Abstract
Background
Acne vulgaris is a very common skin problem that presents with blackheads, whiteheads, and inflamed spots. It frequently results in physical scarring and may cause psychological distress. The use of oral and topical treatments can be limited in some people due to ineffectiveness, inconvenience, poor tolerability or side‐effects. Some studies have suggested promising results for light therapies.
Objectives
To explore the effects of light treatment of different wavelengths for acne.
Search methods
We searched the following databases up to September 2015: the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase and LILACS. We searched ISI Web of Science and Dissertation Abstracts International (from inception). We also searched five trials registers, and grey literature sources. We checked the reference lists of studies and reviews and consulted study authors and other experts in the field to identify further references to relevant randomised controlled trials (RCTs). We updated these searches in July 2016 but these results have not yet been incorporated into the review.
Selection criteria
We included RCTs of light for treatment of acne vulgaris, regardless of language or publication status.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 71 studies, randomising a total of 4211 participants.
Most studies were small (median 31 participants) and included participants with mild to moderate acne of both sexes and with a mean age of 20 to 30 years. Light interventions differed greatly in wavelength, dose, active substances used in photodynamic therapy (PDT), and comparator interventions (most commonly no treatment, placebo, another light intervention, or various topical treatments). Numbers of light sessions varied from one to 112 (most commonly two to four). Frequency of application varied from twice daily to once monthly.
Selection and performance bias were unclear in the majority of studies. Detection bias was unclear for participant‐assessed outcomes and low for investigator‐assessed outcomes in the majority of studies. Attrition and reporting bias were low in over half of the studies and unclear or high in the rest. Two thirds of studies were industry‐sponsored; study authors either reported conflict of interest, or such information was not declared, so we judged the risk of bias as unclear.
Comparisons of most interventions for our first primary outcome 'Participant's global assessment of improvement' were not possible due to the variation in the interventions and the way the studies' outcomes were measured. We did not combine the effect estimates but rated the quality of the evidence as very low for the comparison of light therapies, including PDT to placebo, no treatment, topical treatment or other comparators for this outcome. One study which included 266 participants with moderate to severe acne showed little or no difference in effectiveness for this outcome between 20% aminolevulinic acid (ALA)‐PDT (activated by blue light) versus vehicle plus blue light (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.72 to 1.04, low‐quality evidence). A study (n = 180) of a comparison of ALA‐PDT (activated by red light) concentrations showed 20% ALA was no more effective than 15% (RR 1.05, 95% CI 0.96 to 1.15) but better than 10% ALA (RR 1.22, 95% CI 1.05 to 1.42) and 5% ALA (RR 1.47, 95% CI 1.19 to 1.81). The number needed to treat for an additional beneficial outcome (NNTB) was 6 (95% CI 3 to 19) and 4 (95% CI 2 to 6) for the comparison of 20% ALA with 10% and 5% ALA, respectively.
For our second primary outcome 'Investigator‐assessed changes in lesion counts', we combined three RCTs, with 360 participants with moderate to severe acne and found methyl aminolevulinate (MAL) PDT (activated by red light) was no different to placebo cream plus red light with regard to change in inflamed lesions (ILs) (mean difference (MD) ‐2.85, 95% CI ‐7.51 to 1.81), percentage change in ILs (MD ‐10.09, 95% CI ‐20.25 to 0.06), change in non‐inflamed lesions (NILs) (MD ‐2.01, 95% CI ‐7.07 to 3.05), or in percentage change in NILs (MD ‐8.09, 95% CI ‐21.51 to 5.32). We assessed the evidence as moderate quality for these outcomes meaning that there is little or no clinical difference between these two interventions for lesion counts.
Studies comparing the effects of other interventions were inconsistent or had small samples and high risk of bias. We performed only narrative synthesis for the results of the remaining trials, due to great variation in many aspects of the studies, poor reporting, and failure to obtain necessary data. Several studies compared yellow light to placebo or no treatment, infrared light to no treatment, gold microparticle suspension to vehicle, and clindamycin/benzoyl peroxide combined with pulsed dye laser to clindamycin/benzoyl peroxide alone. There were also several other studies comparing MAL‐PDT to light‐only treatment, to adapalene and in combination with long‐pulsed dye laser to long‐pulsed dye laser alone. None of these showed any clinically significant effects.
Our third primary outcome was 'Investigator‐assessed severe adverse effects'. Most studies reported adverse effects, but not adequately with scarring reported as absent, and blistering reported only in studies on intense pulsed light, infrared light and photodynamic therapies. We rated the quality of the evidence as very low, meaning we were uncertain of the adverse effects of the light therapies.
Although our primary endpoint was long‐term outcomes, less than half of the studies performed assessments later than eight weeks after final treatment. Only a few studies assessed outcomes at more than three months after final treatment, and longer‐term assessments are mostly not covered in this review.
Authors' conclusions
High‐quality evidence on the use of light therapies for people with acne is lacking. There is low certainty of the usefulness of MAL‐PDT (red light) or ALA‐PDT (blue light) as standard therapies for people with moderate to severe acne.
Carefully planned studies, using standardised outcome measures, comparing the effectiveness of common acne treatments with light therapies would be welcomed, together with adherence to the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Plain language summary
The use of light as a therapy for acne
What is the aim of this review?
The aim of this Cochrane Review was to find out whether treatment using lasers and other light sources improves the whiteheads and blackheads, and inflamed spots that people with acne have. We also wanted to know how people with acne assessed their own improvement, and whether they found that these therapies caused unpleasant effects like blistering or scarring. Cochrane researchers collected and analysed all relevant studies to answer these questions and found 71 studies, with a total of 4211 participants.
What was studied in this review?
Acne is a common skin problem. It causes blackheads, whiteheads and inflamed spots, and may lead to scarring. Current treatment options are limited in their effectiveness and convenience, and may cause side‐effects. We investigated lasers and other light sources, which are used as an alternative therapy, either on their own or in combination with a chemical that makes the skin more sensitive to the light source (photodynamic therapy (PDT)). We compared different light therapies with other treatment options, no treatment, or placebo.
Most studies included people with mild to moderate acne in their twenties. Light treatments in these studies varied greatly in many important aspects, such as wavelength of light used, duration of treatment, chemicals used in photodynamic therapy, and others.
Over half of the studies were industry sponsored; study authors reported either conflict of interest, or such information was not declared.
Key messages
We are unable to draw firm conclusions from the results of our review, as it was not clear whether the light therapies (including PDT) assessed in these studies were more effective than the other comparators tested such as placebo, no treatment, or treatments rubbed on the skin, nor how long the possible benefits lasted.
What are the main results of this review?
We investigated how people with acne assessed their own improvement, but it was not clear whether the light therapies in the studies had a beneficial effect. Evidence on how investigators assessed changes in numbers of blackheads, whiteheads and inflamed spots in people with acne was also limited for most types of light therapies, due to variation in the way the studies were conducted and measured.
Most studies reported side‐effects, but not adequately. Scarring was reported as absent, and blistering was reported in studies on intense pulsed light, infrared light and on PDT.
Three studies, with a total of 360 participants with moderate to severe acne, showed that photodynamic therapy with methyl aminolevulinate (MAL), activated by red light, had a similar effect on changes in numbers of blackheads, whiteheads and inflamed spots when compared with placebo cream with red light. We judged the quality of this evidence moderate.
Future well planned studies comparing the effectiveness of common acne treatments with light therapies are needed to assess the true clinical effects and side‐effects of light therapies for acne.
How up to date is this review?
This review included studies up to September 2015.
Summary of findings
Summary of findings for the main comparison. Light therapies (including photodynamic therapy) compared to placebo, no treatment, topical treatment and other comparators for acne vulgaris.
| Light therapies (including photodynamic therapy) for acne vulgaris | ||||||
|
Patient or population: Mild, moderate and severe acne vulgaris
Settings: Single and multicentre, worldwide
Intervention: Light therapies including photodynamic therapy Comparison: Placebo, no treatment, topical treatment and other comparators | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Light therapies | |||||
| Participant's global assessment of improvement Non‐standardised scales Follow‐up: up to 24 weeks after final treatment | See comment | See comment | Not estimable | 1033 (23 studies) | ⊕⊝⊝⊝ very low1,2,3 | We decided not to combine the effect estimates from the different interventions. We instead rated the quality of the evidence based on the GRADE considerations. The direction and size of effect across the individual study results across the 38 different comparisons were inconsistent. 13 studies used Likert or Likert‐like scales, 5 visual analogue scales, 3 other methods and in 2 studies it was unclear which method was used. In many studies last evaluation at final treatment, timing of assessment unclearly reported or not reported. 13 studies had split‐face design, 8 parallel‐group design, 2 split faces within parallel‐group design.4,5 |
| Investigator‐assessed change in lesion counts Lesion counts Follow‐up: up to 12 months after final treatment | See comment | See comment | Not estimable | 2242 (51 studies) | ⊕⊝⊝⊝ very low1,2,3 | We decided not to combine the effect estimates from the different interventions. We instead rated the quality of the evidence based on the GRADE considerations. The direction and size of effect across the individual study results across the 76 different comparisons were inconsistent. Different methods for lesion counting reported including change or percentage change from baseline in the number of individual or various aggregates of counts of inflamed lesions, non‐inflamed lesions, nodules and cysts. 22 studies had split‐face design, 1 split‐face or back design, 2 split‐back design, 19 parallel‐group design, 7 split‐face within parallel‐group design.4,5 |
| Investigator‐assessed severe adverse effects Blistering or scarring Follow‐up: up to 12 months after final treatment | See comment | See comment | Not estimable | 3945 (66 studies) | ⊕⊝⊝⊝ very low1,2,3 | We decided not to combine the effect estimates from the different interventions. We rated the quality of the evidence based on the GRADE considerations. In most studies it was reported that adverse effects were recorded, without stating explicit intent to record blistering and scarring. No reports of scarring in any of the studies. No reports of blistering in 56 studies with a total of 3378 participants. Blistering was reported in two studies on infrared light and one study on intense pulsed light6, as well as in seven studies on photodynamic therapies (PDT)7. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded by one level because of risk of bias: unclear to high overall risk of bias in the majority of studies. 2 We downgraded by one level because of indirectness: lack of comparisons with conventional treatments. Limited generalisation due to variation of participants (such as Fitzpatrick skin types, severity of acne etc.). 3 We downgraded by one level because of imprecision: small sample sizes (median of 24 for 'Participant's global assessment of improvement', and median of 30 for studies on each of the other two outcomes), power calculations not reported, often unclear assignment to groups or face sides. 4 We have not downgraded further because of inconsistency, but there was heterogeneity across studies due to diversity of populations, interventions, comparators and methods of outcome assessment. 5 We have not downgraded further because of publication bias, however our searches identified considerable number of unpublished studies, but with no available data. 6 Three split‐face trials; one included two reports on the infrared treated sides 2/46 (4.3%) and no reports on the untreated sides (0%); one included one report on the single pass 1450 nm laser‐treated side 1/11 (9%) and no reports on the double pass 1450 nm laser‐treated sides (0%); one study included one report on the intense pulsed light (IPL)‐treated sides 1/10 (10%) and no reports on the untreated sides (0%). 7 Three studies on methyl aminolevulinate (MAL)‐PDT, (one of which is presented in Summary of findings table 2), the second was a split‐face within parallel‐group trial included one report on the 37 J/cm² 80 mg/g MAL‐PDT with occlusion 1/22 (4.5%) sides and no reports on the 37 J/cm² 80 mg/g MAL‐PDT without occlusion sides (0%), nor on the 25 J/cm² 80 mg/g MAL‐PDT with or without occlusion sides (0%). Further split‐face study included one report on 160 mg/g MAL‐PDT sides 1/30 (3%), and no reports on red‐light‐only control sides. Four 20% aminolevulinic acid (ALA )‐PDT studies: one split‐face trial included one report 1/44 (2.3%) on the sides with pulsed dye laser (PDL) used for activation and no reports on the untreated sides. One split‐back within parallel‐group included one report 1/11 (9%) in the single‐treatment group on back sites with 550–700 nm light used for activation, and no reports in the multiple treatment groups on the ALA‐PDT, nor ALA alone, light alone or untreated back sites in any of the groups. One parallel‐group trial included one report in the arm which used a combination of IPL of 580–980 nm and bipolar radiofrequency energies for activation, and no reports in the arms which used 517 nm light or IPL‐alone (600–850 nm) for activation; the number of participants per group unclear. One parallel‐group trial included one report in the arm which used 20% ALA 1/45 (2%) and no reports (0%) in arms with 5%, 10% nor 15% ALA activated by 633 nm light.
Summary of findings 2. MAL‐PDT compared to red light only for acne vulgaris.
| MAL‐PDT compared to red light only for acne vulgaris | ||||||
| Patient or population: Moderate and severe acne vulgaris Settings: Multicentre, USA and Canada Intervention: 80 mg/g methyl aminolevulinate (MAL) PDT activated by red light Comparison: Placebo cream with red light | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Red light only | MAL‐PDT | |||||
| Paticipant's global assessment of improvement ‐ Not measured | ‐ | ‐ | Not estimable | ‐ | ‐ | ‐ |
| Investigator‐assessed change in inflamed lesions (ILs) Lesion counts Follow‐up: 6 weeks after final treatment | Baseline mean ILs count in the red‐light‐only groups was 39.9; the mean investigator‐assessed change in ILs in the red‐light‐only groups was ‐10.6 | Baseline mean ILs count in the MAL‐PDT group was 39.2; the mean investigator‐assessed change in ILs in the MAL‐PDT groups was 2.85 lower (7.51 lower to 1.81 higher) | ‐ | 360 (3 studies) | ⊕⊕⊕⊝ moderate1 | Two additional trials not included due to clinical and methodological heterogeneity. Assumed risk is based on weighted average of the mean ILs counts in the control groups and the corresponding risk on weighted average of the mean ILs counts in the intervention groups of the three studies2,3,4 |
| Investigator‐assessed change in non‐inflamed lesions (NILs) Lesion counts Follow‐up: 6 weeks after final treatment | Baseline mean NILs count in the red‐light‐only groups was 47.6; the mean investigator‐assessed change in NILs in the red‐light‐only groups was ‐10.8 | Baseline mean NILs count in the MAL‐PDT group was 45.6; the mean investigator‐assessed change in NILs in the MAL‐PDT groups was 2.01 lower (7.07 lower to 3.05 higher) | ‐ | 360 (3 studies) | ⊕⊕⊕⊝ moderate1 | Two additional trials not included due to clinical and methodological heterogeneity. Assumed risk is based on weighted average of the mean NILs counts in the control groups and the corresponding risk on the weighted average of the mean NILs counts in the intervention groups of the three studies2,3,4 |
| Investigator‐assessed percentage change in ILs Lesion counts Follow‐up: 6 weeks after final treatment | Baseline mean ILs count in the red‐light‐only groups was 39.9; the mean investigator‐assessed percentage change in ILs in the red‐light‐only groups was ‐25.7% | Baseline mean ILs count in the MAL‐PDT group was 39.2; the mean investigator‐assessed percentage change in ILs in the MAL‐PDT groups was 10.09 lower (20.25 lower to 0.06 higher) | ‐ | 360 (3 studies) | ⊕⊕⊕⊝ moderate1 | Two additional trials not included due to clinical and methodological heterogeneity. Assumed risk is based on weighted average of the mean ILs counts in the control groups and the corresponding risk on the weighted average of the mean ILs counts in the intervention groups of the three studies2,3,4 |
| Investigator‐assessed percentage change in NILs Lesion counts Follow‐up: 6 weeks after final treatment | Baseline mean NILs count in the red‐light‐only groups was 47.6; the mean investigator‐assessed percentage change in NILs in the red‐light‐only groups was ‐16.6% | Baseline mean ILs count in the MAL‐PDT group was 45.6; the mean investigator‐assessed percentage change in NILs in the MAL‐PDT groups was 8.09 lower (21.51 lower to 5.32 higher) | ‐ | 360 (3 studies) | ⊕⊕⊕⊝ moderate1 | Two additional trials not included due to clinical and methodological heterogeneity. Assumed risk is based on weighted average of the mean NILs counts in the control groups and the corresponding risk on the weighted average of the mean NILs counts in the intervention groups of the three studies2,3,4 |
|
Investigator‐assessed severe adverse effects Application site blister Follow‐up: during whole study period |
Study population | Not estimable | 360 (3 studies) | ⊕⊕⊕⊝ moderate1 | Scarring was not reported. Two additional trials not included due to clinical and methodological heterogeneity. Due to the lack of events occurring in both groups, the relative risk is unreliable2,3,4 | |
| Application site blister rates in the red‐light‐only groups were 0/158 (0%) | Application site blister rates in the MAL‐PDT groups were 1/202 (0.5 %) | |||||
| Investigator's global assessment (IGA) of improvement Treatment 'success' as defined by IGA score decrease5 Follow‐up: 6 weeks after final treatment | Study population | RR 1.74 (1.11 to 2.74) | 360 (3 studies) | ⊕⊕⊕⊝ moderate1 | The absolute effect was 89 more per 1000 (95% CI 13 more to 209 more). The number needed to treat for an additional treatment 'success' was 7 (95% CI 5 to 11).2,3,4 An additional trial not included due to clinical and methodological heterogeneity. |
|
| 120 per 1000 | 209 per 1000 (133 to 329) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded by one level because of indirectness: comparisons with no treatment, placebo or conventional treatments not included. 2 We have not downgraded because of risk of bias. Please note that these were industry‐sponsored studies, so we judged 'other bias' as unclear. NCT00594425 had high attrition and selective reporting bias. Low risk in all other bias domain for all three studies. 3 We have not downgraded because of inconsistency. There was some clinical heterogeneity across studies to take into account, in one study only participants with severe acne were included, in the other two studies participants with both moderate and severe acne were included (less than 20% of the included participants had severe acne in those trials). 4 The three studies included 53, 53,and 52 participants in the control group and 100, 54 and 48 participants in the intervention group respectively. 5 1 = almost clear; 2 = mild severity; 3 = moderate severity; 4 = severe. Success defined as improvement of at least two grades from baseline.
Summary of findings 3. ALA‐PDT compared to blue light only for acne vulgaris.
| ALA‐PDT compared to blue light only for acne vulgaris | ||||||
| Patient or population: Moderate and severe acne vulgaris Setting: Multicentre, USA Intervention: 20% aminolevulinic acid (ALA) activated by 500 s and 1000 s blue light Comparison: Vehicle plus 500 s and 1000 s blue light | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with blue light only | Risk with ALA‐PDT | |||||
|
Participant's global assessment of improvement Non‐standardised scale5 Follow up: 6 weeks |
Study population | RR 0.87 (0.72 to 1.04) | 266 (1 study) | ⊕⊕⊝⊝ low1,2 |
Results for 500 s ALA and 1000 s ALA groups combined under 'Intervention', as our analyses found no statistically significant difference between them. 1000 s vehicle plus blue light and 500 s vehicle plus blue light groups combined in 'Comparison' , as our analyses found no statistically significant difference between them. | |
| 602 per 1000 | 523 per 1000 (433 to 626) | |||||
| Investigator‐assessed change in inflamed lesions (ILs) Lesion counts Follow up: 6 weeks | Not estimable. See comment. | Not estimable. See comment. | Not estimable | 266 (1 study) | ⊕⊝⊝⊝ very low1,3 |
Means not reported nor provided upon request. The median investigator‐assessed change (standard deviation, SD) in ILs was ‐21.0 (23.63) in the vehicle 1000 s, ‐17.0 (26.71) in the vehicle 500 s group, ‐18.5 (30.15) in the ALA 1000 s and ‐13.0 (28.74) in the ALA 500 s group. |
|
Investigator‐assessed percentage change in ILs Lesion counts Follow up: 6 weeks |
Not estimable. See comment. | Not estimable. See comment. | Not estimable | 266 (1 study) | ⊕⊝⊝⊝ very low1,3 |
Means not reported nor provided upon request. The median investigator‐assessed percentage change (SD) in ILs was ‐48.4 (32.81) in the vehicle 1000 s, ‐45.2 (50.15) in the vehicle 500 s group, ‐34.4 (37.8) in the ALA 1000 s group and ‐29.0 (42.57) in the ALA 500 s group. |
|
Investigator‐assessed severe adverse effects Application site blister Follow‐up: during whole study period |
Study population | Not estimable | 266 (1 study) | ⊕⊝⊝⊝ very low1,4 |
"Oozing/Vesiculation/Crusting" were evaluated at baseline, and were then assessed pre‐ and post‐treatment & 48 h after treatment at each treatment session, as well as 3 and 6 weeks after final treatment. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Investigator's global assessment (IGA) of improvement Treatment 'success' as defined by IGA score decrease6 Follow up: 6 weeks | Study population | RR 0.81 (0.51 to 1.29) | 266 (1 study) | ⊕⊕⊝⊝ low1,2 |
Results for 1000 s ALA and 500 s ALA groups combined under 'Intervention', as our analyses found no statistically significant difference between them. 1000 s vehicle plus blue light and 500 s vehicle plus blue light groups combined in 'Comparison', as our analyses found no statistically significant difference between them. | |
| 195 per 1000 | 158 per 1000 (100 to 252) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded by one level because of indirectness: comparisons with no treatment, placebo or conventional treatments not included. 2 We have downgraded by one level because of risk of bias. 3 We have downgraded by two levels because of risk of bias. Means and 95% CIs were not reported. 4 We have downgraded by two levels because of risk of bias. There were no reports of application site blisters among adverse effects, however it is possible that some occurred, but it is impossible to separate those as they were reported together with oozing and crusting under "Oozing/ Vesiculation/Crusting". 5 Excellent = very satisfied; good = moderately satisfied; fair = slightly satisfied; poor = not satisfied at all. Success defined as improvement of at least two grades from baseline. 6 0 = clear skin with no ILs or NILs; almost clear; rare NILs with no more than a few small ILs; Mild; > Grade 1 = some NILs with some ILs (papules/pustules only; no nodules); Moderate; > Grade 2 = up to many NILs and a moderate number of ILs but no more than one small nodule; Severe; > Grade 3 = up to many NILs and ILs, but no more than a few nodules. Success was defined as a two‐point or more improvement on the IGA scale since baseline'
Background
Description of the condition
Acne is a very common inflammatory skin condition that affects the face of over 90% of people some point in their lives, the chest in 60% of people, and the back in 15% (Cunliffe 1989). The condition usually starts in adolescence and frequently resolves by the mid‐twenties (Bhate 2013; Burton 1971).
Acne is characterised by an increase in sebum production; the formation of lesions called open and closed comedones (which appear as blackheads and whiteheads); raised red spots, known as papules and pustules and in more severe cases nodules; deep pustules; and cysts (Degitz 2007; Nast 2012). Acne can range from a mild form, with a few of these lesions, to more severe forms embracing multiple lesions over the face and trunk (O'Brien 1998).
Mild acne is more prevalent than the severe form (Kilkenny 1998). In some cases, acne persists, or initially starts, in adulthood, and in this situation, it is seen more commonly in adult women than men (Choi 2011; Dreno 2013; Preneau 2012; Williams 2006).
Impact
Acne results in a significant burden. One study from the USA indicated that the prevalence by the mid‐teens was virtually 100% (Stern 1992). A more recent European study estimated a rate to be 82.4% in 10 to 12 year olds and identified that over 40% of people sought treatment (Amado 2006).
The duration of acne can be anything from 5 to 10 years (Cunliffe 1979). In most people, acne has resolved by the age of 25 years (Cunliffe 1979). Between 7% and 17% of those affected have clinical acne beyond this time (Goulden 1997).
Acne can produce significant psychological and social problems, and those having acne may be affected by lower self‐esteem, anxiety, depression, and low mood (Baldwin 2002; Tan 2004; Thomas 2004). Scarring is a very common problem, and treatment is extremely difficult (Jordan 2000; Layton 1994; Tan 2010); scarring can also result in significant psychological and social problems (Hayashi 2015).
The treatments available for acne may result in adverse effects, which may limit their use (Nast 2012; Williams 2012). The complex pathophysiology of acne often results in the need for multiple treatments within any given regimen, and this can have impact on adherence (Dreno 2010; Krejci‐Manwaring 2006). There is increasing concern about the use of antibiotics in the management of acne due to emerging bacterial resistance (Coates 2002).
Causes
Acne usually presents around puberty and arises as a result of an increase in hormone levels, particularly androgen hormones (Thiboutot 2004; Zouboulis 2004). This leads to enlargement of the sebaceous (grease) glands and an increased cell turnover resulting in blockage and plugging of the duct that carries the sebum to the skin, which leads to the formation of a comedone (whiteheads and blackheads, Cunliffe 2004). Skin bacteria, in particular Propionibacterium acnes (P acnes), become trapped within the duct, and an intense inflammatory reaction ensues, which results in the inflamed skin lesions characteristic to acne, that is, the pustules, papules, and in the worst cases, nodules and cysts (Degitz 2007; Nast 2012). Insulin resistance is one factor implicated in the development of severe acne and is a common complaint of women with polycystic ovarian syndrome (Archer 2004; Pfeifer 2005).
Conventional treatments
First‐line treatments in Europe include fixed combinations of benzoyl peroxide (BPO) with adapalene or clindamycin for mild‐to‐moderate papulopustular acne, whereas isotretinoin is recommended for more severe forms of acne (Nast 2012). Recent guidelines published by the American Academy of Dermatology (AAD) also recommend BPO or topical retinoid, or topical combination therapy including BPO with or without antibiotic for mild acne, however separate components, as well as fixed combination products may be prescribed (Zaenglein 2016). Topical combination therapy for moderate acne may also be prescribed together with an oral antibiotic for moderate and severe acne as a first line treatment (Zaenglein 2016). As in Europe, isotretinoin is only recommended for more severe forms of acne as a first line treatment (Zaenglein 2016). Systemic antibiotics in combination with adapalene, azelaic acid, or a fixed combination of adapalene and BPO are recommended for more severe forms of acne (Nast 2012).
For mild‐to‐moderate acne, second‐line treatments in Europe include topical treatments such as azelaic acid, BPO, or topical retinoids; however, systemic antibiotics in combination with adapalene can also be considered (Nast 2012). Alternative treatment suggested by the AAD guidelines for mild forms of acne include adding topical retinoid or BPO if they have not been part of the combination already, and considering alternate retinoid or topical dapsone (Zaenglein 2016). Alternative treatment for moderate forms of acne include alternating combination therapies, whereas, for both moderate and severe acne, changes in oral antibiotics, adding combined oral contraceptive or oral spironolactone for women, as well as oral isotretinoin may be considered (Zaenglein 2016).
Topical treatments target the plugged follicle and the bacteria implicated in acne as well as inflammation (Nast 2012). It is now recommended that topical antibiotics should not be used alone as they can lead to antibiotic resistance (Nast 2012). All antibiotics employed for acne should be used alongside anti‐resistant agents in the treatment of moderate acne, that is, agents that reduce antibiotic‐resistant strains of P acnes and avoid emergence of novel resistant strains (Nast 2012).
Women with acne may be prescribed hormone therapies, which are also used as combined oral contraceptives (Arowojolu 2012; Zaenglein 2016). Oral isotretinoin, which is a synthetic form of vitamin A, is very effective for moderate nodular and severe papulopustular acne (Nast 2012). For the majority of people following a course of isotretinoin, their skin clears fully by the end of a course of therapy; however, in some cases, the acne will recur (White 1998). Side‐effects from oral isotretinoin include dry lips, eyes, skin, and mucous membranes (Charakida 2004). Isotretinoin is also teratogenic, meaning that if a woman becomes pregnant whilst taking isotretinoin, it is likely to cause birth defects (Lammer 1985). This limits its use in women of childbearing age (Abroms 2006; Stern 1989).
Description of the intervention
Light therapies utilise light with different properties (wavelength, intensity, coherent or incoherent light) with the aim of achieving a beneficial result for those with acne (Haedersdal 2008a; Mariwalla 2005). Lasers (Light amplification by stimulated emission of radiation) (Leinwoll 1965) are the most common light sources that have been used for acne therapy. Lasers produce a high‐energy beam of light of a precise wavelength range, which can be focused accurately (Haedersdal 2008a; Mariwalla 2005). Several different delivery systems are used, incorporating timing controls for safety, and cooling systems to reduce discomfort during treatment (Haedersdal 2008a; Hamilton 2009; Mariwalla 2005).
How the intervention might work
The exact mechanisms of action for light therapies are still not fully understood, but three components of the intervention are considered crucial: light, photosensitisers (i.e. molecules that absorb and are then activated by light), and oxidative stress resulting from their activation (Fritsch 1998; Mariwalla 2005; Sakamoto 2010). Photosensitisers can be produced endogenously or applied exogenously (Fritsch 1998). Probable biological consequences of oxidative stress include damaging bacteria and sebaceous glands, together with reduction of follicular obstruction and hyperkeratosis (Mariwalla 2005; Sakamoto 2010). Possible interference with the immunological response, not necessarily mediated by photosensitisers, are also believed to be important (Sakamoto 2010).
Different wavelengths have different effects on P acnes bacterial colonies in vitro (Cho 2006). However, the evidence on in vivo reduction of P acnes is limited, although different light therapies have had different effects on outcomes in clinical trials (Haedersdal 2008a; Hamilton 2009).
P acnes produces endogenous porphyrins, which absorb light to form a highly reactive singlet oxygen, which destroys the bacteria (Mariwalla 2005). The peak absorption occurs at blue light wavelengths, providing a rationale for selecting blue light as a logical wavelength when using physical therapy for acne (Mariwalla 2005). However, red light is also absorbed by porphyrins and can penetrate deeper into the skin where it may directly affect inflammatory mediators (Mariwalla 2005; Ross 2005). Other light therapies, including infra‐red lasers, low energy pulsed‐dye lasers (PDL), and radiofrequency devices (Mariwalla 2005), are directed towards damaging sebaceous glands, reducing their size and thus sebum output (Lloyd 2002). Photodynamic therapy (PDT) uses specific light‐activating topical products, consisting of various porphyrin precursors, most commonly 5‐aminolevulinic acid (ALA) and its methyl‐ester methyl‐aminolevulinate (MAL) (Sakamoto 2010a). These are absorbed into the skin and amplify the response to light therapy, but in so doing, tend to produce more side‐effects (Sakamoto 2010a).
Since the 1970s the mechanism of action of PDT has been better known for the treatment of malignancies than for other uses in dermatology (Fritsch 1998; Sharma 2012). Photosensitisers used in PDT probably accumulate inside gram‐positive bacteria (such as P acnes), and when activated, a type I reaction is induced, producing hydroxyl radicals, a leak‐out of cellular contents, and death of the microbial cells (Sharma 2012). Differences in pharmacokinetic characteristics of drugs used in PDT, their incubation time, whether they were administered under occlusion or not, their ability to penetrate the intrafollicular duct, alongside wavelengths and doses of light used for activation, as well as care applied before and after the treatment, are all confounding factors likely to affect clinical results (Sakamoto 2010a). Sakamoto et al suggested two dose‐related PDT mechanisms of action: 'low dose' PDT ('low drug concentration, low light fluence, short incubation time between drug application and light exposure, use of blue light with minimal penetration depth, and/or various pulsed source exposures') is probably mainly based on transient antimicrobial or immunomodulatory effects, whereas 'high dose' PDT ('prolonged application of high ALA concentration followed by high fluence red light') is based mainly on damaging sebaceous glands (Sakamoto 2010). Optimal regimens have not yet been established (Sakamoto 2010a). There is an ongoing debate on whether lack of selectivity of the photosensitisers could lead to substantial damage to the surrounding tissue and subsequent necrosis (Sharma 2012).
Why it is important to do this review
Current treatment options may be limited in effectiveness or acceptability due to adverse effects, poor tolerability and the inconvenience of using them on a regular and prolonged basis (Nast 2012; Williams 2012; Zaenglein 2016). Conventional treatments have limitations. Most oral and topical treatments are less effective than oral isotretinoin, but the latter has significant adverse effects (Nast 2012; Williams 2012). Combination regimens, which are required for the treatment of acne, are often complex for a person to use, are time‐consuming, and can result in poor adherence (Dreno 2010). Increasing concern about the use of antibiotics for acne has emerged due to the rise in antibiotic‐resistant bacteria (Nast 2012). If we were able to identify alternative therapies that addressed some of these issues, it would clearly be advantageous to patients, the wider community, and prescribers. This is highlighted by the fact that the Acne Priority Setting Partnership, which received responses from over 8000 clinicians, patients, and carers placed the question of safety and effectiveness of physical therapies, including lasers and other light‐based treatments, in treating acne among the top 10 research priorities (Layton 2015). Light therapies seem to be increasingly popular, and many light sources are now offered for people to purchase directly using the Internet. Therefore, there is a lot of public interest in this treatment, as well as interest from health service commissioners.
To date, the evidence regarding the efficacy of light and laser interventions is not robust (Nast 2012; Zaenglein 2016 ). There have been few studies comparing lasers and light therapies with conventional acne treatments, or studies using physical therapies in severe acne, or any evaluation of the long‐term benefit of these treatments (Hamilton 2009), and so there is still uncertainty and controversy (Sanclemente 2014; Williams 2012). European guidelines (Nast 2012) gave negative recommendations for artificial ultraviolet (UV) radiation in mild, moderate, and severe papulopustular acne and for visible light as monotherapy in severe papulopustular acne. Blue light monotherapy is recommended with a low strength of recommendation for treatment of mild to moderate papulopustular acne (Nast 2012). Because of a lack of evidence, Nast 2012 left recommendations open for visible light of other wavelengths as monotherapy, lasers with infrared wavelengths, intense pulsed light (IPL), and PDT for mild to moderate and severe papulopustular acne. This is somewhat contradictory to the European guidelines for topical PDT, where inflammatory and infectious dermatoses are seen as an "emerging indication", and acne has the highest strength of recommendation, with the evidence rated as of highest possible quality (Morton 2013). Recently updated American guidelines included lasers and PDT as a new clinical question, but are not explicit in stating the strength of their recommendation, nor levels of underlying evidence (Zaenglein 2016). The study authors concluded that there was "limited evidence to recommend the use and benefit of physical modalities for the routine treatment, including pulsed dye laser..." and that "Some laser and light devices may be beneficial for acne, but additional studies are needed" (Zaenglein 2016). Zaenglein 2016 have also included clinical trials of lasers and light‐based therapies as one of the most important current research and knowledge gaps to address in acne treatment.
The worldwide market potential for anti‐acne skin preparations alone was estimated to be USD 3300 million in 2013 (GMR Data 2013). The growing market and the willingness of people to take up treatments that have not been clinically proven to be effective means that research into the use and marketing of novel treatments, such as light therapies, is important. If light therapies prove effective, they could offset the cost of acne‐related treatments. If, however, light therapies are ineffective, their use should be stopped.
Hence, establishing the evidence to support treatment of acne with light of different wavelengths is critical. The plans for this review were published as a protocol 'Light therapies for acne' (Car 2009).
Objectives
To explore the effects of light treatment of different wavelengths for acne.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), which were of two types: those which compared two groups of participants where one group was randomised to receive treatment and the other served as the control group; and those which applied treatment randomly to one part of a participant's body compared with another part which served as the control (such as split‐face studies).
We did not include cross‐over trials because an intervention for acne may have had a lasting effect that could have carried over to subsequent periods of the trial.
Types of participants
Anyone with a diagnosis of mild, moderate, or severe acne vulgaris defined by any classification system.
Types of interventions
We searched for any therapy based on the healing properties of light for the treatment of acne vulgaris. We also accepted therapies that combined light with other treatments to boost the effect of the light. We focused on a comparison between the effectiveness of treatment with light of different properties ‐ coherence, wavelength, and intensity.
Types of outcome measures
Primary outcomes
Participant's global assessment of improvement. This was recorded using a Likert or Likert‐like scale (for instance, selecting from the following categories the extent of change of their acne after treatment: acne has worsened a lot; worsened a little; stayed the same; improved a little; or improved a lot) or other scales.
-
Investigator‐assessed change in lesion count.
-
The change or percentage change from baseline in the number of:
inflamed lesions (ILs) (papules or pustules or both);
non‐inflamed lesions (NILs) (blackheads or whiteheads or both); or
nodules and cysts (for nodulocystic acne only
-
If individual lesion counts were not available, then the change or percentage change from baseline in the number of:
ILs and NILs; or
combined count of all lesion types.
-
Investigator‐assessed severe adverse effects. If blistering or scarring of the skin followed treatment with light therapy then, if possible, we reported on the severity of the adverse effect and whether it resolved in the short‐term or was permanent.
Secondary outcomes
Investigator‐assessed change in acne severity. The change in acne severity from baseline, using a published grading scale (like the Leeds grading system, which involves counting lesions and weighting them according to severity to give a combined grade) or a severity index determined by the lesion count.
Investigator's global assessment of improvement recorded using a Likert or Likert‐like scale or other scales.
Changes in quality of life assessed using a recognised tool.
Other adverse outcomes
We recorded the incidence and, when possible, severity of all other adverse events reported in the included studies. We used the system organ classes (SOCs) defined in MedDRA (MedDRA 2010), version 15.1. MedDRA® ('the Medical Dictionary for Regulatory Activities, terminology is the international medical terminology developed under the auspices of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). MedDRA® trademark is owned by the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) on behalf of ICH').
Timing of outcome assessment
We considered short‐term (two to four weeks after final treatment), medium‐term (five to eight weeks after final treatment), and long‐term (longer than eight weeks after final treatment) follow‐up periods. The long‐term data were the primary endpoint, but we were also interested in short‐term data, indicating early improvement, which may have encouraged participants to continue with treatment.
Exclusion criteria
Studies which were not RCTs.
Studies not focused on the healing properties of light in the management of acne.
Studies on light therapies for acne scars.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 29 September 2015:
the Cochrane Skin Specialised Register using the following terms: acne and (laser* or sunlight or phototherap* or photolysis or photochemotherapy or “ ultraviolet therap*” or “photosensitizing agent*” or “light therap*” or “photodynamic therap*” or “photosensitising agent*”);
the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library 2015, Issue 8) using the search strategy in Appendix 1;
MEDLINE via Ovid (from 1946) using the strategy in Appendix 2;
Embase via Ovid (from 1974) using the strategy in Appendix 3; and
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 4.
We searched the following databases up to 28 September 2015:
ISI Web of Science using the strategy in Appendix 5; and
Dissertation Abstracts International (1861) using the strategy in Appendix 6.
Trials registers
We searched the following trials registers up to 28 September 2015:
The metaRegister of Controlled trials (isrctn.com/) using the strategy in Appendix 7.
The U.S. National Institutes of Health Ongoing Trials Register (clinicaltrials.gov) using the strategy in Appendix 8.
The Australian and New Zealand Clinical Trials Registry (anzctr.org.au) using the strategy in Appendix 9.
The World Health Organization International Clinical Trials Registry Platform (who.int/ictrp/en/) using the strategy in Appendix 10.
The EU Clinical Trials Register (clinicaltrialsregister.eu/) using the strategy in Appendix 11.
This review fully incorporates the results of searches conducted up to September 2015. A search update conducted in July 2016 identified a further 15 reports of trials, which we have added to ‘Studies awaiting classification’ and will incorporate into the review at the next update. See Characteristics of studies awaiting classification.
Searching other resources
Grey literature
We attempted to find unpublished studies by searching the following grey literature:
Google Scholar using the strategy in Appendix 12 up to 7 October 2015; and
OpenGrey using the strategy in Appendix 13 up to 29 September 2015.
We also used Internet search engines such as Google.
We consulted trial authors of included and excluded trials published in the last 15 years and other experts in the field of optical therapies for acne, in order to identify further unpublished RCTs.
Reference lists
We checked the bibliographies of published studies and reviews for further references to relevant trials.
Adverse effects
We did not perform a separate search for adverse effects of the target intervention. We recorded adverse effects reported in the included trials and discussed the implications of those adverse outcomes.
Data collection and analysis
We followed the protocol for this review (Car 2009). When this was not possible, we clearly stated and further clarified it in the Differences between protocol and review section.
Selection of studies
Two review authors (JB and RA, PP or MC) screened the titles and abstracts of studies identified by the searches. If studies did not address the study of a light therapy for acne, we excluded them. If any of the review authors felt that a paper could have been relevant, we retrieved the full text, and each author independently checked that it met the pre‐defined selection criteria. We resolved differences of opinion by discussion with the review team.
Data extraction and management
Two review authors (JB and RA or MC) independently recorded data using a specially designed data extraction form. When data were available only in graph or figure format, two review authors (JB and RA or MC) extracted them independently. A third team member (JC or LG) resolved any differences of opinion. One author (JB) inserted the data into Review Manager (RevMan) (RevMan 2014). Two review authors (MC and LG, RA or PP) cross‐checked the data for accuracy.
We defined treatment success as anything above the first category of improvement on a Likert scale or more than 50% improvement from baseline on a continuous scale for participant's global assessment of improvement (primary outcome 1) and secondary outcomes 1, 2, and 3. When individual patient data were not available, we extracted summary data as they were reported. Effects of interventions on investigator‐assessed change in lesion count (primary outcome 2) were recorded as the actual or percentage change from baseline.
In addition we reported on the following:
the baseline and comparisons of the participants for age, sex, duration, location, and severity of acne;
light source identity, dose, duration of treatment, and adequacy of instructions if self‐administered;
whether outcome measures were described and their assessment was standardised;
whether previous acne treatment was discontinued in a timely manner prior to the trial;
whether concomitant acne treatment was permitted and if so, whether standardised; and
the use and appropriateness of statistical analyses, where data were not reported appropriately in the original publication.
Assessment of risk of bias in included studies
Two review authors (JB and RA or MC) used Cochrane's tool for assessing risk of bias, described in section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), to independently assess the methodological quality of each included study. We assessed the following as 'low risk of bias', 'high risk of bias', or 'unclear risk of bias':
how the randomisation sequence was generated;
whether allocation was adequately concealed;
whether participants, clinicians, or outcome assessors were blinded as appropriate, who was blinded and not blinded (participants, clinicians, outcome assessors) if this was appropriate;
incomplete outcome data and how it was addressed;
possible selective outcome reporting; and
possible other bias.
We compared the assessments and discussed and resolved any disagreements in the gradings between the review authors. We also contacted the corresponding researchers for clarification or additional data when necessary.
Measures of treatment effect
We expressed the results as risk ratio (RR) and 95% confidence intervals (CIs) for dichotomous outcomes. When the relative risk was unreliable due to the lack of events occurring in control groups or body sites, we provided event rates instead of RR and calculated risk differences (RD) with 95% CI. We clarified this in the Effects of interventions section, under 'Primary outcome 3'. Although there were no cases where standardised mean differences were needed, we would have computed them if cases existed where comparable measures on different scales had been used across trials. We used only mean differences where appropriate (Deeks 2011). We expressed the results as 'number needed to treat for an additional beneficial outcome' (NNTB) and 'number needed to treat for an additional harmful outcome' (NNTH) for dichotomous outcomes where appropriate, following guidance in section 12.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a).
Unit of analysis issues
Where there were multiple intervention groups within a trial, we made pair‐wise comparisons of light therapies with different wavelengths versus no treatment, placebo, and conventional treatment. When the level of clinical and methodological heterogeneity was acceptable, we considered pooling studies that had a split‐face or split‐back design with studies that had a parallel‐group design in a meta‐analysis using the inverse variance method, described in the Cochrane Handbook for Systematic Reviews of Interventions section 9.4.3 (Deeks 2011). However, we did not pool studies with different designs due to the nature of the results, as there was considerable methodological and clinical heterogeneity, which is outlined in the Effects of interventions section.
Dealing with missing data
If participant drop‐out led to missing data, we conducted an intention‐to‐treat (ITT) analysis. We contacted trial authors or sponsors of studies that were less than 15 years old to provide missing statistics, such as standard deviations. For dichotomous outcomes, we regarded participants with missing outcome data as treatment failures (to be conservative) and included these in the analysis as an imputed value. For continuous outcomes, we imputed missing outcomes by carrying forward the last recorded value for participants with missing outcome data (Higgins 2011b).
Assessment of heterogeneity
We followed updated guidance in sections 9.4.1 and 9.5.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011) on the appropriateness of meta‐analysis. To determine whether it would be clinically meaningful to quantitatively combine results of different studies, we considered differences in interventions (wavelengths, doses, active substances used in PDT, number of light sessions, and frequency of application) together with differences in comparator interventions (no treatment, placebo, other light interventions, and various topical treatments and their various combinations). For comparisons where no substantial clinical diversity existed with regard to the above, we assessed statistical heterogeneity using the I² statistic (Higgins 2003) and synthesised data using meta‐analysis techniques when appropriate (i.e. when I² statistic was lower than 50%) following guidance in section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Assessment of reporting biases
We planned to test publication bias by the use of a funnel plot when adequate data were available for similar light therapies, following guidance in section 10.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011). However, we were unable to implement this method in the current review and test publication bias by the use of a funnel plot due to the nature of our results.
Data synthesis
For studies with acceptable levels of clinical and methodological heterogeneity, we performed a meta‐analysis to calculate a weighted treatment effect across trials, using a random‐effects model. Where it was not possible to perform a meta‐analysis due to substantial clinical and methodological heterogeneity, we narratively synthesised the results, following guidance in section 11.7.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011b).
Subgroup analysis and investigation of heterogeneity
If substantial statistical heterogeneity (I² statistic greater than 50%) existed between studies for the primary outcome, we looked for the reasons for this, such as differences in disease severity, exposure, and duration of treatment. We planned to undertake further subgroup analysis if sufficient information was given. The groups were to include those with different severity or onset of acne and the age of participants (child or adult). However, subgroup analyses were not performed in the current review due to the nature of the results of the meta‐analyses (the I² statistic was lower than 50% for primary outcomes).
Sensitivity analysis
We intended to undertake sensitivity analyses to determine the effects of excluding the poorer quality trials and those with an unclear or high risk of bias as defined in theCochrane Handbook of Systematic Reviews of Interventions (Deeks 2011).
Adverse outcomes
We described:
whether the methods used to record adverse events were appropriate; and
whether reporting of adverse outcomes was adequate.
Other
Where necessary, we contacted the trial authors for clarification.
We created 'Summary of findings' tables using GRADEpro Guideline Developement Tool (GRADEpro GDT 2015).
Results
Description of studies
Please see the Characteristics of included studies tables, Characteristics of excluded studies tables, Characteristics of studies awaiting classification tables, and Characteristics of ongoing studies tables in this review.
Results of the search
The 'Study flow diagram' summarises the results of our incorporated searches up to September 2015 (see Figure 1). We identified 862 records through searching the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase and LILACS. We identified a further 907 records through searching ISI Web of Science and Dissertation Abstracts International. We identified 51 records through other searches. (Please see 'Clinical trials registers and 'Grey literature searches' section below for details.)
1.

Study flow diagram.
Our searches retrieved a total of 1820 records. We removed 1018 duplicates leaving 802 records. We excluded 648 records based on the titles and abstracts. We obtained full text copies of the remaining 154 records when appropriate. After assessing full texts, we excluded 25 records (corresponding to 24 studies) for reasons outlined in the Characteristics of excluded studies tables.
We included a total of 98 records in a narrative synthesis (corresponding to 71 studies). We were unable to obtain enough information to include or exclude 28 records (corresponding to 23 studies), which we listed in the Characteristics of studies awaiting classification tables. A further three studies are ongoing (EU 2014‐005235‐13; NCT02217228; NCT02431494).
We included three studies in a quantitative meta‐analysis (NCT00594425; NCT00933543; Pariser 2013).
We only included final results of the clinical trials registers and grey literature searches in the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) chart for reasons of clarity (Figure 1; Moher 2009).
Our final searches in July 2016 identified 13 additional studies (14 references): Demina 2015; Du 2015; Elgendy 2015; Ganceviciene 2015; Kwon 2016; Lekakh 2015; Moftah 2016; NCT02647528; Nestor 2016; Park 2015; Sadick 2016; Voravutinon 2016; Wang 2016. We have added a further report as a secondary reference to a previously identified study (Pariser 2013). We will incorporate the additional studies into the next update of this review.
Clinical trials registers and grey literature searches
Clinical trials registers and Open Grey returned a total of 377 records. Of these, 33 identifiers were relevant for the review. We matched 12 identifiers to 11 included studies identified through searches of other databases (Bissonnette 2010; Darne 2011; Haedersdal 2008; Hörfelt 2006; Karsai 2010; McGill 2008; Orringer 2007; Orringer 2010; Pariser 2013 (two identifiers); Uebelhoer 2007; Wiegell 2006b), while one identifier was matched to two separate studies, one included (Paithankar 2015) and one excluded (Owczarek 2014). We matched two identifiers to one study awaiting classification (Shaheen 2011). We were unable to match 18 identifiers with any of the studies identified through searches of other databases. They corresponded to 17 studies, as one study (NCT00237978) was registered in two different registers. We excluded one of these studies after contacting the study authors for clarification (NCT00613444). We obtained full results for three studies (NCT00594425; NCT00673933; NCT00933543) and results of one study were available in the register (NCT00706433), so we included them in our analysis. Nine are among studies awaiting classification (NCT00237978 (two identifiers); NCT00814918;NCT01245946; NCT01472900; NCT01584674; NCT01689935; ISRCTN73616060; ISRCTN78675673; ISRCTN95939628). Three studies are ongoing (EU 2014‐005235‐13; NCT02217228; NCT02431494).
A search of Google Scholar retrieved 963 records, and after screening, we found nine records of potentially relevant studies not identified through searches of the other databases.
We identified nine additional records through other sources (including authors' suggestions, reference lists of papers, and a Google search).
We have described our attempts to contact the authors of individual studies in the 'Notes' sections of the Characteristics of included studies tables, Characteristics of studies awaiting classification tables, Characteristics of ongoing studies tables, or Characteristics of excluded studies tables.
Included studies
We included 71 studies, with a total of 4211 included participants, of which 40 were studies of light therapies, excluding comparisons with photodynamic therapy (PDT) and randomised a total of 2485 participants, and 31 were studies of PDT (including comparisons with light therapies) which included a total of 1726 participants. Please see the Characteristics of included studies tables for details.
Design
All included studies were RCTs. Most had a parallel‐group design (40 studies), or a split‐face design (28 studies), two had a split‐back design (NCT00673933; Pollock 2004), and one had a split‐face and split‐back design (Barolet 2010).
Eleven of the 40 studies above had a parallel‐group design, but within each group, a different intervention was administered to each side of the face or other body part; six studies with such a design randomised both groups and face sides (Bissonnette 2010; Oh 2009; Orringer 2004; Seaton 2003; Yeung 2007; Yilmaz 2011); two studies randomised groups, but not face sides (Liu 2014; Yin 2010); three other studies randomised participants to groups, but it was unclear whether within those groups, treatments were also randomly applied to one part of a participant's body compared with another part that served as control (Genina 2004; Hongcharu 2000; Sami 2008).
Most studies reported, or study authors later provided information that ethical approval was obtained, but this was unclear in 22 studies (Baugh 2005; Bernstein 2007; Bowes 2003; Cheng 2008; Elman 2003; Fadel 2009; Genina 2004; Gold 2011; Hongcharu 2000; Jih 2006; Kim 2009; Ling 2010; Liu 2014; NCT00706433; Ou 2014; Papageorgieu 2000; Sadick 2010a; Taub 2007; Tzung 2004; Zhang 2009a; Zhang 2013a; Zhang 2013b).
The majority of studies reported, or later provided information regarding sponsorship and conflict of interest, but this remained unclear for 20 studies (Bernstein 2007; Borhan 2014; Bowes 2003; Chen 2015; Cheng 2008; de Arruda 2009; Elman 2003; Hong 2013; Ling 2010; Liu 2011; McGill 2008; Na 2011; Ou 2014; Papageorgieu 2000; Pollock 2004; Sami 2008; Tzung 2004; Zhang 2009a; Zhang 2013a; Zhang 2013b). The authors of 20 studies declared no conflict of interest and no commercial sponsors (Anyachukwu 2014; Chang 2007; Choi 2010; Fadel 2009; Ianosi 2013; Jung 2009; Jung 2012; Karsai 2010; Kim 2009; Lee 2010; Leheta 2009; Liu 2014; Mei 2013; Na 2007; Oh 2009; Song 2014; Wiegell 2006a; Wiegell 2006b; Yilmaz 2011; Yin 2010). In 25 studies, the authors declared some sort of conflict of interest or were industry sponsored (Ash 2015; Barolet 2010; Baugh 2005; Bissonnette 2010; Darne 2011; Genina 2004; Gold 2005; Gold 2011; Haedersdal 2008; Hongcharu 2000; Hörfelt 2006; Jih 2006; NCT00594425; NCT00673933; NCT00706433; NCT00933543; Orringer 2004; Orringer 2007; Paithankar 2015; Pariser 2013; Seaton 2003; Taub 2007; Uebelhoer 2007; Wang 2006; Yeung 2007). In five studies, the authors declared that they had no conflicts of interest, but it was unclear who provided the device or the sham device (Kwon 2013) or whether there was commercial sponsorship (Moneib 2014; Ragab 2014; Sadick 2010a; Sadick 2010b). One study had non‐commercial sponsors but it was unclear whether the authors had some sort of conflict of interest (Orringer 2010).
Only 18 studies clearly performed power calculations (Ash 2015; Barolet 2010; Bissonnette 2010; Darne 2011; Gold 2005; Hörfelt 2006; Karsai 2010; Ling 2010; NCT00594425; NCT00933543; Orringer 2004; Orringer 2007; Orringer 2010; Pariser 2013; Sadick 2010b; Seaton 2003; Wiegell 2006b; Yeung 2007).
Sample sizes
Individual sample sizes varied from 7 to 738, with an average sample size of 59 participants and median size of 31 participants. Studies of light‐only therapies, excluding comparisons with PDT, had an average sample size of 62 and median size of 36.5 participants. Studies of PDT (including comparisons with light therapies) had an average sample size of 56 and median size of 25 participants.
Twelve studies randomised more than 100 participants (Ianosi 2013; Ling 2010; Liu 2014; NCT00594425; NCT00706433; NCT00933543; Papageorgieu 2000; Pariser 2013; Yin 2010; Zhang 2009a; Zhang 2013a, Zhang 2013b); five studies randomised 60 to 90 participants (Cheng 2008; de Arruda 2009; Karsai 2010; Ou 2014; Sadick 2010b).
Setting
Most studies were performed in a single centre or it was unclear whether they were single or multicenter. Only 13 studies were clearly multicenter (Gold 2005; Hörfelt 2006; Kwon 2013; Ling 2010; NCT00594425; NCT00673933; NCT00706433; NCT00933543; Paithankar 2015; Pariser 2013; Sadick 2010b; Tzung 2004; Uebelhoer 2007).
Twenty‐seven studies were performed in Asia, 21 in North America, 14 in Europe, seven in Africa, and one in South America (de Arruda 2009). No studies were conducted in Australia. One multicenter study, Sadick 2010b, was conducted in North America and Asia.
Study authors reported several means of recruitment. The most common way was through outpatient clinics and dermatology departments ‐ reported in 33 studies. Around one third of studies (23) did not describe recruitment methods.
Participants
The lowest age as an inclusion criterion was nine years. The age of included participants ranged from 11 to 59 years. In 46 studies, the mean age of included participants was between 20 and 30 years, and 38 of these studies also reported age ranges of included participants (means of age ranges were 17 to 37 years, medians of age ranges 18 to 37.5 years). Seven studies had a mean age lower than 20 (de Arruda 2009; Elman 2003; Hörfelt 2006; Karsai 2010; NCT00933543; Pariser 2013; Ragab 2014) and three, higher than 30 (Gold 2005; McGill 2008; Wang 2006).
Two studies reported no data on age (Bowes 2003; Na 2011), three reported only the inclusion criterion (Ash 2015; Fadel 2009; Wiegell 2006a), one study reported on median age and inclusion criterion only (Ianosi 2013), six reported only the age range (Genina 2004; Hong 2013; Kwon 2013; Pollock 2004; Seaton 2003; Zhang 2013a), and two reported the age range and inclusion criterion (Haedersdal 2008; Leheta 2009).
Most studies enrolled both male and female participants. One study was female only (Chang 2007), and one was male only (Anyachukwu 2014). Sex of participants was unclear in 10 studies (Bowes 2003; Fadel 2009; Jung 2009; Jung 2012; Leheta 2009; Na 2011; Taub 2007; Tzung 2004; Wiegell 2006a; Wiegell 2006b).
All studies included participants with clinically evident acne. Most studies included participants with mild to moderate acne (27 studies) or moderate to severe acne (18 studies). Four studies did not report severity of acne assessment when including the participants (Bernstein 2007; Jung 2012; Na 2011; Orringer 2010).
Most studies defined severity by various grading scores (34 studies). Twelve studies defined severity using lesion counts (Gold 2005; Haedersdal 2008; Ianosi 2013; Jih 2006; NCT00673933; Papageorgieu 2000; Sadick 2010b; Uebelhoer 2007; Wiegell 2006a; Wiegell 2006b; Yeung 2007; Yilmaz 2011), and eleven studies used both grading scores and lesion counts (Barolet 2010; Bissonnette 2010; Darne 2011; Hörfelt 2006; NCT00594425; NCT00706433; NCT00933543; Paithankar 2015; Pariser 2013; Seaton 2003; Taub 2007). It was unclear how ten studies performed severity assessment when including participants (Baugh 2005; Bowes 2003; Elman 2003; Fadel 2009; Genina 2004; Kim 2009; Leheta 2009; Na 2007; Tzung 2004; Wang 2006).
Studies included participants with different skin responses to sun exposure, that is, different phototypes. According to the commonly used Fitzpatrick's classification, phototypes range from type I (pale white skin which always burns and never tans) to type VI (deeply pigmented dark brown to black skin which never burns and tans very easily) (Fitzpatrick 1988). Ten studies included participants with Fitzpatrick Skin Types (FPTs) I to III (Barolet 2010; Baugh 2005; Bernstein 2007; Haedersdal 2008; Hörfelt 2006; Karsai 2010; McGill 2008; Paithankar 2015; Sadick 2010a; Yilmaz 2011), and five studies, FPT I to IV (Bissonnette 2010; Gold 2011; Hongcharu 2000; Ianosi 2013; NCT00594425). Eight studies included FPT III to IV (Borhan 2014; Chang 2007; Liu 2011; Oh 2009; Sami 2008; Song 2014; Tzung 2004; Yin 2010), and four studies included participants with FPTs III to V ( Choi 2010; Jung 2012; Kwon 2013; Ragab 2014). Three studies included FPT II‐IV (Mei 2013; Taub 2007; Wang 2006), two included FPT V to VI (Anyachukwu 2014; NCT00673933), two included FPT IV to V (Hong 2013; Yeung 2007), one included only FPT III (Lee 2010) and 12 studies included participants with 4 or more different FPTs from I to VI (Ash 2015; Darne 2011; Jih 2006; NCT00706433; NCT00933543; Orringer 2007; Orringer 2010; Pariser 2013; Pollock 2004; Sadick 2010b; Wiegell 2006b). Twenty‐four studies did not report FPTs.
Interventions
We observed a substantial heterogeneity in interventions. To present them in a clearer way, we first separated studies of light‐only therapies (excluding comparisons with PDT and studies of PDT (including comparisons with light‐only therapies)). We then made subgroups according to comparison interventions (such as placebo or no treatment, topical treatments, and other comparisons) and wavelengths used in light interventions. To describe light of different wavelengths, we used corresponding colours ('green light' for wavelengths 495 to 570 nm, 'yellow light' for wavelengths 570 to 590 nm etc.). We additionally grouped PDT studies according to active substances used: methyl aminolevulinate (MAL), aminolevulinic acid (ALA), MAL versus ALA, and other active substances.
Below we have listed light‐only studies from 1 to 3 and PDT studies from 4 to 7, as well as their subgroups. If a study had more than one comparison, we listed it for every comparison it included.
1. Light versus placebo or no treatment
a) Green light versus placebo: three studies (Baugh 2005; Bowes 2003; Yilmaz 2011) b) Yellow light versus placebo or no treatment: two studies (Orringer 2004; Seaton 2003) c) Infrared light versus no treatment: three studies (Darne 2011; Moneib 2014; Orringer 2007) d) Blue light versus placebo or no treatment: three studies (Elman 2003; Gold 2011; Tzung 2004) e) Red light versus no treatment: one study (Na 2007) f) Blue‐red light versus placebo: two studies (Kwon 2013; Papageorgieu 2000) g) Broad spectrum light versus placebo: one study (Sadick 2010b) h) Intense pulsed light (IPL) versus no treatment: one study (McGill 2008)
2. Light versus topical treatment
a) Light versus benzoyl peroxide (BPO): three studies; one blue light (de Arruda 2009) and two blue‐red light (Chang 2007; Papageorgieu 2000) b) Light versus clindamycin: two studies (Gold 2005; Lee 2010) c) Light and other topical treatments: seven studies (Anyachukwu 2014; Ash 2015; Borhan 2014; Ianosi 2013; Karsai 2010; Leheta 2009; Zhang 2009a)
3. Light versus other comparators
a) Comparison of light therapies of different wavelengths: seven studies (Cheng 2008; Choi 2010; Jung 2009; Liu 2011; Liu 2014; Papageorgieu 2000; Sami 2008) b) Comparison of light therapies of different doses: four studies (Bernstein 2007; Jih 2006; NCT00706433; Uebelhoer 2007) c) Comparison of light therapies of different treatment application intervals: one study (Yilmaz 2011) d) Light alone versus combined with microdermoabrasion: one study (Wang 2006) e) Light in combination with carbon lotion (topical carbon suspension) versus no treatment: one study (Jung 2012) f) Light in combination with oral therapy versus other comparators: four studies (Ling 2010; Ou 2014; Zhang 2009a; Zhang 2013b) g) Intense pulsed light (IPL) alone versus IPL in combination with vacuum: one study (Ianosi 2013)
4. MAL‐PDT versus other comparators
a) MAL‐PDT versus red light alone: five studies (Hörfelt 2006; NCT00594425; NCT00673933; NCT00933543; Pariser 2013) b) MAL‐PDT versus yellow light alone: one study (Haedersdal 2008) c) MAL‐PDT versus placebo or no treatment: one study (Wiegell 2006b) d) MAL‐PDT other: four studies (Bissonnette 2010; Hong 2013; NCT00594425; Yeung 2007)
5. ALA‐PDT versus other comparators
a) ALA‐PDT versus red light alone: three studies (Chen 2015; Pollock 2004; Zhang 2013a) b) ALA‐PDT versus blue light alone: one study (NCT00706433) c) ALA‐PDT versus blue‐red light alone: one study (Liu 2014) d) ALA‐PDT versus IPL alone: four studies (Liu 2014; Mei 2013; Oh 2009; Ragab 2014). (Please note that different filters were used.) e) ALA‐PDT versus green light alone: one study (Sadick 2010a) f) ALA‐PDT versus placebo or no treatment: two studies (Orringer 2010; Pollock 2004) g) ALA‐PDT other: six studies (Barolet 2010; Hongcharu 2000; NCT00706433; Pollock 2004; Taub 2007; Yin 2010)
6. MAL‐PDT versus ALA‐PDT
a) One study compared these interventions (Wiegell 2006a)
7. Other (non‐MAL, non‐ALA) PDT versus other comparators
a) Indocyanine green (ICG) PDT: two studies (Genina 2004; Kim 2009) b) Indole‐3‐acetic acid (IAA) PDT: one study (Na 2011) c) Topical liposomal methylene blue (TLMB) PDT: one study (Fadel 2009) d) Chlorophyll‐a (CHA) PDT: one study (Song 2014) e) Gold microparticles PDT: one study (Paithankar 2015)
Seven studies had a single light treatment session in one of the interventions (Barolet 2010; Genina 2004; Hongcharu 2000; Kim 2009; Orringer 2004; Seaton 2003; Wiegell 2006a).
Most interventions had two to four sessions, two studies had five sessions (Ianosi 2013; McGill 2008), two studies had six sessions (Leheta 2009; Ou 2014), 12 studies had eight sessions (Anyachukwu 2014; de Arruda 2009; Elman 2003; Genina 2004; Gold 2005; Lee 2010; Ling 2010; Liu 2011; Song 2014; Tzung 2004, Zhang 2009a; Zhang 2013b), one study had up to 24 sessions (Cheng 2008), one study had 28 sessions (Ash 2015) and one study had 84 sessions (Papageorgieu 2000). Two self‐administered interventions had a total of 56 (Kwon 2013) and 112 sessions (Na 2007).
Four studies included endpoints, such as time to resolution or interventions in which treatments were applied until a certain improvement threshold was reached (Gold 2011; Liu 2014; Sadick 2010b; Sami 2008), so the number of light sessions differed between study arms. Please see the Characteristics of included studies tables for details.
The frequency of application varied from twice a day to once a month.
Outcome assessment
Timing of outcome assessment
The majority of studies (52) conducted short‐term assessments, two to four weeks after the final treatment. The most common assessment time point was four weeks after final treatment (42 studies), followed by two weeks after final treatment (16 studies), with some of these studies containing assessments at both time points.
About a third of studies (27) conducted medium‐term assessments, five to eight weeks after final treatment (Bernstein 2007; Borhan 2014; Chen 2015; Choi 2010; Elman 2003; Fadel 2009; Jung 2009; Kwon 2013; Lee 2010; Leheta 2009; Mei 2013; NCT00594425; NCT00706433; NCT00933543; Oh 2009; Orringer 2004; Orringer 2007; Orringer 2010; Paithankar 2015; Pariser 2013; Ragab 2014; Sadick 2010a; Seaton 2003; Wang 2006; Wiegell 2006a; Wiegell 2006b; Zhang 2013a). The most common assessment time point was eight weeks after final treatment (18 studies), followed by six weeks after final treatment (12 studies).
About a third of studies (25) conducted assessments longer than eight weeks after final treatment (Bissonnette 2010; Darne 2011; Fadel 2009; Haedersdal 2008; Hongcharu 2000; Hörfelt 2006; Jih 2006; Leheta 2009; McGill 2008; Mei 2013; Moneib 2014; NCT00594425; Oh 2009; Orringer 2004; Orringer 2010; Ou 2014; Paithankar 2015; Sadick 2010a; Seaton 2003; Taub 2007; Uebelhoer 2007; Wang 2006; Wiegell 2006b; Yeung 2007; Yin 2010), but the majority at no longer than three months after final treatment. The most common assessment time point was 12 weeks after final treatment (18 studies).
Please note that we listed studies multiple times if they assessed outcomes at multiple time points corresponding to the short‐, medium‐, or long‐term time points defined by our protocol.
We included four studies which had a final evaluation at last treatment (de Arruda 2009; Ianosi 2013; Na 2007; Papageorgieu 2000) and reported their results at the final assessment. In three studies, the time points of assessments were unclear (Anyachukwu 2014, Borhan 2014; Leheta 2009). Comparison of interventions and the outcomes at time points as defined by our protocol was not possible for studies with time‐to‐resolution or time to a pre‐defined improvement threshold (Gold 2011; Liu 2014; Sadick 2010b; Sami 2008), apart from comparison for primary outcome 3, 'Investigator‐assessed severe adverse effects', as well as 'Other adverse effects'.
Primary outcome measures
Primary outcome measure 1: Participant's global assessment of improvement
A total of 23 studies addressed this outcome. Of these 13 used Likert or Likert‐like scales (Bernstein 2007; Chang 2007; Choi 2010; Darne 2011; Haedersdal 2008; Lee 2010; Moneib 2014; NCT00706433; Oh 2009; Papageorgieu 2000; Ragab 2014; Wiegell 2006b; Yin 2010). Five used visual analogue scales (VAS) (Hong 2013; Jung 2009; Jung 2012; Kwon 2013; Na 2007). In three studies other methods were used (Baugh 2005; Kim 2009; Orringer 2007), and in two studies, it was unclear which method was used (Liu 2011; Taub 2007).
In an additional split‐face study, this outcome was also addressed, but not for separate face sides (Jih 2006).
Primary outcome measure 2: Investigator‐assessed change in lesion count
The majority of studies (51) addressed this outcome.
Primary outcome measure 3: Investigator‐assessed severe adverse effects and other adverse effects
Please note that methods used for assessment of 'Investigator‐assessed severe adverse effects' and 'Other adverse effects' are listed under 'Adverse effects', in the 'Outcomes' sections of the Characteristics of included studies tables.
Five studies did not record or report on adverse effects (Bowes 2003; Cheng 2008; Ling 2010; Orringer 2004; Tzung 2004).
Seventeen studies that reported on adverse effects did not report the method they used to record them (Chang 2007; Elman 2003; Jung 2009; Kwon 2013; Moneib 2014; Na 2007; Na 2011; Orringer 2007; Orringer 2010; Ou 2014; Paithankar 2015; Papageorgieu 2000; Song 2014; Taub 2007; Zhang 2009a; Zhang 2013a; Zhang 2013b).
Secondary outcome measures
Secondary outcome measure 1: Investigator‐assessed change in acne severity
A total of 30 studies addressed this outcome. The most commonly used scale was the Leeds revised grading scale (O'Brien 1998), reported in 12 studies (Darne 2011; Fadel 2009; Ianosi 2013; Jung 2009; Leheta 2009; McGill 2008; Orringer 2004; Orringer 2007; Orringer 2010; Seaton 2003; Wiegell 2006a; Wiegell 2006b) and an additional five studies used the same scale referring to it as Cunliffe's grading system (Choi 2010; Hong 2013; Jung 2012; Kim 2009; Song 2014). Five studies, Baugh 2005; Bowes 2003; Hongcharu 2000; Tzung 2004; Yilmaz 2011, used the Michaëlsson grading score (Michaelsson 1977). Two studies, Bernstein 2007; Uebelhoer 2007, used the Allen‐Smith scale (Allen 1982). One study used the Korean Acne grading system (Chang 2007), one (Bissonnette 2010) used the Global Acne Grading System, and four used non‐standardised grading scales (Gold 2005; Hörfelt 2006; NCT00706433; Taub 2007).
Secondary outcome measure 2: Investigator's global assessment of improvement
A total of 32 studies addressed this outcome. The most commonly used scale was the Investigators' Global Assessment (IGA) suggested by the U.S. Food and Drug Administration (FDA) guidance for developing drugs for the treatment of acne vulgaris (FDA 2005), used in six studies (Borhan 2014; Kwon 2013; NCT00594425; NCT00933543; Paithankar 2015; Pariser 2013); eight studies used various Likert or Likert‐like scales (Barolet 2010; Baugh 2005; Bernstein 2007; Gold 2005; Karsai 2010; Sadick 2010a; Uebelhoer 2007; Wiegell 2006b), and 17 studies used various per cent improvement scales (Chen 2015; Cheng 2008; Fadel 2009; Hongcharu 2000; Ianosi 2013; Leheta 2009; Ling 2010; Liu 2011; Mei 2013; Moneib 2014; Oh 2009; Ou 2014; Papageorgieu 2000; Yin 2010; Zhang 2009a; Zhang 2013a; Zhang 2013b). In one study, it was unclear which method was used (Taub 2007).
Secondary outcome measure 3: Changes in quality of life
Only three studies recorded this outcome, two using the Dermatology Life Quality Index (DLQI) (McGill 2008; Karsai 2010) and one using the Cardiff Acne Disability Index (CADI) (Ianosi 2013).
Excluded studies
We excluded a total of 24 studies (25 records). Please see the reasons for exclusion in the Characteristics of excluded studies tables. We excluded 16 studies because they were not RCTs. Three studies started as RCTs but then did not follow the protocol and no longer met our inclusion criteria thereafter (Alexiades‐Armenakas 2006; Morton 2005; Tuchin 2003). Three studies were not focusing on direct effects of light therapies for acne (Shin 2012; Yang 2013; Zhan 1997). One RCT had a cross‐over design (Owczarek 2014), and one was focusing on acne scars (Yoon 2014). Please see the Characteristics of excluded studies tables and Methods for details.
Studies awaiting classification
Please see the Characteristics of studies awaiting classification tables. We identified 23 studies we were unable to include or exclude. Clinical trials registers recorded four studies as completed, but results were not published (NCT01245946; NCT01472900; NCT01584674; ISRCTN78675673). Four studies were discontinued or terminated (Berson 2006; NCT00237978; NCT00814918; ISRCTN73616060), and one was completed, but the author confirmed that data were not available (ISRCTN95939628). Two studies were pilot studies, and three studies were conference proceedings without enough information provided to include or exclude them (Kim 2012; Lee 2012; Passeron 2011; Song 2012; Troilius 2005), and for an additional study it was unclear to us whether it was a RCT (Faghihi 2011). We were unsuccessful when we attempted to contact the responsible parties and obtain further information and results of these studies. Responsible parties of two studies provided information that the trials had been completed, but they were aiming at publishing the results and therefore couldn't provide the data (Sakamoto 2012; Shaheen 2011). One of the clinical trial records (NCT01689935) could correspond to Sakamoto 2012, but we were unable to confirm this with the study authors. One study was completed, but there was ambiguity regarding the randomisation method, and the raw data we obtained were unclear and we were not able to interpret it (Edwards 2006). For two studies published in Mandarin, we were unable to obtain full texts (Lin 2011; Zhang 2009b). We were unable to obtain the full text of one study in Spanish (Pinto 2011); attempts to contact the study authors were unsuccessful. Similarily, we were unsuccessful in obtaining the full text and additional information on a study we identified in a reference list and through grey literature searches (Nataloni 2003).
Ongoing studies
Please see Characteristics of ongoing studies for details about the three ongoing studies we identified (EU 2014‐005235‐13; NCT02217228; NCT02431494).
Risk of bias in included studies
Selection bias was unclear for the majority of studies, with about half of studies describing adequate methods of random sequence generation and less than a third of studies describing adequate allocation concealment methods. Performance bias was also unclear in more than half of studies, high in about a quarter, and unclear in the remaining studies. Out of 26 studies which included participant‐assessed outcomes, detection bias was low in only two studies, high in 10 studies, and unclear in the remaining studies. Detection bias was low in over half of studies for investigator‐assessed outcomes and unclear in most of the rest. Attrition bias was low in over half of studies, high in about a quarter, and unclear in a few studies only. Reporting bias was similar. Other risk of bias was low in about a third of studies. Two thirds of studies had unclear risk because of possible conflicts of interest or sponsorship, or both, were not declared; they were industry‐sponsored; or they reported some sort of conflict of interest, and a few studies had a high risk due to other reasons, such as baseline imbalances and concomitant treatment.
Please see Figure 2 for details. Please note that studies which did not include participant‐assessed outcomes also have 'Detection bias for patient‐assessed outcomes' marked as 'unclear' in Figure 2. It is therefore not possible to distinguish them in Figure 2 alone from studies which included such outcomes, but had 'unclear' risk of bias. In the corresponding 'Risk of bias' tables for the individual studies, we have clearly stated when studies did not include participant‐assessed outcomes.
2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Please see the Characteristics of included studies tables for details on risk of bias in individual studies.
Allocation
Random sequence generation
We judged the risk of bias as low in 34 studies in which study authors reported or later clarified how they generated the allocation sequence; four using coin toss (Barolet 2010; Baugh 2005; Moneib 2014; Uebelhoer 2007); 13 using computer software (Bissonnette 2010; Darne 2011; Genina 2004; Ianosi 2013; Karsai 2010; Kwon 2013; NCT00594425; NCT00673933; NCT00933543; Ou 2014; Papageorgieu 2000; Seaton 2003; Yin 2010); 10 using 'randomised code' (Ash 2015; Choi 2010; Oh 2009; Orringer 2004; Orringer 2007; Orringer 2010; Pariser 2013; Sadick 2010b; Song 2014; Yeung 2007); and seven using drawing lots (Anyachukwu 2014; Haedersdal 2008; Lee 2010; Liu 2014; McGill 2008; Mei 2013; Wiegell 2006b). We judged the risk of bias as unclear in 37 reports which did not include the method used to generate the allocation sequence.
Allocation concealment
We judged the risk of bias as low in 19 studies. Authors of 15 studies reported, or later clarified, that they used sealed envelopes or boxes to conceal the allocation sequence (Anyachukwu 2014; Ash 2015; Darne 2011; Haedersdal 2008; Lee 2010; Liu 2014; McGill 2008; NCT00594425; NCT00673933; NCT00933543; Oh 2009; Ou 2014; Ragab 2014; Seaton 2003; Uebelhoer 2007). One study used kits with randomised codes (Pariser 2013). One study communicated patient allocation via phone by an independent investigator prior to enrolment of each participant (Ianosi 2013). One study reported that assignments were concealed by securing randomisation codes until all data were entered (Kwon 2013) and 'by blinded sponsor numerical allocation' in another study (Sadick 2010b).
Fifty‐one studies did not specifically report the intention or method (or both) of concealing the allocation sequence, so we judged the risk of bias as unclear. We judged the risk of bias as high in one study as the study authors clarified that they did not conceal allocation (Mei 2013).
Blinding
Performance bias
We judged the risk of bias as low in 11 studies. In eight studies, the authors described or later clarified blinding of both participants and personnel that we judged as adequate (Hörfelt 2006; Kwon 2013; Mei 2013; NCT00594425; NCT00673933; NCT00933543; Pariser 2013; Wiegell 2006a). Three split‐face trials described blinding of participants that we judged as adequate, with unclear blinding of performing clinicians (Baugh 2005; Bowes 2003; Na 2011), but systematic differences between face sides in the care that was provided or in exposure to factors other than the interventions of interest were unlikely.
We judged the risk of bias as unclear in 40 studies, most of which did not report intention to blind participants or performing clinicians, or both, and did not present evidence that participants or clinicians were blinded. Given the nature of the interventions, it is not likely that participants or performing clinicians were blinded in those studies, but without the necessary information, we were unable to clearly judge the risk based on these assumptions.
We judged the risk of bias as high in 20 studies. In 14 studies, the authors specifically reported or later clarified that they did not blind both participants and performing clinicians (Bissonnette 2010; Darne 2011; Gold 2005; Karsai 2010; Kim 2009; Leheta 2009; Na 2007; Oh 2009; Orringer 2004; Orringer 2007; Orringer 2010; Sadick 2010a; Wang 2006; Wiegell 2006b). One study was an open trial (de Arruda 2009). One study described an adequate blinding of performing clinicians, but inadequate blinding of participants (Lee 2010). Four studies described blinding of participants that we judged as ineffective, with unclear blinding of performing clinicians (Elman 2003; Haedersdal 2008; Papageorgieu 2000; Seaton 2003).
Detection bias
Participant‐assessed outcomes
Please note that 45 studies which did not include participant‐assessed outcomes (participant's global assessment of improvement and changes in quality of life, or both) have 'Detection bias' for participant‐assessed outcomes marked as 'unclear' in Figure 2. It is therefore not possible to distinguish them in Figure 2 alone from 14 studies which included such outcomes and had 'unclear' risk of bias (Bernstein 2007; Chang 2007; Choi 2010; Hong 2013; Ianosi 2013; Jung 2009; Jung 2012; Liu 2011; McGill 2008; Moneib 2014; NCT00706433; Ragab 2014; Taub 2007; Yin 2010). In the corresponding 'Risk of bias' tables for the individual studies, we clearly state when studies did not include participant‐assessed outcomes. Two studies described blinding of participants that we judged as adequate (Baugh 2005; Kwon 2013) and the risk of bias as low. We judged the risk of bias as high in 10 studies. In nine studies, the authors specifically reported that they did not blind participants (Darne 2011; Karsai 2010; Kim 2009; Lee 2010; Na 2007; Oh 2009; Orringer 2007; Papageorgieu 2000; Wiegell 2006b). In one study, the authors reported that they unsuccessfully attempted to blind the participants (Haedersdal 2008).
Investigator‐assessed outcomes
We judged the risk of bias as low in 41 studies. Authors of 20 studies reported blinding by use of photographs (Barolet 2010; Bernstein 2007; Borhan 2014; Darne 2011; Hong 2013; Hongcharu 2000; Ianosi 2013; Jung 2009; Karsai 2010; Liu 2011; McGill 2008; Na 2007; Na 2011; Oh 2009; Orringer 2004; Orringer 2007; Sadick 2010b; Song 2014; Wang 2006; Yeung 2007); 20 studies reported assessment by blinded investigators who did not participate in treatment and were unaware of the intervention status, or both (Ash 2015; Anyachukwu 2014; Bissonnette 2010; Haedersdal 2008; Kwon 2013; Lee 2010; Leheta 2009; Mei 2013; NCT00594425; NCT00673933; NCT00933543; Orringer 2010; Pariser 2013; Pollock 2004; Sami 2008; Seaton 2003; Tzung 2004; Uebelhoer 2007; Wiegell 2006a; Wiegell 2006b); and one study reported blinding of participants and performing clinicians (i.e. those treating the participants) who did outcome assessment that we judged as adequate (Hörfelt 2006).
We judged the risk of bias as unclear in 27 studies. Seven studies stated that they blinded the assessors, but did not describe the method (Chang 2007; Choi 2010; Elman 2003; Fadel 2009; Gold 2005; NCT00706433; Papageorgieu 2000). Four studies reported that they used photographs for evaluation of outcomes, but it was unclear whether they blinded dermatologists (e.g. not performing the treatment and unaware of the intervention status), so we judged the risk of bias as unclear (Chen 2015; Genina 2004; Moneib 2014; Ragab 2014). In 16 studies, there was no report of intended blinding of outcome assessors, and study authors did not provide evidence that they blinded assessors (Baugh 2005; Bowes 2003; Cheng 2008; Gold 2011; Jih 2006; Jung 2012; Kim 2009; Ling 2010; Liu 2014; Ou 2014; Taub 2007; Yilmaz 2011; Yin 2010; Zhang 2009a; Zhang 2013a; Zhang 2013b).
We judged the risk of bias as high in three studies; two studies were open trials (de Arruda 2009; Sadick 2010a) and one study performed both blinded and unblinded assessment (Paithankar 2015).
Incomplete outcome data
We judged the risk of bias as low in 43 studies which reported outcomes for 80% or more of participants randomised for prespecified time points, with reasons for missing data (if there were any) balanced in numbers across intervention groups and unlikely to be related to true outcome.
We judged the risk of bias as unclear in 11 studies. Ten studies did not report number of withdrawals, losses to follow‐up, and final number of evaluable participants (Bowes 2003; Elman 2003; Gold 2011; Liu 2011; Liu 2014; Moneib 2014; Na 2011; Ragab 2014; Sami 2008; Song 2014) and reported in a way that did not permit a clear judgement of bias in one study (Genina 2004).
We judged the risk of bias as high in 17 studies which reported outcomes for less than 80% of participants randomised at some of the predefined time points (Anyachukwu 2014; Baugh 2005; Bissonnette 2010; Darne 2011; Fadel 2009; Gold 2005; Ianosi 2013; McGill 2008; Na 2007; NCT00594425; Orringer 2004; Orringer 2007; Orringer 2010; Papageorgieu 2000; Taub 2007; Wiegell 2006b; Yeung 2007). Three studies imputed missing data using various methods that we judged as appropriate (Orringer 2004; Orringer 2007; Orringer 2010). However, we still judged the risk of bias as high in those studies, as we could not obtain information on when the last observation that was carried forward was recorded and there was a high dropout rate. We believe this introduced uncertainty although study authors handled missing data using imputation.
Selective reporting
We judged the risk of bias as low in 44 studies in which prespecified outcomes and those mentioned in the methods section appeared to have been reported at predefined time points or study authors provided them upon our request.
We judged the risk as unclear in eight studies in which baseline data were not reported, or results were reported in graph or figure format or in a way different from prespecified for some outcomes, or both (Chang 2007; Hörfelt 2006; Kim 2009; Liu 2011; Liu 2014; Ragab 2014; Song 2014; Wiegell 2006b).
We judged the risk of bias as high in 19 studies. Thirteen studies did not report results for prespecified outcomes, or results for prespecified time points, or both (Borhan 2014; Fadel 2009; Gold 2005; Gold 2011; Moneib 2014; Na 2007; NCT00594425; Paithankar 2015; Papageorgieu 2000; Pollock 2004; Sadick 2010a; Taub 2007; Uebelhoer 2007). Three studies reported results in graph or figure format only for most outcomes or in a way different from those prespecified (Kwon 2013; Na 2011; Tzung 2004). Two studies did not clearly prespecify the outcomes in the 'Methods' section (Ash 2015; Anyachukwu 2014). In one study, we were unable to obtain statistical data regarding differences between groups to which participants were initially randomised (Orringer 2004).
Other potential sources of bias
We identified no additional sources of bias and judged the risk of bias as low in 23 studies.
We judged the risk of bias as unclear in 44 studies. In 21 studies, we judged the risk of bias as unclear because possible conflicts of interest or sponsorship, or both, were not declared (Bernstein 2007; Bowes 2003; Chen 2015; de Arruda 2009; Elman 2003; Hong 2013; McGill 2008; Moneib 2014; Na 2011; Papageorgieu 2000; Pollock 2004; Ragab 2014; Sadick 2010a; Sami 2008; Tzung 2004). In six of these studies (Cheng 2008; Ling 2010; Ou 2014; Zhang 2009a; Zhang 2013a; Zhang 2013b), additional bias might also have been introduced as these studies were in Mandarin and only one person performed data extraction. In two studies, the authors declared that they had no conflicts of interest, but it was unclear who provided the device or the sham device (Kwon 2013) and whether there was commercial sponsorship (Sadick 2010b). In 21 studies, we judged the risk as unclear because the study authors declared potential conflict of interest or had a commercial sponsor, or both, and it was unclear whether this affected the results (Barolet 2010; Baugh 2005; Bissonnette 2010; Darne 2011; Genina 2004; Gold 2005; Gold 2011; Haedersdal 2008; Hörfelt 2006; Jih 2006; NCT00594425; NCT00673933; NCT00706433; NCT00933543; Paithankar 2015; Pariser 2013; Seaton 2003; Taub 2007; Uebelhoer 2007; Wang 2006; Yeung 2007).
In four studies, we judged the risk of bias as high due to baseline imbalances and concomitant treatment (Anyachukwu 2014; Ash 2015; Borhan 2014; Liu 2011). For two of these studies (Borhan 2014; Liu 2011), sponsorship was also unclear, and in one it was unclear whether potential conflicts of interest might have affected the results (Ash 2015).
Effects of interventions
See: Table 1; Table 2; Table 3
We used GRADEpro GDT (GRADEpro GDT 2015) to create a 'Summary of findings' table (Table 1) for our primary outcomes Participant's global assessment of improvement, Investigator‐assessed change in lesion count, and Investigator‐assessed severe adverse effects.
The aim was to illustrate the nature of the results of this review and different aspects of heterogeneity that we took into account when interpreting the results of the included studies. We judged that pooling the results of most of the studies was inappropriate, due to methodological and clinical heterogeneity, including the following:
differences of included participants (Fitzpatrick skin types and acne severity);
differences in design (parallel groups, split‐face or split‐back studies, and designs combining them);
differences in interventions (wavelengths, doses, different active substances used in PDT and their pharmacokinetic characteristics, incubation time and whether they were administered under occlusion or not, number of light sessions and frequency of application, pre‐ and post‐treatment care);
differences in comparator interventions (most common being no treatment, placebo, other light interventions, and various topical treatments, but also their various combinations);
differences in outcomes assessed, as well as methods and timing of outcome assessment; and
poor reporting and failure to obtain necessary data.
To make it easier for the reader to follow the effects of interventions of the studies we included, we grouped the studies by our outcome (primary and secondary) and then by comparison, as previously described in the Included studies section (under 'Interventions'). For clarity, we used five additional tables to present the effects of interventions (Table 4, Table 5, Table 6, Table 7; Table 8). We reported effects of interventions using the statistics and methods described in the Methods section. When such reporting was not possible, we reported results the way they were available and clarified our reasons for our inability to report them as planned.
1. Participant's global assessment of improvement.
| Study | Participants | Intervention(s) and control(s) | Participant's global assessment of improvement |
| 1. Light versus placebo or no treatment | |||
| Green light versus placebo | |||
| Baugh 2005 | 25 (4 M, 21 F) aged 19‐41 years (mean 27.8), diagnosed with mild to moderate inflammatory facial acne; FPT I–III | 532 nm pulsed laser vs sham in a split‐face trial, both with skin cooling system; two exposures ⁄ week for 2 weeks. Assessed at 1 and 4 weeks post‐treatment | Non‐standardised scale (overall treatment satisfaction in intervals of 10 percentage points) was used for evaluation. At 4 weeks 4.8% participants reported 30% to 39% satisfaction, 9.5% reported 50% to 59% satisfaction, 23.8% reported 60% to 69% satisfaction, 47.6% reported 70% to 79% satisfaction, 9.5% reported 80% to 89% satisfaction and 4.8 reported 90% to 100% satisfaction. Further data were not provided. |
| Infrared light versus no treatment | |||
| Darne 2011 | 38 (7 M, 31 F), aged 18‐47 years (mean 28), with moderate‐severe facial acne; FPT I‐V | 1450 nm laser (8‐9 J/cm²) in a split‐face trial, 3 treatments monthly, assessed monthly for 4 months, then at 3‐monthly intervals for 12 months after final treatment | Non‐standardised scale ('highly satisfied', 'satisfied', 'neutral' or 'unsatisfied' and 'would recommend to a friend') was used for evaluation. At 4 weeks 6/25 (24%) of participants were 'highly satisfied', 9/25 (36%) were 'satisfied', 6/25 (24%) were 'neutral' and 4/25 (16%) reported the treatment to be 'unsatisfactory'. 21/25 (84%) reported that they would 'recommend the treatment to a friend'. |
| Moneib 2014 | 24 (5 M, 19 F), aged 15‐8 years (mean 21.5), with moderate‐severe acne; FPT II‐V | Fractional Erbium Glass 1559 nm laser, in a split‐face trial, 4 treatments, at 2‐week intervals, assessed every 3 months for 1 year after final treatment | Non‐standardised scale (0 = no improvement; < 25% = mild improvement; 26% to 50% = moderate improvement; 51% to 75% = good improvement; 76% to 100% = excellent improvement) was used for evaluation. Reported in graph format and for treatment face sides only, and at unclear time point. Our interpretation of the graph was that 5% of participants assessed their improvement to be mild, 5% to be moderate, 20% to be good and 70% to be excellent. |
| Orringer 2007 | 46 (10 M, 36 F) enrolled, 30 completed, mean age 23.9 years (range not reported) with clinically apparent active facial acne; FPT II–VI | 1320 nm Nd:YAG laser in a split‐face trial with cooling; 3 treatments at 3‐week intervals; assessed at weeks 7 and 14 | Non‐standardised scale (details not given) was used for evaluation. At final treatment, 29/37 of participants who completed the treatments (78%) "indicated that their acne was at least mildly improved on the treated side of the face as compared with baseline", and 16/37 participants (43%) indicated "moderate or better" improvement. Data for non‐treated sides were not given, but 22/37 (59%) of participants reported that "their acne had improved at least mildly when compared with the untreated skin". |
| Red light versus no treatment | |||
| Na 2007 | 30 (7 M, 23 F) aged 19‐33 years (mean 23.6) with mild‐moderate acne; skin types not documented | 635–670 nm portable red light device in a split‐face trial, self‐administered to the treatment side twice daily for 8 weeks; assessed at weeks 1, 2, 4 and 8 | VAS: 0‐5, none to very severe was used for evaluation. Score (unclear whether mean or median) decreased from baseline 3.9 to 1.8 at final treatment on the treated and from 3.9 to 2.9 on the control side respectively, with significant difference between the sides (P < 0.005). This outcome was not evaluated after final treatment and no further data were provided. |
| Blue‐red light versus placebo | |||
| Papageorgieu 2000 | 30, mean age 24.8 years in blue–red light group; 25 participants, mean age 25.6 years in white light control group; randomised from the original 107 recruited (33 M, 74 F, age 14‐50 years), all with mild‐moderate acne; skin types not stated | 415 nm plus 660 nm light vs cool white light; treated daily for 12 weeks; assessed every 4 weeks for the 12‐week treatment period | Non‐standardised scale: 'worse' (≤ ‐10%), 'unchanged' (‐9% to 9%), 'mild improvement' (10% to 39%), 'moderate improvement' (40% to 59%), 'marked improvement' (60% to 89%) or 'clearance' (≥ 90%) was used for evaluation, but reported only in graph format and no details were provided. Not evaluated after final treatment. Our interpretation of the graph was that around 4% of participants reported 'clearance', 70% reported 'marked improvement', 20% 'moderate improvement' and 4% reported 'mild improvement' in the blue‐red light group, whilst in the white light group around 70% of participants reported 'unchanged' or 'mild improvement', 20% 'moderate improvement' and 8% 'marked improvement'. Further data were not provided. We dichotomised the data to 27/30 of 'success' outcomes in the blue‐red and 7/25 in the white light group. Blue red‐light was superior to white light with RR (95% CI) of 3.21 (1.70, 6.09), P = 0.0003, and the NNTB was 2 (95% CI 1 to 3) |
| Kwon 2013 | 35 (11 M, 24 F); aged 20‐27 years (mean not given), with mild‐moderate acne, FPT III‐V; 18 participants in the blue‐red light group, 17 in the placebo group | 420 nm plus 660 nm home use LED device vs home‐use sham device; self‐treatment twice daily for 4 weeks in a split‐face trial; assessed 4 and 8 weeks after final treatment | VAS was used for evaluation (10 = same as before the first treatment; 0 = no acne). Mean VAS score 10 at baseline in both groups decreased to 4.3 in the blue‐red light group, and stayed at 10 or above in the placebo group (extracted from graph) at 8 weeks after final treatment. No further data (SDs) were provided in text nor in graph format. |
| 2. Light versus topical treatment | |||
| Light versus benzoyl peroxide (BPO) | |||
| Chang 2007 | 30 women aged 23–32 years (mean 25 ± 7) with mild‐moderate acne; FPT III‐IV | IPL with 530–750 nm filter with cooling gel in a split‐face trial, 3 sessions, 3 weeks apart, BPO gel used on both sides of the face. Assessed 3 weeks after final treatment | Non‐standardised scale (highly satisfied, satisfied, neutral, or dissatisfied) was used for evaluation. At 3 weeks participants were "uniformly satisfied with their treatment, but IPL treatment did not give any additional benefit". No further data were reported. |
| Papageorgieu 2000 | 30 participants, mean age 24.8 years in blue–red light group and 25 participants, mean age 23.4 years in the BPO group, randomised from the original 107 recruited (33 M, 74 F, age 14‐50 years) all with mild‐moderate acne; skin types not stated | 415 nm plus 660 nm light vs 5% BPO, parallel groups, treated daily; assessed every 4 weeks for the 12‐week treatment period | Non‐standardised scale: 'worse' (≤ ‐10%), 'unchanged' (‐9% to 9%), 'mild improvement' (10% to 39%), 'moderate improvement' (40% to 59%), 'marked improvement' (60% to 89%) or 'clearance' (≥ 90%) was used for evaluation, but reported only in graph format and no details were provided. Not evaluated after final treatment. Our interpretation of the graph was that around 4% of participants reported 'clearance', 70% reported 'marked improvement', 20% 'moderate improvement' and 4% reported 'mild improvement' in the blue‐red light group, whilst around 35% of participants showed 'marked improvement', 45% 'moderate improvement', 10% 'mild' improvement' and 10% 'unchanged' in the BPO group. Further data were not provided. We dichotomised the data to 27/30 of 'success' outcomes in the blue‐red and 20/25 in the BPO group. The difference was non significant, with RR (95% CI) of 1.13 (0.89, 1.42), P = 0.31 |
| Light versus clindamycin | |||
| Lee 2010 | 9, with inflammatory acne (other characteristics not given) | Full‐spectrum light twice a week vs 1% clindamycin twice a day, in a split‐face trial, for 4 weeks, evaluation weekly whilst on treatment and 2, 4 and 8 weeks after final treatment | Non‐standardised scale ('worse', 'no change', 'fair', 'good' and 'excellent') was used for evaluation. Participants rated the treatment as 'good' or 'excellent' (unclear for which intervention and at what time point). Further data were not reported. |
| Light and other topical treatments | |||
| Ash 2015 | 41 (M/F not reported, study authors clarified "a 50/50 split"), 26 in the intervention, 15 in control group, aged 16–45 years (mean not reported) with mild‐moderate acne (Leeds grade); FPT not given: "Caucasian, Asian and mixed Afro‐Caribbean ethnic groups" |
Pre‐treatment facial wash/weak chemical peel (containing salicylic acid, glycolic acid, lactic acid) followed by treatment with blue light device and then post treatment facial moisturiser (containing salicylic acid, glycolic acid, lactic acid, menthol, niacin) versus unclear control in a parallel group trial, 28 sessions in total, every other day for 8 weeks. Assessed at 12 weeks (4 weeks after final treatment?) | Details on scale used for evaluation not given. Results reported as "the majority of subjects reporting that they were satisfied, very satisfied, or extremely satisfied with treatment" in the treatment group. Results were not reported for the control group. No further data were reported. |
| 3. Light versus other comparators | |||
| Comparison of light therapies of different wavelengths | |||
| Choi 2010 | 20 (1 M, 19 F, aged 20‐37 years, mean age 26); all with acne (Cunliffe severity grade 2‐4), FPT types III‐V | 585 nm PDL vs 530‐750 nm IPL, 4 treatments at 2‐week intervals, in a split‐face trial, assessed 4 and 8 weeks after last treatment | Non‐standardised rating scale (from 0‐10, neutral to highly satisfied) was used for evaluation. No statistically significant difference in improvement of scores between the two treatments (P > 0.05) was found. They increased from baseline 0 for both to 3.3 for IPL and 3.7 for PDL at 4 weeks after treatment and then to 4.7 for IPL and 5.2 for PDL at eight weeks after treatment. Further data were not reported. |
| Jung 2009 | 18 enrolled, 16 completed (5 M, 11 F, aged 20‐31 years, mean age 26); with mild‐moderate acne (Cunliffe severity grade 2‐5), skin types not given | 585 nm PDL vs combined 585/1064 nm PDL, in a split‐face trial, 3 treatments at 2‐week intervals, assessed at 8 and 12 weeks after initial treatment | VAS (0‐10, worst imaginable acne state to disease free) was used for evaluation; please note that opposite VAS was used in Jung 2012. Mean scores on the PDL sides and on the 585/1,064‐nm laser sides increased from 3.3 and 3.7 at baseline to 6.63 (P = 0.002) and 6.60 (P = 0.001) at 8 weeks respectively. At 12 weeks, they declined to 6.12 at both sides. Further data were not reported. |
| Liu 2011 | 20 (6 M/14 F) completed the study, number of randomised participants not reported, 10 completed in the blue‐light, 10 in the red‐light group, aged 19–28 years (mean 23.6 years) with mild‐moderate acne (Global Acne Grading System); FPT III‐IV | Blue (405 ± 10 nm) vs red (630 ± 10 nm) LED portable device treatments, about 20 cycles of illumination and the corresponding light doses received in each session were 7.2 J/cm² and 11.52 J/cm², in a parallel‐group trial, 8 sessions in total, twice weekly for 4 weeks; assessed at 4 weeks after final treatment and at each treatment session | "Subjective evaluation was based on the observations of face skin and communications between the patient and researcher (for the follow‐ups)." Further details on scale used for evaluation not given. Results reported as "A few participants reported that fresh new acne lesions came out, while the total number of lesions decreased slightly." No further data were reported. |
| Papageorgieu 2000 | 30 participants, mean age 24.8 years in blue–red light group and 27 participants, mean age 23.4 years in the blue‐light group, randomised from the original 107 recruited (33 M, 74 F, age 14–50 years) all with mild‐moderate acne; skin types not stated | 415 nm plus 660 nm light vs 415 nm light, parallel groups, treated daily for 12 weeks; assessed every 4 weeks for the 12‐week treatment period | Non‐standardised scale: 'worse' (≤ ‐10%), 'unchanged' (‐9% to 9%), 'mild improvement' (10% to 39%), 'moderate improvement' (40% to 59%), 'marked improvement' (60% to 89%) or 'clearance' (≥ 90%) was used for evaluation, but reported only in graph format and no details were provided. Not evaluated after final treatment. Our interpretation of the graph was that around 4% of participants reported 'clearance', 70% reported 'marked improvement', 20% 'moderate improvement' and 4% reported 'mild improvement' in the blue‐red light group, whilst in the blue‐light group around 4% of participants experienced 'clearance', 50% of participants 'marked improvement', 30% 'moderate improvement', 8% 'mild' improvement' and 8% 'unchanged'. Further data were not provided. We dichotomised the data to 27/30 of 'success' outcomes in the blue‐red and 23/27 in the blue‐light group. The difference was non significant, with RR (95% CI) of 1.06 (0.87, 1.29), P = 0.59. |
| Comparison of light therapies of different doses | |||
| Bernstein 2007 | 7 enrolled, 6 completed (1 M, 4 F, aged 23‐41 years, mean age 29), all with active papular acne, FPT I‐III | Comparison of two 1450 nm laser treatments; single‐pass, high‐energy (13–14 J/cm²) vs double‐pass, low energy (8–11 J/cm²); 4 treatments at monthly intervals, assessed 1 month following each treatment and 2 months after final treatment | Non‐standardised rating scale (0= worsening, 1= no change, 2= mild improvement , 3= moderate improvement, 4= marked improvement) was used for evaluation. At 8 weeks average score on the single‐pass side was 2.3 (range 1‐4) and on the double‐pass side 2.3 (range 2‐4). |
| Jih 2006 | 20 (10 M, 10 F) age 18‐39 years (mean 23) with active inflammatory facial acne; FPT II–VI | 1450 nm diode laser in a split‐face trial using anaesthetic cream and 14 J/cm² in one group and 16 J/cm² in the second, with 3 treatments given at 3–4 week intervals, assessed at 1, 3, 6 and 12 months after final treatment | Non‐standardised rating scale (0 = worsening, 1 = no change, 2 = mild improvement , 3 = moderate improvement, 4 = marked improvement) was used for evaluation. The majority of participants reported moderate to marked improvement, 85.3% at the 1‐month, 67.7% at the 3‐month, 60.0% at the 6‐month and 82.1% at the 12‐month assessments. No separate data for different doses given. |
| NCT00706433 | 266 (128 M, 138 F), 68 in the ALA 1000 s group, 65 in the ALA 500 s group, 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group, mean age 20.1 years, inclusion criterion 12 > years, with moderate and severe acne (IGA score 3 and 4, with at least 20 ILs); FPT I‐VI | 20% ALA (45 min incubation) plus blue 1000 s light vs 20% ALA (45 min incubation) plus 500 s blue light vs vehicle (45 min incubation) plus blue 1000 s light vs vehicle (45 min incubation) plus 500 s blue light; in a parallel‐group trial; up to 4 treatments at 3 weeks intervals, assessed 3 and 6 weeks after the final treatment | Non‐standardised scale ('subject satisfaction score'; excellent‐very satisfied; good‐moderately satisfied; fair‐slightly satisfied; poor‐not satisfied at all) was used for evaluation. At 6 weeks after final treatment 20/67 participants in the vehicle 1000 s and 23/66 in the vehicle 500 s group assessed their improvement as 'good'; 23/67 in the vehicle 1000 s and 26/66 in the vehicle 500 s group assessed their improvement as 'excellent'. We dichotomised the data to 43/67 of 'success' outcomes in the vehicle 1000 s and 49/66 in the vehicle 500 s group. The difference between vehicle 1000 s blue light and vehicle 500 s blue light groups was non significant, with RR (95% CI) of 0.86 (0.69, 1.09), P = 0.21. |
| Light in combination with carbon lotion versus no treatment | |||
| Jung 2012 | 22 (4 M, 18 F), 20 completed (2 M, 18 F, aged 19‐34 years, mean age 25.4), FPT III‐IV, acne severity not given | Carbon lotion plus quasi‐long pulse and Q‐switched 1064 nm Nd:YAG laser vs non‐treated control, in a split‐face trial, 3 treatments over 4 weeks, evaluation every 2 weeks whilst on treatment and then every 4 weeks | VAS (0‐10, disease free to initial visit acne status) was used for evaluation; please note that opposite VAS was used in Jung 2009. At 4 weeks after final treatment participants assessed significantly greater improvement on the laser‐treated compared to the untreated side (P < 0.05). VAS score mean (SDs not given) decreased from initial 10 at both sides to 5.9 (P < 0.001) on the laser‐treated and to 9.2 (P = 0.007) on the untreated side. |
| 4. MAL‐PDT versus other comparators | |||
| MAL‐PDT versus orange light alone | |||
| Haedersdal 2008 | 15 (5 M, 10 F) age 18‐31 years (median 18), with at least 12 facial inflammatory acne lesions; FPT I–III | Split‐face design with non purpuric LPDL 595 nm full‐face treatment and MAL cream applied to randomised side of the face for 3 h before laser exposure, with dynamic cooling device; three treatments at 2‐week intervals; assessed 4 and 12 weeks after final treatment | Non‐standardised numerical scale (0‐10, no satisfaction to best imaginable satisfaction) was used for evaluation. Median (25‐75 percentiles) score (range) was significantly higher for MAL‐LPDL treatment than for LPDL treatment alone at both 4 weeks after final treatment (P = 0.031); 7 (4.75 to 8) vs 6 (3.75 to 8), and at 12 weeks after final treatment (P = 0.034); 8 (6.25 to 9) vs 7.5 (5 to 8.75). |
| MAL‐PDT versus placebo or no treatment | |||
| Wiegell 2006b | 36 participants: 21 in treatment group aged 23 ± 5 years (9 M, 10 F analysed) and 15 in control group aged 24 ± 5 years (3 M, 9 F analysed), with > 12 inflammatory acne lesions; FPT II–V | Comparison of MAL plus 630 nm with no treatment in a parallel‐group trial; two treatments, 2 weeks apart, assessed every 4 weeks for 12 weeks after treatment | Non‐standardised grading scale (0‐4; acne worse, no change, slight improvement, moderate improvement, marked improvement) was used for evaluation. Results were reported in graph format and no details were provided. Our interpretation of the graph was that at 4, 8 and 12 weeks after final treatment median improvement scores were 3, 2 and 3 in the MAL‐PDT group and 1.5, 1 and 1 in the control group respectively. |
| MAL‐PDT other | |||
| Hong 2013 | 22 (2 M, 20 F), age 19‐35 years (mean not given), "at least grade 2 (Cunliffe acne grading system)", FPT IV‐V | MAL plus 630 nm light vs MAL plus 530‐750 nm light in a split‐face trial, 3 treatments in total, 2‐week intervals, assessed at 4 weeks after treatment | VAS scale (10‐0, 10 = same as before the first treatment; 0 = no acne) was used for evaluation. Mean VAS score decreased from baseline 10 on both sides to 5.0 at the red light side, and 4.9 at the IPL side at 4 weeks after final treatment, with no significant difference between the 2 sides. Further data were not provided. |
| 5. ALA‐PDT versus other comparators | |||
| ALA‐PDT versus blue light alone | |||
| NCT00706433 | 266 (128 M, 138 F), 68 in the ALA 1000 s group, 65 in the ALA 500 s group, 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group, mean age 20.1 years, inclusion criterion 12 > years, with moderate and severe acne (IGA score 3 and 4, with at least 20 ILs); FPT I‐VI | 20% ALA (45 min incubation) plus blue 1000 s light vs 20% ALA (45 min incubation) plus 500 s blue light vs vehicle (45 min incubation) plus blue 1000 s light vs vehicle (45 min incubation) plus 500 s blue light; in a parallel‐group trial; up to 4 treatments at 3‐week intervals, assessed 3 and 6 weeks after the final treatment | Non‐standardised scale ('subject satisfaction score'; excellent‐very satisfied; good‐moderately satisfied; fair‐slightly satisfied; poor‐not satisfied at all) was used for evaluation. At 6 weeks after final treatment 18/68 participants in ALA 1000 s, 28/65 in the ALA 500 s, 20/67 in the vehicle 1000 s and 23/66 in the vehicle 500 s group assessed their improvement as 'good'; 23/68 participants in ALA 1000 s, 11/65 in the ALA 500 s, 23/67 in the vehicle 1000 s and 26/66 in the vehicle 500 s group assessed their improvement as 'excellent'. We dichotomised the data to 41/68 of 'success' outcomes in ALA 1000 s, 39/65 in the ALA 500 s, 43/67 in the vehicle 1000 s and 49/66 in the vehicle 500 s group. The difference between ALA 1000 s and vehicle 1000 s groups was non significant, with RR (95% CI) of 0.94 (0.72, 1.22), P = 0.64, and it was non significant between ALA 500 s and vehicle 500 s groups, with RR (95% CI) 0.81 (0.63, 1.03), P = 0.09. |
| ALA‐PDT versus IPL alone | |||
| Oh 2009 | 20 (4 M, 16F), aged 18‐30 years, 9 in the short incubation group (3M, 6F, mean age ± SD 23 ± 4.12 years) and 10 in the long incubation group (1 M, 9 F and 23 ± 5.53 years), with moderate and severe acne (Evaluator Global Severity Score 3 and 4); FPT II‐IV | 20% ALA plus 590 nm IPL; 2 parallel groups: short incubation (30 min) vs long incubation (3 h), one half of the face within each treated with IPL alone; 3 treatments at 4‐week intervals, assessed 4 weeks after each treatment and 8 and 12 weeks after the third treatment | Non‐standardised (significant improvement (> 75%), moderate improvement (50% to 75%), mild improvement (25% to 50%), no improvement (0% to 25%), worse (< 0%) relative to baseline) was used for evaluation. At 12 weeks 6/9 (66.7%) participants assessed their improvement as mild and 3/9 (33.3%) as moderate in the short incubation group; 4/11 (36.4%) as mild, 6/11 (54.5%) as moderate and 1/11 (9.1%) as significant in the long incubation group. We dichotomised the data to 3/9 of 'success' outcomes in the short incubation and 7/11 in the long incubation group. The difference was non significant, with RR (95% CI) 0.52 (0.19, 1.46), P = 0.22. |
| Ragab 2014 | 25 (1 M, 24 F), aged 14‐39 years, 15 in the ALA‐IPL group (mean 19.7) and 10 in the IPL alone group (mean age 19.0), "with mild‐moderate facial acne"; FPT III‐V | 20% ALA plus 560? nm IPL versus 560 nm IPL alone; in a parallel‐group trial; two treatments at two weeks intervals, assessed 2 and 8 weeks after final treatment | Non‐standardised scale (marked improvement = 3; moderate improvement = 2; no change = 1; acne worsened = 0) was used for evaluation. At 8 weeks 5/15 (33%) participants assessed their improvement as moderate and 10/15 (67%) as marked in the ALA‐IPL group, whereas 3/10 (30%) of participants assessed their improvement as marked, 4/10 (40%) as mild and 1/10 (10%) as "slight" (a non pre‐specified category) in the IPL alone group. 2/10 (20%) of participants in the IPL alone group assessed that there was no change. We dichotomised the data to 10/15 'success' outcomes in the ALA‐PDT group and 3/10 in the IPL alone group. The difference was non significant, with RR (95% CI) 2.22 (0.81, 6.11), P = 0.12. |
| ALA‐PDT other | |||
| NCT00706433 | 266 (128 M, 138F), 68 in the ALA 1000 s group, 65 in the ALA 500 s group, 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group, mean age 20.1 years, inclusion criterion 12 > years, with moderate and severe acne (IGA score 3 and 4, with at least 20 ILs); FPT I‐VI | 20% ALA (45 min incubation) plus blue 1000 s light vs 20% ALA (45 min incubation) plus 500 s blue light vs vehicle (45 min incubation) plus blue 1000 s light vs vehicle (45 min incubation) plus 500 s blue light; in a parallel‐group trial; up to 4 treatments at 3‐week intervals, assessed 3 and 6 weeks after the final treatment | Non‐standardised scale ('subject satisfaction score'; excellent ‐ very satisfied; good ‐ moderately satisfied; fair ‐ slightly satisfied; poor ‐ not satisfied at all) was used for evaluation. We dichotomised the data to 41/68 of 'success' outcomes in ALA 1000 s and 39/65 in the ALA 500 s group. The difference between ALA 1000 s and ALA 500 s groups was non significant, with RR (95% CI) 1.00 (0.76, 1.33), P = 0.97. |
| Taub 2007 | 22 recruited, 19 participated, mean ± SD age 26.5 ± 9.1 years, 7 M, 12 F, with moderate‐severe acne and > 10 inflammatory acne lesions; FPT not given | Comparison of PDT with different light sources for activation: ALA activated by IPL (600–850 nm), or a combination of IPL (580–980 nm) and bipolar radiofrequency energies, or blue light (417 nm) in a parallel‐group trial; 3 treatments at 2‐week intervals; follow up at 1 and 3 months after final treatment | The method used for evaluation was unclear. At 1 month after treatments differences among groups were not statistically significant (P = 0.3210); median percentage improvement score (96.9% CI) was 58.75 (5‐70) in the IPL group, 20 (0‐80) in the IPL‐RF group and 15 (0‐87.5) in the blue‐light group. At three months data were only reported for IPL and blue light only groups 72.3 (range 42.5) versus 15 (range 27.5), so the analysis was not possible. |
| Yin 2010 | 180 (83 M, 97 F), aged 18‐38, mean 25.8, with moderate‐severe facial acne (Pillsbury), FPT III‐IV, 45 participants in each group | 633 ± 3 nm (red light) plus different ALA concentrations (5%, 10%, 15% and 20%) vs red light alone, 4 treatments every 10 days, 4 parallel groups, each treated with a different concentration on the right side and placebo agent on the left side; assessments at 2, 4, 12 and 24 weeks after last treatment | Non‐standardised scale ('marked improvement', 'moderate improvement', 'no charge' or 'acne worse') was used for evaluation. At 24 weeks after treatment a majority of the participants assessed that their acne had improved on both ALA‐PDT and control cheeks. In the 20% ALA group 44/45 of participants (98%, 1 drop‐out due to adverse effects) experienced a 'marked improvement' in their acne at ALA‐PDT sites, 42/45 (95%) in the 15% ALA and 36/40 (90%) in 10% ALA groups. Other data were not reported in text, but in graph format only. Our interpretation of the graph was that 30 participants in the 5% ALA group (67%) reported 'marked improvement', 3/45 (87%) of participants in the 15% ALA, 5/45 (11%) in the 10% ALA, and 9/45 (20%) in the 5% ALA group reported 'moderate improvement'. One participant, 1/45 (2%) in the 10% ALA group, as well as 3/45 (7%) in the 5% ALA group reported 'no change', whereas 3/45 (7%) of participants in both 10% and 5% ALA reported 'acne worse'. We dichotomised the data to 44/45 'success' outcomes in the 20% ALA group, 42/45 in the 15% ALA group, 36/45 in the 10% ALA group and 30/45 in the 5% ALA group. 20% ALA was not superior to 15% ALA with RR (95% CI) of 1.05 (0.96, 1.15) and P = 0.3. However, 20% ALA was more effective than 10% ALA with RR (95% CI) of 1.22 (1.05, 1.42) and P = 0.01, and more effective than 5% ALA with RR (95% CI) of 1.47 (1.19, 1.81) and P = 0.0004. The NNTB were 6 (95% CI 3 to 19) and 4 (95% CI 2 to 6) for the comparison of 20% ALA with 10% and 5% ALA respectively. However, there is no calculable NNTB for the comparison of 20% to 15% ALA since the 95% CI for the risk difference contains zero (i.e. no effect), and this corresponds to an infinite upper 'limit' for the 95% CI for the NNTB, which indicates that there is no true boundary on how large the NNTB could be for this comparison. |
| 7. Other (non‐MAL, non‐ALA) PDT versus other comparators | |||
| ICG‐PDT | |||
| Kim 2009 | 16 (7 M, 9 F, aged 16‐34 years, mean age 25 ± 3.09) with mild‐moderate acne, skin types not given, 9 in single, 7 in multiple treatment group | 2 groups randomised: single treatment vs multiple treatments (once weekly over 3 weeks); right cheek of each patient indocyanine green plus 805 nm light, left cheek light only and forehead "spontaneous resolution" control, evaluated 2 and 4 weeks after final treatment, multiple group also at final treatment | –100 to +100 scale scoring was used for evaluation, no details were reported. At both 2 and 4 weeks after final treatment difference between PDT and light‐only side was statistically significant only in the multiple treatment group (P < 0.05 at all assessment time points). Further data were not reported. Our interpretation of the graph was that at 4 weeks after final treatment mean VAS score was 20 for both PDT and light‐only side in the single treatment group; whereas in the multiple treatment group 50 on the light‐only side and 60 on the PDT side. SDs not presented in the graph. |
ALA = 5‐aminolevulinic acid BPO = benzoyl peroxide CHA = chlorophyll‐a FPT = Fitzpatrick's Skin Types: based on different reactions to sun exposure and range from type I ('pale white skin which always burns and never tans') to type VI ('deeply pigmented dark brown to black skin which never burns and tans very easily') (Fitzpatrick 1988) GAAS = Global acne assessment scoring scale IAA = indole 3‐acetic acid IGA = Investigator global assessment score ILs = inflamed lesions IPL = intense pulsed light IR = infrared ITT = intention‐to‐treat analysis LPDL = long pulsed dye laser LOCF = last observation carried forward LLT = lower level term MAL = methyl‐aminolevulinate NILs = non‐inflamed lesions NNTB = number needed to treat for an additional beneficial outcome OFI = optical fibre intra‐tissue irradiation PDL = pulsed‐dye laser PDT = photodynamic therapy PT = preferred term RCT = randomised controlled trial SD = standard deviation SE = standard error SPF = Sun protection factor TER = total effective rate TLMB = topical liposomal methylene blue
Change from baseline i.e. absolute change is calculated by subtracting baseline count from count assessed at certain time point. Percentage change is calculated by dividing the absolute change with baseline count and then multiplying that value by 100 to get percentages.
Unless specified differently, results presented as reported in the published papers, without performing independent analysis. Please see Characteristics of included studies for details on withdrawals and drop‐outs of participants for each study.
