Abstract
Fear extinction, an inhibitory learning that suppresses a previously learned fear memory, is diminished during adolescence. Earlier studies have shown that this suppressed fear extinction during adolescence involves an altered glutamatergic plasticity in infralimbic medial prefrontal cortical (IL-mPFC) pyramidal neurons. However, it is unclear whether the excitability of IL-mPFC pyramidal neurons plays a role in this development-dependent suppression of fear extinction. Therefore, we examined whether fear conditioning and extinction affect the active and passive membrane properties of IL-mPFC layer 5 pyramidal neurons in pre-adolescent, adolescent and adult mice. Both pre-adolescent and adult mice exhibited a bidirectional modulation of the excitability of IL-mPFC layer 5 pyramidal neurons following fear conditioning and extinction, i.e., fear conditioning reduced membrane excitability whereas fear extinction reversed this effect. However, the fear conditioning-induced suppression of excitability was not reversed in adolescent mice following fear extinction training. Neither fear conditioning nor extinction affected GABAergic transmission in IL-mPFC layer 5 pyramidal neurons suggesting that GABAergic transmission did not play a role in experience-dependent modulation of neuronal excitability. Our results suggest that the extinction-specific modulation of excitability is impaired during adolescence.
Keywords: infralimbic medial prefrontal cortex, pyramidal neurons, excitability, GABA, fear extinction
Graphical abstract
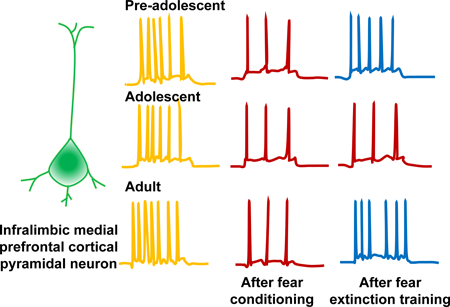
Fear learning suppresses excitability of infralimbic medial prefrontal cortical pyramidal neurons. Consistent with the impairment of fear extinction during adolescence, fear extinction training fails to restore infralimbic medial prefrontal cortical pyramidal neuron excitability in adolescent mice.
Introduction
The majority of adults who are diagnosed with anxiety and fear-related disorders exhibit symptoms during their childhood and adolescent years (Kim-Cohen et al., 2003; Pollack et al., 1996). The dearth of knowledge about the mechanism underlying increased anxiety and trauma-related disorders during adolescence poses a significant challenge to the development of appropriate therapeutic strategies in both young and adult subjects. Therefore, understanding the development-dependent changes in the brain that are pertinent to anxiety- and trauma-related behaviors is critical for the prevention and management of anxiety disorders across the lifespan. Adolescence is a time of increased susceptibility to anxiety disorders and other mental illnesses (Kessler et al., 2005; Paus et al., 2008). Correspondingly, adolescent humans and rodents show a diminished fear extinction, an inhibitory learning necessary to suppress a previously learned fear (McCallum et al., 2010; Pattwell et al., 2012). Since adolescence is a developmental period that demands the initiation of behaviors to attain independence from the protected period of early life, an interaction between stressful life challenges, a diminished ability to attenuate fear and potential genetic risk factors is likely to exacerbate anxiety symptoms. Earlier studies to understand the mechanism underlying diminished fear extinction during adolescence revealed that the synaptic potentiation in the infralimbic medial prefrontal cortex (IL-mPFC) is suppressed during adolescence (Pattwell et al., 2012). This experience-dependent potentiation of glutamatergic synapses in the IL-mPFC involves synaptic insertion of calcium permeable AMPA receptors (Sepulveda-Orengo et al., 2013). It is highly likely that this fear extinction-dependent potentiation of glutamatergic synapses in the IL-mPFC allows a top-down regulation of the amygdala to suppress fear memory (Burgos-Robles et al., 2007; Gabbott and Bacon, 1996; Likhtik et al., 2005; McDonald, 1998; McDonald et al., 1996; Milad and Quirk, 2002; Pare et al., 2004; Peters et al., 2009; Quirk et al., 2006; Quirk et al., 2003; Russchen, 1982; Santini et al., 2004; Vertes, 2004; Vertes, 2006).
Apart from the aforementioned synaptic plasticity in the IL-mPFC, there have been studies that demonstrated fear behavior-dependent modulation of intrinsic excitability of IL-mPFC pyramidal neurons (Santini et al., 2008). Therefore, a modulation of intrinsic excitability of IL-mPFC pyramidal neurons could contribute to the top-town regulation of amygdala and hence behavior. Subsequent studies have shown that M-type K+ channels play an important role in changes in the intrinsic excitability of IL-mPFC pyramidal neurons and fear expression (Santini and Porter, 2010; Santini et al., 2012). Given the suppression of fear extinction during adolescence, it is important to know whether there are adolescence-specific deficits in intrinsic plasticity in the IL-mPFC.
Our current study examined whether the intrinsic plasticity in the IL-mPFC neurons involved in fear learning- and extinction is altered during adolescence. As reported previously, adolescent mice exhibited a diminished fear extinction compared to pre-adolescent and adult mice (McCallum et al., 2010; Pattwell et al., 2012). While fear conditioning suppressed the membrane excitability of the layer 5 pyramidal neurons of the IL-mPFC, fear extinction reversed this suppression of excitability in both pre-adolescent and adult mice. Although adolescent mice exhibited a fear conditioning-induced suppression of the excitability of the IL-mPFC neurons, this suppressed excitability was not reversed by extinction training. Our results suggest that the adolescent IL-mPFC pyramidal neurons lack the ability to restore fear conditioning-induced suppression of intrinsic excitability, which might contribute to the diminished fear extinction during adolescence.
