Abstract
Background
Gastrointestinal paralysis, nausea and vomiting and pain are major clinical problems following abdominal surgery. Anaesthetic and analgesic techniques that reduce pain and postoperative nausea and vomiting (PONV), while preventing or reducing postoperative ileus, may reduce postoperative morbidity, duration of hospitalization and hospital costs. This review was first published in 2001 and was updated by new review authors in 2016.
Objectives
To compare effects of postoperative epidural analgesia with local anaesthetics versus postoperative systemic or epidural opioids in terms of return of gastrointestinal transit, postoperative pain control, postoperative vomiting, incidence of anastomotic leak, length of hospital stay and costs after abdominal surgery.
Search methods
We identified trials by conducting computerized searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 12), MEDLINE (from 1950 to December 2014) and EMBASE (from 1974 to December 2014) and by checking the reference lists of trials retained. When we reran the search in February 2016, we added 16 potential new studies of interest to the list of ‘Studies awaiting classification' and will incorporate these studies into formal review findings during the next review update.
Selection criteria
We included parallel randomized controlled trials comparing effects of postoperative epidural local anaesthetic versus regimens based on systemic or epidural opioids.
Data collection and analysis
We rated the quality of studies by using the Cochrane 'Risk of bias' tool. Two review authors independently extracted data and judged the quality of evidence according to the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) scale.
Main results
We included 128 trials with 8754 participants in the review, and 94 trials with 5846 participants in the analysis. Trials included in the review were funded as follows: charity (n = 19), departmental resources (n = 8), governmental sources (n = 15) and industry (in part or in total) (n = 15). The source of funding was not specified for the other studies.
Results of 22 trials including 1138 participants show that an epidural containing a local anaesthetic will decrease the time required for return of gastrointestinal transit as measured by time to first flatus after an abdominal surgery (standardized mean difference (SMD) ‐1.28, 95% confidence interval (CI) ‐1.71 to ‐0.86; high quality of evidence; equivalent to 17.5 hours). The effect is proportionate to the concentration of local anaesthetic used. A total of 28 trials including 1559 participants reported a decrease in time to first faeces (stool) (SMD ‐0.67, 95% CI ‐0.86 to ‐0.47; low quality of evidence; equivalent to 22 hours). Thirty‐five trials including 2731 participants found that pain on movement at 24 hours after surgery was also reduced (SMD ‐0.89, 95% CI ‐1.08 to ‐0.70; moderate quality of evidence; equivalent to 2.5 on scale from 0 to 10). From findings of 22 trials including 1154 participants we did not find a difference in the incidence of vomiting within 24 hours (risk ratio (RR) 0.84, 95% CI 0.57 to 1.23; low quality of evidence). From investigators in 17 trials including 848 participants we did not find a difference in the incidence of gastrointestinal anastomotic leak (RR 0.74, 95% CI 0.41 to 1.32; low quality of evidence). Researchers in 30 trials including 2598 participants noted that epidural analgesia reduced length of hospital stay for an open surgery (SMD ‐0.20, 95% CI ‐0.35 to ‐0.04; very low quality of evidence; equivalent to one day). Data on costs were very limited.
Authors' conclusions
An epidural containing a local anaesthetic, with or without the addition of an opioid, accelerates the return of gastrointestinal transit (high quality of evidence). An epidural containing a local anaesthetic with an opioid decreases pain after abdominal surgery (moderate quality of evidence). We did not find a difference in the incidence of vomiting or anastomotic leak (low quality of evidence). For open surgery, an epidural containing a local anaesthetic would reduce the length of hospital stay (very low quality of evidence).
Plain language summary
Epidural local anaesthetics for prevention of postoperative gastrointestinal paralysis, vomiting and pain after abdominal surgery
Background
Pain and gut paralysis (movement failure) commonly occur after abdominal surgery. Following laparotomy, laparoscopic cholecystectomy and colectomy, approximately 10.3% of patients will have temporary gut paralysis. This may prolong length of hospital stay and may increase costs of the procedure. Among the possible ways to treat pain after abdominal surgery are an epidural and injections of opioids (morphine‐like substances or pain killers). An epidural consists of inserting a catheter (a narrow tube) into the epidural space (the virtual space surrounding the membrane that contains cerebrospinal fluid and the spinal cord) and infusing a solution of local anaesthetic (substance that cuts pain transmission to the brain) (alone or in combination with opioids) to anaesthetize the abdomen. This Cochrane review compares the effects of an epidural containing a local anaesthetic with those of an opioid‐based regimen on the postoperative course after abdominal surgery.
Search dates
The evidence is current to December 2014. When we reran the search in February 2016, we added 16 potential new studies of interest to the list of ‘Studies awaiting classification' and will incorporate them into formal review findings during the next review update.
Study characteristics
We included 128 trials with 8754 participants of both sexes aged between 33 and 76 years in the review and 94 trials with 5846 participants in the analysis. Three trials reported that their trial was officially registered.
Study funding sources
Trials included in the review were funded as follows: charity (n = 19), departmental resources (n = 8), governmental sources (n = 15) and industry (in part or in total) (n = 15). The source of funding was not specified for the other trials.
Key results
We found that an epidural containing a local anaesthetic reduces the time required for return of gut function compared with an opioid‐based regimen (equivalent to 17 hours). An epidural providing a local anaesthetic and an opioid also reduce pain (equivalent to a reduction of 2.5 on a scale from 0 to 10 for pain on movement at 24 hours after surgery) and time spent in hospital for open surgery (equivalent to one day). We found no evidence that an epidural with a local anaesthetic would affect the incidence of vomiting or poor healing of the gut.
Quality of evidence
We rated the quality of the evidence as high for return of gastrointestinal function, moderate for pain treatment, low for no effect on vomiting or healing of the gut and very low for reduced time spent in the hospital after open surgery.
Summary of findings
Summary of findings for the main comparison. Epidural local anaesthetic compared with opioid‐based regimen for adults.
| Epidural local anaesthetic compared with opioid‐based regimen for adults | ||||||
| Patient or population: adults Settings: Trials were performed in Australia (n = 4); Canada (n = 19); China (n = 6); Czech Republic (n = 1); Denmark (n = 8); Egypt (n = 3); Finland (n = 4); France (n = 5); Germany (n = 10); Greece (n = 2); India (n = 3); Israel (n = 2); Italy (n = 10); Japan (n = 1); Korea (n = 1); Lithuania (n = 1); Romania (n = 2); Russia (n = 1); Serbia (n = 1); Spain (n = 1); Sweden (n = 6); Switzerland (n = 2); The Netherlands (n = 1); Turkey (n = 8); United Kingdom (n = 6); United States of America (n = 17); and Uruguay (n = 1) Intervention: epidural local anaesthetic Comparison: opioid‐based regimen | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Opioid‐based regimen | Epidural local anaesthetic | |||||
| Time required to observe first flatus | Mean time required to observe first flatus in the intervention groups was 1.28 standard deviations lower (1.71 to 0.86 lower) | 1138 (22 studies) | ⊕⊕⊕⊕ higha,b,c,d,e,f,g,h | Effect size was proportionate to the concentration of local anaesthetic used Pooled reduction is equivalent to 17.5 hours | ||
| Time required to observe first faeces | Mean time required to observe first faeces in the intervention groups was 0.67 standard deviations lower (0.86 to 0.47 lower) | 1559 (28 studies) | ⊕⊕⊝⊝ lowc,d,e,g,i,j,k,l | Pooled reduction is equivalent to 22 hours | ||
| VAS scores on movement at 24 hours | Mean VAS scores on movement at 24 hours in the intervention groups was 0.85 standard deviations lower (1.04 to 0.67 lower) | 2731 (35 studies) | ⊕⊕⊕⊝ moderatea,b,c,d,e,f,g,l | Pooled reduction is equivalent to 2.5 on a scale from 0 to 10 | ||
| Vomiting during first 24 hours | Study population | RR 0.84 (0.57 to 1.23) | 1154 (22 studies) | ⊕⊕⊝⊝ lowc,e,g,i,k,l,m,n | ||
| 170 per 1000 | 143 per 1000 (97 to 210) | |||||
| Low | ||||||
| 50 per 1000 | 42 per 1000 (28 to 62) | |||||
| High | ||||||
| 250 per 1000 | 210 per 1000 (142 to 308) | |||||
| Anastomotic leak | Study population | RR 0.74 (0.41 to 1.32) | 848 (17 studies) | ⊕⊕⊝⊝ lowc,e,g,i,k,l,m,n | ||
| 53 per 1000 | 39 per 1000 (22 to 70) | |||||
| Low | ||||||
| 30 per 1000 | 22 per 1000 (12 to 40) | |||||
| High | ||||||
| 100 per 1000 | 74 per 1000 (41 to 132) | |||||
| Length of hospital stay | Mean length of hospital stay in the intervention groups was 0.20 standard deviations lower (0.35 to 0.04 lower) | 2598 (30 studies) | ⊕⊝⊝⊝ very lowa,c,d,j,k,l,o,p | Pooled reduction is equivalent to 1 day | ||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aAllocation concealment and/or blinding of outcome assessors rated as unclear or high risk for 75% or more of included studies for this outcome bWe did not downgrade the quality of evidence on the basis of inconsistency because a reasonable explanation was found for heterogeneity cDirect comparisons performed on the population of interest and not a surrogate marker dOptimal information size achieved eNo evidence of a publication bias, or applying a correction for the possibility of publication bias would not modify the conclusion fLarge effect size (SMD≥ 0.8) gNo evidence of confounding factors to justify upgrading hEffect size was proportionate to the local anaesthetic concentration i50% or more of included studies were rated as unclear or high risk for allocation concealment and/or blinding of outcome assessors jWe downgraded the level of evidence on inconsistency owing to a moderate amount of heterogeneity kNo evidence of a large effect lNo evidence of a dose‐response effect mNo heterogeneity or heterogeneity less than 25% nOptimal information size not achieved oCorrecting for the possibility of publication bias would modify the conclusion pLength of hospital stay may not adequately reflect readiness for discharge, as actual discharge may be delayed for various reasons
Background
This is an update of a previously published Cochrane review (Jorgensen 2001).
Description of the condition
In 2011, nearly 29% of hospital stays and 48% of hospital costs in the United States (USA) involved operating room procedures (http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb165.jsp). Among the 15 procedures most commonly performed in the USA were cholecystectomy and common bile duct exploration (129.4 per 100,000 population), abdominal and vaginal hysterectomy (99.4 per 100,000 population), colorectal resection (97.4 per 100,000 population), excision or lysis of peritoneal adhesions (97.4 per 100,000 population), appendicectomy (93.3 per 100,000 population) and oophorectomy (71.3 per 100,000 population). Thus abdominal surgery represents a significant proportion of hospital stays and costs. Gastrointestinal paralysis and postoperative pain are two major issues that need to be taken care of after abdominal surgery.
Gastrointestinal paralysis following abdominal surgery may result in prolonged hospital stay and increased costs. Following laparotomy, laparoscopic cholecystectomy and colectomy, approximately 10.3% of patients will have an ileus (Gan 2015). An ileus occurs more frequently in colectomy than cholecystectomy and more often when performed by laparotomy. Patients with ileus receiving opioids will have an increased length of hospital stay (ranging from 4.8 to 5.7 days), greater total costs (from USD 9,945 to USD 13,055) and a higher 30‐day all‐cause readmission rate (2.3% to 5.3% higher) compared with patients without an ileus (Gan 2015).
In 2000, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) suggested that pain should be considered as the fifth vital sign, and that under‐treatment of pain would constitute abrogation of a fundamental human right (White 2007). After this statement was issued, an increase in the use of opioids for acute postoperative pain treatment was observed, as was an increase in their side effects (White 2007). It was also noted that postoperative critical respiratory events were encountered more frequently during the first 24 hours after opioid therapy was introduced (Ramachandran 2011).
Description of the intervention
Epidural anaesthesia or analgesia or both consist of injection of a local anaesthetic into the spine outside the dura mater. Epidural local anaesthetic may be used during abdominal surgery, sometimes as a replacement for general anaesthesia but most commonly as a supplement to general anaesthesia for surgery and for postoperative analgesia.
How the intervention might work
Epidural analgesia has been claimed to facilitate many of the steps through which a patient must go before returning to his or her preoperative functional level after a major abdominal surgery, including motility of the gastrointestinal tract (Thörn 1996). As summarized in their review, Holte and Kehlet considered that "the pathogenesis of postoperative ileus is multifactorial, and includes activation of inhibitory reflexes, inflammatory mediators and opioids (endogenous and exogenous)" (Holte 2002). Epidural analgesia may promote a faster return to intestinal transit through various mechanisms including a reduction in opioid administration (Guay 2006), a blockade of sympathetic gut innervation (creating a relative parasympathetic predominance) and a direct effect of systemic local anaesthetics (McCarthy 2010). Thorn et al included 14 participants and demonstrated that the gastrointestinal electromyographic activity of participants who received epidurally administered bupivacaine was different from that of participants who received epidurally administered morphine (Thörn 1996). Oral acetaminophen absorption (as demonstrated by the area under the curve of acetaminophen blood concentrations from zero to 60 minutes) was also greater among participants who received bupivacaine than among those given morphine (Thörn 1996). Thus an epidural containing a local anaesthetic may promote faster gastrointestinal transit return than is attained with systemic opioids.
Among undisturbed participants receiving patient‐controlled morphine analgesia after surgery, abnormal breathing patterns with cyclical airway obstruction are extremely common (Drummond 2013). Provided that pain relief would be at least equivalent to that achieved with opioid therapy, decreasing the quantity of opioids administered (Guay 2006) would make epidural analgesia with a local anaesthetic appear as an interesting alternative in the treatment of acute postoperative pain for the first days after abdominal surgery ‐ the time when pain is most intense. Reducing the quantity of opioids administered after surgery may reduce the rare, but serious, adverse respiratory events associated with administration of opioids for the treatment of postoperative pain.
Why it is important to do this review
This is an update of a previously published Cochrane review (Jorgensen 2001) in which review authors concluded that an epidural with a local anaesthetic reduced the time to return of gastrointestinal transit but with substantial heterogeneity. The effect of additional epidural opioid on gastrointestinal function was unclear. We undertook this review to look for new studies, to update methods and to re‐explore heterogeneity.
Objectives
To compare effects of postoperative epidural analgesia with local anaesthetics versus postoperative systemic or epidural opioids in terms of return of gastrointestinal transit, postoperative pain control, postoperative vomiting, incidence of anastomotic leak, length of hospital stay and costs after abdominal surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel randomized controlled trials (RCTs) in which an epidural containing a local anaesthetic was added to general anaesthesia and was continued or not for postoperative analgesia or was used for postoperative analgesia in one group, and this group was compared with another group given systemic or epidural opioid‐based regimens. We excluded quasi‐randomized trials (e.g. even/odd day of birth, chart number), and we applied no language or publication status restrictions.
Types of participants
We included adult (≥ 16 years old accepted) patients undergoing any abdominal surgery (open or laparoscopic) under general anaesthesia. We excluded trials performed on children, trials performed outside the perioperative period (chronic pain, labour analgesia) and trials in which participants underwent surgery at other surgical sites (i.e. not abdominal surgery).
Types of interventions
Treatment groups received epidural anaesthesia/analgesia containing a local anaesthetic with or without added opioids.
Control groups received an opioid‐based regimen administered by the systemic route or through an epidural.
General anaesthesia was used for all participants during surgery.
We excluded trials comparing various types or various concentrations of local anaesthetics when investigators included no control group without a local anaesthetic (different intervention).
Some substances are not universally accepted as safe for injection in the epidural space. Therefore, we did not retain in the analysis any trial (or subgroup) in which anything other than an opioid or a local anaesthetic or epinephrine was injected into the epidural space (e.g. midazolam, ketamine, tramadol).
Types of outcome measures
Primary outcomes
Postoperative paralytic ileus as measured by first passage of flatus.
Secondary outcomes
Postoperative paralytic ileus as measured by first passage of faeces (stool).
Pain scores (any ascending scale) at rest and on movement at six to eight hours, 24 hours, 48 hours and 72 hours.
Incidence of postoperative vomiting: number of participants who had experienced vomiting on day one.
Anastomotic leak.
Length of hospital stay (LOS).
Hospital costs.
Search methods for identification of studies
Figure 1 presents the flow chart for study selection.
1.
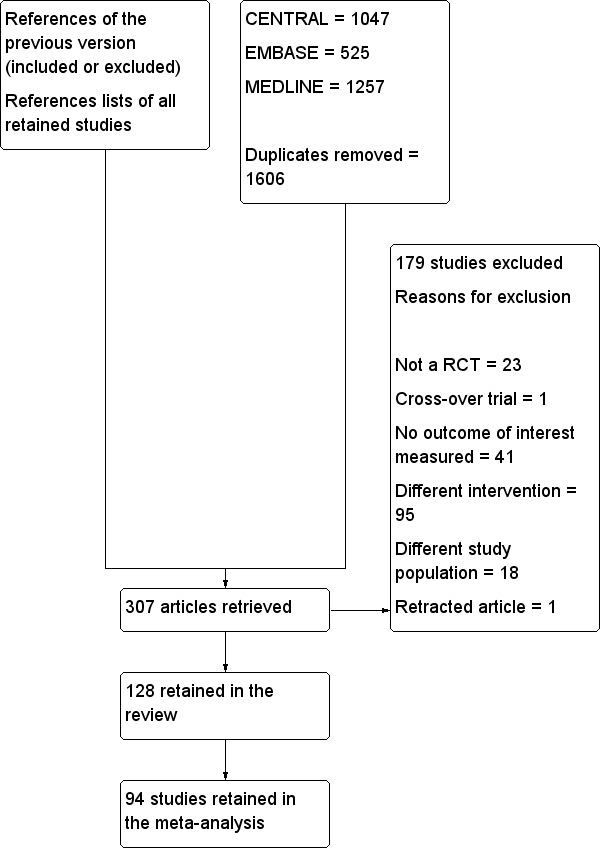
Flow diagram. Study selection from the 2014 search. We reran the search in February 2016, and added 16 potential new studies of interest to the list of ‘Studies awaiting classification'. These studies will be incorporated into the formal review findings during the next review update. We also found one ongoing trial.
Electronic searches
We conducted a comprehensive literature search to identify all published and unpublished RCTs with no language restriction. We searched the following electronic databases to identify potential studies: Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 12) (Appendix 1); MEDLINE (OVID) 1950 to December 2014 (Appendix 2); and EMBASE (OVID) 1974 to December 2014 (Appendix 3). We reran the search in February 2016 and added 16 potential new studies of interest to the list of Studies awaiting classification; we will incorporate these studies into formal review findings during the next review update. We added one study to the Ongoing studies section.
Searching other resources
We also looked at PsycINFO as a source of grey literature in March 2015 (Appendix 4).
Data collection and analysis
Selection of studies
Two review authors (JG and DNN (DNN left the review before its completion) or MN) scanned the titles and abstracts of all reports identified by electronic searching and retrieved full texts of articles for potential inclusion. We excluded duplicate publications by comparing sites and dates of data collection. We stated reasons for excluding retrieved studies under Characteristics of excluded studies and in Figure 1.
Data extraction and management
Two review authors (JG and DNN (DNN left the review before its completion) or MN) independently extracted data. We resolved disagreements by discussion and did not require assistance from a third review author. We extracted events and total number of participants in each group for dichotomous data when available. We extracted mean, standard deviation (SD) and number of participants in each group for continuous data when available. If results were not available in our favoured format or were provided on different scales, we extracted data as P values and number of participants for each group. When we were not able to extract data in any of these formats, we contacted study authors to obtain additional information from their trials. We did not use medians as estimates for means and did not estimate variances from interquartile or range. We extracted sites and dates of data collection (for exclusion of duplicate publications) and factors required for exploration of heterogeneity (see Assessment of heterogeneity). After we had reached agreement, data were entered into RevMan (http://ims.cochrane.org/revman/about‐revman‐5) and into Comprehensive Meta Analysis Version 2.2.044 (www.Meta‐Analysis.com) (for exploration of heterogeneity and assessment of small‐study effects and publication bias) by one review author (JG).
Assessment of risk of bias in included studies
Two review authors (JG, DNN or MN) evaluated the methodological quality of selected studies with no assumption using the risk of bias assessment tool of The Cochrane Collaboration (Higgins 2011). We resolved disagreements by discussion. We rated as unclear elements for which the report provided insufficient information to allow us to make a clear judgement.
Generation of the allocation sequence of interventions: We considered randomization adequate if it was generated by a computer or a random number table algorithm. We judged other processes, such as tossing of a coin, adequate if the whole sequence was generated before the start of the trial. We considered the trial as quasi‐randomized if a non‐random system, such as dates, names or identification numbers, was used.
Concealment of allocation: We considered concealment adequate if the process that was used prevented patient recruiters, investigators and participants from knowing the intervention allocation of the next participant to be enrolled in the study. We considered concealment inadequate if the allocation method allowed patient recruiters, investigators or participants to know the treatment allocation of the next participant to be enrolled in the study.
Blinding of participants and personnel: We considered blinding adequate if the participant and personnel taking care of the participant were blinded to the intervention. We considered blinding inadequate if the participant or personnel were not blinded to the intervention. We rated as unclear trials for which this was only partially adequately addressed (personnel blinded but not participants or vice versa, etc.).
Blinding of outcome assessment: We considered blinding adequate if the outcome assessor was blinded to the intervention. We considered blinding inadequate if the outcome assessor was not blinded to the intervention.
Incomplete outcome data (attrition bias): We considered the trial adequate if all dropouts or withdrawals were accounted for, and if the number of dropouts was small (< 20%), was similar for both interventions and reasons for dropping out seemed reasonable. We considered the trial inadequate for this specific item if reasons for dropping out of patients were not stated or did not sound reasonable, the number was high (≥ 20%) or the number was highly different between groups.
Selective reporting (reporting bias): We considered the trial at low risk of bias if all measurements stated in the Methods section were included in the Results section. We rated the trial as having unclear risk of bias when some of the results were missing or insufficient information was provided (conference abstract). We rated the trial as having high risk of bias when important results (taking study author objectives into account) were mentioned in the Methods section but were not given in the Results section.
Any other risk of bias: We considered any other reason that may have influenced study results. Per‐protocol (not intention‐to‐treat) results were considered as introducing potential risk, and we rated these as having unclear risk. Differences between study groups in demographic characteristics were rated as presenting unclear or high risk, depending on their potential influence on review results. We rated study protocols at high risk when other treatment modalities differed markedly (e.g. high steroid dose was given to one group only, epidural local anaesthetic was part of a fast track programme applied to one group only).
Measures of treatment effect
We reported results as risk ratios (RRs) and their 95% confidence intervals (CIs) for dichotomous data (vomiting and gastrointestinal anastomotic leak). Odds ratios (ORs) are not easily understood by clinicians (McColl 1998). All continuous data (time to first flatus, time to first faeces, pain scores, length of hospital stay and costs) included items entered as P values. Therefore it was not possible to provide results as differences in means and their 95% CIs. Instead we provided results as standardized mean differences (SMDs) and their CIs. For SMDs, we considered 0.2 a small difference, 0.5 a medium difference and 0.8 a large difference (Pace 2011). For clinical correspondence, we multiplied the standard deviation (SD) of the control group of a study at low risk of bias, and when a typical SD was available, by the SMD. When we noted an effect, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH) on the basis of the odds ratio, as this value is less likely to be influenced by the side (benefit or harm) on which data have been entered (Deeks 2002) (http://www.nntonline.net/visualrx/). When we observed no effect, we calculated optimal size information (number of participants needed for inclusion in a simple large trial) to justify a conclusion based on absence of effect (Pogue 1998) (http://www.stat.ubc.ca/˜rollin/stats/ssize/).
Unit of analysis issues
The unit of analysis was a participant who was individually randomized to the treatment group (intervention or control) in RCTs selected for this review.
Dealing with missing data
We contacted study authors for additional information when published articles did not provide enough information for extraction of data. We made no imputation.
Assessment of heterogeneity
We considered clinical heterogeneity before pooling results and examined statistical heterogeneity before carrying out any meta‐analysis. We quantified statistical heterogeneity by using the I2 statistic. We qualified the amount as low (< 25%), moderate (50%) or high (≥ 75%) depending on the value obtained for the I2 statistic (Higgins 2003).
Assessment of reporting biases
We assessed publication bias by using a funnel plot, followed by Duval and Tweedie's trim and fill technique (Borenstein 2009; Duval 2000; Duval 2000a). This technique not only assesses whether publication bias is likely, it also yields an estimate of effect size after correction for the possibility of publication bias when such bias is suspected.
Data synthesis
We analysed data with RevMan (http://ims.cochrane.org/revman/about‐revman‐5) and Comprehensive Meta Analysis Version 2.2.044 (www.Meta‐Analysis.com) by using fixed‐effect (I2 < 25%) or random‐effects models (I2 > 25%). For continuous data, all analyses provided data that we could not enter in our favoured format (mean, SD and number of participants). In these situations, we chose not to consider a median as equivalent to a mean and did not estimate SD from quartiles. Instead we entered data into Comprehensive Meta Analysis as P values and numbers of participants. In such cases, mean differences cannot be obtained. We then transferred data in RevMan as generic variance and presented our results as standardized mean differences (SMDs). For SMDs, we considered 0.8 as the cutoff limit for a large effect (Pace 2011). For clinical equivalents, we multiplied the SMD by the SD of a study at low risk of bias, and when a typical SD on a clinical scale was provided (Higgins 2011). For dichotomous data, we provided results as risk ratios (values best understood by clinicians; McColl 1998). For results in which the intervention produced an effect, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) by using the odds ratio (http://www.nntonline.net/visualrx/). When results were negative, we also calculated optimal information size to ensure that enough participants were included in the retained studies to justify a conclusion based on absence of effect (Pogue 1998) (www.stat.ubc.ca/˜rollin/stats/ssize/b2.html).
Subgroup analysis and investigation of heterogeneity
We explored any amount of heterogeneity > 25% by using Egger's regression intercept (Comprehensive Meta Analysis; to eliminate a small‐study effect), sensitivity analysis, subgrouping or meta‐regression (Comprehensive Meta Analysis) as appropriate. A priori factors for heterogeneity consisted of:
level of the epidural (thoracic vs lumbar);
type of drug used (local anaesthetic alone (concentration in lidocaine equivalent potency calculated as follows: lidocaine = 1, bupivacaine = 4, chloroprocaine = 1.5, dibucaine = 4, etidocaine = 4, levobupivacaine = 3.9, mepivacaine = 0.8, prilocaine = 0.9, procaine = 0.5, ropivacaine = 3 and tetracaine = 4; Berde 2009)) versus local anaesthetic plus opioid (and type of opioid);
timing (pre‐surgical vs post‐surgical incision) and duration of administration (intraoperative only, < 48 hours vs ≥ 48 hours);
site of surgery (bowel surgery; gynaecological, urological or vascular surgery);
type of surgery (open vs laparoscopic);
mean group age;
American Society of Anestheiologists (ASA) physical status; and
substance used and route of administration of analgesia in the control group (intravenous (with or without use of a patient‐controlled analgesia device) vs epidural (with or without use of a patient‐controlled analgesia device) vs other routes).
Although forest plots for all outcomes were examined while studies were placed in order for all potential heterogeneity factors, to avoid multiple comparisons, we performed analysis (sensitivity, subgrouping or meta‐regression) only when forest plots suggested a statistically significant effect, or when reviewers made the request (open vs laparoscopic surgery). All analysis performed are reported. Analysis included as forest plots in RevMan (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13; Analysis 1.14; Analysis 1.15; Analysis 1.17; Analysis 1.18) are those chosen as most interesting for each outcome (i.e. subgrouped to offer maximal possibility of showing subgroup differences).
1.1. Analysis.
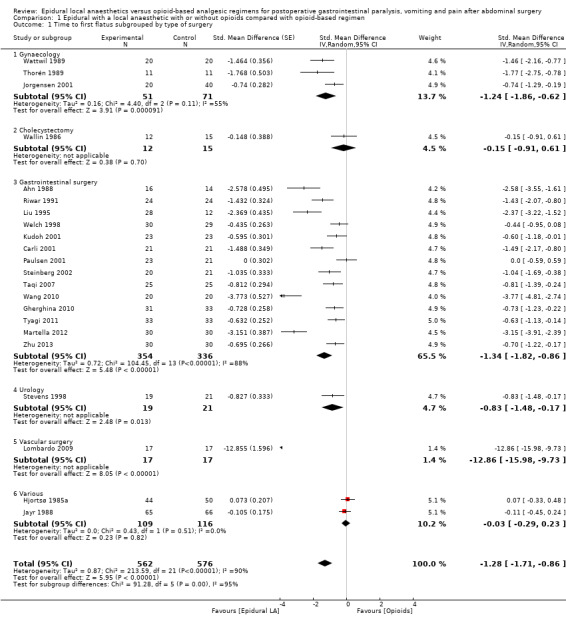
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 1 Time to first flatus subgrouped by type of surgery.
1.2. Analysis.
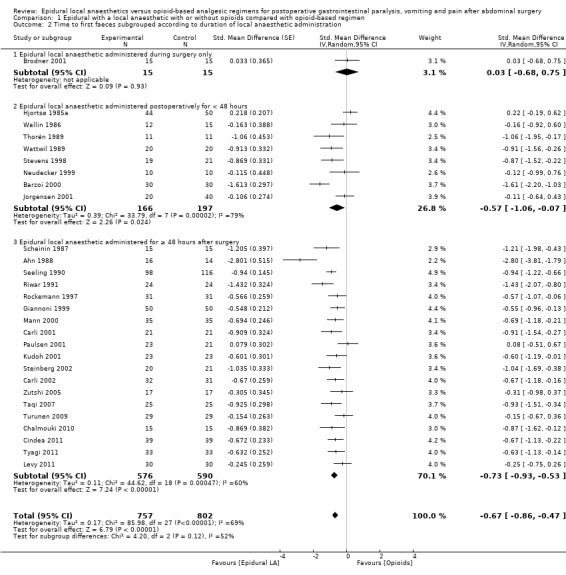
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 2 Time to first faeces subgrouped according to duration of local anaesthetic administration.
1.3. Analysis.
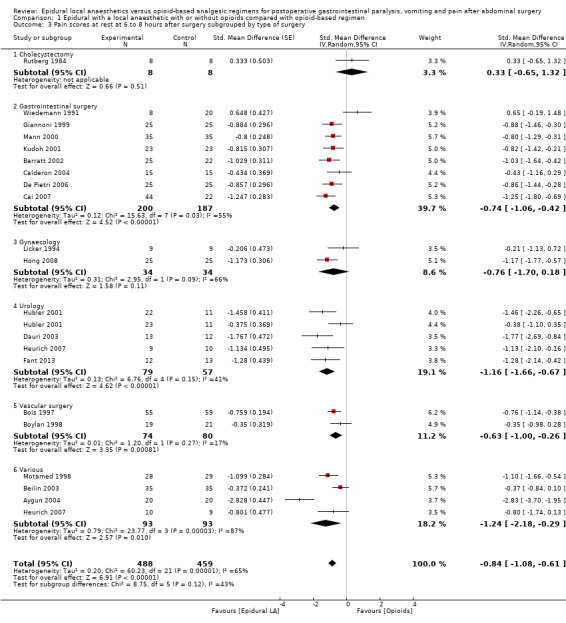
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 3 Pain scores at rest at 6 to 8 hours after surgery subgrouped by type of surgery.
1.4. Analysis.
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 4 Pain scores on movement at 6 to 8 hours after surgery subgrouped by type of opioid in the control group.
1.5. Analysis.
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 5 Pain scores at rest at 24 hours subgrouped by type of opioid in the epidural.
1.6. Analysis.
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 6 Pain scores at rest at 24 hours subgrouped by opioid in the control group.
1.7. Analysis.
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 7 Pain scores on movement at 24 hours subgrouped by type of surgery.
1.8. Analysis.
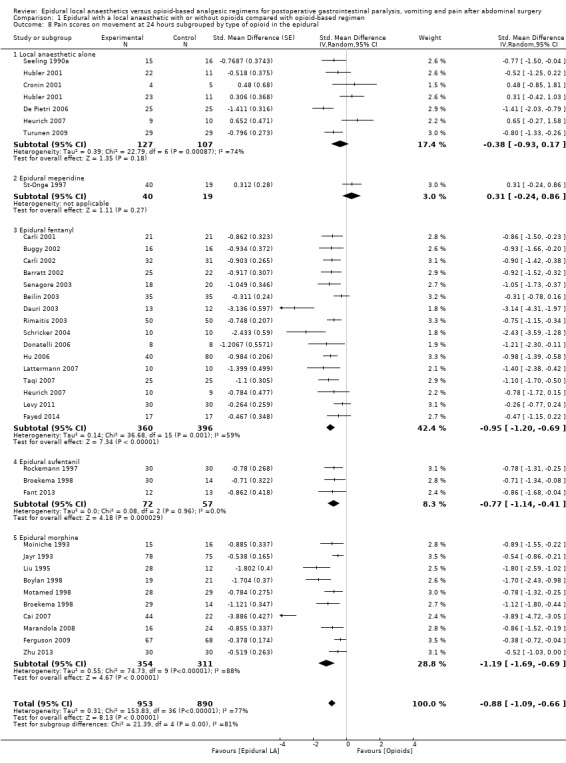
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 8 Pain scores on movement at 24 hours subgrouped by type of opioid in the epidural.
1.9. Analysis.
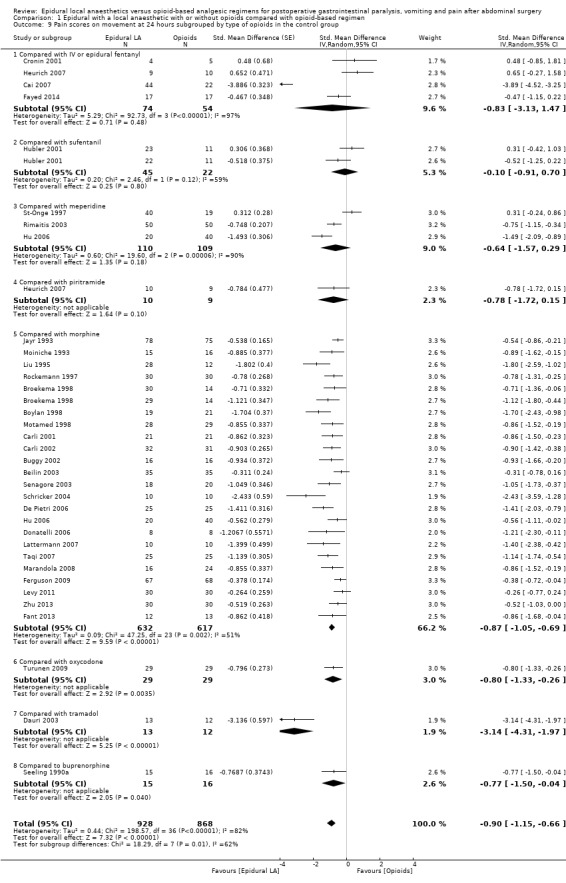
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 9 Pain scores on movement at 24 hours subgrouped by type of opioids in the control group.
1.10. Analysis.
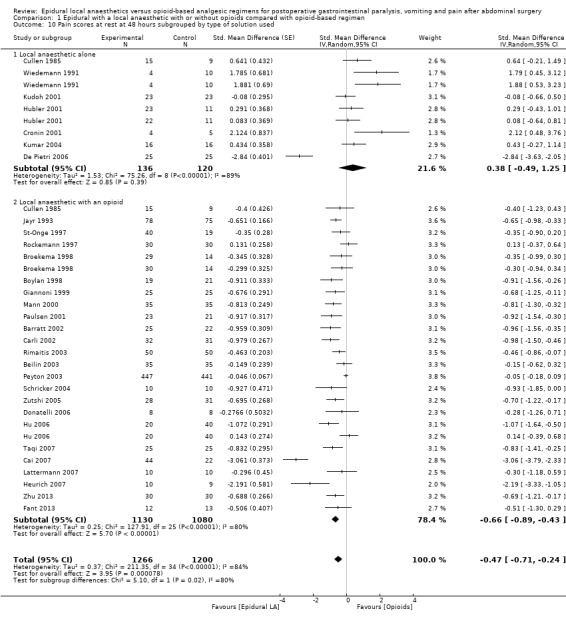
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 10 Pain scores at rest at 48 hours subgrouped by type of solution used.
1.11. Analysis.
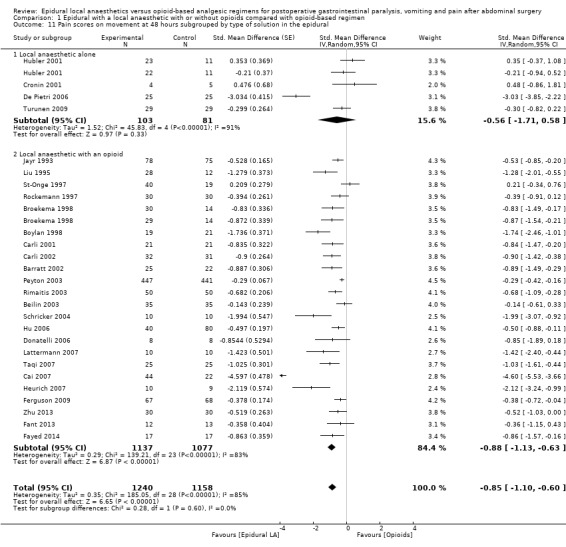
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 11 Pain scores on movement at 48 hours subgrouped by type of solution in the epidural.
1.12. Analysis.
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 12 Pain scores at rest at 72 hours subgrouped by type of solution used.
1.13. Analysis.
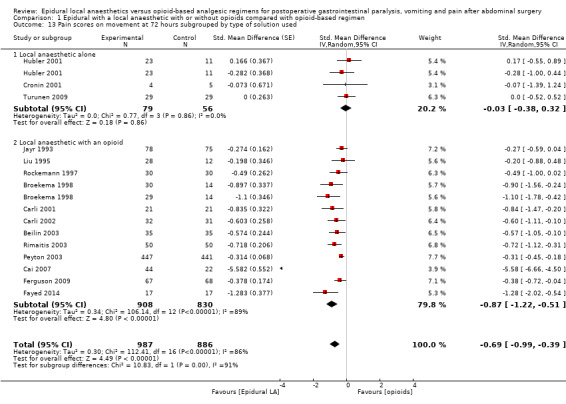
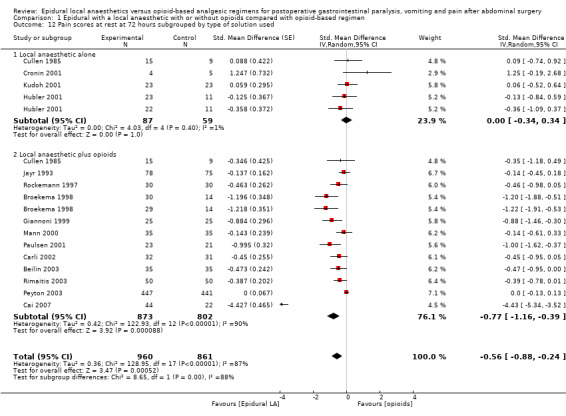
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 13 Pain scores on movement at 72 hours subgrouped by type of solution used.
1.14. Analysis.
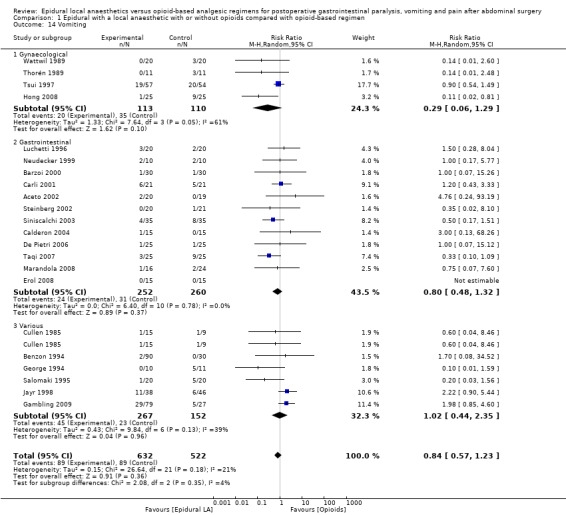
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 14 Vomiting.
1.15. Analysis.
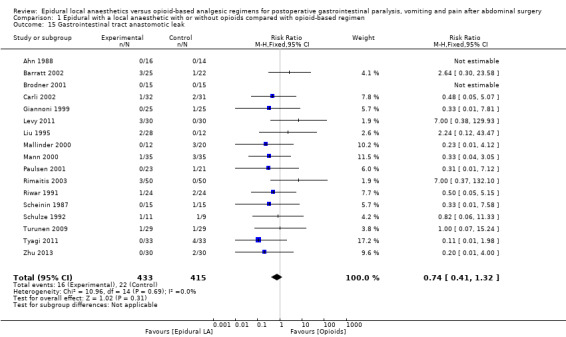
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 15 Gastrointestinal tract anastomotic leak.
1.17. Analysis.
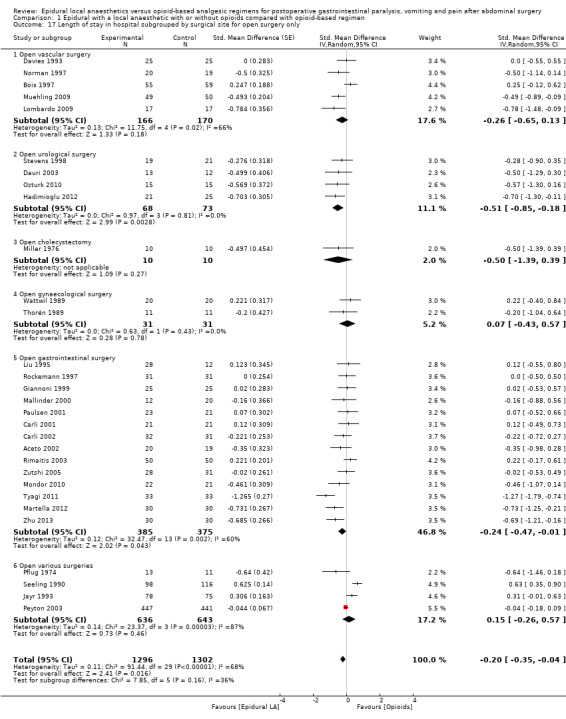
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 17 Length of stay in hospital subgrouped by surgical site for open surgery only.
1.18. Analysis.
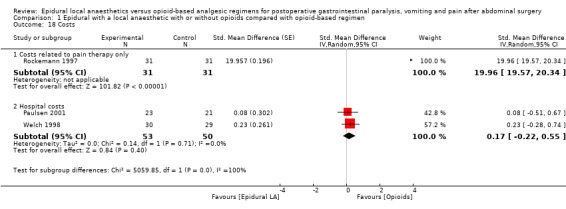
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 18 Costs.
Sensitivity analysis
We performed sensitivity analysis (defined as excluding a study on its risk of bias or because it appeared as an outlier on a forest plot).
Quality of evidence
We judged the quality of a body of evidence according to the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) system (Guyatt 2011a) and presented this assessment in Table 1 (ims.cochrane.org/revman/gradepro) for all outcomes. For pain, we chose scores on movement at postoperative day one. For risk of bias, we judged the quality of evidence as high when most information was derived from studies at low risk of bias, and downgraded quality by one level when most information was obtained from studies at high or unclear risk of bias (allocation concealment and blinding of outcome assessors), or by two levels when the proportion of information obtained from studies at high risk of bias was sufficient to affect interpretation of results. For inconsistency, we downgraded the quality of evidence by one when the I2 statistic was 50% or higher without satisfactory explanation, and by two levels when the I2 statistic was 75% or higher without explanation. We did not downgrade the quality of evidence for indirectness, as all outcomes were based on direct comparisons were performed on the population of interest and were not surrogate markers (Guyatt 2011b). For imprecision (Guyatt 2011c), we downgraded the quality of evidence by one level when the CI around the effect size was large or overlapped an absence of effect and failed to exclude an important benefit or harm, or when the number of participants was less than the optimal information size; and we downgraded the quality by two levels when the CI was very wide and included both appreciable benefit and harm. For publication bias, we downgraded the quality of evidence by one level when correcting for the possibility of publication bias as assessed by Duval and Tweedie’s fill and trim analysis changed the conclusion. We upgraded the quality of evidence by one when the effect size was large (RR < 0.5 or > 2.0), and by two levels when the effect size was very large (RR < 0.2 or > 5) (Guyatt 2011d). We applied the same rules for OR when basal risk was less than 20%. For SMD, we used 0.8 as the cutoff point for a large effect (Pace 2011). We also upgraded quality by one level when we found evidence of a dose‐related response. We upgraded quality by one level when the possible effect of confounding factors would reduce a demonstrated effect or suggest a spurious effect when results show no effect. When the quality of the body of evidence is high, further research is very unlikely to change our confidence in the estimate of effect. When quality is moderate, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. When quality is low, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. When the quality is very low, any estimate of effect is very uncertain (Guyatt 2008).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of studies awaiting classification.
Results of the search
We reviewed all included and excluded studies presented in the previous version of the review (Jorgensen 2001). The electronic search yielded 1047 abstracts from The Cochrane Library, 525 from EMBASE and 1257 from MEDLINE (Figure 1). After removal of duplicates, we screened 1606 abstracts. From studies in the previous version of the review, the list of abstracts obtained by the electronic search and the reference lists of relevant studies, we selected 307 studies (of which 216 were new studies) for further evaluation. We excluded 179 studies for various reasons: not a randomized controlled trial (n = 23), cross‐over trial (n = 1), no outcome of interest measured for the actual review (n = 41), different intervention (n = 95), different study population (n = 18) or retracted (n = 1).
Included studies
Sixteen studies are awaiting classification and one is ongoing. We retained 128 trials with 8754 participants (4426 in epidural local anaesthetic groups and 4328 in control groups) in the review and 94 studies with 5846 participants (3010 in epidural local anaesthetic groups and 2836 in opioid‐based regimen groups) in the analysis (Figure 1). We included 34 trials in the review but not in the analysis: 17 because data could not be extracted (Addison 1974; Alpaslan 2010; Beilin 2008; Bellolio 2012; Bisgaard 1990; Carli 1997; Cuschieri 1985; Elkaradawy 2011; Kentner 1996; Lugli 2008; Lugli 2010; Malenkovic 2003; O'Connor 2006; Schulze 1988; Seeling 1991; Tuman 1991; Yeager 1987), 15 because pain scores were the only outcomes of interest measured and local anaesthetics were administered during surgery only or for an unspecified duration (Doruk 2003; El‐Refai 2003; Handley 1997; Katz 2003; Limberi 2003; Liuboshevskii 2012; Ozcan 2004; Ozdilmac 2003; Park 2001; Rockemann 1996; Schricker 2000; Schricker 2002; Schumann 2003; Subramaniam 2000; Watters 1993), one because no outcomes were available at our selected time points (Scheinin 1982) and one because investigators provided insufficient information (Voylenko 2013). The 128 included trials enrolled participants with a mean age between 33 and 76 years of age, with a mean ASA score between 1.41 and 3.45 or from 1 to 2 to 1 to 5, and were published between 1974 and 2014. Researchers in three studies reported that their trial was registered: Fant 2013 (www.ClinicalTrials.gov; identifier: NCT01367418), Levy 2011 (www.ClinicalTrials.gov; identifier: NCT 18926278) and Muehling 2009 (www.ClinicalTrials.gov; identifier: NCT 00615888).
Trials were performed in Australia (n = four: Barratt 2002; Davies 1993; Handley 1997; Peyton 2003); Canada (n = 19; Bois 1997; Boylan 1998; Carli 1997; Carli 2001; Carli 2002; Donatelli 2006; Katz 2003; Lattermann 2007; Lugli 2008; Lugli 2010; Miller 1976; Mondor 2010; O'Connor 2006; Schricker 2000; Schricker 2002; Schricker 2004; St‐Onge 1997; Taqi 2007; Watters 1993); China (n = six; Cai 2007; Hu 2006; Tsui 1997; Wang 2010; Zeng 2003; Zhu 2013); Czech Republic (n = 1; Voylenko 2013); Denmark (n = eight; Bisgaard 1990; Brodner 2001; Hjortsø 1985a; Hjortsø 1985b; Jorgensen 2001; Moiniche 1993; Schulze 1988; Schulze 1992); Egypt (n = three; Elkaradawy 2011; El‐Refai 2003; Fayed 2014); Finland (n = four; Salomaki 1995; Scheinin 1982; Scheinin 1987; Turunen 2009); France (n = five; Jayr 1988; Jayr 1993; Jayr 1998; Mann 2000; Motamed 1998); Germany (n = 10; Heurich 2007; Hubler 2001; Kentner 1996; Muehling 2009; Neudecker 1999; Rockemann 1996; Rockemann 1997; Seeling 1990; Seeling 1990a; Seeling 1991); Greece (n = two; Chalmouki 2010; Limberi 2003); India (n = three; Kumar 2004; Subramaniam 2000; Tyagi 2011); Israel (n = two; Beilin 2003; Beilin 2008); Italy (n = 10; Aceto 2002; Barzoi 2000; Dauri 2003; De Pietri 2006; Giannoni 1999; Lombardo 2009; Luchetti 1996; Marandola 2008; Martella 2012; Siniscalchi 2003); Japan (n = one; Kudoh 2001); Korea (n = one; Hong 2008); Lithuania (n = one; Rimaitis 2003), Romania (n = two; Cindea 2011; Gherghina 2010); Russia (n = one; Liuboshevskii 2012), Serbia (n = 1; Malenkovic 2003); Spain (n = one; Calderon 2004); Sweden (n = six; Ahn 1988; Fant 2013; Rutberg 1984; Thorén 1989; Wallin 1986; Wattwil 1989); Switzerland (n = two; Licker 1994; Riwar 1991); The Netherlands (n = one; Broekema 1998); Turkey (n = eight; Alpaslan 2010; Aygun 2004; Doruk 2003; Erol 2008; Hadimioglu 2012; Ozcan 2004; Ozdilmac 2003; Ozturk 2010); United Kingdom (n = six; Addison 1974; Buggy 2002; Cuschieri 1985; George 1994; Levy 2011; Mallinder 2000); United States of America (n = 17; Benzon 1994; Cronin 2001; Cullen 1985; Ferguson 2009; Gambling 2009; Liu 1995; Norman 1997; Park 2001; Paulsen 2001; Pflug 1974; Schumann 2003; Senagore 2003; Steinberg 2002; Stevens 1998; Welch 1998; Yeager 1987; Zutshi 2005); and Uruguay (n = one; Bellolio 2012).
The funding source of 56/128 (44%) included trials was known. Those trials were funded by charity (n = 19; Beilin 2008; Buggy 2002; Carli 2001; Carli 2002; Cronin 2001; Cullen 1985; Cuschieri 1985; Donatelli 2006; Handley 1997; Jayr 1988; Jorgensen 2001; Mann 2000; Salomaki 1995; Scheinin 1987; Schricker 2000; Schricker 2002; Schricker 2004; Schulze 1992; Taqi 2007), departmental resources (n = eight; Cai 2007; Ferguson 2009; Hu 2006; Levy 2011; Mondor 2010; Tyagi 2011; Wang 2010; Zeng 2003), governmental sources (n = 15; Barratt 2002; Beilin 2003; Boylan 1998; Fant 2013; Heurich 2007; Katz 2003; Lugli 2008; Lugli 2010; Miller 1976; Park 2001; Peyton 2003; Pflug 1974; Schumann 2003; Thorén 1989; Watters 1993) and industry (in part or in total) (n = 15; Benzon 1994; Bois 1997; Gambling 2009; Hjortsø 1985a; Hjortsø 1985b; Jayr 1993; Jayr 1998; Liu 1995; Mallinder 2000; Moiniche 1993; Rutberg 1984; St‐Onge 1997; Schulze 1988; Steinberg 2002; Wallin 1986).
Surgeries included bariatric surgery (Schumann 2003); cholecystectomy (Addison 1974; Cuschieri 1985; Elkaradawy 2011; Erol 2008; Miller 1976; Moiniche 1993; Rutberg 1984; Schulze 1988; Wallin 1986); surgery of the gastrointestinal tract (Aceto 2002; Ahn 1988; Barratt 2002; Barzoi 2000; Bisgaard 1990; Cai 2007; Calderon 2004; Carli 1997; Carli 2001; Carli 2002; De Pietri 2006; Donatelli 2006; Fayed 2014; Gherghina 2010; Giannoni 1999; Handley 1997; Hjortsø 1985b; Kudoh 2001; Lattermann 2007; Levy 2011; Liu 1995; Liuboshevskii 2012; Luchetti 1996; Lugli 2008; Lugli 2010; Mallinder 2000; Mann 2000; Marandola 2008; Martella 2012; Mondor 2010; Neudecker 1999; Ozdilmac 2003; Paulsen 2001; Rimaitis 2003; Riwar 1991; Rockemann 1997; Scheinin 1987; Schricker 2000; Schricker 2002; Schricker 2004; Senagore 2003; Siniscalchi 2003; Steinberg 2002; Schulze 1992; Taqi 2007; Turunen 2009; Tyagi 2011; Wang 2010; Watters 1993; Welch 1998; Zhu 2013; Zutshi 2005); gynaecological surgery (Cronin 2001; El‐Refai 2003; Ferguson 2009; Hong 2008; Jorgensen 2001; Katz 2003; Licker 1994; Ozcan 2004; Thorén 1989; Tsui 1997; Wattwil 1989); liver surgery (Bellolio 2012); urological surgery (Brodner 2001; Chalmouki 2010; Dauri 2003; Doruk 2003; Fant 2013; Hadimioglu 2012; Hubler 2001; Kentner 1996; O'Connor 2006; Ozturk 2010; Voylenko 2013); vascular surgery (Bois 1997; Boylan 1998; Davies 1993; Lombardo 2009; Muehling 2009; Norman 1997; Tuman 1991); and various abdominal surgeries (Alpaslan 2010; Aygun 2004; Beilin 2003; Beilin 2008; Benzon 1994; Broekema 1998; Buggy 2002; Cindea 2011; Cullen 1985; Gambling 2009; George 1994; Hjortsø 1985a; Hu 2006; Jayr 1993; Jayr 1998; Kumar 2004; Limberi 2003; Malenkovic 2003; Motamed 1998; Park 2001; Peyton 2003; Pflug 1974; Rockemann 1996; Salomaki 1995; Scheinin 1982; Seeling 1990; Seeling 1990a; Seeling 1991; St‐Onge 1997; Subramaniam 2000; Yeager 1987; Zeng 2003). These surgeries were performed by laparoscopy (Hong 2008; Levy 2011; Luchetti 1996; Neudecker 1999; Senagore 2003; Taqi 2007; Turunen 2009); or by open laparotomy (Aceto 2002; Addison 1974; Ahn 1988; Alpaslan 2010; Aygun 2004; Barratt 2002; Barzoi 2000; Beilin 2003; Beilin 2008; Bellolio 2012; Benzon 1994; Bisgaard 1990; Bois 1997; Boylan 1998; Brodner 2001; Broekema 1998; Buggy 2002; Cai 2007; Calderon 2004; Carli 1997; Carli 2001; Carli 2002; Chalmouki 2010; Cindea 2011; Cronin 2001; Cullen 1985; Cuschieri 1985; Dauri 2003; Davies 1993; De Pietri 2006; Donatelli 2006; Doruk 2003; Elkaradawy 2011; El‐Refai 2003; Erol 2008; Fant 2013; Fayed 2014; Ferguson 2009; Gambling 2009; George 1994; Gherghina 2010; Giannoni 1999; Hadimioglu 2012; Handley 1997; Heurich 2007; Hjortsø 1985a; Hjortsø 1985b; Hu 2006; Hubler 2001; Jayr 1988; Jayr 1993; Jayr 1998; Jorgensen 2001; Katz 2003; Kentner 1996; Kudoh 2001; Kumar 2004; Lattermann 2007; Licker 1994; Limberi 2003; Liu 1995; Liuboshevskii 2012; Lombardo 2009; Lugli 2008; Lugli 2010; Malenkovic 2003; Mallinder 2000; Mann 2000; Marandola 2008; Martella 2012; Miller 1976; Moiniche 1993; Mondor 2010; Motamed 1998; Muehling 2009; Norman 1997; O'Connor 2006; Ozcan 2004; Ozdilmac 2003; Ozturk 2010; Park 2001; Paulsen 2001; Peyton 2003; Pflug 1974; Rimaitis 2003; Riwar 1991; Rockemann 1996; Rockemann 1997; Rutberg 1984; Salomaki 1995; Scheinin 1982; Scheinin 1987; Schricker 2000; Schricker 2002; Schricker 2004; Schulze 1988; Schulze 1992; Schumann 2003; Seeling 1990; Seeling 1990a; Seeling 1991; Siniscalchi 2003; Steinberg 2002; Stevens 1998; St‐Onge 1997; Subramaniam 2000; Thorén 1989; Tsui 1997; Tuman 1991; Tyagi 2011; Voylenko 2013; Wallin 1986; Wang 2010; Watters 1993; Wattwil 1989; Welch 1998; Wiedemann 1991; Yeager 1987; Zeng 2003; Zhu 2013; Zutshi 2005).
