Abstract
Background
There have been a number of studies with conflicting results which have examined the effect of anti‐tuberculous therapy in Crohn's disease. A meta‐analysis was performed to evaluate the use of anti‐tuberculous therapy for the maintenance of remission in Crohn's disease.
Objectives
To evaluate the effects of anti‐tuberculous therapy for the maintenance of remission in patients with Crohn's disease.
Search methods
We searched MEDLINE, EMBASE, the Cochrane LIbrary, and the Cochrane IBD Group Specialized Register from inception to June 22, 2015.
Selection criteria
Randomized controlled trials (RCTs) of anti‐tuberculous therapy compared to placebo or another active therapy in patients with quiescent Crohn's disease were considered for inclusion.
Data collection and analysis
At least two authors independently extracted data and assessed the quality of included studies using the Cochrane risk of bias tool. We calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for dichotomous outcomes.. The primary outcome was relapse. Secondary outcomes included adverse events, withdrawals due to adverse events and serious adverse events. All data were analyzed on an intention‐to‐treat basis. The overall quality of the evidence supporting the primary and secondary outcomes was evaluated using the GRADE criteria.
Main results
Four placebo‐controlled RCTs including 206 participants were included. Three trials included an 8 to 16 week induction phase with tapering corticosteroids (prednisone, prednisolone or methylprednisolone) as induction therapy. Anti‐tuberculous therapy included monotherapy with clofazimine, combination therapy with clofazimine, rifampin, ethambutol, and dapsone or combination therapy with clarithromycin, rifabutin and clofazimine. All of the studies were rated as unclear risk of bias for allocation concealment, three were rated as unclear risk of bias for random sequence generation and two were rated as unclear risk of bias for blinding or participants and personnel. There was a statistically significant difference in relapse rates favoring anti‐tuberculous therapy over placebo. Thirty‐nine per cent (44/112) of patients in the anti‐tuberculous therapy group relapsed at 9 months to 2 years compared to 67% (63/94) of placebo patients (RR 0.58, 95% CI 0.45 to 0.75, I2 = 47%). A GRADE analysis indicates that the overall quality of the evidence supporting this outcome was very low due to unknown risk of bias and sparse data. Adverse events occurred more frequently in the anti‐tuberculous therapy group (37/159) compared to the placebo group (14/163) with a pooled RR of 2.57 (95% CI 1.45 to 4.55; N=322; studies=4, I2=64%). A GRADE analysis indicates that the overall quality of the evidence supporting this outcome was very low due to unknown risk of bias, unexplained heterogeneity and sparse data. There was no difference in withdrawals due to adverse events. Nine per cent (14/159) of anti‐tuberculous therapy patients withdrew due to adverse events compared to 7% (11/163) of placebo patients (RR 1.29, 95% CI 0.60 to 2.77, I2 = 0%). Common adverse events included increased skin pigmentation and rashes. No serious adverse events were reported in any of the included studies.
Authors' conclusions
Anti‐tuberculous therapy may provide a benefit over placebo for the prevention of relapse in participants with Crohn's disease in remission. However, this result is very uncertain due to unclear study quality and the small numbers of patients assessed. Further studies are needed to provide better quality evidence for the use of anti‐tuberculous therapy for maintaining remission in people with quiescent Crohn's disease.
Keywords: Humans, Secondary Prevention, Antitubercular Agents, Antitubercular Agents/adverse effects, Antitubercular Agents/therapeutic use, Clarithromycin, Clarithromycin/therapeutic use, Clofazimine, Clofazimine/therapeutic use, Crohn Disease, Crohn Disease/drug therapy, Crohn Disease/prevention & control, Ethambutol, Ethambutol/therapeutic use, Glucocorticoids, Glucocorticoids/therapeutic use, Maintenance Chemotherapy, Maintenance Chemotherapy/methods, Methylprednisolone, Methylprednisolone/therapeutic use, Prednisone, Prednisone/therapeutic use, Randomized Controlled Trials as Topic, Recurrence, Remission Induction, Rifabutin, Rifabutin/therapeutic use, Rifampin, Rifampin/therapeutic use
Plain language summary
Anti‐tuberculous therapy for maintaining remission in Crohn's disease
Tuberculous bacteria have been suggested as a possible cause of Crohn's disease due to a similarity between Crohn's and tuberculous lesions when viewed under a microscope. Four studies examined the use of anti‐tuberculous therapy to reduce the chance of the disease recurring in patients with non‐active Crohn's disease. The results of these studies suggest that this treatment might be effective for this purpose. However, this finding has not been definitively proven, and anti‐tuberculous therapy should not be used to treat Crohn's disease without further study.
What is Crohn's disease?
Crohn's disease is a chronic inflammatory disorder that can occur in any part of the gastrointestinal tract and can affect people of any age. Common symptoms include weight loss, diarrhoea and abdominal pain. When people with Crohn's disease experience these symptoms the disease is active. When patients no longer experience disease symptoms their disease is said to be in remission.
Review question
Prevention of relapse (recurrence of symptoms) is an important objective in the management of Crohn's disease. There is no current treatment available that completely prevents relapse and is without significant side‐effects. Tuberculous bacteria have been suggested as a possible cause of Crohn's disease due to a similarity between Crohn's and tuberculous lesions when viewed under a microscope. We wanted to see if anti‐tuberculous therapy is better than placebo for maintaining remission in patients with Crohn's disease
What is anti‐tuberculous therapy?
Anti‐tuberculous therapy generally consists of combinations of antibiotic and antibacterial type drugs.
What did the researchers investigate?
The researchers studied whether anti‐tuberculous therapy maintains remission in patients with Crohn's disease and whether it causes any harms (side effects). The investigators searched the medical literature extensively up to 22 June 2015.
What did the researchers find?
The researchers identified four studies that included a total of 206 participants. All of the studies were small in size and were judged to be of unclear quality. The studies compared anti‐tuberculous therapy (i.e. clofazimine or combination therapy with clofazimine, rifampin, ethambutol, and dapsone or combination therapy with clarithromycin, rifabutin and clofazimine) to placebo (inactive pills or tablets). Anti‐tuberculous therapy may provide a benefit over placebo for the prevention of relapse in participants with Crohn's disease in remission. However, this result is very uncertain due to unclear study quality and the small numbers of patients assessed. Participants receiving anti‐tuberculous drugs had more side effects than placebo participants. Common side effects included reversible pink skin discolouration and rash. No serious side effects were reported in the four studies. Further studies are needed to provide better quality evidence for the use of anti‐tuberculous therapy for maintaining remission in people with inactive Crohn's disease.
