Abstract
To reduce the risk of salt-induced hypertension, medical authorities have emphasized dietary guidelines promoting high intakes of potassium and low intakes of salt that provide molar ratios of potassium to salt of ~1:1 or greater. However, over the past several decades, relatively few people have changed their eating habits sufficiently to reach the recommended dietary goals for salt and potassium. Thus, new strategies that reduce the risk of salt-induced hypertension without requiring major changes in dietary habits would be of considerable medical interest. In the current studies in a widely used model of salt-induced hypertension, the Dahl salt-sensitive rat, we found that supplemental dietary sodium nitrate confers substantial protection from initiation of salt-induced hypertension when the molar ratio of added nitrate to added salt is only ~1:170. Provision of a low molar ratio of added nitrate to added salt of ~1:110 by supplementing the diet with beetroot also conferred substantial protection against salt-induced increases in blood pressure. The results suggest that on a molar basis and a weight basis, dietary nitrate may be ~100 times more potent than dietary potassium with respect to providing substantial resistance to the pressor effects of increased salt intake. Given that leafy green and root vegetables contain very large amounts of inorganic nitrate, these findings raise the possibility that fortification of salty food products with small amounts of a nitrate-rich vegetable concentrate may provide a simple method for reducing risk for salt-induced hypertension.
Keywords: salt, hypertension, salt-sensitivity, sodium, beetroot, nitrate, nitric oxide
INTRODUCTION
Recently, it has been proposed that the amount of inorganic nitrate naturally present in diets rich in leafy green and root vegetables may be an important factor contributing to the antihypertensive effects of the Dietary Approaches to Stop Hypertension (DASH) diet. 1–6 The blood pressure lowering effects of supplemental inorganic nitrate and of nitrate-rich vegetable products such as beetroot juice have been widely-discussed and studied.2–4, 7–11 For example, in randomized, double-blind, placebo-controlled studies in hypertensive subjects, Kapil and colleagues found that acute and chronic administration of beetroot juice significantly reduced systolic and diastolic arterial pressure whereas nitrate-depleted beetroot juice did not.11 An extensive body of research has demonstrated that dietary nitrate can be endogenously converted to the potent vasodilator nitric oxide (NO), and based on the work of many experts in the field, it is believed that the beneficial effects of supplemental dietary nitrate on blood pressure are largely mediated by increases in NO activity.7, 10 It is important to note, however, that the mechanisms mediating the blood pressure effects of dietary nitrate are subject to ongoing investigations.
In the DASH-Sodium study, the pressor effect of increasing salt intake by 100 mmol/day was substantially lower in subjects consuming the DASH diet than in subjects consuming an American-style control diet.12 This raises the question: is the amount of supplemental nitrate provided by the original DASH study diet sufficient to largely account for the substantial capacity of that diet to protect against salt-induced increases in blood pressure ? According to an analysis by Keller and colleagues,6 the original DASH study diet increased nitrate intake approximately 1 mmol/day over the level of nitrate provided by a typical American-style diet. It would be remarkable and medically significant if a 1 mmol/day increase in dietary intake of a single electrolyte could substantially protect against the pressor effects of a 100 mmol/day or greater increase in salt intake.
Studies by Carlstrom and colleagues indicate that in a surgically-induced model of salt sensitivity (unilaterally nephrectomized Sprague Dawley rats), a molar ratio of added dietary nitrate to added salt of ~1:35 can protect against the hypertensinogenic effects of a high salt diet.13 However, it is unknown whether significant resistance to the pressor effects of a high salt diet can be conferred by the much lower ratio of added nitrate to added salt as used in the DASH-Sodium trial (~1:100) or even lower. It is also unknown whether supplemental nitrate can protect against initiation of salt-induced hypertension in spontaneous forms of salt sensitivity. If so, this would suggest new approaches for reducing the risk for salt-induced hypertension that do not depend on major alterations in dietary habits, large increases in nitrate intake, or substantial decreases in food salt content. Accordingly, in the most widely studied model of spontaneous salt sensitivity, the Dahl salt sensitive rat, we investigated whether the pressor effects of a very large increase in salt intake can be substantially attenuated by a relatively small increase in nitrate intake.
Methods
The data supporting the findings of this study are available within the article and its Online Supplement, and from the corresponding author on appropriate request.
Animal Model
Inbred, male Dahl salt-sensitive rats were obtained as weanlings from a colony maintained at the Institute of Physiology of the Czech Academy of Sciences in Prague, Czech Republic that was originally established with breeding pairs of inbred Dahl SS/Jr rats provided courtesy of Professor John P. Rapp (hereafter referred to as Dahl SS rats). Experiments were performed in conformance with the Animal Protection Law of the Czech Republic, the Guide to the Care and Use of Laboratory Animals (8th edition) of the US National Research Council, and were approved by the Ethics Committee of the Institute of Physiology, Academy of Sciences of the Czech Republic, Prague.
A detailed discussion of the study design and methods used is available in the online-only Data Supplement.
Statistical Analysis
The following null hypothesis was tested: a relatively small increase in nitrate intake, achieved by oral administration of either a nitrate-rich vegetable product (beetroot juice) or sodium nitrate, does not attenuate increases in mean arterial pressure otherwise induced by a very large increase in dietary sodium chloride. To investigate the primary null hypothesis, we used one-way analysis of variance (ANOVA) and the Holm-Sidak procedure to test for differences in the salt-induced changes in mean arterial pressure between the control group and the beetroot-treated and nitrate-treated experimental groups. ANOVA with Holm-Sidak testing was also used in an exploratory analysis of the effects of nitrate supplementation or beetroot supplementation on salt-induced changes in heart rate and locomotor activity. An unpaired t test was used in the analysis of a separate exploratory study of the effects of sodium nitrate supplementation on sodium balance. The results are expressed as means ± standard errors of the means (SEM), and P values are adjusted for the multiple group comparisons (group 1 treated with beetroot plus salt, and group 2 treated with sodium nitrate plus salt, each compared to the control group treated with salt alone). Statistical significance was defined as P < 0.05 and statistical analyses were performed with GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA).
