Abstract
Background
Admission avoidance hospital at home provides active treatment by healthcare professionals in the patient's home for a condition that otherwise would require acute hospital inpatient care, and always for a limited time period. This is the third update of the original review.
Objectives
To determine the effectiveness and cost of managing patients with admission avoidance hospital at home compared with inpatient hospital care.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, two other databases, and two trials registers on 2 March 2016. We checked the reference lists of eligible articles. We sought unpublished studies by contacting providers and researchers who were known to be involved in the field.
Selection criteria
Randomised controlled trials recruiting participants aged 18 years and over. Studies comparing admission avoidance hospital at home with acute hospital inpatient care.
Data collection and analysis
We followed the standard methodological procedures expected by Cochrane and the Effective Practice and Organisation of Care (EPOC) Group. We performed meta‐analysis for trials that compared similar interventions and reported comparable outcomes with sufficient data, requested individual patient data from trialists, and relied on published data when this was not available. We used the GRADE approach to assess the certainty of the body of evidence for the most important outcomes.
Main results
We included 16 randomised controlled trials with a total of 1814 participants; three trials recruited participants with chronic obstructive pulmonary disease, two trials recruited participants recovering from a stroke, six trials recruited participants with an acute medical condition who were mainly elderly, and the remaining trials recruited participants with a mix of conditions. We assessed the majority of the included studies as at low risk of selection, detection, and attrition bias, and unclear for selective reporting and performance bias. Admission avoidance hospital at home probably makes little or no difference on mortality at six months' follow‐up (risk ratio (RR) 0.77, 95% confidence interval (CI) 0.60 to 0.99; P = 0.04; I2 = 0%; 912 participants; moderate‐certainty evidence), little or no difference on the likelihood of being transferred (or readmitted) to hospital (RR 0.98, 95% CI 0.77 to 1.23; P = 0.84; I2 = 28%; 834 participants; moderate‐certainty evidence), and may reduce the likelihood of living in residential care at six months' follow‐up (RR 0.35, 95% CI 0.22 to 0.57; P < 0.0001; I2 = 78%; 727 participants; low‐certainty evidence). Satisfaction with healthcare received may be improved with admission avoidance hospital at home (646 participants, low‐certainty evidence); few studies reported the effect on caregivers. When the costs of informal care were excluded, admission avoidance hospital at home may be less expensive than admission to an acute hospital ward (287 participants, low‐certainty evidence); there was variation in the reduction of hospital length of stay, estimates ranged from a mean difference of ‐8.09 days (95% CI ‐14.34 to ‐1.85) in a trial recruiting older people with varied health problems, to a mean increase of 15.90 days (95% CI 8.10 to 23.70) in a study that recruited patients recovering from a stroke.
Authors' conclusions
Admission avoidance hospital at home, with the option of transfer to hospital, may provide an effective alternative to inpatient care for a select group of elderly patients requiring hospital admission. However, the evidence is limited by the small randomised controlled trials included in the review, which adds a degree of imprecision to the results for the main outcomes.
Plain language summary
'Hospital at home' services to avoid admission to hospital
What is the aim of this review?
The aim of this Cochrane review was to find out if providing healthcare in an admission avoidance hospital at home setting improves patient health outcomes and reduces cost to the health service.
Key messages
Admission avoidance hospital at home probably makes little or no difference to patient health outcomes, may increase the chances of living at home at six months' follow‐up, and may be slightly less expensive. However, the findings are not precise due to the small size of the studies included in the review.
What was studied in this review?
There continues to be more demand for acute hospital beds than there are beds. One way to reduce reliance on hospital beds is to provide people with acute health care at home, sometimes called 'hospital at home'. We systematically reviewed the literature on the effect of providing hospital at home services to avoid hospital admission for adults.
What are the main results of this review?
Admission avoidance hospital at home, with the option of transfer to hospital, may provide an effective alternative to inpatient care for a select group of elderly patients requiring hospital admission. We found 16 studies, of which six were identified for this update. Three studies recruited participants with chronic obstructive (lung) disease, two recruited participants recovering from a stroke, six recruited participants with a (sudden or short‐term) medical condition who were mainly elderly, and the remaining studies recruited participants with a mix of conditions. The studies showed that when compared to in‐hospital care, admission avoidance hospital at home services probably make little or no difference to patient health outcomes or to the likelihood of being taken to hospital, and may increase the chances of living at home at six months' follow‐up. Patients who receive care at home may be more satisfied than those who are in hospital, but it is not known how this type of health care affects the caregivers who support them. With respect to costs, it is uncertain if hospital at home services reduce or increase length of stay or cost to the health service; when the costs for caregivers are taken into account any difference in cost may disappear.
How up to date is the review?
The review authors searched for studies published up to March 2016.
Summary of findings
Summary of findings for the main comparison. Admission avoidance hospital at home compared with inpatient admission for older people requiring admission to hospital.
| Admission avoidance hospital at home compared with inpatient admission for older people requiring admission to hospital | |||||
|
Patient or population: Older people requiring hospital admission Settings: Home Intervention: Admission avoidance hospital at home Comparison: Inpatient care | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Inpatient admission | Admission avoidance hospital at home | ||||
|
Transfer (or readmission) to hospital for patients with a medical condition using individual patient data and published data (3 to 12 months' follow‐up) |
Study population |
RR 0.98 (0.77 to 1.23) |
834 (7 RCTs) | ⊕⊕⊕o1 Moderate | |
| 254 per 1000 | 249 per 1000 (195 to 312) | ||||
| Medium‐risk population | |||||
| 340 per 1000 | 333 per 1000 (262 to 418) | ||||
|
Mortality at 6 months' follow‐up (using data from trialists and published data) |
Study population |
RR 0.77 (0.60 to 0.99) |
912 (6 RCTs) | ⊕⊕⊕o1 Moderate | |
| 240 per 1000 | 185 per 1000 (144 to 237) | ||||
| Medium‐risk population | |||||
| 196 per 1000 | 151 per 1000 (118 to 194) | ||||
|
Living in an institutional setting at 6 months' follow‐up |
Study population |
RR 0.35 (0.22 to 0.57) |
727 (5 RCTs) | ⊕⊕oo2 Low |
|
| 161 per 1000 | 56 per 1000 (35 to 92) | ||||
| Medium‐risk population | |||||
| 151 per 1000 | 53 per 1000 (33 to 86) | ||||
| Patient satisfaction | Patients allocated to hospital at home care reported higher levels of satisfaction, range 8% to 40% | ‐ | 646 (5 RCTs) |
⊕⊕oo3 Low | |
| Hospital and hospital at home length of stay | Length of stay ranged from a mean reduction of ‐8.09 days (95% CI ‐14.34 to ‐1.85) to a mean increase of 15.90 days (95% CI 8.10 to 23.70) | ‐ | 714 (7 RCTs) |
⊕⊕oo2 Low |
|
| Cost | Estimates (boot strapped mean difference) of health service cost varied, from a lower cost at 3 months' follow‐up for hospital at home of GBP 210.90 (95% CI GBP ‐1025 to GBP 635.47), to a mean difference (lower cost for hospital at home) per episode of immediate care of GBP ‐304.72, 95% CI GBP ‐1112.35 to GBP 447.89 | ‐ | 287 (2 RCTs) |
⊕⊕oo4 Low | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||
1We downgraded the certainty of the evidence due to lack of precision. 2We downgraded the certainty of the evidence due to lack of precision and inconsistency. 3We downgraded the certainty of the evidence, as only 31% of studies reported this outcome, and there is risk of detection bias due to subjective reporting of this outcome. 4We downgraded the certainty of the evidence, as only two studies reported a full cost analysis.
Background
For several decades there has been an emphasis by health systems around the world on avoiding admission to hospital, and in the last 10 years this has gained some momentum, reflecting the changing demographic and also the relatively limited gain from discharging patients early after a stay in hospital, given the universal trend for shorter hospital lengths of stay. Cutting costs by avoiding admission to hospital in the first instance is the central goal of such schemes. Other perceived benefits include reducing the risk of adverse events associated with time in hospital and the potential benefit of receiving rehabilitation within the home environment (Brennan 2004). The type of patient treated in hospital at home services varies between schemes, as does the use of technology. Some schemes are designed to care for specific conditions, such as chronic obstructive pulmonary disease, or provide specific skills, such as parenteral nutrition (Mughal 1986). These schemes usually have close ties with acute hospitals and may be encouraged by the different structure of incentives in insurance‐based systems of health care. However, many other hospital at home schemes lack such a clear function and have an 'open‐door' policy covering a large range of conditions.
Description of the condition
The demographic shift of a rising number of older people, combined with the relative reduction in the number of working‐age adults contributing to the economy, make the provision of sustainable safe health care for older adults a major health concern for the 21st century. For example, in the UK nearly two‐thirds (65%) of people admitted to hospital are over 65 years old, and over the last 10 years there has been a 65% increase in the number of people aged over 75 who have required secondary care (Cornwall 2012). Healthcare decision‐makers in a number of countries are attempting to reconfigure services to deal with the year‐on‐year increase in hospital admissions, often with an inadequate evidence base (Nolte 2008). These changes have raised concerns that the pressure of delivering health care to greater numbers may be at odds with the provision of person‐centred, high‐quality care (Royal College of Physicians 2012).
Description of the intervention
Admission avoidance hospital at home provides co‐ordinated, multidisciplinary care in the home for people who would otherwise be admitted to hospital. People are admitted to admission avoidance hospital at home after assessment in the community by their primary care physician, in the emergency department or a medical admissions unit. Hospital at home may also provide hospital‐level care following early discharge from hospital (we have conducted a parallel systematic review of early discharge hospital at home, Shepperd 2009).
How the intervention might work
One aim of admission avoidance hospital at home is to reduce the demand for acute hospitals beds; a second aim is to lower the risk of functional decline from limited mobility that can occur during an admission to hospital, particularly in frail older people, by providing co‐ordinated health care in a less restrictive environment and thereby giving patients the opportunity for continued involvement in activities of daily living (Covinsky 2003).
Why it is important to do this review
Despite a policy emphasis on care closer to home (Monitor 2015), it is not known if people admitted to admission avoidance hospital at home have better or equivalent health outcomes compared with those receiving inpatient hospital care. It is also unknown whether the provision of hospital at home results in a reduction or increase in health service costs.
Objectives
To determine the effectiveness and cost of managing patients with admission avoidance hospital at home compared with inpatient hospital care.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
This review included evaluations of admission avoidance hospital at home schemes involving people aged 18 years and over. We did not include people with long‐term care needs unless they required admission to hospital for an acute episode of care. We excluded evaluations of obstetric, paediatric, and mental health hospital at home schemes from the review since our preliminary literature searches suggested that separate reviews would be justified for each of these groups. For the purpose of this review, we defined older patients as those aged 65 years and older.
Types of interventions
Studies comparing admission avoidance hospital at home with acute hospital inpatient care. The admission avoidance hospital at home studies may admit patients directly from the community thereby avoiding physical contact with the hospital, or may admit from the emergency room. We used the following definition to determine if studies should be included in the review: hospital at home is a service that can avoid the need for hospital admission by providing active treatment by healthcare professionals in the patient's home for a condition that otherwise would require acute hospital inpatient care, and always for a limited time period. In particular, hospital at home has to offer a specific service to patients in their home requiring healthcare professionals to take an active part in the patients' care. If hospital at home were not available, then the patient would be admitted to an acute hospital ward. We have therefore excluded the following services from this review:
services providing long‐term care;
services provided in outpatient settings or postdischarge from hospital; and
self care by the patient in their home such as self administration of an intravenous infusion.
Types of outcome measures
Main outcomes
Mortality
Transfer (or readmission) to hospital
Other outcomes
Functional status
Quality of life or self reported health status
Cognitive function
Depression
Clinical outcomes
Place of residence at follow‐up (living in a residential setting)
Patient satisfaction
Caregiver outcomes
Health professionals' views
Length of stay in hospital and hospital at home
Cost
Use of other health services and informal care
Search methods for identification of studies
Electronic searches
We searched the following databases on 2 March 2016 for references published since the last version of this review.
Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 2 of 12, 2016), including the Effective Practice and Organisation of Care (EPOC) Group Specialised Register, Wiley (search date 2 March 2016).
MEDLINE (1946 to 2 March 2016), MEDLINE and MEDLINE In‐Process and Other Non‐Indexed Citations, OvidSP.
EMBASE (1974 to 1 March 2016), OvidSP.
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1980 to 2 March 2016), EBSCOhost.
EconLit (1886 to 2 March 2016), ProQuest.
There were no restrictions to publication status or language of publication. See Appendix 1 and Appendix 2 for details of the search strategies used.
Searching other resources
We checked the reference lists of articles identified electronically for evaluations of hospital at home and obtained potentially relevant articles. We conducted a citation search of all included studies in the previous version of this review using the Science Citation Index (search date 22 April 2015). We searched clinical trial registries using the terms 'hospital at home' and 'admission' for open, interventional trials that recruited adults and older adults (ClinicalTrials.gov) and the term 'hospital at home' (who.int/ictrp).
Data collection and analysis
Selection of studies
Two review authors (SS, DGB) read all the abstracts in the records retrieved by the electronic searches to identify potentially eligible publications. We retrieved full‐text papers for these publications, and two review authors (SS and SI, or SS and DGB) independently assessed their eligibility. We selected studies for the review according to the prespecified inclusion criteria and resolved disagreements by discussion.
Data extraction and management
Two review authors (SS and SI, or SS and DGB) independently completed data extraction using a good‐practice extraction form developed by Cochrane that was modified and amended for the purposes of this review (EPOC 2015a).
Assessment of risk of bias in included studies
Two review authors (SS and SI, or SS and DGB) independently assessed the risk of bias of the included studies using the suggested 'Risk of bias' criteria for EPOC reviews (EPOC 2015b):
random sequence generation;
allocation concealment;
baseline outcome measurement;
baseline characteristics;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective reporting of outcomes.