Summary of findings
Summary of findings for the main comparison. Anti‐TB therapy versus placebo for maintenance of remission in Crohn's disease.
| Anti‐TB therapy versus placebo for maintenance of remission in Crohn's disease | ||||||
| Patient or population: patients with maintenance of remission in Crohn's disease Settings: Intervention: Anti‐TB therapy versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Anti‐TB therapy versus placebo | |||||
| Relapse | 670 per 10001 | 389 per 1000 (302 to 503) | RR 0.58 (0.45 to 0.75) | 206 (4 studies) | ⊕⊝⊝⊝ very low2,3 | |
| Adverse events | 86 per 10001 | 221 per 1000 (125 to 391) | RR 2.57 (1.45 to 4.55) | 322 (4 studies) | ⊕⊝⊝⊝ very low2,4,5 | |
| Withdrawals due to adverse events | 67 per 10001 | 87 per 1000 (40 to 187) | RR 1.29 (0.6 to 2.77) | 322 (4 studies) | ⊕⊝⊝⊝ very low2,6 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. 2 Downgraded two levels due to unknown risk of bias for random sequence generation and allocation concealment for all 4 studies in the pooled analysis and unknown risk of bias for blinding for 3 studies in the pooled analysis. 3 Downgraded one level due to sparse data (107 events) 4 Downgraded one level due to unexplained heterogeneity (I2 = 64%) 5 Downgraded one level due to sparse data (51 events) 6 Downgraded two levels due to very sparse data (25 events)
Background
The etiology of Crohn's disease remains unknown. A variety of possible causes have been proposed, including mycobacterial infection. Dalziel first noted similarities between Crohn's disease and tuberculous gastroenteritis in 1913 (Dalziel 1913). However, attempts to culture mycobacteria from Crohn's disease patients were unsuccessful until 1978 when Burnham reported the culture of Mycobacterium kansasii from lymph nodes of a Crohn's patient (Burnham 1978). More recently, Sanderson has used PCR and a specific DNA probe to detect Mycobacterium paratuberculosis in up to two thirds of Crohn's disease tissue (Sanderson 1991a, Sanderson 1991b). Of interest is a histopathological similarity between Crohn's disease and Johne's disease, an infectious enteritis in ruminants caused by Mycobacterium paratuberculosis (Morgan 1987).
A number of studies examined the effect of anti‐tuberculous therapy in Crohn's disease, reporting conflicting results. Therefore, a meta‐analysis was performed to evaluate the use of anti‐tuberculous therapy for maintenance of remission in Crohn's disease. This systematic review is an update of a previously published Cochrane systematic review (Borgaonkar 2015).
Objectives
The primary objectives were to evaluate the efficacy and safety of anti‐tuberculous therapy for maintenance of remission in patients with quiescent Crohn's disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials were considered for inclusion.
Types of participants
Patients with quiescent Crohn's disease diagnosed by conventional clinical, radiological and endoscopic criteria (as defined by the individual studies) were considered for inclusion. Patients with active disease were excluded.
Types of interventions
Interventions that assess the efficacy of anti‐tuberculous therapy compared to placebo or an active control therapy were considered for inclusion. Adjunct therapies were also permitted.
Types of outcome measures
Primary outcomes
The primary outcome was the proportion of patients who relapsed as defined by the included studies (e.g. Crohn's disease activity index (CDAI) > 150)).
Secondary outcomes
Secondary outcomes included the proportion of patients with any adverse event, withdrawals due to adverse events and serious adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases from inception to June 22, 2015:
MEDLINE (Ovid);
EMBASE (Ovid); and
CENTRAL.
We searched the Cochrane IBD Group Specialized Register to identify studies published in abstract form. Conference proceedings were also searched to identify additional studies.
The electronic search strategies are described in Appendix 1.
Data collection and analysis
Selection of studies
Study titles and abstracts identified by the electronic literature searches were reviewed and potentially relevant studies were identified for full text evaluation. The studies selected for full text review were then independently assessed for inclusion by two authors (PHP and JKM). Any disagreements regarding inclusion were resolved by discussion and consensus or by consulting a third author (NC). Reasons for exclusion were documented in the review.
Data extraction and management
Standardized data extraction sheets were prepared and two authors independently extracted data from the included studies. Any disagreement in data extraction was resolved by discussion and consensus.
Assessment of risk of bias in included studies
Two authors independently assessed study quality (PHP and JKM) using the Cochrane risk of bias tool (Higgins 2011). The following items were assessed:
Random sequence generation;
Allocation concealment;
Blinding of participants, personnel and assessment of outcome;
Incomplete outcome data;
Selective reporting; and
Other biases.
Each item was evaluated as low, high or unclear risk of bias and justification for this judgment was provided in the Characteristics of included studies table. Any disagreements were resolved by discussion and consensus or by consulting with a third author (NC).
We utilized the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) criteria to assess the overall quality of evidence supporting the primary and secondary outcomes. Evidence from randomized controlled trials begin as high quality evidence but can be downgraded due to: risk of bias, indirect evidence, inconsistency (unexplained heterogeneity), imprecision, and publication bias. The overall quality of evidence for each outcome was ascertained and classified as high quality (i.e. further research is very unlikely to change our confidence in the estimate of effect); moderate quality (i.e. further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate); low quality (i.e. further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate); and very low quality (i.e. we are very uncertain about the estimate) (Guyatt 2008; Schünemann 2011).
Measures of treatment effect
For dichotomous outcomes we calculated the risk ratio (RR) and corresponding 95% confidence interval (CI). RevMan was used for data analysis.
Unit of analysis issues
When studies reported multiple observations for the same outcome, the outcomes were combined for fixed intervals of follow‐up (e.g., relapse at 52 weeks). Cross‐over trials were only included if data were available from the first phase of the study (i.e., before any cross‐over). Separate analyses were to be conducted for comparisons between anti‐tuberculous therapy versus placebo as well as comparisons with active comparators. Where studies allocated subjects to more than one anti‐tuberculous treatment arm, the arms would be pooled for the primary analysis. When possible, subgroup analyses were to be performed to compare efficacy and safety among different doses of anti‐tuberculous drugs. Although some studies may report more than one efficacy or safety event per patient, the primary analysis considered the proportion of patients who experienced at least one event. Studies with induction phases were considered for inclusion, but analysis for relapse only included participants who entered the maintenance phase. Analysis of adverse events, withdrawals as a result of adverse events and serious adverse events were performed using all participants across all study phases.
Dealing with missing data
We attempted to contact study authors to obtain missing data. All data were analyzed on an intention‐to‐treat basis whereby all missing participants were considered to be treatment failures.
Assessment of heterogeneity
We assessed heterogeneity using the Chi2 test. A P value of 0.1 was considered to be statistically significant. The degree of statistical heterogeneity was quantified using the I2 statistic. Heterogeneity was considered to be moderate if I2 was > 50%, and high if I2 was > 75%. We explored potential explanations for heterogeneity by visual inspection of forest plots and by sensitivity analysis excluding any outlier studies. To account for the heterogeneity, we also examined the characteristics of potential outlier studies to see if these studies differed in any way from the other studies in the pooled analysis.
