Abstract
Introduction: Non-thyroidal illness (NTI), which occurs with fasting and in response to illness, is characterized by thyroid hormone inactivation with low triiodothyronine (T3) and high reverse T3 (rT3), followed by suppressed thyrotropin (TSH). Withholding supplemental parenteral nutrition early in pediatric critical illness (late-PN), thus accepting low/no macronutrient intake up to day 8 in the pediatric intensive care unit (PICU), accelerated recovery compared to initiating supplemental parenteral nutrition early (early-PN). Whether NTI is harmful or beneficial in pediatric critical illness and how it is affected by a macronutrient deficit remains unclear. This study investigated the prognostic value of NTI, the impact of late-PN on NTI, and whether such impact explains or counteracts the outcome benefit of late-PN in critically ill children.
Methods: This preplanned secondary analysis of the Early versus Late Parenteral Nutrition in the Pediatric Intensive Care Unit randomized controlled trial quantified serum TSH, total thyroxine (T4), T3, and rT3 concentrations in 982 patients upon PICU admission versus 64 matched healthy children and in 772 propensity score–matched early-PN and late-PN patients upon admission and at day 3 or last PICU day for shorter PICU stay. Associations between thyroid hormone concentrations upon admission and outcome, as well as impact of late-PN on NTI in relation with outcome, were assessed with univariable analyses and multivariable logistic regression, linear regression, or Cox proportional hazard analysis, adjusted for baseline risk factors.
Results: Upon PICU admission, critically ill children revealed lower TSH, T4, T3, and T3/rT3 and higher rT3 than healthy children (p < 0.0001). A more pronounced NTI upon admission, with low T4, T3, and T3/rT3 and high rT3 was associated with higher mortality and morbidity. Late-PN further reduced T4, T3, and T3/rT3 and increased rT3 (p ≤ 0.001). Statistically, the further lowering of T4 by late-PN reduced the outcome benefit (p < 0.0001), whereas the further lowering of T3/rT3 explained part of the outcome benefit of late-PN (p ≤ 0.004). This effect was greater for infants than for older children.
Conclusion: In critically ill children, the peripheral inactivation of thyroid hormone, characterized by a decrease in T3/rT3, which is further accentuated by low/no macronutrient intake, appears beneficial. In contrast, the central component of NTI attributable to suppressed TSH, evidenced by the decrease in T4, seems to be a harmful response to critical illness. Whether treating the central component with TSH releasing hormone infusion in the PICU is beneficial requires further investigation.
Keywords: pediatrics, critical illness, non-thyroidal illness syndrome, nutrition, prognostic value
Introduction
As in adults, critical illness in children evokes pronounced changes in the thyroid axis (1–7). A low serum concentration of triiodothyronine (T3) and a rise in reverse T3 (rT3) are typically observed in the acute phase of critical illness, possibly reflecting an attempt to reduce energy expenditure. Such peripheral inactivation of thyroid hormone is mainly explained by a decrease in type-1 deiodinase (D1) activity in the liver and kidney and an increase in type-3 deiodinase (D3) activity in the liver and muscle (8,9). In the face of these peripheral changes, an acute short-lived rise in thyrotropin (TSH)—and thyroxine (T4)—has been documented in response to the acute stress of surgery/illness (10). However, when critical illness persists, pulsatile TSH secretion becomes suppressed, which results in the lowering of serum T4 (11–13). Together, these changes are referred to as the low-T3 or non-thyroidal illness syndrome (NTI). Although the severity of NTI has been associated with adverse clinical outcomes in adults (14,15) and children (4–7,16–18), it remains elusive as to what extent the various components of NTI reflect beneficial adaptations to stress or represent instead a maladaptive and harmful response to illness.
The NTI of critical illness closely mimics the response of the thyroid axis to fasting in healthy individuals. Indeed, fasting also leads to low T3 and high rT3 concentrations in the presence of altered deiodinase activity (19). When fasting is prolonged, this is followed by low T4 without a compensatory rise in TSH. During critical illness, nutrient intake is often insufficient due to anorexia, a dysfunctional gastrointestinal tract, or interruptions of enteral nutrition (20,21). Hence, a reduced nutrient intake may also play a role in NTI of critical illness.
Recently, the effect of accepting low or even no macronutrient intake in the early phase of critical illness with delaying the initiation of supplemental parenteral nutrition (PN) until beyond the first week in adults and children admitted to the intensive care unit (ICU) was studied. The withholding of early supplemental PN reduced the risk of acquiring a new infection and accelerated recovery compared to providing early full nutrition by giving PN to supplement any degree of insufficient enteral nutrition (22,23). The clinical benefit of not using PN up to day 8 in the ICU was greater for critically ill children than it was for adults. Interestingly, in adult patients, accepting low or no macronutrient intake was found to aggravate NTI further (24). In the adult population, statistical analyses suggested that the more pronounced peripheral inactivation of thyroid hormone with accepting the low/no macronutrient intake in the acute phase of illness reflects a beneficial adaptation to enhance recovery (24). In contrast, the aggravation of the central component of the NTI, with further lowering of T4, could delay recovery (24). Most nutrition guidelines recommend more aggressive feeding for critically ill children than for adult patients (25). It was hypothesized that not using PN up to day 8 in the pediatric ICU (PICU) aggravates NTI even further than in adult patients. It was also hypothesized that more pronounced NTI may explain why the clinical benefits of not using PN up to day 8 in the ICU were also larger for critically ill children than for adults.
This study first investigated the prognostic value of NTI upon PICU admission for 90-day mortality, for the time to discharge from PICU, and for the risk of acquiring a new infection in the PICU. Next, it documented the impact of accepting low or no macronutrient intake up to day 8 of critical illness in children on the change of the NTI from admission to day 3 or last day for patients discharged earlier. It also investigated to what extent any such impact offers a statistical explanation for the higher likelihood of an earlier discharge, and the lower risk of acquiring a new infection brought about by not using PN up to day 8 in the PICU.
Methods
Patients
This study was a preplanned secondary analysis of the Early versus Late Parenteral Nutrition in the Pediatric Intensive Care Unit (PEPaNIC) randomized controlled trial (RCT) (23). PEPaNIC investigated the clinical outcomes of withholding supplemental PN up to day 8 in the PICU, further referred to as “late-PN,” although for the majority of patients this strategy meant no PN at all, compared to early supplemental PN whenever enteral nutrition alone was insufficient to reach the caloric target, referred to as “early-PN,” in children admitted to the PICU. All children aged 0–17 years were eligible for inclusion if a stay of ≥24 hours in the PICU was expected, if they had a moderate or severe risk of malnutrition (score of ≥2 on the Screening Tool for Risk on Nutritional Status and Growth [STRONGkids]), and if none of the predefined exclusion criteria were met (23). In addition, healthy children who had never been critically ill and from whom blood was drawn immediately after intravenous catheterization prior to minor elective surgery were included for comparison with the patients. Written informed consent was obtained from parents or legal guardians. The institutional ethical review board at each participating center approved the study protocol and consent forms (ML8052, NL38772.000.12, and Pro00038098). The detailed study protocol and primary results have been published elsewhere (23,26).
The study was conducted in three centers that used early-PN as the standard of care: Leuven (Belgium), Rotterdam (the Netherlands), and Edmonton (Canada). Enteral nutrition was started as soon as possible in both groups. In the early-PN group, supplemental PN was initiated within 24 hours after PICU admission, whereas any PN was withheld up to the morning of day 8 in the PICU for patients in the late-PN group. In the late-PN group, a mixture of intravenous dextrose (5%) and saline was administered to match the intravenous fluid load given to the early-PN patients. Patients in both groups received intravenous trace elements, minerals and vitamins, and blood glucose control with insulin according to local targets. Blood was systematically sampled upon admission and then daily at 6:00am until PICU discharge or death. After clotting and centrifugation, serum was stored at −80°C until analysis.
Serum analyses
Serum TSH concentrations were quantified with a commercially available TSH immunoradiometric assay (TSH IRMA kit; Beckman Coulter, Prague, Czech Republic). Serum total T4, T3, and rT3 concentrations were quantified with commercially available radioimmunoassays (RIA; total T4 RIA kit and total T3 RIA kit; Beckman Coulter; RIAZEN Reverse T3; ZenTech s.a., Liège, Belgium). Free thyroid hormone concentrations were not measured, given that the blood samples had been drawn via heparinized lines, which induce artifacts in free hormone quantification (27).
Statistical analyses
Data for univariable analyses are presented as numbers and percentages, means ± standard errors, or medians and interquartile ranges. Univariable differences were assessed with the chi-square test for proportions and Mann–Whitney U-test for continuous data.