MATERIALS AND METHODS
Animals
All mice [(homozygous PV-Cre (stock #017320) and homozygous Ai32 (stock #024109)] were initially ordered from Jackson Laboratory and subsequently bred in the New York University Medical School animal facility. Mice were housed in the New York University Medical School animal facility on a 12:12 light-dark cycle at 23 °C with access to food and water ad libitum. Male mice were used in all experiments and all procedures were approved by the Institutional Animal Care and Use Committee of the New York University School of Medicine.
Behavior
We followed the age categorization suggested by Spear which defines adolescence in mice to span from P28 until P45 (Spear, 2000). Fear conditioning (FC) or tone alone (TA) exposure was performed on P22 for juveniles, on P27 for adolescents or between P60–118 for adults (Table 1). For conditioning, a mouse was placed in the conditioning chamber (context A) within a soundproof box (Coulbourn Instruments). After a 2 min exploration, mice received two habituation tones (5 kHz, 30 dB, 30 sec duration) followed by three tones (30 sec duration) that co-terminated with foot shocks (2 sec duration, 0.5 mA, 30 sec interval). Mice were removed from the box and returned to the home cage 30 sec after the final tone-shock pairing. The tone alone group received the same tone presentations as the fear conditioned group but without foot shocks. Fear extinction (FE) training was performed 24 hr after conditioning and consisted of 30 tone presentations (30 sec duration) at 30 sec interval (context B). For all groups, fear memory was tested 48 hr after the initial conditioning by exposing the animal to three tones (30 sec duration) at 30 sec interval (context B) (Table 1). Freezing was measured using the software provided by Coulbourn Instruments.
Table1.
Experimental design for behavior and brain slice preparation for electrophysiology. P22 (pre-adolescent), P27 (adolescent) or P60+ (adult) mice were grouped into tone alone, fear condition and fear extinction groups. Brain slices were prepared for electrophysiological recordings 1 hr after fear memory test on day 3.
| Groups | Habituation and conditioning (Day 1) [P22 (pre-adolescent), P27 (adolescent), P60–118 (adult)] | Extinction (Day 2) | Fear memory test (Day 3) | Preparation of brain slices (1 hr. after fear memory test) |
|---|---|---|---|---|
| Tone alone | 2 habituation tones +3 tones in conditioning context | Home cages | 3 tones | ✓ |
| Fear conditioning | 2 habituation tones +3-tone-shocks in conditioning context | Home cages | 3 tones | ✓ |
| Fear extinction | 2 habituation tones +3-tone-shocks in conditioning context | 30 tones in extinction context | 3 tones | ✓ |
Electrophysiology
Following the fear memory testing (∼1 hr), mice were anesthetized with pentobarbital (120 mg/kg, i.p.) and transcardially perfused with ice cold, oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl (118), glucose (10), KCl (2.5), NaH2PO4 (1), CaCl2 (1) and MgSO4 (1.5) (325 mOsm, pH 7.4). The brain was then rapidly removed and coronal brain slices of 300 µm thickness were prepared using a vibrating microtome (Campden Instruments). Slices were left to recover for at least 1 hr at room temperature. After recovery, one slice was transferred to the recording chamber and superfused with the aforementioned ACSF containing 2.5 mM CaCl2 at 32 °C at 2–3 ml/min. The IL-mPFC was located using a 4x objective and layer 5 pyramidal neurons were visualized using a 40x water immersion objective and video-enhanced differential interference contrast microscopy. Layer 5 pyramidal neurons were identified by their morphology and accommodating action potential firing characteristics. Glass pipettes of 3 – 5 MΩ resistance were filled with internal solution containing (in mM): K-gluconate (130), KCl (10), MgCl2 (5), MgATP (5), NaGTP (0.2), EGTA (0.5), HEPES (5), pH adjusted to 7.4 with KOH. Electrophysiological measurements were performed with a Multiclamp 700B amplifier connected to a Digidata 1550A (Molecular Devices, CA, USA). Signals were sampled at 20 – 100 kHz and filtered at 2 kHz. Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded in the presence of DNQX (10 µM) and D-2-Amino-5-phosphonovaleric acid (APV, 50 μM) at −60mV. Neuronal excitability was measured in current clamp mode by injecting currents from −50 to 200 pA (10 pA increments) in the presence of glutamate receptor blockers, DNQX and AP5.
Data Analysis
Neuronal firing and passive membrane properties were analyzed using Clampfit 10.5. Passive membrane properties were measured from the membrane voltage response to hyperpolarizing current injections of −50 to −10 pA. Input resistance (Rin) was calculated as the slope of the current-voltage relationship from −50 to 0 pA and membrane time constant tau was calculated by fitting the initial change in membrane voltage in response to a −50 pA step to a single exponential function. Membrane capacitance (Cm) was calculated from tau and Rin. After-hyperpolarization (AHP) was calculated by measuring the difference between the action potential threshold and the maximal hyperpolarization after the first action potential. All statistical analyses and graph preparations were performed in GraphPad Prism 7 (GraphPad Software, Inc). One-way ANOVA with Tukey’s post hoc test or Kruskal-Wallis with Dunn’s post hoc test were used to compare passive membrane properties, spontaneous neurotransmission and fear extinction memory. Two-way ANOVA with Tukey’s post hoc test was used to analyze neuronal excitability.