Epidurals were placed at the thoracic level (Aceto 2002; Addison 1974; Barratt 2002; Barzoi 2000; Bois 1997; Brodner 2001; Broekema 1998; Cai 2007; Carli 1997; Carli 2001; Carli 2002; Chalmouki 2010; Cronin 2001; Cuschieri 1985; Dauri 2003; Davies 1993; De Pietri 2006; Donatelli 2006; Elkaradawy 2011; Erol 2008; Fant 2013; Fayed 2014; Ferguson 2009; George 1994; Hubler 2001; Jayr 1988; Jayr 1993; Jayr 1998; Jorgensen 2001; Kudoh 2001; Kumar 2004; Lattermann 2007; Levy 2011; Liu 1995; Liuboshevskii 2012; Luchetti 1996; Lugli 2008; Lugli 2010; Mann 2000; Marandola 2008; Martella 2012; Moiniche 1993; Mondor 2010; Motamed 1998; Muehling 2009; Neudecker 1999; Norman 1997; Paulsen 2001; Pflug 1974; Rimaitis 2003; Rockemann 1996; Rockemann 1997; Rutberg 1984; Salomaki 1995; Scheinin 1982; Schricker 2000; Schricker 2002; Schricker 2004; Schulze 1988; Schulze 1992; Schumann 2003; Seeling 1990; Seeling 1990a; Senagore 2003; Siniscalchi 2003; Steinberg 2002; Stevens 1998; St‐Onge 1997; Taqi 2007; Thorén 1989; Turunen 2009; Tyagi 2011; Wallin 1986; Wattwil 1989; Zhu 2013; Zutshi 2005), at the lumbar level (Ahn 1988; Alpaslan 2010; Aygun 2004; Beilin 2003; Beilin 2008; Bisgaard 1990; Boylan 1998; Calderon 2004; Doruk 2003; El‐Refai 2003; Giannoni 1999; Hadimioglu 2012; Handley 1997; Hjortsø 1985a; Hong 2008; Hu 2006; Katz 2003; Kentner 1996; Licker 1994; Malenkovic 2003; Mallinder 2000; Miller 1976; Ozcan 2004;Ozdilmac 2003; Riwar 1991; Subramaniam 2000; Tsui 1997; Watters 1993) or at the thoracic or lumbar level (Benzon 1994; Buggy 2002; Gherghina 2010; Heurich 2007; Limberi 2003; O'Connor 2006; Park 2001; Peyton 2003; Tuman 1991; Yeager 1987), or they were placed at an unspecified level (Bellolio 2012; Cindea 2011; Cullen 1985; Gambling 2009; Hjortsø 1985b; Lombardo 2009; Ozturk 2010; Scheinin 1987; Seeling 1991; Voylenko 2013; Wang 2010; Welch 1998; Zeng 2003).
Local anaesthetics were administered only for surgery (Brodner 2001; Gambling 2009; Hadimioglu 2012; Handley 1997; Jayr 1988; Katz 2003; Limberi 2003; Luchetti 1996; Mallinder 2000; Mondor 2010; Norman 1997; Ozcan 2004; Ozdilmac 2003; Park 2001; Rockemann 1996; Subramaniam 2000; Watters 1993), for less than 48 hours (Alpaslan 2010; Aygun 2004; Barzoi 2000; Beilin 2008; Benzon 1994; Buggy 2002; Calderon 2004; Cuschieri 1985; Dauri 2003; Elkaradawy 2011; George 1994; Hjortsø 1985a; Hjortsø 1985b; Jayr 1998; Jorgensen 2001; Kentner 1996; Licker 1994; Lombardo 2009; Marandola 2008; Miller 1976; Moiniche 1993; Neudecker 1999; Rutberg 1984; Salomaki 1995; Scheinin 1982; Seeling 1990a; Senagore 2003; Stevens 1998; Thorén 1989; Wallin 1986; Wang 2010; Wattwil 1989; Zeng 2003), for 48 hours or longer (Aceto 2002; Addison 1974; Ahn 1988; Barratt 2002; Beilin 2003; Bisgaard 1990; Bois 1997; Boylan 1998; Broekema 1998; Cai 2007; Carli 1997; Carli 2001; Carli 2002; Chalmouki 2010; Cindea 2011; Cronin 2001; Cullen 1985; Davies 1993; De Pietri 2006; Donatelli 2006; Erol 2008; Fant 2013; Fayed 2014; Ferguson 2009; Gherghina 2010; Giannoni 1999; Hong 2008; Hu 2006; Hubler 2001; Jayr 1993; Kudoh 2001; Kumar 2004; Lattermann 2007; Levy 2011; Liu 1995; Lugli 2008; Lugli 2010; Malenkovic 2003; Mann 2000; Motamed 1998; Paulsen 2001; Peyton 2003; Pflug 1974; Rimaitis 2003; Riwar 1991; Rockemann 1997; Scheinin 1987; Schricker 2004; Schulze 1988; Schulze 1992; Seeling 1990; Seeling 1991; Steinberg 2002; St‐Onge 1997; Taqi 2007; Tsui 1997; Tuman 1991; Turunen 2009; Tyagi 2011; Zhu 2013; Zutshi 2005) or for an unspecified duration (Bellolio 2012; Doruk 2003; El‐Refai 2003; Liuboshevskii 2012; Martella 2012; Muehling 2009; O'Connor 2006; Ozturk 2010; Schricker 2000; Schricker 2002; Schumann 2003; Siniscalchi 2003; Voylenko 2013; Welch 1998; Yeager 1987). For Heurich 2007, local anaesthetic was administered for 24 hours to participants in the second portion of the trial and for 48 hours to participants in the first portion. The concentration of local anaesthetic varied between 2 and 30 milligrams per millilitre in lidocaine equivalent. Fentanyl (Aygun 2004; Barratt 2002; Beilin 2003; Beilin 2008; Benzon 1994; Bois 1997; Buggy 2002; Calderon 2004; Carli 2001; Carli 2002; Cindea 2011; Dauri 2003; Donatelli 2006; Elkaradawy 2011; Erol 2008; Fayed 2014; George 1994; Hu 2006; Katz 2003; Lattermann 2007; Levy 2011; Licker 1994; Liuboshevskii 2012; Lugli 2008; Lugli 2010; O'Connor 2006; Ozcan 2004; Ozturk 2010; Paulsen 2001; Rimaitis 2003; Salomaki 1995; Schricker 2000; Schricker 2002; Schricker 2004; Seeling 1990; Senagore 2003; Steinberg 2002; Taqi 2007; Tsui 1997; Tuman 1991; Tyagi 2011; Zeng 2003; Zutshi 2005), meperidine (Schumann 2003; St‐Onge 1997), fentanyl or meperidine (Peyton 2003), fentanyl or no opioid (Heurich 2007), morphine (Barzoi 2000; Bisgaard 1990; Boylan 1998; Cai 2007; Ferguson 2009; Giannoni 1999; Hjortsø 1985a; Hjortsø 1985b; Hong 2008; Jayr 1993; Liu 1995; Luchetti 1996; Marandola 2008; Moiniche 1993; Motamed 1998; Ozdilmac 2003; Rockemann 1996; Schulze 1992; Seeling 1991; Stevens 1998; Subramaniam 2000; Wang 2010; Welch 1998; Zhu 2013), extended‐release morphine (Gambling 2009), sufentanil (Aceto 2002; Fant 2013; Gherghina 2010; Lombardo 2009; Mann 2000; Martella 2012; Muehling 2009; Rockemann 1997), morphine or sufentanil (Broekema 1998), morphine or no opioid (Cullen 1985; Doruk 2003) or no opioid (Addison 1974; Ahn 1988; Alpaslan 2010; Brodner 2001; Chalmouki 2010; Cronin 2001; Cuschieri 1985; Davies 1993; De Pietri 2006; El‐Refai 2003; Hadimioglu 2012; Handley 1997; Hubler 2001; Jayr 1988; Jayr 1998; Jorgensen 2001; Kentner 1996; Kudoh 2001; Kumar 2004; Limberi 2003; Malenkovic 2003; Mallinder 2000; Miller 1976; Mondor 2010; Neudecker 1999; Norman 1997; Park 2001; Pflug 1974; Riwar 1991; Rutberg 1984; Scheinin 1982; Scheinin 1987; Schulze 1988; Seeling 1990a; Siniscalchi 2003; Thorén 1989; Turunen 2009; Voylenko 2013; Wallin 1986; Watters 1993; Wattwil 1989; Yeager 1987) was added to the epidural.
Epidural administration of local anaesthetic was started before (Aceto 2002; Addison 1974; Ahn 1988; Alpaslan 2010; Barratt 2002; Barzoi 2000; Beilin 2008; Bellolio 2012; Bisgaard 1990; Boylan 1998; Brodner 2001; Broekema 1998; Buggy 2002; Cai 2007; Carli 1997; Carli 2001; Carli 2002; Chalmouki 2010; Cronin 2001; Cuschieri 1985; Dauri 2003; Davies 1993; De Pietri 2006; Donatelli 2006; Doruk 2003; Elkaradawy 2011; El‐Refai 2003; Erol 2008; Fant 2013; Ferguson 2009; Gambling 2009; George 1994; Gherghina 2010; Giannoni 1999; Hadimioglu 2012; Handley 1997; Heurich 2007; Hjortsø 1985a; Hjortsø 1985b; Hong 2008; Hubler 2001; Jayr 1988; Jayr 1993; Jorgensen 2001; Kentner 1996; Kudoh 2001; Lattermann 2007; Levy 2011; Licker 1994; Limberi 2003; Liu 1995; Liuboshevskii 2012; Lombardo 2009; Luchetti 1996; Lugli 2008; Lugli 2010; Malenkovic 2003; Mallinder 2000; Mann 2000; Marandola 2008; Moiniche 1993; Mondor 2010; Muehling 2009; Neudecker 1999; Norman 1997; O'Connor 2006; Ozcan 2004; Ozdilmac 2003; Ozturk 2010; Park 2001; Peyton 2003; Rimaitis 2003; Riwar 1991; Rockemann 1997; Rutberg 1984; Scheinin 1982; Schricker 2000; Schricker 2002; Schricker 2004; Schulze 1988; Schulze 1992; Schumann 2003; Seeling 1990; Seeling 1991; Senagore 2003; Siniscalchi 2003; Steinberg 2002; Stevens 1998; St‐Onge 1997; Taqi 2007; Thorén 1989; Tsui 1997; Tuman 1991; Turunen 2009; Tyagi 2011; Wallin 1986; Wang 2010; Watters 1993; Wattwil 1989; Yeager 1987; Zeng 2003) or after the surgical incision was made (Aygun 2004; Beilin 2003; Benzon 1994; Bois 1997; Calderon 2004; Cullen 1985; Fayed 2014; Jayr 1998; Hu 2006; Kumar 2004; Martella 2012; Miller 1976; Motamed 1998; Ozcan 2004; Paulsen 2001; Pflug 1974; Salomaki 1995; Scheinin 1987; Seeling 1990a; Welch 1998; Zhu 2013; Zutshi 2005), or before for one group and after for another (Rockemann 1996; Subramaniam 2000). Timing was unclear for two trials (Cindea 2011; Voylenko 2013).
The control group received opioids by the epidural (Barzoi 2000; Benzon 1994; Bisgaard 1990; Cronin 2001; Cullen 1985; Gambling 2009; Hubler 2001; Kumar 2004; Liu 1995; Rutberg 1984; Salomaki 1995; Scheinin 1982; Scheinin 1987; St‐Onge 1997; Subramaniam 2000; Thorén 1989), intrathecal (De Pietri 2006), intramuscular (Addison 1974; Ahn 1988; Broekema 1998; Hjortsø 1985a; Jorgensen 2001; Liuboshevskii 2012; Miller 1976; Moiniche 1993; Ozcan 2004; Ozturk 2010; Rimaitis 2003; Seeling 1990; Wallin 1986; Wattwil 1989; Welch 1998), intravenous (Aceto 2002; Aygun 2004; Barratt 2002; Beilin 2003; Beilin 2008; Bois 1997; Boylan 1998; Brodner 2001; Buggy 2002; Cai 2007; Carli 2001; Carli 2002; Chalmouki 2010; Dauri 2003; Davies 1993; Donatelli 2006; Doruk 2003; Elkaradawy 2011; El‐Refai 2003; Fant 2013; Fayed 2014; Ferguson 2009; George 1994; Gherghina 2010; Giannoni 1999; Hadimioglu 2012; Handley 1997; Hong 2008; Jayr 1998; Kentner 1996; Kudoh 2001; Lattermann 2007; Licker 1994; Limberi 2003; Liu 1995; Lombardo 2009; Luchetti 1996; Lugli 2008; Lugli 2010; Mallinder 2000; Mann 2000; Marandola 2008; Martella 2012; Mondor 2010; Motamed 1998; Muehling 2009; Neudecker 1999; Norman 1997; Ozdilmac 2003; Paulsen 2001; Peyton 2003; Pflug 1974; Riwar 1991; Rockemann 1996; Rockemann 1997; Schricker 2000; Schricker 2002; Schricker 2004; Schumann 2003; Seeling 1990a; Senagore 2003; Siniscalchi 2003; Steinberg 2002; Stevens 1998; Taqi 2007; Tsui 1997; Tyagi 2011; Zeng 2003; Zhu 2013; Zutshi 2005), epidural or intravenous (Heurich 2007; Seeling 1991), intravenous or intramuscular (Cuschieri 1985; Hu 2006; Park 2001; Turunen 2009; Watters 1993; Yeager 1987) or subcutaneous route (Carli 1997; Jayr 1988; Jayr 1993). For Calderon 2004; Hjortsø 1985b; Malenkovic 2003; Schulze 1988; Schulze 1992; and Wang 2010, the exact route of administration of the opioid in the control group was not specified. The opioid administered in the control group was buprenorphine (Kudoh 2001; Malenkovic 2003; Seeling 1990a), fentanyl (Benzon 1994; Cai 2007; Cronin 2001; Erol 2008; Fayed 2014; Hong 2008; Luchetti 1996; Salomaki 1995), fentanyl or piritramide (Heurich 2007), ketobemidone (Wattwil 1989), meperidine (Addison 1974; Liuboshevskii 2012; Miller 1976; Ozcan 2004; Rimaitis 2003; St‐Onge 1997), meperidine or tramadol (Wang 2010), morphine (Barratt 2002; Barzoi 2000; Beilin 2003; Beilin 2008; Bisgaard 1990; Bois 1997; Boylan 1998; Broekema 1998; Buggy 2002; Carli 2001; Carli 2002; Chalmouki 2010; Cindea 2011; Cullen 1985; Cuschieri 1985; Davies 1993; De Pietri 2006; Donatelli 2006; Elkaradawy 2011; El‐Refai 2003; Fant 2013; Ferguson 2009; George 1994; Gherghina 2010; Giannoni 1999; Hadimioglu 2012; Handley 1997; Hjortsø 1985a; Hjortsø 1985b; Jayr 1988; Jayr 1993; Jayr 1998; Jorgensen 2001; Katz 2003; Kumar 2004; Lattermann 2007; Levy 2011; Licker 1994; Limberi 2003; Liu 1995; Lombardo 2009; Lugli 2008; Lugli 2010; Mallinder 2000; Mann 2000; Marandola 2008; Martella 2012; Moiniche 1993; Mondor 2010; Motamed 1998; Neudecker 1999; Norman 1997; Ozdilmac 2003; Ozturk 2010; Park 2001; Pflug 1974; Rockemann 1996; Rockemann 1997; Rutberg 1984; Scheinin 1982; Scheinin 1987; Schricker 2000; Schricker 2002; Schricker 2004; Schulze 1992; Schumann 2003; Seeling 1991; Senagore 2003; Siniscalchi 2003; Steinberg 2002; Stevens 1998; Subramaniam 2000; Taqi 2007; Thorén 1989; Tsui 1997; Zeng 2003; Zhu 2013), morphine or meperidine (Hu 2006; Paulsen 2001; Watters 1993; Welch 1998), morphine or tramadol (Doruk 2003), extended‐release morphine (Gambling 2009), nicomorphine (Schulze 1988), oxycodone (Turunen 2009), papaveratum (Carli 1997), pentazocine (Ahn 1988; Riwar 1991; Wallin 1986), piritramide (Brodner 2001; Kentner 1996; Muehling 2009; Seeling 1990), sufentanil (Hubler 2001) or tramadol (Aceto 2002; Aygun 2004; Dauri 2003; Tyagi 2011). The type of opioid used in the control group was not specified for Peyton 2003; Tuman 1991; Yeager 1987 and Zutshi 2005. The comparator was unclear for Alpaslan 2010; Bellolio 2012; O'Connor 2006 and Voylenko 2013.
Excluded studies
We excluded 179 studies because they were not randomized controlled trials (n = 23), were cross‐over trials (n = 1), had no outcomes of interest measured for the actual review (n = 41), studied a different intervention (n = 95), studied a different population (n = 18) or retracted the report (n = 1). The characteristics of excluded studies and reasons for exclusion are listed under Characteristics of excluded studies and in Figure 1. Some of the studies included in the previous version of this systematic review (Jorgensen 2001) did not include a group without local anaesthetics, as all groups received a neuraxial block with a local anaesthetic during surgery. For this reason (see Characteristics of excluded studies for individual studies), some studies included in the previous version of this review do not appear among our included studies. Likewise, as the result of a different method of data extraction and redefinition of outcomes, some studies excluded in the previous version of this systematic review (Jorgensen 2001) may be included in the current version.
Ongoing studies
We did not specifically look for ongoing studies, but we found one ongoing trial when we reran the search in February 2016. We will search trial registers for the next update.
Studies awaiting classification
We found 16 trials of potential interest when we reran the search in February 2016, five of which contained our selected outcomes. We will evaluate those studies further at the next update.
Risk of bias in included studies
Among the included trials, we identified the following as the most common flaws: insufficient description of the method of randomization, absence of details of allocation concealment and absence of blinding or of information on blinding (Figure 2; Figure 3).
2.
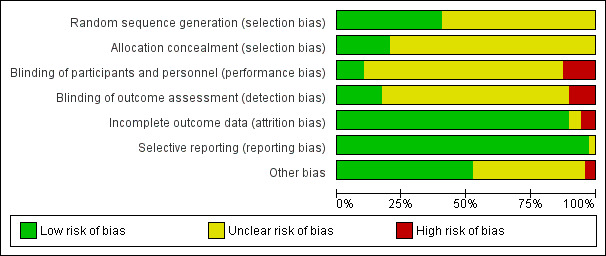
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged allocation concealment as appropriate for less than 25% of included trials and marked this as unclear for all other trials (Figure 2).
Blinding
We rated blinding of participants and personnel taking care of the participants or assessing participants' outcomes as adequate for less than 25% of included trials (Figure 2). Despite the difficulty of the exercise, the following trials succeeded in blinding outcome assessors: Barzoi 2000; Benzon 1994; Broekema 1998; Cronin 2001; Cullen 1985; De Pietri 2006; Elkaradawy 2011; El‐Refai 2003; Gambling 2009; Hubler 2001; Jayr 1988; Jayr 1993; Katz 2003; Kumar 2004; Liu 1995; Luchetti 1996; Malenkovic 2003; Mondor 2010; O'Connor 2006; Salomaki 1995; St‐Onge 1997; Subramaniam 2000.
Incomplete outcome data
We determined that complete data were provided for more than 75% of included trials (Figure 2).
Selective reporting
We judged selective reporting as problematic for less than 25% of included trials (Figure 2).
Other potential sources of bias
We judged more than 50% of included trials as having low risk for other potential bias (Figure 2).
Effects of interventions
See: Table 1
Primary outcome
Return of gastrointestinal transit
Postoperative paralytic ileus as measured by first passage of flatus
In 22 trials that included 1138 participants (Ahn 1988; Carli 2001; Gherghina 2010; Hjortsø 1985a; Jayr 1988; Jorgensen 2001; Kudoh 2001; Liu 1995; Lombardo 2009; Martella 2012; Paulsen 2001; Riwar 1991; Steinberg 2002; Stevens 1998; Taqi 2007; Thorén 1989; Tyagi 2011; Wallin 1986; Wang 2010; Wattwil 1989; Welch 1998; Zhu 2013), an epidural containing a local anaesthetic reduced the time required for return of gastrointestinal transit after abdominal surgery, as measured by time required before first flatus was observed: standardized mean difference (SMD) ‐1.28, 95% confidence interval (CI) ‐1.71 to ‐0.86; I2 = 90%.(random‐effects model) (Analysis 1.1). Egger's regression intercept showed that part of the heterogeneity might be due to a small‐study effect (P value < 0.0001; two‐tailed). Duval and Tweedie's trim and fill analysis calculated that two trials might be missing to left of mean for an adjusted SMD of ‐1.47 (95% CI ‐1.94 to ‐1.00) (random‐effects model). Keeping only trials in which local anaesthetic was continued after completion of surgery (Ahn 1988; Carli 2001; Gherghina 2010; Hjortsø 1985a; Jorgensen 2001; Kudoh 2001; Liu 1995; Lombardo 2009; Paulsen 2001; Riwar 1991; Steinberg 2002; Stevens 1998; Taqi 2007; Thorén 1989; Tyagi 2011; Wallin 1986; Wang 2010; Wattwil 1989; Zhu 2013) would not affect statistical heterogeneity (I2 = 89%). When this was done, the effect size was similar whether an opioid was (SMD ‐1.14, 95% CI ‐1.73 to ‐0.56; 11 trials including 575 participants) or was not (SMD ‐1.19, 95% CI ‐1.72 to ‐0.66; seven trials including 273 participants) added to the epidural infusion (mixed‐effects analysis): P value for heterogeneity between the two subgroups = 0.92 (Q = 0.011). For the same trials (local anaesthetic continued after surgery), when trials were subgrouped by type of surgery performed, a large effect (SMD ≥ 0.8) was seen for gastrointestinal (SMD ‐1.26, 95% CI ‐1.72 to ‐0.80), abdominal aortic repair (SMD ‐12.86, 95% CI ‐15.98 to ‐9.73), gynaecological (SMD ‐1.24, 95% CI ‐1.86 to ‐0.62) and urological (SMD ‐0.83, 95% CI ‐1.47 to ‐0.18) surgeries only (mixed‐effects analysis). Although a high (gastrointestinal surgery) or moderate (gynaecological surgery) amount of heterogeneity is seen, this heterogeneity comes from the amplitude in effect, because no trial favoured the opioid‐based regimen over the epidural regimen with a local anaesthetic. If the following potency equivalences are assumed ‐ lidocaine = 1, mepivacaine = 0.8, ropivacaine = 3, levobupivacaine = 3.9 and bupivacaine = 4 ‐ for participants undergoing gastrointestinal, abdominal aortic repair, gynaecological or urological surgery, for whom the infusion was used after surgery, higher concentrations (in mg/mL) of local anaesthetic infusion after surgery would increase the amplitude of effect (P value = 0.0008) (Figure 4). For laparoscopic surgery (one study with 50 participants; Taqi 2007), the SMD would be ‐0.81 (95% CI ‐1.39 to ‐0.23). Liu 1995 (mean and SD of the control group 71 ± 13.86 hours, respectively) revealed that 54 participants (27 per group) could eliminate a 15% difference in a simple trial (alpha = 0.05; beta = 0.2; two‐sided test).
4.
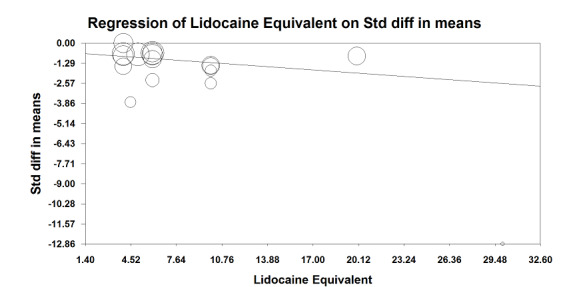
Meta‐regression of effects of the concentration of local anaesthetic used (mg/mL) after surgery on the standardized mean difference for return of gastrointestinal transit as measured by the time required to obtain first flatus. P value = 0.0008.
If a trial at low risk of bias (Liu 1995) is used as the standard (SD of the control group 13.86 hours), the difference in gastrointestinal surgery would be equivalent to 17.5 hours.
Quality of evidence
We downgraded evidence by two levels for risk of bias because 75% or more of the studies included for this outcome were rated as having unclear or high risk of bias for allocation concealment and blinding of outcome assessors. We did not downgrade evidence on the basis of inconsistency because a reasonable explanation was found for heterogeneity. We did not downgrade for indirectness because we included direct comparisons for the population of interest, and we did not consider time to first flatus as a surrogate marker for clinical evidence of gastrointestinal transit. We did not downgrade level of evidence for imprecision because the optimal information size was achieved. We did not downgrade evidence for publication bias because applying a correction would not modify the conclusion. We upgraded evidence by one level for a large effect size (SMD > 0.8). We found no evidence of confounding factors to justify upgrading. We upgraded the level of evidence by one for a dose response (increasing the concentration of the local anaesthetic increased the effect size). We rated the level of evidence as high.
Secondary outcomes
Postoperative paralytic ileus as measured by first passage of stool
This outcome was available for 28 trials that included 1559 participants (Ahn 1988; Barzoi 2000; Brodner 2001; Carli 2001; Carli 2002; Chalmouki 2010; Cindea 2011; Giannoni 1999; Hjortsø 1985a; Jorgensen 2001; Kudoh 2001; Levy 2011; Mann 2000; Neudecker 1999; Paulsen 2001; Riwar 1991; Rockemann 1997; Scheinin 1987; Seeling 1990; Steinberg 2002; Stevens 1998; Taqi 2007; Thorén 1989; Turunen 2009; Tyagi 2011; Wallin 1986; Wattwil 1989; Zutshi 2005) in which the duration of local anaesthetic infusion after surgery was known (Analysis 1.2). An epidural with a local anaesthetic infusion after surgery reduces the time required before observation of first faeces (stool): SMD ‐0.67, 95% CI ‐0.86 to ‐0.47; I2 = 69% (random‐effects model). This effect was not seen in one trial in which the local anaesthetic was administered only during surgery (Analysis 1.2). We excluded this trial (Brodner 2001) from the rest of the analysis. When this trial was excluded, effect size (SMD ‐0.71, 95% CI ‐0.90 to ‐0.51) and heterogeneity remained the same (I2 = 69%). Egger's regression intercept showed no significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis showed no evidence of publication bias. The addition of an opioid (SMD ‐0.66, 95% CI ‐0.89 to ‐0.44; 16 trials for infusion of a local anaesthetic with an opioid; Barzoi 2000; Carli 2001; Carli 2002; Cindea 2011; Giannoni 1999; Hjortsø 1985a; Levy 2011; Mann 2000; Paulsen 2001; Rockemann 1997; Seeling 1990; Stevens 1998; Steinberg 2002; Taqi 2007; Tyagi 2011; Zutshi 2005) did not significantly modify the amplitude of the effect size (vs SMD ‐0.80, 95% CI ‐1.21 to ‐0.40; 11 trials; Ahn 1988; Chalmouki 2010; Jorgensen 2001; Kudoh 2001; Neudecker 1999; Riwar 1991; Scheinin 1987; Thorén 1989; Turunen 2009; Wallin 1986; Wattwil 1989; P value for heterogeneity between the two subgroups = 0.56, Q = 0.337, mixed=effects analysis). The effect was seen for gastrointestinal (SMD ‐0.78, 95% CI ‐1.03 to ‐0.53; Ahn 1988; Barzoi 2000; Carli 2001; Carli 2002; Kudoh 2001; Levy 2011; Mann 2000; Neudecker 1999; Paulsen 2001; Riwar 1991; Rockemann 1997; Scheinin 1987; Steinberg 2002; Taqi 2007; Turunen 2009; Tyagi 2011; Zutshi 2005), gynaecological (SMD ‐0.62, 95% CI ‐1.23 to ‐0.01; Jorgensen 2001; Thorén 1989; Wattwil 1989) and urological surgery (SMD ‐0.87, 95% CI ‐1.36 to ‐0.38; Chalmouki 2010; Stevens 1998; mixed‐effects analysis) without a statistically significant difference between those subgroups (P value for heterogeneity between subgroups = 0.82, Q = 0.400). This outcome was not available for aortic abdominal surgery. Investigators used thoracic epidural anaesthesia for 21 of these trials and a lumbar epidural for three trials; this information was not available for two trials (Cindea 2011; Scheinin 1987). The concentration of local anaesthetic used after surgery did not influence effect size. For laparoscopic surgery (four studies with 188 participants; Levy 2011; Neudecker 1999; Taqi 2007; Turunen 2009), the SMD would be ‐0.37 (95% CI ‐0.75 to ‐0.00; I2 = 36%; random‐effects model). Kudoh 2001 revealed (mean and SD of control group 114.5 and 28.2 hours, respectively), that 86 participants (43 per group) could eliminate a 15% difference (alpha = 0.05; beta = 0.2; two‐sided test).
If a trial at low risk of bias (Kudoh 2001) is taken as the standard (SD of control group 28.2 hours), the difference in gastrointestinal surgery would be equivalent to 22 hours.
Quality of evidence
We downgraded the quality level by one for risk of bias because 50% or more of included studies were rated as having unclear or high risk for allocation concealment and/or blinding of outcome assessors. We downgraded the level of evidence for inconsistency on the basis of a moderate amount of heterogeneity. We did not downgrade for indirectness, as we included direct comparisons on the population of interest and did not consider time to first faeces as a surrogate marker for clinical evidence of gastrointestinal transit. We did not downgrade for imprecision because the optimal information size was achieved. We found no evidence of publication bias nor of large effect size, confounding factors to justify upgrading or dose‐response effect. We rated the level of evidence as low.
Pain scores at rest and on movement at six to eight hours, 24 hours, 48 hours and 72 hours
Pain scores at rest and on movement at six to eight hours
Findings of 20 trials that included 947 participants (Aygun 2004; Barratt 2002; Beilin 2003; Bois 1997; Boylan 1998; Cai 2007; Calderon 2004; Dauri 2003; De Pietri 2006; Fant 2013; Giannoni 1999; Heurich 2007; Hong 2008; Hubler 2001; Kudoh 2001; Licker 1994; Mann 2000; Motamed 1998; Rutberg 1984; Wiedemann 1991) showed that an epidural containing a local anaesthetic infused after abdominal surgery decreases pain scores at rest at six to eight hours after surgery: SMD ‐0.84, 95% CI ‐1.08 to ‐0.61 (random‐effects model); I2 = 86%. Egger's regression intercept showed no evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis provided no evidence of publication bias. Trials were subgrouped by type of surgery (Analysis 1.3). The effect was seen for gastrointestinal surgery (SMD ‐0.74, 95% CI ‐1.06 to ‐0.42), urological surgery (SMD ‐1.16, 95% CI ‐1.66 to ‐0.67) and aortic abdominal repair (SMD ‐0.63, 95% CI ‐1.00 to ‐0.26) with no statistically significant differences between those three subgroups (Q = 3.604; P value = 0.17) (mixed‐effects analysis). For a trial at low risk of bias with an average SD of 2.75 (Hong 2008), this would be equivalent to a decrease in visual/verbal analogue (VAS) score of 2.3 on a scale from 0 to 10.
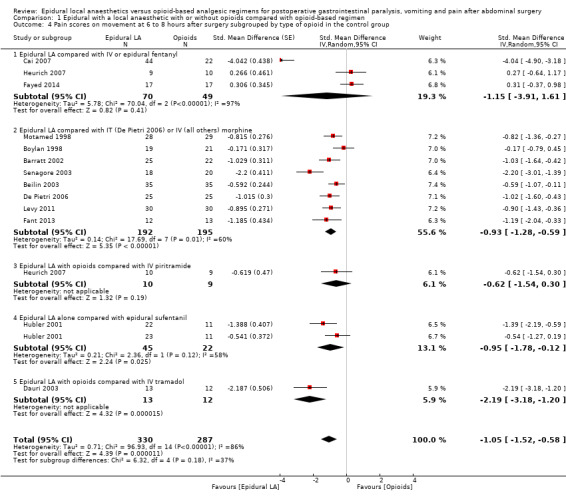
Thirteen trials with 617 participants (Barratt 2002; Beilin 2003; Boylan 1998; Cai 2007; Dauri 2003; De Pietri 2006; Fant 2013; Fayed 2014; Heurich 2007; Hubler 2001; Levy 2011; Motamed 1998; Senagore 2003) reported that an epidural containing a local anaesthetic infused after abdominal surgery decreases pain scores on movement (or coughing) at six to eight hours after surgery: SMD ‐1.05, 95% CI ‐1.52 to ‐0.58; I2 = 86%. Egger's regression intercept showed the possibility of a small‐study effect (P value = 0.047; two‐sided test) as part of the heterogeneity. Duval and Tweedie's trim and fill analysis calculated that two trials might be missing to left of mean for an adjusted point of estimate: SMD ‐1.32, 95% CI ‐1.84 to ‐0.79 (random‐effects model). Trials were subgrouped by type of opioid in the control group (Analysis 1.4). An effect was seen for trials in which an epidural with a local anaesthetic was compared with morphine (SMD ‐0.93, 95% CI ‐1.28 to ‐0.59), sufentanil (SMD ‐0.95, 95% CI ‐1.78 to ‐0.12) or tramadol (SMD ‐2.19, 95% CI ‐3.18 to ‐1.20), without a statistically significant difference between morphine and sufentanil (Q = 5.711, P value = 0.06). For a trial at low risk of bias with an average SD of 2.24 (Senagore 2003), the decrease in VAS score would be equivalent to 2.4.
Pain scores at rest and on movement at 24 hours
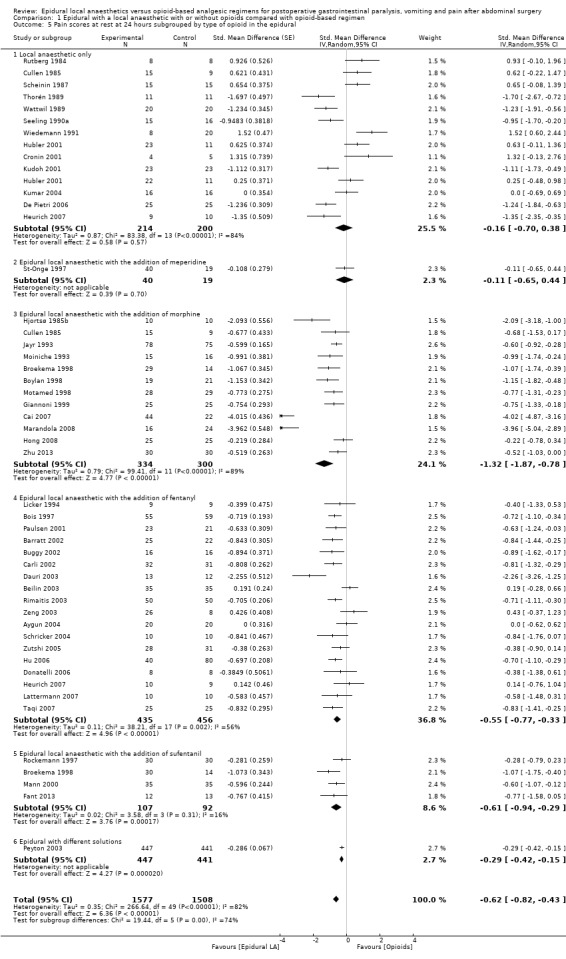
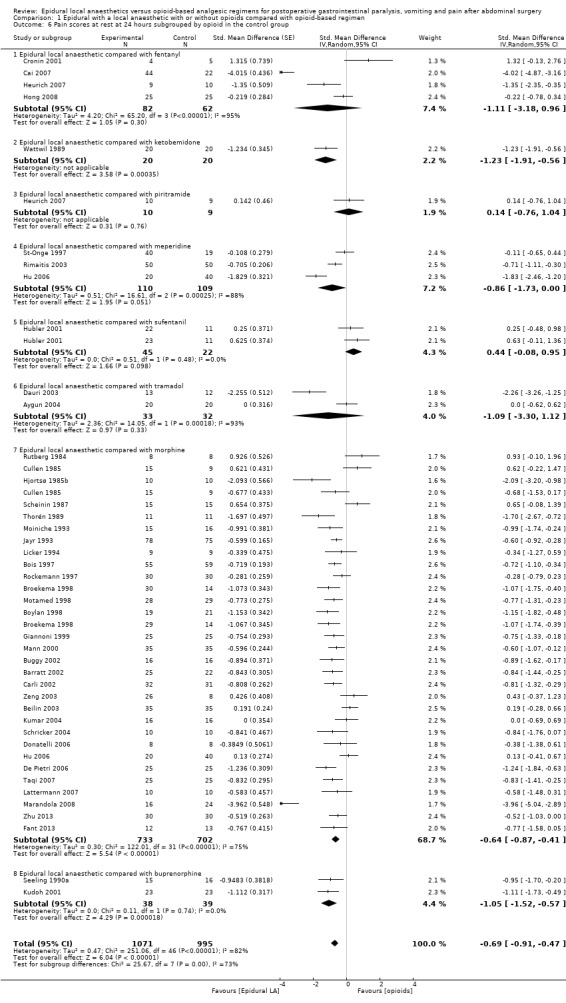
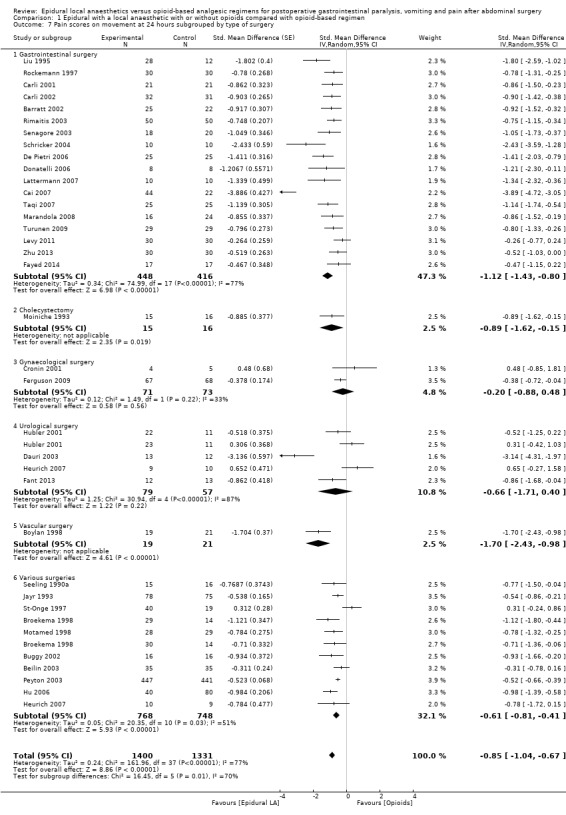
If the local anaesthetic is continued after surgery, 46 trials that included 3085 participants (Aygun 2004; Barratt 2002; Beilin 2003; Bois 1997; Boylan 1998; Broekema 1998; Buggy 2002; Cai 2007; Carli 2002; Cronin 2001; Cullen 1985; Dauri 2003; De Pietri 2006; Donatelli 2006; Fant 2013; Giannoni 1999; Heurich 2007; Hjortsø 1985b; Hong 2008; Hu 2006; Hubler 2001; Jayr 1993; Kudoh 2001; Kumar 2004; Lattermann 2007; Licker 1994; Mann 2000; Marandola 2008; Moiniche 1993; Motamed 1998; Paulsen 2001; Peyton 2003; Rimaitis 2003; Rockemann 1997; Rutberg 1984; Scheinin 1987; Schricker 2004; Seeling 1990a; St‐Onge 1997; Taqi 2007; Thorén 1989; Wattwil 1989; Wiedemann 1991; Zeng 2003; Zhu 2013; Zutshi 2005) indicated that an epidural with a local anaesthetic decreases pain scores at rest at 24 hours: SMD ‐0.62, 95% CI ‐0.82 to ‐0.43; I2 = 82% (random‐effects model). Egger's regression intercept showed no statistically significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis calculated that 13 trials might be missing to left of mean for an adjusted point of estimate: SMD ‐1.01, 95% CI ‐1.22 to ‐0.78 (random‐effects model). Trials were subgrouped by type of opoid included in the epidural (Analysis 1.5). An epidural with a local anaesthetic decreased VAS scores at rest at 24 hours only when morphine (SMD ‐1.32, 95% CI ‐1.87 to ‐0.78), fentanyl (SMD ‐0.55, 95% CI ‐0.77 to ‐0.33) or sufentanil (SMD ‐0.61, 95% CI ‐0.94 to ‐0.29) was added to the epidural infusion. Morphine was more effective than fentanyl (Q = 5.222; P value = 0.02) (mixed‐effects analysis). Trials were subgrouped by type of opioid in the control group (Analysis 1.6). An effect was seen when an epidural with a local anaesthetic was compared with buprenorphine (SMD ‐1.05, 95% CI ‐1.52 to ‐0.57), ketobemidone (SMD ‐1.23, 95% CI ‐1.91 to ‐0.56) or morphine (SMD ‐0.64, 95% CI ‐0.87 to ‐0.41). Investigators reported no statistically significant differences between these three subgroups (Q = 3.86; P value = 0.145) (mixed‐effects analysis). For a trial at low risk of bias (Peyton 2003; SD of the control group 2.5), the decrease in VAS score would be equivalent to 1.6.
Findings of 35 trials including 2731 participants (Barratt 2002; Beilin 2003; Boylan 1998; Broekema 1998; Buggy 2002; Cai 2007; Carli 2001; Carli 2002; Cronin 2001; Dauri 2003; De Pietri 2006; Donatelli 2006; Fant 2013; Fayed 2014; Ferguson 2009; Heurich 2007; Hu 2006; Hubler 2001; Jayr 1993; Lattermann 2007; Levy 2011; Liu 1995; Marandola 2008; Moiniche 1993; Motamed 1998; Peyton 2003; Rimaitis 2003; Rockemann 1997; Schricker 2004; Seeling 1990a; Senagore 2003; St‐Onge 1997; Taqi 2007; Turunen 2009; Zhu 2013) showed that an epidural infusion with a local anaesthetic after abdominal surgery decreases pain scores with movement at 24 hours: SMD ‐0.89, 95% CI ‐1.08 to ‐0.70; I2 = 78% (random‐effects model). Egger's regression intercept showed the possibility of a small‐study effect (P value = 0.004; two‐sided test). Duval and Tweedie's trim and fill analysis showed that six trials might be missing to left of mean for an adjusted point of estimate: SMD ‐1.07, 95% CI ‐1.29 to ‐0.87 (random‐effects model). Trials were subgrouped by type of surgery (Analysis 1.7). An epidural with a local anaesthetic decreases VAS scores on movement at 24 hours after abdominal surgery for a cholecystectomy (SMD ‐0.89, 95% CI ‐1.62 to ‐0.15), gastrointestinal surgery (SMD ‐1.12, 95% CI ‐1.43 to ‐0.80) or abdominal aortic repair (SMD ‐1.70, 95% CI ‐2.43 to ‐0.98). The effect on these three types of surgery was not different (Q = 2.75; P value = 0.25) (mixed‐effects analysis). Trials were subgrouped by type of opioid added to the epidural infusion (Analysis 1.8). An effect was seen when fentanyl (SMD ‐0.95, 95% CI ‐1.20 to ‐0.69), morphine (SMD ‐1.19, 95% CI ‐1.69 to ‐0.69) or sufentanil (SMD ‐0.77, 95% CI ‐1.14 to ‐0.41) was added to the solution (random‐effects model). Researchers reported no statistically significant differences between these three subgroups (Q = 1.22; P value = 0.54) (mixed‐effects analysis). Trials were subgrouped by type of opioid in the control group (Analysis 1.9). An effect was seen when an epidural containing a local anaesthetic was compared with morphine (SMD ‐0.87, 95% CI ‐1.05 to ‐0.69), oxycodone (SMD ‐0.80, 95% CI ‐1.33 to ‐0.26) or tramadol (SMD ‐3.14, 95% CI ‐4.31 to ‐1.97) (random‐effects model). These three subgroups differed (Q = 14.09; P value = 0.001) (mixed‐effects analysis). For laparoscopic surgery (four studies with 206 participants; Levy 2011; Senagore 2003; Taqi 2007; Turunen 2009), the SMD would be ‐0.78 (95% CI ‐1.18 to ‐0.38; I2 = 49%; random‐effects model). Peyton 2003 (mean and SD of the control group: 5.5 and 2.8, respectively on a scale from 0 to 10) required 248 participants (124 per group) to eliminate a difference of 1 in a simple trial (alpha = 0.05; beta = 0.2; two‐sided test).
For a trial at low risk of bias (Peyton 2003; SD of the control group 2.8), the decrease would be equivalent to 2.5.
Quality of evidence for VAS scores on movement at 24 hours
We downgraded level of evidence by two levels for risk of bias because 75% or more of the included studies were rated as having unclear or high risk of bias for allocation concealment and/or blinding of outcome assessors. We did not downgrade for inconsistency because we found reasonable explanations for heterogeneity. We used direct comparisons only with studies performed on the population of interest, and we did not consider pain scores to be surrogate markers for clinical pain. We did not downgrade for imprecision because the optimal information size was achieved. We did not downgrade the level of evidence for the possibility of publication bias because applying a correction for the possibility of one would not modify the conclusion. We upgraded level of evidence by one level for a large effect size (SMD > 0.8). We found no evidence of confounding factors or dose‐response effect to justify upgrading. We rated the level of evidence as moderate.
Pain scores at rest and on movement at 48 hours
If the local anaesthetic was continued for 48 hours or longer, 30 trials with 2466 participants (Barratt 2002; Beilin 2003; Boylan 1998; Broekema 1998; Cai 2007; Carli 2002; Cronin 2001; Cullen 1985; De Pietri 2006; Donatelli 2006; Fant 2013; Giannoni 1999; Heurich 2007; Hu 2006; Hubler 2001; Jayr 1993; Kudoh 2001; Kumar 2004; Lattermann 2007; Mann 2000; Paulsen 2001; Peyton 2003; Rimaitis 2003; Rockemann 1997; Schricker 2004; St‐Onge 1997; Taqi 2007; Wiedemann 1991; Zhu 2013; Zutshi 2005) reported that an epidural containing a local anaesthetic reduces pain scores at rest at 48 hours after surgery: SMD ‐0.47, 95% CI ‐0.71 to ‐0.24; I2 = 80% (random‐effects model). Egger's regression intercept showed no significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis calculated that 10 trials might be missing to left of mean for an adjusted point of estimate to SMD ‐0.82 (95% CI ‐1.09 to ‐0.55) (random‐effects model). An epidural infusion with a local anaesthetic alone did not decrease pain scores at rest at 48 hours: SMD 0.38, 95% CI ‐0.49 to 1.25, but an epidural infusion of a local anaesthetic with an opioid did: SMD ‐0.66, 95% CI ‐0.89 to ‐0.43 (random‐effects model) (Analysis 1.10). A meta‐regression of the mean age of participants included in the trial showed that older participants benefit more from an epidural containing a local anaesthetic at 48 hours after abdominal surgery (P value = 0.0002) (Figure 5). The route of administration of the opioid in the control group also influenced the effect size. An epidural containing a local anaesthetic did not improve pain scores at rest at 48 hours when compared with an epidural without a local anaesthetic: SMD 0.24, 95% CI ‐0.22 to 0.70, but it did so when compared with IV (SMD ‐0.74, 95% CI ‐1.04 to ‐0.44; IM (SMD ‐0.40, 95% CI ‐0.70 to ‐0.10) and subcutaneous routes (SMD ‐0.65, 95% CI ‐0.98 to ‐0.33) (Q value for the difference between these four subgroups = 32.46; P value < 0.001) (mixed‐effects analysis). For a trial with low risk of bias (SD 2.1; Peyton 2003), the decrease would be equivalent to 1.0.
5.
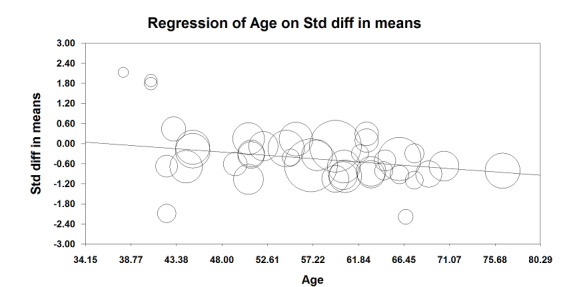
Meta‐regression of the effects of mean age of participants included in the study on VAS scores at rest at 48 hours (P value = 0.0002). Older participants benefit more from an epidural containing a local anaesthetic for an abdominal surgery.
Findings of 27 trials that included 2398 participants (Barratt 2002; Beilin 2003; Boylan 1998; Broekema 1998; Cai 2007; Carli 2001; Carli 2002; Cronin 2001; De Pietri 2006; Donatelli 2006; Fant 2013; Fayed 2014; Ferguson 2009; Heurich 2007; Hu 2006; Hubler 2001; Jayr 1993; Lattermann 2007; Liu 1995; Peyton 2003; Rimaitis 2003; Rockemann 1997; Schricker 2004; St‐Onge 1997; Taqi 2007; Turunen 2009; Zhu 2013) showed that an epidural containing a local anaesthetic decreased pain scores on movement at 48 hours: SMD ‐0.85, 95% CI ‐1.10 to ‐0.60; I2 = 85%. Egger's regression intercept showed that a small‐study effect might be present (P value = 0.002; two‐sided test). Duval and Tweedie's trim and fill analysis calculated that four trials might be missing left of mean for an adjusted point of estimate: SMD ‐1.06, 95% CI ‐1.34 to ‐0.77 (random‐effects model). An effect was not seen (SMD ‐0.56, 95% CI ‐1.71 to 0.58) when a local anaesthetic alone was used, but an epidural containing both a local anaesthetic and an opioid decreased pain scores on movement at 48 hours: SMD ‐0.88, 95% CI ‐1.13 to ‐0.63 (random‐effects model) (Analysis 1.11). A meta‐regression of the mean age of participants included in the trial showed that older participants benefit more from an epidural containing a local anaesthetic at 48 hours after abdominal surgery (P value = 0.002) (Figure 6). An epidural containing a local anaesthetic did not improve pain scores on movement at 48 hours when compared with an epidural without a local anaesthetic: SMD ‐0.17, 95% CI ‐0.88 to 0.54. An epidural containing a local anaesthetic improved pain scores when it was compared with pain treatment administered by intravenous (SMD ‐1.04, 95% CI ‐1.39 to ‐0.68), intramuscular (SMD ‐0.76, 95% CI ‐088 to ‐0.54) and subcutaneous (SMD ‐0.53, 95% CI ‐0.85 to ‐0.21) routes, or by a single intrathecal injection (SMD ‐3.03, 95% CI ‐3.85 to ‐2.22) (Q value for the difference between these four subgroups = 32.92; P value < 0.001) (mixed‐effects analysis). For a trial at low risk of bias with the typical SD of 2.6 (Peyton 2003), the decrease would be equivalent to 2.2.
6.
Meta‐regression of the effects of mean age of participants included in the study on VAS scores on movement at 48 hours (P value = 0.002). Older participants benefit more from an epidural containing a local anaesthetic for an abdominal surgery.
Pain scores at rest and on movement at 72 hours
Fifteen trials that included 1821 participants (Beilin 2003; Broekema 1998; Cai 2007; Carli 2002; Cronin 2001; Cullen 1985; Giannoni 1999; Hubler 2001; Jayr 1993; Kudoh 2001; Mann 2000; Paulsen 2001; Peyton 2003; Rimaitis 2003; Rockemann 1997) showed that an epidural with a local anaesthetic decreases VAS scores at rest at 72 hours: SMD ‐0.56, 95% CI ‐0.88 to ‐0.24; I2 = 90% (random‐effects model) (Analysis 1.12). Egger's regression intercept showed that a small‐study effect might be present (P value = 0.04). Duval and Tweedie's trim and fill analysis calculated that six trials might be missing to left of mean for an adjusted point of estimate of ‐0.99 (95% CI ‐1.45 to ‐0.53) (random‐effects model). We found no effect with a local anaesthetic alone on pain scores at rest at 72 hours (SMD 0.00, 95% CI ‐0.34 to 0.34), but an epidural containing both a local anaesthetic and an opioid decreased pain scores at rest at 72 hours (SMD ‐0.77, 95% CI ‐1.16 to ‐0.39) (random‐effects model). For a trial with low risk of bias (SD 1.7; Peyton 2003), the decrease would be equivalent to 1.0.
Findings of 15 trials with 1873 participants (Beilin 2003; Broekema 1998; Cai 2007; Carli 2001; Carli 2002; Cronin 2001; Fayed 2014; Ferguson 2009; Hubler 2001; Jayr 1993; Liu 1995; Peyton 2003; Rimaitis 2003; Rockemann 1997; Turunen 2009) showed that an epidural with a local anaesthetic decreased pain scores on movement at 72 hours: SMD ‐0.69, 95% CI ‐0.99 to ‐0.39; I2 = 86% (random‐effects model) (Analysis 1.13). Egger's regression intercept showed no significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis calculated that six trials might be missing left of mean for an adjusted point of estimate: SMD ‐1.14, 95% CI ‐1.56 to ‐0.72 (random‐effects model). We found no effect when a local anaesthetic alone was infused through the epidural catheter (SMD ‐0.03, 95% CI ‐0.38 to 0.32), but an epidural infusion containing both a local anaesthetic and an opioid decreased pain scores on movement at 72 hours (SMD ‐0.87, 95% CI ‐1.22 to ‐0.51) (random‐effects model). For a trial at low risk of bias (SD 2.5; Peyton 2003), the decrease would be equivalent to 1.7.
Incidence of postoperative vomiting: number of participants who experienced vomiting on day one
From a total of 22 trials with 1154 participants (Aceto 2002; Barzoi 2000; Benzon 1994; Calderon 2004; Carli 2001; Cullen 1985; De Pietri 2006; Erol 2008; Gambling 2009; George 1994; Hong 2008; Jayr 1998; Luchetti 1996; Marandola 2008; Neudecker 1999; Salomaki 1995; Siniscalchi 2003; Steinberg 2002; Taqi 2007; Thorén 1989; Tsui 1997; Wattwil 1989), we did not find a difference in the number of participants who will experience vomiting during the first 24 hours after abdominal surgery performed under general anaesthesia: risk ratio (RR) 0.84, 95% CI 0.57 to 1.23; I2 = 21% (Analysis 1.14). Egger's regression intercept showed no statistically significant small‐study effect. Duval and Tweedie's trim and fill analysis showed that five studies might be missing to right of mean for an adjusted point of estimate: RR 1.05, 95% CI 0.68 to 1.60 (random‐effects model). For laparoscopic surgery (four studies with 160 participants; Hong 2008; Luchetti 1996; Neudecker 1999; Taqi 2007), RR would be 0.50 (95% CI 0.18 to 1.38; I2 = 39% (random‐effects model). For gynaecological surgery, three studies used a high lidocaine equivalent concentration (10 mg/mL; Hong 2008; Thorén 1989; Wattwil 1989) and one study used a low lidocaine equivalent concentration (2.5 mg/mL; Tsui 1997). The incidence was reduced when a high lidocaine equivalent concentration was used (three trials with 112 participants: RR 0.13, 95% CI 0.03 to 0.52; I2 = 0%). For this subgroup, Tweedie's trim and fill analysis showed that two studies might be missing to left of mean for an adjusted point of estimate: RR 0.12, 95% CI 0.04 to 0.37. If an incidence of 32% is assumed, the NNTB for an epidural containing a lidocaine equivalent of 10 mg/mL would be 4 (95% CI 4 to 7).
