Abstract
Background
Historically, whole brain radiation therapy (WBRT) has been the main treatment for brain metastases. Stereotactic radiosurgery (SRS) delivers high‐dose focused radiation and is being increasingly utilized to treat brain metastases. The benefit of adding SRS to WBRT is unclear. This is an updated version of the original Cochrane review published in Issue 6, 2010.
Objectives
To assess the efficacy of WBRT plus SRS versus WBRT alone in the treatment of brain metastases.
Search methods
In the original review we searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 2, 2009), MEDLINE (1966 to 2009), EMBASE (1980 to 2009), and CancerLit (1975 to 2009) in order to identify trials for inclusion in this review.
In this update we searched the following electronic databases in May 2012: Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 5, 2012), MEDLINE (2009 to May week 4 2012), and EMBASE (2009 to 2012 week 21) in order to identify trials for inclusion in the review.
Selection criteria
The review was restricted to randomized controlled trials (RCTs) that compared use of WBRT plus SRS versus WBRT alone for upfront treatment of adult patients with newly diagnosed metastases (single or multiple) in the brain resulting from any primary, extracranial cancer.
Data collection and analysis
The Generic Inverse Variance method, random‐effects model in RevMan 5 was used for the meta‐analysis.
Main results
A meta‐analysis of two trials with a total of 358 participants, found no statistically significant difference in overall survival (OS) between WBRT plus SRS and WBRT alone groups (hazard ratio (HR) 0.82; 95% confidence interval (CI) 0.65 to 1.02). For patients with one brain metastasis median survival was significantly longer in WBRT plus SRS group (6.5 months) versus WBRT group (4.9 months; P = 0.04). Patients in the WBRT plus SRS group had decreased local failure compared to patients who received WBRT alone (HR 0.27; 95% CI 0.14 to 0.52). Furthermore, a statistically significant improvement in performance status scores and decrease in steroid use was seen in the WBRT plus SRS group. Unchanged or improved Karnofsky Performance Scale (KPS) at 6 months was seen in 43% of patients in the combined therapy group versus only 28% in WBRT group (P = 0.03). Overall, risk of bias in the included studies was unclear.
Authors' conclusions
Since the last version of this review no new studies were found that met the inclusion criteria. Given the unclear risk of bias in the included studies, the results of this analysis have to be interpreted with caution. Analysis of all included patients, SRS plus WBRT, did not show a survival benefit over WBRT alone. However, performance status and local control were significantly better in the SRS plus WBRT group. Furthermore, significantly longer OS was reported in the combined treatment group for recursive partitioning analysis (RPA) Class I patients as well as patients with single metastasis.
Keywords: Adult, Humans, Brain Neoplasms, Brain Neoplasms/mortality, Brain Neoplasms/radiotherapy, Brain Neoplasms/secondary, Brain Neoplasms/surgery, Combined Modality Therapy, Combined Modality Therapy/methods, Combined Modality Therapy/mortality, Cranial Irradiation, Cranial Irradiation/methods, Cranial Irradiation/mortality, Radiosurgery, Radiosurgery/methods, Radiosurgery/mortality, Randomized Controlled Trials as Topic
Is adding focused radiation (radiosurgery) to whole brain radiation therapy beneficial to patients with brain metastases?
We identified three RCTs that looked at whether adding focused radiation (radiosurgery) to whole brain radiation therapy (WBRT) is beneficial to patients with brain metastases. Most of our conclusions are based on the results of one large trial with unclear prejudice and therefore, we cautiously make the following remarks: we found that when radiosurgery is added to WBRT, there was no evidence to suggest that patients lived any longer than if they had WBRT alone, except for patients with only one brain metastasis (who may live longer if they receive the combination treatment). Patients in the combination treatment also seemed to function better in daily life, their treated tumors were associated with having less chance of growing back, and they had to take less steroid medication. The side effects of combined therapy and WBRT alone were similar.
Summary of findings
Summary of findings for the main comparison.
Summary of findings table
| WBRT + SRS versus WBRT for the treatment of brain metastases | ||||||
|
Patient or population: patients with the treatment of brain metastases Settings: inpatients or outpatient Intervention: WBRT + SRS versus WBRT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | WBRT + SRS versus WBRT | |||||
| Overall survival Follow‐up: 12 months1 | Study population | HR 0.82 (0.65 to 1.02) | 358 (2 studies3) | ⊕⊕⊕⊝ moderate2 | ||
| 762 per 1000 | 692 per 1000 (607 to 769) | |||||
| Medium‐risk population | ||||||
| 773 per 1000 | 704 per 1000 (619 to 780) | |||||
| Overall survival Follow‐up: 24 months4 | Study population | HR 0.82 (0.65 to 1.02) | 358 (2 studies3) | ⊕⊕⊕⊝ moderate2 | ||
| 912 per 1000 | 864 per 1000 (794 to 916) | |||||
| Medium‐risk population | ||||||
| 952 per 1000 | 917 per 1000 (861 to 955) | |||||
| Death owing to brain metastasis | Study population | RR 0.92 (0.64 to 1.32) | 286 (1 study3) | ⊕⊕⊕⊝ moderate2 | ||
| 309 per 1000 | 284 per 1000 (198 to 408) | |||||
| Medium‐risk population | ||||||
| 309 per 1000 | 284 per 1000 (198 to 408) | |||||
| Local tumor control Follow‐up: 12 months1 | Study population | HR 0.27 (0.14 to 0.52) | 129 (2 studies3) | ⊕⊕⊕⊝ moderate2 | ||
| 439 per 1000 | 145 per 1000 (78 to 260) | |||||
| Medium‐risk population | ||||||
| 644 per 1000 | 243 per 1000 (135 to 416) | |||||
| Functionally independent survival (KPS) Follow‐up: 6 months | Study population | RR 0.78 (0.61 to 1) | 145 (1 study3) | ⊕⊕⊕⊝ moderate2 | ||
| 725 per 1000 | 565 per 1000 (442 to 725) | |||||
| Medium‐risk population | ||||||
| 725 per 1000 | 565 per 1000 (442 to 725) | |||||
| Steroid use Follow‐up: 6 months | Study population | RR 0.64 (0.42 to 0.97) | 118 (1 study3) | ⊕⊕⊝⊝ low2 | ||
| 545 per 1000 | 349 per 1000 (229 to 529) | |||||
| Medium‐risk population | ||||||
| 546 per 1000 | 349 per 1000 (229 to 530) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; KPS: Karnofsky Performance Status; RR: risk ratio; SRS: stereotactic radiosurgery. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 12 months was used to calculate baseline rates, since we used a HR in the main analysis.
2 Estimate is imprecise as there is a fair degree of uncertainty in the pooled estimate as indicated by 95% confidence interval.
3 Downgraded to moderate quality of evidence because, "further research is very unlikely to change our confidence in the estimate of effect", may not be true. Evidence from more relevant trials would be welcome.
4 24 months was used to calculate baseline rates, since we used an HR in the main analysis.
Background
This review is an update of a previously published review in The Cochrane Library (Issue 6, 2010) on whole brain radiation therapy (WBRT) alone versus WBRT plus stereotactic radiosurgery (SRS) for the treatment of brain metastases.
