Abstract
Background
Panic disorder is common and deleterious to mental well‐being. Psychological therapies and pharmacological interventions are both used as treatments for panic disorder with and without agoraphobia. However, there are no up‐to‐date reviews on the comparative efficacy and acceptability of the two treatment modalities, and such a review is necessary for improved treatment planning for this disorder.
Objectives
To assess the efficacy and acceptability of psychological therapies versus pharmacological interventions for panic disorder, with or without agoraphobia, in adults.
Search methods
We searched the Cochrane Common Mental Disorders Group Specialised Register on 11 September 2015. This register contains reports of relevant randomised controlled trials from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (1950 to present), Embase (1974 to present), and PsycINFO (1967 to present). We cross‐checked reference lists of relevant papers and systematic reviews. We did not apply any restrictions on date, language, or publication status.
Selection criteria
We included all randomised controlled trials comparing psychological therapies with pharmacological interventions for panic disorder with or without agoraphobia as diagnosed by operationalised criteria in adults.
Data collection and analysis
Two review authors independently extracted data and resolved any disagreements in consultation with a third review author. For dichotomous data, we calculated risk ratios (RR) with 95% confidence intervals (CI). We analysed continuous data using standardised mean differences (with 95% CI). We used the random‐effects model throughout.
Main results
We included 16 studies with a total of 966 participants in the present review. Eight of the studies were conducted in Europe, four in the USA, two in the Middle East, and one in Southeast Asia.
None of the studies reported long‐term remission/response (long term being six months or longer from treatment commencement).
There was no evidence of a difference between psychological therapies and selective serotonin reuptake inhibitors (SSRIs) in terms of short‐term remission (RR 0.85, 95% CI 0.62 to 1.17; 6 studies; 334 participants) or short‐term response (RR 0.97, 95% CI 0.51 to 1.86; 5 studies; 277 participants) (very low‐quality evidence), and no evidence of a difference between psychological therapies and SSRIs in treatment acceptability as measured using dropouts for any reason (RR 1.33, 95% CI 0.80 to 2.22; 6 studies; 334 participants; low‐quality evidence).
There was no evidence of a difference between psychological therapies and tricyclic antidepressants in terms of short‐term remission (RR 0.82, 95% CI 0.62 to 1.09; 3 studies; 229 participants), short‐term response (RR 0.75, 95% CI 0.51 to 1.10; 4 studies; 270 participants), or dropouts for any reason (RR 0.83, 95% CI 0.53 to 1.30; 5 studies; 430 participants) (low‐quality evidence).
There was no evidence of a difference between psychological therapies and other antidepressants in terms of short‐term remission (RR 0.90, 95% CI 0.48 to 1.67; 3 studies; 135 participants; very low‐quality evidence) and evidence that psychological therapies did not significantly increase or decrease the short‐term response over other antidepressants (RR 0.96, 95% CI 0.67 to 1.37; 3 studies; 128 participants) or dropouts for any reason (RR 1.55, 95% CI 0.91 to 2.65; 3 studies; 180 participants) (low‐quality evidence).
There was no evidence of a difference between psychological therapies and benzodiazepines in terms of short‐term remission (RR 1.08, 95% CI 0.70 to 1.65; 3 studies; 95 participants), short‐term response (RR 1.58, 95% CI 0.70 to 3.58; 2 studies; 69 participants), or dropouts for any reason (RR 1.12, 95% CI 0.54 to 2.36; 3 studies; 116 participants) (very low‐quality evidence).
There was no evidence of a difference between psychological therapies and either antidepressant alone or antidepressants plus benzodiazepines in terms of short‐term remission (RR 0.86, 95% CI 0.71 to 1.05; 11 studies; 663 participants) and short‐term response (RR 0.95, 95% CI 0.76 to 1.18; 12 studies; 800 participants) (low‐quality evidence), and there was no evidence of a difference between psychological therapies and either antidepressants alone or antidepressants plus benzodiazepines in terms of treatment acceptability as measured by dropouts for any reason (RR 1.08, 95% CI 0.77 to 1.51; 13 studies; 909 participants; very low‐quality evidence). The risk of selection bias and reporting bias was largely unclear. Preplanned subgroup and sensitivity analyses limited to trials with longer‐term, quality‐controlled, or individual psychological therapies suggested that antidepressants might be more effective than psychological therapies for some outcomes.
There were no data to contribute to a comparison between psychological therapies and serotonin–norepinephrine reuptake inhibitors (SNRIs) and subsequent adverse effects.
Authors' conclusions
The evidence in this review was often imprecise. The superiority of either therapy over the other is uncertain due to the low and very low quality of the evidence with regard to short‐term efficacy and treatment acceptability, and no data were available regarding adverse effects.
The sensitivity analysis and investigation of the sources of heterogeneity indicated three possible influential factors: quality control of psychological therapies, the length of intervention, and the individual modality of psychological therapies.
Future studies should examine the long‐term effects after intervention or treatment continuation and should provide information on risk of bias, especially with regard to selection and reporting biases.
Plain language summary
Psychological therapies versus medication for panic disorder with or without agoraphobia in adults
Why is this review important?
Panic disorder is common and harmful to mental well‐being. Psychological therapies and medication (usually antidepressants or benzodiazepines) are effective against panic disorder. However, there are no up‐to‐date reviews on the superiority and acceptability of these two forms of treatment, and such a review is necessary to improve treatment planning for this disorder.
Who will be interested in this review?
Adult patients, professionals working in mental health services, and general practitioners.
What questions does this review aim to answer?
The present review aims to answer the following questions:
• Are psychological therapies more effective than antidepressants or benzodiazepines?
• Are psychological therapies more acceptable than antidepressants or benzodiazepines?
Which studies were included in the review?
We searched databases to find all trials published between 1950 and September 2015 comparing psychological therapies with antidepressants or benzodiazepines for the treatment of panic disorder with or without agoraphobia in adults. To be included in the review, the studies had to be randomised controlled trials and had to include people with a clear diagnosis of panic disorder with or without agoraphobia.
We included 16 studies with a total of 966 participants in the review. We rated the overall quality of the evidence from these studies as very low to moderate.
What does the evidence from the review tell us?
The superiority or inferiority of psychological therapies over drugs remains unclear with regard to treatment effectiveness and acceptability.
None of the studies reported information regarding long‐term effectiveness and quality of life.
What should happen next?
The review authors recommend that future research should use less biased psychological therapies, evaluate long‐term outcomes, and report information related to the risk of study bias.
Summary of findings
Summary of findings for the main comparison. All psychological therapies compared to SSRI for panic disorder with or without agoraphobia in adults.
| All psychological therapies compared to SSRI for panic disorder with or without agoraphobia in adults | ||||||
| Patient or population: adults with panic disorder with or without agoraphobia Settings: outpatient Intervention: all psychological therapies Comparison: SSRI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| SSRI | All psychological therapies | |||||
| Short‐term remission | 618 per 1000 | 525 per 1000 (383 to 723) | RR 0.85 (0.62 to 1.17) | 334 (6 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| Short‐term response | 447 per 1000 | 434 per 1000 (228 to 831) | RR 0.97 (0.51 to 1.86) | 277 (5 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| Dropouts for any reason | 176 per 1000 | 234 per 1000 (141 to 390) | RR 1.33 (0.8 to 2.22) | 334 (6 studies) | ⊕⊕⊝⊝ low1,3 | |
| Long‐term remission/response | Study population | Not estimable | 0 (0) | See comment** | ||
| See comment** | See comment** | |||||
| Moderate | ||||||
Short‐term improvement
Scale used (range; lower is better):
|
‐ | The mean short‐term improvement in the intervention groups was 0.20 standard deviations lower (1.09 lower to 0.69 higher) | ‐ | 242 (5 studies) | ⊕⊕⊕⊝ moderate1 | Scores estimated using a standardised mean difference |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; RR: risk ratio; SSRI: selective serotonin reuptake inhibitor ** No study reported long‐term remission/response | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one point for risk of bias due to a high risk of bias for blinding and an unclear risk of bias for several domains in many studies. 2Downgraded one point for inconsistency due to substantial heterogeneity. 3Downgraded one point for imprecision based on the fact that the 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit.
Summary of findings 2. All psychological therapies compared to tricyclic antidepressants for panic disorder with or without agoraphobia in adults.
| All psychological therapies compared to tricyclic antidepressants for panic disorder with or without agoraphobia in adults | ||||||
| Patient or population: adults with panic disorder with or without agoraphobia Settings: outpatient Intervention: all psychological therapies Comparison: tricyclic antidepressants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Tricyclic antidepressants | All psychological therapies | |||||
| Short‐term remission | 525 per 1000 | 430 per 1000 (326 to 572) | RR 0.82 (0.62 to 1.09) | 229 (3 studies) | ⊕⊕⊝⊝ low1,2 | |
| Short‐term response | 322 per 1000 | 241 per 1000 (164 to 354) | RR 0.75 (0.51 to 1.10) | 270 (4 studies) | ⊕⊕⊝⊝ low1,2 | |
| Dropouts for any reason | 271 per 1000 | 225 per 1000 (143 to 352) | RR 0.83 (0.53 to 1.3) | 430 (5 studies) | ⊕⊕⊝⊝ low1,2 | |
| Long‐term remission/response | Study population | Not estimable | 0 (0) | See comment** | ||
| See comment** | See comment** | |||||
| Moderate | ||||||
|
Short‐term improvement Scale used (range; lower is better):
|
‐ | The mean short‐term improvement in the intervention groups was 0.20 standard deviations higher (0.03 lower to 0.43 higher) | ‐ | 414 (5 studies) | ⊕⊕⊕⊝ moderate1 | Scores estimated using a standardised mean difference |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; RR: risk ratio ** No study reported long‐term remission/response | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one point for risk of bias due to a high risk of bias for blinding and an unclear risk of bias for several domains in many studies. 2Downgraded one point for imprecision based on the fact that the 95% confidence interval around the pooled estimate of effect included both no effect and appreciable benefit.
Summary of findings 3. All psychological therapies compared to other antidepressants for panic disorder with or without agoraphobia in adults.
| All psychological therapies compared to other antidepressants for panic disorder with or without agoraphobia in adults | ||||||
| Patient or population: adults with panic disorder with or without agoraphobia Settings: outpatient Intervention: all psychological therapies Comparison: other antidepressants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other antidepressants | All psychological therapies | |||||
| Short‐term remission | 576 per 1000 | 518 per 1000 (276 to 962) | RR 0.90 (0.48 to 1.67) | 135 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| Short‐term response | 705 per 1000 | 677 per 1000 (472 to 966) | RR 0.96 (0.67 to 1.37) | 128 (3 studies) | ⊕⊕⊝⊝ low2,3 | |
| Dropouts for any reason | 191 per 1000 | 296 per 1000 (174 to 506) | RR 1.55 (0.91 to 2.65) | 180 (3 studies) | ⊕⊕⊝⊝ low2,3 | |
| Long‐term remission/response | Study population | Not estimable | 0 (0) | See comment** | ||
| See comment** | See comment** | |||||
| Moderate | ||||||
Short‐term improvement
Scale used (range; lower is better):
|
‐ | The mean short‐term improvement in the intervention groups was 0.43 standard deviations lower (1.36 lower to 0.51 higher) | ‐ | 27 (2 studies) | ⊕⊕⊕⊝ moderate2 | Scores estimated using a standardised mean difference |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; RR: risk ratio ** No study reported long‐term remission/response | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one point for inconsistency due to the existence of substantial heterogeneity. 2Downgraded one point for imprecision based on the fact that the 95% confidence interval around the pooled estimate of effect included both no effect and appreciable benefit. 3Downgraded one point for risk of bias due to a high risk of bias for blinding and an unclear risk of bias for several domains in many studies.
Summary of findings 4. All psychological therapies compared to benzodiazepines for panic disorder with or without agoraphobia in adults.
| All psychological therapies compared to benzodiazepines for panic disorder with or without agoraphobia in adults | ||||||
| Patient or population: adults with panic disorder with or without agoraphobia Settings: outpatient Intervention: all psychological therapies Comparison: benzodiazepines | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Benzodiazepines | All psychological therapies | |||||
| Short‐term remission | 404 per 1000 | 437 per 1000 (283 to 667) | RR 1.08 (0.70 to 1.65) | 95 (3 studies) | ⊕⊝⊝⊝ very low1,2 | |
| Short‐term response | 261 per 1000 | 412 per 1000 (183 to 934) | RR 1.58 (0.7 to 3.58) | 69 (2 studies) | ⊕⊕⊝⊝ very low1,2 | |
| Dropouts for any reason | 227 per 1000 | 255 per 1000 (123 to 536) | RR 1.12 (0.54 to 2.36) | 116 (3 studies) | ⊕⊝⊝⊝ very low1,2 | |
| Long‐term remission/response | Study population | Not estimable | 0 (0) | See comment** | ||
| See comment** | See comment** | |||||
| Moderate | ||||||
|
Short‐term improvement Scale used (range; lower is better):
|
‐ | The mean short‐term improvement in the intervention groups was 0.23 standard deviations lower (0.69 lower to 0.23 higher) | ‐ | 89 (3 studies) | ⊕⊝⊝⊝ very low1,2 | Scores estimated using a standardised mean difference |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; RR: risk ratio; STAI: State‐Trait Anxiety Inventory ** No study reported long‐term remission/response | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one point for risk of bias due to a high risk of bias for blinding and an unclear risk of bias for several domains in many studies. 2Downgraded two points for very serious imprecision due to the very small sample size and small number of studies.
Summary of findings 5. All psychological therapies compared to antidepressants alone or antidepressants and benzodiazepines for panic disorder with or without agoraphobia in adults.
| All psychological therapies compared to antidepressants alone or antidepressants and benzodiazepines for panic disorder with or without agoraphobia in adults | ||||||
| Patient or population: adults with panic disorder with or without agoraphobia Settings: outpatient Intervention: all psychological therapies Comparison: antidepressants alone or antidepressants and benzodiazepines | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Antidepressants alone or antidepressants and benzodiazepines | All psychological therapies | |||||
| Short‐term remission | 585 per 1000 | 503 per 1000 (415 to 614) | RR 0.86 (0.71 to 1.05) | 663 (11 studies) | ⊕⊕⊝⊝ low1,2 | |
| Short‐term response | 474 per 1000 | 460 per 1000 (365 to 578) | RR 0.95 (0.76 to 1.18) | 800 (12 studies) | ⊕⊕⊝⊝ low1,2 | |
| Dropouts for any reason | 217 per 1000 | 234 per 1000 (167 to 328) | RR 1.08 (0.77 to 1.51) | 909 (13 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| Long‐term remission/response | Study population | Not estimable | 0 (0) | See comment** | ||
| See comment** | See comment** | |||||
| Moderate | ||||||
|
Short‐term improvement Scale used (range; lower is better):
|
‐ | The mean short‐term improvement in the intervention groups was 0.01 standard deviations higher (0.34 lower to 0.35 higher) | ‐ | 638 (11 studies) | ⊕⊕⊝⊝ low1,3 | Scores estimated using a standardised mean difference |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: confidence interval; RR: risk ratio; STAI: State‐Trait Anxiety Inventory ** No study reported long‐term remission/response | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one point for risk of bias due to a high risk of bias for blinding and an unclear risk of bias for several domains in many studies. 2Downgraded one point for imprecision based on the fact that the 95% confidence interval around the pooled estimate of effect included both no effect and appreciable benefit. 3Downgraded one point for publication bias judged by the funnel plot asymmetry.
Background
Description of the condition
A panic attack is a discrete period of fear or anxiety that has a rapid onset and reaches a peak within 10 minutes. According to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR) (APA 2000), it includes at least four out of 13 characteristic symptoms. These are bodily symptoms, such as a racing heart, chest pain, sweating, shaking, dizziness, flushing, stomach‐churning, faintness, and breathlessness, and fearful cognitions, such as the fear of collapse, going mad or dying, and derealisation (unreal perception). Panic disorder is characterised by recurrent, spontaneous, and unexpected panic attacks, at least one of which has been followed by one month (or more) of persistent concern about having additional attacks, worry about the implications of the attack (or its consequences), or a significant change in behaviour related to the attacks (APA 2000). In around a quarter of patients, panic disorder is accompanied by agoraphobia (avoidance of situations where escape would be difficult when panic attacks occur) (Kessler 2006). The presence of agoraphobia is associated with increased severity and worse outcome (Kessler 2006).
Panic disorder is a very common disorder that presents across the spectrum of severity, with very large numbers of people suffering considerable handicaps as a result. Panic disorder is common in the general population, with a lifetime prevalence of 1% to 4% (Bijl 1998; Eaton 1994). In primary care settings, the prevalence rises to around 10% (King 2008). It is also highly comorbid with other psychiatric disorders, such as drug dependence, major depression, bipolar I disorder, social phobia, specific phobia, and generalised anxiety disorder (Grant 2006). It is estimated that generalised anxiety disorder co‐occurs in 68% of people with panic disorder (Starcevic 2009), and only 38.6% of people with major depression (Wittchen 2002). People suffering from panic disorder exhibit considerably poorer overall mental well‐being than individuals with cancer, diabetes, heart disease, arthritis, hypertension, and a host of other chronic physical conditions (Comer 2011). Having panic disorder is also related to poorer quality of life and physical function (Sherbourne 2010); the risk of attempting suicide is also higher (Sareen 2005). These effects are similar to or greater than those associated with major depression (Markowitz 1989). A study has reported that nearly one‐third of these patients in primary care had seen three or more healthcare professionals, and almost one‐fifth had visited general hospital emergency departments (Swinson 1992).
Description of the intervention
Psychological therapies
There are various psychological therapies for panic disorder. Whilst they may all be variously used in the treatment of panic disorder in everyday clinical practice, only approaches informed by the principles of cognitive behavioural therapy are recommended in clinical guidelines for panic disorder (NICE 2011).
Psychoeducation consists of providing information to patients on things such as symptoms and theories of psychological therapy (Schotte 2006). Supportive psychotherapy is the non‐specific component of psychological therapies and involves warm, empathic, and non‐directive emotional support (Winston 2004). Physiological therapy uses physical techniques such as breathing retraining, relaxation, and biofeedback with the goal of helping patients to control the physiological symptoms of panic disorder (Mauret 2010). Behaviour therapy is characterised by exposure aimed at habituation; patients are gradually exposed to their phobic situations (in vivo exposure, virtual reality exposure) or bodily sensations (interoceptive exposure) in order to reduce their anxiety in such environments through habituation (Marks 1981). Cognitive therapy is composed of cognitive restructuring, in which the patient and therapist co‐operate to find and reconstruct irrational or maladaptive thoughts (Barlow 1988). Cognitive behavioural therapy (CBT) is the combination of behaviour and cognitive therapy, and often includes psychoeducation and physiological therapy elements, such as breathing retraining and progressive muscle relaxation. The third‐wave therapies are a group of treatments that share some features of CBT, but focus more on mindfulness, acceptance, the patient's values in life, and relationships (Ost 2008). Examples are acceptance and commitment treatment, cognitive behavioural analysis, dialectical behavioural therapy, and mindfulness‐based cognitive therapy (Kahl 2012). Psychodynamic therapy is represented by psychoanalysis and its derivatives, which deal with the unconscious anger that patients with panic disorder are hypothesised to have, and the defence mechanisms of reaction formation, undoing and denial, which are frequently employed by people with panic disorder, compromising the formations which panic attacks represent, the pleasure principle, and transference (Busch 1999).
Pharmacological interventions
Different types of antidepressants augment the function of chemicals in the brain, in particular serotonin, noradrenaline, or both. Tricyclic antidepressants and serotonin‐noradrenaline reuptake inhibitors inhibit both serotonin and noradrenaline reuptake. Selective serotonin reuptake inhibitors (SSRIs) inhibit the reuptake of serotonin into nerve terminals by inhibiting serotonin transporters. Selective noradrenaline reuptake inhibitors inhibit noradrenaline transporter. Monoamine oxidase inhibitors inhibit enzymes that oxidise monoamines such as serotonin, noradrenaline, and dopamine. Other types of antidepressants include bupropion, which is a weak inhibitor of the neuronal uptake of norepinephrine and dopamine but has no effect on serotonin; trazodone and nefazodone, which block serotonin receptors; and mirtazapine, which increases central noradrenergic and serotonergic neurotransmission (by inhibiting the negative feedback from serotonin and noradrenaline that has already been released by the neuron), but also has antagonist and partial agonist actions on certain serotonin receptors (Baudry 2011). Benzodiazepines have been used for panic disorder (Ravindran 2010). However, recent guidelines recommend SSRIs as the first‐line medication (APA 2009; BAP 2014), as benzodiazepines may lead to dependence, and tricyclic antidepressants and monoamine oxidase inhibitors have more unfavourable adverse effects than SSRIs (Freire 2011).
How the intervention might work
Psychological therapy can be broadly conceived and defined as therapeutic interaction aimed at the alleviation of mental suffering through verbal and non‐verbal communication between a professional and a client, based on a certain theoretical model of how that mental suffering occurs and is maintained. Psychoeducation aims to help people acquire the competence to manage their symptoms and preserve their mental health (Daele 2012). Supportive psychological therapy helps patients see things more clearly by supporting reality testing, challenging unrealistic ideas, and demonstrating more effective, less costly ways of defending while supporting adaptive defences (Douglas 2008). Physiological therapy is based on the suffocation alarm theory, which postulates that a monitor in the human central nervous system sets off the alarm for imminent suffocation when its regulatory threshold is exceeded (Klein 1993). Physiological therapy, such as respiratory training, tries to treat panic disorder by controlling pCO2 (partial pressure of carbon dioxide), which is the key to the suffocation alarm. Applied relaxation, one element of physiological therapy, is to teach the patient to observe the very first signs of a panic attack and to apply a rapid and effective relaxation technique to cope with, and eventually abort, these symptoms before they develop into a panic attack. Behavioural therapy focuses on the maintenance of fear through avoidance and treats agoraphobia by exposure to feared stimuli and habituation of fear responses (Marks 1981). Cognitive therapy postulates that panic disorder occurs through a catastrophising misinterpretation of bodily sensations, which should therefore be corrected through cognitive restructuring with or without behavioural experiments (Barlow 1988). The third‐wave therapies focus more on contact with internal events, acceptance, and mindfulness; emphasis is given to contacting the present moment (Hayes 2004). Mindfulness alters the function of thoughts without first altering their form (Teasdale 2002). Psychodynamic therapy assumes that a panic attack occurs when the defence mechanism against anxiety is broken. According to this theory, the loss of parents or experience of separation anxiety in childhood is regarded as the cause of agoraphobia (Sadock 2003).
A range of psychotropic agents have been shown to be effective against panic disorder. Medications affecting the serotonin system, such as tricyclic antidepressants and SSRIs, have anxiolytic effects (Michelson 1998); some studies also suggest that they have a regulatory effect on respiration (Eldridge 1981). The frontal cortex is the most consistently reported site that responds to antidepressant treatment, which induces normalisation of its overactivity and underactivity (Goldapple 2004). Other supposed regions affected by antidepressant treatment include specific subcortical (brain stem, hypothalamus), limbic‐paralimbic (hippocampus, posterior cingulate), and striatal (caudate, globus pallidus) nodes (Mayberg 2000). The site and direction of regional changes also suggest mechanisms for changes in mood and motor, cognitive, and somatic symptoms of depression (Mayberg 1999). Benzodiazepines enhance gamma‐aminobutyric acid (GABA) transmission by acting as agonists at a specific site on the GABA‐A receptor, resulting in anxiolytic, hypnotic, and anticonvulsant effects. They are effective for rapid relief of symptoms, but have disadvantages, such as sedation and the potential for abuse and formation of tolerance (Zohar 2000). Decreased benzodiazepine receptor binding in people with panic disorder has been suggested in one study (Malizia 1998), which is consistent with the idea that panic disorder may be due to defective brain inhibition that leads to paroxysmal elevations in anxiety during panic attacks.
Some authors have suggested that the biological mechanisms of action of psychological therapies and antidepressants may not be entirely mutually exclusive (Goldapple 2004).
Why it is important to do this review
Various randomised controlled trials (RCTs) and meta‐analyses have demonstrated the efficacy of psychological therapies in comparison with waiting list, psychological placebo, or pharmacological placebo controls (Sanchez‐Meca 2010), and the efficacy of pharmacotherapy in comparison with placebo (Bradwejn 2005). Several meta‐analyses have also examined the relative efficacy of psychological therapies and pharmacotherapy.
A meta‐analysis of combined psychological therapy and antidepressant medication, compared to either treatment administered as monotherapy, concluded that combined therapy is better than antidepressants and that psychological therapy is as effective as combined therapy (Furukawa 2007). Considering this evidence, it may be the case that psychological therapy is better than antidepressants. However, to draw any firm and direct conclusions we would need to analyse head‐to‐head comparisons of psychological therapy alone and antidepressants alone.
Three previous meta‐analyses have included studies directly comparing psychological therapy with pharmacotherapy, but they have some critical limitations (Clum 1993; Gould 1995; Mitte 2005). Two of these reviews used data from 1964 to 1990 and from 1974 to 1994, and are therefore out of date. Moreover, only one out of the 29 included studies in Clum 1993, and three out of the 43 included studies in Gould 1995, directly compared pharmacotherapy with psychological therapies, and their only pharmacological intervention was imipramine. Given that between 30 to 40 antidepressants are now available, with different modes of action (such as selective serotonin uptake inhibitors and noradrenergic and specific serotonergic antidepressants), meta‐analyses including a wide range of medications should be carried out.
A more recent meta‐analysis analysed different therapy approaches including pharmacotherapy and psychological therapies, but only cognitive behavioural therapy or behaviour therapy were included within the latter category (Mitte 2005). Other psychotherapies have been shown to be effective for panic disorder, such as psychoanalytic therapy (Milrod 2007).
There is therefore a need for an up‐to‐date and more comprehensive review that directly compares different types of psychological therapy and pharmacotherapy to better inform treatment choices.
Objectives
To assess the effects of psychological therapies versus pharmacological interventions for panic disorder, with or without agoraphobia, in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant RCTs in the review. We planned to include cluster‐randomised studies in which the effects of clustering have been taken into account, as well as trials using a cross‐over design, however we found no cluster‐randomised or cross‐over trials. We excluded quasi‐randomised trials, such as those allocating participants using alternate days of the week.
Types of participants
Age
The participants had to be 18 years of age or older. We did not include studies focusing on adolescents. There is a Cochrane review on cognitive behavioural therapy for anxiety disorders in children and adolescents (James 2013). However, we included studies with participants younger that 18 in the sample as long as at least 80% of participants were aged 18 or older.