With a basal rate of 17% in the trial population (Table 1), 1732 participants (866 per group) were required, to eliminate a decrease of 25% in the incidence of vomiting (alpha = 0.05, beta = 0.2; one‐sided test) (http://www.stat.ubc.ca/˜rollin/stats/ssize/b2.html).
Quality of evidence for postoperative vomiting
We downgraded level of evidence by one level for risk of bias because 50% or more of the included studies were rated as having unclear or high risk of bias for allocation concealment and/or blinding of outcome assessors. Heterogeneity was lower than 25%. We included direct comparisons of the population of interest, and we did not consider vomiting as a surrogate marker for food tolerance. We downgraded level of evidence by one level for imprecision because the number of participants included was below the optimal information size. Correcting for publication bias would not modify the conclusion. We found no evidence of a large effect size and identified no confounding factors to justify upgrading. We noted no dose‐response effect when all studies were included. We rated the quality of evidence as low.
Gastrointestinal tract anastomotic leak
From findings of 17 trials with 848 participants (Ahn 1988; Barratt 2002; Brodner 2001; Carli 2002; Giannoni 1999; Levy 2011; Liu 1995; Mallinder 2000; Mann 2000; Paulsen 2001; Rimaitis 2003; Riwar 1991; Scheinin 1987; Schulze 1992; Turunen 2009; Tyagi 2011; Zhu 2013), we did not find a difference in the incidence of anastomotic leak: RR 0.74, 95% CI 0.41 to 1.32; I2 = 0% (Analysis 1.15). Egger's regression intercept showed no significant small‐study effect. Duval and Tweedie's trim and fill analysis calculated that one study might be missing to right of mean for an adjusted point of estimate: RR 0.79, 95% CI 0.40 to 1.55. For laparoscopic surgery (two studies with 118 participants; Levy 2011; Turunen 2009), RR would be 2.47 (95% CI 0.34 to 18.12; I2 = 0%).
With a basal rate of 6% in the study population (Table 1), 5466 participants (2733 per group) were required, to eliminate a decrease of 25% in the incidence of anastomotic leak (alpha = 0.05, beta = 0.2; one‐sided test) in a simple trial (http://www.stat.ubc.ca/˜rollin/stats/ssize/b2.html).
Quality of evidence
We downgraded level of evidence for risk of bias by one level because 50% or more of the included studies were rated as having unclear or high risk of bias for allocation concealment and/or blinding of outcome assessors. We observed no heterogeneity. We included direct comparisons of the population of interest and this is not a surrogate marker. We downgraded level of evidence by one level for imprecision because the number of participants included was below the optimal information size. We found no evidence of publication bias nor of a large effect, confounding factors to justify upgrading or dose‐response effect. We rated the level of evidence as low.
Length of hospital stay
Investigators in 34 trials with 2774 participants (Aceto 2002; Bois 1997; Carli 2001; Carli 2002; Dauri 2003; Davies 1993; Giannoni 1999; Hadimioglu 2012; Jayr 1993; Levy 2011; Liu 1995; Lombardo 2009; Mallinder 2000; Martella 2012; Miller 1976; Mondor 2010; Muehling 2009; Neudecker 1999; Norman 1997; Ozturk 2010; Paulsen 2001; Peyton 2003; Pflug 1974; Rimaitis 2003; Rockemann 1997; Seeling 1990; Senagore 2003; Stevens 1998; Thorén 1989; Turunen 2009; Tyagi 2011; Wattwil 1989; Zhu 2013; Zutshi 2005) reported that an epidural with a local anaesthetic does not reduce length of hospital stay: SMD ‐0.13, 95% CI ‐0.29 to 0.02; I2 = 69% (random‐effects model) (Analysis 1.16). Egger's regression intercept showed no statistically significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis calculated that nine trials might be missing to right of mean for an adjusted point of estimate: SMD 0.05, 95% CI ‐0.11 to 0.21. Epidural analgesia reduced length of hospital stay for open surgery (SMD ‐0.20, 95% CI ‐0.35 to ‐0.04; I2 = 68%; 30 studies with 2598 participants (Analysis 1.17) but not for laparoscopic surgery (four studies with 176 participants; Levy 2011; Neudecker 1999; Senagore 2003; Turunen 2009): SMD 0.38, 95% CI ‐0.06 to 0.82; I2 = 49%; random‐effects model). For open surgery, a small‐study effect might occur (P value = 0.03; two‐sided test for Egger's regression intercept). and Duval and Tweedie's trim and fill analysis showed that nine studies might be missing to right of mean for an adjusted point of estimate: SMD 0.00, 95% CI ‐0.16 to 0.16; random‐effects model. Carli 2002 (SD in the control group 5 days) indicated that the reduction for an open surgery would be equivalent to one day. In the same study, 786 participants (393 per group) would be required for a simple trial to eliminate a difference of one day (from eight to seven days) (alpha = 0.05; beta = 0.2; two‐sided test).
1.16. Analysis.
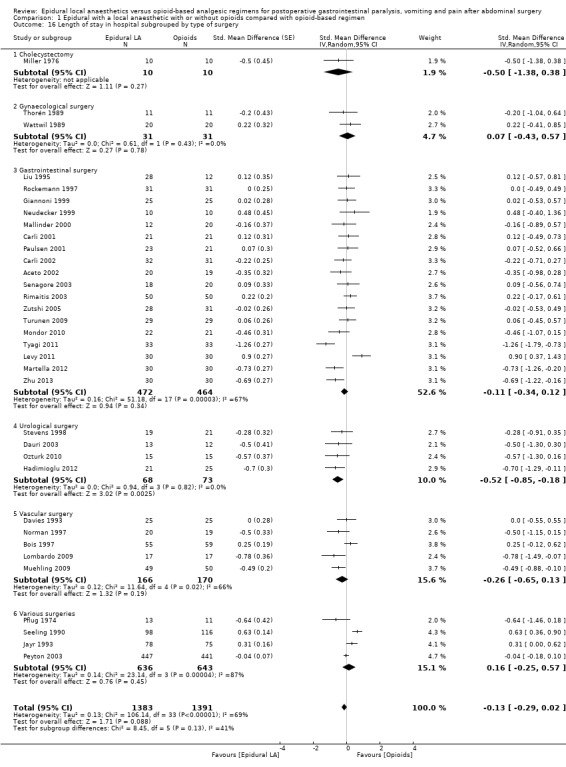
Comparison 1 Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen, Outcome 16 Length of stay in hospital subgrouped by type of surgery.
Quality of evidence for length of hospital stay for open surgery
We downgraded level of evidence by two levels for risk of bias because 75% or more of the studies included for this outcome were rated as having unclear or high risk of bias for allocation concealment and/or blinding of outcome assessors. We downgraded level of evidence for inconsistency on the basis of a moderate amount of heterogeneity. We included direct comparisons of the population of interest and did not consider length of hospital stay as a surrogate marker. The optimal information size was achieved. We downgraded for publication bias because correcting for the possibility of one would modify the conclusion (absence of effect). We found no evidence of a large effect size nor of a dose‐response effect. We upgraded level of evidence by one level for possible confounding factors, as hospital discharge may not actually reflect readiness for discharge. Participants must be ready to be discharged, but not all participants ready for discharge are discharged at that specific time. Delays may happen for various reasons (e.g. number of days in hospital fixed for each type of surgery at certain centres, no one available to sign for participant discharge, lack of help/assistance with assisted care at home). We found no evidence of a dose‐response effect. We rated the quality of evidence as very low.
Hospital costs
Data for cost were available for two small trials only (Paulsen 2001; Welch 1998) (Analysis 1.18). One trial provided costs related to pain therapy only (Rockemann 1997). These three trials studied participants undergoing open abdominal surgery.
Discussion
An epidural containing a local anaesthetic will decrease the time required for return of gastrointestinal transit, as measured by the time required to observe first flatus after abdominal surgery (Analysis 1.1) (high quality of evidence; equivalent to 17.5 hours; Table 1). The effect is seen for almost every type of abdominal surgery and is proportionate to the concentration of local anaesthetic administered after surgery (Figure 4). The optimal concentration required to obtain the maximal effect without impeding ambulation by producing motor blockade of the lower limbs may need to be determined in future trials/reviews. Adding an opioid to the mixture does not reduce the benefit. The exact duration of administration required may also need to be determined in future trials/reviews. The effect of an epidural with a local anaesthetic on time between surgery and first faeces was not as clear (quality of evidence low; equivalent to 22 hours; Table 1). Administration of the local anaesthetic only during the intraoperative period might not be sufficient to produce an effect. A reduced incidence of vomiting was seen only at relatively high local anaesthetic concentrations and for gynaecological surgeries. Return of gastrointestinal transit is an important goal to achieve after abdominal surgery, as it is generally considered to be required before hospital discharge, and because postoperative ileus will increase hospital costs (Gan 2015). Return of gastrointestinal transit as measured by time to first flatus or bowel movement is often chosen as an outcome measure in clinical trials evaluating recovery after surgery (Neville 2014). However, some study authors consider that passage of flatus or stool may reflect rectal emptying rather than effective gastrointestinal motility. A combination of food tolerance plus defecation has recently been reported as having a good positive predictive value to identify recovery of gastrointestinal transit compared with scintigraphic assessment (van Bree 2014). In the present review, positive effects on gastrointestinal transit translated to an interesting shortening of hospital stay for participants undergoing open surgery (very low quality of evidence). Data on cost were too limited to allow us to make any comment on them.
In 2000, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) suggested that pain should be considered as the fifth vital sign, and that under‐treatment of pain should constitute abrogation of a fundamental human right (White 2007). After this statement was issued, an increase in the use of opioids for treatment of patients with acute postoperative pain was observed, as was an increase in opioid side effects (White 2007). Regional blockade interrupts pain transmission to the brain and may be used during the surgery itself as a replacement for general anaesthesia (regional anaesthesia), or for treatment of postoperative pain (regional analgesia). In adults, regional analgesic techniques decrease postoperative opioid consumption (Guay 2006), making them a potentially interesting alternative or adjunct to opioid‐based regimens for treatment of postoperative pain, provided of course that the efficacy would be at least equivalent. An epidural with a local anaesthetic has a clinically relevant effect on pain scores (moderate quality of evidence for pain on movement at 24 hours after surgery; Table 1). Not every patient will benefit to the same extent from an epidural with a local anaesthetic. Many factors will increase or decrease the beneficial effects. For patients themselves, the older the patient, the greater the difference in pain scores between an epidural with a local anaesthetic and an opioid‐based regimen (Figure 5; Figure 6). For type of surgery, patients undergoing abdominal aortic repair, gastrointestinal surgery or urological surgery seem to be the ones for which an epidural containing a local anaesthetic would be most beneficial. The type of solution used also matters. Administering only a local anaesthetic through the epidural catheter will not produce a decrease in pain scores, at least from 24 hours and after, and this applies to pain scores both at rest and on movement. Fortunately, as mentioned above, adding an opioid to the mixture does not seem to decrease the benefit of the local anaesthetic for acceleration of gastrointestinal transit return. For the opioid added to the local anaesthetic, morphine, fentanyl and sufentanil seem to be equally effective for pain on movement. When an opioid is administered in the epidural space, blood concentrations achieved will vary according to the type of opioid used. A systemic effect of the opioid is to be expected. The type of opioid used in the control group as well as the route of administration may increase or decrease the difference in pain scores between an epidural containing a local anaesthetic and an opioid‐based regimen. Pain reduction was noted regardless of the type of approach used (open vs laparoscopic). Thus the decision to use epidural analgesia after abdominal surgery versus other modalities has to be considered on a case‐by‐case basis, taking into account patient age, associated co‐morbidities, relative contraindications and type of surgery performed.
When evaluating the benefit of an intervention, one must also consider potential side effects. Because they may increase the risk of hypotension (Davies 2006), some clinicians have hypothesized that secondary extra fluid administration could increase sutures, oedema and anastomotic dehiscence. We did not find a difference in the incidence of anastomotic leak (low quality of evidence). Severe complications such as paraplegia or death related to epidurals when used for perioperative pain treatment in adults are fortunately very rare (1.0 to 6.1 per 100,000 procedures; Cook 2009) and are best evaluated by large prospective trials, as they are rarely reported in randomized controlled trials using neuraxial blocks (Guay 2014).
Summary of main results
An epidural with a local anaesthetic will accelerate the return of gastrointestinal transit by approximately 17 hours. The effect is proportional to the local anaesthetic concentration. To be effective, the solution may need to be administered after surgery ‐ not solely intraoperatively. The effect of an epidural containing a local anaesthetic on gastrointestinal transit will translate to shorter hospital stay for open surgery only. An epidural with a local anaesthetic and an opioid improves pain scores (open or laparoscopic surgeries). Adding an opioid to the solution of local anaesthetic will improve pain scores without affecting gastrointestinal transit. When all studies were included, we did not find an effect on the incidence of vomiting or evidence to support any effect of an epidural with a local anaesthetic on the incidence of anastomotic leak of the gastrointestinal tractus.
Overall completeness and applicability of evidence
Given the high number of participants and studies included in this review, we are confident that our conclusions are valid.
Quality of the evidence
We rated the quality of evidence as high for reduced time to first flatus, and as moderate for pain scores on movement at 24 hours. We rated the quality of evidence as low for absence of effect on vomiting, for reduced time to first faeces and for absence of effect on the incidence of anastomotic leaks. We rated the quality as very low for reduced length of hospital stay for open surgery.
Potential biases in the review process
Our search was quite extensive, and we applied no language restrictions. We are therefore confident that our review reflects actual available information on this topic. We reran the search in February 2016 and found six studies with our selected outcomes. We added five studies to a list of Characteristics of studies awaiting classification (Chen 2015; Khoronenko 2014; Satsuta 2015; Sidiropoulou 2014; Xu 2014) and will incorporate them into formal review findings during the next review update. We added one study under Characteristics of ongoing studies (Li 2015a).
Agreements and disagreements with other studies or reviews
We agree with the previous version of this review (Jorgensen 2001) that an epidural with a local anaesthetic decreases time to return of gastrointestinal tract transit, as measured by time to first flatus after surgery. We found that heterogeneity observed in the previous version can be explained by the variety of local anaesthetic concentrations used in the included studies. We agree that an epidural that would contain only local anaesthetic offers no clear advantage over other modalities of treatment in terms of pain reduction. The epidural solution must contain a mixture of local anaesthetic and opioids to be more effective than other modalities of pain treatment after abdominal surgery.
Authors' conclusions
Implications for practice.
We found evidence of high quality suggesting that an epidural containing a local anaesthetic with or without the addition of an opioid will decrease the time required for return of gastrointestinal transit. We found evidence of moderate quality indicating that an epidural containing a mixture of local anaesthetic and opioids will reduce pain on movement at 24 hours after abdominal surgery (open or laparoscopic). We found evidence of very low quality showing that an epidural containing a local anaesthetic will reduce length of hospital stay for open surgery. The decision to use epidural analgesia versus other modalities after abdominal surgery must be made on a case‐by‐case basis, taking into account patient age, associated co‐morbidities, relative contraindications and type of surgery.
Implications for research.
The optimal concentration of local anaesthetic required to hasten the return of gastrointestinal transit without impeding ambulation and the optimal duration of administration may need to be determined in futures studies or reviews.
What's new
| Date | Event | Description |
|---|---|---|
| 3 January 2017 | Amended | Co‐publication of review in Anesthesia and Analgesia (see Guay 2016) |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 9 February 2016 | New citation required and conclusions have changed | Three new review authors have updated this review (Joanne Guay, Mina Nishimori and Sandra Kopp) We re‐evaluated all studies from the previous version of this review (included or excluded) for inclusion in this updated version. We evaluated 216 new studies. In total, our updated review contains 128 included studies We changed conclusions as a result of inclusion of new studies |
| 9 February 2016 | New search has been performed | We converted the review to RevMan 5 We rewrote the Introduction We redefined the Objectives We ran the search in December 2014 and updated the search in February 2016; we added 16 potential new studies of interest to the list of Studies awaiting classification We assessed risk of bias and extracted data again We included full risk of bias tables and Table 1 We repeated the analysis We rewrote the Discussion section |
| 1 September 2000 | New citation required and conclusions have changed | We have made substantive amendments to the review |
Notes
August 2015
To include maximal data with no assumption, we entered data expressed as median and range via another software, using the exact P value and the number of participants included in each group (a function not provided by RevMan). We then transferred data to RevMan as standardized mean difference (SMD) and standard error (SE). For this reason, results provided in the text (exact calculations from www.Meta‐Analysis.com) may sometimes differ by a few decimal places from those noted in the Figures (automatically recalculated in RevMan from SMD and SE entered). Conclusions (effects found or not found) were never affected by these small differences.
Acknowledgements
The review authors wish to thank the University of Quebec in Abitibi Temiscamingue, the University of Sherbrooke and the University of Montreal for providing access to electronic databases and medical journals. We also wish to thank Dat Nhut Nguyen, who participated in data extraction and gradation of the level of evidence but preferred to withdraw from the review on May 28, 2015, because of lack of academic time. We also thank Jia Jiang for the translation of Chinese studies (Cai 2007; Han 2005; Hu 2006; Liu 2005; Wang 2010; Zeng 2003). We extend our thanks to Honario T Benzon (Benzon 1994), Joergen B. Dahl (Bisgaard 1990), Joel Katz (Boylan 1998), Franco Carli (Carli 1997; Lugli 2008; Lugli 2010), Enrique Calderón (Calderon 2004), Joergen B Dahl (Dahl 1992), Christian Jayr (Jayr 1998; Motamed 1998), Rainer Kentner (Kentner 1996), Leonidas Grigorakos (Limberi 2003), Luc Massicotte (Mondor 2010), Michael G. Rockemann (Rockemann 1997), Henrik Kehlet (Schulze 1988; Schulze 1992), Roman Schumann (Schumann 2003), Charles Gibson (Steinberg 2002) and S.L. Tsui (Tsui 1997), who provided additional information on their studies or took the time to reply when data were no longer available, and Nesrine El‐Refai (El‐Refai 2003), who graciously provided a copy of his article.
We also wish to thank Mark Neuman (content editor), Cathal Walsh (statistical editor), Paul S. Myles, Fahad Javaid Siddiqui, James Paul (peer reviewers), Shunjie Chua (consumer referee) for their help and editorial advice during the preparation of this updated systematic review.
Appendices
Appendix 1. CENTRAL, The Cochrane Library, search strategy
#1 MeSH descriptor: [Analgesia, Epidural] explode all trees #2 MeSH descriptor: [Anesthetics, Local] explode all trees #3 ((epidural or local) near (analg* or an?esth*)):ti,ab #4 MeSH descriptor: [Analgesics, Opioid] explode all trees #5 ((systemic or epidural) near opioid*):ti,ab #6 (#1 or #2 or #3) and (#4 or #5) #7 MeSH descriptor: [Postoperative Period] explode all trees #8 MeSH descriptor: [Pain, Postoperative] explode all trees #9 MeSH descriptor: [Postoperative Care] explode all trees #10 MeSH descriptor: [Postoperative Nausea and Vomiting] explode all trees #11 ((gastrointestinal near (transit or paralysis)) or (post?operative near (abdominal surgery or nausea or vomiting or pain))) #12 #7 or #8 or #9 or #10 or #11 #13 #6 and #12
Appendix 2. MEDLINE (OVID SP) search strategy
1. (Analgesia, Epidural/ or Anesthetics, Local/ or ((epidural or local) adj4 (analg* or an?esth*)).ti,ab.) and (Analgesics, Opioid/ or ((systemic or epidural) adj4 opioid*).ti,ab.) 2. Postoperative Period/ or Pain, Postoperative/ or Postoperative Care/ or "Postoperative Nausea and Vomiting"/ or (gastrointestinal adj3 (transit or paralysis)).ti,ab. or (post?operative adj3 (abdominal surgery or nausea or vomiting or pain)).ti,ab. 3. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 3. EMBASE search strategy
1. (epidural anesthesia/ or local anesthetic agent/ or ((epidural or local) adj4 (analg* or an?esth*)).ti,ab.) and (narcotic analgesic agent/ or ((systemic or epidural) adj4 opioid*).ti,ab.) 2. postoperative period/ or postoperative pain/ or postoperative care/ or postoperative nausea/ or postoperative vomiting/ or (gastrointestinal adj3 (transit or paralysis)).ti,ab. or (post?operative adj3 (abdominal surgery or nausea or vomiting or pain)).ti,ab. 3. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Appendix 4. PsycINFO
1. Postoperative ileus AND epidural
2. Postoperative pain AND epidural
Appendix 5. MEDLINE(R) (OVID SP) 2014 to March Week 1 2016, and CENTRAL, The Cochrane Library, 2014 to February 2016, search strategy
Limit to Humans
1. Epidural.mp.[mp=ti, ab, ot, nm, hw, kw, kf, px, rx, ui, an, sh]
2. surgery.mp.[mp=ti, ab, ot, nm, hw, kw, kf, px, rx, ui, an, sh]
3, 1 AND 2
4. Remove duplicate
Data and analyses
Comparison 1. Epidural with a local anaesthetic with or without opioids compared with opioid‐based regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to first flatus subgrouped by type of surgery | 22 | 1138 | Std. Mean Difference (Random, 95% CI) | ‐1.28 [‐1.71, ‐0.86] |
| 1.1 Gynaecology | 3 | 122 | Std. Mean Difference (Random, 95% CI) | ‐1.24 [‐1.86, ‐0.62] |
| 1.2 Cholecystectomy | 1 | 27 | Std. Mean Difference (Random, 95% CI) | ‐0.15 [‐0.91, 0.61] |
| 1.3 Gastrointestinal surgery | 14 | 690 | Std. Mean Difference (Random, 95% CI) | ‐1.34 [‐1.82, ‐0.86] |
| 1.4 Urology | 1 | 40 | Std. Mean Difference (Random, 95% CI) | ‐0.83 [‐1.48, ‐0.17] |
| 1.5 Vascular surgery | 1 | 34 | Std. Mean Difference (Random, 95% CI) | ‐12.86 [‐15.98, ‐9.73] |
| 1.6 Various | 2 | 225 | Std. Mean Difference (Random, 95% CI) | ‐0.03 [‐0.29, 0.23] |
| 2 Time to first faeces subgrouped according to duration of local anaesthetic administration | 28 | 1559 | Std. Mean Difference (Random, 95% CI) | ‐0.67 [‐0.86, ‐0.47] |
| 2.1 Epidural local anaesthetic administered during surgery only | 1 | 30 | Std. Mean Difference (Random, 95% CI) | 0.03 [‐0.68, 0.75] |
| 2.2 Epidural local anaesthetic administered postoperatively for < 48 hours | 8 | 363 | Std. Mean Difference (Random, 95% CI) | ‐0.57 [‐1.06, ‐0.07] |
| 2.3 Epidural local anaesthetic administered for ≥ 48 hours after surgery | 19 | 1166 | Std. Mean Difference (Random, 95% CI) | ‐0.73 [‐0.93, ‐0.53] |
| 3 Pain scores at rest at 6 to 8 hours after surgery subgrouped by type of surgery | 20 | 947 | Std. Mean Difference (Random, 95% CI) | ‐0.84 [‐1.08, ‐0.61] |
| 3.1 Cholecystectomy | 1 | 16 | Std. Mean Difference (Random, 95% CI) | 0.33 [‐0.65, 1.32] |
| 3.2 Gastrointestinal surgery | 8 | 387 | Std. Mean Difference (Random, 95% CI) | ‐0.74 [‐1.06, ‐0.42] |
| 3.3 Gynaecology | 2 | 68 | Std. Mean Difference (Random, 95% CI) | ‐0.76 [‐1.70, 0.18] |
| 3.4 Urology | 4 | 136 | Std. Mean Difference (Random, 95% CI) | ‐1.16 [‐1.66, ‐0.67] |
| 3.5 Vascular surgery | 2 | 154 | Std. Mean Difference (Random, 95% CI) | ‐0.63 [‐1.00, ‐0.26] |
| 3.6 Various | 4 | 186 | Std. Mean Difference (Random, 95% CI) | ‐1.24 [‐2.18, ‐0.29] |
| 4 Pain scores on movement at 6 to 8 hours after surgery subgrouped by type of opioid in the control group | 13 | 617 | Std. Mean Difference (Random, 95% CI) | ‐1.05 [‐1.52, ‐0.58] |
| 4.1 Epidural LA compared with IV or epidural fentanyl | 3 | 119 | Std. Mean Difference (Random, 95% CI) | ‐1.15 [‐3.91, 1.61] |
| 4.2 Epidural LA compared with IT (De Pietri 2006) or IV (all others) morphine | 8 | 387 | Std. Mean Difference (Random, 95% CI) | ‐0.93 [‐1.28, ‐0.59] |
| 4.3 Epidural LA with opioids compared with IV piritramide | 1 | 19 | Std. Mean Difference (Random, 95% CI) | ‐0.62 [‐1.54, 0.30] |
| 4.4 Epidural LA alone compared with epidural sufentanil | 1 | 67 | Std. Mean Difference (Random, 95% CI) | ‐0.95 [‐1.78, ‐0.12] |
| 4.5 Epidural LA with opioids compared with IV tramadol | 1 | 25 | Std. Mean Difference (Random, 95% CI) | ‐2.19 [‐3.18, ‐1.20] |
| 5 Pain scores at rest at 24 hours subgrouped by type of opioid in the epidural | 46 | 3085 | Std. Mean Difference (Random, 95% CI) | ‐0.62 [‐0.82, ‐0.43] |
| 5.1 Local anaesthetic only | 13 | 414 | Std. Mean Difference (Random, 95% CI) | ‐0.16 [‐0.70, 0.38] |
| 5.2 Epidural local anaesthetic with the addition of meperidine | 1 | 59 | Std. Mean Difference (Random, 95% CI) | ‐0.11 [‐0.65, 0.44] |
| 5.3 Epidural local anaesthetic with the addition of morphine | 12 | 634 | Std. Mean Difference (Random, 95% CI) | ‐1.32 [‐1.87, ‐0.78] |
| 5.4 Epidural local anaesthetic with the addition of fentanyl | 18 | 891 | Std. Mean Difference (Random, 95% CI) | ‐0.55 [‐0.77, ‐0.33] |
| 5.5 Epidural local anaesthetic with the addition of sufentanil | 4 | 199 | Std. Mean Difference (Random, 95% CI) | ‐0.61 [‐0.94, ‐0.29] |
| 5.6 Epidural with different solutions | 1 | 888 | Std. Mean Difference (Random, 95% CI) | ‐0.29 [‐0.42, ‐0.15] |
| 6 Pain scores at rest at 24 hours subgrouped by opioid in the control group | 42 | 2066 | Std. Mean Difference (Random, 95% CI) | ‐0.69 [‐0.91, ‐0.47] |
| 6.1 Epidural local anaesthetic compared with fentanyl | 4 | 144 | Std. Mean Difference (Random, 95% CI) | ‐1.11 [‐3.18, 0.96] |
| 6.2 Epidural local anaesthetic compared with ketobemidone | 1 | 40 | Std. Mean Difference (Random, 95% CI) | ‐1.23 [‐1.91, ‐0.56] |
| 6.3 Epidural local anaesthetic compared with piritramide | 1 | 19 | Std. Mean Difference (Random, 95% CI) | 0.14 [‐0.76, 1.04] |
| 6.4 Epidural local anaesthetic compared with meperidine | 3 | 219 | Std. Mean Difference (Random, 95% CI) | ‐0.86 [‐1.73, 0.00] |
| 6.5 Epidural local anaesthetic compared with sufentanil | 1 | 67 | Std. Mean Difference (Random, 95% CI) | 0.44 [‐0.08, 0.95] |
| 6.6 Epidural local anaesthetic compared with tramadol | 2 | 65 | Std. Mean Difference (Random, 95% CI) | ‐1.09 [‐3.30, 1.12] |
| 6.7 Epidural local anaesthetic compared with morphine | 30 | 1435 | Std. Mean Difference (Random, 95% CI) | ‐0.64 [‐0.87, ‐0.41] |
| 6.8 Epidural local anaesthetic compared with buprenorphine | 2 | 77 | Std. Mean Difference (Random, 95% CI) | ‐1.05 [‐1.52, ‐0.57] |
| 7 Pain scores on movement at 24 hours subgrouped by type of surgery | 35 | 2731 | Std. Mean Difference (Random, 95% CI) | ‐0.85 [‐1.04, ‐0.67] |
| 7.1 Gastrointestinal surgery | 18 | 864 | Std. Mean Difference (Random, 95% CI) | ‐1.12 [‐1.43, ‐0.80] |
| 7.2 Cholecystectomy | 1 | 31 | Std. Mean Difference (Random, 95% CI) | ‐0.89 [‐1.62, ‐0.15] |
| 7.3 Gynaecological surgery | 2 | 144 | Std. Mean Difference (Random, 95% CI) | ‐0.20 [‐0.88, 0.48] |
| 7.4 Urological surgery | 4 | 136 | Std. Mean Difference (Random, 95% CI) | ‐0.66 [‐1.71, 0.40] |
| 7.5 Vascular surgery | 1 | 40 | Std. Mean Difference (Random, 95% CI) | ‐1.70 [‐2.43, ‐0.98] |
| 7.6 Various surgeries | 10 | 1516 | Std. Mean Difference (Random, 95% CI) | ‐0.61 [‐0.81, ‐0.41] |
| 8 Pain scores on movement at 24 hours subgrouped by type of opioid in the epidural | 34 | 1843 | Std. Mean Difference (Random, 95% CI) | ‐0.88 [‐1.09, ‐0.66] |
| 8.1 Local anaesthetic alone | 6 | 234 | Std. Mean Difference (Random, 95% CI) | ‐0.38 [‐0.93, 0.17] |
| 8.2 Epidural meperidine | 1 | 59 | Std. Mean Difference (Random, 95% CI) | 0.31 [‐0.24, 0.86] |
| 8.3 Epidural fentanyl | 16 | 756 | Std. Mean Difference (Random, 95% CI) | ‐0.95 [‐1.20, ‐0.69] |
| 8.4 Epidural sufentanil | 3 | 129 | Std. Mean Difference (Random, 95% CI) | ‐0.77 [‐1.14, ‐0.41] |
| 8.5 Epidural morphine | 10 | 665 | Std. Mean Difference (Random, 95% CI) | ‐1.19 [‐1.69, ‐0.69] |
| 9 Pain scores on movement at 24 hours subgrouped by type of opioids in the control group | 33 | 1796 | Std. Mean Difference (Random, 95% CI) | ‐0.90 [‐1.15, ‐0.66] |
| 9.1 Compared with IV or epidural fentanyl | 4 | 128 | Std. Mean Difference (Random, 95% CI) | ‐0.83 [‐3.13, 1.47] |
| 9.2 Compared with sufentanil | 1 | 67 | Std. Mean Difference (Random, 95% CI) | ‐0.10 [‐0.91, 0.70] |
| 9.3 Compared with meperidine | 3 | 219 | Std. Mean Difference (Random, 95% CI) | ‐0.64 [‐1.57, 0.29] |
| 9.4 Compared with piritramide | 1 | 19 | Std. Mean Difference (Random, 95% CI) | ‐0.78 [‐1.72, 0.15] |
| 9.5 Compared with morphine | 23 | 1249 | Std. Mean Difference (Random, 95% CI) | ‐0.87 [‐1.05, ‐0.69] |
| 9.6 Compared with oxycodone | 1 | 58 | Std. Mean Difference (Random, 95% CI) | ‐0.80 [‐1.33, ‐0.26] |
| 9.7 Compared with tramadol | 1 | 25 | Std. Mean Difference (Random, 95% CI) | ‐3.14 [‐4.31, ‐1.97] |
| 9.8 Compared to buprenorphine | 1 | 31 | Std. Mean Difference (Random, 95% CI) | ‐0.77 [‐1.50, ‐0.04] |
| 10 Pain scores at rest at 48 hours subgrouped by type of solution used | 30 | 2466 | Std. Mean Difference (Random, 95% CI) | ‐0.47 [‐0.71, ‐0.24] |
| 10.1 Local anaesthetic alone | 7 | 256 | Std. Mean Difference (Random, 95% CI) | 0.38 [‐0.49, 1.25] |
| 10.2 Local anaesthetic with an opioid | 24 | 2210 | Std. Mean Difference (Random, 95% CI) | ‐0.66 [‐0.89, ‐0.43] |
| 11 Pain scores on movement at 48 hours subgrouped by type of solution in the epidural | 27 | 2398 | Std. Mean Difference (Random, 95% CI) | ‐0.85 [‐1.10, ‐0.60] |
| 11.1 Local anaesthetic alone | 4 | 184 | Std. Mean Difference (Random, 95% CI) | ‐0.56 [‐1.71, 0.58] |
| 11.2 Local anaesthetic with an opioid | 23 | 2214 | Std. Mean Difference (Random, 95% CI) | ‐0.88 [‐1.13, ‐0.63] |
| 12 Pain scores at rest at 72 hours subgrouped by type of solution used | 15 | 1821 | Std. Mean Difference (Random, 95% CI) | ‐0.56 [‐0.88, ‐0.24] |
| 12.1 Local anaesthetic alone | 4 | 146 | Std. Mean Difference (Random, 95% CI) | ‐0.00 [‐0.34, 0.34] |
| 12.2 Local anaesthetic plus opioids | 12 | 1675 | Std. Mean Difference (Random, 95% CI) | ‐0.77 [‐1.16, ‐0.39] |
| 13 Pain scores on movement at 72 hours subgrouped by type of solution used | 15 | 1873 | Std. Mean Difference (Random, 95% CI) | ‐0.69 [‐0.99, ‐0.39] |
| 13.1 Local anaesthetic alone | 3 | 135 | Std. Mean Difference (Random, 95% CI) | ‐0.03 [‐0.38, 0.32] |
| 13.2 Local anaesthetic with an opioid | 12 | 1738 | Std. Mean Difference (Random, 95% CI) | ‐0.87 [‐1.22, ‐0.51] |
| 14 Vomiting | 22 | 1154 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.57, 1.23] |
| 14.1 Gynaecological | 4 | 223 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.29] |
| 14.2 Gastrointestinal | 12 | 512 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.48, 1.32] |
| 14.3 Various | 6 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.44, 2.35] |
| 15 Gastrointestinal tract anastomotic leak | 17 | 848 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.41, 1.32] |
| 16 Length of stay in hospital subgrouped by type of surgery | 34 | 2774 | Std. Mean Difference (Random, 95% CI) | ‐0.13 [‐0.29, 0.02] |
| 16.1 Cholecystectomy | 1 | 20 | Std. Mean Difference (Random, 95% CI) | ‐0.5 [‐1.38, 0.38] |
| 16.2 Gynaecological surgery | 2 | 62 | Std. Mean Difference (Random, 95% CI) | 0.07 [‐0.43, 0.57] |
| 16.3 Gastrointestinal surgery | 18 | 936 | Std. Mean Difference (Random, 95% CI) | ‐0.11 [‐0.34, 0.12] |
| 16.4 Urological surgery | 4 | 141 | Std. Mean Difference (Random, 95% CI) | ‐0.52 [‐0.85, ‐0.18] |
| 16.5 Vascular surgery | 5 | 336 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.65, 0.13] |
| 16.6 Various surgeries | 4 | 1279 | Std. Mean Difference (Random, 95% CI) | 0.16 [‐0.25, 0.57] |
| 17 Length of stay in hospital subgrouped by surgical site for open surgery only | 30 | 2598 | Std. Mean Difference (Random, 95% CI) | ‐0.20 [‐0.35, ‐0.04] |
| 17.1 Open vascular surgery | 5 | 336 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.65, 0.13] |
| 17.2 Open urological surgery | 4 | 141 | Std. Mean Difference (Random, 95% CI) | ‐0.51 [‐0.85, ‐0.18] |
| 17.3 Open cholecystectomy | 1 | 20 | Std. Mean Difference (Random, 95% CI) | ‐0.50 [‐1.39, 0.39] |
| 17.4 Open gynaecological surgery | 2 | 62 | Std. Mean Difference (Random, 95% CI) | 0.07 [‐0.43, 0.57] |
| 17.5 Open gastrointestinal surgery | 14 | 760 | Std. Mean Difference (Random, 95% CI) | ‐0.24 [‐0.47, ‐0.01] |
| 17.6 Open various surgeries | 4 | 1279 | Std. Mean Difference (Random, 95% CI) | 0.15 [‐0.26, 0.57] |
| 18 Costs | 3 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 18.1 Costs related to pain therapy only | 1 | 62 | Std. Mean Difference (Random, 95% CI) | 19.96 [19.57, 20.34] |
| 18.2 Hospital costs | 2 | 103 | Std. Mean Difference (Random, 95% CI) | 0.17 [‐0.22, 0.55] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aceto 2002.
| Methods | RCT Informed consent obtained Setting: Italy Funding: unspecified |
|
| Participants | 40 patients, aged 28 to 70 years, with ASA physical status 1 to 3, undergoing colorectal surgery for cancer | |
| Interventions |
Treatment group: thoracic epidural analgesia (TEA) (T6‐T9) with ropivacaine 0.2% and sufentanil 0.75 mcg/mL for 72 hours (n = 20) Control group: tramadol and ketorolac IV infusion plus IV morphine PCA for 48 hours (n = 20) General anaesthesia for all participants |
|
| Outcomes | Vomiting Length of hospital stay |
|
| Notes | No address available for contacting study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient excluded because of failure of the PCA device |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Addison 1974.
| Methods | RCT Informed consent not reported Setting: United Kingdom Funding: unspecified |
|
| Participants | 50 patients undergoing open cholecystectomy Excluded were heavy cigarette smokers and patients with severe pre‐existing pulmonary disease |
|
| Interventions |
Treatment group: thoracic epidural catheter (T5; inserted 4 to 5 cm caudally; 4 mL of "plain bupivacaine" injected at insertion) inserted after anaesthesia induction for postoperative 6 mL boluses of "plain bupivacaine" administered as required for 48 hours (n = 25) Control group: weight‐related intramuscular pethidine on demand (n = 25) General anaesthesia for all participants |
|
| Outcomes | Pain scores expressed as percentage of pain relief of 30%, 30% to 69% and 70% or higher | |
| Notes | Lack of details on results, therefore not extractable to satisfy our objectives. No address available to contact study authors Therefore, this study is included in the review but not in the analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "divided at random", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Ahn 1988.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Sweden Funding: unspecified |
|
| Participants | 30 patients undergoing left colonic or rectal surgery | |
| Interventions | Treatment group: lumbar epidural analgesia (LEA) (L2‐L3). Repeated bolus of bupivacaine 2.5 mg/mL intermittent 8 to 15 mL for 48 hours (n = 16) Control group: postoperative intermittent IV injections of pentazocine 30 to 60 mg (n = 14) | |
| Outcomes | Time of first flatus Time of first stool Anastomotic leakage | |
| Notes | Sensory level maintained between T5 and T10 as measured at 24 hours. Various degrees of accompanying lower limb motor blockade "Insertion of the epidural catheter was not accompanied by any complications" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants followed until all outcomes had occurred No drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Alpaslan 2010.
| Methods | RCT Informed consent not mentioned Setting: Turkey Funding: unspecified |
|
| Participants | 36 patients undergoing lower abdominal surgery | |
| Interventions |
Treatment group: lumbar epidural analgesia started before induction with 10 mL of 0.25 bupivacaine followed by an infusion at 4 mL/h for 24 hours (n = 16) Control group: n = 20 General anaesthesia for all participants |
|
| Outcomes | Time to first flatus Time to first faeces |
|
| Notes | Conference abstract Lack of details on results (gastrointestinal functions normalized at similar times in the 2 groups (P value > 0.05)), therefore not extractable to satisfy our objectives. No address available to contact study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "divided randomly", no details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | Limited information on group characteristics |
Aygun 2004.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Turkey Funding: unspecified |
|
| Participants | 80 ASA 1 and 2 patients aged 18 to 60 years undergoing elective lower abdominal surgery | |
| Interventions |
Treatment group: LEA (catheter inserted 3 to 4 cm) with ropivacaine 0.125% plus fentanyl 2 mcg/mL for 24 hours (Gr 4; n = 20) Control groups: IV tramadol for 24 hours (Gr 1; n = 20) General anaesthesia for all participants |
|
| Outcomes | VAS at 6 and 24 hours (unclear, therefore taken as at rest). A value of 0.001 has been entered as SD when the SD was reported as 0 | |
| Notes | Study also includes 2 groups that were not retained: 1 group with epidurally injected tramadol and 1 group with IV fentanyl infusion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Barratt 2002.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Australia Funding: governmental |
|
| Participants | 47 adult patients, aged 18 to 80 years, undergoing major open upper abdominal surgery for which the medical management plan called for a period of gut rest for the first 10 to 14 postoperative days. Surgical blocks included Whipple procedure, gastrojejunostomy, hepato‐biliary surgery,
gastrectomy and others. All required midline incisions from T7 to T11 dermatomes Excluded were patients with significant cardiac disease (severe angina, congestive cardiac failure, recent acute myocardial infarction); respiratory disease (preoperative PaO2 50 mm Hg (room air), PaCO2 50 mm Hg (room air)); renal disease (plasma creatinine 0.2 mmol/L); musculoskeletal or neurological disease; haematological disease; drug dependency disorder; or psychiatric disease |
|
| Interventions |
Treatment groups: TEA T7‐T8, T8‐T9, or T9‐T10 interspace, and a block was established to T4 using bupivacaine 0.5%. Intraoperative block was maintained with bupivacaine 0.5% and was continued postoperatively for a minimum of 48 hours, with an infusion of bupivacaine 0.25% with fentanyl 2.5 mcg/mL at 5 to 10 mL/h. With (n = 12) or without (n =13) intravenous parenteral nutrition. At the conclusion of anaesthesia, 20 to 30 mg ketorolac tromethamine was administered intramuscularly,
and 10 to 15 mg was administered every 6 hours up to 48 hours Control group: intravenous PCA with fentanyl (n = 1) or morphine. With (n = 10) or without (n =12) intravenous parenteral nutrition General anaesthesia for all participants |
|
| Outcomes | VAS scores at rest at 6, 24 and 48 hours VAS scores on movement at 6, 24 and 48 hours Anastomotic leak Length of hospital stay |
|
| Notes | Study authors contacted on 6 April 2015, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated cards in sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | "randomly allocated cards in sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | Ketorolac for 48 hours in the epidural group only |
Barzoi 2000.
| Methods | RCT Setting: Italy Funding: unspecified |
|
| Participants | 60 ASA 2 or 3 patients scheduled for major surgery with hepato‐biliary neoplastic disease | |
| Interventions |
Control group: TEA (T6‐T7; catheter inserted 3 cm passed the needle tip) with bupivacaine 0.125% plus morphine for 36 hours (n = 30) Treatment group: TEA (T6‐T7; catheter inserted 3 cm passed the needle tip) with morphine alone for 36 hours (n = 30) General anaesthesia for all participants |
|
| Outcomes | Time to first faeces Vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Treatment was administered by some study authors (GB, SC, BB) and results were analysed by others (GM, SV, GC), who were unaware of the type of analgesic treatment that had been used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Beilin 2003.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Israel Funding: governmental |
|
| Participants | 115 patients (ASA physical status 1 to 3) scheduled for elective lower abdominal surgery | |
| Interventions |
Treatment group: LEA (T12‐L1 or L1‐L2 with the catheter inserted 3 to 4 cm) with bupivacaine 0.1% and fentanyl 2 mcg/mL (n = 35) Control groups: IV patient‐controlled analgesia (PCA), morphine (n = 35) General anaesthesia for all participants. Exact duration of treatment unclear, taken at ≥ 48 hours (participants received 429 mL epidurally at a rate at 6 mL/h) |
|
| Outcomes | VAS at rest at 8, 24, 48 and 72 hours VAS on coughing (taken as on movement) at 8, 24, 48 and 72 hours |
|
| Notes | Study also includes a group with on demand intramuscular pethidine that was not retained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assigned to" |
| Allocation concealment (selection bias) | Unclear risk | "On the preoperative visit" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | Groups well balanced "Patients requiring blood transfusion during the perioperative period were not included in this study". Therefore, not in intention‐to‐treat |
Beilin 2008.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Israel Funding: charity |
|
| Participants | 82 ASA 1 to 3 patients, aged 20 to 72 years, undergoing general, gynaecological, urological or orthopaedic surgery Exclusion criteria were not fluent in Hebrew, serious hearing or visual impairment precluding neuropsychological testing, absence of consent, history of head trauma, neurological disease, alcoholism, drug abuse and consumption of psychotropic drugs or antidepressants |
|
| Interventions |
Treatment group: patient‐controlled lumbar (L2‐L4; catheter advanced 3 to 4 cm cephalad) epidural analgesia with 3 mL of 2% lidocaine as a test followed by 12 mL of 0.5% bupivacaine and 50 to 100 mcg of fentanyl 15 minutes before surgical incision, and bupivacaine 0.1% and fentanyl 2 mcg/mL for 24 hours (n = 30) Control group: IV patient‐controlled analgesia with morphine for 24 hours (n = 30) General anaesthesia for all participants |
|
| Outcomes | Pain: "Pain intensity was significantly greater in patients of the IV group throughout the observation period, both at rest and during coughing" | |
| Notes | Data not extractable for abdominal surgery only. Study authors contacted 23 February 2016, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned", "by a schedule based on a random‐numbers table" |
| Allocation concealment (selection bias) | Unclear risk | "Preoperative evaluation was performed by an anaesthesiologist, 3 to 7 days before surgery", "Additional
information, including age, weight, demographic status, and education, were documented for patients who consented to participate in the study", "randomized immediately prior to surgery", "by a schedule based on a random‐numbers table" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of drop‐outs: "A total of 22 patients dropped from the study because of failure to complete the second (postoperative) testing session for various reasons: A total of 6 patients did not feel well enough to cooperate; 5 were disconnected from the patient‐controlled pump early due to nausea and vomiting; and 11 had other reasons (fever, lack of optical glasses, noisy setting, refusal to participate in second testing, etc.)" |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced: "The groups were similar in demographic characteristics, including body weight, age, male‐to‐female ratio, years of education, type and duration of surgery" |
Bellolio 2012.
| Methods | RCT Informed consent obtained Setting: Uruguay Funding: unspecified |
|
| Participants | 21 patients undergoing liver surgery | |
| Interventions |
Treatment group: epidural local anaesthetic before general anaesthesia (n = 10) Control group: intrathecal morphine (n = 11) General anaesthesia for all participants |
|
| Outcomes | Pain: "Pain relief was better in the epidural group (P = 0.019), in the first postoperative day" | |
| Notes | Conference abstract Data not extractable. No address available to contact study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "we assigned", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced: "Demographic and surgical characteristic were similar in both groups" |
Benzon 1994.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: United States of America Funding: industry |
|
| Participants | 120 patients who underwent major abdominal or genitourinary procedures. Gastrointestinal procedures included gastrectomy, abdominoperineal resection and small bowel and colon resection. Genitourinary procedures included nephrectomy, radical nephrectomy and radical prostatectomy with pelvic lymph node dissection | |
| Interventions |
Treatment group: epidural fentanyl 10 mcg/mL with 0.1% (n = 30), 0.15% (n = 30) or 0.2% (n = 30) bupivacaine for 24 hours Control group: epidural fentanyl 10 mcg/mL in preservative‐free saline (n = 30) Epidural catheter was placed as close to the site of surgical incision as possible (i.e. low thoracic level for nephrectomy or gastrectomy, and lumbar level for prostatectomy and pelvic lymph node dissection). Initial rate of 5 mL/h adjusted to maintain a VAS ≤ 3. General anaesthesia for all participants |
|
| Outcomes | Vomiting | |
| Notes | "One in Group I (Epidural fentanyl only) with postoperative numbness was diagnosed with a lumbar plexopathy secondary to postoperative intrapelvic hematoma" Study authors contacted on 23 February 2014, replied to our request on 24 February 2014, that additional information is not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomized, via a random number generator" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All drugs for infusion were prepared by the hospital pharmacy just before use and were labelled simply "study drug" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All drugs for infusion were prepared by the hospital pharmacy just before use and were labelled simply "study drug" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Bisgaard 1990.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Denmark Funding: unspecified |
|
| Participants | 30 ASA 1 or 2 patients, aged 32 to 77 years, scheduled for elective major abdominal surgery | |
| Interventions |
Treatment group: continuous lumbar (L2‐L3 or L3‐L4; catheters inserted 10 cm) epidural analgesia with bupivacaine 0.5% 12 to 24 mL for sensory block from T4 to S5 followed by bupivacaine 0.25% plus morphine 0.06 mg/mL at 9 mL/h for 48 hours and epidural morphine for 3 to 6 days thereafter (n = 14) Control group: epidural morphine 4 to 6 mg every 4 to 6 hours for 48 hours (n = 15) General anaesthesia and postoperative supplemental intravenous pethidine as required for all participants |
|
| Outcomes | Time to first flatus: "median 4.8, range 2 to 8 days versus median 4.1, range 4 to 10 days" Time to first faeces: "median 6.8, range 4 to 10 days versus median 6.5, range 4 to 11 days" Pain: "The combination of bupivacaine plus morphine provided significantly superior analgesia compared with epidural morphine alone" Colonic motility (radio‐opaque markers instilled into the stomach and carried at least 1 colonic segment): "No difference in colonic motility was observed" |
|
| Notes | Results not extractable. Study authors replied to our request that the original data are no longer available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated by the closed‐envelope method" |
| Allocation concealment (selection bias) | Low risk | "randomly allocated by the closed‐envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One patient in Group epidural bupivacaine with a non‐functioning epidural catheter was excluded from the study", "Another patient in Group epidural bupivacaine had a non‐lethal pulmonary embolism on the second post‐operative day; data until then are included in the study" |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups similar for sex distribution, age, weight, height and duration of surgery Not in intention‐to‐treat |
Bois 1997.
| Methods | RCT Approved by the institution and informed consent obtained Setting: Canada Funding: industry |
|
| Participants | 124 patients scheduled for elective abdominal aortic surgery were recruited | |
| Interventions |
Treatment group: TEA (T6‐T7 or T7‐T8) with bupivacaine 0.125% and fentanyl 10 mcg/mL adjusted for VAS scores ≤ 3 for 48 hours (n = 55) Control group: IV PCA with morphine for 48 hours (n = 59) General anaesthesia for all participants |
|
| Outcomes | VAS scores at rest at 8 and 24 hours Hospital LOS (days) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "prospectively randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants with TEA and 6 with PCA were excluded because of failure of Holter monitoring or epidural analgesia, or because use of analgesia was not included in the protocol |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat One participant was initially ascribed to the epidural group and received postoperative PCA instead because of a failed epidural (2 dural punctures during performance of the epidural). This participant was not excluded from the study |
Boylan 1998.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Canada Funding: governmental |
|
| Participants | 40 ASA 2 or 3 patients coming for elective open aorto‐bifemoral bypass | |
| Interventions |
Treatment group: LEA at L2‐L3 or L3‐L4 with bupivacaine 0.125% and morphine 0.1 mg/mL for 48 hours (n = 19) Control group: IV PCA with morphine for 48 hours (n = 21) General anaesthesia for all participants |
|
| Outcomes | VAS scores at rest at 8, 24 and 48 hours VAS scores on movement at 8, 24 and 48 hours |
|
| Notes | Study authors contacted for additional information on 18 July 2014; replied that original data were no longer available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", no details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Opened design" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Opened design" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No failed epidural mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced In intention‐to‐treat |
Brodner 2001.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Denmark Funding: unspecified |
|
| Participants | 30 patients undergoing radical cystectomy with formation of an ileal neobladder | |
| Interventions |
Treatment group: TEA (T9‐T11) with ropivacaine 0.5% during the intraoperative period and 10 mL of ropivacaine 0.2% at the end of surgery. IV piritramide thereafter (n = 15) Control group: IV piritramide (n = 15) All participants received general anaesthesia |
|
| Outcomes | VAS on movement at 24, 48 and 72 hours Time to first faeces Anastomotic leak |
|
| Notes | Study includes a third group collected after the first 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced except for blood losses |
Broekema 1998.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: The Netherlands Funding: unspecified |
|
| Participants | 90 patients, 18 to 76 years of age, ASA physical status 1 to 3, scheduled for elective major abdominal surgery (abdominal aortic surgery, pancreaticoduodenectomy, extended hepato‐biliary surgery, colonic or other upper abdominal) | |
| Interventions |
Treatment groups: TEA (catheter inserted 4 to 6 cm passed the needle tip) with bupivacaine and morphine (n = 29) or sufentanil (n = 30) intraoperatively and postoperatively for > 48 hours. Rate adjusted according to pain (4 at rest and 6 on movement) Control group: IM morphine (n = 28) General anaesthesia for all participants. Co‐analgesia with paracetamol ± diclofenac |
|
| Outcomes | VAS scores (scale from 0 to 10) at rest at 24, 48 and 72 hours VAS scores (scale from 0 to 10) on movement at 24, 48 and 72 hours |
|
| Notes | Technical difficulties or complications of the epidural technique recorded. No neurological sequelae Control group divided into 2 groups for comparisons with each treatment group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to 1 of 3 groups: no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both participant and investigator were informed about the nature of the treatment ‐ IM or epidural |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Route of administration unknown to observers (3 medical students), who assessed postoperative analgesia and side effects. Participants in the IM group received a sham epidural catheter on the skin of the back. This catheter was connected to an empty syringe in an infusion pump, which was covered postoperatively to shield its contents from the observer. The same cover was used for participants in the epidural groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were excluded: 2 in the IM and 1 in the EM group. Participant 7 (IM group) developed sepsis after surgery and was mechanically ventilated for 4 days. Participant 22 (EM group) lost 22 L of blood and died from multiple‐organ failure 8 days after surgery. Participant 80 (IM group) developed acute respiratory distress syndrome (ARDS); he was tracheally extubated 5 days after surgery |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced All analyses performed on an intention‐to‐treat basis. This principle was applied if the epidural technique failed and in cases of dysfunction of the epidural catheter, premature removal or dislocation of the epidural catheter |
Buggy 2002.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: United Kingdom Funding: charity |
|
| Participants | Patients (20 to 80 years) having extensive abdominal or pelvic surgery involving a midline abdominal incision (open colon or rectal excision, radical gastrectomy or nephrectomy, total abdominal hysterectomy with bilateral salpingo‐oophorectomy, ovarian cystectomy) | |
| Interventions |
Treatment group: PCEA (TEA or LEA) with bupivacaine 0.125% and fentanyl 4 mcg/mL for 24 hours (n = 16) Control group: IV PCA with morphine (n = 16) All participants received general anaesthesia |
|
| Outcomes | VAS at rest at 24 hours VAS on movement at 24 hours |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each participant was randomly assigned to 1 of 2 groups via blocked randomization from a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Assignments were kept in sealed, sequentially numbered envelopes until use |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Patients were not told whether their patient controlled analgesia system was epidural or intravenous." Participants in the morphine PCA group were positioned for epidural anaesthesia but received skin infiltration of local anaesthetic only, and had an epidural catheter attached along their back with adhesive tape, as a placebo mock epidural. "Anesthetists directly caring for the patients during surgery were, of course, aware of the group allocation, but they took no part in subsequent data collection. Investigators collecting postoperative data were also aware of group allocation, but patients were blind" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Anesthetists directly caring for the patients during surgery were, of course, aware of the group allocation, but they took no part in subsequent data collection. Investigators collecting postoperative data were also aware of group allocation, but patients were blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced The sole epidural participant who required intravenous PCA received it 6 hours postoperatively, but this participant's data were treated as from the epidural group, consistent with intention‐to‐treat analysis |
Cai 2007.