Certainty of the evidence
We graded our confidence in the evidence by creating a 'Summary of findings' table using the approach recommended by the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Working Group, in Guyatt 2008, and the specific guidance developed by EPOC (EPOC 2015c). We included the main outcomes of mortality and hospital readmission, as well as place of residence at follow‐up, patient satisfaction, and costs. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and risk of bias) to assess the certainty of the evidence as it relates to the main outcomes (Guyatt 2008). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
We conducted an individual patient data (IPD) meta‐analysis in a subgroup of trials evaluating specific outcomes in the more homogeneous populations. We contacted the investigators of 10 of the included trials by email or telephone, inviting them to contribute data to the hospital at home admission avoidance collaborative review. We sent up to four reminders. We excluded five trials from the IPD meta‐analysis (n = 518 participants), though included these trials in the review, as they recruited participants who differed substantially from the populations in the other trials, in terms of having an acute short‐term condition (community‐acquired pneumonia, Richards 2005), having significant cognitive impairment (Tibaldi 2004), cellulitis (Corwin 2005), experiencing febrile neutropenia as a result of chemotherapy (Talcott 2011), and having neuromuscular disease and a respiratory infection (Vianello 2013). One small trial was published as a meeting abstract only; we tried to contact the authors for further information but did not receive a reply (Andrei 2011, n = 45).
For the IPD meta‐analysis, where at least one event was reported in both study groups in a trial, we used Cox regression models to calculate the log hazard ratio and its standard error for mortality for each data set. We included randomisation group (admission avoidance hospital at home versus control), age (above or below the median), and gender in the models. We combined the calculated log hazard ratios using fixed‐effect inverse variance meta‐analysis (Deeks 2001). The pooled effect is expressed as the hazard ratio for hospital at home compared with usual hospital care. If there were no events in one group, we used the Peto odds ratio method to calculate a log odds ratio from the sum of the log‐rank test 'O‐E' statistics from a Kaplan‐Meier survival analysis. This method does not require corrections for zero cell counts, and thus it performs well when events are rare (Deeks 1998). Throughout the analyses, we took statistical significance at the two‐sided 5% level (P < 0.05), presenting data as the estimated effect with 95% confidence intervals. For the original review, we used SPSS version 14.0 and Stata for all the analyses (SPSS 2006; Stata 2005), undertaking the meta‐analysis in Review Manager version 4.2. For this update, we conducted analysis using Review Manager version 5.3 (RevMan 2014).
Our statistical analyses sought to include all randomised participants, using intention‐to‐treat. We relied on published data when the IPD did not include the relevant outcomes. When combining outcome data was not possible because of differences in the reporting of outcomes, we presented the data from individual studies in summary tables. For each comparison using published data for dichotomous outcomes we calculated risk ratios using a fixed‐effect model to combine data. Comparison between health outcomes was restricted by the different measurement tools used in the included trials. Although planned, we did not attempt a direct comparison of costs because the trials collected data on different resources and used different methods to calculate costs.
Dealing with missing data
In two data sets contributing to the IPD meta‐analysis (Davies 2000; Kalra 2000), some dates were missing for known events, and so we gave the missing event a time at the midpoint between randomisation and last follow‐up, or as the midpoint between follow‐up times if these were known. For one trial where follow‐up was 90 days, we set the time to event as 45 days for three cases in the admission avoidance hospital at home arm and for one case in the control group where we knew death had occurred but we did not have a date (Davies 2000). For the other trial, we gave a time to event of 14 days if the participant was known to have died some time between randomisation and one‐month follow‐up, and at 59 days if they were known to have died between one and three months' follow‐up (Kalra 2000).
Assessment of heterogeneity
We quantified heterogeneity by Cochran's Q and the I2 statistic (Cochran 1954), the latter quantifying the percentage of the total variation across studies that is due to heterogeneity rather than chance (Higgins 2003); smaller percentages suggest less observed heterogeneity.
Assessment of reporting biases
We created one funnel plot, for mortality at six months' follow‐up, recognising that when the number of trials is small these plots are not necessarily indicative of publication bias.
Results
Description of studies
We identified 16 trials that randomised individual participants (N = 1814), of which six were identified in this update. We invited 10 trialists to contribute data to the IPD meta‐analysis (Caplan 1999; Davies 2000; Harris 2005; Kalra 2000; Mendoza 2009; Nicholson 2001; Ricauda 2004; Ricauda 2008; Tibaldi 2009; Wilson 1999), of which six contributed data (n = 921/1206; 76%) (Davies 2000; Harris 2005; Kalra 2000; Mendoza 2009; Ricauda 2004; Wilson 1999). We contacted the authors of a study published as a meeting abstract for further information, but did not receive a reply (Andrei 2011, n = 45). We did not contact five trialists as their studies included participants who were significantly different from the remaining populations, for example populations with short‐term acute conditions or advanced dementia (Corwin 2005; Richards 2005; Talcott 2011; Tibaldi 2004; Vianello 2013). We used published data when we did not have access to IPD. Follow‐up times varied across the different trials, ranging from 1 week to 12 months.
Results of the search
For this update the search retrieved 1364 records, of which 1350 were ineligible. We obtained full texts for the remaining 14 records, seven of which fulfilled the inclusion criteria (six trials, seven records), bringing the total number of trials included in the review to 16 (Figure 1). We also identified two ongoing trials (ISRCTN29082260; ISRCTN60477865).
1.

Study flow diagram.
Included studies
Study populations
Three trials recruited participants with chronic obstructive pulmonary disease (COPD) (Davies 2000; Nicholson 2001; Ricauda 2008), two trials recruited participants recovering from a moderately severe stroke who were clinically stable (Kalra 2000; Ricauda 2004), and six trials recruited participants with an acute medical condition who were mainly elderly (Andrei 2011; Caplan 1999; Harris 2005; Mendoza 2009; Tibaldi 2009; Wilson 1999). There was one trial each for participants with cellulitis (Corwin 2005), community‐acquired pneumonia (Richards 2005), fever and neutropenia (Talcott 2011), frail elderly participants with dementia (Tibaldi 2004), and neuromuscular disease (Vianello 2013). The 16 trials were conducted in seven countries: Australia (two trials), Italy (five trials), New Zealand (three trials), Romania (one trial), Spain (one trial), the UK (three trials), and the US (one trial).
Interventions
In 12 of the trials included in this review participants were admitted to hospital at home from the emergency room (Andrei 2011; Caplan 1999; Corwin 2005; Davies 2000; Mendoza 2009; Nicholson 2001; Ricauda 2004; Ricauda 2008; Richards 2005; Tibaldi 2004; Tibaldi 2009; Vianello 2013), in three directly from the community following referral by their primary care physician (Harris 2005; Kalra 2000; Wilson 1999), and in one from an outpatient department (Talcott 2011). For participants allocated to hospital at home, health care was provided by a hospital outreach team (Caplan 1999; Harris 2005; Mendoza 2009; Ricauda 2004; Ricauda 2008; Talcott 2011; Tibaldi 2004; Tibaldi 2009), a mix of outreach and community staff (Davies 2000; Kalra 2000; Nicholson 2001; Vianello 2013), or by the general practitioner (GP) and community nursing staff (Corwin 2005; Richards 2005; Wilson 1999). For one of the trials it was not clear who provided care (Andrei 2011). In two trials, the intervention was provided by Pegasus Health, an independent association of GPs (Corwin 2005; Richards 2005). One trial was a three‐group comparison of stroke unit care, inpatient stroke team, and hospital at home (Kalra 2000); we selected the inpatient stroke team as the comparison group, as this was most similar to the comparator in the other trials.
Physiotherapy care was described in seven of the interventions (Harris 2005; Kalra 2000; Nicholson 2001; Ricauda 2004; Ricauda 2008; Tibaldi 2004; Wilson 1999), and occupational therapist care in four of the interventions (Harris 2005; Kalra 2000; Nicholson 2001; Wilson 1999). A social worker was part of the hospital at home team in seven of the interventions (Davies 2000; Harris 2005; Kalra 2000; Ricauda 2004; Talcott 2011; Tibaldi 2004; Wilson 1999), and a counsellor in one (Talcott 2011). Access to a speech therapist was described in three of the interventions (Kalra 2000; Ricauda 2004; Wilson 1999). One trial described access to a cultural link worker (Wilson 1999). The intervention in one trial included the use of a portable ventilator; a respiratory therapist made daily visits for the first three days of home care, and district nurses and caregivers were trained in the application of the device and on assisting with coughing (Vianello 2013). District nurses visited daily until recovery from the respiratory tract infection; participants also had telephone access to pulmonary specialists (Vianello 2013).
Excluded studies
See the Characteristics of excluded studies table. We excluded three studies: one was a cross‐over randomised controlled trial (King 2000), one a non‐randomised comparison (Wade 1985), and in the third study the intervention did not substitute for inpatient care (Wolfe 2000).
Risk of bias in included studies
Allocation
In 11 studies concealment of allocation was adequate (Caplan 1999; Corwin 2005; Davies 2000; Harris 2005; Kalra 2000; Mendoza 2009; Ricauda 2008; Richards 2005; Talcott 2011; Tibaldi 2009; Wilson 1999) (Figure 2; Figure 3), and in eight studies sequence generation was adequately described. (Caplan 1999; Harris 2005; Kalra 2000; Mendoza 2009; Ricauda 2004; Ricauda 2008; Richards 2005; Talcott 2011)
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
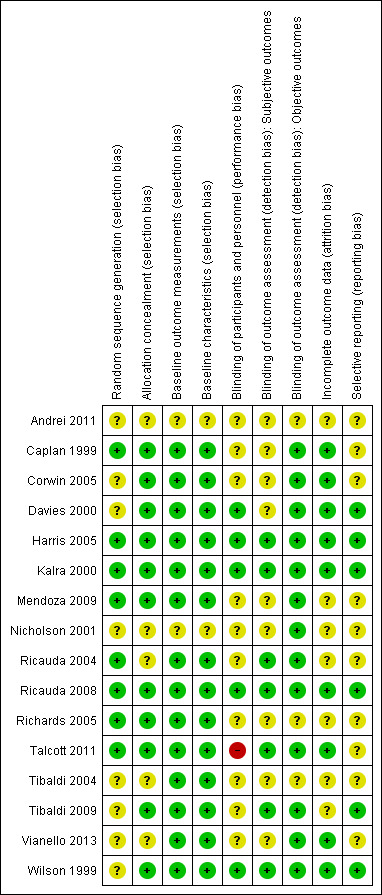
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Blinding
All but one study used reliable measures of outcome (Vianello 2013), and four studies reported blinded assessment of outcome (Kalra 2000; Ricauda 2004; Ricauda 2008; Tibaldi 2009). We assessed 13 studies as at low risk of bias for the measurement of objective outcomes, and seven at low risk of bias for the measurement of subjective outcomes (Figure 3).
Incomplete outcome data
Most of the studies had a low risk of attrition bias, with seven studies having an unclear risk (Andrei 2011; Mendoza 2009; Nicholson 2001; Ricauda 2004; Richards 2005; Tibaldi 2004; Tibaldi 2009).
Selective reporting
Six studies were at low risk of bias for selective reporting (Davies 2000; Harris 2005; Kalra 2000; Ricauda 2008; Tibaldi 2009; Wilson 1999).
Effects of interventions
See: Table 1
See Table 1 for the main comparison admission avoidance hospital at home compared with inpatient admission for older people requiring admission to hospital.
Mortality
We combined IPD, adjusted for age and sex, for mortality at three months' follow‐up for five studies (risk ratio (RR) 0.77, 95% confidence interval (CI) 0.54 to 1.09; P = 0.15; 833 participants; moderate‐certainty evidence) (Davies 2000; Harris 2005; Kalra 2000; Ricauda 2004; Wilson 1999) (Analysis 1.1); and combined published data from three studies, Caplan 1999, Ricauda 2008, and Tibaldi 2009, with data received from trialists for three studies, Kalra 2000, Ricauda 2004, and Wilson 1999, for mortality at six months (RR 0.77, 95% CI 0.60 to 0.99; P = 0.04; 912 participants; moderate‐certainty evidence) (Analysis 1.2; Figure 4).
1.1. Analysis.
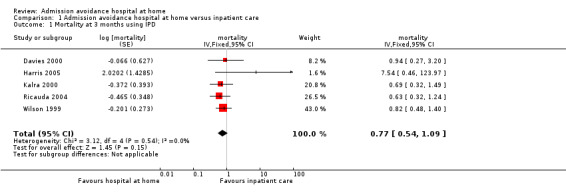
Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 1 Mortality at 3 months using IPD.
1.2. Analysis.

Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 2 Mortality at 6 months' follow‐up (using data from trialists, apart from Caplan).
4.
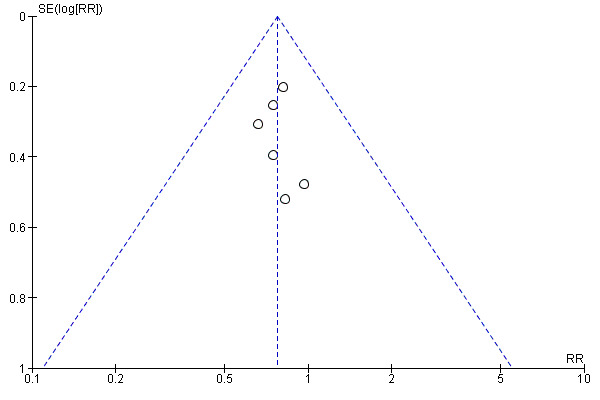
Funnel plot of admission avoidance hospital at home versus inpatient care, for mortality at 6 months' follow‐up (using data from trialists and published data from one study).
Transfer (or readmission) to hospital
We analysed the effect of admission avoidance hospital at home on hospital readmission or transfer at three months' follow‐up using data received from four trialists, Davies 2000, Harris 2005, Mendoza 2009, and Wilson 1999, and published data from three studies (Caplan 1999; Ricauda 2008; Tibaldi 2009). Results indicated that admission avoidance hospital at home may make little to no difference for hospital readmission or transfer (RR 0.98, 95% CI 0.77 to 1.23; P = 0.84; 834 participants; moderate‐certainty evidence) (Analysis 1.4).
1.4. Analysis.

Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 4 Readmission to hospital for patients with a medical condition at an average of 5 months' follow‐up.