Diagnosis
We included studies that have enlisted participants with a primary diagnosis of panic disorder, with or without agoraphobia, according to any of the following criteria: Feighner (Feighner 1972), Research Diagnostic Criteria (Spitzer 1978), Diagnostic and Statistical Manual of Mental Disorders (DSM)‐III (APA 1980), DSM‐III‐R (APA 1987), DSM‐IV (APA 2000), or International Classification of Diseases, Tenth Edition (ICD‐10) (WHO 1992). We anticipated there would be few, if any, studies that use DSM‐5 diagnostic criteria, owing to its relatively recent publication. As such, we planned not to include DSM‐5 criteria in this version of the review. DSM‐5 considers panic disorder and agoraphobia as independent conditions; in future revisions of the review, we will include studies based on DSM‐5 if they focus on panic disorder, with or without agoraphobia.
If studies focused on agoraphobia, rather than panic disorder, we included them if participants were operationally diagnosed according to the above criteria and when it could be safely assumed that some of the participants were suffering from panic disorder as defined by the above criteria. In other words, we planned to exclude studies that focused on agoraphobia without panic disorder. There is evidence that over 95% of people with agoraphobia seen clinically also suffer from panic disorder (Goisman 1995). We planned to examine the effect of the inclusion of these studies (i.e. studies in which some, but possibly not all, of the participants had panic disorder) in a sensitivity analysis. However, we found no such studies.
We excluded studies where panic was induced.
Setting
Participants must have been outpatients at the time of enrolment.
Previous treatment
We included both treatment‐naive patients and patients who had already undergone some previous treatment (either psychological or pharmacological), as long as they satisfied the above inclusion criteria.
Comorbidities
We included studies in which participants may have other anxiety disorders (e.g. generalised anxiety disorder, specific phobias) or with subthreshold panic disorder if: 1) separate results for participants with panic disorder were available; and 2) randomisation was stratified by specific diagnosis. Stratification by diagnosis was not required if the total sample included at least 40 participants with panic disorder.
We excluded studies in which all participants had a concurrent primary diagnosis of Axis I or II disorders other than panic disorder or agoraphobia.
We included studies in which the participants may have physical comorbidities. However, we excluded studies that explicitly focused on panic disorder or agoraphobia among people with a specific physical comorbidity, which can be a confounding factor.
Types of interventions
For this review, we chose to focus on the most representative psychological therapy schools (i.e. CBT and its components or developments, psychodynamic psychological therapy, and supportive psychological therapy) and their control conditions.
We included trials if they compared a psychological therapy with a pharmacological intervention.
Psychological therapies
Psychoeducation, defined as sessions in which patients are provided with information about their disease (Daele 2012).
Supportive psychological therapy, defined as sessions in which patients are administered an active, although non‐specific, psychological treatment (Douglas 2008).
Physiological therapies that use some kind of physical training (e.g. breathing retraining, progressive muscle relaxation, applied relaxation) in order to reduce the physiological manifestations of anxiety (Klein 1993).
Behaviour therapy, with or without physiological components, aiming at patients' habituation to anxiety‐provoking situations and sensations through some kind of exposure (e.g. interoceptive, in vivo) (Marks 1981).
Cognitive therapy, with or without physiological components and behavioural experiments, aiming at the modification of maladaptive thoughts through some kind of cognitive restructuring (Barlow 1988).
Cognitive behavioural therapy (CBT), with or without physiological components, comprising both cognitive and behavioural therapy elements.
Third‐wave therapies, including acceptance and commitment therapy, mindfulness‐based therapy, and other so‐called third‐wave therapies administered with or without other CBT components (e.g. exposure, cognitive restructuring, breathing retraining, muscle relaxation) (Hayes 2004).
Psychodynamic therapy, focused on revealing and resolving intrapsychic or unconscious conflicts (Sadock 2003).
As therapies could be of any length, we accepted those given in a single session.
We included both individual and group therapies.
Therapies must be administered face‐to‐face. We excluded therapies administered in their self help (e.g. book, computer, internet) or remote (e.g. telephone, video conference) versions. In the case of psychoeducation, we did not consider the simple provision of informational material without any face‐to‐face session to be an active intervention, but rather a comparator intervention (such as no psychological treatment or waiting list).
We excluded family therapy, couple therapy, and other psychosocial interventions whose intervention focus is not the individual but rather the family system or couple as a whole.
We included so‐called component studies (e.g. dismantling studies) as long as each arm could be regarded as any of the above‐defined experimental interventions compared against another experimental or comparator treatment. We ultimately regarded study arms as giving information about the same experimental intervention and thus combined them.
We excluded any other psychological therapies (such as interpersonal therapy, eye movement desensitisation and reprocessing (EMDR), and Morita therapy) on the grounds that they did not meet the criteria described above for a CBT (and its components and developments), psychodynamic psychological therapy, or supportive psychological therapy approach.
When psychoeducation or psychological support (or both) were accompanied by any other psychological intervention, we classified study arms according to the latter and regarded psychoeducation and psychological support as components of that intervention.
Pharmacotherapy
Tricyclic and heterocyclic antidepressants: imipramine, amitriptyline, clomipramine, desipramine, nortriptyline, doxepin, dosulepin/dothiepin, trimipramine, lofepramine, protriptyline, amineptine.
Selective serotonin reuptake inhibitors (SSRIs): citalopram, escitalopram, fluvoxamine, fluoxetine, paroxetine, sertraline.
Monoamine oxidase inhibitors (MAOIs): phenelzine, isocarboxazid, tranylcypromine, moclobemide, brofaromine.
Serotonin‐noradrenaline reuptake inhibitors (SNRIs): desvenlafaxine, duloxetine, milnacipran, venlafaxine.
Noradrenergic and specific serotonergic antidepressants: mirtazapine.
Selective noradrenergic reuptake inhibitors: reboxetine.
Other antidepressants: agomelatine, bupropion, trazodone, nefazodone, mianserin, maprotilin, vortioxetine, vilazodone, viloxazine.
Benzodiazepines: adinazolam, alprazolam, bentazepam, bromazepam, brotizolam, camazepam, chlordiazepoxide, clobazam, clonazepam, clorazepate, clotiazepam, cloxazolam, diazepam, etizolam, flutoprazepam, halazepam, ketazolam, loflazepate, lorazepam, lormetazepam, medazepam, metaclazepam, mexazolam, midazolam, nordazepam, oxazepam, prazepam, propazepam, quazepam, ripazepam, tofisopam.
Combination pharmacotherapy
We included studies in which regular use of antidepressants and irregular use of benzodiazepines took place and regarded them as antidepressant monotherapy. We also included studies in which regular use of antidepressants and benzodiazepines took place but regarded them as combination therapy of antidepressants plus regular benzodiazepines. We excluded other combination pharmacotherapy.
Types of outcome measures
Primary outcomes
-
Efficacy
Short‐term* 'remission', i.e. satisfactory end‐state as defined by the global judgement of the original investigators, such as 'panic free' or 'no or minimal symptoms' according to the Clinical Global Impression (CGI) Severity Scale (Guy 1976), or a Panic Disorder Severity Scale (PDSS) score of seven or below (Shear 1997), or a Fear Questionnaire Agoraphobia Subscale score of nine or below (Marks 1979).
Short‐term* 'response' (a dichotomised outcome). 'Response' is intended to be a substantial improvement from baseline as defined by the global judgement of the original authors, such as 'very much' or 'much' improvement according to the CGI Change Scale, a more than 40% reduction in the PDSS, or a more than 50% reduction in the Fear Questionnaire Agoraphobia Subscale.
-
Adverse effects
Total number of dropouts for any reason, as a proxy measure of treatment acceptability.
(*) within six months from treatment commencement. When multiple time point measures were available, we gave preference to measures at approximately three months after treatment commencement.
Secondary outcomes
-
Efficacy
Long‐term remission/response of panic disorder, with or without agoraphobia, either after treatment discontinuation or on continued treatment. We defined long term as six months or longer after treatment commencement. When multiple time point measures were available, we gave preference to measures at approximately 12 to 15 months after treatment commencement.
-
Short‐term improvement of panic disorder, with or without agoraphobia, as measured on a continuous scale. Examples of continuous scales are the PDSS (total score 0 to 28), Panic and Agoraphobia Scale (total score 0 to 45), CGI Severity Scale (1 to 7), CGI Change Scale (1 to 7), etc. When multiple measures were used, we gave preference in the following order:
PDSS > Panic and Agoraphobia Scale (Bandelow 1995) > Anxiety Sensitivity Index‐Revised (Deacon 2003) > Anxiety Sensitivity Index (Reiss 1986) > Agoraphobic Cognition Questionnaire (Chambless 1984) > Body Sensation Questionnaire (Chambless 1984) > other scales specific to panic disorder.
CGI Severity Scale > CGI Improvement Scale > Global Assessment Scale (Endicott 1976) > Global Assessment of Functioning (APA 2000) > other global scales.
Fear Questionnaire Agoraphobia Subscale > Fear Questionnaire Global Phobia Rating> Mobility Inventory for Agoraphobia (Chambless 1985) > other scales specific to agoraphobia only.
Panic frequency > panic severity > other scales specific to panic attacks only.
Beck Anxiety Inventory (Beck 1988) > Hamilton Anxiety Rating Scale (Hamilton 1959) > State‐Trait Anxiety Inventory (Spielberger 1989) > other general anxiety scales.
When both self and observer‐rated assessments were available, we gave preference to the latter. We indicated the actual measure entered into the meta‐analysis at the top of the listings in the Characteristics of included studies table.
Short‐term improvement of quality of life as measured on a continuous scale. Examples are the 36‐Item Short Form Health Survey (SF‐36), in Ware 1992, or EuroQoL (Kind 1996).
-
Adverse effects
Suicide and suicide attempts.
Number of dropouts due to adverse effects.
Number of participants experiencing at least one adverse effect.
Timing of outcome assessment
Short‐term timing of outcome assessment was within six months from treatment commencement, and long‐term assessment was six months or longer.
Search methods for identification of studies
Specialised Register of the Cochrane Common Mental Disorders Group (CCMDCTR)
The Cochrane Common Mental Disorders Group maintains a specialised register of RCTs, the CCMDCTR. This register contains over 40,000 reference records (reports of RCTs) for anxiety disorders, depression, bipolar disorder, eating disorders, self harm, and other mental disorders within the scope of this Group. The CCMDCTR is a partially studies‐based register with more than 50% of reference records tagged to c12,500 individually PICO coded study records. Reports of trials for inclusion in the register are collated from (weekly) generic searches of MEDLINE (1950‐), Embase (1974‐), and PsycINFO (1967‐), quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), and review‐specific searches of additional databases. Reports of trials are also sourced from international trial registries, drug companies, the handsearching of key journals, conference proceedings, and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) can be found on the Group's website; an example of the core MEDLINE search is displayed in Appendix 1.
Electronic searches
CCMDCTR‐Studies Register
The Cochrane Common Mental Disorders Group's Information Specialist searched the CCMDCTR‐Studies Register (to 11 September 2015) using the following terms:
Condition/Co‐morbidity = panic AND Intervention = (attention* or behav* or biblio* or biofeedback or cognitive or collaborative or contact or counsel* or desensiti* or educat* or expos* or feedback or 'group” or imag* or interpersonal or intervention or management or panic or prevention or psycho* or relaxation or self* or stress* or support* or *therap* or *train* or treatment or unclear or 'not stated')
Studies comparing a psychological therapy with a pharmacological intervention were manually selected for the authors to screen.
The CCMDCTR‐References Register
The CCMDCTR‐References Register was searched (to 11 September 2015) using a more sensitive set of terms to identify additional untagged/uncoded reports of RCTs (Appendix 2).
Anxiety Disorders Not Otherwise Specified (ADNOS)
A further search of the CCMDCTR was conducted at this time to identify ADNOS studies. The CCMDCTR‐Studies Register and References Register were cross‐searched using the following terms:
("anxiety disorder*" not (agoraphobi* or panic or (social and (anxi* or phobi*)) or generalised or generalized or obsessive or compulsive or OCD or PTSD or post‐trauma* or "post trauma*” or posttrauma* or "specific phobia*" or "spider phobi*")) + ('psychotherapies' as listed in Appendix 2).
Supplementary searches
We conducted a complementary search in PubMed (Appendix 3), as well as in trials registries such as the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/) and ClinicalTrials.gov (clinicaltrials.gov/).
We applied no restrictions on date, language, or publication status to the searches.
Searching other resources
Reference search
We checked the reference lists of all included studies and relevant systematic reviews to identify additional studies missed from the original electronic searches.
Citation search
We conducted a citation search on Web of Science to identify articles citing any of the included studies.
Personal communication
We contacted trialists and subject experts for information on unpublished or ongoing studies or to request additional trial data.
Data collection and analysis
Selection of studies
Two review authors independently examined titles and abstracts of references identified by the electronic search strategies described above to determine whether the study was likely to be relevant, using the following three simple criteria.
Randomised trial.
Panic disorder as diagnosed by operationalised criteria.
Comparison between psychological therapies and pharmacotherapy.
Next we obtained the full texts of the studies identified as potentially relevant by at least one review author. Two out of three review authors (HI, AT, PC) then independently inspected the full texts with regard to the strict eligibility criteria. The strict eligibility criteria consisted of five items which operationalised the above‐listed criteria for considering studies. In case of disagreements, we made the final rating by discussion or by involving a third review author (TAF) where necessary. The inter‐rater reliability (kappa) of these judgements was 0.81. We performed this assessment without being blinded to the author names, journal name, or year of publication.
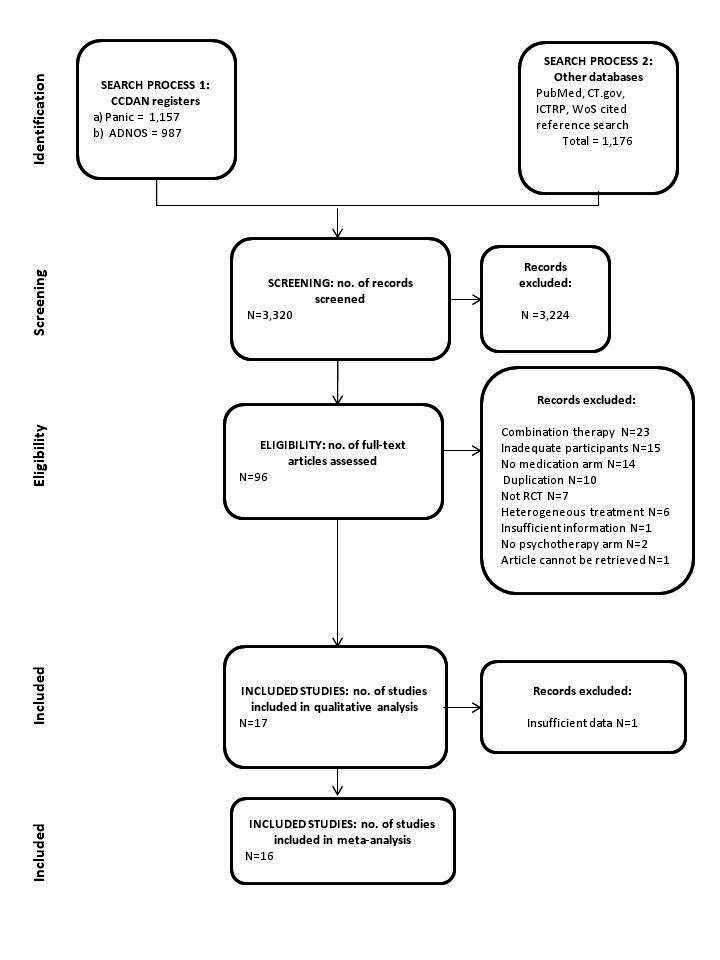
Figure 1 shows the study selection process (along with the number of references and studies, and reasons for exclusion of studies).
Data extraction and management
Two out of three review authors (HI, AT, PC) independently extracted data from the original reports using the pilot‐tested, prespecified data extraction forms. We extracted information about study design, participants (diagnostic criteria, percentage of agoraphobic participants), interventions (format and timing of psychological therapies and control condition, therapist training, intervention components and comparators, name of medication, dosing schedule of medication and range and intended mean dose), outcomes, and risk of bias. Any disagreements were resolved by consensus between the two review authors in consultation with a third review author (TAF). If necessary we contacted authors of the original studies to obtain further clarification.
Main comparisons
All psychological therapies versus SSRIs.
All psychological therapies versus SNRIs.
All psychological therapies versus tricyclic antidepressants.
All psychological therapies versus other antidepressants.
All psychological therapies versus benzodiazepines.
All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines,
Assessment of risk of bias in included studies
Two review authors independently assessed the methodological quality of the selected trials. Any disagreements were resolved by consensus between the two review authors or, if necessary, in consultation with a third review author. The criteria for quality assessment were based on the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following domains.
Random sequence generation and allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessors (detection bias).
Incomplete outcome data reporting (attrition bias).
Selective reporting (reporting bias).
-
Other sources of bias.
Researcher allegiance (post‐hoc assessment)
Therapist allegiance (post‐hoc assessment)
Sponsorship bias (post‐hoc assessment)
We assessed each potential risk of bias and assigned one of three categories: low, high, or unclear risk of bias (when there was insufficient information to permit judgement of high or low risk), and described this in the 'Risk of bias' table for each trial. We made the assessments according to prespecified criteria, mainly based on the Cochrane Handbook.
Measures of treatment effect
Dichotomous data
We calculated risk ratios (RRs) and their 95% confidence intervals (CIs) using a random‐effects model. RRs using a random‐effects model have been shown to be superior for clinical interpretation and external generalisability compared to fixed‐effect models and odds ratios (ORs) or risk differences (RDs) (Furukawa 2002).
Continuous data
We calculated standardised mean differences (SMDs) and their 95% CIs, because we expected different measures to have been used to measure the same construct.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials only if the intracluster correlation coefficient (ICC) was reported or could be borrowed from similar trials. We planned to estimate the effect size in cluster‐randomised trials using the ICC to adjust for cluster effects, however there were no such trials.
Cross‐over trials
We planned to use the first active treatment phase for our analysis, however there were no such trials.
Multiple‐armed trials
We combined arms as long as they could be regarded as subtypes of the same treatment. If this was not the case, we treated each arm as a separate comparator and subdivided the sample size of the common comparator arm for pairwise meta‐analysis.
Dealing with missing data
Dichotomous data
We contacted the original study authors for missing data. We calculated responders to treatment and remitters on a strict intention‐to‐treat (ITT) basis and thus included all dropouts in the analyses. Where participants had been excluded from a trial before the endpoint, we assumed that they had experienced a negative outcome by the end of the trial (e.g. failure to respond to treatment). We examined the validity of this decision in sensitivity analyses by applying worst‐ and best‐case scenarios (i.e. missing data are assumed either as responder or non‐responder in the corresponding sensitivity analysis).
When dichotomous outcomes were not reported, but baseline mean, endpoint mean, and standard deviations (SDs) of the scores were provided, we converted continuous outcome data expressed as mean and SD into the number of responding and remitting participants, according to a validated imputation method (Furukawa 2005). We examined the validity of this imputation in the sensitivity analyses.
Continuous data
We contacted the original study authors for missing data. When there were missing data and the method of 'last observation carried forward' (LOCF) had been used to perform an ITT analysis, then we used the LOCF data.
When only the standard error (SE) or t‐statistics or P values were reported, we planned to calculate SDs according to Altman (Altman 1996). Where SDs were not reported, we planned to contact the trial authors and ask them to supply the data; in the absence of data from the authors, we planned to borrow SDs from other studies in the review (Furukawa 2006). We also planned to examine the validity of this imputation in the sensitivity analyses; however there were no trials where SDs were not reported.
Assessment of heterogeneity
We assessed heterogeneity between trials qualitatively, by visual inspection of the forest plots, and quantitatively using the Chi2 test and the I2 statistic. Due to the known low sensitivity of the Chi2 test, we took a P value of less than 0.10 to be suggestive of heterogeneity. We interpreted the I2 value according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% may represent considerable heterogeneity) (Higgins 2011), while keeping in mind that the importance of the observed value of I2 depends on (i) the magnitude and direction of effects; and (ii) the strength of evidence for heterogeneity (e.g. a confidence interval for I2) (Higgins 2003). If we noted significant heterogeneity, we investigated its sources.
Assessment of reporting biases
We entered data from the included studies into a funnel plot (trial effect against trial variance) to investigate small‐study effects. We used the tests for funnel plot asymmetry only when there were at least 10 studies included in the meta‐analysis, and we interpreted the results cautiously, with visual inspection of the funnel plots (Higgins 2011). When we identified evidence of small‐study effects, we investigated possible reasons for funnel plot asymmetry, including publication bias.
Data synthesis
We used a random‐effects model to calculate the intervention effect, as it assumes heterogeneity of intervention effects. Although the random‐effects model gives a more conservative estimate than the fixed‐effect model, it gives weight to small studies, which can increase or decrease the effect size. Taking this into consideration, we also used a fixed‐effect model for the primary outcome to see if there was a large difference between the two models.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses at the study level, where possible, for the following a priori reasons.
For each type of psychological therapy separately (CBT, cognitive therapy, behaviour therapy, psychodynamic therapy, and others), because they may not have the same degree of efficacy.
For studies including agoraphobic participants and those excluding them, because the same treatment may have varying efficacy with regard to panic and agoraphobia.
For studies including participants with and without depressive disorder, because it is likely that antidepressants may confer more benefit on those patients with panic disorder comorbid with depression.
We added the following post‐hoc subgroup analyses to investigate heterogeneity: studies excluding group psychological therapies; outcomes using the same scales; and studies without extreme results.
Sensitivity analysis
We conducted the following sensitivity analyses.
Limiting to participants without any comorbid physical or mental disorders.
-
Limiting to trials where quality control of the psychological therapies administered was adequate, because we consider therapists' adherence to the model of psychological therapy to be important in establishing its true effects. We assessed the adequacy of the psychological therapies administered as follows. We carried out a sensitivity analysis by limiting to trials with a rating of 'A' or 'B'.
A: When the conduct of the psychological therapies was examined by a third author through audiotapes, etc., and when the authors gave sufficiently detailed description of the therapy procedure in the text or provided information about the treatment manual.
B: When the trial fulfilled either of the two conditions stated above.
C: When none of these applied.
Limiting to trials that met both of the following criteria: allocation concealment is rated as at low risk of bias, and outcome assessors are rated as at low risk of bias.
Calculating ORs and their 95% CIs instead of RRs and their 95% CIs for the primary outcomes (dichotomous data) in order to investigate whether our choice of RRs is critical to the conclusions of the review.
Limiting to studies including participants with agoraphobia.
Excluding studies in which some, but possibly not all, of the participants had panic disorder.
We added the following post‐hoc sensitivity analyses: calculating primary outcomes using worst‐case and best‐case scenarios; and limiting to studies without imputed data.
'Summary of findings' tables
'Summary of findings' tables included a list of all important outcomes, RRs for dichotomous outcomes and SMDs for continuous outcomes, numbers of participants and studies addressing these outcomes, and a rating of the overall quality of the evidence, using the GRADE approach. The included population were people with panic disorder with or without agoraphobia.
Results
Description of studies
Results of the search
Our search identified 3320 references. We excluded 3224 studies after assessment of the titles and abstracts. We retrieved 96 full‐text papers for full inspection. Of these, we excluded 79 studies. We finally included 17 reports, but information for one report was insufficient, and we were unable to obtain further information from the study authors. As a result, we included 16 reports with a total of 966 participants in our meta‐analysis. Cohen's kappa among the three review authors for the selection from 96 full‐text papers was 0.81, which means an excellent agreement. We contacted authors of the eight studies (Ataoglu 2000; Azhar 2000; Bressi 2010; Dannon 2004; Prasko 2004; Taylor 1982; Tyrer 1988; van Apeldoorn 2008) for additional information, and three authors responded (Bressi 2010; Spinhoven 1996; Tyrer 1988). See Figure 1 for a PRISMA flow diagram depicting the study selection process (Moher 2009).
1.
Included studies
Seventeen studies were eligible for this review, but one study did not report extractable data. We finally included 16 studies in the analyses, with characteristics as follows (see also Characteristics of included studies).
Design
All of the included studies were parallel‐group, individually randomised controlled trials. Two studies were two‐armed (Ataoglu 2000; Bressi 2010), and the other studies were multi‐armed.
Sample sizes
The mean sample size per arm was 28 (range 6 to 83). One study recruited fewer than 10 participants overall (Prasko 2004).
Setting
All trials were conducted in the outpatient setting. Eight studies were carried out in European countries, five in the USA, two in the Middle East, and one in Southeast Asia.
Participants
The proportion of women ranged from 19% to 82%. One study had a proportion of less than 30% women (Clark 1994), and two studies had proportions of more than 30% women (Black 1993; Klosko 1988; Sharp 1996). Two studies did not report the percentage of women with panic disorder (Azhar 2000; Bressi 2010; Tyrer 1988).
The mean ages of the participants were in the 30s in the majority of studies (12 out of 15 studies). The mean age was in the 40s in one study (Dannon 2004), and in the 60s in one study (Hendriks 2010). One study reported the mean age of all the participants, including participants with panic disorder and other disorders, but not exclusively for participants with panic disorder (Tyrer 1988).
Interventions
We found seven studies comparing CBT with SSRI/SNRI (three studies versus fluvoxamine (Azhar 2000; Black 1993; Sharp 1996), two studies versus paroxetine (Dannon 2004; Hendriks 2010), two studies versus non‐specific SSRI/SNRI where clinician could choose any SSRI/SNRI (Prasko 2004; van Apeldoorn 2008)); one study comparing CBT with tricyclic antidepressant (imipramine) (Barlow 2000); two studies comparing CBT with benzodiazepine (alprazolam) (Ataoglu 2000; Klosko 1988); one study comparing psychodynamic psychological therapy with non‐specific SSRI/SNRI (Bressi 2010); and one study comparing physiological therapy with benzodiazepine (diazepam) (Taylor 1982). Four studies included multiple comparisons: one study comparing CBT with SSRI (paroxetine) or tricyclic antidepressant (clomipramine) (Bakker 1999); one study comparing CBT with tricyclic antidepressant (dosulepin) or benzodiazepine (diazepam) (Tyrer 1988); one study comparing CBT or behaviour therapy with tricyclic antidepressant (imipramine) (Clark 1994); one study comparing CBT or supportive psychological therapy with tricyclic antidepressant (imipramine) (Shear 2000).
Outcomes
Eleven studies reported short‐term remission, of which six studies used panic‐free as the definition of remission. Four studies reported short‐term response, of which two studies used an improvement in Clinical Global Impression (CGI), one study used a reduction in Panic Disorder Severity Scale (PDSS), and one study used a clinically relevant improvement. All of the included studies reported the number of dropouts. Of our secondary outcomes only information on short‐term improvement was available. Fourteen studies reported continuous outcomes at post‐treatment; however, two of these studies lacked the number of participants assessed at post‐treatment. Among the 14 studies with continuous outcomes, four studies used CGI, three studies used PDSS, two studies used the Agoraphobic Cognitions Questionnaire, and two studies used the State‐Trait Anxiety Inventory. The other studies used the Hamilton Anxiety Scale, Hopkins Symptom Checklist, the Brief Scale for Anxiety, and global clinical severity.
