following diagnostic criteria for infantile colic: all of the following in infants from birth to four months of age: paroxysms of irritability, fussing or crying that start and stop without obvious cause; episodes lasting three or more hours per day and occurring at least three days per week for at least one week; and no failure to thrive.
Moreover, accurate instruments to register outcomes, such as video or audio taping and actigraphy (Martin‐Martinez 2010), should be considered. Investigators should establish a minimum set of outcomes, assessed in all clinical trials in infants with colic, in a standardised manner, and reported in the final publication (Steutel 2014). Researchers should consider family dynamics when assigning a treatment plan for infants with colic, and should acknowledge that evaluation of parental satisfaction is an important aspect of treatment efficacy.
Acknowledgements
Many thanks to:
Margaret Anderson, Information Specialist of the Cochrane Developmental, Psychosocial and Learning Problems Group, for invaluable assistance in refining the search strategy;
Geraldine Macdonald, Co‐ordinating Editor of the Cochrane Developmental, Psychosocial and Learning Problems Group, for assistance in preparing the manuscript;
Laura MacDonald, former Managing Editor of the Cochrane Developmental, Psychosocial and Learning Problems Group, for support and guidance provided throughout the process;
Joanne Wilson, Managing Editor of the Cochrane Developmental, Psychosocial and Learning Problems Group, for support and guidance provided throughout the process;
Silvia Minozzi, Method Editor of the Cochrane Drugs and Alcohol Group, for methodological support provided; and
Eva Nelson (Sweden) for assistance in translating the Blomquist article from Swedish to English (Blomquist 1983).
Appendices
Appendix 1. Record of searches
| Record of searches up to 27 March 2015 | ||||
| Database | Search date | Issue or date Range of database | Number of Records | Limits |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library* | 3 April 2012 | 2012, Issue 3 | 106 | No limits |
| 14 April 2014 | 2014, Issue 3 | 4 | 2012‐2014 | |
| 27 March 2015 | 2015, Issue 2 | 3 | 2014‐2015 | |
| 16 May 2016 | 2016, Issue 4 | 3 | 2015‐2016 | |
| Ovid MEDLINE (R) | 3 April 2012 | 1946 to March week 3 2012 | 190 | No limits |
| 11 April 2014 | 1946 to April week 1 2014 | 4 | ed=2012041‐20140411 | |
| 27 March 2015 | 1946 to March week 4 2015 | 4 | ed=20140401‐20150319 | |
| 16 May 2016 | 1946 to May week 1 2016 | 3 | ed=20150319‐20160505 | |
| Ovid Medline In‐Process & Other Non‐Indexed Citations | 27 March 2015 | 26 March 2015 | 6 | No limits |
| 16 May 2016 | 13 May 2016 | 6 | No limits | |
| Embase Ovid | 3 April 2012 | 1980 to 2012 week 13 | 292 | No limits |
| 11 April 2014 | 1980 to 2014 week 14 | 11 | em=201213‐201414 | |
| 27 March 2015 | 1980 to 2015 week 12 | 8 | em=201414‐201512 | |
| 16 May 2016 | 1980 to 2016 week 20 | 10 | em=201512 ‐201619 | |
| PsycINFO Ovid | 3 April 2012 | 1967 to March week 3 2012 | 31 | No limits |
| 11 April 2014 | 1806 to April week 2 2014 | 3 | up=20120402‐20140407 | |
| 27 March 2015 | 1806 to March week 4 2015 | 1 | up=20140407‐20150323 | |
| 16 May 2016 | 1806 to May week 2 2016 | 3 | up=20150330‐20160509 | |
| CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) | 3 April 2012 | 1937 to current | 36 | No limits |
| 14 April 2014 | 1937 to current | 6 | EM 20120401‐ | |
| 27 March 2015 | 1937 to current | 5 | EM 20140401‐ | |
| 16 May 2016 | 1937 to current | 5 | EM 20150301‐ | |
| Science Citation Index Web of Science (SCI) | 3 April 2012 | 1970 to 30 March 2012 | 242 | No limits |
| Social Science Citation Index Web of Science (SSCI) | 3 April 2012 | 1970 to 30 March 2012 | 15 | No limits |
| Science Citation Index (SCI) & Social Science Citation Index (SSCI) | 14 April 2014 | 1970 to 11 April 2014 | 12 | 2012 ‐2014 |
| 27 March 2015 | 1970 to 26 March 2015 | 6 | 2014‐2015 | |
| 17 May 2016 | 1970 to 16 May 2016 | 5 | 2015‐2016 | |
| Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S); Conference Proceedings Citation Index ‐ Social Sciences & Humanities Web of Science (CPCI‐SS&H) |
03 April 2012 | 1990 to 30 March 2012 | 0 | No limits |
| 14 April 2014 | 1990 to 11 April 2014 | 0 | 2012‐2014 | |
| 27 March 2015 | 1990 to 26 March 2015 | 0 | 2014‐2015 | |
| 17 May 2016 | 1970 to 16 May 2016 | 0 | 2015‐2016 | |
| Cochrane Database of Systematic Reviews (CDSR) in the Cochrane Library | 03 April 2012 | 2012 Issue 3 | 26 | No limits |
| 14 April 2014 | 2014 Issue 4 | 0 | 2012‐2014 | |
| 27 March 2015 | 2015 Issue 3 | 0 | 2014‐2015 | |
| 16 May 2016 | 2016 Issue 5 | 0 | 2015‐2016 | |
| Database of Abstracts of Reviews of Effectiveness (DARE) in the Cochrane Library | 3 April 2012 | 2012 Issue 1 | 20 | No limits |
| 14 April 2014 | 2014 Issue 1 | 0 | 2012‐2014 | |
| 27 March 2015 | 2015 issue 1 | 0 | 2014‐2015 | |
| 16 May 2016 | 2016 Issue 2 | 0 | 2015‐2016 | |
| WorldCat (worldcat.org) | 4 April 2012 | All available years | 2 | Limited to theses |
| 14 April 2014 | All available years | 1 | Limited to theses; 2012‐2014 | |
| 27 March 2015 | All available years | 1 | Limited to theses; 2014‐2015 | |
| 17 May 2016 | all available years | 0 | Limited to theses; 2015‐2016 | |
| HOMEOINDEX (VHL; bvsalud.org/en) | 02 April 2012 | All available years | 27 | No limits |
| 14 April 2014 | All available years | 1 | Compared with previous records | |
| 27 March 2015 | All available years | 0 | Compared with previous records | |
| 17 May 2016 | all available years | 3 | 2015‐2016 | |
| LILACS (VHL; lilacs.bvsalud.org/en) | 2 April 2012 | All available years | 17 | No limits |
| 15 April 2014 | All available years | 0 | 2012‐2014 | |
| 27 March 2015 | All available years | 0 | 2014‐2015 | |
| 17 May 2016 | All available years | 2 | 2015‐2016 | |
| Networked Digital Library of Theses and Dissertations SCIRUS (NDLTD) | 3 April 2012 | All available years | 20 | Email attachment |
| 15 April 2014 | No longer available | No longer available | No longer available | |
| Networked Digital Library of Theses and Dissertations (NDLTD) (search.ndltd.org/index.php) | 17 May 2016 | All available years | 1 | ‐ |
| IBECS (VHL; bvsalud.org/en) | 02 April 2012 | All available years | 7 | No limits |
| 15 April 2014 | All available years | 0 | 2012‐2014 | |
| 27 March 2015 | All available years | 7 | 2014‐2015 | |
| 17 May 2016 | All available years | 2 | 2015‐2016 | |
| ClinicalTrials.gov (clinicaltrials.gov) | 3 April 2012 | All available years | 18 | No limits |
| 15 April 2014 | All available years | 19 | Trials received from 04/01/2012 to 04/15/2014 | |
| 27 March 2015 | All available years | 0 | Trials received from 04/01/2014 to 03/27/2015 | |
| 17 May 2016 | All available years | 11 | Trials received from 03/01/2015 to 05/17/2015 | |
| World Health Organization International Clinical Trials Registry (WHO ICTRP; apps.who.int/trialsearch) | 03 April 2012 | All available years | 43 | No limits |
| 15 April 2014 | All available years | 15 | Trials registered from 04/01/2012 to 04/15/2014 | |
| 27 March 2015 | All available years | 21 | Trials registered in 2014 or 2015 | |
| 17 May 2016 | all available years | 22 | Trials registered in 2015 or 2016 | |
| Australasian Theses (TROVE; trove.nla.gov.au) | 3 April 2012 | All available years | 0 | No records |
| 15 April 2014 | All available years | 0 | No records | |
| 27 March 2015 | All available years | 0 | No records | |
| Not searched as not productive | Not searched as not productive | Not searched as not productive | Not searched as not productive | |
| DART‐Europe E‐theses Portal (www.dart‐europe.eu/basic‐search.php) | 3 April 2012 | All available years | 0 | No records |
| 15 April 2014 | All available years | 0 | No records | |
| 27 March 2015 | All available years | 0 | No records | |
| Not searched as not productive | Not searched as not productive | Not searched as not productive | Not searched as not productive | |
| Total number of records | 1230 | |||
*includes the Cochrane Developmental, Psychosocial and Learning Problems Group Specialised Register.
Appendix 2. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, which includes the Cochrane Developmental, Psychosocial and Learning Problems Group Specialised Register
CENTRAL strategy used for 2012 searches
#1MeSH descriptor Crying, this term only #2MeSH descriptor Colic, this term only #3cry or crying or cries #4colic* #5((stomach or abdominal or abdomen*) NEAR/3 (spasm* or pain* or cramp*)) in Cochrane Reviews and Other Reviews #6((gastric or gastro*) NEAR/3 (spasm* or pain* or cramp*)) #7(#1 OR #2 OR #3 OR #4 OR #5 OR #6) #8MeSH descriptor Dicyclomine, this term only #9MeSH descriptor Simethicone, this term only #10Dic*clomine #11simethicon* #12cimetropium* #13bentyl* or bentylol or merbentyl or di‐cyclonex or dibent or dicycloverin or di‐spaz or florizel or lomine or "or‐tyl" or spascol #14MeSH descriptor Plant Extracts, this term only #15MeSH descriptor Plants, Medicinal, this term only #16MeSH descriptor Phytotherapy, this term only #17phytotherap* #18(herbal NEAR/3 (agent* or formulation* or medicine* or remed*)) #19plant extract* #20MeSH descriptor Chamomile explode all trees #21chamomile OR camomile #22MeSH descriptor Melissa, this term only #23(lemonbalm or lemon‐balm or melissa*) #24MeSH descriptor Foeniculum, this term only #25Foeniculum* #26fennel #27(#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26) #28(#7 AND #27)
CENTRAL strategy with age filter used for searches 2014 onwards
#1MeSH descriptor: [Crying] this term only #2MeSH descriptor: [Colic] this term only #3(cry or crying or cries) #4colic* #5((stomach or abdominal or abdomen*) near/3 (spasm* or pain* or cramp*)) #6((gastric or gastro*) near/3 (spasm* or pain* or cramp*)) #7#1 or #2 or #3 or #4 or #5 or #6 #8MeSH descriptor: [Infant] 1 tree(s) exploded #9(infant* or baby or babies or child* or neonat* or p*ediatric*) #10#8 or #9 #11#7 and #10 #12MeSH descriptor: [Dicyclomine] this term only #13MeSH descriptor: [Simethicone] this term only #14Dic*clomine #15simethicon* #16cimetropium* #17bentyl* or bentylol or merbentyl or di‐cyclonex or dibent or dicycloverin or di‐spaz or florizel or lomine or "or‐tyl" or spascol #18MeSH descriptor: [Plant Extracts] this term only #19MeSH descriptor: [Plants, Medicinal] this term only #20MeSH descriptor: [Phytotherapy] this term only #21phytotherap* #22(herbal near/3 (agent* or formulation* or medicine* or remed*)) #23plant extract* 4105 #24MeSH descriptor: [Chamomile] explode all trees #25chamomile or camomile #26MeSH descriptor: [Melissa] this term only #27(lemonbalm or lemon‐balm or melissa*) #28MeSH descriptor: [Foeniculum] this term only #29Foeniculum* #30fennel #31#12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 #32#11 and #31
Ovid MEDLINE (R)
Ovid MEDLINE strategy used for 2012 searches
1 crying/ (1886) 2 (cry or crying or cries).tw. 3 colic/ 4 colic$.tw. 5 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).tw. 6 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw. 7 or/1‐6 8 dicyclomine/ 9 Dic#clomine.tw. 10 simethicone/ 11 simethicon$.tw. 12 cimetropium$.tw. 13 (bentyl$ or bentylol or merbentyl or di‐cyclonex or dibent or dicycloverin or di‐spaz or florizel or lomine or "or‐tyl" or "spascol").tw. 14 Herbal medicine/ 15 Plant extracts/ 16 Plants,medicinal/ 17 Phytotherapy/ 18 phytot?erap$.tw. 19 (herbal adj3 (agent$ or formulation$ or medicine$ or remed$)).tw. 20 plant extract$.tw. 21 exp Chamomile/ 22 c?amomile.tw. 23 Melissa/ 24 (lemonbalm or lemon‐balm or melissa$).tw. 25 Foeniculum/ 26 Foeniculum$.tw. 27 fennel.tw. 28 or/8‐27 29 randomized controlled trial.pt. 30 controlled clinical trial.pt. 31 randomi#ed.ab. 32 placebo$.ab. 33 drug therapy.fs. 34 randomly.ab. 35 trial.ab. 36 groups.ab. 37 or/29‐36 38 exp animals/ not humans.sh. 39 37 not 38 40 7 and 28 and 39
Lines 29 to 39 comprise the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE (Lefebvre 2008)
Ovid MEDLINE strategy limited to infant age group used for searches 2014 onwards
1 crying/ 2 (cry or crying or cries).tw. 3 colic/ 4 colic$.tw. 5 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).tw. 6 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw. 7 or/1‐6 8 exp infant/ 9 (baby or babies or child$ or infant$ or newborn$ or neonat$).tw. 10 or/8‐9 11 7 and 10 12 dicyclomine/ 13 Dic#clomine.tw. 14 simethicone/ 15 simethicon$.tw. 16 cimetropium$.tw. 17 (bentyl$ or bentylol or merbentyl or di‐cyclonex or dibent or dicycloverin or di‐spaz or florizel or lomine or "or‐tyl or spascol").tw. 18 Herbal medicine/ 19 Plant extracts/ 20 Plants,medicinal/ 21 Phytotherapy/ 22 phytot?erap$.tw. 23 (herbal adj3 (agent$ or formulation$ or medicine$ or remed$)).tw. 24 plant extract$.tw. 25 exp Chamomile/ 26 c?amomile.tw. 27 Melissa/ 28 (lemonbalm or lemon‐balm or melissa$).tw. 29 Foeniculum/ 30 Foeniculum$.tw. 31 fennel.tw. 32 or/12‐31 33 randomized controlled trial.pt. 34 controlled clinical trial.pt. 35 randomi#ed.ab. 36 placebo$.ab. 37 drug therapy.fs. 38 randomly.ab. 39 trial.ab. 40 groups.ab. 41 or/33‐40 42 exp animals/ not humans.sh. 43 41 not 42 44 11 and 32 and 43
Lines 33 to 43 comprise the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE (Lefebvre 2008).