Description of the condition
Approximately 20% to 40% of patients with cancer will go on to develop brain metastases (Andrews 2004; Hasegawa 2003). Primary tumor histologies most commonly include nonsmall cell lung cancer, breast cancer, melanoma, colon cancer, and renal cell carcinoma (Chidel 2000; Flickinger 1994; Hasegawa 2003; Pirzkall 1998). The median survival of patients after diagnosis of brain metastases is less than six months (Li 2000).
Description of the intervention
Historically, WBRT has been utilized as the main treatment modality for the management of brain metastases (Hasegawa 2003; Sneed 1999). Before WBRT, survival rates averaged one to two months with the administration of corticosteroids (Andrews 2004; Pirzkall 1998; Tsao 2012). The addition of WBRT to steroids extended median survival to three to six months (Andrews 2004; Flickinger 1994; Hasegawa 2003; Kondziolka 1999; Sneed 1999). However, in the last decade there has been mounting evidence enumerating the toxic effects of WBRT especially, serious neuro‐cognitive impairments (Hasegawa 2003). Two randomized controlled trials (RCTs) of patients with solitary brain metastasis have shown that combined treatment of surgical resection (craniotomy) with WBRT improved survival rates and led to greater local tumor control than WBRT alone (Flickinger 1994; Pirzkall 1998). It has since been suggested WBRT and SRS together can produce similar results (Sneed 1999). SRS, developed by Swedish neurosurgeon Lars Leksell in 1951, is a technique that focuses high‐dose radiation at precise intracranial targets (Andrews 2004). Radiosurgical procedures are non‐invasive, provide excellent local tumor control, and can be used to treat multiple tumors with minimal dose overlapping (Fuller 1992; Kondziolka 1999).
Why it is important to do this review
In the past, WBRT has been the standard treatment for brain metastases; however, SRS is being increasingly used for the management of brain metastases. How and in what situations these two treatments should be combined or used individually remains to be definitively answered. Therefore, defining the role of SRS in the management of patients with brain metastases has become critical.
Objectives
To assess the efficacy of WBRT plus SRS versus WBRT alone in the treatment of adult patients with brain metastases.
Methods
Criteria for considering studies for this review
Types of studies
RCTs
Types of participants
Adult patients (over 18 years of age) with newly diagnosed metastases (single or multiple) in the brain resulting from any primary, extracranial cancer were included. Patients who had received previous cranial radiation were excluded.
Types of interventions
Intervention:
WBRT with SRS for upfront treatment of single or multiple brain metastases.
Comparison:
WBRT alone.
Salvage treatments (i.e. treatments after initial treatment failure) should follow clinical protocol.
Types of outcome measures
Primary outcomes
Overall survival (OS): death from all causes from time of randomization.
Disease‐specific survival (DSS): death from metastases of the brain.
Functionally independent survival (FIS): as measured using a Karnofsky Performance Scale (KPS) (Karnofsky 1949) baseline or some equivalent system of measurement.
Secondary outcomes
Local tumor control: as defined by either a complete response, partial response, or stable response of all metastases known at time of randomization.
Adverse events (radiation necrosis, new neurologic deficit, peritumoral edema).
Neurologic performance.
Quality of life (QoL), measured using a validated scale.
Steroid requirement.
Search methods for identification of studies
Papers in all languages were sought and translations carried out where necessary.
Electronic searches
In the original review the following electronic databases were searched in the following order to identify trials for inclusion in this review: MEDLINE (1966 to 2009) (Appendix 1) including CancerLit (1975 to 2003); Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 2, 2009) (Appendix 2); and EMBASE (1980 to 2009) (Appendix 2).
The following subsequent electronic databases were searched in May 2012 to identify new trials for inclusion in this updated review: CENTRAL (Issue 5, 2012) (Appendix 3), MEDLINE (2009 to May week 4 2012) (Appendix 4), and EMBASE (2009 to 2012 week 21) (Appendix 5). A standard strategy was employed to search each electronic database. Three separate search buckets were independently created using the 'OR' operator. These buckets focused on identifying RCTs, diseases of interest, and interventions of interest. All three buckets were then combined using the 'AND' operator to yield the final data bucket. Duplicates and non‐human applications were then eliminated from this bucket. Please Note: elements of the search strategies have been adopted from those detailed in Hart 2004.
For MEDLINE (1966 to 2009) and (2009 to May week 4 2012) search strategies terms one to 10 were originally devised and have been revised by Carol Lefebvre and Steve McDonald at the UK Cochrane Centre for the identification of all randomized and clinical controlled trials. For further source detail, please see Higgins 2011.
All relevant articles found were identified on PubMed and using the 'related articles' feature, a further search was carried out for newly published articles.
The search strategies for the original review were developed and executed by the review author team. For this update the search strategies were revised and run by Jane Hayes, Information Manager for the Cochrane Gynecological Cancer Group.
Searching other resources
Unpublished and gray literature
Meta‐Register, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov, and www.cancer.gov/clinicaltrials were searched for ongoing trials. The main investigators of any relevant ongoing trials were contacted for further information, as were any major co‐operative trials groups active in this area.
Reference lists and correspondence
The citation lists of all included trials were checked and experts in the field contacted to identify further reports of trials.
Data collection and analysis
Selection of studies
All titles and abstracts retrieved by electronic searching were downloaded to the reference management database Endnote, duplicates were removed, and the remaining references were examined by two review authors (KP, CP) independently. Review authors were not blinded to the authors or affiliations of the studies. Those studies that clearly did not meet the inclusion criteria were excluded and copies of the full text of potentially relevant references were obtained. The eligibility of retrieved papers was assessed independently by two review authors (KP, CP). Disagreements were resolved by discussion between the two review authors. Reasons for exclusion were documented.
Data extraction and management
For included trials, data were abstracted as recommended in Chapter 7 of Higgins 2011. This included data on the following:
author, year of publication, and journal citation (including language);
country;
setting;
inclusion and exclusion criteria;
study design, methodology;
-
study population:
total number enrolled;
patient characteristics;
age;
sex;
comorbidities;
previous treatment;
neurologic performance;
primary cancer type;
-
brain metastases details at diagnosis:
size of metastases (including largest);
number of brain metastases;
tumor histology;
-
intervention details:
-
details of SRS;
type,
dose,
fractions,
maximum radiosurgical dose (Dmax),
dose to the tumor margin and isodose line,
duration;
-
details of WBRT;
type,
dose,
fractions,
duration;
-
risk of bias in study (Assessment of risk of bias in included studies);
duration of follow‐up;
-
outcomes included OS, FIS, local tumor control, cause of death, steroid requirement, and adverse events:
-
OS:
definition: OS was measured from date of randomization until death or last follow‐up,
unit of measurement: months;
-
FIS;
-
assessed via the KPS. The KPS score runs from 100 to 0, where 100 is perfect health and 0 is death:
100% ‐ normal, no complaints, no signs of disease;
90% ‐ capable of normal activity, few symptoms or signs of disease;
80% ‐ normal activity with some difficulty, some symptoms or signs;
70% ‐ caring for self, not capable of normal activity or work;
60% ‐ requiring some help, can take care of most personal requirements;
50% ‐ requires help often, requires frequent medical care;
40% ‐ disabled, requires special care and help;
30% ‐ severely disabled, hospital admission indicated but no risk of death;
20% ‐ very ill, urgently requiring admission, requires supportive measures or treatment;
10% ‐ moribund, rapidly progressive fatal disease processes;
0% ‐ death;
-
-
local tumor control:
defined as decrease or no change in tumor size as judged by serial post‐treatment magnetic resonance imaging (MRI) scans;
-
DSS:
definition: death owing to neurologic cause that is because of brain metastasis
-
steroid requirement:
steroid requirement was measured as unchanged, improved, or worsened;
patients with brain metastases are often managed with steroids to decrease cerebral edema. Longer steroid use has been implicated in many medical complications including worsened sugar control and increased cardiovascular risk;
-
adverse events:
-
treatment toxicities were classified in the trial of Andrews 2004 as:
acute (within 90 days of radiation treatment) or
late toxicities and included nausea/vomiting, hearing loss, skin, neurologic, and other toxicities. These were graded as per the Radiation Therapy Oncology Group (RTOG) central nervous system (CNS) toxicity criteria (Appendix 6).