| Methods | RCT Setting: China Funding: departmental |
|
| Participants | 62 ASA 1 to 2 patients, aged 33 to ˜60 years, weighing 42 to ˜56 kg, scheduled for elective gastrectomy Exclusion criteria were abnormal heart, lung, liver, kidney and coagulation function and history of recent use of hormone or non‐steroidal anti‐inflammatory drugs |
|
| Interventions |
Treatment groups: thoracic epidural analgesia (T7‐T8; catheter advanced 3 to 4 cm passed the needle tip, test dose 3 mL of 1% lidocaine) with ropivacaine 0.25% (for sensory level to T4) and morphine 2 mg (before surgical incision), combined with postoperative epidural analgesia with ropivacaine 0.15% and morphine 1.5 mcg/mL at 2 mL/h for 72 hours (n = 22) or ropivacaine 0.25% 10 mL and morphine 2 mg (before surgical incision, postoperative epidural analgesia with ropivacaine 0.15% and morphine 1.5 mcg/mL at 2 mL/h for 72 hours (n = 22) Control group: postoperative intravenous analgesia with fentanyl 0.1 mg IV at the end of surgery, followed by continuous intravenous fentanyl 0.25 mcg//mL and droperidol 0.05 mg/mL at a rate of 2 mL/h for 72 hours (n = 22) General anaesthesia with propofol, fentanyl isoflurane and vecuronium for all participants |
|
| Outcomes | Pain scores at rest and on coughing (taken as on movement) at 8, 12, 24, 48 and 72 hours after surgery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Calderon 2004.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Spain Funding: unspecified |
|
| Participants | 30 ASA 2 to 3 patients aged 30 to 75 years, scheduled for abdominal surgery (pancreatico‐duodenectomy or hemicolectomy) Exclusion criteria were cardiovascular disease or disease of the central nervous system, allergy to opioids or non‐steroidal anti‐inflammatory drugs, long‐term use of opioids or psychotropic drugs, history of addiction to drugs or alcohol abuse and contraindications to epidural analgesia |
|
| Interventions |
Treatment group: lumbar epidural analgesia (L1‐L2 inserted before surgery), bupivacaine 0.25% 15 mL and fentanyl 1 mcg/kg 40 minutes after surgery followed by 1.5 mL/h of bupivacaine 0.25% and fentanyl 1 mcg/h for 24 hours (n = 15) Control group: morphine 0.15 mg/kg and ketorolac 30 mg IV followed by an infusion of tramadol 300 mg/24 h and ketorolac 90 mg/24 h plus morphine as rescue (n = 15) General anaesthesia with propofol, remifentanil, sevoflurane and rocuronium. Prophylaxis with ondansetron |
|
| Outcomes | Pain scores (0 to 3) at 6 hours after surgery Vomiting (first 24 hours) |
|
| Notes | Study authors contacted on 25 June 2015, sent additional data on 13 July 2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Carli 1997.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Canada Funding: unspecified |
|
| Participants | 12 patients undergoing elective gastrointestinal surgery for resection of non‐metastatic adenocarcinoma of the rectosigmoid colon Exclusion criteria were anaemia, diabetes mellitus, morbid obesity and severe cardiovascular disease |
|
| Interventions |
Treatment group: thoracic (T8) epidural analgesia with 10 to 15 mL of 0.75% bupivacaine for a sensory block from T3 to S5 followed by 5 mL every 90 minutes during surgery and an infusion of 0.25% bupivacaine at 8 to 12 mL/h for 48 hours. Intramuscular papaveratum as required (n = 6) Control group: subcutaneous infusion of papaveratum at 3 to 8 mg/h (n = 6) General anaesthesia for all participants |
|
| Outcomes | Pain: Postoperative pain scores in the epidural group ranged from 0.6 to 1.6 at rest and from 2.3 to 4.1 on coughing. Pain scores in the control group ranged from 0.8 to 2.1 at rest and from 2.4 to 6.3 on coughing. No significant difference in pain scores at rest was observed between the 2 groups. In contrast, pain scores on coughing were lower in the epidural group (P value = 0.021) | |
| Notes | Data not extractable; study authors contacted on 4 May 2015; informed us that data are no longer available Participants in the epidural group could not move on first postoperative day owing to a motor block |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Carli 2001.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Canada Funding: charity |
|
| Participants | 42 patients undergoing elective colorectal surgery Exclusion criteria included malnutrition, severe cardiopulmonary disease, sepsis, inflammatory bowel disease, chemotherapy or radiotherapy 6 months before surgery and inability to communicate and understand the aim of the project |
|
| Interventions |
Treatment group: TEA (T8‐T9) with bupivacaine 0.1% and fentanyl 2 mcg/mL for 4 ± 2 days after surgery (n = 21) Control group: IV PCA with morphine (n = 21) All participants received general anaesthesia |
|
| Outcomes | VAS on movement at 24, 48 and 72 hours Vomiting Time to first flatus Time to first faeces Hospital LOS |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | On the day of surgery, participants were allocated at random to 1 of 2 groups |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Carli 2002.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Canada Funding: charity |
|
| Participants | 64 adult patients undergoing elective colorectal surgery for non‐metastatic conditions | |
| Interventions |
Treatment group: TEA (T8 or T9 interspace) with bupivacaine 0.1% and fentanyl 2 mcg/mL or morphine 0.1 mg/mL for 4 days (n = 32) Control group: IV PCA with morphine for 3 to 4 days (n = 32) All participants received general anaesthesia |
|
| Outcomes | VAS at rest at 24, 48 and 72 hours VAS on movement at 24, 48 and 72 hours Time to transit taken at time to first faeces Anastomotic leak Hospital LOS |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", no details |
| Allocation concealment (selection bias) | Low risk | "On the morning of the surgical procedure, the group to which the subject had been randomly assigned was revealed" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The patients were not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced If the epidural block did not provide adequate analgesia, the participant continued to be included in the intention‐to‐treat analysis but was excluded from analysis addressing efficacy |
Chalmouki 2010.
| Methods | Unclear Setting: Greece Funding: unspecified |
|
| Participants | 30 male patients, aged 51 to 72 years, ASA 1 to 3, undergoing radical prostatectomy | |
| Interventions |
Treatment group: TEA with ropivacaine 0.2% for 48 hours (n = 15) Control group: IV morphine infusion for 48 hours (n = 15) General anaesthesia for all participants |
|
| Outcomes | Time to first faeces (hours) | |
| Notes | Conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | No details, abstract |
Cindea 2011.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Romania Funding: unspecified |
|
| Participants | 78 ASA 2 to 3 elderly patients (> 75 years) with good mental status, scheduled for major abdominal surgery under general anaesthesia | |
| Interventions |
Treatment group: PCEA with 0.1% ropivacaine and 5 mcg/mL of fentanyl (n = 39). Taken as 72 hours, as participants were followed during this time Control group: IV PCA with morphine (n = 39) |
|
| Outcomes | Time to first faeces ("return of gastrointestinal function") | |
| Notes | Conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | No details |
Cronin 2001.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: United States of America Funding: charity |
|
| Participants | 10 women undergoing minor gynaecological procedures through a low abdominal incision | |
| Interventions |
Treatment group: TEA (T10‐T11 or T11‐T12) with bupivacaine 0.125% for 48 hours (n = 4) Control group: TEA (T10‐T11 or T11‐T12) with fentanyl 0.75 to 1 mcg/kg/h for 48 hours (n = 6) General anaesthesia for all participants and IV ketorolac on request |
|
| Outcomes | VAS at rest at 24, 48 and 72 hours VAS on coughing (taken as on movement) at 24, 48 and 72 hours |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants blinded to treatment, but not personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pain assessed by participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant withdrawn from fentanyl group |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced One participant withdrawn from the fentanyl group; therefore not in intention‐to‐treat |
Cullen 1985.
| Methods | RCT Setting: United States of America Funding: charity |
|
| Participants | 81 patients undergoing major abdominal surgery were enrolled | |
| Interventions | Treatment groups: epidural bupivacaine 0.1%, 3 to 4 mL/h for 72 hours, alone (n = 15) or with morphine (n = 15) Control group: epidural morphine 0.1 mg/mL, 3 to 4 mL/h for 72 hours (n = 18) | |
| Outcomes | VAS scores taken as at rest (not clearly mentioned) Vomiting (time point unclear) |
|
| Notes | Epidural catheter placed at middle dermatome crossed by surgical incision 2 groups (epidural saline (n = 15) and no catheter (n = 18)) of participants not included in this analysis. Instead, the epidural morphine group was divided into 2 groups to serve as comparisons for the 2 treatment subgroups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 22 participants withdrawn: 7 for mechanical problems, 4 for pain/discomfort, 2 for paraesthesia (bupivacaine alone), 3 for wet tap or hypotension and 6 for incomplete data |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups said to be equivalent Not in intention‐to‐treat |
Cuschieri 1985.
| Methods | RCT Informed consent not mentioned Setting: United Kingdom Funding: charity |
|
| Participants | 75 patients undergoing open cholecystectomy | |
| Interventions |
Treatment group: low thoracic epidural analgesia (catheters inserted after induction and loaded with an age‐related dose of bupivacaine 0.5%) followed by intermittent boluses of 0.5% bupivacaine for 12 hours followed by intramuscular morphine on request (n = 25) Control group: intermittent intramuscular on request (n = 25) or continuous intravenous morphine for 60 hours (n = 25) General anaesthesia for all participants |
|
| Outcomes | Pain: Participants receiving epidural bupivacaine for 12 hours had better analgesia than those receiving morphine (P value < 0.001) | |
| Notes | No serious complications occurred in the epidural group Data not extractable. Study authors contacted on 18 July 2014, but did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned, "assessed daily during the postoperative period by a single observer" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. 4 failed attempts at catheter insertion kept as intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Dauri 2003.
| Methods | RCT Written informed consent obtained Setting: Italy Funding: unspecified |
|
| Participants | 25 patients undergoing cadaveric renal transplantation | |
| Interventions |
Treatment group: TEA (T12‐L1) with ropivacaine 0.2% and fentanyl 2 mcg/mL for 24 hours (n = 13) Control group: IV tramadol (n = 12) |
|
| Outcomes | VAS at rest at 6 and 24 hours VAS on movement at 6 and 24 hours Hospital LOS |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated into two groups", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Davies 1993.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Australia Funding: unspecified |
|
| Participants | 50 patients undergoing elective aortic abdominal surgery | |
| Interventions |
Treatment group: TEA (T9‐T10) with bupivacaine 0.5% for 62 (± 26) hours (n = 25) Control group: IV morphine infusion (n = 25) General anaesthesia for all participants |
|
| Outcomes | Hospital LOS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 failed epidural |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced In intention‐to‐treat |
De Pietri 2006.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Italy Funding: unspecified |
|
| Participants | 50 adult patients of both sexes, ASA 1 and 2, scheduled for liver surgery. | |
| Interventions |
Treatment group: TEA (T9‐T10 or T10‐T11) with ropivacaine 0.2% for 96 hours or longer (n = 25) Control group: intrathecal morphine 0.2 mg followed by IV PCA with morphine (n = 25) General anaesthesia for all participants |
|
| Outcomes | VAS at rest at 8, 24 and 48 hours VAS on coughing (taken as on movement) at 8, 24 and 48 hours |
|
| Notes | "Patients were evaluated for post‐dural puncture headache and radicular back pain; muscle weakness and sensory deficit, as early signs of spinal cord compression caused by hematoma, were also evaluated" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated to 2 groups by a computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Postoperative clinical monitoring of participants and evaluation of VAS were managed by investigators blinded to the analgesic technique used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Postoperative clinical monitoring of participants and evaluation of VAS were managed by investigators blinded to the analgesic technique used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned: "no patient was excluded from the study" |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Donatelli 2006.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Canada Funding: charity |
|
| Participants | 16 patients undergoing elective colorectal surgery Exclusion criteria were more than 20% loss of body weight in the past 6 months, evidence of metastatic disease, severe cardiac and respiratory diseases, diabetes with albumin < 35 g/L and anaemia (haemoglobin < 100 g/L) |
|
| Interventions |
Treatment group: thoracic (T9‐T11) epidural loaded with 15 mL of 0,5% bupivacaine for a sensory block from T4 to S5, maintained with 5 mL of 0.25% bupivacaine every hour during surgery followed by an infusion of 0.1% bupivacaine plus fentanyl 2 mcg/mL at 8 to 15 mL/h for 48 hours after surgery (n = 8) Control group: IV patient‐controlled analgesia with morphine (n = 8) General anaesthesia for all participants |
|
| Outcomes | Pain scores at rest and on movement (coughing) at 24 and 48 hours | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using a computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced except for age; participants in the epidural group were older (65 vs 59 years) |
Doruk 2003.
| Methods | RCT Approved by the ethics committee Setting: Turkey Funding: unspecified |
|
| Participants | 65 ASA 1 to 3 patients undergoing open urological surgery | |
| Interventions |
Treatment group: lumbar (L2 to L3 or L3 to L4) epidural patient‐controlled analgesia with bupivacaine (20 mL of 0.125% bupivacaine as a loading dose; n = 13) or bupivacaine plus morphine (20 mL of 0.125% bupivacaine plus morphine 2 mg as the loading dose; n = 13) Control group: IV patient‐controlled analgesia with morphine (n = 13) or tramadol (n = 13) |
|
| Outcomes | Pain: Pain scores were lower with epidural bupivacaine plus morphine than with morphine or tramadol | |
| Notes | Study measured pain scores at 8 and 24 hours. Details in text insufficient for data extraction. Copy received via interlibrary loan contains no figures or tables ‐ just text. Journal website starts at 2011. No address given to contact study authors Study also includes a group given epidural bupivacaine plus tramadol (n = 13) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not enough information for review authors to judge the report |
| Other bias | Unclear risk | Not enough information for review authors to judge the report |
El‐Refai 2003.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Egypt Funding: unspecified |
|
| Participants | 30 ASA 1 or 2 women undergoing total abdominal hysterectomy Exclusion criteria were history of hepatic or renal disease, myocardial infarction within the previous 6 months and general anaesthesia within the previous 3 months |
|
| Interventions |
Treatment group: lumbar (L3‐L4 or L4‐L5) epidural with 3 mL of 2% lidocaine as a test dose followed by 9 mL of 0.25% bupivacaine for a sensory level at T10, and 5 mL every hour thereafter (n = 15) Control group: IV patient‐controlled analgesia with morphine (n = 15) General anaesthesia for all participants |
|
| Outcomes | Pain at 30 minutes after surgery: 1.4 ± 1.3 vs 2.4 ± 1.4 | |
| Notes | No outcomes of interest measured at our selected time points | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Low risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Elkaradawy 2011.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Egypt Funding: unspecified |
|
| Participants | 50 ASA 2 type 2 diabetic patients undergoing open cholecystectomy with negative stress exercise test and at least 2 cardiac risk factors preoperatively Exclusion criteria were history of coronary heart disease; hypertension; respiratory, renal or hepatic insufficiency; or contraindication to epidural analgesia |
|
| Interventions |
Treatment group: thoracic (T7‐T8 and advanced 3 cm) epidural analgesia with 15 mL of 0.2% ropivacaine and fentanyl 2 mcg/mL followed by 5 to 8 mL/h of 0.1% ropivacaine plus fentanyl 1 mcg/mL at 5 to 8 mL/h for 24 hours (n = 25) Control group: IV patient‐controlled analgesia with morphine (n = 25) General anaesthesia for all participants |
|
| Outcomes | Pain: less pain with epidural analgesia | |
| Notes | Results not extractable. Study authors contacted on 15 June 2014, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "block‐wise balanced randomization" |
| Allocation concealment (selection bias) | Low risk | "on cards sealed into opaque envelopes", "opened after taking decision to operate" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "neutral observer blinded with anaesthetic and analgesic techniques" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Erol 2008.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Turkey Funding: unspecified |
|
| Participants | 30 ASA 1 or 2 patients aged between 18 and 60 years scheduled for laparoscopic cholecystectomy | |
| Interventions |
Treatment group: TEA (T11‐T12) with bupivacaine 0.125% and fentanyl for 48 hours (n = 15) Control group: IV fentanyl infusion (n = 15) General anaesthesia and nausea/vomiting prophylaxis for all participants |
|
| Outcomes | Vomiting. No participants in either group experienced vomiting (effect not estimable) | |
| Notes | No incidence of motor blockade from epidural infusions Study authors contacted for additional information on 15 June 2014, but did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided into 2 groups", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Fant 2013.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Sweden Funding: governmental |
|
| Participants | 26 patients (ASA physical status 1 to 2) in the age group 50 to 75 years, undergoing elective radical retropubic prostatectomy | |
| Interventions |
Treatment group: TEA (T10‐T12) with ropivacaine 0.2% and sufentanil 1 mcg/mL for 48 hours (n = 12) Control group: IV PCA with morphine (n = 14) General anaesthesia for all participants |
|
| Outcomes | VAS scores at rest at 8, 24 and 48 hours VAS scores on movement at 8, 24 and 48 hours |
|
| Notes |
NCT01367418 All participants in Group E received an epidural catheter successfully; no complications were associated with catheter insertion |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group randomization and concealed allocation was done using cards inserted into opaque, sealed envelopes by an independent person not involved in the study" |
| Allocation concealment (selection bias) | Low risk | "Group randomization and concealed allocation was done using cards inserted into opaque, sealed envelopes by an independent person not involved in the study" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The study was only blinded to laboratory personnel involved in biochemical assays" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The study was only blinded to laboratory personnel involved in biochemical assays" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 opioid based regimen patient switched to non steroid anti‐inflammatory drugs |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Fayed 2014.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Egypt Funding: unspecified |
|
| Participants | 34 ASA 1 or 2 Child A cirrhotic patients aged 25 years or older, undergoing major liver resection for tumour removal Exclusion criteria included failure of surgery to proceed as planned, development of postoperative complications limiting assessment, objection to an epidural catheter or inability to use PCA pump, postoperative need for mechanical ventilation, contraindication to regional technique such as infection and anatomical spinal abnormality and pre‐existing severe pulmonary or psychiatric disease |
|
| Interventions |
Treatment group: patient‐controlled thoracic (T11‐T12) epidural with bupivacaine 0.125% and fentanyl 2 mcg/mL, basal infusion 6 mL/h plus 15‐minute boluses of 3 mL on demand, started within 2 hours of induction (n = 17). Mean time for epidural catheter stay was 5.88 ± 1.27 days before removal Control group: IV PCA with fentanyl (n = 17) All surgeries were performed with participants under standardized general anaesthesia |
|
| Outcomes | VAS scores on movement (cough) at 8, 24, 48 and 72 hours after surgery | |
| Notes | 4 out of 17 participants in the epidural group reported bilateral lower limb numbness during the first postoperative day in the PCEA group, owing to the established epidural block, but only 1 participant developed moderate motor block; in this sole case, the epidural infusion was stopped with close follow‐up of motor status. No clinical manifestations suggesting epidural haematoma Study authors contacted for additional information on 12 April 2015, but did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated" (abstract) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Ferguson 2009.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: United States of America Funding: departmental |
|
| Participants | Women 18 years of age and older undergoing abdominal surgery by laparotomy for a gynaecological disorder | |
| Interventions |
Treatment group: PCEA (TEA at T6‐T12) with bupivacaine 0.05% and morphine 0.1 mg/mL (n = 67) Control group: IV PCA with morphine (n = 68) General anaesthesia and IV ketorolac for 48 hours for all participants. Exact duration not specified |
|
| Outcomes | VAS scores on movement (cough taken as movement) at 24, 48 and 72 hours | |
| Notes | Study was stopped early because the stopping criteria for efficacy were met by our primary endpoint Overall malfunction rate of thoracic epidural in this study was only 1.5% Study authors contacted for additional information on 15 June 2014, but did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was not blinded because of ethical issues involved in placing a “sham” epidural catheter in half of study participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study was not blinded because of ethical issues involved in placing a “sham” epidural catheter in half of study participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 women were randomized, but data were discarded (11 PCEA group and 7 PCA group). Reasons data were discarded included the following: 7 (5 PCEA group and 2 PCA group) withdrew from the study after randomization, and 8 did not undergo initial planned surgical intervention (4 PCEA group and 4 PCA group). In addition, 3 participants were not eligible because they did not meet the inclusion criteria for the following reasons: underwent laparoscopic surgery (2 participants) and had a history of alcohol abuse (1 participant) |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced Groups were followed for all primary and secondary endpoints and were analysed initially as randomized (intent‐to‐treat principle). Only 3 women were switched to an alternative medication or intervention for pain (2 epidural local anaesthetic group; 1 opioid group) |
Gambling 2009.
| Methods | RCT Approved by ethics committees (multi‐centre) and written informed consent obtained Setting: United States of America Funding: industry |
|
| Participants | Adult patients aged 18 years or older with ASA 1 to 3, who were scheduled to undergo total abdominal hysterectomy, prostatectomy or colon resection under general anaesthesia Exclusion criteria were pregnancy or lactation, any present or past disease or condition or prior post‐surgical complication that might increase the risk associated with surgery or risk of post‐surgical complications, sleep disorder (e.g. sleep apnoea, narcolepsy, excessive daytime sleepiness) and history of substance abuse. Patients who underwent treatment with clonidine or daily opioids for longer than 7 days before enrolment or who used any long‐acting opioid, ketorolac or cyclo‐oxygenase 2 inhibitor for 48 hours before surgery were excluded |
|
| Interventions |
Treatment group: epidural catheter at an unspecified level. Test dose with 3 mL of 1.5% lidocaine with epinephrine 5 mcg/mL. Bupivacaine 0.25% 20 mL before surgical incision plus extended‐release morphine 15 mg injected 15 (28 enrolled/28 analysed), 30 (30 enrolled/28 analysed) or 60 minutes (29 enrolled/22 analysed) after bupivacaine dose. Catheters were removed at the end of surgery. IV PCA with fentanyl after surgery Control group: epidural catheter at an unspecified level. Test dose at the discretion of the attending anaesthesiologist. Extended‐release epidural morphine 15 mg. Catheters were removed at the end of surgery. IV PCA with fentanyl after surgery (29 enrolled/27 analysed) General anaesthesia for all participants |
|
| Outcomes | VAS scores on movement at 8, 24 and 48 hours after surgery Vomiting |
|
| Notes | Study also included a group given bupivacaine alone not retained for this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computerized randomization system" |
| Allocation concealment (selection bias) | Low risk | "Patient numbers were assigned by the study‐site pharmacist using an interactive voice recognition system" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study medications were prepared and administered by unblinded pharmacists and anaesthesiologists, respectively, none of whom were involved in study assessments |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 participants withdrew from the study early because of withdrawal of consent (n = 2), loss to follow‐up (n = 1), change in surgery (n = 2) or other reasons (n = 11, all unique reasons such as bleeding risk, investigator withdrawal and unsuccessful epidural catheterization). 15 of these participants did not receive study drug (extended‐release morphine or placebo) and were excluded from analyses |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
George 1994.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: United Kingdom Funding: unspecified |
|
| Participants | 21 adult patients with ASA status 1 or 2, aged between 20 and 74 years and undergoing upper abdominal surgery | |
| Interventions |
Treatment group: TEA (T7‐T8 or T8‐T9 and catheter threaded 3 cm in) with bupivacaine 0.2% and fentanyl 10 mcg/mL for 24 hours (n = 10) Control group: IV PCA with morphine (n = 11) General anaesthesia for all participants |
|
| Outcomes | Vomiting | |
| Notes | No motor blockade Study authors contacted for additional information on 18 July 2014, but did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Gherghina 2010.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Romania Funding: unspecified |
|
| Participants | 70 patients older than 70 years of age and undergoing major abdominal surgery (gastrointestinal mainly). Criteria for inclusion of patients in the study were age over 70 years, ASA physical status 1 to 3, major abdominal surgery, normal preoperative neurological status and Abbreviated Mental Test score ≥ 8 | |
| Interventions |
Treatment group: TEA/LEA with bupivacaine 0.125% and sufentanil 5 mcg/mL started before surgery and kept for 79 hours (n = 35 randomized; 33 analysed) Control group: IV PCA with morphine (n = 35 randomized; 31 analysed) General anaesthesia with propofol, sufentanil, sevoflurane and atracurium. Rescue analgesia (VAS > 3) with paracetamol (1 G IV) or ketoprofen 100 mg IV |
|
| Outcomes | Time to return of intestinal transit (taken as time to first flatus) | |
| Notes | Study authors contacted on 23 June 2015, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Six participants lost to follow‐up: 4 in the epidural group and 2 in the opioid‐based regimen group |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Giannoni 1999.
| Methods | Randomized prospective study Written informed consent obtained Setting: Italy Funding: unspecified |
|
| Participants | 50 ASA 2 to 4 patients undergoing gastrointestinal abdominal surgery for colorectal cancer | |
| Interventions |
Treatment group: continuous epidural thoraco‐lumbar anaesthesia for 96 hours with bupivacaine 0.125% and morphine during the first 48 hours, and bupivacaine 0.125% only in the following 48 hours (n = 25) Control group: continuous analgesic intravenous therapy for 96 hours (with morphine and ketorolac in the first 48 hours and ketorolac only in the following 48 hours) (n = 25). General anaesthesia for surgery for all participants with thiopental, fentanyl, nitrous oxide, isoflurane and pancuronium (Group general anaesthesia) or atracurium (Group general anaesthesia plus epidural) |
|
| Outcomes | Time to first faeces Postoperative analgesia VAS scores at 6, 24, 48 and 72 hours. Results at 72 hours for one group were 0 for the mean and 0 for SD; a value of 0.001 for SD was entered for analysis purposes. At 96 hours, the result was 0 for all participants (not included in the analysis) Vomiting (during hospital stay) Anastomotic leak Length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Hadimioglu 2012.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Turkey Funding: unspecified |
|
| Participants | 46 non‐diabetic patients aged between 18 and 65 years who were scheduled for living‐related renal transplantation | |
| Interventions |
Treatment group: LEA (L1‐L2) with 14 to 18 mL of bupivacaine 0.5% during surgery and morphine after surgery (n = 21) Control group: IV PCA with morphine (n = 25) General anaesthesia for all participants |
|
| Outcomes | Hospital LOS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reason for exclusion provided |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Participants who needed a blood transfusion owing to extreme haemorrhage or with severe haemodynamic instability intraoperatively, as well as those with problems with the epidural catheter, were excluded from the study |
Handley 1997.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Australia Funding: charity |
|
| Participants | 30 consecutive patients aged 18 to 74 years, ASA 1 to 3, undergoing elective abdominal (gastrointestinal) surgery and suitable for epidural anaesthesia | |
| Interventions |
Treatment group: LEA with bupivacaine 0.5% intraoperatively only, IV PCA with morphine after surgery (n = 15) Control group: IV PCA with morphine after surgery (n = 15) General anaesthesia for all participants |
|
| Outcomes | VAS scores at rest at 24 hours VAS scores on coughing at 24 hours |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized by computer" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "A sham epidural was not performed in the general anaesthesia group, hence neither group of patients was blinded to treatment", "At the end of surgery, the epidural catheter was removed from those patients in the general anaesthesia‐epidural group so that all assessments in the post anaesthetic period" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "A sham epidural was not performed in the general anaesthesia group, hence neither group of patients was blinded to treatment", "At the end of surgery, the epidural catheter was removed from those patients in the general anaesthesia‐epidural group so that all assessments in the post anaesthetic period" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced except for age |
Heurich 2007.
| Methods | RCT Study was conducted with approval of the local ethics committee, and written informed consent was obtained from patients Setting: Germany Funding: governmental |
|
| Participants | 55 ASA 1 to 3 patients, 18 years of age and older, scheduled for elective major abdominal surgery via midline incision (part I: prostatectomy, cystectomy, hysterectomy, hemicolectomy (n = 30); part II: prostatectomy (n = 25)) were included | |
| Interventions | Part I: epidural versus intravenous analgesia Treatment group: TEA (T11–L1) or LEA (L1–L3) with the catheter advanced 2 to 3 cm into the epidural space. Bupivacaine 0.5% during surgery and bupivacaine 0.0625% to 0.125% and fentanyl 2 to 4 mcg/mL for 48 hours, adjusted for VAS scores at rest of 3 or less (n = 10) Control group: IV PCA with piritramide (n = 9) All participants received general anaesthesia Part II Treatment group: TEA or LEA (T10–L2) with bupivacaine 0.25% to .0.5% during surgery and PCEA with bupivacaine 0.125% for 24 hours after surgery (n = 9) Control group: epidural fentanyl for 24 hours (n = 10) All participants received general anaesthesia |
|
| Outcomes | Part I VAS scores at rest and on movement at 6, 24 and 48 hours Part II VAS scores at rest and on movement at 6 and 24 hours |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomization list was generated by our department of statistics, together with numbered envelopes |
| Allocation concealment (selection bias) | Low risk | Numbered envelopes. "After obtaining written informed consent from the patients by the investigator, the patient was allocated to one of two treatments according to the randomization" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The investigator assessing histological outcomes was blinded to the treatment regimen |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Large number of exclusions (see below) |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | During each part of the study, the 2 groups of participants were similar with respect to weight, age, sex and duration of surgical procedures Not in intention‐to‐treat Part I: 11/36 excluded from analysis Part II: 6/29 excluded from analysis Participants with dislocated catheters were excluded |
Hjortsø 1985a.
| Methods | RCT Approved by the ethics committee and written informed consent obtained. Setting: Denmark Funding: industry |
|
| Participants | 100 patients aged 50 years or older and scheduled for elective major abdominal procedures | |
| Interventions |
Treatment group: LEA (L1 to L2) with etidocaine intraoperatively and bupivacaine 0.5% for 24 hours after surgery. Morphine for 3 to 72 hours after surgery (n = 44) Control group: systemic IM morphine (4 to 8 mg every 4 to 6 hours) postoperatively (n = 50) General anaesthesia for all participants |
|
| Outcomes | Time to first flatus Time to first faeces |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No other loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat: For 2 participants, it was not possible to place the catheter, dura puncture was accidentally performed in 2 participants and another 2 participants lost their catheters during the immediate postoperative period |
Hjortsø 1985b.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Denmark Funding: industry |
|
| Participants | 20 patients undergoing elective major abdominal surgery (bowel or gastric resection and 1 cholecystectomy) | |
| Interventions |
Treatment group: epidural anaesthesia with 1.5% etidocaine during surgery and bupivacaine 0.5% bolus for 24 hours after surgery. In addition, extradural morphine 4 mg was administered every 12 hours from 3 to 72 hours after skin incision (n = 10) Control group: systemic morphine (n = 10) |
|
| Outcomes | VAS at 24, 48 and 72 hours (average pain experienced during the preceding 24 hours) taken as at rest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized into two groups", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or epidural failure mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Hong 2008.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Korea Funding: unspecified |
|
| Participants | 50 ASA 1 or 2 female patients, aged 29 to 57 years, scheduled for elective laparoscopic total hysterectomy | |
| Interventions |
Treatment group: LEA (L1‐L2 with catheter inserted 5 cm cranially) and PCEA with lidocaine 1% and morphine 0.1 mg/mL during surgery and for 48 hours after surgery (n = 25) Control group: IV PCA with fentanyl and ketorolac for 48 hours (n = 25) General anaesthesia for all participants |
|
| Outcomes | VAS 6 and 24 hours (unspecified, taken as at rest) Vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation schedule was computer‐generated" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Hu 2006.
| Methods | RCT Informed consent obtained Setting: China Fundong: departmental resources |
|
| Participants | 120 patients scheduled for lower abdominal surgery under general anaesthesia Exclusion criteria were reoperation, severe inflammation and immunological disease |
|
| Interventions |
Treatment group: epidural analgesia (T12‐L1; installed before surgery) with bupivacaine 0.1% and fentanyl 2 mcg/mL through patient‐controlled epidural analgesia: bolus 3 mL, lockout time 10 minutes, basal rate 6 mL/h (n = 40), duration 48 hours Control groups: IM pethidine on request (n = 40) or IV PCA with morphine (n = 40) General anaesthesia for all participants |
|
| Outcomes | Pain scores at rest and on coughing (taken as on movement) at 24 and 48 hours | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. No failed epidural mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Hubler 2001.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Germany Funding: unspecified |
|
| Participants | 121 patients aged 18 to 80 years with ASA physical status grade 1 to 3 who were scheduled to undergo major urological surgery (radical prostatovesiculectomy, retroperitoneal or transperitoneal nephrectomy, cystectomy, retroperitoneal lymphadenectomy) | |
| Interventions |
Treatment groups: TEA (T8‐T12) with 0.25% bupivacaine (group B; n = 22) or 0.2% ropivacaine (group R; n = 23) for 72 hours Control group: 0.5 mcg/mL of sufentanil only (group S; n = 22) for 72 hours. This group was split in half for comparison General anaesthesia for all participants |
|
| Outcomes | VAS scores at rest at 8, 24, 48 and 72 hours VAS scores on coughing (taken as on movement) at 8, 24, 48 and 72 hours |
|
| Notes | For VAS scores, results are provided as means and pooled SDs. Those pooled SDs were retained Study includes 2 other groups: 0.25% bupivacaine with 0.5 mcg/mL sufentanil (group BS), and 0.2% ropivacaine with 0.5 mcg/mL sufentanil (group RS), not retained "A high percentage of patients in the groups who received the local anaesthetic bupivacaine experienced some degree of muscular impairment", "Drowsiness was very common in all groups during the first 24 hours and highest in the groups that received epidural sufentanil (groups BS, RS and S)" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "a staff anaesthesiologist not directly involved in the study prepared study solutions" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "a staff anaesthesiologist not directly involved in the study prepared study solutions" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on participants who withdrew their consent to participate in the study before the follow‐up period was completed were included in the analysis up to the time point of their withdrawal. 12 were excluded because of protocol violations |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Retained groups well balanced Data on participants who withdrew their consent to participate in the study before the follow‐up period was completed were included in the analysis up to the time point of their withdrawal. 12 were excluded because of protocol violations Not in intention‐to‐treat |
Jayr 1988.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: France Funding: charity |
|
| Participants | 150 patients scheduled for major abdominal cancer surgery through a midline incision were included | |
| Interventions |
Treatment group: TEA (T11‐T12) with bupivacaine intraoperatively and morphine alone postoperatively until postoperative day 5 (n = 74) Control group: morphine 10 mg SC every 4 hours on demand (n = 72) General anaesthesia for all participants |
|
| Outcomes | Time to first flatus VAS scores at 24, 48 and 72 hours (during the prior 24 hours, taken as at rest) |
|
| Notes | Study authors contacted 1 July 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocated to one of two treatment groups". To avoid imbalance between the numbers of participants with a previous history of respiratory disease in the 2 treatment groups, randomization was stratified for this factor |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | An epidural catheter was inserted into the subcutaneous tissue in such a way that it could not be distinguished from an epidural catheter positioned in the epidural space. Saline solution injections (2 mL) were given through this catheter twice daily. Saline solution and morphine syringes were filled and labelled by the hospital pharmacy. Nurses in charge of participants did not know the nature of the contents of the syringes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An epidural catheter was inserted into the subcutaneous tissue in such a way that it could not be distinguished from an epidural catheter positioned in the epidural space. Saline solution injections (2 mL) were given through this catheter twice daily. Saline solution and morphine syringes were filled and labelled by the hospital pharmacy. Nurses in charge of participants did not know the nature of the contents of the syringes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants were excluded for the following reasons: early surgical complications (n = 2), unsuccessful respiratory weaning (n = 1), associated thoracotomy (n = 1) |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Jayr 1993.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: France Funding: charity (plus a contribution from the industry) |
|
| Participants | 163 patients undergoing elective major abdominal surgery for cancer via midline or bilateral subcostal incision | |
| Interventions |
Treatment group: TEA (T7‐T11) inserted before surgery. Used intraoperatively and postoperatively. Bupivacaine for surgery and bupivacaine 0.125% plus morphine for postoperative analgesia for 5 days (n = 78) Control group: morphine infusion through a subcutaneous catheter for 5 days (n = 75) General anaesthesia for all participants |
|
| Outcomes | VAS scores at rest at 24, 48 and 72 hours VAS scores on coughing at 24, 48 and 72 hours (taken as on movement) Hospital LOS |
|
| Notes | Recovery of intestinal gas transit occurred earlier in the EP group than in the SC group (P value < 0.05). Data expressed as cumulative % of participants with recovery from day 2 to day 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by history of bronchopulmonary disease |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Subcutaneous catheter located and dressed as an epidural. Solutions filled and labelled by the pharmacy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Subcutaneous catheter located and dressed as an epidural. Solutions filled and labelled by the pharmacy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 participants excluded from analysis: early postoperative complications (n = 4), ventilator dependence (n = 1), associated thoracotomy (n = 1), intraoperative anaphylaxis (n = 1) and surgery cancelled after randomization (n = 3) ‐ 4 from the epidural group |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced except that more smokers were included in the subcutaneous group Not in intention‐to‐treat |
Jayr 1998.
| Methods | RCT Ethics committee approval and written informed consent obtained from each patient Setting: France Funding: industry |
|
| Participants | 141 ASA 1 to 3 patients aged 18 to 75 years and weighing 50 to 110 kg undergoing elective open major abdominal (urological, gynaecological or gastrointestinal) | |
| Interventions |
Treatment group: TEA (T12‐L1; level according to surgical site) with 0.2% ropivacaine started after surgery and kept for 24 hours (n = 38) Control group: IV PCA with morphine (n = 46) General anaesthesia for all participants |
|
| Outcomes | Vomiting | |
| Notes | A third group with epidural ropivacaine plus IV morphine was not retained Study authors contacted for additional information; referred us to AstraZeneca, which did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation code envelopes" |
| Allocation concealment (selection bias) | Low risk | Envelopes opened just before preparation for anaesthesia |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | More women in the PCA group Not in intention‐to‐treat |
Jorgensen 2001.
| Methods | RCT Approved by the ethics committee and written informed consent obtained from each patient Setting: Denmark Funding: charity |
|
| Participants | 60 ASA 1 or 2 women aged 18 to 75 years undergoing elective abdominal hysterectomy through a Phannenstiel or median incision | |
| Interventions |
Treatment groups: TEA with lidocaine intraoperatively only (n = 20) or TEA with lidocaine intraoperatively and bupivacaine 0.2% for 24 hours after surgery (n = 20) Control group: no epidural (n = 20) General anaesthesia, paracetamol, ketorolac and IM (?) morphine for all participants |
|
| Outcomes | Time to first flatus Time to first faeces |
|
| Notes | Study authors contacted on 27 June 2014, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding between the 2 epidural groups only |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding between the 2 epidural groups only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants excluded after randomization; replaced with new sealed envelopes |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Katz 2003.
| Methods | RCT Approval was obtained from the research ethics board. All patients gave written informed consent to participate before enrolling in the study Setting: Canada Funding: governmental |
|
| Participants | Patients with American Society of Anesthesiologists (ASA) physical status 1 to 3, age between 19 and 75 years, weight between 45 and 90 kg, height between 150 and 175 cm, body mass index ≤ 30, able to speak and read English, scheduled for major gynaecological surgical procedures by laparotomy (horizontal or midline incision) | |
| Interventions |
Treatment groups: LEA (L2‐L3 or L3‐L4 with the catheter advanced 3 to 4 cm past the tip of the needle) with lidocaine 2%, epinephrine 5 mcg/mL and fentanyl 4 mcg/kg before (n = 45) or after (n = 49) the incision Control group: sham epidural procedure (n = 47). This group was split in half for comparison with each subgroup General anaesthesia and IV PCA with morphine for 48 hours for all participants |
|
| Outcomes | VAS scores at rest at 6, 24 and 48 hours VAS scores on movement at 24 and 48 hours |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomization schedule was computer‐generated by a biostatistician (not otherwise involved in the study) and was provided to the hospital pharmacist, who prepared and dispensed drugs for clinical trials. The randomization schedule specified the group (1, 2 or 3) to which each prospective participant would be allocated upon enrolment in the trial |
| Allocation concealment (selection bias) | Low risk | An opaque envelope containing participant number and group assignment was prepared, sealed and numbered for each participant by the hospital pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | A standard volume of lidocaine, fentanyl and preservative‐free saline for epidural and intravenous administration was prepared in separate syringes, coded for blinding purposes, numbered and dispensed by the hospital pharmacy on the day of surgery. The pharmacist who dispensed study medications was not involved in any other aspect of the study. The anaesthesiologist in charge of the case was aware of group allocation for control group participants and was not involved in postoperative management or data collection. Sham epidural catheter: "The anaesthesiologist went through all the motions of placing an epidural catheter, including prepping and cleansing the skin, infiltrating the skin and interspinous regions with 2–3 ml lidocaine (2%), applying pressure as if inserting the needle, simulating loss of resistance, and threading of the catheter. The epidural needle was removed, and the exposed length of catheter was wrapped in gauze and taped to the patient’s back. A test dose of 3–5 ml normal saline was injected into the catheter that drained into the gauze" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants and personnel involved in participant management and data collection were unaware of the group to which the participant had been allocated. The anaesthesiologist in charge of the case was aware of group allocation for control group participants and was not involved in postoperative management or data collection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 withdrawn from the analysis ‐ 5 could not have the epidural catheter placed. In addition, 7 participants were withdrawn during surgery owing to intraoperative protocol violations, and 4 were withdrawn after surgery owing to apnoea and chest wall rigidity upon extubation requiring reintubation, faulty PCA equipment and nausea and back pain |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced, except larger number of participants with pain history in the post‐incisional group Not in intention‐to‐treat |
Kentner 1996.
| Methods | RCT Approved by the ethics committee Setting: Germany Funding: unspecified |
|
| Participants | 74 patients undergoing urological surgery through a lower abdominal incision | |
| Interventions |
Treatment group: lumbar (L3‐L4; catheter advanced 3 to 4 cm) epidural analgesia with 4 mL of mepivacaine 2% plus epinephrine as a test dose followed by 10 to 16 mL of 0.5% bupivacaine plus 5 to 8 mL every 90 to 120 minutes intraoperatively, and 0.25% bupivacaine for 6 hours after surgery followed by 0.175% bupivacaine at 8 mL/h thereafter for 36 hours plus IV patient‐controlled analgesia with piritramide (n = 37) Control group: IV patient‐controlled analgesia with priritramide (n = 37) |
|
| Outcomes | Time to first faeces: First bowel movement occurred between postoperative days 3 and 5 with no differences noted between the 2 groups Pain on a 6‐degree scale: Participants in the epidural group had lower pain scores during the first 8 hours |
|
| Notes | Data not extractable; study authors contacted on 23 June 2015, and informed us that original data were no longer available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant given epidural analgesia excluded for catheter dislodgement; 1 participant given opioid analgesia excluded for incomplete data |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Kudoh 2001.
| Methods | RCT Approved by the medical ethics committee and informed consent obtained from all patients and their families Setting: Japan Funding: unspecified |
|
| Participants | 46 patients, ranging in age from 36 to 77 years, who were diagnosed as having schizophrenia by Diagnostic and Statistical Manual of Mental Disorders and scheduled for elective lower abdominal surgery including colectomy, hemicolectomy or sigmoidectomy for malignant tumours | |
| Interventions |
Treatment group: TEA (T9‐T10 with the catheter threaded 3 cm passed the needle tip) with bupivacaine 0.25%. A bolus was given before surgery; the infusion was started after surgery and was maintained for 72 hours (n = 23) Control group: IV 4 mcg/kg buprenorphine and continuous infusion of 0.6 mcg/kg/h buprenorphine for 96 hours (n = 23) General anaesthesia for all participants |
|
| Outcomes | VAS scores at 6, 24, 48 and 72 hours (taken as at rest) Time to first flatus (hours) Time to first faeces (hours) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed via computer‐generated codes |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Kumar 2004.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: India Funding: unspecified |
|
| Participants | 48 ASA 1 or 2 patients aged between 20 and 70 years undergoing major abdominal surgery | |
| Interventions |
Treatment group: TEA (T12‐L1 with the catheter advanced 3 cm passed the needle tip) with bupivacaine 0.25% started after the end of surgery (n = 16) Control group: TEA with morphine 0.02 mg/mL (n = 16) General anaesthesia for all participants |
|
| Outcomes | VAS (visual analogue) scores at 22 (taken as 24 hours) and 40 (taken as 48 hours) (taken as at rest) | |
| Notes | One group with a combination of bupivacaine and morphine ‐ not retained Duration of treatment unspecified ‐ taken as 48 hours (i.e. equivalent to measurements) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "at random", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Type of surgery not mentioned Unilateral sensory impairment and motor weakness in 3/16 participants, retained in their group |
Lattermann 2007.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Canada Funding: unspecified |
|
| Participants | 20 patients undergoing elective open resection of localized, non‐metastatic colorectal carcinoma Exclusion criteria were evidence of metastatic disease, congestive heart failure, hepatic disease or diabetes; serum albumin < 35 g/L or anaemia (haemoglobin 100 g/L); and drugs known to have metabolic effects, such as corticosteroids or beta‐blockers |
|
| Interventions |
Treatment group: thoracic epidural analgesia (T9‐T11) with bupivacaine 0.5% for a sensory level from T4 to L3 before the surgical incision, bupivacaine 0.25% during surgery and bupivacaine 0.1% plus fentanyl 2 mcg/mL after surgery for at least 48 hours (n = 10) Control group: IV PCA with morphine (n = 10) General anaesthesia for all participants |
|
| Outcomes | VAS scores at rest and on movement at 24 and 48 hours | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Both anaesthesiologist (TS, RL) and surgeon (SM) were aware of the individual patient’s group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Both anaesthesiologist (TS, RL) and surgeon (SM) were aware of the individual patient’s group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No missing information |
| Other bias | Low risk | Both groups were homogeneous with respect to type of surgery (epidural group: 8 sigmoid colectomy, 2 hemicolectomy; intravenous analgesia group: 7 sigmoid colectomy, 2 hemicolectomy) |
Levy 2011.
| Methods | RCT Approved by the ethics committee and consent obtained Registration number: NCT 18926278 Setting: United Kingdom Funding: departmental |
|
| Participants | Patients with colorectal disease (benign or malignant) who required a laparoscopic large bowel resection that did not involve a stoma or perineal dissection were considered for the trial | |
| Interventions |
Treatment group: TEA (T9‐T12) with bupivacaine 0.15% and fentanyl 2 mcg/mL for 48 hours (n = 30) Control group: IV PCA with morphine (n = 30) General anaesthesia for all participants |
|
| Outcomes | Time to first faeces VAS scores on movement/coughing (whichever worst), evening (taken as 6 to 8 hours), 24 hours Leak Hospital LOS (from the start of surgery) |
|
| Notes | Study also includes a group with spinal analgesia, not retained in our analysis Study authors contacted for additional information on 21 July 2014, but did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization code was created by an independent company, which used a computer randomization programme to generate the sequence, then placed the appropriate analgesic regimen in sequentially numbered opaque envelopes |
| Allocation concealment (selection bias) | Low risk | The randomization code, which was kept in an opaque envelope in an off‐site building, was opened after consent was obtained |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 99 included and 91 completed the trial |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced except higher body weight for the opioids group Not in intention‐to‐treat |
Licker 1994.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Switzerland Funding: unspecified |
|
| Participants | 19 women with ASA 1 to 2 undergoing abdominal hysterectomy | |
| Interventions |
Treatment group: lumbar epidural analgesia with bupivacaine and fentanyl adjusted to keep the participant free of pain (n = 9) Control group: IV morphine PCA (n = 9) All participants were given a propofol‐based general anaesthesia |
|
| Outcomes | VAS scores at 8 and 24 hours (taken as at rest; not clearly written) | |
| Notes | No complications occurred in any participants during the hospital stay | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Initially, 10 participants in the epidural group; 1 excluded because of failure of a device required for the main objective of the study |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Initially, 10 participants in the epidural group; 1 excluded because of failure of a device required for the main objective of the study. Therefore, not in intention‐to‐treat |
Limberi 2003.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Greece Funding: unspecified |
|
| Participants | 50 patients with coronary artery disease scheduled for elective upper abdominal surgery for non‐vascular disease Exclusion criteria were preoperative electrocardiogram that interfered with accurate diagnosis of myocardial ischaemia (bundle branch block, left ventricular hypertrophy with strain pattern, ventricular ectasia), valvular heart disease, prior digoxin therapy, contraindication to insertion of an epidural catheter, localized infection, septicaemia, preoperative coagulopathy, neurological disease) and partial oxygen pressure < 60 mm Hg |
|
| Interventions |
Treatment group: thoracic or lumbar (T12‐L1 or L1‐L2) epidural analgesia tested with 2 mL of 2% lidocaine with epinephrine 5 mcg/mL, followed by 7 to 15 mL of 0.5% bupivacaine for a sensory level from T4 to L5 at 20 minutes before surgical incision and morphine 2 mg at skin closure (n = 25) Control group: IV morphine (n = 25) General anaesthesia for all participants |
|
| Outcomes | Pain: Epidural group had better pain control | |
| Notes | No outcome of interest available at our selected time points. VAS scores provided are computed scores for 24‐hour periods. Study authors contacted on 23 February 2016. Data are no longer available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Liu 1995.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: United States of America Funding: charity (plus a contribution from industry) |
|
| Participants | 54 ASA 1 to 3 patients undergoing elective partial resection of the colon | |
| Interventions |
Treatment group: TEA (T8‐T9 or T9‐T10; catheter threaded 3 cm) with bupivacaine 0.15%, 10 mL/h for various times (n = 14) or combinations of epidural morphine 0.03 mg/mL + bupivacaine 0.1%, 10 mL/h for various times (n = 14)
Control group: postoperative epidural morphine 0.05 mg/mL 10 mL/h for various times (n = 12) Analgesia at rest was titrated to a verbal pain score < 5/10 (0 = no pain, 10 = worst possible pain) with an epidural injection of fentanyl 50 micrograms followed by an increase in the epidural infusion of 2 mL/h every hour as needed. Therefore, all participants received epidural opioids, and the 2 groups with bupivacaine were fused to be compared with the control group Length of epidural infusion taken as ≥ 48 hours, although not clearly stipulated |
|
| Outcomes | Time of first flatus VAS scores at movement at 24, 48 and 72 hours Hospital LOS Anastomotic leakage |
|
| Notes | One group with postoperative IV PCA morphine not retained for analysis Groups B and MB were ready for discharge an average of 35 hours earlier (95% confidence interval for difference ranges from 27 to 49 hours) than groups M and P |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Separate randomization tables were prepared for each institution. Randomization was stratified by planned left vs right colonic anastomosis because left colonic anastomosis may result in greater postoperative ileus |
| Allocation concealment (selection bias) | Unclear risk | Unclear (randomized on arrival to the preoperative holding area) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Epidural groups (MB, M and B) were double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Epidural groups (MB, M and B) were double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No other loss to follow‐up (apart from the 2 withdrawn because a catheter could not be inserted) |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat: Epidural catheters could not be placed in 2 participants, who were withdrawn from the protocol |
Liuboshevskii 2012.
| Methods | RCT Setting: Russia Funding: unspecified |
|
| Participants | 120 patients scheduled to undergo elective gastrointestinal low‐abdominal surgery Patients with coagulation/haemostasis abnormalities including liver failure were excluded |
|
| Interventions |
Treatment group: TEA (T8) with 0.75% ropivacaine for surgery followed by ropivacaine 0.2% plus fentanyl 2 mcg/mL 6 to 10 mL/h and systemic ketorolac administration (n = 40). Exact duration not specified Control group: IM trimeperidine 20 mg every 4 to 6 hours and ketorolac 30 mg every 8 hours (n = 40) All participants had total intravenous anaesthesia based on propofol, fentanyl and mechanical ventilation |
|
| Outcomes | VAS scores at 6, 24 and 48 hours | |
| Notes | Study includes a third group with general anaesthesia plus spinal anaesthesia not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "divided", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Lombardo 2009.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Italy Funding: unspecified |
|
| Participants | 34 patients (all male: 28 with abdominal aortic aneurysm, 6 with obstructive aorto‐iliac disease; mean age: 68 ± 7 years) | |
| Interventions |
Treatment group: epidural catheter (level unspecified) with ropivacaine 1% 8.8 to 13.2 mg/h plus sufentanil 0.8 to 1.2 μg/h (n = 17). Epidural analgesics were discontinued after surgery upon return to normal gastrointestinal function
when the participant was able to tolerate oral analgesics (taken as 24 hours, mean time for first flatus in this group) Control group: IV morphine, ketorolac and tramadol (n = 17) General anaesthesia (that included a remifentanil infusion) for all participants. Ketoprofene at 200 mg and fraxiparine at 4000 to 8000 UI |
|
| Outcomes | Time to first flatus Length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants failed to complete the postoperative work‐up for technical reasons |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Luchetti 1996.
| Methods | RCT Approved by the institution and informed consent obtained Setting: Italy Funding: unspecified |
|
| Participants | 40 ASA 2 or 3 patients scheduled for laparoscopic cholecystectomy | |
| Interventions |
Treatment group: TEA (T12‐L1; cranially directed for about 5 cm) with bupivacaine 0.5% (initial bolus) plus 0.25% and morphine for surgery (n = 20) Control group: fentanyl IV during surgery and ketorolac for the first 4 hours after surgery (n = 20) Propofol‐based general anaesthesia for all participants |
|
| Outcomes | Vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All data were collected in a blinded manner by a physician who was unaware as to the anaesthesia technique used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Lugli 2008.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Canada Funding: governmental |
|
| Participants | 12 type 2 diabetic patients undergoing elective colorectal surgery Exclusion criteria were severe cardiac, hepatic, renal or metabolic disorders; diabetes mellitus type 1; plasma albumin concentration < 35 g/L; > 10% weight loss over the preceding 3 months; anaemia (hematocrit < 30%); use of steroids; previous spine surgery limiting the use of an epidural catheter; and pregnancy |
|
| Interventions |
Treatment group: thoracic (T8‐T11) epidural analgesia with 0.5% bupivacaine for a sensory level from T4 to S1 and maintained with an infusion of 0.25% bupivacaine during surgery and 0.1% bupivacaine plus fentanyl 3 mcg/mL for 48 hours after surgery (n = 6) Control group: IV patient‐controlled analgesia with morphine (n = 6) General anaesthesia for all participants |
|
| Outcomes | Pain: "Pain scores measured by visual analogical scale at rest, at 12 and 24 hours after surgery, and during the study on the second postoperative day never exceeded the value of 4, and no patient reported severe pain in either group" | |
| Notes | Data not extractable. Study authors contacted, replied in May 2015 that they would send the data but never did so | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated by a computer generated schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Lugli 2010.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Canada Funding: governmental |
|
| Participants | 24 patients undergoing elective colorectal surgery Exclusion criteria were severe cardiac, hepatic, renal or metabolic disorders; diabetes mellitus type 1 with plasma albumin concentration < 35 g/L; more than 10% weight loss during the preceding 3 months; anaemia (haematocrit level < 30%); use of steroids; previous spine surgery limiting use of an epidural catheter; and pregnancy |
|
| Interventions |
Treatment group: thoracic (T8‐T10) epidural analgesia with 0.5% bupivacaine for a sensory level from T4 to S1, maintained with an infusion of 0.25% bupivacaine during surgery and 0.1% bupivacaine plus fentanyl 3 mcg/mL for 48 hours after surgery (n = 12) Control group: IV patient‐controlled analgesia with morphine (n = 12) |
|
| Outcomes | Pain: Pain scores at rest, at 12 and 24 hours after surgery and during the study on the second postoperative day never exceeded the value of 4, and no participant in either group reported severe pain | |
| Notes | Data not extractable enough. Study authors contacted; replied on 4 May 2015, that they would send the data but never did so | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated by a computer‐generated schedule" and "stratified for presence/absence of diabetes type 2" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Malenkovic 2003.