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations
Ovid MEDLINE In‐Process strategy used for 2015 searches
1 (cry or crying or cries).tw. 2 colic$.tw. 3 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).tw. 4 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw. 5 (baby or babies or child$ or infant$ or newborn$ or neonat$).tw. 6 Dic#clomine.tw. 7 simethicon$.tw. 8 cimetropium$.tw. 9 (bentyl$ or bentylol or merbentyl or di‐cyclonex or dibent or dicycloverin or di‐spaz or florizel or lomine or "or‐tyl or spascol").tw. 10 phytot?erap$.tw. (154) 11 (herbal adj3 (agent$ or formulation$ or medicine$ or remed$)).tw. 12 plant extract$.tw. 13 c?amomile.tw. 14 (lemonbalm or lemon‐balm or melissa$).tw. 15 Foeniculum$.tw. 16 fennel.tw. 17 or/1‐4 18 5 and 17 19 or/6‐16 20 18 and 19
Embase Ovid
Embase strategy used for 2012 searches.
1 crying/ 2 (cry or crying or cries).tw. 3 colic/ 4 colic$.tw. 5 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).tw. 6 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw. 7 or/1‐6 8 dicycloverine/ 9 simethicone/ 10 Dic#clomine.tw. 11 simethicon$.tw. 12 cimetropium$.tw. 13 (bentyl$ or bentylol or merbentyl or di‐cyclonex or dibent or dicycloverin or di‐spaz or florizel or lomine or "or‐tyl" or spascol).tw. 14 herbal medicine/ 15 plant extract/ 16 medicinal plant/ 17 phytotherapy/ 18 phytot?erap$.tw. 19 (herbal adj3 (agent$ or formulation$ or medicine$ or remed$)).tw. 20 plant extract$.tw. 21 chamomile/ 22 digestive tract agent/ 23 c?amomile.tw. 24 Melissa officinalis/ 25 (lemonbalm or lemon‐balm or melissa$).tw. 26 fennel/ 27 Foeniculum/ 28 Foeniculum$.tw. 29 fennel oil/ 30 fennel.tw. 31 or/8‐30 32 exp Clinical trial/ 33 Randomized controlled trial/ 34 Randomization/ 35 Single blind procedure/ 36 Double blind procedure/ 37 Crossover procedure/ 38 Placebo/ 39 Randomi#ed.tw. 40 RCT.tw. 41 (random$ adj3 (allocat$ or assign$)).tw. 42 randomly.ab. 43 groups.ab. 44 trial.ab. 45 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 46 Placebo$.tw. 47 Prospective study/ 48 (crossover or cross‐over).tw. 49 prospective.tw. 50 or/32‐49 51 7 and 31 52 50 and 51
Embase strategy limited to infant age group used for searches 2014 onwards.
1 crying/ 2 (cry or crying or cries).tw. 3 colic/ 4 colic$.tw. 5 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).tw. 6 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw. 7 or/1‐6 8 exp infant/ 9 (baby or babies or child$ or infant$ or newborn$ or neonat$).tw. 10 or/8‐9 11 7 and 10 12 dicycloverine/ 13 simethicone/ 14 Dic#clomine.tw. 15 simethicon$.tw. 16 cimetropium$.tw. 17 (bentyl$ or bentylol or merbentyl or di‐cyclonex or dibent or dicycloverin or di‐spaz or florizel or lomine or "or‐tyl" or spascol).tw. 18 herbal medicine/ 19 plant extract/ 20 medicinal plant/ 21 phytotherapy/ 22 phytot?erap$.tw. 23 (herbal adj3 (agent$ or formulation$ or medicine$ or remed$)).tw. 24 plant extract$.tw. 25 chamomile/ 26 digestive tract agent/ 27 c?amomile.tw. 28 Melissa officinalis/ 29 (lemonbalm or lemon‐balm or melissa$).tw. 30 fennel/ 31 Foeniculum/ 32 Foeniculum$.tw. 33 fennel oil/ 34 fennel.tw. 35 or/12‐34 36 exp Clinical trial/ 37 Randomized controlled trial/ 38 Randomization/ 39 Single blind procedure/ 40 Double blind procedure/ 41 Crossover procedure/ 42 Placebo/ 43 Randomi#ed.tw. 44 RCT.tw. (13384) 45 (random$ adj3 (allocat$ or assign$)).tw. 46 randomly.ab. 47 groups.ab. 48 trial.ab. 49 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 50 Placebo$.tw. 51 Prospective study/ 52 (crossover or cross‐over).tw. 53 prospective.tw. 54 or/36‐53 55 11 and 35 and 54
PsycINFO Ovid
1 exp Crying/ 2 (cry or crying or cries).tw. 3 colic$.tw. 4 ((stomach or abdominal or abdomen$) adj3 (spasm$ or pain$ or cramp$)).tw. 5 ((gastric or gastro$) adj3 (spasm$ or pain$ or cramp$)).tw. 6 or/1‐5 7 Dic#clomine.tw. 8 simethicon$.tw. 9 cimetropium$.tw. 10 (bentyl$ or bentylol or merbentyl or di‐cyclonex or dibent or dicycloverin or di‐spaz or florizel or lomine or "or‐tyl" or spascol).tw. 11 "medicinal herbs and plants"/ 12 "plants (botanical)"/ 13 folk medicine/ 14 alternative medicine/ 15 phytot?erap$.tw. 16 (herbal adj3 (agent$ or formulation$ or medicine$ or remed$)).tw. 17 plant extract$.tw. 18 c?amomile.tw. 19 (lemonbalm or lemon‐balm or melissa$).tw. 20 Foeniculum$.tw. 21 fennel.tw. 22 or/7‐21 23 6 and 22
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature)
S42 S27 and S41 S41 S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 S40 placebo* S39 crossover* or "cross over*" S38 (MH "Crossover Design") S37 (tripl* N3 mask*) or (tripl* N3 blind*) S36 (trebl* N3 mask*) or (trebl* N3 blind*) S35 (doubl* N3 mask*) or (doubl* N3 blind*) S34 (singl* N3 mask*) or (singl* N3 blind*) S33 (clinic* N3 trial*) or (control* N3 trial*) S32 (random* N3 allocat* ) or (random* N3 assign*) S31 randomis* or randomiz* S30 (MH "Meta Analysis") S29 (MH "Clinical Trials+") S28 MH random assignment 5 S27 S7 and S26 S26 S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 S25 Foeniculum* or fennel* S24 (MH "Fennel") S S23 (lemonbalm or lemon‐balm or melissa*) S22 (MH "Lemon Balm") S S21 c#amomile S20 (MH "Chamomile") S19 plant extract* S S18 (herbal N3 (agent* or formulation* or medicine* or remed*)) S17 phytotherap* S16 (MH "Medicine, Herbal") S15 (MH "Plants, Medicinal") S14 (MH "Plant Extracts") S13 (bentyl* or bentylol or merbentyl or di‐cyclonex or dibent or dicycloverin or di‐spaz or florizel or lomine or "or‐tyl" or spascol) S12 cimetropium* S11 simethicon* S10 (MH "Simethicone") S9 Dic?clomine S8 (MH "Dicyclomine") S7 S1 or S2 or S3 or S4 or S5 or S6 S6 ((gastric or gastro*) N3 (spasm* or pain* or cramp*)) S5 ((stomach or abdominal or abdomen*) N3 (spasm* or pain* or cramp*)) S4 colic* S3 (cry or crying or cries) S2 (MH "Crying") S1 (MH "Infant Colic")
Web of Science databases
Science Citation Index (SCI) Social Science Citation Index (SSCI) Conference Proceedings Citation Index ‐ Science (CPCI‐S) Conference Proceedings Citation Index ‐ Social Sciences & Humanities (CPCI‐SS&H)
#11#10 AND #4 DocType=All document types; Language=All languages; #10#9 OR #8 OR #7 OR #6 OR #5 DocType=All document types; Language=All languages; #9TS= (chamomile Or camomile OR Melissa OR lemonbalm or lemon‐balm OR melissa* OR Foeniculum* OR fennel*) DocType=All document types; Language=All languages; #8TS= (herbal NEAR/3 (agent* or formulation* or medicine* or remed*)) DocType=All document types; Language=All languages; #7TS=phytotherap* DocType=All document types; Language=All languages; #6TS=(plant* NEAR/3 (extract* OR medicinal)) DocType=All document types; Language=All languages; #5TS=(dicyclomin* OR simethicon* OR cimetropium* OR bentyl* or bentylol or merbentyl or di‐cyclonex or dibent or dicycloverin or di‐spaz or florizel or lomine or "or‐tyl" or spascol) DocType=All document types; Language=All languages; #4#3 OR #2 OR #1 DocType=All document types; Language=All languages; #3TS= ((gastric or gastro*) NEAR/3 (spasm* or pain* or cramp*)) DocType=All document types; Language=All languages; #2TS= ((stomach or abdominal or abdomen*) NEAR/3 (spasm* or pain* or cramp*)) DocType=All document types; Language=All languages; #1TS=(cry or crying or cries or colic*) DocType=All document types; Language=All languages;
Cochrane Database of Systematic Reviews (CDSR) in the Cochrane Library
#1MeSH descriptor: [Crying] this term only #2MeSH descriptor: [Colic] this term only #3(cry or crying or cries):ti,ab #4colic* #5((stomach or abdominal or abdomen*) near/3 (spasm* or pain* or cramp*)):ti,ab #6((gastric or gastro*) near/3 (spasm* or pain* or cramp*)):ti,ab #7#1 or #2 or #3 or #4 or #5 or #6 #8MeSH descriptor: [Infant] 1 tree(s) exploded #9(infant* or baby or babies or child* or neonat* or p*ediatric*):ti,ab #10#8 or #9 #11#7 and #10 #12MeSH descriptor: [Dicyclomine] this term only #13MeSH descriptor: [Simethicone] this term only #14Dic*clomine:ti,ab #15simethicon*:ti,ab #16cimetropium*:ti,ab #17(bentyl* or bentylol or merbentyl or di‐cyclonex or dibent or dicycloverin or di‐spaz or florizel or lomine or "or‐tyl" or spascol):ti,ab #18MeSH descriptor: [Plant Extracts] this term only #19MeSH descriptor: [Plants, Medicinal] this term only #20MeSH descriptor: [Phytotherapy] this term only #21phytotherap*:ti,ab #22(herbal near/3 (agent* or formulation* or medicine* or remed*)):ti,ab #23plant next extract*:ti,ab #24MeSH descriptor: [Chamomile] explode all trees #25(chamomile or camomile):ti,ab #26MeSH descriptor: [Melissa] this term only #27(lemonbalm or lemon‐balm or melissa*):ti,ab #28MeSH descriptor: [Foeniculum] this term only #29Foeniculum*:ti,ab #30fennel:ti,ab #31#12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 #32#11 and #31
Database of Abstracts of Reviews of Effects (DARE) in the Cochrane Library
#1MeSH descriptor: [Crying] this term only #2MeSH descriptor: [Colic] this term only #3(cry or crying or cries):ti,ab #4colic* #5((stomach or abdominal or abdomen*) near/3 (spasm* or pain* or cramp*)):ti,ab #6((gastric or gastro*) near/3 (spasm* or pain* or cramp*)):ti,ab #7#1 or #2 or #3 or #4 or #5 or #6 #8MeSH descriptor: [Infant] 1 tree(s) exploded #9(infant* or baby or babies or child* or neonat* or p*ediatric*):ti,ab #10#8 or #9 #11#7 and #10 #12MeSH descriptor: [Dicyclomine] this term only #13MeSH descriptor: [Simethicone] this term only #14Dic*clomine:ti,ab #15simethicon*:ti,ab #16cimetropium*:ti,ab #17(bentyl* or bentylol or merbentyl or di‐cyclonex or dibent or dicycloverin or di‐spaz or florizel or lomine or "or‐tyl" or spascol):ti,ab #18MeSH descriptor: [Plant Extracts] this term only #19MeSH descriptor: [Plants, Medicinal] this term only #20MeSH descriptor: [Phytotherapy] this term only #21phytotherap*:ti,ab #22(herbal near/3 (agent* or formulation* or medicine* or remed*)):ti,ab #23plant next extract*:ti,ab #24MeSH descriptor: [Chamomile] explode all trees #25(chamomile or camomile):ti,ab #26MeSH descriptor: [Melissa] this term only #27(lemonbalm or lemon‐balm or melissa*):ti,ab #28MeSH descriptor: [Foeniculum] this term only #29Foeniculum*:ti,ab #30fennel:ti,ab #31#12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30
WorldCat
su:infant colicLimited to theses/dissertations
HomeoIndex (Virtual Health Library)
("COLIC" or "CRYING") [Subject descriptor] or colic$ or cry$ or cries [Words]
LILACS (Virtual Health Library)
tw:(colic* OR cries OR crying )) OR (mh:("colic")) OR (mh:("crying")) AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
Networked Digital Library of Theses and Disserations SCIRUS (NDLTD)
Not searched after 2012 because SCIRUS no longer functioning.