To assess the association between serum concentrations of thyroid hormones upon PICU admission and patient outcomes, death at 90 days, length of PICU stay, and risk of acquiring a new infection in the PICU were studied. First, univariable analyses were performed for the association of TSH, T4, T3, rT3, and T3/rT3 upon PICU admission and outcome. Subsequently, multivariable analyses were performed to assess independent associations of thyroid hormones that were significantly associated with outcome in univariable analyses, where baseline risk factors were adjusted for (treatment center, risk of malnutrition [STRONGkids score], age, diagnosis upon admission, severity of illness [PeLOD reflecting degree of organ failure], and PIM2 score estimating risk of death) (28–30). Multivariable logistic regression was used to assess associations with 90-day mortality and risk of acquiring a new infection, and multivariable linear regression analysis was used to assess length of PICU stay. Effect sizes are reported as odds ratios (OR) and β estimates, respectively, and confidence intervals (CI). Multicollinearity was excluded based on the multicollinearity diagnostics tools in IBM SPSS Statistics for Macintosh v24.0 (IBM Corp., Armonk, NY), with tolerance ≥0.3, variance inflation factor <3.5, and condition index <30 for all variables entered in the models (31).
To evaluate the effect of late-PN versus early-PN on the early central (TSH, T4) and peripheral (T3/rT3) components of the thyroid axis, a subgroup of patients was selected by propensity score matching (SPSS R-menu R3.1; Foundation for Statistical Computing) in IBM SPSS Statistics v23.0 (IBM Corp.). Logistic regression was used to estimate propensity scores with the baseline risk factors sex, treatment center, stratification group, severity of illness (PeLOD and PIM2 score), risk of malnutrition (STRONGkids score), height and weight as percentiles of population norms, infection upon admission, need for mechanical ventilation, and need for extracorporeal membrane oxygenation or other assist devices as covariates. It was hypothesized that a further reduction of the T3/rT3 ratio by ±15% would occur in the late-PN group compared to the early-PN group. This effect size was based upon a similar impact of another metabolic intervention in PICU patients, namely targeting strict age-adjusted fasting blood glucose levels (18). To detect such a difference, with 80% power and 95% certainty, 386 patients per group were needed. The required number of patients was obtained with use of a caliper of 0.35 for one-to-one nearest-neighbor matching. In this propensity score–matched subset, the changes in hormone concentrations from PICU admission to PICU day 3 or to the last PICU day for patients discharged earlier (denoted as ΔTSH, ΔT4, ΔT3, ΔrT3, and ΔT3/rT3) were compared between early-PN and late-PN patients. Differences in these changes from baseline between both groups were analyzed with Mann–Whitney U-test and with multivariable linear regression analysis adjusted for the baseline risk factors described above.
To investigate whether any of the observed changes in the central and peripheral components of the thyroid axis (ΔTSH, ΔT4, and ΔT3/rT3) played a role in the beneficial effects of late-PN on time to discharge from PICU (accounting for death as competing risk by censoring non-surviving patients beyond all survivors at 91 days) and risk of new infection, which were the primary endpoints of the PEPaNIC study, multivariable Cox proportional hazard and logistic regression analyses were performed, as described above. In the first step, the model included the baseline risk factors and the randomized intervention. In the second explanatory step, the model was further adjusted for the changes from baseline in the thyroid hormone parameters that were affected by the randomized intervention. A change from baseline of a thyroid hormone would thus statistically explain (part of) the beneficial impact of late-PN on outcome when adjusting for this change would reduce the effect of late-PN on outcome. Vice versa, when adjusting for a change from baseline of a hormone would increase the significant impact of late-PN on outcome, this change could be interpreted as a deleterious side effect of late-PN. Finally, in order to determine whether there were interactions between the effect of late-PN versus early-PN and the age group (infants <1 year old vs. older children) of the patients, we calculated interaction p-values in all multivariable models.
Statistical analyses were performed with JMP Pro v13.0.0 (SAS Institute, Cary, NC). Statistical significance was set at a p-value of ≤0.05.
Results
Thyroid hormone concentrations upon PICU admission and predictive value for outcomes
PICU admission samples from 982 patients were available for thyroid hormone measurements (Fig. 1). The baseline characteristics of these patients are described in Table 1. In addition, 64 healthy children were studied, matched with the patients for age and sex (Table 1).
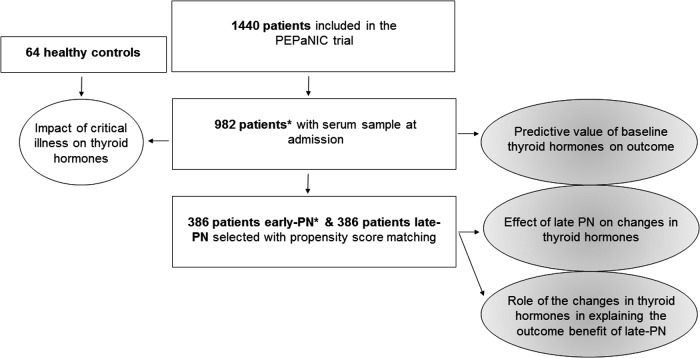
FIG. 1.
Diagram of the study design. A PICU admission serum sample was available for 982/1440 patients included in the PEPaNIC trial. Those 982 admission samples were used to compare thyroid hormones in critically ill children to those in healthy control children, and to study the association of baseline thyroid hormone concentrations with clinical outcome. A subgroup of early-PN and late-PN patients was selected with propensity score matching to investigate the effect of the randomized intervention on the thyroid axis, as well as the impact of these changes on the outcome benefit of late-PN. *Data were incomplete for one early-PN patient as not enough serum was available to perform the complete analysis. Results of another early-PN patient were retrospectively excluded because of severely aberrant thyroid hormone concentrations after radioactive iodine metaiodobenzoguanidine therapy. PN, parenteral nutrition.
Table 1.
Baseline Characteristics of Patients and Healthy Controls Used to Study the Impact of Critical Illness on the Thyroid Axis
| Baseline characteristics | Patients | Controls | p |
|---|---|---|---|
| N = 982 | N = 64 | ||
| Age (years), median (IQR) | 1.92 (0.35–7.57) | 2.00 (0.73–6.66) | 0.28 |
| Age <1 year, n (%) | 402 (40.94) | 25 (39.06) | 0.76 |
| Male sex, n (%) | 567 (57.74) | 37 (57.81) | 0.99 |
| Weight (kg), median (IQR) | 11.75 (5.40–22.00) | ||
| Standard deviation score, median (IQR)a | −0.51 (−1.40 to 0.46) | ||
| Height (cm), median (IQR) | 86 (60–120) | ||
| Standard deviation score, median (IQR)a | −0.30 (−1.38 to 0.77) | ||
| STRONGkids risk level, n (%) | |||
| Medium | 895 (91.14) | ||
| High | 87 (8.86) | ||
| PeLOD score, first 24 hours in PICU, median (IQR) | 22 (12–32) | ||
| PIM2 score, median (IQR) | −2.75 (−3.65 – −1.46) | ||
| PIM2-calculated risk of death (%), median (IQR) | 0.06 (0.03–0.19) | ||
| Emergency admission, n (%) | 424 (43.18) | ||
| Diagnostic group, n (%) | |||
| Type of illness | |||
| Surgical | |||
| Abdominal | 35 (3.56) | ||
| Burns | 6 (0.61) | ||
| Cardiac | 480 (48.88) | ||
| Neurosurgery-traumatic brain injury | 95 (9.67) | ||
| Thoracic | 38 (3.87) | ||
| Transplantation | 18 (1.83) | ||
| Orthopedic surgery-trauma | 49 (4.99) | ||
| Other | 21 (2.14) | ||
| Medical | |||
| Cardiac | 32 (3.26) | ||
| Gastrointestinal-hepatic | 2 (0.20) | ||
| Oncologic-hematologic | 8 (0.81) | ||
| Neurologic | 53 (5.40) | ||
| Renal | 1 (0.10) | ||
| Respiratory | 88 (8.96) | ||
| Other | 56 (5.70) | ||
| Condition on admission, n (%) | |||
| Mechanical ventilation required | 882 (89.82) | ||
| ECMO or other assist device required | 33 (3.36) | ||
| Infection | 328 (33.40) | ||
| Outcomes | |||
| 90-day mortality, n (%) | 65 (6.62) | ||
| Length of PICU stay (days), median (IQR) | 3 (2–7) | ||
| New infection during PICU stay, n (%) | 143 (14.56) | ||
| Thyroid hormones upon PICU admission, median (IQR) | |||
| TSH (mIU/L) | 1.74 (0.91–3.26) | 2.61 (1.93–3.52) | <0.0001 |
| T4 (nmol/L) | 59.56 (44.67–78.30) | 98.02 (85.94–110.33) | <0.0001 |
| T3 (nmol/L) | 1.15 (0.84–1.55) | 2.38 (2.04–2.66) | <0.0001 |
| rT3 (nmol/L) | 0.42 (0.23–0.73) | 0.24 (0.19–0.30) | <0.0001 |
| T3/rT3 | 2.93 (1.35–5.91) | 9.39 (7.47–13.67) | <0.0001 |
Age- and sex-specific standard deviation scores were calculated with the use of reference data from the World Health Organization growth charts: www.bcchildrens.ca/Services/SpecializedPediatrics/EndocrinologyDiabetesUnit/ForProfessionals/AnthropometricCalculators.htm
ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; PeLOD, Pediatric Logistic Organ Dysfunction score; PICU, pediatric intensive care unit; PIM2, Pediatric Risk of Mortality 2 score; STRONGkids, Screening Tool for Risk On Nutritional Status and Growth (score of 0 indicating a low risk of malnutrition, a score of 1–3 indicating medium risk, and a score of 4–5 indicating high risk); TSH, thyrotropin; T4, thyroxine; T3, triiodothyronine; rT3, reverse triiodothyronine.