| Methods | RCT Setting: Serbia Funding: unspecified |
|
| Participants | 67 ASA 1 to 5 patients undergoing laparotomy for various surgeries | |
| Interventions |
Treatment group: combined spinal (4.5 mg of 0.25% bupivacaine plus 0.2 mg of morphine) lumbar (L1‐L2 or L2‐L3; catheter tested with 3 mL of 2% lidocaine) epidural analgesia loaded with 10 mL of 0.25% bupivacaine followed by 3 to 5 mL of 0.25% bupivacaine every hour during surgery and bupivacaine 0.125% to 0.25% 15 mL every 8 to 12 hours started on postoperative day 2 and kept for 2 or 3 days (n = 34) Control group: acetaminophen and butorphanol (n = 33) General anaesthesia for all participants |
|
| Outcomes | Intestinal motility: "faster for epidural group" Pain at rest and on movement Length of hospital stay |
|
| Notes | Pain scores, intestinal motility and length of hospital stay measured but results not provided; no corresponding address for postal mail and invalid email address given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind", no sham block |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Results not provided |
| Other bias | High risk | Participants of the epidural group also had spinal anaesthesia with bupivacaine and morphine. This is different from the other studies |
Mallinder 2000.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: United Kingdom Funding: industry |
|
| Participants | 40 ASA 1 to 3 patients scheduled for elective colorectal surgery | |
| Interventions |
Treatment group: LEA (L2‐L3 or L3‐L4) with bupivacaine 0.25% during surgery (n = 12) Control group: IV morphine (n = 20) General anaesthesia for all participants |
|
| Outcomes | Anastomotic leak Hospital LOS |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Technical monitoring difficulties meant that complete data were collected on only 32 participants (12 epidural and 20 morphine). |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Trend towards longer operating time (143 (54) vs 130 (45) minutes) and greater blood loss (1045 (753) vs 785 (887) mL) in the morphine group, but this did not reach significance Not in intention‐to‐treat |
Mann 2000.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: France Funding: charity |
|
| Participants | 70 patients older than 70 years of age, ASA status 1 or 2 (age criterion taken away), normal preoperative mental status defined by a modified Abbreviated Mental Test score, undergoing elective major abdominal surgery for cancer via midline or bi‐subcostal incision | |
| Interventions |
Treatment group: TEA (PCEA) with bupivacaine 0.125% and sufentanil 0.5 mcg/mL (mean 79 hours) (n = 35) Control group: IV morphine PCA (n = 35) General anaesthesia for all participants |
|
| Outcomes | Time to first faeces (hours) VAS scores at rest at 6 to 8 (postop 0, evening), 24, 48 and 72 hours Anastomotic leak |
|
| Notes | Subcutaneous abscess or neurological complications related to the epidural catheter did not develop in any participant Study authors contacted on 8 July 2014, for additional information but did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...the patients were assigned to receive, as determined by a table of random numbers, either general anaesthesia and postoperative morphine (PCA group) or general anaesthesia combined with epidural bupivacaine sufentanil anaesthesia (PCEA group)" |
| Allocation concealment (selection bias) | Low risk | "The day before surgery and after obtaining written informed consent, all subjects received written and verbal instructions for use of PCA or PCEA and were instructed to balance analgesia against sedation. Then, the patients were assigned..." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants were assessed for return of gastrointestinal function 2 times a day by a physician who systematically questioned participants and consulted nurse observations until the return of flatus, faeces and eating without nausea |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6 participants did not complete the postoperative study and were excluded from postoperative data analysis because of absence of surgical resection (2 in each group) or refusal to use the patient‐controlled device with requirement of conventional analgesia (2 in the PCEA group and 0 in the PCA group) |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Marandola 2008.
| Methods | RCT Setting: Italy Funding: unspecified |
|
| Participants | 40 patients enrolled to undergo surgical procedure for pancreatic or periampullary cancer | |
| Interventions |
Treatment group: TEA (T9–T10 and catheter inserted approximately 4 to 5 cm into the epidural space in a presumed
cranial direction) with ropivacaine 0.2% and morphine 0.05 mg/mL (n = 16). Duration unspecified but taken as 42 hours because participants received 420 mg of 0.2% at 5 mL/h Control group: IV morphine infusion (n = 24) General anaesthesia for all participants; rescue analgesia provided by IV infusion of tramadol every 6 hours if required |
|
| Outcomes | VAS scores at rest at 24 hours VAS scores on movement at 24 hours Vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly divided", no details and groups unequal (16 vs 24) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups highly unequal in number (16 vs 24) |
Martella 2012.
| Methods | RCT Setting: Italy Funding: unspecified |
|
| Participants | 60 consecutive patients (ASA 1 to 2) undergoing elective colorectal surgery | |
| Interventions |
Treatment group: TEA with ropivacaine 0.1% and sufentanil 1 mcg/mL (n = 30) Control group: IV ketorolac 90 mg/d and morphine 0.01 mg/kg/h (n = 30) General anaesthesia for all participants. Rescue analgesia with intravenous tramadol 0.15 mg/kg was administered in both groups whenever the VAS score was > 3 at rest |
|
| Outcomes | Time to first flatus (gastrointestinal recovery) Hospital LOS |
|
| Notes | Conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prospectively randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | No details, conference abstract only |
Miller 1976.
| Methods | RCT Setting: Canada Funding: governmental |
|
| Participants | Adults > 30 years scheduled for elective (open?) cholecystectomy. Some patients had received additional interventions. No cardiopulmonary disease but 30% smokers | |
| Interventions |
Treatment group: LEA (T12‐L3). Repeated epidural lidocaine bolus (n = 10) Control group IM meperidine (n = 10) |
|
| Outcomes | Length of hospital stay | |
| Notes | No specific a priori discharge criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Moiniche 1993.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Denmark Funding: industry |
|
| Participants | 31 ASA 1 to 2 patients scheduled for elective cholecystectomy performed through a mini‐laparotomy | |
| Interventions |
Treatment group: TEA (T7‐T8) with bupivacaine and morphine intraoperatively and for 38 hours after surgery (n =15) Control group: from 2 to 24 hours postoperatively morphine 0.125 mg/kg IM was administered every 6 hours, and from 24 to 48 hours postoperatively every 8 hours (n = 16) |
|
| Outcomes | Pain scores at rest at 24 and 48 hours (6‐point scale) Pain scores on movement at 24 and 48 hours (6‐point scale) |
|
| Notes | In all participants, subcutaneous infiltration of the surgical field with plain bupivacaine 0.25% 15 mL was performed immediately before surgical incision | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One of these participants was excluded because of preoperatively observed orthostatic hypotension (i.e. fall in systolic BP > 20 mm Hg (2.8 kPa), in association with symptoms of dizziness); no other loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat: 1 participant was excluded because of preoperatively observed orthostatic hypotension (i.e. fall in systolic BP > 20 mm Hg (2.8 kPa), in association with symptoms of dizziness) |
Mondor 2010.
| Methods | RCT Approved by the ethics committee and signed informed consent given by each patient Setting: Canada Funding: departmental |
|
| Participants | 44 patients scheduled for major liver resection | |
| Interventions |
Treatment group: TEA (T7‐T8 or T8‐T9) with 0.5% bupivacaine during surgery only (n = 22) Control group: sham epidural (n = 22) All participants were administered an intrathecal injection of morphine 0.5 mg and fentanyl 15 mcg at L2 to L3 or L3 to L4 plus general anaesthesia and IV PCA morphine after surgery |
|
| Outcomes | VAS scores at rest at 6, 24 and 48 hours obtained from study authors VAS scores on movement at 6, 24 and 48 hours obtained from study authors Hospital LOS |
|
| Notes | No post‐dural puncture headache or neurological complications reported in relation to the intrathecal or epidural technique Additional information received from study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized (by computer)" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "sham epidural" ‐ In the placebo group, the skin was punctured with the Tuohy needle, but it was not advanced beyond the subcutaneous tissue. The epidural catheter was then taped in a sponge on the patient’s back, and NaCl 0.9% infusion at 3 mL/h replaced the local anaesthetic infusion. After the real or sham epidural, the anaesthesiologist who performed the technique was replaced by the anaesthesiologist in charge of the case. At the end of surgery, a 3‐mL bolus of bupivacaine 0.5% was injected via the epidural catheter in the epidural group, or 3 mL of NaCl 0.9% in the placebo group, and the thoracic epidural catheter was removed by the same anaesthesiologist who inserted it (M.E.M.) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "sham epidural" ‐ All outcome measures were recorded by the same research nurses at 6, 9, 12, 18, 24, 36 and 48 hours after regional anaesthesia. PaCO2 also was recorded at 6 hours after intrathecal morphine injection" "Patient randomization was not known by any of the patients; anesthesiologists in charge of the cases and their assistants (residents, respiratory therapists); surgeons; nurses in the operating room, in the PACU, in the intensive care unit, or on the floor; and the research nurse. Only 1 person had information on patient randomization (M.E.M.)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was excluded because he died of a massive thrombotic cerebrovascular accident 12 hours postoperatively |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Motamed 1998.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: France Funding: unspecified |
|
| Participants | 60 ASA 1 or 2 patients aged between 18 and 70 years undergoing midline or bi‐subcostal incision | |
| Interventions |
Treatment group: TEA (T9‐T11) with bupivacaine 0.25% and morphine 0.25 mg/mL started after surgery for 48 hours (n = 28) Control group: IV PCA with morphine (n = 29) General anaesthesia for all participants |
|
| Outcomes | VAS scores at rest at 8 and 24 hours VAS scores on movement at 8 and 24 hours |
|
| Notes | Additional information received from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants excluded for severe desaturation |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Participants in the epidural group who required supplemental analgesia were withdrawn from the study |
Muehling 2009.
| Methods | RCT Approved by the local ethics committee and written informed consent obtained from all patients clinicaltrials.gov identifier NCT 00615888 Setting: Germany Funding: unspecified |
|
| Participants | All patients admitted with indications for elective open repair of an infrarenal aortic aneurysm were eligible for the study | |
| Interventions |
Treatment group: TEA (T7 and T10) in PCEA with ropivacaine 0.2% and sufentanil 2 mcg/mL (n = 50). Used intraoperatively and postoperatively for an unspecified duration Control group: IV PCA with piritramide (n = 49) General anaesthesia for all participants |
|
| Outcomes | Hospital LOS | |
| Notes | Study authors contacted on 21 July 2014, for additional information but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized block design prepared by the Department of Biometry, University of Ulm" |
| Allocation concealment (selection bias) | Unclear risk | "After they gave written informed consent, patients were randomly assigned" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant in each group withdrew written informed consent and was excluded, leaving 50 participants in the traditional group and 49 in the fast‐track group, who were studied in an intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Other treatment modalities differ between the 2 groups as per protocol Analysed in intention‐to‐treat |
Neudecker 1999.
| Methods | RCT Approved by the ethics committee and written informed consent obtained from all patients Setting: Germany Funding: unspecified |
|
| Participants | 20 patients scheduled for elective laparoscopic bowel resection | |
| Interventions |
Treatment group: TEA (T9‐T12) with ropivacaine 0.2% during surgery for 24 hours (n = 10) Control group: no epidural (n = 10) General anaesthesia and IV PCA morphine for 4 days for all participants |
|
| Outcomes | Time to first faeces Vomiting Hospital LOS |
|
| Notes | Lower limb motor blockade in 2/10 patients Study authors contacted for additional information on 9 July 2014, but did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized controlled trial", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Norman 1997.
| Methods | RCT Approved by the ethics committee and written informed consent obtained from all patients Setting: United States of America Funding: unspecified |
|
| Participants | 42 male patients undergoing elective abdominal aortic replacement | |
| Interventions |
Treatment group: TEA (T9‐T10 or T10‐T11) with bupivacaine intraoperatively only and morphine thereafter (n = 22) Control group: IV PCA with morphine (n = 20) General anaesthesia for all participants |
|
| Outcomes | Hospital LOS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants eliminated for protocol violation |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat: 2‐failed epidurals excluded |
O'Connor 2006.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Canada Funding: unspecified |
|
| Participants | 102 ASA 1 to 3 patients undergoing radical prostatectomy Exclusion criteria were history of a bleeding diathesis, aortic or mitral stenosis, uncontrolled hypertension (diastolic blood pressure > 110 mm Hg), myocardial infarction within a year preoperatively, previous cerebrovascular accident, transient Ischaemic attack within 6 months, extensive spinal surgery, hematocrit < 0.39 and serum creatinine > 150 micromol/L |
|
| Interventions |
Treatment group: thoracic or lumbar epidural analgesia tested with 3 mL of 1.5% lidocaine with epinephrine 5 mcg/mL, loaded with ropivacaine 0.5% titrated on mean arterial blood pressure target and followed by an infusion of ropivacaine 0.2% plus fentanyl 2 mcg/mL (n = 49) Control group: no epidural (n = 50) General anaesthesia for all participants |
|
| Outcomes | Length of stay in hospital: percentages of participants who stayed longer than 5 days were 49% and 68% for epidural and control groups, respectively | |
| Notes | Data not extractable; study authors contacted on 5 May 2015, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "based on a computer‐generated table of random numbers, participants were block randomized (block size = 10)" |
| Allocation concealment (selection bias) | Low risk | "using blinded study envelopes which were opened immediately prior surgery" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "single‐blind trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "single‐blind trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants excluded from analysis (1 of control group erroneously enrolled (high preoperative creatinine) and 2 failed epidural insertion attempts) |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Ozcan 2004.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Turkey Funding: unspecified |
|
| Participants | 60 ASA 1 to 2 women undergoing abdominal hysterectomy through a Pfannenstiel incision Patients with systemic disease, alcohol and opioid dependency or chronic pain and/or those with contraindication to regional anaesthesia were excluded |
|
| Interventions |
Treatment groups: lumbar epidural analgesia (L2‐L3 or L3‐L4; catheter advanced 4 to 5 cm passed the needle tip) with 10 mL of 0.25% bupivacaine and 2 mcg/kg of fentanyl 20 minutes before (n = 20) or after surgical incision (n = 20) Control group: 10 mL saline through epidural catheter before surgical incision (n = 20) All participants had an epidural catheter installed and tested with 3 mL of 2% lidocaine. Anesthesia was induced with thiopental 7 mg/kg and vecuronium 0.02 mg/kg and maintained with nitrous oxide 50%, isoflurane 0.5% to 1.5% |
|
| Outcomes | Pain scores (0 to 10) at 6, 24 and 48 hours after surgery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "sealed envelope technique" |
| Allocation concealment (selection bias) | Low risk | "sealed envelope technique" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Ozdilmac 2003.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Turkey Funding: unspecified |
|
| Participants | 30 ASA 1 to 2 physical status patients undergoing major abdominal surgery (colon/rectum) | |
| Interventions |
Treatment group: lumbar epidural (L2‐L3), test dose 3 mL of 2% lidocaine and 10 mL of 0.25% bupivacaine before induction. Patient‐controlled epidural analgesia with morphine infusion after surgery (n = 15) Control group: IV PCA with morphine (n = 15) General anaesthesia with propofol, morphine, nitrous oxide, desflurane and atracurium |
|
| Outcomes | Pain scores at 6 and 24 hours | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly divided" with a "random number generator" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Ozturk 2010.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Turkey Funding: unspecified |
|
| Participants | 30 ASA 1 to 2 patients aged 18 to 78 years scheduled for nephrectomy Exclusion criteria were liver or heart or neurological disease, uncontrolled hypertension, coagulopathy, immunodeficiency, allergy to local anaesthetics and contraindications to regional anaesthesia |
|
| Interventions |
Treatment group: epidural anaesthesia (T8‐L1; catheter advanced 3 to 4 cm passed the needle tip) inserted 1 hour before surgery with 8 to 10 mL of levobupivacaine 1.25 mg/mL and fentanyl 2 mcg/mL followed by an infusion at 6 to 8 mL/h during surgery and patient‐controlled epidural analgesia (basal rate 6 to 8 mL/h, bolus 6 mL, lock‐out time 20 minutes); exact duration unspecified (n = 15) Control group: IM meperidine (n = 15) General anaesthesia for all participants with fentanyl, etomidate, nitrous oxide, sevoflurane and rocuronium |
|
| Outcomes | Length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "divided random", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Park 2001.
| Methods | RCT Approved by the VA Cooperative Studies Program and local institutional review boards at 15 participating medical centres. Patients gave informed consent before they entered into the study Setting: United States of America Funding: governmental |
|
| Participants | 1021 patients who required anaesthesia for 1 of the intra‐abdominal aortic, gastric, biliary or colon operations | |
| Interventions |
Treatment group: TEA/LEA with bupivacaine 0.5% plus epinephrine intraoperatively only and morphine only after surgery (n = 514) Control group: systemic (IV or IM) opioids (n = 507) All participants had general anaesthesia for surgery |
|
| Outcomes | VAS scores at rest at 24 and 72 hours | |
| Notes | Study authors contacted for additional information on 10 July 2014, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Adaptive randomization scheme, 13 within each of the 15 sites, we allocated patients to one of two treatment groups to balance between the groups the following prognostic variables: surgical type (aortic, gastric, biliary, or colon); age (younger than 50 years, 50–70 years, older than 70 years); and Goldman index14 (#12, 13 and over)" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "After randomization, surgeons cancelled the operations of 26 patients, and 11 patients withdrew from the study. Finally, 495 patients in group 1 and 489 patients in group 2 underwent surgery. We completed 30‐day follow‐ups for all but 11 study patients" |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced Intenton‐to‐treat |
Paulsen 2001.
| Methods | RCT Approved by the Institutional Review Board and informed consent obtained Setting: United States of America Funding: unspecified |
|
| Participants | 49 patients (men and women) aged 18 years or older who were scheduled to undergo elective small bowel or colon resection with a primary anastomosis | |
| Interventions |
Treatment group: TEA (T10‐T12) with bupivacaine 0.1% and fentanyl 5 mcg/mL for 3.7 days as a mean (n = 23) Control group: IV PCA with morphine or meperidine (n = 21) General anaesthesia for all participants |
|
| Outcomes | Time to first flatus Time to first faeces VAS scores (taken as at rest) at 24, 48 and 72 hours Leak Hospital length of stay Cost (analgesia plus room) |
|
| Notes | 4% of participants (n = 1) in the EPI arm required removal of bupivacaine from the epidural solution secondary to lower extremity paraesthesia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "the study was not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "the study was not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 49 participants were enrolled into the study. 5 of these participants were removed after enrolment (1 (EPI) and required mechanical ventilation for 24 hours after surgery, 3 (PCA) were not able to provide pain scores and 1 (PCA) was found to have extensive bowel necrosis at laparotomy and did not undergo resection |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | More diabetic participants in the opioids group. The type of surgical procedure performed was similar between the 2 groups Not in intention‐to‐treat |
Peyton 2003.
| Methods | RCT Approved by the human investigation committee at each of 25 participating centres, and written informed consent was obtained from all randomized participants Setting: Australia Funding: governmental |
|
| Participants | 888 patients (920 randomized) undergoing elective, open and major abdominal surgery (including oesophagectomy) at high risk of an adverse outcome from having 1 or more important pre‐existing co‐morbidities | |
| Interventions |
Treatment group: TEA/LEA epidural block 2 spinal segments above the upper end of the participant's wound, intraoperative loading with local anaesthetic (bupivacaine or ropivacaine) and continuation by infusion of local anaesthetic and opioid (pethidine or fentanyl) after surgery for 72 hours or longer (n = 447) Control group: participant‐ or physician‐controlled IV opioid infusions initially (n = 441) All participants had general anaesthesia |
|
| Outcomes | VAS at rest at 24, 48 and 72 hours (morning) VAS on movement (coughing) at 24, 48 and 72 hours (morning) Hospital LOS |
|
| Notes | 225/447 fully compliant with protocol (183 removed before 72 hours) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "By permuted random blocks with stratification by study centre" |
| Allocation concealment (selection bias) | Low risk | Eligible patients were identified preoperatively by nurses or anaesthetists in collaborating hospitals and, after informed consent had been obtained, they were allocated by a central 24‐hour randomization service to control or epidural groups by permuted random blocks with stratification by study centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No sham catheters; "deemed masking to be unethical in very sick patients" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Relevant data were collected by study nurses at each participating institution, but whether particular endpoints had occurred was defined by a computer algorithm at the time of data entry by staff of the Trial Secretariat who were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "23 patients whose surgery was cancelled after randomisation and four who were randomised for an ineligible procedure were also excluded from analysis. 19 patients who were listed for an eligible procedure at the time of randomisation subsequently underwent a non‐eligible operation. By the intention‐to‐treat principle, these patients were included in the primary analysis" |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Comparison of treatment groups showed no statistically significant differences in the distribution of any inclusion criterion "23 patients whose surgery was cancelled after randomisation and four who were randomised for an ineligible procedure were also excluded from analysis. 19 patients who were listed for an eligible procedure at the time of randomisation subsequently underwent a non‐eligible operation. By the intention‐to‐treat principle, these patients were included in the primary analysis" |
Pflug 1974.
| Methods | RCT Informed consent obtained from each participant before the operation Funding: governmental |
|
| Participants | 40 patients scheduled for upper abdominal (n = 24) or hip fracture surgery (n = 16) were studied for 72 hours Inclusion criteria included absence of hypoxaemia, normal chest x‐ray, ambulatory before illness and no uncontrolled systemic disease We retained participants undergoing abdominal surgery only (n = 24) |
|
| Interventions |
Treatment group: postoperative peridural analgesia with (n = 8) or without pulmonary therapy (n = 5). Catheters were inserted at T10‐T11 under general anaesthesia, and an infusion of bupivacaine 0.5% (day of surgery) followed by bupivacaine 0.25% (thereafter) was started in the post‐anaesthesia care unit at 3 to 5 mL/h (6 to 8 dermatoma) for 72 hours. Concentrations were adjusted to allow walking and coughing without discomfort Control group: IM morphine analgesia (10 to 15 mg every 3 to 6 hours; participants comfortable but not obtunded) with (n = 5) or without pulmonary therapy (n = 6) All participants received general anaesthesia with nitrous oxide, halothane 0.5% to 1.5% and neuromuscular blocking agents. No prophylactic antibiotics given. Pain not measured |
|
| Outcomes | Hospital LOS | |
| Notes | Study authors contacted 24 February 2016, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random selection according to a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned; no catheters in the IM group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results mentioned in the Methods section are provided in the Results section |
| Other bias | Low risk | Groups well balanced (age 46.5 ± 4.6 vs 42.7 ± 4.9, for epidural bupivacaine and IM morphine, respectively) |
Rimaitis 2003.
| Methods | RCT Approved by the local ethics committee of our institution; informed written consent was obtained from each patient Setting: Lithuania Funding: unspecified |
|
| Participants | 100 patients (ASA 1 to 3) scheduled to undergo elective colorectal cancer surgery | |
| Interventions |
Treatment group: TEA (T10‐L1 with the catheter advanced 4 cm passed the needle tip) with bupivacaine 0.1% and fentanyl 5 mcg/mL for 72 hours (n = 50) Control group: IM pethidine analgesia (n = 50) All participants received general anaesthesia |
|
| Outcomes | VAS at rest at 24, 48 and 72 hours VAS on movement (coughing) at 24, 48 and 72 hours Vomiting (first day) Leak Hospital LOS |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The epidural catheter was retracted 1.5 cm for 1 participant in the EA group, who had a sufficient block level only unilaterally. Another participant in the EA group had accidental withdrawal of an epidural catheter on the morning of the third postoperative day, and systemic analgesia was started |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Riwar 1991.
| Methods | RCT Setting: Switzerland Funding: unspecified |
|
| Participants | Average age 66 years; scheduled for open gastrointestinal resection for colorectal carcinoma (n = 33) or diverticulosis (n = 24) | |
| Interventions |
Treatment group: lumbar epidural analgesia (L2‐L3), prior induction of anaesthesia and subsequently every 90 to 120 minutes during surgery, bupivacaine 0.25% at 6 to 12 mL/h for 48 hours after surgery (targeted sensory level T4‐T8) (n = 24) Control group: IV infusion of pentazocine 10 mg/h (n = 24) General anaesthesia with nitrous oxide, isoflurane |
|
| Outcomes | Time to first flatus Time to first faeces Anastomotic leak |
|
| Notes | Study authors contacted 23 June 2015, no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "urn principle" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Rockemann 1996.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Germany Funding: unspecified |
|
| Participants | 142 patients undergoing various types of major abdominal surgery Exclusion criteria were age > 65 years, creatinine level > 140 micromol/L, long‐term treatment with analgesics or corticosteroids, allergy against 1 of the study substances and contraindications against epidural puncture |
|
| Interventions |
Treatment group: thoracic (T8‐T10; inserted on the day before surgery at 7 cm; position confirmed by epidurography and tested with 5 mL of mepivacaine 1%) epidural injected with 0.2 mL/kg of 1% mepivacaine plus 75 mcg/kg of morphine before surgical incision (n = 48) or before wound closure (n = 48) Control group: no epidural analgesia (n = 46) General anaesthesia for surgery and IV patient‐controlled analgesia with morphine for postoperative analgesia for 5 days for all participants |
|
| Outcomes | Pain scores: "Median visual analogue scale pain intensities were < 3 cm and did not differ among the groups" | |
| Notes | For this study, for our selected outcomes, an exact P value (0.001) was given only for the difference between group 2 and group 3 at 8 AM on postoperative day 1; all other values were said to be not statistically significant. Retaining this single value would have been viewed as "selective reporting" by us Study authors contacted on 26 July 2014, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "prerandomized list unknown to the investigator recruiting the patients" |
| Allocation concealment (selection bias) | Low risk | "prerandomized list unknown to the investigator recruiting the patients" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Participants and personnel involved in the postoperative care and data collection were blinded regarding the allocation of the patients to groups 1 and 2 (i.e. pre‐ or post‐incision administration of study drugs). Group 3 participants did not receive catheters and consequently could not be blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Participants and personnel involved in the postoperative care and data collection were blinded regarding the allocation of the patients to groups 1 and 2 (i.e. pre‐ or post‐incision administration of study drugs). Group 3 participants did not receive catheters and consequently could not be blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants in the 2 epidural groups (n = 3) were excluded from the analysis if they received epidural bupivacaine after surgery |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Rockemann 1997.
| Methods | RCT Approved by the ethics committee Setting: Germany Funding: unspecified |
|
| Participants | 62 patients undergoing abdominal surgery (gastrointestinal mainly) Exclusion criteria were allergy to 1 of the substances used, age > 65 years, long‐term opioid or corticosteroid use, coagulation disorder and systemic infection |
|
| Interventions |
Treatment group: thoracic epidural analgesia (T7‐T8 to T10‐T11, catheter introduced 5 to 7 cm passed the needle tip, median approach) with bupivacaine 0.25% and sufentanil 2 mcg/mL (0.05 mL/kg; lockout time 10 minutes) (n = 31) Control group: IV PCA with morphine 2 mg, lockout time 10 minutes (n = 31) General anaesthesia with propofol, sufentanil, nitrous oxide, enflurane and pancuronium. To increase intestinal motility, participants received metoclopramide 10 mg 3 times daily from postoperative day 1, and prostigmine 1 mg as a short infusion from postoperative day 3. Duration of pain treatment was 101 ± 3 hours |
|
| Outcomes | Time to first faeces Pain scores (0 to 10) at rest and on coughing (taken as on movement) at 24, 48 and 72 hours after surgery Length of hospital stay Costs |
|
| Notes | Addiitonal data provided by study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Rutberg 1984.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Sweden Funding: governmental (plus a contribution from the industry) |
|
| Participants | 24 female patients undergoing cholecystectomy | |
| Interventions |
Treatment group: TEA (T9‐T10 or T10‐T11) with bupivacaine 0.5% before surgery and 0.25% to 0.375%, 5 to 8 mL to maintain segmental blockade from T4 to L3 (n = 8) for 24 hours
Control group: epidural morphine 4 mg in 7 mL of saline, repeated every 10 hours (n = 8), and postoperative IV morphine 2.5 mg as required (n = 8) General anaesthesia for all participants |
|
| Outcomes | VAS scores at 6 and 24 hours (not clearly mentioned, taken as at rest) | |
| Notes | Study includes a third group referred to as "control" group and not retained for the purpose of this meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up and no epidural failure mentioned |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Salomaki 1995.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Finland Funding: charity |
|
| Participants | 40 ASA 1 or 2 adult patients scheduled for elective major abdominal surgery | |
| Interventions |
Treatment group: TEA ("mainly thoracic") with bupivacaine 0.1% and fentanyl for 18 hours after surgery (n = 20) Control group: TEA ("mainly thoracic") with fentanyl (n = 20) All catheters tested with 4 mL of 0.5% bupivacaine before surgery and at arrival to the recovery room. In both groups, the rate was adjusted for a VAS score ≤ 2 General anaesthesia for all participants |
|
| Outcomes | Vomiting (cumulative for 18 hours taken as 24 hours) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated" with sealed envelopes |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, nurses, surgeons, anaesthesiologists, and investigators were blinded to the type of epidural infusion. Infusion syringes were prepared with a fentanyl solution with a concentration of 10 mcg/mL with or without an addition of 1 mg/mL (0.1%) bupivacaine. Solutions were prepared by a trained nurse not involved in the study or patient care" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients, nurses, surgeons, anaesthesiologists, and investigators were blinded to the type of epidural infusion. Infusion syringes were prepared with a fentanyl solution with a concentration of 10 mcg/mL with or without an addition of 1 mg/mL (0.1%) bupivacaine. Solutions were prepared by a trained nurse not involved in the study or patient care" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up and no failed epidurals mentioned |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Scheinin 1982.
| Methods | RCT Informed consent obtained Setting: Finland Funding: unspecified |
|
| Participants | 40 ASA 1 to 3 patients undergoing upper abdominal surgery | |
| Interventions |
Treatment group: thoracic (catheter tip at T9‐T10) epidural with 2 injections of 10 mL of bupivacaine 0.5% at 4 hours apart (n = 10) Control group: epidural with 2 (n = 10) or 4 mg of morphine (n = 10) |
|
| Outcomes | Pain at 4 hours after anaesthesia | |
| Notes | No outcomes of interest available for our selected time points Study also includes a group without epidural |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "treated double‐blind: the anaesthetist performing the epidural injection was unaware of which drug or dose was injected" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study authors did not specify the identity of the outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | High risk | A considerable difference in duration of surgery was noted among groups. Participants' demographics (such as age and ASA) were not fully reported, so we cannot evaluate whether the backgrounds of participants were well balanced |
Scheinin 1987.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Finland Funding: charity |
|
| Participants | 60 patients undergoing colonic surgery (right or left hemicolectomy or anterior resection) 21 males, 39 females | |
| Interventions |
Treatment group: epidural bupivacaine 0.25%, 4 to 6 mL/h, for 48 hours (n = 15)
Control group: postoperative epidural morphine continuously 2 to 6 mg/24 h for 48 h (n = 15)
This study also included a group given parenteral oxycodone 0.15 mg/kg on request (n = 15) and a group given postoperative epidural bolus morphine 2 to 6 mg/24 h (n = 15) not retained for this meta‐analysis. Therefore, we retained only groups numbered II and IV. The catheter was inserted with its tip at a level corresponding to the middle of the planned incision General anaesthesia for all participants |
|
| Outcomes | Time to first faeces VAS scores at 24 hours (not specified, taken as at rest) Anastomotic leakage |
|
| Notes | Epidural catheter inserted "with its tip at a level responding to the middle of the planned incision" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Schricker 2000.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Canada Funding: charity |
|
| Participants | 16 patients with localized non‐metastatic adenocarcinoma of the rectosigmoid colon scheduled for elective
colorectal surgery No patient had developed recent weight loss or had a plasma albumin concentration of 35 G/L |
|
| Interventions |
Treatment group: thoracic epidural anaesthesia/analgesia (T10‐T12) with bupivacaine 0.5% for a sensory level from T4 to S5, and bupivacaine 0.25% during surgery followed by bupivacaine 0.1% with fentanyl 2 mcg/mL after surgery (n = 8). Exact duration unspecified Control group: IV PCA with morphine (n = 8) All participants received general anaesthesia |
|
| Outcomes | Pain at rest at 48 hours | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were allocated according to a computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced No cross‐over |
Schricker 2002.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Canada Funding: charity |
|
| Participants | 16 patients with localized colorectal carcinoma scheduled for elective colorectal surgery None of the patients suffered from cardiac, hepatic, renal or metabolic disease |
|
| Interventions |
Treatment group: thoracic epidural anaesthesia/analgesia (T10‐T12) with bupivacaine 0.5% for a sensory level from T4 to S5 before surgery, bupivacaine 0.25% during surgery and bupivacaine 0.1% with fentanyl 2 mcg/mL after surgery (n = 8). Exact duration unspecified Control group: IV PCA with morphine (n = 8) All participants also received general anaesthesia |
|
| Outcomes | Pain scores at rest at 24 hours | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were allocated according to a computer‐generated randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant in the opioid group had to be excluded from further analysis because he inadvertently did not receive amino acids (because of an error made by pharmacy preparing the feeding solutions) |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | Groups well balanced No cross‐over mentioned Not in intention‐to‐treat |
Schricker 2004.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Canada Funding: charity |
|
| Participants | 20 patients undergoing elective resection of colorectal carcinoma Excluded were patients with evidence of metastatic disease, congestive heart failure, hepatic disease or diabetes; those who had serum albumin < 35 g/L or had anaemia (haemoglobin 100 G/L); and those receiving drugs known to have metabolic effects, such as corticosteroids or blockers |
|
| Interventions |
Treatment group: thoracic epidural anaesthesia/analgesia (T9‐T11) with bupivacaine 0.5% for a sensory level from T4 to S5 before surgery, bupivacaine 0.25% during surgery and bupivacaine 0.1% with fentanyl 2 mcg/mL for 48 hours after surgery (n = 10) Control group: IV PCA with morphine (n = 10) All participants also received general anaesthesia |
|
| Outcomes | VAS scores at rest and on movement at 24 and 48 hours | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed by the same investigator using a sealed envelope with a computer‐generated random allocation" |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed by the same investigator using a sealed envelope with a computer‐generated random allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Both anaesthesiologist (T.S.) and surgeon (S.M.) were aware of the individual patient’s group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced No cross‐over |
Schulze 1988.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Denmark Funding: industry (in part) |
|
| Participants | 24 patients undergoing elective open cholecystectomy Exclusion criteria were signs of cardiopulmonary, endocrinological, renal hepatic or immunological disease or infection within 1 week |
|
| Interventions |
Treatment group: thoracic epidural analgesia with plain bupivacaine 0.5% for 24 hours, then 0.25% for another 24 hours (T4‐L1) and epidural morphine 4 mg every 8 hours thereafter for 96 hours plus systemic indomethacin 100 mg every 8 hours for 96 hours (n = 12) Control group: intermittent nicomorphine (10 to 15 mg) and acetaminophen (1 G) on request (n = 12) General anaesthesia for all participants |
|
| Outcomes | Pain at rest and on coughing at 6, 24 and 48 hours: "The patients in the opioids group had significantly more pain during the postoperative course (P < 0.001)" | |
| Notes | Data not extractable. Study authors contacted on 3 June 2016. Replied that data are no longer available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants requiring bile duct exploration were excluded |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Schulze 1992.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Denmark Funding: charity |
|
| Participants | 25 patients aged 67 to 79 years undergoing elective left‐side colonic resection Exclusion criteria were signs of endocrinological, renal, hepatic, immunological or severe cardiopulmonary disease or infection within 2 weeks before the operation |
|
| Interventions |
Treatment group: thoracic epidural analgesia with lidocaine for surgery and bupivacaine plus morphine for 48 hours after surgery plus intrathecal 5% lidocaine and 30 mg/kg of methylprednisolone IV before surgery plus routine systematic indomethacin after surgery (n = 11) Control group: intermittent morphine and acetaminophen on request (n = 9) |
|
| Outcomes | Time to first faeces Pain at rest and during coughing at 6, 24, 48 and 72 hours Gastointestinal anastomotic leakage Length of hospital stay |
|
| Notes | Email sent 30 May 2016, for additional information; study authors replied that data are no longer available; therefore, we could extract only gastrointestinal leakage data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Excluded if blood losses exceeded 1 L, or if left colonic flexure mobilization was required Five participants were excluded ‐ 2 because of excessive bleeding (> 1 L), 2 because of excessive surgery due to tumour invasion and 1 because no tumour was found at operation |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | High risk | Treatment group also received a high dose of steroid and routine systemic co‐analgesia Not in intention‐to‐treat |
Schumann 2003.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: United States of America Funding: governmental |
|
| Participants | 114 patients, aged 18 to 80 years, undergoing gastric bypass surgery as a treatment for obesity Exclusion criteria were significant cardiovascular, hepatic, pulmonary, renal, hematological, neurological or psychiatric disease; known hypersensitivity to any of the study drugs; history of drug or alcohol abuse within the previous year; pre‐existing long‐ or short‐term pain and previous abdominal surgery or any surgery in the previous 3 months |
|
| Interventions |
Treatment group: thoracic epidural analgesia started during surgery, followed by an infusion of 0.1% bupivacaine and 1 mg/mL of meperidine after surgery (n = 39) Control group: IV patient‐controlled analgesia with morphine (n = 36) General anaesthesia and postoperative co‐analgesia with non‐steroidal anti‐inflammatory drugs available for all participants |
|
| Outcomes | Pain Length of stay in hospital: "was equivalent between the three groups" |
|
| Notes | Study also includes a group with wound infiltration Data not extractable. Study authors replied to our request that additional information is not available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Successive envelopes containing allocation codes were opened by one investigator when patients were in the preoperative holding area, after consent had been obtained" |
| Allocation concealment (selection bias) | Low risk | "Successive envelopes containing allocation codes were opened by one investigator when patients were in the preoperative holding area, after consent had been obtained" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "open‐label" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "open‐label" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Decreases in the number of patients contributing data points across time were not because of additional dropouts but instead reflected incomplete capture or documentation of pain scores by ward nursing staff (80% of subjects at time 0 versus 51% at 48 hours)" 3 of 39 participants randomized to the epidural group were dropped because the epidural catheter was not placed because an intraoperative significant adverse event occurred and one of the cases was a revision Additionally, 4 participants from the IV group were dropped from the study because of unexpected intraoperative or perioperative complications not attributable to the analgesic regimen |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | "All three treatment groups were equivalent with respect to the demographic characteristics of age, sex, and body mass index" Not in intention‐to‐treat |
Seeling 1990.
| Methods | RCT Written consent obtained Setting: Germany Funding: unspecified |
|
| Participants | 214 patients undergoing infrarenal aortic bypass operation, gastric resection, gastrectomy, Whipple's operation or duodenum‐preserving pancreatic resection | |
| Interventions |
Treatment group: TEA (T7‐T11; installed the day before surgery when possible) with bupivacaine 0.25% (sensory level T4‐T5 to L1‐L2 before surgery) and fentanyl 2 mcg/mL started intraoperatively and continued for 76 hours after surgery; rate 10 to 15 mL/h (n = 98/124) Control group: IM piritramide 10 to 15 mg (n = 116/123) General anaesthesia for all participants |
|
| Outcomes | Time to first faeces Hospital LOS |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seven drop‐outs in the opioid group and 26 in the epidural group |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Participants with failed epidurals were included in the study, but study authors did not include their data in the analysis |
Seeling 1990a.
| Methods | RCT Setting: Germany Funding: unspecified |
|
| Participants | 75 participants undergoing major abdominal surgery | |
| Interventions |
Treatment group: thoracic epidural analgesia started after surgery with bupivacaine 0.5% for the first bolus and 0.25% for subsequent doses given as 0.15 mL/kg every 2 hours, until 19H00 the day after surgery (n = 15) Control group: IV buprenorphine (n = 16) General anaesthesia for all participants |
|
| Outcomes | Pain at rest and on coughing at 24 hours after surgery | |
| Notes | Study also includes 2 other groups: Group III: buprenorphine given epidurally. This group was not retained, as something other than just local anaesthetic, opioid or epinephrine, was injected epidurally Group IV: bupivacaine plus buprenorphine given epidurally, also excluded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Owing to technical problems, 11 participants were withdrawn from the study: insufficient epidural extension (n = 4); inability to deal with the pain scale (n = 2); operative and postoperative complications (n = 4); other (n = 1). Their specific group allocation is not mentioned |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Not in intention‐to‐treat |
Seeling 1991.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Germany Funding: unspecified |
|
| Participants | Patients undergoing infrarenal abdominal aortic aneurysm; gastric or bladder cancer cystectomy; pancreatic head resection; or Whipple's procedure | |
| Interventions |
Treatment group: thoracic or lumbar (according to surgical site; catheter inserted and tested the day before surgery when possible) epidural analgesia with 0.1 mL/kg (ideal body weight) of 0.25% bupivacaine plus morphine 60 mcg/mL up to postoperative day 3 (n = 95) Control group: thoracic epidural analgesia with morphine 0.05 mg/kg in 10 mL of isotonic saline every 8 hours as needed (n = 90) or IV patient‐controlled analgesia with morphine (n = 107) General anaesthesia for all participants |
|
| Outcomes | Time to first faeces: "We were able to capture this event for 85 participants of group epidural bupivacaine plus morphine, 86 of epidural morphine and 104 of IV morphine group. There was no difference between groups: 1.33 hours versus 1.30 and 1.30 hours; alpha = 0.15 > 0.0167" Pain at rest and on coughing at 8H00, 12H00, 16H00 and 20H00 on postoperative days 1, 2 and 3: "patients in the epidural bupivacaine plus morphine group had lower pain scores on maximal coughing compared with the two other groups", "the difference between the epidural bupivacaine morphine group and the two other groups for all time points until 12H00 of postoperative day 3 were lower than 0.0063", "Pain at rest at 8H00 on postoperative day 1 was lower for epidural bupivacaine morphine compared with epidural morphine and IV (P < 0.01) and remained so until 16H00. The difference was no longer seen at 20H00. The difference was significant again in the mornings of postoperative days 2 and 3" Length of stay in hospital: epidural bupivacaine plus morphine 19: 1.69 days vs 18: 1.61 days and 19; 1.65 days: alpha 0.52 > 0.025; means and standard deviations for normally distributed values or medians and standard deviation factors |
|
| Notes | Data not extractable; letter sent to study authors on 23 June 2015. We received no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number Geigy tables and stratified for age > or < 60 years, gender and types of operations |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers of drop‐outs = 20 for epidural bupivacaine with morphine, 18 for epidural morphine and 9 for IV |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Not in intention‐to‐treat: failed catheter epidural insertion and failed analgesia were not included in the analysis |
Senagore 2003.
| Methods | RCT Approved by the Institutional Review Board and informed consent obtained Setting: United States of America Funding: unspecified |
|
| Participants | 38 patents scheduled for segmental laparoscopic colectomy | |
| Interventions |
Treatment group: TEA (T8‐T9 or T9‐T10) with bupivacaine 0.1% and fentanyl 20 mcg/mL for 18 hours (n = 18) Control group: IV PCA with morphine for 18 hours (n = 20) General anaesthesia, diclofenac 50 mg orally every 8 hours beginning the evening before surgery and continued after operation, ketorolac 30 mg within 30 minutes of completion of surgery and antiemetic prophylaxis of dexamethasone 8 mg and ondansetron 4 mg for all participants |
|
| Outcomes | VAS scores at movement at 6 and 24 hours Hospital length of stay (Criteria for discharge from hospital in both groups of participants included tolerance of 3 consecutive general meals without nausea or vomiting, adequate pain control with oral analgesics and passage of flatus. Length of hospital stay was defined as the number of nights spent in hospital from day of operation until discharge) |
|
| Notes | Investigators reported no instances of TEA catheter malfunction or complications that required early removal of the catheter | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random number after giving informed consent" |
| Allocation concealment (selection bias) | Low risk | "computer‐generated random number after giving informed consent" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "There were no instances of thoracic epidural catheter malfunction or complications that required early removal of the catheter" "Forty‐seven patients were randomized during the study but four (two thoracic epidural and two IV opioids) had no resection or a second surgical procedure during their hospital stay, and five (three TEA and two PCA) had protocol violations and were excluded from subsequent analysis" |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat: "whose operation was converted to open surgery after randomization were excluded from analysis" |
Siniscalchi 2003.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Italy Funding: unspecified |
|
| Participants | 70 ASA 1 to 3 patients (aged 18 to 70 years) undergoing liver or gallbladder cancer surgery through right subcostal incision | |
| Interventions |
Treatment group: TEA (T9‐T10 with the catheter inserted 5 cm passed the needle tip) with ropivacaine 0.2% 7 mL/h intraoperatively and 5 mL boluses for an unspecified duration after surgery (n = 35) Control group: IV morphine (n = 35) General anaesthesia for all participants |
|
| Outcomes | Vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
St‐Onge 1997.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Canada Funding: industry (in part) |
|
| Participants | 60 ASA 1 to 3 patients (aged 18 to 75 years) undergoing elective abdominal surgery, vascular or bowel resection surgery, requiring a midline incision | |
| Interventions |
Treatment group: TEA (T10‐L1) with bupivacaine 0.05% (n = 20) or 0.1% (n = 20) and meperidine 1 mg/mL adjusted for VAS score, 4/10. These 2 groups were fused. Exact duration unspecified, taken as 48‐hour duration of data collection for epidurally administered meperidine Control group: TEA with meperidine 1 mg/mL only (n = 19) General anaesthesia for all participants |
|
| Outcomes | VAS scores at rest 24 (period 24 to 36 hours taken as 24 hours) and 48 hours (period 36 to 48 hours taken as 48 hours) VAS scores on movement (sitting) (period 24 to 36 hours taken as 24 hours) and at 48 hours (period 36 to 48 hours taken as 48 hours) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind" ‐ The infusion solution was prepared by the hospital pharmacy, and the anaesthetist and the investigator were blinded to which solution was used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐blind" ‐ The infusion solution was prepared by the hospital pharmacy and the anaesthetist, and investigators were blinded to which solution was used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was excluded because of complications unrelated to the investigation in the immediate postoperative period. Six participants did not complete the study, but each 12 hours of completed data was kept for analysis. Of the 6 participants, 1 had the technique interrupted because of surgical complications (haemorraghic shock), 2 had epidural catheter dislodgement and 3 (2 in the 0% and 1 in the 0.10% group) did not complete the 48 hours because of unsatisfactory analgesia in spite of a well‐positioned catheter |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Supported, in part, by Faulding (Canada) Inc, Vaudreuil, Quebec, and Nellcor Inc, Hayward, California Not in intention‐to‐treat |
Steinberg 2002.
| Methods | RCT Protocol approved by the ethics committee at each of the 5 centres and informed written consent obtained Setting: United States of America Funding: industry |
|
| Participants | ASA physical status classification 4, age 18 to 80 years, weight 50 to 110 kg patients undergoing elective partial colon resection at 5 institutions | |
| Interventions |
Treatment group: TEA ( T7‐T10; PCEA with catheter inserted 3 to 5 cm cephalad) with ropivacaine 0.2% and fentanyl 2 mcg/mL adjusted for resting VAS scores < 5/10 until the predetermined discharge criterion of adequate pain control with oral medication was met, or a maximum of 6 days (n = 20) Control group: IV PCA with morphine (n = 21) General anaesthesia for all participants. Ketorolac was given intramuscularly or IV to both groups as a supplementary analgesic. Treatment for relief of nausea or vomiting was administered at the discretion of the investigator |
|
| Outcomes | Time to first flatus Time to first bowel movement Vomiting (first 24 hours) |
|
| Notes | Because of the slow accrual of participants, study was terminated before enrolment of the desired 120 participants Time to achieved discharge milestones approximately 1 day sooner than in the IV PCA group (P value < 0.002). Standardized criteria required that participants be afebrile (T 37.7°C), able to ingest sufficient fluid PO to maintain hydration, maintain adequate pain control with oral medications and achieve recovery from ileus (passage of flatus). Participants were assessed twice daily, usually concurrently with ambulation and pain assessments, for completion of discharge criteria Study authors contacted for additional information on 22 July 2014; referred us to AstraZeneca, which did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "open" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "open" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 48 participants were randomized to the study, and 41 completed the protocol |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Supported by a grant from AstraZeneca LP, Sodertalje, Sweden Not in intention‐to‐treat |
Stevens 1998.
| Methods | RCT Approved by the Institutional Review Board and written informed consent obtained Setting: United States of America Funding: unspecified |
|
| Participants | 40 ASA 1 to 3 patients with adenocarcinoma of the prostate undergoing prostatectomy | |
| Interventions |
Treatment group: TEA (T10‐T11 or T11‐T12) with bupivacaine 0.5% during surgery and for 24 hours after surgery plus a bolus of morphine 4 mg (single bolus at the end of surgery) (n = 19) Control group: IV PCA with morphine (n = 21) General anaesthesia for all participants. Ketorolac (30 mg) was given intravenously to participants in both groups at the end of surgery |
|
| Outcomes | Time to first flatus (hours) Time to first bowel movement (hours) Hospital LOS (When the participant had consumed 2 clear liquid meals without vomiting, a general (solid food) diet was begun. When the participant had eaten 2 solid meals without vomiting, had acceptable analgesia (VAS pain score < 3 out of 10) when taking hydrocodone/acetaminophen, was haemodynamically stable, could ambulate without assistance, had no ongoing surgical or anaesthetic complications, had a temperature less than 38°C and had normal healing of the surgical incision, the participant was deemed to have met discharge criteria |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced except more ASA 3 participants (6 vs 1) in the epidural group |
Subramaniam 2000.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: India Funding: unspecified |
|
| Participants | 80 ASA 1 to 3 patients undergoing upper abdominal and thoracic surgery Exclusion criteria were history of drug or alcohol abuse, daily intake of opioids and major systemic illnesses and chronic pain |
|
| Interventions |
Treatment group: lumbar epidural (L2‐L3 or L3‐L4) morphine 50 mcg/kg plus bupivacaine 10 mg in 10 mL saline given before (n = 20) or after surgery (n = 20) Control group: lumbar epidural (L2‐L3 or L3‐L4) morphine 50 mcg/kg in 10 mL saline given before (n = 20) or after surgery (n = 20) General anaesthesia and supplemental epidural morphine as needed after surgery for all participants |
|
| Outcomes | Pain at rest and on coughing: "The pain scores postoperatively were comparable for all four subgroups. All the groups had median pain scores of less than or equal to four in the first three days" | |
| Notes | Thoracic and abdominal surgery. Study authors contacted 23 February 2016, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized", "using a random generator table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind", "10 mL syringes were given to the anaesthetist, one for preoperative and the other for postoperative administration. One of the study investigators, blinded to the administered drug, was responsible for drug administration and postoperative follow‐up" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐blind", "10 mL syringes were given to the anaesthetist, one for preoperative and the other for postoperative administration. One of the study investigators, blinded to the administered drug, was responsible for drug administration and postoperative follow‐up" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Taqi 2007.
| Methods | RCT Approved by the ethics board and written consent obtained from all patients Setting: Canada Funding: charity |
|
| Participants | 50 patients scheduled for elective laparoscopic colorectal surgery for benign and malignant colorectal lesions | |
| Interventions |
Treatment group: TEA (T8‐T9) with bupivacaine 0.5% and 0.25% during surgery and bupivacaine 0.1% plus fentanyl 3 mcg/mL for 72 hours after surgery (n = 25) Control group: IV PCA with morphine (n = 25) General anaesthesia for all participants. Both groups also received 500 mg naproxen twice a day orally or rectally for 4 days, and acetaminophen 1 gram 4 times a day for 4 days |
|
| Outcomes | Time to first flatus Time to first faeces Vomiting VAS at rest and on movement (walking) at 24 and 48 hours |
|
| Notes | Study authors contacted for additional information on 12 July 2014, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "patients were not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All the patients enrolled in the research project completed the study" "There were no epidural failures (dislodgement, leak, disconnection) during the postoperative period" |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Demographic characteristics and clinical data related to preoperative health status, diagnosis and type of surgery, and preoperative nutritional status, were similar in the 2 groups |
Thorén 1989.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: Sweden Funding: governmental |
|
| Participants | 22 ASA 1 or 2 female patients undergoing hysterectomy | |
| Interventions |
Treatment group: TEA (T12‐L1 with the catheter inserted 2 to 3 cm into the epidural space) with bupivacaine 0.5% during surgery, and 0.25% 8 mL/h for 42 hours (n = 11)
Control group: TEA (T12‐L1 with the catheter inserted 2 to 3 cm into the epidural space) with morphine 4 mg bolus, 2 mg on request for 42 hours (n = 11) All participants operated under general anaesthesia |
|
| Outcomes | Time to first flatus (hours) Time to first faeces (hours) VAS scores at day 1 (taken as at rest) Length of hospital stay (days) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Before operation, participants were randomly allocated to 2 groups |
| Allocation concealment (selection bias) | Unclear risk | Before operation, participants were randomly allocated to 2 groups |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Low risk | Groups well balanced |
Tsui 1997.
| Methods | RCT Approved by the ethics committee and written informed consent obtained from all patients Setting: China Funding: unspecified |
|
| Participants | 120 ASA class 1 or 2 female patients scheduled for gynaecological lower abdominal operations through a vertical midline incision | |
| Interventions |
Treatment group: LEA (L2‐L3 or L3‐L4) with bupivacaine 0.0625% and fentanyl 3.3 mcg/mL adjusted for VAS scores < 3/10 at rest for 48 hours (n = 57) Control group: IV PCA with morphine adjusted for VAS scores < 3/10 at rest (n = 54) General anaesthesia for all participants |
|
| Outcomes | Vomiting | |
| Notes | 32% of participants in the epidural group had limb weakness Study authors contacted for additional information. Replied that original data are no longer available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | "Before the preoperative visit, patients were randomly allocated" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 dropped out owing to blockage of the IV cannula for PCA administration (n = 5), extrusion of the epidural catheter during the study period (n = 2) or failure to receive a vertical midline incision (n = 2) |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Tuman 1991.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: United States of America Funding: unspecified |
|
| Participants | 80 adult patients undergoing major vascular surgery of the abdominal aorta and lower extremities Exclusion criteria were preoperative coagulopathies or liver disease, receiving anticoagulant or antiplatelet medications and any contraindications to use of an epidural catheter, or any of the standard anaesthetic/analgesic agents employed |
|
| Interventions |
Treatment group: high lumbar or low thoracic (L3‐T10) tested with 3 mL of 1.5% lidocaine with epinephrine 5 mcg/mL, lidocaine 1.5% during surgery (12 to 20 mL) followed by an infusion with 0.1% bupivacaine and fentanyl 0.001% after surgery for an average duration of 2.40 ± 0.98 postoperative days (n = 40) Control group: no epidural and parenteral and/or oral opioid analgesics as requested for pain relief after surgery (n = 40) General anaesthesia for all participants |
|
| Outcomes | Pain: "The average pain scores in the epidural local anaesthetic group on the first three postoperative days were 2.3 ± 2.6, 1.1 ± 1.4, and 0.8 ± 1.0, respectively, as measured on a visual analogue scale from 0 to 10" Length of hospital stay: "The duration of hospital stay after surgical procedures performed under combined general/epidural anaesthesia was 10.7 ± 6.8 days compared with 13.2 ± 11.7 days in those receiving general anaesthesia alone (P = 0.565)" |
|
| Notes | Possibly included surgery on lower extremities. Requested aortic abdominal surgery participant data (for another Cochrane review: Guay J, Kopp S. Epidural pain relief versus systemic opioid‐based pain relief for abdominal aortic surgery. Cochrane Database of Systematic Reviews 2016, Issue 1. Art. No.: CD005059), but study authors did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned: "The physicians and nurses involved in the care of the patients were unaware of the outcome variables that were being monitored" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned: "Data were collected by a full‐time data manager" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | High risk | "The study groups are similar in terms of preoperative characteristics, chronic drug therapy, and surgical variables, except for a greater incidence of diabetes mellitus and prior myocardial infarction in the epidural group" |
Turunen 2009.