Excluded studies
Of the 96 references retrieved for more detailed evaluation, 79 references did not meet our inclusion criteria and were excluded or are awaiting classification. Twenty‐three studies dealt with combination therapy, 15 studies included participants who did not meet our inclusion criteria, 14 studies had no pharmacological intervention arm, 10 studies were duplicate report, seven studies were not a randomised controlled trial, six studies had a heterogeneous treatment arm, and two studies had no psychological therapy arm. Among the studies with inappropriate participants, three studies included participants with panic disorder; however, stratified randomisation was not performed (Borkovec 1988; Huppert 2004; Schuurmans 2006), and participants of one study had agoraphobia without panic attacks (Echeburua 1993). Among the studies with a heterogeneous treatment arm, doctors chose treatment in a control arm or a pharmacological treatment arm in two studies (Echeburua 2006; Lang 2006), and one study permitted regular benzodiazepine use in the psychological therapy arm, and some of the participants in this arm used benzodiazepine, which is regarded as a mixture of combination therapy and psychological therapy (Spinhoven 1996). (See also Characteristics of excluded studies.)
Studies awaiting classification
One study lacked information required for inclusion (Prasko 2004b), and the full text of one study could not be retrieved (Takriti 1989).
Ongoing studies
We did not find any ongoing studies.
Risk of bias in included studies
For details of the 'Risk of bias' judgements for each study, see Characteristics of included studies. Graphical representations of the overall risk of bias in included studies are presented in Figure 2 and Figure 3.
2.
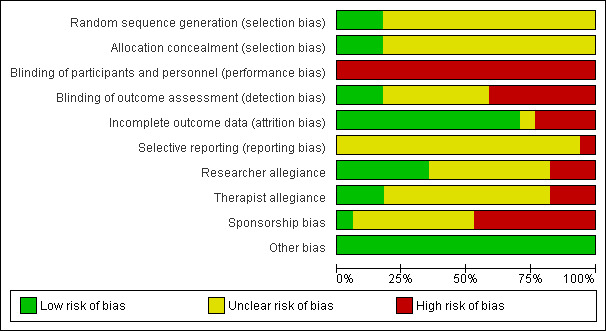
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
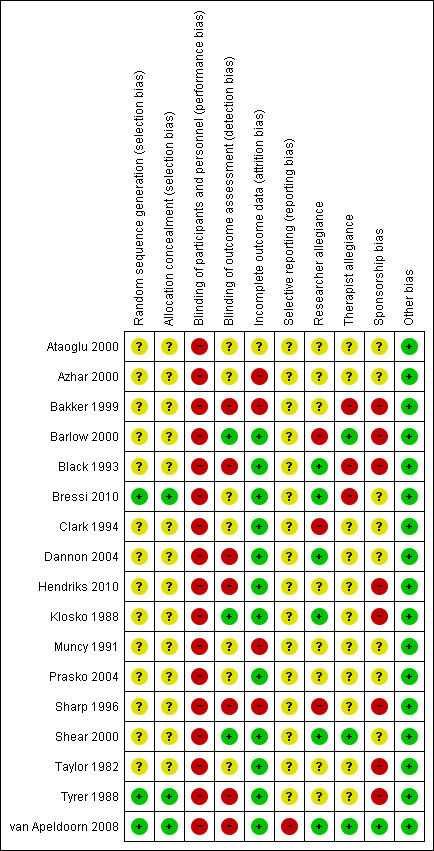
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only three studies reported sufficient details regarding allocation concealment (Bressi 2010; Tyrer 1988; van Apeldoorn 2008). The agreement between the two independent raters in the assessment of risk of allocation bias was 100%.
Blinding
Performance bias
Participants and personnel were aware of the intervention, as all studies included psychological therapies. We judged that all of the included studies were at high risk of performance bias.
Detection bias
Asessors in seven studies were not blinded (Bakker 1999; Black 1993; Dannon 2004; Hendriks 2010; Sharp 1996; Tyrer 1988; van Apeldoorn 2008); those in three studies were blinded (Barlow 2000; Klosko 1988; Shear 2000); and seven studies did not provide information regarding the blinding of the assessors (Ataoglu 2000; Azhar 2000; Bressi 2010; Clark 1994; Muncy 1991; Prasko 2004; Taylor 1982). The agreement between two independent raters in the assessment of risk of bias due to lack of blinding was 66%. The inter‐rater reliability (quadratic weighted kappa) of these judgements was 0.53.
Incomplete outcome data
Twelve studies had no or few dropouts or included all the participants in the analysis (Barlow 2000; Black 1993; Bressi 2010; Clark 1994; Dannon 2004; Hendriks 2010; Klosko 1988; Prasko 2004; Shear 2000; Taylor 1982; Tyrer 1988; van Apeldoorn 2008). Four studies had a dropout rate of more than 20% of the randomised participants (Azhar 2000; Bakker 1999; Muncy 1991; Sharp 1996). One study did not report the number of participants assessed post‐treatment (Ataoglu 2000). The agreement between two independent raters in the assessment of allocation bias was 61%. The inter‐rater reliability (quadratic weighted kappa) of these judgements was 0.48.
Selective reporting
One study did not report some outcomes that had been indicated in the trial registration (van Apeldoorn 2008). Other studies did not include the study protocol. The agreement between two independent raters in the assessment of risk of bias due to selective reporting was 87%. The inter‐rater reliability (quadratic weighted kappa) of these judgements was ‐0.063.
Other potential sources of bias
Researcher allegiance
We rated the risk of bias due to researcher allegiance as high risk if the authors had allegiance to one of the treatment arms, such as authors who had created a treatment manual for psychological therapy or authors with connections to the manufacturer of the medication that was used. We rated 47% of the studies as having an unclear risk of bias, 35% as having a low risk of bias, and 18% as having a high risk of bias. Researchers in two studies had an allegiance to the psychological therapy because of involvement in the creation of a treatment manual (Barlow 2000; Clark 1994), and another researcher had an allegiance to the pharmacological intervention because of employment with the manufacturer of the medication (Sharp 1996). The agreement between two independent raters in the assessment of risk of bias due to researcher allegiance was 47%. The inter‐rater reliability (quadratic weighted kappa) of these judgements was 0.36.
Therapist allegiance
We rated the risk of bias due to therapist allegiance as high risk if therapists had allegiance to one of the treatments, such as a psychological therapist who was a specialist in the treatment or a psychopharmacologist who conducted the medical intervention. We rated 65% of the studies as having an unclear risk of bias, 18% as having a low risk of bias, and 18% as having a high risk of bias. The therapists in three studies had an allegiance to the psychological therapy (Bakker 1999; Black 1993; Bressi 2010). The agreement between the two independent raters in the assessment of risk of bias due to therapist allegiance was 70%. The inter‐rater reliability (quadratic weighted kappa) of these judgements was 0.41.
Sponsorship bias
Eight studies were sponsored by the manufacturer of the medication involved (Bakker 1999; Barlow 2000; Black 1993; Hendriks 2010; Klosko 1988; Sharp 1996; Taylor 1982; Tyrer 1988). One study reported no conflict of interest (van Apeldoorn 2008). The other studies did not mention conflicts of interest. The agreement between two independent raters in the assessment of risk of bias due to sponsorship was 65%. The inter‐rater reliability (quadratic weighted kappa) of these judgements was 0.41.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
The agreement between two independent raters in the primary efficacy outcomes was 71% for short‐term remission and 82% for short‐term response, and 71% for the treatment acceptability.
In the results shown below, the psychological therapies were favoured with a RR over 1.0 for short‐term remission and response and a negative SMD for short‐term improvement. Pharmacological interventions were favoured with a RR over 1.0 for dropouts for any reason.
Comparison 1: All psychological therapies versus SSRI
See: Table 1
Primary outcomes
1.1 Short‐term remission
There was no evidence of a difference between psychological therapies and SSRI in terms of short‐term remission (RR 0.85, 95% CI 0.62 to 1.17; 6 studies; 334 participants) (very low‐quality evidence) (Analysis 1.1). There was substantial heterogeneity in the study results (I2 = 69%, Tau2 = 0.09) and a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
1.1. Analysis.
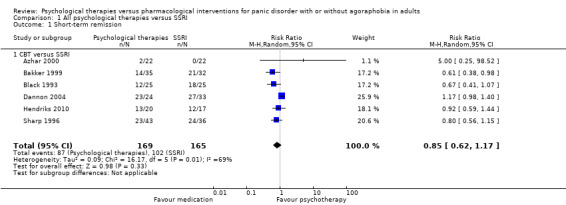
Comparison 1 All psychological therapies versus SSRI, Outcome 1 Short‐term remission.
1.2 Short‐term response
There was no evidence of a difference between psychological therapies and SSRI in terms of short‐term response (RR 0.97, 95% CI 0.51 to 1.86; 5 studies; 277 participants) (very low‐quality evidence) (Analysis 1.2). There was substantial heterogeneity in the study results (I2 = 78%, Tau2 = 0.42) and a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
1.2. Analysis.
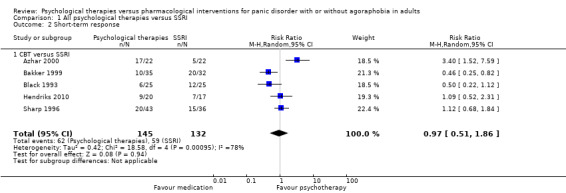
Comparison 1 All psychological therapies versus SSRI, Outcome 2 Short‐term response.
1.3 Dropouts for any reason
There was no evidence of a difference between psychological therapies and SSRI in terms of dropouts for any reason (RR 1.33, 95% CI 0.80 to 2.22; 6 studies; 334 participants) (low‐quality evidence) (Analysis 1.3). The heterogeneity was negligible (I2 = 15%, Tau2 = 0.06). There was a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
1.3. Analysis.
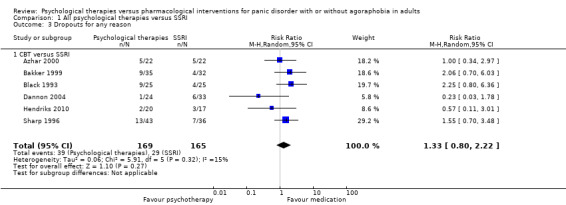
Comparison 1 All psychological therapies versus SSRI, Outcome 3 Dropouts for any reason.
Secondary outcomes
Only short‐term improvement was available among the secondary outcomes.
1.4 Short‐term improvement
There was no evidence of a difference between psychological therapies and SSRI in terms of short‐term improvement (SMD ‐0.20, 95% CI ‐1.09 to 0.69; 5 studies; 242 participants) (moderate‐quality evidence) (Analysis 1.4). There was considerable heterogeneity in the study results (I2 = 91%, Tau2 = 0.92) and a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
1.4. Analysis.
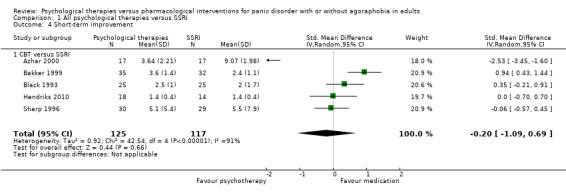
Comparison 1 All psychological therapies versus SSRI, Outcome 4 Short‐term improvement.
Comparison 2: All psychological therapies versus SNRIs
There were no data to contribute to this comparison.
Comparison 3: All psychological therapies versus tricyclic antidepressants
See: Table 2
Primary outcomes
3.1 Short‐term remission
There was no evidence of a difference between psychological therapies and tricyclic antidepressants in terms of short‐term remission (RR 0.82, 95% CI 0.62 to 1.09; 3 studies; 229 participants) (low‐quality evidence) (Analysis 2.1). The heterogeneity was negligible (I2 = 0%, Tau2 = 0.00). There was a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
2.1. Analysis.
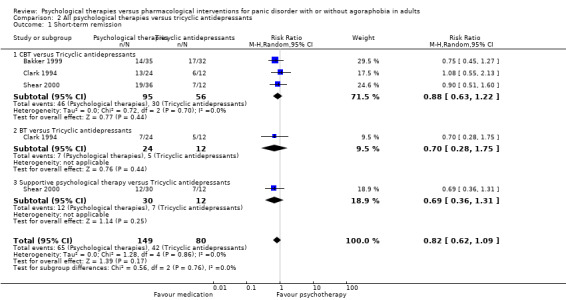
Comparison 2 All psychological therapies versus tricyclic antidepressants, Outcome 1 Short‐term remission.
3.2 Short‐term response
There was no evidence of a difference between psychological therapies and tricyclic antidepressants in terms of short‐term response (RR 0.75, 95% CI 0.51 to 1.10; 4 studies; 270 participants) (low‐quality evidence) (Analysis 2.2). The heterogeneity was negligible (I2 = 0%, Tau2 = 0.00). There was a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
2.2. Analysis.
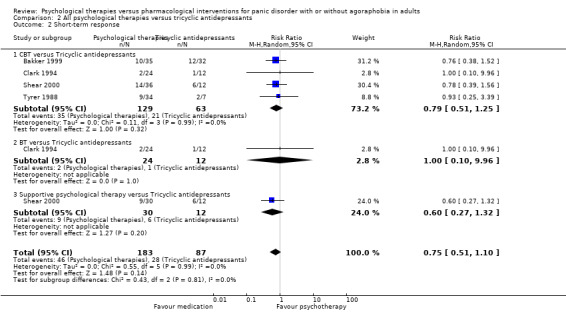
Comparison 2 All psychological therapies versus tricyclic antidepressants, Outcome 2 Short‐term response.
3.3 Dropouts for any reason
There was no evidence of a difference between psychological therapies and tricyclic antidepressants in terms of dropouts for any reason (RR 0.83, 95% CI 0.53 to 1.30; 5 studies; 430 participants) (low‐quality evidence) (Analysis 2.3). The heterogeneity was negligible (I2 = 25%, Tau2 = 0.07). There was a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
2.3. Analysis.
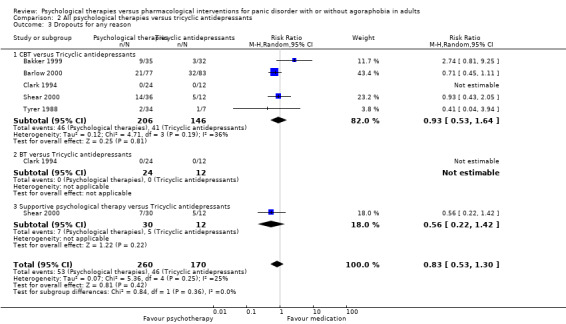
Comparison 2 All psychological therapies versus tricyclic antidepressants, Outcome 3 Dropouts for any reason.
Secondary outcomes
Only short‐term improvement was available among the secondary outcomes.
3.4 Short‐term improvement
There was no evidence of a difference between psychological therapies and tricyclic antidepressants in terms of short‐term improvement (SMD 0.20, 95% CI ‐0.03 to 0.43; 5 studies; 414 participants) (moderate‐quality evidence) (Analysis 2.4). The heterogeneity was negligible (I2 = 12%, Tau2 = 0.01).
2.4. Analysis.
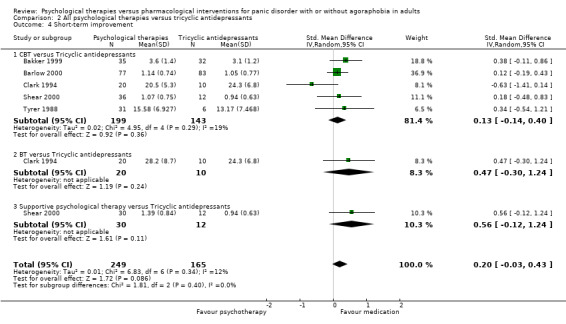
Comparison 2 All psychological therapies versus tricyclic antidepressants, Outcome 4 Short‐term improvement.
Comparison 4: All psychological therapies versus other antidepressants
See: Table 3
Primary outcomes
4.1 Short‐term remission
There was no evidence of a difference between psychological therapies and other antidepressants in terms of short‐term remission (RR 0.90, 95% CI 0.48 to 1.67; 3 studies; 135 participants) (very low‐quality evidence) (Analysis 3.1). There was substantial heterogeneity (I2 = 61%, Tau2 = 0.19) and a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
3.1. Analysis.
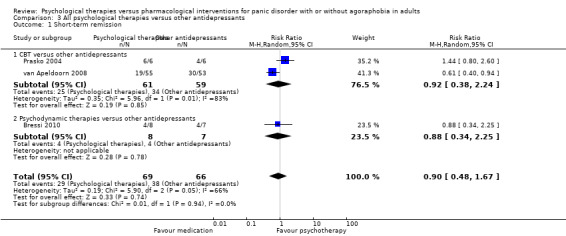
Comparison 3 All psychological therapies versus other antidepressants, Outcome 1 Short‐term remission.
4.2 Short‐term response
There was no evidence of a difference between psychological therapies and other antidepressants in terms of short‐term response (RR 0.96, 95% CI 0.67 to 1.37; 3 studies; 128 participants) (low‐quality evidence) (Analysis 3.2). The heterogeneity was negligible (I2 = 33%, Tau2 = 0.04). There was a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
3.2. Analysis.
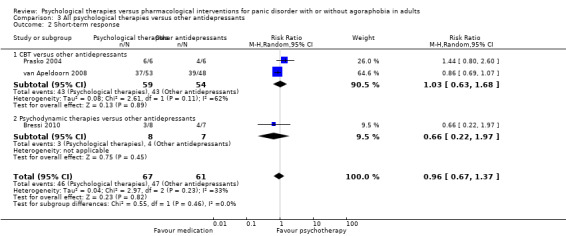
Comparison 3 All psychological therapies versus other antidepressants, Outcome 2 Short‐term response.
4.3 Dropouts for any reason
There was no evidence of a difference between psychological therapies and other antidepressants in terms of dropouts for any reason (RR 1.55, 95% CI 0.91 to 2.65; 3 studies; 180 participants) (low‐quality evidence) (Analysis 3.3). The heterogeneity was negligible (I2 = 1%, Tau2 = 0.00). There was a lack of precision.
3.3. Analysis.
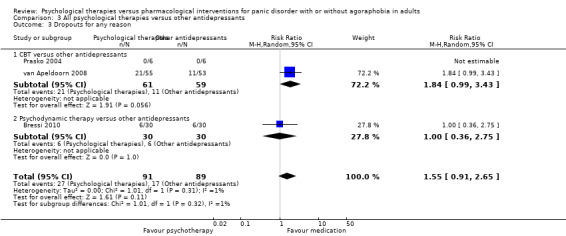
Comparison 3 All psychological therapies versus other antidepressants, Outcome 3 Dropouts for any reason.
Secondary outcomes
Only short‐term improvement was available among the secondary outcomes.
4.4 Short‐term improvement
There was no evidence of a difference between psychological therapies and other antidepressants in terms of short‐term improvement (SMD ‐0.43, 95% CI ‐1.36 to 0.51; 2 studies; 27 participants) (moderate‐quality evidence) (Analysis 3.4). The heterogeneity was negligible (I2 = 29%, Tau2 = 0.14). There was a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
3.4. Analysis.
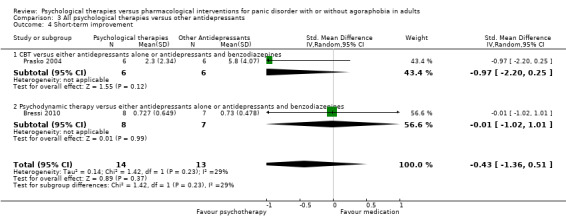
Comparison 3 All psychological therapies versus other antidepressants, Outcome 4 Short‐term improvement.
Comparison 5: All psychological therapies versus benzodiazepines
See: Table 4
Primary outcomes
5.1 Short‐term remission
There was no evidence of a difference between psychological therapies and benzodiazepines in terms of short‐term remission (RR 1.08, 95% CI 0.70 to 1.65; 3 studies; 95 participants) (very low‐quality evidence) (Analysis 4.1). The heterogeneity was negligible (I2 = 0%, Tau2 = 0.00). There was a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
4.1. Analysis.
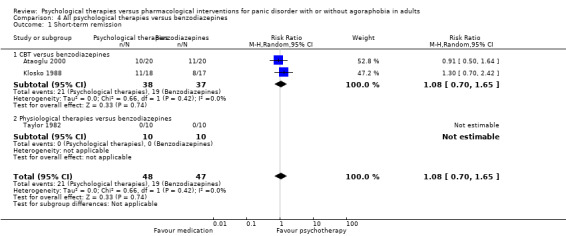
Comparison 4 All psychological therapies versus benzodiazepines, Outcome 1 Short‐term remission.
5.2 Short‐term response
Two studies including 69 participants contributed data. There was no evidence of a difference between psychological therapies and benzodiazepines in terms of short‐term response (RR 1.58, 95% CI 0.70 to 3.58; 2 studies; 69 participants) (very low‐quality evidence) (Analysis 4.2). The heterogeneity was negligible (I2 = 0%, Tau2 = 0.00). There was a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
4.2. Analysis.
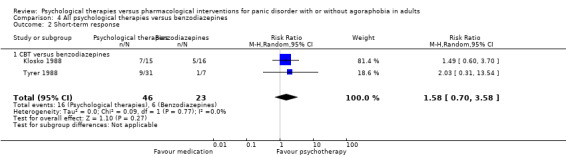
Comparison 4 All psychological therapies versus benzodiazepines, Outcome 2 Short‐term response.
5.3 Dropouts for any reason
Three studies including 116 participants contributed data. There was no evidence of a difference between psychological therapies and benzodiazepines in terms of dropouts for any reason (RR 1.12, 95% CI 0.54 to 2.36; 3 studies; 116 participants) (very low‐quality evidence) (Analysis 4.3). The heterogeneity was negligible (I2 = 0%, Tau2 = 0.00). There was a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
4.3. Analysis.
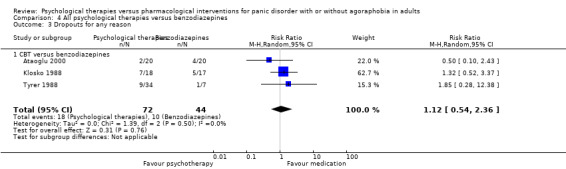
Comparison 4 All psychological therapies versus benzodiazepines, Outcome 3 Dropouts for any reason.
Secondary outcomes
Only short‐term improvement was available among the secondary outcomes.
5.4 Short‐term improvement
There was no evidence of a difference between psychological therapies and benzodiazepines in terms of short‐term improvement (SMD ‐0.23, 95% CI ‐0.69 to 0.23; 3 studies; 89 participants) (very low‐quality evidence) (Analysis 4.4). The heterogeneity was negligible (I2 = 0%, Tau2 = 0.00). There was a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm.
4.4. Analysis.
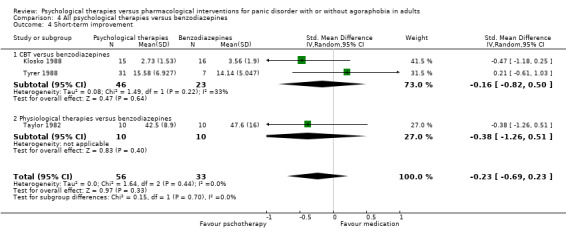
Comparison 4 All psychological therapies versus benzodiazepines, Outcome 4 Short‐term improvement.
Comparison 6: All psychological therapies versus either antidepressant alone or antidepressants and benzodiazepines
See: Table 5
Primary outcomes
6.1 Short‐term remission
There was no evidence of a difference between psychological therapies and either antidepressant alone or antidepressants and benzodiazepines in terms of short‐term remission (RR 0.86, 95% CI 0.71 to 1.05; 11 studies; 663 participants) (low‐quality evidence) (Analysis 5.1). There was moderate heterogeneity (I2 = 46%, Tau2 = 0.05) and a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm. The Egger's test for funnel plot asymmetry was non‐significant (P = 0.44).
5.1. Analysis.
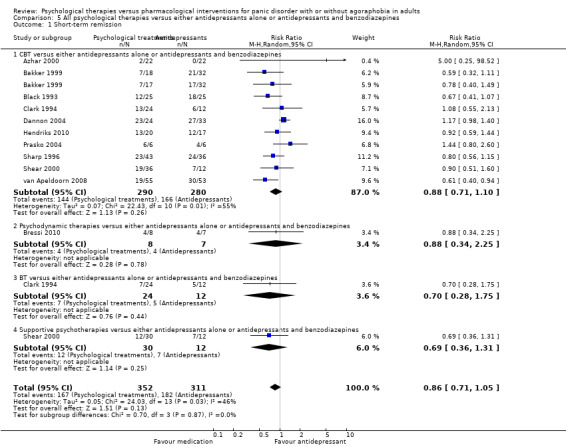
Comparison 5 All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 1 Short‐term remission.
6.2 Short‐term response
There was no evidence of a difference between psychological therapies and either antidepressant alone or antidepressants and benzodiazepines in terms of short‐term response (RR 0.95, 95% CI 0.76 to 1.18; 12 studies; 800 participants) (low‐quality evidence) (Analysis 5.2). The heterogeneity was negligible (I2 = 37%, Tau2 = 0.05). There was a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm. The Egger's test for funnel plot asymmetry was non‐significant (P = 0.78).
5.2. Analysis.
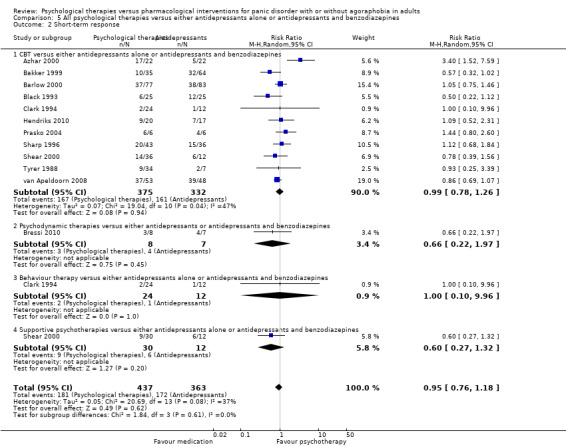
Comparison 5 All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 2 Short‐term response.
6.3 Dropouts for any reason
There was no evidence of a difference between psychological therapies and either antidepressant alone or antidepressants and benzodiazepines in terms of dropouts for any reason (RR 1.08, 95% CI 0.77 to 1.51; 13 studies; 909 participants) (very low‐quality evidence) (Analysis 5.3). The heterogeneity was negligible (I2 = 36%, Tau2 = 0.12). There was a lack of precision. The 95% confidence interval around the pooled estimate of the effect included both no effect and an appreciable benefit or appreciable harm. The Egger's test for funnel plot asymmetry was significant (P = 0.047) (Figure 4). However, contour‐enhanced funnel plot did not suggest publication bias (Figure 5).