As a group, critically ill children presented upon PICU admission with low serum concentrations of TSH, T4, and T3 and high rT3 compared to healthy children, resulting in a low T3/rT3 ratio (Table 1). The critical illness–induced rise in rT3 was more pronounced among infants and resulted in a lower T3/rT3 ratio than among older children (Fig. 2).
FIG. 2.
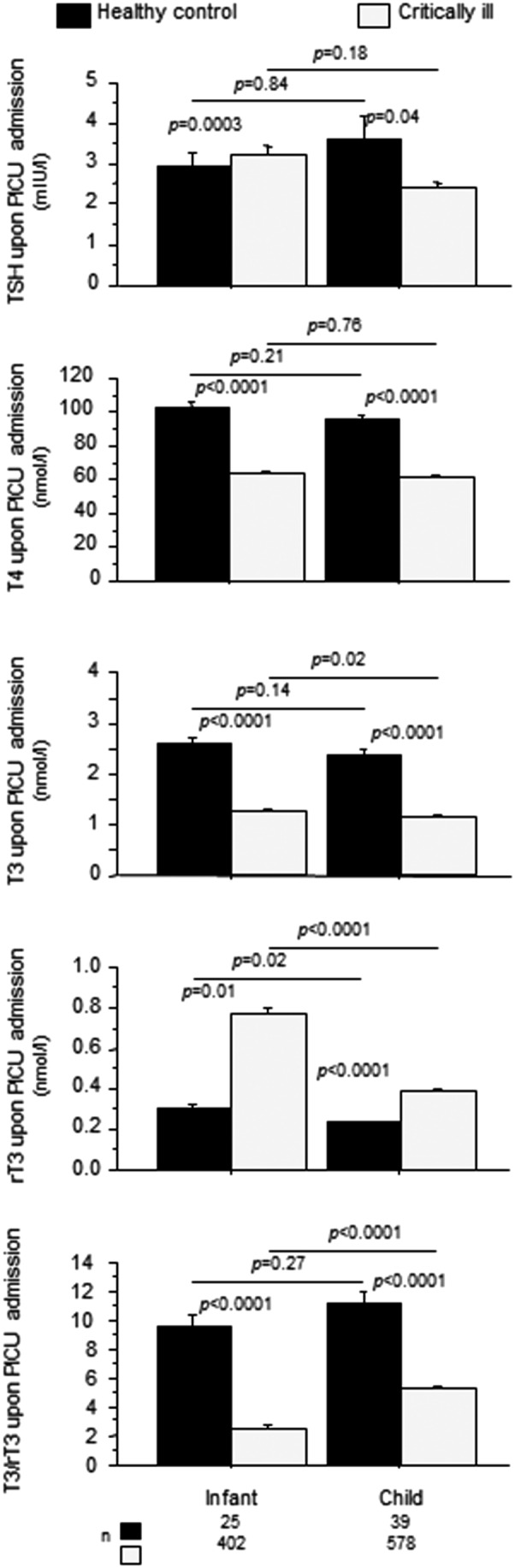
Impact of critical illness on thyroid hormone concentrations upon PICU admission in infants and children. Infants are younger than one year old. Bars represent means, and whiskers represent the standard error (SE). The black boxplots represent healthy children, and the light-gray boxes represent critically ill patients.
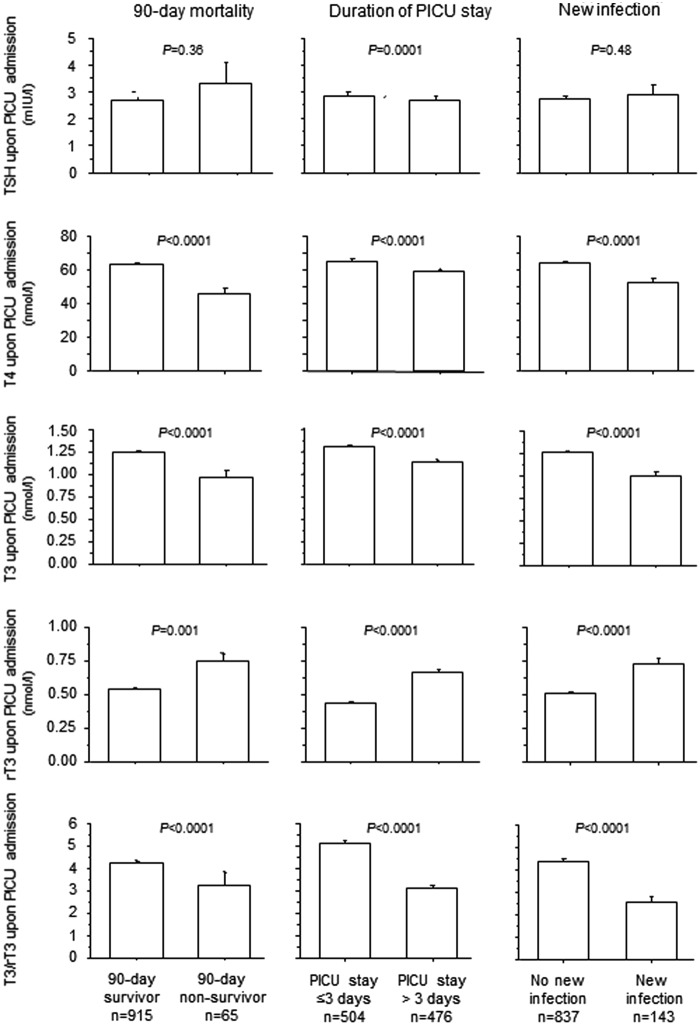
In univariable analysis, a lower TSH upon admission was only associated with a PICU stay longer than the median of three days, but no correlation was found with PICU stay as a continuous variable (p = 0.79). A lower T4, T3, and T3/rT3 ratio and a higher rT3 upon admission were associated with a higher mortality at 90 days, a longer PICU stay, and the acquisition of a new infection (Fig. 3).
FIG. 3.
Univariable analyses for the association between upon admission thyroid hormones and outcome. Bars represent means, and whiskers represent the SE.
In multivariable analyses including all thyroid hormones significantly associated with the outcomes in univariable analysis, a lower T4 and higher rT3 were systematically associated with unfavorable outcomes, whereas a lower T3/rT3 was only associated with a longer PICU stay and a higher risk of acquiring a new infection. The performance of the models adjusted for the thyroid hormones was low compared to that of the models for the respective outcomes adjusted for the baseline risk factors treatment center, risk of malnutrition, age, type and severity of illness, as reflected by lower R2 of the former models (Table 2). Model performance slightly improved when combining the baseline risk factors with the thyroid hormones upon PICU admission. Adjusted for baseline risk factors, a lower T4 upon admission was independently associated with a higher risk of death at 90 days and a higher risk of acquiring a new infection. A lower T3/rT3 ratio was independently associated with a longer PICU stay and a higher risk of a new infection.
Table 2.
