Abstract
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (2010, Issue 9 and 2013, Issue 6). Epithelial ovarian cancer accounts for about 90% of all cases of ovarian cancer. Debulking surgery and six courses of platinum‐based chemotherapy results in complete clinical remission (CCR) in up to 75% of cases. However, 75% of the responders will relapse within a median time of 18 to 28 months and only 20% to 40% of women will survive beyond five years. It has been suggested that maintenance chemotherapy could assist in prolonging remission. To date, there has not been a systematic review on the impact of maintenance chemotherapy for epithelial ovarian cancer.
Objectives
To assess the effectiveness and toxicity of maintenance chemotherapy for epithelial ovarian cancer and to evaluate the impact on quality of life (QoL).
Search methods
In the original review we searched the Cochrane Gynaecological Cancer Review Group Specialised Register, Cochrane Central Register of Controlled Trails (CENTRAL, the Cochrane Library 2009, Issue 1), MEDLINE, Embase, PubMed, CBMdisc, CNKI and VIP (to May 2009). We collected information from ongoing trials, checked reference lists of published articles and consulted experts in the field. For the first update the searches were extended to October 2012 and for this update to February 2017.
Selection criteria
Randomised controlled trials (RCTs) comparing maintenance chemotherapy with no further intervention, maintenance radiotherapy or other maintenance therapy.
Data collection and analysis
Two review authors independently assessed trials for eligibility and quality and extracted data. We analysed overall survival (OS) and progression‐free survival (PFS) rates as dichotomous variables. Toxicity and QoL data were extracted where present. All analyses were based on intention‐to‐treat (ITT) on the endpoint of survival. We also analysed data by subgroups of drugs.
Main results
No new studies were found for inclusion in this update from the latest searches. We included eight trials (1644 women). When all chemotherapy regimens were combined, meta‐analysis indicated no significant difference in three‐, five‐ and 10‐year OS or PFS. For five‐year OS, the combined risk ratio (RR) was 1.03 (95% confidence interval (CI) 0.96 to 1.10; 4 studies, 899 participants; moderate‐certainly evidence) and for the five‐year PFS, the combined RR was 1.06 (95% CI 0.97 to 1.17; 3 studies, 761 participants; moderate‐certainly evidence). Results were very similar when trials of different regimens were analysed. Comparing chemotherapy with radiotherapy, only the RR for 10‐year PFS in pathological complete remission (PCR) was in favour of whole abdominal radiotherapy 0.51 (95% CI 0.27 to 1.00), while three‐ and five‐year OS rates have no significant difference between the two groups.
Authors' conclusions
There is no evidence to suggest that the use of platinum agents, doxorubicin or paclitaxel used as maintenance chemotherapy is more effective than observation alone. Further investigations regarding the effect of paclitaxel used as maintenance chemotherapy are required.
Plain language summary
Maintenance chemotherapy for ovarian cancer
Background
Of all the gynaecological cancers, ovarian cancer has the highest death rate and epithelial ovarian cancer accounts for about 90% of all cases. Surgery and six courses of platinum‐based chemotherapy is the standard treatment and 75% of the women may not have any evidence of disease at the end of this treatment. However, 75% of the women who respond to initial treatment will relapse within 18 to 28 months and only 20% to 40% of all women will survive beyond five years.Some doctors suggest giving maintenance chemotherapy for epithelial ovarian cancer. Maintenance chemotherapy refers to the chemotherapy given to women who have achieved remission after initial surgery and induction chemotherapy.The aim of maintenance chemotherapy is to prolong the duration of remission and improve the overall length of survival. Some studies indicate that maintenance chemotherapy can improve the time without cancer progression, while others do not show any effect.
The aim of the review
The aim of this review was to estimate whether using maintenance chemotherapy is better than observation alone for women with epithelial ovarian cancer.
What are the main findings?
We identified eight trials that used different types of chemotherapy (e.g. platinum agents, doxorubicin, topotecan or paclitaxel) but there was not sufficient evidence to prove any of the drugs were better than observation alone. An important consideration for women with advanced disease is the balance between the benefit of treatment and the harms or adverse effects that these treatments may cause. There were insufficient data to comment on the overall impact of the maintenance chemotherapy on clinical benefit from the women's perspective.
Quality of the evidence
We tried to identify all trials and both published and unpublished data in this review; thereby minimising the influence of publication bias. The included trials are graded as moderate quality but this meta‐analysis currently provides a reliable assessment of the average treatment effect of platinum and doxorubicin among women with advanced epithelial ovarian cancer.
What are the conclusions?
Use of platinum agents, doxorubicin or paclitaxel used as maintenance chemotherapy has not proved effective to prolong the life time of women with epithelial ovarian cancer. Further investigations regarding the effect of paclitaxel used as maintenance chemotherapy are required.
Summary of findings
Background
This review is an update of a previously published Cochrane review (Mei 2010; Mei 2013).
Description of the condition
Worldwide, approximately 238,719 women are diagnosed with ovarian cancer and about 151,917 die from this disease each year. Ovarian cancer is the seventh most common cancer among women. (GLOBOCAN 2012). A woman's cumulative risk of developing ovarian cancer by age 65 years is 0.5%: 0.4% in less developed countries and 0.7% in more developed countries. It is less common in women under the age of 35 years, and its incidence increases with age (GLOBOCAN 2002).
Of all the malignant gynaecological tumours, ovarian cancer has the highest mortality rate because ovarian cancer often does not cause symptoms until it has become widespread (Poveda 2003). Despite, however, good responses to chemotherapy, there is a high recurrence rate (Ozols 2006). Epithelial ovarian cancer accounts for about 90% of all cases of ovarian cancer (Thigpen 2004). Debulking surgery and six courses of platinum‐based chemotherapy results in complete clinical remission (CCR) in up to 75% of cases (Thigpen 2004). However, 75% of the responders will relapse within the median time of 18 to 28 months (Stuart 2003) and only 20% to 40% women will survive beyond five years (Kikuchi 2005). Studies have shown that more than six courses of induction chemotherapy does not improve progression‐free survival (PFS) or overall survival (OS) but increases toxicity (Bertelsen 1993; Hakes 1992; Lambert 1997). Women receiving prolonged courses of chemotherapy therefore may gain little survival benefit while suffering from more adverse effects.
Description of the intervention
It has been suggested that maintenance or consolidation chemotherapy may be administered for epithelial ovarian cancer. Maintenance chemotherapy refers to chemotherapy given after women have achieved CCR or pathological complete remission (PCR), after initial surgery and induction chemotherapy.
How the intervention might work
The aim of the intervention is to prolong the interval of remission and improve the OS. Some clinicians differentiate maintenance chemotherapy from consolidation chemotherapy, as high‐dose or relatively short‐term chemotherapy given after CCR or PCR (Ozols 2004). The aim in this setting is to prevent recurrence rather than delay recurrence. Currently there are no specific definitions for these concepts, so we will consider consolidation and maintenance chemotherapy as the same, as long as it is applied after the women have achieved CCR or PCR and it will be referred to as maintenance chemotherapy in this review. CCR is defined as a patient with a normal CA‐125 blood test according to the local laboratory parameters, having no cancer‐related symptoms, a normal physical examination and a negative CT scan of the abdomen and/or pelvis and chest x‐ray (Markman 2003). PCR is defined as a patient with CCR confirmed as tumour‐negative by the second‐look surgery (Varia 2003).
Why it is important to do this review
Some studies have indicated that maintenance chemotherapy can improve PFS (Markman 2003), while others did not show any effect. To date, there have not been any published systematic reviews on the impact of maintenance chemotherapy for epithelial ovarian cancer.
Objectives
To assess the effectiveness and toxicity of maintenance chemotherapy for epithelial ovarian cancer and to evaluate the impact on quality of life (QoL) of maintenance chemotherapy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Women with epithelial ovarian cancer who have achieved CCR or PCR after initial surgery and chemotherapy.
Types of interventions
Maintenance chemotherapy versus no further intervention
Maintenance chemotherapy versus maintenance radiotherapy