Functional status
Nine trials reported measures of functional ability, for which higher scores indicate greater independence (see Analysis 1.5 for specific details on the scales used). Caplan 1999 reported scores for instrumental activities of daily living between admission and discharge (mean difference (MD) ‐0.23, P = 0.04), and the Barthel Index (hospital at home (T): 0.37 (0.27), hospital (C): ‐0.04 (0.27)). A trial that recruited participants with dementia reported that fewer participants in the hospital at home group had problems with sleep (difference 34%, P < 0.001), agitation and aggression (difference 32.5%, P < 0.001), and feeding (difference 31%, P < 0.001) (Tibaldi 2004). One trial recruiting participants who had a stroke reported the number of participants with a favourable outcome measured by the Barthel (score of 15 to 20) at three months (T: 106/145 (73%), C: 106/151 (70%), RR 0.96 (95% CI 0.83 to 1.11), P = 0.58) (Kalra 2000); and Ricauda 2004, which also recruited participants with a stroke, reported activities of daily living (scale 0 to 6) at six months (median (interquartile range (IQR)), T: 4 (2 to 5), C: 4 (2 to 6); P = 0.57). Two trials recruiting participants with COPD reported follow‐up data: Ricauda 2008 reported activities of daily living at six months: T: 0.12 (standard deviation (SD) 0.64), C: 0.08 (SD 0.73), P = 0.81, and Davies 2000 reported forced expiratory volume in 1 second (FEV1) at three months' follow‐up (T: 41.5%, 95% CI 8.2% to 74.8%; C: 41.9%, 95% CI 6.2% to 77.6%). Two studies recruiting participants with heart failure reported change in activities of daily living measured by the Barthel Index at six months' follow‐up (mean T: ‐1.95 (SD 9.61), C: ‐0.30 (SD 10.12) (Tibaldi 2009); and at one year (T: 4.0, 95% CI ‐0.9 to 8.9; C: 4.7, 95% CI ‐2.2 to 11.5; P = 0.21), adjusted for baseline differences (Mendoza 2009). Wilson 1999, which recruited older people with a mix of conditions, assessed functional ability at three months using the Barthel Index (median (IQR) T: 16 (13 to 19), C: 16 (12 to 20)) (Analysis 1.5).
1.5. Analysis.
Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 5 Functional status.
| Functional status | ||
|---|---|---|
| Study | Functional ability | Results |
| Admission avoidance patients with a medical condition ‐ functional ability | ||
| Caplan 1999 | Change in Barthel score from admission to discharge (high score=greater independence) Instrumental activities of daily living score from admission to discharge (higher score=greater independence) | Mean (SEM) T= 0.37 (0.27), C= ‐0.04 (0.27), NS Mean (SEM) T= 0.65 (0.23), C= ‐0.88 (0.26), P = 0.037 |
| Davies 2000 | St Georges' respiratory questionnaire (to a random sub‐group of 90 participants). High score indicates poorer health related quality of life. A minimum change in score of 4 units is clinically relevant. Forced expiratory volume in one second (FEV1) |
Baseline scores
T= 71.5 (43.4 to 99.6), C= 71.0 (43.4 to 98.6)
Mean (SD) change at 3 months
T= 0.48 (16.92) C= 3.13 (14.02) Forced expiratory volume in 1 second (FEV1) At 3 months: T= 41.5% (95% CI 8.2% to 74.8%) C= 41.9% (95% CI 6.2% to 77.6%) |
| Kalra 2000 | Modified Rankin scale 0‐3 (measure of dependence: 0=independent and 3=dependent). Number independent and require minor assistance for day to day activities. Barthel 0‐20 (higher score=greater independence) |
Modified Rankin At 3 months T= 107/145 (74%) C= 111/151 (74%) RR 1.00 (0.86, 1.15), P = 0.96 At 12 months T= 102/144 (71%) C= 99/149 (66%) RR 0.94 (0.81, 1.09), P = 0.42 Barthel 15‐20 (number with favourable outcome) At 3 months T= 106/145 (73%) C= 106/151 (70%) RR 0.96 (0.83, 1.11), P = 0.58 At 12 months T= 102/144 (71%) C= 102/149 (69%) RR 0.97 (0.85, 1.11), P = 0.65 |
| Mendoza 2009 | Activities of daily living |
Mean score Barthel Index at 1 year (adjusted for baseline differences) T= 4.0 (‐0.9 to 8.9) C= 4.7 (‐2.2 to 11.5) P = 0.21 |
| Ricauda 2004 | Activities of daily living (number of functions lost, score 0 to 6).
Functional impairment measure (level of independence, range 28 to 126. High score =greater independence Canadian Neurological Scale Score (higher score=improvement, range 0‐10) National Institute of Health Stroke Scale Score (low score =improvement; range 0‐36) |
Activities of daily living At 6 months (Median (IQR)) T= 4 (2‐5), C= 4 (2‐6), P = 0.57 (Mann Whitney) Functional impairment measure At 6 months (Median (IQR)) T= 106 (67.5‐121.5), C= 96.5 (56.5‐116.5), P = 0.26 (Mann Whitney) Canadian Neurological Scale Score At 6 months (Median (IQR)) T= 10 (8.5‐10.0), C= 9.5 (7.0‐10.0), P = 0.39 (Mann Whitney National Institute of Health Stroke Scale Score At 6 months (Median (IQR)) T= 8 (4‐26), C= 8 (6‐24), P = 0.37 (Mann Whitney) |
| Ricauda 2008 | Change in ADL (score 0 to 6) | At 6 months, mean (SD) T= 0.12 (0.64), C= 0.08 (0.73), P = 0.81 |
| Tibaldi 2004 | Behavioural disturbances | Sleeping disorders T= 5/56 (9%), C= 23/53 (43%), MD: ‐34%, 95% CI ‐50% to ‐19%, P < 0.001 Agitation/aggressiveness T= 5 /56 (9%), C= 22/53 (41.5%), MD ‐33% 95% CI ‐48% to ‐17%, P<0.001 Feeding disorders T= 5 /56 (9%), C= 21/53 (40%), MD ‐31% 95% CI ‐46% to ‐16%, P < 0.001 |
| Tibaldi 2009 | Activities of daily living Barthel Index |
ADL at 6 months mean change T= ‐1.95 (9.61) N=48, C= ‐0.30 (10.12) N=53, |
| Wilson 1999 | Barthel Index |
Barthel Index At 3 months (Median (IQR)) T= 16 (13‐19), C= 16 (12‐20) Barthel Index ‐ number (%) not assessed: T= 21 (28%), C= 18 (28%) Sickness Impact Profile: At 3 months (Median (IQR)) T= 24 (20‐31), C= 26 (20‐31) Sickness Impact Profile ‐ no (%) not assessed T= 31 (41%), C= 30 (46%) |
Quality of life or self reported health status
Seven trials assessed health status or quality of life using different measures (Analysis 1.6). One trial that recruited people with cellulitis reported 36‐Item Short Form Health Survey (SF‐36) scores at six days' follow‐up (physical component scale MD ‐5.2, 95% CI ‐13.7 to 3.2; role physical scale MD 2.2, 95% CI ‐10.7 to 15.1; pain scale MD ‐3.8, 95% CI ‐10.6 to 3.0) (Corwin 2005). A second trial measuring health status with the SF‐36 reported follow‐up data at one year for the physical component scale (T: 3.6 (‐0.5 to 7.7), C: 2.2 (‐1.9 to 6.4); P = 0.47) and the mental component scale (T: 4.0 (‐0.9 to 8.9), C: 2.8 (‐2.4 to 8.0); P = 0.38) (Mendoza 2009). One trial measured quality of life with the SF‐12 at six weeks' follow‐up and reported similar scores for each group on the physical component (T: 42.2, C: 45.8; P = 0.18) and mental component scale (T: 50.4, C: 51.0; P = 0.81) (Richards 2005). Two trials assessed quality of life using the Nottingham Health Profile at six months' follow‐up (T: +1.09 (2.57) n = 48, C: +0.18 (SD 1.94); P = 0.046) (Tibaldi 2009), and (T: 3.6 (SD 7.9), C: 0.8 (SD 4.5); P = 0.04) (Ricauda 2008). One trial assessed a change from baseline in quality of life when a participant had a health event using the EORTC QLQ‐C30 (T: 0.58, C: 0.78, P = 0.05; emotional function hospital at home 3.27, hospital ‐6.94, P = 0.04) (Talcott 2011). One trial reported median values at three months' follow‐up for the Sickness Impact Profile (T: 24 (IQR 20 to 31), C: 26 (IQR 20 to 31); MD ‐2, 95% CI ‐4 to 4; P = 0.73) and the EuroQol (T: 0.64, n = 73, C: 0.63, n = 96; MD 0.01, 95% CI ‐0.12 to 0.09; P = 0.94) (Wilson 1999).
1.6. Analysis.
Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 6 Quality of life/health status.
| Quality of life/health status | |||
|---|---|---|---|
| Study | Outcomes | Results | Notes |
| Admission avoidance quality of life | |||
| Corwin 2005 | SF 36 Physical functioning Role physical Pain | SF 36 Physical functioning Day 3 T= 37 (29.1), C= 41 (28.3) Mean difference ‐1.9, 95% CI ‐10.7 to 6.9 Day 6 T=50.7 (33.7), C=50.9 (31.6) Mean difference ‐5.2, 95% CI ‐13.7 to 3.2 Role physical Day 3 T= 5.4 (18.8), C=5.5 (19.7) Mean difference ‐1.8 95% CI ‐13.1 to 9.4 Day 6 T=21.1 (36.9), C=18.4 (36.5) Mean difference 2.2, 95% CI ‐10.7 to 15.1 Pain Day 3 T=57 (28.8), C=55.9 (25.4) Mean difference ‐2.5 95% CI ‐10.1 to 5.1 Day 6 T=69.8 (26.4), C=64.8 (25.6) Mean difference ‐3.8 95% CI ‐10.6 to 3.0 | Differences calculated on absolute differences between day 0 & day 3, or day 0 & day 6.
Numbers vary due to missing data (high score=better health) |
| Mendoza 2009 | SF 36 Physical component Mental component |
Physical component T= 3.6 (‐0.5 to 7.7), C= 2.2 (‐1.9 to 6.4), P = 0.47 Mental component T= 4.0 (‐0.9 to 8.9), C= 2.8 (‐2.4 to 8.0), P = 0.38 |
Score at 1 year (adjusted for baseline differences) |
| Ricauda 2008 | Nottingham Health Profile | 6 months, mean (SD) T= 3.6 (7.9), C= 0.8 (4.5), P = 0.04 |
Changes at 6 months |
| Richards 2005 | SF‐12 Mean physical and mental component score |
Physical component At 2 weeks T= 38.1, C= 40.2, P = 0.45 At 6 weeks T= 42.2, C=45.8, P = 0.18 Mental component At 2 weeks T=48.3, C=48.6, P = 0.91 At 6 weeks T = 50.4, C=51.0, P = 0.81 |
higher score=better health |
| Talcott 2011 | Quality of life EORTC QLQ C‐30 |
Role Function T= 0.58, C= 0.78, P = 0.05 Emotional Function T= 3.27, C= ‐6.94, P = 0.04 |
Quality of life data were collected at the time of consent to join the study, as soon as possible after the resolution of the episode. Data were collected for the first study episode. Change score |
| Tibaldi 2009 | Nottingham Health Profile | 6 months, mean (SD) T= +1.09 (2.57), C= +0.18 (1.94), P = 0.046 |
|
| Wilson 1999 | Sickness Impact Profile (SIP) Euroqol | SIP, median (IQR) T= 24 (20‐31), C= 26 (20‐31) Difference ‐2 (95% CI ‐4 to 4), P = 0.73 Euroqol, median T= 0.64, C= 0.63 Difference 0.01 (95% CI ‐0.12 to 0.09), P = 0.94 | At 3 months follow‐up |
Cognitive function and depression
Five trials measured cognitive function and depression (Analysis 1.7). One trial that recruited participants recovering from a stroke reported that hospital at home may lead to lower scores on the Geriatric Depression Scale (GDS) (lower scores = fewer symptoms) (MD 7 points, on a scale of 0 to 30, P < 0.001) (Ricauda 2004). A second trial, which recruited participants with COPD, reported a greater improvement for those allocated to hospital at home from baseline on the GDS at six months (T: ‐3.1 (SD 4.7), C: 0.7 (SD 3.2), P < 0.0001) (Ricauda 2008). One trial that recruited participants with acute chronic heart failure reported fewer depressive symptoms at six months follow‐up (measured by the GDS) for those allocated to admission avoidance hospital at home (mean change T: 1.48 (SD 1.86), C: 0.12 (SD 3.36); P = 0.02) (Tibaldi 2009). Wilson 1999 reported median (IQR) scores for the Philadelphia Geriatric Morale Scale at three months, with little to no difference between groups (T: 37 (30 to 42), C: 37 (31 to 43); MD 0, 95% CI ‐4.1 to 4.1).
1.7. Analysis.
Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 7 Cognitive function and depression.
| Cognitive function and depression | ||
|---|---|---|
| Study | Outcomes | Results |
| admission avoidance ‐ cognitive function/well being | ||
| Caplan 1999 | Mental status questionnaire score from admission to discharge (maximum score 10); Number with confusion | Mean (SEM) T= 0.43 (0.12), C= 0.27 (0.12), NS Number with confusion T=0/51, C=10/49 |
| Ricauda 2004 | Geriatric Depression Scale score (range 0‐30)
higher scores indicate depression Change in Mini Mental State Exam (score 0 to 30) |
At 6 months, median (IQR)
T= 10 (5‐15), C=17 (13‐20), P<0.001 (Mann Whitney) At 6 months, mean (SD) T= ‐0.4 (4.0), C=‐0.5 (1.8), P = 0.88 |
| Ricauda 2008 | Change in geriatric Depression Scale score (range 0‐30) higher scores indicate depression | At 6 months, mean (SD) T= ‐3.1 (4.7), C=0.7 (3.2), P < 0.0001 |
| Tibaldi 2009 | Mini Mental State Exam (MMSE) Geriatric Depression Scale |
At 6 months, mean change (SD) T= +0.07 (1.38), C= +0.08 (1.36), P = 0.97 At 6 months, mean change (SD) T= +1.48 (1.86), C= +0.12 (3.36), P = 0.02 |
| Wilson 1999 | Philadelphia Geriatric Morale Scale | At 3 months, median (IQR) T= 37 (30‐42), C= 37 (31‐43), Difference 0, 95% CI ‐4.1 to 4.1 |
Two trials used the mini‐mental state examination to assess cognitive functioning at six months' follow‐up and reported little to no difference between groups (T: ‐0.4 (SD 4.0), C: ‐0.5 (SD 1.8); P = 0.88) (Ricauda 2004); and (T: 0.07 (SD 1.38), C: 0.08 (SD 1.36); P = 0.97) (Tibaldi 2009). One trial that recruited participants with a mix of conditions reported cognitive function scores: mean T: 0.43 (standard error of the mean (SEM) 0.12), C: 0.27 (SEM 0.12), and that fewer people receiving hospital at home care experienced short‐term confusion during an episode of care (MD ‐20.4%, 95% CI ‐32% to ‐9%) (Caplan 1999).