Multivariable Analyses of Predictive Value for Outcome of Thyroid Hormone Concentrations Upon PICU Admission
| 90-day mortality | Length of PICU stay | Acquisition of a new infection | ||||
|---|---|---|---|---|---|---|
| Model 1: baseline risk factors only | R2 = 0.358 | R2 = 0.081 | R2 = 0.091 | |||
| Model 2: upon admission thyroid hormones only | R2 = 0.085 | R2 = 0.038 | R2 = 0.065 | |||
| OR [CI] | p | β estimate [CI] | p | OR [CI] | p | |
| Admission T3 | 1.002 [0.470–2.224] | 0.95 | 9.228 [−2.385 to 5.143] | 0.47 | 1.197 [0.693–2.066] | 0.52 |
| Admission T4 | 0.968 [0.953–0.983] | <0.0001 | −0.049 [−6.995 to 1.032] | 0.008 | 0.983 [0.973–0.993] | 0.0006 |
| Admission rT3 | 2.383 [1.384–4.104] | 0.001 | 2.347 [0.989–10.529] | 0.01 | 1.725 [1.095–2.717] | 0.01 |
| Admission T3/rT3 | 1.051 [0.960–1.151] | 0.29 | −0.298 [−7.052 to 0.441] | 0.02 | 0.898 [0.815–0.989] | 0.01 |
| Model 3: baseline risk factors and thyroid hormones | R2 = 0.398 | R2 = 0.156 | R2 = 0.154 | |||
|---|---|---|---|---|---|---|
| OR [CI] | p | β estimate [CI] | p | OR [CI] | p | |
| Admission T3 | 1.847 [0.611–5.584] | 0.28 | 0.865 [−2.428 to 5.768] | 0.42 | 1.425 [0.736–2.759] | 0.29 |
| Admission T4 | 0.972 [0.953–0.992] | 0.004 | −0.030 [−5.563 to 0.679] | 0.12 | 0.987 [0.976–0.998] | 0.02 |
| Admission rT3 | 1.528 [0.727–3.213] | 0.29 | −0.655 [−6.830 to 3.616] | 0.54 | 1.359 [0.815–2.266] | 0.24 |
| Admission T3/rT3 | 0.885 [0.775–1.011] | 0.06 | −0.349 [−7.807 to 0.978] | 0.01 | 0.850 [0.763–0.947] | 0.0009 |
Baseline risk factors: treatment center, risk of malnutrition (STRONGkids score), age, diagnosis upon admission, severity of illness (PeLOD and PIM2 score). Statistically significant values are shown in bold.
CI, confidence interval; OR, odds ratio.
Effect of late-PN versus early-PN on thyroid hormone concentrations
The propensity score–matched subgroups of 386 late-PN and 386 early-PN patients, selected to evaluate the differences between late-PN and early-PN (Fig. 1), were comparable for baseline characteristics (Table 3). According to the study protocol, caloric intake was lower in the late-PN group than in the early-PN group (Fig. 4).
Table 3.
Baseline Characteristics of the Propensity Score–Matched Patients Selected to Evaluate the Effect of Late-PN Versus Early-PN on Thyroid Hormone Concentrations
| Early-PN | Late-PN | p | |
|---|---|---|---|
| Baseline characteristics | N = 386 | N = 386 | |
| Age (years), median (IQR) | 2.05 (0.38–7.26) | 2.08 (0.38–7.92) | 0.63 |
| Age <1 year, n (%) | 159 (41.2) | 159 (41.2) | >0.99 |
| Age <4 weeks, n (%) | 29 (7.51) | 31 (8.03) | 0.78 |
| Male sex, n (%) | 217 (56.2) | 230 (59.6) | 0.34 |
| Weight (kg), median (IQR) | 11.60 (5.61–22.00) | 12.00 (5.58–23.13) | 0.60 |
| Standard deviation score, median (IQR)a | −0.56 (−1.45 to 0.42) | −0.52 (−1.50 to 0.41) | 0.62 |
| Height (cm), median (IQR) | 85.50 (61.00–118.00) | 88.00 (60.75–122.00) | 0.54 |
| Standard deviation score, median (IQR)a | −0.37 (−1.59 to 0.57) | −0.34 (−1.28 to 0.67) | 0.35 |
| STRONGkids risk level, n (%) | 0.55 | ||
| Medium | 364 (94.3) | 360 (93.3) | |
| High | 22 (5.7) | 26 (6.7) | |
| PeLOD score, first 24 hours in PICU, median (IQR) | 23 (21–32) | 31 (21–32) | 0.81 |
| PIM2 score, median (IQR) | −2.89 (−3.72 to −1.51) | −2.84 (−3.68 to −1.64) | 0.94 |
| PIM2-calculated risk of death (%), median (IQR) | 0.05 (0.02–0.18) | 0.05 (0.02–0.16) | 0.94 |
| Emergency admission, n (%) | 134 (34.72) | 152 (39.38) | 0.17 |
| Diagnostic group, n (%) | 0.60 | ||
| Surgical | |||
| Abdominal | 10 (2.6) | 8 (2.1) | |
| Burns | 1 (0.3) | 2 (0.5) | |
| Cardiac | 215 (55.7) | 209 (54.2) | |
| Neurosurgery-traumatic brain injury | 39 (10.1) | 32 (8.3) | |
| Thoracic | 14 (3.6) | 16 (4.2) | |
| Transplantation | 5 (1.3) | 10 (2.6) | |
| Orthopedic surgery-trauma | 24 (6.2) | 20 (5.2) | |
| Other | 7 (1.8) | 10 (2.6) | |
| Medical | |||
| Cardiac | 11 (2.9) | 12 (3.1) | |
| Gastrointestinal-hepatic | 1 (0.3) | 1 (0.3) | |
| Oncologic-hematologic | 0 (0.0) | 5 (1.3) | |
| Neurologic | 16 (4.2) | 17 (4.4) | |
| Renal | 0 (0) | 0 (0) | |
| Respiratory | 24 (6.2) | 25 (6.5) | |
| Other | 19 (4.9) | 19 (4.9) | |
| Condition on admission, n (%) | |||
| Mechanical ventilation required | 351 (90.9) | 346 (89.6) | 0.54 |
| ECMO or other assist device required | 8 (2.1) | 10 (2.6) | 0.63 |
| Infection | 112 (29.0) | 108 (28.0) | 0.74 |
| Outcomes | |||
| 90-day mortality, n (%) | 24 (6.22) | 16 (4.15) | 0.19 |
| Length of PICU stay (days), median (IQR) | 3 (2–7) | 3 (1.75–6) | 0.11 |
| New infection during PICU stay, n (%) | 56 (14.51) | 37 (9.59) | 0.03 |
| Thyroid hormones upon PICU admission, median (IQR) | |||
| TSH (mIU/L) | 1.98 (1.04–3.46) | 1.79 (0.94–3.23) | 0.21 |
| T4 (nmol/L) | 58.30 (44.32–75.73) | 60.98 (45.91–79.55) | 0.28 |
| T3 (nmol/L) | 1.18 (0.87–1.58) | 1.18 (0.86–1.53) | 0.95 |
| rT3 (nmol/L) | 0.39 (0.22–0.67) | 0.41 (0.21–0.70) | 0.57 |
| T3/rT3 ratio | 3.29 (1.53–6.05) | 3.01 (1.49–6.19) | 0.52 |
Statistically significant values are shown in bold.
Age- and sex-specific standard deviation scores were calculated with the use of reference data from the World Health Organization growth charts: www.bcchildrens.ca/Services/SpecializedPediatrics/EndocrinologyDiabetesUnit/ForProfessionals/AnthropometricCalculators.htm
PN, parenteral nutrition.
FIG. 4.
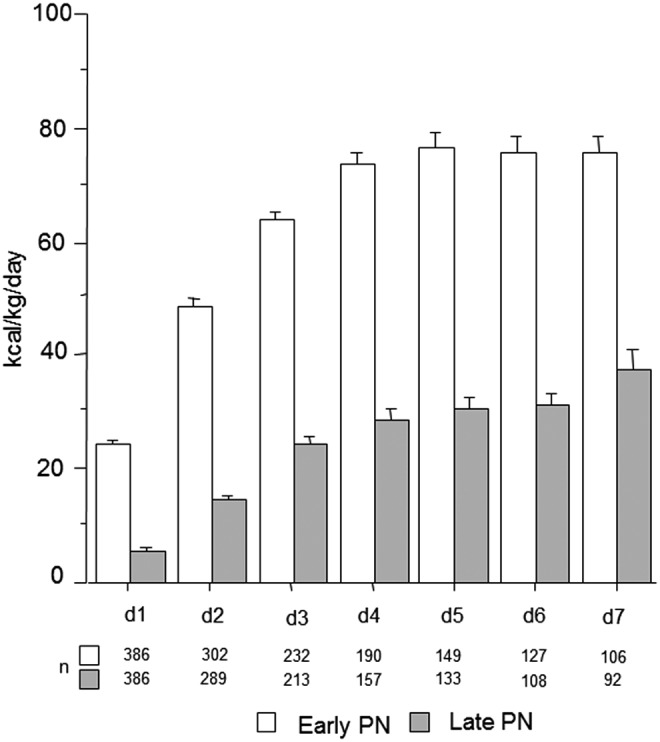
Total daily caloric intake of the patients in the propensity score matched subgroup. Bars show the mean daily amount of energy (kilocalories/kg/day) provided by the combination of the enteral and parenteral route, with whiskers representing the SE. The open and filled bars represent the patients randomized to the early-PN and late-PN groups, respectively.