Maintenance chemotherapy versus other maintenance therapy except chemotherapy and radiotherapy (e.g. biotherapy, immunotherapy)
Types of outcome measures
Primary outcomes
PFS and OS rates
Secondary outcomes
Adverse effect events (nausea‐vomiting, diarrhoea, ileus, bone marrow toxicity, neurotoxicity, mucositis, renal toxicity, hepatic toxicity, bladder toxicity etc) and
QoL (if a validated scale had been used)
Search methods for identification of studies
Electronic searches
For the original review we searched the following databases, The Cochrane Gynaecological Cancer Review Group Specialised Register, The Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Library, 2009, Issue 1, MEDLINE (from 1950 to May 2009), Embase (from 1966 to May 2009), PubMed (May 2009), CBMdisc (1978 to May 2009), CNKI (1979 to May 2009) and VIP (1989 to May 2009). For the first update the searches were extended to October 2012.
For this update we extended the search to: CENTRAL Issue 2, 2017, MEDLINE (January Week 4, 2017), Embase (week 6, 2017), PubMed (to March 2017),CNKI (to March 2017), CBMdisc (to March 2017) and VIP (to March 2017).
For MEDLINE, the subject search used a combination of vocabulary (MeSH terms) and free text terms (Appendix 1). We adapted the search strategy accordingly for CENTRAL, Embase, PubMed, CBMdisc,CNKI and VIP. The search strategies can be found in Appendix 2, Appendix 3, Appendix 4.
For MEDLINE, Embase and PubMed, there were no language restrictions placed on the search.
The search strategies used were developed and executed by the author team.
Searching other resources
We checked the reference lists of obtained articles to check for other related published and unpublished studies.
-
We searched relevant web sites for ongoing trials:
Personal communication: In addition, we contacted authors of included RCTs to identify any additional published and unpublished materials.
Data collection and analysis
Selection of studies
Two review authors (ML and WDM) scanned the titles and abstracts from the initial search in order to exclude those that did not meet the inclusion criteria. The full text of potentially relevant studies were obtained for independent assessment of eligibility by two review authors (ML and CH). Any disagreements were resolved through discussion with a third review author (FF) if necessary.
Data extraction and management
Two review authors (XHY and WX) independently extracted data using a previously specified form listing the following:
study characteristics (randomisation process, allocation concealment, blinding, attrition bias and intention‐to‐treat (ITT) analysis);
basic information of the participants (number of the women, mean age, age range);
base‐line data of the participants (FIGO stages, histological type, pathological grade and response to the first‐line treatment);
intervention (drug, dose and courses); and
outcome (OS after three, five and 10 years, PFS after three, five and 10 years, the incidence and severity of toxicity such as nausea‐vomiting, mucositis, leucopenia, thrombocytopenia, neutropenia, neurotoxicity, hepatic toxicity and renal toxicity and QoL score).
We resolved any disagreements by referring to the trial report or by consulting a third review author (FF). We contacted the trial authors for additional information if data from the trial reports were insufficient or missing.
Assessment of risk of bias in included studies
We assessed and reported the methodological risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), which recommends the explicit reporting of the following individual elements for RCTs.
Selection bias: random sequence generation and allocation concealment
Performance bias: blinding of participants and personnel (i.e. treatment providers) [blinding may only be applicable to outcome assessors, see point below]
Detection bias: blinding of outcome assessment
Attrition bias: incomplete outcome data
Reporting bias: selective reporting of outcomes
other possible sources of bias (e.g. baseline imbalance)
Two review authors (ML and WDM) independently applied the 'Risk of bias' tool and resolved differences by discussion or by appeal to a third review author (FF). We judged each item as being at high, low or unclear risk of bias as set out in the criteria provided by Higgins 2011, and provide a quote from the study report or a statement as justification for the judgement for each item in the risk of bias table or both. We summarised results in both a 'Risk of bias' graph and a risk of bias summary. When interpreting treatment effects and meta‐analyses, we took into account the 'Risk of bias' for the studies that contribute to that outcome.
Measures of treatment effect
When sufficient, clinically similar trials were available, we pooled the results in meta‐analyses. For dichotomous outcomes, we calculated the risk ratio (RR) for each study and pooled them. For continuous outcomes, we planned to pool the mean differences (or standardised mean differences) between the treatment arms at the end of follow‐up.
Dealing with missing data
Whenever possible, we contacted the original investigators to request missing data.
Data synthesis
When sufficient, clinically similar trials were available, we pooled their results in meta‐analyses. We analysed the data using Review Manager 5. We used RR and its 95% confidence interval (CI) to estimate the combined effect of OS, PFS and certain adverse effect rate. If the effect could not be combined, we described the outcome separately. If QoL had been reported by continuous data, we would have pooled the mean differences between the treatment arms.
We presented the overall quality of the evidence for each outcome according to the GRADE approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity such as directness of results (Langendam 2013). We created 'Summary of findings' tables based on the methods described the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and using GRADEpro GDT. We used the GRADE checklist and GRADE Working Group quality of evidence definitions (Meader 2014). We downgraded the evidence from 'high' quality by one level for serious (or by two for very serious) concerns for each limitation.
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis by type of regimen, such as platinum‐based chemotherapy and doxorubicin‐based chemotherapy. We tested heterogeneity using both the Chi2 test and the I2 test. A significance level of less than 0.10 of Chi2 was interpreted as evidence of heterogeneity. I2 was used to estimate total variation across studies, where less than 30% is considered as low level of heterogeneity and higher than 50% as high level (Higgins 2002). If there was evidence of substantial heterogeneity, we investigated and reported the possible reasons for this.
Sensitivity analysis
We intended that if the eligibility of some studies in the meta‐analysis had been dubious, sensitivity analysis might involve undertaking the meta‐analysis twice: firstly including all studies and secondly only excluding studies that were of high risk of bias and had unadjusted results. We planned to report the sensitivity analyses in a summary table.
Results
Description of studies
Results of the search
Since the last version of this review no new studies were identified for inclusion. In the last version search identified 718 citations and initially 559 were excluded through title and abstract screening. We then obtained full‐text articles for the remaining 159 trials for further scrutiny. For this update, we identified an additional 722 citations trials but none of them were identified for inclusion. The flow chart on how the selection of studies was made can be found in Figure 1.
1.
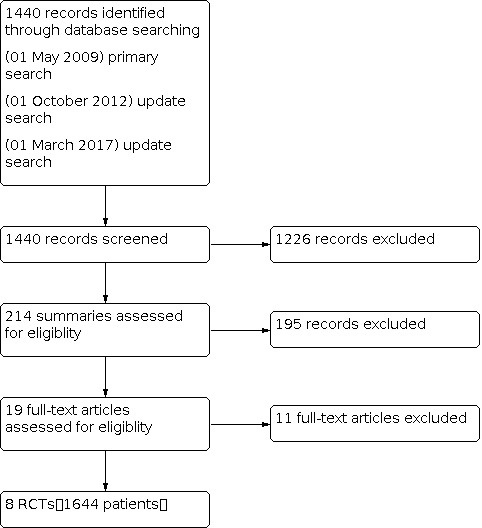
Studies Selection
Included studies
We identified eight RCTs and included data from 1644 women in this review. Seven trials compared maintenance chemotherapy with no further treatment (Bolis 2006; Cheng 2006; Mannel 2011; Nicoletto 2004; Pecorelli 2009; Piccart 2003; Placido 2004).
One study (Sorbe 2003) was a three‐arm study comparing maintenance chemotherapy, maintenance radiotherapy and no further treatment. A total of 172 women were included, 98 with pathological complete remission (PCR) and 74 with complete clinical remission (CCR). The included women had endometrial ovarian cancer ranging from stage ⅠC to stage Ⅳ. Women with stage Ⅲ to Ⅳ accounted for 89.9% of the total number of women included.
In addition, Piccart 2003 included women with non‐epithelial cancer, but we included it because the percentage of participants with non‐epithelial cancer was very small and there was no significant heterogeneity when compared with the other studies.
Excluded studies
Eleven trials were initially identified as potentially eligible for inclusion but were subsequently found to be ineligible and therefore excluded (Abaid 2010; Cure 2001; Lesnock 2011; Mannel 2010; Markman 2003; Markman 2009; Scarfone 2002Bois 2014; Gordon 2011; Lee 2006; Suidan 2014). Reasons for exclusions are listed in the table of Characteristics of excluded studies.
Risk of bias in included studies