Assessment of reporting biases
Protocols were not available for the included studies. We evaluated reporting bias by comparing the reported outcomes listed in the methods section of the manuscript to those described in the results section. There were less than 10 included studies and therefore publication bias could not be evaluated using funnel plots.
Data synthesis
For dichotomous outcomes, we calculated the pooled risk ratio (RR) and corresponding 95% CI using a fixed‐effect model. Data from individual trials were pooled for meta‐analysis if included studies were similar in regards to patients, treatments and outcomes (determined by consensus). If significant heterogeneity was identified, we used a random‐effects model for meta‐analysis. If heterogeneity was substantially high (I2 > 75%) and a single study that was causing the heterogeneity was identified, it was excluded from pooled meta‐analysis. The primary analysis was to be confined to those trials which were fully published. A sensitivity analysis was to be carried out which included trials in abstract form. A subgroup analysis was to be performed on those trials in which patients were initially brought into remission with corticosteroids. However, only one small study reported as an abstract was available and almost all patients were induced into remission with corticosteroids, so these analyses were not performed as they would not have affected the results.
Results
Description of studies
Results of the search
A literature search conducted on June 22, 2015 identified 576 studies. After duplicates were removed a total of 411 studies remained for review of titles and abstracts. Nineteen studies of anti‐tuberculous therapy for maintenance of remission in Crohn's were selected for full text review (See Figure 1). Twelve reports of 11 studies were excluded because they did not meet the inclusion criteria. Six reports of four studies (total 206 participants) met the inclusion criteria and were included in the review (Afdhal 1991; Kelleher 1982; Prantera 1994; Selby 2007).
1.
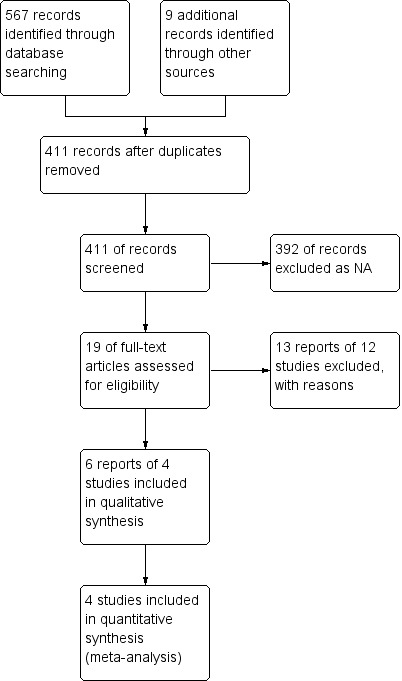
Study flow diagram.
Included studies
Afdhal 1991 conducted a randomized, double‐blind, placebo‐controlled, three phase trial to assess the efficacy of clofazimine in combination with prednisone compared to placebo with prednisone in 49 patients with active Crohn's disease ((Disease Activity Score (DAS) >10) to induce remission (phase 1) and maintain remission (phase 2 and 3). Remission was defined as a DAS score of < 5. The dose of clofazimine was kept constant at 100 mg daily throughout all three phases, while prednisone was used at an initial dose of 45 mg daily and then tapered to zero over the twelve weeks of phase one. Patients were excluded from the study if they had indications for surgery or were pregnant. Phase 1 (intervention group (n = 25) and placebo group (n = 24)) used a DAS score to evaluate the primary outcome of remission (DAS < 5) induction after 12 weeks of therapy with 100 mg daily clofazimine and tapered prednisone or placebo and tapered prednisone. Failure of phase 1 in either group occurred if the patient did not enter remission or could not be tapered off steroids, and these patients were prohibited from inclusion in the next phase. Phase 2 (intervention group (n = 16) and placebo group (n = 12)) continued the patients with 100 mg daily clofazimine or matched daily placebo for another month with the primary outcome of maintaining remission (DAS < 5). Failure of phase 2 occurred if the patients relapsed within the time frame, which prohibited their inclusion in phase 3. Phase 3 (intervention group (n = 15) and placebo group (n = 12)) continued the patients on 100 mg daily clofazimine or matched daily placebo for another 8 months with the primary outcome of maintaining remission (DAS < 5). Failure of phase 3 occurred if the patients relapsed within the time frame. The effect disease location on outcomes and adverse effects of clofazimine were also reported.
Kelleher 1982 conducted a randomized, placebo controlled trial of 20 patients with Crohn's disease in remission to evaluate the effectiveness of clofazimine (dose regimen was not described) at maintaining remission. Patients discontinued any pre‐existing treatment for the first four weeks of the six month study period. Disease activity was monitored as the primary outcome using the CDAI. The primary outcome was relapse. Secondary outcomes included toxic effects of clofazimine monitored by biochemical and hematological screening and clinical adverse effects of treatment.
Prantera 1994 conducted a nine month randomized, double‐blind, matched placebo‐controlled trial that evaluated the effectiveness of a combination of antimycobacterial therapies [clofazimine (100 mg every 2 days for 9 months), rifampin (600 mg, 1 dose taken at trial entry), ethambutol (15 mg/kg/day for 9 months) and dapsone (100 mg, 6 days per week for 9 months)] compared to an identically matched placebo, both paired with an eight week‐tapered course of methylprednisolone for inducing remission in active Crohn's disease. Patients (N = 40) selected for the study had to have active disease with CDAI > 250 and had been on specific steroid regimes, but were excluded if < 18 years old; pregnant or breastfeeding; had intestinal stenosis (with obstructive symptoms); diabetes mellitus; significant hepatic or renal disease; had glucose‐6‐phosphate dehydrogenase deficiency; had TPN or elemental diet; or had radiographic findings of active pulmonary TB. Patients who entered remission were entered into the maintenance phase where the patients were continued on their previously started drug regime. Failure, at any time, was defined as a need for corticosteroids, other drug therapy or surgery based on clinical symptoms and signs. Placebo patients who failed were offered open‐label antimycobacterial therapy, including the eight week tapered course of corticosteroids. The primary outcomes were induction of remission and maintenance of remission of CD, without symptoms or complications and endoscopic or radiologic disappearance or active inflammatory lesions. Other evaluated variables included visual acuity changes, compliance, side effects, toxicity and follow‐up.