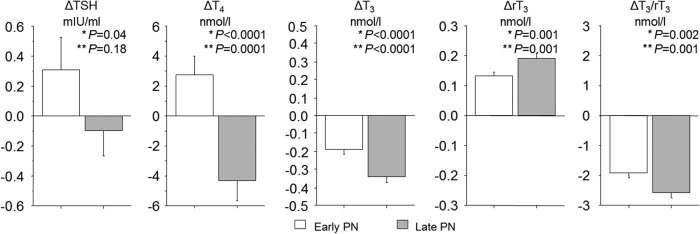
The serum concentrations of TSH, T4, T3, and rT3 and the T3/rT3 ratio determined at admission were comparable to those in the total population and were not different for the late-PN and early-PN groups (Table 3). In univariable analysis, late-PN further lowered serum TSH concentrations between admission and day 3 or last PICU day compared to the early-PN group (p = 0.04). However, in multivariable analysis adjusting for baseline risk factors, this effect was no longer significant (p = 0.18; Fig. 5). Late-PN also lowered serum T4 compared to the early-PN group, both in univariable analysis (p < 0.0001) and after adjustment for risk factors in the multivariable model (p = 0.0001). Late-PN also further lowered the T3/rT3 ratio (univariable p = 0.002; multivariable p = 0.001) due to both a lowering of T3 (univariable and multivariable p < 0.0001) and a rise in rT3 (univariable and multivariable p = 0.001). The impact of late-PN versus early-PN on the changes from baseline in T4, T3, and rT3 and in the T3/rT3 ratio was larger among children than among infants (Supplementary Fig. S1).
FIG. 5.
Effect of early-PN versus late-PN on the thyroid axis. Bars (mean ± SE) represent the changes (referred to as Δ) from the admission values to day 3 in the PICU (or to the last day for patients with shorter PICU stay) in serum thyrotropin (TSH), thyroxine (T4), triiodothyronine (T3), reverse T3 (rT3), and the T3/rT3 ratio. The open and filled bars represent the patients randomized to the early-PN and late-PN groups, respectively. Infants are younger than one year old. *p-Values obtained with univariable analysis; **p-values obtained with multivariable analysis after adjustment for baseline risk factors (treatment center, risk of malnutrition [STRONGkids score], age, diagnosis upon admission, and severity of illness [PeLOD and PIM2 score]). PeLOD, Pediatric Logistic Organ Dysfunction score; PIM2, Pediatric Risk of Mortality 2 score; STRONGkids, Screening Tool for Risk On Nutritional Status and Growth (score of 0 indicating a low risk of malnutrition, a score of 1–3 indicating medium risk, and a score of 4–5 indicating high risk).
Role of the changes in thyroid hormones in explaining the outcome benefit of late-PN
In the propensity score–matched subset, similarly to what has been reported previously for the total PEPaNIC cohort (23), patients in the late-PN group had a higher likelihood of being discharged earlier from PICU than patients in the early-PN group (hazard ratio [HR] = 1.173 [CI 1.013–1.357]; p = 0.03) and had a lower risk of acquiring a new infection (OR = 0.571 [CI 0.347–0.925]; p = 0.02; Table 4).
Table 4.
Role of Independent Associations of the Changes from Baseline in the Thyroid Hormones with Clinical Outcome in Explaining the Outcome Benefit with Late-PN
| HR or OR [CI] | p | |
|---|---|---|
| Time to discharge from PICU | ||
| 1. Randomization to late-PN versus early-PN | 1.173 [1.013–1.357] | 0.03 |
| 2. Randomization to late-PN versus early-PN | 1.209 [1.042–1.401] | 0.01 |
| ΔT4 (per nmol/L added) | 1.010 [1.007–1.013] | <0.0001 |
| ΔT3/rT3 (per unit added) | 0.945 [0.921–0.969] | <0.0001 |
| Risk of new infection | ||
| 1. Randomization to late-PN versus early-PN | 0.571 [0.350–0.930] | 0.02 |
| 2. Randomization to late-PN versus early-PN | 0.539 [0.325–0.892] | 0.01 |
| ΔT4 (per nmol/L added) | 0.978 [0.968–0.989] | <0.0001 |
| ΔT3/rT3 (per unit added) | 1.148 [1.037–1.270] | 0.004 |
Model 1: Model adjusted for the baseline risk factors treatment center, risk of malnutrition (STRONGkids score), age, diagnosis upon admission, and severity of illness (PeLOD and PIM2 score). Model 2: The changes from baseline of the thyroid hormones that were significantly affected by late-PN in multivariable analyses were added to model 1 to study their independent association with outcome and to assess any potential implication in explaining the clinical outcome benefit with late-PN. Statistically significant values are shown in bold.
HR, hazard ratio.
To investigate whether the observed acute changes in thyroid hormones by late-PN statistically explained (part of) these benefits, the change from baseline in T4 (reflecting the impact of late-PN on the central component of the thyroid axis) and in the T3/rT3 ratio (reflecting the impact of late-PN on the peripheral conversion) were added to the multivariable Cox proportional hazard and logistic regression models. This somewhat increased the size of the effect of late-PN on the likelihood of an earlier discharge from PICU to a HR of 1.209 ([CI 1.042–1.401]; p = 0.01; Table 4). In the adjusted model, a rise in T4 from admission to day 3 or last PICU day was independently associated with a higher likelihood of being discharged from PICU (HR = 1.010 [CI 1.007–1.013]) per nmol/L increase; p < 0.0001), whereas a rise in the T3/rT3 ratio was independently associated with a lower likelihood (HR = 0.945 [CI 0.921–0.969] per unit increase; p < 0.0001).
With regard to acquisition of a new infection, addition of the observed changes in thyroid hormones by late-PN to the multivariable model slightly increased the effect size of late-PN on the risk of acquiring a new infection (OR = 0.539 [CI 0.323–0.887]; p = 0.01; Table 4). In the adjusted model, a rise in T4 was independently associated with a lower risk of infection (OR = 0.978 [CI 0.967–0.989] per nmol/L increase; p < 0.0001), whereas a rise in the T3/rT3 ratio was independently associated with a higher risk of new infection (OR = 1.148 [CI 1.042–1.277] per unit increase; p = 0.004).
Finally, a significant interaction was found between the change from baseline in the T3/rT3 ratio and the age groups (infants and children) in relation to the outcomes affected by late-PN. Indeed, the contribution of a lowered T3/rT3 ratio to a higher likelihood of an earlier discharge from PICU (interaction p = 0.04) and to a decreased risk of new infection (interaction p = 0.01) was larger for infants than for older children (HR per unit T3/rT3 ratio increase in infants of 0.873 [CI 0.827–0.925] and 0.968 [CI 0.940–0.997] in older children for time to PICU discharge; OR per unit increase in T3/rT3 ratio of 1.601 [CI 1.119–2.346] in infants and 1.057 [CI 0.954–1.183] in older children for acquiring a new infection). No such interaction with age was observed for the change from baseline in T4.
Discussion
In a large group of critically ill children admitted to the PICU after various life-threatening insults, first the development of NTI was confirmed, with its severity being predictive for adverse clinical outcomes. Second, it was demonstrated that the severity of NTI is affected by the nutritional management, since children for whom low or no macronutrient intake was accepted during the first week of critical illness showed a further drop in T4 and T3 concentrations and in the T3/rT3 ratio, while rT3 increased further compared to children who received early full nutrition. The accentuated decrease in T4, reflecting the central component of NTI, appeared to counteract the outcome benefit of late-PN in terms of early discharge from PICU and risk of new infection. In contrast, the peripheral inactivation of thyroid hormone, reflected in the further decline in the T3/rT3 ratio, statistically explained part of the outcome benefit of late-PN. Interestingly, whereas the reduction in the T3/rT3 ratio was larger in older children than in infants, the contributory effect on outcome of a decrease in the T3/rT3 ratio was more pronounced for critically ill infants than it was for older children.
The presence of NTI with a decrease in T4, T3, and T3/rT3 and an increase in rT3 and its association with adverse short-term outcomes in this large cohort of almost 1000 critically ill children confirmed the findings of earlier smaller studies (4–7,16–18). In multivariable analysis adjusted for baseline risk factors, the association between T4 and/or T3/rT3 at admission remained significant. By adding the thyroid hormones at admission to the baseline risk factors, the performance of the models slightly increased, which suggests a contributing effect of T4 and/or T3/rT3 at admission to the prediction of clinical outcomes, in addition to the other baseline risk factors.