"infant colic" and random*
Networked Digital Library of Theses and Disserations (NDLTD)
(search.ndltd.org/index.php)
"infant colic" and random*
IBECS (Virtual Health Library)
(tw:(colic* OR cries OR crying OR cry)) OR (mh:( "colic" OR "crying" )) AND (instance:"regional") AND ( db:("IBECS") AND type_of_study:("clinical_trials"))
ClinicalTrials.gov
Advanced search: Condition= infant colic AND Study type= intervention
World Health Organisation International Clinical Trials Registry Platform (WHO ICTRP)
Basic search: infant AND crying OR infant AND cries OR infant AND colic
TROVE
KEYWORD This phrase: infant colic and KEYWORD random*
DART‐Europe E‐theses Portal
"infant colic" and random*
Appendix 3. Criteria for judging risk of bias
| Item | Judgement | Description |
| Random sequence generation (selection bias) | Low risk | Investigators describe a random component in the sequence generation process such as random numbers table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation. |
| High risk | Investigators describe a non‐random component in the sequence generation process such as odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention. | |
| Unclear risk | Information about the sequence generation process was insufficient to permit judgement of low or high risk. | |
| Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. |
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed or were non‐opaque or were not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear risk | Information was insufficient to permit judgement of low or high risk. This is usually the case if the method of concealment is not described or is not described in sufficient detail to allow a definitive judgement. | |
| Blinding of participants and personnel (performance bias) | Low risk | No blinding or incomplete blinding, but review authors judge that the outcome is not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured, and unlikely that blinding could have been broken |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that blinding could have been broken, and the outcome is likely to be influenced by lack of blinding | |
| Unclear risk | Information was insufficient to permit judgement of low or high risk | |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes | Low risk | No blinding of outcome assessment, but review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; or blinding of outcome assessment ensured and unlikely that blinding could have been broken |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that blinding could have been broken and the outcome measurement is likely to be influenced by lack of blinding | |
| Unclear risk | Information was insufficient to permit judgement of low or high risk. | |
| Incomplete outcome data (attrition bias). For all outcomes except retention in treatment or dropout | Low risk | No missing outcome data; or percentage of missing data ≤ 10% of the overall sample and (1) reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias), (2) missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups, (3) for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate and (4) for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; or missing data > 10% but (1) they have been imputed using appropriate methods, or (2) all randomised participants are reported/analysed in the group to which they were allocated by randomisation irrespective of non‐compliance and co‐interventions (intention to treat) |
| High risk | Percentage of missing data > 10% or missing data unbalanced across groups; or reason for missing outcome data likely to be related to true outcome, with imbalance in numbers or reasons for missing data across intervention groups; or, for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; or, for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; or ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation |
|
| Unclear risk | Information insufficient to permit judgement of low or high risk (e.g. number randomised not stated, no reasons for missing data provided; number of dropouts not reported for each group) | |
| Selective outcome reporting (reporting bias) | Low risk | The study protocol is available, and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way; or the study protocol is not available but all study outcomes declared in the Methods section were reported in the Results |
| High risk | Not all of the study’s prespecified primary outcomes have been reported; or ≥ 1 primary outcome is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified; or ≥ 1 reported primary outcome was not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); or ≥ 1 outcome of interest in the review is reported incompletely, so cannot be entered into a meta‐analysis; or the study report fails to include results for a key outcome that would be expected to have been reported for such a study | |
| Unclear risk | Information insufficient to permit judgement of low or high risk | |
| Other bias | Low risk | The study appeared to be free of other sources of bias such as being stopped early because of a data‐dependent process or having a baseline imbalance between groups. For cross‐over studies, we assessed if a washout period ≥ 1 day was provided. |
| High risk | Baseline imbalance among groups; or no washout period for cross‐over studies | |
| Unclear risk | Information insufficient to permit judgement of low or high risk; study authors contacted for clarification, but the information was not forthcoming |
Appendix 4. Methods reported in the protocol and not used in the review
| Analysis | Method |
| Dealing with missing data | For studies missing > 40% of their data, we intended to conduct sensitivity analysis to explore the nature of the missing data, when available data permitted. |
| Subgroup analysis and investigation of heterogeneity | We planned to conduct the following subgroup analyses:
These analyses would have been exploratory as they involved non‐experimental (cross‐study) comparisons, and given the large numbers of subgroup analyses, they may lead to misleading conclusions (Yusuf 1991; Oxman 1992). |
| Sensitivity analysis | We planned to assess the sensitivity of findings for any imputed data to explore the possible impact of missing data (Savino 2012). |
Data and analyses
Comparison 1. Simethicone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in crying duration | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Responders | 2 | 220 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.73, 1.23] |
Comparison 2. Herbal agents versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in crying duration | 3 | 279 | Mean Difference (IV, Random, 95% CI) | 1.33 [0.71, 1.96] |
| 2 Sensitivity: reduction in crying duration | 2 | 209 | Mean Difference (IV, Random, 95% CI) | 1.09 [0.11, 2.08] |
| 3 Responders | 3 | 277 | Risk Ratio (IV, Random, 95% CI) | 2.05 [1.56, 2.70] |
Comparison 3. Sugar versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in crying duration | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Dicyclomine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Responders | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Cimetropium bromide versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in crying duration | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Responders | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3 Reduction in frequency of crying episodes | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
5.1. Analysis.
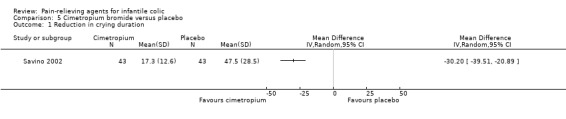
Comparison 5 Cimetropium bromide versus placebo, Outcome 1 Reduction in crying duration.
5.2. Analysis.
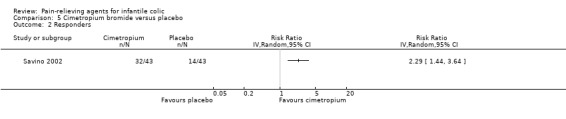
Comparison 5 Cimetropium bromide versus placebo, Outcome 2 Responders.
Comparison 6. Cimetropium bromide 1.2 mg/kg versus cimetropium bromide 2.0 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in frequency of crying episodes | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 7. Simethicone versus herbal agents (Mentha piperita).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in crying duration | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Responders | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Reduction in frequency of crying episodes | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 8. Sugar versus herbal agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in crying duration | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akçam 2006.
| Methods | Randomised, double‐blind, cross‐over, placebo‐controlled trial with 2 treatments groups | |
| Participants |
Sample size: 30 infants with typical infantile colic (minimum of 3 hours of crying per day, 3 days per week for the last 3 weeks). 5 dropped out (2 for urinary infections, 1 for otitis and 2 because of loss of contact) Setting: recruited from public healthcare clinics, general practitioners and the paediatric outpatient clinic at the Maternity Hospital Sex: boys (40%) Mean age: 9 (SD 5.9) weeks; range not reported Mean weight: 5046 (SD 1296) grams Mean duration of colic: 7.1 (SD 5.4) weeks Mean crying: 3.9 (SD 0.8) hours per day Feeding: breast fed (10 purely breast fed; 25 partially breast fed) Birth order: not reported Inclusion criteria:
Exclusion criteria: not reported |
|
| Interventions |
Intervention (25 infants): glucose solution (30%) prepared for intravenous usage Placebo (25 infants): distilled water Administration: Parents received oral and written instructions to give 1 mL of the distributed solution by medicine dropper over 15 to 20 minutes, while holding the infants in their arms, when the infant continued to cry after attempts of consoling by feeding, changing the nappy or carrying had failed. Repeat visits were scheduled for the fourth and eighth days after the first visit. Same patients used 1 drug for 4 days, and then used other drug for another 4 days. Duration of the study: 8 days Washout period: not planned. Same patients used 1 drug for 4 days, then used other drug for another 4 days. |
|
| Outcomes | At each visit, parents described the effect of the last treatment using a 6‐point scale: 0 = 'getting worse', 1 = ' no improvement', 2 = 'mild improvement', 3 = 'moderate improvement', 4 = 'marked improvement', 5 = 'completely well after each dose'. Study authors considered infants with an improvement of 2, 3 or 4 as responders. | |
| Notes |
Country: Turkey Funding source: Study authors did not report whether the study received funding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No information was reported about the method used to generate randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: 1 bottle containing glucose solution and 1 containing distilled water were prepared by a pharmacist, who also arranged and kept the coding and distributed the bottles; they were arranged in numbered pairs, and within the pairs, glucose and placebo were randomly designated with the letters A and B. Each infant was randomised to a number, and to the pair of bottles to be tried first, by 2 separate draws through the sealed envelope technique. Not specified whether the envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Glucose as 30% solution and placebo as distilled water were arranged in identical coloured glass bottles. No information was given to parents regarding the contents of either bottle. The study was conducted as double‐blind ‐ only pharmacist knew the bottle coding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: No information was given to parents regarding the contents of either bottle. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: A total of 25 of 30 infants (83.3%) completed the study. 5 infants were excluded (2 for urinary infections, 1 for otitis and 2 because of loss of contact) for reasons that seemed not related to the outcome. Not reported during which period they dropped out. |
| Selective reporting (reporting bias) | High risk | Comment: Study authors reported data about the outcome declared in the Methods section for all 25 infants who completed the study, but they did not report results for each treatment by study period, neither the number of infants who improved with both treatments. |
| Other bias | High risk | Comment: Same infants used 1 drug for 4 days, then used other drug for another 4 days. Washout period was not planned. |
Alexandrovich 2003.
| Methods | Randomised, double‐blind, placebo‐controlled trial with 2 treatments groups | |
| Participants |
Sample size: 125 infants diagnosed with colic. Infants enrolled into the study were not tested for milk allergy, and continued their diets. 4 participants dropped out Setting: recruited at 2 large multi‐specialty clinics of Kalinin district of St Petersburg Sex: boys (45.5%) Mean age: 30 (SD 7) days. Range 2 to 12 weeks Mean weight: 3868 (SD 295) grams Mean duration of colic: not reported Mean crying: 13.2 (SD 1.7) hours per week Feeding: breast fed (45.5%) Birth order: not reported Inclusion criteria: infants were included in the study if they met the criteria offered by Wessel et al Exclusion criteria:
|
|
| Interventions |
Intervention (65 infants): fennel seed oil emulsion (water emulsion of 0.1% fennel seed oil and 0.4% polysorbate‐80) Placebo (60 infants): 0.4% polysorbate in water Administration: A pharmacist dispensed both the fennel preparation and the placebo into quantities sufficient to last 1 week. Parents were instructed to administer a minimum of 5 mL and a maximum of 20 mL of fennel seed oil emulsion or placebo up to 4 times a day, orally before meals, and at the onset of colic episodes. In addition, parents were instructed to limit consumption to about 12 mL/kg/d, which would provide about 12 mg/kg/d of fennel seed oil. Duration of the study: 7 days (+ 7 days follow‐up) |
|
| Outcomes | Calculated cumulative crying for a week. Relief of colic symptoms, which was defined as decrease in cumulative crying to < 9 hours per week Each family received a diary with instructions to enter data on a daily basis. Diaries were completed for 21 days: 7 days before the trial, during the 7‐day trial, and 7 days after the trial |
|
| Notes |
Country: Russia Funding source: This study was supported by institutional funds. The fennel seed oil emulsion and placebo were provided by Lev Laboratories (Glencoe, IL) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Randomisation was achieved by a computer‐generated number with the use of Microsoft Excel (Microsoft, Redmond, WA, USA). |
| Allocation concealment (selection bias) | Low risk | Comment: A pharmacist dispensed both the fennel preparation and placebo in quantities sufficient to last 1 week. The pharmacist was unaware of which parents received the preparation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: The fennel preparation and the placebo appeared identical on visual examination and were bottled in plastic 6 oz (180 mL) nursing bottles by a laboratory technician. Neither the observing paediatrician nor parents were aware of the content of the bottles. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Neither the observing paediatrician nor the parents were aware of the content of the bottles. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: A total of 121 infants completed the study (97%). 1 infant in the treatment group was withdrawn from the study because his parents failed to complete the diary. Because of a relocation, 2 infants (twins) in the treatment group were lost to follow‐up. 1 infant in the control group was withdrawn from the study because of a severe cold. Percentage of drop‐outs < 10%. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcomes were not clearly specified in the Methods section. |
| Other bias | Low risk | Comment: Intervention and control groups did not differ significantly in baseline infant characteristics. No significant difference in cumulative crying was noted between the 2 groups before the start of treatment. |
Alves 2012.
| Methods | Randomised, double blind, cross‐over trial | |
| Participants |
Sample size: 30 infants, diagnosed with infantile colic (Wessel criteria). No withdrawals from the study Setting: recruited at the Instituto de Medicina Integral by Professor Bernardo Figueira, Receife Sex: boys (45.5%) Mean age: 33 (SD 11) days. Range 8 to 56 days Mean weight: 4650 (SD 415) grams Mean duration of colic: not reported Mean crying: 3.2 (SD 0.8) hours per day Feeding: breast fed (100%) Birth order: not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (30 infants): simethicone ‐ liquid drops (2.5 mg/kg body weight), daily for a period of 7 days Control (30 infants):Mentha piperita ‐ liquid drops (1 drop/kg body weight) daily for a period of 7 days Administration: Repeated visits were scheduled for the 7th and 17th days after the first visit. On the 7th day visit, the medication was returned to the hospital, and another pair of medication bottles were distributed. When patients did not return to the hospital on the 7th day, a home visit was conducted by a researcher. During visits, the infant was clinically examined. Duration of the study: 14 days Washout period: 3 days. After the first 7 days of the study and a period of washout for 3 days, all infants had their medication alternated and were followed for another 7 days. During the washout period, parents were oriented to use paracetamol for colic treatment |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes |
Country: Brazil Funding source: This study was sponsored by INFAN, Brazil. The funding sponsor had no involvement in the design, analysis or writing process. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Allocation sequence and randomisation list were computer‐generated by the “randomised” programme (randomised.com). |
| Allocation concealment (selection bias) | Unclear risk | Comment: Information reported was insufficient to permit a judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment:Mentha piperita and simethicone were identical in weight, smell, colour, taste and package. All researchers and parents were unaware of the treatment administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: All researchers and parents were unaware of the treatment administered. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No withdrawals from the study were reported. |
| Selective reporting (reporting bias) | High risk | Comment: Study authors did not report clearly the information about time spent crying (outcome declared as primary in the Methods section) by treatment period but reported colic duration by treatment. Moreover, they did not report results on secondary outcomes. |
| Other bias | Low risk | Comment: A 3‐day washout period was planned. |
Arikan 2008.
