Abstract
Background
Randomized trials investigating the efficacy of aminosalicylates for the treatment of mildly to moderately active Crohn's disease have yielded conflicting results. A systematic review was conducted to critically examine current available data on the efficacy of sulfasalazine and mesalamine for inducing remission or clinical response in these patients.
Objectives
To evaluate the efficacy of aminosalicylates compared to placebo, corticosteroids, and other aminosalicylates (alone or in combination with corticosteroids) for the treatment of mildly to moderately active Crohn's disease.
Search methods
We searched PubMed, EMBASE, MEDLINE and the Cochrane Central Library from inception to June 2015 to identify relevant studies. There were no language restrictions. We also searched reference lists from potentially relevant papers and review articles, as well as proceedings from annual meetings (1991‐2015) of the American Gastroenterological Association and American College of Gastroenterology.
Selection criteria
Randomized controlled trials that evaluated the efficacy of sulfasalazine or mesalamine in the treatment of mildly to moderately active Crohn's disease compared to placebo, corticosteroids, and other aminosalicylates (alone or in combination with corticosteroids) were included.
Data collection and analysis
Data extraction and assessment of methodological quality was independently performed by the investigators and any disagreement was resolved by discussion and consensus. We assessed methodological quality using the Cochrane risk of bias tool. The overall quality of the evidence supporting the outcomes was evaluated using the GRADE criteria. The primary outcome measure was a well defined clinical endpoint of induction of remission or response to treatment. Secondary outcomes included mean Crohn's disease activity index (CDAI) scores, adverse events, serious adverse events and withdrawal due to adverse events. For dichotomous outcomes we calculated the pooled risk ratio (RR) and corresponding 95% confidence interval (CI) using a random‐effects model. For continuous outcomes we calculated the mean difference (MD) and 95% CI using a random‐effects model. Sensitivity analyses based on a fixed‐effect model and duration of therapy were conducted where appropriate.
Main results
Twenty studies (2367 patients) were included. Two studies were judged to be at high risk of bias due to lack of blinding. Eight studies were judged to be at high risk of bias due to incomplete outcomes data (high drop‐out rates) and potential selective reporting. The other 10 studies were judged to be at low risk of bias. A non‐significant trend in favour of sulfasalazine over placebo for inducing remission was observed, with benefit confined mainly to patients with Crohn's colitis. Forty‐five per cent (63/141) of sulfasalazine patients entered remission at 17‐18 weeks compared to 29% (43/148) of placebo patients (RR 1.38, 95% CI 1.00 to 1.89, 2 studies). A GRADE analysis rated the overall quality of the evidence supporting this outcome as moderate due to sparse data (106 events). There was no difference between sulfasalazine and placebo in adverse event outcomes. Sulfasalazine was significantly less effective than corticosteroids and inferior to combination therapy with corticosteroids (RR 0.64, 95% CI 0.47 to 0.86, 1 study, 110 patients). Forty‐three per cent (55/128) of sulfasalazine patients entered remission at 17 to 18 weeks compared to 60% (79/132) of corticosteroid patients (RR 0.68, 95% CI 0.51 to 0.91; 2 studies, 260 patients). A GRADE analysis rated the overall quality of the evidence supporting this outcome as moderate due to sparse data (134 events). Sulfasalazine patients experienced significantly fewer adverse events than corticosteroid patients (RR 0.43, 95% CI 0.22 to 0.82; 1 study, 159 patients). There was no difference between sulfasalazine and corticosteroids in serious adverse events or withdrawal due to adverse events. Olsalazine was less effective than placebo in a single trial (RR 0.36, 95% CI 0.18 to 0.71; 91 patients). Low dose mesalamine (1 to 2 g/day) was not superior to placebo for induction of remission. Twenty‐three per cent (43/185) of low dose mesalamine patients entered remission at week 6 compared to 15% (18/117) of placebo patients (RR = 1.46, 95% CI 0.89 to 2.40; n = 302). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to risk of bias (incomplete outcome data) and sparse data (61 events). There was no difference between low dose mesalamine and placebo in the proportion of patients who had adverse events (RR 1.33, 95% CI 0.91 to 1.96; 3 studies, 342 patients) or withdrew due to adverse events (RR 1.21, 95% CI 0.75 to 1.95; 3 studies, 342 patients). High dose controlled‐release mesalamine (4 g/day) was not superior to placebo, inducing a clinically non significant reduction in CDAI (MD ‐19.8 points, 95% CI ‐46.2 to 6.7; 3 studies, 615 patients), and was also inferior to budesonide (RR 0.56, 95% CI 0.40 to 0.78; 1 study, 182 patients, GRADE = low). While high dose delayed‐release mesalamine (3 to 4.5 g/day) was not superior to placebo for induction of remission (RR 2.02, 95% CI 0.75 to 5.45; 1 study, 38 patients, GRADE = very low), no significant difference in efficacy was found when compared to conventional corticosteroids (RR 1.04, 95% CI 0.79 to 1.36; 3 studies, 178 patients, GRADE = moderate) or budesonide (RR 0.89, 95% CI 0.76 to 1.05; 1 study, 307 patients, GRADE = moderate). However, these trials were limited by risk of bias (incomplete outcome data) and sparse data (small numbers of events). There was a lack of good quality clinical trials comparing sulfasalazine with other mesalamine formulations. Adverse events that were commonly reported included headache, nausea, vomiting, abdominal pain and diarrhea.
Authors' conclusions
Sulfasalazine is only modestly effective with a trend towards benefit over placebo and is inferior to corticosteroids for the treatment of mildly to moderately active Crohn's disease. Olsalazine and low dose mesalamine (1 to 2 g/day) are not superior to placebo. High dose mesalamine (3.2 to 4 g/day) is not more effective than placebo for inducing response or remission. However, trials assessing the efficacy of high dose mesalamine (4 to 4.5 g/day) compared to budesonide yielded conflicting results and firm conclusions cannot be made. Future large randomized controlled trials are needed to provide definitive evidence on the efficacy of aminosalicylates in active Crohn's disease.
Keywords: Humans; Aminosalicylic Acids; Aminosalicylic Acids/therapeutic use; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/therapeutic use; Budesonide; Budesonide/therapeutic use; Crohn Disease; Crohn Disease/drug therapy; Delayed‐Action Preparations; Gastrointestinal Agents; Gastrointestinal Agents/therapeutic use; Induction Chemotherapy; Induction Chemotherapy/methods; Mesalamine; Mesalamine/therapeutic use; Randomized Controlled Trials as Topic; Sulfasalazine; Sulfasalazine/therapeutic use
Plain language summary
Aminosalicylates for treatment of active Crohn's disease
What is Crohn's disease?
Crohn's disease is a chronic inflammatory disease of the intestines. Although Crohn's disease is often found in the ileum (the lower part of the small intestine), it can occur in any part of the digestive tract, from the mouth to the anus. The most common symptoms of Crohn's disease are diarrhea and abdominal pain which often occurs in the lower right region of the abdomen.
What are aminosalicylates?
Aminosalicylates are a family of medications with various formulations that deliver the active ingredient, mesalamine, to target sites. Aminosalicylates are thought to treat Crohn's disease by reducing the inflammation of the intestines caused by the disease.
What did the researchers investigate?
The researchers investigated whether aminosalicylates produce remission or alleviate disease severity in individuals with mildly to moderately active Crohn's disease, and whether they cause any harms (side effects). The researchers searched the medical literature extensively up to June 10, 2015.
What did the researchers find?
The researchers identified twenty studies including a total of 2367 participants. Ten studies were judged to be of moderate to high quality, while the other ten studies were judged to be of low quality. The studies compared aminosalicylates (sulfasalazine, mesalazine and mesalamine) with placebo (inactive pills or tablets), corticosteroids or budesonide (a steroid that is rapidly metabolized by the body and has less side‐effects than traditional corticosteroids).
The researchers found that, comparing to placebo, sulfasalazine provides only a modest benefit for the treatment of mild to moderately active Crohn's disease and is inferior to corticosteroids for treatment of active Crohn's disease. Sulphasalazine differs from other aminosalicylates in that it contains a sulpha portion that has been eliminated in the other preparations.
Mesalazine and mesalamine formulations are not effective for inducing remission in active Crohn's disease. Budesonide was compared to high dose mesalamine (4 to 4.5 g/day) but results were conflicting. One study found mesalamine to be inferior to budesonide and the other study found no difference in effectiveness between mesalamine and budesonide.
Side effects are generally mild in nature and typically include headache, nausea, vomiting, abdominal pain and diarrhea.
In conclusion, sulfasalazine is only modestly effective for the treatment of active Crohn's disease. However, the existing data show little benefit for mesalamine.
Summary of findings
Summary of findings for the main comparison. Sulfasalazine compared to placebo for induction of remission or response in Crohn's disease.
| Sulfasalazine compared to placebo for induction of remission or response in Crohn's disease | ||||||
| Patient or population: patients with induction of remission or response in Crohn's disease Settings: Inpatient/Outpatient Intervention: Sulfasalazine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Sulfasalazine | |||||
| Induction of remission (CDAI <150), therapeutic response (VHI decrease >=25%) or clinical improvement Follow‐up: 17‐26 weeks | 291 per 10001 | 442 per 1000 (276 to 706) | RR 1.52 (0.95 to 2.43) | 289 (3 studies) | ⊕⊕⊝⊝ low2,3 | |
| Induction of remission (CDAI <150) (Random Effects Model) Follow‐up: 17‐18 weeks | 311 per 10001 | 429 per 1000 (311 to 588) | RR 1.38 (1 to 1.89) | 263 (2 studies) | ⊕⊕⊕⊝ moderate4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of meta‐analysis, based on included trials. 2 Dowgraded one level due to sparse data (106 events). 3 Dowgraded one level due heterogeneity (I2 = 41%). 4 Downgraded one level due to sparse data (97 events).
Summary of findings 2. Sulfasalazine compared to Corticosteroids for induction of remission or response in Crohn's disease.
| Sulfasalazine compared to Corticosteroids for induction of remission or response in Crohn's disease | ||||||
| Patient or population: patients with induction of remission or response in Crohn's disease Settings: Inpatient/Outpatient Intervention: Sulfasalazine Comparison: Corticosteroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroids | Sulfasalazine | |||||
| Induction of remission (CDAI <150) Follow‐up: 17‐18 weeks | 598 per 10001 | 407 per 1000 (305 to 545) | RR 0.68 (0.51 to 0.91) | 260 (2 studies) | ⊕⊕⊕⊝ moderate2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to sparse data (134 events).
Summary of findings 3. Sulfasalazine compared to Sulfasalazine and corticosteroids for induction of remission or response in Crohn's disease.
| Sulfasalazine compared to Sulfasalazine and corticosteroids for induction of remission or response in Crohn's disease | ||||||
| Patient or population: patients with induction of remission or response in Crohn's disease Settings: Inpatient/Outpatient Intervention: Sulfasalazine Comparison: Sulfasalazine and corticosteroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sulfasalazine and corticosteroids | Sulfasalazine | |||||
| Induction of remission Follow‐up: 18 weeks | 786 per 10001 | 503 per 1000 (369 to 676) | RR 0.64 (0.47 to 0.86) | 110 (1 study) | ⊕⊕⊕⊝ moderate2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of the included study 2 Downgraded one level due to sparse data (71 events)
Summary of findings 4. Controlled‐release mesalamine (1 ‐ 2 g/day) compared to Placebo for induction of remission or response in Crohn's disease.
| Controlled‐release mesalamine (1 ‐ 2 g/day) compared to Placebo for induction of remission or response in Crohn's disease | ||||||
| Patient or population: patients with induction of remission or response in Crohn's disease Settings: Outpatient Intervention: Controlled‐release mesalamine (1 ‐ 2 g/day) Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Controlled‐release mesalamine (1 ‐ 2 g/day) | |||||
| Decrease in CDAI >=50, HBI >=2 or improvement/remission (as defined by Tvede et al) Follow‐up: 6‐16 weeks | 350 per 10001 | 375 per 1000 (280 to 498) | RR 1.07 (0.8 to 1.42) | 342 (3 studies) | ⊕⊕⊝⊝ low2,3 | |
| Induction of remission (CDAI <=150 + decrease of >=50 or as defined by Tvede et al) Follow‐up: 16 weeks | 444 per 10001 | 649 per 1000 (396 to 1000) | RR 1.46 (0.89 to 2.4) | 302 (2 studies) | ⊕⊕⊝⊝ low2,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of meta‐analysis, based on included trials. 2 Downgraded one level because two studies in the pooled analysis were rated as high risk of bias for incomplete outcome data. 3 Downgraded one level; due to sparse data (127 events). 4 Downgraded one level due to sparse data (61 events).
Summary of findings 5. Controlled‐release mesalamine (4 g/day) compared to Placebo for Induction of remission or response in Crohn's disease.
| Controlled‐release mesalamine (4 g/day) compared to Placebo for Induction of remission or response in Crohn's disease | ||||||
| Patient or population: patients with Induction of remission or response in Crohn's disease Settings: Outpatient Intervention: Controlled‐release mesalamine (4 g/day) Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Controlled‐release mesalamine (4 g/day) | |||||
| Mean change in baseline CDAI (Random effects model) Follow‐up: 16 weeks | The mean mean change in baseline cdai (random effects model) in the intervention groups was 19.76 lower (46.22 lower to 6.7 higher) | 615 (3 studies) | ⊕⊕⊝⊝ low1,2 | |||
| Mean change in baseline CDAI (Fixed effects model) Follow‐up: 16 weeks | The mean mean change in baseline cdai (fixed effects model) in the intervention groups was 17.54 lower (35 to 0.08 lower) | 615 (3 studies) | ⊕⊕⊝⊝ low1,2 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded one level due to moderate heterogeneity (I2 = 54%). 2 Downgraded one level because all three studies in the pooled analysis were rated as high risk of bias for incomplete outcome data.
Summary of findings 6. Azo‐bonded and delayed‐release mesalamine (2 ‐ 3.2 g/day) compared to Placebo for Induction of remission or response in Crohn's disease.
| Azo‐bonded and delayed‐release mesalamine (2 ‐ 3.2 g/day) compared to Placebo for Induction of remission or response in Crohn's disease | ||||||
| Patient or population: patients with Induction of remission or response in Crohn's disease Settings: Outpatient Intervention: Azo‐bonded and delayed‐release mesalamine (2 ‐ 3.2 g/day) Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Azo‐bonded and delayed‐release mesalamine (2 ‐ 3.2 g/day) | |||||
| Induction of remission or clinical improvement ‐ Olsalazine (2 g/day) Follow‐up: 16 weeks | 489 per 10001 | 176 per 1000 (88 to 347) | RR 0.36 (0.18 to 0.71) | 91 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| Induction of remission or clinical improvement ‐ Asacol (3.2 g/day) Follow‐up: 16 weeks | 222 per 10001 | 600 per 1000 (236 to 1000) | RR 2.7 (1.06 to 6.88) | 38 (1 study) | ⊕⊝⊝⊝ very low2,4 | |
| Induction of remission (CDAI < 150 + decrease >=70) ‐ Asacol (3.2 g/day) Follow‐up: 16 weeks | 222 per 10001 | 451 per 1000 (167 to 1000) | RR 2.03 (0.75 to 5.45) | 38 (1 study) | ⊕⊝⊝⊝ very low2,5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of the included study. 2 Downgraded one level because the study was rated as high risk of bias for incomplete outcome data. 3 Downgraded two levels due to very sparse data (30 events). 4 Downgraded two levels due to very sparse data (16 events). 5 Downgraded two levels due to very sparse data (13 events).
Summary of findings 7. Delayed‐release mesalamine (3 ‐ 4.5 g/day) compared to Corticosteroids for Induction of remission or response in Crohn's disease.
| Delayed‐release mesalamine (3 ‐ 4.5 g/day) compared to Corticosteroids for Induction of remission or response in Crohn's disease | ||||||
| Patient or population: patients with Induction of remission or response in Crohn's disease Settings: Outpatient Intervention: Delayed‐release mesalamine (3 ‐ 4.5 g/day) Comparison: Corticosteroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroids | Delayed‐release mesalamine (3 ‐ 4.5 g/day) | |||||
| Induction of remission (CDAI < or =150 with or without decrease of at least 60 points) Follow‐up: 8‐12 weeks | 526 per 10001 | 547 per 1000 (416 to 716) | RR 1.04 (0.79 to 1.36) | 178 (3 studies) | ⊕⊕⊕⊝ moderate2 | |
| Induction of remission (CDAI < or =150 with or without decrease of at least 60 points) ‐ 3 g/day Follow‐up: 12 weeks | 429 per 10003 | 407 per 1000 (210 to 793) | RR 0.95 (0.49 to 1.85) | 50 (1 study) | ⊕⊕⊝⊝ low4 | |
| Induction of remission (CDAI < or =150 with or without decrease of at least 60 points) ‐ 2.4 g/day Follow‐up: 12 weeks | 600 per 10003 | 600 per 1000 (366 to 984) | RR 1 (0.61 to 1.64) | 50 (1 study) | ⊕⊕⊕⊝ moderate5 | |
| Induction of remission (CDAI < or =150 with or without decrease of at least 60 points) ‐ 4 g/day microgranules Follow‐up: 12 weeks | 625 per 10003 | 788 per 1000 (512 to 1000) | RR 1.26 (0.82 to 1.92) | 44 (1 study) | ⊕⊕⊕⊝ moderate6 | |
| Induction of remission (CDAI < or =150 with or without decrease of at least 60 points) ‐ 4.5 g/day Follow‐up: 8 weeks | 529 per 10003 | 355 per 1000 (159 to 773) | RR 0.67 (0.3 to 1.46) | 34 (1 study) | ⊕⊕⊝⊝ low7 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of meta‐analysis, based on included trials. 2 Downgraded one level due to sparse data (98 events). 3 Control group risk comes from control arm of the included study. 4 Downgraded two levels due to very sparse data (21 events). 5 Downgraded one level due to sparse data (40 events). 6 Downgraded one level due to sparse data (41 events). 7 Downgraded two levels due to very sparse data (15 events).
Summary of findings 8. Mesalamine (4 ‐ 4.5 g/day) compared to Budesonide for Induction of remission or response in Crohn's disease.
| Mesalamine (4 ‐ 4.5 g/day) compared to Budesonide for Induction of remission or response in Crohn's disease | ||||||
| Patient or population: patients with Induction of remission or response in Crohn's disease Settings: Outpatient Intervention: Mesalamine (4 ‐ 4.5 g/day) Comparison: Budesonide | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Budesonide | Mesalamine (4 ‐ 4.5 g/day) | |||||
| Induction of remission (CDAI < or = 150) ‐ Pentasa (4 g/day) Follow‐up: 16 weeks | 602 per 10001 | 337 per 1000 (241 to 470) | RR 0.56 (0.4 to 0.78) | 182 (1 study) | ⊕⊕⊝⊝ low2,3 | |
| Induction of remission (CDAI < or = 150) ‐ Salofalk (4.5 g/day) Follow‐up: 8 weeks | 695 per 10001 | 618 per 1000 (528 to 730) | RR 0.89 (0.76 to 1.05) | 307 (1 study) | ⊕⊕⊕⊝ moderate | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of the included study. 2 Downgraded one level because the study was rated as high risk of bias for incomplete outcome data. 3 Downgraded one level due to sparse data (86 events). 4 Downgraded one level due to Ssparse data (202 events).
Summary of findings 9. Mesalamine compared to Sulfasalazine (alone or in combination with corticosteroids) for Induction of remission or response in Crohn's disease.
| Mesalamine compared to Sulfasalazine (alone or in combination with corticosteroids) for Induction of remission or response in Crohn's disease | ||||||
| Patient or population: patients with Induction of remission or response in Crohn's disease Settings: Outpatient Intervention: Mesalamine Comparison: Sulfasalazine (alone or in combination with corticosteroids) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Sulfasalazine (alone or in combination with corticosteroids) | Mesalamine | |||||
| Induction of remission (CDAI < 150) or clinical improvement ‐ Salofalk (1.5 g/day) Follow‐up: 8 weeks | 733 per 10001 | 865 per 1000 (601 to 1000) | RR 1.18 (0.82 to 1.7) | 30 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| Induction of remission (CDAI < 150) or clinical improvement ‐ Salofalk (3.0 g/day) Follow‐up: 12 weeks | 885 per 10001 | 832 per 1000 (663 to 1000) | RR 0.94 (0.75 to 1.18) | 50 (1 study) | ⊕⊕⊝⊝ low2,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of the included study. 2 Downgraded one level because the study was rated as high risk for blinding. 3 Downgraded two levels due to very sparse data (24 events). 4 Downgraded one level due to sparse data (43 events).
Background
Crohn's disease is a chronic inflammatory disease characterized by focal, asymmetric, transmural and granulomatous inflammation of the gastrointestinal tract. Although Crohn's disease can affect any part of the gut, it primarily involves the ileum and colon. The majority of patients suffer from chronic relapsing symptoms that are associated with decreased quality of life. In North America, Crohn's disease has a prevalence ranging 26 to 199 cases per 100,000 and an incidence of 3.1 to 14.6 cases per 100,000 person‐years. Approximately 630,000 North Americans are affected (Loftus 2004). Pharmacotherapy remains the cornerstone of treatment, with most patients requiring lifelong therapy due to the chronicity of the disease and its typical onset before 30 years of age. Surgery is reserved for medical refractory disease and specific complications (Hanauer 2003).
Aminosalicylates may beused in the treatment of active mild to moderate Crohn's disease. The exact mechanism of action of aminosalicylates remains unknown, but is thought to be related to a topical effect on the gastrointestinal mucosa rather than a systemic one. Aminosalicylates have a wide range of anti‐inflammatory and immunomodulatory actions. Aminosalicylates block production of interleukin (IL)‐1 and tumor necrosis factor (TNF)‐α, and prevent TNF‐α binding to its receptor. Aminosalicylates are potent inhibitors of cyclooxygenase and 5‐lipoxygenase, and they block production and the proinflammatory activity and chemotactic action of prostaglandin E2 and leukotrienes respectively. Aminosalicylates possess potent anti‐oxidant and free‐radical‐scavenger properties, and also inhibit antigen presentation,T‐cell proliferation, antibody production by B‐cells, cytotoxic T‐cells, natural killer cells and the activation and expression of adhesion molecules on endothelial cells. The inhibitory effects of aminosalicylates on multiple inflammatory pathways may be explained by the inhibition of nuclear factor kappa B (NFκB) activation, a pivotal transcription factor that regulates gene expression for many pro‐inflammatory cytokines, chemokines, adhesion molecules and inflammatory mediators (MacDermott 2000). Most recently mesalamine has been demonstrated to induce activation of PPARg in epithelial cells and lamina propria lymphocytes, resulting in inhibition of NFkB signalling pathway (Dubuquoy 2006; Rousseaux 2005).
Sulfasalazine in daily doses of 3‐6 g is recommended for the treatment of ileocolonic or colonic disease (Hanauer 2001; Sandborn 2003). However, as many as 30‐40% of patients, particularly slow acetylators, are intolerant of high doses of sulfasalazine due to systemic absorption of the sulfapyridine carrier molecule. The discovery of the therapeutically active moiety, 5‐aminosalicylic acid (5‐ASA), in sulfasalazine (Azad 1977) has led to the development of newer sulfa‐free 5‐ASA formulations, that deliver higher concentrations of 5‐ASA without the dose‐limiting side effects of sulfasalazine. PH dependent release formulations include a Eudragit‐S coated mesalamine formulation (Asacol®) that releases 5‐ASA in the terminal ileum and cecum at pH 7, while Eudragit‐L coated mesalamine formulations (Salofalk®, Mesasal® and Claversal®) releases in the mid‐ileum at pH 6. A formulation of mesalamine microgranules enclosed within a semi‐permeable membrane of ethylcellulose (Pentasa®) is designed for time‐dependent release throughout the small and large intestine, beginning in the duodenum. Newer azo‐bonded formulations designed for release in the colon include 5‐ASA dimer, olsalazine (Dipentum®), and balsalazide (Colazal®), composed of 5‐ASA linked to 4‐aminobenzoyl‐b alanine.