-
-
Data on outcomes were be extracted as below
For time to event (e.g. OS, DSS, and local tumor control rates) data, we extracted the log of the hazard ratio [log(HR)] and its standard error from trial reports; if these were not reported, we attempted to estimate them from other reported statistics using the methods of Parmar 1998.
For dichotomous outcomes (e.g. adverse events or deaths if it was not possible to use a HR), we extracted the number of patients in each treatment arm who experienced the outcome of interest and the number of patients assessed at end point, in order to estimate a risk ratio (RR).
For continuous outcomes (e.g. QoL measures), we extracted the final value and standard deviation (SD) of the outcome of interest and the number of patients assessed at end point in each treatment arm at the end of follow‐up, in order to estimate the mean difference (if trials measured outcomes on the same scale) or standardized mean differences (if trials measured outcomes on different scales) between treatment arms and its standard error.
Where possible, all data extracted were those relevant to an intention‐to‐treat (ITT) analysis, in which participants were analyzed in groups to which they were assigned.
The time points at which outcomes were collected and reported were noted.
Data were abstracted independently by two review authors (KP, CP) onto a data abstraction form specially designed for the review. Differences between review authors were resolved by discussion or by appeal to a third review author if necessary.
Assessment of risk of bias in included studies
Risk of bias in included RCTs was assessed using the following questions and criteria (see Chapter 8 of Higgins 2011):
Sequence generation
Was the allocation sequence adequately generated?
Yes, for example a computer‐generated random sequence or a table of random numbers.
No, for example date of birth, clinic id number or surname.
Unclear, for example not reported.
Allocation concealment
Was allocation adequately concealed?
Yes, for example where the allocation sequence could not be foretold.
No, for example allocation sequence could be foretold by patients, investigators, or treatment providers.
Unclear, for example not reported.
Blinding
Assessment of blinding was restricted to blinding of outcome assessors, since it would not be possible to blind participants and treatment providers to the different interventions.
Was knowledge of the allocated interventions adequately prevented during the study?
Yes.
No.
Unclear.
Incomplete reporting of outcome data
We recorded the proportion of participants whose outcomes were not reported at the end of the study.
Were incomplete outcome data adequately addressed?
Yes, if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms.
No, if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms.
Unclear if loss to follow‐up was not reported.
Selective reporting of outcomes
Are reports of the study free of suggestion of selective outcome reporting?
Yes, for example if review reported all outcomes specified in the protocol.
No, otherwise.
Unclear, if insufficient information available.
Other potential threats to validity
Was the study apparently free of other problems that could put it at a high risk of bias?
Yes.
No.
Unclear.
The 'Risk of bias' tool was applied independently by two review authors (KP, CP) and differences were resolved by discussion. Results are presented in both a 'Risk of bias' graph and a 'Risk of bias' summary. Results of meta‐analyses were interpreted in light of the findings with respect to risk of bias.
Measures of treatment effect
We used the following measures of the effect of treatment:
for time to event data, we used the HR, where possible;
for dichotomous outcomes, we used the RR;
for continuous outcomes (e.g. QoL measures), we used the mean difference between treatment arms.
Dealing with missing data
We did not impute missing outcome data. For the primary outcome, if data were missing or only imputed data were reported, we contacted trial authors to request data on the outcomes among participants who were assessed.
Assessment of heterogeneity
Heterogeneity between studies was assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials that cannot be ascribed to sampling variation (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, the possible reasons for this were investigated and reported.
Assessment of reporting biases
Reporting biases were not assessed as there was an insufficient number of included trials in which to compute funnel plots to assess the potential for small study effects such as publication bias.
Data synthesis
If sufficient, clinically similar studies were available their results were pooled in meta‐analyses.
For time‐to‐event data, HRs were pooled using the generic inverse variance facility of RevMan 5 (RevMan 2011).
For dichotomous outcomes, the RR was calculated for each study and these were then pooled.
For continuous outcomes, the mean differences between the treatment arms at the end of follow‐up were pooled if all trials measured the outcome on the same scale, otherwise standardized mean differences were pooled.
Random‐effects models with inverse variance weighting were used for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
Factors such as age, number of metastases, and length of follow‐up, were considered in interpretation of any heterogeneity.
Sensitivity analysis
Sensitivity analysis was not performed as there was an insufficient number of trials in the review.
Results
Description of studies
Results of the search
The search strategy identified 482 references in MEDLINE including Cancer‐Lit, 1961 in EMBASE and 38 in CENTRAL. Reference lists and correspondence did not produce any additional studies. A total of seven articles were retrieved in full. The full‐text screening of these seven references excluded four studies for the reasons described in the table Characteristics of excluded studies. The remaining three RCTs (two full articles and one abstract) met our inclusion criteria and are described in the table Characteristics of included studies, but only two were included in the analysis. Searches of the gray literature did not identify any additional relevant trials.
Included studies
Three RCTs met our inclusion criteria. Chougule 2000 was presented in abstract form only and included 109 patients who were randomized into WBRT‐only, WBRT plus SRS and SRS‐only groups. No difference in overall median survival was reported in the WBRT‐only and WBRT plus SRS groups. Local control was reported as being superior in the WBRT plus SRS group (91%) versus 62% in the WBRT‐only group. No other outcomes were evaluated in this trial. The abstract only reported median survival and local control in the different groups without providing P values or Kaplan‐Meier analysis. Further details about the trial could not be obtained from the authors. Hence, this RCT was not included in the current meta‐analysis.
Our meta‐analysis included two trials (Andrews 2004; Kondziolka 1999) that randomized 358 participants all of whom were assessed at the end of the trials. Andrews 2004 was by far the largest and only Phase III multi‐institutional RCT to compare outcomes in patients who received WBRT plus SRS (n = 164) versus WBRT‐only (n = 167). This trial included adults with one to three brain metastases with KPS > 70. Outcomes reported included OS, local control, KPS, cause of death, steroid requirement, and neurologic performance. OS was stratified for patients with one metastasis and more than one metastasis. In addition Andrews 2004 stratified survival according to recursive partitioning analysis (RPA) class. RPA class prognosticates survival and outcomes in patients with brain metastases. RPA Class 1 patients are those who have a KPS ≥ 70, controlled primary status, age < 65 years, and have no extracranial disease. Andrews 2004 analyzed RPA Class I patients separately and reported significantly longer survival in the WBRT plus SRS group (11.6 months) versus WBRT (9.6 months) (P = 0.045). No such stratification was available in the other studies.