Selby 2007 conducted a three year, three phase, prospective, parallel, randomized, double‐blind, placebo‐controlled trial to evaluate the potential long‐term benefits of two years of treatment with a combination of antibiotics for maintaining remission after an initial remission induction phase with tapering adjunct corticosteroids. The phases included: induction phase (16 weeks), maintenance phase (16‐104 weeks) and follow‐up phase (104‐156 weeks). The combination therapy was clarithromycin (750 mg/day), rifabutin (450 mg/day), and clofazimine (50 mg/day), which each had an initial step‐wise dosing regimen to prevent adverse events. Two‐hundred and thirteen adult patients (age > 18 years) with active Crohn's disease (CDAI > 200) diagnosed with standard criteria, from 20 Australian centres were selected. Patients were excluded if they met the following conditions: isolated upper gastrointestinal or isolated perianal disease or a stoma; need for intravenous corticosteroids at initial assessment; expected need for surgery during the first four months of the study; antibiotic use for Crohn’s disease within one month of study entry; prior use of infliximab. Primary outcomes of the study were the proportions of patients with at least 1 relapse at 12, 24 and 36 months. Secondary outcomes included percentage of patients in remission (CDAI < 150) at week 16; number of patients who relapsed by predetermined time periods; length of time to first relapse; intervention and placebo safety profiles; other outcomes (improvements in Crohn's Disease Endoscopic Index of Severity (CDEIS) scores, proportion of subjects requiring Crohn's related‐surgery); laboratory profile changes (serum albumin, erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP)); and quality of life scores (questionnaires). Other outcomes monitored were the proportion of patients in endoscopic remission at week 156; patient compliance with the drug regimen; and maintenance of blinding.
Excluded studies
Excluded studies and justification for exclusion are reported in the Characteristics of excluded studies tables.
Risk of bias in included studies
The risk of bias analysis is summarized in Figure 2.
2.
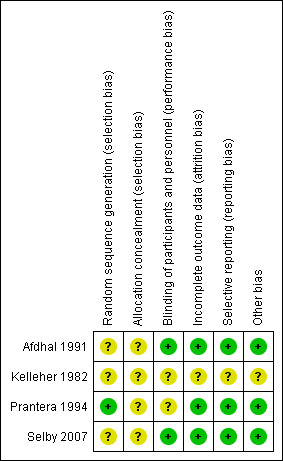
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Prantera 1994 performed randomization using a random number table and this study was rated a low risk of bias for random sequence generation. The other three studies did not describe the method used for randomization and these studies were rated as unclear risk of bias. None of the four studies described the procedures used for allocation concealment and all were rated as unclear risk of bias for allocation concealment.
Blinding
Afdhal 1991 was judged to be at low risk of bias for blinding as the study described the use of double blinding with matching placebo. Kelleher 1982 was assigned an unclear risk of bias for blinding as the blinding method was not described. Prantera 1994 was judged to be an unclear risk of bias for blinding as one researcher had access to the treatment assignments. The role of this individual played in the study was not clearly described. All other participants were described as being adequately blinded. Selby 2007 was judged to be at low risk of bias for blinding as blinding as an identical placebo was used. Blinding was monitored throughout the study to ensure it was maintained for personnel and participants.
Incomplete outcome data
Afdhal 1991 was judged to be at low risk of bias for incomplete outcomes data as withdrawals and reasons for withdrawal were balanced across clofazimine and placebo groups. Kelleher 1982 was judged to be at unclear risk of bias for incomplete outcome data because there was no description of any missing data. Prantera 1994 was assessed to be at low risk of bias for incomplete outcome data. All missing patient data was adequately recorded. Selby 2007 was assessed to be at low risk for attrition bias because drop‐outs were balanced across intervention groups with similar reasons for withdrawal.
Selective reporting
Afdhal 1991 was assigned a low risk for reporting bias as all expected outcomes were reported. Kelleher 1982 was deemed to be at unclear risk of reporting bias. Relapses were the only outcome data reported and there was no description of the inclusion, selection or monitoring of the patient groups. Prantera 1994 was judged to be at low risk of bias for selective reporting because all expected outcomes were reported. Selby 2007 was assigned a low risk of reporting bias as all primary and secondary outcomes were reported according to study protocol.
Other potential sources of bias
Kelleher 1982 was judged to be at unclear risk of bias for other bias because baseline patient characteristics for each group were not reported. The other three studies were judged to be at low risk of bias for other bias.
Effects of interventions
See: Table 1
Pooled analysis was performed evaluating the studies for relapse, and safety data, including adverse events, withdrawal as a result of adverse events, and serious adverse events. Interventions in the studies included anti‐tuberculous treatments as mono or combined therapies, in isolation or following adjunct therapy with corticosteroids to induce remission. Relapse was defined by the individual studies.
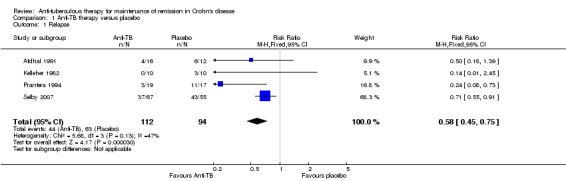
Occurence of relapse during maintenance therapy There was a statistically significant difference in relapse rates favoring anti‐tuberculous therapy over placebo. Thirty‐nine per cent (44/112) of patients in the anti‐tuberculous therapy group relapsed at 9 months to 2 years compared to 67% (63/94) of placebo patients (RR 0.58, 95% CI 0.45 to 0.75, I2 = 47%). A GRADE analysis indicates that the overall quality of the evidence supporting this outcome was very low due to unknown risk of bias and sparse data (See Table 1).
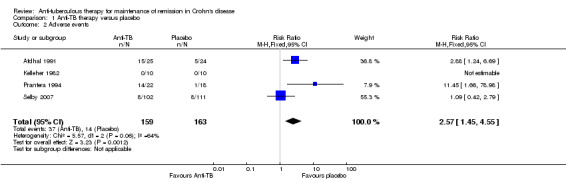
Adverse events, withdrawals due to adverse events, and serious adverse events Adverse events occurred more frequently in the anti‐tuberculous therapy group (37/159) compared to the placebo group (14/163) with a pooled RR of 2.57 (95% CI 1.45 to 4.55; N=322; studies=4, I2=64%). A GRADE analysis indicates that the overall quality of the evidence supporting this outcome was very low due to unknown risk of bias, unexplained heterogeneity and sparse data (See Table 1). Common adverse events included increased skin pigmentation and rashes. No serious adverse events were reported in any of the included studies. There was no statistically significant difference in withdrawals due to adverse events. Nine per cent (14/159) of patients in the anti‐tuberculous therapy group withdrew due to adverse events compared to 7% (11/163) of placebo patients (RR 1.29, 95% CI 0.60 to 2.77; N = 322; studies = 4; I2 = 0%). A GRADE analysis indicates that the overall quality of the evidence supporting this outcome was very low due to unknown risk of bias and very sparse data (See Table 1).
Afdhal 1991 reported that adverse events were mild and self‐limiting. Twelve patients in the clofazimine group had increased skin pigmentation compared to five patients in the placebo group. Three patients in the clofazimine group developed skin rashes and no patients had abnormal eye exams. Two patients in each group asked to be withdrawn without specific reasons given, but were not included as failures. No serious adverse events were reported.
Kelleher 1982: reported no adverse events other than reversible pink skin discolouration (proportions not reported).