Aminosalicylates (mesalamine 3.2 to 4 g or sulfasalazine 3 to 6 g daily in divided doses) have been recommended by some experts as first‐line therapy for mildly to moderately active Crohn's disease (Hanauer 2001). However, the efficacy of mesalamine has been called into question and first line therapy with sulfasalazine or budesonide was proposed as alternative first line strategies (Sandborn 2003). Early studies demonstrated the efficacy of sulfasalazine in inducing remission in mildly to moderately active Crohn's disease (Summers 1979; Malchow 1984). Much enthusiasm has surrounded the newer 5‐ASA formulations as they were expected to be as efficacious as sulfasalazine. A meta‐analysis of three large trials of Pentasa in active Crohn's disease demonstrated a statistically significant benefit over placebo in reducing the Crohn's disease activity index (CDAI; (Weighted Mean Difference, WMD, ‐18 points; 95% CD ‐35 to ‐1) (Hanauer 2004). Although this benefit was statistically significant, it is of questionable clinical significance because the minimum detectable difference in CDAI that a physician or patient can detect is approximately 50 points (Brant 1999; Feagan 2004). This systematic review critically examines the current available data regarding the efficacy of sulfasalazine and mesalamine for induction of remission or clinical response in patients with mildly to moderately active Crohn's disease and is an update of a previously published Cochrane review (Lim 2010). Where possible, data from comparable trials were pooled together in meta‐analyses to obtain a more precise estimate of the treatment effect.
Objectives
To evaluate the efficacy of aminosalicylates compared to placebo, corticosteroids, and other aminosalicylates (alone or in combination with corticosteroids) for the treatment of mildly to moderately active Crohn's disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials that evaluated the efficacy sulfasalazine or mesalamine for the treatment of active Crohn's disease.
Types of participants
Adults with mildly to moderately active Crohn's disease.
Types of interventions
Comparison of oral sulfasalazine or mesalamine alone to placebo, corticosteroids, and other aminosalicylates (alone or in combination with corticosteroids).
Types of outcome measures
The primary outcome measure was a well defined clinical endpoint of induction of remission or response to treatment. Secondary outcomes included mean Crohn's disease activity index (CDAI) scores, adverse events, serious adverse events and withdrawal due to adverse events.
Search methods for identification of studies
We searched PubMed, EMBASE, MEDLINE and Cochrane Central Library from inception to June 10, 2015 to identify relevant studies. There were no language restrictions. The search strategy is reported in Appendix 1. In addition, manual searches of the reference list from potentially relevant papers and review articles, as well as proceedings from annual meetings of the American Gastroenterological Association and American College of Gastroenterology from 1991 to 2015 were performed.
Data collection and analysis
Data Collection Using the above search strategy, two investigators (W.C.L and S.B.H) independently reviewed the titles and abstracts of all identified citations. Manuscripts of potentially relevant studies that evaluated the efficacy of sulfasalazine or 5‐ASA for the treatment of Crohn's disease were retrieved and reviewed and only studies that fulfilled the a priori defined inclusion criteria were selected. The following exclusion criteria were applied: (1) Non‐randomized, uncontrolled, open‐label trials, (2) studies involving pediatric population, (3) patients with severely active Crohn's disease, (4) Crohn's disease in remission (medically‐ or surgically‐ induced), (5) ulcerative colitis, (6) rectal, or intestinal lavage drug delivery, (7) studies evaluating efficacy of aminosalicylates in combination with other treatments, (8) studies with no comparators, (9) studies that compared 5‐ASA to antibiotics, immunosuppressants, nutritional therapy, herbs, yeast, or surgery, (10) studies assessing effect on fecal flora, and (11) crossover studies that do not provide data prior to the first crossover. The results of each study were independently extracted using a form that was developed by the investigators. Disagreement was resolved by consensus. A recent literature search and citation review were performed by Y.W and J.K.M to modernize a previous update by W.C.L and S.B.H.
Statistical Methods Results using intention‐to‐treat (ITT) analysis were reported according to the a priori definition of the primary endpoint described in each study. Dropouts from studies were regarded as treatment failures regardless of treatment group. When an ITT analysis was not possible, per‐protocol‐analysis results were reported instead. Where appropriate, results of comparable studies with similar doses of medication and outcomes were pooled and analyzed. The random effects model of DerSimonian and Laird (DerSimonian 1986) was used to calculate pooled effects estimates. Sensitivity analyses based on the fixed effect model and duration of therapy were also conducted. Heterogeneity between trials was assessed by calculating the chi square test of heterogeneity and a P value of < 0.10 was used to indicate statistically significant heterogeneity between trials; the I2 statistic was also used to quantify heterogeneity: a value of > 30% was considered moderate heterogeneity. The risk ratio (RR), 95% confidence intervals (CI), absolute benefit increase (ABI) and numbers needed to treat (NNT) were calculated for each statistically significant outcome. The Cochrane Collaboration Review Manager (RevMan) software (Version 5.3.5) was used for data analysis.
Quality assessment The methodological quality of the included studies was evaluated using the Cochrane risk of bias tool (Higgins 2011). Each trial was rated as high, low, or unclear risk for each of the following criteria:
Randomization sequence generation;
Allocation concealment;
Blinding;
Missing data and attrition;
Outcome reporting; and
Other sources of bias.
The overall quality of the evidence supporting the primary outcomes was evaluated using the GRADE approach (Guyatt 2008; Schünemann 2011). Randomized trials are considered to provide high quality evidence, but may be downgraded due to: (1) risk of bias, (2) indirectness of evidence, (3) inconsistency (unexplained heterogeneity), (4) imprecision (sparse data), and (5) reporting bias (publication bias). The different quality ratings are interpreted as the likelihood that future research would change the effect estimate. Further research is unlikely to change the effect estimate if the evidence is high quality. If the overall evidence is of moderate quality further research may have an impact on our confidence in the effect estimate and may change the estimate. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate when the evidence is rated as low quality. Very low quality research means that we are very uncertain about the finding (Guyatt 2008; Schünemann 2011).
Results
Description of studies
A literature search conducted on 10 June 2015 identified 1961 studies. Five additional studies were identified through searching of references. After duplicates were removed a total of 1177 reports remained for review of titles and abstracts. Two authors independently reviewed the titles and abstracts of these studies and 101 reports were selected for full text review (See Figure 1). Seventy reports of 68 studies were excluded (See Characteristics of excluded studies and additional Table 10). Thirty‐one reports of 20 studies involving a total of 2367 patients, were selected for inclusion (See Characteristics of included studies).
1.
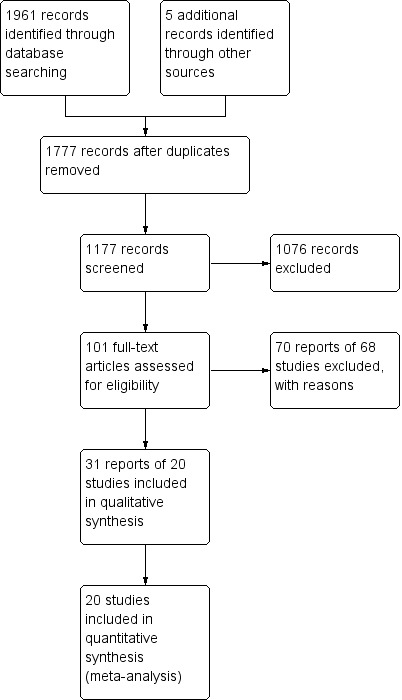
Study flow diagram.
1. Characteristics of Excluded Studies.
| Study ID | Comparators | Endpoint | Study design | Patient Population | Exclusion reasons |
| Anonymous 1985 | SASP 1 g/15 kg /day alone | Clinical response | Uncontrolled | Active CD | 1, 5 |
| Anonymous 1990 | 5‐ASA 1.5 g/day versus Placebo | Clinical relapse | Randomized controlled | CD in remission (Medical/Surgical) | 2, 6 |
| Anthonisen 1974 | SASP (1.5 g for 3 days followed by 3 g/day) versus Placebo | Clinical improvement | Double‐blind placebo controlled cross‐over | Active CD | 7 |
| Arber 1995 | 5‐ASA 1 g/day versus Placebo | Clinical relapse | Randomized controlled | CD in remission | 2, 6 |
| Ardizzone 2004 | 5‐ASA 3 g/day versus Azathioprine | Clinical and surgical relapse | Open label, randomized | CD in remission (Surgical) | 2, 5, 6 |
| Beck 1988 | 5‐ASA versus SASP | Clinical response | Uncontrolled | Active CD | 1 |
| Bergman 1976 | SASP + CS versus No Treatment | Clinical relapse | Randomized controlled | CD in remission (Surgical) | 2, 4, 6 |
| Blichfeldt 1978 | SASP versus prednisolone (Metronidazole/ placebo cross‐over) | Clinical improvement | Double‐blind cross‐over | Active CD | 4, 5 |
| Bresci 1994 | 5‐ASA 2.4 g/day versus No Specific Therapy | Clinical relapse | Randomized controlled | CD in remission (Medical) | 2, 6 |
| Brignola 1992 | 5‐ASA 2 g/day versus Placebo | Clinical relapse | Randomized controlled | CD in remission | 2, 6 |
| Brignola 1995 | 5‐ASA 3 g/day versus Placebo | Endoscopic relapse | Double‐blind placebo controlled | CD in remission (Surgical) | 2, 6 |
| Caprilli 1994 | 5‐ASA 2.4 g/day versus No Treatment | Endoscopic relapse | Randomized controlled | CD in remission (Surgical) | 2, 6 |
| Caprilli 2003 | 5‐ASA 2.4 g/day versus 5‐ASA 4 g/day | Clinical and endoscopic relapse | Randomized controlled | CD in remission (Surgical) | 2, 6 |
| Cezard 2009 | 5‐ASA versus Placebo | Clinical relapse | Double‐blind placebo‐controlled | CD in remission (Paediatric) | 2, 6 |
| Cohen 2000 | 5ASA versus Placebo | Endoscopic recurrence | Randomized, controlled | CD in remission (Surgical) | 2, 6 |
| Colombel 1999 | 5‐ASA versus Antibiotic | Remission | Randomized controlled | Active CD | 5 |
| de Franchis R 1997 | 5‐ASA 3 g/day versus Placebo | Clinical relapse | Randomized controlled | CD in remission (Steroid‐induced) | 2, 6 |
| Del Corso 1995 | 5‐ASA 2.4 g/day versus No Treatment | Clinical relapse | Controlled trial | CD in remission (Medical/Surgical) | 2, 6 |
| Dirks 1989 | SASP + CS versus Surgery | Clinical relapse | Uncontrolled | CD in remission | 1,2,4,5,6 |
| Ewe 1976 | SASP versus Placebo | Relapse | Double‐blind | CD in remission | 2, 6 |
| Ewe 1984 | SASP, radical versus restricted surgery | Clinical relapse | Partially randomized, double‐blind | CD in remission (Surgical) | 2,6 |
| Ewe 1986 | SASP, radicality of surgery | Clinical relapse | CD in remission (Surgical) | 2,6 | |
| Ewe 1989 | SASP versus Placebo | Clinical relapse | Randomized controlled | CD in remission (Surgical) | 2, 6 |
| Fiasse 1990 | 5‐ASA versus Placebo | Relapse | Double‐blind placebo‐controlled | CD in remission (Surgical) | 2, 6 |
| Florent 1996 | 5‐ASA 3 g/day versus Placebo | Endoscopic relapse | Double‐blind placebo‐controlled | CD in remission (Surgical) | 2, 6 |
| Gendre 1993 | 5‐ASA 2 g/day versus Placebo | Clinical relapse | Randomized controlled | CD in remission | 2, 6 |
| Gerhardt 2001 | 5‐ASA versus Boswellia serrata extract H15 | Change in CDAI | Randomized controlled | Active CD | 5 |
| Goldstein 1987 | SASP alone | Clinical response | Retrospective | Active small bowel CD | 1, 5 |
| Griffiths 1993 | 5‐ASA 50 mg/kg versus Placebo | Change in CDAI, VHI | Randomized controlled | Active small bowel CD (Paediatric) | 2 |
| Guslandi 2000 | 5‐ASA 3 g/day versus 5‐ASA 2 g/day + Saccharomyces boulardii (yeast) | Clinical relapse | Randomized controlled | CD in remission | 2, 5, 6 |
| Hanauer 1993 | 5‐ASA 4g/day alone | Clinical response | Uncontrolled | Active CD and CD in remission | 1, 5 |
| Hanauer 2004b | 5‐ASA 3 g/day versus 6‐MP 50 mg/day versus placebo | Clinical, endoscopic and radiographic relapse | Randomized controlled | CD in remission (Surgical) | 2, 6 |
| Howaldt 1993 | 5‐ASA 1.5 g/day versus 4‐ASA 1.5 g/day | Clinical relapse | Randomized controlled | CD in remission | 2, 6 |
| Klein 1995 | 5‐ASA 1.5 g/day versus Placebo | Endoscopic relapse | Controlled trial | CD in remission (Surgical) | 2, 6 |
| Klotz 1980 | SASP versus Sulfapyridine versus Rectal 5‐ASA | Activity index, stool quality, remission rate | Randomized controlled | Active CD and UC | 3, 5 |
| Lennard‐Jones 1977 | SASP versus Placebo | Clinical relapse | Double‐blind placebo‐controlled | CD in remission (Medical/Surgical) | 2, 6 |
| Lichtenstein 2009a | 5‐ASA alone | Clinical relapse | Prospective, uncontrolled | CD in remission | 1, 2, 5, 6 |
| Lichtenstein 2009b | 5‐ASA alone | Clinical remission | Prospective, uncontrolled | Active CD | 1, 5 |
| Lochs 1991 | SASP 3 g/day + CS versus Enteral Nutrition | Clinical remission | Randomized controlled | Active CD | 4, 5 |
| Lochs 2000 | 5‐ASA 4 g/day versus Placebo | Clinical relapse | Randomized controlled | CD in remission (Surgical) | 2, 6 |
| Mahmud 2001 | 5‐ASA 2 g/day versus Placebo | Clinical relapse | Randomized controlled | CD in remission | 2, 6 |
| Malchow 1990 | SASP + CS versus Enteral Nutrition | Clinical remission | Randomized controlled | Active CD | 4, 5 |
| Mantzaris 2003 | 5‐ASA 3 g/day versus Budesonide 6 mg/day | Clinical relapse and quality of life | Randomized controlled | CD in remission (Steroid‐dependent) | 2, 6 |
| Mate‐Jimenez 2000 | 5‐ASA 3g/day versus MTX 15 mg/week versus 6‐MP 1.5 mg/kg/day | Clinical remission and relapse | Randomized controlled | CD and UC (Steroid‐dependent) | 2, 5, 6 |
| McLeod 1995 | 5‐ASA 3g/day versus Placebo | Clinical relapse | Randomized controlled | CD in remission (Surgical) | 2, 6 |
| Modigliani 1996 | 5‐ASA 4g/day versus Placebo | Clinical relapse, steroid weaning | Randomized controlled | CD in remission (Steroid‐induced) | 2,6 |
| Orlando 2012 | 5‐ASA alone | Endoscopic recurrence | Prospective, uncontrolled | CD in remission (Surgical) | 1, 2, 5, 6 |
| Papi 2009 | 5‐ASA alone vs No Treatment | Clinical and surgical relapse | Retrospective | CD in remission (Surgical) | 1, 2, 6 |
| Prantera 1992 | 5‐ASA 2.4 g/day versus placebo | Clinical relapse | Randomized controlled | CD in remission | 2, 6 |
| Rasmussen 1983 | 5‐ASA 1.5 g/day alone | Clinical response | Uncontrolled | Active CD | 1, 5 |
| Reinisch 2010 | 5‐ASA versus Azathioprine | Therapeutic failure | Duoble‐blind, Double‐dummy, Randomized controlled | CD in remission, moderate/severe endoscopic recurrence | 2, 5, 6 |
| Romano 2005 | 5‐ASA+omega‐3 FA versus 5‐ASA | Clinical relapse | Randomized controlled, double‐blind | CD in remission (Paediatric) | 2, 4, 6 |
| Rosen 1982 Ursing1982 | SASP 3 g/day versus Metronidazole | Remission | Randomized controlled | Active CD | 5 |
| Savarino 2013 | 5ASA versus Azathioprine versus Adalimumab | Endoscopic and clinical recurrence | Randomized controlled | CD in remission (Surgical) | 2, 5, 6 |
| Schneider 1985 | Metronidazole versus CS + SASP +/‐ Metronidazole | Clinical response | Randomized controlled | Active CD or discharging fistulae | 4, 5 |
| Schreiber 1994 | 5‐ASA 1.5 g/day versus 4‐ASA 1.5 g/day | Clinical relapse | Randomized controlled | CD in remission | 2, 6 |
| Singleton 1979 | SASP 1 g/15 kg + CS versus CS alone | Clinical remission and response | Randomized controlled | Active CD | 4 |
| Stober 1983 | SASP+CS versus Elementary Diet + SASP +/‐ CS | Laboratory parameters, body weight | Active CD (Paediatric) | 2, 4, 5, 6 | |
| Sutherland 1997 | 5‐ASA 3g/day versus Placebo | Clinical relapse | Randomized controlled | CD in remission (Medical or Surgical) | 2,6 |
| Tao 2009 | 5‐ASA versus Tripterygium wilfordii | Clinical relapse | Randomized controlled | CD in remission (Surgical) | 2, 5, 6 |
| Terranova 2001 | 5ASA + Enteral Nutrition versus 5‐ASA + CS | Clinical improvement, biohumoral markers | Randomized controlled | Active CD and UC | 4, 5 |
| Terrin 2002 | 5‐ASA + CS versus Semi‐Elemental Diet | Clinical remission | Randomized controlled | Active CD | 4, 5 |
| Thomson 1995 | 5‐ASA 3g/day versus Placebo | Clinical relapse | Randomized controlled | CD in remission | 2, 6 |
| Triantafillidis 2010 | 5‐ASA vs Modulen ®IBD | Clinical relapse | Randomized controlled | CD in remission | 2, 5, 6 |
| Wellman 1986 | TPN + steroids with or without 5‐ASA lavage | Endotoxemia, clinical response | Randomized controlled | Active CD | 3, 4, 5 |
| Wellmann 1988 | 5‐ASA versus Placebo | Clinical relapse | Double‐blind placebo‐controlled | CD in remission | 2, 6 |
| Wenckert 1978 | SASP versus Placebo | Clinical relapse | Double‐blind placebo‐controlled | CD in remission (Surgical) | 2, 6 |
| Yamamoto 2009 | 5‐ASA versus Azathioprine versus Infliximab | Clinical relapse | Prospective | CD in remission (Surgical) | 1, 2, 5, 6 |
1=Inappropriate study design (Uncontrolled, open‐label), 2= Inappropriate study population (pediatric, CD in remission, severe CD), 3= Inappropriate route of drug delivery (rectal, lavage), 4= combined therapy, 5= inappropriate comparator, 6= inappropriate endpoint, 7=cross‐over studies that did not provide data prior to first crossover. Numbers in bold indicate primary reason for exclusion.
Two studies included more than two treatments arms (Malchow 1984; Summers 1979). Comparisons are described below.
a. Three studies compared the efficacy of sulfasalazine with placebo (Summers 1979; Van Hees 1981; Malchow 1984). b. Two studies compared the efficacy of sulfasalazine with corticosteroids (Summers 1979; Malchow 1984). c. Two studies examined the efficacy of sulfasalazine either alone or in combination with corticosteroids (Malchow 1984; Rijk 1991). d. Eight studies compared the efficacy of mesalamine with placebo (Saverymuttu 1986; Rasmussen 1987; Mahida 1990; Singleton 1993; Singleton 1994; Crohn's III 1997; Tremaine 1994; Wright 1995). e. Four studies compared the efficacy of mesalamine with conventional corticosteroids (Martin 1990; Scholmerich 1990; Gross 1995; Prantera 1999). f. Two studies compared the efficacy of mesalamine with budesonide (Thomsen 1998, Tromm 2011). g. Two studies compared the efficacy of mesalamine with sulfasalazine (either alone or in combination with corticosteroids) (Maier 1985; Maier 1990).
One German article was translated with the assistance of an interpreter (Maier 1985).
Risk of bias in included studies
The risk of bias results were summarized in Figure 2. Four of the 20 included studies were rated as low risk of bias for all six items (Malchow 1984; Prantera 1999; Summers 1979; Tromm 2011). The authors were unable to assess the risk of bias for 2 studies, because they were not fully published (Crohn's III 1997; Singleton 1994). The risk of bias was high for 2 studies that did not use blinding (Maier 1985; Maier 1990) or unclear for some quality items in 11 studies (due to inadequate descriptions of methods used for sequence generation and/or allocation concealment; Gross 1995; Mahida 1990; Martin 1990; Rasmussen 1987; Rijk 1991; Saverymuttu 1986; Scholmerich 1990;Singleton 1993; Tremaine 1994; Van Hees 1981; Wright 1995). Two studies (Rijk 1991; Wright 1995) scored high risk of bias for incomplete outcome data and selective reporting. In addition, six other studies were rated as high risk for attrition bias (Crohn's III 1997; Rasmussen 1987; Singleton 1993; Singleton 1994; Thomsen 1998; Tremaine 1994).
2.
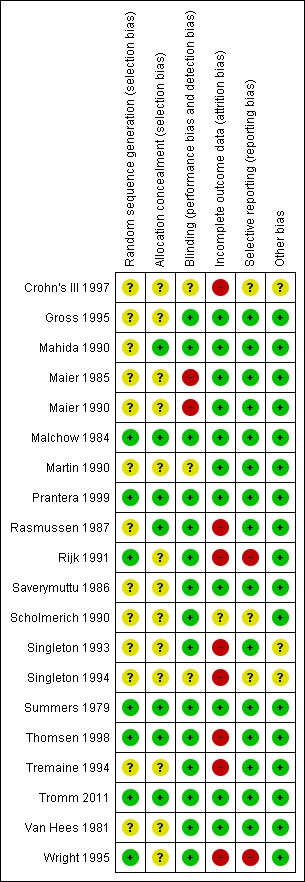
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Sulfasalazine a. Sulfasalazine versus placebo Three trials compared the efficacy of sulfasalazine with placebo (Van Hees 1981; Summers 1979; Malchow 1984). Due to a lack of available data in these trials, only per protocol results were reported in this review.
Van Hees 1981 Van Hees 1981 randomly assigned 27 patients with active Crohn's disease to receive sulfasalazine 4 to 6 g/day (n = 13) or placebo (n = 13) for 26 weeks. More sulfasalazine‐treated patients responded (≥ 25% decrease in baseline Van Hees Activity Index [VHAI]) than those assigned to placebo (61.5% [8/13] versus 7.7% [1/13], P = 0.03; RR 8; 95% CI 1.16 to 55.2), ABI = 53.8%, NNT = 2). Firm conclusions cannot be made due to the small sample size of this study.