Kondziolka 1999 was a single‐institution RCT that was stopped following an interim analysis of 27 patients that revealed a significant benefit in the rate of local control in the WBRT plus SRS group. This trial included patients with two to four brain metastases that were 25 mm or less. Local tumor control was the primary outcome and OS was also evaluated. No other outcomes were assessed. Follow‐up MRI scans were read by an independent blinded observer. This trial found a statistically significant difference in local control of tumors in the WBRT plus SRS group compared to WBRT‐only group. Survival was similar in both groups.
See Characteristics of included studies for details.
Excluded studies
The full text was obtained for five additional references, but all were excluded from the review for the reasons given in Characteristics of excluded studies.
None of the five excluded studies were RCTs. Feng 2002, Sanghavi 2001, and Sneed 2002 were retrospective studies, while Li 2000 and Minniti 2010 were prospective non‐RCTs.
Risk of bias in included studies
All three included trials (Andrews 2004; Chougule 2000; Kondziolka 1999) were at high risk of bias: they satisfied at most only two of the criteria that were used to assess risk of bias. The trial of Chougule 2000 was at extremely high risk of bias as it was only in abstract form and did not satisfy any of the criteria (Figure 1; Figure 2).
Figure 1.
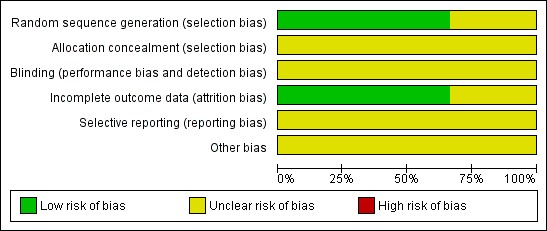
Figure 2.

Two trials (Andrews 2004; Kondziolka 1999) reported the method of generation of the sequence of random numbers used to allocate women to treatment arms, but concealment of this allocation sequence from patients and healthcare professionals involved in the trial was not reported. In the trial of Chougule 2000 it was unclear whether the method of assigning patients to treatment groups was carried out using an adequate method of sequence generation and it was also unclear whether an attempt to conceal the allocation was made. None of the trials reported whether the outcome assessors were blinded. In two of the trials (Andrews 2004; Kondziolka 1999) 100% of patients who were enrolled were assessed at end point, but this was unclear in the trial of Chougule 2000. There was insufficient information to permit judgment as to whether any of the trials reported all the outcomes that they assessed.
Other potential sources of bias: performance bias
The trials of Andrews 2004 and Kondziolka 1999 both indicate that participants were allowed to pursue further treatment upon tumor recurrence or progression, or both. Kondziolka 1999 presents outcomes of patients initially assigned to WBRT alone who later were treated with delayed salvage SRS as a third treatment group. Aside from this discreet cohort, neither trial clearly elaborates the number of patients who required further interventions or the extent of successive interventions. These successive treatments may confound interpretation of survival data. It was not certain whether any other bias may have been present in any of the three trials.
Effects of interventions
See: Table 1
Overall survival
Using an HR to compare the survival experience of women in the two treatment groups, a meta‐analysis of two trials (Andrews 2004; Kondziolka 1999), assessing 358 participants, found no statistically significant difference in OS between the WBRT plus SRS and the WBRT‐alone groups (HR 0.82; 95% CI 0.65 to 1.02; Analysis 1.1). The percentage of the variability in effect estimates that was because of heterogeneity rather than by chance was not important (I2 = 0%).
Analysis 1.1.

Comparison 1 WBRT plus radiosurgery versus WBRT, Outcome 1 Overall survival.
Subgroup analysis for overall survival
Only the trial of Andrews 2004 included and analyzed patients with one brain metastasis. For patients with one brain metastasis, median survival was significantly longer in the WBRT plus SRS group (6.5 months) versus the WBRT‐only group (4.9 months) (P = 0.04). Similarly, Andrews 2004 analyzed RPA Class I patients separately and reported significantly longer survival in the WBRT plus SRS group (11.6 months) versus WBRT‐only group (9.6 months) (P = 0.045). No such stratification was available in the other trials.
Disease‐specific survival
Only Andrews 2004 reported data on DSS. Cause of death was ascertained in 149 out of 167 participants in the WBRT group and 137 out of 164 patients in the WBRT plus SRS group. They found no significant difference in the risk of death from metastases of the brain in the WBRT plus SRS group (28%) compared to the WBRT‐only group (31%) (RR 0.92; 95% CI 0.64 to 1.32 (Analysis 1.2).
Analysis 1.2.
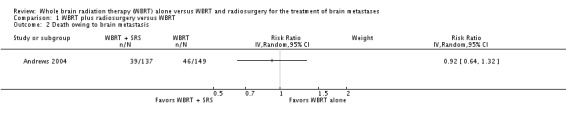
Comparison 1 WBRT plus radiosurgery versus WBRT, Outcome 2 Death owing to brain metastasis.
Local tumor control/failure
Local control was defined as unchanged or improved post‐treatment MRI scans. When a treated tumor increased in size on follow‐up MRI scan, it was deemed a local failure. Local control was assessed in 135 participants each in both treatment groups in the trial of Andrews 2004 and in all participants in the Kondziolka 1999 trial. The addition of SRS to WBRT increased local control of tumors in both the included studies. Meta‐analysis of two trials (Andrews 2004; Kondziolka 1999), assessing 358 participants, found patients receiving WBRT plus SRS had less chance of local failure than patients who received WBRT alone (HR 0.27; 95% CI 0.14 to 0.52) (Analysis 1.3). The percentage of the variability in effect estimates that was because of heterogeneity rather than by chance was not important (I2 = 0%).
Analysis 1.3.
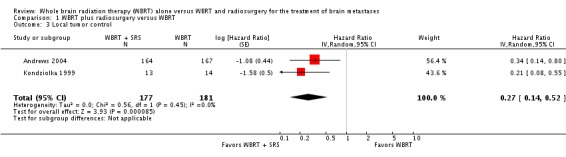
Comparison 1 WBRT plus radiosurgery versus WBRT, Outcome 3 Local tumor control.
Functionally independent survival
Only the trial of Andrews 2004 reported on functional or performance status. This trial compared KPS scores before and six months after treatment (WBRT plus SRS or WBRT only). At six months 75 participants in WBRT‐only group and 79 in WBRT plus SRS group were available for outcome assessment. KPS was assessed in 69 out of 75 participants at six months in the WBRT‐only group (six missing) and in 76 out of 79 participants in the WBRT plus SRS group (three missing). Patients who received WBRT plus SRS for treatment of brain metastases were associated with significantly (borderline) less chance of a worse KPS score at six months compared to those who received WBRT only (RR 0.78; 95% CI 0.61 to 1.00; P = 0.05), although statistical significance was only marginally significant at the 5% level (Analysis 1.4).