2. Investigator‐assessed change in lesion count, studies of light‐only therapies (excluding comparisons with photodynamic therapy).
| Study | Participants | Intervention(s) and control(s) | Investigator‐assessed change in lesion counts |
| 1. Light versus placebo or no treatment | |||
| Yellow light versus placebo or no treatment | |||
| Seaton 2003 | 41, 31 randomised to treatment, 10 to control group; with mild‐moderate acne, other characteristics not given | 585 nm pulsed dye laser vs sham laser, parallel‐group trial, single treatment, assessed at 2, 4, 8 and 12 weeks after treatment | Significantly greater improvement from baseline in ILs and total lesion counts (P = 0.024 and 0.023 respectively) in laser‐treated group than in placebo group at 12 weeks, whereas the difference in improvement in NILs was non significant (P = 0.14). ILs median (interquartile range) improvement from baseline in the treatment group was 49% (30% to 75%) versus 10% (‐8% to 49%) in the placebo group, NILs 40% (0% to 75%) versus ‐13% (‐42% to 23%), total lesion 53% (19% to 64%) versus 9% (‐16% to 38%). |
| Orringer 2004 | 40 (24 M, 16 F) enrolled, 26 completed, mean age 20.7 years (range not reported), with facial acne Leeds score > 2; FPT not given ("28 whites, 7 Asians, 2 blacks, 3 unknown") | 585 nm PDL in a split‐face trial, single treatment and 2 treatment groups (2 weeks apart), serially assessed for 12 weeks after final treatment | Non significant differences in changes in means of papules (P = 0.08), pustules (P = 0.12), comedones (P = 0.63) and cysts (P > 0.99) at 12 weeks between treated and untreated face sides. Changes in means (95% CIs) of papules, pustules, comedones and cysts at 12 weeks ‐4.2 (‐7.7 to ‐0.6), 0 (−1.4 to 1.4), 2.9 (−4.0 to 9.7) and 0 (‐0.5 to 0.5) on the treated sides respectively; and −2.2 (−5.3 to 0.9), ‐1 (‐2 to ‐0.01), 1.6 (‐5.2 to 8.4) and 0 (‐0.6 to 0.6) on the untreated sides respectively. LOCF method was used for analysis (n = 38). Our analyses using LOCF data (n = 38) confirmed no significant differences in means between treated and untreated face sides at 12 weeks, MD (95% CIs) for investigator‐assessed change in ILs (papules) was ‐2.00 (‐6.60, 2.60), P = 0.39, for investigator‐assessed change in ILs (pustules) 1.00, P = 0.24, and for investigator‐assessed change in NILs 1.30 (‐8.00, 10.60), P = 0.78 and for investigator‐assessed change in cysts 0.00 (‐0.76, 0.76), P = 1.00. Please note that we based all the calculations from the values provided in the table reported, and we double and triple checked the values using both RevMan and R statistical software, but some of our P values did not match up with the ones presented by the study authors. |
| Infrared light versus no treatment | |||
| Darne 2011 | 38 (7 M, 31 F), aged 18‐47 years (mean 28), with moderate‐severe facial acne; FPT I‐V | 1450 nm laser (8‐9 J/cm²) in a split‐face trial, 3 treatments monthly, assessed monthly for 4 months, then at 3‐monthly intervals for 12 months after final treatment | Similar reduction in ILs at 1 and 12 months on both sides; treated sides median 0 (95% CI ‐4 to 2) and untreated sides median 0 (95% CI ‐3.7 to 0). |
| Orringer 2007 | 46 (10 M, 36 F) enrolled, 30 completed, mean age 23.9 years (range not reported) with clinically apparent active facial acne; FPT II–VI | 1320 nm Nd:YAG laser in a split‐face trial with cooling; 3 treatments at 3‐week intervals; assessed at weeks 7 and 14 | No significant differences in changes in papules (P = 0.62), pustules (P = 0.39), open (P = 0.09), nor closed comedones (P = 0.20) between the treated and untreated sides at week 14. Difference in changes in cyst counts was significant (P = 0.04). Mean (SE) changes in papules, pustules, open comedones, closed comedones and cysts reported at week 14: ‐1.57 (0.95), ‐2.54 (1.45), ‐1.08 (1.91), ‐8.19 (3.48) and 0 (0.08) on the treated sides respectively; and ‐1.03 (1.31), ‐1.86 (1.16), 1.84 (1.85), ‐1.24 (7.45) and 0.43 (0.17) on the untreated sides respectively. LOCF method was used for analysis (n = 37, 9 participants withdrew prior to any clinical endpoint evaluation, and were not included in the analysis). Our analyses using LOCF data (n = 37) confirmed no significant differences in means between treated and untreated face sides at week 14 (i.e. 8 weeks after final treatment), MD (95% CIs) for investigator‐assessed change in ILs (papules) was ‐0.54 (‐3.71, 2.63), P = 0.74, for investigator‐assessed change in ILs (pustules) ‐0.73 (‐4.37, 2.91), P = 0.69, for investigator‐assessed change in NILs (open comedones) ‐2.92 (‐8.13, 2.29), P = 0.27, for investigator‐assessed change in NILs (closed comedones) ‐6.95 (‐23.07, 9.17), P = 0.40. The difference in means for investigator‐assessed change in cysts was significant (MD ‐0.43, 95% CI ‐0.80 to ‐0.06), P = 0.02. Please note that we based all the calculations from the values provided in the table reported, and we double and triple checked the values using both RevMan and R statistical software, but some of our P values did not match up with the ones presented by the study authors. |
| Moneib 2014 | 24 (5 M, 19 F), age 15‐38 years (mean 21.5), with moderate‐severe acne; FPT II‐V | Fractional Erbium Glass 1559 nm laser, in a split‐face trial, 4 treatments at 2‐week intervals; assessed every 3 months for 1 year after final treatment | Follow‐up time point unclear. At treated sides mean papules counts (SD) reduced from baseline 15.42 (14.38) to 0.88 (3.35), mean pustules count from baseline 2.58 (3.32) to 0.46 (1.38), open comedones from 4.25 (7.59) to 1.25 (3.07), closed comedones from 1.75 (3.45) to 0.33 (1.01) and nodules from baseline 1.00 (1.87) to 0.08 (0.41) at "follow up". At control sides mean papules counts (SD) changed from baseline 12.83 (10.89) to 14.08 (12.93), mean pustules count from baseline 3.17 (5.21) to 4.21 (7.40), open comedones from baseline 2.58 (3.37) to 2.88 (3.54), closed comedones from baseline 1.79 (3.75) to 1.21 (2.50) and nodules from baseline 0.92 (1.61) to 1.79 (2.00) at "follow up". |
| Blue light versus placebo or no treatment | |||
| Elman 2003 | 23 (11 M, 12 F), mean age 18.8 years (range not given) with mild‐severe papulopustular acne; skin types not documented | 405–420 nm laser with skin cooling in a split‐face trial, twice weekly for 4 weeks, assessed at each treatment and at 2, 4, and 8 weeks after treatment | ILs percentage change median reduction of 30% at final treatment on untreated sides, other data not available. ILs percentage change median reduction at 2, 4 and 8 weeks post treatment 59%, 61% and 53% respectively on treated sides (P = 0.01 at 8 weeks compared to untreated sides, using McNemar test; other statistical data not provided) |
| Red light versus no treatment | |||
| Na 2007 | 30 (7 M, 23 F) aged 19–33 years (mean 23.6) with mild‐moderate acne; skin types not documented | 635–670 nm portable red light device in a split‐face trial, self‐administered to the treatment side twice daily for 8 weeks; assessed at weeks 1, 2, 4 and 8, and then for 8 weeks after final treatment | At week 8, NILs percentage change ‐59% on treatment sides versus 3% increase on control sides (P < 0.005), ILs percentage change ‐66% on treatment side vs 74% increase in ILs on control sides (P < 0.005). Further data not given. At 4 weeks after final treatment 10/25 (40%) of followed‐up participants were reported to have "showed an increase in acne lesions", and at 8 weeks 21/22 (95%) were reported to "have complained of acne exacerbation compared with their status during treatment period". Further data were not provided. |
| Blue‐red light versus placebo | |||
| Papageorgieu 2000 | 30 participants, mean age 24.8 years in blue–red light group; 25 participants, mean age 25.6 years in white light control group; randomised from the original 107 recruited (33 M, 74 F, age 14‐50 years), all with mild‐moderate acne; skin types not stated | 415 nm plus 660 nm light vs cool white light; treated daily for 12 weeks; assessed every 4 weeks for the 12‐week treatment period | Blue‐red light superior at all time points, differences in mean percentage improvements (95% CI) 50.3 (40.1 to 60.5) for ILs and 66.5 (56.0 to 77.0) for comedones at week 12 (final treatment). |
| Kwon 2013 | 35 participants (11 M, 24 F); aged 20‐27 years (mean not given), with mild‐moderate acne, FPT III‐V; 18 participants in the blue‐red light group, 17 in the placebo group | 420 plus 660 nm home use LED device vs home use sham device; self‐treatment twice daily for 4 weeks in a split‐face trial; assessed 4 and 8 weeks after final treatment | Mean IL counts reduced from baseline 22.8 to 5.3 (by 76.7%, P < 0.01) and mean NILs counts reduced from baseline 51.2 to 23.5 (by 53.3%, P < 0.01) at eight weeks after final treatment in the blue‐red light group. Mean reduction of ILs and NILs counts in the placebo group was not statistically significant at eight weeks after final treatment (both P > 0.05). Results reported as percentage improvements in graph format (means and SDs not presented). |
| 2. Light versus topical treatment | |||
| Light versus BPO | |||
| de Arruda 2009 | 60 (34 M, 26 F, mean age 17.3, range not given), all with Brasilian Group of Acne grade II or III, skin types described as mixed Brazilians (11), caucasian (47) and unknown (2). | 407 nm‐420 nm (blue light) twice weekly for 4 weeks vs 5% BPO, self‐administered, twice daily; parallel groups, assessed 4 weeks after initial treatment and 2weeks after end treatment | No statistically significant difference in decrease of means of ILs (P = 0.500) and NILs (P = 0.177) between the blue light and 5% BPO group. In the blue‐light group ILs means (SD) reduced from baseline 27.87 (18.08) to 23.33 (15.10) at 4 weeks. NILs means (SD) reduced from baseline 111.6 (45.03) to 85.92 (57.78) at 4 weeks. In the BPO group ILs means (SD) reduced from baseline 35.37 (22.16) to 19.14 (17.95) at 4 weeks. NILs means (SD) reduced from baseline 128.67 (90.8) to 93.50 (69.74) at 4 weeks. We calculated that at 4 weeks the mean difference (95% CI) in changes in NILs was 9.49 (‐10.84, 29.82); however, the mean difference in changes in ILs was 0 (and since the P value the study authors presented was 0.5, then there are infinitely many possibilities for the standard error, hence the lack of a 95% CI provided for ILs). |
| Papageorgieu 2000 | 30 participants, mean age 24 ± 8 years in blue–red light group and 25 participants, mean age 26 ± 7 years in the BPO group, randomised from the original 107 recruited (33 M, 74 F, age 14‐50 years) all with mild‐moderate acne; skin types not stated | 415 nm plus 660 nm light vs 5% BPO, parallel groups, treated daily; assessed every 4 weeks for the 12‐week treatment period | Blue–red light superior to BPO at week 12 (P = 0.006). Difference in mean percentage improvements (95% CI) at week 12 was 17.6 (7.5 to 27.6) for IL counts and 0.9 (‐9.4 to 11.3) for comedones. |
| Chang 2007 | 30 women aged 23‐32 years (mean 25 ± 7); with mild‐moderate acne; FPT III‐IV | IPL with 530–750 nm filter with cooling gel in a split‐face trial, 3 sessions, 3 weeks apart, BPO gel used on both sides of the face. Assessed 3 weeks after final treatment | No significant difference between IPL‐treated and untreated sides of the face for changes in mean papule and pustule counts (‐3.2 vs ‐3.1; P > 0.05). Further data not reported |
| Light versus clindamycin | |||
| Gold 2005 | 34 (25 completed the trial, 3 M and 22 F) aged 13–55 years (mean 31 ± 0) with mild‐moderate acne; skin types described: caucasian (16), African‐American (7), American‐Indian (1), Chinese (1); 13 participants in clindamycin group and 12 in blue light group | 417 nm (blue light) twice weekly for 4 weeks vs self‐administered topical clindamycin 1%, twice daily, parallel groups, assessed at 4 weeks after final treatment | NILs & ILs count "averages" (ranges) in the blue‐light group were 29.4 (9 to 120) and 22.6 (16 to 34) at baseline and 21.4 (8 to 40) and 11.1 (0 to 24) 4 weeks after final treatment respectively. NILs & ILs count "averages" (ranges) in the clindamycin group were 29 (9 to 95) and 17.4 (12 to 32) at baseline and 12 (4 to 38) and 10.4 (4 to 19) 4 weeks after final treatment respectively. |
| Lee 2010 | 9, with inflammatory acne (other characteristics not given) | Full‐spectrum light twice a week vs 1% clindamycin twice a day, in a split‐face trial, for 4 weeks, evaluation weekly whilst on treatment and 2, 4 and 8 weeks after final treatment | Reduction in IL counts by 76.8% at light and 25.5% at clindamycin‐treated side (time point and other data not given) |
| Light and other topical treatments | |||
| Karsai 2010 | 89 randomised, 80 evaluated (38 M, 42 F, 13.3‐43.8 years, mean ± SD age 19.7 ± 5.9 years), with mild‐moderate acne (Investigator's Static Global Assessment ‐ISGA score 2‐4), FPT I‐III | Clindamycin 1% BPO 5% hydrating gel (C ⁄ BPO) alone, once daily "throughout the observation period" vs in combination with two 585 nm PDL treatments. Parallel groups, assessed at 2 and 4 weeks after initial treatment | In the C/BPO group there was a 36.3% reduction in number of ILs and 9.2% reduction in total lesion count 4 weeks after initial treatment. In the C/BPO plus light group there was a 36.9% reduction in number of ILs and 9.0% reduction in total lesion count. Means and SD reported in graph format. Our interpretation of the graph was that ILs (SD) in the C/BPO group reduced from baseline 37.5 (20) to 25 (15), and in the C/BPO plus light group from 50 (30) to 30 (25) at 4 weeks after initial treatment. Total lesions reduced from baseline 127.5 (70) to 115 (70) in the C/BPO group, and from 175 (105) to 150 (100) in the C/BPO plus light group at 4 weeks after initial treatment. We judged further analyses would be biased due to lack of precise data, so we did not perform them. |
| Anyachukwu 2014 | 40 (all M), 20 randomised to the light group, 20 to the placebo group, mean age 22 ± 4 years (range not reported), Global Acne Grading System (GAGS) > 19, FPTs not given | 905 nm light combined with "self‐management topical agents" ("antibiotic cream", "medicated soap", "talcum powder" or "personal hygiene"), 8 light treatments, twice weekly over 4 weeks, in a parallel‐group trial, control group treated with placebo‐non radiating light probe combined with "self‐management topical agents", details of topical treatment not given, unclear frequency of application; assessed within treatment and 3 days after final treatment | Mean percentage change from baseline in combined number of lesions (SD) was 54.98 (16.297) in the laser group and 17.97 (16.472) in the control group 3 days after final treatment. Mean percentage changes from baseline in combined number of lesions at 3 days after final treatment were 70.37, 61.90, 71.43, 71.43 in the laser combined with "antibiotic cream", "medicated soap", "talcum powder" and "personal hygiene" subgroups respectively. Mean percentage change from baseline in combined number of lesions at 3 days after final treatment were 38.71, 45.00, 10.34 and 12.50 in the placebo plus "antibiotic cream", "medicated soap", "talcum powder" and "personal hygiene" subgroups respectively. Further data were not given. |
| Ash 2015 | 41 (M/F not reported, study authors clarified "a 50/50 split"), 26 in the intervention, 15 in control group, aged 16–45 years (mean not reported) with mild‐moderate acne (Leeds grade); FPT not given: "Caucasian, Asian and mixed Afro‐Caribbean ethnic groups" | Pre‐treatment facial wash/weak chemical peel (containing salicylic acid, glycolic acid, lactic acid) followed by treatment with blue‐light device and then post‐treatment facial moisturiser (containing salicylic acid, glycolic acid, lactic acid, menthol, niacin) versus unclear control in a parallel‐group trial, 28 sessions in total, every other day for 8 weeks. Assessed at 12 weeks (4 weeks after final treatment?) | At 12 weeks (4 weeks after final treatment?) mean lesion counts reduced by 50.08% (P = 0.002) in the treatment group. In the control group, mean lesion counts increased by 2.45% (P = 0.0029). Further data not given |
| Borhan 2014 | 40 (8 M, 12 F in the light group, 9 M, 11 F in the control group), mean age 21.3 ± 2.0 in the intervention and 21.05 ± 2.18 in the control group (range 18‐25 years), with mild‐moderate acne vulgaris (Burton scale), FPT III‐IV | 595 nm light plus "traditional topical antibiotic medication" versus "traditional topical antibiotic medication" alone in a parallel‐group trial, 3 light treatments in total, at 4‐week intervals, details of topical treatment not given, unclear frequency of application; assessed at week 4, 8 and 12 (final evaluation 4 weeks after final treatment) | At week 12 combined number of lesions, reported as "acnes number", (SD) changed from baseline 25.7 (5.88) to 8.75 (2.91) in the laser + topical antibiotics group, and from baseline 25.75 (6.71) to 17.7 (5.14) in the topical antibiotics‐alone group ( P = 0.0001). |
| 3. Light versus other comparators | |||
| Comparison of light therapies of different wavelengths | |||
| Papageorgieu 2000 | 30 participants, mean age 24.8 years in blue–red light group and 27 participants, mean age 23.4 years in the blue‐light group, randomised from the original 107 recruited (33 M, 74 F, age 14–50 years) all with mild‐moderate acne; skin types not stated | 415 nm plus 660 nm light vs 415 nm light, parallel groups, treated daily for 12 weeks; assessed every 4 weeks for the 12‐week treatment period | There was no significant difference between the treatments in ILs at week 12 (P = 0.1), nor in comedone count (P value not given). Difference in mean percentage improvements (95% CI) at week 12 was 13.1 (3.0 to 23.1) for ILs counts and 12.9 (2.5 to 23.2) for comedones. |
| Liu 2011 | 20 (6M/14F) completed the study, number of randomised participants not reported, 10 completed in the blue light, 10 in the red‐light group, aged 19–28 years (mean 23.6 years) with mild‐moderate acne (Global Acne Grading System); FPT III‐IV | Blue (405 ± 10 nm) vs red (630 ± 10 nm) LED portable device treatments, about 20 cycles of illumination and the corresponding light doses received in each session were 7.2 J/cm² and 11.52 J/cm², in a parallel‐group trial, 8 sessions in total, twice weekly for 4 weeks; assessed at 4 weeks after final treatment and at each treatment session | In the blue‐light group, the mean ILs count (papules and pustules) dropped from baseline 19.2 to 5.5 (by 71.4%) at final treatment and in the red‐light group from baseline 8.2 to 6.6 at final treatment (by 19.5%). SDs and further data not given |
| Choi 2010 | 20 (1 M, 19 F, age 20‐37, mean age 26); all with acne (Cunliffe severity grade 2‐4), FPT III‐V | 585 nm PDL vs 530‐750 nm IPL, 4 treatments at 2‐week intervals, in a split‐face trial, assessed 4 and 8 weeks after last treatment | 4 weeks after final treatment greater reductions on PDL sides versus IPL treatment sides for NILs (47% versus 33% reduction), but lower for ILs (62% versus 66%). 8 weeks after final treatment significantly greater improvements on PDL sides versus IPL treatment sides for both ILs (86% versus 35% reductions) and NILs (59% versus 43% reduction). Individual participant data reported at baseline and 8 weeks (n = 17). We calculated means (SD): IPL at baseline: ILs 6.17 (3.67) NILs 15 (8.51), at 8 weeks ILs 2.23 (2.19) NILs 6.52 (4.15) PDL at baseline: ILs 6.76 (4.08) NILs 14.64 (8.65), at 8 weeks ILs 0.82(1.13) NILs 5.41(3.93). Mean differences (95% CI) between the 2 treatments at 8 weeks using t‐distribution were 2.00 (‐0.85, 4.85), P = 0.178, t = 1.431 for changes in ILs and 0.77 (‐3.65, 5.19), P = 0.735, t = 0.355 for changes in NILs. MDs (95% CI) between the two treatments at 8 weeks using normal distribution were 2.00 (‐0.74, 4.74), P = 0.15 for changes in ILs and 0.77 (‐3.49, 5.03), P = 0.72 for changes in NILs. |
| Jung 2009 | 18 enrolled, 16 completed (5 M, 11 F, aged 20‐31 years, mean age 26); with mild‐moderate acne (Cunliffe severity grade 2‐5), skin types not given | 585 nm PDL vs combined 585/1064 nm PDL, in a split‐face trial, 3 treatments at 2‐week intervals, assessed at 8 and 12 weeks after initial treatment | ILs and NILs reduced by 86% and 69% respectively on the PDL sides and by 89% and 64% on the 585/1,064 nm laser sides respectively at final evaluation (P values reported as < 0.05 "compared with baseline"). No significant difference in the effect of the two interventions (P values and further data not provided) |
| Comparison of light therapies of different doses | |||
| Bernstein 2007 | 7 enrolled, 6 completed (1 M, 6 F, aged 23‐41 years, mean age 29), all with active papular acne, FPT I‐III | Comparison of two 1450 nm laser treatments; single‐pass, high‐energy (13–14 J/cm²) vs double‐pass, low‐energy (8–11 J/cm²); 4 treatments at monthly intervals, assessed 1 month following each treatment and 2 months after final treatment | ILs counts means (SD) dropped from 19.5 (11.9) to 4.2 (4.7) on the single‐pass face side, and from 16.2 (6.0) to 5.2 (4.5) on the double‐pass face side. Individual participant data reported (n = 6). We calculated mean difference (95% CI) of ‐4.33, 95% CI ‐13.4 to 4.74, P = 0.372, t = ‐1.063 using t‐distribution and MD (95% CI) of ‐4.33 (‐12.31, 3.65), P = 0.29 using normal distribution |
| Jih 2006 | 20 (10 M, 10 F), aged 18‐39 years (mean 23) with active inflammatory facial acne; FPT II–VI | 1450 nm diode laser in a split‐face trial using anaesthetic cream and 14 J/cm² in one group and 16 J/cm² in the second, with three treatments given at 3–4 week intervals, assessed at 1, 3, 6 and 12 months after final treatment | Baseline mean IL counts 16.1 for the 14/J cm² and 16.8 for the 16 J/cm² side (SDs not reported). At 1, 3, 6 and 12 month follow‐up percentage reductions were 75.1%, 88.6%, 81.6% and 76.1% on the 14 J/cm² and 70.6%, 81.5%, 84.1% and 70.5% on the 16 J/cm² face side respectively (P < 0.001). There was no significant difference in reduction between the different light intensities. Sponsors provided detailed data and our analyses confirmed that. The mean differences (95% CI) in changes in ILs and percentage changes in ILs calculated using t‐distribution were ‐2.40 (‐6.46, 1.66), P = 0.26, t = ‐1.203 and ‐3.40 (‐14.21, 7.41), P = 0.54, t = ‐ 0.641 respectively at 1 month; ‐3.20 (‐7.43, 1.03), P = 0.15, t = 1.541 and ‐7.05 (‐16.05, 1.95), P = 0.13, t = ‐1.596 respectively at 3 months; ‐2.00 (‐5.87, 1.87), P = 0.32, t = ‐1.053 and 2.49 (‐6.37, 11.35), P = 0.59, t = 0.572 respectively at 6 months; and ‐2.40 (‐7.13, 2.33), P = 0.33, t = ‐1.034 and ‐5.59 (‐26.07, 14.89), P = 0.60, t = ‐0.556 respectively at 12 months. The MDs (95% CI) in changes in ILs and percentage changes in ILs calculated using normal distribution were ‐2.40 (‐6.31, 1.51), P = 0.23 and ‐3.40 (‐13.80, 7.00), P = 0.52 respectively at 1 month; ‐3.20 (‐7.27, 0.87), P = 0.12 and ‐7.05 (‐15.71, 1.61), P = 0.11 respectively at 3 months; ‐2.00 (‐5.72, 1.72), P = 0.29 and 2.49 (‐6.04, 11.02), P = 0.57 respectively at 6 months; and ‐2.40 (‐6.95, 2.15), P = 0.30 and ‐5.59 (‐25.30, 14.12), P = 0.58 respectively at 12 months |
| Uebelhoer 2007 | 11 (2 M, 9 F, age 19‐39 years, mean age 26), 9 completed, all with ≥ 10 inflammatory papules on each side of the face and Allen‐Smith grade ≥ 3 and ≤ 5; skin types not given | 1450 nm laser single‐pass treatment consisting of stacked double pulses vs a double‐pass treatment of single pulses; in a split‐face trial, treated every 3 weeks for a total of 3 treatments, assessed before each follow‐up treatment, and at 3 months after the final treatment | Statistically significant reduction of mean acne lesion counts on both the single‐pass side and double‐pass side of 57.6% (P = 0.02) and 49.8% (P = 0.02), respectively. Further details not given |
| NCT00706433 | 266 (128 M, 138F), 68 in the ALA 1000 s group, 65 in the ALA 500 s group, 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group, mean age 20.1 years, inclusion criterion 12 > years, with moderate and severe acne (IGA score 3 and 4, with at least 20 ILs); FPT I‐V | 20% ALA (45 min incubation) plus blue 1000 s light vs 20% ALA (45 min incubation) plus 500 s blue light vs vehicle (45 min incubation) plus blue 1000 s light vs vehicle (45 min incubation) plus 500 s blue light; in a parallel‐group trial; up to 4 treatments at 3‐week intervals, assessed 3 and 6 weeks after the final treatment | At 3 weeks after final treatment investigator‐assessed median change in ILs (SD) was ‐19.0 (22.8) in the vehicle 1000 s and ‐14.5 (24.0) in the vehicle 500 s group; investigator‐assessed median percentage change in ILs (SD) was ‐41.7 (38.82) in the vehicle 1000 s and ‐37.0 (40.23) in the vehicle 500 s group. At 6 weeks after final treatment investigator‐assessed median change in ILs (SD) was ‐21.0 (23.63) in the vehicle 1000 s and ‐17.0 (26.71) in the vehicle 500 s group; investigator‐assessed median percentage change in ILs (SD) was ‐48.4 (32.81) in the vehicle 1000 s and ‐45.2 (50.15) in the vehicle 500 s group. Statistical tests to determine whether any changes were significant could not be performed due to the study authors’ use of median changes rather than the typical mean changes required for significance testing in order to make appropriate comparisons with other included studies. Furthermore, it is not clearly stated whether the study authors implemented an ITT analysis or a LOCF approach to handling missing data. |
| Light alone versus combined with microdermoabrasion | |||
| Wang 2006 | 20 (7 M, 13 F) age 19–59 years (mean 34 ± 3) with active inflammatory facial acne; FPT II–IV | 1450 nm diode laser plus microdermoabrasion in a split‐face design with light treatment on the control side of the face with topical anaesthetic to whole face; 4 treatments, 3 weeks apart; assessed at 6 and 12 weeks after the final treatment | Microdermabrasion plus light treatment decreased the mean acne lesion count by 52.8% by 6 weeks and 54.4% by 12 weeks (P <0.02 compared with baseline counts). Light treatment alone reduced the counts by 53.5% by 6 weeks and 61.1% by 12 weeks (P <0.05 compared with baseline counts). No statistically significant difference between the two treatments at any point |
| Light in combination with carbon lotion versus no treatment | |||
| Jung 2012 | 22 (4 M, 18F), 20 completed (2 M, 18F, aged 19‐34 years, mean age 25.4), FPT III‐IV, acne severity not given | Carbon lotion plus quasi‐long pulse and Q‐switched 1064 nm Nd:YAG laser vs non treated control, in a split‐face trial, 3 treatments over 4 weeks, evaluation every 2 weeks whilst on treatment and then every 4 weeks | Difference in means of both ILs and NILs statistically significant between treated and untreated side (P < 0.001), but clear data for non treated side not given. Both ILs and NILs reduced to 58.6% (P < 0.001) and to 52.4% (P < 0.001) respectively on the laser‐treated side |
ALA = 5‐aminolevulinic acid BPO = benzoyl peroxide CHA = chlorophyll‐a FPT = Fitzpatrick's Skin Types: based on different reactions to sun exposure and range from type I ('pale white skin which always burns and never tans') to type VI ('deeply pigmented dark brown to black skin which never burns and tans very easily') (Fitzpatrick 1988) GAAS = Global acne assessment scoring scale IAA = indole 3‐acetic acid IGA = Investigator global assessment score ILs = inflamed lesions IPL = intense pulsed light IR = infrared ITT = intention‐to‐treat analysis LPDL = long pulsed dye laser LOCF = last observation carried forward LLT = lower level term MAL = methyl‐aminolevulinate NILs = non‐inflamed lesions NNTB = number needed to treat for an additional beneficial outcome OFI = optical fibre intra‐tissue irradiation PDL = pulsed‐dye laser PDT = photodynamic therapy PT = preferred term RCT = randomised controlled trial SD = standard deviation SE = standard error SPF = Sun protection factor TER = total effective rate TLMB = topical liposomal methylene blue
Change from baseline i.e. absolute change is calculated by subtracting baseline count from count assessed at certain time point. Percentage change is calculated by dividing the absolute change with baseline count and then multiplying that value by 100 to get percentages.
Unless specified differently, results presented as reported in the published papers, without performing independent analysis. Please see Characteristics of included studies for details on withdrawals and drop‐outs of participants for each study.
3. Investigator‐assessed change in lesion count, studies of photodynamic therapy (including comparisons with light‐only therapies).
| Study | Participants | Intervention(s) and control(s) | Investigator‐assessed change in lesion counts |
| 4. MAL‐PDT versus other comparators | |||
| MAL‐PDT versus red light alone | |||
| Pariser 2013 | 153 participants (87 M/66 F), 100 in the 80 mg/g MAL‐PDT group, 53 in the placebo group, aged 12‐35 years (mean 18.6), with severe facial acne vulgaris, IGA score 4, 25‐75 ILs and 20‐100 NILs on the face, FPT I‐VI | 80 mg/g MAL‐PDT under occlusion followed by illumination with 632 nm 37 J/cm²red light vs placebo cream plus 632 nm 37 J/cm² light in a parallel‐group trial, 4 treatments at 2‐week intervals, assessed at 6 weeks after final treatment | 15 withdrawals from the MAL‐PDT group, 4 withdrawals and 1 lost to follow‐up from the placebo group. ITT analysis was performed. Our analyses for the individual study showed that at 6 weeks after final treatment 80 mg/g MAL‐PDT was superior to placebo cream plus red light in change in ILs (MD ‐7.80, 95% CI ‐14.39 to ‐1.21), in percentage change in ILs (MD ‐21.10, 95% CI ‐37.69 to ‐4.51), but was not superior in change in NILs (MD ‐1.10, 95% CI ‐8.11 to 5.91), nor in percentage change in NILs (MD ‐3.70, 95% CI ‐19.30 to 11.90). Please note that the results of this study were combined with those of NCT00933543 and NCT00594425 for the same comparison. |
| NCT00933543 | 107 participants (48 M/59 F), 54 in the 80 mg/g MAL‐PDT group, 53 in the placebo group, aged 11‐35 years (mean 17.2), with moderate‐severe facial acne vulgaris, IGA score 3‐4, 20‐100 ILs and 30‐120 NILs on the face, FPT I‐VI | 80 mg/g MAL‐PDT (without occlusive dressing) followed by illumination with 632 nm 37 J/cm² red light vs placebo cream plus 632 nm 37 J/cm² light (without occlusive dressing) in a parallel‐group trial, 4 treatments at 2‐week intervals, assessed at 6 weeks after final treatment | 3 withdrawals in MAL‐PDT group, 6 withdrawals and 1 lost to follow‐up in placebo group. ITT analysis was performed. Our analyses for the individual study showed that at 6 weeks after final treatment 80 mg/g MAL‐PDT was not superior to placebo cream plus red light in change in ILs (MD ‐0.20, 95% CI ‐8.19 to 7.79), in percentage change in ILs (MD ‐5.60, 95% CI ‐21.50 to 10.30), in change in NILs (MD 2.80, 95% CI ‐7.13 to 12.73), nor in percentage change in NILs (MD ‐1.60, 95% CI ‐18.83 to 15.63). Please note that the results of this study were combined with those of Pariser 2013 and NCT00594425 for the same comparison. |
| NCT00594425 | 150 participants (59 M/91 F), 50 in the 40 mg/g MAL‐PDT group, 48 in the 80 mg/g MAL‐PDT group, 52 in the placebo group, aged 15‐40 years (mean 21.3), with moderate‐severe acne, IGA score 3‐4, 20‐100 ILs and up to 200 NILs on the face, FPT I‐IV | 80 mg/mL MAL under occlusion (1.5h) plus 632 nm 37 J/cm² light vs 40 mg/mL MAL under occlusion (1.5 h) plus 632 nm 37 J/cm² light vs placebo cream plus 632 nm 37 J/cm² light in a parallel‐group trial, 4 treatments at 2‐week intervals, assessed at 2, 3, 6, 12 and 24 weeks after final treatment | 43 participants completed in the 40 mg/g group, 34 in the 80 mg/g group and 42 participants completed in the placebo‐cream group, ITT analysis was performed. Our analyses showed that at 6 weeks after final treatment 40 mg/g MAL‐PDT was not superior to placebo cream plus red light in change in ILs (MD ‐3.00, 95% CI ‐7.76 to 1.76), P = 0.22, in percentage change in ILs (MD ‐7.90, 95% CI ‐22.33 to 6.53), P = 0.28, in change in NILs (MD ‐7.50, 95% CI ‐16.07 to 1.07), P = 0.09, while there was a borderline superiority in percentage change in NILs (MD ‐25.80, 95% CI ‐51.69 to 0.09), P = 0.05. Our analyses for the individual study showed that at 6 weeks after final treatment 80 mg/g MAL‐PDT was not superior to placebo cream plus red light in change in ILs (MD ‐0.80, 95% CI ‐5.61 to 4.01), in percentage change in ILs (MD ‐4.80, 95% CI ‐19.85 to 10.25), in change in NILs (MD ‐6.90, 95% CI ‐15.38 to 1.58), while there was a borderline superiority in percentage change in NILs (MD ‐27.50, 95% CI ‐52.76 to ‐2.24). Please note that the results of this study were combined with those of Pariser 2013 and NCT00933543 for the same comparison. |
| NCT00673933 | 20 (11 M, 9 F), age 14‐40 years (mean 26 years) with moderate‐severe acne, FPT V‐VI | 80 mg/mL MAL plus 653 nm light vs placebo cream plus 653 nm light, in a split‐back trial, 2 sessions, 2 weeks apart, assessed 4 weeks after final treatment | Baseline ILs means (full range) were 5.9 (5 to 11) in the PDT group and 6.0 (5 to 10) in the placebo group. Mean change from baseline in number of ILs ± SD at 4 weeks was ‐3.70 ± 2.43 in MAL‐PDT group and ‐3.90 ± 2.07 in the placebo plus red light group. Baseline NILs means (full range) were 6.5 (1 to 21) in the PDT group and 5.4 (2 to 17) in the placebo group. Mean change from baseline in number of NILs ± SD at 4 weeks was ‐2.95 ± 4.84 in MAL‐PDT group and ‐2.50 ± 2.65 in the placebo plus red light group. Using t‐distribution, we calculated that at 4 weeks after final treatment the mean difference (95% CI) in changes in lesion counts on back sides treated with MAL‐PDT and those treated with placebo cream plus red light was non significant for ILs 0.20 (‐1.24, 1.64), P = 0.79, t = 0.280, as well as for NILs ‐0.45 (‐2.95, 2.05), P = 0.73, t = ‐0.365. 17 participants completed the study, results reported for 20, ITT analysis performed. MD (95% CI) in changes in lesion counts on back sides treated with MAL‐PDT and those treated with placebo cream plus red light was non significant for ILs 0.20 (‐1.20, 1.60), P = 0.78, as well as for NILs ‐0.45 (‐2.87, 1.97), P = 0.72 in the analyses using normal distribution. |
| Hörfelt 2006 | 30 (25 M, 5 F), 27 completed, aged 15‐28 years (mean 18) with moderate‐severe inflammatory facial acne (Leeds score 5–10); FPT types I–III | 635 nm light plus MAL vs placebo cream and light in a split‐face trial, two treatments, 2 weeks apart, assessed at 4 and 10 weeks after treatment | MAL–PDT significantly more effective than light alone for IL: median percentage reduction 63% (95% CI 50% to 71%) versus 28% (95% CI 19% to 47%) at 4 weeks (P = 0.0004), and 54% (95% CI 35% to 64%) versus 20% (95% CI 8% to 50%) at 10 weeks (P = 0.0006). No statistically significant difference in treating NILs observed between two interventions (open comedones P = 0.6875, closed comedones P = 1.00). Intention‐to‐treat analysis (last observation carried forward method) results reported (n = 30). Study authors provided further data on changes and percentage changes in ILs (ITT population). Changes in means (SD) in ILs were 9.4 (7.4) at 4 weeks and 8.2 (7.4) at 10 weeks after final treatment in the MAL‐PDT group and 6.8 (7.8) at 4 weeks and 5.7 (8.7) at 10 weeks respectively in the placebo cream plus light group. Percentage changes in means (SD) in ILs were 53.6% (29.1) at 4 weeks and 45.7 (34.5) at 10 weeks after final treatment in the MAL‐PDT group and 29.7% (30.7) at 4 weeks and 26.6% (38.6%) at 10 weeks respectively in the placebo cream plus light group. We calculated that MAL‐PDT was not superior to placebo cream plus light in change in ILs at 4 weeks nor at 12 weeks, with mean differences (95% CI) of ‐2.60 (‐6.45, 1.25), P = 0.19 and ‐2.50 (‐6.59, 1.59), P = 0.23 respectively. Howewer, it was superior in percentage change in ILs at 4 weeks and percentage change in ILs at 10 weeks, with mean differences (95% CI) of ‐23.90 (‐39.04, ‐8.76), P = 0.002 and ‐19.10 (‐37.63, ‐0.57), P = 0.04 respectively. |
| MAL‐PDT versus yellow light alone | |||
| Haedersdal 2008 | 15 (5 M, 10 F) age 18‐31 years (median 18), with at least 12 facial inflammatory acne lesions; FPT I–III | Split‐face design with non‐purpuric LPDL 595 nm full‐face treatment and MAL cream applied to randomised side of the face for 3 h before laser exposure, with dynamic cooling device; 3 treatments at 2‐week intervals; assessed 4 and 12 weeks after final treatment | Median percentage reduction in IL counts was significantly greater with MAL–LPDL than with LPDL at 4 weeks (70% versus 50%, P = 0.03) and 12 weeks (80% versus 67%, P = 0.004). Median percentage reduction in NILs lesions was significantly greater on the MAL–LPDL side at 4 weeks (P = 0.035) but difference between the treatments (53% versus 42%) did not achieve statistical significance at final follow‐up (P = 0.158). Median IL counts (25% to 75% percentiles) at baseline, 4 and 12 weeks were 21.0 (16‐36), 7 (4.75‐15) and 3.5 (2‐9.5) on the MAL‐LPDL side, and 22 (14‐36), 10 (6.5‐16) and 7 (2‐9.5) on the LPDL side respectively. Median NIL counts (25% to 75% percentiles) at baseline, 4 and 12 weeks were 33 (26‐41), 23 (17‐40) and 15 (9‐21) on the MAL‐LPDL side, and 32 (25‐41), 26 (17‐33) and 20 (12‐27) on the LPDL side respectively |
| MAL‐PDT versus placebo or no treatment | |||
| Wiegell 2006b | 36 participants: 21 in treatment group age 23 ± 5 years (9 M, 10 F analysed) and 15 in control group age 24 ± 5 years (3 M, 9 F analysed), with > 12 inflammatory acne lesions; FPT II–V | Comparison of MAL plus 630 nm with no treatment in a parallel‐group trial; two treatments, 2 weeks apart, assessed every 4 weeks for 12 weeks after treatment | A significantly greater median reduction in ILs in the treatment group at 8 weeks (P = 0.023) and 12 weeks (P = 0.0023) at 12 weeks. Median ILs change from baseline (range) at 12 weeks was 24 (‐4 to 55) in the MAL‐PDT group and 0 (‐39 to 19) in the control group. Median ILs count (range) at baseline, 4 , 8 and 12 weeks were 46 (13 to 99), 24 (9 to 68), 22 (8 to 83) and 14 (4 to 44) in the MAL‐PDT group and 32 (13 to 99), 32 (8 to 128), 42 (9 to 109) and 40 (13 to 80) in the control group. Non significant difference in median change in NILs between the MAL‐PDT and control group (P = 0.90) at 12 weeks. Median NILs change from baseline (range) at 12 weeks was 6 (‐15 to 18) in the MAL‐PDT group and 2 (‐14 to 35) in the control group. Median NILs count (range) at baseline, 4, 8 and 12 weeks were 17 (2 to 73), 22 (0 to 56), 24 (6 to 59) and 24 (9 to 74) in the MAL‐PDT group and 24 (2 to 64), 19 (0 to 76), 21 (2 to 81) and 31 (5 to 59) in the control group. |
| MAL‐PDT other | |||
| Bissonnette 2010 | 44 participants, 33 completed (M/F not stated), aged 18‐40 years (mean 24.4), 22 randomised to each group,10 ≥ ILs on each side of the face and a Global Acne Severity score 3 ≥, FPT I‐IV | 80 mg/mL MAL plus 630 nm 25 J/cm² light vs 80 mg/mL MAL plus 630 nm 37 J/cm² light in a parallel‐group trial, split‐face randomisation within each group to occlusion or no occlusion, 4 treatments at 2‐week intervals, assessed at 4 and 12 weeks after final treatment | ILs means (95% CIs) changed from baseline 16.7 (11.8 to 21.5), 16.6 (12.6 to 20.5), 14.9 (12.3 to 17.1) and 15.7 (13.17 to 18.8) on the non‐occluded 25 J/cm², occluded 25 J/cm², non‐occluded 37 J/cm² and occluded 37 J/cm² face sides, respectively to 11.0 (8.7 to 13.4), 9.4 (6.3 to 12.4), 8.6 (5.2 to 11.9) and 8.9 (5.5 to 11.8) respectively at 12 weeks after final treatment. NILs means (95% CIs) changed from baseline 10.8 (7.0 to 14.6), 11.3 (7.9 to 14.7), 14.6 (7.8 to 21.4) and 15.1 (8.9 to 21.3) on the non‐occluded 25 J/cm², occluded 25 J/cm², non‐occluded 37 J/cm² and occluded 37 J/cm² face sides, respectively to 8.6 (5.7 to 11.5), 7.5 (4.9 to 10.1), 12.7 (5.8 to 19.6) and 12.2 (5.8 to 18.6) respectively at 12 weeks after final treatment. The number of ILs was significantly lower than baseline on all face sides but the non‐occluded 25 J/cm² (based on non‐overlapping 95% CI). No statistically significant difference in mean reduction of ILs between face sides with and without occlusion, for both 25 J/cm² and 37 J/cm². No statistically significant difference in NILs mean change from baseline between the treatments at 12 weeks follow‐up. ITT analysis (LOCF method) results reported |
| Hong 2013 | 22 (2 M, 20 F), aged 19‐35 years (mean not given), "at least grade 2 (Cunliffe acne grading system)", FPT IV‐V | MAL plus 630 nm light vs MAL plus 530‐750 nm light in a split‐face trial, 3 treatments in total, 2‐week intervals, assessed at 4 weeks after treatment | At 4 weeks after treatment, there was no statistically significant difference between red‐light and IPL‐treated sides in mean percentage reduction of ILs (69.5% versus 72.0% respectively) and NILs (43.4% versus 46.3% respectively). Further data not provided |
| NCT00594425 | 150 participants (59 M/91 F), 50 in the 40 mg/g MAL‐PDT group, 48 in the 80 mg/g MAL‐PDT group, 52 in the placebo group, aged 15‐40 years (mean 21.3), with moderate‐severe acne, IGA score 3‐4, 20‐100 ILs and up to 200 NILs on the face, FPT I‐IV | 80 mg/mL MAL under occlusion (1.5 h) plus 632 nm 37 J/cm² light vs 40 mg/mL MAL under occlusion (1.5 h) plus 632 nm 37 J/cm² light vs placebo cream plus 632 nm 37 J/cm² light in a parallel‐group trial, 4 treatments at 2‐week intervals, assessed at 2, 3, 6, 12 and 24 weeks after final treatment | 37 participants completed in the 80 mg/g group, and 43 completed in the 40 mg/g group, ITT analysis was performed. Our analyses showed that at 6 weeks after final treatment 80 mg/g MAL‐PDT was not superior to 40 mg/g MAL‐PDT in change in ILs (MD 2.20, 95% CI ‐2.57 to 6.97), P = 0.37, in percentage change in ILs (MD 3.10 95% CI ‐11.8 to 17.38), P = 0.67, in change in NILs (MD 0.6, CI 95% ‐6.36 to 7.56), P = 0.87, nor in percentage change in NILs (MD ‐1.7, 95% CI ‐20.67 to 17.27), P = 0.94 |
| Yeung 2007 | 30 participants (8 M, 15 F ) aged 18‐41 years (mean 25) with moderate facial acne; FPT IV–V | All participants used topical adapalene 0.1% gel at night and were randomised to 2 split‐face treatment groups: 530–750 nm light with contact cooling gel plus MAL vs IPL only; and IPL with contact cooling gel vs topical adapalene‐only control; 4 treatments with intervals of 3 weeks, assessed after each treatment and at 4 and 12 weeks post‐treatment | Only the control face side showed a statistically significant mean reduction (P =0.01) in IL counts. At 4 weeks and 12 weeks IL counts means (SE) were reported to be reduced by 52.7% (52.5) and 64.5% (54.8) on the MAL–PDT face sides; 22.1% (55.3) and 22.9% (52.2) on the light‐only face sides; and 72.4% (19.9) and 88% (12.5) on the control face sides. A significant reduction in comedones on the MAL–PDT (P = 0.05) and light‐only (P = 0.01) face sides at 12 weeks compared with the control face sides. At 4 weeks and 12 weeks NIL counts means (SE) reduced by 51.6 (26.1) and 38 (53.5) on the MAL–PDT face sides; 15.5 (42.3) and 43.6 (26.5) on the light‐only face sides. 4 weeks after final treatment NIL counts means (SE) reduced by 13.8% (34) on the control face sides, but increased by 15.1% (SE) 12 weeks after final treatment. We performed analyses based on t‐distribution and found that MAL‐PDT was not superior to IPL alone in percentage change in ILs at both 4 weeks and at 12 weeks, with mean differences (95% CI) of ‐30.60 (‐70.37, 9.17), P = 0.141, t = ‐1.567 and ‐41.60 (‐81.90, ‐1.30), P = 0.052, t = ‐2.103 respectively. However, we found a transient superior effect on percentage change in NILs at 4 weeks, which was lost at 10 weeks, with mean differences (95% CI) of ‐36.10 (‐60.18, ‐12.02), P = 0.006, t = ‐3.054 and 5.60 (‐29.13, 40.33), P = 0.754, t = 0.328 respectively. We found no difference in effect between adapalene and MAL‐PDT in percentage change in ILs at both 4 weeks and at 12 weeks, with mean differences (95% CI) of 19.70 (‐15.32, 54.72), P = 0,283, t = 1.170 and 23.50 (‐11.68, 58.68), P = 0.205, t = 1.390 respectively. However, MAL‐PDT also had a transient superior effect to adapalene on percentage change in NILs at 4 weeks, which was lost at 10 weeks, with mean differences (95% CI) of ‐37.80 (‐63.97, ‐11.63), P = 0.01, t = ‐3.005 and ‐53.10 (‐119.64, 13.44), P = 0.133, t = ‐1.660 respectively. Results of our analyses based on normal distribution were not substantially different and also showed that MAL‐PDT was not superior to IPL alone in percentage change in ILs at both 4 weeks and at 12 weeks, with mean differences (95% CI) of ‐30.60 (‐68.86, 7.66), P = 0.133 and ‐41.60 (‐80.38, ‐2.82), P = 0.0401 respectively. We also found a transient superior effect on percentage change in NILs at 4 weeks, which was lost at 10 weeks, with mean differences (95% CI) of ‐36.10 (‐59.27, ‐12.93), P = 0.014 and 5.60 (‐27.82, 39.02), P = 0.683 respectively. We also found no difference in effect between adapalene and MAL‐PDT in percentage change in ILs at both 4 weeks and at 12 weeks, with mean differences (95% CI) of 19.70 (‐13.30, 52.70), P = 0.240 and 23.50 (‐9.65, 56.65), P = 0.162 respectively. We also found a transient superior effect of MAL‐PDT as compared to adapalene in percentage change in NILs at 4 weeks, which was lost at 10 weeks, with mean differences (95% CI) of ‐37.80 (‐62.46, ‐13.14), P = 0.007 and ‐53.10 (‐115.80, 9.60), P = 0.120 respectively |
| 5. ALA‐PDT versus other comparators | |||
| ALA‐PDT versus red light alone | |||
| Pollock 2004 | 10 (9 M, 1 F) age 16–40 years (mean 26.9) with mild‐moderate acne of the back, Leeds grades 2‐4; FPT I‐V | Four equal 30 cm² areas on the back: 635 nm light plus ALA vs light alone; ALA alone; untreated control; treated weekly for 3 weeks, assessed at each treatment and 3 weeks after final treatment | Statistically significant reduction from baseline in ILs counts from second treatment (P < 0.005) at the ALA–PDT site but not the other sites: reduction in acne was 69% at 21 days' follow‐up. Further data reported in graph format, mean ILs at baseline 8.3 and 11.6 at light alone and ALA‐PDT areas respectively decreased to 6.1 and 3.6 respectively at 3 weeks' follow‐up. Other data not given |
| ALA‐PDT versus blue light alone | |||
| NCT00706433 | 266 (128 M, 138F), 68 in the ALA 1000 s group, 65 in the ALA 500 s group, 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group, mean age 20.1 years, inclusion criterion 12 > years, with moderate and severe acne (IGA score 3 and 4, with at least 20 ILs); FPT I‐V | 20% ALA (45 min incubation) plus blue 1000 s light vs 20% ALA (45 min incubation) plus 500 s blue light vs vehicle (45 min incubation) plus blue 1000 s light vs vehicle (45 min incubation) plus 500 s blue light; in a parallel‐group trial; up to 4 treatments at 3‐week intervals, assessed 3 and 6 weeks after the final treatment | At 3 weeks after final treatment investigator‐assessed median change in ILs (SD) was ‐18.0 (26.3) in ALA 1000 s, ‐14.0 (26.8) in the ALA 500 s, ‐19.0 (22.8) in the vehicle 1000 s and ‐14.5 (24.0) in the vehicle 500 s group; investigator‐assessed median percentage change in ILs (SD) was ‐37.5 (38.79) in ALA 1000 s, ‐29.2 (46.68) in the ALA 500 s, ‐41.7 (38.82) in the vehicle 1000 s and ‐37.0 (40.23) in the vehicle 500 s group. At 6 weeks after final treatment investigator‐assessed median change in ILs (SD) was ‐18.5 (30.15) in ALA 1000 s, ‐13.0 (28.74) in the ALA 500 s, ‐21.0 (23.63) in the vehicle 1000 s and ‐17.0 (26.71) in the vehicle 500 s group; investigator‐assessed median percentage change in ILs (SD) was ‐34.4 (37.8) in ALA 1000 s, ‐29.0 (42.57) in the ALA 500 s, ‐48.4 (32.81) in the vehicle 1000 s and ‐45.2 (50.15) in the vehicle 500 s group. Statistical tests to determine whether any changes were significant could not be performed due to the study authors’ use of median changes rather than the typical mean changes required for significance testing in order to make appropriate comparisons with other included studies. Furthermore, it is not clearly stated whether the study authors implemented an ITT analysis or a LOCF approach to handling missing data. |
| ALA‐PDT versus IPL alone | |||
| Oh 2009 | 20 (4 M, 16 F) , aged 18‐30 years, 9 in the short incubation group (3 M, 6 F, mean age ± SD 23 ± 4.12 years) and 10 in the long incubation group (1 M, 9 F and 23 ± 5.53 years), with moderate and severe acne (Evaluator Global Severity Score 3 and 4); FPT II‐IV | 20% ALA plus 590 nm IPL; 2 parallel groups: short incubation (30 min) vs long incubation (3 h), one half of the face within each treated with IPL alone; 3 treatments at 4‐week intervals, assessed 4 weeks after each treatment and 8 and 12 weeks after the third treatment | Mean reduction of ILs 84.4% in the long‐incubation‐time group, 72.6% in the short‐incubation‐time group and 65.9% on the face sides treated with IPL alone at 4 weeks (P < 0.001 in all cases). Mean reduction of ILs 89.5% in the long‐incubation‐time group, 83.0% in the short‐incubation‐time group and 74.0% on the face sides treated with IPL alone at 12 weeks (P < 0.001 in all cases). Mean reduction significantly greater in the long‐incubation sides versus the IPL‐alone sides (P = 0.01). The difference was not statistically significant between short‐incubation and placebo‐treated sides (P = 0.21). Further data not given |
| Mei 2013 | 41 (24 M, 17 F), mean age 24 years, 21 in the ALA‐IPL PDT group, 20 in the placebo cream‐IPL group, II–IV Pillsbury grade acne; FPT II‐IV | 10% ALA plus 420–950 nm light versus placebo cream plus 420–950 nm light in a parallel‐group trial, 4 treatments in total, weekly, assessed 4, 8 and 12 weeks after treatment | ILs counts (% mean ± SE) reduced by 76.3 ± 3.7, 81.5 ± 4.6 and 83.6 ± 4.1 at 4, 8 and 12 weeks after final treatment respectively in the ALA‐IPL group and by 64.9 ± 4.1, 68.3 ± 4.4 and 69.8 ± 4.6 respectively in the IPL‐only group. Mean NILs counts (% mean ± SE) reduced by 44.9 ± 5.2, 49.9 ± 6.6 and 57.5 ± 6.8 at 4, 8 and 12 weeks after final treatment respectively in the ALA‐IPL group and by 29.3 ± 5.6, 30.7 ± 6.7 and 30.7 ± 6.7 in the IPL only group respectively. Our analyses based on t‐distribution showed that ALA‐PDT was superior to light alone in percentage changes in ILs, with mean differences (95% CI) of 13.80 (1.34, 26.26), P = 0.04, t = 2.240 and in percentage changes in NILs, with MDs (95% CIs) of 24.10 (4.65, 43.55), P = 0.02, t = 2.506. Analyses based on normal distribution showed similar results; ALA‐PDT was superior to light alone in percentage changes in ILs, with mean differences (95% CI) of 13.80 (1.72, 25.88), P = 0.03 and in percentage changes in NILs, with MDs (95% CIs) of 24.10 (5.25, 42.95), P = 0.01 |
| Ragab 2014 | 25 (1 M, 24 F), age 14‐39 years, 15 in the ALA‐IPL group (mean 19.7) and 10 in the IPL alone group (mean age 19.0), "with mild‐moderate facial acne" ; FPT III‐V | 20% ALA plus 560? nm IPL versus 560 nm IPL alone; in a parallel‐group trial; 2 treatments at 2‐week intervals, assessed 2 and 8 weeks after final treatment | Mean ILs counts decreased from baseline 15.7 to 7.7 and 5.4 at 2 and 8 weeks respectively in the ALA‐IPL group; and from baseline 9.6 to 5.2 and 4.4 at 2 and 8 weeks respectively in the IPL‐alone group. Mean NILs (comedones) counts decreased from baseline 50.9 to 36.9 and 31.3 at 2 and 8 weeks respectively in the ALA‐IPL group; and from baseline 41.8 to 23.8 and 24.4 at 2 and 8 weeks respectively in the IP‐ alone group. Mean combined lesion counts decreased from baseline 66.6 to 35.7 at 8 weeks in the ALA‐IPL group; and from baseline 51.4 to 28.8 at 8 weeks in the IPL‐alone group. SDs were not reported. Mean percentage reductions from baseline at 8 weeks in ALA‐IPL group compared with IPL alone were reported to be 73.4 versus 18.9% (P = 0.012) for ILs, 33.6 versus 29.8% (P = 0.739) for NILs (comedones) and 45.6 versus 27.8% (P = 0.202) for combined lesion counts respectively. |
| ALA‐PDT versus no treatment | |||
| Orringer 2010 | 99 screened, 44 enrolled (14 M, 30 F), age range 15‐50, mean age 25, all with clinically evident facial acne, all FPT included | 20% ALA plus PDL compared with no treatment in a split‐face trial, 3 treatments at 2‐week intervals, evaluated every 2 weeks for a total of 16 weeks. | No statistically significant differences reported between treated and untreated control skin in papules (P = 0.62), pustules (P = 0.85), cysts (P = 0.49), closed (P = 0.21) and open comedones (P = 0.27) at week 16. Transient statistically significant decrease from baseline in mean papule counts on treated sides when compared with untreated sides (P = 0.01) at week 10. No statistically significant difference between treated and untreated control sides in all other lesion counts at week 10. At week 12 mean changes from baseline (95% CIs) in papules, pustules, cysts, closed and open comedones were ‐1.79 (‐5.98 to 2.39), ‐2.72 (‐6.65 to 1.20), 0.38 (‐0.20 to 0.96), ‐6.97 (‐13.30 to ‐0.63) and ‐4.79 (‐11.62 to 2.04) on the treated sides respectively, and ‐0.97 (‐4.32 to 2.39), ‐2.62 (‐6.25 to 1.01), 0.24 (‐0.33 to 0.82), ‐4.07 (‐9.12 to 0.98) and ‐6.79 (‐13.88 to 0.29) on the untreated sides respectively. Our analyses using LOCF data (n = 44) confirmed transient statistically significant decrease from baseline in investigator‐assessed change in ILs (papules) on treated sides when compared with untreated sides at week 10 of the study (i.e. 4 weeks after final treatment), with MD ‐4.50 (95% CI ‐8.28 to ‐0.72), P = 0.02. We found no significant differences in means between treated and untreated face sides for investigator‐assessed change in ILs (pustules) ‐0.60 (‐5.09, 3.89), P = 0.79, for investigator‐assessed change in NILs (open comedones) ‐0.37 (‐7.76, 7.02), P = 0.92, for investigator‐assessed change in NILs (closed comedones) ‐3.90 (‐12.05, 4.25), P = 0.35, and for cysts 0.03 (‐0.53, 0.59), P = 0.92. Our analyses also confirmed no significant differences in means between treated and untreated face sides at week 16 (i.e. 10 weeks after final treatment), MD (95% CIs) for investigator‐assessed change in ILs (papules) was ‐0.82 (‐6.03, 4.39), P = 0.76, for investigator‐assessed change in ILs (pustules) ‐0.10 (‐5.29, 5.09), P = 0.97, for investigator‐assessed change in NILs (open comedones) 2.00 (‐7.51, 11.51), P = 0.68, for investigator‐assessed change in NILs (closed comedones) ‐2.90 (‐10.78, 4.98), P = 0.47, and for cysts 0.14 (‐0.66, 0.94), P = 0.73. Please note that we based all the calculations from the values provided in the table reported, and we double and triple checked the values using both RevMan and R statistical software, but some of our P values did not match up with the ones presented by the study authors. |
| Pollock 2004 | 10 (9 M, 1 F) age 16–40 years (mean 26.9) with mild‐moderate acne of the back, Leeds grades 2‐4; FPT I‐V | Four equal 30 cm² areas on the back: 635 nm light plus ALA vs light alone; ALA alone; untreated control; treated weekly for 3 weeks, assessed at each treatment and 3 weeks after final treatment | Statistically significant reduction from baseline in ILs counts from second treatment (P < 0.005) at the ALA–PDT site but not the other sites: reduction in acne was 69% at 21 days' follow‐up. Further data reported in graph format, mean ILs at baseline 11.6 and 10.1 at ALA‐PDT and no treatment control areas respectively decreased to 3.6 and 6.3 respectively at 3 weeks' follow‐up. Other data not given |
| ALA‐PDT other | |||
| Barolet 2010 | 10 (7M, 3F, aged 13‐54, mean age 26.2), with mild‐moderate acne, with ≥10 acne lesions, FPT I‐III | 970 nm IR pre‐treatment plus ALA and 630 nm PDT vs ALA‐PDT alone, 1 treatment in a split‐face or split‐back design, evaluated after 4 weeks | Significantly greater improvement in IL medians on the IR pre‐treated versus control side 4 weeks after treatment (P < 0.0001). Median percentage reduction (95% CI for mean?) in ILs was 73% (51% to 81%) on the IR pre‐treated side versus 38% (8% to 55%) on the control side. Further data not provided, 95% CI reported for means, but means were not given |
| NCT00706433 | 266 (128 M, 138 F), 68 in the ALA 1000 s group, 65 in the ALA 500 s group, 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group, mean age 20.1 years, inclusion criterion 12 > years, with moderate and severe acne (IGA score 3 and 4, with at least 20 ILs); FPT I‐V | 20% ALA (45 min incubation) plus blue 1000 s light vs 20% ALA (45 min incubation) plus 500 s blue light vs vehicle (45 min incubation) plus blue 1000 s light vs vehicle (45 min incubation) plus 500 s blue light; in a parallel‐group trial; up to 4 treatments at 3‐week intervals, assessed 3 and 6 weeks after the final treatment | At 3 weeks after final treatment investigator‐assessed median change in ILs (SD) was ‐18.0 (26.3) in ALA 1000 s, and ‐14.0 (26.8) in the ALA 500 s, ‐19.0 (22.8) group; investigator‐assessed median percentage change in ILs (SD) was ‐37.5 (38.79) in ALA 1000 s and ‐29.2 (46.68) in the ALA 500 s group. At 6 weeks after final treatment investigator‐assessed median change in ILs (SD) was ‐18.5 (30.15) in ALA 1000 s and ‐13.0 (28.74) in the ALA 500 s group; investigator‐assessed median percentage change in ILs (SD) was ‐34.4 (37.8) in ALA 1000 s and ‐29.0 (42.57) in the ALA 500 s group. Statistical tests to determine whether any changes were significant could not be performed due to the study authors’ use of median changes rather than the typical mean changes required for significance testing in order to make appropriate comparisons with other included studies. Furthermore, it is not clearly stated whether the study authors implemented an ITT analysis or a LOCF approach to handling missing data. |
| Pollock 2004 | 10 (9 M, 1 F) age 16–40 years (mean 26.9) with mild‐moderate acne of the back, Leeds grades 2‐4; FPT I‐V | Four equal 30 cm² areas on the back: 635 nm light plus ALA vs light alone; ALA alone; untreated control; treated weekly for 3 weeks, assessed at each treatment and 3 weeks after final treatment | Statistically significant reduction from baseline in ILs counts from second treatment (P < 0.005) at the ALA–PDT site but not the other sites: reduction in acne was 69% at 21 days' follow up. Further data reported in graph format, mean ILs at baseline 6.6 and 11.6 at light‐alone, ALA‐alone, ALA‐PDT and no‐treatment control areas respectively decreased to 4.6 and 3.6 respectively at 3 weeks follow‐up. Other data not given |
| Taub 2007 | 22 recruited, 19 participated, mean ± SD age 26.5 ± 9.1 years, 7 M, 12 F, with moderate‐severe acne and > 10 inflammatory acne lesions; FPT not given | Comparison of PDT with different light sources for activation: ALA activated by IPL (600–850 nm), or a combination of IPL (580–980 nm) and bipolar radiofrequency energies, or blue light (417 nm) in a parallel‐group trial; 3 treatments at 2‐week intervals; follow‐up at 1 and 3 months after final treatment | Reductions in counts in all 3 groups, highest in the IPL activation group and lowest in the blue‐light group, but the difference was not statistically significant (P values not given). Median lesion count percentage reductions (96.9% CI) at 1 month after treatment were 76.8 (12.5 to 86.4) in the IPL group, 47 (8.3 to 82.2) in the IPL‐RF group and 52.8 (‐88.9 to 66.7) in the blue‐light group. At 3 months after treatment, median lesion count percentage reduction (range, defined as "difference between the upper and lower ends of 96.9% CI, indicated when <5 data points are available") was 73.2 (72.4) in the IPL group, 41.6 (167.5%) in the IPL‐RF group and ‐88.9 (123.3) in the blue‐light group |
| Yin 2010 | 180 ( 83 M, 97 F), aged 18‐38 years, mean 25.8, with moderate‐severe facial acne (Pillsbury), FPT III‐IV | 633 ± 3 nm (red light) plus different ALA concentrations (5%, 10%, 15% and 20%) vs red light alone, 4 treatments every 10 days, 4 parallel groups, each treated with a different concentration on the right side and placebo agent on the left side; assessments at 2, 4, 12 and 24 weeks after last treatment | Greater reduction in both IL and NIL counts at sides treated by ALA‐PDT of all concentrations compared with the controls treated by red light alone at 2 weeks (P < 0.001), 4 weeks (P < 0.05), 12 weeks (P < 0.001) and 24 weeks (P < 0.001). Combined data from all follow‐up visits, the higher‐concentration ALA treatment groups showed more improvement than the lower‐concentration groups (P<0.01). Means (SD) reported in graph format only. Our interpretation of the graph was that ILs reduced from baseline 21 (5), 20.5 (5.5), 19 (5), 21 (5) and 20 (4) in the 20% ALA group, 15% ALA group, 10% ALA group, 5% ALA group and control face sides respectively to 1 (0.5), 1.3 (0.5), 3.3 (1), 4 (1) and 5 (1) in the 20% ALA group, 15% ALA group, 10% ALA group, 5% ALA group and control face sides respectively. NILs reduced from baseline 12.9 (4.5), 13 (3.5), 13 (4), 12.5 (3.5) and 11.5 (4) in the 20% ALA group, 15% ALA group, 10% ALA group, 5% ALA group and control face sides respectively to 1.4 (1), 1.4 (0.5),1.5 (0.5), 2.5 (0.5) and 5.5 (1.5) in the 20% ALA group, 15% ALA group, 10% ALA group, 5% ALA group and control face sides respectively at 24 weeks after final treatment. We judged further analyses would be biased due to lack of precise data, so we did not perform them. |
| 6. MAL‐PDT versus ALA‐PDT | |||
| Wiegell 2006a | 15 participants > 18 years but age range not given, with > 12 inflammatory acne lesions; FPT not stated | Comparison of MAL and ALA creams: 620 nm light with split‐face design; one full‐face PDT treatment with MAL on one side and ALA on the other, assessed at 6 and 12 weeks after treatment | No significant differences in reductions of ILs between ALA‐ and MAL‐treated sides at 6 weeks' (P = 0.061) and 12 weeks' (P = 0.08) follow‐up. Baseline differences in ILs counts (P = 0.0049). Median ILs counts (inter‐quartile range) at baseline, 6 and 12 weeks after treatment were 19 (13 to 27), 8 (6 to 14) and 8 (3 to 11) on the MAL‐treated sides and 16 (11 to 22), 5 (3 to 11) and 5 (3 to11) on the ALA‐treated sides respectively. No significant differences in reductions of NIL between ALA‐ and MAL‐treated sides at 6 weeks' (P = 0.18) and 12 weeks' (P = 0.052) follow‐up. Median NILs counts (inter‐quartile range) at baseline, 6 and 12 weeks after treatment were 14 (6 to 22), 21 (17 to 31) and 17 (9 to 29) on the MAL‐treated sides and 17 (7 to 21), 18 (13 to 29) and 20 (17 to 38) on the ALA‐treated sides respectively. |
| 7. Other (non‐MAL, non‐ALA) PDT versus other comparators | |||
| Indocyanine green‐PDT | |||
| Genina 2004 | 12 (5 M, 7 F) aged 17‐27 years (mean age not given) with light‐severe acne on the face or back; FPT not given | 803 nm low‐intensity diode laser ± indocyanine green (ICG), single (8 participants) and multiple (4 participants) treatment groups, multiple treatment group had 2 treatments weekly for 4 weeks, assessed 1 week and 1 month after treatment | IL counts improved by 23% at 4 weeks for the single treatment groups and by 7% for control at ICG plus light sites; 80% improvement at 4 weeks for the multiple treatment group versus no improvement for control. More improvement was seen in participants with severe acne. |
| Kim 2009 | 16 (7 M, 9 F) aged 16‐34 years, mean age 25 ± 3.09, with mild‐moderate acne, skin types not given, 9 in single, 7 in multiple treatment group, FPT not given | 2 groups randomised: single treatment vs multiple (once‐weekly over 3 weeks); right cheek of each patient ICG plus 805 nm light, left cheek light only and forehead "spontaneous resolution" control, evaluated 2 and 4 weeks after final treatment, multiple group also at final treatment | Significant improvement only in mean number of closed comedones at PDL‐treated side at all assessment periods, and at light‐only side at 4 weeks post‐treatment when compared to "spontaneous resolution" control (P < 0.05 in all cases). ILs improved at all sites, but non significantly (other data not given). Not reported whether there were differences between the two groups. Further data not given and part of the results reported in graph format. Our interpretation of the graph was that mean counts of closed comedones reduced from baseline 15 to 9 on the PDT sides and from 16 to 14 on the light‐only sides respectively at final evaluation in the single treatment group, and from baseline 12 to 8 on the PDT sides and from 13 to 10 on the light‐only sides in the multiple treatment group respectively. |
| Indole 3‐acetic acid (IAA)‐PDT | |||
| Na 2011 | 14 participants with inflammatory acne, sex, age, acne severity and FPT not given | 520 nm green light plus 0.015% IAA vs placebo cream plus green light, split‐face trial, 3 treatments at 2‐week intervals, assessed 0, 2, 4 and 6 weeks of treatment | Improvement in ILs count was observed on both sides. Difference between treatment and control group statistically significant from week 4 after final treatment (P < 0.05). Further data not given and reported in graph format. Our interpretation of the graph was that mean? ILs counts reduced from baseline 16.5 to 15.2 on the control sides, and from 16.3 to 14 on the treatment sides |
| Topical liposomal methylene blue (TLMB)‐PDT | |||
| Fadel 2009 | 20 (M/F not stated), age not stated (> 18 years), with mild‐moderate acne, FPT not given | TLMB plus 650 nm light vs no treatment in a split‐face trial, 2 treatments in total, weekly, assessed every 2 weeks for 3 months after treatment. | At 4 weeks IL counts decreased by 83.3% and NILs by 63.6% on the treated sides. Results for control sides not reported in narrative form. At 12 weeks reduction was also significant for ILs (P < 0.01) and NILs (P < 0.01). Further data not given |
| Chlorophyll‐a (CHA)‐PDT | |||
| Song 2014 | 24 (14 M, 10 F), mean age 23.4 ± 3.5 years; range 18‐32 years, "acne on both sides of the face", Cunliffe grades 2‐4, FPT III‐IV | 430 plus 660 nm light combined with CHA vs 430 plus 660 nm light alone in a split‐face trial, 8 treatments in total, twice weekly, final assessment 2 weeks after last treatment | 2 weeks after final treatment papule counts reduced from baseline 13.0 to 5.1 on the CHA plus light sides and from baseline 13.1 to 8.6 on the light‐only sides (P = 0.030, SDs not given); pustule counts reduced from baseline 3.8 to 1.3 on the CHA‐plus‐light sides and from baseline 4.2 to 3.0 on the light‐only sides (P < 0.001, precise P value not given, SDs not given); open comedone counts reduced from baseline 9.0 to 4.2 on the CHA‐plus‐light sides and from baseline 9.1 to 6.7 on the light‐only sides (P = 0.011, SDs not given); closed comedones counts reduced from baseline 18.4 to 8.5 on the CHA‐plus‐light sides and from baseline 18.4 to 13.3 on the light‐only sides (P = 0.014, SDs not given); nodules & cyst counts reduced from baseline 0.6 to 0.1 on the CHA‐plus‐light sides and from baseline 0.55 to 0.3 on the light‐only sides (P value not given, data extracted from figure). Further data were not given |
| Gold microparticle PDT versus other comparators | |||
| Paithankar 2015 | 51 (14 M, 37 F), mean age 21.4 years, age range 16‐26 years, IGA scores 3–4 with at least 25 total papules and pustules on face, FPT I‐III | Gold microparticle suspension plus light (details not given) vs microparticle suspension vehicle (without light‐absorbing particles) plus light (details not given) in a parallel‐group trial, 3 treatments in total, weekly, assessed at 6, 10 and 14 weeks after final treatment | At 6 weeks after final treatment, the mean percentage change in inflammatory lesion count was −44.0% and −14.0% for the active treatment and sham arms, respectively. At 10 weeks after final treatment, the mean percentage change in inflammatory lesion count was −49.0% and −21.7% for the active treatment and sham arms, respectively (P = 0.015). At 14 weeks after final treatment changes were −53% and −30% for the active treatment and sham arms, respectively (P = 0.04). Other data were not given |
ALA = 5‐aminolevulinic acid BPO = benzoyl peroxide CHA = chlorophyll‐a FPT = Fitzpatrick's Skin Types: based on different reactions to sun exposure and range from type I ('pale white skin which always burns and never tans') to type VI ('deeply pigmented dark brown to black skin which never burns and tans very easily') (Fitzpatrick 1988) GAAS = Global acne assessment scoring scale IAA = indole 3‐acetic acid IGA = Investigator global assessment score ILs = inflamed lesions IPL = intense pulsed light IR = infrared ITT = intention‐to‐treat analysis LPDL = long pulsed dye laser LOCF = last observation carried forward LLT = lower level term MAL = methyl‐aminolevulinate NILs = non‐inflamed lesions NNTB = number needed to treat for an additional beneficial outcome OFI = optical fibre intra‐tissue irradiation PDL = pulsed‐dye laser PDT = photodynamic therapy PT = preferred term RCT = randomised controlled trial SD = standard deviation SE = standard error SPF = Sun protection factor TER = total effective rate TLMB = topical liposomal methylene blue
Change from baseline i.e. absolute change is calculated by subtracting baseline count from count assessed at certain time point. Percentage change is calculated by dividing the absolute change with baseline count and then multiplying that value by 100 to get percentages.
Unless specified differently, results presented as reported in the published papers, without performing independent analysis. Please see Characteristics of included studies for details on withdrawals and drop‐outs of participants for each study.
4. Adverse effects.
| Study | SOC skin and subcutaneous tissue disorders | SOC general disorders and application site conditions | Other SOCs |
| 1. Light versus placebo or no treatment | |||
| Green light versus placebo | |||
| Baugh 2005 | None reported | None reported | None reported |
| Yilmaz 2011 | None reported | None reported | None reported |
| Yellow light versus placebo or no treatment | |||
| Seaton 2003 | In the yellow‐light group: 2/31 (6.4%) pain of skin, 1/31 (3.2%) purpura, 1/31 (3.2%) pruritus, 2/31 (6.4%) dry skin. In the placebo group: 1/10 (10%) pruritus, 2/10 (20%) dry skin | None reported | In the yellow‐light group: SOC Eye disorders: 1/31 (3.2%) lacrimation increased |
| Infrared light (IR) versus no treatment | |||
| Darne 2011 | None reported | Application site erythema in "most" of 38 participants | None reported |
| Moneib 2014 | 2/24 (83%) dry skin (in the nasolabial fold), 2/24 (83%) pustular rash (LLT pustular skin eruption). Unclear whether treated or untreated face sides | Application site erythema, and "decreased oiliness" in "all" of 24 participants. Unclear whether treated or untreated face sides | None reported |
| Orringer 2007 | IR sides: 2/46 (4.4%) post‐inflammatory pigmentation change (LLT post‐inflammatory hyperpigmentation) | IR sides: application site discomfort: 34/46 (74%) moderate, 12/46 (24%) substantial causing 2 withdrawals; 2/46 (4.4%) application site vesicle (LLT application site blister)2 | IR sides: SOC Psychiatric disorders: 1/46 (2.2%) panic attack, caused one withdrawal |
| Blue light versus placebo or no treatment | |||
| Elman 2003 | None reported | None reported | None reported |
| Red light versus no treatment | |||
| Na 2007 | None reported | None reported | Red‐light sides: SOC Nervous systems 1/30 (3.3%) burning sensation |
| Blue‐red light versus placebo | |||
| Papageorgieu 2000 | In the blue‐red light group: 2/30 (6.6%) acne (LLT acne exacerbation), 1/30 (3.3%) dry skin and pruritus, 2/30 (6.6%) rash (LLT facial rash). In the placebo group: 2/25 (8%) acne (LLT acne exacerbation), 2/25 (8%) dry skin and pruritus | None reported | In the blue‐red light group: SOC Nervous system disorders: 1/30 (3.3%) headache |
| Kwon 2013 | 2/18 (11%) dry skin 1/18 (6%) erythema and skin exfoliation (unclear in which group) | None reported | None reported |
| Broad spectrum light versus placebo | |||
| Sadick 2010b | None reported | None reported | None reported |
| IPL versus no treatment | |||
| McGill 2008 | 1/10 (10%) acne (LLT acne exacerbation), on both IPL and control sides, reported as "A further patient experienced an acne flare‐up following the first treatment. However, this was bilateral and so was felt to be unrelated to the IPL treatment." | IPL sides: 1/10 (10%) application site vesicle (LLT application site blister); "One patient developed minor blistering after the fifth treatment, which resolved without scarring. This occurred in areas where double passing treatment was carried out, and were most likely due to the second pass taking place too quickly after the first." | None reported |
| 2. Light versus topical treatment | |||
| Light versus benzoyl peroxide (BPO) | |||
| de Arruda 2009 | In the BPO group: 28/30 (93.3%) "some level of erythema, desquamation, dryness or burning". In the blue‐light group: 7/30 (23.3%) skin exfoliation and dry skin, reported as "all of mild intensity". | None reported | None reported |
| Chang 2007 | Post‐inflammatory pigmentation change (LLT post‐inflammatory hyperpigmentation) 3/30 (10%) (unclear on which face‐sides) | None reported | None reported |
| Papageorgieu 2000 | In the blue‐red light group: 2/30 (6.6%) acne (LLT acne exacerbation), 1/30 (3.3%) dry skin and pruritus, 2/30 (6.6%) rash (LLT facial rash). In the BPO group: 2/25 (8%) acne (LLT acne exacerbation), 8/25 (32%) dry skin and pruritus, 2/25 (8%) rash (LLT facial rash) | None reported | In the blue‐red light group: SOC Nervous system disorders: 1/30 (3.3%) headache |
| Light versus clindamycin | |||
| Gold 2005 | None reported | None reported | None reported |
| Lee 2010 | No "significant" adverse effects | No "significant" adverse effects | No "significant" adverse effects |
| Light and other topical treatments | |||
| Ash 2015 | None reported | None reported | None reported |
| Ianosi 2013 | 11/180 (6%) scab, reported as "Eleven patients with dark III and IV phototypes presented with hematic crusts", unclear in which group, 34/60 (57%) in the vacum‐IPL group and 4/60 (7%) reported as "sebum secretion increase" | Application site erythema in light treatment groups, reported as "persistent erythema during a period of 24 h was noted in almost all patients", lasting for 72 h in 10/60 (17%) in the vacum‐IPL group and 3/60 (5%) in the IPL‐only group. 12/60 (20%) application site ecchymosis in the vacum‐IPL group | None reported |
| Karsai 2010 | In the C/BPO plus laser group: 1/51 (2%) purpura; reported as "one case of mild purpura lasting 3 days (incidence 2%)" | None reported | None reported |
| Zhang 2009a | None reported | In the blue and red light in combination with antibiotics group: 120/508 (23.6%) application site discomfort (reported as "participants from the blue and red light in combination with antibiotics found the red light ‘too intense’"; exact effects not specified); 2/508 (0,4%) withdrew due to application site discomfort |
None reported |
| 3. Light versus other comparators | |||
| Comparison of light therapies of different wavelengths | |||
| Choi 2010 | None reported | Mild application site oedema, mild application site erythema (unclear on which face sides, exact numbers not given) | None reported |
| Jung 2009 | Pain of skin, dry skin, skin exfoliation; reported as "one patient reported mild dryness that disappeared after a few days. All participants tolerated pain well" (unclear whether PDL or combined 585/1,064 nm laser‐treated sides) | Application site erythema, reported as "All patients reported mild erythema" (unclear whether PDL or combined 585/1,064 nm laser‐treated sides). | None reported |
| Liu 2011 | Dry skin. Reported as "A few patients stated certain dryness of skin after exposure to light sources for 20‐min session… The result (shown in Fig. 5) demonstrated that there was no obvious change in skin color." Further details not given | None reported | None reported |
| Liu 2014 | None reported | In the IPL group: 28/50 (56%) application site erythema. In the LED group: 3/50 (6%) application site erythema | SOC Nervous system disorders: 28/50 (56%) in the IPL group and 3/50 (6%) in the LED group paraesthesia (reported as "slight stinging sensations immediately after the procedures that disappeared within approximately 2 h") |
| Papageorgieu 2000 | In the blue‐red light group: 2/30 (6.6%) acne (LLT acne exacerbation), 1/30 (3.3%) dry skin and pruritus, 2/30 (6.6%) rash (LLT facial rash). In the blue light‐only group: 3/27 (11.1%) acne (LLT acne exacerbation), 3/27 (11.1%) dry skin and pruritus, 1/27 (3.7%) rash (LLT facial rash) | None reported | In the blue‐red light group: SOC Nervous system disorders: 1/30 (3.3%) headache |
| Sami 2008 | In the PDL group: 3/15 (20%) post‐inflammatory pigmentation change (LLLT post‐inflammatory hyperpigmentation), "mild purpura" (participants' numbers unclear) | In the PDL group: application site discomfort (participants' numbers unclear). In the IPL group: application site erythema (participants' numbers unclear). In the LED group application site warmth (participants' numbers unclear). | In the IPL group SOC Nervous system disorders: paraesthesia (participants' numbers unclear) |
| Comparison of light therapies of different doses | |||
| Bernstein 2007 | None reported | Application site oedema 12/30 (41.6%) on the single‐pass side and 7/30 (23%) on the double‐pass side; application site erythema 30/30 (100%) on both sides | None reported |
| Jih 2006 | None reported | Application site oedema, application site erythema, pain of skin ("most common side effects", numbers not given) | None reported |
| Uebelhoer 2007 | None reported | 11/11 (100%) application site oedema and 11/11 (100%) application site erythema on both sides. On the single‐pass side: 1/11 (9%) application site discolouration (LLT Application site hyperpigmentation).1/11 (9%) application site vesicle (LLT application site blister) reported as "We also experienced a cryogen failure that resulted in a single blister that resolved completely with proper wound care" | None reported |
| Light alone versus combined with microdermoabrasion | |||
| Wang 2006 | Pain of skin (numbers reported unclearly), 1/20 (5%) post‐inflammatory pigmentation change (post inflammatory hypopigmentation) on the laser plus microdermoabrasion side | Application site oedema, application site erythema, application site papules (numbers reported unclearly) | None reported |
| Light in combination with carbon lotion versus no treatment | |||
| Jung 2012 | Mild pain of skin (numbers reported unclearly), 15/22 (75%) dry skin, skin exfoliation reported as "mild dryness and desquamation of the treated side" | 22/22 (100%) application site erythema | None reported |
| Light in combination with oral therapy versus other comparators | |||
| Ou 2014 | In the Yinhua decoction with electric light synergy group: 1/43 who completed (2.3%) dry skin; in the Yinhua decoction in combination with red and blue light treatment group 7/40 who completed (17.5%) dry skin and pruritus. Number of participants randomised to each group unclear | None reported | In the Yinhua decoction in combination with red and blue light treatment group: SOC Gastrointestinal disorders 1/40 who completed (2.5%) LLT Diarrhoea (reported as: "after having yinhua concoction – side effects subsided after participant changed to having yinhua concoction after meals"). None reported in the intervention group |
| Zhang 2013b | None reported | In the red‐blue combined with jinhua xiaocuo pills and chloramphenicol tincture group 2/60 (3.3%) subjects reported "mild facial erythema, itching and scaling". No adverse effects were reported in the jinhua xiaocuo pills and chloramphenicol tincture alone group | None reported |
| 4. MAL‐PDT versus other comparators | |||
| MAL‐PDT versus red light alone | |||
| NCT005944251 | None reported | In the 40 mg/g MAL plus red light group: 3/50 (6%) application site discolouration, 1/50 (2%) application site dryness, 40/50 (80%) application site erythema, 3/50 (6%) application site exfoliation, 31/50 (62%) application site irritation, 32/50 (64%) application site pain, 3/50 (6%) application site paraesthesia, 13/50 (26%) application site pruritus, 3/50 (6%) application site scab, 2/50 (4%) application site warmth. In the 80 mg/g MAL plus red light group: 7/48 (16%) application site discolouration, 3/48 (6%) application site dryness, 35/48 (73%) application site erythema, 3/48 (6%) application site exfoliation, 26/48 (54%) application site irritation, 31/48 (65%) application site pain, 9/48 (19%) application site paraesthesia, 10/48 (21%) application site pruritus, 3/48 (6%) application site scab, 0/48 (0%) application site warmth. In the placebo cream plus red light group: 3/52 (6%) application site discolouration, 0/52 (0%) application site dryness, 17/52 (33%) application site erythema, 0/52 (0%) application site exfoliation, 4/52 (8%) application site irritation, 5/52 application site pain, 4/52 (8%) application site paraesthesia, 5/52 (10%) application site pruritus, 0/52 (0%) application site scab, 4/52 (8%) application site warmth. We only included treatment‐related adverse effects in this table. Frequency threshold above which adverse effects were reported was 2%. Sponsors confirmed that there were no reports of application site blisters. | In the 40 mg/g MAL plus red light group: SOC Nervous system disorders 2/50 (4%) headache. In the 80 mg/g MAL plus red light group: SOC Nervous system disorders: 2/48 (4%) headache. In the placebo cream plus red light group: SOC Nervous system disorders 0/52 (0%) headache |
| NCT009335431 | In the 80 mg/g MAL plus red light group: 26/54 (48%) erythema, 14/54 (26%) pruritus, 12/54 (23%) skin burning sensation, 4/54 (7.4%) skin irritation. In the placebo cream plus red light group: 9/53 (17%) erythema, 8/53 (16%) pruritus, 4/53 (8%) skin burning sensation, 0/54 (0%) skin irritation | In 80 mg/g MAL plus red light group: 4/54 (7%) facial pain, 2/54 (4%) "feeling hot", 27/54 (50%) pain. In placebo cream plus red light group: 4/53 (8%) facial pain, 2/53 (4%) 'feeling hot', 6/53 (11%) pain. Sponsors confirmed that there were no reports of application site blisters | In 80 mg/g MAL plus red light group: SOC Infections and infestations: 10/54 (19%) nasopharyngitis, SOC Nervous disorders: 9/54 (17%) paraesthesia. In placebo cream plus red light group: SOC Infections and Infestations: 5/53 (9%) nasopharyngitis, SOC Nervous disorders: 1/53 (2%) paraesthesia |
| Pariser 20131 | In the 80 mg/g MAL plus red light group: 17/100 (17%) pain of skin, 15/100 (15%) skin burning sensation, 8/100 (8%) pruritus, 4/100 (4%) erythema, 2/100 (2%) rash, 2/100 (2%) scab, 2/100 (2%) skin hyperpigmentation. In the placebo cream plus red light group: 0/53 (0%) pain of skin, 0/53 (0%) skin burning sensation, 1/53 (2%) pruritus, 0/53 (0%) erythema, 1/53 (2%) rash, 0/53 (0%) scab, 0/53 (0%) skin hyperpigmentation. Data provided by sponsors for adverse events experienced by more than one participant in each treatment group | In the 80 mg/g MAL plus red light group: 1/100 (1%) application site blister. In the placebo cream plus red light group: None reported | In 80 mg/g MAL plus red light group: SOC Musculoskeletal and connective tissue disorders: 2/100 (2%) back pain. In placebo cream plus red light group: None reported. We only included treatment‐related adverse effects in this table |
| NCT006739331 | On the MAL‐PDT area: 4/20 (20%) erythema, 1/20 (5%) pain of skin, 5/20 (25%) pruritus, 4/20 (20%) skin burning sensation, 7/20 (35%) skin warmth. On the placebo cream plus red light area: 0/20 (0%) erythema, 0/20 (0%) pain of skin, 1/20 (5%) pruritus, 4/20 (20%) skin burning sensation, 5/20 (25%) skin warmth. Frequency threshold above which adverse effects were reported was 5% | None reported. Sponsors confirmed that there were no reports of application site blisters | On the MAL‐PDT area: SOC Nervous system disorders: 1/20 (5%) paraesthesia. SOC Vascular disorders: 1/20 (5%) hematoma. On the placebo cream plus red light area: SOC Nervous system disorders: 3/20 (15%) paraesthesia. SOC Vascular disorders: 1/20 (5%) haematoma |
| Hörfelt 2006 | 9/30 (30%) pain of skin (unclear on which face side) | 8/30 (27%) application site erythema, 5/30 (17%) application site oedema (unclear on which face side); 1/30 application site blister (3%) on the MAL‐PDT side | None reported |
| MAL‐PDT versus yellow light alone | |||
| Haedersdal 2008 | On the MAL‐LPDL side 15/15 (100%) pain of skin, 1/15 (6.6%) MAL‐LPDL scab (LLT crust). On the LPDL only side: 4/15 (26.6%) pain of skin | 15/15 (100%) MAL‐LPDL, 12/15 (80%) LPDL application site erythema; 15/15 (100%) MAL‐LPDL, 4/15 (26.6%) LPDL application site oedema; 12/15 (80%) MAL‐LPDL, 5/15 (33.3%) LPDL application site pustules/rash pustular (LLT pustular skin eruption) | None reported |
| (4c) MAL‐PDT versus placebo or no treatment | |||
| Wiegell 2006b | Pain of skin, pustular rash (LLT pustular skin eruption) ("in almost all patients", number of participants unclear); scab (LLT crust) "a third of patients", skin exfoliation ("in some patients"; numbers and groups unclear | Application site erythema, application site oedema (number of participants unclear) | SOC Social conditions: "Approximately half of the patients did not go to school or work for between 1 day and 1 week after treatment due to their appearance" (we were unable to find an appropriate MedDRA PT) |
| MAL‐PDT other | |||
| Bissonnette 20101 | None reported | Please note that total unit of analyses numbers were reported as n = 154 in the 25 J/cm² group, and n = 169 in the 37 J/cm² group. 22 participants randomised to each group initially (2 withdrew after an adverse event from the 25 J/cm² group (first because of a pustular eruption on the face following MAL‐PDT; second due to pain during light exposure). We were unable to obtain further explanations by the study authors. On the non‐occluded 25 J/cm² side: 15/154 (9.7%) erythema, 39/154 pain (25.3%), 2/154 pruritus (1.3%), 1/154 (0.6%) scab, 1/154 (0.6%) pustular eruption and 3/154 (2%) paraesthesia. On the occluded 25 J/cm² face sides: 1/154 (0.6%) dryness, 5/154 (3.2%) erythema (3.2%), 60/154 pain, 2/154 pruritus (1.3%), 1/154 (0.6%) scab, 1/154 (0.6%) pustular eruption, 4/154 (2.6%) paraesthesia and 1/154 (0.6%) desquamation. On the non‐occluded 37 J/cm² group there were 14/169 (8.2%) reports of erythema, 48/169 (28.4%) pain and 6/169 (3.5%) paraesthesia. On the occluded 37 J/cm² face sides 7/169 (4.1%) reports of erythema and 59/169 (35%) pain, 6/169 (3.5%) paraesthesia and 1/169 blister (0.6%). "Other adverse events" 18/154 (11.7%) in the 25 J/cm² group, 28/169 (16.6%) in the 37 Jcm² group. Regarding application site blisters, sponsors provided information that there was "1 report from 44 Visonac treated patients" 1/44 (2%) | None reported |
| Hong 2013 | On the MAL plus 630 nm light side: 1/22 (4.5%) erythema, On the MAL plus 530‐750 nm light side: 1/22 (4.5%) post‐inflammatory pigmentation change (LLT post inflammatory hyperpigmentation) | 22/22 (100%) application site pain (both sides) | None reported |
| Yeung 2007 | 1/11 (9%) scab and skin hyperpigmentation in the MAL PDT group, 2/12 (16%) scab and skin hyperpigmentation in IPL‐only group; dermatitis acneiform (LLT rash acneiform) "in some patients", details not provided. Unclear reporting | Application site stinging, application site oedema, and application site erythema, caused 4? withdrawals ('MAL‐PDT side', details not given) | SOC Nervous system disorders: 4? withdrawals because of paraesthesia (LLTs skin burning sensation). Unclear reporting |
| 5. ALA‐PDT versus other comparators | |||
| ALA‐PDT versus red light alone | |||
| Chen 2015 | None reported | In the ALA‑PDT group: 7/25 (28%) combination of application site erythema, application site oedema, application site pain and application site paraesthesia ("burning sensations"); 3/25 (12%) application site discolouration (LLT application site hyperpigmentation); 2/25 (8%) application site pustules/rash pustular (LLT pustular skin eruption; reported as "Two patients developed small pimples and were diagnosed with acute acneform lesions, which were topically treated successfully with mupirocin ointment". In the control group 2/25 (8%) application site erythema and application site dryness (reported as "two patients experienced flushing and dryness of the face") | None reported |
| Pollock 2004 | On the ALA‐PDT site: 10/10 (100%) urticaria (LLT erythema urticarial, reported as "urticated erythema"), 10/10 (100%) post‐inflammatory pigmentation change (post‐inflammatory hyperpigmentation), resolved within 1 month in 9/10 (90%), and within 3 months in 1/10 (10%), FPT V | On the ALA‐PDT site: 1/10 (10%) application site discomfort | On the ALA‐PDT site: SOC Nervous system disorders: 10/10 (100%) paraesthesia (LLTs skin tingling, tingling sensation, skin burning sensation), 4/10 (40%) perifollicular eruption (we were unable to find an appropriate MedDRA PT) |
| Zhang 2013a | None reported | In the ALA‐PDT group: unclear reporting, 6/63 (9.5%) (LLT application site blister)?, together with varying degrees of application site oedema, application site erythema, application site burning sensation?. Reported as:"Three days after treatment, 6 participants experienced varying degrees of erythema, burning heat sensation, swelling, water blisters. These side effects disappeared after cold compress within 5 to 10 days in 5 of these participants. The side effects in the other participant disappeared 3 days after taking metacortandracin and undergoing cold compress." Not reported for the red‐light‐only group | None reported |
| ALA‐PDT versus blue light alone | |||
| NCT007064331 | None reported | Reported as "Injury, poisoning and procedural complications". In the ALA 1000 s group: "Stinging/Burning" 17/68 (25.00%), "Dry skin" 7/68 (10.29%), "Erythema" 13/68 (19.12%), "Itching of face" 14/68 (20.59%), "Scabbing" 4/68 (5.88%), "Peeling of skin" 6/68 (8.82%), "Tightness of skin" 4/68 (5.88%), "Facial pain" 4/68 (5.88%) In the ALA 500 s group: "Stinging/Burning" 17/65 (26.15%), "Dry skin" 4/65 (6.15%), ‘Erythema’ 5/65 (7.69%), "Itching of face" 20/65 (30.77%), "Peeling of skin" 5/65 (7.69%), "Tightness of skin’ 6/65 (9.23%), ‘Facial pain’ 6/65 (9.23%). In the vehicle 1000 s group: ‘Stinging/Burning’ 6/67 (8.96%), ‘Dry skin’3/67 (4.48%), ‘Erythema’ 1/67 (1.49%), ‘Itching of face’ 6/67 (8.96%), ‘Peeling of skin" 1/67 (1.49%), "Tightness of skin" 2/67 (2.99%). In the vehicle 500 s group:"Stinging/Burning" 5/66 (7.58%), "Dry skin" 4/66 (6.06%), "Erythema" 1/66 (1.52%), "Itching of face" 6/66 (9.09%), "Peeling of skin" 2/66 (3.03%), "Tightness of skin" 1/66 (1.52%)’ | In the ALA 1000 s group: Gastrointestinal disorders:1/68 (1.47%) vomiting; Infections and infestations: 5/68 (7.35%) Upper respiratory tract infection; Nervous system disorders: Headache 5/68 (7.35%); Respiratory, thoracic and mediastinal disorders: Nasopharyngitis 6/68 (8.82%). In the ALA 500 s group Gastrointestinal disorders: 1/65 (1.54%) vomiting; Infections and infestations: 2/65 (3.08%) Upper respiratory tract infection; Nervous system disorders: Headache 4/65 (6.15%); Respiratory, thoracic and mediastinal disorders: Nasopharyngitis 7/65 (10.77%). In the vehicle 1000 s group: Gastrointestinal disorders 1/67 (1.49%) vomiting; Nervous system disorders: Headache 3/67 (4.48%); Respiratory, thoracic and mediastinal disorders: Nasopharyngitis 4/67 (5.97%), Sinus congestion 1/67 (1.49%) In the vehicle 500 s group: 3/66 (4.55%) vomiting; Infections and infestations: 5/66 (7.58%) Upper respiratory tract infection; Nervous system disorders: Headache 2/66 (3.03%); Respiratory, thoracic and mediastinal disorders: Nasopharyngitis 4/66 (6.06%), Sinus congestion 4/66 (6.06%) |
| ALA‐PDT versus blue‐red light alone | |||
| Liu 2014 | In the ALA‐PDT group: 2/50 (4%) post‐inflammatory pigmentation change (LLT post‐inflammatory hyperpigmentation); 10/50 (20%) "brightening of skin tone and improvements of skin texture after treatment" (we were unable to find an appropriate MedDRA PT) | In the ALA‐PDT group 46/50 (92%) combination of application site pain, application site erythema and application site oedema. In the LED group: 3/50 (6%) application site erythema | SOC Nervous system disorders: 3/50 (6%) in the LED group paraesthesia (reported as "slight stinging sensations immediately after the procedures that disappeared within approximately 2 h") |
| ALA‐PDT versus IPL alone | |||
| Oh 2009 | Short incubation ALA‐PDT side: 1/9 (11.1%) dermatitis acneiform (LLT acneiform eruption or rash acneiform) | Short incubation ALA‐PDT side: 1/9 (11.1%) application site discolouration (LLT application site hyperpigmentation). Application site erythema and application site oedema (unclear reporting) | None reported |
| Liu 2014 | In the ALA‐PDT group: 2/50 (4%) post‐inflammatory pigmentation change (LLT post‐inflammatory hyperpigmentation); 10/50 (20%) "brightening of skin tone and improvements of skin texture after treatment" (we were unable to find an appropriate MedDRA PT) | In the IPL group: 28/50 (56%) application site erythema. | SOC Nervous system disorders: 28/50 (56%) in the IPL group paraesthesia (reported as "slight stinging sensations immediately after the procedures that disappeared within approximately 2 h") |
| Mei 2013 | In the ALA‐IPL group: 3/21 (14%) erythema, 3/21 (14%) dermatitis acneiform (LLT acneiform eruption) | In the ALA‐IPL group: 21/21 (100%) application site pain | None reported |
| Ragab 2014 | In the ALA‐IPL group: 15/15 (100%) pain of skin, of which 4/15 mild, 8/15 moderate and 3/15 severe. In the IPL‐alone group: 10/10 (100%) pain of skin, of which 8/10 mild and 2/10 moderate | In the ALA‐IPL group: 4/15 (27%) application site discolouration (LLT application site hyperpigmentation); 10/15 (67%) application site exfoliation, of which 5/15 mild and 5/15 moderate; 14/15 (93%) application site erythema, of which 6/15 mild, 6/15 moderate and 2/15 severe. In the IPL‐alone group: 1/10 (10%) application site discolouration (LLT application site hyperpigmentation); 2/10 (20%) application site exfoliation, of which 2/2 moderate; 8/10 (80%) application site erythema, of which 7/10 mild and 1/10 moderate | None reported |
| ALA‐PDT versus green light alone | |||
| Sadick 2010a | None reported | None reported | None reported |
| ALA‐PDT versus placebo or no treatment | |||
| Orringer 2010 | On the ALA‐PDT side: 2/44 participants (4.5%) skin desquamation, 2/44 (4.5%) post inflammatory pigmentation change (LLT post inflammatory hyperpigmentation). Both of these participants withdrew from trial | 1/44 patient (2.3%) application site vesicle (LLT application site blister). Resolved without permanent consequences | None reported |
| ALA‐PDT other | |||
| Barolet 2010 | Scab (exact numbers of participants not given). 2/10 (20%) participants had acneiform folliculitis (we were unable to find an adequate term in MedDRA). Not clear whether this refers to IR‐LED treatment or PDT | Application site erythema. Not clear whether this refers to IR‐LED treatment or PDT | SOC Nervous system disorders: paraesthesia (LLTs skin burning sensation). Not clear whether this refers to IR‐LED treatment or PDT |
| Hongcharu 2000 | Pain of skin, pruritus, skin burning sensation, post‐inflammatory pigmentation change (post‐inflammatory hyperpigmentation), lasting more than 20 weeks in 55% of multiple treatment group participants; numbers and group those participants were assigned to reported unclearly. Acne (LLT exacerbation of acne; reported as "acute eruption of inflammatory acneiform lesions") in all participants | Application site erythema, application site oedema, numbers reported unclearly. 1/11? (9%) application site vesicle (LLT application site blister) reported as: "one subject in the single PDT group developed severe blistering in the PDT site after vigorous aerobic exercise while wearing a tight outfit the day after treatment". | Transient purpura in 10% of multiple treatment participants (following "superficial but very prominent exfoliation"). Numbers reported unclearly |
| NCT007064331 | SeeALA‐PDT versus blue light alone above | See ALA‐PDT versus blue light alone above. | See ALA‐PDT versus blue light alone above |
| Taub 2007 | In the IPL group: 1 severe erythema and skin exfoliation, 1 alopecia; in the IPL‐RF group: 1 severe erythema and skin exfoliation, 1 acne (LLT exacerbation of acne) 1 contusion (LLT bruise). In ALA‐PDT plus blue light 1 acne (LLT exacerbation of acne). Numbers of participants per group were not stated | In the IPL‐RF group: 1 application site vesicle (LLT application site blister). Numbers of participants per group were not stated | None reported |
| Yin 2010 | In 5%, 10%, 15% and 20% ALA groups 1/45 (2%), 2/45 (4%), 2/45 (4%), 5/45 (11%) respectively combination of mild dry skin and skin exfoliation. In the 20% ALA‐PDT group 5/45 (%) marked dry skin and skin exfoliation | In the 20% ALA group: 30/45 (67%) application site discomfort, 3/45 (7%) severe application site oedema and application site erythema, scab (exact numbers of participants not given), 1/45 (2%) combination of application site erythema, application site oedema and application site vesicle (LLT application site blister);"treated with systemic glucocorticoids and resolution took place in 2 weeks, with no persistent clinical sequelae or permanent scarring". 2/45 (4%), 5/45 (11%), 7/45 (16%), 10/45 (22%) application site discolouration (LLT application site hyperpigmentation) in 5%, 10%, 15% and 20% ALA‐PDT groups respectively | SOC Nervous system disorders: paraesthesia (LLTs skin burning sensation): "occurred in almost all the patients, but seldom led to considerable pain and normally disappeared within 5 min". |
| 6. MAL‐PDT versus ALA‐PDT | |||
| Wiegell 2006a | Pain of skin, reported as "The two treatments were equally painful during illumination"; ALA sides: 6/19 (31.5%), scab (LLT crust) reported as "yellow crusting", treated with antibiotics to avoid infection | Application site oedema, application site erythema, pustular rash (LLT pustular skin eruption), reported as "After illumination edema and severe inflammation were seen in the treatment area. In the following days, a pustular eruption and epithelial exfoliation occurred." Participants' numbers not given, 12/15 (80%) of adverse effects more prominent on the ALA side as compared to MAL. 3/15 (20%) no differences between the sides in adverse effects | SOC Social conditions: "Approximately half of the patients did not go to school or work the following days due to their appearance" (we were unable to find an appropriate MedDRA PT) |
| 7. Other (non MAL, non ALA) PDT versus other comparators | |||
| Indocyanine green‐PDT | |||
| Genina 2004 | None reported | None reported | None reported |
| Kim 2009 | None reported | 2/16 (12.5%) application site oedema and application site erythema (1 in single and 1 in multiple treatment 'group') 1/7 (14.3%) (in the multiple treatment 'group') application site discolouration (LLT application site hyperpigmentation) and scab (LLT crust). Please see 'Notes' in the Characteristics of Included Studies | None reported |
| Indole 3‐Acetic Acid‐PDT | |||
| Na 2011 | None reported | None reported | None reported |
| Topical liposomal methylene blue‐PDT | |||
| Fadel 2009 | Pain of skin 7/20 (35%) | 10/20 (50%) application site erythema, 3/20 (15%) skin erythema, 3/20 (15%) application site discolouration (LLT application site hyperpigmentation), reported to be 'transient'. | None reported |
| Chlorophyll‐a (CHA)‐PDT | |||
| Song 2014 | None reported | None reported | None reported |
| Gold microparticle PDT versus other comparators | |||
| Paithankar 2015 | None reported | Application site pain (reported as: "Treatment was well tolerated, with a mean pain score of 3.5 in the active treatment group.", further information not given) | None reported |
1We reported the adverse events as provided by the study authors or sponsors (we did not perform coding ourselves).
2'Investigator‐assessed severe adverse effects' are presented in bolded text.
ALA = 5‐aminolevulinic acid BPO = benzoyl peroxide FPT = Fitzpatrick's Skin Types: based on different reactions to sun exposure and range from type I ('pale white skin which always burns and never tans') to type VI ('deeply pigmented dark brown to black skin which never burns and tans very easily') (Fitzpatrick 1988) GAAS = Global Acne Assessment Scoring ILs = inflamed lesions IPL = intense pulsed light IR = Infrared ITT = Intention‐to‐treat analysis LLT = Lower Level Term MAL = methyl‐aminolevulinate NILs = non‐inflamed lesions OFI = optical fibre intra‐tissue irradiation PDL = pulsed‐dye laser PDT = photodynamic therapy PT = Preferred term RCT = randomised controlled trial SD = standard deviation SOC* = System Organ Class SPF = sun protection factor
*MedDRA®, the Medical Dictionary for Regulatory Activities, terminology is the international medical terminology developed under the auspices of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). MedDRA® trademark is owned by the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) on behalf of ICH.