We summarised the risk of bias in included studies in Figure 2 and Figure 3.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All eight studies used randomised allocation, but only four described the randomisation method (Bolis 2006; Mannel 2011; Pecorelli 2009; Placido 2004) and two (Bolis 2006; Mannel 2011) had adequate allocation concealment. According to the assessment criteria, all could be judged with low risk of bias for sequence generation and unclear risk of bias for allocation concealment.
Blinding
None of the included studies used blinding, but this is not likely to have influenced the results as the outcomes were overall survival (OS) and progression‐free survival (PFS). We therefore judged the studies as having low risk of bias for blinding.
Incomplete outcome data
Two studies lost one patient to follow‐up (Nicoletto 2004; Piccart 2003), but both used intention‐to‐treat (ITT) analysis and for OS and PFS the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect. One study had 32 patients who withdrew during the follow‐up (Mannel 2011), but it was balanced between the treatment and the control arms, therefore, they were graded with low risk of bias.
Selective reporting
All expected outcomes were reported. There was no selective reporting identified for any of the studies.
Other potential sources of bias
Piccart 2003 had a high risk of bias as "the study was closed prematurely in view of a disappointing recruitment rate. …". The remaining studies appeared to be free of other sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Maintenance chemotherapy versus observation.
| Maintenance chemotherapy vs. observation | ||||||
| Patient or population: patients with epithelial ovarian cancer Intervention: maintenance chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Maintenance chemotherapy | |||||
| 3‐year PFS Follow‐up: median 43.5 months | Study population | RR 1.07 (0.91 to 1.25) | 541 (4 studies) | ⊕⊕⊕⊝ moderate1 | There was no statistically significant difference in the three‐, five‐ and 10‐year PFS or OS. For the five‐year PFS the combined risk ratio (RR) was 1.06 (95% confidence interval (CI) 0.97 to 1.17 ) and for five‐year OS, the combined RR was 1.03 (95% CI, 0.96 to 1.10) . | |
| 521 per 1000 | 557 per 1000 (474 to 651) | |||||
| 5‐year PFS Follow‐up: mean 88.5 months | Study population | RR 1.06 (0.97 to 1.17) | 761 (3 studies1) | ⊕⊕⊕⊝ moderate1 | ||
| 664 per 1000 | 704 per 1000 (644 to 777) | |||||
| 10‐year PFS Follow‐up: median 96.7 months | Study population | RR 0.96 (0.65 to 1.41) | 219 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 327 per 1000 | 314 per 1000 (213 to 461) | |||||
| 3‐year OS Follow‐up: median 43.5 months | Study population | RR 1.00 (0.92 to 1.08) | 679 (5 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 795 per 1000 | 795 per 1000 (731 to 858) | |||||
| 5‐year OS Follow‐up: median 6.7 years | Study population | RR 1.03 (0.96 to 1.10) | 899 (4 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 746 per 1000 | 768 per 1000 (716 to 821) | |||||
| 10‐year OS Follow‐up: median 96.7 months | Study population | RR 1.08 (0.78 to 1.49) | 219 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 383 per 1000 | 414 per 1000 (299 to 571) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OS: overall survival; PFS: progression‐free survival; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded as allocation concealment is unclear
Summary of findings 2. Platin‐based maintenance chemotherapy versus observation.
| Platin‐based maintenance chemotherapy for epithelial ovarian cancer | ||||||
| Patient or population: patients with epithelial ovarian cancer Intervention: platin‐based maintenance chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Platin‐based maintenance chemotherapy | |||||
| 3‐year PFS Follow‐up: median 96.7 months | Study population | RR 1.04 (0.86 to 1.24) | 341 (3 studies) | ⊕⊕⊕⊝ moderate1 | There was no consistent effect in the three‐, five‐ and 10‐year PFS or OS. For the five‐year PFS the combined risk ratio (RR) was 1.18 (95% confidence interval (CI) 0.88 to 1.58 ) and for five‐year OS, the combined RR was 1.07 (95% CI, 0.88 to 1.31) . | |
| 571 per 1000 | 594 per 1000 (491 to 709) | |||||
| 5‐year PFS Follow‐up: median 96.7 months | Study population | RR 1.18 (0.88 to 1.58) | 219 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 411 per 1000 | 485 per 1000 (362 to 650) | |||||
| 10‐year PFS Follow‐up: median 96.7 months | Study population | RR 0.96 (0.65 to 1.41) | 219 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 327 per 1000 | 314 per 1000 (213 to 461) | |||||
| 3‐year OS Follow‐up: median 96.7 months | Study population | RR 1.06 (0.94 to 1.18) | 341 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 762 per 1000 | 808 per 1000 (716 to 899) | |||||
| 5‐year OS Follow‐up: median 96.7 months | Study population | RR 1.07 (0.88 to 1.31) | 219 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 617 per 1000 | 660 per 1000 (543 to 808) | |||||
| 10‐year OS Follow‐up: median 96.7 months | Study population | RR 1.08 (0.78 to 1.49) | 219 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 383 per 1000 | 414 per 1000 (299 to 571) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OS: overall survival; PFS: progression‐free survival; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded as allocation concealment is unclear
Summary of findings 3. Doxorubicin‐based maintenance chemotherapy versus observation.
| Doxorubicin‐based maintenance chemotherapy for epithelial ovarian cancer | ||||||
| Patient or population: patients with epithelial ovarian cancer Intervention: Doxorubicin‐based maintenance chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Doxorubicin‐based maintenance chemotherapy | |||||
| 3‐year OS Follow‐up: median 40 months | Study population | RR 1.00 (0.86 to 1.15) | 204 (2 studies) | ⊕⊕⊕⊝ moderate1 | There was no consistent effect for doxorubicin‐based maintenance chemotherapy. Overall survival for three and five years RR was 1.00 (95% CI 0.86 to 1.15 and 0.79 to 1.27, respectively) | |
| 781 per 1000 | 781 per 1000 (672 to 898) | |||||
| 5‐year OS Follow‐up: median 40 months | Study population | RR 1.00 (0.79 to 1.27) | 204 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 571 per 1000 | 571 per 1000 (451 to 726) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OS: overall survival; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded as allocation concealment is unclear
Summary of findings 4. Maintenance chemotherapy compared to maintenance radiotherapy for epithelial ovarian cancer.
| Maintenance chemotherapy compared to maintenance radiotherapy for epithelial ovarian cancer | ||||||
| Patient or population: epithelial ovarian cancer Intervention: maintenance chemotherapy Comparison: maintenance radiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Maintenance radiotherapy | Maintenance chemotherapy | |||||
| 3‐year PFS | Study population | RR 1.07 (0.75 to 1.54) | 141 (1 study) | ⊕⊕⊝⊝ low1,2 | The results showed no statistical difference between chemotherapy and radiotherapy groups.The 5‐year OS RR was 0.97 (95% CI 0.70 to 1.35). | |
| 420 per 1000 | 450 per 1000 (315 to 647) | |||||
| Moderate | ||||||
| 5‐year PFS | Study population | RR 0.86 (0.55 to 1.37) | 141 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 348 per 1000 | 299 per 1000 (191 to 477) | |||||
| Moderate | ||||||
| 10‐year PFS | Study population | RR 0.89 (0.51 to 1.55) | 141 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 261 per 1000 | 232 per 1000 (133 to 404) | |||||
| Moderate | ||||||
| 3‐year OS | Study population | RR 1.06 (0.85 to 1.32) | 141 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 652 per 1000 | 691 per 1000 (554 to 861) | |||||
| Moderate | ||||||
| 5‐year OS | Study population | RR 0.97 (0.70 to 1.35) | 141 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 493 per 1000 | 478 per 1000 (345 to 665) | |||||
| Moderate | ||||||
| 10‐year OS | Study population | RR 0.94 (0.58 to 1.52) | 141 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 304 per 1000 | 286 per 1000 (177 to 463) | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OS: overall survival; PFS: progression‐free survival; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded as allocation concealment is unclear 2 Downgraded as the baseline is imbalanced
Maintenance chemotherapy versus observation
Data were available on 1221 women from six of the included trials (Bolis 2006; Mannel 2011; Nicoletto 2004; Pecorelli 2009; Piccart 2003; Sorbe 2003). One trial used cisplatin alone (Piccart 2003), another studied epidoxorubicin (Bolis 2006), a third cisplatin‐based combination chemotherapy (Nicoletto 2004), another trial used the regimen of cisplatin and doxorubicin (Sorbe 2003) and another two studies used paclitaxel (Mannel 2011; Pecorelli 2009). The intended number of courses ranged from three to six. Except for Mannel 2011, the other included studies had maintenance chemotherapy scheduled to start after the women had achieved PCR or CCR. There was no significant heterogeneity within each category of drugs. In addition, there was no difference in the three‐, five‐ and 10‐year PFS or OS. For the five‐year PFS the combined risk ratio (RR) was 1.06 (95% confidence interval (CI) 0.97 to 1.17; 3 studies; 761 participants; moderate‐certainty evidence) (Analysis 1.2) and for five‐year OS, the combined RR was 1.03 (95% CI, 0.96 to 1.10; 4 studies, 899 participants; moderate‐certainly evidence) (Analysis 1.5) (Table 1)
1.2. Analysis.