Analysis 1.4.
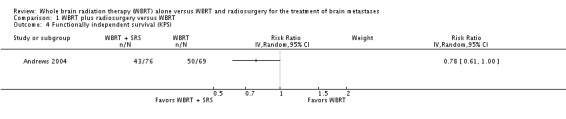
Comparison 1 WBRT plus radiosurgery versus WBRT, Outcome 4 Functionally independent survival (KPS).
Quality of life
None of the RCTs assessed or reported a QoL measure.
Steroid requirement
The trial of Andrews 2004 studied the need for steroids six months after treatment in both groups. Steroid requirement was assessed in 55 out of 75 participants at six months in the WBRT‐only group (20 missing) and in 63 out of 79 participants in the WBRT plus SRS group (13 missing). This trial found that patients who received WBRT plus SRS for treatment of brain metastases were associated with significantly less chance of prolonged steroid use compared to those who received WBRT only (RR 0.64; 95% CI 0.42 to 0.97; P = 0.03) (Analysis 1.5).
Analysis 1.5.

Comparison 1 WBRT plus radiosurgery versus WBRT, Outcome 5 Steroid use.
Adverse events
One trial (Andrews 2004) reported treatment toxicities after WBRT plus SRS versus WBRT‐only only. Acute and late toxicities were assessed in 166 and 112 participants, respectively, in the WBRT group and 160 and 113 participants in the WBRT plus SRS group. Acute toxicities (within 90 days of treatment) were similar in the WBRT plus SRS group versus WBRT‐only group. They most commonly included skin changes, nausea or vomiting, and CNS deficit or toxicity. In the WBRT plus SRS group 43% of patients reported Grade I toxicity, 18% reported Grade 2 toxicity, 2% Grade 3 toxicity and 1% Grade 4 toxicity. In comparison, 36% of patients with WBRT only reported Grade I toxicity and 26% reported Grade 2 toxicity. Similarly, late toxicities did not differ between treatment groups and most commonly included CNS deficit/toxicity. The study concluded that acute and late toxicities did not increase significantly with the addition of SRS (Appendix 6).
Kondziolka 1999 reported no neurologic or systemic morbidity related to SRS and only commented that WBRT was associated with mild scalp erythema and hair loss.
Discussion
Summary of main results
Overall, WBRT plus SRS did not significantly improve survival in patients with brain metastases as compared to WBRT alone. Analysis of all included patients in neither trial showed a survival benefit from the addition of SRS to WBRT but there was a survival benefit reported by the trial of Andrews 2004 for patients with a single metastasis and for patients that were RPA Class I. The large, multicenter cohort in the trial of Andrews 2004 showed that WBRT plus SRS statistically improved median survival in patients with single, unresectable metastatic foci as compared to WBRT alone. Of note, only Andrews 2004 included and analyzed patients with single brain metastases. For patients with one brain metastasis, median survival was significantly longer in the WBRT plus SRS group (6.5 months) versus the WBRT group (4.9 months). Additionally, Andrews 2004 analyzed RPA Class I patients separately and reported significantly longer survival in the WBRT plus SRS group (11.6 months) versus WBRT (9.6 months). Patients with unresectable lesions (either located in deep gray matter or in areas of eloquent cortex) typically are treated with WBRT alone thereby missing the known advantage conferred by surgical resection plus WBRT. However, the data from this RCT suggests WBRT followed by radiosurgical boost similarly improves median survival in this oncologic niche.
In the analysis of all included patients, combined therapy improved local tumor control. Compared with WBRT alone, addition of SRS to WBRT increased local control of tumors in both the included studies. When a treated tumor increased in size on follow‐up MRI scan, it was deemed a local failure. Kondziolka 1999 discontinued their control treatment arm (WBRT alone) after interim analysis performed at the 60% accrual mark showed markedly improved local control in the combined treatment group. Similarly, Andrews 2004 reported a 43% greater risk of local recurrence with WBRT alone. Our analysis showed that patients receiving WBRT plus SRS had significantly lower local failures compared to WBRT alone.
One of the most important clinical measures of treatment efficacy is performance status or functional outcome. Andrews 2004 compared KPS scores before and six months after treatment (WBRT plus SRS or WBRT‐only). Statistically significant improvement in KPS scores was reported in the WBRT plus SRS group compared to the WBRT only group. Forty‐three per cent of patients in the WBRT plus SRS group had unchanged or improved KPS at six months post‐treatment versus only 28% in WBRT group. And although none of the trials indicated patient‐reported measures of QoL, Andrews 2004 assessed the need for long‐term steroid three months status post‐intervention. They found that 65% of patients in the WBRT plus SRS group had decreased steroid use (and most were not taking steroids) compared to 45% with decreased steroid use in the WBRT group. Decreased steroid requirement likely diminishes the associated comorbidities of long‐term steroid use including weight gain, poor glycemic control, and successive increase in cardiovascular risk and may contribute to a better QoL or functional status.
Treatment‐related morbidity did not change significantly with the addition of SRS to WBRT. Kondziolka 1999 reported "no neurologic or systemic morbidity related to SRS" and only mild scalp erythema and hair loss associated with WBRT. Andrews 2004, reported similar rates of acute toxicities (within 90 days of treatment) across treatment groups. Most commonly reported side effects included skin changes, nausea/vomiting, and CNS deficit/toxicity. Similarly, late toxicities did not differ between treatment groups and most commonly included CNS deficit/toxicity. Andrews 2004 concluded that neither acute nor late toxicities increase significantly with the addition of SRS, further validating the addition of radiosurgical boost to WBRT without significant risk of harm to the patient.
Overall completeness and applicability of evidence
The Kondziolka 1999 and Andrews 2004 trials were aimed at evaluating the precise question we were trying to answer in this review: Is the addition of upfront SRS to WBRT better than WBRT alone? Kondziolka 1999 focused on local control as their primary outcome and their study was stopped because of the benefit seen in the WBRT plus SRS group. Therefore, their study was not powered to detect a difference in OS or any other outcomes. They did not assess functional outcome or QoL, which are extremely important primary outcomes in any palliative treatment. Andrews 2004 conducted a large well‐designed multicenter RCT and appropriately evaluated many key outcomes including, OS, local control, performance status, steroid requirement, and cause of death. Neurocognitive performance and overall QoL was not assessed adequately in either trial and needs to be the focus of future investigations. Since SRS and WBRT may have different effects on cognition, especially in long‐term survivors, it is imperative that future trials use neurocognitive performance as one of their primary end points. These results should change current practice of WBRT‐only for all patients with multiple brain metastases and SRS should be added as upfront treatment for selected patients.
Quality of the evidence
Two RCTs, one large multicenter RCT (Andrews 2004) and one small single‐institution RCT (Kondziolka 1999), form the basis of our systematic review and its conclusions. Overall both studies had an unclear risk of bias and they satisfied at most only two of the criteria that we used to assess risk of bias. Given this risk of bias, the results and conclusions of our review have to be interpreted in the context of this uncertainty.