Prantera 1994 reported on adverse events due to dapsone or clofazimine. Two patients withdrew (at 1 and 5 months) due to moderate anemia, while six others developed mild anemia, but did not discontinue treatment; all of these events were attributed to dapsone. Two patients withdrew from active therapy for "feeling sick". Clofazimine was responsible for four cases of increased skin pigmentation. No adverse events were attributed to the other study drugs and no serious adverse events were reported.
Selby 2007 reported that the treatments were well‐tolerated. Sixteen patients (eight in each of the placebo and intervention groups) had adverse events and withdrew from the study. Adverse events included abnormal liver function, vaginal candidiasis, abdominal distention, myalgia and urine discolouration, during the induction phase; and arthralgia and tooth discolouration during in the maintenance phase. No serious adverse events were reported.
Discussion
Summary of main results
The electronic search of the databases resulted in six reports of four studies (N=206), which were included in the meta‐analysis, to evaluate the effect of anti‐tuberculous therapy for the maintenance of remission in patients with Crohn's disease. Pooled risk ratios were calculated for relapse and safety outcomes (adverse events, withdrawals as a result of adverse events and serious adverse events). Four studies examined anti‐tuberculous therapy for maintenance of remission in patients with Crohn's disease. The pooled RR for relapse was 0.58 (95% CI 0.45‐0.75; N=206; studies=4, I2=47%), suggesting a potential benefit for anti‐tuberculous therapy compared to placebo. Although patients in the anti‐tuberculous therapy group were significantly more likely than placebo patients to experience an adverse event, there was no difference in withdrawals due to adverse events. Common adverse events included increased skin pigmentation and rashes. None of the included studies reported any serious adverse events.
Quality of the evidence
The included studies were rated as unclear risk of bias for several important quality indicators. None of the included studies adequately described methods used for allocation concealment and the studies were rated as unclear risk of bias for that item. Only one study described the method used for random sequence generation and this study was rated as low risk of bias. The other three studies were rated as unclear risk of bias for random sequence generation. Two studies did not describe procedures used for double‐blinding and were rated as unclear risk of bias for blinding. GRADE analyses indicated that the overall quality of the evidence supporting the outcomes assessed in this systematic review (i.e. relapse and safety outcomes) was very low due to unclear risk of bias, serious imprecision and unexplained heterogeneity.
Potential biases in the review process
In an attempt to reduce the potential for bias in the review process we performed a comprehensive literature search to identify all eligible studies. Two authors independently assessed studies for inclusion, extracted data and assessed study quality. There are some limitations to this review. The studies were small, underpowered and of unclear methodological quality. Futhermore the overall quality of the evidence supporting the outcomes assessed in this review was very low. Thus any conclusions generated from this review need to be interpreted with caution.
Agreements and disagreements with other studies or reviews
Two other systematic reviews that examined the effectiveness of anti‐tuberculous therapy for maintenance of remission in Crohn's disease suggested a potential benefit for this treatment, and had similar judgements for study quality (Feller 2010; Khan 2011). Other types of antibiotics that are not used for the treatment of Mycobacterium may provide a benefit for induction of remission in Crohn's disease (Feller 2010; Khan 2011). However, these antibiotics have not been tested extensively in patients with quiescent Crohn's disease (Herfarth 2013; Jigaranu 2014; Manosa 2013).
Authors' conclusions
Implications for practice.
Very low quality evidence suggests that anti‐tuberculous therapy may be effective for maintenance of remission in patients with Crohn's disease. Most of the included patients were initially treated with a course of corticosteroids combined with anti‐tuberculous drugs to induce remission. It is unknown if anti‐tuberculous therapy would be effective in patients brought into remission with other medical or surgical therapies.
Implications for research.
Although the results are not definitive, they do support further trials using anti‐tuberculous drugs for Crohn's disease, including studies using a combination of corticosteroids and anti‐tuberculous agents to induce remission followed by maintenance therapy with anti‐tuberculous agents, as well as other medical and surgical therapies used to induce remission followed by maintenance therapy with anti‐tuberculous agents. These studies will provide better quality evidence for the use of anti‐tuberculous therapy for maintaining remission in people with quiescent Crohn's disease.
What's new
| Date | Event | Description |
|---|---|---|
| 22 June 2015 | New search has been performed | New literature searches conducted on June 22, 2015. New study added. |
| 22 June 2015 | New citation required and conclusions have changed | Substantively updated review with new conclusions and authors. |
Acknowledgements
Partial funding for the Cochrane IBD Group (April 1, 2016 ‐ March 31, 2018) has been provided by Crohn's and Colitis Canada (CCC).
Appendices
Appendix 1. Search Strategies
MEDLINE/EMBASE
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. exp Crohn disease/ or crohn*.mp.
22. inflammatory bowel disease*.mp.
23. IBD.mp.
24. (anti‐tuberculosis or anti tuberculosis).mp.
25. tuberculosis management.mp.
26. exp antimycobacterial/or antimycobacterial*.mp.
27. (Isonicotinyl hydrazine or Isonicotinic acid hydrazide or INH or Isoniazid or Laniazid or Nydrazid or isonicotinylhydrazine).mp.
28. (Pyrazinamide or PZA).mp.
29. (Rifampicin or rifampin or rifamycin or RMP or RIF or rofact or rifaldazine or Tubocin or Sinerdol or Rifadin or Rimactan or Rifater or Rifinah or Rimactazid or Rifadin or Rifater or Rimactane or Rifadine or Rimycin or Rimactan or Eremfat).mp.
30. bactericidal*.mp.
31. (aminoglycoside* or streptomycin or tobramycin, or apramycin or arbekacin or gentamicin or kanamycin or neomycin or netilmicin or paromomycin or rhodostreptomycin).mp.
32. (capreomycin or viomycin or enviomycin).mp.
33. polypeptide*.mp.
34. fluoroquinolone*.mp.
35. thioamide*.mp.
36. (rifabutin or macrolide* or linezolid or lzd or thioacetone or thioridazine or arginine or vitamin d or R207910).mp.
37. (Clofazimine or Lamprene or Dapsone or diamino‐diphenyl sulfone).mp.
38. anti‐tb.mp.
39. 21 or 22 or 23
40. 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38
41. 20 and 39 and 40
Cochrane Library
#1 crohn* or IBD or (inflammatory bowel disease*)
#2 anti‐tuberculosis or anti tuberculosis or tuberculosis management or antimycobacterial or Isonicotinyl hydrazine or Isonicotinic acid hydrazide or INH or Isoniazid or Laniazid or Nydrazid or isonicotinylhydrazine or Pyrazinamide or PZA or rifampicin or rifampin or rifamycin or RMP or RIF or rofact or rifaldazine or Tubocin or Sinerdol or Rifadin or Rimactan or Rifater or Rifinah or Rimactazid or Rifadin or Rifater or Rimactane or Rifadine or Rimycin or Rimactan or Eremfat or bactericidal or aminoglycoside* or streptomycin or tobramycin or apramycin or arbekacin or gentamicin or kanamycin or neomycin or netilmicin or paromomycin or rhodostreptomycin or capreomycin or viomycin or enviomycin or polypeptide or fluoroquinolone or thioamide or rifabutin or macrolide* or linezolid or lzd or thioacetone or thioridazine or arginine or vitamin d or R207910 or clofazimine or lamprene or dapsone or diamino‐diphenyl sulfone or anti‐tb
#3 #1 and #2
Data and analyses
Comparison 1. Anti‐TB therapy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse | 4 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 2 Adverse events | 4 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [1.45, 4.55] |
| 3 Withdrawals due to adverse events | 4 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.60, 2.77] |
| 4 Serious adverse events | 4 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Anti‐TB therapy versus placebo, Outcome 1 Relapse.