Fasting in healthy individuals decreases serum concentrations of thyroid hormones. A decrease in serum concentrations of leptin and downregulation of hypothalamic TRH neurons are involved, contributing to persistently low serum TSH concentrations (32,33). Furthermore, reduced D1 activity and increased D3 activity with fasting reduce the conversion of T4 to active T3 while favoring formation and hampering clearance of inactive rT3 (32,34). Accepting low or no macronutrient intake during the first week in PICU, thus mimicking (virtual) fasting superimposed on the illness, aggravated NTI in critically ill children, reflected by a further drop in T4, T3, and T3/rT3 and a further rise in rT3. This is in agreement with findings in critically ill adult patients (24,35–37). A small observational study of PICU patients, all fed according to a similar enteral/parenteral feeding strategy, could not find an association between the amount of nutrients delivered and the degree of NTI (38). However, the impact of early nutrition on thyroid hormone parameters in critically ill children had not been previously investigated in the context of a RCT. Interestingly, under early supplemental PN, further accentuation of the peripheral inactivation of thyroid hormone was also observed with tight glycemic control to age-adjusted normal fasting glycemia in critically ill children, which was found to reduce morbidity and mortality in a large RCT of PICU patients (39). Indeed, this intervention further decreased T3 and further increased rT3 during the first days of critical illness, though without an effect on T4 (18). Hence, this intervention partly mimicked the fasting response of the thyroid axis, an effect that was interpreted as being caused by the fasting levels of blood glucose achieved by tight glycemic control. The low or absent macronutrient intake that was accepted with the late-PN strategy in the present study, combined with lower blood glucose concentrations under glycemic control, had a greater impact, with an additional aggravation of NTI at a central level (i.e., resulting in a further lowering of T4).
Statistical analysis suggests that the more pronounced suppression of the central component of NTI in the condition of low/absent macronutrient intake, with further lowering of T4 from baseline, might be deleterious. Indeed, this effect statistically counteracted the outcome benefit of early macronutrient restriction with regard to likelihood of earlier PICU discharge and risk of acquiring a new infection in the PICU. This is in line with the rise in T4 achieved by TRH infusion during a five-day period combined with a GH secretagogue in prolonged critically ill adults. This intervention evoked an anabolic/anti-catabolic response in muscle and bone, in which the normalization of T4 played a key role (40). However, in the sickest individuals in this previous study, the combined secretagogue infusion increased not only T3 but also rT3 concentrations, which suggests that the distinction between the central and the peripheral component may not be absolute. In contrast, the peripheral component with more pronounced inactivation of T3 to rT3 might be a beneficial adaptation, as it statistically contributed to the outcome benefit of the intervention. This contribution was more pronounced with regard to the lower risk of acquiring a new infection compared to the higher likelihood of an earlier live PICU discharge. In theory, lowered energy expenditure with lower T3 availability brought about by the lack of macronutrients may have contributed to faster recovery (41). Interestingly, the lower risk of infection associated with a lowered T3/rT3 ratio could be explained by optimized bacterial killing capacity mediated by increased D3 activity and elevated rT3 locally within granulocytes (42). However, recent research also pointed to an anti-inflammatory effect of low intracellular T3 action in macrophages (43). Hence, one could speculate that the increased rT3 in particular, rather than the decreased T3, may have contributed to the lowered infection risk with not using early-PN in the PICU.
Interestingly, compared to older children, infants showed a more pronounced peripheral inactivation of thyroid hormone upon PICU admission, resulting in a much lower T3/rT3 ratio, whereas the further decrease in T3/rT3 toward day 3 or last PICU day was less pronounced. However, the contributing effect of this peripheral thyroid hormone inactivation to an earlier discharge from PICU and decreased risk of new infection with late-PN was greater for infants than it was for older children. These findings might indicate a more appropriate response to the acute stress of critical illness in infants than in older children. Several explanations may be invoked, though they remain speculative. Evolutionary, infants may need a stronger fasting response as part of a survival strategy, since they are entirely dependent on external help for nutrient provision. This is in line with the faster increase in ketone body concentrations and a faster decrease in glucose concentrations in response to fasting in healthy younger children compared to older children (44). Furthermore, the pronounced peripheral inactivation of T3 might be related to a more efficient postnatal reactivation of D3 expression during critical illness, considering that fetal and placental D3 levels are physiologically high to protect against maternal thyroid hormone (45). Finally, the targets for age-adjusted normal blood glucose concentrations, particularly in the Leuven center (23), which are lower for infants than for older children (46), or a larger difference in total macronutrient intake between the early-PN and late-PN groups in infants versus older children may theoretically play a role, since they might mimic a more pronounced fasting response in infants compared to older children. The different thyroidal axis responses to critical illness in different age groups stress the importance of this distinction for further research.
The present findings suggest important clinical implications, since the distinction of the changes in the thyroid axis during critical illness as beneficial or harmful is embedded in the controversy about whether to treat NTI (47,48). If NTI is harmful, hormone substitution (central or peripherally) might improve clinical outcomes. However, if NTI reflects a beneficial adaptation of the critically ill body to reduce energy expenditure and to prevent infections, treatment could be deleterious. In several small RCTs, T3 treatment of critically ill adult patients did not improve short-term outcomes or alter mortality (49–51), nor did treatment with T4 (52). T3 infusion in critically ill children undergoing surgery for congenital heart disease appeared to have a slight positive inotropic effect on cardiac function but did not improve other clinical outcomes either (53–56). The lack of efficacy of T3 treatment might be due to the iatrogenic suppression of TSH secretion and hereby lowering of the T4 availability, which could hamper fast normalization of thyroid function upon recovery (52). Moreover, the administration of thyroid hormone is potentially harmful, as excessive levels may lead to coronary ischemia, myocardial infarction, hypertension, arrhythmia, and death (54,55,57,58). Although hypothyroidism in children specifically raises concerns about long-term neurodevelopment and growth (59,60), prophylactic thyroid hormone therapy given to preterm infants (58,61) and to children after cardiopulmonary bypass (62) with NTI did not improve neurodevelopmental outcomes. The lack of efficacy of T3 treatment is in line with the present study's finding that the peripheral conversion of T3 to rT3 may be a beneficial adaptation to critical illness in children. Since the present study suggests that the central component of NTI may not be beneficial, TRH infusion as treatment could be considered rather than treatment with thyroid hormone. This treatment could also be safer, as the negative feedback exerted by thyroid hormones on the TSH-producing pituitary cells is maintained, and thereby excessively elevated thyroid hormone levels are avoided (12,63). However, RCTs evaluating TRH treatment in critically ill adults and children with NTI are needed to ascertain whether this translates to improved clinically relevant outcomes.
This study has some limitations. First, because blood samples were taken via heparinized lines, it was not possible to quantify free hormone concentrations, since these would be distorted by the heparin (27). Second, as accepting low/no macronutrient intake early during critical illness accelerated recovery, only the short-term impact of nutritional management on changes in thyroid hormones in the first few days of critical illness was studied. This was necessary, as otherwise the findings could have been biased, since no samples were collected from recovered patients after PICU discharge. Finally, although the statistical models point to a potentially harmful central and potentially beneficial peripheral component of NTI in critically ill children, the biological mechanisms for such effects remain to be further investigated.
In conclusion, accepting low or even absent macronutrient intake early during critical illness in children further aggravated NTI compared to early full feeding. The effect on the peripheral but not the central component of NTI with accentuated conversion of T3 into rT3 explains part of the outcome benefit of accepting such virtual fasting during critical illness, and thus appears to reflect a beneficial adaptation in this condition.
Acknowledgments
We acknowledge the work of the research nurses and nurses and doctors of the PICU who collected and entered the data. We would also like to thank the healthy children, the patients admitted to the PICU, and their parents for participating in the study.
Author Disclosure Statement
S.V. received funding from the Research Foundation, Flanders (FWO) as a PhD fellow. S.V. received funding from Fonds NutsOhra, Sophia Research Foundation (SSWO), Stichting Agis Zorginnovatie, and the Erasmus Trustfonds. I.V., L.L., and G.V.d.B. received funding from the Methusalem program of the Flemish Government (Belgium, METH14/06). G.V.d.B. further received funding from the Flemish Agency for Innovation through Science and Technology (IWT-TBM110685) and the European Research Council (Advanced Grant AdvG-2012-321670 & AdvG-2017-785809). The remaining authors state that they do not have any potential conflicts of interest.