5.3. Analysis.
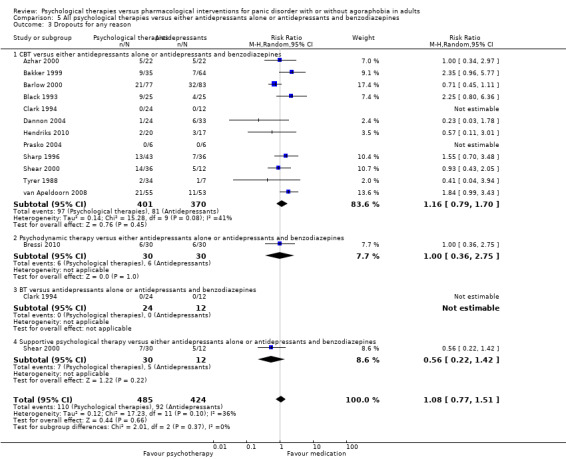
Comparison 5 All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 3 Dropouts for any reason.
4.
Funnel plot of comparison: 5 All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, outcome: 5.3 Dropouts for any reason.
5.
Contour‐enhanced funnel plot: 5 All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, outcome: 5.3 Dropouts for any reason.
Secondary outcomes
Only short‐term improvement was available among the secondary outcomes.
6.4 Short‐term improvement
There was no evidence of a difference between psychological therapies and either antidepressant alone or antidepressants and benzodiazepines in terms of short‐term improvement (SMD 0.01, 95% CI ‐0.34 to 0.35; 11 studies; 638 participants) (low‐quality evidence) (Analysis 5.4). There was substantial heterogeneity (I2 = 73%, Tau2 = 0.30). The Egger's test for funnel plot asymmetry was non‐significant (P = 0.36).
5.4. Analysis.
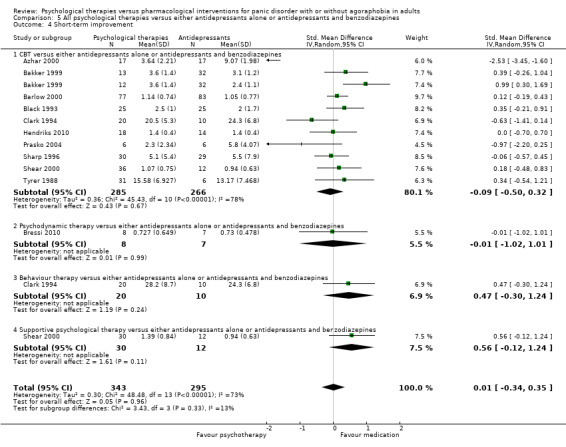
Comparison 5 All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 4 Short‐term improvement.
Subgroup analyses
1. For each type of psychological therapy separately
1.1 Psychological therapies versus SSRI
The studies included only CBT.
1.2. Psychological therapies versus tricyclic antidepressants
The studies included CBT, behaviour therapy (BT), and supportive psychological therapy. However, there was only one study each for BT or supportive psychological therapy (Clark 1994; Shear 2000). There was no significant difference in the efficacy of CBT and tricyclic antidepressants as measured using short‐term remission (RR 0.88, 95% CI 0.63 to 1.22; 3 studies; 151 participants) (Analysis 2.1), short‐term response (RR 0.79, 95% CI 0.51 to 1.25; 4 studies; 192 participants), or short‐term improvement (SMD 0.13, 95% CI ‐0.14 to 0.40; 5 studies; 342 participants) or in treatment acceptability as measured using dropouts for any reason (RR 0.93, 95% CI 0.53 to 1.64; 5 studies; 352 participants) (Analysis 2.2).
1.3. Psychological therapies versus other antidepressants
The studies included CBT and psychodynamic therapies. However, only one study examined psychodynamic therapies (Bressi 2010). There was no significant difference in the efficacy of CBT and other antidepressants as measured using short‐term remission (RR 0.92, 95% CI 0.38 to 2.24; 2 studies; 120 participants) (Analysis 3.1.1), or short‐term response (RR 1.03, 95% CI 0.63 to 1.68; 2 studies; 113 participants) (Analysis 3.2.1), or in treatment acceptability as measured using dropouts for any reason (RR 1.84, 95% CI 0.99 to 3.43; 2 studies; 120 participants) (Analysis 3.3.1). Only one study including CBT was available for short‐term improvement.
1.4. Psychological therapies versus benzodiazepines
The studies included CBT and physiological therapies. However, only one study examined physiological therapies (Taylor 1982). There was no significant difference in the efficacy of CBT and benzodiazepines as measured using short‐term remission (RR 1.08, 95% CI 0.70 to 1.65; 2 studies; 75 participants) (Analysis 4.1.1), short‐term response (RR 1.58, 95% CI 0.70 to 3.58; 2 studies; 69 participants) (Analysis 4.2.1), or short‐term improvement (SMD ‐0.16, 95% CI ‐0.82 to 0.50; 2 studies; 69 participants) (Analysis 4.4.1). All psychological therapies used in the studies included in the analysis of dropouts for any reason were CBT.
1.5. Psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines
The studies included CBT, BT, psychodynamic therapies, and supportive psychological therapy. However, there was only one study each for BT, psychodynamic therapies, or supportive psychological therapy (Bressi 2010; Clark 1994; Shear 2000). There was no significant difference between CBT and either antidepressants alone or antidepressants and benzodiazepines in terms of efficacy as measured using short‐term remission (RR 0.88, 95% CI 0.71 to 1.10; 10 studies; 570 participants) (Analysis 5.1.1), short‐term response (RR 0.99, 95% CI 0.78 to 1.26; 11 studies; 707 participants) (Analysis 5.2.1), short‐term improvement (SMD ‐0.09, 95% CI ‐0.50 to 0.32; 10 studies; 551 participants) (Analysis 5.4.1) or in treatment acceptability as measured using dropouts for any reason (RR 1.16, 95% CI 0.79 to 1.70; 12 studies; 771 participants) (Analysis 5.3.1).
2. For participants with and without agoraphobia
We found no study where all the participants were without agoraphobia, while almost all of the participants had panic disorder with agoraphobia in one study (100% in SSRI arm, 97% in tricyclic antidepressant arm, and 91% in CBT arm) (Bakker 1999). It was therefore not possible to execute this subgroup analysis.
3. For participants with and without depressive disorder
Two studies included only participants without depressive disorder (Dannon 2004; Prasko 2004), and we were able to conduct a subgroup analysis for this subtype, i.e. participants without depressive disorder only. The comparison between all psychological therapies and either antidepressant alone or antidepressants and benzodiazepines included these studies with 69 participants. There was high‐quality evidence that either antidepressant alone or antidepressants and benzodiazepines were marginally more effective than psychological therapies as measured using the short‐term response (RR 1.19, 95% CI 1.00 to 1.42; 2 studies; 69 participants) (Analysis 6.1). However, there was no difference in treatment acceptability as measured using dropouts for any reason (RR 0.23, 95% CI 0.03 to 1.78; 2 studies; 69 participants) (Analysis 6.2).
6.1. Analysis.
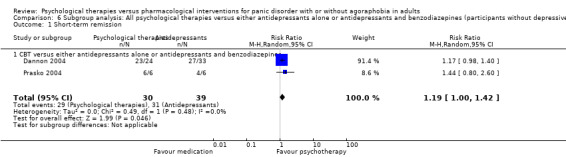
Comparison 6 Subgroup analysis: All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines (participants without depressive disorder), Outcome 1 Short‐term remission.
6.2. Analysis.
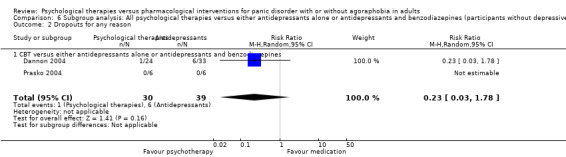
Comparison 6 Subgroup analysis: All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines (participants without depressive disorder), Outcome 2 Dropouts for any reason.
All of the other studies included both participants with and without depressive disorder.
4. For length of intervention (investigation of heterogeneity)
4.1. All psychological therapies versus other antidepressants (short‐term remission)
Two studies were relatively consistent (Bressi 2010; van Apeldoorn 2008), while the results of Prasko 2004 were opposite. The studies had different study periods (36 weeks in van Apeldoorn 2008, 44 weeks in Bressi 2010, and 12 weeks in Prasko 2004) and risk of bias (low risk of selection bias in Bressi 2010 and van Apeldoorn 2008 and unclear risk of selection bias in Prasko 2004). An analysis of the two studies with longer duration of intervention indicated that other antidepressants were more effective than all psychological therapies (RR 0.65, 95% CI 0.44 to 0.96; 2 studies; 123 participants). There was no evidence of heterogeneity (I2 = 0%, Tau2 = 0.00) (Analysis 8.1).
8.1. Analysis.
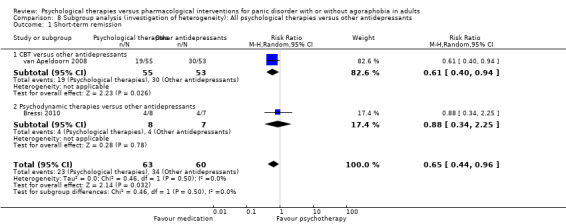
Comparison 8 Subgroup analysis (investigation of heterogeneity): All psychological therapies versus other antidepressants, Outcome 1 Short‐term remission.
5. For studies excluding group psychological therapies (investigation of heterogeneity, post‐hoc analysis)
5.1. All psychological therapies versus SSRI (short‐term remission)
Four studies indicated that SSRI tended to be more effective than psychological therapies (Bakker 1999; Black 1993; Hendriks 2010; Sharp 1996), whereas the results of one study tended to show the opposite (Dannon 2004). The former studies used individual psychological therapy, whereas the latter study used group psychological therapy. The analysis with the four former studies with individual psychological therapy indicated that SSRI was more effective than psychological therapies (RR 0.79, 95% CI 0.62 to 0.99; 5 studies; 275 participants). The results of the three studies were homogeneous (I2 = 0%, Tau2 = 0.01) (Analysis 7.1).
7.1. Analysis.

Comparison 7 Subgroup analysis (investigation of heterogeneity): All psychological therapies versus SSRI, Outcome 1 Short‐term remission.
5.2. All psychological therapies versus either antidepressant alone or antidepressants and benzodiazepines (short‐term remission)
The results of one study seemed to be inconsistent with those of the other studies (Dannon 2004). The study used group psychological therapy, while the others did not, which might have been the source of the heterogeneity. An analysis excluding this one study indicated that either antidepressant alone or antidepressants and benzodiazepines were more effective than all psychological therapies (RR 0.80, 95% CI 0.69 to 0.94; 10 studies; 606 participants). The results of the nine studies were homogeneous (I2 = 0%, Tau2 = 0.00) (Analysis 9.1).
9.1. Analysis.
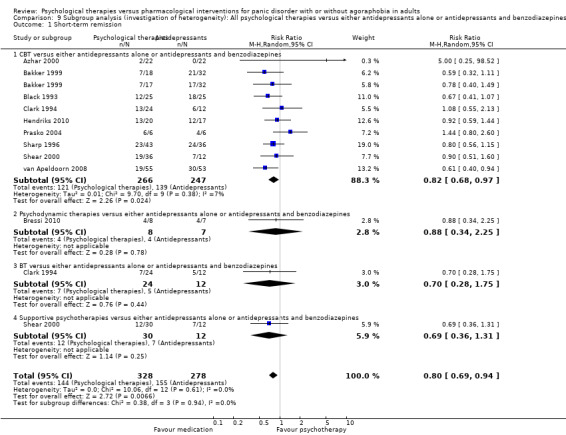
Comparison 9 Subgroup analysis (investigation of heterogeneity): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 1 Short‐term remission.
6. For outcome using same scales (investigation of heterogeneity, post‐hoc analysis)
6.1. All psychological therapies versus SSRI (short‐term response)
The results of two studies, Bakker 1999 and Black 1993, were inconsistent with those of three studies (Azhar 2000; Hendriks 2010; Sharp 1996). This discrepancy could be because the response was defined by the same scale (Clinical Global Impression) in the former two studies, whereas it was defined by different scales in the latter three studies (panic frequency in Azhar 2000, Fear Questionnaire in Sharp 1996, and Agoraphobic Cognitions Questionnaire in Hendriks 2010). An analysis excluding the latter three studies indicated that psychological therapies were more effective than SSRI (RR 0.47, 95% CI 0.29 to 0.76; 2 studies; 117 participants). The results of the two studies were consistent (I2 = 0%, Tau2 = 0.00) (Analysis 7.2).
7.2. Analysis.
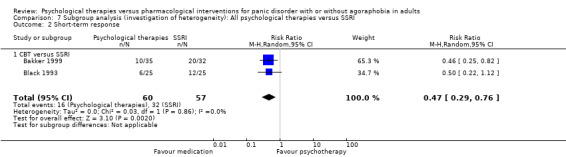
Comparison 7 Subgroup analysis (investigation of heterogeneity): All psychological therapies versus SSRI, Outcome 2 Short‐term response.
7. For studies without extreme results (investigation of heterogeneity, post‐hoc analysis)
7.1. All psychological therapies versus either antidepressant alone or antidepressants and benzodiazepines (short‐term improvement)
One study had an extreme result (Azhar 2000), which might have caused heterogeneity. The results excluding this study indicated that there was no significant difference between all psychological therapies versus either antidepressant alone or antidepressants and benzodiazepines (SMD 0.19, 95% CI ‐0.03 to 0.40; 10 studies; 604 participants). The results of the 10 studies were relatively homogeneous (I2 = 29%, Tau2 = 0.04) (Analysis 9.2).
9.2. Analysis.
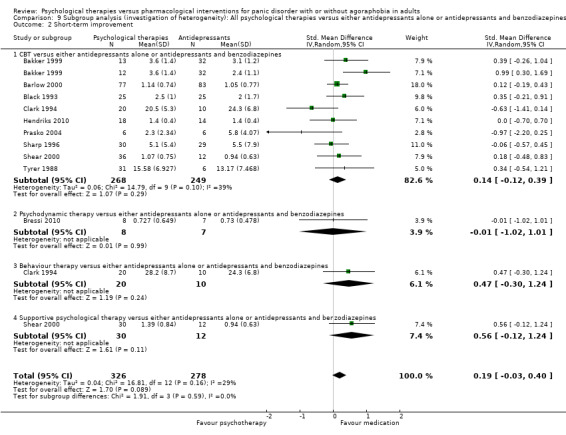
Comparison 9 Subgroup analysis (investigation of heterogeneity): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 2 Short‐term improvement.
Sensitivity analyses
1. Limited to participants without any comorbid physical or mental disorders
As all of the studies included some comorbid physical or mental disorders, it was not possible to execute this preplanned sensitivity analysis.
2. Limited to trials where quality control of the psychological therapies was adequate
Eleven studies reported the provision of a treatment manual or supervision of the psychological therapies, or both. Among these studies, eight studies included CBT only (Bakker 1999; Barlow 2000; Black 1993; Hendriks 2010; Klosko 1988; Tyrer 1988; van Apeldoorn 2008); one included CBT and BT (Clark 1994); one included CBT and supportive psychological therapy (Shear 2000); and one included only psychodynamic therapy (Bressi 2010).
2.1 All psychological therapies versus SSRI
Psychological therapies produced a significantly lower short‐term remission than SSRI (RR 0.75, 95% CI 0.61 to 0.94; 4 studies; 233 participants) (Analysis 10.1). However, there was no significant difference in efficacy in terms of the short‐term response (RR 0.72, 95% CI 0.46 to 1.12; 4 studies; 233 participants) (Analysis 10.2), short‐term improvement (SMD 0.33, 95% CI ‐0.15 to 0.80; 4 studies; 208 participants) (Analysis 10.4), or in treatment acceptability as measured using dropouts for any reason (RR 1.65, 95% CI 0.98 to 2.78; 4 studies; 233 participants) (Analysis 10.3).
10.1. Analysis.
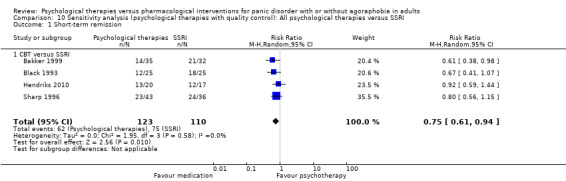
Comparison 10 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus SSRI, Outcome 1 Short‐term remission.
10.2. Analysis.
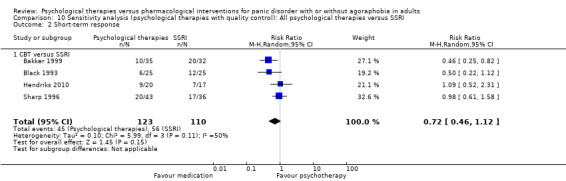
Comparison 10 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus SSRI, Outcome 2 Short‐term response.
10.4. Analysis.
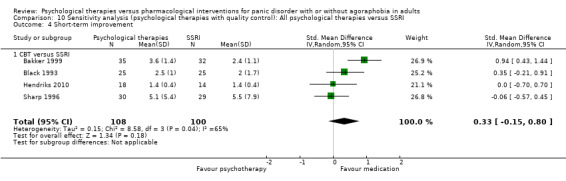
Comparison 10 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus SSRI, Outcome 4 Short‐term improvement.
10.3. Analysis.
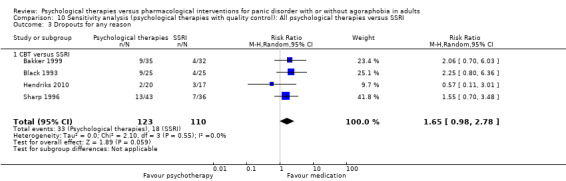
Comparison 10 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus SSRI, Outcome 3 Dropouts for any reason.
2.2. All psychological therapies versus tricyclic antidepressants
All of the studies included in this comparison dealt with CBT with quality control for the psychological therapies, therefore the results were the same as in the main analyses (Analysis 2.1; Analysis 2.2; Analysis 2.3).
2.3. All psychological therapies versus other antidepressants
Other antidepressants were more effective than all psychological therapies in terms of short‐term remission (RR 0.65, 95% CI 0.44 to 0.96; 2 studies; 123 participants) (Analysis 11.1). However, there was no significant difference in efficacy between the two arms as measured using short‐term response (RR 0.85, 95% CI 0.68 to 1.06; 2 studies; 116 participants) (Analysis 11.2) or in treatment acceptability as measured using dropouts for any reason (RR 1.55, 95% CI 0.91 to 2.65; 2 studies; 168 participants) (Analysis 11.3). Only one study with data on short‐term improvement was available, and there was no significant difference between the two groups in terms of short‐term improvement (SMD ‐0.01, 95% CI ‐1.02 to 1.01; 1 study; 15 participants).
11.1. Analysis.
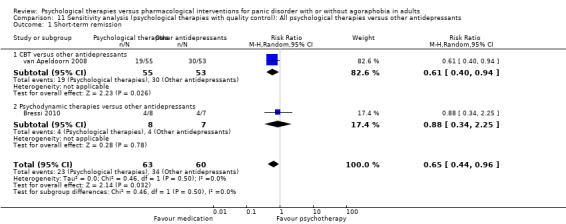
Comparison 11 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus other antidepressants, Outcome 1 Short‐term remission.
11.2. Analysis.
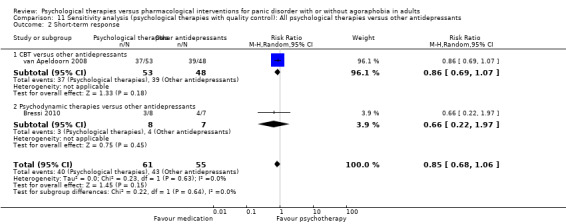
Comparison 11 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus other antidepressants, Outcome 2 Short‐term response.
11.3. Analysis.
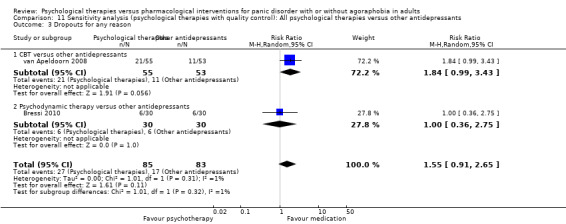
Comparison 11 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus other antidepressants, Outcome 3 Dropouts for any reason.
2.4. All psychological therapies versus benzodiazepines
There was no significant difference in efficacy between all psychological therapies and benzodiazepines as measured using short‐term response (RR 1.41, 95% CI 0.61 to 3.27; 2 studies; 76 participants) (Analysis 12.1) or in treatment acceptability as measured using dropouts for any reason (RR 1.41, 95% CI 0.61 to 3.27; 2 studies; 76 participants) (Analysis 12.2). Only one study was included in the analysis of short‐term remission. The studies included in the analysis of short‐term improvement were the same as those included in Analysis 4.4.1.
12.1. Analysis.
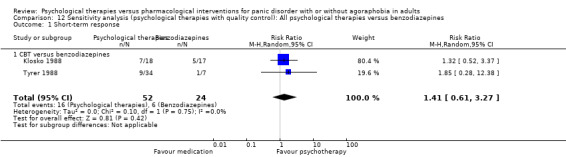
Comparison 12 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus benzodiazepines, Outcome 1 Short‐term response.
12.2. Analysis.
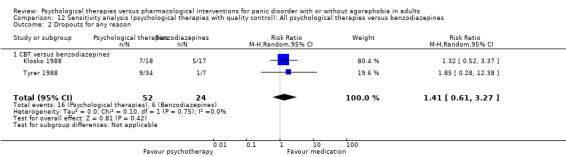
Comparison 12 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus benzodiazepines, Outcome 2 Dropouts for any reason.
2.5. All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines
Antidepressants alone and antidepressants plus benzodiazepines were more effective than all of the psychological therapies in terms of short‐term remission (RR 0.76, 95% CI 0.65 to 0.90; 8 studies; 550 participants) (Analysis 13.1) and short‐term improvement (SMD 0.21, 95% CI 0.02 to 0.41; 9 studies; 592 participants) (Analysis 13.4). However, there were no significant differences in efficacy between all psychological therapies and either antidepressants alone or antidepressants and benzodiazepines as measured using short‐term response (RR 0.89, 95% CI 0.77 to 1.03; 10 studies; 724 participants) (Analysis 13.2) or in treatment acceptability as measured using dropouts for any reason (RR 1.13, 95% CI 0.79 to 1.62; 10 studies; 796 participants) (Analysis 13.3).
13.1. Analysis.
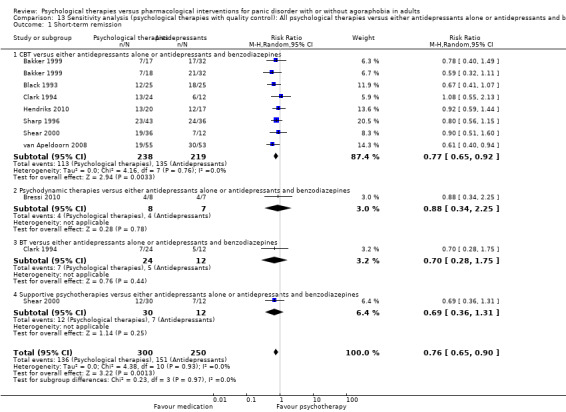
Comparison 13 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 1 Short‐term remission.
13.4. Analysis.
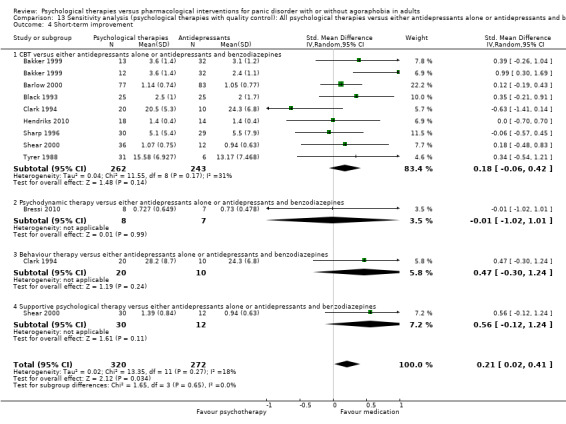
Comparison 13 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 4 Short‐term improvement.
13.2. Analysis.
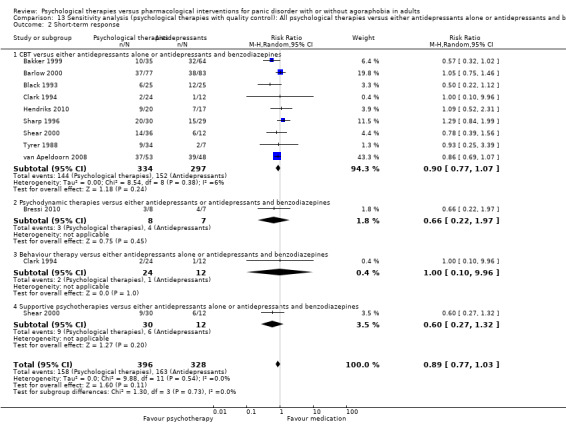
Comparison 13 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 2 Short‐term response.
13.3. Analysis.
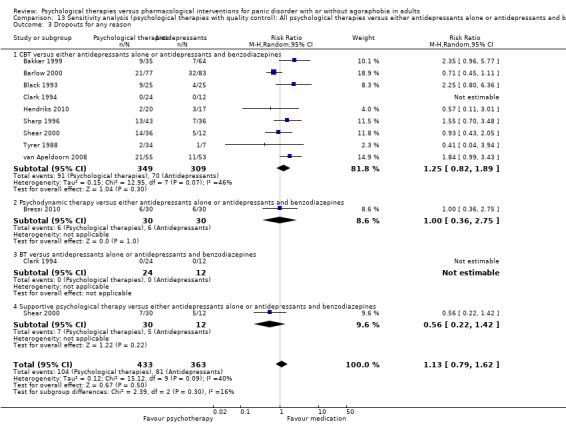
Comparison 13 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 3 Dropouts for any reason.
3. Limited to trials that met both of the following criteria as described in the 'Assessment of risk of bias in included studies' section: allocation concealment rated as low risk of bias, and detection bias rated as low risk of bias
No study met this criteria.
4. Calculating ORs and their 95% CIs instead of RRs and their 95% CIs for the primary outcomes (dichotomous data), in order to investigate whether our choice of RRs was critical to the conclusions of the review
4.1. All psychological therapies versus SSRI
There were no significant differences in efficacy between psychological therapies and SSRI as measured using short‐term remission (OR 0.64, 95% CI 0.32 to 1.28; 6 studies; 334 participants), short‐term response (OR 1.01, 95% CI 0.30 to 3.37; 5 studies; 277 participants), or in treatment acceptability as measured using dropouts for any reason (OR 1.38, 95% CI 0.71 to 2.69; 6 studies; 334 participants).
4.2: All psychological therapies versus tricyclic antidepressants
There were no significant differences in efficacy between psychological therapies and tricyclic antidepressants as measured using short‐term remission (OR 0.67, 95% CI 0.38 to 1.17; 3 studies; 229 participants), short‐term response (OR 0.66, 95% CI 0.36 to 1.21; 4 studies; 270 participants), or in treatment acceptability as measured using dropouts for any reason (OR 0.80, 95% CI 0.40 to 1.57; 5 studies; 430 participants).