Clinical outcomes
One trial measured clinical complications, with fewer participants allocated to hospital at home reporting bowel complications (difference ‐22.5%, 95% CI ‐34% to ‐10.8%) or urinary complications (difference ‐14.4%, 95% CI ‐25.4% to ‐3.3%) (Caplan 1999). In a trial recruiting participants with dementia, fewer participants in the hospital at home group were prescribed antipsychotic drugs at discharge (difference ‐14%, 95% CI ‐28% to 0.3%) (Tibaldi 2004). One trial that recruited people with cellulitis reported risk of advancement of cellulitis (hazard ratio (HR) 0.98, 95% CI 0.73 to 1.32) (Corwin 2005), and one trial recruiting participants with COPD reported that more participants were prescribed an antibiotic if they were allocated to hospital at home (difference 18%, 95% CI 1.4% to 34.6%) (Davies 2000). Talcott 2011 reported the difference in major complications during the episode of care (difference 1%, 95% CI ‐10 to 13%) (Analysis 1.8).
1.8. Analysis.
Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 8 Clinical outcomes.
| Clinical outcomes | ||
|---|---|---|
| Study | Outcomes | Results |
| Clinical outcomes | ||
| Corwin 2005 | No advancement of cellulitis (indelible line drawn around peripheral margin of the cellulitis and dated) |
Mean (SD) days T= 1.5 (0.11), C= 1.49 (0.10), Mean difference 0.01 days, 95% CI ‐0.3 to 0.28 Days of no advancement of cellulites HR 0.98, 95% CI 0.73 to 1.32, P = 0.90 Days on intravenous antibiotics HR 0.84, 95% CI 0.63 to 1.12, P = 0.23 Days to discharge HR 0.93, 95% CI 0.70 to 1.23, P = 0.60 Days on oral antibiotics HR 1.09, 95% CI 0.82 to 1.45, P = 0.56 |
| Davies 2000 | Proportion of patients prescribed an antibiotic at 3 months | T= 56/100 (56%), C= 19/50 (38%), Difference 18%, 95% CI 1.4 to 34.6% |
| Talcott 2011 | Major medical complications during care in hospital at home or hospital | T= 4/47 (9%), C= 5/66 (8%), Difference 1%, 95% CI ‐10 to13% |
| Tibaldi 2004 | Use of antipsychotic drugs | On admission T= 26/56 (46.4%), C= 18/56 (32%), Difference 14.3%, 95% CI ‐3.7% to 31.1% On discharge T= 6/56 (11%), C = 13/53 (25%), Difference 14%, 95% CI ‐28% to 0.3% |
Place of residence at follow‐up (living in a residential setting)
Admission avoidance may reduce the likelihood of living in residential care, measured at discharge to six months' follow‐up (RR 0.35, 95% CI 0.22 to 0.57; P < 0.0001; I2 = 78%; 5 trials; low‐certainty evidence) (Kalra 2000; Ricauda 2004; Ricauda 2008; Tibaldi 2004; Tibaldi 2009). We downgraded the certainty of evidence due to the high level of statistical heterogeneity and imprecision (Analysis 1.9).
1.9. Analysis.

Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 9 Place of residence at follow‐up (living in residential care).
Patient satisfaction
Admission avoidance may increase patient satisfaction with the health care received. Participants allocated to hospital at home care reported higher levels of patient satisfaction across a range of different conditions (5 studies; 646 participants; low‐certainty evidence). For participants with cellulitis, 27% (P < 0.0001) more participants in the hospital at home group reported increased satisfaction with their location of care compared with those admitted to hospital (Corwin 2005), and 40% (P < 0.001) more participants with community‐acquired pneumonia allocated to hospital at home reported that they were happy with their care (Richards 2005). Two trials (recruiting mainly elderly participants with a mix of medical conditions) also reported increased levels of satisfaction for those allocated to hospital at home care (median difference of 3 on a 0‐to‐18‐point scale, P < 0.0001) (Wilson 1999), and a MD of 0.9 on a 4‐point scale (P < 0.0001) (Caplan 1999). However, there was a low response rate for the control group in the latter trial: 40% compared with 78% in the hospital at home group (Caplan 1999). Some participants (6/101; 6%) refused hospital at home care and were admitted to hospital, and a greater number of participants allocated to hospital care (23/97; 24%) were not admitted because of refusal by the participant, caregiver, or general practitioner (Wilson 1999). One trial recruiting participants with COPD reported the number assessing satisfaction with care as very good or excellent (hospital at home 49/52 (94%), hospital 46/52 (88%); P = 0.83) (Ricauda 2008) (Analysis 1.10).
1.10. Analysis.
Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 10 Patient satisfaction.
| Patient satisfaction | |||
|---|---|---|---|
| Study | Outcomes | Results | Notes |
| Caplan 1999 | Satisfaction rated on a 4 point scale: 1=excellent, 2=good, 3=fair, 4=poor. | Mean score T= 1.1, C= 2.0, P < 0.0001 | Response rates were 78% for the treatment group, and 40% for the control. |
| Corwin 2005 | Patient satisfaction questionnaire (not described) |
Overall
T= 87/91 (96%), C=87/96 (96%), P = 0.12
Satisfaction with location of care
T= 85/91 (93%), C= 59/88 (66%), P < 0.0001
Location preference
In the hospital
T= 5/91 (5%), C= 27/88 (31%)
In the community
T= 78/91 (86%), C= 31/88 (35%)
No preference
T= 8/91 (9%), C= 30/88 (34%) P < 0.0001 |
Numbers for control group vary between 88 and 91 due to missing data Proportion of participants satisfied or very satisfied |
| Ricauda 2008 | Patient satisfaction questionnaire (not described) | T= 49/52 (94%), C= 46/52 (88%), P = 0.83 | Proportion of participants rating satisfaction as very good/excellent at discharge |
| Richards 2005 | Outcome not described | T= 24/24 (100%), C= 14/24 (60%), P = 0.001 | Proportion of patients very happy with care |
| Wilson 1999 | Patient satisfaction, scale 0 to 18 | Median (IQR) T= 15 (13 to 16.5), C= 12 (11 to 14), P < 0.0001 | At 2 weeks, or discharge |
Caregiver outcomes
One trial reported that caregivers in the hospital at home group had significantly higher levels of satisfaction compared with those in the hospital group (difference ‐0.8 on a 4‐point scale, P < 0.0001) (Caplan 1999), although the response rate was 27% in the hospital group and 55% in the hospital at home group. A second trial assessed caregiver satisfaction through semi‐structured interviews; caregivers reported that although hospital would potentially relieve them from caring, the upheaval of visiting hospital and the accompanying anxiety was a less satisfactory option (Wilson 1999). One trial recruiting participants with COPD reported change in relatives' stress at six months (mean scores (SD) T: 4.6 (5.6), C: 2.6 (6.1); P = 0.16) (Ricauda 2008) (Analysis 1.11).
1.11. Analysis.
Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 11 Care giver outcomes.
| Care giver outcomes | |||
|---|---|---|---|
| Study | Outcomes | Results | Notes |
| Care giver satisfaction | |||
| Caplan 1999 | Carer satisfaction | Mean score T= 1.1, C= 1.9, P < 0.0001 | Satisfaction rated on a 4 point scale: 1=excellent, 2=good, 3=fair, 4=poor |
| Ricauda 2008 | Change in Relative’s Stress Scale Score |
At 6 months, mean (SD) T= 4.6 (5.6), C= 2.6 (6.1), P = 0.16 |
|
Health professionals' views
One trial evaluated general practitioners' satisfaction with the service (T: 1.17, C: 1.8, score of 1 to 4, with high score being excellent, low score poor); the response rate was poor: 63% in the hospital at home group and 37% in the control group (Caplan 1999) (Analysis 1.12).
1.12. Analysis.
Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 12 Views of health professionals.
| Views of health professionals | |||
|---|---|---|---|
| Study | Outcomes | Results | Notes |
| Caplan 1999 | GP satisfaction | Mean score (95% CI) T= 1.7 (1.4 to 2.0), C= 1.8 (1.4 to 2.2), Difference: NS | Higher scores indicate higher satisfaction Response rate: T: 63%, C: 37% |
Length of stay in hospital and hospital at home
Seven trials reported the effect of admission avoidance hospital at home on length of hospital stay and hospital at home, with differing results (Analysis 1.13). Length of stay varied from a mean reduction of ‐8.09 days (95% CI ‐14.34 to ‐1.85) in a trial recruiting older people with varied health problems (Wilson 1999), to a mean increase of 15.90 days (95% CI 8.10 to 23.70) in a study that recruited patients recovering from a stroke (Ricauda 2004). One trial recruiting participants that were recovering from a stroke reported that 51 out of 153 (33%) of participants allocated to hospital at home received inpatient care within two weeks of randomisation, with a mean length of stay of 49 days; this exceeded the mean length of stay for those allocated to an inpatient hospital stroke team by 17 days (95% CI 7.9 to 25.3) (Kalra 2000).
1.13. Analysis.
Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 13 Length of stay.
| Length of stay | |||
|---|---|---|---|
| Study | Results | Outcomes | Notes |
| Hospital and hospital at home length of stay | |||
| Davies 2000 | Hospital length of stay | Median (IQR) 5 days (4 to 7) N=100 Mean (SD) 6.72 days (4.3) N=100 |
Data for the control group only |
| Harris 2005 | Average length of stay for the index episode until discharge from hospital or hospital at home (days) |
T= 11.33 days (SD 11.14) N=39, C= 7.83 days (7.35) N=37 Mean difference 3.5 95% CI ‐0.80 to 7.80 | IPD |
| Kalra 2000 | Average length of stay for readmission within two weeks of discharge (T) or index episode (C) (days) |
T= 48.6 (SD 26.7) N=51/146, C= 29.5 (SD 40.1) N=151 Difference 16.6, 95% CI 7.9 to 25.3 |
Data for inpatients treated by a stroke team |
| Mendoza 2009 | Average length of stay for the index episode (days) | T= 10.9 (SD 5.9) N=37, C= 7.9 (SD 3.0), P = 0.01 N=34 | |
| Ricauda 2004 | Average length of treatment (days) | T= 38.1 (SD 28.6) N=60, C= 22.2 (11.5) N=60 Difference 15.90, 95% CI 8.10 to 23.70 |
|
| Ricauda 2008 | Hospital at home and hospital length of stay (days) Total length of stay to include hospital transfers for the hospital at home group |
Total days of care (hospital plus hospital at home), mean (SD) T= 15.5 (SD 9.5) N=52, C= 52 (SD 7.9) Difference 4.50, 95% CI 1.14, 7.86 |
|
| Richards 2005 | Median number of days to discharge | T=4 (range 1‐14) N=24, C= 2 (range 0‐10) N=25 | |
| Tibaldi 2009 | Time in the emergency department (hours) Length of stay (days) |
Time in ED, mean (SD) T= 14.6 (3.4), C= 16.3 (3.0) Length of treatment, mean (SD) T= 20.7 (6.9) N=48, C= 11.6 (10.7) N=53, P = 0.001 |
|
| Wilson 1999 | Length of stay | Treatment N=102 Control N=97 Length of hospital stay in days, median T= 5.1 (13.53), C= 18.5 (18.51) days, P = 0.026 Total days of care (hospital plus hospital at home), median T= 9, C= 16 days; P = 0.031 Total days of care (hospital plus hospital at home and readmission days), mean (SD) T= 13.33 (17.26), C= 21.42 (25.46) Difference ‐8.09 95% CI ‐14.34 to ‐1.85 |
|
Cost
Two trials reported a full evaluation of healthcare resources and costs (Patel 2004 for Kalra 2000; Wilson 1999); one of these trials included informal‐care costs (Patel 2004 for Kalra 2000). Admission avoidance hospital at home may slightly decrease treatment costs, although this benefit is offset when the costs of informal care are considered (287 participants, low‐certainty evidence).
Elderly participants with a medical condition
One trial reported a cost minimisation analysis (Wilson 1999), finding an initial increase in the mean cost per day for hospital at home (difference GBP 99.71, P < 0.001) and little or no difference in cost at three months' follow‐up (GBP ‐210.9, 95% CI GBP ‐1025 to GBP 635.47). When participants refusing their allocated place of care (T: n = 6/101, C: n = 23/97) were removed from the analysis, there was a reduction in costs for those receiving hospital at home for the initial episode of care (difference GBP ‐1070.53, 95% CI GBP ‐1843.2 to GBP ‐245.73) and at three months' follow‐up (difference GBP ‐1063.45, 95% CI GBP ‐2043 to GBP ‐162.7). The difference in mean cost per day between hospital at home and hospital care was reduced, although hospital at home care remained more costly per day (GBP 206.68 versus GBP 133.7, MD GBP 72.98, P < 0.001).
Another trial, recruiting mainly elderly participants with a mix of conditions, examined health service costs (Board 2000 secondary publication to Caplan 1999), using average costs and reported reduced health service costs for the intervention group (T: AUD 1764 (SD AUD 1253), C: AUD 3775 (SD AUD 2496) for an episode of care, MD per episode AUD ‐2011) and cost per day (T: AUD 191 (SD AUD 58), C: AUD 484 (SD AUD 67.23); MD AUD 293). The costs of the nurse co‐ordinator and hospital doctor involved were excluded from this analysis (see Analysis 1.14.1). Mendoza 2009 reported the mean (SD) cost at one‐year follow‐up (T: EUR 2541 (1334), C: EUR 4502 (2153); difference EUR 1961, P < 0.001).