Results
Bidirectional modulation of IL-mPFC layer 5 pyramidal neuron excitability by fear conditioning and extinction in pre-adolescent mice
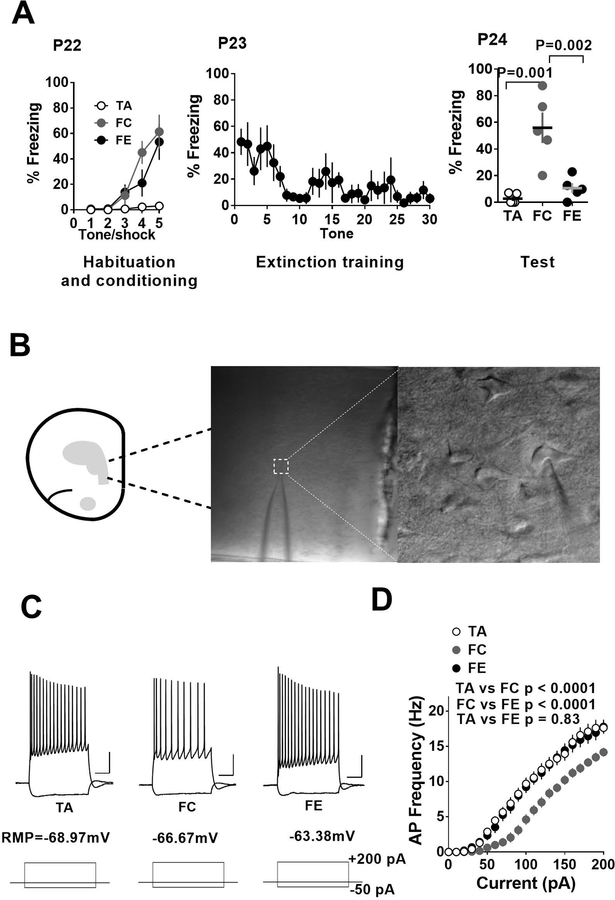
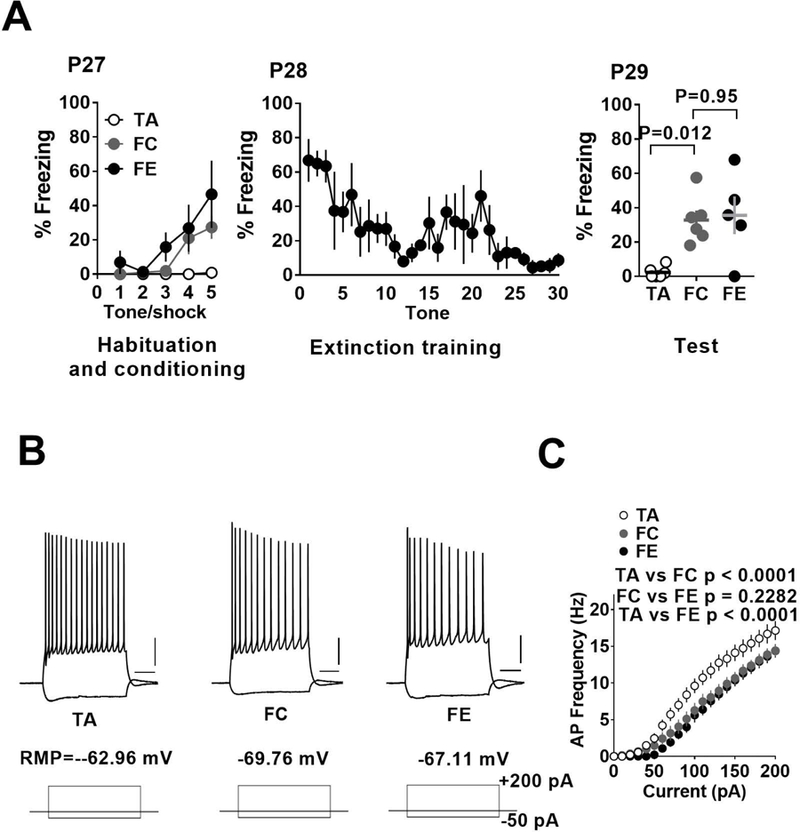
Given the earlier demonstration of a bidirectional modulation of IL-mPFC pyramidal neuron excitability by fear conditioning and extinction (Santini et al., 2008), first we asked whether pre-adolescent (P24) mice exhibit a similar bidirectional change in membrane excitability following fear conditioning and extinction (Table 1). Fear conditioned pre-adolescent mice exhibited an increased freezing compared to the pre-adolescent tone alone group (Figure 1A). In addition, pre-adolescent mice that underwent fear extinction training exhibited a significant decrease in freezing compared to fear conditioned group (Figure 1A) (F (2, 12) = 16.7, p < 0.0001; tone alone vs fear condition: p < 0.001; tone alone vs fear extinction: p = 0.9; fear condition vs fear extinction: p < 0.002). Electrophysiological experiments to measure action potentials in layer 5 pyramidal neurons in response current injection revealed a reduction in the frequency of action potentials in fear conditioned group compared to tone alone group suggesting a suppression of neuronal excitability following fear learning (Figure 1C,D). Furthermore, fear extinction training restored the neuronal excitability to values comparable to the tone alone group (effect of treatment: F (2, 985) = 141.1, p < 0.0001; tone alone vs fear condition: p < 0.0001; tone alone vs fear extinction: p = 0.83; fear condition vs fear extinction: p < 0.0001; two-way ANOVA with Tukey’s post hoc test) (Figure 1C,D).