5. Secondary outcomes other than adverse effects.
| Study | Participants | Intervention(s) and control(s) | Secondary outcomes other than adverse effects |
| 1. Light versus placebo or no treatment | |||
| Green light versus placebo | |||
| Baugh 2005 | 25 (4 M, 21 F) aged 19‐41 years (mean 27.8), diagnosed with mild‐moderate inflammatory facial acne; FPT I–III | 532 nm pulsed laser vs sham in a split‐face trial, both with skin cooling system; 2 exposures a week for 2 weeks. Assessed at 1 and 4 weeks post treatment | At week 4 mean Michaelsson acne severity score decreased from baseline 42.9 to 34.1 (by 21%) on the treated side and increased from baseline 41.2 to 51.4 (by 25%) on the control side (P = 0. 089, SDs not given). At 4 weeks investigators assessed that 14.3% of participants had 50% to 59% improvement, 14.3% had 60% to 69% improvement, 57.1% had 70% to 79% and 14.3% had 80% to 89% improvement. Results for control sides not given |
| Bowes 2003 | 11 (M ⁄ F proportion not given) with mild‐moderate acne; skin types not given | 532 nm pulsed laser vs sham in a split‐face trial, both with skin cooling system; 2 treatments weekly for 2 weeks; assessed at 1 week and 1 month after final treatment | At 4 weeks Michaelsson acne severity score decreased by 35.9% on the treated and increased by 1.8% on the untreated side (SDs not given) |
| Yilmaz 2011 | 44; 38 completed, 20 participants in the once‐weekly group (12 M, 8 F) and 18 in twice weekly group (12 M, 6 F); mean ages (± standard deviation) of the participants were 21.0 ± 3.5 and 20.7 ± 2.7 in each group respectively; all with ≥ 4 inflammatory acne lesions, FPT I‐III | 532 nm KTP laser, 2 randomised groups, application once weekly for 4 weeks vs twice weekly for 2 weeks. Within each group 1 side of the face randomised to assigned treatment and the other to no treatment; evaluated at 0, 1 and 4 weeks after final treatment | Both sides improved, but decrease in Michaelsson severity score was significantly greater on the treated side ‐ 31% versus 6% (P = 0.005) in once‐weekly group and by 40% versus 13% in twice‐weekly group (P < 0.001). Means and SDs were not given, further data not given |
| Yellow light versus placebo or no treatment | |||
| Seaton 2003 | 41, 31 randomised to treatment, 10 to control group; with mild‐moderate acne, other characteristics not given | 585 nm PDL vs sham laser, parallel‐group trial, single treatment, assessed at 2, 4, 8 and 12 weeks after treatment | Median (inter quartile range) improvements in Leeds score were 1.9 (1.8) in the treated group and 0.1 (1.4) in the placebo group (P = 0.007) |
| Orringer 2004 | 40 (24 M, 16 F) enrolled, 26 completed, mean age 20.7 years (range not reported), with facial acne Leeds score > 2; FPT not given ("28 whites, 7 Asians, 2 blacks, 3 unknown") | 585 nm PDL in a split‐face trial, single treatment and 2 treatment (2 weeks apart) groups, serially assessed for 12 weeks after final treatment | Changes in means (SE) Leeds scores were not statistically significant at week 4 (P = 0.56) nor at week 12 (P > 0.99) for both treated and untreated sides. Changes in means (SE) were 0.07 (0.17) on the treated and 0.01 (0.10) on the untreated side at week 4 and 0.04 (0.15) and 0.04 (0.09) at week 12 on each side respectively. ITT analysis (LOCF method) reported |
| Infrared light versus no treatment | |||
| Darne 2011 | 38 (7 M, 31 F), aged 18‐47 years (mean 28), with moderate‐severe facial acne; FPT I‐V | 1450 nm laser (8‐9 J/cm²) in a split‐face trial, 3 treatments monthly, assessed monthly for 4 months, then at 3‐monthly intervals for 12 months after final treatment | Similar reduction in Leeds grade on both treated and untreated sides at 1 and at 12 months after final treatment with median difference between sides 0 (95% CI ‐1 to 0) and 0 (95% CI ‐1 to 0.7) respectively |
| Orringer 2007 | 46 (10 M, 36 F) enrolled, 30 completed, mean age 23.9 years (range not reported) with clinically apparent active facial acne; FPT II–VI | 1320 nm Nd:YAG laser in a split‐face trial with cooling; 3 treatments at 3‐week intervals; assessed at weeks 7 and 14 | Modified Leeds acne severity scale was used. At week 7, both sides graded as slightly worse, by 0.07 (0.23) units for the treated side and by 0.18 (0.22) units for the untreated side (P = 0.46) for 37 participants who completed, and had similar baseline scores with means (SE of the mean) of 2.97 (0.26) and 2.99 (0.26) for treated and untreated sides, respectively. At week 14 both sides graded as slightly improved, but not statistically significant; by 0.20 (0.21) and 0.23 (0.18) units for treated and untreated sides, respectively (P = 0.85) for 32 participants who completed, and had similar baseline means (SE) of 2.88 (0.29) and 2.85 (0.28) for treated and untreated sides, respectively |
| Moneib 2014 | 24 (5 M, 19 F), age 15‐38 years (mean 21.5), with moderate‐severe acne; FPT II‐V | Fractional Erbium Glass 1559 nm laser, in a split‐face trial, 4 treatments at 2‐week intervals, assessed every 3 months for 1 year after final treatment | Non‐standardised scale (0 = no improvement; < 25% = mild improvement; 26% to 50% = moderate improvement; 51% to 75% = good improvement; 76% to 100% = excellent improvement) was used for evaluation. Reported in graph format and for treatment face sides only, and at unclear time point. Our interpretation of the graph was that investigators assessed 5% participants had moderate, 25% good and 70% excellent improvement |
| Blue light versus placebo or no treatment | |||
| Tzung 2004 | 31 (28 completed: 10 M, 18 F) age15–32 years (mean 20.79) with mild‐moderate acne; all Taiwanese; FPT III–IV | 420 nm light in a split‐face trial, twice‐weekly for 4 consecutive weeks, assessed after each treatment and at 1 month after final treatment | Michaelsson modified grade percentage improvement light compared to control was reported as 52% and 12% respectively at 8 weeks, P = 0.009. Unclear whether mean or median |
| Blue‐red light versus placebo | |||
| Papageorgieu 2000 | 30 participants, mean age 24.8 years in blue–red light group; 25 participants, mean age 25.6 years in white light control group; randomised from the original 107 recruited (33 M, 74 F, aged 14‐50 years), all with mild‐moderate acne; skin types not stated | 415 nm plus 660 nm light vs cool white light; treated daily for 12 weeks; assessed every 4 weeks for the 12‐week treatment period | Non‐standardised scale: 'worse' (≤ ‐10%), 'unchanged' (‐9% to 9%), 'mild improvement' (10% to 39%), 'moderate improvement' (40% to 59%), 'marked improvement' (60% to 89%) or 'clearance' (≥ 90%) was used for evaluation, but reported only in graph format and no details were provided. Not evaluated after final treatment. Our interpretation of the graph was that in the blue‐red light group 4% of participants were reported to have their acne as 'unchanged', 4% as 'mild improvement', 25% as 'moderate improvement', 55% as 'marked improvement' and 6% as 'clearance'. In the white light group 38% of participants were reported as 'unchanged', 38% as 'mild improvement', 15% as 'moderate improvement' and 9% as 'marked improvement'. We dichotomised the data to 26/30 'success' outcomes in the blue‐red group and 6/25 in the white light group. Blue red‐light was superior to white light with RR (95% CI) of 3.61 (1.77, 7.36), P = 0.0004 and the 'number needed to treat for an additional beneficial outcome' (NNTB) was 2 (95% CI 1 to 3) |
| Kwon 2013 | 35 participants (11 M, 24 F); aged 20‐27 years (mean not given), with mild‐moderate acne, FPT III‐V; 18 participants in the blue‐red light group, 17 in the placebo group | 420 nm plus 660 nm home use LED device vs home use sham device; self‐treatment twice daily for 4 weeks in a split‐face trial; assessed 4 and 8 weeks after final treatment | No difference in the distribution of IGA‐score between 2 groups at baseline (P > 0.05). At 8 weeks after final treatment 14/18 participants (77.8%) in the blue‐red light group and 2/17 (11.8%) in the placebo group had grade 0 (clear) or grade 1 (almost clear) and the difference in distribution of participants was statistically significant (P < 0.01) |
| Intense pulsed light (IPL) versus no treatment | |||
| McGill 2008 | 10 (3 M, 7 F), 7 completed, 5 evaluated, aged 18‐47 years (mean 30), with mild‐moderate facial acne; FPT I‐II | IPL, ‘upper’ and ‘lower’ halves of face sides treated with different filters; 550‐1100 nm filter ("585 filter"), and the "Dual band" filter (blue light), versus no treatment? (unclear intervention on control half‐sides), in a split‐face trial, 5 treatments at 2‐week intervals, assessed at 1, 3 and 6 months after final treatment | Leeds grade reduced from baseline 3.1 ± 1.7 on the 585 half‐sides (n = 10) to 1.6 ± 1.1 at 1 month (n = 8), 1.9 ± 1.4 at 3 months (n = 7) and 2.2 ± 1.8 at 6 months (n = 5); from baseline 2.4 ± 1.8 on the 585 control half‐sides to 1.9 ± 1.9 at 1 month, 1.3 ± 1.3 at 3 months and 1.6 ± 1.5 at 6 months; from baseline 3.1 ± 1.7 on the blue‐light half‐sides to 1.9 ± 1.1 at 1 month, 1.9 ± 1.2 at 3 months and 2.2 ± 1.8 at 6 months; and from baseline 2.5 ± 1.8 on the blue‐light control half‐sides to 2.0 ± 1.8 at 1 month, 1.6 ± 1.0 at 3 months and 1.8 ± 1.3 at 6 months. At 6 months after final treatment, our calculations using t‐distribution showed that there were no significant differences in changes in Leeds grade between 585 half sides and control sides (MD 0.60, 95% CI ‐1.88 to 3.08), P = 0.64, nor between blue‐light and control sides (MD 0.40, 95% CI ‐1.95 to 2.75), P = 0.74. Mean (± SD) pretreatment Dermatology Life Quality Index (DLQI) scores were 11 ± 5 (range 3 to 19). At 1 month DLQI score had decreased to 6 ± 5 (range 0 to 12), at 3 months to 5 ± 2 (range 2 to 7) and at 6 months it increased to 7 ± 4 (range 4 to 12). Not reported for separate face half‐sides |
| 2. Light versus topical treatment | |||
| Light versus benzoyl peroxide (BPO) | |||
| Papageorgieu 2000 | 30 participants, mean age 24 ± 8 years in blue–red light group and 25 participants, mean age 26 ± 7 years in the BPO group, randomised from the original 107 recruited (33 M, 74 F, age 14–50 years) all with mild‐moderate acne; skin types not stated | 415 nm plus 660 nm light vs 5% BPO parallel groups, treated daily; assessed every 4 weeks for the 12‐week treatment period | Non‐standardised scale: 'worse' (≤ ‐10%), 'unchanged' (‐9% to 9%), 'mild improvement' (10% to 39%), 'moderate improvement' (40% to 59%), 'marked improvement' (60% to 89%) or 'clearance' (≥ 90%) was used for evaluation, but reported only in graph format and no details were provided. Not evaluated after final treatment. Our interpretation of the graph was that in the blue‐red light group 4% of participants were reported to have their acne as 'unchanged', 4% as 'mild improvement', 25% as 'moderate improvement', 55% as 'marked improvement' and 6% as 'clearance'. In the BPO group 10% of participants were reported as 'unchanged', 25% as 'mild improvement', 30% as 'moderate improvement', 30% as 'marked improvement' and 4% as 'clearance'. We dichotomised the data to 26/30 'success' outcomes in the blue‐red group and 16/25 in the BPO group.The difference was non significant, with RR (95% CI) of 1.35 (0.98, 1.88), P = 0.07 |
| Light versus clindamycin | |||
| Gold 2005 | 34 (25 completed the trial, 3 M and 22 F) aged 13‐55 years (mean 31 ± 0) with mild‐moderate acne; skin types described: caucasian (16), African‐American (7), American‐Indian (1), Chinese (1); 13 participants in clindamycin group and 12 in blue light group | 417 nm (blue light) twice weekly for 4 weeks vs self‐administered topical clindamycin 1% twice daily, parallel groups, assessed at 4 weeks after final treatment | Investigator‐assessed change in acne severity and global assessment of improvement reported as similar for both groups (figures not given in paper) |
| Light and other topical treatments | |||
| Borhan 2014 | 40 (8 M, 12 F in the light group, 9 M, 11 F in the control group), mean age 21.3 ± 2.0 in the intervention and 21.05 ± 2.18 in the control group (range 18‐25 years), with mild‐moderate acne vulgaris (Burton scale), FPT III‐IV | 595 nm light plus "traditional topical antibiotic medication" versus "traditional topical antibiotic medication" alone in a parallel‐group trial, 3 light treatments in total, at 4‐week intervals, details of topical treatment not given, unclear frequency of application; assessed at week 4, 8 and 12 (final evaluation 4 weeks after final treatment) | At week 12 investigators assessed that 19/20 participants (95%) had marked and 1/20 (5%) had moderate improvement in the laser combined with topical antibiotics group. In the topical antibiotics‐alone group 19/20 participants (95%) had mild improvement and 1/20 (5%) had moderate improvement |
| Ianosi 2013 | 180 participants (56 M, 124 F), aged 24 years (median), 60 in each group, with mild‐moderate acne, FPT I‐IV | 500‐1200 nm light plus vacuum vs IPL alone 400–700 nm and 870–1200 nm vs anti‐acne micellar solution, light applied once a week for 5 weeks, micellar solution unclear, final assessment at final treatment | Greater reduction in Leeds score in light‐treatment groups compared to micellar‐solution group reported in graph format and no further data provided. Significantly greater effect on quality of life (using Cardiff Acne Disability Index) in vacuum plus IPL group compared to micellar solution group (P = 0.004). Further data not given |
| Karsai 2010 | 89 randomised, 80 evaluated (38 M, 42 F, aged 13.3‐43.8 years, mean ± SD age 19.7 ± 5.9 years), with mild‐moderate acne (Investigator's Static Global Assessment ‐ISGA score 2‐4), FPT I‐III | Clindamycin 1%–BPO 5% hydrating gel (C ⁄ BPO) alone, once daily "throughout the observation period" vs in combination with 2 585 nm PDL treatments. Parallel groups, assessed at 2 and 4 weeks after initial treatment | Similar reduction in investigator's global assessment of improvement in both groups (27.1% versus 24.6%), measured by Investigator's Static Global Assessment Score (ISGA). ISGA score means (SD) in the C/BPO group were 3.17 (0.76) at baseline and 2.31 (0.54) 4 weeks after initial treatment. They were similar in the C/BPO with light group, 3.37 (0.60) at baseline and 2.54 (0.72) 4 weeks after initial treatment. Dermatology Life Quality Index (DLQI) was used for evaluation of life quality (0‐1 = no effect at all on patient's life, 2‐5 = small effect on patient's life, 6‐10 = moderate effect on patient's life, 11‐20 = very large effect on patient's life, 21‐30 = extremely large effect on patient's life). Significant DLQI points reduction of 2.31 points (54.5%) in the C/BPO only group and 3.06 points (42.5%) in the C/BPO with light group, with no significant difference in reduction between the groups. Means and SD reported in graph format. Our interpretation of the graph was that mean (SD) in the C/BPO group reduced from baseline 4.3 (3.5) to 2 (2) at 4 weeks after initial treatment, and in the C/BPO from baseline 7.1 (6) to 4 (4) at 4 weeks after initial treatment |
| Leheta 2009 | 75 screened, 45 randomised, aged 18‐30 years (mean not reported). 13 (6 M, 7 F, mean age ± SD 24.2 ± 4.6 years) completed the study in the PDL group, 13 (8 M, 5 F, 23.2 ± 4.2 years) in the tretinoin and BPO group, 15 (7 M, 8 F, 24.8 ± 3.8 years) in the chemical peeling group; all with mild‐moderate acne, FPT II‐IV | 585 nm PDL, 6 treatments at 2‐week intervals vs daily self‐administered topical 5% BPO and 0.1% tretinoin (treatment duration not specified) vs chemical peeling with 25% trichloroacetic acid, 6 treatments at 2‐week intervals + monthly during the follow‐up period. Parallel groups, assessed at the end of the treatment period (3 months) | Leeds score means (SD) in the PDL group were 1.673 (0.926) at baseline and 0.557 (0.573) 3 months after initial treatment. In the T/BPO group 2.019 (1.012) at baseline and 0.648 (0.469) 3 months after initial treatment. In the TCAA group 2.083 (0.948) at baseline and 0.680 (0.497) 3 months after initial treatment. Investigator's global assessment of improvement was evaluated using "degree of clinical improvement": marked response (> 75% improvement), moderate response (51% to 75% improvement), mild response (25% to 50% improvement), minimal response (< 25% improvement), no change, or worsening. In the PDL group 6 (46.2%) participants had been assessed to have marked and 7 (53.8%) moderate improvement; in the T/BPO 5 (38.5%) participants had marked improvement participants and 8 (61.5%) had moderate improvement; in the TCAA 6 (40%) participants had marked and 9 (60%) participants moderate improvement. We dichotomised the data to 13/15 'success' outcomes in the PDL group, 13/15 in T/BPO group and 15/15 in the TCAA group. PDL was not superior to T/BPO with RR (95% CI) of 1.00 (0.76, 1.32), P = 1.00, nor to TCAA, RR (95% CI) of 0.87 (0.69, 1.09), P = 0.24 |
| Zhang 2009a | 738 randomised, 508 (247 M/261 F) in the intervention group, and 230 (112 M/118 F) in the control group, aged 12–53 years (mean not reported), with mild‐severe acne (Pillsbury grades I‐IV); FPT not given | 415 ± 5 nm blue and 633 ± 6 red light in combination with clindamycin gel, azithromycin, antisterone or cimetidine versus clindamycin gel, azithromycin, antisterone or cimetidine alone, in a parallel‐group trial, 8 light treatments in total, twice weekly, clindamycin gel twice per day and azithromycin 0.5 g/day (on days without light therapy when in the group with light treatments), assessed at 4 weeks after final treatment | Non‐standardised method used for evaluation, based on percentage change in combined lesion counts. Percentage change in lesion count = (lesion count before treatment – lesion count after treatment)/lesion count before treatment × 100%; scale based on lesion count percentage change: ≥ 90% improvement = 'full recovery'; 60% to 89% = 'good improvement'; 30% to 59% = 'effective improvement'; ≤ 29% = 'no effect'; Total effective rate (TER) = (number of fully recovered + good improvement)/total number of participants x 100%. At 4 weeks after final treatment TERs were 65.6% in the treatment group and 54.4% in the control group respectively, with a significant difference between the groups (P value reported as < 0.05). In the intervention group 142 participants were reported to have ‘fully recovered’, 190 had ‘good improvement’ and 151 had ‘effective improvement’. In the control group 44 participants ‘fully recovered’, 81 had ‘good improvement’ and 87 had ‘effective improvement’. We dichotomised the data following our protocol and ITT approach to 332/508 ‘success’ outcomes in the intervention and 125/230 ‘success’ outcomes in the control group. Antibiotic treatment in combination with blue‐red light was superior to antibiotic treatment alone with RR (95% CI) of 1.20 (1.05, 1.38), P = 0.006 . The NNTB was 10 (95% CI 6 to 20) |
| 3. Light other comparators | |||
| Comparison of light therapies of different wavelengths | |||
| Cheng 2008 | 36 participants (29 M, 7 F) in the blue‐light group, 28 participants (19 M, 9 F) in the blue‐red light group, aged 14‐36 years (mean 22.6 years), all with mild‐moderate acne, FPT not reported | 400‐410 nm light versus 400‐410 nm plus 660 nm light, 2 treatments a week, duration depending on Pillsbury grade; 4 weeks for grade I in a parallel‐group trial, up to 12 weeks for Pillsbury III, evaluated at 1 and 4 weeks after treatment | Investigators assessed improvement using the following scale based on lesion count percentage change: ≥ 90% improvement = 'full recovery'; 70% to 89% = 'good improvement'; 30% to 69% = 'effective improvement'; ≤ 30% = 'no effect'. In the blue‐light group in 7/36 (19.4%) participants there was no improvement, in 3/36 (8.3%) participants the improvement was good and 26/36 (72.2%) participants have 'fully recovered'. In the blue‐red light group there was no improvement in 3/28 (10.7%) participants, in 10/28 (35.7%) participants the improvement was good and 15/28 (53.5%) have 'fully recovered'. We dichotomised the data to 15/28 'success' outcomes in the blue‐red group and 26/36 in the blue light alone group. The difference was non significant with RR (95% CI) of 0.74 (0.50, 1.11) and P = 0.14 |
| Choi 2010 | 20 (1 M, 19 F, age 20‐37, mean age 26); all with acne (Cunliffe severity grade 2‐4), FPT III‐V | 585 nm PDL vs 530‐750 nm IPL, 4 treatments at 2‐week intervals, in a split‐face trial, assessed 4 and 8 weeks after last treatment | No statistically significant difference in improvement of Cunliffe scores between the two treatments (P > 0.05); decrease from baseline 2.5 for both to 1.2 for IPL and 1.3 for PDL at 4 weeks and to 1.2 for IPL and to 1.0 for PDL at 8 weeks after treatment |
| Jung 2009 | 18 enrolled, 16 completed (5 M, 11 F, aged 20‐31 years, mean age 26); with mild‐moderate acne (Cunliffe severity grade 2‐5), skin types not given | 585 nm PDL vs combined 585/1064 nm PDL, in a split‐face trial, 3 treatments at 2‐week intervals, assessed at 8 and 12 weeks after initial treatment | Baseline mean Cunliffe grades of 2.43 on the PDL sides and 2.19 on the 585/1,064‐nm laser sides decreased to 0.77 (P < 0.001) and 0.91 (P = 0.001) at the final visit respectively. Further data not given |
| Liu 2011 | 20 (6 M/14 F) completed the study, number of randomised participants not reported, 10 completed in the blue light, 10 in the red‐light group, aged 19–28 years (mean 23.6 years) with mild‐moderate acne (Global Acne Grading System); FPT III‐IV | Blue (405 ± 10 nm) vs red (630 ± 10 nm) LED portable device treatments, about 20 cycles of illumination and the corresponding light doses received in each session were 7.2 J/cm² and 11.52 J/cm², in a parallel‐group trial, 8 sessions in total, twice weekly for 4 weeks; assessed at 4 weeks after final treatment and at each treatment session | Non standardised scale used for investigator's global assessment of improvement (‘reduction ≥ 90% = ‘full recovery’; 60% to 89% reduction = ‘significant improvement’, 40% to 59% reduction = ‘moderate improvement’, 20% to 39% reduction = ‘mild improvement’, and ≤ 19% reduction = ‘non‐ improvement or aggravation’). In the blue‐light group 2 participants ‘fully recovered’, 5 had ‘significant improvement’, 1 ‘moderate improvement’, 1 ‘mild improvement’, and 1 ‘non‐improvement or aggravation’. In the red‐light group there were 4 participants with ‘significant improvement’, 1 ‘moderate improvement’, 1 ‘mild improvement’, and 4 ‘non‐improvement or aggravation’. We dichotomised the data following our protocol to 8/10 ‘success’ outcomes in the blue light and 5/10 in the red‐light group. The difference was non significant with RR (95% CI) of 1.60 (0.80, 3.20), P = 0.18 |
| Papageorgieu 2000 | 30 participants, mean age 24.8 years in blue–red light group and 27 participants, mean age 23.4 years in the blue‐light group, randomised from the original 107 recruited (33 M, 74 F, aged 14‐50 years) all with mild‐moderate acne; skin types not stated | 415 nm plus 660 nm light vs 415 nm light, parallel groups, treated daily for 12 weeks; assessed every 4 weeks for the 12‐week treatment period | Non‐standardised scale: 'worse' (≤ ‐10%), 'unchanged' (‐9% to 9%), 'mild improvement' (10% to 39%), 'moderate improvement' (40% to 59%), 'marked improvement' (60% to 89%) or 'clearance' (≥ 90%) was used for evaluation, but reported only in graph format and no details were provided. Not evaluated after final treatment. Our interpretation of the graph was that in the blue‐red light group 4% of participants were reported to have their acne as 'unchanged', 4% as 'mild improvement', 25% as 'moderate improvement', 55% as 'marked improvement' and 6% as 'clearance'. In the blue‐light group 25% of participants were reported to have their acne as 'unchanged', 4% as 'mild improvement', 30% as 'moderate improvement', 35% as 'marked improvement' and 4% as 'clearance'. We dichotomised the data to 26/30 'success' outcomes in the blue‐red group and 19/27 in the blue light alone group. The difference was non significant, with RR (95% CI) of 1.23 (0.93, 1.63), P = 0.15 |
| Comparison of light therapies of different doses | |||
| Bernstein 2007 | 7 enrolled, 6 completed (1 M, 4 F, aged 23‐41 years, mean age 29), all with active papular acne, FPT I‐III | Comparison of two 1450 nm laser treatments; single‐pass, high‐energy (13–14 J/cm²) vs double‐pass, low‐energy (8–11 J/cm²); 4 treatments at monthly intervals, assessed 1 month following each treatment and 2 months after final treatment | Allen‐Smith acne severity score mean (SD) dropped from 3.1 (1.1) to 1 (1.1) on the single‐pass face side and from 3.2 (0.7) to 1 (1.1) on the double‐pass face side. Single‐pass mean (SD) investigator‐assessed improvement score mean (SD) was 1.6 (1.1) on the single‐pass side of the face and 2.4 (0.9) on the double‐pass side of the face |
| Uebelhoer 2007 | 11 (2 M, 9 F, aged 19‐39 years, mean age 26), 9 completed, all with ≥ 10 inflammatory papules on each side of the face and Allen‐Smith grade ≥ 3 and ≤ 5; skin types not given | 1450 nm laser single‐pass treatment consisting of stacked double pulses vs a double‐pass treatment of single pulses; in a split‐face trial, treated every 3 weeks for a total of 3 treatments, assessed before each follow‐up treatment, and at 3 months after the final treatment | Decrease in acne severity in 8/9 subjects (89%); the mean acne severity scores decreased to 2.1 (range 0 to 5) on the single‐pass sides and 2.2 (range 1 to 5) on the double‐pass sides from 3.3 (range to 3–5) at baseline. One subject's grade increased from 3 to 5. Data not reported at any time point for Investigator's global assessment of improvement |
| NCT00706433 | 266 (128 M, 138 F), 68 in the ALA 1000 s group, 65 in the ALA 500 s group, 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group, mean age 20.1 years, inclusion criterion 12 > years, with moderate and severe acne (IGA score 3 and 4, with at least 20 ILs); FPT I‐V | 20% ALA (45 min incubation) plus blue 1000 s light vs 20% ALA (45 min incubation) plus 500 s blue light vs vehicle (45 min incubation) plus blue 1000 s light vs vehicle (45 min incubation) plus 500 s blue light; in a parallel‐group trial; up to 4 treatments at 3‐week intervals, assessed 3 and 6 weeks after the final treatment | Investigator Global Assessment (IGA) was used for evaluation (0; clear skin with no ILs or NILs; almost clear; rare NILs with no more than a few small ILs; Mild; > Grade 1; some NILs with some ILs (papules/pustules only; no nodules); Moderate; > Grade 2; up to many NILs and a moderate number of ILs but no more than one small nodule; Severe; > Grade 3; up to many NILs and ILs, but no more than a few nodules); success was defined as a 2 point or more improvement on the IGA scale since baseline. At 3 weeks after final treatment there were 15/67 of 'success' outcomes in the vehicle 1000 s and 11/66 in the vehicle 500 s group. The difference between vehicle 1000 s and vehicle 500 s groups was non significant, with RR (95% CI) of 1.34 (0.67, 2.70), P = 0.43. At 6 weeks after final treatment there were 16/67 of 'success' outcomes in the vehicle 1000 s and 16/66 in the vehicle 500 s group. The difference between vehicle 1000 s and vehicle 500 s groups was non significant, with RR (95% CI) 0.99 (0.54, 1.80), P = 0.96 |
| Comparison of light therapies of different treatment application intervals | |||
| Yilmaz 2011 | 44; 38 completed, 20 participants in the once‐weekly group (12 M, 8 F) and 18 in twice‐weekly group (12 M, 6 F); mean ages (± standard deviation) of the participants were 21.0 ± 3.5 years and 20.7 ± 2.7 years in each group respectively; all with ≥ 4 inflammatory acne lesions, FPT I‐III | 532 nm KTP laser, 2 randomised groups, application once weekly for 4 weeks vs twice weekly for 2 weeks. Within each group 1 side of the face randomised to assigned treatment and the other to no treatment; evaluated at 0, 1 and 4 weeks after final treatment | At 4 weeks there was no statistically significant difference in decrease of acne severity between the treated sides among the 2 groups. Michaelson acne severity scores of treated sides of the face dropped by 41% in once‐weekly treatment group and by 40% in twice‐weekly group. Differences in Michaelson acne severity score means (SD) of the treated face sides at baseline and at 4 weeks were ‐5.9 (7.9) in the once‐weekly group and ‐9.3 (7.5) in the twice‐weekly group |
| Light in combination with carbon lotion versus no treatment | |||
| Jung 2012 | 22 (4 M, 18 F), 20 completed (2 M, 18 F, aged 19‐34 years, mean age 25.4), FPT III‐IV, acne severity not given | Carbon lotion plus quasi‐long pulse and Q‐switched 1064 nm Nd:YAG laser vs non treated control, in a split‐face trial, 3 treatments over 4 weeks, evaluation every 2 weeks whilst on treatment and then every 4 weeks | Cunliffe severity grade decreased significantly from 3.2 to 1.7 (P < 0.001) on the laser‐treated side and from 2.7 to 2.6 (P < 0.05) on the non‐treated side. The difference between the 2 treatments was significant (P = 0.04) |
| Light in combination with oral therapy versus other comparators | |||
| Ling 2010 | 120 (68 M, 52 F), aged 12‐32 years, means given for individual groups 21‐22 years); 30 in each group, moderate‐severe acne according to Pillsbury classification, FPT not reported | 415 nm plus 630 nm light in combination with sulfotanshinone vs sulfotanshinone alone vs 415 nm plus 630 nm light in combination with sulfotanshinone and prednisolone vs sulfotashinone and prednisolone; blue‐red light applied twice weekly, sulfotanshinone 4 times daily and prednisolone 3 times daily in a parallel‐group trial, assessed 4 weeks after treatment | Investigators assessed improvement using the following scale based on lesion count percentage change: ≥ 95% improvement = 'full recovery'; 60% to 95% = 'good improvement'; 20% to 59% = 'effective improvement'; ≤ 20% = 'no effect'. In the blue‐red light plus sulfotanshinone group 19/30 (63.3%) participants fully recovered, 7/30 (23.3%) had good improvement, in 3/30 (10%) the treatment was effective and 1/30 (3.33%) there was no effect. In the sulfotanshinone‐alone group 9/30 (30%) participants fully recovered, 10/30 (33.33%) had good improvement, in 7/30 (23.3%) the treatment was effective and 4/30 (13.33%) there was no effect. In the blue ‐red light plus sulfotanshinone plus prednisolone group 8/30 (26.6%) participants fully recovered, 8/30 (26.6%) had good improvement, in 7/30 (23.3%) the treatment was effective and 7/30 (23.3%) that there was no effect. In the sulfotanshinone plus prednisolone group 6/30 (20%) participants fully recovered, 7/50 (23.3%) had good improvement, in 8/30 (26.6%) the treatment was effective and 9/30 (30%) that there was no effect. We dichotomised the data to 26/30 'success' outcomes in the blue‐red light plus sulfotanshinone group,19/30 in the sulfotanshinone alone group, 16/30 in the blue‐red light plus sulfotanshinone plus prednisolone group and 13/30 in the sulfotanshinone plus prednisolone group. Blue and red light plus sulfotanshinone was superior to sulfotanshinone alone with RR (95% CI) with 1.37 (1.01, 1.86), P = 0.04; to blue and red light plus sulfotanshinone plus prednisolone with RR (95% CI) of 1.63 (1.13, 2.34), P = 0.009; and to sulfotanshinone plus prednisolone with RR (95% CI) of 2.00 (1.30, 3.08), P = 0.002. The NNTB were 3 (95% CI 1 to 9) and 3 (95% CI 1 to 5) for the latter two comparisons with blue‐red light plus sulfotanshinone respectively. However, there is no calculable NNTB for the comparison of blue‐red light plus sulfotanshinone to sulfotanshinone alone since the 95% CI for the risk difference contains zero (i.e. no effect), and this corresponds to an infinite upper 'limit' for the 95% CI for the NNTB, which indicates that there is no true boundary on how large the NNTB could be for this comparison: this is also seen in the marginal effect seen with the RR |
| Ou 2014 | 90 randomised; number of participants per group not reported (M/F not reported, 43 in the intervention, 40 in the control group), 83 completed (13 M/70 F), aged 18–38 years (mean 25.1), with moderate acne (grade II‐III according to the Chinese Acne Treatment Guidelines); FPT not given | Yinhua decoction (term as presented in the English translation of the abstract provided by the journal where full text was published in Mandarin) with electric light synergy versus Yinhua decoction in combination with red and blue light treatment, in a parallel‐group trial, 6 treatments in total, applied every 2 weeks, assessed at 12 weeks after final treatment | Non‐standardised method used for evaluation, based on percentage change in combined lesion counts. Percentage change in lesion count = (lesion count before treatment – lesion count after treatment)/lesion count before treatment × 100%; Fully recovered: percentage change in lesion count ≥ 90%; Good improvement: percentage change in lesion count 60% to 89%; Effective: percentage change in lesion count 30% to 59%; No effect: percentage change in lesion count ≤ 29%; Total effective rate (TER) = (number of fully recovered + good improvement)/total number of participants x 100%. At 12 weeks after final treatment the study authors reported TERs of 70% in the treatment group and of 37.5% in the control group respectively, with a reported significant difference between the groups (P = 0.002). In the intervention group 6 participants ‘fully recovered’, 24 had ‘good improvement’ and 10 had ‘effective improvement’. In the control group no participants ‘fully recovered’, 15 had ‘good improvement’ and 20 had ‘effective improvement’. 43 participants completed the trial in the intervention group and 40 completed in the control group. We dichotomised the data to 30/43 (69.7% of those who completed) 'success' outcomes in the intervention arm, and 15/40 (37.5% of those who completed) in the control arm. Numbers of randomised participants in each group were not reported, and so we were unable to use ITT approach. YD plus "electric light synergy" were superior to YD in combination with blue‐red light with RR (95% CI) of 1.86 (1.19, 2.91), P=0.006. The NNTB was 4 (95% CI 2 to 10) |
| Zhang 2009a | 738 randomised, 508 (247 M/261 F) in the intervention group, and 230 (112 M/118 F) in the control group, aged 12–53 years (mean not reported), with mild‐severe acne (Pillsbury grades I‐IV); FPT not given | 415 ± 5 nm blue and 633 ± 6 red light in combination with clindamycin gel, azithromycin, antisterone or cimetidine versus clindamycin gel, azithromycin, antisterone or cimetidine alone, in a parallel‐group trial, 8 light treatments in total, twice weekly, clindamycin gel twice per day and azithromycin 0.5g/day (on days without light therapy when in the group with light treatments), assessed at 4 weeks after final treatment | Please see results under Light and other topical treatments as this study could be placed under both comparisons. We were unable to perform subgroup analyses |
| Zhang 2013b | 120 (59 M/61 F), 60 in each group, aged 14–40 years (mean 22.1 in the intervention, 23.6 in the control group), with mild‐moderate acne (Pillsbury grades I‐III); FPT not given | 415 ± 5 nm (blue) and 633 ± 3 nm (red) light combined with jinhua xiaocuo (term as presented in the English translation of the abstract provided by the journal where full text was published in Mandarin) pills and chloramphenicol tincture versus Jinhua xiaocuo pills and chloramphenicol tincture alone, in a parallel‐group trial, 8 treatments, applied twice weekly; Jinhua xiaocuo pills 4 g orally 3 times/day, Chloramphenicol tincture 10 mg/mL (applied once in the day once at night), assessed 4 weeks after final treatment |
Non‐standardised method used for evaluation, based on percentage change in combined lesion counts. Percentage change in lesion count = (lesion count before treatment – lesion count after treatment)/lesion count before treatment × 100%; Fully recovered: percentage change in lesion count ≥ 90%; Good improvement: percentage change in lesion count 60% to 89%; Effective: percentage change in lesion count 30% to 59%; No effect: percentage change in lesion count ≤ 29%; Total effective rate (TER) = (number of fully recovered + good improvement)/total number of participants x 100%). At 4 weeks after final treatment TERs were reported to be 91.7% in the treatment group and 65% in the control group respectively, with a reported significant difference between the groups (P value reported as < 0.05). In the intervention group 25 participants ‘fully recovered’, 30 had ‘good improvement’ and 5 had ‘effective improvement’. In the control group 17 participants ‘fully recovered’, 22 had ‘good improvement’ and 11 had ‘effective improvement’. We dichotomised the data following our protocol to 55/60 ‘success’ outcomes in the intervention and 39/60 ‘success’ outcomes in the control group. Jinhua xiaocuo pills and chloramphenicol tincture in combination with blue‐red light were superior to jinhua xiaocuo pills and chloramphenicol tincture alone with RR (95% CI) of 1.41 (1.15, 1.72), P = 0.0008. The NNTB was 4 (95% CI 3 to 9) |
| IPL alone versus IPL in combination with vacuum | |||
| Ianosi 2013 | 180 participants (56 M, 124 F), aged 24 years (median), 60 in each group, with mild‐moderate acne, FPT I‐IV | 500‐1200 nm light plus vacuum vs IPL alone 400–700 nm and 870–1200 nm vs anti‐acne micellar solution, light applied once a week for 5 weeks, micellar solution unclear, final assessment at final treatment | Changes in lesion counts reported as scores 1 = insignificant result (lesion count reduction 0% to 25%) to 4 = very good result (lesion count reduction 76% to 100%). No significant differences found between treatments at final assessment in reduction score of papules and pustules (P reported as 'NS'). Significantlly greater reduction score of comedones in vacuum plus IPL group (P < 0.001). Greater reduction in Leeds score in IPL‐only group reported in graph format and no further data provided. Significantly greater effect on quality of life (using Cardiff Acne Disability Index) in vacuum plus IPL group (P = 0.004). Further data not given |
| 4. MAL‐PDT versus other comparators | |||
| MAL‐PDT versus red light alone | |||
| Pariser 2013 | 153 participants (87 M/66 F), 100 in the 80 mg/g MAL‐PDT group, 53 in the placebo group, aged 12‐35 years (mean 18.6), with severe facial acne vulgaris, IGA score 4, 25‐75 ILs and 20‐100 NILs on the face, FPT I‐VI | 80 mg/g MAL‐PDT under occlusion followed by illumination with 632 nm 37J/cm² red light vs placebo cream plus 632 nm 37 J/cm² light in a parallel‐group trial, 4 treatments at 2‐week intervals, assessed at 6 weeks after final treatment | 15 withdrawals from the MAL‐PDT group, 4 withdrawals and 1 lost to follow‐up from the placebo group. ITT analysis was performed. At 6 weeks after final treatment 'success' outcomes as defined by the IGA score were found in 44/100 participants in the 80 mg/g group and 14/53 in the placebo cream group. Our analyses showed borderline superiority of 80 mg/g MAL‐PDT to placebo cream activated by red light, with RR 1.67 (95% CI 1.01 to 2.75). Please note that the results of this study were combined with those of NCT00933543 and NCT00594425 for the same comparison |
| NCT00933543 | 107 participants (48 M/59 F), 54 in the 80 mg/g MAL‐PDT group, 53 in the placebo group, aged 11‐35 years (mean 17.2), with moderate‐severe facial acne vulgaris, IGA score 3‐4, 20‐100 ILs and 30‐120 NILs on the face, FPT I‐VI | 80 mg/g MAL‐PDT (without occlusive dressing) followed by illumination with 632 nm 37 J/cm²red light vs placebo cream plus 632 nm 37 J/cm² light (without occlusive dressing) in a parallel‐group trial, 4 treatments at 2‐week intervals, assessed at 6 weeks after final treatment | 3 withdrawals in MAL‐PDT group, 6 withdrawals and 1 lost to follow‐up in placebo group. ITT analysis was performed. At 6 weeks after final treatment 'success' outcomes as defined by the IGA score were found in 5/54 participants in the 80 mg/g group and 1/53 in the placebo‐cream group. Our analyses showed that 80 mg/g MAL‐PDT was not superior to placebo cream activated by red light, with RR 4.91 (95% CI 0.59 to 40.61). Please note that the results of this study were combined with those of Pariser 2013 and NCT00594425 for the same comparison |
| NCT00594425 | 150 participants (59 M/91 F), 50 in the 40 mg/g MAL‐PDT group, 48 in the 80 mg/g MAL‐PDT group, 52 in the placebo group, aged 15‐40 years (mean 21.3), with moderate‐severe acne, IGA score 3‐4, 20‐100 ILs and up‐200 NILs on the face, FPT I‐IV | 80 mg/mL MAL under occlusion (1.5 h) plus 632 nm 37 J/cm² light vs 40 mg/mL MAL under occlusion (1.5 h) plus 632 nm 37 J/cm² light vs placebo cream plus 632 nm 37 J/cm² light in a parallel‐group trial, 4 treatments at 2‐week intervals, assessed at 2, 3, 6, 12 and 24 weeks after final treatment | 43 participants completed in the 40 mg/g group, 34 completed in the 80 mg/g group and 42 completed in the placebo‐cream group, ITT analysis was performed (LOCF method). At 6 weeks after final treatment 'success' outcomes as defined by the IGA score were found in 6/50 participants in the 40 mg/g group and 4/52 in the placebo‐cream group. Our analyses showed that 40 mg/g MAL‐PDT was not superior to placebo cream activated by red light, with RR 1.56 (95% CI 0.47 to 5.20), P = 0.47. At 6 weeks after final treatment 'success' outcomes as defined by the IGA score were found in 6/48 participants in the 80 mg/g group and 4/52 in the placebo cream group. Our analyses showed that 80 mg/g MAL‐PDT was not superior to placebo cream activated by red light, with RR 1.63 (95% CI 0.49 to 5.41). Please note that the results of this study were combined with those of Pariser 2013 and NCT00933543 for the same comparison. |
| Hörfelt 2006 | 30 (25 M, 5 F), 27 completed, aged 15‐28 years (mean 18) with moderate‐severe inflammatory facial acne (Leeds score 5–10); FPT types I–III | 635 nm light plus MAL vs placebo cream and light in a split‐face trial, 2 treatments, 2 weeks apart, assessed at 4 and 10 weeks after treatment | At 12 weeks investigator‐assessed change in acne severity (global severity assessment clear or almost clear) observed in 9/30 participants (30%) for the MAL‐PDT side and in 3/30 participants (10%) on the light‐only side. Significantly greater improvement on the MAL‐PDT side than on placebo‐PDT side (P = 0.0143). 12 (40%) participants improved in more than one category on the MAL‐PDT side versus 7 (23%) on the placebo‐PDT side. We dichotomised the data to 12/30 'success' outcomes on the MAL‐PDT sides and 7/30 on the placebo‐PDT sides. The difference was non significant, with RR (95% CI) of 1.71 (0.78, 3.75), P = 0.18 |
| MAL‐PDT versus placebo or no treatment | |||
| Wiegell 2006b | 36 participants: 21 in treatment group age 23 ± 5 years (9 M, 10 F analysed) and 15 in control group age 24 ± 5 years (3 M, 9 F analysed), with > 12 inflammatory acne lesions; FPT II–V | Comparison of MAL plus 630 nm with no treatment in a parallel‐group trial; 2 treatments, 2 weeks apart, assessed every 4 weeks for 12 weeks after treatment | No significant difference was observed in reduction in Leeds grade between the two groups (P = 0.24). Median score (range) at 12 weeks was 1 (0 to 5) in the MAL‐PDT and 2 (0 to 8) in the control group. In the MAL‐PDT group median improvement score was 2 at 4 weeks, 2 at 8 weeks and 3 at 12 weeks. In the control group median improvement score was 1 at 4 weeks, 0 at 8 weeks and 1 at 12 weeks after treatment (results were reported in graph form, our interpretation given). No further data were provided |
| MAL‐PDT other | |||
| NCT00594425 | 150 participants (59 M/91 F), 50 in the 40 mg/g MAL‐PDT group, 48 in the 80 mg/g MAL‐PDT group, 52 in the placebo group, aged 15‐40 years (mean 21.3), with moderate to severe acne, IGA score 3‐4, 20‐100 ILs and up‐200 NILs on the face, FPT I‐IV | 80 mg/mL MAL under occlusion (1.5 h) plus 632 nm 37 J/cm² light vs 40 mg/mL MAL under occlusion (1.5 h) plus 632 nm 37 J/cm² light vs placebo cream plus 632 nm 37 J/cm² light in a parallel‐group trial, 4 treatments at 2 weeks intervals, assessed at 2, 3, 6, 12 and 24 weeks after final treatment | 37 participants completed in the 80 mg/g group, and 43 completed in the 40 mg/g group, ITT analysis was performed (LOCF method). At 6 weeks after final treatment 'success' outcomes as defined by the IGA score were found in 6/48 participants in the 80 mg/g group and 6/50 in the 40 mg/g group. Our analyses showed that 80 mg/g MAL‐PDT was not superior to 40 mg/g MAL‐PDT, with RR 1.04 (95% CI 0.36 to 3.01), P = 0.94 |
| Bissonnette 2010 | 44 participants, 33 completed (M/F not stated), aged 18‐40 years (mean 24.4), 22 randomised to each group,10 ≥ ILs on each side of the face and a Global Acne Severity score 3 ≥, FPT I‐IV | 80 mg/mL MAL plus 630 nm 25 J/cm² light vs 80 mg/mL MAL plus 630 nm 37 J/cm² light in a parallel‐group trial, split‐face randomisation within each group to occlusion or no occlusion, 4 treatments at 2‐week intervals, assessed at 4 and 12 weeks after final treatment | At 4 weeks after treatment Global Acne Severity score 0 or 1 ('success') was found on 1/16 (6.3%) of face sides with occlusion and on 0/16 (0%) face sides without occlusion in the 25 J/cm² group; and on 0/17 (0%) of face sides with occlusion and on 1/17 (5.9%) of the face sides without occlusion in the 37 J/cm² group. At 12 weeks ('success') was found on 0/20 (0%) of face sides with occlusion and on 0/20 (0%) face sides without occlusion in the 25 J/cm² group; and on 1/20 (5.6%) of face sides with occlusion and 2/20 (11.1%) of the face sides without occlusion in the 37 J/cm² group. Detailed data provided by the study authors. ITT analysis results reported (LOCF method). At 12 weeks the difference for comparison 37 J/cm² treatment with occlusion versus 37 J/cm² treatment without occlusion was non significant, with RR (95% CIs) 0.50 (0.05, 5.12) |
| Hong 2013 | 22 (2 M, 20 F), aged 19‐35 years (mean not given), 'at least grade 2 (Cunliffe acne grading system)', FPT IV‐V | MAL plus 630 nm light vs MAL plus 530‐750 nm light in a split‐face trial, 3 treatments in total, 2‐week intervals, assessed at 4 weeks after treatment | At 4 weeks after treatment there was no significant difference in the improvement in acne Cunliffe grade between the red‐light side (1.9) and IPL side (2.0). Baseline means extracted from graph as 3.6 on the red‐light side and 3.75 on the IPL side. Further data were not provided |
| 5. ALA‐PDT versus other comparators | |||
| ALA‐PDT versus red light alone | |||
| Chen 2015 | 50, 47 completed (25 M/22 F), 24/25 in the intervention, 23/25 in control group, aged 18–33 years (mean 23.6 in the intervention, 24.1 in the control group), with mild‐severe acne (non‐standardised scale); FPT not given | 20% ALA (90 min under plastic film occlusion) plus 633 ± 10 nm red light for 20 min versus 633 ± 10 nm red light for 20 min alone in a parallel‐group trial, 3 treatments in total, weekly, assessed at 2, 4 and 6 weeks after final treatment | Non‐standardised method used for evaluation, TER (‘Reduction rate was calculated as follows: Reduction rate (%) = (numbers of comedones before treatment ‐ numbers of comedones after treatment)/number of comedones before treatment x 100. Skin lesions with ≥ 90% improvement were classified as cured, skin lesions with 60%‑89% improvement were classified as excellent effect, skin lesions with 30%‑59% improvement were classified as fair effect and skin lesions with < 30% improvement or exacerbations were classified as no effect. TER was computed as follows: TER (%) = (number of cured cases + excellent effect cases)/total number of cases x 100). TERs in the treatment group were 54.2% at 2 weeks, 75.0% at 4 weeks and 83.3% at 6 weeks, whereas those in the control group were 26.1% at 2 weeks, 43.5% at 4 weeks and 56.5% at 6 weeks. P‑values reported for differences between the 2 groups were P = 0.050 at 2 weeks, P = 0.028 at 4 weeks and P = 0.045 at 6 weeks. In the ALA‐PDT group 3, 11 and 15 participants were reported to be ‘cured’ at 2, 4 and 6 weeks after final treatment respectively; 10, 7 and 5 had ‘excellent effect’ at 2, 4 and 6 weeks after final treatment respectively. In the red‐light group 1, 4 and 6 participants were ‘cured’ at 2, 4 and 6 weeks after final treatment respectively; 5, 6 and 7 had ‘excellent effect’ at 2, 4 and 6 weeks after final treatment respectively; 1 participant dropped out from the ALA‐PDT group, and 2 from the red‐light‐only group, and we treated them as treatment failures as per our protocol. We dichotomised the data following our protocol to 13/25 ‘success’ outcomes at 2 weeks, 18/25 at 4 weeks and 20/25 at 6 weeks in the intervention group, whereas in the control group there were 6/25 ‘success’ outcomes at 2 weeks, 10/25 at 4 weeks and 13/25 at 6 weeks. ALA‐PDT was not superior to red light alone with RR (95% CI) of 1.54 (1.01, 2.35), P = 0.05 at 6 weeks. We combined the results of this study with those of Zhang 2013a for assessments at 2 and 4 weeks |
| Zhang 2013a | 116 (47 M/59 F) randomised, 63 in the intervention, 53 in control group, aged 16–47 years (mean 24 years in the intervention, 23 years in the control group), with moderate‐severe acne (Pillsbury grade II‐IV); FPT not given | Unclear % of 5‐ALA plus 630 ± 5 nm red light versus 630±5 nm red light alone, in a parallel‐group trial, 3 treatments in total, weekly, assessed at 2, 4 and 8 weeks after final treatment |
Non‐standardised method used for evaluation, based on percentage change in combined lesion counts. Percentage change in lesion count = (lesion count before treatment – lesion count after treatment)/lesion count before treatment × 100%; Fully recovered: percentage change in lesion count ≥ 90%; Good improvement: percentage change in lesion count 60% to 89%; Effective: percentage change in lesion count 20% to 59%; No effect: percentage change in lesion count ≤ 19%; total percentage effectiveness = (number of fully recovered + good improvement)/total number of participants x100%). TERs were 44.4%, 58,7% and 79.4%, in the treatment group at 2, 4 and 8 weeks after final treatment respectively. TERs in the control group were 13.2%, 28.3% and 41.5% at 2, 4 and 8 weeks after final treatment respectively. In the intervention group 5, 12 and 24 participants ‘fully recovered’ at 2, 4 and 8 weeks after final treatment respectively; 23, 25 and 26 had ‘good improvement’ at 2, 4 and 8 weeks after final treatment respectively; and 33, 26, 13 had ‘effective improvement’ at 2, 4 and 8 weeks after final treatment respectively. In the control group no participants ‘fully recovered’ at 2, 4 nor at 8 weeks after final treatment; 7, 15 and 22 had ‘good improvement’ at 2, 4 and 8 weeks after final treatment respectively; and 3, 21 and 19 had ‘effective improvement’ at 2, 4 and 8 weeks after final treatment respectively. We dichotomised the data following our protocol to 28/63, 37/63, and 50/63 ‘success’ outcomes in the intervention group at 2, 4 and 8 weeks after final treatment respectively; and 7/53, 15/53, and 22/53 ‘success’ outcomes in the control group at 2, 4 and 8 weeks after final treatment respectively. ALA‐PDT was superior to red light alone with RR (95% CI) of 1.91 (1.36, 2.70), P = 0.0002 at 8 weeks. The NNTB was 3 (95% CI 2 to 5) at 8 weeks. We combined the results of this study with those of Chen 2015 for assessments at 2 and 4 weeks |
| ALA‐PDT versus blue light alone | |||
| NCT00706433 | 266 (128 M, 138 F), 68 in the ALA 1000 s group, 65 in the ALA 500 s group, 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group, mean age 20.1 years, inclusion criterion 12 > years, with moderate and severe acne (IGA score 3 and 4, with at least 20 ILs); FPT I‐V | 20% ALA (45 min incubation) plus blue 1000 s light vs 20% ALA (45 min incubation) plus 500 s blue light vs vehicle (45 min incubation) plus blue 1000 s light vs vehicle (45 min incubation) plus 500 s blue light; in a parallel‐group trial; up to 4 treatments at 3 weeks intervals, assessed 3 and 6 weeks after the final treatment | IGA was used for evaluation (0; clear skin with no ILs or NILs; almost clear; rare NILs with no more than a few small ILs; mild; > Grade 1; some NILs with some ILs (papules/pustules only; no nodules); moderate; > grade 2; up to many NILs and a moderate number of ILs but no more than one small nodule; severe; > Grade 3; up to many NILs and ILs, but no more than a few nodules); success was defined as a 2 point or more improvement on the IGA scale since baseline. At 3 weeks after final treatment there were 13/68 of 'success' outcomes in ALA 1000 s, 11/65 in the ALA 500 s, 15/67 in the vehicle 1000 s and 11/66 in the vehicle 500 s group. The difference between ALA 1000 s and vehicle 1000 s groups was non significant, with RR (95% CI) 0.85 (0.44, 1.65), P = 0.64, and it was non significant between ALA 500 s and vehicle 500 s groups, with RR (95% CI) 1.02 (0.47, 2.18), P = 0.97. At 6 weeks after final treatment there were 15/68 of 'success' outcomes in ALA 1000 s, 11/65 in the ALA 500 s, 16/67 in the vehicle 1000 s and 16/66 in the vehicle 500 s group. The difference between ALA 1000 s and vehicle 1000 s groups was non significant, with RR (95% CI) 0.92 (0.50, 1.71), P = 0.80, and it was non significant between ALA 500 s and vehicle 500 s groups, with RR (95% CI) 0.70 (0.35, 1.39), P = 0.31 |
| ALA‐PDT versus IPL alone | |||
| Oh 2009 | 20 (4 M, 16 F) , aged 18‐30 years, 9 in the short‐incubation group (3 M, 6 F, mean age ± SD 23 ± 4.12 years) and 10 in the long‐incubation group (1 M, 9 F and 23 ± 5.53 years), with moderate and severe acne (Evaluator Global Severity Score 3 and 4); FPT II‐IV | 20% ALA plus 590 nm IPL; 2 parallel groups: short incubation (30 min) vs long incubation (3 h), half of the face within each treated with IPL alone; 3 treatments at 4 weeks intervals, assessed 4 weeks after each treatment and 8 and 12 weeks after the third treatment | At 12 weeks investigators assessed improvement as mild in 3/9 participants (33.3%) and as moderate in 6/9 participants (66.7%) in the short incubation group; as mild in 2/11 participants (18.2%), as moderate in 5/11 participants (45.4%) and as significant in 4/11 participants (36.4%) in the long incubation group. We dichotomised the data to 6/9 'success' outcomes in short incubation group and 9/11 in the long incubation group. The difference was non significant, with RR (95% CI) of 0.44 (0.06, 3.51), P = 0.44 |
| Mei 2013 | 41 (24 M, 17 F), mean age 24 years, 21 in the ALA‐IPL PDT group, 20 in the placebo cream‐IPL group, II–IV Pillsbury grade acne; FPT II‐IV. | 10% ALA plus 420–950 nm light versus placebo cream plus 420–950 nm light in a parallel‐group trial, 4 treatments in total, weekly, assessed 4, 8 and 12 weeks after treatment. | At 12 weeks after final treatment investigators assessed an improvement of 75% to 100% in all lesions in 13/21, of 50% to 75% in 5/21 and of 25% to 50% in 2/21 participants and no improvements 1/21 participants in the ALA‐IPL group. In the control group an improvement of 75% to 100% in all lesions was achieved in 3/20, of 50% to 75% in 9/20, of 25% to 50% in 6/20 and no improvements in 2/20 participants. We dichotomised the data to 18/21 'success' outcomes in the ALA‐IPL group and 12/20 in IPL‐alone group. The difference was non significant, with RR (95% CI) of 1.43 (0.96, 2.13), P = 0.08 |
| ALA‐PDT versus green light alone | |||
| Sadick 2010a | 10 randomised (M/F not reported), 8 (2 M,6 F) completed, all > 18 years, mean age and age range not reported, all with moderate‐severe acne IGA 3‐4, FPT I‐III | 20% ALA plus KTP 532 nm laser compared with KTP 532 nm laser alone in a split‐face trial, 3 treatments spaced at 3‐4 week intervals, evaluated after each treatment and at 2, 6 and 12 weeks after final treatment | IGA was used for evaluation (Grade 0 = clear skin, no inflammatory lesions; grade 1 = almost clear, rare non‐inflammatory lesions, few small inflammatory lesions; grade 2 = mild severity, some non‐inflammatory lesions, some inflammatory lesions (papules, pustules, no nodular lesions); grade 3 = moderate severity, many non‐inflammatory and moderate inflammatory lesions, no more than one nodular lesion; grade 4 = severe, many non‐inflammatory and inflammatory lesions, nodular lesions are present). On the ALA‐PDT sides IGA score (mean ± SE) reduced from baseline 3.50 ± 0.19 to 2.29 ± 0.29 (35% improvement) after first treatment and to 2.13 ± 0.40 (39% improvement) after second treatment. On the light‐only sides IGA score (mean ± SE) reduced from baseline 3.63 ± 0.18 to 2.42 ± 0.30 (33% improvement) after first treatment and to 2.38 ± 0.33 (34% improvement) after second treatment. Further details and results of evaluations after final treatment were not given (reported as "Similar results were recorded after the third treatment session that was evaluated at week 12") |
| ALA‐PDT versus placebo or no treatment | |||
| Orringer 2010 | 99 screened, 44 enrolled (14 M, 30 F) aged 15‐50 years, mean 25, all with clinically evident facial acne, all FPT included | 20% ALA plus PDL compared with placebo in a split‐face trial, 3 treatments spaced at 2‐week intervals, evaluated every 2 weeks for a total of 16 weeks | Statistically significant improvement (P = 0.01) in mean Leeds score on treated skin versus untreated skin at week 16. Mean change in score from baseline (95% CI) was ‐1.07 (‐1.69 to ‐0.45) on the treated sides and ‐0.52 (‐1.07 to 0.04) on the control sides |
| ALA‐PDT other | |||
| Barolet 2010 | 10 (7 M, 3 F), aged 13‐54 years, mean age 26.2, with mild‐moderate acne, with ≥ 10 acne lesions, FPT I‐III | 970 nm IR pre‐treatment plus ALA and 630 nm PDT vs ALA‐PDT alone, one treatment in a split‐face or split‐back design, evaluated after 4 weeks | 4 weeks after treatment greater improvement in Global Severity Assessment Score medians on the IR pre‐treated (1, 95% CIs 0.74 to 1.34) versus control side (2, 95% CIs 1.17 to 1.72). 95% CI reported for means, but means were not given |
| Hongcharu 2000 | 22 participants, aged 18‐44 years; 11 in single‐treatment group, mean age 30 years, 9 M, 2 F; 11 in multiple‐treatment group, mean age 27 years, 8 M, 3 F; all with mild‐moderate acne of the back; FPT I–IV | 4 areas on the back of each participant: 550–700 nm light source used. ALA–light; ALA alone; light alone; untreated control. Single and multiple treatment groups, assessed at 1, 2, 3, 10 and 20 weeks | Change from baseline in Michaelsson acne severity score was significantly better in ALA‐PDT than other three areas at 3, 10 and 20 weeks after single treatment (P values not given) and at all visits after multiple treatment (P < 0.05). ALA–PDT and multiple ALA treatment sites showed more improvement than single treatment (P < 0.001 and P = 0.007, respectively). Investigator's global assessment of improvement scores also significantly better for the ALA‐PDT areas than other 3 areas where some improvement was also observed in both single and multiple treatment groups. These comparisons, as well as comparison between single and multiple treatment groups were reported in an unclear way |
| NCT00706433 | 266 (128 M, 138 F), 68 in the ALA 1000 s group, 65 in the ALA 500 s group, 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group, mean age 20.1 years, inclusion criterion 12 > years, with moderate and severe acne (IGA score 3 and 4, with at least 20 ILs); FPT I‐V | 20% ALA (45 min incubation) plus blue 1000 s light vs 20% ALA (45 min incubation) plus 500 s blue light vs vehicle (45 min incubation) plus blue 1000 s light vs vehicle (45 min incubation) plus 500 s blue light; in a parallel‐group trial; up to 4 treatments at 3 weeks intervals, assessed 3 and 6 weeks after the final treatment | IGA was used for evaluation (0; clear skin with no ILs or NILs; almost clear; rare NILs with no more than a few small ILs; mild; > grade 1; some NILs with some ILs (papules/pustules only; no nodules); moderate; > grade 2; up to many NILs and a moderate number of ILs but no more than one small nodule; severe; > grade 3; up to many NILs and ILs, but no more than a few nodules); success was defined as a 2 point or more improvement on the IGA scale since baseline. At 3 weeks after final treatment there were 13/68 of 'success' outcomes in ALA 1000 s and 11/65 in the ALA 500 s group. The difference between ALA 1000 s and ALA 500 s groups was non significant, with RR (95% CI) 1.13 (0.55, 2.34), P = 0.33. At 6 weeks after final treatment there were 15/68 of 'success' outcomes in ALA 1000 s and 11/65 in the ALA 500 s group. The difference between ALA 1000 s and ALA 500 s groups was non significant, with RR (95% CI) 1.30 (0.65, 2.62), P = 0.74 |
| Taub 2007 | 22 recruited, 19 participated, mean ± SD aged 26.5 ± 9.1 years, 7 M, 12 F, with moderate‐severe acne and > 10 inflammatory acne lesions; FPT not given | Comparison of PDT with different light sources for activation: ALA activated by IPL (600–850 nm), or a combination of IPL (580–980 nm) and bipolar radiofrequency energies, or blue light (417 nm) in a parallel‐group trial; 3 treatments at 2‐week intervals; follow up at 1 and 3 months after final treatment | Median acne grade score (96.9% CI) at baseline, and 1 month after treatment were 2.75 (2.5‐4.0) and 1.5 (1.0‐2.5) in the IPL group, 2.5 (2.0‐4.0) and 2.25 (1.5‐3.5) in the IPL‐RF group and 3.25 (2.5‐3.5) and 1.50 (1.0‐3.5) in the blue‐light group. At 3 months after treatment median acne grade score (range) was 1.75 (1.5) in the IPL group, 1.5 (2) in the IPL‐RF group and 2.00 (1) in the blue‐light group. Investigator‐assessed improvement was highest with IPL activation and lowest with blue light, and the differences between groups reached borderline statistical significance at 3 months (P = 0.0498). At 1 month after treatment median percentage improvement score (96.9% CI) was 56.25 (27.5‐85.0) in the IPL group, 23.75 (2.5‐85.0) in the IPL‐RF group and 20 (0‐62.5) in the blue‐light group. At 3 months after treatment median percentage improvement score (range) was 72.5 (42.5) in the IPL group, 50 (47.5) in the IPL‐RF group and 25 (40) in the blue‐light group |
| Yin 2010 | 180 (83 M, 97 F), aged 18‐38 years, mean 25.8, with moderate‐severe facial acne (Pillsbury), FPT III‐IV, 45 participants in each group | 633 ± 3 nm (red light) plus different ALA concentrations (5%, 10%, 15% and 20%) vs red light alone, 4 treatments every 10 days, 4 parallel groups, each treated with a different concentration on the right side and placebo agent on the left side; assessments at 2, 4, 12 and 24 weeks last after treatment | Assessed by a grading scale that was defined as ‐3 for > 50% exacerbation, ‐2 for 25% to 50% exacerbation, ‐1 for 1% to 25% exacerbation, 0 if unchanged, 1 for 1% to 25% improvement, 2 for 25% to 50% improvement, 3 for 50% to 75% improvement, 4 for 75% to 99% improvement, and 5 for 100% improvement, compared with baseline. Significant difference among the different ALA concentration groups (P values not given), with a clear positive correlation between global improvement score and ALA concentration (P < 0.05). Further data were expressed in graph format. Our interpretation of the graph was that there were mean improvements (SE) of 3.9 (0.2), 4 (0.5), 3.9 (0.5), 3 (1) and 1.9 (1.5) in the 20% ALA group, 15% ALA group, 10% ALA group, 5% ALA group and control face sides respectively at 24 weeks after last treatment |
| 6. MAL‐PDT versus ALA‐PDT | |||
| Wiegell 2006a | 15 participants > 18 years but age range not given, with > 12 inflammatory acne lesions; FPT not stated | Comparison of MAL and ALA creams: 620 nm light with split‐face design; one full‐face PDT treatment with MAL on one side and ALA on the other, assessed at 6 and 12 weeks after treatment | The median of Leeds revised acne global severity grade reduced from 2 before treatment to 1 at 12‐week follow‐up in both the MAL‐PDT‐ and ALA‐PDT‐treated sides of the face. Thre were no significant differences between the two treatments (P = 0.250) |
| 7. Other (non‐MAL, non‐ALA) PDT versus other comparators | |||
| Indocyanine green‐PDT | |||
| Kim 2009 | 16 (7 M, 9 F, aged 16‐34 years, mean age 25 ± 3.09) with mild‐moderate acne, skin types not given, 9 in single, 7 in multiple treatment group | 2 groups randomised: single treatment vs multiple (once weekly over 3 weeks); right cheek of each participant indocyanine green plus 805 nm light, left cheek light only and forehead "spontaneous resolution" control, evaluated 2 and 4 weeks after final treatment, multiple group also at final treatment | Significant improvement in Cunliffe acne severity score in both groups at 2 and 4 weeks after final treatment (P < 0.05). Not reported whether there were differences between the 2 groups. Part of the results reported in graph format. Our interpretation of the graph was that Cunliffe grade reduced from baseline 3.8 to 2.5 on the single‐treatment sides, and from baseline 3.5 to 2.1 on the multiple‐treatment sides respectively at final evaluation. Results not reported for sides treated only with light |
| Topical liposomal methylene blue‐PDT | |||
| Fadel 2009 | 20 (M/F not stated), age not stated (> 18 years), with mild‐moderate acne, FPT not given | Topical liposomal methylene blue plus 650 nm light vs no treatment in a split‐face trial, 2 treatments in total, weekly, assessed every 2 weeks for 3 months after treatment | At 12 weeks median Leeds severity grade on the treated side was 1 (range 0‐2) and on the untreated side 3 (range 2‐4). No baseline data given. At 12 weeks 7/13 (54%) participants had marked, 4/13 (31%) participants had moderate and 2/13 (15%) participants had slight improvement. "Approximately the same improvements" after 4 weeks and 8 weeks. Control areas reported to have no change or worsening of acne with no details provided |
| Chlorophyll‐a (CHA)‐PDT | |||
| Song 2014 | 24 (14 M, 10 F), mean age 23.4 ± 3.5 years; range 18‐32 years, "acne on both sides of the face", Cunliffe grades 2‐4, FPT III‐IV | 430 plus 660 nm light combined with chlorophyll‐a (CHA) vs 430 plus 660 nm light alone in a split‐face trial, 8 treatments in total, twice weekly, final assessment 2 weeks after last treatment | 2 weeks after final treatment Cunliffe grade reduced from baseline 3.1 to 1.8 on the CHA plus light sides and from baseline 3.1 to 2.2 on the light‐only sides (P = 0.027). Further data were not given |
| Gold microparticle PDT versus other comparators | |||
| Paithankar 2015 | 51 (14 M, 37 F), mean age 21.4 years, age range 16‐26 years, IGA scores 3–4 with at least 25 total papules and pustules on face, FPT I‐III | Gold microparticle suspension plus light (details not given) vs microparticle suspension vehicle (without light‐absorbing particles) plus light (details not given) in a parallel‐group trial, 3 treatments in total, weekly, assessed at 6, 10 and 14 weeks after final treatment | At 10 weeks after final treatment, "40% of subjects in the treatment arm, whereas none in the sham arm, showed Investigator’s Global Assessment (IGA) score reduction in two or higher". Further data were not given |
ALA = 5‐aminolevulinic acid BPO = benzoyl peroxide CHA = chlorophyll‐a FPT = Fitzpatrick's Skin Types: based on different reactions to sun exposure and range from type I ('pale white skin which always burns and never tans') to type VI ('deeply pigmented dark brown to black skin which never burns and tans very easily') (Fitzpatrick 1988) GAAS = Global acne assessment scoring scale IAA = indole 3‐acetic acid IGA = Investigator global assessment score ILs = inflamed lesions IPL = intense pulsed light IR = infrared ITT = intention‐to‐treat analysis LPDL = long pulsed dye laser LOCF = last observation carried forward LLT = lower level term MAL = methyl‐aminolevulinate NILs = non‐inflamed lesions NNTB = number needed to treat for an additional beneficial outcome OFI = optical fibre intra‐tissue irradiation PDL = pulsed‐dye laser PDT = photodynamic therapy PT = preferred term RCT = randomised controlled trial SD = standard deviation SE = standard error SPF = Sun protection factor TER = total effective rate TLMB = topical liposomal methylene blue
Unless specified differently, results presented as reported in the published papers, without performing independent analysis. Please see Characteristics of included studies for details on withdrawals and drop‐outs of participants for each study.
Change from baseline i.e. absolute change is calculated by subtracting baseline count from count assessed at certain time point. Percentage change is calculated by dividing the absolute change with baseline count and then multiplying that value by 100 to get percentages.
We tried to present our analyses in numerical order but this was not always possible due to the nature of the many comparisons and our desire to present the outcomes for a particular comparison together.
We identified three studies of 80 mg/g MAL‐PDT in combination with red light compared with red light alone (NCT00594425; NCT00933543; Pariser 2013) where no substantial statistical heterogeneity existed for primary outcomes (I² statistic was 39% for change in inflamed lesions (ILs), 19% for percentage change in ILs, 11% for change in non‐inflamed lesions (NILs), and 35% for percentage change in NILs), and although there was some clinical heterogeneity, we synthesised data using meta‐analysis techniques. We have presented the results in Table 2. We have not performed subgroup analyses because the I² statistic was lower than 50%, the threshold defined in our protocol.
In the following section, we provide details on why pooling data was not possible for each outcome and intervention subgroup, together with a narrative synthesis of the effects of interventions for individual studies where appropriate.
Primary outcome 1: Participant's global assessment of improvement
We have presented the details of participants, interventions, and the effects of interventions for this outcome in Table 4.
1. Participant's global assessment of improvement: 1. Light versus placebo or no treatment
1.1.a. Green light versus placebo
One split‐face study, Baugh 2005, of four treatments included 18 participants (FPT I to III, with mild to moderate acne). A non‐standardised scale (overall treatment satisfaction in intervals of 10 percentage points) was used for evaluation. At four weeks, 4.8% of participants reported 30% to 39% satisfaction, 9.5% reported 50% to 59% satisfaction, 23.8% reported 60% to 69% satisfaction, 47.6% reported 70% to 79% satisfaction, 9.5% reported 80% to 89% satisfaction, and 4.8% reported 90% to 100% satisfaction. Further data were not provided.
1. Participant's global assessment of improvement: 1. Light versus placebo or no treatment
1.1.c. Infrared light versus no treatment
One split‐face study, Darne 2011, of three treatments randomised 38 participants (FPT I to V, with moderate to severe or mild but treatment‐resistant acne). A non‐standardised scale ('highly satisfied', 'satisfied', 'neutral' or 'unsatisfied' and 'would recommend to a friend') was used for evaluation. At four weeks, 6/25 (24%) of participants were 'highly satisfied', 9/25 (36%) were 'satisfied', 6/25 (24%) were 'neutral', and 4/25 (16%) reported the treatment to be 'unsatisfactory'. A total of 21/25 (84%) reported that they would "recommend the treatment to a friend".
A split‐face study of four treatments, Moneib 2014, randomised 24 participants (FPT II to V, with moderate to severe acne). A non‐standardised scale (0 = no improvement; less than 25% = mild improvement; 26% to 50% = moderate improvement; 51% to 75% = good improvement; 76% to 100% = excellent improvement) was used for evaluation. Results were reported at an unclear time point, in graph format, and for treated face sides only. Our interpretation of the graph was that 5% of participants assessed their improvement to be mild, 5% to be moderate, 20% to be good, and 70% to be excellent.
Another split‐face study of three treatments, Orringer 2007, randomised 46 participants (FPT II to VI, with clinically active facial acne). A non‐standardised scale (details not given) was used for evaluation. At final treatment, 29/37 of participants who completed the treatments (78%) "indicated that their acne was at least mildly improved on the treated side of the face as compared with baseline", and 16/37 participants (43%) indicated "moderate or better" improvement. Data for non‐treated sides were not given, but 22/37 (59%) of participants reported that "their acne had improved at least mildly when compared with the untreated skin".
1. Participant's global assessment of improvement: 1. Light versus placebo or no treatment
1.1.e. Red light versus no treatment
One split‐face study of 122 self‐administered treatments (twice daily for eight weeks) randomised 30 participants (FPT not reported, with mild to moderate acne) (Na 2007). Visual analogue scale (VAS) (0 to 5, none to very severe) was used for evaluation. Score (unclear whether mean or median) decreased from baseline 3.9 to 1.8 at final treatment on the treated and from 3.9 to 2.9 on the control side, respectively, with significant difference between the sides (P < 0.005). The study did not evaluate this outcome after final treatment, and no further data were provided.
1. Participant's global assessment of improvement: 1. Light versus placebo or no treatment
1.1.f. Blue‐red light versus placebo
Two parallel‐group studies, Kwon 2013; Papageorgieu 2000, included this comparison for this outcome but we were unable to pool data due to substantial methodological heterogeneity (84 versus 56 treatments, different scales and timings of outcome assessment).
Kwon 2013, with 56 treatments, randomised 18 patients to the blue‐red light group and 17 to the placebo group (FPT III to V, with mild to moderate acne). A VAS scale was used for evaluation (10 = same as before the first treatment; 0 = no acne). Mean VAS score 10 at baseline in both groups decreased to 4.3 in the blue‐red light group and stayed at 10 or above in the placebo group (extracted from graph) at eight weeks after final treatment. No further data (standard deviations (SDs)) were provided in text or in graph format.
Papageorgieu 2000, with 84 treatments, randomised 30 participants to the blue‐red light group and 25 to the white light group (FPTs not reported, all with mild to moderate acne). A non‐standardised scale ('worse', ‐10% or less; 'unchanged', ‐9% to 9%; 'mild improvement', 10% to 39%; 'moderate improvement', 40% to 59%; 'marked improvement', 60% to 89%; or 'clearance', 90% or above) was used for evaluation. At final treatment the assessments were "in favour of blue‐red light", but reported only in graph format, and no details were provided. Final evaluation was performed at final treatment. We extracted the data from the graph and dichotomised them to 27/30 of 'success' outcomes in the blue‐red and 7/25 in the white light group. Blue‐red light was superior to white light with RR 3.21, 95% CI 1.70 to 6.09, P = 0.0003 (Analysis 1.1), and the 'number needed to treat for an additional beneficial outcome' (NNTB) was 2 (95% CI 1 to 3).
1.1. Analysis.
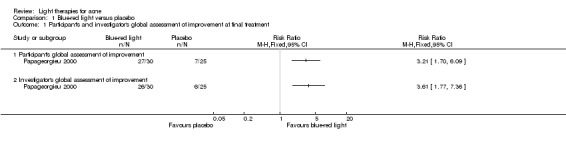
Comparison 1 Blue‐red light versus placebo, Outcome 1 Participant's and investigator's global assessment of improvement at final treatment.
1. Participant's global assessment of improvement: 2. Light versus topical treatment
1.2.a Light versus benzoyl peroxide (BPO)
One split‐face, Chang 2007, and one parallel‐group study, Papageorgieu 2000, included this outcome for this comparison, so we did not perform quantitative synthesis. Light interventions had different light sources, numbers, and frequency of sessions. Timing of outcome assessment was also different.
Chang 2007 compared a combination of BPO and three sessions of 530 nm to 750 nm light with BPO alone and included 30 women (FPT III to IV, with mild to moderate acne). A non‐standardised scale (highly satisfied, satisfied, neutral, or dissatisfied) was used for evaluation. At three weeks participants were "uniformly satisfied with their treatment, but intense pulsed light (IPL) treatment did not give any additional benefit". No further data were reported.
Papageorgieu 2000 randomised 30 participants to the blue‐red light group and 25 to the BPO group (FPTs not reported, all with mild to moderate acne). A non‐standardised scale was used for evaluation (please see above) and reported in graph format only. We extracted the data from the graph and dichotomised them to 27/30 of 'success' outcomes in the blue‐red and 20/25 in the BPO group. The difference was non significant, RR 1.13, 95% CI 0.89 to 1.42), P = 0.31 (Analysis 2.1).
2.1. Analysis.
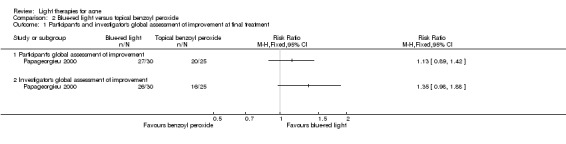
Comparison 2 Blue‐red light versus topical benzoyl peroxide, Outcome 1 Participant's and investigator's global assessment of improvement at final treatment.
1. Participant's global assessment of improvement: 2. Light versus topical treatment
1.2.b. Light versus clindamycin
One split‐face study (Lee 2010) compared eight full‐spectrum light treatments to 1% clindamycin twice daily over four weeks and randomised nine participants (FPT III, with moderate to severe acne). A non‐standardised scale ('worse', 'no change', 'fair', 'good', and 'excellent') was used for evaluation. Participants rated the treatment as 'good' or 'excellent' (it is unclear for which intervention and at what time point). Further data were not reported.
1. Participant's global assessment of improvement: 2. Light versus topical treatment
1.2.c. Light and other topical treatments
One parallel‐group study (Ash 2015), randomised 26 participants to the blue‐light group (28 sessions in total) and 15 to the control group with unclear (probably topical treatment) intervention (FPTs I‐V, all with mild to moderate acne). A non‐standardised scale was used for evaluation. Results reported as "the majority of subjects reporting that they were satisfied, very satisfied, or extremely satisfied with treatment" in the blue‐light group, and not reported for the control group. No further data were reported nor supplied upon request.
1. Participant's global assessment of improvement: 3. Light versus other comparators
1.3.a. Comparison of light therapies of different wavelengths
Two split‐face studies (Choi 2010; Jung 2009) and two parallel‐group studies (Liu 2011; Papageorgieu 2000) included this comparison for this outcome, but we were unable to pool data due to substantial methodological heterogeneity (different wavelengths used as comparators, different number of sessions, and different evaluation scales).
Choi 2010 compared three sessions of 585 nm pulsed‐dye lasers (PDL) with combined 585/1064 nm PDL and included 20 participants (FPT III to V, with mild to moderate acne). A non‐standardised rating scale (from 0 to 10, neutral to highly satisfied) was used for evaluation. No statistically significant difference in improvement of scores between the two treatments (P > 0.05) was found. They increased from baseline 0 for both to 3.3 for IPL and 3.7 for PDL at four weeks after treatment and then to 4.7 for IPL and 5.2 for PDL at eight weeks after treatment. Further data were not reported.
Jung 2009 compared three sessions of 585 nm PDL with combined 585/1064 nm PDL and included 18 participants (FPT not reported, with mild to moderate acne). A VAS (0 to 10, worst imaginable acne state to disease free) was used for evaluation; please note that the opposite VAS was used in Jung 2012. Mean scores on the PDL sides and on the 585/1064 nm‐laser sides increased from 3.3 and 3.7 at baseline to 6.63 (P = 0.002) and 6.60 (P = 0.001) at eight weeks respectively. At 12 weeks, they declined to 6.12 at both sides. Further data were not reported.
Liu 2011 included results for 20 participants (FPTs III‐IV, all with mild to moderate acne) who completed the trial of eight sessions of blue light in one group (405 ± 10 nm, power of 30 mW/cm²) and red light (630 ± 10 nm, power of 48 mW/cm²) in the other group. A non‐standardised scale was used for evaluation. Results were reported as, "A few patients reported that fresh new acne lesions came out, while the total number of lesions decreased slightly". Further data were not reported.
Papageorgieu 2000 randomised 30 participants to the blue‐red light group and 27 to the blue‐light group (FPTs not reported, all with mild to moderate acne). A non‐standardised scale was used for evaluation (please see above) and reported in graph format only. We extracted the data from the graph and dichotomised them to 27/30 of 'success' outcomes in the blue‐red and 23/27 in the blue‐light group. The difference was non significant, RR 1.06, 95% CI 0.87 to 1.29, P = 0.59 (Analysis 3.1).
3.1. Analysis.
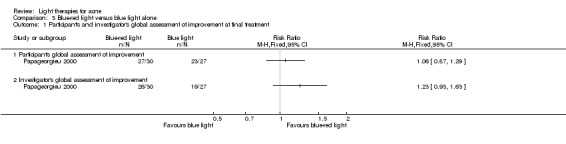
Comparison 3 Blue‐red light versus blue light alone, Outcome 1 Participant's and investigator's global assessment of improvement at final treatment.
1. Participant's global assessment of improvement: 3. Light versus other comparators
1.3.b. Comparison of light therapies of different doses
Two split‐face trials (Bernstein 2007; Jih 2006) compared different numbers of sessions, passes and doses of 1450 nm lasers, in participants with different FPT and different timings of outcome assessment, so we did not perform a meta‐analysis.
Bernstein 2007 compared four sessions of 1450 nm laser treatments; single‐pass, high‐energy (13 to 14 J/cm²) versus double‐pass, low‐energy (8 to 11 J/cm²) and included seven participants (all with active papular acne, FPT I to III). A non‐standardised rating scale (0 = worsening, 1 = no change, 2 = mild improvement , 3 = moderate improvement, 4 = marked improvement) was used for evaluation. At eight weeks, the average score on the single‐pass side was 2.3 (range 1 to 4) and on the double‐pass side 2.3 (range 2 to 4).
Jih 2006 also compared three sessions of 14 J/cm² and 16 J/cm² 1450 nm laser and included 20 participants (all with active inflammatory facial acne, FPT II to VI). A non‐standardised rating scale (0 = worsening, 1 = no change, 2 = mild improvement , 3 = moderate improvement, 4 = marked improvement) was used for evaluation. The majority of participants reported moderate to marked improvement, 85.3% at the one‐month, 67.7% at the three‐month, 60.0% at the six‐month, and 82.1% at the 12‐month assessments. No separate data for different doses were reported.
One parallel‐group trial (NCT00706433) compared four interventions:
20% ALA (45 min incubation) plus 1000 s of blue light;
20% ALA (45 min incubation) plus 500 s of blue light;
vehicle (45 min incubation) plus 1000 s of blue light; and
vehicle (45 min incubation) plus 500 s of blue light.
The study included a total of 266 participants (FPT I‐VI, with moderate to severe acne, IGA score 3 and 4, with at least 20 ILs); 67 in the vehicle‐1000 s group and 66 in the vehicle‐500 s group. A non‐standardised scale ('subject satisfaction score'; excellent = very satisfied; good = moderately satisfied; fair = slightly satisfied; poor = not satisfied at all) was used for evaluation. At six weeks after final treatment 20/67 participants in the vehicle‐1000 s and 23/66 in the vehicle‐500 s group assessed their improvement as 'good'; 23/67 participants in the vehicle‐1000 s and 26/66 in the vehicle‐500 s group assessed their improvement as 'excellent'. We dichotomised the data to 43/67 of 'success' outcomes in the vehicle‐1000 s and 49/66 in the vehicle‐500 s group. The difference between vehicle‐1000 s blue light and vehicle‐500 s blue light groups was non significant, with RR 0.86, 95% CI 0.69 to 1.09, P = 0.21 (Analysis 4.1).
4.1. Analysis.
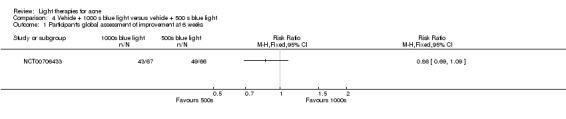
Comparison 4 Vehicle + 1000 s blue light versus vehicle + 500 s blue light, Outcome 1 Participant's global assessment of improvement at 6 weeks.
1. Participant's global assessment of improvement: 3. Light versus other comparators
1.3.e. Light in combination with carbon lotion versus no treatment
One split‐face study (Jung 2012) compared three sessions of quasi‐long pulse and Q‐switched 1064 nm Nd:YAG laser plus carbon lotion with non‐treated control and included 22 participants (FPT III to V, unclear severity). A VAS (0 to 10, disease‐free to initial visit acne status) was used for evaluation (please note that the opposite VAS was used in Jung 2009). At four weeks after final treatment, participants assessed significantly greater improvement on the laser‐treated side compared with the untreated side (P < 0.05). The VAS score mean (SDs not given) decreased from an initial 10 at both sides to 5.9 (P < 0.001) on the laser‐treated side and to 9.2 (P = 0.007) on the untreated side.
1. Participant's global assessment of improvement: 4. MAL‐PDT versus other comparators
1.4.b. MAL‐PDT versus yellow light alone
One split‐face study (Haedersdal 2008) compared three sessions of 595 nm long‐pulsed dye laser (LPDL) plus methyl aminolevulinate (MAL) with LPDL only and included 15 participants (FPT I to III, with at least 12 facial ILs). A non‐standardised numerical scale (0 to 10, no satisfaction to best imaginable satisfaction) was used for evaluation. Median (25 to 75 percentiles) score (range) was significantly higher for MAL‐LPDL treatment than for LPDL treatment alone at both four weeks after final treatment (P = 0.031); 7 (4.75 to 8) versus 6 (3.75 to 8), and at 12 weeks after final treatment (P = 0.034); 8 (6.25 to 9) versus 7.5 (5 to 8.75).
1. Participant's global assessment of improvement: 4. MAL‐PDT versus other comparators
1.4.c. MAL‐PDT versus placebo or no treatment
A parallel‐group study (Wiegell 2006b) of two treatments of 630 nm plus 160 mg/g MAL included 21 participants in the treatment group and 15 in the control group (FPT II to V, with at least 12 facial ILs). A non‐standardised grading scale (0 to 4; acne worse, no change, slight improvement, moderate improvement, marked improvement) was used for evaluation. Results were reported in graph format, and no details were provided. Our interpretation of the graph was that at 4, 8, and 12 weeks after final treatment, median improvement scores were 3, 2, and 3 in the MAL‐PDT group and 1.5, 1, and 1 in the control group respectively.
1. Participant's global assessment of improvement: 4. MAL‐PDT versus other comparators
1.4.d. MAL‐PDT other
One split‐face study (Hong 2013) compared three sessions of 160 mg/g MAL plus red light with three sessions of MAL plus IPL and included 22 participants (FPT IV to V). The VAS scale (10 to 0, 10 = same as before the first treatment; 0 = no acne) was used for evaluation. Mean VAS score decreased from baseline 10 on both sides to 5.0 at the red light side, and 4.9 at the IPL side at four weeks after final treatment, with no significant difference between the two sides. Further data were not provided.
1. Participant's global assessment of improvement: 5. ALA‐PDT versus other comparators
1.5.b. ALA‐PDT versus blue light alone
One parallel‐group trial (NCT00706433) compared four interventions:
20% ALA (45 min incubation) plus 1000 s of blue light;
20% ALA (45 min incubation) plus 500 s of blue light;
vehicle (45 min incubation) plus 1000 s of blue light; and
vehicle (45 min incubation) plus 500 s of blue light.
The study included a total of 266 participants (FPT I‐VI, with moderate to severe acne, IGA score 3 and 4, with at least 20 ILs); 68 in the ALA‐1000 s group, 65 in the ALA‐500 s group, 67 in the vehicle‐1000 s group and 66 in the vehicle‐500 s group. A non‐standardised scale ('subject satisfaction score'; excellent = very satisfied; good = moderately satisfied; fair = slightly satisfied; poor = not satisfied at all) was used for evaluation. At six weeks after final treatment 18/68 participants in ALA‐1000 s, 28/65 in the ALA‐500 s, 20/67 in the vehicle‐1000 s and 23/66 in the vehicle‐500 s group assessed their improvement as 'good'; 23/68 participants in ALA‐1000 s, 11/65 in the ALA‐500 s, 23/67 in the vehicle‐1000 s and 26/66 in the vehicle‐500 s group assessed their improvement as 'excellent'. We dichotomised the data to 41/68 of 'success' outcomes in ALA‐1000 s, 39/65 in the ALA‐500 s, 43/67 in the vehicle‐1000 s and 49/66 in the vehicle‐500 s group. The difference between ALA‐1000 s and vehicle‐1000 s groups was non significant, with RR 0.94, 95% CI 0.72 to 1.22, P = 0.64 (Analysis 5.1), and it was non significant between ALA‐500 s and vehicle‐500 s groups, with RR 0.81, 95% CI 0.63 to 1.03, P = 0.09 (Analysis 5.1). The difference between ALA‐PDT and vehicle plus blue light was non significant when we combined results for the 1000 s and 500 s subgroups using a random‐effects model, with RR 0.87, 95% CI 0.72 to 1.04, P = 0.12 (Analysis 5.1). See Table 3 where we rated the evidence for this outcome as low quality.
5.1. Analysis.
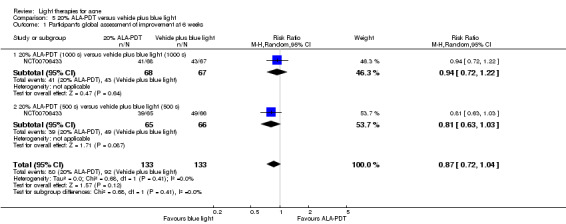
Comparison 5 20% ALA‐PDT versus vehicle plus blue light, Outcome 1 Participant's global assessment of improvement at 6 weeks.
1. Participant's global assessment of improvement: 5. ALA‐PDT versus other comparators
1.5.d ALA‐PDT versus IPL alone
One split‐face study (Oh 2009) and one parallel‐group study (Ragab 2014) included this comparison for this outcome. We were unable to combine their results due to methodological (different outcome assessment methods) and clinical differences (including numbers of treatment, application intervals, wavelengths used, incubation times).
Oh 2009 compared three sessions of 20% aminolevulinic acid (ALA) plus IPL (one face side randomised to either 30 minutes' or three hours' incubation) with IPL‐only and included 20 participants (FPT III to IV, with moderate to severe acne). A non‐standardised scale (significant improvement (over 75%), moderate improvement (50% to 75%), mild improvement (25% to 50%), no improvement (0% to 25%), worse (less than 0%) relative to baseline) was used for evaluation. We dichotomised the data to 3/9 of 'success' outcomes in the short‐incubation and 7/11 in the long‐incubation group. The difference was non significant, with RR 0.52, 95% CI 0.19 to 1.46, P = 0.22 (Analysis 6.1). Results were not reported for IPL‐only sides.
6.1. Analysis.
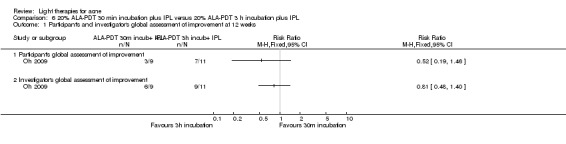
Comparison 6 20% ALA‐PDT 30 min incubation plus IPL versus 20% ALA‐PDT 3 h incubation plus IPL, Outcome 1 Participant's and investigator's global assessment of improvement at 12 weeks.
Ragab 2014 (FPT III to V, with mild to moderate facial acne) compared two treatments of 20% ALA‐PDT plus IPL (15 participants randomised) with IPL alone (10 participants randomised). A non‐standardised scale (marked improvement = 3; moderate improvement = 2; no change = 1; acne worsened = 0) was used for evaluation. We dichotomised the data at eight weeks to 10/15 'success' outcomes in the ALA‐PDT group and 3/10 in the IPL alone group. The difference was non significant, with RR 2.22, 95% CI 0.81 to 6.11, P = 0.12. (Analysis 7.1).
7.1. Analysis.
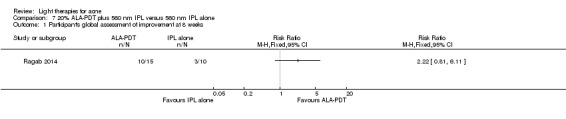
Comparison 7 20% ALA‐PDT plus 560 nm IPL versus 560 nm IPL alone, Outcome 1 Participant's global assessment of improvement at 8 weeks.
1. Participant's global assessment of improvement: 5. ALA‐PDT versus other comparators
1.5.g. ALA‐PDT other
Three parallel‐group studies (NCT00706433; Taub 2007; Yin 2010) included this comparison for this outcome but we were unable to pool data due to substantial methodological heterogeneity (different number of treatments, different ALA concentrations, different light wavelengths used for activation). Methods (scales and timings of outcome assessment) were unclear in one study, and we were unable to obtain additional data and clarification.
One parallel‐group trial (NCT00706433) compared four interventions:
20% ALA (45 min incubation) plus 1000 s of blue light;
20% ALA (45 min incubation) plus 500 s of blue light;
vehicle (45 min incubation) plus 1000 s of blue light; and
vehicle (45 min incubation) plus 500 s of blue light.
The study included a total of 266 participants (FPT I‐VI, with moderate to severe acne, IGA score 3 and 4, with at least 20 ILs); 68 in the ALA 1000 s group, 65 in the ALA 500 s group, 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group. A non‐standardised scale was used for evaluation (please see above). We dichotomised the data to 41/68 of 'success' outcomes in ALA‐1000 s and 39/65 in the ALA‐500 s group. The difference between ALA‐1000 s and ALA‐500 s groups was non significant, with RR 1.00, 95% CI 0.76 to 1.33, P = 0.97 (Analysis 8.1).
8.1. Analysis.
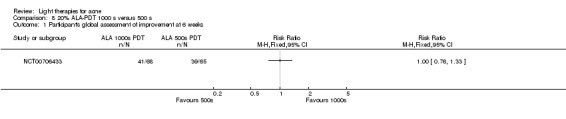
Comparison 8 20% ALA‐PDT 1000 s versus 500 s, Outcome 1 Participant's global assessment of improvement at 6 weeks.
Taub 2007 compared three ALA‐PDT treatments with different light sources for activation: IPL (600 nm to 850 nm) versus a combination of IPL (580 nm to 980 nm) and bipolar radiofrequency (RF) energies versus blue light (417 nm) and included 19 participants (FPT II to IV, with > 10 facial ILs, moderate to severe acne). The method used for evaluation was unclear. One month after the treatments, differences among the groups were not statistically significant (P = 0.3210); the median percentage improvement score was 58.75 (96.9% CI 5 to 70) in the IPL group, 20 (96.9% CI 0 to 80) in the IPL‐RF group, and 15 (96.9% CI 0 to 87.5) in the blue‐light group. At three months, data were only reported for IPL and blue‐light‐only groups 72.3 (range 42.5) versus 15 (range 27.5), so analysis was not possible.
Yin 2010 compared four red light ALA‐PDT treatments with different ALA concentrations: 5% versus 10%, versus 15% versus 20% and included 180 participants (FPT III to IV, with moderate to severe acne). A non‐standardised scale ('marked improvement', 'moderate improvement', 'no charge', or 'acne worse') was used for evaluation. At 24 weeks after treatment, a majority of the participants assessed that their acne had improved on both the ALA‐PDT‐treated and control cheeks. We dichotomised the data to 44/45 'success' outcomes in the 20% ALA group, 42/45 in the 15% ALA group, 36/45 in the 10% ALA group, and 30/45 in the 5% ALA group. 20% ALA was not superior to 15% ALA with RR 1.05, 95% CI 0.96 to 1.15 and P = 0.3 (Analysis 9.1). However, 20% ALA was more effective than 10% ALA with RR 1.22, 95% CI 1.05 to 1.42 and P = 0.01 (Analysis 10.1) and more effective than 5% ALA with RR 1.47, 95% CI 1.19 to 1.81 and P = 0.0004 (Analysis 11.1). The NNTB were 6 (95% CI 3 to 19) and 4 (95% CI 2 to 6) for the comparison of 20% ALA with 10% and 5% ALA, respectively. However, there was no calculable NNTB for the comparison of 20% to 15% ALA since the 95% CI for the risk difference contained zero (i.e. no effect), and this corresponded to an infinite upper 'limit' for the 95% CI for the NNTB, which indicated that there was no true boundary on how large the NNTB could be for this comparison.
9.1. Analysis.
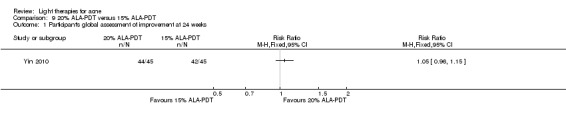
Comparison 9 20% ALA‐PDT versus 15% ALA‐PDT, Outcome 1 Participant's global assessment of improvement at 24 weeks.
10.1. Analysis.
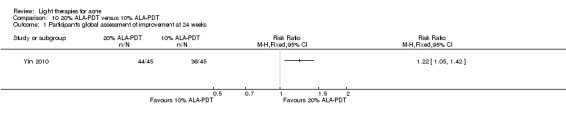
Comparison 10 20% ALA‐PDT versus 10% ALA‐PDT, Outcome 1 Participant's global assessment of improvement at 24 weeks.
11.1. Analysis.
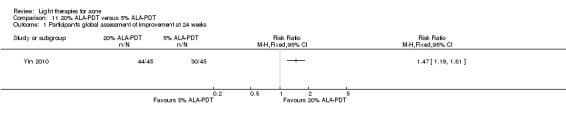
Comparison 11 20% ALA‐PDT versus 5% ALA‐PDT, Outcome 1 Participant's global assessment of improvement at 24 weeks.
1. Participant's global assessment of improvement: 6. MAL‐PDT versus ALA‐PDT
No studies reported results for this outcome for this comparison.
1. Participant's global assessment of improvement: 7. Other (non‐MAL, non‐ALA) PDT versus other comparators
1.7.a. ICG‐PDT versus other comparators
One parallel‐group study (Kim 2009) of a single treatment of topical application of indocyanine green (ICG) dye applied to the right cheek compared with three treatments of indocyanine green plus 805 nm light (right cheek), 805 nm light alone (left cheek), and 'spontaneous resolution' control (forehead) included 16 participants (FPTs not reported, with mild to moderate acne). A VAS score on a scale from –100 to +100 was used for evaluation; no details were reported. At both two and four weeks after final treatment, the difference between the PDT and light‐only sides was statistically significant only in the multiple treatment group (P < 0.05 at all assessment time points). Further data were not reported. Our interpretation of the graph was that at four weeks after final treatment, mean VAS score was 20 for both the PDT and the light‐only side in the single treatment group whereas in the multiple treatment group, mean VAS score was 50 on the light‐only side and 60 on the PDT side. SDs were not presented in the graph format.
Primary outcome 2: Investigator‐assessed change in lesion count: the change or percentage change from baseline in number of lesions
We have presented the details of participants, interventions, and the effects of interventions for this outcome in Table 5 for studies of light only therapies (excluding comparisons with PDT) and in Table 6 for studies of PDT (including comparisons with light‐only therapies). Please note that we calculated change from baseline (absolute change) by subtracting baseline count from count assessed at a certain time point. We calculated percentage change by dividing the absolute change with baseline count and then multiplying that value by 100 to get percentages.
2. Investigator‐assessed change in lesion count: 1. Light versus placebo or no treatment
2.1.b. Yellow light versus placebo or no treatment
Two studies included this comparison for this outcome, one parallel‐group trial with a total of 41 participants (FPT not reported, with mild to moderate acne), which compared a single light treatment with placebo (Seaton 2003), and another split‐face trial with a total of 40 participants (FPT not reported, Leeds severity greater than 2) which compared single or two light treatments with no treatment (Orringer 2004). Orringer 2004 initially randomised participants into single‐treatment and two‐treatment groups, with split‐face design within each group. The study authors reported only combined group data and were unable to provide separate data for the groups. Seaton 2003 reported medians of lesion counts, and we were unable to obtain original data, so we were unable to combine the results in a meta‐analysis. Results of the two studies were inconsistent.
Seaton 2003 found significantly greater improvement from baseline in ILs and total lesion counts in the laser‐treated group than in the placebo group at 12 weeks: ILs median (interquartile range) improvement from baseline in the treatment group was 49% (30% to 75%) versus 10% (‐8% to 49%) in the placebo group P = 0.024, and total lesions 53% (19% to 64%) versus 9% (‐16% to 38%) in the placebo group P = 0.023. NILs median (interquartile range) improvement from baseline in the treatment group was 40% (0% to 75%) versus ‐13% (‐42% to 23%) in the placebo group, with non significant difference between the groups (P = 0.14).
However Orringer 2004 reported non significant differences in changes in means of papules, pustules, comedones, and cysts at 12 weeks between the treated and untreated sides of the face. Our analyses using last observation carried forward (LOCF) data (n = 38) confirmed no significant differences in means between the treated and untreated sides of the face at 12 weeks: investigator‐assessed change in ILs (papules) was MD ‐2.00, 95% CI ‐6.60 to 2.60, P = 0.39 (Analysis 12.1); investigator‐assessed change in ILs (pustules) MD 1.00, 95% CI ‐0.66 to 2.66, P = 0.24 (Analysis 12.1); investigator‐assessed change in NILs, MD 1.30, 95% CI ‐8.00 to 10.60, P = 0.78 (Analysis 12.1); and investigator‐assessed change in cysts MD 0.00, 95% CI ‐0.76 to 0.76, P = 1.00 (Analysis 12.1).
12.1. Analysis.
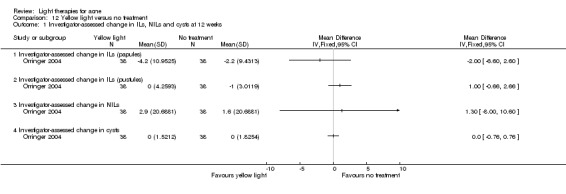
Comparison 12 Yellow light versus no treatment, Outcome 1 Investigator‐assessed change in ILs, NILs and cysts at 12 weeks.
2. Investigator‐assessed change in lesion count: 1. Light versus placebo or no treatment
2.1.c. Infrared light versus no treatment
Two split‐face trials (Darne 2011; Orringer 2007) of three treatments included a total of 84 participants (FPT I to VI, with mild to moderate acne). Meta‐analysis was not possible for this outcome because of timings and methods of outcome assessment and because the report of one of them included only medians of lesion counts. However, both studies had consistent results.
Darne 2011 randomised 38 participants (FPT I to V, with moderate to severe or mild but treatment‐resistant acne) and found similar reduction in ILs at one and 12 months on both sides; the treated sides' median was 0 (95% CI ‐4 to 2) and untreated sides' median was 0 (95% CI ‐3.7 to 0).
Orringer 2007 randomised 46 participants (FPT II to VI, with clinically active facial acne) and reported no significant differences in changes in papules, pustules, and open or closed comedones between the treated and untreated sides at week 14. Difference in changes in cyst counts was reported to be significant. Our analyses using LOCF data (n = 37, 9 participants withdrew prior to any clinical endpoint evaluation, and were not included in the analysis) confirmed no significant differences in means between treated and untreated face sides at week 14 (i.e. eight weeks after final treatment): investigator‐assessed change in ILs (papules) was MD ‐0.54, 95% CI ‐3.71 to 2.63, P = 0.74 (Analysis 13.1); investigator‐assessed change in ILs (pustules) MD ‐0.73, 95% CI ‐4.37 to 2.91, P = 0.69 (Analysis 13.1); investigator‐assessed change in NILs (open comedones) MD ‐2.92, 95% CI ‐8.13 to 2.29, P = 0.27 (Analysis 13.1); investigator‐assessed change in NILs (closed comedones) MD ‐6.95, 95% CI ‐23.07 to 9.17, P = 0.40 (Analysis 13.1). The difference in means for investigator‐assessed change in cysts was significant, favouring infrared light (MD ‐0.43, 95% CI ‐0.80 to ‐0.06, P = 0.02) (Analysis 13.2).
13.1. Analysis.
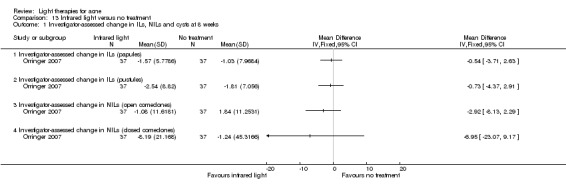
Comparison 13 Infrared light versus no treatment, Outcome 1 Investigator‐assessed change in ILs, NILs and cysts at 8 weeks.
13.2. Analysis.
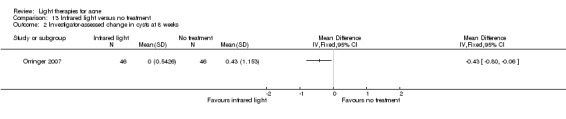
Comparison 13 Infrared light versus no treatment, Outcome 2 Investigator‐assessed change in cysts at 8 weeks.
Another smaller split‐face trial (Moneib 2014) of four treatments, randomised 24 participants (FPT II to V, with moderate to severe acne), but the time point of reported assessment was unclear. Results were inconsistent with Darne 2011 and Orringer 2007 (above). On the treated sides, mean papule counts (SD) reduced from a baseline of 15.42 (14.38) to 0.88 (3.35), mean pustule counts from a baseline of 2.58 (3.32) to 0.46 (1.38), open comedones from a baseline of 4.25 (7.59) to 1.25 (3.07), closed comedones from a baseline of 1.75 (3.45) to 0.33 (1.01), and nodules from a baseline of 1.00 (1.87) to 0.08 (0.41) at 'follow‐up'. On the control sides mean papule counts (SD) changed from baseline 12.83 (10.89) to 14.08 (12.93), mean pustule counts from a baseline of 3.17 (5.21) to 4.21 (7.40), open comedones from a baseline of 2.58 (3.37) to 2.88 (3.54), closed comedones from a baseline of 1.79 (3.75) to 1.21 (2.50), and nodules from a baseline of 0.92 (1.61) to 1.79 (2.00) at 'follow‐up'.
2. Investigator‐assessed change in lesion count: 1. Light versus placebo or no treatment
2.1.d. Blue light versus no treatment
One split‐face study (Elman 2003) of eight treatments included 23 participants with mild to severe acne and unclear FPT. ILs percentage change median reduction at two, four, and eight weeks post‐treatment were 59%, 61%, and 53%, respectively on treated sides (P = 0.01 at eight weeks compared with untreated sides, using McNemar test; other statistical data not provided). ILs percentage change median reduction was 30% at final treatment on untreated sides; other data were not available.
2. Investigator‐assessed change in lesion count: 1. Light versus placebo or no treatment
2.1.e. Red light versus no treatment
One split‐face study (Na 2007) of 122 self‐administered treatments (twice daily for eight weeks) included 30 participants (FPT not reported, with mild to moderate acne). At week eight, NILs percentage change ‐59% on treatment sides versus 3% increase on control sides (P < 0.005), ILs percentage change was ‐66% on treatment side versus 74% increase in ILs on control sides (P < 0.005). Further data were not given. At four weeks after final treatment 10/25 (40%) of followed‐up participants were reported to have "showed an increase in acne lesions", and at eight weeks 21/22 (95%) were reported to "have complained of acne exacerbation compared with their status during treatment period". Further data were not provided.
2. Investigator‐assessed change in lesion count: 1. Light versus placebo or no treatment
2.1.f. Blue‐red light versus placebo
Two parallel‐group studies (Kwon 2013; Papageorgieu 2000) included this comparison for this outcome but we were unable to pool data due to substantial methodological heterogeneity (84 versus 56 treatments, different timings of outcome assessment). We were also unable to obtain additional data and clarifications.
Kwon 2013, with 56 treatments, randomised 18 participants to the blue‐red light group and 17 to the placebo group (FPT III‐V, with mild to moderate acne). Mean IL counts reduced from baseline 22.8 to 5.3 (by 76.7%, P < 0.01) and mean NIL counts reduced from baseline 51.2 to 23.5 (by 53.3%, P < 0.01) at eight weeks after final treatment in the blue‐red light group. Mean reduction of IL and NIL counts in the placebo group was not statistically significant at eight weeks after final treatment (both P > 0.05). Results were reported as percentage improvements in graph format (means and SDs not presented).
Papageorgieu 2000, with 84 treatments, randomised 30 participants to the blue‐red light group and 25 to the white light group (FPTs not reported, all with mild to moderate acne). Blue‐red light was reported to be superior at all time points, differences in mean percentage improvements 50.3 (95% CI 40.1 to 60.5) for ILs and 66.5 (95% CI 56.0 to 77.0) for comedones at week 12 (final treatment).
2. Investigator‐assessed change in lesion count: 2. Light versus topical treatment
2.2.a. Light versus benzoyl peroxide (BPO)
Two parallel‐group trials included comparison of blue (de Arruda 2009) and blue‐red light (Papageorgieu 2000) with 5% BPO. A total of 115 participants were included (FPTs not reported, with mild to moderate acne). We did not carry out meta‐analysis due to differences in light wavelengths (blue versus blue‐red light), number of light treatment sessions (eight versus 84), number of daily applications of BPO (single versus twice daily), different outcomes recorded and timing of their assessment. We did not combine them with results of a split‐face study (Chang 2007) which compared a combination of BPO and three sessions of 530–750 nm light with BPO alone and included 30 women (FPT III‐IV, with mild to moderate acne). The results of these studies were inconsistent.
de Arruda 2009, with eight treatments, randomised 60 participants (unclear FPT, Brazilian group of Acne Grade II‐III) to two groups and found no statistically significant difference in decrease of means of ILs (P = 0.500) and NILs (P = 0.177) between the blue light and 5% BPO group. We calculated that at four weeks the MD in changes in NILs was 9.49, 95% CI ‐10.84 to 29.82; however, the MD in changes in ILs was 0 (and since the P value the study authors presented was 0.5, there are infinitely many possibilities for the standard error (SE), hence, the lack of a 95% CI provided for ILs).
Papageorgieu 2000, 84 treatments in total, randomised 30 participants to the blue‐red light group and 25 to the BPO group (FPTs not reported, all with mild to moderate acne). Blue–red light was reported to be superior to BPO at week 12 (P = 0.006). Difference in mean percentage improvements at week 12 was 17.6 (95% CI 7.5 to 27.6) for IL counts and 0.9 (95% CI ‐9.4 to 11.3) for comedones.
Chang 2007 compared a combination of BPO and three sessions of 530–750 nm light with BPO alone and included 30 women (FPT III‐IV, with mild to moderate acne and found no significant difference between IPL‐treated and untreated sides of the face for changes in mean papule and pustule counts (‐3.2 versus ‐3.1; P > 0.05). Further data were not reported.
2. Investigator‐assessed change in lesion count: 2. Light versus topical treatment
2.2.b. Light versus clindamycin
One parallel‐group trial (Gold 2005) compared eight sessions of 417 nm blue light with self‐administered topical clindamycin and included 34 participants (FPT not reported, with mild to moderate acne). This study found that NILs & ILs counts' 'averages' (ranges) in the blue‐light group were 29.4 (9 to 120) and 22.6 (16 to 34) at baseline and 21.4 (8 to 40) and 11.1 (0 to 24) four weeks after final treatment respectively. NILs & ILs counts' 'averages' (ranges) in the clindamycin group were 29 (9 to 95) and 17.4 (12 to 32) at baseline and 12 (4 to 38) and 10.4 (4 to 19) 4 weeks after final treatment respectively.
One split‐face trial (Lee 2010) compared eight treatments of full‐spectrum light with 1% clindamycin twice daily and included nine participants (FPT III, with moderate to severe acne).
We were unable to combine the results of these two trials quantitatively due to clinical and methodological differences and unclear reporting of timings of outcome assessment in one of the studies (Lee 2010).
2. Investigator‐assessed change in lesion count: 2. Light versus topical treatment
2.2.c. Light and other topical treatments
Four parallel‐group studies included this comparison, but they all had different topical treatments or combinations of topical treatments comparisons, so we did not perform a meta‐analysis.
Karsai 2010 compared clindamycin 1%–benzoyl peroxide 5% hydrating gel (C/BPO) alone with C/BPO in combination with two 585 nm PDL treatments and included 89 participants (FPT I‐III, with mild to moderate acne). C/BPO was applied daily over four weeks. In the C/BPO group, there was a 36.3% reduction in the number of ILs and 9.2% reduction in total lesion count four weeks after initial treatment (at final treatment). In the C/BPO plus light group, there was a 36.9% reduction in number of ILs and 9.0% reduction in total lesion count. Means and SD were reported in graph format. Our interpretation of the graph was that ILs (SD) in the C/BPO group reduced from baseline 37.5 (20) to 25 (15), and in the C/BPO plus light group from 50 (30) to 30 (25) at four weeks after initial treatment. Total lesions reduced from baseline 127.5 (70) to 115 (70) in the C/BPO group, and from 175 (105) to 150 (100) in the C/BPO plus light group. We judged further analyses would be biased due to lack of precise data, so we did not perform them.
There were three studies where details of topical treatments that were used were not specified or the control intervention was unclear.
Anyachukwu 2014 randomised 40 men (FPT unclear, Global Acne Grading System > 19) either to eight treatments of 905 nm light combined with 'self‐management topical agents' (including 'antibiotic cream', 'medicated soap', 'talcum powder' or 'personal hygiene'), or to the control group, who were treated with placebo‐non radiating light probe combined with 'self‐management topical agents'. Mean percentage change from baseline in combined number of lesions (SD) was 54.98 (16.297) in the laser group and 17.97 (16.472) in the control group three days after final treatment. Mean percentage changes from baseline in combined number of lesions at three days after final treatment were 70.37, 61.90, 71.43, 71.43 in the laser combined with 'antibiotic cream', 'medicated soap', 'talcum powder' and 'personal hygiene' subgroups respectively. Mean percentage change from baseline in combined number of lesions at three days after final treatment were 38.71, 45.00, 10.34 and 12.50 in the placebo plus 'antibiotic cream', 'medicated soap', 'talcum powder' and 'personal hygiene' subgroups respectively. Further data were not provided.
Ash 2015 randomised 26 participants to the blue‐light group (28 sessions in total) and 15 to the control group with an unclear (probably topical treatment) intervention (FPTs not reported I‐V, all with mild to moderate acne). At 12 weeks (four weeks after final treatment) mean lesion counts reduced by 50.08% (P = 0.002) in the treatment group and increased by 2.45% in the control group (P = 0.0029). Further data not given nor supplied upon request.
The other study (Borhan 2014) compared three treatments of 595 nm light plus "traditional topical antibiotic medication" with "traditional topical antibiotic medication" alone. A total of 40 participants were randomised (FPT III‐IV, with mild to moderate acne). At week 12 the combined number of lesions, reported as "acnes number" (SD) changed from a baseline of 25.7 (5.88) to 8.75 (2.91) in the laser combined with topical antibiotics group, and from a baseline of 25.75 (6.71) to 17.7 (5.14) in the topical antibiotics‐alone group (P = 0.0001).
2. Investigator‐assessed change in lesion count: 3. Light versus other comparators
2.3.a. Comparison of light therapies of different wavelengths
Four trials included different comparisons; blue and red light (Liu 2011); blue and blue‐red light (Papageorgieu 2000); 585 nm pulsed dye laser (PDL) with four 530‐750 nm IPL (Choi 2010) and 585 nm PDL with combined 585/1064nm PDL (Jung 2009), so we did not perform quantitative synthesis.
Papageorgieu 2000 (parallel‐group trial) had 84 treatments in total and randomised 30 participants to the blue‐red light group and 27 to the blue‐light group (FPTs not reported, all with mild to moderate acne). There was no significant difference between the treatments in ILs at week 12 (P = 0.1), nor in comedone count (P value not given). Difference in mean percentage improvements at week 12 was 13.1 (95% CI 3.0 to 23.1) for IL counts and 12.9 (95% CI 2.5 to 23.2) for comedones.
Liu 2011 (parallel‐group study) included results for 20 participants (FPTs III‐IV, all with mild to moderate acne) who completed the trial of eight sessions of blue light in one group (405 ± 10 nm, power of 30 mW/cm²) and red light (630 ± 10 nm, power of 48 mW/cm²) in the other group. In the blue‐light group, the mean ILs count dropped from baseline 19.2 to 5.5 (by 71.4%) at final treatment and in the red‐light group from baseline 8.2 to 6.6 at final treatment (by 19.5%). SDs and further details were not given.
Choi 2010 (split‐face trial) compared four sessions of 585 nm PDL with four 530‐750 nm IPL sessions and included 20 participants (FPT III‐V, with mild to moderate acne). Individual participant data were given in the paper (n = 17). Our analyses based on t‐distributions showed that at eight weeks PDL was not superior to IPL in changes in ILs (MD 2.00, 95% CI ‐0.85 to 4.85, P = 0.178, t = 1.431 Analysis 14.1) nor in changes in NILs (MD 0.77, 95% CI ‐3.65 to 5.19, P = 0.735, t = 0.355 Analysis 14.1). Results of the analyses using t‐distribution did not substantially differ from the ones in which we used normal distribution (Analysis 14.2).
14.1. Analysis.
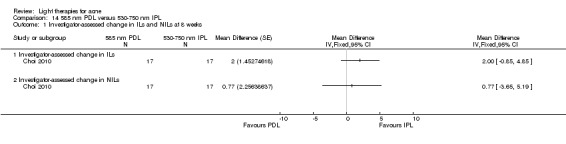
Comparison 14 585 nm PDL versus 530‐750 nm IPL, Outcome 1 Investigator‐assessed change in ILs and NILs at 8 weeks.
14.2. Analysis.
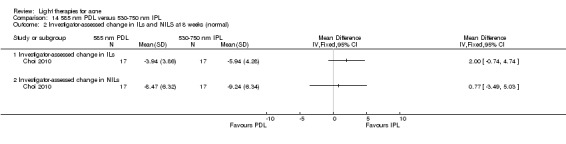
Comparison 14 585 nm PDL versus 530‐750 nm IPL, Outcome 2 Investigator‐assessed change in ILs and NILS at 8 weeks (normal).
Jung 2009 (split‐face trial) compared three sessions of 585 nm PDL with combined 585/1064nm PDL and included 18 participants (FPT not reported, with mild to moderate acne). ILs and NILs reduced by 86% and 69% respectively on the PDL sides and by 89% and 64% on the 585/1,064‐nm laser sides respectively at final evaluation (P values reported as < 0.05 "compared with baseline"). There was no significant difference in the effect of the two interventions (P values and further data not provided).
2. Investigator‐assessed change in lesion count: 3. Light versus other comparators
2.3.b. Comparison of light therapies of different doses
Three split‐face trials (Bernstein 2007; Jih 2006; Uebelhoer 2007) compared different numbers of sessions, passes and doses of 1450 nm lasers, in participants with different FPT and different timings of outcome assessment, so we did not perform a meta‐analysis.
Bernstein 2007 compared four sessions of two 1450 nm laser doses: single‐pass, high‐energy (13 to 14 J/cm²) and double‐pass, low‐energy (8 to 11 J/cm²) and included 30 participants (FPT I‐III, with mild to moderate acne). Individual participant data were given in the paper (n = 6). We found no significant difference at eight weeks, with MD ‐4.33, 95% CI ‐13.4 to 4.74, P = 0.372, t = ‐1.063 (Analysis 15.1). Results of the analyses using t‐distribution did not substantially differ from the ones in which we used normal distribution (MD ‐4.33, 95% CI ‐12.31 to 3.65) (Analysis 15.2).
15.1. Analysis.
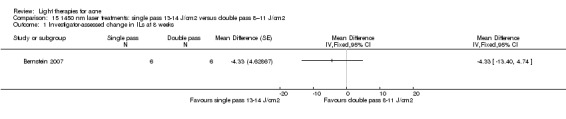
Comparison 15 1450 nm laser treatments: single pass 13‐14 J/cm2 versus double pass 8–11 J/cm2, Outcome 1 Investigator‐assessed change in ILs at 8 weeks.
15.2. Analysis.
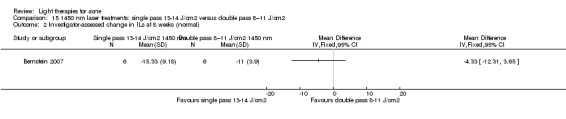
Comparison 15 1450 nm laser treatments: single pass 13‐14 J/cm2 versus double pass 8–11 J/cm2, Outcome 2 Investigator‐assessed change in ILs at 8 weeks (normal).
Jih 2006 compared three sessions of different (infrared) light intensities of 1450 nm diode laser: 14 J/cm² and 16 J/cm² and included 20 participants (FST II‐VI, with at least 20 ILs). Sponsors provided detailed data and our analyses confirmed no significant difference in reduction between the different light intensities. The MDs in changes in ILs and percentage changes in ILs see (Analysis 16.1) were: MD ‐2.40, 95% CI ‐6.46 to 1.66, P = 0.26, t = ‐1.203 and MD ‐3.40, 95% CI ‐14.21 to 7.41, P = 0.54, t = ‐ 0.641 respectively at one month; MD ‐7.05, 95% CI ‐16.05 to 1.95, P = 0.13, t = ‐1.596 and MD ‐3.20, 95% CI ‐7.43 to 1.03, P = 0.15, t = 1.541 respectively at three months; MD ‐2.00, 95% CI ‐5.87 to 1.87, P = 0.32, t = ‐1.053 and MD 2.49, 95% CI ‐6.37 to 11.35, P = 0.59, t = 0.572 respectively at six months; and MD ‐2.40, 95% CI ‐7.13 to 2.33, P = 0.33, t = ‐1.034 and MD ‐5.59, 95% CI ‐26.07 to 14.89, P = 0.60, t = ‐0.556 respectively at 12 months. Results of the analyses using t‐distribution did not substantially differ from the ones in which we used normal distribution (Analysis 16.2).
16.1. Analysis.
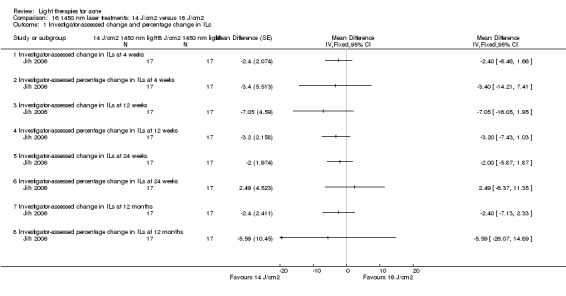
Comparison 16 1450 nm laser treatments: 14 J/cm2 versus 16 J/cm2, Outcome 1 Investigator‐assessed change and percentage change in ILs.
16.2. Analysis.
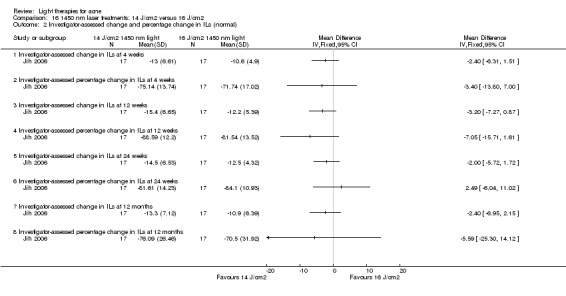
Comparison 16 1450 nm laser treatments: 14 J/cm2 versus 16 J/cm2, Outcome 2 Investigator‐assessed change and percentage change in ILs (normal).
Uebelhoer 2007 compared three sessions of single‐pass with double‐pass of 1450 nm laser treatment and included 11 participants (FPT not given, with at least 10 ILs on each side of the face). There was a statistically significant reduction of mean acne lesion counts on both the single‐pass side and double‐pass side of 57.6% (P = 0.02) and 49.8% (P = 0.02), respectively. Further details were not given.
One parallel‐group trial (NCT00706433) compared four interventions:
20% ALA (45 min incubation) plus 1000 s of blue light;
20% ALA (45 min incubation) plus 500 s of blue light;
vehicle (45 min incubation) plus 1000 s of blue light; and
vehicle (45 min incubation) plus 500 s of blue light.
The study included a total of 266 participants (FPT I‐VI, with moderate to severe acne, IGA score 3 and 4, with at least 20 ILs); 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group. At three weeks after final treatment investigator‐assessed median change in ILs (SD) was ‐19.0 (22.8) in the vehicle 1000 s and ‐14.5 (24.0) in the vehicle 500 s group; investigator‐assessed median percentage change in ILs (SD) was ‐41.7 (38.82) in the vehicle 1000 s and ‐37.0 (40.23) in the vehicle 500 s group. At six weeks after final treatment investigator‐assessed median change in ILs (SD) was ‐21.0 (23.63) in the vehicle 1000 s and ‐17.0 (26.71) in the vehicle 500 s group; investigator‐assessed median percentage change in ILs (SD) was ‐48.4 (32.81) in the vehicle 1000 s and ‐45.2 (50.15) in the vehicle 500 s group. We could not perform statistical tests to determine whether any changes were significant due to the study authors’ use of median changes rather than the typical mean changes required for significance testing in order to make appropriate comparisons with other included studies. Furthermore, it is not clearly stated whether the study authors implemented an ITT analysis or a LOCF approach to handling missing data.
2. Investigator‐assessed change in lesion count: 3. Light versus other comparators
2.3.d. Light alone versus combined with microdermoabrasion
One split‐face trial (Wang 2006) compared four sessions of 1450 nm diode laser plus microdermoabrasion with 1450 nm diode laser therapy alone. The trial included 20 participants (FPT II‐IV, with moderate to severe acne). Microdermoabrasion plus light treatment decreased the mean acne lesion count by 52.8% by six weeks and 54.4% by 12 weeks (P < 0.02 compared with baseline counts). Light treatment alone reduced the counts by 53.5% by six weeks and 61.1% by 12 weeks (P < 0.05 compared with baseline counts). There was no statistically significant difference between the two treatments at any point.
2. Investigator‐assessed change in lesion count: 3. Light versus other comparators
2.3.e Light in combination with carbon lotion versus no treatment
One split‐face trial (Jung 2012) compared three sessions of quasi‐long pulse and Q‐switched 1064 nm Nd:YAG laser plus carbon lotion with non‐treated control and included 22 participants (FPT III‐V, with unclear severity of acne). The difference in means of both ILs and NILs was statistically significant between treated and untreated sides (P < 0.001), but clear data for non treated sides were not given. Both ILs and NILs reduced to 58.6% (P < 0.001) and to 52.4% (P < 0.001), respectively on the laser‐treated side.
2. Investigator‐assessed change in lesion count: 4. MAL‐PDT versus other comparators
We have presented the details of participants, studies of PDT (including comparisons with light‐only therapies), and the effects of interventions for this outcome in Table 6.
2. Investigator‐assessed change in lesion count: 4. MAL‐PDT versus other comparators
2.4.a. MAL‐PDT versus red light alone
We combined results of three parallel‐group studies (NCT00594425; NCT00933543; Pariser 2013) comparing four sessions of red light plus MAL with placebo cream and red light, with a final evaluation at six weeks after the last treatment. We combined and compared two groups from these studies: 80 mg/g MAL‐PDT groups (a total of 202 participants) and placebo cream groups (a total of 158 participants). The participants had FPT I‐VI and moderate to severe acne. NCT00594425 had an additional group of 50 participants treated with 40 mg/g MAL‐PDT whom we did not include in the meta‐analysis (see below). The statistical heterogeneity across studies was not substantial, that is, the I² statistic fitted the criteria we stated in our protocol (I² statistic had to be lower than 50%). I² was 39% for change in ILs, 19% for percentage change in ILs, 11% for change in NILs and 35% for percentage change in NILs. Therefore we judged it was appropriate to combine the results. However, there was some clinical heterogeneity across studies to take into account. We have narratively summarised it here, please check Characteristics of included studies tables of each study for details.
Pariser 2013 included only people with severe acne, whilst NCT00594425 and NCT00933543 included people with both severe and moderate acne (the sponsor later provided information that less than 20% of the included participants had severe acne in those two trials). Pariser 2013 and NCT00933543 included all skin types, whilst NCT00594425 included only skin types I‐IV. Occlusive dressing was used during incubation in Pariser 2013 and NCT00594425, but was not used in NCT00933543. Sponsors later clarified that investigators used the Aktilite lamp in NCT00594425, and the Nedax lamp in Pariser 2013 and NCT00933543. Both lamps produce a wavelength of 632 nm, but the illumination field is four times larger with the Nedax lamp. The angle between the LED panel and the face is also different (90° for the Aktilite and 60° for the Nedax lamp).
Meta‐analysis of these three studies (n = 360), using a random‐effects model, showed that MAL‐PDT was not superior to red light alone for: change in ILs (MD ‐2.85, 95% CI ‐7.51 to 1.81) (Analysis 17.1); percentage change in ILs (MD ‐10.09, 95% CI ‐20.25 to 0.06) (Analysis 17.2); change in NILs (MD ‐2.01, 95% CI ‐7.07 to 3.05) (Analysis 17.3); nor for percentage change in NILs (MD ‐8.09, 95% CI ‐21.51 to 5.32). (Analysis 17.4). See Table 2 where we rated the evidence as moderate quality for these outcomes. Please note that these studies are not presented in Table 6.
17.1. Analysis.
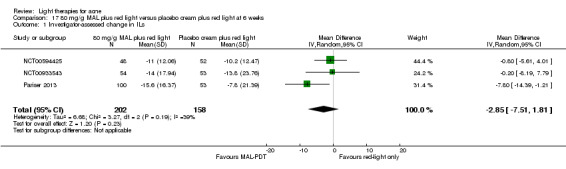
Comparison 17 80 mg/g MAL plus red light versus placebo cream plus red light at 6 weeks, Outcome 1 Investigator‐assessed change in ILs.
17.2. Analysis.
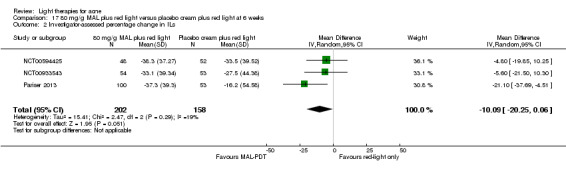
Comparison 17 80 mg/g MAL plus red light versus placebo cream plus red light at 6 weeks, Outcome 2 Investigator‐assessed percentage change in ILs.
17.3. Analysis.
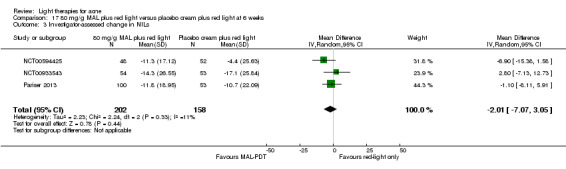
Comparison 17 80 mg/g MAL plus red light versus placebo cream plus red light at 6 weeks, Outcome 3 Investigator‐assessed change in NILs.
17.4. Analysis.
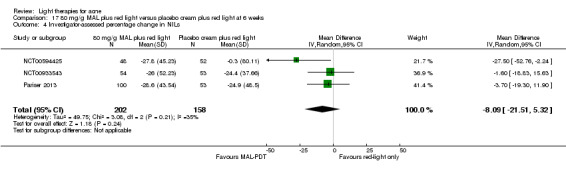
Comparison 17 80 mg/g MAL plus red light versus placebo cream plus red light at 6 weeks, Outcome 4 Investigator‐assessed percentage change in NILs.
NCT00594425 was a three‐arm parallel‐group trial, which also randomised 50 participants in the 40 mg/g MAL‐PDT group (FPT I‐IV, with moderate to severe acne, IGA score 3 to 4, 20 to 100 ILs and up to 200 NILs on the face). Four treatments at two‐week intervals were applied; 43/50 participants completed treatment in the 40 mg/g group and 42/52 completed treatment in the placebo (vehicle cream) group. We used the data as provided by the sponsors, who used both ITT and the LOCF method to account for missing data within their analyses. Our analyses showed that at six weeks after final treatment 40 mg/g MAL‐PDT was not superior to placebo cream plus red light in change in ILs (MD ‐3.00, 95% CI ‐7.76 to 1.76, P = 0.22) (Analysis 18.1), in percentage change in ILs (MD ‐7.90, 95% CI ‐22.33 to 6.53, P = 0.28) (Analysis 18.2), and in change in NILs (MD ‐7.50, 95% CI ‐16.07 to 1.07, P = 0.09) (Analysis 18.3), while there was a borderline superiority in percentage change in NILs (MD ‐25.80, 95% CI ‐51.69 to 0.09, P = 0.05) (Analysis 18.4).
18.1. Analysis.
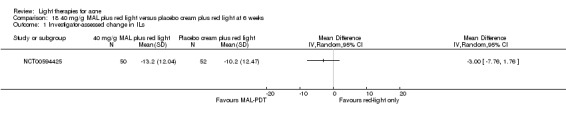
Comparison 18 40 mg/g MAL plus red light versus placebo cream plus red light at 6 weeks, Outcome 1 Investigator‐assessed change in ILs.
18.2. Analysis.
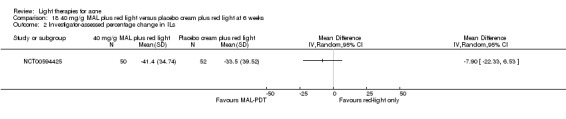
Comparison 18 40 mg/g MAL plus red light versus placebo cream plus red light at 6 weeks, Outcome 2 Investigator‐assessed percentage change in ILs.
18.3. Analysis.
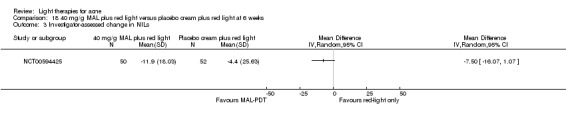
Comparison 18 40 mg/g MAL plus red light versus placebo cream plus red light at 6 weeks, Outcome 3 Investigator‐assessed change in NILs.
18.4. Analysis.
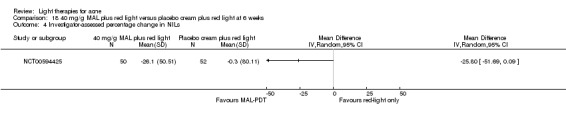
Comparison 18 40 mg/g MAL plus red light versus placebo cream plus red light at 6 weeks, Outcome 4 Investigator‐assessed percentage change in NILs.
Two more trials included this comparison for these outcomes, but we were unable to combine their results quantitatively because one was a split‐back trial (two 8 m² x 8 cm² areas) which included only participants with FPT V‐VI (NCT00673933) and the other was a split‐face trial, which compared only two sessions of 635 nm light plus 160 mg/g MAL with placebo cream and light (Hörfelt 2006). Both of these studies were assessed at different time points.
NCT00673933 compared two sessions of red light plus 80 mg/g MAL with placebo cream and red light. It included a total of 20 participants (FPT V‐VI, with moderate to severe acne). Our analyses based on t‐distributions showed that at four weeks after final treatment MAL‐ PDT was not superior in changing the ILs count (MD 0.20 CI 95% ‐1.24 to 1.64, P = 0.79, t = 0.280) (Analysis 19.1) nor the NILs count (MD ‐0.45 CI 95% ‐2.95 to 2.05, P = 0.73, t = ‐0.365) (Analysis 19.1). ITT analysis results were given (n = 20). Results of the analyses using t‐distribution did not substantially differ from the ones in which we used normal distribution (Analysis 19.2)
19.1. Analysis.

Comparison 19 80 mg/g MAL plus red light versus placebo cream plus red light at 4 weeks, Outcome 1 Investigator‐assessed change in ILs and NILs.
19.2. Analysis.
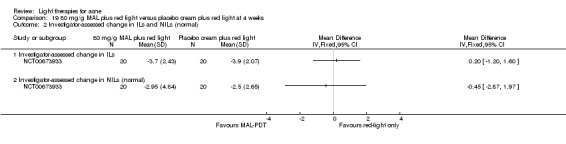
Comparison 19 80 mg/g MAL plus red light versus placebo cream plus red light at 4 weeks, Outcome 2 Investigator‐assessed change in ILs and NILs (normal).
Hörfelt 2006 compared two sessions of 635 nm light plus 160 mg/g MAL with placebo cream and light. The trial included 30 participants (FPT I‐III, with moderate to severe acne). MAL–PDT was reported to be significantly more effective than light alone for ILs: median percentage reduction 63% (95% CI 50% to 71%) versus 28% (95% CI 19% to 47%) at four weeks (P = 0.0004), and 54% (95% CI 35% to 64%) versus 20% (95% CI 8% to 50%) at 10 weeks (P = 0.0006). No statistically significant difference in treating NILs was observed between two interventions (open comedones P = 0.6875, closed comedones P = 1.00). The study authors used the LOCF method to account for missing data for three participants who dropped out due to adverse effects. The study authors stated that they used both ITT and LOCF, in this way, within their analyses. Study authors provided further data on changes and percentage changes in ILs. We calculated that MAL‐PDT was not superior to placebo cream plus light in change in ILs at four weeks nor at 10 weeks, with MD ‐2.60, 95% CI ‐6.45 to 1.25, P = 0.19 (Analysis 20.1) and MD ‐2.50, 95% CI ‐6.59 to 1.59, P = 0.23 (Analysis 20.1) respectively. However, it was superior in percentage change in ILs at four weeks and percentage change in ILs at 10 weeks, with MD ‐23.90, 95% CI ‐39.04 to ‐8.76, P = 0.002 (Analysis 20.2) and MD ‐19.10, 95% CI ‐37.63 to ‐0.57, P = 0.04 (Analysis 20.2), respectively.
20.1. Analysis.
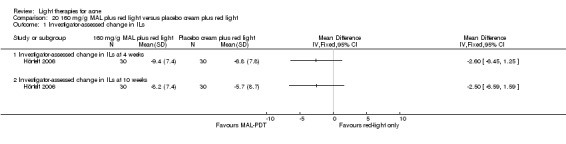
Comparison 20 160 mg/g MAL plus red light versus placebo cream plus red light, Outcome 1 Investigator‐assessed change in ILs.
20.2. Analysis.
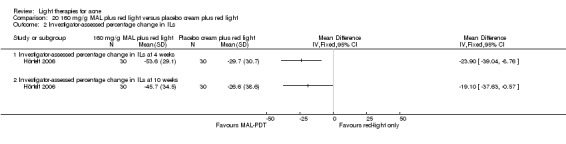
Comparison 20 160 mg/g MAL plus red light versus placebo cream plus red light, Outcome 2 Investigator‐assessed percentage change in ILs.
2. Investigator‐assessed change in lesion count: 4. MAL‐PDT versus other comparators
2.4.b. MAL‐PDT versus yellow light alone
One split‐face study (Haedersdal 2008) compared three sessions of 595 nm LPDL plus 160 mg/g MAL with LPDL only and included 15 participants (FPT I to III, with at least 12 facial ILs). Median percentage reduction in IL counts was significantly greater with MAL–LPDL than with LPDL alone at four weeks (70% versus 50%, P = 0.03) and 12 weeks (80% versus 67%, P = 0.004). Median percentage reduction in NILs lesions was significantly greater on the MAL–LPDL side at four weeks (P = 0.035), but the difference between the treatments (53% versus 42%) did not achieve statistical significance at final follow‐up (P = 0.158). Median IL counts (25% to 75% percentiles) at baseline, four and 12 weeks were 21.0 (16 to 36), 7 (4.75 to 15) and 3.5 (2 to 9.5) on the MAL‐LPDL side, and 22 (14 to 36), 10 (6.5 to 16) and 7 (2 to 9.5) on the LPDL side respectively. Median NIL counts (25% to 75% percentiles) at baseline, four and 12 weeks were 33 (26 to 41), 23 (17 to 40) and 15 (9 to 21) on the MAL‐LPDL side, and 32 (25 to 41), 26 (17 to 33) and 20 (12 to 27) on the LPDL side respectively.
2. Investigator‐assessed change in lesion count: 4. MAL‐PDT versus other comparators
2.4.c. MAL‐PDT versus placebo or no treatment
This was a parallel‐group study (Wiegell 2006b) of two treatments of 630 nm plus 160 mg/g MAL which included 21 participants in the treatment group and 15 in the control group (FPT II to V, with at least 12 facial ILs). There was a significantly greater median reduction in ILs in the treatment group at eight weeks (P = 0.023) and 12 weeks (P = 0.0023). Median ILs change from baseline (range) at 12 weeks was 24 (‐4 to 55) in the MAL‐PDT group and 0 (‐39 to 19) in the control group. Median ILs count (range) at baseline, 4, 8 and 12 weeks were 46 (13 to 99), 24 (9 to 68), 22 (8 to 83) and 14 (4 to 44) in the MAL‐PDT group and 32 (13 to 99), 32 (8 to 128), 42 (9 to 109) and 40 (13 to 80) in the control group. There was a non‐significant difference in median change in NILs between the MAL‐PDT and control group (P = 0.90) at 12 weeks. Median NILs change from baseline (range) at 12 weeks was 6 (‐15 to 18) in the MAL‐PDT group and 2 (‐14 to 35) in the control group. Median NILs count (range) at baseline, 4, 8 and 12 weeks were 17 (2 to 73), 22 (0 to 56), 24 (6 to 59) and 24 (9 to 74) in the MAL‐PDT group and 24 (2 to 64), 19 (0 to 76), 21 (2 to 81) and 31 (5 to 59) in the control group.
2. Investigator‐assessed change in lesion count: 4. MAL‐PDT versus other comparators
2.4.d. MAL‐PDT other
Due to substantial clinical and methodological heterogeneity of four studies with different interventions and comparators (Bissonnette 2010; Hong 2013; NCT00594425; Yeung 2007) we did not perform quantitative synthesis of their results.
Bissonnette 2010 (parallel‐group trial) randomised 44 participants (FPT I to IV, with 10 or more ILs on each face side) to compare 80 mg/g MAL with or without occlusion followed by different red light intensity exposure; participants randomised in four groups with 25 J/cm² or 37 J/cm² and with or without occlusion; four treatments, assessed at four and 12 weeks after the final treatment.
ILs means changed from baseline 16.7 (95% CI 11.8 to 21.5), 16.6 (95% CI 12.6 to 20.5), 14.9 (95% CI 12.3 to 17.1) and 15.7 (95% CI 13.17 to 18.8) on the non‐occluded 25 J/cm², occluded 25 J/cm², non‐occluded 37 J/cm² and occluded 37 J/cm² face sides, respectively to 11.0 (95% CI 8.7 to 13.4), 9.4 (95% CI 6.3 to 12.4), 8.6 (95% CI 5.2 to 11.9) and 8.9 (95% CI 5.5 to 11.8) respectively at 12 weeks after final treatment.
NILs means changed from baseline 10.8 (95% CI 7.0 to 14.6), 11.3 (95% CI 7.9 to 14.7), 14.6 (95% CI 7.8 to 21.4) and 15.1 (95% CI 8.9 to 21.3) on the non‐occluded 25 J/cm², occluded 25 J/cm², non‐occluded 37 J/cm² and occluded 37 J/cm² face sides, respectively to 8.6 (95% CI 5.7 to 11.5), 7.5 (95% CI 4.9 to 10.1), 12.7 (95% CI 5.8 to 19.6) and 12.2 (95% CI 5.8 to 18.6) respectively at 12 weeks after final treatment. The number of ILs was significantly lower than baseline on all face sides except the non‐occluded 25 J/cm² (based on non‐overlapping 95% CI). There was no statistically significant difference in mean reduction of ILs between face sides with and without occlusion, for both 25 J/cm² and 37 J/cm². There was no statistically significant difference in NILs mean change from baseline between the treatments at 12 weeks follow‐up, based on overlapping CIs. The study authors stated using both ITT and LOCF within their analyses, please see the 'Risk of bias' table of this study for details.
Hong 2013 (split‐face study) compared three sessions of 160 mg/g MAL plus red light with three sessions of MAL plus IPL and included 22 participants (FPT IV to V). At four weeks after treatment, there was no statistically significant difference between red light and IPL treated sides in mean percentage reduction of ILs (69.5% versus 72.0% respectively) and NILs (43.4% versus 46.3% respectively). Further data were not provided.
NCT00594425 (three‐arm parallel‐group trial) randomised 48 participants to the 80 mg/g MAL‐PDT arm and 50 participants to the 40 mg/g MAL‐PDT arm (FPT I to IV, with moderate to severe acne, IGA score 3 to 4, 20 to 100 ILs and up to 200 NILs on the face). Four treatments at two‐week intervals were applied, 37 participants completed treatment in the 80 mg/g group, and 43 completed treatment in the 40 mg/g group. Our analyses showed that at six weeks after final treatment 80 mg/g MAL‐PDT was not superior to 40 mg/g MAL‐PDT in change in ILs (MD 2.20, 95% CI ‐2.57 to 6.97, P = 0.37) (Analysis 21.1), in percentage change in ILs (MD 3.10, 95% CI ‐11.8 to 17.38, P = 0.67) (Analysis 21.2), in change in NILs (MD 0.6, CI 95% ‐6.36 to 7.56, P = 0.87) (Analysis 21.3), nor in percentage change in NILs (MD ‐1.7, 95% CI ‐20.67 to 17.27, P = 0.94) (Analysis 21.4).
21.1. Analysis.
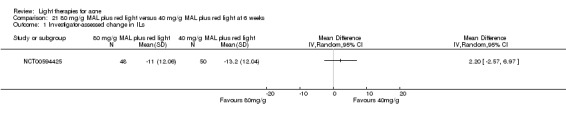
Comparison 21 80 mg/g MAL plus red light versus 40 mg/g MAL plus red light at 6 weeks, Outcome 1 Investigator‐assessed change in ILs.
21.2. Analysis.
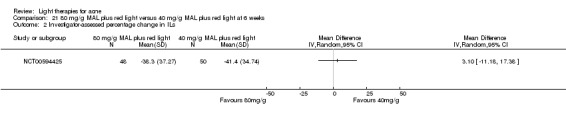
Comparison 21 80 mg/g MAL plus red light versus 40 mg/g MAL plus red light at 6 weeks, Outcome 2 Investigator‐assessed percentage change in ILs.
21.3. Analysis.
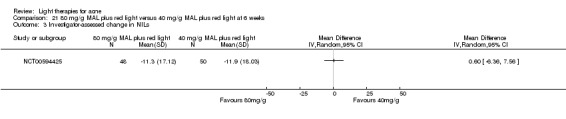
Comparison 21 80 mg/g MAL plus red light versus 40 mg/g MAL plus red light at 6 weeks, Outcome 3 Investigator‐assessed change in NILs.
21.4. Analysis.
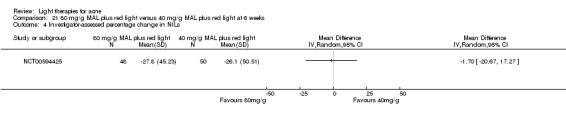
Comparison 21 80 mg/g MAL plus red light versus 40 mg/g MAL plus red light at 6 weeks, Outcome 4 Investigator‐assessed percentage change in NILs.
Yeung 2007 30 participants (FPT IV to V, with moderate acne) used topical adapalene 0.1% gel at night and were randomised to two split‐face treatment groups: 530 nm to 750 nm light plus 160 mg/g MAL versus IPL light (11 participants completed treatment) or IPL versus adapalene‐only control (12 participants completed treatment). Four light treatments were applied. We performed analyses based on t‐distribution and found that MAL‐PDT was not superior to IPL alone in percentage change in ILs at both four weeks and at 12 weeks, with MD ‐30.60, 95% CI ‐70.37 to 9.17, P = 0.141, t = ‐1.567 (Analysis 22.1) and MD ‐41.60, 95% CI ‐81.90 to ‐1.30, P = 0.052, t = ‐2.103 (Analysis 22.1) respectively. However, we found a transient superior effect on percentage change in NILs at four weeks, which was lost at 12 weeks, with MD ‐36.10, 95% CI ‐60.18 to ‐12.02, P = 0.006, t = ‐3.054 (Analysis 22.1) and MD 5.60, 95% CI ‐29.13 to 40.33, P = 0.754, t = 0.328 (Analysis 22.1) respectively. Results of the analyses using t‐distribution did not substantially differ from the ones in which we used normal distribution (Analysis 22.2).
22.1. Analysis.
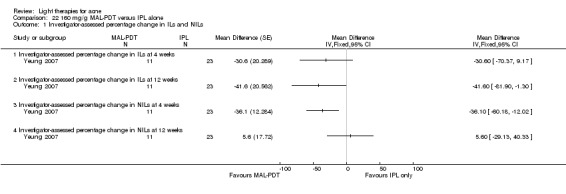
Comparison 22 160 mg/g MAL‐PDT versus IPL alone, Outcome 1 Investigator‐assessed percentage change in ILs and NILs.
22.2. Analysis.
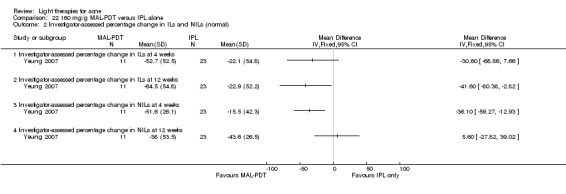
Comparison 22 160 mg/g MAL‐PDT versus IPL alone, Outcome 2 Investigator‐assessed percentage change in ILs and NILs (normal).
We found no difference in effect between adapalene and MAL‐PDT in percentage change in ILs at both four weeks and at 12 weeks, with MD 19.70, 95% CI ‐15.32 to 54.72, P = 0.283, t = 1.170 (Analysis 23.1) and MD 23.50, 95% CI ‐11.68, 58.68), P = 0.205, t = 1.390 (Analysis 23.1) respectively. However, MAL‐PDT also had a transient superior effect to adapalene on percentage change in NILs at four weeks, which was lost at 12 weeks, with MD ‐37.80, 95% CI ‐63.97 to ‐11.63, P = 0.01, t = ‐3.005 (Analysis 23.1) and MD ‐53.10, 95% CI ‐119.64 to 13.44, P = 0.133, t = ‐1.660 (Analysis 23.1) respectively. Results of the analyses using t‐distribution did not substantially differ from the ones in which we used normal distribution (Analysis 23.2).
23.1. Analysis.
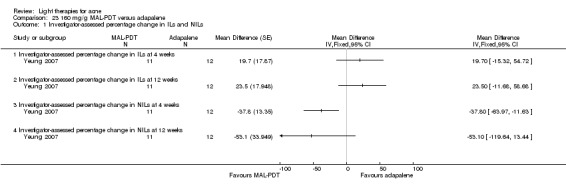
Comparison 23 160 mg/g MAL‐PDT versus adapalene, Outcome 1 Investigator‐assessed percentage change in ILs and NILs.
23.2. Analysis.
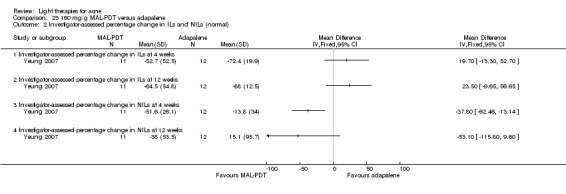
Comparison 23 160 mg/g MAL‐PDT versus adapalene, Outcome 2 Investigator‐assessed percentage change in ILs and NILs (normal).
2. Investigator‐assessed change in lesion count: 5. ALA‐PDT versus other comparators
2.5.a. ALA‐PDT versus red light alone
One split‐back trial (Pollock 2004) compared three sessions of 635 nm light plus 20% ALA with light alone, ALA alone and untreated control. The trial included 10 participants (FPT I to III and V, with mild to moderate acne). There was a statistically significant reduction from baseline in IL counts from the second treatment (P < 0.005) at the ALA–PDT site but not the other sites: reduction in acne was 69% at 21 days' follow‐up. Further data was reported in graph format. Mean baseline IL counts were 8.3, and 11.6 respectively at the light‐alone and ALA‐PDT areas. At three weeks' follow‐up IL counts at the light‐alone and ALA‐PDT areas decreased to 6.1 and 6.3 respectively. Other data were not given.