1.14. Analysis.
Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 14 Resources and costs.
| Resources and costs | |||
|---|---|---|---|
| Study | Outcomes | Results | Notes |
| Health service resources and costs | |||
| Caplan 1999 | Cost | Average cost per episode, mean (SD)
T= $1,764 ($1,253), C= $3,775 ($2,496)
Mean difference per episode $‐2011
Cost per day, mean (SD)
T= $191 ($58), C= $484 ($67.23) Mean difference per day ‐$293 |
Cost data financial year 1995/1996 |
| Corwin 2005 | Days on oral antibiotics | HR 1.09 (0.82 to 1.45), P = 0.56 | |
| Mendoza 2009 | Cost | Mean (SD) T= €2,541 (1,334), C= €4,502 (2,153) Difference €1,961 P < 0.0001 |
Difference attributed to fewer investigations. Costs include health service costs used during follow‐up period of 1 year, excludes informal care. |
| Nicholson 2001 | Costs | Cost per episode, mean (95% C) T= $745 ($595 to $895),C= $2543 ($1766 to $3321) Difference $1798, P < 0.01 Hospital at home costs 29% of the average hospital managed patient episode. Reported cost effectiveness ratio of 3:1 T + C costs GP 10% of costs, Domiciliary allied health 21% of costs, community nursing 28% of costs = 59% of costs and hospital care 41% of costs. If C=$895 then T= $1287 (59% of costs) Total costs=$2182 per patient episode of care |
Costs based on financial year 99/00; Used average DRG costs (Australian $), patient data for ED costs, and modelled costs for OPD clinic visits. HAH care individual costs, included direct and non direct costs. GP costs at $91.00 per hour. |
| Ricauda 2004 | Mean total cost (EUR converted to US$ 1 Euro=$1.3) | T= $6 413.5 per patient, C= $6 504.8 per patient Cost per patient per day (SD) T= $163 (20.5), C= $275.6 (27.7) P < 0.001 | |
| Ricauda 2008 | Hospital at home resources Total costs |
Nursing visits (range) T= 14.1 (3 to 38) Physician visits T= 9.9 (2 to 28) Visits to hospital for diagnosis T= 11 Total mean cost per patient T=$1,175.9, C= $1,390.9, P = 0.38 Total mean cost per day (SD) T= $101.4 (61.3), C= 151.7 (96.4) |
|
| Richards 2005 | Cost based on DRGs for control and actual cost for intervention | Mean cost per patient NZ$ T= $1157.9, C= $1556.28 | |
| Wilson 1999 | Cost | Cost of initial episode (95% CI) T= £2,568.9 (2,089.3 to 2,972.1) C= £2,880.6 (2,316.1 to 3,547.8) Difference ‐311.7, P > 0.43 Bootstrap difference using 1000 subsamples: ‐304.72 (‐1,112.4 to 447.9). Mean cost per day (95% CI) T= £204.6 (91.5 to 118.4) C= £104.9 £ (181.1 to 228.22) Mean difference £99.71 P < 0.001 Cost at 3 months (95% CI) T= £3,671.3 (3,140.5 to 4,231.3) C= £3,876.9 (3,224.51 to 4,559.6) Difference ‐205.7, P > 0.65 Bootstrap difference using 1000 subsamples: ‐210.9 (‐1,025 to 635.5) COSTS EXCLUDING REFUSERS Cost of initial episode, mean (95% CI) T= £2,594.4 (£2,170.36 to £3,143.5) C= £3,659.20 (£3,140.46 to £4,231.28) Mean difference ‐£1,064.79, P < 0.01. Bootstrap mean difference £1070.53, (95% CI‐£1843.2 to ‐£245.73) 95% CI derived using bootstrap method with 1000 subsamples Cost per day, mean (95% CI) T= £206.68 (£183.21 to £230.14) C= £133.7 (£124.6 to £142.8) Mean difference £72.98, P < 0.001 Cost at 3 months, mean (95% CI) T= £3,697.5 (£3136.13 to £4330.66) C= £4,761.3 (£4105.6 to £5476.6) Mean difference ‐£1,063.8, p = 0.025 Bootstrap mean difference: £1,063.45 (95% CI ‐£2043.8 to ‐£162.7) | Cost data financial year 1995/1996 BNF for medicines 1995 |
| Use of other social services | |||
| Davies 2000 | While receiving hospital at home care, or on discharge from hospital | Referred for increased social support T= 24/100 (24%), C= 3/50 (6%) Difference 18%, 95% CI 7.3% to 28.6% |
|
| Informal care inputs | |||
| Kalra 2000 | Informal care inputs | Received informal care: T= 100/140 (71%), C= 98/147 (67%), Difference 4.8%, 95% CI ‐5.9% to 15.3% Total from co residents over 12 months, hours (SD) T= 899.18 (1760), C= 718 (6778), P = 0.75 Total hours per average week from co residents (SD) T=46.38 (48.15), C= 33.71 (44.35), P = 0.02 Total hours from nonresidents over 12 months (SD) T= 79.7 (283), C= 127.44 (348), P = 0.27 Total average hours per week from non residents T= 4.79 (16.51), C= 5.03 (11.54), P = 0.88 Total hours over 12 months (SD) T= 979 (1749), C= 846 (1549), P = 0.49 | |
Participants recovering from a stroke
A trial recruiting participants recovering from a stroke compared stroke unit care, inpatient stroke team care, and hospital at home. In terms of immediate care, hospital at home care was less costly than inpatient stroke team care (MD GBP ‐2096, 95% CI GBP ‐3272 to GBP ‐920). The inclusion of costs of informal care, based on the minimum wage, resulted in a MD of GBP ‐2216 (95% CI GBP ‐4771 to GBP 339) (Patel 2004 for Kalra 2000) (Analysis 1.14). In another trial recruiting participants with a stroke, a small reduction in mean cost per patient was reported for those allocated to hospital at home (USD 6413.5 versus USD 6504.8) (Ricauda 2004), which translated to a lower cost per day for hospital at home of USD 112.00 (USD 163.0, SD 20.5 versus USD 275.6, SD 27.7; P < 0.001).
COPD and community‐acquired pneumonia
One trial recruiting participants with COPD reported a lower mean health service cost for participants allocated to hospital at home; hospital costs were based on an average DRG (a diagnostic‐related group categorised by resource use) cost per bed day (cost per episode MD GBP ‐1798, P < 0.01) (Nicholson 2001). Another trial recruiting participants with community‐acquired pneumonia, again using DRG costs for the control and actual resource use for costing the intervention, reported a reduced cost for those allocated to hospital at home (mean cost per patient T: NZD 1157.9, C: NZD 1556.28) (Richards 2005). Ricauda 2008 reported the total mean cost per patient (T: USD 1175.9, C: USD 1390.9, P = 0.38) and the total mean cost per day (T: USD 101.4 (SD 61.3), C: USD 151.7 (SD 96.4)).
Use of other health services and informal care
Davies 2000 reported an increase in referrals for social support for participants with COPD who were allocated to hospital at home. This occurred during the time they were receiving hospital at home or when the control group had been discharged from hospital (24% versus 6%, difference 18%, 95% CI 7.3% to 28.6%) (Analysis 1.14.2). One trial recruiting participants recovering from a stroke reported that 71% (100/140) of those allocated to hospital at home received informal care, compared with 67% (98/147) receiving care from the inpatient stroke team (Patel 2004). This translated into 979 hours (SD 1749) versus 846 hours (SD 1549) of care over a 12‐month period (Analysis 1.14.3).
Discussion
Summary of main results
Admission avoidance hospital at home, with the option of transfer to hospital, may provide an effective alternative to inpatient care for a select group of elderly patients requiring hospital admission. Admission avoidance hospital at home probably makes little or no difference to the risk of death at six months' follow‐up or transfer to hospital (moderate‐certainty evidence); it may increase the likelihood of living at home (low‐certainty evidence). The increased satisfaction reported by patients allocated to hospital at home (low‐certainty evidence) must be balanced against the lack of evidence on the views of caregivers. Interviews with patients reveal that the most‐valued aspects of hospital at home care are the quality of communication and personal care received (Wilson 1999).
The total costs for the initial episode of care were estimated at 3 and 12 months after randomisation. We were not able to combine cost data due to the different ways costs had been calculated, and only two trials conducted a full economic evaluation (Jones 1999 for Wilson 1999; Patel 2004 for Kalra 2000). Hospital at home may decrease treatment costs slightly when compared with admission to an acute hospital ward (low‐certainty evidence). However, caregiver costs may offset this difference.
There was some variation in the way the admission avoidance hospital at home schemes operated. Admission avoidance hospital at home schemes admitted patients directly from the community (Harris 2005; Kalra 2000; Vianello 2013; Wilson 1999), outpatients (Talcott 2011), and from an accident and emergency department (Andrei 2011; Caplan 1999; Corwin 2005; Davies 2000; Mendoza 2009; Nicholson 2001; Ricauda 2004; Ricauda 2008; Richards 2005; Tibaldi 2004; Tibaldi 2009). Three trials evaluated interventions where the patient could be living alone, and five trials required a caregiver to be either living with the patient or nearby. Nonetheless, there were some important common features, which included care being co‐ordinated in each of the schemes by a multidisciplinary team; the provision of 24‐hour cover if required, with access to a doctor; and a safe home environment.
Overall completeness and applicability of evidence
The evidence indicates that admission avoidance hospital at home can provide an effective alternative to inpatient care for a select group of patients requiring hospital admission. However, determining the type of patients who are most likely to benefit is not simple. The majority of the included trials recruited participants who were elderly with a medical event, including stroke, COPD, and heart failure, that required admission to hospital. Their average age ranged from 70 to over 80 years. The patients opting to participate in the trials may prefer treatment at home, this may be why high levels of satisfaction are reported for those receiving healthcare at home. All trials but one, Andrei 2011, were conducted in high‐income countries.
Half of the trials excluded patients who did not have continuous family support (Caplan 1999; Mendoza 2009; Ricauda 2004; Ricauda 2008; Richards 2005; Tibaldi 2004; Tibaldi 2009; Vianello 2013). Although none of the included trials specifically looked into the socioeconomic characteristics of the excluded patients, it has been suggested that more disadvantaged areas have a higher percentage of informal caregivers (Young 2005). Three trials included participants from ethnic minorities, ranging from 14% to 20% of all recruited participants (Corwin 2005; Richards 2005; Talcott 2011); the authors did not report subgroup analysis for these participants.
Quality of the evidence
While we assessed the overall risk of bias as low, the studies included in this review were small, with the largest study recruiting 197 participants who contributed to the analysis. The meta‐analysis for the main outcome included a subgroup of six trials recruiting participants with similar conditions (older patients with a mix of medical conditions) to limit heterogeneity. A further concern is the role of chance and the fact that risk of publication bias cannot be ruled out.
Potential biases in the review process
We limited publication bias by conducting an extensive search that included different databases of published articles and sources of unpublished literature. Over the last 20 years we have established an international network of people working in this field who alert us to new randomised controlled trials. Two people screened all search results in order to reduce the risk of missing a study for inclusion, and the review authors discussed studies for possible inclusion to check that the inclusion criteria had been consistently applied.
Agreements and disagreements with other studies or reviews
Other reviews have analysed the effect of hospital at home schemes for patients with specific conditions, such as COPD and heart failure (Jeppesen 2012; Qaddoura 2015). For patients with COPD, it was reported that hospital at home reduced the number of readmissions when compared with hospital care, with inconclusive evidence for mortality, health‐related quality of life, cost, and clinical outcomes (Jeppesen 2012). The findings of a review of a small number of studies that specifically recruited patients with heart failure indicated a slight increase of time to readmission, improved health‐related quality of life, and reduced index costs, with limited evidence for mortality for those allocated to hospital at home. The authors judged these studies to be of modest quality (Qaddoura 2015).
Authors' conclusions
Implications for practice.
Problems can arise when comparisons are made between countries. The 16 trials included in this review came from Australia, Italy, New Zealand, Romania, Spain, the UK, and the US. Although the health systems in these countries vary with respect to the way healthcare financing is structured, the policy objectives are the same, with admission avoidance hospital at home being provided to control costs and reduce demand for inpatient hospital beds (Naik 2006). The level of existing primary care in a country, and the enthusiasm of local clinicians and healthcare managers, may determine the degree to which admission avoidance hospital at home operates as an outreach model or is run by supplementing existing primary care services. The way health care for the control group is organised will have an impact on outcome. The entry criteria required patients to be clinically stable and not requiring specialist diagnostic investigation or emergency interventions. Patients eligible for the trials included in this review did not include those whose condition was so severe that death was an expected outcome. Furthermore, patients whose condition unexpectedly deteriorated, or who could no longer be managed at home, had access to hospital admission. Interestingly, fewer behavioural problems were reported for those allocated to admission avoidance hospital at home in the trial recruiting patients with dementia, despite them experiencing serious cognitive and functional decline (Tibaldi 2004).
Although admission avoidance hospital at home provides an alternative to inpatient admission for some patients, the volume of such patients recruited to the included trials was low, and some of these patients will require access to hospital services, thus making the closure of a ward or hospital in favour of hospital at home an unrealistic option. Furthermore, the effectiveness of admission avoidance hospital at home may be reduced if hospital admission incorporates aspects of care known to be effective for these groups of patients, such as stroke unit care or comprehensive geriatric assessment (Stroke Unit Trialist; Stuck 1993).
It should be noted that admission avoidance hospital at home does not totally substitute for hospital, in that admission to hospital remains an option if required. One trial recruiting participants recovering from a stroke reported that 51 out of 153 (33%) of the patients allocated to hospital at home received inpatient care within two weeks of randomisation (Kalra 2000). In another trial, a small proportion (6 out of 101; 6%) refused hospital at home care and were admitted to hospital (Wilson 1999).
Implications for research.
Over the last 15 years the randomised evidence has grown from 1 to 16 trials, despite the practical difficulties of conducting randomised controlled trials of service innovations, which is encouraging. However, the continuing lack of functional outcome measures, failure to record place of residence at follow‐up, and absence of economic evaluations are disappointing. Future research of admission avoidance hospital at home should continue to measure mortality, readmission (with particular attention to the transfer of patients between admission avoidance hospital at home and inpatient care), and place of residence at follow‐up. In addition, clinical and dependency data of recruited patients should be collected using standardised measures to facilitate the application of evidence. Trials should also include a formal, planned economic analysis using costs that are sensitive to the different resources used during an episode of care. Comparisons with other ways of organising inpatient care, for example incorporating aspects of case management and comprehensive assessment, would also improve the evidence supporting decisions about how services should be organised to maximise health outcomes. The effect of the intervention for patient groups considered to be at higher risk of hospital admission should also be considered. Some of the factors to take into account are age, social circumstances, and comorbidities. Finally, the role of advanced portable medical devices and communication technologies in admission avoidance hospital at home could be explored in pilot studies.
What's new
| Date | Event | Description |
|---|---|---|
| 2 March 2016 | New search has been performed | New searches performed. Six new trials identified. |
| 2 March 2016 | New citation required but conclusions have not changed | Daniela C Gonçalves‐Bradley is a new author; the review includes 16 trials (6 trials included in this update). Methods have been updated to align with current Cochrane guidance. |
History
Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 6 July 2011 | Amended | Reference revised to published review. |
| 8 June 2011 | Amended | Title changed for consistency and changes to published notes. |
| 17 February 2010 | Amended | Change to published notes. |
| 1 August 2008 | New search has been performed | This review is an updated search and partial update from the original review (Shepperd 1998). Shepperd 1998 has been split into three reviews, of which this is one. |
| 10 July 2008 | Amended | Converted to new review format. |
Notes
This review is the third update; the original review was first published in Issue 1, 1998 of the Cochrane Library (Shepperd 1998).The original review has been separated into three distinct reviews: Hospital at home admission avoidance, Hospital at home early discharge, and Hospital at home: home‐based end‐of‐life care. The titles have been changed for consistency. Hospital at home early discharge, Shepperd 2009, and Hospital at home: home‐based end‐of‐life care, Shepperd 2011, are published in the Cochrane Library.