Figure 1.
Fear conditioning and extinction result in a bidirectional modulation of excitability in IL-mPFC layer 5 pyramidal neurons of pre-adolescent mice. A) Average freezing during exposure to tone alone or tone-shock pairing on day 1, during extinction training on day 2 and fear memory test on day 3. B) Schematic presentation and an example of whole cell recording in IL-mPFC layer 5 pyramidal neurons. C) Example traces of voltage responses to hyperpolarizing (−50 pA) and depolarizing (+200 pA) current steps in pyramidal neurons from tone alone (TA), fear condition (FC) and fear extinction (FE) groups. Scale: 250 ms/20 mV. D) Current vs. action potential (AP) frequency plot in tone alone (16 neurons/5 mice), fear condition (18 neurons/5 mice) and fear extinction (19 neurons/5 mice) groups. The fear condition group showed fewer action potentials compared to tone alone group and this effect was reversed by fear extinction.
Adolescent mice exhibit diminished fear extinction and intrinsic plasticity involved in fear extinction
Similar to the pre-adolescent mice, the fear conditioned adolescent group exhibited an increased freezing compared to the adolescent tone alone group (Figure 2A). However, pre-adolescent mice that underwent fear extinction training did not show a significant decrease in freezing compared to fear conditioned group (Figure 2A) (F (2, 14) = 8.06, p = 0.005; tone alone vs fear condition: p = 0.012; tone alone vs fear extinction: p = 0.009; fear condition vs fear extinction: p = 0.954). These results are consistent with the previous reports showing an attenuation of fear extinction during adolescence (McCallum et al., 2010; Pattwell et al., 2012). Consistent with the comparable fear conditioning in pre-adolescent and adolescent groups, fear conditioned adolescent mice exhibited a significant suppression of action potential frequency compared to the tone alone adolescent group (Figure 2B,C). However, the diminished fear extinction in adolescent group was associated with an attenuated restoration of neuronal excitability following fear extinction training (effect of treatment: F (2, 950) = 65.88, p < 0.0001; tone alone vs fear condition: p < 0.0001; tone alone vs fear extinction: p < 0.0001; fear condition vs fear extinction: p = 0.2282) (Figure 2B,C).
Figure 2.
Fear extinction fails to reverse fear conditioning-induced suppression of excitability in IL-mPFC layer 5 pyramidal neurons of adolescent mice. A) Average freezing during exposure to tone alone or tone-shock pairing on day 1, during extinction training on day 2 and fear memory test on day 3. B) Example traces of voltage responses to hyperpolarizing (−50 pA) and depolarizing (+200 pA) current steps in pyramidal neurons from tone alone, fear condition and fear extinction groups. Scale: 250 ms/20 mV. C) Current vs. action potential (AP) frequency plot in tone alone (18 neurons/5 mice), fear condition (19 neurons/5 mice) and fear extinction (13 neurons/5 mice) groups. The fear condition group showed fewer action potentials compared to tone alone group and this effect remained unaffected by fear extinction.
Bidirectional modulation of IL-mPFC layer 5 pyramidal neuron excitability by fear conditioning and extinction in adult mice
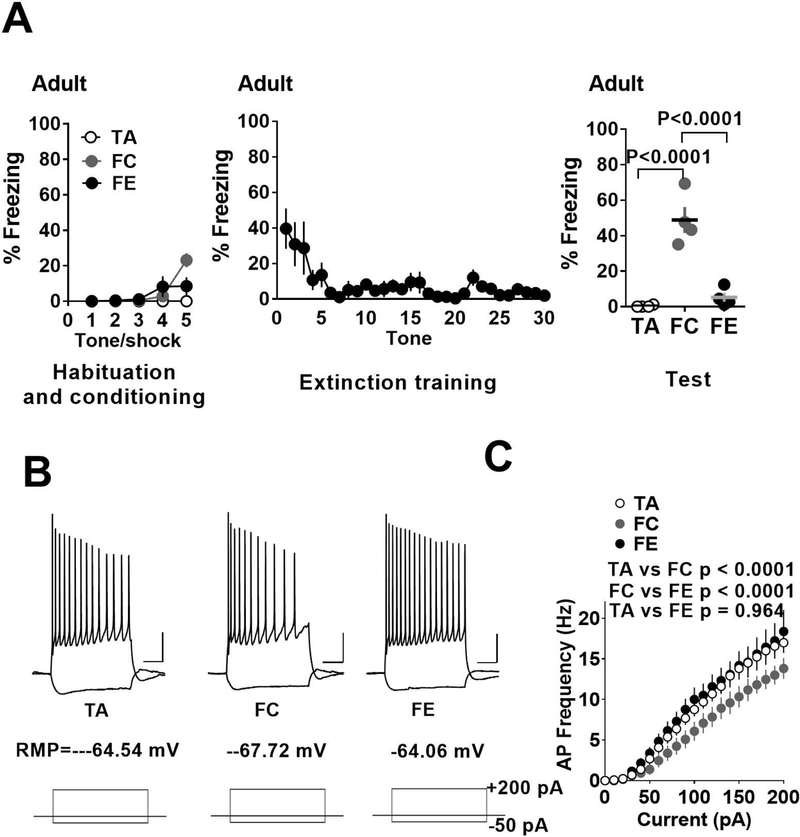
As in the case of both pre-adolescent and adolescent mice, fear conditioned adult mice also exhibited an increased freezing compared to the adult tone alone group (Figure 3A). In addition, adult mice that underwent fear extinction training exhibited a significant decrease in freezing compared to fear conditioned group (Figure 3A) (F (2, 9) = 35.7, p < 0.0001; tone alone vs fear condition: p < 0.0001; tone alone vs fear extinction: p = 0.726; fear condition vs fear extinction: p < 0.0001). Similar to both pre-adolescent and adolescent mice, fear conditioned adult mice showed a reduction in the frequency of action potentials compared to tone alone group suggesting a suppression of neuronal excitability following fear learning (Figure 3B,C). However, unlike the adolescent mice, fear extinction training restored the neuronal excitability to values comparable to the tone alone group (effect of treatment: F (2, 710) = 40.45, p < 0.0001; tone alone vs fear condition: p < 0.0001; tone alone vs fear extinction: p = 0.964; fear condition vs fear extinction: p < 0.0001) (Figure 3B,C).