1.2. Analysis.
Comparison 1 Anti‐TB therapy versus placebo, Outcome 2 Adverse events.
1.3. Analysis.
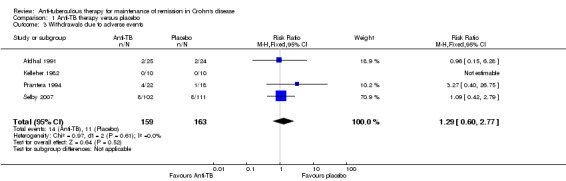
Comparison 1 Anti‐TB therapy versus placebo, Outcome 3 Withdrawals due to adverse events.
1.4. Analysis.
Comparison 1 Anti‐TB therapy versus placebo, Outcome 4 Serious adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Afdhal 1991.
| Methods | Randomized with substratification by disease site, double‐blinded, placebo‐controlled trial | |
| Participants | Patients, N = 49 (34 women, 15 men), with active Crohn's Disease confirmed by standard clinical, radiological, and histological criteria. Study participant exclusionary criteria: indication for surgery, including strictures causing bowel obstruction, fistulae, and abscess formation; and pregnancy. active CD Disease Activity Score (DAS) > 10 (Lancet 1978, 2: 955‐57) Phase 1: Intervention group (n = 25) and placebo group (n = 24) Phase 2: Intervention group (n = 16) and placebo group (n = 12) Phase 3: Intervention group (n = 15) and placebo group (n = 12) |
|
| Interventions | Clofazimine 100 mg daily Adjunct therapy included prednisone (initial dose of 45 mg daily) tapered over 3 months for both intervention and placebo groups; Phase 1 ‐ steroids tapered over 12 weeks + 100 mg Clofazimine vs. steroids tapered over 12 weeks + placebo; Phase 2 ‐ Clofazimine 100 mg daily vs placebo for 4 weeks; Phase 3 ‐ Clofazimine 100 mg daily vs placebo for 8 months | |
| Outcomes | Remission induction (phase 1) and maintenance of remission (phases 2 & 3); with remission defined as DAS < 5 | |
| Notes | Reference in Lancet to DAS score DAS score was reported for the end of each phase of the trial Side effects experience in each group was provided Disease site effect on outcome was discussed, but no calculation was provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not described. Contains substratification for disease site; method not described, but matched in control and intervention groups |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded, control and intervention groups matched for treatment regime (taken once daily), including clofazimine and matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were approximately balanced between intervention and placebo groups across the 3 Phases of the trial, with similar reasons provided, including phase failure, loss to follow‐up or requested withdrawal. Phase 1: Intervention group (n= 25) and placebo group (n= 24) Phase 2: Intervention group (n= 16) and placebo group (n= 12) Phase 3: Intervention group (n= 15) and placebo group (n= 12) Analysis was performed on an intention to treat basis |
| Selective reporting (reporting bias) | Low risk | Expected outcomes (DAS scores, failures) for each phase were reported, including substratification effects and treatment side effects |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kelleher 1982.
| Methods | Randomized, placebo‐controlled trial | |
| Participants | Pateints (N=20) with Crohn's disease in remission No discussion of placebo or intervention group breakdown, patient characteristics, or previous therapy Exclusion criteria not described |
|
| Interventions | Clofazimine for 6 months (dosing regime not described) Pre‐existing medications were withdrawn within the first 4 weeks of clofazimine or placebo therapy | |
| Outcomes | Changes in the patients' disease activity were monitored using the Crohn's Disease Acitivity Index (CDAI) Failure was considered disease relapse Secondary outcomes include toxic effects of clofazimine, monitored by biochemical and haematological screening and major side effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described No discussion of placebo matching or blinded parties |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Relapses were the only outcome data reported and there was no description of the inclusion, selection or monitoring of the patient groups Not described The only data described were failures in both placebo and intervention groups Secondary outcome was mentioned without discussion as to the number of patients effected Effect on 4 cases severe acute ulcerative colitis described as outcome results without discussion of when or how patients were selected or included No statistical methods or analyses were described |
| Other bias | Unclear risk | Not described Baseline patient characteristics were not reported for either treatment group |
Prantera 1994.
| Methods | Randomized, double‐blinded, placebo‐controlled trial with cross‐over to intervention group if placebo failure occurred | |
| Participants | Pateints (> 18 yrs old) with clinically, radiologically, and/or histologically confirmed Crohn's present in any bowel site were considered for inclusion Inclusion criteria (must satisfy one of the following):
Exclusion criteria: < 18 years old; Pregnant or breastfeeding; Intestinal stenosis (with obstructive symptoms); Diabetes Mellitus; Significant hepatic or renal disease; glucose‐6‐phosphate dehydrogenase deficiency; TPN or elemental diet; and/or Radiographic findings of active pulmonary TB. N = 40 (15 males) Induction Phase: intervention (n = 22) and placebo (n = 18) Maintenance Phase: intervention (n = 19) and placebo (n = 17) Cross‐over phase (after relapse on placebo, re‐enters to receive intervention): 7/10 patients who relapsed on placebo during the trial and 2 more patients receiving placebo that had symptoms upon trial completion re‐entered the study as part of a open‐label intervention group |
|
| Interventions | All other CD active drugs ceased before study began Total study duration: 9 months (not including re‐entry patients) Interventions Evaluated: clofazimine (100 mg q2days for 9 months), rifampin (600 mg, 1 dose taken at trial entry), ethambutol (15 mg/kg/day for 9 months) and dapsone (100 mg, 6 days per week for 9 months) vs. identically matched placebo Induction Phase: 8 weeks of a tapering dose of once daily IV methylprednisolone; initial dose 0.70‐1.0 mg/kg/day; route of admission IV to oral, switched at various times within the 8 weeks for each individual + intervention (listed above) Maintenance Phase: Interventions continued for remaining 7 months Cross‐over: failed placebo patients that re‐entered as cross‐over study participants restarted the trial at the beginning of the induction phase as an open‐label 9 month course |
|
| Outcomes | Primary outcomes:
‐ Failure defined as need for steroids, other drug therapy or surgery based on clinical symptoms and signs Patients examined at entry and at 4 week intervals by three researchers and at 3 month intervals by the PI: clinical assessments using CDAI, physical examination and laboratory measurements (CBC, ESR, CRP, serum protein electrophoresis, seromucoids). Toxicity was also monitored (Serum Cr, ALT, AST, GGT, ALP and urinalysis) Visual acuity was assessed every 12 weeks or if visual disturbances were noted Small bowel x‐ray by enteroclysis with a nasogastric tube, or total colonoscopy was performed before entry and repeated within 2 weeks of trial completion; completed by endoscopists and radiologists, designated as unchanged or improved |
|
| Notes | Compliance, side effects and a follow‐up (at mean 8.6±5 months) were also evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using a random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Randomization was performed by one researcher who was aware of treatment assignment and also supervised drug supplies and monitored compliance and side effects, but did not evaluate patients Procedures for allocation concealment were not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinded; both patients and evaluating researchers were blinded Placebo was identical to intervention in dose regime and physical appearance Endoscopologists and radiologists performing and evaluating the small bowel x‐rays and colonoscopies were also blinded Hospital pharmacy was responsible for packaging and labelling the drug supplies However, the researcher who monitored compliance and side effects was aware of the treatment assignment. No information provided whether this researcher was involved in data analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All missing patient data was accounted for with description and justification for failure, removal or withdrawal from study Data was reasonably balanced for removal and withdrawal from study, but was significantly different between intervention group and placebo group for treatment failure |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported Not all lab measurements for each assessment were reported: only CDAI, CRP, seromucoids and hematocrit were reported for the beginning and end of the trial |
| Other bias | Low risk | No other bias appears to be present |
Selby 2007.