Summers 1979 The National Cooperative Crohn's Disease Study (NCCDS) compared the efficacy of sulfasalazine, prednisone and azathioprine to placebo in a two‐part, multicenter trial (Summers 1979). Six hundred and four patients with Crohn's disease were randomized into Part I or Part II of the study. Thirty‐five randomized patients were excluded from the final analysis due to an erroneous diagnosis (20), inappropriate entry (12) or administrative error during the conduct of the trial (3). In part I of the study, data from 295 patients with active Crohn's disease, randomized to 1 g/15kg sulfasalazine (n = 74), 0.25 to 0.75 mg/kg prednisone (dose adjusted according to disease activity, n = 85), 2.5 mg/kg azathioprine (n = 59) or placebo (n = 77) for 17 weeks were analyzed. The dose of sulfasalazine ranged from 2 to 5 g/day (mean 4 g/day). Remission (CDAI < 150) was achieved in 38% (28/74) of sulfasalazine‐treated patients versus 26% (20/77) in the placebo group (P = 0.12). Patients with Crohn's colitis (with [P = 0.027] or without [P = 0.006] small bowel disease) or those who were treatment‐naive at entry (P = 0.01) were more likely to respond. These data suggest that patients who continued to have active disease despite prior treatment with steroids or sulfasalazine were unlikely to respond to further sulfasalazine therapy. There was no statistically significant difference in the proportion of patients who experienced an adverse event or serious adverse event. Fourteen per cent of patients sulfasalazine experienced at least one adverse event during the induction study compared to 6% of placebo patients (RR 2.08, 95% CI 0.75 to 5.80). There were no serious adverse events in the sulfasalazine group compared to one event in the placebo group (RR 0.35, 95% CI 0.01 to 8.38). Adverse events reported in the sulfasalazine group included skin rash, nausea and vomiting, headache and leukopenia. Adverse events reported in the placebo group included depression, abscess, candidiasis of mouth and duo ulcer (serious adverse event).
Malchow 1984 In the European Cooperative Crohn's Disease Study (ECCDS) (Malchow 1984) randomized 455 patients with Crohn's disease to receive sulfasalazine, 6‐methylprednisolone, combination therapy (sulfasalazine with 6‐methylprednisolone) or placebo. Three patients were excluded after randomization due to an incorrect diagnosis. Of 452 patients, 215 had active disease (CDAI ≥ 150) were treated with sulfasalazine 3 g/day (n = 54), 6‐methylprednisolone (48 mg/day tapered weekly to 12 mg/day, n = 47), combination therapy (n = 56) or placebo (n = 58) for 6 weeks. Patients could be re‐treated with the same drug regimen once or twice if induction therapy was not successful. Although there were significantly fewer "treatment failures and relapses" in the sulfasalazine group compared to placebo (P < .05), particularly in patients with colonic disease (P < .01), the difference in proportions of patients in remission (CDAI < 150) before the end of 18 weeks was not significant (sulfasalazine: 27/54, 50%; placebo: 22/58, 38%; P = 0.20). Common adverse events reported in the sulfasalazine group included nausea, headache, infection, hypertension, anorexia, back pain, and skin rash. Common adverse events reported in the placebo group included nausea, headache, infection, hypertension, anorexia, back pain, skin rash and acne.
Pooled analysis In a combined analysis of three trials (n = 289), sulfasalazine was not superior to placebo for inducing remission or response at 17 to 26 weeks of follow‐up (See Analysis 1.1) Forthy‐five per cent (63/141) of sulfasalazine patients entered remission or responded compared to 29% (43/148) of placebo patients (RR 1.52; 95% CI 0.95 to 2.43; P = 0.08). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to sparse data and heterogeneity (See Table 1). However, moderate heterogeneity was observed (test of heterogeneity chi2 = 3.38, P = 0.18, I2 = 41%) for this comparison. A visual inspection of the forest plot indicated that the Van Hees 1981 study was the likely source of this heterogeneity and this study employed different measures of treatment response and duration of therapy than the larger trials (Summers 1979; Malchow 1984). A sensitivity analysis combining data from only the NCCDS and ECCDS (n = 263) that employed similar efficacy measures, therapeutic endpoints and duration of therapy (See Analysis 1.2; Analysis 1.3) reduced the I2 value to zero. A trend in favour of sulfasalazine over placebo for inducing remission was observed at 17‐18 weeks follow‐up (random‐effects model: RR 1.38; 95% CI 1.00 to 1.89, P = 0.05; fixed‐effect model: RR 1.38; 95% CI 1.01‐1.90; P = 0.05, ABI = 12%, NNT = 8). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to sparse data (See Table 1). A pooled analysis of three studies (n = 289 patients) found no difference in the proportion of patients who withdrew due to adverse events. Seven per cent (10/141) of sulfasalazine patients withdrew due to an adverse event compared to 6% (9/148) of placebo patients (RR 1.00, 95% CI 0.26 to 3.83).
1.1. Analysis.
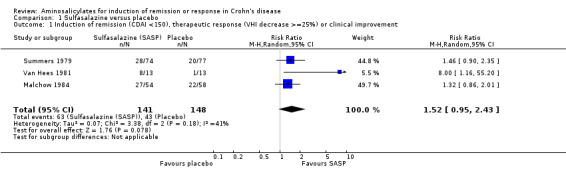
Comparison 1 Sulfasalazine versus placebo, Outcome 1 Induction of remission (CDAI <150), therapeutic response (VHI decrease >=25%) or clinical improvement.
1.2. Analysis.
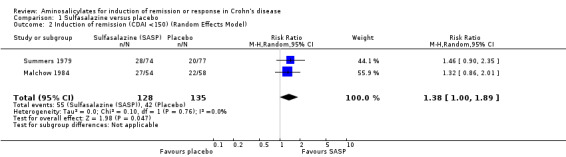
Comparison 1 Sulfasalazine versus placebo, Outcome 2 Induction of remission (CDAI <150) (Random Effects Model).
1.3. Analysis.
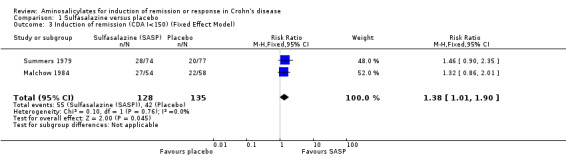
Comparison 1 Sulfasalazine versus placebo, Outcome 3 Induction of remission (CDA I<150) (Fixed Effect Model).
b. Sulfasalazine versus corticosteroids
Two trials, the NCCDS (Summers 1979) and ECCDS (Malchow 1984), compared the efficacy of sulfasalazine with corticosteroids. Per‐protocol results were reported due to lack of data.
Summers 1979 In the NCCDS, 38% (28/74) of sulfasalazine‐treated patients achieved remission compared to 47% (40/85) in the prednisone group (P = 0.25). Common adverse events reported in the prednisone group included acne, ecchymosis, moon face, psychic disturbances, peptic symptoms and hypertension.
Malchow 1984 In the ECCDS, less sulfasalazine‐treated patients achieved remission compared to 6‐methylprednisolone (50% [27/54] versus 83% [39/47], P = 0.001). Common adverse events reported in the 6‐methylprednisolone group included acne, moon face, headache, hypertension and infection.
Pooled analysis Combining results from these two trials (n = 260), sulfasalazine was clearly inferior to corticosteroids at 17 to 18 weeks of follow‐up (See Analysis 2.1). Forty‐three per cent (55/128) of sulfasalazine patients entered remission compared to 60% (79/132) corticosteroid patients (RR 0.68; 95% CI 0.51 to 0.91, P = 0.009. A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to sparse data (See Table 2). A sensitivity analysis using a fixed‐effect model had minimal impact: RR 0.70; 95% CI 0.55 to 0.88. A sensitivity analysis based on duration of therapy was not carried out, as the two trials had a similar duration of treatment.
2.1. Analysis.
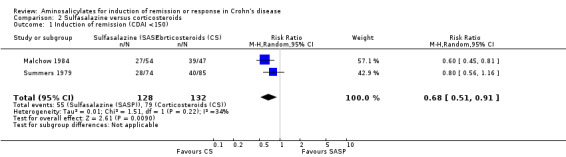
Comparison 2 Sulfasalazine versus corticosteroids, Outcome 1 Induction of remission (CDAI <150).
c. Sulfasalazine versus combination therapy with sulfasalazine and corticosteroids Two trials examined the efficacy of sulfasalazine either alone or in combination with corticosteroids. Per‐protocol results were reported due to the lack of data.
Malchow 1984 In the ECCDS (Malchow 1984), 50% (27/54) of sulfasalazine‐treated patients achieved remission at 18 weeks compared with 79% (44/56) in the combination therapy group (RR 0.64; 95% CI 0.47 to 0.86; P = 0.003). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to sparse data (See Table 3). In those that achieved remission, the CDAI score decreased to approximately 32% (sulfasalazine group) and 29% (combination group) of its initial value at the end of 18 weeks (See Comparison 3, Outcome 01; Analysis 3.1). The overall final CDAI in each treatment group was not reported. Common adverse events reported in the combination therapy group included acne, moon face, headache, back pain, hypertension and infection.
3.1. Analysis.
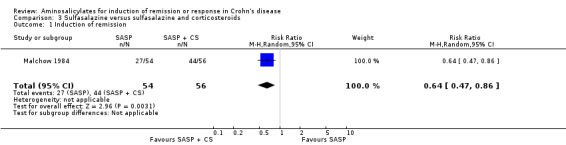
Comparison 3 Sulfasalazine versus sulfasalazine and corticosteroids, Outcome 1 Induction of remission.
Rijk 1991 Rijk 1991 randomly assigned 71 patients (11 dropouts) with active CD to receive higher doses of sulfasalazine at 4 to 6 g/day alone (n = 30) or in combination with lower doses of corticosteroids, prednisone 30 mg/day, equivalent to methylprednisolone 24 mg/day (n = 30, tapered 5 mg/2‐weeks and maintained at 10 mg/day) for 16 weeks. Therapeutic response in the initial 6 weeks (initial response) and the last 4 weeks (final response) were assessed using the Van Hees Activity Index (VHAI) and CDAI. Based on VHAI scores, a significantly greater and more rapid initial response was observed with combination therapy (median 30% decrease versus 13%, P = 0.001). This advantage remained statistically significant only in the a priori subgroup analysis of patients with severe disease at entry (VHAI ≥175) in the final response (median decrease 58% versus 30%, P = 0.02). A greater but insignificant decline in CDAI scores was seen with combination therapy (initial response: decrease of 35% versus 25%, P > 0.2; final response: decrease of 35% versus 24%, P = 0.19). The proportion of patients in remission was not reported in this trial. Although three of 11 drop‐outs withdrew due sulfasalazine‐related adverse events, adverse events were not reported on as an outcome.
Although results from these 2 trials could not be pooled together as they employed differing dosages, treatment regimens and endpoints, the results are consistent: sulfasalazine monotherapy was inferior to combination therapy with corticosteroids, particularly in patients with severe disease.
Mesalamine
a. Mesalamine versus placebo
Eight placebo‐controlled trials evaluated the efficacy of different dosages of controlled‐release mesalamine (Pentasa), delayed‐release mesalamine (Asacol) and olsalazine (Dipentum) for the treatment of mildly to moderately active Crohn's disease.
Controlled‐release Mesalamine 1 to 2 g/day Saverymuttu 1986 Saverymuttu 1986 provided some evidence that controlled‐release mesalamine reduced gut inflammation in mildly‐moderately active Crohn's colitis. Twelve patients were randomized to receive 1.5 g/day of Pentasa (n = 6) or placebo (n = 6) for 10 days. The primary outcome of the study was assessment of disease activity at the end of the study period with fecal granulocyte excretion, CDAI and erythrocyte sedimentation rate (ESR). Fecal granulocyte excretion was significantly reduced in all Pentasa‐treated patients (5% decrease, P < 0.01) but not in the placebo group (2.1% decrease, P = NS). No significant changes in CDAI or ESR were observed in this small study. One placebo patient withdrew due to an adverse event (nausea). No other adverse events were reported.
Three subsequent published studies (Rasmussen 1987; Mahida 1990; Singleton 1993) and one unpublished studies (Singleton 1994) examined the therapeutic efficacy of controlled‐release mesalamine at 1 to 2 g/day. Results based on ITT analysis are reported in this review.
Rasmussen 1987 Sixty‐seven patients with active Crohn's disease were randomized to Pentasa 1.5 g/day (n = 30) or placebo (n = 37) for 16 weeks (Rasmussen 1987). There was no significant difference between the treatment groups in the proportion of patients who achieved remission (as defined by Tvede 1983) or improvement (Pentasa 13/30, 43.3% versus placebo 9/37, 24.3%; RR 1.78; 95% CI 0.88 to 3.59). In addition, the cumulative proportion of patients achieving a > 33% reduction in CDAI did not differ between the 2 groups: 26% (Pentasa) versus 24% (placebo), P > 0.5. Common adverse events included headache, nausea and vomiting and itching. No serious adverse events were reported.
Mahida 1990 Similarly, Mahida and colleagues (Mahida 1990) did not find any therapeutic benefit of Pentasa 1.5 g/day in a pilot trial in which 40 patients with active Crohn's disease were randomly assigned to Pentasa 1.5 g/day (n = 20) or placebo (n = 20) for 6 weeks; 40% (8/20) and 35% (7/20) in the Pentasa and placebo group achieved "improvement" respectively, defined as a reduction of Harvey Bradshaw Index (HBI) by = 2 points (P = 0.74). Seven patients in the Pentasa group withdrew due to adverse events including deteriorating Crohn's disease (4 patients), abdominal distension and pain (1 patient) and malaise (2 patients). Four patients in the placebo group withdrew due to adverse events including deteriorating Crohn's disease (3 patients) and nausea (1 patient).
Singleton 1993 In the third trial, Singleton and colleagues (Singleton 1993) compared three daily doses of Pentasa at 1 g (n = 80), 2 g (n = 75) and 4 g (n = 75) with placebo (n = 80) in 310 patients with active CD for 16 weeks. Mean CDAI reductions (baseline to final study visit) in patients taking the 1 g/day (‐8) and 2 g/day (‐29) doses did not differ significantly from placebo‐treated patients (‐21). Remission (defined as CDAI ≤ 150 with > 50‐points reduction) and therapeutic benefit (defined as ≥ 50‐points reduction) was achieved in 22.5% (18/80) and 36.3% (29/80) in the 1‐g group, 24% (18/75) and 38.7% (29/75) in the 2‐g group, 17.5% (14/80) and 40% (32/80) in the placebo group respectively (P > 0.05). Results for the higher 4 g/day dose will be discussed in the next section. Common adverse events included nausea or vomiting, headache, abdominal pain, diarrhea and rash.
Singleton 1994 In a second trial by the same investigator (Singleton 1994, not fully published), 232 patients with active Crohn's disease were randomized to receive Pentasa 2 g/day (n = 82), 4 g/day (n = 75), or placebo (n = 75) for 16 weeks. Remission rates were not reported. There were no significant differences in CDAI scores between the Pentasa 2 g, 4 g and placebo groups (P > 0.05). The actual CDAI values were not available for the 2 g group; results on the 4 g group are discussed in the next section.
Pooled analysis Result from these three studies (Rasmussen 1987; Mahida 1990; Singleton 1993), were combined and analyzed (n =342). Pentasa at 1 to 2 g/day was not superior to placebo for inducing a therapeutic benefit defined by improvement in disease activity. Thirty‐eight per cent (79/205) of Pentasa patients improved compared to 35% (48/137) of placebo patients (RR 1.07; 95% CI 0.80 to 1.42; P = 0.65; See Analysis 4.1). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to sparse data and risk of bias (See Table 4). For the endpoint of induction of remission, results from Rasmussen 1987 and Singleton 1993 were pooled (n = 302). Similarly, Pentasa 1 to 2 g/day was not superior to placebo at 16 weeks follow‐up (See Analysis 4.2). Twenty‐three per cent (43/185) of Pentasa patients entered remission compared to 15% (18/117) of placebo patients (RR 1.46; 95% CI 0.89 to 2.40; P = 0.14). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to sparse data and risk of bias (See Table 4). Sensitivity analyses using a fixed‐effect model had minimal impact on point estimates with with a pooled RR of 1.08 for clinical improvement (95% CI 0.81 to 1.43) and 1.46 for clinical remission (95% CI 0.89 to 2.40) respectively. Sensitivity analysis based on duration of therapy, performed by excluding Mahida 1990, yielded similar RR of 1.08 (95% CI 0.75 to 1.54) and 1.46 (95% CI 0.89 to 2.40) respectively. A pooled analysis of two studies (342 patients) showed no statistically significant difference in the proportion of patients who had an adverse event or withdrew due to adverse events. Twenty‐eight per cent (58/205) of Pentasa patients had an adverse event compared to 23% (31/137) of placebo patients (RR 1.33, 95% CI 0.91 to 1.96). Twenty per cent (41/205) of Pentasa patients withdrew due to an adverse event compared to 15% (21/137) of placebo patients (RR 1.21, 95% CI 0.75 to 1.95).
4.1. Analysis.
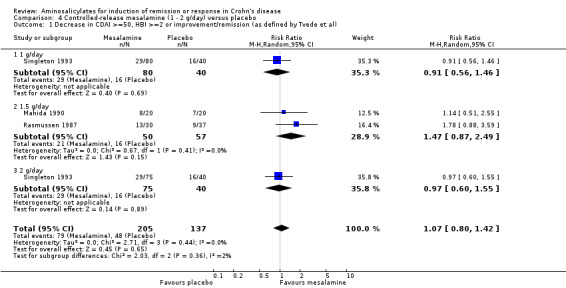
Comparison 4 Controlled‐release mesalamine (1 ‐ 2 g/day) versus placebo, Outcome 1 Decrease in CDAI >=50, HBI >=2 or improvement/remission (as defined by Tvede et al).
4.2. Analysis.
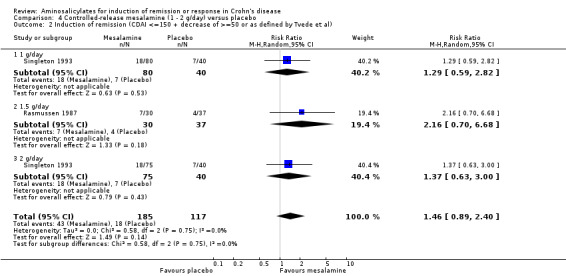
Comparison 4 Controlled‐release mesalamine (1 ‐ 2 g/day) versus placebo, Outcome 2 Induction of remission (CDAI <=150 + decrease of >=50 or as defined by Tvede et al).
Controlled‐release Mesalamine 4 g/day The efficacy of higher doses of Pentasa at 4 g/day was evaluated in three similarly designed, randomized, double‐blind, placebo‐controlled trials (Singleton 1993, Singleton 1994, Crohn's III 1997). Results based on ITT analysis are reported here. The proportion of patients in remission was only available for Singleton 1993. An attempt to provide additional information regarding the efficacy of high dose controlled release 5‐ASA in a meta‐analysis was performed by Hanauer 2004 using individual patient data from the three trials. This analysis revealed a small difference in reduction of CDAI between Pentasa and placebo treated patients of only 18 points (P = 0.04).
Singleton 1993 In the first Singleton trial (described in the previous section), Pentasa 4 g/day significantly reduced baseline CDAI (‐72 versus ‐21, P = 0.005). A greater proportion of patients in the Pentasa 4 g/day group achieved remission (42.7% [32/75] versus 17.5% [14/80], P = 0.001; RR 2.44 [95% CI 1.42 to 4.20], ABI = 25% and NNT = 4) and therapeutic benefit (64% [48/75] versus 40% [32/80], P = 0.004; RR 1.6 [95% CI 1.16 to 2.20], ABI = 24% and NNT = 4) when compared to placebo. The largest CDAI reduction was observed in those with isolated ileal disease (Singleton 1993). There was no statistically significant difference in the proportion of patients who experienced an adverse event or withdrew due to an adverse event. Twenty‐seven per cent (20/75) of Pentasa 4 g/day patients had an adverse event compared to 19% (15/80) of placebo patients (RR 1.42, 95% CI 0.79 to 2.57). Twelve per cent (9/75) of Pentasa 4 g/day patients withdrew due to an adverse event compared to 19% (15/80) of placebo patients (RR 0.64, 95% CI 0.30 to 1.37). Common adverse events included nausea or vomiting, headache, abdominal pain, diarrhea and rash.
Singleton 1994 In the second Singleton trial (described in the previous section), there were no significant differences in reduction of CDAI scores between the Pentasa 4 g/day and placebo groups (Pentasa 4 g/day ‐41 versus placebo ‐35; WMD ‐6; 95% CI ‐39 to ‐27) (Singleton 1994). Remission rates were not reported.
Crohn III 1997 In this third, unpublished, trial, Hanauer and colleagues (Crohn's III 1997), randomly assigned 310 patients to receive Pentasa 4 g/day (n = 154) or placebo (n = 156) for 16 weeks. There were no statistically significant differences in CDAI scores between the Pentasa 4 g/day and placebo groups (Pentasa 4/g day ‐72 versus placebo ‐64; WMD ‐8; 95% CI ‐33 to ‐17). The proportions of patients that achieved remission or therapeutic benefit were not reported.
Pooled analysis Data from these three studies were combined using an ITT approach (n = 615). A non‐significant mean difference (Pentasa ‐ placebo) in CDAI reduction of ‐19.8 (95% CI ‐46.2 to 6.7, P = 0.14) points was obtained (See Analysis 5.1). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to heterogeneity and risk of bias (See Table 5). However, a sensitivity analysis based on a fixed‐effect model yielded a mean difference in CDAI reduction of ‐17.5 (95% CI ‐35 to ‐0.1, P = 0.05; See Analysis 5.2). This difference is of questionable clinical significance because the minimum detectable difference in CDAI that a patient can detect is approximately 50 points (Brant 1999; Feagan 2004).
5.1. Analysis.
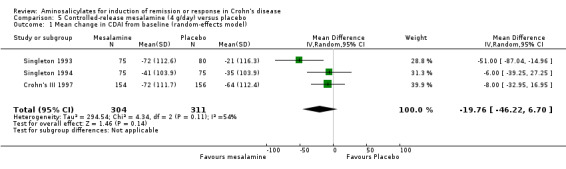
Comparison 5 Controlled‐release mesalamine (4 g/day) versus placebo, Outcome 1 Mean change in CDAI from baseline (random‐effects model).
5.2. Analysis.
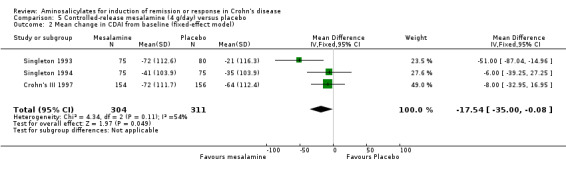
Comparison 5 Controlled‐release mesalamine (4 g/day) versus placebo, Outcome 2 Mean change in CDAI from baseline (fixed‐effect model).
Delayed‐release mesalamine
Tremaine 1994 Thirty‐eight patients with active Crohn's disease were randomly assigned to Asacol 3.2 g/day (n = 20) or placebo (n = 18) for 16 weeks (Tremaine 1994). On ITT analysis, more patients in the Asacol group (12/20, 60%) achieved 'complete success' (CDAI < 150 with ≥ 70‐points reduction) or 'partial success' (CDAI ≥ 150 with ≥ 70‐points reduction) compared to only 22.2% (4/18) in the placebo group (RR 2.70; 95% CI 1.06 to 6.88; P = 0.04; ABI = 37.8%; NNT = 3). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to high risk of bias due to incomplete outcome data and very sparse data (See Table 6). The difference in proportions of patients with 'complete success' (i.e. clinical remission) was not statistically significant (Asacol: 9/20, 45%; placebo: 4/18, 22.2%; RR 2.02, 95% CI 0.75 to 5.45; P = 0.16). Given the small sample size, a type II error has to be considered (See Analysis 6.1; Analysis 6.2). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to high risk of bias due to incomplete outcome data and very sparse data (See Table 6). Common adverse events included arthralgias, headache, abdominal cramps, nausea and dizziness.