Figure 3.
Fear conditioning and extinction result in a bidirectional modulation of excitability in IL-mPFC layer 5 pyramidal neurons of adult mice. A) Average freezing during exposure to tone alone or tone-shock pairing on day 1, during extinction training on day 2 and fear memory test on day 3. B) Example traces of voltage responses to hyperpolarizing (−50 pA) and depolarizing (+200 pA) current steps in pyramidal neurons from tone alone, fear condition and fear extinction groups. Scale: 250 ms/20 mV. D) Current vs. action potential (AP) frequency plot in tone alone (13 neurons/4 mice), fear condition (13 neurons/4 mice) and fear extinction (13 neurons/4 mice) groups. The fear condition group showed fewer action potentials compared to tone alone group and this effect was reversed by fear extinction.
Effect of fear conditioning and extinction on passive membrane properties in pre-adolescent, adolescent and adult mice
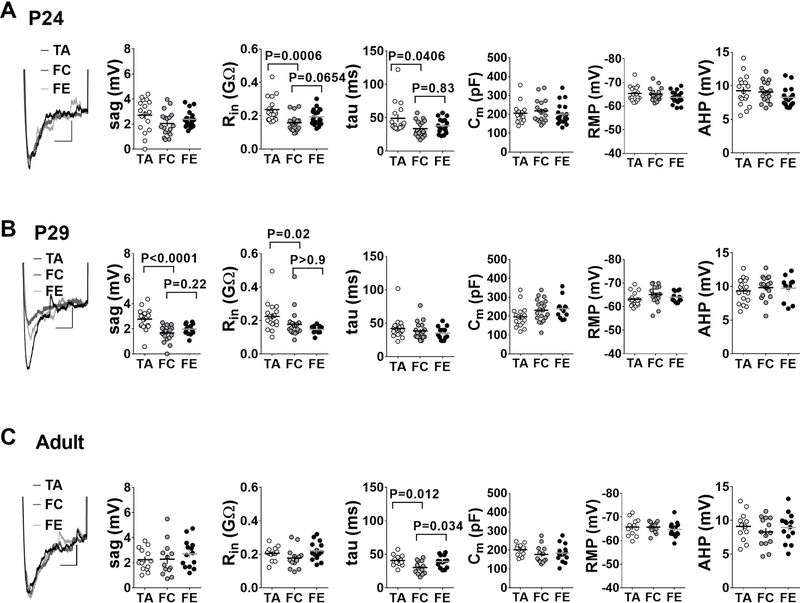
To identify potential mechanisms underlying the experience-driven changes in excitability, we measured passive membrane properties of IL-mPFC layer 5 pyramidal neurons in tone alone, fear condition, and fear extinction groups across the three developmental stages. In the pre-adolescent mice, the fear conditioned mice showed significantly lower input resistance and time constant values compared to the tone alone group (Figure 4A). However, despite the fear extinction-mediated restoration of neuronal excitability, both input resistance and time constant remained unaffected after fear extinction. Neither fear conditioning nor extinction affected membrane capacitance, sag, resting membrane potential (RMP) or after-hyperpolarization (AHP) values (Figure 4A). In adolescent mice, both input resistance and sag values were significantly reduced in fear condition and fear extinction groups compared with the tone alone group (Figure 4B). Fear conditioning and extinction did not affect time constant, membrane capacitance, RMP or AHP values. In adult mice, passive membrane properties remained largely unaffected by fear conditioning and extinction with the exception of significantly lower time constant values in the fear condition group compared to the tone alone and fear extinction groups (Figure 4C). The differential modulation of passive membrane properties could be due to a development- and experience-dependent release of several neuromodulators. Nevertheless, both fear conditioning and extinction modulate membrane excitability in a bidirectional and development-specific manner. Interestingly, an irreversible suppression of sag values in adolescent mice following fear conditioning suggests the possible involvement of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in fear extinction impairment during adolescence.
Figure 4.
Passive membrane properties of IL-mPFC layer 5 pyramidal neurons in tone alone, fear condition and fear extinction groups from the three developmental stages. Passive membrane properties are measured in same neurons described in figures 1–3. A) Passive membrane properties in pre-adolescent group. The fear condition group exhibited significantly lower input resistance (Rin) and membrane time constant tau values compared with the tone alone group. B) Passive membrane properties in adolescent group. The fear condition and fear extinction groups exhibited significantly reduced Rin and sag values compared with the tone alone group. C) Passive membrane properties in adult group. Passive membrane properties remained largely unaffected, with the exception of significantly reduced tau values in the fear condition group. Left panels show example traces for sag response (Scale: 250 ms/1 mV).