Comparison 1: Maintenance chemotherapy versus observation, Outcome 2: 5‐year PFS
1.5. Analysis.

Comparison 1: Maintenance chemotherapy versus observation, Outcome 5: 5‐year OS
Two trials (Mannel 2011; Pecorelli 2009) that used paclitaxel were not combined in the analysis because there was significant heterogeneity between the stage of disease. Mannel 2011 included high‐risk early‐staged disease (stage I‐A or B (grade 3 or clear cell), all I‐C or II epithelial ovarian cancer) whereas, Pecorelli 2009 mainly included advanced staged disease. In addition, the regimens used in each study were different; Mannel 2011 used weekly low‐dose paclitaxel (40 mg/m²) and Pecorelli 2009 used six courses of paclitaxel (175 mg/m²) at three‐week intervals. Neither study indicated if paclitaxel could decrease the recurrence rate or increase the OS rate.
One trial (Cheng 2006) used a regimen of cisplatin and cyclophosphamide or taxol was also not included in the meta‐analysis as it used different outcomes. The results indicated maintenance chemotherapy could prolong the time of progression‐free interval (P = 0.033), while it had little effect on prolonging survival time (P = 0.22).
Another trial (Placido 2004) used topotecan and was not included in the meta‐analysis because the follow‐up duration was shorter and the outcomes could not be combined with other studies. It indicated that the one‐year PFS was 60.4% and 65.4% in the topotecan and control arms, respectively and there was no significant difference between the arms.
Trials using cisplatin‐based regimens
We analysed three studies including 341 women (Nicoletto 2004; Piccart 2003; Sorbe 2003) comparing cisplatin alone or combined with other drugs with no further treatment. Results were not conclusive and the 95% CI for absolute difference in OS was consistent with a 12% detriment to a 31% benefit of chemotherapy at five years. Similarly, the 95% CI for absolute difference in PFS is consistent with a 12% detriment to a 58% benefit at five years (Table 2).
Trials using doxorubicin‐based regimens
We undertook a subgroup analysis of two studies including 204 women (Bolis 2006; Sorbe 2003) comparing doxorubicin‐based maintenance chemotherapy with observation. Overall survival for three and five years was RR 1.00 (95% CI 0.86 to 1.15 and 0.79 to 1.27 respectively; 2 studies, 204 participants; moderate‐certainty evidence) (Analysis 3.1; Analysis 3.2; Table 3).
3.1. Analysis.