4.3. All psychological therapies versus other antidepressants
There were no significant differences in efficacy between psychological therapies and other antidepressants as measured using short‐term remission (OR 0.67, 95% CI 0.20 to 2.29; 3 studies; 135 participants), short‐term response (OR 0.66, 95% CI 0.23 to 1.88; 3 studies; 128 participants), or in treatment acceptability as measured using dropouts for any reason (OR 1.75, 95% CI 0.79 to 3.90; 3 studies; 180 participants).
4.4. All psychological therapies versus benzodiazepines
There were no significant differences in efficacy between psychological therapies and benzodiazepines as measured using short‐term remission (OR 1.17, 95% CI 0.47 to 2.91; 3 studies; 95 participants), short‐term response (OR 2.07, 95% CI 0.61 to 7.07; 2 studies; 69 participants), or in treatment acceptability as measured using dropouts for any reason (OR 1.13, 95% CI 0.42 to 3.07; 3 studies; 116 participants).
4.5: All psychological therapies versus either antidepressant alone or antidepressants and benzodiazepines
There were no significant differences in efficacy between psychological therapies and either antidepressant alone or antidepressants and benzodiazepines as measured using short‐term response (OR 0.87, 95% CI 0.55 to 1.40; 12 studies; 800 participants) or in treatment acceptability as measured using dropouts for any reason (OR 1.10, 95% CI 0.70 to 1.73; 13 studies; 909 participants). However, either antidepressant alone or antidepressants plus benzodiazepines was more effective than psychological therapies in terms of short‐term remission (OR 0.60, 95% CI 0.42 to 0.85; 11 studies; 663 participants).
5. Limited to participants with agoraphobia
We examined this analysis in the subgroup analysis.
6. Excluding studies in which some, but possibly not all, of the participants had panic disorder
No such studies were included in the analysis. It was therefore not meaningful to execute this preplanned sensitivity analysis.
7. Calculating primary outcomes using worst‐ and best‐case scenarios (post‐hoc analysis)
Analyses 14‐23 showed the sensitivity analyses by calculating the primary outcomes using the best‐case (favouring psychological therapies) and worst‐case (favouring pharmacological intervention) scenarios. Psychological therapies were more effective than SSRI in terms of short‐term remission in the best‐case scenario (RR 1.18, 95% CI 1.05 to 1.32; 6 studies; 334 participants), and tricyclic antidepressants were more effective than psychological therapies in terms of short‐term remission in the worst‐case scenario (RR 0.59, 95% CI 0.45 to 0.79; 3 studies; 229 participants). However, there were no considerable differences between these extreme scenarios and the original results in other comparisons. For example, there was no significant difference between all psychological therapies and SSRI in terms of short‐term response either in the best‐case scenario (RR 1.01, 95% CI 0.53 to 1.95; 5 studies; 277 participants) or in the worst‐case scenario (RR 0.91, 95% CI 0.48 to 1.69; 5 studies; 277 participants). In other words, the dropouts did not appear to influence the overall study results substantively.
8. Limited to studies without imputed data (post‐hoc analysis)
As none of the data on dropouts for any reason were missing, we carried out this sensitivity analysis for short‐term remission and response.
8.1. All psychological therapies versus SSRI
There were no significant differences in efficacy between psychological therapies and SSRI as measured using short‐term remission (RR 0.84, 95% CI 0.60 to 1.16; 5 studies; 290 participants) (Analysis 24.1). As there was only one study without imputed data for the short‐term response, it was not meaningful to execute the sensitivity analysis for this outcome.
24.1. Analysis.
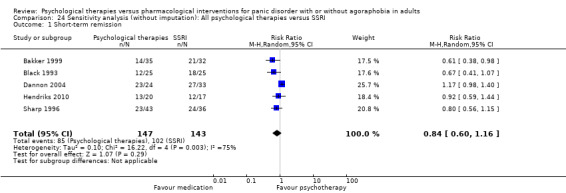
Comparison 24 Sensitivity analysis (without imputation): All psychological therapies versus SSRI, Outcome 1 Short‐term remission.
8.2: All psychological therapies versus tricyclic antidepressants
All of the studies used the imputed data of this comparison, therefore it was not meaningful to execute the sensitivity analysis for this comparison.
8.3. All psychological therapies versus other antidepressants
There were no significant differences in efficacy between psychological therapies and other antidepressants as measured using short‐term remission (RR 0.65, 95% CI 0.44 to 0.96; 2 studies; 123 participants) (Analysis 25.1). As there was only one study without imputed data for the short‐term response, it was not possible to execute the sensitivity analysis for this outcome.
25.1. Analysis.
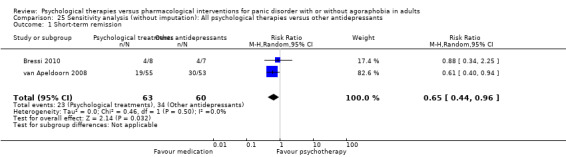
Comparison 25 Sensitivity analysis (without imputation): All psychological therapies versus other antidepressants, Outcome 1 Short‐term remission.
8.4. All psychological therapies versus benzodiazepines
There were no significant differences in efficacy between psychological therapies and benzodiazepines as measured using short‐term remission (RR 1.08, 95% CI 0.70 to 1.65; 2 studies; 75 participants) (Analysis 26.1). As no study was without imputed data for the short‐term response, it was not meaningful to execute the sensitivity analysis for this outcome.
26.1. Analysis.
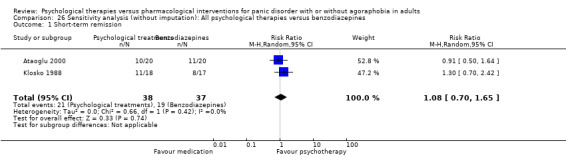
Comparison 26 Sensitivity analysis (without imputation): All psychological therapies versus benzodiazepines, Outcome 1 Short‐term remission.
8.5: All psychological therapies versus either antidepressant alone or antidepressants and benzodiazepines
There were no significant differences in efficacy between psychological therapies and either antidepressants alone or antidepressants and benzodiazepines as measured using short‐term remission (RR 0.83, 95% CI 0.67 to 1.01; 9 studies; 652 participants) (Analysis 27.1) and short‐term response (RR 0.97, 95% CI 0.80 to 1.17; 3 studies; 305 participants) (Analysis 27.2).
27.1. Analysis.
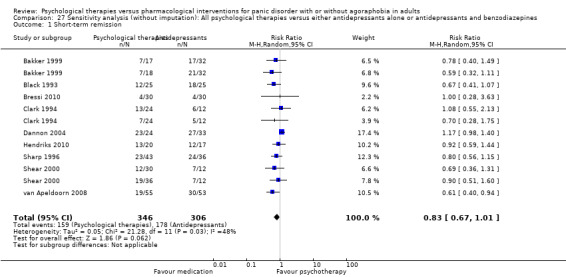
Comparison 27 Sensitivity analysis (without imputation): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 1 Short‐term remission.
27.2. Analysis.
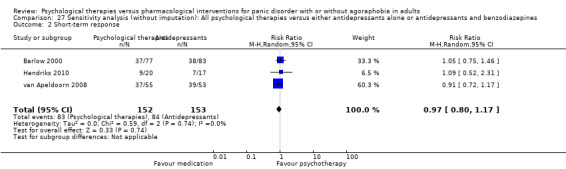
Comparison 27 Sensitivity analysis (without imputation): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 2 Short‐term response.
9. Calculating primary outcomes with fixed‐effect model
9.1. All psychological therapies versus SSRI
There were no significant differences in efficacy between psychological therapies and SSRI as measured using short‐term remission (RR 0.85, 95% CI 0.72 to 1.01; 6 studies; 334 participants), short‐term response (RR 0.96, 95% CI 0.72 to 1.26; 5 studies; 277 participants), or in treatment acceptability as measured using dropouts for any reason (RR 1.29, 95% CI 0.83 to 2.00; 6 studies; 334 participants).
9.2: All psychological therapies versus tricyclic antidepressants
There were no significant differences in efficacy between psychological therapies and tricyclic antidepressants as measured using short‐term remission (RR 0.81, 95% CI 0.61 to 1.08; 3 studies; 229 participants), short‐term response (RR 0.75, 95% CI 0.51 to 1.10; 4 studies; 270 participants), or in treatment acceptability as measured using dropouts for any reason (RR 0.84, 95% CI 0.60 to 1.17; 5 studies; 430 participants).
9.3. All psychological therapies versus other antidepressants
There were no significant differences in efficacy between psychological therapies and other antidepressants as measured using short‐term remission (RR 0.73, 95% CI 0.52 to 1.03; 3 studies; 135 participants), short‐term response (RR 0.89, 95% CI 0.73 to 1.10; 3 studies; 128 participants), or in treatment acceptability as measured using dropouts for any reason (RR 1.55, 95% CI 0.91 to 2.62; 3 studies; 180 participants).
9.4. All psychological therapies versus benzodiazepines
There were no significant differences in efficacy between psychological therapies and benzodiazepines as measured using short‐term remission (RR 1.08, 95% CI 0.70 to 1.65; 3 studies; 95 participants), short‐term response (RR 1.63, 95% CI 0.70 to 3.77; 2 studies; 69 participants), or in treatment acceptability as measured using dropouts for any reason (RR 1.10, 95% CI 0.53 to 2.29; 3 studies; 116 participants).
9.5: All psychological therapies versus either antidepressant alone or antidepressants and benzodiazepines
Either antidepressant alone or antidepressants and benzodiazepines was more effective than psychological therapies in terms of short‐term remission (RR 0.83, 95% CI 0.72 to 0.95; 11 studies; 663 participants). However, there were no significant differences in efficacy between psychological therapies and either antidepressant alone or antidepressants and benzodiazepines as measured using short‐term response (RR 0.94, 95% CI 0.81 to 1.10; 12 studies; 800 participants) or in treatment acceptability as measured using dropouts for any reason (RR 1.07, 95% CI 0.84 to 1.36; 13 studies; 909 participants).
Reporting bias
We could not perform a funnel plot analysis for most of the comparisons. Only the comparisons of all psychological therapies versus either antidepressants alone or antidepressants plus benzodiazepines included more than 10 studies. We observed a statistically significant funnel plot asymmetry for dropouts for any reason in a comparison of all psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines. A contour‐enhanced funnel plot did not suggest a publication bias.
Discussion
Summary of main results
We included a total of 16 RCTs in this review; however, the number of studies included in each comparison ranged from 2 (27 participants) to 13 (909 participants). The results of the main comparisons did not indicate any superiority or inferiority in short‐term efficacy and treatment acceptability between psychological therapies as a whole and SSRI, tricyclic antidepressants, other antidepressants, benzodiazepines, or either antidepressants alone or antidepressants and benzodiazepines. None of the studies reported long‐term outcomes.
The studies available for subgroup analyses comparing each psychological therapy and pharmacological intervention were limited. Only studies that included CBT were available for a meta‐analysis, and the results did not indicate any superiority or inferiority in efficacy or treatment acceptability. There were also no significant differences in the other comparisons. Other psychological therapies examined in the RCTs included behaviour therapy, psychodynamic therapy, supportive psychological therapy, and physiological therapy. However, only one study was available for each comparison.
The sensitivity analysis and investigation of the sources of heterogeneity indicated three possible influential factors: quality control of psychological therapies, the length of intervention, and the individual modality of psychological therapies.
Three of the five comparisons in the sensitivity analyses limited to studies with quality control for psychological therapies showed a superior efficacy of pharmacological intervention over psychological therapies in terms of short‐term remission. These comparisons were psychological therapies versus SSRI, other antidepressants, and either antidepressants alone or antidepressants plus benzodiazepines.
We found a significant heterogeneity of the results for efficacy for comparisons that included antidepressants. The heterogeneity in the comparison between all psychological therapies and other antidepressants seemed to be caused by the length of intervention: trials with a longer duration suggested that the pharmacological interventions tended to be superior. The heterogeneity in the comparison between all psychological therapies and SSRI and either antidepressants alone or antidepressants plus benzodiazepines seemed to be caused by the individual modality of psychological therapies: SSRI and either antidepressants alone or antidepressants plus benzodiazepines were more effective than psychological therapies in terms of short‐term remission.
Overall completeness and applicability of evidence
We were able to perform a meta‐analysis for all of the primary outcomes in all of the comparisons we declared except for the comparison between psychological therapies and SNRIs. However, the number of included studies was small for all of the comparisons but one, which was all psychological therapies versus either antidepressants or antidepressants with benzodiazepines. Furthermore, the psychotherapies intervention included in the analyses was mostly CBT, and there were few studies examining other psychotherapies. We could not find any trial dealing with psychoeducation, cognitive therapy, or third‐wave therapies. The possible reasons for the scarcity of trials other than those examining CBT and BT are that other psychological therapies are regarded as non‐promising or time and personnel consuming. In fact, one short‐term psychodynamic therapy that we included required 40 weekly sessions of 45 minutes in duration, and the therapist had 10 to 15 years of experience with psychodynamic therapy (Bressi 2010). As for third‐wave therapies, such treatments are relatively new and may not be available for the treatment of panic disorder. Although we performed a thorough search, it is possible that unpublished studies exist. If there are unpublished trials, we assume that the reasons for the lack of publication may vary: some trials may have had negative results, and other trials may have been influenced by the allegiances of researchers to their sponsors or their therapeutic preferences.
Among the secondary outcomes, only information on the short‐term improvement of panic disorder was available. We could not retrieve other secondary outcomes, including long‐term remission/response, short‐term improvements in quality of life, and adverse events. Adverse events arising from psychological therapies are often not reported because they are difficult to recognise and study (Linden 2013). The nature of adverse events arising from psychological therapies also differs from those caused by pharmacological intervention. Nevertheless, adverse events are an important outcome for decision making, so the use of a structured checklist for the adverse events of psychological therapies and comparison with adverse events according to severity may be useful when reporting adverse events.
Quality of the evidence
The quality of the evidence for each outcome ranged from low to moderate. In all of the studies participants were not blinded to treatment because of the psychological therapy arm.
Many of the obtained results demonstrated substantial heterogeneity, some of which seemed to be related to the length of psychological therapy and one particular study with extreme results.
As the number of participants included in the meta‐analysis was 909 at most, the evidence tended to be imprecise.
The number of studies included in each comparison for the detection of publication bias was small. The comparison between all psychological therapies versus antidepressants alone or antidepressants and benzodiazepines had a sufficient number of studies for a funnel plot analysis, and we suspected publication bias for dropouts for any reason and short‐term improvement.
Most of the included studies had an unclear risk of bias for several domains. Only three studies reported adequate information concerning random sequence generation and allocation concealment. Blinding of the participants and personnel was difficult as all the comparisons included psychological therapy. However, the use of psychological and pharmacological placebo could mitigate the performance bias. The blinding of outcome assessments was not appropriately reported or broken in 14 studies. Selective outcome reporting was unclear, as the study protocol was only available for one study. The attrition in many studies was negligible. We evaluated researcher and therapist allegiance and sponsorship bias as other potential sources of bias. Researcher allegiance was balanced between the arms in many studies; however, many studies lacked information regarding therapist allegiance. Many studies had a sponsorship bias, or conflicts of interest were not appropriately reported.
Potential biases in the review process
Study‐level limitations
Quality of interventions
We performed a sensitivity analysis limited to studies with quality control (adequate supervision and the use of a treatment manual) for the psychological therapies, which changed the results, favouring pharmacological interventions in some comparisons. This may be because studies with good quality control against psychological therapies are less biased to psychological therapies. We evaluated adequate supervision and the use of a manual in the sensitivity analysis, whereas we did not consider the components of psychological therapies, and psychological therapies under the same category included different components. For example, in one study CBT included breathing retraining, but in another study it did not. We did not consider the quality control of pharmacological treatments. All pharmacological treatments adopted a flexible dose. However, some studies did not report the mean dose administered during the trials. These factors may affect the results.
Length of intervention
The length of the interventions ranged from 6 to 40 weeks. Investigation of the sources of heterogeneity suggested that it might be due to the length of the intervention. Longer trials tended to favour pharmacological intervention in one comparison. Previous reports have suggested that the duration of psychological therapy is not related to its effect (Baldwin 2009; Stiles 2015). On the other hand, pharmacological intervention may be more effective with longer duration (Quitkin 1984; Tedeschini 2011). Considering these facts, pharmacological therapy may be beneficial in long term duration.
Modalities of the psychological therapies
One study used group‐based psychological therapy, and the efficacy was inconsistent with studies using individual psychological therapies; the former study favoured psychological therapy, and the latter studies favoured pharmacological intervention. According to this result, the therapeutic modality could be a potential source of bias.
Number of intervention arms
The number of intervention arms varied among the included studies, ranging from two to five. Greenberg reported that the effect sizes of three‐arm studies including an antidepressant to be tested, a standard antidepressant, and a placebo showed a smaller difference between the standard antidepressant and the placebo than those studies comparing two arms only (Greenberg 1992). Considering this fact, the various numbers of intervention arms may have influenced the present results.
Omission of DSM‐5 in the study inclusion criteria
The DSM‐5 was not among the diagnostic criteria that we had listed as being eligible for inclusion in our original study protocol. However, we did not find any literature that used the DSM‐5 and met our other inclusion criteria. In the DSM‐5, as in the ICD‐10, panic disorder and agoraphobia are separate diagnosable disorders. We found only one study where almost all of the participants had agoraphobia. We planned to exclude studies that focused on agoraphobia without panic disorder, but no such studies were found. The omission of the DSM‐5 criteria in the present review therefore did not affect our results. However, we should consider this factor in future reviews.
Outcome‐level limitations
Assessment
By global judgement of the original investigators our primary outcomes were remission and response, which included several different scales, including the number of panic attacks, CGI, and PDSS. The investigation of heterogeneity in the comparison between psychological therapies and SSRI suggested that using different scales might have been a source of heterogeneity.
Risk of bias
Information related to risk of bias was not reported in many studies. In particular, many studies had an unclear risk of bias for random sequence generation, allocation concealment, and selective outcome reporting. Blinding of participants was impossible for the psychological therapies but was possible for the pharmacological interventions, which could have led to psychological therapies being favoured in studies with a pharmacological placebo arm.
There is controversy as to whether industry sponsorship is an extra factor to be rated within 'other potential sources of bias'. A Cochrane methodology review provides empirical evidence indicating that industry‐sponsored drug and device studies are more often favourable to the sponsor's products compared with non–industry‐sponsored drug and device studies because of biases that cannot be explained by standard 'Risk of bias' assessment tools (Lundh 2012). We therefore chose to be transparent in presenting sponsorship bias that may distort the meta‐analyses.
As many risks of bias were unclear, and blinding of participants was at high risk of bias, we downgraded the quality of the evidence by one point for this reason.
Review‐level limitations
The inter‐rater reliability was relatively fair. This was because studies that did not report information related to risk of bias were usually judged as having an unclear risk of bias, but some studies were judged as having low or high risk of bias depending on the context. This caused the disagreement between two raters.
Agreements and disagreements with other studies or reviews
Three reviews included a comparison between psychological therapies and pharmacological interventions (Clum 1993; Gould 1995; Mitte 2005). Two old reviews showed psychological therapies to be more effective than pharmacological intervention (Clum 1993; Gould 1995). Clum 1993 indicated that exposure with a drug placebo was more effective than antidepressants (effect size = 0.4), but this result was found in only one study, and the control consisted of combination therapy using exposure and a drug placebo. Gould 1995 included two studies comparing CBT with pharmacological intervention and indicated that CBT was more effective and had fewer dropouts than pharmacological intervention; we included these studies in our meta‐analysis. Mitte 2005 compared pharmacological intervention with psychological therapies in terms of anxiety, depression, and clinical significance, and the results were compatible with our results: effect size of 0.27 (95% CI ‐0.07 to 0.62) for anxiety, 0.21 (95% CI ‐0.34 to 0.75) for depression, and 0.09 (95% CI ‐0.30 to 0.56) for clinical significance.
Furukawa 2007 compared combination therapy with psychotherapy or pharmacotherapy. Their findings suggested that the effect of psychological treatment lasts longer than that of pharmacological treatment, since psychological therapy plus pharmacological intervention did not increase the long‐term remission/response after the discontinuation of treatment compared with psychological therapy plus a placebo (RR 0.96, 95% CI 0.79 to 1.16), whereas combination therapy was superior to pharmacological intervention alone in terms of remission/response after the discontinuation of treatment (RR 1.61, 95% CI 1.23 to 2.11). These results seem to be incongruous with the present review, which suggested that pharmacological intervention tends to be superior to psychological intervention. However, the number of studies included in Furukawa's review was small; the former comparison included one study, and the latter included eight studies. Additionally, the combination therapy was superior to pharmacological intervention alone (RR 1.63, 95% CI 1.21 to 2.19) or psychological therapy plus placebo (RR 1.29, 95% CI 1.03 to 1.62) in terms of the long‐term remission/response on continued treatment. Considering these findings, the results are inconclusive. More studies are needed to determine the superiority in a head‐to‐head comparison between psychological therapies and pharmacological intervention in longer‐term outcomes.
Authors' conclusions
Implications for practice.
We found no evidence for the superiority of one form of therapy over another with regard to short‐term efficacy and treatment acceptability. However, psychological therapies with longer‐term duration, quality control, or an individual modality may be less effective than pharmacological intervention in terms of short‐term efficacy. No information was available about long‐term efficacy or quality of life.
The evidence in this review ranged between very low and moderate, and was often imprecise. In the absence of high‐quality evidence, decisions to use pharmacotherapy or psychotherapy may depend on the costs, time, and labour required, as well as the availability of qualified personnel in each clinical setting in conjunction with the individual patient's preferences.
Implications for research.
Future studies should examine the influence of accompanying agoraphobia. We found only one study where almost all of the participants had agoraphobia. It would be clinically meaningful to examine which approach is more or less effective and tolerable for patients with agoraphobia or with panic disorder.
Many studies have investigated CBT. However, other psychological therapies, such as psychodynamic psychological therapy, BT, and physiological therapy, need further investigation. The quality of psychological therapies should be well controlled and should be reported in publications. The influence of the length of intervention should be analysed, and the long‐term effect after intervention should be tested, especially in psychological therapies.
Adequately controlled (i.e. pill placebo and non‐specific psychological control) and powered studies are important. Outcomes that can be appropriately blinded are desirable. The method of randomisation and allocation concealment should be clearly reported in all publications. Trial registration or a detailed study protocol should be made available before commencing the trial.
There is a need for larger studies that are adequately powered to detect differences between two active and effective treatments.
Long‐term response or remission and outcome related to quality of life should be reported. Other items that should be reported include the outcome as it is related to adverse events such as suicide or suicide attempts, the number of dropouts because of side effects, and the number of participants experiencing at least one adverse effect.
Acknowledgements
Cochrane Review Group Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. CCMD’s core MEDLINE search strategy used to inform the Group’s specialised register
OVID Medline ‐ weekly search alert (condition + RCT filter only) 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.3. [RCT filter]: (controlled clinical trial.pt. or randomized controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomized controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)4. (1 and 2 and 3)
Records are de‐duplicated and screened for reports of RCTs which fall within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs are tagged to the appropriate study record.