Lack of experience-dependent modulation of GABAergic input to the IL-mPFC layer 5 pyramidal neurons
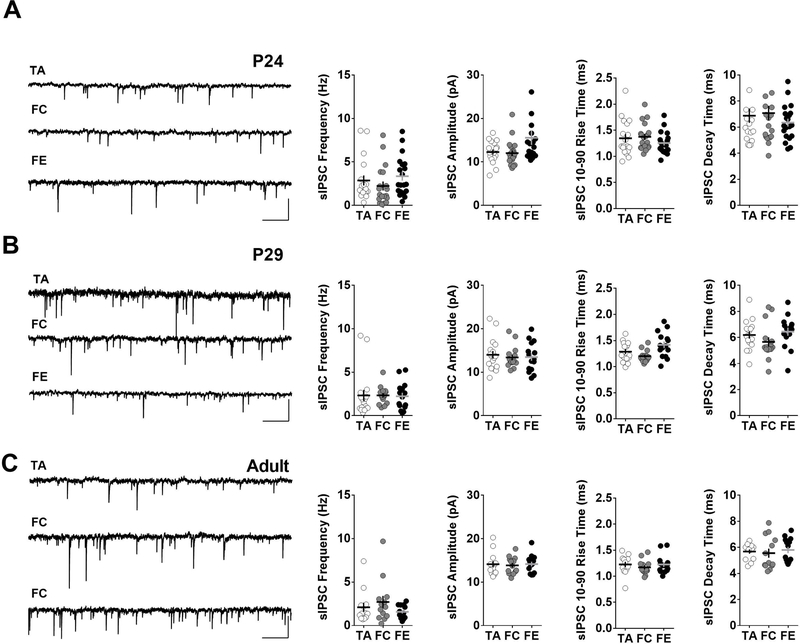
Since GABAergic transmission is critical for cortical processing and function (Isaacson and Scanziani, 2011), we examined whether an experience-dependent change in GABAergic input to IL-mPFC pyramidal neurons contributes to the lack of bidirectional intrinsic plasticity during adolescence. We recorded spontaneous inhibitory post-synaptic currents (sIPSCs) in IL-mPFC layer 5 pyramidal neurons. A comparison of the frequency, amplitude, rise time and decay time of sIPSCs in tone alone, fear condition and fear extinction groups revealed that fear learning or extinction does not affect total GABAergic input to IL-mPFC pyramidal neurons at all three developmental stages (Figure 5A,B,C). Therefore, the total GABAergic input does not play a role in the lack of bidirectional intrinsic plasticity during adolescence.
Figure 5.
Fear conditioning or extinction did not affect GABAergic input IL-mPFC layer 5 pyramidal neurons. A) Left panel shows example sIPSC traces from IL-mPFC layer 5 neurons of pre-adolescent tone alone, fear condition and fear extinction groups. Scale: 1 s / 20 pA. Right panels show average frequency, amplitude, rise time and decay time of sIPSCs from pre-adolescent tone alone (18 neurons/5 mice), fear condition (18 neurons/5 mice) and fear extinction (20 neurons/5 mice) groups. B) Left panel shows example sIPSC traces from IL-mPFC layer 5 neurons of adolescent tone alone, fear condition and fear extinction groups. Scale: 1 s / 20 pA. Right panels show average frequency, amplitude, rise time and decay time of sIPSCs from adolescent tone alone (17 neurons/5 mice), fear condition (15 neurons/5 mice) and fear extinction (15 neurons/5 mice) groups. C) Left panel shows example sIPSC traces from IL-mPFC layer 5 neurons of adult tone alone, fear condition and fear extinction groups. Scale: 1 s / 20 pA. Right panels show average frequency, amplitude, rise time and decay time of sIPSCs from adult tone alone (13 neurons/4 mice), fear condition (13 neurons/4 mice) and fear extinction (14 neurons/4 mice) groups.
Discussion
In this study, we report an adolescence-specific lack of intrinsic plasticity in the IL-mPFC layer 5 pyramidal neurons, which is involved in fear extinction. Consistent with the previous studies, we observed a diminished fear extinction in adolescent mice compared to pre-adolescent and adult mice (McCallum et al., 2010; Pattwell et al., 2012). An earlier study reported a bidirectional modulation of membrane excitability in IL-mPFC layer 5 pyramidal neurons following fear conditioning and extinction in rats (Santini et al., 2008). We observed similar results in pre-adolescent and adult mice. However, consistent with the diminished fear extinction during adolescence, adolescent mice failed to exhibit a restoration of membrane excitability following fear extinction trials. The bidirectional regulation of membrane excitability is particularly noteworthy, as fear extinction is believed to be a new learning rather than a modification of learned fear. The fear extinction-induced reversal of suppressed IL-mPFC pyramidal neuron excitability might be a key mechanism in suppressing a previously learned fear. It is plausible that distinct neuromodulatory systems cause suppression of neuronal excitability following fear learning and its reversal following fear extinction. Therefore, a deficit in neuromodulatory process involved in restoring IL-mPFC pyramidal neuron excitability might be responsible for suppressed fear extinction in adolescent mice. Unlike the suppressive effect of delay fear conditioning on IL-mPFC pyramidal neuron excitability, trace fear conditioning enhances the excitability of IL-mPFC pyramidal neurons (Song et al., 2015), suggesting distinct roles for IL-mPFC pyramidal neurons in delay and trace fear conditioning.