6.1. Analysis.
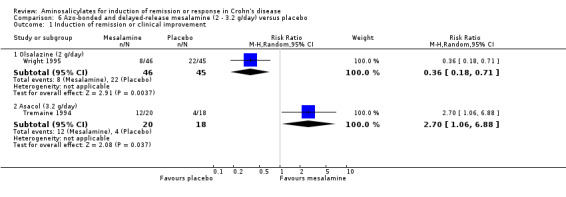
Comparison 6 Azo‐bonded and delayed‐release mesalamine (2 ‐ 3.2 g/day) versus placebo, Outcome 1 Induction of remission or clinical improvement.
6.2. Analysis.
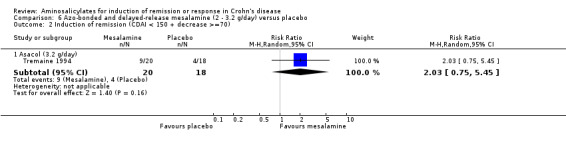
Comparison 6 Azo‐bonded and delayed‐release mesalamine (2 ‐ 3.2 g/day) versus placebo, Outcome 2 Induction of remission (CDAI < 150 + decrease >=70).
Azo‐bonded mesalamine: Olsalazine
Wright 1995 Ninety‐one patients with active Crohn's disease were randomized to receive olsalazine 2 g/day (n = 46) or placebo (n = 45) for 16 weeks (Wright 1995). A high withdrawal rate was observed: 35 of 46 (76.1%) patients taking olsalazine and 24 of 45 (53.3%) patients taking placebo. Although withdrawal rates for uncontrolled active disease were similar (28.3% versus 33.3% respectively, P = 0.6), a significant proportion of patients in the olsalazine group withdrew because of diarrhea (22% versus 4% respectively, P = 0.015). On ITT analysis, only 17.4% (8/46) olsalazine‐treated patients entered remission or had symptomatic improvement compared with 48.8% (22/45) placebo‐treated patients (RR 0.36, 95% CI 0.18 to 0.71; P = 0.004). However, this study was limited by high withdrawal rates and the small number of patients that actually completed the study (See Analysis 6.1). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to high risk of bias due to incomplete outcome data and very sparse data (See Table 6). Common adverse events included diarrhea, vomiting, pain, anorexia and itching rash.
b. Mesalamine versus corticosteroids
Delayed‐release mesalamine versus conventional corticosteroids
Four published trials compared delayed‐release mesalamine with a tapering dose of conventional corticosteroids.
Scholmerich 1990 In the first trial, Schölmerich and colleagues (Scholmerich 1990), randomized 62 patients with active Crohn's disease to Salofalk 2 g/day (n = 30) or 6‐methylprednisolone 48 mg/day (n = 32, tapered to 8 mg/day over 5 weeks) for 24 weeks: 73% (22/30) Salofalk‐treated patients stopped treatment due to "insufficient efficacy" compared to 34% (11/32) in the 6‐methylprednisolone group (P = 0.002), with corresponding lesser reduction in median CDAI scores (‐58 versus ‐151 respectively, P < .001).
Three subsequent trials evaluated the efficacy of higher doses of delayed‐release mesalamine (3 to 4.5 g/day) compared to conventional corticosteroids, with a total of 178 patients. Results based on ITT analysis are reported here.
Martin 1990 Martin and colleagues compared the efficacy of Salofalk 3 g/day (n = 22) with prednisone 40 mg (n = 28, 4 mg/week taper from third week) in 50 patients with active Crohn's disease (60% with isolated ileitis) over 12 weeks (Martin 1990). At week 12, remission (CDAI < 150) was achieved in 40.9% (9/22) Salofalk group and 42.9% (12/28) prednisone group (RR 0.95, 95% 0.49 to 1.85; P = 0.89). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (See Table 7). Final mean CDAI reductions (‐144 versus ‐147 respectively) were similar, although initial CDAI reduction was more rapid int the prednisone group. Post hoc subgroup analysis revealed that prednisone was significantly better at reducing CDAI at almost all time points in patients with ileocolitis; however, no difference in efficacy was found in patients with ileitis alone. Although a higher proportion of patients in the prednisone group experienced at least one adverse event (16/28) compared to the Salofalk group (6/22), the difference was not statistically significant (P = 0.05). Adverse events reported in the Salofalk group included insomnia, headache, edema and nausea. Adverse events reported in the prednisone group included hyperactivity, insomnia, headache, tiredness, edema, acne, and candidiasis. There was no difference in the proportion of patients who experienced a serious adverse event (P = 0.85). There were two serious adverse events in the Salofalk group (one patient with viral hepatitis and one patient with headaches and continuous vomiting) compared to three in the prednisone group (one patient with severe headache, one patient with severe intercostal herpes zoster and patient with severe cushingoid symptoms).
Gross 1995 In the second trial, 34 patients with active Crohn's disease (majority ileocolitis) were randomized to Salofalk 4.5 g/day (n = 17) or 6‐methylprednisolone 48 mg/day (n = 17, 8 mg weekly taper) for 8 weeks (Gross 1995). At 8 weeks, 35.3% (6/17) and 52.9% (9/17) patients achieved remission (CDAI < 150 with ≥ 60‐points decrease) respectively (RR 0.67, 95% CI 0.30 to 1.46; P = 0.3). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (See Table 7). Median change in CDAI: ‐85 (Salofalk) versus ‐122 (6‐methylprednisolone), P = 0.74. Although a trend towards higher efficacy with 6‐methyprednisolone was observed, a true difference may have been missed given the small size of this study. There was no statistically significant difference in the proportion of patients who had an adverse event. Sixty‐five per cent (11/17) of patients in the Salofalk group had an adverse event compared to 58% (10/17) of 6‐methylprednisolone patients (P = 0.72).
Prantera 1999 In the third and largest trial, 94 patients with active Crohn's disease (distal ileum or ileocecal region) were randomly assigned to treatment with Asacol tablets (n = 35) or Asacol microgranules (n = 28) at 4 g/day (tapered to 2.4 g/day), or 6‐methylprednisolone 40 mg/day (n = 31, 4mg weekly taper from third week) for 12 weeks (Prantera 1999). Stringent entry criteria led to recruitment of only 43% of the original planned sample size. At 12 weeks, remission (CDAI ≤ 150) was achieved in 60% (21/35), 78.6% (22/28) and 61.3% (19/31), with median CDAI reduction of 113.5, 123 and 154 in the Asacol tablets, Asacol microgranules and 6‐methylprednisolone groups respectively (P = 0.27 and P = 0.07). The RR for the comparison of Asacol tablets to corticosteroids was 1.00 (95% CI 1.00, 95% CI 0.61 to 1.64). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to sparse data (See Table 7). The RR for the comparison of Asacol granules to corticosteroids was 1.26 (95% CI 0.82 to 1.92). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to sparse data (See Table 7). A significantly higher proportion of adverse events was reported in the 6‐methylprednisolone group (14/31) compared to the Asacol tablets (5/35) and Asacol microgranules (3/28) groups. Adverse events thought to be related to steroids included acne, moon faces, hypertension, insomnia and excitability. Adverse events thought to be related to mesalamine included acute pancreatitis. A higher proportion of serious adverse events was reported in the 6‐methylprednisolone group (5/31) compared to the Asacol tablets (0/35) and Asacol microgranules (1/28) groups.
Pooled analysis The results of these three trials examining higher doses of delayed‐release mesalamine were combined and analyzed (n = 178). No significant difference in efficacy between delayed‐release mesalamine and conventional steroids was found. Fifty‐seven per cent (58/102) of mesalamine patients achieved remission compared to 53% of corticosteroid patients (RR 1.04, 95% CI 0.79 to 1.36; See Analysis 7.1). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to sparse data (See Table 7). Sensitivity analysis based on the fixed effect model and duration of therapy (by excluding Gross et al) had minimal effects on the results: RR 1.00 (95% CI 0.75 to 1.31) and 1.10 (95% CI 0.82 to 1.47) respectively. When the three studies were pooled to assess adverse events there was no statistically significant difference in the proportion of patients who experienced at least one adverse event, a serious adverse event, or withdrawal due to adverse events. Twenty‐four per cent (25/102) of mesalamine patients had at least one adverse event compared to 53% (40/76) of corticosteroid patients (RR 0.49, 95% CI 0.23 to 1.05; P = 0.07). Three per cent (3/102) of mesalamine patients had a serious adverse event compared to 10% (8/76) of corticosteroid patients (RR 0.35, 95% 0.10 to 1.27; P = 0.11). Four per cent (4/102) of mesalamine patients withdrew due to an adverse event compared to 13% (10/76) of corticosteroid patients (RR 0.39, 95% 0.13 to 1.15; P = 0.09).
7.1. Analysis.
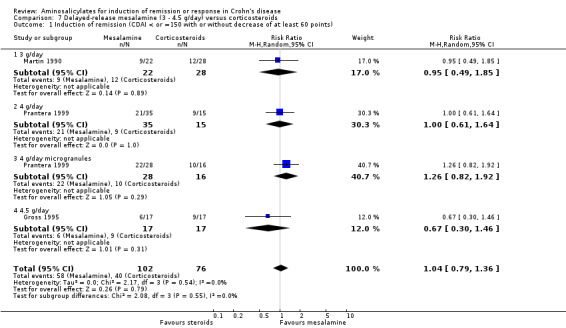
Comparison 7 Delayed‐release mesalamine (3 ‐ 4.5 g/day) versus corticosteroids, Outcome 1 Induction of remission (CDAI < or =150 with or without decrease of at least 60 points).
Mesalamine versus budesonide
Two studies evaluated the efficacy of controlled‐release (Pentasa) and delayed‐release (Salofalk) mesalamine compared with budesonide in patients with mildly to moderately active Crohn's disease. Thomsen 1998 The first trial compared Pentasa with budesonide (Entocort®) (Thomsen 1998). One hundred and eighty‐two patients with active Crohn's disease (disease limited to distal ileum and ascending colon) were randomly assigned to receive Pentasa 4 g/day (n = 89) or budesonide 9 mg/day (n = 93) for 16 weeks. Pentasa was significantly less effective than budesonide for inducing remission (CDAI ≤ 150): 33.7% (30/89) Pentasa group versus 60.2% (56/93) budesonide group at 16 weeks (RR 0.56, 95% CI 0.40 to 0.78; P = 0.0007; See Analysis 8.1). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to high risk of bias due to incomplete outcome data and sparse data (See Table 8). For budesonide, the calculated RR was 1.79 (95% CI 1.28‐2.50), ABI = 26.5% and a NNT = 4. Lower remission rates were observed for patients with more severe disease at entry (CDAI > 300, 11% Pentasa versus 41% budesonide, P = 0.01, RR 0.26 [95% CI 0.08 to 0.84]) and in those with colonic involvement (23% versus 56% respectively, P = 0.03, RR 0.41 [95% CI 0.21 to 0.77]) although budesonide was still more effective than Pentasa. The median time to remission was numerically but not significantly longer with mesalamine treatment (28 versus 58 days, P = 0.12) (Thomsen 2001). There was no statistically significant difference in the proportion of patients who had an adverse event or serious adverse event. Seventy‐two per cent (64/89) of Pentasa patients experienced at least one adverse event compared to 63% (59/93) of budesonide patients (RR 1.13, 95% CI 0.93 to 1.39; P = 0.22). Nineteen per cent (17/89) of Pentasa patients had a serious adverse event compared to 12% (11/93) of budesonide patients (RR 1.61, 95% CI 0.80 to 3.25; P = 0.18). Significantly more Pentasa patients withdrew due to an adverse event compared to budesonide patients. Thirty‐nine per cent (35/89) of Pentasa patients withdrew due to an adverse event compared to 14% (13/93) of budesonide patients (RR 2.81, 95% CI 1.60 to 4.96; P = 0.0003). Common adverse events included headache, abdominal pain, enteritis, nausea, back pain, dizziness. vomiting, anemia, depression and flatulence.
8.1. Analysis.
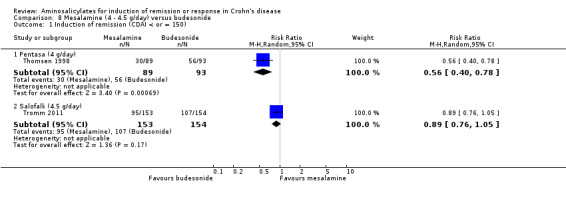
Comparison 8 Mesalamine (4 ‐ 4.5 g/day) versus budesonide, Outcome 1 Induction of remission (CDAI < or = 150).
Tromm 2011
Tromm 2011 compared Salofalk to budesonide (Budenofalk) in a study that was originally designed to assess the superiority of budesonide over mesalamine, but was converted to a non‐inferiority study due to a higher than expected response in the mesalamine arm. Three hundred and nine patients with mildly to moderately active Crohn's disease confined to the terminal ileum and/or ascending colon (84%) or distal colon (16%) were randomly assigned to receive Salofalk 4.5 g/day (n = 153) or budesonide 9 mg/day (taken 9 mg once daily [n = 76] or 3 mg three times daily [n = 78]; 2 patients were excluded from the analysis as baseline CDAI was less than 150) for 8 weeks. Remission (CDAI ≤ 150) was achieved in 62.1% (95/153) in those who received Salofalk compared to 69.5% (107/154) in the budesonide group (RR 0.89; 95% CI 0.76 to 1.05; P = 0.17; See Analysis 8.1). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was moderate due to sparse data (See Table 8). The median time to remission (mesalamine 16 days; budesonide 14 days) and mean change in CDAI scores from baseline (mesalamine ‐130; budesonide ‐149) also did not differ significantly between the two treatment groups. However, budesonide was more efficacious at inducing remission in patients with high baseline erythrocyte sedimentation rate (> 20 mm/hr) while a trend in favour of budesonide was also observed in patients with high baseline CRP (> 10 mg/L) and CDAI scores (> 300), suggesting that budesonide was more effective than delayed‐release mesalamine in patients with more severe inflammation. Clinical remission and response rates did not differ significantly between the two budesonide groups. There was no statistically significant difference in the proportion of patients who had an adverse event or withdrew due to adverse events. Forty‐seven per cent (72/153) of Salofalk patients experienced at least one adverse event compared to 43% (66/154) of budesonide patients (RR 1.10, 95% CI 0.86 to 1.41; P = 0.46). Five per cent (8/153) of Salofalk patients withdrew due to an adverse event compared to 3% (4/154) of budesonide patients (RR 2.01, 95% CI 0.62 to 6.55; P = 0.24). Common adverse events included abdominal pain, worsening Crohn's disease, vomiting, pyrexia, viral infection, decreased blood cortisol (in budesonide patients), back pain and headache.
As the two studies evaluated different formulations of mesalamine, results were not pooled for analysis.
c. Mesalamine versus sulfasalazine alone or in combination with corticosteroids
Two trials evaluated the efficacy of delayed‐release mesalamine compared to sulfasalazine (alone or in combination with corticosteroids).
Maier 1985 In an early small trial, Maier and colleagues (Maier 1985), randomly assigned 30 patients with active Crohn's disease to receive either Salofalk 1.5 g/day (n = 15) or sulfasalazine 3 g/day (n = 15) for 8 weeks. Compared to baseline values, mean CDAI decreased significantly in both groups (Salofalk: ‐189, P < .0001, Sulfasalazine: ‐148, P = 0.0001). There was no difference in clinical improvement rates. Eighty‐seven per cent of (13/15) Salofalk patients improved clinically compared to 73.3% (11/15) of sulfasalazine patients (RR 1.18, 95% CI 0.82 to 1.70; P = ‐0.37; See Analysis 9.1). The addition of corticosteroid therapy (Salofalk: 6; Sulfasalazine: 7) to patients who were still symptomatic after 5 days of aminosalicylates therapy, probably led to these impressive results. A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was very low due to risk of bias due to blinding and very sparse data (See Table 9). The authors reported no adverse events in the Salofalk group and four adverse events in the sulfasalazine group (RR 0.11, 95% CI 0.01 to 1.90; P = 0.13). Sulfasalazine had to be withdrawn in four patients due to intolerance.
9.1. Analysis.
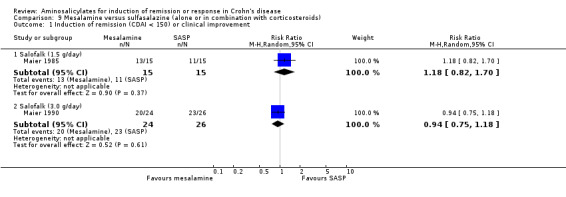
Comparison 9 Mesalamine versus sulfasalazine (alone or in combination with corticosteroids), Outcome 1 Induction of remission (CDAI < 150) or clinical improvement.
Maier 1990 In a subsequent trial, the same authors randomized 54 patients (4 dropouts) with active Crohn's disease to Salofalk 3 g/day (n = 24) or combination therapy with sulfasalazine 3 g/day and 6‐methylprednisolone 40 mg/day (n = 26, reductions of 4 mg/week) for 12 weeks (Maier 1990). At week 12, 83.3% (20/24) Salofalk‐treated patients and 88.5% (23/26) in the combination group achieved remission (CDAI < 150) (RR 0.94, 95% 0.75 to 1.18; P = 0.61, See Analysis 9.1). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to risk of bias due to blinding and very sparse data (See Table 9). There was a mean change in CDAI of ‐148 and ‐146 respectively (P = 0.90). There was no difference in the proportion of patients who experienced an adverse event. Twelve per cent (3/24) of Salofalk patients experienced an adverse event compared to 23% (6/26) of sulfasalazine patients (RR 0.54, 95% CI 0.15 to 1.93). It should be noted that Maier 1985 and Maier 1990 were not designed as formal equivalence or non‐inferiority trials. Future trials would require the randomization of large numbers of patients.
Discussion
The focus of this review was broad, and all information pertaining to the efficacy of sulfasalazine or 5‐ASA alone for the treatment of mildly to moderately active Crohn's disease when compared to placebo, corticosteroids, and other aminosalicylates (alone or in combination with corticosteroids) was sought. To ensure that effect estimates were interpretable and clinically meaningful in this review where trials were anticipated to be diverse with different aminosalicylate formulations, dosages, comparators and outcome, each combination was considered separately and only results from similar trials were synthesized. A random‐effects model was used for pooling data as it allowed between‐trial variability to be accounted for in the overall effect estimate, producing more conservative results with wider 95% confidence intervals. Results based on a fixed‐effect model and duration of therapy were also presented in sensitivity analyses planned a priori and these did not significantly alter the results obtained with the random‐effects model or when trials with different duration of treatment were included.
The results showed that sulfasalazine at 3 to 6 g/day had only modest efficacy for inducing remission, and a trend towards benefit of sulfasalazine over placebo was observed (pooled RR 1.38, random‐effects model 95% CI 1.00 ‐ 1.89, fixed‐effect model 95% CI 1.01‐1.90). This benefit was limited to patients with Crohn's colitis. Patients with small bowel disease or those who continued to have active disease despite previous corticosteroid and sulfasalazine treatment were not likely to benefit. Sulfasalazine was less effective than corticosteroids with a pooled RR of 0.68, that is, sulfasalazine‐treated patients had 32% less chance of achieving remission than corticosteroid‐treated patients. In addition, sulfasalazine monotherapy was less effective than combination therapy with corticosteroids. The question of whether sulfasalazine is a useful adjunct to corticosteroid therapy, while not the primary focus of this review, was addressed in the TAS (Trial of adjunctive sulfasalazine in Crohn's disease) study (Singleton 1979) and ECCDS: 74% (34/56) and 83% (39/47) in the corticosteroid group versus 58% (25/43) and 79% (44/56) in the combination group achieved remission (CDAI < 150) at the end of 8 weeks and 18 weeks respectively (P = 0.12 and 0.57 respectively) with a pooled RR of 1.12 (95% CI 0.94 to 1.33) (See Figure 3), demonstrating that sulfasalazine was not a useful adjunct to corticosteroid therapy. These data suggest that sulfasalazine may be considered for patients with mildly to moderately active Crohn's colitis, reserving the more potent corticosteroids for patients failing sulfasalazine therapy. This is consistent with the pharmacology of sulfasalazine, an azo‐bonded prodrug that requires colonic bacteria azo‐reductases for the release and targeted delivery of active 5‐ASA moiety to the colon. In contrast, olsalazine, a new azo‐bonded 5‐ASA dimer, was shown to lack therapeutic effect, with a significant proportion developing worsening diarrhoea (Wright 1995). This is a common and unique dose‐related complication of olsalazine therapy, a consequence of increased ileal secretion and gastrointestinal transit (Rao 1987; Wadworth 1991; Sandborn 2002a).
3.
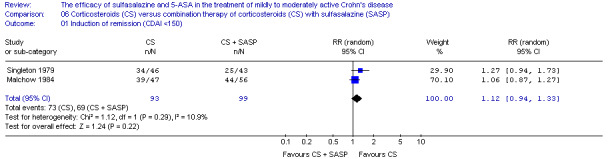
While mesalamine has similar efficacy to sulfasalazine when equimolar doses are used in ulcerative colitis (3 to 6 g of sulfasalazine is equivalent to 1.2 to 2.4 g of mesalamine), low dose controlled‐release mesalamine (Pentasa at 1 to 2 g/day) was not more effective than placebo for inducing remission in active Crohn's disease (Analysis 4.2). Predictably, delayed‐release mesalamine (Salofalk at 2 g/day) was less efficacious than corticosteroids (Scholmerich 1990).
Trials assessing higher doses of mesalamine produced conflicting results. Controlled‐release mesalamine (Pentasa) at 4 g/day produced a clinically insignificant reduction in CDAI compared to placebo (Singleton 1993; Singleton 1994; Crohn's III 1997, Comparison 05, Outcome 01 and 02; Analysis 5.1; Analysis 5.2), and was inferior to budesonide for inducing remission in active Crohn's disease (Thomsen 1998; Analysis 8.1). The positive effect of Pentasa at 4 g/day in one study (Singleton 1993), was in contrast to the lack of effect in similarly designed trials (Singleton 1994; Crohn's III 1997). Moderate heterogeneity and rather large placebo effects could have reduced the power to detect a statistically significant difference between placebo and mesalamine, should one exist. However, placebo remission and response rates were not reported for the Singleton 1994 and Crohn's III 1997 studies and only changes in CDAI scores were available. Based on a mean difference in CDAI scores of approximately 10 points these studies would have needed to randomize a very large number of patients to have the statistical power to detect a significant difference between placebo and controlled‐release mesalamine. The Singleton 1994 and Crohn's III 1997 studies were therefore too small and lacked the statistical power to detect any significant difference between placebo and controlled‐release mesalamine. A meta‐analysis of clinical trials in active Crohn's disease has estimated placebo remission and response rates at 18% (95% CI 14 to 24%) and 19% (95% CI 13 to 28%) respectively (Su 2004). High placebo response rates may reduce the power to detect a small but statistically significant difference between placebo and active treatment. Randomized, placebo‐controlled trials assessing biologic therapy in active Crohn's disease exhibited placebo response rates of 23.5% and 35.6% respectively (Sandborn 2004; Schreiber 2005). In post hoc subgroup analyses of patients with elevated C‐reactive protein (CRP) at baseline the placebo remission rates were 15.5% and 17.9% respectively (Sandborn 2004; Schreiber 2005), improving the apparent efficacy of active study drug. High CRP, an indicator of active inflammation, appeared to improve the 'efficiency' of clinical trials by separating placebo from drug responders. Therein lies the limitations of using CDAI as a measure of disease activity and efficacy endpoint in clinical trials: it is an indicator of 'illness' rather than inflammation. The CDAI may not correlate with pathology and laboratory markers (Cellier 1994), and is heavily weighted to 'intensity of abdominal pain' and 'general well being', subjective items that rely substantially on patients' perception of their disease (Sandborn 2002b). High placebo response rates may result from the inclusion of patients with mild disease or predominantly functional symptoms, frequent study visits and intense contact with health care providers during the trial period (Su 2004).