| Methods | Randomised, controlled trial with 4 treatment groups | |
| Participants |
Sample size: 175 infants. No withdrawals from the study Setting: recruited at public healthcare clinics and Department of Pediatrics at the Yakutiye Research Hospital, Atatürk University Sex: boys (55%) Mean age: 2.15 (SD 0.7) months. Range not reported Mean weight: 5250 (SD 1039) grams Mean duration of colic: not reported Mean crying: 5.14 (SD 1.59) hours/d Feeding: breast fed (80%) Birth order: not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention 1 (35 infants): massage (chiropractic spinal manipulation). Parents were advised to administer massage twice a day for 25 minutes during symptoms of colic. This time period and method were used successfully in a previous study (Huhtala et al. 2000). Intervention 2 (35 infants): sucrose solution (12%). Sucrose administered at a dose of 2 mL of 12% solution twice a day at 5 PM and 8 PM. This concentration and volume were chosen because they had been used successfully in a previous study on pain in newborn infants (Haourai et al. 1995). Intervention 3 (35 infants): herbal tea (fennel tea). Herbal tea administered at a dose of 35 mL (maximum dose of 150 mL), 3 times a day (Weizman et al. 1993). Intervention 4 (35 infants): hydrolysed formula. In the group receiving breast feeding, it was thought that it would be wrong to discontinue breast feeding. Therefore, only infants fed standard formula were assigned to the group to receive hydrolysed formula. Control (35 infants): no intervention. No nursing intervention was administered to the control group. Administration: The same paediatrician and nurse were in contact with all study parents, each of whom was visited and trained in the scoring system. Parents were educated by researchers about their assigned regimen. Mothers were trained in massage technique and were given brochures with written illustrated instructions. Duration of the study: 7 days |
|
| Outcomes | Crying time: Before starting the study, parents were given a structured questionnaire about their infants’ behaviour, temperament, sleeping and eating habits and history of colic symptoms. Parent participants were then given a 1‐week diary in which to record crying times and durations (onset of crying time, when the intervention was administered, end of crying time, side effects observed during week of therapy). Crying was quantified by length of crying in hours per day for 1 week before and 1 week during the intervention. | |
| Notes |
Country: Turkey Funding source: Study authors received no financial support for this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: Information reported on the group receiving breast feeding was insufficient; it was thought that it would be wrong to discontinue breast feeding. Therefore, only infants fed with standard formula were assigned to the group to receive hydrolysed formula. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Because of the design of the study, blinding was not applied. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: No blinding was applied. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Study authors reported study results for all randomised infants. |
| Selective reporting (reporting bias) | High risk | Comment: Parents wrote a diary to record crying duration and side effects observed during therapy. Side effects results were not reported. |
| Other bias | High risk | Comment: Intervention groups and control group did not differ significantly in baseline infant characteristics, except for crying duration. |
Blomquist 1983.
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over trial | |
| Participants |
Sample size: 18 infants. No withdrawals from the study Setting: recruited from 9 child health centres (CHCs) in Umeå and the surrounding area Sex: boys (44%) Mean age: not reported (SD not reported). Range 2 to 14 weeks Mean weight: not reported Mean duration of colic: not reported Mean crying: not reported Feeding: not reported Birth order: not reported Inclusion criteria:
Exclusion criteria: not reported |
|
| Interventions |
Intervention (18 infants): dicyclomine hydrochloride 100 mg in 100 mL of solution. As the mixture has a sweet and sour flavour, sugar and lemon lime were added. Control (18 infants): placebo. Dosage was 5 mL of the respective solution, given 20 minutes before afternoon and evening meals. Parents were asked to refrain from changing the infant’s diet during treatment. Administration: After 1 week, parents returned to the CHC for follow‐up, which included a weight check. At this time, parents made an overall assessment of the treatment week. Parents returned the first bottle and were given the new code‐labelled bottle. After an additional week, parents made a new overall assessment in connection with a new visit to the CHC. Duration of study: 2 weeks Washout period: no washout period planned |
|
| Outcomes | The CHC distributed a diary containing data entry pages for 15 days of treatment. In the diary, parents recorded, for each day, times of meals, colic attacks, bowel movements and colic medications. Parents also commented on how severe they considered the child’s colic attacks to have been during the past 24‐hour period (none, mild, moderate, severe, very severe). In addition, parents assessed during which week treatment showed the highest efficacy. | |
| Notes |
Country: Sweden Funding source: Study authors did not specify whether they received financial support. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Information was insufficient to permit a judgement of low risk. Order of use between the 2 treatment alternatives was randomly assigned. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Two code‐labelled bottles ‐ 1 of which contained dicyclomine hydrochloride, and the other, the solution without dicyclomine hydrochloride ‐ were given to each infant. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Two code‐labelled bottles ‐ 1 of which contained dicyclomine hydrochloride, and the other, the solution without dicyclomine hydrochloride ‐ were given to each infant. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Study authors reported study results for all randomised infants. |
| Selective reporting (reporting bias) | High risk | Comment: Outcomes were not clearly prespecified, and details of results were not reported by study period. |
| Other bias | High risk | Comment: No washout period was planned. |
Danielsson 1985.
| Methods | Double‐blind, placebo‐controlled, cross‐over trial | |
| Participants |
Sample size: Study included 32 infants with colic: 5 infants dropped out after 2 to 7 days owing to parents' decisions (2 taking simethicone, and 3 placebo). Data from this study were derived from 27 infants. Setting: baby clinics near Gothenburg Sex: boys (44%) Mean age: 4.8 (SD not reported) weeks; range 2 to 8 weeks Mean weight: not reported Mean duration of colic: not reported Mean crying: not reported Feeding: breast fed (all mothers, except 1, were breast feeding); received bottle supplements (3 mothers gave their infants occasional bottle supplements) Birth order: not reported Inclusion criteria: Crying was diagnosed as infantile colic if it lasted longer than 3 hours a day and occurred more than 3 days a week. Exclusion criteria: not specified |
|
| Interventions |
Intervention (27 infants): simethicone: 0.3 mL (10 drops) of simethicone solution (94 mg/mL) administered to each infant before each meal Control (27 infants): placebo; designed to have the same taste, smell, colour and texture as the active solution Administration: The same assistant visited each family twice. The first treatment was administered from day 1 to day 7; this was followed by a 3‐day washout period. The second treatment was administered from day 11 to day 17. Duration of study: 7 days of treatment, 3 days of washout and 7 days of another treatment Washout period: 3 days |
|
| Outcomes | Parents were interviewed before and after treatment to obtain background data and to evaluate treatment efficacy. Parents kept 24‐hour records about how long their infants had been crying or fussing, and how often their infants had eaten and passed stools. | |
| Notes |
Country: Sweden Funding source: This research was supported by a grant from the First of May Flower Annual Campaign for Children's Health (Sweden). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: It is not clearly reported whether treatment order was assigned by randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Information was insufficient to permit a judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Medicine bottles were coded by the manufacturer; placebo and tested drug had the same smell, taste, colour and texture. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Medicine bottles were coded by the manufacturer; placebo and tested drug had the same smell, taste, colour and texture. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Five infants dropped out owing to parents' decisions (2 taking simethicone, and 3 placebo). 15% were lost. Balanced reasons for dropout were reported. |
| Selective reporting (reporting bias) | High risk | Comment: Outcomes were not clearly reported in the Methods section, and results were not reported by study period. |
| Other bias | Low risk | Comment: Three‐day washout period was planned. |
Gomirato 1989.
| Methods | Randomised, controlled, parallel‐group trial | |
| Participants |
Sample size: 40 infants. Information on dropouts was not clearly reported. Setting: not reported Sex: boys (40%) Mean age: 4.4 (SD not reported) weeks; range not reported Mean weight: not reported (SD not reported) Mean duration of colic: ≥ 1 week Mean crying: longer than 90 consecutive minutes Feeding: not reported Birth order: not reported Inclusion criteria:
Exclusion criteria: infants with known organic disorders or positive to faecal blood test |
|
| Interventions |
Intervention 1 (20 infants): 1.2 mg/kg cimetropium bromide Intervention 2 (20 infants): 2.0 mg/kg cimetropium bromide Administration: Both drugs were administered 1 hour before bottle feeding. Treatment lasted 2 weeks, with 3 visits during this period to evaluate general conditions, symptoms and adverse effects. Parents could use pacifiers such as abdominal massage, clyster or a trip in a car. No diet modifications were performed. Duration of study: 2 weeks |
|
| Outcomes | During the visits, evaluation was based on the diary. The mother had to report the following every day for 2 weeks: number of crying crises, duration of longest crisis, weight changes and number of evacuations. Improvement in symptoms was classified by the following scale: '‐ 2' the longest crisis increased by more than 60 minutes compared with basal; '‐ 1' the longest crisis increased by 30 to 60 minutes compared with basal; '0' no improvement compared with basal; '+ 1' the shortest crying episode reduced by 30 to 60 minutes compared with basal; '+ 2' the shortest crying episode reduced by more than 60 minutes compared with basal. Parental satisfaction was evaluated during the second visit. Child temperament and treatment safety (heart frequency [heart rate], body temperature, number of episodes of diarrhoea) were evaluated during treatment. | |
| Notes |
Country: Italy Funding source: Study authors reported no information about funding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Information was insufficient to permit a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: No information was reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: No information was reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Four infants receiving the drug at the higher dosage (2 mg/kg) showed constipation and stopped treatment before the end of the study period, but study authors did not explain how they considered these infants in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcomes were not clearly prespecified in the Methods section. |
| Other bias | Unclear risk | Comment: Information was insufficient to permit a judgement of baseline balance between the 2 groups. |
Grunseit 1977.
| Methods | Randomised, double‐blind, cross‐over trial | |
| Participants |
Sample size: 25 infants. 3 dropouts Setting: not reported Sex: boys (41%) Mean age: 5.4 (SD not reported) weeks at baseline; range 3 to 12 weeks at baseline Mean weight: not reported Mean duration of colic: not reported Mean crying: not reported Feeding: not reported Birth order: 16 first‐born, 4 second‐born, 2 fourth‐born Inclusion criteria: infants suffering from infant colic. Diagnostic criteria for inclusion were postprandial attacks of screaming and crying that were unabated by maternal comforting, vomiting and sleep disturbance. Exclusion criteria:
|
|
| Interventions |
Intervention (22 infants): dicyclomine hydrochloride syrup, 5 mg per 5 mL Control (22 infants): placebo; appeared identical to dicyclomine Administration: Parents were instructed to give 5 mL of syrup to the baby 15 to 20 minutes before 4 feeds/d. Infants received treatment or placebo for 1 week. Duration of study: 2 weeks Washout period: none |
|
| Outcomes | Colic symptoms: postprandial crying, postprandial vomiting, sleep disturbance. If symptoms were assessed as mild, they were rated '1', moderately severe '2' and severe '3'. If absent, '0'. The mother was provided a diary card to record symptoms, time, severity and medication used. At the end of 1 week, the baby was brought back to the physician for assessment of each symptom and completion of the scoring form. After the second week of therapy, the baby was brought back for final assessment. | |
| Notes |
Country: Australia Funding source: Study authors did not specify whether they had received financial support for the study. Drug and placebo were supplied by the company (Richardson‐Merrell). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Information was insufficient to permit a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: The mother was provided dicyclomine hydrochloride syrup or identical appearing placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: The mother was provided dicyclomine hydrochloride syrup or identical appearing placebo. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: A total of 22 of 25 infants completed the study: 3 infants were excluded, 2 failed to return for the final visit (1 taking dicyclomine and 1 taking placebo) and a third infant was excluded because his condition worsened during the placebo phase, requiring hospitalisation. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Study outcomes were not clearly prespecified. |
| Other bias | High risk | Comment: No washout period was applied. |
Hwang 1985.
| Methods | Placebo‐controlled, cross‐over trial | |
| Participants |
Sample size: 30 infants with colic. Dropouts/withdrawals not reported Setting: not reported Sex: not reported Mean age: 4.5 (SD not reported) weeks; range not reported Mean weight: not reported Mean duration of colic: not reported Mean crying: 4.9 (SD 3.7) hours Feeding: not reported Birth order: not reported Inclusion criteria: Crying was diagnosed as infantile colic if it lasted longer than 3 hours a day and occurred more than 3 days a week. Exclusion criteria: not reported |
|
| Interventions |
Intervention (30 infants): dicyclomine hydrochloride, 5 mg 4 times/d Control (30 infants): placebo; designed to have the same taste, smell, colour and texture as the active solution Administration: Infants were given placebo and dicyclomine, each for 1 week, with the order counterbalanced. Duration of study: 2 weeks Washout period: no information |
|
| Outcomes |
Crying time: mean daily hours of crying (based on crying diary completed by parents). Parents were interviewed before and after treatment to obtain background data and to evaluate therapeutic efficacy. In addition, parents kept 24‐hour records about their infants' crying, irritability and sleeping. The article also reported sleeping time, number of feeds and number of stools (before and during treatment). |
|
| Notes |
Country: Sweden Funding source: This study was supported by a grant from the First of May Flower Annual Campaign for Children's Health (Sweden). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: It is not clearly reported whether treatment order was assigned by randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Placebo was designed to have the same taste, smell, colour and texture as the active solution. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Placebo was designed to have the same taste, smell, colour and texture as the active solution. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Information was insufficient to permit a judgement. |
| Selective reporting (reporting bias) | High risk | Comment: Study outcomes were not clearly specified, and treatment results were not reported by study period. |
| Other bias | Unclear risk | Comment: No information about washout period was reported. |
Illingworth 1959.