Supplementary Material
References
- 1. Faber J, Kirkegaard C, Rasmussen B, Westh H, Busch-Sorensen M, Jensen IW. 1987. Pituitary–thyroid axis in critical illness. J Clin Endocrinol Metab 65:315–320 [DOI] [PubMed] [Google Scholar]
- 2. Murzi B, Iervasi G, Masini S, Moschetti R, Vanini V, Zucchelli G, Biagini A. 1995. Thyroid hormones homeostasis in pediatric patients during and after cardiopulmonary bypass. Ann Thorac Surg 59:481–485 [DOI] [PubMed] [Google Scholar]
- 3. Van den Berghe G, de Zegher F, Lauwers P. 1994. Dopamine suppresses pituitary function in infants and children. Crit Care Med 22:1747–1753 [PubMed] [Google Scholar]
- 4. Uzel N, Neyzi O. 1986. Thyroid function in critically ill infants with infections. Pediatr Infect Dis 5:516–519 [DOI] [PubMed] [Google Scholar]
- 5. Allen DB, Dietrich KA, Zimmerman JJ. 1989. Thyroid hormone metabolism and level of illness severity in pediatric cardiac surgery patients. J Pediatr 114:59–62 [DOI] [PubMed] [Google Scholar]
- 6. Bettendorf M, Schmidt KG, Tiefenbacher U, Grulich-Henn J, Heinrich UE, Schonberg DK. 1997. Transient secondary hypothyroidism in children after cardiac surgery. Pediatr Res 41:375–379 [DOI] [PubMed] [Google Scholar]
- 7. den Brinker M, Dumas B, Visser TJ, Hop WC, Hazelzet JA, Festen DA, Hokken-Koelega AC, Joosten KF. 2005. Thyroid function and outcome in children who survived meningococcal septic shock. Intensive Care Med 31:970–976 [DOI] [PubMed] [Google Scholar]
- 8. Peeters RP, Wouters PJ, Kaptein E, van Toor H, Visser TJ, Van den Berghe G. 2003. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab 88:3202–3211 [DOI] [PubMed] [Google Scholar]
- 9. Rodriguez-Perez A, Palos-Paz F, Kaptein E, Visser TJ, Dominguez-Gerpe L, Alvarez-Escudero J, Lado-Abeal J. 2008. Identification of molecular mechanisms related to nonthyroidal illness syndrome in skeletal muscle and adipose tissue from patients with septic shock. Clin Endocrinol (Oxf) 68:821–827 [DOI] [PubMed] [Google Scholar]
- 10. Michalaki M, Vagenakis AG, Makri M, Kalfarentzos F, Kyriazopoulou V. 2001. Dissociation of the early decline in serum T(3) concentration and serum IL-6 rise and TNFalpha in nonthyroidal illness syndrome induced by abdominal surgery. J Clin Endocrinol Metab 86:4198–4205 [DOI] [PubMed] [Google Scholar]
- 11. Shih JL, Agus MS. 2009. Thyroid function in the critically ill newborn and child. Curr Opin Pediatr 21:536–540 [DOI] [PubMed] [Google Scholar]
- 12. Van den Berghe G, de Zegher F, Baxter RC, Veldhuis JD, Wouters P, Schetz M, Verwaest C, Van der Vorst E, Lauwers P, Bouillon R, Bowers CY. 1998. Neuroendocrinology of prolonged critical illness: effects of exogenous thyrotropin-releasing hormone and its combination with growth hormone secretagogues. J Clin Endocrinol Metab 83:309–319 [DOI] [PubMed] [Google Scholar]
- 13. Fliers E, Guldenaar SE, Wiersinga WM, Swaab DF. 1997. Decreased hypothalamic thyrotropin-releasing hormone gene expression in patients with nonthyroidal illness. J Clin Endocrinol Metab 82:4032–4036 [DOI] [PubMed] [Google Scholar]
- 14. Rothwell PM, Udwadia ZF, Lawler PG. 1993. Thyrotropin concentration predicts outcome in critical illness. Anaesthesia 48:373–376 [DOI] [PubMed] [Google Scholar]
- 15. Peeters RP, Wouters PJ, van Toor H, Kaptein E, Visser TJ, Van den Berghe G. 2005. Serum 3,3′,5′-triiodothyronine (rT3) and 3,5,3′-triiodothyronine/rT3 are prognostic markers in critically ill patients and are associated with postmortem tissue deiodinase activities. J Clin Endocrinol Metab 90:4559–4565 [DOI] [PubMed] [Google Scholar]
- 16. Marks SD, Haines C, Rebeyka IM, Couch RM. 2009. Hypothalamic–pituitary–thyroid axis changes in children after cardiac surgery. J Clin Endocrinol Metab 94:2781–2786 [DOI] [PubMed] [Google Scholar]
- 17. Angelousi AG, Karageorgopoulos DE, Kapaskelis AM, Falagas ME. 2011. Association between thyroid function tests at baseline and the outcome of patients with sepsis or septic shock: a systematic review. Eur J Endocrinol 164:147–155 [DOI] [PubMed] [Google Scholar]
- 18. Gielen M, Mesotten D, Wouters PJ, Desmet L, Vlasselaers D, Vanhorebeek I, Langouche L, Van den Berghe G. 2012. Effect of tight glucose control with insulin on the thyroid axis of critically ill children and its relation with outcome. J Clin Endocrinol Metab 97:3569–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boelen A, Wiersinga WM, Fliers E. 2008. Fasting-induced changes in the hypothalamus–pituitary–thyroid axis. Thyroid 18:123–129 [DOI] [PubMed] [Google Scholar]
- 20. Kerklaan D, Fivez T, Mehta NM, Mesotten D, van Rosmalen J, Hulst JM, Van den Berghe G, Joosten KF, Verbruggen SC. 2016. Worldwide survey of nutritional practices in PICUs. Pediatr Crit Care Med 17:10–18 [DOI] [PubMed] [Google Scholar]
- 21. Mehta NM, McAleer D, Hamilton S, Naples E, Leavitt K, Mitchell P, Duggan C. 2010. Challenges to optimal enteral nutrition in a multidisciplinary pediatric intensive care unit. JPEN J Parenter Enteral Nutr 34:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, Vlasselaers D, Debaveye Y, Desmet L, Dubois J, Van Assche A, Vanderheyden S, Wilmer A, Van den Berghe G. 2011. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 365:506–517 [DOI] [PubMed] [Google Scholar]
- 23. Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I, Debaveye Y, Vlasselaers D, Desmet L, Casaer MP, Garcia Guerra G, Hanot J, Joffe A, Tibboel D, Joosten K, Van den Berghe G. 2016. Early versus late parenteral nutrition in critically ill children. N Engl J Med 374:1111–1122 [DOI] [PubMed] [Google Scholar]
- 24. Langouche L, Vander Perre S, Marques M, Boelen A, Wouters PJ, Casaer MP, Van den Berghe G. 2013. Impact of early nutrient restriction during critical illness on the nonthyroidal illness syndrome and its relation with outcome: a randomized, controlled clinical study. J Clin Endocrinol Metab 98:1006–1013 [DOI] [PubMed] [Google Scholar]
- 25. Mehta NM, Skillman HE, Irving SY, Coss-Bu JA, Vermilyea S, Farrington EA, McKeever L, Hall AM, Goday PS, Braunschweig C. 2017. Guidelines for the provision and assessment of nutrition support therapy in the pediatric critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. Pediatr Crit Care Med 18:675–715 [DOI] [PubMed] [Google Scholar]
- 26. Fivez T, Kerklaan D, Verbruggen S, Vanhorebeek I, Verstraete S, Tibboel D, Guerra GG, Wouters PJ, Joffe A, Joosten K, Mesotten D, Van den Berghe G. 2015. Impact of withholding early parenteral nutrition completing enteral nutrition in pediatric critically ill patients (PEPaNIC trial): study protocol for a randomized controlled trial. Trials 16:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stockigt JR, Lim CF. 2009. Medications that distort in vitro tests of thyroid function, with particular reference to estimates of serum free thyroxine. Best Pract Res Clin Endocrinol Metab 23:753–767 [DOI] [PubMed] [Google Scholar]
- 28. Hulst JM, Zwart H, Hop WC, Joosten KF. 2010. Dutch national survey to test the STRONGkids nutritional risk screening tool in hospitalized children. Clin Nutr 29:106–111 [DOI] [PubMed] [Google Scholar]
- 29. Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J, Hubert P, Lacroix J, Leclerc F. 2003. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet 362:192–197 [DOI] [PubMed] [Google Scholar]
- 30. Slater A, Shann F, Pearson G, Paediatric Index of Mortality Study G 2003. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 29:278–285 [DOI] [PubMed] [Google Scholar]