Similar weekly search alerts are also conducted on OVID EMBASE and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
Appendix 2. CCMDCTR‐References search (psychotherapies and panic)
#1. (*therapy or *therapies or therapist*):ti,ab or psychotherapy:kw,ky,emt,mh
#2. (acceptance* or commitment* or adlerian or art or aversion or brief or “client cent*” or color or colour or “compassion* focus*” or compassionate or conjoint or conversion or conversational or couples or dance or desensitization or desensitisation or dialectic* or diffusion or distraction or eclectic or (emotion NEAR focus*) or existential or experiential or exposure or expressive or family or “focus oriented” or freudian or gestalt or "group" or humanistic or implosive or "insight oriented" or integrative or interpersonal or jungian or kleinian or logo or marital or metacognitive or “meta cognitive” or milieu or morita or multimodal or “multi modal” or music or narrative or nondirective or “non directive” or nonspecific or “non specific” or “object relations” or “personal construct” or “person cent*” or persuasion or play or primal or “problem focus*” or “problem sol*” or “process experiential” or “rational emotive” or reality or “reciprocal inhibition” or relationship* or reminiscence or restructuring or rogerian or schema* or "short term" or sex or “social effectiveness” or “social skill*” or “socio environment*” or “solution focus*” or “time limited” or transference or transdiagnostic or transtheoretical)
#3. (abreaction or “acting out” or “activity scheduling” or “age regression” or ((assertive* or attention or autogenic or mind or sensitivity) and train*) or autosuggestion or “balint group” or ((behavior* or behaviour*) NEAR (activation or *therap* or treatment or contracting or modification)) or bibliotherap* or biofeedback or "breathing retrain*" or catharsis or “brief *therap*” or *cognitive* or *CBT* or counsel* or “contingency management” or countertransference or “covert sensitization” or “crisis intervention” or “dream analysis” or education* or “emotional freedom” or “eye movement desensiti*” or EMDR or flooding or “free association” or “functional analys*” or griefwork or “group *therap*” or "guided mastery" or hypno* or imagery or meditation* or “mental healing” or mindfulness* or "panic management" or “panic program*” or "paradoxical intention" or ((pleasant or pleasing) NEAR event*) or psychoanaly* or psychodrama or psychodynamic or psychoeducat* or “psycho educat*” or “psycho* support*” or "psychological therap*" or psychotherap* or relaxation or “role play*” or “self analysis” or "self control" or “self esteem” or "self help" or "self master*" or "self monitor*" or “sensitivity training” or "stress inoculation" or “stress management” or “support* group*” or “supportive *therap*“ or “therapeutic technique*” or therapist or “third wave” or “transactional analysis” or “validation therap” or "virtual reality")
#4. ((1 and 2) or 3)
#5. (panic or agoraphobi*)
#6. (4 and 5)
Appendix 3. PubMed search strategy
(((((((((((trial[Title])) OR (randomly[Title/Abstract])) OR (clinical trials as topic[MeSH Terms])) OR (randomized[Title/Abstract])) OR (controlled clinical trial[Publication Type])) OR (randomized controlled trial[Publication Type]))) NOT ((animals[MeSH Terms]) NOT humans[MeSH Terms]))) AND ((((((("Behavior Therapy"[Mesh]) OR "Bibliotherapy"[Mesh]) OR "Feedback, Psychological"[Mesh]) OR "Nondirective Therapy"[Mesh]) OR "Psychoanalytic Therapy"[Mesh]) OR "Psychotherapy, Brief"[Mesh]) OR "Psychotherapy, Rational‐Emotive"[Mesh])) AND (("Agoraphobia"[Mesh]) OR "Panic Disorder"[Mesh])
Data and analyses
Comparison 1. All psychological therapies versus SSRI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 6 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.17] |
| 1.1 CBT versus SSRI | 6 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.62, 1.17] |
| 2 Short‐term response | 5 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.51, 1.86] |
| 2.1 CBT versus SSRI | 5 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.51, 1.86] |
| 3 Dropouts for any reason | 6 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.80, 2.22] |
| 3.1 CBT versus SSRI | 6 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.80, 2.22] |
| 4 Short‐term improvement | 5 | 242 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.09, 0.69] |
| 4.1 CBT versus SSRI | 5 | 242 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.09, 0.69] |
Comparison 2. All psychological therapies versus tricyclic antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.62, 1.09] |
| 1.1 CBT versus Tricyclic antidepressants | 3 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.22] |
| 1.2 BT versus Tricyclic antidepressants | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.28, 1.75] |
| 1.3 Supportive psychological therapy versus Tricyclic antidepressants | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.31] |
| 2 Short‐term response | 4 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.51, 1.10] |
| 2.1 CBT versus Tricyclic antidepressants | 4 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.25] |
| 2.2 BT versus Tricyclic antidepressants | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.10, 9.96] |
| 2.3 Supportive psychological therapy versus Tricyclic antidepressants | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.27, 1.32] |
| 3 Dropouts for any reason | 5 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.53, 1.30] |
| 3.1 CBT versus Tricyclic antidepressants | 5 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.53, 1.64] |
| 3.2 BT versus Tricyclic antidepressants | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Supportive psychological therapy versus Tricyclic antidepressants | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.22, 1.42] |
| 4 Short‐term improvement | 5 | 414 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.03, 0.43] |
| 4.1 CBT versus Tricyclic antidepressants | 5 | 342 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.14, 0.40] |
| 4.2 BT versus Tricyclic antidepressants | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.30, 1.24] |
| 4.3 Supportive psychological therapy versus Tricyclic antidepressants | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [‐0.12, 1.24] |
Comparison 3. All psychological therapies versus other antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.48, 1.67] |
| 1.1 CBT versus other antidepressants | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.38, 2.24] |
| 1.2 Psychodynamic therapies versus other antidepressants | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.34, 2.25] |
| 2 Short‐term response | 3 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.67, 1.37] |
| 2.1 CBT versus other antidepressants | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.63, 1.68] |
| 2.2 Psychodynamic therapies versus other antidepressants | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.22, 1.97] |
| 3 Dropouts for any reason | 3 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.91, 2.65] |
| 3.1 CBT versus other antidepressants | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.99, 3.43] |
| 3.2 Psychodynamic therapy versus other antidepressants | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.36, 2.75] |
| 4 Short‐term improvement | 2 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.36, 0.51] |
| 4.1 CBT versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 12 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐2.20, 0.25] |
| 4.2 Psychodynamic therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐1.02, 1.01] |
Comparison 4. All psychological therapies versus benzodiazepines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 3 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.70, 1.65] |
| 1.1 CBT versus benzodiazepines | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.70, 1.65] |
| 1.2 Physiological therapies versus benzodiazepines | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Short‐term response | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.70, 3.58] |
| 2.1 CBT versus benzodiazepines | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.70, 3.58] |
| 3 Dropouts for any reason | 3 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.54, 2.36] |
| 3.1 CBT versus benzodiazepines | 3 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.54, 2.36] |
| 4 Short‐term improvement | 3 | 89 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.69, 0.23] |
| 4.1 CBT versus benzodiazepines | 2 | 69 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.82, 0.50] |
| 4.2 Physiological therapies versus benzodiazepines | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.26, 0.51] |
Comparison 5. All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 11 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.71, 1.05] |
| 1.1 CBT versus either antidepressants alone or antidepressants and benzodiazepines | 10 | 570 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.71, 1.10] |
| 1.2 Psychodynamic therapies versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.34, 2.25] |
| 1.3 BT versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.28, 1.75] |
| 1.4 Supportive psychotherapies versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.31] |
| 2 Short‐term response | 12 | 800 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.18] |
| 2.1 CBT versus either antidepressants alone or antidepressants and benzodiazepines | 11 | 707 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.78, 1.26] |
| 2.2 Psychodynamic therapies versus either antidepressants or antidepressants and benzodiazepines | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.22, 1.97] |
| 2.3 Behaviour therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.10, 9.96] |
| 2.4 Supportive psychotherapies versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.27, 1.32] |
| 3 Dropouts for any reason | 13 | 909 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.77, 1.51] |
| 3.1 CBT versus either antidepressants alone or antidepressants and benzodiazepines | 12 | 771 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.79, 1.70] |
| 3.2 Psychodynamic therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.36, 2.75] |
| 3.3 BT versus antidepressants alone or antidepressants and benzodiazepines | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Supportive psychological therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.22, 1.42] |
| 4 Short‐term improvement | 11 | 638 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.34, 0.35] |
| 4.1 CBT versus either antidepressants alone or antidepressants and benzodiazepines | 10 | 551 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.50, 0.32] |
| 4.2 Psychodynamic therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐1.02, 1.01] |
| 4.3 Behaviour therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.30, 1.24] |
| 4.4 Supportive psychological therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [‐0.12, 1.24] |
Comparison 6. Subgroup analysis: All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines (participants without depressive disorder).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.00, 1.42] |
| 1.1 CBT versus either antidepressants alone or antidepressants and benzodiazepines | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.00, 1.42] |
| 2 Dropouts for any reason | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.03, 1.78] |
| 2.1 CBT versus either antidepressants alone or antidepressants and benzodiazepines | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.03, 1.78] |
Comparison 7. Subgroup analysis (investigation of heterogeneity): All psychological therapies versus SSRI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 5 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 0.99] |
| 1.1 CBT versus SSRI | 5 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 0.99] |
| 2 Short‐term response | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.29, 0.76] |
| 2.1 CBT versus SSRI | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.29, 0.76] |
| 3 Short‐term improvement | 2 | 91 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.45, 0.37] |
| 3.1 CBT versus SSRI | 2 | 91 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.45, 0.37] |
7.3. Analysis.
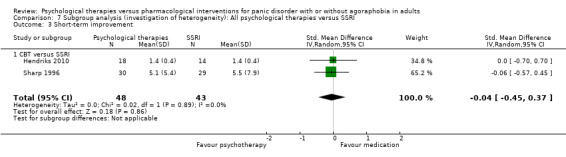
Comparison 7 Subgroup analysis (investigation of heterogeneity): All psychological therapies versus SSRI, Outcome 3 Short‐term improvement.
Comparison 8. Subgroup analysis (investigation of heterogeneity): All psychological therapies versus other antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 2 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.96] |
| 1.1 CBT versus other antidepressants | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.40, 0.94] |
| 1.2 Psychodynamic therapies versus other antidepressants | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.34, 2.25] |
Comparison 9. Subgroup analysis (investigation of heterogeneity): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 10 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.69, 0.94] |
| 1.1 CBT versus either antidepressants alone or antidepressants and benzodiazepines | 9 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.68, 0.97] |
| 1.2 Psychodynamic therapies versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.34, 2.25] |
| 1.3 BT versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.28, 1.75] |
| 1.4 Supportive psychotherapies versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.31] |
| 2 Short‐term improvement | 10 | 604 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.03, 0.40] |
| 2.1 CBT versus either antidepressants alone or antidepressants and benzodiazepines | 9 | 517 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.12, 0.39] |
| 2.2 Psychodynamic therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐1.02, 1.01] |
| 2.3 Behaviour therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.30, 1.24] |
| 2.4 Supportive psychological therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [‐0.12, 1.24] |
Comparison 10. Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus SSRI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 4 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.61, 0.94] |
| 1.1 CBT versus SSRI | 4 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.61, 0.94] |
| 2 Short‐term response | 4 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.46, 1.12] |
| 2.1 CBT versus SSRI | 4 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.46, 1.12] |
| 3 Dropouts for any reason | 4 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.98, 2.78] |
| 3.1 CBT versus SSRI | 4 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.98, 2.78] |
| 4 Short‐term improvement | 4 | 208 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.15, 0.80] |
| 4.1 CBT versus SSRI | 4 | 208 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.15, 0.80] |
Comparison 11. Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus other antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 2 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.96] |
| 1.1 CBT versus other antidepressants | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.40, 0.94] |
| 1.2 Psychodynamic therapies versus other antidepressants | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.34, 2.25] |
| 2 Short‐term response | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.68, 1.06] |
| 2.1 CBT versus other antidepressants | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.69, 1.07] |
| 2.2 Psychodynamic therapies versus other antidepressants | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.22, 1.97] |
| 3 Dropouts for any reason | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.91, 2.65] |
| 3.1 CBT versus other antidepressants | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.99, 3.43] |
| 3.2 Psychodynamic therapy versus other antidepressants | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.36, 2.75] |
Comparison 12. Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus benzodiazepines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term response | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.61, 3.27] |
| 1.1 CBT versus benzodiazepines | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.61, 3.27] |
| 2 Dropouts for any reason | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.61, 3.27] |
| 2.1 CBT versus benzodiazepines | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.61, 3.27] |
| 3 Short‐term improvement | 2 | 69 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.82, 0.50] |
| 3.1 CBT versus benzodiazepines | 2 | 69 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.82, 0.50] |
12.3. Analysis.
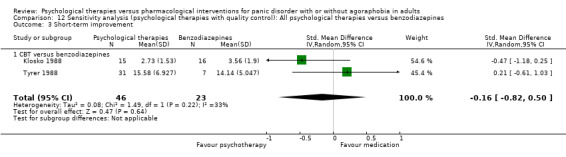
Comparison 12 Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus benzodiazepines, Outcome 3 Short‐term improvement.
Comparison 13. Sensitivity analysis (psychological therapies with quality control): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 8 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.65, 0.90] |
| 1.1 CBT versus either antidepressants alone or antidepressants and benzodiazepines | 7 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.65, 0.92] |
| 1.2 Psychodynamic therapies versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.34, 2.25] |
| 1.3 BT versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.7 [0.28, 1.75] |
| 1.4 Supportive psychotherapies versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.31] |
| 2 Short‐term response | 10 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.77, 1.03] |
| 2.1 CBT versus either antidepressants alone or antidepressants and benzodiazepines | 9 | 631 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.77, 1.07] |
| 2.2 Psychodynamic therapies versus either antidepressants or antidepressants and benzodiazepines | 1 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.22, 1.97] |
| 2.3 Behaviour therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.10, 9.96] |
| 2.4 Supportive psychotherapies versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.6 [0.27, 1.32] |
| 3 Dropouts for any reason | 10 | 796 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.79, 1.62] |
| 3.1 CBT versus either antidepressants alone or antidepressants and benzodiazepines | 9 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.82, 1.89] |
| 3.2 Psychodynamic therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.36, 2.75] |
| 3.3 BT versus antidepressants alone or antidepressants and benzodiazepines | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Supportive psychological therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.22, 1.42] |
| 4 Short‐term improvement | 9 | 592 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [0.02, 0.41] |
| 4.1 CBT versus either antidepressants alone or antidepressants and benzodiazepines | 8 | 505 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.06, 0.42] |
| 4.2 Psychodynamic therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐1.02, 1.01] |
| 4.3 Behaviour therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.30, 1.24] |
| 4.4 Supportive psychological therapy versus either antidepressants alone or antidepressants and benzodiazepines | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [‐0.12, 1.24] |
Comparison 14. Sensitivity analysis (best case): All psychological therapies versus SSRI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 6 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [1.05, 1.32] |
| 2 Short‐term response | 5 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.53, 1.95] |
14.1. Analysis.
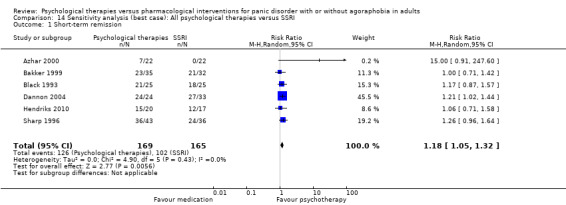
Comparison 14 Sensitivity analysis (best case): All psychological therapies versus SSRI, Outcome 1 Short‐term remission.
14.2. Analysis.

Comparison 14 Sensitivity analysis (best case): All psychological therapies versus SSRI, Outcome 2 Short‐term response.
Comparison 15. Sensitivity analysis (worst case): All psychological therapies versus SSRI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 6 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.49, 1.08] |
| 2 Short‐term response | 5 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.48, 1.69] |
15.1. Analysis.
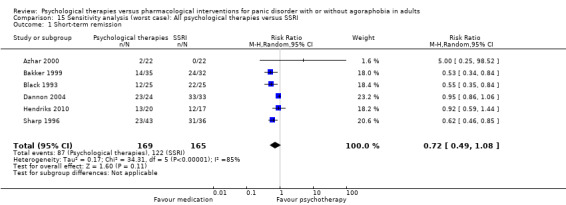
Comparison 15 Sensitivity analysis (worst case): All psychological therapies versus SSRI, Outcome 1 Short‐term remission.
15.2. Analysis.
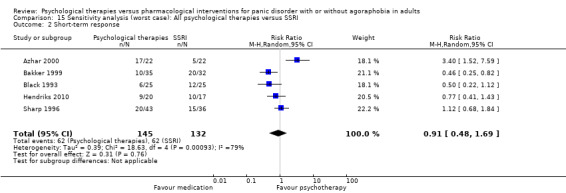
Comparison 15 Sensitivity analysis (worst case): All psychological therapies versus SSRI, Outcome 2 Short‐term response.
Comparison 16. Sensitivity analysis (best case): All psychological therapies versus tricyclic antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.94, 1.55] |
| 2 Short‐term response | 4 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.53, 1.15] |
16.1. Analysis.
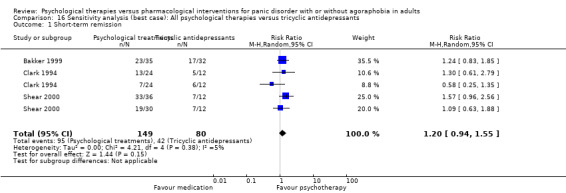
Comparison 16 Sensitivity analysis (best case): All psychological therapies versus tricyclic antidepressants, Outcome 1 Short‐term remission.
16.2. Analysis.
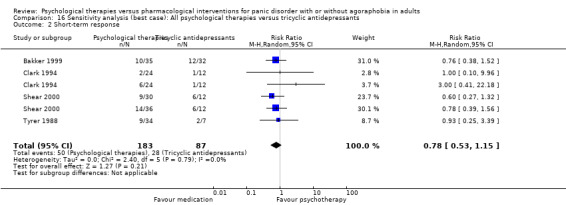
Comparison 16 Sensitivity analysis (best case): All psychological therapies versus tricyclic antidepressants, Outcome 2 Short‐term response.
Comparison 17. Sensitivity analysis (worst case): All psychological therapies versus tricyclic antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.45, 0.79] |
| 2 Short‐term response | 4 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.51, 1.10] |
17.1. Analysis.
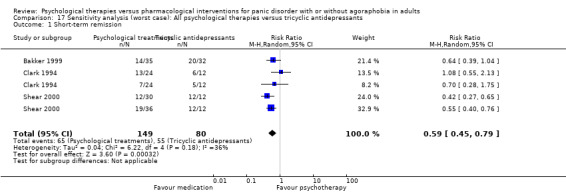
Comparison 17 Sensitivity analysis (worst case): All psychological therapies versus tricyclic antidepressants, Outcome 1 Short‐term remission.
17.2. Analysis.
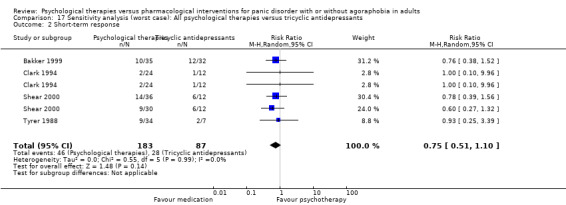
Comparison 17 Sensitivity analysis (worst case): All psychological therapies versus tricyclic antidepressants, Outcome 2 Short‐term response.
Comparison 18. Sensitivity analysis (best case): All psychological therapies versus other antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.48, 1.67] |
| 2 Short‐term response | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.35] |
18.1. Analysis.
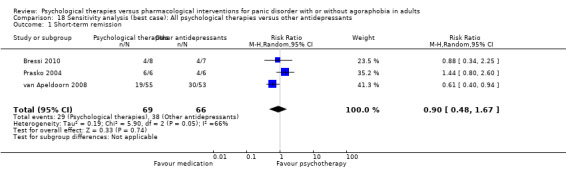
Comparison 18 Sensitivity analysis (best case): All psychological therapies versus other antidepressants, Outcome 1 Short‐term remission.
18.2. Analysis.
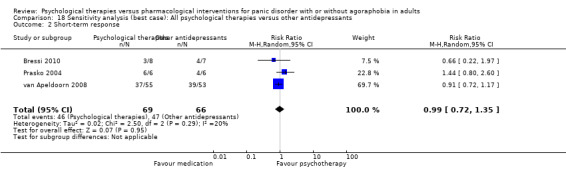
Comparison 18 Sensitivity analysis (best case): All psychological therapies versus other antidepressants, Outcome 2 Short‐term response.
Comparison 19. Sensitivity analysis (worst case): All psychological therapies versus other antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.48, 1.67] |
| 2 Short‐term response | 3 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.72, 1.35] |
19.1. Analysis.
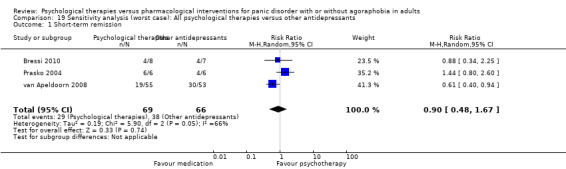
Comparison 19 Sensitivity analysis (worst case): All psychological therapies versus other antidepressants, Outcome 1 Short‐term remission.
19.2. Analysis.
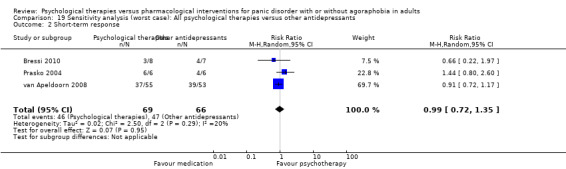
Comparison 19 Sensitivity analysis (worst case): All psychological therapies versus other antidepressants, Outcome 2 Short‐term response.
Comparison 20. Sensitivity analysis (best case): All psychological therapies versus benzodiazepines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 3 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.99, 2.06] |
20.1. Analysis.
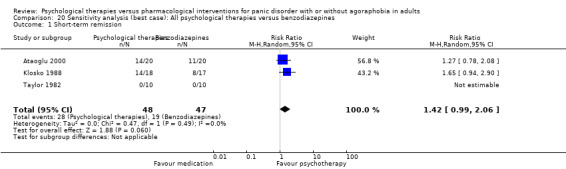
Comparison 20 Sensitivity analysis (best case): All psychological therapies versus benzodiazepines, Outcome 1 Short‐term remission.
Comparison 21. Sensitivity analysis (worst case): All psychological therapies versus benzodiazepines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 3 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.63, 1.38] |
21.1. Analysis.
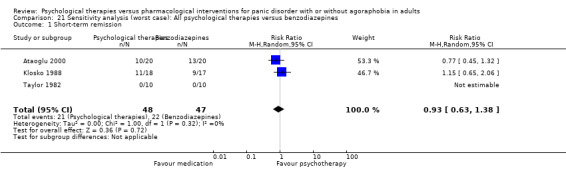
Comparison 21 Sensitivity analysis (worst case): All psychological therapies versus benzodiazepines, Outcome 1 Short‐term remission.
Comparison 22. Sensitivity analysis (best case): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 11 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.98, 1.27] |
| 2 Short‐term response | 11 | 757 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.89, 1.46] |
22.1. Analysis.
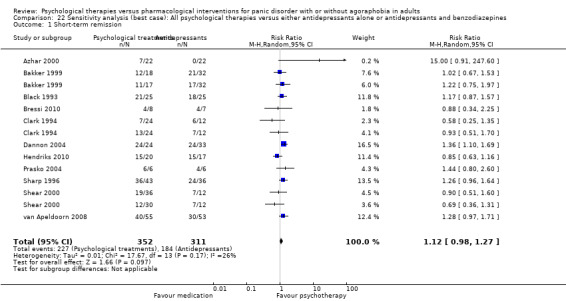
Comparison 22 Sensitivity analysis (best case): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 1 Short‐term remission.
22.2. Analysis.
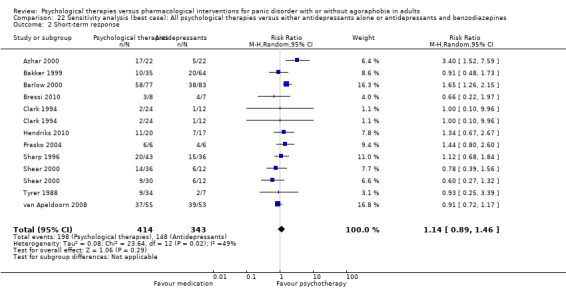
Comparison 22 Sensitivity analysis (best case): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 2 Short‐term response.
Comparison 23. Sensitivity analysis (worst case): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 11 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.72, 1.10] |
| 2 Short‐term response | 11 | 757 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.80, 1.23] |
23.1. Analysis.
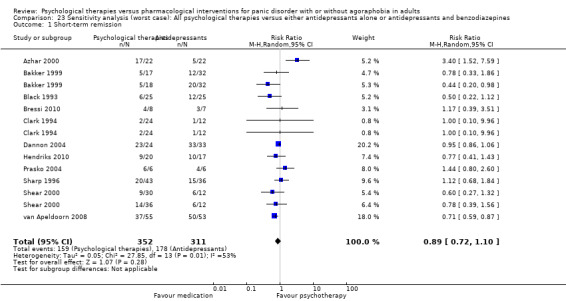
Comparison 23 Sensitivity analysis (worst case): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 1 Short‐term remission.
23.2. Analysis.
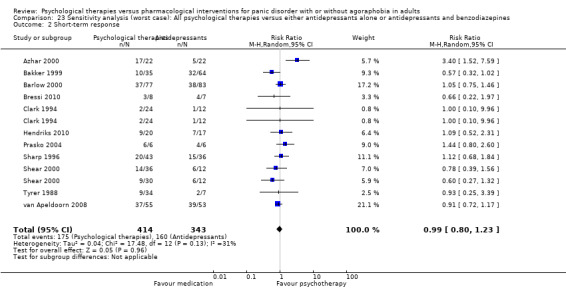
Comparison 23 Sensitivity analysis (worst case): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines, Outcome 2 Short‐term response.