| Methods | Randomised, double‐blind, cross‐over trial | |
| Participants |
Sample size: 20 infants, but 4 infants did not have the inert substance, and 1 did not have the active drug Setting: well‐baby clinic at Jessop Hospital, Sheffield Mean age: not reported (SD not reported); range not reported. Simply states that all infants were younger than 8 weeks Mean weight: not reported Mean duration of colic: not reported Mean crying: not reported Feeding: not reported Birth order: not reported Inclusion criteria:
Exclusion criteria: not specified |
|
| Interventions |
Intervention (20 infants): dicyclomine hydrochloride, half teaspoon (approximately 5 mg) before the 6 PM feed Control (16 infants): placebo, half teaspoon (approximately 5 mg) before the 6 PM feed Administration: The doctor prescribed dicyclomine hydrochloride, and the pharmacist dispensed dicyclomine or inert substance by random sampling. After 1 week, the medicine was prescribed again, and the pharmacist gave the opposite of what she had given before. Only the pharmacist knew which material (drug or placebo) the child had received. Duration of the study: 2 weeks Washout period: no information |
|
| Outcomes | The child was seen 1 week after the start of treatment, and the mother's word about colic was reported. Results were graded from '‐ 3' to '+ 3' as follows: '+ 1' child slightly better, '+ 2' definitively better but still with some discomfort, '+ 3' infant with very great improvement and free from symptoms, '‐ 1' infant slightly worse | |
| Notes |
Country: England Funding source: Study authors did not specify whether they had received financial support for the study; drug and placebo were supplied by the company, Merrell National (UK). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No information about random sequence generation was reported. |
| Allocation concealment (selection bias) | Low risk | Comment: Pharmacist dispensed dicyclomine or an inert substance by random sampling. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Parental blinding was performed. Only the pharmacist knew which material the child had received. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Parental and paediatrician blinding was performed. Only the pharmacist knew which material the child had received. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Four infants did not have the inherited substance, and 1 did not have the active drug. |
| Selective reporting (reporting bias) | High risk | Comment: Study authors did not report the results of each study period by treatment group (dicyclomine or placebo). |
| Other bias | Unclear risk | Comment: No information about washout period was reported. |
Markestad 1997.
| Methods | Randomised, double‐blind, cross‐over trial | |
| Participants |
Sample size: 20 consecutive infants; 1 infant was excluded because of organic disease Setting: recruited from public healthcare clinics Sex: 13 boys, 6 girls Mean age: 7.3 (SD 3.4) weeks; range not reported Mean weight: 3502 (SD 570) grams Mean duration of colic: 5.2 (SD 3.0) weeks Mean crying: 5.7 (SD 2.6) hours Feeding: breast fed (17 purely breast fed, 2 partially breast fed) Birth order: not reported Inclusion criteria:
Exclusion criteria: not specified |
|
| Interventions |
Intervention (19 infants): sucrose, 12% solution in distilled water Placebo (19 infants): distilled water Administration: One bottle containing sucrose and 1 containing placebo were arranged in numbered pairs. Parents received oral and written instructions to give 2 mL of the distributed solution by syringe over 30 to 60 seconds, while holding the infant in their arms, when the infant continued to cry after attempts of consoling by feeding, by changing the nappy or by carrying had failed. Repeat visits were scheduled 3 to 4 and 6 to 8 days after the first visit, and a telephone interview was conducted 3 to 4 days after the last visit. On each visit, the bottle was returned, and the other of the pair was distributed. Duration of study: 8 days Washout period: no information |
|
| Outcomes | The infant was examined clinically at repeated visits, and on each contact (visit or telephone contact), the parent described the effect of the last treatment on a 5‐point scale: ‘getting worse’ to ‘no improvement’, ‘some improvement’, ‘marked improvement’, and ‘complete stop of crying after each dose’. | |
| Notes |
Country: Norway Funding source: Study authors did not report whether the study had received support. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Comment: Study authors did not describe the randomisation method. Quote: "each infant was randomised to a number and which of the pair of bottles to be tried first by two separate draws using the sealed envelope technique". |
| Allocation concealment (selection bias) | Unclear risk | Comment: sealed envelope technique; not reported whether the envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Sucrose as a 12% solution in distilled water and placebo as distilled water were prepared by a pharmacist, who also arranged and kept the coding, and distributed treatment in identical coloured glass bottles. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Only the pharmacist knew which material the child had received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 19 infants completed the study and were analysed. |
| Selective reporting (reporting bias) | High risk | Comment: Study authors did not report results by study period. |
| Other bias | Unclear risk | Comment: No information about washout period was reported. |
Metcalf 1994.
| Methods | Randomised, double‐blind, cross‐over trial | |
| Participants |
Sample size: 92 infants enrolled; 9 infants excluded: 8 for failure to keep follow‐up visits and 1 for infection. Total of 83 infants were included in the analysis Setting: 3 general paediatric practices in distinct geographic regions Sex: boys (49.4%) Mean age: not reported (SD not reported); range 2 to 8 weeks Mean weight: not reported Mean duration of colic: not reported; 24% had severe colic at baseline Mean crying: not reported Feeding: breast fed (26.5%) Birth order: not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (83 infants): simethicone; 0.3 cc of simethicone solution before each meal Control (83 infants): placebo Administration: Trial consisted of 2 study periods, each of approximately 1 week (minimum 3 days; maximum 10 days). Infants first received simethicone or placebo according to a schedule determined by random number tables, followed by the alternate substance for the second study period. Carers were given a bottle of coded medication and were instructed to give 0.3 mL with each feeding. Duration of study: 2 weeks Washout period: 1 day |
|
| Outcomes | Treatment efficacy was measured by interviews, 3‐ to 4‐hour behavioural observations and 24‐hour records in which parents described infants’ crying, fussing, eating and stools. Parents were asked to record in the daily diary each administration of medication and to provide written comments on events deemed noteworthy, including any modifications in dietary habits or feeding schedule. At the end of each day, parents were to rate their child’s colic compared with when they had first sought treatment for the infant. They used a 5‐point scale to identify the child’s symptoms as follows: '+ 2', definitely better or symptom‐free; '+ 1', possibly better; '0', the same; '‐ 1', possibly worse; '‐ 2', definitely worse. After the first study period, carers returned the diary and any unused medication to the physician’s office, or they gave these items to a nurse study co‐ordinator during a home visit. Responders to simethicone or to placebo were infants judged by the carer to have had a positive response (+ 1, + 2) only to simethicone or only to placebo. | |
| Notes |
Country: United States Funding source: This study was supported by a grant from Smart Pharmaceuticals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Infants first received either simethicone or placebo, based on a schedule determined by random number tables, followed by the alternate substance for the second study period". |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Caregivers were given a bottle of coded medication". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Caregivers were given a bottle of coded medication". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Information was insufficient to permit a judgment; researchers reported only withdrawals ‐ 8 infants were excluded for failure to keep follow‐up visits, and 1 child developed upper air respiratory infection and was excluded. |
| Selective reporting (reporting bias) | High risk | Comment: Study authors did not report results by study period. |
| Other bias | Low risk | Comment: One‐day washout was planned. |
Montaseri 2013.
| Methods | Randomised, double‐blind, multi‐centre, placebo‐controlled trial | |
| Participants |
Sample size: 60 infants. 17% of infants were withdrawn from the study for non‐attendance at clinic or an incomplete booklet. Setting: 6 clinics affiliated with Shiraz University of Medical Sciences Sex: boys (50%) Mean age: not reported (SD not reported); range 1 to 4 months. The largest age group consisted of 2‐month‐old infants in both treatment and control groups. Mean weight: not reported Mean duration of colic: not reported Mean crying: not reported Feeding: not reported Birth order: first born (intervention 50%, control 58%) Inclusion criteria: infants with colic pain (selected and examined by a physician) Exclusion criteria: infants with other problems/disorders |
|
| Interventions |
Intervention (26 infants):Fumaria extract 2.5 cc was prescribed to be taken 3 times a day for a week. Control (24 infants): placebo Mothers were required to refrain from using any other type of medicine or treatment during this period. Fumaria extract and placebo were prepared and encoded according to the table of random numbers. These codes were kept secret until data collection by a pharmacist was complete, and they were revealed after analysis was performed and labels on reports were prepared. Administration: 2.5 cc of Fumaria extract was prescribed to be taken by infants 3 times a day for a week. Duration of study: 1 week |
|
| Outcomes | Treatment efficacy was measured by mothers via a booklet diary. Parents were asked to come to the clinic after the treatment period to hand in the booklet and, at the same time, evaluate colic pain in their infant. Information recorded by mothers included frequency and length of crying and frequency of waking up owing to colic pain per day. | |
| Notes |
Country: Iran Funding source: This study was supported by the Vice Chancellor of Shiraz University of Medical Sciences. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment:Fumaria extract and placebo were prepared and encoded according to the table of random numbers. After written informed consent was obtained from the mothers of the infants, control and treatment groups were established on the basis of labels. |
| Allocation concealment (selection bias) | Low risk | Comment:Fumaria extract and placebo were prepared and encoded according to the table of random numbers. These codes were kept secret until data collection by a pharmacist was complete, and they were revealed after analysis was performed and labels on reports were prepared. After written informed consent was obtained from the mothers of the infants, control and treatment groups were established on the basis of these labels. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Information was insufficient to permit a judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Information was insufficient to permit a judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 17% of infants were withdrawn from the study for non‐attendance at clinic or an incomplete booklet. The number of infants randomised to each group was not reported, nor was the number of dropouts in each group. |
| Selective reporting (reporting bias) | Low risk | Comment: Study authors reported results for outcomes declared in the Methods section. |
| Other bias | Low risk | Comment: At baseline, no significant differences in demographic characteristics and colic criteria were evident between the 2 groups. |
Savino 2002.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Sample size: 97 infants with colic. Of these 97 infants, 11 (5 from the treatment group and 6 from the control group) dropped out; 7 did not come to the second visit, 2 were excluded because of fever and 2 were excluded because of gastroenteritis. Setting: Children's Hospital Regina Margherita Sex: not reported Mean age: not reported (SD not reported); range 15 to 60 days Mean weight: not reported Mean duration of colic: not reported Mean crying: cimetropium bromide 17.3 (SD 12.6) minutes; placebo 47.5 (SD 28.5) minutes Feeding: exclusively breast fed Birth order: not reported Inclusion criteria:
Exclusion criteria: not reported |
|
| Interventions |
Intervention (43 infants): cimetropium bromide 3 drops/kg (1.2 mg/kg) Control (43 infants): placebo; solution with the same colour, smell, taste and package but with no pharmacological properties Administration: Treatment should be administered at the onset of each crisis, defined as inconsolable full‐force crying with typical characteristics of infantile colic (legs flexed over the abdomen, fists closed, meteorism) and no response to common consolation procedures, such as pacifier use, rocking or dull continuous background noise. Duration of study: not reported |
|
| Outcomes | Therapy was considered efficacious if crying ended within 15 minutes of administration of the compounds. Responders were children who stopped crying within this time. Parents received a structured diary in which they recorded daily (1) time crying began, time medication was given and time crying ended; and (2) side effects observed (meteorism, vomiting, sleepiness, restlessness, inappetence, cutaneous reactions, constipation, diarrhoea, respiratory distress or apnoea). | |
| Notes |
Country: Italy Funding source: This study was not supported; the drug and placebo were provided by Pharma S.P. A., Carugate (Milan). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Sequentially to recruitment each infant was assigned randomly to one of the two groups". Insufficient information was reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Comment: Placebo was a solution with the same colour, smell, taste and package but with no pharmacological properties. Quote: "Neither doctors nor parents knew which infants received treatment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither doctors nor parents knew which infants received treatment". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 11 infants (5 from the treatment group and 6 from the control group) dropped out. 7 did not come to the second visit, 2 were excluded because of fever and 2 were excluded because of gastroenteritis. Reasons for missing data could be related to treatment, but study authors did not report reasons for missing data by arm. |
| Selective reporting (reporting bias) | Low risk | Comment: Study authors reported results for all outcomes declared in the Methods section. |
| Other bias | Low risk | Comment: Before the study began, the average number of crises each day and average crying duration were similar in both groups. |
Savino 2005.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Sample size: 93 colicky infants. Of these 93 infants, 5 (2 from the treatment group and 3 from the control group) dropped out: 2 did not come to the second visit, 3 were excluded because of fever. Nobody withdrew because of problems related to the trial; therefore, the study population may be considered homogeneous. Setting: recruited from patients seen at the Department of Pediatrics of the Regina Margherita Children’s Hospital, University of Turin Sex: boys (46.6%) Mean age: herbal agents 4.2 (SD 1.4) months, placebo 4.4 (SD 1.6) months; range not reported Mean weight: herbal agents 3420 (SD 390) grams, placebo 3510 (SD 330) grams Mean duration of colic: not reported (SD not reported) Mean crying: herbal agents 201.2 (SD 18.3) minutes, placebo 198.7 (SD 16.9) minutes Feeding: not specified Birth order: not specified Inclusion criteria:
Exclusion criteria: infants receiving any medication that could affect abdominal symptoms, such as antibiotic and probiotic drugs |
|
| Interventions |
Intervention 1 (41 infants): phytotherapeutic agent (extracts of Matricaria recutita, Foeniculum vulgare and Melissa officinalis). Each dose of herbal agent consisted of 1 bottle with tank cap containing Foeniculum vulgare Miller Var. Dulce (164.29 mg), Matricaria recutita L. (177.69 mg), Melissa officinalis L. (96.89 mg), vitamin B1 (0.85 mg), calcium pantothenate (3.24 mg), vitamin B6 (1.20 mg), maltodextrin (dose not specified) and Syloid 244 FP (dose not specified) (ColiMil, Milte‐Milan, Italy). At the administered dosage, the herbal agent provides Matricaria recutita L. 71.10 mg/kg/d, Foeniculum vulgare Miller Var. Dulce 65.71 mg/kg/d and Melissa officinalis L. 38.75 mg/kg/d. Control (47 infants): placebo. Placebo looking like the phytotherapeutic agent with regard to colour, smell, taste and package, but containing only vitamins. Each dose of placebo consisted of 1 identical bottle with tank cap containing water obtained by inverted osmosis, fructose, pineapple flavour, citric acid and sorbate potassium. Administration: Both herbal agent and placebo were administered twice a day at 5 PM and 8 PM, some minutes before feeding, at a dosage of 2 mL/kg/d. Infants had to take treatment consecutively for 7 days. Duration of study: 21 days |
|
| Outcomes | Parents wrote a daily structured diary, recording (1) the start of crying time ‐ when the medication was administered, (2) the end of crying time and (3) any side effects they observed for the 7 days of therapy and until day 21 from enrolment (vomiting, sleepiness, restlessness, inappetence, cutaneous reactions, constipation, diarrhoea). Before starting treatment, parents were invited to record data concerning daily crying time for 3 days (day − 2; day − 1; day 0). At days 1 and 7, infants were seen in the department, and parents gave the diary to researchers. At day 21 after baseline, mothers were asked to complete a questionnaire about crying time during the observation period. To ensure that all parents noted crying time in a uniform way, and to ensure that infants were given medication correctly, 1 researcher was always available by phone to help parents. Therapy was considered efficacious if crying time was reduced by ≥ 50% per day; responders were infants who showed such a reduction in crying time. | |
| Notes |
Country: Italy Funding source: Study authors did not report whether the study received any support. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Information was insufficient to permit a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Comment: Placebo looked like the phytotherapeutic agent with regard to colour, smell, taste and package. Quote: "Neither doctors nor parents knew whether the infants received treatment or not". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither doctors nor parents knew whether the infants received treatment or not". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 5 infants (2 from the treatment group and 3 from the placebo group) dropped out ‐ 2 did not come to the second visit, and 3 were excluded because of fever, but nobody withdrew because of problems related to the trial. |
| Selective reporting (reporting bias) | Low risk | Comment: Study authors reported results for all outcomes declared in the Methods section. |
| Other bias | Low risk | Comment: No significant differences between groups at baseline were reported. |
Sethi 1988.