Results
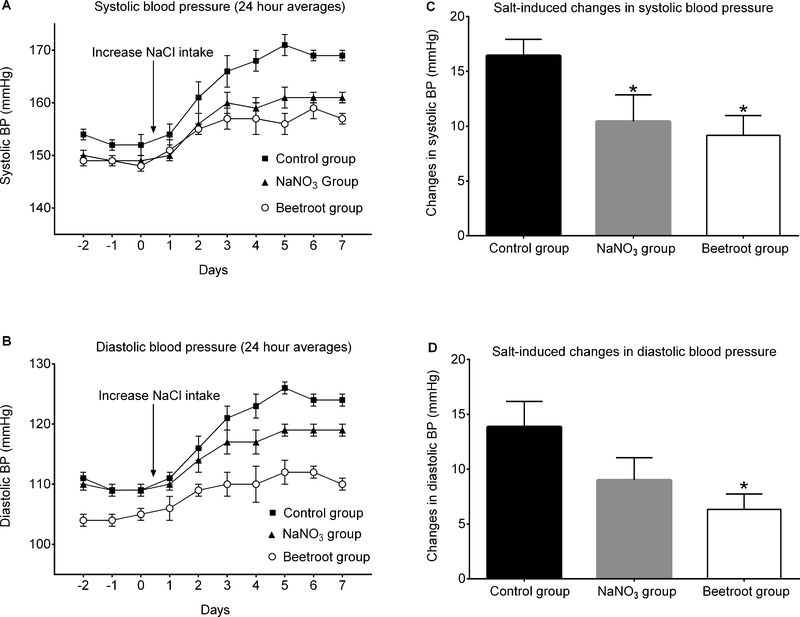
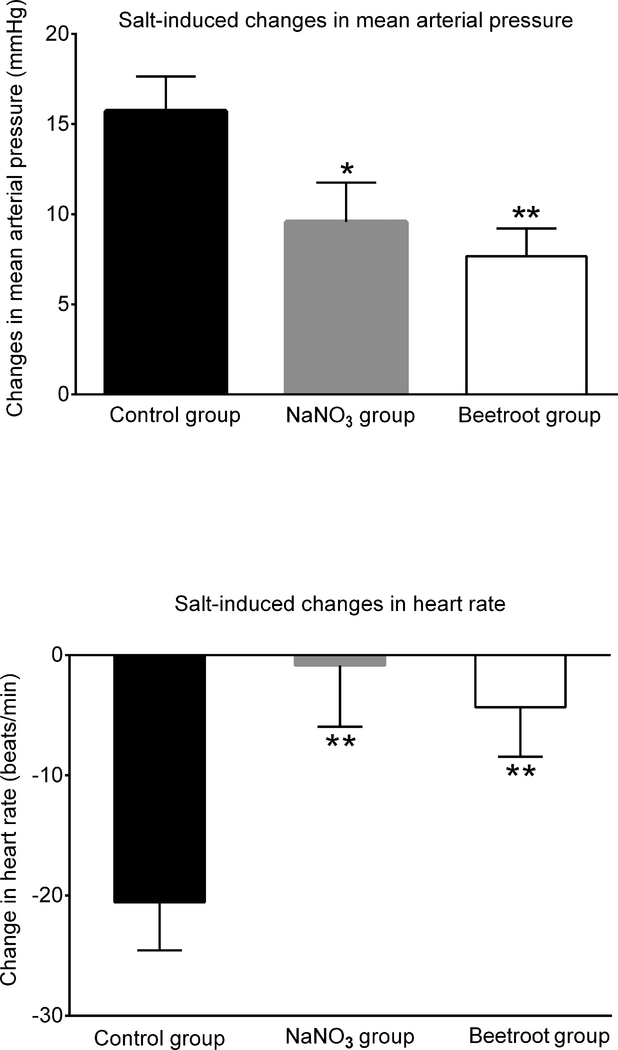
Figure 1 presents the time course of 24-hour averages of systolic arterial pressure (panel A) and diastolic arterial pressure (panel B) for the 3 experimental groups. Salt-loading rapidly induced substantial increases in arterial pressure that were attenuated by treatment with sodium nitrate or beetroot. Administration of sodium nitrate in a molar ratio of added nitrate to added salt of ~1:170 significantly protected against salt-induced increases in systolic arterial pressure as did administration of beetroot and salt which provided a nitrate to salt ratio of ~1:110 (Figure 1, panel C). Administration of sodium nitrate or beetroot also attenuated salt-induced increases in diastolic blood pressure, but after adjustment for multiple comparisons, the effect achieved statistical significance only in the beetroot-treated group (Figure 1, panel D). Salt-induced increases in mean arterial pressure were significantly attenuated by treatment with either sodium nitrate or beetroot (Figure 2, top panel). Based on these results showing that a very small molar ratio of added nitrate to added salt provides significant protection against salt-induced increases in mean arterial pressure, the null hypothesis is rejected.
Figure 1.