Comparison 24. Sensitivity analysis (without imputation): All psychological therapies versus SSRI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 5 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.60, 1.16] |
Comparison 25. Sensitivity analysis (without imputation): All psychological therapies versus other antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 2 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.96] |
Comparison 26. Sensitivity analysis (without imputation): All psychological therapies versus benzodiazepines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 2 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.70, 1.65] |
Comparison 27. Sensitivity analysis (without imputation): All psychological therapies versus either antidepressants alone or antidepressants and benzodiazepines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term remission | 9 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.67, 1.01] |
| 2 Short‐term response | 3 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.17] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ataoglu 2000.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R Panic disorder with no or mild agoraphobic avoidance Method of diagnosis: not mentioned Age: for benzodiazepine (alprazolam) arm, M = 31.4 (SD = no data); for CBT arm, M = 30.8 (SD = no data); range = 18 to 65 Sex: for benzodiazepine (alprazolam) arm, 56% women; 44% men; for CBT arm, 43% women, 56% men Location: Turkey Comorbidities: excluded if individuals had current drug or alcohol abuse/dependency, principal diagnosis of major depression, and any signs of psychosis or organic brain syndrome Adjunctive therapy: excluded if individuals had begun taking benzodiazepines or antidepressants within the previous 6 months Adjunctive medication: no data |
|
| Interventions | Participants were randomly assigned to either: (1) Benzodiazepine (alprazolam) arm
(2) CBT arm
|
|
| Outcomes |
Time points for assessment: pre‐ and post‐treatment (2 months) and 3 and 4 months' follow‐up Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: not mentioned Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel could know the intervention allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no description about who assessed the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors did not report the number of participants assessed at post‐treatment. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. |
| Researcher allegiance | Unclear risk | Not mentioned |
| Therapist allegiance | Unclear risk | Not mentioned |
| Sponsorship bias | Unclear risk | Conflict of interest and funding were not mentioned. |
| Other bias | Low risk | We found no other bias. |
Azhar 2000.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV Panic disorder Method of diagnosis: not mentioned Age: for CBT arm, M = 31.7 (SD = 8.1); for fluvoxamine arm, M = 31.4 (SD = 7.5); range = 18 to 44 Sex: no data Location: Malaysia Comorbidities: excluded if individuals had other psychiatric disorders Adjunctive therapy: not mentioned Adjunctive medication: no data |
|
| Interventions | Participants were randomly assigned to either: (1) Fluvoxamine arm
(2) CBT arm
(3) CBT plus fluvoxamine arm |
|
| Outcomes |
Time points for assessment: pre‐ and post‐treatment (9 weeks) Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: not mentioned Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel could know the intervention allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Subjective scale was used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over 20% of outcome data was lost. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | Unclear risk | Not mentioned |
| Therapist allegiance | Unclear risk | Not mentioned |
| Sponsorship bias | Unclear risk | Conflict of interest and funding were not mentioned. |
| Other bias | Low risk | We found no other bias. |
Bakker 1999.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R Panic disorder Method of diagnosis: not mentioned Age: paroxetine arm, M = 34.7 (SD = 8.9); clomipramine arm, M = 35.3 (SD = 9.3); CBT arm, M = 33.7 (SD = 8.1); range = 18 to 70 Sex: paroxetine arm, female 68%; clomipramine arm, female, 56%; CBT arm, female 66% Location: the Netherlands Comorbidities: severe somatic diseases were excluded Adjunctive therapy: not mentioned Adjunctive medication: individuals who used antidepressants, neuroleptics, or benzodiazepines were included if they were able to stop taking the medications before the placebo run‐in period |
|
| Interventions | Participants were randomly assigned to either: (1) Paroxetine arm
(2) Clomipramine arm
(3) CBT arm
(4) Placebo arm |
|
| Outcomes |
Time points for assessment: pre‐ and post‐treatment (12 weeks) Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: SmithKline Beecham Phamaceuticals Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Medication was provided in a double‐blinded fashion, however psychological therapy could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Subjective scale was used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over 20% of outcome data was lost. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | Unclear risk | Not mentioned |
| Therapist allegiance | High risk | Cognitive therapy was provided by psychologists and psychiatrists with broad experience in cognitive behavioural treatments, whereas medication was administered by residents in psychiatry. |
| Sponsorship bias | High risk | The study was funded by manufacturer of the pharmacological intervention. |
| Other bias | Low risk | We found no other bias. |
Barlow 2000.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R Panic disorder with or without mild agoraphobia Method of diagnosis: Anxiety Disorder Interview Schedule‐Revised Age: imipramine arm, M = 35.5 (SD = 9.7); CBT arm, M = 37.5 (SD = 10.9); range = 18 to 65 Sex: imipramine arm, female 60%; CBT arm, female, 63% Location: USA Comorbidities: bipolar, significant medical illness, suicidally, significant substance abuse were excluded. Patients with comorbid unipolar depression were included unless suicidal. Adjunctive therapy: not mentioned Adjunctive medication: benzodiazepines were permitted up to 20 doses during baseline and acute treatment. |
|
| Interventions | Participants were randomly assigned to either: (1) Imipramine arm
(2) CBT arm
(3) Placebo arm (4) CBT plus imipramine arm (5) CBT plus placebo arm |
|
| Outcomes |
Time points for assessment: pre‐ and post‐treatment (12 weeks) Outcomes:
|
|
| Notes |
Date of study: from May 1991 to April 1998 Funding source: National Institute of Mental Health grant. Imipramine and matching placebo were provided by Teva Pharmaceuticals USA. Declarations of interest among the primary researchers: Dr. Barlow has received research support from Pfizer and the National Institute of Mental Health and has served as a consultant for or received honoraria or royalties from Guilford Press, The Psychological Corporation, and Wyeth‐Ayerst Pharmaceuticals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Medication was provided in a double‐blinded fashion, however psychological therapy could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blind to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were entered into the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | High risk | One of the authors made a treatment manual of one arm. |
| Therapist allegiance | Low risk | Both treatments were followed by treatment manuals. |
| Sponsorship bias | High risk | The study was supported by the manufacturer of the pharmacological intervention. |
| Other bias | Low risk | We found no other bias. |
Black 1993.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R Panic disorder Method of diagnosis: Structured Clinical Interview for DSM‐III‐R Age: fluvoxamine arm, M = 35.1 (SD = 10.4); CBT arm, M = 38.7 (SD = 12.4); range = 18 to 65 Sex: fluvoxamine arm, female 60%; CBT arm, female, 88% Location: USA Comorbidities: individuals with current or past major depression were included. Individuals who were psychotic, suicidal, or demented, or who had significant medical illness were excluded. Adjunctive therapy: additional psychotherapies were not allowed. Adjunctive medication: additional psychotropic medication was not allowed. |
|
| Interventions | Participants were randomly assigned to either: (1) Fluvoxamine arm
(2) CBT arm
(3) Placebo arm |
|
| Outcomes |
Time points for assessment: pre‐treatment, 4 weeks, post‐treatment (8 weeks) Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: the study was sponsored in part through a grant from Reid‐Rowell Pharmaceuticals Inc. Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Medication was provided in a double‐blinded fashion, however psychological therapy could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The assessor was not blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were entered into the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | Low risk | Researchers used the existing treatment manual made by others. |
| Therapist allegiance | High risk | Cognitive therapy was performed by professional cognitive therapist. |
| Sponsorship bias | High risk | The study was funded by manufacturer of the pharmacological intervention. |
| Other bias | Low risk | We found no other bias. |
Bressi 2010.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV‐TR Anxiety disorder (panic disorder, generalised anxiety disorder, social phobia) or depressive disorder (major depressive disorder, dysthymic disorder) Method of diagnosis: Structured Clinical Interview for DSM‐IV‐TR Age: not shown for participants with panic disorder. As a whole, for psychodynamic therapy arm, M = 35.8 (SD = 9.3); for non‐specific SSRI/SNRI arm, M = 38.7 (SD = 9.3); range = 18 to 60 Sex: not shown for participants with panic disorder. As a whole, for psychodynamic therapy arm, female 77%; for non‐specific SSRI/SNRI arm, female 77% Location: Italy Comorbidities: individuals with evidence of mental retardation, lifetime history of organic mental disorders, schizophrenic disorders, bipolar disorders, substance abuse, severe axis II psychopathology were excluded. Adjunctive therapy: not allowed Adjunctive medication: benzodiazepines at a maximum of 3 mg lorazepam equivalent per day and zolpidem tartrate (5 mg to 10 mg) were allowed during the first 3 weeks of the study. |
|
| Interventions | Participants were randomly assigned to either: (1) Psychodynamic therapy arm
(2) Non‐specific SSRI/SNRI arm
|
|
| Outcomes |
Time points for assessment: pre‐ and post‐treatment (12 weeks) Outcomes:
|
|
| Notes |
Date of study: October 2005 to October 2007 Funding source: not mentioned Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers were used. |
| Allocation concealment (selection bias) | Low risk | Allocation was made by the computer at the same time as random number generation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel could know the intervention allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcomes were few. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | Low risk | Researchers used existing treatment manual made by others. |
| Therapist allegiance | High risk | The therapists were psychiatrists who were trained in psychodynamic psychotherapy and who had long experience in practicing the intervention. |
| Sponsorship bias | Unclear risk | Conflict of interest and funding were not mentioned. |
| Other bias | Low risk | We found no other bias. |
Clark 1994.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R Panic disorder with no, mild, or moderate agoraphobic avoidance Method of diagnosis: Structured Clinical Interview for DSM‐III‐R Age: for included participants, M = 34.6 (SD = 9.2); range = 18 to 60 Sex: 19% female Location: UK Comorbidities: individuals with organic mental disorder, schizophrenia, alcohol or drug dependence, cardiovascular disease, asthma, epilepsy were excluded. Adjunctive therapy: not mentioned Adjunctive medication: CBT arm, 35%; applied relaxation arm, 40%; imipramine arm, 30% |
|
| Interventions | Participants were randomly assigned to either: (1) CBT arm
(2) Applied relaxation arm
(3) Imipramine arm
(4) Waiting list |
|
| Outcomes |
Time points for assessment: pre‐ and post‐treatment (12 weeks) Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: funded by grants from the Medical Research Council of the UK, the North Atlantic Treaty Organization, and the Wellcome Trust Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel could know the intervention allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Subjective scale was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcomes were few. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | High risk | One of the researchers made treatment manual for psychological treatment. |
| Therapist allegiance | Unclear risk | Not mentioned |
| Sponsorship bias | Unclear risk | Conflict of interest was not mentioned. |
| Other bias | Low risk | We found no other bias. |
Dannon 2004.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV‐TR Panic disorder or panic disorder with agoraphobia Method of diagnosis: authors said that a semi‐structured psychiatric interview was used, however they did not mention the method. Age: paroxetine arm, M = 45.1 (SD = 11.9); CBT arm, M = 44.8 (SD = 13.1); range = 18 to Sex: paroxetine arm, 59% female; CBT arm, 57% female Location: Israel Comorbidities: individuals with comorbid psychiatric diagnosis were excluded. Adjunctive therapy: not mentioned Adjunctive medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: (1) CBT arm
(2) Paroxetine arm
|
|
| Outcomes |
Time points for assessment: pre‐treatment, 4 weeks, and post‐treatment (12 weeks) Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: partially supported by a personal grant to Dr. Pinchas Dannon from the Chief Scientist Office, the Ministry of Health, Israel Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel could know the intervention allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Subjective scale was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcomes were few. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | Low risk | Researcher used existing treatment manual made by others. |
| Therapist allegiance | Unclear risk | Insufficient information |
| Sponsorship bias | Unclear risk | Conflict of interest was not mentioned. |
| Other bias | Low risk | We found no other bias. |
Hendriks 2010.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV‐TR Panic disorder or panic disorder with agoraphobia Method of diagnosis: Anxiety Disorders Interview Schedule‐Revised Age: paroxetine arm, M = 68.8 (SD = 4.3); CBT arm, M = 69.6 (SD = 5.0); range = 60 to Sex: paroxetine arm, 58.8% female; CBT arm, 55.0% female Location: the Netherlands Comorbidities: individuals with comorbid severe psychiatric disorder, dementia, substance abuse, and severe somatic condition were excluded. Adjunctive therapy: not mentioned Adjunctive medication: (oxazepam 10 mg): for paroxetine arm, 58.8%; for CBT arm, 55.0% |
|
| Interventions | Participants were randomly assigned to either: (1) CBT arm
(2) Paroxetine arm
(3) Waiting list arm |
|
| Outcomes |
Time points for assessment: pre‐treatment, 8 weeks, and post‐treatment (14 weeks) Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: one of the authors received an unconditional research grant from GlaxoSmithKline. Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel could know the intervention allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self report questionnaire was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcomes were few. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | Unclear risk | Not mentioned |
| Therapist allegiance | Unclear risk | Not mentioned |
| Sponsorship bias | High risk | One of the authors received an unconditional research grant from the manufacturer of the pharmacological intervention. |
| Other bias | Low risk | We found no other bias. |
Klosko 1988.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III Panic disorder Method of diagnosis: Anxiety Disorders Interview Schedule‐Revised (ADIS‐R) was used. Age: alprazolam arm, M = 38.3 (SD = 6.9); CBT arm, M = 31.3 (SD = 8.0); range = 18 to 65 Sex: alprazolam arm, 75.0% female; CBT arm, 73.3% female Location: USA Comorbidities: individuals with major depression were excluded only if depression predominated over panic disorder at the time of presentation and if depression preceded panic disorder chronologically. Adjunctive therapy: not allowed Adjunctive medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: (1) CBT arm
(2) Paroxetine arm
(3) Placebo arm (4) Waiting list arm |
|
| Outcomes |
Time points for assessment: pre‐treatment and post‐treatment (15 weeks) Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: the study was supported in part by grants from the National Institute of Mental Health and the Upjohn Company. Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Medication was provided in a double‐blinded fashion, however psychological therapy could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was blind to the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcomes were few. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | Low risk | Researcher used existing treatment manual made by others. |
| Therapist allegiance | Unclear risk | Not mentioned |
| Sponsorship bias | High risk | The study was supported by the manufacturer of the pharmacological intervention. |
| Other bias | Low risk | We found no other bias. |
Muncy 1991.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R Panic disorder or agoraphobia with panic Method of diagnosis: not mentioned Age: p106 for G1 arm, M = 33.1 (SD = 6.0); for G2 arm, M = 36.3 (SD = 11.7); for G3 arm, M = 31.6 (SD = 3.7); for G4 arm, M = 42.3 (SD = 10.0) Sex: for G1 arm, 71.4% female; for G2 arm, 62.5% female; for G3 arm, 100% female; for G4 arm, 75.0% female Location: USA Comorbidities: not mentioned Adjunctive therapy: not allowed Adjunctive medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: (1) CBT arm
(2) CBT + biofeedback arm
(3) Imipramine arm
(4) No‐treatment arm |
|
| Outcomes |
Time points for assessment: pre‐treatment and post‐treatment (15 weeks) Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: not mentioned Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel could know the intervention allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over 20% of outcome data was lost. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned |
| Researcher allegiance | Unclear risk | Not mentioned |
| Therapist allegiance | Unclear risk | Not mentioned |
| Sponsorship bias | Unclear risk | Conflict of interest was not mentioned. |
| Other bias | Low risk | We found no other bias. |
Prasko 2004.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV Panic disorder with or without agoraphobia Method of diagnosis: the MINI Interview was used. Age: CBT arm, M = 31.8; SSRI or SNRI arm, M = 31; range = 21 to 44 Sex: CBT arm, 50.0% female; SSRI or SNRI arm, 50.5% female Location: Czech Republic Comorbidities: individuals with serious physical illness or psychiatric disorder other than panic disorder and agoraphobia were excluded. Adjunctive therapy: not allowed Adjunctive medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: (1) CBT arm
(2) SSRI or SNRI arm
|
|
| Outcomes |
Time points for assessment: pre‐treatment, 2, 4, 6 weeks, and post‐treatment (12 weeks) Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: The study was supported by the Internal Grant Agency of Ministry of Health. Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel could know the intervention allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | Unclear risk | Not mentioned |
| Therapist allegiance | Unclear risk | Not mentioned |
| Sponsorship bias | Unclear risk | Conflict of interest was not mentioned. |
| Other bias | Low risk | We found no other bias. |
Sharp 1996.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R Panic disorder with or without agoraphobia Method of diagnosis: not mentioned Age: CBT arm, M = 33.2; fluvoxamine arm, M = 36.6; range = 18 to 70 Sex: CBT arm, 82.1% female; fluvoxamine arm, 73.3% female Location: Scotland Comorbidities: individuals with obsessive‐compulsive disorder, psychotic disorder, manic disorder, substance dependence, and severe somatic disease were excluded. Adjunctive therapy: not allowed Adjunctive medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: (1) CBT arm
(2) Fluvoxamine arm
(3) Fluvoxamine plus CBT arm (4) Placebo plus CBT arm (5) Placebo arm |
|
| Outcomes |
Time points for assessment: pre‐treatment, 6 weeks, post‐treatment (12 weeks), and follow‐up (6 months) Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: the study was supported in part by Duphar Laboratories Ltd. Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Medication was provided in a double‐blinded fashion, however psychological therapy could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self report questionnaire was used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over 20% of outcome data was lost. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | High risk | One of the authors was an employee of the manufacturer of the medication. |
| Therapist allegiance | Unclear risk | Insufficient information |
| Sponsorship bias | High risk | The study was supported by the manufacturer of the pharmacological intervention. |
| Other bias | Low risk | We found no other bias. |
Shear 2000.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R Panic disorder with no more than mild agoraphobia Method of diagnosis: the Anxiety Disorders Interview Schedule Revised was used. Age: CBT arm, M = 35.2 (SD = 11.2); psychological support arm, M = 39.2 (SD = 9.4); imipramine arm, M = 36.7 (SD = 11.3) Sex: CBT arm, 52.8% female; psychological support arm, 76.7% female; imipramine arm, 54.2% female Location: USA Comorbidities: individuals with psychotic disorder, bipolar disorder, significant substance abuse, or significant medical illness were excluded. Adjunctive therapy: not allowed Adjunctive medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: (1) CBT arm
(2) Psychological support arm
(3) Imipramine arm
(4) Placebo arm |
|
| Outcomes |
Time points for assessment: pre‐treatment, 1, 4, 8, 11 weeks, and follow‐up (1, 2, 3, 4, 5, 6 months) Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: the study was supported by National Institute of Mental Health grants and Intervention Center grant. Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Medication was provided in a double‐blinded fashion, however psychological therapy could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor was independent evaluator. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were entered into the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | Low risk | Researcher used existing treatment manual made by others. |
| Therapist allegiance | Low risk | Therapists in both treatment arms were trained adequately. |
| Sponsorship bias | Unclear risk | Conflict of interest was not mentioned. |
| Other bias | Low risk | We found no other bias. |
Taylor 1982.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III Panic disorder Method of diagnosis: not mentioned Age: not mentioned for diazepam arm and relaxation arm Sex: not mentioned for diazepam arm and relaxation arm Location: USA Comorbidities: not mentioned Adjunctive therapy: not mentioned Adjunctive medication: not allowed |
|
| Interventions | Participants were randomly assigned to either: (1) Relaxation arm
(2) Diazepam arm
(3) Control arm (4) Placebo arm |
|
| Outcomes |
Time points for assessment: pre‐ and post‐treatment Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: the study was supported by Biomedical Research Support Grant. Hoffman‐La Roche supplied diazepam and placebo. Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Medication was provided in a double‐blinded fashion, however psychological therapy could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self report questionnaire was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were entered into the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | Unclear risk | Not mentioned |
| Therapist allegiance | Unclear risk | Not mentioned |
| Sponsorship bias | High risk | The study was supported by the manufacturer of the pharmacological intervention. |
| Other bias | Low risk | We found no other bias. |
Tyrer 1988.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III generalised anxiety disorder, dysthymic disorder, or panic disorder Method of diagnosis: the Structured Clinical Interview for DSM‐III was used. Age: not shown for participants with panic disorder; range = 17 to 71 Sex: not shown for participants with panic disorder Location: UK Comorbidities: individuals with major depressive episode or other psychiatric disorder were excluded. Adjunctive therapy: during the study other treatments were discouraged. However, participants who underwent additional treatment were included in the analysis. Adjunctive medication: if further sedative treatment was necessary, the antihistamine drug promethazine was prescribed in a small dose 810 mg to 30 mg daily). |
|
| Interventions | Participants were randomly assigned to either: (1) CBT arm
(2) Diazepam arm
(3) Dosulepin arm
(4) Placebo arm (5) Self help arm |
|
| Outcomes |
Time points for assessment: pre‐treatment and 2, 4, 6, 10 weeks Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: the study was supported by grants from Trent Regional Health Authority, Jessie Spencer Trust, and Boots Pure Drug Company, who also provided the matching diazepam, dosulepin, and placebo capsules. Declarations of interest among the primary researchers: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was made by computer. |
| Allocation concealment (selection bias) | Low risk | The randomised treatments were indicated by opening a sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Medication was provided in a double‐blinded fashion, however psychological therapy could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self report questionnaire was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were entered into the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. |
| Researcher allegiance | Unclear risk | Not mentioned |
| Therapist allegiance | Unclear risk | Not mentioned |
| Sponsorship bias | High risk | The study was supported by the manufacturer of the pharmacological intervention. |
| Other bias | Low risk | We found no other bias. |
van Apeldoorn 2008.
| Methods | Study design: randomised controlled trial | |
| Participants |
Diagnosis: DSM‐IV Panic disorder with or without agoraphobia Method of diagnosis: the MINI was used. Age: CBT arm, M = 39.4 (SD = 10.2); SSRI arm, M = 38.5 (SD = 10.5); range = 18 to 65 Sex: CBT arm, 62.3% female; SSRI arm, 54.2% female Location: the Netherlands Comorbidities: psychotic or severely depressed patients were excluded. Adjunctive therapy: during the study other treatments were discouraged. However, participants who underwent additional treatment were included in the analysis. Adjunctive medication: small dose of benzodiazepines was allowed (maximum the equivalent of 20 mg of oxazepam per day). |
|
| Interventions | Participants were randomly assigned to either: (1) CBT arm
(2) SSRI or SNRI arm
|
|
| Outcomes |
Time points for assessment: pre‐treatment and 2, 4, 6, 10 weeks Outcomes:
|
|
| Notes |
Date of study: not mentioned Funding source: the study was funded by the Dutch Health Insurance Board. Declarations of interest among the primary researchers: no financial or commercial involvements that might present a conflict of interest in connection with the study were present. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Raffle tickets were used. |
| Allocation concealment (selection bias) | Low risk | The random number was contained in an envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel could know the intervention allocated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self report questionnaire was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were entered into the analysis. |
| Selective reporting (reporting bias) | High risk | Some registered outcomes were not reported. |
| Researcher allegiance | Low risk | Researcher used existing treatment manual made by others. |
| Therapist allegiance | Low risk | Therapists in both treatment arms were trained adequately. |
| Sponsorship bias | Low risk | No financial and commercial involvements were present. |
| Other bias | Low risk | We found no other bias. |
CBT: cognitive behavioural therapy DSM: Diagnostic and Statistical Manual of Mental Disorders M: mean SD: standard deviation SNRI: serotonin–norepinephrine reuptake inhibitor SSRI: selective serotonin reuptake inhibitor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Borkovec 1988 | Participants were heterogeneous, however randomisation was not stratified. |
| Echeburua 1993 | Participants were patients with agoraphobia but without panic attacks. |
| Echeburua 2006 | Psychiatrist decided treatment in control arm. |
| Huppert 2004 | Participants were heterogeneous, however randomisation was not stratified. |
| Lang 2006 | Primary care provider decided treatment in control arm. |
| Schuurmans 2006 | Participants were heterogeneous, however randomisation was not stratified. |
| Spinhoven 1996 | Participants were permitted regular benzodiazepine use in the psychological therapy arm, and some of the participants in the arm used benzodiazepine, which is regarded as a mixture of combination therapy and psychological therapy alone. |
Characteristics of studies awaiting assessment [ordered by study ID]
Prasko 2004b.
| Methods | Study design: randomised controlled trial |
| Participants |
Diagnosis: panic disorder Method of diagnosis: not stated Age: not stated Sex: 77% female Location: not stated Comorbidities: not stated Adjunctive therapy: not stated Adjunctive medication: not stated |
| Interventions | Participants were randomly assigned to either: (1) CBT arm
(2) Paroxetine arm
|
| Outcomes |
Time points for assessment: every week Outcomes:
|
| Notes | We contacted the author to ask for details, but have not yet received an answer. |
Takriti 1989.
| Methods | Study design: randomised controlled trial |
| Participants |
Diagnosis: panic disorder with or without agoraphobia Method of diagnosis: not stated Age: not stated Sex: 77% female Location: not stated Comorbidities: not stated Adjunctive therapy: not stated Adjunctive medication: not stated |
| Interventions | Participants were randomly assigned to either: (1) CBT arm
(2) Alprazolam arm
|
| Outcomes |
Time points for assessment: every week Outcomes:
|
| Notes | We were unable to retrieve the article and could only refer to abstract. |
CBT: cognitive behavioural therapy DSM: Diagnostic and Statistical Manual of Mental Disorders
Differences between protocol and review
Our preplanned subgroup analysis for classes of antidepressants was deleted because this was performed in the main analysis.
We added the following subgroup analyses for the investigation of heterogeneity: studies excluding group psychological therapies; outcome using the same scales; and studies without extreme results.
We added the following sensitivity analyses to confirm the rigidity of the results: calculating primary outcomes using worst‐case and best‐case scenarios, and limited to studies without imputed data.
We assessed researcher and therapist allegiance as other sources of potential bias, because these factors could bias the results (Falkenstrom 2013; Munder 2011).
Contributions of authors
TAF and HI devised the idea. TAF, HI, AT, PC, and AP worked on the first draft of the protocol. All authors reviewed and approved the final version of the protocol.
Declarations of interest
HI reports no competing interests.
AT has received honoraria for speaking at a meeting sponsored by Eli Lilly.
PC reports no competing interests.
AP reports no competing interests.
TAF has received honoraria for speaking at continuing medical education meetings sponsored by Asahi Kasei, Eli Lilly, GlaxoSmithKline, Mochida, MSD, Otsuka, Pfizer, Shionogi, and Mitsubishi Tanabe. He is diplomate of the Academy of Cognitive Therapy. He has received royalties from Igaku‐Shoin, Seiwa Shoten, and Nihon Bunka Kagakusha. He is on the advisory board for Sekisui Chemical, and Takeda Science Foundation. The Japanese Ministry of Education, Science and Technology, the Japanese Ministry of Health, Labor and Welfare, and the Japan Foundation for Neuroscience and Mental Health have funded his research projects.