| Methods | Double‐blind, randomised, placebo‐controlled, cross‐over trial | |
| Participants |
Sample size: 26 children; no dropouts/withdrawals Setting: not reported Sex: boys (40%) Mean age: not reported (SD not reported); range 1 week to 3 months Mean weight (SD): not reported Mean duration of colic: not reported (SD not reported); states simply that no differences in frequency of symptoms at the beginning of treatment were noted between groups Mean crying: not reported Feeding: not reported Birth order: not reported Inclusion criteria: infants with diagnosis of colic Exclusion criteria: not specified |
|
| Interventions |
Intervention (n = 26): simethicone; 25 participants received 1 dropper load of medication (20 mg) and 11 received 2 droppers (dose not reported) before evening feeds, since symptoms were nocturnal Control (n = 26): placebo Administration: Therapy was administered as a suspension containing 40 mg/mL simethicone and as a matching placebo suspension. Parents were issued a coded trial medication, and after 1 week, the trial medication was returned; parents then were issued the alternative trial medication for the coming week. Duration of study: 2 weeks Washout period: none |
|
| Outcomes | Parents were asked to record (1) daily frequency of crying and (2) amplitude for crying attacks, using a 4‐point rating scale. Parents were also asked to record (3) the number, nature and consistency of infant stools and (4) any perceived adverse effects. | |
| Notes |
Country: England Funding source: Study authors did not report whether the study received support. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Information was insufficient to permit a judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Parents were issued with a coded trial medication". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Parents were issued with a coded trial medication". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All infants completed the study. |
| Selective reporting (reporting bias) | High risk | Comment: Study authors did not clearly report results for all outcomes and did not report results of each treatment by study period. |
| Other bias | High risk | Comment: No washout period was planned. |
Weissbluth 1984.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Sample size: 48 infants; 6 families did not complete the treatment period (1 dicyclomine hydrochloride, 5 placebo) Setting: Infants received general paediatric care from a physician on staff at Children's Memorial Hospital. Sex: boys (58%) Mean age: 4 (SD not reported) months; range not reported Mean weight: 4.4 (SD not reported) kilograms Mean duration of colic: 5 weeks Mean crying: not reported Feeding: not reported Birth order: 50% first born Inclusion criteria: infants with colic defined as unexplained irritability, agitation, fussiness, crying lasting > 3 hours per day and occurring more than 3 days/week for longer than 3 weeks (Wessel definition) Exclusion criteria: not specified |
|
| Interventions |
Intervention (24 infants): dicyclomine hydrochloride Control (24 infants): placebo Administration: Researchers dispensed 180 mL (6 oz) of cherry syrup (placebo) or dicyclomine hydrochloride in identical appearing bottles. Each family received a syringe‐type measuring device to administer the study colic medicine. Dosage instructions were derived from a pilot study and were based on minimising apparent dose‐related adverse effects observed during the study. Medicine was given for a minimum of 14 days and was started at the lowest dose listed, given once in the morning, once at noon and once in the evening. Investigators reported no baseline differences between treatment groups. 42 of the 48 eligible infants received the medication for 14 days or longer. Duration of study: 14 days |
|
| Outcomes | The family received a 14‐day diary in which to record daily hours of colic, numbers of night awakenings and side effects. The definition of drug treatment outcome was based on analysis of the second week of study treatment. The categorical definition of colic was the same as for the first eligibility criterion of the study. Thus, infants with spells of unexplained crying for less than 3 hours/d (based on information reported in the diary) or longer than 3 hours/d on fewer than 3 days/week were considered to not have colic. | |
| Notes |
Country: USA Funding source: Study authors did not report whether the study received any support. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Information was insufficient to permit a judgement. |
| Allocation concealment (selection bias) | Low risk | Comment: Pharmacists at Children's Memorial Hospital randomly assigned the group allocation as each eligible infant was enrolled in the study. During the entire study, the code‐determining group was known only to the pharmacist. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Placebo or dicyclomine was dispensed in identical appearing bottles labelled only "Colic study medicine". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Placebo or dicyclomine was dispensed in identical appearing bottles labelled only "Colic study medicine". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 6 families did not complete the 14‐day treatment period because they noted "no improvement" and were considered as treatment failures before group allocation was known. |
| Selective reporting (reporting bias) | Low risk | Comment: Study authors reported results for all outcomes declared in the Methods section. |
| Other bias | Low risk | Comment: No group differences were noted between infants given drug or placebo in terms of any baseline characteristic. |
Weizman 1993.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants |
Sample size: 72 infants (36 herbal tea, 36 placebo) were enrolled in the study. 4 infants were excluded during the study: 3 for acute illness (2 from tea arm, 1 from placebo arm), and 1 because of poor parental compliance. 68 were included in the analysis. Setting: primary community‐based paediatric clinics in the Beer‐Sheva area, in Israel Sex: boys (38% of 68) Mean age: herbal tea 21.1 (SD 9.3) days, placebo 24.6 (SD 7.6) days; range not reported Mean weight: herbal tea 3116 (SD 1060) grams, placebo 3201 (SD 1088) grams Mean duration of colic: not reported Mean crying: not reported Feeding: breast fed (herbal tea 72%, placebo 67%) Birth order: first child (herbal tea 59%, placebo 66%) Inclusion criteria: infants with colic according to Wessel definition Exclusion criteria:
|
|
| Interventions |
Intervention (33 infants): herbal tea preparation (Calma Baby Bonomelli) containing fennel, chamomile, vervain, licorice, balm mint Control (35 infants): placebo. The placebo preparation consisted of an instant powder of glucose and natural flowers only, with no herbs. The smell and taste of the placebo and the tea were similar. Administration: Tea was offered to infants with every episode of colic, up to 150 mL/dose, but no more than 3 times a day. Tea powder was dissolved in water according to the manufacturer's instructions. Treatment was administered for 1 week. No significant differences were noted between 2 treatment arms. |
|
| Outcomes | Evaluation of the 2 groups was based on the following 3 measures:
Families received a diary in which to record total daily hours of colic, numbers of night awakenings, a 5‐grade improvement score and adverse effects. Parental diaries covered 7 days with no therapy and 7 days with treatment. |
|
| Notes |
Country: Israel Funding source: This study was supported by Materna Laboratories, Maabarot, Israel. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "infants were randomly assigned to receive either tea or placebo". Comment: Information was insufficient. |
| Allocation concealment (selection bias) | Low risk | Comment: During the entire study, the code determining group allocation was known only to the pharmacists. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The smell and taste of the placebo and the tea were similar; both were packed in identical appearing cans; the code of group allocation was known only by the pharmacist". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The code was known only by the pharmacist". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 4 infants were excluded during the study: 3 (2 tea and 1 placebo) because of acute illness, and 1 (tea) because of poor parental compliance. Study authors excluded these infants from the analyses because of exclusion criteria. |
| Selective reporting (reporting bias) | Low risk | Comment: Study authors reported study results for all outcomes. |
| Other bias | Low risk | Comment: Analysis of infant characteristics (gender, age, feeding method) and colic severity after 7 days of no‐treatment revealed no significant differences between the 2 groups. |
CHC: child health centre. SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barr 1999 | Case‐control study (not an experimental study) |
| Becker 1988 | Not a comparative study. A control arm was not planned; all participants were assigned to simethicone treatment. |
| Benjamins 2013 | Cross‐sectional study (not an experimental study) |
| Koonce 2011 | Commentary on a clinical study (not an experimental study) |
| NCT00655083 | Not a comparative study (phase I study) |
| NCT01532518 | Not eligible participants (infants with feeding intolerance) |
| NCT02708238 | Not an eligible comparison. This study compared Matricaria chamomilla L., Melissa officinalis L. and tyndallised L. Acidophilus (H122) and L reuteri DSM 17938 (108 CFU) with simethicone. |
| Oggero 1994 | Not an eligible comparison. This study compared dicyclomine with dietary modifications. |
| Savino 2007 | Not an eligible comparison. This study compared simethicone with Lactobacillus. |
Characteristics of ongoing studies [ordered by study ID]
NCT01258153.
| Trial name or title | Preliminary Efficacy and Safety Study of Oral Nepadutant in Infant Colic (no‐cry) ClinicalTrials.gov identifier: NCT01258153 Double‐blind, randomised, placebo‐controlled, parallel‐group study to evaluate the efficacy and safety of oral administration of nepadutant in infant colic |
| Methods |
Study type: intervention Study design: randomised, double‐blind |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Number of arms: 3
Administration: oral; once daily for 7 days |
| Outcomes |
Primary outcome measure:
Secondary outcome measures:
|
| Starting date | November 2010 |
| Contact information | Study chair: Sybille Koletzko, Dr. v. Haunersches Kinderspital Ludwig Maximilians University D‐80337 München, Germany |
| Notes | This phase IIa study is designed as a multi‐centre, multi‐national, randomised, double‐blind, placebo‐controlled study in 3 parallel groups, with the aim to evaluate the efficacy and safety of nepadutant given at 2 oral doses once daily for 7 days vs placebo in the treatment of infantile colic. |
Differences between protocol and review
We have partially modified the Methods section, as explained below.
First, in the Types of interventions subsection, we added the example of "sucrose or glucose".
Second, in the Types of outcome measures subsection, we:
replaced point 2 (worsening of symptoms) with "Responders", under Primary outcomes, because we noted that most studies evaluated symptoms as the percentage of infants with improvement, not with deterioration (i.e. worsening);
modified the outcome "Reduction in frequency of crying episodes (post‐treatment vs baseline)*", under Secondary outcomes, from "dichotomous" to "data available may be continuous, for example, hours per day, or dichotomous, for example, reduction under a threshold defined by trialists"; and
removed "Presence/absence of colic after treatment” from our list of Secondary outcomes because it was already captured by the outcome “Numbers of responders”.
Third, in the Searching other resources subsection, we deleted the sentences on searching Google and Google Scholar, as these searches were not performed.
Fourth, in the Assessment of risk of bias in included studies subsection, we moved to Appendix 3 our description of how we assessed each study for risk of bias across each of the seven domains.
Fifth, in the Unit of analysis issues subsection, under the heading "Cross‐over studies", we added details on the method used to analyse cross‐over studies. This section now reads as follows: "We used the inverse variance method, as recommended by Elbourne 2002, to include data from cross‐over studies with an adequate washout period. To take account of the correlation between the two study periods, we calculated the correlation co‐efficient between periods for each study (Savino 2012). When the correlation co‐efficient could not be obtained, we used data from the first period only. For continuous data, no studies reported the SD of a paired t‐test, and for binary data, only one of the included studies with a planned washout period reported the number of participants who responded to both treatments (Metcalf 1994). Consequently, we decided to analyse cross‐over trials as if they were parallel‐group trials. This approach, even if it is not the most correct, is conservative, as it overestimates the variability between study periods. Furthermore, we conducted separate meta‐analyses for cross‐over and parallel‐group trials, thus avoiding the unit of analysis error. For cross‐over studies with an inadequate washout period, we used data from the first period only. If data from the first period were not available, we did not incorporate these studies into a meta‐analysis."
Sixth, in the Assessment of heterogeneity section, we added that we "used Tau² to assess between‐study variability".
Finally, we added the following paragraph to a new subsection entitled, "Summary of findings table", beneath the Data synthesis subsection:
"We summarised the evidence in 'Summary of findings' tables and provided summary estimates of absolute and relative effects (see Summary of findings table 1; Summary of findings table 2; Summary of findings table 3; and Summary of findings table 4). We included a rating (ranging from very low to high) of our confidence in the estimate of effect for the overall quality of evidence for each outcome as assessed via the GRADE approach (Guyatt 2008; Guyatt 2013). We used an iterative, electronic correspondence discussion process to reach consensus on factors that affect confidence in the estimate of effects, including risk of bias (i.e. design and study limitations), imprecision, indirectness (directness in the GRADE approach includes generalisability and applicability), inconsistency of results (i.e. heterogeneity), magnitude of effect and issues of residual plausible confounding; and in evidence rating."
Contributions of authors
Elena Biagioli performed the statistical analyses and wrote the Results and Discussion sections. Valentina Tarasco wrote the protocol and contributed to the write‐up of the Results section of the full review. Carla Lingua added the references. Lorenzo Moja wrote the protocol and reviewed the Results and Discussion sections. Francesco Savino has primary responsibility for the review. He wrote the protocol, evaluated the studies, extracted data from articles and contributed to the write‐up of the Results and Discussion sections.
Sources of support
Internal sources
-
Department of Pediatrics, Regina Margherita Children's Hospital, Turin, Italy.
Logistical support
External sources
None, Other.