Effects of supplemental sodium nitrate or beetroot on salt-induced increases in blood pressure (BP). A. Time course of 24-hour averages of systolic arterial pressure. B. Time course of 24-hour averages of diastolic arterial pressure. C. Mean changes in systolic arterial pressure induced by salt loading. D. Mean changes in diastolic arterial pressure induced by salt loading. The salt-induced changes in arterial pressure were determined by subtracting the average results over the last 3 days on the low salt diet from the average results over the last 3 days on the high salt diet. Statistical analysis of the salt-induced changes in blood pressure was performed by ANOVA with Holm Sidak testing to adjust for multiple comparisons against the control group. * denotes adjusted P <0.05 compared to the salt-loaded control group.
Figure 2.
Changes in 24-hour averages for mean arterial pressure and heart rate induced by salt-loading. Top panel: Changes in mean arterial pressure induced by salt loading. Bottom panel: Changes in heart rate induced by salt loading. The salt-induced changes in mean arterial pressure and heart rate were determined by subtracting the average results over the last 3 days on the low salt diet from the average results over the last 3 days on the high salt diet. Statistical significance was determined by ANOVA with Holm Sidak testing to adjust for multiple comparisons. * denotes adjusted P <0.05 compared to the salt-loaded control group. ** denotes adjusted P< 0.025 compared to the salt-loaded control group. The salt-induced changes in mean arterial pressure were 15.7 ± 1.9 mmHg in the control group, 9.5 ± 2.1 mmHg in the sodium nitrate group, and 7.6 ± 1.5 mmHg in the beetroot group.
In the control rats (no treatment with either sodium nitrate or beetroot), salt-loading induced small reductions in heart rate perhaps mediated by arterial baroreflex responses to the substantial salt-induced increases in blood pressure. Specifically, in the control rats, the salt-induced decrease in heart rate, - 21 ± 4 beats per min, was significantly greater than in sodium nitrate-treated rats, −1 ± 5 beats per min, and beetroot-treated rats, −4 ± 5 beats per min (both P <0.025 compared to control) (Figure 2, bottom panel). Salt-loading had no significant effect on locomotor activity in any of the groups.
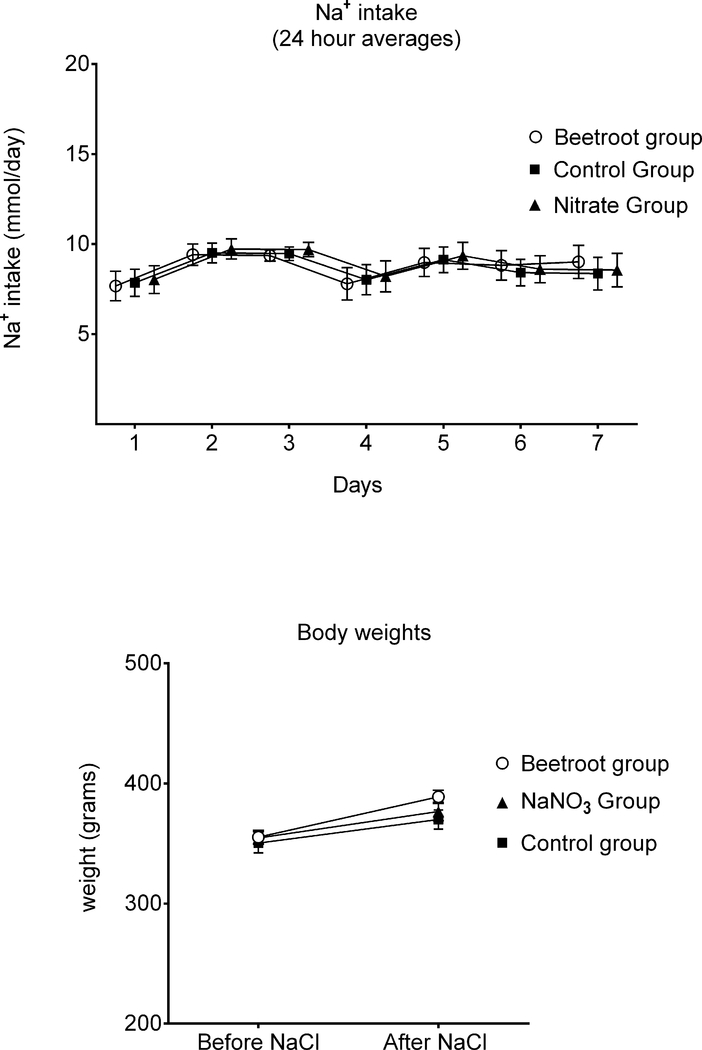
The total intake of nitrate provided by the drinking fluids averaged 0.016 ± 0.0005 mmol/day in the control group, 0.066 ± 0.0019 mmol/day in the sodium nitrate group, and 0.095 ± 0.0029 mmol/day in the beetroot group. Figure 3 (top panel) shows that the different groups of rats consumed similar amounts of sodium throughout salt loading. The rats treated with sodium nitrate or beetroot did not gain less weight than the control rats (Figure 3, bottom panel). Thus, in the sodium nitrate group and the beetroot group, the protection against salt-induced increases in blood pressure was not related to lower salt intake or body weight. If anything, the rats in group 1 (salt + beetroot) gained more weight than those in group 2 (salt + sodium nitrate) or those in group 3 (controls treated with salt alone).
Figure 3.
Sodium intakes and body weights before and after salt-loading. Top panel: Daily sodium intakes were nearly identical in all groups throughout salt loading. Bottom panel: Body weights before and after salt loading were similar among the groups. Greater blood pressure in the salt-loaded control rats was not associated with greater sodium intake or greater body weight in salt-loaded control rats.