Acknowledgements
Dr Roger Harris and Joanne Broad for contributing data from the New Zealand trial to the individual patient data meta‐analysis. Robert M Angus for collaborating on previous versions of the review. Nia Roberts and Paul Miller for updating and conducting the searches, and Julia Worswick for editorial support. We would also like to thank the peer reviewers for their helpful comments: Craig Ramsay, Paul Miller, Orlaith Burke, Christopher Cates, Julia Worswick, and Tomas Pantoja (internal Effective Practice and Organisation of Care (EPOC) editor). This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding and a Cochrane programme grant to the EPOC Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Search strategies
Ovid MEDLINE(R) and Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations (1946 to 2 March 2016)
1 (hospital adj2 home).tw.
2 home based versus hospital based.tw.
3 home hospitalization.tw.
4 exp Home Care Services/
5 exp Hospitalization/
6 4 and 5
7 1 or 2 or 3 or 6
8 randomized controlled trial.pt.
9 controlled clinical trial.pt.
10 randomized.ab.
11 placebo.ab.
12 drug therapy.fs.
13 randomly.ab.
14 trial.ab.
15 groups.ab.
16 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
17exp animals/ not humans.sh.
18 16 not 17
19 7 and 18
EMBASE (1974 to 1 March 2016)
1 (hospital adj2 home).tw.
2 home hospitalization.tw.
3 home based versus hospital based.tw.
4 exp home care/
5 hospitalization/
6 4 and 5
7 1 or 2 or 3 or 6
8 clinical trial/
9 randomization/
10 randomized controlled trial/
11 crossover procedure/
12 double blind procedure/
13 single blind procedure/
14 (randomised or randomized).tw.
15 placebo/
16 (controlled adj study).tw.
17 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16
18 7 and 17
19 nonhuman/
20 18 not 19
CINAHL ‐ Cumulative Index to Nursing & Allied Health Literature (1982 to 2 March 2016)
1 TI hospital N2 home OR AB hospital N2 home
2 TI Home‐based versus hospital‐based OR AB Home‐based versus hospital‐based
3 TI Home hospitalization OR AB Home hospitalization
4 (MH "Home Health Care")
5 (MH "Hospitalization")
6 4 AND 5
7 1 OR 2 OR 3 OR 6
8 (MH "Clinical Trials+")
9 PT clinical trial
10 TI ( controlled trial or controlled study ) OR AB ( controlled trial or controlled study )
11 TI ( randomised or randomized ) OR AB ( randomised or randomized )
12 TI ( (random* N1 (allocat* or assign*)) ) OR AB ( (random* N1 (allocat* or assign*)) )
13 8 OR 9 OR 10 OR 11 OR 12
14 6 AND 13
Cochrane Central Register of Controlled Trials (Issue 2 of 12, 2016)
#1 hospital near/2 home:ti,ab,kw (Word variations have been searched)
#2 home hospitalization:ti,ab,kw (Word variations have been searched)
#3 Home‐based versus hospital‐based :ti,ab,kw (Word variations have been searched)
#4 #1 or #2 or #3
EconLit (1969 to 2 March 2016)
S5 (hospital NEAR/2 home) OR "home hospitalization" OR "home based versus hospital based"Limits applied
S4 (hospital NEAR/2 home) OR "home hospitalization" OR "home based versus hospital based"
S3 "home based versus hospital based"
S2 "home hospitalization"
S1 hospital NEAR/2 home
Appendix 2. Search strategy for EPOC register
21 February 2007
home‐based near in‐patient*
homecare near in‐patient*
hospital‐at‐home near in‐patient*
home‐based near hospital*
homecare or home‐care near hospital*
home hospital* and in‐patient*
21 January 2008 revision
home‐based and in‐patient*
homecare and in‐patient*
hospital‐at‐home and in‐patient*
home‐based and hospital*
homecare and hospital*
home‐care and hospital*
home hospital* and in‐patient*
Data and analyses
Comparison 1. Admission avoidance hospital at home versus inpatient care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at 3 months using IPD | 5 | mortality (Fixed, 95% CI) | 0.77 [0.54, 1.09] | |
| 2 Mortality at 6 months' follow‐up (using data from trialists, apart from Caplan) | 6 | 912 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.99] |
| 3 Readmission or transfer to hospital while receiving hospital at home | Other data | No numeric data | ||
| 4 Readmission to hospital for patients with a medical condition at an average of 5 months' follow‐up | 7 | 834 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.23] |
| 4.1 Readmission for older patients with a medical condition using IPD and published data | 7 | 834 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.23] |
| 5 Functional status | Other data | No numeric data | ||
| 5.2 Admission avoidance patients with a medical condition ‐ functional ability | Other data | No numeric data | ||
| 6 Quality of life/health status | Other data | No numeric data | ||
| 6.1 Admission avoidance quality of life | Other data | No numeric data | ||
| 7 Cognitive function and depression | Other data | No numeric data | ||
| 7.1 admission avoidance ‐ cognitive function/well being | Other data | No numeric data | ||
| 8 Clinical outcomes | Other data | No numeric data | ||
| 8.1 Clinical outcomes | Other data | No numeric data | ||
| 9 Place of residence at follow‐up (living in residential care) | 5 | 727 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.22, 0.57] |
| 9.1 With a medical condition (6 months follow up) | 5 | 727 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.22, 0.57] |
| 10 Patient satisfaction | Other data | No numeric data | ||
| 11 Care giver outcomes | Other data | No numeric data | ||
| 11.1 Care giver satisfaction | Other data | No numeric data | ||
| 12 Views of health professionals | Other data | No numeric data | ||
| 13 Length of stay | Other data | No numeric data | ||
| 13.1 Hospital and hospital at home length of stay | Other data | No numeric data | ||
| 14 Resources and costs | Other data | No numeric data | ||
| 14.1 Health service resources and costs | Other data | No numeric data | ||
| 14.2 Use of other social services | Other data | No numeric data | ||
| 14.3 Informal care inputs | Other data | No numeric data |
1.3. Analysis.
Comparison 1 Admission avoidance hospital at home versus inpatient care, Outcome 3 Readmission or transfer to hospital while receiving hospital at home.
| Readmission or transfer to hospital while receiving hospital at home | ||
|---|---|---|
| Study | Outcomes | Results |
| Corwin 2005 | Transfer to hospital | T= 11/98, C=3/96 |
| Kalra 2000 | Readmission within 2 weeks of randomisation | T= 51/149 |
| Ricauda 2008 | Transferred to acute hospital | T= 3/52 |
| Richards 2005 | T= 2/24, C= 1/25 | |
| Talcott 2011 | Readmission to hospital while receiving hospital at home | T= 4/47, C= 0/66 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andrei 2011.
| Methods | Parallel RCT | |
| Participants | Setting: Romania People with chronic heart failure that had deteriorated at a minimum of 1 week prior to recruitment. Number of participants in each group was not reported, a total of 45 participants recruited |
|
| Interventions | Admission avoidance hospital at home; the first 48 hours of treatment was in the emergency department | |
| Outcomes | Mortality, biological measures, and cost | |
| Notes | Follow‐up at 1, 3, 6, and 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was published as an abstract, details not reported |
| Allocation concealment (selection bias) | Unclear risk | The study was published as an abstract, details not reported |
| Baseline outcome measurements (selection bias) | Unclear risk | The study was published as an abstract, details not reported |
| Baseline characteristics (selection bias) | Unclear risk | The study was published as an abstract, details not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Methods not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study was published as an abstract, details not reported |
| Selective reporting (reporting bias) | Unclear risk | The study was published as an abstract, details not reported |
Caplan 1999.
| Methods | Parallel RCT | |
| Participants | Setting: Australia Range of acute conditions requiring admission to hospital; participants recruited from casualty. Number recruited: hospital at home = 51; hospital = 49 |
|
| Interventions | Hospital community outreach team Type of service: Hospital community outreach team. Clinical responsibility by GP or hospital doctor if GP declined |
|
| Outcomes | Functional status, mental status, clinical complications, patient and caregiver satisfaction, GP views | |
| Notes | Follow‐up: 1 and 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers, stratified by participant's residence and if they had a deep vein thrombosis |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for functional and mental status, and diagnoses; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of treatment and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Unclear risk for functional status, mental status, clinical complications, patient and caregiver satisfaction, GP view |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for mortality, readmission |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Corwin 2005.
| Methods | Parallel RCT | |
| Participants | Setting: New Zealand Patients with cellulitis Number recruited: treatment: 98, control: 96 Ages mean (SD): T: 54.6 (20.6), C: 48.4 (19) European: T: 77/98 (79%), C: 78/96 (81%) Maori: T: 10/98 (10%), C: 5/96 (5%) Pacific: T: 2/98 (2%), C: 1/96 (1%) Other: T: 9/98 (9%), C: 13/96 (12%) |
|
| Interventions | Hospital at home admission avoidance from the emergency department. Run by Pegasus Health, an independent practitioner's association for 230 GPs in Christchurch, New Zealand. Care provided by GP and community care nursing staff. Patients required IV antibiotics for cellulitis | |
| Outcomes | Advancement of cellulitis, readmission, days on IV antibiotics, functional outcomes (SF‐36), patient satisfaction | |
| Notes | Follow up: 3 and 6 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation list produced by SAS code |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation service |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for functional outcomes; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of treatment and control groups are reported and similar for main characteristics |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Unclear risk for pain, functional and physical health (SF‐36), and satisfaction |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for advancement of cellulitis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 from each group excluded at follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Davies 2000.
| Methods | Parallel RCT | |
| Participants | Setting: UK Patients with chronic obstructive airways disease Number recruited: hospital at home: 100, hospital: 50 |
|
| Interventions | Hospital at home Type of service: admission avoidance from accident and emergency department. Care provided by outreach specialist nurses and GP and community nurses if required |
|
| Outcomes | Respiratory function, readmission, quality of life | |
| Notes | Few details on measure of quality of life | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Ratio of 2:1 (hospital at home: hospital) |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for respiratory function; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel not possible Hospital readmission was a primary outcome, with decision to admit made by hospital staff |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | A small group of participants completed the St George's Respiratory Questionnaire |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for admission to hospital, changes in FEV1 score |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: hospital at home: 7/100; hospital: 5/50 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (received trial data set) |
Harris 2005.
| Methods | Parallel RCT | |
| Participants | Setting: New Zealand
Patients had a broad range of diagnoses: fractures (28%); miscellaneous medical problems (18%); respiratory problems (16%); stroke and neurological diagnoses (14%); falls and injuries (11%); cardiac diagnoses (8%); and rehabilitation and other problems (5%). Number recruited: hospital at home: 39, hospital: 37 |
|
| Interventions | Operated as a hospital outreach programme under the management of Auckland Hospital from the emergency department or acute assessment ward. A nurse‐led multidisciplinary team (physiotherapy, occupational therapy, social work) co‐ordinated care and rehabilitation for the patient within the patient's own home. There was a daily nursing review. Clinical responsibility was held by a dedicated hospital at home registrar, a consultant geriatrician, and in some cases the patient's GP, with 24‐hour on‐call medical cover. The service provided care 7 days a week with 10 hours nursing care a day available, and a 24‐hour live‐in home caregiver if required. There was a daily nursing review, and a discharge hand over to ongoing support services | |
| Outcomes | Activities of daily living, cognitive function, instrumental activities of daily living | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation service |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation service |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for activities of daily living, instrumental activities of daily living, and cognitive functioning; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel not possible Research staff did not make decision to admit to hospital |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Trained researchers not involved in patient care assessed participants for functional independence (FIM), cognitive function (MMSE), and instrumental activities of daily living (OARS) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for mortality, readmission, and measurement and valuation of costs |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants withdrawing: hospital at home: 4/143; hospital: 10/124 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (received trial data set) |
Kalra 2000.
| Methods | Parallel RCT | |
| Participants | Setting: UK Patients recovering from a moderately severe stroke Number recruited: hospital at home: 153; stroke unit care: 152; hospital care: 152 Median age (IQR): T: 75 (72 to 84), C: 77.7 (67 to 83) Living alone: T: 50/148 (34%), C: 50/149 (34%) |
|
| Interventions | Hospital outreach admission avoidance multidisciplinary with joint care from community services | |
| Outcomes | Mortality, institutionalisation, level of independence, activities of daily living, treatment inputs, readmission, hospital length of stay, cost | |
| Notes | Follow‐up: 3, 6, and 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for level of independence and activities of daily living; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and personnel not possible Primary outcome: death |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | A trained researcher, independent of the health care provided and unaware of treatment allocation, assessed functional status (Barthel and Rankin scale) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for mortality, institutionalisation, resource use and cost |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: hospital at home: 9/144; hospital: 3/152 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (received trial data set) |
Mendoza 2009.
| Methods | Parallel RCT | |
| Participants | Setting: Spain Patients in A&E with acute decompensation of chronic heart failure. Inclusion criteria: > 65 years of age, with heart failure for 12 at least months prior to study, NYHA functional class II or III prior to acute episode, all‐day supervision available, telephone, < 10k from hospital. Age > 65 years, mean age 79. 29% female in hospital arm, 51% female in hospital at home arm. Number recruited (between May 2006 and March 2007): hospital at home: 37; hospital: 34 |
|
| Interventions | Admission avoidance hospital at home, patients admitted from the emergency departments, hospital outreach model. Hospital at home nurse visited within 12 to 24 hours of admission to hospital at home and then daily visits. Care available between 8 am and 9 pm; patients called emergency services outside these hours. Hospital specialist visited daily or every other day, depending on the patient’s condition. Control group: admitted to hospital |
|
| Outcomes | Mortality, readmission, functional status, general health status, length of stay, costs | |
| Notes | Follow‐up 1 year after discharge | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation described as "externally generated sequence." |
| Allocation concealment (selection bias) | Low risk | Random sequence hidden from the physician until patient had consented to participate |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for functional status and general health status; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | A trained researcher, independent of the health care provided, assessed functional status (Barthel Index), health‐related quality of life (SF‐36) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for death, readmission |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 of 80 did not complete the study (2 in hospital at home group, 7 in hospital group) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Nicholson 2001.