Declarations of interest
Elena Biagioli ‐ none known. Valentina Tarasco ‐ none known. Carla Lingua ‐ none known. Lorenzo Moja ‐ none known. Francesco Savino ‐ has received fees for scientific consultancy from Nestlé Italia Milano, Italy; HiPP GmbH & Co Vertrieb KG, Germany and Danone Trading BV, Amsterdam. FS receives royalties from Springer for the book Nutrizione Parenterale in Pediatria. FS declares that the book does not cover any interventions investigated in the review and is outside the submitted work. FS received payment from Federazione Italiana Medici Pediatrici Piemonte, Torino, Italy, to attend a conference to present a clinical update. FS has received travel grants/accomodation/meeting expenses from Pharmaceutical Laboratories Cana Iraklio, Attica; Radio Televisione Italiana Roma, Italy; BioGaia AB, Stockholm, Sweden; Noos, Roma, Italy; Nestlé Italy and France. FS has received personal fees from Mead Johnson Nutrition Italy; Cana, Thessaloniki, Greece; Nutricia ‐ part of Group Danone, Dubai, Kuwait; HiPP GmbH & Co Vertrieb KG, Germany; Menarini Farmaceutica, Firenze, Italy. These organisations have had no input nor involvement in any aspect of the review process during this, or previous, systematic reviews carried out by FS. FS declares that none of these companies have a real or vested interest in the findings of the review. FS is an author of two included studies (Savino 2002 and Savino 2005). Two independent review authors (EB and VT) evaluated those studies.
New
References
References to studies included in this review
Akçam 2006 {published data only}
- Akçam M, Yilmaz A. Oral hypertonic glucose solution in the treatment of infantile colic. Pediatrics International 2006;48(2):125‐7. [PUBMED: 16635169] [DOI] [PubMed] [Google Scholar]
Alexandrovich 2003 {published data only}
- Alexandrovich I, Rakovitskaya O, Kolmo E, Sidorova T, Shushunov S. The effect of fennel (Foeniculum vulgare) seed oil emulsion in infantile colic: a randomized, placebo‐controlled study. Alternative Therapies in Health and Medicine 2003;9(4):58‐61. [PUBMED: 12868253] [PubMed] [Google Scholar]
Alves 2012 {published data only}
- Alves JG, De Brito Rde CC, Cavalcanti TS. Effectiveness of Mentha piperita in the treatment of infantile colic: a crossover study. Evidence‐Based Complementary and Alternative Medicine 2012; Vol. 2012:981352. [DOI: 10.1155/2012/981352] [DOI] [PMC free article] [PubMed]
Arikan 2008 {published data only}
- Arikan D, Alp H, Gözüm S, Orbak Z, Cifçi EK. Effectiveness of massage, sucrose solution, herbal tea or hydrolysed formula in the treatment of infantile colic. Journal of Clinical Nursing 2008;17(13):1754‐61. [PUBMED: 18592627] [DOI] [PubMed] [Google Scholar]
Blomquist 1983 {published data only}
- Blomquist HK, Mjörndal, Tiger G. Dicycloverin chloride solution ‐ a remedy for severe infantile colic [Dicykloverinkloridlösning hjälp vid svår spädbarnskolik]. Läkartidningen 1983;80(3):116‐8. [PUBMED: 6343737] [PubMed] [Google Scholar]
Danielsson 1985 {published data only}
- Danielsson B, Hwang CP. Treatment of infantile colic with surface active substance (simethicone). Acta Paediatrica Scandinavica 1985;74(3):446‐50. [PUBMED: 3890465] [DOI] [PubMed] [Google Scholar]
Gomirato 1989 {published data only}
- Gomirato G, Bonomi A, Bensi G, Crosato M. Effect of cimetropium bromide in the treatment of colic in the first 3 months of life. Randomized study. Minerva Pediatrica 1989;41(5):259‐62. [PUBMED: 2796881] [PubMed] [Google Scholar]
Grunseit 1977 {published data only}
- Grunseit F. Evaluation of the efficacy of dicyclomine hydrochloride ('Merbentyl') syrup in the treatment of infant colic. Current Medical Research and Opinion 1977;5(3):258‐61. [PUBMED: 162663] [DOI] [PubMed] [Google Scholar]
Hwang 1985 {published data only}
- Hwang CP, Danielsson B. Dicyclomine hydrochloride in infantile colic. BMJ (Clincal Research Ed.) 1985 ;291(6501):1014. [PUBMED: 3931770] [DOI] [PMC free article] [PubMed] [Google Scholar]
Illingworth 1959 {published data only}
- Illingworth RS. Evening colic in infants: a double‐blind trial of dicyclomine hydrochloride. Lancet 1959;274(7112):1119‐20. [DOI: 10.1016/S0140-6736(59)90102-3] [DOI] [Google Scholar]
Markestad 1997 {published data only}
- Markestad T. Use of sucrose as a treatment for infant colic. Archives of Disease in Childhood 1997;76(4):356‐7. [DOI: 10.1136/adc.76.4.356] [DOI] [PMC free article] [PubMed] [Google Scholar]
Metcalf 1994 {published data only}
- Metcalf TJ, Irons TG, Sher LD, Young PC. Simethicone in the treatment of infant colic: a randomized, placebo‐controlled, multicenter trial. Pediatrics 1994;94(1):29‐34. [PUBMED: 8008533] [PubMed] [Google Scholar]
Montaseri 2013 {published data only}
- Montaseri S, Pourarian S, Montaseri H. Effects of fumaria extract on colic pain in 3‐16 weeks infants. Iranian Journal of Neonatology 2013;4(2):10‐5. [Google Scholar]
Savino 2002 {published data only}
- Savino F, Brondello C, Cresi F, Oggero R, Silvestro L. Cimetropium bromide in the treatment of crisis in infantile colic. Journal of Pediatric Gastroenterology and Nutrition 2002;34(4):417‐9. [PUBMED: 11930101] [DOI] [PubMed] [Google Scholar]
Savino 2005 {published data only}
- Savino F, Cresi F, Castagno E, Silvestro L, Oggero R. A randomized double‐blind placebo‐controlled trial of a standardized extract of Matricaria recutita, Foeniculum vulgare and Melissa officinalis (ColiMil) in the treatment of breastfed colicky infants. Phytotherapy Research 2005;19(4):335‐40. [PUBMED: 16041731] [DOI] [PubMed] [Google Scholar]
Sethi 1988 {published data only}
- Sethi KS, Sethi JK. Simethicone in the management of infant colic. Practitioner 1988;232(1448):508. [PUBMED: 3064072] [PubMed] [Google Scholar]
Weissbluth 1984 {published data only}
- Weissbluth M, Christoffel KK, Davis AT. Treatment of infantile colic with dicyclomine hydrochloride. Journal of Pediatrics 1984;104(6):951–5. [PUBMED: 6374085] [DOI] [PubMed] [Google Scholar]
Weizman 1993 {published data only}
- Weizman Z, Alkrinawi S, Goldfarb D, Bitran C. Efficacy of herbal tea preparation in infantile colic. Journal of Pediatrics 1993;122(4):650‐2. [PUBMED: 8463920] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barr 1999 {published data only}
- Barr RG, Young SN, Wright JH, Gravel R, Alkawaf R. Differential calming responses to sucrose taste in crying infants with and without colic. Pediatrics 1999;103(5):e68. [DOI] [PubMed] [Google Scholar]
Becker 1988 {published data only}
- Becker N, Lombardi P, Sidoti E, Katkin LS. Mylicon drops in the treatment of infant colic. Clinical Therapeutics 1988;10(4):401‐5. [PubMed] [Google Scholar]
Benjamins 2013 {published data only}
- Benjamins LJ, Gourishankar A, Yataco‐Marquez V, Cardona EH, Ybarrondo L. Honey pacifier use among an indigent pediatric population. Pediatrics 2013;131(6):e1838‐41. [PUBMED: 23650307] [DOI] [PubMed] [Google Scholar]
Koonce 2011 {published data only}
- Koonce T, Mounsey A, Rowland K. Colicky baby? Here's a surprising remedy. Journal of Family Practice 2011;60(1):34‐6. [PMC free article] [PubMed] [Google Scholar]
NCT00655083 {unpublished data only}
- NCT00655083. A phase I study to evaluate the oral absorption of nepadutant in infants. www.clinicaltrials.gov (accessed 7 October 2015).
NCT01532518 {unpublished data only}
- NCT01532518. Preliminary efficacy, safety and pharmacokinetics study of nepadutant in infant with feeding intolerance. www.clinicaltrials.gov (accessed 7 October 2015).
NCT02708238 {unpublished data only}
- NCT02708238. Efficacy of a standardized extract of Matricaria chamomilla L., Melissa officinalis L. and tyndallized Lactobacillus acidophilus (H122) compared with Lactobacillus reuteri (DSM 17938) and with simethicone for the treatment of infantile colic. www.clinicaltrials.gov (accessed 8 June 2016).
Oggero 1994 {published data only}
- Oggero R, Garbo G, Savino F, Mostert M. Dietary modifications versus dicyclomine hydrochloride in the treatment of severe infantile colics. Acta Paediatrica 1994;83(2):222‐5. [DOI: 10.1111/j.1651-2227.1994.tb13055.x] [DOI] [PubMed] [Google Scholar]
Savino 2007 {published data only}
- Savino F, Pelle E, Palumeri E, Oggero R, Miniero R. Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics 2007;119(1):e124‐30. [PUBMED: 17200238] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01258153 {unpublished data only}
- NCT01258153. Preliminary efficacy and safety study of oral nepadutant in infant colic (no‐cry). www.clinicaltrials.gov (accessed 7 October 2015).
Additional references
Akman 2006
- Akman I, Kusçu K, Ozdemir N, Yurdakul Z, Solakoglu M, Orhan L, et al. Mothers' postpartum psychological adjustment and infantile colic. Archives of Disease in Childhood 2006;91(5):417‐9. [PUBMED: 16452109] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bassotti 1987
- Bassotti G, Imbimbo BP, Gaburri M, Daniotti S, Morelli A. Transverse and sigmoid colon motility in healthy humans: effects of eating and of cimetropium bromide. Digestion 1987;37(1):59‐64. [DOI: 10.1159/000199488] [DOI] [PubMed] [Google Scholar]
Canivet 2000
- Canivet C, Jakobsson I, Hagander B. Infantile colic. Follow‐up at four years of age: still more "emotional". Acta Paediatrica 2000;89(1):13‐7. [DOI: 10.1111/j.1651-2227.2000.tb01179.x] [DOI] [PubMed] [Google Scholar]
Canivet 2008
- Canivet CA, Östergren PO, Jakobsson IL, Dejin‐Karlsson E, Hagander BM. Infantile colic, maternal smoking and infant feeding at 5 weeks of age. Scandinavian Journal of Public Health 2008;36(3):284‐91. [DOI: 10.1177/1403494807086981] [DOI] [PubMed] [Google Scholar]
Capasso 2007
- Capasso R, Savino F, Capasso F. Effects of the herbal formulation ColiMil on upper gastrointestinal transit in mice in vivo. Phytotherapy Research 2007;21(10):999‐1101. [PUBMED: 17582592] [DOI] [PubMed] [Google Scholar]
Carbajal 1999
- Carbajal R, Chauvet X, Couderc S, Oliver‐Martin M. Randomised trial of analgesic effects of sucrose, glucose and pacifiers in term neonates. BMJ 1999;319:1393‐7. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen‐Silver 2009
- Cohen‐Silver J, Ratnapalan S. Management of infantile colic: a review. Clinical Pediatrics 2009;48(1):14‐7. [DOI: 10.1177/0009922808323116] [DOI] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Review of Interventions. Chichester: John Wiley & Sons, 2008:243–96. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9.4.6: Combining dichotomous and continuous outcomes. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Dobson 2012
- Dobson D, Lucassen PLBJ, Miller JJ, Vlieger AM, Prescott P, Lewith G. Manipulative therapies for infantile colic. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD004796.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
DTB 2013
- Drug, Therapeutics Bulletin. Management of infantile colic. Drug and Therapeutics Bulletin 2013;347:f4102. [DOI: 10.1136/bmj.f4102] [DOI] [PubMed] [Google Scholar]
Edwards 1984
- Edwards PDL. Dicyclomine in babies. BMJ 1984;288(6425):1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] [DOI] [PubMed] [Google Scholar]
Fiocchi 2010
- Fiocchi A, Brozek J, Schünemann H, Bahna SL, Berg A, Beyer K, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. World Allergy Organization Journal 2010;3:57‐161. [DOI: 10.1097/WOX.0b013e3181defeb9; PUBMED: 23268426] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freedman 2009
- Freedman SB, Al‐Harthy N, Thull‐Freedman J. The crying infant: diagnostic testing and frequency of serious underlying disease. Pediatrics 2009;123(3):841‐8. [PUBMED: 19255012] [DOI] [PubMed] [Google Scholar]
Garriott 1984
- Garriott JC, Rodriquez R, Norton LE. Two cases of death involving dicyclomine in infants. Measurement of therapeutic and toxic concentrations in blood. Journal of Toxicology: Clinical Toxicology 1984;22(5):455‐62. [DOI: 10.3109/15563658408992576] [DOI] [PubMed] [Google Scholar]
Garrison 2000
- Garrison MM, Christakis DA. Early childhood: colic, child development, and poisoning prevention: a systematic review of treatments for infant colic. Pediatrics 2000;106(1 (Suppl 1)):184‐90. [PubMed] [Google Scholar]
Goldman 2004
- Goldman MH. Letters: dicycloverine for persistent crying in babies: dicycloverine is contraindicated in infants. BMJ 2004;328(7445):956. [DOI: 10.1136/bmj.328.7445.956-b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2007
- Gupta SK. Update on infantile colic and management options. Current Opinion in Investigational Drugs 2007;8(11):921‐6. [PUBMED: 17979025] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI: 10.1136/bmj.39489.470347.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2013
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables‐binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [PUBMED: 22609141] [DOI] [PubMed] [Google Scholar]