To test the effects of sodium nitrate supplementation on sodium balance, we conducted a separate metabolic study in which rats treated with salt plus sodium nitrate were compared with rats treated with salt alone. The amount of sodium retained by rats given salt plus sodium nitrate was significantly greater than that retained by rats given salt alone (Figure S1). This observation suggests that the capacity of nitrate to attenuate salt-induced increases in blood pressure does not necessarily require the attenuation of salt-induced increases in sodium balance. As expected, urinary excretion of nitrate was greater in rats treated with sodium nitrate and sodium chloride than control rats treated with sodium chloride alone (online supplemental methods).
Discussion
In the most widely studied animal model of spontaneous salt sensitivity, we found that supplemental dietary sodium nitrate confers significant and substantial protection from the pressor effects of increased salt intake when the molar ratio of added nitrate to added salt is only ~ 1:170. Furthermore, provision of a low molar ratio of added nitrate to added salt of ~ 1:110 by supplementing the diet with beetroot and a large amount of salt, also conferred significant and substantial protection against salt-induced increases in blood pressure. Beetroot treatment reduced the effect of salt-loading on mean arterial pressure by 60%. To our knowledge, no other dietary ingredient has been identified that can provide this degree of protection against salt-induced increases in blood pressure when added to the diet in such low molar amounts relative to that of added salt. The present findings suggest that the greater amount of nitrate provided in the original DASH diet (~ 160 mg/day)6 compared to that provided in a typical American-style diet (~100 mg/day)14, a difference of just 1 mmol/day, may be sufficient to substantially account for the capacity of the DASH diet to protect against the pressor effects of a 100 mmol increase in salt intake (from 50 mmol/day to 150 mmol/day).12
To put the current findings in perspective, it is instructive to consider the capacity of supplemental dietary potassium to protect against the pressor effects of salt loading. In classic studies conducted in salt sensitive rats over 40 years ago, Dahl and colleagues found that a molar ratio of added dietary potassium to added dietary salt of ~ 1:1 was required for supplemental potassium to strongly protect against the pressor effects of a large increase in salt intake.15 In the present study, we found that a much lower molar ratio of added dietary nitrate to added dietary salt in the range of ~ 1:170 to 1:110 affords substantial protection against the pressor effects of a large increase in salt intake. These findings suggest that on a molar basis or a weight basis, dietary nitrate may be ~ 100 times more potent than dietary potassium with respect to providing substantial protection from the pressor effects of increased salt intake.
Various medical and governmental authorities including the World Health Organization (WHO) have issued guidelines recommending high intakes of potassium and low intakes of salt that provide molar ratios of dietary potassium to salt of ~ 1:1 or greater.16–19 However, only small fractions of populations in the United States and around the world are meeting joint guidelines on potassium intake and salt intake.20, 21 Because substantial changes in dietary habits are required to meet the joint guidelines, the feasibility of achieving the recommended molar ratios of potassium intake to salt intake has come into question.20, 21 In addition, while the DASH diet provides substantial protection against salt-induced increases in blood pressure, sustained adherence to the DASH diet can be challenging.22–25 Thus, additional strategies for preventing salt-induced hypertension, beyond the use of potassium supplementation/salt restriction, and or the DASH diet, would be of considerable medical interest. For individuals who are unable or unwilling to adequately reduce salt intake or follow the original DASH diet, increasing daily intake of inorganic nitrate by ~ 1 mmol, a relatively small amount that can be safely provided by just 2 additional ounces of a raw, nitrate-rich vegetable,8 may afford significant protection against salt-induced increases in blood pressure. This amount of nitrate is well within the WHO limit for the acceptable daily intake of nitrate (~ 262 mg/day for a 70 kg human), and the reference dose for nitrate set by the U.S. Environmental Protection Agency (~490 mg/day for a 70 kg human).26–28
We found that small amounts of added dietary nitrate can substantially protect against the pressor effects of relatively large amounts of added dietary salt without reducing salt consumption or weight gain. In addition, we found that administration of sodium nitrate did not attenuate salt-induced increases in sodium balance (Figure S1). Thus, the current findings suggest that the capacity of dietary nitrate to protect against salt-induced increases in blood pressure is not mediated by reductions in salt intake, sodium balance, or body weight. An extensive body of evidence has indicated that the blood pressure lowering effects of supplemental dietary nitrate may be largely mediated by increases in NO activity.7 As discussed by Carlstrom and colleagues, NO can be endogenously generated by reduction of nitrite derived from dietary or non-dietary sources of nitrate.7 Based on the studies of Gao and colleagues, it appears that the renal microvasculature may be a primary target for blood pressure regulation by nitrite and nitrate because preglomerular resistance vessels are particularly sensitive to the capacity of nitrite to promote vasodilation and to inhibit vasoconstriction induced by angiotensin II.29 Thus, NO-mediated renal vasodilation and associated reductions in renal vascular resistance may help protect against salt-induced increases in blood pressure.30, 31 However, it has also been proposed that NO enhances renal “pressure natriuresis” and sodium excretion32 which may limit salt-induced increases in blood volume, cardiac output, and blood pressure. The usual mechanistic pathways through which changes in NO activity and other factors protect against salt-induced hypertension are controversial and are discussed in detail elsewhere.30–35
The present findings are consistent with the results of seminal studies by Chen and Sanders over 25 years ago in which they found that in Dahl SS rats, oral administration of large amounts of L-arginine, the substrate for enzymatic generation of nitric oxide by NO synthase, prevents salt-induced hypertension.36 However, the use of L-arginine, or its precursor L-citrulline, for regulating blood pressure in humans has gained limited traction possibly due, in part, to the relatively large doses required to achieve an antihypertensive effect (~4,000 to 10,000 mg/day).37, 38 The utility of these agents could also be limited because in patients with endothelial dysfunction, the capacity to convert L-arginine to nitric oxide may be impaired.39 Moreover, as noted by Higashi and colleagues, the ability of L-arginine to increase endothelial NO synthesis appears to be attenuated by a high salt diet.40 In contrast to supplemental L-arginine or L-citrulline, the antihypertensive effects of supplemental inorganic nitrate do not depend on NO synthase activity, and much lower amounts of nitrate may be sufficient to substantially reduce the risk for salt-induced hypertension. Recent studies by Chien et al suggest that in spontaneously hypertensive rats (SHR) consuming normal rat chow, oral administration of sodium nitrate in amounts sufficient to provide a molar ratio of nitrate to salt of ~ 1:10 may attenuate the development of increased blood pressure.41 However, it remains to be determined if the much lower ratios of nitrate to salt used in the current study will also attenuate development of spontaneous hypertension in SHR consuming a normal salt diet.