While delayed‐release mesalamine was not superior to placebo (Tremaine 1994), no difference in efficacy was found when compared to corticosteroids (Gross 1995; Martin 1990; Prantera 1999), or budesonide (Tromm 2011), for inducing remission in patients with mildly to moderately active Crohn's disease. While no difference for induction of remission relative to placebo was demonstrated for delayed‐release mesalamine (Asacol) at a dosage of 3.2 g/day (Analysis 6.2), no significant difference was found between corticosteroids and delayed‐release mesalamine (Asacol, Salofalk) at doses ranging 3 to 4.5 g/day (Analysis 7.1). Subgroup analyses found that systemic corticosteroids appeared to be more effective than delayed‐release mesalamine in patients with more extensive ileocolonic disease (Martin 1990, Gross 1995). There was no difference in efficacy when the disease was confined to the ileum or ileocecal region (Martin 1990, Prantera 1999). However, these results must be interpreted with caution as: (a) these trials were limited by the relatively small number of patients (4 studies ranging from 34 to 94 patients) and were thus underpowered, increasing the probability of a type II error, (b) a fixed dose of mesalamine was compared with a tapering dose of corticosteroids, which may have obscured any differences between the two agents. If higher doses of corticosteroids had been used a difference may have been found, (c) studies comparing delayed‐release mesalamine versus corticosteroids were small in size and were not designed as formal equivalence or non‐inferiority trials, (d) subgroup analyses involved small numbers of patients and results should be interpreted with care, and (e) the heterogeneous spectrum of clinical patterns in Crohn's disease could also account for the difference in response to therapy in these trials, and focusing on a more homogenous group of patients should be considered in future trials of active Crohn's disease. Furthermore, budesonide has been shown to be superior to controlled‐release mesalamine (Thomsen 1998), and conventional corticosteroids have been shown in other studies to be somewhat superior to budesonide (Rutgeerts 1994; Gross 1996; Campieri 1997; Bar‐Meir 1998). In contrast to Thomsen 1998, delayed‐release mesalamine (Salofalk) at 4.5 g/day was at least as effective as budesonide in patients with mildly to moderately active ileocolonic Crohn's disease (Tromm 2011), although budesonide still appeared to be more effective in those with more severe disease, characterized by high ESR, CRP and CDAI >300. It was postulated that this unexpected result may be accounted for by the inclusion of patients with less previous resections, shorter duration of disease and a slightly higher dose of mesalamine using a formulation designed to release mesalamine in the terminal ileum with greater mesalamine absorption (compared to Thomsen 1998). However, the relatively small size of this non‐inferiority study and the inclusion of 50‐60% of patients with normal inflammatory markers (CRP < 5 mg/L, ESR < 20 mm/hr) does not allow any firm conclusions to be made on the efficacy of delayed‐release mesalamine in active Crohn's disease (Levesque 2012).
Several methodological weaknesses may limit the generalizability and precision of effect estimates in this review. First, two trials rated as being of poor quality were included and the results of these studies should be interpreted with caution (Maier 1985; Maier 1990). Second, the use of different scoring systems and definitions of endpoints have added to the difficulty of comparing and pooling results. In this review, pooled risk ratios of trials comparing controlled‐release mesalamine (1 to 2 g/day) with placebo and delayed‐release mesalamine with corticosteroids were calculated despite these deficiencies and should be interpreted with a degree of caution. Recent recommendations to adopt common definitions of endpoints in clinical trials for Crohn's disease will aid in the comparison and synthesis of results in future conducted trials (Sandborn 2002b). Third, the number of patients whose results were pooled in each analysis was relatively small and the precision of effect estimate was thus less than ideal. Lastly, the possibility of publication bias could not be excluded as visual interpretation of funnel plots have limited power to detect bias in this review due to the small number of studies in each analysis (Munafo 2004). In addition, we did not contact other inflammatory bowel disease investigators or manufacturers of sulfasalazine and other 5‐ASA formulations for unpublished papers. These limitations are reflected in the GRADE analyses which rate the overall quality of the evidence supporting the outcomes assessed in this review as very low (See Table 6; Table 9), low (See Table 1; Table 4; Table 5; Table 7; Table 8; Table 9), or moderate quality (See Table 1;Table 2; Table 3; Table 7; Table 8).
Authors' conclusions
Implications for practice.
In conclusion, current available data suggests that for the treatment of mildly to moderately active Crohn's disease: 1. Sulfasalazine at 3 to 6 g/day was only modestly effective (trend towards benefit over placebo), with benefit confined to those with colitis. 2. Sulfasalazine was inferior and not a useful adjunct to corticosteroid therapy. 3. Olsalazine and mesalamine at 1 to 2 g/day were ineffective and not superior to placebo. 4. Higher doses of controlled‐release mesalamine at 4 g/day: a. Have resulted in statistically significant but clinically non significant changes in CDAI scores but have not been consistently shown to be effective for induction of remission in mild to moderately active Crohn's disease. b. Are inferior to budesonide.
5. Higher doses of delayed‐release mesalamine at 3‐4.5 g/day may be as effective as budesonide in those with mildly to moderately active ileocolonic disease. Although the superiority of conventional steroids has not been consistently demonstrated, in the absence of a sufficiently powered formal equivalence or non‐inferiority study, it is likely that delayed‐release mesalamine would be inferior to conventional steroids. 6. There was a lack of good quality clinical trials comparing sulfasalazine with other mesalamine formulations.
Implications for research.
There has been an evolution of clinical trials over the past 30 plus years, during which aminosalicylates have been evaluated for the treatment of mild‐moderate Crohn's disease. The heterogeneity in clinical trials with respect to patients enrolled, entry and exclusion criteria, end‐points, duration, dose, delivery system and placebo responses contribute to the inconsistent trial outcomes and interpretations of a large body of evidence pertaining to aminosalicylates in mild‐moderate Crohn's disease. To date, while a body of evidence suggests a small modest benefit for aminosalicylates on clinical indices used to assess Crohn's disease activity, there is insufficient evidence to indicate that they are effective for induction of remission or mucosal healing. Future large randomized controlled trials are needed to provide definitive evidence on the efficacy of aminosalicylates in active Crohn's disease. As mesalamine is likely to be most effective in the terminal ileum and proximal colon, future trials should examine its efficacy in patients with this disease distribution.
What's new
| Date | Event | Description |
|---|---|---|
| 10 June 2015 | New search has been performed | New literature search performed on June 10, 2015. One new study was added. |
| 10 June 2015 | New citation required and conclusions have changed | Updated review with minor changes to conclusions and new authors |
Acknowledgements
The authors would like to thank Ms. Claire Parker for her assistance in literature search and Dr. Thomas Bockle for his assistance in translating the German‐language article.
Partial funding for the Cochrane IBD Group (April 1, 2016 ‐ March 31, 2018) has been provided by Crohn's and Colitis Canada (CCC).
Appendices
Appendix 1. Search Strategies
MEDLINE on PUBMED was searched using the following search terms:
| #1 crohn* #2 sulphasalazine OR sulfasalazine OR salazosulphapyr* OR salazosulfapyr* OR salicylazosulphapyr* OR salicylazosulfapyr* OR salazopyrin #3 mesalamine OR mezalamine OR aminosalicylate* OR aminosalicylic acid OR 5‐aminosalicylate* OR 5‐aminosalicylic acid OR 5‐ASA #4 #2 OR #3 #5 #4 AND #1 #6 singl* OR doubl* OR tripl* OR trebl* OR blind* OR mask* OR placebo* OR single‐blind* OR double‐blind* OR triple‐blind* OR random* OR (controlled clinical) #7 #5 AND #6 |
EMBASE database was searched using the following search terms:
| #1 random$.tw. #2 factorial$.tw. #3 (crossover$ or cross over$ or cross‐over$).tw. #4 placebo$.tw. #5 single blind.mp. #6 double blind.mp. #7 triple blind.mp. #8 (singl$ adj blind$).tw. #9 (double$ adj blind$).tw. #10 (tripl$ adj blind$).tw. #11 assign$.tw. #12 allocat$.tw. #13 crossover procedure/ #14 double blind procedure/ #15 single blind procedure/ #16 triple blind procedure/ #17 randomized controlled trial/ #18 or/1‐17 #19 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) #20 #18 not #19 #21 exp salazosulfapyridine/ #22 (sulphasalazine or sulfasalazine or salazosulphapyr* or salazosulfapyr* or salicylazosulphapyr* or salicylazosulfapyr* or salazopyrin*).tw. #23 mesalamine.tw. or exp mesalazine/ #24 exp aminosalicylic acid/ #25 aminosalicylate*.tw. or exp aminosalicylic acid derivative/ or exp aminosalicylic acid/ #26 (mesalazine or aminosalicylic acid or 5‐aminosalicylate* or 5‐aminosalicylic acid or 5‐ASA or olsalazine).tw. #27 or/21‐26 #28 exp Crohn disease/ or crohn*.tw. #29 #20 and #27 and #28 |
OVID MEDLINE(R) database was searched using the following search terms:
| #1 random$.tw. #2 factorial$.tw. #3 (crossover$ or cross over$ or cross‐over$).tw. #4 placebo$.tw. #5 single blind.mp. #6 double blind.mp. #7 triple blind.mp. #8 (singl$ adj blind$).tw. #9 (double$ adj blind$).tw. #10 (tripl$ adj blind$).tw. #11 assign$.tw. #12 allocat$.tw. #13 crossover procedure/ #14 double blind procedure/ #15 single blind procedure/ #16 triple blind procedure/ #17 randomized controlled trial/ #18 or/1‐17 #19 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) #20 #18 not #19 #21 exp salazosulfapyridine/ #22 (sulphasalazine or sulfasalazine or salazosulphapyr* or salazosulfapyr* or salicylazosulphapyr* or salicylazosulfapyr* or salazopyrin*).tw. #23 mesalamine.tw. or exp mesalazine/ #24 exp aminosalicylic acid/ #25 aminosalicylate*.tw. or exp aminosalicylic acid derivative/ or exp aminosalicylic acid/ #26 (mesalazine or aminosalicylic acid or 5‐aminosalicylate* or 5‐aminosalicylic acid or 5‐ASA or olsalazine).tw. #27 or/21‐26 #28 exp Crohn disease/ or crohn*.tw. #29 #20 and #27 and #28 |
Cochrance Central Library database was searched using the following search terms:
| #1 crohn* #2 sulphasalazine or sulfasalazine or salazosulphapyr* or salazosulfapyr* or salicylazosulphapyr* or salicylazosulfapyr* #3 mesalamine or mezalamine or aminosalicylate* or aminosalicylic acid or 5‐aminosalicylate* or 5‐aminosalicylic acid or 5‐ASA #4 #2 or #3 #5 #1 and #4 |
The Cochrane IBD‐FBD Specialized Register was searched using the following terms:
| #1 (sulpha or sulfa or sala or salicyl or mesala or aminosal or 5‐aminosal or 5‐ASA or olsal).ti. #2 Crohn.ti. #3 1 and 2 |
Data and analyses
Comparison 1. Sulfasalazine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction of remission (CDAI <150), therapeutic response (VHI decrease >=25%) or clinical improvement | 3 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.95, 2.43] |
| 2 Induction of remission (CDAI <150) (Random Effects Model) | 2 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.00, 1.89] |
| 3 Induction of remission (CDA I<150) (Fixed Effect Model) | 2 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.01, 1.90] |
| 4 Adverse events | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.75, 5.80] |
| 5 Serious adverse events | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.01, 8.38] |
| 6 Withdrawal due to adverse events | 3 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.26, 3.83] |
1.4. Analysis.
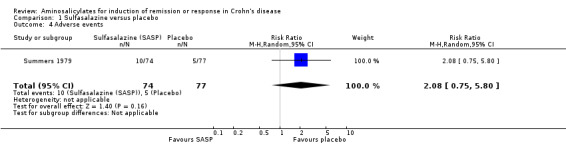
Comparison 1 Sulfasalazine versus placebo, Outcome 4 Adverse events.
1.5. Analysis.
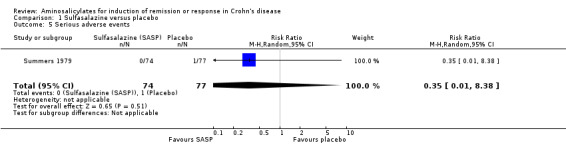
Comparison 1 Sulfasalazine versus placebo, Outcome 5 Serious adverse events.
1.6. Analysis.
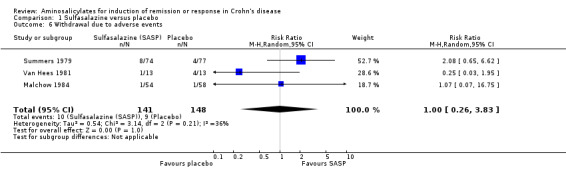
Comparison 1 Sulfasalazine versus placebo, Outcome 6 Withdrawal due to adverse events.
Comparison 2. Sulfasalazine versus corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction of remission (CDAI <150) | 2 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.51, 0.91] |
| 2 Adverse events | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.22, 0.82] |
| 3 Serious adverse events | 1 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.01, 3.12] |
| 4 Withdrawal adverse events | 2 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.33, 1.59] |
2.2. Analysis.
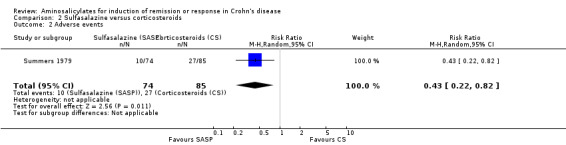
Comparison 2 Sulfasalazine versus corticosteroids, Outcome 2 Adverse events.
2.3. Analysis.
Comparison 2 Sulfasalazine versus corticosteroids, Outcome 3 Serious adverse events.
2.4. Analysis.
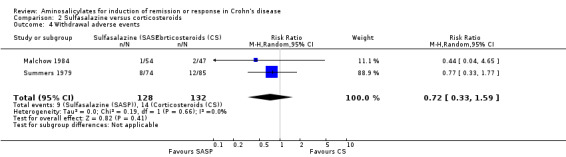
Comparison 2 Sulfasalazine versus corticosteroids, Outcome 4 Withdrawal adverse events.
Comparison 3. Sulfasalazine versus sulfasalazine and corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction of remission | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.47, 0.86] |
| 2 Withdrawal due to adverse events | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.05, 5.55] |
3.2. Analysis.
Comparison 3 Sulfasalazine versus sulfasalazine and corticosteroids, Outcome 2 Withdrawal due to adverse events.
Comparison 4. Controlled‐release mesalamine (1 ‐ 2 g/day) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Decrease in CDAI >=50, HBI >=2 or improvement/remission (as defined by Tvede et al) | 3 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.80, 1.42] |
| 1.1 1 g/day | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.56, 1.46] |
| 1.2 1.5 g/day | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.87, 2.49] |
| 1.3 2 g/day | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.60, 1.55] |
| 2 Induction of remission (CDAI <=150 + decrease of >=50 or as defined by Tvede et al) | 2 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.89, 2.40] |
| 2.1 1 g/day | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.59, 2.82] |
| 2.2 1.5 g/day | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [0.70, 6.68] |
| 2.3 2 g/day | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.63, 3.00] |
| 3 Adverse events | 3 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.91, 1.96] |
| 3.1 1 g/day | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.55, 2.69] |
| 3.2 1.5 g/day | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.73, 2.24] |
| 3.3 2 g/day | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.76, 3.11] |
| 4 Withdrawal due to adverse events | 3 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.75, 1.95] |
| 4.1 1 g/day | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.48, 2.42] |
| 4.2 1.5 g/day | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.56, 3.86] |
| 4.3 2 g/day | 1 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.57, 2.51] |
4.3. Analysis.
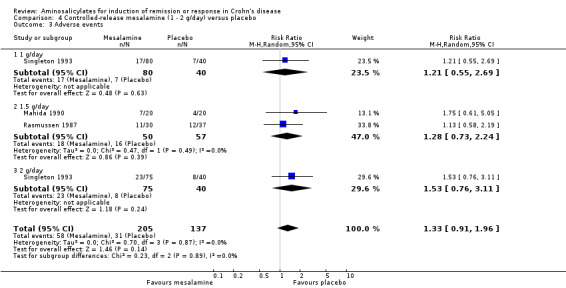
Comparison 4 Controlled‐release mesalamine (1 ‐ 2 g/day) versus placebo, Outcome 3 Adverse events.
4.4. Analysis.
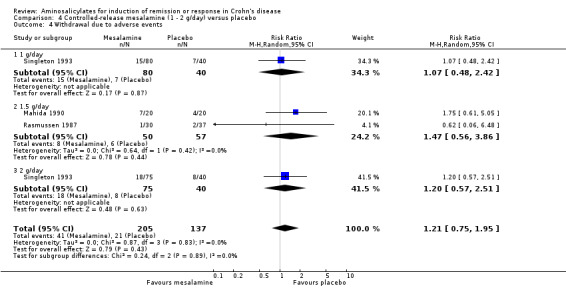
Comparison 4 Controlled‐release mesalamine (1 ‐ 2 g/day) versus placebo, Outcome 4 Withdrawal due to adverse events.
Comparison 5. Controlled‐release mesalamine (4 g/day) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in CDAI from baseline (random‐effects model) | 3 | 615 | Mean Difference (IV, Random, 95% CI) | ‐19.76 [‐46.22, 6.70] |
| 2 Mean change in CDAI from baseline (fixed‐effect model) | 3 | 615 | Mean Difference (IV, Fixed, 95% CI) | ‐17.54 [‐33.00, ‐0.08] |
| 3 Adverse events | 1 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.79, 2.57] |
| 4 Withdrawal due to adverse events | 1 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.30, 1.37] |
5.3. Analysis.
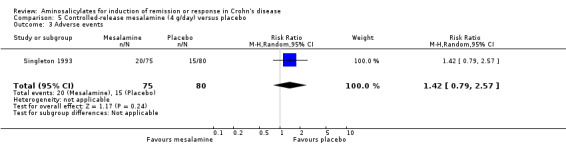
Comparison 5 Controlled‐release mesalamine (4 g/day) versus placebo, Outcome 3 Adverse events.
5.4. Analysis.
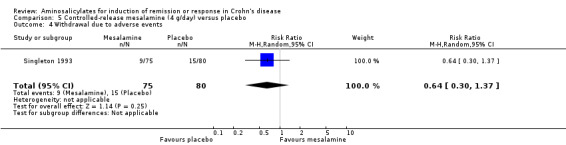
Comparison 5 Controlled‐release mesalamine (4 g/day) versus placebo, Outcome 4 Withdrawal due to adverse events.
Comparison 6. Azo‐bonded and delayed‐release mesalamine (2 ‐ 3.2 g/day) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction of remission or clinical improvement | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Olsalazine (2 g/day) | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.18, 0.71] |
| 1.2 Asacol (3.2 g/day) | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 2.70 [1.06, 6.88] |
| 2 Induction of remission (CDAI < 150 + decrease >=70) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Asacol (3.2 g/day) | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.75, 5.45] |
| 3 Adverse events | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.9 [0.68, 1.18] |
| 4 Withdrawal due to adverse events | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.99, 2.24] |
6.3. Analysis.
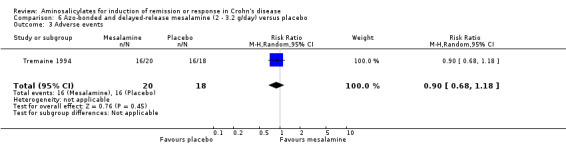
Comparison 6 Azo‐bonded and delayed‐release mesalamine (2 ‐ 3.2 g/day) versus placebo, Outcome 3 Adverse events.
6.4. Analysis.
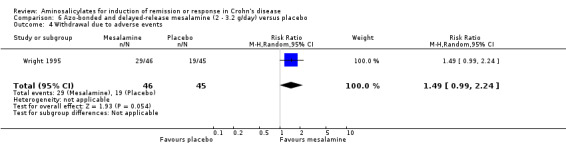
Comparison 6 Azo‐bonded and delayed‐release mesalamine (2 ‐ 3.2 g/day) versus placebo, Outcome 4 Withdrawal due to adverse events.
Comparison 7. Delayed‐release mesalamine (3 ‐ 4.5 g/day) versus corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction of remission (CDAI < or =150 with or without decrease of at least 60 points) | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.79, 1.36] |
| 1.1 3 g/day | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.49, 1.85] |
| 1.2 4 g/day | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.61, 1.64] |
| 1.3 4 g/day microgranules | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.82, 1.92] |
| 1.4 4.5 g/day | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.30, 1.46] |
| 2 Adverse events | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.23, 1.05] |
| 2.1 3 g/day | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.22, 1.01] |
| 2.2 4 g/day | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.12, 0.81] |
| 2.3 4 g/day microgranules | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.07, 0.82] |
| 2.4 4.5 g/day | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 1.1 [0.65, 1.87] |
| 3 Serious adverse events | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.10, 1.27] |
| 3.1 3 g/day | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.16, 4.64] |
| 3.2 4 g/day | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.00, 1.75] |
| 3.3 4 g/day microgranules | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.68] |
| 3.4 4.5 g/day | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Withdrawal due to adverse events | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.13, 1.15] |
| 4.1 3 g/day | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.16, 4.64] |
| 4.2 4 g/day | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.00, 1.75] |
| 4.3 4 g/day microgranules | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.68] |
| 4.4 4.5 g/day | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.01] |
7.2. Analysis.
Comparison 7 Delayed‐release mesalamine (3 ‐ 4.5 g/day) versus corticosteroids, Outcome 2 Adverse events.
7.3. Analysis.
Comparison 7 Delayed‐release mesalamine (3 ‐ 4.5 g/day) versus corticosteroids, Outcome 3 Serious adverse events.
7.4. Analysis.
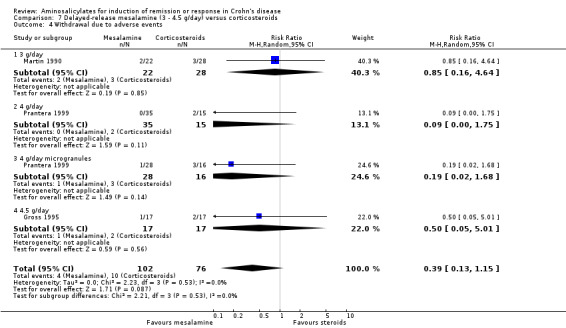
Comparison 7 Delayed‐release mesalamine (3 ‐ 4.5 g/day) versus corticosteroids, Outcome 4 Withdrawal due to adverse events.
Comparison 8. Mesalamine (4 ‐ 4.5 g/day) versus budesonide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction of remission (CDAI < or = 150) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Pentasa (4 g/day) | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.40, 0.78] |
| 1.2 Salofalk (4.5 g/day) | 1 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.76, 1.05] |
| 2 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Pentasa (4 g/day) | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.93, 1.39] |
| 2.2 Salofalk (4.5 g/day) | 1 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.86, 1.41] |
| 3 Serious adverse events | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.80, 3.25] |
| 4 Withdrawal due to adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Pentasa (4 g/day) | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [1.60, 4.96] |
| 4.2 Salofalk (4.5 g/day) | 1 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.62, 6.55] |
8.2. Analysis.
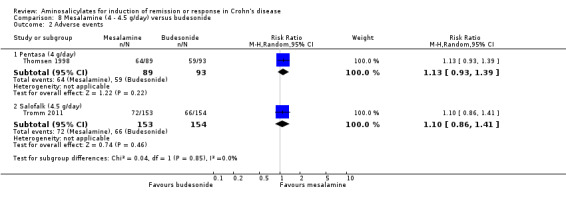
Comparison 8 Mesalamine (4 ‐ 4.5 g/day) versus budesonide, Outcome 2 Adverse events.