2. Investigator‐assessed change in lesion count: 5. ALA‐PDT versus other comparators
2.5.b. ALA‐PDT versus blue light alone
One parallel‐group trial (NCT00706433) compared four interventions:
20% ALA (45 min incubation) plus 1000 s of blue light;
20% ALA (45 min incubation) plus 500 s of blue light;
vehicle (45 min incubation) plus 1000 s of blue light; and
vehicle (45 min incubation) plus 500 s of blue light.
The study included a total of 266 participants (FPT I to VI, with moderate to severe acne, IGA score 3 and 4, with at least 20 ILs); 68 in the ALA 1000 s group, 65 in the ALA 500 s group, 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group. At three weeks after final treatment investigator‐assessed median change in ILs (SD) was ‐18.0 (26.3) in ALA 1000 s, ‐14.0 (26.8) in the ALA 500 s, ‐19.0 (22.8) in the vehicle 1000 s and ‐14.5 (24.0) in the vehicle 500 s group; investigator‐assessed median percentage change in ILs (SD) was ‐37.5 (38.79) in ALA 1000 s, ‐29.2 (46.68) in the ALA 500 s, ‐41.7 (38.82) in the vehicle 1000 s and ‐37.0 (40.23) in the vehicle 500 s group. At six weeks after final treatment investigator‐assessed median change in ILs (SD) was ‐18.5 (30.15) in ALA 1000 s, ‐13.0 (28.74) in the ALA 500 s, ‐21.0 (23.63) in the vehicle 1000 s and ‐17.0 (26.71) in the vehicle 500 s group; investigator‐assessed median percentage change in ILs (SD) was ‐34.4 (37.8) in ALA 1000 s, ‐29.0 (42.57) in the ALA 500 s, ‐48.4 (32.81) in the vehicle 1000 s and ‐45.2 (50.15) in the vehicle 500 s group. Statistical tests to determine whether any changes were significant could not be performed due to the study authors’ use of median changes rather than the typical mean changes required for significance testing in order to make appropriate comparisons with other included studies. Furthermore, it was not clearly stated whether the study authors implemented an ITT analysis or a LOCF approach to handling missing data. See Table 3 where we rated the evidence as very low quality for this comparison.
2. Investigator‐assessed change in lesion count: 5. ALA‐PDT versus other comparators
2.5.d. ALA‐PDT versus IPL alone
Three trials included this comparison, but one had a split‐face design (Oh 2009) and included three treatments with different incubation times in participants with moderate to severe acne, whilst the other two were parallel‐group trials, of different ALA doses, numbers of treatments, application intervals and incubation times, and included participants of different acne severity (Mei 2013, Ragab 2014). We did not combine results because of this heterogeneity.
Oh 2009 compared three sessions of 20% ALA plus IPL (one side of the face randomised to either 30 minutes' or three hours' incubation) with IPL only and included 20 participants (FPT III to IV, with moderate to severe acne). Mean reduction of ILs was 84.4% in the long‐incubation time group, 72.6% in the short‐incubation time group and 65.9% on the sides of the face treated with IPL alone at four weeks (P < 0.001 in all cases). Mean reduction of ILs was 89.5% in the long incubation time group, 83.0% in the short incubation time group and 74.0% for the sides of the face treated with IPL alone at 12 weeks (P < 0.001 in all cases). Mean reduction was significantly greater in the group where the sides of the face were treated for the long incubation time compared to the IPL‐alone treated sides (P = 0.01). The difference was not statistically significant between short incubation and placebo‐treated sides (P = 0.21). Further data were not given.
Mei 2013 (FPT II to IV, with severe acne) compared four treatments of 10% ALA plus IPL (21 participants randomised) to placebo cream plus IPL (20 participants randomised). Our analyses based on t‐distribution showed that ALA‐PDT was superior to light alone in percentage changes in ILs, with MD 13.80, 95% CI 1.34 to 26.26, P = 0.04, t = 2.240 (Analysis 24.1) and in percentage changes in NILs, with MD 24.10, 95% CI 4.65 to 43.55, P = 0.02, t = 2.506 (Analysis 24.1). Results of the analyses using t‐distribution did not substantially differ from the ones in which we used normal distribution (Analysis 24.2).
24.1. Analysis.
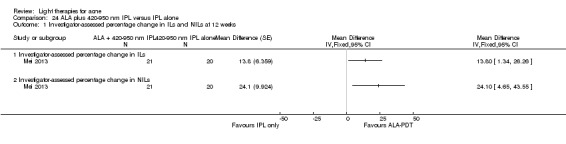
Comparison 24 ALA plus 420‐950 nm IPL versus IPL alone, Outcome 1 Investigator‐assessed percentage change in ILs and NILs at 12 weeks.
24.2. Analysis.
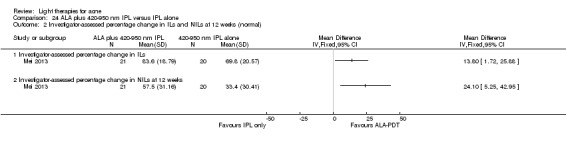
Comparison 24 ALA plus 420‐950 nm IPL versus IPL alone, Outcome 2 Investigator‐assessed percentage change in ILs and NILs at 12 weeks (normal).
Ragab 2014 (FPT III to V, with mild to moderate facial acne) compared two treatments of 20% ALA plus IPL (15 participants randomised) with IPL alone (10 participants randomised). Mean IL counts decreased from a baseline of 15.7 to 7.7 and 5.4 at two and eight weeks respectively in the ALA‐IPL group; and from a baseline of 9.6 to 5.2 and 4.4 at two and eight weeks respectively in the IPL alone group. Mean NIL (comedones) counts decreased from a baseline of 50.9 to 36.9 and 31.3 at two and eight weeks respectively in the ALA‐IPL group; and from a baseline of 41.8 to 23.8 and 24.4 at two and eight weeks respectively in the IPL alone group. Mean combined lesion counts decreased from a baseline of 66.6 to 35.7 at eight weeks in the ALA‐IPL group; and from a baseline of 51.4 to 28.8 at eight weeks in the IPL alone group. SDs were not reported.
2. Investigator‐assessed change in lesion count: 5. ALA‐PDT versus other comparators
2.5.f. ALA‐PDT versus placebo or no treatment
One split‐face trial (Orringer 2010) compared three sessions of 20% ALA plus PDL with untreated control. The trial included 44 participants (all FPTs, severity of acne unclear). The study authors reported no statistically significant difference between treated and untreated control skin in papules, pustules, cysts, closed and open comedones at week 16, but there was a transient statistically significant decrease from baseline in mean papule counts on treated sides when compared with untreated sides at week 10. There was no statistically significant difference between treated and untreated control sides in all other lesion counts at week 10. Our analyses using LOCF data (n = 44) confirmed a transient statistically significant decrease from baseline in investigator‐assessed change in ILs (papules) on treated sides when compared with untreated sides at week 10 of the study (i.e. four weeks after final treatment) see (Analysis 25.1), with MD ‐4.50, 95% CI ‐8.28 to ‐0.72, P = 0.02. We found no significant differences in means between treated and untreated sides of the face for investigator‐assessed change in ILs (pustules) MD ‐0.60, 95% CI ‐5.09 to 3.89, P = 0.79, for investigator‐assessed change in NILs (open comedones) MD ‐0.37, 95% CI ‐7.76 to 7.02, P = 0.92, for investigator‐assessed change in NILs (closed comedones) MD ‐3.90, 95% CI ‐12.05 to 4.25, P = 0.35, and for cysts MD 0.03, 95% CI ‐0.53 to 0.59, P = 0.92. Our analyses also confirmed no significant differences in means between treated and untreated sides of the face at week 16 (i.e. 10 weeks after final treatment): investigator‐assessed change in ILs (papules) was MD ‐0.82, 95% CI ‐6.03 to 4.39, P = 0.76; investigator‐assessed change in ILs (pustules) MD ‐0.10, 95% CI ‐5.29 to 5.09, P = 0.97; investigator‐assessed change in NILs (open comedones) MD 2.00, 95% CI ‐7.51 to 11.51, P = 0.68; investigator‐assessed change in NILs (closed comedones) MD ‐2.90, 95% CI ‐10.78 to 4.98, P = 0.47; and cysts MD 0.14, 95% CI ‐0.66 to 0.94, P = 0.73.
25.1. Analysis.
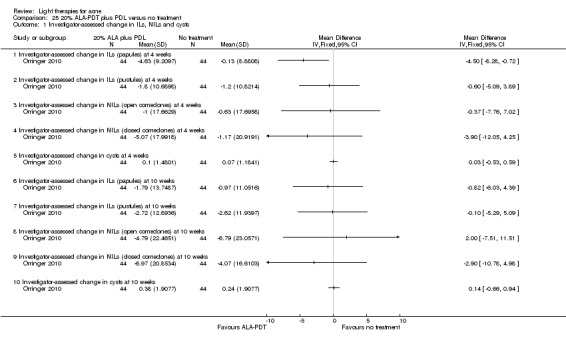
Comparison 25 20% ALA‐PDT plus PDL versus no treatment, Outcome 1 Investigator‐assessed change in ILs, NILs and cysts.
One split‐back trial (Pollock 2004) compared three sessions of 635 nm light plus 20% ALA with light alone, ALA alone and untreated control. The trial included 10 participants (FPT I to III and V, with mild to moderate acne). There was a statistically significant reduction from baseline in IL counts from the second treatment (P < 0.005) at the ALA–PDT site but not the other sites: reduction in acne was 69% at 21 days follow up. Further data was reported in graph format. Mean baseline IL counts were 11.6 and 10.1 respectively at the ALA‐PDT and untreated control areas. At three weeks' follow‐up, IL counts at the ALA‐PDT and untreated control areas decreased to 3.6 and 6.3 respectively. Other data were not given.
2. Investigator‐assessed change in lesion count: 5. ALA‐PDT versus other comparators
2.5.g. ALA‐PDT other
Due to substantial clinical and methodological heterogeneity of five studies with different interventions and comparators (Barolet 2010; NCT00706433; Pollock 2004; Taub 2007; Yin 2010) we did not perform quantitative synthesis of their results.
Barolet 2010 (split‐face or split‐back trial) compared a single treatment of 970 nm IR (radiant infrared) pre‐treatment plus 20% ALA and 630 nm PDT with ALA‐PDT alone. The trial included 10 participants (FPT I to III, with mild to moderate acne). There was a significantly greater improvement in IL medians on the IR pre‐treated versus control side four weeks after treatment (P < 0.0001). Median percentage reduction (95% CI for mean, as reported) in ILs was 73% (95% CI 51% to 81%) on the IR pre‐treated side versus 38% (95% CI 8% to 55%) on the control side. Further data were not provided, 95% CI reported for means, but means were not given.
One parallel‐group trial (NCT00706433) compared four interventions:
20% ALA (45 min incubation) plus 1000 s of blue light;
20% ALA (45 min incubation) plus 500 s of blue light;
vehicle (45 min incubation) plus 1000 s of blue light; and
vehicle (45 min incubation) plus 500 s of blue light.
The study included a total of 266 participants (FPT I to VI, with moderate to severe acne, IGA score 3 and 4, with at least 20 ILs); 68 in the ALA 1000 s group, 65 in the ALA 500 s group. At three weeks after final treatment investigator‐assessed median change in ILs (SD) was ‐18.0 (26.3) in ALA 1000 s and ‐14.0 (26.8) in the ALA 500 s group; investigator‐assessed median percentage change in ILs (SD) was ‐37.5 (38.79) in ALA 1000 s group and ‐29.2 (46.68) in the ALA 500 s group. At six weeks after final treatment investigator‐assessed median change in ILs (SD) was ‐18.5 (30.15) in ALA 1000 s, and ‐13.0 (28.74) in the ALA 500 s group; investigator‐assessed median percentage change in ILs (SD) was ‐34.4 (37.8) in ALA 1000 s and ‐29.0 (42.57) in the ALA 500 s group. We could not perform statistical tests to determine whether any changes were significant due to the study authors’ use of median changes rather than the typical mean changes required for significance testing in order to make appropriate comparisons with other included studies. Furthermore, it was not clearly stated whether the study authors implemented an ITT analysis or a LOCF approach to handling missing data.
Pollock 2004 (split‐back trial) compared three sessions of 635 nm light plus 20% ALA with light alone, ALA alone and untreated control. The trial included 10 participants (FPT I to III and V, with mild to moderate acne). There was a statistically significant reduction from baseline in IL counts from the second treatment (P < 0.005) at the ALA–PDT site but not the other sites: reduction in acne was 69% at 21 days' follow‐up. Further data was reported in graph format. Mean baseline IL counts were 6.6 and 11.6 respectively at the ALA‐alone and ALA‐PDT areas. At three weeks' follow‐up IL counts at the ALA alone and ALA‐PDT areas decreased to 4.6 and 3.6 respectively. Other data were not given.
Taub 2007 (parallel‐group trial) compared three 20% ALA‐PDT treatments with different light sources for activation: IPL (600 nm to 850 nm) versus a combination of IPL (580 nm to 980 nm) and bipolar RF energies versus blue light (417 nm) and included 19 participants (FPT II to IV, with more than 10 facial ILs, moderate to severe acne). Reductions in counts were found in all three groups, with the highest in the IPL‐activation group and the lowest in the blue‐light group, but the difference was not statistically significant (P values not given). Median lesion count percentage reductions at one month after treatment were 76.8 (96.9% CI 12.5 to 86.4) in the IPL group, 47 (96.9% CI 8.3 to 82.2) in the IPL‐RF group and 52.8 (96.9% CI ‐88.9 to 66.7) in the blue‐light group. At three months after treatment, median lesion count percentage reduction (range, defined as "difference between the upper and lower ends of 96.9% CI, indicated when <5 data points are available") was 73.2 (72.4) in the IPL group, 41.6 (167.5%) in the IPL‐RF group and ‐88.9 (123.3) in the blue‐light group.
Yin 2010 (parallel‐group trial) compared four red light ALA‐PDT treatments with different ALA concentrations: 20%, 15%, 10% and 5%, and included a total of 180 participants (FPT III to IV, with moderate to severe acne). Each participant was treated with the assigned concentration on the right side and placebo agent on the left side of the face. Greater reduction in both IL and NIL counts was found at sides treated by ALA‐PDT of all concentrations compared with the controls treated by red light alone at two weeks (P < 0.001), four weeks (P < 0.05), 12 weeks (P < 0.001) and 24 weeks (P < 0.001). Combined data from all follow‐up visits showed more improvement in the higher‐concentration ALA treatment groups than the lower‐concentration groups (P < 0.01).
Means (SD) were reported in graph format only. Our interpretation of the graph was that ILs reduced from a baseline of 21 (5), 20.5 (5.5), 19 (5), 21 (5) and 20 (4) in the 20% ALA group, 15% ALA group, 10% ALA group, 5% ALA group and control face sides, respectively to 1 (0.5), 1.3 (0.5), 3.3 (1), 4 (1) and 5 (1) in the 20% ALA group, 15% ALA group, 10% ALA group, 5% ALA group and control face sides, respectively. NILs reduced from a baseline of 12.9 (4.5), 13 (3.5), 13 (4), 12.5 (3.5) and 11.5 (4) in the 20% ALA group, 15% ALA group, 10% ALA group, 5% ALA group and control face sides, respectively to 1.4 (1), 1.4 (0.5),1.5 (0.5), 2.5 (0.5) and 5.5 (1.5) in the 20% ALA group, 15% ALA group, 10% ALA group, 5% ALA group and control face sides, respectively at 24 weeks after final treatment. We judged further analyses would be biased due to lack of precise data, so we did not perform them. The study authors reported that at 24 weeks for ILs "a significant statistical difference was found in multiple comparisons between 5%, 10%, 15% and 20% ALA (P < 0.05), except between 15% and 20% ALA (P = 0.148)" and for NILs "a significant statistical difference was found in multiple comparisons between 5%, 10%, 15% and 20% ALA (P < 0.05), except for 5% ALA vs. control (P = 1.734) and 15% vs. 20% ALA (P = 0.327)."
2. Investigator‐assessed change in lesion count: 6. MAL‐PDT versus ALA‐PDT
2.6.a. MAL‐PDT versus ALA‐PDT
One split‐face trial (Wiegell 2006a) compared single 620 nm PDT treatments with different creams: 20% ALA versus 160 mg/g MAL. The trial included 19 participants (FPT not given, with more than 12 ILs). There were no significant differences in reductions of ILs between ALA‐treated and MAL‐treated sides at six weeks' (P = 0.061) and 12 weeks' (P = 0.08) follow‐up. Baseline differences in IL counts (P = 0.0049). Median IL counts (inter‐quartile range) at baseline, six and 12 weeks after treatment were 19 (13 to 27), 8 (6 to 14) and 8 (3 to 11) on the MAL‐treated sides and 16 (11 to 22), 5 (3 to 11) and 5 (3 to 11) on the ALA treated sides respectively. There were no significant differences in reductions of NILs between ALA‐treated and MAL‐treated sides at six weeks' (P = 0.18) and 12 weeks' (P = 0.052) follow‐up. Median NIL counts (inter‐quartile range) at baseline, six and 12 weeks after treatment were 14 (6 to 22), 21 (17 to 31) and 17 (9 to 29) on the MAL‐treated sides and 17 (7 to 21), 18 (13 to 29) and 20 (17 to 38) on the ALA‐treated sides respectively.
2. Investigator‐assessed change in lesion count: 7. Other (non‐MAL, non‐ALA) PDT versus other comparators
2.7.a. Indocyanine green (ICG)‐PDT versus other comparators
Two parallel‐group trials (Genina 2004; Kim 2009) included these comparisons, but Genina 2004 evaluated single and multiple treatments whilst Kim 2009 compared a single treatment with three treatments. We were unable to quantitatively combine the results because of different study designs and lack of data.
Genina 2004 compared single and multiple treatments with 803 nm low‐intensity diode laser in combination with ICG. An area of each participant's face or back was then assigned to be treated with ICG, and the other area was used as 'control'. Twelve participants were included (FPT not given, with mild to moderate acne). IL counts improved by 23% at four weeks for the single treatment groups and by 7% for control at ICG plus light sites; 80% improvement at four weeks for the multiple treatment group versus no improvement for control. More improvement was seen in participants with severe acne.
Kim 2009 compared a single treatment with three treatments of ICG plus 805 nm light (right cheek), 805 nm light alone (left cheek) and 'spontaneous resolution' control (forehead). The study included 16 participants (FPT, with mild to moderate acne). Participants were evaluated two and four weeks after final treatment. Significant improvement was found only in the mean number of closed comedones on the PDL‐treated side at all assessment periods, and on the light‐only side at four weeks post‐treatment when compared to 'spontaneous resolution' control (P < 0.05 in all cases). ILs improved at all sites, but non significantly (other data not given). The study did not report whether there were differences between the two groups. Further data were not given and part of the results were reported in graph format. Our interpretation of the graph was that mean counts of closed comedones reduced from a baseline of 15 to 9 on the PDT sides and from 16 to 14 on the light‐only sides, respectively at final evaluation in the single treatment group, and from a baseline of 12 to 8 on the PDT sides and from 13 to 10 on the light‐only sides in the multiple treatment group, respectively.
2. Investigator‐assessed change in lesion count: 7. Other (non‐MAL, non‐ALA) PDT versus other comparators
2.7.b. Indole 3‐acetic acid (IAA)‐PDT versus other comparators
One split‐face trial (Na 2011) compared three sessions of 520 nm green light plus IAA with green light plus placebo cream. The trial included 14 participants (FPT not reported, severity not specified). Improvement in IL counts was observed on both sides. The difference between the treatment and control groups was statistically significant from week four after final treatment (P < 0.05). Further data was not given and was reported only in graph format. Our interpretation of the graph was that mean (we were unsure that this was a measurement of the mean) IL counts reduced from baseline 16.5 to 15.2 on the control sides, and from 16.3 to 14 on the treatment sides.
2. Investigator‐assessed change in lesion count: 7. Other (non‐MAL, non‐ALA) PDT versus other comparators
2.7.c. Topical liposomal methylene blue (TLMB)‐PDT versus other comparators
One split‐face trial (Fadel 2009) compared two sessions of TLMB plus 650 nm light with no treatment. The trial included 20 participants (FPT not reported, with mild to moderate acne). At four weeks IL counts decreased by 83.3% and NILs by 63.6% on the treated sides. Results for control sides were not reported in narrative form. At 12 weeks the reduction was also significant for ILs (P < 0.01) and NILs (P < 0.01). Further data were not given.
2. Investigator‐assessed change in lesion count: 7. Other (non‐MAL, non‐ALA) PDT versus other comparators
2.7.d. Chlorophyll‐a (CHA)‐PDT versus other comparators
One split‐face trial (Song 2014) compared 430 plus 660 nm light combined with CHA with 430 plus 660 nm light alone and included 24 participants (FPT III to IV, acne of Cunliffe grades 2 to 4). Two weeks after final treatment papule counts reduced from baseline 13.0 to 5.1 on the CHA plus light sides and from baseline 13.1 to 8.6 on the light‐only sides (P = 0.030, SDs not given); pustule counts reduced from baseline 3.8 to 1.3 on the CHA plus light sides and from baseline 4.2 to 3.0 on the light‐only sides (P < 0.001, precise P value not given, SDs not given ); open comedone counts reduced from baseline 9.0 to 4.2 on the CHA plus light sides and from baseline 9.1 to 6.7 on the light‐only sides (P = 0.011, SDs not given ); closed comedone counts reduced from baseline 18.4 to 8.5 on the CHA plus light sides and from baseline 18.4 to 13.3 on the light‐only sides (P = 0.014, SDs not given ); nodules & cysts' counts reduced from baseline 0.6 to 0.1 on the CHA plus light sides and from baseline 0.55 to 0.3 on the light‐only sides (P value not given, data extracted from figure). Further data were not given.
2. Investigator‐assessed change in lesion count: 7. Other (non‐MAL, non‐ALA) PDT versus other comparators
2.7.e. Gold microparticle PDT versus other comparators
One parallel‐group trial (Paithankar 2015) compared three sessions (applied one week apart) of gold microparticle suspension plus light (details not given) with vehicle (without light‐absorbing particles) plus light (details not given) control. The trial included 51 participants (FPT I to III, with IGA scores 3 to 4 with at least 25 total papules and pustules on the face). At six weeks after final treatment, the mean percentage change in inflammatory lesion count was −44.0% and −14.0% for the active treatment and sham arms, respectively. At 10 weeks after final treatment, the mean percentage change in inflammatory lesion count was −49.0% and −21.7% for the active treatment and sham arms, respectively (P = 0.015). At 14 weeks after final treatment changes were −53% and −30% for the active treatment and sham arms, respectively (P = 0.04). Other data were not given.
Primary outcome 3: Investigator‐assessed severe adverse effects
We have presented the adverse effects of interventions in (Table 7). There is no separate additional table for 'Investigator‐assessed severe adverse effects', but this outcome is included in Table 7 together with other adverse effects that were reported.
Adverse effects were reported as defined in MedDRA (MedDRA 2010) and coded into System Organ Classes (SOCs) in only a few studies. To report them uniformly in this review, we coded adverse effects reported in other studies using MedDRA lowest level terms (LLTs) where possible and corresponding SOCs, as prespecified in our protocol.
Most studies of light‐only therapies and PDT therapies did not report blistering and there were no reports of scarring. Ten studies (two studies of infrared light, one study on intense pulsed light, two studies of 80 mg/g MAL plus red light, one study of 160 mg/g MAL plus red light, four studies of 20% ALA plus 635 nm light) reported "application site vesicle" (that is, blister; lower level term (LLT): "application site blister") as an adverse effect. Two of them were studies of infrared light (Orringer 2007; Uebelhoer 2007), one was a study of intense pulsed light (McGill 2008), three MAL‐PDT activated by red light (Bissonnette 2010; Hörfelt 2006; Pariser 2013) and four of ALA‐PDT (Hongcharu 2000; Orringer 2010; Taub 2007; Yin 2010). However none of these studies reported the severity adequately (number and size of blisters). Five of the ten studies that reported blistering as an adverse effect (McGill 2008; Hongcharu 2000; Orringer 2007; Orringer 2010; Taub 2007) reported that there was no long‐term scarring.
We have only presented details of effects of interventions for comparisons which included at least one report of 'investigator‐assessed severe adverse effect' in this section. Many studies used very different light sources and applied photosensitisers with different vehicles for variable durations which may have influenced penetration into the follicle. In addition time for and between treatments on different sites challenged comparisons as there are many more pilosebaceous follicles on the face compared to the trunk so one might expect different outcomes with such heterogeneity. All of these sources of clinical and methodological heterogeneity led us to refrain from performing a meta‐analysis, as substantial bias would, indeed, be incurred, hence jeopardising the validity and reliability of any combined results.
The relative risk was unreliable for comparisons in studies which included a report of blister due to the lack of events occurring in control groups or body sites. We were unable to calculate for the same reason. We provided application site blister rates instead and calculated risk differences (RD) with 95% CI for individual studies that included reports of blisters and the comparison in which we were able to combine three studies quantitatively.
3. Investigator‐assessed severe adverse effects: 1. Light versus placebo or no treatment
3.1.c. Infrared light versus no treatment
Two split‐face trials of 38 participants (FPT I to V, with moderate to severe or mild but treatment resistant acne; Darne 2011) and 24 participants (FPT II to V, with mild to severe acne; Moneib 2014) reported 0% application site blisters on either the treatment or control sides.
One split‐face trial of three treatments and application intervals of three weeks (Orringer 2007) randomised 46 participants (FPT II to VI, with clinically active facial acne). There were two reports of application site vesicle (LLT application site blister) on the treated side 2/46 (4.3%) and no reports on the untreated sides (0%), with RD 0.04, 95% CI ‐0.03 to 0.11, P = 0.23 (Analysis 13.3).
13.3. Analysis.

Comparison 13 Infrared light versus no treatment, Outcome 3 Investigator‐assessed severe adverse effects.
We did not combine the studies due to different laser characteristics (1450 nm laser (8‐9 J/cm²) (Orringer 2007), 1320 nm Nd:YAG laser (Darne 2011) and 1550 nm Fractional Erbium Glass Laser (Moneib 2014)). There were also differences in number of treatments, and time intervals between treatments and different application intervals (four versus three weeks).
3. Investigator‐assessed severe adverse effects: 1. Light versus placebo or no treatment
3.1.h. Intense pulsed light (IPL) versus no treatment
One split‐face trial (McGill 2008) randomised ten participants (FPT I to II, with mild to moderate facial acne). IPL was applied, with ‘upper’ and ‘lower’ halves of face sides treated with different filters; 550 nm to 1100 nm filter (‘585 filter’), and the ‘dual band’ filter (blue light), whereas the other face half‐sides served as control. Intervention on the control face sides was unclear, but it was most likely no‐treatment control. Five treatments were applied at two weeks intervals. There was a report of application side blister (LLT application site blister) on the IPL sides, 1/10 (10%), reported as, "One patient developed minor blistering after the fifth treatment, which resolved without scarring. This occurred in areas where double passing treatment was carried out, and were most likely due to the second pass taking place too quickly after the first." We calculated RD 0.10, 95% CI ‐0.14 to 0.34, P = 0.41 (Analysis 26.1).
26.1. Analysis.
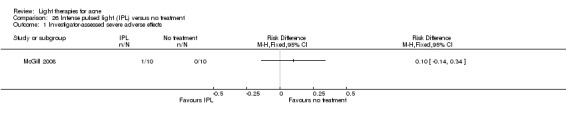
Comparison 26 Intense pulsed light (IPL) versus no treatment, Outcome 1 Investigator‐assessed severe adverse effects.
3. Investigator‐assessed severe adverse effects: 2. Light versus topical treatment
There were no results for this outcome for this comparison.
3. Investigator‐assessed severe adverse effects: 3. Light versus other comparators
3.3.b. Comparison of light therapies of different doses
One split‐face trial (Uebelhoer 2007) compared three sessions of single‐pass with double‐pass of 1450 nm infrared laser treatment and included 11 participants (FPT not given, with at least 10 ILs on each side of the face). There was a report of application site vesicle (LLT application site blister) on the single‐pass side, 1/11 (9%), reported as, "We also experienced a cryogen failure that resulted in a single blister that resolved completely with proper wound care". We calculated RD 0.09, 95% CI ‐0.13 to 0.31, P = 0.42 (Analysis 27.1).
27.1. Analysis.
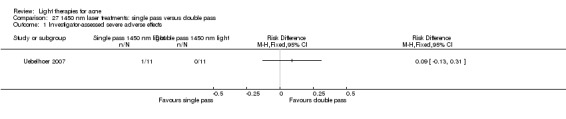
Comparison 27 1450 nm laser treatments: single pass versus double pass, Outcome 1 Investigator‐assessed severe adverse effects.
We were unable to quantitatively combine this study with other studies of infrared light (such as Darne 2011, Orringer 2007 and Moneib 2014) due to substantial clinical heterogeneity in interventions and their comparators.
3. Investigator‐assessed severe adverse effects: 4. MAL‐PDT versus other comparators
3.4.a. MAL‐PDT versus red light alone
We combined results of three parallel‐group studies (NCT00594425; NCT00933543; Pariser 2013) comparing four sessions of red light plus 80 mg/g MAL with placebo cream and red light. NCT00594425 had a group of 50 participants treated with 40 mg/g MAL‐PDT whom we did not include in the meta‐analysis. We have presented the results in Analysis 17.5 and Table 2. We took into account different aspects of methodological, clinical and statistical heterogeneity of the combined studies when considering meta‐analysis, described previously under primary outcome 2 for this comparison. Application site blister rates in the red light‐only groups were 0/158 (0%) and in the MAL‐PDT groups were 1/202 (0.5 %), RD 0.00, 95% CI ‐0.02 to 0.02, P = 0.73.
17.5. Analysis.
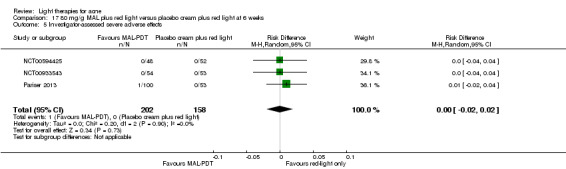
Comparison 17 80 mg/g MAL plus red light versus placebo cream plus red light at 6 weeks, Outcome 5 Investigator‐assessed severe adverse effects.
We also considered combining results of a split‐back study (n = 20) on 80 mg/g MAL‐PDT (NCT00673933) for this outcome, but we did not include it as only two sessions were applied, only participants of FPT V and VI were included, and the treatment was applied on the back, where there are fewer pilosebaceous follicles than on the face.
An additional split‐face trial (Hörfelt 2006) (n = 30) was not included in the meta‐analysis because 160 mg /g MAL was used with 635 nm light and it also included only two sessions. Sponsors provided information that there was one report of application site blister on the MAL‐PDT treated sides in that study; 1/30 (30%). We found RD 0.03, 95% CI ‐0.05 to 0.12, P = 0.4958 (Analysis 20.3).
20.3. Analysis.

Comparison 20 160 mg/g MAL plus red light versus placebo cream plus red light, Outcome 3 Investigator‐assessed severe adverse effects.
3. Investigator‐assessed severe adverse effects: 4. MAL‐PDT versus other comparators
3.4.d. MAL‐PDT other
A parallel‐group trial (Bissonnette 2010) which randomised 44 participants (FPT I to IV, with 10 ILs or more on each face side) to compare 80 mg/g MAL with or without occlusion followed by different red light intensity exposure. Participants were randomised in four groups with 25 J/cm² or 37 J/cm², with or without occlusion on different sides of the face. It included one report of application site blister, 1/22 (4.5%) on the occluded 37 J/cm² face sides; and 0/22 (0%) on the non‐occluded 37 J/cm² face sides, 0/22 (0%) on the occluded and 0/22 (0%) on the non‐occluded 22 J/cm² sides respectively. For 37 J/cm² with and 37 J/cm² without occlusion face sides, we calculated RD 0.05, 95% CI ‐0.07 to 0.16, P = 0.45 (Analysis 28.1).
28.1. Analysis.
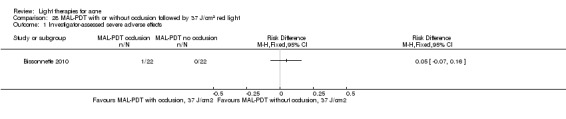
Comparison 28 MAL‐PDT with or without occlusion followed by 37 J/cm2 red light, Outcome 1 Investigator‐assessed severe adverse effects.
3. Investigator‐assessed severe adverse effects: 5. ALA‐PDT versus other comparators
3.5.f. ALA‐PDT versus placebo or no treatment
One split‐face trial (Orringer 2010) compared three sessions of 20% ALA plus PDL with untreated control. The trial included 44 participants (all FPTs, severity of acne unclear). There was one report of application site vesicle (LLT application site blister); 1/44 participants (2.3%). It resolved without permanent consequences. We calculated RD 0.02, 95% CI ‐0.04 to 0.08, P = 0.46 (Analysis 25.2).
25.2. Analysis.
Comparison 25 20% ALA‐PDT plus PDL versus no treatment, Outcome 2 Investigator‐assessed severe adverse effects.
3. Investigator‐assessed severe adverse effects: 5. ALA‐PDT versus other comparators
3.5.g. ALA‐PDT other
One study (Hongcharu 2000) included 22 participants (FPT I to IV, with mild to moderate acne) and randomised 11 of them to the single treatment group, and the other 11 to the multiple treatment group. Four areas on the back of each participant were treated with 20% ALA–plus 550 nm to 700 nm light; or 20% ALA alone; or 550 nm to 700 nm light alone and the fourth area served as an untreated control. There was one report 1/11 (9%) of application site vesicle (LLT application site blister) in the single treatment group on the ALA‐PDT site, "...after vigorous aerobic exercise while wearing a tight outfit [a] day after treatment. This area healed without scarring in three weeks". We calculated RD 0.09, 95% CI ‐0.13 to 0.31, P = 0.42 (Analysis 29.1).
29.1. Analysis.
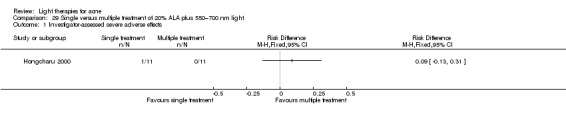
Comparison 29 Single versus multiple treatment of 20% ALA plus 550–700 nm light, Outcome 1 Investigator‐assessed severe adverse effects.
One parallel‐group trial (Taub 2007) compared three 20% ALA‐PDT treatments with different light sources for activation: IPL (600 nm to 850 nm) versus a combination of IPL (580 nm to 980 nm) and bipolar RF energies versus blue light (417 nm) and included 19 participants (FPT II‐IV, with > 10 facial ILs, moderate to severe acne). There was one report of application site vesicle (LLT application site blister) in the IPL‐RF group, but the numbers of participants per group were not stated, so we were unable to perform further analyses.
One parallel‐group trial (Yin 2010) compared four red light (633 nm) ALA‐PDT treatments with different ALA concentrations: 5%, 10%, 15% and 20% and included a total of 120 participants (FPT III to IV, with moderate to severe acne). Each participant was treated with the assigned concentration on the right side and placebo agent on the left side of the face. In the 20% ALA group there was one report 1/45 (2%) of a combination of application site erythema, application site oedema and application site vesicle (LLT application site blister); "treated with systemic glucocorticoids and resolution took place in 2 weeks, with no persistent clinical sequelae or permanent scarring". No reports of adverse effects were made for the other concentrations of ALA. We calculated RD 0.02, 95% CI ‐0.04 to 0.08, P = 0.46 for all three comparisons (Analysis 9.2; Analysis 10.2; Analysis 11.2).
9.2. Analysis.
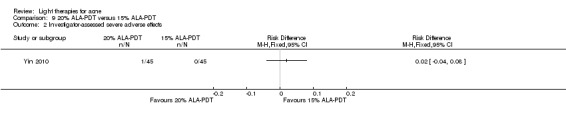
Comparison 9 20% ALA‐PDT versus 15% ALA‐PDT, Outcome 2 Investigator‐assessed severe adverse effects.
10.2. Analysis.
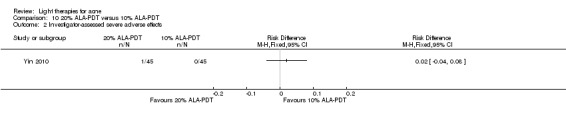
Comparison 10 20% ALA‐PDT versus 10% ALA‐PDT, Outcome 2 Investigator‐assessed severe adverse effects.
11.2. Analysis.
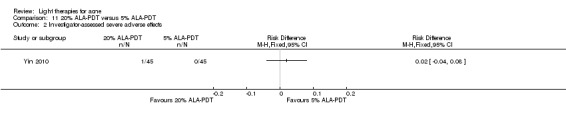
Comparison 11 20% ALA‐PDT versus 5% ALA‐PDT, Outcome 2 Investigator‐assessed severe adverse effects.
We considered combining the results of the above ALA‐PDT studies, together with one more split‐back study of 10 participants (Pollock 2004), as well as one parallel‐group trial of 20 participants (Oh 2009). However, we judged this was inappropriate due to substantial clinical heterogeneity including different pre‐ and post‐treatment care which was applied, incubation times, occlusion regimens, wavelengths and doses used for activation, numbers of treatment sessions, intervals between them etc.
Secondary outcomes 1: Investigator‐assessed change in acne severity; 2: Investigator's global assessment of improvement; and 3: Changes in quality of life
We have presented the details of participants, interventions and the effects of interventions for these outcomes in (Table 8). For studies which had no reports of our primary outcome 2 (Investigator‐assessed change in lesion count) and for which we were therefore unable to provide narrative summary in the previous section, we provide it here (Baugh 2005; Bowes 2003; Chen 2015; Cheng 2008; Hongcharu 2000; Ianosi 2013; Leheta 2009; Ling 2010; McGill 2008; Ou 2014; Sadick 2010a; Tzung 2004; Yilmaz 2011; Zhang 2009a; Zhang 2013a; Zhang 2013b). For studies which included both primary outcome 2 and secondary outcomes 1, 2 and 3, please find the full details on secondary outcomes in Table 8. Where appropriate, we also clarified why we did not perform meta‐analysis.
Secondary outcomes: 1. Light versus placebo or no treatment
Secondary outcomes 1.a. Green light versus placebo
Three split‐face trials (Baugh 2005; Bowes 2003; Yilmaz 2011) of four treatments included a total of 80 participants (FST I to III or not reported, with mild to moderate acne or more than 4 facial ILs). All three studies used the Michaelsson score (where a decrease in the score signifies a decrease in acne severity, Michaelsson 1977) for acne severity evaluation, but meta‐analysis of change in acne severity was not possible because necessary data were not reported nor provided upon request. All three studies reported greater decreases on light‐treated sides at four weeks after final treatment.
The Michaelsson score decreased from a baseline of 42.9 to 34.1 (by 21%) on the treated side and increased from a baseline of 41.2 to 51.4 (by 25%) on the control side (P = 0. 089, SDs not given) in Baugh 2005, and in Bowes 2003 decreased by 35.9% on the treated side and increased by 1.8% on the untreated side (SDs not given). In Yilmaz 2011 (split‐face within a parallel‐group design), which also compared single and multiple treatment groups, both sides improved, but decrease in Michaelsson severity score was significantly greater on the treated side ‐ 31% versus 6% (P = 0.005) in the once‐weekly group and by 40% versus 13% in twice‐weekly group (P < 0.001). Means and SDs were not given; further data were not given.
Secondary outcomes 1.b. Yellow light versus placebo or no treatment
We were unable to pool results of two studies. One parallel‐group study (Seaton 2003) reported median (interquartile range) improvements in Leeds grade whilst the other, split‐face study (Orringer 2004), reported changes in means with SEs of Leeds scores. We were unable to obtain additional data.
Secondary outcomes 1.c. Infrared light versus no treatment
Two split‐face trials (Darne 2011; Orringer 2007) of three treatments included a total of 84 participants (FST I to VI, with mild to moderate acne). Meta‐analysis was not possible because of different types of lasers used, different application intervals and timings of outcome assessment, although both used the Leeds score and reported it with 95% confidence interval (Darne 2011) and SE (Orringer 2007). Another split‐face trial (Moneib 2014) included the outcome 'Investigator's global assessment of improvement', using a non‐standardised scale and reported assessments at an unclear time point.
Secondary outcomes 1.d. Blue light versus no treatment
One split‐face study (Tzung 2004) randomised 31 participants (FPT III to IV, with mild to moderate acne). The Michaelson modified grade percentage improvement in the blue‐light group compared to the control group was reported as 52% and 12% respectively at eight weeks, P = 0.009.
Secondary outcomes 1.f. Blue‐red light versus placebo
Two parallel‐group studies included this comparison; one (Papageorgieu 2000) included 'Investigator‐assessed change in acne severity', another (Kwon 2013) included 'Investigator's global assessment of improvement'. We were therefore unable to pool data. One study (Papageorgieu 2000) randomised 30 participants to the blue‐red‐light group and 25 to the white‐light group (FPTs not reported, all with mild to moderate acne). A non‐standardised scale was used for evaluation (please see Table 8) and reported in graph format only. We extracted the data from the graph and dichotomised them to 26/30 'success' outcomes in the blue‐red group and 6/25 in the white‐light group. Blue‐red light was superior to white light with RR 3.61, 95% CI 1.77 to 7.36, P = 0.0004 (Analysis 1.1) and the NNTB was 2 (95% CI 1 to 3).
Secondary outcomes 1.h. Intense pulsed light (IPL) versus no treatment
One split‐face trial (McGill 2008) randomised ten participants (FPT I to II, with mild to moderate facial acne). IPL was applied, with ‘upper’ and ‘lower’ halves of face sides treated with different filters; 550 nm to 1100 nm filter (‘585 filter’), and the ‘dual band’ filter (blue light), whereas the other half served as control. Intervention on the control face sides was unclear, but it was most likely no‐treatment control. Five treatments were applied at two‐week intervals, and assessed at one, three and six months after final treatment. Seven participants completed the study, and five were evaluated. At six months after final treatment for the outcome 'Investigator‐assessed change in acne severity', our calculations using t‐distribution showed that there were no significant differences in changes in the Leeds grade between 585 half sides and control sides (MD 0.60, 95% CI ‐1.88 to 3.08), P = 0.64 (Analysis 26.2), nor between blue‐light and control sides (MD 0.40, 95% CI ‐1.95 to 2.75), P = 0.74 (Analysis 26.2). Results of the analyses using t‐distribution did not substantially differ from the ones in which we used normal distribution (Analysis 26.3).
26.2. Analysis.
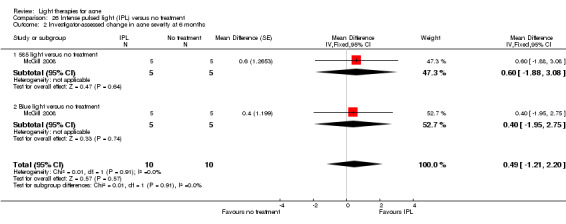
Comparison 26 Intense pulsed light (IPL) versus no treatment, Outcome 2 Investigator‐assessed change in acne severity at 6 months.
26.3. Analysis.
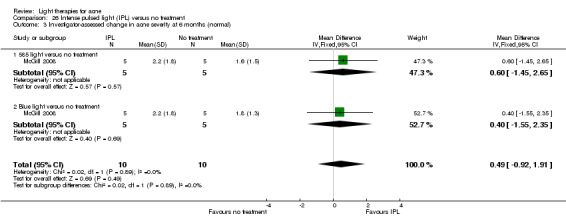
Comparison 26 Intense pulsed light (IPL) versus no treatment, Outcome 3 Investigator‐assessed change in acne severity at 6 months (normal).
Our third secondary outcome was 'Changes in quality of life'. Mean (± SD) pretreatment Dermatology Life Quality Index (DLQI) scores were 11 ± 5 (range 3 to 19). At one month DLQI score had decreased to 6 ± 5 (range 0 to 12), at three months to 5 ± 2 (range 2 to 7) and at six months it increased to 7 ± 4 (range 4 to 12). Not reported for separate face half‐sides.
Secondary outcomes: 2. Light versus topical treatment.
Secondary outcomes 2.a. Light versus benzoyl peroxide (BPO)
Only one study (Papageorgieu 2000) included this outcome (Investigator's global assessment of improvement) for this comparison, which randomised 30 participants to the blue‐red light group and 25 to the BPO group (FPTs not reported, all with mild to moderate acne). A non‐standardised scale was used for evaluation (please see above) and reported in graph format only. We extracted the data from the graph and dichotomised them to 26/30 'success' outcomes in the blue‐red group and 16/25 in the BPO group. The difference was non significant, with RR 1.35, 95% CI 0.98 to 1.88) P = 0.07 (Analysis 2.1).
Secondary outcomes 2.b. Light versus clindamycin
Only one study (Gold 2005) included the outcomes 'investigator‐assessed change in acne severity' and 'global assessment of improvement' for this comparison. It was a parallel‐group trial that compared eight sessions of 417 nm blue light with self‐administered topical clindamycin (34 participants, FPT not reported, with mild to moderate acne). Investigator‐assessed change in acne severity and global assessment of improvement were reported as similar for both groups (figures were not given in the paper).
Secondary outcomes 2.c. Light and other topical treatments
Five parallel‐group studies included this comparison, but their interventions included different modalities of light and topical treatments, so we were unable to combine their results (Borhan 2014; Ianosi 2013; Karsai 2010; Leheta 2009; Zhang 2009a ). The outcomes they assessed also differed.
Ianosi 2013 included 180 participants (FPT I to IV, with mild to moderate acne) and randomised 60 participants to 500nm to 1200 nm light plus vacuum group, 60 participants to IPL alone group (400nm to 700 nm and 870 nm to 1200 nm) and 60 participants to anti‐acne micellar solution. Light treatments were applied once a week for five weeks, and final evaluation was done at the last treatment. There was a greater reduction in the Leeds score in the light‐treatment groups compared to the micellar‐solution group, which was reported only in graph format and no further data were provided. There was also a significantly greater effect on quality of life (using the Cardiff Acne Disability Index) in vacuum plus IPL group compared to the micellar solution group (P = 0.004). Further data were not given.
Leheta 2009 (parallel‐group study). We dichotomised the data for 'investigator's global assessment of improvement' to 3/15 'success' outcomes in the PDL group, 13/15 in 5% BPO in combination with tretinoin (T/BPO) group and 15/15 in the 0.025% retinoic acid cream combined with trichloroacetic acid peeling (TCAA) group. PDL was not superior to T/BPO with RR 1.00, 95% CI 0.76 to 1.32, P = 1.00 (Analysis 30.1), nor to TCAA, RR 0.87, 95% CI 0.69 to 1.09, P = 0.24 (Analysis 31.1).
30.1. Analysis.
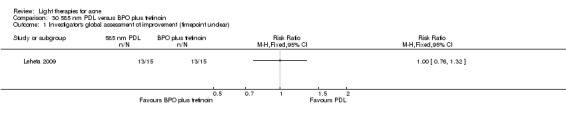
Comparison 30 585 nm PDL versus BPO plus tretinoin, Outcome 1 Investigator's global assessment of improvement (timepoint unclear).
31.1. Analysis.
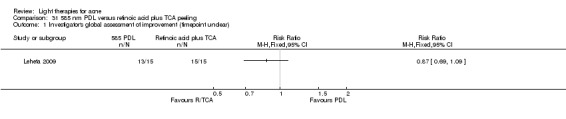
Comparison 31 585 nm PDL versus retinoic acid plus TCA peeling, Outcome 1 Investigator's global assessment of improvement (timepoint unclear).
Zhang 2009a (parallel‐group trial) compared blue and red light in combination with clindamycin gel, azithromycin, antisterone or cimetidine with clindamycin gel, azithromycin, antisterone or cimetidine alone. The trial included 738 participants (FPT not given, with mild to severe acne, Pillsbury grades I to IV). Evaluation was performed four weeks after treatment. Investigators assessed improvement using the following scale based on lesion count percentage change: 90% improvement or above = 'full recovery'; 60% to 89% = 'good improvement'; 30% to 59% = 'effective improvement'; 29% or less = 'no effect'. We dichotomised the data following our protocol and using the ITT approach to present the outcome 'investigator's global assessment of improvement' as 332/508 ‘success’ outcomes in the intervention and 125/230 ‘success’ outcomes in the control group. Antibiotic treatment in combination with blue‐red light was superior to antibiotic treatment alone with RR 1.20, 95% CI 1.05 to 1.38, P = 0.006 (Analysis 32.1). The NNTB was 10 (95% CI 6 to 30).
32.1. Analysis.
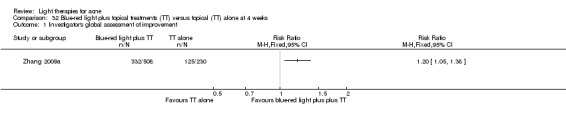
Comparison 32 Blue‐red light plus topical treatments (TT) versus topical (TT) alone at 4 weeks, Outcome 1 Investigator's global assessment of improvement.
Secondary outcomes: 3. Light versus other comparators
Secondary outcomes 3.a. Comparison of light therapies of different wavelengths
Two parallel‐group trials (Cheng 2008; Papageorgieu 2000) included comparison of blue and blue‐red light. Meta‐analysis was not done because of the differences in the number of sessions (84 versus 8 to 24) and timing of their assessment. Another three trials compared different interventions, namely eight sessions of blue LED with eight sessions of red LED (Liu 2011); four sessions of 585 nm PDL compared with four sessions of 530 to 750 nm IPL (Choi 2010) and three sessions of 585 nm PDL compared with three sessions of combined 585/1064 nm PDL (Jung 2009), so quantitative synthesis was not appropriate.
Cheng 2008 (secondary outcomes only reported) included 64 participants (FPT not reported, with mild to moderate acne), who were randomised to the 400 nm to 410 nm light group or to the 400 nm to 410 nm plus 660 nm light group. Investigators assessed improvement using the following scale based on lesion count percentage change: 90% improvement or above = 'full recovery'; 70% to 89% = 'good improvement'; 30% to 69% = 'effective improvement'; 30% or less = 'no effect'. We dichotomised the data to present the outcome 'investigator's global assessment of improvement' as 15/28 'success' outcomes in the blue‐red group and 26/36 in the blue‐light‐alone group. The difference was non significant with RR 0.74, 95% CI 0.50 to 1.11, P = 0.14 (Analysis 33.1).
33.1. Analysis.
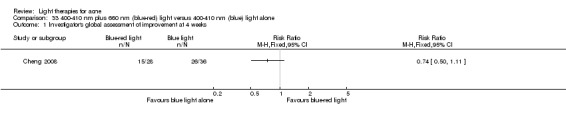
Comparison 33 400‐410 nm plus 660 nm (blue‐red) light versus 400‐410 nm (blue) light alone, Outcome 1 Investigator's global assessment of improvement at 4 weeks.
Liu 2011 (parallel‐group study) compared blue with red light and included results for 20 participants (FPTs III to IV, all with mild to moderate acne), who completed the trial of eight sessions of blue light in one group (405 ± 10 nm, power of 30 mW/cm²) and red light (630 ± 10 nm, power of 48 mW/cm² ) in the other group. Investigators assessed improvement using the following scale based on lesion count percentage change: reduction 90% or above = ‘full recovery’; 60% to 89% reduction= ‘significant improvement’, 40% to 59% reduction = ‘moderate improvement’, 20% to 39% reduction = ‘mild improvement’, and 19% reduction or below = ‘non‐ improvement or aggravation’. We dichotomised the data to present the outcome 'investigator's global assessment of improvement' as 8/10 ‘success’ outcomes in the blue‐light and 5/10 in the red‐light group. The difference was non significant with RR 1.60, 95% CI 0.80 to 3.20, P = 0.18 (Analysis 34.1).
34.1. Analysis.
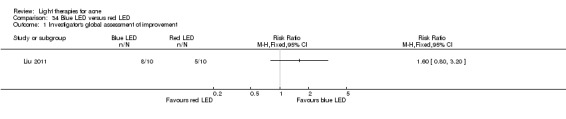
Comparison 34 Blue LED versus red LED, Outcome 1 Investigator's global assessment of improvement.
Papageorgieu 2000 randomised 30 participants to the blue‐red light group and 27 to the blue‐light group (FPTs not reported, all with mild to moderate acne). A non‐standardised scale was used for evaluation (please see above) and reported in graph format only. We extracted the data from the graph and dichotomised them to present the outcome 'investigator's global assessment of improvement' as 26/30 'success' outcomes in the blue‐red group and 19/27 in the blue‐light‐alone group. The difference was non significant, with RR 1.23, 95% CI 0.93 to 1.63, P = 0.15. (Analysis 3.1).
Secondary outcomes 3.b. Comparison of light therapies of different doses
Two split‐face (Bernstein 2007; Uebelhoer 2007) trials compared single and double passes of 1450 nm lasers, but had different numbers of sessions and timings of outcome assessment so we did not quantitatively combine the data.
One parallel‐group trial (NCT00706433) compared four interventions:
20% ALA (45 min incubation) plus 1000 s of blue light;
20% ALA (45 min incubation) plus 500 s of blue light;
vehicle (45 min incubation) plus 1000 s of blue light; and
vehicle (45 min incubation) plus 500 s of blue light.
The study included a total of 266 participants (FPT I to VI, with moderate to severe acne, IGA score 3 and 4, with at least 20 ILs); 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group. At three weeks after final treatment there were 15/67 'success' outcomes in the vehicle 1000 s and 11/66 in the vehicle 500 s group. The difference between vehicle 1000 s and vehicle 500 s groups for the outcome 'investigator's global assessment of improvement' was non significant, with RR 1.34, 95% CI 0.67 to 2.70, P = 0.43 (Analysis 4.2). At six weeks after final treatment there were 16/67 'success' outcomes in the vehicle 1000 s and 16/66 in the vehicle 500 s group. The difference between vehicle 1000 s and vehicle 500 s groups was non significant, with RR 0.99, 0.54 to 1.80, P = 0.96 (Analysis 4.2).
4.2. Analysis.
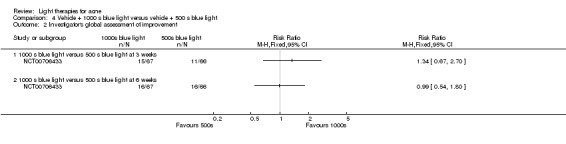
Comparison 4 Vehicle + 1000 s blue light versus vehicle + 500 s blue light, Outcome 2 Investigator's global assessment of improvement.
Secondary outcomes 3.c. Comparison of light therapies of different treatment application intervals
Only one study (Yilmaz 2011) included this comparison for this outcome. This was a parallel‐group RCT (split‐face within groups) which randomised two groups; application of 532 nm (green) light once weekly for four weeks versus twice weekly for two weeks. Within each group one side of the face was randomised to assigned treatment and the other to no treatment. It included a total of 44 participants (FST I to III, with more than 4 facial ILs). Differences in Michaelson acne severity score means (SD) of the treated face sides at baseline and at four weeks were ‐5.9 (7.9) in the once‐weekly group and ‐9.3 (7.5) in the twice‐weekly group.
Secondary outcomes 3.e. Light in combination with carbon lotion versus no treatment
Only one study (Jung 2012) included this outcome for this comparison. This was a split‐face trial that compared three sessions of quasi‐long pulse and Q‐switched 1064 nm Nd:YAG laser plus carbon lotion with non‐treated control and (22 participants, FPT III to V, with unclear severity of acne). The Cunliffe severity grade decreased significantly from 3.2 to 1.7 (P < 0.001) on the laser‐treated side and from 2.7 to 2.6 (P < 0.05) on the non‐treated side. The difference between the two treatments was significant (P = 0.04).
Secondary outcomes 3.f. Light in combination with oral therapy versus other comparators
Four parallel‐group studies included this comparison, but their interventions included different modalities and combinations of light, oral and topical treatments, so we were unable to combine their results (Ling 2010; Ou 2014; Zhang 2009a; Zhang 2013b).
Ling 2010 compared blue and red light plus sulfotanshinone, versus sulfotanshinone alone, versus blue and red light plus sulfotanshinone plus prednisolone, versus sulfotanshinone plus prednisolone. The trial included 30 participants in each of the four groups (FPT not given, with moderate to severe acne). Evaluation was performed four weeks after treatment. Investigators assessed improvement using the following scale based on lesion count percentage change: 95% improvement or above = 'full recovery'; 60% to 95% = 'good improvement'; 20% to 59% = 'effective improvement'; 20% or less = 'no effect'. We dichotomised the data to 26/30 'success' outcomes in the blue‐red light plus sulfotanshinone group, 19/30 in the sulfotanshinone‐alone group, 16/30 in the blue‐red light plus sulfotanshinone plus prednisolone group and 13/30 in the sulfotanshinone plus prednisolone group. Blue and red light plus sulfotanshinone was superior (marginally) to sulfotanshinone alone for the outcome 'investigator's global assessment of improvement' with RR 1.37, 95% CI 1.01 to 1.86, P = 0.04 (Analysis 35.1); to blue and red light plus sulfotanshinone plus prednisolone with RR 1.63, 95% CI 1.13 to 2.34, P = 0.009 (Analysis 36.1); and to sulfotanshinone plus prednisolone with RR 2.00, 95% CI 1.30 to 3.08, P = 0.002 (Analysis 37.1).The NNTBs were 3 (95% CI 1 to 9) and 3 (95% CI 1 to 5) for the latter two comparisons with blue‐red light plus sulfotanshinone respectively. However, there is no calculable NNTB for the comparison of blue‐red light plus sulfotanshinone to sulfotanshinone alone since the 95% CI for the risk difference contains zero (i.e. no effect), and this corresponds to an infinite upper 'limit' for the 95% CI for the NNTB, which indicates that there is no true boundary on how large the NNTB could be for this comparison: this is also seen in the marginal effect seen with the RR.
35.1. Analysis.
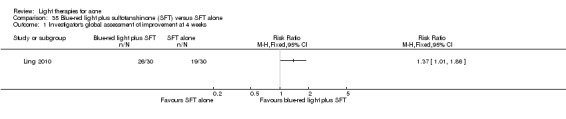
Comparison 35 Blue‐red light plus sulfotanshinone (SFT) versus SFT alone, Outcome 1 Investigator's global assessment of improvement at 4 weeks.
36.1. Analysis.
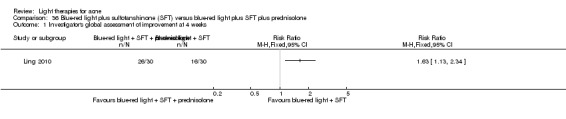
Comparison 36 Blue‐red light plus sulfotanshinone (SFT) versus blue‐red light plus SFT plus prednisolone, Outcome 1 Investigator's global assessment of improvement at 4 weeks.
37.1. Analysis.
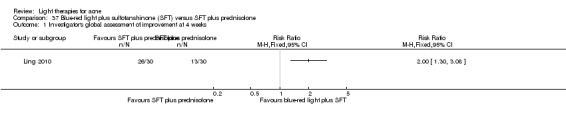
Comparison 37 Blue‐red light plus sulfotanshinone (SFT) versus SFT plus prednisolone, Outcome 1 Investigator's global assessment of improvement at 4 weeks.
Ou 2014 compared Yinhua decoction (YD, term as presented in the English translation of the abstract provided by the journal where full text was published in Mandarin) with 'electric light synergy' versus YD in combination with red and blue light treatment. The trial included 90 participants, and 83 completed the trial (FPT not given, with moderate acne grade II to III Chinese Acne Treatment Guidelines). Evaluation was performed twelve weeks after final treatment. Investigators assessed improvement using the following scale based on lesion count percentage change: 90% improvement or above = 'full recovery'; 60% to 89% = 'good improvement'; 30% to 59% = 'effective improvement'; 29% or under = 'no effect'. We dichotomised the data for the outcome 'investigator's global assessment of improvement' 30/43 (69.7% of those who completed) success outcomes in the intervention arm, and 15/40 (37.5% of those who completed) in the control arm. Numbers of randomised participants in each group were not reported, and so we were unable to use ITT approach. YD plus 'electric light synergy' were superior to YD in combination with blue‐red light with RR 1.86, 95% CI 1.19 to 2.91, P = 0.006 (Analysis 38.1). The NNTB was 4 (95% CI 2 to 10).
38.1. Analysis.
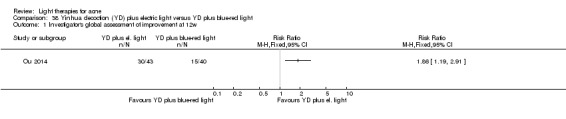
Comparison 38 Yinhua decoction (YD) plus electric light versus YD plus blue‐red light, Outcome 1 Investigator's global assessment of improvement at 12w.
Zhang 2013b compared blue and red light plus Jinhua xiaocuo (term as presented in the English translation of the abstract provided by the journal where full text was published in Mandarin) pills and chloramphenicol tincture versus Jinhua xiaocuo pills and chloramphenicol tincture alone. The trial included 60 in each group (FPT not given, with mild to moderate acne, Pillsbury grades I to III). Evaluation was performed four weeks after final treatment. Investigators assessed improvement using the following scale based on lesion count percentage change: 90% improvement or above = 'full recovery'; 60% to 89% = 'good improvement'; 30% to 59% = 'effective improvement'; 29% or under = 'no effect'. We dichotomised the data following our protocol to 55/60 ‘success’ outcomes in the intervention and 39/60 ‘success’ outcomes in the control group. Jinhua xiaocuo pills and chloramphenicol tincture in combination with blue‐red light were superior to jinhua xiaocuo pills and chloramphenicol tincture alone with RR 1.41, 95% CI 1.15 to 1.72, P = 0.0008 (Analysis 39.1). The NNTB was 4 (95% CI 3 to 9).
39.1. Analysis.

Comparison 39 Blue‐red light plus oral plus topical treatments (OT plus TT) versus OT plus TT alone at 4 weeks, Outcome 1 Investigator's global assessment of improvement.
Zhang 2009a compared blue and red light in combination with clindamycin gel, azithromycin, antisterone or cimetidine with clindamycin gel, azithromycin, antisterone or cimetidine alone. Please see (Analysis 32.1), further details and the results under Secondary outcomes 2.c. Light and other topical treatments, as this study could be placed under both comparisons.
Secondary outcomes 3.g. IPL alone versus IPL in combination with vacuum
One parallel‐group trial (Ianosi 2013) randomised a total of 180 participants (FPT I to IV, with mild to moderate acne) to 500 nm to 1200 nm light plus vacuum group or to an IPL‐alone group (400 nm to 700 nm and 870 nm to 1200 nm). Changes in lesion counts were reported as scores 1 = insignificant result (lesion count reduction 0% to 25%) to 4 = very good result (lesion count reduction 76% to 100%). No significant differences were found between the two treatments at final assessment in a reduction of the score of papules and pustules (P reported as 'NS'). There was a significantly greater reduction in the score of comedones in the vacuum plus IPL group (P < 0.001). There was a greater reduction in the Leeds score in the IPL‐only group reported in graph format and no further data provided. There was a significantly greater effect on quality of life (using Cardiff Acne Disability Index) in the vacuum plus IPL group (P = 0.004). Further data were not given.
Secondary outcomes: 4. MAL‐PDT versus other comparators
Secondary outcomes 4.a. MAL‐PDT versus red light alone
We combined results of three parallel‐group studies (NCT00594425; NCT00933543; Pariser 2013) comparing four sessions of red light plus MAL with placebo cream and red light, with final evaluation at six weeks after last treatment (please see above for details). Meta‐analysis showed that MAL‐PDT was superior to red light alone in IGA score improvement ('success' outcome defined by decrease in the IGA score by at least two grades from baseline), with RR 1.74, 95% CI 1.11 to 2.74 (Analysis 17.6), moderate quality evidence (Table 2). There were 120 reports of treatment 'success' as defined by IGA score decrease per 1000 study population in the red‐light‐alone group, and 209 per 1000 study population (95% CI 133 to 329) in the MAL‐PDT group. The absolute effect was 89 more treatment 'success' outcomes per 1000 (95% CI from 13 more to 209 more). The NNTB was 7 (95% CI 5 to 11). Please see Analysis 17.6 and Table 2 for details. Please note that these studies are not presented in Table 8.
17.6. Analysis.
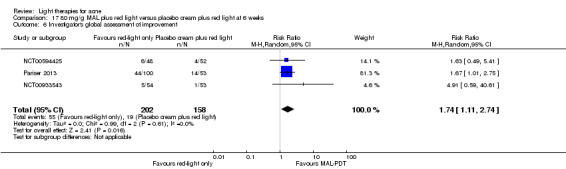
Comparison 17 80 mg/g MAL plus red light versus placebo cream plus red light at 6 weeks, Outcome 6 Investigator's global assessment of improvement.
NCT00594425 (three‐arm trial) randomised 50 participants in the 40 mg/g MAL‐PDT group (FPT I to V, with moderate to severe acne, IGA score 3 to 4, 20 to 100 ILs and up to 200 NILs on the face). Four treatments at two‐week intervals were applied. At six weeks after final treatment 'success' outcomes (defined by decrease in IGA score by at least two grades from baseline) were found in 6/50 participants in the 40 mg/g group and 4/52 in the placebo‐cream group. Our analyses showed that 40 mg/g MAL‐PDT was not superior to placebo cream activated by red light for the outcome 'investigator's global assessment of improvement', with RR 1.56, 95% CI 0.47 to 5.20, P = 0.47 (Analysis 18.5).
18.5. Analysis.
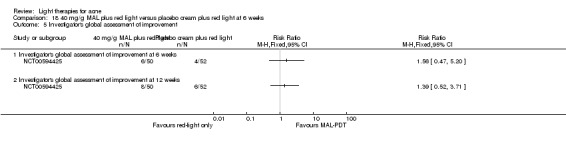
Comparison 18 40 mg/g MAL plus red light versus placebo cream plus red light at 6 weeks, Outcome 5 Investigator's global assessment of improvement.
We were unable to combine results from one more split‐face trial, which compared only two sessions of 635 nm light plus 160 mg/g MAL with placebo cream and light and also had different assessment time points (Hörfelt 2006). In that study, we dichotomised the data to 12/30 'success' outcomes on the MAL‐PDT sides and 7/30 on the placebo‐PDT sides. The difference was non significant, with RR 1.71, 95% CI 0.78 to 3.75, P = 0.18 (Analysis 20.4; Table 8).
20.4. Analysis.
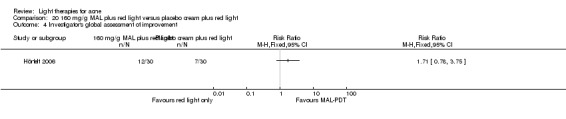
Comparison 20 160 mg/g MAL plus red light versus placebo cream plus red light, Outcome 4 Investigator's global assessment of improvement.
Secondary outcomes 4.c. MAL‐PDT versus placebo or no treatment
Only one study (Wiegell 2006b) included this comparison for this outcome. This was a parallel‐group study of two treatments of 630 nm plus 160 mg/g MAL (21 participants in the treatment group and 15 in the control group; FPT II to V, with at least 12 facial ILs). No significant difference was observed in reduction in the Leeds grade between the two groups (P = 0.24).
Secondary outcomes 4.d. MAL‐PDT other
Due to substantial clinical and methodological heterogeneity of three studies with different interventions and comparators (Bissonnette 2010; Hong 2013; NCT00594425) we did not perform quantitative synthesis of their results.
NCT00594425 (three‐arm parallel‐group trial) randomised 48 participants to the 80 mg/g MAL‐PDT arm and 50 participants to the 40 mg/g MAL‐PDT arm (FPT I to IV, with moderate to severe acne, IGA score 3 to 4, 20 to 100 ILs and up to 200 NILs on the face). Four treatments at two‐week intervals were applied, and 37 participants completed treatment in the 80 mg/g group, and 43 completed in the 40 mg/g group. Our analyses showed that at six weeks after final treatment 80 mg/g MAL‐PDT was not superior to 40 mg/g MAL‐PDT by the 'investigator's assessment of improvement' (a 'success' outcome was defined by a decrease in the IGA score by at least two grades from baseline), (RR 1.04, 95% CI 0.36 to 3.01; n = 98, P = 0.94) (Analysis 21.5).
21.5. Analysis.
Comparison 21 80 mg/g MAL plus red light versus 40 mg/g MAL plus red light at 6 weeks, Outcome 5 Investigator's global assessment of improvement.
Bissonnette 2010 (parallel‐group trial) randomised 44 participants (FPT I to IV, with 10 or more ILs on each face side) to compare 80 mg/g MAL with or without occlusion followed by different red light intensity exposure; participants were randomised in four groups with 25 J/cm² or 37 J/cm² and with or without occlusion; there were four treatments, assessed at four and 12 weeks after the final treatment. At 12 weeks for the outcome 'investigator's assessment of improvement' the difference in 'success' outcomes (defined by decrease in the IGA score by at least two grades from baseline) was non significant for the comparison 37 J/cm² treatment with occlusion versus 37 J/cm² treatment without occlusion, (RR 0.50, 95% CI 0.05 to 5.12; n = 44) (Analysis 28.2).
28.2. Analysis.
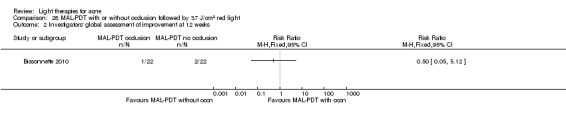
Comparison 28 MAL‐PDT with or without occlusion followed by 37 J/cm2 red light, Outcome 2 Investigators' global assessment of improvement at 12 weeks.
Hong 2013 (split‐face study) compared three sessions of 160 mg/g MAL plus red light with three sessions of MAL plus IPL and included 22 participants (FPT IV to V). At four weeks after treatment there was no significant difference in the improvement in acne Cunliffe grade between the red light side (1.9) and IPL side (2.0).
Secondary outcomes: 5. ALA‐PDT versus other comparators
Secondary outcomes 5.a. ALA‐PDT versus red light alone
One parallel‐group trial (Chen 2015) compared three red light (633 nm) 20% ALA‐PDT treatments with three treatments of red light alone and included a total of 50 participants (FPT not given, with mild to severe acne). A non‐standardised method was used for the investigators' evaluation (90% or above improvement = 'cured', 60% to 89% improvement = 'excellent effect', 30% to 59% improvement = 'fair effect', 30% improvement or exacerbations or less = 'no effect'). One participant dropped out from the ALA‐PDT group, and two dropped out from the red‐light only group, so we treated them as treatment failures as per our protocol. We dichotomised the data following our protocol ('success' defined as anything above the first category of improvement) to 13/25 ‘success’ outcomes at two weeks, 18/25 at four weeks and 20/25 at six weeks in the intervention group, whereas in the control group there were 6/25 ‘success’ outcomes at two weeks, 10/25 at four weeks and 13/25 at six weeks.
Another parallel‐group trial (Zhang 2013a) compared three red light ALA‐PDT treatments with three treatments of red light alone and included a total of 116 participants (FPT not given, with moderate to severe acne, Pillsbury grade II to IV). Evaluation was performed two, four and eight weeks after final treatment. Investigators assessed improvement using the following scale based on lesion count percentage change: 90% improvement or above = 'full recovery'; 60% to 89% = 'good improvement'; 20% to 59% = 'effective improvement'; 19% or below = 'no effect'. We dichotomised the data following our protocol ('success' defined as anything above the first category of improvement) to 28/63, 37/63, and 50/63 ‘success’ outcomes in the intervention group at two, four and eight weeks after final treatment respectively; and 7/53, 15/53, and 22/53 ‘success’ outcomes in the control group at two, four and eight weeks after final treatment respectively.
We judged it was appropriate to combine the results of the above two parallel‐group studies (Chen 2015; Zhang 2013a). We have presented details of the data and results as reported by the authors of these studies in Table 8. Treatments were applied in weekly intervals in both studies. Both studies also had evaluation time points at two and four weeks after last treatment, but final evaluation was done at six weeks after last treatment in Chen 2015, and eight weeks after last treatment in Zhang 2013a. The statistical heterogeneity across studies was not substantial, I² was 0% at both two weeks and four weeks, and fitted the criteria we stated in our protocol (I² had to be lower than 50%). Therefore we judged it was appropriate to combine the results. However, there was some clinical heterogeneity across studies to take into account. We have narratively summarised it here, please check Characteristics of included studies tables of each study for details. While Chen 2015 included all acne severity grades (mild to severe), Zhang 2013a included only moderate to severe acne. FPTs were not reported in either of the studies. Both studies had the same ALA supplier, however it is unclear whether the same ALA percentage was used. Characteristics of red light also differed, but not substantially.
Meta‐analysis, using a random‐effects model, showed that ALA‐PDT was superior to red light alone in improving the 'investigator global assessment of improvement' score at two weeks with RR 2.74, 95% CI 1.59 to 4.71 (Analysis 40.1), as well as at four weeks with RR 1.95, 95% CI 1.36 to 2.79 (Analysis 40.1). The NNTB was 4 (95% CI 3 to 7) at two weeks, as well as at four weeks. However, results Chen 2015 also showed that at six weeks ALA‐PDT was no longer superior to red light alone with RR 1.54, 95% CI 1.01 to 2.35, P = 0.05 (Analysis 40.1). Zhang 2013a did not include six weeks as an assessment time point, but found that ALA‐PDT was still superior to red light alone at eight weeks after final treatment with RR 1.91, 95% CI 1.36 to 2.70, P = 0.0002 (Analysis 40.1). The NNTB was 3 (95% CI 2 to 5) at eight weeks.
40.1. Analysis.
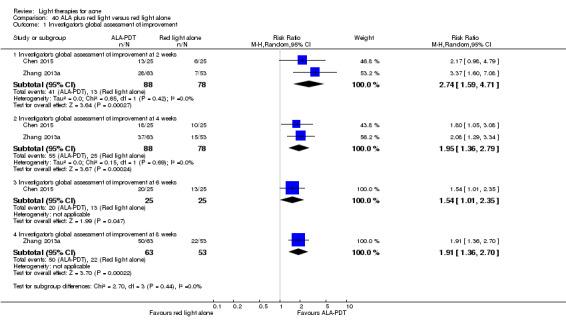
Comparison 40 ALA plus red light versus red light alone, Outcome 1 Investigator's global assessment of improvement.
Secondary outcomes 5.b. ALA‐PDT versus blue light alone
One parallel‐group trial (NCT00706433) compared four interventions:
20% ALA (45 min incubation) plus 1000 s of blue light;
20% ALA (45 min incubation) plus 500 s of blue light;
vehicle (45 min incubation) plus 1000 s of blue light; and
vehicle (45 min incubation) plus 500 s of blue light.
The study included a total of 266 participants (FPT I to VI, with moderate to severe acne, IGA score 3 and 4, with at least 20 ILs). The difference in the; 'investigator global assessment (IGA) of improvement' ('success' outcome defined as a 2 point or more improvement on the IGA scale from baseline) between ALA 1000 s and vehicle 1000 s groups was non significant at three weeks after final treatment, with RR 0.85, 95% CI 0.44 to 1.65, P = 0.64, and it was also non significant between ALA 500 s and vehicle 500 s groups, with RR 1.02, 95% CI 0.47 to 2.18, P = 0.97 (Analysis 5.2; Table 8). At six weeks after final treatment, the difference between ALA 1000 s and vehicle 1000 s groups remained non significant, with RR 0.92, 95% CI 0.50 to 1.71), P = 0.80, and it also remained non significant between ALA 500 s and vehicle 500 s groups, with RR 0.70, 95% CI 0.35 to 1.39, P = 0.31 (Analysis 5.3; Table 8). The difference between ALA‐PDT and vehicle plus blue light was non significant when we combined results for the 1000 s and 500 s subgroups using a random‐effects model, with RR 0.92, 95% CI 0.56 to 1.52, P = 0.74 at 3 weeks and RR 0.81, 95% CI 0.51 to 1.29, P = 0.38 at six weeks after final treatment respectively (Analysis 5.3). See Table 3 where we rated the evidence as low quality for this outcome.
5.2. Analysis.
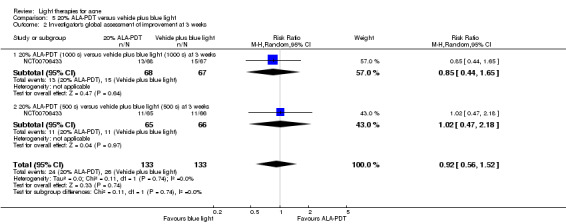
Comparison 5 20% ALA‐PDT versus vehicle plus blue light, Outcome 2 Investigator's global assessment of improvement at 3 weeks.
5.3. Analysis.
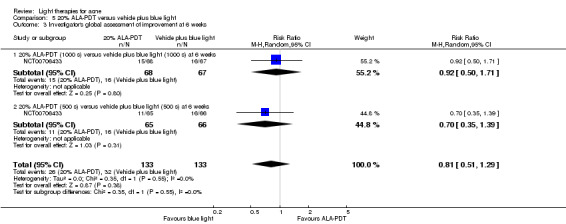
Comparison 5 20% ALA‐PDT versus vehicle plus blue light, Outcome 3 Investigator's global assessment of improvement at 6 weeks.
Secondary outcomes 5.d. ALA‐PDT versus IPL alone
Two trials included this comparison, but one had a split‐face design (Oh 2009), and included three treatments with different incubation times in participants with moderate to severe acne, whilst the other was a parallel‐group trial, of four treatments and included participants with severe acne (Mei 2013). Different scales were used for assessment. We did not combine results because of this heterogeneity and calculated RR with 95% CI for individual studies.
Oh 2009 compared three sessions of 20% ALA plus IPL (one face side randomised to either 30 minutes' or three hours' incubation) with IPL only and included 20 participants (FPT III to IV, with moderate to severe acne). The difference was non significant, (RR 0.81, 95% CI 0.48 to 1.40) (Analysis 6.1). Results were reported for IPL‐only sides.
Mei 2013: the investigators assessed there was no significant difference in improvement between the 10% ALA‐PDT and IPL‐alone group at 12 weeks after final treatment, (RR 1.43, 95% CI 0.96 to 2.13, P = 0.08) (Analysis 24.3).
24.3. Analysis.
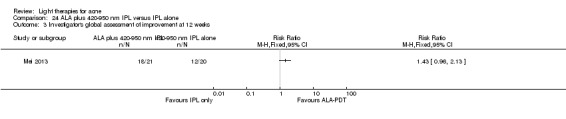
Comparison 24 ALA plus 420‐950 nm IPL versus IPL alone, Outcome 3 Investigator's global assessment of improvement at 12 weeks.
Secondary outcomes 5.e. ALA‐PDT versus green light alone
Only one split‐face trial (Sadick 2010a) compared three 20% ALA (30 min incubation) plus 532 nm potassium titanyl phosphate (KTP) laser light with KTP laser alone. The study included a total of 10 participants (FPT I to III, with moderate to severe acne, IGA score 3 and 4). IGA was also used for evaluation (see above). On the ALA‐PDT sides IGA score (mean ± standard error) reduced from baseline 3.50 ± 0.19 to 2.29 ± 0.29 (35% improvement) after the first treatment and to 2.13 ± 0.40 (39% improvement) after the second treatment. On the light‐only sides IGA score (mean ± standard error) reduced from baseline 3.63 ± 0.18 to 2.42 ± 0.30 (33% improvement) after the first treatment and to 2.38 ± 0.33 (34% improvement) after the second treatment. Further details and results of evaluations after the final treatment were not given (reported as "Similar results were recorded after the third treatment session that was evaluated at week 12").
Secondary outcomes 5.f. ALA‐PDT versus placebo or no treatment
Only one study (Orringer 2010) included investigator‐assessed change in acne severity for this comparison. This was a split‐face trial that compared three sessions of 20% ALA plus PDL with untreated control. The trial included 44 participants (all FPTs, severity of acne unclear). There was a statistically significant difference in decrease (i.e. improvement, P = 0.01) in the mean Leeds score on treated skin versus untreated skin at week 16 (i.e. 10 weeks after final treatment). Mean change in score from baseline was ‐1.07, (95% CI ‐1.69 to ‐0.45) on the treated sides and ‐0.52 (95% CI ‐1.07 to 0.04) on the control sides.
Secondary outcomes 5.g. ALA‐PDT other
Due to substantial clinical and methodological heterogeneity of five studies with different interventions and comparators (Barolet 2010; Hongcharu 2000; NCT00706433; Taub 2007; Yin 2010), we did not perform quantitative synthesis of their results. Please see Table 8 and Analysis 8.2 for details.
8.2. Analysis.
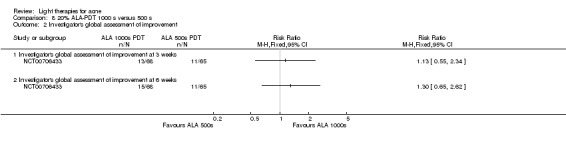
Comparison 8 20% ALA‐PDT 1000 s versus 500 s, Outcome 2 Investigator's global assessment of improvement.
Barolet 2010 (split‐face or split‐back trial) compared a single treatment of 970 nm IR pre‐treatment plus 20% ALA and 630 nm PDT with ALA‐PDT alone. The trial included 10 participants (FPT I to III, with mild to moderate acne). At four weeks after treatment there was greater improvement in Global Severity Assessment Score medians on the IR pre‐treated (1, 95% CI 0.74 to 1.34) versus control side (2, 95% CI 1.17 to 1.72). Further data were not provided, 95% CI reported for means, but means were not given.
Hongcharu 2000 randomised 22 participants (FPT I to IV, with mild to moderate acne) into single and multiple treatment groups, with four areas on the back of each participant treated with ALA plus 550 nm to 700 nm light, ALA alone, or 550 nm to 700 nm light, or untreated as control. Change from baseline in Michaelsson acne severity score was significantly better in ALA‐PDT than the other three areas at 3, 10 and 20 weeks after single treatment (P values not given) and at all visits after multiple treatment (P < 0.05). ALA–PDT and multiple ALA treatment sites showed more improvement than single treatment (P < 0.001 and P = 0.007, respectively). Investigator's global assessment of improvement scores was also significantly better for the ALA‐PDT areas than the other three areas where some improvement has also been observed in both single and multiple treatment groups. These comparisons, as well as comparison between single and multiple treatment groups were reported in an unclear way.
One parallel‐group trial (NCT00706433) compared four interventions:
20% ALA (45 min incubation) plus 1000 s of blue light;
20% ALA (45 min incubation) plus 500 s of blue light;
vehicle (45 min incubation) plus 1000 s of blue light; and
vehicle (45 min incubation) plus 500 s of blue light.
The study included a total of 266 participants (FPT I to VI, with moderate to severe acne, IGA score 3 and 4, with at least 20 ILs); 68 in the ALA 1000 s group, 65 in the ALA 500 s group, 67 in the vehicle 1000 s group and 66 in the vehicle 500 s group. The improvement of the Investigator Global Assessment (IGA) score at three weeks after final treatment between ALA 1000 s and ALA 500 s groups was non significant, (RR 1.13, 95% CI 0.55 to 2.34, n=143, P = 0.33) (Analysis 8.2), and it remained non significant at 6 weeks after final treatment, (RR 1.30, 95% CI 0.65 to 2.62, n=143, P = 0.74) (Analysis 8.2).
Taub 2007 compared three ALA‐PDT treatments with different light sources for activation: IPL (600 nm to 850 nm) versus a combination of IPL (580 nm to 980 nm) and bipolar RF energies versus blue light (417 nm) and included 19 participants (FPT II to IV, with more than 10 facial ILs, moderate to severe acne). Investigator‐assessed improvement was highest with IPL activation and lowest with blue light, and the differences between groups reached borderline statistical significance at three months (P = 0.0498). At one month after treatment median percentage improvement score was 56.25 (96.9% CI 27.5 to 85.0) in the IPL group, 23.75 (96.9% CI 2.5 to 85.0) in the IPL‐RF group and 20 (96.9% CI 0 to 62.5) in the blue‐light group. At three months after treatment median percentage improvement score (range) was 72.5 (42.5) in the IPL group, 50 (47.5) in the IPL‐RF group and 25 (40) in the blue‐light group.
Yin 2010 compared four red light ALA‐PDT treatments with different ALA concentrations: 5%, 10%, 15% and 20% and included 180 participants (FPT III to IV, with moderate to severe acne). A non‐standardised scale was used for evaluation. At 24 weeks after treatment, a significant difference among the different ALA concentration groups (P values not given) was reported, with a clear positive correlation between global improvement score and ALA concentration (P < 0.05). Further data were expressed in graph format, please see Table 8 for details.
Secondary outcomes: 6. MAL‐PDT versus ALA‐PDT
Secondary outcomes 6.a. MAL‐PDT versus ALA‐PDT
Only one study (Wiegell 2006a) included investigator‐assessed change in acne severity for this comparison. This was a split‐face trial that compared single 620 nm PDT treatments with different creams: 20% ALA versus 160 mg/g MAL. The trial included 19 participants (FPT not given, with more than 12 ILs). Median of the Leeds revised acne global severity grade reduced from 2 before treatment to 1 at 12‐week follow‐up in both the MAL‐PDT and ALA‐PDT treated sides of the face. There were no significant differences between the two treatments (P = 0.250).
Secondary outcomes: 7. Other (non MAL, non ALA) PDT versus other comparators
Secondary outcomes 7.a. Indocyanine green (ICG)‐PDT versus other comparators
Only one study (Kim 2009) included investigator‐assessed change in acne severity for this comparison. This was a parallel‐group study of a single treatment with three treatments of ICG plus 805 nm light (right cheek), 805 nm light alone (left cheek) and 'spontaneous resolution' control (forehead). The study included 16 participants (FPT, with mild to moderate acne). There was significant improvement in the Cunliffe acne severity score in both groups at two and four weeks after final treatment (P < 0.05). It was not reported whether there were differences between the two groups.
Secondary outcomes 7.c. Topical liposomal methylene blue (TLMB)‐PDT versus other comparators
Only one study (Fadel 2009) included investigator‐assessed change in acne severity for this comparison.This was a split‐face trial that compared two sessions of TLMB plus 650 nm light with no treatment. The trial included 20 participants (FPT not reported, with mild to moderate acne). At 12 weeks the median Leeds severity grade on the treated side was 1 (range 0 to 2) and on the untreated side 3 (range 2 to 4). No baseline data given. At 12 weeks 7/13 (54%) participants had marked improvement, 4/13 (31%) participants had moderate and 2/13 (15%) participants had slight improvement. "Approximately the same improvements" after four weeks and eight weeks. Study authors reported that control areas had no change or worsening of acne with no details provided.
Secondary outcomes 7.e. Gold microparticle PDT versus other comparators
Only one parallel‐group trial (Paithankar 2015) compared three sessions of gold microparticle suspension plus light (details not given) with vehicle (without light‐absorbing particles) plus light (details not given) control. The trial included 51 participants (FPT I to III, with IGA scores 3 to 4 with at least 25 total papules and pustules on the face). At 10 weeks after the final treatment, the study authors stated "40% of subjects in the treatment arm, whereas none in the sham arm, showed Investigator’s Global Assessment (IGA) score reduction in two or higher". Further data were not given.
Other adverse effects
Most commonly reported adverse effects were application site erythema, application site oedema and pain of skin. Please see Table 7 for details and other adverse effects and their incidence reported in individual studies.
Adverse effects were reported inadequately in most studies and most studies did not quantify adverse effects in each intervention group or report them separately for the sides of the face or back assigned to different interventions. Six studies did not explicitly report whether participants experienced any adverse effects (Bowes 2003; Cheng 2008; Gold 2011; Ling 2010; Orringer 2004; Tzung 2004) and ten studies reported that they recorded adverse effects but no adverse effects were observed (Ash 2015; Baugh 2005; Elman 2003; Genina 2004; Gold 2005; Lee 2010; Na 2011; Sadick 2010b; Song 2014; Yilmaz 2011).
Discussion
Summary of main results
We included 71 studies with a total of 4211 participants, of which 40 were studies of light‐only therapies with a total of 2485 participants, and 31 were studies of photodynamic therapy (PDT) with a total of 1726 participants. Most studies had a parallel‐group design, split‐face design, or a design that combined split‐face and parallel groups. The majority had small sample sizes (median 31, mean 59). Most studies were single centre and did not report on funding sources, or were sponsored by industry if multicentre. Most studies included participants with a mean age of between 20 and 30 years, of both sexes, with mild to moderate acne. Many studies did not report on Fitzpatrick Skin Types (FPTs) and a great proportion of studies which did, included up to three FPTs, typically I to III or III to V. Light interventions differed greatly in wavelengths, doses, active substances used in PDT and comparator interventions (most common being no treatment, placebo, other light interventions and various topical treatments). The number of light sessions of the interventions varied from one to 112, with two to four sessions being the most common. Frequency of application varied from twice a day to once a month.
We have summarised the comparison of light therapies (including PDT) compared to placebo, no treatment, topical treatment and other comparators in Table 1 for our primary outcomes. Twenty‐three studies addressed our first primary outcome, 'participant's global assessment of improvement'. Most of them had small sample sizes (median sample size 24), used non‐standardised scales, were poorly reported, and only a few assessed this outcome at times after the final treatment. We decided not to combine the effect estimates from the different interventions for this outcome, but rated the evidence based on the GRADE considerations as very low quality, as we were uncertain whether light therapies improve acne compared to placebo, no treatment, topical treatment and other comparators.
For our second primary outcome, Investigator‐assessed change in lesion counts, 51 studies with 2242 participants addressed this outcome. Here too we were unable to combine the effect estimates from the different interventions and rated the quality of the evidence as very low, so we are uncertain whether light therapies improve lesion counts compared to placebo, no treatment, topical treatment or other comparators.
For our third primary outcome, 'investigator‐assessed severe adverse effects', adverse effects were reported inadequately in most studies. Six studies did not report whether any adverse effects were experienced by participants. Adverse effects were reported as defined in MedDRA (MedDRA 2010) and coded into System Organ Classes (SOCs) in a few studies only. There were no reports of scarring in any of the studies and no reports of blistering (application site blister) in 56 studies with a total of 3378 participants. Here too we were unable to combine the effect estimates from the different interventions and rated the quality of the evidence as very low, so we are uncertain whether light therapies caused more adverse effects compared to placebo, no treatment, topical treatment and other comparators.
Please see Table 2, where MAL‐PDT (methyl aminolevulinate‐photodynamic therapy) activated by red light was compared to red light only for acne vulgaris. Our primary outcome which was ‘participants’ global assessment of improvement was not addressed by these studies. Meta‐analysis of results from three studies comparing four treatments of 80 mg/g MAL plus red light with placebo cream and red light in a total of 360 participants with moderate to severe acne showed that at six weeks after final treatment MAL‐PDT was not superior in reducing the counts or the percentage change in inflamed or non‐inflamed lesions as assessed by the investigator, which was our second primary outcome. We rated this evidence as of moderate quality and so of moderate certainty. The outcome, Investigator‐assessed severe adverse effects found a lack of adverse events, such as application site blisters in the red‐light‐alone group (0/158, 0%), while there was one in the MAL‐PDT group (1/202, 0.5%). For our secondary outcome, ‘investigators’ global assessment of improvement’ we combined three studies (n = 360) which gave statistically significantly greater improvement in the MAL‐PDT groups (moderate‐quality evidence). The number needed to treat for an additional treatment 'success' was 7 (95% CI 5 to 15) which we did not interpret as a clinically significant result.
The largest clinical trial we identified, with 266 participants, compared ALA‐PDT (20% aminolevulinic acid (ALA) activated by 500 s and 1000 s blue light) with vehicle plus 500 s and 1000 s blue light, and found no difference for our outcome ‘participants’ global assessment of improvement’ at six weeks after final treatment (Table 3). Similarly, for the outcome of 'investigator‐assessed treatment 'success' at three and at six weeks after final treatment there was no significant difference between the treatments. Both of these were rated as low‐quality evidence, meaning we have low certainty in the result and that future studies may alter this evidence. For our outcomes ‘investigator‐assessed change' or 'percentage change in inflamed lesions', or 'severe adverse effects’, we assessed the certainty of the evidence as very low.
We were unable to quantitatively combine the data for most comparisons due to great variation in many aspects of the studies, poor reporting and failure to obtain necessary data. We therefore performed a narrative synthesis of the results for most of the studies.
Briefly, studies comparing the effects of other interventions were inconsistent or had small samples and high risk of bias. We performed only narrative synthesis for the results of the remaining trials, due to great variation in many aspects of the studies, poor reporting, and failure to obtain necessary data. Several studies compared yellow light to placebo or no treatment, infrared light to no treatment, gold microparticle suspension to vehicle, and clindamycin/benzoyl peroxide combined with pulsed dye laser to clindamycin/benzoyl peroxide alone. There were also several other studies comparing MAL‐PDT to light‐only treatment, to adapalene and in combination with long‐pulsed dye laser to long‐pulsed dye laser alone. None of these showed any clinically significant effects.
Overall completeness and applicability of evidence
The studies we included were performed in different geographical and cultural settings, which might prevent generalisation of the results to some extent because of factors such as differences in exposure to natural sunlight or impact on non‐validated scales for participants' assessment of improvement of their acne. More importantly, this implies that participants of various FPTs may have been included although not reported (Fitzpatrick 1988). This challenges the applicability of evidence to all FPTs, and in particular to FPTs V and VI, which are known to have a greater risk of adverse effects compared to other skin types when applying light therapies (Alexis 2013). In studies which reported FPTs they were, unsurprisingly, different among studies from Europe, Asia and North America. Other important factors which should be considered in the context of limited generalisability are participants' sex and age, with possible differences in the underlying subtypes of acne and their response to treatment (Choi 2011; Dreno 2013; Preneau 2012).
Most studies included participants with mild to moderate acne, but some did not report the severity of the acne. This limits generalisation, as the effect of light therapies in those with severe acne is less clear.
Participants with acne refractory to antibiotic treatments have often been included in the comparison of different modalities of light therapies. When light therapies were compared with topical treatment, it was often unclear whether there was initial resistance to topical antibiotics in acne patients included in topical antibiotic arms of trials. Initial resistance might have caused antibiotic treatments to prove less effective in these participants, but this would not necessarily be the case in other participants who did not have a resistance problem.
Many studies had a split‐face design. It is unclear whether there are possible systemic effects that light and other therapies used in such studies could have on the side of the face used as the control, even if it is not treated directly.
A variety of interventions regarding different wavelengths, fluences, numbers of sessions, as well as frequency of application have been included in this review. However, there are still a lot of possibilities in combining different modalities which were not performed in the studies we included. There were only a few studies using the conventional treatments documented in guidelines (Nast 2012; Zaenglein 2016) for acne as a control. Only a few studies had systemic therapy as a comparator. Combination of light therapies with topical therapies, and particularly systemic therapy have rarely been explored.
Our primary endpoint was long term outcomes, but less than half of studies performed assessments later than eight weeks after final treatment. Clinically, if a treatment did not give at least three months' resolution it could arguably be a failure. Only a few studies assessed outcomes at more than three months after final treatment, and longer‐term assessments are mostly not covered in this review. Although long‐term data were our primary endpoint, we were also interested in short‐term data, indicating early improvement which may have encouraged participants to continue with the treatment and we therefore considered follow‐ups of two to eight weeks after final treatment, reported in the majority of studies. We also reported results recorded at final treatment for studies which did not include follow‐up thereafter. Possibly, some interventions may have an early transitional effect on outcomes which our review did not cover, as we only considered follow‐ups after final treatment (or at final treatment for studies which did not include evaluations after final treatment). Timing of outcome assessment should be taken into account when interpreting our results, as effects may be different at different time points, some of which are not covered by our review.
Only three studies addressed changes in quality of life (Ianosi 2013; Karsai 2010; McGill 2008) making it the most under‐investigated outcome in our review.
Quality of the evidence
The body of evidence we identified did not allow a robust conclusion on the effectiveness of light therapies for acne. We included 71 studies with a total of 4211 participants. The overall quality of evidence was very low, as presented in Table 1. We decided not to combine the effect estimates from the different interventions. Instead we rated the quality of the evidence based on the GRADE considerations for our three primary outcomes, taking into account factors that decrease the quality level of a body of evidence outlined in the Cochrane Handbook for Systematic Reviews of Interventions section 12.2.2 (Schünemann 2011a).
Studies addressing 'participant's global assessment of improvement' (23 studies, 1033 participants included) used non‐standardised scales, were poorly reported and only a few assessed this outcome later than at final treatment (Table 1). The evidence for the effectiveness of light therapies on changes and percentage changes of lesion counts was somewhat more robust in terms of numbers of studies and included participants (51 studies, 2242 participants included) and consistency of methods used for outcome assessment. Most studies (66 studies with 3945 included participants) assessed adverse effects and we presented their results for our third primary outcome (‘investigator‐assessed severe adverse effects'). We downgraded the body of evidence for all of these outcomes for several reasons. Firstly, most of the evidence came from studies with unclear or high overall risk of bias, and for primary outcome 1 detection bias was high or unclear in all but two studies. Secondly, quality was limited by inconsistency in the results of individual studies and heterogeneity across studies due to diversity of populations, interventions, comparators and methods of outcome assessment. Thirdly, only a few studies included comparisons with standard treatments, and rarely included comparisons with placebo or no treatment, and so their results did not answer our review question directly, and were further limited by variation of participants who had been included (in terms of Fitzpatrick skin types, severity of acne etc.). Furthermore, most studies had small sample sizes, with medians of 24, 30 and 30 for primary outcomes 1, 2 and 3 respectively. For comparisons where individual studies had randomised fewer than 30 participants per arm, we used t‐distribution for analyses of continuous outcomes to account for the sample size. However, substantial imprecision should be taken into consideration when assessing the quality of evidence, in particular when assessing the quality of the evidence for comparisons where only such small studies were available. We also downgraded the evidence because our searches identified a number of unpublished studies but with no available data, which we believe raises questions of whether those trials suggested no benefit.
Quantitative synthesis of several studies was only possible for the comparison of MAL‐PDT with red light. We graded the body of evidence for that comparison as moderate (Table 2). Studies did not include comparisons with conventional treatments documented in guidelines (Nast 2012; Zaenglein 2016), placebo or no treatment, and we judged this was a reason to downgrade the quality level of evidence on the basis of indirectness. Although the following were not reasons for downgrading the evidence, we did consider clinical heterogeneity across studies, such as differences among included participants (Fitzpatrick skin types and severity of acne), as well as differences in interventions (use of occlusive dressing during incubation and different lamps). The studies had low overall risk of bias, so we did not downgrade the evidence on that basis, but we did consider the possible impact of high attrition and selective reporting bias in one study and the fact that the studies were industry sponsored.
We also graded the evidence from a single study with 266 participants for comparison of ALA‐PDT with blue light as low for 'participant's global assessment of improvement' and for 'investigator's global assessment of improvement' (Table 3). The study did not include comparisons with standard treatments, placebo or no treatment, and we judged this was a reason to downgrade the quality level of the evidence on the basis of indirectness. We also downgraded the evidence for all outcomes by one level because of risk of bias, as the study had unclear risk of bias in most of the domains. We considered the possible impact of non‐standardised scales which were used to measure these outcomes, but have not further downgraded the evidence on that basis. We graded the evidence as very low for 'investigator‐assessed change in ILs' and 'investigator‐assessed percentage change in ILs' (Table 3). Only medians with standard deviations of changes for these continuous outcomes were reported, and means were not provided upon request, so we were unable to perform further analyses. This was an additional reason to downgrade the quality of evidence by one level, along with the reasons listed for the evidence on the above outcomes. We also graded the quality of evidence as very low for our third primary outcome 'Investigator‐assessed severe adverse effects' (Table 3). There were no reports of application site blisters among adverse effects, however it is possible that some occurred, but it is impossible to separate those as they were reported together with "Oozing/ Vesiculation/Crusting", so we downgraded it by two levels because of risk of bias.
As previously described, the quality of evidence for other interventions was fairly limited since we were unable to quantitatively combine the data. Individual studies we identified did not present conclusive evidence of high quality.
Potential biases in the review process
To avoid bias, we followed the protocol for this study (Car 2009). However, considerable time has passed since the protocol was produced in 2009 and we had to make a few minor changes, mostly related to updates in Cochrane methodology. Please see Differences between protocol and review for details.
We tried to minimise bias in the review process through a comprehensive search for all eligible studies, irrespective of language in which they were published or publication status. Seven out of 12 studies with the largest samples (more than 100 participants) were identified through grey literature searches or were not in English (Ling 2010; NCT00594425; NCT00706433; NCT00933543; Zhang 2009a; Zhang 2013a; Zhang 2013b). We intended to test for publication bias by the use of a funnel plot for similar light therapies, however we were unable to create funnel plots because most studies were too heterogeneous to be combined. Two studies we did combine in meta‐analyses were not published (we identified NCT00594425 and NCT00933543 in clinical trials registers only) so we did not construct a funnel plot for these not‐yet published works. According to trial register records, the final data collection date for primary outcome measures for these studies was 2008 (NCT00594425), and 2010 (NCT00933543). Some bias was probably introduced because we were unable to obtain reports or full results of 36 studies which may possibly meet our inclusion criteria in the future. Please see 'Characteristics of studies awaiting classification' section for details. We therefore believe that despite the fact that our efforts to identify unpublished studies were successful to some extent, publication bias may have still affected the results our review.
Further skewing of the results in our review might be due to unclear selection and performance bias in most studies, together with unclear to high overall detection bias for participant‐reported outcomes. Most studies which had unclear to low overall bias, good methodological quality and larger sample sizes were industry sponsored, or study authors had reported some sort of conflict of interest, so additional bias might have been introduced. Non industry‐sponsored studies, on the other hand, were in general of lower methodological quality, had unclear to high overall bias and smaller sample sizes.
At least two review authors independently assessed studies for eligibility and extracted data. English translations were obtained for studies in other languages when that was possible. For one study in Portuguese that we included (de Arruda 2009) two review authors extracted data independently from an English translation. However only one person screened full texts of studies which were originally in Mandarin. Six of these studies were included in the review (Cheng 2008; Ling 2010; Ou 2014; Zhang 2009a; Zhang 2013a; Zhang 2013b) and this sole person extracted the data from them.
Poor reporting in general may have introduced some bias in our assessment of some studies, as well as our failure to obtain the additional data we needed to clarify ambiguities resulting from such poor reporting. As we were unable to obtain Individual Patient Data for most (or almost none of the) studies, we considered chapter 18 (18.4.2) of the Cochrane Handbook for Systematic Reviews of Interventions (Stewart 2009). We believe we have minimised bias by reporting results in the original papers with the additional limited data obtained from the study authors or sponsors, rather than not reporting results of the majority of studies at all. However, the results we presented should be interpreted with the potential bias such reporting has introduced in mind. Unclear reporting issues, if there were any, are given specifically for each study within Characteristics of included studies and Characteristics of excluded studies when appropriate. Some bias was probably introduced because we had to code adverse effects from most studies in MedDRA (MedDRA 2010) ourselves in order to uniformly report them.
Agreements and disagreements with other studies or reviews
An overview of systematic reviews on treatments for acne (Smith 2011) identified three systematic reviews addressing laser and light therapies from 2009 (Hamilton 2009; Riddle 2009; Taylor 2009). We considered several other systematic reviews (Erceg 2013; Haedersdal 2008a; Wat 2014) and a recent narrative review (Pei 2015). Our conclusions are somewhat different from those of previous reviews. This is partly because we included studies published several years after some of the above reviews were done. We also screened out studies of non‐RCT design due to our rigorous assessment of studies against criteria in our protocol. Our search was also more comprehensive as we included studies in languages other than English. Additionally, our extensive grey literature search identified several unpublished studies.
The conclusions of the previous reviews (Haedersdal 2008a; Hamilton 2009; Pei 2015) are in line with our conclusions regarding the general direction of evidence for green light, blue light, blue‐red light and infrared light. The authors of earlier reviews emphasised the need for larger studies of better quality, in particular those comparing light therapies to standard treatments, or evaluating possible increased benefit of standard therapies in combination with light as compared to standard therapies alone, which is in agreement with our findings.
Our conclusions regarding the efficacy of pulsed‐dye lasers PDL (i.e. yellow light) for acne are different to those of a recent systematic review on the efficacy of PDL for inflammatory skin diseases (Erceg 2013). The authors identified two RCTs included in our review (Orringer 2004; Seaton 2003), together with several non‐RCT studies, and acknowledged design of such studies as the main limitation to the conclusions in their review. Erceg et al. graded the evidence according to the Oxford Center for Evidence‐based Medicine Levels of Evidence (OCEBM 2011). The authors suggested a B level of recommendation (based on 'studies with consistent evidence from systematic reviews of cohort studies, individual cohort studies, including low quality RCTs, systematic reviews of case‐control studies, individual case control studies or extrapolation from systematic reviews of RCTs or individual RCTs') and concluded that 'PDL seems to be an effective treatment for acne vulgaris' (Erceg 2013; OCEBM 2011). As the two RCTs identified in our review presented inconsistent results (Orringer 2004; Seaton 2003), and there is a paucity of further RCTs we believe that the grade of recommendation should be D ‐'a recommendation based on case reports or expert opinions or troubling, inconsistent or inconclusive studies of any level' (OCEBM 2011).
For similar reasons, our conclusions are different to those of a recent systematic review on intense pulsed light (IPL) for treatment of different dermatologic conditions, which included acne vulgaris (Wat 2014). We considered RCTs only, so we screened out many studies Wat et al included. We found that the evidence is still inconclusive, as opposed to 'treatment of acne vulgaris with IPL alone has the potential to achieve significant improvement in clinical severity and patient satisfaction' and 'IPL‐PDT is a good treatment option for acne vulgaris' (Wat 2014). Furthermore, we rigorously assessed risk of bias using the Cochrane tool and found the overall risk of bias to be unclear or high in most of the studies. That, together with consideration of sample sizes and heterogeneity (regarding populations, interventions, controls and outcomes) prevented us from reaching firm conclusions. Additionally, we grouped interventions not only according to whether an active substance was used prior to illumination (IPL alone versus IPL‐PDT), but also taking into account filters used to narrow the spectrum to selected wavelengths, as these varied across studies. Although the 530 nm to 750 nm filter ('the acne filter') was used most commonly, there were examples where different filters were used in different interventions even within the same study (Taub 2007). We believe filters introduce considerable heterogeneity and it would thus be inappropriate to lose sight of them when reaching conclusions on the effectiveness of IPL.
Our conclusions regarding the effectiveness of photodynamic therapies (PDT) are different to those of reviews on PDT‐only studies (Riddle 2009; Sakamoto 2010; Taylor 2009), broader systematic reviews (Haedersdal 2008a; Hamilton 2009) and a recent narrative review (Pei 2015). We included several new studies on PDT. New studies with larger samples and better quality showed that MAL‐PDT was not more effective than red light alone. We presented a larger and more conclusive body of evidence for that comparison. Similarly, the largest study on ALA‐PDT in our review was identified through grey literature searches, included a total of 266 participants, and showed that ALA‐PDT was not more effective than blue light alone. Recent studies on ALA‐PDT activated by red light were also included in our review, including one originally in Mandarin, with 116 participants. Furthermore, new evidence has emerged on PDT modalities other than MAL‐PDT and ALA‐PDT.
We also found that severe adverse effects as defined in our protocol (blistering) were reported in studies on infrared light, IPL, 37J/cm² MAL‐PDT with occlusion and ALA‐PDT, whereas previous reviews mostly reported on non‐severe adverse effects.
Like other Cochrane Reviews on treatments for acne (Arowojolu 2012; Cao 2015; Garner 2012), we found that many of the included studies had methodological, as well as reporting flaws and identified a lack of standardised outcome measures as an important problem. Previous reviews on core outcome measures in acne have highlighted this problem (Barratt 2009; Tan 2008). Lack of studies comparing light therapies with standard acne treatments is in line with general lack of evidence on comparative effectiveness of common acne therapies (Williams 2012).
Authors' conclusions
Implications for practice.
Due to limited evidence, we are unable to draw firm conclusions from the results of our review. In particular, the lack of long‐term outcomes was a major drawback because if a treatment does not give at least three months’ benefit, it could arguably be considered a treatment failure.
We identified the greatest body of moderate‐quality evidence for the comparison of MAL‐PDT and red light only. However, current evidence does not support the use of MAL‐PDT as a standard therapy for people with moderate to severe acne.
The use of 20% ALA‐PDT activated by blue light as a standard therapy for people with moderate to severe acne, was not supported by the evidence (low and very low quality) as this treatment did not show superior effectiveness in comparison with blue light alone. However, the overall evidence suggests that using lower ALA doses (15% and 10%), together with light modalities other than blue light may be of benefit. This is because several studies found that 20% ALA had more adverse effects (including blistering), whereas individual studies also found that, for example, 20% ALA activated by red light was not more effective than 15% ALA activated by red light, and 10% ALA activated by IPL was more effective than IPL alone.
Although the body of evidence on photodynamic therapies other than MAL‐PDT and ALA‐PDT has increased, it is still inconclusive, and so we could not draw firm conclusions.
We did not identify additional studies on blue light which would suggest recommending blue light as monotherapy with a greater strength of recommendation. Red light alone has shown promising results in several studies, but these were of high overall risk of bias. The new studies we included in our review also suggest greater effectiveness of blue‐red light to that of blue light alone or placebo. Green light was more effective than placebo or no treatment, however these studies were very small.
Although the evidence was not conclusive and we were unable to combine it quantitatively, studies with a larger number of participants and of high overall risk of bias showed that infrared light was not more effective than placebo or no treatment and had more side effects, including severe ones.
Some of the studies in Characteristics of studies awaiting classification may alter the conclusions of the review once fully assessed.
Implications for research.
Acne is a common, non‐life‐threatening condition. Assessment of different therapies are amenable to being tested by randomised controlled trials. However we found that the majority of trials were not properly randomised, with an overall unclear to high risk of bias and were poorly reported. It is well recognised that acne trials are often of poor methodological quality and also affected by poor reporting standards (Ingram 2010).
Methodological issues
Development of detailed guidance for clinical studies as well as standardisation of factors that influence the clinical evaluation of light therapies for acne is needed for future production of high‐quality evidence. Several studies have adhered to FDA guidance for developing drugs for the treatment of acne vulgaris (FDA 2005), which is arguably the best available source for this purpose to date. However this is not specifically designed for light therapies and there have been marked technological advancements in the field since 2005.
A range of different assessment methods in acne trials often prevent, complicate and prolong collection, interpretation, extraction and synthesis of data. Economic impact and, more importantly, the impact this has on patient care needs to be addressed.
Although consensus and recommendations on a consistent use of investigator‐assessed outcome measures would minimise this problem, consensus has still not been reached in the USA (Zaenglein 2016) or in Europe (Nast 2012). Further evaluation of validity, reliability and reproducibility of current outcome measures is needed to come up with the most appropriate ones to agree upon. This should be complemented by exploring relevant information technology and basic medical research advancements in developing innovative techniques for this purpose.
A minority of studies included participant‐assessed outcomes. As with investigator‐assessed outcomes, a variety of measures with questionable validity and reliability were used, particularly in trials with a split‐face design and long follow‐up periods. In individual trials participants commonly assessed their outcomes less often than investigators. This lack of monitoring of the participant perspective on treatment effects prevents adequate comparisons with the investigator perspective. Also, participants were not blinded in most trials, although the investigator assessors were. Due to the nature of interventions and adverse effects, blinding of participants and clinicians is challenging. Even when the participants do not evaluate the effects themselves, their awareness of the intervention may lead to systematic differences in the outcomes unrelated to the effects of interventions of interest (due to possible confounding factors, e.g. different care applied to different face sides or sleeping on the untreated side etc.). Attempts to blind the participants (or lack of such intentions) were not clearly reported in most studies, and so it seems that performance bias has often been overlooked in the studies we included. Future development of participant assessment methods need to be addressed and how they correspond to investigator assessment and compliance. Participant assessment should be performed with similar frequency to investigator assessment in future trials.
Only three studies included a quality‐of‐life assessment. We believe this important participant‐assessed outcome should also be consistently incorporated into future trial protocols. Specific acne quality of life (QoL) instruments for adults and children have been developed (Tan 2008), but need further assessment and validation.
In this review we considered short‐term (two to four weeks), medium (five to eight weeks) and long‐term (longer than eight weeks) follow‐up periods. Standardisation of time points for short, medium and long term assessment after final treatment is needed to enable synthesis of trial data. Furthermore, although of primary interest in this review, long‐term data were scarce, similar to evidence for other acne treatments (Williams 2012), indicating a need to incorporate those assessment time points in the protocols of future trials. As patients are often treated at a young age, a way should also be sought to address follow‐up and possible unwanted effects of light therapies decades after treatment.
Recent initiatives, such as The Cochrane Skin Group Outcomes Research Initiative (CSG‐COUSIN, Schmitt 2016) and the Acne Core Outcomes Research Network (ACORN) (ACORN 2013) may accelerate improvement and standardisation of outcome measurement.
Reporting issues
Tools developed to improve reporting of randomised control trials are freely available, but have not been used in a majority of the reports of included studies. Recommendations of the CONSORT Statement (Schulz 2010) and its extension for non‐pharmacologic treatment (NPT) interventions should be applied to all future reports. The following specific aspects of light therapies and acne trials should be reported:
Light source identity including wavelength, fluence, pulse duration and spot size
Total number and frequency of treatments as well as duration of single light treatment
Definition of time of year (months) when treatment was administered
Instructions given and compliance monitoring method if self‐administered
Whether sun protection advice was given if appropriate
Whether previous acne treatment was stopped and when
Whether concomitant acne treatment was permitted, and if so whether standardised
Baseline measures of the participants for age, sex, Fitzpatrick skin types, duration and location of acne
Initial severity of condition assessment measured by published grading system or preferably by lesion counts. Initial lesion counts should be reported separately for face sides in split‐face trials.
How many investigators performed assessment and their educational background or training
Adverse effects should be reported using lowest level terms (LLTs) as defined in the latest version of MedDRA (MedDRA 2010) and in accordance with CONSORT Statement extension on reporting of harms (Ioannidis 2004). Future studies should also adequately code adverse events into SOCs (System Organ Class) to enable adverse effects to be combined properly in future reviews. Adverse effects such as oedema, erythema, discolouration etc. which occur locally on the laser application site should consistently be coded using LLTs, which reflect the information that the reaction occurred locally at the application site (e.g. in SOC general disorders and application site conditions or in both that SOC and SOC skin and subcutaneous tissue disorders, and not solely in SOC skin and subcutaneous tissue disorders), taking into account directions set out in the MedDRA (MedDRA 2010).
Full results of a number of studies presented in conferences or registered in trials' registers were not published and study authors were unable to provide the full data, or reasons for their early termination. We believe that these details should be added to trials registers' records when appropriate or reported in the form of short communications to journals. Establishing a database for full results of acne clinical trials to enable storing data in a timely manner could also be considered.
Many study authors did not respond to our requests or were unable to provide original data when it was appropriate to combine them with results from other studies. Full results tables should be added as online supplementary material in journals when possible. Adequate data on participants' FPTs, sex, age and severity of acne would enable subgroup analyses and aid identification of differences in the treatment response of acne subtypes in future updates of this review. Furthermore, overcoming of reporting flaws together with standardisation of methodological aspects would enable multiple‐treatment (network) meta‐analyses of different light and other therapies for acne (Caldwell 2005).
Therapies
We have prioritised clinical outcomes in this review. However, further research on the underlying mechanisms of action, (including impact on seborrhoea, effects on sebocytes and sebaceous gland function, antimicrobial and immunomodulatory effects) are required to inform and guide future decisions about the conduct of clinical trials as well as clinical practice in treating acne with light therapies.
Future research must take into account the methodological and reporting issues, as well as whether the following have implications for practice: the possible superior effectiveness of MAL‐PDT in those with severe acne; the use of blue light, red light, blue‐red light and green light alone; 15% ALA‐PDT activated by red or blue‐red light; as well as PDT modalities other than MAL‐ and ALA‐PDT, compared to conventional treatments, placebo or no treatment.
In summary, more robust, well planned studies with greater sample sizes comparing the effectiveness of common acne treatments with light therapies and their effect on reducing lesion counts would be welcomed together with prospective trial registration and adherence to the CONSORT guidelines.
Notes
The protocol was published in 2009 (Car 2009). This is the first version of this review.
Acknowledgements
We wish to thank those trial authors and sponsors who responded to our request for additional information; Elizabeth Doney, Nikolaos Mastellos, Tim Reeves, and Maggie Yin for their support in searches; Dr Quan Yang and Elicia Toon Yuan Ni for their help with screening of studies in Mandarin and data extraction from such studies we subsequently included; Marijan Sember and Dr Adriana Andric for their help with MedDRA; Toby Lasserson, Matthew Grainge and Dr Monika Semwal for their helpful comments and suggestions. We also wish to thank Finola Delamere, Laura Prescott, Hywel Williams and Helen Scott from Cochrane Skin for their continuous support during work on this review.
The Cochrane Skin editorial base wish to thank Sue Jessop, who was the Cochrane Dermatology Editor for this review; Ben Carter, who was the statistical editor; Esther van Zuuren, who was the methods editor; the clinical referees, Brigitte Dréno and Gloria Sanclemente; and the consumer referee, Jack Tweed, as well as Denise Mitchell, who copy‐edited the review.
Appendices
Appendix 1. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor Acne Vulgaris explode all trees #2 (acne):ti,ab,kw #3 (#1 OR #2) #4 MeSH descriptor Lasers explode all trees #5 MeSH descriptor Sunlight explode all trees #6 MeSH descriptor Ultraviolet Therapy explode all trees #7 MeSH descriptor Photolysis explode all trees #8 MeSH descriptor Phototherapy explode all trees #9 MeSH descriptor Photochemotherapy explode all trees #10 MeSH descriptor Photosensitizing Agents explode all trees #11 MeSH descriptor Laser Therapy explode all trees #12 (laser* or sunlight or photolysis or phototherap* or photochemotherapy):ti,ab,kw #13 "ultraviolet therap*" or "Photosensitizing Agent*" or "Photosensitising Agent*" or "light therap*" or "photodynamic therap*":ti,ab,kw #14 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13) #15 (#3 AND #14)
Appendix 2. MEDLINE (Ovid) search strategy
1. exp Acne Vulgaris/ 2. acne.ti,ab. 3. 1 or 2 4. laser$.ti,ab. or exp Lasers/ 5. sunlight.ti,ab. or exp Sunlight/ 6. ultraviolet therap$.ti,ab. or exp Ultraviolet Therapy/ 7. photolysis.ti,ab. or exp Photolysis/ 8. phototherap$.ti,ab. or exp Phototherapy/ 9. photochemotherapy.ti,ab. or exp Photochemotherapy/ 10. photosensiti#ing agent$.ti,ab. or exp Photosensitizing Agents/ 11. light therap$.ti,ab. 12. exp Laser Therapy/ 13. photodynamic therap$.ti,ab. 14. or/4‐13 15. randomized controlled trial.pt. 16. controlled clinical trial.pt. 17. randomized.ab. 18. placebo.ab. 19. clinical trials as topic.sh. 20. randomly.ab. 21. trial.ti. 22. 15 or 16 or 17 or 18 or 19 or 20 or 21 23. (animals not (humans and animals)).sh. 24. 22 not 23 25. 3 and 14 and 24
[Lines 15‐24: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 3. Embase (Ovid) search strategy
1. exp acne vulgaris/ 2. acne.mp. 3. 1 or 2 4. exp phototherapy/ 5. light therap$.ti,ab. 6. exp photodynamic therapy/ 7. photodynamic therap$.ti,ab. 8. exp photochemotherapy/ 9. photochemotherap$.ti,ab. 10. exp sunlight/ 11. sunlight.ti,ab. 12. phototherap$.ti,ab. 13. exp photosensitizing agent/ 14. photosensitizing agent$.ti,ab. 15. photosensitising agent$.ti,ab. 16. ultraviolet therap$.ti,ab. 17. exp photolysis/ 18. photolysis.ti,ab. 19. exp laser/ 20. laser$.ti,ab. 21. or/4‐20 22. random$.mp. 23. factorial$.mp. 24. (crossover$ or cross‐over$).mp. 25. placebo$.mp. or PLACEBO/ 26. (doubl$ adj blind$).mp. 27. (singl$ adj blind$).mp. 28. (assign$ or allocat$).mp. 29. volunteer$.mp. or VOLUNTEER/ 30. Crossover Procedure/ 31. Double Blind Procedure/ 32. Randomized Controlled Trial/ 33. Single Blind Procedure/ 34. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 35. 3 and 21 and 34
Appendix 4. LILACS search strategy
(Acne and (laser$ or sunlight or "luz solar" or phototherap$ or fototerapia or photolysis or photochemotherapy or fotoquimioterapia or ((ultraviolet or photodynamic or light) and therap$) or ((photosensitizing or photosensitising) and agent$)))
These terms combined with the Controlled clinical trials topic‐specific query filter within LILACS.