Comparison 3: Doxorubicin‐based maintenance chemotherapy versus observation, Outcome 1: 3‐year OS
3.2. Analysis.

Comparison 3: Doxorubicin‐based maintenance chemotherapy versus observation, Outcome 2: 5‐year OS
Maintenance chemotherapy versus maintenance radiotherapy
One trial (Sorbe 2003) randomised 141 women into the chemotherapy and whole abdominal radiotherapy group. Sixty‐seven women achieved PCR and the other 74 women CCR. There was considerable diversity of results across the two subgroups. The test for heterogeneity was significant for the combined RR for three‐ and 10‐year PFS and 10‐year OS. Ten‐year PFS in the PCR group was in favour of whole abdominal radiotherapy (RR 0.51, 95% CI 0.27 to 1.00; low‐certainty evidence) (Analysis 4.3) (Table 4). The other results showed no statistical difference between chemotherapy and radiotherapy in either group.
4.3. Analysis.

Comparison 4: Maintenance chemotherapy versus maintenance radiotherapy, Outcome 3: 10‐year PFS
Maintenance chemotherapy versus other maintenance therapy
We found no eligible RCTs for this comparison.
Toxicity of maintenance chemotherapy
Seven trials described the toxicities of maintenance chemotherapy (Bolis 2006; Mannel 2011; Nicoletto 2004; Pecorelli 2009; Piccart 2003; Placido 2004; Sorbe 2003), but only one (Mannel 2011) made comparison between intervention and control groups. It reported that the incidence of grade 2 or worse peripheral neuropathy, infection or fever, and dermatologic events was significantly higher among patients treated on the maintenance weekly paclitaxel regimen (P < 0.001). There was also a slight incidence of grade 2 or worse cardiovascular events (P = 0.044) among those on the maintenance regimen. Grade 3 or 4 peripheral neuropathy was reported in 0.7% of the observation group compared to 4.4% of the maintenance paclitaxel group (P = 0.012).
One trial (Piccart 2003) used intraperitoneal cisplatin and reported that the main side effect was bowel obstruction due to intraperitoneal catheters. The most common toxicities of cisplatin were vomiting (82% grade 2) and renal toxicity (45% grade 2).
Another trial (Bolis 2006) using epidoxorubicin reported neutropenia, anaemia and thrombocytopenia and 41.9% of women had severe grade 4 neutropenia .
In the trial that used topotecan (Placido 2004), data were available for 112 out of 117 women in the experimental arm. Grade 3 to 4 neutropenia was recorded in 58% of women and 21% had grade 3 thrombocytopenia. Nausea and vomiting were the most frequent non‐haematologic toxicities.
When comparing chemotherapy with radiotherapy, more side effects were recorded in the radiotherapy group, notably adverse gastro‐intestinal effects. In the radiotherapy arm,14.5% of women had severe diarrhoea, bloody stools or bowel obstruction compared to 4.2% of women in the chemotherapy arm (P = 0.034).
Discussion
Summary of main results
To date, there are no data which adequately support the use of platinum, doxorubicin or paclitaxel as maintenance chemotherapy for ovarian cancer.
When comparing maintenance chemotherapy with maintenance radiotherapy, the number of included women was too small to give an accurate results. However, from the subgroup analysis we may speculate that maintenance chemotherapy is more appropriate for women in complete clinical remission (CCR), while women in pathological complete remission (PCR) will benefit from maintenance radiotherapy.
The toxicity from platin was tolerable but the clinical application of doxorubicin and topotecan may be limited due to the severe bone marrow toxicity induced.
Overall completeness and applicability of evidence
This review was unable to address the issue of impact on quality of life (QoL) of maintenance chemotherapy due to a lack of trials reporting data relating to QoL. Therefore, it could not be addressed in the meta‐analysis.
This review was unable to report on the results of meta‐analysis of toxicity of maintenance chemotherapy as only one of the included trials (Mannel 2011) made comparison between the chemotherapy and observed groups. It was only possible to describe the observed toxicities during treatment.
This review was also unable to address the issue of whether maintenance chemotherapy is more effective than other maintenance therapy, especially biologic therapy because so far no RCT has focused on this topic.
Quality of the evidence
This meta‐analysis is based on data from 1644 women, from eight RCTs that compared platinum‐, doxorubicin‐ or paclitaxel‐based maintenance chemotherapy with no further treatment in epithelial ovarian cancer. We employed a number of methods to try to identify all trials and included both published and unpublished data in this review; thereby minimising the influence of publication bias. Most of the included trials were downgraded as allocation concealment was unclear, therefore we downgraded the evidence to moderate quality. Although the number of included women is small, this meta‐analysis currently provides a reliable assessment of the average treatment effect of platinum and doxorubicin among women with advanced epithelial ovarian cancer. For the comparison of maintenance chemotherapy with maintenance radiotherapy, the baseline is imbalanced so we downgraded the evidence to low quality. Further researches may change the estimate results..
Potential biases in the review process
Current practice prescribes that maintenance therapy begins after a woman achieves PCR or CCR but does not define how long the woman must be in remission before maintenance therapy is commenced. None of the trials included in this review described the exact start time of maintenance chemotherapy. It is widely accepted that the remission phase of platin‐sensitive cases usually lasts more than six months. Therefore, if maintenance chemotherapy was started earlier than this, some platin non‐sensitive cases may be included, which may introduce bias into the review.
Agreements and disagreements with other studies or reviews
The meta‐analysis indicated that there has not been sufficient evidence to prove that maintenance chemotherapy using platin or doxorubicin for advanced epithelial ovarian cancer can improve the progression‐free survival (PFS) or overall survival (OS). According to Markman 2003, 12 courses of paclitaxel can significantly prolong PFS rather than three courses. However, the control arm used three courses of maintenance chemotherapy but not observation and the trial was closed prematurely. So far, only one trial (Pecorelli 2009), studied paclitaxel as maintenance chemotherapy for advanced epithelial ovarian cancer but the results showed no survival benefit. One ongoing trial using paclitaxel as maintenance chemotherapy may provide new evidence (NCT00108745).
Authors' conclusions
Implications for practice.
Since the last version of this review no new studies have been found, so there is still insufficient evidence to support the use of platin, doxorubicin or paclitaxel used as maintenance chemotherapy and is more effective than observation alone.
Implications for research.
Considering the wide use of paclitaxel and its effectiveness during the induction phase of chemotherapy for advanced epithelial ovarian cancer, further studies on the effect of paclitaxel as maintenance chemotherapy should be investigated.
Larger treatment effects are needed before there is convincing evidence that maintenance chemotherapy is beneficial. Any future high‐quality trials should include QoL as this is an important consideration when prescribing maintenance chemotherapy.
What's new
| Date | Event | Description |
|---|---|---|
| 12 January 2022 | Amended | Author by‐line corrected. |
| 12 January 2022 | Review declared as stable | This review will be superseded by updates of the following reviews: Angiogenesis inhibitors for the treatment of ovarian cancer [ 10.1002/14651858.CD007930.pub2] and Poly(ADP‐ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer [10.1002/14651858.CD007929.pub3]. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 9, 2010
| Date | Event | Description |
|---|---|---|
| 6 November 2017 | New citation required but conclusions have not changed | No new studies identified for inclusion. Text updated as required and summary of findings tables added. |
| 7 February 2017 | New search has been performed | New searches run. |
| 5 June 2013 | Amended | Minor amendment to PLS |
| 29 May 2013 | New citation required but conclusions have not changed | Two studies added but conclusions remain unchanged. |
| 5 May 2013 | New search has been performed | Review updated, new searches run and text revised. |
| 5 August 2010 | Amended | EMBASE search strategy added. |
Acknowledgements
We would like to thank Hui Chen, Xu Han, Xun Wang and Dong Mei Wei for their contribution to the orginal review.
We would like to thank Jo Morrsion, Clare Jess, Joanne Platt and Tracey Harrison of the Editoral base of the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers for their contribution to the editorial process.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
#1 exp Ovarian Neoplasms/ #2 (ovar* adj5 (cancer* or tumor* or tumour* or malignan* or neoplas* or carcinoma*)).mp. #3 1 or 2 #4 drug therapy.fs. #5 exp Antineoplastic Agents/ #6 Antineoplastic Combined Chemotherapy Protocols/ #7 chemotherap*.mp. #8 4 or 5 or 6 or 7 #9 (maintain or maintenance or consolidat*).mp. #10 3 and 8 and 9 #11 randomized controlled trial.pt. #12 controlled clinical trial.pt. #13 randomized.ab. #14 placebo.ab. #15 drug therapy.fs. #16 randomly.ab. #17 trial.ab. #18 groups.ab. #19 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 #20 10 and 19 key: mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier pt=publication type fs=floating subheading ab=abstract
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Ovarian Neoplasms] explode all trees #2 ovar* near/5 (cancer* or tumor* or tumour* or malignan* or neoplas* or carcinoma*) #3 #1 or #2 #4 Any MeSH descriptor with qualifier(s): [Drug therapy ‐ DT] in all MeSH products #5 MeSH descriptor: [Antineoplastic Agents] explode all trees #6 MeSH descriptor: [Antineoplastic Combined Chemotherapy Protocols] this term only #7 chemotherap* #8 #4 or #5 or #6 #9 maintain or maintenance or consolidat* #10 #3 and #8 and #9
Appendix 3. Embase search strategy
#1 exp ovary tumor/ #2 (ovar* adj5 (cancer* or tumor* or tumour* or malignan* or neoplas* or carcinoma*)).mp. #3 1 or 2 #4 dt.fs. #5 exp antineoplastic agent/ #6 chemotherap*.mp. #7 4 or 5 or 6 #8 (maintain or maintenance or consolidat*).mp. #9 3 and 7 and 8 #10 crossover procedure/ #11 double‐blind procedure/ #12 randomized controlled trial/ #13 single‐blind procedure/ #14 random*.mp. #15 factorial*.mp. #16 (crossover* or cross over* or cross‐over*).mp. #17 placebo*.mp. #18 (double* adj blind*).mp. #19 (singl* adj blind*).mp. #20 assign*.mp. #21 allocat*.mp. #22 volunteer*.mp. #23 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 #24 9 and 23 key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Appendix 4. CNKI,VIP and CBMdisc
#1 ovarian cancer #2 ovarian tumor #3 maintenance therapy #4 maintenance chemotherapy #5 maintenance radiotherapy #6 consolidation therapy #7 consolidation chemotherapy #8 consolidation radiotherapy #9 or/1 2 #10 or/3 8 #11 9 and 10
Data and analyses
Comparison 1. Maintenance chemotherapy versus observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 3‐year PFS | 4 | 541 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.91, 1.25] |
| 1.2 5‐year PFS | 3 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.97, 1.17] |
| 1.3 10‐year PFS | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.41] |
| 1.4 3‐year OS | 5 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.08] |
| 1.5 5‐year OS | 4 | 899 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.10] |
| 1.6 10‐year OS | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.78, 1.49] |
1.1. Analysis.