Prevention Versus Reversal of Hypertension
In the present study, we focused on using very small molar ratios of added nitrate to added salt to attenuate the initiation of salt-induced hypertension. The current observations are not intended to imply that small amounts of supplemental nitrate or nitrate-rich vegetables are likely to be sufficient for reducing blood pressure in individuals with established hypertension. As noted by Sanders and colleagues in their studies in Dahl SS rats, supplemental arginine was effective in attenuating salt-induced increases in blood pressure but not in reversing established hypertension.36 Similarly, we observed that while supplemental intake of a relatively small amount of nitrate relative to salt can provide substantial protection from initiation of salt-induced hypertension, it did not attenuate or reverse the course of salt-induced hypertension that was already well underway (Figure S2). It is possible that larger doses of nitrate are required for reversal of hypertension than for prevention of salt-induced hypertension. In rats with established renal artery hypertension, Montenegro and colleagues reported that administration of large amounts of nitrite in the drinking water (5 – 50 mmol/l sodium nitrite) can decrease blood pressure.42 In the randomized, placebo-controlled trial conducted in the United Kingdom (UK) by Kapil and colleagues in humans with established hypertension,11 supplemental intake of a relatively large amount of nitrate (~ 4 times more than the usual dietary intake of 100 mg/day) was used to reduce blood pressure.11 Assuming the normal subjects in the study of Kapil et al ingested the average amount of salt consumed in the UK (~ 9 grams/day),43 the total amount of nitrate ingested provided a molar ratio of nitrate to salt of ~ 1:20. Interestingly, in some subjects consuming a DASH-style diet, intake of nitrate may be very high and exceed 1000 mg/day, depending on the extent to which nitrate-rich vegetables are incorporated into the diet.5
Limitations
The present studies investigated whether a very low molar ratio of added dietary nitrate to added dietary salt protects against the pressor effects of short-term salt loading and did not involve testing with long-term salt loading. However, the studies of Carlstrom and colleagues and Chen and colleagues indicate that protection by supplemental nitrate or arginine against the pressor effects of short-term salt loading is predictive of protection by these agents against the pressor effects of long-term salt loading.13, 44 In addition, the current studies focused on testing the antihypertensive effects of a very low ratio of added nitrate to added salt and did not investigate the effects of other ratios of added nitrate to salt. In future studies, it would be of interest to test the effects of additional ratios of nitrate to salt on salt-induced changes in blood pressure in a variety of animal models of salt sensitivity and in salt sensitive humans. Although extrapolation of findings in animal models to humans should always be done with caution, it should be noted that the Dahl SS rat is considered to be an excellent model of human salt sensitivity and shows blood pressure responses to dietary interventions consistent with observations made in clinical studies. 15, 45–47
The results of the present studies are consistent with the view that the antihypertensive effects of beetroot may largely be mediated by nitrate. However, it should be recognized that beetroot also contains a variety of other compounds that could be influencing blood pressure including flavonoids, phenolic acids and amides, carotenoids, betacyanin, betaxanthin, ascorbic acid, and potassium.48 With respect to potassium, the amount of potassium added by the beetroot in the current studies was very small relative to the amount of added salt and is unlikely to account for the protection against salt-induced increases in blood pressure.15 In addition, in humans with hypertension, it has been reported that nitrate-depleted beetroot juice has relatively little or no effect on blood pressure compared to beetroot juice that has not been depleted of nitrate.11 While these observations suggest that nitrate may be necessary for the beneficial effects of beetroot on blood pressure, we cannot exclude the possibility that other ingredients may also be influencing the capacity of beetroot to protect against salt-induced hypertension. Finally, it should be pointed out that natural products such as beetroot can vary considerably in their biochemical makeup (including nitrate levels) depending on the cultivar type, soil conditions, time of harvesting, use of fertilizers, etc.49 Thus, when studying beetroot or other nitrate-rich vegetable ingredients, it is important that investigators check the nitrate levels to insure consistency of the products and to consider the use of relevant control groups in their experiments (e.g., groups treated specifically with a nitrate salt itself and or groups treated with a nitrate-depleted product).
Perspectives and Conclusions
Over the past few decades, medical and governmental authorities worldwide have called for extensive efforts to reduce salt intake at the population level with the hope that such efforts would reduce the risk for salt-induced hypertension and related cardiovascular diseases.50–54 In most countries, however, average salt intakes have remained well-above recommended targets, and in some population subgroups (e.g., hypertensive subjects in the USA), salt intake appears to have increased.43, 53, 55, 56 In addition, prominent scientists continue to raise questions about the wisdom of attempts to reduce salt intake in the population as a whole.57–62 Thus, new approaches to reducing the risk for salt-induced hypertension are of considerable medical and scientific interest.