Appendix 5. ISI Web of Science search strategy
1. acne=Topic 2. laser$=Topic 3. sunlight=Topic 4. phototherap*=Topic 5. photolysis=Topic 6. photochemotherapy=Topic 7. “ultraviolet therapy”=Topic 8. “ultraviolet therapies”=Topic 9. “photosensitising agent$”=Topic 10. “photosensitizing agent$”=Topic 11. “light therapy”=Topic 12. “light therapies”=Topic 13. “photodynamic therapy”=Topic 14. “photodynamic therapies”=Topic 15. 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 16. random*=Topic 17. trial$=Topic 18. placebo$=Topic 19. factorial$=Topic 20. crossover*=Topic 21. cross‐over*=Topic 22. doubl* NEAR/1 blind*=Topic 23. singl* NEAR/1 blind*=Topic 24. assign*=Topic 25. allocate*=Topic 26. volunteer*=Topic 27. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 28. 1 and 15 and 27
Appendix 6. Dissertation Abstracts International search strategy
ALL(Acne) and ALL(laser? or sunlight or phototherapy* or photolysis or photochemotherapy or "ultraviolet therapy" or "ultraviolet therapies" or "photosensitizing agent" or "photosensitizing agents" or "photosensitising agent" or "photosensitising agents" or "light therapy" or "light therapies" or "photodynamic therapy" "photodynamic therapies")
Appendix 7. MetaRegister of Controlled Trials search strategy
Acne AND (laser OR lasers OR sunlight OR phototherapy OR photolysis OR photochemotherapy OR therapy OR therapies OR agent OR agents)
Appendix 8. U.S. National Institutes of Health Ongoing Trials Register search strategy
Acne AND (laser OR lasers OR sunlight OR phototherapy OR photolysis OR photochemotherapy OR "ultraviolet therapy" OR "ultraviolet therapies" OR "photosensitizing agent" OR "photosensitizing agents" OR "photosensitising agent" OR "photosensitising agents" OR "light therapy" OR "light therapies" OR "photodynamic therapy" OR "photodynamic therapies")
Please note that a character limit for searches (100 characters) was introduced by the registry, and we replaced the above search strategy (used up to 28.9.2015) for searches on 27.07.2016 with:
Acne AND (laser* OR sunlight OR phototherapy OR photolysis OR photochemotherapy OR "ultraviolet therap*" OR "photosensitizing agent" OR "photosensitising agent*" OR "light therap*" OR "photodynamic therap*"
Appendix 9. Australian and New Zealand Clinical Trials Registry search strategy
Acne
Appendix 10. World Health Organization International Clinical Trials Registry Platform search strategy
Acne AND (laser* OR sunlight OR Photo* OR therap*)
Appendix 11. EU Clinical trials register search strategy
Acne AND (laser OR lasers OR sunlight OR phototherapy OR photolysis OR photochemotherapy OR therapy OR therapies OR agent OR agents)
Appendix 12. Google Scholar search strategy
Advanced search
With all the words: Acne
With at least one of the words: laser OR lasers OR sunlight OR phototherapy OR photolysis OR photochemotherapy OR therapy
Where my words occur: in the title of the article
Appendix 13. OpenGrey search strategy
Acne
Appendix 14. Glossary
| Acronym | Full term |
| ALA | 5‐aminolevulinic acid |
| BPO | benzoyl peroxide |
| C/BPO | clindamycin/1%–benzoyl peroxide 5% hydrating gel |
| CADI | Cardiff Acne Disability Index |
| CHA | chlorophyll‐a |
| CI | confidence interval |
| DLQI | Dermatology Life Quality Index |
| FDA | the U.S. Food and Drug Administration |
| FPT | Fitzpatrick's Skin Types: based on different reactions to sun exposure and range from type I ('pale white skin, which always burns and never tans') to type VI ('deeply pigmented dark brown to black skin, which never burns and tans very easily') (Fitzpatrick 1988) |
| IAA | indole‐3‐acetic acid |
| ICG | indocyanine green |
| IGA | Investigators' Global Assessment |
| ILs | inflamed lesions, includes papules or pustules or both |
| IPL | intense pulsed light |
| ITT | intention‐to‐treat analysis |
| KTP | potassium titanyl phosphate |
| LOCF | last observation carried forward |
| LPDL | long‐pulsed dye laser |
| MAL | methyl‐aminolevulinate |
| MASS | Michaëlsson acne severity grading score |
| MD | mean difference |
| NILs | non‐inflamed lesions, includes blackheads or whiteheads or both |
| NNTB | number needed to treat for an additional beneficial outcome |
| NNTH | number needed to treat for an additional harmful outcome |
| OFI | optical fibre intra‐tissue irradiation |
| P acnes | Propionibacterium acnes |
| PDL | pulsed‐dye laser |
| PDT | photodynamic therapy |
| RCT | randomised controlled trial |
| RD | risk difference |
| RF | radiofrequency |
| RR | risk ratio |
| SD | standard deviation |
| SE | standard error |
| SPF | sun protection factor |
| TLMB | topical liposomal methylene blue |
| UV | ultraviolet |
| VAS | visual analogue scale |
| YD | Yinhua decoction |
Data and analyses
Comparison 1. Blue‐red light versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant's and investigator's global assessment of improvement at final treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Participant's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Blue‐red light versus topical benzoyl peroxide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant's and investigator's global assessment of improvement at final treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Participant's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Blue‐red light versus blue light alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant's and investigator's global assessment of improvement at final treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Participant's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Vehicle + 1000 s blue light versus vehicle + 500 s blue light.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant's global assessment of improvement at 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Investigator's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 1000 s blue light versus 500 s blue light at 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 1000 s blue light versus 500 s blue light at 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. 20% ALA‐PDT versus vehicle plus blue light.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant's global assessment of improvement at 6 weeks | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.04] |
| 1.1 20% ALA‐PDT (1000 s) versus vehicle plus blue light (1000 s) | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 1.2 20% ALA‐PDT (500 s) versus vehicle plus blue light (500 s) | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.03] |
| 2 Investigator's global assessment of improvement at 3 weeks | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.56, 1.52] |
| 2.1 20% ALA‐PDT (1000 s) versus vehicle plus blue light (1000 s) at 3 weeks | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.44, 1.65] |
| 2.2 20% ALA‐PDT (500 s) versus vehicle plus blue light (500 s) at 3 weeks | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.47, 2.18] |
| 3 Investigator's global assessment of improvement at 6 weeks | 1 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.51, 1.29] |
| 3.1 20% ALA‐PDT (1000 s) versus vehicle plus blue light (1000 s) at 6 weeks | 1 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.50, 1.71] |
| 3.2 20% ALA‐PDT (500 s) versus vehicle plus blue light (500 s) at 6 weeks | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.35, 1.39] |
Comparison 6. 20% ALA‐PDT 30 min incubation plus IPL versus 20% ALA‐PDT 3 h incubation plus IPL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant's and investigator's global assessment of improvement at 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Participant's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. 20% ALA‐PDT plus 560 nm IPL versus 560 nm IPL alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant's global assessment of improvement at 8 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. 20% ALA‐PDT 1000 s versus 500 s.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant's global assessment of improvement at 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Investigator's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Investigator's global assessment of improvement at 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Investigator's global assessment of improvement at 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. 20% ALA‐PDT versus 15% ALA‐PDT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant's global assessment of improvement at 24 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Investigator‐assessed severe adverse effects | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. 20% ALA‐PDT versus 10% ALA‐PDT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant's global assessment of improvement at 24 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Investigator‐assessed severe adverse effects | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 11. 20% ALA‐PDT versus 5% ALA‐PDT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant's global assessment of improvement at 24 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Investigator‐assessed severe adverse effects | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 12. Yellow light versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed change in ILs, NILs and cysts at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Investigator‐assessed change in ILs (papules) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator‐assessed change in ILs (pustules) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Investigator‐assessed change in NILs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Investigator‐assessed change in cysts | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. Infrared light versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed change in ILs, NILs and cysts at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Investigator‐assessed change in ILs (papules) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator‐assessed change in ILs (pustules) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Investigator‐assessed change in NILs (open comedones) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Investigator‐assessed change in NILs (closed comedones) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Investigator‐assessed change in cysts at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Investigator‐assessed severe adverse effects | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 14. 585 nm PDL versus 530‐750 nm IPL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed change in ILs and NILs at 8 weeks | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 1.1 Investigator‐assessed change in ILs | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator‐assessed change in NILs | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Investigator‐assessed change in ILs and NILS at 8 weeks (normal) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Investigator‐assessed change in ILs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Investigator‐assessed change in NILs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. 1450 nm laser treatments: single pass 13‐14 J/cm2 versus double pass 8–11 J/cm2.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed change in ILs at 8 weeks | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Investigator‐assessed change in ILs at 8 weeks (normal) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 16. 1450 nm laser treatments: 14 J/cm2 versus 16 J/cm2.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed change and percentage change in ILs | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 1.1 Investigator‐assessed change in ILs at 4 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator‐assessed percentage change in ILs at 4 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Investigator‐assessed change in ILs at 12 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Investigator‐assessed percentage change in ILs at 12 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Investigator‐assessed change in ILs at 24 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Investigator‐assessed percentage change in ILs at 24 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Investigator‐assessed change in ILs at 12 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Investigator‐assessed percentage change in ILs at 12 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Investigator‐assessed change and percentage change in ILs (normal) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Investigator‐assessed change in ILs at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Investigator‐assessed percentage change in ILs at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Investigator‐assessed change in ILs at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Investigator‐assessed percentage change in ILs at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Investigator‐assessed change in ILs at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Investigator‐assessed percentage change in ILs at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Investigator‐assessed change in ILs at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Investigator‐assessed percentage change in ILs at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 17. 80 mg/g MAL plus red light versus placebo cream plus red light at 6 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed change in ILs | 3 | 360 | Mean Difference (IV, Random, 95% CI) | ‐2.85 [‐7.51, 1.81] |
| 2 Investigator‐assessed percentage change in ILs | 3 | 360 | Mean Difference (IV, Random, 95% CI) | ‐10.09 [‐20.25, 0.06] |
| 3 Investigator‐assessed change in NILs | 3 | 360 | Mean Difference (IV, Random, 95% CI) | ‐2.01 [‐7.07, 3.05] |
| 4 Investigator‐assessed percentage change in NILs | 3 | 360 | Mean Difference (IV, Random, 95% CI) | ‐8.09 [‐21.51, 5.32] |
| 5 Investigator‐assessed severe adverse effects | 3 | 360 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.02] |
| 6 Investigator's global assessment of improvement | 3 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.11, 2.74] |
Comparison 18. 40 mg/g MAL plus red light versus placebo cream plus red light at 6 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed change in ILs | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Investigator‐assessed percentage change in ILs | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Investigator‐assessed change in NILs | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Investigator‐assessed percentage change in NILs | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Investigator's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Investigator's global assessment of improvement at 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Investigator's global assessment of improvement at 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 19. 80 mg/g MAL plus red light versus placebo cream plus red light at 4 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed change in ILs and NILs | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 1.1 Investigator‐assessed change in ILs | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator‐assessed change in NILs | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Investigator‐assessed change in ILs and NILs (normal) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Investigator‐assessed change in ILs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Investigator‐assessed change in NILs (normal) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 20. 160 mg/g MAL plus red light versus placebo cream plus red light.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed change in ILs | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Investigator‐assessed change in ILs at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator‐assessed change in ILs at 10 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Investigator‐assessed percentage change in ILs | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Investigator‐assessed percentage change in ILs at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Investigator‐assessed percentage change in ILs at 10 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Investigator‐assessed severe adverse effects | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Investigator's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 21. 80 mg/g MAL plus red light versus 40 mg/g MAL plus red light at 6 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed change in ILs | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Investigator‐assessed percentage change in ILs | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Investigator‐assessed change in NILs | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Investigator‐assessed percentage change in NILs | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Investigator's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 22. 160 mg/g MAL‐PDT versus IPL alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed percentage change in ILs and NILs | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 1.1 Investigator‐assessed percentage change in ILs at 4 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator‐assessed percentage change in ILs at 12 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Investigator‐assessed percentage change in NILs at 4 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Investigator‐assessed percentage change in NILs at 12 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Investigator‐assessed percentage change in ILs and NILs (normal) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Investigator‐assessed percentage change in ILs at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Investigator‐assessed percentage change in ILs at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Investigator‐assessed percentage change in NILs at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Investigator‐assessed percentage change in NILs at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 23. 160 mg/g MAL‐PDT versus adapalene.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed percentage change in ILs and NILs | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 1.1 Investigator‐assessed percentage change in ILs at 4 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator‐assessed percentage change in ILs at 12 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Investigator‐assessed percentage change in NILs at 4 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Investigator‐assessed percentage change in NILs at 12 weeks | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Investigator‐assessed percentage change in ILs and NILs (normal) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Investigator‐assessed percentage change in ILs at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Investigator‐assessed percentage change in ILs at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Investigator‐assessed percentage change in NILs at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Investigator‐assessed percentage change in NILs at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 24. ALA plus 420‐950 nm IPL versus IPL alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed percentage change in ILs and NILs at 12 weeks | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 1.1 Investigator‐assessed percentage change in ILs | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator‐assessed percentage change in NILs | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Investigator‐assessed percentage change in ILs and NILs at 12 weeks (normal) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Investigator‐assessed percentage change in ILs | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Investigator‐assessed percentage change in NILs at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Investigator's global assessment of improvement at 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 25. 20% ALA‐PDT plus PDL versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed change in ILs, NILs and cysts | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Investigator‐assessed change in ILs (papules) at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Investigator‐assessed change in ILs (pustules) at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Investigator‐assessed change in NILs (open comedones) at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Investigator‐assessed change in NILs (closed comedones) at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Investigator‐assessed change in cysts at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Investigator‐assessed change in ILs (papules) at 10 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Investigator‐assessed change in ILs (pustules) at 10 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Investigator‐assessed change in NILs (open comedones) at 10 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Investigator‐assessed change in NILs (closed comedones) at 10 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Investigator‐assessed change in cysts at 10 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Investigator‐assessed severe adverse effects | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 26. Intense pulsed light (IPL) versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed severe adverse effects | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Investigator‐assessed change in acne severity at 6 months | 1 | 20 | Mean Difference (Fixed, 95% CI) | 0.49 [‐1.21, 2.20] |
| 2.1 585 light versus no treatment | 1 | 10 | Mean Difference (Fixed, 95% CI) | 0.6 [‐1.88, 3.08] |
| 2.2 Blue light versus no treatment | 1 | 10 | Mean Difference (Fixed, 95% CI) | 0.4 [‐1.95, 2.75] |
| 3 Investigator‐assessed change in acne severity at 6 months (normal) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.49 [‐0.92, 1.91] |
| 3.1 585 light versus no treatment | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐1.45, 2.65] |
| 3.2 Blue light versus no treatment | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐1.55, 2.35] |
Comparison 27. 1450 nm laser treatments: single pass versus double pass.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed severe adverse effects | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 28. MAL‐PDT with or without occlusion followed by 37 J/cm2 red light.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed severe adverse effects | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Investigators' global assessment of improvement at 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 29. Single versus multiple treatment of 20% ALA plus 550–700 nm light.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator‐assessed severe adverse effects | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 30. 585 nm PDL versus BPO plus tretinoin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator's global assessment of improvement (timepoint unclear) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 31. 585 nm PDL versus retinoic acid plus TCA peeling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator's global assessment of improvement (timepoint unclear) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 32. Blue‐red light plus topical treatments (TT) versus topical (TT) alone at 4 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 33. 400‐410 nm plus 660 nm (blue‐red) light versus 400‐410 nm (blue) light alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator's global assessment of improvement at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 34. Blue LED versus red LED.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 35. Blue‐red light plus sulfotanshinone (SFT) versus SFT alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator's global assessment of improvement at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 36. Blue‐red light plus sulfotanshinone (SFT) versus blue‐red light plus SFT plus prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator's global assessment of improvement at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 37. Blue‐red light plus sulfotanshinone (SFT) versus SFT plus prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator's global assessment of improvement at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 38. Yinhua decoction (YD) plus electric light versus YD plus blue‐red light.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator's global assessment of improvement at 12w | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 39. Blue‐red light plus oral plus topical treatments (OT plus TT) versus OT plus TT alone at 4 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator's global assessment of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 40. ALA plus red light versus red light alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Investigator's global assessment of improvement | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Investigator's global assessment of improvement at 2 weeks | 2 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 2.74 [1.59, 4.71] |
| 1.2 Investigator's global assessment of improvement at 4 weeks | 2 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.36, 2.79] |
| 1.3 Investigator's global assessment of improvement at 6 weeks | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.01, 2.35] |
| 1.4 Investigator's global assessment of improvement at 8 weeks | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [1.36, 2.70] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anyachukwu 2014.
| Methods | This was a parallel‐group RCT Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared, none. Quote (page 70): "No conflicts of interest, no funding sources." Setting: Single centre, Nsukka (Nigeria) Recruitment: "drawn from an indefinite 6 stratified faculties population (equal numbers of patients were enrolled from each faculties of the campus (UNEC) and screened after meeting the eligibility criteria)" Duration: 3 months, May 2012 to July 2012 |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; 22 ± 4 years; not stated Clinically evident acne: Yes Severity of condition assessment: Moderate to severe? acne "GAGS severity level rating >19" (Global Acne Grading System) Fitzpatrick skin types: V‐VI Other: "Male student of University of Nigeria Enugu Campus (UNEC), general good health, .. willingness and convenience to follow up treatment regime"; "self‐management topical agents" allowed, differed among groups. Excluded "Being under acne systemic therapy or other microbial for at least 1month ago, presenting acne fulminans or follicular occlusion triad, female subjects and male subjects below 16 years under stress, severely photosensitivity or on steroid drugs for at least 6 month to the study." Enrolled: 40 (all male), 20 in the light group, 20 in the placebo group Randomised: 40 Withdrawals/drop‐outs: 4 withdrew (3 not treated, 1 "tight schedule") and 1 lost to follow‐up in the light treatment group, no withdrawals/dropouts in the placebo group Final number and proportion of participants evaluable: 15/20 (75%) in the light treatment group, 20/20 (100%) in the placebo group. Intention‐to‐treat analysis: No |
|
| Interventions |
Intervention 1 Infrared non ablative laser combined with "self‐management topical agents" Number and frequency of treatments: 8 in total, 2 weekly over 4 weeks Wavelength/Fluence/Duration/Spot size: 905 nm/5 J/cm²/pulse 120 nm, duration 12 min Supplier: CARCI – Lasermed 4098 Instructions to participants: Not applicable Intervention 2 Placebo‐non radiating probe combined with "self‐management topical agents" Number and frequency of treatments: 8 in total, 2 weekly over 4 weeks Wavelength/Fluence/Duration/Spot size: Not applicable Supplier: CARCI – Lasermed 4098 Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: Unclear (assessed at each session whilst on treatment and 3 days after final treatment?) Primary outcomes of review interest recorded 1. Percentage change from baseline of combined number of lesions? Methods of assessing primary outcomes 1. "The face was arbitrarily divided into four (25 cm²) quadrants (Global Acne Grading System – GAGS severity level rating > 19) to assess baseline distribution (number, type and the mean density of acne lesions of comedones, papule, pustule, nodules) face map pattern, also the frequency severity of facial acne. The clearance rate was calculated and recorded mean density of acne was calculated and recorded at base line after treatment for the four consecutive week treatment sessions. Density = n/25 cm² (Initial Density − Present Density = Level of Clearance)." Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. "10 min observation on subjects for any possible adverse reaction post‐treatment"; "Participants undergo treatment for about 4 weeks (8 sessions), participants were monitored for 10 min after each session for; erythema, rashes, pigmentation, inflammation, itching or any subjective complaints." (study authors' clarification). |
|
| Notes | Language: English. "Self‐management topical agents" allowed, possible bias introduced due to baseline differences. Final evaluation performed less than 2 weeks post treatment. The study authors were contacted and provided additional information on power calculation, Fitzpatrick skin types, ITT analysis, number and frequency of treatments, supplier of placebo device, primary outcomes, methods of assessing adverse effects, blinding of performing clinicians, participants and outcome assessors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 67): "Balloting by an independent physician after another independent clinician had generated the random allocation tags and numbers concealed in a uniform brown envelopes.These were thoroughly mixed in an opaque container, and subjects picked numbers assigning them to their respective intervention groups." Comment: We judged this as adequate and at a low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Please see quote above. We judged this as adequate and risk of bias as low. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 67): "All participants and the assessors (physicians) were blocked and blinded to the planned interventions during the randomization."; 'control group patients were treated with a placebo‐non radiating probe". Further clarification provided by the study authors: "Same device was used (CARCI – Lasermed 4098). The 905 nm is not visible to all participants, the probe when used in placebo is set off and lock out in the user interface only the researcher/treatment physician who was not blinded can determine if the probe is in treatment or placebo mode by using the inbuilt sensor on the device." Comment: Performing clinicians were not blinded. An attempt to blind participants by placebo‐non radiating probe, not enough details provided to evaluate whether it was successful. We judged the risk of bias as unclear. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 67): "All participants and the assessors (physicians) were blocked and blinded to the planned interventions during the randomization." Study authors further clarified that the physicians doing the assessment were not allowed to know which group participants belonged to nor access to the participants’ tags or the treatment room. Comment: We judged this as adequate and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome measures obtained for 15/20 (75%) participants randomised to intervention group, which is less than 80%, so we judged the risk of bias as high. ITT analysis was not performed (study authors' clarification). |
| Selective reporting (reporting bias) | High risk | Outcomes were not pre‐specified in the methods section, so we judged this as at high risk of bias. Study authors clarified it as "the percentage clearance rate, density of old and new eruption (vigor) and change in severity using GAGS". |
| Other bias | High risk | "Self‐management topical agents" allowed, possible bias introduced due to baseline differences, so we judged risk of bias as high. |
Ash 2015.
| Methods | This was a parallel‐group RCT Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Yes, details not reported Sponsorship and conflict of interest: Funded by The Dezac Group Ltd, Cheltenham UK; "Anna Harrison and Rebecca Whittall have no conflict of interest. Caerwyn Ash and Samantha Drew receive salary from The Dezac Group Ltd. The sponsors of this study had no role in the study design, data collection, data analysis, interpretation, or writing of the report." Setting: The Dezac Group Headquarters, Cheltenham, UK Recruitment: Advertisements at Gloucester University, doctors' surgeries, schools (sixth form), colleges Duration: 2 months (December 2012–January 2013) |
|
| Participants |
Included Age (inclusion criterion; mean; range): 16‐45 years; not stated; not stated Clinically evident acne: mild to moderate inflammatory facial acne Severity of condition assessment: Leeds grading Fitzpatrick skin types: I‐V Other: "…male and female; able to give informed consent…Cohorts consisted of Caucasian, Asian and mixed Afro‐Caribbean ethnic groups" Excluded "History of photosensitivity and pregnancy or lactation within the previous 3 months; subjects who had excessive facial exposure to sunlight or artificial UV‐light within three months prior the study were excluded; psoriasis or sandpaper acne; participated in any clinical study during the previous month; migraines or seizures triggered by light; topical anti‐spot medications, topical antibiotics or topical steroid usage; washout periods for previous treatments were 8 weeks for oral antibiotics and topical treatments, 12 weeks for contraceptives containing cyproteroneacetate, 52 weeks for oral Isotretinoin." Enrolled: 41 Randomised: 41 in total, 26 to treatment group, 15 to control group (M/F not reported) Withdrawals/drop‐outs: 3 due to "employment contractions"; 2 "removed from the study due to exposure to sunlight..." (group assignment unclear) Final number and proportion of participants evaluable: 36/41 (89%) ITT analysis: Yes |
|
| Interventions |
Intervention 1 Pre‐treatment facial wash/weak chemical peel (containing salicylic acid, glycolic acid, lactic acid) followed by treatment with blue light device and then post treatment facial moisturiser (containing salicylic acid, glycolic acid, lactic acid, menthol, niacin) Number and frequency of treatments: "Treatment was performed every other day for 8 weeks" (28? treatments in total, every other day over 8 weeks) Wavelength/Fluence/Duration/Spot size: 414nm/220 J over 6 cm²/3 min/ not applicable Supplier: The Dezac Group Ltd, Cheltenham, UK Instructions to participants: "Subjects were instructed to cleanse their face daily with a facial cleanser containing glycolic, salicylic, and lactic acids, which was provided by the sponsor. Subjects in the treatment group were required to adopt the specified facial skin care regimen and avoid using any other facial skin care products, for the duration of the study… After the first consultation, screened subjects in the treatment group watched a short video on how to use the device and creams, and were given a diary card, indicating treatment days and days for photographic assessment at the clinical office". Additional information provided by the author: "Each patient was contacted on a weekly sometimes biweekly interval to ensure compliance" Intervention 2 Not reported. Author's clarification: "Initially they were given a sham device, however due to the nature of treatment of visible blue light, subjects identified early that they were control" Number and frequency of treatments: Unclear Instructions to participants: "The control group was given a diary card for photographic assessment dates, and a list of non‐conformance medication and over the counter (OTC) products." Additional information provided by the author: "Each patient was contacted on a weekly sometimes biweekly interval to ensure compliance" |
|
| Outcomes | Evaluation time points of review interest: Unclear, 4 weeks after final treatment? ("evaluated at 1, 2, 4, 8 and 12 weeks after start of treatment, final evaluation 4 weeks after final treatment… All participants completed questionnaires at baseline, 3 months, and 6 months") Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement? 2. Investigator‐assessed change in lesion count (IL change and percentage change from baseline) Methods of assessing primary outcomes 1. "The investigators and subjects overall assessment of the treatment was recorded"… "All participants completed questionnaires at baseline, 3 months, and 6 months." 2. "The acne was quantified in this study by lesion counts using custom software developed by the authors" Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Unclear |
|
| Notes | Language: English. The study authors were contacted and provided additional information on power calculation, Fitzpatrick skin types, ITT, control intervention, assessment of compliance and adverse effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 3): "At recruitment, patients were randomised to either treatment or control by sequential numbers in sealed envelopes in a 4:1 ratio.’ Comment: We judged this as adequate and risk of bias as low. |
| Allocation concealment (selection bias) | Low risk | Quote (page 3): "Allocations were concealed from assessors and patients throughout the study and revealed only to the investigator (CA)." Comment: See above. We judged this as adequate and risk of bias as low. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear what the exact control intervention was, so it is not possible to evaluate risk of bias for this domain. Author's clarification: "Initially they were given a sham device, however due to the nature of treatment of visible blue light, subjects identified early that they were control". We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | Unclear whether such outcomes were recorded. Author's clarification: "Initially they were given a sham device, however due to the nature of treatment of visible blue light, subjects identified early that they were control" |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 3): "The acne was quantified in this study by lesion counts using custom software developed by the authors (figure 3). The two assessors were blinded by the subjects cohort and assessment interval". Comment: We judged this as adequate and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures obtained for 36/41 (88%) participants. We therefore judged the bias to be low. |
| Selective reporting (reporting bias) | High risk | Quote (page 4): "This improvement also correlated into an improvement in their social confidence and self appearance… the majority of subjects reporting that they were satisfied, very satisfied, or extremely satisfied with treatment". Comment: Participant‐assessed outcomes not reported with quantitative data, so we judged the risk of bias to be high. |
| Other bias | High risk | Quote (page 6): "Anna Harrison and Rebecca Whittall have no conflict of interest. Caerwyn Ash and Samantha Drew receive salary from The Dezac Group Ltd. The sponsors of this study had no role in the study design, data collection, data analysis, interpretation, or writing of the report." Comment: Unclear control intervention. Unclear whether groups comparable at baseline. Author clarified that the participants were comparable at baseline, but has not provided additional data. The study was funded by The Dezac Group Ltd., Cheltenham. U.K. Lead author and one co‐author receive salary from the company which funded the study, which might have introduced additional bias, although the role of the sponsor was clarified as above. Due to all of the above reasons we judged the risk of other bias as high. |
Barolet 2010.
| Methods | This was a split‐face or back RCT. Unit of randomisation: Left or right face or back Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (page 171): "Conflict of interest: Intellectual Property disclosure related to the radiant IR pre‐PDT method by the first author. Contract grant sponsor: RoseLab Skin Optics Laboratory." Setting: Single centre, Montreal (Quebec, Canada) Recruitment: Dr Daniel Barolet Clinic Duration: 6 months, September 2007 to February 2008 |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; 26.2 years; 13‐54 years Clinically evident acne: Yes Severity of condition assessment: Mild to moderate acne (Combined Acne Severity Classification); lesion count > 10 Fitzpatrick skin types: I‐III Other: Otherwise healthy participants. Excluded "Patients taking cortisone (Prednisone), anticoagulant therapy, or any drug known to increase photosensitivity, In addition, during the 12 months preceding the study, patients were required not to have used isotretinoin (Accutane), or applied topical steroids on the site to be treated. Moreover, oral antibiotics use, laser or topical anti‐acne medication at the to‐be‐treated site were not permitted for 8 weeks prior to the study." Enrolled: 10 (7M/3F) Randomised: 10 Withdrawals/drop‐outs: 1 lost to follow‐up. Final number and proportion of participants evaluable: 9 (90%) ITT analysis: No |
|
| Interventions |
Intervention 1 Infrared light emitting diode (LED) pre‐treatment followed by 20% ALA‐PDT Number and frequency of treatments: Single treatment Wavelength/Fluence/Duration/Spot size: Infrared (970 nm/72 J/cm²/ pre‐treatment duration 15 min/ not reported) followed by those as in intervention 2 Supplier: ALA (20% Levulan Kerastick, DUSA Pharmaceuticals) and PDT LumiPhase‐ R/BTM, OPUSMED Inc, Montreal, Canada) Instructions to participants: Not applicable Intervention 2 20% ALA‐PDT Number and frequency of treatments: Single treatment Wavelength/Fluence/Duration/Spot size: 630 nm; 70 J/cm²; ALA incubation 60 min, light treatment 23 min; not reported Supplier: ALA (20% Levulan Kerastick, DUSA Pharmaceuticals) and PDT LumiPhase‐R/BTM, OPUSMED Inc, Montreal, Canada) Instructions to participants: Not applicable. |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final (single) treatment Primary outcomes of review interest recorded 1. Percentage change from baseline in IL count (papules, pustules and nodules reported separately) 2. Percentage change from baseline in NIL count (open and closed comedones reported separately) Methods of assessing primary outcomes 1.& 2. Lesion counts were performed based on digital photos Secondary outcomes of review interest recorded 1. Investigator's global assessment of improvement (Clinical Global Severity Assessment) 2. Adverse effects Methods of assessing secondary outcomes 1. 6‐point rating scale (0 = Clear, 1 = almost clear, 2 = mild, 3 = moderate ,4 = severe, 5 = very severe) 2. Monitored during study (signs of erythema, oedema, scaling/crusting, bronzing, textural changes, hyper and hypo‐pigmentation documented) |
|
| Notes | Language: English. Significant difference between treated and untreated side in papule counts (P = 0.037); median (SD) 12 (26) versus 6 (13). No significant difference for other lesion counts. Quote (page 174): "For the secondary efficacy variable of NILs the percent change from baseline for the IR‐treated side was found to be statistically superior to that of the control side (P < 0.037)." This is not in line with the data given in Table 3 on page 175 for total NIL median changes (0.00 for both sides). Results for lesion counts reported as medians (95% CIs for means). We contacted the study author but he was unable to provide additional information to clarify these issues. Sponsors were not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 173): "One side was randomly (using a coin flip) assigned to receive IR pre‐treatment and ALA–PDT, and the other ALA–PDT alone to serve as control." Comment: We judged this as adequate and at a low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No evidence that blinding of participants/personnel was carried out. Given that one side of the face was pre‐treated with IR then it is unlikely that participants/personnel were blinded. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quotes (page 173): "Lesion counts were performed based on the digital photographs by two non treating physicians who were blinded to the treatment regimen (IR‐treated or control side) and to the timing of the photographs (baseline or post‐treatment)"; "The global severity of acne was assessed at the end of the study by the three non treating physicians..." Comment: We judged this as adequate and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures obtained for 90% of randomised participants. One patient was lost to follow‐up. Comment: We judged this as at a low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | All outcome measures mentioned in the 'Materials and Methods' section were reported, so we judged this as at a low risk of bias. |
| Other bias | Unclear risk | Commercial sponsorship declared, which might have introduced some bias. No other possible sources of bias were identified. Insufficient information was given to permit a clear judgement. |
Baugh 2005.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Declared. Quote (page 1290): "Funding for this research was provided by a grant from Laseroscope, San Jose, CA." Setting: Single centre (California, USA) Recruitment: Through local advertisements and physician referrals Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; 27.8 (± 7.5) years; 19 ‐41 years Clinically evident acne: Yes Severity of condition assessment: "clinically evaluated with mild to moderate acne" (page 1290) Fitzpatrick skin types: I‐III Other: Before the commencement of the treatment phase, each subject was exposed to a test spot laser treatment, in an area other than the designated treatment site, to assess any adverse reactions. Excluded "Individuals who have been treated with systemic antibiotics within 8 weeks prior to treatment and subjects who have been treated with topical acne medications within 2 weeks prior to treatment, including benzoyl peroxides, salicylates, retinoids, antibiotics,and astringents. Additionally, subjects with a medical history of keloid scar formation and a history of oral retinoid ingestion within 6 months prior to treatment were also excluded from study enrolment." Enrolled: 25 (4 M/21 F) Randomised: 25 Withdrawals/drop‐outs: 2 withdrawals (unclear whether they withdrew pre or post randomisation; one due to personal reasons before treatment phase), 5 drop‐outs ("Five subjects (19%) voluntarily discontinued the study before completion of the final follow‐up"; reasons not stated) Final number and proportion of participants evaluable: Unclear whether 16/23 or 18/25 participants. ITT analysis: Not stated |
|
| Interventions |
Intervention 1 KTP 532 nm laser with continuous cooling Number and frequency of treatments: 4 treatments in total, twice a week, with no fewer than 72 h apart Wavelength/Fluence/Duration/Spot size: 532 nm; 12 J/cm²; pulse width 30‐40 ms; not reported Supplier: Aura KTP 532 nm pulsed laser system, Laserscope, San Jose Instructions to participants: Not applicable Intervention 2 Treatments with continuous cooling without laser Number and frequency of treatments: 4 treatments in total, twice a week, with no fewer than 72h apart Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment (also assessed at 1 week after final treatment) Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement Methods of assessing primary outcomes 1. Non‐standardised overall treatment percent satisfaction scale (overall treatment satisfaction in intervals of 10 percentage points) Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Investigator’s global assessment of improvement 3. Adverse effects Methods of assessing secondary outcomes 1. The number of comedones, papules, pustules, and infiltrated lesions recorded and scored as a severity index (using Michaëlsson acne severity grading score) 2. Non‐standardised overall treatment percent satisfaction scale (baseline photographs used) 3. Monitored during study |
|
| Notes | Language: English. We attempted to contact the study authors for clarification, but were not successful. Sponsors were not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1291): "The half of the face to receive the laser exposure was chosen randomly for each subject by using a coin toss with a 25‐cent piece (heads = right side of face; tails = left side of the face)." Comment: We judged this as adequate and the risk of bias as low. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 1291): "To mimic a laser exposure, the entire facial area of the control site was exposed to contact cooling without delivery of laser irradiance." Comment: We judged that the participants were adequately blinded based on the above evidence. Given that laser was used on one side of the face and contact cooling was used on the other side of the face then personnel were probably not blinded, but we judged it was unlikely that there were systematic differences between face sides in the care that was provided, or in exposure to factors other than the interventions of interest. We therefore judged the risk of bias as low. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Low risk | Participants were adequately blinded (please see above), so we judged the risk of bias as low. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Not stated whether the clinicians applying the treatment were outcome assessors as well. No intended blinding of outcome assessors reported. No evidence that assessors were blinded provided. We judged this as at unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Final number and proportion of participants evaluable unclear. However, outcome measures reported for less than 80% of randomised participants. We judged this as at a high risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | The protocol for this study was not available. Outcome measures mentioned were reported, so we judged this as a low risk of bias. |
| Other bias | Unclear risk | Commercial sponsorship might have introduced some bias. Insufficient information to permit a clear judgement. |
Bernstein 2007.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Single centre (Pennsylvania, USA) Recruitment: Not stated Duration: 6 months. Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; 29 years; 23‐41 years Clinically evident acne: Yes Severity of condition assessment: "...with active papular acne..." (page 193) Fitzpatrick skin types: I‐III Excluded Not stated. Enrolled: 7 (1 M/7 F) Randomised: 7 Withdrawals/drop‐outs: 1 withdrawal (to continue treatment at a tanning salon after the first treatment) Final number and proportion of participants evaluable: 6 (86%) Intention to treat analysis: No |
|
| Interventions |
Intervention 1 4% lidocaine applied for 40 minutes, then washed and cleaned with 3% hydrogen peroxide (HPO), then single‐pass, high‐energy laser treatment Number and frequency of treatments: 4 treatments, monthly Wavelength/Fluence/Duration/Spot size: 1450 nm/13‐14 J/cm²/4 x 50 ms/6 mm² Supplier: Smoothbeam, Candela Corp., Wayland, MA Instructions to participants: Not applicable Intervention 2 4% lidocaine applied for 40 minutes, then washed and cleaned with 3% HPO, double‐pass, low‐energy laser treatment Number and frequency of treatments: 4 treatments, monthly Wavelength/Fluence/Duration/Spot size: 1450 nm/8‐11 J/cm²/4 x 50 ms/6 mm² Supplier: Smoothbeam, Candela Corp., Wayland, MA Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 8 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Change from baseline in ILs count Methods of assessing primary outcomes 1. A 0–4 scale, with 0 being worse, 1 being no change, 2 being mild improvement, 3 being moderate improvement, and 4 being marked improvement 2. Acne papules and pustules counted in each cosmetic unit Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Investigator’s global assessment of improvement 3. Adverse effects (pain, erythema, edema, blistering, hyper‐pigmentation, hypo‐pigmentation, and scarring) Methods of assessing secondary outcomes 1. Allen‐Smith acne severity scale, a 0–8 scale, with 0 representing no acne and 8 representing involvement virtually the entire face by acne 2. A 0–4 scale, with 0 being worse, 1 being no change, 2 being mild improvement, 3 being moderate improvement, and 4 being marked improvement (treating physician) 3. Participants assessed pain on a 0‐10 scale, treating physicians assessed other adverse effects on a 0‐3 scale |
|
| Notes | Language: English. We contacted the study authors for clarification, but were unsuccessful in obtaining additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 194): "..were randomised as to which side of the face received which treatment." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No evidence that participants/clinicians were blinded provided. Given that one side of the face was treated with "double pass" laser and the other with single pass laser then it is unlikely that participants/ personnel were blinded. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | No evidence that participants were blinded was given, so we judged the risk of bias as unclear for participant‐assessed outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 194): "Three physicians blinded as to the treatment parameters compared pre‐ and post‐treatment photographs in a blinded fashion." Comment: We judged this as adequate and at a low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for 6 out of 7 randomised participants (86%). We judged this as at a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Unclear risk | There was no declaration of sponsorship or potential conflicts of interest. Insufficient information was given to permit a clear judgement. |
Bissonnette 2010.
| Methods | This was a parallel‐group RCT (split face within groups). Participants were randomised to treatment with 25 or 37 J/cm² light, and their face sides to treatment with or without occlusion. Unit of randomisation: Whole person and left or right face. Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (page 1352): "This study was funded by Photocure ASA, Oslo, Norway". Study authors also disclosed possible conflicts interest (page 1352). Setting: Single centre (2 site locations, Montreal, Quebec, Canada) Recruitment: Through single centre (two site locations) Duration: 12 months, July 2007‐ July 2008 |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 18 years; 24.4 ± 5.9; 18‐40 years Clinically evident acne: Yes Severity of condition assessment: Severe acne. At least 10 ILs on each side of the face and a Global Acne Severity score of at least 3 Fitzpatrick skin types: I‐IV Excluded Washout periods for topical treatments 2 weeks, systemic antibiotics and phototherapy 4 weeks, isotretinoin 1 year Enrolled: 55 (M/F not stated) Randomised: 44 (22 in group 1, 22 in group 2) Withdrawals/drop‐outs: In group 1: 6 participants discontinued treatment, 2 withdrew after an adverse event (one because of a pustular eruption on the face following MAL‐PDT; second participant due to pain during light exposure), 1 because of compliance issues, 1 no longer available for evaluation; In group 2: 5 participants discontinued treatment, 2 no longer available for evaluation Final number and proportion of participants evaluable: In group 1: 16 (72%), in group 2: 17 (77%). ITT analysis: Yes |
|
| Interventions |
Intervention 1 MAL 80 mg/mL and 25 J/cm² red light with no occlusion Number and frequency of treatments: 4 treatments at 2‐week intervals Wavelength/Fluence/Duration/Spot size: 630 nm/ 25 J/cm²/ 90 min (MAL)/ Further details not reported Supplier: Visonac™ Photocure ASA, Oslo, Norway; Aktilite CL 128 Instructions to participants: Not applicable Intervention 2 MAL 80 mg/mL and 25 J/cm² red light with occlusion Number and frequency of treatments: 4 treatments at 2‐week intervals Wavelength/Fluence/Duration/Spot size: 630 nm/ 25 J/cm²/ 90 min (MAL)/further details not reported Supplier: Visonac™ Photocure ASA, Oslo, Norway; Aktilite CL 128 Instructions to participants: Not applicable Intervention 3 MAL 80 mg/mL and 37 J/cm² red light with no occlusion Number and frequency of treatments: 4 treatments at 2‐week intervals Wavelength/Fluence/Duration/Spot size: 630 nm/ 37 J/cm²/ 90 min (MAL)/further details not reported Supplier: Visonac™ Photocure ASA, Oslo, Norway; Aktilite CL 128 Instructions to participants: Not applicable Intervention 4 MAL 80 mg/mL and 37 J/cm² red light with occlusion Number and frequency of treatments: 4 treatments at 2‐week intervals Wavelength/Fluence/Duration/Spot size: 630 nm/ 37 J/cm²/ 90 min (MAL)/further details not reported Supplier: Visonac™ Photocure ASA, Oslo, Norway; Aktilite CL 128 Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4 and 12 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Change from baseline in ILs count 2. Change from baseline in NILs count Methods of assessing primary outcomes 1. Counting of ILs (papules, pustules and nodules) acne lesions on each side of the face. 2. Counting of NILs (open and closed comedones) acne lesions on each side of the face. Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. Using Global Acne Severity Scale (5‐point global assessment). Success (0 or 1) or failure (2, 3, 4). 2. Monitored during study |
|
| Notes | Language: English. The study authors were contacted and provided additional information on participants' mean age and age range, detailed data on Global Acne Severity Scores and assessor blinding methods. Sponsor provided additional data on application site blister rates ("1 report from 44 Visonac treated participants") | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1347): "The randomisation list was created with a computer software (SAS, version 9.1.3)." Comment: We judged this as adequate and at a low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 1347): "Only the acne assessor was blinded to treatment assignment". Comment: No blinding of participants and/or personnel was carried out. We judged this as at high risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 1347): "Only the acne assessor was blinded to treatment assignment". Study authors provided additional information that the acne assessor performed lesion counts and assessed acne severity only and did not have any other interaction with the subjects. Comment: We judged this as at low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote (page 1347): ‘The intent‐to treat (ITT) and safety populations consist of all patients enrolled in the study who received at least one MAL application. All efficacy analyses presented were conducted on the ITT population. The last observation was carried forward (LOCF) for missing data." Comment: Outcome measures were obtained for 72% of subjects randomised in group 1, and 77% randomised in group 2. Although ITT analysis was performed (the study authors state using both ITT and LOCF within their analyses), we judged the risk of bias as high. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were reported except form Global Acne Severity score, but study authors provided data on request. |
| Other bias | Unclear risk | Commercial sponsorship and possible conflicts of interest declared, which might have introduced some bias. No other possible sources of bias were identified. Insufficient information to permit a clear judgement |
Borhan 2014.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Unclear, not declared Setting: Single centre, Giza (Egypt) Recruitment: Unclear Duration: Start and end dates were not reported |
|
| Participants |
Included Age (inclusion criterion; mean; range): 18‐25; 21.3 ± 2.0 intervention group; 21.05 ± 2.18 control group; 18‐25 in both groups Clinically evident acne: Yes Severity of condition assessment: "with acne vulgaris in one or more of the following areas: face, back and upper arms"; "with mild to moderate acne vulgaris according to scale stated by Burton et al." Fitzpatrick skin types: III‐IV Other: "non smoker, not alcohol drinker and had no systemic diseases" Excluded "Patients who had skin malignancy, history of diabetes, circulatory or sensory disorders, mental or psychological disorders and any systemic diseases specially that might interfere with objectives of the study as pulmonary, cardiac or vascular diseases. Patients who received radiotherapy, chemotherapy or photosensitive drugs. Patients who had photosensitivity or have a history of frequent sunburns and patients with any dermatological condition rather than acne vulgaris." Enrolled: 40, 20 (8 M /12 F) in the PDT plus 'topical antibiotics' group, 20 (9 M/11 F ) in the 'topical antibiotics‐alone' group Randomised: 40: 20 in the PDT plus 'topical antibiotics' group; 20 in the 'topical antibiotics‐alone' group Withdrawals/drop‐outs: None Final number and proportion of participants evaluable: 20/20 (100%) in each of the groups ITT analysis: No |
|
| Interventions |
Intervention 1 PDL combined with "traditional topical antibiotic medication" (unclear what specifically) Number and frequency of treatments: 2 in total, every 4 weeks (for PDL), unclear for "traditional topical antibiotic medication" Wavelength/Fluence/Duration/Spot size: 595 nm/4 J/cm²/pulse duration 350 ms, treatment duration 2‐3 min/5 or 7 mm² Supplier: Unclear Instructions to participants: Unclear whether appropriate for topical antibiotics Intervention 2 "Traditional topical antibiotic medication" alone (unclear what specifically) Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Not applicable Supplier: Unclear Instructions to participants: Unclear |
|
| Outcomes | Evaluation time points of review interest: Unclear (reported as "at 4th, 8th and 12th week"; at final laser treatment and 4 and 8 weeks after final laser treatment?) Primary outcomes of review interest recorded 1. Percentage change from baseline of combined number of lesions Methods of assessing primary outcomes 1. "Photographic picture were taken to every patient at the base line, and at 12th week after the first treatment." Secondary outcomes of review interest recorded 1. Investigator's global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. "IGA was taken to every patient at the baseline, 4, 8 and 12th week after the first treatment, the comparison was done each time to the initial acnes count" 2. "The patients were also instructed to report any side effects during the treatment sessions." |
|
| Notes | Language: English. Unclear whether previous treatment was stopped, and concomitant treatment was allowed. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 67): "Forty patients with acne vulgaris were randomly divided into two equal groups (PDL group and control group)." (from abstract) Comment: Method used for randomisation was not described, so we judged this as at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. Given the nature of the interventions involved then blinding is unlikely. We judged the risk of bias as unclear. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 68): "Photographic picture were taken to every patient at the baseline, and at 12th week after the first treatment." Comment: Outcome assessors were blinded by photographs for lesion counts, unclear whether they were blinded for IGA assessment. We judged this as adequate and risk of bias as low for lesion counts and unclear for IGA. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures obtained for 20/20 (100%) of participants randomised to each group, so we judged the risk of bias as low. |
| Selective reporting (reporting bias) | High risk | IGA not reported for all time points. Adverse effects not reported at any time point. We judged this as at high risk of bias. |
| Other bias | High risk | Sponsorship and conflict of interest not reported. Unclear whether previous treatment was stopped and whether concomitant treatment was allowed. In both intervention and control group "traditional topical antibiotic medication" was included, but unclear what specifically. Baseline imbalances suspected. We judged the risk of bias as high. |
Bowes 2003.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Single centre (Boston, Massachusetts, USA) Recruitment: Not stated Duration: Start and end dates were not reported |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "with mild to moderate acne vulgaris" Fitzpatrick skin types: Not reported Other: Otherwise healthy volunteers Excluded Not stated Enrolled: Not reported Randomised: 11 (M/F not reported) Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: Not reported ITT analysis: Not stated |
|
| Interventions |
Intervention 1 KTP laser Number and frequency of treatments: 4 in total, for 2 consecutive weeks Wavelength/Fluence/Duration/Spot size: 532 nm/7‐9 J/cm² per pulse, cumulative 20‐50 J/cm²/pulse duration 20 ms/ 4 mm² Supplier: Aura, Laserscope, Palo Alto, CA Instructions to participants: Not applicable Intervention 2 Contact cooling only Number and frequency of treatments: 4 in total, for 2 consecutive weeks Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment (also assessed at 1 week after final treatment) Primary outcomes of review interest recorded None Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity Methods of assessing secondary outcomes 1. Michaelson acne count score |
|
| Notes | Language: English This was a conference proceeding report. The study authors were contacted in 2008, but were unable to provide additional information. We have attempted to contact the study authors again but were not successful in obtaining additional information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Suggestion that blinding of participants may have been attempted as one side of the face was treated with laser and the other side with contact cooling, and we judged it as adequate. Given that laser was used on one side of the face and contact cooling was used on the other side of the face then personnel were probably not blinded, but we judged it was unlikely that there were systematic differences between face sides in the care that was provided, or in exposure to factors other than the interventions of interest. We therefore judged the risk of bias as low. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | No intended blinding of outcome assessors reported. No evidence that assessors were blinded provided. Insufficient information was given to permit a clear judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition and exclusions from the analysis were not reported. Insufficient information given to permit a clear judgement. |
| Selective reporting (reporting bias) | Low risk | The protocol for this study was not available. Outcome measures mentioned were reported, so we judged this as a low risk of bias. |
| Other bias | Unclear risk | Sponsorship or potential conflicts of interest were not declared. Insufficient information was given to permit a clear judgement. |
Chang 2007.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (page 676): "The authors have indicated no significant interest with commercial supporters". Setting: Single centre, (Seoul, Korea) Recruitment: Dermatology outpatient clinic Duration: Start and end dates were not reported |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; 25.7 years; 23‐32 years Clinically evident acne: Yes Severity of condition assessment: Mild‐moderate, Grade 2 of the Korean Grading system Fitzpatrick skin types: III‐IV Excluded Previous oral anti acne medication in less than 1 month before this IPL trial. Enrolled: 30 (0 M/30 F) Randomised: 30 Withdrawals/drop‐outs: None Final number and proportion of participants evaluable: 30/30 (100%) ITT analysis: Not stated |
|
| Interventions |
Intervention 1 BPO gel with PR filter (acne filter) of IPL Number and frequency of treatments: 3 sessions 3 weeks apart Wavelength/Fluence/Duration/Spot size: 530‐750 nm/8 (FPT III), 7.15 (FPT IV) – skin type dependent J/cm²/pulse duration 2.5 ms/further details not reported Supplier: I2PL, Ellipse Flex, DDD, Horsholm, Denmark Instructions to participants: Adequate. "Patients were instructed to use topical benzoyl peroxide (BP) gel on the lesions of both sides of face once a day." (page 677) Intervention 2 Benzoyl peroxide (BPO) gel Number and frequency of treatments: Applied once per day, for 9 weeks Instructions to participants: Adequate. See above |
|
| Outcomes | Evaluation time points of review interest: 3 weeks after final treatment Primary outcomes of review interest recorded 1. Participant's global assessment of improvement 2. Change from baseline in ILs count (papules and pustules recorded separately) Methods of assessing primary outcomes 1. Questionnaire ranking the degree of satisfaction as highly satisfied, satisfied, neutral, or dissatisfied at baseline and at each visit 2. Papule and pustule counts Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. Korean Acne grading system 2. Not reported |
|
| Notes | Language: English. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 677): "While using the BP gel, randomly selected side of the face was treated with the IPL". Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No evidence that blinding of participants/ performing clinicians was carried out. Given that one side of the face was treated with IPL then it is unlikely that participants/ personnel were blinded. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | No evidence that participants were blinded was given, so we judged the risk of bias as unclear for participant‐assessed outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Quote (page 677 ): "For lesion counts at baseline and 3 weeks after the third session, two blinded raters (dermatologic residents) did lesion counts and means were recorded." Comment: Evidence that assessors were blinded, but method used was not described, so we judged this at an unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 677): "All patients experienced a reduction in inflammatory lesion counts on both sides of the face." Comment: The above implies that outcome measures were obtained for 100% of randomised participants. No withdrawals were reported. We judged this as at a low risk of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Quotes (page 677): "Evaluation of patient's subjective response to treatment was performed by a questionnaire ranking the degree of satisfaction as highly satisfied, satisfied, neutral, or dissatisfied at baseline and at each visit."; "Although patients were uniformly satisfied with their treatment, IPL treatment did not give any additional benefit to reduction of papules and pustules." Comment: Baseline data was not given. Precise results not reported for the outcomes predefined in the methods section, including lesion counts, Korean severity scores and participants' subjective response to treatment. We judged this as at unclear risk of bias. |
| Other bias | Low risk | No other sources of potential bias identified. |
Chen 2015.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes, "approval from the Ethics Committee of Fuzhou General Hospital (Fuzhou, China)" Sponsorship and conflict of interest: Not declared Setting: Probably Fuzhou General Hospital (Fuzhou, China), however unclear Recruitment: Unclear Duration: 19 months (June 2011‐December 2012) |
|
| Participants |
Included Age: for those completing treatment in intervention group range 18‐33 years, mean age 23.57 years; those completing in control group 19‐32 years, mean age 24.12 years Clinically evident acne: Yes Severity of condition assessment: "The acne vulgaris of the patients was graded in terms of property and severity as follows: Low grade, which presented with only acne; moderate grade, which was acne that presented with inflammatory papules and pustules; and severe grade, which is acne that presented with inflammatory papules, nodules, cysts and scars." Fitzpatrick skin types: Not reported Other: Both sexes Excluded "i) use of any topical antibiotics within 2 weeks of the study or intake of systemic oral antibiotics within 4 weeks of the study; ii) use of systemic retinoids within 6 months of the study; iii) porphyria or facial atopic dermatitis; iv) pregnancy or lactation; v) history of keloid or photosensitivity disorders; vi) photosensitive eczema or autoimmune diseases; and vii) use of anti‑acne medication such as prophylactics, glucocorticoid and photosensitizers." Enrolled: 50 Randomised: 50, 25 in treatment group, 25 in control group Withdrawals/drop‐outs: 1 in treatment group (undisclosed reason), 2 in control group ("because of side‑effects and/or poor effect") Final number and proportion of participants evaluable: 47/50 (94%) in total completed, 24/25 (96%) in the intervention, 23/25 (92%) in the control group ITT analysis: Not reported |
|
| Interventions |
Intervention 1 "Prior to ALA application, the skin was cleansed with 70% isopropyl alcohol. Then, 20% topical ALA was applied for 90 min under plastic film occlusion and exposed three times for 20 min to red light… once a week." Number and frequency of treatments: Once a week, for 3 weeks Wavelength/Fluence/Duration/Spot size: 633 ± 10 nm/10 mW/cm2; 120 J/cm² Supplier: 5% ALA solution; Shanghai Fudan‑Zhangjiang Bio‑Pharmaceutical Co. Ltd. (Shanghai, China); "LED‐IB photodynamic therapy instrument, Wuhan Yage Optic and Electronic Technique Co. Ltd, Wuhan, China" Instructions to participants: Not applicable. Intervention 2 Three 20 min doses of infrared radiation without 5‑ALA Number and frequency of treatments: Once a week for 3 weeks Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Unclear Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 2, 4 and 6 weeks after final treatment? Also assessed at each treatment session. Primary outcomes of review interest: not recorded Secondary outcomes of review interest: 1. Investigator's global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. "The acne of each patient was evaluated using an inflammatory acne score modified from previously described criteria (8). The classifications used in this study accounted for both the number and the size of the lesions. The number of comedones, inflammatory comedones, papules, pustules, nodules and cysts in each test area were recorded. The effects were evaluated in terms of the reduction rate of the acne lesions. Reduction rate was calculated as follows: Reduction rate (%) = (numbers of comedones before treatment ‑ numbers of comedones after treatment)/number of comedones before treatment x 100. Skin lesions with ≥ 90% improvement were classified as cured, skin lesions with 60‑89% improvement were classified as excellent effect, skin lesions with 30‑59% improvement were classified as fair effect and skin lesions with < 30% improvement or exacerbations were classified as no effect. The total effective rate (TER) was computed as follows: TER (%) = (number of cured cases + excellent effect cases)/total number of cases x 100.…Clinical photographs were taken prior to and following treatment and at every follow‑up every 2 weeks for 6 weeks." 2. "Side‐effects, including itching, pain, erythema, hyperpigmentation and exfoliation, were recorded during the course of treatment." |
|
| Notes | Language: English. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1195) : "A total of 50 patients were randomly divided equally into a control group and a therapy group." Comment: Methods used for randomisation not given. We judged this as at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Quote (page 1195): "Clinical photographs were taken prior to and following treatment and at every follow‑up every 2 weeks for 6 weeks." Comment: Photographs were used for evaluation, but it was not specifically reported whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 24/25 (96%) participants in the treatment, and 23/25 (92%) participants in the control group were included in the analysis so we judged the risk of bias as low. |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified in the methods section reported. |
| Other bias | Unclear risk | Sponsorship or potential conflicts of interest were not declared in the paper. |
Cheng 2008.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Single centre (Guangdong, China) Recruitment: Not reported Duration: 36 months, May 2004‐May 2007 |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 22.6 years; 14‐36 years Clinically evident acne: Yes Severity of condition assessment: Mild to severe, Pillsbury classification I‐III Fitzpatrick skin types: Not reported Other: Not allowed to take any oral or topical antibiotics 1 week prior to the light treatment. Given informed consent Excluded Light‐sensitive skin Enrolled: 36 (29 M/7 F) in group 1, 28 (19 M/9 F) in group 2 Randomised: 36 in group 1, 28 in group 2 Withdrawals/drop‐outs: None Final number and proportion of participants evaluable: 36 (100%) in group 1, 28 (100%) in group 2 ITT analysis: Not stated |
|
| Interventions |
Intervention 1 Blue light only. Participants had their eyes covered during treatment. Distance from the light source to face was 25 cm. Number and frequency of treatments: 2 treatments a week. For Pillsbury I participants 1 cycle; Pillsbury II‐III 1‐3 cycles (a cycle consisting of 4 weeks) Wavelength/Fluence/Duration/Spot size: 400‐410 nm/not given/12 minutes for Pillsbury I and 15‐20 minutes for Pillsbury II‐III participants/not given Supplier: Medilite Blue from Inner act Ltd Instructions to participants: Not applicable Intervention 2 Blue and red light. Participants had their eyes covered during treatment. Distance from the light source to face was 25 cm. Number and frequency of treatments: 2 treatments a week. For Pillsbury I participants 1 cycle; Pillsbury II‐III, 1‐3 cycles (a cycle consisting of 4 weeks) Wavelength/Fluence/Duration/Spot size: 400‐410 nm and 660 nm/not given/12 minutes for Pillsbury I and 15‐20 minutes for Pillsbury II‐III participants/not given Supplier: Medilite Blue from Inner act Ltd Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment (also assessed at 1 week after final treatment) Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator's global assessment of improvement Methods of assessing secondary outcomes 1. Non standard scale based on percentage change in lesion counts. 90% ≥ improvement = "full recovery"; 70 to 89% = "good improvement"; 30 to 69% = "effective improvement"; ≤ 30% = "no effect" |
|
| Notes | Language: Mandarin. English translation was not available. Data extraction was done by one native speaker (QY) from the original paper. We have not attempted to contact the study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1): "The patients were randomised into two groups:..." Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | No intended blinding of outcome assessors reported. No evidence that assessors were blinded provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised participants in each group. We judged this as at a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the methods section were reported. |
| Other bias | Unclear risk | Sponsorship and/or potential conflicts of interest were not declared. Insufficient information to permit clear judgement. The study was in Mandarin and potential bias has been introduced by the fact that we have only been able to do single rather than double data extraction. |
Choi 2010.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared. Study authors declared no potential conflict of interest (page 773). Setting: Single centre (Seoul, Korea) Recruitment: Not stated Duration: 9 months, May 2007‐January 2008 |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 15 years; 26 years; 20‐37 years Clinically evident acne: Yes Severity of condition assessment: Acne severity grade of 2–4, as defined by Cunliffe's grading system Fitzpatrick skin types: III‐V Other: General good health, the ability to comply with the study Excluded A history of keloid, a photosensitive disorder, oral retinoid use within 6 months of study commencement, microdermoabrasion on the face within 3 months of study commencement, the use of oral topical antibiotics, topical retinoid or alpha‐hydroxyl acid within 1 month of study commencement, or dermabrasion or laser resurfacing of facial skin. Enrolled: 20 (1 M/19 F) Randomised: 20 Withdrawals/drop‐outs: 3 (1 due to pregnancy, 2 to schedule conflict) Final number and proportion of participants evaluable: 17/20 (85%) ITT analysis: Not reported |
|
| Interventions |
Intervention 1 IPL, triple light pulse with 9 ms interval , 2 passes, cooling gel applied before IPL Number and frequency of treatments: 4 treatments, 2‐week intervals Wavelength/Fluence/Duration/Spot size: 530‐750 nm/ 7.5‐8.3 J/cm²/pulse duration 2.5 ms/other details not given Supplier: Ellipse Flex System; DDD, Horsholm, Denmark Instructions to participants: Not applicable Intervention 2 PDL Number and frequency of treatments: 4 treatments, 2‐week intervals Wavelength/Fluence/Duration/Spot size: 585 nm/ 8‐10 J/cm²/2 passes 40 ms/10mm² Supplier: Cynergy; Cynosure, Inc. Chelmsford, MA, USA Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4 and 8 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Participant's global assessment of improvement 2. Change from baseline in ILs and NILs count Methods of assessing primary outcomes 1. Questionnaires rating degree of satisfaction from 0 (neutral) to 10 (highly satisfied) 2. Numbers of ILs and NILs were counted before each treatment and at 4 and 8 weeks after final sessions. Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. Cunliffe's grading system, standardised digital photographs taken during each treatment visit 2. Monitored during study |
|
| Notes | Language: English. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 774): "A randomised code was used to determine treatment sides." Comment: We judged this as adequate and at a low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No evidence that participants and personnel were blinded. Given that one side of the face was treated with IPL and the other with PDL then it is unlikely that participants/performing clinicians were blinded. We judged this as at an unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | No evidence that participants were blinded was given, so we judged the risk of bias as unclear for participant‐assessed outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Although the study was described as "single blind" on page 774, no measures used for blinding of outcome assessors was described. Insufficient information to permit a clear judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 774): "Twenty patients, one man and 19 women….were enrolled. ….Of these, 17 completed the study, three female patients withdrew. The reasons for withdrawal were schedule conflict for two patients and pregnancy for one patient." Comment: Outcome measures obtained for 17/20 (85%) of subjects randomised, with reasons for withdrawal reported. We judged this as at a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcome measures prespecified in the methods section were reported, so we judged this as a low risk of bias. |
| Other bias | Low risk | No other potential sources of bias identified. |
Darne 2011.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (page 1256): "The 1450 nm Smoothbeam diode laser was provided by Candela (Cwmbran, UK) for the purpose of research." The study authors declared no conflict of interest. Setting: Single centre (Middlesbrough, UK) Recruitment: By a single dermatologist in secondary care at the outpatient dermatology department at a university hospital Duration: 31 months, August 2006‐February 2009 |
|
| Participants |
Included: Age (inclusion criterion; mean; range): > 16 years; 28 years; 18‐47 years Clinically evident acne: Yes Severity of condition assessment: "moderate to severe acne", "mild but treatment resistant acne", lesion counts and Leeds Revised Acne Grading Scale Fitzpatrick skin types: I‐V Excluded History of severe depression, pregnant or breastfeeding, dermal fillers or ablative laser treatment in the previous 3 months, systemic isotretinoin in the previous 12 months. Other: "Participants continued to use their prescribed acne treatment, which would have had an equivalent effect on both the treated and control sides of the face." Enrolled: 38 (7M/31F) Randomised: 38 Withdrawals/drop‐outs: 4 participants did not attend for their laser treatments. The reason for their withdrawal was not ascertained in the 2 who dropped out after the first treatment. The 2 who did not attend after their second treatment had "changed their minds" and it was not due to an adverse effect of the laser. 2 participants unable to attend the appointment 1 month after final treatment (reasons not documented). 9 participants did not attend assessment appointments (one pregnant, reasons for the rest not documented). Final number and proportion of participants evaluable: 32 (84%) at 1 month after final treatment; 23 (60%) at 12 months after final treatment ITT analysis: No |
|
| Interventions |
Intervention 1 Candela smooth beam laser; "double‐pass technique (treatment was performed twice on the appropriate side)"; participants could choose to use topical local anaesthetic (EMLA cream; AstraZeneca, London, UK) applied 1 h prior to treatment Number and frequency of treatments: 3 treatments, applied monthly Wavelength/Fluence/Duration/Spot size: 1450 nm/8‐9 J/cm²/210 ms/6 mm² Supplier: Candela, Cwmbran, UK Instructions to participants: Not applicable Intervention 2 Nil |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment , then at 3‐monthly intervals for 12 months after the last treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Participant's global assessment of improvement 2. Change from baseline in ILs count (papules and pustules not reported separately) Methods of assessing primary outcomes 1. Non‐standardised questionnaire relating to the lasered side of the face ("highly satisfied", "satisfied", "neutral" or "unsatisfied" and "would recommend to a friend" ) 2. Spot counts using a transparent sheet with the assessor tracing and counting the ILs on each side of the face. The nose was excluded as sebaceous hyperplasia can be difficult to distinguish from acne lesions. Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. Leeds Revised Acne Grading Scale on photographs taken by the medical photography department using standardised conditions 2. "All participants were given written information about the possible adverse effects of the laser. Monitoring of these reactions took place at each assessment, prior to treatment which was monthly for the first 12 weeks of the study. Participants were assessed by the blinded observer in the dermatology department and asked specifically about adverse effects at this point, which were documented. Participants then proceeded to a separate location to receive their laser treatment. In addition, participants were given twenty‐four hour open access via telephone for concerns about serious adverse effects." |
|
| Notes | Language: English. Concomittant treatment allowed. "Eighteen participants had previously received a course of oral isotretinoin, nine of whom had had two previous courses of oral isotretinoin." Study authors were contacted and provided additional data on reasons for withdrawal/drop‐out, duration of the study, methods of monitoring adverse effects, methods for blinding of performing and assessing investigators, timing of patient satisfaction assessment and ITT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1258): "The side of the face to be treated was randomised as ‘left' or ‘right' using a computer‐generated sequence." Comment: We judged this as adequate. |
| Allocation concealment (selection bias) | Low risk | Quote (page 1258): "This was put into a sealed envelope by an individual not involved in the trial. The envelope was opened by the participant once they had left the department." Comment: We judged this as adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors clarified that participants and performing clinicians were not blinded, so we judged the risk of bias as high. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | High risk | Study authors clarified that participants were not blinded, so we judged the risk of bias as high for participant‐assessed outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quotes (page 1257): "Participants were assessed by a single blinded investigator (S.D.) just prior to their treatment so that the assessor was not biased by post‐laser erythema on the treated side."; "The photographs were also graded (the right and left sides of the face separately) by D.C.S. who was blinded as to the treatment allocation." Quote (page 1258) "Assessments were made by a single investigator (S.D.) who was blinded as to the side of the face being treated. Participants were specifically directed not to disclose which side of the face was being treated to the assessor. Participants had the allocated side of the face treated at a separate location by a third investigator (E.L.H.) who was not involved in assessments." Comment: We judged this as at a low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quotes (page 1259): "At visit 4 (primary endpoint) 32 participants remained in the study.", "At visit 8 (12 months after the last treatment) 23 participants remained in the study". Comment: 32/38 (84.2%) randomised participants included in the analysis at visit 4, however only 23/38 (60.52%) at visit 8. We judged this as at a low risk of bias at 1 month after final treatment, but high risk of bias at 12 months after final treatment. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were reported. The time point for the evaluation of participants for the "Participant's global assessment of improvement" was not given in the report. Study authors clarified that the patient satisfaction data were collected 4 weeks after final laser treatment. |
| Other bias | Unclear risk | The study authors declared no conflict of interest, but commercial sponsorship might have introduced some bias. Insufficient information to permit a clear judgement. |
de Arruda 2009.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Not declared Setting: Single centre, (Campinas, SP, Brazil) Recruitment: Not stated Duration: 11 months, November 2006 to September 2007 |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 17.3 years ;not reported Clinically evident acne: Yes Severity of condition assessment: "Acne lesions grades II or III, according to the classification of the Brazilian Group of Acne" Fitzpatrick skin types: Not reported Other: "They were all healthy and had no other comorbidities or used any medication that could have interfered in the progression or patient compliance to treatment."; "Eleven were mixed Brazilians, 47 were Caucasian, and two had no reference to race in the chart." Excluded Enrolled: 16 M/14 F in group 1; 18 M/12 F in group 2; 34 M/26 F in total Randomised: 60 in total, 30 in each group Withdrawals/drop‐outs: 6 in group 1, 3 in group 2; 9 in total. Reasons not stated. Unclear whether withdrawal or lost to follow‐up Final number and proportion of participants evaluable: 24 (80%) in group 1, 27 (90%) in group 2; 51 (85%) in total ITT analysis: Not stated |
|
| Interventions |
Intervention 1 Facial hygiene soap and sun protection lotion SPF15 daily and blue light therapy Number and frequency of treatments: 8 treatments in total, twice weekly with minimum intervals of 48 h Wavelength/Fluence/Duration/Spot size: 407‐420 nm/(40 mW/cm²)/15 minutes/not given Supplier: Soret Blue Light (EVTECH and Komlux Fibras Opticas) Instructions to participants: Not applicable Intervention 2 Facial hygiene soap and sun protection lotion SPF15 daily and 5% benzoyl peroxide Number and frequency of treatments: Twice daily. Length of treatment not clearly stated, presumed to be the same as Intervention 1 i.e. 4 weeks. Supplier: Manufactured by the reference laboratory of the Service of Dermatology. Instructions to participants: Unclear whether participants were given adequate instructions |
|
| Outcomes | Evaluation time points of review interest: None (assessed at 2 and 4 weeks whilst on treatment, final evaluation at final treatment) Primary outcomes of review interest recorded 1. Change from baseline in ILs & NILs count (papules, pustules and comedones not recorded separately) Methods of assessing primary outcomes 1. Counting the total number of ILs (papules and nodules) and NILs (comedones) on the face and documented by photos. Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Participants were asked about the occurrence of erythema, dryness, desquamation and burning during treatment in all visits. |
|
| Notes | Language: Portuguese. Data was extracted from the English translation, available from the journal's web‐site, by two review authors. Table 1 in the translation did not correspond to the one in the original Portuguese version and was translated separately. In some participants benzoyl peroxide treatment was reduced to once a day due to adverse effects. Results at 4 weeks whilst on treatment only. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 464): "They were randomly divided into two groups, ..." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open trial (as stated in the title), so we judged the risk of bias as high. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | High risk | This was an open trial (as stated in the title), so we judged the risk of bias as high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures obtained for 85% of randomised subjects; 80% of participants randomised in the blue‐light group and 90% of participants in the benzyl‐peroxide group. We judged this as at low risk of bias. |
| Selective reporting (reporting bias) | Low risk | Quote (page 465): "The last visit (V4) was carried out just for follow‐up purpose after the end of treatment, and we did not use it to analyse efficacy by counting the number of lesions. Visits V1 and V2 served as a control to check patients' compliance and adverse events." Comment: Outcomes not reported at 2 weeks post treatment (visit V4). Reason for lack of outcomes at 2 weeks was justified in text, so we judged this as at low risk of bias. |
| Other bias | Unclear risk | Sponsorship or potential conflicts of interest were not declared. Insufficient information was given to permit a clear judgement. |
Elman 2003.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared. One of the study authors was employed by the company supplying the laser. Setting: Not reported (Israel?) Recruitment: Not stated Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 18 years; 18.8 years; not stated Clinically evident acne: Yes Severity of condition assessment: "with mild to severe papulo‐pustular acne" Fitzpatrick skin types: Not reported Other: participants had a wash‐out period of at least 4 weeks from topical or oral anti‐acne medications Excluded More than two deep cysts or less than 10 ILs Enrolled: 23 (11 M/12 F) Randomised: 23 Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: It was not stated whether outcomes were obtained for all randomised participants. ITT analysis: Unclear |
|
| Interventions |
Intervention 1 Half of face treated with ClearLight Therapy System Number and frequency of treatments: 8 treatments, twice a week for 4 weeks Wavelength/Fluence/Duration/Spot size: 405‐420 nm/15 min/other data not given Supplier: ClearLight Therapy System (CureLight Ltd, distributed by Lumenis) Instructions to participants: Not applicable Intervention 2 Half of face covered with black cloth during treatment of the other side of the face |
|
| Outcomes | Evaluation time points of review interest: 2, 4 and 8 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Percentage change in ILs (papules and pustules not reported separately) Methods of assessing primary outcomes 1. ILs on the treated and untreated sides were counted and photographed at each treatment and at follow‐ups 2, 4 and 8 weeks after the end of therapy Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Not reported |
|
| Notes | Language: English. Table with baseline data reported, which didn't include ILs counts of irradiated and non‐irradiated sides of the face. Data on "median percent reduction of inflammatory acne lesions" expressed in graph format. McNemar analysis was used we judged as appropriate. The study authors were contacted and additional data requested, but they were unable to provide them. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 112): "One side of the face was randomly chosen to be the treated side, and the other side was covered by black cloth." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 112): "One side of the face was randomly chosen to be the treated side, and the other side was covered by black cloth." Comment: Given that one side of the face was treated with light and the other was covered with a black cloth it is unlikely that participants and personnel were blinded. We therefore judged the risk of bias as high. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Quote (page 112): "Results were evaluated by a trained physician blinded to the treatment side." Comment: Method of blinding not described, so we judged this as at and unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of withdrawals and lost to follow‐up not reported, final number of evaluable participants not reported. Insufficient information was given to permit a clear judgement. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were reported. |
| Other bias | Unclear risk | Sponsorship or potential conflicts of interest were not declared. No other sources of bias were identified. Insufficient information was given to permit a clear judgement. |
Fadel 2009.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Declared. There were no commercial sponsors or potential conflicts of interest. Setting: Single centre (Cairo, Egypt) Recruitment: Dermatology Unit, University of Cairo Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 18 years; not stated, not stated Clinically evident acne: Yes Severity of condition assessment: "mild to moderate acne" Fitzpatrick skin types: Not reported Excluded Oral retinoids within 1 year, systemic antibiotics within 1 month, topical acne treatment within 2 weeks, pregnancy, lactation Enrolled: Not stated (M/F not stated) Randomised: 20 Withdrawals/drop‐outs: 5 (before treatment; did not meet inclusion criteria) and 2 (after 1st treatment; personal reasons) Final number and proportion of participants evaluable: 13 (65%) ITT analysis: Not stated |
|
| Interventions |
Intervention 1 Topical liposomal methylene blue applied to half face and covered for 15 min then treated with laser Number and frequency of treatments: 2 treatments in total, weekly Wavelength/Fluence/Duration/Spot size: 650 nm/other data not given Supplier: Mesh‐Tel‐Division of Intelite Inc, Santa Monica, CA Instructions to participants: Not applicable. "After treatment patients had to avoid sun exposure...or use sunscreen of > 50 SPF and only an emollient soap could be used" Intervention 2 Nil |
|
| Outcomes | Evaluation time points of review interest: every two weeks for three months after treatment (also assessed every two weeks whilst on treatment) Primary outcomes of review interest recorded 1. Percentage change from baseline of ILs (papules and pustules not reported separately) 2. Percentage change from baseline of NILs (open and closed comedones not reported separately) Methods of assessing primary outcomes 1. & 2. Lesion count Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Investigator's global assessment of improvement 3. Adverse effects Methods of assessing secondary outcomes 1. Leeds revised acne grading system 2. Responses graded: 0 = acne worse, 1 = no change, 2 = slight improvement, 3 = moderate improvement, 4 = marked improvement (page 985‐986) 3. Post treatment pain, erythema, edema and hyperpigmentation if present were graded on a 5‐point scale (1 = none, 2 = slight, 3 = moderate, 4 = severe and 5 = intolerable) |
|
| Notes | Language: English. No baseline data given for Leeds severity score. Data on baseline lesion count and lesion count results presented in graph format. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 987): "Twenty patients were randomised to participate in the study..." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. Given that one side of the face was treated with liposomal methylene blue applied and then laser it is unlikely that participants/personnel were blinded. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Quote (page 986): "The evaluating dermatologist was blinded to the treatment/ control side." Comment: The method used for blinding was not described and we judged this as at unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome measures obtained for 65% of randomised subjects. We judged this as at high risk of bias. |
| Selective reporting (reporting bias) | High risk | Quote (page 985): "At each treatment and follow‐up visit the patients and the evaluating dermatologists also had to decide whether or not the patients condition had improved. Responses were graded...". Comment: Participant's global assessment of improvement results were not reported, although they were assessed. Other outcomes were reported at 4 and 12 weeks only, although assessment was made every 2 weeks. We judged this as at high risk of reporting bias. |
| Other bias | Low risk | No other sources of bias were identified. |
Genina 2004.
| Methods | This was a parallel‐group RCT (single or multiple treatment groups). Within each group split‐face or split‐back design (different interventions) Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Declared. Quote (page 833): "The authors are grateful to Palomar Medical Products, Inc. for funding this work and providing the diode IR laser and Nikon Coolpix 990 digital camera" Setting: Single centre (Saratov, Russia) Recruitment: "volunteers", more information not reported Duration: 5 months, February 2001‐ June 2001 |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not given; not given; 17‐27 years Clinically evident acne: Yes Severity of condition assessment: "with acne vulgaris ranging from light to severe forms…" Fitzpatrick skin types: Not reported Excluded "persons who expected to have excessive sun exposure, or with a history of keloid or photosensitivity disorder, pregnant and lactating women, and mentally handicapped persons were also excluded." Enrolled: 8 (3 M/5 F) in group 1; 4 (2 M/2 F) in group 2 Randomised: 8 in group 1, 4 in group 2 Withdrawals/drop‐outs: Not specifically reported in the paper, but study authors provided further data that there were no withdrawals and lost‐to‐follow‐ups. Final number and proportion of participants evaluable: 100% in both groups (8/8 in the single treatment group and 4/4 in the multiple treatment group) ITT analysis: Unclear |
|
| Interventions |
Intervention 1 Topical application of indocyanine green 5 min before near infrared diode laser treatment Number and frequency of treatments: Single treatment Wavelength/Fluence/Duration/Spot size: 803 nm/15 and 30 J/cm²/5 min for the participants with a light form of acne and 10 min for the participants with moderate to severe/10 cm² Supplier: OPC–BO15–MMM–FCTS, Opto Power Corp., Tucson, Arizona Instructions to participants: Not applicable. Intervention 2 Topical application of indocyanine green 5 min before near infrared diode laser treatment Number and frequency of treatments: 8 treatments in total, applied twice a week, for four weeks Wavelength/Fluence/Duration/Spot size: 803 nm/15 and 30 J/cm²/5 min for the participants with a light form of acne and 10 min for the participants with moderate to severe/10cm² Supplier: OPC–BO15–MMM–FCTS, Opto Power Corp., Tucson, Arizona Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment (also assessed at 1 week after final treatment) Primary outcomes of review interest recorded 1. Change from baseline in number ILs & NILs count 2. Percentage change from baseline of ILs & NILs count Methods of assessing primary outcomes 1.& 2 Photographs, given to dermatologists who counted who counted the number of "elements" (comedones, papules, pustules and nodules). "The number of active elements was averaged". Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. After each treatment and at follow‐up investigators assessed erythema, edema, hypo‐ and hyper‐pigmentation. |
|
| Notes | Language: English. The study authors were contacted and provided additional data in 2008, but we were unsuccessful in contacting them afterwards. They clarified that they also recorded adverse effects and the methods they used for that. They also provided additional data regarding actual lesion counts pre‐ and post treatment, as well as withdrawals, lost‐to‐follow‐ups and random sequence generation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 829): "The subjects were randomly divided into single‐treatment and multiple‐treatment groups." Comment: Method used to generate the allocation sequence was not stated in the paper, but the study authors were contacted and clarified that they used computer software. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. Given that one group received multiple treatments and the other received a single treatment then it is unlikely that the personnel were blinded. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Quote (page 830): "To estimate the state of a volunteer's skin impartially, photographs of both treated and control sites were given to two dermatologists for the analysis..." Comment: Unclear whether outcome assessment was blinded. We judged this as at an unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Results reported for 100% of included participants. However, it was not clearly stated that there were no withdrawals or lost‐to‐follow‐up participants. It is not clear why there are different numbers between groups and so it is likely that some participants dropped out. We judged this as an unclear risk of bias. |
| Selective reporting (reporting bias) | Low risk | Outcomes were not clearly stated. Study authors provided complete data after they were contacted. We judged this as at low risk of bias. |
| Other bias | Unclear risk | Commercial sponsorship declared, which might have introduced some bias. No other possible sources of bias were identified. Insufficient information was given to permit a clear judgement. |
Gold 2005.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Not declared in the paper. The study authors later clarified that the study was funded by Dusa Pharmaceuticals. Setting: Multicenter (Nashville,TN and La Jolla, CA) USA Recruitment: Not reported Duration: Start and end dates were not reported (2006‐2007) |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not given; 31.0 years; 13‐55 years Clinically evident acne: Yes Severity of condition assessment: "mild to moderate inflammatory acne lesions"; "lesion counting by board‐certified dermatologists". Fitzpatrick skin types: Not reported Other: evaluated participants: 16 white, 7 African‐American, 1 American Indian, 1 of Chinese origin Excluded Previous light therapy of any kind. "patients had to be off systemic antibiotics for 4 weeks and systemic retinoids for 6 months prior to the study"; "during the 1 week washout phase, the patients refrained from using any medicated topical products to treat their facial acne vulgaris except for a standard facial cleanser" Enrolled: 34 (M/F not stated, 3 M/22 F continued to follow‐up) Randomised: 34, 17 in each group Withdrawals/drop‐outs: 5 withdrawals and 3 lost to follow‐up in group 1; 4 withdrawals and 4 lost to follow‐up in group 2. Reasons for withdrawal not reported. Final number and proportion of participants evaluable: 9 (51%) in group 1, 9 (51%) in group 2 ITT analysis: No |
|
| Interventions |
Intervention 1 Blue light Number and frequency of treatments: 8 treatments in total, twice per week during 4 weeks Wavelength/Fluence/Duration/Spot size: 16 min, 40 s (1000 sec)/other data not given Supplier: Blu‐U. Blue light Photodynamic Therapy Illuminator Model 4170. Dusa Pharmaceuticals Instructions to participants: Not applicable Intervention 2 Topical clindamycin 1% solution Number and frequency of treatments: Applied at home twice daily for 4 weeks Supplier: Cleocin T, UpJohn Pharmaceuticals, Wilmington, MA Instructions to participants: Unclear whether participants were given adequate instructions |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment (also assessed at 4 weeks whilst on treatment) Primary outcomes of review interest recorded 1. Change from baseline in ILs & NILs count (papules, pustules and comedones not reported separately) Methods of assessing primary outcomes 1. Lesion count Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Investigator's global assessment of improvement 3. Adverse effects Methods of assessing secondary outcomes 1. Using "global severity score analysis" 2. Using an "overall improvement score" 3. "noted and documented at all times during the time period of this trial" |
|
| Notes | Language: English. The study authors were contacted and provided additional information on power calculation, sponsorship/possible conflicts of interest, recruitment, acne severity assessment method, ITT analysis, study duration and methods used for random sequence generation, allocation concealment and blinding. They clarified that "averages" reported stand for "means" and that compliance assessment of participants on topical clindamycin was undertaken by "collection of bottles used". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 65 ): "During the 4‐week treatment phase, patients were randomised to receive either:..." Comment: Method used to generate the allocation sequence was not stated. The study authors were contacted but were unable to provide additional data on the method used. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported in the paper. The study authors clarified that the allocation was concealed but were unable to provide additional data on the method used. We therefore judged the risk of bias as unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study authors clarified that the participants and performing clinicians were not blinded. We judged this as at high risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Quote (page 65 ): "A blinded investigator evaluated the patient and performed acne vulgaris lesion counts, a global severity score analysis, and an overall improvement score." Comment: The method of blinding not described, so we judged this as at unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome measures were obtained for 53% of subjects randomised and we judged this as at a high risk of bias. |
| Selective reporting (reporting bias) | High risk | Quote (page 67): "Improvement scores and global improvement scores were similar between both groups of patients". Comment: Outcomes were not specifically reported for global severity score nor overall improvement score. We judged this as at a high risk of reporting bias. |
| Other bias | Unclear risk | Sponsorship or potential conflicts of interest were not declared in the paper. The study authors later clarified that the study was funded by Dusa Pharmaceuticals. Insufficient information to permit a clear judgement. |
Gold 2011.
| Methods | This was a split‐face RCT. Unit of randomisation: Lesion. Quote (page 309): "For each subject, 2 similar lesions (either papules or pustules of similar eruption status and age), one of each side of the face were identified by the physician and were randomly assigned to treatment of either the active or sham device." Power calculation: Unclear Ethical approval: Unclear. Informed consent was obtained. Quote (page 309): "… were included in the study after signing the informed consent form approved by the auspices of an institutional review board (IRB)." Sponsorship and conflict of interest: Sponsorship not declared. Conflict of interest declared: "Dr. Gold is a consultant to Pharos Life, a division of Syneron‐Candela, speaks on their behalf and performs research." Setting: Single centre, Nashville, Tennessee (USA) Recruitment: Tennessee Clinical Research Center Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; 22 ± 4 years; not stated Clinically evident acne: Yes Severity of condition assessment: Mild‐moderate acne, Burton scale Fitzpatrick skin types: I‐IV Other: willing and able to comply with treatment, willing and able to give consent (for subjects under 18 years of age the legal guardian willing to give consent); female participants of childbearing potential negative urine pregnancy test result at baseline and a reliable method of contraception throughout the study Excluded "...received treatment to their face with an investigational device or drug within 30 ... had excessive facial exposure to sunlight or artificial UV‐light within one month prior the study."; skin type V or VI; severe acne vulgaris requiring prescription medications; use of topical or systemic steroids or NSAIDs (e.g. pain or skin conditions); clinically infected lesions requiring systemic antibiotics and/or local antiseptics and/or other treatment; pregnant or nursing women; known history of poor compliance with medical treatment Enrolled: 30 (2 M/28 F) Randomised: 30 Withdrawals/drop‐outs: Unclear Final number and proportion of participants evaluable: Unclear ITT analysis: Unclear |
|
| Interventions |
Intervention 1 Blue LED treatment. "Upon pressing the start button, the treatment device provides a short one second vibration to signal the start of treatment, and then the LEDs are illuminated and vibration provided throughout the treatment cycle." Number and frequency of treatments: 4 in total, in two consecutive days, 2 per day Wavelength/Fluence/Duration/Spot size: 414 nm/unclear/duration 2 min Supplier: Tanda Zap (TZ) device, Pharos Life Corp., a subsidiary of Syneron – Candela, Ontario, Canada Instructions to participants: Unclear Intervention 2 Placebo (sham device) Number and frequency of treatments: 4 in total, in two consecutive days, 2 per day Wavelength/Fluence/Duration/Spot size: Not applicable Supplier: Unclear Instructions to participants: Unclear |
|
| Outcomes | Evaluation time points of review interest: None, please see 'Notes' (assessed "up to 10 days post the first treatment or until the lesions resolved") Primary outcomes of review interest: not recorded. Please see 'Notes'. Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. "Adverse events were monitored, and patients were photographed on each visit." |
|
| Notes | Language: English. Comparison of interventions and the outcomes at time points as defined by our protocol was not possible because of different time points of final evaluation, as each "participant was followed for up to 10 days post the first treatment or until the lesions resolved". Improvement evaluated at lesion level. Quote (page 310): "The two inflammatory lesions, similar in their appearance and severity were evaluated by both the physician and the subject pre and post each treatment in order to measure the difference over baseline in lesions treated with the active TZ vs. lesions that were treated with the sham. Lesions were evaluated using the following criteria: lesion size (not raised, slight, moderately or severely raised) and erythema (none, trace, moderate, severe)." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 309): "For each subject, 2 similar lesions (either papules or pustules of similar eruption status and age), one of each side of the face were identified by the physician and were randomly assigned to treatment of either the active or sham device." Comment: Method used to generate the allocation sequence was not stated. We judged this as at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quotes (page 309): "The subjects served as their own controls and all were treated by the Principal Investigator (PI) or his designated staff with both the active and sham devices."; "Upon pressing the start button, the treatment device provides a short one second vibration to signal the start of treatment, and then the LEDs are illuminated and vibration provided throughout the treatment cycle. The sham device has a completely similar look to the active device, but it does not deliver any therapeutic light and does not vibrate throughout the treatment cycle; it only provides a short vibration at the start and end of treatment to signal a complete cycle." Comment: Unclear whether adequate blinding of participants and performing clinicians was achieved. The sham device did not vibrate nor emit light therefore likely that participants would have been able to identify treatment device. Intention and/or method to blind the performing clinicians not described. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes of interest for this review. Please see 'Notes' above. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Intention and/or method to blind the assessing physicians were not specifically reported. We judged this as at unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported whether there were participants who withdrew or were lost to follow‐up. We judged this as at unclear risk of bias. |
| Selective reporting (reporting bias) | High risk | Outcomes not clearly pre‐specified. Adverse effects not reported, although prespecified in the methods section. We judged this as at high risk of bias. |
| Other bias | Unclear risk | Sponsorship unclear. Unclear who provided the sham device. Conflicts of interest reported. We judged this as at unclear risk of bias. |
Haedersdal 2008.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (page 387): "Disclosure: V‐beam Perfecta was borrowed from Candela Laser Corp., Wayland, Mass (M. H.). Lectures given for PhotoCure as part of an educational program (by M. H., S. R. W., H. C. W.)." Setting: Single centre, (Copenhagen) Denmark Recruitment: participants were recruited from advertisements and among participants referred to the Department of Dermatology, Bispebjerg Hospital. Duration: 5 months, November 2006‐March 2007 |
|
| Participants |
Included Age (inclusion criterion; mean; range): 18 > years; not given; 18‐31 years Clinically evident acne: Yes Severity of condition assessment: "at least a total of 12 inflammatory symmetrically distributed facial acne lesions " Fitzpatrick skin types: I‐III Excluded History of topical acne treatments within 2 weeks of study initiation, oral antibiotic treatment within 4 weeks, oral retinoid treatment within 6 months of study initiation. Contraceptive pills with anti‐androgenetic efficacy were not to be instituted within 12 weeks of study initiation. Pregnant or lactating woman and participants with a known history of melasma were excluded. Enrolled: 15 (5 M/10 F) Randomised: 15 Withdrawals/drop‐outs: 1 withdrew after 3rd treatment (personal reasons); 2 were lost to follow‐up at weeks 6 and 7 because of need for topical treatment Final number and proportion of participants evaluable: 14 (93%) at week 4 and 12 (80%) at week 12 ITT analysis: Not stated |
|
| Interventions |
Intervention 1 Long PDL (two passes), with pre‐operative MAL cream (approximately 2 g) applied 3 h before laser exposure; "covered with light impermeable dressing" Number and frequency of treatments: 3 in total, every 2 weeks Wavelength/Fluence/Duration/Spot size: 595 nm/7.5 J/cm²/10 ms (pulse width)/10 mm² Supplier: Metvix, Photocure ASA, Oslo, Norway; V‐beam Perfecta, 595 nm, Candela Laser Corp., Wayland, Mass Instructions to participants: Not applicable Intervention 2 Long PDL (two passes) Number and frequency of treatments: 3 in total, every 2 weeks Wavelength/Fluence/Duration/Spot size: 595 nm/7.5 J/cm²/10 ms (pulse width)/10 mm² Supplier: V‐beam Perfecta, 595 nm, Candela Laser Corp., Wayland, Mass Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4 and 12 weeks after final treatment Primary outcomes of review interest recorded 1. Participant's global assessment of improvement 2. Change from baseline in ILs counts (papules and pustules not reported separately) 3. Change from baseline in NILs count (open and closed comedones not reported separately) Methods of assessing primary outcomes 1. Numerical scale ranging from 0‐10 (0 = no satisfaction and 10 = best imaginable satisfaction) 2. & 3. Lesion counts (A dermatologist counted the number of different acne lesions at on‐site visits, counts were taken separately from the left and right sides by a face‐counting template) Secondary outcomes of review interest recorded 1. Adverse effects (pain, erythema, edema, pustules, crusting, and oozing skin areas, hyperpigmentation, hypopigmentation and scarring) Methods of assessing secondary outcomes 1. Pain was assessed using a numeric scale ranging from 0‐10 (0 = no pain and 10 = worst imaginable pain). Erythema, edema, pustules, crusting, and oozing skin areas were evaluated the day after first treatment (4‐point scale) and participants filled in a questionnaire concerning the duration of skin reactions. Adverse effects of hyperpigmentation, hypopigmentation and scarring were evaluated before second and third laser treatments and at subsequent visits up to 3 months after final treatment. |
|
| Notes | Language: English. We attempted to contact the study authors, but were not successful. Sponsors were not contacted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 388): "The randomisation was carried out by patients drawing lots between opaque sealed envelopes containing cards with 'LPDL' or 'MAL‐LPDL' representing the treatments for right and left split‐face sides." Comment: We judged this as adequate. |
| Allocation concealment (selection bias) | Low risk | Quote (page 388): "The randomisation was carried out by patients drawing lots between opaque sealed envelopes containing cards with 'LPDL' or 'MAL‐LPDL' representing the treatments for right and left split‐face sides." Comment: We judged this as adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. Given that one side of the face was treated with MAL cream for 3 h before laser treatment then it is unlikely that personnel were blinded, and that participants were blinded successfully (see below). We therefore judged the risk of bias as high. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | High risk | Quote (page 389 ): "...the blinding was not ideal as two patients spontaneously told which side was preoperatively treated with MAL." Comment: We judged this as at a high risk of bias for participant‐assessed outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 389 ): "The evaluating dermatologist was not the same as the treating dermatologist and case record forms were not available when the clinical assessments were performed. However, the blinding was not ideal as two patients spontaneously told which side was preoperatively treated with MAL." Comment: We judged this as at a low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures obtained for 93.33% randomised participants at 4 weeks of follow up, and for 80% at 12 weeks of follow‐up. We judged this as at a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were reported. |
| Other bias | Unclear risk | Some bias might have been introduced by conflicts of interests the study authors have declared. No other possible sources of bias were identified. Insufficient information to permit a clear judgement. |
Hong 2013.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Not declared Setting: Single centre, Seoul (Korea) Recruitment: Seoul National University Hospital, details not provided Duration: Start and end dates were not reported |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; not stated; 19‐35 years Clinically evident acne: Yes Severity of condition assessment: "at least grade 2 (Cunliffe acne grading system)" Fitzpatrick skin types: IV‐V Excluded "...history of keloid, photosensitive disorders, taking medication such as oral contraceptives, oral antibiotics, and topical agents within four weeks, treatment with oral isotretinoin within the past six months, or pregnant and/or lactating women." Enrolled: 22 (2 M/20 F) Randomised: 22 Withdrawals/drop‐outs: 2 withdrawals due to side effects after applying 37 J/cm²; "pain, severe erythema, and considerable edema until five days after treatment. Furthermore, postinflammatory hyperpigmentation persisted for four weeks after treatment."; 22 J/cm² was used for the remaining 20 participants Final number and proportion of participants evaluable: 20 (2 F/18 M) (91%) ITT analysis: Unclear |
|
| Interventions |
Intervention 1 2 g of MAL applied, 3 h incubation time, MAL "removed with a mild soap and 70% alcohol", followed by red light application Number and frequency of treatments: 3 in total, 2‐week intervals Wavelength/Fluence/Duration/Spot size: 630 nm/22 J/cm²/other not reported Supplier: Metvix; Galderma, Watford, UK; Aktilite CL 128; PhotoCure ASA, Oslo, Norway Instructions to participants: Not applicable Intervention 2 2 g of MAL applied, 3 h incubation time, MAL "removed with a mild soap and 70% alcohol", followed by IPL application Number and frequency of treatments: 3 in total, 2‐week intervals Wavelength/Fluence/Duration/Spot size: 530‐750 nm; 8‐10 J/cm²/2 x 2.5 ms/ 10 x 48 mm² Supplier: Metvix; Galderma, Watford, UK; Ellipse Flex system; Danish Dermatologic Development, Horsholm, Denmark Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Participant's global assessment of improvement 2. Percentage change from baseline in ILs count (papules, pustules and nodules not reported separately) 3. Percentage change from baseline in NILs count (open and closed comedones not reported separately) Methods of assessing primary outcomes 1. "The patients assessed improvement subjectively, using a visual analogue scale from 10 to 0 (in which 10 was the same as before the first treatment and 0 meant currently with no acne)." 2. & 3. "During each visit, standardized digital photographs were taken. The number of inflammatory and non‐inflammatory acne lesions on both sides was counted by two independent dermatologists, blinded to the subject's condition, before each treatment and at four weeks after the last treatment." Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. "The acne grade was assessed according to the Cunliffe acne grading system." 2. "Complications, including erythema and hyperpigmentation, were also assessed at each visit…Subjects were asked to grade the pain during illumination with light sources and after treatment. Pain was also assessed by means of a visual analogue scale from 0 to 10 in which 0 was 'no pain' and 10 'pain as bad as it could be'." |
|
| Notes | Language: English. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 2): "The application side of the two different methods was randomised before the first treatment." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method used to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Intention and/or method used to blind performing clinicians and/or participants were not specifically reported. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | Intention and/or method used to blind participants were not specifically reported. We therefore judged the risk of bias as unclear. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 2): "During each visit, standardized digital photographs were taken. The number of inflammatory and non‐inflammatory acne lesions on both sides was counted by two independent dermatologists, blinded to the subject's condition, before each treatment and at four weeks after the last treatment." Comment: We judged this as adequate for investigator‐assessed outcomes and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures obtained for over 80% of randomised participants. We judged the risk of bias as low. |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the methods section were reported. |
| Other bias | Unclear risk | Sponsorship or potential conflicts of interest were not declared. Insufficient information was given to permit a clear judgement. |
Hongcharu 2000.
| Methods | This was a parallel‐group RCT (single vs multiple treatments). Within each group participants' backs were split into 4 areas to which different interventions were applied. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Declared. Quote (page 190): "We thank DUSA Pharmaceuticals Inc. for donating the ALA supply. DUSA did not fund this research, and none of the study authors has any financial interest in DUSA or ALA‐PDT for acne". Setting: Single centre (Boston, Massachusetts, USA) Recruitment: Not reported Duration: 6 months, October 1998‐March 1999 |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; 30 in single treatment group, 27 in multiple treatment group; range 18‐44 (whole sample) Clinically evident acne: Yes Severity of condition assessment: Mild‐moderate, Burke & Cunliffe grades 1‐4 (Leeds acne grading system) Fitzpatrick skin types: I‐IV Excluded "Topical acne treatment, systemic antibiotics in the past 2 weeks, or systemic retinoids in the past year, medication that may exacerbate or alleviate acne, planning to have excessive sunlight exposure, history of keloid or photosensitivity disorder, Fitzpatrick photo type V‐VI; pregnant and lactating women." Enrolled: 23 Randomised: 23 Withdrawals/drop‐outs: "One was dropped from the study because his asthma necessitated systematic steroid treatment, which is one of the exclusion criteria" (page 185). It was unclear which group that participant was randomised to. Final number and proportion of participants evaluable: 22/23 (96%); 11 (9 M/2 F) in the single treatment group and 11 (8 M/3 F) completed in the multiple treatment group completed. ITT analysis: No |
|
| Interventions |
Intervention 1 Skin cleaned with 70% propyl‐alcohol, 20 % ALA in hydroalcoholic vehicle applied for 3 h under occlusion with plastic film (Saran wrap) + red light Number and frequency of treatments: Single treatment or multiple treatments (four in total, once a week in four consecutive weeks) Wavelength/Fluence/Duration/Spot size: 550‐700 nm/150 J/cm²/for 3 h/not reported Supplier: Levulan, DUSA Pharmaceuticals; laser supplier not reported Instructions to participants: Not applicable Intervention 2 Skin cleaned with 70% propyl‐alcohol, 20% ALA in hydroalcoholic vehicle applied for 3 h under occlusion with plastic film (Saran wrap) Number and frequency of treatments: Single treatment or multiple treatments (4 in total, once a week in 4 consecutive weeks) Supplier: Levulan, DUSA Pharmaceuticals Instructions to participants: Not applicable Intervention 3: Red light alone Number and frequency of treatments: Single treatment or multiple treatments (4 in total, once a week in 4 consecutive weeks) Wavelength/Fluence/Duration/Spot size: 550‐700 nm/150 J/cm²/ for 3 h/not reported Supplier: Not reported Instructions to participants: Not applicable Intervention 4 Untreated control |
|
| Outcomes | Evaluation time points of review interest: 2, 3, 10 and 20 weeks after final treatment (also assessed at 1 week after final treatment) Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Investigator's global assessment of improvement 3. Adverse effects Methods of assessing secondary outcomes 1. Modified Michaelson grade score 2. Non‐standardised grading scale: ‐3 for over 50% exacerbation, ‐2 for 25% to 50% exacerbation, ‐1 for 25% to 0% exacerbation, 0 if unchanged, 1 for 1% to 25% improvement, 2 for 25% to 50% improvement, 3 for 50% to 75% improvement, 4 for 75% to 99% improvement and 5 for 100% improvement, compared with the baseline, using photographs 3. Scored by clinical evaluation of erythema, edema, loss of epidermis, hyperpigmentation, haemorrhage, vesiculation, exfoliation on a VAS from 0‐3 (0 = absent,1 = mild, 2 = moderate, 3 = severe) |
|
| Notes | Language: English. Mean + SEM results data reported in graph‐format for 1.& 2. secondary outcome of review interest. It appears that statistical tests were used to compare the different treatments used within each group rather than between the two randomised groups. The study authors were contacted in 2008, but were unable to provide additional information. We have not attempted to contact the study authors again. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 184): "Subjects were randomly divided into single‐treatment and multiple‐treatment groups." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 184): "Clinical improvement was globally assessed by three dermatologists unaware of the status of the treatment, who blindly graded changes in acne from fixed‐magnification clinical photographs, after being shown a small set of standardized series of slides, not used in the data evaluation." Comment: We judged this as adequate and at a low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 95.6% of randomised participants were evaluated. We judged this as adequate. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were reported. |
| Other bias | Low risk | Study authors declared that industry donated the ALA supply, but the study authors clarified their role in the study and we judged this was unlikely to affect the results. We therefore judged the risk to be low. |
Hörfelt 2006.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quotes (page 608): "Conflicts of interest: A‐M.W has received fees from Photocure for giving lectures and for organizing education."; (page 612) "Acknowledgements: This study was funded by PhotoCure ASA, Oslo, Norway" Setting: Multicenter (Gothenburg and Stockholm, Sweden; Moss, Norway) Recruitment: By outpatient dermatology clinics in 2 centres in Sweden (male participants only) and 1 centre in Norway Duration: 8 months, October 2004‐May 2005 |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 15 years; 18; 15‐28 Clinically evident acne: Yes Severity of condition assessment: "active inflammatory acne" ; "Leeds score 5‐10" (moderate and severe) ; "Moderate inflammatory facial acne vulgaris was defined as at least 10 inflammatory lesions (papules and pustules) and 15‐100 non‐inflammatory lesions (open and closed comedones), excluding the nose" Fitzpatrick skin types: I‐III Excluded Not stated Other: Acne treatments were discontinued up to 3 months before the study. Enrolled: 30 (25 M/5 F) Randomised: 30 Withdrawals/drop‐outs: 2 withdrawals due to moderate erythema, 1 due to moderate pain. No drop‐outs Final number and proportion of participants evaluable: 27 (90%) ITT analysis: Yes |
|
| Interventions |
Intervention 1 MAL cream 160 mg/g applied to side of face in 1 mm thick layer (above the jaw line) excluding the nose and a 1 cm periocular area and covered with an adhesive occlusive dressing. Nodular or cystic lesions were prepared using a cannula to facilitate cream penetration. After 3 h the cream was wiped off both sides immediately before illumination with non coherent red light. Number and frequency of treatments: 2 in total, every 2 weeks Wavelength/Fluence/Duration/Spot size: 635 nm/ 37 J/cm²/other data not given Suppliers: Aktilite CL 128 lamp; MAL cream: Metvix, Photocure ASA, Oslo, Norway; occlusive dressing: 3M Tegaderm Beirsdorf A/S, Birkhoed, Denmark OR Opsite, Smith & Nephew, Hull, UK Instructions to participants: Not applicable Intervention 2 Placebo cream applied to side of face in 1 mm thick layer (above the jaw line) excluding the nose and a 1 cm periocular area and covered with an adhesive occlusive dressing. Nodular or cystic lesions were prepared using a cannula to facilitate cream penetration. After 3 h the cream was wiped off both sides immediately before illumination with non coherent red light. Number and frequency of treatments: 2 in total, every 2 weeks Wavelength/Fluence/Duration/Spot size: 635 nm/37 J/cm²/other data not given Suppliers: Aktilite CL 128 lamp; occlusive dressing: 3M Tegaderm Beirsdorf A/S, Birkhoed, Denmark OR Opsite, Smith & Nephew, Hull, UK Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4 and 10 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Percentage change from baseline of ILs counts (papules and pustules not reported separately) 2. Percentage change from baseline of NILs lesion counts (open and closed comedones reported separately) Methods of assessing primary outcomes 1. Lesion count "recorded by the investigator in the clinic by marking with a pen each lesion on the face that was counted to make sure each lesion was counted only once" 2. Lesion count (see above) Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. 6‐point rating scale ("1 – Almost clear: A few scattered comedones and a few (less than five) small papules 2 – Mild: Easily recognizable; less than half the face is involved. Many comedones and many papules and pustules 3 – Moderate: More than half of the face is involved. Numerous comedones, papules and pustules 4 – Severe: Entire face is involved. Covered with comedones, numerous papules and pustules and few nodules and cysts 5 – Very Severe: Highly inflammatory acne covering the face; with nodules and cysts present") 2. All adverse events were assessed, pain after illumination using VAS |
|
| Notes | Language: English. This was a split‐face trial but the last paragraph on page 610 describes results in "MAL‐PDT" and "placebo‐PDT" groups which is a bit confusing. It was not specified on which side of the face adverse events causing drop‐out occurred. The study authors were contacted and provided additional data in 2008 (results' details), but we were unsuccessful in contacting them afterwards. Sponsors were contacted regarding rates of application site blisters, and provided information as follows: "1 report from 30 Metvix (double concentration of Visonac) treated patients". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 609): "Each patient was randomly assigned to placebo and MAL cream, each to be applied to one side of the face." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quotes (page 609): "The application was done in a double‐blinded manner (blinded to both patient and investigator) as the MAL cream and the placebo cream were of the same colour and consistency."; Quote (MD thesis, page 42): "However, an experienced observer could tell the difference since the MAL cream gave obvious side effects such as pain shortly after onset of illumination." Comment: Despite the fact that some bias might have been introduced, we judged it as at a low risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quotes (page 609): "The double‐blind design of the study allowed the same investigator to perform the counting and severity scoring as well as performing the treatment. Each side of the face was photographed to document the patient's participation in the study, and to support the clinically assessed outcomes."; Quote (MD thesis, page 42): "However, an experienced observer could tell the difference since the MAL cream gave obvious side effects such as pain shortly after onset of illumination." Comment: Despite the fact that some bias might have been introduced, we judged it as at a low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 609): "Efficacy and safety analyses were performed on the intention‐to‐treat population (including all 30 participants) using the last observation carried forward method for missing data." Comment: Although an ITT analysis was performed, three of the 30 participants dropped out of the study due to adverse effects between the first and second PDT treatments. The study authors used the LOCF method to account for missing data for these three patients. The study authors state using both ITT and LOCF, in this way, within their analyses. In a full ITT analysis, the 3 participants’ latter observations would be missing, however according to the study authors, they use the LOCF values for the missing data of these 3 individuals. As outcome measures were obtained for 90% of subjects randomised, we judged this as at a low risk of bias.) |
| Selective reporting (reporting bias) | Unclear risk | Baseline data reported for both ILs and NILs as absolute counts. Results reported for NILs as absolute counts, whilst as percentage changes only for ILs. Acne severity score non reported for 4 weeks post‐treatment assessment. We judged this as at unclear risk of bias. |
| Other bias | Unclear risk | Commercial sponsorship might have introduced some bias. We judged this as at an unclear risk of bias. |
Ianosi 2013.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (pages 248‐249) "study organized under the license No.169/2011 from the Ministry of Health of Romania". Setting: Single centre (Craiova, Romania) Recruitment: "an outpatient laser clinic in Craiova (Medical Center Dr. Ianosi) with high expertise in acne treatment in the Oltenia region (which has over 2.5 million inhabitants)." Duration: 6 months, March 2012‐August 2012 |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 18 years; median 24.1 years; not reported Clinically evident acne: Yes Severity of condition assessment: "...with mild to moderate acne vulgaris, with one or more inflammatory lesions" Fitzpatrick skin types: I‐IV Excluded Quote (page 249): "Open lesions, broken and extremely dry skin; Any active infections; History of skin cancer or precancerous lesions, herpes type I or II, lupus erythematous, porphyria, endocrine disorders; Patients who have used Accutane within the last 6 months or photosensitive medications; Patients who were recently tanned; Pregnant or nursing women" Enrolled: Not reported, M/F unclear Randomised: 180 (60 in each group), 56 M/124 F Withdrawals/drop‐outs: Unclear. "A total of 57 patients were removed from the study: 23 patients breached protocol, 12 patients were not able to continue the treatment, and 22 patients refused to continue the study due to absence of therapeutic response (all from control group)." Final number and proportion of participants evaluable: 123/180 (68%, 37 M/86 F); 43/60 (72%), 44/60 (73%), 36/60 (60%) ITT analysis: No |
|
| Interventions |
Intervention 1 IPL + vacuum. Before each visit the participants were exposed to steam for 10 min Number and frequency of treatments: Once a week for 5 weeks Wavelength/Fluence/Duration/Spot size: 500‐1200 nm; "Two passes were performed on each patient with energy level 6, vacuum V3 for chins and S3 for forehead, double pulse, 3 ms pulse width and 750 ms pulse delay for skin type II and light III. For dark III and IV phototypes, we used energy level 4, vacuum V2 for chins and S1 or S2 for forehead, double pulse, 25 ms pulse width and 750 ms pulse delay" Supplier: Acleara ™ Acne Clearing System, manufactured by Theravant, Inc. for Palomar Medical Technologies, Burlington, VT, USA Instructions to participants: Adequate Intervention 2 IPL Number and frequency of treatments: Once a week for 5 weeks Wavelength/Fluence/Duration/Spot size: 400–700 nm and 870–1200 nm/100 ms pulse width and 10–12 J/cm² fluence for the first pass and 20 ms pulse width and 8–10 J/cm² fluence for the second. For later visits, pulse width and fluence gradually increased according to FPT Supplier: StarLux System Lux V Pulsed Light Handpiece, Palomar Medical Technologies, Burlington, VT, USA Instructions to participants: Adequate Intervention 3 Anti‐acne micellar solution Number and frequency of treatments: For 5 weeks, frequency unclear Supplier: Bioderma Laboratoire Dermatologique Instructions to participants: Unclear |
|
| Outcomes | Evaluation time points of review interest: None (assessed at each session whilst on treatment, final evaluation at final treatment) Primary outcomes of review interest: not assessed Secondary outcomes of review interest recorded 1. Investigator's assessment of change in acne severity 2. Investigator's global assessment of improvement 3. Changes in quality of life 4. Adverse effects Methods of assessing secondary outcomes 1. Leeds revised acne grading system; standardised photographs 2. Based on "evolution of papules, pustules and comedones"; "Insignificant result (1)‐lesion numbers and erythema reduction between 0% and 25%; Moderate result (2)‐lesion numbers and erythema reduction between 26% and 50%; Good result (3)‐lesion numbers and erythema reduction between 51% and 75%; Very good result (4)‐lesion numbers and erythema reduction between 76% and 100%" 3. Cardiff Acne Disability Index (CADI) 4. "Pain during treatment was evaluated as painless (0), light pain (1), moderate pain (2), and severe pain (3)." |
|
| Notes | Language: English. Groups comparable at baseline. No mention of systemic nor topical treatment wash‐out periods (only for Accutane). The intervention changed according to skin type. Last evaluation at final treatment. Results in graph format. We attempted to contact the study authors but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 254): "For allocation of the participants, a computer generated list of random numbers was used." Comment: We judged this as adequate and risk of bias as low. |
| Allocation concealment (selection bias) | Low risk | Quote (page 254): "Prior to every enrolment, patient allocation to one group or another was transmitted through phone to the principal investigator by a computer specialist not involved in this study." Comment: We judged this as adequate and risk of bias as low. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Control group didn't have a 'placebo' intervention. Due to nature of intervention it is hard to blind participants/personnel. No evidence and details of blinding of participants and personnel. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | Control group didn't have a 'placebo' intervention. Due to nature of intervention it is hard to blind participants/personnel. No evidence and details of blinding of participants and personnel. Comment: We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 254): "Standardized digital photos of each patient were taken prior to starting a treatment session and after every visit using a Cannon G9 Power Shot 12.6 M pixels Camera. Two observers, not involved in the recruitment of patients in order to maintain the concealment of the allocated interventions, evaluated each patient weekly." Comment: We judged this as adequate and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome measures reported for less than 80% of participants randomised in total and for each group, so we judged this as at high risk of bias. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes of interest to this review were reported. |
| Other bias | Low risk | No other possible source of bias identified. |
Jih 2006.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Declared. Quote (page 80): "Funding sources: Laser and patient stipend provided by Candela Corporation. Disclosure: Dr Friedman has been a paid investigator for Candela Corporation." Setting: Single centre, (Houston, Texas, USA) Recruitment: Not reported Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 23 years; 18‐39 years Clinically evident acne: Yes Severity of condition assessment: "at least 20 active inflammatory acne lesions" Fitzpatrick skin types: II‐VI Excluded Pregnancy, treatment with oral isotretinoin within 6 months, commencement or alteration in the use of oral contraceptives during the previous 3 months, use of oral antibiotics in the previous 4 weeks, use of laser/light based acne treatments within 6 months, tanned skin, recent excess sun exposure Enrolled: 20 (10 M/10 F) Randomised: 20 Withdrawals/drop‐outs: None Final number and proportion of participants evaluable: 20 (100%) ITT analysis: No |
|
| Interventions |
Intervention 1 Topical lidocaine 5% 1 hour before laser treatment with non overlapping single pulses of diode laser with an integrated dynamic cooling device Number and frequency of treatments: 3 treatments, every 3‐4 weeks Wavelength/Fluence/Duration/Spot size: 1450 nm/14 J/cm²/not reported/6 mm² Supplier: Smoothbeam, Candela Corp., Wayland, Mass Instructions to participants: Not applicable. "Patients were counselled to avoid sun exposure after the laser treatment and counselled to use a sunscreen with a sun protection factor of 30 daily." Intervention 2 Topical lidocaine 5% 1 hour before laser treatment with non overlapping single pulses of diode laser Number and frequency of treatments: 3 treatments, every 3‐4 weeks Wavelength/Fluence/Duration/Spot size: 1450 nm/16 J/cm²/not reported/6mm² Supplier: Smoothbeam, Candela Corp., Wayland, Mass Instructions to participants: Not applicable. "Patients were counselled to avoid sun exposure after the laser treatment and counselled to use a sunscreen with a sun protection factor of 30 daily." |
|
| Outcomes | Evaluation time points of review interest: 1, 3, 6 and 12 months after final treatment Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Percentage change in ILs count Methods of assessing primary outcomes 1. Non‐standardised rating scale (0 = worsening, 1 = no change, 2 = mild improvement, 3 = moderate improvement, 4 = marked improvement) at 1, 3, 6, 12 months follow‐up 2. ILs were counted at baseline and before each treatment and at each follow‐up visit ("photographs were obtained by means of standardized settings and lighting with a stereotactic device and a 35‐mm film camera (Canfield Scientific, Fairfield, NJ) at baseline and before each treatment and at each follow‐up visit from the front and left and right sides at 45 degrees") Secondary outcomes of review interest recorded 1. Adverse effects (pain scores related to treatment and complications) Methods of assessing secondary outcomes 1. Pain scores related to laser treatment based on a VAS at each treatment visit using a scale of 0 (no pain) to 10 (worst pain). Complications were assessed at each visit. |
|
| Notes | Language: English. Patient assessment of acne was not scored for split sides of face. The sponsors were contacted in 2008 and provided additional information (detailed results). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 81): "Split face comparisons of two laser fluences were performed by randomising patients to one of two fluences (14 or 16 J/cm²) administered to the right or left side of the face." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | Patient assessment of acne was not scored for split sides of face, so we did not include the results in our report. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | No intended blinding of outcome assessors reported. No evidence that assessors were blinded provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 81): "The data for all patients were used in the statistical analysis and none were excluded from the analysis." Comment: 100% of randomised participants included in the analysis. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were reported. |
| Other bias | Unclear risk | Commercial sponsorship and declared conflicts of interest might have introduced additional bias. Not enough information provided to make a clear judgment. |
Jung 2009.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared. No commercial sponsors and no conflict of interest declared (page 1181) Setting: Single centre (Seoul, Korea) Recruitment: Not reported Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 26 years; 20 ‐31 years Clinically evident acne: Yes Severity of condition assessment: "acne severity grade 2‐5, as defined using the Cunliffe grading system" (page 1182) Fitzpatrick skin types: Not reported Excluded Pregnancy, prior acne therapy, including isotretinoin therapy within 12 months, systemic antibiotic therapy (for any indication) within 1 months, any topical acne preparations or intralesional steroid injections within 2 weeks of starting laser treatment Enrolled: Unclear (M/F unclear) Randomised: 18 Withdrawals/drop‐outs: 2 withdrawals, reasons not stated ("personal reasons"). No lost to follow‐up Final number and proportion of participants evaluable: 16 (88%) ITT analysis: Not stated |
|
| Interventions |
Intervention 1 Single pass of a combined 585/1064nm laser on half of the face Number and frequency of treatments: 3 treatments, every 2 weeks Wavelength/Fluence/Duration/Spot size: 585/1064 nm/7‐9/40‐50 J/cm²/40 ms (pulse duration)/7 mm² Supplier: Not stated Instructions to participants: Not applicable Intervention 2 Single pass of PDL alone on half of the face Number and frequency of treatments: 3 treatments, every 2 weeks Wavelength/Fluence/Duration/Spot size: 585 nm/ 7‐9 J/cm²/40 ms (pulse duration)/ 7 mm² Supplier: Not stated Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4 and 8 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Participant's global assessment of improvement 2. Change from baseline in number of ILs & NILs counts Methods of assessing primary outcomes 1. VAS that ranged from 0 (worst imaginable acne state) to 10 (disease free) 2. Lesion counts, using photographs Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. Leeds acne grading system, using photographs 2. Unclear |
|
| Notes | Language: English. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1182): "The 16 participants were randomised to receive PDL treatment on half of the face and combined 585/1,064‐nm laser treatment on the other half." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study described as "double‐blind" (page 1181), however not stated as to whether participants/ clinicians were blinded and how. We judged this as at an unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | No evidence that participants were blinded was given, so we judged the risk of bias as unclear for participant‐assessed outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 1182): "Two independent dermatologists performed clinical assessments using clinical photographs." Comment: Adequate for outcomes assessed by blinded dermatologists. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 1182): "Of the 18 subjects initially enrolled, 16 (5 men, 11 women) completed the study; two dropped out for personal reasons. The 16 participants were randomised to receive PDL treatment on half of the face and combined 585/1,064‐nm laser treatment on the other half." Comment: Outcomes obtained for 16/18 (88.8%) participants. Reasons for withdrawal reported. We judged this as at low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were reported. |
| Other bias | Low risk | No other possible source of bias identified. |
Jung 2012.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared. No funding sources and conflicts of interest (page 626) Setting: Single centre (Seoul, Korea) Recruitment: Not reported Duration: Start and end dates were not reported |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 25.4 years; 19‐34 years Clinically evident acne: Yes Severity of condition assessment: Unclear Fitzpatrick skin types: III‐V Excluded Pregnancy and prior acne therapy, including isotretinoin therapy within 6 months, systemic antibiotic therapy (for any indication) within 1 month, and topical acne preparations or intralesional steroid injections within 1 month of starting laser treatment Enrolled: Unclear (M/F unclear) Randomised: 22 Withdrawals/drop‐outs: 2 withdrawals due to personal reasons Final number and proportion of participants evaluable: 20 (91%) ITT analysis: Not stated |
|
| Interventions |
Intervention 1 Face washed with cleanser, carbon lotion applied for 20 minutes followed by single pass of quasi long‐pulse Nd:Yag laser followed by 3 passes of Q‐switched Nd:Yag laser Number and frequency of treatments: 3 treatments in total, applied every 3 weeks Wavelength/Fluence/Duration/Spot size: 1064 nm/1.8‐2.3 J/cm²/not reported/7 mm² Supplier: Spectra VRMIII, Lutronic, Ilsan, Korea Instructions to participants: Not applicable Intervention 2 No treatment |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Percentage change from baseline of ILs & NILs counts Methods of assessing primary outcomes 1. VAS, ranging from 0 (disease‐free status) to 10 (initial visit acne status). When the acne was aggravated compared to the initial visits, the VAS scores exceeded 10 2. Lesion counts, digital photographs Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. Cunliffe’s grading system, digital photographs 2. Adverse reactions were recorded at every visit |
|
| Notes | Language: English. Quotes (page 629): "The laser treated side had statistically more acne lesions than the non‐treated side at baseline."; "Mean baseline acne grades of laser‐treated and control sides were 3.2 and 2.7 respectively. Statistically the laser‐treated side showed more severe acne status than the non‐treated side did at baseline (P = 0.003)" Comment: Difference in mean acne grades at baseline not corrected for in analysis. Quote (page 627): "Subjects were not allowed to use any systemic, topical, or phototherapy‐based acne treatment during this study" Comment: No mention of hormonal treatment for acne. Unclear whether hormonal treatment is covered by 'systemic treatment'. We attempted to contact the study authors, but were not successful. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 628): "The patients were randomised to receive laser treatment on one half of the face, whereas the other side of the face was observed." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. Given that one side of the face was treated with a laser then it is unlikely that participants/ personnel were blinded. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | No evidence that participants were blinded was given, so we judged the risk of bias as unclear for participant‐assessed outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Quote (page 628): "clinical assessments were performed by two independent dermatologists...". Comment: It was unclear whether assessors were blinded. Insufficient information was given to permit a clear judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over 90% of randomised participants included in the analysis, so we judged it as at a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | No other possible source of bias identified. We judged this as at a low risk of bias. |
Karsai 2010.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Declared. No conflicts of interest declared (page 395) Setting: Single centre (Karlsruhe, Germany) Recruitment: Regional treatment centre for aesthetic laser surgery (Laserklinik Karlsruhe) on a "first come – first served" basis Duration: 7 months, October 2008‐April 2009 |
|
| Participants |
Included Age (inclusion criterion; mean; range): "adolescents and adults"; 19.7 years; 13.3‐43.8 years Clinically evident acne: Yes Severity of condition assessment: mild to moderate inflammatory acne vulgaris, Investigator’s Static Global Assessment (ISGA) score 2‐4 Fitzpatrick skin types: I‐III Other: The ability and willingness to comply with the requirements of the protocol Excluded Quote (page 396): "(i) atopic dermatitis (because of the irritating potential of BPO 26); (ii) a history of regional enteritis, Crohn’s disease or antibiotics‐associated colitis; (iii) oral antibiotics during the last 4 weeks prior to enrolment; (iv) oral isotretinoin during the last 52 weeks prior to enrolment; (v) oral contraceptives during the last 26 weeks prior to enrolment; (vi) topical acne treatment during the last 4 weeks prior to enrolment (including artifical or natural ultraviolet therapy); (vii) laser surgery interventions within the treatment region during the last 12 weeks prior to enrolment; (viii) coagulation disorders or anticoagulant treatment; (ix) photosensitising medication (e.g. tetracycline, gold); and (x) pregnancy" Enrolled: 134 screened for eligibility, M/F unclear Randomised: 89, M/F not reported Withdrawals/drop‐outs: 2 withdrawals (due to noncompliance ‐ discontinuation of C/BPO or sunbathing), 7 lost to follow‐up. Intervention group for withdrawals and lost to follow‐up not reported Final number and proportion of participants evaluable: 80 (90%) (38 M/42 F) ITT analysis: No |
|
| Interventions |
Intervention 1 Fixed‐combination clindamycin 1%‐benzoyl peroxide 5% hydrating gel (C/BPO) Number and frequency of treatments: Applied at night and left on overnight for 4 weeks Supplier: Duac Akne Gel; Stiefel Laboratorium GmbH, Offenbach, Germany Instructions to participants: Adequate Intervention 2 Fixed combination clindamycin 1%‐benzoyl peroxide 5% gel ‐ applied at night and left on overnight for 4 weeks and PDL Number and frequency of treatments: Gel applied at night and left on overnight for 4 weeks; 2 laser treatments in total, second after 2 weeks Wavelength/Fluence/Duration/Spot size: 585 nm/3 J/cm²/0.35 ms/7 mm² Supplier: Duac Akne Gel; Stiefel Laboratorium GmbH, Offenbach, Germany; NLite V; Medical Bio Care, Berlin, Germany Instructions to participants: Adequate |
|
| Outcomes | Evaluation time points of review interest: 2 weeks after final laser treatment (also assessed at 2 weeks after initial treatment) Primary outcomes of review interest recorded 1. Change from baseline in number of ILs (papules and pustules not reported separately) 2. Change from baseline in total number of acne lesions (including papules, pustules, open and closed comedones) Methods of assessing primary outcomes 1. & 2. The number of ILs (papules and pustules) and the total number of lesions (including open and closed comedones) on the whole face (except the nose) counted on site. Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Changes in quality of life 3. Adverse effects Methods of assessing secondary outcomes 1. Investigator’s Static Global Assessment (ISGA) score; standardised photographs 2. Dermatology Life Quality Index (DLQI) 3. Active questions about side‐effects (erythema, oedema, purpura, blisters, crusts, bleeding, hyper‐ or hypopigmentation, scars, atrophy, pain, paraesthesia) were recorded by a medical assistant not otherwise involved in the trial |
|
| Notes | Language: English. Study authors stated that their primary endpoints were ISGA score and lesion count, however means and SDs not reported for lesion counts. Significant difference in baseline lesion counts (P < 0.05) between the two groups for all lesions (Figure 2, page 398). Unclear whether compliance assessment was performed. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 396): "..patients were assigned to treatment groups in a 1:2 ratio using a computer‐generated randomisation schedule." Comment: We judged this as adequate. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 396): "It was not possible to blind either the patient or the therapist..." Comment: We judged this as at a high risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | High risk | Quote (page 396): "It was not possible to blind either the patient or the therapist..." Comment: We judged this as at a high risk of bias. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 396): "Photographs were taken..." (for ISGA score); "were counted on site by a fourth independent investigator who was blinded with regard to group assignment and time point" (for lesion counts). Comment: We judged this as adequate and as at low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 196): "89 patients fulfilled the inclusion and exclusion criteria and agreed to participate…overall, 80 patients eventually completed the trial." Data does not include 9 participants who withdrew or were lost to follow‐up. Outcome measures reported for 90% of participants randomised, so we judged this as at a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified in the methods section reported. |
| Other bias | Low risk | No other possible source of bias identified. We judged this as at a low risk of bias. |
Kim 2009.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Declared. No conflict of interest (page 216) Setting: Not reported (Seoul, Korea) Recruitment: "volunteers", other information not given Duration: Start and end dates were not reported |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 25 years; 16‐34 years Clinically evident acne: Yes Severity of condition assessment: Mild to moderate facial acne Fitzpatrick skin types: Not reported Other: Otherwise healthy Excluded History of medical or surgical treatment during the last 6 months Enrolled: 16 (7 M/9 F) Randomised: 9 in group 1, 7 in group 2 Withdrawals/drop‐outs: None Final number and proportion of participants evaluable: 16 (100%) ITT analysis: Not stated |
|
| Interventions |
Intervention 1 Topical application of indocyanine green dye applied to the right cheek and washed off after 30 minutes. Gel applied prior to treatment with near infrared diode laser. Number and frequency of treatments: Single treatment Wavelength/Fluence/Duration/Spot size: 805 nm/12 J/cm²/pulse duration 30 ms/not reported Supplier: LightSheer; Lumenis, Santa Clara, CA, USA Instructions to participants: Not applicable Intervention 2 Topical application of indocyanine green dye applied to the right cheek and washed off after 30 minutes. Gel applied prior to treatment with near infrared diode laser. Number and frequency of treatments: Multiple treatments (3 in total, weekly) Wavelength/Fluence/Duration/Spot size: 805 nm/12 J/cm²/pulse duration 30 ms/not reported Supplier: LightSheer; Lumenis, Santa Clara, CA, USA Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 2 and 4 weeks after final treatment Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement ("Subjective satisfaction") 2. Change from baseline in number of ILs & NILs Methods of assessing primary outcomes 1. –100 to +100 scale scoring 2. Lesion counts (open and closed comedones, papules and pustules) Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. Cunliffe acne grading system 2. "Checked" at each visit |
|
| Notes | Language: English. Data expressed in graph format only. No details of baseline ILs and NILs data reported, so it is unclear whether the groups were comparable at baseline. Single versus multiple treatment groups only randomised, 3 interventions non randomly applied to the facial areas of the same individual. Results reported as "ICG combined with laser group" and "laser only group" although the assigned groups were single verus multiple treatments, and described treatments were applied to different areas of the face of the same individual and not 'group'. Unclear how Cunliffe score can be assessed for 3 different treatments applied to different areas of the same face. We attempted to contact the study authors but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 16): "…16 volunteers were randomly assigned to two groups". Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and performing clinicians were not blinded which is likely to have introduced bias. We judged this as at a high risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | High risk | participants were not blinded, so we judged the risk of bias as high for participant‐assessed outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | It was unclear whether assessors were blinded. Insufficient information was given to permit a clear judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis, so we judged this as at a low risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | No details of baseline data reported. Detailed report only for open comedones and no other outcomes predefined in the 'Methods' was given. Other outcomes reported as graphs, no figures were given. Insufficient information was given to permit a clear judgement. |
| Other bias | Low risk | We did not identify other possible sources of bias. |
Kwon 2013.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared. None, it is however unclear who provided the device. Setting: Multicenter, Seoul (Korea) Recruitment: Department of Dermatology, Seoul National University Hospital and Department of Dermatology and Chonnam National University Medical School Duration: 4 months, December 2011‐March 2012 |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; not stated; 20‐27 years Clinically evident acne: Yes Severity of condition assessment: "with mild to moderate acne as defined by IGA scale 2 to 4" Fitzpatrick skin types: III‐V Other: "not allowed to use any systemic, topical, or light‐based acne treatment during the course of this study" Excluded "pregnancy, mental illness, intake of oral isotretinoin within 6 months, and application of the other oral and topical acne medications, chemical peeling and light based treatments within 6 weeks" Enrolled: 35 (11 M/24 F) Randomised: 18 in light group, 17 in sham group Withdrawals/drop‐outs: "three dropped out for personal reasons, and there was no patient dropout because of serious side effects or inconvenience to use the device during the study period" Final number and proportion of participants evaluable: 32/35 (91.4%), 16/18 (89%) in the light group, 16/17 (94%) in the sham group ITT analysis: Unclear |
|
| Interventions |
Intervention 1 Home use light emitting diode (LED) device (blue and red light) Number and frequency of treatments: 56 in total, twice a day for 4 weeks Wavelength/Fluence/Duration/Spot size: 420 + 660 nm/0.91 + 1.22 J/cm² per 2.5 min treatment Supplier: OCimple Light Therapy System MP 200 (Ceragemmedisys, Cheonan, Korea) Instructions to participants: "All patients were instructed to turn on the LED machines after closely contacting the light emitting plane to the acne lesions of forehead and both cheeks twice a day for 4 weeks. It takes 5 minutes in one irradiation session (2.5 min per each wavelength). participants were also educated to keep the usage record to check out the compliance." Intervention 2 Home‐use sham device Number and frequency of treatments: 56 in total, twice a day for 4 weeks Wavelength/Fluence/Duration/Spot size: Supplier: Unclear Instructions to participants: Please see above |
|
| Outcomes | Evaluation time points of review interest: 4 and 8 weeks after final treatment (also assessed at 2 and 4 weeks within treatment). Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Percentage change from baseline in ILs count (papules, pustules and nodules reported separately) 3. Percentage change from baseline in NILs count (open and closed comedones reported separately) Methods of assessing primary outcomes 1. VAS. "Disease‐free state was designated as 0, and acne state at the initial visit was set as 10. If patients felt that their acne had been aggravated in relation to the first visit, they could choose scores of greater than 10 for grading to allow the recording of any acne deterioration during clinical trial." 2. & 3. Acne assessments were conducted using individual lesion counts in the entire face ranging from hairline to jaw line. Secondary outcomes of review interest recorded 1. Investigator global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. "The IGA score was used for clinical grading, and dermatological assessments were performed blind by three independent dermatologists. To ensure the reliability of our evaluation, standardized digital photographs were taken prior to the initiation of the LED treatment and at each follow‐up visit using identical camera settings (Nikon D70, Nikon Corp., Tokyo, Japan)." 2. Unclear |
|
| Notes | Language: English "Usage compliance was also periodically (twice weekly) monitored via telephone interviews and electronic mail during whole study period." Results for all outcomes other than IGA reported in graph format. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A blocked random allocation sequence was created by computer generated random numbers, and allocation to the either one of the two groups was performed by a research nurse." Comment: We judged this as adequate and risk of bias as low. |
| Allocation concealment (selection bias) | Low risk | Quote: "All dermatologists, research nurse, and patients were unaware of the group assignments. Randomization codes were secured until all data entry was complete." Comment: We judged this as adequate and risk of bias as low. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "It has a completely similar look to the active device, but does not deliver any therapeutic light."; "All dermatologists, research nurse, and patients were unaware of the group assignments." Comment: We judged this as adequate and the risk of bias as low. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Low risk | Please see above. We judged the risk of bias as low. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Please see above. We judged the risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures obtained for over 80% of participants in each group so we judged the risk of bias as low. |
| Selective reporting (reporting bias) | High risk | The protocol for the study was not available. Results for all outcomes other than IGA reported in graph format. We judged the risk of bias as high. |
| Other bias | Unclear risk | Study authors declared no conflicts of interest, it is however unclear who provided the device. Insufficient information to permit clear judgement. |
Lee 2010.