- 31. Midi H, Sarkar SK, Rana S. 2010. Collinearity diagnostics of binary logistic regression model. J Interdiscip Math 13:253–267 [Google Scholar]
- 32. Fliers E, Bianco AC, Langouche L, Boelen A. 2015. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol 3:816–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fekete C, Lechan RM. 2014. Central regulation of hypothalamic–pituitary–thyroid axis under physiological and pathophysiological conditions. Endocr Rev 35:159–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mebis L, Eerdekens A, Guiza F, Princen L, Derde S, Vanwijngaerden YM, Vanhorebeek I, Darras VM, Van den Berghe G, Langouche L. 2012. Contribution of nutritional deficit to the pathogenesis of the nonthyroidal illness syndrome in critical illness: a rabbit model study. Endocrinology 153:973–984 [DOI] [PubMed] [Google Scholar]
- 35. Richmand DA, Molitch ME, O'Donnell TF. 1980. Altered thyroid hormone levels in bacterial sepsis: the role of nutritional adequacy. Metabolism 29:936–942 [DOI] [PubMed] [Google Scholar]
- 36. Chourdakis M, Kraus MM, Tzellos T, Sardeli C, Peftoulidou M, Vassilakos D, Kouvelas D. 2012. Effect of early compared with delayed enteral nutrition on endocrine function in patients with traumatic brain injury: an open-labeled randomized trial. JPEN J Parenter Enteral Nutr 36:108–116 [DOI] [PubMed] [Google Scholar]
- 37. Ouchi K, Matsubara S, Matsuno S. 1991. Effects of supplementary parenteral nutrition on thyroid hormone patterns in surgical patients with liver cirrhosis. Nutrition 7:189–192 [PubMed] [Google Scholar]
- 38. Hulst JM, van Goudoever JB, Visser TJ, Tibboel D, Joosten KF. 2006. Hormone levels in children during the first week of ICU-admission: is there an effect of adequate feeding? Clin Nutr 25:154–162 [DOI] [PubMed] [Google Scholar]
- 39. Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G. 2009. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet 373:547–556 [DOI] [PubMed] [Google Scholar]
- 40. Van den Berghe G, Wouters P, Weekers F, Mohan S, Baxter RC, Veldhuis JD, Bowers CY, Bouillon R. 1999. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab 84:1311–1323 [DOI] [PubMed] [Google Scholar]
- 41. Van den Berghe G. 2016. On the neuroendocrinopathy of critical illness. Perspectives for feeding and novel treatments. Am J Respir Crit Care Med 194:1337–1348 [DOI] [PubMed] [Google Scholar]
- 42. Boelen A, Boorsma J, Kwakkel J, Wieland CW, Renckens R, Visser TJ, Fliers E, Wiersinga WM. 2008. Type 3 deiodinase is highly expressed in infiltrating neutrophilic granulocytes in response to acute bacterial infection. Thyroid 18:1095–1103 [DOI] [PubMed] [Google Scholar]
- 43. van der Spek AH, Surovtseva OV, Jim KK, van Oudenaren A, Brouwer MC, Vandenbroucke-Grauls C, Leenen PJM, van de Beek D, Hernandez A, Fliers E, Boelen A. 2018. Regulation of intracellular triiodothyronine is essential for optimal macrophage function. Endocrinology 159:2241–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Veen MR, van Hasselt PM, de Sain-van der Velden MG, Verhoeven N, Hofstede FC, de Koning TJ, Visser G. 2011. Metabolic profiles in children during fasting. Pediatrics 127:e1021–1027 [DOI] [PubMed] [Google Scholar]
- 45. Huang SA. 2005. Physiology and pathophysiology of type 3 deiodinase in humans. Thyroid 15:875–881 [DOI] [PubMed] [Google Scholar]
- 46. Brown TC, Connelly JF, Dunlop ME, McDougall PN, Tibballs J. 1980. Fasting plasma glucose in children. Aust Paediatr J 16:28–29 [DOI] [PubMed] [Google Scholar]
- 47. Utiger RD. 1995. Altered thyroid function in nonthyroidal illness and surgery. To treat or not to treat? N Engl J Med 333:1562–1563 [DOI] [PubMed] [Google Scholar]
- 48. Van den Berghe G. 2000. Euthyroid sick syndrome. Curr Opin Anaesthesiol 13:89–91 [DOI] [PubMed] [Google Scholar]
- 49. Klemperer JD, Klein I, Gomez M, Helm RE, Ojamaa K, Thomas SJ, Isom OW, Krieger K. 1995. Thyroid hormone treatment after coronary-artery bypass surgery. N Engl J Med 333:1522–1527 [DOI] [PubMed] [Google Scholar]
- 50. Becker RA, Vaughan GM, Ziegler MG, Seraile LG, Goldfarb IW, Mansour EH, McManus WF, Pruitt BA, Jr, Mason AD., Jr 1982. Hypermetabolic low triiodothyronine syndrome of burn injury. Crit Care Med 10:870–875 [DOI] [PubMed] [Google Scholar]
- 51. Sirlak M, Yazicioglu L, Inan MB, Eryilmaz S, Tasoz R, Aral A, Ozyurda U. 2004. Oral thyroid hormone pretreatment in left ventricular dysfunction. Eur J Cardiothorac Surg 26:720–725 [DOI] [PubMed] [Google Scholar]
- 52. Brent GA, Hershman JM. 1986. Thyroxine therapy in patients with severe nonthyroidal illnesses and low serum thyroxine concentration. J Clin Endocrinol Metab 63:1–8 [DOI] [PubMed] [Google Scholar]
- 53. Chowdhury D, Ojamaa K, Parnell VA, McMahon C, Sison CP, Klein I. 2001. A prospective randomized clinical study of thyroid hormone treatment after operations for complex congenital heart disease. J Thorac Cardiovasc Surg 122:1023–1025 [DOI] [PubMed] [Google Scholar]
- 54. Portman MA, Fearneyhough C, Ning XH, Duncan BW, Rosenthal GL, Lupinetti FM. 2000. Triiodothyronine repletion in infants during cardiopulmonary bypass for congenital heart disease. J Thorac Cardiovasc Surg 120:604–608 [DOI] [PubMed] [Google Scholar]
- 55. Mackie AS, Booth KL, Newburger JW, Gauvreau K, Huang SA, Laussen PC, DiNardo JA, del Nido PJ, Mayer JE, Jr, Jonas RA, McGrath E, Elder J, Roth SJ. 2005. A randomized, double-blind, placebo-controlled pilot trial of triiodothyronine in neonatal heart surgery. J Thorac Cardiovasc Surg 130:810–816 [DOI] [PubMed] [Google Scholar]
- 56. Bettendorf M, Schmidt KG, Grulich-Henn J, Ulmer HE, Heinrich UE. 2000. Tri-iodothyronine treatment in children after cardiac surgery: a double-blind, randomised, placebo-controlled study. Lancet 356:529–534 [DOI] [PubMed] [Google Scholar]
- 57. Bhasin S, Wallace W, Lawrence JB, Lesch M. 1981. Sudden death associated with thyroid hormone abuse. Am J Med 71:887–890 [DOI] [PubMed] [Google Scholar]
- 58. van Wassenaer-Leemhuis A, Ares S, Golombek S, Kok J, Paneth N, Kase J, LaGamma EF. 2014. Thyroid hormone supplementation in preterm infants born before 28 weeks gestational age and neurodevelopmental outcome at age 36 months. Thyroid 24:1162–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dugbartey AT. 1998. Neurocognitive aspects of hypothyroidism. Arch Intern Med 158:1413–1418 [DOI] [PubMed] [Google Scholar]
- 60. Hanley P, Lord K, Bauer AJ. 2016. Thyroid disorders in children and adolescents: a review. JAMA Pediatr 170:1008–1019 [DOI] [PubMed] [Google Scholar]
- 61. Osborn DA 2001 Thyroid hormones for preventing neurodevelopmental impairment in preterm infants. Cochrane Database Syst Rev CD001070 [DOI] [PubMed]
- 62. Mittnacht J, Choukair D, Kneppo C, Brunner R, Parzer P, Gorenflo M, Bettendorf M. 2015. Long-term neurodevelopmental outcome of children treated with tri-iodothyronine after cardiac surgery: follow-up of a double-blind, randomized, placebo-controlled study. Horm Res Paediatr 84:130–136 [DOI] [PubMed] [Google Scholar]
- 63. Van den Berghe G, Baxter RC, Weekers F, Wouters P, Bowers CY, Iranmanesh A, Veldhuis JD, Bouillon R. 2002. The combined administration of GH-releasing peptide-2 (GHRP-2), TRH and GnRH to men with prolonged critical illness evokes superior endocrine and metabolic effects compared to treatment with GHRP-2 alone. Clin Endocrinol (Oxf) 56:655–669 [DOI] [PubMed] [Google Scholar]