The results of the present study indicate that fortifying salty food products with surprisingly small amounts of vegetable products naturally rich in inorganic nitrate may provide a safe and simple strategy for reducing the risk for salt-induced hypertension. Because relatively low concentrations of an added vegetable concentrate could provide the desired amount of nitrate, this approach might be implemented in many cases without affecting the taste or physical properties of the salty food product of interest, and without requiring a change in eating habits. For example, soy sauces, including “reduced sodium” soy sauces, typically have very high concentrations of salt ranging from ~ 10 – 15% and in Japan, soy sauce is the major source of salt in the diet.63, 64 Given that vegetables such as beetroot or arugula can have very high concentrations of inorganic nitrate, the desired molar ratio of nitrate to salt in soy sauce could be achieved by adding a few grams of an appropriate vegetable concentrate to 100 mL of soy sauce. In other sauces and condiments that contain less salt than soy sauce, even smaller amounts of a nitrate-rich vegetable product could be added to achieve the desired ratio of nitrate to sodium chloride. This approach need not interfere with conventional strategies for reducing the risk for salt-induced hypertension and would not require a change in dietary habits or the need to purchase and consume a salt substitute or a separate dietary supplement.
Supplementary Material
Novelty and Significance.
What Is New?
In the Dahl salt sensitive rat, a widely used model of salt-induced hypertension, a very low molar ratio of added dietary nitrate to added dietary salt of ~ 1:170, affords substantial protection against the pressor effects of a large increase in salt intake.
This finding suggests that on a molar basis and on a weight basis, dietary nitrate may be ~ 100 times more potent than dietary potassium with respect to providing substantial resistance to the pressor effects of increased salt intake.
What Is Relevant?
The present results are relevant for identifying new strategies for the prevention of salt-induced hypertension that may not require restricting salt intake or making major changes in dietary habits.
Summary
A small increase in dietary inorganic nitrate can substantially protect against salt-induced increases in blood pressure. This finding suggests that fortification of salty food products with small amounts of a nitrate-rich vegetable ingredient may provide a safe and simple method for reducing risk for salt-induced hypertension.
Acknowledgments
Sources of funding
National Center for Research Resources, M0 RR-00079, US Public Health Service; National Institutes of Health/National Heart, Lung and Blood Institute grant RO1-HL64230; Praemium Academiae award of the Czech Academy of Sciences to MP; and gifts from the Saw Island Foundation, the Antel Foundation, and the Maier Family Foundation.
Footnotes
Disclosures
T. W. Kurtz is a board member and stockholder of Mission Salt, Inc. that has filed patent applications for salty food compositions which contain nitrate-rich vegetable ingredients.
References
- 1.Lundberg JO, Feelisch M, Bjorne H, Jansson EA, Weitzberg E. Cardioprotective effects of vegetables: is nitrate the answer? Nitric Oxide. 2006;15:359–362. [DOI] [PubMed] [Google Scholar]
- 2.Gee LC, Ahluwalia A. Dietary Nitrate Lowers Blood Pressure: Epidemiological, Pre-clinical Experimental and Clinical Trial Evidence. Curr Hypertens Rep. 2016;18:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khatri J, Mills CE, Maskell P, Odongerel C, Webb AJ. It is Rocket Science - Why dietary nitrate is hard to beet! Part I: Twists and turns in the realisation of the nitrate-nitrite-NO pathway. Br J Clin Pharmacol. 2017;83:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills CE, Khatri J, Maskell P, Odongerel C, Webb AJ. It is rocket science - why dietary nitrate is hard to Beet! part II: further mechanisms and therapeutic potential of the nitrate-nitrite-NO pathway. Br J Clin Pharmacol. 2017;83:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10. [DOI] [PubMed] [Google Scholar]
- 6.Keller RM, Beaver L, Prater MC, Hord NG. Dietary Nitrate and Nitrite Concentrations in Food Patterns and Dietary Supplements. Nutrition Today. 2017; doi: 10.1097/NT.0000000000000253 [DOI] [Google Scholar]
- 7.Carlstrom M, Lundberg JO, Weitzberg E. Mechanisms underlying blood pressure reduction by dietary inorganic nitrate. Acta Physiol (Oxf). 2018:e13080. [DOI] [PubMed] [Google Scholar]
- 8.Lidder S, Webb AJ. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br J Clin Pharmacol. 2013;75:677–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahluwalia A, Gladwin M, Coleman GD, Hord N, Howard G, Kim-Shapiro DB, Lajous M, Larsen FJ, Lefer DJ, McClure LA, Nolan BT, Pluta R, Schechter A, Wang CY, Ward MH, Harman JL. Dietary Nitrate and the Epidemiology of Cardiovascular Disease: Report From a National Heart, Lung, and Blood Institute Workshop. J Am Heart Assoc. 2016;5: e003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, Karanja N, Lin PH, Group DA-SCR. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 13.Carlstrom M, Persson AE, Larsson E, Hezel M, Scheffer PG, Teerlink T, Weitzberg E, Lundberg JO. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc Res. 2011;89:574–585. [DOI] [PubMed] [Google Scholar]
- 14.Gangolli SD, van den Brandt PA, Feron VJ, Janzowsky C, Koeman JH, Speijers GJ, Spiegelhalder B, Walker R, Wisnok JS. Nitrate, nitrite and N-nitroso compounds. Eur J Pharmacol. 1994;292:1–38. [DOI] [PubMed] [Google Scholar]
- 15.Dahl LK, Leitl G, Heine M. Influence of dietary potassium and sodium-potassium molar ratios on the development of salt hypertension. J.Exp.Med. 1972;136:318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute of Medicine (U.S.). Panel on Dietary Reference Intakes for Electrolytes and Water DRI, dietary reference intakes for water, potassium, sodium, chloride, and sulfate. Washington, D.C.: National Academies Press; 2005. [Google Scholar]
- 17.US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 2015–2020. https://health.gov/dietaryguidelines/2015/guidelines/ (accessed September 24, 2018).