Hall 2012
- Hall B, Chesters J, Robinson A. Infantile colic: a systematic review of medical and conventional therapies. Journal of Paediatric Child Health 2012;48(2):128‐37. [DOI: 10.1111/j.1440-1754.2011.02061.x] [DOI] [PubMed] [Google Scholar]
Heine 2006
- Heine RG. Gastroesophageal reflux disease, colic and constipation in infants with food allergy. Current Opinion in Allergy and Clinical Immunology 2006;6(3):220‐5. [PUBMED: 16670518] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011c
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hill 2000
- Hill DJ, Hosking CS. Infantile colic and food hypersensitivity. Journal of Pediatric Gastroenterology and Nutrition 2000;30 Suppl:S67‐76. [PUBMED: 10634302] [DOI] [PubMed] [Google Scholar]
Hyman 2006
- Hyman PE, Milla PJ, Benninga MA, Davidson GP, Fleisher DF, Taminiau J. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology 2006;130(5):1519‐26. [DOI: 10.1053/j.gastro.2005.11.065] [DOI] [PubMed] [Google Scholar]
Iacono 1991
- Iacono G, Carroccio A, Montalto G, Cavataio F, Bragion E, Lorello D, et al. Severe infantile colic and food intolerance: a long‐term prospective study. Journal of Pediatric Gastroenterology and Nutrition 1991;12(3):332‐5. [PUBMED: 2072224] [DOI] [PubMed] [Google Scholar]
Iacono 2005
- Iacono G, Merolla R, D'Amico D, Bonci E, Cavataio F, Prima L, et al. Gastrointestinal symptoms in infancy: a population‐based prospective study. Digestive and Liver Disease 2005;37(6):432‐8. [DOI] [PubMed] [Google Scholar]
Imbimbo 1986
- Imbimbo BP, Daniotti S, Vidi A, Foschi D, Saporiti F, Ferrante L. Discontinuous oral absorption of cimetropium bromide, a new antispasmodic drug. Journal of Pharmaceutical Sciences 1986;75(7):680‐4. [DOI: 10.1002/jps.2600750713] [DOI] [PubMed] [Google Scholar]
Kanabar 2001
- Kanabar D, Randhawa M, Clayton P. Improvement of symptoms in infant colic following reduction of lactose load with lactase. Journal of Human Nutrition and Dietetics 2001;14(5):359–63. [DOI] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chaper 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chicester: John Wiley & Sons, 2008:95‐147. [Google Scholar]
Lehtonen 1994
- Lehtonen L, Korvenranta H, Eerola E. Intestinal microflora in colicky and non colicky infants: bacterial cultures and gas‐liquid chromatography. Journal of Pediatric Gastroenterology and Nutrition 1994;19(3):310‐4. [PUBMED: 7815263] [DOI] [PubMed] [Google Scholar]
Lehtonen 1994b
- Lehtonen L, Korhonen T, Korvenranta H. Temperament and sleeping patterns in colicky infants during the first year of life. Journal of Developmental and Behavioral Pediatrics 1994;15(6):416‐20. [DOI: 10.1097/00004703-199412000-00004] [DOI] [PubMed] [Google Scholar]
Lothe 1987
- Lothe L, Ivarsson SA, Lindberg T. Motilin, vasoactive intestinal peptide and gastrin in infantile colic. Acta Paediatrica Scandinavica 1987;76(2):316‐20. [PUBMED: 3591297] [DOI] [PubMed] [Google Scholar]
Lothe 1990
- Lothe L, Ivarsson SA, Ekman R, Lindberg T. Motilin and infantile colic. A prospective study. Acta Paediatrica Scandinavica 1990;79(4):410‐6. [DOI] [PubMed] [Google Scholar]
Lucassen 1998
- Lucassen PL, Assendelft WJ, Gubbels JW, Eijk JT, Geldrop WJ, Neven AK. Effectiveness of treatments for infantile colic: systematic review. BMJ 1998;316(7144):1563–9. [PUBMED: 9596593] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucassen 2001
- Lucassen PL, Assendelft WJ, Eijk JT, Gubbels JW, Douwes AC, Geldrop WJ. Systematic review of the occurrence of infantile colic in the community. Archives of Disease in Childhood 2001;84(5):398‐403. [PUBMED: 11316682] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucassen 2015
- Lucassen P. Colic in infants. BMJ Clinical Evidence 2015:309. [PMC free article] [PubMed]
Martin‐Martinez 2010
- Martin‐Martinez D, Casaseca‐de‐la‐Higuera P, Vegas‐Sanchez‐Ferrero G, Cordero‐Grande L, Andres‐de‐Llano JM, Garmendia‐Leiza JR, et al. Characterization of activity epochs in actimetric registries for infantile colic diagnosis: identification and feature extraction based on wavelets and symbolic dynamics. Proceedings of the 32nd Annual International Conference of the IEEE EMBS; 2010 August 31 ‐ September 4; Buenos Aires, Argentina. 2010:2383‐6. [DOI] [PubMed]
Miller‐Loncar 2004
- Miller‐Loncar C, Bigsby R, High P, Wallach M, Lester B. Infant colic and feeding difficulties. Archives of Disease in Childhood 2004;89(10):908‐12. [PUBMED: 15383432] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nocerino 2015
- Nocerino R, Pezzella V, Cosenza L, Amoroso A, Scala C, Amato F, et al. The controversial role of food allergy in infantile colic: evidence and clinical management. Nutrients 2015;7(3):2015‐25. [DOI: 10.3390/nu7032015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oxman 1992
- Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Annals of Internal Medicine 1992;116(1):78–84. [PUBMED: 1530753] [DOI] [PubMed] [Google Scholar]
Pauli‐Pott 2000
- Pauli‐Potta U, Becker K, Mertesacker T, Beckmann D. Infants with “colic” ‐ mothers’ perspectives on the crying problem. Journal of Psychosomatic Research 2000;48(2):125‐32. [DOI: 10.1016/S0022-3999(99)00084-7] [DOI] [PubMed] [Google Scholar]
Perry 2011
- Perry R, Hunt K, Ernst E. Nutritional supplements and other complementary medicines for infantile colic: a systematic review. Pediatrics 2011;127(4):720‐33. [DOI: 10.1542/peds.2010-2098] [DOI] [PubMed] [Google Scholar]
Praveen 2014
- Praveen V, Praveen S, Deshpande G, Patole SK. Oral probiotics for infantile colic. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD010986] [DOI] [Google Scholar]
Randall 1986
- Randall B, Gerry G, Rance F. Dicyclomine in the sudden infant death syndrome (SIDS) ‐ a cause of death or an incidental finding?. Journal of Forensic Sciences 1986;31(4):1470‐4. [PubMed] [Google Scholar]
Reijneveld 2002
- Reijneveld SA, Brugman E, Hirasing RA. Excessive infant crying: definitions determine risk groups. Archives of Disease in Childhood 2002;87:43‐4. [DOI: 10.1136/adc.87.1.43] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhoads 2009
- Rhoads JM, Fatheree NY, Norori J, Liu Y, Lucke JF, Tyson JE, et al. Altered fecal microflora and increased fecal calprotectin in infants with colic. Journal of Pediatrics 2009;155(6):823‐8.e1. [DOI: 10.1016/j.jpeds.2009.05.012] [DOI] [PubMed] [Google Scholar]
Roberts 2004
- Roberts DM, Ostapchuk M, O'Brien JG. Infantile colic. American Family Physician 2004;70(4):735‐40. [PubMed] [Google Scholar]
Räihä 2002
- Räihä H, Lehtonen L, Huhtala V, Saleva K, Korvenranta H. Excessively crying in the family: mother‐infant, father‐infant and mother‐father interaction. Child: Care, Health and Development 2002;28(5):419–29. [PUBMED: 12296876] [DOI] [PubMed] [Google Scholar]
Sadeh 2009
- Sadeh A, Sivan Y. Clinical practice: sleep problems during infancy. European Journal of Pediatrics 2009;168(10):1159–64. [PUBMED: 19343361] [DOI] [PubMed] [Google Scholar]
Sagrada 1989
- Sagrada A, Schiavone A, Cefalà A, Micheletti R. Cimetropium bromide: in vitro and in vivo evaluation of spasmolytic activity on human and dog colon. Digestion 1989;42(3):143‐50. [DOI: 10.1159/000199839] [DOI] [PubMed] [Google Scholar]
Savino 2004
- Savino F, Cresi F, Pautasso S, Palumeri E, Tullio V, Roana J, et al. Intestinal microflora in breastfed colicky and non‐colicky infants. Acta Paediatrica 2004;93(6):825‐9. [PUBMED: 15244234] [PubMed] [Google Scholar]
Savino 2005b
- Savino F, Bailo E, Oggero R, Tullio V, Roana J, Carlone N, et al. Bacterial counts of intestinal Lactobacillus species in infants with colic. Pediatric Allergy Immunology 2005;16(1):72‐5. [PUBMED: 15693915] [DOI] [PubMed] [Google Scholar]
Savino 2006
- Savino F, Grassino EC, Guidi C, Oggero R, Silvestro L, Miniero R. Ghrelin and motilin concentration in colicky infants. Acta Paediatrica 2006;95(6):738‐41. [PUBMED: 16754557] [DOI] [PubMed] [Google Scholar]
Savino 2007b
- Savino F. Focus on infantile colic. Acta Paediatrica 2007;96(9):1259‐64. [DOI: 10.1111/j.1651-2227.2007.00428.x] [DOI] [PubMed] [Google Scholar]
Savino 2009
- Savino F, Cordisco L, Tarasco V, Calabrese R, Palumeri E, Matteuzzi D. Molecular identification of coliform bacteria from colicky breastfed infants. Acta Paediatrica 2009;98(10):1582–8. [DOI: 10.1111/j.1651-2227.2009.01419.x; PUBMED: 19604166] [DOI] [PubMed] [Google Scholar]
Savino 2010
- Savino F, Tarasco V. New treatments for infant colic. Current Opinion in Pediatrics 2010;22(6):791‐7. [DOI: 10.1097/MOP.0b013e32833fac24.; PUBMED: 20859207] [DOI] [PubMed] [Google Scholar]
Savino 2014
- Savino F, Ceratto S, Marco A, Cordero di Montezemolo L. Looking for new treatments of infantile colic. Italian Journal of Pediatrics 2014;40:53. [DOI: 10.1186/1824-7288-40-53] [DOI] [PMC free article] [PubMed] [Google Scholar]
Savino 2014b
- Savino F, Tarasco V, Sorrenti M, Lingua C, Moja L, Gordon M, et al. Dietary modifications for infantile colic. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD011029] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scarpignato 1985
- Scarpignato C, Bianchi Porro G. Cimetropium bromide, a new antispasmodic compound: pharmacology and therapeutic perspectives. International Journal of Clinical Pharmacology Research 1985;5(6):467‐77. [PUBMED: 3912339] [PubMed] [Google Scholar]
Schiavone 1985
- Schiavone A, Schiavi GB, Conti L, Micheletti R, Sagrada A, Hammer R, et al. Cimetropium: characterization of antimuscarinic and spasmolytic properties. Arzneimittel‐Forschung 1985;35(5):796‐9. [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:335–57. [Google Scholar]
Sferra 1996
- Sferra TJ, Heitlinger LA. Gastrointestinal gas formation and infantile colic. Pediatric Clinics of North America 1996;43(2):489‐510. [PUBMED: 8614612] [DOI] [PubMed] [Google Scholar]
Skogsdal 1997
- Skogsdal Y, Eriksson M, Schollin J. Analgesia in newborns given oral glucose. Acta Pediatrica 1997;86(2):217‐20. [DOI: 10.1111/j.1651-2227.1997.tb08872.x] [DOI] [PubMed] [Google Scholar]
Steinherz 2004
- Steinherz R. Dicycloverine for persistent crying in babies: beware recommending dicycloverine treatment in babies. BMJ 2004;328(7445):956. [DOI: 10.1136/bmj.328.7445.956-c] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steutel 2014
- Steutel NF, Benninga MA, Langendam MW, Kruijff I, Tabbers MM. Reporting outcome measures in trials of infant colic. Journal of Paediatric Gastroenterology and Nutrition 2014;59(3):341‐6. [DOI: 10.1097/MPG.0000000000000412] [DOI] [PubMed] [Google Scholar]
Sung 2014
- Sung V, Hiscock H, Tang MLK, Mensah FK, Nation ML, Satzke C, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ 2014;348:g2107. [DOI: 10.1136/bmj.g2107] [DOI] [PMC free article] [PubMed] [Google Scholar]
Treem 1994
- Treem WR. Infant colic. A pediatric gastroenterologist's perspective. Pediatric Clinics of North America 1994;41(5):1121‐38. [PUBMED: 7936776] [DOI] [PubMed] [Google Scholar]
Van den Berg 2009
- Berg MP, Ende J, Crijnen AAM, Jaddoe VWV, Moll HA, Mackenbach JP, et al. Paternal depressive symptoms during pregnancy are related to excessive infant crying. Pediatrics 2009;124(1):e96‐103. [DOI: 10.1542/peds.2008-3100] [DOI] [PubMed] [Google Scholar]
Vandenplas 2015
- Vandenplas Y, Abkari A, Bellaiche M, Benninga M, Chouraqui JP, Çokura F, et al. Prevalence and health outcomes of functional gastrointestinal symptoms in infants from birth to 12 months of age. Journal of Pediatric Gastroenterology and Nutrition 2015;61(5):531‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vik 2009
- Vik T, Grote V, Escribano J, Socha J, Verduci E, Fritsch M, et al. Infantile colic, prolonged crying and maternal postnatal depression. Acta Paediatrica 2009;98(8):1344‐8. [DOI: 10.1111/j.1651-2227.2009.01317.x] [DOI] [PubMed] [Google Scholar]
Walker 1988
- Walker AM, Romieu I. Infant medication and sudden infant death syndrome. American Journal of Preventive Medicine 1988;4(2 Suppl):29‐31. [PubMed] [Google Scholar]
Wessel 1954
- Wessel MA, Cobb JC, Jackson EB, Harris GS Jr, Detwiler AC. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics 1954;14(5):421‐35. [PubMed] [Google Scholar]
Williams 1984
- Williams J, Watkins‐Jones R. Dicyclomine: worrying symptoms associated with its use in some small babies. BMJ 1984;288(6421):901. [DOI: 10.1136/bmj.288.6421.901] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yusuf 1991
- Yusuf S, Wittes J, Probsfield K, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266(1):93–8. [PUBMED: 2046134] [PubMed] [Google Scholar]
References to other published versions of this review
Savino 2012
- Savino F, Tarasco V, Lingua C, Moja L, Ricceri F. Pain‐relieving agents for infant colic. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009999] [DOI] [PMC free article] [PubMed] [Google Scholar]