| Methods | RCT Approved by the ethics committee and written informed consent obtained from each patient Setting: Finland Funding: unspecified |
|
| Participants | 60 ASA physical status score of 1 to 3 consecutive elective patients with complicated diverticular disease (1 acute episode in patients younger than 50 years, and 2 in older patients, or a preoperative stricture) undergoing laparoscopic sigmoid resection | |
| Interventions |
Treatment group: TEA (T10‐T11) with ropivacaine 0.5% during surgery and ropivacaine 0.2% for 48 hours after surgery (n = 29) Control group: IV or IM oxycodone (n = 29) General anaesthesia for all participants. All participants received daily doses of ketoprofen including three 100‐mg doses administered intravenously (IV) or orally, 4 paracetamol 1‐g doses given IV or orally and, if needed, oxycodone 0.05 mg/kg IV or 0.15 mg/kg administered intramuscularly |
|
| Outcomes | Time to first flatus VAS scores on movement at 24, 48 and 72 hours Leak |
|
| Notes | Study authors contacted for additional information on 12 July 2014, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization in blocks of 10" |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant from each group was excluded because of a missing self care questionnaire |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced. "The data were analyzed on an intention‐to‐treat basis, which means that patients with unsuccessful epidural analgesia were counted as epidural analgesia patients" |
Tyagi 2011.
| Methods | RCT Approved by Institutional Revie\v Board and informed written consent obtained from all patients Setting: India Funding: departmental resources only |
|
| Participants | 66 patients of ASA physical status 2 to 3, aged 18 to 65 years, scheduled for emergency laparotomy in view of peritonitis due to perforation in the small intestine | |
| Interventions |
Treatment group: TEA (T8‐T9 or T9‐T10 and catheter advanced 3 cm passed the needle tip) with bupivacaine 0.125% plus fentanyl during surgery and bupivacaine 0.125% thereafter for 48 hours (n = 33) Control group: IV tramadol (n = 33) General anaesthesia for all participants |
|
| Outcomes | Time to first flatus Time to first faeces Leak Hospital LOS |
|
| Notes | "None of the patients in group GT developed epidural abscess or meningitis post‐operatively" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sealed opaque envelopes" |
| Allocation concealment (selection bias) | Low risk | "Sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | For the 2 participants with failed epidural blockade, missing data were replaced by the "worst possible recorded outcome in group GT" The 2 groups were similar with regard to demographic profiles and baseline haemodynamic parameters, signs of SIRS, ASA status 2 and 3 , modified APACHE score and MPI score 2 failed‐block analysed in intention‐to‐treat |
Voylenko 2013.
| Methods | RCT Setting: Czech Republic Funding: unspecified |
|
| Participants | 41 patients undergoing nephron sparing surgery for renal cell carcinoma through transabdominal approach without central ischaemia Exclusion criteria were procedures involving the kidney pelvic system and significant co‐morbidities |
|
| Interventions |
Treatment group: intraoperatively: epidural anaesthesia with 0.125% bupivacaine 8 to 9 mL/h, postoperatively: use of epidural analgesics (exact number unclear) Control group: not clearly mentioned (exact number unclear) Unclear whether general anaesthesia was used for all participants Number of participants was entered as 20 in each group for calculation of number of participants included in the review |
|
| Outcomes | Pain: "pain levels in the study group were insignificantly lower to control: 2.9 ± 1,1 points versus 3.5 ± 1,6 points (T‐test; P = 0.21)" Length of hospital stay: "Postoperative hospital stay was significantly shorter for study group 4.1 ± 0.8 days versus 6.8 ± 1.1 days (T‐test; P < 0.001)" |
|
| Notes | Conference abstract The abstract does not clearly state whether participants in the control group had an epidural. No contact address is provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised control trial", no detail |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Limited information |
| Other bias | High risk | Groups well balanced: "Patients age was 52.8 ± 10.5 and 51 ± 12.8 respectively in study and control groups. Mean tumour size was 3.4 ± 0.5 cm versus 3.5 ± 0.4 cm; mean glomerular filtration rate 90.1 ± 178 versus 89.7 ± 18.2 mL/min and "ECOG (not defined in the abstract)" status 0.67 ± 0,5 versus 0.72 ± 0.57 for study and control group respectively" Epidural was part of a "fast track" programme that included preoperatively: no bowel preparation, nutrition with high‐carb diet shakes 5 to 7 hours before surgery and cancellation of premedication with narcotic analgesics; intraoperatively: epidural anaesthesia with 0.125% bupivacaine 8 to 9 mL/h, use of non‐steroidal anti‐inflammatory drugs, minimally invasive approach and no drain policy; postoperatively: early (6 hours) start of oral nutrition, early participant mobilization, use of epidural analgesics and no narcotics policy |
Wallin 1986.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Sweden Funding: governmental (plus a contribution from industry) |
|
| Participants | 30 patients scheduled for elective cholecystectomy; 3 loss to follow‐up (27 analysed: 17 female and 10 male) | |
| Interventions |
Treatment group: TEA (T12‐L1; catheter inserted 3 to 4 cm passed the needle tip) with bupivacaine 0.5% started before the incision, and 0.25% intermittent injection of 10 to 14 mL every 3 hours for 24 hours after surgery (n = 12)
Control group: postoperative IM pentazocine 30 to 60 mg on request (n = 15) General anaesthesia for all participants |
|
| Outcomes | Time of first flatus (hours) Time of first stool (hours) |
|
| Notes | Study authors contacted for additional information on 23 July 2014, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the Mudy was randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 in the epidural group lost to follow‐up (failed epidural) |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat (n = 12 for epidural in the results) |
Wang 2010.
| Methods | RCT Informed consent obtained Setting: China Funding: departmental resources |
|
| Participants | 40 patients undergoing radical colon resection for cancer (right hemicolectomy/transverse colon resection/descending colon resection/sigmoid resection) | |
| Interventions |
Treatment group: epidural analgesia as the main anaesthetic method followed by bupivacaine 1.125 mg/mL and morphine 0.04 mg/mL at the rate of 2.0 mL/h for less than 24 hours (n = 20) Control group: meperidine or tramadol (n = 20) General anaesthesia for all participants |
|
| Outcomes | Time to first flatus ("functional recovery of the intestinal tract") | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "divided in two groups", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | Unclear, abstract only. Other modalities of therapy might have differed |
Watters 1993.
| Methods | RCT Approved by the research ethics committee and informed written consent obtained from each participant Setting: Canada Funding: governmental |
|
| Participants | 20 consecutive adult patients of either sex who were scheduled to undergo elective colorectal resection | |
| Interventions |
Treatment group: LEA (lower lumbar level) with 2% lidocaine and epinephrine 5 mcg/mL intraoperatively only and epidural morphine after surgery (n = 12) Control group: IV/IM morphine/meperidine (n = 8) General anaesthesia for all participants |
|
| Outcomes | VAS scores (unspecified, taken as at rest) at 6, 24 and 48 hours | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sealed envelopes prepared from a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes prepared from a table of random numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up "All epidural catheters were judged clinically by the attending anaesthetist to be functioning well" |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Wattwil 1989.
| Methods | RCT Approved by the ethics committee and informed consent obtained Setting: Sweden Funding: unspecified |
|
| Participants | 40 ASA 1 or 2 women undergoing hysterectomy | |
| Interventions |
Treatment group: TEA (T12‐L1) with bupivacaine 0.5% started before the surgical incision, and 0.25% 8 mL/h for 26 to 30 hours (n = 20). Bolus to maintain T6 sensory level
Control group: IM ketobemidone 5 mg (n = 20) General anaesthesia for all participants |
|
| Outcomes | Time to first flatus Time to first stool VAS scores at day 1 (taken as at rest although not clearly specified). Length of hospital stay |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Before operation, the patients were randomly allocated to two groups", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No sham catheter mentioned (data collected by ward nurses) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Welch 1998.
| Methods | RCT Approved by the institutional review committee and written informed consent obtained Setting: United States of America Funding: unspecified |
|
| Participants | 59 ASA class 1 or 2 patients undergoing elective major colorectal surgery under general anaesthesia | |
| Interventions |
Treatment group: bupivacaine 0.1% and morphine 0.1 mg/mL started in PACU for an unspecified duration (n = 30) Control group: IM morphine/meperidine (n = 29) All participants received general anaesthesia |
|
| Outcomes | Time to first faeces (bowel function) Time to first flatus (ileus) Cost |
|
| Notes | No catheter‐related complications or episodes of respiratory depression were reported Study authors contacted for additional information on 26 July 2014, but did not reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | No details |
Wiedemann 1991.
| Methods | RCT Informed consent obtained Setting: Germany Funding: unspecified |
|
| Participants | 28 male patients undergoing upper abdominal surgery (mainly proximal vagotomy for duodenal ulcer) | |
| Interventions |
Treatment group: thoracic epidural (T7‐T9, inserted the day before surgery through a paramedian approach) with 12 to 14 mL of 0.5% bupivacaine followed by 12 to 14 mL/h of bupivacaine (0.25% for 12 to 18 hours, then 0.125%) started before induction and continued for 48 hours (targeted sensory level T4) (n = 8) Control groups: thoracic epidural morphine 4 to 5 mg 30 minutes before induction and every 12 hours (n = 10) or IM piritramide Neuroleptanaesthesia with etomidate, succinylcholine, fentanyl, droperidol, nitrous oxide and pancuronium |
|
| Outcomes | Pain scores at rest at 6, 24 and 48 hours (scale 0 to 4) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned No failed epidural mentioned but groups unequal (8 vs 10 and 10) |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Yeager 1987.
| Methods | RCT Approved by the ethics committee and written informed consent obtained Setting: United States of America Funding: unspecified |
|
| Participants | 55 adult patients undergoing intra‐abdominal or intrathoracic surgery requiring intensive care unit admission Exclusion criteria were contraindication to insertion of an epidural catheter (localized infection, septicaemia, preoperative coagulopathy) |
|
| Interventions |
Treatment group: thoracic or lumbar (according to surgical site) epidural (catheter inserted before surgery) analgesia with intraoperative administration of local aesthetics in sufficient dose and concentration to achIeve and maintain surgical anaesthesia and muscle relaxation (bupivacaine 0.75% or lidocaine 1.5% with epinephrine 5 mcg/mL) Postoperatively, physicians caring for participants utilized the epidural catheter for pain relief with analgesic concentrations of local anaesthetics and/or epidural administration of narcotics, avoiding use of parenteral narcotics for an average duration of 31 hours (range: 8 to 79 hours) (n = 28) Control group: intermittent opioids as required administered parenterally during intensive care unit stay and parenterally or orally thereafter (n = 25) General anaesthesia for all participants |
|
| Outcomes | Length of hospital stay: "11.4 ± 4.6 days in hospital for epidural group participants compared with 15.8 ± 12.3 days in hospital for opioids group participants" Total cost: "The average hospital cost for group epidural patients ($11,218 ± 5,738) was also significantly less than the average cost for group opioids patients ($20,380 ± 20,343) (P = 0.02). The average physician costs for group epidural patients ($3,801 ± 1,342) was less than group opioids patients ($5,134 ± 2,939) (P = 0.05)" |
|
| Notes | Participants undergoing intrathoracic, intra‐abdominal or major (non‐cerebral) vascular surgery. Data not extractable separately. Study authors contacted on 23 February 2016, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized from a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 (opioid group) participants dropped after randomization because surgery was cancelled "Three patients in group epidural did not have a functioning epidural catheter; one, due to technical failure, and two, because a catheter was never inserted by independent decision of the anaesthesiologist in charge of the case"; those participants were kept for analysis |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced Intention‐to‐treat |
Zeng 2003.
| Methods | RCT Setting: China Funding: departmental resources |
|
| Participants | 42 ASA 1 to 2 patients, aged 37 to 70 years, with no preoperative immune or endocrine disease and scheduled for elective abdominal surgery of an expected duration < 4 hours |
|
| Interventions |
Treatment groups: TEA or LEA (according to surgical site) inserted before induction and tested with 4 mL of lidocaine 2%, bupivacaine 0.33% 4 to 6 mL during surgery followed by ropivacaine 0.2% and fentanyl 2 mcg/mL as patient‐controlled analgesia (background infusion 4 mL/h; bolus 2 mL; lockout time 20 minutes) (n = 9) or bupivacaine 0.12% plus fentanyl 2 mcg/mL (same settings) (n = 8) or bupivacaine 0.12% plus morphine 0.08 mg/mL (same settings) (n = 9) for 24 hours Control group: IV morphine (n = 8) General anaesthesia with propofol, fentanyl, nitrous oxide, isoflurane and vecuronium |
|
| Outcomes | Pain scores at 24 hours after surgery (taken as at rest) | |
| Notes | Study includes a fourth treatment group, with intraoperative bupivacaine followed by IV morphine after surgery. This group was not retained in the analysis. Owing to the small number of participants in the control group (n = 8), the 3 treatment groups were fused for analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Low risk | Groups well balanced |
Zhu 2013.
| Methods | RCT Approved by the ethics committees and written informed consent obtained for all patients Setting: China Funding: unspecified |
|
| Participants | 67 patients undergoing D2 (systematic dissection of lymph nodes in the second tier with clear histological margins.) radical gastrectomy for gastric cancer | |
| Interventions |
Treatment group: TEA (T8‐T9) PCEA with bupivacaine 0.05% and morphine 0.1 mg/mL for 48 hours (n = 34) Control group: IV PCA with morphine (n = 33) General anaesthesia for all participants; pethidine was used as a supplemental drug for breakthrough pain in both groups |
|
| Outcomes | Time to first flatus (hours) VAS at rest at 24 and 48 hours VAS on movement (coughing) at 24 and 48 hours Anastomotic leak Hospital LOS (Predetermined discharge criteria were used to measure length of hospital stay after the operation. Criteria were defined as eating a normal diet, tolerating clear fluids for 24 hours, having no complaints of pain, providing no evidence of complications for 24 hours and obtaining consent from the participant) |
|
| Notes | Study authors contacted for additional information on 13 July 2014, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants excluded from analysis |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Zutshi 2005.
| Methods | RCT Approved by the institutional review board and informed consent obtained Setting: United States of America Funding: unspecified |
|
| Participants | Patients undergoing elective segmental intestinal resection by laparotomy. Reoperative cases and patients with co‐morbidities were included | |
| Interventions |
Treatment group: TEA (PCEA) (T8‐T9 or T9‐T10) with bupivacaine and fentanyl for 48 hours (n = 28). Concentrations unspecified Control group: IV PCA (n = 31). Drug unspecified General anaesthesia plus ketorolac and antiemetic prophylaxis for all participants |
|
| Outcomes | Time to first faeces VAS scores (taken as at rest) at 24 and 48 hours Hospital LOS (Before discharge, all participants passed flatus or stool, were comfortable on oral analgesia, could stand and walk independently and had tolerated 3 successive solid meals) Costs |
|
| Notes | Study authors contacted for additional information on 26 July 2014, but did not reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed using sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data are missing for some outcomes |
| Selective reporting (reporting bias) | Low risk | All results provided |
| Other bias | Unclear risk | Groups well balanced Lack of details on the content of epidural solution and of IV opioids Intention‐to‐treat outcomes retained for this review |
APACHE: Acute Physiology and Chronic Health Evaluation; ARDS: adult respiratory distress syndrome (acute lung injury); ASA: American Society of Anesthesiologists; B: bupivacaine; BP: blood pressure; d:day; EA: epidural anaesthesia or analgesia; EM: epidural morphine; G: gram; Gr: group; IM: intramuscular; IV: intravenous; kg: kilogram; L: litre; LEA: lumbar epidural anaesthesia or analgesia; LOS: length of stay; M: morphine; mcg: microgram; mL: millilitre; MPI: Mannheim Peritonitis Index score; NCT: number of clinical trial; NSAID: non‐steroidal anti‐inflammatory drug; PCA: patient‐controlled analgesia; PCEA: patient‐controlled epidural analgesia; RCT: randomized controlled trial; SD: standard deviation; SIRS: systemic inflammatory response syndrome; T: thoracic; TEA: thoracic epidural anaesthesia or analgesia; VAS: visual or verbal analogue pain score; y: year
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akarsu 2012 | No outcome of interest measured |
| Ali 2010 | Different population: participants undergoing thoracic or thoraco‐abdominal surgery |
| Aono 1998 | No outcome of interest measured |
| Aribogan 2003 | Different intervention: tramadol injected epidurally |
| Asantila 1991 | Different intervention: all participants received 20 mL of 0.5% bupivacaine at the insertion of the catheter, and randomization started after surgery only |
| Axelsson 2005 | Different study population: knee surgery |
| Badner 1994 | Different intervention: Some participants in all groups (fentanyl alone or with a local anaesthetic) might have received local anaesthetic during surgery: "Intraoperatively, the epidural catheter was used for anaesthesia at the discretion of the attending anaesthetist", and participants were randomized at wound closure |
| Baron 1991 | Different intervention: Participants from both groups may have received epidural local anaesthetic postoperatively |
| Basse 2002 | Different intervention: Both groups had an epidural with a local anaesthetic |
| Beeby 1984 | Different intervention: cesarean section operated under epidural anaesthesia; therefore, all participants received local anaesthetic epidurally |
| Bigler 1989 | Different intervention: 2 groups with local anaesthetics: epidural vs paravertebral blockade |
| Bloch 1989 | Different intervention: epidural anaesthesia vs general anaesthesia for surgery |
| Bowdle 1997 | Not an RCT: post hoc analysis of individuals who had participated in a multi‐centre trial |
| Brandt 1976 | Not an RCT: Participants were divided into 2 groups, but the word 'randomized' was not found anywhere in the report |
| Brandt 1978 | Different intervention: epidural anaesthesia without general anaesthesia vs general anaesthesia |
| Bredtmann 1990 | Not an RCT: Randomization was performed by allotting participants who had surgery on odd‐numbered days to the group receiving thoracic epidural analgesia plus general anaesthesia (group I, n = 57), and participants scheduled for surgery on even‐numbered days to the group receiving general anaesthesia without epidural block (group II, n = 59) |
| Bredtmann 1991 | Different intervention: no group without local anaesthetic |
| Bridenbaugh 1976 | Different intervention: no group without local anaesthetics |
| Brodner 2000 | Different intervention: all participants received a local anaesthetic through an epidural catheter |
| Bromage 1955 | Cross‐over trial |
| Bromage 1971 | Different study population: thoracic and abdominal surgery. No outcomes of interest measured |
| Brownridge 1985 | Different intervention: all participants received local anaesthetics for surgery |
| Buckley 1978 | Different intervention: study of different solutions of epidural etidocaine to participants undergoing gynaecological surgery. Excluded as epidural local anaesthetic was not compared with an opioid‐based regimen |
| Buckley 1982 | No outcome of interest measured |
| Carli 1991 | No outcome of interest measured: study on the effect of perioperative epidural local anaesthetic on whole body protein turnover and urinary excretion of urea nitrogen, adrenaline, noradrenaline and cortisol. Excluded as the study was not relevant to this review |
| Carli 1995 | No outcome of interest measured |
| Casati 2002 | No outcome of interest measured |
| Catley 1985 | Not an RCT: "allocated" to 2 groups (the word 'random' does not appear in the report). Groups were "matched" for age, weight, height and type of surgery; the word 'prospective' does not appear in the report |
| Chestnut 1986 | Different intervention: all participants had surgery under epidural anaesthesia with local anaesthetics |
| Cooper 1996 | Different intervention: all participants operated under epidural anaesthesia and randomized after surgery |
| Cowen 1982 | Different intervention: all participants received epidural local anaesthetics during surgery |
| Crews 1999 | Different intervention: all participants received epidural analgesia containing levobupivacaine during surgery and were randomized to 3 groups only after surgery |
| Dahl 1992 | Different intervention: all participants received an epidural with bupivacaine and opioids during surgery |
| De Kock 1999 | Different intervention: not compared with an opioid‐based regimen |
| Delilkan 1993 | Different intervention: compared with epidurally administered tramadol |
| Dupont 1987 | Different population: children |
| Dyer 1992 | Different intervention: all participants received intraoperative local anaesthetics |
| Engquist 1977 | No outcome of interest measured |
| Engquist 1980 | Not an RCT: The word 'randomized' is not mentioned anywhere in the report |
| Etches 1997 | Different intervention: all participants received local anaesthetics during surgery |
| Feo 2009 | Not an RCT: retrospective |
| Frings 1982 | Different intervention: epidural opioids vs systemic opioids |
| Fujita 2011 | Different intervention: both groups received postoperative epidural ropivacaine and fentanyl |
| Garnett 1996 | No outcome of interest measured |
| Geddes 1991 | Different intervention: all participants received epidural local anaesthetics for surgery |
| Gelman 1977 | Not an RCT: participants were divided into 3 groups, but the word 'randomized' is not mentioned anywhere in the report |
| Gelman 1980 | No outcome of interest measured |
| George 1992 | Different intervention: all participants received 8 mL of 0.5% bupivacaine at catheter insertion and were randomized after surgery |
| Gold 1994 | No outcome of interest measured |
| Gow 1979 | Not an RCT |
| Grass 1992 | Not an RCT: retrospective study |
| Grass 1993 | Different intervention: participants received epidural fentanyl with or without ketorolac. Excluded as no group received epidural local anaesthetic |
| Gulucu 2009 | Different intervention: no group given a local anaesthetic |
| Gupta 2006 | Different intervention: all participants received local anaesthetics during the intraoperative period |
| Han 2005 | Different intervention: all groups with an epidural received something other than a local anaesthetic or an opioid in the epidural (phenergan, droperidol and ondansetron or tramadol) |
| Harukuni 1995 | No outcome of interest measured |
| Hashimoto 1995 | No outcome of interest measured |
| Hendolin 1982 | No outcome of interest measured |
| Hendolin 1987a | No outcome of interest measured |
| Hendolin 1987b | No outcome of interest measured |
| Her 1990 | Not an RCT: participants assigned to general anaesthesia with or without an epidural according to their preference for having an epidural or not |
| Hjortso 1986 | Different intervention: postoperative epidural bupivacaine with or without morphine |
| Houweling 1992 | No outcome of interest measured: study comparing intraoperative haemodynamic changes in epidural bupivacaine vs epidural sufentanil. Excluded as no postoperative outcomes were presented |
| Hull 1991 | Different intervention: study comparing 2 surgical techniques |
| Inoue 2005 | Different intervention: all participants received epidural local anaesthetics during surgery |
| Jones 2013 | Different intervention: all participants received an epidural with local anaesthetics for surgery |
| Jorgensen 1978 | Different intervention: all participants received epidural local anaesthetics |
| Kabon 2003 | No outcome of interest measured |
| Kajiyama 2004 | Not an RCT: this is an observational study. No abdominal surgery participants included. Not eligible |
| Kapral 1996 | Different intervention: study compares intraoperative gastric intramucosal CO2 as a measure of visceral perfusion to obtain an indirect measure of surgical stress response |
| Kausalya 1994 | Different intervention: general anaesthesia vs regional anaesthesia |
| Kilbride 1992 | Different intervention: no group was given epidural local anaesthetic |
| Kiya 2003 | Different intervention: all participants received local anaesthetics during surgery |
| Koganemaru 1996 | Different intervention: all participants received epidural mepivacaine |
| Korinek 1985 | Different intervention: all participants received epidural local anaesthetics for surgery |
| Kossman 1982 | No outcome of interest measured |
| Kouraklis 2000 | No outcome of interest measured |
| Krane 1987 | Different study population: children |
| Krane 1989 | Different study population: children |
| Kumar 1993 | Different study population: children |
| Lattermann 2001 | No outcome of interest measured |
| Lattermann 2002 | No outcome of interest measured |
| Lattermann 2003 | No outcome of interest measured |
| Lee 1988 | Different intervention: all participants received 20 mL of 2% lidocaine at catheter insertion plus 10 mL of 0.5% bupivacaine 1 hour later or at wound closure if earlier and were randomized at the end of surgery |
| Lee 1991 | Different intervention: all participants received local anaesthetics |
| Licker 1995 | No outcome of interest measured |
| Liu 2005 | Different intervention: droperidol added to the epidural solution |
| Lowson 1994 | Different intervention: all participants received an epidural with 0.5% bupivacaine during surgery |
| Madej 1992 | Different intervention: all participants received 0.5% bupivacaine during surgery |
| Manikian 1988 | Not an RCT |
| Marco 1989 | Different study population: children |
| Matsunaga 1996 | Different intervention: all participants received epidural buprenorphine with bupivacaine |
| Mellbring 1983 | No outcome of interest measured |
| Modig 1981 | Different study population: hip replacement |
| Moine 1992 | Different study population: children |
| Moller 1982 | Not randomized? The word 'random' is not mentioned in the report. No outcome of interest measured |
| Morley 2002 | No outcome of interest measured |
| Moselli 2011 | Different intervention: all participants received epidural analgesia with a local anaesthetic after surgery |
| Moskovitz 1986 | Not an RCT |
| Muneyuki 1968 | Not an RCT: participants are said to have been divided into 2 groups, but the word 'randomized' does not appear in the report |
| Murakami 2009 | Different intervention: participants form group general anaesthesia/epidural anaesthesia/analgesia received droperidol in the epidural space |
| Murrat 1988 | Different study population: children |
| Mushambi 1992 | Different intervention: study on gastric emptying (paracetamol absorption test) after general anaesthesia for minor gynaecological surgery. Excluded as no participants had epidural local anaesthetic |
| Naesh 1994 | No outcome of interest measured |
| Nandate 2003 | No outcome of interest measured |
| Niiyama 2005 | Different intervention: all participants received epidural local anaesthetics intraoperatively |
| Nimmo 1978 | Not an RCT |
| Nishikawa 2007 | Different intervention: buprenorphine in the epidural solution |
| Nishiyama 1991 | Different intervention: this study compares postoperative epidural midazolam with and without local anaesthetics |
| Noreng 1987 | Not an RCT? The word 'random' does not appear in the report. No outcome of interest measured |
| Norris 2001 | Different intervention: all participants received epidural local anaesthetics intraoperatively. "Patients who received general anaesthesia intraoperatively received 6 ml of 0.25% bupivacaine" |
| O'Connor 2001 | Not an RCT: "Limitations include retrospective nature of this study" |
| Ohtaka 1991 | Different intervention: this study compares postoperative epidural buprenorphine with and without local anaesthetics for upper abdominal surgery |
| Olofsson 1997 | Different intervention: all participants received neuraxial local anaesthetics |
| Omar 2009 | Different intervention: excluded because the study provided epidurally administered ketamine |
| Osipova 2002 | No outcome of interest measured |
| Paech 1994 | Different intervention: both groups received bupivacaine to induce a T4 level for surgery |
| Parker 1992 | Different intervention: all participants operated under epidural anaesthesia |
| Petring 1984 | Different population: surgeries on the extremities |
| Poopalalingam 2003 | Different intervention: all participants received local anaesthetic during surgery and in the post‐anaesthesia care unit. Randomization started thereafter only |
| Porter 1997 | Different population: labor analgesia |
| Pouzeratte 2001 | Different intervention: the 3 groups received an epidural with local anaesthetics during surgery |
| Rademaker 1992 | Not an RCT: quasi‐randomized: "on an alternating basis" |
| Randalls 1991 | Different intervention: all participants received local anaesthetic for surgery |
| Rawal 1984 | Different intervention: epidural local anaesthetics were given to all participants during surgery and to none after surgery |
| Reinhart 1989 | No outcome of interest measured |
| Reiz 1982 | No outcome of interest measured |
| Renck 1975 | Different intervention: all participants received local anaesthetics |
| Rucci 1985 | Different intervention: all participants received local anaesthetics |
| Ryan 1992 | Different intervention? Mode of analgesia in the control group unspecified |
| Saito 1995 | No outcome of interest measured |
| Sakaguchi 1995 | Different population (includes participants 12 years of age and older) and different intervention (all participants may have received epidural local anaesthetics during surgery) |
| Salman 2013 | Not an RCT: quasi‐randomized trial: "randomized as follows: each patient was given a number according to chronological order beginning from 1. Intravenous analgesia with meperidine was used in odd numbered patients (n=40), and epidural analgesia with bupivacaine was used in even numbered patients (n=40)" |
| Schug 1996 | Different intervention: all participants received an epidural with local anaesthetics during surgery |
| Schulte 2008 | Different intervention: both groups received an epidural after surgery |
| Schurizek 1982 | Different intervention: excluded as no group received only epidural local anaesthetic |
| Scott 1989 | Different intervention: both groups received an epidural with a local anaesthetic |
| Scott 1995 | Different intervention: all participants received epidural ropivacaine during surgery |
| Scott 1999 | Different intervention: both groups had local anaesthetics |
| Seeling 1984 | No outcome of interest measured |
| Seeling 1985 | No outcome of interest measured. Excluded as no postoperative assessments were performed |
| Seow 1976 | Different intervention: all participants received local anaesthetics |
| Seow 1982 | Different intervention: all participants received local anaesthetics |
| Shir 1995 | Different intervention: all participants received local anaesthetic in the epidural after surgery |
| Sinclair 1984 | Different intervention: all participants received local anaesthetics |
| Smeets 1993 | No outcome of interest measured |
| Smith 1996 | Different intervention: all participants received epidural bupivacaine during surgery |
| Spence 1971 | No outcome of interest measured |
| Stamenkovic 2008 | Different intervention: all participants received epidurally injected bupivacaine 0.25% during surgery |
| Stamenkovic 2009 | Different intervention: all participants received epidurally injected bupivacaine 0.25% after surgery |
| Stehr‐Zirngibl 1997 | Different intervention: all participants had an epidural with local anaesthetic during surgery |
| Stenseth 1994 | No outcome of interest measured |
| Sutcliffe 1996 | Different study population: includes participants undergoing laryngectomy. No outcome of interest measured |
| Suttner 2005 | Retracted article |
| Takahashi 2005 | Different intervention: all participants received epidural ropivacaine during surgery |
| Thörn 1992 | Different intervention: both groups received an epidural with local anaesthetics during surgery |
| Thörn 1996 | No outcome of interest measured |
| Tikuisis 2009 | No outcome of interest measured |
| Torda 1995 | Different intervention: cross‐over trial |
| Traynor 1982 | Different intervention: participants with an epidural also had a vagal block with lidocaine |
| Tsuji 1983 | Different intervention: general anaesthesia vs epidural anaesthesia |
| Tsuji 1983a | Different intervention: general anaesthesia vs regional blockade (combination of epidural anaesthesia and splanchnic nerve blockade) |
| Tsuji 1987 | Different intervention: general anaesthesia vs epidural anaesthesia |
| Uchida 1988 | Different intervention: general anaesthesia vs epidural anaesthesia |
| Vedrinne 1989 | Not an RCT? The word 'random' is not mentioned in the report |
| Virlos 2010 | Not an RCT: observational study |
| von Ungern‐Sternberg 2005 | Not an RCT: "After making a free choice between epidural analgesia and opioids", "the allocation to the different analgesic regimens was not randomized" |
| Wang 2013 | No outcome of interest measured |
| Wessen 1994 | No outcome of interest measured |
| White 1979 | Different study population: peripheral vascular surgery |
| Wiebalck 1997 | Different intervention: all participants received local anaesthetics |
| Wolf 1993 | Different population: children |
| Wright 1992 | Different population: labour analgesia |
| Wu 2000 | Different intervention: all participants received an epidural with a local anaesthetic after surgery |
| Yeh 2005 | Different intervention: all participants received an epidural containing a local anaesthetic after surgery |
| Yorozu 1996 | Different intervention: all participants received an epidural containing a local anaesthetic after surgery |
| Yorozu 1997 | Different intervention: all participants received an epidural containing a local anaesthetic after surgery |
| Yuceyar 2004 | No outcome of interest measured |
| Zingg 2009 | Different intervention: not randomized for epidural analgesia |
mL: millilitre
RCT: randomized controlled trial
T: thoracic
TEA: thoracic epidural anaesthesia or analgesia
VAS: visual or verbal analogue pain scores
Characteristics of studies awaiting assessment [ordered by study ID]
Baumunk 2014.
| Methods | RCT |
| Participants | 235 participants undergoing radical retropubic prostatectomy |
| Interventions |
Intervention: thoracic epidural anaesthesia with continuous administration of 0.25% bupivacaine (n = 116) Control: intravenous analgesia with fentanyl (intubation: 2 mcg/kg; maintenance: 0.1 to 0.3 mg) General anaesthesia for all participants |
| Outcomes | Blood loss Transfusion rate |
| Notes |
Chen 2015.
| Methods | RCT |
| Participants | 53 ASA 1 or 2 patients undergoing surgery for surgical tumour resection for colon cancer |
| Interventions |
Intervention: epidural anaesthesia (n = 26) Control: n = 27 General anaesthesia for all participants |
| Outcomes | Times to first flatus Times to tolerate a full diet Immunosuppression |
| Notes |
Enohata 2014.
| Methods | RCT Funding: none reported |
| Participants | 20 ASA 1 or 2 patients scheduled for gynaecological surgery between May and December 2012 Exclusions were previous history of renal failure, cardiovascular disease or diabetes mellitus; participants taking medications that have effects on the autonomic nervous system; participants who had more than 1000 mL blood loss/received blood transfusion during surgery |
| Interventions |
Intervention: thoracic epidural anaesthesia (T12‐L1) with test dose followed by 1% mepivacaine Control: remifentanil during surgery General anaesthesia and epidural analgesia with 0.375% ropivacaine after surgery for all participants |
| Outcomes | Superoxide dismutase Myeloperoxidase Adrenaline and noradrenaline |
| Notes | Study will likely be excluded, as all participants received epidural analgesia |
Khoronenko 2014.
| Methods | RCT |
| Participants | 127 women with oncogynaecological pathology |
| Interventions |
Intervention: epidural anaesthesia (n = 40) Control: no epidural (n = 43) All participants received general anaesthesia and standard prophylactics for postoperative nausea and vomiting with ondansetron 8 mg and dexamethasone 8 mg intravenously |
| Outcomes | Postoperative nausea and vomiting |
| Notes | Study also includes another group with general anaesthesia and droperidol (n = 44) |
Kun 2014.
| Methods | RCT |
| Participants | 71 ASA 1 to 3 patients undergoing radical resection of a gastric cancer |
| Interventions |
Intervention: epidural anaesthesia (n = 35) Control: no epidural (n = 36) General anaesthesia for all participants |
| Outcomes | Immunosuppression |
| Notes |
Li 2015.
| Methods | RCT |
| Participants | 85 patients undergoing radical resection of cervical carcinoma |
| Interventions |
Intervention: epidural anaesthesia (n = 35) Control: no epidural (n = 36) General anaesthesia for all participants |
| Outcomes | Immunosuppression |
| Notes |
Orsolya 2015.
| Methods | RCT |
| Participants | 40 patients undergoing robotic urogenital oncosurgery |
| Interventions |
Intervention: epidural anaesthesia (n = 16) Control: no epidural (n = 24) General anaesthesia for all participants |
| Outcomes | Acute kidney injury Neutrophil gelatinase‐associated lipocalin |
| Notes |
Pan 2015.
| Methods | RCT |
| Participants | 40 patients undergoing retroperitoneal laparoscopic surgery for adrenal tumours |
| Interventions |
Intervention: pre‐emptive epidural anaesthesia at T10‐T11 with 0.2% bupivacaine 5 to 10 mL to maintain anaesthesia level at T4 (n = 20) Control: no epidural (n = 20) General anaesthesia for all participants |
| Outcomes | Endothelin Calcitonin gene‐related peptide |
| Notes |
Satsuta 2015.
| Methods | RCT |
| Participants | 40 ASA 3 patients undergoing abdominal surgery for peritonitis |
| Interventions |
Intervention: epidural blockade for 74 hours Control: no epidural General anaesthesia for all participants |
| Outcomes | Time to return of gastrointestinal function |
| Notes | Conference abstract, numbers for each group not provided, no contact address |
Sayan 2015.
| Methods | RCT |
| Participants | 40 ASA 1 or 2 patients undergoing right hepatectomy for living‐donor liver transplantation |
| Interventions |
Intervention: epidural anaesthesia at T6‐T8 (n = 20) Control: no epidural (n = 20) General anaesthesia for all participants |
| Outcomes | Liver blood flow Prothrombin time International normalized ratio Total bilirubin Direct bilirubin Albumin Aspartate transaminase Alanine transaminase |
| Notes |
Sen 2014.
| Methods | RCT |
| Participants | 50 ASA 2 or 3 chronically ill end‐stage renal disease adult patients scheduled for elective live‐related kidney transplantation |
| Interventions |
Intervention: epidural anaesthesia at T12‐L1 with fentanyl and bupivacaine (n = 25) Control: epidural anaesthesia with fentanyl (n = 25) General anaesthesia and epidural analgesia with 0.125% bupivacaine at 4 to 8 mL per hour after surgery for all participants |
| Outcomes | Perioperative haemodynamics and vasopressor requirements Early graft function |
| Notes | Study will likely be excluded, as all participants received epidural analgesia |
Sidiropoulou 2014.
| Methods | RCT |
| Participants | 60 ASA 1 or 2 patients undergoing laparoscopic cholecystectomy |
| Interventions |
Intervention: epidural blockade (n = 30) Control: IV patient‐controlled analgesia (n = 30) General anaesthesia for all participants |
| Outcomes | Pain at rest at 6 and 24 hours after surgery Cortisol Human growth hormone Prolactin Glucose C‐reactive protein |
| Notes |
Watanabe 2014.
| Methods | RCT |
| Participants | 60 patients undergoing laparoscopic colectomy |
| Interventions |
Intervention: thoracic epidural anaesthesia (n = 20) Control: high‐dose remifentanil (n = 20) Control group: low‐dose remifentanil (n = 20) General anaesthesia for all participants |
| Outcomes | Adrenocorticotropic hormone Cortisol Antidiuretic hormone Catecholamines |
| Notes |
Xiang 2014.
| Methods | RCT |
| Participants | 66 adult, ASA 1 or 2 patients undergoing elective abdominal surgery |
| Interventions |
Intervention: lumbar epidural lidocaine (n = 16) Intervention: thoracic epidural lidocaine group (n = 16) Control: epidural saline (n = 16) General anaesthesia and patient‐controlled epidural analgesia after surgery for all participants |
| Outcomes | Time taken for the bispectral index to decrease to 60 |
| Notes | Study also includes an IV lidocaine group (n = 18). This study will likely be excluded, as all participants received epidural analgesia |
Xu 2014.
| Methods | RCT |
| Participants | 40 participants undergoing colon cancer surgery |
| Interventions |
Intervention: thoracic epidural anaesthesia/analgesia with ropivacaine and sufentanil for 72 hours (n = 20) Control: IV patient‐controlled analgesia with sufentanil (n = 20) General anaesthesia for all participants |
| Outcomes | Pain at rest and during coughing at 2, 24 and 48 hours after operation Vascular endothelial growth factor C Cytokines |
| Notes | ChiCTR.org ID ChiCTR‐TRC‐13003146 |
Zhao 2015.
| Methods | RCT |
| Participants | 64 patients undergoing radical resection of gastric antral carcinoma |
| Interventions |
Intervention: epidural anaesthesia/analgesia Control: no epidural General anaesthesia for all participants |
| Outcomes | Tumour necrosis factor α Interleukins 6 and 8 T‐lymphocyte subsets Natural killer cells |
| Notes |
ASA: American Society of Anesthesiologists physical status; mcg/kg: micrograms per kilogram of body weight; N: number; RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
Li 2015a.
| Trial name or title | Effects of 2 different anesthesia‐analgesia methods on incidence of postoperative delirium in elderly patients undergoing major thoracic and abdominal surgery: study rationale and protocol for a multicenter randomized controlled trial |
| Methods | RCT |
| Participants | 1800 elderly participants (age range: 60 to 90 years) scheduled to undergo major thoracic or abdominal surgery |
| Interventions |
Intervention: combined epidural‐general anaesthesia plus postoperative epidural analgesia Control: general anaesthesia plus postoperative intravenous analgesia |
| Outcomes | 7‐Day incidence of postoperative delirium Duration of postoperative delirium Pain during the first 3 days after surgery 30‐Day incidence of postoperative non‐delirium complications Length of stay in hospital after surgery 30‐Day all‐cause mortality |
| Starting date | November 2011 |
| Contact information | Department of Anesthesiology and Critical Care Medicine, Peking University First Hospital, No.8 Xishiku Street, Xicheng District, Beijing, 100034, China |
| Notes | ClinicalTrials.gov NCT01661907 and Chinese Clinical Trial Registry ChiCTR‐TRC‐12002371 |
RCT: randomized controlled trial
Differences between protocol and review
This is an update. We made the following modifications to the previous version.
Study selection, types of participants: We also included laparoscopic abdominal surgery.
Outcomes:
We limited the total number to seven.
We deleted the following.
Paracetamol absorption test as a measure of gastric emptying.
Passage of barium sulphate through the large intestine.
Nausea.
Surgical complications.
We added the following.
Gastrointestinal anastomotic leak.
Length of stay in hospital.
Cost.
Data collection and analysis
Quality of study: We assessed study quality with the new Cochrane tool as presenting low risk, unclear risk or high risk of bias for randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting and other bias.
We presented results for dichotomous outcomes as risk ratios. We provided continuous data measured on different scales or entered as P values as standardized mean differences. When this happened, we provided clinical equivalence. We assessed small‐study effects with Eger's regression intercept and publication bias with Duval and Tweedie's trim and fill analysis. We calculated the number needed for an additional beneficial or harmful outcome when appropriate. We calculated optimal information size when appropriate. We added meta‐regressions for exploration of heterogeneity. We assessed the quality of the body of evidence according to GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) recommendations. We added a 'Summary of findings' table.
Contributions of authors
Conceiving of the review: Joanne Guay (JG) and Sandra Kopp (SK).
Co‐ordinating the review: JG.
Screening search results: JG.
Screening retrieved papers against inclusion criteria: JG and Mina Nishimori (MN).
Appraising the quality of papers: JG and MN.
Abstracting data from papers: JG and MN.
Managing data for the review: JG.
Entering data into Review Manager: JG.
Analysing RevMan statistical data: JG.
Performing other statistical analysis not using RevMan: JG.
Interpreting data: JG, MN and SK.
Making statistical inferences: JG.
Writing the review: JG, MN and SK.
Securing funding for the review: departmental resources only.
Performing previous work that was the foundation of the present study: JG and SK.
Serving as guarantor for the review: JG.
Taking responsibility for reading and checking the review before submission: JG, MN and SK.
Sources of support
Internal sources
-
University of Quebec en Abitibi Temiscamingue (UQAT), Canada.
UQAT provided the articles (in part)
-
University of Montreal, Canada.
University of Montreal provided access to databases and some of the articles
External sources
No sources of support supplied
Declarations of interest
Joanne Guay: I have had no direct relationship with any pharmaceutical company or equipment manufacturer in the past five years. I have not acted as a witness expert in the past five years. I am not an author of any of the included or excluded studies. I do not hold stock other than mutual funds. I am the editor of a multi‐author textbook on anaesthesia (including notions on general and regional anaesthesia). I receive fees for a course on airway management at University of Quebec, in Abitibi‐Temiscamingue.
Mina Nishimori: no conflicts of interest.
Sandra Kopp: no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Aceto 2002 {published data only}
- Aceto P, Clemente A, Sicilia F, Cosmo G. Comparison between intravenous patient‐controlled analgesia and epidural analgesia in patients undergoing lower abdominal surgery. Acta Medica Romana 2002;40(1):3‐11. [EMBASE: AN: 2003250043 ] [Google Scholar]
Addison 1974 {published data only}
- Addison NV, Brear FA, Budd K, Whittaker M. Epidural analgesia following cholecystectomy. The British Journal of Surgery 1974;61(10):850‐2. [PUBMED: 4413267] [DOI] [PubMed] [Google Scholar]
Ahn 1988 {published data only}
- Ahn H, Johansson A, Ygge H, Lindhagen J. Effect of continuous postoperative epidural analgesia on intestinal motility. British Journal of Surgery 1988;75:1176‐8. [DOI] [PubMed] [Google Scholar]
Alpaslan 2010 {published data only}
- Alpaslan S, Baysal A, Turhan M, Kocak T. The comparison of the effects of general anaesthesia versus combined general/epidural anaesthesia on cardiac enzymes and surgical stress response in lower abdominal surgery. Journal of Cardiothoracic and Vascular Anesthesia 2010;24(3 Suppl 1):S42. [EMBASE: 70157197] [Google Scholar]
Aygun 2004 {published data only}
- Aygun S, Kocoglu H, Goksu S, Karaca M, Oner U. Postoperative patient‐controlled analgesia with intravenous tramadol, intravenous fentanyl, epidural tramadol and epidural ropivacaine+fentanyl combination. European Journal of Gynaecological Oncology 2004;25(4):498‐501. [PUBMED: 15285314] [PubMed] [Google Scholar]
Barratt 2002 {published data only}
- Barratt SM, Smith RC, Kee AJ, Mather LE, Cousins MJ. Multimodal analgesia and intravenous nutrition preserves total body protein following major upper gastrointestinal surgery. Regional Anaesthesia and Pain Medicine 2002;27(1):15‐22. [PUBMED: 11799500] [DOI] [PubMed] [Google Scholar]
Barzoi 2000 {published data only}
- Barzoi G, Carluccio S, Bianchi B, Vassia S, Colucci G, Mangiante GL. Morphine plus bupivacaine vs. morphine peridural analgesia in abdominal surgery: the effects on postoperative course in major hepatobiliary surgery. HPB Surgery 2000;11(6):393‐9. [PUBMED: 10977118] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beilin 2003 {published data only}
- Beilin B, Shavit Y, Trabekin E, Mordashev B, Mayburd E, Zeidel A, et al. The effects of postoperative pain management on immune response to surgery. Anesthesia and Analgesia 2003;97(3):822‐7. [PUBMED: 12933409] [DOI] [PubMed] [Google Scholar]
- Yardeni IZ, Shavit Y, Bessler H, Mayburd E, Grinevich G, Beilin B. Comparison of postoperative pain management techniques on endocrine response to surgery: a randomised controlled trial. International Journal of Surgery (London, England) 2007;5(4):239‐43. [PUBMED: 17660130] [DOI] [PubMed] [Google Scholar]
Beilin 2008 {published data only}
- Beilin B, Hoofien D, Poran R, Gral I, Grinevich G, Butin B, et al. Comparison of two patient‐controlled analgesia techniques on neuropsychological functioning in the immediate postoperative period. Journal of Clinical and Experimental Neuropsychology 2008;30(6):674‐82. [PUBMED: 18612876] [DOI] [PubMed] [Google Scholar]
Bellolio 2012 {published data only}
- Bellolio C, Montedeonico C, Hernandez A, Harguindeguy M, Leites A, Rando K. Hemodynamic changes in liver surgery with intrathecal morphine versus epidural local anesthetics: a prospective comparative study. British Journal of Anaesthesia. 2012; Vol. 108:ii246‐7. [EMBASE: 70719358 ]
Benzon 1994 {published and unpublished data}
- Benzon HT. Information on our trial [personal communication]. Email to: J Guay 24 February 2014.
- Benzon HT, Wong CA, Wong HY, Brooke C, Wade L. The effect of low‐dose bupivacaine on postoperative epidural fentanyl analgesia and thrombelastography. Anesthesia and Analgesia 1994;79(5):911‐7. [PUBMED: 7978409] [DOI] [PubMed] [Google Scholar]
Bisgaard 1990 {published and unpublished data}
- Bisgaard C, Mouridsen P, Dahl JB. Continuous lumbar epidural bupivacaine plus morphine versus epidural morphine after major abdominal surgery. European Journal of Anaesthesiology 1990;7:219‐25. [Google Scholar]
- Dahl JB. Information on our trial [personal communication]. Email to: J Guay 25 August 2014.
Bois 1997 {published data only}
- Bois S, Couture P, Boudreault D, Lacombe P, Fugere F, Girard D, et al. Epidural analgesia and intravenous patient‐controlled analgesia result in similar rates of postoperative myocardial ischemia after aortic surgery. Anesthesia and Analgesia 1997;85(6):1233‐9. [PUBMED: 9390586] [DOI] [PubMed] [Google Scholar]
Boylan 1998 {published and unpublished data}
- Boylan JF, Katz J, Kavanagh BP, Klinck JR, Cheng DC, DeMajo WC, et al. Epidural bupivacaine‐morphine analgesia versus patient‐controlled analgesia following abdominal aortic surgery: analgesic, respiratory, and myocardial effects. Anesthesiology 1998;89(3):585‐93. [PUBMED: 9743393] [DOI] [PubMed] [Google Scholar]
- Katz J. Information on our trial [personal communication]. Email to: J Guay 2 October 2014. [Google Scholar]
Brodner 2001 {published data only}
- Brodner G, Aken H, Hertle L, Fobker M, Eckardstein A, Goeters C, et al. Multimodal perioperative management ‐ combining thoracic epidural analgesia, forced mobilization, and oral nutrition ‐ reduces hormonal and metabolic stress and improves convalescence after major urologic surgery. Anesthesia and Analgesia 2001;92(6):1594‐600. [PUBMED: 11375853] [DOI] [PubMed] [Google Scholar]
Broekema 1998 {published data only}
- Broekema AA, Veen A, Fidler V, Gielen MJ, Hennis PJ. Postoperative analgesia with intramuscular morphine at fixed rate versus epidural morphine or sufentanil and bupivacaine in patients undergoing major abdominal surgery. Anesthesia and Analgesia 1998;87(6):1346‐53. [PUBMED: 9842825] [PubMed] [Google Scholar]
Buggy 2002 {published data only}
- Buggy DJ, Doherty WL, Hart EM, Pallett EJ. Postoperative wound oxygen tension with epidural or intravenous analgesia: a prospective, randomized, single‐blind clinical trial. Anesthesiology 2002;97(4):952‐8. [PUBMED: 12357164] [DOI] [PubMed] [Google Scholar]
Cai 2007 {published data only}
- Cai XH, Wang SP, Chen XT, Peng SL, Cao MH, Ye XJ, et al. [Comparison of three analgesic methods for postoperative pain relief and their effects on plasma interleukin‐6 concentration following radical surgery for gastric carcinoma]. Nan Fang Yi Ke Da Xue Xue Bao = Journal of Southern Medical University 2007;27(3):387‐9. [PUBMED: 17426001] [PubMed] [Google Scholar]
Calderon 2004 {published and unpublished data}
- Calderon E, Roman MD, Pernia A, Aragon MC, Vidal M, Torres LM. Analgesic transition after remifentanyl‐based anaesthesia in major abdominal surgery: morphine‐ketorolac versus epidural analgesia [Transicion analgesica tras anestesia basada en remifentanilo en cirugia abdominal mayor: morfina‐ketorolaco versus analgesia epidural]. Revista de la Sociedad Espanola del Dolor 2004;11(1):9‐14. [EMBASE: 2004182344] [Google Scholar]
- Calderón E. Information on our trial [personal communication]. Email to: J Guay 13 July 2015. [Google Scholar]
Carli 1997 {published and unpublished data}
- Carli F. Information on our trial [personal communication]. Email to: J Guay 4 May 2015.