- 18.World Health Organization. WHO issues new guidance on dietary salt and potassium. http://www.who.int/mediacentre/news/notes/2013/salt_potassium_20130131/en/ (accessed September 2018).
- 19.Whelton PK. Sodium and Potassium Intake in US Adults. Circulation. 2018;137:247–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drewnowski A, Maillot M, Rehm C. Reducing the sodium-potassium ratio in the US diet: a challenge for public health. The American Journal of Clinical Nutrition. 2012;96:439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drewnowski A, Rehm CD, Maillot M, Mendoza A, Monsivais P. The feasibility of meeting the WHO guidelines for sodium and potassium: a cross-national comparison study. BMJ Open. 2015;5: e006625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168:308–314. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Andrade FCD. Diagnostic status of hypertension on the adherence to the Dietary Approaches to Stop Hypertension (DASH) diet. Preventive Medicine Reports. 2016;4:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwan MW, Wong MC, Wang HH, Liu KQ, Lee CL, Yan BP, Yu CM, Griffiths SM. Compliance with the Dietary Approaches to Stop Hypertension (DASH) diet: a systematic review. PLoS One. 2013;8:e78412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein DE, Sherwood A, Smith PJ, Craighead L, Caccia C, Lin P-H, Babyak MA, Johnson JJ, Hinderliter A, Blumenthal JA. Determinants and Consequences of Adherence to the DASH Diet in African American and White Adults with High Blood Pressure: Results from the ENCORE Trial. Journal of the Academy of Nutrition and Dietetics. 2012;112:1763–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hord NG, Conley MN. Regulation of Dietary Nitrate and Nitrite: Balancing Essential Physiological Roles with Potential Health Risks In: Bryan NS, Loscalzo J, eds. Nitrite and Nitrate in Human Health and Disease. 2nd ed. Cham: Springer International Publishing; 2017:153–162. [Google Scholar]
- 27.Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services. Nitrate and nitrite toxicity. What are U.S. standards and regulations for nitrates and nitrites exposure? https://www.atsdr.cdc.gov/csem/csem.asp?csem=28&po=8 (accessed September 25, 2018).
- 28.European Food Safety Authority. Nitrate in vegetables: scientific opinion of the panel on contaminants in the food chain. European Food Safety Authority Journal. 2008;689:1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao X, Yang T, Liu M, Peleli M, Zollbrecht C, Weitzberg E, Lundberg JO, Persson AE, Carlstrom M. NADPH oxidase in the renal microvasculature is a primary target for blood pressure-lowering effects by inorganic nitrate and nitrite. Hypertension. 2015;65:161–170. [DOI] [PubMed] [Google Scholar]
- 30.Kurtz TW, DiCarlo SE, Pravenec M, Schmidlin O, Tanaka M, Morris RC. An alternative hypothesis to the widely held view that renal excretion of sodium accounts for resistance to salt-induced hypertension. Kidney Int. 2016;90:965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurtz TW, DiCarlo SE, Pravenec M, Morris RC Jr. The pivotal role of renal vasodysfunction in salt sensitivity and the initiation of salt-induced hypertension. Curr Opin Nephrol Hypertens. 2018;27:83–92. [DOI] [PubMed] [Google Scholar]
- 32.Granger JP, Alexander BT. Abnormal pressure-natriuresis in hypertension: role of nitric oxide. Acta Physiol Scand. 2000;168:161–168. [DOI] [PubMed] [Google Scholar]
- 33.Hall JE. Renal dysfunction, rather than non-renal vascular dysfunction, mediates salt-induced hypertension. Circulation. 2016;133:894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris RC, Schmidlin O, Sebastian A, Tanaka M, Kurtz TW. Vasodysfunction that involves renal vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt-induced hypertension. Circulation. 2016;133:881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manning RD Jr., Hu L, Tan DY, Meng S. Role of abnormal nitric oxide systems in salt-sensitive hypertension. Am J Hypertens. 2001;14:68s–73s. [DOI] [PubMed] [Google Scholar]
- 36.Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88:1559–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siani A, Pagano E, Iacone R, Iacoviello L, Scopacasa F, Strazzullo P. Blood pressure and metabolic changes during dietary L-arginine supplementation in humans. Am J Hypertens. 2000;13:547–551. [DOI] [PubMed] [Google Scholar]
- 38.Mahboobi S, Tsang C, Rezaei S, Jafarnejad S. Effect of l-citrulline supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Journal of Human Hypertension. 2019; 33: 10–21. [DOI] [PubMed] [Google Scholar]
- 39.Zand J, Lanza F, Garg HK, Bryan NS. All-natural nitrite and nitrate containing dietary supplement promotes nitric oxide production and reduces triglycerides in humans. Nutr Res. 2011;31:262–269. [DOI] [PubMed] [Google Scholar]
- 40.Higashi Y, Oshima T, Watanabe M, Matsuura H, Kajiyama G. Renal response to L-arginine in salt-sensitive patients with essential hypertension. Hypertension. 1996;27:643–648. [DOI] [PubMed] [Google Scholar]
- 41.Chien SJ, Lin KM, Kuo HC, Huang CF, Lin YJ, Huang LT, Tain YL. Two different approaches to restore renal nitric oxide and prevent hypertension in young spontaneously hypertensive rats: l-citrulline and nitrate. Translational research: the journal of laboratory and clinical medicine. 2014;163:43–52. [DOI] [PubMed] [Google Scholar]