Both trials are consistent in showing that local control is superior in the WBRT plus SRS group compared to WBRT‐only group and that survival is similar in the two groups. The trial of Andrews 2004 assessed other outcomes such as performance status, steroid requirement, and cause of death. Conclusions based on these outcome measures are derived solely from this large multicenter RCT and may be prone to bias. For example, performance status was only assessed six months after treatment and hence may not accurately represent the performance status at other time points. Kondziolka 1999 only used local control as their primary end point in a small RCT and did not investigate functional outcome, cognitive outcome, or QoL. Hence, the majority of the results and conclusions are based on a single large RCT (Andrews 2004), which limits the internal validity of this systematic review.
Potential biases in the review process
A comprehensive search was performed, including a thorough search of the gray literature and all studies were sifted and data extracted by at least two review authors independently. We restricted the included studies to RCTs as they provide the strongest level of evidence available. Hence, we have attempted to reduce bias in the review process. The greatest threat to the validity of the review is likely to be the possibility of publication bias, that is studies that did not find the treatment to have been effective may not have been published. We were unable to assess this possibility as the analyses were restricted to meta‐analyses of a small number of trials or single trials.
Despite our best efforts, we were not able to get detailed data on one RCT (Chougule 2000), which was published in abstract form. Therefore, data from this trial were not available for meta‐analysis.
Agreements and disagreements with other studies or reviews
Sanghavi 2001 reported improved survival in patients treated with WBRT plus SRS compared to WBRT alone in a large retrospective multi‐institutional analysis. Patients with WBRT plus SRS and RPA Class I had median survival of 16.1 months versus 7.1 months (P < 0.05). This result is in disagreement with our review and all three RCTs included in this review. It is very likely that there was a strong selection bias in this retrospective analysis. No other outcomes, such as local control, were evaluated.
Li 2000, in a prospective non‐RCT, evaluated outcomes in patients with single lung cancer metastasis. Three treatment groups, WBRT only, SRS only, and WBRT plus SRS, were compared. Similar to the Sanghavi 2001 study, Li 2000 reported longer median survival in patients who received WBRT plus SRS (10.6 months) versus WBRT only (5.7 months) (P < 0.0001). Li 2000 reports superior local control and KPS along with a lower neurologic death rate in the WBRT plus SRS group compared to WBRT alone.
One retrospective study also report a similar survival and local tumor control advantage in the WBRT plus SRS group compared to WBRT only (Feng 2002).
The OS advantage seen in these retrospective studies is again likely to be because of a strong selection bias in a non‐RCT setting. Local control, KPS, and cause of death data appear to agree with the results of the Andrews 2004 trial.
Authors' conclusions
The conclusions we have presented are based on only two RCTs. Since the last version of this review no new studies were found. The risk of bias in both these trials was unclear. Therefore, our results and conclusions have to be interpreted in the context of unclear study bias. In an analysis of all included patients SRS plus WBRT did not show a survival benefit over WBRT alone. However, local control and functional outcome were significantly better in the SRS plus WBRT group. Furthermore, significantly longer OS was reported in the combined treatment group for RPA Class I patients as well as patients with single metastasis. Finally, there was no increase in treatment toxicity with the addition of SRS to WBRT. Therefore, we conclude the following:
patients with a single unresectable brain metastases should be treated with SRS plus WBRT;
patients who are RPA Class I should be treated with SRS plus WBRT;
patients with two to four brain metastases should be treated with SRS plus WBRT on the basis of better functional outcome, local control, and decreased steroid requirement.
Further trials designed to have a low risk of bias and sufficient sample size are needed to affirm the results and conclusions of this systematic review. Future trials should also rigorously compare the QoL and cognitive performance of patients undergoing WBRT plus SRS versus WBRT alone. Also, knowing the significant neurocognitive side effects of WBRT in long‐term survivors, trials that omit upfront WBRT are being conducted.
Acknowledgements
Thanks to:
Clare Jess (Gynaecological Cancer Cochrane Group) for her enthusiasm, support, and guidance throughout the process;
Gail Quinn, for her help in the earlier stages of the process;
Dr Heather Dickinson, for her help in developing the statistical methods;
Lauren Maggio (Lane Medical Library), for her search expertise in the original review;
Jane Hayes, Information Manager for the Cochrane Gynaecological Cancer Group, for the revising and running the searches in this update;
Our peer reviewers, for their constructive criticisms and helpful feedback;
Dr Chris Williams, for his editorial guidance.
Appendices
Appendix 1. MEDLINE original search strategy
1966 to 2009
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
animals.sh. not (humans.sh. and animals.sh.)
9 NOT 10
exp central nervous system neoplasm/
exp cerebral cortex/ab,pa,an,cy,su
exp Neoplasm Metastasis/
brain metastas$.mp.
intracranial tumo$.mp.
cerebral metastas$.mp.
(single adj3 metastas$).mp.
(solitary adj3 metastas$).mp.
12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19
radiosurgery/
radiosurg$.mp.
"stereotactic radiotherapy".mp.
"stereotactic surgery".mp
"stereotaxic technique$".mp.
21 OR 22 OR 23 OR 24 OR 25
exp radiotherapy/
radiotherapy.mp.
radiation therapy.mp.
irradiation.mp.
WBRT.mp.
27 OR 28 OR 29 OR 30 OR 31
11 AND 20 AND 26 AND 32
Appendix 2. CENTRAL and EMBASE original search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) Issue 2, 2009
exp central‐nervous‐system‐neoplasms.tw.
metastasis.tw.
metastases.tw.
secondary.tw.
secondaries.tw.
OR/1‐5
exp radiosurgery.tw.
radiosurg$.tw.
Stereotactic surgery.tw.
stereotaxic‐techniques.tw.
stereotactic radiotherapy.tw.
OR 7‐11
exp radiotherapy.tw.
radiation therapy.tw.
radiotherapy.tw.
irradiation.tw.
WBRT.tw.
OR 13‐17
6 AND 12
18 AND 19
EMBASE (1980 to 2009) search strategy
clinical trial/
controlled clinical trial/
multicenter study/
phase 2 clinical trial/
phase 3 clinical trial/
phase 4 clinical trial/
randomized controlled trial/
controlled study/
meta analysis/
crossover procedure/
double blind procedure/
single blind procedure/
randomization/
clinical study/
(clin$ adj25 trial$).tw.
((singl$ or doubl$ or triple$ or treb$) adj25 (blind$ or mask$)).tw.
random$.tw
control$.tw
OR/1‐18
limit 19 to human
brain neoplasm/
exp central nervous system tumor/
exp brain cortex/di,su
brain tumo?r.tw.