| Methods | This was a split‐face RCT. Unit of randomisation: Left and right face Power calculation: No Ethical approval: Yes Sponsorship and conflict of interest: Sponsorship not declared, however further information provided that study was not funded by commercial sponsors. Setting: Single centre (Seoul, Korea) Recruitment: By posters to the public Duration: Start and end dates were not reported |
|
| Participants |
Included Age (inclusion criterion; mean; range): 18‐40 years, 23 years, 19‐28 years Clinically evident acne: Yes Severity of condition assessment: "...with inflammatory acne"; "...in the treatment of moderate to severe inflammatory acne vulgaris" (Burton grade 3‐5) Fitzpatrick skin types: III Excluded Topical acne treatment or systemic antibiotics 2 weeks prior to the trial; systemic retinoids 3 months prior to the trial; a history of photosensitivity or recent use of photosensitising drugs; any skin disease that could interfere with the assessment of the acne; systemic diseases which could affect the severity of acne by themselves or by any medicine prescribed for their treatment; a history of the use of systemic steroids; any change in the use of oral contraceptive pills or antiinflammatory drugs 3 months prior to the trial; pregnant or lactating women; subjects likely to show poor compliance with the protocol. Enrolled: 9 (M/F 4/5) Randomised: 9 Withdrawals/drop‐outs: 0 Final number and proportion of participants evaluable: 100% ITT analysis: Yes |
|
| Interventions |
Intervention 1 Full‐spectrum light generated by high‐energy electrical discharge between carbon arc rods Number and frequency of treatments: Twice a week for 4 weeks Wavelength/Fluence/Duration/Spot size: Not applicable Supplier: BMC Korea, Anyang, South Korea Instructions to participants: Not applicable Intervention 2 1% clindamycin topically Number and frequency of treatments: Twice a day, duration: 4 weeks Supplier: Not reported Instructions to participants: Demonstrated how to apply |
|
| Outcomes | Evaluation time points of review interest: 2, 4 and 8 weeks after final treatment (also assessed every week within treatment) Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Percentage change from baseline in number of ILs Methods of assessing primary outcomes 1. Subjects rated the treatment on a non‐standardised scale. Values 'worse', 'no change', 'fair', 'good', 'excellent'. 2. Lesion counts Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. "Patients were asked about any adverse effects or feelings (e.g. burning sensation, itching, redness, tingling...etc) and also examined by the medical staff for any side effects (e.g. erythema, hyperpigmentation, etc..). All patients were also asked to report any long‐term side effect throughout the follow‐up period." |
|
| Notes | Language: English. This was a conference proceeding. Study authors were contacted and additional data supplied about power calculation, ethical approval, recruitment, exclusion criteria, age, severity of condition assessment, Fitzpatrick skin types, sex, withdrawals, ITT, duration of treatment with clindamycin, instructions to the participants, funding, study protocol, methods of assessing primary outcome and adverse effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment and control sides were allocated at random." Comment: Method used to generate the allocation sequence was not stated. Additional data provided: "same numbers of folded papers that was written as either 'left' or 'right' were well mixed in a black box. Subjects were asked to pick one paper from the box and gave it to a research nurse who was temporarily hired for the study." |
| Allocation concealment (selection bias) | Low risk | Intention and/or method to conceal the allocation sequence were not specifically reported. Additional data was provided as stated above. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No intended blinding of participants/performing clinicians reported. Additional data provided: "the research nurse wrote in a note which side of the subject’s face would be treated and performed the light treatment to the subjects. The note was kept in a locked drawer which only that nurse could access. Subjects could not be blinded to which side was 'treatment' side; subjects used clindamycin themselves to one side of their face (control side)." The author also stated "this treatment is not performer‐dependent (this is not a laser). To treat, the research staff only needed to place a patient in front of the light device and switch the device on." We judged this as high risk. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | High risk | Participants were not blinded so we judged the risk of bias as high for participant‐assessed outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | It was unclear whether assessors were blinded. Additional data provided: "the research nurse performed the treatment in a separate area of the building and was not able to communicate with the assessors according to the study policy. Two dermatologists who did the assessment were blinded to which side was treated with the light therapy. They did not do the treatment themselves and could not access the note that contained the information of which side was treated on which patient." We judged the risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Insufficient information was given to permit a clear judgement. Additional data provided and there was no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Insufficient information was given to permit a clear judgement. Additional data provided on outcomes and all of them appear to be reported. |
| Other bias | Low risk | No other possible source of bias identified. |
Leheta 2009.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared. No conflicts of interest and commercial sponsorships (page 124) Setting: Single centre (Cairo, Egypt) Recruitment: Dermatology outpatient clinic, Faculty of Medicine, Cairo University Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 18 years; not given; 18‐30 years Clinically evident acne: Yes Severity of condition assessment: "mild to moderate facial acne" Fitzpatrick skin types: Not reported Other: General good health, willingness and ability to comply with the requirements of the protocol. Oral and topical treatments stopped 4 weeks prior to the study commencement. Excluded Pregnant or lactating females, nodulocystic acne, active infection, herpes simplex or zoster, bacterial folliculitis, use of isotretinoin in the last 12 months, history of keloid scarring, and pigmentation abnormalities in the treatment areas Enrolled: 75 screened for eligibility (M/F not reported) Randomised: 45 randomised (15 in each group) Withdrawals/drop‐outs: 2 in Intervention 1 group (neither received laser treatment: 1 was lost to follow‐up, 1 disqualified by taking prohibited medications), 2 in Intervention 2 (neither received topical therapy, reasons not stated), none in Intervention 3 group. Final number and proportion of participants evaluable: Intervention 1: 13 (87%); Intervention 2: 13 (90%) Intervention 3: 15 (100%); Total: 41 (91%) ITT analysis: No |
|
| Interventions |
Intervention 1 PDL, non‐overlapping pulses in a "painting" motion Number and frequency of treatments: 6 in total, applied every 2 weeks Wavelength/Fluence/Duration/Spot size: 585 nm/3 J/cm²/pulse duration 350 µs/7 mm² Supplier: RegenLite Instructions to participants: Not applicable Intervention 2 5% benzoyl peroxide cream applied each morning and Tretinoin 0.1% cream applied every evening Number and frequency of treatments: Frequency stated above, duration unclear Supplier: Not given Instructions to participants: Adequate Intervention 3 Retinoic acid cream (0.025%) "at bedtime" for 2 weeks prior to trichloroacetic acid (TCA) peeling – face cleaned with alcohol and then degreased with acetone. TCA 25% was then applied quickly with a cotton‐tipped applicator – repeated every 2 weeks for 6 sessions then monthly during the follow up period (for 8 months?). Number and frequency of treatments: Number and frequency of TCA peeling stated above Supplier: Not given Instructions to participants: Adequate |
|
| Outcomes | Evaluation time points of review interest: Monthly for 8 months after (final laser?) treatment (also assessed within treatment, time points unclear). Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Investigator’s global assessment of improvement 3. Adverse effects Methods of assessing secondary outcomes 1. Leeds acne scoring system 2. Global response to treatment was rated as: marked response (> 75% improvement), moderate response (51%–75% improvement), mild response (25%–50% improvement), minimal response (< 25% improvement), no change, or worsening, using photographs 3. 5 grades (0–4) as: none (0), trace (1), mild (2), moderate (3), marked, or severe (4) |
|
| Notes | Language: English. Not reported whether assessment of compliance was performed. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 120): "These 45 patients were randomly equally divided into three groups." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes (page 119): "Because of the three different interventions used, blinding of study participants could not be achieved."; "Treatments were performed by a single physician, who did not participate in the clinical evaluation of patients." Comment: We judged this as at a high risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quotes (page 119): "Assessors were blinded to the intervention status of participants."; "A blinded evaluator performed the clinical assessment from baseline through the 8 months of follow‐up.". Comment: We judged the this as at low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 91.1% participants randomised in the whole trial were included in the analysis (86.6% of randomised participants in the first group, 86.6% in the second and 100% in the third. We judged this as at a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported. |
| Other bias | Low risk | No other possible source of bias identified. We judged this as at a low risk of bias. |
Ling 2010.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Multicenter (Wujiang and Suzhou, China) Recruitment: Not reported Duration: 8 months, January 2010‐August 2010 |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; mean and range reported for groups and not the whole sample. Group 1 (12‐32 years, mean 22); Group 2 (15‐31 years, mean 21); Group 3 (17‐26 years, mean 21); Group 4 (18‐27 years, mean 22) Clinically evident acne: Yes Severity of condition assessment: "Moderate or severe acne" using Pillsbury classification Fitzpatrick skin types: Not given Excluded Pregnant or breastfeeding, light‐sensitive skin, internal organ diseases such as liver, kidney or blood disease, taking any other medication during treatment Enrolled: 30 (14 M/16 F) in group 1; 30 (16 M/14 F) in group 2; 30 (20 M/10 F) in group 3; 30 (18 F/12 M) in group 4 Randomised: 30 in each group, 120 in total Withdrawals/drop‐outs: None Final number and proportion of participants evaluable: 30 (100%) in each group; 120 (100%) in total ITT analysis: Not stated |
|
| Interventions |
Intervention 1 (A) Blue and red light + sulfotanshinone 4 tablets 3 times a day orally Number and frequency of treatments: Twice weekly, for 4 weeks Wavelength/Fluence/Duration/Spot size: 415 + 3 and 633 + 3 nm/105 mW/cm² and 126 J/cm² (red light); 40 mW/cm² and 48 J/cm² (blue light)/duration 20 minutes/spot size not given Supplier: Omnilux Instructions to participants: Unclear Intervention 2 (B) Sulfotanshinone 4 tablets three times a day orally, no light treatment Number and frequency of treatments: Not specifically reported, presumably same as in Intervention 1 Supplier: Not reported Instructions to participants: Unclear Intervention 3 (C) Blue and red light + sulfotanshinone 4 tablets three times a day + prednisolone 5 mg 3 times a day Number and frequency of treatments: Twice weekly, for 4 weeks Wavelength/Fluence/Duration/Spot size: 415 + 3 and 633 + 3 nm/105 mW/cm² and 126 J/cm² (red light); 40 mW/cm² and 48 J/cm² (blue light)/duration 20 minutes/spot size not given Supplier: Omnilux Instructions to participants: Unclear Intervention 4 (D) Sulfotanshinone 4 tablets 3 times a day + prednisolone 5 mg 3 times a day Number and frequency of treatments: Not specifically reported, presumably same as in Intervention 1 Supplier: Not reported Instructions to participants: Unclear |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement Methods of assessing secondary outcomes 1. Non‐standard scale based on percentage change in combined lesion counts (Full recovery: improvement percentage > 95%; good improvement: improvement percentage 60% to 95%; effective: improvement percentage 20% to 59%; no effect: improvement percentage < 20%) |
|
| Notes | Language: Mandarin. English translation was not available. Data extraction was done by native speaker Quan Yang from the original paper. We have not attempted to contact the study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1): "..patients with mid‐severity acne all given Sulfotanshinone 4 tablets three times a day orally; patients were then randomised into either group A (1. With additional blue and red light treatment) or group B with medical treatment only. Patients with severe acne all given Sulfotanshinone 4 tablets three times a day + prednisolone 5 mg three times a day; Patients were then randomised into group C (3. blue and red light + drugs) and D (medication only).." Comment: The method used to generate the allocation sequence not described. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/ performing clinicians reported. No evidence that participants/clinicians were blinded provided. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | It was unclear whether assessors were blinded. Insufficient information was given to permit a clear judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were obtained for all randomised participants. |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the methods section were reported. |
| Other bias | Unclear risk | Sponsorship and/or potential conflicts of interest were not declared. Insufficient information to permit clear judgement. The study was in Mandarin and potential bias has been introduced by the fact that we have only been able to do single rather than double data extraction. |
Liu 2011.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes. "The study protocol was approved by the ethics committee of Institutes of Biomedical Sciences of Fudan University" Sponsorship and conflict of interest: Not declared Setting: Unclear whether single or multicenter, unclear location, Shanghai? (China) Recruitment: "Patients were recruited by advertising the experiment publicly" Duration: Unclear |
|
| Participants |
Included Age (inclusion criterion; mean; range): 18‐40 years; 23.6 years; 19‐28 years Clinically evident acne: Yes Severity of condition assessment: "mild to moderate level of acne vulgaris in GAGS (Global Acne Grading System)" Fitzpatrick skin types: III‐IV Excluded "…pregnancy, lactation, history of allergic to sunlight or any other photosensitizer, oral contraceptive medication during the past six months, systemic disease with complications with dermatological diseases, systemic and/or topical antibiotic treatment during the past two weeks, and treatment of other medication against acne vulgaris during the past four weeks." Enrolled: Unclear (M/F unclear), unclear how many participants in each group Randomised: Unclear Withdrawals/drop‐outs: Unclear Final number and proportion of participants evaluable: 20 (6 M/14 F) in total, 10 (4 M/6 F) in the blue light and 10 (2 M/8 M) in the red‐light group. Proportions unclear as initial numbers of enrolled/randomized participants were not reported. ITT analysis: Unclear |
|
| Interventions |
Intervention 1 Blue LED portable device; "the power of 30 mW/cm² (at the distance of 2 cm away from the face)"; "with the illumination area of about 10 cm²"; "Eucerin Cleanse Gel was used to cleanse face before exposure to light sources…After wearing the protective glasses, patients held the light sources to illuminate different facial areas moving in the repeating sequence of forehead, left cheek, chin, right cheek, and T‐shape area (nose). It took about 10 s for each area, and 20 min for one session. In each session, there were about 20 cycles of illumination and the corresponding light doses received in each session were 7.2 J/cm² and 11.52 J/cm²." Number and frequency of treatments: 8 in total, twice a week (two days interval) over four weeks Wavelength/Fluence/Duration/Spot size: 405 ± 10 nm/ see above Supplier: Rainbow Communications Corp. (CA, USA); Eucerin, Germany Instructions to participants: "Patients were asked not to put up make‐ups before treatment… Before the first session, researchers taught patients how to use the device correctly." Intervention 2 Red LED portable device; "the power of 48 mW/cm² (at the distance of 2 cm away from the face)"; "with the illumination area of about 10 cm²"; "Eucerin Cleanse Gel was used to cleanse face before exposure to light sources…After wearing the protective glasses, patients held the light sources to illuminate different facial areas moving in the repeating sequence of forehead, left cheek, chin, right cheek, and T‐shape area (nose). It took about 10 s for each area, and 20 min for one session. In each session, there were about 20 cycles of illumination and the corresponding light doses received in each session were 7.2 J/cm² and 11.52 J/cm²." Number and frequency of treatments: 8 in total, twice a week (two days interval) over 4 weeks Wavelength/Fluence/Duration/Spot size: 630 ± 10 nm/ see above Supplier: Rainbow Communications Corp. (CA, USA); Eucerin, Germany Instructions to participants: "Patients were asked not to put up make‐ups before treatment… Before the first session, researchers taught patients how to use the device correctly." |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment (also assessed at each treatment session) Primary outcomes of review interest recorded 1. Participant's global assessment of improvement 2. Change and percentage change from baseline in ILs count (papules and pustules) Methods of assessing secondary outcomes 1. "Subjective evaluation was based on the observations of face skin and communications between the patient and researcher (for the follow‐ups)." Further details not given. 2. "Photographs of patients' faces were captured by the camera of Canon IXUS 90, under the mode of macrophotography and non flashing.’…Photographs taken as above were evaluated by skilled observer to count lesions in different areas of face, which were forehead, left and right cheeks, chin, and nose. Inflammatory lesions were divided into papules and pustules." Secondary outcomes of review interest recorded 1. Investigator's global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Non standardised scale (reduction ≥ 90% = ‘full recovery’; 60% to 89% reduction = ‘significant improvement’, 40% to 59% reduction = ‘moderate improvement’, 20% to 39% reduction = ‘mild improvement’, and ≤ 19% reduction = ‘non‐ improvement or aggravation’) 2. "The patients were questioned about the side effects (erythema, pain, hyperpigmentation, dryness, etc.)." |
|
| Notes | Language: English. We attempted to contact the study authors but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 46): "…and then divided into blue and red groups randomly, equal for each group." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants reported. No evidence that participants were blinded provided. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | No evidence that participants were blinded provided. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quotes (page 46): "Photographs of patients' faces were captured by the camera of Canon IXUS 90, under the mode of macrophotography and non flashing."…(page 47): "Photographs taken as above were evaluated by skilled observer to count lesions in different areas of face, which were forehead, left and right cheeks, chin, and nose. Inflammatory lesions were divided into papules and pustules. All evaluations were conducted by one observer blindly to decrease random errors." Comment: We judged this as adequate and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only number of participants who completed the study reported. Not reported whether there were participants who withdrew or were lost to follow‐up. We judged this as at unclear risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | Results not reported for primary outcome participants’ global assessment of improvement nor for adverse effects for both groups separately. We judged this as at unclear risk. |
| Other bias | High risk | Funding and possible conflicts of interest unclear. Significant baseline imbalances (mean number of ILs in the blue‐light group was 19.2, whereas in the red light only 8.2). The study authors defined and calculated efficacy differently for the blue‐light group and the red‐light group. For blue light, they included all those with a moderate or above improvement; while, in the red‐light group, they considered all those with a mild or above improvement in the calculation. |
Liu 2014.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person. Participants were randomised to 3 different light treatments. "In each patient, the right side of the face was treated and the left side remained untreated as a control." Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Declared. "This work was supported by grants from the Foundation of Capital Medical Development and Research (No. 2007–3027) and the Second Five‐Year Plan of Military Medical Science and Technology Research Foundation (No. CWS11J218)". Setting: Single centre, Beijing (China) Recruitment: "from the outpatient clinic at the Department of Dermatology, General Hospital of Beijing Military Region of People’s Liberation Army (PLA)" Duration: 27 months, July 2009 to October 2011 |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; 26.8 years; 16 to 36 years Clinically evident acne: Yes Severity of condition assessment: "moderate to severe facial acne, according to the Burton classification" Fitzpatrick skin types: Not reported. Excluded "the use of any topical acne treatment or systemic antibiotics within 2 weeks or the use of systemic retinoids within 3 months before the start of the study; a history of photosensitivity or the use of photosensitizing drugs in the 3 months prior to the study; any other skin diseases that could interfere with the assessment of acne; any other systemic diseases or treatments that could affect the severity of acne; previous use of systemic steroids; any change in the use of oral contraceptive pills or anti‐inflammatory drugs within the 3 months before the study; pregnancy or lactation in women; and a likelihood of poor compliance with the protocol" Enrolled: 150 (92M/58F), 50 in each group Randomised: 150 Withdrawals/drop‐outs: Unclear Final number and proportion of participants evaluable: Unclear ITT analysis: Unclear |
|
| Interventions |
Intervention 1 5% ALA‐PDT, skin was cleaned with water and ALA "in a matrix that was applied topically to acne lesions for 1 h and covered by a light‐shielding dressing" Number and frequency of treatments: treatments were continued until ≥ 90% clearance of lesions was achieved (3 ± 1.52 treatments), applied weekly Wavelength/Fluence/Duration/Spot size: 633 ± 6 nm/126 J/cm²/duration 20 min Supplier: Shanghai Fudan Zhangjiang Bio‐Pharmaceutical Co., Ltd, Shanghai, China; Omnilux Revive system (Photo Therapeutics Ltd, Fazeley, UK) Instructions to participants: Not applicable Intervention 2 IPL, "Before IPL irradiation, a water‐based gel was applied to the target areas." Number and frequency of treatments: treatments were continued until ≥ 90% clearance of lesions was achieved (6 ± 2.15 treatments), applied weekly Wavelength/Fluence/Duration/Spot size: 420 nm/11‐15 J/cm²/30‐40 ms (pulse duration)/unclear Supplier: Harmony AFT laser handpiece (Alma Lasers, Caesarea, Israel) Instructions to participants: Not applicable Intervention 3 Blue–red light‐emitting diode (LED). During each treatment, blue light at 415 ± 5 nm was administered first for 20 min, followed by red light at 633 ± 6 nm for 20 min. Number and frequency of treatments: treatments were continued until ≥ 90% clearance of lesions was achieved (9 ± 3.34 treatments), applied weekly Wavelength/Fluence/Duration/Spot size: 415 ± 5 nm first and then 633 ± 6 nm/40 mW/cm² for the blue light and 105 mW/cm² for the red light/20 minutes for each wavelength (see above)/unclear Supplier: Omnilux Blue and Omnilux Revive systems (Photo Therapeutics Ltd) Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: None, please see 'Notes' (at 4 weeks within treatment and 3 months after final treatment) Primary outcomes of review interest: not recorded. Please see 'Notes' Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. "Patients were also asked about any symptoms of adverse side effects at the end of each treatment session." |
|
| Notes | Language: English. Comparison of interventions and outcomes at time points as defined by our protocol was not possible. Duration and number of treatments differed among the groups, as participants were treated "until ≥ 90% clearance of lesions was achieved". We attempted to contact the study authors but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 247): "Patients were randomly assigned in equal numbers to the three phototherapy groups. The randomisation was carried out by patients drawing lots between opaque sealed envelopes that contained cards with 'PDT', 'IPL' or 'LED' to represent the three different phototherapy treatment groups." Comment: We judged this as adequate and at a low risk of bias. |
| Allocation concealment (selection bias) | Low risk | See above. Opaque sealed envelopes were used. We judged this as adequate and risk of bias as low. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes of interest for our review. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Intention and/or method to blind the assessing physicians were not specifically reported. We judged this as at unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported whether there were participants who withdrew or were lost to follow‐up. We judged this as at unclear risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | Numbers of participants who withdrew or were lost to follow‐up was not reported. We judged this as at unclear risk of attrition bias. |
| Other bias | Low risk | We did not identify other possible sources of bias. |
McGill 2008.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear. Quote (page 244): "…we aimed to recruit 40 patients for the study. However, over an 18‐month period only 14 patients were referred for the study from Dermatology out‐patients. Out of these 14 participants, 4 failed to meet the above inclusion criteria, which left 10 participants to undergo treatment in the study" Ethical approval: Yes, "local hospital ethics committee approved the study" Sponsorship and conflict of interest: Not declared Setting: Unclear (Aberdeen, Scotland, UK?) Recruitment: "Patient recruitment for this study took place via Dermatology outpatient departments in the West of Scotland. A letter, and subsequent reminder, was sent out to each Consultant Dermatologist asking them to consider acne patients attending their outpatient clinics for recruitment to our study." Duration: Unclear, 18 months? |
|
| Participants |
Included Age (inclusion criterion; mean; range): Unclear: 30 years; 18‐47 years Clinically evident acne: Yes Severity of condition assessment: "with mild to moderate acne", Leeds scale used Fitzpatrick skin types: I‐III inclusion criterion (only I‐II recruited) Other: "at least a year since cessation of treatment with Isotretinoin; patients either on no treatment or on long term antibiotics" Excluded "…patients with severe acne; Fitzpatrick skin types IV‐VI; patients either currently being treated with Isotretinoin, or who have taken Isotretinoin within the last year; patients either starting or stopping antibiotic treatment within two weeks of starting the study, or during the study or follow‐up period" Enrolled: 14 Randomised: 10 (3 M/7 F) Withdrawals/drop‐outs: 2 withdrew ("failed to complete the treatment side of the study"), 1 lost to follow‐up at 3 months, 2 lost to follow‐up at 10 months. Final number and proportion of participants evaluable: 8/10 (80%) at 1 month, 7/10 (70%) at 3 months and 5/10 (50%) at 10 months ITT analysis: Not reported |
|
| Interventions |
Intervention 1 IPL, ‘upper’ and ‘lower’ halves of face sides treated with different filters; 550‐1100 nm filter (‘585 filter’), and the ‘Dual band’ filter (blue light); "epidermal cooling was achieved using a thin layer of ECG gel and air cooling" Number and frequency of treatments: 5 treatments, 2‐weekly intervals Wavelength/Fluence/Duration/Spot size: 500‐1100 nm, 400‐700 nm and 800‐1200 nm filters/for both filters the fluence was increased, as tolerated by the participant, during the course of treatment; 12‐22J/cm² (for the ‘585 filter’) and 8‐12J/cm² (for the ‘Dual band’)/2 pulses at a 20 ms delay between pulses /3 x 1 cm quartz block Supplier: Lynton Lasers Ltd., Cheshire, England; Cryo 5, Zimmer MedizinSystems, Irvine, Ca Instructions to participants: Not applicable Intervention 2 No treatment? |
|
| Outcomes | Evaluation time points of review interest: 1, 3, and 6 months after final treatment Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Changes in quality of life 3. Adverse effects Methods of assessing secondary outcomes 1. "The revised Leeds Acne scale (O’Brien et al 1998) was used to assess clinical photographs" 2. "The outcome of treatment was assessed using patient questionnaires and assessment of clinical photographs. The questionnaire used in this study was the Dermatology Life Quality Index (DLQI), designed by Finlay and Khan (1994)... Patients were asked to complete the questionnaire before treatment started and then at each of the follow‐up points (1, 3 and 6 months), to assess any changes in quality of life after treatment." 3. 'In addition to these outcome measures, any side effects of treatment were recorded during the course of treatment" |
|
| Notes | Language: English. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 246): "Envelopes were made up randomising the IPL treatment to either ‘right’ or ‘left’, to denote the side of the face to be treated, and also into ‘upper’ and ‘lower’ halves to denote the half of the face to be treated with the 585 filter and hence the other half to be treated with the Dual‐Band filter. The envelopes were opened immediately prior to laser treatment." Comment: We judged this as adequate and risk of bias as low. |
| Allocation concealment (selection bias) | Low risk | See above. We judged this as adequate and risk of bias as low. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Intention and/or method used to blind performing clinicians and/or participants were not specifically reported. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | Intention and/or method used to blind participants were not specifically reported. We therefore judged the risk of bias as unclear. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 246): "Response to treatment was then measured at 1, 3 and 6 months after the final treatment using photographs and repeat DLQI questionnaires…Photographs were taken pre‐treatment and at 1, 3 and 6 months posttreatment. A blinded observer assessed the photographs, with the photographs in random order to reduce the chances of bias in interpretation." Comment: We judged this as adequate for investigator‐assessed outcomes and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 80% of randomised participants were included in the analysis at 1 month follow‐up, but only 70% at 3 month follow‐up and 50% at 10 month follow‐up, so we judged this as at high risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the methods section were reported. |
| Other bias | Unclear risk | Sponsorship or potential conflicts of interest were not declared. Insufficient information was given to permit a clear judgement. |
Mei 2013.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: No Ethical approval: Yes Sponsorship and conflict of interest: Declared. No conflicts of interest and no commercial sponsors. Fudan Biopharmaceuticals as a supplier is the university spin off Setting: Single centre, Shanghai (China) Recruitment: "...were selected for the treatment from the dermatology clinic of Tongji Hospital" Duration: 6 months, March 2012‐August 2012 |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; 24 years; not stated Clinically evident acne: Yes Severity of condition assessment: "II–IV facial acne according Pillsbury grade" (moderate to severe) Fitzpatrick skin types: II‐IV Other: Concomitant treatment was not permitted Excluded "...exposed to systemic retinoid treatment in last 6 months, systemic antibiotics treatment or contraceptive and photosensitive drugs in last 1 month, local acne drug treatment in the last 2 weeks, patients with a tendency to form keloids or with a history of photosensitivity, and women in pregnancy or breastfeeding" Enrolled: 41 (24 M/17 F) Randomised: 21 in the ALA‐IPL‐PDT group, 20 in the placebo cream + IPL group Withdrawals/drop‐outs: None Final number and proportion of participants evaluable: 41 (100%) ITT analysis: No |
|
| Interventions |
Intervention 1 Facial skin cleaning, 10% ALA emulsion application, 1 h occlusion with plastic film followed by IPL illumination Number and frequency of treatments: 4 in total, applied weekly Wavelength/Fluence/Duration/Spot size: 420–950 nm/10‐13 J/cm²/30‐50 ms pulse width/15 x 40 mm² Supplier: Shanghai Fudan‐Zhangjiang BioPharmaceutical Co., Ltd., Shanghai, China; LovelyI, Alma Lasers, Caesarea, Israel Instructions to participants: Not applicable Intervention 2 Placebo oil‐in‐water emulsion application, 1 h occlusion with plastic film followed by IPL illumination Number and frequency of treatments: 4 in total, applied weekly Wavelength/Fluence/Duration/Spot size: 420–950 nm/10‐13 J/cm²/30‐50ms/15 x 40 mm² Supplier: LovelyI, Alma Lasers, Caesarea, Israel Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4, 8 and 12 weeks after final treatment (also assessed 1 week after each session whilst on treatment) Primary outcomes of review interest recorded 1. Percentage change from baseline in IL count (papules, pustules and nodules reported separately) 2. Percentage change from baseline in NIL count (open and closed comedones reported separately) Methods of assessing primary outcomes 1. & 2. "..the numbers of acne lesions were recorded by the same dermatologist separately from the forehead, the left and right cheeks, and the chin above the jaw line." Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. "Clinical improvement was assessed by a global rating scale: significant improvement (> 75%), moderate improvement (50–75%), mild improvement (25–50%), and no improvement (0–25%) relative to baseline." 2. "All adverse events including pruritus, pain, vesicles, erythema, hyperpigmentation, exfoliation, and exacerbation of lesions were recorded in detail at each treatment and follow‐up visit." |
|
| Notes | Language: English. The study authors were contacted and provided additional information on power calculation, concomitant treatment, study duration, withdrawals/lost‐to‐follow‐ups, ITT analysis, concealment of allocation sequence, blinding of participants, performing clinicians and outcome assessors. The study authors also clarified that means of ILs and NILs and SEs were reported on page 92 for time point 12 weeks after final treatment and that percentage reductions were reported in table 1 on page 92. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page ): "All patients were randomly divided into IPL plus ALA group (13 males and 8 females) or IPL only group (11 males and 9 females) by drawing lots." Comment: We judged this as adequate and risk of bias as low. |
| Allocation concealment (selection bias) | High risk | The study authors clarified that the allocation sequence was not concealed. We judged this as at high risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study authors clarified that the participants and performing clinicians were blinded as "ALA in an oil‐in‐water emulsion and only oil‐in‐water emulsion were respectively applied to acne lesions of participants in the IPL plus ALA group as well as the IPL only group". We judged this as adequate and risk of bias as low. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | The study authors clarified that assessors were unaware of the treatment status. We judged this as adequate and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results were reported for all participants and we judged the risk of bias as low. |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the methods section were reported. |
| Other bias | Low risk | We did not identify other possible sources of bias. |
Moneib 2014.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Sponsorship not declared. No conflicts of interest (page 1191) Setting: Unclear whether single or multicenter; Cairo? (Egypt) Recruitment: "…patients were included…upon their request due to failure of other treatments" Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): unclear; 21.5 ± 6.09 years; 15 to 38 years Clinically evident acne: Yes Severity of condition assessment: Burton grade 2 > Fitzpatrick skin types: II‐V Other: "instructed to avoid using any systemic, topical, or other light based acne treatment during the course of the study" Excluded "Exclusion criteria for previous acne therapy included isotretinonin therapy within 6 months, systemic antibiotic therapy (for any indication) within 1 month, and topical acne preparations of intralesional steroid injections within 2 weeks of the start of laser treatment. Patients with active eczema, history of facial eczema, suspected hypersensitivity to lidocaine, pregnancy, and high exposure to sunlight or intraviolet light (tanning) were also excluded." Enrolled: 24 (5 M/19 F) Randomised: 24 Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: Unclear ITT analysis: Unclear |
|
| Interventions |
Intervention 1 Fractional Erbium Glass Laser, "2 passes in stamping mode and 1 pass in moving mode". Cooling with ice between passes. EMLA cream (lidocaine 2.5% and prilocaine 2.5%) was applied under occlusion on the treated side 30 minutes before each session. Number and frequency of treatments: 4 in total, at 2‐week intervals Wavelength/Fluence/Duration/Spot size: 1550 nm; unclear/unclear/50 x 50 mm² Supplier: Sellas Dinona, Deajeon, South Korea Instructions to participants: "Patients were instructed to apply sunscreen during treatment and for 3 months after the end of treatment" Intervention 2 No treatment |
|
| Outcomes | Evaluation time points of review interest: Every 3 months for 1 year after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Participant's global assessment of improvement 2. Change from baseline in IL count (papules, pustules and nodules reported separately) 2. Change from baseline in NIL count (open and closed comedones reported separately) Methods of assessing primary outcomes 1. "Standardized photographs were taken at baseline...; ...expressed the degree of improvement in percentages" 0 = no improvement; < 25% = mild improvement; 26% to 50% = moderate improvement; 51% to 75% = good improvement; 76% to 100% = excellent improvement 2. & 3. "Standardized photographs were taken at baseline, at every session, and at the end of treatment… Treatment efficacy was evaluated by lesion counts" Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. "Standardized photographs were taken at baseline...; ...expressed the degree of improvement in percentages" 0 = no improvement; < 25% = mild improvement; 26% to 50% = moderate improvement; 51% to 75% = good improvement; 76% to 100% = excellent improvement 2. Unclear |
|
| Notes | Language: English. Participants' and investigators' assessments of improvement not reported separately for treated and control face sides. We have not contacted the study authors for clarification (there was no contact e‐mail of the corresponding author and we were unable to find it through Google search). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1192): "The control side of the face was selected randomly by tossing a coin". Comment: We judged this as adequate and at a low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Intention and/or method to blind participants and personnel were not specifically reported. As anaesthetic cream was applied to the treated side of the face, they were probably not blinded. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | Intention and/or method to blind participants and personnel were not specifically reported. As anaesthetic cream was applied to the treated side of the face, they were probably not blinded. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Quote (page 1193): "Standardized photographs were taken at baseline, at every session, and at the end of treatment…"; "Patients and investigator were blinded to each other's answers during the study, not to influence one another" Comment: Unclear whether outcome assessors were blinded to the treatments side. We judged this as at unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Possible withdrawals and lost to follow‐ups were not reported. We judged this as at a unclear risk of attrition bias. |
| Selective reporting (reporting bias) | High risk | Evaluation was done every 3 months after treatment for a year, but results reported at only one "follow‐up" time‐point. Participants' and investigators' assessments of improvement not reported separately for treated and control face sides. We judged this as at high risk of bias. |
| Other bias | Unclear risk | Sponsorship not declared. We judged this as at unclear risk of bias. |
Na 2007.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face. Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (page 1128): "Authors ..have indicated no significant interest with commercial supporters" Setting: Single centre (Seoul, Korea) Recruitment: Dermatology Department, Seoul National University College of Medicine Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 23.6 years, 19‐33 years Clinically evident acne: Yes Severity of condition assessment: "mild to moderate acne" Fitzpatrick skin types: Not reported Excluded Pregnancy; use of oral contraceptives; treatment with oral antibiotics, topical agents, or chemical peels during the previous 4 weeks; oral retinoids during previous 6 months, eye problems, cystic acne Enrolled: 30 (7 M/23 F) Randomised: 30 Withdrawals/drop‐outs: 2 withdrawals, 6 drop‐outs. "Personal reasons" for withdrawal, reasons for drop‐out not stated Final number and proportion of participants evaluable: 28 (93%) 8 weeks within treatment, 25 (83%) 4 weeks post‐treatment, 22 (73%) 8 weeks post‐treatment ITT analysis: Not stated |
|
| Interventions |
Intervention 1 Portable device red light therapy Number and frequency of treatments: 112 treatments in total, twice a day during 8 weeks Wavelength/Fluence/Duration/Spot size: 635‐670 nm/cumulative dose of 604.8 J/cm²/other data not given Supplier: Softlaser SL30, Beurer GmbH &Co., Ulm, Germany Instructions to participants: Unclear whether adequate. "The patient was instructed to perform phototherapy only to the treatment side for 15 minutes twice a day for 8 weeks." (page 1229) Intervention 2 Nil |
|
| Outcomes | Evaluation time points of review interest: None (assessed at 1, 2, 4 and 8 weeks whilst on treatment, final evaluation at final treatment) Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Percentage change from baseline in number of ILs, NILs and combined lesions Methods of assessing primary outcomes 1. VAS: 0 (none) to 5 (very severe) 2. Lesion counts: open comedones, closed comedones, papules, nodules, pustules Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Not reported |
|
| Notes | Language: English. Final evaluation at final treatment, but included participants' assessments of improvement, showing early encouragement to continue with the treatment so we judged they met inclusion criteria. Results reported in a graph format. Baseline lesion counts for participants and face sides not reported; stated only ".. actual lesion counts varied significantly from patient to patient. For example, the number of closed comedones varied from 10 to 51 on one side". VAS results: unclear whether means were reported. Lesion counts reported in mean percentage changes, but no SDs. Timing of assessment for VAS out of our scope: at 8 weeks whilst on treatment (that is less than 2 weeks post treatment). We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1229): "The right or left side of the face was randomised to either treatment or control side." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 1229): "The patient was instructed to perform phototherapy only to the treatment side for 15 minutes twice a day for 8 weeks." Comment: No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. Participants were unblinded for the treatment side, and, given the nature of the intervention then it is unlikely that the personnel were blinded. We therefore judged the risk of bias as high. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | High risk | Participants were not blinded so we judged the risk of bias high for participant‐assessed outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 1229): "Clinical photographs were taken and lesion counts were performed on each side of the face, at baseline and at subsequent visits, by two independent investigators who were unaware of the treated side." Comment: We judged this as adequate and at low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 weeks whilst on treatment outcome measures reported for 93.3% of subjects randomised. Follow‐up outcomes reported for 83.3% randomised participants at week 4 post‐treatment and 70% at week 8 post‐treatment. We judged this as at high risk of bias. |
| Selective reporting (reporting bias) | High risk | Quote (page 1229): "The patients were followed for up to 8 weeks after discontinuation of red light treatment. Of the 25 patients examined for 4 weeks after treatment, 10 patients (40%) showed an increase in acne lesions. Of the 22 patients followed for 8 weeks after treatment, 21 patients (95%) complained of acne exacerbation compared with their status during the treatment period." Comment: Full reports of post‐treatment follow up not reported. |
| Other bias | Low risk | No other sources of bias identified. We judged this as at a low risk of bias. |
Na 2011.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Not declared Setting: Single centre, (Seoul, Korea) Recruitment: Dermatology Department, Seoul National University College of Medicine Duration: Start and end dates were not reported |
|
| Participants |
Included Age (inclusion criterion; mean; range): No data reported Clinically evident acne: Yes Severity of condition assessment: "with inflammatory acne", severity not specified Fitzpatrick skin types: Not reported Excluded Oral antibiotics, topical agents, or chemical peeling during the previous 4 weeks or oral retinoids during the previous 6 months Enrolled: 14 (M/F not reported) Randomised: 14 Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: Not reported ITT analysis: Not stated |
|
| Interventions |
Intervention 1 Indole‐3‐acetic acid (0.015%) was applied on one side of the face for 15 minutes and then green light was irradiated on the face for 15 minutes. Number and frequency of treatments: 3 treatments in total, applied every 2 weeks Wavelength/Fluence/Duration/Spot size: 520 nm/9 J/cm²/other data not given Supplier: Unclear. Possibly HL‐2000‐HP (OceanOptics Co., Dunedin, FL) – mentioned in first (different) study using green light in same paper Instructions to participants: Not applicable Intervention 2 Control base gel was applied on one side of the face for 15 minutes and then green light was irradiated on the face for 15 minutes Number and frequency of treatments: 3 treatments in total, applied every 2 weeks Wavelength/Fluence/Duration/Spot size: 520 nm/9 J/cm²/other data not given Supplier: Unclear. Possibly HL‐2000‐HP (OceanOptics Co., Dunedin, FL) – mentioned in first (different) study using green light in same paper Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 2 weeks after final treatment (also assessed at 2 and 4 weeks whilst on treatment) Primary outcomes of review interest recorded 1. Change from baseline of ILs (papules and pustules not reported separately) Methods of assessing primary outcomes 1. Lesion counts using photographs Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Not reported |
|
| Notes | Language: English. Data for primary outcomes presented in graph format only. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 201 ): "The treatment side was randomly determined." Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 201 ): "IAA (0.015%) was applied on one side of the face and control base was applied on the other." Comment: The study was described as "double blind" (page 202). Control gel was applied to other side of face, therefore participants were probably blinded. It was not clear whether clinicians were blinded, but we judged it was unlikely that there were systematic differences between face sides in the care that was provided, or in exposure to factors other than the interventions of interest. We therefore judged the risk of bias as low. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 201 ): "Evaluation was conducted at 0, 2, 4, 6 weeks by two dermatologists who did not know the treatment side, with clinical photographs." Comment: We judged this as adequate and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote (page 201 ): "Fourteen (14) patients with inflammatory acne were enrolled." Comment: Further data regarding withdrawals, lost‐to‐follow‐ups and the number of participants included in the analysis were not reported. We judged this as at an unclear risk of bias. |
| Selective reporting (reporting bias) | High risk | All outcomes were reported, but in graph format only, so we judged the risk of bias as high. |
| Other bias | Unclear risk | Sponsorship was not declared. Potential commercial sponsors, if there were any, might have introduced some bias. We judged this at an unclear risk of bias. |
NCT00594425.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Sponsored by Photocure Setting: Multicenter (15 centres: San Diego and Vallejo, California; Naperville, Illinois; Clinton, Michigan; Albuquerque, New Mexico; Rochester, New York; Norman, Oklahoma; Portland, Oregon; Dallas, Houston and San Antonio Texas; Salt Lake City, Utah; Lynchberg and Norfolk, Virginia, USA) Recruitment: "Advertisements and doctors internal database" Duration: 18 months (February 2007‐September 2008) |
|
| Participants |
Included Age (inclusion criterion; mean; range): 15‐40 years; 21.3; 15‐37 years Clinically evident acne: Yes Severity of condition assessment: "with moderate to severe facial acne vulgaris (IGA score 3‐4) ", "with 20 to 100 inflammatory lesions (papules, pustules, and nodules) on the face excluding lesions on the nose and in the periocular area", "with up to 200 non inflammatory lesions (open and closed comedones) on the face", "with no more than 2 nodular lesions on the face". The sponsor later clarified that over 80% of included participants had moderate acne. Fitzpatrick skin types: I‐IV Other: "surgically sterile, postmenopausal, abstinent, or willing to use an adequate means of contraception including birth control pills, or barrier methods and spermicide for at least 14 days prior to Day 0. Participants using birth control pills must have used the same product and dose for at least 3 months and must agree to stay with the same product and dose for an additional 3 months; willing and capable of following study instructions to the extent and degree required by the protocol; must sign the approved informed consent form prior to any study procedures; willing to be photographed; willing to sign a photography consent form." Excluded Allergy to MAL or similar PDT compound or to excipients of the cream; "participation in other clinical studies either concurrently or within the last 30 days"; "patients who have a condition or who are in a situation, which, in the investigator's opinion, may put the patient at risk, may confound the study results, or may interfere with the patient's participation in the study"; visible light sensitivity, porphyria or porphyrin sensitivity; UVB phototherapy within the last 30 days; topical treatments for acne in the last 14 days, oral in the last month, oral isotretinoin in the last 6 months, "with a beard or other facial hair that might interfere with study assessments" Enrolled: 150 (59 M/91 F) Randomised: 40 mg/g MAL‐PDT group: 50 (22 M/28 F); 80 mg/g MAL‐PDT group: 48 (21 M/27 F); placebo group: 52 (16 M/36 F) Withdrawals/drop‐outs: 7 in 40 mg/g MAL‐PDT group, 14 in 80 mg/g MAL‐PDT group, 10 in placebo group; "0 in the 40 mg group, 4 in the 80 mg group and 1 in the vehicle group were lost to follow up. 5, 8 and 9 respectively withdrew their consent" Further information not available ITT analysis: Yes |
|
| Interventions |
Intervention 1 40 mg/g MAL‐PDT, "in a thin layer on a clean skin", under occlusion for 1.5 h, followed by illumination with red light Number and frequency of treatments: 4 in total, 2 weeks apart Wavelength/Fluence/Duration/Spot size: 632 nm/37J/cm² Supplier: Photocure, Aktilite CL 128, Galderma Instructions to participants: Not applicable Intervention 2 80 mg/g MAL‐PDT, "in a thin layer on a clean skin", under occlusion for 1.5 h, followed by illumination with red light Number and frequency of treatments: 4 in total, 2 weeks apart Wavelength/Fluence/Duration/Spot size: 632 nm/37 J/cm² Supplier: Photocure, Aktilite CL 128, Galderma Instructions to participants: Not applicable Intervention 3 Placebo cream under occlusion for 1.5 h, "in a thin layer on a clean skin", followed by illumination with red light Number and frequency of treatments: 4 in total, 2 weeks apart Wavelength/Fluence/Duration/Spot size: 632 nm/37 J/cm² Supplier: Aktilite CL 128, Galderma Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 2, 3, 6, 12 and 24 weeks after final treatment (adverse effects also assessed whilst on treatment) Primary outcomes of review interest recorded 1. Change and percentage change from baseline in number of ILs (including nodules, papules, and pustules) 2. Percentage change from baseline in number of NILs (open and closed comedones) 3. Change from baseline in number of total lesions Methods of assessing primary outcomes 1., 2., & 3. "Live assessment done by trained assessor" Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Live assessment. "Proportion of patients with success according to dichotomised IGA scale based on facial assessments (success defined as an improvement of at least 2 grades from baseline score) at 6 weeks post‐treatment" 2. "Erythema score, hyperpigmentation score, hypopigmentation score, other local and non local adverse events"; details not given |
|
| Notes | Language: English. Photocure provided results preview of clinicaltrials.gov record. After extracting data from that record, Photocure provided additional information regarding ethical approval, recruitment, age of included participants, acne severity, mean baseline lesion counts, withdrawals/lost to follow‐ups, intervention details, outcome assessment methods, as well as information needed for selection, performance and detection bias assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An electronic randomisation list was generated using the SAS system." We judged this as adequate and risk of bias as low. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used for this purpose. We judged this as adequate and risk of bias as low. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Blinded packaging" was used to ensure that participants and performing investigators cannot distinguish between MAL and placebo cream."; "Each site had 2 investigators, the investigator responsible for the efficacy evaluations could not be involved in the treatment procedure or the safety assessments." We judged this as adequate and risk of bias as low. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | Such outcomes were not assessed in this study. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | "Each site had 2 investigators, the investigator responsible for the efficacy evaluations could not be involved in the treatment procedure or the safety assessments." We judged this as adequate and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome measures obtained for 71% (less than 80%) of participants randomised in 80 mg/g MAL group and for over 80% in other two groups. ITT analysis was performed. Please note the sponsors used both LOCF and ITT within their analyses to account for missing data. |
| Selective reporting (reporting bias) | High risk | 24 weeks after final treatment pre‐specified time point in the study protocol for percentage reduction in ILs, NILs and total lesion counts assessment but not reported. Absolute change in NILs prespecified as the study outcome in the protocol but not reported. Responsible parties clarified this as follows: "Among the treatment successes at week 12 it was optional to continue for further follow up. Data on 24 weeks were not presented due to the low number of patients (14) that were followed from week 12 to 24." We judged this as at high risk of bias. |
| Other bias | Unclear risk | Funded by Photocure. Insufficient information to judge whether additional bias was introduced. |
NCT00673933.
| Methods | This was a split‐back RCT. Unit of randomisation: 8 x 8 cm² back areas Power calculation: No Ethical approval: Yes Sponsorship and conflict of interest: Sponsored by Photocure Setting: Multicenter (Naperville, Illinois and Albuquerque, New Mexico; USA) Recruitment: "Advertisements and doctors internal database" Duration: 8 months (May 2008 to December 2008) |
|
| Participants |
Included Age (inclusion criterion; mean; range): 15‐40 years; 26; 14‐41 years Clinically evident acne: Yes Severity of condition assessment: "two areas of each 8x8 cm² on the back that include at least 5 inflammatory lesions (papules, pustules, and nodules) each...the minimum distance between the two areas should be at least 4 cm...no more than 2 nodular lesions in any of the two areas of each 8x8 cm² on the back" Fitzpatrick skin types: V and VI Other: "surgically sterile, postmenopausal, abstinent, or willing to use an adequate means of contraception including birth control pills, or barrier methods and spermicide for at least 14 days prior to Day 0. Patients using birth control pills must have used the same product and dose for at least 3 months and must agree to stay with the same product and dose for an additional 3 months; willing and capable of following study instructions to the extent and degree required by the protocol; must sign the approved informed consent form prior to any study procedures; willing to be photographed; willing to sign a photography consent form." Excluded Allergy to MAL or similar PDT compound or to excipients of the cream; "participation in other clinical studies either concurrently or within the last 30 days"; "patients who have a condition or who are in a situation, which, in the investigator's opinion, may put the patient at risk, may confound the study results, or may interfere with the patient's participation in the study"; visible light sensitivity, porphyria or porphyrin sensitivity; UVB phototherapy within the last 30 days; topical treatments for acne in the last 14 days, oral in the last month, oral isotretinoin in the last 6 months Enrolled: 20 (11 M/9 F) Randomised: 20 Withdrawals/drop‐outs: 1 "patient consent withdrawal" ITT analysis: No |
|
| Interventions |
Intervention 1 MAL 80 mg/g (MAL cream 8%) applied for 1.5 h, followed by red light illumination Number and frequency of treatments: 2 in total, 2 weeks apart Wavelength/Fluence/Duration/Spot size: 632 nm/37 J/cm² Supplier: Visionac, Photocure; Aktilite CL128, Galderma Instructions to participants: Not applicable Intervention 2 Placebo cream applied for 1.5 h, followed by red light illumination Number and frequency of treatments: 2 in total, 2 weeks apart Wavelength/Fluence/Duration/Spot size: 632 nm/ 37 J/cm² Supplier: Not reported, Aktilite CL 128, Galderma Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment (adverse effects also assessed whilst on treatment) Primary outcomes of review interest recorded 1. Change from baseline in number of ILs 2. Change from baseline in number of NILs Methods of assessing primary outcomes 1. & 2. Live assessment done by trained assessor Secondary outcomes of review interest recorded 1. Adverse effects (Erythema score and other local and non‐local adverse events, Hypopigmentation and hyperpigmentation score assessed after treatment) Methods of assessing secondary outcomes 1. Hypopigmentation and hyperpigmentation score assessed after treatment, "Standard spontaneous reporting." |
|
| Notes | Language: English. Photocure provided results preview of clinicaltrials.gov record. After extracting data from that record, Photocure provided additional information regarding ethical approval, power calculation, recruitment, age of included participants, withdrawals/lost to follow‐ups, intervention details, outcome assessment methods, as well as information needed for selection, performance and detection bias assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An electronic randomisation list was generated using the SAS system." Comment: We judged this as adequate and risk of bias as low. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used for this purpose. Comment: We judged this as adequate and risk of bias as low. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Blinded packaging" was used to ensure that patients and performing investigators cannot distinguish between MAL and placebo cream."Each site had 2 investigators, the investigator responsible for the efficacy evaluations could not be involved in the treatment procedure or the safety assessments." Comment: We judged this as adequate and risk of bias as low. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | Such outcomes were not assessed in this trial. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | "Each site had 2 investigators, the investigator responsible for the efficacy evaluations could not be involved in the treatment procedure or the safety assessments." Comment: We judged this as adequate and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures obtained for 85% of randomised participants. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes at all pre‐specified time points reported. |
| Other bias | Unclear risk | Funded by Photocure. Insufficient information to judge whether additional bias was introduced. |
NCT00706433.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Sponsored by DUSA Pharmaceuticals, Inc. Setting: Multicenter (14 centres: Hot Springs, Arkansas; San Diego, California; Denver, Colorado; West Palm Beach, Florida; Snellville, Georgia; Carmel, Indiana; Louisville, Kentucky; Fridley, Minnesota; Brooklyn, New York; Hershey, Pennsylvania; Nashville, Tennessee, Austin and Dallas, Texas; Salt Lake City, Utah, USA) Recruitment: Unclear, through medical clinics? Duration: recruitment 12 months (March 2007‐March 2008) |
|
| Participants |
Included Age (inclusion criterion; mean; range): 12 years of age or older; 20.1; range not reported Clinically evident acne: Yes Severity of condition assessment: "Subject has moderate to severe facial acne vulgaris (including the nose), with at least 20 inflammatory lesions (papules, pustules, nodules); Subject has moderate to severe acne as defined by an Investigator Global Assessment of 3 or 4 [0 (clear) to 4 (severe) scale]." Fitzpatrick skin types: I‐VI Other: "Subject is male or non‐pregnant female…; Females must be post‐menopausal, surgically sterile or using a medically acceptable form of birth control, with a negative urine pregnancy test at the Baseline visit; Subject has provided written and verbal informed consent. A subject under 18 years of age must be accompanied by the parent or legal guardian at the time of assent/consent signing. The parent or legal guardian must also provide informed consent for the subject; Subject has a history of recurrent herpes simplex labialis infection in the treatment area AND has had an outbreak within the last 12 months must be placed on antiviral prophylaxis as specified in the protocol; Subject is willing to comply with study instructions and return to the clinic for required visits; Subject must have used the same type and brand of make‐up, other facial products and hair products (e.g. shampoo, gel, hair spray, mousse, etc.) for at least 1 month prior to the Baseline Visit (General Skin & Hair Care). Upon enrolment, all subjects must a) use exclusively an Investigator approved facial cleanser and b) agree to continue their other General Skin & Hair Care for the entire study" Excluded "Subject is pregnant, lactating, or is planning to become pregnant during the study. Subject has a history of cutaneous photosensitization, porphyria, hypersensitivity to porphyrins or photodermatosis; Subject has any skin pathology or condition that could interfere with the evaluation of the test product or requires the use of interfering topical or systemic therapy; Subject has greater than 4 facial nodules (nodule = lesion greater than or equal 0.5 cm in diameter); Subject has an uncorrected coagulation defect or concurrently uses anticoagulants (except aspirin); Subject has any condition which, in the investigator's opinion, would make it unsafe for the subject to participate in this research study; Subject is currently enrolled in an investigational drug or device study; Subject has received an investigational drug or been treated with an investigational device within 30 days prior to the initiation of treatment (baseline); Subject has facial hair that could interfere with the study assessments in the opinion of the investigator; Subject is unable to communicate or cooperate with the investigator due to language problems, poor mental development, or impaired cerebral function; Subject may be unreliable for the study including subjects who engage in excessive alcohol intake or drug abuse, or subjects who are unable to return for scheduled follow‐up visits; Subject has a known sensitivity to one or more of the vehicle components (ethyl alcohol, isopropyl alcohol, laureth 4, polyethylene glycol); Subject has used photosensitizing drugs, e.g. declomycin, tetracycline, sulfa antibiotics, phenothiazines, etc. within a timeframe where photosensitization from these drugs may still be present; Subject has used OTC acne medicated cleansers or soaps within 2 weeks of the initiation of treatment; Subject has the need or plans to be exposed to artificial tanning devices or excessive sunlight during the trial. Subject has used any of the following topical anti‐acne preparations on the face: a.) Topical anti‐acne treatments including benzoyl peroxide, antibiotics, azelaic acid, corticosteroids and salicylic acid within 2 weeks of the initiation of treatment b.) Retinoids, including tazarotene, adapalene, tretinoin within 4 weeks of the initiation of treatment. c.) Light treatments, microdermabrasion or chemical peels within 8 weeks of the initiation of treatment; Subject has used any of the following systemic anti‐acne medications: a.) Corticosteroids (including intramuscular and intralesional injections) within 4 weeks of the initiation of treatment. Inhaled corticosteroids are allowed if use is stable (stable use is defined as dose and frequency unchanged for at least 2 weeks prior to the initiation of treatment). b.) Antibiotics within 4 weeks of the initiation of treatment. c.) Nicotinamide containing products within 4 weeks of the initiation of treatment. d.) Spironolactone within 8 weeks of the initiation of treatment. d.) Retinoid therapy within 6 months of the initiation of treatment." Enrolled: 266 (138 F/128 M) Randomised: 266 in total ("no enrolled participants excluded from the trial before assignment to groups"); 68 (30 F/38 M ) randomised to ALA 1000 s group; randomised to ALA 500 s group; randomised to Vehicle 1000 s group; randomised to Vehicle 500 s group Withdrawals/drop‐outs: 2 withdrawals (1 "by subject" and 1 "protocol violation") and 3 lost to follow‐up in ALA 1000 s group; 2 withdrawals (2 "by subject") and 1 lost to follow up in ALA 500 s group; 4 withdrawals (4 "by subject" and 1 "adverse event") and 6 lost to follow‐up in Vehicle 1000 s group; 2 withdrawals (1 "by subject" and 1 "new job") and 0 lost to follow‐up in Vehicle 500 s group Final number and proportion of participants evaluable: 246/266 (93%) in total; 63/68 (93%) in ALA 1000 s group; 62/65 (95%) in ALA 500 s group; 57/67 (85%) in Vehicle 1000 s group; 64/66 (97%) in Vehicle 500 s group ITT analysis: Yes, LOCF method |
|
| Interventions |
Intervention 1 Aminolevulinic acid HCL (ALA) applied to the entire facial area 45 minutes prior to blue light treatment for 1000 s (16 min and 40 s) Number and frequency of treatments: "up to four treatments at three week (± 2 days) intervals" Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Levulan® Kerastick® containing 20% aminolevulinic acid HCL (ALA), light source? Instructions to participants: Not applicable Intervention 2 Aminolevulinic acid HCL (ALA) applied to the entire facial area 45 minutes prior to blue light treatment for 500 s (8 min and 20 s) Number and frequency of treatments: "up to four treatments at three week (± 2 days) intervals" Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Levulan® Kerastick® containing 20% aminolevulinic acid HCL (ALA), light source? Instructions to participants: Not applicable Intervention 3 Vehicle applied to the entire facial area 45 minutes prior to blue light treatment for 1000 s (16 min and 40 s) Number and frequency of treatments: "up to four treatments at three week (± 2 days) intervals" Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Levulan® Kerastick® containing vehicle ingredients only, light source? Instructions to participants: Not applicable Intervention 4 Vehicle applied to the entire facial area 45 minutes prior to blue light treatment for 500 s (8 min 20 s) Number and frequency of treatments: "up to four treatments at three week (± 2 days) intervals" Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Levulan® Kerastick® containing vehicle ingredients only, light source? Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 3 and 6 weeks after final treatment; adverse effects also assessed at each treatment session. Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Investigator‐assessed change in lesion count (ILs) 3. Investigator‐assessed percentage change in lesion count (ILs) Methods of assessing primary outcomes 1. Subject satisfaction score; excellent (very satisfied), good (moderately satisfied), fair (slightly satisfied), poor (not satisfied at all) 2. Unclear 3. Unclear Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. "Investigator Global Assessment of Acne Severity Successes", "Scale consists of Grade 0 (clear skin) to Grade 4 (severe: up to many non‐inflammatory and inflammatory lesions, but no more than a few nodular lesions) This assessment uses a dichotomised success/failure assessment ‐ with success defined as a 2 point or more improvement on the IGA scale since baseline."; "0 Clear skin with no inflam or non‐inflam lesions; Almost clear; rare non‐inflam lesions with no more than a few small inflam lesions; Mild; > Grade 1; some non‐inflam lesions with some inflam lesions (papules/pustules only; no nodules); Moderate; > Grade 2; up to many non‐inflam lesions and a moderate number of inflam lesions but no more than one small nodule; Severe; > Grade 3; up to many non‐inflam and inflam lesions, but no more than a few nodules" 2. "Safety will be evaluated by adverse events and local skin responses reported during the study." |
|
| Notes | Language: English. The sponsors were contacted and replied, but were unable to provide additional data and clarifications, apart from those contained in the clinicaltrials.gov record for this study. Please note that ‘Hyperpigmentation’, ‘Hypopigmentation’, ‘Oozing/ Vesiculation/Crusting’, ‘Scaling and Dryness’, and ‘Stinging/Burning’ were evaluated at baseline, and were then assessed pre‐ and post‐ treatment and 48 h after treatment at each treatment session, as well as 3 and 6 weeks after final treatment. Detailed results can be found in the ‘Study results’ section of the clinicaltrials.gov record for this study (clinicaltrials.gov/ct2/show/results/NCT00706433). We only included outcomes reported as adverse effects in our report (Additional Table 5). The reported threshold above which other adverse events are reported was 5%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects will be randomized to one of the following four treatment groups (1:1:1:1) to receive topical Levulan® Kerastick® containing 20% aminolevulinic acid HCL (ALA, active study drug) or the Kerastick® containing vehicle ingredients only (VEH)." Comment: Method used for randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specifically reported, presumably not blinded, as per the official title stating only "Evaluator‐blinded". |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | Unclear whether participants were blinded, presumably not blinded, as per the official title stating only "Evaluator‐blinded". |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Investigators assessing outcomes were presumably blinded, as per the official title "Evaluator‐blinded", but the method was not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures obtained for 246/266 (93%) of all participants, and over 80% in each group, so we judged the risk as low. |
| Selective reporting (reporting bias) | Low risk | Results reported for all outcomes prespecified in the protocol at all time points, so we judged the risk as low. However, means were not reported nor provided upon request for investigator‐assessed changes and percentage changes in ILs. There were no reports of application site blisters among adverse effects, however it is possible that some occurred, but it is impossible to separate those as they were reported together with oozing and crusting under ‘Oozing/ Vesiculation/Crusting’. |
| Other bias | Unclear risk | Possibly different number of treatments applied ("up to four treatments at three week (± 2 days) intervals"). Role of sponsor unclear. |
NCT00933543.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Sponsored by Photocure. Setting: Multicenter (USA: San Diego, California; Chicago and Naperville, Illinois; Fridley, Minnesota, Rochester, New York; Hershey, Pennsylvania; Norfolk, Virginia; Madison, Wisconsin Canada: Windsor, Ontario; Montreal and Quebec, Quebec) Recruitment: 11 dermatology clinics/research centres in the USA and Canada, "Recruitment from September to December 2009, Dermatology Clinics with paediatric patients" Duration: 8 months (August 2009 to March 2010) |
|
| Participants |
Included Age (inclusion criterion; mean; range): 9‐35 years; 17.2; 11‐35 years Clinically evident acne: Yes Severity of condition assessment: "with moderate to severe facial acne vulgaris (IGA score 3‐4); with 20 to 100 inflammatory lesions (papules, pustules, and nodules) on the face", "with 30 to 120 non‐inflammatory lesions (open and closed comedones) on the face", "no more than 2 nodular lesions on the face". The sponsor later clarified that over 80% were people with moderate acne. Fitzpatrick skin types: I‐VI Other:"Female patients who are surgically sterile, pre‐menstrual, postmenopausal, abstinent, or willing to use an adequate means of contraception including birth control pills, or barrier methods and spermicide for at least 14 days prior to T1. Patients using birth control pills must have used the same product and dose for at least 6 months and must agree to stay with the same product and dose for an additional 6 months.", "Signed and verified informed consent form. For subjects under age of 18, an assent form in conjunction with an informed consent form, signed and verified by parent/guardian." Excluded "Patient is the investigator or any sub investigator, research assistant, pharmacist, study coordinator, other staff or relative thereof directly involved in the conduct of the protocol"; "unlikely to comply with the protocol, pregnancy, oral contraceptives not used as in inclusion criterion, pregnancy, systemic hormonal treatment of any kind,"; "hormonal contraceptives solely for control of acne"; "Allergy to MAL or similar PDT compound or to excipients of the cream;" "participation in other clinical studies either concurrently or within the last 30 days"; "visible light sensitivity, porphyria or porphyrin sensitivity; UVB phototherapy within the last 30 days; topical treatments for acne in the last 14 days, oral in the last month, oral isotretinoin in the last 6 months, melanoma or dysplastic naevi in the treatment area, UVB phototherapy or sunbed usage within last 30 days, PDT within 12 weeks prior to first treatment." Enrolled: 107 (48 M/59 F) Randomised: 54 (22 M/32 F) in MAL‐PDT group, 53 (26 M/27 F) in placebo group Withdrawals/drop‐outs: 3 withdrawals (1 severe pain and moderate erythema, 1 moderate photosensitivity reactions, 1 unknown) in MAL‐PDT group, 6 withdrawals (1 mild anxiety over the use of the goggles, 3 lack of efficacy, 1 "withdrawal by subject", 1 unknown) and 1 lost to follow‐up in placebo group ITT analysis: Yes |
|
| Interventions |
Intervention 1 80 mg/g MAL‐PDT followed by illumination with red light (without occlusive dressing) Number and frequency of treatments: 4 in total, every 2 weeks Wavelength/Fluence/Duration/Spot size: 632 nm/37 J/cm² Supplier: Visonac, Photocure, Nedax lamp Instructions to participants: Not applicable Intervention 2 Placebo cream followed by illumination with red light (without occlusive dressing) Number and frequency of treatments: 4 in total, every 2 weeks Wavelength/Fluence/Duration/Spot size: 632 nm/37 J/cm² Supplier: Nedax lamp Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 6 weeks after final treatment (adverse effects also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Change from baseline in number of ILs (nodules, papules and pustules) 2. Change from baseline in number of NILs (open and closed comedones) 3. Percentage change from baseline in number of ILs (nodules, papules and pustules) 4. Percentage change from baseline in number of NILs (open and closed comedones) Methods of assessing primary outcomes 1., 2., 3. and 4. "Live by trained assessor" Secondary outcomes of review interest recorded 1. Investigator's global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Live assessment. "Proportion of patients with success according to dichotomised IGA scale based on facial assessments (success defined as an improvement of at least 2 grades from baseline score) at 6w post‐treatment" 2. Pain assessed using VAS 0‐10 (0 = no pain, 10 = worst pain imaginable) directly after each treatment, blood samples pre‐treatment, 1 week after first treatment, 1 week after final treatment.; "Standard spontaneous reporting" for other outcomes |
|
| Notes | Language: English. Photocure provided results preview of clinicaltrials.gov record. After extracting data from that record, Photocure provided additional information regarding ethical approval, power calculation, age of included participants, severity of acne, mean baseline lesion counts, lamps used, withdrawals/lost to follow‐ups, outcome assessment methods, as well as information needed for selection, performance and detection bias assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An electronic randomisation list was generated using the SAS system." Comment: We judged this as adequate and risk of bias as low. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used for this purpose. We judged this as adequate and risk of bias as low. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Blinded packaging" was used to ensure that participants and performing investigators could not distinguish between MAL and placebo cream. "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)"; "Each site had 2 investigators, the investigator responsible for the efficacy evaluations could not be involved in the treatment procedure or the safety assessments." Comment: We judged this as adequate and risk of bias as low. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | Such outcomes were not assessed in this study. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)"; "Each site had 2 investigators, the investigator responsible for the efficacy evaluations could not be involved in the treatment procedure or the safety assessments." Comment: We judged this as adequate and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures obtained for over 80% of participants randomised in all groups. |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes reported at all time points. |
| Other bias | Unclear risk | Funded by Photocure. Insufficient information to judge whether additional bias was introduced. |
Oh 2009.
| Methods | This was a parallel‐group RCT with split face within groups "half of each patient’s face was randomly assigned to the short incubation group with ALA plus IPL (30 minutes) or long incubation group with ALA plus IPL (3 h), and the other half was treated with IPL only." Unit of randomisation: Left or right face Power calculation: No Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (page 1918): "The authors have indicated no significant interest with commercial supporters." Setting: Single centre (Seoul, Korea) Recruitment: Department of Dermatology, Yonsei University College of Medicine, Seoul Duration: 13 months (August 2007‐August 2008) |
|
| Participants |
Included Age (inclusion criterion; mean; range): 18‐30 years; 23 ± 4.12 in short incubation group and 23 ± 5.53 in long incubation group (not given for the whole sample); range 18‐30 years Clinically evident acne: Yes Severity of condition assessment: "with moderate to severe acne", using Evaluator Global Severity score Fitzpatrick skin types: III‐IV Excluded Oral antibiotics or isotretinoin within 6 months, systemic disease, tendency to keloid/photosensitivity, pregnancy Other: No other treatments allowed during the study af follow‐up period. Enrolled: 20 (4 M/16 F) Randomised: 20 in total, 9 in short incubation group (3 M/6 F), 11 in long incubation group (1 M/10 F) Withdrawals/drop‐outs: None Final number and proportion of participants evaluable: 20 (100%) ITT analysis: No |
|
| Interventions |
Intervention 1 Short incubation (30 min) with 5‐ALA, "occlusive technique with foil", plus IPL Number and frequency of treatments: 3 treatments in total, applied every 4 weeks Wavelength/Fluence/Duration/Spot size: 590 nm/12‐15 J/cm²/pulse duration 30 ms/not reported Supplier: ALA hydrochloride (Levulan Kerastick, Dusa Pharmaceuticals, Wilmington, MA) IPL device (BBL, Sciton Inc., Palo, Alto, CA) Instructions to participants: Not applicable Intervention 2 Long incubation (3 h) with ALA, "occlusive technique with foil", plus IPL Number and frequency of treatments: 3 treatments in total, applied every 4 weeks Wavelength/Fluence/Duration/Spot size: 590 nm/12‐15 J/cm²/pulse duration 30 ms/not reported Supplier: ALA hydrochloride (Levulan Kerastick, Dusa Pharmaceuticals, Wilmington, MA) IPL device (BBL, Sciton Inc., Palo, Alto, CA) Instructions to participants: Not applicable Intervention 3 IPL only Number and frequency of treatments: 3 treatments in total, applied every 4 weeks Wavelength/Fluence/Duration/Spot size: 590 nm/12‐15 J/cm²/pulse duration 30 ms/not reported Supplier: BBL, Sciton Inc., Palo, Alto, CA Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4, 8 and 12 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Percentage change from baseline in number of ILs (papules and pustules not reported separately) Methods of assessing primary outcomes 1. Non‐standardised scale: Significant improvement (> 75%), moderate improvement (50% to 75%), mild improvement (25% to 50%), no improvement (0% to 25%), worse (< 0%) relative to baseline 2. Lesion counts (using photographs) Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Non‐standardised scale: Significant improvement (> 75%), moderate improvement (50% to 75%), mild improvement (25% to 50%), no improvement (0% to 25%), worse (< 0%) relative to baseline, using photographs 2. Erythema and hyper‐ and hypopigmentation were assessed at each treatment. |
|
| Notes | Language: English. There were differences in baseline lesion count means (SD) between short and long incubation groups: 11 (6.2) versus 15.7 (7.1) (reported in Table 1 on page 1920). Each participant's half‐face treated with PDL only or IPL, but results presented as "3 intervention groups". We contacted study authors who provided additional information about power calculation, inclusion criterion regarding participants' age, start and end dates of the study, clarification of number of participants in each group, randomisation method, allocation concealment method, blinding of participants and assessing clinicians and SDs for mean reduction of lesion counts at 4, 8 and 12 weeks after final treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1919): "Half of each patient’s face was randomly assigned to the short incubation group with ALA plus IPL (30 minutes, n = 9) or long incubation group with ALA plus IPL (3 h, n = 11), and the other half was treated with IPL only (n = 20)" Comment: Method used to generate the allocation sequence was not stated in the report. Study authors clarified that randomisation was done via tables of random numbers. Firstly participants were randomly assigned to short or long incubation group. Face side to be treated with PDT was also randomly assigned. |
| Allocation concealment (selection bias) | Low risk | Intention and/or method to conceal the allocation sequence were not specifically reported. Study authors clarified that a sealed box was used to conceal the allocation sequence. Comment: We judged this as adequate and risk of bias as low. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided in the report. Study authors clarified that the participants and performing clinicians were not blinded due to the nature of the intervention. We judged risk of bias as high. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | High risk | Participants were not blinded, so we judged the risk of bias as high for participant‐assessed outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 1920): "Two blinded dermatologists independently examined the split‐face images of each patient in chronological order and separately conducted objective clinical assessments of acne." Comment: We judged this as adequate and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | According to 'Subject Characteristics' table (page 1920) there were 1 male and 9 female participants in the long incubation group. In 'Methods' section 11 participants were included in that group and outcomes are later also reported for 11 participants. No withdrawals or lost to follow‐up data reported, so it is not clear how many randomised participants were actually analysed. Implicitly from the tables 100%, as outcomes were reported for 20 participants in total. Study authors clarified the discrepancy between the numbers (mistyping in Table 1) and confirmed 20 participants were included and result were reported for all of them. We judged this as at low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | No other bias identifiable |
Orringer 2004.
| Methods | This was a parallel‐group RCT, with split‐face design within each group. Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (page 2839): "The research for this article was supported by the Babcock Endowment for Dermatological Research at the University of Michigan, ICN Pharmaceuticals.."; "ICN Pharmaceuticals Inc donated the NLite lasers. During the course of this study, ICN Pharmaceuticals divested itself of its Photonics subsidiary, which was responsible for the NLite device. However, we were permitted to retain the lasers to complete our study. At the time of this divestiture, ICN Pharmaceuticals had no knowledge of the outcomes" Setting: Single centre (Ann Arbor, Michigan, USA) Recruitment: Dept. of Dermatology, University of Michigan Medical School, Ann Arbor, newspaper and online advertising, flyers Duration: 4 months, June 2003‐September 2003 (recruitment from August 2002) |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 13 years, 20.7 years; 13‐31 years Clinically evident acne: Yes Severity of condition assessment: Leeds acne severity scale rating > 2 Fitzpatrick skin types: Not reported Other: 28 white, 7 Asian, 2 black, 3 unknown, "general good health, willingness and ability to comply with the requirements of the protocol" Excluded: Leeds acne grade < 2, oral retinoids within 12 months, other systemic/topical therapies within 1 month, alpha hydroxyl acid/glycolic acid use within 1 month, microdermoabrasion of the face within 3 months, < 13 years, history of prior dermabrasion/laser resurfacing, NSAIDS within 10 d prior or for 2 weeks after laser treatment Enrolled: 40 (24 M/16 F) Randomised: 40, 20 in each group Withdrawals/drop‐outs: 3 withdrawals (2 dissatisfied with improvement, 1 schedule conflict) and 3 lost to follow‐up in Intervention 1 group. 6 withdrawals (2 did not receive treatment (1 lost to follow‐up and 1 took prohibited medication), 2 dissatisfied with improvement, 2 unable to continue visits) and 2 lost to follow‐up in Intervention 2 group. Final number and proportion of participants evaluable: 14 (70%) Intervention 1 and 12 (60%) in Intervention 2 ITT analysis: Yes; 20 included in the analysis (Intervention 1); 19 included in the analysis (Intervention 2) |
|
| Interventions |
Intervention 1 Non purpuric PDL treatment to half of the face. "Non overlapping pulses were delivered in a 'painting' motion" Number and frequency of treatments: Single treatment Wavelength/Fluence/Duration/Spot size: 585 nm/3 J/cm²/pulse duration 350 µs/7 mm² Supplier: NLite laser (ICN Pharmaceuticals Inc, Costa Mesa, Ca) Instructions to participants: Not applicable Intervention 2 Non purpuric PDL treatment to half of the face. "Non overlapping pulses were delivered in a 'painting' motion" Number and frequency of treatments: 2, second treatment 2 weeks later Wavelength/Fluence/Duration/Spot size: 585 nm/3 J/cm²/pulse duration 350 µs/7 mm² Supplier: NLite laser (ICN Pharmaceuticals Inc, Costa Mesa, Ca) Instructions to participants: Not applicable Intervention 3 Nil |
|
| Outcomes | Evaluation time points of review interest: 2, 4, 6, 8 and 12 weeks after initial treatment (data for single and 2‐treatments groups combined) Primary outcomes of review interest recorded 1. Change from baseline in number of ILs (papules and pustules reported separately) 2. Change from baseline in number of NILs (open and closed comedones not reported separately) 3. Change from baseline in number of cysts Methods of assessing primary outcomes 1., 2. & 3. live lesion counts performed by a single physician comedones, and erythematous macules (as representative of resolving previously inflammatory lesions) Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity Methods of assessing secondary outcomes 1. Leeds acne severity scale for both treated and untreated sides of the face, using participants' photographs |
|
| Notes | Language: English. The study authors were contacted and were unable to provide separate data for single treatment group. They confirmed that the secondary reference listed for this study was an abstract from a poster presentation of the same study results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes (page 2853): "Patients were randomised to 1 of 2 treatment groups using a table of random numbers."; "A randomised code was used to determine the side of the face that would receive laser therapy as well as the number of treatments the patient would receive." Comment: We judged this as adequate and risk of bias as low. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes (page 2853): "Evaluating physicians were blinded to treatment assignment and regimen. Patients were specifically instructed not to tell the evaluating physician which side of the face was treated." Comment: The above suggests that participants and performing clinicians were not blinded, so we judged the risk of bias as high. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 2837): "Bilateral facial photographs obtained at baseline, week 4, and week 12 were graded by a panel of 3 dermatologists using the Leeds acne severity scale. These evaluators did not perform the laser treatments or the clinical lesion counts and were blinded to which images included treated compared with untreated skin." (Page 2835): "The randomisation of the side of the face receiving treatment was meant to...and helped to ensure that evaluators were unaware of the side in which an individual had received the laser treatment, thereby minimizing the potential for evaluator bias. Evaluating physicians were blinded to treatment assignment and regimen. Patients were specifically instructed not to tell the evaluating physician which side of the face was treated." Comment: We judged this as adequate and at a low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26/40 (65%) completed study however 38/40 (95%) included in analysis (LOCF Method used). We judged this as at high risk of bias as it was unclear when the last observations were made, which might have introduced a great degree of bias since less than 80% of outcome data were obtained. |
| Selective reporting (reporting bias) | High risk | Quote (page 2836): "When comparing patients randomised to receive either 1 or 2 laser treatment sessions, no statistically significant differences in efficacy at any time point or for any subtype of acne lesion were demonstrated. Thus, the data from these groups were combined to provide summary statistics of patient responses to laser therapy (provided as either 1 or 2 treatment sessions" Comment: No statistical data given regarding differences between 1 and 2‐treatment sessions groups which participants were initially randomised to. Study authors were contacted, but unable to provide separate data for single treatment group. We therefore judged risk of selective reporting as high. |
| Other bias | Low risk | Sponsorship was declared, there were commercial sponsors along with non commercial funding but we judged the risk of bias as low as study authors clarified their role. No other sources of bias identified. |
Orringer 2007.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quotes (page 432): "Supported by the University of Michigan Department of Dermatology Laser Research Fund. Conflicts of interest: None."; (page 438): "ICN Pharmaceuticals Inc donated the CoolTouch II laser that was used during the project. During the course of this study, ICN Pharmaceuticals divested itself of its Photonics subsidiary, which was responsible for the CoollTouch II device. However, we were permitted to retain the laser to complete our study. Of note, at the time of this divestiture, ICN Pharmaceuticals had no knowledge of the outcomes."; (page 432) Supported by the University of Michigan Department of Dermatology Laser Research Fund". Setting: Single centre (Michigan, USA) Recruitment: Medical Dermatology clinics, University of Michigan and newspaper advertising; participants reimbursed a small sum at each visit to cover travel expenses and other incidental costs of participation. Duration: 28 months, June 2003 to September 2005 |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 13 years; 24.8 years; range not reported Clinically evident acne: Yes Severity of condition assessment: "The presence of clinically‐apparent facial acne of at least a Leeds acne severity scale rating of 2 (on a 12‐point ordinal scale)" Fitzpatrick skin types: II‐VI Other: General good health, the ability to comply with the study protocol; participants entered into the study 'washed out' from any systemic antibiotic use or any topical anti‐acne therapy for 1 month before study entry. Excluded Oral retinoid use within 1 year of study entry, age younger than 13 years, microdermoabrasion of the face within 3 months of study entry, alpha hydroxy acid or glycolic acid use within 1 month of study entry, and a history of dermabrasion or laser resurfacing of the facial skin Enrolled: 46 (10 M/36 F) Randomised: 46 Withdrawals/drop‐outs: 1 participant did not receive treatment (did not qualify after wash out). 5 withdrawals (2 too much discomfort, 1 adverse event ‐ panic attack, 1 protocol violation ‐ began other acne treatment), 4 lost to follow‐up Final number and proportion of participants evaluable: 30 (65%) ITT analysis: Yes; 30 completed the 12‐week study, 37 included in the analysis |
|
| Interventions |
Intervention 1 LMX 4% anaesthetic cream applied over entire face for 30‐45 min followed by Nd:YAG laser, 2 passes to one half of the face from hair line to jaw line (non overlapping) Number and frequency of treatments: 3 treatments, every 3 weeks Wavelength/Fluence/Duration/Spot size: 1320 nm/not reported/2 x 30 ms/ 10 mm² Supplier: Ferndale Laboratories, Ferndale, Michigan; Nd:YAG laser (CoolTouch II) Instructions to participants: Not applicable Intervention 2 LMX 4% anaesthetic cream applied over entire face for 30‐45 min Number and frequency of treatments: 3 treatments, every 3 weeks Supplier: Ferndale Laboratories, Ferndale, Michigan Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 8 weeks after final treatment (also assessed at 1 week after final treatment) Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Change from baseline in number of ILs, NILs and cystic lesions (papules, pustules, open comedones, closed comedones and cysts reported separately) Methods of assessing primary outcomes 1. "Patients' impressions of the treatment and the associated results in terms of their acne severity and the degree of oiliness of their skin were surveyed at the completion of the treatment phase of the study." Further details not given 2. Evaluations included formal counts of papules, pustules, cysts, and comedones ("Patients were clinically assessed at baseline and weeks 7 and 14. Evaluations included formal counts of papules, pustules, cysts, and comedones…. and image analysis utilizing ImagePro Plus software (Media Cybernetics, Silver Spring, Md) at baseline and weeks 7 and 14") Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. Facial photographs, Leeds acne severity scale for both treated and untreated sides of the face 2. Not reported |
|
| Notes | Language: English. participants' impressions were assessed using survey at the completion of the treatment phase of the study (details were not given). The study authors were contacted, and provided additional information on power calculation and adverse effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 433): "Patients were randomised to receive a series of 3 laser treatments to one half of the face with the contralateral facial skin remaining untreated and serving as a control. A randomised code was used to determine which side of the face was to be treated." Comment: We judged this as adequate. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 433): "Treatments were performed at 3‐week intervals by a single physician (J. S. O.) who did not participate in the clinical evaluation of subjects." Comment: Study was described as "single blind" (page 432). No intended blinding of participants/performing clinicians reported, so we judged this at high risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | High risk | Quote (page 435): "Fifty‐nine percent of patients (22 of 37) thought that their acne had improved at least mildly when compared with the untreated skin." Comment: Study was also described as ‘single blind’. Participants we not blinded, so we judged the risk of bias as high. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 433): "Bilateral facial photographs obtained...were assessed by a panel of 3 dermatologists who were neither involved in the treatment of study participants nor performed the live clinical lesion counts...The evaluators were blinded as to whether images depicted treated or untreated sides of the face." Comment: We judged this as at a low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote (page 433‐434): "Because of the substantial number of early withdrawals, separate analyses were performed with the cohort of subjects that completed through week 7 only (n = 37), and the cohort that completed through week 14 (n = 30). In addition, for the primary efficacy data (lesion counts), a carry‐forward analysis was done using each subjects’ last available data values for the week 14 time point (n = 37). The data were analysed by this carry‐forward method in which each early‐terminated subject’s last available data points are carried forward to the final visit (thereby allowing them to be included in the end‐of‐study analysis) in order to control for attrition bias—that is, results appearing more favourable because of responders completing the study and non‐responders dropping out." Comment: Outcome measures were available on 37/46 (80%) participants at week 7 and only 30/46 (65%) at week 14. Although ITT analysis was performed as described, we judged this as at high risk of bias, as less than 80% of outcome data were obtained at week 14. |
| Selective reporting (reporting bias) | Low risk | All outcome measures pre‐specified in the methods section reported. |
| Other bias | Low risk | Study authors reported no conflicts of interest. Commerical sponsor was reported separately, however we judged it was unlikely that bias was introduced. No other sources of bias identifiable. |
Orringer 2010.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Unit of analysis: Lesion Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (page 33): "This study was supported by the University of Michigan Department of Dermatology Laser Research Fund." Setting: Single centre (Michigan, USA) Recruitment: Dermatology Department, University of Michigan Medical School Duration: 42 months, January 2005‐July 2008 |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 13 years; 25 years; 15‐50 years Clinically evident acne: Yes Severity of condition assessment: "clinically evident facial acne" Fitzpatrick skin types: All types included Other: Generally good health and willing and able to comply with the requirements of the protocol. Excluded Oral retinoid within 1 year, systemic acne therapies (such as oral antibiotics) within 4 weeks, or topical acne therapies including OTC products or prescription medications (retinoids, antibiotics) within 2 weeks of entry into the study. Microdermabrasion or superficial chemical peels at the sites to be treated within 2 months of entry into the study or dermabrasion or laser resurfacing at the sites to be treated at any time. Incompliant participants, those with a significant medical history or concurrent illness condition that the investigators felt was not safe for study participation, and pregnant or nursing participants were also excluded. History of frequent herpes simplex infections of the face or with clinical evidence of active herpes simplex infection, those with a history of keloid scar formation, participants with a known allergy or hypersensitivity to topical photosensitising agents, and those with known photosensitivity disorders. Enrolled: 99 screened for eligibility Randomised: 44 (14 M/30 F) Withdrawals/drop‐outs: 5 withdrew prior week 6 evaluation (1 adverse event ‐ hyperpigmentation, 3 declined to continue treatment, 1 non compliance) and 3 after (1 adverse event – hyperpigmentation, 1 declined to continue treatment, 1 non‐compliance) Final number and proportion of participants evaluable: 29 (66%) ITT analysis: Yes "mixed model fitting.. was used to obtain predicted values where data were missing." (page 30) |
|
| Interventions |
Intervention 1 Acetone scrubs, followed by application of 20% 5‐ALA for 60‐90 min prior to PDL single pass Number and frequency of treatments: 3 treatments in total, applied every 3 weeks Wavelength/Fluence/Duration/Spot size: Not reported/6.6‐7.5 J/cm²/10 ms pulse duration/10 mm² Supplier: 20% ALA: Levulan, DUSA Pharmaceuticals, Inc, Willmington, MA, USA; Laser: VBeam, Candela Corp., Wayland, MA, USA Instructions to participants: Not applicable Intervention 2 Nil |
|
| Outcomes | Evaluation time points of review interest: 2, 4, 6, 8 and 10 weeks after final treatment (evaluated at baseline and every two weeks for a total of 16 weeks during and after treatment) Primary outcomes of review interest recorded 1. Change from baseline in number of ILs (papules and pustules reported separately) 2. Change from baseline in number of NILs (open and closed comedones reported separately) 3. Change from baseline in number of cystic lesions Methods of assessing primary outcomes 1. Evaluations included formal counts of papules, pustules, cysts, and comedones (live lesion counts) Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. Leeds acne severity scale for both treated and untreated sides of the face 2. Not reported |
|
| Notes | Language: English. The study authors were contacted, and provided additional information on power calculation and adverse effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 29): "A randomised code determined which side of each patient’s face was to be treated." Comment: Adequate sequence generation method used. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 29): "The study was a randomised, controlled, split‐face, single‐blind clinical trial."; "The treating physicians (JS Orringer and DL Sachs) were not involved in clinical evaluations of the patients." Comment: The above suggests that participants and performing clinicians were not blinded, so we judged the risk of bias as high. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 29): "The study was a randomised, controlled, split‐face, single‐blind clinical trial."; " The treating physicians (JSO and DLS) were not involved in clinical evaluations of the patients". Comment: The above suggests that outcome assessors were blinded to treatment, so we judged this as at a low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote (page 30): "Mixed model fitting the lesion count with covariates age, gender, race, severity rating, continuous time, treatment, and time–treatment interaction, with random
intercept and time was used to obtain predicted values where data were missing." Comment: 29 of 44 randomised participants completed the 16 weeks study, only 65.9% of randomised subjects available for evaluation. Method used to obtain predicted values when data was missing described. We judged this as at a high risk of bias, although the method used for ITT was adequate. |
| Selective reporting (reporting bias) | Low risk | All outcome measures pre‐specified in the methods section reported. |
| Other bias | Low risk | Sponsorship declared, apparently no commercial interest. No other risk of bias sources likely. |
Ou 2014.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Unit of analysis: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Single centre (Xinjiang, China) Recruitment: Not reported Duration: 4 months, July 2012‐October 2012 |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 25.1 years; 18‐38 years Clinically evident acne: Yes Severity of condition assessment: Moderate acne, grade II‐III according to the Chinese Acne Treatment Guidelines Fitzpatrick skin types: Not reported Excluded Breast‐feeding mothers; allergic to prescribed medications in intervention and control groups; potential exposure to strong sunlight/UV rays during treatment process; taken medication for acne within the last 30 days; major chronic diseases; mental disorders Enrolled: 90 (M/F not reported) Randomised: 90 Withdrawals/drop‐outs: 7, further details not reported Final number and proportion of participants evaluable: 83/90 (92%), 13 M/70 F ITT analysis: Not reported |
|
| Interventions |
Intervention 1 Yinhua decoction twice daily, with electric light synergy Number and frequency of treatments: 6 treatments, applied every 2 weeks for 12 weeks Wavelength/Fluence/Duration/Spot size: 18 J/cm²/individual treatments applied "until the cheeks appeared to be slightly red", further details not reported Supplier: Not reported Instructions to participants: Yes, adequate Intervention 2 Yinhua decoction twice daily, with red and blue light treatment Number and frequency of treatments: 6 treatments, applied every 2 weeks for 12 weeks Wavelength/Fluence/Duration/Spot size: 610 and 415 nm/10 min/further details not reported Supplier: Not reported Instructions to participants: Yes, adequate |
|
| Outcomes | Evaluation time points of review interest: 12 weeks after final treatment Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Non‐standard scale based on percentage change in combined lesion counts. Percentage change in lesion count = (lesion count before treatment – lesion count after treatment)/ lesion count before treatment × 100%; Fully recovered: percentage change in lesion count ≥ 90%; Good improvement: percentage change in lesion count 60% to 89%; Effective: percentage change in lesion count 30% to 59%; No effect: percentage change in lesion count ≤ 29%; Total percentage effectiveness = (no. of fully recovered + good improvement)/total no. of participants x 100% 2. Not reported |
|
| Notes | Language: Mandarin. English translation was not available. Data extraction was done by native speaker Elicia Toon Yuan Ni from the original paper. We have not attempted to contact the study authors. We used the 'Yinhua decoction' term as presented in the English translation of the abstract provided by the journal where full text was published in Mandarin. As clarified by native Mandarin speakers, 'Yinhua decoction' is different from 'Jinhua Xiaocuo' (used in Zhang 2013b study), although both used the same main ingredients (honeysuckle flower). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page1279): "Randomisation done using SAS software." Comment: We judged this as adequate. |
| Allocation concealment (selection bias) | Low risk | Quote (page 1279): "Numbers assigned to participants were placed in envelopes" Comment: We judged this as adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | No intended blinding of outcome assessors reported. No evidence that outcome assessors were blinded provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results reported for 83/90 (92%) randomised participants, so we judged the risk as low. |
| Selective reporting (reporting bias) | Low risk | All outcomes predefined in the ‘Methods’ section were reported. |
| Other bias | Unclear risk | Sponsorship and conflicts of interest unclear. Insufficient information to permit a clear judgment. The study was in Mandarin and potential bias was introduced by the fact that we were only able to do single rather than double data extraction. |
Paithankar 2015.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes, "Trial 2 (Clinicaltrials.gov identifier NCT02219074) was a separate, independent Ethics Committee approved study in which the sites and inclusion/exclusion criteria were unchanged from Trial 1.", further details not provided (Please note that 'Trial 1' refers to Owczarek 2014) Sponsorship and conflict of interest: "DP, RB, TM, and LF are employees of and/or have financial interests in Sebacia. AK, JL, and RRA have consulting relationships with Sebacia. The remaining study authors state no conflict of interest."; "This research was sponsored by Sebacia, Duluth, GA. We acknowledge Apostolos G. Doukas, Stephanie Beall, and Anthony Lando for help with various stages of the project." Setting: Two centre?, "conducted at two sites", Poland, further details not reported Recruitment: Unclear Duration: Unclear: According to clinicaltrials.gov record NCT02219074 (accessed on September 26, 2015) study start date was June 2011 and estimated study completion date October 2015) |
|
| Participants |
Included Age: inclusion criterion 16‐35 years; mean 21.4; age range 16‐26 years Clinically evident acne: Yes Severity of condition assessment: "moderate‐to‐severe inflammatory facial acne, IGA (scale from (Solodyn, 2006)) scores 3–4 with at least 25 total papules and pustules present on face" Fitzpatrick skin types: I‐III Excluded "systemic medications for acne, oral retinoid therapy, or treatment with Intense Pulsed Lights or lasers within the past 12 months." Subjects were randomised after entry to receive either control or treatment. Enrolled: 51 Randomised: 51 (37 F; 14 M), 27 in treatment group, 24 in control group Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: in the intervention group 26/27 (96%) at 6 weeks, 25/27 (92%) at 10 weeks, 24/27 (89%) at 14 weeks after final treatment; in the control group 24/24 (100%) at 6 weeks, 21/24 (88%) at 10 weeks, 19/24 (79%) at 14 weeks after final treatment. ITT analysis: Unclear |
|
| Interventions |
Intervention 1 "On treatment days, face was washed and 3 ml of particle suspension was massaged as described above for 10 minutes. Superficial suspension was wiped; two laser passes were performed with a 9 × 9 mm handpiece with contact cooling and ˜ 10% overlap." The mean laser radiant exposure was 33.4 J/cm². Number and frequency of treatments: 3 treatments, 1 week apart Wavelength/Fluence/Duration/Spot size: Unclear Supplier: "Gold microparticles were manufactured by Nanospectra (Houston, TX) and were placed in suspension at Dow Pharmaceutical Sciences (Petaluma, CA)."? Further details not reported. Intervention 2 Quote (page 1733): "Subjects in the ‘sham’ arm were treated similarly, but instead of the microparticle suspension vehicle (without light‐absorbing particles) was used with a fluence of 10 J/cm²" Wavelength/Fluence/Duration/Spot size: Unclear Number and frequency of treatments: 3 treatments, 1 week apart Supplier: Not specified |
|
| Outcomes | Evaluation time points of review interest: 6, 10 and 14 weeks after final treatment Primary outcomes of review interest recorded 1. Change from baseline in combined number of lesions 2. Percentage change from baseline in combined number of lesions Methods of assessing primary outcomes 1. "Lesion counts and IGA scores were performed 'live' by unblinded assessors, and, in parallel, by a single blinded reviewer (also 'live' and unaware of the assignment to groups) at each site to assess bias (if any). High correlation between blinded and unblinded assessment (r = 0.95) was noted. Thus, data pooled from all five unblinded investigators assessments are reported as these same five investigators conducted assessments in the prior study eliminating intra‐rater variability that might otherwise be introduced if using the different, albeit blinded, investigator assessments. Percent change in inflammatory lesion count from baseline as well as a fraction of subjects showing improvement in IGA score of two or better were compared in the two arms. Response rate calculation (positive response upon 50% or higher reduction in inflammatory lesions) was performed at each follow‐up point." 2. Please see 1. above Secondary outcomes of review interest recorded 1. Investigator's global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Please see 'Methods of assessing primary outcomes' above. 2. Unclear |
|
| Notes | Language: English. We were unable to identify additional information, although it was reported to be presented in Supplementary Information ('The CONSORT flowchart for this trial appears in the Supplementary Information online.’, accessed on September 26, 2015). We have contacted the study authors and sponsors, who clarified that this trial (reported as Trial 2 in the primary reference for this study) was not part of the cross‐over trial we previously identified (Owczarek 2014), but an independent trial. Both studies are registered under the same clinicaltrials.gov Identifier (NCT02219074). They clarified that this study was also presented at the ASLMS 2015 meeting ("there was a late breaking abstract and presentation (LB7) at the 2015 ASLMS meeting, which contained data from Trial 2 (as referred to in the JID article)". We were unable to identify the abstract. Study authors and sponsors also provided information as follows "The JID paper describes two independent clinical trials conducted in Poland". We did not obtain further clarifications and requested information described as 'unclear' or 'not reported' in this table. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1733): "Fifty‐one subjects (37 females) were enrolled with 27 in the active treatment arm." Comment: Method used for randomisation not reported. We judged this as at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not specifically reported. Reported as "Single Blind (Outcomes Assessor)" in the NCT record. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | High risk | Quote (page 1733): "Lesion counts and IGA scores were performed 'live' by unblinded assessors, and, in parallel, by a single blinded reviewer (also 'live' and unaware of the assignment to groups) at each site to assess bias (if any). High correlation between blinded and unblinded assessment (r = 0.95) was noted. Thus, data pooled from all five unblinded investigators assessments are reported as these same five investigators conducted assessments in the prior study eliminating intra‐rater variability that might otherwise be introduced if using the different, albeit blinded, investigator assessments." Reported as ‘Single Blind (Outcomes Assessor)’ in the NCT record. Comment: One assessor blinded but then results pooled with 5 unblinded assessors. We judged this as at high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | More than 80% of randomised participants were included in the analysis at all time points of review interest, apart from control group at 14 weeks after final treatment (79%). Although ITT analysis was not done, we judged this as at low risk of bias. |
| Selective reporting (reporting bias) | High risk | Quote (page 1733): "The analysis for Trial 2 differed slightly; percent change in inflammatory lesion count was used." Percentage changes, and not changes in lesion counts reported, although primary outcomes as per NCT record read: "Change in inflammatory lesion count". Study registered with clinicaltrials.gov in August 2014 after trial start date in June 2011. Comment: We judged this as at high risk of bias. |
| Other bias | Unclear risk | Unclear role of the sponsor in collection and analysis of data. |
Papageorgieu 2000.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person. Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared. Setting: Single centre (London, UK) Recruitment: "Patients attending the dermatology out‐patients clinic at the Hammersmith Hospital were asked to participate in this study with full written consent." Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; not given for the whole sample – groups 1, 2, 3 and 4, 24.8 years, 23.4 years, 26.7 years, 25.6 years respectively; 14‐50 years Clinically evident acne: Yes Severity of condition assessment: Mild to moderate acne Fitzpatrick skin types: Not reported Excluded Pregnant, on oral contraceptives, had taken oral antibiotics during the previous 2 weeks, and participants whose acne was assessed as very mild (with fewer than 5 inflammatory lesions) or severe (cystic). Withdrawal criteria during the study included pregnancy, use of any acne treatment other than that issued, or any intake of oral antibiotics. Enrolled: Blue‐red light 30 (9 M/21 F); blue light 27(8 M/19 F); BPO 25 (9 M/16 F); white light 25 (7 M/18 F); in total 107 (33 M/74 F) Randomised: Total of 107 participants (blue‐red light 30, blue light 27, BPO 25 and white light 25) Withdrawals/drop‐outs: Blue‐red light: acne flare (2), unclear (5); blue light: acne flare (3), unclear (4); BPO: acne flare (2), unclear (3); white light: acne flare (2), unclear (4) Final number and proportion of participants evaluable: blue‐red light 23 (77%), blue light 20 (74%), BPO 20 (80%), white light 19 (76%) ITT analysis: Not stated |
|
| Interventions |
Intervention 1 A mixture of blue (415 nm) and red (660 nm) light, 25 cm from the light source (fluorescent lamps in reflector fixtures) Number and frequency of treatments: Daily for 12 weeks Wavelength/Fluence/Duration/Spot size: 660 ± 10 nm and 415 (+20, ‐15) nm/cumulative dose: 320 and 202 J/cm²/irradiation time 15 min daily/not reported Supplier: Type HF 885, Osram Sylvania, Brussels, Belgium Instructions to participants: Not applicable Intervention 2 Blue light (415 nm), 25 cm from light source (fluorescent lamps in reflector fixtures) Number and frequency of treatments: Daily for 12 weeks Wavelength/Fluence/Duration/Spot size: 415 (+20, ‐15) nm/cumulative dose 320 J/cm²/irradiation time 15 minutes daily/ not reported Supplier: Type HF 885, Osram Sylvania, Brussels, Belgium Instructions to participants: Not applicable Intervention 3 5% benzoyl peroxide cream Number and frequency of treatments: Not stated Instructions to participants: Adequate instructions were probably given to participants. "Written instructions on how to use each treatment were also issued" (page 974) Intervention 4 Cool white light Number and frequency of treatments: Daily for 12 weeks Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: None (evaluated every 4 weeks whilst on treatment, final evaluation at final treatment session) Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Percentage change from baseline in number of ILs (papules and pustules not reported separately) and NILs Methods of assessing primary outcomes 1. Non‐standardised scale: 'worse' (≤ ‐10%), 'unchanged' (‐9% to 9%), 'mild improvement' (10% to 39%), 'moderate improvement' (40% to 59%), 'marked improvement' (60% to 89%) or 'clearance' (≥ 90%) 2. Lesion counts Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Non‐standardised scale: 'worse' (≤ ‐10%), 'unchanged' (‐9% to 9%), 'mild improvement' (10% to 39%), 'moderate improvement' (40% to 59%), 'marked improvement' (60% to 89%) or 'clearance' (≥ 90%) 2. Unclear |
|
| Notes | Language: English. Final evaluation at final treatment, but included participants' assessments of improvement, showing early encouragement to continue with the treatment so we judged they met inclusion criteria. Results reported in graph‐format only for Participant’s global assessment of improvement and Investigator‐assessed change in acne severity. No mention of oral retinoid washout period. Higher baseline numbers of ILs and comedones in the blue‐light group (Table 1, page 974). We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 974): "Patients were exposed to one of the three light sources in a single blind fashion or were treated with 5% benzoyl peroxide cream (unable to be blinded) using a computerized randomisation list." Comment: Adequate sequence generation method described |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 974): "Patients were exposed to one of the three light sources in a single blind fashion or were treated with 5% benzoyl peroxide cream (unable to be blinded) ..." Comment: It is stated that participants using the light sources were ‘blinded’ although no further details were given. Also participants using benzoyl peroxide were not blinded. No evidence that the clinicians were blinded. We judged this as at a high risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | High risk | No evidence that participants were blinded (please see above), so we judged this as at high risk of bias for participant‐assessed outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Quote (page 974): "Assessments were made blind by two assessors." Comment: No details given and we judged it as at unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome measures were obtained for 77% of subjects randomised. Reasons for withdrawals were not clear. We judged this as at a high risk of bias. |
| Selective reporting (reporting bias) | High risk | Investigator and participant‐assessed severity was not reported. All other predefined outcomes were reported. We judged this as at a high risk of bias. |
| Other bias | Unclear risk | Sponsorship was not declared. Insufficient information was given to permit a clear judgement. |
Pariser 2013.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Sponsored by Photocure Setting: Multicentre (Oceanside and San Diego, California; Jaksonville, Florida; Arlington Heights and Napervile, Illinois; Evansville, Indiana; Haverhill, Massachusetts: Fort Gratiot and Troy, Michigan, Hershey, Pennsylvania, Johnston, Rhode Island, Austin and San Antonio, Texas, Norfolk, Virginia, Spokane, Washington; USA) Recruitment: "Clinics, own database and advertising (papers, radio and TV)" Duration: 26 months (May 2011‐May 2013) |
|
| Participants |
Included Age (inclusion criterion; mean; range): 12‐35 years; 18.6; not reported Clinically evident acne: Yes Severity of condition assessment: "with severe facial acne vulgaris (IGA score 4 on IGA scale)", "with 25 to 75 inflammatory lesions (papules, pustules, and nodules) on the face"; "with 20 to 100 non‐inflammatory lesions (open and closed comedones) on the face" Fitzpatrick skin types: I‐VI Other: "Female patients who are surgically sterile, pre‐menstrual, postmenopausal, abstinent, or willing to use an adequate means of contraception including birth control pills, or barrier methods and spermicide for at least 14 days prior to T1. Patients using birth control pills must have used the same product and dose for at least 3 months and must agree to stay with the same product and dose for an additional 3 months."; "Signed and verified informed consent form and photo consent form. For subjects under age of 18, an assent form in conjunction with an informed consent form, signed and verified by parent/guardian." Excluded "acne conglobata, acne fulminans, secondary acne...more than 3 nodules on the face…investigator or any sub investigator, research assistant, pharmacist, study coordinator, other staff or relative thereof directly involved in the conduct of the study…unlikely to comply with the protocol…female patients with childbearing potential... and sexually active, not willing to use a medically accepted contraceptive regimen (as described under inclusion criteria) while on treatment...pregnancy…nursing…participation in other clinical studies either currently or within the last 30 days…porphyria…cutaneous photosensitivity…allergy to MAL, to a similar PDT compound, or to excipients of the cream…using testosterone, any other systemic hormonal treatment or hormonal contraceptives solely for control of acne"; topical treatments within last 14 days, oral antibiotics within last month, oral isotretinoin within the last 6 months, facial procedures like dermabrasion, chemical or laser peels within the last 1 month, testosterone, any systemic hormonal treatment for other reasons than acne treatment and has not been on the same product and dose for at least 3 months; moderate, severe or very severe facial acne scarring, a beard that might interfere with study assessments, melanoma or dysplastic nevi in the treatment area, UVB phototherapy, sun tanning salons within the last 30 days, PDT within 12 weeks before first treatment. Enrolled: 153 (87 M/66 F) Randomised: 100 (56 M/44 F) in MAL‐PDT group, 53 (31 M/22 F) in placebo group Withdrawals/drop‐outs: 15/100 (15%) withdrew from the MAL‐PDT group (12 due to AEs of which 6 were pain‐related, 2 withdrew consent and 3 "other") and 4/53 (7.5%) withdrew from the placebo group (3 withdrew consent and 1 "other"). 1 patient from the placebo group was lost to follow‐up. ITT analysis: Yes |
|
| Interventions |
Intervention 1: 80mg/g MAL‐PDT under occlusion followed by illumination with red light Number and frequency of treatments: 4 in total, 2 weeks apart Wavelength/Fluence/Duration/Spot size: 632 nm/ 37J/cm² Supplier: Visonac, Photocure, Nedax lamp Instructions to participants: Not applicable Intervention 2: Placebo cream under occlusion followed by illumination with red light Number and frequency of treatments: 4 in total, 2 weeks apart Wavelength/Fluence/Duration/Spot size: 632 nm/ 37J/cm² Supplier: Nedax lamp Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 6 weeks after final treatment (adverse effects also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Absolute change from baseline in ILs count (nodules, papules, and pustules) 2. Absolute change from baseline in facial NILs count (open and closed comedones) 3. Percent change from baseline in facial ILs count (nodules, papules, and pustules) 4. Percent change from baseline in facial NILs count (open and closed comedones) Methods of assessing primary outcomes 1., 2., 3. and 4.: "Lesion count and IGA scoring done by trained assessors." Secondary outcomes of review interest recorded 1. Investigator's global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. "Proportion of patients with success according to IGA scale based on the facial assessment at 12 weeks after the first treatment. One scale will be used including inflammatory and non‐inflammatory lesions. Success is defined as an improvement of at least 2 grades from the baseline score." 2. "Pain during illumination using a Visual Analogue Scale (VAS) from 0 to 10, where 0 indicates no pain and 10 indicates the worst pain imaginable."; "Percent of patients with local (facial and non‐facial treatment site) and non‐local adverse events."; "Erythema score"; "Scarring at week 12"; "Local (facial and non‐facial treatment site) and non‐local adverse events" |
|
| Notes | This was a conference report. Language: English. Sponsored by Photocure. The sponsors were contacted and provided additional information on power calculation, ethical approval, recruitment, patient age and gender, lamp used, number of participants randomised in each group, mean baseline lesion counts, number and reasons of withdrawals, lost to follow‐ups, ITT details, methods of assessing primary outcomes and adverse effects, randomisation method, allocation concealment and blinding methods, detailed results and adverse effects, including application site blisters specifically. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sponsors provided the following information: "Patients were randomised to Visonac PDT or vehicle PDT (2:1) in accordance with a pre‐specified block randomisation list produced by Almac (clinical supply service)." Comment: We judged this as adequate and risk of bias as low. |
| Allocation concealment (selection bias) | Low risk | The sponsors provided the following information: "Each kit are numbered with a randomisation number according to the randomisation list described above. Each site was instructed to allocate kits from a lowest to highest order. Scratch cards was provide to all sites for emergency unbinding." Comment: We judged this as adequate and risk of bias as low. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The tubes are only identified by a randomisation number." Comment: We judged this as adequate and risk of bias as low. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | The sponsors provided the following information: "The tubes are only identified by a randomisation number. The person responsible for assessing efficacy (IGA and lesion count) was not allowed to evaluate safety. Source data had to be kept separate." Comment: We judged this as adequate and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data obtained for over 80% of participants in each of the groups. We judged this as adequate and risk of bias as low. LOCF Method was used in ITT analysis. We judged this as appropriate, as more than 80% of outcome data were obtained, although it is unclear when the last observations were made, which might have introduced some bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes predefined in the study protocol identified in Clinicaltrials.gov register were provided upon request for all time points, so we judged the risk of bias as low. |
| Other bias | Unclear risk | The study was commercially funded, insufficient information to judge whether additional bias was introduced. |
Pollock 2004.
| Methods | This was a split‐back RCT. Unit of randomisation: Quadrant of back (30 cm² areas in the back) Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Not declared Setting: Single centre (Leeds, UK) Recruitment: Department of Dermatology, Leeds General Infirmary Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; not reported; 16‐40 years Clinically evident acne: Yes Severity of condition assessment: "mild to moderate acne on their backs (Leeds grades 2–4)" Fitzpatrick skin types: I‐III, V Other: Age and previous systemic treatments of each participant reported in a table. All participants were asked to stop any treatment for at least 4 weeks prior to PDT. No participants had previously been treated with isotretinoin. Excluded Not reported Enrolled: 10 (9 M/1 F) Randomised: 10 Withdrawals/drop‐outs: None Final number and proportion of participants evaluable: 10 (100%) ITT analysis: Yes |
|
| Interventions |
Intervention 1 ALA cream (20% in Unguentum Merck) was applied under occlusion for 3 h followed by red light from a diode laser. Number and frequency of treatments: 3 treatments in total, applied weekly Wavelength/Fluence/Duration/Spot size: 635 nm/15 J/cm²/10 min per site/not reported Supplier: CeramOptec GmbH, Bonn, Germany ALA cream (20% in Unguentum Merck), Tegaderm (3M, Loughborough, UK) occlusion Instructions to participants: Not applicable Intervention 2 Red light from a diode laser Number and frequency of treatments: 3 treatments in total, applied weekly Wavelength/Fluence/Duration/Spot size: 635 nm/15 J/cm²/10 min per site/not reported Supplier: CeramOptec GmbH, Bonn, Germany Instructions to participants: Not applicable Intervention 3 ALA cream (20% in Unguentum Merck) alone was applied under occlusion for 3 h. Number and frequency of treatments: 3 treatments in total, applied weekly Supplier: ALA cream (20% in Unguentum Merck), Tegaderm (3M, Loughborough, UK) occlusion Instructions to participants: Not applicable Intervention 4 Untreated control |
|
| Outcomes | Evaluation time points of review interest: 3 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Change from baseline in number of ILs, NILs and nodular lesions Methods of assessing primary outcomes 1. Lesion counts Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Recorded during study |
|
| Notes | Language: English. Substantial differences in mean IL counts at baseline across study groups (Figure 4: means were 8.3, 6.6, 11.6 and 10.1 in each group). We contacted the study authors in 2008, but they were unable to provide additional data. We have not attempted to contact the study authors since. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 617): "Each 30 cm² area was randomly allocated to either ALA‐PDT treatment, light alone, ALA alone or an untreated control site." Comment: Method used to generate the allocation sequence was not described |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. Given the nature of the interventions involved then blinding of participants/personnel was unlikely, so we judged risk of bias as unclear. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 618): "The acne counts were performed in all cases by the same clinician who was blinded to the treatment status of the site and to the previous results." Comment: This was probably done and we judged it at a low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 619): "No subjects failed to complete the study." Comment: All randomised participants were included in the analysis and we therefore judged the risk of bias as low. |
| Selective reporting (reporting bias) | High risk | Quote (page 619): "There was also a reduction in non‐inflamed lesion counts at the ALA‐PDT site but there were insufficient numbers of lesions for statistical analysis.' Comment: Non‐inflammed lesion counts were not reported. We judged this as at a high risk of bias. |
| Other bias | Unclear risk | Sponsorship not declared, unclear whether this might have had introduced bias. Insufficient information was given to permit a clear judgement. |
Ragab 2014.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Sponsorship not declared. No conflicts of interest (page 179) Setting: Single centre, Alexandria (Egypt) Recruitment: "selected from the attendants of the Dermatology Outpatient Clinic of the Alexandria University Hospital" Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): < 14; 19.7 ± 5.9 in the ALA‐IPL group, 19.0 ± 4.4 IPL alone group; 14‐39 years Clinically evident acne: Yes Severity of condition assessment: "'with mild to moderate facial acne’; ‘Global Severity Score of 2 or 3'" Fitzpatrick skin types: III‐V Other: Both sexes Excluded "therapy with oral isotretinoin in the past 6 months, the use of topical or systemic antibiotics 2 weeks before the study, photosensitive dermatoses, pregnancy, or lactation" Enrolled: 25 (1 M/24 F), 15 in ALA‐IPL group (1 M/14 F), 10 in the IPL alone group (10 F) Randomised: 25 Withdrawals/drop‐outs: Not specifically reported, results presented for 25 participants Final number and proportion of participants evaluable: 25/25 (100%)? Unclear ITT analysis: Unclear |
|
| Interventions |
Intervention 1 "...the entire face was cleansed with soap and 70% alcohol. Freshly prepared 20% topical ALA …mixed in an oil‐in‐water emulsion was then applied...applied on the whole face excluding the nose and a 1 cm periocular area. After occlusion with a plastic film for 1 h, ALA was completely removed with soap and water, and the whole face was exposed to IPL...The 560 hand piece was used throughout the study, and patients received two passes at each treatment session. During the treatment, patients’ eyes were protected with eye goggles." Number and frequency of treatments: "2 in total, every two weeks" Wavelength/Fluence/Duration/Spot size: 560 nm/10‐12 J/cm²/"double pulse (width was 4–5 ms with a 20 ms delay)"/45.8 x 10.8 mm² Supplier: Acros Organics, Morris Plains, New Jersey, USA; AngeLite‐SDC (ALA) ATL Co., Shanghai, China (IPL) Instructions to participants: "Patients were instructed to avoid sun exposure for the first 48 h after treatment and to use regular sunblock." Intervention 2 "Before treatment, the entire face was cleansed with soap and 70% alcohol. …..the whole face was exposed to IPL...The 560 hand piece was used throughout the study, and patients received two passes at each treatment session. During the treatment, patients’ eyes were protected with eye goggles." Number and frequency of treatments: "2 in total, every two weeks" Wavelength/Fluence/Duration/Spot size: 560 nm/10‐12 J/cm²/"double pulse (width was 4–5 ms with a 20 ms delay)"/45.8 x 10.8 mm² Supplier: AngeLite‐SDC (ALA) ATL Co., Shanghai, China Instructions to participants: "Patients were instructed to avoid sun exposure for the first 48 h after treatment and to use regular sunblock." |
|
| Outcomes | Evaluation time points of review interest: 2 and 8 weeks after final treatment Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Change from baseline in number of individual lesions (ILs, NILs or N & Cs) 3. Percentage change from baseline of individual lesions (ILs, NILs or N & Cs) 4. Percentage change from baseline in combined number of lesions Methods of assessing primary outcomes 1. At 8 weeks after the last treatment, participants were asked to assess their improvement. Non standardised scale was used for evaluation: marked improvement = 3; moderate improvement = 2; no change = 1; acne worsened = 0 2.‐4. "The evaluation of efficacy was based on photographs taken before the first treatment and at follow‐up visits. Inflammatory lesions and comedones were counted." Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes "All adverse effects including vesicles, erythema, hyperpigmentation, edema, crusts, erosions, exfoliation, burning/stinging, and pain were recorded in detail at each treatment and follow‐up visit. Adverse effects were recorded according to the severity using a four‐point scale (0, absent; 1, mild; 2, moderate; 3, severe). Patients were instructed to contact the investigator if they experienced any problems between study visits." |
|
| Notes | Language: English. Baseline imbalances between groups regarding lesion counts including both NILs (50.9 versus 41.8) and ILs (15.7 versus 9.6). We attempted to contact the study authors but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 174): "This was a randomised controlled clinical trial (using the sealed‐envelope system)." Comment: Method used to generate the allocation sequence was not stated. We judged this as at unclear risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote (page 174): "This was a randomised controlled clinical trial (using the sealed‐envelope system)." Comment: We judged this as adequate and risk of bias as low. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Intention and/or method to blind participants and personnel were not specifically reported. Given the nature of the intervention and control, it is possible that the participants were blinded, but outcome assessors were not, which might have introduced some bias. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | Intention and/or method to blind participants and personnel were not specifically reported. Given the nature of the intervention and control, it is possible that the participants were blinded. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Quote (page 174): "The evaluation of efficacy was based on photographs taken before the first treatment and at follow‐up visits. Inflammatory lesions and comedones were counted." Comment: Unclear whether outcome assessors were blinded. We judged this as at unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether there were participants who withdrew or were lost to follow up. We judged this as at a unclear risk of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Unclear why results at both 2 and 8 weeks were not reported for all outcomes. Not specifically reported whether there were reports of blisters. We judged this as at a unclear risk of reporting bias. |
| Other bias | Unclear risk | Sponsorship was not declared. We judged this as at unclear risk of bias. |
Sadick 2010a.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Sponsorship unclear. No relevant disclosures to report (page 232) Setting: Single centre, New York (New York, USA) Recruitment: Unclear Duration: Unclear |
|
| Participants |
Included Age (inclusion criterion; mean; range): 18 > years; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "moderate to severe acne… score 3‐4 on the Investigator Global Assessment (IGA) scale" Fitzpatrick skin types: I‐III Other: "A two‐week washout period was required for any candidates who had used two or more topical or systemic therapies. One topical and/or systemic treatment was allowed." Excluded "…if they had used oral retinoids in the past three months. Subjects who were pregnant, planning to become pregnant, or breast‐feeding, and those with a previous diagnosis of facial psoriasis, seborrhetic dermatitis, perioral dermatitis or papulo‐pustular rosacea were excluded from participation. The study also eliminated those subjects with active infections, known photosensitivity, DUSA Pharmaceuticals, Wilmington, MA, porphyria or HIV/AIDS." Enrolled: 10 (M/F not reported) Randomised: 10 Withdrawals/drop‐outs: 2, unclear whether they withdrew or were lost to follow‐up Final number and proportion of participants evaluable: 8 (2 M/ 6F)/10 (80%) ITT analysis: Unclear |
|
| Interventions |
Intervention 1 10% acetone cleanser applied to face, followed by ALA application for 30 minutes. Subsequently, combination anaesthetic agent was applied (benzocaine 20%, lidocaine 4%, tetracaine 5%) for 30 min and removed with a gentle cleanser. Water‐based ultrasound gel was applied. Eye protection was applied, followed by KTP laser treatment. Number and frequency of treatments: 3 in total, 3‐4 weeks apart Wavelength/Fluence/Duration/Spot size: 532 nm/7 J/cm²/pulse duration 30 ms, 2 passes/10 mm² Supplier: Levulan Kerastick, Dusa Pharmaceuticals Instructions to participants: Not applicable Intervention 2 10% acetone cleanser applied to face, followed by combination anaesthetic agent (benzocaine 20%, lidocaine 4%, tetracaine 5%) for 30 min and removed with a gentle cleanser. Water‐based ultrasound gel was applied. Eye protection was applied, followed by KTP laser treatment. Number and frequency of treatments: 3 in total, 3‐4 weeks apart Wavelength/Fluence/Duration/Spot size: 532 nm/7 J/cm²/pulse duration 30 ms, two passes/10 mm² Supplier: Levulan Kerastick, Dusa Pharmaceuticals Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 2, 6 and 12 weeks after final treatment (also assessed at each treatment) Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes: 1. Grade 0 = clear skin, no inflammatory lesions; grade 1 = almost clear, rare non‐inflammatory lesions, few small inflammatory lesions; grade 2 = mild severity, some non‐inflammatory lesions, some inflammatory lesions (papules, pustules, no nodular lesions); grade 3 = moderate severity, many non‐inflammatory and moderate inflammatory lesions, no more than one nodular lesion; grade 4 = severe, many non‐inflammatory and inflammatory lesions, nodular lesions are present. "At follow up visits, photographs were taken and all subjects were queried about adverse events and changes in concomitant medications" 2. See 1. above |
|
| Notes | Language: English. We attempted to contact the study author but were not successful. Concomitant treatment allowed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 229): "a randomized, split‐face study"; (page 230): "…prior to randomized application of ALA (Levulan Kerastick) for 30 minutes." Comment: Method used to generate the allocation sequence was not stated in the report, so we judged this as at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not stated in the report so we judged this as at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 229): "An open‐label, split‐face study…." (title) Comment: We judged this as at high risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes of interest for our review. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | High risk | Quote (page 229): "An open‐label, split‐face study…" (title); (page 230): "At follow‐up visits photographs were taken…" Comment: Photographs were used, but investigators were not blinded, so we judged the risk of bias as high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures obtained for 80% of randomised participants, so we judged the risk of bias as low. |
| Selective reporting (reporting bias) | High risk | Quote (page 230): "Follow‐up visits occurred at two, six and 12 weeks after the third treatment." (page 231): "Similar results were recorded after the third treatment session that was evaluated at week 12." Comment: Results not reported at time points pre‐specified in the methods section, so we judged the risk of bias as high |
| Other bias | Unclear risk | No information on sponsorship in the report. We judged the risk of bias as unclear. |
Sadick 2010b.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: The study was sponsored – no further details provided. Study authors declared no conflicts of interest (page 283). Setting: Multicentre, New York (New York, USA) and Tel Aviv (Israel) Recruitment: Department of Dermatology, Weill Medical College of Cornell University, New York, NY, USA and Zahava Laver Dermatology Clinic, Tel Aviv, Israel Duration: 3 months, April 2008‐June 2008 |
|
| Participants |
Included Age (inclusion criterion; mean; range): 14 >; 23.6 years; 14‐47 years Clinically evident acne: Yes Severity of condition assessment: "with at least four inflamed lesions (papules or pustules) on the face" Fitzpatrick skin types: II‐VI Other: "Only subjects who were at least 14 years old, who met all inclusion and exclusion criteria, who were not on any other acne treatment regimen and who signed the informed consent form were enrolled." No further details reported. Excluded Other acne treatment regimen (see above). Details unclear Enrolled: 63 (16 M/47 F) in total; 32 (6 M/26 F) in the light group, 31 (10 M/21 F) in the placebo group Randomised: 63 Withdrawals/drop‐outs: 2 withdrew early in the placebo device group: 1 due to "non‐compliance with the treatment regimen" and the other "due to consent withdrawal". Final number and proportion of participants evaluable: 61/63 (97%) in total; 32/32 (100%) in the light group, 29/31 (94%) in the placebo group ITT analysis: Unknown (study author's reply) |
|
| Interventions |
Intervention 1 "...small, hand‐held device intended for the treatment of individual mild‐to‐moderate inflammatory acne lesions (papules and pustules)", "each treatment included 2 passes of the device on each lesion. Number and frequency of treatments: 8 in total, twice a day for 4 days, once a day at home and once a day at the clinic in front of an unblinded observer Wavelength/Fluence/Duration/Spot size: 450–2000 nm/6 J/cm² per treatment cycle/unclear Supplier: The no!no! Skin ™ device (Radiancy Inc.) Instructions to participants: Printed out instruction sheets to take home Intervention 2 Placebo device provided by same supplier. Looked the same as the treatment device but emitted no energy. Number and frequency of treatments: 8 in total, twice a day for 4 days, once a day at home and once a day at the clinic in front of an unblinded observer Wavelength/Fluence/Duration/Spot size: Not applicable Supplier: The no!no! Skin ™ device (Radiancy Inc.) Instructions to participants: Printed out instruction sheets to take home |
|
| Outcomes | Evaluation time points of review interest: None, please see 'Notes' (study author’s reply: assessed at "Baseline, visit 2, 3, 4, and 5 (D0‐D4)") Primary outcomes of review interest: not recorded. Please see 'Notes' Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes: 1. "Safety was assessed based upon daily evaluation by the subjects and the unblinded observer of any possible side effects, such as erythema, edema, crusting, blistering or pigmentary changes. Subjects were also asked to report any subjective side effects such as pain, heat sensation, itching, skin dryness or tightness." |
|
| Notes | Language: English. Comparison of interventions and the outcomes at time points as defined by our protocol was not possible. Final evaluation on the 5th day, primary endpoints were defined as time to improvement and time to resolution. Possible baseline imbalances: "No statistically significant difference was found between the study arms with regard to the number of lesions, anatomical site and global acne assessment; however, a difference was found in the type of lesion. The active arm had a higher percentage of pustules (45% vs 26%) and a lower percentage of papules (55% vs 74%) compared with the placebo arm ( P = 0.0012)." We contacted the study authors who provided additional information on power calculation, sponsorship, ITT analysis, evaluation time points, instructions to participants, random sequence generation and allocation concealment, primary outcomes of the study and whether blistering or scarring was reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 277): "Thirty‐one subjects were randomly assigned to the treatment group where all lesions were treated with the active device, while 32 subjects were randomly assigned to the control group where all lesions were treated with placebo devices. Lesions assigned for treatment, in both groups, were designated by the subjects themselves." Comment: Method used to generate the allocation sequence was not stated in the report but the author clarified that participants were "randomly assigned by blinded sponsor numerical allocation." We judged this as appropriate and the risk of bias as low. |
| Allocation concealment (selection bias) | Low risk | Intention and/or method to conceal the allocation sequence were not stated in the report but the study author clarified that participants were "randomly assigned by blinded sponsor numerical allocation." We judged this as appropriate and the risk of bias as low. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 277): "Treatments were self‐administered twice a day for 4 days: once a day at home and once a day at the clinic in front of an unblinded observer." Comment: Performing clinicians unblinded. Details of the sham device were not given in the report, but the author clarified that the sham device "was provided by same supplier and looked the same as the treatment device but emitted no energy". Unclear whether blinding of participants was successful. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes of interest for our review. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 277‐278): "All lesions were photographed at baseline and at each of the daily clinic visits….The unblinded observer also maintained a daily log to record treatments and any adverse events…at the end of the study treatment results were assessed by the blinded investigator and a blinded independent evaluator, each assessing the effect of treatment on each treated lesion based on the macro photographs of the lesions." Comment: Outcome assessors were blinded adequately. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures obtained for more than 80% in each of the groups (for 94% of participants in the active device group and 100% in the placebo device group), so we judged the risk of bias as low. |
| Selective reporting (reporting bias) | Low risk | Outcomes were not pre‐specified clearly in the 'Methods' section. Study authors clarified that primary outcomes were "the efficacy of the OTC device defined as lesion time to improvement and time to resolution as well as safety of device." The study author also provided results for the outcomes of review interest. We therefore judged the risk as low. |
| Other bias | Unclear risk | No information on sponsorship in the report, the study author clarified that the study was sponsored, but gave no further details. We judged this as at unclear risk of bias. |
Sami 2008.