Comparison 1: Maintenance chemotherapy versus observation, Outcome 1: 3‐year PFS
1.3. Analysis.

Comparison 1: Maintenance chemotherapy versus observation, Outcome 3: 10‐year PFS
1.4. Analysis.

Comparison 1: Maintenance chemotherapy versus observation, Outcome 4: 3‐year OS
1.6. Analysis.

Comparison 1: Maintenance chemotherapy versus observation, Outcome 6: 10‐year OS
Comparison 2. Platin‐based maintenance chemotherapy versus observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 3‐year PFS | 3 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.86, 1.24] |
| 2.2 5‐year PFS | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.88, 1.58] |
| 2.3 10‐year PFS | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.41] |
| 2.4 3‐year OS | 3 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.94, 1.18] |
| 2.5 5‐year OS | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.88, 1.31] |
| 2.6 10‐year OS | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.78, 1.49] |
2.1. Analysis.

Comparison 2: Platin‐based maintenance chemotherapy versus observation, Outcome 1: 3‐year PFS
2.2. Analysis.

Comparison 2: Platin‐based maintenance chemotherapy versus observation, Outcome 2: 5‐year PFS
2.3. Analysis.

Comparison 2: Platin‐based maintenance chemotherapy versus observation, Outcome 3: 10‐year PFS
2.4. Analysis.

Comparison 2: Platin‐based maintenance chemotherapy versus observation, Outcome 4: 3‐year OS
2.5. Analysis.

Comparison 2: Platin‐based maintenance chemotherapy versus observation, Outcome 5: 5‐year OS
2.6. Analysis.

Comparison 2: Platin‐based maintenance chemotherapy versus observation, Outcome 6: 10‐year OS
Comparison 3. Doxorubicin‐based maintenance chemotherapy versus observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 3‐year OS | 2 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.15] |
| 3.2 5‐year OS | 2 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.27] |
Comparison 4. Maintenance chemotherapy versus maintenance radiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 3‐year PFS | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.75, 1.54] |
| 4.1.1 PCR | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.11] |
| 4.1.2 CCR | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.99, 4.64] |
| 4.2 5‐year PFS | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.55, 1.37] |
| 4.2.1 PCR | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.12] |
| 4.2.2 CCR | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.59, 3.79] |
| 4.3 10‐year PFS | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.51, 1.55] |
| 4.3.1 PCR | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 1.00] |
| 4.3.2 CCR | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [0.91, 17.59] |
| 4.4 3‐year OS | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.85, 1.32] |
| 4.4.1 PCR | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.16] |
| 4.4.2 CCR | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.84, 1.94] |
| 4.5 5‐year OS | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.70, 1.35] |
| 4.5.1 PCR | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.57, 1.20] |
| 4.5.2 CCR | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.68, 2.29] |
| 4.6 10‐year OS | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.58, 1.52] |
| 4.6.1 PCR | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.41, 1.20] |
| 4.6.2 CCR | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.66, 6.07] |
4.1. Analysis.

Comparison 4: Maintenance chemotherapy versus maintenance radiotherapy, Outcome 1: 3‐year PFS
4.2. Analysis.

Comparison 4: Maintenance chemotherapy versus maintenance radiotherapy, Outcome 2: 5‐year PFS
4.4. Analysis.

Comparison 4: Maintenance chemotherapy versus maintenance radiotherapy, Outcome 4: 3‐year OS
4.5. Analysis.

Comparison 4: Maintenance chemotherapy versus maintenance radiotherapy, Outcome 5: 5‐year OS
4.6. Analysis.