| Methods | Prospective, parallel, stratified to thiopurine usage, randomized, double‐blind, placebo‐controlled, 3 year trial with induction phase (16 weeks), maintenance phase (16‐104 weeks) and follow‐up phase (104‐156 weeks) | |
| Participants | Patients (over 18 years old) with active Crohn's Disease (CD Activity Index (CDAI) > 200) diagnosed with standard criteria enrolled from 20 Australian centres. Exclusion Criteria: patients with: isolated upper gastrointestinal or isolated perianal disease or a stoma; patients requiring: intravenous corticosteroids at initial assessment and/or those thought likely to require surgery during the first 4 months of the study; patients who had received antibiotics for Crohn’s disease within 1 month of entry to the study or had used infliximab N = 213; Induction Phase: Intervention (n = 111) vs Placebo (n = 102) Maintenace Phase:
Follow‐up Phase: Intervention (n = 34) vs Placebo (n = 20) |
|
| Interventions | Combination therapy with clarithromycin, rifabutin, and clofazimine or placebo Adjunct therapy included a 16‐week tapering course of oral prednisolone (starting with 40 mg/day to 0 mg/day) for both intervention and placebo groups Intervention combination:
Induction Phase (0‐16 weeks): Combination therapy or placebo; coupled with adjunct therapy Maintenance Phase (17‐104 weeks): Combination therapy or placebo maintained Follow‐up Phase (105‐156 weeks): Trial medications ceased |
|
| Outcomes | Primary outcomes: proportion of patients with at least 1 relapse at months 12, 24 and 36 Secondary outcomes: percentage of patients in remission (CDAI < 150) at week 16; number of patients who relapsed by predetermined time periods; length of time to first relapse; intervention and placebo safety profiles; other outcomes (improvements in CDEIS scores, proportion of subjects requiring Crohn's related‐surgery); laboratory profile changes (serum albumin, erythrocyte sedimentation rate and CRP); and quality of life scores (questionnaires) |
|
| Notes | Other outcomes monitored included proportion of patients in endoscopic remission at week 156; patient compliance with the drug regime; and maintenance of blinding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind (both patients and physicians) Placebo was identically matched to the intervention regime and included re‐encapsulated clofazimine using gelatine capsules Blinding was also monitored to ensure it was maintained at various points throughout the study for both patients and physicians Compliance was monitored by pharmacy returns |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs balanced across intervention groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcome data were reported according to study protocol. Quality of life questionnaires were not analysed, but rational was provided |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Basilisco 1989 | Patients had active disease |
| Elliott 1982 | Patients had active disease |
| Goodgame 2001 | Patients had active or quiescent disease at entry. Separate results were not provided for patients in remission |
| Graham 1995 | Induction of remission study |
| Gui 1997 | Patients had active disease |
| Inoue 2007 | Patients had active disease |
| Leiper 2000 | Patients had active disease |
| Leiper 2008 | Patients had active disease |
| Prantera 1996 | Patients had active disease |
| Shaffer 1984 | Cross‐over study which did not provide outcome data before the first cross‐over |
| Swift 1994 | Patients had active disease |
| Thayer 1996 | Unclear if patients had active or quiescent disease |
Declarations of interest
Petrease H Patton: None known.
Claire E Parker: None known.
John K MacDonald: None known.