| Methods | This was a parallel‐group RCT. Reported that participants were treated "unilaterally", so possibly split‐face within parallel groups. Details not provided Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Not declared Setting: Unclear whether single or multicenter; Cairo? (Egypt) Recruitment: Not reported Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; 29 years; 20‐38 years Clinically evident acne: Yes Severity of condition assessment: "moderate to severe facial acne according to Burton classification" Fitzpatrick skin types: III‐IV Excluded History of photosensitivity; pregnancy, topical acne treatment or systemic antibiotics within 2 weeks; systemic steroids, anti‐inflammatory drugs or systemic retinoids within 6 months Enrolled: 45 (18 M/27 F) in total, 15 in each group Randomised: 45 Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: Unclear ITT analysis: Unclear |
|
| Interventions |
Intervention 1 PDL Number and frequency of treatments: treatments were continued until ≥ 90% clearance of lesions was achieved (4.1 ± 1.39 treatments), applied weekly Wavelength/Fluence/Duration/Spot size: 595 nm/6‐8 J/cm²/0.5 ms/7 mm² Supplier: Vbeam®, Candela Corp., Wayland MA with cryogen spray DCD, Candela Corp.) Instructions to participants: Not applicable Intervention 2 IPL Number and frequency of treatments: treatments were continued until ≥ 90% clearance of lesions was achieved (6 ± 2.05 treatments), applied weekly Wavelength/Fluence/Duration/Spot size: 550‐1200 nm/22 J/cm²/30 ms/11.25 cm² Supplier: EPI‐C/plus®, Espansione Group, Bologna, Italy Instructions to participants: Not applicable Intervention 3 Combined blue‐red light emitting diode (LED) Number and frequency of treatments: treatments were continued until ≥ 90% clearance of lesions was achieved (10 ± 3.34 treatments), applied twice a week Wavelength/Fluence/Duration/Spot size: 470 nm/10 mW/cm² (first session) followed by 623 nm 40 mW/cm² (second session)/20 min (continuous), 10 min (pulsed) Supplier: Young Again®, Espansione Group Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: None, please see 'Notes' (evaluated at 4 weeks after final session) Primary outcomes of review interest: not recorded. Please see 'Notes'. Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. "Patients were also asked about any symptoms or signs of adverse effects at each treatment session" |
|
| Notes | Language: English. Comparison of interventions and the outcomes at time points as defined by our protocol was not possible. Duration and number of treatments differed among the groups, as participants were treated once a week "until > 90% clearance of lesions was achieved" (in group 1 4.1 ± 1.39, group 2 6 ± 2.05, group 3 10 ± 3.34 sessions). Assessment was done at 4 weeks and after the final session, which was different for each group. We attempted to contact the study authors but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 627): "Patients were randomly divided into 3 equal treatment groups. Treatment was carried out unilaterally…" Comment: Method used to generate the allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes of interest for our review. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 629): "Evaluating physicians were blinded to the treatment assignment with consideration that each patient characteristics were noted by the same physician" Comment: We judged this as at low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported whether there were participants who withdrew or were lost to follow‐up. We judged this as at unclear risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcome measures prespecified in the 'Methods' section reported |
| Other bias | Unclear risk | Unclear sponsorship. We judged this as at unclear risk of bias. |
Seaton 2003.
| Methods | This was a parallel‐group and split‐face RCT. Unit of randomisation: Whole person and left or right face Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (page 1352): "Since completion of the trial, EDS has started laboratory research into the mechanism of action of PDL therapy in acne at the Department of Dermatology, Imperial College, London, UK, with financial support from EUPhotonics. RMC was an academic employee of EUPhotonics, and contributed to development of the laser and trial conception, but not to detailed trial design, data collection, data analysis, or interpretation of the results. The other authors have no conflict of interest."; "EUPhotonics (Swansea, Wales) provided the laser."; Quote (page 1349): "The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report." Setting: Single centre (London, UK) Recruitment: Individuals were recruited through a public request for participants or because of referral to the dermatology clinic. Recruitment from Nov 2001, and April 2002, to avoid confounding effects of summer sunlight. "26 laser‐allocated patients and nine controls had volunteered for the trial independently, whereas the remainder were recruited by the investigators after referrals to the dermatology outpatient clinic." Duration: Recruitment November 2001 to April 2002. Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; not reported; 18‐45 years Clinically evident acne: Yes Severity of condition assessment: "mild‐to‐moderate facial inflammatory acne defined as the presence of at least ten acne papules or pustules between the brow and jaw line and an acne severity score of between 2 and 7 on the Leeds revised acne grading system" Fitzpatrick skin types: Not reported Excluded Not stated Enrolled: Intervention 1: 31 (1 1M/20 F); Intervention 2: 10 (4 M/6 F) Randomised: Intervention 1: 31; Intervention 2: 10 Withdrawals/drop‐outs: Intervention 1: 2 participants by 8 weeks and 1 by 4 weeks, all 3 of whom left the locality. Another patient withdrew by 4 weeks after needing systemic antibiotic treatment for worsening truncal acne. Intervention 2: 1 patient withdrew because of dissatisfaction with clinical response Final number and proportion of participants evaluable: Intervention 1: 27 (87%) Intervention 2: 9 (90%) ITT analysis: Yes |
|
| Interventions |
Intervention 1 PDL. Participants were randomly allocated to receive 1.5 J/cm² on one side of the midline and 3 J/cm² on the other. Number and frequency of treatments: Single treatment. Wavelength/Fluence/Duration/Spot size: 585 nm/1.5 or 3.0 J/cm²/350 µs pulse duration/5 mm² Supplier: Nlite system, EUPhotonics, Swansea, Wales, UK Instructions to participants: Not applicable Intervention 2 Sham laser Number and frequency of treatments: Single treatment Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 2, 4, 8 and 12 weeks after treatment (single session) Primary outcomes of review interest recorded 1. Percentage change from baseline in number of ILs & NILs Methods of assessing primary outcomes 1. Total lesion counts (ILs and NILs), ILs counts (papules and pustules), and NILs counts (open and closed comedones) Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse events Methods of assessing secondary outcomes 1. Leeds revised grading system 2. Possible adverse events were assessed by direct questioning of participants and by review of daily diary sheets that all participants were asked to complete |
|
| Notes | Language: English. We attempted to contact the study authors, but were not successful. We did not contact sponsors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1348): "At recruitment, patients were randomised to either laser or a sham treatment by a computer‐generated sequence." Comment: Adequate and at a low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote (page 1348): "Allocations were contained in opaque, sequentially numbered, sealed envelopes and were concealed from assessors and patients throughout the study and revealed only to the investigator (EDS, AC, or ACC) who was assigned to treat the patient." Comment: Adequate and at a low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 1348): "Controls were treated with a disconnected laser hand piece that was moved across the face in an identical manner to that for the PDL group. All patients wore opaque goggles during treatment to protect their eyes and to ensure that they were unaware of the therapy they received. Treatment was given in a locked room with no windows." Comment: We judged this as inadequate. PDL usually emits a sound with each pulse and so any participants that have received PDL previously may have been aware that they were receiving sham treatment. We therefore judged blinding of participants as ineffective. No evidence that clinicians were blinded, and blinding was unlikely given the nature of the intervention. We judged the risk of bias as high. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 1348): "Investigators were not included in preliminary or post‐treatment assessments of patients that they had treated." Comment: We judged this as at low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures obtained for over 80% of randomised participants in each group. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were reported. |
| Other bias | Unclear risk | Commercial sponsorship might have introduced some bias. Insufficient information to permit a clear judgement. |
Song 2014.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared, none (page 764) Setting: Single centre, Seoul (Korea) Recruitment: Unclear Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): 18‐35 years; 23.4 ± 3.5 years; 18‐32 years Clinically evident acne: Yes Severity of condition assessment: Mild to moderate "acne on both sides of the face", included participants had Cunliffe grades 2‐4 Fitzpatrick skin types: III‐IV Other: "Equivalent severity between the 2 sides", ability to comply with the study protocol Excluded "Use of any topical acne treatment or systemic antibiotics within 6 weeks before study initiation; use of a systemic retinoid within 9 months before study initiation; use of a systemic steroid within 9 months before study initiation; history of photosensitivity; recent use of photosensitizing drugs within 6 weeks before study initiation; presence of any other skin disease that could interfere with the assessment of the acne, such as folliculitis or rosacea; presence of any other systemic disease that could affect the acne severity by its presence, such as polycystic ovarian syndrome, or by any medication prescribed for the treatment of the systemic diseases; presence of any change in the use of oral contraceptive pills or anti‐inflammatory drugs within 3 months before study initiation; pregnancy or lactation; presence of evidence indicating likely poor compliance with the protocol.’ Enrolled: 24 (14 M/10 F) Randomised: 24 Withdrawals/drop‐outs: Not reported. In abstract 24 completed Final number and proportion of participants evaluable: Unclear ITT analysis: Unclear |
|
| Interventions |
Intervention 1 Sheets of chlorophyll‐a incubated without occlusion for 30 minutes plus combined red and blue light emitting diode (LED) irradiation Number and frequency of treatments: 8 in total, twice weekly Wavelength/Fluence/Duration/Spot size: 430 + 660 nm; 1170 + 1080 J/cm² over 30 min Supplier: Biolight LT‐560, Beautech, Seoul, Korea; Virta‐Healer, Aseptica, Moscow, Russia Instructions to participants: "Subjects were treated in the late afternoon, and instructed to avoid sun exposure until the following morning’s sunlight. There was no restriction placed with respect to other forms of ambient lighting." Intervention 2 Combined red and blue LED irradiation Number and frequency of treatments: 8 in total, twice weekly Wavelength/Fluence/Duration/Spot size: 430 + 660 nm; 1170 + 1080 J/cm² over 30 min Supplier: Biolight LT‐560, Beautech, Seoul, Korea Instructions to participants: "Subjects were treated in the late afternoon, and instructed to avoid sun exposure until the following morning’s sunlight. There was no restriction placed with respect to other forms of ambient lighting." |
|
| Outcomes | Evaluation time points of review interest: 2 weeks after final treatment (also evaluated at baseline and at "follow‐up visits after the second (week 1), fourth (week 2), sixth (week 3), and eighth (week 4) sessions") Primary outcomes of review interest recorded 1. Change and percentage change from baseline in IL count (papules, pustules and nodules reported separately) 2. Change and percentage change from baseline in NIL count (open and closed comedones reported separately) Methods of assessing primary outcomes 1. & 2. "Facial photographs were taken at each visit… acne lesion counts (closed/open comedone, papule, pustule, and nodule or cyst) by a dermatologist who was blinded to the treatment received." Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. "Facial photographs were taken at each visit. Assessments were conducted by evaluating the acne severity based on the Cunliffe grading system.." 2. Unclear |
|
| Notes | Language: English. We attempted to contact the study authors, but were not successful. Possibly the same study as Song 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 766): "According to a predetermined randomization table using a random permuted block method, one side of the face received chlorophyll‐a PDT, whereas the other side underwent LED phototherapy as a control." Comment: We judged this as adequate and at a low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study was reported as single blind. No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. Given that one side of the face was treated with chlorophyll‐a applied and then laser it is unlikely that participants/personnel were blinded. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 766): "Facial photographs were taken at each visit. Assessments were conducted by evaluating the acne severity based on the Cunliffe grading system and acne lesion counts (closed/open comedone, papule, pustule, and nodule or cyst) by a dermatologist who was blinded to the treatment received." Comment: We judged this as adequate and risk of bias as low. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Possible withdrawals and lost to follow‐ups were not reported. We judged this as at unclear risk of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Data not reported for cysts and nodules at final assessment the way it was for other lesion counts, only in graph format. We judged the risk of bias to be unclear. |
| Other bias | Low risk | No other possible sources of bias were identified. |
Taub 2007.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Declared. Quote (page 1016): "Dr. Taub received no funding for this investigation. She is a consultant to Dusa Pharmaceuticals Inc. She receives educational honoraria and research grants from Syneron and Cutera." Setting: Single centre (Lincolnshire IL, USA) Recruitment: Not reported Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 26.5 ± 9.1 years; not reported Clinically evident acne: Yes Severity of condition assessment: Grade 3‐4 acne (1 = mild, 2 = mild to moderate, 3 = moderate, 4 = severe) and at least 10 ILs Fitzpatrick skin types: II‐IV Excluded Not stated Enrolled: 22 (M/F not reported) Randomised: 22 Withdrawals/drop‐outs: 2 withdrawals (reasons not reported); 1 missed 1 month follow‐up; 6 missed 3 month follow‐up Final number and proportion of participants evaluable: 18 (81%) at 4 weeks' follow‐up and 13 (59%) at 12 weeks' follow‐up ITT analysis: Not stated |
|
| Interventions |
Intervention 1 20% ALA‐PDT with IPL. Treated areas scrubbed with acetone before ALA application, ALA incubated for 30 min, and removed with alcohol and water immediately after irradiation. Half of participants received 5% ferric chloride lotion to apply hourly for 48 h after treatment Number and frequency of treatments: 3 treatments in total, applied every 2 weeks Wavelength/Fluence/Duration/Spot size: 600‐850 nm/8‐12 J/cm²/other not reported Supplier: 20% ALA, Levulan Kerastick, DUSA Pharmaceuticals; Xeo OPS, Cutera, Inc Instructions to participants: Not applicable Intervention 2 20% ALA‐PDT with IPL and bipolar radiofrequency energies. Treated areas scrubbed with acetone before ALA application, ALA incubated for 30 min, and removed with alcohol and water immediately after irradiation. Half of participants received 5% ferric chloride lotion to apply hourly for 48 h after treatment. Number and frequency of treatments: 3 treatments in total, applied every 2 weeks Wavelength/Fluence/Duration/Spot size: 580‐980 nm/16‐36 J/cm²/other not reported Supplier: 20% ALA, Levulan Kerastick, DUSA Pharmaceuticals; Aurora SR, Syneron Medical Ltd. Instructions to participants: Not applicable Intervention 3 20% ALA‐PDT with blue light. Treated areas scrubbed with acetone before ALA application, ALA incubated for 30 min, and removed with alcohol and water immediately after irradiation. Half of participants received 5% ferric chloride lotion to apply hourly for 48 h after treatment. Number and frequency of treatments: 3 treatments in total, applied every 2 weeks Wavelength/Fluence/Duration/Spot size: 417 nm/not reported/6‐10 minutes exposures/not reported Supplier: 20% ALA, Levulan Kerastick, DUSA Pharmaceuticals; BLU‐U, DUSA Pharmaceuticals Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 4 and 12 weeks after final treatment Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Change from baseline in combined number of lesions Methods of assessing primary outcomes 1. Not stated 2. Details not provided Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Investigator’s global assessment of improvement 3. Adverse effects Methods of assessing secondary outcomes 1. Non‐standard grading scale (1 = mild, 2 = mild to moderate, 3 = moderate, 4 = severe) 2. Unclear 3. Unclear |
|
| Notes | Language: English. Quote: (page 1010): "patients received not topical or systemic acne treatment…during the study period and 3 months after the final treatment" Comment: there was no assessment of compliance with this statement. Number of participants in each group unclear. Full data not reported at 3 months; 96.9% CI were not reported, but ranges "(difference between the upper and lower ends of 96.9% CI) indicated when < 5 data points are available." as described in the Table 1, page 1011. We contacted the study authors but they were unable to provide further clarification. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 1010): "Patients were randomly assigned to receive..." Comment: Method used to generate the allocation sequence was not described. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. Given the nature of the interventions involved then blinding of personnel is unlikely. We judged the risk of bias as unclear. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | No intended blinding of participants reported. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | No intended blinding of outcome assessors reported. No evidence that assessors were blinded provided. Comment: We judged this as at unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome measures obtained for 81% at 1 month and 59% at 3 months. We judged this as at high risk of bias. |
| Selective reporting (reporting bias) | High risk | Full data were not reported at 3 months if there were less than 5 participants in the group. Data reported in Table 1. (page 1011) different from data in Table 2. (page 1013). We judged this as at high risk of bias. |
| Other bias | Unclear risk | Study authors declared conflicts of interest. Insufficient information to judge whether additional bias was introduced |
Tzung 2004.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face. Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Multicenter (Kaohsiung and Hsing‐Chu, Taiwan) Recruitment: Departments of Dermatology, Veterans General Hospital Kaohsiung and Chu‐Tung Veterans Hospital, Hsing‐Chu, Taiwan Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 20.79 years; 15‐32 years Clinically evident acne: Yes Severity of condition assessment: "mild‐to‐moderate severe acne vulgaris symmetrically on the face" Fitzpatrick skin types: III‐IV Excluded Use of topical/systemic treatment within 2 weeks, on medication that exacerbates/alleviates acne, planning excessive sun exposure, pregnancy/lactation Other: Other active treatment was not allowed during the treatment and within 1 month after the treatment completion Enrolled: 31 (M/F not reported) Randomised: 31 Withdrawals/drop‐outs: 3 (2 unsatisfactory results, 1 reason not stated) Final number and proportion of participants evaluable: 28 (90%) ITT analysis: Not stated |
|
| Interventions |
Intervention 1 After gentle facial wash and eye protection with goggles, participants were irradiated with blue light on one side of the face twice weekly for four consecutive weeks. Number and frequency of treatments: 8 treatments in total, twice weekly for 4 consecutive weeks Wavelength/Fluence/Duration/Spot size: 420+/‐ 20 nm/40 J/cm² per treatment/other not reported Supplier: F‐36 W/Blue V, Waldmann, Villingen‐ Schwenningen, Germany Instructions to participants: Not applicable Intervention 2 The other half of the face was left untreated as a control. |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity Methods of assessing secondary outcomes 1. Acne score modified from that previously described by Michaelson et al: each type of lesion was given a severity index as follows: 0.5 for comedo, 1 for papule (1‐55 mm), 2 for pustule, 3 for nodule (> 5 mm) and 4 for inflammatory cyst |
|
| Notes | Language: English. Table with baseline data reported, which didn't include ILs counts of irradiated and non‐irradiated sides of the face. Results reported in graph format. Analysis of arbitrarily divided groups. We contacted study authors but they were unable to provide requested data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 266): "The side of irradiation was randomly assigned for each patient." Comment: Method of randomisation was not stated |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. Given the nature of the interventions involved then blinding of participants/personnel is unlikely. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 267): "All above‐mentioned evaluations were assessed by two dermatologists unaware of the status of treatment" Comment: We judged this as at low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures were obtained for 90% of subjects randomised. We judged this as at a low risk of bias. |
| Selective reporting (reporting bias) | High risk | Quote (page 268): "To further analyse the effect of blue light irradiation on differently weighted acne lesions in all 28 patients, three groups were arbitrarily divided, including comedones, papulopustules, and nodulocysts." Comment: No baseline data for acne severity scores of irradiated and non‐irradiated sides. Results for arbitrarily divided groups reported which were not pre‐specified in the methods section. We judged this as at a high risk of selective reporting. |
| Other bias | Unclear risk | Sponsorship was not declared which might have introduced bias. Also, there is no mention of the untreated side of the face being covered and so the control side may have received some irradiation. Insufficient information was given to permit a clear judgement. We contacted the study authors in 2008, but they were unable to provide requested data and clarifications. |
Uebelhoer 2007.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (page 552): "The laser used in this study was loaned by Candela Corp. Funding for the study was also provided by Candela." Setting: Multicenter (USA) Recruitment: Not stated – study authors from multiple centres; "volunteers" (page 552) Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 26 years; 19‐39 years Clinically evident acne: Yes Severity of condition assessment: "with at least 10 inflammatory papules on each side of the face" (page 553) Fitzpatrick skin types: Not reported Excluded Use of oral retinoids or systemic corticosteroids within the past 6 months and the use of all prescription topical or systemic anti acne medications 4 weeks before initiation of the study. Enrolled: 11(2 M/9 F) Randomised: 11 Withdrawals/drop‐outs: No withdrawals, 2 lost to follow‐up Final number and proportion of participants evaluable: 9 (82%) ITT analysis: Not stated |
|
| Interventions |
Intervention 1 Single pass treatment where each spot was pulse stacked with 2 pulses at 1 Hz and separated from subsequent spots by a distance of 0.5 to 1 cm. Number and frequency of treatments: 3 treatments, every 3 weeks Wavelength/Fluence/Duration/Spot size: 1450 nm/9.5‐ 11 J/cm² (as tolerated)/210 ms/6 mm² Supplier: Smoothbeam, Candela Corp. Instructions to participants: Not applicable Intervention 2 Using the same 0.5 cm to 1 cm spacing, the other side of the face received a double pass treatment where each spot was single pulsed, followed 2 min later by a second pass covering the same area but not necessarily the same precise spots. Number and frequency of treatments: 3 treatments, every 3 weeks Wavelength/Fluence/Duration/Spot size: 1450 nm/9.5‐ 11 J/cm² (as tolerated)/210 ms/6 mm² Supplier: Smoothbeam, Candela Corp. Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 12 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Percentage change in lesion count from baseline in number of combined lesions Methods of assessing primary outcomes 1. Lesion counts Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Investigator’s global assessment of improvement 3. Adverse effects Methods of assessing secondary outcomes 1. Allen & Smith grading system 2. Non‐standardised 0‐3 scale: 0, none; 1, mild; 2, moderate; 3, marked improvement, using photographs 3. "Immediate clinical assessment of all side effects including erythema, edema, papules or blistering was recorded. At each follow up, an evaluation of textural defects, hyper and hypopigmentation was performed". |
|
| Notes | Language: English. We attempted to contact the study authors but were not successful. We did not contact sponsors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 553): "Selection of technique for the right or left side was determined before study initiation by the toss of a coin." Comment: We judged this as adequate. |
| Allocation concealment (selection bias) | Low risk | Quote (page 553): "This predetermined treatment scheme for each of the subjects was placed in a sealed envelope that was opened after subject enrolment and prior to the treatment." Comment: We judged this as adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. Given the nature of the interventions involved then blinding of participants/ personnel is unlikely. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 553): "The primary investigator performed acne lesion counts, classification of acne lesion type and evaluation of acne severity at baseline, before each follow‐up treatment and at 3 months after the final treatment….an assessor blinded to the treatment regimen also performed acne lesion counts and acne severity grade assessment on each subject at the same time intervals." Comment: Outcomes of blinded assessor reported. We judged this as adequate and risk as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures were reported for 82% of subjects randomised. We judged this as at a low risk of bias. |
| Selective reporting (reporting bias) | High risk | Outcomes for acne severity score were not reported at 3, 6 and 9 weeks. Global assessments of improvement not reported at any time point. We judged this as at a high risk of bias. |
| Other bias | Unclear risk | Funding from the company producing the laser used in the study might have introduced bias. No other sources of bias identified. Insufficient information to permit a clear judgement. |
Wang 2006.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: No Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (page 249): "This study was partially funded through a research grant from the Candela Corporation." Setting: Single centre (Minneapolis, Minnesota, USA) Recruitment: "recruited from the clinic population"; Department of Dermatology, University of Minnesota, Minneapolis Duration: 18 months, April 2003‐September 2004 |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 18 years, 34.3 years, 19‐59 years Clinically evident acne: Yes Severity of condition assessment: "active inflammatory acne" and "moderate to severe acne vulgaris on the face" Fitzpatrick skin types: II‐IV Other: willingness to follow the treatment schedule and post‐treatment care requirements; willingness to discontinue use of topical or systemic anti acne medications 3 weeks before the first treatment during the study period. Excluded "(1) presence of scars over the area to be treated, (2) known photosensitivity, (3) ingestion of medication known to induce photosensitivity in the previous 3 months, (4) used topical or oral antibiotics or other topical anti acne treatments in the previous 4 weeks, (5) received Accutane in the previous 6 months, and (6) currently pregnant or lactating." Enrolled: 20 (7 M/13 F) Randomised: 20 Withdrawals/drop‐outs: Not reported. However, it is not stated whether any participants were LFU and the results are expressed as mean lesion counts. Final number and proportion of participants evaluable: 19 (95%) ITT analysis: No |
|
| Interventions |
Intervention 1 Microdermabrasion (6 passes at the full setting) and laser (topical lidocaine 5% applied for 30 min, cleansed, then treated with the smooth beam 1450 nm laser) Number and frequency of treatments: 4 treatments every 3 weeks Wavelength/Fluence/Duration/Spot size: 1450 nm/13.5‐14 J/cm²/not reported/6 mm² Supplier: microdermoabrasion (Vibraderm, Dermatherm, Irving, TX); laser (Candela Corp., MA) Instructions to participants: "Each subject was given instructions on post‐treatment care, including sun avoidance instruction." Intervention 2 Laser (topical lidocaine 5% applied for 30 min, cleansed, then treated with the smooth beam 1450nm laser) Number and frequency of treatments: 4 treatments every 3 weeks Wavelength/Fluence/Duration/Spot size: 1450 nm/13.5‐14 J/cm²‐/not reported/6 mm² Supplier: Candela Corp., MA Instructions to participants: "Each subject was given instructions on post‐treatment care, including sun avoidance instruction." |
|
| Outcomes | Evaluation time points of review interest: 6 and 12 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Change in lesion count from baseline in number of combined lesions Methods of assessing primary outcomes 1. Lesion counts Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Pain assessed by participants using VAS (0 = no pain, 10 = worst possible pain), other adverse effects assessed by investigators using 4‐point scale: 0 (absent), 1 (mild), 2 (moderate), 3 (severe) |
|
| Notes | Language: English. We contacted the study authors who provided further information on power calculation, ITT analysis, study duration and selection bias. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 250): "The subjects were randomly assigned to receive the combination treatment (i.e. microdermabrasion and the 1,450 nm diode laser) on one side of the face. The other side of the face served as the control, receiving only the laser treatment." Comment: Method used to generate the allocation sequence was not stated. The study authors were contacted but were unable to provide additional data on the method used. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported and study authors were unable to provide further details of allocation process. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and performing clinicians were not blinded, so we judged the risk of bias as high. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 251): "An independent observer counted the acne lesions from the photographs and was not aware of the level of treatment (i.e. a 1,450 nm laser alone versus a 1,450 nm laser plus microdermabrasion) that each side of the face received." Comment: We judged this as at low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 251): "Of the 20 subjects, 19 patients completed all four treatments." Comment: Outcomes obtained for 95% of the participants and we judged this as at low risk of bias. However, it is not stated whether any participants were lost to follow‐up and the results are expressed as mean lesion counts. |
| Selective reporting (reporting bias) | Low risk | All outcome measures pre‐specified in the methods section reported. |
| Other bias | Unclear risk | Sponsorship by the company producing the laser used in the study might have introduced bias. No other sources of bias identified. Insufficient information to permit a clear judgement. |
Wiegell 2006a.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Unit of analysis: Lesion Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared. No commercial/financial interest reported by the study authors (page 647). Setting: Single centre (Copenhagen, Denmark) Recruitment: Department of Dermatology, Bispebjerg Hospital, Copenhagen Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 18 years; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: More than 12 inflammatory acne lesions Fitzpatrick skin types: Not reported Excluded History of topical or oral acne treatment within 4 months of study initiation, oral retinoid treatment within 1 year Enrolled: 15 (M/F not reported) Randomised: 15 Withdrawals/drop‐outs: 2 lost to follow‐up (1 did not appear at any follow‐up, one at 12 weeks' follow‐up) Final number and proportion of participants evaluable: 13 (87%) ITT analysis: Not stated |
|
| Interventions |
Intervention 1 2 g of MAL applied and covered with light impermeable dressings for 3 h followed by illumination with red light. Number and frequency of treatments: Single treatment Wavelength/Fluence/Duration/Spot size: 620 nm/37 J/cm²/other not reported Supplier: Commercial MAL cream (Metvix, PhotoCure ASA, Oslo, Norway); Aktilite, PhotoCure ASA Instructions to participants: Not applicable Intervention 2 2 g of ALA applied and covered with light impermeable dressings for 3 h followed by illumination with red light. Number and frequency of treatments: Single treatment Wavelength/Fluence/Duration/Spot size: 620 nm/37 J/cm²/other not reported Supplier: ALA cream produced by hospital pharmacy as a 20% d‐aminolevulinic acid hydrochloride (Sigma Chemical Company, St Louis, Mo) in a Metvix‐placebo cream. Aktilite, PhotoCure ASA Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 6 and 12 weeks after final treatment (single treatment in both interventions) Primary outcomes of review interest recorded 1. Change from baseline in number of ILs and NILs Methods of assessing primary outcomes 1. Lesion counts, using a face‐counting template, excluding the nose, lips, and the areas surrounding the eye, assessed live by a dermatologist Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. Global grade of acne severity (Leeds revised scale), assessed live by a dermatologist 2. Pain assessed by a numeric scale ranging from 0‐10, in which 0 is no pain and 10 is worst imaginable pain, method not stated for other adverse effects |
|
| Notes | Language: English. Quote (page 648): "Before treatment there was significantly more inflammatory lesions in the MAL‐treated side of the face than in the ALA‐treated side (P = .0049). This was a coincidence since the creams were randomised to either side of the face by lot before treatment." Comment: Baseline lesion counts provided, however, substantial differences across face sides treated with different creams. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 648): "The application side of the two creams was randomised before the study." Comment: Method used to generate the allocation sequence was not described. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (page 648): "We used a commercial MAL cream (Metvix, PhotoCure ASA, Oslo, Norway). The ALA cream was produced by our hospital pharmacy as a 20% d‐aminolevulinic acid hydrochloride (Sigma Chemical Company, St Louis, Mo) in a Metvix‐placebo cream. The application side of the two creams was randomised before the study. The patients and the primary investigator were blinded to the creams." Comment: We judged this as adequate and at a low risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 648): "The evaluating dermatologist was blinded to the creams." Comment: This was probably the case and we judged it as at low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome measures were obtained for 87% of subjects randomised. Comment: We judged this as at low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcome measures pre‐specified in the methods section reported. |
| Other bias | Low risk | No other possible source of bias identified. We judged this as a low risk of bias. |
Wiegell 2006b.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Declared. No conflict of interest (page 969) Setting: Single centre (Copenhagen, Denmark) Recruitment: By newspaper advertising/Dermatology Department, Bispebjerg Hospital, Copenhagen Duration: 6 months, November 2004 (recruitment), December 2004 (treatment) to March 2005 |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 18 years; 23 ± 5 treatment group, 24 ± 5 control group (not reported for the whole sample); not reported Clinically evident acne: Yes Severity of condition assessment: More than 12 ILs in the face Fitzpatrick skin types: II‐V Other: With general good health Excluded Skin type VI (black skin), pregnant or lactating women, history of oral retinoid use within 1 year of study entry, systemic antibiotics within 1 month, topical acne treatment within 2 weeks. Enrolled: 36 (M/F not reported) Randomised: 21 in the treatment group and 15 in the control group Withdrawals/drop‐outs: 5 left the study before the first visit. 2 participants did not receive allocated treatment because of personal reasons in the treatment group. Seven participants in the treatment group did not receive the second treatment due to adverse effects during and after the first treatment (2 due to pain during first treatment, 4 due to side effects after first treatment, 1 due to dissatisfaction with response). In the control group 1 patient withdrew because of pregnancy and 2 because of personal reasons. Final number and proportion of participants evaluable: 12/21 (57%) in the treatment group and 11/15 (73%) in the control group; 23/36 (64%) in total ITT analysis: Yes (see figure 1 flow chart page 971 ‐ all participants who received the first treatment included in the analysis) 23 completed, 31 analysed for primary outcome |
|
| Interventions |
Intervention 1 2 g of MAL cream applied to face after gentle curettage of the skin, and covered with occlusive dressing for 3 h. The remaining cream was removed and the face was illuminated with red light. No curettage was performed before the second treatment. Number and frequency of treatments: 2 treatments in total, 2 weeks apart Wavelength/Fluence/Duration/Spot size: 630 nm/total dose 37 J/cm²/9 min/not reported Supplier: Metvix; Photocure ASA, Oslo, Norway. Tegaderm and 3M Micropore; Beiersdorf A S, Birkeroed, Denmark. Aktilite CL 128; Photocure ASA Instructions to participants: Not applicable Intervention 2 No treatment |
|
| Outcomes | Evaluation time points of review interest: 4, 8 and 12 weeks after final treatment (adverse effects also assessed whilst on treatment) Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Change from baseline in number of ILs & NILs Methods of assessing primary outcomes 1. Non‐standardised grading scale: 0, acne worse; 1, no change; 2, slight improvement; 3, moderate improvement; 4, marked improvement 2. Lesion counts (excluding the nose, lips and around the eyes) performed live by evaluating dermatologist Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Investigator’s global assessment of improvement 3. Adverse effects Methods of assessing secondary outcomes 1. Revised Leeds acne grading system, assessed live by dermatologists 2. Non‐standardised grading scale: 0 = acne worse; 1 = no change; 2 = slight improvement; 3 = moderate improvement; 4 = marked improvement (comparing the patient’s condition with a baseline photograph) 3. Recorded during study, the day after the first treatment and before the second treatment. Pain was assessed by a VAS (0 = no pain and 10 = worst imaginable pain) |
|
| Notes | Language: English. Median scores for Participant’s global assessment of improvement and Investigator’s global assessment of improvement reported in a graph‐format (Figure 4 on page 972). Unclear what was the Investigator’s global assessment of improvement median score for control group at 8 weeks. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 970): "The patients were randomised to the treatment group or control group by lot (4 : 3)." Comment: We judged this as adequate and as at a low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (page 970): "The evaluating dermatologist was blinded to treatment control and was not allowed to communicate with the patients about their disease. The patients were instructed not to reveal if they had been treated or not." Comment: Clinicians performing the treatment, as well as participants were not blinded, so we judged the risk of bias as high. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | High risk | Quote (page 970): "The patients were instructed not to reveal if they had been treated or not." Comment: participants were not blinded so we judged this as at high risk of bias. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 970): "The evaluating dermatologist was blinded to treatment control and was not allowed to communicate with the patients about their disease. The patients were instructed not to reveal if they had been treated or not." Comment: Adequate for outcomes assessed by clinicians. We judged it as at low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome measures obtained for 75% of subjects randomised. We judged this as at a high risk of bias |
| Selective reporting (reporting bias) | Unclear risk | All primary outcomes reported, however data not reported at all time points for secondary outcome. We judged this as at an unclear risk of bias. |
| Other bias | Low risk | No other possible source of bias identified. We judged this as a low risk of bias. |
Yeung 2007.
| Methods | This was a parallel‐group RCT (split‐face within groups). Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Declared. Quote (page 1): "C.K.Y., P.B., and H.H.C. have disclosed potential financial conflict of interests with this study." Setting: Single centre (Hong Kong, China) Recruitment: "Volunteers were recruited from our patient pool" Duration: 9 months (December 2004‐August 2005) |
|
| Participants |
Included Age (inclusion criterion; mean; range): > 18 years, 25 years, 18‐41 years Clinically evident acne: Yes Severity of condition assessment: "moderate acne of more than 10 inflammatory acne lesions" Fitzpatrick skin types: IV‐V Excluded Oral isotretinoin for the past 6 months, topical or systemic antibiotics 2 weeks before the treatment, photosensitive dermatoses, pregnancy and lactation Enrolled: 30 (8 M/15 F) Randomised: 30 (Number and gender of participants randomised into each group unclear). Withdrawals/drop‐outs: 4 due to significant stinging/burning/erythema after MAL‐PDT and 1 due to non‐medical reason in MAL‐PDT group; 2 due to non‐medical reasons in IPL group Final number and proportion of participants evaluable: 23 (77%). Unclear. Data not presented for separate groups. 30 participants used topical adapalene 0.1% gel at night and were randomised to 2 split‐face treatment groups: 530–750 nm light plus MAL versus IPL light (11 participants completed) or IPL versus adapalene‐only control (12 participants completed). Study authors clarified that "11 participants completed in PDT group, 23 in IPL group and 12 in control group". ITT analysis: Not stated |
|
| Interventions |
Intervention 1 Half‐face treatment with IPL, with the other side of the face serving as a control through the use of topical adapalene only Number and frequency of treatments: 4 treatments in total, applied every 3 weeks Wavelength/Fluence/Duration/Spot size: 530‐750 nm/7‐9 J/cm²/2.5 s (double pulses)/10 x 48 mm² Supplier: Ellipse Flex system (Danish Dermatologic Development (DDD), HØrsholm, Denmark Instructions to participants: Not applicable Intervention 2 Full‐facial IPL exposure after the topical application of 16% MAL cream on half of the face for 30 min. The non‐MAL treated side was used as an IPL‐treated side. Number and frequency of treatments: 4 treatments in total, applied every 3 weeks Wavelength/Fluence/Duration/Spot size: 530‐750 nm/7‐9 J/cm²/2.5 s (double pulses)/10 x 48 mm² Supplier: Metvix, Galderma, France; Ellipse Flex system (Danish Dermatologic Development (DDD), HØrsholm, Denmark Instructions to participants: Not applicable Intervention 3 Adapalene only Number and frequency of treatments: 4 treatments in total, applied every 3 weeks Instructions to participants: Unclear whether adequate. "Patients were advised to avoid sun exposure for 48 h after the treatment, and to use regular sunblock" |
|
| Outcomes | Evaluation time points of review interest: 4 and 12 weeks after final treatment (also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Percentage change from baseline in number of ILs (papules and pustules not reported separately) 2. Percentage change from baseline in number of NILs (open and closed comedones not reported separately) Methods of assessing primary outcomes 1. & 2. Lesion counts based on photographs Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Evaluation of side effects including pigmentary disturbance, oedema, burning, stinging, crusting, atrophy, scarring unclear |
|
| Notes | Language: English. ILs and NILs mean reductions reported for "MAL‐PDT, IPL and control groups" in tables 1 and 2 (pages 3 and 4) although presumably the study authors were comparing 11 MAL‐PDT‐treated face sides, 23 IPL‐treated face sides and 12 adapalene‐only control face sides. Assessment of compliance was probably not undertaken. No baseline IL and NIL counts data for face‐sides reported. We contacted the study authors and they provided information on power calculation, ethical approval, study duration, recruitment methods, sex of included participants, details on random sequence generation and allocation concealment, as well as outcome assessment blinding. They also clarified that the values reported as standard errors (SE) in Table 1 (page 3) and Table 2 (page 4) were actually standard deviations, so we used them as such for our analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 2): "The subjects were randomised to half‐facial treatments with MAL plus IPL, IPL alone, or as controls in the ratio of 1:2:1." Comment: Method used to generate the allocation sequence was not described, but the author clarified that randomisation codes were used and sent detailed data. We therefore judged the risk of bias to be low. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. Given the nature of the interventions involved then blinding of participants/personnel is unlikely. We judged this as at unclear risk of bias. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Low risk | Quote (page 2 ): "The images are stored in Canfield’s mirror software and were assessed by two blinded investigators who did not participate in the treatment of the subjects." Comment: We judged this as adequate and at a low risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome measures were obtained for 77% of subjects randomised. We judged this as at a high risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcome measures pre‐specified in the methods section reported. |
| Other bias | Unclear risk | Study authors declared conflicts of interest, which might have introduced some bias. No other possible source of bias identified. Insufficient information was given to permit a clear judgement. |
Yilmaz 2011.
| Methods | This was a parallel‐group RCT (split‐face within groups) Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared. Study authors reported no conflict of interest (page 307) Setting: Single centre (Samsun, Turkey) Recruitment: Dermatology Dept, School of Medicine, Ondokuz Mayis University, Samsun, Turkey Duration: Start and end dates were not reported. |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 21.0 ± 3.5 (group 1) and 20.7 ± 2.7 (group 2); not reported Clinically evident acne: Yes Severity of condition assessment: "Active inflammatory acne.." and "who had at least four inflammatory lesions" Fitzpatrick skin types: I‐III Excluded Systemic retinoid treatment for last 6 months, treated with microdermoabrasion within last 3 months, systemic treatment for acne within last 2 months/ topical treatment for acne within last month, prone to hypertrophic scar and keloid formation, seizures/ AID/ pregnancy / lactation Enrolled: 44 (M/F not reported) Randomised: 44 (number of participants in each group not reported) Withdrawals/drop‐outs: 6 (reasons for withdrawal and which group those participants belonged to not reported) Final number and proportion of participants evaluable: Group I: 20 (12 M/8 F); Group II: 18 (12 M/6 F); Total: 38 (24 M/14 F; 86%) ITT analysis: No |
|
| Interventions |
Intervention 1 KTP laser treatment to half of the face after application of cooling gel. Number and frequency of treatments: 4 treatments once weekly Wavelength/Fluence/Duration/Spot size: 532 nm/5‐12 J/cm²/pulse duration 20‐40 ms/4 mm² Supplier: Gemini Laser (Laserscope, San Jose, CA, USA) Instructions to participants: "Informed about photo‐protection and recommended to apply at least SPF‐30 sunblock." Intervention 2 KTP laser treatment to half of the face after application of cooling gel. Number and frequency of treatments: 4 treatments twice weekly Wavelength/Fluence/Duration/Spot size: 532 nm/5‐12 J/cm²/pulse duration 20‐40 ms/4 mm² Supplier: Gemini Laser (Laserscope, San Jose, CA, USA) Instructions to participants: "Informed about photo‐protection and recommended to apply at least SPF‐30 sunblock." Intervention 3 Placebo |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment (also assessed at 1 week after final treatment) Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. Michaëlsson acne severity grading score (MASS) 2. Erythema, edema, burning sensation, colour changes and scar formation observed by participants were apparently assessed, but the method was not clearly stated. |
|
| Notes | Language: English. Quote (page 305): "MASSs at the beginning (MASS1) were significantly higher in group II for both sides of the face (P = 0.018). Since evaluation was based on decrease in MASS, this difference was not taken into consideration." We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 304): "Patients were randomly divided into two groups. Group I was treated once weekly for four weeks. Group II was treated twice weekly for two weeks. Both groups were treated with total of four treatment sessions. Laser treatment was applied to half of the face, and the other half remained as untreated….Side of face to be treated was selected randomly." Comment: Method used to generate the group nor face side allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. Unclear whether it was the same clinician performing the treatment was also doing the assessment. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Quote (page 304): "Evaluation of the patients was performed clinically by the same dermatologist according to Michaëlsson acne severity grading score (MASS), at the beginning, i.e. zero (MASS 1), one (MASS 2) and four (MASS 3) weeks after the last treatment session." Comment: Unclear whether it was the same clinician performing the treatment, or an independent one. We judged it as at unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 305): "Forty‐four patients were included but only 38 (24 male 63%, 14 female 37%) of them completed the study." Comment: Number of participants randomised in each group not reported. No reasons for withdrawal provided. Outcome measures obtained for 86.36% of randomised participants. |
| Selective reporting (reporting bias) | Low risk | All outcome measures pre‐specified in the methods section reported. |
| Other bias | Low risk | No other possible source of bias identified. |
Yin 2010.
| Methods | This was a parallel‐group RCT (split‐face within groups). Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared. Study authors declared no conflict of interest (page 1064) Setting: Single centre (Chongqing, China) Recruitment: Department of Dermatology, Southwest Hospital, Third Military Medical University, Chongqing Duration: 8 months (June 2007‐January 2008) |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 25.8 years, 18‐38 years Clinically evident acne: Yes Severity of condition assessment: "with facial inflammatory acne vulgaris (moderate to severe grade according to Pillsbury et al.)" Fitzpatrick skin types: III‐IV Excluded Topical retinoic acid, glucocorticoids, antibiotics and other drugs within 2 weeks; using medication that may exacerbate or alleviate acne, planning to become pregnant, currently pregnant or lactating, history of photosensitivity disorder, planning to have prolonged exposure to sunlight, herpes simplex outbreak Enrolled: 180 (83 M/97 F) in total; Intervention 1 (5%) ‐ 45 (21 M/24 F); Intervention 2 (10%) ‐ 45 (24 M/21 F) Intervention 3 (15%) ‐ 45 (20 M/25 F); Intervention 4 (20%) ‐ 45 (18 M/27 F) Randomised: 180 in total; 45 participants in each group Withdrawals/drop‐outs: Only one drop‐out because of severe adverse effects after the first treatment in the 20% group Final number and proportion of participants evaluable: 44 (98%) in the 20% group, 45 (100%) in other groups. 179 (99%) in total ITT analysis: Not stated |
|
| Interventions |
Intervention 1 Skin cleaned with 70% isopropyl alcohol then 5% ALA applied to the right side of the face in an oil‐in‐water emulsion and oil‐in‐water emulsion applied to the left side of the face. Following occlusion with plastic film for 1.5 h, participants were exposed to red light Number and frequency of treatments: Once every 10 days for 4 sessions Wavelength/Fluence/Duration/Spot size: 633 +/‐ 3 nm/126 J/cm²/20 min/not reported Supplier: Ominlux Revive, Photo Therapeutics, Carlsbad, CA, USA Instructions to participants: Not applicable Intervention 2 Skin cleaned with 70% isopropyl alcohol then 10% ALA applied to the right side of the face in an oil‐in‐water emulsion and oil‐in‐water emulsion applied to the left side of the face. Following occlusion with plastic film for 1.5 h, participants were exposed to red light Number and frequency of treatments: Once every 10 days for 4 sessions Wavelength/Fluence/Duration/Spot size: 633 +/‐ 3 nm/126 J/cm²/20 min/not reported Supplier: Ominlux Revive, Photo Therapeutics, Carlsbad, CA, USA Instructions to participants: Not applicable Intervention 3 Skin cleaned with 70% isopropyl alcohol then 15% ALA applied to the right side of the face in an oil‐in‐water emulsion and oil‐in‐water emulsion applied to the left side of the face. Following occlusion with plastic film for 1.5 h, participants were exposed to red light Number and frequency of treatments: Once every 10 days for 4 sessions Wavelength/Fluence/Duration/Spot size: 633+/‐3 nm/ 126 J/cm²/ 20 min/ Not reported Supplier: Ominlux Revive, Photo Therapeutics, Carlsbad, CA, USA Instructions to participants: Not applicable. Intervention 4: Skin cleaned with 70% isopropyl alcohol then 20% ALA applied to the right side of the face in an oil in water emulsion and oil in water emulsion applied to the left side of the face. Following occlusion with plastic film for 1.5 h, participants were exposed to red light Number and frequency of treatments: Once every 10 days for 4 sessions Wavelength/Fluence/Duration/Spot size: 633 +/‐ 3 nm/126 J/cm²/20 min/not reported Supplier: Ominlux Revive, Photo Therapeutics, Carlsbad, CA, USA Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 2, 4, 12 and 24 weeks after final treatment (adverse effects also assessed at each session whilst on treatment) Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Change from baseline in number of ILs 2. Change from baseline in number of NILs Methods of assessing primary outcomes 1. Non‐standardised scale: 'marked improvement', 'moderate improvement', 'no change' or 'acne worse' 2. Lesion counts Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Grading scale that was defined as 3 for > 50% exacerbation, 2 for 25%–50% exacerbation, 1 for 1%–25% exacerbation, 0 if unchanged, 1 for 1%–25% improvement, 2 for 25%–50% improvement, 3 for 50%–75% improvement, 4 for 75%–99% improvement, and 5 for 100% improvement, compared with baseline. 2. Adverse effects including pruritus, pain, pustules, vesicles, erythema, hyperpigmentation, loss of epidermis and exfoliation were recorded in detail at each treatment and follow‐up visit. Adverse effects were recorded according to persistence, time to resolve, severity (0, absent; 1, mild; 2, moderate; 3, severe), treatment measure and outcome. |
|
| Notes | Language: English. Data expressed in graph format for primary outcomes and Investigator's global assessment of improvement. We attempted to contact the study authors, but were not successful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1065 ): "Utilizing SAS software (SAS Institute, Cary, NC, U.S.A.), eligible patients were randomly divided into four groups for treatment with four different concentrations of topical ALA: 5%, 10%, 15% and 20%, respectively." Comment: We judged this as adequate and at a low risk. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (page 1066): "Following cleaning of the skin with 70% isopropyl alcohol, patients received topical ALA in an oil‐in‐water emulsion on skin lesions at the right side of the face and only oil‐ in‐water emulsion at the left side."; (page 1065): "a randomised, single‐blind and self‐controlled clinical trial". Comment: Unclear whether participants and clinicians were blinded for the concentration of ALA. We judged that the risk of bias is unclear. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | Unclear whether participants assessing their improvement were blinded for the ALA concentration used for their treatment, so we judged the risk of bias as unclear. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | Quotes (page 1065): "a randomised, single‐blind and self‐controlled clinical trial"; "Briefly, the numbers of skin lesions including ... were recorded simultaneously by three dermatologists." Comment: Unclear whether the dermatologists in question were blinded or not. We judged this as an unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 1066): "Of the 180 patients 179 completely finished the whole therapy scheme, with only one dropout because of severe adverse effects after the first treatment. All cases were successfully followed up through regular clinical consultation." Comment: Outcome measures obtained for 97% of the participants randomised. Unclear which concentration group the dropout belonged to in the methods section, reported only in the 'Results' section under 'Adverse Effects'. We judged this as at a low risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section were reported. |
| Other bias | Low risk | No other possible source of bias identified. |
Zhang 2009a.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Single centre (Jiangxi, China) Recruitment: Not reported Duration: November 2007‐May 2008 |
|
| Participants |
Included Age (inclusion criterion; mean; range): not reported; not reported; 12‐53 years Clinically evident acne: Yes Severity of condition assessment: Mild to severe; Pillsbury grades I‐IV Fitzpatrick skin types: Not reported Excluded Light allergy; taking medication for light allergy; pregnant women Enrolled: 738 Randomised: 738, 508 (247 M/261 F) in the intervention group, and 230 (112 M/118 F) in the control group Withdrawals/drop‐outs: 2 participants withdrew from the intervention group due to adverse effects, there were no lost to follow‐ups. No withdrawals/lost to follow‐ups in the control group Final number and proportion of participants evaluable: 736/738 in total (99.7%); 506/508 (99.6%) in the intervention group, 230/230 (100%) in the control group ITT analysis: Not stated |
|
| Interventions |
Intervention 1 Blue and red light phototherapy with clindamycin gel, azithromycin, antisterone or cimetidine Number and frequency of treatments: 8 treatments, applied twice per week for 4 weeks, clindamycin gel twice per day on days without light therapy, azithromycin 0.5 g/day . Wavelength/Fluence/Duration/Spot size: 415 ± 5 nm (blue) 633 ± 6 nm (red)/48 J/cm² (blue) and 126 J/cm² (red)/20 min alternating between red and blue light/not reported Supplier: Not reported Instructions to participants: Not applicable Intervention 2 Clindamycin gel, azithromycin, antisterone or cimetidine Number and frequency of treatments: Clindamycin gel twice per day, azithromycin 0.5 g/day Supplier: Not reported Instructions to participants: Not reported |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Non‐standard scale based on percentage change in combined lesion counts. Percentage change in lesion count = (lesion count before treatment – lesion count after treatment)/ lesion count before treatment × 100%; Fully recovered: percentage change in lesion count ≥ 90%; Good improvement: percentage change in lesion count 60% to 89%; Effective: percentage change in lesion count 30% to 59%; No effect: percentage change in lesion count ≤ 29%; Total percentage effectiveness = (no. of fully recovered + good improvement)/total no. of participants x 100% 2. Not reported |
|
| Notes | Language: Mandarin. English translation was not available. Data extraction was done by native speaker Elicia Toon Yuan Ni from the original paper. We have not attempted to contact the study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 218): "Participants were randomized into 2 groups" Comment: The method used to generate the allocation sequence not described |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | No intended blinding of outcome assessors reported. No evidence that outcome assessors were blinded provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes for 99.7% of randomized participants included in the analysis, so we judged the risk as low. |
| Selective reporting (reporting bias) | Low risk | All outcomes predefined in the ‘Methods’ section were reported. |
| Other bias | Unclear risk | Sponsorship and conflicts of interest unclear. Insufficient information to permit a clear judgment. The study was in Mandarin and potential bias has been introduced by the fact that we were only able to do single rather than double data extraction. |
Zhang 2013a.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Single centre (Beijing, China) Recruitment: Department of Dermatology, Peking University Shenzhen Hospital Duration: 2008‐2010, months not reported |
|
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; 24 years in the intervention, 23 years in the control group; 16‐47 years Clinically evident acne: Yes Severity of condition assessment: Moderate‐severe, Pillsbury grade II‐IV Fitzpatrick skin types: Not reported Excluded Taken medication (either by application or orally) containing Vitamin A within the last month; breast‐feeding mothers; mental disorder; alcoholics; use of drugs; systemic disease; severe skin disease; light allergy; keloidal scar Enrolled: 116 (47 M/59 F) Randomised: 116, 63 in the intervention group, 53 in the control group Withdrawals/drop‐outs: Not reported, however results given for all of the 116 randomised participants Final number and proportion of participants evaluable: 116/116 (100%) ITT analysis: Unclear |
|
| Interventions |
Intervention 1 5‐ALA plus red light Number and frequency of treatments: 3 treatments, applied weekly Wavelength/Fluence/Duration/Spot size: 630 ± 5 nm/80‐100 J/cm²/20 min/not reported Supplier: Fudan‐Zhangjiang BioPharmaceutical Co., Ltd., Shanghai, China Instructions to participants: Not applicable Intervention 2 Red light alone Number and frequency of treatments: 3 treatments, applied weekly Wavelength/Fluence/Duration/Spot size: 630 ± 5 nm/80‐100 J/cm²/20 min/not reported Supplier: Not reported Instructions to participants: Not applicable |
|
| Outcomes | Evaluation time points of review interest: 2, 4 and 8 weeks after final treatment Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Non‐standard scale based on percentage change in combined lesion counts. Percentage change in lesion count = (lesion count before treatment – lesion count after treatment)/ lesion count before treatment × 100%; Fully recovered: percentage change in lesion count ≥ 90%; Good improvement: percentage change in lesion count 60% to 89%; Effective: percentage change in lesion count 20% to 59%; No effect: percentage change in lesion count ≤ 19%; Total percentage effectiveness = (no. of fully recovered + good improvement)/total no. of participants x 100% 2. Not reported |
|
| Notes | Language: Mandarin. English translation was not available. Data extraction was done by native speaker Elicia Toon Yuan Ni from the original paper. We have not attempted to contact the study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 314): ‘Participants were randomised into 2 groups’ Comment: The method used to generate the allocation sequence not described. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | No intended blinding of outcome assessors reported. No evidence that outcome assessors were blinded provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (page 314): "116 participants were randomised …" It was not reported whether there were participants who withdrew, or were lost to follow up, but the results were reported for 116 participants (100%), so we judged the risk as low. |
| Selective reporting (reporting bias) | Low risk | All outcomes predefined in the ‘Methods’ section were reported. |
| Other bias | Unclear risk | Sponsorship and conflicts of interest unclear. Insufficient information to permit a clear judgment. Possible baseline imbalances between the groups, (baseline data were not reported). The study was in Mandarin and potential bias was introduced by the fact that we were only able to do single rather than double data extraction. |
Zhang 2013b.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Single centre (Zhengzhou, China) Recruitment: Not reported Duration: 4 months, June 2009‐December 2013 |
|
| Participants |
Included Age (inclusion criterion; mean; range): not reported; 22.1 years in the intervention group, 23.6 in the control group; 14‐40 years Clinically evident acne: Yes Severity of condition assessment: Mild to moderate, Pillsbury grades I‐III Fitzpatrick skin types: Not reported Excluded Light allergy; taken antibiotics within the last 4 weeks; breast‐feeding mothers Enrolled: 120 (59 M/61 F) Randomised: 120, 60 in the intervention group and 60 in the control group Withdrawals/drop‐outs: None Final number and proportion of participants evaluable: 120/120 (100%) ITT analysis: No |
|
| Interventions |
Intervention 1 Red‐blue phototherapy combined with jinhua xiaocuo pills and chloramphenicol tincture Number and frequency of treatments: 8 treatments, applied twice weekly over 4 weeks; Jinhua xiaocuo pills 4 g orally 3 times/day Wavelength/Fluence/Duration/Spot size: 415 ± 5 nm (blue) 633 ± 3 nm (red)/48 J/cm²/(blue) 126 J/cm²/(red)/20 min blue, followed by 10 min red/not reported Supplier: Wu Han JiuTouNiao Medical Instruments Development Co., LTD; Jinhua xiaocuo pills supplied by Kunming Traditional Chinese Medicine Factory Co., Ltd. Chloramphenicol tincture made by the clinic themselves. Instructions to participants: Unclear Intervention 2 Jinhua xiaocuo pills and chloramphenicol tincture Number and frequency of treatments: Jinhua xiaocuo pills 4 g orally 3 times/day Chloramphenicol tincture 10 mg/mL (applied once in the day once at night) Instructions to participants: Unclear |
|
| Outcomes | Evaluation time points of review interest: 4 weeks after final treatment Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Non‐standard scale based on percentage change in combined lesion counts. Percentage change in lesion count = (lesion count before treatment – lesion count after treatment)/ lesion count before treatment × 100%; Fully recovered: percentage change in lesion count ≥ 90%; Good improvement: percentage change in lesion count 60% to 89%; Effective: percentage change in lesion count 30% to 59%; No effect: percentage change in lesion count ≤ 29%; Total percentage effectiveness = (no. of fully recovered + good improvement)/total no. of participants x 100% 2. Not reported |
|
| Notes | Language: Mandarin. English translation was not available. Data extraction was done by native speaker Elicia Toon Yuan Ni from the original paper. We have not attempted to contact the study authors. We used the 'Jinhua Xiaocuo' term as presented in the English translation of the abstract provided by the journal where full text was published in Mandarin. As clarified by native Mandarin speakers, 'Jinhua Xiaocuo' is different from 'Yinhua decoction' (used in Ou 2014 study), although both used the same main ingredients (honeysuckle flower). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 304): ‘Participants were randomised into 2 groups’ Comment: The method used to generate the allocation sequence not described. |
| Allocation concealment (selection bias) | Unclear risk | Intention and/or method to conceal the allocation sequence were not specifically reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No intended blinding of participants/performing clinicians reported. No evidence that participants/clinicians were blinded provided. |
| Blinding of outcome assessment (detection bias) Participant‐assessed outcomes | Unclear risk | This study did not address such outcomes. |
| Blinding of outcome assessment (detection bias) Investigator‐assessed outcomes | Unclear risk | No intended blinding of outcome assessors reported. No evidence that outcome assessors were blinded provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results reported for all randomised participants, so we judged the risk as low. |
| Selective reporting (reporting bias) | Low risk | All outcomes predefined in the ‘Methods’ section were reported. |
| Other bias | Unclear risk | Sponsorship and conflicts of interest unclear. Insufficient information to permit a clear judgment. The study was in Mandarin and potential bias was introduced by the fact that we were only able to do single rather than double data extraction. |
ALA = 5‐aminolevulinic acid BPO = benzoyl peroxide FPT = Fitzpatrick's Skin Types: based on different reactions to sun exposure and range from type I ('pale white skin which always burns and never tans') to type VI ('deeply pigmented dark brown to black skin which never burns and tans very easily') (Fitzpatrick 1988) GAAS = Global Acne Assessment Scoring ILs = inflamed lesions IPL = intense pulsed light IR = (radiant) infrared ITT = Intention‐to‐treat analysis MAL = methyl‐aminolevulinate MASS = Michaëlsson acne severity grading score NILs = non inflamed lesions OFI = optical fibre intra‐tissue irradiation PDL = pulsed‐dye laser PDT = photodynamic therapy RCT = randomised controlled trial SD = standard deviation SPF = sun protection factor
Change from baseline i.e. absolute change is calculated by subtracting baseline count from count assessed at certain time‐point. Percentage change is calculated by dividing the absolute change with baseline count and then multiplying that value by 100 to get percentages.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alam 2003 | We contacted the study author who provided information that part of the study had been published by Glaich 2006, which was not a RCT. |
| Alexiades‐Armenakas 2006 | This study compared 14 ALA‐PDT patients with 4 control patients on a conventional therapy (topical medications, oral antibiotics and/or oral contraceptives). Not reported if they were assigned to these groups at random. Within ALA‐PDL group, they were randomised to treatment with laser energy or blue light. Only one participant was treated with blue light. Quote (page 46): "..however, due to the superior efficacy of the LP PDL group, all subsequent patients were treated with LP PDL". |
| Aziz‐Jalali 2012 | This was reported as split‐face RCT ("This study was a single‐blind randomized clinical trial.") but face sides were not allocated at random: "Right and left sides of the face were exposed to red LLLT (R‐LLLT) and infrared LLLT (IR‐LLLT), respectively." |
| De Leeuw 2010 | This was not a RCT, although this was stated in abstract |
| Goldman 2003 | This was not a RCT |
| Hong 2005 | This was not a RCT |
| Kim 2008 | This was not a RCT |
| Lee 2007 | This was not a RCT |
| Ma 2013 | This was not a RCT |
| Morton 2005 | This was an open study. 30 participants were enrolled. Initially, 14 participants were randomised to receive 10 (24 J/cm²) or 20 min (48 J/cm²) light exposure. "As no significant differences in adverse effects were observed the remaining 16 subjects were treated for 20 minutes (48 J/cm²)". Results were reported on 30 participants and not individual groups. |
| NCT00613444 | This was a non‐randomised study of PDT in the treatment of acne vulgaris using non‐coherent red light (Derm 590). According to the study record, which was last updated on 27 December, 2012, "This study has been withdrawn prior to enrolment." |
| Owczarek 2014 | Cross‐over study design |
| Pinto 2013 | This was not a RCT |
| Rojanamatin 2006 | This was not a RCT |
| Santos 2005 | This was not a RCT |
| Shin 2012 | This was a RCT comparing fractional microneedle radiofrequency treatment and C02 laser therapy. We judged that this study was not focusing on healing properties of light but on thermal effects |
| Tuchin 2003 | This was a RCT named "a pilot study of ICG laser therapy of acne vulgaris: photodynamic and photothermolysis treatment." It compared single and multiple treatments of acne with indocyanine green dye followed by near‐infrared laser‐diode light (803 or 809 nm). Each area treated was also divided (not randomly) into a 'treatment' and a 'control' area. The multiple treatment group was treated twice per week for 4 consecutive weeks. Outcome measures were investigator‐assessed change in acne lesions weekly for 1 month and at 2 months after the first treatment. 22 participants were recruited but only descriptive data were reported. The study authors were contacted in 2007 and data on 4/22 participants were provided: at 30 days: mean change ‐5.75, SD 3.59 (treatment group); ‐1.75, SD 3.10 (control group). This study was excluded because the results provided were for the non‐randomised part of the study. |
| Wang 2012 | This was not a RCT |
| Yang 2013 | This study included acne conglobata patients and was not focusing on direct light therapies for acne |
| Yao 2009 | This paper was published in Chinese and was not a RCT. One native Chinese speaker assessed the full text of this study |
| Yoon 2014 | Study of acne scars |
| Zhan 1997 | This paper was published in Chinese. We excluded it on the basis that He‐Ne laser was used on 'earpoints' not on acne lesions, thus not focusing on direct light therapies for acne. |
| Zhong 2007 | This was a paper published in Chinese and was not a RCT. One native Chinese speaker assessed the full text of this study |
| Zhu 2009 | This was a paper published in Chinese and was not a RCT. One native Chinese speaker assessed the full text of this study |
RCT = randomised controlled trial. SD = standard deviation.
Characteristics of studies awaiting assessment [ordered by study ID]
Berson 2006.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Declared Setting: Multicenter (USA) Recruitment: Not reported Duration: Start and end dates were not reported. |
| Participants |
Included Age (inclusion criterion; mean; range): > 16 years; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "moderate to severe facial acne (cysts ≤ 2)" Fitzpatrick skin types: I‐IV Excluded Pregnant women. Spironolactone treatment within 8 weeks, Accutane within 6 months Enrolled: 72 enrolled (M/F not reported) Randomised: 72, it states that there were 24 in each group (different incubation times), however the randomisation ratio is 3:1 (vehicle:ALA) Withdrawals/drop‐outs: Unclear Final number and proportion of participants evaluable: Not reported ITT analysis: Not reported |
| Interventions |
Intervention 1 Topical ALA for 15/60/120 minutes followed by blue light Number and frequency of treatments: Up to 4, every 2 weeks Wavelength/Fluence/Duration/Spot size: ? nm, 5 J/cm², not reported, not reported Supplier: Not reported Instructions to participants: Not applicable Intervention 2 Vehicle (?) for 15/ 60/ 120 minutes followed by blue light Number and frequency of treatments: Up to 4, every 2 weeks Wavelength/Fluence/Duration/Spot size: ? nm, 5 J/cm², not reported, not reported Supplier: Not reported Instructions to participants: Not applicable |
| Outcomes | Evaluation: 2 days after each (up to 4) PDT treatment and 4 and 8 weeks after last PDT treatment Primary outcomes of review interest recorded Unclear Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse events Methods of assessing secondary outcomes 1.Global Acne Severity Score 2.Recorded during study |
| Notes | Language: English. This was a conference abstract of an industry‐sponsored study. We contacted the study author who replied that the study was not completed. |
Demina 2015.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Unclear; single centre? (Moscow?, Russian Federation) Recruitment: Unclear Duration: Unclear |
| Participants |
Included Age (inclusion criterion; mean; range): not reported; not reported; 16‐44 years Clinically evident acne: Yes Severity of condition assessment: "…with various forms of acne; most of them had severe manifestations of acne (n = 126, or 45.6%) and duration of the disease of 1‐5 years (n = 157, 56.9%)’. Further details not given Fitzpatrick skin types: Not reported Excluded: Not reported Enrolled: 276 enrolled (M/F not reported) Randomised: 276, 237 (M/F not reported) in the "phased low‐level laser therapy (LLLT) and PDT group", 39 (M/F not reported) in the "conventional combination therapy" group Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: "…follow‐up during 1 year (n = 144), 2 years (n = 128), 3 years (n = 128), 4 years, and 5 years (n = 104)’, unclear whether this refers to the whole sample or ‘LLLT‐PDT’ group ITT analysis: Not reported |
| Interventions |
Intervention 1 "administered with phased LLLT and PDT therapy based on a proprietary method" Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Unclear Instructions to participants: Unclear Intervention 2 ‘…conventional combination therapy’, details not given Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Unclear Instructions to participants: Unclear |
| Outcomes | Evaluation: "follow‐up during 1 year (n = 144), 2 years (n = 128), 3 years (n = 128), 4 years, and 5 years (n = 104)." Details not given. Primary outcomes of review interest recorded 1. Unclear whether any were assessed Methods of assessing primary outcomes 1. Unclear Secondary outcomes of review interest recorded 1. Unclear whether any were assessed Methods of assessing secondary outcomes 1. Unclear |
| Notes | Language: Russian. This was a study identified in our final searches. We were unable to obtain full text. We extracted data in this table from the abstract in English. We will attempt to obtain the full text in the update of this review. We have also identified MD thesis by the same author through Google search, also in Russian. We have not attempted to contact the study authors. Correspondence: Contact details not identified. |
Du 2015.
| Methods | This was a parallel‐group RCT Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Unclear (China?) Recruitment: Unclear Duration: Unclear |
| Participants |
Included Age (inclusion criterion; mean; range): not reported; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "severe acne", details not given Fitzpatrick skin types: Not reported Excluded: Not reported Enrolled: 61 enrolled (M/F not reported) Randomised: 30 in the ALA‐PDT alone group, 31 in the oral Tanshinone capsules plus ALA‐PDT group Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: Not reported ITT analysis: Not reported |
| Interventions |
Intervention 1 ALA‐PDT; "given topical Metronidazole gel" Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Unclear Instructions to participants: Unclear Intervention 2 Oral Tanshinone capsules plus ALA‐PDT; "given topical Metronidazole gel" Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Unclear Instructions to participants: Unclear |
| Outcomes | Evaluation: Unclear; "followed up for 3 months" Primary outcomes of review interest recorded 1. Unclear whether these were recorded Methods of assessing primary outcomes 1. Unclear Secondary outcomes of review interest recorded 1. Unclear whether these were recorded. Investigator’s global assessment of improvement (referred to as "curative effect"?) 2. Adverse events Methods of assessing secondary outcomes 1. Investigator’s Global Assessment of Improvement (IGA) score, see above 2. Unclear |
| Notes | Language: Mandarin. This was a study identified in our final searches. We were unable to obtain full text. We extracted data in this table from the abstract in English. We will attempt to obtain the full text in the update of this review. We have not attempted to contact the study authors. Correspondence: Contact details not identified |
Edwards 2006.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear (not mentioned in raw data) Ethical approval: Unclear (it states in the raw data (p.29) that, "ethical approval will be obtained") Sponsorship and conflict of interest: Declared Setting: Single centre ( Newport, UK) Recruitment: Dermatology outpatients, Royal Gwent Hospital, Newport Duration: Start and end dates were not reported |
| Participants |
Included Age (inclusion criterion; mean; range): 16‐51 years; not reported; not reported (unable to calculate from raw data due to missing data) Clinically evident acne: "mild to moderate facial acne" Severity of condition assessment: Unclear Fitzpatrick skin types: I‐IV (from raw data) Excluded
Enrolled: 32 Randomised: 32 Withdrawals/drop‐outs: 7 Final number and proportion of participants evaluable: 25/32 (78%) ITT analysis: Unclear |
| Interventions |
Intervention 1 Intense yellow light phototherapy Number and frequency of treatments: 8 treatments, twice weekly Wavelength/Fluence/Duration/Spot size: 570‐600 nm, 1.5 J/cm², not reported, not applicable Supplier: Enfis Ltd Instructions to participants: Not applicable Intervention 2 Intense yellow light phototherapy Number and frequency of treatments: 8 treatments, twice weekly Wavelength/Fluence/Duration/Spot size: 570‐600 nm, 3.0 J/cm², not reported, not applicable Supplier: Enfis Ltd Instructions to participants: Not applicable Intervention 3 "Sham" Intense yellow light phototherapy Number and frequency of treatments: 8 treatments, twice weekly Wavelength/Fluence/Duration/Spot size: 570‐600 nm, < 0.1 J/cm², not reported, not applicable Supplier: Enfis Ltd Instructions to participants: Not applicable |
| Outcomes | Evaluation: Immediately after 4‐week course of treatment and then 2, 4 and 6 weeks after treatment Primary outcomes of review interest recorded 1. Participant's global assessment of improvement 2. Investigator‐assessed change in lesion count Methods of assessing primary outcomes 1. Unclear 2. Lesion count Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Investigator's global assessment of improvement, using global assessment of improvement 3. Changes in quality of life 4. Adverse events Methods of assessing secondary outcomes 1. Leeds acne severity score 2. Global assessment score 3. DLQI 4. Monitoring |
| Notes | Language: English. This was a conference abstract of an industry‐sponsored study which reported data for 20/32 participants. We contacted the study authors who provided further information on the randomisation method, as well as the raw data for 25/32 participants who completed the study, however the raw results data were unclear and therefore we did not extract any results. |
Elgendy 2015.
| Methods | This was probably a parallel‐group RCT, randomisation only mentioned in the abstract Unit of randomisation: Whole person? Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Single‐center (Cairo, Egypt) Recruitment: Al Dar Hospital Dermatology outpatient clinic Duration: 23 months, February 2013‐December 2014 (recruitment) |
| Participants |
Included Age (inclusion criterion; mean; range): > 12 years; not reported; 16‐32 years Clinically evident acne: Yes Severity of condition assessment: "mild to moderate acne", "Investigator’s Global Assessment (IGA) scale" Fitzpatrick skin types: not reported Other: both sexes, "who failed to respond to the classic topical treatment and patients willing to undergo treatment and follow ups"; "patients were on no medications for 4 weeks prior to the study." Excluded "Exclusion criteria for blue light therapy included the following: known light sensitivity; history of phototoxicity and history of herpes simplex virus or cold sores on the treatment area. Exclusion criteria for isotretinoin therapy were patients with age less than 12 years, or those having severe facial acne vulgaris. Also pregnant women or who were planning to become pregnant during the course of treatment were excluded." Enrolled: 60 enrolled (26 M/24 F) Randomised: 60, 30 (12 M/18 F) in the blue‐light group, 30 (14 M/16 F) in the low‐dose isotretinoin group Withdrawals/drop‐outs: 3 discontinued in the blue‐light group, 5 in the isotretinoin group Final number and proportion of participants evaluable: 27/30 (90%) in the blue‐light group, 25/30 in the isotretinoin group (83%) ITT analysis: Not reported |
| Interventions |
Intervention 1 "Blue light group, a high intensity, enhanced, narrowband, blue light source" Number and frequency of treatments: Twice a week, over 6 weeks Wavelength/Fluence/Duration/Spot size: 405‐420 nm/90 mw/cm2/30 minutes' exposure time (15 minutes for each half of the face)/not reported Supplier: "cure light, Iclear XL" Instructions to participants: "Subjects were instructed to cleanse their face before each treatment with an unscented soap or nonirritant facial cleanser. They were also instructed to apply a moisturising non‐comedogenic sunscreen with SPF 32 after each morning treatment as needed (for sun protection and to mitigate potential dryness and/or irritation)." Intervention 2 Isotretinoin, 0.3 mg/kg/d in divided doses for 6 months Number and frequency of treatments: For 6 months. Supplier: Not reported Instructions to participants: Unclear |
| Outcomes | Evaluation: At baseline and weeks 2, 6, 10, 16 and 24. Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement? ‘Patient satisfaction’ 2. Change from baseline in total number of lesions (ILs and NILs) Methods of assessing secondary outcomes 1. ‘satisfied’ or ‘non satisfied’ 2. ‘First criterion of assessment was counting the number of lesions (comedones, papules, pustules and total sum of the lesions)’; ‘Clinical photographs were obtained for evaluation every 4 weeks.’ Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse events Methods of assessing secondary outcomes 1. ‐1 = worsened, 0 = unchanged, 1 = improved, 2 = markedly improved, and 3 = resolved; "Clinical photographs were obtained for evaluation every 4 weeks." 2. "Safety was assessed by asking patients about any symptoms of adverse reactions and laboratory changes especially in isotretinoin group." ; "Safety was assessed during the study by the reporting of adverse events and laboratory changes." |
| Notes | Language: English. This was a study identified in our final searches. It will be included and the results fully incorporated in the update of this review if judged eligible. We have not attempted to contact the study authors. Correspondence: Ayman Elgendy, Professor of Dermatology and Venereology, Benha University, Egypt, Tel: +966507364687; E‐mail: aymanelgendy91@yahoo.com |
Faghihi 2011.
| Methods | Unclear whether this was a parallel‐group or a split‐face trial, and whether it was a RCT. Unit of randomisation: Unclear Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared. One of the study authors employed by industry. Quote (page 183): "We appreciate all the staff of SAAIRAN OPTICS Co. involved in this project." Setting: Multicenter, Noor?, Alzahra? and Shahid Beheshti? (Iran) Recruitment: "the outpatient clinics at educational centers" Duration: Start and end dates were not reported. |
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; 21.6 years; 14‐50 years Clinically evident acne: Yes Severity of condition assessment: "with mild to moderate acne"; "Each patient's acne was assessed by a spot count of both inflamed and non‐inflamed lesions." Fitzpatrick skin types: II‐IV Excluded "pregnancy, use of isotretinoin or other photosensitizer drugs e.g. thiazides, tetracyclines, benzodiazepines, use of any acne treatment other than that issued, or any intake of oral antibiotics, oral contraceptives, immigration, un cooperativeness and unwillingness to continue the treatment." Enrolled: 38 (M/F not reported) Randomised: 38, 32 completed (7 M/25 F) Withdrawals/drop‐outs: 6, "because of undesirable results and experience of deterioration and discomfort, though none of the patients showed any harmful direct side effects from filtered blue light phototherapy such as burns, pigmented macules, keratoses etc. One patient dropped out after two sessions of irradiation and the other three dropped out after four to five sessions because of unsatisfactory results as claimed by the patients themselves. Meanwhile, 2 patients refused from continuing the trial, as they did not like to use erythromycin due to undesirable smell and stinging sensation." Final number and proportion of participants evaluable: 32/38 (84%) ITT analysis: Unclear |
| Interventions |
Intervention 1 Blue filtered light Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: 415 nm/ "The portable light‐ weighted filter was touching the face for 15 minutes once daily at mid‐day time"; further details not reported Supplier: SAAIRAN Optics® Instructions to participants: Not applicable Intervention 2 Topical erythromycin 4% in 70% ethanol solution Number and frequency of treatments: Unclear how many treatments in total, twice daily Supplier: Unclear Instructions to participants: Unclear |
| Outcomes | Evaluation: Unclear ("The patients were followed up to 12 weeks."; "…at baseline and after each visit up to 4 weeks after cessation of the treatment period") Primary outcomes of review interest recorded 1. Participant's global assessment of improvement? Methods of assessing primary outcomes 1. Unclear Secondary outcomes of review interest recorded 1. Investigator's assessment of change in acne severity? 2. Investigator's global assessment of improvement? 3. Adverse effects Methods of assessing secondary outcomes 1. "Acne severity index (ASI) score was estimated by such formula: 0.25 x comedone number + 1 x papule number + 2 x pustule number = ASI score." 2. VAS (0 = none to 5 = very severe)? 3. Unclear |
| Notes | Language: English. It was unclear whether this was a RCT, although this was stated in the title. According to the abstract, there was no left‐right side randomisation, but it is unclear whether groups were randomised. We contacted the study authors but they did not reply. |
Ganceviciene 2015.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Not declared Setting: Single centre (Vilnius, Lithuania) Recruitment: the General and Aesthetic Clinic of Dermatology in Vilnius, Lithuania Duration: Not reported |
| Participants |
Included Age (inclusion criterion; mean; range): > 18 years; 20.4 years; 18‐30 years Clinically evident acne: Yes Severity of condition assessment: "mild to moderate facial acne vulgaris"; "a simple 4‐group clinical classification of patients (Nast et al. 2012) was used, which is based on EU Guidelines (1 – Comedonal acne, 2 ‐ Mild–moderate papulopustular acne, 3 ‐ Severe papulopustular acne, moderate nodular acne, 4 ‐ Severe nodular acne, conglobate acne)"; "Patients were identified with mild to moderate facial acne, severity ‐ grade 2." Fitzpatrick skin types: I‐III inclusion criterion, I‐II included Other: "Patients were interviewed for past skin diseases and were not allowed to use any systemic, topical, or phototherapy‐based acne treatment during the course of this study." Excluded "underage, pregnancy and lactation, prior therapy with isotretinoin within 6 months, systemic antibiotic therapy (for any indication) within 1 month or use of topical acne preparation/intra‐lesional steroid injection within 1 month before the laser treatment" Enrolled: 19 enrolled (M/F not reported) Randomised: 19 Withdrawals/drop‐outs: 17/19 completed (2 M/15 F), "two dropped out for personal reasons" Final number and proportion of participants evaluable: 17/19 (89%) ITT analysis: Not reported |
| Interventions |
Intervention 1 Nd:YAG laser, using an S11 scanner with optimal scanning pattern; "Treatment was performed on one side of the face, with one pass without overlapping the single pulses. Cold air cooling was used throughout the treatment and moisturising cream and/or sunscreen was applied immediately after treatment to ensure comfort and safety at the highest level." Number and frequency of treatments: 5 treatments in total, at 1‐week intervals Wavelength/Fluence/Duration/Spot size: 1064 nm/30‐50 J/ cm2/25‐40 ms pulse duration/6 mm Supplier: Fotona SP Dynamis, Ljubljana, Slovenia Instructions to participants: Not reported Intervention 2 Not specified – no treatment control? Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Not reported Instructions to participants: Not reported |
| Outcomes | Evaluation: At baseline and at each treatment session (weekly for 5 weeks), and then 1 and 4 weeks after final treatment Primary outcomes of review interest recorded 1. Change from baseline in ILs count (papules and pustules) 2. Change from baseline in NILs count (comedones) 3. Change from baseline in total lesion count Methods of assessing primary outcomes 1., 2. & 3. "The clinical outcome was assessed also by inflammatory and non‐inflammatory acne counts by an independent dermatologist." Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse events Methods of assessing secondary outcomes 1. "a simple 4‐group clinical classification of patients (Nast et al. 2012) was used, which is based on EU Guidelines (1 – Comedonal acne, 2 ‐ Mild–moderate papulopustular acne, 3 ‐ Severe papulopustular acne, moderate nodular acne, 4 ‐ Severe nodular acne, conglobate acne. The progress of treatment and acne lesion counts were evaluated by standardized high‐ resolution digital photographs (MVC‐FD97, Sony, Tokyo, Japan), which were taken before each treatment, at every laser treatment, as well as 1 week (and also 1 month in some patients) after the last treatment session (total 6 visits), with the same settings and lighting conditions throughout the study." 2. "The duration and type of adverse reactions, such as erythema, edema, exfoliation or hyper‐ and hypo‐pigmentation, were documented at every follow‐up visit with a 1‐5 VAS scale (1‐none, 2‐mild, 3‐moderate, 4‐severe, 5‐very severe), which was used also for self‐assessment of the pain level during and after the treatment (stinging, burning, itching, dryness)." |
| Notes | Language: English. This was a study identified in our final searches. We will attempt to obtain further details and fully incorporate them in the update of this review if judged eligible. We have not attempted to contact the study authors. Correspondence details not specified |
ISRCTN73616060.
| Methods | RCT, parallel‐group? Details were not provided. Unit of randomisation: Unclear, whole person? Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: The Department of Health Setting: Single centre (Leeds, UK) Recruitment: Unclear Duration: 25 months (January 2004‐January 2006, when the trial was stopped) |
| Participants |
Included Age (inclusion criterion; mean; range): Unclear Clinically evident acne: Yes Severity of condition assessment: "with mild to moderate facial acne" Fitzpatrick skin types: Not reported Excluded "Patients with acne conglobata, acne fulminans and secondary care, with underlying diseases or other dermatological conditions that require the use of interfering topical therapy, with photosensitive disorders." Enrolled: Not reported, target number of participants 48 in each group Randomised: Unclear Withdrawals/drop‐outs: Unclear Final number and proportion of participants evaluable: Unclear Intention‐to‐treat analysis: See 'Notes'. |
| Interventions |
Intervention 1 PDL Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Unclear Instructions to participants: Unclear. Intervention 2 "Standard practice Number and frequency of treatments: Unclear Supplier: Not reported Instructions to participants: Unclear |
| Outcomes | Evaluation: Unclear Primary outcomes of review interest recorded 1. Investigator‐assessed change in ILs, NILs and total lesion counts Methods of assessing primary outcomes 1. Unclear Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity Methods of assessing secondary outcomes 1. Unclear |
| Notes | Language: English. This was a trial register record. The title of the study suggests an observational study: "A preliminary observational study on the effect of pulsed dye laser treatment in patients with facial acne vulgaris." According to trial register record this trial was stopped in January 2006 due to poor recruitment. The study authors clarified this was a RCT which was stopped due to poor recruitment. They were unable to provide further details. |
ISRCTN78675673.
| Methods | RCT, details not provided Unit of randomisation: Unclear Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Unclear Setting: Single centre (London, UK) Recruitment: Primary care, details not provided Duration: 9 months (February 2005 to October 2005) |
| Participants |
Included Age (inclusion criterion; mean; range): 16‐45; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "with moderate active inflammatory acne vulgaris" Fitzpatrick skin types: Not reported. Excluded Not stated Enrolled: Not reported, target number of participants 40 Randomised: 28 Withdrawals/drop‐outs: Unclear Final number and proportion of participants evaluable: Unclear ITT analysis: Unclear |
| Interventions |
Intervention 1 PDL Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: 585 nm, other not reported Supplier: Not reported Instructions to participants: Not applicable Intervention 2 Lymecycline orally & isotretinoin gel topically Number and frequency of treatments: Unclear Supplier: Not reported Instructions to participants: Not reported |
| Outcomes | Evaluation: 3 months after treatment Primary outcomes of review interest recorded 1. Participant's global assessment of improvement ("patient satisfaction") Methods of assessing primary outcomes 1. Unclear Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity Methods of assessing secondary outcomes 1. Leeds score |
| Notes | Language: English This was a trial register record. The study is recorded as completed, but no results were published. Compliance and cost were also assessed. We tried to contact the responsible party, but were unsuccessful. |
ISRCTN95939628.
| Methods | This was a split‐face RCT. Unit of randomisation: Not reported Power calculation: Not reported Ethical approval: Not reported Sponsorship and conflict of interest: Not reported Setting: Single centre (Birmingham, UK) Recruitment: Not reported Duration: Unclear (anticipated May 2005–August 2006) |
| Participants |
Included Age (inclusion criterion; mean; range): not reported; not reported; not reported Clinically evident acne: Not reported Severity of condition assessment: mild‐moderate inflammatory acne vulgaris Fitzpatrick skin types: Not reported Excluded Not stated Enrolled: Target number 30 Randomised: Not reported Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: Not reported ITT analysis: Unclear |
| Interventions |
Intervention 1 Vbeam PDL Number and frequency of treatments: Not reported Wavelength/Fluence/Duration/Spot size: Not reported Supplier: Not reported Instructions to participants: Unclear Intervention 2 Omnilux blue/red light phototherapy system Number and frequency of treatments: Not reported Supplier: Not reported Instructions to participants: Not reported |
| Outcomes | Evaluation: Unclear Primary outcomes of review interest recorded Not reported Secondary outcomes of review interest recorded Not reported |
| Notes | Language: English. This was a trial register record. The study is recorded as completed, but no results were published. We tried to contact the responsible party who confirmed that the study was completed but was unable to provide any further data. |
Kim 2012.
| Methods | This was a split‐face RCT. Unit of randomisation: Left and right face Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Declared Setting: Single centre (Seoul, Korea) Recruitment: Not reported Duration: Start and end dates were not reported. |
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "...with mild to moderate acne" Fitzpatrick skin types: Not reported Excluded "(i) age under 18 years; (ii) cystic acne; (iii) photosensitivity; (iv) recent use of photosensitising agents; (v) use of oral acne treatments within 4 weeks or topical acne treatments within 2 weeks, use of isotretinoin within 6 months; and (vi) pregnancy or lactation." Enrolled: 4 (3 M/1 F) Randomised: 4 Withdrawals/drop‐outs: None Final number and proportion of participants evaluable: 4 (100%) ITT analysis: No |
| Interventions |
Intervention 1 Topical application of 19% a, b‐chlorophyll solution prior to treatment with IPL Number and frequency of treatments: 3 treatments in total, weekly Wavelength/Fluence/Duration/Spot size: 530‐750 nm/6‐8 J/cm²/2.5 ms/not reported Supplier: Delta‐blue; Korea Rub, Korea, Ellipse‐Flex ; DDD, Denmark Instructions to participants: Not applicable Intervention 2 IPL only Number and frequency of treatments: 3 treatments in total, weekly Wavelength/Fluence/Duration/Spot size: 530‐750 nm/6‐8 J/cm²/2.5 ms/not reported Supplier: Ellipse‐Flex ; DDD, Denmark Instructions to participants: Not applicable |
| Outcomes | Evaluation: 1 month after final treatment Primary outcomes of review interest recorded 1. Participant's global assessment of improvement ("Subjective satisfaction") 2. Change from baseline in total lesion count Methods of assessing primary outcomes 1. 0–25%, 25%–50%, 50%–75%, and 75%–100% (poor, fair, good, and excellent, respectively)? 2. Unclear Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse effects Methods of assessing secondary outcomes 1. 'Global Severity score' 2. Unclear |
| Notes | Language: English. This was a pilot study and a 'Letter to the Editor'. We contacted the study authors for further information but got no reply. |
Kwon 2016.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Not declared, "Seoul National University Hospital" (as per the NCT record) Setting: Multicenter (Seoul and Chonnam, Korea) Recruitment: Seoul National University Hospital and Chonnam National University Hospital Duration: 8 months, September 2014‐April 2015 |
| Participants |
Included Age (inclusion criterion; mean; range): not reported; 22.4 in the ALA group, 23.1 in the control group (unclear whether means); not reported Clinically evident acne: Yes Severity of condition assessment: "mild to moderate acne", "Investigator’s Global Assessment (IGA) scale in the range of 2–4" Fitzpatrick skin types: not reported Excluded "The exclusion criteria were pregnancy, mental illness, and prior acne therapy including isotretinoin therapy within 6 months, systemic antibiotic therapy and topical agents within 6 weeks of baseline. Enrolled: 46 enrolled (24 M/22 F) Randomised: 46, 23 (13 M/10 F) in the ALA group, 23 (11 M/12 F) in the control group Withdrawals/drop‐outs: 45/46 completed, "1 dropped out for personal reasons" Final number and proportion of participants evaluable: 45/46 (98%) ITT analysis: Not reported |
| Interventions |
Intervention 1 1.5% ALA‐bu gel. "All patients were educated to apply each allocated agent to all acne lesions on the face for 12 weeks (Fig. 1). They were instructed to apply approximately one fingertip unit of assigned gel around their acne lesions on the face looking in the mirror in the morning (6.00–9.00 h) per every other day." Number and frequency of treatments: Every other day, over 12 weeks Wavelength/Fluence/Duration/Spot size: Daylight Supplier: Ez’P®gel; J care, Gwangju, Korea Instructions to participants: See above Intervention 2 Vehicle gel. "All patients were educated to apply each allocated agent to all acne lesions on the face for 12 weeks (Fig. 1). They were instructed to apply approximately one fingertip unit of assigned gel around their acne lesions on the face looking in the mirror in the morning (6.00–9.00 h) per every other day." Number and frequency of treatments: Every other day, over 12 weeks Wavelength/Fluence/Duration/Spot size: Daylight Supplier: Not reported Instructions to participants: see above |
| Outcomes | Evaluation: At baseline and weeks 2, 4, 8 and 12. Evaluations were not performed after final treatment Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Change and percentage change from baseline in ILs count 3. Change and percentage from baseline in NILs count Methods of assessing primary outcomes 1. "At the final visit, patients’ subjective self‐assessments of efficacies were rated on a visual analog scale (VAS) (ranging 0 = “not effective at all” to 10 = “more effective than any other treatment ever”)." 2. & 3. "Both individual acne lesion counts around the face and IGA score were evaluated by two independent dermatologists. To ensure the reliability of evaluation, standardized digital photographs were taken at baseline and each follow‐up visit using identical camera settings." Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement (referred to as acne severity?) 2. Adverse events Methods of assessing secondary outcomes 1. Investigator’s Global Assessment of Improvement (IGA) score, see above 2. Recorded during study, "through dermatologists’ skin examinations per each visit", "Patients’ subjective evaluations for discomfort related to both sun exposure and gel applications were also evaluated on a VAS (ranging 0 = “none” to 10 = “impossible to apply because of side‐ effects”) with rigorous dermatologists’ physical examinations." |
| Notes | Language: English. This was a study identified in our final searches. It will be included and the results fully incorporated in the update of this review. Registered at ClinicalTrials.gov (NCT02313467). We have not attempted to contact the study authors. Correspondence: Dae Hun Suh MD, PhD, Department of Dermatology, Seoul National University College of Medicine, 28 Yongon‐dong, Chongno‐gu, Seoul 110‐744, Korea. Email: daehun@snu.ac.kr and Jee Bum Lee MD, PhD, Department of Dermatology, Chonnam National University Medical School, 42 Jebong‐ro, Dong‐gu, Gwangju 501‐757, Korea. Email: jbmlee@jnu.ac.kr. Participants not allowed to use any acne treatment during the course of this study |
Lee 2012.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared Setting: Single centre, Daegu (Korea) Recruitment: Unclear Duration: Start and end dates were not reported. |
| Participants |
Included Age (inclusion criterion; mean; range): Not stated; not stated; not stated Clinically evident acne: Yes Severity of condition assessment: "with mild to moderate acne" Fitzpatrick skin types: Not reported Excluded "pregnancy, mental illness, intake of oral isotretinoin within 6 months, and application of the other oral and topical acne medications, chemical peeling and light based treatments within 6 weeks" Enrolled: 18 (M/F not reported) Randomised: 8 in ALA‐PDT group, 10 in untreated control group Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: Not reported ITT analysis: Unclear |
| Interventions |
Intervention 1 3% liposomal ALA emulsion + IPL Number and frequency of treatments: 3 treatments in total, weekly Wavelength/Fluence/Duration/Spot size: 560–950 nm/17 J/cm²/4 ms 10 ms delay/not reported Supplier: Sigma, St Louis, MO, USA Instructions to participants: Not applicable Intervention 2 No treatment |
| Outcomes | Evaluation: 1 week after final treatment Primary outcomes of review interest recorded 1. Participant's global assessment of improvement? 2. Change from baseline in number of ILs (papules and pustules) Methods of assessing primary outcomes 1. Unclear 2. Unclear Secondary outcomes of review interest recorded 1. Investigator's assessment of change in acne severity 2. Investigator's global assessment of improvement? 3. Adverse effects Methods of assessing secondary outcomes 1. Korean Acne Grading System (Grade 1 < 10 papules; Grade 2 11–30 papules; Grade 3 < 31 papules < 10 nodules; Grade 4 11–20 nodules; Grade 5 21–30 nodules; Grade 6 31 nodules) 2. Excellent, good, poor 3. Unclear |
| Notes | Language: English. This was a pilot study and a 'Letter to the Editor'. The timing of outcome assessment was less than 2 weeks after final treatment. It was unclear whether Participant's global assessment of improvement or Investigator's global assessment of improvement was recorded. We contacted the study authors but they did not reply. |
Lekakh 2015.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Sponsorship unclear, conflicts of interest declared (None, p. 170) Setting: Single centre (Illinois, USA) Recruitment: "Loyola University Health System, Division of Dermatology in LaGrange Park, IL" Duration: Start and end dates were not reported. |
| Participants |
Included Age (inclusion criterion; mean; range): > 18 years; 26.3 years; 18‐52 years Clinically evident acne: Yes Severity of condition assessment: "baseline moderate to severe acne vulgaris as defined by grades 3, 4, or 5 on the Global Evaluation Acne (GEA) scale" Fitzpatrick skin types: I‐III Other: "Subjects in good health" Excluded "..pregnancy or lactation, current smokers, previous or current isotretinoin treatment, cosmetic procedures within 3 months of enrollment in the study, active infection on the face excluding acne, allergy to salicylates or petroleum jelly, and a history of bleeding disorders." Enrolled: 19 enrolled (M/F not reported) Randomised: 19 Withdrawals/drop‐outs: "one dropout secondary to time commitment issues" Final number and proportion of participants evaluable: 18(4 M/14 F)/19 (95%) ITT analysis: Not reported |
| Interventions |
Intervention 1 "…the subject’s face was initially cleansed with 70% alcohol. Afterwards, half of the subject’s face was treated with laser, utilizing the PDL (595 nm) [VBeam Perfecta, Syneron‐Candela Inc, Irvine, CA] at laser settings of 7 mm spot size, energy 10 Joules, 10 millisecond pulse duration, cooling setting 2. Finally, two coats of a 30% SA peeling solution [Delasco Dermatologic Lab and Supply, Council Bluffs, IA] (with large cotton‐tipped applicators) were applied to the subject’s entire face and remained in place for 3‐5 minutes. Once a white crystallization appeared, cool washcloths were applied for subject comfort, and the face was wiped clean with water. Triamcinolone acetonide (0.1%) was then applied to the entire face." Number and frequency of treatments: 3 treatments, every 3 weeks Wavelength/Fluence/Duration/Spot size: See above Supplier: See above Instructions to participants: Not applicable Intervention 2 See Intervention 1 above Number and frequency of treatments: See Intervention 1 above Wavelength/Fluence/Duration/Spot size: Not applicable Supplier: See Intervention 1 above Instructions to participants: Not reported |
| Outcomes | Evaluation of review interest: 3 weeks after last treatment (also evaluated at each treatment) Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Changes in quality of life 3. Adverse events Methods of assessing secondary outcomes 1. Global Evaluation Acne (GEA) scale, "It consists of a visual analog scale ranging from 0‐5 (“clear to very severe acne”) in the clinical assessment of acne severity."; "…patients were photographed and a blinded clinician used the GEA acne evaluation scales to numerically (0‐5) grade each side of the patient’s face" 2. "At the weeks 0 and 9 visits, patients completed the Dermatology Life Quality Index (DLQI) questionnaire which is a simple 10‐question dermatology‐specific quality of life questionnaire that is widely used in dermatology clinical trials." 3. Unclear ("There were no reported or observed minor or serious adverse events in either treatment arm.") |
| Notes | Language: English. This was a study identified in our final searches. It will be included and the results fully incorporated in the update of this review. We have not attempted to contact the study authors. Correspondence to: Olga Lekakh, BS; Loyola University Medical Center, Division of Dermatology 2160 S. First Avenue Bldg. 54, Room 101 Maywood, IL 60153, USA. Tel: +708‐2166533; Fax: +708‐2162444; Email: olekakh@luc.edu |
Lin 2011.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Unclear Setting: Single centre (Hunan, China) Recruitment: Department of Medical Cosmetology, Xiangtan Central Hospital Duration: Start and end dates were not reported. |
| Participants |
Included Age (inclusion criterion; mean; range): not reported; not reported; not reported Clinically evident acne: moderate‐severe Severity of condition assessment: Pillsbury classification II/III Fitzpatrick skin types: Not reported Excluded Not stated Enrolled: 92 Randomised: 92 Withdrawals/drop‐outs: Unclear Final number and proportion of participants evaluable: Unclear ITT analysis: Unclear |
| Interventions |
Intervention 1 Red and blue light combined with Chen's Acne Clear Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: not reported Supplier: Not reported Instructions to participants: Not reported Intervention 2 Chen's Acne Clear Number and frequency of treatments: Unclear Supplier: Not reported Instructions to participants: Not reported |
| Outcomes | Evaluation: Unclear Primary outcomes of review interest recorded Unclear Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Unclear |
| Notes | Language: Chinese. This was an English abstract. We were unable to obtain the full Chinese text. |
Moftah 2016.
| Methods | This was a split‐back RCT. Unit of randomisation: Left or right back Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Not declared Setting: Multicenter (Cairo, Egypt) Recruitment: "Outpatient Clinics of the Dermatology and Venereology Department, Al‐Zahraa University Hospital and Cairo Hospital (Al‐Haud Al‐Marsoud)" Duration: 10 months, November 2012‐August 2013 |
| Participants |
Included Age (inclusion criterion; mean; range): > 13 years; 23.7 years; not reported Clinically evident acne: Yes Severity of condition assessment: "using lesion counting and Burton’s acne severity scale" Fitzpatrick skin types: II‐V Excluded Participants taking concomitant acne medication, history of topical or systemic therapy use for the past 6 months, history of photosensitivity reactions, and pregnant and lactating women Enrolled: 35 enrolled (21 M/14 F) Randomised: 35 Withdrawals/drop‐outs: None Final number and proportion of participants evaluable: 35/35 (100%) ITT analysis: Not applicable |
| Interventions |
Intervention 1 "Each subject was advised to wash the treatment area with soap and water. The treatment area was then degreased with isopropyl alcohol… On the one side of the back, topical liposomal methylene blue hydrogel was applied under occlusion by a silver reflective plastic wrap for 60 min. The remaining gel was removed before being illuminated by the IPL …A single pass of IPL... Ice packs were applied to the treated area after treatment to alleviate discomfort and minimize swelling" Number and frequency of treatments: Once a week over 3 weeks, every 2 weeks Wavelength/Fluence/Duration/Spot size: 550–1200 nm/13–16 J/cm2 "according to patient’s skin type"/pulse duration 30 ms/8 cm2 Supplier: Raylife, Asclepion, Germany Instructions to participants: See above Intervention 2 "The other side was treated directly by the IPL alone." (see above) Number and frequency of treatments: Once a week over 3 weeks, every 2 weeks Wavelength/Fluence/Duration/Spot size: 550–1200 nm/13–16 J/cm2 "according to patient’s skin type"/pulse duration 30 ms/8 cm2 Supplier: Raylife, Asclepion, Germany Instructions to participants: See above |
| Outcomes | Evaluation: 4 weeks after final session (also evaluated at each treatment visit) Primary outcomes of review interest recorded 1. Change and percentage change from baseline in ILs count 2. Change and percentage from baseline in NILs count Methods of assessing secondary outcomes 1. & 2. "Total lesion count on both sides of the back was performed; non‐inflammatory lesions (black and white comedones) and inflammatory lesions (papules, pustules, nodules and cysts) were counted separately before commencement of treatment and at the visit 1 month after the last session." Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Changes in quality of life 3. Adverse events Methods of assessing secondary outcomes 1. Burton’s acne severity scale; "Each patient was photographed using the same digital camera Canon (30‐2, shimomaruko, 3‐chome, Ohta‐ ku, Tokyo 146‐8501, Japan) set at a fixed distance from the patient’s back to compare the left and right sides of the back. The degree of acne severity improvement was assessed as follows: a change of 1 grade was considered mild improvement, 2 grades was considered moderate improvement and 3 grades was considered marked improvement." 2. "Patient satisfaction was assessed using the Cardiff Acne Disability Index (CADI), which is a questionnaire consisting of 5 questions with a maximum score of 15 and a minimum score of 0. The questionnaire has a translated and validated Arabic version that was presented to the patients, who answered it prior to the treatment, 1 and 3 months after the last treatment session." 3. "A pain score was conducted to evaluate if the photo‐sensitizer caused more pain, patients were asked to rate the pain induced by the IPL pulse on a scale of 1–10 on each side of the back. They were also asked in the second, third sessions and the fourth visit if they noticed the presence of any of the following side effects; itching, stinging, skin flaking or staining after the previous session." |
| Notes | Language: English. This was a study identified in our final searches. It will be included and the results fully incorporated in the update of this review. We have not attempted to contact the study authors. Correspondence to: Dr Shady Mahmoud Ibrahim, Drshadyaly@yahoo.com; Drshadyaly@azhar.edu. |
Nataloni 2003.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Unclear Setting: Unclear Recruitment: Unclear Duration: Unclear |
| Participants |
Included Age (inclusion criterion; mean; range): Unclear Clinically evident acne: Yes Severity of condition assessment: "with acne", further details not available Fitzpatrick skin types: Not reported Excluded: Unclear Enrolled: 175 (M/F not reported) Randomised: 175 in total, 25 in ‘Intervention 1’ group, 25 in ‘Intervention 2’ group and 125 in ‘Intervention 3’ group Withdrawals/drop‐outs: Not reported ITT analysis: Not reported |
| Interventions |
Intervention 1 "532‐nm variable pulsed laser"… "laser treatment alone" (n = 25) Supplier: Unclear Instructions to participants: Unclear Intervention 2 "…laser treatment plus cleansers and topical antiacne agents (topical retinoids and salicylic acid) after completing 6 laser treatments" Supplier: Unclear Instructions to participants: Unclear Intervention 3 "…laser treatment with cleanser and topical acne therapy for the entire study duration" Supplier: Unclear Instructions to participants: Unclear |
| Outcomes | Evaluation: Unclear, reported as more than 4 months after treatment Primary outcomes of review interest recorded Unclear Secondary outcomes of review interest recorded Unclear |
| Notes | Language: English. This study was identified through searching reference lists of Thiboutot 2009 as: Nataloni R. Laser treatment comparable to oral antibiotics: 532 nm laser addresses multiple acne pathogens. In: Dermatology Times. Danvers (MA): Advanstar Communications; 2003. We attempted to contact the Dermatology Times for details as we had no access to full text, but were unsuccessful. Our Google searches also did not identify e‐mail contacts of the authors of this study. Results reported as follows in Thiboutot 2009: "The results showed that combination therapy involving both laser treatment and topical therapy was most effective. The time to response was slower in the group treated with laser therapy alone; in addition this group had faster relapse rates compared with patients using combination therapy. Of those treated with both medical and laser therapy, more than 50% of patients maintained results for longer than 4 months without requiring another treatment." |
NCT00237978.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Unclear Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Unclear Setting: Single centre (Dresden, Germany) Recruitment: Unclear Duration: Unclear (estimated September 2006‐September 2009) |
| Participants |
Included Age (inclusion criterion; mean; range): ≥ 14 years; not reported; not reported Clinically evident acne: Mild‐moderate acne Severity of condition assessment: Burton Scale Stage 3‐4, at least 5 inflammatory and 5 non‐inflammatory lesions in the face Fitzpatrick skin types: Not reported Excluded Pregnant and nursing women, antiandrogen therapy, therapy with antibiotics within the last 4 weeks, therapy with retinoids within the last 6 months, natural or artificial UV‐therapy within the last 4 weeks, severe acne papulopustulosa according to Burton Scale 5 or 6, severe systemic condition, secondary acne Enrolled: 60 estimated Randomised: Not reported Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: Not reported ITT analysis: Unclear |
| Interventions |
Intervention 1 Visible light with waterfiltered IR Number and frequency of treatments: Not reported Wavelength/Fluence/Duration/Spot size: Not reported Supplier: Not reported Instructions to participants: Not reported Intervention 2 Visible light with waterfiltered IR and adapalene gel (Differin) Number and frequency of treatments: Not reported Supplier: Not reported Instructions to participants: Not reported |
| Outcomes | Evaluation: Unclear (8 weeks after start of treatment?) Primary outcomes of review interest recorded 1. Investigator‐assessed change in lesion count Methods of assessing primary outcomes 1. Not reported Secondary outcomes of review interest recorded 1. Unclear |
| Notes | Language: English. This was a trial register record. We contacted the responsible party who reported that the study had been terminated due to lack of recruitment. |
NCT00814918.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Unclear Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Unclear Setting: Single centre (Chicago, USA) Recruitment: Unclear Duration: Start and end dates were not reported. |
| Participants |
Included Age (inclusion criterion; mean; range): 18‐79 years; not reported; not reported Clinically evident acne: Unclear Severity of condition assessment: Unclear Fitzpatrick skin types: Not reported Excluded Participants who have had Isotretinoin therapy less that 1 year prior to this ALA‐PDT procedure, participants who have an adverse reaction to light exposure (for example photo‐exacerbated seizures), participants with a history of porphyria Enrolled: 10 (estimated) Randomised: Not reported Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: Not reported ITT analysis: Unclear |
| Interventions |
Intervention 1 20% 5‐ALA Levulan Kerastick with Blu‐U light Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: 417 nm, other not reported Supplier: DUSA Instructions to participants: Not applicable Intervention 2 20% 5‐ALA Levulan Kerastick with Candela V‐beam PDL Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: 595 nm, other not reported Supplier: Not reported Instructions to participants: Not reported |
| Outcomes | Evaluation: Unclear Primary outcomes of review interest recorded Unclear Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Unclear |
| Notes | Language: English. This was a trial register record. We contacted the responsible party who reported that the study had been terminated early in 2009. |
NCT01245946.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Unclear Sponsorship and conflict of interest: Declared Setting: Single centre (Santiago, Chile) Recruitment: Departamento de Dermatología, Centro Médico San Joaquín, Pontificia Universidad Católica de Chile Duration: 8 months (October 2010‐May 2011) |
| Participants |
Included Age (inclusion criterion; mean; range): 18‐30 years; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "with moderate inflammatory acne" Fitzpatrick skin types: Not reported Excluded Topical treatment in the last 3 months before or systemic in 6 months prior to the study, pregnant or breastfeeding, history of photosensitivity or autoimmune disease, a history or active TB disease or HIV, unwilling to participate in the study Enrolled: 46 (M/F not reported) Randomised: 46, 23 in each group Withdrawals/drop‐outs: Not reported ITT analysis: Not reported |
| Interventions |
Intervention 1 "Photodynamic therapy: 2 sessions separated by 2 weeks of TDF with topical ALA 20% for 1.5 h, then irradiated with red light (Waldmann lamp) at a fluence of 37 J/cm2 for 7‐9 min. From the sixth week will begin adapalene 0.1% gel until 12 weeks" Supplier: Not reported Instructions to participants: Not reported Intervention 2 "Topical adapalene gel 0.1% at night for 12 weeks plus doxycycline 100 mg/day for 6 weeks." Supplier: Not reported Instructions to participants: Not reported |
| Outcomes | Evaluation: 6 and 12 weeks after treatment Primary outcomes of review interest recorded 1. Change from baseline in number of ILs 2. Change from baseline in number of NILs Methods of assessing primary outcomes 1. Lesion counts, unclear whether live or using photographs 2. Lesion counts, unclear whether live or using photographs Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Investigator's global assessment of improvement 3. Changes in quality of Life Methods of assessing secondary outcomes 1. Unclear 2. Unclear |
| Notes | Language: English. This was a trial register record. The study is recorded as completed, but no results were published. Compliance and cost were also assessed. We attempted to contact the responsible party, but were unsuccessful. |
NCT01472900.
| Methods | The design of this RCT is unclear. Unit of randomisation: Unclear Power calculation: Yes Ethical approval: Unclear Sponsorship and conflict of interest: Declared Setting: Single centre (Bangkok, Thailand) Recruitment: Chulalongkorn University Duration: 19 months (October 2010‐April 2012) |
| Participants |
Included Age (inclusion criterion; mean; range): 18‐45 years; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "Mild to moderate severity of acne vulgaris with at least 5 active inflammatory acne lesions on each side of the face and less than 25% difference in lesion count between each side of face" Fitzpatrick skin types: I‐IV Excluded Hypertrophic scar or keloid, photo‐aggravated skin diseases, oral isotretinoin 6 months prior to enrolment, topical retinoid or oral antibiotics 4 weeks prior to enrolment Enrolled: estimated 25 (M/F not reported) Randomised: Not reported Withdrawals/drop‐outs: Not reported ITT analysis: Not reported |
| Interventions |
Intervention 1 2 passes of Er:YAG laser Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: 2940 nm/other not reported Supplier: DualisXS M002‐2A, Fotona®, Fotona d.d, Ljubljana, Slovenia Instructions to participants: Not applicable Intervention 2 2.5% BPO gel Number and frequency of treatments: twice daily, duration unclear Supplier: Not reported Instructions to participants: Unclear |
| Outcomes | Evaluation: Unclear Primary outcomes of review interest recorded 1. Participant's global assessment of improvement ("Patient satisfaction') 2. Percentage change from baseline in number of ILs Methods of assessing primary outcomes 1. "Self‐evaluation of patient satisfaction" 2. Lesion counts, unclear whether live or using photographs Secondary outcomes of review interest recorded 1. Investigator's global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Using photographs, details not provided 2. "Adverse events (AEs) include types of AEs (erythema, pain/burning sensation, dryness/excessive scaling, pigmentary change), timing, intensity, outcome and action taking regarding to study procedure particular subject" |
| Notes | Language: English This was a trial register record. The study is recorded as completed, but no results were published. Compliance and cost were also assessed. We attempted to contact the responsible party but were unsuccessful. |
NCT01584674.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face? Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: KLOX Technologies Inc Setting: Multicenter (Athens and Thessaloniki, Greece) Recruitment: Not reported Duration: 14 months (March 2012‐April 2013) |
| Participants |
Included Age (inclusion criterion; mean; range): 16‐30 years; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "Moderate is defined as a patient with an IGA of 3 with 20 to 40 inflammatory lesions (papules and pustules) and no more than 1 nodule. Severe is defined as a patient with an IGA of 4 with a greater than 40 inflammatory lesions with the presence of no more than 2 nodules and/or inflammatory scaring type lesion. Also note that all patients should have a similar disease stage on both sides of their face." Fitzpatrick skin types: I‐IV Other: Both male and female; "Known medical history of active acne vulgaris for at least 6 months."; "The patient must have a clinical examination prior to treatment."; "The patient must have signed the consent form."; "The patient must be willing to return for follow‐up visits."; "Females of child bearing potential must have a negative pregnancy test result at baseline and both male and female patients must be willing to adhere to a birth control method." Excluded "Active skin infection on the face. Patient must not have active, localized or systemic infection."; "Facial aesthetic procedure, including laser therapy and injectables within the last 6 months."; "Enrollment in another acne study or other dermatological study using light therapy including tanning beds within 120 days of enrollment. Patients must not take part or intend to take part in another study liable to interfere with this study whatever the region of the body considered for 30 days prior to the study start and 30 days following completion of the study."; "History of head and/or neck irradiation."; "Use of a hormonal contraception is prohibited unless the birth control has been stable for the past 3 months. Note that patients that are presently taking or have taken in past 30 days Cyproterone Acetate + Ethinyl Estradiol (Diane‐35) are not eligible for this study."; "Any facial dermatological conditions that could hinder or interfere with clinical assessments."; "Immunosuppression and/or cortisone therapy in the past 4 months."; "Bleeding diathesis."; "Medications or supplements affecting coagulation."; "Isotretinoin within the last 24 weeks."; "Pregnant, breast‐feeding or pregnancy planned during the trial."; "History of facial nerve palsy or marked facial asymmetry."; "History of neuromuscular disorder."; "Prior facial surgery that alters subcutaneous tissues (e.g., rhytidectomy)."; "Use of non‐acne topical medication that could interfere with study treatment."; "Physical or psychiatric condition the investigator deems would preclude participation in the study. (e.g. Polycystic Ovary disease)"; "Unwillingness to refrain from excess sun exposure or tanning beds during the healing process". Enrolled: 98? (M/F not reported) Randomised: Unclear Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: Not reported ITT analysis: Not reported |
| Interventions |
Intervention 1 KLOX Biophotonic System (KLOX KLGA0105‐01 photo‐converter gel and KLOX THERA lamp) Number and frequency of treatments: 12 in total, twice a week Wavelength/Fluence/Duration/Spot size: Not reported Supplier: KLOX Technologies Inc Instructions to participants: Not reported Intervention 2 No treatment ("No treatment will be administered on the control hemiface") |
| Outcomes | Evaluation: 6 and 12 weeks (at final treatment and at 6 weeks after final treatment?) Primary outcomes of review interest recorded 1. Participant's global assessment of improvement ("patient satisfaction") 2. Change from baseline in number of ILs Methods of assessing primary outcomes 1. Questionnaire. Further details not given 2. Lesion counts. Further details not given Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Proportion of participants achieving a total reduction of at least 2 grades in the Investigator's Global Assessment (IGA) scale 2. Safety evaluations (treatment‐emergent and treatment‐related adverse events) (Time Frame: 12 weeks) |
| Notes | Language: English. We attempted to contact the sponsors, but were unsuccessful. |
NCT01689935.