(metastasis).tw.
brain cancer/ or brain stem tumo$/ or brain tumo$/ or intracranial tumo$/ or posterior cranial fossa tumo$/
OR/21‐26
stereotactic radiosurgery/ or stereotaxic surgery/
SRT/
radiosurgery/
gamma knife radiosurgery/
radiosurg$.tw
stereotactic radiotherapy.tw
OR/28‐33
exp/radiotherapy/
irradiation/
WBRT/
OR/35‐37
27 AND 34
38 AND 39
20 AND 40
CancerLit (1975 to 2009) search strategy This database was searched with the strategy outlined for MEDLINE
Appendix 3. CENTRAL updated search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) Issue 5, 2012
1. MeSH descriptor Central Nervous System Neoplasms explode all trees 2. ((brain* or cerebr* or intracranial or intra‐cranial) adj5 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or metasta* or secondar*)) 3. (#1 OR #2) 4. MeSH descriptor Radiotherapy explode all trees 5. Any MeSH descriptor with qualifier: RT 6. (radiotherap* or radiat* or irradiat*) 7. WBRT 8. (#4 OR #5 OR #6 OR #7) 9. MeSH descriptor Stereotaxic Techniques explode all trees 10. (radiosurg* or (stereota* and (technique* or surg* or radiotherap*))) 11. (#9 OR #10) 12. (#3 AND #8 AND #11)
Appendix 4. MEDLINE updated search strategy
2009 to May week 4 2012
1. exp Central Nervous System Neoplasms/ 2. ((brain* or cerebr* or intracranial or intra‐cranial) adj5 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or metasta* or secondar*)).mp. 3. 1 or 2 4. exp Radiotherapy/ 5. radiotherapy.fs. 6. (radiotherap* or radiat* or irradiat*).mp. 7. WBRT.mp. 8. 4 or 5 or 6 or 7 9. exp Stereotaxic Techniques/ 10. (radiosurg* or (stereota* and (technique* or surg* or radiotherap*))).mp. 11. 9 or 10 12. 3 and 8 and 11 13. randomized controlled trial.pt. 14. controlled clinical trial.pt. 15. randomized.ab. 16. placebo.ab. 17. clinical trials as topic.sh. 18. randomly.ab. 19. trial.ti. 20. 13 or 14 or 15 or 16 or 17 or 18 or 19 21. 12 and 20 key: mp = title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier pt = publication type ab = abstract ti = title
Appendix 5. EMBASE updated search strategies
EMBASE search strategy
2009 to 2012 week 21
1. exp central nervous system tumor/ 2. ((brain* or cerebr* or intracranial or intra‐cranial) adj5 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or metasta* or secondar*)).mp. 3. 1 or 2 4. exp radiotherapy/ 5. rt.fs. 6. (radiotherap* or radiat* or irradiat*).mp. 7. WBRT.mp. 8. 4 or 5 or 6 or 7 9. exp radiosurgery/ 10. exp stereotactic procedure/ 11. (radiosurg* or (stereota* and (technique* or surg* or radiotherap*))).mp. 12. 9 or 10 or 11 13. 3 and 8 and 12 14. crossover procedure/ 15. double‐blind procedure/ 16. randomized controlled trial/ 17. single‐blind procedure/ 18. random*.mp. 19. factorial*.mp. 20. (crossover* or cross over* or cross‐over*).mp. 21. placebo*.mp. 22. (double* adj blind*).mp. 23. (singl* adj blind*).mp. 24. assign*.mp. 25. allocat*.mp. 26. volunteer*.mp. 27. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 28. 13 and 27
key: [mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Appendix 6. Central nervous system toxicity grading
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Motor | No weakness or no change | Subjective weakness/no objective findings | Mild objective weakness without significant impairment of function | Objective weakness with impairment of function |
| Sensory | None or no change | Mild paresthesias or loss of deep tendon reflexes | Mild to moderate objective sensory loss/paresthesias | Severe objective sensory loss or paresthesias that interfere with function |
Data and analyses
Comparison 1.
WBRT plus radiosurgery versus WBRT
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 2 | 358 | Hazard Ratio (Random, 95% CI) | 0.82 [0.65, 1.02] |
| 2 Death owing to brain metastasis | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3 Local tumor control | 2 | 358 | Hazard Ratio (Random, 95% CI) | 0.27 [0.14, 0.52] |
| 4 Functionally independent survival (KPS) | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5 Steroid use | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
What's new
Last assessed as up‐to‐date: 7 August 2012.
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 6, 2010
| Date | Event | Description |
|---|---|---|
| 24 February 2015 | Amended | Contact details updated. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 7 August 2012 | New citation required but conclusions have not changed | 1. Since the last version of this review no new studies were found; therefore, changes to this update were minimal. 2. The search was updated to include studies published from 2009 to 2012 from the following electronic databases: CENTRAL, MEDLINE, and EMBASE. 3. One new excluded study was added in this review: Minniti 2010. This is a prospective, non‐RCT and does not meet the current study's inclusion criteria. 4. There are no additional participants that are part of the review. 5. No further analyses were necessary in this review. 6. The updated search has not altered the conclusions from the last publication of this review. Given that no new RCTs were included in this review, we feel that it is low‐priority for previous readers of the review to re‐read this update. |
| 5 July 2012 | New search has been performed | 1. Electronic search methods section updated. 2. Added appendices 3 and 4. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andrews 2004
| Methods | Multi‐institutional, RCT Power = 0.8: study was designed to detect a 50% improvement in median survival for patients in the WBRT plus SRS group |
|
| Participants | Inclusion criteria: patients 18 years of age or older with no previous cranial radiation. MRI confirmed contrast enhancing, 1 to 3 metastatic brain tumors < 4 cm in diameter Exclusion criteria: KPS < 70, previous cranial radiation, brain stem metastasis or metastasis within 1 cm of optic apparatus, treatment of systemic cancer within 1 month, platelet count < 50,000 cells/μL, hemoglobin < 80 g/L, and absolute neutrophil count of < 1000 cells/μL This was the largest Phase III, multi‐institutional trial with 331 total patients randomized to WBRT plus SRS or WBRT only. Patients were stratified by number of brain metastases (1 versus 2 to 3) and extent of extracranial disease (none versus present) |
|
| Interventions | All patients received 37.5 Gy in 2.5‐Gy daily fractions WBRT plus SRS: 164 patients included in analysis, 31 patients did not receive SRS. SRS dose prescribed per RTOG 90‐05 trial WBRT: dose 37.5 Gy and all patients completed treatment |
|
| Outcomes | Primary outcome: median OS after randomization Secondary outcomes: 1. local control; 2. adverse events; 3. change in KPS; 4. cause of death; 5. steroid requirement |
|
| Notes | 15% of patients allocated to the SRS group did not receive SRS (all patients in both groups received WBRT) At 3 months, in the WBRT‐only group (n = 167), 32 patients had died, 57 cases did not have appropriate follow‐up scans and hence MRIs for only 78 patients (58%) were reviewed. In the WBRT plus SRS group (n = 164), 29 patients had died at 3 months, 60 patients did not have appropriate follow‐up scans, leaving 75 MRI (55%) sets for analysis Reporting bias is possible given cause of death and intracranial tumor progression was assessed by the treating physician at each participating institution |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation within strata by permutated blocks was done by use of computerized techniques at RTOG headquarters when member institutions telephoned to enrol eligible patients" |
| Allocation concealment (selection bias) | Unclear risk | There is no mention of allocation concealment in the manuscript |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % Analyzed in primary analyses: 331 out of 331 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Chougule 2000
| Methods | Single institution, RCT | |