- Carli F, Halliday D. Continuous epidural blockade arrests the postoperative decrease in muscle protein fractional synthetic rate in surgical patients. Anesthesiology 1997;86(5):1033‐40. [PUBMED: 9158351] [DOI] [PubMed] [Google Scholar]
Carli 2001 {published data only}
- Carli F, Trudel JL, Belliveau P. The effect of intraoperative thoracic epidural anesthesia and postoperative analgesia on bowel function after colorectal surgery: a prospective, randomized trial. Diseases of the Colon and Rectum 2001;44(8):1083‐9. [PUBMED: 11535845] [DOI] [PubMed] [Google Scholar]
Carli 2002 {published data only}
- Carli F, Mayo N, Klubien K, Schricker T, Trudel J, Belliveau P. Epidural analgesia enhances functional exercise capacity and health‐related quality of life after colonic surgery: results of a randomized trial. Anesthesiology 2002;97(3):540‐9. [PUBMED: 12218518] [DOI] [PubMed] [Google Scholar]
Chalmouki 2010 {published data only}
- Chalmouki G, Sarridou D, Tsoni I, Moustaka A, Skarpa N, Kostaki S. The advantages of continuous postoperative thoracic epidural analgesia for major urologic surgery. Regional Anesthesia and Pain Medicine 2010;35(5):E177. [EMBASE: 70287668] [Google Scholar]
Cindea 2011 {published data only}
- Cindea I, Balcan A, Gherghina V, Nicolae G, Samoila B, Costea D. Postoperative pain management after major abdominal surgery in elderly patients. European Journal of Anaesthesiology 2011;28:221‐2. [EMBASE: 70681683 ] [Google Scholar]
Cronin 2001 {published data only}
- Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO. Postoperative sleep disturbance: influences of opioids and pain in humans. Sleep 2001;24(1):39‐44. [PUBMED: 11204052] [DOI] [PubMed] [Google Scholar]
Cullen 1985 {published data only}
- Cullen ML, Staren ED, El‐Ganzouri A, Logas WG, Ivankovich AD, Economou SG. Continuous infusion for analgesia after major abdominal operations: a randomized, prospective, double‐blind study. Surgery 1985;98:718‐28. [PubMed] [Google Scholar]
Cuschieri 1985 {published data only}
- Cuschieri RJ, Morran CG, Howie JC, McArdle CS. Postoperative pain and pulmonary complications: comparison of three analgesic regimens. British Journal of Surgery 1985;72:495‐8. [DOI] [PubMed] [Google Scholar]
Dauri 2003 {published data only}
- Dauri M, Costa F, Servetti S, Sidiropoulou T, Fabbi E, Sabato AF. Combined general and epidural anesthesia with ropivacaine for renal transplantation. Minerva Anestesiologica 2003;69(12):873‐84. [PUBMED: 14743119] [PubMed] [Google Scholar]
Davies 1993 {published data only}
- Davies MJ, Silbert BS, Mooney PJ, Dysart RH, Meads AC. Combined epidural and general anaesthesia versus general anaesthesia for abdominal aortic surgery: a prospective randomised trial. Anaesthesia and Intensive Care 1993;21(6):790‐4. [PUBMED: 8122735] [DOI] [PubMed] [Google Scholar]
De Pietri 2006 {published data only}
- Pietri L, Siniscalchi A, Reggiani A, Masetti M, Begliomini B, Gazzi M, et al. The use of intrathecal morphine for postoperative pain relief after liver resection: a comparison with epidural analgesia. Anesthesia and Analgesia 2006;102(4):1157‐63. [PUBMED: 16551916] [DOI] [PubMed] [Google Scholar]
Donatelli 2006 {published data only}
- Donatelli F, Schricker T, Mistraletti G, Asenjo F, Parrella P, Wykes L, et al. Postoperative infusion of amino acids induces a positive protein balance independently of the type of analgesia used. Anesthesiology 2006;105(2):253‐9. [PUBMED: 16871058] [DOI] [PubMed] [Google Scholar]
Doruk 2003 {published data only}
- Doruk N, Aribogan A, Atici S, Camdeviren H. Comparison of the analgesic effect of morphine, tramadol, bupivacaine and various combinations of these drugs in patient‐controlled epidural analgesia [Morfin, Tramadol, Bupivakain ve Kombinasyonlarinin Postoperatif Analjezik Etkinliklerinin Epidural Hasta Kontrollu Analjezi Yontemiyle Karsilastirilmasi]. Turk Anesteziyoloji ve Reanimasyon Dernegi Dergisi 2003;31(6):284‐9. [EMBASE: 2003420704] [Google Scholar]
Elkaradawy 2011 {published data only}
- Elkaradawy SA, Elbanawy HS, Mahmoud K. Silent myocardial ischaemia in diabetic patients after general anaesthesia with 24 h intravenous opioids or with epidural analgesia. Egyptian Journal of Anaesthesia 2011;27(4):279‐96. [EMBASE: 2011673293] [Google Scholar]
El‐Refai 2003 {published data only}
- El‐Refai N, Mandour E. Effects of low‐flow sevoflurane anaesthesia combined with epidural block on stress response, hepatic and renal functions in patients undergoing total abdominal hysterectomy. Egyptian Journal of Anaesthesia 2003;19:155‐61. [EMBASE: 2003448422] [Google Scholar]
Erol 2008 {published data only}
- Erol DD, Yilmaz S, Polat C, Arikan Y. Efficacy of thoracic epidural analgesia for laparoscopic cholecystectomy. Advances in Therapy 2008;25(1):45‐52. [EMBASE: 2009054551] [DOI] [PubMed] [Google Scholar]
Fant 2013 {published data only}
- Fant F, Tina E, Sandblom D, Andersson SO, Magnuson A, Hultgren‐Hornkvist E, et al. Thoracic epidural analgesia inhibits the neuro‐hormonal but not the acute inflammatory stress response after radical retropubic prostatectomy. British Journal of Anaesthesia 2013;110(5):747‐57. [PUBMED: 23295713] [DOI] [PubMed] [Google Scholar]
Fayed 2014 {published data only}
- Fayed NA, Abo El‐Wafa HB, Gab‐Alla NM, Yassen KA, Lotfy ME. Comparison between intravenous patient controlled analgesia and patient controlled epidural analgesia in cirrhotic patients after hepatic resection. Middle East Journal of Anaesthesiology 2014;22(5):467‐76. [PUBMED: 25137863] [PubMed] [Google Scholar]
Ferguson 2009 {published data only}
- Ferguson SE, Malhotra T, Seshan VE, Levine DA, Sonoda Y, Chi DS, et al. A prospective randomized trial comparing patient‐controlled epidural analgesia to patient‐controlled intravenous analgesia on postoperative pain control and recovery after major open gynecologic cancer surgery. Gynecologic Oncology 2009;114(1):111‐6. [PUBMED: 19395071] [DOI] [PubMed] [Google Scholar]
Gambling 2009 {published data only}
- Gambling DR, Hughes TL, Manvelian GZ. Extended‐release epidural morphine (DepoDur) following epidural bupivacaine in patients undergoing lower abdominal surgery: a randomized controlled pharmacokinetic study. Regional Anesthesia and Pain Medicine 2009;34(4):316‐25. [PUBMED: 19574865] [DOI] [PubMed] [Google Scholar]
George 1994 {published data only}
- George KA, Wright PM, Chisakuta AM, Rao NV. Thoracic epidural analgesia compared with patient controlled intravenous morphine after upper abdominal surgery. Acta Anaesthesiologica Scandinavica 1994;38(8):808‐12. [PUBMED: 7887102] [DOI] [PubMed] [Google Scholar]
Gherghina 2010 {published data only}
- Gherghina V, Nicolae Gh, Cindea I, Balcan A, Cosofret M, Sirbu V. Efficacy and adverse effects of intravenous vs epidural patient controlled analgesia in the elderly patients after major abdominal surgery [[Eficacitatea si efectele adverse ale analgeziei controlate de pacient pe cale intravenoasa sau peridurala dup chirurgia abdominal major la pacientii varstnici]]. Jurnalul Roman de Anestezie Terapie Intensiva 2010;17(2):103‐9. [EMBASE: 2010598012] [Google Scholar]
Giannoni 1999 {published data only}
- Giannoni S, Vitale GG, Grancio R, Tinagli F, Pappagallo S, Brancella R, et al. Combined epidural and general anesthesia with epidural analgesia vs general anesthesia with ev analgesia for colorectal surgery [Anestesia combinata con terapia antalgica peridurale vs anestesia generale con terapia antalgica ev nel carcinoma del colon‐retto]. Anaesthesiologica Italica / Anaesthesia and Intensive Care in Italy 1999;50(3):169‐78. [EMBASE: 2000203604] [Google Scholar]
Hadimioglu 2012 {published data only}
- Hadimioglu N, Ulugol H, Akbas H, Coskunfirat N, Ertug Z, Dinckan A. Combination of epidural anesthesia and general anesthesia attenuates stress response to renal transplantation surgery. Transplantation Proceedings 2012;44(10):2949‐54. [PUBMED: 23195004] [DOI] [PubMed] [Google Scholar]
Handley 1997 {published data only}
- Handley GH, Silbert BS, Mooney PH, Schweitzer SA, Allen NB. Combined general and epidural anesthesia versus general anesthesia for major abdominal surgery: postanesthesia recovery characteristics. Regional Anesthesia 1997;22(5):435‐41. [PUBMED: 9338905] [DOI] [PubMed] [Google Scholar]
Heurich 2007 {published data only}
- Heurich M, Mousa SA, Lenzner M, Morciniec P, Kopf A, Welte M, et al. Influence of pain treatment by epidural fentanyl and bupivacaine on homing of opioid‐containing leukocytes to surgical wounds. Brain, Behavior, and Immunity 2007;21(5):544‐52. [PUBMED: 17174527] [DOI] [PubMed] [Google Scholar]
Hjortsø 1985a {published data only}
- Hjortsø NC, Neumann P, Frøsig F, Andersen T, Lindhard A, Rogon E, et al. A controlled study on the effect of epidural analgesia with local anaesthetics and morphine on morbidity after abdominal surgery. Acta Anaesthesiologica Scandinavica 1985;29:790‐6. [DOI] [PubMed] [Google Scholar]
Hjortsø 1985b {published data only}
- Hjortsø NC, Christensen NJ, Andersen T, Kehlet H. Effects of the extradural administration of local anaesthetic agents and morphine on the urinary excretion of cortisol, catecholamines and nitrogen following abdominal surgery. British Journal of Anaesthesia 1985;57:400‐6. [DOI] [PubMed] [Google Scholar]
Hong 2008 {published data only}
- Hong JY. Haemodynamic and ventilatory effects of preoperative epidural analgesia during laparoscopic hysterectomy using NICO. Singapore Medical Journal 2008;49(3):233‐8. [PUBMED: 18363006] [PubMed] [Google Scholar]
Hu 2006 {published data only}
- Hu Y, Wang Y, Li Y‐T. Effects of different analgesic methods on immune function after lower abdominal surgery [Original title available in Chinese characters only]. Chinese Journal of Clinical Rehabilitation 2006;10(4):62‐4. [EMBASE: 2006192077] [Google Scholar]
Hubler 2001 {published data only}
- Hubler M, Litz RJ, Sengebusch KH, Kreinecker I, Frank MD, Hakenberg OW, et al. A comparison of five solutions of local anaesthetics and/or sufentanil for continuous, postoperative epidural analgesia after major urological surgery. European Journal of Anaesthesiology 2001;18(7):450‐7. [PUBMED: 11437873] [DOI] [PubMed] [Google Scholar]
Jayr 1988 {published data only}
- Jayr C, Mollie A, Bourgain JL, Alarcon J, Masselot J, Lasser P, et al. Postoperative pulmonary complications: general anesthesia with postoperative parenteral morphine compared with epidural analgesia. Surgery 1988;104(1):57‐63. [PUBMED: 3388180] [PubMed] [Google Scholar]
Jayr 1993 {published data only}
- Jayr C, Thomas H, Rey A, Farhat F, Lasser P, Bourgain JL. Postoperative pulmonary complications. Epidural analgesia using bupivacaine and opioids versus parenteral opioids. Anesthesiology 1993;78(4):666‐76; discussion 22A. [PUBMED: 8466067] [DOI] [PubMed] [Google Scholar]
Jayr 1998 {published and unpublished data}
- Jayr C. Information on our trial [personal communication]. Email to: J Guay 1 July 2014.
- Jayr C, Beaussier M, Gustafsson U, Leteurnier Y, Nathan N, Plaud B, et al. Continuous epidural infusion of ropivacaine for postoperative analgesia after major abdominal surgery: comparative study with i.v. PCA morphine. British Journal of Anaesthesia 1998;81(6):887‐92. [PUBMED: 10211014] [DOI] [PubMed] [Google Scholar]
Jorgensen 2001 {published data only}
- Jorgensen H, Fomsgaard JS, Dirks J, Wetterslev J, Andreasson B, Dahl JB. Effect of peri‐ and postoperative epidural anaesthesia on pain and gastrointestinal function after abdominal hysterectomy. British Journal of Anaesthesia 2001;87(4):577‐83. [PUBMED: 11878727] [DOI] [PubMed] [Google Scholar]
Katz 2003 {published data only}
- Katz J, Cohen L, Schmid R, Chan VW, Wowk A. Postoperative morphine use and hyperalgesia are reduced by preoperative but not intraoperative epidural analgesia: implications for preemptive analgesia and the prevention of central sensitization. Anesthesiology 2003;98(6):1449‐60. [PUBMED: 12766657] [DOI] [PubMed] [Google Scholar]
Kentner 1996 {published and unpublished data}
- Kentner R. Information on our trial [personal communication]. Email to: J Guay 12 August 2015.
- Kentner R, Heinrichs W, Dick W. [Combination of intravenous patient‐controlled analgesia with epidural anesthesia for postoperative pain therapy] [Kombination einer intravenosen Patientenkontrollierten Analgesie mit Epiduralanasthesie zur postoperativen Schmerztherapie]. Anaesthesiologie und Reanimation 1996;21(3):69‐75. [PUBMED: 8766398] [PubMed] [Google Scholar]
Kudoh 2001 {published data only}
- Kudoh A, Katagai H, Takazawa T. Effect of epidural analgesia on postoperative paralytic ileus in chronic schizophrenia. Regional Anesthesia and Pain Medicine 2001;26(5):456‐60. [PUBMED: 11561267] [DOI] [PubMed] [Google Scholar]
Kumar 2004 {published data only}
- Kumar R, Prasanna A. Post operative analgesia with continuous epidural infusion. Middle East Journal of Anesthesiology 2004;17(5):899‐912. [PUBMED: 15449747] [PubMed] [Google Scholar]
Lattermann 2007 {published data only}
- Lattermann R, Wykes L, Eberhart L, Carli F, Meterissian S, Schricker T. A randomized controlled trial of the anticatabolic effect of epidural analgesia and hypocaloric glucose. Regional Anesthesia and Pain Medicine 2007;32(3):227‐32. [PUBMED: 17543818] [DOI] [PubMed] [Google Scholar]
Levy 2011 {published data only}
- Levy BF, Scott MJ, Fawcett W, Fry C, Rockall TA. Randomized clinical trial of epidural, spinal or patient‐controlled analgesia for patients undergoing laparoscopic colorectal surgery. The British Journal of Surgery 2011;98(8):1068‐78. [PUBMED: 21590762] [DOI] [PubMed] [Google Scholar]
Licker 1994 {published data only}
- Licker M, Suter PM, Krauer F, Rifat NK. Metabolic response to lower abdominal surgery: analgesia by epidural blockade compared with intravenous opiate infusion. European Journal of Anaesthesiology 1994;11(3):193‐9. [PUBMED: 7519554] [PubMed] [Google Scholar]
Limberi 2003 {published and unpublished data}
- Grigorakos L. Information on our trial [personal communication]. Email to: J Guay 4 March 2016.
- Limberi S, Markou N, Sakayianni K, Vourliotou A, Kremastinou F, Savari E, et al. Coronary artery disease and upper abdominal surgery: impact of anesthesia on perioperative myocardial ischemia. Hepato‐gastroenterology 2003;50(54):1814‐20. [PUBMED: 14696412] [PubMed] [Google Scholar]
Liu 1995 {published data only}
- Liu SS, Carpenter RL, Mackey DC, Thirlby RC, Rupp SM, Shine TSJ, et al. Effects of perioperative analgesic technique on rate of recovery after colon surgery. Anesthsiology 1995;83:757‐65. [DOI] [PubMed] [Google Scholar]
Liuboshevskii 2012 {published data only}
- Liuboshevskii PA, Artamonova NI, Ovechkin AM. [Haemostasis disturbances as the component of the surgical stress‐response and possibilities of their correction]. Anesteziologiia i Reanimatologiia 2012;57(3):44‐8. [PUBMED: 22993923] [PubMed] [Google Scholar]
Lombardo 2009 {published data only}
- Lombardo L, Ruggia O, Crocella L, Masoero G, Foti M, Mambrini S, et al. Epidural plus general anesthesia vs general anesthesia alone for elective aortic surgery: effects on gastric electrical activity and serum gastrin secretion. Minerva Anestesiologica 2009;75(3):109‐15. [PUBMED: 19221543] [PubMed] [Google Scholar]
Luchetti 1996 {published data only}
- Luchetti M, Palomba R, Sica G, Massa G, Tufano R. Effectiveness and safety of combined epidural and general anesthesia for laparoscopic cholecystectomy. Regional Anesthesia 1996;21(5):465‐9. [PUBMED: 8896010] [PubMed] [Google Scholar]
Lugli 2008 {published and unpublished data}
- Carli F. Information on our trial [personal communication]. Email to: J Guay 4 May 2015.
- Lugli AK, Donatelli F, Schricker T, Wykes L, Carli F. Epidural analgesia enhances the postoperative anabolic effect of amino acids in diabetes mellitus type 2 patients undergoing colon surgery. Anesthesiology 2008;108(6):1093‐9. [PUBMED: 18497611] [DOI] [PubMed] [Google Scholar]
Lugli 2010 {published and unpublished data}
- Carli F. Information on our trial [personal communication]. Email to: J Guay 4 May 2015. [Google Scholar]
- Lugli AK, Donatelli F, Schricker T, Kindler C, Wykes L, Carli F. Protein balance in nondiabetic versus diabetic patients undergoing colon surgery: effect of epidural analgesia and amino acids. Regional Anesthesia and Pain Medicine 2010;35(4):355‐60. [PUBMED: 20607877] [DOI] [PubMed] [Google Scholar]
Malenkovic 2003 {published data only}
- Malenkovic V, Zoric S, Randelovic T. [Advantage of combined spinal, epidural and general anesthesia in comparison to general anesthesia in abdominal surgery] [Prednosti primene kombinovane spinalne, epiduralne i opste anestezije u odnosu na opstu anesteziju u abdominalnog hirurgiji.]. Srpski Arhiv za Celokupno Lekarstvo 2003;131(5‐6):232‐7. [PUBMED: 14692130] [DOI] [PubMed] [Google Scholar]
Mallinder 2000 {published data only}
- Mallinder PA, Hall JE, Bergin FG, Royle P, Leaper DJ. A comparison of opiate‐ and epidural‐induced alterations in splanchnic blood flow using intra‐operative gastric tonometry. Anaesthesia 2000;55(7):659‐65. [PUBMED: 10919421] [DOI] [PubMed] [Google Scholar]
Mann 2000 {published data only}
- Mann C, Pouzeratte Y, Boccara G, Peccoux C, Vergne C, Brunat G, D, et al. Comparison of intravenous or epidural patient‐controlled analgesia in the elderly after major abdominal surgery. Anesthesiology 2000;92:433‐41. [PUBMED: 10691230] [DOI] [PubMed] [Google Scholar]
Marandola 2008 {published data only}
- Marandola M, Cilli T, Alessandri F, Tellan G, Caronna R, Chirletti P, et al. Perioperative management in patients undergoing pancreatic surgery: the anesthesiologist's point of view. Transplantation Proceedings 2008;40(4):1195‐9. [PUBMED: 18555147] [DOI] [PubMed] [Google Scholar]
Martella 2012 {published data only}
- Martella N, Cazzato MT, Vitale Di Maio F, Olivieri C, Marusco I, Sollazzi L. Epidural analgesia and fast‐track protocols in colorectal surgery: what benefits for the patient?. European Journal of Anaesthesiology. 2012; Vol. 29:29‐30. [EMBASE: 71084087]
Miller 1976 {published data only}
- Miller L, Gertel M, Fox GS, MacLean LD. Comparison of effect of narcotic and epidural analgesia on postoperative respiratory function. American Journal of Surgery 1976;131(3):291‐4. [PUBMED: 1259099] [DOI] [PubMed] [Google Scholar]
Moiniche 1993 {published data only}
- Moiniche S, Hjortso NC, Blemmer T, Dahl JB, Kehlet H. Blood pressure and heart rate during orthostatic stress and walking with continuous postoperative thoracic epidural bupivacaine/morphine. Acta Anaesthesiologica Scandinavica 1993;37(1):65‐9. [PUBMED: 8424297] [DOI] [PubMed] [Google Scholar]
Mondor 2010 {published and unpublished data}
- Massicotte L. Information on our trial [personal communication]. Email to: J Guay 10 July 2014.
- Mondor ME, Massicotte L, Beaulieu D, Roy JD, Lapointe R, Dagenais M, et al. Long‐lasting analgesic effects of intraoperative thoracic epidural with bupivacaine for liver resection. Regional Anaesthesia and Pain Medicine 2010;35(1):51‐6. [PUBMED: 20048658] [DOI] [PubMed] [Google Scholar]
Motamed 1998 {published and unpublished data}
- Jay C. Information on our trial [personal communication]. Email to: J Guay 10 July 2014.
- Jayr C. Information on our trial [personal communication]. Email to: J Guay 5 august 2014.
- Motamed C, Spencer A, Farhat F, Bourgain JL, Lasser P, Jayr C. Postoperative hypoxaemia: continuous extradural infusion of bupivacaine and morphine vs patient‐controlled analgesia with intravenous morphine. British Journal of Anaesthesia 1998;80(6):742‐7. [PUBMED: 9771300] [DOI] [PubMed] [Google Scholar]
Muehling 2009 {published data only}
- Muehling B, Schelzig H, Steffen P, Meierhenrich R, Sunder‐Plassmann L, Orend KH. A prospective randomized trial comparing traditional and fast‐track patient care in elective open infrarenal aneurysm repair. World Journal of Surgery 2009;33(3):577‐85. [PUBMED: 19137363] [DOI] [PubMed] [Google Scholar]
Neudecker 1999 {published data only}
- Neudecker J, Schwenk W, Junghans T, Pietsch S, Bohm B, Muller JM. Randomized controlled trial to examine the influence of thoracic epidural analgesia on postoperative ileus after laparoscopic sigmoid resection. The British Journal of Surgery 1999;86(10):1292‐5. [PUBMED: 10540136] [DOI] [PubMed] [Google Scholar]
Norman 1997 {published data only}
- Norman JG, Fink GW. The effects of epidural anesthesia on the neuroendocrine response to major surgical stress: a randomized prospective trial. The American Surgeon 1997;63(1):75‐80. [PUBMED: 8985076] [PubMed] [Google Scholar]
O'Connor 2006 {published data only}
- O'Connor PJ, Hanson J, Finucane BT. Induced hypotension with epidural/general anesthesia reduces transfusion in radical prostate surgery. Canadian Journal of Anaesthesia 2006;53(9):873‐80. [PUBMED: 16960264] [DOI] [PubMed] [Google Scholar]
- Tsui BC, Rashiq S, Schopflocher D, Murtha A, Broemling S, Pillay J, et al. Epidural anaesthesia and cancer recurrence rates after radical prostatectomy. Canadian Journal of Anaesthesia 2010;57(2):107‐12. [PUBMED: 19911247] [DOI] [PubMed] [Google Scholar]
Ozcan 2004 {published data only}
- Ozcan S, Tabuk M, Baltaci B, Unal N. [Is epidural preemptive analgesia effective in lower abdominal surgery?] [Alt abdominal cerrahi girisimlerde epidural preemptif analjezi etkili midir?]. Agri: Agri (Algoloji) Dernegi'nin Yayin Organidir 2004;16(1):58‐63. [PUBMED: 15152589] [PubMed] [Google Scholar]
Ozdilmac 2003 {published data only}
- Ozdilmac I, Altintas F, Salihoglu Z, Demiroluk S, Aydin S, Uzun H. The effect of general anesthesia and epidural combined with general anesthesia on the response of surgical stress during abdominal surgery. [[Alt batin cerrahisinde genel anestezi ile epidural+genel anestezi uygulamasinin stres yanita etkileri]]. Anesteziyoloji ve Reanimasyon Uzmanları Derneği 2003;11(3):195‐200. [EMBASE: 2003409523] [Google Scholar]
Ozturk 2010 {published data only}
- Ozturk N, Uluer PD, Saydam GS, Topcu DI, Gulapoglu H, Cebi YA, et al. The effect of peroperative epidural analgesia with levobupivacaine on the response of oxidative stress during nefrectomy operations. [[Nefrektomi olgularinda levobupivakain ile saglanan peroperatif epidural analjezinin oksidatif stres uzerine etkisi]]. Anesteziyoloji ve Reanimasyon Uzmanları Derneği 2010;18(1):43‐9. [EMBASE: 2010277555] [Google Scholar]
Park 2001 {published data only}
- Park WY, Thompson JS, Lee KK. Effect of epidural anesthesia and analgesia on perioperative outcome: a randomized, controlled Veterans Affairs cooperative study. Annals of Surgery 2001;234(4):560‐9; discussion 569‐71. [PUBMED: 11573049] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paulsen 2001 {published data only}
- Paulsen EK, Porter MG, Helmer SD, Linhardt PW, Kliewer ML. Thoracic epidural versus patient‐controlled analgesia in elective bowel resections. American Journal of Surgery 2001;182(6):570‐7. [PUBMED: 11839319] [DOI] [PubMed] [Google Scholar]
Peyton 2003 {published data only}
- Myles PS, Peyton P, Silbert B, Hunt J, Rigg JR, Sessler DI, ANZCA Trials Group Investigators. Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence‐free survival: randomised trial. BMJ 2011;342(March 29):d1491. [PUBMED: 21447587 ] [DOI] [PubMed] [Google Scholar]
- Peyton PJ, Myles PS, Silbert BS, Rigg JA, Jamrozik K, Parsons R. Perioperative epidural analgesia and outcome after major abdominal surgery in high‐risk patients. Anesthesia and Analgesia 2003;96(2):548‐54. [PUBMED: 12538211] [DOI] [PubMed] [Google Scholar]
- Rigg JR, Jamrozik K, Myles PS, Silbert B, Peyton P, Parsons RW, et al. Design of the multicenter Australian study of epidural anaesthesia and analgesia in major surgery: the MASTER trial. Controlled Clinical Trials 2000;2(3):244‐56. [PUBMED: 10822122 ] [DOI] [PubMed] [Google Scholar]
- Rigg JR, Jamrozik K, Myles PS, Silbert BS, Peyton PJ, Parsons RW, et al. MASTER Anaethesia Trial Study Group. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet 2002;13(359):1276‐82. [PUBMED: 11965272 ] [DOI] [PubMed] [Google Scholar]
Pflug 1974 {published data only}
- Pflug AE, Murphy TM, Butler SH, Tucker GT. The effects of postoperative peridural analgesia on pulmonary therapy and pulmonary complications. Anesthesiology 1974;41(1):8‐17. [PUBMED: 4599641] [DOI] [PubMed] [Google Scholar]
Rimaitis 2003 {published data only}
- Rimaitis K, Marchertiene I, Pavalkis D. [Comparison of two different methods of analgesia. Postoperative course after colorectal cancer surgery] [Skirtingu skausmo malsinimo metodu veiksmingumo ivertinimas bei ju itaka pooperacinei eigai po storosios zarnos vezio rezekciniu operaciju]. Medicina (Kaunas, Lithuania) 2003;39(2):129‐37. [PUBMED: 12626865] [PubMed] [Google Scholar]
Riwar 1991 {published data only}
- Riwar A, Schär B, Grötzinger U. Effect of continuous postoperative analgesia with bupivacaine on intestinal motility following colorectal resection [Effekt der kontinuierlichen postoperativen analgesie mit bupivacain peridural auf die darmmotilität nach kolorektalen resektionen]. Helvetica Chirurgica Acta 1991;58:729‐33. [PubMed] [Google Scholar]
Rockemann 1996 {published data only}
- Rockemann MG, Seeling W, Bischof C, Borstinghaus D, Steffen P, Georgieff M. Prophylactic use of epidural mepivacaine/morphine, systemic diclofenac, and metamizole reduces postoperative morphine consumption after major abdominal surgery. Anesthesiology 1996;84(5):1027‐34. [PUBMED: 8623995] [DOI] [PubMed] [Google Scholar]
Rockemann 1997 {published and unpublished data}
- Rockemann MG. Information on our trial [personal communication]. Email to: J Guay 6 July 2015.
- Rockemann MG. Information on our trial [personal communication]. Email to: J Guay 8 July 2015.
- Rockemann MG, Seeling W, Goertz AW, Konietzko I, Steffen P, Georgieff M. [Effectiveness, side effects and costs of postoperative pain therapy: intravenous and epidural patient‐controlled analgesia (PCA)] [Wirksamkeit, Nebenwirkungen und Kosten postoperativer Schmerztherapie: Intravenose und epidurale patientenkontrollierte Analgesie (PCA)]. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie: AINS 1997;32(7):414‐9. [PUBMED: 9340029] [DOI] [PubMed] [Google Scholar]
Rutberg 1984 {published data only}
- Håkanson E, Rutberg H, Jorfeldt L, Mårtensson J. Effects of the extradural administration of morphine, or bupivacaine, on the metabolic response to upper abdominal surgery. British Journal of Anaesthesia 1985;57(4):394‐9. [PUBMED: 3986067] [DOI] [PubMed] [Google Scholar]
- Rutberg H, Håkanson E, Anderberg B, Jorfelt L, Mårtensson J, Schildt B. Effects of the extradural administration of morphine, or bupivacaine, on the endocrine response to upper abdominal surgery. British Journal of Anaesthesia 1984;56:233‐8. [DOI] [PubMed] [Google Scholar]
Salomaki 1995 {published data only}
- Salomaki TE, Laitinen JO, Vainionpaa V, Nuutinen LS. 0.1% bupivacaine does not reduce the requirement for epidural fentanyl infusion after major abdominal surgery. Regional Anesthesia 1995;20(5):435‐43. [PUBMED: 8519722] [PubMed] [Google Scholar]
Scheinin 1982 {published data only}
- Scheinin B, Rosenberg PH. Effect of prophylactic epidural morphine or bupivacaine on postoperative pain after upper abdominal surgery. Acta Anaesthesiologica Scandinavica 1982;26(5):474‐8. [PUBMED: 6815974] [DOI] [PubMed] [Google Scholar]
Scheinin 1987 {published data only}
- Scheinin B, Asantila R, Orko R. The effect of bupivacaine and morphine on pain and bowel function after colonic surgery. Acta Anaesthesiologica Scandinavica 1987;31:161‐4. [DOI] [PubMed] [Google Scholar]
Schricker 2000 {published data only}
Schricker 2002 {published data only}
- Schricker T, Wykes L, Eberhart L, Lattermann R, Mazza L, Carli F. The anabolic effect of epidural blockade requires energy and substrate supply. Anesthesiology 2002;97(4):943‐51. [PUBMED: 12357163] [DOI] [PubMed] [Google Scholar]
Schricker 2004 {published data only}
- Schricker T, Meterissian S, Wykes L, Eberhart L, Lattermann R, Carli F. Postoperative protein sparing with epidural analgesia and hypocaloric dextrose. Annals of Surgery 2004;240(5):916‐21. [PUBMED: 15492576] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulze 1988 {published data only}
- Kehlet H. Information on our trial [personal communication]. Email to: J Guay 3 June 2016.
- Schulze S, Roikjaer O, Hasselstrom L, Jensen NH, Kehlet H. Epidural bupivacaine and morphine plus systemic indomethacin eliminates pain but not systemic response and convalescence after cholecystectomy. Surgery 1988;103(3):321‐7. [PUBMED: 3344486] [PubMed] [Google Scholar]
Schulze 1992 {published data only}
- Kehlet H. Information on our trial [personal communication]. Email to: J Guay 31 May 2016.
- Schulze S, Sommer P, Bigler D, Honnens M, Shenkin A, Cruickshank AM, et al. Effect of combined prednisolone, epidural analgesia, and indomethacin on the systemic response after colonic surgery. Archives of Surgery 1992;127(3):325‐31. [PUBMED: 1550481] [DOI] [PubMed] [Google Scholar]
Schumann 2003 {published and unpublished data}
- Schumann R. Information on our trial [personal communication]. Email to: J Guay 6 August 2014. [Google Scholar]
- Schumann R, Shikora S, Weiss JM, Wurm H, Strassels S, Carr DB. A comparison of multimodal perioperative analgesia to epidural pain management after gastric bypass surgery. Anesthesia and Analgesia 2003;96(2):469‐74. [PUBMED: 12538198] [DOI] [PubMed] [Google Scholar]
Seeling 1990 {published data only}
- Seeling W, Bruckmooser KP, Hufner C, Kneitinger E, Rigg C, Rockemann M. [No reduction in postoperative complications by the use of catheterized epidural analgesia following major abdominal surgery] [Keine Verminderung postoperativer Komplikationen durch Katheterepiduralanalgesie nach grossen abdominellen Eingriffen]. Der Anaesthesist 1990;39(1):33‐40. [PUBMED: 2407144] [PubMed] [Google Scholar]
Seeling 1990a {published data only}
- Seeling W, Kustermann J, Schneider E. [Postoperative peridural analgesia via catheter following abdominal surgery. Peridural bupivacaine versus buprenorphine] [Postoperative Katheterperiduralanalgesie nach abdominellen Eingriffen. Bupivacain versus Buprenorphin peridural.]. Regional‐Anaesthesie 1990;13(3):78‐87. [PUBMED: 2192406] [PubMed] [Google Scholar]
Seeling 1991 {published data only}
- Seeling W, Bothner U, Eifert B, Rockemann M, Schreiber M, Schurmann W, et al. [Patient‐controlled analgesia versus epidural analgesia using bupivacaine or morphine following major abdominal surgery. No difference in postoperative morbidity] [Patientenkontrollierte Analgesie versus Epiduralanalgesie mit Bupivacain oder Morphin nach grossen abdominellen Eingriffen. Kein Unterschied in der postoperativen Morbiditat.]. Der Anaesthesist 1991;40(11):614‐23. [PUBMED: 1755532] [PubMed] [Google Scholar]
Senagore 2003 {published data only}
- Senagore AJ, Delaney CP, Mekhail N, Dugan A, Fazio VW. Randomized clinical trial comparing epidural anaesthesia and patient‐controlled analgesia after laparoscopic segmental colectomy. The British Journal of Surgery 2003;90(10):1195‐9. [PUBMED: 14515286] [DOI] [PubMed] [Google Scholar]
Siniscalchi 2003 {published data only}
- Siniscalchi A, Begliomini B, Matteo G, Pietri L, Pasetto A. Intraoperative effects of combined versus general anesthesia during major liver surgery. Minerva Anestesiologica 2003;69(12):885‐95. [PUBMED: 14743120] [PubMed] [Google Scholar]
Steinberg 2002 {published and unpublished data}
- Gibson C. Information on our trial [personal communication]. Email to: J Guay 7 August 2014.
- Steinberg RB, Liu SS, Wu CL, Mackey DC, Grass JA, Ahlen K, et al. Comparison of ropivacaine‐fentanyl patient‐controlled epidural analgesia with morphine intravenous patient‐controlled analgesia for perioperative analgesia and recovery after open colon surgery. Journal of Clinical Anesthesia 2002;14(8):571‐7. [PUBMED: 12565114] [DOI] [PubMed] [Google Scholar]
Stevens 1998 {published data only}
- Stevens RA, Mikat‐Stevens M, Flanigan R, Waters WB, Furry P, Sheikh T, et al. Does the choice of anesthetic technique affect the recovery of bowel function after radical prostatectomy?. Urology 1998;52(2):213‐8. [PUBMED: 9697784] [DOI] [PubMed] [Google Scholar]
St‐Onge 1997 {published data only}
- St‐Onge S, Fugere F, Girard M. Bupivacaine decreases epidural meperidine requirements after abdominal surgery. Canadian Journal of Anaesthesia 1997;44(4):360‐6. [PUBMED: 9104516] [DOI] [PubMed] [Google Scholar]
Subramaniam 2000 {published data only}
- Subramaniam B, Pawar DK, Kashyap L. Pre‐emptive analgesia with epidural morphine or morphine and bupivacaine. Anaesthesia and Intensive Care 2000;28(4):392‐8. [PUBMED: 10969365] [DOI] [PubMed] [Google Scholar]
Taqi 2007 {published data only}
- Taqi A, Hong X, Mistraletti G, Stein B, Charlebois P, Carli F. Thoracic epidural analgesia facilitates the restoration of bowel function and dietary intake in patients undergoing laparoscopic colon resection using a traditional, nonaccelerated, perioperative care program. Surgical Endoscopy 2007;21(2):247‐52. [PUBMED: 17160649] [DOI] [PubMed] [Google Scholar]
Thorén 1989 {published data only}
- Thorén T, Sundberg A, Wattwil M, Garvill JE, Jürgensen U. Effects of epidural bupivacaine and epidural morphine on bowel function and pain after hysterectomy. Acta Anaesthesiologica Scandinavica 1989;33:181‐5. [DOI] [PubMed] [Google Scholar]
Tsui 1997 {published and unpublished data}
- Tsui SL. Information on our trial [personal communication]. Email to: J Guay 4 September 2014.
- Tsui SL, Lee DK, Ng KF, Chan TY, Chan WS, Lo JW. Epidural infusion of bupivacaine 0.0625% plus fentanyl 3.3 micrograms/ml provides better postoperative analgesia than patient‐controlled analgesia with intravenous morphine after gynaecological laparotomy. Anaesthesia and Intensive Care 1997;25(5):476‐81. [PUBMED: 9352758] [DOI] [PubMed] [Google Scholar]
Tuman 1991 {published data only}
- Tuman KJ, McCarthy RJ, March RJ, DeLaria GA, Patel RV, Ivankovich AD. Effects of epidural anesthesia and analgesia on coagulation and outcome after major vascular surgery. Anesthesia and Analgesia 1991;73(6):696‐704. [PUBMED: 1952169] [DOI] [PubMed] [Google Scholar]
Turunen 2009 {published data only}
- Turunen P, Carpelan‐Holmstrom M, Kairaluoma P, Wikstrom H, Kruuna O, Pere P, et al. Epidural analgesia diminished pain but did not otherwise improve enhanced recovery after laparoscopic sigmoidectomy: a prospective randomized study. Surgical Endoscopy 2009;23(1):31‐7. [PUBMED: 18814016] [DOI] [PubMed] [Google Scholar]
Tyagi 2011 {published data only}
- Tyagi A, Seelan S, Sethi AK, Mohta M. Role of thoracic epidural block in improving post‐operative outcome for septic patients: a preliminary report. European Journal of Anaesthesiology 2011;28(4):291‐7. [PUBMED: 21119517] [DOI] [PubMed] [Google Scholar]
Voylenko 2013 {published data only}
- Voylenko O, Vitruk IV, Stakhovskyi O, Kotov VA, Stakhovsky E. Fast track surgery in partial resection for renal cell Carcinoma. EAU 13th Central European Meeting, CEM and the EAU 9th South Eastern European Meeting. 2013; Vol. 12:e1374.
Wallin 1986 {published data only}
- Wallin G, Cassuto J, Högström S, Rimbäck G, Faxén A, Tolleson PO. Failure of epidural anaesthesia to prevent postoperative paralytic ileus. Anesthesiology 1986;65:292‐7. [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang TB, Shi HP, Lin WH, Ye ZJ, Zhang CG. Clinical practice of fast track surgery in colon carcinoma operation [Original title available in Chinese characters only]. Journal of Cancer Prevention and Treatment 2010;17(22):1868‐70. [EMBASE: 2011264312] [Google Scholar]
Watters 1993 {published data only}
- Watters JM, March RJ, Desai D, Monteith K, Hurtig JB. Epidural anaesthesia and analgesia do not affect energy expenditure after major abdominal surgery. Canadian Journal of Anaesthesia 1993;40(4):314‐9. [PUBMED: 8485790] [DOI] [PubMed] [Google Scholar]
Wattwil 1989 {published data only}
- Wattwil M, Thorén T, Hennerdal S, Garvill JE. Epidural analgesia with bupivacaine reduces postoperative paralytic ileus after hysterectomy. Anesthesia and Analgesia 1989;68:353‐8. [PubMed] [Google Scholar]
Welch 1998 {published data only}
- Welch JP, Cohen JL, Vignati PV, Allen LW, Morrow JS, Carter JJ. Pain control following elective gastrointestinal surgery: is epidural anaesthesia warranted?. Connecticut Medicine 1998;62(8):461‐4. [PUBMED: 9753804] [PubMed] [Google Scholar]
Wiedemann 1991 {published data only}
- Wiedemann B, Leibe S, Katzel R, Grube U, Landgraf R, Bierwolf B. [The effect of combination epidural anesthesia techniques in upper abdominal surgery on the stress reaction, pain control and respiratory mechanics] [Der Einfluss epiduraler Kombinationsanaesthesieverfahren bei Oberbaucheingriffen auf Stressreaktion, Schmerzverhalten und Atemmechanik]. Der Anaesthesist 1991;40(11):608‐13. [PUBMED: 1755531] [PubMed] [Google Scholar]
Yeager 1987 {published data only}
- Yeager MP, Glass DD, Neff RK, Brinck‐Johnsen T. Epidural anesthesia and analgesia in high‐risk surgical patients. Anesthesiology 1987;66:729‐36. [PUBMED: 3296854] [DOI] [PubMed] [Google Scholar]
Zeng 2003 {published data only}
- Zeng L, Wu X, Ma Q, Su Y. Effect of different methods for postoperative pain management on catecholamine response to abdominal surgery [Original title available in Chinese characters only]. Beijing da Xue Xue Bao. Yi Xue Ban 2003;35(2):187‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Zhu 2013 {published data only}
- Zhu Z, Wang C, Xu C, Cai Q. Influence of patient‐controlled epidural analgesia versus patient‐controlled intravenous analgesia on postoperative pain control and recovery after gastrectomy for gastric cancer: a prospective randomized trial. Gastric Cancer 2013;16(2):193‐200. [PUBMED: 22806415] [DOI] [PubMed] [Google Scholar]
Zutshi 2005 {published data only}
- Zutshi M, Delaney CP, Senagore AJ, Mekhail N, Lewis B, Connor JT, et al. Randomized controlled trial comparing the controlled rehabilitation with early ambulation and diet pathway versus the controlled rehabilitation with early ambulation and diet with preemptive epidural anaesthesia/analgesia after laparotomy and intestinal resection. American Journal of Surgery 2005;189(3):268‐72. [PUBMED: 15792748] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akarsu 2012 {published data only}
- Akarsu Ayazotlu T, Eseotlu I, Candan A, Ozensoy A, Otut A. Combined epidural anesthesia + TIVA vs TIVA for major abdominal surgery. Regional Anesthesia and Pain Medicine 2012;37(5 Suppl 1):E221. [EMBASE: 70880924] [Google Scholar]
Ali 2010 {published data only}
- Ali M, Winter DC, Hanly AM, O'Hagan C, Keaveny J, Broe P. Prospective, randomized, controlled trial of thoracic epidural or patient‐controlled opiate analgesia on perioperative quality of life. British Journal of Anaesthesia 2010;104(3):292‐7. [PUBMED: 20124282] [DOI] [PubMed] [Google Scholar]
Aono 1998 {published data only}
- Aono H, Takeda A, Tarver SD, Goto H. Stress responses in three different anaesthetic techniques for carbon dioxide laparoscopic cholecystectomy. Journal of Clinical Anaesthesia 1998;10(7):546‐50. [PUBMED: 9805694] [DOI] [PubMed] [Google Scholar]
Aribogan 2003 {published data only}
- Aribogan A, Doruk N, Aridogan A, Akin S, Balcioglu O. Patient‐controlled epidural analgesia after major urologic surgeries. A comparison of tramadol with or without bupivacaine. Urologia Internationalis 2003;71(2):168‐75. [PUBMED: 12890955] [DOI] [PubMed] [Google Scholar]
Asantila 1991 {published data only}
- Asantila R, Eklund P, Rosenberg PH. Continuous epidural infusion of bupivacaine and morphine for postoperative analgesia after hysterectomy. Acta Anaesthesiologic Scandinavia 1991;35:513‐7. [DOI] [PubMed] [Google Scholar]
Axelsson 2005 {published data only}
- Axelsson K, Johanzon E, Essving P, Weckstrom J, Ekback G. Postoperative extradural analgesia with morphine and ropivacaine. A double‐blind comparison between placebo and ropivacaine 10 mg/h or 16 mg/h. Acta Anaesthesiologica Scandinavica 2005;49(8):1191‐9. [PUBMED: 16095462] [DOI] [PubMed] [Google Scholar]
Badner 1994 {published data only}
- Badner NH, Bhandari R, Komar WE. Bupivacaine 0.125% improves continuous postoperative epidural fentanyl analgesia after abdominal or thoracic surgery. Canadian Journal of Anaesthesia 1994;41(5 Pt 1):387‐92. [PUBMED: 8055605] [DOI] [PubMed] [Google Scholar]
Baron 1991 {published data only}
- Baron JF, Bertrand M, Barre E, Godet G, Mundler O, Coriat P, et al. Combined epidural and general anesthesia versus general anesthesia for abdominal aortic surgery. Anesthesiology 1991;75(4):611‐8. [PUBMED: 1928770] [DOI] [PubMed] [Google Scholar]
Basse 2002 {published data only}
- Basse L, Raskov HH, Hjort Jakobsen D, Sonne E, Billesbolle P, Hendel HW, et al. Accelerated postoperative recovery programme after colonic resection improves physical performance, pulmonary function and body composition. The British Journal of Surgery 2002;89(4):446‐53. [PUBMED: 11952586] [DOI] [PubMed] [Google Scholar]
Beeby 1984 {published data only}
- Beeby D, MacIntosh KC, Bailey M, Welch DB. Postoperative analgesia for caesarean section using epidural methadone. Anaesthesia 1984;39:61‐3. [DOI] [PubMed] [Google Scholar]
Bigler 1989 {published data only}
- Bigler D, Dirkes W, Hansen R, Rosenberg J, Kehlet H. Effects of thoracic paravertebral block with bupivacaine versus combined thoracic epidural block with bupivacaine and morphine on pain and pulmonary function after cholecystectomy. Acta Anaesthesiologica Scandinavica 1989;33(7):561‐4. [PUBMED: 2683543] [DOI] [PubMed] [Google Scholar]
Bloch 1989 {published data only}
- Bloch P, Modiano P. [A prospective randomized study comparing peridural anaesthesia and general anesthesia in sus‐mesocolic digestive surgery] [Etude prospective randomisee comparant anesthésie peridurale (APD) et anesthésie générale (AG) en chirurgie digestive sus‐mesocolique]. Annales de Chirurgie. 1989; Vol. 43, issue 9:768. [CENTRAL: CN‐00335077 ]
Bowdle 1997 {published data only}
- Bowdle TA, Ready LB, Kharasch ED, Nichols WW, Cox K. Transition to post‐operative epidural or patient‐controlled intravenous analgesia following total intravenous anaesthesia with remifentanil and propofol for abdominal surgery. European Journal of Anaesthesiology 1997;14(4):374‐9. [PUBMED: 9253564] [DOI] [PubMed] [Google Scholar]
Brandt 1976 {published data only}
- Brandt MR, Kehlet H, Skovsted L, Hansen JM. Rapid decrease in plasma‐triiodothyronine during surgery and epidural analgesia independent of afferent neurogenic stimuli and of cortisol. Lancet 1976;2(7999):1333‐6. [PUBMED: 63806] [DOI] [PubMed] [Google Scholar]
Brandt 1978 {published data only}
- Brandt MR, Fernades A, Mordhorst R, Kehlet H. Epidural analgesia improves postoperative nitrogen balance. British Medical Journal 1978;1(6120):1106‐8. [PUBMED: 638618] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bredtmann 1990 {published data only}
- Bredtmann RD, Herden HN, Teichmann W, Moecke HP, Kniesel B, Baetgen R, et al. Epidural analgesia in colonic surgery: results of a randomized prospective study. British Journal of Surgery 1990;77:638‐42. [DOI] [PubMed] [Google Scholar]
Bredtmann 1991 {published data only}
- Bredtmann RD, Kniesel B, Herden HN, Teichmann W. [The effect of continuous thoracic peridural anesthesia on the pulmonary function of patients undergoing colon surgery. Results of a randomized study of 116 patients] [Einfluss der kontinuierlichen thorakalen Periduralanaesthesie auf die Lungenfunktion kolonchirurgischer Patienten. Ergebnisse einer randomisierten Untersuchung an 116 Patienten]. Regional‐Anaesthesie 1991;14(1):2‐8. [PUBMED: 2006346] [PubMed] [Google Scholar]
Bridenbaugh 1976 {published data only}
- Bridenbaugh PO, Balfour RI, Bridenbaugh LD, Lysons DF. Bupivacaine and etidocaine for lumbar epidural anesthesia for intra‐abdominal pelvic surgery, a double‐blind study. Anesthesiology 1976;45(5):560‐4. [PUBMED: 788557] [PubMed] [Google Scholar]
Brodner 2000 {published data only}
- Brodner G, Mertes N, Aken HV, Möllhoff T, Zahl M, Wirtz S, et al. What concentration of sufentanil should be combined with ropivacaine 0.2% wt/vol for postoperative patient‐controlled epidural analgesia?. Anesthesia and Analgesia 2000;90:649‐57. [DOI] [PubMed] [Google Scholar]
Bromage 1955 {published data only}
- Bromage PR. Spirometry in assessment of analgesia after abdominal surgery; a method of comparing analgesic drugs. British Medical Journal 1955;2(4939):589‐93. [PUBMED: 13240186] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bromage 1971 {published data only}
- Bromage PR, Shibata HR, Willoughby HW. Influence of prolonged epidural blockade on blood sugar and cortisol responses to operations upon the upper part of the abdomen and the thorax. Surgery, Gynecology and Obstetrics 1971;132(6):1051‐6. [PUBMED: 5578429] [PubMed] [Google Scholar]
Brownridge 1985 {published data only}
- Brownridge P, Frewin DB. A comparative study of techniques of postoperative analgesia following caesarean section and lower abdominal surgery. Anaesthesia and Intensive Care Journal 1985;13:123‐30. [DOI] [PubMed] [Google Scholar]
Buckley 1978 {published data only}
- Buckley FP, Littlewood DG, Covino BG, Scott DB. Effects of adrenaline and the concentration of solution on extradural block with etidocaine. British Journal of Anaesthesia 1978;50:171‐5. [DOI] [PubMed] [Google Scholar]
Buckley 1982 {published data only}
- Buckley FP, Kehlet H, Brown NS, Scott DB. Postoperative glucose tolerance during extradural analgesia. British Journal of Anaesthesia 1982;54(3):325‐31. [PUBMED: 7039645] [DOI] [PubMed] [Google Scholar]
Carli 1991 {published data only}
- Carli F, Webster J, Pearson J, Bartlett S, Bannister P, Halliday D. Protein metabolism after abdominal surgery: effect of 24‐h extradural block with local anaesthetic. British Journal of Anaesthesia 1991;67:729‐34. [DOI] [PubMed] [Google Scholar]
Carli 1995 {published data only}
- Carli F, Kulkarni P, Webster JD, MacDonald IA. Post‐surgery epidural blockade with local anaesthetics attenuates the catecholamine and thermogenic response to perioperative hypothermia. Acta Anaesthesiologica Scandinavica 1995;39(8):1041‐7. [PUBMED: 8607306] [DOI] [PubMed] [Google Scholar]
Casati 2002 {published data only}
- Casati L, Fernandez‐Galinski S, Barrera E, Pol O, Puig MM. Isoflurane requirements during combined general/epidural anesthesia for major abdominal surgery. Anesthesia and Analgesia 2002;94(5):1331‐7. [PUBMED: 11973215] [DOI] [PubMed] [Google Scholar]
Catley 1985 {published data only}
- Catley DM, Thornton C, Jordan C, Lehane JR, Royston D, Jones JG. Pronounced, episodic oxygen desaturation in the postoperative period: its association with ventilatory pattern and analgesic regimen. Anesthesiology 1985;63(1):20‐8. [PUBMED: 4014768] [DOI] [PubMed] [Google Scholar]
Chestnut 1986 {published data only}
- Chestnut DH, Choi WW, Isbell TJ. Epidural hydromorphone for postcesarean analgesia. Obstetrics and Gynecology 1986;68(1):65‐9. [PUBMED: 2425315] [PubMed] [Google Scholar]
Cooper 1996 {published data only}
- Cooper DW, Ryall DM, McHardy FE, Lindsay SL, Eldabe SS. Patient‐controlled extradural analgesia with bupivacaine, fentanyl, or a mixture of both, after caesarean section. British Journal of Anaesthesia 1996;76:611‐5. [DOI] [PubMed] [Google Scholar]
Cowen 1982 {published data only}
- Cowen MJ, Bullingham RE, Paterson GM, McQuay HJ, Turner M, Allen MC, et al. A controlled comparison of the effects of extradural diamorphine and bupivacaine on plasma glucose and plasma cortisol in postoperative patients. Anesthesia and Analgesia 1982;61(1):15‐8. [PUBMED: 7198407] [PubMed] [Google Scholar]
Crews 1999 {published data only}
- Crews JC, Hord AH, Denson DD, Schatzman C. A comparison of the analgesic efficacy of 0.25% levobupivacaine combined with 0.005% morphine, 0.25% levobupivacaine alone, or 0.005% morphine alone for the management of postoperative pain in patients undergoing major abdominal surgery. Anesthesia and Analgesia 1999;89(6):1504‐9. [PUBMED: 10589637] [DOI] [PubMed] [Google Scholar]
Dahl 1992 {unpublished data only}
- Dahl JB, Rosenberg J, Hansen BL, Hjortso NC, Kehlet H. Differential analgesic effects of low‐dose epidural morphine and morphine‐bupivacaine at rest and during mobilization after major abdominal surgery. Anesthesia and Analgesia 1992;74(3):362‐5. [PUBMED: 1539815] [DOI] [PubMed] [Google Scholar]
De Kock 1999 {published data only}
- Kock M, Gautier P, Pavlopoulou A, Jonniaux M, Lavand'homme P. Epidural clonidine or bupivacaine as the sole analgesic agent during and after abdominal surgery: a comparative study. Anesthesiology 1999;90(5):1354‐62. [PUBMED: 10319784] [DOI] [PubMed] [Google Scholar]
Delilkan 1993 {published data only}
- Delilkan AE, Vijayan R. Epidural tramadol for postoperative pain relief. Anaesthesia 1993;48:328‐31. [DOI] [PubMed] [Google Scholar]
Dupont 1987 {published data only}
- Dupont D, Velin P, Cabour F, Candito M, Rives E. Effect of caudal anaesthesia on catacholamine secretion in children [Influence de l'anesthésie caudale sur la sécrétion des catécholamines chez l'enfant]. Annales Françaises d'Anesthésie et de Réanimation 1987;6:156‐8. [DOI] [PubMed] [Google Scholar]
Dyer 1992 {published data only}
- Dyer RA, Camden‐Smith K, James MFM. Epidural lidocaine with sufentanil and epinephrine for abdominal hysterectomy under general anaesthesia: respiratory deprssion and postoperative analgesia. Canadian Journal of Anaesthesia 1992;39:220‐5. [DOI] [PubMed] [Google Scholar]
Engquist 1977 {published data only}
- Engquist A, Brandt MR, Fernandes A, Kehlet H. The blocking effect of epidural analgesia on the adrenocortical and hyperglycemic responses to surgery. Acta Anaesthesiologica Scandinavica 1977;21(4):330‐5. [PUBMED: 906788] [DOI] [PubMed] [Google Scholar]
Engquist 1980 {published data only}
- Engquist A, Fog‐Moller F, Christiansen C, Thode J, Vester‐Andersen T, Madsen SN. Influence of epidural analgesia on the catecholamine and cyclic AMP responses to surgery. Acta Anaesthesiologica Scandinavica 1980;24(1):17‐21. [PUBMED: 6246705] [DOI] [PubMed] [Google Scholar]
Etches 1997 {published data only}
- Etches RC, Writer WD, Ansley D, Nydahl PA, Ong BY, Lui A, et al. Continuous epidural ropivacaine 0.2% for analgesia after lower abdominal surgery. Anesthesia and Analgesia 1997;84(4):784‐90. [PUBMED: 9085958] [DOI] [PubMed] [Google Scholar]
Feo 2009 {published data only}
- Feo CV, Lanzara S, Sortini D, Ragazzi R, Pinto M, Pansini GC, et al. Fast track postoperative management after elective colorectal surgery: a controlled trial. The American Surgeon 2009;75(12):1247‐51. [PUBMED: 19999921] [PubMed] [Google Scholar]
Frings 1982 {published data only}
- Frings N, Bormann Bv, Kroh U, Lennartz H. Peridural anesthesia and analgesia results in general surgery [Peridurale anaesthesie‐ und analgesieverfahren in der allgemeinchirurgie]. Chirurg 1982;53:184‐8. [PubMed] [Google Scholar]
Fujita 2011 {published data only}
- Fujita Y, Nakamura K, Horiguchi Y, Ikeda D, Kaneko M, Tomioka K, et al. [Effect of different perioperative analgesic methods on postoperative cognitive dysfunction in elderly patients undergoing upper abdominal surgery]. Masui. The Japanese Journal of Anesthesiology 2011;60(10):1153‐8. [PUBMED: 22111354] [PubMed] [Google Scholar]
Garnett 1996 {published data only}
- Garnett RL, MacIntyre A, Lindsay P, Barber GG, Cole CW, Hajjar G, et al. Perioperative ischaemia in aortic surgery: combined epidural/general anaesthesia and epidural analgesia vs general anaesthesia and i.v. analgesia. Canadian Journal of Anaesthesia 1996;43(8):769‐77. [PUBMED: 8840054] [DOI] [PubMed] [Google Scholar]