Comparison 4: Maintenance chemotherapy versus maintenance radiotherapy, Outcome 6: 10‐year OS
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bolis 2006.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 138 women with epithelial ovarian cancer achieved PCR or CCR Stage 2c, 3 and 4 Mean age was 55.6 years old Median follow‐up time was 40 months |
|
| Interventions | Epidoxorubicin versus observation | |
| Outcomes | 3‐year OS and 5‐year OS; toxic events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Quote: "..according to a computer‐generated list, by phone at the coordinating center" Comment: Probably done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding, but the outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Cheng 2006.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 44 women with epithelial ovarian cancer achieved CCR Stage 3 and 4 Mean age was 53.8 years old in maintenance chemotherapy group and 53.7 in observation group Mean follow‐up time was 39.6 months in maintenance chemotherapy group and 33.2 months in observation group |
|
| Interventions | Platin + CTX/Taxel versus observation | |
| Outcomes | Recurrence rate, disease‐free survival | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " patients were randomly allocated" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding, but the outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Mannel 2011.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 542 women with early stage of epithelial ovarian cancer. stage ⅠA or B grade 3 or clear cell subtype, or any stage IC, or stage II disease. Mean age was 55.1 years old in maintenance chemotherapy group and 56 in observation group Mean follow‐up time was 6.7 years |
|
| Interventions | Weekly paclitaxel 40 mg/m² × 24 weeks versus observation | |
| Outcomes | OS, PFS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "treatment was randomly assigned through the GOG Statistical and Data Center prior to receiving any chemotherapy." Comment: Probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "The treatment assignment was not revealed until after the patient was successfully registered onto the study" Comment: Probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding, but the outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "18 patients randomized to the additional 24 weeks of paclitaxel withdrew during the follow‐up,14 patients of the control group withdrew during the follow‐up." Comment: It is balanced between the two groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Nicoletto 2004.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 122 women with epithelial ovarian cancer achieved PCR Stage 1c, 2b, 2c, 3 and 4 Mean age was 55 years old Median follow‐up time was not reported |
|
| Interventions | 5‐Fu + cisplatin versus observation | |
| Outcomes | 3‐year OS and 3‐year PFS; toxic events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…at time of randomization" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding, but the outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "one patient refused treatment entirely after randomization and is therefore not evaluable" Comment: For OS and PFS, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Pecorelli 2009.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 200 women with epithelial ovarian cancer Stage ⅡB‐Ⅳ with PCR or CCR Mean age was 59 years old in maintenance chemotherapy group and 58 in observation group Median follow‐up time was 43.5 months |
|
| Interventions | 6 cycles of paclitaxel 175 mg/m² every 3 weeks versus observation | |
| Outcomes | OS and PFS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A system of random permuted blocks within strata was used." Comment: Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding, but the outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "6% never started treatment, and a total of 17% stopped treatment early because of toxicity (9%), progression/death (3%), patient refusal (3%), or other reasons (2%)." Comment: For OS and PFS, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Piccart 2003.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 153 women with ovarian cancer achieved PCR Stage 2b, 2c, 3 and 4 Mean age was 55 years old Median follow‐up time was 96.7 months |
|
| Interventions | Cisplatin versus observation | |
| Outcomes | 3‐, 5‐, 8‐, 10‐year OS and PFS; toxic events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization took place…" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding, but the outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "one patient no follow‐up forms. No statistically significant difference between the two groups" |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | High risk | Quote: "the study was closed prematurely in view of a disappointing recruitment rate…" |
Placido 2004.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 273 women with epithelial ovarian cancer achieved CCR or partial clinical remission Stage 1c, 2, 3 and 4 Mean age was 55 years old in maintenance chemotherapy group and 56 in observation group Median follow‐up time was 28 months |
|
| Interventions | Topotecan versus observation; toxic events | |
| Outcomes | 1‐year PFS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…by means of a computer‐driven minimization procedure" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding, but the outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sorbe 2003.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 172 women with epithelial ovarian cancer achieved PCR and CCR Stage 3 and 4 Mean age was 55 years old Median follow‐up time was not reported |
|
| Interventions | Cisplatin + doxorubicin/epidoxorubicin versus observation | |
| Outcomes | 3‐, 5‐, 10‐year OS and PFS; toxic events | |
| Notes | This study is a 3‐arm study comparing maintenance chemotherapy, maintenance radiotherapy and no further treatment. The total partIcipants were 172 women with 98 of PCR and 74 of CCR. The women with PCR were divided into chemotherapy, radiotherapy and observation groups while the women with CCR were divided into chemotherapy and radiotherapy groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients…were entered in a prospective, randomized, multicenter trial" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding, but the outcome and the outcome measurement are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
CCR: complete clinical remission GOG: Gynecologic Oncology Group OS: overall survival PCR: pathological complete remission PFS: progression‐free survival RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abaid 2010 | The follow‐up of Markman 2003 and the results were of high potential bias |
| Bois 2014 | It compared pazopanib and placebo but pazopanib is not a chemotherapy drug. |
| Cure 2001 | It compared high‐dose chemotherapy combined with PBSC versus normal dose maintenance chemotherapy |
| Gordon 2011 | The randomisation was before induced chemotherapy and maintenance therapy was prescribed based on the intention of patients. |
| Lee 2006 | Not RCT |
| Lesnock 2011 | A cost‐effect analysis of three GOG studies |
| Mannel 2010 | The same trial with the Mannel 2011 but not the final result |
| Markman 2003 | It compared short‐ versus long‐duration maintenance chemotherapy |
| Markman 2009 | The follow‐up of Markman 2003 and the results were of high potential bias |
| Scarfone 2002 | The original article or data are unavailable |
| Suidan 2014 | Retrospective study of GOG172, not a RCT. |
GOG: Gynecologic Oncology Group PBSC: peripheral blood stem cell RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT00108745.
| Study name | Paclitaxel or polyglutamate paclitaxel or observation in treating women with stage III or stage IV ovarian epithelial or peritoneal cancer |
| Methods | RCT |
| Participants | Women with advanced ovarian or primary peritoneal cancer who achieve a complete clinical response to primary platinum/taxane chemotherapy |
| Interventions |
In arms I and II, treatment repeats every 28 days for up to 12 courses in the absence of disease progression or unacceptable toxicity |
| Outcomes | Primary outcome measures: overall survival Secondary outcome measures: Peripheral neuropathy by Gynecologic Oncology Group (GOG) NTX4 at 6 months after study enrolment General quality of life by Functional Assessment of Cancer Therapy‐Ovarian‐Trial Outcome Index (FACT‐O‐TOI) at 6 months after study enrolment Exploratory assessment of several tissue and serum angiogenic markers for prognosis by immunohistochemistry and antibody array prior to treatment in courses 1 and 2 Exploratory time‐dependent assessment of quality of life and peripheral neuropathy by FACT‐O‐TOI and GOG‐NTX4 monthly during year 1 and then every 3 months for 2 years |
| Starting date | March 2005 |
| Contact information | Study Chair: Maurie Markman, MD; M.D. Anderson Cancer Center |
| Notes |
IV: intravenous RCT: randomised controlled trial
Differences between protocol and review
For the assessment of risk of bias in included studies, we changed the original five criteria to six criteria according to the guidelines of Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)
Contributions of authors
Mei Ling and Chen Hui were responsible for searching for studies, quality assessment, data extraction, data analysis and review development. Fang Fang offered clinical expertise and took part in the development of this review. Wei Dongmei, Zou Juan and Han Xu undertook searching for studies and quality assessment. Feng Dan, Xie Huanyu, Wang Xun and Chen Hui participated in data extraction and analysis. Liu Guan Jian offered methodological expertise. For the update, Mei Ling and Wei Dongmei were responsible for searching for studies, quality assessment, data extraction and data analysis. Mei Ling was in charge of revising the text.
Sources of support
Internal sources
West China Second Hospital, China
External sources
No sources of support provided
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bolis 2006 {published data only}