| Participants | Patients with MRI confirmed 1 to 3 brain metastases, tumor volume < 30 cc and minimum of 3‐month life expectancy | |
| Interventions | WBRT alone: 31 patients received 30 Gy in 10 fractions WBRT plus SRS: 37 patients, 30 Gy WBRT in 10 fractions plus GK SRS 20 Gy to the tumor margin |
|
| Outcomes | Primary outcome: median OS Secondary outcome: local control |
|
| Notes | Abstract form only. No difference in median OS was reported in the WBRT only and WBRT plus SRS groups. Local control was reported as being superior in the WBRT plus SRS group (91%) versus 62% in the WBRT‐only group. No other outcomes were evaluated in this trial. The abstract only reported median survival and local control in the different groups without providing P values or Kaplan‐Meier analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Kondziolka 1999
| Methods | Single institution RCT Power = 0.8: study was designed to detect a 40% increase in local control after WBRT plus SRS |
|
| Participants | Inclusion criteria: patients with 2 to 4 MRI‐confirmed contrast‐enhancing brain metastases with a biopsy‐confirmed primary tumor. Tumor size ≤ 25 mm and > 5 mm from the optic chiasm. KPS ≥ 70 Exclusion criteria: KPS < 70 |
|
| Interventions | WBRT only: 14 patients received 30 Gy in 12 fractions WBRT plus SRS: 13 patients received 30‐Gy WBRT plus 16‐Gy SRS to tumor margin |
|
| Outcomes | Primary: local tumor control Secondary: OS |
|
| Notes | The study was stopped at 60% accrual at interim evaluation. The interim analysis revealed a "significant benefit in the rate of local tumour control" after WBRT plus SRS. Local control was assessed at 1.5, 3, 6, 9, 12, 15, and 18 months. The rate of local failure was 100% at 1 year in the WBRT alone group, "but only 8% in surviving patients who had SRS plus WBRT". No difference in OS was noted in both groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The method of randomization consisted of a coin toss at the initial clinic visit" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "The data were collated and reviewed by an investigator independent from each treatment arm." It is unclear if the investigator assessing outcomes was blinded, it only notes that the investigator was independent |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % Analyzed in primary analyses: 27 out of /27 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
GK: Gamma Knife; KPS: Karnofsky Performance Status; MRI: magnetic resonance imaging; OS: overall survival; RCT: randomized controlled trial; RTOG: Radiation Therapy Oncology Group; SRS: stereotactic radiosurgery; WBRT: whole brain radiation therapy.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Feng 2002 | Retrospective study, not an RCT |
| Li 2000 | Prospective non‐RCT. Evaluated outcomes in patients with single lung cancer metastasis. 3 treatment groups: WBRT only, SRS only, and WBRT plus SRS |
| Minniti 2010 | Prospective non‐RCT |
| Sanghavi 2001 | Retrospective multi‐institutional study, not an RCT |
| Sneed 2002 | Retrospective cohort study, not an RCT. Evaluated SRS alone versus SRS plus WBRT |
Contributions of authors
CP had the original idea for the protocol and helped review initial drafts of the protocol and prepared the final review. SG designed and wrote the protocol in collaboration with CP and helped prepare the final review. KP helped with the search and in preparing the final review. AB helped with the analysis and preparation of the final review. JS helped with the search and in preparing the final review. KB was the senior mentor who helped CP with the initial drafts of the review, gave expert opinion, and helped edit the review.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
- Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665‐72. [DOI] [PubMed] [Google Scholar]
- Chougule PB, Burton‐Williams M, Saris S, Zheng Z, Ponte B, Noren G, et al. Randomized treatment of brain metastasis with gamma knife radiosurgery, whole brain radiotherapy or both. International Journal of Radiation Oncology, Biology, Physics 2000;48(3S):114. [Google Scholar]
- Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. International Journal of Radiation Oncology, Biology, Physics 1999;45:427‐34. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Feng J, Reng Q, CHong‐ming XU. An analysis of treatment result for brain metastasis of stereotactic radiosurgery plus radiotherapy. Chinese Journal of Cancer Research on Prevention and Treatment 2002;29:394‐5. [Google Scholar]
- Li B, Yu J, Suntharalingam M, Kennedy AS, Amin PP, Chen Z, et al. Comparison of three treatment options for single brain metastasis from lung cancer. International Journal of Cancer 2000;90(1):37‐45. [PubMed] [Google Scholar]
- Minniti G, Salvati M, Muni R, Lanzetta G, Osti MF, Clarke E, et al. Stereotactic radiosurgery plus whole‐brain radiotherapy for treatment of multiple metastases from non‐small cell lung cancer. Anticancer Research 2010;30(7):3055‐61. [PubMed] [Google Scholar]
- Sanghavi SN, Miranpuri SS, Chappell R, Buatti JM, Sneed PK, Suh JH, et al. Radiosurgery for patients with brain metastases: a multi‐institutional analysis, stratified by the RTOG recursive partitioning analysis method. International Journal of Radiation Oncology, Biology, Physics 2001;51(2):426‐34. [DOI] [PubMed] [Google Scholar]
- Sneed PK, Suh JH, Goetsch SJ, Sanghavi SN, Chappell R, Buatti JM, et al. A multi‐institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. International Journal of Radiation Oncology, Biology, Physics 2002;53(3):519‐26. [DOI] [PubMed] [Google Scholar]
Additional references
- Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. International Journal of Radiation Oncology, Biology, Physics 2000;47:993‐9. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. Egger M, Davey Smith G, Altman DG (eds). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
- Flickinger JC, Kondziolka D, Lunsford LD, Coffey RJ, Goodman ML, Shaw EG, et al. A multi‐institutional experience with stereotactic radiosurgery for solitary brain metastases. International Journal of Radiation Oncology, Biology, Physics 1994;28:797‐802. [DOI] [PubMed] [Google Scholar]
- Fuller BG, Kaplan ID, Adler J, Cox RS, Bagshaw MA. Stereotaxic radiosurgery for brain metastases: the importance of adjuvant whole brain irradiation. International Journal of Radiation Oncology, Biology, Physics 1992;23:413‐8. [DOI] [PubMed] [Google Scholar]
- Hart MG, Grant R, Walker M, Dickinson H. Surgical resection and whole brain radiation therapy versus whole brain radiation therapy alone for single brain metastases. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD003292.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: An alternative to whole brain radiotherapy?. Neurosurgery 2003;52:1318‐25. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM editor(s). Evaluation of Chemotherapeutic Agents. New York: Columbia University Press, 1949:196. [Google Scholar]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
- Pirzkall A, Debus J, Lohr F, Fuss M, Rhein B, Engenhart‐Cabillic R, et al. Radiosurgery alone or in combination with whole‐brain radiotherapy for brain metastases. Journal of Clinical Oncology 1998;16:3563‐9. [DOI] [PubMed] [Google Scholar]
- Sneed PK, Lamborn KR, Forstner JM, McDermott MW, Chang S, Park E, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary?. International Journal of Radiation Oncology, Biology, Physics 1999;43:549‐58. [DOI] [PubMed] [Google Scholar]
- Tsao MN, Lloyd N, Wong RKS, Chow E, Rakovitch E, Laperriere N, et al. Whole brain radiotherapy for the treatment of multiple brain metastases. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD003869.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Patil CG, Pricola K, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD006121.pub2] [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.