8.3. Analysis.
Comparison 8 Mesalamine (4 ‐ 4.5 g/day) versus budesonide, Outcome 3 Serious adverse events.
8.4. Analysis.
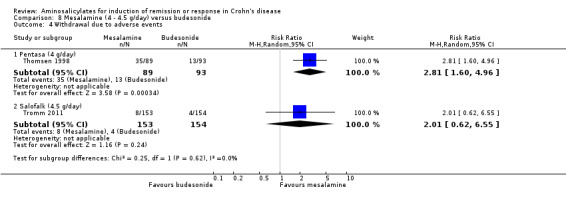
Comparison 8 Mesalamine (4 ‐ 4.5 g/day) versus budesonide, Outcome 4 Withdrawal due to adverse events.
Comparison 9. Mesalamine versus sulfasalazine (alone or in combination with corticosteroids).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Induction of remission (CDAI < 150) or clinical improvement | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Salofalk (1.5 g/day) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.82, 1.70] |
| 1.2 Salofalk (3.0 g/day) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.75, 1.18] |
| 2 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Salofalk (1.5 g/day) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.90] |
| 2.2 Salofalk (3.0 g/day) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.15, 1.93] |
9.2. Analysis.
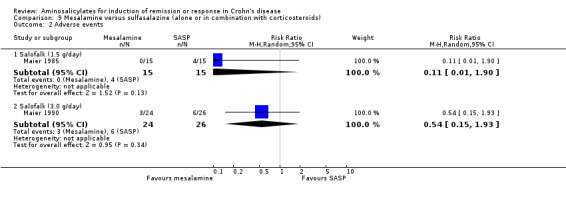
Comparison 9 Mesalamine versus sulfasalazine (alone or in combination with corticosteroids), Outcome 2 Adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Crohn's III 1997.
| Methods | Multicenter study. Randomized. Double blind. Parallel design. Methods of randomization/ blinding, concealment of allocation, adequate follow up could not be assessed as study has not been fully published | |
| Participants | Male and female patients, adults with mildly to moderately active Crohn's disease, entry CDAI 200 to 400. Disease location: ileum and/or colon. Pentasa 4 g/day: mean age 42 years, mean CDAI 265; placebo: mean age 39, mean CDAI 265 | |
| Interventions | Pentasa 4 g/day versus placebo for 16 weeks. n = 310; allocation: 154 to Pentasa 4 g/day, 156 to placebo | |
| Outcomes | Primary endpoint: change in CDAI from baseline to final study visit | |
| Notes | Study has not been fully published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 50/154 patients in the Pentasa group and 60/156 patients in the placebo group did not complete the study/ |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess |
| Other bias | Unclear risk | Unable to assess |
Gross 1995.
| Methods | Multicenter study. Randomized, method of randomization not described. Double‐blind with use of double‐dummy. Parallel design. Follow up described: yes | |
| Participants | Male and female patients, entry CDAI 150 to 350. Disease location: ileum and/or colon. Mean age 26.4 years, 31.9 years and mean entry CDAI 251.5, 236.2 for Salofalk and 6‐Methylprednisolone respectively | |
| Interventions | Salofalk 4.5 g/day versus 6‐methylprednisolone 48mg (tapering doses) for 8 weeks. n = 34; allocation: 17 to Salofalk, 17 to 6‐methylprednisolone | |
| Outcomes | Remission rates after 8 weeks of treatment. Remission defined as CDAI < 150 and decrease > 60 points | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/17 in the 5‐ASA group and 7/17 in the 6‐methylprednisone group discontinued the study for various reasons; intent‐to‐treat and per‐protocol analyses were performed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported in the published study |
| Other bias | Low risk | No other sources of bias were identified |
Mahida 1990.
| Methods | Single center study. Randomized using block randomization methods. Double blind with use of identical placebo. Parallel design. Follow ups described: yes | |
| Participants | Male and female patients, age > 18 years, with active Crohn's disease not requiring steroids. Disease location: ileum and/or colon. Pentasa group: mean age 32.7 (18 to 50) years, HBI 5.2 +/‐1.8; placebo group: mean age 35 (19 to 74) years, HBI 5.0 +/‐ 1.3 | |
| Interventions | Pentasa 1.5g/day versus placebo for 6 weeks. n = 40; allocation: 20 to Pentasa group, 20 to placebo group. 11 dropouts: 7 (Pentasa group), 4 (placebo group) | |
| Outcomes | Improvement, defined as a reduction of HBI by > 2 points | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised to receive Pentasa or dummy tablets according to a pre‐arranged schedule in blocks of 6 patients |
| Allocation concealment (selection bias) | Low risk | Centralized randomization, where a pre‐arranged schedule was held by the hospital pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind with use of identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/20 patients in the Pentasa group and 4/20 patients in the placebo group failed to complete the 6‐week study; intent‐to‐treat analyses were performed |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported in the published report |
| Other bias | Low risk | No other sources of bias were identified |
Maier 1985.
| Methods | Randomized, method of randomization not clearly stated. Not double‐blind. Parallel design. Follow ups described: no | |
| Participants | Male and female patients. Median age and CDAI: 29.5 years, 308 (Salofalk); 26.9 years, 310 (SASP) | |
| Interventions | Salofalk 1.5 g/day versus SASP 3 g/day for 8 weeks. n = 30, allocation: 15 to Salofalk, 15 to SASP. 6‐methylprednisolone added if clinical symptoms persisted after 5 days at 40 mg/day followed by 5 mg weekly reductions | |
| Outcomes | Improvement in CDAI at 8 weeks | |
| Notes | Additional corticosteroid therapy was necessary in 13 patients: 6 (Salofalk group), 7 (SASP group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients, investigators and assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were accounted for at the end of the study |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are reported in the published report |
| Other bias | Low risk | No other sources of bias were identified |
Maier 1990.
| Methods | Single center. Randomized, method of randomization not described. Not blinded. Parallel design. Follow ups not adequately described | |
| Participants | Male and female patients. Mean age and entry CDAI: 32 years, 264 (Salofalk group) and 30 years, 281 (SASP + 6‐methylprednisolone group). Disease location: small bowel and/or colon | |
| Interventions | Salofalk 3 g/day versus Sulfasalazine 3 g/d + 6‐methylprednisolone 40 mg (4 mg weekly taper) for 12 weeks. n = 54, 4 dropouts; allocation: 24 to Salofalk group, 26 to combination therapy group | |
| Outcomes | Remission defined as CDAI < 150 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients, investigators and assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/54 enrolled patients discontinued the study and were not included the data analyses |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported in the published report. |
| Other bias | Low risk | No other sources of bias were identified |
Malchow 1984.
| Methods | Multicenter study. Randomized, method of randomization described. Double‐blind with use of identical placebo. Parallel design. Follow ups described: yes | |
| Participants | 452 male and female patients with Crohn's disease randomized to receive SASP and 6‐methylprednisolone alone or in combination, and placebo. of these, 215 patients had active Crohn's disease (CDAI > 150), and received acute phase treatment. Disease location: small bowel and/or colon. Mean CDAI 265 (SASP), 243 (6‐methylprednisolone), 241 (combination) and 241 (placebo) | |
| Interventions | Active phase treatment: SASP 3 g/day versus 6‐Methylprednisolone 48 mg/d versus SASP + 6‐Methylprednisolone versus placebo for 6 weeks. 6‐methylprednisolone tapered weekly over 6 weeks to 12 mg/day. Patients were retreated with the same regimen at most twice if CDAI fails to fall below 150 (maximum 18 weeks treatment). Allocation: 54 to SASP, 47 to 6‐Methylprednisolone, 56 to combination therapy, 58 to placebo | |
| Outcomes | Remission as defined by CDAI < 150 within 18 weeks | |
| Notes | Maintenance drug regime (for those with entry CDAI < 150 or achieved remission after active phase treatment): SASP 3 g/day, 6‐methylprednisolone 8 mg/day, SASP + 6‐methylprednisolone or placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomization described |
| Allocation concealment (selection bias) | Low risk | Centralized randomization: tablets were packaged centrally and labelled with a code number to keep both physician and patient unaware of what treatment was actually given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind with use of identical placebo: the placebo looked like the corresponding active drug |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled patients were accounted for in the study. Only 3 patients excluded from the analysis due to unconfirmed diagnosis. |
| Selective reporting (reporting bias) | Low risk | The published reported included all expected outcomes. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Martin 1990.
| Methods | Multicenter study. Randomized, randomization method not described. Described as double‐blind. Method of blinding not described. Parallel group design. Follow up described: yes | |
| Participants | Male and female patients, age >18 years, entry CDAI 200 to 450. Disease location: ileum or ileocolon. Mean age 29.2 years, 30.6 years and mean CDAI 295, 291 in the Salofalk and prednisolone group respectively | |
| Interventions | Salofalk 3 g/day versus Prednisone 40 mg (4 mg weekly taper from 3rd week) for 12 weeks. n = 50, allocation: 22 to Salofalk, 28 to prednisone | |
| Outcomes | Remission defined as CDAI < 150 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/22 patients in the Salofalk group and 2/28 the prednisone group were excluded from efficacy and safety analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported in the published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Prantera 1999.
| Methods | Multicenter study. Randomized, using computerized randomization in balanced blocks performed centrally for each center. Double‐blind, double‐dummy. Parallel design. Follow up described: yes | |
| Participants | Male and female, age > 18 years, mild to moderately active Crohn's disease (CDAI 180 to 350) limited to distal ileum or distal ileum plus cecum. Mean age 36.8 years, 32.4 years, 38.3 years and median entry CDAI 220, 222 and 233 for Asacol tablets, Asacol microgranules and 6‐Methylprednisolone respectively | |
| Interventions | Asacol tablets 4 g/day versus Asacol microgranules 4 g/day versus 6‐methylprednisolone 40 mg/day for 12 weeks. Mesalamine doses were tapered to 3.2 g/day after 7 weeks and 2.4 g/day after 10 weeks. Steroid doses tapered at 4 mg weekly from the 3rd week reaching 4 mg/day after 11 weeks. n = 94, allocation: 35 to Asacol tablets, 28 to Asacol microgranules, 31 to 6‐methylprednisolone | |
| Outcomes | Remission rates at 12 weeks, defined as CDAI < 150 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11/35 patients in the mesalamine tablets group, 4/28 patients in the mesalamine microgranular group, and 12/31 patients in the 6‐methylprednisolone group withdrew, Intent‐to‐treat analyses were performed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were presented in the published report |
| Other bias | Low risk | The study appears to be free of other sources of biases |
Rasmussen 1987.
| Methods | Multicenter study. Randomized but method of randomization not described. Double blind with use of identical placebo. Parallel design. Follow ups described: Yes | |
| Participants | Male and female patients, mildly to moderately active Crohn's disease as defined by Binder 1982 Disease location: small bowel +/‐ colon. Age ranged 14‐79 years. Median CDAI at entry 153 (Pentasa), 177 (placebo) |
|
| Interventions | Pentasa 1.5 g/day versus placebo for 16 weeks. n = 67; allocation: 30 to Pentasa, 37 to placebo. Dropouts: 7 from Pentasa group, 10 from placebo | |
| Outcomes | Improvement or remission according to Tvede 1983 at 16 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind with identical placebo. The control group received the same number of tablets without 5‐ASA but identical in appearance, weight, and taste. Efficacy variables were evaluated before breaking the code. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5/30 patients in the 5‐ASA group and 12/37 patients in the placebo group left the study prematurely and they were excluded from the analysis. |
| Selective reporting (reporting bias) | Low risk | The published study reported all expected outcomes. |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Rijk 1991.
| Methods | Multicenter study. Randomized, randomization using Begg and Iglewicz method. Double‐blind with use of identical placebo. Parallel design. Follow ups described: Not adequately | |
| Participants | Male and female patients, active Crohn's disease with Van Hees activity index > 140. Disease location: small bowel and/or colon. SASP + prednisone group: mean age 29.4 years, mean VHAI 178.4/ CDAI 304.9; SASP alone: mean age 27.9 years, VHAI 173.2 / CDAI 254.8 | |
| Interventions | SASP 4 to 6 g/day versus SASP 4 to 6 g/day + prednisone 30 mg for 16 weeks. Prednisone tapered in steps of 5 mg/ 2 weeks to 10 mg/day after 8 weeks at maintained until end of study. 71 patients randomized with 11 dropouts (not stated from which arm). 60 patients were analyzed (30 in each treatment group) | |
| Outcomes | Therapeutic response as measured by the change in VHAI and CDAI in the initial 6 weeks and last 4 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization using Begg and Iglewicz method |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind with use of identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/71 patients dropped out of the study and were not included in the analyses. |
| Selective reporting (reporting bias) | High risk | Baseline characteristics excluded individuals who dropped out of the study. Four patients with premature treatment failure (1 from SASP + prednisone and 3 from SASP + placebo) were excluded from the trial. |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Saverymuttu 1986.
| Methods | Single center study. Randomized, method of randomization not described. Double‐blind, placebo identical in size and shape. Parallel design. Follow ups described: yes | |
| Participants | Male and female, mild to moderately active Crohn's colitis, age 23 to 64 years. Mean CDAI 116 (Pentasa), 155 (Placebo) | |
| Interventions | Pentasa 1.5 g/day versus placebo for 10 days. n = 12; allocation: 6 to Pentasa group, 6 to placebo group. 1 withdrawal from placebo group from nausea | |
| Outcomes | Change in Fecal 111In granulocyte excretion, CDAI and ESR after 10 days of treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, placebo identical in size and shape |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/6 patient from the placebo group and 0/6 patient from the 5‐ASA group withdrew from the study. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were presented in the published report. |
| Other bias | Low risk | No other sources of bias were identified. |
Scholmerich 1990.
| Methods | Multicenter study. Randomized, method of randomization not described. Double‐blind with use of double‐dummy. Parallel design. Follow ups described: yes | |
| Participants | Male and female patients, entry CDAI 150 to 350 or VHAI > 200 + CDAI < 350, disease location: small bowel and/or colon. Mean age, CDAI and VHAI: 32 years, 241 and 177 (Salofalk); 30 years, 241 and 184 (6‐methylprednisolone) | |
| Interventions | Salofalk 2 g/day versus 6‐methylprednisolone 48 mg/day (taper to 8mg/day) for 24 weeks. n = 62; allocation: 30 to Salofalk group, 32 to 6‐methylprednisolone group | |
| Outcomes | Cessation of treatment due to "insufficient efficacy" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described in detail |
| Selective reporting (reporting bias) | Unclear risk | Unable to confirm the reporting of all outcomes |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Singleton 1993.
| Methods | Multicenter study. Randomized, method of randomization not well described. Double blind with use of identical placebo and blister card packaging. Parallel design. Follow ups described: yes | |
| Participants | Male and female patients, age > 18 years old, mildly to moderately active Crohn's disease with CDAI between 151 and 400. Disease location: ileum and/or colon. Pentasa 1 g/day: mean age 36 years, mean CDAI 271; Pentasa 2 g/day: mean age 36 years, mean CDAI 265; Pentasa 4 g/day: mean age 37 years, mean CDAI 260; Placebo: mean age 37 years, mean CDAI 277 | |
| Interventions | Pentasa 1 g/day versus 2 g/day versus 4 g/day versus placebo for 16 weeks. n = 310. Allocation: 80 to 1 g/day, 75 to 2 g/day, 75 to 4 g/day and 80 to placebo. Early termination in 154 patients for various reasons | |
| Outcomes | Primary endpoint: change in CDAI from baseline to final study visit. Remission defined as CDAI < 151 and > 50 points decrease; therapeutic benefit defined as > 50 points decrease in CDAI | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind with use of identical placebo and blister card packaging |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 41/80 patients in the placebo group, 48/80 in the 1 g/day group, 39/75 in the 2 g/day group, and 26/75 in the 4 g/day group did not complete the study. All randomized patients were analysed on the intent‐to‐treat basis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are presented in the published report, |
| Other bias | Unclear risk | The study appeared to be free of other sources of bias |
Singleton 1994.
| Methods | Multicenter study. Randomized. Double blind. Parallel design. Methods of randomization/ blinding, concealment of allocation, adequate follow up could not be assessed as study has not been fully published | |
| Participants | Male and female patients, adults with mildly to moderately active Crohn's disease, entry CDAI 200 to 400. Disease location: ileum and/or colon. Pentasa 4 g/day: mean age 37 years, mean CDAI 248; placebo: mean age 38 years, mean CDAI 255 | |
| Interventions | Pentasa 2 g/day versus 4 g/day versus placebo for 16 weeks. n = 232: allocation: 82 to Pentasa 2 g/day, 75 to Pentasa 4 g/day, 75 to placebo | |
| Outcomes | Primary endpoint: change in CDAI from baseline to final study visit. Remission defined as CDAI < 151 and > 50 points decrease; therapeutic benefit defined as > 50 points decrease in CDAI | |
| Notes | Study has not been fully published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 35/75 patients in the Pentasa group and 34/75 patients in the placebo group terminated the study early |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Not described |
Summers 1979.
| Methods | Multicenter study. Described as randomized, method of randomization described. Double‐blind with use of identical placebo. Parallel design. Follow ups not adequately described | |
| Participants | Two part study: part I (active Crohn's disease), part II (quiescent Crohn's disease). 604 patients randomized; 35 excluded from final analysis due to erroneous diagnosis (20), inappropriate entry (12) or administrative error during the conduct of the trial (3); 295 patients in part I and 274 patients in part II. Part I entry criteria 150 < CDAI < 450. Male and female patients, mean age 33.7 years (placebo), 29.6 years (SASP), 31.8 years (prednisone). Disease location: small bowel and/or colon, mean CDAI at randomization , 256 (SASP), 243 (prednisone), 241 (azathioprine), 242 (placebo) | |
| Interventions | SASP 1 g/15kg (max dose 5 g/day) versus prednisone 0.25 to 0.75 mg/kg (according to disease activity, max dose 60 mg/day) versus azathioprine 2.5 mg/kg versus placebo for 17 weeks. Allocation: 74 to SASP, 85 to prednisone, 59 to azathioprine, 77 to placebo | |
| Outcomes | Remission as defined by CDAI < 150 and maintained through rest of study period | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomization described |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind with use of identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were accounted for in the study |
| Selective reporting (reporting bias) | Low risk | Outcomes were incorporated into the ranking scheme using the Wilcoxon Rank Sum method. |
| Other bias | Low risk | The study appeared to be free of other sources of biases. |
Thomsen 1998.
| Methods | Multicenter study. Randomized, in permuted blocks of four performed at each center with sealed, opaque treatment‐code envelopes. Double‐blind, double‐dummy. Parallel design. Follow ups described: yes | |
| Participants | Male and female, mild to moderately active Crohn's disease confined to distal ileum, ileocecal and ascending colon, entry CDAI 200 to 400. Pentasa group: median age 31 years, median CDAI 278; budesonide group: median age 34 years, median CDAI 266 | |
| Interventions | Pentasa 4 g/day versus budesonide 9 mg/day for 16 weeks. n = 182, allocation: 89 to Pentasa, 93 to budesonide | |
| Outcomes | Remission rates at 16 weeks. Remission defined as CDAI < 150 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque treatment‐code envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16/93 patients in the budesonide group and 49/89 patients in the mesalamine group withdrew from the study. Intention‐to‐treat analyses were performed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported in the published study. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Tremaine 1994.
| Methods | Single center study. Randomized. Patients stratification by disease location, baseline CDAI and use of steroids and randomized within strata. Double blind with use of identical placebo. Parallel design. Follow up described: yes | |
| Participants | Male and female adult, mildly to moderately active Crohn's disease, CDAI 150 to 450. Disease location: colon or ileocolon. Asacol: median age 31 years, mean CDAI 231.7; Placebo: median age 38 years, mean CDAI 204.8 | |
| Interventions | Asacol 3.2 g/day versus placebo for 16 weeks. n = 38; allocation: 20 to Asacol, 18 to placebo. 2 dropouts, 1 from each arm | |
| Outcomes | "Complete success" (remission) defined as CDAI < 150 and > 70 points decrease. "Partial success" (response) defined as CDAI > 150 and > 70 points decrease | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, identical appearing placebo tablets |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/20 patients in the Asacol group and 10/18 patient in the placebo group did not complete the study protocol. Intent‐to‐treat analyses were performed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were included in the published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Tromm 2011.
| Methods | Multicentre study. Randomized: randomization list generated by computer in blocks of 4 (RANDCODE 3.6; IDV, Gauting, Germany); study drug was then dispensed to investigating centre according to each patient's unique randomization number. Double‐blind, double‐dummy. Parallel design. Follow ups described: yes. | |
| Participants | Male and female patients with mildly to moderately active Crohn's disease confined to the terminal ileum and/or ascending colon or distal colon. Entry CDAI 200 to 400. Salofalk group: mean age 37.8 years, mean CDAI 267, 49.7% with CRP < 5mg/L; budesonide group: mean age 36.8 years, mean CDAI 266, 48.7% with CRP < 5mg/L. | |
| Interventions | Salofalk 4.5 g/day (n = 153) versus budesonide 9mg/ day (given 9mg once daily [n = 76] or 3mg tid [n = 78]) | |
| Outcomes | Remission as defined by CDAI < 150 at 8 weeks. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 51/154 patients in the budesonide group and 34/153 patients in the mesalazine group did not complete the study, Both intent‐to‐treat and per‐protocol analyses were performed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported in the published study. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Van Hees 1981.
| Methods | 2 center study. Described as randomized, allocation via minimization method. Described as double‐blind, with use of identical placebo. Parallel design. Follow‐up not adequately described | |
| Participants | Male and female patients, active Crohn's disease with Van Hees activity index > 140. Disease location: small bowel and/or colon. SASP group: mean age 34.3 years, VHAI 185 +/‐ 30; placebo group: mean age 32.9 years, VHAI 165 +/‐ 22 | |
| Interventions | SASP 6 g/day (reduction to 4 g/day allowed if side effects occurred) versus placebo for 26 weeks. n = 27, 13 allocated to SASP, 13 allocated to placebo, 1 dropout | |
| Outcomes | Favorable response at 26 weeks defined as a decrease in Van Hees activity index > 25% | |
| Notes | 8 patients had dose reduced to 4 g/d because of adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, identical placebo: Sulphasalazine and placebo tablets were identical in internal and external appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/13 patients in the sulphasalazine group and 4/13 patients in the placebo group withdrew early from the study due to uncontrolled disease. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were presented in the published report. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Wright 1995.