Our earlier study on the effect of fear conditioning and extinction on glutamatergic transmission revealed that fear conditioning does not affect glutamatergic transmission in IL-mPFC layer 5 pyramidal neurons (Pattwell et al., 2012). However, fear extinction results in a potentiation of glutamatergic synapses in IL-mPFC layer 5 pyramidal neurons of pre-adolescent and adult mice but not adolescent mice (Pattwell et al., 2012). It was shown that a synaptic insertion of calcium permeable AMPA receptors is responsible for this fear extinction-dependent synaptic potentiation in IL-mPFC layer 5 pyramidal neurons (Pattwell et al., 2012; Sepulveda-Orengo et al., 2013). Therefore, both the increase in glutamatergic input and intrinsic excitability following fear extinction training might allow a robust top-down inhibition of the amygdala to suppress learned fear. Recent studies have demonstrated the causal role of amygdala projecting IL-mPFC neurons in fear extinction (Bloodgood et al., 2018). Both human and animal studies have strongly suggested that the medial prefrontal cortex-mediated top-down regulation of the amygdala is involved in fear extinction (Amano et al., 2010; Bremner et al., 1999; Milad and Quirk, 2002; Morgan and LeDoux, 1995; Phelps et al., 2004; Quirk et al., 2006; Quirk et al., 2000; Santini et al., 2004; Santini et al., 2008). The enhanced IL-mPFC output facilitates the feed-forward inhibition of the central amygdala, resulting in decreased output to the brain stem, and hence decreased fear response (Burgos-Robles et al., 2007; Milad and Quirk, 2002; Peters et al., 2009; Quirk et al., 2006; Quirk et al., 2003; Santini et al., 2004). A recent study showed that the IL-mPFC inhibits basomedial amygdala to enhance fear extinction (Adhikari et al., 2015). Therefore, a diminished IL-mPFC plasticity might play a role in increased anxiety- and trauma-related behaviors during adolescence.
Analysis of passive membrane properties after experience-driven excitability changes across ages suggests that the fear conditioning-mediated reduction in IL-mPFC pyramidal neuronal excitability is driven through reductions in input resistance (Santini et al., 2008). The impairment in the restoration of control excitability levels after fear extinction training in adolescents appears to underlie at least two mechanisms. First, the reversal of input resistance values to that of control mice following fear extinction was absent in adolescent mice. Second, an irreversible modulation of sag ratio suggests HCN channel involvement in the alteration of adolescent IL-mPFC excitability (Dickson et al., 2000). Reduced sag values point towards reduced HCN channel conductance after fear conditioning only during the adolescent period. A recent study reported a reduction in hyperpolarization-activated cation current and a diminished intrinsic persistent activity following alcohol consumption during adolescence (Salling et al., 2018). HCN channels are known modulators of neuronal excitability and play a critical role in dendritic integration of prefrontal and hippocampal pyramidal neurons (Aponte et al., 2006; Day et al., 2005; Magee, 1998). Interestingly, mice that lack HCN1 channels show enhanced hippocampal LTP and spatial memory (Nolan et al., 2004). Therefore, an irreversible suppression of HCN activity involved in fear conditioning might play an important role in impaired fear extinction during adolescence. In contrast to our finding of overall reduced excitability after adolescent fear conditioning and extinction, a reduction in HCN channel conductance is often associated with increased input resistances and neuronal excitability (Brager and Johnston, 2007; Magee, 1998; Rosenkranz and Johnston, 2006; Yi et al., 2016). Thus, further studies will be required to evaluate the involvement of IL-mPFC HCN channels in adolescent fear learning.
GABAergic neurons show significant diversity and distinct classes of these neurons show specific roles in cortical oscillations, orchestrating synapse development and plasticity (Fishell and Rudy, 2011; Le Magueresse and Monyer, 2013). Increase in GABAergic transmission has been demonstrated to inhibit plasticity in the mPFC (Couey et al., 2007). However, measurement of spontaneous GABAergic transmission in IL-mPFC layer 5 pyramidal neurons did not reveal statistically significant difference in any of GABAergic properties measured in pre-adolescent, adolescent and adult mice. It is also plausible that behavioral experience, fear conditioning or extinction, could affect these properties and hence modulate intrinsic plasticity in glutamatergic neurons. However, our data show that nether fear learning nor extinction affected GABAergic transmission in IL-mPFC layer 5 pyramidal neurons in any of the developmental stages. Although total GABAergic transmission is not affected, it is possible that differences in the development or plasticity in any of the specific classes of GABAergic neurons could influence intrinsic plasticity in the principal neurons.
In conclusion, our studies demonstrate a specific plasticity in the IL-mPFC involved in fear extinction, which is lacking during adolescence. It is plausible that the adaptive mechanisms that attenuate conditioned fear might involve an enhanced IL-mPFC activity. Future studies will be necessary to understand mechanisms underlying such adaptive processes and its alteration during adolescence, which might provide opportunities for managing anxiety- and trauma-related disorders in both adolescents and adults.
ACKNOWLEDGEMENTS
This work was supported by NIH (HD076914 to I.N.).
Footnotes
The authors declare no biomedical financial interests or potential conflicts of interest.