Nilesh Chande has received funds from AbbVie, Ferring, and Actavis for consulting; payment for lectures from Abbvie; payment for development of educational presentations from AbbVie; has stock or stock options in Pfizer, Glaxo Smith Kline, Procter and Gamble, and Johnson and Johnson; and has received travel support from Merck. All of these activities are outside the submitted work.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Afdhal 1991 {published data only}
- Afdhal NH, Long A, Lennon J, Crowe J, O’Donoghue DP. Controlled trial of antimycobacterial therapy in Crohn's disease. clofazimine versus placebo. Digestive Diseases and Sciences 1991;36(4):449‐53. [DOI] [PubMed] [Google Scholar]
- Afdhal NH, Long A, Lennon J, Crowe J, O’Donoghue DP. Controlled trial of clofazimine in Crohn`s disease. Gut 1987;28:A1391. [Google Scholar]
Kelleher 1982 {published data only}
- Kelleher D, O'Brien S, Weir DG. Preliminary trial of clofazimine in chronic inflammatory bowel disease. Gut 1982;23:A449. [Google Scholar]
Prantera 1994 {published data only}
- Kohn A, Prantera C, Mangiarotti R, Luzi C, Andreoli A. Antimycobacterial therapy and Crohn's disease: a randomized placebo controlled trial. Gastroenterology 1992;102(4 Part 2):A647. [PubMed] [Google Scholar]
- Prantera C, Kohn A, Mangiarotti R, et al. Antimycobacterial therapy in Crohn's disease: results of a controlled, double‐blind trial with a multiple antibiotic regimen. American Journal of Gastroenterology 1994;89:513‐8. [PubMed] [Google Scholar]
Selby 2007 {published data only}
- Selby W, Pavli P, Crotty B, Florin T, Radford‐Smith G, Gibson P, et al. Two‐year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn's disease. Gastroenterology 2007;132(7):2313‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Basilisco 1989 {published data only}
- Basilisco G, Ranzi T, Campanini MC, Piodi L, Bianchi PA. Controlled trial of rifabutin in Crohn's disease. Current Therapeutic Research, Clinical and Experimental 1989;46(2):245‐50. [Google Scholar]
Elliott 1982 {published data only}
- Elliott PR, Burnham WR, Berghouse LM, Lennard‐Jones JE, Langman MJ. Sulphadoxine‐pyrimethamine therapy in Crohn's disease. Digestion 1982;23(2):132‐4. [DOI] [PubMed] [Google Scholar]
Goodgame 2001 {published data only}
- Goodgame RW, Kimball K, Akram S, Ike E, Ou CN, Sutton F, et al. Randomized controlled trial of clarithromycin and ethambutol in the treatment of Crohn's disease. Alimentary Pharmacology and Therapeutics 2001;15(12):1861‐6. [DOI] [PubMed] [Google Scholar]
Graham 1995 {published data only}
- Graham DY, Al‐Assi MT, Robinson M. Prolonged remission in Crohn's disease following therapy for Mycobacterium paratuberculosis infection. Gastroenterology 1995;108:A826. [Google Scholar]
Gui 1997 {published data only}
- Gui GP, Thomas PR, Tizard ML, Lake J, Sanderson JD, Hermon‐Taylor J. Two‐year‐outcomes analysis of Crohn's disease treated with rifabutin and macrolide antibiotics. Journal of Antimicrobial Chemotherapy 1997;39(3):393‐400. [DOI] [PubMed] [Google Scholar]
Inoue 2007 {published data only}
- Inoue S, Nakase H, Matsuura M, Ueno S, Uza N, Kitamura H, et al. Open label trial of clarithromycin therapy in Japanese patients with Crohn's disease. Journal of Gastroenterology and Hepatology 2007;22(7):984‐8. [DOI] [PubMed] [Google Scholar]
Leiper 2000 {published data only}
- Leiper K, Morris AI, Rhodes JM. Open label trial of oral clarithromycin in active Crohn's disease. Alimentary Pharmacology and Therapeutics 2000;14(6):801‐6. [DOI] [PubMed] [Google Scholar]
Leiper 2008 {published data only}
- Leiper K, Martin K, Ellis A, Watson AJ, Morris AI, Rhodes JM. Clinical trial: randomized study of clarithromycin versus placebo in active Crohn's disease. Alimentary Pharmacology and Therapeutics 2008;27(12):1233‐9. [DOI] [PubMed] [Google Scholar]
Prantera 1996 {published data only}
- Prantera C, Zannoni F, Scribano ML, Berto E, Andreoli A, Kohn A, et al. An antibiotic regimen for the treatment of active Crohn's disease: a randomized, controlled clinical trial of metronidazole plus ciprofloxacin. American Journal of Gastroenterology 1996;91(2):328‐32. [PubMed] [Google Scholar]
Shaffer 1984 {published data only}
- Shaffer JL, Hughes S, Linaker BD, Baker RD, Turnberg LA. Controlled trial of rifampicin and ethambutol in Crohn's disease. Gut 1984;25(2):203‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swift 1994 {published data only}
- Swift GL, Srivastava ED, Stone R, Pullan RD, Newcombe RG, Rhodes J, et al. Controlled trial of anti‐tuberculous chemotherapy for two years in Crohn's disease. Gut 1994;35(3):363‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thayer 1996 {published data only}
- Thayer WR, Reinert SE, Natarajan R, Szaro J. Rifabutin/streptomycin in the treatment of Crohn’s disease. A double‐blind controlled trial. DDW 1996. [Google Scholar]
- To KW, Tsiaras WG, Thayer WR. Rifabutin use in inflammatory bowel disease. Archives of Ophthalmology 1995;113(11):1354. [DOI] [PubMed] [Google Scholar]
Additional references
Burnham 1978
- Burnham WR, Lennard‐Jones JE, Stanford JL, et al. Mycobacteria as a possible cause of inflammatory bowel disease. Lancet 1978;2:693‐6. [DOI] [PubMed] [Google Scholar]
Dalziel 1913
- Dalziel TK. Chronic intestinal enteritis. British Medical Journal 1913;2:1068‐70. [Google Scholar]
Feller 2010
- Feller M, Huwiler K, Schoepfer A, Shang A, Furrer H, Egger M. Long‐term antibiotic treatment for Crohn's disease: systematic review and meta‐analysis of placebo‐controlled trials. Clin Infect Dis 2010;50(4):473‐80. [DOI: 10.1086/649923; PUBMED: 20067425 ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herfarth 2013
- Herfarth HH, Katz JA, Hanauer SB, Sandborn WJ, Loftus EV Jr, Sands BE, et al. Ciprofloxacin for the prevention of postoperative recurrence in patients with Crohn's disease: a randomized, double‐blind, placebo‐controlled pilot study. Inflammatory Bowel Diseases 2013;19(5):1073‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Jigaranu 2014
- Jigaranu AO, Nedelciuc O, Blaj A, Badea M, Mihai C, Diculescu M, et al. Is rifaximin effective in maintaining remission in Crohn's disease?. Digestive Diseases (Basel, Switzerland) 2014;32(4):378‐83. [DOI] [PubMed] [Google Scholar]
Khan 2011
- Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A, Marshall JK, Talley NJ, Moayyedi P. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta‐analysis. Am J Gastroenterol 2011;106(4):661‐73. [DOI: 10.1038/ajg.2011.72; PUBMED: 21407187 ] [DOI] [PubMed] [Google Scholar]
Manosa 2013
- Manosa M, Cabre E, Bernal I, Esteve M, Garcia‐Planella E, Ricart E, et al. Addition of metronidazole to azathioprine for the prevention of postoperative recurrence of Crohn's disease: a randomized, double‐blind, placebo‐controlled trial. Inflammatory Bowel Diseases 2013;19(9):1889‐95. [DOI] [PubMed] [Google Scholar]
Morgan 1987
- Morgan KL. Johne's and Crohn's. Chronic inflammatory bowel diseases of infectious aetiology?. Lancet 1987;I:1017‐19. [DOI] [PubMed] [Google Scholar]
Sanderson 1991a
- Sanderson JD, Malik Z, Tizard MLV, et al. Polymerase chain reaction (PCR) directly reports Mycobacterium paratuberculosis genomes in Crohn's disease tissue DNA extracts [abstract]. Gastroenterology 1991;100:A247. [Google Scholar]
Sanderson 1991b
- Sanderson JD, Moss M, Malik Z, Tizard M, Green EP, Hermon‐Taylor J. Polymerase chain reaction detects Mycobacterium paratuberculosis in Crohn's disease tissue extracts [abstract]. Gut 1991;32:A572. [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
References to other published versions of this review
Borgaonkar 2015
- Borgaonkar M, MacIntosh D, Fardy J, Simms L. Anti‐tuberculous therapy for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD000299.pub2] [DOI] [PubMed] [Google Scholar]