| Methods | This was a split‐face and split‐back RCT. Unit of randomisation: Left or right face/back? Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Massachusetts General Hospital Setting: Single‐centre (Boston, Massachusetts, USA) Recruitment: "at Massachusetts General Hospital (MGH)" Duration: Unclear (November 2009‐December 2013?) |
| Participants |
Included Age (inclusion criterion; mean; range): 14‐50 years; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "Healthy subjects with difficult to treat moderate or severe acne on the face or back are eligible to enroll...Subjects with severe acne lesions (one or more nodules or cysts present) on their backs or face…Presence of moderate acne on the back and/or face that has been recalcitrant to previous treatments. Recalcitrant acne is acne with no or mild/temporary (less than 3 months) improvement after using: Accutane® for at least one completed treatment cycle, and/or; Oral antibiotic for ≥ 3 months; and/or; Topical prescription retinoids (tretinoin ‐ retinoic acid, adapalene, tazarotene or other derivatives) for ≥ 3 months, and/or; Topical benzoyl peroxide 2.5% or higher concentrations for ≥ 3 months; Hormonal treatments** for ≥ 3 months.' Fitzpatrick skin types: Not reported Other: Both male and female; 'Willingness to participate in the study; Willingness to receive ALA‐PDT treatment; Informed consent agreement signed by the subject; Willingness to follow the treatment schedule and post treatment care requirements; Willingness to not use topical or systemic (oral) anti‐acne medications including medicated shampoo or soap during the study period." Excluded "Subjects receiving concurrent oral retinoids or antibiotics; ** Subjects with chronic use of antibiotics may be included if proven that its use has not changed the severity of their acne. AND *** Chronic use of antibiotic is considered ≥ 2 years of continuous use; Scarring or infection of the area to be treated; Known photosensitivity; Presence of suntan in the area to be treated; Subjects who have taken medication known to induce photosensitivity in the previous 3 months; Subjects who have had prior oral retinoid (Accutane®) use within 6 months of entering the study; Prior oral antibiotic use within 1 month of entering the study (see exclusion #1); Topical antibiotic or other topical anti‐acne treatments use within 2 weeks of entering the study; Known anticoagulation or thromboembolic condition; Subjects who are immunosuppressed; Subject is unable to comply with treatment, home care or follow‐up visits; Subject is pregnant or breast feeding; Subject has a history of being on photosensitive medications (thiazides [used to treat high blood pressure], tetracyclines, fluoroquinolones griseofulvin or sulfonamides [used to treat infections], sulfonylureas [used to treat diabetes], calcium channel blockers [used to treat hypertension]. phenothiazines [used to treat serious emotional problems]); Known skin sensitivity to blue light; Porphyria (a disorder of the metabolism that can lead to sensitivity to light); Allergies to chemicals called porphyrins; Subjects who started hormonal treatment (for medical conditions or birth control) within less than 3 months." Enrolled: 35 (estimated enrolment) Randomised: Unclear Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: Not reported ITT analysis: Not reported |
| Interventions |
Intervention 1 Drug ‐ topical 20% ALA followed by red light irradiation ‐ conventional PDT Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Not reported Supplier: Levulan® Kerastic® (Dusa Pharmaceuticals, Inc, Wilmington, MA, USA) (Dusa Pharmaceuticals) Instructions to participants: Not reported Intervention 2 Drug ‐ topical 20% ALA followed by inhibitory light during incubation time, then red light for PDT Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Not reported Supplier: Levulan® Kerastic® (Dusa Pharmaceuticals, Inc, Wilmington, MA, USA) (Dusa Pharmaceuticals); Omnilux Blue; 415 nm LED (Phototherapeutics, Cheshire, UK) Instructions to participants: Not reported Intervention 3 Red light only ‐ no drug Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Not reported Supplier: Omnilux Revive; 635 nm ‐ LED (Phototherapeutics, Cheshire, UK) Instructions to participants: Not reported Intervention 4 Blue light only ‐ no drug Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Not reported Supplier: Omnilux Blue; 415 nm LED (Phototherapeutics, Cheshire, UK) Instructions to participants: Not reported |
| Outcomes | Evaluation: "1, 3 and 6 months after treatment" Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Proportion of participants achieving a total reduction of at least 2 grades in the Investigator's Global Assessment (IGA) scale or 'Clear' or 'Almost clear' (Grades 0 or 1) at 12 weeks 2. "Evaluation of overall side‐effects of each test site" |
| Notes | Language: English. Possibly same study as Sakamoto 2012. We attempted to contact the study authors for clarification, but were unsuccessful. |
NCT02647528.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Unclear Sadick Research Group Setting: Unclear. Single centre (New York, NY, USA) Recruitment: Sadick Research Group? Duration: Estimated 24 months, March 2016‐March 2018 |
| Participants |
Included Age (inclusion criterion; mean; range): 18‐65 years; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "with a clinical diagnosis of mild to moderate facial acne vulgaris"; "Subject must have at least eight and not more than fifty inflammatory facial lesions (i.e. papules/pustules) and no nodules on the face. For the purposes of study treatment and evaluation, these lesions should be limited to the facial treatment area including those present on the nose. Lesions involving the eyes, and scalp should be excluded from the count." Fitzpatrick skin types: Not reported Other: "· Subjects who are able to give voluntary, written informed consent to participate in this study and from whom consent has been obtained including HIPAA authorization. · Healthy male or non‐pregnant female 18‐65 years‐of‐age with a clinical diagnosis of mild to moderate facial acne vulgaris. · Subject must be in general good health and free from any clinically significant disease, other than acne, that might interfere with the study evaluations. · Female subjects of childbearing potential (excluding women who are surgically sterilized or postmenopausal for at least 1 year), in addition to having a negative urine pregnancy test, must be willing to use an acceptable form of birth control during the study from the day of the first dose administration to 30 days after the last administration of study drug. · Subjects who use make‐up must have used the same brands/types of make‐up for a minimum period of 14 days prior to study entry and must agree to use the same make‐up brand/type and frequency of use throughout the study. · Subjects must agree not to have any other procedures affecting skin quality (microdermoabrasion, peels, acne treatments, etc.) for the duration of the study. · Subjects must understand the study and be able to follow study instructions as well as attend the required study visits. · Subjects who agree to be photographed for research purposes and their identity may not be concealed in these photographs." Excluded "· Subjects whom are pregnant, planning to become pregnant or breastfeeding. A urine pregnancy test will be done to rule out pregnancy. · Subjects of child‐bearing potential who are not using an approved method of birth control (oral contraceptives, IUD, contraceptive implant, barrier methods with spermicide or abstinence). Females of non‐childbearing potential are defined as post‐menopausal (absence of menstrual bleeding for one year), hysterectomy or bilateral oophorectomy. · Subjects who cannot understand or are not willing to comply with the requirements of the study. · Presence of any skin condition on the face that would interfere with the diagnosis or assessment of acne. · Excessive facial hair (e.g. beards, sideburns, moustaches, etc.) that would interfere with diagnosis or assessment of acne. · The use within 6 months prior to baseline of oral retinoids (e.g. Accutane®) or therapeutic vitamin A supplements of greater than 10,000units/day (multivitamins are allowed). · The use of estrogens or oral contraceptives for less than 3 months prior to baseline. · The use within 1 month prior to baseline of: ‐ topical retinoids to the face; ‐ systemic antibiotics known to have an impact on the severity of facial acne (e.g., containing tetracycline and its derivatives, erythromycin and its derivatives, sulfamethoxazole, or trimethoprim); ‐ systemic corticosteroids (Note: intranasal and inhalational corticosteroids do not require a washout and maybe used throughout the trial if the subject is on a stable dose). · Use within 2 weeks prior to baseline of: ‐ topical corticosteroids; ‐ topical antibiotics; ‐ topical medications for acne (e.g., metronidazole). · Subjects with moderate or severe rhinophyma, dense telangiectases (score 3, severe), or plaque‐like facial edema. · Ocular rosacea (e.g., conjunctivitis, blepharitis, or keratitis) of sufficient severity to require topical or systemic antibiotics. · A subject who has used a sauna during the 2 weeks prior to study entry and during the study. · Subjects who have performed wax epilation of the face within 14 days prior to baseline. · A subject with bacterial folliculitis. · A subject who consumes excessive alcohol, abuses drugs or has a condition that could compromise the subject's ability to comply with study requirements. · Subjects who engage in activities that involve excessive or prolonged exposure to sunlight or weather extremes, such as wind or cold. · A subject who has any clinically significant condition or situation, other than the condition being studied that, in the opinion of the Investigator, would interfere with the study evaluations or optimal participation in the study. · A subject who has used any topical azelaic acid therapy within 30 days of baseline visit. · Subjects who have participated in an investigational drug study (i.e. subjects have been treated with an Investigational Drug) within 30 days prior to baseline will be excluded from study participation. Subjects who are participating in non‐treatment studies such as observational studies or registry studies can be considered for inclusion. · Subjects who have been previously enrolled in this study. · Subjects who have had laser therapy (for telangiectasia or other conditions), electrodessication and phototherapy to the facial area within 180 days prior to study entry. · Subjects who have had cosmetic procedures (e.g., facials) which may affect the efficacy and safety profile of the Investigational Product within 14 days prior to study entry. · Subjects who have had any kind of facial dermabrasion, chemical peel, laser, IPL or any other treatment that could influence the skin quality in the past 6 months or for the duration of the study · Subjects who do not agree to avoid using tanning beds or intensive exposure to the sun two weeks prior to each office visit. · Subjects who have any known cancer including skin cancers (basal cell carcinoma, squamous cell carcinoma and melanoma) in the treatment area. · Subjects who are currently involved in any injury litigation claims." Enrolled: 0, 30 participants anticipated (as per the record archive 2016_01_05); "This study has been withdrawn prior to enrollment." |
| Interventions |
Intervention 1 Chromogenix Regenlite Transform Treatment, PDL Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Not reported/3‐3.6 J/cm2/Not reported/7 mm Supplier: Unclear Instructions to participants: Not reported Intervention 2 Chromogenix Regenlite Transform Placebo, PDL Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Not reported/0 J/cm2/Not reported/7 mm Supplier: Unclear Instructions to participants: Not reported |
| Outcomes | Evaluation: "Through study completion, an average of 6 months"; further details were not given Primary outcomes of review interest: not to be recorded Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Unclear whether adverse events were to be recorded Methods of assessing secondary outcomes 1. ‘Global Acne Assessment Scoring’, details unclear 2. Unclear |
| Notes | Language: English. Title: A Randomized, Blinded, Single‐Centered, Placebo‐Controlled Trial of Pulse Dyed Laser (Chromogenex Regenlite Transform) in the Treatment of Inflammatory Acne Vulgaris. Possibly the same study as Sadick 2016. The record was last updated on July 28, 2016 stating that "This study has been withdrawn prior to enrolment (funding withdrawn)". This was a study identified in our final searches. We have not attempted to contact the study author. |
Nestor 2016.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared p. 25 "Dr. Nestor is a consultant to La Lumiere LLC, Cleveland, Ohio, and received a research grant for this study. The authors acknowledge the editorial assistance of Dr. Carl S. Hornfeldt, Apothekon, Inc., with funding provided by La Lumiere.’" Setting: Single centre? (USA) Recruitment: Not reported Duration: Start and end dates were not reported. |
| Participants |
Included Age (inclusion criterion; mean; range): 12‐35 years; not reported; 12‐33 years Clinically evident acne: Yes Severity of condition assessment: "mild‐ to‐moderate facial acne vulgaris, defined as 20 to 140 total lesions, with 10 to 90 non inflammatory and 10 to 50 inflammatory facial lesions, but no nodules or cysts (Investigator’s Global Assessment Score of 2, 2.5, 3, or 3.5 using the Modified Cook’s Scale)" Fitzpatrick skin types: I‐VI Other: "Each subject expressed a willingness to comply with the requirements of the study, which included avoiding excessive sun exposure and tanning beds, artificial tanning creams, and facial spray tans." Excluded "…a known allergy to any ingredients in the test products; presence of severe acne or acne conglobate; pre‐existing or dormant facial dermatologic conditions, such as psoriasis, rosacea, rashes, many or severe excoriations that could interfere with the outcome of the study; use of prescription topical antibiotics, such as clindamycin or topical retinoids within the past two weeks or the use of oral retinoids within the past six months; use of oral antibiotics within the past four weeks; use of topical acne medications containing BPO or salicylic acid within the past two week; excessive facial hair, including beard, mustache or goatee, or scars that could interfere with imaging or evaluations; or participation in any other clinical study during the past four weeks." Enrolled: 105 enrolled (74 M/31 F) Randomised: 105, 35 in each group Withdrawals/drop‐outs: Unclear; "The most common reason for not completing the study was being unable to comply with visit schedule (n = 8)" Final number and proportion of participants evaluable: 92/105 (88%) in total, 27/35 in the ‘MASK’ group, 33/35 in the BPO group, 32/35 in the ‘MASK‐SA’ group ITT analysis: Yes |
| Interventions |
Intervention 1 ‘MASK’ described as "The acne light therapy device uses LED technology to emit red (630 nm) and blue (445 nm) light… The arrays of LEDs are designed as a lightweight mask that is worn by the user."; "‘MASK’ group: Neutrogena® Ultra‐Gentle Foaming Cleanser (Johnson and Johnson Consumer, Inc., New Brunswick, New Jersey) and the MASK treatment. The cleanser was used to wash the face each morning and evening. The MASK treatment was applied once daily after the facial cleansing. A non‐medicated moisturizer was permitted as needed." Number and frequency of treatments: Total number unclear, once daily for 15 min (over 12 weeks?) Wavelength/Fluence/Duration/Spot size: 630nm + 445 nm, not reported, not reported, not reported Supplier: illuMask® La Lumiere, LLC, Cleveland, Ohio (for ‘MASK’) Instructions to participants: "Study subjects assigned to use the light mask were instructed to place the mask over the face and turn the device on. The device turns off automatically after each 15‐ minute treatment."; "Subjects received pre‐weighed containers of their assigned test product and written and verbal instructions on their product use and were instructed to bring the product to each clinic visit. The initial product application was performed by each subject in the clinic under the supervision of trained study staff. Each subject also received a diary for recording daily product applications."; "Subjects were instructed to cleanse their face with their customary non‐medicated facial cleanser and to remove all facial and eye makeup at least 30 minutes but not more than two hours prior to each clinic visit". Intervention 2 "Neutrogena® Ultra‐Gentle Foaming Cleanser and Neutrogena® Complete Acne Therapy System Overnight Acne Control Lotion (2.5% benzoyl peroxide) (Johnson and Johnson Consumer, Inc.). The cleanser was used to wash the face each morning and evening. The acne treatment was applied to the entire face in a thin layer each morning and evening. The product was allowed to dry before applying any additional facial products. A non‐ medicated moisturizer was permitted not more than twice daily as needed." Number and frequency of treatments: Duration unclear (over 12 weeks?), each morning and evening Supplier: See above Instructions to participants: See Intervention 1 above Intervention 3 "MASK‐SA group: Neutrogena® Ultra‐Gentle Foaming Cleanser and Neutrogena® All‐in‐1 Acne Control Facial Treatment (1% salicylic acid plus retinol) (Johnson and Johnson Consumer, Inc.) and the MASK treatment. The cleanser was used to wash the face each morning and evening. The acne treatment was applied to the entire face in a thin layer each morning. The product was allowed to dry before applying any additional facial products. The light mask treatment was applied once daily after the facial cleansing. A non‐medicated moisturizer was permitted not more than twice daily as needed. In the evening, moisturizer was not to be applied until after the mask treatment was complete." Number and frequency of treatments: Total number unclear, once daily for 15 min (over 12 weeks?) Wavelength/Fluence/Duration/Spot size: 630 nm + 445 nm, not reported, not reported, not reported Supplier: illuMask® La Lumiere, LLC., Cleveland, Ohio (for ‘MASK’) Instructions to participants: See Intervention 1 above |
| Outcomes | Evaluation: Unclear "Day 1, Weeks 1, 2, 4, 8, and 12 (Visits 2, 3, 4, 5, and 6), or at the time of study withdrawal" Final evaluation at final treatment? Primary outcomes of review interest recorded 1. Change from baseline in ILs count 2. Change from baseline in NILs count Methods of assessing primary outcomes 1. & 2. "Full facial acne counts were performed on the forehead, left and right cheeks, chin, upper lip, and nose. Each count including inflammatory and non inflammatory lesions was repeated…" Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse events Methods of assessing secondary outcomes 1."Investigator’s Global Assessment Score…using the Modified Cook’s Scale"; "Treatment Responders were defined as individuals showing improvement in two of the three of the primary endpoints of IGA, inflammatory and noninflammatory lesions at Week 12 while Full Responders were defined as individuals showing improvement in all three primary endpoints." 2. "A grading scale was used by the Investigator for the following objective treatment tolerance assessments: Erythema, Edema, Dryness, and Peeling. A similar grading scale was used by subjects for the following subjective treatment tolerance assessments: Burning/Stinging, Itching and Dryness/Tightness."; "Subjects were queried about potential AEs during each clinic visit and were encouraged to report possible AEs to the Investigator at any time. The Investigator examined the treated area at each visit for evidence of any possible treatment‐related AEs." |
| Notes | Language: English. This was a study identified in our final searches. It will be included and the results fully incorporated in the update of this review. We have not attempted to contact the study authors. Correspondence to: Mark S. Nestor, MD, PhD; E‐mail: nestormd@admcorp.com |
Park 2015.
| Methods | This was a parallel‐group trial, unclear whether randomised Unit of randomisation: Unclear, whole person? Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Unclear (Korea) Recruitment: Unclear Duration: Unclear |
| Participants |
Included Age (inclusion criterion; mean; range): not reported; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "who had acne on their face at the level of mild or comedonal" Fitzpatrick skin types: not reported Other: Female only, "undergraduate" Excluded Unclear Enrolled: 24 enrolled (24 F) Randomised: Unclear how many per group Withdrawals/drop‐outs: Unclear Final number and proportion of participants evaluable: Unclear ITT analysis: Unclear |
| Interventions |
Intervention 1 No treatment Instructions to participants: Unclear Intervention 2 420 nm of blue visible light Number and frequency of treatments: "irradiated with visible light for 20 minutes per week for 6 weeks." Wavelength/Fluence/Duration/Spot size: 420 nm/not reported/not reported/not reported Supplier: Not reported Instructions to participants: Unclear Intervention 3 660 nm of red visible light Number and frequency of treatments: "irradiated with visible light for 20 minutes per week for 6 weeks." Wavelength/Fluence/Duration/Spot size: 660 nm/ not reported/ not reported/ not reported Supplier: Not reported Instructions to participants: Unclear Intervention 4 Blue and red visible light Number and frequency of treatments: "irradiated with visible light for 20 minutes per week for 6 weeks." Wavelength/Fluence/Duration/Spot size: 420 + 660 nm/not reported/not reported/not reported Supplier: Not reported Instructions to participants: Unclear |
| Outcomes | Evaluation: Unclear Primary outcomes of review interest recorded 1. Unclear, change and percentage change from baseline in ILs count (papules and pustules)? Methods of assessing secondary outcomes 1. Unclear Secondary outcomes of review interest recorded 1. Unclear whether these were assessed Methods of assessing secondary outcomes 1. Unclear |
| Notes | Language: Korean. This was a study identified in our final searches. We were unable to obtain full text in English. We extracted data in this table from the abstract in English. We have not attempted to contact the study authors. Correspondence to: Seon‐Nam Park (Hoseo Univ.) Tel: +82‐10‐3401‐1679 email: skinnancy@naver.com |
Passeron 2011.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Single centre (Nice, France) Recruitment: Not reported Duration: Start and end dates were not reported. |
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "patients with severe acne or moderate acne who resisted to topical treatment associated with oral antibiotics" Fitzpatrick skin types: Not reported Excluded Not reported Enrolled: 20 (M/F not reported) Randomised: 20 Withdrawals/drop‐outs: 4 lost to follow‐up, timing and reasons not reported Final number and proportion of participants evaluable: 16 (80%) ITT analysis: Not reported |
| Interventions |
Intervention 1 PDL + diode laser Number and frequency of treatments: PDL ‐ 1 session followed by diode laser 2 weeks later for 3 sessions (1 per month) Wavelength/Fluence/Duration/Spot size: Not reported/10 J/cm2/10 ms/10 mm for PDL and 1450 nm/14 J/cm2/DCD 40/6 mm2 for diode laser Supplier: VBeam, Candela and Smoothbeam, Candela Instructions to participants: Not applicable Intervention 2 Diode laser only Number and frequency of treatments: 3 treatments in total, applied monthly Wavelength/Fluence/Duration/Spot size: 1450 nm/14 J/cm²/DCD 40/6 mm² Supplier: Smoothbeam, Candela Instructions to participants: Not applicable |
| Outcomes | Evaluation: 1, 6 and 12 months after treatment Primary outcomes of review interest recorded 1. Participant's global assessment of improvement ("patient satisfaction") 2. Change from baseline in number of total lesions Methods of assessing primary outcomes 1. VAS 2. Lesion counts (photographs) Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Recorded during study; pain ‐ VAS |
| Notes | Language: English. This was a conference proceeding. We attempted to contact the study authors but were unsuccessful. |
Pinto 2011.
| Methods | Unclear. Please see 'Notes' |
| Participants | Unclear. Please see 'Notes' |
| Interventions | Unclear. Please see 'Notes' |
| Outcomes | Unclear. Please see 'Notes' |
| Notes | Language: Spanish. Full text not obtained (comparison of MAL‐PDT with red light alone), possibly same as Pinto 2013 (same study authors, same comparison) which was not reported as RCT, although this was stated in the abstract we've identified on‐line in both English and Spanish. |
Sadick 2016.
| Methods | This was a RCT of unclear design. Unit of randomisation: Unclear Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Unclear Setting: Unclear. Single centre (New York, USA)? Recruitment: Not reported Duration: Start and end dates were not reported. |
| Participants |
Included Age (inclusion criterion; mean; range): not reported; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "with mild to moderate acne, as determined by PI assessments, GAAS and lesion counts" Fitzpatrick skin types: Not reported Excluded Not reported Enrolled: 30 enrolled (M/F not reported) Randomised: 30 Withdrawals/drop‐outs: Unclear Final number and proportion of participants evaluable: Not reported ITT analysis: Not reported |
| Interventions |
Intervention 1 Non‐ablative PDL (Chromogenex Regenlite Transform) Number and frequency of treatments: 3 in total, 4 weeks apart Wavelength/Fluence/Duration/Spot size: Not reported Supplier: See above Instructions to participants: Not applicable Intervention 2 Unclear Number and frequency of treatments: Unclear Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Unclear Instructions to participants: Unclear |
| Outcomes | Evaluation: "a 16 week post treatment follow up period to evaluate the effect of NA‐PDL treatments on acne"; further detail were not given Primary outcomes of review interest recorded 1 Participant’s global assessment of improvement 2 Lesion counts, unclear? Methods of assessing primary outcomes 1. &2. Unclear, "Improvement of acne was demonstrated in all subjects, as assessed by patient self‐assessment."; "lesion counts"? Secondary outcomes of review interest recorded 1. Investigator’s global assessment of improvement 2. Adverse events Methods of assessing secondary outcomes 1. GAAS, details unclear 2. Unclear |
| Notes | Language: English. This was a conference abstract. This was a study identified in our final searches. It will be included and the results fully incorporated in the update of this review if judged eligible. We have not attempted to contact the study authors. |
Sakamoto 2012.
| Methods | This was a parallel‐group (and split‐face or split‐back) RCT. Unit of randomisation: Whole person (and back quadrants and left or right face) Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared Setting: Single centre (Boston, Massachusetts, USA) Recruitment: Not reported Duration: Start and end dates were not reported |
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "with moderate‐severe, recalcitrant acne on the face or back" Fitzpatrick skin types: Not reported Excluded Not stated Enrolled: 28 (M/F not reported) Randomised: 28 Withdrawals/drop‐outs: 4 participants were not compliant, 1 dropped out because of severe pain on ALA‐PDT site, and 1 did not have clinical improvement. To date 18 completed the study Final number and proportion of participants evaluable: Unclear ITT analysis: Not reported |
| Interventions |
Intervention 1 i‐PDT very low level light exposure during the period of ALA metabolism (3 h of incubation with 20% topical ALA) Number and frequency of treatments: 4 treatments, 1 month apart Wavelength/Fluence/Duration/Spot size: 633 nm/200 J/cm²/other not reported Supplier: Not reported Instructions to participants: Not applicable Intervention 2 Conventional ALA‐PDT (3 h of incubation with 20% topical ALA) Number and frequency of treatments: 4 treatments, 1 month apart Wavelength/Fluence/Duration/Spot size: 633 nm/200 J/cm²/other not reported Supplier: Not reported Instructions to participants: Not applicable Intervention 3 Control and/or Blue and red light alone No further details provided |
| Outcomes | Evaluation: 1, 3 and 6 months after treatment Primary outcomes of review interest recorded 1. Change from baseline in number of total lesions Methods of assessing primary outcomes 1. Total lesion counts Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Adverse events Methods of assessing secondary outcomes 1. IGA severity scores. Unclear what IGA stands for 2. Recorded during the study |
| Notes | Language: English. This was a conference proceeding. The author was contacted and shared data on randomisation but did not provide any results data. Possibly same study as NCT01689935 (we attempted to contact the study authors for clarification, but were unsuccessful). |
Shaheen 2011.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Declared Setting: Single centre (Cardiff, Wales, UK) Recruitment: Not stated Duration: March 2010 to October 2011 |
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; not reported; 18‐45 years Clinically evident acne: Yes Severity of condition assessment: "mild to moderate facial acne" Fitzpatrick skin types: Not reported Excluded Not stated Enrolled: 37 Randomised: 37 Withdrawals/drop‐outs: 7 (3 IPL‐MAL; 1 IPL‐placebo; 3 adapalene) Final number and proportion of participants evaluable: 30/37 (81%) ITT analysis: results not supplied |
| Interventions |
Intervention 1 IPL‐MAL Number and frequency of treatments: 4 treatments, every 2 weeks Wavelength/Fluence/Duration/Spot size: 530‐950 nm/20‐40 J/cm²/5 ms/50 x 10 mm (2 passes) Supplier: UltraPlus VPL System, Energist Ltd, Swansea, UK Instructions to participants: Not applicable Intervention 2 IPL‐placebo Number and frequency of treatments: 4 treatments, every 2 weeks Wavelength/Fluence/Duration/Spot size: 530‐950 nm/20‐40 J/cm²/5 ms/50 x 10 mm² (2 passes) Supplier: UltraPlus VPL System, Energist Ltd, Swansea, UK Instructions to participants: Not applicable Intervention 3 Adapalene 0.1% Number and frequency of treatments: Nightly for 12 weeks Supplier: Galderma UK Ltd, Watford Instructions to participants: Unclear whether adequate |
| Outcomes | Evaluation: 1, 4 and 9 weeks after treatment with IPL; 3 weeks after treatment in the adapalene group Primary outcomes of review interest recorded 1. Percentage change from baseline in non‐inflammatory and inflammatory lesions Methods of assessing primary outcomes 1. Lesion counts Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Investigator asked for adverse effects at each follow‐up |
| Notes | Language: English. This was a conference proceeding. Study authors contacted but declined to share results data because it has not yet been published |
Song 2012.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Single centre (Hwaseong, Korea) Recruitment: Not reported Duration: Start and end dates were not reported |
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "with mild to moderate acne with papulo pustules and comedones" Fitzpatrick skin types: Not reported Excluded Not stated Enrolled: 24 (M/F not reported) Randomised: 24 Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: Unclear ITT analysis: Not stated |
| Interventions |
Intervention 1 Chlorophyll A + LED Number and frequency of treatments: 8 treatments in total, over 4 weeks Wavelength/Fluence/Duration/Spot size: Not reported Supplier: Not reported Instructions to participants: Not applicable Intervention 2 LED (same wavelength) only Number and frequency of treatments: 8 treatments in total, over 4 weeks Wavelength/Fluence/Duration/Spot size: Not reported Supplier: Not reported Instructions to participants: Not applicable |
| Outcomes | Evaluation: 2, 4 and 8 weeks after final treatment Primary outcomes of review interest recorded 1. Change from baseline in number of lesion counts (not specified) Methods of assessing primary outcomes 1. Unclear Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Unclear |
| Notes | Language: English. This was a conference abstract. We attempted to contact the study authors, but were unsuccessful. Possibly the same study as Song 2014. |
Troilius 2005.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Not declared Setting: Single centre (Malmo, Sweden) Recruitment: Not reported Duration: Start and end dates were not reported. |
| Participants |
Included Age (inclusion criterion; mean; range): Not reported; not reported; not reported Clinically evident acne: Yes Severity of condition assessment: "with mild to moderate acne with papulo pustules and comedones" Fitzpatrick skin types: Not reported Excluded Not stated Enrolled: 15 (M/F not reported) Randomised: 15 Withdrawals/drop‐outs: Not reported Final number and proportion of participants evaluable: Unclear ITT analysis: Not stated |
| Interventions |
Intervention 1 Adapalene 0.1% Number and frequency of treatments: daily, on the whole face, presumably for 12 weeks, but this was not clearly stated Supplier: Differin Instructions to participants: Not reported Intervention 2 Adapalene 0.1% + IPL Number and frequency of treatments: 4 treatments in total, applied at 3‐week intervals Wavelength/Fluence/Duration/Spot size: As Intervention 1 + 535‐750 nm/7‐8 J/cm²/2.5 ms double pulse, delay 10 ms/not reported Supplier: Ellipse (Danish Dermatologic Development) Instructions to participants: Not reported |
| Outcomes | Evaluation: 1 month after treatment Primary outcomes of review interest recorded 1. Change from baseline in number of ILs (papules and pustules not reported separately) 2. Change from baseline in number of NILs (open and closed comedones not reported separately) Methods of assessing primary outcomes 1. & 2. Lesion counts Secondary outcomes of review interest: not recorded |
| Notes | Language: English. This was a conference abstract. We attempted to contact the study authors but were unsuccessful. |
Voravutinon 2016.
| Methods | This was a split‐face RCT. Unit of randomisation: Left or right face Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Declared p.403 ("The authors have indicated no significant interest with commercial supporters.") Setting: Single centre (Bangkok, Thailand) Recruitment: outpatient department of the Institute of Dermatology of Thailand Duration: 11 months, November 2007‐September 2008 |
| Participants |
Included Age (inclusion criterion; mean; range): 18‐45 years; 22.32; 16‐43 years (discrepancy with inclusion criterion) Clinically evident acne: Yes Severity of condition assessment: "moderate to severe facial acne"; "Leeds revised acne grading system with a photographic standard of at least grade 6.0 was used" Fitzpatrick skin types: II‐V Other: "with general good health, willingness and ability to comply with the requirements of the protocol"; "if photosensitive dermatitis, keloid, or herpes simplex disease had occurred in the affected areas previously" Excluded "…previous laser treatments, pregnancy, a history of oral retinoid use within 6 months of study entry, other topical or systemic acne therapies (including oral contraceptives) within 1 month, topical alpha hydroxy acid use or glycolic acid use, microdermabrasion to the face within 3 months, the use of nonsteroidal anti‐inflammatory medications within 10 days, and a history of oral contraceptive use within 3 months."; "if photosensitive dermatitis, keloid, or herpes simplex disease had occurred in the affected areas previously". Enrolled: 62 enrolled (22 M/40 F) Randomised: 62 Withdrawals/drop‐outs: "Seven subjects were removed from study because of incomplete follow‐up" Final number and proportion of participants evaluable: 55/62 (87%) ITT analysis: Not reported |
| Interventions |
Intervention 1 "Pretreatment preparation included facial washing and skin shaving. The affected areas of the face were first treated with nonoverlapping single pulses of the 595‐nm PDL (Vbeam; Candela Corporation, Irvine, CA) with a second pass completed only on inflammatory acne lesions. An integrated dynamic‐ cooling device was not used to avoid any confounders which could possibly affect treatment efficacy." Number and frequency of treatments: 4 in total, at 3‐week intervals Wavelength/Fluence/Duration/Spot size: 595 nm, 4 J/cm², pulse duration 6 ms, 10 mm Supplier: See above Instructions to participants: "All patients were asked to use bland facial wash and broad spectrum SPF 30 sunscreen to the affected areas throughout the study. Patients were told to refrain from using any additional facial products or performing any procedures to the face during the study." Intervention 2 See Intervention 1 above. Different PDL parameters used (see below) Number and frequency of treatments: 4 in total, at 3‐week intervals Wavelength/Fluence/Duration/Spot size: 595 nm, 6‐7.5 J/cm², "adjusted according to the patients’ skin type and clinical end point", pulse duration 6 ms, 10 mm Supplier: See Intervention 1 above Instructions to participants: See Intervention 1 above |
| Outcomes | Evaluation: "…every 4 weeks throughout the 3 months of the follow‐up period"; "clinically assessed in the 6 visits after baseline for a total of 21 weeks' Primary outcomes of review interest recorded 1. Participant’s global assessment of improvement 2. Change from baseline in ILs count 3. Change from baseline in NILs count Methods of assessing primary outcomes 1. "patient satisfaction scale measuring the perceived degree of improvement of acne lesions and redness (0 for no improvement, 4 for greatest improvement)." 2. & 3. Standardised bilateral facial photographs; "each participant’s photographs were viewed in a random order by a dermatologist" Secondary outcomes of review interest recorded 1. Adverse events Methods of assessing secondary outcomes 1."Evaluation during each visit included a patient self‐report of complications related to treatment" |
| Notes | Language: English. This was a study identified in our final searches. It will be included and the results fully incorporated in the update of this review. We have not attempted to contact the study authors. Correspondence to: Murad Alam, MD, Department of Dermatology, 676 N. St. Clair Street, Suite 1600, Chicago, IL 60611, or e‐mail: m‐alam@northwestern.edu |
Wang 2016.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: p.362 "This work was supported by the Fund for Scientific and Technological Transformation of Sichuan Province (14010134)", conflicts of interest not declared Setting: Single centre (Chengdu, Sichuan, China) Recruitment: "Institute of Dermatology and Venereology, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, Chengdu, Sichuan, P.R. China" Duration: 18 months, January 2014‐June 2015 |
| Participants |
Included Age (inclusion criterion; mean; range): not reported; 26.8 and 27.3 in each of the groups; 12‐44 years Clinically evident acne: Yes Severity of condition assessment: "moderate to severe acne"; "Cunliffe method"; "These classifications areas are: light (mainly whiteheads and blackheads), medium (mainly inflammatory papules and pustules), and severe (inflammatory papules, nodules, and inflammatory cysts)" Fitzpatrick skin types: Unclear Excluded "(1) internal or external use of antibiotics within the last 4 weeks; (2) systemic use of a retinoid in the last 6 months; (3) photosensitive or keloid history; (4) pregnant or liver function unusual; (5) cannot complete the course; (6) staff directly participating in the study; and (7) participants currently in other clinical studies or who participated in another study within the last 3 months." Enrolled: 60 enrolled (28 M/32 F) Randomised: 60, 30 in each group Withdrawals/drop‐outs: None Final number and proportion of participants evaluable: 60/60 (100%) ITT analysis: Not applicable |
| Interventions |
Intervention 1 Optical fibre intra‐tissue irradiation (OFI) ALA PDT; "3.6% aminolevulinic acid was evenly applied to the rashes and the surrounding 0.5 to 1.0 cm of normal skin. After 1.5 h of incubation shielded from light, we wiped off the remaining photosensitizer, disinfected, inserted disposable optical fiber needles into the skin lesions with inflammatory papules and nodules, and imported the red light irradiation to the tissues located 3 mm below the follicular orifice (including the sebaceous glands) for 5 min. We used 633±3 nm wave length. For irradiation, a dose of 4.5 J/cm2 (dose at skin, detected by a VLP‐200 laser power meter, Changchun Feimiao Tech., Ltd) was given for the first time...and was adjusted to 3–24 J/cm2 in the following irradiations according to adverse reactions. Dark glasses were used to protect patients’ eyes during irradiation. The skin was sterilized again after irradiation and avoided strong light for 3 days." Number and frequency of treatments: 6 treatments, every 7‐10 days Wavelength/Fluence/Duration/Spot size: See above Supplier: See above Instructions to participants: See above Intervention 2 "Traditional ALA‐PDT"; "The traditional skin surface irradiation was used. For irradiation, a dose of 54 J/cm2 at skin was used with a fixed‐power density of 45 mW/cm2 for 20 min, and the distance between the light panel and patient’s apex nasi was set at 10 cm.’ Number and frequency of treatments: 6 treatments, every 7‐10 days Wavelength/Fluence/Duration/Spot size: Unclear, 54 J/cm², a fixed‐power density of 45 mW/cm2 for 20 min, not reported Supplier: Not reported Instructions to participants: Unclear |
| Outcomes | Evaluation: 4, 8, and 16 weeks after final treatment Primary outcomes of review interest: not recorded Secondary outcomes of review interest recorded 1. Investigator's global assessment of improvement 2. Adverse events Methods of assessing secondary outcomes 1. “Cure” was 90% or more of the skin lesions disappeared; “remarkably effective” was 60% to 89% of the skin lesions disappeared; “effective” was 20% to 59% of skin lesions disappeared; and “invalid” was less than 20% of skin lesions disappeared. Effective rate was the percentage of cured cases plus remarkable cases divided by the total cases 2. "Treatment effects and adverse reactions were recorded during each treatment, before the next treatment and in the subsequent follow‐up period. These adverse reactions include itching, pain, pustules, blisters, edematous erythema, pigmentation, reactive acne, and desquamation. We recorded the appearing and fading away time, severity, and actions used to combat these adverse reactions." |
| Notes | Language: English. This was a study identified in our final searches. It will be included and the results fully incorporated in the update of this review. We have not attempted to contact the study authors. Correspondence to: Wei Liu, e‐mail: weiliu_077@163.com |
Zhang 2009b.
| Methods | This was a parallel‐group RCT. Unit of randomisation: Unclear Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Unclear Setting: Single centre (Shanghai, China) Recruitment: Unclear Duration: Start and end dates were not reported. |
| Participants |
Included Age (inclusion criterion; mean; range): not reported; not reported; not reported Clinically evident acne: Moderate to severe acne Severity of condition assessment: Unclear Fitzpatrick skin types: Not reported Excluded Not stated Enrolled: Not reported Randomised: 70 Withdrawals/drop‐outs: Unclear Final number and proportion of participants evaluable: Unclear ITT analysis: Unclear |
| Interventions |
Intervention 1 Topical ALA‐PDT Number and frequency of treatments: 1‐3 sessions, fortnightly Wavelength/Fluence/Duration/Spot size: Not reported Supplier: Not reported Instructions to participants: Not applicable Intervention 2 Oral isotretinoin 10 mg twice daily for 6 weeks Instructions to participants: Not reported |
| Outcomes | Evaluation: 2, 4 6 weeks (after initial treatment) and at 3 months afterwards (to monitor adverse events) Primary outcomes of review interest recorded Unclear Secondary outcomes of review interest recorded 1. Adverse effects Methods of assessing secondary outcomes 1. Unclear |
| Notes | Language: Chinese. The abstract is in English. We were unable to obtain the Chinese full text. |
ALA = 5‐aminolevulinic acid BPO = benzoyl peroxide FPT = Fitzpatrick's Skin Types: based on different reactions to sun exposure and range from type I ('pale white skin which always burns and never tans') to type VI ('deeply pigmented dark brown to black skin which never burns and tans very easily') (Fitzpatrick 1988) GAAS = Global Acne Assessment Scoring ILs = inflamed lesions IPL = intense pulsed light IR = Infrared ITT = Intention‐to‐treat analysis MAL = methyl‐aminolevulinate NILs = non‐inflamed lesions OFI = optical fibre intra‐tissue irradiation PDL = pulsed‐dye laser PDT = photodynamic therapy RCT = randomised controlled trial SD = standard deviation SPF = sun protection factor
Change from baseline i.e. absolute change is calculated by subtracting baseline count from count assessed at certain time point. Percentage change is calculated by dividing the absolute change with baseline count and then multiplying that value by 100 to get percentages.
Characteristics of ongoing studies [ordered by study ID]
EU 2014‐005235‐13.
| Trial name or title | Therapy‐treatment (PDT) on acne, a study to optimise the number of treatments, the right light‐dose and the right pre‐treatment in order to obtain a long‐term remission‐time |
| Methods | This is a parallel‐group, double blind RCT. Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Yes Sponsorship and conflict of interest: Funded by Sahlgrenska University Hospital of Gothenburg, Department of Dermatology Setting: Single centre? Sahlgrenska University Hospital of Gothenburg, Department of Dermatology Recruitment: Unclear |
| Participants |
Included Age (inclusion criterion): 18‐25 years Clinically evident acne: "Patients with papulopustular acne" Severity of condition assessment: Unclear Fitzpatrick skin types: Not reported Other: "who has signed a written informed consent" Excluded "Pregnant or breast‐feeding; Patients who have been treated with tetracycline up to less than a month before PDT treatment; Local acne treatment until up to one week before PDT treatment; Conditions associated with poor protocol compliance, e.g. excessive use of alcohol or drug abuse." Estimated enrolment: 46 |
| Interventions | Duration: estimated 15 months (Month 2015 to Month Year) Evaluation: 20 weeks after final treatment Intervention 1 160 mg/g MAL‐PDT, 4‐treatment regime Number and frequency of treatments: 4, frequency unclear Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Metvix, Galderma Intervention 2 160 mg/g MAL‐PDT, placebo cream? 2‐treatment regime Number and frequency of treatments: 2, frequency unclear Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Metvix, Galderma |
| Outcomes |
Primary outcomes of review interest recorded 1. Unclear Methods of assessing primary outcomes 1. Unclear Secondary outcomes of review interest recorded 1. Unclear 2. Adverse Effects Methods of assessing secondary outcomes 1. Unclear 2. Unclear |
| Starting date | Unclear |
| Contact information | Sahlgrenska University Hospital of Gothenburg, Department of Dermatology; Gröna stråket 16, Gothenburg, 41345, Sweden. +46(0)313429415; carin.sandberg@vgregion.se |
| Notes | Language: English. We attempted to contact the study author, but were not successful. |
NCT02217228.
| Trial name or title | A randomized, prospective, multicenter, controlled study with blinded assessment to determine the safety and effectiveness of the sebacia acne treatment system in the treatment of inflammatory acne vulgaris |
| Methods | This is a parallel‐group RCT, single blind (outcome assessor) Unit of randomisation: Whole person Power calculation: Yes Ethical approval: Yes Sponsorship and conflict of interest: Funded by Sebacia, Inc Setting: Multicenter (23 centres: Scottsdale, Arizona; Sacramento, California; Washington, District of Columbia; Miami, Florida; Snellville, Georgia; Naperville, Illinois; Hunt Valley, Maryland; Newton, Massachusetts; Clarkston, Michigan; Henderson, Nevada; Hackensack, New Jersey; New York, New York, Charlotte, North Carolina; Youngstown, Ohio; Yardley, Pennsylvania; Charleston, South Carolina; Nashville, Tennessee; Bellaire and Houston, Texas; Salt Lake City, Utah; Spokane, Washington, USA) Recruitment: Combination of medical practice patients and external sources (advertisement) |
| Participants |
Included Age (inclusion criterion): 15‐35 years Clinically evident acne: Moderate‐severe Severity of condition assessment: Investigator's Global Assessment 3‐4; "25 to 75 inflammatory lesions on the cheeks, chin and forehead, not concentrated in one area" Fitzpatrick skin types: I‐III Other: male and female; "able to provide informed consent/assent; minors will provide assent while parent or legal guardian will provide consent"; "in good health, willing to participate and able to comply with protocol requirements" Excluded "Severe acne (Investigator's Global Assessment 5) with significant scarring potential and greater than 2 nodular lesions; Clinically relevant history of keloids; Facial tattoos; Acne conglobata, acne fulminans, chloracne, drug‐induced acne; Active concomitant skin disease, excessive scarring or excess facial hair; Heavily tanned skin; unable or unwilling to avoid tanning beds/excessive sun exposure"; "Acne medication and therapy restrictions" ("Oral retinoids ‐ 12 months; Other systemic medications ‐ 4 weeks; Topical retinoids ‐ 4 weeks; Other topical therapy ‐ 2 weeks; Light treatments, microdermabrasion and/or peels ‐ 8 weeks; Intense pulsed light or laser treatment ‐ 12 weeks; Investigational drug, biologic or device ‐ 30 days' prior to treatment); Gold therapy of any type for any reason;" "Pregnant, lactating, nursing or planning to become pregnant during the study period; Known allergy to gold, ethanol, diisopropyl adipate, Polysorbate 80; Clinically relevant condition that makes participation unsafe or that would interfere with study treatment and assessment" Estimated enrolment: 300 |
| Interventions | Duration: 11 months (estimated September 2014‐July 2015) Evaluation: 12 weeks after start of treatment Intervention 1 Gold microparticle suspension + laser treatment Number and frequency of treatments: 3, over the course of 2 weeks Wavelength/Fluence/Duration/Spot size: Unclear Supplier: Sebacia, Inc, laser (missing information) Intervention 2 Vehicle suspension and laser Number and frequency of treatments: 3, over the course of 2 weeks Supplier: Not reported Intervention 3 Gold microparticle suspension treatment Number and frequency of treatments: 3, over the course of 2 weeks Supplier: Sebacia, Inc, laser (missing information) |
| Outcomes |
Primary outcomes of review interest recorded 1. Investigator‐assessed change in lesion count (IL change and percentage change from baseline) Methods of assessing primary outcomes 1. By blinded evaluator trained at the outset of the study Secondary outcomes of review interest recorded 1. Investigator's global assessment of improvement 2. Adverse effects Methods of assessing secondary outcomes 1. Success "defined as 2‐point decrease from baseline IGA" 2. By unblinded evaluator in accordance with standard practice and applicable regulation |
| Starting date | September 2014 |
| Contact information | Gretchen S Richards, MS; 508‐341‐8110; gretchen@sebacia.com; web site: http://severeacnestudy.com/ |
| Notes | Language: English. We contacted the study author who shared some information on methodology, however further details on the interventions were withheld |
NCT02431494.
| Trial name or title | Safety and preliminary efficacy of combination blue light phototherapy and microcurrent therapy for the treatment of acne vulgaris |
| Methods | This is a parallel‐group, open label RCT Unit of randomisation: Whole person Power calculation: Unclear Ethical approval: Unclear Sponsorship and conflict of interest: Funded by Nova Southeastern University Setting: Multicenter (2 centres: Fort Lauderdale and Hollywood, Florida, USA) Recruitment: Unclear |
| Participants |
Included Age (inclusion criterion): 18‐30 years Clinically evident acne: Mild‐moderate facial acne Severity of condition assessment: Unclear Fitzpatrick skin types: II‐V Other: male and female; "Be able to understand written and/or spoken English"; "Be able to provide written informed consent." Excluded "Have been treated with oral retinoids in the past 6 months; Have been treated with oral antibiotic within the last 30 days; Have received topical acne treatment (i.e. retinoids, antibiotics and anti‐inflammatory agents or chemical peeling) within the last 30 days; Pregnant or lactating; Have history of photo‐sensitive dermatitis; Have previously received light therapy; Taking oral contraceptive pills (OCP); Have pacemaker" Estimated enrolment: 60 |
| Interventions | Duration: 27 months (estimated February 2015‐March 2017) Evaluation: Time points of review interest: 1 and 4 weeks after final treatment (also assessed at week 1, 3 and 5) Intervention 1 Blue light phototherapy (BLP). "The duration of each session will be approximately 20 minutes. At each session, the affected areas of the participant's face will be exposed to a light source using blue light phototherapy machine between 15 to 20 minutes." Number and frequency of treatments: 5 in total, at 1‐week interval Supplier: BLU‐U Blue Light Photodynamic Therapy Illuminator manufactured by DUSA Pharmaceutical Intervention 2 Microcurrent therapy (MCT). "The duration of each session will be approximately 45 minutes. The investigators will place one electrode in one of the regional areas of the lymph nodes or affected area (i.e. the forehead) and move the second electrode systematically from the affected area towards the stationary electrode. Once the entire affected area has been covered, the investigators will move the first electrode to another regional area of the lymph nodes or affected area and the process will be repeated. This will continue until all of the affected areas have been treated." Number and frequency of treatments: 5 in total, at 1‐week interval Supplier: Micro Current Electro‐Device with gloves and carrying case, SKU:DSE‐X1008 Classic Spa Collection Intervention 3 Combination of BLP and microcurrent. "At each session, participants will receive MCT portion as described in above followed by BLP portion as described above. These visits will last approximately 65 minutes." Number and frequency of treatments: 5 in total, at 1‐week interval Supplier: Same devices as described above |
| Outcomes |
Primary outcomes of review interest recorded 1. Investigator‐assessed change in lesion count (papules and pustules) Methods of assessing primary outcomes 1. "The investigators will conduct a systematic count of acne lesions (papules and pustules) present in all of the affected areas of the face." Secondary outcomes of review interest recorded 1. Investigator‐assessed change in acne severity 2. Changes in quality of life Methods of assessing secondary outcomes 1. "The investigators will use the acne counts and the digital photographs of the affected areas to compute the acne severity level following the procedures established by Hayashi et al. (2008). The investigators will not print the digital photographs. The investigators will visually inspect the digital photographs and assign a preliminary severity score to each half of the face using the following classification guide: 0‐5 papules and/pustules for mild acne; 6‐20 for moderate acne, 21‐50 for severe acne; and more than 50 for very severe. The investigators will examine each half of the face separately. The most severe classification obtained for either side of the face will be the assigned severity score." 2. "The investigators will use the Dermatology Life Quality Index (DLQI) developed by A. Y. Finlay and G. K. Khan (1992), one of the most widely used, dermatologic specific quality of life measures in the published literature to assess quality of life. The DLQI consists of 10 Likert type items; 9 of these items have 4 response categories scored from 0 to 3 with "very much" being "3" to "not at all" being "0". Item 7 "Over the last week, has participant's skin prevented participant from working or studying" uses dichotomous responses; where "yes" is scored as a "3" and a "no" requires answering an additional sub‐question, "Over the past week how much has participant's skin been a problem at work or studying". Responses for this sub‐question range from "a lot" coded a "2", to "not at all" coded a "0." The DLQI is calculated by summing the response to each question; the maximum score is "30" and the minimum score is "0". The higher the score, the lower the dermatologic quality of life." |
| Starting date | February 2015 |
| Contact information | Sergey Arutyunyan, M.S.; (305) 860‐8710; sa1096@nova.edu |
| Notes | Language: English. We attempted to contact the sponsor, but were not successful. |
FPT = Fitzpatrick's Skin Types: based on different reactions to sun exposure and range from type I ('pale white skin which always burns and never tans') to type VI ('deeply pigmented dark brown to black skin which never burns and tans very easily') (Fitzpatrick 1988) RCT = randomised controlled trial
Change from baseline i.e. absolute change is calculated by subtracting baseline count from count assessed at certain time point. Percentage change is calculated by dividing the absolute change with baseline count and then multiplying that value by 100 to get percentages.
Differences between protocol and review
The protocol for this study was published in 2009 (Car 2009).
There were changes in authorship. Two protocol authors, Fiona Hamilton and Colin Lyons did not contribute to this review. Four new authors were added (JB, RA, PP and LG). This led to differences in the authors who were originally selected to perform tasks as published in the protocol.
We updated the Background section with recent findings and relevant studies.
We made the following changes in the Methods section.
We made minor edits to the inclusion criteria according to the Cochrane Style Guide.
We have not excluded studies on the basis of inadequate description of intervention or lack of validated outcome, but have used those only as elements to judge study quality. We have therefore removed the possibly misleading sentences from the Types of interventions paragraph.
We included 'Investigator‐assessed severe adverse effects' as a primary outcome, although it was listed under secondary outcomes in the protocol. This was done for the review to be in accordance with the Cochrane Handbook for Systematic Reviews of Interventions section 5.4.2 (O'Connor 2011) and the requirement of including at least one undesirable outcome among primary outcomes.
Although we planned to use only validated scales for 'participant's global assessment of improvement', 'investigator‐assessed change in acne severity' and for 'investigator's global assessment of improvement', we had to include other scales that differed from the original protocol, as these were the methods RCTs used to report such outcomes. We clearly state that the scales were non‐standardised where appropriate.
We intended to disregard the 'investigator's global assessment of improvement' if it was performed without using baseline photographs. We decided to include studies where it was unclear whether researchers used baseline photographs. We also included studies where blinded investigators performed live assessments of IGA scores. We included these, as the FDA defines IGA as "a static evaluation of qualitative overall acne severity" (FDA 2005). Evaluations can thus be performed independently of baseline assessment and FDA recommends photographs mainly for verification and auditing purposes (FDA 2005).
We used an updated version of MedDRA (MedDRA 2010).
We added "after final treatment" to follow‐up periods in 'Timing of outcome assessment' to make it clearer as different interpretations of the initial wording were possible.
We planned to search the PsycINFO and CINAHL databases but decided on reflection that their subject areas were unlikely to yield further relevant studies for this review. We also planned to search the Ongoing Skin Trials register nottingham.ac.uk/ongoingskintrials but this resource is now an archive rather than a database of ongoing trials. We searched the EU Clinical Trials Register (clinicaltrialsregister.eu/) instead. Science Citation Index Expanded database is part of ISI Web of Science, which we searched. We therefore concluded that there is no need to search ISI Science Citation Index (on BIDS) separately as we first planned. All Copernicus publications are indexed on Web of Science and Google Scholar, so we decided not to search it separately. MetaRegister of Controlled Trials (mRCT) service was under review when we searched on 28 September 2015 and 27 July 2016. Previous searches of that registry were done up to up to 5 November 2014 and results included. Search of The World Health Organization International Clinical Trials Registry Platform (int/ictrp/en/) was performed on 28 September 2015 and 27 July 2016, as suggested alternate registry on the mRCT website. U.S. National Institutes of Health Ongoing Trials Register has introduced a character limit for searches (100 characters), and our search strategy in place up to 28 September 2015 was too long. We therefore replaced it with modified, shortened strategy for searches up to 27 July 2016 (Appendix 8).
The protocol version of some parts of the Data collection and analysis section was sometimes inadequate for the results we obtained and so we had to make minor changes to what we had initially planned. It is clearly stated and further clarified if, when and why this was the case in the appropriate sections.
In the Data extraction and management section we added details on inserting and checking the data. We clarified that the treatment success had been defined as anything above the first category of improvement on a Likert scale, or more than 50% improvement from baseline on a continuous scale for primary outcome 1 and secondary outcomes 1, 2 and 3, whereas the primary outcomes 2 were recorded as the actual or percentage change from baseline. This was done because different interpretations of the initial version were possible. Additional items (further information on participants, interventions, outcome measures, previous treatment, concomitant treatment, the use and appropriateness of statistical analyses), initially reported under Assessment of risk of bias in included studies was moved to this section, where they are more relevant.
The Assessment of risk of bias in included studies section was updated in accordance with section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). This included updating bias domains following the guidance provided by the Cochrane 'Tool for assessing risk of bias' in Table 8.5a (Higgins 2011a). Domains originally reported under d) and e) of this section in our protocol were considered under a single domain ‘Attrition bias’ (incomplete outcome data and how it was addressed), as suggested by the Table 8.5a (Higgins 2011a) and reported in 'Risk of bias' tables, under ‘Attrition bias’ domain for included studies. 'Possible selective outcome reporting' ('Selection bias' domain) and 'possible other bias' ('Other sources of bias' domain) were also added and reported for included studies, following guidance in Table 8.5a (Higgins 2011a). We have also moved additional items (further information on participants, interventions, outcome measures, previous treatment, concomitant treatment, the use and appropriateness of statistical analyses), initially reported under ‘Assessment of risk of bias in included studies' to Data extraction and management where they are more relevant.
The Measures of treatment effect section was updated according to section 12.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a); 'Numbers needed to treat' in the protocol version was replaced with ‘number needed to treat for an additional beneficial outcome' (NNTB) and ‘number needed to treat for an additional harmful outcome' (NNTH). When the relative risk was unreliable due to the lack of events occurring in control groups or body sites, we provided event rates instead of RR and calculated risk differences (RD) with 95% CI. We clarified this in the Effects of interventions section, under primary outcome 3. For comparisons where individual studies had randomised fewer than 30 participants per arm, we used t‐distribution for analyses of continuous outcomes to account for the sample size, along with analyses defined in our protocol. In such cases, we used generic inverse variance with adjusted SEs, as RevMan does not automatically account for sample sizes. We clearly state when such analyses were done. Summary assessments of the risk of bias for each outcome (across domains) in individual studies were performed according to Table 8.7a (Higgins 2011a).
In the Unit of analysis issues section, although this was not initially planned, we considered pooling results of studies which had split‐face or split‐back design with studies which had parallel‐group design in a meta‐analysis using the inverse variance method, described in the Cochrane Handbook for Systematic Reviews of Interventions section 9.4.3 (Deeks 2011), as we judged this was appropriate. However, due to the nature of the results, we did not pool studies with different designs, as there was considerable methodological and clinical heterogeneity outlined in the Effects of interventions section. We therefore removed the following sentence, which was originally in the protocol: "We will analyse internally controlled trials using appropriate techniques for paired designs and these studies will not be pooled with studies of other designs." from the Unit of analysis issues section.
We added a reference to the Cochrane Handbook for Systematic Reviews of Interventions in the Assessment of reporting biases (Sterne 2011) and Data synthesis (Schünemann 2011b) sections.
The Assessment of heterogeneity section was updated in accordance with sections 9.4.1, 9.5.1 and 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). This included details on assessment of clinical heterogeneity which was not defined in our protocol, together with definition of acceptable statistical heterogeneity.
The Assessment of reporting biases was updated in accordance with section 10.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011). However, we were unable to implement this method in the current review and test publication bias by the use of a funnel plot due to the nature of our results (most studies were too heterogeneous to be combined in meta‐analyses, whereas two of the three studies we did combine in meta‐analyses were not published).
Subgroup analysis and investigation of heterogeneity was included in the protocol, with the threshold defined as I² statistic greater than 50% (Higgins 2003). We did not perform subgroup analyses in the current review due to the nature of the results of the meta‐analyses (the I² statistic was lower than 50% for primary outcomes).
The Sensitivity analysis section was updated in accordance with the Cochrane Handbook for Systematic Reviews of Interventions table 8.7a (Higgins 2011a). In our current review we intended to exclude studies with unclear or high overall risk of bias, as suggested by the table 8.7a, instead of 'moderate or high risk of bias' as originally defined in our protocol. However, we have not performed sensitivity analysis in the current review since the three studies included in meta‐analysis were of similar quality and comparable risk of bias.
Contributions of authors
JC conceived the review and was the contact person with the editorial base.
JB co‐ordinated contributions from the co‐authors, and wrote the final draft of the review.
JB, RA, PP and MC screened papers against eligibility criteria.
JB, RA and PP obtained data on ongoing and unpublished studies.
JB, RA and MC appraised the quality of papers.
JB, RA, MC and PP extracted data for the review and sought additional information about papers.
JB entered data into RevMan. RA, MC, PP and LG cross‐checked this data for accuracy.
JB and LG analysed and interpreted data.
LG, JB and JC worked on the methods sections.
AML and JB drafted the clinical sections of the background and responded to the clinical comments of the referees.
LG, JB and JC responded to the methodology and statistics comments of the referees.
MC was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers.
JB is the guarantor of the review.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
-
Members of the Department of Primary Care and Social Medicine, Imperial College, London, UK.
Access to libraries and MEDLINE, IT and statistical support, advice, and time to write this protocol.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Jelena Barbaric: nothing to declare. Rachel Abbott: nothing to declare. Pawel Posadzki: nothing to declare. Mate Car: nothing to declare. Laura H Gunn: nothing to declare. Alison M Layton: "Over the last five years, the following companies have invited advice, supported educational events, provided unrestricted research grants, or I have acted as CI/PI for their clinical trials: Galderma, GlaxoSmithKline, MEDA, LeoPharma, Indentis, Valeant, Dermira, Pfizer, Novartis, Wyeth and L'Oreal.
I have received remuneration from several different pharmaceutical companies in support of the following:
research projects (funding has been provided as unrestricted educational grants for basic science research);
as an honorarium for lecturing at educational meetings (content of talks unrestricted);
as an honorarium to support work done in an advisory capacity e.g. member of drug monitoring committee or on advisory board.
I am not affiliated to or hold shares in any one specific company." Azeem Majeed: nothing to declare. Josip Car: nothing to declare.
Gloria Sanclemente (an external content expert who peer‐refereed this review) has designed and performed trials with MAL (Metvix®) + Red Light in which Galderma Laboratories has provided the medication and placebo. She has also received honoraria, speaker fees and meeting sponsorship from this pharmaceutical lab.
Brigitte Dréno (an external content expert who also peer‐refereed this review) is a member of an international board for Galderma, and has a grant for clinical studies with drugs in acne and daylight PDT, but no clinical trials with MAL–PDT.
New
References
References to studies included in this review
Anyachukwu 2014 {published data only (unpublished sought but not used)}
- Anyachukwu CC, Onyeso OKK. Efficacy of adjunct (laser) therapy to topical agents among Southern Nigerian acne vulgaris patients. Acupuncture and Related Therapies 2014;2(4):66‐70. [EMBASE: 2014893901] [Google Scholar]
Ash 2015 {published and unpublished data}
- Ash C, Drew S, Harrison A, Grant T, Mills D, Herbert K. Home use efficacy and safety study for mild to moderate acne using high intensity 414 nm solid state diode arrays. Conference: British Medical Laser Association, BMLA 2013 Manchester United Kingdom. Conference Start: 20130515 Conference End: 20130517. Lasers in Medical Science 2013;28(5):1216. [EMBASE: 71355738] [Google Scholar]
- Ash C, Harrison A, Drew S, Whittall R. A randomized controlled study for the treatment of acne vulgaris using high‐intensity 414 nm solid state diode arrays. Journal of Cosmetic and Laser Therapy 2015;17(4):170‐6. [PUBMED: 25594129] [DOI] [PubMed] [Google Scholar]
Barolet 2010 {published data only (unpublished sought but not used)}
- Barolet D, Boucher A. Radiant near infrared light emitting diode exposure as skin preparation to enhance photodynamic therapy inflammatory type acne treatment outcome. Lasers in Surgery and Medicine 2010;42(2):171‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baugh 2005 {published data only (unpublished sought but not used)}
- Baugh WP, Kucaba WD. Nonablative phototherapy for acne vulgaris using the KTP 532 nm laser. Dermatologic Surgery 2005;31(10):1290‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bernstein 2007 {published data only (unpublished sought but not used)}
- Bernstein EF. A pilot investigation comparing low‐energy, double pass 1,450 nm laser treatment of acne to conventional single‐pass, high‐energy treatment. Lasers in Surgery and Medicine 2007;39(2):193‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bissonnette 2010 {published and unpublished data}
- Bissonnette R, Maari C, Nigen S, Provost N, Bolduc C. Photodynamic therapy with methylaminolevulinate 80 mg/g without occlusion improves acne vulgaris. Journal of Drugs in Dermatology 2010;9(11):1347‐52. [PUBMED: 21061756] [PubMed] [Google Scholar]
- Bissonnette R, Maari C, Nigen S, Provost N, Bolduc C, Fuglerud P. Photodynamic therapy with methylaminolevulinate 80 mg/g without occlusion improves acne vulgaris [Poster P22]. innovaderm.ca/images/Inno‐6005.pdf (accessed 6 February 2013).
Borhan 2014 {published data only (unpublished sought but not used)}
- Borhan WH, Hamed HA, Aboelnour NH. Efficacy of pulsed dye laser on acne vulgaris. Journal of American Science 2014;10(3):‐. [Google Scholar]
Bowes 2003 {published data only (unpublished sought but not used)}
- Bowes LE, Manstein D, Rox Andersen R. Effects of 532 nm KTP laser exposure on acne and sebaceous glands (019). Joint International Laser Conference ‐ abstracts. Lasers in Medical Science 2003;18(Suppl 1):S6‐7. [DOI: 10.1007/s10103-003-0277-3] [DOI] [Google Scholar]
Chang 2007 {published data only (unpublished sought but not used)}
- Chang SE, Ahn SJ, Rhee DY, Choi JH, Moon KC, Suh HS, et al. Treatment of facial acne papules and pustules in Korean patients using an intense pulsed light device equipped with a 530‐ to 750‐nm filter. Dermatologic Surgery 2007;33(6):676‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen X, Song X, Chen H, Zhang S, Niu, J Liu G. Clinical efficacy of 5‐aminolevulinic acid photodynamic therapy in the treatment of moderate to severe facial acne vulgaris. Experimental and Therapeutic Medicine 2015;10(3):1194‐8. [PUBMED: 26622463] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2008 {published data only}
- Cheng Z, Li Q, Liu H, Jiang S, Chen K, Chen C. Clinical study of combination blue and red LED phototherapy in the treatment of mild to severe acne vulgaris. Chinese Journal of Aesthetic Medicine 2008;7:1041‐3. [Google Scholar]
Choi 2010 {published data only (unpublished sought but not used)}
- Choi YS, Suh HS, Yoon MY, Min SU, Lee DH, Suh DH. Intense pulsed light vs. pulsed‐dye laser in the treatment of facial acne: a randomized split‐face trial. Journal of the European Academy of Dermatology & Venereology 2010;24(7):773‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Darne 2011 {published and unpublished data}
- Darne S, Hiscutt E, Seukeran DC. Treatment of inflammatory acne with a 1450‐nm smoothbeam diode laser: a split‐face randomized single‐blinded controlled trial (DS‐13). British Society for Dermatological Surgery: Summaries of Papers. British Journal of Dermatology 2009;161(s1):106. [DOI: 10.1111/j.1365-2133.2009.09192.x] [DOI] [Google Scholar]
- Darne S, Hiscutt EL, Seukeran DC. Evaluation of the clinical efficacy of the 1,450 nm laser in acne vulgaris: a randomized split‐face, investigator‐blinded clinical trial. British Journal of Dermatology 2011;165(6):1256‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Arruda 2009 {published data only (unpublished sought but not used)}
- Arruda LH, Kodani V, Bastos Filho A, Mazzaro CB. A prospective, randomized, open and comparative study to evaluate the safety and efficacy of blue light treatment versus a topical benzoyl peroxide 5% formulation in patients with acne grade II and III [Estudo clinico, prospectivo, aberto, randomizado e comparativo para avaliar a seguranca e a eficacia da luz azul versus peroxido de benzoila 5% no tratamento da acne inflamatoria graus II e III]. Anais Brasileiros de Dermatologia 2009;84(5):463‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elman 2003 {published data only (unpublished sought but not used)}
- Elman M, Slatkine M, Harth Y. The effective treatment of acne vulgaris by a high‐intensity, narrow band 405‐420 nm light source. Journal of Cosmetic & Laser Therapy 2003;5(2):111‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Fadel 2009 {published data only (unpublished sought but not used)}
- Fadel M, Salah M, Samy N, Mona S. Liposomal methylene blue hydrogel for selective photodynamic therapy of acne vulgaris. Journal of Drugs in Dermatology 2009;8(11):983‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Genina 2004 {published and unpublished data}
- Genina EA, Bashkatov AN, Simonenko GV, Odoevskaya OD, Tuchin VV, Altshuler GB. Low‐intensity indocyanine‐green laser phototherapy of acne vulgaris: Pilot study. Journal of Biomedical Optics 2004;9(4):828‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gold 2005 {published and unpublished data}
- Gold MH, Rao J, Goldman MP, Bridges TM, Bradshaw VL, Boring MM, et al. A multicenter clinical evaluation of the treatment of mild to moderate inflammatory acne vulgaris of the face with visible blue light in comparison to topical 1% clindamycin antibiotic solution. Journal of Drugs in Dermatology 2005;4(1):64‐70. [MEDLINE: ] [PubMed] [Google Scholar]
Gold 2011 {published data only (unpublished sought but not used)}
- Gold MH, Sensing W, Biron JA. Clinical efficacy of home‐use blue‐light therapy for mild‐to moderate acne. Journal of Cosmetic & Laser Therapy 2011;13(6):308‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haedersdal 2008 {published data only (unpublished sought but not used)}
- Haedersdal M, Togsverd‐Bo K, Wiegell SR, Wulf HC. Long‐pulsed dye laser versus long‐pulsed dye laser‐assisted photodynamic therapy for inflammatory acne. A randomized controlled trial. Journal of the American Academy of Dermatology 2008;58(3):387‐94. [PUBMED: 18280335] [DOI] [PubMed] [Google Scholar]
Hong 2013 {published data only (unpublished sought but not used)}
- Hong JS, Jung JY, Yoon JY, Suh DH. Acne treatment by methyl aminolevulinate photodynamic therapy with red light vs. intense pulsed light. International Journal of Dermatology 2013;52(5):614‐9. [PUBMED: 23046014] [DOI] [PubMed] [Google Scholar]
Hongcharu 2000 {published data only (unpublished sought but not used)}
- Hongcharu W, Taylor CR, Chang Y, Aghassi D, Suthamjariya K, Anderson RR. Topical ALA‐photodynamic therapy for the treatment of acne vulgaris. Journal of Investigative Dermatology 2000;115(2):183‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hörfelt 2006 {published data only (unpublished sought but not used)}
- Hörfelt C. Photodynamic therapy for treatment of Acne Vulgaris in clinical studies: dose response and mode of action [PhD thesis] 2009. University of Gothenburg. gupea.ub.gu.se/bitstream/2077/19061/1/gupea_2077_19061_1.pdf. Gothenburg, Sweden, (accessed 6 February 2013).
- Hörfelt C, Funk J, Frohm‐Nilsson M, Edström DW, Wennberg AM. Topical methyl aminolaevulinate photodynamic therapy for treatment of facial acne vulgaris: results of a randomized, controlled study. British Journal of Dermatology 2006;155(3):608‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ianosi 2013 {published data only (unpublished sought but not used)}
- Ianosi S, Neagoe D, Calbureanu M, Ianosi G. Investigator‐blind, placebo‐controlled, randomized comparative study on combined vacuum and intense pulsed light versus intense pulsed light devices in both comedonal and papulopustular acne. Journal of Cosmetic & Laser Therapy 2013;15(5):248‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jih 2006 {published and unpublished data}
- Jih MH, Friedman PM, Goldberg LH, Robles M, Glaich AS, Kimyai‐Asadi A. The 1450‐nm diode laser for facial inflammatory acne vulgaris: dose‐response and 12‐month follow‐up study. Journal of the American Academy of Dermatology 2006;55(1):80‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jung 2009 {published data only (unpublished sought but not used)}
- Jung JY, Choi YS, Yoon MY, Min SU, Suh DH. Comparison of a pulsed dye laser and a combined 585/1,064‐nm laser in the treatment of acne vulgaris. Dermatologic Surgery 2009;35(8):1181‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jung 2012 {published data only (unpublished sought but not used)}
- Jung JY, Hong JS, Ahn CH, Yoon JY, Kwon HH, Suh DH. Prospective randomized controlled clinical and histopathological study of acne vulgaris treated with dual mode of quasi‐long pulse and Q‐switched 1064‐nm Nd:YAG laser assisted with a topically applied carbon suspension. Journal of the American Academy of Dermatology 2012;66(4):626‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jung JY, Yoon MY, Hong JS, Suh DH. Treatment of acne vulgaris with a low fluence 1064‐nm Nd: YAG laser after applying carbon suspension (90305). Conference: 1st Eastern Asia Dermatology Congress, EADC2010 Fukuoka Japan. Conference Start: 20100930 Conference End: 20101003. Journal of Dermatology 2010;37(Suppl 1):16. [DOI: 10.1111/j.1346-8138.2010.01024.x] [DOI] [Google Scholar]
Karsai 2010 {published data only}
- Karsai S, Schmitt L, Raulin C. The pulsed‐dye laser as an adjuvant treatment modality in acne vulgaris: a randomized controlled single‐blinded trial. British Journal of Dermatology 2010;163(2):395‐401. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only (unpublished sought but not used)}
- Kim BJ, Lee HG, Woo SM, Youn JI, Suh DH. Pilot study on photodynamic therapy for acne using indocyanine green and diode laser. Journal of Dermatology 2009;36(1):17‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kwon 2013 {published data only}
- Kwon HH, Lee JB, Yoon JY, Park SY, Ryu HH, Park BM, et al. The clinical and histological effect of home‐use, combination blue‐red LED phototherapy for mild to moderate acne vulgaris in Korean patients: a double blind, randomized controlled trial. British Journal of Dermatology 2013;168(5):1088‐94. [PUBMED: 23278295] [DOI] [PubMed] [Google Scholar]
- Kwon HH, Park BM, Suh DH, Lee JB, Yoon JY, Park SY, et al. The clinical and histologic effect of home‐use, combination blue‐red LED phototherapy for mild to moderate acne vulgaris in Korean patients: a double‐blind, randomized, sham device controlled trial. Conference: 71st Annual Meeting of the American Academy of Dermatology Miami Beach, FL United States. Conference Start: 20130301 Conference End: 20130305. Journal of the American Academy of Dermatology 2013;68(4 Suppl 1):AB18. [EMBASE: 70997159] [Google Scholar]
- Kwon HH, Yoon YI, Park SY, Ryu HH, Park BM, Kim JY, et al. A randomized, controlled trial of home‐use, combination blue‐red LED phototherapy for acne vulgaris with clinical and histological effectiveness (PO68). Proceedings of Dermatology (Korean) 2012; Vol. 64, issue 3:290. [210.101.116.28/W_files/kiss8/27728281_pv.pdf]
Lee 2010 {published and unpublished data}
- Lee SY. The efficacy of full‐spectrum light generated by electrical discharge between two carbon arc rods for the treatment of acne compared to 1% topical clindamycin. Conference: 30th Annual Conference of the American Society for Laser Medicine and Surgery Phoenix, AZ United States. Conference Start: 20100416 Conference End: 20100418. Lasers in Surgery & Medicine 2010;42:72. [EMBASE: 70420843] [Google Scholar]
Leheta 2009 {published data only (unpublished sought but not used)}
- Leheta TM. Role of the 585‐nm pulsed dye laser in the treatment of acne in comparison with other topical therapeutic modalities. Journal of Cosmetic & Laser Therapy 2009;11(2):118‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ling 2010 {published data only}
- Ling X, Chen L, Ji J, Shi X. Clinical comparative observation of red and blue LED light combination phototherapy in the treatment of medium and severe acne. Chinese Journal of Aesthetic Medicine 2010;12:1835‐6. [Google Scholar]
Liu 2011 {published data only}
- Liu G, Pan C, Li K, Tan Y, Wei X. Phototherapy for mild to moderate acne vulgaris with portable blue and red LED. Journal of Innovative Optical Health Sciences 2011;04(01):45‐52. [Google Scholar]
Liu 2014 {published data only (unpublished sought but not used)}
- Liu H, Fan X, An YX, Zhang J, Wang CM, Yang RY. Randomized trial of three phototherapy methods for the treatment of acne vulgaris in Chinese patients. Photodermatology, Photoimmunology & Photomedicine 2014;30(5):246‐53. [PUBMED: 24313686] [DOI] [PubMed] [Google Scholar]
McGill 2008 {published data only}
- McGill DJ. Current understanding of cutaneous laser treatment: a need for improved outcome and objective methods of assessing results [MD thesis] 2008. University of Aberdeen. digitool.abdn.ac.uk:80/webclient/DeliveryManager?application=DIGITOOL‐3&owner=resourcediscovery&custom_att_2=simple_viewer&pid=25020. Aberdeen, Scotland, UK, (accessed 13 October 2015).
Mei 2013 {published and unpublished data}
- Mei X, Shi W, Piao Y. Effectiveness of photodynamic therapy with topical 5‐aminolevulinic acid and intense pulsed light in Chinese acne vulgaris patients. Photodermatology, Photoimmunology & Photomedicine 2013;29(2):90‐6. [PUBMED: 23458393] [DOI] [PubMed] [Google Scholar]
Moneib 2014 {published data only}
- Moneib H, Tawfik AA, Youssef SS, Fawzy MM. Randomized split‐face controlled study to evaluate 1550‐nm fractionated Erbium Glass Laser for treatment of acne vulgaris‐an image analysis evaluation. Dermatologic Surgery 2014;40(11):1191‐200. [DOI: 10.1097/DSS.0000000000000167; PUBMED: 25310750] [DOI] [PubMed] [Google Scholar]
Na 2007 {published data only (unpublished sought but not used)}
- Na JI, Suh DH. Red light phototherapy alone is effective for acne vulgaris: randomized, single‐blinded clinical trial. Dermatologic Surgery 2007;33(10):1228‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Na 2011 {published data only (unpublished sought but not used)}
- Na JI, Kim JH, Kim SY, Park K‐C, Youn S‐W, Huh C‐H, et al. A new photosensitizer for acne photodynamic therapy: Indole‐3‐acetic acid. Conference: 29th Annual Conference of the American Society for Laser Medicine and Surgery National Harbor, MD United States. Conference Start: 20090401 Conference End: 20090405. Lasers in Surgery & Medicine 2009;41:30‐1. [EMBASE: 70332704] [Google Scholar]
- Na JI, Kim SY, Kim JH, Youn SW, Huh CH, Park KC. Indole‐3‐acetic acid: a potential new photosensitizer for photodynamic therapy of acne vulgaris. Lasers in Surgery and Medicine 2011;43(3):200‐5. [PUBMED: 21412803] [DOI] [PubMed] [Google Scholar]
NCT00594425 {published and unpublished data}
- NCT00594425. Photodynamic therapy (PDT) with methyl aminolevulinate (MAL) cream in moderate to severe acne. clinicaltrials.gov/ct2/show/NCT00594425?term=NCT00594425 (accessed 6 February 2013).
NCT00673933 {published and unpublished data}
- NCT00673933. Photodynamic therapy (PDT) with methyl aminolevulinate (MAL) cream in patients with skin type V or IV with acne vulgaris. clinicaltrials.gov/ct2/show/NCT00673933?term=NCT00673933 (accessed 6 February 2013).
NCT00706433 {published data only}
- NCT00706433. Light dose ranging study of photodynamic therapy (PDT) with Levulan + blue light versus vehicle + blue light in aevere dacial Acne. clinicaltrials.gov/ct2/show/NCT00706433 (accessed 29 September 2015).
NCT00933543 {published and unpublished data}
- NCT00933543. Efficacy and safety study with visonac photodynamic therapy (PDT). clinicaltrials.gov/ct2/show/NCT00933543?term=NCT00933543 (accessed 6 February 2013).
Oh 2009 {published and unpublished data}
- Oh SH, Ryu DJ, Han EC, Lee KH, Lee JH. A comparative study of topical 5‐aminolevulinic acid incubation times in photodynamic therapy with intense pulsed light for the treatment of inflammatory acne. Dermatologic Surgery 2009;35(12):1918‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orringer 2004 {published and unpublished data}
- Orringer J, Kang S, Cho S, Schumacher W, Karimipour D, Voorhees J. NLite pulsed dye laser therapy for acne vulgaris: a randomized clinical trial. Abstract 157‐01. American Academy of Dermatology 63rd Annual Meeting February 18‐22, 2005. Journal of the American Academy of Dermatology 2005;53(3 Suppl):AB11. [Google Scholar]
- Orringer J, Kang S, Cho S, Schumacher W, Karimipour D, Voorhees JJ. NLite pulsed dye laser therapy for acne vulgaris: a randomized clinical trial (Abstract 305). SID 2004 Meeting Abstracts. Journal of Investigative Dermatology 2004;122(3):A51. [DOI: 10.1111/j.1523-1747.2004..x] [DOI] [Google Scholar]
- Orringer JS, Kang SW, Hamilton T, Schumacher W, Cho SY, Hammerberg C, et al. Treatment of acne vulgaris with a pulsed dye laser ‐ a randomized controlled trial. JAMA 2004;291(23):2834‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orringer 2007 {published and unpublished data}
- Orringer JS, Kang S, Maier L, Johnson TM, Sachs DL, Karimipour DJ, et al. A randomized, controlled, split‐face clinical trial of 1320‐nm Nd:YAG laser therapy in the treatment of acne vulgaris. Journal of the American Academy of Dermatology 2007;56(3):432‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orringer 2010 {published and unpublished data}
- Orringer JS, Sachs DL, Bailey E, Kang S, Hamilton T, Voorhees JJ. Photodynamic therapy for acne vulgaris: a randomized, controlled, split‐face clinical trial of topical aminolevulinic acid and pulsed dye laser therapy. Journal of Cosmetic Dermatology 2010;9(1):28‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ou 2014 {published data only}
- Ou Yun, Liu Hong Xia, Ji Yan. Clinical observation of electric light combined with Yinhua Decoction on moderate acne (Feiweiyunre Syndrome). Chinese Journal of Dermatovenereology 2014;28:1279‐81. [Google Scholar]
Paithankar 2015 {published and unpublished data}
- Paithankar DY, Sakamoto FH, Farinelli WA, Kositratna G, Blomgren RD, Meyer TJ, et al. Acne treatment based on selective photothermolysis of sebaceous follicles with topically delivered light‐absorbing gold microparticles. Journal of Investigative Dermatology 2015;135(7):1727‐34. [PUBMED: 25748556] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papageorgieu 2000 {published data only (unpublished sought but not used)}
- Papageorgiou P, Katsambas A, Chu A. Phototherapy with blue (415 nm) and red (660 nm) light in the treatment of acne vulgaris. British Journal of Dermatology 2000;142(5):973‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pariser 2013 {published and unpublished data}
- Pariser D. Photodynamic therapy with methylaminolevulinate 80 mg/g and red light demonstrates significant efficacy in acne. Conference: 71st Annual Meeting of the American Academy of Dermatology Miami Beach, FL United States. Conference Start: 20130301 Conference End: 20130305. Journal of the American Academy of Dermatology 2013;68(4 Suppl 1):AB17. [EMBASE: 70997156] [Google Scholar]
- Pariser DM, Eichenfield LF, Bukhalo M, Waterman G, Jarratt M. Photodynamic therapy with methyl aminolaevulinate 80 mg g(‐1) for severe facial acne vulgaris: a randomized vehicle‐controlled study. British Journal of Dermatology 2016;174(4):770‐7. [PUBMED: 26663215] [DOI] [PubMed] [Google Scholar]
Pollock 2004 {published data only (unpublished sought but not used)}
- Pollock B, Turner D, Goulden V, Stables GS, Cunliffe WJ. Aminolaevulinic acid (ALA) photodynamic therapy (PDT) for the treatment of acne vulgaris Abstract. British Journal of Dermatology 2001;145(Suppl 59):66. [DOI: 10.1046/j.1365-2133.2001.040ff.x] [DOI] [PubMed] [Google Scholar]
- Pollock B, Turner D, Stringer MR, Bojar RA, Goulden V, Stables GI, et al. Topical aminolaevulinic acid‐photodynamic therapy for the treatment of acne vulgaris: a study of clinical efficacy and mechanism of action. British Journal of Dermatology 2004;151(3):616‐22. [EMBASE: 2004430542] [DOI] [PubMed] [Google Scholar]
Ragab 2014 {published data only (unpublished sought but not used)}
- Ragab MA, Hussein TM, Salem MA. Photodynamic therapy using 5‐aminolevulinic acid and intense pulsed light against intense pulsed light alone in the treatment of acne vulgaris. Journal of the Egyptian Women's Dermatologic Society 2014;11(3):173‐8. [DOI: 10.1097/01.EWX.0000452298.99232.bf] [DOI] [Google Scholar]
Sadick 2010a {published data only}
- Sadick N. An open‐label, split‐face study comparing the safety and efficacy of Levulan Kerastick (aminolevulonic acid) plus a 532 nm KTP laser to a 532 nm KTP laser alone for the treatment of moderate facial acne. Journal of Drugs in Dermatology 2010;9(3):229‐33. [PUBMED: 20232583] [PubMed] [Google Scholar]
Sadick 2010b {published and unpublished data}
- Sadick N, Laver Z, Laver L. Treatment of mild to moderate acne vulgaris using a combined light and heat energy device: home‐use clinical study conference: 30th Annual Conference of the American Society for Laser Medicine and Surgery Phoenix, AZ United States. Conference Start: 20100416 Conference End: 20100418. Lasers in Surgery and Medicine 2010;42:35. [EMBASE: 70420734] [Google Scholar]
- Sadick NS, Laver Z, Laver L. Treatment of mild‐to‐moderate acne vulgaris using a combined light and heat energy device: home‐use clinical study. Journal of Cosmetic and Laser Therapy 2010;12(6):276‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sami 2008 {published data only (unpublished sought but not used)}
- Sami NA, Attia AT, Badawi AM. Phototherapy in the treatment of acne vulgaris. Journal of Drugs in Dermatology 2008;7(7):627‐32. [EMBASE: 18664153] [PubMed] [Google Scholar]
Seaton 2003 {published data only (unpublished sought but not used)}
- Seaton E, Charakida A, Mouser PE, Grace I, Clement RM, Chu AC. Pulsed dye laser treatment in inflammatory acne vulgaris: a randomized controlled trial (Abstract RF‐6). British Association of Dermatologists 83rd Annual Meeting. British Journal of Dermatology 2003;149(Suppl 64):11‐2. [DOI: 10.1046/j.1365-2133.149.s64.9.x] [DOI] [Google Scholar]
- Seaton ED, Charakida A, Mouser PE, Grace I, Clement RM, Chu AC. Pulsed‐dye laser treatment for inflammatory acne vulgaris: randomised controlled trial. Lancet 2003;362(9393):1347‐52. [EMBASE: 2003440785] [DOI] [PubMed] [Google Scholar]
Song 2014 {published data only (unpublished sought but not used)}
- Kwon I, Ku S, Lee D. Photodynamic therapy using chlorophyll‐a in the treatment of acne vulgaris: a randomized, single‐blind, split‐face study. Conference: 44th Annual Meeting of the European Society for Dermatological Research Copenhagen Denmark. Conference Start: 20140910 Conference End: 20140913. Journal of Investigative Dermatology 2014;134:S87. [EMBASE: 71606808] [Google Scholar]
- Song BH, Lee DH, Kim BC, Ku SH, Park EJ, Kwon IH, et al. Photodynamic therapy using chlorophyll‐a in the treatment of acne vulgaris: a randomized, single‐blind, split‐face study. Journal of the American Academy of Dermatology 2014;71(4):764‐71. [PUBMED: 24930587] [DOI] [PubMed] [Google Scholar]
Taub 2007 {published data only (unpublished sought but not used)}
- Taub AF. A comparison of intense pulsed light, combination radiofrequency and intense pulsed light, and blue light in photodynamic therapy for acne vulgaris. Journal of Drugs in Dermatology 2007;6(10):1010‐6. [EMBASE: 17966178] [PubMed] [Google Scholar]
Tzung 2004 {published data only (unpublished sought but not used)}
- Tzung TY, Wu KH, Huang ML. Blue light phototherapy in the treatment of acne. Photodermatology, Photoimmunology & Photomedicine 2004;20(5):266‐9. [EMBASE: 2004437889] [DOI] [PubMed] [Google Scholar]
Uebelhoer 2007 {published data only (unpublished sought but not used)}
- Uebelhoer NS, Bogle MA, Dover JS, Arndt KA, Rohrer TE. Comparison of stacked pulses versus double‐pass treatments of facial acne with a 1,450‐nm laser. Dermatologic Surgery 2007;33(5):552‐9. [EMBASE: 2007206610] [DOI] [PubMed] [Google Scholar]
Wang 2006 {published and unpublished data}
- Wang SQ, Counters JT, Flor ME, Zelickson BD. Treatment of inflammatory facial acne with the 1,450 nm diode laser alone versus microdermabrasion plus the 1,450 nm laser: A randomized, split‐face trial. Dermatologic Surgery 2006;32(2):249‐55. [EMBASE: 2006136765] [PubMed] [Google Scholar]
Wiegell 2006a {published data only (unpublished sought but not used)}
- Wiegell SR, Wulf HC. Photodynamic therapy of acne vulgaris using 5‐aminolevulinic acid versus methyl aminolevulinate. Journal of the American Academy of Dermatology 2006;54(4):647‐51. [EMBASE: 2006129707] [DOI] [PubMed] [Google Scholar]
Wiegell 2006b {published data only (unpublished sought but not used)}
- Wiegell SR, Wulf HC. Photodynamic therapy of acne vulgaris using methyl aminolaevulinate: a blinded, randomized, controlled trial. British Journal of Dermatology 2006;154(5):969‐76. [EMBASE: 2006178830] [DOI] [PubMed] [Google Scholar]
Yeung 2007 {published and unpublished data}
- Yeung CK, Shek SY, Bjerring P, Yu CS, Kono T, Chan HH. A comparative study of intense pulsed light alone and its combination with photodynamic therapy for the treatment of facial acne in Asian skin. Lasers in Surgery and Medicine 2007;39(1):1‐6. [EMBASE: 2007083339] [DOI] [PubMed] [Google Scholar]
Yilmaz 2011 {published data only (unpublished sought but not used)}
- Yilmaz O, Senturk N, Yuksel EP, Aydin F, Ozden MG, Canturk T, et al. Evaluation of 532‐nm KTP laser treatment efficacy on acne vulgaris with once and twice weekly applications. Journal of Cosmetic and Laser Therapy 2011;13(6):303‐7. [EMBASE: 2011636520] [DOI] [PubMed] [Google Scholar]
Yin 2010 {published data only (unpublished sought but not used)}
- Yin R, Hao F, Deng J, Yang XC, Yan H. Investigation of optimal aminolaevulinic acid concentration applied in topical aminolaevulinic acid‐photodynamic therapy for treatment of moderate to severe acne: a pilot study in Chinese subjects. British Journal of Dermatology 2010;163(5):1064‐71. [EMBASE: 2010621844] [DOI] [PubMed] [Google Scholar]
Zhang 2009a {published data only}
- Zhang Y, Yang L, Zhou J‐z, Song Q‐h, Liu B, Lei Q, et al. Clinical study of Omnilux blue and red LED phototherapy in the treatment of acne. Chinese Journal of Dermatovenereology 2009;4:013. [Google Scholar]
Zhang 2013a {published data only}
- Zhang J, Yu B, Zhong QL, Shao Y, Chen BC, Ye TL. Therapeutic effect and follow‐up analysis of photodynamic therapy in the treatment of moderate to severe acne. Journal of Clinical Dermatology 2013;42(5):314‐316. [EMBASE: 2013442382] [Google Scholar]
Zhang 2013b {published data only}
- Zhang Y‐H. The clinical efficacy of blue red LED phototherapy combined with jinhua xiaocuo pills and chloramphenicol tincture on the treatment of acne vulgaris. Chinese Journal of Dermatovenereology 2013;27:304‐5. [Google Scholar]
References to studies excluded from this review
Alam 2003 {published data only}
- Alam M, Peterson SR, Silapunt S, Chopra K, Friedman PM, Goldberg LH. Comparison of 1450 nm diode laser and 595 nm pulsed‐dye laser for treatment of facial acne: A left‐right randomized trial of efficacy and adverse effects. Lasers in Surgery and Medicine 2003;32(1):30. [Google Scholar]
Alexiades‐Armenakas 2006 {published data only}
- Alexiades‐Armenakas M. Long‐pulsed dye laser‐mediated photodynamic therapy combined with topical therapy for mild to severe comedonal, inflammatory, or cystic acne. Journal of Drugs in Dermatology 2006;5(1):45‐55. [PUBMED: 16468292] [PubMed] [Google Scholar]
Aziz‐Jalali 2012 {published data only}
- Aziz‐Jalali MH, Tabaie SM, Djavid GE. Comparison of red and infrared low‐level laser therapy in the treatment of acne vulgaris. Indian Journal of Dermatology 2012;57(2):128‐30. [PUBMED: 22615511] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Leeuw 2010 {published data only}
- Leeuw J, Beek N, Bjerring P, Neumann HA. Photodynamic therapy of acne vulgaris using 5‐aminolevulinic acid 0.5% liposomal spray and intense pulsed light in combination with topical keratolytic agents. Journal of the European Academy of Dermatology & Venereology 2010;24(4):460‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goldman 2003 {published data only}
- Goldman MP, Boyce SM. A single‐center study of aminolevulinic acid and 417 NM photodynamic therapy in the treatment of moderate to severe acne vulgaris. Journal of Drugs in Dermatology 2003;2(4):393‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Hong 2005 {published data only}
- Hong SB, Lee MH. Topical aminolevulinic acid‐photodynamic therapy for the treatment of acne vulgaris. Photodermatology, Photoimmunology & Photomedicine 2005;21(6):322‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2008 {published data only}
- Kim S. The dual treatment of acne vulgaris using two kinds of ELOS (TM) (electro optical synergy) system: A simultaneous split‐face trial. Journal of Cosmetic & Laser Therapy 2008;10(4):213‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2007 {published data only}
- Lee JH, Kim SE, Park K, Son SJ. Topical aminolaevulinic acid‐photodynamic therapy for the treatment of mild to moderate inflammatory acne. Annals of Dermatology 2007;19(2):55‐9. [Google Scholar]
Ma 2013 {published data only}
- Ma Li, Xiang LH, Yu B, Yin R, Chen L, Wu Y, et al. Low‐dose topical 5‐aminolevulinic acid photodynamic therapy in the treatment of different severity of acne vulgaris. Photodiagnosis and Photodynamic Therapy 2013;10(4):583‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morton 2005 {published data only}
- Morton CA, Scholefield RD, Whitehurst C, Birch J. An open study to determine the efficacy of blue light in the treatment of mild to moderate acne. Journal of Dermatological Treatment 2005;16(4):219‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT00613444 {published data only}
- NCT00613444. Photodynamic therapy in the treatment of acne. clinicaltrials.gov/ct2/show/NCT00613444?term=NCT00613444 (accessed 6 February 2013).
Owczarek 2014 {published data only}
- Owczarek W, Wydrzyska A, Lebkowska K, Nawrocka A, Paluchowska E, Podolec K, et al. Treatment of acne with selective photothermolysis of the sebaceous follicle with gold‐coated microparticles, a clinical study. Conference: 34rd Annual Conference of the American Society for Laser Medicine and Surgery, ASLMS 2014 Phoenix, AZ United States. Conference Start: 20140402 Conference End: 20140406. Lasers in Surgery and Medicine 2014;46:3. [DOI: 10.1002/lsm.22229; EMBASE: 71594490] [DOI] [Google Scholar]
- Paithankar DY, Sakamoto FH, Farinelli WA, Kositratna G, Blomgren RD, Meyer TJ, et al. Acne treatment based on selective photothermolysis of sebaceous follicles with topically delivered light‐absorbing gold microparticles. Journal of Investigative Dermatology 2015;135(7):1727‐34. [PUBMED: 25748556] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinto 2013 {published data only}
- Pinto C, Schafer F, Orellana JJ, Gonzalez S, Hasson A. Efficacy of red light alone and methyl‐aminolaevulinate‐photodynamic therapy for the treatment of mild and moderate facial acne. Indian Journal of Dermatology, Venereology and Leprology 2013;79(1):77‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rojanamatin 2006 {published data only}
- Rojanamatin J, Choawawanich P. Treatment of inflammatory facial acne vulgaris with intense pulsed light and short contact of topical 5‐aminolevulinic acid: a pilot study. Dermatologic Surgery 2006;32(8):991‐6, 996‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Santos 2005 {published data only}
- Santos MA, Belo VG, Santos G. Effectiveness of photodynamic therapy with topical 5‐aminolevulinic acid and intense pulsed light versus intense pulsed light alone in the treatment of acne vulgaris: comparative study. Dermatologic Surgery 2005;31(8 Pt 1):910‐5. [EMBASE: 16042935] [DOI] [PubMed] [Google Scholar]
Shin 2012 {published data only}
- Shin JU, Lee SH, Jung JY, Lee JH. A split‐face comparison of a fractional microneedle radiofrequency device and fractional carbon dioxide laser therapy in acne patients. Journal of Cosmetic & Laser Therapy 2012;14(5):212‐7. [PUBMED: 23016530] [DOI] [PubMed] [Google Scholar]
Tuchin 2003 {published data only}
- Tuchin VV, Altshuler GB, Genina EA, Bashkatov AN, Simonenko GV, Odoevskaya OD, et al. ICG laser therapy of acne vulgaris. Laser Interaction with Tissue and Cells XV 2004;5319:363‐77. [DOI: 10.1117/12.531010] [DOI] [Google Scholar]
- Tuchin VV, Genina EA, Bashkatov AN, Simonenko GV, Odoevskaya OD, Altshuler GB. A pilot study of ICG laser therapy of acne vulgaris: photodynamic and photothermolysis treatment. Lasers in Surgery and Medicine 2003; Vol. 33, issue 5:296‐310. [EMBASE: 2004006279] [DOI] [PubMed]
Wang 2012 {published data only}
- Wang HW, Lv T, Zhang LL, Guo MX, Stepp H, Yang K, et al. Prospective study of topical 5‐aminolevulinic acid photodynamic therapy for the treatment of moderate to severe acne vulgaris in Chinese patients. Journal of Cutaneous Medicine and Surgery 2012;16(5):324‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yang 2013 {published data only}
- Yang GL, Zhao M, Wang JM, He CF, Luo Y, Liu HY, et al. Short‐term clinical effects of photodynamic therapy with topical 5‐aminolevulinic acid for facial acne conglobata: an open, prospective, parallel‐arm trial. Photodermatology, Photoimmunology & Photomedicine 2013;29(5):233‐8. [PUBMED: 24001378] [DOI] [PubMed] [Google Scholar]
Yao 2009 {published data only}
- Yao Y, Min Z. Efficacy of phototherapy combined with traditional Chinese medicine on acne vulgaris. Chinese Journal of Dermatology 2009; Vol. 42, issue 3:175‐7.
Yoon 2014 {published data only}
- Yoon JH, Park EJ, Kwon IH, Kim CW, Lee GS, Hann SK, et al. Concomitant use of an infrared fractional laser with low‐dose isotretinoin for the treatment of acne and acne scars. Journal of Dermatological Treatment 2014;25(2):142‐6. [DOI] [PubMed] [Google Scholar]
Zhan 1997 {published data only}
- Zhan AR, Chen FR, Yang XD. He‐Ne laser irradiation on ear points for 370 cases with acne patients. Chinese Journal of Physical Therapy 1997;20(3):161‐3. [Google Scholar]
Zhong 2007 {published data only}
- Zhong JQ, Xian DH, Chen DY. The clinical efficacy of narrowband blue light in the treatment of acne vulgaris. Journal of Clinical Dermatology 2007; Vol. 36, issue 2:115‐6.
Zhu 2009 {published data only}
- Zhu X, Li Y, Wu Y, Jia L, Xu T, Wu H, et al. Efficacy and safety of intense pulsed light therapy in combination with ALA‐PDT in the treatment of inflammatory acne papules. Chinese Journal of Aesthetic Medicine 2009;8:1136‐9. [Google Scholar]
References to studies awaiting assessment
Berson 2006 {published data only}
- Berson D, Bruce S, Horne D, Rich P. A Phase II, randomized, investigator‐blinded, parallel study of photodynamic therapy of topical aminolevulinic acid in the treatment of moderate to severe acne rising various drug incubation times. Journal of the American Academy of Dermatology 2006;54(3):AB23. [Google Scholar]
Demina 2015 {published data only}
- Demina O. [ИННОВАЦИОННЫЕ ПАТОГЕНЕТИЧЕСКИЕ И ТЕРАПЕВТИЧЕСКИЕ АСПЕКТЫ УГРЕВОЙ БОЛЕЗНИ)]. Innovative pathogenetic and therapeutic aspects of acne [Masters thesis]. Moscow, Russian Federation: Pirogov Russian National Research Medical University (RNRMU), 2016. [rsmu.ru/fileadmin/rsmu/img/about_rsmu/disser/2016/demina_om/a_demina_om.pdf] [Google Scholar]
- Demina OM, Kaptelishev AV, Potekaev NN. The efficacy of innovative technologies in the combination therapy of patients with acne. Klinicheskaya Dermatologiya i Venerologiya 2015;14(5):17‐23. [Google Scholar]
Du 2015 {published data only}
- Du H, Sun YL, He CF, Luo Y, Long CQ, Gao J, et al. Clinical research of Tanshinone capsule combined with photodynamic therapy in the treatment of 31 cases severe acne. Chinese Journal of Dermatovenereology 2015;29(8):805‐7. [Google Scholar]
Edwards 2006 {published and unpublished data}
- Edwards C, Hill S, Anstey A. A safe and effective yellow light‐emitting diode treatment for mild to moderate acne: a within‐patient half‐face dose ranging study (Abstract P105). American Academy of Dermatology 64th Annual Meeting March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54(3):AB15. [Google Scholar]
Elgendy 2015 {published data only}
- Elgendy A, Khalil K, Alshawadfy E, Wadea N, Alkady O. Blue light therapy versus low dose isotretinoin in mild to moderate acne. Global Dermatology 2015;2(3):131‐134. [DOI: 10.15761/GOD.1000138] [DOI] [Google Scholar]
Faghihi 2011 {published data only}
- Faghihi G, Vali A, Asilian A, Reza Radan M, Esteki H, Elahidoost M. Comparative efficacy of filtered blue light (emitted from sunlight) and topical erythromycin solution in acne treatment: A randomized controlled clinical trial. Journal of Pakistan Association of Dermatologists 2011;21(3):179‐84. [EMBASE: 2011610276] [Google Scholar]
Ganceviciene 2015 {published data only}
- Ganceviciene R, Meskauskas R, Berzanskyte A. Treatment of acne vulgaris with 1064 nm Nd: YAG Laser. Journal of the Laser and Health Academy 2015. [http://www.laserandhealthacademy.com/media/objave/academy/priponke/ganceviciene_laha_2015_onlinefirst.pdf]
ISRCTN73616060 {published data only}
- ISRCTN73616060. A preliminary observational study on the effect of pulsed dye laser treatment in patients with facial acne vulgaris.. www.controlled‐trials.com/ISRCTN73616060 (accessed 10 October 2015).
ISRCTN78675673 {published data only (unpublished sought but not used)}
- ISRCTN78675673. Laser versus conventional treatment for active acne in primary care. controlled‐trials.com/ISRCTN78675673 (accessed 6 February 2013).
ISRCTN95939628 {published and unpublished data}
- ISRCTN95939628. Comparative study of the pulsed dye laser and the Omnilux blue/red light phototherapy system in the treatment of mild‐moderate inflammatory acne vulgaris. controlled‐trials.com/ISRCTN95939628 (accessed 6 February 2013).
Kim 2012 {published data only}
- Kim JE, Hwang JI, Lee JI, Cho BK, Park HJ. Pilot study on photodynamic therapy for acne using chlorophyll: evaluator‐blinded, split‐face study. Journal of Dermatological Treatment 2012;23(1):35‐6. [EMBASE: 2012042231] [DOI] [PubMed] [Google Scholar]
Kwon 2016 {published data only}
- Kwon HH, Moon KR, Park SY, Yoon JY, Suh DH, Lee JB. Daylight photodynamic therapy with 1.5% 3‐butenyl 5‐aminolevulinate gel as a convenient, effective and safe therapy in acne treatment: a double‐blind randomized controlled trial. Journal of Dermatology 2016;43(5):515‐21. [PUBMED: 26660491] [DOI] [PubMed] [Google Scholar]
- NCT02313467. Topical PDT Cream Without Irradiation Source in the Acne Treatment [Topical application of 1.5% butenyl ALA without light source irradiation in the treatment of acne: a double blinded randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT02313467 Date first received: 8 December 2014.
Lee 2012 {published data only}
- Lee WJ, Jung HJ, Kim JY, Lee SJ, Kim DW. Effect of photodynamic therapy on inflammatory acne using 3% liposomal 5‐aminolevulinic acid emulsion and intense‐pulsed light: a pilot study. Journal of Dermatology 2012;39(8):728‐9. [EMBASE: 2012448005] [DOI] [PubMed] [Google Scholar]
Lekakh 2015 {published data only}
- Lekakh O, Mahoney AM, Novice K, Kamalpour J, Sadeghian A, Mondo D, et al. Treatment of acne vulgaris with salicylic acid chemical peel and pulsed dye laser: a split face, rater‐blinded, randomized controlled rrial. Journal of Lasers in Medical Sciences 2015;6(4):167‐70. [PUBMED: 26705462] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2011 {published data only}
- Lin X, Li W. Therapeutic effect of acne treated by red and blue light combine with Chen's Acne Clear. Chinese Journal of Aesthetic Medicine 2011;9:102‐3. [Google Scholar]
Moftah 2016 {published data only}
- Moftah NH, Ibrahim SM, Wahba NH. Intense pulsed light versus photodynamic therapy using liposomal methylene blue gel for the treatment of truncal acne vulgaris: a comparative randomized split body study. Archives of Dermatological Research 2016;308(4):263‐8. [PUBMED: 26993345] [DOI] [PubMed] [Google Scholar]
Nataloni 2003 {published and unpublished data}
- Nataloni R. Laser treatment comparable to oral antibiotics: 532 nm laser addresses multiple acne pathogens. Dermatology Times 2003 business.highbeam.com/137923/article‐1G1‐111035596/laser‐treatment‐comparable‐oral‐antibiotics‐532‐nm (accessed 10 January 2016).
NCT00237978 {published and unpublished data}
- NCT00237978. Control of therapy of acne papulopustulosa by visible light with waterfiltered infrared and / or adapalen (Differin gel). clinicaltrials.gov/ct2/show/NCT00237978 (accessed 6 February 2013).
NCT00814918 {published and unpublished data}
- NCT00814918. Topical 5‐ALA‐PDT with BLU‐U therapy versus topical 5‐ALA with pulse dye laser In treating recalcitrant acne Vulgaris. clinicaltrials.gov/ct2/show/NCT00814918?term=NCT00814918 (accessed 6 February 2013).
NCT01245946 {published data only (unpublished sought but not used)}
- NCT01245946. Photodynamic therapy compared to adapalene 0.1% gel plus doxycycline in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT01245946?term=NCT01245946 (accessed 6 February 2013).
NCT01472900 {published data only (unpublished sought but not used)}
- NCT01472900. 2940nm Er:YAG laser versus benzoyl peroxide gel for the treatment of inflammatory acne. clinicaltrials.gov/ct2/show/NCT01472900?term=NCT01472900 (accessed 6 February 2013).
NCT01584674 {published data only}
- NCT01584674. A clinical trial evaluating the safety and efficacy of the KLOX Biophotonic System in moderate to severe acne. clinicaltrials.gov/ct2/show/NCT01584674 (accessed 10 November 2015).
NCT01689935 {published data only}
- NCT01689935. Reduced side‐effects of photodynamic therapy for the treatment of moderate to severe acne (i‐PDT). clinicaltrials.gov/ct2/show/NCT01689935 (accessed 10 November 2015).
NCT02647528 {published data only}
- NCT02647528. A trial of pulse dyed laser (Chromogenex Regenlite Transform) in the treatment of inflammatory acne vulgaris (chromogenix) [A randomized, blinded, single‐centered, placebo‐controlled trial of pulse dyed laser (Chromogenex Regenlite Transform) in the treatment of inflammatory acne vulgaris]. clinicaltrials.gov/ct2/show/NCT02647528 Date first received: 31 December 2015.
Nestor 2016 {published data only}
- Nestor MS, Swenson N, Macri A, Manway M, Paparone P. Efficacy and tolerability of a combined 445nm and 630nm over‐the‐counter light therapy mask with and without topical salicylic acid versus topical benzoyl peroxide for the treatment of mild‐to‐moderate acne vulgaris. Journal of Clinical and Aesthetic Dermatology 2016;9(3):25‐35. [PUBMED: 27354885] [PMC free article] [PubMed] [Google Scholar]
Park 2015 {published data only}
- Park SN, Kim KY. Effects of photodynamics therapy on the acne skin without light sensitive material [광민감물질이 배제된 광선역학요법이 여드름 피부에 미치는 효과]. Journal of the Korea Academia‐Industrial Cooperation Society 2015;16(5):3131‐8. [DOI: 10.5762/KAIS.2015.16.5.3131] [DOI] [Google Scholar]
Passeron 2011 {published data only}
- Passeron T, Hughes R, Tsilika K, Ortonne JP, Lacour JP. Treatment of severe or refractory acne with non‐ablative 1,450nm diode laser. Conference: 31st Annual Conference of the American Society for Laser Medicine and Surgery, ASLMS 2011 Grapevine, TX United States. Conference Start: 20110330 Conference End: 20110403. Lasers in Surgery and Medicine 2011;43:944‐45. [EMBASE: 70640256] [Google Scholar]
Pinto 2011 {published data only}
- Pinto CS, Hasson AN, Gonzalez SB, Montoya JD, Droppelmann KD, Inostroza EM, et al. Effectiveness of photodynamic therapy with methyl aminolevulinate (MAL) compared with red light alone in the treatment of acne [Efecto de la terapia fotodinamica con metil‐aminolevulinato (MAL) comparada con luz roja en el tratamiento de acne inflamatorio leve a moderado]. Revista Chilena da Dermatologia 2011;27(2):177‐87. [pesquisa.bvsalud.org/portal/resource/en/lil‐645027] [Google Scholar]
Sadick 2016 {published data only}
- Sadick N, Dorizas A, Bonhert K. A randomized, blinded, single‐centered, placebo‐controlled trial of pulse dyed laser (Chromogenex Regenlite Transform) in the treatment of inflammatory acne vulgaris. Lasers in Surgery and Medicine 2016;48:26. [Google Scholar]
Sakamoto 2012 {published and unpublished data}
- Sakamoto FH, Izikson L, Lewis W, Ferrick B, Doukas A, Farinelli WA, et al. Preliminary results of a pilot prospective randomized double‐blinded control study to compare a new inhibitory PDT method to conventional ALA‐PDT for the treatment of recalcitrant moderate‐severe acne. Conference: 32nd Annual Conference of the American Society for Laser Medicine and Surgery, ASLMS 2012 Kissimmee, FL United States. Conference Start: 20120418 Conference End: 20120422. Lasers in Surgery and Medicine 2012;44:8. [EMBASE: 70716650] [Google Scholar]
Shaheen 2011 {published and unpublished data}
- Shaheen B, Gonzalez ML. Randomized, controlled, double‐blind, clinical trial evaluating the mechanism of action, efficacies and safety of methylaminolaevulinate photodynamic therapy (PDT) and intense pulsed light, administered as placebo‐PDT, compared with adapalene 0‐1% gel in the treatment of adults with mild to moderate acne vulgaris. Conference: 91st Annual Meeting of the British Association of Dermatologists London United Kingdom. Conference Start: 20110705 Conference End: 20110707. British Journal of Dermatology 2011;165:103. [EMBASE: 70479588] [Google Scholar]
Song 2012 {published data only}
- Song BH, Ku SH, Park EJ, Kim KH. Efficacy and safety of chlorophyll A photodynamic therapy in acne vulgaris patients. Proceedings of Dermatology (Korean) 2012;64(3):304. [210.101.116.28/W_files/kiss8/27728317_pv.pdf] [Google Scholar]
Troilius 2005 {published data only}
- Troilius A. Acne ‐ intense pulsed light and laser therapies as antibiotic‐sparing strategy in combination with medical management [Abstract SSS09.15]. The 14th Congress of the European Academy of Dermatology and Venereology, London, UK. 12‐15th October 2005. Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 2):17. [Google Scholar]
Voravutinon 2016 {published data only}
- Voravutinon N, Rojanamatin J, Sadhwani D, Iyengar S, Alam M. A comparative split‐face study using different mild purpuric and subpurpuric fluence level of 595‐nm pulsed‐dye laser for treatment of moderate to severe acne vulgaris. Dermatologic Surgery 2016;42(3):403‐9. [PUBMED: 26890802] [DOI] [PubMed] [Google Scholar]
Wang 2016 {published data only}
- Wang Q, Yuan D, Liu W, Chen J, Lin X, Cheng S, et al. Use of optical fiber imported intra‐tissue photodynamic therapy for treatment of moderate to severe acne vulgaris. Medical Science Monitor 2016;22:362‐6. [PUBMED: 26839152] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2009b {published data only}
- Zhang L, Wang X, Wang H, Su L, Guo M. Topical aminolevulinic acid‐photodynamic therapy in acne. Chinese Journal of Dermatology 2009;42(2):78‐80. [Google Scholar]
References to ongoing studies
EU 2014‐005235‐13 {published data only}
- EU 2014‐005235‐13. Therapy‐treatment (PDT) on acne, a study to optimize the number of treatments, the right light‐dose and the right pre‐treatment in order to obtain a longterm remission‐time. clinicaltrialsregister.eu/ctr‐search/trial/2014‐005235‐13/SE (accessed 5 October 2015).
NCT02217228 {published and unpublished data}
- NCT02217228. Pivotal study of sebacia microparticles in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT02217228 (accessed 12 November 2014).
NCT02431494 {published data only}
- NCT02431494. Safety and preliminary efficacy of combination therapy for the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT02431494 (accessed 12 November 2014).
Additional references
Abroms 2006
- Abroms L, Maibach E, Lyon‐Daniel K, Feldman SR. What is the best approach to reducing birth defects associated with isotretinoin?. PLoS Medicine 2006;3(11):e483. [PUBMED: 17121451] [DOI] [PMC free article] [PubMed] [Google Scholar]
ACORN 2013
- Acne Core Outcomes Research Network (ACORN). sites.psu.edu/acnecoreoutcomes/ (accessed March 16 2015). [sites.psu.edu/acnecoreoutcomes/]
Alexis 2013
- Alexis AF. Lasers and light‐based therapies in ethnic skin: treatment options and recommendations for Fitzpatrick skin types V and VI. British Journal of Dermatology 2013;169(Suppl 3):91‐7. [PUBMED: 24098905] [DOI] [PubMed] [Google Scholar]
Allen 1982
- Allen BS, Smith JG. Various parameters for grading acne vulgaris. Archives of Dermatology 1982;118(1):23‐5. [PUBMED: 6460474] [PubMed] [Google Scholar]
Amado 2006
- Amado JM, Matos ME, Abreu AM, Loureiro L, Oliveira J, Verde A, et al. The prevalence of acne in the north of Portugal. Journal of the European Academy of Dermatology & Venereology 2006;20(10):1287‐95. [PUBMED: 17062047] [DOI] [PubMed] [Google Scholar]
Archer 2004
- Archer JS. Hirsutism and acne in polycystic ovary syndrome. Bailliere's Best Practice & Research Clinical Obstetrics & Gynaecology. Vol. 18, Bailliere Tindall, 2004:737. [DOI] [PubMed] [Google Scholar]
Arowojolu 2012
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD004425.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baldwin 2002
- Baldwin HE. The interaction between acne vulgaris and the psyche. Cutis 2002;70(2):133‐9. [EMBASE: 12234161] [PubMed] [Google Scholar]
Barratt 2009
- Barratt H, Hamilton F, Car J, Lyons C, Layton A, Majeed A. Outcome measures in acne vulgaris: systematic review. British Journal of Dermatology 2009;160(1):132‐6. [PUBMED: 19067711] [DOI] [PubMed] [Google Scholar]
Bhate 2013
- Bhate K, Williams HC. Epidemiology of acne vulgaris. British Journal of Dermatology 2013;168(3):474‐85. [PUBMED: 23210645] [DOI] [PubMed] [Google Scholar]
Burton 1971
- Burton JL, Cunliffe WJ, Stafford I, Shuster S. The prevalence of acne vulgaris in adolescence. British Journal of Dermatology 1971;85(2):119‐26. [PUBMED: 4255129] [DOI] [PubMed] [Google Scholar]
Caldwell 2005
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ (Clinical Research Ed.) 2005;331(7521):897‐900. [PUBMED: 16223826] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cao 2015
- Cao H, Yang G, Wang Y, Liu JP, Smith CA, Luo H, et al. Complementary therapies for acne vulgaris. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD009436.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Car 2009
- Car J, Car M, Hamilton F, Layton A, Lyons C, Majeed A. Light therapies for acne. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007917] [DOI] [Google Scholar]
Charakida 2004
Cho 2006
- Cho YJ, Suh DH. Study of the photoinactivation effect on Propionibacterium acnes after light irradiation with variable wavelengths. Korean Journal of Dermatology 2006;44(11):1332–1338. [Google Scholar]
Choi 2011
- Choi CW, Lee DH, Kim HS, Kim BY, Park KC, Youn SW. The clinical features of late onset acne compared with early onset acne in women. Journal of the European Academy of Dermatology and Venereology: JEADV 2011;25(4):454‐61. [PUBMED: 20659307] [DOI] [PubMed] [Google Scholar]
Coates 2002
- Coates P, Vyakrnam S, Eady EA, Jones CE, Cove JH, Cunliffe WJ. Prevalence of antibiotic‐resistant propionibacteria on the skin of acne patients: 10‐year surveillance data and snapshot distribution study. British Journal of Dermatology 2002;146(5):840‐8. [PUBMED: 12000382] [DOI] [PubMed] [Google Scholar]
Cunliffe 1979
- Cunliffe WJ, Gould DJ. Prevalence of facial acne vulgaris in late adolescence and in adults. British Medical Journal 1979;1(6171):1109‐10. [PUBMED: 156054] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunliffe 1989
- Cunliffe WJ. The Acnes. London: Martin Dunitz Ltd, 1989. [Google Scholar]
Cunliffe 2004
- Cunliffe WJ, Holland DB, Jeremy A. Comedone formation: etiology, clinical presentation, and treatment. Clinical Dermatology 2004;22(5):367‐74. [EMBASE: 2004499743] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Degitz 2007
- Degitz K, Placzek M, Borelli C, Plewig G. Pathophysiology of acne. Journal der Deutschen Dermatologischen Gesellschaft 2007;5(4):316‐23. [PUBMED: 17376098] [DOI] [PubMed] [Google Scholar]
Dreno 2010
- Dreno B, Thiboutot D, Gollnick H, Finlay AY, Layton A, Leyden JJ, et al. Large‐scale worldwide observational study of adherence with acne therapy. International Journal of Dermatology 2010;49(4):448‐56. [PUBMED: 20465705] [DOI] [PubMed] [Google Scholar]
Dreno 2013
- Dreno B, Layton A, Zouboulis CC, Lopez‐Estebaranz JL, Zalewska‐Janowska A, Bagatin E, et al. Adult female acne: a new paradigm. Journal of the European Academy of Dermatology and Venereology: JEADV 2013;27(9):1063‐70. [PUBMED: 23302006] [DOI] [PubMed] [Google Scholar]
Erceg 2013
- Erceg A, Jong EMJG, Kerkhof PCM, Seyger MMB. The efficacy of pulsed dye laser treatment for inflammatory skin diseases: a systematic review. Journal of the American Academy of Dermatology 2013;69(4):609‐15.e8. [EMBASE: 2013590539] [DOI] [PubMed] [Google Scholar]
FDA 2005
- U.S. Department of Health and Human Services, Food, Drug Administration. Center for Drug Evaluation and Research. Guidance for Industry. Acne vulgaris: developing drugs for treatment September 2005 Draft. fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071292.pdf (accessed 27 November 2013).
Fitzpatrick 1988
- Fitzpatrick TB. The validity and practicality of sun‐reactive skin types I through VI. Archives of Dermatology 1988;124(6):869‐71. [PUBMED: 3377516] [DOI] [PubMed] [Google Scholar]
Fritsch 1998
- Fritsch C, Goerz G, Ruzicka T. Photodynamic therapy in dermatology. Archives of Dermatology 1998;134(2):207‐14. [PUBMED: 9487213] [DOI] [PubMed] [Google Scholar]
Garner 2012
- Garner SE, Eady A, Bennett C, Newton JN, Thomas K, Popescu CM. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD002086.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glaich 2006
- Glaich AS, Friedman PM, Jih MH, Goldberg LH. Treatment of inflammatory facial acne vulgaris with combination 595‐nm pulsed‐dye laser with dynamic‐cooling‐device and 1,450‐nm diode laser. Lasers in surgery and medicine 2006;38(3):177‐80. [PUBMED: 16180221] [DOI] [PubMed] [Google Scholar]
GMR Data 2013
- MarketResearch.com. GMR Data Publisher Sample 2013. marketresearch.com/product/sample‐7912090.pdf (accessed prior to 1 August 2016):3.
Goulden 1997
- Goulden V, Clark SM, Cunliffe WJ. Post‐adolescent acne: a review of clinical features. British Journal of Dermatology 1997;136(1):66‐70. [PUBMED: 9039297] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Copenhagen: GRADE Working Group, McMaster University, 2015.
Haedersdal 2008a
- Haedersdal M, Togsverd‐Bo K, Wulf HC. Evidence‐based review of lasers, light sources and photodynamic therapy in the treatment of acne vulgaris. Journal of the European Academy of Dermatology and Venereology: JEADV 2008;22(3):267‐78. [PUBMED: 18221341] [DOI] [PubMed] [Google Scholar]
Hamilton 2009
- Hamilton FL, Car J, Lyons C, Car M, Layton A, Majeed A. Laser and other light therapies for the treatment of acne vulgaris: systematic review. British Journal of Dermatology 2009;160(6):1273‐85. [PUBMED: 19239470] [DOI] [PubMed] [Google Scholar]
Hayashi 2015
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ingram 2010
- Ingram JR, Grindlay DJ, Williams HC. Problems in the reporting of acne clinical trials: a spot check from the 2009 Annual Evidence Update on Acne Vulgaris. Trials 2010;11:77. [PUBMED: 20624287] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [PUBMED: 15545678] [DOI] [PubMed] [Google Scholar]
Jordan 2000
- Jordan RE, Cummins CL, Burls AJE, Seukeran DC. Laser resurfacing for facial acne scars. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD001866] [DOI] [PubMed] [Google Scholar]
Kilkenny 1998
- Kilkenny M, Merlin K, Plunkett A, Marks R. The prevalence of common skin conditions in Australian school students: 3. Acne vulgaris. British Journal of Dermatology 1998;139(5):840‐5. [EMBASE: 1998385934] [DOI] [PubMed] [Google Scholar]
Krejci‐Manwaring 2006
- Krejci‐Manwaring J, McCarty MA, Camacho F, Carroll CL, Johnson K, Manuel J, et al. Adherence with topical treatment is poor compared with adherence with oral agents: implications for effective clinical use of topical agents. Journal of the American Academy of Dermatology 2006; Vol. 54, issue 5 Suppl:S235‐6. [PUBMED: 16631951] [DOI] [PubMed]
Lammer 1985
- Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, et al. Retinoic acid embryopathy. New England Journal of Medicine 1985;313(14):837‐41. [PUBMED: 3162101] [DOI] [PubMed] [Google Scholar]
Layton 1994
- Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clinical and Experimental Dermatology 1994;19(4):303‐8. [EMBASE: 1994232732] [DOI] [PubMed] [Google Scholar]
Layton 2015
- Layton A, Eady EA, Peat M, Whitehouse H, Levell N, Ridd M, et al. Identifying acne treatment uncertainties via a James Lind Alliance Priority Setting Partnership. BMJ 2015;5(7):e008085. [PUBMED: 26187120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leinwoll 1965
- Leinwoll S. Understanding Lasers and Masers. London: Iliffe Books Ltd, 1965. [Google Scholar]
Lloyd 2002
- Lloyd JR, Mirkov M. Selective photothermolysis of the sebaceous glands for acne treatment. Lasers in Surgery and Medicine 2002;31(2):115‐20. [EMBASE: 2002318362] [DOI] [PubMed] [Google Scholar]
Mariwalla 2005
- Mariwalla K, Rohrer TE. Use of lasers and light‐based therapies for treatment of acne vulgaris. Lasers in Surgery and Medicine 2005;37(5):333‐42. [EMBASE: 2006071670] [DOI] [PubMed] [Google Scholar]
MedDRA 2010
- MedDRA MSSO. Medical Dictionary for Regulatory Activities Terminology, Version 15.1 (MedDRA). meddramsso.com/ (accessed 16 January 2013).
Michaelsson 1977
- Michaelsson G, Juhlin L, Vahlquist A. Effects of oral zinc and vitamin A in acne. Archives of Dermatology 1977;113(1):31‐6. [PUBMED: 137693] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morton 2013
- Morton CA, Szeimies RM, Sidoroff A, Braathen LR. European guidelines for topical photodynamic therapy part 2: emerging indications ‐ field cancerization, photorejuvenation and inflammatory/infective dermatoses. Journal of the European Academy of Dermatology and Venereology : JEADV 2013;27(6):672‐9. [PUBMED: 23181556] [DOI] [PubMed] [Google Scholar]
Nast 2012
- Nast A, Dreno B, Bettoli V, Degitz K, Erdmann R, Finlay AY, et al. European evidence‐based (S3) guidelines for the treatment of acne. Journal of the European Academy of Dermatology and Venereology: JEADV 2012;26(Suppl 1):1‐29. [PUBMED: 22356611] [DOI] [PubMed] [Google Scholar]
O'Brien 1998
- O’Brien SC, Lewis JB, Cunliffe WJ. The Leeds revised acne grading system. Journal of Dermatological Treatment 1998;9(4):215‐220. [EMBASE: 1999025622] [Google Scholar]
O'Connor 2011
- O’Connor D, Green S, Higgins JPT (editors). Chapter 5: Defining the review question and developing criteria for including studies. In: Higgins JPT, Green S (editors), Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
OCEBM 2011
- Oxford Centre for Evidence‐Based Medicine Working Group. The Oxford Levels of Evidence v2. cebm.net/index.aspx?o=5653 (accessed 20 May 2014).
Pei 2015
- Pei S, Inamadar AC, Adya KA, Tsoukas MM. Light‐based therapies in acne treatment. Indian Dermatology Online Journal 2015;6(3):145‐57. [PUBMED: 26009707] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfeifer 2005
- Pfeifer SM. Polycystic ovary syndrome in adolescent girls. Seminars in Pediatric Surgery 2005;14(2):111‐7. [EMBASE: 2005180997] [DOI] [PubMed] [Google Scholar]
Preneau 2012
- Preneau S, Dreno B. Female acne ‐ a different subtype of teenager acne?. Journal of the European Academy of Dermatology and Venereology: JEADV 2012;26(3):277‐82. [PUBMED: 21848892] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riddle 2009
- Riddle CC, Terrell SN, Menser MB, Aires DJ, Schweiger ES. A review of photodynamic therapy (PDT) for the treatment of acne vulgaris. Journal of Drugs in Dermatology 2009;8(11):1010‐9. [PUBMED: 19894368] [PubMed] [Google Scholar]
Ross 2005
- Ross EV. Optical treatments for acne. Dermatologic Therapy 2005;18(3):253‐66. [EMBASE: 2005554880] [DOI] [PubMed] [Google Scholar]
Sakamoto 2010
- Sakamoto FH, Lopes JD, Anderson RR. Photodynamic therapy for acne vulgaris: a critical review from basics to clinical practice: part I. Acne vulgaris: when and why consider photodynamic therapy?. Journal of the American Academy of Dermatology 2010;63(2):183‐93; quiz 193‐4. [PUBMED: 20633796] [DOI] [PubMed] [Google Scholar]
Sakamoto 2010a
- Sakamoto FH, Torezan L, Anderson RR. Photodynamic therapy for acne vulgaris: a critical review from basics to clinical practice: part II. Understanding parameters for acne treatment with photodynamic therapy. Journal of the American Academy of Dermatology 2010;63(2):195‐211; quiz 211‐2. [PUBMED: 20633797] [DOI] [PubMed] [Google Scholar]
Sanclemente 2014
- Sanclemente G, Acosta JL, Tamayo ME, Bonfill X, Alonso‐Coello P. Clinical practice guidelines for treatment of acne vulgaris: a critical appraisal using the AGREE II instrument. Archives of Dermatological Research 2014;306(3):269‐77. [PUBMED: 23925586] [DOI] [PubMed] [Google Scholar]
Schmitt 2016
- Schmitt J, Deckert S, Alam M, Apfelbacher C, Barbaric J, Bauer A, et al. Report from the kick‐off meeting of the Cochrane Skin Group Core Outcome Set Initiative (CSG‐COUSIN). British Journal of Dermatology 2016;174(2):287‐95. [PUBMED: 26779929] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010;340(7748):c332. [EMBASE: 2010199168] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sharma 2012
- Sharma SK, Mroz P, Dai T, Huang YY, Denis TG, Hamblin MR. Photodynamic therapy for cancer and for infections: what is the difference?. Israel Journal of Chemistry 2012;52(8‐9):691‐705. [PUBMED: 23248387] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2011
- Smith EV, Grindlay DJ, Williams HC. What's new in acne? An analysis of systematic reviews published in 2009‐2010. Clinical & Experimental Dermatology 2011;36(2):119‐22; quiz 123. [PUBMED: 20738323] [DOI] [PubMed] [Google Scholar]
Stern 1989
- Stern RS. When a uniquely effective drug is teratogenic. The case of isotretinoin. New England Journal of Medicine 1989;320(15):1007‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stern 1992
- Stern RS. The prevalence of acne on the basis of physical examination. Journal of the American Academy of Dermatology 1992;26(6):931‐5. [PUBMED: 1535079] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stewart 2009
- Stewart LA, Tierney JF, Clarke M. Chapter 19: Reviews of individual patient data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Tan 2004
- Tan JK. Impact of acne vulgaris: evaluating the evidence. Skin Therapy 2004;9(7):1‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Tan 2008
- Tan JK. Current measures for the evaluation of acne severity. Expert Review of Dermatology 2008;3(5):595‐603. [Google Scholar]
Tan 2010
- Tan JK, Tang J, Fung K, Gupta AK, Richard Thomas D, Sapra S, et al. Development and validation of a Scale for Acne Scar Severity (SCAR‐S) of the face and trunk. Journal of Cutaneous Medicine and Surgery 2010;14(4):156‐60. [PUBMED: 20642983] [DOI] [PubMed] [Google Scholar]
Taylor 2009
- Taylor MN, Gonzalez ML. The practicalities of photodynamic therapy in acne vulgaris. British Journal of Dermatology 2009;160(6):1140‐8. [PUBMED: 19239465] [DOI] [PubMed] [Google Scholar]
Thiboutot 2004
- Thiboutot D. Acne: hormonal concepts and therapy. Clinics in Dermatology 2004;22(5):419‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thiboutot 2009
- Thiboutot D, Gollnick H, Bettoli V, Dreno B, Kang S, Leyden JJ, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. Journal of the American Academy of Dermatology 2009;60(5 Suppl):S1‐50. [PUBMED: 19376456] [DOI] [PubMed] [Google Scholar]
Thomas 2004
- Thomas DR. Psychosocial effects of acne. Journal of Cutaneous Medicine and Surgery 2004;8(Suppl 4):3‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wat 2014
- Wat H, Wu DC, Rao J, Goldman MP. Application of intense pulsed light in the treatment of dermatologic disease: a systematic review. Dermatologic Surgery 2014;40(4):359‐77. [PUBMED: 24495252] [DOI] [PubMed] [Google Scholar]
White 1998
Williams 2006
- Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. American Journal of Clinical Dermatology 2006;7(5):281‐90. [PUBMED: 17007539] [DOI] [PubMed] [Google Scholar]
Williams 2012
- Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet 2012;379(9813):361‐72. [PUBMED: 21880356] [DOI] [PubMed] [Google Scholar]
Zaenglein 2016
- Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. Journal of the American Academy of Dermatology 2016;74(5):945‐73. [PUBMED: 26897386] [DOI] [PubMed] [Google Scholar]
Zouboulis 2004
- Zouboulis CC. Acne and sebaceous gland function. Clinics in Dermatology 2004;22(5):360‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