| Methods | Parallel RCT | |
| Participants | Setting: Australia Patients with COPD Inclusion criteria: age > 45 years, COPD, current or ex‐smoker, FEV1 < 60% predicted, admission requested by GP or OPD clinic staff or ED staff, telephone at home Number recruited: hospital at home: 13; hospital: 12 |
|
| Interventions | Hospital at home (discharge from emergency department) Patients retained in patient status and received clinical supervision from hospital specialist, and hospital had legal and financial responsibility; also received care from GP, community nursing, and domiciliary care. Hospital medical staff provided 24‐hour telephone support |
|
| Outcomes | Cost to the health service | |
| Notes | Follow‐up: duration of care in hospital at home or inpatient care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Baseline outcome measurements (selection bias) | Unclear risk | Baseline outcome measurements not reported |
| Baseline characteristics (selection bias) | Unclear risk | Baseline characteristics of the study and control groups not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | None reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for resource use and cost |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to allocate low or high risk |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Ricauda 2004.
| Methods | Parallel RCT | |
| Participants | Setting: Italy Patients recovering from a stroke Eligibility criteria: patients admitted to hospital within 24 hours of the onset of symptoms and evaluated for at least 24 hours Age (IQR): 76 to 88, median 82 Number recruited: hospital at home: 60; hospital: 60 |
|
| Interventions | Hospital outreach admission avoidance. 24‐hour care available multidisciplinary team: physiotherapist, occupational therapist, nursing, hospital geriatrician, social worker, speech therapist, psychologist |
|
| Outcomes | Length of treatment, mortality, activities of daily living, functional impairment, depression, costs | |
| Notes | Follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for activities of daily living, functional impairment, and depression; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Outcomes assessor blinded to the study allocation, and used validated measures of outcome |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcomes assessor blinded to the study allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Ricauda 2008.
| Methods | Parallel RCT | |
| Participants | Setting: Italy Patients requiring acute hospitalisation for acute exacerbation of COPD; care supervision at home; telephone connection; living in the catchment area; family or social support Age (SD): T: 80.1 (3.2), C: 79.2 (3.1) Number recruited: hospital at home: 52; inpatient: 52 |
|
| Interventions | Physician‐led admission avoidance hospital outreach service; geriatric home hospitalisation service (GHHS) of a regional hospital. The home care program emphasised patient and caregiver education about the disease, advice about smoking cessation, nutrition, management of activities of daily living and energy conservation, understanding and use of drugs, health maintenance, and early recognition of triggers of exacerbation that required medical intervention. In the first days after admission to GHHS, physicians and nurses visited each patient at home daily, then daily visits by the nurse and visits by the doctor every 2 to 3 days or less. Blood tests, electrocardiogram, spirometry, pulse oximetry, oxygen, IV fluids, antimicrobials and other medications, blood transfusions, surgical treatment for pressure ulcers were available. Control group: inpatient hospital care |
|
| Outcomes | Mortality, readmission, health status, satisfaction, residential care, length of stay, resource use and cost, caregiver outcomes | |
| Notes | Follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | The project manager randomly allocated participants using a numbered set of sealed envelopes |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for health status; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Data collected by independent postgraduate physicians who were not involved in the care of patients or the research team |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Outcomes assessed by a postgraduate doctor not involved with delivery of health care, and blinded to the study allocation |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcomes assessed by a postgraduate doctor not involved with delivery of health care, and blinded to the study allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Standard set of outcomes reported |
Richards 2005.
| Methods | Parallel RCT | |
| Participants | Setting: New Zealand Patients with community‐acquired pneumonia Age: T: 50.1, C: 49.8 Number recruited: hospital at home: 24; hospital: 25 |
|
| Interventions | Hospital at home: admission avoidance from emergency room. Run by Pegasus Health, an independent practitioner's association for 230 GPs in Christchurch, New Zealand. Care provided by GP and community care nursing staff | |
| Outcomes | Median number of days to discharge, days of IV antibiotics, functional outcomes, mortality, readmission, patient satisfaction, costs | |
| Notes | Follow‐up: 2 and 6 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for functional outcomes; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Patient‐rated symptoms, satisfaction |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Days on IV antibiotics, admissions extracted from clinical records |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 exclusions after randomisation, no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Talcott 2011.
| Methods | Parallel RCT | |
| Participants | Setting: US Participants: outpatients with postchemotherapy febrile neutropenia and assessed as low risk Age 20 to 81 years, median 47 years Number recruited: hospital at home: 47; inpatient: 66 |
|
| Interventions | Admission avoidance hospital at home, patients recruited from outpatient clinic. Provided by commercial home care provider, daily visits by a home care nurse who followed a protocol/standard checklist and contacted the primary care physician if there were abnormal findings. Daily blood tests. 24‐hour care available. Hospital specialist examined the patient 2 to 4 days following discharge and then at least weekly. Home IV available. Control group received care in oncology units in general hospitals |
|
| Outcomes | Major medical complications, readmission to hospital, quality of life | |
| Notes | Folllow‐up time for each episode was the resolution of fever, neutropenia, and any complications arising during the episode. Quality‐of‐life data were collected at the time of consent to join the study and as soon as possible after the resolution of the episode | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocks of random numbers stratified by colony‐stimulating factors, institution, and whether random assignment occurred on weekends, holidays, or after hours |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for clinical characteristics and quality of life; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar for all main characteristics |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Any medical event requiring urgent diagnostic or therapeutic intervention. Predefined complications included systemic hypotension (systolic blood pressure > 90 mmHg), respiratory failure (partial pressure of oxygen < 60 torr adjusted for hyperventilation) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Predefined medical complications using blinded review |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for mortality and cost |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up, 5 withdrawn/excluded (3 withdrew consent, 2 excluded as neutropenia had resolved at recruitment) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Tibaldi 2004.
| Methods | Parallel RCT | |
| Participants | Setting: Italy Patients: elderly with advanced dementia Mean age (SD): T: 82.9 (7.9), C: 84.1 (7.5) Number recruited: hospital at home: 56; inpatient: 53 |
|
| Interventions | Hospital at home run by S. Giovanni Battista Hospital, Turin, Italy: geriatric home hospitalisation service, patients referred from emergency department. 24‐hour‐a‐day care available, home nursing multidisciplinary care, rapid access to equipment |
|
| Outcomes | Behavioural disturbances, number of patients treated with antipsychotic drugs on admission and on discharge, mortality, length of stay, place of discharge (home or to a nursing home) | |
| Notes | Follow‐up: to discharge from service 4 admitted from hospital at home to hospital for new medical problems |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for cognitive status, severity of disease, and activities of daily living; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Assessment method not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Low risk for mortality |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals not reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Tibaldi 2009.
| Methods | Parallel RCT | |
| Participants | Setting: Italy People with acute decompensation of chronic heart failure recruited within 12 to 24 hours of admission to the emergency department. Care supervision possible at home, telephone at home, need for IV infusions, living in catchment area, at least 1 previous admission for chronic heart failure Age: 75 years and over, mean age 81 Number recruited: hospital at home: 48; hospital: 53 |
|
| Interventions | Admission avoidance hospital at home, hospital outreach (hospital maintains legal and financial responsibility); 24‐hour care available 7 days a week; 4 specialist geriatricians, home care nurses, physiotherapist, social worker, counsellor, IV infusions available. Control group: admission to San Giovanni Battista Hospital, Turin, Italy |
|
| Outcomes | Mortality, readmission, length of stay, residential care, health status, psychological well‐being | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed envelopes |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for health status and psychological well‐being; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Outcomes assessed by a postgraduate doctor not involved with delivery of health care, and blinded to the study allocation |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcomes assessed by a postgraduate doctor not involved with delivery of health care, and blinded to the study allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4% loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Standard set of outcomes reported |
Vianello 2013.
| Methods | Parallel RCT | |
| Participants | Setting: Italy Patients with neuromuscular disease and who had an acute respiratory tract infection and required hospital admission; recruited between January 2009 and December 2011. Number recruited: hospital at home: 26; inpatient hospital: 27 |
|
| Interventions | The use of a portable ventilator; a respiratory therapist made daily visits for the first 3 days of home care, and district nurses and caregivers were trained in the application of the device and on assisting with coughing. District nurses visited daily until recovery from the respiratory tract infection, participants also had telephone access to pulmonary specialists | |
| Outcomes | Recovery from exacerbation defined as relief of respiratory distress and return of SpO2 level | |
| Notes | 3 months' follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for respiratory distress and SpO2 level; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Subjective outcomes not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for mortality, need for intubation, and cost |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up data reported in Table 3, page 2066; the authors did not report loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to allocate low or high risk |
Wilson 1999.
| Methods | Parallel RCT | |
| Participants | Setting: UK
Patients with a mix of conditions (majority elderly) referred by GP to Bed Bureau Number recruited: hospital at home: 102; inpatient: 97 |
|
| Interventions | Hospital at home (admission avoidance) Type of service: multidisciplinary team (nurses, therapy, generic health workers, cultural link worker) Maximum of 5 patients at a time Control group: inpatient hospital care |
|
| Outcomes | Mortality, readmission, functional status, quality of life, patient satisfaction | |
| Notes | Follow‐up: 3 days, 2 weeks, 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed envelopes |
| Baseline outcome measurements (selection bias) | Low risk | Baseline outcome measurements done prior to intervention for functional status and quality of life; no relevant differences found |
| Baseline characteristics (selection bias) | Low risk | Baseline characteristics of the study and control groups are reported and are similar |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Outcome assessments by independent research staff, decision to admit made by hospital staff, not research team |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Low risk for health status (Sickness Impact Profile 68), cognitive function (Clifton Assessment Procedures for the Elderly), functional status (Barthel Index), and quality of life (EuroQol) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Low risk for mortality, readmission, resource use and cost |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: hospital at home: 8/87; hospital: 5/80 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported (received trial data set) |
A&E: accident & emergency department C: control COPD: chronic obstructive pulmonary disease ED: emergency department FEV1: forced expiratory volume at 1 second FIM: Functional Independence Measure GHHS: geriatric home hospitalisation service GP: general practitioner IQR: interquartile range IV: intravenous MMSE: mini–mental state examination NYHA: New York Heart Association OARS: Older Americans' Resources and Services OPD: outpatient department RCT: randomised controlled trial SD: standard deviation SF‐12: 12‐Item Short Form Health Survey SF‐36: 36‐Item Short Form Health Survey T: treatment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| King 2000 | Cross‐over randomised controlled trial evaluating chemotherapy provided in a home setting with an outpatient clinic setting |
| Wade 1985 | Clinical controlled trial Compared 2 districts, 1 with a domiciliary stroke service and 1 without |
| Wolfe 2000 | Intervention does not substitute for inpatient care |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN29082260.
| Trial name or title | Randomised controlled trial of hospital at home compared to standard inpatient management of patients with an acute exacerbation of chronic obstructive pulmonary disease (AECOPD), triaged for hospital admission by Accident and Emergency and with low mortality risk according to the novel DECAF score (HOT DECAF) |
| Methods | Parallel RCT; economic evaluation; qualitative interviews |
| Participants | Adults >= 35 years admitted with acute exacerbation of COPD and assessed as low risk by a clinical scoring system |
| Interventions | Patients allocated to hospital at home will remain under the care of the hospital team, with 24/7 on‐call support. Home treatment will comprise of twice daily respiratory specialist nurse visits supervised by a respiratory consultant, with additional input from physiotherapy, occupational therapy, and formal social care as required. Comparison: usual care |
| Outcomes | Main outcomes: health and social care costs over 90 days (non‐inferiority analysis) Other outcomes: survival; all‐cause and respiratory readmission rates; bed days over a) acute period of care, and b) postdischarge to 90 days; caregiver and patient preference; COPD exacerbations; unplanned health resource use; hospital anxiety and depression score; quality of life; caregiver burden; perceptions of health care of patients and their caregivers and health professionals with regards to the use of the clinical score for group allocation |
| Starting date | 28 April 2014 |
| Contact information | |
| Notes | ISRCTN29082260 |
ISRCTN60477865.
| Trial name or title | A multi‐centre randomised controlled trial to compare the effectiveness of admission avoidance hospital at home with comprehensive geriatric assessment vs. inpatient comprehensive geriatric assessment on the number of frail older people ‘living at home’ (RCT of comprehensive geriatric assessment in a hospital at home setting) |
| Methods | Parallel RCT; economic evaluation; qualitative interviews |
| Participants | Older people with markers of frailty or prior dependence who have been referred to admission avoidance hospital at home for an acute medical event |
| Interventions | Intervention: specialist‐led service providing assessment and a tailored management plan, multidisciplinary management, and co‐ordinated care in the patient’s own home. Comparison: care as usual |
| Outcomes | Main outcome: living at home (the inverse of death or living in a residential care setting) Follow‐up at 6 and 12 months Other outcomes: activities of daily living; cognitive impairment; delirium; mortality; new long‐term residential care; quality of life; resource use; transfer to hospital |
| Starting date | 30 November 2014 |
| Contact information | |
| Notes | ISRCTN60477865 |
DECAF: Dyspnea, Eosinopenia, Consolidation, respiratory Acidosis and atrial Fibrillation RCT: randomised controlled trial
Differences between protocol and review
We made a posteriori decisions on how to analyse missing data. We updated the methods to align with current Cochrane guidance (MECIR 2012). The authorship has changed since the publication of the protocol, with the addition of Helen Doll, Mike J Clarke, Lalit Kalra, Nicoletta Aimonino Ricauda, Andrew D Wilson, and Daniela C Gonçalves‐Bradley.
Contributions of authors
SS co‐ordinated the review, screened records, extracted data, analysed the results (with HD), and lead the writing of the review.
SI screened records, extracted data, and commented on each draft of the review.
DGB screened records, extracted data, and contributed to the writing of the review.
HD, MJC, LK, and ADW all contributed to interpretation of data and writing the review.
Two of the review authors (LK, AW) contributed trial data to the individual patient data meta‐analysis.
Sources of support
Internal sources
No sources of support supplied
External sources
NIHR Research Scientist in Evidence Synthesis Award to Sasha Shepperd supported this work, Other.
Declarations of interest
SS None known.
SI None known.
HD None known.
MC None known.
LK None known.
AW None known.