References
- Adhikari A, Lerner TN, Finkelstein J, Pak S, Jennings JH, Davidson TJ, Ferenczi E, Gunaydin LA, Mirzabekov JJ, Ye L, Kim SY, Lei A, Deisseroth K. 2015. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 527(7577):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Unal CT, Pare D. 2010. Synaptic correlates of fear extinction in the amygdala. Nature neuroscience 13(4):489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Lien CC, Reisinger E, Jonas P. 2006. Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. The Journal of physiology 574(Pt 1):229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood DW, Sugam JA, Holmes A, Kash TL. 2018. Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Translational psychiatry 8(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Johnston D. 2007. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in I(h) in hippocampal CA1 pyramidal neurons. J Neurosci 27(51):13926–13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. 1999. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological psychiatry 45(7):806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. 2007. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron 53(6):871–880. [DOI] [PubMed] [Google Scholar]
- Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, Mansvelder HD. 2007. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron 54(1):73–87. [DOI] [PubMed] [Google Scholar]
- Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. 2005. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. The Journal of neuroscience : the official journal of the Society for Neuroscience 25(38):8776–8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson CT, Magistretti J, Shalinsky MH, Fransen E, Hasselmo ME, Alonso A. 2000. Properties and role of I(h) in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. Journal of neurophysiology 83(5):2562–2579. [DOI] [PubMed] [Google Scholar]
- Fishell G, Rudy B. 2011. Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annual review of neuroscience 34:535–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ. 1996. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. The Journal of comparative neurology 364(4):609–636. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. 2011. How inhibition shapes cortical activity. Neuron 72(2):231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry 62(6):593–602. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. 2003. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Archives of general psychiatry 60(7):709–717. [DOI] [PubMed] [Google Scholar]
- Le Magueresse C, Monyer H. 2013. GABAergic interneurons shape the functional maturation of the cortex. Neuron 77(3):388–405. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. 2005. Prefrontal control of the amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience 25(32):7429–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. 1998. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 18(19):7613–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum J, Kim JH, Richardson R. 2010. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology 35(10):2134–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. 1998. Cortical pathways to the mammalian amygdala. Progress in neurobiology 55(3):257–332. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. 1996. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71(1):55–75. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. 2002. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420(6911):70–74. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. 1995. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral neuroscience 109(4):681–688. [DOI] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. 2004. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell 119(5):719–732. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. 2004. New vistas on amygdala networks in conditioned fear. Journal of neurophysiology 92(1):1–9. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, Soliman F, Glatt CE, Casey BJ, Ninan I, Lee FS. 2012. Altered Fear Learning Across Development in Both Mouse and Human. Proceedings of the National Academy of Sciences of the United States of America 109(40):16318–16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. 2008. Why do many psychiatric disorders emerge during adolescence? Nature reviews 9(12):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. 2009. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & memory (Cold Spring Harbor, NY 16(5):279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. 2004. Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43(6):897–905. [DOI] [PubMed] [Google Scholar]
- Pollack MH, Otto MW, Sabatino S, Majcher D, Worthington JJ, McArdle ET, Rosenbaum JF. 1996. Relationship of childhood anxiety to adult panic disorder: correlates and influence on course. Am J Psychiatry 153(3):376–381. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. 2006. Prefrontal mechanisms in extinction of conditioned fear. Biological psychiatry 60(4):337–343. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. 2003. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 23(25):8800–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. 2000. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 20(16):6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D. 2006. Dopaminergic regulation of neuronal excitability through modulation of Ih in layer V entorhinal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 26(12):3229–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russchen FT. 1982. Amygdalopetal projections in the cat. I. Cortical afferent connections. A study with retrograde and anterograde tracing techniques. The Journal of comparative neurology 206(2):159–179. [DOI] [PubMed] [Google Scholar]
- Salling MC, Skelly MJ, Avegno E, Regan S, Zeric T, Nichols E, Harrison NL. 2018. Alcohol Consumption during Adolescence in a Mouse Model of Binge Drinking Alters the Intrinsic Excitability and Function of the Prefrontal Cortex through a Reduction in the Hyperpolarization-Activated Cation Current. J Neurosci 38(27):6207–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. 2004. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci 24(25):5704–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Porter JT. 2010. M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction. J Neurosci 30(37):12379–12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. 2008. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 28(15):4028–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Sepulveda-Orengo M, Porter JT. 2012. Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction. Neuropsychopharmacology 37(9):2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda-Orengo MT, Lopez AV, Soler-Cedeno O, Porter JT. 2013. Fear Extinction Induces mGluR5-Mediated Synaptic and Intrinsic Plasticity in Infralimbic Neurons. J Neurosci 33(17):7184–7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Ehlers VL, Moyer JR Jr., 2015. Trace Fear Conditioning Differentially Modulates Intrinsic Excitability of Medial Prefrontal Cortex-Basolateral Complex of Amygdala Projection Neurons in Infralimbic and Prelimbic Cortices. J Neurosci 35(39):13511–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. 2000. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews 24(4):417–463. [DOI] [PubMed] [Google Scholar]
- Vertes RP. 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse (New York, NY 51(1):32–58. [DOI] [PubMed] [Google Scholar]
- Vertes RP. 2006. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142(1):1–20. [DOI] [PubMed] [Google Scholar]
- Yi F, Danko T, Botelho SC, Patzke C, Pak C, Wernig M, Sudhof TC. 2016. Autism-associated SHANK3 haploinsufficiency causes Ih channelopathy in human neurons. Science (New York, NY) 352(6286):aaf2669. [DOI] [PMC free article] [PubMed] [Google Scholar]