| Methods | Multicenter study. Randomized using computer‐generated series of random numbers. Double blind with use of identical placebo. Parallel design. Follow ups described: yes | |
| Participants | Male and female adult patients, age > 18 years, mildly to moderately active Crohn's disease as defined by De Dombal 1974 Disease location: ileum and/or colon. Olsalazine: median age 33 years, HBI 7; placebo: median age 32, HBI 7 |
|
| Interventions | Olsalazine 2 g/day versus placebo for 16 weeks. n = 91; allocation: 46 to Olsalazine, 45 to placebo | |
| Outcomes | Remission or improved symptoms by physician assessment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, identical placebo: Those taking placebo received the same number of tablets with an identical appearance containing dicalcium phosphate and riboflavin as a colouring agent |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 35/46 patients in olsalazine group and 24/45 patients in placebo group withdrew from the study. |
| Selective reporting (reporting bias) | High risk | Only HBI was reported in the published manuscript |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 1985 | Uncontrolled trial with no comparator group |
| Anonymous 1990 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Anthonisen 1974 | Crossover study, only overall results are provided. No data before the first crossover are available |
| Arber 1995 | Crohn's disease in remission. Primary outcome was clinical relapse |
| Ardizzone 2004 | Crohn's disease in remission. The comparator was azathioprine. The primary outcomes were clinical and surgical relapse |
| Beck 1988 | Uncontrolled study |
| Bergman 1976 | Crohn's disease in surgical remission. Combination therapy (Sulfasalazine + corticosteroids). The primary outcome was clinical relapse |
| Blichfeldt 1978 | Double‐blind cross‐study of metronidazole or placebo in active CD patients on treatment with SASP or prednisolone. Combination therapy included SASP + metronidazole versus prednisolone. Inappropriate comparator included SASP versus prednisolone + metronidazole. |
| Bresci 1994 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Brignola 1992 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Brignola 1995 | Crohn's disease in surgical remission. The primary outcome was endoscopic relapse |
| Caprilli 1994 | Crohn's disease in surgical remission. The primary outcome was endoscopic relapse |
| Caprilli 2003 | Crohn's disease in surgical remission. The primary outcome was endoscopic relapse |
| Cezard 2009 | Pediatric Crohn's disease in surgical remission. The primary outcome was clinical relapse |
| Cohen 2000 | Crohn's disease in surgical remission. The primary outcome was endoscopic recurrence. |
| Colombel 1999 | The comparator was ciprofloxacin |
| de Franchis 1997 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Del Corso 1995 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Dirks 1989 | Uncontrolled trial of patients with Crohn's disease in remission. Combination therapy included 5‐ASA + corticosteroids versus surgery. The primary outcome was clinical relapse |
| Ewe 1976 | Crohn's disease in remission. The primary outcome was relapse |
| Ewe 1984 | Crohn's disease in surgical remission. The primary outcome was clinical relapse |
| Ewe 1986 | Crohn's disease in surgical remission. The primary outcome was clinical relapse |
| Ewe 1989 | Crohn's disease in surgical remission. The primary outcome was clinical relapse |
| Fiasse 1990 | Crohn's disease in surgical remission. The primary outcome was relapse |
| Florent 1996 | Crohn's disease in surgical remission. The primary outcome was endoscopic relapse |
| Gendre 1993 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Gerhardt 2001 | The comparator was Bowsellia serrata extract H15 |
| Goldstein 1987 | Retrospective study design. No comparator group. |
| Griffiths 1993 | Pediatric study population |
| Guslandi 2000 | Crohn's disease in remission. The comparator was Saccharomyces boulardii. The primary outcome was clinical relapse |
| Hanauer 1993 | Uncontrolled trial with no comparator or placebo group |
| Hanauer 2004b | Crohn's disease in surgical remission. The primary outcomes were clinical, endoscopic and radiographic relapse |
| Howaldt 1993 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Klein 1995 | Crohn's disease in surgical remission. The primary outcome was endoscopic relapse |
| Klotz 1980 | Sulfasalazine was compared to sulfapyridine or rectal 5‐ASA |
| Lennard‐Jones 1977 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Lichtenstein 2009a | Crohn's disease in remission. Prospective, uncontrolled study. No comparator or placebo group. Primary endpoint was clinical relapse. |
| Lichtenstein 2009b | Prospective, uncontrolled study. No comparator or placebo group. |
| Lochs 1991 | Combination therapy included sulfasalazine + corticosteroids versus enteral nutrition |
| Lochs 2000 | Crohn's disease in surgical remission. The primary outcome was clinical relapse |
| Mahmud 2001 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Malchow 1990 | Combination therapy included sulfasalazine + corticosteroids versus enteral nutrition |
| Mantzaris 2003 | Crohn's disease in remission. Primary outcomes were clinical relapse and quality of life |
| Mate‐Jimenez 2000 | Study population included steroid dependent Crohn's disease and ulcerative colitis. Comparator groups included methotrexate or 6‐mercaptopurine. The primary outcomes were clinical remission and relapse |
| McLeod 1995 | Crohn's disease in surgical remission. The primary outcome was clinical relapse |
| Modigliani 1996 | Crohn's disease in steroid‐induced remission. The primary outcomes were clinical relapse and steroid weaning |
| Orlando 2012 | Crohn's disease in remission (surgical). Prospective uncontrolled study with no comparator group. The primary outcome was endoscopic recurrence. |
| Papi 2009 | Crohn's disease in surgical remission. Retrospective study design. The primary outcome was clinical/ surgical relapse. |
| Prantera 1992 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Rasmussen 1983 | Uncontrolled trial with no comparator or placebo group |
| Reinisch 2010 | Crohn's disease in remission. The primary outcome was therapeutic failure. 5‐ASA was compared to azathioprine. |
| Romano 2005 | Crohn's disease in remission. Paediatric population. Combination therapy of 5‐ASA + omega‐3 fatty acids vs 5‐ASA alone. The primary outcome was clinical relapse. |
| Rosen, Ursing 1982 | Comparator group was metronidazole |
| Savarino 2013 | Crohn's disease in surgical remission. The primary outcome was endoscopic and clinical relapse. 5‐ASA was compared to azathioprine and adalimumab. |
| Schneider 1985 | Combination therapy included sulfasalzine + corticosteroids +/‐ metronidazole versus metronidazole |
| Schreiber 1994 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Singleton 1979 | Combination therapy included sulfasalazine + corticosteroids versus corticosteroids alone |
| Stober 1983 | Pediatric study population. Combination therapy included sulfasalazine + corticosteroids versus sulfasalzine + elemental diet +/‐ corticosteroids. The primary outcomes included laboratory parameters and body weight |
| Sutherland 1997 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Tao 2009 | Crohn's disease in surgical remission. The primary outcome was clinical relapse. 5‐ASA was comparted to Tripterygium wilfordii. |
| Terranova 2001 | Combination therapy included 5‐ASA + enteral nutrition versus 5‐ASA + corticosteroids |
| Terrin 2002 | Combination therapy included 5‐ASA + corticosteroids versus semi‐elemental diet. |
| Thomson 1995 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Triantafillidis 2010 | Crohn's disease in remission. The primary outcome was clinical relapse. 5‐ASA was compared to Modulen®IBD. |
| Wellmann 1986 | Combination therapy included total parenteral nutrition + corticosteroids +/‐ 5‐ASA lavage |
| Wellmann 1988 | Crohn's disease in remission. The primary outcome was clinical relapse |
| Wenckert 1978 | Crohn's disease in surgical remission. The primary outcome was clinical relapse |
| Yamamoto 2009 | Crohn's disease in surgical remission. Prospective, non‐randomized. The primary outcome was clinical relapse. 5‐ASA was compared to azathioprine and infliximab. |
Declarations of interest
Wee‐Chian Lim: Wee‐Chian Lim's institution has received funds from Takeda Pharmaceuticals (Asia Pacific) Pte. Ltd, JANSSEN ASIA PACIFIC, a division of Johnson & Johnson Pte Ltd, and Abbvie Pte Ltd for participant in Advisory Boards. All of these financial activities are outside of the scope of the present review.
Yongjun Wang: None known
John MacDonald: None known
Stephen Hanauer: Stephen Hanauer has received funds from Abbvie, Cellgene, Janssen, Ferring, Takeda, Pfizer, Merk, UCB, Actavis, and Shire for consultancy; and fees from Abbvie, Takeda and Janssen as payment for lectures; and travel expenses from Falk Foundation. All of these financial activities are outside of the scope of the present review.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Crohn's III 1997 {published data only}
- Hanauer SB, Stromberg U. Oral Pentasa in the treatment of active Crohn's disease: A meta‐analysis of double‐blind, placebo‐controlled trials. Clinical Gastroenterology and Hepatology 2004;2(5):379‐88. [DOI] [PubMed] [Google Scholar]
- Hoechst Marion Roussel Inc. Clinical study report: efficacy and safety of oral Pentasa in the treatment of active Crohn's disease (Crohn's III). January 28, 1997.
Gross 1995 {published data only}
- Gross V, Andus T, Fischbach W, Weber A, Gierend M, Hartmann F, et al. Comparison between high dose 5‐aminosalicylic acid and 6‐methylprednisolone in active Crohn's ileocolitis. A multicenter randomized double‐blind study. German 5‐ASA Study Group. Zeitschrift fur Gastroenterologie 1995;33(10):581‐4. [PubMed] [Google Scholar]
Mahida 1990 {published data only}
- Mahida YR, Jewell DP. Slow‐release 5‐amino‐salicylic acid (Pentasa) for the treatment of active Crohn's disease. Digestion 1990;45(2):88‐92. [DOI] [PubMed] [Google Scholar]
Maier 1985 {published data only}
- Maier K, Fruhmorgen P, Bode JC, Heller T, Gaisberg U, Klotz U. Successful acute treatment of chronic inflammatory intestinal diseases with oral 5‐aminosalicylic acid. Deutsche Medizinische Wochenschrift 1985;110(10):363‐8. [DOI] [PubMed] [Google Scholar]
Maier 1990 {published data only}
- Maier K, Frick HJ, Gaisberg U, Teufel TH, Klotz U. Clinical efficacy of oral mesalamine in Crohn's disease. Can J Gastroenterol 1990;4(1):13‐8. [Google Scholar]
Malchow 1984 {published data only}
- Malchow H, Ewe K, Brandes JW, Goebell H, Ehms H, Sommer H, et al. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology 1984;86(2):249‐66. [PubMed] [Google Scholar]
Martin 1990 {published data only}
- Martin F, Sutherland L, Beck IT, Anderson AH, Williams CN, Saibil F, et al. Oral 5‐ASA versus prednisolone in short term treatment of Crohn's disease: A multicentre controlled trial. Canadian Journal of Gastroenterology 1990;4(7):452‐7. [Google Scholar]
Prantera 1999 {published data only}
- Prantera C, Cottone M, Pallone F, Annese V, Franze A, Cerutti R, et al. Mesalamine in the treatment of mild to moderate active Crohn's ileitis: results of a randomized, multicenter trial. Gastroenterology 1999;116(3):521‐6. [DOI] [PubMed] [Google Scholar]
Rasmussen 1987 {published data only}
- Rasmussen SN, Lauritsen K, Tage‐Jensen U, Nielsen OH, Bytzer P, Jacobsen O, et al. 5‐Aminosalicylic acid in the treatment of Crohn's disease. A 16‐week double‐blind, placebo‐controlled, multicentre study with Pentasa. Scandinavian Journal of Gastroenterology 1987;22(7):877‐83. [DOI] [PubMed] [Google Scholar]
Rijk 1991 {published data only}
- Rijk MC, Hogezand RA, Lier HJ, Tongeren JH. Sulphasalazine and prednisone compared with sulphasalazine for treating active Crohn disease. A double‐blind, randomized, multicenter trial. Annals of Internal Medicine 1991;114(6):445‐50. [DOI] [PubMed] [Google Scholar]
Saverymuttu 1986 {published data only}
- Saverymuttu SH, Gupta S, Keshavarzian A, Donovan B, Hodgson HJ. Effect of a slow‐release 5'‐aminosalicylic acid preparation on disease activity in Crohn's disease. Digestion 1986;33(2):89‐91. [DOI] [PubMed] [Google Scholar]
Scholmerich 1990 {published data only}
- Jenss H, Hartmann F, Schölmerich J, und Deutsche 5‐ASA Studiengruppe. 5‐Aminosalicylic acid versus methylprednisolone in the therapy of acute Crohn's disease ‐ results of a double‐blind multicentric clinical study [5‐aminosalicylsäure versus methylprednisolon in der therapie des aktiven morbus Crohn ‐ ergebnisse einer doppelblinden multizentrischen klinischen studie]. Klinische Wochenschrift 1990;68(Suppl 19):34. [Google Scholar]
- Scholmerich J, Jenss H, Hartmann F, Dopfer H. Oral 5‐aminosalicylic acid versus 6‐methylprednisolone in active Crohn's disease. Canadian Journal of Gastroenterology 1990;4(7):446‐51. [Google Scholar]
Singleton 1993 {published data only}
- Singleton JW, Hanauer S, Robinson M. Quality‐of‐life results of double‐blind, placebo‐controlled trial of mesalamine in patients with Crohn's disease. Digestive Diseases and Sciences 1995;40(5):931‐5. [DOI] [PubMed] [Google Scholar]
- Singleton JW, Hanauer SB, Gitnick GL, Peppercorn MA, Robinson MG, Wruble LD, et al. Mesalamine capsules for the treatment of active Crohn's disease: results of a 16‐week trial. Pentasa Crohn's Disease Study Group. Gastroenterology 1993;104(5):1293‐301. [DOI] [PubMed] [Google Scholar]
Singleton 1994 {published data only}
- Nordic Research Inc. Clinical research report: efficacy and safety of oral Pentasa in the treatment of active Crohn's disease (Crohn's II). October 23, 1991.
- Singleton J. Second trial of mesalamine therapy in the treatment of active Crohn's disease. Gastroenterology 1994;107(2):632‐3. [DOI] [PubMed] [Google Scholar]
Summers 1979 {published data only}
- Singleton JW. Results of treatment with sulfasalazine in the American Multicenter Study on the treatment of Crohn disease (National Cooperative Crohn's Disease Study) [Ergebnisse der behandlung mit sulfasalazine der amerikanischen multcienter‐studie zur behandlung des morbus Crohn (National Cooperative Crohn's Disease Study [NCCDS])]. Zeitschrift fur Gastroenterologie ‐ Verhandlungsband 1981 Jun;19:38‐40. [PubMed] [Google Scholar]
- Singleton JW, Law DH, Kelley ML Jr, Mekhjian HS, Sturdevant RA. National Cooperative Crohn's Disease Study: adverse reactions to study drugs. Gastroenterology 1979;77(4 Pt 2):870‐82. [PubMed] [Google Scholar]
- Summers RW, Singleton JW. National Cooperative Crohn's Disease Study (NCCDS): a controlled prospective trial of three drugs vs placebo. Gut 1977;18(11):A972‐3. [Google Scholar]
- Summers RW, Switz DM, Sessions JT Jr, Becktel JM, Best WR, Kern F Jr, et al. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology 1979;77(4 Pt 2):847‐69. [PubMed] [Google Scholar]
Thomsen 1998 {published data only}
- Thomsen OO, Cortot A, Jewell D, Wright JP, Winter T, Veloso FT, et al. A comparison of budesonide and mesalamine for active Crohn's disease. International Budesonide‐Mesalamine Study Group. New England Journal of Medicine 1998;339(6):370‐4. [DOI] [PubMed] [Google Scholar]
- Thomsen OO, Cortot A, Jewell D, Wright JP, Winter T, Veloso FT, et al. Budesonide and mesalazine in active Crohn's disease: a comparison of the effects on quality of life. American Journal of Gastroenterology 2002 Mar;97(3):649‐53. [DOI] [PubMed] [Google Scholar]
Tremaine 1994 {published data only}
- Tremaine WJ, Schroeder KW, Harrison JM, Zinsmeister AR. A randomized, double‐blind, placebo‐controlled trial of the oral mesalamine (5‐ASA) preparation, Asacol, in the treatment of symptomatic Crohn's colitis and ileocolitis. Journal of Clinical Gastroenterology 1994;19(4):278‐82. [DOI] [PubMed] [Google Scholar]
Tromm 2011 {published data only}
- Tromm A, Bungani I, Tomsová E, Tulassay Z, Luká M, Kykal J, Bátovský M, Fixa B, Gabalec L, Safadi R, Kramm H, J.Altorjay I, Löhr H, Koutroubakis I, Bar‐Meir S, Stimac D, Schäffeler E, Glasmacher C, Dilger K, Mohrbacher R, Greinwald R. Budesonide 9 mg is at least as effective as mesalamine 4.5 g in patients with mildly to moderately active Crohn's disease. Gastroenterology 2011;140(2):425‐34. [DOI] [PubMed] [Google Scholar]
- Tromm A, Bunganic I, Tomsová E, Tulassay Z, Lukas M, Kykal J, et al. Both budesonide (9mg) as well as high‐dose eudragit‐l‐coated mesalamine(4.5g) lead to high remission rates in moderately active Crohn's disease patients: a double‐blind, double‐dummy, randomized, multicenter study. Gastroenterology 2009;136(5 supplement 1):A65. [Google Scholar]
Van Hees 1981 {published data only}
- Hees PA, Lier HJ, Elteren PH, Driessen M, Hogezand RA, Velde GP, et al. Effect of sulphasalazine in patients with active Crohn's disease: a controlled double‐blind study. Gut 1981;22(5):404‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hees P, Velde G, Hogezand R, Driessen W, Bakker J, Lier H, et al. Effect of sulphasalazine in patients with Crohn's disease. a controlled double‐blind trial. Hepato‐Gastroenterology 1980;27(SUPPL.):E38.5. [Google Scholar]
- van HeesP, Hogezand R, Velde G. Effect of sulphasalazine in patients with active Crohn's disease. a controlled double‐blind trial. Netherlands Journal of Medicine 1981;24(4):157. [Google Scholar]
Wright 1995 {published data only}
- Wright JP, Jewell DP, Modigliani R, Malchow H. A randomized, double‐blind, placebo‐controlled trial of olsalazine for active Crohn's disease. Inflammatory Bowel Diseases 1995;1(4):241‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 1985 {published data only}
- Salazopyrin in the management of Crohn's disease. The Japanese Research Committee for Crohn's disease. Gastroenterologia Japonica 1985;20(1):71‐81. [PubMed] [Google Scholar]
Anonymous 1990 {published data only}
- Coated oral 5‐aminosalicylic acid versus placebo in maintaining remission of inactive Crohn's disease. International Mesalazine Study Group. Alimentary Pharmacology and Therapeutics 1990;4(1):55‐64. [PubMed] [Google Scholar]
Anthonisen 1974 {published data only}
- Anthonisen P, Barany F, Folkenborg O, Holtz A, Jarnum S, Kristensen M, et al. The clinical effect of salazosulphapyridine (Salazopyrin r) in Crohn's disease. A controlled double‐blind study. Scandinavian Journal of Gastroenterology 1974;9(6):549‐54. [PubMed] [Google Scholar]
- Anthonisen P, Bárány F, FolkenborgO, Holtz A, Jarnum S, KristensenM, et al. The clinical effect of salazosulphapyridine (Salazopyrin) in Crohn's disease. Report of a controlled double blind investigation. Ugeskrift for Laeger 1974;136(32):1798‐802. [PubMed] [Google Scholar]
Arber 1995 {published data only}
- Arber N, Odes HS, Fireman Z, Lavie A, Broide E, Bujanover Y, et al. A controlled double blind multicenter study of the effectiveness of 5‐aminosalicylic acid in patients with Crohn's disease in remission. Journal of Clinical Gastroenterology 1995;20(3):203‐6. [DOI] [PubMed] [Google Scholar]
Ardizzone 2004 {published data only}
- Ardizzone S, Maconi G, Sampietro GM, Russo A, Radice E, Colombo E, et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn's disease. Gastroenterology 2004;127(3):730‐40. [DOI] [PubMed] [Google Scholar]
Beck 1988 {published data only}
- Beck IT, Hudacin J, Paterson WG, Depew WT, Simon JB, Groll A. Mesalazine in the treatment of active Crohn's disease. Canadian Journal of Gastroenterology 1988;2(Suppl A):63A‐70A. [Google Scholar]
Bergman 1976 {published data only}
- Bergman L, Krause U. Postoperative treatment with corticosteroids and salazosulphapyridine (Salazopyrin) after radical resection for Crohn's disease. Scandinavian Journal of Gastroenterology 1976;11(7):651‐6. [PubMed] [Google Scholar]
Blichfeldt 1978 {published data only}
- Blichfeldt P, Blomhoff JP, Myhre E, Gjone E. Metronidazole in Crohn's disease. A double blind cross‐over clinical trial. Scandinavian Journal of Gastroenterology 1978;13(1):123‐7. [DOI] [PubMed] [Google Scholar]
Bresci 1994 {published data only}
- Bresci G, Parisi G, Banti S. Long‐term therapy with 5‐aminosalicylic acid in Crohn's disease: is it useful? Our four years experience. International Journal of Clinical Pharmacology Research 1994;14(4):133‐8. [PubMed] [Google Scholar]
Brignola 1992 {published data only}
- Brignola C, Iannone P, Pasquali S, Campieri M, Gionchetti P, Belluzzi A, et al. Placebo‐controlled trial of oral 5‐ASA in relapse prevention of Crohn's disease. Digestive Diseases and Sciences 1992;37(1):29‐32. [DOI] [PubMed] [Google Scholar]
Brignola 1995 {published data only}
- Brignola C, Cottone M, Pera A, Ardizzone S, Scribano ML, Franchis R, et al. Mesalamine in the prevention of endoscopic recurrence after intestinal resection for Crohn's disease. Italian Cooperative Study Group. Gastroenterology 1995;108(2):345‐9. [DOI] [PubMed] [Google Scholar]
Caprilli 1994 {published data only}
- Caprilli R, Andreoli A, Capurso L, Corrao G, D'Albasio G, Gioieni A, et al. Oral mesalazine (5‐aminosalicylic acid; Asacol) for the prevention of post‐operative recurrence of Crohn's disease. Gruppo Italiano per lo Studio del Colon e del Retto (GISC). Alimentary Pharmacology and Therapeutics 1994;8(1):35‐43. [DOI] [PubMed] [Google Scholar]
Caprilli 2003 {published data only}
- Caprilli R, Cottone M, Tonelli F, Sturniolo G, Castiglione F, Annese V, et al. Two mesalazine regimens in the prevention of the post‐operative recurrence of Crohn's disease: a pragmatic, double‐blind, randomized controlled trial. Alimentary Pharmacology and Therapeutics 2003;17(4):517‐23. [DOI] [PubMed] [Google Scholar]
Cezard 2009 {published data only}
- Cezard JP, Munck A, Mouterde O, Morali A, Lenaerts C, Lachaux A, et al. Prevention of relapse by mesalazine (Pentasa) in pediatric Crohn's disease: a multicenter, double‐blind, randomized, placebo‐controlled trial. Gastroenterologie Clinique et Biologique 2009;33(1 pt 1):31‐40. [DOI] [PubMed] [Google Scholar]
Cohen 2000 {published data only}
- Cohen Z. Crohn's disease ‐ Outcome of surgery. Drugs of Today 2000;36(Suppl G):51‐7. [Google Scholar]
Colombel 1999 {published data only}
- Colombel JF, Lemann M, Cassagnou M, Bouhnik Y, Duclos B, Dupas JL, et al. A controlled trial comparing ciprofloxacin with mesalazine for the treatment of active Crohn's disease. Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives (GETAID). American Journal of Gastroenterology 1999;94(3):674‐8. [DOI] [PubMed] [Google Scholar]
de Franchis 1997 {published data only}