- 42.Montenegro MF, Amaral JH, Pinheiro LC, Sakamoto EK, Ferreira GC, Reis RI, Marcal DM, Pereira RP, Tanus-Santos JE. Sodium nitrite downregulates vascular NADPH oxidase and exerts antihypertensive effects in hypertension. Free Radic Biol Med. 2011;51:144–152. [DOI] [PubMed] [Google Scholar]
- 43.Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, Engell RE, Lim SS, Danaei G, Mozaffarian D, Global Burden of Diseases N, Chronic Diseases Expert G. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen PY, St.John PL, Kirk KA, Abrahamson DR, Sanders PW. Hypertensive nephrosclerosis in the Dahl/Rapp rat: Initial sites of injury and effect of dietary L-arginine supplementation. 1993;68:174–184. [PubMed] [Google Scholar]
- 45.Adrogue HJ, Madias NE. The impact of sodium and potassium on hypertension risk. Semin Nephrol. 2014;34:257–272. [DOI] [PubMed] [Google Scholar]
- 46.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, Cowley AW Jr. Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiol Genomics. 2004;16:194–203. [DOI] [PubMed] [Google Scholar]
- 47.Abais-Battad JM, Mattson DL. The Influence of dietary protein on Dahl salt-sensitive hypertension: a potential role for gut microbiota. Am J Physiol Regul Integr Comp Physiol. 2018. doi: 10.1152/ajpregu.00399.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chhikara N, Kushwaha K, Sharma P, Gat Y, Panghal A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food chemistry. 2019;272:192–200. [DOI] [PubMed] [Google Scholar]
- 49.Keeton JT. History of nitrite and nitrate in food In: Bryan NS, Loscalzo J, eds. Nitrite and Nitrate in Human Health and Disease. 2nd ed. Cham: Springer International; 2017:85–97. [Google Scholar]
- 50.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. [DOI] [PubMed] [Google Scholar]
- 51.Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW Jr., Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000;102:2284–2299. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization. Diet, Nutrition and the Prevention of Chronic Disease Report of a Joint WHO/FAO Expert Consultation. Geneva: WHO, 2003. [Google Scholar]
- 53.Barberio AM, Sumar N, Trieu K, Lorenzetti DL, Tarasuk V, Webster J, Campbell NRC, McLaren L. Population-level interventions in government jurisdictions for dietary sodium reduction: a Cochrane Review. International Journal of Epidemiology. 2017;46:1551–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell NR, Lackland DT, Chockalingam A, Lisheng L, Harrap SB, Touyz RM, Burrell LM, Ramirez AJ, Schmieder RE, Schutte AE, Prabhakaran D, Schiffrin EL. The International Society of Hypertension and World Hypertension League call on governments, nongovernmental organizations and the food industry to work to reduce dietary sodium. J Hypertens. 2014;32:446–447. [DOI] [PubMed] [Google Scholar]
- 55.Dolmatova EV, Moazzami K, Bansilal S. Dietary sodium intake among US adults with hypertension, 1999–2012. J Hypertens. 2018;36:237–242. [DOI] [PubMed] [Google Scholar]
- 56.Meyer KA, Harnack LJ, Luepker RV, Zhou X, Jacobs DR, Steffen LM. Twenty-two-year population trends in sodium and potassium consumption: the Minnesota Heart Survey. J Am Heart Assoc. 2013;2:e000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mente A, O’Donnell M, Rangarajan S, McQueen M, Dagenais G, Wielgosz A, Lear S, Ah STL, Wei L, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Mony P, Szuba A, Iqbal R, Yusuf R, Mohammadifard N, Khatib R, Yusoff K, Ismail N, Gulec S, Rosengren A, Yusufali A, Kruger L, Tsolekile LP, Chifamba J, Dans A, Alhabib KF, Yeates K, Teo K, Yusuf S. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: a community-level prospective epidemiological cohort study. Lancet. 2018;392:496–506. [DOI] [PubMed] [Google Scholar]
- 58.Mente A, O’Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Li W, Lu Y, Yi S, Rensheng L, Iqbal R, Mony P, Yusuf R, Yusoff K, Szuba A, Oguz A, Rosengren A, Bahonar A, Yusufali A, Schutte AE, Chifamba J, Mann JF, Anand SS, Teo K, Yusuf S. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388:465–475. [DOI] [PubMed] [Google Scholar]
- 59.Graudal N, Hubeck-Graudal T, Jurgens G, McCarron DA. The significance of duration and amount of sodium reduction intervention in normotensive and hypertensive individuals: a meta-analysis. Adv Nutr. 2015;6:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stolarz-Skrzypek K, Staessen JA. Reducing salt intake for prevention of cardiovascular disease--times are changing. Adv Chronic Kidney Dis. 2015;22:108–115. [DOI] [PubMed] [Google Scholar]
- 61.Alderman MH. Dietary Sodium: Where Science and Policy Diverge. Am J Hypertens. 2016;29:424–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graudal N, Jurgens G. Conflicting Evidence on Health Effects Associated with Salt Reduction Calls for a Redesign of the Salt Dietary Guidelines. Prog Cardiovasc Dis. 2018;61:20–26. [DOI] [PubMed] [Google Scholar]
- 63.Okuda N, Okayama A, Miura K, Yoshita K, Saito S, Nakagawa H, Sakata K, Miyagawa N, Chan Q, Elliott P, Ueshima H, Stamler J. Food sources of dietary sodium in the Japanese adult population: the international study of macro-/micronutrients and blood pressure (INTERMAP). Eur J Nutr. 2017;56:1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asakura K, Uechi K, Masayasu S, Sasaki S. Sodium sources in the Japanese diet: difference between generations and sexes. Public Health Nutrition. 2016;19:2011–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.