Geddes 1991 {published data only}
- Geddes SM, Thorburn J, Logan RW. Gastric emptying following caesarean section and the effect of epidural fentanyl. Anaesthesia 1991;46:1016‐8. [DOI] [PubMed] [Google Scholar]
Gelman 1977 {published data only}
- Gelman S, Feigenberg Z, Dintzman M, Levy E. Electroenterography after cholecystectomy. Archives of Surgery 1977;112:580‐3. [DOI] [PubMed] [Google Scholar]
Gelman 1980 {published data only}
- Gelman S, Laws HL, Potzick J, Strong S, Smith L, Erdemir H. Thoracic epidural vs balanced anesthesia in morbid obesity: an intraoperative and postoperative hemodynamic study. Anesthesia and Analgesia 1980;59(12):902‐8. [PUBMED: 7192509] [PubMed] [Google Scholar]
George 1992 {published data only}
- George KA, Chisakuta AM, Gamble JAS, Browne GA. Thoracic epidural infusion for postoperative pain relief following abdominal aorta surgery: bupivacaine, fentanyl or a mixture of both?. Anaesthesia 1992;47:388‐94. [DOI] [PubMed] [Google Scholar]
Gold 1994 {published data only}
- Gold MS, DeCrosta D, Rizzuto C, Ben‐Harari RR, Ramanathan S. The effect of lumbar epidural and general anesthesia on plasma catecholamines and hemodynamics during abdominal aortic aneurysm repair. Anesthesia and Analgesia 1994;78(2):225‐30. [PUBMED: 8311273] [DOI] [PubMed] [Google Scholar]
Gow 1979 {published data only}
- Gow NM, Cochrane JP. The effect of epidural analgesia on postoperative sodium balance. The British Journal of Surgery 1979;66(12):864‐7. [PUBMED: 509060] [DOI] [PubMed] [Google Scholar]
Grass 1992 {published data only}
- Grass JA, Sakima NT. Epidural anaesthesia and analgesia results ln shorter hospital stay after total abdominal hysterectomy. Regional Anesthesia and Pain Medicine 1992;17(Suppl 1):77. [Google Scholar]
Grass 1993 {published data only}
- Grass JA, Sakima NT, Valley M, Fischer K, Jackson C, Walsh P, et al. Assessment of ketorolac as an adjuvant to fentanyl patient‐controlled epidural analgesia after radical retropubic prostatectomy. Anesthesiology 1993;78:642‐8. [PubMed] [Google Scholar]
Gulucu 2009 {published data only}
- Gulucu C, Yilmaz AA, Yalcin S, Can OS, Demiralp S, Alkis N. [The effects of patient controlled analgesia methods with intravenous and epidural morphine on cognitive functions in geriatric patients] [Geriatrik hastalarda intravezoz ve epidural morfin ile hasta kontrollu analjeziuygulamasinin kognitif fonksiyonlar uzerine etkisi]. Anesteziyoloji ve Reanimasyon Uzmanları Derneği 2009;17(1):41‐8. [CENTRAL: CN‐00754806 ] [Google Scholar]
Gupta 2006 {published data only}
- Gupta A, Fant F, Axelsson K, Sandblom D, Rykowski J, Johansson JE, et al. Postoperative analgesia after radical retropubic prostatectomy: a double‐blind comparison between low thoracic epidural and patient‐controlled intravenous analgesia. Anesthesiology 2006;105(4):784‐93. [PUBMED: 17006078] [DOI] [PubMed] [Google Scholar]
Han 2005 {published data only}
- Han C, Zhou X, Qian Y, Yu L, Hao X. Effects of different analgesic techniques on blood glucose, insulin and cortisol in post‐hysterectomy patients [Original title available only in Chinese characters]. Journal of Xi'an Jiaotong University (Medical Sciences) 2005;26(2):191‐4. [EMBASE: 2005214273] [Google Scholar]
Harukuni 1995 {published data only}
- Harukuni I, Yamaguchi H, Sato S, Naito H. The comparison of epidural fentanyl, epidural lidocaine, and intravenous fentanyl in patients undergoing gastrectomy. Anesthesia and Analgesia 1995;81:1169‐74. [DOI] [PubMed] [Google Scholar]
Hashimoto 1995 {published data only}
- Hashimoto T, Hashimoto S, Hori Y, Nakagawa H, Hosokawa T. Epidural anaesthesia blocks changes in peripheral lymphocytes subpopulation during gastrectomy for stomach cancer. Acta Anaesthesiologica Scandinavica 1995;39(3):294‐8. [PUBMED: 7793203] [DOI] [PubMed] [Google Scholar]
Hendolin 1982 {published data only}
- Hendolin H, Tuppurainen T, Lahtinen J. Thoracic epidural analgesia and deep vein thrombosis in cholecystectomized patients. Acta Chirurgica Scandinavica 1982;148(5):405‐9. [PUBMED: 6758456] [PubMed] [Google Scholar]
Hendolin 1987a {published data only}
- Hendolin H, Lahtinen J, Länsimies E, Tuppurainen T, Partanen K. The effect of thoracic epidural analgesia on respiratory function after cholecystectomy. Acta Anaesthesiologica Scandinavia 1987;31:645‐51. [DOI] [PubMed] [Google Scholar]
Hendolin 1987b {published data only}
- Hendolin H, Lahtinen J, Lansimies E, Tuppurainen T. The effect of thoracic epidural analgesia on postoperative stress and morbidity. Annales Chirurgiae et Gynaecologiae 1987;76(4):234‐40. [PUBMED: 3434997] [PubMed] [Google Scholar]
Her 1990 {published data only}
- Her C, Kizelshteyn G, Walker V, Hayes D, Lees DE. Combined epidural and general anesthesia for abdominal aortic surgery. Journal of Cardiothoracic Anesthesia 1990;4(5):552‐7. [PUBMED: 2132133] [DOI] [PubMed] [Google Scholar]
Hjortso 1986 {published data only}
- Hjortso NC, Lund C, Mogensen T, Bigler D, Kehlet H. Epidural morphine improves pain relief and maintains sensory analgesia during continuous epidural bupivacaine after abdominal surgery. Anesthesia and Analgesia 1986;65(10):1033‐6. [PUBMED: 3752551] [PubMed] [Google Scholar]
Houweling 1992 {published data only}
- Houweling PL, Ionescu TI. Epidural bupivacaine versus epidural sufentanil anesthesia: hemodynamic differences during induction of anesthesia and abdominal dissection in aortic surgery. Acta Anaesthesiologica Belgica 1992;43(4):227‐33. [PUBMED: 1300856] [PubMed] [Google Scholar]
Hull 1991 {published data only}
- Hull DB, Varner MW. A randomized study of closure of the peritoneum at cesarean delivery. Obstetrics and Gynecology 1991;77(6):818‐21. [PUBMED: 2030849] [PubMed] [Google Scholar]
Inoue 2005 {published data only}
- Inoue S, Mitsuhata H, Kawakami T, Shimohata K, Hirabayashi Y, Seo N. Addition of 0.1% bupivacaine to buprenorphine and droperidol in patient‐controlled epidural analgesia improved postoperative pain scores on coughing after gynecological surgery. Journal of Clinical Anesthesia 2005;17(3):167‐71. [PUBMED: 15896581] [DOI] [PubMed] [Google Scholar]
Jones 2013 {published data only}
- Jones C, Kelliher L, Dickinson M, Riga A, Worthington T, Scott MJ, et al. Randomized clinical trial on enhanced recovery versus standard care following open liver resection. The British Journal of Surgery 2013;100(8):1015‐24. [PUBMED: 23696477] [DOI] [PubMed] [Google Scholar]
Jorgensen 1978 {published data only}
- Jörgensen H. Epidural anaesthesia with bupivacaine 0.75% in comparison with bupivacaine 0.5% and mepivacaine 1.5% with adrenaline [Epiduralanaesthesie mit bupivacain 0.75% im vergleich bupivacain 0.5% und mepivacain‐adrenalin 1.5%]. Regional‐Anaesthesie 1978;1:11‐5. [PubMed] [Google Scholar]
Kabon 2003 {published data only}
- Kabon B, Fleischmann E, Treschan T, Taguchi A, Kapral S, Kurz A. Thoracic epidural anesthesia increases tissue oxygenation during major abdominal surgery. Anesthesia and Analgesia 2003;97(6):1812‐7. [PUBMED: 14633566] [DOI] [PubMed] [Google Scholar]
Kajiyama 2004 {published data only}
- Kajiyama S, Kobayashi M, Okada Y. [Criteria for postoperative discharge of the patients managed by anesthesiologists in an ambulatory surgery unit]. Masui. The Japanese Journal of Anesthesiology 2004;53(8):882‐7. [PUBMED: 15446676] [PubMed] [Google Scholar]
Kapral 1996 {published data only}
- Kapral S, Gollmann G, Lehofer F. Gastric tonometry as a visceral perfusion monitoring during thoracic epidural anaesthesia. Acta Anaesthesiologic Scandinavia 1996;109(Suppl):178‐80. [PubMed] [Google Scholar]
Kausalya 1994 {published data only}
- Kausalya R, Jacob R. Efficacy of low‐dose epidural anaesthesia in surgery of anal canal ‐ A randomised controlled trial. Anaesthesia and Intensive Care Journal 1994;22:161‐4. [DOI] [PubMed] [Google Scholar]
Kilbride 1992 {published data only}
- Kilbride MJ, Senagore J, Patric Mazier W, Ferguson C, Ufkes T. Epidural analgesia. Surgery, Gynaecology & Obstetrics 1992;174:137‐40. [PubMed] [Google Scholar]
Kiya 2003 {published data only}
- Kiya T, Fujimura N, Okanuma M, Namiki A. [Effect of continuous extradural infusion of bupivacaine on diaphragmatic function after upper abdominal surgery]. Masui. The Japanese Journal of Anesthesiology 2003;52(5):500‐4. [PUBMED: 12795130] [PubMed] [Google Scholar]
Koganemaru 1996 {published data only}
- Koganemaru M, Nakamura T, Takasaki M. Postoperative pain management with continuous epidural infusion of fentanyl‐bupivacaine mixture. Hiroshima Journal of Anesthesia 1996;32(3):267‐9. [CENTRAL: 00352104 ] [Google Scholar]
Korinek 1985 {published data only}
- Korinek AM, Languille M, Bonnet F, Thibonnier M, Sasano P, Lienhart A, et al. Effect of postoperative extradural morphine on ADH secretion. British Journal of Anaesthesia 1985;57:407‐11. [DOI] [PubMed] [Google Scholar]
Kossman 1982 {published data only}
- Kossman B, Volk E, Spilker ED, Maier V, Fehm HL. Influence of thoracic epidural analgesia on glucose, cortisol, insulin, and glucagon responses to surgery. Regional Anesthesia and Pain Medicine 1982;7(3):107‐9. [Google Scholar]
Kouraklis 2000 {published data only}
- Kouraklis G, Glinavou A, Raftopoulos L, Alevisou V, Lagos G, Karatzas G. Epidural analgesia attenuates the systemic stress response to upper abdominal surgery: a randomized trial. International Surgery 2000;85(4):353‐7. [PUBMED: 11589607] [PubMed] [Google Scholar]
Krane 1987 {published data only}
- Krane EJ, Jacobson LE, Lynn AM, Parrot C, Tyler D. Caudal morphine for postoperative analgesia in children: a comparison with caudal bupivacaine and intravenous morphine. Anesthesia and Analgesia 1987;66:647‐53. [PubMed] [Google Scholar]
Krane 1989 {published data only}
- Krane EJ, Tyler DC, Jacobsen LE. The dose response of caudal morphine in children. Anesthesiology 1989;71:48‐52. [DOI] [PubMed] [Google Scholar]
Kumar 1993 {published data only}
- Kumar TP, Jacob R. A comparison of caudal epidural bupivacaine with adrenaline and bupivacaine with adrenaline and pethidine for operative and postoperative analgesia in infants and children. Anaesthesia and Intensive Care Journal 1993;21:424‐8. [DOI] [PubMed] [Google Scholar]
Lattermann 2001 {published data only}
- Lattermann R, Schricker T, Wachter U, Goertz A, Georgieff M. Intraoperative epidural blockade prevents the increase in protein breakdown after abdominal surgery. Acta Anaesthesiologica Scandinavica 2001;45(9):1140‐6. [PUBMED: 11683666] [DOI] [PubMed] [Google Scholar]
Lattermann 2002 {published data only}
- Lattermann R, Carli F, Wykes L, Schricker T. Epidural blockade modifies perioperative glucose production without affecting protein catabolism. Anesthesiology 2002;97(2):374‐81. [PUBMED: 12151927] [DOI] [PubMed] [Google Scholar]
Lattermann 2003 {published data only}
- Lattermann R, Carli F, Wykes L, Schricker T. Perioperative glucose infusion and the catabolic response to surgery: the effect of epidural block. Anesthesia and Analgesia 2003;96(2):555‐62. [PUBMED: 12538212] [DOI] [PubMed] [Google Scholar]
Lee 1988 {published data only}
- Lee A, Simpson D, Whitfield A, Scott DB. Postoperative analgesia by continuous extradural infusion of bupivacaine and diamorphine. British Journal of Anaesthesia 1988;60:845‐50. [DOI] [PubMed] [Google Scholar]
Lee 1991 {published data only}
- Lee A, McKeown D, Brockway M, Bannister J, Wildsmith JAW. Comparison of extradural and intravenous diamorphine as a supplement to extradural bupivacaine. Anaesthesia 1991;46:447‐50. [DOI] [PubMed] [Google Scholar]
Licker 1995 {published data only}
- Licker M, Farinelli C, Klopfenstein CE. Cardiovascular reflexes during anesthesia induction and tracheal intubation in elderly patients: the influence of thoracic epidural anesthesia. Journal of Clinical Anesthesia 1995;7(4):281‐7. [PUBMED: 7546753] [DOI] [PubMed] [Google Scholar]
Liu 2005 {published data only}
- Liu X‐R, Wang Y‐L, Wang C‐Y. Effects of different patient‐controlled analgesic methods on circulative function in hypertensive patients after upper abdominal surgery [Original title available in Chinese characters only]. Chinese Journal of Clinical Rehabilitation 2005;9(2):29‐31. [EMBASE: 2005272688] [Google Scholar]
Lowson 1994 {published data only}
- Lowson SM, Alexander JI, Black AM, Bambridge AD. Epidural diamorphine infusions with and without 0.167% bupivacaine for post‐operative analgesia. European Journal of Anaesthesiology 1994;11(5):345‐52. [PUBMED: 7988577] [PubMed] [Google Scholar]
Madej 1992 {published data only}
- Madej TH, Wheatley RG, Jackson IJ, Hunter D. Hypoxaemia and pain relief after lower abdominal surgery: comparison of extradural and patient‐controlled analgesia. British Journal of Anaesthesia 1992;69(6):554‐7. [PUBMED: 1467095] [DOI] [PubMed] [Google Scholar]
Manikian 1988 {published data only}
- Manikian B, Cantineau JP, Bertrand M, Kieffer E, Sartene R, Viars P. Improvement of diaphragmatic function by a thoracic extradural block after upper abdominal surgery. Anesthesiology 1988;68(3):379‐86. [PUBMED: 3344992] [DOI] [PubMed] [Google Scholar]
Marco 1989 {published data only}
- Marco VJ, Mabrok MM, Arqués Teixidor P, González B, Vidal Claramunt JM, Banõs JE. Caudal morphine for postoperative analgesia in children: randomized study and double‐blind evaluation with bupivacaine and control [Analgesia postoperatoria con morfina por vía caudal cirugía pediádrica: esudio aleatorio y a doble ciego comparado con bupivacaína]. Revista Española de Anestesiología y Reanimación 1989;36:88‐92. [PubMed] [Google Scholar]
Matsunaga 1996 {published data only}
- Matsunaga M, Akashi K, Sugano H, Gotoh H, Dan K. Postoperative pain relief and epidural analgesia for open cholecystectomy, laparoscopic cholecystectomy, and gastrectomy. Anesthesia and Resuscitation 1996;32(3):257‐61. [EMBASE: 1997228179] [Google Scholar]
Mellbring 1983 {published data only}
- Mellbring G, Dahlgren S, Reiz S, Sunnegardh O. Thromboembolic complications after major abdominal surgery: effect of thoracic epidural analgesia. Acta Chirurgica Scandinavica 1983;149(3):263‐8. [PUBMED: 6613461] [PubMed] [Google Scholar]
Modig 1981 {published data only}
- Modig J, Paalzow L. A comparison of epidural morphine and epidural bupivacaine for postoperative pain relief. Acta Anaesthesiologica Scandinavica 1981;25:437‐41. [DOI] [PubMed] [Google Scholar]
Moine 1992 {published data only}
- Moine P, Ecoffey C. Caudal block in children: analgesic and ventilatory effects of bupivacaine combined with fentanyl [Bloc caudal chez l'enfant: analgésie et effets respiratoires de l'association bupivacaine‐fentanyl]. Annales Françaises d'Anesthésie et de Réanimation 1992;11:141‐4. [DOI] [PubMed] [Google Scholar]
Moller 1982 {published data only}
- Moller W, Rem J, Brandt R, Kehlet H. Effect of posttraumatic epidural analgesia on the cortisol and hyperglycaemic response to surgery. Acta Anaesthesiologica Scandinavica 1982;26(1):56‐8. [PUBMED: 7072474] [DOI] [PubMed] [Google Scholar]
Morley 2002 {published data only}
- Morley AP, Derrick J, Seed PT, Tan PE, Chung DC, Short TG. Isoflurane dosage for equivalent intraoperative electroencephalographic suppression in patients with and without epidural blockade. Anesthesia and Analgesia 2002;95(5):1412‐8, table of contents. [PUBMED: 12401635] [DOI] [PubMed] [Google Scholar]
Moselli 2011 {published data only}
- Moselli NM, Baricocchi E, Ribero D, Sottile A, Suita L, Debernardi F. Intraoperative epidural analgesia prevents the early proinflammatory response to surgical trauma. Results from a prospective randomized clinical trial of intraoperative epidural versus general analgesia. Annals of Surgical Oncology 2011;18(10):2722‐31. [PUBMED: 21479690] [DOI] [PubMed] [Google Scholar]
Moskovitz 1986 {published data only}
- Moskovitz B, Bolkier M, Ginesin Y, Levin DR, Rosenberg B. Epidural morphine: a new approach to combined anesthesia and analgesia in urological patients. European Urology 1986;12:171‐3. [DOI] [PubMed] [Google Scholar]
Muneyuki 1968 {published data only}
- Muneyuki M, Ueda Y, Urabe N, Takeshita H, Inamoto A. Postoperative pain relief and respiratory function in man: comparison between intermittent intravenous injections of meperidine and continuous lumbar epidural analgesia. Anesthesiology 1968;29(2):304‐13. [DOI] [PubMed] [Google Scholar]
Murakami 2009 {published data only}
- Murakami T, Okuda Y, Ishii M, Kobayashi A, Kawamura M. [Comparison of intravenous fentanyl analgesia and epidural analgesia for postoperative pain relief]. Masui. The Japanese Journal of Anesthesiology 2009;58(9):1149‐53. [PUBMED: 19764439] [PubMed] [Google Scholar]
Murrat 1988 {published data only}
- Murrat I, Walker J, Esteve C, Nahoul K, Saint‐Maurice C. Effect of lumbar epidural anaesthesia on plasma cortisol levels in children. Canadian Journal of Anaesthesia 1988;35:20‐4. [DOI] [PubMed] [Google Scholar]
Mushambi 1992 {published data only}
- Mushambi MC, Rowbotham DJ, Bailey SM. Gastric emptying after minor gynaecological surgery. Anaesthesia 1992;47:297‐9. [DOI] [PubMed] [Google Scholar]
Naesh 1994 {published data only}
- Naesh O, Hindberg I, Friis J, Christiansen C, Pedersen T, Trap‐Jensen J, et al. Platelet activation in major surgical stress: influence of combined epidural and general anaesthesia. Acta Anaesthesiologica Scandinavica 1994;38(8):820‐5. [PUBMED: 7887105] [DOI] [PubMed] [Google Scholar]
Nandate 2003 {published data only}
- Nandate K, Ogata M, Nishimura M, Katsuki T, Kusuda S, Okamoto K, et al. The difference between intramural and arterial partial pressure of carbon dioxide increases significantly during laparoscopic cholecystectomy: the effect of thoracic epidural anesthesia. Anesthesia and Analgesia 2003;97(6):1818‐23. [PUBMED: 14633567] [DOI] [PubMed] [Google Scholar]
Niiyama 2005 {published data only}
- Niiyama Y, Kawamata T, Shimizu H, Omote K, Namiki A. The addition of epidural morphine to ropivacaine improves epidural analgesia after lower abdominal surgery. Canadian Journal of Anaesthesia 2005;52(2):181‐5. [PUBMED: 15684260] [DOI] [PubMed] [Google Scholar]
Nimmo 1978 {published data only}
- Nimmo WS, Littlewood DG, Scott DB, Prescott LF. Gastric emptying following hysterectomy with extradural analgesia. British Journal of Anaesthesia 1978;50:559‐61. [DOI] [PubMed] [Google Scholar]
Nishikawa 2007 {published data only}
- Nishikawa K, Kimura S, Shimodate Y, Igarashi M, Namiki A. A comparison of intravenous‐based and epidural‐based techniques for anesthesia and postoperative analgesia in elderly patients undergoing laparoscopic cholecystectomy. Journal of Anesthesia 2007;21(1):1‐6. [PUBMED: 17285405] [DOI] [PubMed] [Google Scholar]
Nishiyama 1991 {published data only}
- Nishiyama T, Odaka Y, Hirasaki A, Mikane T, Kobayashi O, Seto K. [Epidural administration of midazolam with saline or bupivacaine for postoperative pain]. Masui. The Japanese Journal of Anesthesiology 1991;40(10):1525‐30. [PUBMED: 1766101] [PubMed] [Google Scholar]
Noreng 1987 {published data only}
- Noreng MF, Jensen P, Tjellden NU. Per‐ and postoperative changes in the concentration of serum thyreotropin under general anaesthesia, compared to general anaesthesia with epidural analgesia. Acta Anaesthesiologica Scandinavica 1987;31(4):292‐4. [PUBMED: 3591253] [DOI] [PubMed] [Google Scholar]
Norris 2001 {published data only}
- Norris EJ, Beattie C, Perler BA, Martinez EA, Meinert CL, Anderson GF, et al. Double‐masked randomized trial comparing alternate combinations of intraoperative anesthesia and postoperative analgesia in abdominal aortic surgery. Anesthesiology 2001;95(5):1054‐67. [PUBMED: 11684971] [DOI] [PubMed] [Google Scholar]
O'Connor 2001 {published data only}
- O'Connor PJ, Wollin TA, Finucane BT. Controlled Hypotension with combined epidural /general anesthesia reduces bllod loss and transfusion requiremnts compared with general anesthesia alone in radical prostatectomy (conference abstract). Anesthesia and Analgesia. 2001; Vol. 92 S1:S363.
Ohtaka 1991 {published data only}
- Ohtaka K, Matsumoto S, Mitsuhata H, Yabe M. [The effect of continuous epidural infusion of a combination of 1% mepivacaine and buprenorphine for post‐operative pain relief]. Masui. The Japanese Journal of Anesthesiology 1991;40(6):942‐8. [PUBMED: 1875542] [PubMed] [Google Scholar]
Olofsson 1997 {published data only}
- Olofsson Ch, Ekblom A, Sköldefors E, Wåglund B, Irestedt L. Anesthetic quality during cesarean section following subarachnoid or epidural administration of bupivacaine with or without fentanyl. Acta Anaesthesiologica Scandinavica 1997;41:332‐8. [DOI] [PubMed] [Google Scholar]
Omar 2009 {published data only}
- Omar SH, Radwan KG, Youssif MA, Khafagy HF, Kamal NM, El‐Sabae HH, et al. A non opioid fast track anesthetic regimen for colonic resection. Journal of the Egyptian Society of Parasitology 2009;39(3):849‐64. [PUBMED: 20120751] [PubMed] [Google Scholar]
Osipova 2002 {published data only}
- Osipova NA, Petrova VV, Mitrofanov SV, Beresnev VA, Biriukov VI, Bogdanova EV. [Preparations for peripheral and segmental level protection of the patient in the system of general anesthesia and postoperative analgesia] [Sredstva perifericheskogo i segmentarnogo urovnei zashchity patsienta v sisteme obshchei anestezii i posleoperatsionnogo obezbolivaniia.]. Anesteziologiia i Reanimatologiia 2002;47(4):14‐9. [PUBMED: 12462769] [PubMed] [Google Scholar]
Paech 1994 {published data only}
- Paech MJ, Westmore MD. Postoperative epidural fentanyl infusion ‐ is the addition of 0.1% bupivacaine of benefit?. Anaesthesia and Intensive Care 1994;22(1):9‐14. [PUBMED: 8160955] [DOI] [PubMed] [Google Scholar]
Parker 1992 {published data only}
- Parker RK, Sawaki Y, White PF. Epidural patient‐controlled analgesia: influence of bupivacaine and hydromorphone basal infusion on pain control after cesarean delivery. Anesthesia and Analgesia 1992;75(5):740‐6. [PUBMED: 1384397] [DOI] [PubMed] [Google Scholar]
Petring 1984 {published data only}
- Petring OU, Adelhøj B, Erin‐Madsen J, Angelo H, Jelert H. Epidural anaesthesia does not delay early postoperative gastric emptying in man. Acta Anaesthesiologica Scandinavia 1984;28:393‐5. [DOI] [PubMed] [Google Scholar]
Poopalalingam 2003 {published data only}
- Poopalalingam R, Chow MY, Wong LT. Patient‐controlled epidural analgesia after thoracic and upper abdominal surgery using sufentanil with and without bupivacaine 0.125%. Singapore Medical Journal 2003;44(3):126‐30. [PUBMED: 12953725] [PubMed] [Google Scholar]
Porter 1997 {published data only}
- Porter JS, Bonello E, Reynolds F. The influence of epidural administration of fentanyl infusion on gastric emptying in labour. Anaesthesia 1997;52:1151‐6. [PUBMED: 9485967] [DOI] [PubMed] [Google Scholar]
Pouzeratte 2001 {published data only}
- Pouzeratte Y, Delay JM, Brunat G, Boccara G, Vergne C, Jaber S, et al. Patient‐controlled epidural analgesia after abdominal surgery: ropivacaine versus bupivacaine. Anesthesia and Analgesia 2001;93(6):1587‐92. [PUBMED: 11726450] [DOI] [PubMed] [Google Scholar]
Rademaker 1992 {published data only}
- Rademaker BM, Ringers J, Odoom JA, Wit LT, Kalkman CJ, Oosting J. Pulmonary function and stress response after laparoscopic cholecystectomy: comparison with subcostal incision and influence of thoracic epidural analgesia. Anesthesia and Analgesia 1992;75(3):381‐5. [PUBMED: 1387297] [DOI] [PubMed] [Google Scholar]
Randalls 1991 {published data only}
- Randalls B, Broadway JW, Browne DA, Morgan BM. Comparison of four subarachnoid solutions in a needle‐through‐needle technique for elective caesarean section. British Journal of Anaesthesia 1991;66:314‐8. [PUBMED: 2015147] [DOI] [PubMed] [Google Scholar]
Rawal 1984 {published data only}
- Rawal N, Sjostrand U, Christoffersson E, Dahlstrom B, Arvill A, Rydman H. Comparison of intramuscular and epidural morphine for postoperative analgesia in the grossly obese: influence on postoperative ambulation and pulmonary function. Anesthesia and Analgesia 1984;63(6):583‐92. [PUBMED: 6233917] [PubMed] [Google Scholar]
Reinhart 1989 {published data only}
- Reinhart K, Foehring U, Kersting T, Schaefer M, Bredle D, Hirner A, et al. Effects of thoracic epidural anesthesia on systemic hemodynamic function and systemic oxygen supply‐demand relationship. Anesthesia and Analgesia 1989;69(3):360‐9. [PUBMED: 2774232] [PubMed] [Google Scholar]
Reiz 1982 {published data only}
- Reiz S, Haggmark S, Rydvall A, Ostman M. Beta‐blockers and thoracic epidural analgesia. Cardioprotective and synergistic effects. Acta Anaesthesiologica Scandinavica. Supplementum 1982;76:54‐61. [PUBMED: 6152883] [DOI] [PubMed] [Google Scholar]
Renck 1975 {published data only}
- Renck H, Edström H. Thoracic epidural analgesia ‐ a double‐blind study between etidocaine and bupivacaine. Acta Anaesthesiologica Scandinavica 1975;Suppl 60:72‐5. [PUBMED: 1101615] [PubMed] [Google Scholar]
Rucci 1985 {published data only}
- Rucci FS, Cardamone M, Migliori P. Fentanyl and bupivacaine mixtures for extradural blockade. British Journal of Anaesthesia 1985;57:275‐84. [DOI] [PubMed] [Google Scholar]
Ryan 1992 {published data only}
- Ryan P, Schweitzer SA, Woods RJ. Effect of epidural and general anaesthesia compared with general anaesthesia alone in large bowel anastomoses. A prospective study. The European Journal of Surgery 1992;158(1):45‐9. [PUBMED: 1348641] [PubMed] [Google Scholar]
Saito 1995 {published data only}
- Saito Y, Sakura S, Kaneko M, Kosaka Y. Interaction of extradural morphine and lignocaine on ventilatory response. British Journal of Anaesthesia 1995;75(4):394‐8. [PUBMED: 7488475] [DOI] [PubMed] [Google Scholar]
Sakaguchi 1995 {published data only}
- Sakaguchi H, Ikuta Y, Seshita M, Nakano N, Kano T. [Bupivacaine‐fentanyl continuous infusion is superior to morphine bolus injection in postoperative epidural analgesia]. Masui. The Japanese Journal of Anesthesiology 1995;44(6):786‐94. [PUBMED: 7637152] [PubMed] [Google Scholar]
Salman 2013 {published data only}
- Salman N, Durukan AB, Gurbuz HA, Yamali H, Guler L, Ucar HI, et al. Comparison of effects of epidural bupivacaine and intravenous meperidine analgesia on patient recovery following elective abdominal aortic surgery. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 2013;19:347‐52. [PUBMED: 23666275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schug 1996 {published data only}
- Schug SA, Scott DA, Payne J, Mooney PH, Hagglof B. Postoperative analgesia by continuous extradural infusion of ropivacaine after upper abdominal surgery. British Journal of Anaesthesia 1996;76(4):487‐91. [PUBMED: 8652317] [DOI] [PubMed] [Google Scholar]
Schulte 2008 {published data only}
Schurizek 1982 {published data only}
- Schurizek BA, Rybro L, Petersen TK, Wernberg M. Value of epidural morphine in the treatment of postoperative pain following high laparotomies [En evaluering af epidural morfin i postoperativ smertebehandling af høje laparotomier]. Ugeskr Laeger 1982;144:2638‐41. [PubMed] [Google Scholar]
Scott 1989 {published data only}
- Scott NB, Mogensen T, Bigler D, Lund C, Kehlet H. Continuous thoracic extradural 0.5% bupivacaine with or without morphine: effect on quality of blockade, lung function and the surgical stress response. British Journal of Anaesthesia 1989;62:253‐7. [DOI] [PubMed] [Google Scholar]
Scott 1995 {published data only}
- Scott DA, Chamley DM, Mooney PH, Deam RK, Mark AH, Hagglof B. Epidural ropivacaine infusion for postoperative analgesia after major lower abdominal surgery ‐ a dose finding study. Anesthesia and Analgesia 1995;81(5):982‐6. [PUBMED: 7486088] [DOI] [PubMed] [Google Scholar]
Scott 1999 {published data only}
- Scott DA, Blake D, Buckland M, Etches R, Halliwell R, Marsland C, et al. A comparison of epidural ropivacaine infusion alone and in combination with 1, 2, and 4 microg/mL fentanyl for seventy‐two hours of postoperative analgesia after major abdominal surgery. Anesthesia and Analgesia 1999;88(4):857‐64. [PUBMED: 10195538] [DOI] [PubMed] [Google Scholar]
Seeling 1984 {published data only}
- Seeling W, Lotz P, Schröder M. Respiratory function after upper abdominal surgery. Continuous epidural analgesia has no advantage over intramuscular piritramide [Untersuchungen zur postoperativen lungenfunktion nach abdominellen eingriffen]. Anaesthesist 1984;33:408‐16. [PubMed] [Google Scholar]
Seeling 1985 {published data only}
- Seeling W, Ahnefeld FW, Rosenberg G, Heinrich H, Spilker D. Cardiovascular changes associated with epidural combined with general anaesthesia as compared to neuroleptanaesthesia [Aortofemoraler bifurkationsbypass ‐ der einfluss des anaesthesieverfahrens (NLA, thorakale kontinuerliche katheterperiduralanaesthesie) auf kreislauf, atmung und stoffwechsel]. Anaesthesist 1985;34:217‐28. [PubMed] [Google Scholar]
Seow 1976 {published data only}
- Seow LT, Chiu HH, Tye CY. Clinical evaluation of etidocaine in continuous caudal analgesia for pelvic floor repair and post‐operative pain relief. Anaesthesia and Intensive Care 1976;4:239‐44. [DOI] [PubMed] [Google Scholar]
Seow 1982 {published data only}
- Seow LT, Lips FJ, Cousins MJ, Mather LE. Lidocaine and bupivacaine mixtures for epidural blockade. Anesthesiology 1982;56:177‐83. [DOI] [PubMed] [Google Scholar]
Shir 1995 {published data only}
- Shir Y, Raja SN, Frank SM, Brendler CB. Intraoperative blood loss during radical retropubic prostatectomy: epidural versus general anaesthesia. Urology 1995;45(6):993‐9. [PUBMED: 7771032] [DOI] [PubMed] [Google Scholar]
Sinclair 1984 {published data only}
- Sinclair CJ, Scott DB. Comparison of bupivacaine and etidocaine in extradural blockade. British Journal of Anaesthesia 1984;56:147‐53. [DOI] [PubMed] [Google Scholar]
Smeets 1993 {published data only}
- Smeets HJ, Kievit J, Dulfer FT, Kleef JW. Endocrine‐metabolic response to abdominal aortic surgery: a randomized trial of general anesthesia versus general plus epidural anesthesia. World Journal of Surgery 1993;17(5):601‐6; discussion 606‐7. [PUBMED: 8273381] [DOI] [PubMed] [Google Scholar]
Smith 1996 {published data only}
- Smith AJ, Haynes TK, Roberts DE, Harmer M. A comparison of opioid solutions for patient‐controlled epidural analgesia. Anaesthesia 1996;51(11):1013‐7. [PUBMED: 8943590] [DOI] [PubMed] [Google Scholar]
Spence 1971 {published data only}
- Spence AA, Smith G. Postoperative analgesia and lung function: a comparison of morphine with extradural block. British Journal of Anaesthesia 1971;43(2):144‐8. [PUBMED: 5550845] [DOI] [PubMed] [Google Scholar]
Stamenkovic 2008 {published data only}
- Stamenkovic DM, Geric V, Slavkovic Z, Raskovic J, Djordjevic M. Combined spinal‐epidural analgesia vs. intermittent bolus epidural analgesia for pain relief after major abdominal surgery. A prospective, randomised, double‐blind clinical trial. International Journal of Clinical Practice 2008;62(2):255‐62. [PUBMED: 18028385] [DOI] [PubMed] [Google Scholar]
Stamenkovic 2009 {published data only}
- Stamenkovic D, Geric V, Djordjevic M, Raskovic J, Slavkovic Z, Randjelovic T, et al. Subarachnoid morphine, bupivacaine and fentanyl as part of combined spinal‐epidural analgesia for low anterior resection. A prospective, randomised, double‐blind clinical trial. Anaesthesia and Intensive Care 2009;37(4):552‐60. [PUBMED: 19681410] [DOI] [PubMed] [Google Scholar]
Stehr‐Zirngibl 1997 {published data only}
- Stehr‐Zirngibl S, Doblinger L, Neumeier S, Zirngibl H, Taeger K. [Intravenous versus thoracic‐epidural patient‐controlled analgesia following extended abdominal or thoracic surgery] [Intravenose versus thorakale‐epidurale patienten‐kontrollierte Analgesie bei ausgedehnten Oberbauch‐und Thoraxeingriffen]. Der Anaesthesist 1997;46 Suppl 3:S172‐8. [PUBMED: 9412274] [DOI] [PubMed] [Google Scholar]
Stenseth 1994 {published data only}
- Stenseth R, Bjella L, Berg EM, Christensen O, Levang OW, Gisvold SE. Thoracic epidural analgesia in aortocoronary bypass surgery. I: Haemodynamic effects. Acta Anaesthesiologica Scandinavica 1994;38(8):826‐33. [PUBMED: 826‐33] [DOI] [PubMed] [Google Scholar]
- Stenseth R, Bjella L, Berg EM, Christensen O, Levang OW, Gisvold SE. Thoracic epidural analgesia in aortocoronary bypass surgery. II: Effects on the endocrine metabolic response. Acta Anaesthesiologica Scandinavica 1994;38(8):834‐9. [PUBMED: 7887107] [DOI] [PubMed] [Google Scholar]
Sutcliffe 1996 {published data only}
- Sutcliffe NP, Mostafa SM, Gannon J, Harper SJ. The effect of epidural blockade on gastric intramucosal pH in the peri‐operative period. Anaesthesia 1996;51(1):37‐40. [PUBMED: 8669564] [DOI] [PubMed] [Google Scholar]
Suttner 2005 {published data only}
- Suttner S, Lang K, Piper SN, Schultz H, Rohm KD, Boldt J. Continuous intra‐ and postoperative thoracic epidural analgesia attenuates brain natriuretic peptide release after major abdominal surgery. Anesthesia and Analgesia 2005;101(3):896‐903, table of contents. [PUBMED: 16116011] [DOI] [PubMed] [Google Scholar]
- [No authors listed]. Continuous intra‐ and postoperative thoracic epidural analgesia attenuates brain natriuretic peptide release after major abdominal surgery: retraction. Anesthesia and Analgesia 2011;112(5):1109. [PUBMED: 21451068] [DOI] [PubMed] [Google Scholar]
Takahashi 2005 {published data only}
- Takahashi W, Okazaki J, Yamamoto T, Nishino T. [Effects of simultaneous epidural administration of ropivacaine and morphine on the post‐operative pain in the gynecologic patients]. Masui. The Japanese Journal of Anesthesiology 2005;54(2):126‐32. [PUBMED: 15747505] [PubMed] [Google Scholar]
Thörn 1992 {published data only}
- Thörn SE, Wattwil M, Näslund I. Postoperative epidural morphine, but not epidural bupivacaine, delays gastric emptying on the first day after cholecystectomy. Regional Anesthesia 1992;17:91‐4. [PubMed] [Google Scholar]
Thörn 1996 {published data only}
- Thörn SE, Wichbom G, Philipson L, Leissner P, Wattwil M. Myoelectric activity in the stomach and duodenum after epidural administration of morphine or bupivacaine. Acta Anaesthesiologica Scandinavica 1996;40:773‐8. [DOI] [PubMed] [Google Scholar]
Tikuisis 2009 {published data only}
- Tikuisis R, Miliauskas P, Samalavicius NE, Zurauskas A, Sruogis A. Epidural and general anesthesia versus general anesthesia in radical prostatectomy. Medicina (Kaunas, Lithuania) 2009;45(10):772‐7. [PUBMED: 19996663] [PubMed] [Google Scholar]
Torda 1995 {published data only}
- Torda TA, Hann P, Mills G, Leon G, Penman D. Comparison of extradural fentanyl, bupivacaine and two fentanyl‐bupivacaine mixtures for pain relief after abdominal surgery. British Journal of Anaesthesia 1995;74:35‐40. [DOI] [PubMed] [Google Scholar]
Traynor 1982 {published data only}
- Traynor C, Paterson JL, Ward ID, Morgan M, Hall GM. Effects of extradural analgesia and vagal blockade on the metabolic and endocrine response to upper abdominal surgery. British Journal of Anaesthesia 1982;54(3):319‐23. [PUBMED: 7039644] [DOI] [PubMed] [Google Scholar]
Tsuji 1983 {published data only}
- Tsuji H, Asoh T, Takeuchi Y, Shirasaka C. Attenuation of adrenocortical response to upper abdominal surgery with epidural blockade. The British Journal of Surgery 1983;70(2):122‐4. [PUBMED: 6824898] [DOI] [PubMed] [Google Scholar]
Tsuji 1983a {published data only}
- Tsuji H, Shirasaka C, Asoh T, Takeuchi Y. Influences of splanchnic nerve blockade on endocrine‐metabolic responses to upper abdominal surgery. The British Journal of Surgery 1983;70(7):437‐9. [PUBMED: 6307458] [DOI] [PubMed] [Google Scholar]
Tsuji 1987 {published data only}
- Tsuji H, Shirasaka C, Asoh T, Uchida I. Effects of epidural administration of local anaesthetics or morphine on postoperative nitrogen loss and catabolic hormones. The British Journal of Surgery 1987;74(5):421‐5. [PUBMED: 3594144] [DOI] [PubMed] [Google Scholar]
Uchida 1988 {published data only}
- Uchida I, Asoh T, Shirasaka C, Tsuji H. Effect of epidural analgesia on postoperative insulin resistance as evaluated by insulin clamp technique. The British Journal of ´Surgery 1988;75(6):557‐62. [PUBMED: 3293693] [DOI] [PubMed] [Google Scholar]
Vedrinne 1989 {published data only}
- Vedrinne C, Vedrinne JM, Guiraud M, Patricot MC, Bouletreau P. Nitrogen‐sparing effect of epidural administration of local anesthetics in colon surgery. Anesthesia and Analgesia 1989;69(3):354‐9. [PUBMED: 2774231] [PubMed] [Google Scholar]
Virlos 2010 {published data only}
- Virlos I, Clements D, Beynon J, Ratnalikar V, Khot U. Short‐term outcomes with intrathecal versus epidural analgesia in laparoscopic colorectal surgery. The British Journal of Surgery 2010;97(9):1401‐6. [PUBMED: 20603849] [DOI] [PubMed] [Google Scholar]
von Ungern‐Sternberg 2005 {published data only}
- Ungern‐Sternberg BS, Regli A, Reber A, Schneider MC. Effect of obesity and thoracic epidural analgesia on perioperative spirometry. British Journal of Anaesthesia 2005;94(1):121‐7. [PUBMED: 15486001] [DOI] [PubMed] [Google Scholar]
Wang 2013 {published data only}
- Wang P, Wang HW, Zhong TD. Influence of different anesthesia on liver and renal function in elderly patients undergoing laparoscopic colon or rectal resection. Hepato‐Gastroenterology 2013;60(121):79‐82. [PUBMED: 22773302] [DOI] [PubMed] [Google Scholar]
Wessen 1994 {published data only}
- Wessen A, Persson PM, Nilsson A, Hartvig P. Clinical pharmacokinetics of propofol given as a constant‐rate infusion and in combination with epidural blockade. Journal of Clinical Anesthesia 1994;6(3):193‐8. [PUBMED: 7914736] [DOI] [PubMed] [Google Scholar]
White 1979 {published data only}
- White WD, Pearce DJ, Norman J. Postoperative analgesia: a comparison of intravenous on‐demand fentanyl with epidural bupivacaine. British Medical Journal 1979;2(6183):166‐7. [PUBMED: 466335] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiebalck 1997 {published data only}
- Wiebalcht A, Brodner G, Aken HV. The effect of adding sufentanil to bupivacaine for postoperative patient‐controlled epidural analgesia. Anesthesia and Analgesia 1997;85:124‐9. [PUBMED: 9212134] [DOI] [PubMed] [Google Scholar]
Wolf 1993 {published data only}
- Wolf AR, Hughes D. Pain relief for infants undergoing abdominal surgery: comparison of infusions of i.v. morphine and extradural bupivacaine. British Journal of Anaesthesia 1993;70:10‐6. [DOI] [PubMed] [Google Scholar]
Wright 1992 {published data only}
- Wright PMC, Allen RW, Moore J, Donnelly JP. Gastric emptying during lumbar extradural analgesia in labour: effect of fentanyl supplementation. British Journal of Anaesthesia 1992;68:248‐51. [DOI] [PubMed] [Google Scholar]
Wu 2000 {published data only}
- Wu CT, Yeh CC, Yu JC, Lee MM, Tao PL, Ho ST, et al. Pre‐incisional epidural ketamine, morphine and bupivacaine combined with epidural and general anaesthesia provides pre‐emptive analgesia for upper abdominal surgery. Acta Anaesthesiologica Scandinavica 2000;44(1):63‐8. [PUBMED: 10669274] [DOI] [PubMed] [Google Scholar]
Yeh 2005 {published data only}
- Yeh CC, Jao SW, Huh BK, Wong CS, Yang CP, White WD, et al. Preincisional dextromethorphan combined with thoracic epidural anesthesia and analgesia improves postoperative pain and bowel function in patients undergoing colonic surgery. Anesthesia and Analgesia 2005;100(5):1384‐9. [PUBMED: 15845691] [DOI] [PubMed] [Google Scholar]
Yorozu 1996 {published data only}
- Yorozu T, Morisaki H, Kondoh M, Toyoda Y, Miyazawa N, Shigematsu T. Epidural anaesthesia during upper abdominal surgery provides better postoperative analgesia. Journal of Anaesthesia 1996;10(1):10‐5. [PUBMED: 23839545] [DOI] [PubMed] [Google Scholar]
Yorozu 1997 {published data only}
- Yorozu T, Morisaki H, Kondoh M, Tomizawa K, Satoh M, Shigematsu T. Epidural anesthesia during hysterectomy diminishes postoperative pain and urinary cortisol release. Journal of Anesthesia 1997;11(4):260‐4. [EMBASE: 1998011089] [DOI] [PubMed] [Google Scholar]
Yuceyar 2004 {published data only}
- Yuceyar L, Erolcay H, Konukoglu D, Bozkurt AK, Aykac B. Epidural anesthesia may attenuate lipid peroxidation during aorto‐femoral surgery. Canadian Journal of Anaesthesia 2004;51(5):465‐71. [PUBMED: 15128632] [DOI] [PubMed] [Google Scholar]
Zingg 2009 {published data only}
- Zingg U, Miskovic D, Hamel CT, Erni L, Oertli D, Metzger U. Influence of thoracic epidural analgesia on postoperative pain relief and ileus after laparoscopic colorectal resection: benefit with epidural analgesia. Surgical Endoscopy 2009;23(2):276‐82. [PUBMED: 18363059] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Baumunk 2014 {published data only}
- Baumunk D, Strang CM, Kropf S, Schafer M, Schrader M, Weikert S, et al. Impact of thoracic epidural analgesia on blood loss in radical retropubic prostatectomy. Urologia Internationalis 2014;93(2):193‐201. [PUBMED: 24851943] [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen WK, Ren L, Wei Y, Zhu DX, Miao CH, Xu JM. General anesthesia combined with epidural anesthesia ameliorates the effect of fast‐track surgery by mitigating immunosuppression and facilitating intestinal functional recovery in colon cancer patients. International Journal of Colorectal Disease 2015;30(4):475‐81. [PUBMED: 25579161] [DOI] [PubMed] [Google Scholar]
Enohata 2014 {published data only}
- Enohata K, Hasegawa‐Moriyama M, Kuniyoshi T, Kanmura Y. [Plasma levels of anti‐oxidant markers during general anesthesia‐‐a comparison between remifentanil‐ and epidural‐based anesthesia]. Masui. The Japanese Journal of Anesthesiology 2014;63(3):328‐32. [PUBMED: 24724445] [PubMed] [Google Scholar]
Khoronenko 2014 {published data only}
- Khoronenko VE, Baskakov DS. [Role of migraine history in the development of postoperative nausea and vomiting in patients undergoing general and combined general‐epidural anesthesia]. Anesteziologiia i Reanimatologiia 2014;59(2):41‐4. [PUBMED: 25055492] [PubMed] [Google Scholar]
Kun 2014 {published data only}
- Kun L, Tang L, Wang J, Yang H, Ren J. Effect of combined general/epidural anesthesia on postoperative NK cell activity and cytokine response in gastric cancer patients undergoing radical resection. Hepato‐Gastroenterology 2014;61(132):1142‐7. [PUBMED: 26158178] [PubMed] [Google Scholar]
Li 2015 {published data only}
- Li JM, Shao JL, Zeng WJ, Liang RB. General/epidural anesthesia in combination preserves NK cell activity and affects cytokine response in cervical carcinoma patients undergoing radical resection: a cohort prospective study. European Journal of Gynaecological Oncology 2015;36(6):703‐7. [PUBMED: 26775356] [PubMed] [Google Scholar]
Orsolya 2015 {published data only}
- Orsolya M, Attila‐Zoltan M, Gherman V, Zaharie F, Bolboaca S, Chira C, et al. The effect of anaesthetic management on neutrophil gelatinase associated lipocalin (NGAL) levels after robotic surgical oncology. Journal of the Balkan Union of Oncology 2015;20(1):317‐24. [PUBMED: 25778333] [PubMed] [Google Scholar]
Pan 2015 {published data only}
- Pan YS, Hu YF, Tian FB, Xu K. Effects of epidural preemptive analgesia on stress reaction in retroperitoneal laparoscopic adrenalectomy surgery: a randomized controlled study. International Journal of Clinical and Experimental Medicine 2015;8(6):9862‐8. [PUBMED: 26309669] [PMC free article] [PubMed] [Google Scholar]
Satsuta 2015 {published data only}
- Satsuta SV, Bondarev RV. Anesthesia via sevofluranum with prolonged epidural blockade decreasing complications during and after laparoscopic sanitation in patients with abdominal sepsis. Surgical Endoscopy and Other Interventional Techniques 2015;29:598. [CENTRAL: 01080313] [Google Scholar]
Sayan 2015 {published data only}
- Sayan H, Aydogan MS, Bicakcioglu M, Toprak HI, Isik B, Yilmaz S. Effects of thoracic epidural anesthesia on liver blood flow and indocyanine green clearance test in living‐donor liver transplantation: a prospective, randomized, double‐blind study. Transplantation Proceedings 2015;47(5):1462‐5. [PUBMED: 26093743] [DOI] [PubMed] [Google Scholar]
Sen 2014 {published data only}
- Sen I, Thomas S, Arya VK, Minz M. Preinduction hemodynamic fluctuations in renal transplant recipients ‐ comparison of two combined anesthesia regimens. Saudi Journal of Kidney Diseases and Transplantation 2014;25(6):1232‐9. [PUBMED: 25394440] [DOI] [PubMed] [Google Scholar]
Sidiropoulou 2014 {published data only}
- Sidiropoulou I, Tsaousi G, Sidiropoulos A, Tsantilas D, Vasilakos D. Impact of anesthetic technique on the stress response elicited by laparoscopic cholecystectomy. European Journal of Anaesthesiology 2014;31:133‐4. [CENTRAL: 01054861] [Google Scholar]
Watanabe 2014 {published data only}
- Watanabe K, Kashiwagi K, Kamiyama T, Yamamoto M, Fukunaga M, Inada E, et al. High‐dose remifentanil suppresses stress response associated with pneumoperitoneum during laparoscopic colectomy. Journal of Anesthesia 2014;28(3):334‐40. [PUBMED: 24197291] [DOI] [PubMed] [Google Scholar]
Xiang 2014 {published data only}
- Xiang Y, Chen CQ, Chen HJ, Li M, Bao FP, Zhu SM. The effect of epidural lidocaine administration on sedation of propofol general anesthesia: a randomized trial. Journal of Clinical Anesthesia 2014;26(7):523‐9. [PUBMED: 25439415] [DOI] [PubMed] [Google Scholar]
Xu 2014 {published data only}
- Xu YJ, Chen WK, Zhu Y, Wang SL, Miao CH. Effect of thoracic epidural anaesthesia on serum vascular endothelial growth factor C and cytokines in patients undergoing anaesthesia and surgery for colon cancer. British Journal of Anaesthesia 2014;113 Suppl 1:i49‐55. [PUBMED: 24966150] [DOI] [PubMed] [Google Scholar]
Zhao 2015 {published data only}
- Zhao J, Mo H. The impact of different anesthesia methods on stress reaction and immune function of the patients with gastric cancer during peri‐operative period. Journal of the Medical Association of Thailand 2015;98(6):568‐73. [PUBMED: 26219161] [PubMed] [Google Scholar]
References to ongoing studies
Li 2015a {published data only}
- Li YW, Li HJ, Li HJ, Feng Y, Yu Y, Guo XY, et al. Effects of two different anesthesia‐analgesia methods on incidence of postoperative delirium in elderly patients undergoing major thoracic and abdominal surgery: study rationale and protocol for a multicenter randomized controlled trial. BMC Anesthesiology 2015;15:144. [PUBMED: 26459347] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Berde 2009
- Berde CB, Strichartz GR. Local anesthetics. In: Miller RD, Eriksson LI, Fleisher LA, Wiener‐Kronish JP, Young WL editor(s). Miller's Anesthesia. 7th Edition. San Francisco, California, USA: Churchil Livingstone, 2009:Chapter 30. [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Publication bias. In: Borenstein M, Hedges LV, Higgins JPT, Rothstein HR editor(s). Introduction to Meta‐Analysis. 1st Edition. Chichester, UK: Wiley, 2009:277‐92. [Google Scholar]
Cook 2009
- Cook TM, Counsell D, Wildsmith JA. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. British Journal of Anaesthesia 2009;102(2):179‐90. [PUBMED: 19139027] [DOI] [PubMed] [Google Scholar]
Davies 2006
- Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side‐effects of paravertebral vs epidural blockade for thoracotomy ‐ a systematic review and meta‐analysis of randomized trials. British Journal of Anaesthesia 2006;96(4):418‐26. [PUBMED: 16476698] [DOI] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21(11):1575‐600. [PUBMED: 12111921] [DOI] [PubMed] [Google Scholar]
Drummond 2013
- Drummond GB, Bates A, Mann J, Arvind DK. Characterization of breathing patterns during patient‐controlled opioid analgesia. British Journal of Anaesthesia 2013;111(6):971‐8. [PUBMED: 23970443] [DOI] [PubMed] [Google Scholar]
Duval 2000
- Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56(2):455‐63. [PUBMED: 10877304] [DOI] [PubMed] [Google Scholar]
Duval 2000a
- Duval S, Tweedie R. A non parametric 'trim and fill' method accounting for publication bias in meta‐analysis. Journal of the American Statistical Association 2000;95:89‐98. [Google Scholar]
Gan 2015
- Gan TJ, Robinson SB, Oderda GM, Scranton R, Pepin J, Ramamoorthy S. Impact of postsurgical opioid use and ileus on economic outcomes in gastrointestinal surgeries. Current Medical Research and Opinion 2015;31:1‐10. [PUBMED: 25586296] [DOI] [PubMed] [Google Scholar]
Guay 2006
- Guay J. The benefits of adding epidural analgesia to general anesthesia: a metaanalysis. Journal of Anesthesia 2006;20(4):335‐40. [PUBMED: 17072704] [DOI] [PubMed] [Google Scholar]
Guay 2014
- Guay J, Choi P, Suresh S, Albert N, Kopp S, Pace NL. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010108.pub2; PUBMED: 24464831] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed.) 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical research ed.) 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors) editor(s). Cochrane Handbook for Systematic Reviews of Interventions. www.cochrane‐handbook.org: The Cochrane Collaboration, 2011:Chapter 12. [Google Scholar]
Holte 2002
- Holte K, Kehlet H. Postoperative ileus: progress towards effective management. Drugs 2002;62(18):2603‐15. [PUBMED: 12466000] [DOI] [PubMed] [Google Scholar]
McCarthy 2010
- McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs 2010;70(9):1149‐63. [PUBMED: 20518581] [DOI] [PubMed] [Google Scholar]
McColl 1998
- McColl A, Smith H, White P, Field J. General practitioner's perceptions of the route to evidence based medicine: a questionnaire survey. BMJ (Clinical research ed.) 1998;316(7128):361‐5. [PUBMED: 9487174] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neville 2014
- Neville A, Lee L, Antonescu I, Mayo NE, Vassiliou MC, Fried GM, et al. Systematic review of outcomes used to evaluate enhanced recovery after surgery. The British Journal of Surgery 2014;101(3):159‐70. [PUBMED: 24469616] [DOI] [PubMed] [Google Scholar]
Pace 2011
- Pace NL. Research methods for meta‐analyses. Best Practice & Research. Clinical Anaesthesiology 2011;25(4):523‐33. [PUBMED: 22099918] [DOI] [PubMed] [Google Scholar]
Pogue 1998
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet (London, England) 1998;351(9095):47‐52. [PUBMED: 9433436] [DOI] [PubMed] [Google Scholar]
Ramachandran 2011
- Ramachandran SK, Haider N, Saran KA, Mathis M, Kim J, Morris M, et al. Life‐threatening critical respiratory events: a retrospective study of postoperative patients found unresponsive during analgesic therapy. Journal of Clinical Anesthesia 2011;23(3):207‐13. [PUBMED: 21570616] [DOI] [PubMed] [Google Scholar]
van Bree 2014
- Bree SH, Bemelman WA, Hollmann MW, Zwinderman AH, Matteoli G, Temna S, et al. Identification of clinical outcome measures for recovery of gastrointestinal motility in postoperative ileus. Annals of Surgery 2014;259(4):708‐14. [PUBMED: 23657087] [DOI] [PubMed] [Google Scholar]
White 2007
References to other published versions of this review
Guay 2016
- Guay J, Nishimori M, Kopp SL. Epidural local anesthetics versus opioid‐based analgesic regimens for postoperative gastrointestinal paralysis, vomiting, and pain after abdominal surgery: A Cochrane Review. Anesthesia and Analgesia 2016;123(6):1591‐602. [DOI] [PubMed] [Google Scholar]
Jorgensen 2001
- Jorgensen H, Wetterslev J, Moiniche S, Dahl JB. Epidural local anaesthetics versus opioid‐based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001893] [DOI] [PubMed] [Google Scholar]
Jørgensen 2000
- Jørgensen H, Wetterslev J, Møiniche S, Dahl JB. Epidural local anaesthetics versus opioid‐based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001893] [DOI] [PubMed] [Google Scholar]