- Franchis R, Omodei P, Ranzi T, Brignola C, Rocca R, Prada A, et al. Controlled trial of oral 5‐aminosalicylic acid for the prevention of early relapse in Crohn's disease. Alimentary Pharmacology and Therapeutics 1997;11(5):845‐52. [DOI] [PubMed] [Google Scholar]
Del Corso 1995 {published data only}
- Corso L, Moruzzo D, Romanelli AM, Norpoth M, Pentimone F, Bresci G. [5‐Aminosalicylic acid in the prevention of recurrences of Crohn's disease]. Deutsche Medizinische Wochenschrif 1995;120(50):1723‐7. [DOI] [PubMed] [Google Scholar]
Dirks 1989 {published data only}
- Dirks E, Goebell H, Schaarschmidt K, Forster S, Quebe‐Fehling E, Eigler FW. Clinical relapse of Crohn's disease under standardized conservative treatment and after excisional surgery. Digestive Diseases and Sciences 1989;34(12):1832‐40. [DOI] [PubMed] [Google Scholar]
Ewe 1976 {published data only}
- Ewe K, Holtermuller KH, Baas U, Eckhart V, Krieg H, Kutzner J, et al. [Prevention of recurrence by salazosulfapyridine (azulfidine) therapy in Crohn's disease. A double blind study]. Verhandlungen der Deutschen Gesellschaft fur Innere Medizin 1976;82 Pt 1:930‐2. [PubMed] [Google Scholar]
Ewe 1984 {published data only}
- Ewe K, Malchow H, Herfarth, C. Radical operation and recurrence prevention with azulfidine in Crohn disease: a prospective multicenter study‐‐initial results [Operative Radikalitat und Rezidivprophylaxe mit Azulfidine bei M. Crohn: Eine prospektive multizentrische Studie‐‐Erste Ergebnisse.]. Langenbecks Archiv für Chirurgie 1984;364:427‐30. [DOI] [PubMed] [Google Scholar]
Ewe 1986 {published data only}
- Ewe K, Herfarth Ch, Malchow H, Jesdinsky H. Crohn study II: Influence of surgical radicality and salazosulfapyridine prophylaxis on postoperative relapses. Klinische Wochenschrift 1986;64:50‐1. [Google Scholar]
Ewe 1989 {published data only}
- Ewe K, Herfarth C, Malchow H, Jesdinsky HJ. Postoperative recurrence of Crohn's disease in relation to radicality of operation and sulfasalazine prophylaxis: a multicenter trial. Digestion 1989;42(4):224‐32. [DOI] [PubMed] [Google Scholar]
Fiasse 1990 {published data only}
- Fiasse R, Fontaine F, Heuverzwijn R, Biemans M. Prevention of Crohn's disease recurrences after intestinal resection with eudragid‐l‐coated 5‐aminosalicylic acid. An interim report of a one year double‐blind placebo controlled study (ABSTRACT). Acta Gastroenterologica Belgica 1990;53(1):A11. [Google Scholar]
Florent 1996 {published data only}
- Florent C, Cortot A, Quandale P, Sahmound T, Modigliani R, Sarfaty E, et al. Placebo‐controlled clinical trial of mesalazine in the prevention of early endoscopic recurrences after resection for Crohn's disease. Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives (GETAID). European Journal of Gastroenterology and Hepatology 1996;8(3):229‐33. [DOI] [PubMed] [Google Scholar]
Gendre 1993 {published data only}
- Gendre JP, Mary JY, Florent C, Modigliani R, Colombel JF, Soule JC, et al. Oral mesalamine (Pentasa) as maintenance treatment in Crohn's disease: a multicenter placebo‐controlled study. The Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives (GETAID). Gastroenterology 1993;104(2):435‐9. [DOI] [PubMed] [Google Scholar]
Gerhardt 2001 {published data only}
- Gerhardt H, Seifert F, Buvari P, Vogelsang H, Repges R. [Therapy of active Crohn disease with Boswellia serrata extract H 15]. Zeitschrift fur Gastroenterologie 2001;39(1):11‐7. [DOI] [PubMed] [Google Scholar]
Goldstein 1987 {published data only}
- Goldstein F, Farquhar S, Thornton JJ, Abramson J. Favorable effects of sulfasalazine on small bowel Crohn's disease: a long‐term study.". American Journal of Gastroenterology 1987;82(9):848‐853. [PubMed] [Google Scholar]
Griffiths 1993 {published data only}
- Griffiths A, Koletzko S, Sylvester F, Marcon M, Sherman P. Slow‐release 5‐aminosalicylic acid therapy in children with small intestinal Crohn's disease. Journal of Pediatric Gastroenterology and Nutrition 1993;17(2):186‐92. [DOI] [PubMed] [Google Scholar]
Guslandi 2000 {published data only}
- Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Digestive Diseases and Sciences 2000;45(7):1462‐4. [DOI] [PubMed] [Google Scholar]
Hanauer 1993 {published data only}
- Hanauer SB, Krawitt EL, Robinson M, Rick GG, Safdi MA. Long‐term management of Crohn's disease with mesalamine capsules (Pentasa). Pentasa Crohn's Disease Compassionate Use Study Group. American Journal of Gastroenterology 1993;88(9):1343‐51. [PubMed] [Google Scholar]
Hanauer 2004b {published data only}
- Hanauer SB, Korelitz BI, Rutgeerts P, Peppercorn MA, Thisted RA, Cohen RD, et al. Postoperative maintenance of Crohn's disease remission with 6‐mercaptopurine, mesalamine, or placebo: a 2‐year trial. Gastroenterology 2004;127(3):723‐9. [DOI] [PubMed] [Google Scholar]
Howaldt 1993 {published data only}
- Howaldt S, Raedler A, Reinecker HC, Berghaus D, Hoyer S, Kaiser B, et al. Comparative trial of remission prophylaxis in quiescent Crohn's disease with oral 4‐aminosalicylic acid versus 5‐aminosalicylic acid slow release tablets. Can J Gastroenterol 1993;7(2):241‐4. [Google Scholar]
Klein 1995 {published data only}
- Klein O, Colombel JF, Lescut D, Gambiez L, Desreumaux P, Quandalle P, et al. Remaining small bowel endoscopic lesions at surgery have no influence on early anastomotic recurrences in Crohn's disease. Am J Gastroenterol 1995;90(11):1949‐52. [PubMed] [Google Scholar]
Klotz 1980 {published data only}
- Klotz U, Maier K, Fischer C, Heinkel K. Therapeutic efficacy of sulfasalazine and its metabolites in patients with ulcerative colitis and Crohn's disease. New England Journal of Medicine 1980;303(26):1499‐502. [DOI] [PubMed] [Google Scholar]
Lennard‐Jones 1977 {published data only}
- Lennard‐Jones JE. Sulphasalazine in asymptomatic Crohn's disease. A multicentre trial. Gut 1977;18(1):69‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lichtenstein 2009a {published data only}
- Lichtenstein GR, Ramsey D. Experience with delayed‐release mesalamine for maintenance of remission of Crohn's disease (CD). Gastroenterology 2009;136(5):A661‐2. [Google Scholar]
Lichtenstein 2009b {published data only}
- Lichtenstein GR, Ramsey D. Experience with delayed‐release mesalamine for active Crohn's disease (CD). Gastroenterology 2009;136(5):A657‐8. [Google Scholar]
Lochs 1991 {published data only}
- Lochs H, Steinhardt HJ, Klaus‐Wentz B, Zeitz M, Vogelsang H, Sommer H, et al. Comparison of enteral nutrition and drug treatment in active Crohn's disease. Results of the European Cooperative Crohn's Disease Study. IV. Gastroenterology 1991;101(4):881‐8. [DOI] [PubMed] [Google Scholar]
Lochs 2000 {published data only}
- Lochs H, Mayer M, Fleig WE, Mortensen PB, Bauer P, Genser D, et al. Prophylaxis of postoperative relapse in Crohn's disease with mesalamine: European Cooperative Crohn's Disease Study VI. Gastroenterology 2000;118(2):264‐73. [DOI] [PubMed] [Google Scholar]
Mahmud 2001 {published data only}
- Mahmud N, Kamm MA, Dupas JL, Jewell DP, O'Morain CA, Weir DG, et al. Olsalazine is not superior to placebo in maintaining remission of inactive Crohn's colitis and ileocolitis: a double blind, parallel, randomised, multicentre study. Gut 2001;49(4):552‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malchow 1990 {published data only}
- Malchow H, Steinhardt HJ, Lorenz‐Meyer H, Strohm WD, Rasmussen S, Sommer H, et al. Feasibility and effectiveness of a defined‐formula diet regimen in treating active Crohn's disease. European Cooperative Crohn's Disease Study III. Scandinavian Journal of Gastroenterology 1990;25(3):235‐44. [PubMed] [Google Scholar]
Mantzaris 2003 {published data only}
- Mantzaris GJ, Petraki K, Sfakianakis M, Archavlis E, Christidou A, Chadio‐Iordanides H, et al. Budesonide versus mesalamine for maintaining remission in patients refusing other immunomodulators for steroid‐dependent Crohn's disease. Clinical Gastroenterology and Hepatology 2003;1(2):122‐8. [DOI] [PubMed] [Google Scholar]
Mate‐Jimenez 2000 {published data only}
- Mate‐Jimenez J, Hermida C, Cantero‐Perona J, Moreno‐Otero R. 6‐mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid‐dependent inflammatory bowel disease. European Journal of Gastroenterology and Hepatology 2000;12(11):1227‐33. [DOI] [PubMed] [Google Scholar]
McLeod 1995 {published data only}
- McLeod RS, Wolff BG, Steinhart AH, Carryer PW, O'Rourke K, Andrews DF, et al. Prophylactic mesalamine treatment decreases postoperative recurrence of Crohn's disease. Gastroenterology 1995;109(2):404‐13. [DOI] [PubMed] [Google Scholar]
Modigliani 1996 {published data only}
- Modigliani R, Colombel JF, Dupas JL, Dapoigny M, Costil V, Veyrac M, et al. Mesalamine in Crohn's disease with steroid‐induced remission: effect on steroid withdrawal and remission maintenance, Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives. Gastroenterology 1996;110(3):688‐93. [DOI] [PubMed] [Google Scholar]
Orlando 2012 {published data only}
- Orlando A, Mocciaro F, Scimeca D, Rispo A, Scribano ML, Testa A, et al. Early post‐operative endoscopic recurrence in crohn's disease in a large prospective Italian multicenter cohort. Digestive and Liver Disease 2012;44:S81. [Google Scholar]
Papi 2009 {published data only}
- Papi C, Aratari A, Tornatore V, Koch M, Capurso L, Caprilli, R. Long‐term prevention of post‐operative recurrence in Crohn's disease cannot be affected by mesalazine. Journal of Crohn's and Colitis 2009;3(2):109‐14. [DOI] [PubMed] [Google Scholar]
Prantera 1992 {published data only}
- Prantera C, Pallone F, Brunetti G, Cottone M, Miglioli M. Oral 5‐aminosalicylic acid (Asacol) in the maintenance treatment of Crohn's disease. The Italian IBD Study Group. Gastroenterology 1992;103(2):363‐8. [DOI] [PubMed] [Google Scholar]
Rasmussen 1983 {published data only}
- Rasmussen SN, Binder V, Maier K, Bondesen S, Fischer C, Klotz U, et al. Treatment of Crohn's disease with peroral 5‐aminosalicylic acid. Gastroenterology 1983;85(6):1350‐3. [PubMed] [Google Scholar]
Reinisch 2010 {published data only}
- Reinisch W, Angelberger S, Petritsch W, Shonova O, Lukas M, Bar‐Meir S, et al. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn's disease with endoscopic recurrence: efficacy and safety results of a randomised, double‐blind, double‐dummy, multicentre trial. Gut 2010;59(6):752‐9. [DOI] [PubMed] [Google Scholar]
Romano 2005 {published data only}
- Romano C, Cucchiara S, Barabino A, Annese V, Sferlazzas C. Usefulness of omega‐3 fatty acid supplementation in addition to mesalazine in maintaining remission in pediatric Crohn's disease: a double‐blind, randomized, placebo‐controlled study. World Journal of Gastroenterology 2005;11(45):7118‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosen, Ursing 1982 {published data only}
- Rosen A, Ursing B, Alm T, Barany F, Bergelin I, Ganrot‐Norlin K, et al. A comparative study of metronidazole and sulfasalazine for active Crohn's disease: the cooperative Crohn's disease study in Sweden. I. Design and methodologic considerations. Gastroenterology 1982;83(3):541‐9. [PubMed] [Google Scholar]
- Ursing B, Alm T, Barany F, Bergelin I, Ganrot‐Norlin K, Hoevels J, et al. A comparative study of metronidazole and sulfasalazine for active Crohn's disease: the cooperative Crohn's disease study in Sweden. II. Result. Gastroenterology 1982;83(3):550‐62. [PubMed] [Google Scholar]
Savarino 2013 {published data only}
- Savarino E, Bodini G, Dulbecco P, Marabotto E, Assandri L, Bruzzone L, Mazza F, Fazio V, Giambruno E, Gemignani L, Savarino V. Adalimumab Is More Effective Than Azathioprine and Mesalamine At Preventing Postoperative Recurrence of Crohn's Disease ‐ A Randomized Trial. Gastroenterology 2013;144(5):S21. [DOI] [PubMed] [Google Scholar]
Schneider 1985 {published data only}
- Schneider MU, Laudage G, Guggenmoos‐Holzmann I, Riemann JF. [Metronidazole in the treatment of Crohn disease. Results of a controlled randomized prospective study]. Deutsche Medizinische Wochenschrift 1985;110(45):1724‐30. [DOI] [PubMed] [Google Scholar]
Schreiber 1994 {published data only}
- Schreiber S, Howaldt S, Raedler A. Oral 4‐aminosalicylic acid versus 5‐aminosalicylic acid slow release tablets. Double blind, controlled pilot study in the maintenance treatment of Crohn's ileocolitis. Gut 1994;35(8):1081‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singleton 1979 {published data only}
- Singleton JW, Summers RW, Kern F Jr, Becktel JM, Best WR, Hansen RN, et al. A trial of sulfasalazine as adjunctive therapy in Crohn's disease. Gastroenterology 1979;77(4 Pt2):887‐97. [PubMed] [Google Scholar]
Stober 1983 {published data only}
- Stober B, Nutzenadel W, Ullrich F. [Basic diet in Crohn's disease]. Monatsschr Kinderheilkd 1983;131(10):721‐4. [PubMed] [Google Scholar]
Sutherland 1997 {published data only}
- Sutherland LR, Martin F, Bailey RJ, Fedorak RN, Poleski M, Dallaire C, et al. A randomized, placebo‐controlled, double‐blind trial of mesalamine in the maintenance of remission of Crohn's disease. The Canadian Mesalamine for Remission of Crohn's Disease Study Group. Gastroenterology 1997;112(4):1069‐77. [DOI] [PubMed] [Google Scholar]
Tao 2009 {published data only}
- Tao, Q. S.Ren, J. A.Ji, Z. L.Li, J. S.Wang, X. B.Jiang, X. H. Maintenance effect of polyglycosides of Tripterygium wilfordii on remission in postoperative Crohn disease. Zhonghua Wei Chang Wai Ke Za Zhi 2009;12(5):491‐3. [PubMed] [Google Scholar]
Terranova 2001 {published data only}
- Terranova M, Leonello G, Macri A, Versaci A, Scuderi G, Crisafulli C, et al. Enteral nutrition in the short‐term treatment of active inflammatory bowel diseases: A single‐centre experience. Rivista Italiana di Nutrizione Parenterale ed Enterale 2001;19(1):12‐7. [Google Scholar]
Terrin 2002 {published data only}
- Terrin G, Canani RB, Ambrosini A, Viola F, Mesquita MB, Nardo G, Dito L, Cucchiara S. A semielemental diet (Pregomin) as primary therapy for inducing remission in children with active Crohn's disease. Italian Journal of Pediatrics 2002;25(5):401‐5. [Google Scholar]
Thomson 1995 {published data only}
- Thomson AB, Wright JP, Vatn M, Bailey RJ, Rachmilewitz D, Adler M, et al. Mesalazine (Mesasal/Claversal) 1.5 g b.d. vs. placebo in the maintenance of remission of patients with Crohn's disease. Alimentary Pharmacology and Therapeutics 1995;9(6):673‐83. [DOI] [PubMed] [Google Scholar]
Triantafillidis 2010 {published data only}
- Triantafillidis JK, Stamataki A, Karagianni V, Gikas A, Malgarinos G. Maintenance treatment of Crohn's disease with a polymeric feed rich in TGF‐beta. Annals of Gastroenterology 2010;23(2):113‐8. [Google Scholar]
Wellmann 1986 {published data only}
- Wellmann W, Fink PC, Benner F, Schmidt FW. Endotoxaemia in active Crohn's disease. Treatment with whole gut irrigation and 5‐aminosalicylic acid. Gut 1986;27(7):814‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wellmann 1988 {published data only}
- Wellmann W, Schroder U. New oral preparations for maintenance therapy in Crohn's disease (abstract). Canadian Journal of Gastroenterology 1988;2(Supplement A):71A‐2. [Google Scholar]
Wenckert 1978 {published data only}
- Wenckert A, Kristensen M, Eklund AE, Barany F, Jarnum S, Worning H, et al. The long‐term prophylactic effect of salazosulphapyridine (Salazopyrin) in primarily resected patients with Crohn's disease. A controlled double‐blind trial. Scand J Gastroenterol 1978;13(2):161‐7. [DOI] [PubMed] [Google Scholar]
Yamamoto 2009 {published data only}
- Yamamoto T, Umegae S, Matsumoto K. Impact of infliximab therapy after early endoscopic recurrence following ileocolonic resection of Crohn's disease: a prospective pilot study. Inflammatory Bowel Diseases 2009;15(10):1460‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Azad 1977
- Azad Khan AK, Piris J, Truelove SC. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet 1977;2(8044):892‐5. [DOI] [PubMed] [Google Scholar]
Bar‐Meir 1998
- Bar‐Meir S, Chowers Y, Lavy A, Abramovitch D, Sternberg A, Leichtmann G, et al. Budesonide versus prednisone in the treatment of active Crohn's disease. The Israeli Budesonide Study Group. Gastroenterology 1998;115(4):835‐40. [DOI] [PubMed] [Google Scholar]
Binder 1982
- Binder V, Both H, Hansen PK, Hendriksen C, Kreiner S, Torp‐Pedersen K. Incidence and prevalence of ulcerative colitis and Crohn's disease in the County of Copenhagen, 1962 to 1978. Gastroenterology 1982;83(3):563‐8. [PubMed] [Google Scholar]
Brant 1999
- Brant R, Sutherland L, Hilsden R. Examining the minimum important difference. Statistics in Medicine 1999;18(19):2593‐603. [DOI] [PubMed] [Google Scholar]
Campieri 1997
- Campieri M, Ferguson A, Doe W, Persson T, Nilsson LG. Oral budesonide is as effective as oral prednisolone in active Crohn's disease. The Global Budesonide Study Group. Gut 1997;41(2):209‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cellier 1994
- Cellier C, Sahmoud T, Froguel E, Adenis A, Belaiche J, Bretagne JF. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn's disease. A prospective multicentre study of 121 cases. The Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut 1994;35(2):231‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Dombal 1974
- Dombal FT, Burton IL, Clamp SE, Goligher JC. Short‐term course and prognosis of Crohn's disease. Gut 1974;15(6):435‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dubuquoy 2006
- Dubuquoy L, Rousseaux C, Thuru X, Peyrin‐Biroulet L, Romano O, Chavatte P, et al. PPARgamma as a new therapeutic target in inflammatory bowel diseases. Gut 2006;55(9):1341‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feagan 2004
- Feagan BG. 5‐ASA therapy for active Crohn's disease: old friends, old data, and a new conclusion. Clinical Gastroenterology and Hepatology 2004;2(5):376‐8. [DOI] [PubMed] [Google Scholar]
Gross 1996
- Gross V, Andus T, Caesar I, Bischoff SC, Lochs H, Tromm A, et al. Oral pH‐modified release budesonide versus 6‐methylprednisolone in active Crohn's disease. German/Austrian Budesonide Study Group. European Journal of Gastroenterology and Hepatology 1996;8(9):905‐9. [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanauer 2001
- Hanauer SB, Sandborn W. Management of Crohn's disease in adults. American Journal of Gastroenterology 2001;96(3):635‐43. [DOI] [PubMed] [Google Scholar]
Hanauer 2003
- Hanauer SB, Present DH. The state of the art in the management of inflammatory bowel disease. Reviews in Gastroenterological Disorders 2003;3(2):81‐92. [PubMed] [Google Scholar]
Hanauer 2004
- Hanauer SB, Stromberg U. Oral Pentasa in the treatment of active Crohn's disease: A meta‐analysis of double‐blind, placebo‐controlled trials. Clinical Gastroenterology and Hepatology 2004;2(5):379‐88. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors(s) editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Levesque 2012
- Levesque BG, Sandborn WJ. Setting a high threshold for noninferiority: mesalamine and budesonide in Crohn's disease. Inflammatory Bowel Diseases 2012;18(4):795‐6. [DOI] [PubMed] [Google Scholar]
Loftus 2004
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence and environmental influences. Gastroenterology 2004;126(6):1504‐17. [DOI] [PubMed] [Google Scholar]
MacDermott 2000
- MacDermott RP. Progress in understanding the mechanisms of action of 5‐aminosalicylic acid. American Journal of Gastroenterology 2000;95(12):3343‐5. [DOI] [PubMed] [Google Scholar]
Munafo 2004
- Munafo MR, Clark TG, Flint J. Assessing publication bias in genetic association studies: evidence from a recent meta‐analysis. Psychiatry Research 2004;129(1):39‐44. [DOI] [PubMed] [Google Scholar]
Rao 1987
- Rao SS, Read NW, Holdsworth CD. Influence of olsalazine on gastrointestinal transit in ulcerative colitis. Gut 1987;28(11):1474‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rousseaux 2005
- Rousseaux C, Lefebvre B, Dubuquoy L, Lefebvre P, Romano O, Auwerx J, et al. Intestinal antiinflammatory effect of 5‐aminosalicylic acid is dependent on peroxisome proliferator‐activated receptor‐gamma. Journal of Experimental Medicine 2005;201(8):1205‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutgeerts 1994
- Rutgeerts P, Lofberg R, Malchow H, Lamers C, Olaison G, Jewell D, et al. A comparison of budesonide with prednisolone for active Crohn's disease. New England Journal of Medicine 1994;331(13):842‐5. [DOI] [PubMed] [Google Scholar]
Sandborn 2002a
- Sandborn WJ. Rational selection of oral 5‐aminosalicylate formulations and prodrugs for the treatment of ulcerative colitis. American Journal of Gastroenterology 2002;97(12):2939‐41. [DOI] [PubMed] [Google Scholar]
Sandborn 2002b
- Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Lofberg R, Modigliani R, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology 2002;122(2):512‐30. [DOI] [PubMed] [Google Scholar]
Sandborn 2003
- Sandborn WJ, Feagan BG. Review article: mild to moderate Crohn's disease‐‐defining the basis for a new treatment algorithm. Alimentary Pharmacology and Therapeutics 2003;18(3):263‐77. [DOI] [PubMed] [Google Scholar]
Sandborn 2004
- Sandborn WJ, Feagan BG, Radford‐Smith G, Kovacs A, Enns R, Innes A, et al. CDP571, a humanised monoclonal antibody to tumour necrosis factor alpha, for moderate to severe Crohn's disease: a randomised, double blind, placebo controlled trial. Gut 2004;53(10):1485‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schreiber 2005
- Schreiber S, Rutgeerts P, Fedorak RN, Khaliq‐Kareemi M, Kamm MA, Boivin M, et al. A randomized, placebo‐controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology 2005;129(3):807‐18. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors(s) editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Su 2004
- Su C, Lichtenstein GR, Krok K, Brensinger CM, Lewis JD. A meta‐analysis of the placebo rates of remission and response in clinical trials of active Crohn's disease. Gastroenterology 2004;126(5):1257‐69. [DOI] [PubMed] [Google Scholar]
Thomsen 2001
- Thomsen OO. A comparison of budesonide and mesalamine for active Crohn's disease. Correction. New England Journal of Medicine 2001;345(22):1652. [DOI] [PubMed] [Google Scholar]
Tvede 1983
- Tvede M, Bondesen S, Nielsen OH, Rasmussen SN. Serum antibodies to Bacteroides species in chronic inflammatory bowel disease. Scandinavian Journal of Gastroenterology 1983;18(6):783‐9. [DOI] [PubMed] [Google Scholar]
Wadworth 1991
- Wadworth AN, Fitton A. Olsalazine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in inflammatory bowel disease. Drugs 1991;41(4):647‐64. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lim 2010
- Lim WC, Hanauer S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008870] [DOI] [PubMed] [Google Scholar]