- Bolis G, Danese S, Tateo S, Rabaiotti E, D'Agostino G, Merisio C, et al.Epidoxorubicin versus no treatment as consolidation therapy in advanced ovarian cancer:results from a phase IIstudy. International Journal of Gynecology Cancer 2006;16(suppl 1):74-8. [DOI] [PubMed] [Google Scholar]
Cheng 2006 {published data only}
- Cheng NH, Huang HF, Pan LY, Shen K, Wu M, Yang JX.Consolidation chemotherapy in advanced epithelial ovarian carcinoma:a prospective randomized controlled trial. Medical Journal of Chinese People's Liberation Army 2006;31(7):654-6. [Google Scholar]
Mannel 2011 {published data only}
- Mannel RS, Brady MF, Kohn EC, Hanjani P, Hiura M, Lee R, et al.A randomized phase III trial of IV carboplatin and paclitaxel × 3 courses followed by observation versus weekly maintenance low-dose paclitaxel in patients with early-stage ovarian carcinoma: a Gynecologic Oncology Group Study. Gynecology Oncology 2011;122(1):89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicoletto 2004 {published data only}
- Nicoletto MO, Tumolo S, Falci C, Donach M, Visona E, Rosabian A, et al.A randomized study of epithelial ovarian cancer: Is chemotherapy useful after complete remission? International Journal of Medical Sciences 2004;1(2):116-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pecorelli 2009 {published data only}
- Pecorelli S, Favalli G, Gadducci A, Katsaros D, Panici PB, Carpi A, et al, After 6 Italian Cooperative Group.Phase III trial of observation versus six courses of paclitaxel in patients with advanced epithelial ovarian cancer in complete response after six courses of paclitaxel/platinum-based chemotherapy: final results of the After-6 protocol 1. Journal of Clinical Oncology 2009;27(28):4642-8. [DOI] [PubMed] [Google Scholar]
Piccart 2003 {published and unpublished data}
- Piccart MJ, Floquet A, Scarfone G, Willemse PHB, Emerich J, Vergote I, et al.Intraperitoneal cisplatin versus no further treatment: 8-year results of EORTC 55875, a randomized phase 3 study in ovarian cancer patients with a pathologically complete remission after platinum-based intravenous chemotherapy. International Journal of Gynecologic Cancer 2003;13(suppl 2):196-203. [DOI] [PubMed] [Google Scholar]
Placido 2004 {published and unpublished data}
- Placido SD, Scambia G, Vagno GD, Naglieri E, Lombardi VA, Biamonte R, et al.Toptecan compared with no therapy after response to surgery and carboplatin/paclitaxel in patients with ovarian cancer: Multicenter Italian Trials in Ovarian Cancer (MITO-1) randomized study. Journal of Clinical Oncology 2004;22(13):2635-42. [DOI] [PubMed] [Google Scholar]
Sorbe 2003 {published data only}
- Sorbe B.Consolidation treatment of advanced (FIGO stage 3) ovarian carcinoma in complete surgical remission after induction chemotherapy: A randomized, controlled, clinical trial comparing whole abdominal radiotherapy, chemotherapy, and no further treatment. International Journal of Gynecologic Cancer 2003;13(3):278-86. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abaid 2010 {published data only}
- Abaid LN, Goldstein BH, Micha JP, Rettenmaier MA, Brown JV, Markman M.Improved overall survival with 12 cycles of single-agent paclitaxel maintenance therapy following a complete response to induction chemotherapy in advanced ovarian carcinoma. Oncology 2010;78(5-6):389-93. [DOI] [PubMed] [Google Scholar]
Bois 2014 {published data only}
- Bois A, Floquet A, Kim JW, Rau J, Campo JM, Friedlander M, et al.Incorporation of Pazopanib in maintenance therapy of ovarian cancer. Journal of Clinical Oncology 2014;32(30):3374-87. [DOI] [PubMed] [Google Scholar]
Cure 2001 {published data only}
- Cure H, Battista C, Guastalla JP, Fabbro M, Tubiana-Mathieu N, Bourgeois H, et al.Phase 3 randomized trial of high-dose chemotherapy (HDC) and peripheral blood stem cell (PBSC) support as consolidation in patients with advanced ovarian cancer (AOC): 5-year follow-up of a GINECO/FNCLCC/SFGM-TC/Italy study. In: Proceedings of American Society of Clinical Oncology. 2001.
Gordon 2011 {published data only}
- Gordon AN, Teneriello M, Janicek MF, Hines J, Lim PC, Chen MD, et al.Phase III trial of induction gemcitabine or paclitaxel plus carboplatin followedby paclitaxel consolidation in ovarian cancer. Gynecologic Oncology 2011;123:479-85. [DOI] [PubMed] [Google Scholar]
Lee 2006 {published data only}
- Lee SJ, Lee JW, Min JA, Park CS, Kim BG, Lee JH, et al.A pilot study of three-cycle consolidation chemotherapy with paclitaxel and platinum inepithelial ovarian cancer patients with clinical complete response after paclitaxel and platinum chemotherapy. International Journal of Gynecological Cancer 2006;16(1):95-100. [DOI] [PubMed] [Google Scholar]
Lesnock 2011 {published data only}
- Lesnock JL, Farris C, Krivak TC, Smith KJ, Markman M.Consolidation paclitaxel is more cost-effective than bevacizumab following upfront treatment of advanced epithelial ovarian cancer. Gynecology Oncology 2011;122(3):473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mannel 2010 {published data only}
- Mannel R, Brady M, Kohn E, Hanjani P, Hiura M, Lee R, et al. In:Gynecologic Oncology.Conference: 41st Annual Meeting of the Society of Gynecologic Oncologists, SGO San Francisco, CA United States. Vol. 116. March 2010:S2.
Markman 2003 {published data only}
- Markman M, Liu PY, Wilczynski S, Mank B, Copeland LJ, Alvarez RD, et al.Phase 3 randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: A Southwest Oncology Group and Gynecologic Oncology Group Trial. Journal of Clinical Oncology 2003;21(13):2460-5. [DOI] [PubMed] [Google Scholar]
Markman 2009 {published data only}
- Markman M, Liu PY, Moon J, Monk BJ, Copeland L, Wilczynski S, et al.Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel: follow-up of a Southwest Oncology Group and Gynecologic Oncology Group phase 3 trial. Gynecology Oncology 2009;114(2):195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scarfone 2002 {published data only}
- Scarfone G, Merisio C, Garavaglia E, Richiardi G, Tateo S, D'Agostino S, et al.A phase 3 trial of consolidation versus NIHL (NIL) for advanced epithelial ovarian cancer (AEOC) after complete remission (CR). In: Proceedings of American Society of Clinical Oncology. 2002.
Suidan 2014 {published data only}
- Suidan RS, Clair CM, Lee SJ, Barlin JN, Roche KL, Tanner EJ, et al.A comparison of primary intraperitoneal chemotherapy to consolidation intraperitoneal chemotherapy in optimally resected advancedovarian cancer. Gycecologic Oncology 2014;134(3):468-72. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00108745 {published data only}
- NCT00108745.Paclitaxel or polyglutamate paclitaxel or observation in treating women with stage III or stage IV ovarian epithelial or peritoneal cancer. http://clinicaltrials.gov/show/NCT00108745.
Additional references
Bertelsen 1993
- Bertelsen K, Jakobsen A, Strøyer J, Nielsen K, Sandberg E, Andersen JE, et al.A prospective randomized comparison of 6 and 12 cycles of cyclophosphamide, adriamycin, and cisplatin in advanced epithelial ovarian cancer: a Danish Ovarian Study Group trial (DACOVA). Gynecologic Oncology 1993;49(1):30-6. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2002
- Ferlay J, Bray F, Pisani P, Parkin DM.GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase 2004; 2004;(No.5. version 2.0).
GLOBOCAN 2012
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al.GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on 27/11/2017.
Hakes 1992
- Hakes TB, Chalas E, Hoskins WJ, Jones WB, Markman M, Rubin SC.Randomized prospective trial of 5 versus 10 cycles of cyclophosphamide doxorubicin and cisplatin in advanced ovarian carcinoma. Gynecologic Oncology 1992;45(3):284-8. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG.Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Kikuchi 2005
- Kikuchi Y, Kita T, Takano M, Kudoh K, Yamamoto K.Treatment options in the management of ovarian cancer. Expert Opinion on Pharmacotherapy 2005;6(5):743-54. [DOI] [PubMed] [Google Scholar]
Lambert 1997
- Lambert HE, Rustin GJS, Gregory WM, Nelstrop AE.A randomized trial of five versus eight courses of cisplatin or carboplatin in advanced epithelial ovarian carcinoma. Annals of Oncology 1997;8:327-33. [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schunemann HJ.Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;23(2):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al.A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozols 2004
- Ozols RF.Maintenance chemotherapy standard care. In: American Society of Clinical Oncology. 2004.
Ozols 2006
- Ozols RF.Challenges for chemotherapy in ovarian cancer. Annals of Oncology 2006;17(suppl.5):181-7. [DOI] [PubMed] [Google Scholar]
Poveda 2003
- Poveda A.Ovarian cancer treatment: what is new. International Journal of Gynecologic Cancer 2003;13(suppl 2):241-50. [DOI] [PubMed] [Google Scholar]
Stuart 2003
- Stuart GCE.First-line treatment regimens and the role of consolidation therapy in advanced ovarian cancer. Gynecologic Oncology 2003;90:8-15. [DOI] [PubMed] [Google Scholar]
Thigpen 2004
- Thigpen T.First-line therapy for ovarian carcinoma: What's next? Cancer Investigation 2004;22(suppl 2):21-8. [DOI] [PubMed] [Google Scholar]
Varia 2003
- Varia MA, Stehman FB, Bundy BN, Benda JA, Clarke-Pearson DL, Alvarez RD.Intraperitoneal radioactive phosphorus (32P) versus observation after negative second-look laparotomy for stage III ovarian carcinoma: a randomized trial of the Gynecologic Oncology Group. Journal of Clinical Oncology 2003;21:2849-55. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mei 2010
- Mei L, Chen H, Wei DM, Fang F, Liu GJ, Xie HY, et al.Maintenance chemotherapy for ovarian cancer. Cochrane Database of Systematic Reviews 2010, Issue 9. Art. No: CD007414. [DOI: 10.1002/14651858.CD007414.pub2] [DOI] [PubMed] [Google Scholar]
Mei 2013
- Mei L, Chen H, Wei DM, Fang F, Liu GJ, Xie HY, et al.Maintenance chemotherapy for ovarian cancer. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD007414. [DOI: 10.1002/14651858.CD007414.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mel 2008
- Mei L, Fang F, Wei DM, Chen H, Liu GJ, Xie HY, et al.Maintenance chemotherapy for ovarian cancer. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD007414. [DOI: 10.1002/14651858.CD007414] [DOI] [Google Scholar]