New
References
References to studies included in this review
Ataoglu 2000 {published data only}
- Ataoglu A, Ozkan M, Tutkun H, Maras A. Alprazolam and cognitive behavior therapy in treatment of panic disorder. Turkish Journal of Medical Sciences 2000;30:167‐71. [Google Scholar]
Azhar 2000 {published data only}
- Azhar MZ. Comparison of fluvoxamine alone, fluvoxamine and cognitive psychotherapy and psychotherapy alone in the treatment of panic disorder in Kelantan ‐ implications for management by family doctors. Medical Journal of Malaysia 2000;55:402‐8. [PubMed] [Google Scholar]
Bakker 1999 {published data only}
- Bakker A, Spinhoven P, Does AJW, Balkom AJLM, Dyck R. Locus of control orientation in panic disorder and the differential effects of treatment. Psychotherapy and Psychosomatics 2002;71:85‐9. [DOI] [PubMed] [Google Scholar]
- Bakker A, Dyck R, Spinhoven P, Balkom AJLM. Paroxetine, clomipramine, and cognitive therapy in the treatment of panic disorder. Journal of Clinical Psychiatry 1999;60:831‐8. [DOI] [PubMed] [Google Scholar]
- Dusseldorp E, Spinhoven P, Bakker A, Dyck R, Balkom AJLM. Which panic disorder patients benefit from which treatment: cognitive therapy or antidepressants?. Psychotherapy and Psychosomatics 2007;76:254‐61. [DOI] [PubMed] [Google Scholar]
Barlow 2000 {published data only}
- Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive‐behavioral therapy, imipramine, or their combination for panic disorder: a randomized controlled trial. JAMA 2000;283:2529‐36. [DOI] [PubMed] [Google Scholar]
- Hicks TV, Leitenberg H, Barlow DH, Gorman JM, Shear MK, Woods SW. Physical, mental and social catastrophic cognitions as prognostic factors in cognitive‐behavioral and pharmacological treatments for panic disorder. Journal of Consulting and Clinical Psychology 2005;73(3):506‐14. [DOI] [PubMed] [Google Scholar]
- Hoffmann SG, Suvak MK, Barlow DH, Shear MK, Meuret AE, Rosenfield D, et al. Preliminary evidence for cognitive mediation during cognitive‐behavioral therapy of panic disorder. Journal of Consulting and Clinical Psychology 2007;75(3):374‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Shear MK, Barlow DH, Gorman JM, Hershberger D, Patterson M, et al. Effects of panic disorder treatments on personality disorder characteristics. Depression and Anxiety 1998;8:14‐20. [PubMed] [Google Scholar]
- McHugh RK, Otto MW, Barlow DH, Gorman JM, Shear MK, Woods SW. Cost‐efficacy of individual and combined treatments for panic disorder. Journal of Clinical Psychiatry 2007;68:1038‐1044. [DOI] [PubMed] [Google Scholar]
- Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, et al. Multicenter collaborative panic disorder severity scale. American journal of psychiatry 1997;154(11):1571‐1575. [DOI] [PubMed] [Google Scholar]
- Shear MK, Houck P, Greeno C, Masters S. Emotion‐focused psychotherapy for patients with panic disorder. American Journal of Psychiatry 2001;158(12):1993‐1998. [DOI] [PubMed] [Google Scholar]
Black 1993 {published data only}
- Black DW, Monahan P, Wesner R, Gabel J, Bowers W. The effect of fluvoxamine, cognitive therapy, and placebo on abnormal personality traits in 44 patients with panic disorder. Journal of Personality Disorders 1996;10(2):185‐194. [Google Scholar]
- Black DW, Wesner R, Bowers W, Gabel J. A comparison of fluvoxamine, cognitive therapy, and placebo in the treatment of panic disorder. Archives of General Psychiatry 1993;50:44‐50. [DOI] [PubMed] [Google Scholar]
- Black DW, Wesner R, Bowers W, Monahan P, Gabel J. Acute treatment response in outpatients with panic disorder: high versus low depressive symptoms. Annals of Clinical Psychiatry 1995;7(4):181‐188. [DOI] [PubMed] [Google Scholar]
- Black DW, Wesner RB, Gabel J, Bowers W, Patrick M. Predictors of short‐term treatment response in 66 patients with panic disorder. Journal of Affective Disorders 1994;39:233‐241. [DOI] [PubMed] [Google Scholar]
Bressi 2010 {published data only}
- Bressi C, Porcellana M, Marinaccio PM, Nocito EP, Magri L. Short‐term psychodynamic psychotherapy versus treatment as usual for depressive and anxiety disorders. Journal of Nervous and Mental Disease 2010;198(9):647‐652. [DOI] [PubMed] [Google Scholar]
Clark 1994 {published data only}
- Clark DM, Salkovskis PM, Hackmann A, Middleton H, Anastasiades P, Gelder M. A comparison of cognitive therapy, applied relaxation and imipramine in the treatment of panic disorder. British Journal of Psychiatry 1994;164:759‐769. [DOI] [PubMed] [Google Scholar]
Dannon 2004 {published data only}
- Dannon PN, Gon‐usishkin M, Gelbert A, Lowengrub K, Grunhaus L. Cognitive behavioral group therapy in panic disorder patients: the efficacy of CBGT versus drug treatment. Annals of Clinical Psychiatry 2004;16:41‐46. [DOI] [PubMed] [Google Scholar]
Hendriks 2010 {published data only}
- Hendriks GJ, Keijsers GPJ, Kampman M, Hoogduin CAL, Voshaar RCO. Predictors of outcome of pharmacological and psychological treatment of late‐life panic disorder with agoraphobia. International Journal of Geriatric Psychiatry 2012;27:146‐150. [DOI] [PubMed] [Google Scholar]
- Hendriks GJ, Keijsers GPJ, Kampman M, Oude Voshaar RC, Verbraak MJPM, Broekman TG, et al. A randomized controlled study of paroxetine and cognitive‐behavioural therapy for late‐life panic disorder. Acta Psychiatrica Scandinavica 2010;122:11‐19. [DOI] [PubMed] [Google Scholar]
Klosko 1988 {published data only}
- Klosko JS, Barlow DH, Tassinari R, Cerny JA. A comparison of alprazolam and behavior therapy in treatment of panic disorder. Journal of Consulting and Clinical Psychology 1990;58(1):77‐84. [DOI] [PubMed] [Google Scholar]
Muncy 1991 {published data only}
- Muncy SM. Panic: a Comparison of Four Treatment Methods [Doctoral dissertation]. 1990. [Google Scholar]
Prasko 2004 {published data only}
- Prasko J, Horacek J, Zalesky R, Kopecek M, Novak T, Paskova B, et al. The change of regional brain metabolism (18FDG PET) in panic disorder during the treatment with cognitive behavioral therapy or antidepressants. Neuroendocrinology Letters 2004;25:340‐348. [PubMed] [Google Scholar]
Sharp 1996 {published data only}
- Sharp DM, Power KG. Fluvoxamine, placebo, and cognitive behaviour therapy used alone and in combination in the treatment of panic disorder and agoraphobia. Journal of Anxiety Disorders 1996;10(4):219‐242. [Google Scholar]
- Sharp DM, Power KG. Predicting treatment outcome for panic disorder and agoraphobia in primary care. Clinical Psychology and Psychotherapy 1999;6:336‐348. [Google Scholar]
- Sharp DM, Power KG. Psychologist, patient, and general practitioner rating of outcome of pharmacological and psychological treatments for panic disorder and agoraphobia in primary care. Behavioural and Cognitive Psychotherapy 1998;26:13‐27. [Google Scholar]
- Sharp DM, Power KG, Simpson RJ, Swanson V, Anstee JA. Global measure of outcome in a controlled comparison of pharmacological treatment of panic disorder and agoraphobia in primary care. British Journal of General Practice 1997;47:150‐155. [PMC free article] [PubMed] [Google Scholar]
Shear 2000 {published data only}
- Shear MK, Houck P, Greeno C, Masters S. Emotion‐focused psychotherapy for patients with panic disorder. American Journal of Psychiatry 2001;158(12):1993‐1998. [DOI] [PubMed] [Google Scholar]
Taylor 1982 {published data only}
- Taylor CB, Kenigsberg ML, Robinson JM. A controlled comparison of relaxation and diazepam in panic disorder. Journal of Clinical Psychiatry 1982;43(10):423‐425. [PubMed] [Google Scholar]
Tyrer 1988 {published data only}
- Kingdon D, Tyrer P, Seivewright N, Ferguson B, Murphy S. The Nottingham study of neurotic disorder: influence of cognitive therapists on outcome. British Journal of Psychiatry 1996;169:93‐97. [DOI] [PubMed] [Google Scholar]
- Knerer G, Byford S, Johnson T, Seivewright H, Tyrer P. The Nottingham study of neurotic disorder: predictors of 12 year costs. Acta Psychiatrica Scandinavica 2005;112:224‐232. [DOI] [PubMed] [Google Scholar]
- Seivewright H, Tyrer P, Johnson T. Change in personality status in neurotic disorders. Lancet 2002;359:2253‐2254. [DOI] [PubMed] [Google Scholar]
- Seivewright H, Tyrer P, Johnson T. Prediction of outcome in neurotic disorder: 5‐year prospective study. Psychological Medicine 1998;28(5):1149‐1157. [DOI] [PubMed] [Google Scholar]
- Seivewright N, Tyrer P, Ferguson B, Murphy S, Johnson T. Longitudinal study of the influence of life events and personality status on diagnostic change in three neurotic disorders. Depression and Anxiety 2000;11:105‐113. [PubMed] [Google Scholar]
- Tyrer P, Seivewright H, Ferguson B, Johnson T. "Cold calling" in psychiatric follow up studies: is it justified?. journal of Medical Ethics 2003;29:238‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrer P, Seivewright H, Johnson T. The Nottingham study of neurotic disorder: predictors of 12‐year outcome of dysthymic, panic and generalized anxiety disorder. Psychological Medicine 2004;34(8):1385‐1394. [DOI] [PubMed] [Google Scholar]
- Tyrer P, Seivewright H, Simmonds S, Johnson T. Prospective studies of dysthymia (mixed anxiety‐depression): how do they inform clinical practice?. European Archives of Psychiatry and Clinical Neuroscience 2001;251(Suppl 2):II/53‐II/56. [DOI] [PubMed] [Google Scholar]
- Tyrer P, Seivewright N, Ferguson B, Brothwell J, Darling C, Murphy S. Nottingham study of neurotic disorder. Lancet 1988;30(2):235‐240. [DOI] [PubMed] [Google Scholar]
- Tyrer P, Seivewright N, Ferguson B, Murphy S, Johnson AL. The Nottingham study of neurotic disorder. Effect of personality status on response to drug treatment, cognitive therapy and self‐help over two years. British Journal of Psychiatry 1993;162:219‐226. [DOI] [PubMed] [Google Scholar]
- Tyrer P, Seivewright N, Ferguson B, Murphy S, Johnson AL. The Nottingham study of neurotic disorder. Effect of personality status on response to drug treatment, cognitive therapy and self‐help over two years. British journal of psychiatry 1993;162:219‐226. [DOI] [PubMed] [Google Scholar]
- Tyrer P, Seivewright N, Ferguson B, Tyrer J. The general neurotic syndrome: a coaxial diagnosis of anxiety, depression and personality disorder. Acta Psychiatrica Scandinavica 1992;85:201‐206. [DOI] [PubMed] [Google Scholar]
- Tyrer P, Seivewright N, Murphy S, Ferguson B, Kingdon D, Barczak P, et al. The Nottingham study of neurotic disorder: comparison of drug and psychological treatments. Lancet 1988;8605:235‐240. [DOI] [PubMed] [Google Scholar]
- Tyrer P, Seivewright N, Seivewright H. Long‐term outcome of hypochondriacal personality disorder. Journal of Psychosomatic Research 1999;46(2):177‐185. [DOI] [PubMed] [Google Scholar]
van Apeldoorn 2008 {published data only}
- Apeldoorn FJ, Hout WJPJ, Mersch PPA, Huisman M, Slaap BR, Hale WW, et al. Is a combined therapy more effective than either CBT or SSRI alone? Results of a multicenter trial on panic disorder with or without agoraphobia. Acta Psychiatrica Scandinavica 2008;117:260‐270. [DOI] [PubMed] [Google Scholar]
- Apeldoorn PJ, Hout WJPJ, Timmerman ME, Mersch PPA, Boer JA. Rate of improvement during and across three treatments for panic disorder with or without agoraphobia: cognitive behavioral therapy. Journal of Affective Disorders 2013;150:313‐319. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Borkovec 1988 {published data only}
- Borkovec TD, Mathews AM. Treatment of nonphobic anxiety disorders: a comparison of nondirective, cognitive, and coping desensitization therapy. Journal of Consulting and Clinical Psychology 1988;56(6):877‐884. [DOI] [PubMed] [Google Scholar]
Echeburua 1993 {published data only}
- Echeburua E, Corral PD, Bajos EG, Borda M. Interactions between self‐exposure and alprazolam in the treatment of agoraphobia without current panic: an exploratory study. Behavioural and Cognitive Psychotherapy 1993;21:219‐238. [Google Scholar]
Echeburua 2006 {published data only}
- Echeburua E, Salaberria K, Corral PD, Cenea R. Treatment of mixed anxiety‐depression disorder: long‐term outcome. Behavioural and Cognitive Psychotherapy 2006;34:95‐101. [Google Scholar]
Huppert 2004 {published data only}
- Huppert JD, Schultz LT, Foa EB, Barlow DH, Davidson JRT, Gorman JM, et al. Differential response to placebo among patients with social phobia, panic disorder, and obsessive‐compulsive disorder. American Journal of Psychiatry 2004;161:1485‐1487. [DOI] [PubMed] [Google Scholar]
Lang 2006 {published data only}
- Lang AJ, Norman GJ, Casmar PV. A randomized trial of a brief mental health intervention for primary care patients. Journal of Consulting and Clinical Psychology 2006;74(6):1173‐1179. [DOI] [PubMed] [Google Scholar]
Schuurmans 2006 {published data only}
- Schuurmans J, Comijs H, Emmelkamp PMG, Gundy CMM, Weijnen I, Hout MVD, et al. A randomized, controlled trial of the effectiveness of cognitive‐behavioral therapy and sertraline versus a waitlist control group for anxiety disorders in older adults. American Journal of Geriatric Psychiatry 2006;14(3):255‐263. [DOI] [PubMed] [Google Scholar]
Spinhoven 1996 {published data only}
- Spinhoven P, Onstein EJ, Klinkhamer RA, Klein EAM. Panic management, trazodone and a combination of both in the treatment of panic disorder. Clinical Psychology and Psychotherapy 1996;3(2):86‐92. [Google Scholar]
References to studies awaiting assessment
Prasko 2004b {unpublished data only}
- Prasko J, Horacek J, Paskova B. CBT or paroxetine in the treatment of panic disorder. 32nd Congress of the British Association for Behavioural and Cognitive Psychotherapies (jointly with the European Association of Behavioural and Cognitive Therapies); 2004 September 7 ‐ 11; Manchester. 2004.
Takriti 1989 {published data only}
- Tariti AY, Zakaria Z. Panic disorder alprazolam versus cognitive therapy. Arab Journal of Psychiatry 1989;1:12‐21. [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland MJ. Detecting skewness for summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. American Psychiatric Association, 1987. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. American Psychiatric Association, 2000. [Google Scholar]
APA 2009
- American Psychiatric Association. American Psychiatric Association Practice Guidelines for the Treatment of Panic Disorder. American Psychiatric Publishing, 2009. [Google Scholar]
Baldwin 2009
- Baldwin SA, Berkeljon A, Atkins DC, Olsen JA, Nielsen SL. Rates of change in naturalistic psychotherapy: contrasting dose–effect and good‐enough level models of change. Journal of Consulting and Clinical Psychology 2009;77:203‐211. [DOI] [PubMed] [Google Scholar]
Bandelow 1995
- Bandelow B. Assessing the efficacy of treatments for panic disorder and agoraphobia. II. The Panic and Agoraphobia Scale. International Clinical Psychopharmacology 1995;10(2):73‐81. [DOI] [PubMed] [Google Scholar]
BAP 2014
- Baldwin DS, Anderson IM, Nutt DJ, Allgulander C, Bandelow B, Boer JA, et al. Evidence‐based pharmacological treatment of anxiety disorders, post‐traumatic stress disorder and obsessive‐compulsive disorder: revision of the 2005 guidelines from the British Association for Psychopharmacology. Journal of Psychopharmacology 2014;28(5):403‐39. [DOI] [PubMed] [Google Scholar]
Barlow 1988
- Barlow DH. Anxiety and Its Disorders. Guilford Press, 1988. [Google Scholar]
Baudry 2011
- Baudry A, Mouillet‐Richard S, Launay J, Kellermann O. New view on antidepressant action. Current Opinion in Neurobiology 2011;21(6):858‐65. [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988;56(6):893‐7. [DOI] [PubMed] [Google Scholar]
Bijl 1998
- Bijl RV, Ravelli A, Zessen G. Prevalence of psychiatric disorder in the general population: results of The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Social Psychiatry and Psychiatric Epidemiology 1998;33(6):683‐91. [DOI] [PubMed] [Google Scholar]
Bradwejn 2005
- Bradwejn J, Ahokas A, Stein DJ, Salinas E, Emilien G, Whitaker T. Venlafaxine extended‐release capsules in panic disorder: flexible‐dose, double‐blind, placebo‐controlled study. British Journal of Psychiatry 2005;187:352‐9. [DOI] [PubMed] [Google Scholar]
Busch 1999
- Busch FN, Milrod BL, Singer MB. Theory and technique in psychodynamic treatment of panic disorder. Journal of Psychotherapy Practice and Research 1999;8(3):234‐42. [PMC free article] [PubMed] [Google Scholar]
Chambless 1984
- Chambless DL, Caputo GC, Bright P, Gallagher R. Assessment of fear of in agoraphobics: the Body Sensations Questionnaire and the Agoraphobic Cognitions Questionnaire. Journal of Consulting and Clinical Psychology 1984;52(6):1090‐7. [DOI] [PubMed] [Google Scholar]
Chambless 1985
- Chambless DL, Caputo GC, Jasin SE, Gracely EJ, Williams C. The Mobility Inventory for Agoraphobia. Behaviour Research and Therapy 1985;23(1):36‐44. [DOI] [PubMed] [Google Scholar]
Clum 1993
- Clum GA, Surls R. A meta‐analysis of treatments for panic disorder. Journal of Consulting and Clinical Psychology 1993;61(2):317‐26. [DOI] [PubMed] [Google Scholar]
Comer 2011
- Comer JS, Blanco C, Hasin DS, Liu SM, Grant BF, Turner JB, et al. Health‐related quality of life across the anxiety disorders. Journal of Clinical Psychiatry 2011;72(1):43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daele 2012
- Daele T, Hermans D, Audenhove C, Bergh O. Stress reduction through psychoeducation: a meta‐analytic review. Health Education & Behavior 2012;39(4):474‐85. [DOI] [PubMed] [Google Scholar]
Deacon 2003
- Deacon B, Abramowitz J, Woods C, Tolin D. The Anxiety Sensitivity Index ‐ Revised: psychometric properties and factor structure in two nonclinical samples. Behaviour Research and Therapy 2003;41(12):1427‐49. [DOI] [PubMed] [Google Scholar]
Douglas 2008
- Douglas C. Teaching supportive psychotherapy to psychiatric residents. American Journal of Psychiatry 2008;165(4):445‐52. [DOI] [PubMed] [Google Scholar]
Eaton 1994
- Eaton WW, Kessler RC, Wittchen HU, Magee WJ. Panic and panic disorder in the United States. American Journal of Psychiatry 1994;151:413‐20. [DOI] [PubMed] [Google Scholar]
Eldridge 1981
- Eldridge FL, Millhorn DE. Central regulation of respiration by endogenous neurotransmitters and neuromodulators. Annual Review of Physiology 1981;43:121‐35. [DOI] [PubMed] [Google Scholar]
Endicott 1976
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry 1976;33(6):766‐71. [DOI] [PubMed] [Google Scholar]
Falkenstrom 2013
- Falkenstrom F, Markowitz JC, Jonker H, Philips B, Holmqvist R. Can psychotherapists function as their own controls? Meta‐analysis of the crossed therapist design in comparative psychotherapy trials. Journal of Clinical Psychiatry 2013;74(5):482‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feighner 1972
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Archives of General Psychiatry 1972;26(1):57‐63. [DOI] [PubMed] [Google Scholar]
Freire 2011
- Freire RC, Hallak JE, Nardi AE. New treatment options for panic disorder: clinical trials from 2000 to 2010. Expert Opinion Pharmacotherapy 2011;12(9):1419‐28. [DOI] [PubMed] [Google Scholar]
Furukawa 2002
- Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta‐analyses. International Journal of Epidemiology 2002;31(1):72‐6. [DOI] [PubMed] [Google Scholar]
Furukawa 2005
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta‐analysis. International Clinical Psychopharmacology 2005;20(1):49‐52. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta‐analysis. International Clinical Psychopharmacology 2005;20:49‐52. [DOI] [PubMed] [Google Scholar]
Furukawa 2007
- Furukawa TA, Watanabe N, Churchill R. Combined psychotherapy plus antidepressants for panic disorder with or without agoraphobia. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004364.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldapple 2004
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, et al. Modulation of cortical‐limbic pathways in major depression: treatment‐specific effects of cognitive behavior therapy. Archives of General Psychiatry 2004;61(1):34‐41. [DOI] [PubMed] [Google Scholar]
Gould 1995
- Gould RA, Otto MW, Pollack MH. A meta‐analysis of treatment outcome for panic disorder. Clinical Psychology Review 1995;15(8):819‐44. [Google Scholar]
Grant 2006
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Goldstein RB, Smith S, et al. The epidemiology of DSM‐IV panic disorder and agoraphobia in the United States: result from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry 2006;67:363‐74. [DOI] [PubMed] [Google Scholar]
Greenberg 1992
- Greenberg RP, Bornstein RF, Greenberg MD, Fisher S. A meta‐analysis of antidepressant outcome under "blinder" conditions. Journal of Consulting and Clinical Psychology 1992;60(5):664‐669. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. Clinical Global Impression. ECDEU Assessment Manual for Psychopharmacology, revised. National Institute of Mental Health, 1976:218‐22. [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32(1):50‐5. [DOI] [PubMed] [Google Scholar]
Hayes 2004
- Hayes S. Acceptance and commitment therapy, relational frame theory, and the third wave of behavioral and cognitive therapies. Behaviour Therapy 2004;35(4):639‐65. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
James 2013
- James A, James G, Cowdrey F, Soler A, Choke A. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD004690.pub3] [DOI] [PubMed] [Google Scholar]
Kahl 2012
- Kahl KG, Winter L, Schweiger U. The third wave of cognitive behavioural therapies: what is new and what is effective?. Current Opinion in Psychiatry 2012;25(6):522‐8. [DOI] [PubMed] [Google Scholar]
Kessler 2006
- Kessler RC, Chiu WT, Jin R, Ruscio AM, Shear K, Walters EE. The epidemiology of panic attacks, panic disorder, and agoraphobia in the National Comorbidity Survey Replication. Archives of General Psychiatry 2006;63:415‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kind 1996
- Kind P. The EuroQol instrument: an index of health related quality of life. In: Spilker B editor(s). Quality of Life and Pharmacoeconomics in Clinical Trials. 2nd Edition. Philadelphia: Lippincott‐Raven Publishers, 1996:191‐201. [Google Scholar]
King 2008
- King M, Nazareth I, Levy G, Walker C, Morris R, Weich S, et al. Prevalence of common mental disorders in general practice attendees across Europe. British Journal of Psychiatry 2008;192(5):362‐7. [DOI] [PubMed] [Google Scholar]
Klein 1993
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Archives of General Psychiatry 1993;50(4):306‐17. [DOI] [PubMed] [Google Scholar]
Linden 2013
- Linden M. How to define, find and classify side effects in psychotherapy: from unwanted events to adverse treatment reactions. Clinical Psychology and Psychotherapy 2013;20:286‐96. [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012;12:MR000033. [DOI] [PubMed] [Google Scholar]
Malizia 1998
- Malizia AL, Cunningham VJ, Bell CJ, Liddle PF, Jones T. Decreased brain GABAA‐benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Archives of General Psychiatry 1998;55(8):715‐20. [DOI] [PubMed] [Google Scholar]
Markowitz 1989
- Markowitz JS, Weissman MM, Ouellette R, Lish JD, Klerman GL. Quality of life in panic disorder. Archives of General Psychiatry 1989;46(11):984‐92. [DOI] [PubMed] [Google Scholar]
Marks 1979
- Marks IM, Mathew AM. Brief standard self‐rating for phobic patients. Behavior Research and Therapy 1979;17(3):263‐7. [DOI] [PubMed] [Google Scholar]
Marks 1981
- Marks IM. Cure and Care of Neuroses: Theory and Practice of Behavioural Psychotherapy. New York: Wiley, 1981. [Google Scholar]
Mauret 2010
- Mauret AE, Ritz T. Hyperventilation in panic disorder and asthma: empirical evidence and clinical strategies. International Journal of Psychophysiology 2010;78(1):68‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayberg 1999
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic‐cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of Psychiatry 1999;156:675‐82. [DOI] [PubMed] [Google Scholar]
Mayberg 2000
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biological Psychiatry 2000;48:830‐43. [DOI] [PubMed] [Google Scholar]
Michelson 1998
- Michelson D, Lydiard RB, Pollack MH, Tamura RN, Hoog SL, Tepner R, et al. Outcome assessment and clinical improvement in panic disorder: evidence from a randomised controlled trial of fluoxetine and placebo. American Journal of Psychiatry 1998;155(11):1570‐7. [DOI] [PubMed] [Google Scholar]
Milrod 2007
- Milrod B, Leon AC, Busch F, Rudden M, Schwalberg M, Clarkin J, et al. A randomized controlled clinical trial of psychoanalytic psychotherapy for panic disorder. American Journal of Psychiatry 2007;164(2):265‐72. [DOI] [PubMed] [Google Scholar]
Mitte 2005
- Mitte K. A meta‐analysis of the efficacy of psycho‐ and pharmacotherapy in panic disorder with and without agoraphobia. Journal of Affective Disorders 2005;88(1):27‐45. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA group. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. PLoS Medicine 2009;6(6):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munder 2011
- Munder T, Gerger H, Trelle S, Barth J. Testing the allegiance bias hypothesis: a meta‐analysis. Psychotherapy Research 2011;21(6):670‐84. [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Care Excellence. Generalised anxiety disorder and panic disorder (with or without agoraphobia) in adults: Management in primary, secondary and community care (CG113). London: National Institute for Health and Care Excellence 2011.
Ost 2008
- Ost L. Efficacy of the third wave of behavioral therapies: a systematic review and meta‐analysis. Behaviour Research and Therapy 2008;46(3):296‐321. [DOI] [PubMed] [Google Scholar]
Quitkin 1984
- Quitkin FM, Rabkin JG, Ross D, McGrath PJ. Duration of antidepressant drug treatment: what is an adequate trial?. Archives of General Psychiatry 1984;41(3):238‐245. [DOI] [PubMed] [Google Scholar]
Ravindran 2010
- Ravindran LN, Stein MB. The pharmacologic treatment of anxiety disorders: a review of progress. Journal of Clinical Psychiatry 2010;71(7):839‐54. [DOI] [PubMed] [Google Scholar]
Reiss 1986
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency, and the prediction of fearfulness. Behaviour Research and Therapy 1986;24(1):1‐8. [DOI] [PubMed] [Google Scholar]
Sadock 2003
- Sadock BJ, Kaplan HI, Sadock VA (editors). Kaplan & Sadock's Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. Lippincott Williams & Wilkins, 2003. [Google Scholar]
Sanchez‐Meca 2010
- Sanchez‐Meca J, Rosa‐Alcazar AI, Marin‐Martinez F, Gomez‐Conesa A. Psychological treatment of panic disorder with or without agoraphobia: a meta‐analysis. Clinical Psychology Review 2010;30(1):37‐50. [DOI] [PubMed] [Google Scholar]
Sareen 2005
- Sareen J, Cox BJ, Afifi TO, Graaf RD, Asmundson GJG, Have TH, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts. Archives of General Psychiatry 2005;62:1249‐57. [DOI] [PubMed] [Google Scholar]
Schotte 2006
- Schotte C, Bossche B, Doncker D, Claes S, Cosyns P. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depression and Anxiety 2006;23(5):312‐24. [DOI] [PubMed] [Google Scholar]
Shear 1997
- Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SE, et al. Multicenter collaborative panic disorder severity scale. American Journal of Psychiatry 1997;154(11):1571‐5. [DOI] [PubMed] [Google Scholar]
Sherbourne 2010
- Sherbourne C, Sullivana G, Crasked MG, Roy‐Byrnee P, Golinellia D, Rosed RD, et al. Functioning and disability levels in primary care outpatients with one or more anxiety disorders. Psychological Medicine 2010;40(12):2059‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spielberger 1989
- Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State‐Trait Anxiety Inventory. Consulting Psychologists Press, 1983. [Google Scholar]
Spitzer 1978
- Spitzer RL, Robins E. Research diagnostic criteria: rationale and reliability. Archives of General Psychiatry 1978;35(6):773‐82. [DOI] [PubMed] [Google Scholar]
Starcevic 2009
- Starcevic V. Anxiety Disorders in Adults: a Clinical Guide. Oxford University Press, 2009. [Google Scholar]
Stiles 2015
- Stiles WB, Barkham M, Wheeler S. Effect of duration of psychological therapy on recovery and improvement rates: evidence from UK routine practice. British Journal of Psychiatry 2015;207(2):115‐122. [DOI] [PubMed] [Google Scholar]
Swinson 1992
- Swinson RP, Cox BJ, Woszczyna CB. Use of medical services and treatment for panic disorder with agoraphobia and for social phobia. Canadian Medical Association Journal 1992;147(6):878‐83. [PMC free article] [PubMed] [Google Scholar]
Teasdale 2002
- Teasdale JD, Moore RG, Hayhurst H, Pope M, Williams S, Segal ZV. Metacognitive awareness and prevention of relapse in depression: empirical evidence. Journal of Consulting and Clinical Psychology 2002;70(2):275‐87. [DOI] [PubMed] [Google Scholar]
Tedeschini 2011
- Tedeschini E, Fava M, Papakostas GI. Placebo‐controlled, antidepressant clinical trials cannot be shortened to less than 4 weeks' duration: a pooled analysis of randomized clinical trials employing a diagnostic odds ratio–based approach. Journal of Clinical Psychiatry 2011;72(1):98‐113. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short form health survey (SF‐36) I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. The ICD‐10 International Statistical Classification of Diseases and Health Related Problems. Geneva: World Health Organization, 1992. [Google Scholar]
Winston 2004
- Winston A, Rosenthal RN, Pinsker H. Introduction to Supportive Psychotherapy. Washington, DC: American Psychiatric Publishing, 2004. [Google Scholar]
Wittchen 2002
- Wittchen HU. Generalized anxiety disorder: prevalence, burden, and cost to society. Depression and Anxiety 2002;16:162‐71. [DOI] [PubMed] [Google Scholar]
Zohar 2000
- Zohar J, Westenberg HG. Anxiety disorders: a review of tricyclic antidepressants and selective serotonin reuptake inhibitors. Acta Psychiatrica Scandinavica 2000;101(S403):39‐49. [DOI] [PubMed] [Google Scholar]