DGB None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andrei 2011 {published data only}
- Andrei CL, Sinescu CJ, Ianula RM, Popa AI, Chioncel VP, Mischie AN, Adam FC, Dasoveanu M, Mincu DE, Grigorean VT. Can be the home care of the heart failure patients a better economic alternative?. European Journal of Heart Failure Supplements (Abstract). 2011;10(S1):S29. [Google Scholar]
Caplan 1999 {published data only}
- Board N, Brennan N, Caplan G. A randomised controlled trial of the costs of hospital as compared with hospital at home for acute medical patients. Australian and New Zealand Journal of Public Health 2000;24(3):305‐11. [PUBMED: 10937409] [DOI] [PubMed] [Google Scholar]
- Caplan GA, Coconis J, Woods J. Effect of hospital in the home treatment on physical and cognitive function: a randomized controlled trial. Journal of Gerontology 2005;60(8):1035‐8. [PUBMED: 16127109] [DOI] [PubMed] [Google Scholar]
- Caplan GA, Ward JA, Brennan NJ, Coconis J, Board N, Brown A. Hospital in the home: a randomised controlled trial. The Medical Journal of Australia 1999;170(4):156‐60. [PUBMED: 10078179] [PubMed] [Google Scholar]
Corwin 2005 {published data only}
- Corwin P, Toop L, McGeoch G, Than M, Wynn‐Thomas S, Wells JE, et al. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospital. BMJ 2005;330(7483):129. [PUBMED: 15604157] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2000 {published data only}
- Davies L, Wilkinson M, Bonner S, Calverley PM, Angus RM. Hospital at home versus hospital care in patients with exacerbations of chronic obstructive pulmonary disease: prospective randomised controlled trial. BMJ 2000;321(7271):1265‐8. [PUBMED: 11082090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2005 {published and unpublished data}
- Harris R, Ashton T, Broad J, Connolly G, Richmond D. The effectiveness, acceptability and costs of a hospital‐at‐home service compared with acute hospital care: a randomised controlled trial. Journal of Health Services and Research Policy 2005;10(3):158‐66. [PUBMED: 16053592] [DOI] [PubMed] [Google Scholar]
Kalra 2000 {published data only}
- Kalra L, Evans A, Perez I, Knapp M, Donaldson N, Swift CG. Alternative strategies for stroke care: a prospective randomised controlled trial. The Lancet 2000;356(9233):894‐9. [PUBMED: 11036894] [DOI] [PubMed] [Google Scholar]
- Patel A, Knapp M, Perez I, Evans A, Kalra L. Alternative strategies for stroke care: cost effectiveness and cost‐utility analyses from a prospective randomized controlled trial. Stroke 2004;35(1):196‐203. [PUBMED: 14684783] [DOI] [PubMed] [Google Scholar]
Mendoza 2009 {published data only}
- Mendoza H, Martin MJ, Garcia A, Aros F, Aizpuru F, Regalado de Los Cobos J, et al. Hospital at home care model as an effective alternative in the management of decompensated chronic heart failure. European Journal of Heart Failure 2009;11:1208‐13. [DOI] [PubMed] [Google Scholar]
Nicholson 2001 {published data only}
- Nicholson C, Bowler S, Jackson C, Schollay D, Tweeddale M, O'Rourke P. Cost comparison of hospital‐ and home‐based treatment models for acute chronic obstructive pulmonary disease. Australian Health Review 2001;24(4):181‐7. [PUBMED: 11842709] [DOI] [PubMed] [Google Scholar]
Ricauda 2004 {published data only}
- Ricauda NA, Bo M, Molaschi M, Massaia M, Salerno K, Amati D, et al. Home hospitalization service for acute uncomplicated first ischemic stroke in elderly patients: a randomized trial. Journal of the American Geriatrics Society 2004;52(2):278‐83. [PUBMED: 14728641] [DOI] [PubMed] [Google Scholar]
- Ricauda NA, Pla LF, Marinello M, Molaschi M, Fabris F. Feasibility of an acute stroke home care service for elderly patients. Archives of Gerontology and Geriatrics 1998;26(Suppl 6):17‐22. [EMBASE: 1998333220] [Google Scholar]
Ricauda 2008 {published data only}
- Ricauda NA, Tibaldi V, Leff B, Scarafiotti C, Marinello R, Zanocchi M, et al. Substitutive hospital at home versus inpatient care for elderly patients with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Journal of the American Geriatrics Society 2008;56:493‐500. [DOI] [PubMed] [Google Scholar]
Richards 2005 {published data only}
- Richards DA, Toop LJ, Epton MJ, McGeoch RB, Town GI, Wynn‐Thomas SMH, et al. Home management of mild to moderately severe community‐acquired pneumonia: a randomised controlled trial. The Medical Journal of Australia 2005;183(5):235‐8. [PUBMED: 16138795] [DOI] [PubMed] [Google Scholar]
Talcott 2011 {published data only}
- Hendricks AM, Loggers ET, Talcott JA. Costs of home versus inpatient treatment for fever and neutropenia: analysis of a multicenter randomized trial. Journal of Clinical Oncology 2011;29:3984‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talcott JA, Yeap JA, Siegal RD, Loggers ET, Lu C, Godley PA. Safety of early discharge for low risk patients with febrile neutropenia: A multicentre randomised controlled trial. Journal of Clinical Oncology 2011;29:3977‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tibaldi 2004 {published data only}
- Tibaldi V, Aimonino N, Ponzetto M, Stasi MF, Amati D, Raspo S, et al. A randomized controlled trial of a home hospital intervention for frail elderly demented patients: behavioral disturbances and caregiver's stress. Archives of Gerontology and Geriatrics ‐ Supplement 2004;38(9):431‐6. [PUBMED: 15207444] [DOI] [PubMed] [Google Scholar]
Tibaldi 2009 {published data only}
- Tibaldi V, Isaia G, Scarafiotti C, Gariglio F, Zanocchi M, Bo M, et al. Hospital at home for elderly patients with acute decompensation of chronic heart failure: a prospective randomized controlled trial. Archives of Internal Medicine 2009;169:1569‐75. [DOI] [PubMed] [Google Scholar]
Vianello 2013 {published data only}
- Vianello A, Savoia F, Pipitone E, Nordio B, Gallina G, Paladini L, et al. “Hospital at home” for neuromuscular disease patients with respiratory tract infection: a pilot study. Respiratory Care 2013;12(58):2061‐8. [DOI] [PubMed] [Google Scholar]
Wilson 1999 {published and unpublished data}
- Jones J, Wilson A, Parker H, Wynn A, Jagger C, Spiers N, et al. Economic evaluation of hospital at home versus hospital care: cost minimisation analysis of data from randomised controlled trial. BMJ 1999;319(7224):1547‐50. [PUBMED: 10591720] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Parker H, Wynn A, Jagger C, Spiers N, Jones J, et al. Randomised controlled trial of effectiveness of Leicester hospital at home scheme compared with hospital care. BMJ 1999;319(7224):1542‐6. [PUBMED: 10591717] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Wynn A, Parker H. Patient and carer satisfaction with 'hospital at home': quantitative and qualitative results from a randomised controlled trial. British Journal of General Practice 2002;52(474):9‐13. [PUBMED: 11791829] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
King 2000 {published data only}
- King MT, Hall J, Caleo S, Gurney HP, Harnett PR. Home or hospital? An evaluation of the costs, preferences and outcomes of domiciliary chemotherapy. International Journal of Health Services 2000;30:557‐79. [DOI] [PubMed] [Google Scholar]
Wade 1985 {published data only}
- Wade DT, Langton‐Hewer R, Skilbeck CE, Bainton D, Burns‐Cox C. Controlled trial of a home‐care service for acute stroke patients. The Lancet 1985;1(8424):323‐6. [PUBMED: 2857372] [DOI] [PubMed] [Google Scholar]
Wolfe 2000 {published data only}
- Wolfe CDA, Tilling K, Rudd AG. The effectiveness of community‐based rehabilitation for stroke patients who remain at home: a pilot randomized trial. Clinical Rehabilitation 2000;14(6):563‐9. [PUBMED: 11128729] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN29082260 {published data only}
- ISRCTN29082260. A study comparing home treatment of COPD exacerbations to usual hospital care. http://www.isrctn.com/ISRCTN29082260 (date accessed 5 January 2015).
ISRCTN60477865 {published data only}
- ISRCTN60477865. A multi‐centre randomised controlled trial to compare the effectiveness of admission avoidance hospital at home with comprehensive geriatric assessment vs. inpatient comprehensive geriatric assessment on the number of frail older people ‘living at home’. http://www.isrctn.com/ISRCTN60477865 (date accessed 5 January 2015).
Additional references
Board 2000
- Board N, Brennan N, Caplan G. A randomised controlled trial of the costs of hospital as compared with hospital at home for acute medical patients. Australian and New Zealand Journal of Public Health 2000;24.(3):305‐11[CRSREF: 2987213; PubMed: 10937409]. [DOI] [PubMed] [Google Scholar]
Brennan 2004
- Brennan T, Leape L, Laird NM, Herbert L, Localio AR, Lawthers AG, et al. Incidence of adverse events and negligence in hospitalized patients: results of the Harvard Medical Practice Study I. 1991. Quality and Safety in Health Care 2004;13(2):145‐51; discussion 151‐2. [PUBMED: 15069223] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochran 1954
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101‐29. [PsycINFO: 1955‐00087‐001] [Google Scholar]
Cornwall 2012
- Cornwall J, Levenson R, Sonola L, Poteliakhoff E. Continuity of Care for Older Hospital Patients: A Call for Action. London: Kings Fund, 2012. [Google Scholar]
Covinsky 2003
- Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic DM, et al. Loss of independence in activities of daily living in older adults hospitalised with medical illness: increased vulnerability with age. Journal of the American Geriatrics Society 2003;51:51‐8. [DOI] [PubMed] [Google Scholar]
Deeks 1998
- Deeks J, Bradburn M, Bilker W, Localio R, Berlin J. Much ado about nothing: statistical methods for meta‐analysis with rare events. Proceedings of the Sixth Cochrane Colloquium; 1998 22‐26 Oct; Baltimore, USA.. Oxford, 1998, issue Meeting Abstracts.
Deeks 2001
- Deeks JJ, Altman D, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care. Meta‐Analysis in Context. Wiley, 2001:285‐312. [Google Scholar]
EPOC 2015a
- Effective Practice, Organisation of Care (EPOC). Data collection form. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services. Available at: http://epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed September 2015).
EPOC 2015b
- Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services. Available at: http://epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed September 2015).
EPOC 2015c
- Effective Practice, Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services. Available at: http://epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed September 2015).
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ, the GRADE Working Group. What is ‘quality of evidence’ and why is it important to clinicians?. BMJ 2008;336:995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jeppesen 2012
- Jeppesen E, Bruberg KG, Vist GE, Wedzicha JA, Wright JJ, Greenstone M, et al. Hospital at home for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD003573.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
MECIR 2012
- Standards for the reporting of new Cochrane Intervention Reviews. http://editorial‐unit.cochrane.org/sites/editorial‐unit.cochrane.org/files/uploads/MECIR%20Reporting%20standards%201.1_17122012_2.pdf (accessed 19 February 2016).
Monitor 2015
- Monitor: Making the health sector work for patients. Moving healthcare closer to home: Literature review of clinical impacts. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/459268/Moving_healthcare_closer_to_home_clinical_review.pdf Accesed 17 August 2015.
Mughal 1986
- Mughal MM, Irving MH. Home parenteral nutrition in the United Kingdom and Ireland. The Lancet 1986;2(8503):383‐7. [PUBMED: 2874379] [DOI] [PubMed] [Google Scholar]
Naik 2006
- Naik G. Portland hospital gives acutely ill a homecare option. Wall Street Journal (http://www.wsj.com/articles/SB114540442717929396) 19 April 2006.
Nolte 2008
- Nolte E, McKee M. Caring for People With Chronic Conditions: A Health System Perspective. Maidenhead: McGraw Hill Open University Press, 2008. [Google Scholar]
Patel 2004
- Patel A, Knapp M, Perez I, Evans A, Kalra L. Alternative strategies for stroke care: cost effectiveness and cost utility analysis from a prospective randomised controlled trial. Stroke 2004;35(1):196‐203. [PUBMED: 14684783] [DOI] [PubMed] [Google Scholar]
Qaddoura 2015
- Qaddoura A, Yazdan‐Ashoori P, Kabali C, Thabane L, Haynes RB, Connolly SJ, et al. Efficacy of hospital at home in patients with heart failure: A systematic review and meta‐analysis. PLoS One 2015;10(6):e0129282. [DOI: 10.1371/journal.pone.0129282] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royal College of Physicians 2012
- Royal College of Physicians. Hospitals on the edge? The time for action. Royal College of Physicians 2012.
Shepperd 2009
- Shepperd S, Doll H, Broad J, Gladman J, Iliffe S, Langhorne P, et al. Hospital at home early discharge. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD000356.pub3; PUBMED: 19160179] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepperd 2011
- Shepperd S, Wee B, Straus SE. Hospital at home: home‐based end of life care. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009231; PUBMED: 21735440] [DOI] [PMC free article] [PubMed] [Google Scholar]
SPSS 2006 [Computer program]
- SPSS Inc. SPSS for Windows. Version 14.0. Chicago: SPSS Inc., 2006.
Stata 2005 [Computer program]
- StataCorp. Stata Statistical Software: Release 9. College Station, TX: StataCorp LP, 2005.
Stroke Unit Trialist
- Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care after stroke. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000197.pub2] [DOI] [PubMed] [Google Scholar]
Stuck 1993
- Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta‐analysis of controlled trials. The Lancet 1993;342(8878):1032‐6. [PUBMED: 8105269] [DOI] [PubMed] [Google Scholar]
Young 2005
- Young H, Grundy E, Kalogirou S. Who cares? Geographic variation in unpaid caregiving in England and Wales: evidence from the 2001 census. Population Trends 2005;120:23‐34. [PubMed] [Google Scholar]
References to other published versions of this review
Jones 1999
- Jones J, Wilson A, Parker H, Wynn A, Jagger C, Spiers N, et al. Economic evaluation of hospital at home versus hospital care: cost minimisation analysis of data from randomised controlled trial. BMJ 1999;319(7224):1547‐50. [PUBMED: 10591720] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepperd 1998
- Shepperd S, Iliffe S. Effectiveness of hospital at home compared to in‐patient hospital care. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD000356] [DOI] [Google Scholar]
Shepperd 2005
- Shepperd S, Iliffe S. Hospital at home versus in‐patient hospital care. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD000356.pub2; PUBMED: 16034853] [DOI] [PubMed] [Google Scholar]
Shepperd 2008
- Shepperd S, Doll H, Angus RM, Clarke MJ, Illife S, Kalra L, et al. Hospital at home admission avoidance. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007491] [DOI] [PMC free article] [PubMed] [Google Scholar]


